PHYSICS APPLIED TO ANAESTHESIA III: THE PROPERTIES OF LIQUIDS, GASES AND VAPOURS
|
|
|
- Claribel Morris
- 5 years ago
- Views:
Transcription
1 Brit. J. Anaesth. (1965), 37, 967 PHYSICS APPLIED TO ANAESTHESIA III: THE PROPERTIES OF LIQUIDS, GASES AND VAPOURS BY D. W. HILL Research Department of Anaesthetics, Royal College of Surgeons of England, London Density The pressure exerted by a column of liquid or gas depends on its density. Density is defined as mass per unit volume. The density of water is 1 g per ml at 4 C, and that of mercury 13.6 g per ml at 20 G. Density is temperature-dependent and, strictly speaking, a value for a density must always include a statement of the ambient temperature. Although static columns of liquid are much used in manometers for measuring pressure, the majority of applications of liquids and vapours in surgery and anaesthesia are concerned with the movement of these substances. In Part II of this series, the work cost of moving a given volume of gas was calculated. In practice, we are mainly concerned with the movement of gases and liquids through pipes or tubes, and it is necessary to know which properties are important. Viscosity It can be shown that for a given pressure drop developed across a given pipe, the volume of liquid or gas flowing per unit time depends on its viscosity. In a viscous fluid, the velocity of adjacent layers of the fluid will differ. The viscosity of a liquid is defined as the resistance which it exhibits to the flow of one layer over another. The layers in contact with the walls are assumed to be at rest, and the linear velocity of the fluid along the length of the tube increases towards the centre. Consider two layers of area A situated at distances x and (x+dx) from one wall. Let the linear velocities of the planes be V and (V+dV). Due to the internal friction between the layers, there exists a tangential viscous force between them. The value of this force is given by 17 A This is Newton's law for viscous flow (for streamline as opposed to turbulent flow). The quantity T is termed the coefficient of viscosity of the fluid. d V For moderate pressure gradients, the individual molecules of the fluid progress down the pipe in orderly fashion. This is known as laminar or streamline flow. Under these conditions, the volume of fluid passing per second Q is given by the wellknown Poiseuille's formula * Srjl where P is the pressure gradient across the pipe, r is the radius of the pipe, and / is the length. It is seen that for a given gas, pressure gradient, and length of the pipe, the volume of gas that can be passed depends on the fourth power of the radius. Thus doubling the radius will increase the flow of gas by a factor of 2*= 16. It is for this reason that the bore of endotracheal tubes and connectors should be kept as large as possible. For sick patients breathing spontaneously with a small respiratory effort, the length and bore of any breathing tubes may be of importance. A pneumotachograph head is basically a resistance to gas flow, so designed that the pressure drop produced across it is linearly related to the volume flow rate over a stated range. The well-knowa design due to Professor Fleisch of Lausanne consists of a large number of small-bore pipes in parallel. The flow in each is small enough to be streamline. From Poiseuille's equation it is seen that the volume of gas passing in unit time is directly proportional to the pressure drop, providing the flow pattern remains streamline. Increasing the applied pressure above a certain value for the tube will cause the orderly motion of the molecules to break down. At higher values of applied pressure the flow becomes turbulent, the molecules swirling around in eddies. This is a less efficient way of transporting the gas or liquid. In between the fully streamline and fully turbulent conditions, there is a transitional region. The velocity V e at which fully turbulent flow sets in is known as the critical velocity.
2 968 BRITISH JOURNAL OF ANAESTHESIA It can be shown that v c = k -l where k is a constant known as Reynold's Number, r is the radius of the tube, and p is the density of the fluid. At low fluid velocities, when the motion is streamline, the volume flow is proportional to the pressure gradient. When the velocity is increased above the critical value, the volume flow increases less rapidly with pressure. It soon becomes independent of the viscosity of the fluid, and dependent mainly on its density. When fully turbulent, the volume flow is nearly proportional to the square root of the pressure. The pressure is now used to overcome the turbulent motion and in communicating kinetic energy to the fluid. Examples of turbulent flow are to be found in both the respiratory and circulatory systems. The sound heard during quiet breathing in a healthy person, using a stethoscope, constitutes the normal respiratory murnr.ir. The inspiratory murmur is a soft blowing sound of a frequency averaging 350 c.p.s. (Graves and Graves, 1964). It is related to turbulence as air flows from the small air passages into the alveoli. It is heard only because it takes place so near to the stethoscope. In the case of the circulation, turbulent flow is usually seen only at the root of the aorta, and possibly at the heart valves where it may be responsible for the sound of heart murmurs. Elsewhere the flow of blood is streamline, except in the smaller vessels, the arterioles and the capillaries. Here the bloodflowis faster than would be expected on the basis of Poiseuille's equation (Nightingale, 1959). The red cells tend to congregate along the axis of the tube, leaving a layer of plasma along the walls. The plasma has a lower viscosity than does whole blood, and this allows the cells to pass more rapidly than would uniform whole blood. The anomalous viscosity of blood is well discussed by Bayliss (1962). Turbulent flow conditions may arise from the rapid flow of blood through dilated vessels, if the force and speed of the blood passing is sufficient. It may be possible to hear sounds usually referred to as "bruits"; the bruit generally pulses in time with the heartbeat. Vascular bruits arising from the local turbulence may also be produced by such things as sudden changes in the smoothness of the vessel wall or diameter of the vessel. Turbulence occurring when a vessel branches is discussed by Krovetz (1965). Units of viscosity. The units of viscosity can be readily determined by substituting the appropriate dimensions for the other terms in Poiseuille's equation. Thus Q = volume per unit time (L 3 /T), P=pressure, force per unit area, (M.L.T.~ 2 /L 2 ), so that since on substituting the appropriate dimensions we have ML L 4 T _ M. T 2 L 2 0 L = TL The dimensional formula for viscosity is thus M.L^T- 1. This can be rewritten as MLT- 2.T.L.~ 2 or dynes per second per square centimetre. The unit of viscosity on the c.g.s. system is the poise where one poise equals 1 dyne per second per square centimetre. This is further divided into centipoises where 100 centipoises equal 1 poise. A mean value for the viscosity of whole blood in vivo is 2.70 centipoises. That of water is 1 centipose at 20 C. ' The onset of turbulence as expressed in the concept of critical velocity depends on the kinematic viscosity which is given by viscosity _ r\ density p for the gas or liquid. The dimensional formula for kinematic viscosity is L 2 T -1. The unit on the c.g.s. system is the Stokes where 1 Stokes is equal to 1 square centimetre per second. The kinematic viscosity for oxygen is approximately twice that for nitrous oxide, so that for a given tube and pressure drop one would expect that the flow for nitrous oxide would become turbulent at a considerably lower flow rate than would be the case for oxygen. The flow of fluids through orifices. In a pipe or tube, the length is large compared with the radius but in the case of an orifice, the length is less than that of the radius. Theflowof gas through an orifice is always partly turbulent and the rate of flow is dependent on the density of the gas rather than the viscosity. The smaller the density, the less is the pressure drop required across the orifice to maintain a given gas flow. For this reason, a helium-oxygen mixture is sometimes ad-
3 PHYSICS APPLIED TO ANAESTHESIA ffl ministered to patients suffering from respiratory obstruction, since this mixture can be respired with less effort than air. Measuring orifice C0 2 ABsorber Critical orifice To pump Sample Inlet tlg. I Critical orifice carbon dioxide analyzer. The critical orifice. An interesting case arises in that of the critical orifice. If the pressure on one side of a thin, sharpwalled, orifice is less than 0.58 times the absolute pressure on the upstream side of the orifice, the gas passing through the orifice attains the velocity of sound in the gas. The velocity of sound in a gas is given by v = / cm/sec, V p where y is the ratio of the specific heats at constant pressure and constant volume for the gas, P is the pressure at the orifice in dynes per sq.cm and p is the density of the gas in g/ml. If the cross-sectional area of the orifice is A cm2, then the volume flow rate V of gas through the orifice is given by V P A simple carbon dioxide analyzer based on this principle is shown in figure 1 (Mead and Collier, 1959). The critical orifice ensures a constant outflow of gas from the carbon dioxide absorber. The pressure across the inlet, non-critical, orifice depends on the rate at which gas enters the arrangement which in turn depends on the rate of absorption of carbon dioxide. The manometer can thus be calibrated in terms of the carbon dioxide concentration in the gas stream. Surface tension The attractive forces existing between molecules give rise, in liquids, to a phenomenon known as surface tension. In the bulk of the liquid, a mole- 969 cule is surrounded by others on all sides, and the attractive forces between molecules will tend to cancel. However, a molecule at the surface will receive a net inward force from those below it. The fact that a liquid surface contracts spontaneously shows that there is free energy associated with it, and that work must be done to extend the surface. The surface of a liquid acts at all times as though it has a thin membrane stretched over it, and for any specified volume of matter, a sphere has a smaller surface area than any other geometrical figure. Hence droplets of water and soap bubbles assume a spherical shape as they fall through the air. The work done in extending by 1 cm a surface which is pulling with a tension of S dynes per cm is 5 ergs per sq.cm. An erg is a dyne cm, and the c.g.s. unit of surface tension is dynes per cm. For example, the surface tension of water at 20 C is 73 dynes per cm It can be shown that the pressure existing inside a bubble is given by 2S\r dynes per sq.cm, where 5 is the surface tension of the liquid coating of the bubble, and r is the bubble radius in cm. The pressure inside the bubble is raised by the action of the compressive tension of the film. This force can be high when the bubble radius is small, resulting in a stable bubble. Such droplets may cause trouble when it is desired to vaporize a liquid such as halothane in order to produce accurate calibration concentrations of halothane vapour For example, the saturation vapour pressure of halothane cooled to -30 C is 16.8 mm Hg. Assuming a barometric pressure of 760 mm Hg, this will correspond to a halothane concentration of 2.2 per cent v/v. However, a rather higher value is produced due to the formation of droplets unless the issuing vapour is passed through a condensing coil immersed in the coolant bath, to trap out any small droplets of liquid halothane. Without this precaution, the small droplets are swept along with the vapour stream, and evaporate to produce an enhanced concentration. Surface tension plays an important part in the contraction of the lungs. The alveoli can be considered as a large number of bubbles, each communicating with the lung volume via a duct. If the surface tension of the alveolar lining was constant, then the pressure in the smaller alveoli would be higher than that in the larger alveoli. On this basis, one would have an unstable situation,
4 970 BRITISH JOURNAL OF ANAESTHESIA with the smaller alveoli tending to discharge into the larger ones. Brown, Johnson and Clements (1959), and Clements and associates (1961) have shown that the surface tension of the lung is not constant, but decreases markedly on compression resulting from deflation. With changes of surface area of less than 50 per cent the lung surface tension approaches a limiting value of dynes per cm. On re-expansion of the surface, an upper limiting tension of dynes per cm is approached. Considerable hysteresis occurs, and indicates a major alteration of the surface material when compressed beyond 50 per cent. The diminution in surface tension with reduction of area is thought to be due to the action of a lipoprotein which is known as the "anti-atelectatic substance" or "surfactant". Diffusion of gases. Gas molecules will move, by a process known as diffusion, from a place where there is a higher partial pressure of the gas concerned, to one where there is a lower partial pressure. This is a molecular process, and is not to be confused with processes such as convection in which a movement occurs of the gas in bulk. Thus gas molecules can diffuse through pores of a porous membrane to make the partial pressures equal on either side. The oxygenation of the venous blood in the lung capillaries arises from the diffusion of oxygen into the blood from the alveolar air. Similarly, carbon dioxide diffuses from the venous blood into the alveoli. Another example occurs with diffusion respiration. This was reported in the dog by Draper and Whitehead (1944), who found that under certain conditions, blood will be normally oxygenated from the lungs in the absence of respiratory movements and rhythmical pressure changes in the lungs. Draper and Whitehead (1949) explain the phenomenon as follows. The marked affinity of oxygen for haemoglobin causes the amount of oxygen removed from the alveoli during apnoea to exceed the amount of carbon dioxide that simultaneously leaves the blood. The net result is to reduce the pressure in the alveoli to below atmospheric, thus giving rise to a continuous inward flow of the deadspace gas and the external atmosphere. Essential conditions for diffusion respiration are a high percentage of oxygen in the lungs and in the deadspace, a free airway and an adequate circulation. The technique of diffusion respiration has been applied to man by Enghoff, Holmdahl and Risholm (1951), Payne (1962) and others. The quantitative treatment of diffusion is based upon Pick's law of diffusion, which is analogous to Ohm's law of conduction of electricity. Fields law states that the rate of diffusion is proportional to the concentration gradient, that is to the change of concentration per unit length in the direction of diffusion. An important case arises in the diffusion of oxygen and carbon dioxide across the alveolar membrane. Carbon dioxide diffuses rapidly, widi the result that equilibration is normally reached between the alveolar air and the capillary blood. Oxygen may not always achieve equilibrium, and this fact is reflected in the diffusion capacity for oxygen. Fields law may be stated in the form dq = - dt dx where dq is the volume of oxygen diffusing across the alveolar membrane in a time interval dt, k is the diffusion constant for oxygen and the alveolar membrane, 5 is the area of membrane through which the oxygen diffuses, and dc\dx is the concentration gradient across the membrane of thickness dx. For a thin membrane, dc ci cz dx Thus._ -ksfa-c&t By means of the Bunsen solubility coefficient for oxygen, the concentrations c\ and ci can be related to the gas tensions on either side of die membrane, i.e. and B where a is the Bunsen solubility coefficient, B is the barometric pressures and/>i and/>2 are the tensions. Pick's law equation becomes dq -ksa dt fi~ (pl ^ or
5 PHYSICS APPLIED TO ANAESTHESIA 971 where D is called the pulmonary diffusing capacity. In practice, the diffusing capacity for oxygen can be denned as DOz = where V02 is the volume of oxygen diffusing per minute, PAO, is the oxygen tension in the alveoli, and PCo, is the effective oxygen tension in the capillary blood. The units of pulmonary diffusing capacity for oxygen are ml of 02/per minute/mm Hg pressure difference across the lung. A typical value would be 21 ml Oz/min/mm Hg for D02 in a normal adult subject at rest. A clear account of diffusion processes in the lungs is given by Comroe and his colleagues (1962). Graham's law of diffusion states that the rates of diffusion of gases through certain membranes is inversely proportional to the square root of their molecular weight. The molecular weight of oxygen is 32, and that of carbon dioxide 44. The square roots of the molecular weights are in the ratio of 1.2:1, so that oxygen will diffuse faster by a factor of 20 per cent through a dry porous membrane than will carbon dioxide. The nature of the membrane, however, exerts an important influence on the rates of diffusion. For example, carbon dioxide diffuses rather more rapidly than oxygen through a rubber-lined Douglas bag. Mills (1952) reports losses in the range 0.22 per cent v/v to 0.45 per cent v/v of carbon dioxide per day from Douglas bags due to diffusion. As mentioned in the calculation on pulmonary diffusing capacity, the effective tension of gases on either side of the moist alveolar membrane depends on the solubility of the gases involved. The diffusion rate of carbon dioxide across the alveolar membrane is high, due to the marked solubility of carbon dioxide in water (23 times that of oxygen). Thus sufficient excretion of carbon dioxide occurs, even though the difference is small between the mixed venous carbon dioxide tension of 46.5 mm Hg, and the 40 mm Hg partial pressure of alveolar air. Oxygen diffuses in the reverse direction under the influence of the pressure gradient between the mixed venous oxygen tension of 40 mm Hg and the partial pressure of oxygen in the alveolar air of 110 mm Hg, causing the arterial blood to have a normal tension of 95 mm Hg of oxygen. Nitrogen does not diffuse across the alveolar membrane since the tensions in venous blood, arterial blood and the partial pressure in alveolar air are all the same, i.e. 573 mm Hg. REFERENCES Bayliss, L. E. (1962). The rheology of blood; in The Handbook of Physiology, Vol. 2, section 2 (Circulation), p Washington: American Physiological Society. Brown, E. S., Johnson, R. P., and Clements, J. A. (1959). Pulmonary surface tension. J 1. appl. Physiol: 14, 717. Clements, J. A., Hustead, R. F., Johnson, R. P., and Gribetz, I. (1961). Pulmonary surface tension and alveolar stability. J. appl. Physiol., 16, 444. Comroe, J. H., Forster, R. E., Dubois, A. B., Briscoe, W. A., and Carlsen, Elizabeth (1962). The Lung, 2nd ed., p Chicago: Year Book. Draper, W. B., and Whitehead, R. W. (1944). Diffusion respiration in the dog anesthetized with Pentothal sodium. Anesthesiology, 5, 262. (1949). The phenomenon of diffusion respiration. Curr. Res. Anesth. Analg., 28, 307. Enghoff, H., Holmdahl, M. H:Son., and Risholm, L. (1951). Diffusion respiration in man. Nature, 168, 830. Graves, G., and Graves, Valerie (1964). Medical Sound Recording. London: Focal. Krovetz, L. J. (1965). The effect of vessel branching on haemodynamic stability. Phys. in Med. Biol., 10,417. Mead, J., and Collier, C. (1959). Relation of volume history of lungs to respiratory mechanism in anesthetized dogs. J. appl. Physiol., 14, 669. Mills, J. N. (1952). The use of an infra-red analyser in testing the properties of Douglas bags. J. Physiol. (Lond.), 116, 22P. Nightingale, A. (1959). Physics and Electronics in Physical Medicine. London: Bell. Payne, J. P. (1962). Apnoeic oxygenation in anesthetized man. Acta anaesth. scand., 6, 129.
Sign up to receive ATOTW weekly -
 THE PHYSICS OF FLOW ANAESTHESIA TUTORIAL OF THE WEEK 84 9TH APRIL 2008 Paul Clements, SpR in Anaesthetics, Hope Hospital, Salford, UK. Carl Gwinnutt, Consultant Anaesthetist, Hope Hospital, Salford, UK.
THE PHYSICS OF FLOW ANAESTHESIA TUTORIAL OF THE WEEK 84 9TH APRIL 2008 Paul Clements, SpR in Anaesthetics, Hope Hospital, Salford, UK. Carl Gwinnutt, Consultant Anaesthetist, Hope Hospital, Salford, UK.
Collin County Community College. Lung Physiology
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY
 BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY Dr.R.Selvakumar.M.D.D.A.DNB Professor of Anaesthesiology, K.A.P.Viswanatham Govt medical college, Trichy. The current practice of Anaesthesiology demands knowledge
BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY Dr.R.Selvakumar.M.D.D.A.DNB Professor of Anaesthesiology, K.A.P.Viswanatham Govt medical college, Trichy. The current practice of Anaesthesiology demands knowledge
Respiratory System. Part 2
 Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Section Two Diffusion of gases
 Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
I Physical Principles of Gas Exchange
 Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
RESPIRATORY GAS EXCHANGE
 RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
Lung Volumes and Capacities
 Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Section 1 Questions. Physics and Clinical Measurement... 3 Clinical Anaesthesia Physiology Pharmacology... 79
 Section 1 uestions Physics and Clinical Measurement... 3 Clinical Anaesthesia... 35 Physiology... 47 Pharmacology... 79 in this web service in this web service Physics and Clinical Measurement uestions
Section 1 uestions Physics and Clinical Measurement... 3 Clinical Anaesthesia... 35 Physiology... 47 Pharmacology... 79 in this web service in this web service Physics and Clinical Measurement uestions
Respiratory Pulmonary Ventilation
 Respiratory Pulmonary Ventilation Pulmonary Ventilation Pulmonary ventilation is the act of breathing and the first step in the respiratory process. Pulmonary ventilation brings in air with a new supply
Respiratory Pulmonary Ventilation Pulmonary Ventilation Pulmonary ventilation is the act of breathing and the first step in the respiratory process. Pulmonary ventilation brings in air with a new supply
CHEM 355 EXPERIMENT 7. Viscosity of gases: Estimation of molecular diameter
 CHEM 355 EXPERIMENT 7 Viscosity of gases: Estimation of molecular diameter Expressed most simply, the viscosity of a fluid (liquid or gas) relates to its resistance to flow. The viscosity of a gas is determined
CHEM 355 EXPERIMENT 7 Viscosity of gases: Estimation of molecular diameter Expressed most simply, the viscosity of a fluid (liquid or gas) relates to its resistance to flow. The viscosity of a gas is determined
The Physiologic Basis of DLCO testing. Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan
 The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
RSPT 1060 OBJECTIVES OBJECTIVES OBJECTIVES EQUATION OF MOTION. MODULE C Applied Physics Lesson #1 - Mechanics. Ventilation vs.
 RSPT 1060 MODULE C Applied Physics Lesson #1 - Mechanics OBJECTIVES At the end of this module, the student should be able to define the terms and abbreviations used in the module. draw & explain the equation
RSPT 1060 MODULE C Applied Physics Lesson #1 - Mechanics OBJECTIVES At the end of this module, the student should be able to define the terms and abbreviations used in the module. draw & explain the equation
By: Aseel Jamil Al-twaijer. Lec : physical principles of gas exchange
 By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
 Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Chapter 13 Fluids. Copyright 2009 Pearson Education, Inc.
 Chapter 13 Fluids Phases of Matter Density and Specific Gravity Pressure in Fluids Atmospheric Pressure and Gauge Pressure Pascal s Principle Units of Chapter 13 Measurement of Pressure; Gauges and the
Chapter 13 Fluids Phases of Matter Density and Specific Gravity Pressure in Fluids Atmospheric Pressure and Gauge Pressure Pascal s Principle Units of Chapter 13 Measurement of Pressure; Gauges and the
Surface Tension of the Alveoli. Aslı AYKAÇ, PhD. NEU Faculty of Medicine Department of Biophysics
 Surface Tension of the Alveoli Aslı AYKAÇ, PhD. NEU Faculty of Medicine Department of Biophysics Learning Objectives Define surface tension and use Laplace s law to understand how the pressure inside a
Surface Tension of the Alveoli Aslı AYKAÇ, PhD. NEU Faculty of Medicine Department of Biophysics Learning Objectives Define surface tension and use Laplace s law to understand how the pressure inside a
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation
 Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
AIIMS, New Delhi. Dr. K. K. Deepak, Prof. & HOD, Physiology AIIMS, New Delhi Dr. Geetanjali Bade, Asst. Professor AIIMS, New Delhi
 Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
RESPIRATORY REGULATION DURING EXERCISE
 RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
Appendix 2. Basic physical properties applied to the respiratory system
 Appendix 2. Basic physical properties applied to the respiratory system Fluid is a general definition of a state of matter characterized by a weak intermolecular connection (Van der Waal's cohesive forces),
Appendix 2. Basic physical properties applied to the respiratory system Fluid is a general definition of a state of matter characterized by a weak intermolecular connection (Van der Waal's cohesive forces),
2. State the volume of air remaining in the lungs after a normal breathing.
 CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
Essential Skills Course Acute Care Module. Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook
 Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
4/18/12 MECHANISM OF RESPIRATION. Every Breath You Take. Fun Facts
 Objectives MECHANISM OF RESPIRATION Dr Badri Paudel Explain how the intrapulmonary and intrapleural pressures vary during ventilation and relate these pressure changes to Boyle s law. Define the terms
Objectives MECHANISM OF RESPIRATION Dr Badri Paudel Explain how the intrapulmonary and intrapleural pressures vary during ventilation and relate these pressure changes to Boyle s law. Define the terms
The physiological functions of respiration and circulation. Mechanics. exercise 7. Respiratory Volumes. Objectives
 exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
Applied Physics Topics 2
 Applied Physics Topics 2 Dr Andrey Varvinskiy Consultant Anaesthetist Torbay Hospital, UK EDAIC Paper B Lead and Examiner TOPICS 2 Gas Laws Other Laws: Dalton, Avogadro Critical temperature Critical pressure
Applied Physics Topics 2 Dr Andrey Varvinskiy Consultant Anaesthetist Torbay Hospital, UK EDAIC Paper B Lead and Examiner TOPICS 2 Gas Laws Other Laws: Dalton, Avogadro Critical temperature Critical pressure
Human gas exchange. Question Paper. Save My Exams! The Home of Revision. Cambridge International Examinations. 56 minutes. Time Allowed: Score: /46
 Human gas exchange Question Paper Level Subject Exam oard Topic Sub Topic ooklet O Level iology ambridge International Examinations Respiration Human gas exchange Question Paper Time llowed: 56 minutes
Human gas exchange Question Paper Level Subject Exam oard Topic Sub Topic ooklet O Level iology ambridge International Examinations Respiration Human gas exchange Question Paper Time llowed: 56 minutes
Chapter 15 Fluid. Density
 Density Chapter 15 Fluid Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle By Dr. Weining man 1 Units of Chapter 15 Fluid
Density Chapter 15 Fluid Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle By Dr. Weining man 1 Units of Chapter 15 Fluid
Pulmonary Circulation Linda Costanzo Ph.D.
 Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
Lecture Outline Chapter 15. Physics, 4 th Edition James S. Walker. Copyright 2010 Pearson Education, Inc.
 Lecture Outline Chapter 15 Physics, 4 th Edition James S. Walker Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy
Lecture Outline Chapter 15 Physics, 4 th Edition James S. Walker Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy
Vapour pressure of liquids SURFACE TENSION
 Vapour pressure of liquids A liquid in a closed container is subjected to partial vapour pressure due to the escaping molecules from the surface; it reaches a stage of equilibrium when this pressure reaches
Vapour pressure of liquids A liquid in a closed container is subjected to partial vapour pressure due to the escaping molecules from the surface; it reaches a stage of equilibrium when this pressure reaches
IT is frequently desirable to be able to determine the
 NEW INVENTIONS A PORTABLE OXYGEN ANALYSER By A. BRACKEN Research and Development Department, The British Oxygen Company, Limited IT is frequently desirable to be able to determine the oxygen concentration
NEW INVENTIONS A PORTABLE OXYGEN ANALYSER By A. BRACKEN Research and Development Department, The British Oxygen Company, Limited IT is frequently desirable to be able to determine the oxygen concentration
Respiratory Physiology Gaseous Exchange
 Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
Monday, ! Today: Respiratory system! 5/20/14! Transport of Blood! What we ve been covering! Circulatory system! Parts of blood! Heart! tubing!
 Monday, 5.19.14! What we ve been covering! Circulatory system! Parts of blood! Heart! tubing! Transport of Blood! What is transported! Nutrients! Oxygen! Carbon Dioxide! Hormones! Antibodies! What it is/does!
Monday, 5.19.14! What we ve been covering! Circulatory system! Parts of blood! Heart! tubing! Transport of Blood! What is transported! Nutrients! Oxygen! Carbon Dioxide! Hormones! Antibodies! What it is/does!
Respiratory System Physiology. Dr. Vedat Evren
 Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
States of Matter Review
 States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
Applications of Bernoulli s principle. Principle states that areas with faster moving fluids will experience less pressure
 Applications of Bernoulli s principle Principle states that areas with faster moving fluids will experience less pressure Artery o When blood flows through narrower regions of arteries, the speed increases
Applications of Bernoulli s principle Principle states that areas with faster moving fluids will experience less pressure Artery o When blood flows through narrower regions of arteries, the speed increases
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D.
 Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Gas exchange. Tissue cells CO2 CO 2 O 2. Pulmonary capillary. Tissue capillaries
 Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing
 Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
PICU Resident Self-Study Tutorial The Basic Physics of Oxygen Transport. I was told that there would be no math!
 Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
Table of Contents. By Adam Hollingworth
 By Adam Hollingworth Table of Contents Oxygen Cascade... 2 Diffusion... 2 Laws of Diffusion... 2 Diffusion & Perfusion Limitations... 3 Oxygen Uptake Along Pulmon Capillary... 4 Measurement of Diffusing
By Adam Hollingworth Table of Contents Oxygen Cascade... 2 Diffusion... 2 Laws of Diffusion... 2 Diffusion & Perfusion Limitations... 3 Oxygen Uptake Along Pulmon Capillary... 4 Measurement of Diffusing
Respiration (revised 2006) Pulmonary Mechanics
 Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
Physiology Unit 4 RESPIRATORY PHYSIOLOGY
 Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
L 15 Fluids [4] The Venturi Meter. Bernoulli s principle WIND. Basic principles of fluid motion. Why does a roof blow off in high winds?
![L 15 Fluids [4] The Venturi Meter. Bernoulli s principle WIND. Basic principles of fluid motion. Why does a roof blow off in high winds? L 15 Fluids [4] The Venturi Meter. Bernoulli s principle WIND. Basic principles of fluid motion. Why does a roof blow off in high winds?](/thumbs/72/68053820.jpg) Basic principles of fluid motion L 15 Fluids [4] >Fluid flow and Bernoulli s s principle >Airplanes and curveballs >viscosity (real fluids) v 1 Continuity equation: v A = constant A 1 A 2 v 2 Bernoulli
Basic principles of fluid motion L 15 Fluids [4] >Fluid flow and Bernoulli s s principle >Airplanes and curveballs >viscosity (real fluids) v 1 Continuity equation: v A = constant A 1 A 2 v 2 Bernoulli
Worksheet 12 - Partial Pressures and the Kinetic Molecular Theory of Gases
 Worksheet 12 - Partial Pressures and the Kinetic olecular Theory of Gases Dalton's Law of Partial Pressures states that the sums of the pressures of each gas in the mixture add to give the total pressure
Worksheet 12 - Partial Pressures and the Kinetic olecular Theory of Gases Dalton's Law of Partial Pressures states that the sums of the pressures of each gas in the mixture add to give the total pressure
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works.
 Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
AP Physics B Ch 10 Fluids. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Name: Period: Date: AP Physics B Ch 10 Fluids 1) The three common phases of matter are A) solid, liquid, and vapor. B) solid, plasma, and gas. C) condensate, plasma, and gas. D) solid, liquid, and gas.
Name: Period: Date: AP Physics B Ch 10 Fluids 1) The three common phases of matter are A) solid, liquid, and vapor. B) solid, plasma, and gas. C) condensate, plasma, and gas. D) solid, liquid, and gas.
Dalton s Law How is the total pressure of a mixture of gases related to the partial pressures of the component gases?
 Dalton s Law Chapter 4 The Behavior of Gases 4. Properties of Gases 4. The Gas Laws 4. Ideal Gases Dalton s Law How is the total pressure of a mixture of gases related to the partial pressures of the component
Dalton s Law Chapter 4 The Behavior of Gases 4. Properties of Gases 4. The Gas Laws 4. Ideal Gases Dalton s Law How is the total pressure of a mixture of gases related to the partial pressures of the component
 Graham s Law of Diffusion. olecules of gas are in continuous motion. When two or more gases are placed in contact, their molecules mix spontaneously until a homogeneous mixture is formed. ixing of gases
Graham s Law of Diffusion. olecules of gas are in continuous motion. When two or more gases are placed in contact, their molecules mix spontaneously until a homogeneous mixture is formed. ixing of gases
Chapter 4: Ventilation Test Bank MULTIPLE CHOICE
 Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
Lesson 9.1: The Importance of an Organ Delivery System
 Lesson 9.1: The Importance of an Organ Delivery System Animals require a continuous supply of oxygen (O 2 ) for cellular respiration, and they must expel carbon dioxide (CO 2 ), the waste product of this
Lesson 9.1: The Importance of an Organ Delivery System Animals require a continuous supply of oxygen (O 2 ) for cellular respiration, and they must expel carbon dioxide (CO 2 ), the waste product of this
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1
 AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
αo 2 : solubility coefficient of O 2
 Version 2006 Dr. Puntarica Suwanprathes 1) Fick s law of diffusion 2) facts which limit gas transfer 3) diffusion capacity gas volume gaseous phase dissolved gas exert pressure*** Solubility of Gas C =P.
Version 2006 Dr. Puntarica Suwanprathes 1) Fick s law of diffusion 2) facts which limit gas transfer 3) diffusion capacity gas volume gaseous phase dissolved gas exert pressure*** Solubility of Gas C =P.
Name Chemistry Pre-AP
 Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Irrigation &Hydraulics Department lb / ft to kg/lit.
 CAIRO UNIVERSITY FLUID MECHANICS Faculty of Engineering nd Year CIVIL ENG. Irrigation &Hydraulics Department 010-011 1. FLUID PROPERTIES 1. Identify the dimensions and units for the following engineering
CAIRO UNIVERSITY FLUID MECHANICS Faculty of Engineering nd Year CIVIL ENG. Irrigation &Hydraulics Department 010-011 1. FLUID PROPERTIES 1. Identify the dimensions and units for the following engineering
Fluid Flow. Link. Flow» P 1 P 2 Figure 1. Flow Model
 Fluid Flow Equipment: Water reservoir, output tubes of various dimensions (length, diameter), beaker, electronic scale for each table. Computer and Logger Pro software. Lots of ice.temperature probe on
Fluid Flow Equipment: Water reservoir, output tubes of various dimensions (length, diameter), beaker, electronic scale for each table. Computer and Logger Pro software. Lots of ice.temperature probe on
CHAPTER 3: The respiratory system
 CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
Lab 3 Introduction to Quantitative Analysis: Pumps and Measurements of Flow
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641, Spring 2008 Lab 3 Introduction to Quantitative Analysis: Pumps and Measurements of Flow Purpose of Lab 3: 1) To gain a
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641, Spring 2008 Lab 3 Introduction to Quantitative Analysis: Pumps and Measurements of Flow Purpose of Lab 3: 1) To gain a
Lesson 12: Fluid statics, Continuity equation (Sections ) Chapter 9 Fluids
 Lesson : luid statics, Continuity equation (Sections 9.-9.7) Chapter 9 luids States of Matter - Solid, liquid, gas. luids (liquids and gases) do not hold their shapes. In many cases we can think of liquids
Lesson : luid statics, Continuity equation (Sections 9.-9.7) Chapter 9 luids States of Matter - Solid, liquid, gas. luids (liquids and gases) do not hold their shapes. In many cases we can think of liquids
CHAPTER 3: The cardio-respiratory system
 : The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
: The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
States of Matter. Q 7. Calculate the average of kinetic energy, in joules of the molecules in 8.0 g of methane at 27 o C. (IIT JEE Marks)
 Q 1. States of Matter Calculate density of NH 3 at 30 o C and 5 atm pressure Q 2. (IIT JEE 1978 3 Marks) 3.7 g of a gas at 25 o C occupied the same volume as 0.184g of hydrogen at 17 o C and at the same
Q 1. States of Matter Calculate density of NH 3 at 30 o C and 5 atm pressure Q 2. (IIT JEE 1978 3 Marks) 3.7 g of a gas at 25 o C occupied the same volume as 0.184g of hydrogen at 17 o C and at the same
Chapter 10: Gases. Characteristics of Gases
 Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
IV. FROM AQUATIC TO ATMOSPHERIC BREATHING: THE TRACHEA & THE LUNG
 GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
Gas Pressure. Pressure is the force exerted per unit area by gas molecules as they strike the surfaces around them.
 Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Bioreactor System ERT 314. Sidang /2011
 Bioreactor System ERT 314 Sidang 1 2010/2011 Chapter 2:Types of Bioreactors Week 4 Flow Patterns in Agitated Tanks The flow pattern in an agitated tank depends on the impeller design, the properties of
Bioreactor System ERT 314 Sidang 1 2010/2011 Chapter 2:Types of Bioreactors Week 4 Flow Patterns in Agitated Tanks The flow pattern in an agitated tank depends on the impeller design, the properties of
Respiration - Human 1
 Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
You should be able to: Describe Equipment Barometer Manometer. 5.1 Pressure Read and outline 5.1 Define Barometer
 A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
(A) The partial pressure in the lungs is higher than in the blood, and oxygen diffuses out of the lungs passively.
 DAT Biology - Problem Drill 12: The Respiratory System Question No. 1 of 10 1. Which statement about the partial pressure of oxygen inside the lungs is correct? Question #01 (A) The partial pressure in
DAT Biology - Problem Drill 12: The Respiratory System Question No. 1 of 10 1. Which statement about the partial pressure of oxygen inside the lungs is correct? Question #01 (A) The partial pressure in
Chapter 13 The Respiratory System
 Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
These two respiratory media (air & water) impose rather different constraints on oxygen uptake:
 Topic 19: OXYGEN UPTAKE AND TRANSPORT (lectures 29-30) OBJECTIVES: 1. Be able to compare air vs. water as a respiratory medium with respect to oxygen content, diffusion coefficient, viscosity and water
Topic 19: OXYGEN UPTAKE AND TRANSPORT (lectures 29-30) OBJECTIVES: 1. Be able to compare air vs. water as a respiratory medium with respect to oxygen content, diffusion coefficient, viscosity and water
The Kinetic-Molecular Theory of Gases based on the idea that particles are always in motion
 The Kinetic-Molecular Theory of Gases based on the idea that particles are always in motion Five assumptions: 1. Most of the volume occupied dby a gas is empty space 2. Collisions between gas particles
The Kinetic-Molecular Theory of Gases based on the idea that particles are always in motion Five assumptions: 1. Most of the volume occupied dby a gas is empty space 2. Collisions between gas particles
Then the partial pressure of oxygen is x 760 = 160 mm Hg
 1 AP Biology March 2008 Respiration Chapter 42 Gas exchange occurs across specialized respiratory surfaces. 1) Gas exchange: the uptake of molecular oxygen (O2) from the environment and the discharge of
1 AP Biology March 2008 Respiration Chapter 42 Gas exchange occurs across specialized respiratory surfaces. 1) Gas exchange: the uptake of molecular oxygen (O2) from the environment and the discharge of
Circulatory And Respiration
 Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Chemistry Chapter 10 Test
 Chemistry Chapter 10 Test True/False Indicate whether the sentence or statement is true or false. 1. KMT stands for Kinetic Mole Theory. 2. One of the assumptions in the KMT is that the particles are spread
Chemistry Chapter 10 Test True/False Indicate whether the sentence or statement is true or false. 1. KMT stands for Kinetic Mole Theory. 2. One of the assumptions in the KMT is that the particles are spread
Chapter 13. liquids. gases. 1) Fluids exert pressure. a) because they're made up of matter and therefore forces are applied to them
 \ Chapter 13 Fluids 1) Fluids exert pressure a) because they're made up of matter and therefore forces are applied to them liquids gases b) they are made of matter in constant motion colliding with other
\ Chapter 13 Fluids 1) Fluids exert pressure a) because they're made up of matter and therefore forces are applied to them liquids gases b) they are made of matter in constant motion colliding with other
Unit 8: Gases and States of Matter
 Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
More About Solids, Liquids and Gases ASSIGNMENT
 More About Solids, Liquids and Gases ASSIGNMENT 1. Fill in the blank spaces by choosing the correct words from the list given below: List : water, density, altitudes, lateral, intermolecular, force, cohesion,
More About Solids, Liquids and Gases ASSIGNMENT 1. Fill in the blank spaces by choosing the correct words from the list given below: List : water, density, altitudes, lateral, intermolecular, force, cohesion,
Dissolved Oxygen Guide
 Educat i onser i es Di ssol vedoxygengui de Dissolved Oxygen Guide Introduction Dissolved oxygen probes provide a convenient approach to essentially direct measurement of molecular oxygen. The membrane
Educat i onser i es Di ssol vedoxygengui de Dissolved Oxygen Guide Introduction Dissolved oxygen probes provide a convenient approach to essentially direct measurement of molecular oxygen. The membrane
UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT
 UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION
UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION
Static Fluids. **All simulations and videos required for this package can be found on my website, here:
 DP Physics HL Static Fluids **All simulations and videos required for this package can be found on my website, here: http://ismackinsey.weebly.com/fluids-hl.html Fluids are substances that can flow, so
DP Physics HL Static Fluids **All simulations and videos required for this package can be found on my website, here: http://ismackinsey.weebly.com/fluids-hl.html Fluids are substances that can flow, so
Oxygen convulsions are believed by many workers to be caused by an accumulation
 272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT.
 Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
Breathing oxygenates the blood to allow food to be respired
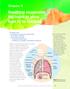 Chapter 6 Breathing oxygenates the blood to allow food to be respired This chapter covers: the structure of the human gas exchange system the mechanism of breathing gas exchange in the alveoli the concept
Chapter 6 Breathing oxygenates the blood to allow food to be respired This chapter covers: the structure of the human gas exchange system the mechanism of breathing gas exchange in the alveoli the concept
Physiology - lecture 3
 Physiology - lecture 3 Residual Volume (RV):the amount of gas remaining in the lung at the end of a maximal exhalation Tidal Volume (TV):the volume of gas inhaled and exhaled during one respiratory cycle.
Physiology - lecture 3 Residual Volume (RV):the amount of gas remaining in the lung at the end of a maximal exhalation Tidal Volume (TV):the volume of gas inhaled and exhaled during one respiratory cycle.
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 11 Respiratory System 2 Pulmonary Ventilation Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session plan o Pulmonary Ventilation
BIOH122 Human Biological Science 2 Session 11 Respiratory System 2 Pulmonary Ventilation Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session plan o Pulmonary Ventilation
PHYS 101 Previous Exam Problems
 PHYS 101 Previous Exam Problems CHAPTER 14 Fluids Fluids at rest pressure vs. depth Pascal s principle Archimedes s principle Buoynat forces Fluids in motion: Continuity & Bernoulli equations 1. How deep
PHYS 101 Previous Exam Problems CHAPTER 14 Fluids Fluids at rest pressure vs. depth Pascal s principle Archimedes s principle Buoynat forces Fluids in motion: Continuity & Bernoulli equations 1. How deep
Question McGraw-Hill Ryerson Limited
 Question 1 Which of the following cannot be explained by considering the empty space between the particles of a gas? A) Gases are more compressible than liquids. B) Gases have lower viscosities than liquids.
Question 1 Which of the following cannot be explained by considering the empty space between the particles of a gas? A) Gases are more compressible than liquids. B) Gases have lower viscosities than liquids.
IASbaba ILP: Value Add Material - Matter MATTER. Iasbaba.com Page 1
 MATTER Iasbaba.com Page 1 LIQUID A. VAPOUR PRESSURE: Pressure of vapour in a closed container Changes majorly with temperature Has an exponential relationship with temperature Increases with increase in
MATTER Iasbaba.com Page 1 LIQUID A. VAPOUR PRESSURE: Pressure of vapour in a closed container Changes majorly with temperature Has an exponential relationship with temperature Increases with increase in
PSI Chemistry: Gases Multiple Choice Review
 PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
Ch. 14 The Behavior of Gases
 Ch. 14 The Behavior of Gases 14.1 PROPERTIES OF GASES Compressibility Compressibility: a measure of how much the volume of matter decreases under pressure Gases are easily compressed because of the spaces
Ch. 14 The Behavior of Gases 14.1 PROPERTIES OF GASES Compressibility Compressibility: a measure of how much the volume of matter decreases under pressure Gases are easily compressed because of the spaces
Chemistry A Molecular Approach. Fourth Edition. Chapter 5. Gases. Copyright 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
 Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Aerobic and Anaerobic Respiration Revision 4
 Aerobic and Anaerobic Respiration Revision 4 64 minutes 64 marks Page of 0 Q. (a) The table shows an athlete s breathing rate after the end of a race. Use the information shown in the table to draw a line
Aerobic and Anaerobic Respiration Revision 4 64 minutes 64 marks Page of 0 Q. (a) The table shows an athlete s breathing rate after the end of a race. Use the information shown in the table to draw a line
Two interconnected rubber balloons as a demonstration showing the effect of surface tension
 Two interconnected rubber balloons as a demonstration showing the effect of surface tension Abstract Science One 28-9 CHEN, Chieh-Shan The two interconnected rubber balloons system is a demonstration widely
Two interconnected rubber balloons as a demonstration showing the effect of surface tension Abstract Science One 28-9 CHEN, Chieh-Shan The two interconnected rubber balloons system is a demonstration widely
Chemistry 20 Unit 2 Gases FITB Notes. Topic A Characteristics of Gases
 Chemistry 20 Unit 2 Gases FITB Notes General Outcome: Topic A Characteristics of Gases We use technologies that were designed with the knowledge of the visible characteristics ( ) of gases ex. SCUBA equipment,
Chemistry 20 Unit 2 Gases FITB Notes General Outcome: Topic A Characteristics of Gases We use technologies that were designed with the knowledge of the visible characteristics ( ) of gases ex. SCUBA equipment,
Ch. 11 Mass transfer principles
 Transport of chemical species in solid, liquid, or gas mixture Transport driven by composition gradient, similar to temperature gradients driving heat transport We will look at two mass transport mechanisms,
Transport of chemical species in solid, liquid, or gas mixture Transport driven by composition gradient, similar to temperature gradients driving heat transport We will look at two mass transport mechanisms,
Physics of Gases and Fluids: A Clinical Perspective
 Physics of Gases and Fluids: A Clinical Perspective 2011 Edition D. John Doyle MD PhD Department of General Anesthesiology Cleveland Clinic Foundation 9500 Euclid Avenue E31 Cleveland, Ohio, USA 44195
Physics of Gases and Fluids: A Clinical Perspective 2011 Edition D. John Doyle MD PhD Department of General Anesthesiology Cleveland Clinic Foundation 9500 Euclid Avenue E31 Cleveland, Ohio, USA 44195
Chapter 15 Fluids. Copyright 2010 Pearson Education, Inc.
 Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle Fluid Flow and Continuity
Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle Fluid Flow and Continuity
RESPIRATORY PHYSIOLOGY. Anaesthesiology Block 18 (GNK 586) Prof Pierre Fourie
 RESPIRATORY PHYSIOLOGY Anaesthesiology Block 18 (GNK 586) Prof Pierre Fourie Outline Ventilation Diffusion Perfusion Ventilation-Perfusion relationship Work of breathing Control of Ventilation 2 This image
RESPIRATORY PHYSIOLOGY Anaesthesiology Block 18 (GNK 586) Prof Pierre Fourie Outline Ventilation Diffusion Perfusion Ventilation-Perfusion relationship Work of breathing Control of Ventilation 2 This image
Fall 2004 Homework Problem Set 9 Due Wednesday, November 24, at start of class
 0.30 Fall 004 Homework Problem Set 9 Due Wednesday, November 4, at start of class Part A. Consider an iron surface which serves as a catalyst for the production of ammonia from nitrogen and hydrogen. The
0.30 Fall 004 Homework Problem Set 9 Due Wednesday, November 4, at start of class Part A. Consider an iron surface which serves as a catalyst for the production of ammonia from nitrogen and hydrogen. The
