Figure. Experimental set up of catalyst, reactants and product syringes.
|
|
|
- Rafe Nicholson
- 5 years ago
- Views:
Transcription
1 Construction and Use of the Gas Reaction Catalyst Tube** As shown in the figures, the experimental apparatus consists of a 60 ml syringe containing the gaseous reactants connected to a gas phase catalyst tube containing elemental palladium on a ceramic support. The other end of the catalyst tube is connected to another 60 ml syringe where the products will be collected. This second picture shows our homemade catalyst tube in more detail. Figure. Experimental set up of catalyst, reactants and product syringes. A 25-cm length of borosilicate glass tubing, 6 mm OD, is fire-polished on one end until the opening becomes slightly constricted. A 90o angle is formed in the tubing approximately 8 cm from one end with an oxygen/natural gas torch. If the bend slightly constricts the inside diameter, that can serve the useful purpose of preventing the Pd beads from escaping. At this point, the tubing should have the shape of the letter L. We will next place Pd-coated alumina beads into the tubing via the longer arm of the L. A disposable wide-step plastic transfer pipet can be formed into a funnel by cutting off the stem so that it can be slipped over the L -shaped glass tubing. The top half of the bulb can be cut off to create the funnel. Wearing gloves, beads of 0.5% Pd * From the Supplemental Material provided in our article: Heterogeneous catalysis: The Horiuti-Polanyi mechanism and alkene hydrogenation. Bruce Mattson*, Wendy Foster, Jaclyn Greimann, Trisha Hoette, Nhu Le, Anne Mirich, Shanna Wankum, Ann Cabri, Claire Reichenbacher, and Erika Schwanke, Journal of Chemical Education, 2013, 90 (5),
2 on alumina are dropped, one-at-a-time, into the tube. In our experience, the larger beads do not fit in the tube. After the beads inside the glass tube extend a length of 4 8 cm, the second 90 o bend can be formed. Again, a slight constriction prevents the escape of the Pd beads. The second end of the U-shaped tube is fire-polished. Allow 30 minutes to construct the catalyst tube. The catalyst tube can be used indefinitely. Volume and residence time Using a separate empty tube, the internal volume of our catalyst tube was established to be 0.1 ml per cm in an empty tube. This was done by filling the glass tube with water, measuring the mass and length of the water in the tube. Mass is converted to volume. The catalyst tube shown above has 8 cm of beads. Knowing these values can be useful in that the reaction only takes place when the gas mixture is in the presence of the catalyst. If 60 ml are passed in 30 s, the rate is 2 ml/s. Given that 0.8 ml gas mixture can be in the presence of the catalyst at any point in time. This gives an average residence time of 0.8 ml/ 2 ml s -1 = 0.4 s. Moles of catalyst The tube pictured contains 0.61 g of 0.5% Pd on alumina, which equals 3.0 mg Pd or 2.9 X 10-5 mol Pd. This amount of catalyst cost $1.42. Working air-free Syringes and the catalyst tube are purged of air with argon. This is done by connecting the syringe to the argon tank equipped with a regulator valve with a needle valve for fine flow control. Latex tubing connects the needle valve to a bubbler. The bubbler is filled to a depth of 3 cm above the opening of the inside tube with mineral oil; this provides a pressure maximum. The tubing to the argon source and the tubing to the equipment (syringes, catalyst tube) are connected to the horizontal T-part of the bubbler; the side arm is left open to the air. An alternative bubbler device can be made with glass tubing, a T-connector, a large test tube and a 2-holed stopper. As a technique, one should keep an eye on the bubbler and always maintain positive pressure. As long as bubbles are present, argon pressure is positive. If argon is being withdrawn too fast (as would happen if the plunger of a syringe were pulled out too fast), the bubbles would stop and the mineral oil would climb inside the inner tube of the bubbler. This indicates negative pressure in the system and should be avoided. 2
3 The argon purge of syringes follows the sequence: The syringe with plunger fully inserted is connected to the argon source tubing, the syringe is filled with argon by withdrawing the plunger to the 60 ml mark, the tubing is disconnected, the argon is discharged into the air by pushing the plunger fully inward, the argon source tubing is reconnected and the process is repeated at least two more times. When not in use, syringes are kept capped. If argon is not available, nitrogen also works. Tubing Short pieces (4 cm) of latex tubing are used to connect syringes to each other and to the catalyst tube. Similar pieces are used for drying tubes. A syringe filled with argon is used to purge the connecting tubes of air just prior to use in connections. Pretreatment of the Catalyst Prior to each experiment, the catalyst tube must be prepared to minimize the presence of water, oxygen and residual substances from previous experiments. With a stream of argon flowing through the glass catalyst tube, the catalyst tube is heated over a Bunsen burner flame for a period of 3 minutes with constant motion to prevent hot spots. The catalyst tube is allowed to cool to room temperature with argon flowing. The catalyst tube is then connected to two syringes that were previously purged of air with argon. Short pieces (4 cm) of latex tubing are used to connect syringes to each other and to the catalyst tube. The treated catalyst tube can be stored by connecting it to two argon-purged syringes. This creates a closed system under argon. Preparation of the Drying Tube If gases are prepared by using one of our in-syringe methods, it may be useful to dry the gases before using them. This is true if the product gases are to be analyzed by mass spectroscopy or NMR spectroscopy, but is generally unnecessary if product analysis is being done by qualitative chemical tests. The drying tube is constructed from a 5 cm length of 1/8-in latex tubing. A tiny cotton plug (from an ordinary cotton ball) is inserted into one end using a plastic pipet as a poker. About 0.5 g of a drying agent is then added to the tube, followed by another cotton plug. We have used either anhydrous, granular Na 2 SO 4 or anhydrous, powdered MgSO 4. Drying tubes are prepared fresh for every experiment. 3
4 Materials Fisher, Flinn and Educational Innovations sell all of the items needed. Fisher has the best prices for syringes bought in boxes of 30. Flinn and Educational Innovations are excellent sources of syringe caps and latex tubing. Reagent Vendor (used for example only) Product number and price (2012) 60 ml plastic syringes Fisher Scientific ; $60.65 for 30; $ for 4 pks x 30 Syringe caps Latex tubing, 1/8-inch (3.175 mm) ID Flinn Educational Innovations Fisher Flinn Educational Innovations AP8958; ten-pack, $2.40; GAS-160, ten-pack, $ case of 50 $64.31 AP2076; 10-ft, $7.15; GAS-220, 5-ft, $5.95 Experimental procedure In a typical reaction, volume stoichiometry dictates a reasonable mixture. For example, equal volumes (30 ml) of each of alkene and H 2 (g) would be used for a hydrogenation reaction (and 30 ml of product gas would be expected). Volumes are measured out in individual argon-flushed syringes and then combined into one of the syringes using a 4 cm latex connecting tube. The reactant syringe is attached to one end of the catalyst using a latex tube. The entire volume is passed through the catalyst tube submerged in a water (or ice) bath over the course of 30 seconds, for example. The reactants are passed in a smooth, continuous motion. Products are collected in another 60 ml syringe attached to the other side of the catalyst tube. Preparation of samples using Gas Chromatography - Mass Spectometry Approximately 10 µl of product is needed for this analysis. The injection syringe is flushed with at least one volume of sample gas and discharged before obtaining a sample for injection. The needle of the 10 µl syringe is directed through the opening in the 60 ml syringe. The large syringe is held with the opening upright if the gas sample is heavier-than-air, such as butane, or with the opening downward for lighter-than-air gases such as methane. The GC-MS is set to mild conditions, only reaching 40ºC in the oven and as low of an ionization energy as possible to minimize fragmentation. 4
5 Preparation and analysis of samples using 1 H-NMR An NMR tube is filled to the appropriate level with deuterochloroform. A thinstem plastic transfer pipette is stretched to form a long, very thin capillary. The bulb and unstretched ends are cut off, leaving only the capillary portion. The tubing is inserted into the 60 ml syringe containing the product gases to be analyzed. Teflon tape is wrapped around the opening of the syringe and the capillary tube to form a gas-tight seal. The other end of the capillary tube is inserted to the bottom of CDCl 3 in the NMR tube. In ten 1 ml increments, the product gas is slowly passed through the solvent forming a stream of small bubbles. After each increment, the NMR tube is capped, and shaken to dissolve product gas above the surface of the solvent. This process is repeated ten times. Some gases such as butane are very soluble and such an elaborate procedure can be simplified considerably. 5
PREPARING GAS SAMPLES IN LARGE PLASTIC SYRINGES
 PREPARING GAS SAMPLES IN LARGE PLASTIC SYRINGES A wide variety of gases can be prepared safely inside a 60 ml plastic syringe. Here you will practice making carbon dioxide, so that you know the technique
PREPARING GAS SAMPLES IN LARGE PLASTIC SYRINGES A wide variety of gases can be prepared safely inside a 60 ml plastic syringe. Here you will practice making carbon dioxide, so that you know the technique
Technical Bulletin AL-134
 Technical Bulletin AL-134 Handling Air-Sensitive Reagents The Aldrich Sure/Seal system Anhydrous solvents and air-sensitive reagents from Aldrich are packaged in our exclusive Sure/Seal bottles which provide
Technical Bulletin AL-134 Handling Air-Sensitive Reagents The Aldrich Sure/Seal system Anhydrous solvents and air-sensitive reagents from Aldrich are packaged in our exclusive Sure/Seal bottles which provide
Boyle s Law VC 09. Experiment 9: Gas Laws. Abstract
 Experiment 9: Gas Laws VC 09 Abstract In this laboratory activity, you will experimentally confirm Boyle s Law, determine absolute zero from Gay-Lussac's Law, and determine the molecular weight of acetone,
Experiment 9: Gas Laws VC 09 Abstract In this laboratory activity, you will experimentally confirm Boyle s Law, determine absolute zero from Gay-Lussac's Law, and determine the molecular weight of acetone,
VACUUM & AIRLESS. Select from Kimble vacuum traps, manifolds, Schlenk tubes, adapters, and bubblers for your vacuum and airless glassware needs.
 VACUUM & AIRLESS Select from Kimble vacuum traps, manifolds, Schlenk tubes, adapters, and bubblers for your vacuum and airless glassware needs. tions precision glassware solutions precision glassware solutions
VACUUM & AIRLESS Select from Kimble vacuum traps, manifolds, Schlenk tubes, adapters, and bubblers for your vacuum and airless glassware needs. tions precision glassware solutions precision glassware solutions
Experiment 18 Properties of Gases
 Experiment 18 Properties of Gases E18-1 E18-2 The Task In this experiment you will investigate some of the properties of gases, i.e. how gases flow, their phase changes and chemical reactivity. Skills
Experiment 18 Properties of Gases E18-1 E18-2 The Task In this experiment you will investigate some of the properties of gases, i.e. how gases flow, their phase changes and chemical reactivity. Skills
UNIT 10 - GASES. Notes & Worksheets - Honors
 Ideal Gas Equation 1 WKSHT 1.) What is the pressure exerted by 2.0 moles of an ideal gas when it occupies a volume of 12.0 L at 373 K? 2.) A flashbulb of volume 2.6 cm 3 contains O 2 gas at a pressure
Ideal Gas Equation 1 WKSHT 1.) What is the pressure exerted by 2.0 moles of an ideal gas when it occupies a volume of 12.0 L at 373 K? 2.) A flashbulb of volume 2.6 cm 3 contains O 2 gas at a pressure
ASTM 3612/TOGA/Dissolved Gas GC Revised October 2013
 The SRI ASTM 3612/TOGA/ Dissolved Gas GC configuration ( TOGA GC ) permits measurement of gasses dissolved in water, oil or other liquids, and is based on the requirements of ASTM method 3612, option C,
The SRI ASTM 3612/TOGA/ Dissolved Gas GC configuration ( TOGA GC ) permits measurement of gasses dissolved in water, oil or other liquids, and is based on the requirements of ASTM method 3612, option C,
Solvent System Walkthrough
 v2.0 1 Solvent System Walkthrough The Contour Glass solvent system is designed to give you air-free anhydrous solvent with minimal effort. However, the system is only as good as its users so please read
v2.0 1 Solvent System Walkthrough The Contour Glass solvent system is designed to give you air-free anhydrous solvent with minimal effort. However, the system is only as good as its users so please read
NMR Service. How to Prepare Samples for NMR
 NMR Service How to Prepare Samples for NMR In NMR, unlike other types of spectroscopy, the quality of the sample has a profound effect on the quality of the resulting spectrum. If you follow a few simple
NMR Service How to Prepare Samples for NMR In NMR, unlike other types of spectroscopy, the quality of the sample has a profound effect on the quality of the resulting spectrum. If you follow a few simple
DEMONSTRATION 2.1 PROPERTIES OF CO 2. Chapter 2: Gases
 DEMONSTRATION 2.1 Chapter 2: Gases PROPERTIES OF CO 2 This demonstration has two aims: firstly, to show that carbon dioxide gas is denser than air; secondly, to show that carbon dioxide will not support
DEMONSTRATION 2.1 Chapter 2: Gases PROPERTIES OF CO 2 This demonstration has two aims: firstly, to show that carbon dioxide gas is denser than air; secondly, to show that carbon dioxide will not support
LABORATORY TECHNIQUES. Pouring Liquids
 LABORATORY TECHNIQUES Working in the chemistry laboratory you will be handling potentially dangerous substances and performing unfamiliar tasks. This section provides you with a guide to the safe laboratory
LABORATORY TECHNIQUES Working in the chemistry laboratory you will be handling potentially dangerous substances and performing unfamiliar tasks. This section provides you with a guide to the safe laboratory
acrolein, acetaldehyde and acetone( cm -1 ); methanol (1306 cm -1 ); ethylene (949 cm -1 ); and isoprene (893 cm -1 ).
 acrolein, acetaldehyde and acetone(1550 1800 cm -1 ); methanol (1306 cm -1 ); ethylene (949 cm -1 ); and isoprene (893 cm -1 ). 5 Figure 4a 6 Figure 4b Figure 4c 7 Figure 5 Questions in Student Handout
acrolein, acetaldehyde and acetone(1550 1800 cm -1 ); methanol (1306 cm -1 ); ethylene (949 cm -1 ); and isoprene (893 cm -1 ). 5 Figure 4a 6 Figure 4b Figure 4c 7 Figure 5 Questions in Student Handout
INSTRUCTIONS: CHEMICAL ANALYSIS OF ACTIVE SULFIDES IN OIL-BASED DRILLING FLUID
 OFI Testing Equipment Chemical Analysis of Active Sulfides Instructions Page 1 of 4 INSTRUCTIONS: CHEMICAL ANALYSIS OF ACTIVE SULFIDES IN OIL-BASED DRILLING FLUID DESCRIPTION: 1. The Garrett Gas Train
OFI Testing Equipment Chemical Analysis of Active Sulfides Instructions Page 1 of 4 INSTRUCTIONS: CHEMICAL ANALYSIS OF ACTIVE SULFIDES IN OIL-BASED DRILLING FLUID DESCRIPTION: 1. The Garrett Gas Train
Students measure the change in pressure by varying the volume of trapped air in a syringe while:
 How Does a Trapped Gas Behave? Teacher Information Objective Students investigate the effect of changes in the volume of a confined gas on pressure at constant temperature. Using the pressure sensor, students
How Does a Trapped Gas Behave? Teacher Information Objective Students investigate the effect of changes in the volume of a confined gas on pressure at constant temperature. Using the pressure sensor, students
Working correctly with gas
 Working correctly with gas When working with a reactive gas in a cylinder, there is an inherent risk. Special care has to be taken to allow safe handling. This example is with HCl. 1) Fixing the gas bottle.
Working correctly with gas When working with a reactive gas in a cylinder, there is an inherent risk. Special care has to be taken to allow safe handling. This example is with HCl. 1) Fixing the gas bottle.
Unit 11 Gas Laws Chapters 13 of your textbook
 Unit 11 Gas Laws Chapters 13 of your textbook Early Booklet E.C.: + 2 Unit 11 Hwk. Pts.: / 19 Unit 11 Lab Pts.: / 20 Late, Incomplete, No Work, No Units Fees? Y / N Learning Targets for Unit 11 1.1 I can
Unit 11 Gas Laws Chapters 13 of your textbook Early Booklet E.C.: + 2 Unit 11 Hwk. Pts.: / 19 Unit 11 Lab Pts.: / 20 Late, Incomplete, No Work, No Units Fees? Y / N Learning Targets for Unit 11 1.1 I can
19. The Grignard Reaction
 19. The Grignard eaction A. Introduction The Grignard reaction is an extremely valuable reaction in organic chemistry because it allows for the formation of carbon-carbon bonds. The reaction was discovered
19. The Grignard eaction A. Introduction The Grignard reaction is an extremely valuable reaction in organic chemistry because it allows for the formation of carbon-carbon bonds. The reaction was discovered
PREPARATION AND PROPERTIES OF ATMOSPHERIC GASES I: Carbon Dioxide and Oxygen
 PREPARATION AND PROPERTIES OF ATMOSPHERIC GASES I: Carbon Dioxide and Oxygen.... :O = C = O:.... :O = O: INTRODUCTION The atmosphere consists predominantly of three gases -- nitrogen (N 2 ) 78%, oxygen
PREPARATION AND PROPERTIES OF ATMOSPHERIC GASES I: Carbon Dioxide and Oxygen.... :O = C = O:.... :O = O: INTRODUCTION The atmosphere consists predominantly of three gases -- nitrogen (N 2 ) 78%, oxygen
Please do not write on this test. Please use the answer sheet.
 AP Chemistry Test (Chapter 5) Multiple Choice (50%) Please do not write on this test. Please use the answer sheet. Form: Snowboard 1) A sample of argon gas is sealed in a rigid metal tank. The temperature
AP Chemistry Test (Chapter 5) Multiple Choice (50%) Please do not write on this test. Please use the answer sheet. Form: Snowboard 1) A sample of argon gas is sealed in a rigid metal tank. The temperature
ExamLearn.ie. The Air & Oxygen
 ExamLearn.ie The Air & Oxygen The Air & Oxygen The air is a mixture of gases, which forms a blanket around the earth. Another name for the air is the atmosphere. *To investigate the percentage of oxygen
ExamLearn.ie The Air & Oxygen The Air & Oxygen The air is a mixture of gases, which forms a blanket around the earth. Another name for the air is the atmosphere. *To investigate the percentage of oxygen
POLYMER TEST KIT (CLAPPER METHOD) OFI PART No
 OFI Testing Equipment Polymer Test Kit (Clapper Method) Part # 295-00 Instructions Page 1 of 4 POLYMER TEST KIT (CLAPPER METHOD) OFI PART No. 295-00 This test determines the Partially Hydrolyzed Polyacrylamide
OFI Testing Equipment Polymer Test Kit (Clapper Method) Part # 295-00 Instructions Page 1 of 4 POLYMER TEST KIT (CLAPPER METHOD) OFI PART No. 295-00 This test determines the Partially Hydrolyzed Polyacrylamide
CO 2 and Mass Chemistry
 CO 2 and Mass Chemistry Background: Many students believe that gases like carbon dioxide (CO 2) do not have mass. The fact is that atmospheric gases like CO 2 and methane (CH 4) have a tremendous amount
CO 2 and Mass Chemistry Background: Many students believe that gases like carbon dioxide (CO 2) do not have mass. The fact is that atmospheric gases like CO 2 and methane (CH 4) have a tremendous amount
Completed ALL 2 Warm-up IC Kinetic Molecular Theory Notes. Kinetic Molecular Theory and Pressure Worksheet
 Name: Unit 10- Gas Laws Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 5 Kinetic Molecular Theory Notes IC 1 6 8 Kinetic Molecular Theory and Pressure Worksheet IC 2 9 10 Gas Law
Name: Unit 10- Gas Laws Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 5 Kinetic Molecular Theory Notes IC 1 6 8 Kinetic Molecular Theory and Pressure Worksheet IC 2 9 10 Gas Law
The Safe Use of Pyrophoric Substances
 The Safe Use of Pyrophoric Substances INTRODUCTION In light of a fatality of a researcher earlier this year at UCLA, The University of Queensland has released a guideline for The Safe Use of Pyrophoric
The Safe Use of Pyrophoric Substances INTRODUCTION In light of a fatality of a researcher earlier this year at UCLA, The University of Queensland has released a guideline for The Safe Use of Pyrophoric
INSTRUCTOR RESOURCES
 Gases: Dalton s Law INSTRUCTOR RESOURCES By Dale A. Hammond, PhD LEARNING OBJECTIVES introduce the concept of ideal gases. experimentally determine the relationship between pressure and amount of gas,
Gases: Dalton s Law INSTRUCTOR RESOURCES By Dale A. Hammond, PhD LEARNING OBJECTIVES introduce the concept of ideal gases. experimentally determine the relationship between pressure and amount of gas,
CHEM254 #4 The Synthesis of a Tertiary Alcohol Using a Pre-Made Grignard Reagent 1
 CHEM254 #4 The Synthesis of a Tertiary Alcohol Using a Pre-Made Grignard Reagent 1 Background In this project, we will perform a Grignard reaction using a pre-made Grignard reagent. Grignard reagents can
CHEM254 #4 The Synthesis of a Tertiary Alcohol Using a Pre-Made Grignard Reagent 1 Background In this project, we will perform a Grignard reaction using a pre-made Grignard reagent. Grignard reagents can
AQUARIUS. Operating Instructions for the. Type 70 Electrolytic Gas Soldering / Welding Unit. Table of Contents
 AQUARIUS Operating Instructions for the Type 70 Electrolytic Gas Soldering / Welding Unit Table of Contents 1. Important General Remarks 2 2. Safety Instructions 2 3. Description of the Soldering Unit
AQUARIUS Operating Instructions for the Type 70 Electrolytic Gas Soldering / Welding Unit Table of Contents 1. Important General Remarks 2 2. Safety Instructions 2 3. Description of the Soldering Unit
FisherPak Instructions
 FisherPak Instructions Installation and Start-Up Procedures 2 FisherPak Dip Tape Level Gauge Operating Instructions 3 Trouble Shooting Guide 3 FisherPak Disconnection Procedure 4 Rupture Disk Replacement
FisherPak Instructions Installation and Start-Up Procedures 2 FisherPak Dip Tape Level Gauge Operating Instructions 3 Trouble Shooting Guide 3 FisherPak Disconnection Procedure 4 Rupture Disk Replacement
Multiple Choice (40%)
 AP Chemistry Test (Chapter 5) Please do not write on this test thank you! Multiple Choice (40%) 1) A sealed rigid container is filled with three ideal gases: A, B and C. The partial pressure of each gas
AP Chemistry Test (Chapter 5) Please do not write on this test thank you! Multiple Choice (40%) 1) A sealed rigid container is filled with three ideal gases: A, B and C. The partial pressure of each gas
You should be able to: Describe Equipment Barometer Manometer. 5.1 Pressure Read and outline 5.1 Define Barometer
 A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
Multiple Gas#5 GC configuration Jan 2016
 History: Unfortunately there is no single column that can separate: Hydrogen Oxygen Nitrogen Methane CO CO2 Ethane Water Propane Butane Pentane Over the years SRI Instruments has devised several solutions
History: Unfortunately there is no single column that can separate: Hydrogen Oxygen Nitrogen Methane CO CO2 Ethane Water Propane Butane Pentane Over the years SRI Instruments has devised several solutions
Please do not write on this test. Please use the answer sheet. 1) Please choose all conditions that would allow a gas sample to behave ideally.
 AP Chemistry Test (Chapter 5) Please do not write on this test. Please use the answer sheet. Multiple Choice (50%) 1) Please choose all conditions that would allow a gas sample to behave ideally. I) Nonpolar
AP Chemistry Test (Chapter 5) Please do not write on this test. Please use the answer sheet. Multiple Choice (50%) 1) Please choose all conditions that would allow a gas sample to behave ideally. I) Nonpolar
EXPERIMENT. Identification of Gases
 EXPERIMENT Identification of Gases Hands-On Labs, Inc. Version 42-0189-00-02 Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before you begin. Take time
EXPERIMENT Identification of Gases Hands-On Labs, Inc. Version 42-0189-00-02 Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before you begin. Take time
Determination of the Gas-Law Constant (R) using CO2
 Determination of the Gas-Law Constant (R) using CO2 EXPERIMENT 11 Prepared by Edward L. Brown and Miranda Raines, Lee University The student will become familiar with ideal gases and how their properties
Determination of the Gas-Law Constant (R) using CO2 EXPERIMENT 11 Prepared by Edward L. Brown and Miranda Raines, Lee University The student will become familiar with ideal gases and how their properties
LAB 06 Organismal Respiration
 LAB 06 Organismal Respiration Objectives: To learn how a respirometer can be used to determine a respiration rate. Identify and explain the effect of seed germination on cell respiration. To design and
LAB 06 Organismal Respiration Objectives: To learn how a respirometer can be used to determine a respiration rate. Identify and explain the effect of seed germination on cell respiration. To design and
Gas Laws. 2 HCl(aq) + CaCO 3 (s) H 2 O(l) + CO 2 (g) + CaCl 2 (aq) HCl(aq) + NaHCO 3 (s) H 2 O(l) + CO 2 (g) + NaCl(aq)
 Gas Laws Introduction: Although we cannot see gases, we can observe their behavior and study their properties. For example, we can watch a balloon filled with helium gas floating in air and conclude that
Gas Laws Introduction: Although we cannot see gases, we can observe their behavior and study their properties. For example, we can watch a balloon filled with helium gas floating in air and conclude that
Chapter 13 Gases. H. Cannon, C. Clapper and T. Guillot Klein High School. Pressure/Temperature Conversions
 Chapter 13 Gases Pressure/Temperature Conversions Convert the following: 1. 3.50 atm = kpa 2. 123 atm = mmhg 3. 970.0 mmhg = torr 4. 870.0 torr = kpa 5. 250.0 kpa = atm 6. 205.0 mmhg = kpa 7. 12.4 atm
Chapter 13 Gases Pressure/Temperature Conversions Convert the following: 1. 3.50 atm = kpa 2. 123 atm = mmhg 3. 970.0 mmhg = torr 4. 870.0 torr = kpa 5. 250.0 kpa = atm 6. 205.0 mmhg = kpa 7. 12.4 atm
Activity Title: Exploring the Ocean with Robots
 BEST OF COSEE HANDS-ON ACTIVITIES Activity Title: Exploring the Ocean with Robots Learning Objectives This lesson will introduce students to robotic submarines, called gliders, including basic properties
BEST OF COSEE HANDS-ON ACTIVITIES Activity Title: Exploring the Ocean with Robots Learning Objectives This lesson will introduce students to robotic submarines, called gliders, including basic properties
Patients Guide for Self Administering Intravenous Antibiotics General points
 Patients Guide for Self Administering Intravenous Antibiotics General points An intravenous line is an open route for infection therefore you need to prevent this by washing your hands before any procedure
Patients Guide for Self Administering Intravenous Antibiotics General points An intravenous line is an open route for infection therefore you need to prevent this by washing your hands before any procedure
The grade 6 English science unit, Gases, meets the academic content standards set in the Korean curriculum, which state students should:
 This area deals with the properties of gases as small collections of particles. Different kinds of intangible and invisible gases attract students curiosity and promote their use of reasoning skills. Students
This area deals with the properties of gases as small collections of particles. Different kinds of intangible and invisible gases attract students curiosity and promote their use of reasoning skills. Students
To derive from experiment the relationships between Pressure (P), Volume (V), Temperature (T), and Water Solubility of gases.
 PROPERTIES OF GASES: PRESSURE, VOLUME, TEMPERATURE, & SOLUBILITY RELATIONSHIPS PURPOSE: To derive from experiment the relationships between Pressure (P), Volume (V), Temperature (T), and Water Solubility
PROPERTIES OF GASES: PRESSURE, VOLUME, TEMPERATURE, & SOLUBILITY RELATIONSHIPS PURPOSE: To derive from experiment the relationships between Pressure (P), Volume (V), Temperature (T), and Water Solubility
Standard Operating Procedure Inert Vacuum Line. Ryan Mulvenna, May 2012
 Standard Operating Procedure Inert Vacuum Line Ryan Mulvenna, May 2012 Description of Process The inert vacuum lines located in the fume hoods one (Conjugated Polymer Synthesis: CPS) and two (ATRP polymer
Standard Operating Procedure Inert Vacuum Line Ryan Mulvenna, May 2012 Description of Process The inert vacuum lines located in the fume hoods one (Conjugated Polymer Synthesis: CPS) and two (ATRP polymer
Gas Laws. 2 HCl(aq) + CaCO 3 (s) H 2 O(l) + CO 2 (g) + CaCl 2 (aq) HCl(aq) + NaHCO 3 (s) H 2 O(l) + CO 2 (g) + NaCl(aq)
 Gas Laws Introduction: Although we cannot see gases, we can observe their behavior and study their properties. For example, we can watch a balloon filled with helium gas floating in air and conclude that
Gas Laws Introduction: Although we cannot see gases, we can observe their behavior and study their properties. For example, we can watch a balloon filled with helium gas floating in air and conclude that
Detector Carrier Gas Comments Detector anode purge or reference gas. Electron Capture Nitrogen Maximum sensitivity Nitrogen Argon/Methane
 Gas requirements Gases for packed columns The carrier gas you use depends upon the type of detector and the performance requirements. Table 520-1 lists gas recommendations for packed column use. In general,
Gas requirements Gases for packed columns The carrier gas you use depends upon the type of detector and the performance requirements. Table 520-1 lists gas recommendations for packed column use. In general,
SOLUBILITY OF A SOLID IN WATER
 1516L Experiment 2 SOLUBILITY OF A SOLID IN WATER Objectives In this experiment you will determine the solubility of potassium nitrate (KNO 3 ) in water at various temperatures. You will prepare a plot
1516L Experiment 2 SOLUBILITY OF A SOLID IN WATER Objectives In this experiment you will determine the solubility of potassium nitrate (KNO 3 ) in water at various temperatures. You will prepare a plot
Cooling Gases Phase Changes and Phase Diagrams
 Cooling Gases Phase Changes and Phase Diagrams SCIENTIFIC Introduction What happens to gases when the temperature is dropped way down? As this activity illustrates, it all depends on the gas. Concepts
Cooling Gases Phase Changes and Phase Diagrams SCIENTIFIC Introduction What happens to gases when the temperature is dropped way down? As this activity illustrates, it all depends on the gas. Concepts
SOLUBILITY OF A SOLID IN WATER
 1516L Experiment 1 SOLUBILITY OF A SOLID IN WATER Objectives In this experiment you will determine the solubility of potassium nitrate (KNO 3 ) in water at various temperatures. You will prepare a plot
1516L Experiment 1 SOLUBILITY OF A SOLID IN WATER Objectives In this experiment you will determine the solubility of potassium nitrate (KNO 3 ) in water at various temperatures. You will prepare a plot
Experiment 8 GAS LAWS
 Experiment 8 GAS LAWS FV 6/25/2017 MATERIALS: Amontons Law apparatus, Boyle s Law apparatus, Avogadro s Corollary apparatus, four beakers (2 L), warm-water bath, ice, barometer, digital thermometer, air
Experiment 8 GAS LAWS FV 6/25/2017 MATERIALS: Amontons Law apparatus, Boyle s Law apparatus, Avogadro s Corollary apparatus, four beakers (2 L), warm-water bath, ice, barometer, digital thermometer, air
Commonwealth of Pennsylvania PA Test Method No. 742 Department of Transportation October Pages LABORATORY TESTING SECTION. Method of Test for
 Commonwealth of Pennsylvania PA Test Method No. 742 Department of Transportation 14 Pages LABORATORY TESTING SECTION Method of Test for BITUMEN CONTENT OF BITUMINOUS CONCRETE MIXTURES (Pennsylvania Pycnometer
Commonwealth of Pennsylvania PA Test Method No. 742 Department of Transportation 14 Pages LABORATORY TESTING SECTION Method of Test for BITUMEN CONTENT OF BITUMINOUS CONCRETE MIXTURES (Pennsylvania Pycnometer
D. De La Matter 2004 Swimming Pool Chemistry STUDENT ACTIVITIES:
 D. De La Matter 2004 Swimming Pool Chemistry STUDENT ACTIVITIES: Good News! Flinn Scientific Inc. has developed a classroom kit of experiments based on these activities. The Kit Catalog # is AP6599. Ordering
D. De La Matter 2004 Swimming Pool Chemistry STUDENT ACTIVITIES: Good News! Flinn Scientific Inc. has developed a classroom kit of experiments based on these activities. The Kit Catalog # is AP6599. Ordering
Research Question How does the concentration of catalase affect the speed of the decomposition reaction of Hydrogen Peroxide into oxygen and water?
 Research Question How does the concentration of catalase affect the speed of the decomposition reaction of Hydrogen Peroxide into oxygen and water? Aim To observe the effect of increasing enzyme (catalase)
Research Question How does the concentration of catalase affect the speed of the decomposition reaction of Hydrogen Peroxide into oxygen and water? Aim To observe the effect of increasing enzyme (catalase)
Gas Laws. Introduction
 Gas Laws Introduction In 1662 Robert Boyle found that, at constant temperature, the pressure of a gas and its volume are inversely proportional such that P x V = constant. This relationship is known as
Gas Laws Introduction In 1662 Robert Boyle found that, at constant temperature, the pressure of a gas and its volume are inversely proportional such that P x V = constant. This relationship is known as
Bauman Machine Inc. COMPRESSED GAS CYLINDER SAFETY
 Bauman Machine Inc. COMPRESSED GAS CYLINDER SAFETY Many industrial and laboratory operations require the use of compressed gases for a variety of different operations. Compressed gases present a unique
Bauman Machine Inc. COMPRESSED GAS CYLINDER SAFETY Many industrial and laboratory operations require the use of compressed gases for a variety of different operations. Compressed gases present a unique
Determination of R: The Gas-Law Constant
 Determination of R: The Gas-Law Constant PURPOSE: EXPERIMENT 9 To gain a feeling for how well real gases obey the ideal-gas law and to determine the ideal-gas-law constant R. APPARATUS AND CHEMICALS: KClO
Determination of R: The Gas-Law Constant PURPOSE: EXPERIMENT 9 To gain a feeling for how well real gases obey the ideal-gas law and to determine the ideal-gas-law constant R. APPARATUS AND CHEMICALS: KClO
Enzyme Activity Lab. Wear safety goggles when handling hydrogen peroxide.
 Enzyme Activity Lab This laboratory involves the use of an enzyme that will react with hydrogen peroxide. The enzyme is catalase and hydrogen peroxide (H2O2) is the substrate. The reaction is as follows:
Enzyme Activity Lab This laboratory involves the use of an enzyme that will react with hydrogen peroxide. The enzyme is catalase and hydrogen peroxide (H2O2) is the substrate. The reaction is as follows:
Chemical Class Standard Operating Procedures. Pyrophoric Chemicals (PYR) & Water Reactive Chemicats (WRC) Diethylzinc
 Chemical Class Standard Operating Procedures Pyrophoric Chemicals (PYR) & Water Reactive Chemicats (WRC) Diethylzinc Department: Chemistry Date SOP was written: April 16, 2014 Principal Investigator: Dr.
Chemical Class Standard Operating Procedures Pyrophoric Chemicals (PYR) & Water Reactive Chemicats (WRC) Diethylzinc Department: Chemistry Date SOP was written: April 16, 2014 Principal Investigator: Dr.
The Decomposition of Potassium Chlorate
 The Decomposition of Potassium Chlorate Small quantities of molecular oxygen (O 2 ) can be obtained from the thermal decomposition of certain oxides, peroxides, and salts of oxoacids. Some examples of
The Decomposition of Potassium Chlorate Small quantities of molecular oxygen (O 2 ) can be obtained from the thermal decomposition of certain oxides, peroxides, and salts of oxoacids. Some examples of
Chemistry Chapter 11 Test Review
 Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
Funsheet [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015
![Funsheet [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015 Funsheet [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015](/thumbs/93/112589015.jpg) Funsheet 7.0 7.1 [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015 1. Convert the following pressures. a) 101 kpa =? atm b) 55 Torr =? psi c) 60. mmhg =? bar d) 45 Torr =? kpa e) 5 psi =? atm f) 0.0056 atm =?
Funsheet 7.0 7.1 [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015 1. Convert the following pressures. a) 101 kpa =? atm b) 55 Torr =? psi c) 60. mmhg =? bar d) 45 Torr =? kpa e) 5 psi =? atm f) 0.0056 atm =?
Name Chemistry Pre-AP
 Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
AR251/AR251-B. Venepuncture and Infusion Arm. Instruction Manual
 AR251/AR251-B Venepuncture and Infusion Arm Instruction Manual Thank you for purchasing this AR251 Venepuncture and Infusion Arm. Cast from life, it shows a well developed male left arm in fine detail.
AR251/AR251-B Venepuncture and Infusion Arm Instruction Manual Thank you for purchasing this AR251 Venepuncture and Infusion Arm. Cast from life, it shows a well developed male left arm in fine detail.
Experimental Procedure
 1 of 6 10/3/2018, 1:37 PM https://www.sciencebuddies.org/science-fair-projects/project-ideas/microbio_p009/microbiology/yeast-metabolism-aerobic-anaerobic (http://www.sciencebuddies.org /science-fair-projects/project-ideas/microbio_p009/microbiology/yeast-metabolism-aerobic-anaerobic)
1 of 6 10/3/2018, 1:37 PM https://www.sciencebuddies.org/science-fair-projects/project-ideas/microbio_p009/microbiology/yeast-metabolism-aerobic-anaerobic (http://www.sciencebuddies.org /science-fair-projects/project-ideas/microbio_p009/microbiology/yeast-metabolism-aerobic-anaerobic)
Standard Operating Procedure
 Standard Operating Procedure Read the EH&S Standard Operating Procedures Fact Sheet before filling out this form. Print out the completed form and keep a readily accessible hard copy in the lab (also keeping
Standard Operating Procedure Read the EH&S Standard Operating Procedures Fact Sheet before filling out this form. Print out the completed form and keep a readily accessible hard copy in the lab (also keeping
EXPERIMENT 7 THE IDEAL GAS LAW AND DENSITY
 EXPERIMENT 7 THE IDEAL GAS LAW AND DENSITY In this experiment you will determine the average molecular mass of air using two different methods, first by measuring the density of air with the density of
EXPERIMENT 7 THE IDEAL GAS LAW AND DENSITY In this experiment you will determine the average molecular mass of air using two different methods, first by measuring the density of air with the density of
USER'S GUIDE. Nanoflow Probe. Red Stripe. 3-4mm. ptfe Sleeve. To Injector. ZU1XC Fused Silica TTF115. Fused Silica. ptfe Sleeve
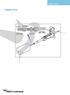 USER'S GUIDE Nanoflow Probe 3-4mm Red Stripe ptfe Sleeve To Injector ZU1XC Fused Silica 20µm 90µm TTF115 ptfe Sleeve Fused Silica 25µm 375µm Micromass UK Limited Floats Road Wythenshawe M23 9LZ Tel: +44
USER'S GUIDE Nanoflow Probe 3-4mm Red Stripe ptfe Sleeve To Injector ZU1XC Fused Silica 20µm 90µm TTF115 ptfe Sleeve Fused Silica 25µm 375µm Micromass UK Limited Floats Road Wythenshawe M23 9LZ Tel: +44
METHOD 204F--VOLATILE ORGANIC COMPOUNDS CONTENT IN LIQUID INPUT STREAM (DISTILLATION APPROACH) 1.1 Applicability. This procedure is applicable for
 METHOD 204F--VOLATILE ORGANIC COMPOUNDS CONTENT IN LIQUID INPUT STREAM (DISTILLATION APPROACH) 1. INTRODUCTION 1.1 Applicability. This procedure is applicable for determining the input of volatile organic
METHOD 204F--VOLATILE ORGANIC COMPOUNDS CONTENT IN LIQUID INPUT STREAM (DISTILLATION APPROACH) 1. INTRODUCTION 1.1 Applicability. This procedure is applicable for determining the input of volatile organic
Chemistry HP Unit 6 Gases. Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases
 Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Setting up and running a column
 Setting up and running a column PART 1: What you need and theory PART 2: Using a bellow and packing the column PART 3: Loading sample to silica PART 4: Running column PART 5: TLC analysis and combining
Setting up and running a column PART 1: What you need and theory PART 2: Using a bellow and packing the column PART 3: Loading sample to silica PART 4: Running column PART 5: TLC analysis and combining
Unblocking the MicroSprayer Aerosolizer Model IA-1B With the High Pressure Reverse Cleaning Adapter. Instructions for Use
 Penn-Century, Inc. 7670 Queen Street Suite 200 Wyndmoor, PA 19038-8031 USA 215-753-6540 Tel 215-220-3040 Fax www.penncentury.com Unblocking the MicroSprayer Aerosolizer Model IA-1B With the High Pressure
Penn-Century, Inc. 7670 Queen Street Suite 200 Wyndmoor, PA 19038-8031 USA 215-753-6540 Tel 215-220-3040 Fax www.penncentury.com Unblocking the MicroSprayer Aerosolizer Model IA-1B With the High Pressure
Unblocking the MicroSprayer Aerosolizer Model IA-1C With the High Pressure Reverse Cleaning Adapter. Instructions for Use
 Penn-Century, Inc. 7670 Queen Street Suite 200 Wyndmoor, PA 19038-8031 USA 215-753-6540 Tel 215-220-3040 Fax www.penncentury.com Unblocking the MicroSprayer Aerosolizer Model IA-1C With the High Pressure
Penn-Century, Inc. 7670 Queen Street Suite 200 Wyndmoor, PA 19038-8031 USA 215-753-6540 Tel 215-220-3040 Fax www.penncentury.com Unblocking the MicroSprayer Aerosolizer Model IA-1C With the High Pressure
Automated Determination of Dissolved Gases in Water Anne Jurek. Abstract: Discussion:
 Automated Determination of Dissolved Gases in Water Anne Jurek Abstract: The RSK-175 standard operating procedure was developed in order to determine the amount of dissolved gas in water. Due to the expansion
Automated Determination of Dissolved Gases in Water Anne Jurek Abstract: The RSK-175 standard operating procedure was developed in order to determine the amount of dissolved gas in water. Due to the expansion
Analysis of a KClO3 Mixture and Determination of R
 Experiment 10 Analysis of a KClO3 Mixture and Determination of R Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise.
Experiment 10 Analysis of a KClO3 Mixture and Determination of R Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise.
Each gas sample has the same A) density B) mass C) number of molecules D) number of atoms
 1. A real gas behaves most like an ideal gas at A) low pressure and high temperature B) average potential energy of its particles C) ionization energy of its particles D) activation energy of its particles
1. A real gas behaves most like an ideal gas at A) low pressure and high temperature B) average potential energy of its particles C) ionization energy of its particles D) activation energy of its particles
Alkylphosphines. Storage and Handling Recommendations
 Alkylphosphines Storage and Handling Recommendations 2 The Phosphine Specialties Group has been producing phosphine and phosphine derivatives on a large scale for a number of years and the handling procedures
Alkylphosphines Storage and Handling Recommendations 2 The Phosphine Specialties Group has been producing phosphine and phosphine derivatives on a large scale for a number of years and the handling procedures
Properties of Gases Observing Atom Pressure of a Gas Measuring Gas Products of Chemical Inferring Molecule Reactions
 It s a Gas! In a gas, molecules or atoms move constantly and spread far apart. If a gas cannot escape its container, it applies pressure on the container. For example, gas pressure inflates a balloon.
It s a Gas! In a gas, molecules or atoms move constantly and spread far apart. If a gas cannot escape its container, it applies pressure on the container. For example, gas pressure inflates a balloon.
4.) There are no forces of attraction or repulsion between gas particles. This means that
 KINETIC MOLECULAR (K-M) THEORY OF MATTER NOTES - based on the idea that particles of matter are always in motion - assumptions of the K-M Theory 1.) Gases consist of large numbers of tiny particles that
KINETIC MOLECULAR (K-M) THEORY OF MATTER NOTES - based on the idea that particles of matter are always in motion - assumptions of the K-M Theory 1.) Gases consist of large numbers of tiny particles that
Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks =
 Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks = Lab Problem 1 One way of using solar energy is to capture heat from the sun in a reservoir to be released later. You have been
Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks = Lab Problem 1 One way of using solar energy is to capture heat from the sun in a reservoir to be released later. You have been
STANDARD OPERATING PROCEDURE ADVANCED INTRAVENOUS TRAINING ARM (S )
 Page 1 of 27 1. Scope This Standard Operating Procedure (SOP) applies to the staff and students using the Advanced Intravenous Training Arm in the Pharmacy Practice Resource Unit (PPRU) at the Pharmacy
Page 1 of 27 1. Scope This Standard Operating Procedure (SOP) applies to the staff and students using the Advanced Intravenous Training Arm in the Pharmacy Practice Resource Unit (PPRU) at the Pharmacy
User s Guide. Vacuum Controller for liquid delivery systems
 Flow Control User s Guide Vacuum Controller for liquid delivery systems Precise Vacuum Control throughout the experiment Flow control Compatible with any perfusion system Ideal for Small Volume Delivery
Flow Control User s Guide Vacuum Controller for liquid delivery systems Precise Vacuum Control throughout the experiment Flow control Compatible with any perfusion system Ideal for Small Volume Delivery
Kinetic-Molecular Theory
 GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
Physics Experiment 17 Ideal Gas Law Qualitative Study
 Physics 210 17-1 Experiment 17 Ideal Gas Law Qualitative Study Note 1: Parts of this lab involve using a laptop computer and the PASCO ScienceWorkshop Interface to collect data. The lab also involves use
Physics 210 17-1 Experiment 17 Ideal Gas Law Qualitative Study Note 1: Parts of this lab involve using a laptop computer and the PASCO ScienceWorkshop Interface to collect data. The lab also involves use
CHEMISTRY - CLUTCH CH.5 - GASES.
 !! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
!! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
Unit 8: Gases and States of Matter
 Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
Generating Calibration Gas Standards
 Technical Note 1001 Metronics Inc. Generating Calibration Gas Standards with Dynacal Permeation Devices Permeation devices provide an excellent method of producing known gas concentrations in the PPM and
Technical Note 1001 Metronics Inc. Generating Calibration Gas Standards with Dynacal Permeation Devices Permeation devices provide an excellent method of producing known gas concentrations in the PPM and
R: The Ideal Gas Constant Pre-Lab Assignment
 R: The Ideal Gas Constant Pre-Lab Assignment Read the entire laboratory investigation and the relevant pages in your textbook, then answers the questions that follow in the space provided below. 1 Describe
R: The Ideal Gas Constant Pre-Lab Assignment Read the entire laboratory investigation and the relevant pages in your textbook, then answers the questions that follow in the space provided below. 1 Describe
Technical Operating Instructions for Magnum Cutting Systems. for online instructions
 Technical Operating Instructions for Magnum Cutting Systems http://www.magnumusa.com/operating-video.html for online instructions In Case of Emergency Immediately Call for Medical Aid. Familiarize yourself
Technical Operating Instructions for Magnum Cutting Systems http://www.magnumusa.com/operating-video.html for online instructions In Case of Emergency Immediately Call for Medical Aid. Familiarize yourself
Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes:
 Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
Birck Nanotechnology Center
 EFFECTIVE DATE: October 20, 2016 PAGE 1 of 8 This instruction covers the set-up and use of the bench-top for annealing and oxidizing samples within the cleanroom. 1. SAFETY REQUIREMENTS 1.1 Safety glasses
EFFECTIVE DATE: October 20, 2016 PAGE 1 of 8 This instruction covers the set-up and use of the bench-top for annealing and oxidizing samples within the cleanroom. 1. SAFETY REQUIREMENTS 1.1 Safety glasses
Lab 5- Cellular Respiration
 Lab 5- Cellular Respiration Background: Many cellular processes require energy. Aerobic cellular respiration supplies energy by the oxidation of glucose. This is a complex process involving a number of
Lab 5- Cellular Respiration Background: Many cellular processes require energy. Aerobic cellular respiration supplies energy by the oxidation of glucose. This is a complex process involving a number of
BS2000 MOTOR ELECTRONICS, INC. PROTECTION INSTRUCTION MANUAL. (407) Phone: Website:
 BS2000 INSTRUCTION MANUAL MOTOR PROTECTION ELECTRONICS, INC. 2464 Vulcan Road Apopka, Florida 32703 Phone: Website: (407) 299-3825 www.mpelectronics.com Revision Date: 6-23-14 RECOMMENDED REPLACEMENT
BS2000 INSTRUCTION MANUAL MOTOR PROTECTION ELECTRONICS, INC. 2464 Vulcan Road Apopka, Florida 32703 Phone: Website: (407) 299-3825 www.mpelectronics.com Revision Date: 6-23-14 RECOMMENDED REPLACEMENT
Gaseous oxygen technical grade Specification
 CDC 09 (5010) P3 Rev. TZS 217:1984 FINALIZED TANZANIA STANDARD Gaseous oxygen technical grade Specification TANZANIA BUREAU OF STANDARDS. 2 nd edition TBS 2017 0. FOREWORD 0.1. This Tanzania Draft Standard
CDC 09 (5010) P3 Rev. TZS 217:1984 FINALIZED TANZANIA STANDARD Gaseous oxygen technical grade Specification TANZANIA BUREAU OF STANDARDS. 2 nd edition TBS 2017 0. FOREWORD 0.1. This Tanzania Draft Standard
MB-SP Series Solvent Purification System
 Operation Manual MB-SP Series Solvent Purification System Table of contents 1. System overview 2 2. Column activation.. 5 3. System purging.. 6 4. Glove box integration.... 7 5. Solvent reservoir installation....
Operation Manual MB-SP Series Solvent Purification System Table of contents 1. System overview 2 2. Column activation.. 5 3. System purging.. 6 4. Glove box integration.... 7 5. Solvent reservoir installation....
AP Biology 12 Cellular Respiration Lab
 AP Biology 12 Cellular Respiration Lab Background: Each individual cell is responsible for the energy exchanges necessary to sustain its ordered structure. Cells accomplish this task by breaking down nutrient
AP Biology 12 Cellular Respiration Lab Background: Each individual cell is responsible for the energy exchanges necessary to sustain its ordered structure. Cells accomplish this task by breaking down nutrient
GASES. Unit #8. AP Chemistry
 GASES Unit #8 AP Chemistry I. Characteristics of Gases A. Gas Characteristics: 1. Fills its container a. no definite shape b. no definite vol. 2. Easily mixes w/ other gases 3. Exerts pressure on its surroundings
GASES Unit #8 AP Chemistry I. Characteristics of Gases A. Gas Characteristics: 1. Fills its container a. no definite shape b. no definite vol. 2. Easily mixes w/ other gases 3. Exerts pressure on its surroundings
INSTRUCTION MANUAL. January 23, 2003, Revision 0
 INSTRUCTION MANUAL Model 810A In-Vitro Test Apparatus for 310B Muscle Lever January 23, 2003, Revision 0 Copyright 2003 Aurora Scientific Inc. Aurora Scientific Inc. 360 Industrial Parkway S., Unit 4 Aurora,
INSTRUCTION MANUAL Model 810A In-Vitro Test Apparatus for 310B Muscle Lever January 23, 2003, Revision 0 Copyright 2003 Aurora Scientific Inc. Aurora Scientific Inc. 360 Industrial Parkway S., Unit 4 Aurora,
A) It cannot be predicted. B) It is squared. C) It is doubled. D) It is halved. E) It does not change.
 AP Chemistry Test (Chapter 5) Class Set Multiple Choice (50%) 1) A sample of argon gas is sealed in a container. The volume of the container is doubled at a constant temperature. What happens to the pressure
AP Chemistry Test (Chapter 5) Class Set Multiple Choice (50%) 1) A sample of argon gas is sealed in a container. The volume of the container is doubled at a constant temperature. What happens to the pressure
TRADE OF HEAVY VEHICLE MECHANIC
 TRADE OF HEAVY VEHICLE MECHANIC PHASE 2 Module 1 Induction/Customer Care/Bench Fitting/Welding UNIT: 5 Table of Contents Aims and Objectives... 1 Learning Outcome:... 1 Setting up an Oxyacetylene torch...
TRADE OF HEAVY VEHICLE MECHANIC PHASE 2 Module 1 Induction/Customer Care/Bench Fitting/Welding UNIT: 5 Table of Contents Aims and Objectives... 1 Learning Outcome:... 1 Setting up an Oxyacetylene torch...
CHEM 355 EXPERIMENT 7. Viscosity of gases: Estimation of molecular diameter
 CHEM 355 EXPERIMENT 7 Viscosity of gases: Estimation of molecular diameter Expressed most simply, the viscosity of a fluid (liquid or gas) relates to its resistance to flow. The viscosity of a gas is determined
CHEM 355 EXPERIMENT 7 Viscosity of gases: Estimation of molecular diameter Expressed most simply, the viscosity of a fluid (liquid or gas) relates to its resistance to flow. The viscosity of a gas is determined
Lab 4: Transpiration
 Lab 4: Transpiration Water is transported in plants, from the roots to the leaves, following a decreasing water potential gradient. Transpiration, or loss of water from the leaves, helps to create a lower
Lab 4: Transpiration Water is transported in plants, from the roots to the leaves, following a decreasing water potential gradient. Transpiration, or loss of water from the leaves, helps to create a lower
Aseptic Techniques. Techniques for Sterile Compounding. Pharmacy Technician Training Systems Passassured, LLC
 Aseptic Techniques Techniques for Sterile Compounding Pharmacy Technician Training Systems Passassured, LLC Aseptic Techniques, Tech for Sterile Compounding PassAssured's Pharmacy Technician Training Program
Aseptic Techniques Techniques for Sterile Compounding Pharmacy Technician Training Systems Passassured, LLC Aseptic Techniques, Tech for Sterile Compounding PassAssured's Pharmacy Technician Training Program
