Guidance for Industry
|
|
|
- Millicent Joseph
- 6 years ago
- Views:
Transcription
1 Guidance for Industry Granularity Document Annex to M4: Organization of the CTD U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) October 2005 ICH
2 Guidance for Industry Granularity Document Annex to M4: Organization of the CTD Additional copies are available from: Office of Training and Communication Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane Rockville, MD (Tel) Office of Communication, Training and Manufacturers Assistance, HFM-40 Center for Biologics Evaluation and Research Food and Drug Administration 1401 Rockville Pike, Rockville, MD U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) October 2005 ICH
3 TABLE OF CONTENTS INTRODUCTION... 1 ANNEX : GRANULARITY DOCUMENT... 2 Definition of a Document... 2 Module Module Module Module Document Pagination and Segregation... 8 Section Numbering Within Documents... 8 Table of Contents Formatting... 8 Module Module Module Module
4 Guidance for Industry 1 Granularity Document Annex to M4: Organization of the CTD This guidance represents the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if that approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the FDA staff responsible for implementing this guidance. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance. INTRODUCTION This is one in a series of guidances that provide recommendations for applicants preparing the Common Technical Document for the Registration of Pharmaceuticals for Human Use (CTD) for submission to the U.S. Food and Drug Administration (FDA). This annex to the M4 guidance on the organization of the CTD was developed by ICH in response to requests for additional information after the harmonized CTD guidance documents were finalized in November The annex is intended to clarify what constitutes a document in the paper CTD and in the ectd, and includes the following information for modules 2 through 5: location and hierararchy of headings within the modules document pagination and segregation section numbering within documents formatting of the table of contents The information provided here reflects the consensus of the ICH parties. The annex will be incorporated in FDA s next revision of the M4 guidance. 1 This guidance was developed within the M4 CTD and M2 ectd Implementation Working Groups of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) and has been subject to consultation by the regulatory parties, in accordance with the ICH process. This document has been endorsed by the ICH Steering Committee at Step 4 of the ICH process in September A revision was signed off by ICH in November 2003, and the annex was corrected in Janaury At Step 4 of the process, the final draft is recommended for adoption to the regulatory bodies of the European Union, Japan, and the United States. 1
5 FDA's guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe the Agency's current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required. ANNEX : GRANULARITY DOCUMENT The CTD specifies many section headings and numbers. Could guidance be provided for all modules on headings in relation to document location and the section headings within those documents? Could guidance also be provided on where in the CTD and ectd multiple documents can be located in the hierarchy? Could guidance be given on how documents should be paginated and on what the module Table of Contents should therefore include? Definition of a Document A document is defined for a paper submission as a set of pages, numbered sequentially and divided from other documents by a tab (see Document Pagination and Segregation section of this Annex). A document can be equated to a file for an electronic submission. The granularity of the paper and electronic submissions should be equivalent, although if a paper submission is updated to be an electronic submission, some changes in granularity could be introduced to facilitate on-going lifecycle management. In an electronic submission, a new file starts at the same point at which, in a paper submission, a tab divides the documents. In deciding whether one or more documents or files are appropriate, it should be considered that once a particular approach has been adopted, the same approach should be used throughout the life of the dossier since it is the intention that replacement documents/files be provided when information is changed. The following tables describe the levels in the CTD/eCTD hierarchy at which documents/files should be placed and whether single or multiple documents are appropriate at each point. This describes all sections of a CTD/eCTD, but for individual submissions, all sections might not be applicable. 2
6 Module 2 Module The TOC is only called for in the paper version of the CTD; there is no entry needed for the ectd 2.2 Introduction 2.3.S Note S S S S S S S P Note P P P P P P P P A 2.3.A A A R Note Note Key Documents rolled up to this level are not considered appropriate One document may be submitted at this level Note 1 : Optionality of granularity for the Quality Overall Summary is provided in order to accommodate different levels of complexity of products. The applicant can choose the level at which the QOS is managed. Note 2 : One document should be submitted for each drug substance. Note 3 : For a drug product supplied with reconstitution diluent(s), the information on the diluent(s) should be provided in a separate part P document. Note 4 : One document for each indication should be submitted, although closely related indications can be within a single document. 3
7 Module 3 Module 3 Note The TOC is only called for in the paper version of the CTD; there is no entry needed for the ectd S Note S S S S P Note S S S S S S P P S S S S S S S S S S S S S S S S P.2.1 Note P.2.2 Note P P P P P P P P P P P P P P P P P P P P P P.5.4 4
8 3.2.A 3.3 One file per reference Note P P P A A A R Note P P P P P.8.3 Key Documents rolled up to this level are not considered appropriate One or multiple documents can be submitted at this level Note 1 : In choosing the level of granularity for this Module, the applicant should consider that, when relevant information is changed at any point in the product's lifecycle, replacements of complete documents/files should be provided in the CTD and ectd. Note 2 : For a drug product containing more than one drug substance, the information requested for part S should be provided in its entirety for each drug substance. Note 3 : For a drug product supplied with reconstitution diluent(s), the information on the diluent(s) should be provided in a separate part P, as appropriate. Note 4 : The lower level of headings included in CTD-Q at this point are unlikely to be individual documents or files. Note 5 : Refer to regional guidances. Note 6 : Literature References should be listed in the tables of contents. 5
9 Module 4 Module The TOC is only called for in the paper version of the CTD; there is no entry needed for the ectd Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note One file per reference Note Studies Note Studies Note Studies Note Studies Note Studies Note 1 Key Documents rolled up to this level are not considered appropriate One or multiple documents can be submitted at this level Note 1 : Typically, a single document should be provided for each study report included in Module 4. However, where the study report is large, (e.g., a carcinogenicity study), the applicant can choose to submit the report as more than one document. In this case, the text portion of the report should be one document and the appendices can be one or more documents. In choosing the level of granularity for these reports, the applicant should consider that, when relevant information is changed at any point in the product's lifecycle, replacements of complete documents/files should be provided. Note 2 : Literature References should be listed in the tables of contents. 6
10 Module 5 Module The TOC is only called for in the paper version of the CTD; there is no entry needed for the ectd Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Studies Note Note Studies Note Studies Note Studies Note Studies Note Studies Note One file per reference Note 3 Key Documents rolled up to this level are not considered appropriate One document can be submitted at this level One or multiple documents can be submitted at this level Note 1 : The applicants should ordinarily provide the study reports as multiple documents (a synopsis, a main body of the study report and appropriate appendices). Appendices should be organized in accordance with the ICH E3 guideline, which describes the content and format of the clinical study report. In choosing the level of granularity for reports the applicant should consider that, when relevant information is changed at any point in the product's lifecycle, replacements of complete documents/files should be provided. Note 2 : For applications in support of more than one indication, this section should be repeated for each indication. Note 3 : Literature References should be listed in the tables of content. 7
11 Document Pagination and Segregation Every document should be numbered starting at page one, except for individual literature references, where the existing journal page numbering is considered sufficient. Applicants need not display the number as '1 of n' where n is the total number of pages in the document. Additionally, all pages of a document should include a unique header or footer that briefly identifies its subject matter. In a paper-based drug submission, a similar identifier should be used on a tab that precedes the document, to facilitate finding that document within the dossier. An abbreviation of the full section number and title can be used. If a section contains more than one document, a specific Table of Contents for that section can be included to identify the chronology and titles of the documents contained therein, e.g., Tab with 3.2.S.4.2 Analytical Procedures o Table of Contents, listing the title of Procedure A, Procedure B, Procedure C Tab with 3.2.S.4.2 Procedure A ; o Procedure A (i.e. document, page 1-n) Tab with 3.2.S.4.2 Procedure B ; o Procedure B (i.e. document, page 1-n) Tab with 3.2.S.4.2 Procedure C ; o Procedure C (i.e. document, page 1-n) If a section contains only a single document (e.g. 3.2.S.1.1 Nomenclature), only a tab identified by 3.2.S.1.1 Nomenclature should precede the document. Section Numbering Within Documents In order to avoid 5 th, 6 th, etc., level subheading numbering (e.g., ) within a document, the applicant can use a shortened numbering string. In this case, the document number and the name (e.g., Toxicology Written Summary) should appear in page headers or footers and then section numbering within the document can be used, for example, 1, 1.1, 2, 3, 3.1, 3.2. Use of the full numbering string (e.g., ) is also considered acceptable. Table of Contents Formatting Module 2 The 2.1 CTD Table of Contents should go down to the third (e.g., 2.3.S) or fourth (e.g., 2.3.S.1) level, depending on how a document is defined for the Quality Overall Summary. (See Definition of a document for Module 2.) 8
12 Module 3 The Table of Contents provided under 3.1 should cover the high-level section numbering, the associated section heading and the Volume number in the order that they appear in the drug submission. This Table of Contents would be used to identify the contents of Module 3 as defined in the M4Q guideline. It should go down to the fifth level only (e.g., 3.2.P.2.1). Note that additional subsections and subheadings are defined in the M4Q guideline beyond this level (e.g., under 3.2.P.2) and this formatting should be used within the dossier, despite not being included in the 3.1 Table of Contents. The lower level Table of Contents described under Document Pagination and Segregation should be excluded from the 3.1 Table of Contents. At the applicant s discretion, a Table of Contents can also be included for a particular section that contains multiple documents, in order to identify the chronology and the document subject matter. If there is a desire to introduce additional headers or subsection numbering beyond those which are defined in the M4Q guideline, these should only be included within a document and should be created neither as a separate document nor as a new subsection. In this case, a specific Table of Contents for that document can be included to identify the chronology and titles of the subsections contained therein. These documents and subsections should not appear in the 3.1 Table of Contents. Furthermore, additional attachments or appendices should not be incorporated into this formatting, except as a document under a section where multiple documents might be provided. In this case, a cross-reference should be made within the relevant section to the attached or appended document. If there is a desire to append or attach additional information to a section that is comprised of only one document, this information should be incorporated within that document. All Table of Contents title entries should either correspond to heading names and section numbering as defined in the M4Q guideline or to identifiers appearing on tabs (for a paper-based drug submission only), preferably by their full title, which should easily identify any abbreviated title that might be used on the corresponding tab. The Table of Contents should not specify any page numbers. Literature References should be listed in a Table of Contents specific for this section. Module 4 The Table of Contents for Module 4 should include all of the numerical items listed in the CTD guideline in order to identify all of the important components of the application (for example, Fertility and early embryonic development) and should continue down to at least the level of the study report. Thus, each study report should be identified in the table of contents. The sections of a study report could be identified in the Module 4 Table of Contents of the dossier or only in the Table of Contents of the individual study report. 9
13 Illustration of part of the Module 4 Table of Contents Repeat-Dose Toxicity Study aa-aaa: 30 day repeat dose toxicity study with Drug C in rat Study bb-bbb: 6 month repeat dose toxicity study with Drug C in rat Study cc-ccc: 30 day repeat dose toxicity study with Drug C in dog Study dd-ddd: 6 month repeat dose toxicity study with Drug C in dog Genotoxicity In vitro Study ee-eee: Ames test with Drug C etc. Module 5 The Table of Contents for Module 5 should include all of the numerical items listed in the CTD guideline in order to identify all of the important components of the application (for example, Placebo Controlled Trials) and should continue down to at least the level of the clinical study report. Thus each clinical study report should be identified in the table of contents. The sections of a clinical study report (E3) could be identified in the Module 5 Table of Contents of the dossier or only in the Table of Contents of the individual clinical study report. Illustration of part of the Module 5 Table of Contents Indication Z - Reports of Efficacy and Safety Studies Indication Z - Study Reports of Controlled Clinical Trials Pertinent to the Claimed Indication Indication Z - Placebo Controlled Trials Study xx-xxx: A double blind, placebo-controlled trial of Drug A in Indication Z Study yy-yyy: A double blind Indication Z - Active Controlled Trials Study zz-zzz: A double blind, active controlled trial of Drug A vs. Drug C in Indication Z Indication Q - Reports of Efficacy and Safety Studies Indication Q - Study Reports of Controlled Clinical Trials Pertinent to the Claimed Indication etc. 10
Information Sheet Guidance For IRBs, Clinical Investigators, and Sponsors
 Information Sheet Guidance For IRBs, Clinical Investigators, and Sponsors FDA Institutional Review Board Inspections Additional copies are available from: Good Clinical Practice Program, HF-34 Office of
Information Sheet Guidance For IRBs, Clinical Investigators, and Sponsors FDA Institutional Review Board Inspections Additional copies are available from: Good Clinical Practice Program, HF-34 Office of
Investigator Manual. If you have questions about whether an activity requires IRB review, contact the IRB Office.
 CM-999 001 1 May 2015 Page 1 of 5 What is the purpose of this manual? This document is designed to guide you through policies and procedures related to the conduct of human research that are specific to
CM-999 001 1 May 2015 Page 1 of 5 What is the purpose of this manual? This document is designed to guide you through policies and procedures related to the conduct of human research that are specific to
Introduction to the NRA Design Manual for Roads and Bridges
 Volume 0 Section 0 Part A 0.0.A Introduction to the NRA Design Manual for Roads and Bridges July 2011 St. Martin s House, Waterloo Road, Dublin 4. Tel:+353 1 660 2511 Fax +353 1 668 0009 Email : info@nra.ie
Volume 0 Section 0 Part A 0.0.A Introduction to the NRA Design Manual for Roads and Bridges July 2011 St. Martin s House, Waterloo Road, Dublin 4. Tel:+353 1 660 2511 Fax +353 1 668 0009 Email : info@nra.ie
UNIVERSITY OF TENNESSEE GRADUATE SCHOOL OF MEDICINE INSTITUTIONAL REVIEW BOARD CONTINUING REVIEW AND REAPPROVAL OF RESEARCH
 UNIVERSITY OF TENNESSEE GRADUATE SCHOOL OF MEDICINE INSTITUTIONAL REVIEW BOARD CONTINUING REVIEW AND REAPPROVAL OF RESEARCH I. PURPOSE This document outlines the required elements of University of Tennessee
UNIVERSITY OF TENNESSEE GRADUATE SCHOOL OF MEDICINE INSTITUTIONAL REVIEW BOARD CONTINUING REVIEW AND REAPPROVAL OF RESEARCH I. PURPOSE This document outlines the required elements of University of Tennessee
Inspections, Compliance, Enforcement, and Criminal Investigations
 Inspections, Compliance, Enforcement, and Criminal Investigations Burzynski Research Institute / IRB Department of Health and Human Services Public Health Service Food and Drug Administration Silver Spring,
Inspections, Compliance, Enforcement, and Criminal Investigations Burzynski Research Institute / IRB Department of Health and Human Services Public Health Service Food and Drug Administration Silver Spring,
OHRP Guidance on Written IRB Procedures
 Office for Human Research Protections (OHRP) Department of Health and Human Services Date: OHRP Guidance on Written IRB Procedures Scope: This document outlines the required elements of written Institutional
Office for Human Research Protections (OHRP) Department of Health and Human Services Date: OHRP Guidance on Written IRB Procedures Scope: This document outlines the required elements of written Institutional
MSC Guidelines Potable Water Systems
 MSC Guidelines Potable Water Systems S. T. Brady, CDR, Chief of Engineering Division References: a. 46 CFR 56.60 (Subchapter F) b. 46 CFR 31.30-1 & 32.40 (Subchapter D) c. 46 CFR 70.20-1, 70.25-15 & 70.20-25
MSC Guidelines Potable Water Systems S. T. Brady, CDR, Chief of Engineering Division References: a. 46 CFR 56.60 (Subchapter F) b. 46 CFR 31.30-1 & 32.40 (Subchapter D) c. 46 CFR 70.20-1, 70.25-15 & 70.20-25
The Complete Stability Testing for a Successful Application. Strategies
 The Complete Staility Testing for a Successful Application, Requirements, Basic Principles Performance, Documents 1 Introduction Ojectives To secure the registration application and the successful marketing
The Complete Staility Testing for a Successful Application, Requirements, Basic Principles Performance, Documents 1 Introduction Ojectives To secure the registration application and the successful marketing
CONTINUING REVIEW 3/7/2016
 DUKE UNIVERSITY HEALTH SYSTEM Human Research Protection Program Introduction CONTINUING REVIEW 3/7/2016 Federal regulations require that DUHS has written procedures which the IRB will follow for (a) conducting
DUKE UNIVERSITY HEALTH SYSTEM Human Research Protection Program Introduction CONTINUING REVIEW 3/7/2016 Federal regulations require that DUHS has written procedures which the IRB will follow for (a) conducting
Guidance for IRBs, Clinical Investigators, and Sponsors IRB Continuing Review after Clinical Investigation Approval
 Guidance for IRBs, Clinical Investigators, and Sponsors IRB Continuing Review after Clinical Investigation Approval Additional copies are available from: Office of Communication Division of Drug Information,
Guidance for IRBs, Clinical Investigators, and Sponsors IRB Continuing Review after Clinical Investigation Approval Additional copies are available from: Office of Communication Division of Drug Information,
MSC Guidelines for Review of Vapor Control Systems Procedure Number: C1-46 Revision Date: 01/08/2018
 T. O. Phillips, CDR, Chief, Tank Vessel & Offshore Division Purpose: The purpose of this document is to provide guidance and information regarding the submission of vapor control system (VCS) piping plans
T. O. Phillips, CDR, Chief, Tank Vessel & Offshore Division Purpose: The purpose of this document is to provide guidance and information regarding the submission of vapor control system (VCS) piping plans
MSC Guidelines for the Review of Vapor Control Systems Procedure Number: C1-46 Revision Date: March 30, 2012
 R. J. LECHNER, CDR, Tank Vessel and Offshore Division Purpose: To outline procedures for review of vapor control system (VCS) piping plans and pressure drop calculations, and for generating a VCS List
R. J. LECHNER, CDR, Tank Vessel and Offshore Division Purpose: To outline procedures for review of vapor control system (VCS) piping plans and pressure drop calculations, and for generating a VCS List
What s New in GCP? FDA, OHRP Issue Harmonized Guidance on Transferring IRB Oversight of Clinical Studies
 Vol. 8, No. 8, August 2012 Happy Trials to You What s New in GCP? FDA, OHRP Issue Harmonized Guidance on Transferring IRB Oversight of Clinical Studies Reprinted from the Guide to Good Clinical Practice
Vol. 8, No. 8, August 2012 Happy Trials to You What s New in GCP? FDA, OHRP Issue Harmonized Guidance on Transferring IRB Oversight of Clinical Studies Reprinted from the Guide to Good Clinical Practice
NRA MANUAL OF CONTRACT DOCUMENTS FOR ROAD WORKS. Introduction to the NRA Manual of Contract Documents for Road Works. Volume 0 Section 0 Part 1
 Volume 0 Section 0 Part 1 NRA SD 01/12 NRA MANUAL OF CONTRACT DOCUMENTS FOR ROAD WORKS Introduction to the NRA Manual of Contract Documents for Road Works February 2012 St. Martin s House, Waterloo Road,
Volume 0 Section 0 Part 1 NRA SD 01/12 NRA MANUAL OF CONTRACT DOCUMENTS FOR ROAD WORKS Introduction to the NRA Manual of Contract Documents for Road Works February 2012 St. Martin s House, Waterloo Road,
NRA MANUAL OF CONTRACT DOCUMENTS FOR ROAD WORKS
 Volume 0 Section 0 Part 1 NRA SD 01/13 NRA MANUAL OF CONTRACT DOCUMENTS FOR ROAD WORKS Introduction to the NRA Manual of Contract Documents for Road Works March 2013 St. Martin s House, Waterloo Road,
Volume 0 Section 0 Part 1 NRA SD 01/13 NRA MANUAL OF CONTRACT DOCUMENTS FOR ROAD WORKS Introduction to the NRA Manual of Contract Documents for Road Works March 2013 St. Martin s House, Waterloo Road,
T. O. PHILLIPS, CDR, Tank Vessel and Offshore Division
 T. O. PHILLIPS, CDR, Tank Vessel and Offshore Division Purpose: To establish a process for reviewing stability calculations for an Oceangoing Tank Barge regulated under 46 CFR Subchapters D, I, O, and/or
T. O. PHILLIPS, CDR, Tank Vessel and Offshore Division Purpose: To establish a process for reviewing stability calculations for an Oceangoing Tank Barge regulated under 46 CFR Subchapters D, I, O, and/or
Guidance on the compilation of safety data sheets Version 1.0 September Guidance on the compilation of safety data sheets
 Guidance on the compilation of safety data sheets Version 1.0 September 2011 LEGAL NOTICE This document contains guidance on REACH explaining the REACH obligations and how to fulfil them. However, users
Guidance on the compilation of safety data sheets Version 1.0 September 2011 LEGAL NOTICE This document contains guidance on REACH explaining the REACH obligations and how to fulfil them. However, users
Safety Assessments Revised Toy Safety Directive 2009/48/EC
 Toy Safety Update Safety Assessments Revised Toy Safety Directive 2009/48/EC Contents 1. Obligations of Economic Operators 2. Introduction 3. Overview 4. How to use this guide 5. Approach to Safety Assessment
Toy Safety Update Safety Assessments Revised Toy Safety Directive 2009/48/EC Contents 1. Obligations of Economic Operators 2. Introduction 3. Overview 4. How to use this guide 5. Approach to Safety Assessment
MSC Guidelines for Gas Carrier Barge Cargo Authority Procedure Number: C1-41 Revision Date: January 27, 2012
 R. J. LECHNER, CDR, Tank Vessel and Offshore Division Purpose To establish the procedures for determining cargo authority for liquefied gas barges and calculating loading constraints for liquefied gas
R. J. LECHNER, CDR, Tank Vessel and Offshore Division Purpose To establish the procedures for determining cargo authority for liquefied gas barges and calculating loading constraints for liquefied gas
Study Management SM STANDARD OPERATING PROCEDURE FOR Interactions with the Institutional Review Board (IRB)
 Study Management SM 302.00 STANDARD OPERATING PROCEDURE FOR Interactions with the Institutional Review Board (IRB) Approval: Nancy Paris, MS, FACHE President and CEO 24 May 2017 (Signature and Date) Approval:
Study Management SM 302.00 STANDARD OPERATING PROCEDURE FOR Interactions with the Institutional Review Board (IRB) Approval: Nancy Paris, MS, FACHE President and CEO 24 May 2017 (Signature and Date) Approval:
Independent Ethics Committees
 GCP Independent Ethics Committees 1 GCP What is an Ethics Committee? An ethics committee is a committee formally designated to review and approve the initiation of a clinical research study involving human
GCP Independent Ethics Committees 1 GCP What is an Ethics Committee? An ethics committee is a committee formally designated to review and approve the initiation of a clinical research study involving human
MSC Guidelines for Review of Inland Tank Barge Stability Procedure Number: C1-13 Revision Date: May 13, 2016
 T. O. PHILLIPS, CDR, Chief, Tank Vessel and Offshore Division Purpose: To establish a process for reviewing stability calculations submitted to the MSC for Inland Tank Barges regulated under 46 CFR Subchapters
T. O. PHILLIPS, CDR, Chief, Tank Vessel and Offshore Division Purpose: To establish a process for reviewing stability calculations submitted to the MSC for Inland Tank Barges regulated under 46 CFR Subchapters
Children s Hospital of Philadelphia Committees for the Protection of Human Subjects Policies and Procedures. IRB Review Processes
 Page: 1 of 8 I. PURPOSE II. III. IV. The purpose of this standard operating procedure is to describe IRB review processes, including pre-review, review, and post-review procedures. POLICY STATEMENT The
Page: 1 of 8 I. PURPOSE II. III. IV. The purpose of this standard operating procedure is to describe IRB review processes, including pre-review, review, and post-review procedures. POLICY STATEMENT The
TITLE 11. DEPARTMENT OF JUSTICE NOTICE OF PROPOSED RULEMAKING
 TITLE 11. DEPARTMENT OF JUSTICE NOTICE OF PROPOSED RULEMAKING NOTICE IS HEREBY GIVEN that the Department of Justice (DOJ) proposes to adopt as permanent regulations the Attorney General s establishment
TITLE 11. DEPARTMENT OF JUSTICE NOTICE OF PROPOSED RULEMAKING NOTICE IS HEREBY GIVEN that the Department of Justice (DOJ) proposes to adopt as permanent regulations the Attorney General s establishment
1. Review and approval of the consent document is a responsibility that FDA assigns to the IRB with jurisdiction
 Medical College of Wisconsin/Froedtert Hospital Guidance on Negotiating Consent Document Language For Clinical Trial Sponsors 1. Review and approval of the consent document is a responsibility that FDA
Medical College of Wisconsin/Froedtert Hospital Guidance on Negotiating Consent Document Language For Clinical Trial Sponsors 1. Review and approval of the consent document is a responsibility that FDA
UNIVERSITY OF TENNESSEE HEALTH SCIENCE CENTER INSTITUTIONAL REVIEW BOARD CONTINUING REVIEW OF RESEARCH
 UNIVERSITY OF TENNESSEE HEALTH SCIENCE CENTER INSTITUTIONAL REVIEW BOARD CONTINUING REVIEW OF RESEARCH I. PURPOSE This document outlines the University of Tennessee Health Science Center Institutional
UNIVERSITY OF TENNESSEE HEALTH SCIENCE CENTER INSTITUTIONAL REVIEW BOARD CONTINUING REVIEW OF RESEARCH I. PURPOSE This document outlines the University of Tennessee Health Science Center Institutional
SOUTH AFRICAN RUGBY UNION - ANTI-DOPING REGULATIONS
 SOUTH AFRICAN RUGBY UNION - ANTI-DOPING REGULATIONS INTRODUCTION 1. SARU Position on Doping SARU condemns doping. It is harmful to the health of players, totally contrary to the spirit of rugby and SARU
SOUTH AFRICAN RUGBY UNION - ANTI-DOPING REGULATIONS INTRODUCTION 1. SARU Position on Doping SARU condemns doping. It is harmful to the health of players, totally contrary to the spirit of rugby and SARU
Standard Operating Policy and Procedures (SOPP) 3:
 Standard Operating Policy and Procedures (SOPP) 3: INITIAL AND CONTINUING REVIEW BY THE IRB: REQUIREMENTS FOR SUBMISSION OF APPLICATIONS, APPROVAL CRITERIA, EXPEDITED AND CONVENED COMMITTEE REVIEW AND
Standard Operating Policy and Procedures (SOPP) 3: INITIAL AND CONTINUING REVIEW BY THE IRB: REQUIREMENTS FOR SUBMISSION OF APPLICATIONS, APPROVAL CRITERIA, EXPEDITED AND CONVENED COMMITTEE REVIEW AND
MSC Guidelines for Sanitary & Sewage Systems
 S. J. Kelly, CDR, Chief of Engineering Division References: Contact Information: a. Title 33 CFR 159; Marine Sanitation Devices (All Vessels) b. Title 46 CFR 56.50-1 & 56.50-95 (Subchapter F) c. Title
S. J. Kelly, CDR, Chief of Engineering Division References: Contact Information: a. Title 33 CFR 159; Marine Sanitation Devices (All Vessels) b. Title 46 CFR 56.50-1 & 56.50-95 (Subchapter F) c. Title
University of Iowa External/Central IRB Reliance Process Standard Operating Procedure (SOP)
 University of Iowa External/Central IRB Reliance Process Standard Operating Procedure (SOP) I. OVERVIEW The purpose of this Standard Operating Procedure is to define a process for all University of Iowa
University of Iowa External/Central IRB Reliance Process Standard Operating Procedure (SOP) I. OVERVIEW The purpose of this Standard Operating Procedure is to define a process for all University of Iowa
CHAPTER 2G. PREFERENTIAL AND MANAGED LANE SIGNS
 2011 Edition - Revision 2 Page 275 Section 2G.01 Scope CHAPTER 2G. PREFERENTIAL AND MANAGED LANE SIGNS 01 Preferential lanes are lanes designated for special traffic uses such as high-occupancy vehicles
2011 Edition - Revision 2 Page 275 Section 2G.01 Scope CHAPTER 2G. PREFERENTIAL AND MANAGED LANE SIGNS 01 Preferential lanes are lanes designated for special traffic uses such as high-occupancy vehicles
(y9. &i?r, JUL t Certified-Return Receipt Requested WARNING LE7TER
 ... (y9 &i?r, DEPARTMENT OF HEALTH a HUMAN SERVICES Public Health Service Food and Drug Admini~ation Center for Biologics Evaluation and Rem 1401 Rockville Pike Rockville MD 20852-1448 JUL t 71997 Certified-Return
... (y9 &i?r, DEPARTMENT OF HEALTH a HUMAN SERVICES Public Health Service Food and Drug Admini~ation Center for Biologics Evaluation and Rem 1401 Rockville Pike Rockville MD 20852-1448 JUL t 71997 Certified-Return
Human Research Protections Program Medical College of Wisconsin and Froedtert Memorial Lutheran Hospital
 Human Research Protections Program Medical College of Wisconsin and Froedtert Memorial Lutheran Hospital To Whom It May Concern: The Medical College of Wisconsin (MCW) has an approved Federal Wide Assurance
Human Research Protections Program Medical College of Wisconsin and Froedtert Memorial Lutheran Hospital To Whom It May Concern: The Medical College of Wisconsin (MCW) has an approved Federal Wide Assurance
CONTINUING REVIEW CRITERIA FOR RENEWAL
 1. POLICY Steering Committee approved / Effective Date: 9/2/15 The IRB conducts continuing review of research taking place within its jurisdiction at intervals appropriate to the degree of risk, but not
1. POLICY Steering Committee approved / Effective Date: 9/2/15 The IRB conducts continuing review of research taking place within its jurisdiction at intervals appropriate to the degree of risk, but not
Via Federal Express Rockville, MD 20850
 DEPARTMENT OF HEAKTH & HUMAN SERVICES JUL 8 -- WARNING LETTER Food and Drug Administration Center for Devices and Radiological Health 2098 Gaither Road Via Federal Express Rockville, MD 20850 Gordon Binder
DEPARTMENT OF HEAKTH & HUMAN SERVICES JUL 8 -- WARNING LETTER Food and Drug Administration Center for Devices and Radiological Health 2098 Gaither Road Via Federal Express Rockville, MD 20850 Gordon Binder
ISO INTERNATIONAL STANDARD. Natural gas Measurement of properties Volumetric properties: density, pressure, temperature and compression factor
 INTERNATIONAL STANDARD ISO 15970 First edition 2008-06-15 Natural gas Measurement of properties Volumetric properties: density, pressure, temperature and compression factor Gaz naturel Mesurage des caractéristiques
INTERNATIONAL STANDARD ISO 15970 First edition 2008-06-15 Natural gas Measurement of properties Volumetric properties: density, pressure, temperature and compression factor Gaz naturel Mesurage des caractéristiques
MSC Guidelines for Liquefied Gas Barge General Arrangements
 T. O. PHILLIPS, CDR, Chief, Tank Vessel and Offshore Division Purpose: To establish a procedure for reviewing general arrangement plans for a liquefied gas barge with independent pressure tanks regulated
T. O. PHILLIPS, CDR, Chief, Tank Vessel and Offshore Division Purpose: To establish a procedure for reviewing general arrangement plans for a liquefied gas barge with independent pressure tanks regulated
Coaches, Parents, Players and Fans
 P.O. Box 865 * Lancaster, OH 43130 * 740-808-0380 * www.ohioyouthbasketball.com Coaches, Parents, Players and Fans Sunday s Championship Tournament in Boys Grades 5th thru 10/11th will be conducted in
P.O. Box 865 * Lancaster, OH 43130 * 740-808-0380 * www.ohioyouthbasketball.com Coaches, Parents, Players and Fans Sunday s Championship Tournament in Boys Grades 5th thru 10/11th will be conducted in
New Albany / Fred Klink Memorial Classic 3rd Grade Championship Bracket **Daylight Saving Time - Turn Clocks Up One Hour**
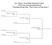 rd Grade Championship Bracket st Pool B nd Pool A 9:00 am New Albany Annex :0 am New Albany Annex st Pool C nd Pool B :0 pm New Albany nd Pool C 0:05 am New Albany Annex :5 pm New Albany Annex st Pool
rd Grade Championship Bracket st Pool B nd Pool A 9:00 am New Albany Annex :0 am New Albany Annex st Pool C nd Pool B :0 pm New Albany nd Pool C 0:05 am New Albany Annex :5 pm New Albany Annex st Pool
Info Sheet - Operations
 DANA-FARBER / HARVARD CANCER CENTER Info Sheet - Operations Office for Human Research Studies Use of Informed Consent Documents Posted to OncPro General Information Upon activation of a research study,
DANA-FARBER / HARVARD CANCER CENTER Info Sheet - Operations Office for Human Research Studies Use of Informed Consent Documents Posted to OncPro General Information Upon activation of a research study,
Policy Number: 42 Title: Investigational Devices Date of Last Revision: 06/12/2008; 07/22/2010; 05/29/2013; 05/01/2016; 10/16/2018
 University of California, Irvine Human Research Protections Standard Operating Policies and Procedures Policy Number: 42 Title: Investigational Devices Date of Last Revision: 06/12/2008; 07/22/2010; 05/29/2013;
University of California, Irvine Human Research Protections Standard Operating Policies and Procedures Policy Number: 42 Title: Investigational Devices Date of Last Revision: 06/12/2008; 07/22/2010; 05/29/2013;
MSC Guidelines for Tank Barge General Arrangements Procedure Number: C1-16 Revision Date: June 1, 2017
 T. O. PHILLIPS, CDR, Tank Vessel and Offshore Division Purpose: The purpose of this document is to provide guidance and information regarding the submission of General Arrangements plans for an Oceangoing
T. O. PHILLIPS, CDR, Tank Vessel and Offshore Division Purpose: The purpose of this document is to provide guidance and information regarding the submission of General Arrangements plans for an Oceangoing
Submitting Continuing Reviews and/or Amendments to the IRB
 Submitting Continuing Reviews and/or Amendments to the IRB Tufts-New England Medical Center Tufts University Health Sciences IRB Education Series 2006 Presentation may only be reused or reprinted with
Submitting Continuing Reviews and/or Amendments to the IRB Tufts-New England Medical Center Tufts University Health Sciences IRB Education Series 2006 Presentation may only be reused or reprinted with
MATERIAL SAFETY DATA SHEET
 Section Section 1: Identification of the substance/mixture and of the company undertaking MATERIAL SAFETY DATA SHEET Product Code: MW113 Details 1.1 Product identifier Product Name: Product Codes: MW113
Section Section 1: Identification of the substance/mixture and of the company undertaking MATERIAL SAFETY DATA SHEET Product Code: MW113 Details 1.1 Product identifier Product Name: Product Codes: MW113
HCT/P Deviations and Reporting
 HCT/P Deviations and Reporting Sharon O Callaghan Consumer Safety Officer Division of Inspections and Surveillance Office of Compliance and Biologics Quality 13 th Annual FDA and the Changing Paradigm
HCT/P Deviations and Reporting Sharon O Callaghan Consumer Safety Officer Division of Inspections and Surveillance Office of Compliance and Biologics Quality 13 th Annual FDA and the Changing Paradigm
Telematics Strategy Implications of Brexit and beyond
 20 th DGRA Annual Congress Telematics Strategy 2025 - Implications of Brexit and beyond Karl Broich President BfArM; Chair of the EU Telematics Management Board Karl Broich DGRA Annual meeting 2018 Telematics
20 th DGRA Annual Congress Telematics Strategy 2025 - Implications of Brexit and beyond Karl Broich President BfArM; Chair of the EU Telematics Management Board Karl Broich DGRA Annual meeting 2018 Telematics
TOXIC GAS DETECTORS IN THE WORKPLACE UK REGULATIONS AND STANDARDS
 SUMMARY TOXIC GAS DETECTORS IN THE WORKPLACE UK REGULATIONS AND STANDARDS In recent articles supplied by the Council of Gas Detection and Environmental Monitoring (CoGDEM) for inclusion in International
SUMMARY TOXIC GAS DETECTORS IN THE WORKPLACE UK REGULATIONS AND STANDARDS In recent articles supplied by the Council of Gas Detection and Environmental Monitoring (CoGDEM) for inclusion in International
SOP 407: PROTOCOL DEVIATIONS AND UNANTICIPATED PROBLEMS
 University of Oklahoma Office of Human Research Participant Protection : PROTOCOL DEVIATIONS AND UNANTICIPATED PROBLEMS 1. POLICY Protocol deviations and unanticipated problems may be discovered in a variety
University of Oklahoma Office of Human Research Participant Protection : PROTOCOL DEVIATIONS AND UNANTICIPATED PROBLEMS 1. POLICY Protocol deviations and unanticipated problems may be discovered in a variety
Organisation Management Services (OMS) operating model
 3 July 2017 EMA/459105/2016 version 3 Information Management Division Organisation Management Services (OMS) operating model Table of contents 1. Executive summary... 2 2. OMS operating model... 2 2.1.
3 July 2017 EMA/459105/2016 version 3 Information Management Division Organisation Management Services (OMS) operating model Table of contents 1. Executive summary... 2 2. OMS operating model... 2 2.1.
Draft Indian Standard SAFES Part 2 Tests for Burglary Resistance (Fifth Revision)
 Draft For Comments Only Draft Indian Standard SAFES Part 2 Tests for Burglary Resistance (Fifth Revision) Not to be reproduced without the permission of Last date for receipt of BIS or used as a STANDARD
Draft For Comments Only Draft Indian Standard SAFES Part 2 Tests for Burglary Resistance (Fifth Revision) Not to be reproduced without the permission of Last date for receipt of BIS or used as a STANDARD
Iteration: while, for, do while, Reading Input with Sentinels and User-defined Functions
 Iteration: while, for, do while, Reading Input with Sentinels and User-defined Functions This programming assignment uses many of the ideas presented in sections 6 and 7 of the course notes. You are advised
Iteration: while, for, do while, Reading Input with Sentinels and User-defined Functions This programming assignment uses many of the ideas presented in sections 6 and 7 of the course notes. You are advised
MSC Guidelines for Review of Passenger Vessel Stability (Subchapters K & H)
 S. E. HEMANN, CDR, Chief, Hull Division References Contact Information a. 46 CFR 170: Stability requirements for all inspected vessels b. 46 CFR 171: Special Rules pertaining to Passenger vessels c. Marine
S. E. HEMANN, CDR, Chief, Hull Division References Contact Information a. 46 CFR 170: Stability requirements for all inspected vessels b. 46 CFR 171: Special Rules pertaining to Passenger vessels c. Marine
MSC Guidelines for Compressed Air Systems
 Procedure Number: E1-04 Revision Date: 12/16/2013 References: a. 46 CFR 54 Pressure Vessels (Subchapter F) P. W. Gooding, CDR, Chief, Engineering Division b. 46 CFR 56 Piping Systems & Appurtenances (Subchapter
Procedure Number: E1-04 Revision Date: 12/16/2013 References: a. 46 CFR 54 Pressure Vessels (Subchapter F) P. W. Gooding, CDR, Chief, Engineering Division b. 46 CFR 56 Piping Systems & Appurtenances (Subchapter
ocr o12011
 Commandant 2100 2na St. S.W Stop 7581 Homeland U.S. Departmento~ Security United States Coast Guard Washington, DC 20593-7581 Staff Symbol: CG-543 United States Phone: (202) 372-1250 Coast Guard Fax: (202)
Commandant 2100 2na St. S.W Stop 7581 Homeland U.S. Departmento~ Security United States Coast Guard Washington, DC 20593-7581 Staff Symbol: CG-543 United States Phone: (202) 372-1250 Coast Guard Fax: (202)
DECISION OF THE EEA JOINT COMMITTEE. No 206/2016. of 30 September amending Annex IX (Financial services) to the EEA Agreement [2017/283]
![DECISION OF THE EEA JOINT COMMITTEE. No 206/2016. of 30 September amending Annex IX (Financial services) to the EEA Agreement [2017/283] DECISION OF THE EEA JOINT COMMITTEE. No 206/2016. of 30 September amending Annex IX (Financial services) to the EEA Agreement [2017/283]](/thumbs/85/93043176.jpg) 23.2.2017 EN Official Journal of the European Union L 46/53 DECISION OF THE EEA JOINT COMMITTEE No 206/2016 of 30 September 2016 amending Annex IX (Financial services) to the EEA Agreement [2017/283] THE
23.2.2017 EN Official Journal of the European Union L 46/53 DECISION OF THE EEA JOINT COMMITTEE No 206/2016 of 30 September 2016 amending Annex IX (Financial services) to the EEA Agreement [2017/283] THE
European Directive 2007/23/EC on the Placing on the Market of Pyrotechnical Articles: Are you concerned?
 Author manuscript, published in "35. International Pyrotechnics Seminar (IPS 2008), Fort Collins : United States (2008)" European Directive 2007/23/EC on the Placing on the Market of Pyrotechnical Articles:
Author manuscript, published in "35. International Pyrotechnics Seminar (IPS 2008), Fort Collins : United States (2008)" European Directive 2007/23/EC on the Placing on the Market of Pyrotechnical Articles:
YOUTH OLYMPIC GAMES 2018: ARCHERY
 Page 1 of 12 YOUTH OLYMPIC GAMES 2018: ARCHERY NOMINATION POLICY The Buenos Aires 2018 YOUTH OLYMPIC GAMES are to be held in Buenos Aires, Argentina from 6 th to 18 th October 2018. This Policy applies
Page 1 of 12 YOUTH OLYMPIC GAMES 2018: ARCHERY NOMINATION POLICY The Buenos Aires 2018 YOUTH OLYMPIC GAMES are to be held in Buenos Aires, Argentina from 6 th to 18 th October 2018. This Policy applies
The Children s Hospital of Philadelphia Committee for the Protection of Human Subjects Policies and Procedures. Cooperative Agreements
 Page: 1 of 8 I. PURPOSE This standard operating procedure describes the IRB s procedures for review and oversight of research conducted under a cooperative agreement with an external (outside) IRB. It
Page: 1 of 8 I. PURPOSE This standard operating procedure describes the IRB s procedures for review and oversight of research conducted under a cooperative agreement with an external (outside) IRB. It
Pressure Equipment Directive PED 2014/68/EU Commission's Working Group "Pressure"
 I. MISCELLANEOUS Guideline I-01 Guideline related to: Article 4 paragraph 3 What is to be understood by "sound engineering practice"? Sound engineering practice means, without prejudice to Article 5, paragraph
I. MISCELLANEOUS Guideline I-01 Guideline related to: Article 4 paragraph 3 What is to be understood by "sound engineering practice"? Sound engineering practice means, without prejudice to Article 5, paragraph
Children s Hospital of Philadelphia Committees for the Protection of Human Subjects Policies and Procedures Continuing Review of Approved Research
 Page: 1 of 6 I. PURPOSE The purpose of this SOP is to describe the submission requirements for investigators and the review requirements for the IRB for the conduct of continuing review in accordance with
Page: 1 of 6 I. PURPOSE The purpose of this SOP is to describe the submission requirements for investigators and the review requirements for the IRB for the conduct of continuing review in accordance with
Appendix C - Guidance for Integrating EFH Consultations with Endangered Species Act Section 7 Consultations
 Appendix C - Guidance for Integrating EFH Consultations with Endangered Species Act Section 7 Consultations C.1 Guidance for Integrating Magnuson-Stevens Fishery Conservation and Management Act EFH Consultations
Appendix C - Guidance for Integrating EFH Consultations with Endangered Species Act Section 7 Consultations C.1 Guidance for Integrating Magnuson-Stevens Fishery Conservation and Management Act EFH Consultations
Original language: English CoP17 Doc CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA
 Original language: English CoP17 Doc. 39.2 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA Seventeenth meeting of the Conference of the Parties Johannesburg (South Africa),
Original language: English CoP17 Doc. 39.2 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA Seventeenth meeting of the Conference of the Parties Johannesburg (South Africa),
Baptist Health Institutional Review Board. Study Closure Report (Expedited Review) IRB #: Study Title:
 Baptist Health Institutional Review Board Study Closure Report (Expedited Review) (Please complete ALL sections of this form. Incomplete forms will be returned) Principal Investigator: E-mail: Phone #:
Baptist Health Institutional Review Board Study Closure Report (Expedited Review) (Please complete ALL sections of this form. Incomplete forms will be returned) Principal Investigator: E-mail: Phone #:
MSC Guidelines for the Review of Mobile Offshore Drilling Unit (MODU) General Arrangement Plans Procedure Number: C2-36 Revision Date: 10/18/2016
 T. O. Phillips, CDR, Chief, Tank Vessel & Offshore Division Purpose: The purpose of this document is to provide guidance and information regarding the submission of MODU general arrangement plans under
T. O. Phillips, CDR, Chief, Tank Vessel & Offshore Division Purpose: The purpose of this document is to provide guidance and information regarding the submission of MODU general arrangement plans under
MSC Guidelines for Review of Cargo and Miscellaneous Vessel Stability (Subchapter I)
 S. E. HEMANN, CDR, Chief, Hull Division Purpose The purpose of this Plan Review Guideline is to provide the submitter with general guidance and information for the preparation and submission of stability
S. E. HEMANN, CDR, Chief, Hull Division Purpose The purpose of this Plan Review Guideline is to provide the submitter with general guidance and information for the preparation and submission of stability
Ch. 870 SUPER 6 LOTTO CHAPTER 870. SUPER 6 LOTTO
 Ch. 870 SUPER 6 LOTTO 61 870.1 CHAPTER 870. SUPER 6 LOTTO Sec. 870.1. Creation. 870.2. Purpose. 870.3. Definitions. 870.4. Ticket sales retailers. 870.5. Ticket price. 870.6. Super 6 Lotto bet slip and
Ch. 870 SUPER 6 LOTTO 61 870.1 CHAPTER 870. SUPER 6 LOTTO Sec. 870.1. Creation. 870.2. Purpose. 870.3. Definitions. 870.4. Ticket sales retailers. 870.5. Ticket price. 870.6. Super 6 Lotto bet slip and
ECHA s support to BPR helpdesks: state of play
 ECHA s support to BPR helpdesks: state of play CA-Dec13-Doc.5.1.f 54 th BPR MSCAs meeting December 2013, Brussels Henna Piha HelpNet Secretary, ECHA 05 December 2013 1 ECHA s support to BPR helpdesks:
ECHA s support to BPR helpdesks: state of play CA-Dec13-Doc.5.1.f 54 th BPR MSCAs meeting December 2013, Brussels Henna Piha HelpNet Secretary, ECHA 05 December 2013 1 ECHA s support to BPR helpdesks:
Traceable calibration of automatic weighing instruments in dynamic operation
 Traceable calibration of automatic weighing instruments in dynamic operation Matej Grum* Metrology Institute of the Republic of Slovenia, Grudnovo nabrežje 17, 1000 Ljubljana, Slovenia Abstract. The article
Traceable calibration of automatic weighing instruments in dynamic operation Matej Grum* Metrology Institute of the Republic of Slovenia, Grudnovo nabrežje 17, 1000 Ljubljana, Slovenia Abstract. The article
ISO 9244 INTERNATIONAL STANDARD. Earth-moving machinery Machine safety labels General principles
 INTERNATIONAL STANDARD ISO 9244 Second edition 2008-06-15 Earth-moving machinery Machine safety labels General principles Engins de terrassement Étiquetage de sécurité de la machine Principes généraux
INTERNATIONAL STANDARD ISO 9244 Second edition 2008-06-15 Earth-moving machinery Machine safety labels General principles Engins de terrassement Étiquetage de sécurité de la machine Principes généraux
SHIP DESIGN AND EQUIPMENT
 E MARITIME SAFETY COMMITTEE 92nd session Agenda item 13 MSC 92/INF.7 5 April 2013 ENGLISH ONLY SHIP DESIGN AND EQUIPMENT Sample form for ship-specific plans and procedures for recovery of persons from
E MARITIME SAFETY COMMITTEE 92nd session Agenda item 13 MSC 92/INF.7 5 April 2013 ENGLISH ONLY SHIP DESIGN AND EQUIPMENT Sample form for ship-specific plans and procedures for recovery of persons from
EMERGENCY USE 03/02/2016
 DUKE UNIVERSITY HEALTH SYSTEM Human Research Protection Program EMERGENCY USE 03/02/2016 Emergency Exemption for an Investigational Drug or Biologic or Unapproved Device Emergency use of an investigational
DUKE UNIVERSITY HEALTH SYSTEM Human Research Protection Program EMERGENCY USE 03/02/2016 Emergency Exemption for an Investigational Drug or Biologic or Unapproved Device Emergency use of an investigational
Safety Data Sheet According to Regulation (EC) No 1907/2006
 Safety Data Sheet According to Regulation (EC) No 1907/2006 Revision: 2012-12-12 01/01/2017 Dove(*) Beauty Cream Bar SECTION 1: Identification of the substance/mixture and of the company/undertaking Version
Safety Data Sheet According to Regulation (EC) No 1907/2006 Revision: 2012-12-12 01/01/2017 Dove(*) Beauty Cream Bar SECTION 1: Identification of the substance/mixture and of the company/undertaking Version
Safety Data Sheet According to Regulation (EC) No 1907/2006
 Safety Data Sheet According to Regulation (EC) No 1907/2006 Soft Care Bac H4 Revision: 2012-09-28 Version 02 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product
Safety Data Sheet According to Regulation (EC) No 1907/2006 Soft Care Bac H4 Revision: 2012-09-28 Version 02 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product
CHAPTER Committee Substitute for Committee Substitute for Committee Substitute for House Bill No. 41
 CHAPTER 2015-101 Committee Substitute for Committee Substitute for Committee Substitute for House Bill No. 41 An act relating to hazardous walking conditions; providing a short title; amending s. 1006.23,
CHAPTER 2015-101 Committee Substitute for Committee Substitute for Committee Substitute for House Bill No. 41 An act relating to hazardous walking conditions; providing a short title; amending s. 1006.23,
Racing Rules of Sailing
 Racing Rules of Sailing 2017-2020 APPENDIX L SAILING INSTRUCTIONS GUIDE This guide provides a set of tested sailing instructions designed primarily for major championship regattas for one or more classes.
Racing Rules of Sailing 2017-2020 APPENDIX L SAILING INSTRUCTIONS GUIDE This guide provides a set of tested sailing instructions designed primarily for major championship regattas for one or more classes.
IRB Training October 22, 2015
 Humanitarian Use Devices & Humanitarian Device Exemptions IRB Training October 22, 2015 What is a Humanitarian Use Device (HUD)? Intended to benefit patients in the treatment or diagnosis of diseases or
Humanitarian Use Devices & Humanitarian Device Exemptions IRB Training October 22, 2015 What is a Humanitarian Use Device (HUD)? Intended to benefit patients in the treatment or diagnosis of diseases or
Safety When Using Liquid Coatings
 Page 1 of 14 Contents: 1. Object 2. System requirements 3. Safety concept structure 4. The explosion protection concept 5. Using the tools 6. Sample explosion protection documents 7. Creating the explosion
Page 1 of 14 Contents: 1. Object 2. System requirements 3. Safety concept structure 4. The explosion protection concept 5. Using the tools 6. Sample explosion protection documents 7. Creating the explosion
BOOK 1 GENERAL REGULATION
 REGULATIONS BOOK 1 GENERAL REGULATION 26 September 2018 1 TABLE OF CONTENTS PART 1 PURPOSE OF GENERAL REGULATION... 3 PART 2 STATUS OF ALL REGULATIONS... 3 PART 3 COMMENCEMENT DATE OF ALL REGULATIONS...
REGULATIONS BOOK 1 GENERAL REGULATION 26 September 2018 1 TABLE OF CONTENTS PART 1 PURPOSE OF GENERAL REGULATION... 3 PART 2 STATUS OF ALL REGULATIONS... 3 PART 3 COMMENCEMENT DATE OF ALL REGULATIONS...
CONTINUING REVIEW CRITERIA FOR RENEWAL
 1. POLICY Steering Committee approved / Effective Date: 9/2/19/19/11 The IRB conducts continuing review of research taking place within its jurisdiction at intervals appropriate to the degree of risk,
1. POLICY Steering Committee approved / Effective Date: 9/2/19/19/11 The IRB conducts continuing review of research taking place within its jurisdiction at intervals appropriate to the degree of risk,
Traffic Impact Analysis (TIA) Process and Procedures Manual. September 2017
 Traffic Impact Analysis (TIA) Process and Procedures Manual Post Office Box 664 101 Huntersville-Concord Road Huntersville, NC 28070 Phone 704-766-2220 Fax 704-992-5528 www.huntersville.org (c) 2017, Town
Traffic Impact Analysis (TIA) Process and Procedures Manual Post Office Box 664 101 Huntersville-Concord Road Huntersville, NC 28070 Phone 704-766-2220 Fax 704-992-5528 www.huntersville.org (c) 2017, Town
INTERIM ADVICE NOTE 150/12. Guidance for Alternative Temporary Traffic Management Techniques for Relaxation Schemes on Dual Carriageways.
 INTERIM ADVICE NOTE 150/12 Guidance for Alternative Temporary Traffic Management Techniques for Relaxation Schemes on Dual Carriageways Summary Guidance for temporary traffic management (TTM), on the approach
INTERIM ADVICE NOTE 150/12 Guidance for Alternative Temporary Traffic Management Techniques for Relaxation Schemes on Dual Carriageways Summary Guidance for temporary traffic management (TTM), on the approach
FINA HIGH DIVING RULES
 PART VI FINA HIGH DIVING RULES 2017 2021 HD 1 HD 2 HD 3 HD 4 HD 5 HD 6 HD 7 HD 8 General Competitions Statement of Dives Competition Procedure Duties of the Referee and Assistant Referees Duties of the
PART VI FINA HIGH DIVING RULES 2017 2021 HD 1 HD 2 HD 3 HD 4 HD 5 HD 6 HD 7 HD 8 General Competitions Statement of Dives Competition Procedure Duties of the Referee and Assistant Referees Duties of the
UK Pavement Management System. Technical Note 45. Data Topic guidance notes for UKPMS Developers. Version Number 2.00 Issue
 UK Pavement Management System Technical Note 45 Data Topic 130-02 guidance notes for UKPMS Developers Version Number 2.00 Issue February 2019 Document Information Title (Sub Title) Technical Note 45 Data
UK Pavement Management System Technical Note 45 Data Topic 130-02 guidance notes for UKPMS Developers Version Number 2.00 Issue February 2019 Document Information Title (Sub Title) Technical Note 45 Data
Understood, Inc. User Guide SCUBA Solutions Version 1.7
 Understood, Inc. User Guide SCUBA Solutions Version 1.7 Table of Contents Revision History... 4 Introduction... 5 Purpose... 5 Scope... 5 Home... 5 Today s Dive Trips [Display]:... 6 Next Dive Trip [Display]:...
Understood, Inc. User Guide SCUBA Solutions Version 1.7 Table of Contents Revision History... 4 Introduction... 5 Purpose... 5 Scope... 5 Home... 5 Today s Dive Trips [Display]:... 6 Next Dive Trip [Display]:...
Section 1 Preparation
 IOC Toolkit for safeguarding athletes from harassment and abuse in sport Section 1 Preparation In the introduction we considered why it is important for sports organisations to develop policies and procedures
IOC Toolkit for safeguarding athletes from harassment and abuse in sport Section 1 Preparation In the introduction we considered why it is important for sports organisations to develop policies and procedures
IRB MEETING ADMINISTRATION
 1. POLICY Steering Committee approved / Effective date: 9/11/15 Except when an expedited review procedure is appropriate or an exemption determination may be made, the IRB will review proposed research
1. POLICY Steering Committee approved / Effective date: 9/11/15 Except when an expedited review procedure is appropriate or an exemption determination may be made, the IRB will review proposed research
S T A T E O F M I C H I G A N BOARD OF COMMISSIONERS OF THE COUNTY OF ALLEGAN. January 14, 2010 EMERGENCY MANAGEMENT APPROVE EMERGENCY OPERATIONS PLAN
 DRAFT 67-232 S T A T E O F M I C H I G A N BOARD OF COMMISSIONERS OF THE COUNTY OF ALLEGAN January 14, 2010 EMERGENCY MANAGEMENT APPROVE EMERGENCY OPERATIONS PLAN WHEREAS, averting the threat of, or minimizing
DRAFT 67-232 S T A T E O F M I C H I G A N BOARD OF COMMISSIONERS OF THE COUNTY OF ALLEGAN January 14, 2010 EMERGENCY MANAGEMENT APPROVE EMERGENCY OPERATIONS PLAN WHEREAS, averting the threat of, or minimizing
JOE NEGRON RICHARD CORCORAN THE FLORIDA LEGISLATURE JOINT ADMINISTRATIVE PROCEDURES COMMITTEE. Representative George R. Moraitis, Jr.
 JOE NEGRON RICHARD CORCORAN President Speaker THE FLORIDA LEGISLATURE JOINT ADMINISTRATIVE PROCEDURES COMMITTEE Senator Kevin Rader, Chair KENNETH J. PLANTE Representative George R. Moraitis, Jr., Vice
JOE NEGRON RICHARD CORCORAN President Speaker THE FLORIDA LEGISLATURE JOINT ADMINISTRATIVE PROCEDURES COMMITTEE Senator Kevin Rader, Chair KENNETH J. PLANTE Representative George R. Moraitis, Jr., Vice
Forty-first Session of the Executive Council UNESCO, Paris, 24 June 1 st July 2008
 Restricted Distribution IOC/EC-XLI/2 Annex 11 Paris, 21 May 2008 Original: English INTERGOVERNMENTAL OCEANOGRAPHIC COMMISSION (of UNESCO) Forty-first Session of the Executive Council UNESCO, Paris, 24
Restricted Distribution IOC/EC-XLI/2 Annex 11 Paris, 21 May 2008 Original: English INTERGOVERNMENTAL OCEANOGRAPHIC COMMISSION (of UNESCO) Forty-first Session of the Executive Council UNESCO, Paris, 24
Articles By-Laws Regulations History
 Articles By-Laws Regulations History Effective 2007-2008 Season www.hockeycanada.ca As adopted at Ottawa, December 4, 1914 and amended to June 2007. HOCKEY CANADA Articles By-Laws Regulations History As
Articles By-Laws Regulations History Effective 2007-2008 Season www.hockeycanada.ca As adopted at Ottawa, December 4, 1914 and amended to June 2007. HOCKEY CANADA Articles By-Laws Regulations History As
2019 Formula SAE Japan Local Rules Number 2 Issued: January 18, 2019
 2019 Formula SAE Japan Local Rules Number 2 Issued: January 18, 2019 The purpose of this document is to inform of the local rules that shall apply to 2019 Formula SAE Japan. Any additional local rules
2019 Formula SAE Japan Local Rules Number 2 Issued: January 18, 2019 The purpose of this document is to inform of the local rules that shall apply to 2019 Formula SAE Japan. Any additional local rules
The Best Use of Lockout/Tagout and Control Reliable Circuits
 Session No. 565 The Best Use of Lockout/Tagout and Control Reliable Circuits Introduction L. Tyson Ross, P.E., C.S.P. Principal LJB Inc. Dayton, Ohio Anyone involved in the design, installation, operation,
Session No. 565 The Best Use of Lockout/Tagout and Control Reliable Circuits Introduction L. Tyson Ross, P.E., C.S.P. Principal LJB Inc. Dayton, Ohio Anyone involved in the design, installation, operation,
SAILING INSTRUCTIONS
 APPENDIX L This guide provides a set of tested sailing instructions designed primarily for major championship regattas for one or more classes. It therefore will be particularly useful for world, continental
APPENDIX L This guide provides a set of tested sailing instructions designed primarily for major championship regattas for one or more classes. It therefore will be particularly useful for world, continental
MSC Guidelines for the Review of OSV Stability
 MSC Guidelines for the Review of OSV Stability Procedure Number: C1-06 Revision Date: February 13, 2013 R. J. LECHNER, CDR, Tank Vessel and Offshore Division Purpose To establish a procedure for reviewing
MSC Guidelines for the Review of OSV Stability Procedure Number: C1-06 Revision Date: February 13, 2013 R. J. LECHNER, CDR, Tank Vessel and Offshore Division Purpose To establish a procedure for reviewing
Safety Data Sheet. acc. to OSHA HCS. 2: Hazard(s) identification. Page 1/6 Printing date 10/26/2015 Reviewed on 10/26/2015. * 1: Identification
 Page 1/6 * 1: Identification 1.1 Product identifier Product Code: 106791 CAS Number: 1680-31-5 EC number: 605-498-9 1.2 Relevant identified uses of the substance or mixture and uses advised against No
Page 1/6 * 1: Identification 1.1 Product identifier Product Code: 106791 CAS Number: 1680-31-5 EC number: 605-498-9 1.2 Relevant identified uses of the substance or mixture and uses advised against No
Canada s Plant Breeders Rights (PBR) Intellectual Property (IP) Framework
 Canada s Plant Breeders Rights (PBR) Intellectual Property (IP) Framework Presented to: Canadian Horticultural Council (CHC) - AGM Date Presented: March 10, 2016 Name of Presenter/: Anthony Parker, PBR
Canada s Plant Breeders Rights (PBR) Intellectual Property (IP) Framework Presented to: Canadian Horticultural Council (CHC) - AGM Date Presented: March 10, 2016 Name of Presenter/: Anthony Parker, PBR
International Convention on Maritime Search and Rescue, 1979
 International Convention on Maritime Search and Rescue, 1979 http://www.imo.org/conventions/contents.asp?doc_id=653&topic_id=257#top Adoption: 27 April 1979 Entry into force: 22 June 1985 Introduction
International Convention on Maritime Search and Rescue, 1979 http://www.imo.org/conventions/contents.asp?doc_id=653&topic_id=257#top Adoption: 27 April 1979 Entry into force: 22 June 1985 Introduction
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 8655-4 First edition 2002-09-15 Piston-operated volumetric apparatus Part 4: Dilutors Appareils volumétriques à piston Partie 4: Diluteurs Reference number ISO 8655-4:2002(E)
INTERNATIONAL STANDARD ISO 8655-4 First edition 2002-09-15 Piston-operated volumetric apparatus Part 4: Dilutors Appareils volumétriques à piston Partie 4: Diluteurs Reference number ISO 8655-4:2002(E)
Managing the TSR lifecycle
 Table of Contents MANAGING THE TSR LIFECYCLE... 1 OVERVIEW... 2 LIFECYCLE FOR PLANNED TSRS... 2 TYPICAL WORKFLOW (PLANNED TSRS)... 3 LIFECYCLE FOR UNPLANNED TSRS... 5 TYPICAL WORKFLOW (UNPLANNED TSRS)...
Table of Contents MANAGING THE TSR LIFECYCLE... 1 OVERVIEW... 2 LIFECYCLE FOR PLANNED TSRS... 2 TYPICAL WORKFLOW (PLANNED TSRS)... 3 LIFECYCLE FOR UNPLANNED TSRS... 5 TYPICAL WORKFLOW (UNPLANNED TSRS)...
DEPARTMENT OF ENVIRONMENTAL PROTECTION Office of Oil and Gas Management
 DEPARTMENT OF ENVIRONMENTAL PROTECTION Office of Oil and Gas Management DOCUMENT NUMBER: 800-0810-003 TITLE: EFFECTIVE DATE: AUTHORITY: POLICY: PURPOSE: APPLICABILITY: DISCLAIMER: Guidelines for Development
DEPARTMENT OF ENVIRONMENTAL PROTECTION Office of Oil and Gas Management DOCUMENT NUMBER: 800-0810-003 TITLE: EFFECTIVE DATE: AUTHORITY: POLICY: PURPOSE: APPLICABILITY: DISCLAIMER: Guidelines for Development
