Varithena Delivery System Instructions for Use
|
|
|
- Sherilyn McKinney
- 6 years ago
- Views:
Transcription
1 Varithena Delivery System Instructions for Use Please read all prescribing information before using the product. The Instructions for Use are for the entire Varithena system. There are 2 packaging configurations: Option A: Bi-Canister box and Administration Pack* Option B: Convenience box (Bi-Canister Box + 3 Ancillary Packs + 3 Varithena transfer units)* *The components in each packaging configuration are to be used only in conjunction with each other for activation of Varithena. Administration Packs can be used for either configuration for further treatment sessions. Always write the activation date and time on the canister and verify the product has not expired prior to use. Once the Varithena canister has been activated, the shelf life for the product is thirty days. Rx Only A canister of Varithena generates 90mL of foam which, following purging instructions contained in this IFU, is sufficient to yield 45mL of usable foam for injection. The gas mix of the foam is 65:35 O 2 :CO 2. WARNINGS: As the foam fills the syringe and before injecting, inspect the syringe full of foam for any visible bubbles. If there are any present, the foam should be emptied into the Varithena transfer unit waste chamber and the syringe refilled. Do not shake Varithena canisters. Always use a fresh pair of sterile gloves when handling the Bi-Canister and Varithena transfer unit. A new Varithena transfer unit must be used for each treatment session. Notes: Use a new sterile syringe after each injection. Never fill a syringe until just before the foam is required. The activated Varithena canister should always be stored with a Varithena transfer unit in place in the upright position at controlled room temperature in an appropriately controlled clean area to limit contamination. Use foam within 75 seconds of generation or discard and generate new foam. Page 1 of 17
2 Unpacking Varithena: Option A: Bi-Canister Box and Administration Pack Gather all the items needed for the generation of foam: the Varithena Bi-Canister box (Figure 1a), Administration Pack (including: Varithena transfer unit, manometer tube, compression pad and silicone-free syringes) (Figure 1b), and the following items that are not supplied: scissors, pen, sterile alcoholic wipes, timer and gloves (Figure 1c). Open the Varithena Bi-Canister box and remove the Varithena Bi-Canister pouch. Open the Administration Pack and remove the components. Inspect the pouch and components for damage (do not use product if there are any visible signs of damage to pouch or components). Figure 1a Varithena Bi-Canister Figure 1b Administration Pack Figure 1c Additional Procedure Items (not supplied) Page 2 of 17
3 Unpacking Varithena: Option B: Convenience Box (Bi-Canister Box + 3 Ancillary Packs + 3 Varithena transfer units) Gather all the items needed for the generation of foam: the Varithena Bi-Canister Box (Figure 2a), Ancillary Pack (including silicone-free syringes, manometer tubing, and compression pads and Varithena transfer unit (Figure 2b), and the following items that are not supplied: scissors, pen, sterile alcoholic wipes, timer and gloves (Figure 2c). Open the Varithena Convenience box and remove all the components. Open the Varithena Bi-Canister box and remove the Varithena Bi-Canister pouch. Open an Ancillary Pack and remove the components. Inspect the pouch and components for damage (do not use product if there are any visible signs of damage to pouch or components). Figure 2a Varithena Bi-Canister Figure 2b Three Ancillary Packs Figure 2c Additional Procedure Items (not Supplied) Page 3 of 17
4 Preparing the Patient Preparations for treating the patient with Varithena should include the following steps: Position the patient comfortably on the treatment table in a supine position with their hip externally rotated to facilitate access to the GSV. Use ultrasound to find the best site for venous access. Using an aseptic technique, infiltrate the skin over the venous access point with local anesthetic. Obtain venous access under ultrasound guidance. IV catheters that are 16 to 22 gauge and 40 - to 50 - mm long or micropuncture sets are recommended for venous access. Prefill the manometer tube with sterile heparinized normal saline solution and connect to the IV catheter. Confirm venous access by aspirating with a syringe, blood should be dark and under low pressure. Flush the IV catheter and manometer tube with heparinized normal saline and secure it to the skin with adhesive tape, leave the saline syringe connected. With the IV catheter in place and secure, place the patient supine and elevate the leg to approximately 45 degrees. Complete all preparation of the patient and preparations for Varithena injectable foam injection before generation of the foam. Page 4 of 17
5 Varithena Preparation 1 2 Wearing appropriate sterile gloves, open Bi- Canister pouch using a pair of scissors. Place canisters upright on a cleaned (sterile wipes) stable surface with the white oxygen canister on top (Figure 3). Discard empty pouch. Figure 3 Figure 4 Remove the safety clip by lifting one corner of the clip out (Figure 4). Discard the safety clip. Page 5 of 17
6 Gas Activation of the Varithena Canister 3 To begin the gas transfer, twist the canisters together clockwise (Figure 5) until they come to a stop and the small indicators/marks on the collars are aligned (Figure 6). You may hear a bubbling sound. While the canisters are activating, keep them upright on the clean flat surface for 1 minute. Use a timing device to keep track of the 1 minute time. Figure 5 Figure 6 Page 6 of 17
7 Gas Activation of the Varithena Canister 4 Note: In order to maintain sterility of the Varithena transfer unit, the following steps must be followed. While waiting 1 minute for the gas transfer, open a new Varithena transfer unit, blister pack, but leave the Varithena transfer unit in the package (Figure 7). The manometer tubing (20 inch) should have been previously filled with sterile heparinized normal saline solution. Figure 7 Page 7 of 17
8 Gas Activation of the Varithena Canister 5 After 1 minute, Twist the two canisters by turning them in the opposite direction (counterclockwise) as before (Figure 8). Pull straight up to separate the oxygen canister from the Varithena canister, as shown (Figure 9). Do not separate canisters until you have a Varithena transfer unit ready to place onto the Varithena canister (See step 6). Put the oxygen canister (with white collar) aside. The Varithena canister (with blue collar) should remain on a clean flat surface, in the upright position. Figure 8 Figure 9 Figure 10 Write today s date and time in the Date and Time of Activation box on the Varithena canister (Figure 10) Page 8 of 17
9 Connecting a new Varithena transfer unit and syringe 6 Remove the Varithena transfer unit from the blister pack, wearing a fresh pair of sterile gloves. Make sure not to touch the sterile underside of the Varithena transfer unit, (discard Varithena transfer unit if contaminated). Immediately place the Varithena transfer unit on top of the blue Varithena canister. Gently rotate the Varithena transfer unit clockwise as indicated (Figure 11) until it drops into the collar threads then twist the Varithena transfer unit (clockwise) until it reaches a stop (Figure 12). Figure 11 Figure 12 The system is now activated and ready for use. Page 9 of 17
10 Connecting a new Varithena transfer unit and syringe 7 Change the Varithena transfer unit immediately before each new treatment session. Once all preparations for injection are complete, i.e., cannula in situ, patient s leg elevated and a good ultrasound view of the saphenofemoral junction (SFJ) obtained, foam may be generated for immediate use. Open a sterile 10mL silicone-free syringe package and keep it in the package until needed. Remove the syringe from the package, and connect it to the Varithena transfer unit as shown (Figure 13). Figure 13 Priming a New Syringe 8 Gently press down the Varithena transfer unit to begin producing foam (Figure 14). Using continuous pressure, allow the silicone-free syringe to fill between 3mL and 5mL. Release the pressure on the Varithena transfer unit and leave the syringe connected. Figure 14 Priming a New Syringe Page 10 of 17
11 9 Push the silicone-free syringe plunger in fully to discard its contents (Figure 15). Do not disconnect the syringe. Note: The foam will automatically be diverted into the waste chamber within the Varithena transfer unit (Figure 16). This process eliminates the small quantity of air in the syringe and Varithena transfer unit. Figure 15 Figure 16 Page 11 of 17
12 Generation of Foam 10 Foam Generation: The technique to produce usable foam requires a single purge cycle before filling the syringe, a process that takes less than 1 second. Important Note: Foam must be generated by pushing down on the Varithena transfer unit continuously without pulling back on the plunger of the syringe (aspirating). While holding the silicone-free syringe plunger in place, gently press down on the Varithena transfer unit to begin the purge cycle (Figure 17). Visually inspect the flowing foam inside the Varithena transfer unit to make sure the visible air bubbles have been expelled (less than 1 second) before releasing the syringe plunger and allowing it to fill to the desired volume (Figure 18). Draw up to 5mL of foam into the syringe. Figure 17 Figure 18 Page 12 of 17
13 Inspecting and Injecting Foam 11 Figure 19 After the silicone-free syringe has filled to the desired volume, wait 10 seconds to allow the pressure to equalize before removing the syringe from the Varithena transfer unit (Figure 19). WARNING: As the foam fills the syringe and before injecting, inspect the syringe full of foam for any visible bubbles (easily seen with the unaided eye at arm s length). If there are any present, empty the foam into the Varithena transfer unit waste chamber and refill the syringe. 12 Remove the syringe from the Varithena transfer unit and inspect it for visible bubbles (Figure 20). Figure 20 If no visible bubbles are present then the foam is ready for use. Use the foam within 75 seconds of generation or discard and generate new foam. WARNING: The total amount of foam injected in any one treatment session must not exceed 15mL, comprised of individual injections of up to 5mL each. Figure 21 After each treatment session, mark-off on the canister label the number of aliquots of up to 5mL of usable foam drawn from the canister per step 11 (Figure 21). 13 Connect a syringe of freshly generated foam to the manometer tubing, which is already connected to the cannula, in preparation for the initial injection. The manometer tubing (20) inch should have been previously filled with sterile heparinized normal saline solution. Page 13 of 17
14 Inspecting and Injecting Foam 14 Inject the foam at approximately 0.5mL to 1.0mL per second through the manometer tubing. Five (5) mls of foam should be injected in approximately 10 seconds. Always inspect the foam as it passes through the manometer tubing for visible bubbles (Figure 22). If any visible bubbles are seen (easily seen with the unaided eye at arm's length) they should be aspirated back into the silicone-free syringe and the syringe contents discarded back into the Varithena transfer unit waste chamber, and a fresh syringe of foam generated. Notes: Use a new sterile syringe after each injection. Figure 22 WARNING: The total amount of foam injected in any one treatment session must not exceed 15mL, comprised of individual injections of up to 5mL each Do not remove Varithena transfer unit if the Varithena canister is to be stored (see Storage) Change sterile gloves appropriately, to limit any contamination of the Varithena transfer unit and Bi- Canister. Compression Pads 15 Once treatment is complete, the Compression Pads should be used: The objective of the pads is to focus the compression forces on the treated vein to keep them as free from blood as possible, thus minimizing retained thrombus. The compression pads supplied should be placed along the course of the treated trunk vein in the thigh, and over raised treated varicose veins above and below the knee. The pads may be shaped to follow the course of the veins. The pads should be placed outside the first layer of limited stretch bandage and held in place by a second layer of bandage. The appropriate length compression stocking is then applied. Page 14 of 17
15 Replacing the Varithena transfer unit Important Note: Do not replace the Varithena transfer unit if the canister is to be stored for future use. The activated Varithena canister should always be stored in an appropriately cleaned area with a Varithena transfer unit in place in the upright position at controlled room temperature. Replace the Varithena transfer unit just prior to the next treatment session. 16 Wearing appropriate new sterile gloves, hold the Varithena canister, twist the Varithena transfer unit counterclockwise and then pull up to separate from the canister (Figure 23). 17 Discard the old Varithena transfer unit and open a new Varithena transfer unit. Make sure not to touch the sterile underside of the Varithena transfer unit. Figure 23 Page 15 of 17
16 Replacing the Varithena transfer unit 18 Swab the uncovered shuttle with a fresh sterile alcohol wipe (Figure 24) and immediately place the Varithena transfer unit on top of the Varithena canister. Gently rotate the Varithena transfer unit clockwise until it drops into the collar threads (Figure 25), then twist the Varithena transfer unit (clockwise) until it reaches a stop (Figure 26). Figure 24 Figure 25 Figure 26 The Varithena device now ready for use for a new treatment session, following the instructions in Steps 7 to 15. Page 16 of 17
17 Storage and Disposal Note: The activated Varithena canister should always be stored with a Varithena transfer unit in place in the upright position at controlled room temperature in an appropriately controlled clean area to limit contamination. Once the Varithena canister has been activated, the shelf life for the product is thirty (30) calendar days. Always write the activation date and time on the canister and verify the product has not expired prior to use. Dispose of Varithena and oxygen canisters following local and state regulations for aerosol disposal. The Varithena transfer unit can be disposed of as non-toxic non-clinical waste. Net Contents: 18ml One canister of Varithena contains: 180mg Polidocanol, ethanol 756mg (96%), disodium hydrogen phosphate dihydrate 43.2mg, potassium dihydrogen phosphate 15.3mg, water for injection. One canister of Varithena generates 90mL of foam which, following purging instructions contained in this IFU, is sufficient to yield 45mL of usable foam for injection. The gas mix of the foam is 65:35 O2:CO2. NDC Varithena Bi-Canister Administration Pack NDC Varithena Convenience Pack Manufactured for Provensis Ltd by: Biocompatibles UK Ltd Chapman House, Weydon Lane, Farnham, Surrey, UK, GU9 8QL Distributed by: Biocompatibles Inc., Five Tower Bridge, Suite 810, 300 Barr Harbor Drive, West Conshohocken, PA, , USA. Varithena is a registered trademark of Provensis Ltd BTG and the BTG roundel logo are registered trademarks of BTG International Ltd Provensis Ltd, and Biocompatibles UK Ltd, are BTG International group companies Page 17 of 17
Varithena Delivery System Instructions for Use INSTRUCTIONS FOR USE Please read the Product Monograph before using the product.
 Varithena Delivery System Instructions for Use INSTRUCTIONS FOR USE Please read the Product Monograph before using the product. The Instructions for Use are for the entire Varithena TM system. There are
Varithena Delivery System Instructions for Use INSTRUCTIONS FOR USE Please read the Product Monograph before using the product. The Instructions for Use are for the entire Varithena TM system. There are
PREPARATION AND SELF-ADMINISTRATION INSTRUCTIONS FOR PATIENTS
 PREPARATION AND SELF-ADMINISTRATION INSTRUCTIONS FOR PATIENTS Hereditary Angioedema Jenny, a real HAE patient TAKE CONTROL OF YOUR HEREDITARY ANGIOEDEMA (HAE) TREATMENT WITH SELF-ADMINISTRATION Choosing
PREPARATION AND SELF-ADMINISTRATION INSTRUCTIONS FOR PATIENTS Hereditary Angioedema Jenny, a real HAE patient TAKE CONTROL OF YOUR HEREDITARY ANGIOEDEMA (HAE) TREATMENT WITH SELF-ADMINISTRATION Choosing
RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS
 RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS TRUST THE POWER OF PREVENTION WITH CINRYZE Whether your patients are new to treatment or are continuing their journey, remind
RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS TRUST THE POWER OF PREVENTION WITH CINRYZE Whether your patients are new to treatment or are continuing their journey, remind
RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS. Hereditary Angioedema
 RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS Hereditary Angioedema TRUST PROVEN PREVENTION WITH CINRYZE Whether your patients are new to treatment or are continuing their
RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS Hereditary Angioedema TRUST PROVEN PREVENTION WITH CINRYZE Whether your patients are new to treatment or are continuing their
PREPARATION AND SELF-ADMINISTRATION INSTRUCTIONS FOR PATIENTS
 PREPARATION AND SELF-ADMINISTRATION INSTRUCTIONS FOR PATIENTS Hereditary Angioedema TAKE CONTROL OF HEREDITARY ANGIOEDEMA (HAE) TREATMENT WITH FLEXIBLE ADMINISTRATION Choosing to treat with CINRYZE (C1
PREPARATION AND SELF-ADMINISTRATION INSTRUCTIONS FOR PATIENTS Hereditary Angioedema TAKE CONTROL OF HEREDITARY ANGIOEDEMA (HAE) TREATMENT WITH FLEXIBLE ADMINISTRATION Choosing to treat with CINRYZE (C1
RECOMBINATE. [Antihemophilic Factor (Recombinant)] (For intravenous use only)
![RECOMBINATE. [Antihemophilic Factor (Recombinant)] (For intravenous use only) RECOMBINATE. [Antihemophilic Factor (Recombinant)] (For intravenous use only)](/thumbs/79/80305643.jpg) Website for more information: http://www.recombinate.com/ INSTRUCTIONS FOR USE RECOMBINATE [Antihemophilic Factor (Recombinant)] (For intravenous use only) Do not attempt to do an infusion to yourself
Website for more information: http://www.recombinate.com/ INSTRUCTIONS FOR USE RECOMBINATE [Antihemophilic Factor (Recombinant)] (For intravenous use only) Do not attempt to do an infusion to yourself
Peripheral Venous Catheter Placement Simulator
 Attention Do not let ink from pens, newspapers, this manual or other sources come in contact with the manikin, as they cannot be cleaned the manikin skin. MW9 Peripheral Venous Catheter Placement Simulator
Attention Do not let ink from pens, newspapers, this manual or other sources come in contact with the manikin, as they cannot be cleaned the manikin skin. MW9 Peripheral Venous Catheter Placement Simulator
STANDARD OPERATING PROCEDURE ADVANCED INTRAVENOUS TRAINING ARM (S )
 Page 1 of 27 1. Scope This Standard Operating Procedure (SOP) applies to the staff and students using the Advanced Intravenous Training Arm in the Pharmacy Practice Resource Unit (PPRU) at the Pharmacy
Page 1 of 27 1. Scope This Standard Operating Procedure (SOP) applies to the staff and students using the Advanced Intravenous Training Arm in the Pharmacy Practice Resource Unit (PPRU) at the Pharmacy
TEV-TROPIN [somatropin (rdna origin) for injection] 5 mg & 10 mg
![TEV-TROPIN [somatropin (rdna origin) for injection] 5 mg & 10 mg TEV-TROPIN [somatropin (rdna origin) for injection] 5 mg & 10 mg](/thumbs/74/70163471.jpg) TEV-TROPIN [somatropin (rdna origin) for injection] 5 mg & 10 mg TEV-TROPIN (somatropin for injection) Information for the Patient or Parent Read all instructions before proceeding. Do not mix (reconstitute)
TEV-TROPIN [somatropin (rdna origin) for injection] 5 mg & 10 mg TEV-TROPIN (somatropin for injection) Information for the Patient or Parent Read all instructions before proceeding. Do not mix (reconstitute)
Venepuncture. Clinical Skills. Venepuncture. Dr Brian Jenkins (Clinical Skills Lead) Sian Williams (Clinical Skills Manager)
 Clinical Skills Venepuncture Dr Brian Jenkins (Clinical Skills Lead) Sian Williams (Clinical Skills Manager) Aims and Objectives Aims and Objectives The aim of this module is to facilitate learning regarding
Clinical Skills Venepuncture Dr Brian Jenkins (Clinical Skills Lead) Sian Williams (Clinical Skills Manager) Aims and Objectives Aims and Objectives The aim of this module is to facilitate learning regarding
limbsandthings.com Advanced Venepuncture Arm User Guide For more skills training products visit Limbs & Things Ltd.
 User Guide Advanced Venepuncture Arm Product No: 00290 (Light) Product No: 00298 (Brown) For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol,
User Guide Advanced Venepuncture Arm Product No: 00290 (Light) Product No: 00298 (Brown) For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol,
Aseptic Techniques. Techniques for Sterile Compounding. Pharmacy Technician Training Systems Passassured, LLC
 Aseptic Techniques Techniques for Sterile Compounding Pharmacy Technician Training Systems Passassured, LLC Aseptic Techniques, Tech for Sterile Compounding PassAssured's Pharmacy Technician Training Program
Aseptic Techniques Techniques for Sterile Compounding Pharmacy Technician Training Systems Passassured, LLC Aseptic Techniques, Tech for Sterile Compounding PassAssured's Pharmacy Technician Training Program
Standard. Standard. Venepuncture Arm. Light. Part No: Brown. Part No: Black. Part No: 00332
 Setup Guide Standard Standard Light. Part No: 000 Brown. Part No: 00 Black. Part No: 00 Part No: 065-964 Issue, June 0 0 Limbs & Things For more skills training products visit limbsandthings.com Limbs
Setup Guide Standard Standard Light. Part No: 000 Brown. Part No: 00 Black. Part No: 00 Part No: 065-964 Issue, June 0 0 Limbs & Things For more skills training products visit limbsandthings.com Limbs
Chapter 40. Fluid, Electrolyte, and Acid Base Balance. Procedures Checklist INTRAVENOUS THERAPY. Procedure 40.1: Starting an Intravenous Infusion
 Procedures Checklist INTRAVENOUS THERAPY Chapter 40 Fluid, Electrolyte, and Acid Base Balance Procedure 40.1: Starting an Intravenous Infusion Performed Preparation Yes No Mastered Comments 1. Assess:
Procedures Checklist INTRAVENOUS THERAPY Chapter 40 Fluid, Electrolyte, and Acid Base Balance Procedure 40.1: Starting an Intravenous Infusion Performed Preparation Yes No Mastered Comments 1. Assess:
Veterinary Science Handling and Restraining Practicum. Restraint of the Cat for Cephalic IV Catheter Placement
 Career Development Event Last updated: //3 Handling and Restraining Practicum Restraint of the Cat for Cephalic IV Catheter Placement. The student places the cat in sternal recumbency on an 6 examination
Career Development Event Last updated: //3 Handling and Restraining Practicum Restraint of the Cat for Cephalic IV Catheter Placement. The student places the cat in sternal recumbency on an 6 examination
Patients Guide for Self Administering Intravenous Antibiotics General points
 Patients Guide for Self Administering Intravenous Antibiotics General points An intravenous line is an open route for infection therefore you need to prevent this by washing your hands before any procedure
Patients Guide for Self Administering Intravenous Antibiotics General points An intravenous line is an open route for infection therefore you need to prevent this by washing your hands before any procedure
The NAMIC Dream Kit. Single Operator Control. Reduce Contrast Waste. Less Force to Inject. Compensator * Manifold. How the Compensator Manifold Works:
 Dream Kit The NAMIC Dream Kit Single Operator Control. Reduce Contrast Waste. Less Force to Inject. For more than 40 years, NAMIC * Fluid Management products have been providing cardiologists, technicians
Dream Kit The NAMIC Dream Kit Single Operator Control. Reduce Contrast Waste. Less Force to Inject. For more than 40 years, NAMIC * Fluid Management products have been providing cardiologists, technicians
PIMA MEDICAL INSTITUTE RADIOGRAPHY PROGRAM ARRT GENERAL PATIENT CARE COMPETENCIES CPR
 CPR STUDENT CLINICAL SITE Perform CPR Demonstrate the proper techniques utilized for CPR Provided preceptor with a copy of current CPR certification card *Not * If a competency is initialed as Not, it
CPR STUDENT CLINICAL SITE Perform CPR Demonstrate the proper techniques utilized for CPR Provided preceptor with a copy of current CPR certification card *Not * If a competency is initialed as Not, it
Procedure 85 Attaching The Humidifier To The Oxygen Flow Meter Or Regulator. Procedure 86 Administering Oxygen Through A Nasal Cannula
 Chapter 12 Respiratory Procedures Procedure 81 Checking Capillary Refill Procedure 82 Using A Pulse Oximeter Procedure 83 Preparing Wall-Outlet Oxygen Procedure 84 Preparing The Oxygen Cylinder Procedure
Chapter 12 Respiratory Procedures Procedure 81 Checking Capillary Refill Procedure 82 Using A Pulse Oximeter Procedure 83 Preparing Wall-Outlet Oxygen Procedure 84 Preparing The Oxygen Cylinder Procedure
Instruction for Use of Balloon Inflator JS-CE-SM-12-01, A/0
 Instruction for Use of Balloon Inflator JS-CE-SM-12-01, A/0 Instruction for Use of Balloon Inflator Product name Balloon Inflator Product structure and components The Balloon Inflator can be divided into
Instruction for Use of Balloon Inflator JS-CE-SM-12-01, A/0 Instruction for Use of Balloon Inflator Product name Balloon Inflator Product structure and components The Balloon Inflator can be divided into
Standard. Bag & Stand Advanced Venepuncture Arm. Light. Part No: Brown. Part No: Black. Part No: 00442
 Setup Guide Bag & Stand Advanced Standard Light. Part No: 00440 Brown. Part No: 0044 Black. Part No: 0044 Part No: 065-0 Issue, May 0 0 Limbs & Things For more skills training products visit limbsandthings.com
Setup Guide Bag & Stand Advanced Standard Light. Part No: 00440 Brown. Part No: 0044 Black. Part No: 0044 Part No: 065-0 Issue, May 0 0 Limbs & Things For more skills training products visit limbsandthings.com
TITLE: Pulse Oximetry COMPETENCY #: Resp #1 NEW COMPETENCY REVISION DATE: 10/18/12 EMPLOYEE NAME. DATE INITIAL RE-EVALUATION ANNUAL (if required) PRN
 : Pulse Oximetry COMPETENCY #: Resp #1 NEW COMPETENCY REVISION : 10/18/12 INITIAL RE-EVALUATION ANNUAL (if required) PRN 1. Verifies physician order. 2. Gathers equipment and supplies. 3. Knocks on door
: Pulse Oximetry COMPETENCY #: Resp #1 NEW COMPETENCY REVISION : 10/18/12 INITIAL RE-EVALUATION ANNUAL (if required) PRN 1. Verifies physician order. 2. Gathers equipment and supplies. 3. Knocks on door
ASAHI Neurovascular Guide Wire
 ASAHI Neurovascular Guide Wire AMK-DT244 Ver.1.00 / 11TS073 Legal manufacturer Consult instructions for use Do not use if package is damaged RX ONLY Caution: Federal (USA) Law restricts this device to
ASAHI Neurovascular Guide Wire AMK-DT244 Ver.1.00 / 11TS073 Legal manufacturer Consult instructions for use Do not use if package is damaged RX ONLY Caution: Federal (USA) Law restricts this device to
Alfred ECMO Circuit Priming and Storage Guide
 Alfred ECMO Circuit Priming and Storage Guide 1 March 2012 Check List Page 2. Assembly, Priming and Storage Page 3. Prepare ECMO trolley and Circuit immediately before use Page 6. Connection to Patient
Alfred ECMO Circuit Priming and Storage Guide 1 March 2012 Check List Page 2. Assembly, Priming and Storage Page 3. Prepare ECMO trolley and Circuit immediately before use Page 6. Connection to Patient
SPEMS SKILLS PROFICIENCY CRITERIA Paramedic
 SPEMS SKILLS PROFICIENCY CRITERIA Paramedic The following skills are required at the Paramedic Level: 1. King Airway 2. IV 3. Endotracheal Intubation 4. Adult EZ IO 5. Pedi EZ IO 6. Pleural Decompression
SPEMS SKILLS PROFICIENCY CRITERIA Paramedic The following skills are required at the Paramedic Level: 1. King Airway 2. IV 3. Endotracheal Intubation 4. Adult EZ IO 5. Pedi EZ IO 6. Pleural Decompression
Nita Newborn Model 1800 User Manual
 Nita Newborn Model 1800 User Manual 308 South Sequoia Parkway, Canby, Oregon 97013 USA ph. 503.651.5050 fax 503.651.5052 email info@vatainc.com www.vatainc.com Meet Nita Newborn TM Nita Newborn is a unique
Nita Newborn Model 1800 User Manual 308 South Sequoia Parkway, Canby, Oregon 97013 USA ph. 503.651.5050 fax 503.651.5052 email info@vatainc.com www.vatainc.com Meet Nita Newborn TM Nita Newborn is a unique
Ultrasound Compatible Lumbar Puncture/ Epidural Simulator
 M43E Ultrasound Compatible Lumbar Puncture/ Epidural Simulator Instruction Manual Contents Please read General information Preparation Before training Training After training P.1 P.2-6 P.7 P.8-10 It includes
M43E Ultrasound Compatible Lumbar Puncture/ Epidural Simulator Instruction Manual Contents Please read General information Preparation Before training Training After training P.1 P.2-6 P.7 P.8-10 It includes
Contacts. Quick Start Guide
 Contacts Clinical Support Specialist: Phone: Cell Phone: Email: Fresenius Renal Technologies A division of Fresenius Medical Care North America 920 Winter Street Waltham, MA 02451 Technical Service Customer
Contacts Clinical Support Specialist: Phone: Cell Phone: Email: Fresenius Renal Technologies A division of Fresenius Medical Care North America 920 Winter Street Waltham, MA 02451 Technical Service Customer
O P E R A T O R S M A N U A L
 Danville Materials a Zest Anchors, LLC company 2875 Loker Avenue East Carlsbad, CA 92010 (1)760-743-7744 From the pioneers in dental air abrasion. www.zestdent.com PrepStart H 2 O TM O P E R A T O R S
Danville Materials a Zest Anchors, LLC company 2875 Loker Avenue East Carlsbad, CA 92010 (1)760-743-7744 From the pioneers in dental air abrasion. www.zestdent.com PrepStart H 2 O TM O P E R A T O R S
Parts identification:
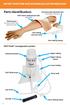 ARTERY PUNCTURE AND INTRAMUSCULAR TRAINING ARM Parts identification: FAST-derm replacement skin (removable) Intramuscular injection pad (removable for cleaning) Training arm (includes one FAST-derm replacement
ARTERY PUNCTURE AND INTRAMUSCULAR TRAINING ARM Parts identification: FAST-derm replacement skin (removable) Intramuscular injection pad (removable for cleaning) Training arm (includes one FAST-derm replacement
HEALTH SERVICES CODE A.6 CATEGORY: RN - SNP
 NURSING PROCEDURE TITLE ARTERIAL BLOOD SAMPLING (NEONATAL) A. Umbilical Catheter Blood Collection B. Radial Arterial Line CATEGORY: RN - SNP PURPOSE To obtain blood samples for laboratory analysis NURSING
NURSING PROCEDURE TITLE ARTERIAL BLOOD SAMPLING (NEONATAL) A. Umbilical Catheter Blood Collection B. Radial Arterial Line CATEGORY: RN - SNP PURPOSE To obtain blood samples for laboratory analysis NURSING
Making Life Easier Easypump II
 Making Life Easier Easypump II The elastomeric pump system for short- and long-term infusion therapy Elastomeric Infusion Systems Technical Guide Easypump II Disposable Elastomeric Infusion Pump System
Making Life Easier Easypump II The elastomeric pump system for short- and long-term infusion therapy Elastomeric Infusion Systems Technical Guide Easypump II Disposable Elastomeric Infusion Pump System
TJF-Q180V Cleaning and Disinfection Checklist
 TJF-Q180V Cleaning and Disinfection Checklist TJF-Q180V Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-Q180V has been performed
TJF-Q180V Cleaning and Disinfection Checklist TJF-Q180V Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-Q180V has been performed
Chest Drains. All Covenant Health Intermediate Care Nursery staff. Needle Aspiration
 Approved by: Chest Drains Gail Cameron Director, Maternal, Neonatal & Child Health Programs Neonatal Nursery Policy & Procedures Manual : April 2013 Next Review April 2016 Dr. Ensenat Medical Director,
Approved by: Chest Drains Gail Cameron Director, Maternal, Neonatal & Child Health Programs Neonatal Nursery Policy & Procedures Manual : April 2013 Next Review April 2016 Dr. Ensenat Medical Director,
AQUADEX FLEXFLOW SYSTEM QUICK REFERENCE GUIDE
 AQUADEX FLEXFLOW SYSTEM QUICK REFERENCE GUIDE AQUADEX FLEXFLOW SYSTEM DISCLAIMER The quick reference guide is not intended to replace CHF Solutions, Inc. Aquadex FlexFlow Direction For Use (DFU). Always
AQUADEX FLEXFLOW SYSTEM QUICK REFERENCE GUIDE AQUADEX FLEXFLOW SYSTEM DISCLAIMER The quick reference guide is not intended to replace CHF Solutions, Inc. Aquadex FlexFlow Direction For Use (DFU). Always
Articulating Legs with Intraosseous Infusion Simulator LF03840U Instruction Manual
 Articulating Legs with Intraosseous Infusion Simulator LF03840U Instruction Manual Products by Nasco C. O. A. D. B. E. M. I. J. G. K. H. F. N. L. About the Simulator Designed to attach easily to any Life/form
Articulating Legs with Intraosseous Infusion Simulator LF03840U Instruction Manual Products by Nasco C. O. A. D. B. E. M. I. J. G. K. H. F. N. L. About the Simulator Designed to attach easily to any Life/form
AR251/AR251-B. Venepuncture and Infusion Arm. Instruction Manual
 AR251/AR251-B Venepuncture and Infusion Arm Instruction Manual Thank you for purchasing this AR251 Venepuncture and Infusion Arm. Cast from life, it shows a well developed male left arm in fine detail.
AR251/AR251-B Venepuncture and Infusion Arm Instruction Manual Thank you for purchasing this AR251 Venepuncture and Infusion Arm. Cast from life, it shows a well developed male left arm in fine detail.
ATI Skills Modules Checklist for Oxygen Therapy
 For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Oxygen Therapy Student s name Date Verify order Patient record Assess for procedure need Identify, gather, and prepare equipment
For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Oxygen Therapy Student s name Date Verify order Patient record Assess for procedure need Identify, gather, and prepare equipment
Blood Pressure Monitoring: Arterial Line
 Approved by: Blood Pressure Monitoring: Arterial Line Gail Cameron Senior Director, Operations, Maternal, Neonatal & Child Health Programs Dr. Ensenat Medical Director, Neonatology Neonatal Nursery Policy
Approved by: Blood Pressure Monitoring: Arterial Line Gail Cameron Senior Director, Operations, Maternal, Neonatal & Child Health Programs Dr. Ensenat Medical Director, Neonatology Neonatal Nursery Policy
TJF-Q180V Cleaning and Disinfection Checklist
 TJF-Q180V Cleaning and Disinfection Checklist TJF-Q180V Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-Q180V has been performed
TJF-Q180V Cleaning and Disinfection Checklist TJF-Q180V Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-Q180V has been performed
Mixing Methacholine Dilutions for Bronchoprovocation Challenge Testing
 powder for solution, for inhalation Mixing Methacholine Dilutions for Bronchoprovocation Challenge Testing Provocholine is a registered trademark of Methapharm Inc. Copyright Methapharm Inc. 2016. All
powder for solution, for inhalation Mixing Methacholine Dilutions for Bronchoprovocation Challenge Testing Provocholine is a registered trademark of Methapharm Inc. Copyright Methapharm Inc. 2016. All
Instructions For Use. Elastomeric Infusion Device
 Instructions For Use Elastomeric Infusion Device Elastomeric Infusion device This brochure is intended as a guide for the proper use of the mobifuser disposable infusion pump. The mobifuser is a safe,
Instructions For Use Elastomeric Infusion Device Elastomeric Infusion device This brochure is intended as a guide for the proper use of the mobifuser disposable infusion pump. The mobifuser is a safe,
Indications for Use: Caution: Note:
 IV 1 2 IV This reference is to be used in conjunction with the Crit-Line IV Monitor User s Guide (P/N CL80050002). Refer to the User s Guide for a complete description of alerts, warnings, cautions, and
IV 1 2 IV This reference is to be used in conjunction with the Crit-Line IV Monitor User s Guide (P/N CL80050002). Refer to the User s Guide for a complete description of alerts, warnings, cautions, and
Dilution Sequence Protocol
 Dilution Sequence Protocol US Package Insert Multi-Patient (2-5 patients; requires 2 vials of Provocholine) 0.025 0.25 2.5 10 25 Provocholine 100 mg/vial (US NDC 64281-100-06) 1 Provocholine (methacholine
Dilution Sequence Protocol US Package Insert Multi-Patient (2-5 patients; requires 2 vials of Provocholine) 0.025 0.25 2.5 10 25 Provocholine 100 mg/vial (US NDC 64281-100-06) 1 Provocholine (methacholine
Installation of Your SprayMaster System
 Installation of Your SprayMaster System 1. At the installation site, remove all equipment from the corrugated box and the polyethylene drum and replace the drum lid. Check the picture to identify each
Installation of Your SprayMaster System 1. At the installation site, remove all equipment from the corrugated box and the polyethylene drum and replace the drum lid. Check the picture to identify each
Dilution Sequence Protocol
 Dilution Sequence Protocol ATS Short Quadrupling Doses 0.0625 0.25 1 4 16 Provocholine 1600 mg/vial (US NDC 64281-016-01) 1 Provocholine (methacholine chloride USP) powder for solution, for inhalation,
Dilution Sequence Protocol ATS Short Quadrupling Doses 0.0625 0.25 1 4 16 Provocholine 1600 mg/vial (US NDC 64281-016-01) 1 Provocholine (methacholine chloride USP) powder for solution, for inhalation,
NEXUS TKO. Specialty Neonatal/Pediatric. Safety Infusion Sets. Blood Reflux Protection 24/7! Anti-Reflux Technology Provides
 Specialty Neonatal/Pediatric Safety Infusion Sets Anti-Reflux Technology Provides Blood Reflux Protection 24/7! NEXUS TKO TECHNOLOGY THAT NEVER SLEEPS! Nexus TKO Neonatal Safety Catheter Nexus Tri-Seal
Specialty Neonatal/Pediatric Safety Infusion Sets Anti-Reflux Technology Provides Blood Reflux Protection 24/7! NEXUS TKO TECHNOLOGY THAT NEVER SLEEPS! Nexus TKO Neonatal Safety Catheter Nexus Tri-Seal
Clinician Guide. Baxter Elastomeric Devices. Promoting patient-centric care
 Clinician Guide Baxter Elastomeric Devices Promoting patient-centric care Portfolio Overview Portfolio Overview Baxter Elastomeric Devices are non-electronic medication devices designed to provide ambulatory
Clinician Guide Baxter Elastomeric Devices Promoting patient-centric care Portfolio Overview Portfolio Overview Baxter Elastomeric Devices are non-electronic medication devices designed to provide ambulatory
EMERGENCY EQUIPMENT TROLLEY DAILY SIGNATURE SHEET. Please refer to the Emergency Equipment stock poster for detailed information
 EMERGENCY EQUIPMENT TROLLEY DAILY SIGNATURE SHEET Please refer to the Emergency Equipment stock poster for detailed information Equipment must be checked daily and immediately following an emergency event
EMERGENCY EQUIPMENT TROLLEY DAILY SIGNATURE SHEET Please refer to the Emergency Equipment stock poster for detailed information Equipment must be checked daily and immediately following an emergency event
Tourniquet System. Ideal for single or bi-lateral orthopaedic surgery and pain management. AT4 Tourniquet System. Introduction.
 Tourniquet System Ideal for single or bi-lateral orthopaedic surgery and pain management Innovative technology practically applied Renowned for innovation and high quality, Anetic Aid listens to customers
Tourniquet System Ideal for single or bi-lateral orthopaedic surgery and pain management Innovative technology practically applied Renowned for innovation and high quality, Anetic Aid listens to customers
RayPette Plus Autoclavable Pipette. User Manual
 RayPette Plus Autoclavable Pipette User Manual CONTENTS 1. YOUR NEW PIPETTE... 1 1.1. Adjustable volume pipettes... 1 1.3 Fully autoclavable... 2 2. UNPACKING... 3 3. INSTALLING THE PIPETTE HOLDER... 4
RayPette Plus Autoclavable Pipette User Manual CONTENTS 1. YOUR NEW PIPETTE... 1 1.1. Adjustable volume pipettes... 1 1.3 Fully autoclavable... 2 2. UNPACKING... 3 3. INSTALLING THE PIPETTE HOLDER... 4
CENTRAL IOWA HEALTHCARE Marshalltown, Iowa
 CENTRAL IOWA HEALTHCARE Marshalltown, Iowa INTENSIVE CARE UNIT POLICY & PROCEDURE Policy Number: R-28/4.22 Subject: Purpose: Policy: Arterial Monitoring Use of intra-arterial pressure monitoring allows
CENTRAL IOWA HEALTHCARE Marshalltown, Iowa INTENSIVE CARE UNIT POLICY & PROCEDURE Policy Number: R-28/4.22 Subject: Purpose: Policy: Arterial Monitoring Use of intra-arterial pressure monitoring allows
Xcela Hybrid PICC. Clinician Reference Tool
 Xcela Hybrid PICC Clinician Reference Tool Xcela * Hybrid PICC Overview ew Three Lumens, Two Valves Two PASV * valved lumens designed to prevent blood reflux that could lead to catheter-related complications
Xcela Hybrid PICC Clinician Reference Tool Xcela * Hybrid PICC Overview ew Three Lumens, Two Valves Two PASV * valved lumens designed to prevent blood reflux that could lead to catheter-related complications
Standard. Abdominal Aortic Aneurysm (AAA) Repair Trainer. Part No: 60610
 User Guide Abdominal Aortic Aneurysm (AAA) Repair Trainer Part No: 6060 Standard Part No: 065-088 Issue, June 005 04 Limbs & Things For more Vascular training products visit limbsandthings.com Limbs &
User Guide Abdominal Aortic Aneurysm (AAA) Repair Trainer Part No: 6060 Standard Part No: 065-088 Issue, June 005 04 Limbs & Things For more Vascular training products visit limbsandthings.com Limbs &
Stretch Net tubular elastic bandage Illustrated Guide
 Stretch Net tubular elastic bandage Illustrated Guide www.deroyal.com SECTION PAGE SIZE Product Listing... 3 ALL Finger... 5 1-2 Foot.... 6 3-4 Elbow, Forearm, or Knee... 7 3-4 Hand... 8 3-4 Head - Full
Stretch Net tubular elastic bandage Illustrated Guide www.deroyal.com SECTION PAGE SIZE Product Listing... 3 ALL Finger... 5 1-2 Foot.... 6 3-4 Elbow, Forearm, or Knee... 7 3-4 Hand... 8 3-4 Head - Full
The pipettes cover a volume range from 0.1µl to 10ml.
 CONTENTS 1. YOUR NEW PIPETTE 1 1.1. Adjustable volume pipettes 1 1.2. Fixed volume pipettes 2 1.3 Fully autoclavable 3 2. UNPACKING 3 3. INSTALLING THE PIPETTE HOLDER 4 4. PIPETTE COMPONENTS 5 5. PIPETTE
CONTENTS 1. YOUR NEW PIPETTE 1 1.1. Adjustable volume pipettes 1 1.2. Fixed volume pipettes 2 1.3 Fully autoclavable 3 2. UNPACKING 3 3. INSTALLING THE PIPETTE HOLDER 4 4. PIPETTE COMPONENTS 5 5. PIPETTE
Operation and Maintenance Manual for GENTEC Model 881VR Continuous/Intermittent Digital Suction Regulators
 Operation and Maintenance Manual for GENTEC Model 881VR Continuous/Intermittent Digital Suction Regulators Genstar Technologies Co., Inc. 4525 Edison Avenue Chino, CA 91710 USA TEL 909-0-272 FAX 909-0-485
Operation and Maintenance Manual for GENTEC Model 881VR Continuous/Intermittent Digital Suction Regulators Genstar Technologies Co., Inc. 4525 Edison Avenue Chino, CA 91710 USA TEL 909-0-272 FAX 909-0-485
INSTRUCTIONS FOR USE - US RaplixaSpray (Raplixa Delivery Device)
 INSTRUCTIONS FOR USE - US RaplixaSpray (Raplixa Delivery Device) INSTRUCTIONS FOR USE - US RaplixaSpray (Raplixa Delivery Device) For the application of Raplixa powder to stop bleeding. Raplixa is a hemostatic
INSTRUCTIONS FOR USE - US RaplixaSpray (Raplixa Delivery Device) INSTRUCTIONS FOR USE - US RaplixaSpray (Raplixa Delivery Device) For the application of Raplixa powder to stop bleeding. Raplixa is a hemostatic
HemoCue Hb Procedure Template
 HemoCue Hb 201 + Procedure Template PURPOSE The HemoCue Hb 201 + System is used for the quantitative determination of hemoglobin in blood using a specially designed analyzer, HemoCue Hb 201 +, and specially
HemoCue Hb 201 + Procedure Template PURPOSE The HemoCue Hb 201 + System is used for the quantitative determination of hemoglobin in blood using a specially designed analyzer, HemoCue Hb 201 +, and specially
ARTERIAL MONITORING. Final Approval: VP Operations, Chief Nursing Officer Distribution: ICU, PACU POLICY:
 Policy Number: R-28 / Care of Patient 4.22 Section: Intensive Care Date Written: June 1988 Date(s) Revised: 8/98, 6/01, 11/03 Date(s) Reviewed: 4/92, 12/93, 7/95, 6/04 Reviewed/Approved by: Dept.Cmt. Date
Policy Number: R-28 / Care of Patient 4.22 Section: Intensive Care Date Written: June 1988 Date(s) Revised: 8/98, 6/01, 11/03 Date(s) Reviewed: 4/92, 12/93, 7/95, 6/04 Reviewed/Approved by: Dept.Cmt. Date
Dilution Sequence Protocol
 Dilution Sequence Protocol ATS Short Quadrupling Doses 0.0625 0.25 1 4 16 Provocholine 100 mg/vial (US NDC 64281-100-06) 1 Provocholine (methacholine chloride USP) powder for solution, for inhalation,
Dilution Sequence Protocol ATS Short Quadrupling Doses 0.0625 0.25 1 4 16 Provocholine 100 mg/vial (US NDC 64281-100-06) 1 Provocholine (methacholine chloride USP) powder for solution, for inhalation,
CVC Insertion Simulator
 M93UB CVC Insertion Simulator Don t mark on the model and other components with pen or leave printed materials contacted on their surface. Ink marks on the models will be irremovable. Instruction Manual
M93UB CVC Insertion Simulator Don t mark on the model and other components with pen or leave printed materials contacted on their surface. Ink marks on the models will be irremovable. Instruction Manual
MANITOBA RENAL PROGRAM
 MANITOBA RENAL PROGRAM SUBJECT Fresenius 5008 Preparation for Hemodialysis using the ONLINEplus System SECTION CODE 30.10.02 30.10 Hemodialysis: Equipment and Procedures AUTHORIZATION Professional Advisory
MANITOBA RENAL PROGRAM SUBJECT Fresenius 5008 Preparation for Hemodialysis using the ONLINEplus System SECTION CODE 30.10.02 30.10 Hemodialysis: Equipment and Procedures AUTHORIZATION Professional Advisory
Digital Display large color coded LED display is easy to read precise measuring shows actual cuff pressure
 Pressure Regulation microprocessor controlled fast cuff inflation and defl ation regulator knob allows very precise regulation limits pressure to max. 600 mmhg Digital Display large color coded LED display
Pressure Regulation microprocessor controlled fast cuff inflation and defl ation regulator knob allows very precise regulation limits pressure to max. 600 mmhg Digital Display large color coded LED display
Presented by ARAMSCO
 Iso-Chamber Setup Instructions Presented by ARAMSCO STC Environmental Specialty Products A Division of Seattle Tarp Company Iso-Chamber, Isolation Chamber United States Patent No. 6,969,346 Effective 11/29/2005
Iso-Chamber Setup Instructions Presented by ARAMSCO STC Environmental Specialty Products A Division of Seattle Tarp Company Iso-Chamber, Isolation Chamber United States Patent No. 6,969,346 Effective 11/29/2005
Patient Sleep Test Hookup
 Patient Sleep Test Hookup Dear Patient, The following information is designed to help you fit the apparatus for your sleep study. It is important that you follow the instructions carefully. 1. Preparation
Patient Sleep Test Hookup Dear Patient, The following information is designed to help you fit the apparatus for your sleep study. It is important that you follow the instructions carefully. 1. Preparation
Cavitron DualSelect. Introduction ENGLISH. Directions For Use. Cavitron DualSelect Dispensing System ENGLISH 1
 ENGLISH Cavitron DualSelect Dispensing System Directions For Use Please read carefully and completely before operating unit. Introduction Cavitron DualSelect Dispensing System Congratulations. Your purchase
ENGLISH Cavitron DualSelect Dispensing System Directions For Use Please read carefully and completely before operating unit. Introduction Cavitron DualSelect Dispensing System Congratulations. Your purchase
Assisting with Insertion. Care of Intraspinal Catheters
 Guidelines included: Assisting with an Insertion Care of Various types of Intraspinal s Care of the Intraspinal Infusion Monitoring Removal of the Short Term Non Assisting with Insertion INR should be
Guidelines included: Assisting with an Insertion Care of Various types of Intraspinal s Care of the Intraspinal Infusion Monitoring Removal of the Short Term Non Assisting with Insertion INR should be
Pipettor. User Manual
 Pipettor User Manual Product Code Description Single Chanel Pipettor Variable 550.002.055 Volume 0.5 to 10ul 550.002.060 2 to 20ul 550.002.065 10 to 100ul 550.002.070 20 to 200ul 550.002.075 100 to 100ul
Pipettor User Manual Product Code Description Single Chanel Pipettor Variable 550.002.055 Volume 0.5 to 10ul 550.002.060 2 to 20ul 550.002.065 10 to 100ul 550.002.070 20 to 200ul 550.002.075 100 to 100ul
Skills & Scenarios 81
 Skills & Scenarios 81 Psychomotor Skills Checklist Skill Lab Clinical Date Initial Date Initial 1. Change I.V. tubing 2. Change I.V solution 3. Regulate I.V. flow rate 4. Flush peripheral lock 5. Change
Skills & Scenarios 81 Psychomotor Skills Checklist Skill Lab Clinical Date Initial Date Initial 1. Change I.V. tubing 2. Change I.V solution 3. Regulate I.V. flow rate 4. Flush peripheral lock 5. Change
Tourniquet Systems Pressure Infusion
 Tourniquet Systems Pressure Infusion VBM Medizintechnik GmbH VBM 1 VBM 2 Summary Tourniquets and Accessories Digital Tourniquets... Compressed Air Tourniquets... Electronic Tourniquets... Application Tourniquets...
Tourniquet Systems Pressure Infusion VBM Medizintechnik GmbH VBM 1 VBM 2 Summary Tourniquets and Accessories Digital Tourniquets... Compressed Air Tourniquets... Electronic Tourniquets... Application Tourniquets...
Tridak Model 1050 User Guide
 Tridak Model 1050 User Guide Syringe Filling System Instructions for Safe Use Setup and Operation Maintenance Ordering Spare Parts and Accessories 2 Tridak Model 1050 Syringe Filling System User Guide
Tridak Model 1050 User Guide Syringe Filling System Instructions for Safe Use Setup and Operation Maintenance Ordering Spare Parts and Accessories 2 Tridak Model 1050 Syringe Filling System User Guide
INSTRUCTION MANUAL Elisa Baby-PICC
 The simulator is ideal for techniques and skills in vascular access and intravenous therapy. The newborn is made of semi-flexible and non-toxic material. It measures approximately 52cm in height and weighs
The simulator is ideal for techniques and skills in vascular access and intravenous therapy. The newborn is made of semi-flexible and non-toxic material. It measures approximately 52cm in height and weighs
Abdominal Aortic Aneurysm (AAA) Repair Trainer User Guide
 Abdominal Aortic Aneurysm (AAA) Repair Trainer User Guide Designed and manufactured by Limbs & Things Limited, Sussex Street, St. Philips, Bristol, BS 0RA, UK. Telephone: +44 (0)7 3 0500 Fax: +44 (0)7
Abdominal Aortic Aneurysm (AAA) Repair Trainer User Guide Designed and manufactured by Limbs & Things Limited, Sussex Street, St. Philips, Bristol, BS 0RA, UK. Telephone: +44 (0)7 3 0500 Fax: +44 (0)7
Elcometer 138. Bresle Kit and Patches. Operating Instructions
 Elcometer 138 English Bresle Kit and Patches Operating Instructions R English The Conductivity Meter model B-173 has been tested in accordance with EU regulations governing Electro-magnetic compliance
Elcometer 138 English Bresle Kit and Patches Operating Instructions R English The Conductivity Meter model B-173 has been tested in accordance with EU regulations governing Electro-magnetic compliance
EASYPUMP II THE FLEXIBLE SOLUTION FOR SHORT AND LONG TERM INFUSION THERAPIES
 THE FLEXIBLE SOLUTION FOR SHORT AND LONG TERM INFUSION THERAPIES CONTENT 4 PATIENT INFORMATION 5 Patient data 6 Treatment monitoring 7 Further notes 8 PRODUCT INFORMATION 9 Easypump II 9 Working principle
THE FLEXIBLE SOLUTION FOR SHORT AND LONG TERM INFUSION THERAPIES CONTENT 4 PATIENT INFORMATION 5 Patient data 6 Treatment monitoring 7 Further notes 8 PRODUCT INFORMATION 9 Easypump II 9 Working principle
INSTRUCTION MANUAL FOR POLYKEG DISPOSABLE KEG
 TABLE OF CONTENTS OF MANUAL Chapters 1.0 Product description 2.0 Safety rules for the utilisation of the keg 3.0 Instructions for manual filling 4.0 Instructions for automatic filling 5.0 Instructions
TABLE OF CONTENTS OF MANUAL Chapters 1.0 Product description 2.0 Safety rules for the utilisation of the keg 3.0 Instructions for manual filling 4.0 Instructions for automatic filling 5.0 Instructions
SomnoSuite FAQ. Setup. Calibration 4. What are the calibration requirements for the SomnoSuite? Settings
 SomnoSuite FAQ V1.3 January 2015 Setup 1. How do I connect the SomnoSuite to my oxygen source? 2. Is there a way to speed up the downward movement of the pusher block when setting the empty position? 3.
SomnoSuite FAQ V1.3 January 2015 Setup 1. How do I connect the SomnoSuite to my oxygen source? 2. Is there a way to speed up the downward movement of the pusher block when setting the empty position? 3.
Ultrasound Guided Thoracentesis Simulator Strap-on Type
 Caution Do not mark on the model and other components with pen nor leave printed materials contacted on surface. Ink marks on the models cannot be removed. MW4A Ultrasound Guided Thoracentesis Simulator
Caution Do not mark on the model and other components with pen nor leave printed materials contacted on surface. Ink marks on the models cannot be removed. MW4A Ultrasound Guided Thoracentesis Simulator
Arterial Punctures Blood Gas Collections (ABG)
 Arterial Punctures Blood Gas Collections (ABG) Danny Grabau, CPT, (NHA) Clinical Core Laboratory Services Phlebotomy Education Assistant Randy Gruhlke, MS, PBT(ASCP) Associate Program Director, Phlebotomy
Arterial Punctures Blood Gas Collections (ABG) Danny Grabau, CPT, (NHA) Clinical Core Laboratory Services Phlebotomy Education Assistant Randy Gruhlke, MS, PBT(ASCP) Associate Program Director, Phlebotomy
Ultrasound Guided Thoracentesis Simulator Strap-on Type
 Do not mark on the model and other components with pen nor leave printed materials contacted on surface. Ink marks on the models cannot be removed. MW4A Ultrasound Guided Simulator Strap-on Type Instruction
Do not mark on the model and other components with pen nor leave printed materials contacted on surface. Ink marks on the models cannot be removed. MW4A Ultrasound Guided Simulator Strap-on Type Instruction
Before you start sampling, be sure to read
 6. DISSOLVED OXYGEN MONITORING: MONITORING: Using the Titration Method Before you start sampling, be sure to read the following pages to familiarize yourself with the equipment and the procedures that
6. DISSOLVED OXYGEN MONITORING: MONITORING: Using the Titration Method Before you start sampling, be sure to read the following pages to familiarize yourself with the equipment and the procedures that
1.0 PURPOSE The purpose of this procedure is to describe the use of basic aseptic technique.
 1.0 PURPOSE The purpose of this procedure is to describe the use of basic aseptic technique. 2.0 RESPONSIBILITY 2.1 It is the responsibility of each employee working in a laboratory setting to follow this
1.0 PURPOSE The purpose of this procedure is to describe the use of basic aseptic technique. 2.0 RESPONSIBILITY 2.1 It is the responsibility of each employee working in a laboratory setting to follow this
Instructions and User Guide. Ultrasound Musculoskeletal Training Model BPMSK-1000 Series. April 26, 2016 Rev 1.0
 Instructions and User Guide Ultrasound Musculoskeletal Training Model BPMSK-1000 Series April 26, 2016 Rev 1.0 1 Overview 1 Giving you the confidence only experience can offer Congratulations on the purchase
Instructions and User Guide Ultrasound Musculoskeletal Training Model BPMSK-1000 Series April 26, 2016 Rev 1.0 1 Overview 1 Giving you the confidence only experience can offer Congratulations on the purchase
New. The high security connection
 L ON Cata C lo gu e O O G Y New The high security connection Preparation devices for medications/solutions Bag qimospike B 7218.01 qimospike B is an IV fluid bag access spike fitted with qimo. Aspiration
L ON Cata C lo gu e O O G Y New The high security connection Preparation devices for medications/solutions Bag qimospike B 7218.01 qimospike B is an IV fluid bag access spike fitted with qimo. Aspiration
AT Kelly Torso. Directions for Use
 AT Kelly Torso Directions for Use Table of Contents Recommends 4 Items Included 5 Skills Taught 5 Preparing the Manikin for Use 6 Head 6 Lungs 6 Abdominal Thrust 6 Instructions for Use 6 Airway Management
AT Kelly Torso Directions for Use Table of Contents Recommends 4 Items Included 5 Skills Taught 5 Preparing the Manikin for Use 6 Head 6 Lungs 6 Abdominal Thrust 6 Instructions for Use 6 Airway Management
AT Kelly Torso. Directions for Use
 AT Kelly Torso Directions for Use 260-00001 2 Table of Contents Recommends 4 Items Included 5 Skills Taught 5 Preparing the Manikin for Use 6 Head 6 Lungs 6 Abdominal Thrust 6 Instructions for Use 6 Airway
AT Kelly Torso Directions for Use 260-00001 2 Table of Contents Recommends 4 Items Included 5 Skills Taught 5 Preparing the Manikin for Use 6 Head 6 Lungs 6 Abdominal Thrust 6 Instructions for Use 6 Airway
COLLECTION OF BLOOD. Types of patients
 COLLECTION OF BLOOD Capillary Sampling The specimen of choice for laboratory analysis is generally a venous specimen. For blood gas analysis, the specimen of choice originates from an arterial draw. When
COLLECTION OF BLOOD Capillary Sampling The specimen of choice for laboratory analysis is generally a venous specimen. For blood gas analysis, the specimen of choice originates from an arterial draw. When
R2S Standard Sampling Assembly OPERATION PROCEDURE
 PURPOSE 1 of 10 To describe the procedure to monitor for viable airborne bacteria with the Remote-Slit- Sampler Assembly (R2S) from EMTEK, LLC. Principle A 100 mm agar based test plate is placed on the
PURPOSE 1 of 10 To describe the procedure to monitor for viable airborne bacteria with the Remote-Slit- Sampler Assembly (R2S) from EMTEK, LLC. Principle A 100 mm agar based test plate is placed on the
Medical oxygen. LIV IQ Digital therapy device.
 Medical oxygen. LIV IQ Digital therapy device. BOC: Living healthcare 02 Cylinder instructions Cylinder instructions 03 Therapy device features. LIV IQ giving you safe guidance during patient treatment.
Medical oxygen. LIV IQ Digital therapy device. BOC: Living healthcare 02 Cylinder instructions Cylinder instructions 03 Therapy device features. LIV IQ giving you safe guidance during patient treatment.
SARASOTA MEMORIAL HOSPITAL NURSING PROCEDURE
 SARASOTA MEMORIAL HOSPITAL NURSING PROCEDURE TITLE: ISSUED FOR: ENDO TRACHEAL SUCTIONING OF THE ADULT PATIENT (suc03) Nursing, Respiratory DATE: REVIEWED: PAGES: 11/82 12/18 1 of 5 RESPONSIBILITY: RN,
SARASOTA MEMORIAL HOSPITAL NURSING PROCEDURE TITLE: ISSUED FOR: ENDO TRACHEAL SUCTIONING OF THE ADULT PATIENT (suc03) Nursing, Respiratory DATE: REVIEWED: PAGES: 11/82 12/18 1 of 5 RESPONSIBILITY: RN,
Title of Manual: Specimen Collection Document Number: GPA.SPC.45.0
 Capillary Blood Page 1 of 5 I. PURPOSE This procedure provides guidelines on the proper technique for collecting diagnostic capillary blood specimens via skin puncture or heel puncture (infant only). Blood
Capillary Blood Page 1 of 5 I. PURPOSE This procedure provides guidelines on the proper technique for collecting diagnostic capillary blood specimens via skin puncture or heel puncture (infant only). Blood
Before using or working with the Grab n Go Heliox system, please read and understand the usage and safety instructions provided in this booklet.
 Operating Instructions for the Before using or working with the Grab n Go Heliox system, please read and understand the usage and safety instructions provided in this booklet. Introduction The Praxair
Operating Instructions for the Before using or working with the Grab n Go Heliox system, please read and understand the usage and safety instructions provided in this booklet. Introduction The Praxair
HAEMOFILTRATION GUIDELINES
 HAEMOFILTRATION GUIDELINES The treatment mode used for Continuous Renal Replacement Therapy on the Cardiff PICU is Continuous Veno-Venous Haemofiltration (CVVH). CVVH facilitates the continuous Convective
HAEMOFILTRATION GUIDELINES The treatment mode used for Continuous Renal Replacement Therapy on the Cardiff PICU is Continuous Veno-Venous Haemofiltration (CVVH). CVVH facilitates the continuous Convective
Personal Safety- S.E.T.U.P.
 First Aid & CPR Personal Safety- S.E.T.U.P. Stop - Pause to identify hazards Environment - Consider your surroundings Traffic - Be careful along roadways Unknown hazards - Consider things that are not
First Aid & CPR Personal Safety- S.E.T.U.P. Stop - Pause to identify hazards Environment - Consider your surroundings Traffic - Be careful along roadways Unknown hazards - Consider things that are not
Techniques To Treat Your Pain At Home (512)
 Techniques To Treat Your Pain At Home (512) 288-5322 7010 W. TX-71 Suite 360 Austin TX, 78735 Arm Only Bird Dog Start in the quadruped position (all fours) with arms and thighs perpendicular to the floor.
Techniques To Treat Your Pain At Home (512) 288-5322 7010 W. TX-71 Suite 360 Austin TX, 78735 Arm Only Bird Dog Start in the quadruped position (all fours) with arms and thighs perpendicular to the floor.
Elcometer 138. Bresle Kit and Patches. Operating Instructions
 Elcometer 138 English Bresle Kit and Patches Operating Instructions R English This product meets the Electromagnetic Compatibility Directive. The product is Class B, Group 1 ISM equipment according to
Elcometer 138 English Bresle Kit and Patches Operating Instructions R English This product meets the Electromagnetic Compatibility Directive. The product is Class B, Group 1 ISM equipment according to
MyoSure Setup Simplified
 MyoSure Setup Simplified This is designed as a quick reference guide only. This guide is not a substitute for the Operator s Manual and/or Instructions for Use (IFU). MyoSure Setup with Aquilex MyoSure
MyoSure Setup Simplified This is designed as a quick reference guide only. This guide is not a substitute for the Operator s Manual and/or Instructions for Use (IFU). MyoSure Setup with Aquilex MyoSure
Skills & Scenarios 81
 Skills & Scenarios 81 Psychomotor Skills Checklist Skill Lab Clinical Date Initial Date Initial 1. Change I.V. tubing 2. Change I.V solution 3. Regulate I.V. flow rate 4. Flush peripheral lock 5. Change
Skills & Scenarios 81 Psychomotor Skills Checklist Skill Lab Clinical Date Initial Date Initial 1. Change I.V. tubing 2. Change I.V solution 3. Regulate I.V. flow rate 4. Flush peripheral lock 5. Change
POWER FLUSH KIT INSTRUCTIONS
 1 GENERAL INFORMATION POWER FLUSH KIT INSTRUCTIONS ACE Power Flush is an ozone-safe solvent engineered specifically for flushing refrigeration and air conditioning systems. Used exactly like the old R-11
1 GENERAL INFORMATION POWER FLUSH KIT INSTRUCTIONS ACE Power Flush is an ozone-safe solvent engineered specifically for flushing refrigeration and air conditioning systems. Used exactly like the old R-11
