Devices and chemical sensing applications of metal oxide nanowires
|
|
|
- Leslie Poole
- 6 years ago
- Views:
Transcription
1 APPLICATION Journal of Materials Chemistry Devices and chemical sensing applications of metal oxide nanowires Guozhen Shen,* Po-Chiang Chen, Koungmin Ryu and Chongwu Zhou* Received 22nd September 2008, Accepted 21st October 2008 First published as an Advance Article on the web 20th November 2008 DOI: /b816543b Metal oxide nanowires, with special physical properties, are ideal building blocks for a wide range of nanoscale electronics, optoelectronics, and chemical sensing devices. This article will describe the stateof-the-art research activities in metal oxide nanowire applications. This paper consists of three main sections categorized by metal oxide nanowire synthesis, electronic and optoelectronic devices applications, and chemical sensing applications. Finally, we will conclude this review with some perspectives and outlook on the future developments in the metal oxide nanowire research area. 1. Introduction Due to their special shapes, compositions, chemical and physical properties, one-dimensional (1-D) metal oxide nanostructures are the focus of current research efforts in nanotechnology since they are the commonest minerals in the earth. 1-D metal oxide nanostructures have now been widely used in many areas, such as ceramics, catalysis, sensors, transparent conductive films, electro-optical and electro-chromic devices. 1 5 Intensive studies have been carried out on the synthesis of metal oxide nanowires as well as the exploration of their novel properties. For example, 1-D ZnO nanostructures with many different shapes, such as nanowires, nanobelts, nanotubes, nanorings, and nanosprings, have been prepared using many synthesis s. High-performance chemical sensors have been fabricated on SnO 2, ZnO, and In 2 O 3 nanowires due to their large surface area to volume ratio. Department of Electrical Engineering, University of Southern California, Los Angeles, CA 90089, USA. gzshen@ustc.edu; guozhens@usc. edu; chongwuz@usc.edu; Fax: ; Tel: This paper is part of a Journal of Materials Chemistry theme issue on Nanotubes and Nanowires. Guest editor: Z. L. Wang. This article will provide a comprehensive review of the stateof-the-art research activities focused on devices and chemical sensing applications of metal oxide nanowires, and can be divided into three main sections. The first section briefly introduces two synthesis strategies, which include top-down approaches and bottom-up approaches, with the focus on bottom-up approaches, for the synthesis of metal oxide nanowires. Next, some important electronic and optoelectronic devices built on metal oxide nanowires are presented, which include field-effect transistors (FETs), transparent electronics, lasers and waveguide, nanogenerators, solar cells and photocatalysts, and field nanoemitters. In the third part, we will discuss recent developments in the chemical sensing area of metal oxide nanowires. The review will then conclude with some perspectives and outlook on the future developments in the metal oxide nanowire research area. 2. Synthesis of metal oxide nanowires Till now, many s have been developed to synthesize 1-D metal oxide nanostructures. Basically, they can be described as two different types: the top-down approaches and the bottom-up approaches. In this section, we will briefly discuss Dr Guozhen Shen received his Ph.D. degree in Chemistry from University of Science and Technology of China in He conducted his postdoctoral research at Hanyang University, Korea in 2004 and then joined National Institute for Materials Science, Japan as a visiting researcher. Currently, he is a research scientist in University of Southern California. He is the author or co-author of more Dr Guozhen Shen than 100 research articles and 5 book chapters. His most recent research interests include the synthesis and characterization of onedimensional nanostructures and their device applications in electronics and optoelectronics. Po-Chiang Chen Po-Chiang Chen holds a B.S. degree in Physics and a M.S. in Optoelectronics. He is currently working toward a Ph.D. degree in Chemical Engineering and Materials Science at the University of Southern California. His research focus is on the device applications based on 1-D nanomaterials, including chemical sensors, transparent electronics, and energy conversion and storage devices. 828 J. Mater. Chem., 2009, 19, This journal is ª The Royal Society of Chemistry 2009
2 these two approaches developed to synthesize metal oxide nanowires Top-down synthesis Top-down approaches usually utilize planar, lithographic techniques to transfer a pre-designed pattern to a substrate which can form complex high density structures in well-defined positions on substrates. 6,7 For example, Im et al. 6 synthesized ZnO nanowires using a complicated nanoscale spacer lithography, which can be used to detect H 2 and CO gases. Top-down approaches have been widely used in the current microelectronic industry. They can produce nanostructures with very uniform shapes and electronic properties. However, as the microelectronic industry advances towards ever smaller devices, top-down approaches will soon reach their physical and economic limits, which motivates global efforts to search for new strategies to meet the expected demand for increased computational power as well as for integrating low-cost and flexible computing in unconventional environments in the future Bottom-up synthesis The bottom-up approaches, in which functional electronic structures are assembled from chemically synthesized nanoscale building blocks, represent flexible alternatives to conventional top-down s. They can go far beyond the limits of top-down technology in terms of future physical and economic limits. 8,9 Table 1 lists a host of 1-D metal oxide nanostructures grown from bottom-up approaches using different techniques To get 1-D metal oxide nanostructures using the bottom-up approaches, one key concept is to break the growth symmetry of materials. A straightforward to break the symmetry for 1-D growth is the use of hard or soft templates, which may include the edges of surface steps, carbon nanotubes, porous membranes, surfactant, or microemulsions. This is conceptually very simple and has been widely used to prepare a variety of metal oxide nanowires. Despite its simplicity, the template-directed is limited by the fact that the synthesized nanowires are usually polycrystalline, which limits their potential applications in many areas. Another general strategy for the bottom-up synthesis is the use of a catalyst to direct the 1-D growth. According to the phases involved in the reaction, this approach can be defined as either vapor-liquid-solid (VLS) growth, 104 or solution-liquid-solid growth (SLS). 105 Fig. 1a illustrates the schematic of a typical VLS process. During this process, a vapor phase reactant is solubilized by a liquid catalyst particle to form solid wire-like structures. In this process, the catalyst is envisioned as a growth site that defines the diameter of nanowires. According to the reaction system, the VLS process can be divided into thermal evaporation, chemical vapor deposition (CVD), metal-organic chemical vapor deposition (MOCVD), laser-ablation and many others. Fig. 1b and c show SEM and HRTEM images of In 2 O 3 nanowires synthesized from the VLS process using a laser-ablation, which exhibit very good crystallinity and are of single crystal nature. The inset is a TEM image of a typical In 2 O 3 nanowire. The catalyst particle can be clearly seen attached to the top of the nanowire, indicating the VLS growth process. Compared with the VLS process, the SLS process adopts a similar idea except that the reactant comes from solution instead of the vapor phase. Though the vapor-solid (VS) process is not as clearly understood as the VLS process, it has already proved to be a very important to synthesize 1-D metal oxide nanowires. 18,28 During VS growth, no catalyst is used and the nanowires are directly grown on the solid particles. This simple has been widely used to synthesize a host of metal oxide nanowires. For example, by heating the metal oxides in a tube furnace at high templerature, Wang et al. synthesized nanobelts of ZnO, SnO 2, CdO, and Ga 2 O 3. 2 Fig. 2 shows several typical TEM images of the VS grown ZnO nanobelts, which have rectangular cross-sections, different with the nanowires with round crosssections. They usually have thickness of nm and width-tothickness ratios of 5 10 nm, respectively. Koungmin Ryu Koungmin Ryu holds a B.S degree in Metallurgy and a M.S degree in Materials Science and Engineering. He is currently a Ph.D. student at the University of Southern California. His research interest covers carbon nanotube synthesis and applications such as nanotube circuits, chemical sensing, and OLED fabricated by carbon nanotube conductive films. He has published 3 journal papers related to carbon nanotube synthesis and OLED fabrication. Dr Chongwu Zhou received his Ph.D. in electrical engineering from Yale University, and then worked as a postdoctoral research fellow at Stanford University. He joined the faculty at University of Southern California in September His research group has been working at the forefront of nanoscience and nanotechnology, including synthesis and applications of carbon nanotubes and nanowires, biosensing, and nano- Dr Chongwu Zhou therapy. He has won a number of awards, including the NSF CAREER Award, the NASA TGiR Award, the USC Junior Faculty Research Award, and the IEEE Nanotechnology Early Career Award. He is currently an Associate Editor for IEEE Transactions on Nanotechnology. This journal is ª The Royal Society of Chemistry 2009 J. Mater. Chem., 2009, 19,
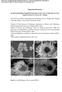
3 Table 1 Summary of 1-D metal oxide nanostructures synthesized using different s Materials Morphology Growth Ref. ZnO Nanowires Vapor-solid 1 Vapor-liquid-solid 10 AAO template-assisted 11 Microemulsion 12 Template-free solution 13 Nanobelts Vapor-solid 2 Vapor-liquid-solid 14 Hydrothermal 15 Nanorods Template-free aqueous 16 Vapor-liquid-solid 17 Vapor-solid 18 Pulsed-laser ablation without catalyst 19 Nanotubes Vapor-solid 20 Vapor-liquid-solid 21 Solution 22 SnO 2 Nanorods Microemulsion 23 Hydrothermal 24 Solution 25 Vapor-liquid-solid 26 Nnanowires Catalyst-assisted laser ablation 27 Vapor-solid 28 Vapor-liquid-solid 29 Solution 3 Nanobelts Thermal oxidation 30 Vapor-solid 31 Laser-ablation 32 Nanotubes Templates hydrothermal 33 Aqueous solution 34 Microemulsion 35 In 2 O 3 Nanowires Catalyst-assisted laser-ablation 4 Vapor-solid 36 Vapor-liquid-solid 37 AAO-templated solution 38 Nanobelts Vapor-solid 39 Nanotubes Thermal evaporation 40 Solvothermal 41 Ga 2 O 3 Nanowires Thermal evaporation 42 Catalyst-assisted arc discharge 43 Laser-ablation 44 Catalyst-assisted vapor 45 Nanobelts Vapor-solid 46 Vapor-liquid-solid 47 Nanotubes Vapor-solid 48 WO 3 Nanowires SBA-15 templated solution 49 Thermal evaporation 50 Hydrothermal 51 Nanobelts Vapor-solid 52 V 2 O 5 Nanowires Hydrothermal 53 Nanobelts Vapor-solid 54 MgO Nanowires Vapor-solid 55 Vapor-liquid-solid 56 Catalyst-assisted laser-ablation 57 nanotubes Vapor-liquid-solid 58 TiO 2 Nanotubes AAO-templated solution 59 Solution 60 Nanowires Hydrothermal 61 Vapor-solid 62 ZrO 2 Nanowires AAO-templated solution 63 Nanorods Precursor thermal decomposition 64 Nanotubes AAO-templated solution 65 Nb 2 O 5 Nanobelts Precursor thermal decomposition 66 nanowires Vapor-liquid-solid 67 Nanotubes Precursor thermal decomposition 5 Ta 2 O 5 Nanotubes Precursor thermal decomposition 5 Table 1 (Contd.) Materials Morphology Growth Ref. MoO 3 Nanotubes Hydrothermal 68 Carbon nanotube templated 69 Vapor-solid 70 Nanowires Thermal evaporation 71 Solution 72 MnO 2 Nanowires Hydrothermal 73 SBA-15 templated synthesis 74 Nanotubes Hydrothermal 75 Fe 2 O 3 Nanowires Thermal oxidation 76 Hydrothermal 77 Nanobelts Thermal oxidation 78 Fe 3 O 4 Nanotubes MgO-templated pulsed-laser 79 deposition Nanowires Magnetic-field-induced hydrothermal 80 Co 3 O 4 Nanowires Thermal oxidation 81 Nanowires Hydrothermal 82 Nanotubes Carbon nanotube templated 83 Nanotubes Solution 84 IrO 2 Nanotubes Metal-organic CVD 85 Nanowires Metal-organic CVD 86 NiO Nanowires Wet chemical route 87 AAO-templated sol-gel 88 Nanotubes AAO-assisted solution 89 Cu 2 O Nanowires Solid-state reduction 90 Surfactant-assisted solution 91 Hydrothermal 92 CuO Nanowires Thermal oxidation 93 AAO-templated deposition 94 Solution 95 Nanobelts Solution 95 CdO Nanowires AAO-assisted electrochemical 96 deposition Chemical bath deposition 97 Nanoneedles Vapor-liquid-solid 98 Al 2 O 3 Nanotubes Pulse anodization 99 Thermal evaporation 100 Surfactant-assisted solution 101 Carbon nanotube-assisted growth 102 Nanowires, nanobelts Vapor-solid 103 Fig. 1 (a) Schematic illustrating the growth process of a VLS process. (b) SEM image and (c) TEM image of In 2 O 3 nanowires grown from VLS process. 830 J. Mater. Chem., 2009, 19, This journal is ª The Royal Society of Chemistry 2009
4 Fig. 2 TEM images of ZnO nanobelts grown from the VS process. Reproduced from ref. 2: Science, 2001, 291, Copyright ª 2001, AAA of Science. After growth, the obtained nanowires were characterized using several techniques, such as X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). Detailed description and analysis of these characterization techniques can be found in some recent review papers and will not be discussed here. 106, Electronic and optoelectronic devices built on metal oxide nanowires Driven by the thrust of fabricating smaller devices to create integrated circuits with improved performance, 1-D metal oxide nanostructures have been exploited as potential building blocks for future nanoelectronics. In this section, we will review some recent works on the electronic and optoelectronic devices built on metal oxide nanowires Field effect transistors The basic field effect transistor (FET) structure fabricated from a single metal oxide nanowire is illustrated in Fig. 3. Basically, the FET is supported on an oxidized p-type silicon substrate with the underlying conducting silicon as the back gate electrode to vary the electrostatic potential of the nanowire. Two metal contacts, corresponding to the source and drain electrodes, are defined by either electron beam lithography or photolithography followed by evaporation of suitable metal contacts. Usually, current (I) vs. source drain voltage (V ds ) and current (I) vs. gate voltage (V g ) are recorded to characterize the nanowire FET. Metal oxides are n-type semiconducting materials. So for the typical I V ds curves recorded from metal oxide nanowire FETs, an increase in conductance for V g > 0 and a decrease in conductance for V g < 0 are obtained. n-type FETs have been Fig. 3 Schematic of an oxide nanowire FET. fabricated on various oxide nanowires, including ZnO, In 2 O 3, SnO 2,Cu 2 O, TiO 2, CdO, etc By introducing suitable dopants, FETs with p-type behavior are obtained for several metal oxide nanowires. For example, Wang fabricated p-type FETs using P-doped ZnO nanowires. 113 Lee et al. obtained p-type FETs by using N-doped ZnO nanowires as the building blocks Transparent electronics Transparent electronics acting as an emerging technology for the next generation of optoelectronic devices have attracted numerous research efforts due to thier great potential to make a significant commercial impact in many areas. 115 Metal oxides are well known transparent conductive semiconductor materials. Using metal oxide nanowires as the building blocks, Janes et al. fabricated fully transparent high-performance In 2 O 3 and ZnO nanowire-based FETs on both glass and flexible plastic substrates. 116 Fig. 4a is the cross-section view of fully transparent and flexible device structure. All the components used are transparent. Optical image, transmission spectrum, and I V curves of the device fabricated on a plastic substrate are shown in Fig. 4b d, exhibiting very good transparency, flexibility, and performance. Transparent devices built on metal oxide nanowires will greatly enhance the performance of transparent and flexible display approaches for heads-up displays and printable/lightweight displays embedded within clothing or equipment. For example, Ju et al. also demonstrated the first transparent active matrix organic light emitting diode (AMOLED) displays driven by transparent devices built on In 2 O 3 nanowire Lasers and waveguides ZnO is a good candidate for room temperature UV lasers as its exciton binding energy is approximately 60 mev, significantly larger than those of widely used short-wavelength semiconductor laser materials, ZnSe (22 mev), GaN (25 mev). Fig. 5a and b show the SEM images of vertically aligned ZnO nanowire arrays grown on sapphire substrates by using a catalyst-assisted vapor phase transport process. 118 Typical nanowires have diameters of nm and lengths of several micrometers. According to the emission spectra taken from ZnO nanowire arrays below and above the lasing threshold, these nanowires are promising miniaturized laser light sources and may have myriad This journal is ª The Royal Society of Chemistry 2009 J. Mater. Chem., 2009, 19,
5 Fig. 4 (a) Cross-section view, (b) photograph, (c) optical transmission spectrum and (d) I ds -V gs characteristics of fully transparent and flexible In 2 O 3 nanowire FETs. Reproduced from ref. 116: Nat. Nanotechnology, 2007, 2, 378. Copyright ª 2007, Nature Publishing Group. Fig. 5 (a,b) SEM images of ZnO nanowire arrays grown on sapphire substrates. (c) Emission spectra from nanowire arrays below and above the lasing threshold. Reproduced from ref. 118: Science, 2001, 292, Copyright ª 2001, AAA of Science. applications in optical computing, information storage, and microanalysis (Fig. 5c). Later, lasers were also observed for ZnO nanostructures with other shapes Because of its near cylindrical geometry and large refractive index (2.0), ZnO is also a natural candidate for optical waveguides. Fig. 6 shows the results of Yang et al., where optically pumped light emission guided by a ZnO nanowire and coupled into an SnO 2 nanoribbon can be clearly seen Piezoelectric nanogenerators Energy harvesting from the ambient environment has been an active research field of nanotechnology in recent years. Wang Fig. 6 (a) An optical microscope image of a ZnO nanowire guiding light into a SnO 2 nanoribbon. (b) SEM image displaying the nanowirenanoribbon junction. Reproduced from ref. 122: Science, 2004, 305, Copyright ª 2004, AAA of Science. et al. made great contributions to this field and ZnO nanowire array based piezoelectric nanogenerators have been demonstrated by them to convert mechanical energy to electricity by utilizing the coupling effect of the semiconducting and piezoelectric properties of ZnO Fig. 7a is a typical SEM image of the aligned ZnO nanowire arrays. 127 The nanowire density was controlled to ensure that the AFM tip can exclusively reach one nanowire without touching another nanowire. When the AFM tip scanned over the nanowires, the corresponding output voltage images across the load were recorded simultaneously: sharp, narrow output peaks were observed, as shown in Fig. 7b. From the results shown in Fig. 7, we can see that the energy output by one nanowire in one discharge event is 0.05 fj, and 832 J. Mater. Chem., 2009, 19, This journal is ª The Royal Society of Chemistry 2009
6 Fig. 7 (a) SEM image of aligned ZnO nanowire arrays. (b) Output voltage image of the nanowire arrays. (c) A series of line profiles of the voltage output signal. (d) Line profiles from the topography and output voltage images across a nanowire. (e) Line profile of the voltage output signal when the AFM tip scans across a vertical nanowire at mm/s. (f) The resonance vibration of a nanowire after being released by the AFM tip. Reproduced from ref. 123: Science, 2006, 312, 242. Copyright ª 2006, AAA of Science. the output voltage on the load is 8 mv. By choosing suitable nanowire density, the power generated may be high enough to drive a single nanowire based device. Further exploration of the piezoelectric nanogenerator concept led to the development of a wide variety of ZnO nanowire based piezotronic devices, including piezoelectric field effect transistors, nanoforce sensors, and gate diodes. 126, Solar cells and photocatalysts In recent years, solar energy conversion devices like solar cells, which directly converse sunlight into electricity, have attracted great research interest to solve the continuously increasing energy problems. Metal oxide nanowires acting as the absorbing layers can be used to build high performance solar cells. They also can be used to replace the conventional quantum dot films in dye-sensitized solar cells (DSSCs). Yang et al. built the first DSSC using aligned ZnO nanowires as shown in Fig. 8. A direct conversion efficiency of 1.5% is demonstrated, which is primarily limited by the surface area of ZnO nanowire arrays. 128 Driven by this work, many DSSCs built on different metal oxide nanowires Fig. 8 (A) Schematic diagram of the ZnO nanowire array DSSC. (B) Traces of current density against voltage for two different DSSCs. Inset is a SEM image of aligned ZnO nanowires. Reproduced from ref. 128: Nat. Mater., 2005, 4, 455. Copyright ª 2005, Nature Publishing Group. with different conversion efficiencies were demonstrated and the used metal oxide nanowires include TiO 2 nanorods, CuO nanorods, core/shell ZnO/Al 2 O 3, ZnO/TiO 2, ZnO/ZnSe nanowires, etc Developing semiconductor photocatalysts for water splitting and degradation of organic pollutants provides another way to solve the urgent energy and environmental issues Metal oxide nanowires gained much attention in this direction to be used as high performance photocatalysts due to their extremely enhanced surface areas D TiO 2 nanostructures, nanorods, nanowires and nanotubes, are the mostly investigated oxide nanostructures that can be used as high performance photocatalysts exhibiting better water splitting and organic degradation properties than bulk materials. 138,139 Several other oxide nanowire photocatalysts include ZnO nanowires, SnO 2 nanowires, VO 2 nanowire arrays, and many ternary oxide nanowires, such as SrSnO 4 nanowires, 142 BiVO 4 nanotubes, and AgIn(WO 4 ) 2 nanotubes. 145 With the development of better synthesis approaches to 1-D metal oxide nanostructures, photocatalysts with greatly improved performance are expected to be obtained Field nanoemitters Field emission, also known as Fowler Nordheim tunneling, is a form of quantum tunneling in which electrons pass from an This journal is ª The Royal Society of Chemistry 2009 J. Mater. Chem., 2009, 19,
7 of electrical conductivity due to the interaction of nanowires with the surrounding environment. The charge transfer process induced by the redox reactions between nanowire surface and tested chemicals determines the conductance of nanowire-based chemical sensors. For example, when a reducing gas (eg. CO) is introduced to a chemical sensor, the following reaction happens: 172 CO + O / CO 2 +e Here, CO reacts with adsorbed oxygen ions on the nanowire surface and thus results in an overall increase of the electrical conductance of metal oxide nanowires. On the other hand, if a chemical sensor is exposed to an oxidation gas (eg. NO 2 ), the following oxidizing reaction may take place: 195 Fig. 9 Field emission properties form vertically aligned ZnO nanowires grown on a Si substrate. Reproduced from ref. 147: Chem. Phys. Lett., 2005, 404, 69. Copyright ª 2005, Sciencedirect. emitting material to the anode through a barrier in the presence of a high electric field. It is one of the main features of metal oxide nanowires, and is of great commercial interest in many areas, such as displays and other electronic devices. Using metal oxide nanowires as field nanoemitters was first reported by Lee in They studied the field emission properties of verticallyaligned ZnO nanowires grown via a VS process and found a turnon voltage of 6.0 V/mm at a current density of 0.1 macm 2 (Fig. 9). 147 Inspired by the work using ZnO nanowires as field nanoemitters, field emission properties of many other kinds of metal oxide nanowires were also studied, including WO 3, IrO 2, RuO 2, CuO, TiO 2, SnO 2, In 2 O 3 nanowires Compared with carbon nanotube based emitteors, metal oxide emitters are more stable in harsh environments and controllable in electrical properties. 4. Chemical sensors built on metal oxide nanowires With large surface-to-volume ratios and a Debye length comparable to their dimensions, metal oxide nanowires have showed great potential to be used as chemical sensors. 106 Recently, the detection of a wide range of chemicals with different nanowire sensor configurations has been reported. For instance, Zhang and coworkers fabricated and tested an In 2 O 3 nanowire mat sensor for which a detection limit of 5 ppb was achieved. 166 Table 2 summarizes a list of typical metal oxide nanowire based chemical sensors with different device configurations, working temperatures, detection limits and response times of different targeted chemicals As one can see, chemical sensors built on SnO 2, ZnO, and In 2 O 3 nanowires have been widely reported due to their easy synthesis, good sensitivity to chemicals, and good stability compared to other metal oxide nanomaterials. In addition, in spite of their sensitivity, selectivity of chemical sensors remains one of the challenging issues in this field. The sensing mechanism of metal oxide nanowires has been discussed in recent publications. Briefly, the working principle of metal oxide nanowire-based chemical sensors relies on changes NO 2 +e / NO 2 NO 2 serve as charge accepting molecules and withdraw electrons from the nanowire, resulted in a reduction of electrical conductance. Based on the above mentioned sensing mechanisms, metal oxide nanowire-based chemical sensors are usually fabricated in two configurations, resistors and FET devices with single or multiple nanowire nanowires. In fact, most reported works in this field are based on these two configurations due to easy fabrication, good reliability, low cost, and easy integration with heat transducers. Instead of the sensors measuring the change of electrical conductance, there are several other kinds of sensors, such as photoluminescence (PL) sensors, and nanostructured ZnO coated quartz crystal microbalance (QCM) sensors. However, compared with the electrical chemical sensors, these sensors are complicated and expensive. Below we will discuss chemical sensors following different sensor configurations, i.e. electrical, optical and nanostructure coated QCM gas sensors and electronic noses, each with one or two examples Electrical based chemical sensors A resistor based sensor is one of the easiest s to carry out chemical sensing experiments by measuring the change of conductance of the sensing element in different surrounding environments. Fig. 10a showed a schematic drawing of a transistor based chemical sensor. Nanowires are dispersed on a SiO 2 / Si substrate followed by patterning source and drain electrodes above the dispersed nanowires. The Si substrate serves as a back gate electrode while the chemical sensor works as FET based sensors. To improve the sensitivity and detect inert gases, several groups reported to integrate MEMS hotplates with chemical sensors. 178 For instances, Fig. 10b is a SEM image of the active area of one chemical sensor chip, where the dashed box represents the SiN membrane and one nanowire is bridged by two electrodes which can be used as a chemical sensor as shown in Fig. 10c. The zigzag shaped micromachined hotplates provide a facile way to control elevated temperatures with low power consumption. With the aid of elevated temperatures, the detection limits of this chemical sensor can be enhanced down to 1 ppm for ethanol as shown in Fig. 10d. 834 J. Mater. Chem., 2009, 19, This journal is ª The Royal Society of Chemistry 2009
8 Table 2 Summary of some typical chemical sensors built on metal oxide nanowires Material Device Diameter (nm) Analytes Detect limit Response time (s) Working temperature Synthesis Ref. In 2 O 3 FET 10 NO 2 NH 3,O 2 CO, H 2 NO 2 5 ppb 5 10 RT a Laser ablation 166 FET 10 NO 2,NH 3 NO ppm RT Laser ablation 167 FET 10 NH % NH3 20 RT Chemical vapor 168 deposition SnO2 Resistor and photoluminescence sensor O2, CO, NO2, ethanol CO 10 ppm; NH3 50 ppm; NO2 1 ppm C Vapor phase deposition 169 FET 60 O 2, CO K Electro-deposition 170 FET 60 O 2, CO K Electro-deposition 171 Resistor 60 O2, CO K Electro-deposition 172 Resistor (AC) 20 CO CO 5 ppm; stability 4% C 173 In-SnO2 Resistor Ethanol 10 ppm C Thermal evaporation 174 Sb-SnO 2 Resistor 40 Ethanol 10 ppm C Thermal evaporation 175 Ru-SnO 2 Resistor NO 2, liquid 50 ppm C Thermal evaporation 176 petroleum gas ZnO FET NH3, NO2 NO2 200 ppb, NH3 0.5% FET O2 Thermal evaporation 178 Resistor 30 Ethanol 50 ppm 60 RT-300 C Thermal evaporation 179 FET 60 O2 10 ppm RT Vapor-liquid-solid 180 Pd, Pt, Au, Ni,Ag,Ti doped ZnO Quartz crystal micro-balance 20 NH ppm 5 RT Thermal evaporation 181 Resistor H 2,O 3 3% O C for H 2 ;RT for O3 MBE 182 Resistor H2 10 ppm 600 RT Thermal evaporation 183 Ga-ZnO Resistor CO 320 C Thermal evaporation 184 Pd-ZnO Resistor H 2 10 ppm 20 RT 185 Pt-ZnO Resistor H ppm 600 RT MBE 186 Ga2O3 Resistor NH3, NO2 NH3 200 ppm, NO2 20 ppm Ga oxidized 187 Resistor Ethanol 1500 ppm 100 C Ga oxidized 188 Several min 250 C Thermal evaporation 189 WO3 Resistor 100 NO2, H2S NO2 50 ppb, H2S 10 ppm TeO 2 Resistor NO 2,NH 3,H 2 S NO 2 10 ppm, NH ppm, H 2 S 50 ppm 600 RT Thermal evaporation 190 V2O5 Resistor He Electro-phoresis sol-gel Resistor 10 1-Butylamine, toluene, 1-propanol 1-Butylamine 30 ppb 500 RT Aqueous solution ZnSnO 3 Resistor Ethanol 1ppm C Thermal evaporation 193 Resistor 50 O 2 Thermal evaporation 194 a RT ¼ Room temperature. This journal is ª The Royal Society of Chemistry 2009 J. Mater. Chem., 2009, 19,
9 based on metal oxide nanowire PL chemical sensors, such as SnO 2, and ZnO, etc. 197,198 After exposure to chemicals, the quenching of PL was observed. 199 Although the microscopic mechanisms are still not clear, the quenching is thought to be related to the change of the oxidation state of the nanowire surface before and after chemical exposure. 197 In addition, the sensing response time and recovery time are fast (merely a few seconds), comparable with the response times of most electrical based chemical sensors. For the QCM based sensor, it is thought to be a mass-sensitive sensor, which can detect the change of mass on a sensing layer. The mass of the sensing layer varies due to the chemical reactions, adsorption, and deposition happening above the surface of the sensing layer, while the sensor is exposed to chemicals. QCM based sensors are also contactless devices. 200 Fig. 10 (a) Schematic diagram of a single nanowire transistor structure. (b) SEM image of the chemical sensor chip integrated of a single In 2 O 3 nanowire and micromachined hotplates, where the dashed box indicates the SiN membrane. (c) SEM image of a sensing device with an In 2 O 3 nanowire bridging two electrodes. (d) Sensing response of an In 2 O 3 nanowire sensor operated at 275 C to four different ethanol concentrations (1, 10, 50, and 100 ppm). Reproduced from ref. 196: Appl. Phys. Lett., 2008, 92, Copyright ª 2007, AIP. Fig. 11 Response of a ZnO nanowire FET exposed to 10 ppm NO 2 gas. Reproduced from ref. 180: Appl. Phys. Lett., 2004, 85, Copyright ª 2004, AIP Electronic noses The idea of electronic noses was inspired by the mechanisms of human olfaction. In general, basic elements of an electronic nose system include an odour sensor array, a data pre-processor, and a pattern recognition (PARC) engine. 201 There are several s to approach this goal, one is to make a chemical sensor array with different nanostructured materials and the other is to make a sensor chip with different material geometric properties and temperature gradients (KAMINA technology). Kolmakov et al. adapted this idea and fabricated a KAMINA sensor chip composed of SnO 2 nanowires with different nanowire densities, which exhibited good selectivity for several chemicals. 202 The achievement not only successfully solved the selectivity issue but also brought nanotechnology a step closer to practical application. Very recently, we developed a new template built with four different semiconducting nanostructures: In 2 O 3 nanowires, SnO 2 nanowires, ZnO nanowires and single-wall C nanotubes (SWNT) as electronic noses to detect different chemicals (Fig. 12 inset). 203 n-type metal oxide nanowires and p-type C nanotubes provide one discrimination factor. The integrated micromachined hot plate enables individual and accurate temperature control of each sensor, which provides the second discrimination factor. In FET based chemical sensors, Fan et al. studied oxygen and NO 2 adsorption on the ZnO nanowire surface by using individual ZnO nanowire field-effect transistors. 177 The results of sensing experiments can be observed in Fig. 11. A considerable variation of conductance was observed when the device was exposed to oxygen or NO 2. In addition, an electrical potential to the back gate electrode was applied, which could help to adjust the sensitivity range of the device or initialize the device completely before exposure to chemicals. This can be attributed to the fact that the Fermi level within the nanowire band gap was manipulated by applying an external gate voltage. In addition, a ZnO chemical sensor was fully refreshed by applying a high negative gate bias of 60 V as shown in Fig Optical and QCM based chemical sensors With the novel characterization of contactless devices, recently several research groups executed chemical sensing experiments Fig. 12 Principal component analysis (PCA) scores and loading plots of a chemical sensor array composed of four different nanostructure materials. 836 J. Mater. Chem., 2009, 19, This journal is ª The Royal Society of Chemistry 2009
10 When this sensor array was exposed to different chemicals, good selectivity was obtained to build up an interesting smell-print library of the detected chemicals (Fig. 12). 5. Summary In summary, we provide a comprehensive review of the state-ofthe-art research activities focused on devices and chemical sensing applications of metal oxide nanowires. The fascinating achievements, till now, towards the device applications of metal oxide nanowires should inspire more and more research efforts to address the remaining challenges in this interesting field. We tried to include the most important topics in this review article. However, due to the tremendous research effort and space limitations, this article is unable to list all the exciting works reported in this field. Although comprehensive efforts have been made towards the synthesis of high quality metal oxide nanowires, there is still plenty of room left unexploited. We believe that future work in the nanowire synthesis direction should continue to focus on generating high quality and large quantity metal oxide nanowires in more controlled, predictable and simple ways. One key issue of metal oxide nanowires is the growth of p-type metal oxide nanowires or the formation of intra-nanowire p-n junctions, which will significantly advance and widen the device application of metal oxide nanowires. One interesting area in the metal oxide nanowire based chemical sensors area is still the development of high quality 1-D metal oxide nanostructures to be used as chemical sensing elements. The sensing issues of extremely high sensitivity, selectivity and stability should be resolved. Though some research groups have successfully detected important chemicals using 1-D metal oxide nanostructures, the selectivity is still quite low. Furthermore, other potential and interesting areas which need further exploration may be the detection of very small amounts of nerve agents such as sarin and soman, or of explosive chemicals for personal health and human security applications. References 1 P. D. Yang, H. Q. Yan, S. Mao, R. Russo, J. Johnson, R. Saykally, N. Morris, J. Pham, R. R. He and H. J. Cho, Adv. Funct. Mater., 2002, 12, Z. W. Pan, Z. R. Dai and Z. L. Wang, Science, 2001, 291, Y. L. Wang, X. C. Jiang and Y. Xia, J. Am. Chem. Soc., 2003, 125, C. Li, D. Zhang, S. Han, X. Liu, T. Tang and C. Zhou, Adv. Mater., 2003, 15, Y. Kobayashi, H. Hata, M. Salama and T. E. Mallouk, Nano Lett., 2007, 7, H. W. Ra, K. S. Choi, J. H. Kim, Y. B. Hahn and Y. H. Im, Small, 2008, 4, F. L. Zhang, T. Nyberg and O. Inganas, Nano Lett., 2002, 2, X. Duan and C. M. Lieber, Adv. Mater., 2000, 12, C. M. Lieber, MRS Bull., 2003, 28, Y. W. Wang, L. D. Zhang, G. Z. Wang, X. S. Peng, Z. Q. Chu and C. H. Liang, J. Cryst. Growth, 2002, 234, Y. Li, G. W. Meng, L. D. Zhang and F. Phillipp, Appl. Phys. Lett., 2000, 76, H. Zhang, D. Yang, Y. J. Ji, X. Y. Ma, J. Xu and D. L. Que, J. Phys. Chem. B, 2004, 108, H. Zhang, D. R. Yang, X. Y. Ma and D. L. Que, J. Phys. Chem. B, 2005, 109, Y. Ding, P. X. Gao and Z. L. Wang, J. Am. Chem. Soc., 2004, 126, X. Y. Zhang, J. Y. Dai, H. C. Ong, N. Wang, H. L. W. Chan and C. L. Choy, Chem. Phys. Lett., 2004, 393, L. Vayssieres, Adv. Mater., 2003, 15, P. X. Gao and Z. L. Wang, J. Phys. Chem. B, 2004, 108, Z. L. Wang, J. Nanosci. Nanotechnl., 2008, 8, A. B. Hartanto, X. Ning, Y. Nakata and T. Okada, Appl. Phys. A, 2004, 78, Y. J. Xing, Z. H. Xi, Z. Q. Xue, X. D. Zhang, J. H. Song, R. M. Wang, J. Xu, Y. Song, S. L. Zhang and D. P. Yu, Appl. Phys. Lett., 2003, 83, X. Kong, X. M. Sun, X. L. Li and Y. D. Li, Mater. Chem. Phys., 2003, 82, Y. Sun, G. M. Fuge, N. A. Fox, D. J. Riley and M. N. R. Ashfold, Adv. Mater., 2005, 17, Y. K. Liu, C. L. Zheng, W. Z. Wang, C. R. Yin and G. H. Wang, Adv. Mater., 2001, 13, D. F. Zhang, L. D. Sun, J. L. Yin and C. H. Yan, Adv. Mater., 2003, 15, B. Cheng, J. M. Russell, W. S. Shi, L. Zhang and E. T. Samulski, J. Am. Chem. Soc., 2004, 126, J. H. He, T. H. Wu, C. L. Hsin, K. M. Li, L. J. Chen, Y. L. Chueh, L. J. Chou and Z. L. Wang, Small, 2006, 2, Z. Liu, D. Zhang, S. Han, C. Li, T. Tang, W. Jin, X. Liu, B. Lei and C. Zhou, Adv. Mater., 2003, 15, S. Luo, P. K. Chu, W. Liu, M. Zhang and C. L. Lin, Appl. Phys. Lett., 2006, 88, Y. Chen, X. Cui, K. Zhang, D. Pan, S. Zhang, B. Wang and J. G. Hou, Chem. Phys. Lett., 2003, 369, S. Sun, G. W. Meng, G. Zhang, T. Gao, B. Geng, L. D. Zhang and J. Zuo, Chem. Phys. Lett., 2003, 376, J. Duan, S. Yang, H. Liu, J. Gong, H. Huang, X. Zhao, R. Zhang and Y. Du, J. Am. Chem. Soc., 2005, 107, J. Hu, Y. Bando, Q. Liu and D. Golberg, Adv. Funct. Mater., 2003, 13, B. Liu and H. C. Zeng, J. Phys. Chem. B, 2004, 108, N. Du, H. Zhang, B. Chen, X. Ma and D. R. Yang, Chem. Commun., 2008, N. Wang, X. Cao and L. Guo, J. Phys. Chem. C, 2008, 112, X. S. Peng, G. W. Meng, J. Zhang, X. F. Wang, Y. W. Wang, C. Z. Wang and L. D. Zhang, J. Mater. Chem., 2002, 12, J. Zhang, X. Qing, F. H. Jiang and Z. H. Dai, Chem. Phys. Lett., 2003, 371, H. Q. Cao, X. Q. Qiu, Y. Liang, Q. Zhu and M. Zhao, Appl. Phys. Lett., 2003, 83, X. Y. Kong and Z. L. Wang, Solid State Commun., 2003, 128, Y. Li, Y. Bando and D. Golberg, Adv. Mater., 2003, 15, C. Chen, D. Chen, X. Jiao and C. Qang, Chem. Commun., 2006, H. Zhang, Y. Kong, Y. Wang, X. Du, Z. Bai, J. Wang, D. P. Yu, Y. Ding, Q. Hang and S. Feng, Solid State Commun., 1999, 109, Y. C. Choi, W. S. Kim, Y. S. Park, S. M. Lee, D. J. Bae, Y. H. Lee, G. S. Park, W. B. Choi, N. S. Lee and J. M. Kim, Adv. Mater., 2000, 12, J. Hu, Q. Li, X. Meng, C. S. Lee and S. T. Lee, J. Phys. Chem. B, 2002, 106, K. W. Chang and J. J. Wu, Appl. Phys. A, 2003, 76, B. Geng, L. D. Zhang, G. W. Meng, T. Xie, X. Peng and Y. Lin, J. Cryst. Growth, 2003, 259, J. Zhagn, F. H. Jiang, Y. Yang and J. Li, J. Phys. Chem. B, 2005, 109, N. Gong, M. Lu, C. Y. Wang, Y. Chen and L. J. Chen, Appl. Phys. Lett., 2008, 92, K. Zhu, H. He, S. Xie, X. Zhang, W. Zhou, S. Jin and B. Yue, Chem. Phys. Lett., 2003, 377, Y. Baek and K. Yong, J. Phys. Chem. C, 2007, 111, X. Song, Y. Zheng, E. Yang and Y. Wang, Mater. Lett., 2007, 61, Y. Li, Y. Bando, D. Golberg and K. Kurashima, Chem. Phys. Lett., 2003, 367, F. Zhou, X. Zhao, Y. Liu, C. Yuan and L. Li, Eur. J. Inorg. Chem., 2008, 16, C. K. Chan, H. Peng, R. D. Twesten, K. Jarausch, X. F. Zhang and Y. Cui, Nano Lett., 2007, 7, Y. Yin, G. Zhang and Y. N. Xia, Adv. Funct. Mater., 2002, 12, H. W. Kim and S. H. Shim, Chem. Phys. Lett., 2006, 422, 165. This journal is ª The Royal Society of Chemistry 2009 J. Mater. Chem., 2009, 19,
11 57 K. Nagashima, T. Yanagida, H. Tanaka and T. Kawai, J. Appl. Phys., 2007, 101, J. H. Zhan, Y. Bando, J. Hu and D. Golberg, Inorg. Chem., 2004, 43, H. Imai, Y. Takei, K. Shimizu, M. Matsuda and H. Hirashima, J. Mater. Chem., 1999, 9, S. Liu, L. Gan, L. Liu, W. Zhang and H. Zeng, Chem. Mater., 2002, 14, Y. Zhang, G. Li, Y. Jin, Y. Zhang, J. Zhang and L. D. Zhang, Chem. Phys. Lett., 2002, 365, J. M. Wu, H. C. Shih and W. T. Wu, Chem. Phys. Lett., 2005, 413, H. Cao, X. Qiu, B. Luo, Y. Liang, Y. Zhang, R. Tan, M. Zhao and Q. Zhu, Adv. Funct. Mater., 2004, 14, L. Li and W. Z. Wang, Solid State Commun., 2003, 127, C. Dae, S. Kim, B. Ahn, J. Kim, M. Sung and H. Shin, J. Mater. Chem., 2008, 18, M. Wei, Z. M. Qi, M. Ichihara and H. Zhou, Acta Mater., 2008, 56, B. Varghese, S. C. Haur and C. T. Lim, J. Phys. Chem. C, 2008, 112, S. Hu and X. Wang, J. Am. Chem. Soc., 2008, 130, B. C. Satishkumar, A. Govindaraj, E. M. Vogl, L. Basumallick and C. N. R. Rao, J. Mater. Res., 1997, 12, Y. Li and Y. Bando, Chem. Phys. Lett., 2002, 364, J. Zhou, S. Deng, N. S. Xu, J. Chen and J. She, Appl. Phys. Lett., 2003, 83, B. Qi, X. Ni, D. Li and H. Zheng, Chem. Lett., 2008, 37, X. Wang and Y. D. Li, J. Am. Chem. Soc., 2002, 124, M. Imperor-Clerc, D. Bazin, M. D. Appay, P. Beaunier and A. Davidson, Chem. Mater., 2004, 16, D. Zheng, S. X. Sun, W. Fan, H. Yu, C. Fan, G. Cao, Z. Yin and X. Song, J. Phys. Chem. B, 2005, 109, Y. Fu, J. Chen and H. Zhang, Chem. Phys. Lett., 2001, 350, Y. Xiong, Y. Xie, Z. Li, R. Zhang, J. Yang and C. Wu, New J. Chem., 2003, 27, X. Wen, S. Wang, Y. Ding, Z. L. Wang and S. Yang, J. Phys. Chem. B, 2005, 109, Z. Liu, D. Zhang, S. Han, C. Li, B. Lei, W. Lu, J. Fang and C. Zhou, J. Am. Chem. Soc., 2005, 127, J. Wang, Q. W. Chen, C. Zeng and B. Y. Hou, Adv. Mater., 2004, 16, Z. Dong, Y. Y. Fu, Q. Han, Y. Xu and H. Zhang, J. Phys. Chem. C, 2007, 111, H. Zhang, J. Wu, C. Zhai, X. Ma, N. Du, J. Tu and D. R. Yang, Nanotechnology, 2008, 19, N. Du, H. Zhang, B. Chen, J. Wu, X. Ma, Z. Liu, Y. Zhang, D. Yang, X. Huang and J. Tu, Adv. Mater., 2007, 19, X. W. Lou, D. Deng, J. Y. Lee, J. Feng and L. A. Archer, Adv. Mater., 2008, 20, R. S. Chen, Y. S. Huang, D. S. Tsai, S. Chattopadhyay, C. T. Wu, Z. H. Lan and K. H. Chen, Chem. Mater., 2004, 16, Y. L. Chen, C. C. Hsu, Y. H. Song, Y. Chi, A. J. Carty, S. M. Peng and G. H. Lee, Chem. Vapor. Dep., 2006, 12, C. K. Xu, K. Q. Hong, S. Liu, G. H. Wang and X. N. Zhao, J. Cryst. Growth, 2003, 255, Q. Yang, J. Sha, X. Y. Ma and D. R. Yang, Mater. Lett., 2005, 59, C. S. Shi, G. Q. Wang, N. Q. Zhao, X. W. Du and J. J. Li, Chem. Phys. Lett., 2008, 454, W. Z. Wang, G. H. Wang, X. S. Wang, Y. J. Zhan, Y. K. Liu and C. L. Zheng, Adv. Mater., 2002, 14, Y. J. Xiong, Z. Q. Li, R. Zhang, Y. Xie, J. Yang and C. Z. Wu, J. Phys. Chem. B, 2003, 107, Y. W. Tan, X. Y. Xue, Q. Peng, H. Zhao, T. H. Wang and Y. D. Li, Nano Lett., 2007, 7, X. C. Jiang, T. Herricks and Y. N. Xia, Nano Lett., 2002, 2, E. Ko, J. Choi, K. Okamoto, Y. Tak and J. Lee, ChemPhysChem., 2006, 7, G. H. Du and G. Van Tendeloo, Chem. Phys. Lett., 2004, 393, X. S. Peng, X. F. Wang, Y. W. Wang, C. Z. Wang, G. W. Meng and L. D. Zhang, J. Phys. D., 2002, 35, L D. S. Dhawale, A. M. More, S. S. Latthe, K. Y. Rajpure and C. D. Lokhande, Appl. Surf. Sci., 2008, 254, X. Liu, C. Li, S. Han, J. Han and C. Zhou, Appl. Phys. Lett., 2003, 82, W. Lee, R. Scholz and U. Gosele, Nano Lett., 2008, 8, Y. B. Li, Y. Bando and D. Golberg, Adv. Mater., 2005, 17, D. B. Kuang, Y. P. Fang, H. Q. Liu, C. Frommen and D. Fenske, J. Mater. Chem., 2003, 13, H. Ogihara, M. Sadakane, Y. Nodasaka and W. Ueda, Chem. Mater., 2006, 18, X. S. Peng, L. D. Zhang, G. W. Meng, X. F. Wang, Y. W. Wang, C. Z. Wang and G. S. Wu, J. Phys. Chem. B, 2002, 106, R. S. Wagner and W. C. Ellis, Appl. Phys. Lett., 1964, 4, T. J. Trentler, K. M. Hickman, S. C. Goel, A. M. Viano, P. C. Gibbons and W. E. Buhro, Science, 1995, 270, J. G. Lu, P. Chang and Z. Fan, Mater. Sci. Eng. R, 2006, 52, Y. Ding and Z. L. Wang, J. Phys. Chem. B, 2004, 108, P. Chang, Z. Fan, D. Wang, W. Tseng, W. Chiou, J. Hong and J. G. Lu, Chem. Mater., 2004, 16, C. Li, D. Zhang, S. Han, X. Liu, T. Tang and C. Zhou, Adv. Mater., 2003, 15, Z. Liu, D. Zhang, S. Han, C. Li, T. Tang, W. Jin, X. Liu, B. Lei and C. Zhou, Adv. Mater., 2003, 15, X. Liu, C. Li, S. Han, J. Han and C. Zhou, Appl. Phys. Lett., 2003, 82, Y. Tan, X. Xue, Q. Peng, H. Zhao, T. Wang and Y. Li, Nano Lett., 2007, 7, B. Xiang, P. Wei, X. Zhang, S. A. Dayeh, D. P. R. Aplin, C. Soci, D. Yu and D. Wang, Nano Lett., 2007, 7, G. D. Yuan, W. J. Zhang, J. Jie, X. Fan, J. A. Zapien, Y. H. Leung, L. B. Luo, P. F. Wang, C. S. Lee and S. T. Lee, Nano Lett., 2008, 8, K. Nomura, H. Ohta, K. Ueda, T. Kamiya, M. Hirano and H. Hosono, Science, 2003, 300, S. Ju, A. Facchetti, Y. Xuan, J. Liu, F. Ishikawa, P. Ye, C. Zhou, T. J. Marks and D. B. Janes, Nat. Nanotech., 2007, 2, S. Ju, J. Li, J. Liu, P. Chen, Y. Ha, F. Ishikawa, H. Chang, C. Zhou, A. Facchetti, D. B. Janes and T. J. Marks, Nano Lett., 2008, 8, M. Huang, S. Mao, H. Feick, H. Yan, Y. Wu, H. Kind, E. Weber, R. Russo and P. Yang, Science, 2001, 292, J. Johnson, H. Yan, R. Schaller, L. Haber, R. Saykally and P. Yang, J. Phys. Chem. B, 2001, 105, H. Kind, H. Yan, M. Law, B. Messer and P. Yang, Adv. Mater., 2002, 14, H. Yan, R. He, J. Johnson, M. Law, R. J. Saykally and P. Yang, J. Am. Chem. Soc., 2003, 125, M. Law, D. Sirbuly, J. Johnson, J. Goldberger, R. Saykally and P. Yang, Science, 2004, 305, Z. L. Wang and J. Song, Science, 2006, 312, X. Wang, J. Song, J. Liu and Z. L. Wang, Science, 2007, 316, Y. Qin, X. Wang and Z. L. Wang, Nature, 2008, 451, X. Wang, J. Zhou, J. Song, J. Liu, N. Xu and Z. L. Wang, Nano Lett., 2006, 6, J. H. He, C. L. Hsin, J. Liu, L. J. Chen and Z. L. Wang, Adv. Mater., 2007, 19, M. Law, L. E. Greene, J. C. Johnson, R. Saykally and P. Yang, Nature Mater., 2005, 4, J. T. Liu, F. M. Wang, S. Isoda and M. Adachi, Chem. Lett., 2005, 34, L. Greene, M. Law, B. Yuhas and P. Yang, J. Phys. Chem. C, 2007, 111, M. Law, L. Greene, A. Radenovic, T. Kuykendall, J. Liphardt and P. Yang, J. Phys. Chem. B, 2006, 110, S. Anandan, X. Wen and S. Yang, Mater. Chem. Phys., 2005, 93, K. Wang, J. Chen, W. Zhou, Y. Zhang, Y. Yan, J. Pern and A. Mascarenhas, Adv. Mater., 2008, 20, A. Fujishimam and K. Honda, Nature, 1972, 238, M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, H. Choi, A. C. Sofranko and D. D. Dionysiou, Adv. Funct. Mater., 2006, 16, S. J. Hwang, C. Petucci and D. Raftery, J. Am. Chem. Soc., 1997, 119, C. Wu, L. Lei, X. Zhu, J. Yang and Y. Xie, Small, 2007, 9, Y. Mao and S. S. Wong, J. Am. Chem. Soc., 2006, 128, G. Wang, W. Lu, J. Li, J. Choi, Y. Jeong, S. Y. Choi, J. B. Park, M. K. Ryu and K. Lee, Small, 2006, 2, J. Mater. Chem., 2009, 19, This journal is ª The Royal Society of Chemistry 2009
12 141 Y. Wang, Z. Zhang, Y. Zhu, Z. Li, R. Vajtai, L. Ci and P. M. Ajayan, ACS Nano, 2008, 2, D. Chen and J. Ye, Chem. Mater., 2007, 19, Y. Yu and D. Xu, Appl. Cata. B, 2007, 73, T. J. Kuo, C. N. Lin, C. L. Kuo and M. H. Huang, Chem. Mater., 2007, 19, L. Zhou, W. Wang, L. Zhang, H. Xu and W. Zhu, J. Phys. Chem. C, 2007, 111, C. J. Lee, T. J. Lee, S. C. Lyu, Y. Zhang, H. Ruh and H. J. Lee, Appl. Phys. Lett., 2002, 81, H. Ham, G. Z. Shen, J. H. Cho, T. J. Lee, S. H. Seo and C. J. Lee, Chem. Phys. Lett., 2005, 404, G. Z. Shen, Y. Bando, B. D. Liu, D. Golberg and C. J. Lee, Adv. Funct. Mater., 2006, 16, Y. K. Tseng, C. J. Huang, H. M. Cheng, I. N. Lin, K. S. Liu and I. C. Chen, Adv. Funct. Mater., 2003, 13, C. X. Xu and X. W. Sun, Appl. Phys. Lett., 2003, 83, Y. B. Li, Y. Bando and D. Golberg, Appl. Phys. Lett., 2004, 84, D. Banerjee, S. H. Jo and Z. F. Ren, Adv. Mater., 2004, 16, Y. H. Baek and K. J. Yong, J. Phys. Chem. C, 2007, 111, Y. B. Li, Y. Bando and D. Golberg, Adv. Mater., 2003, 15, 1294; J. Zhou, L. Gong, S. Z. Deng, J. Chen, J. C. She, N. S. Xu, R. S. Yang and Z. L. Wang, Appl. Phys. Lett., 2005, 87, M. T. Chang, L. J. Chou, Y. L. Chueh, Y. C. Lee, C. H. Hsieh, C. D. Chen, Y. W. Lan and L. J. Chen, Small, 2007, 3, J. G. Liu, Z. J. Zhang, Y. Zhao, X. Su, S. Liu and E. G. Wang, Small, 2005, 1, C. S. Hsieh, D. S. Tsai, R. S. Chen and Y. S. Huang, Appl. Phys. Lett., 2004, 85, C. L. Cheng, Y. F. Chen, R. S. Chen and Y. S. Huang, Appl. Phys. Lett., 2005, 86, Y. W. Zhu, A. M. Moo, T. Yu, X. J. Xu, X. Y. Gao, Y. J. Liu, C. T. Lim, Z. X. Shen, C. K. Ong, A. T. S. Wee, J. T. L. Thong and C. H. Sow, Chem. Phys. Lett., 2006, 419, J. Chen, S. Z. Deng, N. S. Xu, W. X. Zhang, X. G. Wen and S. H. Yang, Appl. Phys. Lett., 2003, 83, J. M. Wu, H. C. Shih and W. T. Wu, Chem. Phys. Lett., 2005, 413, J. H. He, T. H. Wu, C. L. Hsin, K. M. Li, L. J. Chen, Y. L. Chueh, L. J. Chou and Z. L. Wang, Small, 2006, 2, Y. J. Chen, Q. H. Li, Y. X. Liang, T. H. Wang, Q. Zhao and D. P. Yu, Appl. Phys. Lett., 2004, 85, S. Q. Li, Y. X. Liang and T. H. Wang, Appl. Phys. Lett., 2006, 88, S. Kar, S. Chakrabarti and S. Chaudhuri, Nanotechnology, 2006, 17, D. Zhang, Z. Liu, C. Li, T. Tang, X. Liu, S. Han, B. Lei and C. Zhou, Nano Lett., 2004, 4, C. Li, D. Zhang, X. Liu, S. Han, T. Tang, J. Han and C. Zhou, Appl. Phys. Lett., 2003, 82, C. Li, D. Zhang, B. Lei, S. Han, X. Liu and C. Zhou, J. Phys. Chem. B, 2003, 107, C. Baratto, E. Comini, G. Faglia, G. Sberveglieri, M. Zha and A. Zappettini, Sens. Actuators B, 2005, 109, X. Y. Xue, Y. J. Chen, Y. G. Liu, S. L. Shi, Y. G. Wang and T. H. Wang, Appl. Phys. Lett., 2006, 88, Y. Zhang, A. Kolmakov, S. Chretien, H. Metiu and M. Moskovits, Nano Lett., 2004, 3, A. Kolmakov, Y. Zhang, G. Cheng and M. Moskovits, Adv. Mater., 2003, 15, F. Hernandez-Ramirez, A. Tarancon, O. Casals, J. Arbiol, A. Romano-Rodriguez and J. R. Morante, Sens. Actuators B, 2007, 121, X. Y. Xue, Y. J. Chen, Y. G. Liu, S. L. Shi, Y. G. Wang and T. H. Wang, Appl. Phys. Lett., 2006, 88, Q. Wan and T. H. Wang, Chem. Commun., 2005, N. S. Ramgir, I. S. Mulla and K. P. Vijayamohanan, Sens. Actuators B, 2005, 107, Z. Fan and J. G. Lu, Appl. Phys. Lett., 2005, 86, Q. Wan, Q. H. Li, Y. J. Chen, T. H. Wang, X. L. He and J. P. Li, Appl. Phys. Lett., 2004, 85, T.-J. Hsueh, C.-L. Hsu, S.-J. Chang and I. C. Chen, Sens. Actuators B, 2007, 126, Z. Fan, D. Wang, P. Chang, W.-Y. Tseng and J. G. Lu, Appl. Phys. Lett., 2004, 85, X. Wang, J. Zhang and Z. Zhu, Appl. Surf. Sci., 2006, 252, B. S. Kang, Y. W. Heo, L. C. Tien, D. P. Norton, R. Ren, B. P. Gila and S. J. Pearton, Appl. Phys. A, 2005, 80, H. T. Wang, B. S. Kang, F. Ren, P. W. Sadic, D. P. Norton, S. J. Pearton and J. Lin, Appl. Phys. A, 2005, 81, T.-J. Hsueh, Y.-W. Chen, S.-J. Chang, S.-F. Wang, C.-L. Hus, Y.- R. Lin, T.-S. Lin and I. C. Chen, Sens. Actuators B, 2007, 125, H. T. Wang, B. S. Kang, F. Ren, L. C. Tien, P. W. Sadik, D. P. Norton and S. J. Pearton, Appl. Phys. Lett., 2005, 86, L. C. Tien, H. T. Wang, B. S. Kang, F. Ren, P. W. Sadik, D. P. Norton, S. J. Pearton and J. Lin, Electrochem and Solid State Lett., 2005, 9, G Y. Huang, S. Yue, Z. Wang, Q. Wang, C. Shi, Z. Xu, X. D. Bai, C. Tang and C. Gu, J. Phys. Chem. B, 2006, 110, M. F. Yu and M. Z. Atashbar, IEEE Sensors, 2005, 5, A. Ponzoni, E. Comini, G. Sbergeglieli, J. Zhou, S. Z. Deng, N. S. Xu, Y. Ding and Z. L. Wang, Appl. Phys. Lett., 2006, 88, Z. Liu, T. Yamazki, Y. Shen and T. Kikuta, Appl. Phys. Lett., 2007, 90, H. Y. Yu, B. H. Kang, U. H. Pi, C. W. Park and S.-Y. Choi, VAppl. Phys. Lett., 2005, 86, I. Raible, M. Burghard, U. Schlecht, A. Yasuda and T. Vossever, Sens. Actuators B, 2005, 106, X. Y. Xue, Y. J. Chen, Y. G. Wang and T. H. Wang, Appl. Phys. Lett., 2005, 86, X. Y. Xue, P. Feng, Y. G. Wang and T. H. Wang, Appl. Phys. Lett., 2007, 91, C. Baratto, E. Comini, G. Faglia, G. Sberveglieri, M. Zha and A. Zappettini, Sens. Actuators B, 2005, 109, K. Ryu, D. Zhang and C. Zhou, Appl. Phys. Lett., 2008, 92, A. Setaro, A. Bismuto, S. Lettieri, P. Maddalena, E. Comini, S. Bianchi, C. Baratto and Sberveglieri, Sens. Actuators B, 2008, 130, E. Comini, C. Baratto, G. Faglia, M. Ferroni and G. Sberveglieri, J. Phys. D. Appl. Phys., 2007, 40, G. Faglia, C. Baratto, G. Sberveglieri, M. Zha and A. Zappettini, Appl. Phys. Lett., 2005, 86, X. Zhou, J. Zhang, T. Jiang, X. Wang and Z. Zhu, Sens. Actuators A, 2007, 135, M. A. Craven, J. G. Gardner and P. N. Bartlett, Trends in Analytical Chemistry, 1996, 15, V. V. Sysoev, J. Goschnick, T. Schneider, E. Strelcov and A. Kolmakov, Nano Lett., 2007, 7, P. C. Chen, F. N. Ishikawa, H. K. Chang, K. Ryu, and C. Zhou, unpublished results. This journal is ª The Royal Society of Chemistry 2009 J. Mater. Chem., 2009, 19,
Fabrication of Novel Lamellar Alternating Nitrogen-Doped
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Fabrication of Novel Lamellar Alternating Nitrogen-Doped Microporous Carbon
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Fabrication of Novel Lamellar Alternating Nitrogen-Doped Microporous Carbon
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Fe 3 O 4 Quantum Dots Decorated MoS 2 Nanosheet
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Fe 3 O 4 Quantum Dots Decorated MoS 2 Nanosheet
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Metal-organic framework-derived CoSe2/(NiCo)Se2
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Metal-organic framework-derived CoSe2/(NiCo)Se2
Supporting Information. In-Situ Facile Bubble-Templated Fabrication of New-Type Urchin-Like (Li, Mo)- Doped Lix(Mo0.3V0.7)2O5 for Zn 2+ Storage
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information In-Situ Facile Bubble-Templated Fabrication of New-Type
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information In-Situ Facile Bubble-Templated Fabrication of New-Type
Hierachical Nickel-Carbon Structure Templated by Metal-Organic Frameworks for Efficient Overall Water Splitting
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Hierachical Nickel-Carbon Structure
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Hierachical Nickel-Carbon Structure
High performance carbon nanotube based fiber-shaped. supercapacitors using redox additives of polypyrrole and. hydroquinone
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting information High performance carbon nanotube based fiber-shaped
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting information High performance carbon nanotube based fiber-shaped
Interconnected hierarchical NiCo 2 O 4 microspheres as high performance. electrode material for supercapacitor
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Interconnected hierarchical microspheres as high performance electrode material for
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Interconnected hierarchical microspheres as high performance electrode material for
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information 3D-composite structure of FeP nanorods supported
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information 3D-composite structure of FeP nanorods supported
Supporting information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry Please do 2018 not adjust margins Supporting information Self-assembled 3D flower-like
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry Please do 2018 not adjust margins Supporting information Self-assembled 3D flower-like
state asymmetric supercapacitors
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 3D hierarchical CoO@MnO 2 core-shell nanohybrid for high-energy solid state
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 3D hierarchical CoO@MnO 2 core-shell nanohybrid for high-energy solid state
Supplementary Material
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Material Biomass Chitosan Derived Cobalt/Nitrogen Doped Carbon
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Material Biomass Chitosan Derived Cobalt/Nitrogen Doped Carbon
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information High-performance LiTi 2 (PO 4 ) 3
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information High-performance LiTi 2 (PO 4 ) 3
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2019 Supporting Information Bioinspired Pomegranate-like Microflowers Confining
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2019 Supporting Information Bioinspired Pomegranate-like Microflowers Confining
Supporting Information for
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting Information for Core-shell ZnO/ZnFe 2 O 4 @C Mesoporous Nanospheres
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting Information for Core-shell ZnO/ZnFe 2 O 4 @C Mesoporous Nanospheres
Electronic Supplementary Information. Hierarchically porous Fe-N-C nanospindles derived from. porphyrinic coordination network for Oxygen Reduction
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Hierarchically porous Fe-N-C nanospindles
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Hierarchically porous Fe-N-C nanospindles
Facile fabrication of well-defined polyaniline microtubes derived. from natural kapok fiber for supercapacitor with long-term.
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Facile fabrication of well-defined polyaniline microtubes derived from natural kapok fiber
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Facile fabrication of well-defined polyaniline microtubes derived from natural kapok fiber
A reformative oxidation strategy using high concentration nitric acid for. enhancing emission performance of graphene quantum dots
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 A reformative oxidation strategy using high concentration nitric acid for enhancing emission
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 A reformative oxidation strategy using high concentration nitric acid for enhancing emission
Facile synthesis of N-rich carbon quantum dots by spontaneous. polymerization and incision of solvents as efficient bioimaging probes
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2015 Supporting Information Facile synthesis of N-rich carbon quantum dots by spontaneous polymerization
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2015 Supporting Information Facile synthesis of N-rich carbon quantum dots by spontaneous polymerization
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) Nanomat Li-S batteries based on
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) Nanomat Li-S batteries based on
Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2019 Supplementary Information Mo modulation effect on the hydrogen binding
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2019 Supplementary Information Mo modulation effect on the hydrogen binding
Key Laboratory of Material Chemistry for Energy Conversion and Storage (Ministry
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 Supporting Information for Dramatically enhanced visible-light driven H 2 evolution
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 Supporting Information for Dramatically enhanced visible-light driven H 2 evolution
Supporting Information. Metal organic framework-derived Fe/C Nanocubes toward efficient microwave absorption
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting Information Metal organic framework-derived Fe/C Nanocubes toward
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting Information Metal organic framework-derived Fe/C Nanocubes toward
Supporting Information for
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Supporting Information for 3D Hierarchical Ni(PO 3 ) 2 Nanosheet Arrays
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Supporting Information for 3D Hierarchical Ni(PO 3 ) 2 Nanosheet Arrays
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Paragenesis BN/CNTs Hybrid as a Monoclinic Sulfur
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Paragenesis BN/CNTs Hybrid as a Monoclinic Sulfur
Supplementary Information. O-vacancy Enriched NiO Hexagonal Platelets Fabricated on Ni. Foam as Self-supported Electrode for Extraordinary
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information O-vacancy Enriched NiO Hexagonal Platelets Fabricated
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information O-vacancy Enriched NiO Hexagonal Platelets Fabricated
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) Hierarchically porous Mo-doped
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) Hierarchically porous Mo-doped
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Flexible 3D Porous CuO Nanowire Arrays for Enzymeless Glucose
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Flexible 3D Porous CuO Nanowire Arrays for Enzymeless Glucose
Supplementary Information. A synergistic interaction between isolated Au nanoparticles with oxygen vacancies in
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information A synergistic interaction between isolated Au
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information A synergistic interaction between isolated Au
Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information Scalable and Ascendant Synthesis of Coated Carbon
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information Scalable and Ascendant Synthesis of Coated Carbon
noble-metal-free hetero-structural photocatalyst for efficient H 2
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Ni 12 P 5 nanoparticles embedded into porous g-c 3 N 4 nanosheets as a
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Ni 12 P 5 nanoparticles embedded into porous g-c 3 N 4 nanosheets as a
Silver Nanowires Coated on Cotton for Flexible Pressure Sensors. College of Materials Science and Engineering, Key Lab of Guangdong Province for
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2015 Supporting Information Silver Nanowires Coated on Cotton for Flexible Pressure
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2015 Supporting Information Silver Nanowires Coated on Cotton for Flexible Pressure
Tunable CoFe-Based Active Sites on 3D Heteroatom Doped. Graphene Aerogel Electrocatalysts via Annealing Gas Regulation for
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Tunable CoFe-Based Active Sites on 3D Heteroatom Doped Graphene Aerogel
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Tunable CoFe-Based Active Sites on 3D Heteroatom Doped Graphene Aerogel
Supplementary Information. High areal capacity lithium sulfur battery cathode by. site-selective vapor infiltration of hierarchical
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Supplementary Information High areal capacity lithium sulfur battery cathode by site-selective
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Supplementary Information High areal capacity lithium sulfur battery cathode by site-selective
Layered polyaniline/graphene film from sandwich-structured polyaniline/graphene/polyaniline nanosheets for high-performance pseudosupercapacitors
 Supporting Information for: Layered polyaniline/graphene film from sandwich-structured polyaniline/graphene/polyaniline nanosheets for high-performance pseudosupercapacitors Zhongqiu Tong a, Yongning Yang
Supporting Information for: Layered polyaniline/graphene film from sandwich-structured polyaniline/graphene/polyaniline nanosheets for high-performance pseudosupercapacitors Zhongqiu Tong a, Yongning Yang
Pingping zhao, Xing Hua, Wei Xu, Wei Luo,* Shengli Chen,* and Gongzhen Cheng
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information Metal-Organic Framework-Derived Hybrid
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information Metal-Organic Framework-Derived Hybrid
Octahedral Pd Nanocages with Porous Shells Converted by Co(OH) 2 Nanocages with Nanosheet surface as Robust Electrocatalysts for Ethanol Oxidation
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Octahedral Pd Nanocages with Porous Shells Converted by Co(OH) 2 Nanocages
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Octahedral Pd Nanocages with Porous Shells Converted by Co(OH) 2 Nanocages
Supplementary Information
 Supplementary Information Fig S1 a top-down SEM image of PS template. b. cross-sectional SEM of PS template (with diameter of 440 nm). Fig. S2 The cross-sectional SEM of the dense Ge film(a) and 3DOM Ge
Supplementary Information Fig S1 a top-down SEM image of PS template. b. cross-sectional SEM of PS template (with diameter of 440 nm). Fig. S2 The cross-sectional SEM of the dense Ge film(a) and 3DOM Ge
Electronic Supporting Information (ESI)
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Electronic Supporting Information (ESI) Designing a Carbon Nanotubes Interconnected
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Electronic Supporting Information (ESI) Designing a Carbon Nanotubes Interconnected
Supporting Information
 Electronic Supplementary Material (ESI) for Materials Horizons. This journal is The Royal Society of Chemistry 2017 Supporting Information Co/CoP Embedded in Hairy Nitrogen-Doped Carbon Polyhedron as an
Electronic Supplementary Material (ESI) for Materials Horizons. This journal is The Royal Society of Chemistry 2017 Supporting Information Co/CoP Embedded in Hairy Nitrogen-Doped Carbon Polyhedron as an
catalytically deposited Cu current collector patterns for high-performance flexible in-plane micro-size energy
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 018 Electronic Supplementary Information (ESI) In-situ growth of Cu(OH) @FeOOH
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 018 Electronic Supplementary Information (ESI) In-situ growth of Cu(OH) @FeOOH
Supporting Information
 Copyright WILEY VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2016. Supporting Information for Adv. Energy Mater., DOI: 10.1002/aenm.201502539 From Hollow Carbon Spheres to N-Doped Hollow Porous
Copyright WILEY VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2016. Supporting Information for Adv. Energy Mater., DOI: 10.1002/aenm.201502539 From Hollow Carbon Spheres to N-Doped Hollow Porous
Supporting Information. Mitigating the P2 O2 phase transition of high-voltage
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2019 Supporting Information Mitigating the P2 O2 phase transition of high-voltage
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2019 Supporting Information Mitigating the P2 O2 phase transition of high-voltage
Supplementary Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2018 Supplementary Information Core@Shell Structured Co-CoO@NC Nanoparticles Supported on Nitrogen
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2018 Supplementary Information Core@Shell Structured Co-CoO@NC Nanoparticles Supported on Nitrogen
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting Information Template-Preparation of Three-Dimensional Molybdenum
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting Information Template-Preparation of Three-Dimensional Molybdenum
Flexible and Printable Paper Based Strain Sensors for Wearable and Large Area Green Electronics
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Supporting Information Flexible and Printable Paper Based Strain Sensors for Wearable and Large
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Supporting Information Flexible and Printable Paper Based Strain Sensors for Wearable and Large
Supporting Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Supporting Information Dual active nitrogen doped hierarchical porous hollow carbon nanospheres
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Supporting Information Dual active nitrogen doped hierarchical porous hollow carbon nanospheres
Supporting Information
 Supporting Information A General Strategy to Fabricate P as Highly Efficient Cocatalyst via Photo-Reduction Deposition for Hydrogen Evolution Yuming Dong a, *, Linggang Kong a, Pingping Jiang a, Guangli
Supporting Information A General Strategy to Fabricate P as Highly Efficient Cocatalyst via Photo-Reduction Deposition for Hydrogen Evolution Yuming Dong a, *, Linggang Kong a, Pingping Jiang a, Guangli
Supporting information
 Electronic Supplementary Material (ESI) for Inorganic Chemistry Frontiers. This journal is the Partner Organisations 2018 Supporting information Cube-Like CuCoO Nanostructures on Reduced Graphene Oxide
Electronic Supplementary Material (ESI) for Inorganic Chemistry Frontiers. This journal is the Partner Organisations 2018 Supporting information Cube-Like CuCoO Nanostructures on Reduced Graphene Oxide
Supporting Information. High cycling stable supercapacitor through electrochemical. deposition of metal-organic frameworks/polypyrrole positive
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 Supporting Information High cycling stable supercapacitor through electrochemical deposition
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 Supporting Information High cycling stable supercapacitor through electrochemical deposition
Recent advances in energy transfer in bulk and nanoscale. luminescent materials: From spectroscopy to applications
 Electronic Supplementary Material (ESI) for Chemical Society Reviews. This journal is The Royal Society of Chemistry 215 Electronic supplementary information Recent advances in energy transfer in ulk and
Electronic Supplementary Material (ESI) for Chemical Society Reviews. This journal is The Royal Society of Chemistry 215 Electronic supplementary information Recent advances in energy transfer in ulk and
MXene/Graphene Hybrid Fibers for High. Performance Flexible Supercapacitors
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information for MXene/Graphene Hybrid Fibers for High Performance
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information for MXene/Graphene Hybrid Fibers for High Performance
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2019 Electronic Supplementary Information (ESI) FeP@C Nanoarray Vertically Grown on Graphene Nanosheets:
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2019 Electronic Supplementary Information (ESI) FeP@C Nanoarray Vertically Grown on Graphene Nanosheets:
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 018 Electronic Supplementary Information Superhydrophilic amorphous Co-B-P nanosheet
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 018 Electronic Supplementary Information Superhydrophilic amorphous Co-B-P nanosheet
Ultrathin Co-Fe Hydroxide Nanosheet Arrays for Improved
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2017 Supplementary Information Ultrathin Co-Fe Hydroxide Nanosheet Arrays for Improved Oxygen Evolution
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2017 Supplementary Information Ultrathin Co-Fe Hydroxide Nanosheet Arrays for Improved Oxygen Evolution
Journal of Materials Chemistry A. Supporting Information. Cobalt Nickel Boride as an Active Electrocatalyst for Water Splitting
 Electronic Supplementary Material (ESI) for. This journal is The Royal Society of Chemistry Please do 2017 not adjust margins Supporting Information Cobalt Nickel Boride as an Active Electrocatalyst for
Electronic Supplementary Material (ESI) for. This journal is The Royal Society of Chemistry Please do 2017 not adjust margins Supporting Information Cobalt Nickel Boride as an Active Electrocatalyst for
RSC Advances.
 This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after
This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after
Supplementary Information. Flexible crystalline silicon radial junction photovoltaics with vertically aligned tapered microwires
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Supplementary Information Flexible crystalline silicon radial junction photovoltaics
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Supplementary Information Flexible crystalline silicon radial junction photovoltaics
Supporting Information
 Electronic Supplementary Material (ESI) for Sustainable Energy & Fuels. This journal is The Royal Society of Chemistry 2018 Supporting Information Electrospray Synthesis of Si Encapsulated in Graphite/carbon
Electronic Supplementary Material (ESI) for Sustainable Energy & Fuels. This journal is The Royal Society of Chemistry 2018 Supporting Information Electrospray Synthesis of Si Encapsulated in Graphite/carbon
Electronic Supplementary Information for. Highly Stable Mesoporous Silica Nanospheres Embedded with
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 17 Electronic Supplementary Information for Highly Stable Mesoporous Silica Nanospheres Embedded
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 17 Electronic Supplementary Information for Highly Stable Mesoporous Silica Nanospheres Embedded
Supplementary Information. Indole-Based Conjugated Macromolecule as Redox- Mediated Electrolyte for Ultrahigh Power Supercapacitor
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2017 Supplementary Information Indole-Based Conjugated Macromolecule as Redox-
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2017 Supplementary Information Indole-Based Conjugated Macromolecule as Redox-
A Ni 3 N-Co 3 N hybrid nanowire array electrode for high-performance nonenzymatic glucose detection
 Electronic Supplementary Material (ESI) for Analytical Methods. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information A Ni 3 N-Co 3 N hybrid nanowire array electrode
Electronic Supplementary Material (ESI) for Analytical Methods. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information A Ni 3 N-Co 3 N hybrid nanowire array electrode
Porous and High-strength Graphitic Carbon/SiC Three-Dimensional Electrode for Capacitive Deionization and Fuel Cell Applications
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Porous and High-strength Graphitic Carbon/SiC Three-Dimensional
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Porous and High-strength Graphitic Carbon/SiC Three-Dimensional
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Electronic coupling-tunable of cobalt sulfide/carbon
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Electronic coupling-tunable of cobalt sulfide/carbon
3D Yolk-Shelled NiGa 2 S 4 Microspheres Confined with Nanosheets for High Performance Supercapacitors
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information (ESI) 3D Yolk-Shelled NiGa 2 S 4 Microspheres
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information (ESI) 3D Yolk-Shelled NiGa 2 S 4 Microspheres
Electronic Supplementary Information (ESI) for Analyst. A Facile Graphene Oxide-Based Fluorescent Nanosensor for in Situ
 Electronic Supplementary Material (ESI) for Analyst. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) for Analyst A Facile Graphene Oxide-Based Fluorescent
Electronic Supplementary Material (ESI) for Analyst. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) for Analyst A Facile Graphene Oxide-Based Fluorescent
Supporting Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Supporting Information Phytic acid-derivative transition metal phosphides encapsulated in N,P-codoped
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Supporting Information Phytic acid-derivative transition metal phosphides encapsulated in N,P-codoped
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Supporting Information Branched polyethylenimine grafted electrospun polyacrylonitrile
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Supporting Information Branched polyethylenimine grafted electrospun polyacrylonitrile
Curriculum Vitae. Yu (Will) Wang
 Curriculum Vitae Yu (Will) Wang Assistant Research Professor yu.wang3@wsu.edu School of Mechanical and Materials Engineering Washington State University, Pullman, 99164, WA, USA EDUCATION B.S. Sichuan
Curriculum Vitae Yu (Will) Wang Assistant Research Professor yu.wang3@wsu.edu School of Mechanical and Materials Engineering Washington State University, Pullman, 99164, WA, USA EDUCATION B.S. Sichuan
High performing AgNWs transparent conducting electrodes with 2.5Ω/Sq based upon Roll-to- Roll compatible post processing technique
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2019 High performing AgNWs transparent conducting electrodes with 2.5Ω/Sq based upon Roll-to- Roll
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2019 High performing AgNWs transparent conducting electrodes with 2.5Ω/Sq based upon Roll-to- Roll
Optical measurement in carbon nanotubes formation by pulsed laser ablation
 Thin Solid Films 457 (004) 7 11 Optical measurement in carbon nanotubes formation by pulsed laser ablation Tomoaki Ikegami, Futoshi Nakanishi*, Makoto Uchiyama, Kenji Ebihara Graduate School of Science
Thin Solid Films 457 (004) 7 11 Optical measurement in carbon nanotubes formation by pulsed laser ablation Tomoaki Ikegami, Futoshi Nakanishi*, Makoto Uchiyama, Kenji Ebihara Graduate School of Science
Highly enhanced performance of spongy graphene as oil sorbent
 Supporting Information Highly enhanced performance of spongy graphene as oil sorbent Hengchang Bi, a Xiao Xie, a Kuibo Yin, a Yilong Zhou, a Shu Wan, a Rodney S. Ruoff b and Litao Sun* a a SEU-FEI Nano-Pico
Supporting Information Highly enhanced performance of spongy graphene as oil sorbent Hengchang Bi, a Xiao Xie, a Kuibo Yin, a Yilong Zhou, a Shu Wan, a Rodney S. Ruoff b and Litao Sun* a a SEU-FEI Nano-Pico
Supporting Information. Outstanding hydrogen evolution reaction catalyzed by porous nickel diselenide
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2017 Supporting Information Outstanding hydrogen evolution reaction catalyzed
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2017 Supporting Information Outstanding hydrogen evolution reaction catalyzed
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry B. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Experimental 2.1 Chemicals and Materials
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry B. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Experimental 2.1 Chemicals and Materials
Inorganic semiconductor nanostructures and their field-emission applications
 FEATURE ARTICLE www.rsc.org/materials Journal of Materials Chemistry Inorganic semiconductor nanostructures and their field-emission applications Xiaosheng Fang,* Yoshio Bando, Ujjal K. Gautam, Changhui
FEATURE ARTICLE www.rsc.org/materials Journal of Materials Chemistry Inorganic semiconductor nanostructures and their field-emission applications Xiaosheng Fang,* Yoshio Bando, Ujjal K. Gautam, Changhui
General Information. Department of Physics, Kansas State University, 116 Cardwell Hall Manhattan, KS 66506, USA. Education
 General Information Name Gender Birth Citizenship Major Email Address Qing Liao Male 27/02/1983, China Chinese Physical Electronics liaoqing@phys.ksu.edu Department of Physics, Kansas State University,
General Information Name Gender Birth Citizenship Major Email Address Qing Liao Male 27/02/1983, China Chinese Physical Electronics liaoqing@phys.ksu.edu Department of Physics, Kansas State University,
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information From ZIF-8 Polyhedron to Three-Dimensional Nitrogen
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information From ZIF-8 Polyhedron to Three-Dimensional Nitrogen
Supporting Information
 Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2016. Supporting Information for Adv. Energy Mater., DOI: 10.1002/aenm.201600322 Integrated Intercalation-Based and Interfacial Sodium
Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2016. Supporting Information for Adv. Energy Mater., DOI: 10.1002/aenm.201600322 Integrated Intercalation-Based and Interfacial Sodium
A Flexible, Lightweight, and Wearable Triboelectric Nanogenerator for Energy Harvesting and Self- Powered Sensing
 FULL PAPER Triboelectric Nanogenerators A Flexible, Lightweight, and Wearable Triboelectric Nanogenerator for Energy Harvesting and Self- Powered Sensing Fan Wu, Congju Li,* Yingying Yin, Ran Cao, Hui
FULL PAPER Triboelectric Nanogenerators A Flexible, Lightweight, and Wearable Triboelectric Nanogenerator for Energy Harvesting and Self- Powered Sensing Fan Wu, Congju Li,* Yingying Yin, Ran Cao, Hui
Supporting Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Supporting Information Efficient and reliable surface charge transfer doping of black phosphorus
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Supporting Information Efficient and reliable surface charge transfer doping of black phosphorus
A ligand conformation preorganization approach to construct a. copper-hexacarboxylate framework with a novel topology for
 Electronic Supplementary Material (ESI) for Inorganic Chemistry Frontiers. This journal is the Partner rganisations 2018 A ligand conformation preorganization approach to construct a copper-hexacarboxylate
Electronic Supplementary Material (ESI) for Inorganic Chemistry Frontiers. This journal is the Partner rganisations 2018 A ligand conformation preorganization approach to construct a copper-hexacarboxylate
Electronic Supplementary Information for
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information for Al-doping to Synchronously Improve
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information for Al-doping to Synchronously Improve
Three-Dimensional Plasmonic Hydrogel Architecture: Facile Synthesis and Its Macro Scale Effective Space
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 0 Electronic Supporting Information Three-Dimensional Plasmonic Hydrogel Architecture: Facile Synthesis
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 0 Electronic Supporting Information Three-Dimensional Plasmonic Hydrogel Architecture: Facile Synthesis
Nitrogen-Doped Core-Sheath Carbon Nanotube Array for Highly Stretchable Supercapacitor
 www.advancedsciencenews.com Nitrogen-Doped Core-Sheath Carbon Nanotube Array for Highly Stretchable Supercapacitor Zhitao Zhang, Lie Wang, Yiming Li, Yuhang Wang, Jing Zhang, Guozhen Guan, Zhiyong Pan,
www.advancedsciencenews.com Nitrogen-Doped Core-Sheath Carbon Nanotube Array for Highly Stretchable Supercapacitor Zhitao Zhang, Lie Wang, Yiming Li, Yuhang Wang, Jing Zhang, Guozhen Guan, Zhiyong Pan,
Sodium Borohydride Stabilizes Very Active Gold Nanoparticle Catalyst ELECTRONIC SUPPLEMENTARY INFORMATIONS. I. General data..p2
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Sodium Borohydride Stabilizes Very Active Gold Nanoparticle Catalyst Christophe Deraedt, Lionel
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Sodium Borohydride Stabilizes Very Active Gold Nanoparticle Catalyst Christophe Deraedt, Lionel
Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information A novel ball milling technique for room temperature
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information A novel ball milling technique for room temperature
Complex Systems and Applications
 Complex Systems and Applications Volume 1 TABLE OF CONTENTS State Feedback and Pole Assignment for A Class of the Second Order Coupled Generalized Distributed Parameter Systems 1 Z. Q. Ge, G. T. Zhu On
Complex Systems and Applications Volume 1 TABLE OF CONTENTS State Feedback and Pole Assignment for A Class of the Second Order Coupled Generalized Distributed Parameter Systems 1 Z. Q. Ge, G. T. Zhu On
Roles of Nitrogen Functionalities in Enhancing the. Excitation-Independent Green-Color Photoluminescence of
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Roles of Nitrogen Functionalities in Enhancing the Excitation-Independent
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Roles of Nitrogen Functionalities in Enhancing the Excitation-Independent
Identification of a Large Amount of Excess Fe in Superconducting Single- Layer FeSe/SrTiO 3 Films
 Identification of a Large Amount of Excess Fe in Superconducting Single- Layer FeSe/SrTiO 3 Films Yong Hu 1,2,#, Yu Xu 1,2,, Qingyan Wang 1,, Lin Zhao 1, Shaolong He 1, Jianwei Huang 1,2, Cong Li 1,2,
Identification of a Large Amount of Excess Fe in Superconducting Single- Layer FeSe/SrTiO 3 Films Yong Hu 1,2,#, Yu Xu 1,2,, Qingyan Wang 1,, Lin Zhao 1, Shaolong He 1, Jianwei Huang 1,2, Cong Li 1,2,
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information Experimental Section Materials: Carbon
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information Experimental Section Materials: Carbon
Publication List: ( 华中科技大学 ): <2017>
 Publication List: 2010.10-2016( 华中科技大学 ): [101] D. Li, C. Sun, H. Li, H. Shi, X. X. Shai, Q. Sun, J. B. Han( 韩俊波 ), Y. Shen, H. L. Yip*, F. Huang*, and M. K. Wang*, Amino-functionalized conjugated
Publication List: 2010.10-2016( 华中科技大学 ): [101] D. Li, C. Sun, H. Li, H. Shi, X. X. Shai, Q. Sun, J. B. Han( 韩俊波 ), Y. Shen, H. L. Yip*, F. Huang*, and M. K. Wang*, Amino-functionalized conjugated
Room 399 College of Technology Building University of Houston Work ; Fax
 ZHENG FAN, PH.D Room 399 College of Technology Building University of Houston Work 713-743-6978; Fax 713-743-0172 Email: fanzheng@central.uh.edu CURRENT POSITION Assistant Professor in the Department of
ZHENG FAN, PH.D Room 399 College of Technology Building University of Houston Work 713-743-6978; Fax 713-743-0172 Email: fanzheng@central.uh.edu CURRENT POSITION Assistant Professor in the Department of
Takayuki Ieki. Introduction. Feature Article
 F e a t u r e A r t i c l e Feature Article Development of a Large Capacity Liquid Vaporization System Using the Bubbling Method Achieving high accuracy through control of bubble diameter and liquid temperature
F e a t u r e A r t i c l e Feature Article Development of a Large Capacity Liquid Vaporization System Using the Bubbling Method Achieving high accuracy through control of bubble diameter and liquid temperature
A mitochondria-targeted near-infrared probe for colorimetric and. ratiometric fluorescence detection of hypochlorite in living cells
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 216 Electronic Supplementary Information A mitochondria-targeted near-infrared probe for colorimetric
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 216 Electronic Supplementary Information A mitochondria-targeted near-infrared probe for colorimetric
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Continuous fabrication of graphene-confined polypyrrole
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Continuous fabrication of graphene-confined polypyrrole
Journal of Chemical and Pharmaceutical Research, 2016, 8(6): Research Article. Walking Robot Stability Based on Inverted Pendulum Model
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2016, 8(6):463-467 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Walking Robot Stability Based on Inverted Pendulum
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2016, 8(6):463-467 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Walking Robot Stability Based on Inverted Pendulum
Hydrophilic/Hydrophobic Interphase-Mediated. Bubble-Like Stretchable Janus Ultrathin Films. Towards Self-Adaptive and Pneumatic
 1 Supporting Information for Hydrophilic/Hydrophobic Interphase-Mediated Bubble-Like Stretchable Janus Ultrathin Films Towards Self-Adaptive and Pneumatic Multifunctional Electronics Peng Xiao,, Yun Liang,,
1 Supporting Information for Hydrophilic/Hydrophobic Interphase-Mediated Bubble-Like Stretchable Janus Ultrathin Films Towards Self-Adaptive and Pneumatic Multifunctional Electronics Peng Xiao,, Yun Liang,,
Supporting Information. Highly simple and rapid synthesis of ultrathin gold nanowires with
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Highly simple and rapid synthesis of ultrathin gold
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Highly simple and rapid synthesis of ultrathin gold
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Cobalt nanoparticles encapsulated in carbon nanotube-grafted
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Cobalt nanoparticles encapsulated in carbon nanotube-grafted
Proceedings of the Beijing International Workshop on HIGH TEMPERATURE SUPERCONDUCTIVITY
 Progress in High Temperature Superconductivity - Vol. 2 Proceedings of the Beijing International Workshop on HIGH TEMPERATURE SUPERCONDUCTIVITY Beijing, P R China, 29 June 1 July 1987 Editors: Z Z Gan
Progress in High Temperature Superconductivity - Vol. 2 Proceedings of the Beijing International Workshop on HIGH TEMPERATURE SUPERCONDUCTIVITY Beijing, P R China, 29 June 1 July 1987 Editors: Z Z Gan
Nickel Hexacyanoferrate/Carbon Composite as a High-Rate and. Long-Life Cathode Material for Aqueous Hybrid Energy
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Nickel Hexacyanoferrate/Carbon Composite as a High-Rate and Long-Life Cathode Material for Aqueous
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Nickel Hexacyanoferrate/Carbon Composite as a High-Rate and Long-Life Cathode Material for Aqueous
