UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
|
|
|
- Gabriel Rafe Wilkins
- 5 years ago
- Views:
Transcription
1 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education * * CO-ORDINATED SCIENCES 0654/51 Paper 5 Practical Test May/June hours Candidates answer on the Question Paper. Additional Materials: As listed in Instructions to Supervisors READ THESE INSTRUCTIONS FIRST Write your Centre number, candidate number and name on all the work you hand in. Write in dark blue or black pen. You may use a pencil for any diagrams, graphs or rough working. Do not use staples, paper clips, highlighters, glue or correction fluid. DO NOT WRITE IN ANY BARCODES. Answer all questions. Chemistry practical notes for this paper are printed on page 12. At the end of the examination, fasten all your work securely together. The number of marks is given in brackets [ ] at the end of each question or part question Total This document consists of 12 printed pages. IB11 06_0654_51/6RP UCLES 2011 [Turn over
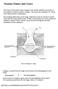
2 2 1 You are going to compare the composition of inhaled and exhaled air. glass beaker candle heat proof tile bench Fig. 1.1 (a) Analysis of inhaled air. Take a 500 cm 3 glass beaker (or glass jar). Light a candle (or night light) and place it onto a heatproof tile. Set the clock to zero. Place the glass beaker over the candle and start the clock (see Fig. 1.1). Record in Table 1.1 the time taken in seconds for the flame to go out. Remove the glass beaker from the candle, leave open to the air for one minute. Repeat the experiment and again record the time for the flame to go out in Table 1.1. blow glass beaker straw bowl water Fig. 1.2 (b) Analysis of exhaled air. Fill the glass beaker (or glass jar) with water and place into a bowl of water, inverted (see Fig. 1.2). Using the straw or tube blow into the glass beaker, until it is full of exhaled air. Light the candle. Using your hand to prevent the loss of air from the glass beaker, transfer the beaker from the bowl and place it over the lit candle. Immediately start the clock. Record in Table 1.1 the time taken in seconds for the flame to go out. Repeat the experiment for exhaled air and again record the time taken for the flame to go out in Table 1.1. UCLES /51/M/J/11
3 3 Table 1.1 inhaled air exhaled air experiment number time taken / s [2] (c) (i) Calculate the average times for the flame to go out in inhaled air and exhaled air. Show all working. average time taken for flame to go out in inhaled air = s average time taken for flame to go out in exhaled air = s (ii) Describe and explain the difference between the results for inhaled and exhaled air. [3] [2] UCLES /51/M/J/11 [Turn over
4 4 (iii) Take two large test-tubes and label them A and B. Place about 10 cm 3 of limewater into each tube. Place a bung over tube A and gently shake it for 30 seconds. This will allow atmospheric air to bubble through the lime water. Using a straw or tube, blow into tube B for 30 seconds. This will allow exhaled air to bubble through the limewater. Describe and explain the results observed in tube A and tube B. [3] (d) Describe an experiment to investigate the effect of exercise on breathing rate. Ensure that it is controlled, reliable and that you explain the need for all equipment. [5] UCLES /51/M/J/11
5 5 2 You are to investigate how an elastic band stretches when acted on by an increasing force. (a) Measure and record the width of the elastic band. width of band = mm [1] Push the pin into the cork leaving just enough showing to enable the elastic band to be hung from it. Clamp the cork firmly and adjust its position to give about 500 mm or more clearance from the bench top as shown in Fig cork and pin elastic band bench Fig. 2.1 (b) Hang the elastic band from the pin in such a way that masses can be attached, causing the band to stretch. If you have a carrier for the masses, this should be attached before the initial length of the elastic band is taken. If no carrier is available, you will need to tie various masses together using thread and tie these to the band. (i) Measure the length of the elastic band without any masses attached. Record this length in Table 2.1. (ii) Attach a mass of 100 g to the band. Measure and record its new length in Table 2.1. (iii) Continue to add masses of 100 g until you have a total of 500 g hanging from the band. Measure the length of the band after each addition and record the values in Table 2.1. (iv) Complete Table 2.1 by converting each mass into a force and also calculating the total increase in length of the band. UCLES /51/M/J/11 [Turn over
6 6 Table 2.1 total mass / g force / N length of band / mm total increase in length / mm [4] (c) Plot a graph of total increase in length (vertical axis) against force (horizontal axis). Before drawing a line, answer question (d). [3] UCLES /51/M/J/11
7 7 (d) Do you expect the line to pass through the origin? Explain your answer. [1] (e) Draw a suitable best fit line using the points plotted, taking into account your answer in (d). [1] (f) your graph to find the total increase in length produced by a mass of 250 g. total increase in length = mm [1] (g) your graph to describe the relationship between the force applied and the total increase in length. [1] (h) A student suggested that the band may have stretched and would not return to its original form when the force is removed. Describe how you could test this suggestion. [2] UCLES /51/M/J/11 [Turn over
8 8 (i) If masses were added beyond 500 g, the elastic band would eventually break. On the axes below, sketch the shape of the graph that would be obtained. [1] UCLES /51/M/J/11
9 9 3 You are provided with a salt Z which contains two cations and one anion. Carry out the following tests. (a) Record the appearance of solid Z. appearance [1] (b) Place about one quarter of solid Z in a hard glass test-tube. Heat strongly. Test any gas given off with litmus paper. Record all your observations. action on litmus paper observations [2] (c) (i) Determine how to make a solution of Z. Make sure you keep some of the solid for test (d). Place about half of the solid Z into a fresh test-tube. Try to dissolve Z in cold water and if it does not dissolve, try cold dilute sulfuric acid and then hot dilute sulfuric acid. Stop as soon as you have found a way of dissolving Z. Note the colour of the solution. Keep the solution for further tests. You will need about 10 cm 3 of solution. Z dissolves in colour of solution [2] (ii) Place about 2 cm 3 of the solution from (c)(i) into a large test-tube. Add dilute sodium hydroxide until alkaline. litmus paper to check that you have added enough. Record your observation. observation [1] Now carefully bring the mixture to the boil and test any gas given off with litmus paper. Record your observation. observation [1] UCLES /51/M/J/11 [Turn over
10 10 (iii) Place a second 2 cm 3 portion of the solution from (c)(i) in a fresh test-tube. Add ammonia solution until in excess. Record your observation. observation [1] (d) Place a small amount of solid Z in a fresh test-tube. Dissolve in about 5 cm 3 of dilute nitric acid. this solution to test for the chloride anion and the sulfate anion. Divide the solution into two portions before carrying out any tests. Describe how you carried out each test and record the result. test for the chloride anion result [2] test for the sulfate anion result [2] UCLES /51/M/J/11
11 11 (e) State the three ions in solid Z, giving a reason for each one. ion 1 reason ion 2 reason ion 3 reason [3] UCLES /51/M/J/11 [Turn over
12 12 CHEMISTRY PRACTICAL NOTES Test for anions anion test test result carbonate (CO 2-3 ) add dilute acid effervescence, carbon dioxide produced chloride (Cl - ) [in solution] nitrate (NO 3 - ) [in solution] sulfate (SO 4 2- ) [in solution] acidify with dilute nitric acid, then add aqueous silver nitrate add aqueous sodium hydroxide then aluminium foil; warm carefully acidify then add aqueous barium chloride or aqueous barium nitrate white ppt. ammonia produced white ppt. Test for aqueous cations cation effect of aqueous sodium hydroxide effect of aqueous ammonia ammonium (NH 4 + ) ammonia produced on warming - copper(ii) (Cu 2+ ) light blue ppt., insoluble in excess light blue ppt., soluble in excess giving a dark blue solution iron(ii) (Fe 2+ ) green ppt., insoluble in excess green ppt., insoluble in excess iron(iii) (Fe 3+ ) red-brown ppt., insoluble in excess red-brown ppt., insoluble in excess zinc (Zn 2+ ) white ppt., soluble in excess giving a colourless solution white ppt., soluble in excess giving a colourless solution Test for gases gas ammonia (NH 3 ) carbon dioxide (CO 2 ) chlorine (Cl 2 ) hydrogen (H 2 ) oxygen (O 2 ) test and test results turns damp red litmus paper blue turns limewater milky bleaches damp litmus paper pops with a lighted splint relights a glowing splint Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included, the publisher will be pleased to make amends at the earliest possible opportunity. University of Cambridge International Examinations is part of the Cambridge Assessment Group. Cambridge Assessment is the brand name of University of Cambridge Local Examinations Syndicate (UCLES), which is itself a department of the University of Cambridge. UCLES /51/M/J/11
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certifi cate of Education Advanced Level
 *0337350796* UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certifi cate of Education Advanced Level CHEMISTRY 9701/53 Paper 5 Planning, Analysis and Evaluation October/November 2012 1 hour
*0337350796* UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certifi cate of Education Advanced Level CHEMISTRY 9701/53 Paper 5 Planning, Analysis and Evaluation October/November 2012 1 hour
COMBINED SCIENCE 0653/6 CO-ORDINATED SCIENCES 0654/6
 Centre Number Candidate Number Candidate Name International General Certificate of Secondary Education UNIVERSITY OF CAMBRIDGE LOCAL EXAMINATIONS SYNDICATE COMBINED SCIENCE 0653/6 CO-ORDINATED SCIENCES
Centre Number Candidate Number Candidate Name International General Certificate of Secondary Education UNIVERSITY OF CAMBRIDGE LOCAL EXAMINATIONS SYNDICATE COMBINED SCIENCE 0653/6 CO-ORDINATED SCIENCES
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 www.xtremepapers.com Cambridge International Examinations Cambridge International General Certificate of Secondary Education *2215383014* CAMBRIGE INTERNATIONAL MATHEMATICS 0607/62 Paper 6 (Extended) October/November
www.xtremepapers.com Cambridge International Examinations Cambridge International General Certificate of Secondary Education *2215383014* CAMBRIGE INTERNATIONAL MATHEMATICS 0607/62 Paper 6 (Extended) October/November
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *3585141648* BIOLOGY 0610/62 Paper 6 Alternative to Practical February/March 2018 1 hour Candidates
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *3585141648* BIOLOGY 0610/62 Paper 6 Alternative to Practical February/March 2018 1 hour Candidates
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0993917174* BIOLOGY 0610/63 Paper 6 Alternative to Practical May/June 2016 1 hour Candidates answer
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0993917174* BIOLOGY 0610/63 Paper 6 Alternative to Practical May/June 2016 1 hour Candidates answer
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level
 www.xtremepapers.com UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level *1732862249* PHYSICS 5054/22 Paper 2 Theory October/November 2012 1 hour 45 minutes
www.xtremepapers.com UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level *1732862249* PHYSICS 5054/22 Paper 2 Theory October/November 2012 1 hour 45 minutes
Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level
 Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level *8637380180* BIOLOGY 9700/53 Paper 5 Planning, Analysis and Evaluation May/June 2018 1 hour 15 minutes
Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level *8637380180* BIOLOGY 9700/53 Paper 5 Planning, Analysis and Evaluation May/June 2018 1 hour 15 minutes
ExamLearn.ie. The Air & Oxygen
 ExamLearn.ie The Air & Oxygen The Air & Oxygen The air is a mixture of gases, which forms a blanket around the earth. Another name for the air is the atmosphere. *To investigate the percentage of oxygen
ExamLearn.ie The Air & Oxygen The Air & Oxygen The air is a mixture of gases, which forms a blanket around the earth. Another name for the air is the atmosphere. *To investigate the percentage of oxygen
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *8269683414* PHYSICS 0625/31 Paper 3 Extended October/November 2014 1 hour 15 minutes Candidates
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *8269683414* PHYSICS 0625/31 Paper 3 Extended October/November 2014 1 hour 15 minutes Candidates
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certifi cate of Secondary Education
 www.xtremepapers.com UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certifi cate of Secondary Education *0008718255* DESIGN AND TECHNOLOGY 0445/41 Paper 4 Systems and Control
www.xtremepapers.com UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certifi cate of Secondary Education *0008718255* DESIGN AND TECHNOLOGY 0445/41 Paper 4 Systems and Control
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *5757795995* PHYSICS 0625/33 Paper 3 Extended October/November 2012 1 hour 15 minutes Candidates
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *5757795995* PHYSICS 0625/33 Paper 3 Extended October/November 2012 1 hour 15 minutes Candidates
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Subsidiary Level and Advanced Level
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Subsidiary Level and Advanced Level *2677524077* MARINE SCIENCE 9693/03 Structured Questions May/June 2013 Paper
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Subsidiary Level and Advanced Level *2677524077* MARINE SCIENCE 9693/03 Structured Questions May/June 2013 Paper
CHAPTER 5 : THE AIR AROUND US
 CHAPTER 5 : THE AIR AROUND US The Composition of Air Air is a mixture consist of Nitrogen 78% Oxygen 21% Carbon dioxide 0.03% Inert gases 0.97% Water vapour Microorganism Dust The percentage of the constituents
CHAPTER 5 : THE AIR AROUND US The Composition of Air Air is a mixture consist of Nitrogen 78% Oxygen 21% Carbon dioxide 0.03% Inert gases 0.97% Water vapour Microorganism Dust The percentage of the constituents
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS Cambridge Checkpoint. Paper 1 For Examination from 2012 SPECIMEN PAPER 45 minutes
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS Cambridge Checkpoint www.xtremepapers.com SCIENCE 1113/01 Paper 1 Examination from 2012 SPECIMEN PAPER 45 minutes Candidates answer on the Question Paper.
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS Cambridge Checkpoint www.xtremepapers.com SCIENCE 1113/01 Paper 1 Examination from 2012 SPECIMEN PAPER 45 minutes Candidates answer on the Question Paper.
Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level
 Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level *8315138911* MARINE SCIENCE 9693/04 Paper 4 A2 Data-Handling and Free-Response May/June 2015 1 hour 15
Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level *8315138911* MARINE SCIENCE 9693/04 Paper 4 A2 Data-Handling and Free-Response May/June 2015 1 hour 15
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *5527988714* ENVIRONMENTAL MANAGEMENT 0680/21 Paper 2 May/June 2018 1 hour 45 minutes Candidates
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *5527988714* ENVIRONMENTAL MANAGEMENT 0680/21 Paper 2 May/June 2018 1 hour 45 minutes Candidates
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Subsidiary Level and Advanced Level
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Subsidiary Level and Advanced Level *5466176767* MARINE SCIENCE 9693/03 Structured Questions May/June 2011 Paper
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Subsidiary Level and Advanced Level *5466176767* MARINE SCIENCE 9693/03 Structured Questions May/June 2011 Paper
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level *2109531537* FISHERIES SCIENCE 5151/01 Paper 1 October/November 2007 1 hour 30 minutes Candidates answer
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level *2109531537* FISHERIES SCIENCE 5151/01 Paper 1 October/November 2007 1 hour 30 minutes Candidates answer
Cambridge International Examinations Cambridge Ordinary Level
 Cambridge International Examinations Cambridge Ordinary Level *8824771695* MARINE SCIENCE 5180/01 Paper 1 Structured October/November 2016 1 hour 30 minutes Candidates answer on the Question Paper. No
Cambridge International Examinations Cambridge Ordinary Level *8824771695* MARINE SCIENCE 5180/01 Paper 1 Structured October/November 2016 1 hour 30 minutes Candidates answer on the Question Paper. No
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level *3373524824* STATISTICS 4040/23 Paper 2 October/November 2013 Candidates answer on the question paper.
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level *3373524824* STATISTICS 4040/23 Paper 2 October/November 2013 Candidates answer on the question paper.
Cambridge International Examinations Cambridge Ordinary Level
 Cambridge International Examinations Cambridge Ordinary Level *5529084743* NEPALI 3202/01 Paper 1 Composition, Translation and Comprehension May/June 2014 Additional Materials: Answer Booklet/Paper READ
Cambridge International Examinations Cambridge Ordinary Level *5529084743* NEPALI 3202/01 Paper 1 Composition, Translation and Comprehension May/June 2014 Additional Materials: Answer Booklet/Paper READ
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *6519612675* MATHEMATICS 0580/32 Paper 3 (Core) February/March 2018 Candidates answer on the Question
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *6519612675* MATHEMATICS 0580/32 Paper 3 (Core) February/March 2018 Candidates answer on the Question
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *2203951931* ENGLISH AS A SECOND LANGUAGE 0510/42 Paper 4 Listening (Extended) October/November 2014
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *2203951931* ENGLISH AS A SECOND LANGUAGE 0510/42 Paper 4 Listening (Extended) October/November 2014
Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level
 Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level *4245615796* MARINE SCIENCE 9693/03 Paper 3 A2 Structured Questions May/June 2015 1 hour 30 minutes Candidates
Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level *4245615796* MARINE SCIENCE 9693/03 Paper 3 A2 Structured Questions May/June 2015 1 hour 30 minutes Candidates
Cambridge International Examinations Cambridge Ordinary Level
 Cambridge International Examinations Cambridge Ordinary Level *7638984299* MARINE SCIENCE 5180/01 Paper 1 Structured October/November 2015 1 hour 30 minutes Candidates answer on the Question Paper. No
Cambridge International Examinations Cambridge Ordinary Level *7638984299* MARINE SCIENCE 5180/01 Paper 1 Structured October/November 2015 1 hour 30 minutes Candidates answer on the Question Paper. No
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level FISHERIES SCIENCE 5151/01
 Centre Number Candidate Number Name UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level FISHERIES SCIENCE 5151/01 Paper 1 Candidates answer on the Question
Centre Number Candidate Number Name UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level FISHERIES SCIENCE 5151/01 Paper 1 Candidates answer on the Question
Cambridge International Examinations Cambridge Ordinary Level
 Cambridge International Examinations Cambridge Ordinary Level *4492835387* MARINE SCIENCE 5180/01 Paper 1 Structured October/November 2017 1 hour 30 minutes Candidates answer on the Question Paper. No
Cambridge International Examinations Cambridge Ordinary Level *4492835387* MARINE SCIENCE 5180/01 Paper 1 Structured October/November 2017 1 hour 30 minutes Candidates answer on the Question Paper. No
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *1089858088* GEOGRAPHY 0460/43 Paper 4 Alternative to Coursework May/June 2016 Candidates answer
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *1089858088* GEOGRAPHY 0460/43 Paper 4 Alternative to Coursework May/June 2016 Candidates answer
Cambridge International Examinations Cambridge Ordinary Level
 Cambridge International Examinations Cambridge Ordinary Level *9399919087* STATISTICS 4040/12 Paper 1 October/November 2015 Candidates answer on the Question Paper. Additional Materials: Pair of compasses
Cambridge International Examinations Cambridge Ordinary Level *9399919087* STATISTICS 4040/12 Paper 1 October/November 2015 Candidates answer on the Question Paper. Additional Materials: Pair of compasses
ADVANCED SUBSIDIARY GCE G622 APPLIED SCIENCE
 ADVANCED SUBSIDIARY GCE APPLIED SCIENCE Unit 3: Monitoring the activity of the human body TUESDAY 5 JUNE 2007 G622 Afternoon *CUP/T21601* Additional materials: Electronic calculator Ruler (cm/mm) Time:
ADVANCED SUBSIDIARY GCE APPLIED SCIENCE Unit 3: Monitoring the activity of the human body TUESDAY 5 JUNE 2007 G622 Afternoon *CUP/T21601* Additional materials: Electronic calculator Ruler (cm/mm) Time:
GRADE 6: Materials 1. UNIT 6M.1 7 hours. Solubility. Resources. About this unit. Previous learning. Expectations. Key vocabulary and technical terms
 GRADE 6: Materials 1 Solubility UNIT 6M.1 7 hours About this unit This is the first of four units on materials in Grade 6. This unit builds on the study of the properties of water in Unit 5M.1. Unit 7M.1
GRADE 6: Materials 1 Solubility UNIT 6M.1 7 hours About this unit This is the first of four units on materials in Grade 6. This unit builds on the study of the properties of water in Unit 5M.1. Unit 7M.1
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *7429369695* ENVIRONMENTAL MANAGEMENT 0680/43 Paper 4 October/November 2016 1 hour 30 minutes Candidates
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *7429369695* ENVIRONMENTAL MANAGEMENT 0680/43 Paper 4 October/November 2016 1 hour 30 minutes Candidates
DEMONSTRATION 2.1 PROPERTIES OF CO 2. Chapter 2: Gases
 DEMONSTRATION 2.1 Chapter 2: Gases PROPERTIES OF CO 2 This demonstration has two aims: firstly, to show that carbon dioxide gas is denser than air; secondly, to show that carbon dioxide will not support
DEMONSTRATION 2.1 Chapter 2: Gases PROPERTIES OF CO 2 This demonstration has two aims: firstly, to show that carbon dioxide gas is denser than air; secondly, to show that carbon dioxide will not support
Worksheet: Solubility
 1. According to your Reference Tables, which substance forms an unsaturated solution when 80 grams of the substance is dissolved in 100 grams of H 2 O at 10 C? (A) KI (B) KNO 3 (C) NaNO 3 (D) NaCl 2. The
1. According to your Reference Tables, which substance forms an unsaturated solution when 80 grams of the substance is dissolved in 100 grams of H 2 O at 10 C? (A) KI (B) KNO 3 (C) NaNO 3 (D) NaCl 2. The
Cambridge International Examinations Cambridge Ordinary Level
 Cambridge International Examinations Cambridge Ordinary Level *3365325931* ENVIRONMENTAL MANAGEMENT 5014/21 Paper 2 May/June 2018 1 hour 30 minutes Candidates answer on the Question Paper. No Additional
Cambridge International Examinations Cambridge Ordinary Level *3365325931* ENVIRONMENTAL MANAGEMENT 5014/21 Paper 2 May/June 2018 1 hour 30 minutes Candidates answer on the Question Paper. No Additional
 Cambridge International Examinations Cambridge International General Certifi cate of Secondary Education *9994985227* MATHEMATICS 0580/33 Paper 3 (Core) May/June 2014 Candidates answer on the Question
Cambridge International Examinations Cambridge International General Certifi cate of Secondary Education *9994985227* MATHEMATICS 0580/33 Paper 3 (Core) May/June 2014 Candidates answer on the Question
CLASS COPY-DO NOT WRITE ON
 Save Our Shells - Central Question: How does carbon dioxide affect salt water? CLASS COPY-DO NOT WRITE ON Overview of experiment: Exhaling carbon dioxide into a beaker of salt water mimics the gas exchange
Save Our Shells - Central Question: How does carbon dioxide affect salt water? CLASS COPY-DO NOT WRITE ON Overview of experiment: Exhaling carbon dioxide into a beaker of salt water mimics the gas exchange
GCSE. Chemistry Practical Manual CCEA GCSE TEACHER GUIDANCE. Unit 3: Practical Skills
 GCSE CCEA GCSE TEACHER GUIDANCE Chemistry Practical Manual Unit 3: Practical Skills C9: Investigate the preparation, properties, tests and reactions of the gases hydrogen, oxygen and carbon dioxide For
GCSE CCEA GCSE TEACHER GUIDANCE Chemistry Practical Manual Unit 3: Practical Skills C9: Investigate the preparation, properties, tests and reactions of the gases hydrogen, oxygen and carbon dioxide For
Core practical 10: Investigate the effects of different wavelengths of light on the rate of photosynthesis
 Core practical 10 Teacher sheet Core practical 10: Investigate the effects of different wavelengths of light on Objectives To understand how to measure by measuring oxygen production To investigate the
Core practical 10 Teacher sheet Core practical 10: Investigate the effects of different wavelengths of light on Objectives To understand how to measure by measuring oxygen production To investigate the
Cambridge International Examinations Cambridge Primary Checkpoint
 Cambridge International Examinations Cambridge Primary Checkpoint CANDIDATE NAME CENTRE NUMBER CANDIDATE NUMBER ENGLISH AS A SECOND LANGUAGE Paper 1 Reading and Usage SPECIMEN PAPER 0837/01 For Examination
Cambridge International Examinations Cambridge Primary Checkpoint CANDIDATE NAME CENTRE NUMBER CANDIDATE NUMBER ENGLISH AS A SECOND LANGUAGE Paper 1 Reading and Usage SPECIMEN PAPER 0837/01 For Examination
SPECIMEN. All items required by teachers and candidates for this task are included in this pack.
 Advanced Subsidiary GCE HUMAN BIOLOGY Unit F223: Practical Skills in Human Biology: Quantitative Task Specimen Task For use from September 2008 to June 2009. F223 All items required by teachers and candidates
Advanced Subsidiary GCE HUMAN BIOLOGY Unit F223: Practical Skills in Human Biology: Quantitative Task Specimen Task For use from September 2008 to June 2009. F223 All items required by teachers and candidates
Studying Carbon Dioxide
 Activity 3 Studying Carbon Dioxide GOALS In this activity you will: Generate CO 2 by various methods, then collect and characterize it. Explore how the volume of a gas varies with temperature. Compare
Activity 3 Studying Carbon Dioxide GOALS In this activity you will: Generate CO 2 by various methods, then collect and characterize it. Explore how the volume of a gas varies with temperature. Compare
PREPARING GAS SAMPLES IN LARGE PLASTIC SYRINGES
 PREPARING GAS SAMPLES IN LARGE PLASTIC SYRINGES A wide variety of gases can be prepared safely inside a 60 ml plastic syringe. Here you will practice making carbon dioxide, so that you know the technique
PREPARING GAS SAMPLES IN LARGE PLASTIC SYRINGES A wide variety of gases can be prepared safely inside a 60 ml plastic syringe. Here you will practice making carbon dioxide, so that you know the technique
Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level
 Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level *9180775193* MARINE SCIENCE 9693/03 Paper 3 A2 Structured Questions May/June 2017 1 hour 30 minutes Candidates
Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level *9180775193* MARINE SCIENCE 9693/03 Paper 3 A2 Structured Questions May/June 2017 1 hour 30 minutes Candidates
Fish Farming Water Quality Test Kit
 DOC326.97.00098 Fish Farming Water Quality Test Kit FF-1A (243002) 11/2017, Edition 1 User Manual Table of Contents General information... 3 Safety information... 3 Use of hazard information...3 Product
DOC326.97.00098 Fish Farming Water Quality Test Kit FF-1A (243002) 11/2017, Edition 1 User Manual Table of Contents General information... 3 Safety information... 3 Use of hazard information...3 Product
3 Solubility and Concentration
 CHAPTER 8 SECTION Solutions 3 Solubility and Concentration KEY IDEAS As you read this section, keep these questions in mind: What is solubility? What happens when you add more solute to a saturated solution?
CHAPTER 8 SECTION Solutions 3 Solubility and Concentration KEY IDEAS As you read this section, keep these questions in mind: What is solubility? What happens when you add more solute to a saturated solution?
OB11 Carry out qualitative tests to compare the carbon dioxide levels of inhaled and exhaled air
 Biology: 5. Respiration and Breathing Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier OB9 Syllabus Describe the process of aerobic respiration
Biology: 5. Respiration and Breathing Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier OB9 Syllabus Describe the process of aerobic respiration
PREPARATION AND PROPERTIES OF ATMOSPHERIC GASES I: Carbon Dioxide and Oxygen
 PREPARATION AND PROPERTIES OF ATMOSPHERIC GASES I: Carbon Dioxide and Oxygen.... :O = C = O:.... :O = O: INTRODUCTION The atmosphere consists predominantly of three gases -- nitrogen (N 2 ) 78%, oxygen
PREPARATION AND PROPERTIES OF ATMOSPHERIC GASES I: Carbon Dioxide and Oxygen.... :O = C = O:.... :O = O: INTRODUCTION The atmosphere consists predominantly of three gases -- nitrogen (N 2 ) 78%, oxygen
clubs STEM BALLOON ROCKETS Focus: Physics AIM Equipment: Instructions: Discuss: Useful Links:
 BALLOON ROCKETS Focus: Physics A balloon provides a simple example of how a rocket engine works. The air trapped inside the balloon pushes out the open end, causing the balloon to move forward. The force
BALLOON ROCKETS Focus: Physics A balloon provides a simple example of how a rocket engine works. The air trapped inside the balloon pushes out the open end, causing the balloon to move forward. The force
Gas Exchange ACTIVITY OVERVIEW SUMMARY KEY CONCEPTS AND PROCESS SKILLS KEY VOCABULARY. Teacher s Guide B-75 L A B O R ATO R Y
 Gas Exchange 17 40- to 2 50-minute sessions ACTIVITY OVERVIEW L A B O R ATO R Y SUMMARY This activity explores the role of the respiratory system in the regulation of gases in the blood. Students investigate
Gas Exchange 17 40- to 2 50-minute sessions ACTIVITY OVERVIEW L A B O R ATO R Y SUMMARY This activity explores the role of the respiratory system in the regulation of gases in the blood. Students investigate
Experiment 18 Properties of Gases
 Experiment 18 Properties of Gases E18-1 E18-2 The Task In this experiment you will investigate some of the properties of gases, i.e. how gases flow, their phase changes and chemical reactivity. Skills
Experiment 18 Properties of Gases E18-1 E18-2 The Task In this experiment you will investigate some of the properties of gases, i.e. how gases flow, their phase changes and chemical reactivity. Skills
Level 3 Cambridge Technical in Engineering 05822/05823/05824/05825/05873 Unit 3: Principles of mechanical engineering
 Level 3 Cambridge Technical in Engineering 05822/05823/05824/05825/05873 Unit 3: Principles of mechanical engineering Monday 16 January 2017 Afternoon Time allowed: 1 hour 30 minutes You must have: the
Level 3 Cambridge Technical in Engineering 05822/05823/05824/05825/05873 Unit 3: Principles of mechanical engineering Monday 16 January 2017 Afternoon Time allowed: 1 hour 30 minutes You must have: the
Noxious Fumes and Gases
 Noxious Fumes and Gases The tissues of the body require oxygen 0 2 for normal metabolic processes (ie. the oxidation of food to produce energy). They must also eliminate CO, which is the waste product
Noxious Fumes and Gases The tissues of the body require oxygen 0 2 for normal metabolic processes (ie. the oxidation of food to produce energy). They must also eliminate CO, which is the waste product
DURING the course of certain investigations it became
 VOLUMETRIC DETERMINATION OF ETHER OR CYCLOPROPANE, CARBON DIOXIDE, NITROUS OXIDE AND OXYGEN IN ANESTHETIC MIXTURES By F. J. PRIME DURING the course of certain investigations it became necessary to be able
VOLUMETRIC DETERMINATION OF ETHER OR CYCLOPROPANE, CARBON DIOXIDE, NITROUS OXIDE AND OXYGEN IN ANESTHETIC MIXTURES By F. J. PRIME DURING the course of certain investigations it became necessary to be able
Tuesday 11 June 2013 Morning
 F Tuesday 11 June 2013 Morning GCSE MATHEMATICS B J567/01 Paper 1 (Foundation Tier) *J517110613* Candidates answer on the Question Paper. OCR supplied materials: None Other materials required: Geometrical
F Tuesday 11 June 2013 Morning GCSE MATHEMATICS B J567/01 Paper 1 (Foundation Tier) *J517110613* Candidates answer on the Question Paper. OCR supplied materials: None Other materials required: Geometrical
General Chemistry I Percent Yield of Hydrogen Gas From Magnesium and HCl
 Introduction For chemical reactions involving gases, gas volume measurements provide a convenient means of determining stoichiometric relationships. A gaseous product is collected in a long, thin graduated
Introduction For chemical reactions involving gases, gas volume measurements provide a convenient means of determining stoichiometric relationships. A gaseous product is collected in a long, thin graduated
EXPERIMENT. Identification of Gases
 EXPERIMENT Identification of Gases Hands-On Labs, Inc. Version 42-0189-00-02 Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before you begin. Take time
EXPERIMENT Identification of Gases Hands-On Labs, Inc. Version 42-0189-00-02 Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before you begin. Take time
The grade 6 English science unit, Gases, meets the academic content standards set in the Korean curriculum, which state students should:
 This area deals with the properties of gases as small collections of particles. Different kinds of intangible and invisible gases attract students curiosity and promote their use of reasoning skills. Students
This area deals with the properties of gases as small collections of particles. Different kinds of intangible and invisible gases attract students curiosity and promote their use of reasoning skills. Students
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *4574868004* GEOGRAPHY 0460/43 Paper 4 Alternative to Coursework October/November 2017 Candidates
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *4574868004* GEOGRAPHY 0460/43 Paper 4 Alternative to Coursework October/November 2017 Candidates
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 ambridge International Examinations ambridge International General ertificate of Secondary Education *2535117741* ENGLISH S SEOND LNGUGE 0510/32 Paper 3 Listening (ore) October/November 2014 pprox. 30
ambridge International Examinations ambridge International General ertificate of Secondary Education *2535117741* ENGLISH S SEOND LNGUGE 0510/32 Paper 3 Listening (ore) October/November 2014 pprox. 30
4/26/16. Section 1 Understanding Solutions. Solutions and Suspensions. Solutions and Suspensions. Solutions and Suspensions. Solvents and Solutes
 Section 1 Understanding Solutions On a hot day, you may think that a cool glass of plain water would be refreshing. However, if the glass were actually filled with plain water, it would taste stale. This
Section 1 Understanding Solutions On a hot day, you may think that a cool glass of plain water would be refreshing. However, if the glass were actually filled with plain water, it would taste stale. This
maximum grams of solute that will dissolve in 100 g of solvent at a given temperature Varies with temperature Sugar is more soluble than salt
 maximum grams of solute that will dissolve in 100 g of solvent at a given temperature Varies with temperature Sugar is more soluble than salt Solids are more soluble at... high temperatures. Gases are
maximum grams of solute that will dissolve in 100 g of solvent at a given temperature Varies with temperature Sugar is more soluble than salt Solids are more soluble at... high temperatures. Gases are
BOYLE S / CHARLES LAW APPARATUS - 1m long
 BOYLE S / CHARLES LAW APPARATUS - 1m long Cat: MF0340-101 (combination Boyle s and Charles without mercury) DESCRIPTION: The IEC Boyle's & Charles Law apparatus is a high quality instrument designed to
BOYLE S / CHARLES LAW APPARATUS - 1m long Cat: MF0340-101 (combination Boyle s and Charles without mercury) DESCRIPTION: The IEC Boyle's & Charles Law apparatus is a high quality instrument designed to
Elementary Science ACTIVITIES
 Elementary Science ACTIVITIES Table of Contents Introduction Properties of Matter Mixture I Mixture II What s Magnetic? Solutions Emulsion Layers Separating a Suspension What Do Liquids Do? Solids, Liquids,
Elementary Science ACTIVITIES Table of Contents Introduction Properties of Matter Mixture I Mixture II What s Magnetic? Solutions Emulsion Layers Separating a Suspension What Do Liquids Do? Solids, Liquids,
Measuring Carbon Dioxide in Breath
 Measuring Carbon Dioxide in Breath OBJECTIVES 1. Measure the partial pressure of carbon dioxide in your breath 2. Estimate the volume of air you exhale per day 3. Estimate the volume and mass of CO2 you
Measuring Carbon Dioxide in Breath OBJECTIVES 1. Measure the partial pressure of carbon dioxide in your breath 2. Estimate the volume of air you exhale per day 3. Estimate the volume and mass of CO2 you
MARK SCHEME for the October/November 2012 series 0610 BIOLOGY. 0610/51 Paper 5 (Practical Test), maximum raw mark 40
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the October/November 2012 series 0610 BIOLOGY 0610/51 Paper 5 (Practical Test), maximum raw
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the October/November 2012 series 0610 BIOLOGY 0610/51 Paper 5 (Practical Test), maximum raw
Supporting inquiry based teaching and learning. Respiration. Student Name: Class:
 Supporting inquiry based teaching and learning Respiration Student Name: Class: Teacher Name: School: Question 1 a) Respiration is the process that the swimmer uses to release energy from the digested
Supporting inquiry based teaching and learning Respiration Student Name: Class: Teacher Name: School: Question 1 a) Respiration is the process that the swimmer uses to release energy from the digested
Equilibrium. Observations
 Equilibrium Observations When you look closely at a rope you will see that it consists of several strands of twine. If you tried to hang a heavy (or massive) object on a single strand of twine it would
Equilibrium Observations When you look closely at a rope you will see that it consists of several strands of twine. If you tried to hang a heavy (or massive) object on a single strand of twine it would
Morning Time: 1 hour 30 minutes
 ADVANCED SUBSIDIARY GCE APPLIED SCIENCE Unit 3: Monitoring the activity of the human body FRIDAY 23 MAY 2008 G622 Morning Time: 1 hour 30 minutes *CUP/T44164* Candidates answer on the question paper Additional
ADVANCED SUBSIDIARY GCE APPLIED SCIENCE Unit 3: Monitoring the activity of the human body FRIDAY 23 MAY 2008 G622 Morning Time: 1 hour 30 minutes *CUP/T44164* Candidates answer on the question paper Additional
Chapter 5 TEST: Gases
 Chapter 5 TEST: Gases 1) Gases generally have A) low density B) high density C) closely packed particles D) no increase in volume when temperature is increased E) no decrease in volume when pressure is
Chapter 5 TEST: Gases 1) Gases generally have A) low density B) high density C) closely packed particles D) no increase in volume when temperature is increased E) no decrease in volume when pressure is
States of Matter. Q 7. Calculate the average of kinetic energy, in joules of the molecules in 8.0 g of methane at 27 o C. (IIT JEE Marks)
 Q 1. States of Matter Calculate density of NH 3 at 30 o C and 5 atm pressure Q 2. (IIT JEE 1978 3 Marks) 3.7 g of a gas at 25 o C occupied the same volume as 0.184g of hydrogen at 17 o C and at the same
Q 1. States of Matter Calculate density of NH 3 at 30 o C and 5 atm pressure Q 2. (IIT JEE 1978 3 Marks) 3.7 g of a gas at 25 o C occupied the same volume as 0.184g of hydrogen at 17 o C and at the same
Properties of Gases Observing Atom Pressure of a Gas Measuring Gas Products of Chemical Inferring Molecule Reactions
 It s a Gas! In a gas, molecules or atoms move constantly and spread far apart. If a gas cannot escape its container, it applies pressure on the container. For example, gas pressure inflates a balloon.
It s a Gas! In a gas, molecules or atoms move constantly and spread far apart. If a gas cannot escape its container, it applies pressure on the container. For example, gas pressure inflates a balloon.
Factors that affect the motion of a vehicle along a surface
 SPECIMEN GENERAL CERTIFICATE OF SECONDARY EDUCATION TWENTY FIRST CENTURY SCIENCE ADDITIONAL SCIENCE A Unit A154: (controlled assessment) PHYSICS A Unit A184 (controlled assessment) A154 A184 Factors that
SPECIMEN GENERAL CERTIFICATE OF SECONDARY EDUCATION TWENTY FIRST CENTURY SCIENCE ADDITIONAL SCIENCE A Unit A154: (controlled assessment) PHYSICS A Unit A184 (controlled assessment) A154 A184 Factors that
Introduction to ChemSense
 Introduction to ChemSense As you may have seen, the ChemSense software allows you to create drawings and animation of chemical phenomena (and non-chemical phenomena for some of you). The software also
Introduction to ChemSense As you may have seen, the ChemSense software allows you to create drawings and animation of chemical phenomena (and non-chemical phenomena for some of you). The software also
GAS LAW WORKSHEET 1 KEY
 377 GAS LAW WORKSHEET 1 KEY 1. A sample of oxygen gas occupies a volume of 436. ml at 1.0 atm. If the temperature is held constant, what would the pressure of this gas be when the gas is compressed to
377 GAS LAW WORKSHEET 1 KEY 1. A sample of oxygen gas occupies a volume of 436. ml at 1.0 atm. If the temperature is held constant, what would the pressure of this gas be when the gas is compressed to
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *5896380298* ENVIRONMENTAL MANAGEMENT 0680/21 Paper 2 October/November 2011 1 hour 45 minutes
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *5896380298* ENVIRONMENTAL MANAGEMENT 0680/21 Paper 2 October/November 2011 1 hour 45 minutes
SOLUBILITY OF A SOLID IN WATER
 1516L Experiment 2 SOLUBILITY OF A SOLID IN WATER Objectives In this experiment you will determine the solubility of potassium nitrate (KNO 3 ) in water at various temperatures. You will prepare a plot
1516L Experiment 2 SOLUBILITY OF A SOLID IN WATER Objectives In this experiment you will determine the solubility of potassium nitrate (KNO 3 ) in water at various temperatures. You will prepare a plot
Gaseous oxygen technical grade Specification
 CDC 09 (5010) P3 Rev. TZS 217:1984 FINALIZED TANZANIA STANDARD Gaseous oxygen technical grade Specification TANZANIA BUREAU OF STANDARDS. 2 nd edition TBS 2017 0. FOREWORD 0.1. This Tanzania Draft Standard
CDC 09 (5010) P3 Rev. TZS 217:1984 FINALIZED TANZANIA STANDARD Gaseous oxygen technical grade Specification TANZANIA BUREAU OF STANDARDS. 2 nd edition TBS 2017 0. FOREWORD 0.1. This Tanzania Draft Standard
Multicellular Organisms. Sub-Topic 2.7 Animal Transport & Exchange Systems
 Multicellular Organisms Sub-Topic 2.7 Animal Transport & Exchange Systems On completion of this sub-topic I will be able to state that: Rings of cartilage keep the main airways open Oxygen and carbon dioxide
Multicellular Organisms Sub-Topic 2.7 Animal Transport & Exchange Systems On completion of this sub-topic I will be able to state that: Rings of cartilage keep the main airways open Oxygen and carbon dioxide
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 ambridge International Examinations ambridge International General ertificate of Secondary Education *4701421727* ENGLISH S SEOND LNGUGE 0510/31 Paper 3 Listening (ore) October/November 2014 pprox. 30
ambridge International Examinations ambridge International General ertificate of Secondary Education *4701421727* ENGLISH S SEOND LNGUGE 0510/31 Paper 3 Listening (ore) October/November 2014 pprox. 30
Blue Thumb Test Procedures
 Blue Thumb Test Procedures Items to take to Creek with you: 1. DO Kit (with scissors & thermometer) 2. Sample Bottle 3. Secchi Disk 4. Clip Board (with pen, instructions, and data sheet) 5. Goggles, Gloves,
Blue Thumb Test Procedures Items to take to Creek with you: 1. DO Kit (with scissors & thermometer) 2. Sample Bottle 3. Secchi Disk 4. Clip Board (with pen, instructions, and data sheet) 5. Goggles, Gloves,
THINKING SKILLS 9694/31 Paper 3 Problem Analysis and Solution May/June hours Additional Materials: Electronic Calculator
 Cambridge International Examinations Cambridge International Advanced Level THINKING SKILLS 9694/31 Paper 3 Problem Analysis and Solution May/June 2016 2 hours Additional Materials: Electronic Calculator
Cambridge International Examinations Cambridge International Advanced Level THINKING SKILLS 9694/31 Paper 3 Problem Analysis and Solution May/June 2016 2 hours Additional Materials: Electronic Calculator
Surface Area: the smaller the particles, the the rate of solubility.
 1. What is a solution? 2. What are the parts of a solution? (Ex. Lemonade) a. Solute: Ex. b. Solvent: Ex. 3. What is Solubility? a. Soluble: b. Insoluble: 4. Factors that Affect the Rate of Solubility:
1. What is a solution? 2. What are the parts of a solution? (Ex. Lemonade) a. Solute: Ex. b. Solvent: Ex. 3. What is Solubility? a. Soluble: b. Insoluble: 4. Factors that Affect the Rate of Solubility:
The Ideal Gas Constant
 Chem 2115 Experiment # 8 The Ideal Gas Constant OBJECTIVE: This experiment is designed to provide experience in gas handling methods and experimental insight into the relationships between pressure, volume,
Chem 2115 Experiment # 8 The Ideal Gas Constant OBJECTIVE: This experiment is designed to provide experience in gas handling methods and experimental insight into the relationships between pressure, volume,
Tuesday 23 May 2017 Morning Time allowed: 1 hour 15 minutes
 Oxford Cambridge and RSA AS Level Physical Education H155/01 Physiological factors affecting performance Tuesday 23 May 2017 Morning Time allowed: 1 hour 15 minutes *6962735173* You may use: A scientific
Oxford Cambridge and RSA AS Level Physical Education H155/01 Physiological factors affecting performance Tuesday 23 May 2017 Morning Time allowed: 1 hour 15 minutes *6962735173* You may use: A scientific
Friday 19 May 2017 Afternoon
 Oxford Cambridge and RSA Friday 19 May 2017 Afternoon LEVEL 1/2 CAMBRIDGE NATIONAL IN SPORT STUDIES R051/01 Contemporary issues in sport *6694906507* Candidates answer on the Question Paper. OCR supplied
Oxford Cambridge and RSA Friday 19 May 2017 Afternoon LEVEL 1/2 CAMBRIDGE NATIONAL IN SPORT STUDIES R051/01 Contemporary issues in sport *6694906507* Candidates answer on the Question Paper. OCR supplied
OUTREACH SPRING QUARTER 2002
 OUTREACH SPRING QUARTER 2002 Tuesday April 9, PSBN 1622 Workshop I: 9 to 10:30 Workshop II: 10:30 to 12 Saturday May 4, PSBN 1652 Workshop I: 10:30 to 12 Workshop II: 1:30 to 3 Tuesday May 7, PSBN 1652
OUTREACH SPRING QUARTER 2002 Tuesday April 9, PSBN 1622 Workshop I: 9 to 10:30 Workshop II: 10:30 to 12 Saturday May 4, PSBN 1652 Workshop I: 10:30 to 12 Workshop II: 1:30 to 3 Tuesday May 7, PSBN 1652
Name Gas Law Date. Version 3
 Name Gas Law Date 1. A real gas behaves least like an ideal gas under the conditions of 1) low temperature and high pressure 2) high temperature and low pressure 3) high temperature and high pressure 4)
Name Gas Law Date 1. A real gas behaves least like an ideal gas under the conditions of 1) low temperature and high pressure 2) high temperature and low pressure 3) high temperature and high pressure 4)
To derive from experiment the relationships between Pressure (P), Volume (V), Temperature (T), and Water Solubility of gases.
 PROPERTIES OF GASES: PRESSURE, VOLUME, TEMPERATURE, & SOLUBILITY RELATIONSHIPS PURPOSE: To derive from experiment the relationships between Pressure (P), Volume (V), Temperature (T), and Water Solubility
PROPERTIES OF GASES: PRESSURE, VOLUME, TEMPERATURE, & SOLUBILITY RELATIONSHIPS PURPOSE: To derive from experiment the relationships between Pressure (P), Volume (V), Temperature (T), and Water Solubility
LABORATORY TECHNIQUES. Pouring Liquids
 LABORATORY TECHNIQUES Working in the chemistry laboratory you will be handling potentially dangerous substances and performing unfamiliar tasks. This section provides you with a guide to the safe laboratory
LABORATORY TECHNIQUES Working in the chemistry laboratory you will be handling potentially dangerous substances and performing unfamiliar tasks. This section provides you with a guide to the safe laboratory
KEY CONCEPT Earth s atmosphere supports life. Living things need food, water, and air Matter can be solid, liquid, or gas
 KEY CONCEPT Earth s atmosphere supports life. BEFORE, you learned Living things need food, water, and air Matter can be solid, liquid, or gas NOW, you will learn Why the atmosphere is important to living
KEY CONCEPT Earth s atmosphere supports life. BEFORE, you learned Living things need food, water, and air Matter can be solid, liquid, or gas NOW, you will learn Why the atmosphere is important to living
CO 2 and Mass Chemistry
 CO 2 and Mass Chemistry Background: Many students believe that gases like carbon dioxide (CO 2) do not have mass. The fact is that atmospheric gases like CO 2 and methane (CH 4) have a tremendous amount
CO 2 and Mass Chemistry Background: Many students believe that gases like carbon dioxide (CO 2) do not have mass. The fact is that atmospheric gases like CO 2 and methane (CH 4) have a tremendous amount
Investigation of Boyle s Law: methods
 Name: Teacher: Class: Investigation of Boyle s Law: methods Your task is to investigate the relationship between volume, pressure and temperature for a gas. You must write detailed methods and select appropriate
Name: Teacher: Class: Investigation of Boyle s Law: methods Your task is to investigate the relationship between volume, pressure and temperature for a gas. You must write detailed methods and select appropriate
1. Harry investigated the effects of fizzy cola drink on his heart rate.
 1. Harry investigated the effects of fizzy cola drink on his heart rate. First he measured his heart rate every minute for 5 minutes when sitting down. Then he drank some cola. He continued to measure
1. Harry investigated the effects of fizzy cola drink on his heart rate. First he measured his heart rate every minute for 5 minutes when sitting down. Then he drank some cola. He continued to measure
[2] Explain how the alveoli create a surface for efficient gaseous exchange
![[2] Explain how the alveoli create a surface for efficient gaseous exchange [2] Explain how the alveoli create a surface for efficient gaseous exchange](/thumbs/89/98827571.jpg) 1 (a) (i) Name the two types of epithelial tissue found in the lungs and airways.... [2] (ii) The epithelial cells in the lungs are arranged into structures called alveoli. Explain how the alveoli create
1 (a) (i) Name the two types of epithelial tissue found in the lungs and airways.... [2] (ii) The epithelial cells in the lungs are arranged into structures called alveoli. Explain how the alveoli create
Solubility Unit. Solubility Unit Teacher Guide L1-3. Introduction:
 Solubility Unit Introduction: In this unit the students will learn about solubility. Students should already be familiar with the basic chemistry concepts. They should know that some substances are soluble
Solubility Unit Introduction: In this unit the students will learn about solubility. Students should already be familiar with the basic chemistry concepts. They should know that some substances are soluble
Finding the water potential of potato tissue INTRODUCTION. Figure 1. Concentrated. Potato core
 General Certificate of Education June 2009 Advanced Subsidiary Examination BIOLOGY Investigative Skills Assignment Task Sheet BIO3T/Q09/task Finding the water potential of potato tissue INTRODUCTION When
General Certificate of Education June 2009 Advanced Subsidiary Examination BIOLOGY Investigative Skills Assignment Task Sheet BIO3T/Q09/task Finding the water potential of potato tissue INTRODUCTION When
SAFETY DATA SHEET. Section 1: Product and Company Identity
 SAFETY DATA SHEET Section 1: Product and Company Identity Name: Gendex Process Chemistry - Developer Product Number: 1.0069274 & 0.8223045 (296-49,296-40) Product Use: Photographic Developer Manufacturer:
SAFETY DATA SHEET Section 1: Product and Company Identity Name: Gendex Process Chemistry - Developer Product Number: 1.0069274 & 0.8223045 (296-49,296-40) Product Use: Photographic Developer Manufacturer:
SOLUBILITY OF A SOLID IN WATER
 1516L Experiment 1 SOLUBILITY OF A SOLID IN WATER Objectives In this experiment you will determine the solubility of potassium nitrate (KNO 3 ) in water at various temperatures. You will prepare a plot
1516L Experiment 1 SOLUBILITY OF A SOLID IN WATER Objectives In this experiment you will determine the solubility of potassium nitrate (KNO 3 ) in water at various temperatures. You will prepare a plot
Title: Solubility of Gas A Daily Experience. Subject: Chemistry. Grade Level: 10 th 12 th
 Title: Solubility of Gas A Daily Experience Subject: Chemistry Grade Level: 10 th 12 th Rational or Purpose: This lesson brings an everyday life experience to students knowledge on solubility of gas in
Title: Solubility of Gas A Daily Experience Subject: Chemistry Grade Level: 10 th 12 th Rational or Purpose: This lesson brings an everyday life experience to students knowledge on solubility of gas in
