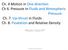
Andrew Carter. Dry Ice: Sublimating underwater, Forming a Cloud of CO 2, and CO 2 gas spilling over an edge.
|
|
|
- George Pierce
- 5 years ago
- Views:
Transcription
1 Flow Visualization MCEN 5151, Spring 2011 Andrew Carter Team Project 3 5/4/11 Dry Ice: Sublimating underwater, Forming a Cloud of CO 2, and CO 2 gas spilling over an edge.
2 Purpose: The objective of this image was to use dry ice as the focus and capture as much physics as possible. This was accomplished by placing a piece of dry ice under water, capturing it sublimating, and having the of gas cloud spill over an edge. Although the set up seams simple it creates an senario in which a lot is happening. First the piece of dry ice is sublimating and forming bubbles. Those bubbles are buoyant and rise to the water s surface where there water s surface tension is not enough force to contain the gas and they burst. The gas originally contained in the bubble then released into the upper area of the container. This gas being denser than air remains contained in the container until CO 2 fills the upper portion of the container. Then the cloud spills over the edge. The image shows this in profile. This set up draws light on to the physics of a cold and denser gas forming, floating, and sinking through other fluids. Flow Apparatus: The flow apparatus used here was a square acrylic container measuring 3 inches to a side and 5 inches tall. Three sides of the container were opaque while the fourth side was clear. This created a window to see what was happening within the container. A different side was slightly lower than the rest and had a notch removed from its top edge. This directed the flow of the CO 2 gas in a relatively +controlled manner. Figure 1 shows a rough schematic of the container. Lower side, w/ Flow control notch. Clear Front Panel. Figure 1, Artistic rendition of the flow apparatus. Flow: Background: Dry ice is a common term for the solid form of carbon dioxide (CO 2 ). At atmospheric pressures and temperatures, dry ice changes straight from solid to gas through a process known as sublimation.[1] This process is described in detail below. Even in gas form CO 2 is still denser than air. [1] Therefore the cloud formed by dry ice sublimation sinks to the lowest confined place. If not confined the CO 2 gas will eventually diffuse into the atmosphere. As the density due of CO 2
3 decreases, due to the increasing temperature, Brownian motion and diffusion driven gradients take over and spread the gas throughout the atmosphere. CO 2 Sublimating: CO 2 s triple point, seen in the phase diagram (figure 2) is positioned such that for standard pressures found in our atmosphere the material will change directly from solid to gas. This process is called sublimation. This phenomenon can be seen in figure 3, and is highlighted by circle 1. Sublimation is happening across the entire surface area of the piece of dry ice. The reason why it only appears to happen at the top is due to CO 2 buoyancy and the surface tension of the water. This is discussed with greater detail in the next section. The phase diagram below shows that at 1 atm CO 2 is solid below ᵒC. [2] Above this temperature it is gas. When a material undergoes a phase change no matter if its from solid to liquid, or liquid to gas, or any combination of two of the three. The material undergoes a rapid change of density. Specifically for CO 2 under goes sublimation it goes from a density of to. [3] This also corresponds to a drastic increase in volume. In fact the change in volume is equal to the inverse ratio of the densities, there for for going from solid to gas the change in volume is: CO 2 will be 555 times larger as a gas. [1], meaning the volume of solid Figure 2, Phase Diagram or CO 2 [2] Figure 3, Highlighting different Dynamics. 1. Sublimation, 2.Bubbles of CO2, 3 Bubbles Bursting, 4. Gas spilling Bubble Rising & Bursting: As discussed above, in standard states found in out atmosphere CO 2 does not melt, its sublimates. [1] This is even true when it is submersed under water (as shown in the image). Therefore the bubbles seen under the water s surface are 100% CO 2. The gas phase of CO 2 is about 500 times less dense than that of H 2 0. [1] This means there is a tremendous buoyancy force acting on the gas. However we also need to consider the surface tension of the water, which at the surface to the dry ice acts against the buoyancy force. Therefore small bubbles of CO 2 only move along the
4 surface of the dry ice. Only when small bubbles join together to form a large enough bubble will the buoyancy force over power the surface tension. This event is highlighted by circle 2, in figure 3. It is a pretty trivial calculation to determine the approximated buoyancy force on a bubble of CO 2. This calculation is outlined below. Approximating the bubble volume which is highlighted by circle #2 in figure 3 to be letting for is: be equal to the acceleration due to gravity the buoyancy force the gas experiences in water ( ) This is a huge force for such a little bubble and causes the bubbles to rise rapidly. When the bubbles reach the surface of the water the bubbles burst. This is highlighted by circle 3, in figure 3. A bubble burst is caused by the internal pressure of the bubble overcoming the surface tension of the water. Because the bubbles are moving so fast there will be a lot of inertial contributions to the internal pressure. This is because when the bubble reaches the surface, the surface tension wants to stop the bubble propagation immediately. However the gas having some mass will want to keep going. Additionally, there is only a certain size bubble that water can form. The bubble circled in figure 3 by circle 2 is much bigger than this limit. Cloud spilling: When the bubbles burst the CO 2 is released into the upper cavity of the container. The gas and being more dense than air, will remained confined in that cavity until the cavity becomes full. Remember that the volume of gas produced by small amount of dry ice is very large. Therefore the amount of dry ice seen had no trouble filling the upper cavity. When the upper cavity was filled the gas then spilled over the edge. This is highlighted by circle 4 in figure 3. It spilled over because, again, the gas is denser than the air. We can calculate the buoyancy force on a similar volume of CO 2 as above. ( ) First notice the negative sign, as anticipated from the image, the CO 2 will sink in the air. Second, notice the magnitude of this buoyancy force compared to that calculated for CO 2 in water. In air it is much smaller. Therefore we can conclude that the gas will sink in air much slower than it will rise in water. This is exactly what was witnessed. Note, that the sinking of the CO 2 happened soon after sublimation. At this time the gas is cold and very dense. At about 15ᵒC (~60ᵒF) the density is approximately. When the gas reaches this density diffusion and Brownian motion dominates over the negative buoyancy force. This is why we don t suffocate at ground level.
5 Interesting Fact: Earths north and south poles impose a constant magnetic field throughout the planet of. [5] At the poles magnetic fields range from micro- to milli-teslas. This is unique compared to Mars, which has negligible magnetic poles, and gaseous planets like Jupiter contain have large magnetic field strengths. [5] Mars unlike Earth has no CO 2 at its poles while large gaswours planets have literally tons of CO 2. [5] This raised the question of whether magnetic fields increase the release of CO 2. [5] The study outlined in Effects of Gradient Magnetic Fields on CO 2 Sublimation in Dry Ice prove that in fact our poles is increasing the rate of CO 2 production on earth, (and gaseous giants) and directly affecting global warming and climate change. [5] Visualization Technique: The image setup is as follows. The container was filled just under half with water. It was then placed in front of a black backdrop and underneath a table. This eliminated the excess glare from the above florescent lights. The camera was then positioned and focused on the front of the container. Then a large piece of dry ice was placed in the container so that it was close to the front, clear side of the container. Several camera shots were taken while the dry ice was sublimating. This image was chosen since it showed the most fluid dynamics. Then a lot of photo editing was done. First priority was to fix the image perspective. The container was made to appear square with the camera. Then the image was cropped to focus the viewer s attention on the flow. Colors were then inverted to try to bring out detail the regular image hides. Finally distracting image impurities like dots, lines and other distracting nuances were removed to give the final image seen below. Figure 4 shows the before edit and post edit, pre-edit is on the left, and post-edit is on the right. All editing was done in Adobe PhotoShop. Meta data for the image can be found in Table 1 below. This data corresponds to the camera settings and other original image data for the original image. Figure 4, Left, Pre-edited photo. Right, Post-edited photo.
6 Image & Camera Info Camera Nikon D70s Lens mm, 4/ Shutter Speed 1/80sec F-Stop f/5.0 ISO 900 Focal Length 35mm Pixel Resolution x 3008 y 2000 Table 1, Meta Data Image: The final image shows a lot happening. The dry ice sublimating, bubbles filled with CO 2 are rising in the water and busting at the surface. The cloud of CO 2 fills the container s top portion. The gas then spills over the edge. As a focus piece of photography I personally found that dry ice to be a very frustrating medium to work with. Phenomenon surrounding dry ice is erratic and often unpredictable this is because there is so much involved. Several tries were taken of different container orientation, dry ice size, and water levels. I was never pleased with the images I captured. However after editing I am very happy with the results. I believe this image to portray the physics of the flow very accurately despite the perspective editing. The perspective change and all other photo editing was done very carefully not to take away or skew any information but to give the clearest and most esthetic image. As a final result I believe the image to be a good composition of a whole bunch of fluid dynamics shown in an artistic yet straight forward manner.
7 References [1] X R Zhang, H. Yamagucho, M Masuda, Study of the CO 2 Solid-Gas Two Phase Flow with Particle Sublimation and iits Basic Applications, American Institute of Physics (2008) l/ [2] Air Liquide, Gas Encyclopaedia, CO 2, [3] Real Science. Theme: Twenty Ten Blog at WordPress.com. 2/ [4] Engineering ToolBox, Density of Air at 5000ft, Density of water. [5] M. Iwasaka, Effects of Gradient Magnetic Fields on CO 2 Sublimation in Dry Ice Journal of Physics: Conference Series 156 (2009)
3/30/2013. Vapor Pressure and Changes of State Phase Diagrams. Chapter 10 Sections 8, 9
 Vapor Pressure and Changes of State Chapter 10 Sections 8, 9 Vapor Pressure In a closed container, liquid evaporates. The vapor starts to condensate. Condensation and evaporation happens simultaneously.
Vapor Pressure and Changes of State Chapter 10 Sections 8, 9 Vapor Pressure In a closed container, liquid evaporates. The vapor starts to condensate. Condensation and evaporation happens simultaneously.
Team First project: Water Tornado experiment Yusen Ji
 Team First project: Water Tornado experiment Yusen Ji Introduction For the first team project, I choose to take a photo of water tornado. Since the water vortex is very interested to me and during the
Team First project: Water Tornado experiment Yusen Ji Introduction For the first team project, I choose to take a photo of water tornado. Since the water vortex is very interested to me and during the
The Science of Boat Design
 1.3 Read The Science of Boat Design matter: anything that has mass and takes up space. density: the amount of matter in a given amount of space. buoyant force: the upward push that keeps objects floating
1.3 Read The Science of Boat Design matter: anything that has mass and takes up space. density: the amount of matter in a given amount of space. buoyant force: the upward push that keeps objects floating
Chapter: Atmosphere Section 3: Air Movement
 Table of Contents Chapter: Atmosphere Section 3: Air Movement We will learn about Air Movement=Wind -Why different latitudes on Earth will receive different amounts of Solar Energy -The Coriolis Effect
Table of Contents Chapter: Atmosphere Section 3: Air Movement We will learn about Air Movement=Wind -Why different latitudes on Earth will receive different amounts of Solar Energy -The Coriolis Effect
LECTURE 16: Buoyancy. Select LEARNING OBJECTIVES:
 Lectures Page 1 Select LEARNING OBJECTIVES: LECTURE 16: Buoyancy Understand that the buoyant force is a result of a pressure gradient within a fluid. Demonstrate the ability to analyze a scenario involving
Lectures Page 1 Select LEARNING OBJECTIVES: LECTURE 16: Buoyancy Understand that the buoyant force is a result of a pressure gradient within a fluid. Demonstrate the ability to analyze a scenario involving
Notes Chapter 3. Buoyancy
 Notes Chapter 3 Buoyancy Pressure in a Fluid 3.2 Pressure and the Buoyant Forces Liquids and gases are fluids materials that can flow and have no definite shape. Objects in a fluid experience a buoyant
Notes Chapter 3 Buoyancy Pressure in a Fluid 3.2 Pressure and the Buoyant Forces Liquids and gases are fluids materials that can flow and have no definite shape. Objects in a fluid experience a buoyant
Water on Earth. How do oceans relate to weather and the atmosphere? Solar Radiation and Convection Currents
 Earth is often called the Blue Planet because so much of its surface (about 71%) is covered by water. Of all the water on Earth, about 96.5% is held in the world s oceans. As you can imagine, these oceans
Earth is often called the Blue Planet because so much of its surface (about 71%) is covered by water. Of all the water on Earth, about 96.5% is held in the world s oceans. As you can imagine, these oceans
Deep Water Currents Lab
 Deep Water Currents Lab Background: Anyone visiting the seashore is struck by the constant motion of water traveling on the surface of the ocean in the form of waves. But beneath the ocean's surface, water
Deep Water Currents Lab Background: Anyone visiting the seashore is struck by the constant motion of water traveling on the surface of the ocean in the form of waves. But beneath the ocean's surface, water
Exam Review Mass, Weight, Density, Buoyancy, States of Matter
 Exam Review Mass, Weight, Density, Buoyancy, States of Matter Volume An object s volume is the amount of space it takes up. The volume of a cup of water can change if you freeze it in to a solid or boil
Exam Review Mass, Weight, Density, Buoyancy, States of Matter Volume An object s volume is the amount of space it takes up. The volume of a cup of water can change if you freeze it in to a solid or boil
Why do things float? Climate and Global Change. Introduction
 Why do things float? Introduction Archimedes of Syracuse (ca. 287-212 B.C.), a physical scientist, is credited with understanding two basic principles: When describing the mechanical advantage gained by
Why do things float? Introduction Archimedes of Syracuse (ca. 287-212 B.C.), a physical scientist, is credited with understanding two basic principles: When describing the mechanical advantage gained by
Dillon Thorse Flow Visualization MCEN 4047 Team Poject 1 March 14th, 2013
 Dillon Thorse Flow Visualization MCEN 4047 Team Poject 1 March 14 th, 2013 1 Introduction I have always been entranced by flight. Recently I have been taking flying lessons, and I have been learning the
Dillon Thorse Flow Visualization MCEN 4047 Team Poject 1 March 14 th, 2013 1 Introduction I have always been entranced by flight. Recently I have been taking flying lessons, and I have been learning the
Chapter 13 Fluids. Copyright 2009 Pearson Education, Inc.
 Chapter 13 Fluids Phases of Matter Density and Specific Gravity Pressure in Fluids Atmospheric Pressure and Gauge Pressure Pascal s Principle Units of Chapter 13 Measurement of Pressure; Gauges and the
Chapter 13 Fluids Phases of Matter Density and Specific Gravity Pressure in Fluids Atmospheric Pressure and Gauge Pressure Pascal s Principle Units of Chapter 13 Measurement of Pressure; Gauges and the
Meteorology. Circle the letter that corresponds to the correct answer
 Chapter 4 Worksheet 3 Meteorology Name: Circle the letter that corresponds to the correct answer 1) Natural convection and turbulence are most likely to occur when: a) temperature decreases rapidly with
Chapter 4 Worksheet 3 Meteorology Name: Circle the letter that corresponds to the correct answer 1) Natural convection and turbulence are most likely to occur when: a) temperature decreases rapidly with
Wind Movement and Global and Local Winds
 Wind Movement and Global and Local Winds In previous lessons, you learned that the uneven heating of Earth s surface by the Sun causes some areas to be warmer than others. This uneven heating of land forms
Wind Movement and Global and Local Winds In previous lessons, you learned that the uneven heating of Earth s surface by the Sun causes some areas to be warmer than others. This uneven heating of land forms
Scott Denning CSU CMMAP 1
 Thermodynamics, Buoyancy, and Vertical Motion Temperature, Pressure, and Density Buoyancy and Static Stability Adiabatic Lapse Rates Dry and Moist Convective Motions Present Atmospheric Composition What
Thermodynamics, Buoyancy, and Vertical Motion Temperature, Pressure, and Density Buoyancy and Static Stability Adiabatic Lapse Rates Dry and Moist Convective Motions Present Atmospheric Composition What
An underwater explosion is an explosion where the point of detonation is below the surface of the water.
 Underwater Explosion 1 Introduction An underwater explosion is an explosion where the point of detonation is below the surface of the water. Underwater explosion are categorized in accordance with their
Underwater Explosion 1 Introduction An underwater explosion is an explosion where the point of detonation is below the surface of the water. Underwater explosion are categorized in accordance with their
Unit 7. Pressure in fluids
 -- Unit 7. Pressure in fluids Index 1.- Pressure...2 2.- Fluids...2 3.- Pressure in fluids...3 4.- Pascal's principle...5 5.- Archimedes principle...6 6.- Atmospheric pressure...7 6.1.- Torricelli and
-- Unit 7. Pressure in fluids Index 1.- Pressure...2 2.- Fluids...2 3.- Pressure in fluids...3 4.- Pascal's principle...5 5.- Archimedes principle...6 6.- Atmospheric pressure...7 6.1.- Torricelli and
Read each slide, some slides have information to record on your organizer. Some slides have numbers that go with the question or red and underlined
 Read each slide, some slides have information to record on your organizer. Some slides have numbers that go with the question or red and underlined to use for answering the questions. Essential Question:
Read each slide, some slides have information to record on your organizer. Some slides have numbers that go with the question or red and underlined to use for answering the questions. Essential Question:
PHYSICS - CLUTCH CH 17: FLUID MECHANICS.
 !! www.clutchprep.com INTRO TO DENSITY LIQUIDS and GASES are types of. So we use the term to refer generally to both Liquids AND Gases. The DENSITY of a material is a measure of how tight the molecules
!! www.clutchprep.com INTRO TO DENSITY LIQUIDS and GASES are types of. So we use the term to refer generally to both Liquids AND Gases. The DENSITY of a material is a measure of how tight the molecules
AP Physics B Ch 10 Fluids. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Name: Period: Date: AP Physics B Ch 10 Fluids 1) The three common phases of matter are A) solid, liquid, and vapor. B) solid, plasma, and gas. C) condensate, plasma, and gas. D) solid, liquid, and gas.
Name: Period: Date: AP Physics B Ch 10 Fluids 1) The three common phases of matter are A) solid, liquid, and vapor. B) solid, plasma, and gas. C) condensate, plasma, and gas. D) solid, liquid, and gas.
OCN201 Spring14 1. Name: Class: Date: True/False Indicate whether the statement is true or false.
 Name: Class: _ Date: _ OCN201 Spring14 1 True/False Indicate whether the statement is true or false. 1. Short residence time elements are uniformly distributed in the oceans 2. Thermohaline circulation
Name: Class: _ Date: _ OCN201 Spring14 1 True/False Indicate whether the statement is true or false. 1. Short residence time elements are uniformly distributed in the oceans 2. Thermohaline circulation
Dec 6 3:08 PM. Density. Over the last two periods we discussed/observed the concept of density. What have we learned?
 Over the last two periods we discussed/observed the concept of density. What have we learned? is a ratio of mass to volume describes how much matter is packed into a space is a property of both solids
Over the last two periods we discussed/observed the concept of density. What have we learned? is a ratio of mass to volume describes how much matter is packed into a space is a property of both solids
Fluid Mechanics. Liquids and gases have the ability to flow They are called fluids There are a variety of LAWS that fluids obey
 Fluid Mechanics Fluid Mechanics Liquids and gases have the ability to flow They are called fluids There are a variety of LAWS that fluids obey Density Regardless of form (solid, liquid, gas) we can define
Fluid Mechanics Fluid Mechanics Liquids and gases have the ability to flow They are called fluids There are a variety of LAWS that fluids obey Density Regardless of form (solid, liquid, gas) we can define
Fluids always move from high pressure to low pressure. Air molecules pulled by gravity = atmospheric pressure
 9.1 Fluids Under Pressure Fluids always move from high pressure to low pressure w Fluids under pressure and compressed gases are used for a variety of everyday tasks Air molecules pulled by gravity = atmospheric
9.1 Fluids Under Pressure Fluids always move from high pressure to low pressure w Fluids under pressure and compressed gases are used for a variety of everyday tasks Air molecules pulled by gravity = atmospheric
Science 8 Chapter 9 Section 1
 Science 8 Chapter 9 Section 1 Forces and Buoyancy (pp. 334-347) Forces Force: anything that causes a change in the motion of an object; a push or pull on an object balanced forces: the condition in which
Science 8 Chapter 9 Section 1 Forces and Buoyancy (pp. 334-347) Forces Force: anything that causes a change in the motion of an object; a push or pull on an object balanced forces: the condition in which
From and
 From http://www.school-for-champions.com/science/fluidpressure.htm and http://www.school-forchampions.com/science/fluidfloating.htm by Ron Kurtus, School for Champions Pressure in Fluids by Ron Kurtus
From http://www.school-for-champions.com/science/fluidpressure.htm and http://www.school-forchampions.com/science/fluidfloating.htm by Ron Kurtus, School for Champions Pressure in Fluids by Ron Kurtus
Flying High. HHJS Science Week Background Information. Forces and Flight
 Flying High HHJS Science Week 2013 Background Information Forces and Flight Flight Background Information Flying is defined as controlled movement through the air. Many things can become airborne but this
Flying High HHJS Science Week 2013 Background Information Forces and Flight Flight Background Information Flying is defined as controlled movement through the air. Many things can become airborne but this
Key Terms Chapter 7. boiling boiling point change of state concentration condensation deposition evaporation flow rate fluid freezing point
 Foldable Activity Using the instructions on page 267 in your textbook on how to make foldables, write a key term on each front tab, and the definition on the inside (see example that I made up). You will
Foldable Activity Using the instructions on page 267 in your textbook on how to make foldables, write a key term on each front tab, and the definition on the inside (see example that I made up). You will
Chapter 4. Convec.on Adiaba.c lapse rate
 Chapter 4 Convec.on Adiaba.c lapse rate 1.Outline: a. air parcel theory, adiabatic processes b. how do we define/determine atmospheric stability? 2.Readings: Chapter 4 VERTICAL STRUCTURE T STRATIFICATION
Chapter 4 Convec.on Adiaba.c lapse rate 1.Outline: a. air parcel theory, adiabatic processes b. how do we define/determine atmospheric stability? 2.Readings: Chapter 4 VERTICAL STRUCTURE T STRATIFICATION
Physics 221, March 1. Key Concepts: Density and pressure Buoyancy Pumps and siphons Surface tension
 Physics 221, March 1 Key Concepts: Density and pressure Buoyancy Pumps and siphons Surface tension Fluids: Liquids Incompressible Gases Compressible Definitions Particle density: Density: Pressure: ρ particle
Physics 221, March 1 Key Concepts: Density and pressure Buoyancy Pumps and siphons Surface tension Fluids: Liquids Incompressible Gases Compressible Definitions Particle density: Density: Pressure: ρ particle
. In an elevator accelerating upward (A) both the elevator accelerating upward (B) the first is equations are valid
 IIT JEE Achiever 2014 Ist Year Physics-2: Worksheet-1 Date: 2014-06-26 Hydrostatics 1. A liquid can easily change its shape but a solid cannot because (A) the density of a liquid is smaller than that of
IIT JEE Achiever 2014 Ist Year Physics-2: Worksheet-1 Date: 2014-06-26 Hydrostatics 1. A liquid can easily change its shape but a solid cannot because (A) the density of a liquid is smaller than that of
Mini-Labs. 7. Movie Fog 8. Jet Power 9. Sauce Pan 10. Magnetic Bubbles 11. Aquarium Magic 12. Hockey Puck 13. Carbon dioxide balloons
 Mini-Labs (13) Mini-Labs 1. Magic Raisins 2. Mysterious Balloons 3. Candle Power 4. Super-cooled Liquid 5. Singing Tongs 6. Film Canister 7. Movie Fog 8. Jet Power 9. Sauce Pan 10. Magnetic Bubbles 11.
Mini-Labs (13) Mini-Labs 1. Magic Raisins 2. Mysterious Balloons 3. Candle Power 4. Super-cooled Liquid 5. Singing Tongs 6. Film Canister 7. Movie Fog 8. Jet Power 9. Sauce Pan 10. Magnetic Bubbles 11.
Density. Chapters 12-14: Phases of Matter. Example: Density. Conceptual Check. Springs 2/27/12. Mass Density vs. Weight Density
 Chapters 12-14: Phases of Matter Density Sequence of increasing molecule motion (and kinetic energy) Solid Liquid Gas The densities of most liquids and solids vary slightly with changes in temperature
Chapters 12-14: Phases of Matter Density Sequence of increasing molecule motion (and kinetic energy) Solid Liquid Gas The densities of most liquids and solids vary slightly with changes in temperature
States of Matter Review
 States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
Chapter 9 Fluids CHAPTER CONTENTS
 Flowing fluids, such as the water flowing in the photograph at Coors Falls in Colorado, can make interesting patterns In this chapter, we will investigate the basic physics behind such flow Photo credit:
Flowing fluids, such as the water flowing in the photograph at Coors Falls in Colorado, can make interesting patterns In this chapter, we will investigate the basic physics behind such flow Photo credit:
Unit Test Study Guide:
 Name: Homeroom: Date: Unit 6: Meteorology Study Guide Unit Test Study Guide: Atmosphere & Weather Use the summary points below as a resource to help you study for our unit test Monday! EARTH S ATMOSPHERE:
Name: Homeroom: Date: Unit 6: Meteorology Study Guide Unit Test Study Guide: Atmosphere & Weather Use the summary points below as a resource to help you study for our unit test Monday! EARTH S ATMOSPHERE:
4. Using the kinetic molecular theory, explain why a gas can be easily compressed, while a liquid and a solid cannot?
 Name Period HW 1 Worksheet (Goals 1-4) - Kinetic Molecular Theory 1. Describe how gases, liquids, and solids compare using the following table. Solids Liquids Gases Volume (definite or indefinite) Molecular
Name Period HW 1 Worksheet (Goals 1-4) - Kinetic Molecular Theory 1. Describe how gases, liquids, and solids compare using the following table. Solids Liquids Gases Volume (definite or indefinite) Molecular
Chapter Five: Density and Buoyancy
 Chapter Five: Density and Buoyancy 5.1 Density 5.2 Buoyancy 5.3 Heat Affects Density and Buoyancy 5.1 Mass and Weight Mass is the amount of matter in an object. Weight is a measure of the pulling force
Chapter Five: Density and Buoyancy 5.1 Density 5.2 Buoyancy 5.3 Heat Affects Density and Buoyancy 5.1 Mass and Weight Mass is the amount of matter in an object. Weight is a measure of the pulling force
Convection Current Exploration:
 Heat on Earth 8.10A RECOGNIZE THAT THE SUN PROVIDES THE ENERGY THAT DRIVES CONVECTION WITHIN THE ATMOSPHERE AND OCEANS, PRODUCING WINDS AND OCEAN CURRENTS [INCORPORATE 6.6B INTO CONVECTION] A few reminders
Heat on Earth 8.10A RECOGNIZE THAT THE SUN PROVIDES THE ENERGY THAT DRIVES CONVECTION WITHIN THE ATMOSPHERE AND OCEANS, PRODUCING WINDS AND OCEAN CURRENTS [INCORPORATE 6.6B INTO CONVECTION] A few reminders
Kinetic-Molecular Theory
 GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
What Causes Wind? Exploration: How Does Air Move When Pressure Builds Up? 4.2 Explore. Predict
 4.2 Explore What Causes Wind? In Learning Set 1, you built an anemometer. You used it to measure wind speed and direction in your community. In the last section, you read about how wind and ocean currents
4.2 Explore What Causes Wind? In Learning Set 1, you built an anemometer. You used it to measure wind speed and direction in your community. In the last section, you read about how wind and ocean currents
Chapter 13 Temperature, Kinetic Theory, and the Gas Laws 497
 Chapter 13 Temperature, Kinetic Theory, and the Gas Laws 497 Figure 13.25 This photograph of Apollo 17 Commander Eugene Cernan driving the lunar rover on the Moon in 1972 looks as though it was taken at
Chapter 13 Temperature, Kinetic Theory, and the Gas Laws 497 Figure 13.25 This photograph of Apollo 17 Commander Eugene Cernan driving the lunar rover on the Moon in 1972 looks as though it was taken at
IASbaba ILP: Value Add Material - Matter MATTER. Iasbaba.com Page 1
 MATTER Iasbaba.com Page 1 LIQUID A. VAPOUR PRESSURE: Pressure of vapour in a closed container Changes majorly with temperature Has an exponential relationship with temperature Increases with increase in
MATTER Iasbaba.com Page 1 LIQUID A. VAPOUR PRESSURE: Pressure of vapour in a closed container Changes majorly with temperature Has an exponential relationship with temperature Increases with increase in
Chapter 9 Solids and Fluids
 2/17/16 Chapter 9 Solids and Fluids Units of Chapter 9 Solids and Elastic Moduli Fluids: Pressure and Pascal s Buoyancy and Archimedes Fluid Dynamics and Bernoulli s Surface Tension, Viscosity, and Poiseuille
2/17/16 Chapter 9 Solids and Fluids Units of Chapter 9 Solids and Elastic Moduli Fluids: Pressure and Pascal s Buoyancy and Archimedes Fluid Dynamics and Bernoulli s Surface Tension, Viscosity, and Poiseuille
Chapter 1, Lesson 5: Air, It s Really There
 Chapter 1, Lesson 5: Air, It s Really There Key Concepts In a gas, the particles (atoms and molecules) have weak attractions for one another. They are able to move freely past each other with little interaction
Chapter 1, Lesson 5: Air, It s Really There Key Concepts In a gas, the particles (atoms and molecules) have weak attractions for one another. They are able to move freely past each other with little interaction
Transverse waves cause particles to vibrate perpendicularly to the direction of the wave's motion (e.g. waves on a string, ripples on a pond).
 Waves Introduction A vibration must be the source of a wave. Waves in turn also cause vibrations. They are intrinsically connected. Waves transmit energy. There are different ways in which waves can be
Waves Introduction A vibration must be the source of a wave. Waves in turn also cause vibrations. They are intrinsically connected. Waves transmit energy. There are different ways in which waves can be
Numerical Simulations of a Train of Air Bubbles Rising Through Stagnant Water
 Numerical Simulations of a Train of Air Bubbles Rising Through Stagnant Water Hong Xu, Chokri Guetari ANSYS INC. Abstract Transient numerical simulations of the rise of a train of gas bubbles in a liquid
Numerical Simulations of a Train of Air Bubbles Rising Through Stagnant Water Hong Xu, Chokri Guetari ANSYS INC. Abstract Transient numerical simulations of the rise of a train of gas bubbles in a liquid
PHYSICS. Light: Sound:
 PHYSICS Light: The speed of light changes as it passes through different things such as air, glass and water. This affects the way we see things underwater with a diving mask. As the light passes through
PHYSICS Light: The speed of light changes as it passes through different things such as air, glass and water. This affects the way we see things underwater with a diving mask. As the light passes through
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/12
 LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
introduce Grade 10 Earth and Dynamics heat transfers 2). Materials: 3. A hot plate. Procedure: heat it to a boil. 3. Remove flask or table) and
 Concept: After students have examined Water Cycles in their unitt on Weather Dynamics introduce this discrepant event in order to expand on prior knowledge and advance students exploration with regards
Concept: After students have examined Water Cycles in their unitt on Weather Dynamics introduce this discrepant event in order to expand on prior knowledge and advance students exploration with regards
Chapter: Atmosphere Section 3: Air Movement
 Table of Contents Chapter: Atmosphere Section 3: Air Movement We will learn about: -Air Movement=Wind -Why different latitudes on Earth will receive different amounts of Solar Energy -The Coriolis Effect
Table of Contents Chapter: Atmosphere Section 3: Air Movement We will learn about: -Air Movement=Wind -Why different latitudes on Earth will receive different amounts of Solar Energy -The Coriolis Effect
Phys101 Lectures Fluids I. Key points: Pressure and Pascal s Principle Buoyancy and Archimedes Principle. Ref: 10-1,2,3,4,5,6,7.
 Phys101 Lectures 21-22 Fluids I Key points: Pressure and Pascal s Principle Buoyancy and Archimedes Principle Ref: 10-1,2,3,4,5,6,7. Page 1 10-1 Phases of Matter The three common phases of matter are solid,
Phys101 Lectures 21-22 Fluids I Key points: Pressure and Pascal s Principle Buoyancy and Archimedes Principle Ref: 10-1,2,3,4,5,6,7. Page 1 10-1 Phases of Matter The three common phases of matter are solid,
Section 1. Global Wind Patterns and Weather. What Do You See? Think About It. Investigate. Learning Outcomes
 Chapter 5 Winds, Oceans, Weather, and Climate Section 1 Global Wind Patterns and Weather What Do You See? Learning Outcomes In this section, you will Determine the effects of Earth s rotation and the uneven
Chapter 5 Winds, Oceans, Weather, and Climate Section 1 Global Wind Patterns and Weather What Do You See? Learning Outcomes In this section, you will Determine the effects of Earth s rotation and the uneven
Density and Buoyancy Notes
 Density and Buoyancy Notes Measuring Mass and Volume 3.1 Density A balance can be used to measure the mass of an object. If the object is a liquid, pour it into a graduated cylinder to measure the volume.
Density and Buoyancy Notes Measuring Mass and Volume 3.1 Density A balance can be used to measure the mass of an object. If the object is a liquid, pour it into a graduated cylinder to measure the volume.
PURE SUBSTANCE. Nitrogen and gaseous air are pure substances.
 CLASS Third Units PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Air is a mixture of several gases, but it is considered to be a pure substance. Nitrogen and
CLASS Third Units PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Air is a mixture of several gases, but it is considered to be a pure substance. Nitrogen and
Chapter 15 Fluid. Density
 Density Chapter 15 Fluid Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle By Dr. Weining man 1 Units of Chapter 15 Fluid
Density Chapter 15 Fluid Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle By Dr. Weining man 1 Units of Chapter 15 Fluid
Investigating the Bubble Behavior in Pool Boiling in Microgravity Conditions Thilanka Munasinghe, Member, IAENG
 Investigating the Bubble Behavior in Pool Boiling in Microgravity Conditions Thilanka Munasinghe, Member, IAENG In space, objects float without falling down. The floating effect happens because of the
Investigating the Bubble Behavior in Pool Boiling in Microgravity Conditions Thilanka Munasinghe, Member, IAENG In space, objects float without falling down. The floating effect happens because of the
Ocean Motion Notes. Chapter 13 & 14
 Ocean Motion Notes Chapter 13 & 14 What is a Wave? Wave: movement of energy through a body of water How are Waves Caused? Caused mostly by wind Wind blowing on the water transmits energy to the water Size
Ocean Motion Notes Chapter 13 & 14 What is a Wave? Wave: movement of energy through a body of water How are Waves Caused? Caused mostly by wind Wind blowing on the water transmits energy to the water Size
Density, Pressure Learning Outcomes
 1 Density, Pressure Learning Outcomes Define density and pressure, and give their units. Solve problems about density and pressure. Discuss pressure in liquids and gases. State Boyle s Law. Demonstrate
1 Density, Pressure Learning Outcomes Define density and pressure, and give their units. Solve problems about density and pressure. Discuss pressure in liquids and gases. State Boyle s Law. Demonstrate
Density, Pressure Learning Outcomes
 Density, Pressure Learning Outcomes Define density and pressure, and give their units. Solve problems about density and pressure. Discuss pressure in liquids and gases. State Boyle s Law. Demonstrate atmospheric
Density, Pressure Learning Outcomes Define density and pressure, and give their units. Solve problems about density and pressure. Discuss pressure in liquids and gases. State Boyle s Law. Demonstrate atmospheric
FC-CIV HIDRCANA: Channel Hydraulics Flow Mechanics Review Fluid Statics
 FC-CIV HIDRCANA: Channel Hydraulics Flow Mechanics Review Fluid Statics Civil Engineering Program, San Ignacio de Loyola University Objective Calculate the forces exerted by a fluid at rest on plane or
FC-CIV HIDRCANA: Channel Hydraulics Flow Mechanics Review Fluid Statics Civil Engineering Program, San Ignacio de Loyola University Objective Calculate the forces exerted by a fluid at rest on plane or
Today: waves. Exam Results. Wave Motion. What is moving? Motion of a piece of the rope. Energy transport
 Exam: Exam scores posted on Learn@UW No homework due next week Exam Results D C BC B AB A Today: waves Have studied Newton s laws, motion of particles, momentum, energy, etc. Laws for describing things
Exam: Exam scores posted on Learn@UW No homework due next week Exam Results D C BC B AB A Today: waves Have studied Newton s laws, motion of particles, momentum, energy, etc. Laws for describing things
Name Class Date. (pp ) Write the letter of the correct answer in the space provided.
 Skills Worksheet Directed Reading A Section: Buoyancy and Density (pp. 412 419) 1. What is the upward force that fluids exert on all matter called? a. pascal force b. atmospheric pressure c. buoyant force
Skills Worksheet Directed Reading A Section: Buoyancy and Density (pp. 412 419) 1. What is the upward force that fluids exert on all matter called? a. pascal force b. atmospheric pressure c. buoyant force
The density of a substance is the same for all samples of that substance.
 8.8.a Density and Buoyancy Students know density is mass per unit volume. P71 Wood Steel The density of a substance is the same for all samples of that substance. 1. The two blocks shown have the same
8.8.a Density and Buoyancy Students know density is mass per unit volume. P71 Wood Steel The density of a substance is the same for all samples of that substance. 1. The two blocks shown have the same
Lecture Outline Chapter 15. Physics, 4 th Edition James S. Walker. Copyright 2010 Pearson Education, Inc.
 Lecture Outline Chapter 15 Physics, 4 th Edition James S. Walker Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy
Lecture Outline Chapter 15 Physics, 4 th Edition James S. Walker Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy
Wednesday, September 20, 2017 Reminders. Week 3 Review is now available on D2L (through Friday) Exam 1, Monday, September 25, Chapters 1-4
 Wednesday, September 20, 2017 Reminders Week 3 Review is now available on D2L (through Friday) Exam 1, Monday, September 25, Chapters 1-4 PLEASE don t memorize equations, but know how to recognize them
Wednesday, September 20, 2017 Reminders Week 3 Review is now available on D2L (through Friday) Exam 1, Monday, September 25, Chapters 1-4 PLEASE don t memorize equations, but know how to recognize them
17.2 and 17.3 Classifying Matter Liquids. Liquids
 17.2 and 17.3 Classifying Matter Liquids Read p.295-301 in book Liquids Liquids have an indefinite shape, but a definite volume. the same shape as their container. particles that are close together, but
17.2 and 17.3 Classifying Matter Liquids Read p.295-301 in book Liquids Liquids have an indefinite shape, but a definite volume. the same shape as their container. particles that are close together, but
EARTH SCIENCE 5.9 (WIND) WEATHER
 EARTH SCIENCE 5.9 (WIND) WEATHER Video Notes Key Points: 1. According to the video, what two factors cause wind: a. b. 2. Fill in the blanks from this quote from the video: Energy from the Sun heats the,
EARTH SCIENCE 5.9 (WIND) WEATHER Video Notes Key Points: 1. According to the video, what two factors cause wind: a. b. 2. Fill in the blanks from this quote from the video: Energy from the Sun heats the,
DEMONSTRATION 2.1 PROPERTIES OF CO 2. Chapter 2: Gases
 DEMONSTRATION 2.1 Chapter 2: Gases PROPERTIES OF CO 2 This demonstration has two aims: firstly, to show that carbon dioxide gas is denser than air; secondly, to show that carbon dioxide will not support
DEMONSTRATION 2.1 Chapter 2: Gases PROPERTIES OF CO 2 This demonstration has two aims: firstly, to show that carbon dioxide gas is denser than air; secondly, to show that carbon dioxide will not support
CARTESIAN DIVER (1 Hour)
 (1 Hour) Addresses NGSS Level of Difficulty: 2 Grade Range: K-2 OVERVIEW In this activity, students will build a Cartesian diver and discover how compression and changes in density cause the diver to mysteriously
(1 Hour) Addresses NGSS Level of Difficulty: 2 Grade Range: K-2 OVERVIEW In this activity, students will build a Cartesian diver and discover how compression and changes in density cause the diver to mysteriously
LAB 7. ROTATION. 7.1 Problem. 7.2 Equipment. 7.3 Activities
 LAB 7. ROTATION 7.1 Problem How are quantities of rotational motion defined? What sort of influence changes an object s rotation? How do the quantities of rotational motion operate? 7.2 Equipment plumb
LAB 7. ROTATION 7.1 Problem How are quantities of rotational motion defined? What sort of influence changes an object s rotation? How do the quantities of rotational motion operate? 7.2 Equipment plumb
The Hydrological Cycle
 Introduction to Climatology GEOGRAPHY 300 The Hydrological Cycle Tom Giambelluca University of Hawai i at Mānoa Atmospheric Moisture Changes of Phase of Water Changes of Phase of Water 1 Changes of Phase
Introduction to Climatology GEOGRAPHY 300 The Hydrological Cycle Tom Giambelluca University of Hawai i at Mānoa Atmospheric Moisture Changes of Phase of Water Changes of Phase of Water 1 Changes of Phase
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT.
 Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
Winds and Ocean Circulations
 Winds and Ocean Circulations AT 351 Lab 5 February 20, 2008 Sea Surface Temperatures 1 Temperature Structure of the Ocean Ocean Currents 2 What causes ocean circulation? The direction of most ocean currents
Winds and Ocean Circulations AT 351 Lab 5 February 20, 2008 Sea Surface Temperatures 1 Temperature Structure of the Ocean Ocean Currents 2 What causes ocean circulation? The direction of most ocean currents
Nadia Naghi. Hung Do. Minh Lu. George Manoli PHYS Lab 12: Archimede s Principle. July 2, 2014
 1 Nadia Naghi Hung Do Minh Lu George Manoli PHYS 2125 Lab 12: Archimede s Principle July 2, 2014 2 ABSTRACT: This experiment studies the principle of density by applying Archimedes principle and calculating
1 Nadia Naghi Hung Do Minh Lu George Manoli PHYS 2125 Lab 12: Archimede s Principle July 2, 2014 2 ABSTRACT: This experiment studies the principle of density by applying Archimedes principle and calculating
Ch. 4 Motion in One direction Ch 6. Pressure in Fluids and Atmospheric Pressure Ch. 7. Up-thrust in Fluids Ch. 8. Floatation and Relative Density
 Ch. 4 Motion in One direction Ch 6. Pressure in Fluids and Atmospheric Pressure Ch. 7. Up-thrust in Fluids Ch. 8. Floatation and Relative Density Physics Class 9 th Copyright 10x10learning.com 1 Acceleration
Ch. 4 Motion in One direction Ch 6. Pressure in Fluids and Atmospheric Pressure Ch. 7. Up-thrust in Fluids Ch. 8. Floatation and Relative Density Physics Class 9 th Copyright 10x10learning.com 1 Acceleration
Chs. 16 and 17 Mechanical Waves
 Chs. 16 and 17 Mechanical Waves The nature of waves A wave is a traveling disturbance that carries energy from one place to another, and even though matter may be disturbed as a wave travels through a
Chs. 16 and 17 Mechanical Waves The nature of waves A wave is a traveling disturbance that carries energy from one place to another, and even though matter may be disturbed as a wave travels through a
Assessment Schedule 2016 Earth and Space Science: Demonstrate understanding of processes in the ocean system (91413)
 NCEA Level 3 Earth & Space Science (91413) 2016 page 1 of 6 Assessment Schedule 2016 Earth and Space Science: Demonstrate processes in the ocean system (91413) Evidence Statement Q Evidence with with Excellence
NCEA Level 3 Earth & Space Science (91413) 2016 page 1 of 6 Assessment Schedule 2016 Earth and Space Science: Demonstrate processes in the ocean system (91413) Evidence Statement Q Evidence with with Excellence
Conceptual Physics Matter Liquids Gases
 Conceptual Physics Matter Liquids Gases Lana Sheridan De Anza College July 25, 2017 Last time atomic structure forms of matter solids density elasticity liquids & pressure Overview liquids pressure surface
Conceptual Physics Matter Liquids Gases Lana Sheridan De Anza College July 25, 2017 Last time atomic structure forms of matter solids density elasticity liquids & pressure Overview liquids pressure surface
Weather & Atmosphere Study Guide
 Weather & Atmosphere Study Guide 1. Draw a simple water cycle diagram using the following words: Precipitation, Evaporation, Condensation, Transpiration 2. In your own words, explain the difference between
Weather & Atmosphere Study Guide 1. Draw a simple water cycle diagram using the following words: Precipitation, Evaporation, Condensation, Transpiration 2. In your own words, explain the difference between
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The concept of pressure involves both 1) A) force and area. B) force and volume. C) area
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The concept of pressure involves both 1) A) force and area. B) force and volume. C) area
STUDENT PACKET # 10. Vocabulary: condensation, convection, convection current, land breeze, sea breeze
 STUDENT PACKET # 10 Name: Date: Student Exploration: Coastal Winds and Clouds Big Idea 7: Earth Systems and Patterns SC.6.E.7.4 Differentiate and show interactions among the geosphere, hydrosphere, cryosphere,
STUDENT PACKET # 10 Name: Date: Student Exploration: Coastal Winds and Clouds Big Idea 7: Earth Systems and Patterns SC.6.E.7.4 Differentiate and show interactions among the geosphere, hydrosphere, cryosphere,
Unit 1 Lesson 5 Fluids and Pressure. Copyright Houghton Mifflin Harcourt Publishing Company
 Feel the Pressure! What are fluids? A fluid is any material that can flow and that takes the shape of its container. A fluid can flow because its particles easily move past each other. Liquids and gases,
Feel the Pressure! What are fluids? A fluid is any material that can flow and that takes the shape of its container. A fluid can flow because its particles easily move past each other. Liquids and gases,
Fluids. James H Dann, Ph.D. Say Thanks to the Authors Click (No sign in required)
 Fluids James H Dann, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive content, visit
Fluids James H Dann, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive content, visit
Take the challenge exam!
 Take the challenge exam! Today is the last day to take it! Read the book Focus on new concepts Answer the questions at the end of each chapter Vocabulary test #1 deadline: Friday 25 Sept. First exam deadline:
Take the challenge exam! Today is the last day to take it! Read the book Focus on new concepts Answer the questions at the end of each chapter Vocabulary test #1 deadline: Friday 25 Sept. First exam deadline:
1. All fluids are: A. gases B. liquids C. gases or liquids D. non-metallic E. transparent ans: C
 Chapter 14: FLUIDS 1 All fluids are: A gases B liquids C gases or liquids D non-metallic E transparent 2 Gases may be distinguished from other forms of matter by their: A lack of color B small atomic weights
Chapter 14: FLUIDS 1 All fluids are: A gases B liquids C gases or liquids D non-metallic E transparent 2 Gases may be distinguished from other forms of matter by their: A lack of color B small atomic weights
Mix and Flow of Matter Unit Test. For each of the following hazardous products match the correct WHMIS symbol
 /40 Student Name Class Section 1.1 WHMIS For each of the following hazardous products match the correct WHMIS symbol 1 Flammable A. 2 Corrosive B. 3 Dangerously Reactive C. Section 1.2 The Many Uses of
/40 Student Name Class Section 1.1 WHMIS For each of the following hazardous products match the correct WHMIS symbol 1 Flammable A. 2 Corrosive B. 3 Dangerously Reactive C. Section 1.2 The Many Uses of
ACTIVITY 1: Buoyancy Problems. OBJECTIVE: Practice and Reinforce concepts related to Fluid Pressure, primarily Buoyancy
 LESSON PLAN: SNAP, CRACKLE, POP: Submarine Buoyancy, Compression, and Rotational Equilibrium DEVELOPED BY: Bill Sanford, Nansemond Suffolk Academy 2012 NAVAL HISTORICAL FOUNDATION TEACHER FELLOWSHIP ACTIVITY
LESSON PLAN: SNAP, CRACKLE, POP: Submarine Buoyancy, Compression, and Rotational Equilibrium DEVELOPED BY: Bill Sanford, Nansemond Suffolk Academy 2012 NAVAL HISTORICAL FOUNDATION TEACHER FELLOWSHIP ACTIVITY
Copy and answer the following in your marble composition book. 1. Which direction is the wind deflected in the northern hemisphere?
 Copy and answer the following in your marble composition book. 1. Which direction is the wind deflected in the northern hemisphere? 2. Which direction is the wind deflected in the southern hemisphere?
Copy and answer the following in your marble composition book. 1. Which direction is the wind deflected in the northern hemisphere? 2. Which direction is the wind deflected in the southern hemisphere?
KEY CONCEPT Earth s atmosphere supports life. Living things need food, water, and air Matter can be solid, liquid, or gas
 KEY CONCEPT Earth s atmosphere supports life. BEFORE, you learned Living things need food, water, and air Matter can be solid, liquid, or gas NOW, you will learn Why the atmosphere is important to living
KEY CONCEPT Earth s atmosphere supports life. BEFORE, you learned Living things need food, water, and air Matter can be solid, liquid, or gas NOW, you will learn Why the atmosphere is important to living
Chapter 12. Properties of Gases
 Properties of Gases Each state of matter has its own properties. Gases have unique properties because the distance between the particles of a gas is much greater than the distance between the particles
Properties of Gases Each state of matter has its own properties. Gases have unique properties because the distance between the particles of a gas is much greater than the distance between the particles
Vacuum P=0. h=76 cm A B C. Barometer
 Recap: Pressure Pressure = Force per unit area (P = F /A; units: Pascals) Density of object = mass / volume (ρ = m /V; units: kg / m 3 ) Pascal s Law:Pressure is transmitted equally in all directions throughout
Recap: Pressure Pressure = Force per unit area (P = F /A; units: Pascals) Density of object = mass / volume (ρ = m /V; units: kg / m 3 ) Pascal s Law:Pressure is transmitted equally in all directions throughout
1 Fluids and Pressure
 CHAPTER 3 1 Fluids and Pressure SECTION Forces in Fluids BEFORE YOU READ After you read this section, you should be able to answer these questions: What are fluids? What is atmospheric pressure? What is
CHAPTER 3 1 Fluids and Pressure SECTION Forces in Fluids BEFORE YOU READ After you read this section, you should be able to answer these questions: What are fluids? What is atmospheric pressure? What is
Physics General Physics. Lecture 19 - Fluids. Fall 2016 Semester Prof. Matthew Jones
 Physics 22000 General Physics Lecture 19 - Fluids Fall 2016 Semester Prof. Matthew Jones 1 2 What s New This Time? Previously, we had ignored the effect of gravity on the gas particles that were described
Physics 22000 General Physics Lecture 19 - Fluids Fall 2016 Semester Prof. Matthew Jones 1 2 What s New This Time? Previously, we had ignored the effect of gravity on the gas particles that were described
3 1 PRESSURE. This is illustrated in Fig. 3 3.
 P = 3 psi 66 FLUID MECHANICS 150 pounds A feet = 50 in P = 6 psi P = s W 150 lbf n = = 50 in = 3 psi A feet FIGURE 3 1 The normal stress (or pressure ) on the feet of a chubby person is much greater than
P = 3 psi 66 FLUID MECHANICS 150 pounds A feet = 50 in P = 6 psi P = s W 150 lbf n = = 50 in = 3 psi A feet FIGURE 3 1 The normal stress (or pressure ) on the feet of a chubby person is much greater than
The Ideal Gas Constant
 Chem 2115 Experiment # 8 The Ideal Gas Constant OBJECTIVE: This experiment is designed to provide experience in gas handling methods and experimental insight into the relationships between pressure, volume,
Chem 2115 Experiment # 8 The Ideal Gas Constant OBJECTIVE: This experiment is designed to provide experience in gas handling methods and experimental insight into the relationships between pressure, volume,
10.4 Buoyancy is a force
 Chapter 10.4 Learning Goals Define buoyancy. Explain the relationship between density and buoyancy. Discuss applications of Archimedes principle. 10.4 Buoyancy is a force Buoyancy is a measure of the upward
Chapter 10.4 Learning Goals Define buoyancy. Explain the relationship between density and buoyancy. Discuss applications of Archimedes principle. 10.4 Buoyancy is a force Buoyancy is a measure of the upward
Section 1 Types of Waves. Distinguish between mechanical waves and electromagnetic waves.
 Section 1 Types of Waves Objectives Recognize that waves transfer energy. Distinguish between mechanical waves and electromagnetic waves. Explain the relationship between particle vibration and wave motion.
Section 1 Types of Waves Objectives Recognize that waves transfer energy. Distinguish between mechanical waves and electromagnetic waves. Explain the relationship between particle vibration and wave motion.
Chapter 7 Weather and Climate
 Chapter 7 Weather and Climate *Describe what weather is, what affects it, and where it occurs. *Explain the connection between air pressure and wind. * *Many factors affect a region s weather. * *atmosphere
Chapter 7 Weather and Climate *Describe what weather is, what affects it, and where it occurs. *Explain the connection between air pressure and wind. * *Many factors affect a region s weather. * *atmosphere
Vertical Motion and Atmospheric Stability
 Lesson 4 Vertical Motion and Atmospheric Stability This lesson describes the vertical structure of the atmosphere, atmospheric stability and the corresponding vertical motion. Adiabatic diagrams are introduced
Lesson 4 Vertical Motion and Atmospheric Stability This lesson describes the vertical structure of the atmosphere, atmospheric stability and the corresponding vertical motion. Adiabatic diagrams are introduced
