AP* Chemistry GASES PROPERTIES OF GASES
|
|
|
- Frederica Morrison
- 6 years ago
- Views:
Transcription
1 AP* Chemistry GASES The gaseous state of matter is the simplest and best-understood state of matter. You inhale approximately 8,500 L of air each day. This amounts to about 25 lbs of air. Breathing is a three-step process: inhaling, gas exchange with the circulatory system, and exhaling. Approximately 80% pressure differences created by your body allow you to breathe. Nearly the Earth s entire atmosphere is made up of only five gases: nitrogen, oxygen, water vapor, argon, and carbon dioxide. PROPERTIES OF GASES Only FOUR quantities are needed to define the state of a gas: 1. the quantity of the gas, n (in moles) 2. the temperature of the gas, T (in KELVINS) 3. the volume of the gas, V (in liters) 4. the pressure of the gas, P (in atmospheres) A gas uniformly fills any container, is easily compressed & mixes completely with any other gas. GAS PRESSURE is a measure of the force that a gas exerts on its container. Force is the physical quantity that interferes with inertia. Gravity is the force responsible for weight. Force = mass acceleration; (Newton s 2 nd Law) The SI units follow: N = kg m/s 2 Pressure-- Force/ unit area; N/m 2 ; which is the definition of 1.0 Pascal Barometer--invented by Evangelista Torricelli in 1643; uses the height of a column of mercury to measure gas pressure (especially atmospheric P) 1 mm of Hg = 1 torr 1.00 atm = mm Hg = torr = in Hg = 14.7 psi = kpa 10 5 Pa At sea level all of the above define STANDARD PRESSURE. The SI unit of pressure is the Pascal (named after Blaise Pascal); 1 Pa = 1 N / m 2 The manometer a device for measuring the pressure of a gas in a container. The pressure of the gas is given by h [the difference in mercury levels] in units of torr (equivalent to mm Hg). a) Gas pressure = atmospheric pressure h b) Gas pressure = atmospheric pressure + h *AP is a registered trademark of the College Board, which was not involved in the production of, and does not endorse, this product by René McCormick. All rights reserved. Some art copyright Pearson Education Inc. 2014
2 Exercise 1 Pressure Conversions The pressure of a gas is measured as 49 torr. Represent this pressure in both atmospheres and Pascals. Exercise 2 Pressure Comparisons Rank the following pressures in decreasing order of magnitude (largest first, smallest last): 75 kpa, 300. torr, 0.60 atm and 350. mm Hg. GAS LAWS: THE EXPERIMENTAL BASIS BOYLE S LAW: Father of Chemistry --the volume of a confined gas is inversely proportional to the pressure exerted on the gas. ALL GASES BEHAVE IN THIS MANNER! Robert Boyle was an Irish chemist. He studied PV relationships using a J-tube set up in the multi-story entryway of his home. (Thus his was MUCH larger than the one shown right.) o P µ 1/V o pressure and volume are inversely proportional o Volume Pressure at constant temperature, the converse is also true o for a given quantity of a gas at constant temperature, the product of pressure and volume is a constant PV = k k 1 Therefore, V = = k P P which is the equation for a straight line of the type y = mx + b, where m = slope, and b is the y-intercept In this case, y = V, x = 1/P and b = 0. Check out the plot on the right (b). The data Boyle originally collected is graphed on (a) above on the right. o P 1 V 1 = P 2 V 2 is the easiest form of Boyle s law to memorize o Boyle s Law has been tested for over three centuries. It holds true only at low pressures. Gases 2
3 Exercise 3 Boyle s Law I Sulfur dioxide (SO 2 ), a gas that plays a central role in the formation of acid rain, is found in the exhaust of automobiles and power plants. Consider a L sample of gaseous SO 2 at a pressure of Pa. If the pressure is changed to Pa at a constant temperature, what will be the new volume of the gas? Ideal Gases At normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many gases such as nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxide can be treated like ideal gases within reasonable tolerances. Generally, a gas behaves more like an ideal gas at higher temperature and lower pressure, as the work which is against intermolecular forces becomes less significant compared with the particles' kinetic energy, and the size of the molecules becomes less significant compared to the empty space between them. An ideal gas is expected to have a constant value of PV, as shown by the dotted line on the graph pictured above. CO 2 shows the largest change in PV, and this change is actually quite small: PV changes from about L atm at 0.25 atm to L atm at 1.00 atm. Thus Boyle s Law is a good approximation at these relatively low pressures. So, why does CO 2 deviate from ideal behavior the most? It has more electrons, thus is more polarizable, thus has higher dispersion forces (a type of intermolecular force a.k.a. London dispersion forces or LDFs), therefore the molecules are more attracted to each other, so carbon dioxide gas deviates from ideal behavior more than oxygen or carbon dioxide do. Gases 3
4 Exercise 4 Boyle s Law II In a study to see how closely gaseous ammonia obeys Boyle s law, several volume measurements were made at various pressures, using 1.0 mol NH 3 gas at a temperature of 0ºC. Using the results listed below; calculate the Boyle s law constant for NH 3 at the various pressures. Calculate the deviation from ideal behavior in each case. Account for the trend apparent in the data. Experiment Pressure (atm) Volume (L) Exercise 5 Boyle s Law III Next, PLOT the values of PV vs. P for the six experiments in Exercise 4. Extrapolate to determine what PV equals at a hypothetical 0.00 atm pressure. Compare it to the PV vs. P graph on page 3 of these lecture notes. What is the value of the y-intercept? Gases 4
5 CHARLES S LAW: If a given quantity of gas is held at a constant pressure, then its volume is directly proportional to the absolute temperature. Must use KELVINS Why? Jacques Charles was a French physicist and the first person to fill a hot air balloon with hydrogen gas and made the first solo hot air balloon flight! o V µ T plot = straight line o V 1 T 2 = V 2 T 1 o Temperature Volume at constant pressure o This figure shows the plots of V vs. T (Celsius) for several gases. The solid lines represent experimental measurements on gases. The dashed lines represent extrapolation of the data into regions where these gases would become liquids or solids. Note that the samples of the various gases contain different numbers of moles. o What is the temperature when the volume extrapolates to zero? Sound familiar? Exercise 6 Charles s Law A sample of gas at 15ºC and 1 atm has a volume of 2.58 L. What volume will this gas occupy at 38ºC and 1 atm? Each of the balloons below contains the same number of particles in the gas phase. This is yet another example of heat em up and speed em up!. As the molecules warm, they gain kinetic energy, move faster, and thus collide with the walls of their container with more energy. (Umph! If you prefer) In this case, the walls of the container are made of rubber which can expand and contract. Gases 5
6 GAY-LUSSAC S LAW of combining volumes: volumes of gases always combine with one another in the ratio of small whole numbers, as long as volumes are measured at the same T and P. - P 1 T 2 = P 2 T 1 - Avogadro s hypothesis: equal volumes of gases under the same conditions of temperature and pressure contain equal numbers of molecules. These balloons each hold 1.0 L of gas at 25 C and 1 atm. Each balloon contains mol of gas, or gas molecules. AVOGADRO S LAW: The volume of a gas, at a given temperature and pressure, is directly proportional to the quantity of gas. - V n - n Volume at constant T & P HERE S AN EASY WAY TO MEMORIZE ALL OF THIS! Start with the combined gas law: P 1 V 1 T 2 = P 2 V 2 T 1 Memorize just this use a simple pattern to figure the rest out: Place the scientist names in alphabetical order. Boyle s Law uses the first 2 variables, Charles Law the second 2 variables & Gay-Lussac s Law the remaining combination of variables. Whichever variable doesn t appear in the formula is being held CONSTANT! Exercise 7 Avogadro s Law Suppose we have a 12.2-L sample containing 0.50 mol oxygen gas (O 2 ) at a pressure of 1 atm and a temperature of 25ºC. If all this O 2 were converted to ozone (O 3 ) at the same temperature and pressure, what would be the volume of the ozone? Gases 6
7 THE IDEAL GAS LAW Four quantities describe the state of a gas: pressure, volume, temperature, and # of moles (quantity). Combine all 3 laws: V µ nt P Replace the µ with a constant, R, and you get: PV = nrt The ideal gas law is an equation of state. R = L atm/mol K also expressed as L atm mol 1 K 1 Useful only at low Pressures and high temperatures! Guaranteed points on the AP Exam! These next exercises can all be solved with the ideal gas law, BUT, you can use another if you like! Exercise 8 Ideal Gas Law I A sample of hydrogen gas (H 2 ) has a volume of 8.56 L at a temperature of 0ºC and a pressure of 1.5 atm. Calculate the moles of H 2 molecules present in this gas sample. Exercise 9 Ideal Gas Law II Suppose we have a sample of ammonia gas with a volume of 3.5 L at a pressure of 1.68 atm. The gas is compressed to a volume of 1.35 L at a constant temperature. Use the ideal gas law to calculate the final pressure. Exercise 10 Ideal Gas Law III A sample of methane gas that has a volume of 3.8 L at 5ºC is heated to 86ºC at constant pressure. Calculate its new volume. Gases 7
8 Exercise 11 Ideal Gas Law IV A sample of diborane gas (B 2 H 6 ), a substance that bursts into flame when exposed to air, has a pressure of 345 torr at a temperature of -15ºC and a volume of 3.48 L. If conditions are changed so that the temperature is 36ºC and the pressure is 468 torr, what will be the volume of the sample? Exercise 12 Ideal Gas Law V A sample containing 0.35 mol argon gas at a temperature of 13ºC and a pressure of 568 torr is heated to 56ºC and a pressure of 897 torr. Calculate the change in volume that occurs. GAS STOICHIOMETRY Use PV = nrt to solve for the volume of one mole of gas at STP: Look familiar? This is the molar volume of a gas at STP. Work stoichiometry problems using your favorite method, dimensional analysis, mole map, the table way just work FAST! Use the ideal gas law to convert quantities that are NOT at STP. Exercise 13 Gas Stoichiometry I A sample of nitrogen gas has a volume of 1.75 L at STP. How many moles of N 2 are present? Gases 8
9 Exercise 14 Gas Stoichiometry II Quicklime (CaO) is produced by the thermal decomposition of calcium carbonate (CaCO 3 ). Calculate the volume of CO 2 at STP produced from the decomposition of 152 g CaCO 3 by the reaction CaCO 3 (s) CaO(s) + CO 2 (g) Exercise 15 Gas Stoichiometry III A sample of methane gas having a volume of 2.80 L at 25ºC and 1.65 atm was mixed with a sample of oxygen gas having a volume of 35.0 L at 31ºC and 1.25 atm. The mixture was then ignited to form carbon dioxide and water. Calculate the volume of CO 2 formed at a pressure of 2.50 atm and a temperature of 125ºC. Gases 9
10 THE DENSITY OF GASES d = m = P(MM) {for ONE mole of gas}= MM AND Molar Mass = MM = drt V RT 22.4 L P Molecular Mass kitty cat all good cats put dirt [drt] over their pee [P]. Corny? Yep! Crude and socially unacceptable? You bet! But you ll thank me later when you re flying through such gas law problems. Just remember that densities of gases are reported in g/l NOT g/ml. What is the approximate molar mass of air expressed in g/l? List 3 gases that float in air: List 3 gases that sink in air: Exercise 16 Gas Density/Molar Mass The density of a gas was measured at 1.50 atm and 27ºC and found to be 1.95 g/l. Calculate the molar mass of the gas. GAS MIXTURES AND PARTIAL PRESSURES The pressure of a mixture of gases is the sum of the pressures of the different components of the mixture: P total = P 1 + P P n John Dalton s Law of Partial Pressures also uses the concept of mole fraction, χ χ A = moles of A. moles A + moles B + moles C +... so now, P A = χ A P total The partial pressure of each gas in a mixture of gases in a container depends on the number of moles of that gas. The total pressure is the SUM of the partial pressures and depends on the total moles of gas particles present, no matter what they are! Gases 10
11 Exercise 17 Dalton s Law I Mixtures of helium and oxygen are used in scuba diving tanks to help prevent the bends. For a particular dive, 46 L He at 25ºC and 1.0 atm and 12 L O 2 at 25ºC and 1.0 atm were pumped into a tank with a volume of 5.0 L. Calculate the partial pressure of each gas and the total pressure in the tank at 25ºC. Exercise 18 Dalton s Law II The partial pressure of oxygen was observed to be 156 torr in air with a total atmospheric pressure of 743 torr. Calculate the mole fraction of O 2 present. Exercise 19 Dalton s Law III The mole fraction of nitrogen in the air is Calculate the partial pressure of N 2 in air when the atmospheric pressure is 760. torr. WATER DISPLACEMENT It is common to collect a gas in the laboratory by water displacement. The confounding factor is that some of the pressure in the collection vessel is due to water vapor collected as the gas was passing through! You must correct for this in order to report the P of dry gas. How? You simply look up the partial pressure due to water vapor at a given temperature and subtract that value from the total pressure. The experiment pictured is a classic! You may have done it with Mg rather than zinc and used a eudiometer or inverted and sealed buret to measure the volume of the gas collected over water. Gases 11
12 Exercise 20 Gas Collection over Water A sample of solid potassium chlorate (KClO 3 ) was heated in a test tube (see the figure above) and decomposed by the following reaction: 2 KClO 3 (s) 2 KCl(s) + 3 O 2 (g) The oxygen produced was collected by displacement of water at 22ºC at a total pressure of 754 torr. The volume of the gas collected was L, and the vapor pressure of water at 22ºC is 21 torr. Calculate the partial pressure of O 2 in the gas collected and the mass of KClO 3 in the sample that was decomposed. KINETIC MOLECULAR THEORY OF GASES Assumptions of the MODEL: 1. All particles are in constant, random, motion. 2. All collisions between particles are perfectly elastic. 3. The volume of the particles in a gas is negligible 4. The average kinetic energy of the molecules is its Kelvin temperature. This theory neglects any intermolecular forces as well. And it is important to note that gases expand to fill their container, solids/liquids do not. And that gases are compressible; solids/liquids are not appreciably compressible. Gases 12
13 This helps explain Boyle s Law: If the volume is decreased that means that the gas particles will hit the wall more often, thus increasing pressure 1 P = ( nrt ) V Constant And also helps explain Charles Law When a gas is heated, the speeds of its particles increase and thus hit the walls more often and with more force. The only way to keep the P constant is to increase the volume of the container. V nr = T P Constant Yep, you guessed it! It also helps explain Gay-Lussac s Law When the temperature of a gas increases, the speeds of its particles increase, the particles are hitting the wall with greater force and greater frequency. Since the volume remains the same this would result in increased gas pressure. nr P = T V Constant And it even helps explain Avogadro s Law An increase in the number of particles at the same temperature would cause the pressure to increase if the volume were held constant. The only way to keep constant P is to vary the V. RT V = n P Constant What about Dalton s Law? The P exerted by a mixture of gases is the SUM of the partial pressures since gas particles are acting independent of each other and the volumes of the individual particles DO NOT matter. Gases 13
14 DISTRIBUTION OF MOLECULAR SPEEDS Plot # of gas molecules having various speeds vs. the speed and you get a curve. Changing the temperature affects the shape of the curve NOT the area beneath it. Change the # of molecules and all bets are off! Maxwell s equation: 2 3RT u = urms = MM Use the energy R or J/K mol for this equation since kinetic energy is involved. Exercise 21 Root Mean Square Velocity Calculate the root mean square velocity for the atoms in a sample of helium gas at 25ºC. If we could monitor the path of a single molecule it would be very erratic. Mean free path the average distance a particle travels between collisions. It s on the order of a tenth of a micrometer. WAAAAY SMALL! Examine the effect of temperature on the numbers of molecules with a given velocity as it relates to temperature. HEAT EM UP, SPEED EM UP!! Drop a vertical line from the peak of each of the three bell shaped curves that point on the x-axis represents the AVERAGE velocity of the sample at that temperature. Note how the bells are squashed as the temperature increases. You may see graphs like this on the AP exam where you have to identify the highest temperature based on the shape of the graph! GRAHAM S LAW OF DIFFUSION AND EFFUSION Effusion is closely related to diffusion. Diffusion is the term used to describe the mixing of gases. The rate of diffusion is the rate of the mixing. Effusion is the term used to describe the passage of a gas through a tiny orifice into an evacuated chamber as shown on the right. The rate of effusion measures the speed at which the gas is transferred into the chamber. Gases 14
15 The rates of effusion of two gases are inversely proportional to the square roots of their molar masses at the same temperature and pressure. Rate of effusion of gas 1 = Rate of effusion of gas 2 MM MM 2 1 REMEMBER rate is a change in a quantity over time, NOT just the time! Exercise 22 Effusion Rates Calculate the ratio of the effusion rates of hydrogen gas (H 2 ) and uranium hexafluoride (UF 6 ), a gas used in the enrichment process to produce fuel for nuclear reactors. Exercise 23 A pure sample of methane is found to effuse through a porous barrier in 1.50 minutes. Under the same conditions, an equal number of molecules of an unknown gas effuses through the barrier in 4.73 minutes. What is the molar mass of the unknown gas? Diffusion This is a classic! (animation) Effusion of a Gas: (actual, but not great) Distance traveled by NH 3 = u rms for NH 3 = Distance traveled by HCl u rms for HCl MM MM HCl NH = = Gases 15
16 The observed ratio is LESS than a 1.5 distance ratio why? This diffusion is slow despite considering the molecular velocities are 450 and 660 meters per second which one is which? This tube contains air and all those collisions slow the process down in the real world. Speaking of real world. REAL, thus NONIDEAL GASES Most gases behave ideally until you reach high pressure and low temperature. (By the way, either of these can cause a gas to liquefy, go figure!) van der Waals Equation--corrects for negligible volume of molecules and accounts for inelastic collisions leading to intermolecular forces (his real claim to fame). n 2 [ P + a( ) ][ V bn ] = nrt V The coefficients a and b are van der Waals constants; no need to work problems, it s the concepts that are important! Notice pressure is increased (intermolecular forces lower real pressure, you re correcting for this) and volume is decreased (corrects the container to a smaller free volume). These graphs are classics and make great multiple choice questions on the AP exam. When PV/nRT = 1.0, the gas is ideal All of these are at 200K. Note the P s where the curves cross the dashed line [ideality]. This graph is just for nitrogen gas. Note that although nonideal behavior is evident at each temperature, the deviations are smaller at the higher Ts. Don t underestimate the power of understanding these graphs. We love to ask question comparing the behavior of ideal and real gases. It s not likely you ll be asked an entire free-response gas problem on the real exam in May. Gas Laws are tested extensively in the multiple choice since it s easy to write questions involving them! You will most likely see PV = nrt as one part of a problem in the free-response, just not a whole problem! GO FORTH AND RACK UP THOSE MULTIPLE CHOICE POINTS!! Gases 16
17 And, just for fun: Collapsing Can: Shall we try a bigger can? How about the biggest can we can find? Boiling water with ice: Cooling gases with liquid nitrogen MIT: Getting a boiled egg into a bottle: Gravity has nothing to do with it! Getting egg OUT of bottle! Peeps in a vacuum: and another: Ideal Gas Law: Diffusion of ammonia and HCl (animation): Diffusion of ammonia and HCl (real deal): Putting it all together: Why R as in PV = nrt? This is the kind of stuff that drives me crazy as well! I went in search of the answer some time ago, so I'll share what I found: As usual, the answer relates to a history lesson. It was Clausius in the mid 1800 s that refined the conversion factor for converting degrees Celsius to Kelvins (from adding 267 to adding 273 to the Celsius temperature. He did so using the careful experimental data of another French scientist, Regnault. Clausius also noted that Regnault s data indicated that the farther the temperature and pressure conditions were from the condensation point of the gas, the more correctly the Ideal Gas Law applies. So, there is speculation that the constant was assigned the letter R to honor Regnault s work. In the spirit of giving credit where credit is due, my source was a Journal of Chem. Ed article written by William B. Jensen Department of Chemistry, University of Cincinnati, Cincinnati, OH Gases 17
AP* Chemistry GASES mm Hg = torr =1.00 atm = kpa 10 5 Pa
 THE PROPERTIES OF GASES Only 4 quantities are needed to define the state of a gas: a) the quantity of the gas, n (in moles) b) the temperature of the gas, T (in KELVINS) c) the volume of the gas, V (in
THE PROPERTIES OF GASES Only 4 quantities are needed to define the state of a gas: a) the quantity of the gas, n (in moles) b) the temperature of the gas, T (in KELVINS) c) the volume of the gas, V (in
You should be able to: Describe Equipment Barometer Manometer. 5.1 Pressure Read and outline 5.1 Define Barometer
 A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
GASES. Unit #8. AP Chemistry
 GASES Unit #8 AP Chemistry I. Characteristics of Gases A. Gas Characteristics: 1. Fills its container a. no definite shape b. no definite vol. 2. Easily mixes w/ other gases 3. Exerts pressure on its surroundings
GASES Unit #8 AP Chemistry I. Characteristics of Gases A. Gas Characteristics: 1. Fills its container a. no definite shape b. no definite vol. 2. Easily mixes w/ other gases 3. Exerts pressure on its surroundings
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works.
 Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
Section 5.1 Pressure. Why study gases? An understanding of real world phenomena. An understanding of how science works.
 Chapter 5 Gases Section 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage Learning. All rights reserved 2 Section 5.1 Pressure
Chapter 5 Gases Section 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage Learning. All rights reserved 2 Section 5.1 Pressure
The Kinetic-Molecular Theory of Gases based on the idea that particles are always in motion
 The Kinetic-Molecular Theory of Gases based on the idea that particles are always in motion Five assumptions: 1. Most of the volume occupied dby a gas is empty space 2. Collisions between gas particles
The Kinetic-Molecular Theory of Gases based on the idea that particles are always in motion Five assumptions: 1. Most of the volume occupied dby a gas is empty space 2. Collisions between gas particles
Name Chemistry Pre-AP
 Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Elements that exist as gases at 25 o C and 1 atmosphere H 2, N 2, O 2, F 2, Cl 2, He, Ne, Ar, Kr, Xe, Rn
 AP Chemistry Chapter 5 Sections 5. 5.9 Note Organizer Pressure, The Gas Laws of Boyle, Charles, and Avogadro, The Ideal Gas Law, Gas Stoichiometry, Dalton s Law of Partial Pressure, The Kinetic olecular
AP Chemistry Chapter 5 Sections 5. 5.9 Note Organizer Pressure, The Gas Laws of Boyle, Charles, and Avogadro, The Ideal Gas Law, Gas Stoichiometry, Dalton s Law of Partial Pressure, The Kinetic olecular
Pressure of the atmosphere varies with elevation and weather conditions. Barometer- device used to measure atmospheric pressure.
 Chapter 12 Section 1 Pressure A gas exerts pressure on its surroundings. Blow up a balloon. The gas we are most familiar with is the atmosphere, a mixture of mostly elemental nitrogen and oxygen. Pressure
Chapter 12 Section 1 Pressure A gas exerts pressure on its surroundings. Blow up a balloon. The gas we are most familiar with is the atmosphere, a mixture of mostly elemental nitrogen and oxygen. Pressure
Gases. Chapter 5: Gas Laws Demonstration. September 10, Chapter 5 Gasses.notebook. Dec 18 10:23 AM. Jan 1 4:11 PM. Crushing 55 gallon drum
 Chapter 5: Gases Dec 18 10:23 AM Gas Laws Demonstration Crushing 55 gallon drum Egg in a bottle Student in a bag Boiling Water Charles gas Law Water in a flask Ballon in a bottle Jan 1 4:11 PM 1 5.1 Pressure
Chapter 5: Gases Dec 18 10:23 AM Gas Laws Demonstration Crushing 55 gallon drum Egg in a bottle Student in a bag Boiling Water Charles gas Law Water in a flask Ballon in a bottle Jan 1 4:11 PM 1 5.1 Pressure
Unit 8: Gases and States of Matter
 Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
Kinetic Molecular Theory imaginary Assumptions of Kinetic Molecular Theory: Problems with KMT:
 AP Chemistry Ms. Ye Name Date Block Kinetic Molecular Theory Explains properties of gases, liquids, and solids in terms of energy using an ideal gas, an imaginary which fits all the assumptions of kinetic
AP Chemistry Ms. Ye Name Date Block Kinetic Molecular Theory Explains properties of gases, liquids, and solids in terms of energy using an ideal gas, an imaginary which fits all the assumptions of kinetic
Gas Pressure. Pressure is the force exerted per unit area by gas molecules as they strike the surfaces around them.
 Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Chapter 10: Gases. Characteristics of Gases
 Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
Chapter 13 Gases and Pressure. Pressure and Force. Pressure is the force per unit area on a surface. Force Area. Pressure =
 Chapter 13 Gas Laws Chapter 13 Gases and Pressure Pressure and Force Pressure is the force per unit area on a surface. Pressure = Force Area Chapter 13 Gases and Pressure Gases in the Atmosphere The atmosphere
Chapter 13 Gas Laws Chapter 13 Gases and Pressure Pressure and Force Pressure is the force per unit area on a surface. Pressure = Force Area Chapter 13 Gases and Pressure Gases in the Atmosphere The atmosphere
4.) There are no forces of attraction or repulsion between gas particles. This means that
 KINETIC MOLECULAR (K-M) THEORY OF MATTER NOTES - based on the idea that particles of matter are always in motion - assumptions of the K-M Theory 1.) Gases consist of large numbers of tiny particles that
KINETIC MOLECULAR (K-M) THEORY OF MATTER NOTES - based on the idea that particles of matter are always in motion - assumptions of the K-M Theory 1.) Gases consist of large numbers of tiny particles that
Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases
![Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases](/thumbs/78/77082586.jpg) Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] Chapter 10 Gases We have talked a little about gases in Chapter 3 and we dealt briefly with them in our stoichiometric calculations in
Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] Chapter 10 Gases We have talked a little about gases in Chapter 3 and we dealt briefly with them in our stoichiometric calculations in
Chem 110 General Principles of Chemistry
 CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
Chapter 5. Nov 6 1:02 PM
 Chapter 5 Nov 6 1:02 PM Expand to fill their containers Fluid motion (they flow) Have low densities (1/1000 the density of equivalent liquids or solids) Compressible Can Effuse and Diffuse Effuse: The
Chapter 5 Nov 6 1:02 PM Expand to fill their containers Fluid motion (they flow) Have low densities (1/1000 the density of equivalent liquids or solids) Compressible Can Effuse and Diffuse Effuse: The
PSI Chemistry: Gases Multiple Choice Review
 PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
Expand to fill their containers, are highly compressible, have extremely low densities.
 Chem150 week6 Handout 1 Gases Characteristics of Gases: Unlike liquids and solids, they Expand to fill their containers, are highly compressible, have extremely low densities. Pressure is the amount of
Chem150 week6 Handout 1 Gases Characteristics of Gases: Unlike liquids and solids, they Expand to fill their containers, are highly compressible, have extremely low densities. Pressure is the amount of
Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes:
 Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
Lecture Presentation. Chapter 10. Gases. John D. Bookstaver St. Charles Community College Cottleville, MO Pearson Education, Inc.
 Lecture Presentation Chapter 10 John D. Bookstaver St. Charles Community College Cottleville, MO Characteristics of Unlike liquids and solids, gases Expand to fill their containers. Are highly compressible.
Lecture Presentation Chapter 10 John D. Bookstaver St. Charles Community College Cottleville, MO Characteristics of Unlike liquids and solids, gases Expand to fill their containers. Are highly compressible.
World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases
![World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases](/thumbs/96/127526823.jpg) World of Chemistry Notes for Students [Chapter 3, page ] Chapter 3 Gases ) Sec 3.8 Kinetic Theory of Gases and the Nature of Gases The Kinetic Theory of Matter says that the tiny particles in all forms
World of Chemistry Notes for Students [Chapter 3, page ] Chapter 3 Gases ) Sec 3.8 Kinetic Theory of Gases and the Nature of Gases The Kinetic Theory of Matter says that the tiny particles in all forms
Chapter 13. Gases. Copyright Cengage Learning. All rights reserved 1
 Chapter 13 Gases Copyright Cengage Learning. All rights reserved 1 Section 13.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage
Chapter 13 Gases Copyright Cengage Learning. All rights reserved 1 Section 13.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage
Kinetic-Molecular Theory
 GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
Chemistry HP Unit 6 Gases. Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases
 Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory
 CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory
 CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
AP TOPIC 6: Gases. Revised August General properties and kinetic theory
 AP OPIC 6: Gases General properties and kinetic theory Gases are made up of particles that have (relatively) large amounts of energy. A gas has no definite shape or volume and will expand to fill as much
AP OPIC 6: Gases General properties and kinetic theory Gases are made up of particles that have (relatively) large amounts of energy. A gas has no definite shape or volume and will expand to fill as much
Chapter 12. The Gaseous State of Matter
 Chapter 12 The Gaseous State of Matter The air in a hot air balloon expands When it is heated. Some of the air escapes from the top of the balloon, lowering the air density inside the balloon, making the
Chapter 12 The Gaseous State of Matter The air in a hot air balloon expands When it is heated. Some of the air escapes from the top of the balloon, lowering the air density inside the balloon, making the
To play movie you must be in Slide Show Mode CLICK HERE EXERCISE! EXERCISE! To play movie you must be in Slide Show Mode CLICK HERE
 Boyle s Law Boyle s law Pressure and volume are inversely related (constant T, temperature, and n, # of moles of gas). PV k (kis a constant for a given sample of air at a specific temperature) P V P V
Boyle s Law Boyle s law Pressure and volume are inversely related (constant T, temperature, and n, # of moles of gas). PV k (kis a constant for a given sample of air at a specific temperature) P V P V
Chemistry A Molecular Approach. Fourth Edition. Chapter 5. Gases. Copyright 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
 Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
temperature and pressure unchanging
 Gas Laws Review I. Variables Used to Describe a Gas A. Pressure (P) kpa, atm, mmhg (torr) -Pressure=force exerted per unit area (force/area) -Generated by collisions within container walls (more collisions=more
Gas Laws Review I. Variables Used to Describe a Gas A. Pressure (P) kpa, atm, mmhg (torr) -Pressure=force exerted per unit area (force/area) -Generated by collisions within container walls (more collisions=more
Chapter 9 Gases: Their Properties and Behavior
 Chapter 9 Gases: Their Properties and Behavior 國防醫學院生化學科王明芳老師 2011-11-15 & 2011-11-22 Chapter 9/1 Gases and Gas Pressure Gas mixtures are homogeneous and compressible. Air-the mixture of gases. Molecular
Chapter 9 Gases: Their Properties and Behavior 國防醫學院生化學科王明芳老師 2011-11-15 & 2011-11-22 Chapter 9/1 Gases and Gas Pressure Gas mixtures are homogeneous and compressible. Air-the mixture of gases. Molecular
Chapter 13: The Behavior of Gases
 Chapter 13: The Behavior of Gases I. First Concepts a. The 3 states of matter most important to us: solids, liquids, and gases. b. Real Gases and Ideal Gases i. Real gases exist, ideal gases do not ii.
Chapter 13: The Behavior of Gases I. First Concepts a. The 3 states of matter most important to us: solids, liquids, and gases. b. Real Gases and Ideal Gases i. Real gases exist, ideal gases do not ii.
Gases and Pressure. Main Ideas
 Gases and Pressure Key Terms pressure millimeters of mercury partial pressure newton atmosphere of pressure Dalton s law of partial pressures barometer pascal In the chapter States of Matter, you read
Gases and Pressure Key Terms pressure millimeters of mercury partial pressure newton atmosphere of pressure Dalton s law of partial pressures barometer pascal In the chapter States of Matter, you read
Chapter 10 Gases. Characteristics of Gases. Pressure. The Gas Laws. The Ideal-Gas Equation. Applications of the Ideal-Gas Equation
 Characteristics of Gases Chapter 10 Gases Pressure The Gas Laws The Ideal-Gas Equation Applications of the Ideal-Gas Equation Gas mixtures and partial pressures Kinetic-Molecular Theory Real Gases: Deviations
Characteristics of Gases Chapter 10 Gases Pressure The Gas Laws The Ideal-Gas Equation Applications of the Ideal-Gas Equation Gas mixtures and partial pressures Kinetic-Molecular Theory Real Gases: Deviations
CHEMISTRY - CLUTCH CH.5 - GASES.
 !! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
!! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
Chapter 5. Pressure. Atmospheric Pressure. Gases. Force Pressure = Area
 Chapter 5 Gases Water for many homes is supplied by a well The pump removes air from the pipe, decreasing the air pressure in the pipe The pressure then pushes the water up the pipe Pressure Atmospheric
Chapter 5 Gases Water for many homes is supplied by a well The pump removes air from the pipe, decreasing the air pressure in the pipe The pressure then pushes the water up the pipe Pressure Atmospheric
Section 8: Gases. The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC (c).
 Section 8: Gases The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 8.01 Simple Gas Laws Chemistry (9)(A) 8.02 Ideal Gas Law Chemistry
Section 8: Gases The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 8.01 Simple Gas Laws Chemistry (9)(A) 8.02 Ideal Gas Law Chemistry
Gilbert Kirss Foster. Chapter 10. Properties of Gases The Air We Breathe
 Gilbert Kirss Foster Chapter 10 Properties of Gases The Air We Breathe Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4
Gilbert Kirss Foster Chapter 10 Properties of Gases The Air We Breathe Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4
Kinetic-Molecular Theory of Matter
 Gases Properties of Gases Gas Pressure Gases What gases are important for each of the following: O 2, CO 2 and/or He? A. B. C. D. 1 2 Gases What gases are important for each of the following: O 2, CO 2
Gases Properties of Gases Gas Pressure Gases What gases are important for each of the following: O 2, CO 2 and/or He? A. B. C. D. 1 2 Gases What gases are important for each of the following: O 2, CO 2
Accelerated Chemistry Study Guide Chapter 13: Gases
 Accelerated Chemistry Study Guide Chapter 13: Gases Terms, definitions, topics Diffusion Kinetic Molecular Theory Atmospheric pressure Barometer Manometer STP Absolute zero Page 1 of 42 Molar volume Partial
Accelerated Chemistry Study Guide Chapter 13: Gases Terms, definitions, topics Diffusion Kinetic Molecular Theory Atmospheric pressure Barometer Manometer STP Absolute zero Page 1 of 42 Molar volume Partial
Chapter 14-Gases. Dr. Walker
 Chapter 14-Gases Dr. Walker State of Matter Gases are one of the four states of matter along with solids, liquids, and plasma Conversion to Gases From liquids Evaporation Example: Boiling water From solids
Chapter 14-Gases Dr. Walker State of Matter Gases are one of the four states of matter along with solids, liquids, and plasma Conversion to Gases From liquids Evaporation Example: Boiling water From solids
Gases and Pressure SECTION 11.1
 SECTION 11.1 Gases and In the chapter States of Matter, you read about the kineticmolecular theory of matter. You were also introduced to how this theory explains some of the properties of ideal gases.
SECTION 11.1 Gases and In the chapter States of Matter, you read about the kineticmolecular theory of matter. You were also introduced to how this theory explains some of the properties of ideal gases.
Unit 8: Kinetic Theory Homework Packet (90 points)
 Name: Key Period: By the end of Unit 8, you should be able to: Kinetic Theory Chapter 13-14 4. Define kinetic theory of gases including collisions 5. Define pressure, including atmospheric pressure, vapor
Name: Key Period: By the end of Unit 8, you should be able to: Kinetic Theory Chapter 13-14 4. Define kinetic theory of gases including collisions 5. Define pressure, including atmospheric pressure, vapor
Name /74. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch 11 Gases STUDY GUIDE Accelerated Chemistry SCANTRON Name /74 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements
Ch 11 Gases STUDY GUIDE Accelerated Chemistry SCANTRON Name /74 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements
Unit 10: Gas Laws. Monday Tuesday Wednesday Thursday Friday. 10 Review for Cumulative Retest. 17 Chem Think Gas Laws Tutorial- Computer Lab-
 Unit 10: Gas Laws Name: Monday Tuesday Wednesday Thursday Friday February 8 Stoichiometry Test Review 9 Stoichiometry Test 10 Review for Cumulative Retest 11 Cumulative Re-Test 12 Pressure & Kinetic Theory
Unit 10: Gas Laws Name: Monday Tuesday Wednesday Thursday Friday February 8 Stoichiometry Test Review 9 Stoichiometry Test 10 Review for Cumulative Retest 11 Cumulative Re-Test 12 Pressure & Kinetic Theory
C h e m i s t r y 1 A : C h a p t e r 5 P a g e 1
 C h e m i s t r y 1 A : C h a p t e r 5 P a g e 1 Chapter 5: Gases Homework: Read Chapter 5. Work out sample/practice exercises Keep up with MasteringChemistry assignments Gas Properties: Ideal Gas: Gases
C h e m i s t r y 1 A : C h a p t e r 5 P a g e 1 Chapter 5: Gases Homework: Read Chapter 5. Work out sample/practice exercises Keep up with MasteringChemistry assignments Gas Properties: Ideal Gas: Gases
Honors Chemistry Unit 7 Gas Laws Notes
 Honors Chemistry Unit 7 Gas Laws Notes Kinetic Molecular Theory 1. List the five assumptions: Assumption Description Extra Info 1 Basically means: the particles themselves have compared to the space between
Honors Chemistry Unit 7 Gas Laws Notes Kinetic Molecular Theory 1. List the five assumptions: Assumption Description Extra Info 1 Basically means: the particles themselves have compared to the space between
To convert to millimeters of mercury, we derive a unit factor related to the equivalent relationship 29.9 in. Hg = 760 mm Hg.
 Example Exercise 11.1 Gas Pressure Conversion Meteorologists state that a falling barometer indicates an approaching storm. Given a barometric pressure of 27.5 in. Hg, express the pressure in each of the
Example Exercise 11.1 Gas Pressure Conversion Meteorologists state that a falling barometer indicates an approaching storm. Given a barometric pressure of 27.5 in. Hg, express the pressure in each of the
Chemistry Chapter 12. Characteristics of Gases. Characteristics of Gases 1/31/2012. Gases and Liquids
 Importance of Gases Chemistry Chapter 12 Gases and Liquids Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide, NaN 3. 2 NaN 3 ---> 2 Na + 3 N 2 THREE STATES
Importance of Gases Chemistry Chapter 12 Gases and Liquids Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide, NaN 3. 2 NaN 3 ---> 2 Na + 3 N 2 THREE STATES
Name Unit 9 Notes: Gas Laws Period. Complete throughout unit. Due on test day!
 Name Unit 9 Notes: Gas Laws Period Skills: 1. Gases and Entropy 2. Distinguish between Ideal and Real gases 3. Understand KMT and Avogadro s Law 4. Identify and Solve Boyle s Law Problems 5. Identify and
Name Unit 9 Notes: Gas Laws Period Skills: 1. Gases and Entropy 2. Distinguish between Ideal and Real gases 3. Understand KMT and Avogadro s Law 4. Identify and Solve Boyle s Law Problems 5. Identify and
Boyle s Law Practice
 Boyle s Law Practice Boyle s Law is an indirect relationship. Most of these problems can be done in your head without showing your work. 1. Herman has 30.0 L of helium gas trapped in a cylinder by a piston.
Boyle s Law Practice Boyle s Law is an indirect relationship. Most of these problems can be done in your head without showing your work. 1. Herman has 30.0 L of helium gas trapped in a cylinder by a piston.
General Properties of Gases
 GASES Chapter 13 Importance of Gases Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide,, NaN 3. 2 NaN 3 ---> > 2 Na + 3 N 2 THREE STATES OF MATTER General
GASES Chapter 13 Importance of Gases Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide,, NaN 3. 2 NaN 3 ---> > 2 Na + 3 N 2 THREE STATES OF MATTER General
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT.
 Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
General Chemistry II CHEM 116
 General Chemistry II CHEM 116 Professor Evans (first two weeks) Professor Satyamurti (last four weeks) Syllabus; 2 exams (22.5 % each) and a final (45 %), lecture attendance 5 %, discussion attendance
General Chemistry II CHEM 116 Professor Evans (first two weeks) Professor Satyamurti (last four weeks) Syllabus; 2 exams (22.5 % each) and a final (45 %), lecture attendance 5 %, discussion attendance
THE GAS STATE. Unit 4. CHAPTER KEY TERMS HOME WORK 9.1 Kinetic Molecular Theory States of Matter Solid, Liquid, gas.
 Unit 4 THE GAS STATE CHAPTER KEY TERMS HOME WORK 9. Kinetic Molecular Theory States of Matter Solid, Liquid, gas Page 4 # to 4 9. Boyles Law P α /V PV = Constant P V = P V Pressure Atmospheric Pressure
Unit 4 THE GAS STATE CHAPTER KEY TERMS HOME WORK 9. Kinetic Molecular Theory States of Matter Solid, Liquid, gas Page 4 # to 4 9. Boyles Law P α /V PV = Constant P V = P V Pressure Atmospheric Pressure
Substances that are liquids or solids under ordinary conditions may also exist as gases. These are often referred to as vapors. Properties of Gases
 Common Student Misconceptions Students need to be told to always use temperature in Kelvin in gas problems. Students should always use units in gas-law problems to keep track of required conversions. Due
Common Student Misconceptions Students need to be told to always use temperature in Kelvin in gas problems. Students should always use units in gas-law problems to keep track of required conversions. Due
NOTES: Behavior of Gases
 NOTES: Behavior of Gases Properties of Gases Gases have weight Gases take up space Gases exert pressure Gases fill their containers Gases are mostly empty space The molecules in a gas are separate, very
NOTES: Behavior of Gases Properties of Gases Gases have weight Gases take up space Gases exert pressure Gases fill their containers Gases are mostly empty space The molecules in a gas are separate, very
Chapter 5 Gases. AP CHEMISTRY Chapter 5 Scotch Plains-Fanwood High School Page 1
 Chapter 5 Gases Kinetic Theory All matter is composed of tiny particles that are in continuous, random motion. Gas Pressure = Force Demo: Test tube/h2o beaker Area Demo: Can AP CHEMISTRY Chapter 5 Scotch
Chapter 5 Gases Kinetic Theory All matter is composed of tiny particles that are in continuous, random motion. Gas Pressure = Force Demo: Test tube/h2o beaker Area Demo: Can AP CHEMISTRY Chapter 5 Scotch
A. What are the three states of matter chemists work with?
 Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
Chapter 10. Physical Characteristics of Gases
 Chapter 10 Physical Characteristics of Gases Kinetic Molecular Theory An understanding of the behavior of atoms that make up matter Ideal gas: an imaginary gas that perfectly fits all assumptions of the
Chapter 10 Physical Characteristics of Gases Kinetic Molecular Theory An understanding of the behavior of atoms that make up matter Ideal gas: an imaginary gas that perfectly fits all assumptions of the
States of Matter Review
 States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
2. Calculate the ratio of diffusion rates for carbon monoxide (CO) and carbon dioxide (CO2). υa = MB = 44 = 1.25
 Gas laws worksheet (2-08) (modified 3/17) Answer key Graham s Law 1. Calculate the ratio of effusion rates for nitrogen (N2) and neon (Ne). υa = MB = 20 = 0.845 υb MA 28 2. Calculate the ratio of diffusion
Gas laws worksheet (2-08) (modified 3/17) Answer key Graham s Law 1. Calculate the ratio of effusion rates for nitrogen (N2) and neon (Ne). υa = MB = 20 = 0.845 υb MA 28 2. Calculate the ratio of diffusion
Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops
 C h e m i s t r y 1 2 C h a p t e r 11 G a s e s P a g e 1 Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops Gas Properties: Gases have high kinetic energy low
C h e m i s t r y 1 2 C h a p t e r 11 G a s e s P a g e 1 Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops Gas Properties: Gases have high kinetic energy low
Kinetic Molecular Theory Gases. Behavior of gases. Postulate two. Postulate one. Postulate three. Postulate four
 Kinetic Molecular Theory Gases Gas particles are so small that their individual volume can be considered to be negligible Gas particles are in constant motion and the collisions of the particles with the
Kinetic Molecular Theory Gases Gas particles are so small that their individual volume can be considered to be negligible Gas particles are in constant motion and the collisions of the particles with the
Chapter 10: Properties of Gases: The Air We Breathe
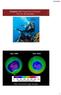 Chapter 10: Properties of Gases: The Air We Breathe Sept, 2006 Sept, 2016 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 The Properties of Gases 10.2 The Kinetic Molecular Theory of Gases* 10.3
Chapter 10: Properties of Gases: The Air We Breathe Sept, 2006 Sept, 2016 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 The Properties of Gases 10.2 The Kinetic Molecular Theory of Gases* 10.3
Unit 9: Gas Laws REGENTS CHEMISTRY
 Name: Unit 9: Gas Laws REGENTS CHEMISTRY 1 Name: Unit 9: Gas Laws The concept of an ideal gas is a model to explain the behavior of gases. A real gas is most like an ideal gas when the real gas is at low
Name: Unit 9: Gas Laws REGENTS CHEMISTRY 1 Name: Unit 9: Gas Laws The concept of an ideal gas is a model to explain the behavior of gases. A real gas is most like an ideal gas when the real gas is at low
Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases.
 Chapter 8 Gases Practice Problems Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases. Summary: In a gas, particles are so far
Chapter 8 Gases Practice Problems Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases. Summary: In a gas, particles are so far
Chapter 11. Recall: States of Matter. Properties of Gases. Gases
 Chapter 11 Gases Recall: States of Matter Solids and Liquids: are closely related because in each case the particles are interacting with each other Gases: Properties of Gases Gases can be compressed Gases
Chapter 11 Gases Recall: States of Matter Solids and Liquids: are closely related because in each case the particles are interacting with each other Gases: Properties of Gases Gases can be compressed Gases
Worksheet 12 - Partial Pressures and the Kinetic Molecular Theory of Gases
 Worksheet 12 - Partial Pressures and the Kinetic olecular Theory of Gases Dalton's Law of Partial Pressures states that the sums of the pressures of each gas in the mixture add to give the total pressure
Worksheet 12 - Partial Pressures and the Kinetic olecular Theory of Gases Dalton's Law of Partial Pressures states that the sums of the pressures of each gas in the mixture add to give the total pressure
8.1 Properties of Gases. Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases.
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8. Chapter 8
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases. Chapter 8. Chapter 8. Gases Properties of Gases. We are surrounded by gases, but we are often
 Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
Gas Law Worksheets - WS: Boyle s and Charles Law
 Gas Law Worksheets - WS: Boyle s and Charles Law Boyle s Law states that the volume of a gas varies inversely with its pressure if temperature is held constant. (If one goes up the, other goes down.) We
Gas Law Worksheets - WS: Boyle s and Charles Law Boyle s Law states that the volume of a gas varies inversely with its pressure if temperature is held constant. (If one goes up the, other goes down.) We
Notes: Gas Laws (text Ch. 11)
 Name Per. Notes: Gas Laws (text Ch. 11) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to get missing information
Name Per. Notes: Gas Laws (text Ch. 11) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to get missing information
Chemistry 101 Chapter 5 GAS MIXTURES
 GAS MIXTURES Consider mixing equal volumes of 3 different gases, all at the same temperature and pressure in a container of the same size. 1 L He 1 L N 2 1 L O 2 1 L mixture t = 0 0 C t = 0 0 C t = 0 0
GAS MIXTURES Consider mixing equal volumes of 3 different gases, all at the same temperature and pressure in a container of the same size. 1 L He 1 L N 2 1 L O 2 1 L mixture t = 0 0 C t = 0 0 C t = 0 0
Kinetic Molecular Theory
 Kinetic Molecular Theory Name Period Unit 7 HW 1 Worksheet (Goals 1 & 2) 1. Describe how gases, liquids, and solids compare using the following table. Volume (definite or indefinite) Molecular Motion (high,
Kinetic Molecular Theory Name Period Unit 7 HW 1 Worksheet (Goals 1 & 2) 1. Describe how gases, liquids, and solids compare using the following table. Volume (definite or indefinite) Molecular Motion (high,
Chemistry 20 Unit 2 Gases FITB Notes. Topic A Characteristics of Gases
 Chemistry 20 Unit 2 Gases FITB Notes General Outcome: Topic A Characteristics of Gases We use technologies that were designed with the knowledge of the visible characteristics ( ) of gases ex. SCUBA equipment,
Chemistry 20 Unit 2 Gases FITB Notes General Outcome: Topic A Characteristics of Gases We use technologies that were designed with the knowledge of the visible characteristics ( ) of gases ex. SCUBA equipment,
Practice MC Test unit D (Ch 10) Gas Laws (pg 1 of 10)
 Practice MC Test unit D (Ch 10) Gas Laws (pg 1 of 10) This is practice - Do NOT cheat yourself of finding out what you are capable of doing. Be sure you follow the testing conditions outlined below. DO
Practice MC Test unit D (Ch 10) Gas Laws (pg 1 of 10) This is practice - Do NOT cheat yourself of finding out what you are capable of doing. Be sure you follow the testing conditions outlined below. DO
Simple Gas Laws. To facilitate comparison of gases, the following standards are used: STP: O C (273 K) and kpa. SATP: 25 C (298 K) and 101.
 Simple Gas Laws To facilitate comparison of gases, the following standards are used: STP: O C (273 K) and 101.3 kpa If assuming 1 mol, V = 22.4L SATP: 25 C (298 K) and 101.3 kpa If assuming 1 mol, V =
Simple Gas Laws To facilitate comparison of gases, the following standards are used: STP: O C (273 K) and 101.3 kpa If assuming 1 mol, V = 22.4L SATP: 25 C (298 K) and 101.3 kpa If assuming 1 mol, V =
Chapter 10: Properties of Gases: The Air We Breathe
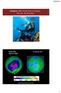 Chapter 10: Properties of Gases: The Air We Breathe South Pole Sept 24, 2006 15 February 2017 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 10.2 10.3 The Properties of Gases Effusion and the Kinetic
Chapter 10: Properties of Gases: The Air We Breathe South Pole Sept 24, 2006 15 February 2017 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 10.2 10.3 The Properties of Gases Effusion and the Kinetic
4. Using the kinetic molecular theory, explain why a gas can be easily compressed, while a liquid and a solid cannot?
 Name Period HW 1 Worksheet (Goals 1-4) - Kinetic Molecular Theory 1. Describe how gases, liquids, and solids compare using the following table. Solids Liquids Gases Volume (definite or indefinite) Molecular
Name Period HW 1 Worksheet (Goals 1-4) - Kinetic Molecular Theory 1. Describe how gases, liquids, and solids compare using the following table. Solids Liquids Gases Volume (definite or indefinite) Molecular
PETER STARODUB - PALOS VERDES PENINSULA HIGH SCHOOL
 STARODUB CHEM. 2AP UNIT 2-2 CH. 5: Gases 1 UNIT 2-2: CH. 5 GASES READ P. 179 214 ASSIGNMENTS: #1 P. 7 #1-12 Pressure Problems #2 P. 14 #1-17 Gas Law Problems #3 P. 17 #1-12 Gas Density, Molar Mass, Stoichiometry
STARODUB CHEM. 2AP UNIT 2-2 CH. 5: Gases 1 UNIT 2-2: CH. 5 GASES READ P. 179 214 ASSIGNMENTS: #1 P. 7 #1-12 Pressure Problems #2 P. 14 #1-17 Gas Law Problems #3 P. 17 #1-12 Gas Density, Molar Mass, Stoichiometry
Gases. Edward Wen, PhD
 Gases Edward Wen, PhD Properties of Gases expand to completely fill their container take the shape of their container low density much less than solid or liquid state compressible when pressure is changed.
Gases Edward Wen, PhD Properties of Gases expand to completely fill their container take the shape of their container low density much less than solid or liquid state compressible when pressure is changed.
Section 10-1: The Kinetic-Molecular Theory of Matter. 1) How does the word kinetic apply to particles of matter?
 Kinetic-Molecular theory of Matter/Ch10, Gases/Ch11 Column notes: Answer all parts of each question IN YOUR OWN WORDS. Use the text, figures and captions as resources. Section 10-1: The Kinetic-Molecular
Kinetic-Molecular theory of Matter/Ch10, Gases/Ch11 Column notes: Answer all parts of each question IN YOUR OWN WORDS. Use the text, figures and captions as resources. Section 10-1: The Kinetic-Molecular
Temperature Temperature
 Temperature Temperature is a measure of how hot or cold an object is compared to another object. indicates that heat flows from the object with a higher temperature to the object with a lower temperature.
Temperature Temperature is a measure of how hot or cold an object is compared to another object. indicates that heat flows from the object with a higher temperature to the object with a lower temperature.
Lab Dates. CRHS Academic Chemistry Unit 11 Gas Laws Notes
 Name Period CRHS Academic Chemistry Unit 11 Gas Laws Notes Quiz Date Lab Dates Exam Date Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Name Period CRHS Academic Chemistry Unit 11 Gas Laws Notes Quiz Date Lab Dates Exam Date Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Chemistry 51 Chapter 7 PROPERTIES OF GASES. Gases are the least dense and most mobile of the three phases of matter.
 ROERIES OF GASES Gases are the least dense and most mobile of the three phases of matter. articles of matter in the gas phase are spaced far apart from one another and move rapidly and collide with each
ROERIES OF GASES Gases are the least dense and most mobile of the three phases of matter. articles of matter in the gas phase are spaced far apart from one another and move rapidly and collide with each
Example: 25 C = ( ) K = 298 K. Pressure Symbol: p Units: force per area 1Pa (Pascal) = 1 N/m 2
 Chapter 6: Gases 6.1 Measurements on Gases MH5, Chapter 5.1 Let s look at a certain amount of gas, i.e. trapped inside a balloon. To completely describe the state of this gas one has to specify the following
Chapter 6: Gases 6.1 Measurements on Gases MH5, Chapter 5.1 Let s look at a certain amount of gas, i.e. trapped inside a balloon. To completely describe the state of this gas one has to specify the following
Chapter 6 10/14/13. Gas Law. Volume change with temperature and pressure.
 Chapter 6 10/14/13 Gas Law 1. Properties of a Gas a. Neither definite shape nor volume i. Uniformly fills any container i Exerts pressure on surroundings Volume change with temperature and pressure. b.
Chapter 6 10/14/13 Gas Law 1. Properties of a Gas a. Neither definite shape nor volume i. Uniformly fills any container i Exerts pressure on surroundings Volume change with temperature and pressure. b.
Chapter 12. Properties of Gases
 Properties of Gases Each state of matter has its own properties. Gases have unique properties because the distance between the particles of a gas is much greater than the distance between the particles
Properties of Gases Each state of matter has its own properties. Gases have unique properties because the distance between the particles of a gas is much greater than the distance between the particles
Chemistry Chapter 11 Test Review
 Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
Name: Chapter 13: Gases
 Name: Chapter 13: Gases Gases and gas behavior is one of the most important and most fun things to learn during your year in chemistry. Here are all of the gas notes and worksheets in two packets. We will
Name: Chapter 13: Gases Gases and gas behavior is one of the most important and most fun things to learn during your year in chemistry. Here are all of the gas notes and worksheets in two packets. We will
CHAPTER 1 KINETIC THEORY OF GASES (PART A)
 For updated version, please click on http://ocw.ump.edu.my BSK1133 PHYSICAL CHEMISTRY CHAPTER 1 KINETIC THEORY OF GASES (PART A) PREPARED BY: DR. YUEN MEI LIAN AND DR. SITI NOOR HIDAYAH MUSTAPHA Faculty
For updated version, please click on http://ocw.ump.edu.my BSK1133 PHYSICAL CHEMISTRY CHAPTER 1 KINETIC THEORY OF GASES (PART A) PREPARED BY: DR. YUEN MEI LIAN AND DR. SITI NOOR HIDAYAH MUSTAPHA Faculty
Chemistry Chapter 10 Test
 Chemistry Chapter 10 Test True/False Indicate whether the sentence or statement is true or false. 1. KMT stands for Kinetic Mole Theory. 2. One of the assumptions in the KMT is that the particles are spread
Chemistry Chapter 10 Test True/False Indicate whether the sentence or statement is true or false. 1. KMT stands for Kinetic Mole Theory. 2. One of the assumptions in the KMT is that the particles are spread
8. Now plot on the following grid the values of T (K) and V from the table above, and connect the points.
 Charles s Law According to Charles s law, the volume of a fixed mass of gas varies directly with its Kelvin temperature if its pressure is constant. The following table contains Celsius temperature and
Charles s Law According to Charles s law, the volume of a fixed mass of gas varies directly with its Kelvin temperature if its pressure is constant. The following table contains Celsius temperature and
Lecture Handout 5: Gases (Online Text Chapter 6)
 Lecture Handout 5: Gases (Online Text Chapter 6) I. The Structure of a Gas A. Gases are composed of particles that are flying around very fast in their container(s). 1. The particles travel in straight
Lecture Handout 5: Gases (Online Text Chapter 6) I. The Structure of a Gas A. Gases are composed of particles that are flying around very fast in their container(s). 1. The particles travel in straight
