TJF-Q180V Cleaning and Disinfection Checklist
|
|
|
- Basil Payne
- 6 years ago
- Views:
Transcription
1 TJF-Q180V Cleaning and Disinfection Checklist
2 TJF-Q180V Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-Q180V has been performed as instructed in the Olympus reprocessing manual. The reprocessing technician should perform cleaning and disinfection of the endoscope in the same way as routine reprocessing without referring to this checklist. The evaluator stands next to the reprocessing technician and checks the reprocessing work using this checklist. After that, the evaluator determines whether or not there was any deviation in the reprocessing process. WARNING: The reprocessing technician must not use this checklist while performing reprocessing as it may pose an infection control risk. Facility name S/N of TJF-Q180V Procedure No. or Patient ID No. Procedure date Reprocessing WARNING: The reprocessing technician must not sign this checklist while performing reprocessing as it may pose an infection control risk. Technician: Print name Signature Date Technician: Print name Signature Date Evaluator: Print name Signature Date 1
3 Contents General Policy... 3 Automated Endoscope Reprocessor... 4 Precleaning (at the patient bed side)... 5 Leakage Testing of the Endoscope... 9 Manually Cleaning the Endoscope and Accessories Manual High-Level Disinfection Rinsing Alcohol Flush Cleaning and Disinfection using an Automated Endoscope Reprocessor
4 General Policy 1 Reprocessing technician should receive sufficient education on the following items: Professional health and safety policies of the facility Structure and handling of endoscope and accessories Handling of pertinent chemicals Cleaning and Disinfection procedure of TJF-Q180V Reprocessing Technician name: Received Not received Reprocessing Technician name: Received Not received Maintain an internal system of identifying contaminated versus reprocessed endoscopes and accessories to prevent both mix-ups and cross-contamination. In the event that the TJF-Q180V has been repaired by a non-olympus facility, contact that repair facility for instructions regarding reprocessing. Instructions provided in the TJF-Q180V REPROCESSING MANUAL are not valid for Olympus devices repaired by a non-olympus facility. The Olympus recommended reprocessing procedures have not been validated for reprocessing devices repaired by a non-olympus facility. Document as performed items such as: local SOPs (standard operating procedures), confirmation of technician training, routine testing of the disinfectant s MEC (minimal effective concentration), confirmation of the disinfectant s use-life, etc. For leakage testing and manual cleaning of the endoscope and accessories, use either fresh, potable tap water or water that has been processed (e.g., filtered, deionized or purified) to improve its chemical and/or microbiological quality. Consult with your hospital s infection control committee. Establish a local policy regarding the method and frequency of cleaning and disinfecting the endoscope storage cabinet, which staff members can access the cabinet, which items can be stored in the cabinet, etc. Evaluation of the General Policy Deviation Comments: Established Not repaired Documented Established No deviation Not established Repaired Not documented Not established Deviation 3
5 Automated Endoscope Reprocessor (AER) Policy 1 Confirm that the manufacturer of the AER has validated compatibility of the AER with the TJF-Q180V. Only Olympus-recommended or Olympus-endorsed AERs have been validated by Olympus. Confirmed Not confirmed N/A 2 Before using an AER, confirm that it is capable of reprocessing the TJF-Q180V including all channels, the forceps elevator recess, and accessories. Capable Not capable If you are uncertain as to the ability of your AER to reprocess the TJF-Q180V including all channels, the forceps elevator recess, and accessories, contact the manufacturer of the AER for specific instructions and information on compatibility and required connectors. 3 Confirm availability of all required connectors. For details concerning appropriate connectors, refer to the instructions of the AER manufacturer. Confirmed Not confirmed Evaluation of the Automated Endoscope Reprocessor Policy Deviation Comments: No deviation Deviation 4
6 Date Precleaning start time Procedure finish time Precleaning technician Precleaning (at the patient bed side) 1 Preparation Immediately following the patient procedure, with the endoscope still connected to the equipment used in the patient procedure (i.e., the light source, video system center, suction pump), turn the video system center and light source OFF. 2 Prepare a clean 500 ml container of water. Wipe the insertion section 3 Dip a clean, lint-free cloth or sponge in the clean water and wipe the entire insertion section of the endoscope. Wipe from the boot at the control section toward the distal end. Aspirate water 4 Turn the suction pump ON. 5 Close the cap on the biopsy valve. Suction valve (MH-443) Cap 6 Lower the forceps elevator by turning the elevator control lever. 7 Immerse the distal end of the insertion section in the clean water. 5
7 8 9 Depress the suction valve (MH-443) on the endoscope and aspirate the water through the endoscope for 30 seconds. While continuing the immersion and the aspiration, raise and lower the forceps elevator three times, by turning the elevator control lever. 30 seconds Raise/Lower 3 times 10 Remove the distal end from the water. 11 Depress the suction valve and aspirate air for 10 seconds. 10 seconds 12 Turn the suction pump OFF. Flush the air/water channel with water and air 13 Turn the light source ON. 14 Switch OFF the airflow regulator on the light source Detach the air/water valve (MH-438) from the endoscope and place it in the detergent solution. Attach the AW channel cleaning adapter (MH-948) to the air/water cylinder of the endoscope. Air/water valve AW channel cleaning adapter 6
8 17 Immerse the distal end of the insertion section in the clean water. 18 Switch the airflow regulator on the light source to maximum output ( HIGH or 3 ). 19 Depress the button of the AW channel cleaning adapter to flush the air/water channel with water from the water container for 30 seconds. 30 seconds 20 Release the button to flush air for 10 seconds. 10 seconds 21 Turn the light source OFF. Detach accessories from the endoscope 22 Detach the videoscope cable (MAJ-1430, MAJ-843, or MH-976) from the electrical connector of the endoscope. 23 Detach the suction tube from the suction connector on the endoscope connector Detach the metal tip of the water container (MAJ-901 or MH-884) from the air/water supply connector on the endoscope connector. Put the metal tip of the water container tube into the receptacle on the lid of the water container. 26 Confirm that the exterior surface of the electrical connector is free from scratches. Attach the water resistant cap (MH-553) 7
9 27 Attach the cap to the electrical connector. Evaluation of the Precleaning Deviation Comments: No deviation Deviation 8
10 Start time Reprocessing technician name Leakage Testing of the Endoscope 1 Detach the endoscope from the light source. 2 Transport the endoscope to the reprocessing area. Use a covered container if required by local policy. 3 Detach the AW channel cleaning adapter (MH-948), the suction valve (MH-443), and the biopsy valve (MB-358) from the endoscope and place them in the detergent solution. 4 Fill a clean, large basin with water. 5 Attach the leakage tester connector of the leakage tester (MB-155) to the output socket of the maintenance unit (MU-1) or the light source. Turn the maintenance unit or the light source ON. Set the light source s airflow regulator switch to its maximum level. 6 Depress the pin located inside the connector cap of the leakage tester and listen to confirm that air is emitted from the connector cap. 7 Confirm that both the connector cap of the leakage tester and the venting connector of the water resistant cap are dry. If not, dry with a clean, lint free cloth. Attach the connector cap to the venting connector by pushing on and rotating clockwise until it stops. 9
11 8 With the leakage tester attached, immerse the endoscope in the water and observe for approximately 30 seconds while deflecting the bending section of the endoscope by turning the endoscope s UP/DOWN and RIGHT/LEFT angulation control knobs, and while raising and lowering the forceps elevator by moving the endoscope s elevator control lever. Confirm that there is no location on the endoscope from which a continuous series of air bubbles emerge. Air bubbles Performed Deflected Raised and lowered Not performed Not deflected Not raised and lowered No leakage Leakage Channel opening Bending section 9 Remove the endoscope from the water with the leakage tester still attached. 10 Turn the maintenance unit or the light source OFF. 11 Detach the leakage tester from the maintenance unit or the light source. 12 Wait 30 seconds, or until the covering of the bending section contracts to its pre-expansion size. Detach the leakage tester from the water resistant cap. 13 Thoroughly dry the leakage tester using a clean, lint-free cloth. Evaluation of the Leakage Testing Deviation Comments: No deviation Deviation 10
12 Start time Reprocessing technician name Manually Cleaning the Endoscope and Accessories 1 Clean the external surface Fill a clean, large basin with the detergent solution at the concentration recommended by the detergent manufacturer. Use a medical-grade, low-foaming, neutral ph detergent. Follow the instructions provided by the detergent manufacturer regarding concentration, temperature, contact time, and expiration date. Actual Condition Detergent Name: Concentration: Temperature: Contact time: Expiration date: 2 Immerse the endoscope in the detergent solution. 3 Thoroughly brush or wipe all external surfaces of the endoscope, using a clean, lint-free cloth, brush or sponge. Pay particular attention to the air/water nozzle opening and the objective lens on the distal end of the insertion section, and ensure that all surfaces of the distal end are thoroughly cleaned. All external surfaces Distal end Air/water nozzle opening Objective lens 11
13 4 Brush the forceps elevator and elevator recess Lower the forceps elevator by turning the elevator control lever in the opposite direction of the tu direction until it stops. Perform the following brushing in the detergent solution. Elevator control lever 5 Straighten the bending section of the endoscope. 6 Brush the forceps elevator including the guidewire-locking groove and the forceps elevator recess with the single use channel-opening cleaning brush (MAJ-1339) or the single use combination cleaning brush (BW-412T) as follows: a) Insert the brush into the forceps elevator recess along the forceps elevator until the brush handle touches the distal end of the endoscope and pull the brush out of the forceps elevator recess in the detergent solution. Brush handle touched b) Brush the guidewire-locking groove in the detergent solution. 12
14 c) Brush both grooves in the detergent solution. First groove Second groove 7 Raise the forceps elevator by turning the elevator control lever in the tu direction until resistance is felt. Elevator control lever 8 While holding the distal end, brush the forceps elevator and the forceps elevator recess with the single-use channel-opening cleaning brush as follows: a) Insert the brush into the forceps elevator recess along the back of the forceps elevator until the distal end of the brush touches the bottom of the forceps elevator recess in the detergent solution. Forceps elevator a Forceps elevator recess b) Insert the brush into the forceps elevator recess and rotate the brush one full revolution in the detergent solution. Insertion Forceps elevator Forceps elevator recess Rotation b 13
15 c) Brush both sides of the forceps elevator in the detergent solution. First side Second side 9 Operate the elevator control lever to raise and lower the forceps elevator in the detergent solution at least three times. 3 times 10 Inspect whether there is debris on the forceps elevator and in the forceps elevator recess (Inspection by the reprocessing technician). confirmed on the elevator confirmed on the elevator confirmed in the recess confirmed in the recess Go to Inspect whether there is debris on the forceps elevator and in the forceps elevator recess (Inspection by the evaluator). WARNING - The evaluator must not touch the endoscope. That may pose an infection control risk. confirmed on the elevator confirmed in the recess confirmed on the elevator confirmed in the recess 14
16 12 Repeat Steps 4 through 11 until no debris is observed upon inspection of the forceps elevator and the forceps elevator recess. 6-a) 6-b) 6-c) 8-a) Not done 8-b) 8-c) 9 (3 times) confirmed on the elevator confirmed on the elevator confirmed in the recess confirmed in the recess 15
17 13 Inspect whether there is debris on the forceps elevator and in the forceps elevator recess (Inspection by the evaluator). WARNING - The evaluator must not touch the endoscope. That may pose an infection control risk. confirmed on the elevator confirmed in the recess confirmed on the elevator confirmed in the recess 14 Lower the forceps elevator by turning the elevator control lever in the opposite direction of the tu direction until it stops. 15 Brush the distal end of the endoscope except the forceps elevator and the forceps elevator recess, using the single use channel-opening cleaning brush, until no debris is observed upon inspection of the brush. Brushing confirmed confirmed 16 Inspect whether no debris is observed on the brush (Inspection by the evaluator). WARNING - The evaluator must not touch the endoscope. That may pose an infection control risk. confirmed confirmed 17 Brush the channels Confirm that no parts remain inside either the instrument channel or the suction channel of the endoscope by carefully passing a new brush through both channels. Instrument channel Instrument channel not Suction channel Suction channel not No parts Parts 16
18 18 Brush from the suction cylinder to the distal end of the insertion section Straighten the bending section of the endoscope Insert the brush at a 45 angle into the opening located in the side wall of the suction cylinder. Using short strokes, feed the brush through the instrument channel until it emerges from the distal end of the endoscope s insertion section. Suction cylinder Instrument channel Distal end Opening Short strokes Emerged Long strokes Not emerged Not brushed Suction channel Suction cylinder 20 Inspect whether there is debris on the bristles when the brush emerges from the distal end. Clean the bristles in the detergent solution using your gloved fingertips to remove any debris. Inspection Inspection not Cleaning Cleaning not 21 Carefully pull the brush back through the channel and out of the suction cylinder. 22 Inspect whether there is debris on the bristles when the brush emerges from the suction cylinder. Clean the bristles in the detergent solution using your gloved fingertips to remove any debris. Inspection Cleaning Inspection not Cleaning not 17
19 23 Repeat steps 19 through 22 until no debris is observed upon inspection of the brush. observed observed observed observed 24 Inspect whether no debris is observed on the brush (Inspection by the evaluator). WARNING - The evaluator must not touch the endoscope. That may pose an infection control risk. observed observed 25 Insert the brush straight into the suction cylinder. Using short strokes, feed the brush through the suction channel until it emerges from the suction connector on the endoscope connector. Suction cylinder Brush from the suction cylinder to the endoscope connector Opening Short strokes Emerged Long strokes Not emerged Not brushed Suction channel Suction cylinder 26 Inspect whether there is debris on the bristles when the brush emerges from the suction connector. Clean the bristles in the detergent solution using your gloved fingertips to remove any debris. Inspection Cleaning Inspection not Cleaning not 18
20 27 Carefully pull the brush back through the channel and out of the suction cylinder. 28 Inspect whether there is debris on the bristles when the brush emerges from the suction cylinder. Clean the bristles in the detergent solution using your gloved fingertips to remove any debris. Inspection Cleaning Inspection not Cleaning not 29 Repeat steps 25 through 28 until no debris is observed upon inspection of the brush. observed observed observed observed Inspect whether no debris is observed on the brush (Inspection by the evaluator). WARNING - The evaluator must not touch the endoscope. That may pose an infection control risk. Insert the single use channel-opening cleaning brush into the suction cylinder, until half of the brush section is inserted. Suction cylinder Brush the suction cylinder observed observed 19
21 32 Rotate the inserted brush one full revolution. 33 Pull the brush out of the cylinder. 34 Inspect whether there is debris on the bristles. Clean the bristles in the detergent solution using your gloved fingertips to remove any debris. Inspection Inspection not Cleaning Cleaning not 35 Repeat steps 31 through 34 until no debris is observed upon inspection of the brush. observed observed observed observed 36 Inspect whether no debris is observed on the brush (Inspection by the evaluator). WARNING - The evaluator must not touch the endoscope. That may pose an infection control risk. observed observed 20
22 Brush the instrument channel port 37Insert the single use channel-opening cleaning brush (MAJ-1339), the channelopening cleaning brush part of the single use combination cleaning brush (BW-412T), into the instrument channel port, until the brush handle touches the Instrument channel opening. channel port 38 Rotate the inserted brush one full revolution. 39 Pull the brush out of the instrument channel port. 40 Inspect whether there is debris on the bristles. Clean the bristles in the detergent solution using your gloved fingertips to remove any debris. Inspection Inspection not Cleaning Cleaning not 41 Repeat Steps 37 through 40 until no debris is observed upon inspection of the brush. observed observed observed observed 21
23 42 Inspect whether no debris is observed on the brush (Inspection by the evaluator). WARNING - The evaluator must not touch the endoscope. That may pose an infection control risk. observed observed 43 Dispose of the single use channel-opening cleaning brush. 44 Remove the endoscope from the detergent solution. 45 Aspirate detergent solution through the instrument channel and the suction channel Attach the suction cleaning adapter (MH-856) to the instrument channel port. 46 Attach the suction tube from the suction pump to the suction connector on the endoscope connector. Suction pump Suction connector Suction tube 47 Turn the suction pump ON. 48 Immerse both the distal end of the insertion section and the weighted end of the suction cleaning adapter in the detergent solution. Suction cylinder Connecting end Suction cleaning adapter Instrument channel port Weighted end Suction pump 22
24 49 Cover the suction cylinder of the endoscope with your gloved finger and aspirate the detergent solution through the instrument channel and the suction channel of the endoscope for approximately 30 seconds. 30 seconds Suction cylinder 50 Turn the suction pump OFF. 51 Detach the suction tube and the suction cleaning adapter from the endoscope. 52 Flush the forceps elevator recess with detergent solution Immerse the distal end in detergent solution. 53 Operate the elevator control lever to raise and lower the forceps elevator at least three times. 3 times 54 Raise the forceps elevator by turning the elevator control lever in the tu direction until resistance is felt. 55 Insert the distal end of a clean 30 ml syringe into the forceps elevator recess and flush the interior of the forceps elevator recess with 30 ml of the detergent solution by pumping the syringe. Detergent solution Insertion Flushing Insertion not Flushing not Insert Syringe Forceps elevator recess 23
25 56 Lower the forceps elevator by turning the elevator control lever in the opposite direction of the tu direction until it stops. 57 Flush the air/water channel with detergent solution Attach the biopsy valve cap of the channel plug (MH-944) to the instrument channel port of the endoscope. Channel plug Instrument channel port Biopsy valve cap 58 Attach the channel plug to the air/water and suction cylinders of the endoscope. 59 Attach the injection tube (MH-946) to the endoscope connector. 60 Immerse the suction port of the injection tube in the detergent solution. 61 Attach a clean 30 ml syringe to the air/water channel port of the injection tube. Air/water channel tube Air/water channel port Syringe Suction channel port 62 Flush the air/water channel with 90 ml of the detergent solution by pumping the syringe at least three times. 3 times 24
26 63 Immerse the endoscope and accessories in detergent solution Wipe all external surfaces of the endoscope, the channel plug (MH-944), and the injection tube (MH-946) to remove debris while they are immersed in the detergent solution, using a clean, lint-free cloth, brush, or sponge. 64 Leave the endoscope with attached accessories immersed in the detergent solution, according to the instructions of the detergent manufacturer. 65 Remove the endoscope with attached accessories from the detergent solution. Remove detergent solution from all channels 66 Fill a clean large basin with water. 67 Immerse the endoscope with attached accessories in the water and gently agitate them to thoroughly rinse. 68 Immerse the suction port of the injection tube (MH-946) in the water. Air and water supply connector Air pipe a Connector plug Injection tube b Air/water channel port Suction connector c Suction channel port 69 Suction channel tube Air pipe port Suction port Attach a clean 30 ml syringe to the suction channel port of the injection tube and flush the suction channel with 90 ml of water (i.e., pump the syringe at least three times). Attaching Attaching not Air/water channel tube Air/water channel port 3 times flushing Flushing not Syringe Suction channel port 25
27 70 Move the syringe to the air/water channel port of the injection tube and flush the air/water channel with 90 ml of water (i.e., pump the syringe at least three times). Moving Moving not Air/water channel tube Air/water channel port 3 Times flushing Flushing not Syringe Suction channel port 71 Remove the endoscope with attached accessories from the water. 72 Place them in a clean basin. 73 Cover the distal end and the control section of the endoscope with a clean, lint-free cloth(s) to prevent splashing from the channel openings. 74 Attach the syringe to the suction channel port of the injection tube and flush the suction channel with 90 ml of air. Air/water channel tube Air/water channel port Syringe Attaching 3 times flushing Attaching not Flushing not Suction channel port 75 Move the syringe to the air/water channel port of the injection tube and flush the air/water channel with 90 ml of air. Moving Moving not Air/water channel tube Air/water channel port Syringe 3 times flushing Flushing not Suction channel port 26
28 76 Remove the cloth(s) from the endoscope. 77 Detach the channel plug and the injection tube from the endoscope. Dry external surfaces 78 Dry the external surfaces of the endoscope, the channel plug, and the injection tube by wiping with a clean, lint-free cloth(s). 79 Inspect all items for residual debris. Should any debris remain, repeat the entire cleaning procedure until all debris is removed. Inspection Inspection not remained remained Repeating Repeating not Inspection Inspection not remained remained 80 Inspect whether debris is observed on the endoscope (Inspection by the evaluator). WARNING - The evaluator must not touch the endoscope. That may pose an infection control risk. remained remained 27
29 Evaluation of the Manually Cleaning the Endoscope and Accessories Deviation Comments: No deviation Deviation 28
30 NOTE - Go to page 46 for using an AER. Start time Reprocessing technician name Manual High-Level Disinfection Preparation 1 Fill a clean large basin with the disinfectant solution. 2 Use a high-level disinfectant cleared by your national regulatory agency for use in reprocessing flexible endoscopes. Follow the disinfectant manufacturer s instructions regarding activation (if required), concentration, temperature, contact time, and expiration date. Actual condition Detergent name: Concentration: Temperature: Contact time: Expiration date: 3 Immerse the endoscope in the disinfectant solution. 29
31 4 Attach the channel plug (MH-944) and the injection tube (MH-946) to the endoscope. Channel plug Suction plug Plug frame Instrument channel port Suction cylinder d b a c Air/water plug Biopsy valve cap Switch 1 Control section Air/water cylinder Air and water supply connector Air pipe a Connector plug Injection tube b Air/water channel port Suction connector c Suction channel port Suction channel tube Air pipe port Suction port 5 Immerse in the disinfectant solution. Flush all channels and the forceps elevator recess with disinfectant solution 6 Confirm that the suction port of the injection tube (MH-946) is immersed in the disinfectant solution. 7 Attach a clean 30 ml syringe to the suction channel port of the injection tube and forcefully flush the suction channel with 180 ml of the disinfectant solution i.e., by pumping the syringe at least six times. Confirm that no air bubbles exit the distal end of the endoscope s insertion section during the sixth flush. If air bubbles still exit, flush the channel with the disinfectant solution until no air bubbles exit. 6 times flushing Confirmation Flushing not Confirmation not No air bubbles Air bubbles Additional flushing Additional flushing not No air bubbles Air bubbles 30
32 8 Move the syringe to the air/water channel port of the injection tube and forcefully flush the air/water channel with 180 ml of the disinfectant solution. Confirm that no air bubbles exit the distal end during the sixth flush. If air bubbles still exit, flush the channel with the disinfectant solution until no air bubbles exit. Moving 6 times flushing Moving not Flushing not Confirmation Confirmation not No air bubbles Air bubbles Additional flushing Additional flushing not No air bubbles Air bubbles 9 Remove the biopsy valve cap of the channel plug (MH-946) from the instrument channel port of the endoscope, leaving the channel plug attached to the air/water and suction cylinders of the endoscope. Forcefully flush the instrument channel with 180ml of the disinfectant solution, using the 30ml syringe - i.e., fill the syringe with the disinfectant solution without air, put the distal end of the syringe in the instrument channel port in the disinfectant solution, and forcefully flush at least six times, minimizing disinfectant solution leakage from the port. Confirm that no air bubbles exit the distal end of the endoscope s insertion section during the sixth flush. If air bubbles still exit, flush the channel with the disinfectant solution until no air bubbles exit. Moving 6 times flushing Confirmation Moving not Flushing not Confirmation not No air bubbles Air bubbles Additional flushing Additional flushing not No air bubbles Air bubbles 10 Raise the forceps elevator by turning the elevator control lever in the tu direction until resistance is felt. 31
33 11 Insert the tip of the 30 ml syringe into the interior of the forceps elevator recess in the disinfectant solution, and flush the interior of the recess with 60 ml of the disinfectant solution. Disinfectant solution Insertion Flushing Insertion not Flushing not Insert Syringe Forceps elevator recess NOTE, When using a luer-lock type syringe, the tip of the syringe may not fit into the interior of the forceps elevator recess. In this case, hold the tip of the syringe over the interior of the recess while the syringe contacts the surface of the endoscope. 12 Lower the forceps elevator by turning the elevator control lever. Insert the tip of the 30 ml syringe into the interior the forceps elevator recess in the disinfectant solution, and flush the interior of the recess with 60 ml of the disinfectant solution. NOTE, When using a luer-lock type syringe, the tip of the syringe may not fit into the interior of the forceps elevator recess. In this case, hold the tip of the syringe over the interior of the recess while the syringe contacts the surface of the endoscope. 32
34 13 14 Turn the elevator control lever to raise and lower the forceps elevator three times, keeping the distal end of the endoscope immersed in the disinfectant solution Forcefully flush the instrument channel with 90ml of the disinfectant solution, using the 30ml syringe - i.e., fill the syringe with the disinfectant solution without air, put the distal end of the syringe in the instrument channel port in the disinfectant solution, and forcefully flush at least three times, minimizing disinfectant solution leakage from the port. Confirm that no air bubbles exit the distal end of the endoscope s insertion section during the third flush. If air bubbles still exit, flush the channel with the disinfectant solution until no air bubbles exit. Repeat the steps above. NOTE, When using a luer-lock type syringe, the tip of the syringe may not fit into the interior of the forceps elevator recess. In this case, hold the tip of the syringe over the interior of the recess while the syringe contacts the surface of the endoscope. 15 Immerse the endoscope and accessories in disinfectant solution Detach the syringe from the accessories. While immersed, detach the channel plug (MH-944) and the injection tube (MH-946) from the endoscope. 16 Confirm that the endoscope and all accessories are ly submerged in the disinfectant solution. 17 Confirm that there are no air bubbles on the surfaces of the endoscope and accessories. If air bubbles adhere to the surfaces, wipe them away using your gloved finger or a clean, lint-free cloth. Confirmed No air bubbles Not confirmed Air bubbles Wiping Wiping not No air bubbles Air bubbles 18 Cover the basin of the disinfectant solution with a tight-fitting lid to minimize the diffusion of disinfectant vapors. 33
35 19 Leave the endoscope, the channel plug, and the injection tube immersed in the disinfectant solution according to the instructions of the disinfectant manufacturer. Confirm the recommended contact time, temperature, and concentration. Use a clock or timer to accurately measure the disinfection contact time. Actual condition Start time and date: Reprocessing technician name: Leaving Using Leaving not Using not Actual immersion time: Expiration date: (From the above leaving start time to the start time of this section) Labeled condition Labeled immersion time: 20 Remove the endoscope and accessories from disinfectant solution Attach the channel plug (MH-944) and the injection tube (MH-946) to the endoscope. Channel plug Suction plug Plug frame Instrument channel port Suction cylinder d b a c Air/water plug Biopsy valve cap Switch 1 Control section Air/water cylinder Air and water supply connector Air pipe a Connector plug Injection tube b Air/water channel port Suction connector c Suction channel port Suction channel tube Air pipe port Suction port 21 Remove the suction port of the injection tube from the disinfectant solution. 34
36 22 Attach a sterile 30 ml syringe to the suction channel port of the injection tube and flush the suction channel with 90 ml of air i.e., pump the syringe at least three times. Air/water channel tube Air/water channel port Attaching 3 times flushing Attaching not Flushing not Syringe Suction channel port 23 Move the syringe to the air/water channel port of the injection tube and flush the air/water channel with 90 ml of air. Moving Air/water channel tube Air/water channel port 3 times flushing Flushing not Syringe Suction channel port 24 Remove the endoscope with attached accessories from the disinfectant solution. Evaluation of the Manual High-Level Disinfection Deviation Comments: No deviation Deviation 35
37 1 Rinsing Use sterile water, either fresh, potable tap water, or water that has been processed (e.g., filtered, deionized, or purified) to improve its chemical and/or microbiological quality, and flush the endoscope and accessories with the alcohol after rinsing. Consult with your hospital s infection control committee regarding local policies on water quality. Actual condition Sterile water Fresh water Potable water Other: Rinse the endoscope and accessories 2 Fill a sterile large basin with the rinse water. 3 Immerse the endoscope with attached accessories in the rinse water. 4 Detach the channel plug (MH-944) and injection tube (MH-946) from the endoscope. 5 Wipe all external surfaces of the endoscope and accessories, using a sterile, lint-free cloth. 6 Attach the channel plug and the injection tube to the endoscope. Instrument channel port Channel plug Suction plug Suction cylinder d b a c Plug frame Air/water plug Biopsy valve cap Switch 1 Control section Air/water cylinder Air and water supply connector Air pipe a Connector plug Injection tube b Air/water channel port Suction connector c Suction channel port Suction channel tube Air pipe port Suction port 36
38 7 Immerse the suction port of the injection tube in the rinse water. 8 Attach a sterile 30 ml syringe to the suction channel port of the injection tube and flush the suction channel with 90 ml of the rinse water i.e., pump the syringe at least three times. Attaching Attaching not Air/water channel tube Air/water channel port 3 times flushing Flushing not Syringe Suction channel port 9 Move the syringe to the air/water channel port of the injection tube and flush the air/water channel with 90 ml of the rinse water. Moving Moving not Air/water channel tube Air/water channel port 3 times flushing Flushing not Syringe Suction channel port 10 Raise the forceps elevator by turning the elevator control lever in the tu direction until resistance is felt. 37
39 11 Insert the tip of the 30 ml syringe into the interior of the forceps elevator recess in the rinse water, and flush the interior of the recess with 30 ml of the rinse water. Insertion Insertion not Flushing Flushing not NOTE, When using a luer-lock type syringe, the tip of the syringe may not fit into the interior of the forceps elevator recess. In this case, hold the tip of the syringe over the interior of the recess while the syringe contacts the surface of the endoscope. 12 Lower the forceps elevator by turning the elevator control lever. Insert the tip of the 30 ml syringe into the interior the forceps elevator recess in the rinse water, and flush the interior of the recess with 30 ml of the rinse water. NOTE, When using a luer-lock type syringe, the tip of the syringe may not fit into the interior of the forceps elevator recess. In this case, hold the tip of the syringe over the interior of the recess while the syringe contacts the surface of the endoscope. 38
40 13 14 Turn the elevator control lever to raise and lower the forceps elevator three times, keeping the distal end of the endoscope immersed in the rinse water. Repeat the step 1-12 above for the necessary number of times, following the rinsing method described in the disinfectant solution manual. By turning the elevator control lever, put the forceps elevator in intermediate position of the range of movement. 3 times 15 Remove the endoscope with attached accessories from the rinse water and place them in a sterile basin. 16 Cover the distal end and the control section of the endoscope with a sterile, lint-free cloth(s) to prevent splashing from the channel openings. 17 Attach the syringe to the suction channel port of the injection tube and flush the suction channel with 90 ml of air. Air/water channel tube Air/water channel port Syringe Attaching 3 times flushing Attaching not Flushing not Suction channel port 18 Move the syringe to the air/water channel port of the injection tube and flush the air/water channel with 90 ml of air. Moving Moving not Air/water channel tube Air/water channel port 3 times flushing Flushing not Syringe Suction channel port 39
41 19 Remove the cloth(s) from the endoscope. 20 Detach only the injection tube from the endoscope. 21 Attach a sterile suction tube from the suction pump to the suction connector on the endoscope connector. Suction pump Suction connector Channel plug 22 Turn the suction pump ON and aspirate air for at least 15 seconds. Air will flow through the instrument channel and the suction channel of the endoscope. Turning Turning not 15 seconds aspiration Aspiration not 23 While continuing the aspiration, raise and lower the forceps elevator three times, by turning the elevator control lever. 3 times 24 Turn the suction pump OFF. 25 Detach the suction tube and the channel plug from the endoscope. 26 Thoroughly dry the external surfaces of the endoscope, the channel plug, and the injection tube, by wiping with a sterile, lint-free cloth(s). 40
42 27 Thoroughly dry the inside of the suction cylinder, the air/water cylinder, the instrument channel port of the endoscope, and forceps elevator recess, using a sterile cotton swab(s). Evaluation of the Rinsing Deviation Comments: No deviation Deviation 41
43 Termination time of alcohol flush Reprocessing technician name Alcohol Flush Use medical-grade 70% ethyl or 70% isopropyl alcohol. 70% ethyl alcohol used 70% isopropyl alcohol used Other used Alcohol Flush 1 Fill a sterile small basin with the alcohol. 2 Attach the channel plug (MH-944) and the injection tube (MH-946) to the endoscope. Channel plug Suction plug Plug frame Instrument channel port Suction cylinder d b a c Air/water plug Biopsy valve cap Switch 1 Control section Air/water cylinder Air and water supply connector Air pipe a Connector plug Injection tube b Air/water channel port Suction connector c Suction channel port Suction channel tube Air pipe port Suction port 3 Immerse the suction port of the injection tube in the alcohol. 4 Cover the distal end and the control section of the endoscope with a sterile, lint-free cloth(s) to prevent splashing alcohol from the channel openings. 42
44 5 Attach a sterile 30 ml syringe to the suction channel port of the injection tube and flush the suction channel with 90 ml of the alcohol i.e., pump the syringe at least three times. Attaching Attaching not Air/water channel tube Air/water channel port 3 times flushing Flushing not Syringe Suction channel port 6 Move the syringe to the air/water channel port of the injection tube and flush the air/water channel with 30 ml of the alcohol. Moving Moving not Air/water channel tube Air/water channel port Flushing 3 times Flushing not Syringe Suction channel port 7 Raise the forceps elevator by turning the elevator control lever in the tu direction until resistance is felt. 8 Insert the tip of the 30 ml syringe into the interior of the forceps elevator recess, and flush the interior of the recess with 30 ml of alcohol. Insertion Insertion not NOTE, When using a luer-lock type syringe, the tip of the syringe may not fit into the interior of the forceps elevator recess. In this case, hold the tip of the syringe over the interior of the recess while the syringe contacts the surface of the endoscope. Flushing Flushing not 43
45 9 Lower the forceps elevator by turning the elevator control lever. Insert the tip of the 30 ml syringe into the interior the forceps elevator recess, and flush the interior of the recess with 30 ml of the alcohol. 10 NOTE, When using a luer-lock type syringe, the tip of the syringe may not fit into the interior of the forceps elevator recess. In this case, hold the tip of the syringe over the interior of the recess while the syringe contacts the surface of the endoscope. Turn the elevator control level to raise and lower the forceps elevator three times 11 Remove the suction port of the injection tube from the alcohol. 12 Attach the syringe to the suction channel port of the injection tube and flush the suction channel with 90 ml of air. Attaching Attaching not Air/water channel tube Air/water channel port 3 times flushing Flushing not Syringe Suction channel port 13 Move the syringe to the air/water channel port of the injection tube and flush the air/water channel with 90 ml of air. Moving Moving not Air/water channel tube Air/water channel port Flushing 3 times Flushing not Syringe Suction channel port 44
46 14 Remove the cloth(s) from the endoscope. 15 Detach the channel plug and the injection tube from the endoscope. 16 Thoroughly dry the external surfaces of the endoscope, the channel plug, and the injection tube, by wiping with a sterile, lint-free cloth(s). 17 Thoroughly dry the inside of the suction cylinder, the air/water cylinder, the instrument channel port of the endoscope, and the forceps elevator recess, using a sterile cotton swab(s). Evaluation of the Alcohol Flush Deviation Comments: No deviation Deviation 45
47 Start time Reprocessing technician name 1 Cleaning and Disinfection using an Automated Endoscope Reprocessor (AER) Put the forceps elevator in intermediate position of the range of movement by moving the elevator control lever and set it in your AER so that the forceps elevator recess can be sufficiently cleaned and disinfected. 2 Inspect whether the forceps elevator is in intermediate position (Inspection by the evaluator). WARNING - The evaluator must not touch the endoscope. That may pose an infection control risk. Intermediate position Not intermediate position 3 Place the endoscope in your AER according to the instructions of the AER manufacturer. 4 Connect any necessary connecting tubes in accordance with the instructions from the AER manufacturer. 5 any terminal steps that are not automatically performed by the AER such as alcohol flush. 6 Thoroughly dry the forceps elevator recess after cleaning and disinfection by your AER. Insufficiently drying may cause the bacterial proliferation and pose an infection control risk. Evaluation of the Cleaning and Disinfection using an Automated Endoscope Reprocessor No deviation Deviation Deviation Comments: 46
48 Overall Evaluation Evaluation of the Cleaning and Disinfection of the TJF-Q180V No deviation Deviation 47
49 This checklist is intended for Americas and Oceania. For other areas, the described steps may be subject to change. Olympus is a registered trademark of Olympus Corporation, Olympus America Inc., and/or their affiliates Corporate Parkway, PO Box 610, Center Valley, PA For more information, contact your Olympus sales representative, or call Olympus America Inc. All rights reserved. Printed in USA OAIGI0115BRO15549
TJF-Q180V Cleaning and Disinfection Checklist
 TJF-Q180V Cleaning and Disinfection Checklist TJF-Q180V Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-Q180V has been performed
TJF-Q180V Cleaning and Disinfection Checklist TJF-Q180V Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-Q180V has been performed
INSTRUCTIONS EVIS EXERA II DUODENOVIDEOSCOPE OLYMPUS TJF TYPE Q180V
 INSTRUCTIONS EVIS EXERA II DUODENOVIDEOSCOPE OLYMPUS TJF TYPE Q180V Refer to the endoscope s companion manual, the OLYMPUS TJF TYPE Q180V REPROCESSING MANUAL, for reprocessing information. USA: CAUTION:
INSTRUCTIONS EVIS EXERA II DUODENOVIDEOSCOPE OLYMPUS TJF TYPE Q180V Refer to the endoscope s companion manual, the OLYMPUS TJF TYPE Q180V REPROCESSING MANUAL, for reprocessing information. USA: CAUTION:
INSTRUCTIONS OLYMPUS GIF TYPE 2TQ260M EVIS LUCERA GASTROINTESTINAL VIDEOSCOPE
 INSTRUCTIONS EVIS LUCERA GASTROINTESTINAL VIDEOSCOPE OLYMPUS GIF TYPE 2TQ260M Refer to the endoscope s companion manual, the OLYMPUS GIF TYPE 2TQ260M REPROCESSING MANUAL for reprocessing information. Contents
INSTRUCTIONS EVIS LUCERA GASTROINTESTINAL VIDEOSCOPE OLYMPUS GIF TYPE 2TQ260M Refer to the endoscope s companion manual, the OLYMPUS GIF TYPE 2TQ260M REPROCESSING MANUAL for reprocessing information. Contents
Endoscope Cleaning & Maintenance Workshop
 Endoscope Cleaning & Maintenance Workshop Table of Contents Introduction 1 CHAPTER 6 CHAPTER 1 Programme 2 Manual cleaning of removable parts 29 Disinfection of removable parts 31 CHAPTER 2 History of
Endoscope Cleaning & Maintenance Workshop Table of Contents Introduction 1 CHAPTER 6 CHAPTER 1 Programme 2 Manual cleaning of removable parts 29 Disinfection of removable parts 31 CHAPTER 2 History of
Endo-Flush Order # ZUTR30004 OPERATION MANUAL. Zutron Medical, LLC W 98 th St #40-27 Lenexa, KS Phone Fax
 OPERATION MANUAL Zutron Medical, LLC 17501 W 98 th St #40-27 Lenexa, KS 66219 Phone 877-343-5873 Fax 913-967-5944 ZUT-Lab-004-30004 REV. 03312017 Table of Contents 2 Introduction 1. Intended Use 2. Labels,
OPERATION MANUAL Zutron Medical, LLC 17501 W 98 th St #40-27 Lenexa, KS 66219 Phone 877-343-5873 Fax 913-967-5944 ZUT-Lab-004-30004 REV. 03312017 Table of Contents 2 Introduction 1. Intended Use 2. Labels,
OPERATION MANUAL. Hand Held Leak Tester Model # ZUTR-10002
 OPERATION MANUAL ZUT-Lab-003-10002 REV. B Table of Contents Page 2 Introduction 1. Intended Use 2. Labels, Symbols and Signal Words Page 3 Page 4 Page 5 Important Safety Information and Cleaning Procedure
OPERATION MANUAL ZUT-Lab-003-10002 REV. B Table of Contents Page 2 Introduction 1. Intended Use 2. Labels, Symbols and Signal Words Page 3 Page 4 Page 5 Important Safety Information and Cleaning Procedure
INSTRUCTIONS. Sterilization lot number
 Single Use 3-Lumen Extraction Balloon V B-V231P-A, B-V231P-B INSTRUCTIONS USA: CAUTION: This Product Contains Natural Rubber Latex Which May Cause Allergic Reactions. Federal law restricts this device
Single Use 3-Lumen Extraction Balloon V B-V231P-A, B-V231P-B INSTRUCTIONS USA: CAUTION: This Product Contains Natural Rubber Latex Which May Cause Allergic Reactions. Federal law restricts this device
The purpose of this DOPs form is to provide a universal training and assessment tool for continuity when training in manual cleaning processes.
 1 Insert company logo Formative DOPS Assessment Form Manual Cleaning of Gastrointestinal Endoscopes Hospital: Trainee s name (print): Job title: Date of assessment: Review date: Manufacturer: Equipment
1 Insert company logo Formative DOPS Assessment Form Manual Cleaning of Gastrointestinal Endoscopes Hospital: Trainee s name (print): Job title: Date of assessment: Review date: Manufacturer: Equipment
REV A International ( ) 2013 MEDIVATORS Inc.
 User Manual FUJINON is a registered trademark of FUJIFILM Corporation MEDIVATORS is a registered trademark of MEDIVATORS Inc. OLYMPUS is a registered trademark of OLYMPUS Corporation. PENTAX is a registered
User Manual FUJINON is a registered trademark of FUJIFILM Corporation MEDIVATORS is a registered trademark of MEDIVATORS Inc. OLYMPUS is a registered trademark of OLYMPUS Corporation. PENTAX is a registered
PENTAX VIDEO COLONOSCOPES
 Only For the Americas INSTRUCTIONS FOR USE (OPERATION) PENTAX VIDEO COLONOSCOPES EC-3890TLK, EC-2990Li, EC-3490Li EC-3890Li, EC-3490TLi EC-3490LK, EC-3890LK EC-3890LZi, EC34-i10L EC38-i10L This instructions
Only For the Americas INSTRUCTIONS FOR USE (OPERATION) PENTAX VIDEO COLONOSCOPES EC-3890TLK, EC-2990Li, EC-3490Li EC-3890Li, EC-3490TLi EC-3490LK, EC-3890LK EC-3890LZi, EC34-i10L EC38-i10L This instructions
Blue River Technologies Port-A-Poly Mixer w/2.5 GPH LMI Pump And Secondary Water Dilution Line INSTALLATION AND OPERATION
 Blue River Technologies Port-A-Poly Mixer w/2.5 GPH LMI Pump And Secondary Water Dilution Line INSTALLATION AND OPERATION Install your Blue River Technologies Port-A-Poly mixing system in a clean dry area.
Blue River Technologies Port-A-Poly Mixer w/2.5 GPH LMI Pump And Secondary Water Dilution Line INSTALLATION AND OPERATION Install your Blue River Technologies Port-A-Poly mixing system in a clean dry area.
Procedure 85 Attaching The Humidifier To The Oxygen Flow Meter Or Regulator. Procedure 86 Administering Oxygen Through A Nasal Cannula
 Chapter 12 Respiratory Procedures Procedure 81 Checking Capillary Refill Procedure 82 Using A Pulse Oximeter Procedure 83 Preparing Wall-Outlet Oxygen Procedure 84 Preparing The Oxygen Cylinder Procedure
Chapter 12 Respiratory Procedures Procedure 81 Checking Capillary Refill Procedure 82 Using A Pulse Oximeter Procedure 83 Preparing Wall-Outlet Oxygen Procedure 84 Preparing The Oxygen Cylinder Procedure
Prestige Endoscopic Graspers Instructions for Use
 Prestige Endoscopic Graspers Instructions for Use DESCRIPTION The Prestige Endoscopic Graspers are designed to grasp soft tissue during endoscopic procedures. The graspers are designed to be used through
Prestige Endoscopic Graspers Instructions for Use DESCRIPTION The Prestige Endoscopic Graspers are designed to grasp soft tissue during endoscopic procedures. The graspers are designed to be used through
Installing ASI Umbilical Delivery Systems
 Rev A 05 Nov 2015 Page 1 of 10 PURPOSE This guide provides an overview of tubing connections into an ASI wall-mounted Junction Box that has already been plumbed with a compressed air connection including
Rev A 05 Nov 2015 Page 1 of 10 PURPOSE This guide provides an overview of tubing connections into an ASI wall-mounted Junction Box that has already been plumbed with a compressed air connection including
Frigitronics CRYO-PLUS
 Frigitronics CRYO-PLUS OPERATING & MAINTENANCE MANUAL REF 2400 R x Only CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. INDICATIONS FOR USE The Cryo-Plus is
Frigitronics CRYO-PLUS OPERATING & MAINTENANCE MANUAL REF 2400 R x Only CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. INDICATIONS FOR USE The Cryo-Plus is
WELLS JOHNSON HIGH VOLUME CANISTERS
 WELLS JOHNSON HIGH VOLUME CANISTERS USER MANUAL DISASSEMBLY OF HARVESTING CANISTERS Please follow the instructions provided to disassemble 5L, 3L, 2L, and 1L, 250mL and 500mL harvesting canisters. 1. Unscrew
WELLS JOHNSON HIGH VOLUME CANISTERS USER MANUAL DISASSEMBLY OF HARVESTING CANISTERS Please follow the instructions provided to disassemble 5L, 3L, 2L, and 1L, 250mL and 500mL harvesting canisters. 1. Unscrew
MyoSure Setup Simplified
 MyoSure Setup Simplified This is designed as a quick reference guide only. This guide is not a substitute for the Operator s Manual and/or Instructions for Use (IFU). MyoSure Setup with Aquilex MyoSure
MyoSure Setup Simplified This is designed as a quick reference guide only. This guide is not a substitute for the Operator s Manual and/or Instructions for Use (IFU). MyoSure Setup with Aquilex MyoSure
User Guide. Pall Laboratory Manifold. For laboratory use. Not for use in a manner other than indicated. Introduction. Regulatory References
 User Guide Pall Laboratory Manifold For laboratory use. Not for use in a manner other than indicated. Introduction Application and Intended Use The membrane filtration (MF) technique is a method of analyzing
User Guide Pall Laboratory Manifold For laboratory use. Not for use in a manner other than indicated. Introduction Application and Intended Use The membrane filtration (MF) technique is a method of analyzing
Shukla Medical. TITLE: Manual Surgical Orthopedic Instruments Recommendations for Care, Cleaning, Maintenance and Sterilization
 Page 1 of 5 1. PROCESSING INSTRUCTIONS These processing instructions are intended to assist the hospital and central supply management in developing procedures for safe and effective reprocessing of both,
Page 1 of 5 1. PROCESSING INSTRUCTIONS These processing instructions are intended to assist the hospital and central supply management in developing procedures for safe and effective reprocessing of both,
Cavitron DualSelect. Introduction ENGLISH. Directions For Use. Cavitron DualSelect Dispensing System ENGLISH 1
 ENGLISH Cavitron DualSelect Dispensing System Directions For Use Please read carefully and completely before operating unit. Introduction Cavitron DualSelect Dispensing System Congratulations. Your purchase
ENGLISH Cavitron DualSelect Dispensing System Directions For Use Please read carefully and completely before operating unit. Introduction Cavitron DualSelect Dispensing System Congratulations. Your purchase
Flexible Endoscope Cleaning System. Instruction Manual CM Rev.B
 Flexible Endoscope Cleaning System Instruction Manual 1 0901136-CM Rev.B TABLE OF CONTENTS Introduction...3 Indication for Use......3 Safety Precautions...3 Specifications...4 Accessory Kit Parts List...5
Flexible Endoscope Cleaning System Instruction Manual 1 0901136-CM Rev.B TABLE OF CONTENTS Introduction...3 Indication for Use......3 Safety Precautions...3 Specifications...4 Accessory Kit Parts List...5
SCOPE BUDDY USER MANUAL ENDOSCOPE FLUSHING AID Rev D
 SCOPE BUDDY ENDOSCOPE FLUSHING AID USER MANUAL 50096-381 Rev D SCOPE BUDDY USER/SERVICE MANUAL FUJINON is a registered trademark of Fujinon Corporation MEDIVATORS is a registered trademark of Minntech
SCOPE BUDDY ENDOSCOPE FLUSHING AID USER MANUAL 50096-381 Rev D SCOPE BUDDY USER/SERVICE MANUAL FUJINON is a registered trademark of Fujinon Corporation MEDIVATORS is a registered trademark of Minntech
Instructions for Assembly, Installation, and Operation of the Gas Addition Kit Accessory with the CEM Discover Systems
 Corporation Issued: 5/09 P/N: 600104 Rev. 2 Instructions for Assembly, Installation, and Operation of the Gas Addition Kit Accessory with the CEM Discover Systems The Gas Addition Accessory permits the
Corporation Issued: 5/09 P/N: 600104 Rev. 2 Instructions for Assembly, Installation, and Operation of the Gas Addition Kit Accessory with the CEM Discover Systems The Gas Addition Accessory permits the
Rejuvenation Instructions
 Rejuvenation Instructions #401 Air Systems UPR This NRI covers the following: Understanding the applications and operation of flow meters. Understand the application and operation of test pressure gauges.
Rejuvenation Instructions #401 Air Systems UPR This NRI covers the following: Understanding the applications and operation of flow meters. Understand the application and operation of test pressure gauges.
spirit of excellence Hygiene Manual Reprocessing of RICHARD WOLF Heat--Sensitive Instruments
 spirit of excellence Manual Reprocessing of RICHARD WOLF Heat--Sensitive Instruments GA -J 050 /en/ Index: 05--09--5.0 / ÄM: PDB 09--3623 Important general instructions for use Reprocessing should be carried
spirit of excellence Manual Reprocessing of RICHARD WOLF Heat--Sensitive Instruments GA -J 050 /en/ Index: 05--09--5.0 / ÄM: PDB 09--3623 Important general instructions for use Reprocessing should be carried
MIC *, MIC-KEY * GASTRIC-JEJUNAL (GJ) FEEDING TUBE PATIENT USE & CARE GUIDE
 MIC *, MIC-KEY * GASTRIC-JEJUNAL (GJ) FEEDING TUBE PATIENT USE & CARE GUIDE CARE OF PATIENT WITH A GASTRIC-JEJUNAL FEEDING TUBE Some patients may benefit from the use of an enteral feeding tube that provides
MIC *, MIC-KEY * GASTRIC-JEJUNAL (GJ) FEEDING TUBE PATIENT USE & CARE GUIDE CARE OF PATIENT WITH A GASTRIC-JEJUNAL FEEDING TUBE Some patients may benefit from the use of an enteral feeding tube that provides
INSTRUCTIONS. Rotatable Clip Fixing Device HX-110LR HX-110QR HX-110UR. Clip HX HX Long Clip HX L.
 INSTRUCTIONS Rotatable Clip Fixing Device HX-110LR HX-110QR HX-110UR Clip HX-610-090 HX-610-135 Long Clip HX-610-090L Short Clip HX-610-090S HX-610-135S Colored Short Clip HX-610-090SC AUTOCLAVABLE Contents
INSTRUCTIONS Rotatable Clip Fixing Device HX-110LR HX-110QR HX-110UR Clip HX-610-090 HX-610-135 Long Clip HX-610-090L Short Clip HX-610-090S HX-610-135S Colored Short Clip HX-610-090SC AUTOCLAVABLE Contents
Rev G Medivators Inc.
 User Manual MEDIVATORS and SCOPE BUDDY are registered trademark of Medivators Inc. OLYMPUS is a registered trademark of Olympus Corporation. PENTAX is a registered trademark of Hoya Corporation. FUJIFILM
User Manual MEDIVATORS and SCOPE BUDDY are registered trademark of Medivators Inc. OLYMPUS is a registered trademark of Olympus Corporation. PENTAX is a registered trademark of Hoya Corporation. FUJIFILM
Read this first. Zetasizer nano series Self installation and Quick start guide MRK825-02
 ! Read this first Zetasizer nano series Self installation and Quick start guide I N S T R U M E N T S MRK825-02 Zetasizer Nano series Self installation and Quick start guide MAN0383 Issue 1.1 July 2007
! Read this first Zetasizer nano series Self installation and Quick start guide I N S T R U M E N T S MRK825-02 Zetasizer Nano series Self installation and Quick start guide MAN0383 Issue 1.1 July 2007
Med Aire Alternating Pressure Pump and Pad System
 User Manual Med Aire Alternating Pressure Pump and Pad System 14002E 14001E Symbols & Statements NOTE Indicates some tips or some information users should be aware of. CAUTION Indicates correct operating
User Manual Med Aire Alternating Pressure Pump and Pad System 14002E 14001E Symbols & Statements NOTE Indicates some tips or some information users should be aware of. CAUTION Indicates correct operating
Ritter AquaBottleSystem
 J1174, V02.00.00 J1174, V02.00.00 Page 0 Concept GmbH Table of Contents 1 General guidelines 2 2 Reference for the disinfection of the dental unit 3 3 Filling of the bottle 4 4 Execution of disinfection
J1174, V02.00.00 J1174, V02.00.00 Page 0 Concept GmbH Table of Contents 1 General guidelines 2 2 Reference for the disinfection of the dental unit 3 3 Filling of the bottle 4 4 Execution of disinfection
USER MANUAL. AirPal Air Supply. (AS-1100 North America) 1488 Limeport Pike, Coopersburg, PA airpal.com fax
 USER MANUAL AirPal Air Supply (AS-1100 North America) 1488 Limeport Pike, Coopersburg, PA 18036 airpal.com 800.633.4725 fax 610.965.2460 Welcome! Thank you for purchasing an AirPal Air Supply. This User
USER MANUAL AirPal Air Supply (AS-1100 North America) 1488 Limeport Pike, Coopersburg, PA 18036 airpal.com 800.633.4725 fax 610.965.2460 Welcome! Thank you for purchasing an AirPal Air Supply. This User
9BCPD24/9BCPD36-TFH BUBBLE TUBE PODIUM INSTRUCTIONS 2017
 The TFH Bubble Tube Podium is made of sturdy MDF painted wood and comes with a padded seat cushion. It is available in two sizes 24 and 36. The purpose of the Bubble/Hurricane Tube Podium is to provide
The TFH Bubble Tube Podium is made of sturdy MDF painted wood and comes with a padded seat cushion. It is available in two sizes 24 and 36. The purpose of the Bubble/Hurricane Tube Podium is to provide
PremAire INSTRUCTIONS FOR VORTEX TUBE MODE OF OPERATION
 DUAL SUPPLY REGULATOR ESCAPE CYLINDER VORTEX MAIN INLET PremAire INSTRUCTIONS FOR VORTEX TUBE MODE OF OPERATION WARNING THIS MANUAL MUST BE READ CAREFULLY BY ALL PERSONS WHO HAVE OR WILL HAVE THE RESPONSIBILITY
DUAL SUPPLY REGULATOR ESCAPE CYLINDER VORTEX MAIN INLET PremAire INSTRUCTIONS FOR VORTEX TUBE MODE OF OPERATION WARNING THIS MANUAL MUST BE READ CAREFULLY BY ALL PERSONS WHO HAVE OR WILL HAVE THE RESPONSIBILITY
Instructions for use Compressed air injector with water trap
 Instructions for use Compressed air injector with water trap Table of Contents Introduction 1. Description of Functions 2. Putting into Operation 3. Instructions for Cleaning and Care 3.1 Disassembling
Instructions for use Compressed air injector with water trap Table of Contents Introduction 1. Description of Functions 2. Putting into Operation 3. Instructions for Cleaning and Care 3.1 Disassembling
1020 Industrial Drive, Orlinda, TN fax
 Operation Manual Tank Distribution System A-UPT Series 615-654-4441 sales@specialtyh2o.com 615-654-4449 fax TABLE OF CONTENTS Section 1 GENERAL 1.2 Warnings and Cautions... 1 1.2 Theory of Operation...
Operation Manual Tank Distribution System A-UPT Series 615-654-4441 sales@specialtyh2o.com 615-654-4449 fax TABLE OF CONTENTS Section 1 GENERAL 1.2 Warnings and Cautions... 1 1.2 Theory of Operation...
Use and Care Guide. PMU and PMU w/over the Patient. Swing Mount. Over the Patient. Side Delivery
 Swing Mount Use and Care Guide PMU and PMU w/over the Patient Over the Patient Side Delivery Table of Contents Page General Information 2 Operation 3-5 Dental Unit Adjustments 6-9 Cleaning, Disinfecting
Swing Mount Use and Care Guide PMU and PMU w/over the Patient Over the Patient Side Delivery Table of Contents Page General Information 2 Operation 3-5 Dental Unit Adjustments 6-9 Cleaning, Disinfecting
Underwater Housing for Panasonic Lumix DMC-ZS60, DMC-TZ80
 Underwater Housing for Panasonic Lumix DMC-ZS60, DMC-TZ80 Product Number 6170.60 Product Registration Please register your product at ikelite.com within 15 days of purchase. Our product registration database
Underwater Housing for Panasonic Lumix DMC-ZS60, DMC-TZ80 Product Number 6170.60 Product Registration Please register your product at ikelite.com within 15 days of purchase. Our product registration database
Gas Lines. Technical Manual. Sumitomo (SHI) Cryogenics of America, Inc Vultee Street Allentown, PA U.S.A.
 Gas Lines Technical Manual Sumitomo (SHI) Cryogenics of America, Inc. 1833 Vultee Street Allentown, PA 18103-4783 U.S.A. Revision F: April 2008 261320A TABLE OF CONTENTS Page DESCRIPTION...1 SPECIFICATIONS...2
Gas Lines Technical Manual Sumitomo (SHI) Cryogenics of America, Inc. 1833 Vultee Street Allentown, PA 18103-4783 U.S.A. Revision F: April 2008 261320A TABLE OF CONTENTS Page DESCRIPTION...1 SPECIFICATIONS...2
R2S Standard Sampling Assembly OPERATION PROCEDURE
 PURPOSE 1 of 10 To describe the procedure to monitor for viable airborne bacteria with the Remote-Slit- Sampler Assembly (R2S) from EMTEK, LLC. Principle A 100 mm agar based test plate is placed on the
PURPOSE 1 of 10 To describe the procedure to monitor for viable airborne bacteria with the Remote-Slit- Sampler Assembly (R2S) from EMTEK, LLC. Principle A 100 mm agar based test plate is placed on the
Syringe, Distribution Valve and Infusion Pump Removal/Replacement ATTENTION SYRINGE REPLACEMENT
 ATTENTION SYRINGE REPLACEMENT Please read through the document completely before starting any repairs. Refer to the proper section in the service manual for complete removal and replacement procedures.
ATTENTION SYRINGE REPLACEMENT Please read through the document completely before starting any repairs. Refer to the proper section in the service manual for complete removal and replacement procedures.
User s Manual. Autoclavable & UV resistant. Multi-channel digital micro pipette. In Vitro Medical Diagnostic Devices (98/79/EC)
 Autoclavable & UV resistant Multi-channel digital micro pipette User s Manual In Vitro Medical Diagnostic Devices (98/79/EC) Annex III self-declared ISO 8655 STANDARD CERTIFIED ISO9001 Thank you very much
Autoclavable & UV resistant Multi-channel digital micro pipette User s Manual In Vitro Medical Diagnostic Devices (98/79/EC) Annex III self-declared ISO 8655 STANDARD CERTIFIED ISO9001 Thank you very much
GLIDERITE RIGID STYLET. Operations & Maintenance Manual
 GLIDERITE RIGID STYLET Operations & Maintenance Manual 0900 4686 00 60 GLIDERITE RIGID STYLET Operations & Maintenance Manual Effective: March 4, 2016 Caution: Federal (United States) law restricts this
GLIDERITE RIGID STYLET Operations & Maintenance Manual 0900 4686 00 60 GLIDERITE RIGID STYLET Operations & Maintenance Manual Effective: March 4, 2016 Caution: Federal (United States) law restricts this
Underwater Housing for Canon EOS M
 Underwater Housing for Canon EOS M User Manual 1 Table of Contents 1. Introduction 2. Specifications 3. Function Controls 4. Set up Instructions 5. Use & Care of Housing 6. Service 7. Warranty 1. Introduction
Underwater Housing for Canon EOS M User Manual 1 Table of Contents 1. Introduction 2. Specifications 3. Function Controls 4. Set up Instructions 5. Use & Care of Housing 6. Service 7. Warranty 1. Introduction
RG1200 Service and Repair Manual
 Dive Rite RG 1200 Regulator Service and Repair Manual Page 1 Text and Photography by Pete Nawrocky Copyright ( ) 1999-2000, Lamartek, Inc., dba Dive Rite RG1200 Service and Repair Manual First Stage.........................................
Dive Rite RG 1200 Regulator Service and Repair Manual Page 1 Text and Photography by Pete Nawrocky Copyright ( ) 1999-2000, Lamartek, Inc., dba Dive Rite RG1200 Service and Repair Manual First Stage.........................................
Cleaning rod: spring steel, stainless steel or carbon fibre cleaning rod - only use a one-piece rod. Avoid using snakes.
 Telemark Biathlon Where performance and precision come together http://telemarkbiathlon.com Rifle Cleaning Date : July 19, 2013 Anschutz Rifle Manual - Click Here Izhmash 7-3 Rifle Manual - still looking
Telemark Biathlon Where performance and precision come together http://telemarkbiathlon.com Rifle Cleaning Date : July 19, 2013 Anschutz Rifle Manual - Click Here Izhmash 7-3 Rifle Manual - still looking
PERFORM Operating Document
 PERFORM Operating Document Use and Maintenance of CO 2 Incubator PC-POD-CA-007-v02 Revision History Version Reason for Revision Date 01 New POD 30-Sep-13 02 Minor revisions for section 2.3, 3.1, 4.3. 14-April-16
PERFORM Operating Document Use and Maintenance of CO 2 Incubator PC-POD-CA-007-v02 Revision History Version Reason for Revision Date 01 New POD 30-Sep-13 02 Minor revisions for section 2.3, 3.1, 4.3. 14-April-16
6900 Maintenance Instruction System Flush
 Equipment Required FA74005 Damper Drain Tube FA16005 Cover Removal Tool FA900005 Beaker 0.25 Litre FA900003 Solvent Cleaning Bottle FA940021 Syringe Polypropylene 50 ml as required FA999045 Gloves Latex
Equipment Required FA74005 Damper Drain Tube FA16005 Cover Removal Tool FA900005 Beaker 0.25 Litre FA900003 Solvent Cleaning Bottle FA940021 Syringe Polypropylene 50 ml as required FA999045 Gloves Latex
Competency Record for Sonic Cleaning
 Competency Record for Sonic Cleaning Name: Competency Achieved: (Date) Evaluator: Learner: Comments: Background information on sonic testing ST 79; 2009A section 7.5.3.3 states this on weekly testing Mechanical
Competency Record for Sonic Cleaning Name: Competency Achieved: (Date) Evaluator: Learner: Comments: Background information on sonic testing ST 79; 2009A section 7.5.3.3 states this on weekly testing Mechanical
Underwater Housing for Canon G7X Mark II
 Underwater Housing for Canon G7X Mark II Product Number 6146.08 Product Registration Please register your product at ikelite.com within 15 days of purchase. Our product registration database is the best
Underwater Housing for Canon G7X Mark II Product Number 6146.08 Product Registration Please register your product at ikelite.com within 15 days of purchase. Our product registration database is the best
NanoSight NS300. NanoSight NS300. Operation instructions. Laser Spectroscopy Labs, UCI
 NanoSight NS300 Operation instructions Injection/flushing brief overview: 1. Do not exceed flow of 1 ml per 20 seconds. 2. Inject two 1 ml syringes with nano-pure or DI water. 3. If the water does not
NanoSight NS300 Operation instructions Injection/flushing brief overview: 1. Do not exceed flow of 1 ml per 20 seconds. 2. Inject two 1 ml syringes with nano-pure or DI water. 3. If the water does not
OPERATING INSTRUCTIONS MANUAL FOR QACV PNEUMATIC DOSING PUMP
 This operating instructions contains safety information that if ignored can endanger life or result in serious injury. They are indicated by this icon. Use of this pump with radioactive chemicals is forbidden!
This operating instructions contains safety information that if ignored can endanger life or result in serious injury. They are indicated by this icon. Use of this pump with radioactive chemicals is forbidden!
Using the Akta Prime plus October 22, 2012
 Some starting precautions: 1. Vacuum filter all buffers. Removes any large particles/debris that may clog your column De-gases the buffers 2. Clarify lysates first by centrifugation and then filtration
Some starting precautions: 1. Vacuum filter all buffers. Removes any large particles/debris that may clog your column De-gases the buffers 2. Clarify lysates first by centrifugation and then filtration
User s Guide. CO2 & O2 Controller for miniature incubators
 CO2 O2 Control User s Guide CO2 & O2 Controller for miniature incubators Precise CO2 & O2 Control throughout the experiment Media ph control Compatible with any perfusion system Miniature incubators for
CO2 O2 Control User s Guide CO2 & O2 Controller for miniature incubators Precise CO2 & O2 Control throughout the experiment Media ph control Compatible with any perfusion system Miniature incubators for
OPERATOR S MANUAL HOTLINE 3 Blood and Fluid Warmer
 l OPERATOR S MANUAL - HOTLINE 3 Blood and Fluid Warmer < HL-390-38 6 s l HOTLINE 3 Blood and Fluid Warmer < HL-390-38 OPERATOR S MANUAL PN 4534013EN Rev. 002 s G e n e r a l I n f o r m a t i o n HOTLINE
l OPERATOR S MANUAL - HOTLINE 3 Blood and Fluid Warmer < HL-390-38 6 s l HOTLINE 3 Blood and Fluid Warmer < HL-390-38 OPERATOR S MANUAL PN 4534013EN Rev. 002 s G e n e r a l I n f o r m a t i o n HOTLINE
OPERATION MANUAL (Preparation and Operation)
 US Endoscope ED-530XT OPERATION MANUAL (Preparation and Operation) Thank you for purchasing our product. Read this manual carefully before use to avoid unexpected accidents and to take full advantage of
US Endoscope ED-530XT OPERATION MANUAL (Preparation and Operation) Thank you for purchasing our product. Read this manual carefully before use to avoid unexpected accidents and to take full advantage of
Parts identification:
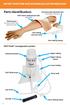 ARTERY PUNCTURE AND INTRAMUSCULAR TRAINING ARM Parts identification: FAST-derm replacement skin (removable) Intramuscular injection pad (removable for cleaning) Training arm (includes one FAST-derm replacement
ARTERY PUNCTURE AND INTRAMUSCULAR TRAINING ARM Parts identification: FAST-derm replacement skin (removable) Intramuscular injection pad (removable for cleaning) Training arm (includes one FAST-derm replacement
Installation of Your SprayMaster System
 Installation of Your SprayMaster System 1. At the installation site, remove all equipment from the corrugated box and the polyethylene drum and replace the drum lid. Check the picture to identify each
Installation of Your SprayMaster System 1. At the installation site, remove all equipment from the corrugated box and the polyethylene drum and replace the drum lid. Check the picture to identify each
REL-510H WARNING NOTICE 12 TON SINGLE ACTING REMOTE HYDRAULIC CRIMPING HEAD
 OPERATORS ORS GUIDE REL-510H 12 TON SINGLE ACTING REMOTE HYDRAULIC CRIMPING HEAD Compatible with U style and RELIABLE R12 shell type 12 ton compression dies. RELIABLE EQUIPMENT & SERVICE CO., INC. 92 Steamwhistle
OPERATORS ORS GUIDE REL-510H 12 TON SINGLE ACTING REMOTE HYDRAULIC CRIMPING HEAD Compatible with U style and RELIABLE R12 shell type 12 ton compression dies. RELIABLE EQUIPMENT & SERVICE CO., INC. 92 Steamwhistle
DIAGNOSTIC CARDIOLOGY
 DIAGNOSTIC CARDIOLOGY For Your Procedural Needs Manufactured to the Highest Standards, Customised to Your Specific Needs MEDLINE PROCEDURE PACKS The items contained within this brochure are perfect for
DIAGNOSTIC CARDIOLOGY For Your Procedural Needs Manufactured to the Highest Standards, Customised to Your Specific Needs MEDLINE PROCEDURE PACKS The items contained within this brochure are perfect for
User s Guide. CO2 Controller for miniature incubators
 CO2 Control User s Guide CO2 Controller for miniature incubators Precise CO2 Control throughout the experiment Media ph control Compatible with any perfusion system Miniature incubators for any microscope
CO2 Control User s Guide CO2 Controller for miniature incubators Precise CO2 Control throughout the experiment Media ph control Compatible with any perfusion system Miniature incubators for any microscope
Model VR6 System. Installation, Operation & Maintenance
 Model VR6 System Installation, Operation & Maintenance General: All Archer Instruments chlorination systems are carefully designed and tested for years of safe, accurate field service. All Archer Instruments
Model VR6 System Installation, Operation & Maintenance General: All Archer Instruments chlorination systems are carefully designed and tested for years of safe, accurate field service. All Archer Instruments
BS ml Bottle Sampling Unit
 BS500 500ml Bottle Sampling Unit User Guide www.mpfiltri.co.uk 200.307-EN Covers Model Numbers BS500 SAFETY WARNING Hydraulic systems contain dangerous fluids at high pressures and temperatures. Installation,
BS500 500ml Bottle Sampling Unit User Guide www.mpfiltri.co.uk 200.307-EN Covers Model Numbers BS500 SAFETY WARNING Hydraulic systems contain dangerous fluids at high pressures and temperatures. Installation,
HemoCue Hb Procedure Template
 HemoCue Hb 201 + Procedure Template PURPOSE The HemoCue Hb 201 + System is used for the quantitative determination of hemoglobin in blood using a specially designed analyzer, HemoCue Hb 201 +, and specially
HemoCue Hb 201 + Procedure Template PURPOSE The HemoCue Hb 201 + System is used for the quantitative determination of hemoglobin in blood using a specially designed analyzer, HemoCue Hb 201 +, and specially
F-330 Instruction manual. Magic Glove Bio Skin Smoother High Frequency Ultrasound Galvanic
 F-330 Instruction manual Magic Glove Bio Skin Smoother High Frequency Ultrasound Galvanic Warning Never under any circumstances attempt to open or inspect the internal components or accessories of the
F-330 Instruction manual Magic Glove Bio Skin Smoother High Frequency Ultrasound Galvanic Warning Never under any circumstances attempt to open or inspect the internal components or accessories of the
PERFORM Operating Document
 PERFORM Operating Document Use and Maintenance of CO 2 Incubator PC-POD-CA-007-v03 Revision History Version Reason for Revision Date 01 New POD 30-Sep-13 02 Minor revisions for section 2.3, 3.1, 4.3. 14-April-16
PERFORM Operating Document Use and Maintenance of CO 2 Incubator PC-POD-CA-007-v03 Revision History Version Reason for Revision Date 01 New POD 30-Sep-13 02 Minor revisions for section 2.3, 3.1, 4.3. 14-April-16
EMT EnvironmentalMonitoringTECHNOLOGIES
 1 of 11.PURPOSE To describe the procedure to monitor for viable airborne bacteria with the R2S Remote Start Sampling Assembly from EMT. Principle A 100 mm agar based test plate is placed on the turntable
1 of 11.PURPOSE To describe the procedure to monitor for viable airborne bacteria with the R2S Remote Start Sampling Assembly from EMT. Principle A 100 mm agar based test plate is placed on the turntable
To provide adequate disinfection for reusable semi-critical equipment other than scopes. Processing of scopes is covered in
 Purpose To provide adequate disinfection for reusable semi-critical equipment other than scopes. Processing of scopes is covered in 01.05.04. Audience Policy Guidelines for High- Level All those in the
Purpose To provide adequate disinfection for reusable semi-critical equipment other than scopes. Processing of scopes is covered in 01.05.04. Audience Policy Guidelines for High- Level All those in the
Contents: 1. Introduction
 Camera Underwater Housing G11 (Canon) User Manual Contents: 1. Introduction 2. Specifications 3. Function Controls 4. Set up Instructions 5. Use & Care of Housing 6. Service 7. Warranty 1. Introduction
Camera Underwater Housing G11 (Canon) User Manual Contents: 1. Introduction 2. Specifications 3. Function Controls 4. Set up Instructions 5. Use & Care of Housing 6. Service 7. Warranty 1. Introduction
Techcon Systems TS1254 Pressure Pot
 Techcon Systems TS1254 Pressure Pot User Guide Copyright OK International, Inc. TABLE OF CONTENT 1. SAFETY. 3 1.1 Intended Use 3 1.2 Safety Precaution 3 2. FEATURES. 5 3. SPECIFICATIONS 4 4. INSTALLATION
Techcon Systems TS1254 Pressure Pot User Guide Copyright OK International, Inc. TABLE OF CONTENT 1. SAFETY. 3 1.1 Intended Use 3 1.2 Safety Precaution 3 2. FEATURES. 5 3. SPECIFICATIONS 4 4. INSTALLATION
Instructions for use Evacuation system compressed air injector SL 20 / SL 20 Max SL 30 / SL 30 Max
 Instructions for use Evacuation system compressed air injector SL 20 / SL 20 Max SL 30 / SL 30 Max Table of Contents Introduction 1. Description of Functions 2. Putting into Operation 3. Instructions for
Instructions for use Evacuation system compressed air injector SL 20 / SL 20 Max SL 30 / SL 30 Max Table of Contents Introduction 1. Description of Functions 2. Putting into Operation 3. Instructions for
EasyFit Lite Nasal Mask, Silicone. Nasal Mask. EasyFit Lite Nasal Mask. Device Description and Instructions for Use. Caution:
 EasyFit Lite Nasal Mask EasyFit Lite Nasal Mask, Silicone Nasal Mask Device Description and Instructions for Use Caution: Federal Law (U.S.A.) restricts this device to sale by or on the order of a physician.
EasyFit Lite Nasal Mask EasyFit Lite Nasal Mask, Silicone Nasal Mask Device Description and Instructions for Use Caution: Federal Law (U.S.A.) restricts this device to sale by or on the order of a physician.
Water Weir Flow Controller. Introduction. Safety Precautions. Mounting the Hardware
 57007-88 Introduction Safety Precautions This instruction sheet describes how to set up and use the Hach (Figure 1). A water weir is a device that raises or diverts water to regulate the flow. Hach s water
57007-88 Introduction Safety Precautions This instruction sheet describes how to set up and use the Hach (Figure 1). A water weir is a device that raises or diverts water to regulate the flow. Hach s water
Operation and Maintenance Manual for GENTEC Model 881VR Continuous/Intermittent Digital Suction Regulators
 Operation and Maintenance Manual for GENTEC Model 881VR Continuous/Intermittent Digital Suction Regulators Genstar Technologies Co., Inc. 4525 Edison Avenue Chino, CA 91710 USA TEL 909-0-272 FAX 909-0-485
Operation and Maintenance Manual for GENTEC Model 881VR Continuous/Intermittent Digital Suction Regulators Genstar Technologies Co., Inc. 4525 Edison Avenue Chino, CA 91710 USA TEL 909-0-272 FAX 909-0-485
AMBLER SURGICAL INSTRUCTIONS FOR USE - OPHTHALMIC DUET-STYLE SCISSORS
 AMBLER SURGICAL INSTRUCTIONS FOR USE - OPHTHALMIC DUET-STYLE SCISSORS Ambler Item # 6112E Duet-style MICS Packer/Chang IOL cutter, 1 3/8", 19 gauge, straight shaft, for use with Ambler #6100T INDICATIONS
AMBLER SURGICAL INSTRUCTIONS FOR USE - OPHTHALMIC DUET-STYLE SCISSORS Ambler Item # 6112E Duet-style MICS Packer/Chang IOL cutter, 1 3/8", 19 gauge, straight shaft, for use with Ambler #6100T INDICATIONS
AIR DISTRIBUTOR. PART NUMBER (Including, but not inclusive) AD28483-_ALB, AD28563-_ALB, AD36097-_ALB, AD36098-_ALB, AD28485-_ALB
 Manual #: MM-AD001 12/5/11 Rev. A Page 1 of 8 PART NUMBER (Including, but not inclusive) AD28483-_ALB, AD28563-_ALB, AD36097-_ALB, AD36098-_ALB, AD28485-_ALB Manual #: MM-AD001 12/5/11 Rev. A Page 2 of
Manual #: MM-AD001 12/5/11 Rev. A Page 1 of 8 PART NUMBER (Including, but not inclusive) AD28483-_ALB, AD28563-_ALB, AD36097-_ALB, AD36098-_ALB, AD28485-_ALB Manual #: MM-AD001 12/5/11 Rev. A Page 2 of
User's Manual. MixRite TF 10. Edition 05.08
 User's Manual MixRite TF 10 Edition 05.08 1 Tefen MixRite TF 10 fertilizer and chemicals Injector Congratulations on your purchase of one of Tefen s high quality products. To get the best results from
User's Manual MixRite TF 10 Edition 05.08 1 Tefen MixRite TF 10 fertilizer and chemicals Injector Congratulations on your purchase of one of Tefen s high quality products. To get the best results from
Air-Mate High Efficiency Powered Air Purifying Respirator (PAPR) Staff Training Module
 Air-Mate High Efficiency Powered Air Purifying Respirator (PAPR) Staff Training Module PRES0389 7/05-1 Powered Air Purifying Respirators (PAPR) Reduce the risk of transmission of infectious agents spread
Air-Mate High Efficiency Powered Air Purifying Respirator (PAPR) Staff Training Module PRES0389 7/05-1 Powered Air Purifying Respirators (PAPR) Reduce the risk of transmission of infectious agents spread
EMERGENCY EQUIPMENT TROLLEY DAILY SIGNATURE SHEET. Please refer to the Emergency Equipment stock poster for detailed information
 EMERGENCY EQUIPMENT TROLLEY DAILY SIGNATURE SHEET Please refer to the Emergency Equipment stock poster for detailed information Equipment must be checked daily and immediately following an emergency event
EMERGENCY EQUIPMENT TROLLEY DAILY SIGNATURE SHEET Please refer to the Emergency Equipment stock poster for detailed information Equipment must be checked daily and immediately following an emergency event
Suction Regulators. Recommendations for Cleaning, Disinfection and Reprocessing: Boehringer 3800 &3900 Series Suction Regulators
 Suction Regulators Recommendations for Cleaning, Disinfection and Reprocessing: Boehringer 3800 &3900 Series Suction Regulators Boehringer Laboratories, LLC 300 Thoms Drive Phoenixville, PA 19460 (800)
Suction Regulators Recommendations for Cleaning, Disinfection and Reprocessing: Boehringer 3800 &3900 Series Suction Regulators Boehringer Laboratories, LLC 300 Thoms Drive Phoenixville, PA 19460 (800)
Pressure Dump Valve Service Kit for Series 2300 Units
 Instruction Sheet Pressure Dump Valve Service Kit for Series 00 Units. Overview The Nordson pressure dump valve is used to relieve hydraulic pressure instantly in Series 00 applicator tanks when the unit
Instruction Sheet Pressure Dump Valve Service Kit for Series 00 Units. Overview The Nordson pressure dump valve is used to relieve hydraulic pressure instantly in Series 00 applicator tanks when the unit
BD Cytopeia Fluidic Kit User s Guide
 BD Cytopeia Fluidic Kit User s Guide For Research Use Only 23-17618-00 9/2015 Becton, Dickinson and Company BD Biosciences 2350 Qume Drive San Jose, CA 95131 USA BD Biosciences European Customer Support
BD Cytopeia Fluidic Kit User s Guide For Research Use Only 23-17618-00 9/2015 Becton, Dickinson and Company BD Biosciences 2350 Qume Drive San Jose, CA 95131 USA BD Biosciences European Customer Support
Drawn up by: / RK Revision: 01 Reviewed: Page: 1 of 23 HI-SR 540 USA. Operating instruction SR 540 Face Shield
 Page: 1 of 23 Operating instruction SR 540 Face Shield Page: 2 of 23 General information The SR 540 Face Shield can be used together with the SR 500 Powered Air-Purifying Respirator system of components
Page: 1 of 23 Operating instruction SR 540 Face Shield Page: 2 of 23 General information The SR 540 Face Shield can be used together with the SR 500 Powered Air-Purifying Respirator system of components
Hydraulic Punch Drivers
 SERVICE MANUAL 7804SB / 7806SB Quick Draw 7704SB / 7706SB Quick Draw Flex Quick Draw Hydraulic Punch Drivers Serial Codes AHJ and YZ Read and understand all of the instructions and safety information in
SERVICE MANUAL 7804SB / 7806SB Quick Draw 7704SB / 7706SB Quick Draw Flex Quick Draw Hydraulic Punch Drivers Serial Codes AHJ and YZ Read and understand all of the instructions and safety information in
6800 Maintenance Instruction System Flush Procedure
 Equipment Required FA74005 FA65318 FA900005 FA900003 Damper Drain Tube 6800 Cover Removal Tool Beaker 0.25 Litre Solvent Cleaning Bottle FA940021 Syringe Polypropylene 50 ml as required FA999045 Gloves
Equipment Required FA74005 FA65318 FA900005 FA900003 Damper Drain Tube 6800 Cover Removal Tool Beaker 0.25 Litre Solvent Cleaning Bottle FA940021 Syringe Polypropylene 50 ml as required FA999045 Gloves
Wolfson Campus Natural Science Department. Biology Laboratory. Student Safety Contract
 Wolfson Campus Natural Science Department Biology Laboratory Student Safety Contract July 2000 Wolfson Campus Student Safety Contract-Biology Laboratory Purpose The Biology laboratory is a hands-on learning
Wolfson Campus Natural Science Department Biology Laboratory Student Safety Contract July 2000 Wolfson Campus Student Safety Contract-Biology Laboratory Purpose The Biology laboratory is a hands-on learning
Metric Porcelain Enamel Powder Pump
 Instruction Sheet P/N 06 83D Metric Porcelain Enamel Powder Pump. Introduction This instruction sheet covers the Metric Porcelain Enamel Powder Pump. The pump is a highly efficient venturi-type powder
Instruction Sheet P/N 06 83D Metric Porcelain Enamel Powder Pump. Introduction This instruction sheet covers the Metric Porcelain Enamel Powder Pump. The pump is a highly efficient venturi-type powder
User Manual POINT SETTER MODEL:
 MITAKA KOHKI Co., Ltd. User Manual POINT SETTER MODEL: PSMS-2 PSMS2-M03 Last update January 1, 2015 INTRODUCTION Thank you very much for purchasing the MITAKA Point Setter (holding system for surgical
MITAKA KOHKI Co., Ltd. User Manual POINT SETTER MODEL: PSMS-2 PSMS2-M03 Last update January 1, 2015 INTRODUCTION Thank you very much for purchasing the MITAKA Point Setter (holding system for surgical
Zero Head Space Extractor. Catalogue No. YT HW. Operation and Maintenance Manual
 Catalogue No. YT30 090 HW Operation and Maintenance Manual ABOUT THE ZERO HEAD SPACE EXTRACTOR The Millipore Zero Head Space Extractor (ZHE) meets the U.S. EPA'S Toxicity Characteristics Leaching Procedure
Catalogue No. YT30 090 HW Operation and Maintenance Manual ABOUT THE ZERO HEAD SPACE EXTRACTOR The Millipore Zero Head Space Extractor (ZHE) meets the U.S. EPA'S Toxicity Characteristics Leaching Procedure
Sunrise Fountain Water Wonders Assembly & Mounting Instructions
 Welcome to the Water Wonders family. A few simple steps will ensure that your Sunrise Fountain remains a soothing, enjoyable fountain that brings the sights and sounds of nature to you. Two important considerations
Welcome to the Water Wonders family. A few simple steps will ensure that your Sunrise Fountain remains a soothing, enjoyable fountain that brings the sights and sounds of nature to you. Two important considerations
SUGGESTED POLICY FOR THE MONITORING OF THE CLEANING EFFICIENCY OF AN ULTRASONIC CLEANERS( SONIC & TOSI) WEEKLY
 SUGGESTED POLICY FOR THE MONITORING OF THE CLEANING EFFICIENCY OF AN ULTRASONIC CLEANERS( SONIC & TOSI) WEEKLY SUBJECT: ULTRASONIC CLEANER MONITORING WEEKLY DEPARTMENT: Central Service APPROVED BY: EFFECTIVE:
SUGGESTED POLICY FOR THE MONITORING OF THE CLEANING EFFICIENCY OF AN ULTRASONIC CLEANERS( SONIC & TOSI) WEEKLY SUBJECT: ULTRASONIC CLEANER MONITORING WEEKLY DEPARTMENT: Central Service APPROVED BY: EFFECTIVE:
Contacts. Quick Start Guide
 Contacts Clinical Support Specialist: Phone: Cell Phone: Email: Fresenius Renal Technologies A division of Fresenius Medical Care North America 920 Winter Street Waltham, MA 02451 Technical Service Customer
Contacts Clinical Support Specialist: Phone: Cell Phone: Email: Fresenius Renal Technologies A division of Fresenius Medical Care North America 920 Winter Street Waltham, MA 02451 Technical Service Customer
KMB 18/28 (BANDMASK) MONTHLY MASK INSPECTION APPENDIX A
 KMB 18/28 (BANDMASK) MONTHLY MASK INSPECTION APPENDIX A2.2 08-01-17 This inspection is the minimum recommended maintenance and should be performed at least ONCE A MONTH with Mask(s) in continuous use (used
KMB 18/28 (BANDMASK) MONTHLY MASK INSPECTION APPENDIX A2.2 08-01-17 This inspection is the minimum recommended maintenance and should be performed at least ONCE A MONTH with Mask(s) in continuous use (used
RECOMBINATE. [Antihemophilic Factor (Recombinant)] (For intravenous use only)
![RECOMBINATE. [Antihemophilic Factor (Recombinant)] (For intravenous use only) RECOMBINATE. [Antihemophilic Factor (Recombinant)] (For intravenous use only)](/thumbs/79/80305643.jpg) Website for more information: http://www.recombinate.com/ INSTRUCTIONS FOR USE RECOMBINATE [Antihemophilic Factor (Recombinant)] (For intravenous use only) Do not attempt to do an infusion to yourself
Website for more information: http://www.recombinate.com/ INSTRUCTIONS FOR USE RECOMBINATE [Antihemophilic Factor (Recombinant)] (For intravenous use only) Do not attempt to do an infusion to yourself
NOVALYNX CORPORATION MODEL RAIN GAUGE CALIBRATOR INSTRUCTION MANUAL
 NOVALYNX CORPORATION MODEL 260-2595 RAIN GAUGE CALIBRATOR INSTRUCTION MANUAL REVISION DATE: August 2018 Receiving and Unpacking Carefully unpack all components and compare to the packing list. Notify NovaLynx
NOVALYNX CORPORATION MODEL 260-2595 RAIN GAUGE CALIBRATOR INSTRUCTION MANUAL REVISION DATE: August 2018 Receiving and Unpacking Carefully unpack all components and compare to the packing list. Notify NovaLynx
Pressure Dump Valve Service Kit for Series 3000 Units
 Instruction Sheet Pressure Dump Valve Service Kit for Series 000 Units. Overview The Nordson pressure dump valve is used to relieve hydraulic pressure instantly in Series 00, 400, 500, and 700 applicator
Instruction Sheet Pressure Dump Valve Service Kit for Series 000 Units. Overview The Nordson pressure dump valve is used to relieve hydraulic pressure instantly in Series 00, 400, 500, and 700 applicator
ShellPa. Standard Mechanical Cell Stretch System Model No: NNMS Serial #: User Manual
 ShellPa Standard Mechanical Cell Stretch System Model No: NNMS Serial #: User Manual To operate the system properly and safely, read the manual before using ShellPa. This system is not a medical device.
ShellPa Standard Mechanical Cell Stretch System Model No: NNMS Serial #: User Manual To operate the system properly and safely, read the manual before using ShellPa. This system is not a medical device.
ESCONDIDO FIRE DEPARTMENT TRAINING MANUAL ENGINE MODULE SCBA PAGE 1 OF 14 Maintenance Revised
 SCBA 10-07-16 PAGE 1 OF 14 The California Occupational Safety and Health Administration requires that: Respirators shall be regularly cleaned after each use or more often if necessary. Those used by more
SCBA 10-07-16 PAGE 1 OF 14 The California Occupational Safety and Health Administration requires that: Respirators shall be regularly cleaned after each use or more often if necessary. Those used by more
CART FOR CENTURION 1500+
 CART FOR CENTURION 1500+ Operation & Maintenance Manual www.ameriwater.com 800-535-5585 AmeriWater 3345 Stop 8 Rd. Dayton, OH 45414 98-0161 Rev A Contents 1. GENERAL INFORMATION... 1 1.1. Features...
CART FOR CENTURION 1500+ Operation & Maintenance Manual www.ameriwater.com 800-535-5585 AmeriWater 3345 Stop 8 Rd. Dayton, OH 45414 98-0161 Rev A Contents 1. GENERAL INFORMATION... 1 1.1. Features...
SC505 Sof Care Inflator. Operating Instructions and Service Manual
 SC505 Sof Care Inflator Gaymar Industries, Inc. 10 Centre Drive Orchard Park, NY 14127 Toll Free +1 800.828.7341 + 1 716.662.2551 Fax +1 800.993.7890 Outside USA +1 716.662.8636 Outside USA Fax +1 716.662.0730
SC505 Sof Care Inflator Gaymar Industries, Inc. 10 Centre Drive Orchard Park, NY 14127 Toll Free +1 800.828.7341 + 1 716.662.2551 Fax +1 800.993.7890 Outside USA +1 716.662.8636 Outside USA Fax +1 716.662.0730
