PROFESSIONAL FITTING GUIDE. for the. HUBBLE Daily. Table of Contents
|
|
|
- Claire Barnett
- 6 years ago
- Views:
Transcription
1 PROFESSIONAL FITTING GUIDE for the HUBBLE Daily CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A LICENSED PRACTICIONER. Table of Contents Description Lens Material Lens Properties Lens Parameters Actions Indications Contraindications Warnings Precautions Adverse reactions Patient selection Fitting procedure Follow-up examinations Replacement and Wear Schedule Monovision fitting guidelines Emergencies Reporting of adverse reactions How supplied
2 DESCRIPTION HUBBLE Daily contact lenses are available in a spherical lens design. The lenses are to be prescribed for single use, daily disposable wear. LENS MATERIAL The lens material is 55% water and 45% methafilcon A polymer (2-hydroxyethyl methacrylate and methacrylic acid crosslinked with ethylene glycol dimethacrylate). For visibility tinted lenses, the color additive copper phthalocyanine is added to the lens material to make them easier to see when handling. A UV absorbing monomer, 2-[3-(2H- Benzotriazol-2y1)-4-hydroxyphenyl] ethyl methacrylate, is incorporated into the lens polymer to block UV radiation. The Hubble (methafilcon A) Soft (hydrophilic) Contact Lenses block an average of 80 % in the UVA range (316 nm to 380 nm) and 95 % in the UVB range (315 nm to 280 nm). 1. Human cornea from a 24 year-old person as described in Lerman, S., Radiant Energy and the Eye, New York, 1980, p. 58, Figure Human crystalline lens from a 25 year-old person as described in Waxler, M., Hitchins, V.M., Optical Radiation and Visual Health, CRC Press, Boca Raton, Florida, 1986, p. 19, Figure The Hubble (methafilcon A) Soft (hydrophilic) Contact Lenses. * Lens transmittance was obtained from measurements taken through the central 3-5 mm portion of the thinnest marketed version of the UV lens. LENS PROPERTIES Refractive index: (hydrated) Light transmittance: >96% (381 nm ~ 780 nm) Oxygen permeability (Dk): 21.4 x (cm 2 /sec) (ml O2 /ml x mm Hg), measured at 35 C (Fatt, edge effect corrected) Water content: 55% by weight in normal saline LENS PARAMETERS HUBBLE Daily are available in the following dimensions: Base curve: 8.6 mm Diameter: 14.2 mm Powers available: -8.00D to +6.00D (0.25D steps); Center thickness: 0.10 mm at -3.00D (varies with power) Tint: Light blue handling tint
3 ACTIONS When hydrated and placed on the cornea HUBBLE Daily contact lenses act as a refracting medium to focus light rays on the retina. INDICATIONS HUBBLE Daily contact lenses are indicated for daily wear for the correction of refractive ametropia (myopia, hyperopia) in aphakic or not-aphakic persons with non-diseased eyes that may exhibit refractive astigmatism up to 2.00 diopters that does not interfere with visual acuity. HUBBLE Daily contact lenses are to be prescribed for single use, daily disposable wear. The lenses are not intended to be cleaned or disinfected and should be discarded after a single use. CONTRAINDICATIONS Do not use HUBBLE Daily when any of the following conditions exists: Acute or subacute inflammation or infection of the anterior chamber of the eye. Any eye disease, injury or abnormality affecting the cornea, conjunctiva, or eyelids that may be exacerbated by contact lens wear. Insufficiency of lacrimal secretion (dry eye) that interferes with contact lens wear. Corneal hypoesthesia (reduced corneal sensitivity). Any systemic disease which may be exacerbated by or interferes with contact lens wear. Allergic reactions or ocular irritation of the ocular surfaces or adnexa that may be caused by or exacerbated by the wearing of contact lenses. Any active corneal infection (bacterial, fungal, or viral). The use of any medication that is contraindicated or interferes with contact lens wear, including eye medications. Patient history of recurring eye or eyelid infections, adverse effects associated with contact lens wear, intolerance or abnormal ocular response to contact lens wear. If eyes become red or irritated. WARNINGS Patients should be advised of the following warnings pertaining to contact lens wear: Problems with contact lenses could result in serious injury to the eye. It is essential that patients follow their eye care professional s directions and all labeling instructions for proper use of their lenses. Eye problems, including corneal ulcers, can develop rapidly and lead to loss of vision. Daily wear lenses are not indicated for overnight wear, and patients should be instructed not to wear lenses while sleeping. Clinical study results have shown that the risk of ulcerative keratitis is nine times greater for daily wear users who wear their lenses overnight (outside the approved indication) compared to those who do not wear them overnight. Studies have shown that contact lens wearers who smoke have an estimated 3 to 8 times greater risk of suffering ulcerative keratitis than those who are nonsmokers. If a patient experiences eye discomfort, excessive tearing, vision changes, redness of the eye, or other problems they should be instructed to immediately remove their lenses and promptly contact their eye care professional. It is recommended that contact lens wearers see their eye care professional regularly as directed. UV-absorbing contact lenses are NOT substitutes for protective UV absorbing eye wear such as UV absorbing goggles or sunglasses because they do not completely cover the eye and surrounding area. The patient should continue to use UV absorbing eye wear as directed. PRECAUTIONS
4 Special Precautions for Eye Care Practitioner: Due to the small number of patients enrolled in the clinical investigation of lenses, all refractive powers, design configurations, or lens parameters available in the lens material are not evaluated in significant numbers. Consequently, when selecting an appropriate lens design and parameters, the eye care professional should consider all characteristics of the lens that can affect lens performance and ocular health, including oxygen permeability, central and peripheral thickness, and optic zone diameter. The potential impact of these factors on the patient s ocular health should be carefully weighed against the patient s need for refractive correction; therefore, the continuing ocular health of the patient and lens performance on the eye should be carefully monitored by the prescribing eye care professional. Fluorescein, a yellow dye, should not be used while the lenses are on the eyes. The lenses absorb this dye and become discolored. Whenever fluorescein is used, the eyes should be flushed thoroughly with sterile saline solution that is recommended for in eye use prior to inserting lenses. Avoid dispensing saline from an aerosol can directly into the eye. Visual requirements vary with the individual and should be considered when selecting the most appropriate type of lens for each patient. Before leaving the eye care professional s office, the patient should be able to promptly remove their lenses or should have someone else available who can remove their lenses for them. Eye care professionals should instruct the patient to remove the lenses immediately if the eye becomes red or irritated. Routine eye examinations are necessary to help assure the continuing health of the patient s eyes. Eye care professionals should make arrangements with the patient for appropriate follow-up visits. Vision Path, Inc. recommends that patients see their eye care professional once each year or as recommended by the eye care professional. Visual changes or changes in lens tolerance may occur during pregnancy or use of oral contraceptives. Caution patients accordingly. Eye Care Professionals should carefully instruct patients about the following safety precautions: Carefully follow the handling, insertion, removal, and wearing instructions in the HUBBLE Daily Wearer s Guide and any additional instructions provided by the eye care professional. Note the correct lens power for each eye to prevent getting them mixed up. Always keep spare lenses available to avoid reusing the lenses. Good hygiene habits help promote safe and comfortable lens wear. Always wash and rinse hands before handling lenses. Shake the blister pack gently prior to opening. Remove the lens from the blister pack by carefully pouring the lens onto the palm of your clean hand. Never use tweezers or other sharp objects such as fingernails to remove the lens from the container to avoid damaging the lens. Eye irritation, infection, or lens damage may result if cosmetics, lotion, soap, cream, hair spray, deodorant, aerosol products or foreign particles come in contact with lenses. If sprays are used, eyes should be kept closed until the spray has settled. Always handle lenses carefully. If a lens is dropped, small particles or fibers may adhere to the lens surface which can irritate the eye. Replace with a sterile fresh, new lens. Never allow contact lenses to come into contact with non-sterile liquids (including tap water and saliva) as microbial contamination can occur, which may lead to permanent eye damage. Consult the eye care professional about wearing lenses during sporting and water related activities. Exposure to water while wearing contact lenses in activities such as swimming, water skiing, and hot tubs may increase the risk of ocular infection, including but not limited to Acanthamoeba keratitis. Avoid all harmful or irritating vapors or fumes while wearing lenses. Promptly remove a lens to avoid serious injury in the event that dust, a foreign body or other contaminant gets between the lens and the eye. Discard any lens which has become dehydrated or damaged. Replace with a sterile fresh, new lens. Patients should be instructed to remove their lenses before sleeping. The lens should move freely on the eye at all times. If the lens sticks (stops moving) on the eye, follow the recommended directions in the section Care for a Sticking Lens. If non-movement of the lens continues, the patient should be instructed to consult their eye care professional immediately. Patients should inform their employer of being a contact lens wearer. Some jobs may require the use of eye protection equipment or restrict the use of contact lenses in certain work environments.
5 Patients should inform their physician that contact lenses are worn and should consult their eye care professional before using any medication in the eye. Do not use lenses beyond the expiration date. Certain medications such as antihistamines, decongestants, diuretics, muscle relaxants, tranquilizers, and those for motion sickness may cause dryness of the eye, increased lens awareness, lens intolerance, blurred vision or visual changes. Patients should be informed of these potential conditions and proper remedial treatment should be prescribed if any of these conditions occur. Depending on the severity of the condition appropriate treatment may include the use of rewetting drops intended for use with soft contact lenses or temporary cessation of contact lens wear until the conditions subsides. It is strongly recommended that patients be provided with a copy of the HUBBLE Daily Wearers Guide available from Vision Path, Inc. and understand its contents prior to dispensing the lenses. The Patient Wearers Guide is available for download at Hubblecontacts.com. ADVERSE REACTIONS Potentially serious complications are usually accompanied by one or more of the following signs or symptoms: Foreign body sensation Excessive watering or other unusual eye secretions including mucopurulent discharge Redness of the eyes Photophobia (sensitivity to light) Burning, stinging, itching or other pain associated with the eyes Comfort is less compared to when the lens was first placed on eye Poor visual acuity (reduced sharpness of vision) Blurred vision, rainbows or halos around objects Feeling of dryness If any of the previous signs or symptoms occur: The patient should IMMEDIATELY REMOVE THE LENS(ES). If the discomfort or problem stops, the patient should discard the lens and replace it with a new one. IF THE PROBLEM CONTINUES AFTER INSERTING A NEW LENS, THE PATIENT SHOULD IMMEDIATELY REMOVE THE LENS(ES) AND CONTACT AN EYE CARE PROFESSIONAL AT ONCE Patients should be informed that a serious condition such as corneal ulcer, infection, corneal vascularization, or iritis may be present and may progress rapidly. Less serious reactions such as abrasions, infiltrates and bacterial conjunctivitis must be managed and treated early to avoid more serious complications. Additionally, contact lens wear may be associated with ocular changes which require consideration of discontinuation or restriction of wear. These include but are not limited to local or generalized corneal edema, epithelial microcysts, epithelial staining, infiltrates, neovascularization, endothelial polymegathism, tarsal papillary changes, conjunctival injection or iritis. PATIENT SELECTION Patient communication is vital. Patients who require visual correction but cannot adhere to the recommended care of the HUBBLE Daily contact lens should not be provided with this lens. All necessary steps in lens care and all precautions and warnings should be discussed and understood by the patient (Review Package Insert with patient). FITTING PROCEDURE Fitting Procedure for the Spherical HUBBLE Daily 1. Pre-fitting Examination 2. Trial Lens Evaluation 3. Lens Fit Assessment
6 4. Final Lens Power Determination 1. Pre-fitting Examination A pre-fitting examination is necessary to: assess the patient s motivation, physical state and willingness to comply with instructions regarding hygiene and wear schedule make ocular measurements for initial contact lens parameter selection collect baseline clinical information to which post-fitting examination results can be compared The pre-fitting examination should include: a thorough case history a spherocylindrical refraction keratometry tear film assessment biomicroscopy 2. Trial Lens Evaluation HUBBLE Daily contact lenses are available in a single base curve/diameter combination of 8.6/14.20 mm. Following initial power selection, a trial lens should be placed on the eye for assessment of lens fit and comfort, and final power verification. Initial Lens Power Selection: Select an initial lens power as close as possible to the patient s spherical equivalent refraction. The spherical equivalent refraction is determined as follows: Spherical Equivalent = Sphere power + Cylinder Power/2 Example: Spectacle Rx: -3.00D x 180 Spherical Equivalent: -3.00D D = -3.50D Remember: If the spherical equivalent is greater than ± 4.00D, a vertex distance correction is necessary to determine the correct lens power at the corneal plane. 3. Lens Fit Assessment HUBBLE Daily should be comfortable immediately upon placement on the eye. Care should be taken to ensure the lens is free of foreign particles such as lint, and is not inverted prior to placement on the eye. Reflex tearing due to an uncomfortable lens may cause the lens to stop moving and give the appearance of a tight fit. Allow the lenses to settle on the eyes for approximately 5 to 10 minutes. This allows time for the patient to adapt to the lenses and time for the lens to equilibrate. A well-fitted HUBBLE Daily has the following characteristics: 1. Good centration with full corneal coverage in all fields of gaze 2. Sufficient movement to allow tear exchange under the lens during the blink; 0.1 to 0.5 mm is generally considered optimal. 3. Satisfactory Push-Up Test o This test is a reliable indicator of a good fit. With the patient looking straight ahead, place your index finger on the patient s lower lid margin and gently nudge the edge of the lens upward. o A well-fitted lens will move freely when pushed upward with fingertip pressure and return quickly to its original position. 4. Good comfort and stable visual response (with over-refraction)
7 Characteristics of a Tight (Steep) Lens Fit A tight or steep lens fi t would display some or all of the following characteristics: 1. Insufficient or no lens movement during the blink in primary or upgaze 2. Unsatisfactory Push-Up Test A tight fitting lens will resist movement. If successfully nudged upward, the lens may remain decentered or return slowly to its original position. 3. Good centration 4. Good comfort 5. Fluctuating vision between blinks Characteristics of a Loose (Flat) Lens Fit A loose or flat lens fit would display some or all of the following characteristics: 1. Reduced comfort, usually accompanied by lower lid sensation 2. Poor centration with limbal exposure on exaggerated eye movement 3. Lens edge standoff 4. Excessive lens movement during the blink in primary or upgaze 5. Unsatisfactory Push-Up Test A loose fitting lens will move easily but may remain decentered or slip under the upper lid. 6. Vision may be blurred after the blink An inverted lens will mimic the characteristics of a loose lens. If any of the above signs occur remove the lens and check to make sure it is not inverted. General Fitting Tips While helpful for monitoring corneal stability over time, keratometry is not a reliable predictor of base curve/fi t relationship. Trial fitting of the individual eye is strongly recommended. A well-fitting lens will show less movement than generally thought, 0.1 to 0.5 mm is considered optimal. A flat base curve/cornea relationship may actually show limited movement. Decentration and excessive lid sensation accompanied by limited movement often indicates the lens is too flat for the given eye. If the criteria for a well-fitted lens cannot be achieved, do not dispense. 4. Final Lens Power Determination After the characteristics of a well-fitted lens have been satisfied, conduct a spherical over-refraction to determine the proper lens power to be dispensed. Example: Trial lens: -4.50D Over-refraction: -0.25D Final Lens Power: -4.75D Use a fresh, new pair of lenses for each trial fitting. Do not attempt to disinfect and re-use trial lenses. FOLLOW-UP EXAMINATIONS Follow-up care is necessary to ensure continued successful contact lens wear. Follow-up examinations should include: Case history, including questions to identify any problems related to contact lens wear Management of specific problems, if any, and A review with the patient of the lens wear and replacement schedule, proper lens handling procedures, and to ensure sufficient supply of spare lenses.
8 Follow-Up Examination Procedures: Prior to a follow-up examination, the contact lenses should be worn for at least four continuous hours. Record patient s symptoms, if any. Measure visual acuity monocularly and binocularly with the contact lenses in place. Perform an over-refraction to check for residual refractive error. With lenses in place, evaluate the fitting performance of the lenses to assure the criteria of a wellfitted lens continue to be satisfied. Examine the lenses closely for surface deposition and/or damage. Remove the lenses and conduct a thorough biomicroscopy examination. Periodically perform keratometry and spectacle refractions and compare the results with the initial measurements. If any observations are abnormal, use professional judgment to manage the problem and restore the eye to optimal conditions. If visual requirements or the criteria of a well-fitted lens are not satisfied during any follow-up examination, the patient should be re-fitted with a more appropriate lens. REPLACEMENT AND WEAR SCHEDULE HUBBLE Daily are intended to be worn once and then discarded at the end of each wearing period. The patient should be instructed to start the next wearing period with a fresh new lens. WEARING SCHEDULE Daily Wear (less than 24 hours, while awake) The maximum daily wearing time should be determined by the eye care professional based upon the patient s physiological eye condition because individual responses to contact lenses vary. There may be a tendency for patients to over wear the lenses initially. The eye care professional should stress the importance of adhering to the initial maximum wearing schedule. Studies have not been conducted to show that HUBBLE Daily are safe to wear during sleep, therefore patients should be advised to remove their lenses while sleeping. Normal daily wear of lenses assumes a minimum of 6 hours of non-lens wear per 24 hour period. Optimum individual wearing schedule will vary. MONOVISION FITTING GUIDELINES 1. Patient Selection A. Monovision Needs Assessment For a good prognosis the patient should have adequately corrected distance and near visual acuity in each eye. The amblyopic patient or the patient with significant astigmatism (greater than 1.50 diopter) in one eye may not be a good candidate for monovision with the HUBBLE Daily. Occupational and environmental visual demands should be considered. If the patient requires critical vision (visual acuity and stereopsis) it should be determined by trial whether this patient can function adequately with monovision. Monovision contact lens wear may not be optimal for such activities as: B. Patient Education Visually demanding situations such as operating potentially dangerous machinery or performing other potentially hazardous activities; and Driving automobiles (e.g., driving at night). Patients who cannot pass their state drivers license requirements with monovision correction should be advised to not drive with this correction, OR may require that additional over-correction be prescribed. All patients do not function equally well with monovision correction. Patients may not perform as well for certain tasks with this correction as they have with bifocal reading glasses. Each patient
9 2. Eye Selection should understand that monovision, as well as other presbyopic contact lenses, or other alternative, can create vision compromise that may reduce visual acuity and depth perception for distance and near tasks. During the fitting process it is necessary for the patient to realize the disadvantages as well as the advantages of clear near vision in straight ahead and upward gaze that monovision contact lenses provide. Generally, the non-dominant eye is corrected for near vision. The following test for eye dominance can be used. A. Ocular Preference Determination Methods Method 1 determine which eye is the sight eye. Have the patient point to and object at the far end of the room. Cover one eye. If the patient is still pointing directly at the object, the eye being used is the dominant (sighting) eye. Method 2 Determine which eye will accept the added power with the latest reduction in vision. Place a trial spectacle near add lens in front of one eye and then the other while the distance refractive error correction is in place for both eyes. Determine whether the patient functions best with the near add lens over the right or left eye. B. Refractive Error Method For anisometropic corrections, it is generally best to fit the more hyperopic (less myopic) eye for distance and the more myopic (less hyperopic) eye for near. C. Visual Demands Method Consider the patient's occupation during the eye selection process to determine the critical vision requirements. If a patient's gaze for near tasks is usually in one direction correct the eye on that side for near. Example: A secretary who places copy to the left side of the desk will usually function best with the near lens on the left eye. 3. Special Fitting Considerations Unilateral Lens Correction There are circumstances where only one contact lens is required. As an example, an emmetropic patient would only require a near lens while a bilateral myope may require only a distance lens. Example: A presbyopic emmetropic patient who requires a diopter add would have a lens on the near eye and the other eye left with a lens. A presbyopic patient requiring a diopter add who is diopters myopic in the right eye and diopters myopic in the left eye may have the right eye corrected for distance and the left uncorrected for near. 4. Near Add Determination
10 Always prescribe the lens power for the near eye that provides optimal near acuity at the midpoint of the patient's habitual reading distance. However, when more than one power provides optimal reading performance, prescribe the least plus (most minus) of the powers. 5. Trial Lens Fitting A trial fitting is performed in the office to allow the patient to experience monovision correction. Lenses are fit according to the directions in the general fitting guidelines and base curve selection described earlier in the guide. Case history and standard clinical evaluation procedure should be used to determine the prognosis. Determine which eye is to be corrected for distance and which eye is to be corrected for near. Next determine the near add. With trial lenses of the proper power in place observe the reaction to this mode of correction. Immediately after the correct power lenses are in place, walk across the room and have the patient look at you. Assess the patient's reaction to distance vision under these circumstances. Then have the patient look at familiar near objects such as a watch face or fingernails. Again assess the reaction. As the patient continues to look around the room at both near and distance objects, observe the reactions. Only after these vision tasks are completed should the patient be asked to read print. Evaluate the patient's reaction to large print (e.g. typewritten copy) at first and then graduate to news print and finally smaller type sizes. After the patient's performance under the above conditions are completed, tests of visual acuity and reading ability under conditions of moderately dim illumination should be attempted. An initial unfavorable response in the office, while indicative of a guarded prognosis, should not immediately rule out a more extensive trial under the usual conditions in which a patient functions. 6. Adaptation Visually demanding situations should be avoided during the initial wearing period. A patient may at first experience some mild blurred vision, dizziness, headaches, and a feeling of slight imbalance. You should explain the adaptation symptoms to the patient. These symptoms may last for a brief minute or for several weeks. the longer these symptoms persist, the poorer the prognosis for successful adaptation. To help in the adaptation process the patient can be advised to first use the lenses in a comfortable familiar environment such as in the home. Some patients feel that automobile driving performance may not be optimal during the adaptation process. This is particularly true when driving at night. Before driving a motor vehicle, it may be recommended that the patient be a passenger first to make sure that their vision is satisfactory for operating an automobile. During the first several weeks of wear (when adaptation is occurring), it may be advisable for the patient to only drive during optimal driving conditions. After adaptation and success with these activities, the patient should be able to drive under other conditions with caution. 7. Other Suggestions The success of the monovision technique may be further improved by having your patient follow the suggestions below. - Having a third contact lens (distance power) to use when critical distance viewing is needed. - Having a third contact lens (near power) to use when critical near viewing is needed. - Having supplemental spectacles to wear over the monovision contact lenses for specific visual tasks may improve the success of monovision correction. this is particularly applicable for those patients who cannot meet state licensing requirements with a monovision correction. - Make use of proper illumination when carrying out visual tasks. Success in fitting monovision can be improved by the following suggestions: - Reverse the distance and near eyes if a patient is having trouble adapting. - Refine the lens powers if there is trouble with adaptation. Accurate lens power is critical for presbyopic patients.
11 - Emphasize the benefits of the clear near vision in straight ahead and upward gaze with monovision. EMERGENCIES * The decision to fit a patient with a monovision correction is most appropriately left to the eye care practitioner in conjunction with the patient after carefully considering the patient's needs. * All patients should be supplied with a copy of the HUBBLE Daily Wearer s Guide. Patients should be informed that if chemicals of any kind (household products, gardening solutions, laboratory chemicals, etc.) are splashed into the eyes, the patient should: Flush eyes immediately with tap water or fresh saline solution, remove and discard the lens, and immediately contact the eye care professional or visit a hospital emergency room without delay. Additional information regarding emergency treatment may be provided on the product container label. REPORTING OF ADVERSE EVENTS If a patient experiences any serious adverse effects associated with the use of HUBBLE Daily, licensed eye care professionals please notify: VISION PATH, INC Broadway, Suite 300, New York, NY USA help@hubblecontacts.com U.S. Toll Free: HOW SUPPLIED HUBBLE Daily contact lenses are supplied sterile and packaged in strips of five foil sealed blister packs containing buffered saline solution. Five blister pack containers are attached to form a single strip. The package is marked with the base curve, diameter, dioptric power, manufacturing lot number and expiration date. Print Date: 05/2017
POLYMACON HYDROPHILIC CONTACT LENSES PRACTITIONER FITTING GUIDE
 POLYMACON HYDROPHILIC CONTACT LENSES PRACTITIONER FITTING GUIDE Part Number: PFG01005 Page 1 of 10 Table of Contents INTRODUCTION:... 3 MATERIAL:... 3 DESIGN:... 4 AVAILABLE LENS PARAMETERS... 4 INDICATIONS:...
POLYMACON HYDROPHILIC CONTACT LENSES PRACTITIONER FITTING GUIDE Part Number: PFG01005 Page 1 of 10 Table of Contents INTRODUCTION:... 3 MATERIAL:... 3 DESIGN:... 4 AVAILABLE LENS PARAMETERS... 4 INDICATIONS:...
Sofmed breathables XW (comfilcon A) Sofmed breathables XW premium (comfilcon A) Sofmed breathables XW toric (comfilcon A)
 Sofmed breathables XW (comfilcon A) Sofmed breathables XW premium (comfilcon A) Sofmed breathables XW toric (comfilcon A) Sofmed breathables XW multifocal (comfilcon A) SOFT (HYDROPHILIC) CONTACT LENSES
Sofmed breathables XW (comfilcon A) Sofmed breathables XW premium (comfilcon A) Sofmed breathables XW toric (comfilcon A) Sofmed breathables XW multifocal (comfilcon A) SOFT (HYDROPHILIC) CONTACT LENSES
PACKAGE INSERT RIGID GAS PERMEABLE CONTACT LENSES FOR ORTHOKERATOLOGY. (hexafocon B)
 PACKAGE INSERT BOSTON XO 2 (hexafocon B) RIGID GAS PERMEABLE CONTACT LENSES FOR ORTHOKERATOLOGY Daily Wear IMPORTANT: Please read carefully and keep this information for future use. This package insert
PACKAGE INSERT BOSTON XO 2 (hexafocon B) RIGID GAS PERMEABLE CONTACT LENSES FOR ORTHOKERATOLOGY Daily Wear IMPORTANT: Please read carefully and keep this information for future use. This package insert
senofilcon A Soft (hydrophilic) Contact Lenses Visibility Tinted with UV Blocker for Daily Disposable Wear
 IMPORTANT: Please read carefully and keep this information for future use. This Package Insert and Fitting Guide is intended for the Eye Care Professional, but should be made available to patients upon
IMPORTANT: Please read carefully and keep this information for future use. This Package Insert and Fitting Guide is intended for the Eye Care Professional, but should be made available to patients upon
Fitting Guide. Minutes-to-Fit 2.0. by SynergEyes Moving Vision Forward
 Fitting Guide Minutes-to-Fit 2.0 by SynergEyes Moving Vision Forward Minutes-to-Fit 2.0 Everyday success made easy! Thank you for making Duette HD, by SynergEyes, a part of your contact lens practice.
Fitting Guide Minutes-to-Fit 2.0 by SynergEyes Moving Vision Forward Minutes-to-Fit 2.0 Everyday success made easy! Thank you for making Duette HD, by SynergEyes, a part of your contact lens practice.
Orthokeratology. What is Orthokeratology? How does it work?
 What is? The goal of, OrthoK or corneal refractive therapy is to use a rigid gas permeable contact lens to reshape the cornea and provides correction for refractive errors. is a non-surgical procedure
What is? The goal of, OrthoK or corneal refractive therapy is to use a rigid gas permeable contact lens to reshape the cornea and provides correction for refractive errors. is a non-surgical procedure
An introduction to modern scleral lenses
 An introduction to modern scleral lenses The subject of scleral lenses seems to divide eye care practitioners more than just about any other type of contact lens. Views range considerably-some believing
An introduction to modern scleral lenses The subject of scleral lenses seems to divide eye care practitioners more than just about any other type of contact lens. Views range considerably-some believing
AIR OPTIX BRAND CONTACT LENSES LENS PARAMETER GUIDE November 2016
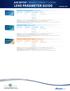 AIR OPTIX BRAND CONTACT LENSES AIR OPTIX plus HydraGlyde Contact Lenses 8.6 14.2 +6.00 to -8.00 +6.50 to +8.00-8.50 to -12.00 Material: Silicone Hydrogel, lotrafilcon B Water Content: 33% Dk/t: 138 @-3.00D
AIR OPTIX BRAND CONTACT LENSES AIR OPTIX plus HydraGlyde Contact Lenses 8.6 14.2 +6.00 to -8.00 +6.50 to +8.00-8.50 to -12.00 Material: Silicone Hydrogel, lotrafilcon B Water Content: 33% Dk/t: 138 @-3.00D
The BE Retainer System Certification Course. Supplement
 The System Certification Course Supplement The BE Retainer System Certification Course The System for Vision Shaping Treatment Certification Course Welcome to the System Certification Course. This handout
The System Certification Course Supplement The BE Retainer System Certification Course The System for Vision Shaping Treatment Certification Course Welcome to the System Certification Course. This handout
Acknowledgments. Paragon Vision Sciences Adoption of CRT certification slide for this generic talk. Material Considerations
 Corneal Reshaping Jeffrey J. Walline, OD PhD The Ohio State University College of Optometry Acknowledgments Paragon Vision Sciences Adoption of CRT certification slide for this generic talk Corneal Refractive
Corneal Reshaping Jeffrey J. Walline, OD PhD The Ohio State University College of Optometry Acknowledgments Paragon Vision Sciences Adoption of CRT certification slide for this generic talk Corneal Refractive
EVERY TREATMENT IS A TRUE ORIGINAL
 EVERY TREATMENT IS A TRUE ORIGINAL : The STAR S4 IR Excimer Laser System and the idesign Refractive Studio is indicated for wavefront-guided laser assisted in situ keratomileusis () to achieve monovision
EVERY TREATMENT IS A TRUE ORIGINAL : The STAR S4 IR Excimer Laser System and the idesign Refractive Studio is indicated for wavefront-guided laser assisted in situ keratomileusis () to achieve monovision
Dr. Thomas R. Reim President, DreimLens, Inc.
 DreamLens Fitting Guide Dr. Thomas R. Reim President, DreimLens, Inc. The introduction of the DreamLens in 1997 has revolutionized orthokeratology and now made it a viable procedure for the general population.
DreamLens Fitting Guide Dr. Thomas R. Reim President, DreimLens, Inc. The introduction of the DreamLens in 1997 has revolutionized orthokeratology and now made it a viable procedure for the general population.
PACKAGE INSERT RIGID GAS PERMEABLE CONTACT LENSES FOR CONTACT LENS CORNEAL REFRACTIVE THERAPY
 PACKAGE INSERT Paragon CRT Contact Lens Manufactured in Paragon HDS (paflufocon B) or Paragon CRT 00 Contact Lens Manufactured in Paragon HDS 00 (paflufocon D) RIGID GAS PERMEABLE CONTACT LENSES FOR CONTACT
PACKAGE INSERT Paragon CRT Contact Lens Manufactured in Paragon HDS (paflufocon B) or Paragon CRT 00 Contact Lens Manufactured in Paragon HDS 00 (paflufocon D) RIGID GAS PERMEABLE CONTACT LENSES FOR CONTACT
Spherical Soft Contact Lens Fitting. J. Perrigin OPTO 6374 Spring 2017
 Spherical Soft Contact Lens Fitting J. Perrigin OPTO 6374 Spring 2017 Reading Assignment Bennett & Henry: Chapter 11 pages 270-286 Tables 11.1 &11.2 Some Advantages of SCLs versus GPs Initial comfort,
Spherical Soft Contact Lens Fitting J. Perrigin OPTO 6374 Spring 2017 Reading Assignment Bennett & Henry: Chapter 11 pages 270-286 Tables 11.1 &11.2 Some Advantages of SCLs versus GPs Initial comfort,
Learn Connect Succeed. JCAHPO Regional Meetings 2017
 Learn Connect Succeed JCAHPO Regional Meetings 2017 Refractometry: Knowledge and Skills for Today s Technicians Matthew L. Parker, PhD, LSSMBB, COMT, CST, OSC U.S. Army (Retired) I have absolutely no financial
Learn Connect Succeed JCAHPO Regional Meetings 2017 Refractometry: Knowledge and Skills for Today s Technicians Matthew L. Parker, PhD, LSSMBB, COMT, CST, OSC U.S. Army (Retired) I have absolutely no financial
Scleral Fitting Guide
 Scleral Fitting Guide Scleral Lens Design...3 Fitting Philosophy...4 Select a Diagnostic Lens...5 Initial Lens Evaluation...6 Assess Scleral Landing Zone (SLZ), Sagittal Depth, Limbal Clearance, Lens Power...7-10
Scleral Fitting Guide Scleral Lens Design...3 Fitting Philosophy...4 Select a Diagnostic Lens...5 Initial Lens Evaluation...6 Assess Scleral Landing Zone (SLZ), Sagittal Depth, Limbal Clearance, Lens Power...7-10
NormalEyes Guidelines For Successful Fitting RIGID GAS PERMEABLE SCLERAL CONTACT LENSES. Manufactured in Paragon HDS 1OO (paflufocon D)
 NormalEyes 15.5 Guidelines For Successful Fitting RIGID GAS PERMEABLE SCLERAL CONTACT LENSES Manufactured in Paragon HDS 1OO (paflufocon D) FOR DAILY WEAR PARAGON VISION SCIENCES Just watch us 2013 Paragon
NormalEyes 15.5 Guidelines For Successful Fitting RIGID GAS PERMEABLE SCLERAL CONTACT LENSES Manufactured in Paragon HDS 1OO (paflufocon D) FOR DAILY WEAR PARAGON VISION SCIENCES Just watch us 2013 Paragon
Rejuvenating Aftercare for Contact Lens Patients
 Rejuvenating Aftercare for Contact Lens Patients Karen Walsh Karen Walsh explores the opportunities that exist in routine contact lens aftercare to enhance patient satisfaction and retention. The title
Rejuvenating Aftercare for Contact Lens Patients Karen Walsh Karen Walsh explores the opportunities that exist in routine contact lens aftercare to enhance patient satisfaction and retention. The title
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Material Identity Product Name: SunnyBrite II Product Code: 9303 Chemical Formula: General or Generic ID: Company Emergency Telephone Number BTR
Page 1 of 5 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Material Identity Product Name: SunnyBrite II Product Code: 9303 Chemical Formula: General or Generic ID: Company Emergency Telephone Number BTR
Material Safety Data Sheet
 Material Safety Data Sheet RMI - 25 Section 1: Identification of the substance/mixture and of the company /undertaking 1:1 Product identifier Product name: RMI-25 Cooling System Treatment 1:2 Relevant
Material Safety Data Sheet RMI - 25 Section 1: Identification of the substance/mixture and of the company /undertaking 1:1 Product identifier Product name: RMI-25 Cooling System Treatment 1:2 Relevant
Pardon the Objection (PTO) Contact Lens Clinical Case Debate (2 hours)
 Pardon the Objection (PTO) Contact Lens Clinical Case Debate (2 hours) Mile Brujic, O.D. Melissa Barnett, O.D. Glenda Secor, O.D. Jeff Sonsino, O.D. Summary The contact lens options that we have continue
Pardon the Objection (PTO) Contact Lens Clinical Case Debate (2 hours) Mile Brujic, O.D. Melissa Barnett, O.D. Glenda Secor, O.D. Jeff Sonsino, O.D. Summary The contact lens options that we have continue
"EYE CARE AND SAFETY"
 MAJOR PROGRAM POINTS "EYE CARE AND SAFETY" Part of the "GENERAL SAFETY SERIES" Quality Safety and Health Products, for Today... and Tomorrow Outline of Major Points Covered in the "Eye Safety" Course The
MAJOR PROGRAM POINTS "EYE CARE AND SAFETY" Part of the "GENERAL SAFETY SERIES" Quality Safety and Health Products, for Today... and Tomorrow Outline of Major Points Covered in the "Eye Safety" Course The
FITTING AND TROUBLESHOOTING SCLERAL LENSES
 FITTING AND TROUBLESHOOTING SCLERAL LENSES ANDREW J. BIONDO, OD, FSLS SCLERAL LENS ADVANTAGES Comfort Vision Centration Stability Health Moisture Easy to fit 1 WHO S A GOOD CANDIDATE Any irregular cornea
FITTING AND TROUBLESHOOTING SCLERAL LENSES ANDREW J. BIONDO, OD, FSLS SCLERAL LENS ADVANTAGES Comfort Vision Centration Stability Health Moisture Easy to fit 1 WHO S A GOOD CANDIDATE Any irregular cornea
SAFETY DATA SHEET (SDS)
 www.newdirectionsaromatics.com SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : December 2,2015 Print Date : September 15, 2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Onion Essential Oil
www.newdirectionsaromatics.com SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : December 2,2015 Print Date : September 15, 2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Onion Essential Oil
This training session discusses eye protection in the workplace. Your vision is vitally important think about what would happen if you lost it.
 This training session discusses eye protection in the workplace. Your vision is vitally important think about what would happen if you lost it. That s why it s so important to understand eye hazards on
This training session discusses eye protection in the workplace. Your vision is vitally important think about what would happen if you lost it. That s why it s so important to understand eye hazards on
Appendix C: Skill Stations Examination - Marking Rubric
 Appendix C: Skill Stations Examination - Marking Rubric This document provides the assessment sheets which are used by the examiners for the Skills Station examination component of the OCANZ Competency
Appendix C: Skill Stations Examination - Marking Rubric This document provides the assessment sheets which are used by the examiners for the Skills Station examination component of the OCANZ Competency
3M Eyewear Fit System
 3M Eyewear Fit System Background Occupational use of safety eyewear must be in compliance with applicable health and safety standards. In the U.S., employers must comply with the OSHA personal protective
3M Eyewear Fit System Background Occupational use of safety eyewear must be in compliance with applicable health and safety standards. In the U.S., employers must comply with the OSHA personal protective
Appendix C: Skill Stations Examination - Marking Rubric
 Appendix C: Skill Stations Examination - Marking Rubric This document provides the assessment sheets which are used by the examiners for the Skills Station examination component of the OCANZ Competency
Appendix C: Skill Stations Examination - Marking Rubric This document provides the assessment sheets which are used by the examiners for the Skills Station examination component of the OCANZ Competency
SAFETY DATA SHEET. Emergency telephone For health information only call Piscataway NJ
 SAFETY DATA SHEET According to Regulation (EC) No 1907/2006, Annex II, as amended by Regulation (EU) No 453/2010 1. Identification Product identifier Product name Product number 3030932/3040932 Recommended
SAFETY DATA SHEET According to Regulation (EC) No 1907/2006, Annex II, as amended by Regulation (EU) No 453/2010 1. Identification Product identifier Product name Product number 3030932/3040932 Recommended
ADDRESS (NUMBER, STREET, CITY, STATE, ZIP): 470 Magnum Dr. NE, Kalkaska, MI 49646
 Magnum Solvent, Inc. MSDS for Ethylene Glycol-Deionized Water 75/25 mix 1 - Site Specific Information Customer: 2 - Section I - General Information MANUFACTURER'S NAME: Magnum Solvent, Inc. EMERGENCY TELEPHONE
Magnum Solvent, Inc. MSDS for Ethylene Glycol-Deionized Water 75/25 mix 1 - Site Specific Information Customer: 2 - Section I - General Information MANUFACTURER'S NAME: Magnum Solvent, Inc. EMERGENCY TELEPHONE
PROFESSIONAL FITTING AND INFORMATION GUIDE. Paragon CRT Manufactured in Paragon HDS (paflufocon B)
 PROFESSIONAL FITTING AND INFORMATION GUIDE Paragon CRT Manufactured in Paragon HDS (paflufocon B) or Paragon CRT 100 Manufactured in Paragon HDS 1OO (paflufocon D) RIGID GAS PERMEABLE CONTACT LENSES FOR
PROFESSIONAL FITTING AND INFORMATION GUIDE Paragon CRT Manufactured in Paragon HDS (paflufocon B) or Paragon CRT 100 Manufactured in Paragon HDS 1OO (paflufocon D) RIGID GAS PERMEABLE CONTACT LENSES FOR
SAFETY DATA SHEET (SDS)
 SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : August 31,2018 Print Date : March 20, 2019 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Ho Wood Essential Oil INCI name : Cinnamomum Camphora
SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : August 31,2018 Print Date : March 20, 2019 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Ho Wood Essential Oil INCI name : Cinnamomum Camphora
SAFETY DATA SHEET BAL BOND
 Revision Date 22.05.15 Revision. SAFETY DATA SHEET BAL BOND SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product name BAL BOND 1.2. Relevant
Revision Date 22.05.15 Revision. SAFETY DATA SHEET BAL BOND SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product name BAL BOND 1.2. Relevant
SAFETY DATA SHEET ARDITEX CL LIQUID
 SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name 1.2. Relevant identified uses of the substance or mixture and uses
SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name 1.2. Relevant identified uses of the substance or mixture and uses
MATERIAL SAFETY DATA SHEET PRODUCT: ENVIRO-GUARD Pre-Charged Antifreezee Revision Date: 04/14/2011
 MATERIAL SAFETY DATA SHEET PRODUCT: ENVIRO-GUARD Pre-Charged Antifreezee Revision Date: 04/14/2011 HAZARD RATING Health: 0 Flammability: 1 Reactivity: 0 * Personal Protection: 0 1. GENERAL Trade Name:
MATERIAL SAFETY DATA SHEET PRODUCT: ENVIRO-GUARD Pre-Charged Antifreezee Revision Date: 04/14/2011 HAZARD RATING Health: 0 Flammability: 1 Reactivity: 0 * Personal Protection: 0 1. GENERAL Trade Name:
SAFETY DATA SHEET BAL PROTECTIVE SEALER
 Revision Date 22.05.15 SAFETY DATA SHEET SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product name 1.2. Relevant identified uses of the substance
Revision Date 22.05.15 SAFETY DATA SHEET SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product name 1.2. Relevant identified uses of the substance
First Aid Treatment for Sulfuric Acid Exposures Including Oleum and Chlorosulfonic Acid
 1 Disclaimer: Please note specifications and technical information are provided for guidance only and are intended to supplement, but not replace, user s design and safety criteria. The information is
1 Disclaimer: Please note specifications and technical information are provided for guidance only and are intended to supplement, but not replace, user s design and safety criteria. The information is
SAFETY DATA SHEET Kilfrost Windscreen Washing Fluid 3 (WWF MOD 3)
 Revision Date 04/08/2014 Revision 2 Supersedes Date 09/03/2013 SAFETY DATA SHEET According to ANSI Z-400. 1-2004 1. IDENTIFICATION Product Name Identified uses Windscreen wash fluid to remove ice, insect
Revision Date 04/08/2014 Revision 2 Supersedes Date 09/03/2013 SAFETY DATA SHEET According to ANSI Z-400. 1-2004 1. IDENTIFICATION Product Name Identified uses Windscreen wash fluid to remove ice, insect
Safety Data Sheet 1. IDENTIFICATION OF THE SUBSTANCE / PREPARATION AND OF THE COMPANY / UNDERTAKING
 Safety Data Sheet Revision date: May 4 th, 2015 Version: D SDS number: 10061607 1. IDENTIFICATION OF THE SUBSTANCE / PREPARATION AND OF THE COMPANY / UNDERTAKING 1.1. Product identifier: Product Name:
Safety Data Sheet Revision date: May 4 th, 2015 Version: D SDS number: 10061607 1. IDENTIFICATION OF THE SUBSTANCE / PREPARATION AND OF THE COMPANY / UNDERTAKING 1.1. Product identifier: Product Name:
SAFETY DATA SHEET (SDS)
 www.newdirectionsaromatics.com SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : April 18,2018 Print Date : April 20, 2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name : French Lavender Fragrance
www.newdirectionsaromatics.com SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : April 18,2018 Print Date : April 20, 2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name : French Lavender Fragrance
Ophthalmology Clinical Pearls A Quick Guide To Crystalens
 Ophthalmology Clinical Pearls A Quick Guide To Crystalens Crystalens Accommodating Posterior Chamber Intraocular Lens BRIEF STATEMENT Rx only. Indications for Use: The Crystalens is intended for primary
Ophthalmology Clinical Pearls A Quick Guide To Crystalens Crystalens Accommodating Posterior Chamber Intraocular Lens BRIEF STATEMENT Rx only. Indications for Use: The Crystalens is intended for primary
SAFETY DATA SHEET CLEENOL HEAVY DUTY CLEANER
 Revision Date 18/06/2010 Revision 1 SAFETY DATA SHEET SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product name 1.2. Relevant identified uses
Revision Date 18/06/2010 Revision 1 SAFETY DATA SHEET SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product name 1.2. Relevant identified uses
International Nutrition, Inc. Safety Data Sheet
 SECTION 1: IDENTIFICATION OF THE SUBSTANCE OR MIXTURE AND OF THE SUPPLIER 1.1 Product Identifier Product form: Free-flowing, fine granular mix Product name: Organic Iodine 40 1.2 Relevant identified uses
SECTION 1: IDENTIFICATION OF THE SUBSTANCE OR MIXTURE AND OF THE SUPPLIER 1.1 Product Identifier Product form: Free-flowing, fine granular mix Product name: Organic Iodine 40 1.2 Relevant identified uses
SAFETY DATA SHEET WINTERHALTER B22S DISHWASHING RINSEAID
 SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name 1.2. Relevant identified uses of the substance or mixture and uses
SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name 1.2. Relevant identified uses of the substance or mixture and uses
DISCLOSURES FITTING AND TROUBLESHOOTING SCLERAL LENSES WHO S A GOOD CANDIDATE SCLERAL LENS ADVANTAGES ANDREW J. BIONDO, OD, FSLS.
 FITTING AND TROUBLESHOOTING SCLERAL LENSES ANDREW J. BIONDO, OD, FSLS DISCLOSURES Disclosures: Paid consultant and lecturer for X Cel Specialty Contacts Paid consultant for SynergEyes Remote Education
FITTING AND TROUBLESHOOTING SCLERAL LENSES ANDREW J. BIONDO, OD, FSLS DISCLOSURES Disclosures: Paid consultant and lecturer for X Cel Specialty Contacts Paid consultant for SynergEyes Remote Education
Density and Stress in Plastics Mary V. McCrary
 SCIENCE EXPERIMENTS ON FILE Revised Edition 5.25-1 Density and Stress in Plastics Mary V. McCrary Topic Density and birefringence of plastics Time Part A: 30 to 45 minutes; Part B: 30 to 45 minutes! Safety
SCIENCE EXPERIMENTS ON FILE Revised Edition 5.25-1 Density and Stress in Plastics Mary V. McCrary Topic Density and birefringence of plastics Time Part A: 30 to 45 minutes; Part B: 30 to 45 minutes! Safety
SAFETY DATA SHEET (SDS)
 www.newdirectionsaromatics.com SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : March 16,2018 Print Date : October 18, 2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Eucalyptus Lemon Organic
www.newdirectionsaromatics.com SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : March 16,2018 Print Date : October 18, 2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Eucalyptus Lemon Organic
SAFETY DATA SHEET DPD No. 3 RAPID TABLETS
 SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product number 66149195, AD-0302, AL 030/100B, AT 030, PK 011, WAG-WE10953-10,
SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product number 66149195, AD-0302, AL 030/100B, AT 030, PK 011, WAG-WE10953-10,
8245 Backlick Rd Unit D, Lorton VA USA. Telephone number 888-WFMED-97 ( ) Emergency phone number 888-WFMED-97 ( )
 www.wfmed.com SAFETY DATA SHEET (SDS) Creation date: 10/28/2016 Revision date: 7/26/2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name Product code Botanical name Coffee Essential Oil EO-COFFE Coffea
www.wfmed.com SAFETY DATA SHEET (SDS) Creation date: 10/28/2016 Revision date: 7/26/2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name Product code Botanical name Coffee Essential Oil EO-COFFE Coffea
SAFETY DATA SHEET (SDS)
 www.newdirectionsaromatics.com SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : April 18,2018 Print Date : June 13, 2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Frankincense & Myrrh Fragrance
www.newdirectionsaromatics.com SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : April 18,2018 Print Date : June 13, 2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Frankincense & Myrrh Fragrance
WHS Guidelines Working with Chemicals
 WHS Guidelines Working with Chemicals 1. Scope These guidelines are applicable to all environments where are used. The purpose of this document is to detail minimum requirements for working with, to list
WHS Guidelines Working with Chemicals 1. Scope These guidelines are applicable to all environments where are used. The purpose of this document is to detail minimum requirements for working with, to list
SAFETY DATA SHEET PRIVATE LABEL BLEACH BASED SANITIZER PLB1037 SPD1032
 SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name 1.2. Relevant identified uses of the substance or mixture and uses
SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name 1.2. Relevant identified uses of the substance or mixture and uses
SAFETY DATA SHEET INSULCAST RTVS 27 LV GRAY PT A
 SAFETY DATA SHEET 1. Identification Product identifier Product name Recommended use of the chemical and restrictions on use Application Casting compound Details of the supplier of the safety data sheet
SAFETY DATA SHEET 1. Identification Product identifier Product name Recommended use of the chemical and restrictions on use Application Casting compound Details of the supplier of the safety data sheet
SAFETY DATA SHEET SECTION 1 : IDENTIFICATION SECTION 2 : HAZARD(S) IDENTIFICATION SECTION 3 : COMPOSITION / INFORMATION ON INGREDIENTS
 SAFETY DATA SHEET IDENTIFICATION NUMBER: 323120-09 PRODUCT NAME: PRODUCT USE: ACTIPHYTE MEADOWSWEET AQUEOUS Cosmetic Ingredient SECTION 1 : IDENTIFICATION MANUFACTURER: ACTIVE ORGANICS, INC 1097 YATES
SAFETY DATA SHEET IDENTIFICATION NUMBER: 323120-09 PRODUCT NAME: PRODUCT USE: ACTIPHYTE MEADOWSWEET AQUEOUS Cosmetic Ingredient SECTION 1 : IDENTIFICATION MANUFACTURER: ACTIVE ORGANICS, INC 1097 YATES
Injector system for implantation of 1stQ foldable intraocular lenses
 Instruction For Use 1st Inject Instrument Injector system for implantation of 1stQ foldable intraocular lenses The IFU is available electronically on our website: www.1stq.eu Content: The package contains
Instruction For Use 1st Inject Instrument Injector system for implantation of 1stQ foldable intraocular lenses The IFU is available electronically on our website: www.1stq.eu Content: The package contains
Safety Data Sheet Prepared on 05/20/2016 This form is subject to changes
 Safety Data Sheet Prepared on 05/20/2016 This form is subject to changes 1. Identification of substance: Product Name: Tungsten Disulfide in solution Product Number(s): WS2-100ml CAS-No. 12138-09-9 Manufacturer/Supplier:
Safety Data Sheet Prepared on 05/20/2016 This form is subject to changes 1. Identification of substance: Product Name: Tungsten Disulfide in solution Product Number(s): WS2-100ml CAS-No. 12138-09-9 Manufacturer/Supplier:
Version /28/2006
 1. PRODUCT AND COMPANY INFORMATION Company :...BASF Corporation 23700 Chagrin Blvd BEACHWOOD, OH 44122 Telephone : 216-839-7500 Emergency telephone number : (800) 424-9300 (703) 527-3887 (Outside Continental
1. PRODUCT AND COMPANY INFORMATION Company :...BASF Corporation 23700 Chagrin Blvd BEACHWOOD, OH 44122 Telephone : 216-839-7500 Emergency telephone number : (800) 424-9300 (703) 527-3887 (Outside Continental
SAFETY DATA SHEET. 1. Identification of the substance/mixture and of the company/undertaking
 1. Identification of the substance/mixture and of the company/undertaking 1.1 Identification of the substance/preparation Product name : FluoroVue Nucleic Acid Gel Stain (10,000X) Product code : NS1000/NS1001
1. Identification of the substance/mixture and of the company/undertaking 1.1 Identification of the substance/preparation Product name : FluoroVue Nucleic Acid Gel Stain (10,000X) Product code : NS1000/NS1001
SAFETY DATA SHEET ADI-FLOW Dry
 SAFETY DATA SHEET 1. Identification Product identifier Product name Product number ERP 5590 Recommended use of the chemical and restrictions on use Application FOR USE IN ANIMAL FEEDSTUFFS ONLY Details
SAFETY DATA SHEET 1. Identification Product identifier Product name Product number ERP 5590 Recommended use of the chemical and restrictions on use Application FOR USE IN ANIMAL FEEDSTUFFS ONLY Details
SAFETY DATA SHEET (SDS)
 www.newdirectionsaromatics.com SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : April 27,2018 Print Date : May 7, 2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Ylang Ylang Essential Oil
www.newdirectionsaromatics.com SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : April 27,2018 Print Date : May 7, 2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Ylang Ylang Essential Oil
Material Safety Data Sheet (MSDS) H100 & H100d Hydrophilic coating
 Material Safety Data Sheet (MSDS) H100 & H100d Hydrophilic coating Document date July 28, 2016 SECTION 1: Identification of the substance/mixture and of the company 1.1 Product identifier Product name:
Material Safety Data Sheet (MSDS) H100 & H100d Hydrophilic coating Document date July 28, 2016 SECTION 1: Identification of the substance/mixture and of the company 1.1 Product identifier Product name:
SAFETY DATA SHEET according to Regulation (EC) No. 1907/2006 Version 1.0 Revision Date
 SAFETY DATA SHEET according to Regulation (EC) No. 1907/2006 Version 1.0 Revision Date 26.06.2011 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING Product name Product Number HEMA
SAFETY DATA SHEET according to Regulation (EC) No. 1907/2006 Version 1.0 Revision Date 26.06.2011 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING Product name Product Number HEMA
SAFETY DATA SHEET TURFCOMPLEX
 SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product number BIO002/10, BIO002/100 1.2. Relevant identified uses
SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product number BIO002/10, BIO002/100 1.2. Relevant identified uses
SAFETY DATA SHEET (SDS)
 www.newdirectionsaromatics.com SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : June 7,2018 Print Date : June 20, 2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Spruce Essential Oil (Hemlock)
www.newdirectionsaromatics.com SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : June 7,2018 Print Date : June 20, 2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Spruce Essential Oil (Hemlock)
Rhodamine 6g Safety Data Sheet
 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form Substance name Product code : Substance : : LV505 1.2. Relevant identified uses of
SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form Substance name Product code : Substance : : LV505 1.2. Relevant identified uses of
Eye Injuries. Chapter 25
 Eye Injuries Chapter 25 Anatomy of the Eye Eye Injuries Can produce severe complications Examine pupil for shape and reaction (if you can see it) Appearance of Eye In a normal, uninjured eye, the entire
Eye Injuries Chapter 25 Anatomy of the Eye Eye Injuries Can produce severe complications Examine pupil for shape and reaction (if you can see it) Appearance of Eye In a normal, uninjured eye, the entire
SAFETY DATA SHEET DPD No. 1 RAPID TABLETS
 SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product number 66149194, AD-0102, AL 003B, AL 004B, AL 010/100B,AL
SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product number 66149194, AD-0102, AL 003B, AL 004B, AL 010/100B,AL
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Ethambutol Hydrochloride Tablets USP 100 mg and 400 mg Lupin Limited Mumbai 400 098 INDIA
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Ethambutol Hydrochloride Tablets USP 100 mg and 400 mg Lupin Limited Mumbai 400 098 INDIA
SAFETY DATA SHEET Confirms to OSHA Hazard Communication Standard (CFR ) HazCom 2012
 Product Identifier Product Name: Product Code: Cool Soak T5 SECTION 1 IDENTIFICATION Recommended Use of the Chemical and Restrictions for Use Recommended Use: Cleaner Restrictions for Use: Use only as
Product Identifier Product Name: Product Code: Cool Soak T5 SECTION 1 IDENTIFICATION Recommended Use of the Chemical and Restrictions for Use Recommended Use: Cleaner Restrictions for Use: Use only as
MATERIAL SAFETY DATA SHEET Gemini VS Poly Diamond Suspension
 1 MATERIAL SAFETY DATA SHEET Gemini VS Poly Diamond Suspension 164330-1643390 1 PRODUCT AND COMPANY IDENTIFICATION Product name Suspension Product Description Use of Substance / Preparation: polishing
1 MATERIAL SAFETY DATA SHEET Gemini VS Poly Diamond Suspension 164330-1643390 1 PRODUCT AND COMPANY IDENTIFICATION Product name Suspension Product Description Use of Substance / Preparation: polishing
Eye and Face Protection
 Eye and Face Protection Latest revised date: October 26, 2011 Page 1 of 9 1.0 Introduction Eye protection shall be worn wherever an individual is exposed to the threat of eye injury from contact with sharp,
Eye and Face Protection Latest revised date: October 26, 2011 Page 1 of 9 1.0 Introduction Eye protection shall be worn wherever an individual is exposed to the threat of eye injury from contact with sharp,
SAFETY DATA SHEET DPD XT TABLETS
 SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product No. AT-0330, AP 033, AT-0331, PM 033, AP 033/1,AL 200 Internal
SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product No. AT-0330, AP 033, AT-0331, PM 033, AP 033/1,AL 200 Internal
Compliance Requirements
 Compliance Requirements The compliance requirements for OSHA s Personal Protective Equipment regulations are very straightforward, and logical. GENERAL REQUIREMENTS (29 CFR 1910.132) The general OSHA Personal
Compliance Requirements The compliance requirements for OSHA s Personal Protective Equipment regulations are very straightforward, and logical. GENERAL REQUIREMENTS (29 CFR 1910.132) The general OSHA Personal
SAFETY DATA SHEET (SDS)
 www.africaimports.com SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : August 31,2018 Print Date : September 28, 2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Clary Sage Essential Oil Botanical
www.africaimports.com SAFETY DATA SHEET (SDS) Version 2.0 Revision Date : August 31,2018 Print Date : September 28, 2018 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Clary Sage Essential Oil Botanical
RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS
 RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS TRUST THE POWER OF PREVENTION WITH CINRYZE Whether your patients are new to treatment or are continuing their journey, remind
RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS TRUST THE POWER OF PREVENTION WITH CINRYZE Whether your patients are new to treatment or are continuing their journey, remind
Safety Data Sheet 1225BR
 Safety Data Sheet 1225BR 1 Material and Supplier Indentification Product Name: Description: Recommended use of the chemical and restrictions: High Gloss Finish Floor Finish Use only for the purpose on
Safety Data Sheet 1225BR 1 Material and Supplier Indentification Product Name: Description: Recommended use of the chemical and restrictions: High Gloss Finish Floor Finish Use only for the purpose on
SAFETY DATA SHEET. TRD Revision 01/30/ PRODUCT AND COMPANY IDENTIFICATION 2. HAZARDOUS IDENTIFICATION
 1. PRODUCT AND COMPANY IDENTIFICATION Product Identifier Recommended use Distributed by: Antifoam DyChem International 560 North 500 West Salt Lake City, UT 84116 (800) 453-4606 Emergency Contact # (24
1. PRODUCT AND COMPANY IDENTIFICATION Product Identifier Recommended use Distributed by: Antifoam DyChem International 560 North 500 West Salt Lake City, UT 84116 (800) 453-4606 Emergency Contact # (24
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET Manufacturer Jack's Magic Products, Inc. Address 6950 112th Circle Largo, FL 33773 Phone Number (for Information) 1-800-348-1656 Emergency Phone Number 1-800-424-9300 (international
MATERIAL SAFETY DATA SHEET Manufacturer Jack's Magic Products, Inc. Address 6950 112th Circle Largo, FL 33773 Phone Number (for Information) 1-800-348-1656 Emergency Phone Number 1-800-424-9300 (international
Refined Glycerine Kosher 99.50% Minimum CAS No
 Material Safety Data Sheet Effectivity Date : 26 August 2008 Page 1 of 6 Revision No. : 01 Refined Glycerine Kosher 99.50% Minimum CAS No. 56-81-5 1. Chemical Identification & Company Information Chemical
Material Safety Data Sheet Effectivity Date : 26 August 2008 Page 1 of 6 Revision No. : 01 Refined Glycerine Kosher 99.50% Minimum CAS No. 56-81-5 1. Chemical Identification & Company Information Chemical
SECTION I - PRODUCT IDENTIFICATION SECTION II - HAZARDOUS INGREDIENTS & OTHER COMPONENTS SECTION III PHYSICAL DATA / CONTENTS WITHOUT PROPELLENT
 Page 1 of 5 WBVA/CR-4 WATERBASE CROSSLINKER (DISCONTINUED) Written by Super User. Posted in MSDS SHEETS SECTION I - PRODUCT IDENTIFICATION Product Name: WBVA/CR-4 WATERBASE CROSSLINKER (DISCONTINUED) Revision:
Page 1 of 5 WBVA/CR-4 WATERBASE CROSSLINKER (DISCONTINUED) Written by Super User. Posted in MSDS SHEETS SECTION I - PRODUCT IDENTIFICATION Product Name: WBVA/CR-4 WATERBASE CROSSLINKER (DISCONTINUED) Revision:
Revision date: 29/02/2016 Revision: 4 Supersedes date: 16/05/2012
 SAFETY DATA SHEET According to the National Code of Practice for the Preparation of Material Safety Data Sheets 2nd Edition [NOHSC:2011(2003)] SECTION 1: Identification of the substance/mixture and of
SAFETY DATA SHEET According to the National Code of Practice for the Preparation of Material Safety Data Sheets 2nd Edition [NOHSC:2011(2003)] SECTION 1: Identification of the substance/mixture and of
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION 1.1 Product Identifiers Product Name(s): Biotin-16-deoxycytidine-5'-triphosphate Synonym: Biotin-16-dCTP Product Number: CC-6003 1.2 Uses Recommended use: Laboratory
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION 1.1 Product Identifiers Product Name(s): Biotin-16-deoxycytidine-5'-triphosphate Synonym: Biotin-16-dCTP Product Number: CC-6003 1.2 Uses Recommended use: Laboratory
DFO (1,8- Diazafluorene-9-one) 50g. Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations
 Revision date:12/26/2012 Supersedes:01/27/2011 Version: SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Substance Substance name
Revision date:12/26/2012 Supersedes:01/27/2011 Version: SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Substance Substance name
Safety Data Sheet MICRO-KILL DISINFECTANT WIPES
 Safety Data Sheet MICRO-KILL DISINFECTANT WIPES Section 1. Identification Product Identifier Synonyms Manufacturer Stock Numbers Recommended use Uses advised against Manufacturer Contact Address MICRO-KILL
Safety Data Sheet MICRO-KILL DISINFECTANT WIPES Section 1. Identification Product Identifier Synonyms Manufacturer Stock Numbers Recommended use Uses advised against Manufacturer Contact Address MICRO-KILL
ENVIRO STAINS (VARIOUS COLORS)
 ENVIRO STAINS (VARIOUS COLORS) Cohills Building Specialties, Inc. 3825 East Anne Street Phoenix, AZ 85040 Product Name: Enviro Stains (Various Colors) Product Use: Mixture for Concrete Staining SECTION
ENVIRO STAINS (VARIOUS COLORS) Cohills Building Specialties, Inc. 3825 East Anne Street Phoenix, AZ 85040 Product Name: Enviro Stains (Various Colors) Product Use: Mixture for Concrete Staining SECTION
Revision date: 29/02/2016 Revision: 8 Supersedes date: 09/12/2014
 SAFETY DATA SHEET According to the National Code of Practice for the Preparation of Material Safety Data Sheets 2nd Edition [NOHSC:2011(2003)] SECTION 1: Identification of the substance/mixture and of
SAFETY DATA SHEET According to the National Code of Practice for the Preparation of Material Safety Data Sheets 2nd Edition [NOHSC:2011(2003)] SECTION 1: Identification of the substance/mixture and of
Revision date: 24/02/2016 Revision: 5 Supersedes date: 14/09/2015
 SAFETY DATA SHEET According to the National Code of Practice for the Preparation of Material Safety Data Sheets 2nd Edition [NOHSC:2011(2003)] SECTION 1: Identification of the substance/mixture and of
SAFETY DATA SHEET According to the National Code of Practice for the Preparation of Material Safety Data Sheets 2nd Edition [NOHSC:2011(2003)] SECTION 1: Identification of the substance/mixture and of
SAFETY DATA SHEET CALCICOL No. 2 TABLETS
 SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product No. AL 200, AL 300, PM 252, SP 709E, SP 725E,AR 790HIL,
SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product No. AL 200, AL 300, PM 252, SP 709E, SP 725E,AR 790HIL,
City of Kalispell RESPIRATOR PROTECTION POLICY October 12, 2015
 A. Executive Summary and Introduction City of Kalispell RESPIRATOR PROTECTION POLICY October 12, 2015 1. Annual fit test is required for all employees who will be required to wear a respirator as a part
A. Executive Summary and Introduction City of Kalispell RESPIRATOR PROTECTION POLICY October 12, 2015 1. Annual fit test is required for all employees who will be required to wear a respirator as a part
0800 POISON A
 Safety Data Sheet Deltaphar 25EC Date of issue: 28 th May, 2014 1) Identification of Substance: Product name: Active Ingredient(s): ACVM Approval: EPA Approval: Distributed by: Emergency Number: Deltaphar
Safety Data Sheet Deltaphar 25EC Date of issue: 28 th May, 2014 1) Identification of Substance: Product name: Active Ingredient(s): ACVM Approval: EPA Approval: Distributed by: Emergency Number: Deltaphar
SAFETY DATA SHEET. CU-8120 Enzyme & bacteria digestive powder. None. (800) (800) Category 5 Category 3 Category 2B
 SAFETY DATA SHEET 1. Identification Product Identifier Other means of identification Product code Recommended use Recommended restrictions Manufacturer information Company name Address Telephone Fax Emergency
SAFETY DATA SHEET 1. Identification Product Identifier Other means of identification Product code Recommended use Recommended restrictions Manufacturer information Company name Address Telephone Fax Emergency
Tableau Wood Soap Ext 5 Office Hours (Mon- Fri 9am-5pm) only.
 Tableau Wood Soap 01424 575131 Ext 5 Office Hours (Mon- Fri 9am-5pm) only. 3.2. Mixtures MULTICOMPONENT ANIONIC/NONIONIC SURFACTANT BLEND 5-10% CAS number: EC number: Classification Skin Irrit. 2 - H315
Tableau Wood Soap 01424 575131 Ext 5 Office Hours (Mon- Fri 9am-5pm) only. 3.2. Mixtures MULTICOMPONENT ANIONIC/NONIONIC SURFACTANT BLEND 5-10% CAS number: EC number: Classification Skin Irrit. 2 - H315
SAFETY DATA SHEET according to 1907/2006/EC, Article 31
 SAFETY DATA SHEET according to 1907/2006/EC, Article 31 NEO 800 Page 1/5 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name NEO 800 1.2
SAFETY DATA SHEET according to 1907/2006/EC, Article 31 NEO 800 Page 1/5 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name NEO 800 1.2
SAFETY DATA SHEET EP2 GREASE
 SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product number XGE003, XGE125, XGE400, XGE500, QHE500 1.2. Relevant
SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product number XGE003, XGE125, XGE400, XGE500, QHE500 1.2. Relevant
PRODUCT SAFETY DATA SHEET
 PRODUCT SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING 1.1. Product Identifiers Product Name: Ready Mixed and Super Ready Mixed Paints (Standard, Fluorescent
PRODUCT SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING 1.1. Product Identifiers Product Name: Ready Mixed and Super Ready Mixed Paints (Standard, Fluorescent
Safety Data Sheet READYBATH SELECT MEDIUM WEIGHT CLEANSING WASHCLOTHS
 Safety Data Sheet READYBATH SELECT MEDIUM WEIGHT CLEANSING WASHCLOTHS Section 1. Identification Product Identifier Synonyms Manufacturer Stock Numbers Recommended use Uses advised against Manufacturer
Safety Data Sheet READYBATH SELECT MEDIUM WEIGHT CLEANSING WASHCLOTHS Section 1. Identification Product Identifier Synonyms Manufacturer Stock Numbers Recommended use Uses advised against Manufacturer
SAFETY DATA SHEET WINTERHALTER C15 CATERING DEGREASER - 5L
 SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name 1.2. Relevant identified uses of the substance or mixture and uses
SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name 1.2. Relevant identified uses of the substance or mixture and uses
SAFETY DATA SHEET Loxeal Grasso 9
 Revision Date 29/03/2013 Revision 1 SAFETY DATA SHEET SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product name 1.2. Relevant identified uses
Revision Date 29/03/2013 Revision 1 SAFETY DATA SHEET SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product name 1.2. Relevant identified uses
Material Safety Data Sheet
 Material Safety Data Sheet SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product number XW557 1.2. Relevant identified
Material Safety Data Sheet SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product number XW557 1.2. Relevant identified
SAFETY DATA SHEET ph 7 BUFFER TABLETS
 SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product number SK 400,SK 500,PT 105/5,PT 105/6,SL 170,SP 301,PT
SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name Product number SK 400,SK 500,PT 105/5,PT 105/6,SL 170,SP 301,PT
