Evaluation of the Critikon 8100 and Spacelabs non-invasive blood pressure monitors using a test simulator
|
|
|
- Noreen Allison
- 6 years ago
- Views:
Transcription
1 Journal of Human Hypertension (1997) 11, Stockton Press. All rights reserved /97 $12.00 Evaluation of the Critikon 8100 and Spacelabs non-invasive blood pressure monitors using a test simulator Department of Medical Physics and Medical Engineering, Royal Infirmary of Edinburgh, Edinburgh, UK The Critikon Dinamap 8100 and the Spacelabs consistency than the Dinamap which showed a higher ambulatory non-invasive blood pressure (NIBP) moni- standard deviation under all the conditions. The relators were evaluated using a test simulator using an tively high standard deviation of the recordings made evaluation protocol which covered a wide range of by the Dinamap could explain the non-systematic errors simulated pressures (with five determinations at each of found in some evaluations. Both instruments recorded six steps from 60/30 to 200/150 mm Hg), pulse rates pressures within 5 mm Hg of the target over the range (from 40 to 200 bpm), artefact levels (simulated motion of pressures and pulse rates, and coped well under conand tremor artefact) and pulse strengths (down to 10% ditions of severe artefact and weak pulsations, either by of the nominal strength). Determinations were made at signalling inability to record or by recording satisfac- 5 min intervals. The average and standard deviation of torily. the five measurements at each condition were calculated. The Spacelabs recorded pressures with a greater Keywords: NIBP monitors; evaluation; simulators Introduction Oscillometric non-invasive blood pressure (NIBP) measuring instruments are in widespread use in health care, with a significant growth in the use of these devices in hospitals in recent years; in our teaching hospital their number, per 100 hospital beds, increased over five-fold from about three in early 1987 to 17 in early While several tech- niques may be used to measure blood pressure (BP), 1 the oscillometric technique is most commonly used for automated NIBP measurements, being found in over 80% of instruments. 2 Oscillometric NIBP monitors record the systolic, mean and diastolic pressures by relating the cuff pressure to the shape of the waveform of low ampli- tude ( 2 mm Hg) pressure oscillations. The oscil- lations are generated in a cuff placed around a limb as its pressure is reduced from above the systolic to below the diastolic pressure. The mean BP is estimated as the cuff pressure corresponding to the maximum amplitude of the oscillometric wave- form. 3 The diastolic and systolic pressures are empirically determined based on the relationship between the cuff pressures corresponding to specific fractions of the peak height of the envelope of the oscillations. Different manufacturers use different algorithms to interpret the oscillometric waveform, 4 Address for correspondence: Dr John Amoore, Department of Medical Physics and Medical Engineering, Royal Infirmary of Edinburgh, Lauriston Place, Edinburgh EH3 9YW, UK Received 30 January 1996; revised 2 January 1997; accepted 6 January 1997 and their own proprietary signal processing techniques to improve the ability of the monitor to record the BP under conditions of low pulse strength and movement artefact. The consequence of the device-specific signal pro- cessing and algorithm is that each different NIBP monitor has to be validated to check if it correctly interprets the BP. Evaluation standards and protocols have been drawn up, 5 7 which compare the NIBP monitor with direct intra-arterial BP readings or with manual auscultatory measurements These clinical trials evaluate the monitor over a wide range of BPs, with multiple readings at each pressure. This is of necessity a costly business requiring the recruitment of many patients. To reduce the need for trained observers a technique has been developed which involves recording on video-tape the measurement process (mercury sphygmomanometer together with the auscultatory signal) using a camcorder. 11 This technique pre- serves the advantages of evaluating an NIBP monitor against the standard auscultatory method and by recording the process allows the measurements to be made and checked later. The development of NIBP test simulators presents the opportunity of developing alternative protocols for evaluating NIBP monitors. 7,12,13 These protocols can supplement, but not replace, clinical trials. We have developed an evaluation protocol using test simulators which has been described elsewhere. 13 Here we show how this protocol can be used to help evaluate two NIBP monitors, providing functional information not readily obtainable during conventional clinical trials.
2 164 Materials and methods affects the measurement of the pressure. 14 We recorded the pressures over a range of pulse rates A number of NIBP simulators, which generate oscillometric from 40 bpm to 200 bpm with the simulated presscommercially pulses in response to cuff inflation, are ure kept fixed at 120/80 (93) mm Hg. Most NIBP available. 7 Waveforms simulating a monitors adjust the rate of change of cuff pressure wide range of pressures and pulse rates may be gen- with the pulse rate to ensure accurate determinations erated, with their repeatability specified to within 1 at low pulse rates, while taking advantage mm Hg. The test simulators enable NIBP monitors of the higher sampling frequency at higher pulse to be tested over a wide variety of conditions such rates to reduce the determination time. We recorded as may be encountered in clinical practice. The the time for each determination. Five determinations simulators also record a number of parameters at each pulse rate were made. describing the determination, such as peak inflation The BP-Pump can be used to test the ability of the pressure, determination time and deflation rate. The NIBP monitor to cope with artefact. A low frequency Bio-Tek BP Pump (Bio-Tek Instruments Inc, High- Motion artefact may be added to the oscillometric land Park, Box 998, Winooski, Vermont waveform in various degrees of severity from 1 to , USA) was used in this study. to 5 and up to 10. (Level 1 artefact is simulated by The cuff hose of the monitor under test was connected adding noise with a peak-to-peak amplitude of to the test simulator which incorporates an 0.2 ml in relation to the signal amplitude of 1.2 ml. internal cuff, designed to simulate the volume and Level 2 is twice the noise amplitude. We tested the compliance of an adult cuff wrapped around a limb. monitors up to noise levels 5, that is noise amplitude The simulator was controlled by a personal computer of 1 ml.) Similar ranges of severity of mixed which recorded the peak inflation pressure low and high frequency Tremor artefact were added. and the determination time of the NIBP monitor as In all cases the simulator was set to generate a press- determined by the simulator. 13 The Critikon 8100 ure of 120/80 mg Hg at a pulse rate of 80 bpm. The was also directly connected to the personal computer average Bias and standard deviation of five success- via its serial link, and each measured pressure ive determinations (at 5-min intervals) were determ- was recorded. The Spacelabs stores its recorded ined at each artefact setting. measurements in its internal memory. Its measure- The ability of a monitor to cope with weak pulsations ments were transferred after the evaluation to a spreadsheet was tested by reducing the amplitude of the and linked to the data from the simulator oscillometric waveform down to 10% of the nominal for analysis. typical amplitude. The Bio-Tek BP-Pump califor We compared the difference (Bias) between the brates its nominal 100% amplitude as a 1.2 ml volume pressure set on the simulator (the measurement displacement. This corresponds to the typical standard for the analysis) and the pressure recorded amplitude of the oscillometric waveform of 2 by the simulator. Even when presented with a fixed mm Hg. simulated waveform NIBP recordings will vary from We demonstrate the role that NIBP test simulators determination to determination. 12,14 Hence, each can play as a complementary tool for the evaluation simulated pressure waveform was repeated five of NIBP monitors by assessing the Critikon Dinamap times (with an interval of 5 min between each 8100 NIBP monitor and the Spacelabs ambu- determination). The averaged Bias was calculated as latory NIBP recorder. The Dinamap 8100 is in widespread was the sample standard deviation. use in health care facilities, and has been The evaluation protocol covered a range of simu- evaluated according to the British Hypertension lated pressures, pulse rates, weak pulsations, and Society guidelines, 9 as has the Spacelabs ambulat- motion and tremor artefact (Table 1). The first part ory monitor. 8 of the protocol tested the monitor at six different pressures ranging from low (60/30 mm Hg) to high (200/100 mm Hg), with five determinations at each Results target pressure. The bias and standard deviation Both NIBP instruments were tested over a range of were calculated at each pressure. We recorded the pressures from 60/30 to 200/150 mm Hg with the peak cuff inflation pressure and calculated the dif- pulse rate fixed at 80 bpm. The average of the difference ference between it and the previous systolic pressure, between the measured pressure and the set ignoring the first determination at each pressure simulator pressure for each of the five determinations step. This difference reflects the ability of the monitor at each pressure (bias) and the standard to adjust its cuff inflation pressure to the pre- deviation were calculated (Figure 1). The standard vious systolic pressure. deviation of the Critikon was greater than the Space- We then presented the monitor with a ramped labs. pressure sequence to test the ability of the monitor We calculated the difference between the peak to follow changing pressure patterns. The pressure cuff inflation pressure and the previous systolic setting of the simulator was ramped up from 60/30 pressure at each of the pressure steps (Figure 2); mm Hg to 255/195 mm Hg and then down to 60/30 both instruments adjusted their peak cuff inflation mm Hg with the sequence repeated. The differences pressure based on the previous systolic. between the measured pressures and the target Both instruments were able to follow a ramped simulator pressures were recorded. pressure changed with the Bias keeping within ±10 The cuff pressure is sampled by the oscillometric mm Hg over the systolic pressure range from waveform at the pulse rate, and hence the pulse rate mm Hg (Figure 3). While we tested both units at a
3 Table 1 NIBP monitor evaluation protocol 165 Test description Simulated pressure Pulse rate Pulse strength (%) Pressure range Tests at six simulated pressures: Fixed pulse rate Fixed at 100% 60/30 (40) 120/80 (93) 80 bpm 80/50 (60) 150/100 (116) 100/65 (76) 200/150 (166) Ramp Pressure ramps from 60/30 to Fixed pulse rate Fixed at 100% (single determination at 255/195 to 60/30 80 bpm each step) Pulse rate Fixed pressure Pulse rates: 40, 60, Fixed at 100% 120/80 (93) 80, 120, 160, 200 Artefact Fixed pressure Fixed pulse rate Fixed at 100% Motion: Low frequency 120/80 (93) 80 bpm noise Tremor: Mixed low and high frequency noise Pulse strength Fixed pressure Fixed pulse rate Pulse strength: 100%, 120/80 (93) 80 bpm 75%, 50%, 25%, 10% Note: Unless indicated otherwise, five successive determinations are made at each setting within each test group. All pressures are in mm Hg. systolic of 255 mm Hg it should be noted that they 60/30 to 200/150 mm Hg. These results suggest that are only specified up to systolic pressures of 250 the relatively high variability of the determinations mm Hg. However, the high systolic pressure was by the Dinamap may account for the non-systematic included in order to establish whether they could errors which caused it to fail the BHS society evalu- successfully record a lower pressure (150 mm Hg) ation protocol. 9 In contrast, the Spacelabs produced after the peak high pressure. consistent measurements, in agreement with earl- Figure 4 shows the pressures recorded by both ier validations. 8 monitors over 25 successive determinations, five at The assessment of an NIBP monitor should each of five pulse rate over the range from 40 include an evaluation of its ability to record accurately 200 bpm. The determination time increased with the patient s BP over a wide range of con- decreases in pulse rate (Figure 5) reflecting the ditions including pressures and pulse rate. Conventionally reduction in deflation rate at the lower pulse rates the assessment is made by testing the in order to maintain consistent measurements. monitor over a range of patients and comparing the Figure 6 shows the Bias and Error of the recorded monitored pressure to the patient s actual pressure systolic pressure from two monitors when presented as determined by an arterial line, or by a gold standard with increasing levels of Motion and Tremor artefact. technique such as the auscultatory method. The target pressure was 120/80 (93) with a NIBP simulators provide an alternative assessment pulse rate of 80 bpm. Figure 6 shows only the systolic tool, but instead of comparing the monitor s deter- pressures; the effects of the artefact on the dias- mination to the actual patient pressure, the moni- tolic and mean pressures were similar. The Critikon tor s determination is compared to the notional notified the operator of unacceptably high levels of pressure of a simulated patient waveform. Figure 1 artefact at Tremor Level 5 (ee error code); the Spacelabs shows the difference between the pressures managed to record pressures at this high arte- recorded by the Critikon 8100 and the Spacelabs fact level, albeit with an increased Bias and variability and the notional pressure of the waveform simulated by the test instrument. Clearly therefore, Both monitors successfully recorded pressures the extent to which the Bias shown in Figure 1 when the pulse strength was reduced to 25% of the matches that determined from clinical trials will nominal 2 mm Hg oscillometric waveform (Figure depend on the extent to which the simulated wave- 7), but neither could record pressures at 10% form matches the waveform of the patient. Nonetheless, pulse strength. the simulated waveform is consistent, and hence should enable a comparative assessment of the various monitors to be made. Discussion The average of the measurements at each simulated We tested both the Critikon Dinamap 8100 and the condition recorded by the Dinamap and the Spacelabs over a wide range of conditions. Spacelabs were within 5 mm Hg of each other. The main finding was the greater consistency of the O Brien et al 9 showed a non-systematic systolic measurements made by the Spacelabs over all the error of 1 ± 7 mm Hg for the Dinamap. The slightly conditions, with comparatively large determination lower standard deviation found in this study may to determination variations recorded by the Dina- reflect the contribution of physiological variations to map. The average bias of each instrument was however the variability observed in the clinical trial. How- within 5 mm Hg of the simulator target and of ever, this study did not find the systematic error in each other over the full range of pressures from the diastolic measurements suggested by the 6 ± 7
4 166 Figure 1 The Bias and standard deviation of five successive pressures recorded by the Critikon Dinamap 8100 and the Spacelabs at each of six pressures covering the range of pressures from 60/30 mm Hg to 200/150 mm Hg. The X axis shows the pressure (in mm Hg) at each simulation. shown how the test simulator correctly interpreted the effects of the different algorithms included in the Nellcor N3100 monitor. 16 It provides two algorithms to accommodate different interpretations of non- invasive BP measurements between Japan and Europe. The adjusted option was included for the Eur- opean market and involves a 2 mm Hg decrease in the systolic pressure and a 5 mm Hg increase in the diastolic pressure. The measurements recorded by the simulator reflected these differences. The simulator enabled us to test the ability of the monitor to follow large abrupt changes in BP, by presenting the ramped change in pressure. This test is useful, as most NIBP monitors base their cuff mm Hg observed in the clinical trial. We do not have an explanation for this, other than to note that another study recorded a non-systematic diastolic error of 0.7 ± 11 mm Hg. 15 Certainly, the results suggests relatively large non-systematic errors in the recordings of the Dinamap. While the simulators are designed to generate realistic waveforms based on recordings from patients, no reports validating the accuracy of the waveforms have been reported. The waveforms are however repeatable, enabling the consistency of measurements made by NIBP monitors to be assessed and to evaluate the relative differences between NIBP monitors. 16 In earlier work we have
5 167 Figure 2 Pressure above the systolic pressure to which the cuff is inflated for Critikon 8100 and the Spacelabs Figure 4 The systolic, mean and diastolic pressures recorded by each monitor as the pulse rate was increased through the range 40, 60, 80, 120, 160 and 200 bpm. Five determinations were made at each pulse rate at intervals of 5 min. The simulated pressure was kept constant at 120/80 (93) mm Hg. inflation pressure according to the previously recorded systolic pressure. In unpublished work we found one monitor which was unable to accurately record low pressures following a high pressure. It remembered the previous high systolic reading and, presented with a lower pressure and not finding the expected high systolic pressure, appeared to increase the gain of its pressure transducer amplifier and consequently interpreted noise as the start of the oscillometric waveform. Controlled artefact can be simulated and the ability of the monitor to accurately record pressures during the presence of artefact can be assessed. This is important as it follows from the very nature of automated NIBP monitors in clinical use that medical staff will not necessarily be observing the patient Figure 3 The systolic pressures recorded by the Dinamap and the Spacelabs as it was presented with two ramped changes of press- ure. The X axis shows the simulated systolic pressure. while the pressure is being recorded. Hence the monitor could be recording pressures while the patient s limb is moving without staff being aware of the movement. A monitor which provides apparently accurate recordings of BP which are significantly influenced by artefact may tend to mislead medical staff. It is much better that the monitor identify in some way the presence of artefact than attempt to provide a reading which is in error. Both in this study and in others 17 an increased variability of the recordings in the presence of simulated artefact has been observed. In addition, we observed that high levels of artefact typically lead to higher readings in monitors which do not cope well with artefact (Figure 6). This is because the presence of artefact superimposed on the cuff pressure leads to the monitor detecting an apparent oscillometric waveform at a higher cuff pressure. The evaluation of the ability of an NIBP monitor to cope with artefact is perhaps particularly relevant for ambulatory instruments. 18 NIBP simulators provide an objective
6 168 Figure 5 The time taken to record pressures at each of six pulse rates from bpm. The average time over five successive recordings was determined. Figure 7 Effects of reducing the pulse strength on the Bias and standard deviation of the recorded systolic pressure. The target pressure was 120 mm Hg. Besides simply comparing the pressure determinations of a particular monitor with standardised waveforms, NIBP simulators can provide other useful measures of performance. The ability of NIBP monitors to rapidly make the measurement and with minimal inflation of the cuff helps to reduce the stress to the patient caused by the cuff inflation. Monitors adjust their rate of cuff deflation (or cuff inflation if the measurement is made during inflation) to maintain reproducibility by ensuring that the changing cuff pressure is adequately sampled by the pressure oscillations at the pulse rate (Figure 5). Technological advances have enabled the determination time to be reduced to s compared to the s of the early generation of monitors such as the Critikon Equally important is the ability to record the pressures without undue inflation of the cuff. Figure Figure 6 Effects of increasing levels of Motion (M1, M2, and M5) 2 compares the pressure to which the cuff is inflated and Tremor (T1, T2, T5) artefact on the Bias and standard deviabove the systolic pressure for the two monitors. ation of the recorded systolic pressure. The target pressure was 120 mm Hg. (ee) indicates that the monitor displayed its inability Both monitors adjusted their peak cuff inflation to record because of high levels of artefact. pressure in relation to the previous systolic peak, with the Dinamap generating lower cuff pressures at method of studying the effects of artefact on the the very low systolic pressures. This may reflect its recordings of pressure by NIBP monitors. In this neonatal mode of operation; the Spacelabs is study the Spacelabs was shown to cope designed for adult only use. extremely well with severe levels of artefact. The We have presented an evaluation of NIBP monitors Bio Tek BP-Pump provides a range of severities of using commercially available test simulators. artefact. Data needs to be collected to determine the We believe that such devices can provide an objective severity of artefacts encountered in the clinical setting. and important and significant adjunct to the clinical evaluation of NIBP monitors. Test simulators The NIBP simulator can also evaluate how well a can assess the consistency of pressure monitor copes with weak pulsations. Again, data measurements, check the response to abrupt needs to be collected to evaluate the amplitude of changes in pressure, examine the ability to cope with artefact and low pulse strengths and record details of the determinations such as the determi- nation time and the peak cuff inflation pressure. By the oscillometric waveform in patients with weak pulsations in order to decide what levels of pulse strength an evaluation protocol should include.
7 enabling an NIBP monitor to be checked over a wide sphygmomanometers. Hypertension 1993; 21: 504 range of pressures and pulse rates they may enable 509. clinical trials to be simplified, perhaps reducing the 7 Ng K-G, Small CF. Review of methods and simulators number of patients required for each evaluation. A for evaluation of non-invasive blood pressure moni- tors. J Clin Eng 1992; 17: further important potential use for the NIBP simu- 8 O Brien E, Mee F, Atkins N, O Malley K. Accuracy of lator will be to reassess the adequacy of NIBP monithe Spacelabs determined by the British Hypertors after prolonged periods of clinical use. tension Society protocol. J Hypertens 1991; 9: Acknowledgements 9 O Brien E, Mee F, Atkins N, O Malley K. Short report: Accuracy of the Dinamap portable monitor, model 8100 determined by the British Hypertension Society The financial support of the Chief Scientists Organprotocol. J Hypertens 1993; 11: isation of the Scottish Office Home and Health 10 Alpert BS. Validation of CAS model 9010 automated Department through the Advisory Panel on Evalu- blood pressure monitor: children/adult and neonatal ation of Medical and Scientific Equipment and studies. Blood Press Monit 1996; 1: Health Service is gratefully acknowledged. We 11 O Brien E et al. A new audiovisual technique for recthank the reviewer for his comments and Dr P Pad- ording blood pressure in research: the Sphygmocorder. field of the Department of Medicine at the Western J Hypertens 1995; 13: General Hospital in Edinburgh for helpful advice in 12 Amoore JN. Assessment of oscillometric non-invasive preparing the paper. blood pressure monitors using the Dynatech Nevada CuffLink analyser. J Med Eng Tech 1993; 17: Geake WB, Amoore JN, Scott DHT. An automated system for the functional evaluation of oscillometric non- References invasive blood pressure monitors. J Med Eng Tech 1 O Brien E, Fitzgerald D. The history of blood pressure 1995; 19: measurement. J Hum Hypertens 1994; 8: Hatsel CP. Cardiac cycle phase uncertainty: another 2 Ng K-G, Small CF. Survey of automated non-invasive source of error in indirect blood pressure measureblood pressure monitors. J Clin Eng 1994; 19: ment. J Med Eng Tech 1992; 16: Ramsey M III. Noninvasive automatic determination of 15 Ornstein S, Makert G, Litchfield I, Zemp L. Evaluation mean arterial blood pressure. Med Biolog Eng Comput of the DINAMAP blood pressure monitor in an ambulatory 1979; 17: care setting. J Fam Pract 1988; 26: Sapinski A. Standard algorithm of blood-pressure 16 Amoore JN, Geake WB, Scott DHT. Oscillometric noninvasive measurement by the oscillometric method (Letter). blood pressure measurements: the influence Med Biolog Eng Comput 1994; 32: 599. of the make of instrument on the reading? Med Biolog 5 O Brien E et al. The British Hypertension Society protocol Eng Comp 1997; 35: for the evaluation of automated and semi-auto- 17 Mieke S et al. Zur Me sicherheit nichtinvasiver oszil- mated blood pressure measuring devices with special lometrischer Blutdruckme geräte. Anaesthesist 1993; reference to ambulatory systems. J Hypertens 1990; 8: 42: West JNW et al. Effect of unrestricted activity on accu- 6 White WB et al. National standard for measurement of racy of ambulatory blood pressure measurement. resting and ambulatory blood pressure with automated Hypertension 1991; 18:
Noninvasive Blood Pressure Measurement. D. J. McMahon rev cewood
 Noninvasive Blood Pressure Measurement D. J. McMahon 141018 rev cewood 2017-10-23 Key Points Noninvasive Blood Pressure Measurement: - ways to measure pressure, direct mechanical measurement: Bourdon Tube
Noninvasive Blood Pressure Measurement D. J. McMahon 141018 rev cewood 2017-10-23 Key Points Noninvasive Blood Pressure Measurement: - ways to measure pressure, direct mechanical measurement: Bourdon Tube
Linear Infl ation Technology Non-invasive Blood Pressure Measurement. inibp Case Report Experiences in OR
 Linear Infl ation Technology Non-invasive Blood Pressure inibp Case Report Experiences in OR C ONTENTS Introduction 2 Technological Description of inibp inibp advantages 2 inibp technology adapts to each
Linear Infl ation Technology Non-invasive Blood Pressure inibp Case Report Experiences in OR C ONTENTS Introduction 2 Technological Description of inibp inibp advantages 2 inibp technology adapts to each
Section 2: Using the Invasive Blood Pressure Module - An Introduction
 Section 2: Using the Invasive Blood Pressure Module - An Introduction IBP Module - General The Invasive Blood Pressure module is a dual function module that can monitor pulsatile pressure waves such as
Section 2: Using the Invasive Blood Pressure Module - An Introduction IBP Module - General The Invasive Blood Pressure module is a dual function module that can monitor pulsatile pressure waves such as
Bloodpressure measurement, improvement of oscillometric method.
 Bloodpressure measurement, improvement of oscillometric method. Øystein Stene, Lars Jørgen Kristoffersen, Waldemar Sulkowski Høgskolen i Narvik, Sivilingeniørutdanningen, postboks 385, N-8501 Narvik Norway,
Bloodpressure measurement, improvement of oscillometric method. Øystein Stene, Lars Jørgen Kristoffersen, Waldemar Sulkowski Høgskolen i Narvik, Sivilingeniørutdanningen, postboks 385, N-8501 Narvik Norway,
BLOOD PRESSURE SENSOR BT17i USER S GUIDE
 BLOOD PRESSURE SENSOR BT17i USER S GUIDE CENTRE FOR MICROCOMPUTER APPLICATIONS http://www.cma-science.nl Short description The Blood Pressure sensor BT17i allows measuring arterial blood pressure. The
BLOOD PRESSURE SENSOR BT17i USER S GUIDE CENTRE FOR MICROCOMPUTER APPLICATIONS http://www.cma-science.nl Short description The Blood Pressure sensor BT17i allows measuring arterial blood pressure. The
3.1.3 Weight. Frequency. Weight is obtained at the baseline examination and annually. Equipment
 The excerpt from the SPRINT Manual of Procedures (MOP) below, dated November 1, 2010, includes instructions about blood pressure measurement. Recruitment for the SPRINT trial began on November 8, 2010;
The excerpt from the SPRINT Manual of Procedures (MOP) below, dated November 1, 2010, includes instructions about blood pressure measurement. Recruitment for the SPRINT trial began on November 8, 2010;
BLOOD PRESSURE SENSOR 0377i
 BLOOD PRESSURE SENSOR 0377i USER S GUIDE CENTRE FOR MICROCOMPUTER APPLICATIONS http://www.cma-science.nl Short description The Blood Pressure sensor 0377i is used to measure arterial blood pressure in
BLOOD PRESSURE SENSOR 0377i USER S GUIDE CENTRE FOR MICROCOMPUTER APPLICATIONS http://www.cma-science.nl Short description The Blood Pressure sensor 0377i is used to measure arterial blood pressure in
NIBP OEM Module. (Model : M_NIBP) Contents. 1. Concept 2. Composition 3. PCB Dimension 4. Specification 5. Protocol 6. Cuff & Electrical Connection
 1 NIBP OEM Module (Model : M_NIBP) Contents 1. Concept 2. Composition 3. PCB Dimension 4. Specification 5. Protocol 6. Cuff & Electrical Connection 2 1. Concept Rev V1.00 Oscillometry : The oscillometric
1 NIBP OEM Module (Model : M_NIBP) Contents 1. Concept 2. Composition 3. PCB Dimension 4. Specification 5. Protocol 6. Cuff & Electrical Connection 2 1. Concept Rev V1.00 Oscillometry : The oscillometric
Declaration of Equivalence Form
 Declaration of Equivalence Form DECLARATION OF BLOOD PRESSURE MEASURING DEVICE EQUIVALENCE 2013 SECTION A - Please complete all items. A SIGNED COPY WILL BE POSTED ON THE www.dableducational.org WEBSITE
Declaration of Equivalence Form DECLARATION OF BLOOD PRESSURE MEASURING DEVICE EQUIVALENCE 2013 SECTION A - Please complete all items. A SIGNED COPY WILL BE POSTED ON THE www.dableducational.org WEBSITE
Sports Wrist Digital Blood Pressure Monitor Item #
 Instruction Manual Sports Wrist Digital Blood Pressure Monitor Item # 04-885-001 Please read this guidebook completely before operating this unit. Limited Five-Year Warranty The warrantor guarantees that
Instruction Manual Sports Wrist Digital Blood Pressure Monitor Item # 04-885-001 Please read this guidebook completely before operating this unit. Limited Five-Year Warranty The warrantor guarantees that
PHY100 s Lab: The Relationship Between Liquid Pressure and Depth as it applies to Blood Pressure. Course & Sec:
 PHY100 s Lab: The Relationship Between Liquid Pressure and Depth as it applies to Blood Pressure Name: Lab Partner: Course & Sec: Date: Disclaimer: The procedures in this lab are not according to proper
PHY100 s Lab: The Relationship Between Liquid Pressure and Depth as it applies to Blood Pressure Name: Lab Partner: Course & Sec: Date: Disclaimer: The procedures in this lab are not according to proper
CONTENTS SPECIFICATIONS GENERAL INFORMATION RECOMMENDED USE OPERATING PRINCIPLE TIPS ON TAKING YOUR BLOOD PRESSURE 3-4 BATTERY INSTALLATION
 IFU SBPMON107 CONTENTS SPECIFICATIONS GENERAL INFORMATION RECOMMENDED USE OPERATING PRINCIPLE TIPS ON TAKING YOUR BLOOD PRESSURE BATTERY INSTALLATION CORRECT POSITION FOR MEASUREMENT POSITIONING THE CUFF
IFU SBPMON107 CONTENTS SPECIFICATIONS GENERAL INFORMATION RECOMMENDED USE OPERATING PRINCIPLE TIPS ON TAKING YOUR BLOOD PRESSURE BATTERY INSTALLATION CORRECT POSITION FOR MEASUREMENT POSITIONING THE CUFF
Semi-Automatic Blood Pressure Monitor with Memory
 INSTRUCTION MANUAL Semi-Automatic Blood Pressure Monitor with Memory 61-268-001 (Adult size cuff) Please read this instruction manual completely before operating this unit. English Spanish Limited Five
INSTRUCTION MANUAL Semi-Automatic Blood Pressure Monitor with Memory 61-268-001 (Adult size cuff) Please read this instruction manual completely before operating this unit. English Spanish Limited Five
Instruction Manual. Auto Inflate Blood Pressure Monitor
 TM Auto Inflate Blood Pressure Monitor Instruction Manual Features: Fuzzy Logic Technology Auto memory for up to 90 readings One-Touch Operation Easy-to-read Display Date and Time indications AC Adapter
TM Auto Inflate Blood Pressure Monitor Instruction Manual Features: Fuzzy Logic Technology Auto memory for up to 90 readings One-Touch Operation Easy-to-read Display Date and Time indications AC Adapter
Analog Front End Design of a Digital Blood Pressure Meter
 Analog Front End Design of a Digital Blood Pressure Meter Santosh Kulkarni Dr.Veena. S. Chakravarthy Vishweshwara Mundkur Dept. of ECE, Professor, Dept. of ECE, Director, BNM Institute of Technology, BNM
Analog Front End Design of a Digital Blood Pressure Meter Santosh Kulkarni Dr.Veena. S. Chakravarthy Vishweshwara Mundkur Dept. of ECE, Professor, Dept. of ECE, Director, BNM Institute of Technology, BNM
Non-Invasive Blood Pressure (NIBP)
 Non-Invasive Blood Pressure (NIBP) October 2007 9650-1214-01 Rev. G The issue date or revision level for this operation guide is shown on the front cover. ZOLL and E Series are registered trademarks of
Non-Invasive Blood Pressure (NIBP) October 2007 9650-1214-01 Rev. G The issue date or revision level for this operation guide is shown on the front cover. ZOLL and E Series are registered trademarks of
MANUAL BLOOD PRESSURE MONITOR BPM 168B Contents Blood Pressure Monitor Intended Use What is blood pressure?
 MANUAL BLOOD PRESSURE MONITOR BPM 168B Contents 01... Blood Pressure Monitor Intended Use 02... 1. What is blood pressure? 02... 2. Why is it useful measure blood pressure at home?... A. WHO blood pressure
MANUAL BLOOD PRESSURE MONITOR BPM 168B Contents 01... Blood Pressure Monitor Intended Use 02... 1. What is blood pressure? 02... 2. Why is it useful measure blood pressure at home?... A. WHO blood pressure
Model AUTOMATIC UPPER ARM Blood Pressure Monitor
 AUTOMATIC UPPER ARM Blood Pressure Monitor Model 1130 Real Fuzzy t e c h n o log y Features: Real Fuzzy Technology Automatic 60 sets of memory One-Touch Operation Easy-to-read Display Table of Contents:
AUTOMATIC UPPER ARM Blood Pressure Monitor Model 1130 Real Fuzzy t e c h n o log y Features: Real Fuzzy Technology Automatic 60 sets of memory One-Touch Operation Easy-to-read Display Table of Contents:
Fully Fuzzy Auto Digital Wrist Blood Pressure Monitor. tensio control. INSTRUCTION MANUAL Model GP-6220
 Fully Fuzzy Auto Digital Wrist Blood Pressure Monitor tensio control INSTRUCTION MANUAL Model GP-6220 Manufactured by: Geratherm Medical AG Fahrenheitstraße 1 98716 Geschwenda Germany Phone: +49 (0) 36205
Fully Fuzzy Auto Digital Wrist Blood Pressure Monitor tensio control INSTRUCTION MANUAL Model GP-6220 Manufactured by: Geratherm Medical AG Fahrenheitstraße 1 98716 Geschwenda Germany Phone: +49 (0) 36205
Mouse and Rat Tail Cuff Blood Pressure Systems from 1-24 Animals. Mouse rat tail cuff blood pressure system components
 Mouse and Rat Tail Cuff Blood Pressure Systems from 1-24 Animals Mouse rat tail cuff blood pressure system components Tail Cuff Method Sensors Rodent Restrainers Software Chart Recorder Amplifier with
Mouse and Rat Tail Cuff Blood Pressure Systems from 1-24 Animals Mouse rat tail cuff blood pressure system components Tail Cuff Method Sensors Rodent Restrainers Software Chart Recorder Amplifier with
Auto Arm Blood Pressure Monitor
 Now you know ys Auto Arm Blood Pressure Monitor Featuring: One-touch operation 85 memories Instruction Manual Table of Contents Important information................... 3 Fuzzy Logic technology.................
Now you know ys Auto Arm Blood Pressure Monitor Featuring: One-touch operation 85 memories Instruction Manual Table of Contents Important information................... 3 Fuzzy Logic technology.................
Upload-pressurized Automatic Sphygmomanometer.
 www.inbody.com Upload-pressurized Automatic Sphygmomanometer. www.inbody.com Measuring blood pressure is one of the fundamental steps for maintaining a healthy lifestyle Blood screening is basic to health
www.inbody.com Upload-pressurized Automatic Sphygmomanometer. www.inbody.com Measuring blood pressure is one of the fundamental steps for maintaining a healthy lifestyle Blood screening is basic to health
OIML R 16-1 RECOMMENDATION. Edition 2002 (E) ORGANISATION INTERNATIONALE INTERNATIONAL ORGANIZATION. Non-invasive mechanical sphygmomanometers
 INTERNATIONAL RECOMMENDATION OIML R 16-1 Edition 2002 (E) Non-invasive mechanical sphygmomanometers Sphygmomanomètres non invasifs mécaniques OIML R 16-1 Edition 2002 (E) ORGANISATION INTERNATIONALE DE
INTERNATIONAL RECOMMENDATION OIML R 16-1 Edition 2002 (E) Non-invasive mechanical sphygmomanometers Sphygmomanomètres non invasifs mécaniques OIML R 16-1 Edition 2002 (E) ORGANISATION INTERNATIONALE DE
REF CH-602B IDENTIFICATION OF PARTS
 English TM INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR REF CH-602B IDENTIFICATION OF PARTS Systolic blood pressure display section Battery cover Diastolic blood pressure / Pulse display section
English TM INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR REF CH-602B IDENTIFICATION OF PARTS Systolic blood pressure display section Battery cover Diastolic blood pressure / Pulse display section
Operation Manual. AccuPulse is a trademark of Clinical Dynamics Corporation PN: Rev 00
 Operation Manual AccuPulse is a trademark of PN: 1500-00-02 Rev 00 10 Capital Drive Wallingford, CT 06492 U.S.A. www.clinicaldynamics.com Phone: 203.269.0090 Facsimile: 203.269.3402 Operation Manual Notices
Operation Manual AccuPulse is a trademark of PN: 1500-00-02 Rev 00 10 Capital Drive Wallingford, CT 06492 U.S.A. www.clinicaldynamics.com Phone: 203.269.0090 Facsimile: 203.269.3402 Operation Manual Notices
Oregon Scientific Wrist Blood Pressure Monitor (BPW211)
 Oregon Scientific Wrist Blood Pressure Monitor (BPW211) User Manual TABLE OF CONTENTS Introduction... 2 Key features... 2 Main unit... 2 Plastic storage container... 2 LCD display symbols... 3 Safety and
Oregon Scientific Wrist Blood Pressure Monitor (BPW211) User Manual TABLE OF CONTENTS Introduction... 2 Key features... 2 Main unit... 2 Plastic storage container... 2 LCD display symbols... 3 Safety and
Vital Signs Monitoring
 Vital Signs Monitoring Practical Skills Teaching Year 2 Medical Students MB BCh 2013-2014 Contents Introduction to workshop... 4 Overall Session Aim... 5 Intended Learning Outcomes... 5 Workshop Structure
Vital Signs Monitoring Practical Skills Teaching Year 2 Medical Students MB BCh 2013-2014 Contents Introduction to workshop... 4 Overall Session Aim... 5 Intended Learning Outcomes... 5 Workshop Structure
Circulation and Respiration: Vital Signs Student Version
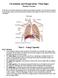 Circulation and Respiration: Vital Signs Student Version In this lab, you will learn about the circulatory and respiratory systems. You will test the capacity of your lungs, measure your blood pressure
Circulation and Respiration: Vital Signs Student Version In this lab, you will learn about the circulatory and respiratory systems. You will test the capacity of your lungs, measure your blood pressure
Declaration of Equivalence Form
 Declaration of Equivalence Form DECLARATION OF BLOOD PRESSURE MEASURING DEVICE EQUIVALENCE 2013 A SIGNED COPY WILL BE POSTED ON THE www.dableducatianal.argwebslte SECTION A - Please complete all items.
Declaration of Equivalence Form DECLARATION OF BLOOD PRESSURE MEASURING DEVICE EQUIVALENCE 2013 A SIGNED COPY WILL BE POSTED ON THE www.dableducatianal.argwebslte SECTION A - Please complete all items.
6833_INSTRUCTIONS MANUAL DIGITAL WIRST BLOOD PRESSURE
 6833_INSTRUCTIONS MANUAL DIGITAL WIRST BLOOD PRESSURE PURCHASE ACKNOWLEDGEMENT JOCCA thanks you for the trust placed in the purchase of our product and we are certain that you will always be satisfied
6833_INSTRUCTIONS MANUAL DIGITAL WIRST BLOOD PRESSURE PURCHASE ACKNOWLEDGEMENT JOCCA thanks you for the trust placed in the purchase of our product and we are certain that you will always be satisfied
Talking Wrist TypeBlood Pressure Monitor. Talking Wrist Type Blood Pressure Monitor Model: BPW810. User Manual
 Talking Wrist TypeBlood Pressure Monitor Talking Wrist Type Blood Pressure Monitor Model: BPW810 User Manual TALKING WRIST TYPE BLOOD PRESSURE MONITOR CONTENTS Model: BPW810 USER MANUAL Deleting the Latest
Talking Wrist TypeBlood Pressure Monitor Talking Wrist Type Blood Pressure Monitor Model: BPW810 User Manual TALKING WRIST TYPE BLOOD PRESSURE MONITOR CONTENTS Model: BPW810 USER MANUAL Deleting the Latest
REF CH-302B IDENTIFICATION OF PARTS
 English TM INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR REF CH-302B IDENTIFICATION OF PARTS Exhaust button Air-release system Bulb Systolic blood pressure display section Diastolic blood pressure/pulse
English TM INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR REF CH-302B IDENTIFICATION OF PARTS Exhaust button Air-release system Bulb Systolic blood pressure display section Diastolic blood pressure/pulse
hlon Invasive Blood Pressure OEM board NIBP2O2O UP
 ": 'PnR Meaüintec'ot'n, r l Teöl1*{-?iflItion I D#.h;;. äii hlon Invasive Blood Pressure OEM board NIBP2O2O UP Hardware-Version: Firmware-Version: A1 6.3 lssued: L. Engel Date: 05.02.2018 Approved: A.
": 'PnR Meaüintec'ot'n, r l Teöl1*{-?iflItion I D#.h;;. äii hlon Invasive Blood Pressure OEM board NIBP2O2O UP Hardware-Version: Firmware-Version: A1 6.3 lssued: L. Engel Date: 05.02.2018 Approved: A.
Blood Pressure Cuffs
 Blood Pressure Cuffs Blood Pressure Cuffs from GBUK Healthcare Measuring and monitoring blood pressure is a vital element in accurate diagnosis and care management. From the simplest manual aneroid sphygmomanometer,
Blood Pressure Cuffs Blood Pressure Cuffs from GBUK Healthcare Measuring and monitoring blood pressure is a vital element in accurate diagnosis and care management. From the simplest manual aneroid sphygmomanometer,
Module No GETTING ACQUAINTED GENERAL GUIDE TIMEKEEPING
 Module No. 2196 2196-1 GETTING ACQUAINTED Congratulations upon your selection of this CASIO Pressure Monitor Watch (BP-1B, Module No. 2196). To get the most out of your purchase, be sure to carefully read
Module No. 2196 2196-1 GETTING ACQUAINTED Congratulations upon your selection of this CASIO Pressure Monitor Watch (BP-1B, Module No. 2196). To get the most out of your purchase, be sure to carefully read
Device Equivalence Evaluation Form Comparison of the Omron RS2 (HEM 6121 E) with the Omron RS3 (HEM 6130 E)
 Device Equivalence Evaluation Form Comparison of the Omron RS2 (HEM 6121 E) with the Omron RS3 (HEM 6130 E) Pictures Validation ESH 2010 Device 1 Criteria Same Criteria Accuracy BP accuracy ± 3 mmhg 1,
Device Equivalence Evaluation Form Comparison of the Omron RS2 (HEM 6121 E) with the Omron RS3 (HEM 6130 E) Pictures Validation ESH 2010 Device 1 Criteria Same Criteria Accuracy BP accuracy ± 3 mmhg 1,
Observing Waves, Their Properties, and Relationships
 Observing Waves, Their Properties, and Relationships Part I: Setting Up the Activity 1. Refer to the material list for materials needed. 2. To successfully conduct this activity, you will need an area
Observing Waves, Their Properties, and Relationships Part I: Setting Up the Activity 1. Refer to the material list for materials needed. 2. To successfully conduct this activity, you will need an area
Declaration of Equivalence Form
 Declaration of Equivalence Form DECLARATION OF BLOOD PRESSURE MEASURING DEVICE EQUIVALENCE 2013 SECTION A - Please complete all items. A SIGNED COPY WILL BE POSTED ON THE www.dableducational.org WEBSITE
Declaration of Equivalence Form DECLARATION OF BLOOD PRESSURE MEASURING DEVICE EQUIVALENCE 2013 SECTION A - Please complete all items. A SIGNED COPY WILL BE POSTED ON THE www.dableducational.org WEBSITE
Kroger Wrist Blood Pressure Monitor Manual Wordpress
 We have made it easy for you to find a PDF Ebooks without any digging. And by having access to our ebooks online or by storing it on your computer, you have convenient answers with kroger wrist blood pressure
We have made it easy for you to find a PDF Ebooks without any digging. And by having access to our ebooks online or by storing it on your computer, you have convenient answers with kroger wrist blood pressure
Model UB-328. Wrist Digital Blood Pressure Monitor. Instruction Manual. Manuel d instructions. Manual de Instrucciones. Manuale di Istruzioni
 Wrist Digital Blood Pressure Monitor Model UB-328 Instruction Manual Manuel d instructions Manual de Instrucciones Manuale di Istruzioni WM+PD4000913 ENGLISH Dear Customers Congratulations. You have purchased
Wrist Digital Blood Pressure Monitor Model UB-328 Instruction Manual Manuel d instructions Manual de Instrucciones Manuale di Istruzioni WM+PD4000913 ENGLISH Dear Customers Congratulations. You have purchased
User Manual DMD1001. Arm Type. Digital Blood Pressure Monitor. version:1.0
 User Manual Digital Blood Pressure Monitor version:1.0 DMD1001 Arm Type WWW.VIVEHEALTH.COM Contact us 24/7 at www.vivehealth.com Thank you very much for selecting VIVE Digital Blood Pressure Monitor DMD1001
User Manual Digital Blood Pressure Monitor version:1.0 DMD1001 Arm Type WWW.VIVEHEALTH.COM Contact us 24/7 at www.vivehealth.com Thank you very much for selecting VIVE Digital Blood Pressure Monitor DMD1001
INSTRUCTION MANUAL. Automatic Blood Pressure Monitor with Fit Cuff. =Fit Cuff=!"#$% IA1B. Model
 IA1B INSTRUCTION MANUAL Automatic Blood Pressure Monitor with Fit Cuff =Fit Cuff=!"#$% Model IA1B Contents Introduction... 2 Notes on Safety... 3 Know Your Unit... 5 Quick Reference Guide... 7 Initial
IA1B INSTRUCTION MANUAL Automatic Blood Pressure Monitor with Fit Cuff =Fit Cuff=!"#$% Model IA1B Contents Introduction... 2 Notes on Safety... 3 Know Your Unit... 5 Quick Reference Guide... 7 Initial
Drilling Efficiency Utilizing Coriolis Flow Technology
 Session 12: Drilling Efficiency Utilizing Coriolis Flow Technology Clement Cabanayan Emerson Process Management Abstract Continuous, accurate and reliable measurement of drilling fluid volumes and densities
Session 12: Drilling Efficiency Utilizing Coriolis Flow Technology Clement Cabanayan Emerson Process Management Abstract Continuous, accurate and reliable measurement of drilling fluid volumes and densities
2015 Guidelines Summary HeartSine samaritan PAD Automated External Defibrillators
 2015 Guidelines Summary HeartSine samaritan PAD Automated External Defibrillators This document provides a summary of the 2015 guidelines and how the HeartSine samaritan PAD range of products complies
2015 Guidelines Summary HeartSine samaritan PAD Automated External Defibrillators This document provides a summary of the 2015 guidelines and how the HeartSine samaritan PAD range of products complies
The RICH LIFE Project: Blood Pressure Training Certification Checklists
 December 2016 to September 2020 The RICH LIFE Project: Blood Pressure Training Certification Checklists Reducing Inequities in Care of Hypertension: Lifestyle Improvement for Everyone PRINCIPAL INVESTIGATORS:
December 2016 to September 2020 The RICH LIFE Project: Blood Pressure Training Certification Checklists Reducing Inequities in Care of Hypertension: Lifestyle Improvement for Everyone PRINCIPAL INVESTIGATORS:
User Manual DMD1001. Arm Type. Digital Blood Pressure Monitor. version:1.0
 User Manual Digital Blood Pressure Monitor version:1.0 DMD1001 Arm Type Thank you very much for selecting VIVE Digital Blood Pressure Monitor DMD1001 WWW.VIVEHEALTH.COM Contact us 24/7 at www.vivehealth.com
User Manual Digital Blood Pressure Monitor version:1.0 DMD1001 Arm Type Thank you very much for selecting VIVE Digital Blood Pressure Monitor DMD1001 WWW.VIVEHEALTH.COM Contact us 24/7 at www.vivehealth.com
Accutorr. Quick Reference Guide
 Accutorr Note: The Accutorr 3 is not intended as a replacement to the Operating Instructions. Prior to operating the equipment, the user must be familiar with the Operating Instructions Manual contents.
Accutorr Note: The Accutorr 3 is not intended as a replacement to the Operating Instructions. Prior to operating the equipment, the user must be familiar with the Operating Instructions Manual contents.
(12) United States Patent
 (12) United States Patent Nissilii et al. USOO6443905B1 (10) Patent No.: (45) Date of Patent: Sep. 3, 2002 (54) METHOD AND ARRANGEMENT FOR BLOOD PRESSURE MEASUREMENT (75) Inventors: Seppo Nissilä; Eija
(12) United States Patent Nissilii et al. USOO6443905B1 (10) Patent No.: (45) Date of Patent: Sep. 3, 2002 (54) METHOD AND ARRANGEMENT FOR BLOOD PRESSURE MEASUREMENT (75) Inventors: Seppo Nissilä; Eija
Instructions for use. 3year. Wrist MOBIL BASIS. The monitor for first users To be used on the wrist. Also suitable for: WARRANTY. Clinically validated
 Instructions for use Wrist MOBIL BASIS The monitor for first users To be used on the wrist Arrhythmia detection Clinically validated 3year WARRANTY Also suitable for: Messgenauigkeit klinisch validiert
Instructions for use Wrist MOBIL BASIS The monitor for first users To be used on the wrist Arrhythmia detection Clinically validated 3year WARRANTY Also suitable for: Messgenauigkeit klinisch validiert
Essential Performance for MED rd ed. PSES San Diego chapter meeting December 11, 2012
 Essential Performance for MED 601-1 3 rd ed. Grant Schmidbauer, Nemko USA, Inc. PSES San Diego chapter meeting December 11, 2012 2012 PSES San Diego Chapter http://www.psessymposium.org/ 1 3.27 Essential
Essential Performance for MED 601-1 3 rd ed. Grant Schmidbauer, Nemko USA, Inc. PSES San Diego chapter meeting December 11, 2012 2012 PSES San Diego Chapter http://www.psessymposium.org/ 1 3.27 Essential
VGP-4300-G. Travel Pulse. Blood Pressure Monitor for Travel
 VGP-4300-G Travel Pulse Blood Pressure Monitor for Travel Congratulations on your Vitagoods purchase. The Travel Pulse Blood Pressure Monitor, will allow you to measure vital blood pressure parameters
VGP-4300-G Travel Pulse Blood Pressure Monitor for Travel Congratulations on your Vitagoods purchase. The Travel Pulse Blood Pressure Monitor, will allow you to measure vital blood pressure parameters
Blease900 Ventilator PATIENT CENTERED VENTILATION
 Blease900 Ventilator PATIENT CENTERED VENTILATIN PATIENT CENTERED VENTILATIN From the first Blease/Manley ventilator sixty years ago, Spacelabs Healthcare has built a heritage of innovative and intuitive
Blease900 Ventilator PATIENT CENTERED VENTILATIN PATIENT CENTERED VENTILATIN From the first Blease/Manley ventilator sixty years ago, Spacelabs Healthcare has built a heritage of innovative and intuitive
Declaration of Equivalence Form
 Declaration of Equivalence Form DECLARATION OF BLOOD PRESSURE MEASURING DEVICE EQUIVALENCE 2013 SECTION A - Please complete all items. A SIGNED COPY WILL BE POSTED ON THE www.dableducational.org WEBSITE
Declaration of Equivalence Form DECLARATION OF BLOOD PRESSURE MEASURING DEVICE EQUIVALENCE 2013 SECTION A - Please complete all items. A SIGNED COPY WILL BE POSTED ON THE www.dableducational.org WEBSITE
Digital Blood Pressure Monitor. Model UA-705. Instruction Manual. Manuel d instructions. Manual de Instrucciones. Manuale di Istruzioni 1WMPD B
 Digital Blood Pressure Monitor Model UA-705 Instruction Manual Manuel d instructions Manual de Instrucciones Manuale di Istruzioni 1WMPD4000988B ENGLISH Contents Dear Customers... 2 Preliminary Remarks...
Digital Blood Pressure Monitor Model UA-705 Instruction Manual Manuel d instructions Manual de Instrucciones Manuale di Istruzioni 1WMPD4000988B ENGLISH Contents Dear Customers... 2 Preliminary Remarks...
Digital Blood Pressure Monitor. Model UA-787
 Digital Blood Pressure Monitor Model UA-787 Instruction Manual Manuel d instructions Manual de Instrucciones Manuale di Istruzioni UA-787EX-C WM:PD4000287 Preliminary Remarks This device conforms to the
Digital Blood Pressure Monitor Model UA-787 Instruction Manual Manuel d instructions Manual de Instrucciones Manuale di Istruzioni UA-787EX-C WM:PD4000287 Preliminary Remarks This device conforms to the
Device Equivalence Evaluation Form
 Device Equivalence Evaluation Form Comparison of the SEJOY BP-1209 with the SEJOY BP-1307 Devices Item 9 SEJOY BP-1209 SEJOY BP-1307 Pictures Display Image Validation ESH 2010 Category Upper arm blood
Device Equivalence Evaluation Form Comparison of the SEJOY BP-1209 with the SEJOY BP-1307 Devices Item 9 SEJOY BP-1209 SEJOY BP-1307 Pictures Display Image Validation ESH 2010 Category Upper arm blood
Identification of the dynamic component of the finger arterial pressure-volume relationship
 Modelling in Medicine and Biology VI 153 Identification of the dynamic component of the finger arterial pressure-volume relationship J. Talts, R. Raamat & K. Jagomägi Department of Physiology, University
Modelling in Medicine and Biology VI 153 Identification of the dynamic component of the finger arterial pressure-volume relationship J. Talts, R. Raamat & K. Jagomägi Department of Physiology, University
Device Equivalence Evaluation Form
 Comparison of the Braun BP6100 with the Transtek TMB-986 Devices Braun BP6100 Transtek TMB-986 Pictures Display Validation ESH 2002 Device 1 Criteria Buttons/Switches Settings Mode 10 Analysis Average
Comparison of the Braun BP6100 with the Transtek TMB-986 Devices Braun BP6100 Transtek TMB-986 Pictures Display Validation ESH 2002 Device 1 Criteria Buttons/Switches Settings Mode 10 Analysis Average
Using an Adaptive Thresholding Algorithm to Detect CA1 Hippocampal Sharp Wave Ripples. Jay Patel. Michigan State University
 Using an Adaptive Thresholding Algorithm to Detect CA1 Hippocampal Sharp Wave Ripples Jay Patel Michigan State University Department of Physics and Astronomy, University of California, Los Angeles 2013
Using an Adaptive Thresholding Algorithm to Detect CA1 Hippocampal Sharp Wave Ripples Jay Patel Michigan State University Department of Physics and Astronomy, University of California, Los Angeles 2013
Device Equivalence Evaluation Form
 Comparison of the Braun BP6200 with the Transtek TMB-986 Devices Braun BP6200 Transtek TMB-986 Pictures Display Validation ESH 2002 Device 1 Criteria Buttons/Switches Settings Mode 10 Analysis Average
Comparison of the Braun BP6200 with the Transtek TMB-986 Devices Braun BP6200 Transtek TMB-986 Pictures Display Validation ESH 2002 Device 1 Criteria Buttons/Switches Settings Mode 10 Analysis Average
ENHANCED PARKWAY STUDY: PHASE 2 CONTINUOUS FLOW INTERSECTIONS. Final Report
 Preparedby: ENHANCED PARKWAY STUDY: PHASE 2 CONTINUOUS FLOW INTERSECTIONS Final Report Prepared for Maricopa County Department of Transportation Prepared by TABLE OF CONTENTS Page EXECUTIVE SUMMARY ES-1
Preparedby: ENHANCED PARKWAY STUDY: PHASE 2 CONTINUOUS FLOW INTERSECTIONS Final Report Prepared for Maricopa County Department of Transportation Prepared by TABLE OF CONTENTS Page EXECUTIVE SUMMARY ES-1
MODEL CH-432B INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR. - Eng 1 - English Español Português Deutch. Italiano Français CH-432B
 English Español Português Deutch INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR MODEL CH-432B CH-432B POWER START Italiano Français - Eng 1 - CLEANING METHOD OF CUFF After cleaning the cuff with
English Español Português Deutch INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR MODEL CH-432B CH-432B POWER START Italiano Français - Eng 1 - CLEANING METHOD OF CUFF After cleaning the cuff with
English Español Português Italiano Deutch Français INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR MODEL CH-656C. - Eng 1 -
 English Español Português Italiano Deutch Français INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR MODEL CH-656C M - Eng 1 - CLEANING METHOD OF CUFF After cleaning the cuff with a neutral detergent,
English Español Português Italiano Deutch Français INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR MODEL CH-656C M - Eng 1 - CLEANING METHOD OF CUFF After cleaning the cuff with a neutral detergent,
Talking Wrist Style Blood Pressure Monitor Model INS-LAB-RevB08
 Talking Wrist Style Blood Pressure Monitor Model 1145 1145-INS-LAB-RevB08 Contents Introduction...2 Key Features... 2 Front View... 2 Rear View... 3 LCD Symbols... 3 Safety and Care Instructions... 4 Safety
Talking Wrist Style Blood Pressure Monitor Model 1145 1145-INS-LAB-RevB08 Contents Introduction...2 Key Features... 2 Front View... 2 Rear View... 3 LCD Symbols... 3 Safety and Care Instructions... 4 Safety
Legendre et al Appendices and Supplements, p. 1
 Legendre et al. 2010 Appendices and Supplements, p. 1 Appendices and Supplement to: Legendre, P., M. De Cáceres, and D. Borcard. 2010. Community surveys through space and time: testing the space-time interaction
Legendre et al. 2010 Appendices and Supplements, p. 1 Appendices and Supplement to: Legendre, P., M. De Cáceres, and D. Borcard. 2010. Community surveys through space and time: testing the space-time interaction
INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR
 INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR Model HEM-412C TABLE OF CONTENTS Introduction 3 Know Your Unit 4 Quick Reference Guide 5 Battery Installation/Replacement 6 How To Apply The Arm
INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR Model HEM-412C TABLE OF CONTENTS Introduction 3 Know Your Unit 4 Quick Reference Guide 5 Battery Installation/Replacement 6 How To Apply The Arm
Non-Invasive Blood Pressure
 Non-Invasive Blood Pressure 9650-0214-01 Rev. G The issue date or revision level for this operation guide is shown on the front cover. ZOLL and M Series are registered trademarks of ZOLL Medical Corporation.
Non-Invasive Blood Pressure 9650-0214-01 Rev. G The issue date or revision level for this operation guide is shown on the front cover. ZOLL and M Series are registered trademarks of ZOLL Medical Corporation.
Hydraulic and Economic Analysis of Real Time Control
 Hydraulic and Economic Analysis of Real Time Control Tom Walski 1, Enrico Creaco 2 1 Bentley Systems, Incorporated, 3 Brian s Place, Nanticoke, PA, USA 2 Dipartimento di Ingegneria Civile ed Architettura,
Hydraulic and Economic Analysis of Real Time Control Tom Walski 1, Enrico Creaco 2 1 Bentley Systems, Incorporated, 3 Brian s Place, Nanticoke, PA, USA 2 Dipartimento di Ingegneria Civile ed Architettura,
Blood Pressure Monitoring: Arterial Line
 Approved by: Blood Pressure Monitoring: Arterial Line Gail Cameron Senior Director, Operations, Maternal, Neonatal & Child Health Programs Dr. Ensenat Medical Director, Neonatology Neonatal Nursery Policy
Approved by: Blood Pressure Monitoring: Arterial Line Gail Cameron Senior Director, Operations, Maternal, Neonatal & Child Health Programs Dr. Ensenat Medical Director, Neonatology Neonatal Nursery Policy
CATALOG NO. FIG DESCRIPTION NIB A Replacement Hose for Critikon-type devices-models 8100, 8100T, 9300XL, & 1846SX. 12
 NIBP REPLACEMENT HOSES NIBP A B C D E F CATALOG NO. FIG DESCRIPTION NIB100012 A Replacement Hose for Critikon-type devices-models 8100, 8100T, 9300XL, & 1846SX. 12 NIB300012 B Replacement Hose for Criticare
NIBP REPLACEMENT HOSES NIBP A B C D E F CATALOG NO. FIG DESCRIPTION NIB100012 A Replacement Hose for Critikon-type devices-models 8100, 8100T, 9300XL, & 1846SX. 12 NIB300012 B Replacement Hose for Criticare
A CO 2 Waveform Simulator For Evaluation and Testing of Respiratory Gas Analyzers
 2011 ROCKY MOUNTAIN NASA SPACE GRANT CONSORTIUM 1 A CO 2 Waveform Simulator For Evaluation and Testing of Respiratory Gas Analyzers Christina Long, and Joseph Orr, Ph.D. Department of Bioengineering, University
2011 ROCKY MOUNTAIN NASA SPACE GRANT CONSORTIUM 1 A CO 2 Waveform Simulator For Evaluation and Testing of Respiratory Gas Analyzers Christina Long, and Joseph Orr, Ph.D. Department of Bioengineering, University
ICS Supersedes EN :1997. English Version
 EUROPEAN STANDARD NORME EUROPÉENNE EUROPÄISCHE NORM EN 1060-3:1997+A2 November 2009 ICS 11.040.55 Supersedes EN 1060-3:1997 English Version Non-invasive sphygmomanometers - Part 3: Supplementary requirements
EUROPEAN STANDARD NORME EUROPÉENNE EUROPÄISCHE NORM EN 1060-3:1997+A2 November 2009 ICS 11.040.55 Supersedes EN 1060-3:1997 English Version Non-invasive sphygmomanometers - Part 3: Supplementary requirements
User Manual DMD1001 1
 User Manual DMD1001 1 TABLE OF CONTENTS PRODUCT DESCRIPTION... WHAT S INCLUDED... PARTS LIST... POWER SUPPLY... REPLACING BATTERIES... MEASUREMENT PRINCIPLE... OPERATING INSTRUCTIONS - SETTING DATE, TIME,
User Manual DMD1001 1 TABLE OF CONTENTS PRODUCT DESCRIPTION... WHAT S INCLUDED... PARTS LIST... POWER SUPPLY... REPLACING BATTERIES... MEASUREMENT PRINCIPLE... OPERATING INSTRUCTIONS - SETTING DATE, TIME,
VER: A00. Model No:BA2010
 VER: A00 Model No:BA2010 Before Using the Monitor Introduction...1 Symbols Use...2 Important Safety Notes...3 Konw Your Unit...45 IHB Introduction...6 Before Taking A Measurement...7 Quick Reference Guide...8
VER: A00 Model No:BA2010 Before Using the Monitor Introduction...1 Symbols Use...2 Important Safety Notes...3 Konw Your Unit...45 IHB Introduction...6 Before Taking A Measurement...7 Quick Reference Guide...8
W. D. A. SMITH Research Department of Anaesthetics, Royal College of Surgeons of England, London
 Brit. 1. Anaesth. (1962), 34, 136 A METHOD OF RECORDING SYSTOLIC BLOOD PRESSURE BY W. D. A. SMITH Research Department of Anaesthetics, Royal College of Surgeons of England, London The method of recording
Brit. 1. Anaesth. (1962), 34, 136 A METHOD OF RECORDING SYSTOLIC BLOOD PRESSURE BY W. D. A. SMITH Research Department of Anaesthetics, Royal College of Surgeons of England, London The method of recording
NASG REMOVAL. Removing the NASG
 NASG REMOVAL Removing the NASG DISCLAIMER: We highly recommend using the training materials as part of a hands-on training program led by an experienced trainer with NASG experience. Neither UCSF, nor
NASG REMOVAL Removing the NASG DISCLAIMER: We highly recommend using the training materials as part of a hands-on training program led by an experienced trainer with NASG experience. Neither UCSF, nor
The British Hypertension Society protocol for the evaluation of blood pressure measuring devices
 The British Hypertension Society protocol for the evaluation of blood pressure measuring devices Eoin O'Brien*, James Petrie*?, William Littler*$, Michael de Swiet*, Paul L. Padfield*l, Douglas G. Altmanu,
The British Hypertension Society protocol for the evaluation of blood pressure measuring devices Eoin O'Brien*, James Petrie*?, William Littler*$, Michael de Swiet*, Paul L. Padfield*l, Douglas G. Altmanu,
DEVELOPMENT AND TESTING OF AIR EXCHANGE RATE METHODS TO IMPROVE COST EFFECTIVENESS WHILE MAINTAINING ACCURACY
 DEVELOPMENT AND TESTING OF AIR EXCHANGE RATE METHODS TO IMPROVE COST EFFECTIVENESS WHILE MAINTAINING ACCURACY Prepared by: Joachim Eberharter (Joachim.Eberharter@tetratech.com) and James Elliot (Tetra
DEVELOPMENT AND TESTING OF AIR EXCHANGE RATE METHODS TO IMPROVE COST EFFECTIVENESS WHILE MAINTAINING ACCURACY Prepared by: Joachim Eberharter (Joachim.Eberharter@tetratech.com) and James Elliot (Tetra
Fully Automatic Blood Pressure Monitor
 Fully Automatic Blood Pressure Monitor User Manual BPM1KTL Series Content Introduction 2 Parts 3 Setting up your Blood Pressure Monitor Loading Battery 4-6 4 Connecting the Cuff 4 Clock and Date Adjustment
Fully Automatic Blood Pressure Monitor User Manual BPM1KTL Series Content Introduction 2 Parts 3 Setting up your Blood Pressure Monitor Loading Battery 4-6 4 Connecting the Cuff 4 Clock and Date Adjustment
Analyses of the Scoring of Writing Essays For the Pennsylvania System of Student Assessment
 Analyses of the Scoring of Writing Essays For the Pennsylvania System of Student Assessment Richard Hill The National Center for the Improvement of Educational Assessment, Inc. April 4, 2001 Revised--August
Analyses of the Scoring of Writing Essays For the Pennsylvania System of Student Assessment Richard Hill The National Center for the Improvement of Educational Assessment, Inc. April 4, 2001 Revised--August
P extremities to apply sufficient pressure to a limb to
 EEE TRANSACTONS ON BOMEDCAL ENGNEERNG, VOL. 35, NO. 4, APRL 1988 22 1 mproved Tracking of Limb Occlusion Pressure for Surgical Tourniquets AbstractConstantpressure tourniquets are widely used to occlude
EEE TRANSACTONS ON BOMEDCAL ENGNEERNG, VOL. 35, NO. 4, APRL 1988 22 1 mproved Tracking of Limb Occlusion Pressure for Surgical Tourniquets AbstractConstantpressure tourniquets are widely used to occlude
HAEMODYNAMIC MONITORING SETUP IN THE CATHETERISATION LABORATORY MAHAT SAPION
 HAEMODYNAMIC MONITORING SETUP IN THE CATHETERISATION LABORATORY MAHAT SAPION Haemodynamic Monitoring Functions of monitoring to detect that there is a problem blood pressure compensatory sympathetic stimulation
HAEMODYNAMIC MONITORING SETUP IN THE CATHETERISATION LABORATORY MAHAT SAPION Haemodynamic Monitoring Functions of monitoring to detect that there is a problem blood pressure compensatory sympathetic stimulation
A Method Quantitatively Evaluating on Technical Progress of Students in Ship Handling Simulator Training ABSTRACT
 A Method Quantitatively Evaluating on Technical Progress of Students in Ship Handling Simulator Training Kinzo INOUE, Hideo USUI, Rong MA and Cemil YURTOREN Kobe University of Mercantile Marine 5-1-1 Fukae-minami
A Method Quantitatively Evaluating on Technical Progress of Students in Ship Handling Simulator Training Kinzo INOUE, Hideo USUI, Rong MA and Cemil YURTOREN Kobe University of Mercantile Marine 5-1-1 Fukae-minami
Critical Gust Pressures on Tall Building Frames-Review of Codal Provisions
 Dr. B.Dean Kumar Dept. of Civil Engineering JNTUH College of Engineering Hyderabad, INDIA bdeankumar@gmail.com Dr. B.L.P Swami Dept. of Civil Engineering Vasavi College of Engineering Hyderabad, INDIA
Dr. B.Dean Kumar Dept. of Civil Engineering JNTUH College of Engineering Hyderabad, INDIA bdeankumar@gmail.com Dr. B.L.P Swami Dept. of Civil Engineering Vasavi College of Engineering Hyderabad, INDIA
ProBP 2400 Digital Blood Pressure Device
 Advancing Frontline Care ProBP 2400 Digital Blood Pressure Device Directions for use EN 1 powered by CAUTION Federal law restricts this device to sale by or on the order of a physician or licensed healthcare
Advancing Frontline Care ProBP 2400 Digital Blood Pressure Device Directions for use EN 1 powered by CAUTION Federal law restricts this device to sale by or on the order of a physician or licensed healthcare
LD22. Digital Blood Pressure Monitor Instruction Manual
 LD22 Digital Blood Pressure Monitor Instruction Manual Ciśnieniomierz elektroniczny półautomatyczny LD do pomiaru ciśnienia tętniczego krwi i pulsu Instrukcja Obsługi POL TABLE OF CONTENTS PARTS AND COMPONENTS............................................3
LD22 Digital Blood Pressure Monitor Instruction Manual Ciśnieniomierz elektroniczny półautomatyczny LD do pomiaru ciśnienia tętniczego krwi i pulsu Instrukcja Obsługi POL TABLE OF CONTENTS PARTS AND COMPONENTS............................................3
Challenges in Measuring CSF Flow with MRI
 Challenges in Measuring CSF Flow with MRI Rafeeque A. Bhadelia, MD HARVARD MEDICAL SCHOOL Background MR imaging of CSF flow is difficult to use in clinical practice This is because: Qualitative visual
Challenges in Measuring CSF Flow with MRI Rafeeque A. Bhadelia, MD HARVARD MEDICAL SCHOOL Background MR imaging of CSF flow is difficult to use in clinical practice This is because: Qualitative visual
ESTABLISHED OVER 150 YEARS FROM Accuracy starts in the name SPHYGMOMANOMETERS
 ESTABLISHED OVER 150 YEARS FROM 1859 SPHYGMOMANOMETERS TM accuracy without mercury None of the fragility of aneroid devices greenlight 300 model Code 0702 confidence without mercury self calibration without
ESTABLISHED OVER 150 YEARS FROM 1859 SPHYGMOMANOMETERS TM accuracy without mercury None of the fragility of aneroid devices greenlight 300 model Code 0702 confidence without mercury self calibration without
Wave Motion. interference destructive interferecne constructive interference in phase. out of phase standing wave antinodes resonant frequencies
 Wave Motion Vocabulary mechanical waves pulse continuous periodic wave amplitude period wavelength period wave velocity phase transverse wave longitudinal wave intensity displacement amplitude phase velocity
Wave Motion Vocabulary mechanical waves pulse continuous periodic wave amplitude period wavelength period wave velocity phase transverse wave longitudinal wave intensity displacement amplitude phase velocity
Oregon Scientific Wrist Blood Pressure Monitor (BPW120)
 Oregon Scientific Wrist Blood Pressure Monitor (BPW120) USER MANUAL TABLE OF CONTENTS Introduction... 2 Key features... 2 Main unit... 2 Plastic storage container... 2 LCD display symbols... 2 Safety and
Oregon Scientific Wrist Blood Pressure Monitor (BPW120) USER MANUAL TABLE OF CONTENTS Introduction... 2 Key features... 2 Main unit... 2 Plastic storage container... 2 LCD display symbols... 2 Safety and
Contents. Safety Notice. Wrist Type
 Wrist Type Contents 1 Safety Notice 2 Safety Notice... 02 Unit Illustration... 05 Important Testing Guidelines... 07 Quick Start... 08 Unit Operation... 10 Battery Installation... 10 Thank you for purchasing
Wrist Type Contents 1 Safety Notice 2 Safety Notice... 02 Unit Illustration... 05 Important Testing Guidelines... 07 Quick Start... 08 Unit Operation... 10 Battery Installation... 10 Thank you for purchasing
Engineering: Measurement Technology Pressure/Level (SCQF level 6)
 National Unit Specification: general information CODE F5KT 12 SUMMARY This Unit can be delivered as part of a National Qualification Group Award but can also be taken as a free-standing Unit. This Unit
National Unit Specification: general information CODE F5KT 12 SUMMARY This Unit can be delivered as part of a National Qualification Group Award but can also be taken as a free-standing Unit. This Unit
Let s talk about Capnography
 Let s talk about Capnography This is one of a series of articles by Keith Simpson BVSc MRCVS MIET (Electronics) discussing the practical aspects of some common monitoring techniques. Capnometry is the
Let s talk about Capnography This is one of a series of articles by Keith Simpson BVSc MRCVS MIET (Electronics) discussing the practical aspects of some common monitoring techniques. Capnometry is the
HHS Public Access Author manuscript Int J Cardiol. Author manuscript; available in PMC 2016 April 15.
 FITBIT : AN ACCURATE AND RELIABLE DEVICE FOR WIRELESS PHYSICAL ACTIVITY TRACKING Keith M. Diaz 1, David J. Krupka 1, Melinda J Chang 1, James Peacock 1, Yao Ma 2, Jeff Goldsmith 2, Joseph E. Schwartz 1,
FITBIT : AN ACCURATE AND RELIABLE DEVICE FOR WIRELESS PHYSICAL ACTIVITY TRACKING Keith M. Diaz 1, David J. Krupka 1, Melinda J Chang 1, James Peacock 1, Yao Ma 2, Jeff Goldsmith 2, Joseph E. Schwartz 1,
LD6. Digital Blood Pressure Monitor Instruction Manual
 LD6 Digital Blood Pressure Monitor Instruction Manual Ciśnieniomierz elektroniczny automatyczny do pomiaru ciśnienia tętniczego krwi i pulsu Instrukcja obsługi POL TABLE OF CONTENTS PARTS AND COMPONENTS.............................................
LD6 Digital Blood Pressure Monitor Instruction Manual Ciśnieniomierz elektroniczny automatyczny do pomiaru ciśnienia tętniczego krwi i pulsu Instrukcja obsługi POL TABLE OF CONTENTS PARTS AND COMPONENTS.............................................
Blood Pressure Monitor Instruction Manual
 Blood Pressure Monitor Instruction Manual BMP-2244BT Manual Ver.: 1.0 Issuing Date: 2016/09/22 2016. All rights reserved. Thank you for selecting iprovén arm type Blood Pressure Monitor BPM-2244BT. The
Blood Pressure Monitor Instruction Manual BMP-2244BT Manual Ver.: 1.0 Issuing Date: 2016/09/22 2016. All rights reserved. Thank you for selecting iprovén arm type Blood Pressure Monitor BPM-2244BT. The
Omron Blood Pressure Monitor Need Manual Bp760 Automatic
 Omron Blood Pressure Monitor Need Manual Bp760 Automatic Omron 7 series upper arm blood pressure monitor is a good monitor with 7 Series have 3 different models BP761, BP760N and BP760 with minor variations.
Omron Blood Pressure Monitor Need Manual Bp760 Automatic Omron 7 series upper arm blood pressure monitor is a good monitor with 7 Series have 3 different models BP761, BP760N and BP760 with minor variations.
Traceable calibration of automatic weighing instruments in dynamic operation
 Traceable calibration of automatic weighing instruments in dynamic operation Matej Grum* Metrology Institute of the Republic of Slovenia, Grudnovo nabrežje 17, 1000 Ljubljana, Slovenia Abstract. The article
Traceable calibration of automatic weighing instruments in dynamic operation Matej Grum* Metrology Institute of the Republic of Slovenia, Grudnovo nabrežje 17, 1000 Ljubljana, Slovenia Abstract. The article
ANZAI AZ-733VI. Respiratory Gating System. since 1976
 ANZAI since 1976 Respiratory Gating System AZ-733VI Specifications AZ-733VI Dimension Configuration Options Sensor Port W:260 D:230 H:126mm Sensor Port Laser Sensor & Fixing Arm Relay Box Wave Monitor
ANZAI since 1976 Respiratory Gating System AZ-733VI Specifications AZ-733VI Dimension Configuration Options Sensor Port W:260 D:230 H:126mm Sensor Port Laser Sensor & Fixing Arm Relay Box Wave Monitor
boso Germany Do you? Premium quality essgeräte für die Selbstmessung I use equipment of BOSCH + SOHN to measure blood pressure.
 I use equipment of BOSCH + SOHN to measure blood pressure. Do you? If you self-test, trust the brand that 77% of all German doctors use. essgeräte für die Selbstmessung Premium quality Premium quality
I use equipment of BOSCH + SOHN to measure blood pressure. Do you? If you self-test, trust the brand that 77% of all German doctors use. essgeräte für die Selbstmessung Premium quality Premium quality
