Gilbert Kirss Foster. Chapter 10. Properties of Gases The Air We Breathe
|
|
|
- Diane Webster
- 5 years ago
- Views:
Transcription
1 Gilbert Kirss Foster Chapter 10 Properties of Gases The Air We Breathe
2 Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4 The Gas Laws 10.5 The Combined Gas Law 10.6 Ideal Gases and the Ideal Gas Law 10.7 Densities of Gases 10.8 Gases in Chemical Reactions 10.9 Mixtures of Gases Solubility of Gases and Henry s Law Gas Diffusion: Molecules Moving Rapidly Real Gases
3 Properties of a Gas Neither definite shape nor definite volume Uniformly fills any container Exerts pressure on surroundings Volume changes with temperature and pressure Mixes completely with other gases Much less dense than solids, liquids
4 Parameters Affecting Gases Pressure (P) Volume (V) Temperature (T) Number of Moles (n)
5 Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4 The Gas Laws 10.5 The Combined Gas Law 10.6 Ideal Gases and the Ideal Gas Law 10.7 Densities of Gases 10.8 Gases in Chemical Reactions 10.9 Mixtures of Gases Solubility of Gases and Henry s Law Gas Diffusion: Molecules Moving Rapidly Real Gases
6 Kinetic Molecular Theory (KMT) Assumes that gas molecules: 1. Have tiny volumes compared with their container s volume 2. Don t interact with other gas molecules 3. Move randomly and constantly 4. Engage in elastic collisions with walls of container and other gas molecules 5. Have average kinetic energy that is proportional to absolute temperature
7 Kinetic Molecular Theory (cont.) Average Kinetic Energy: KEavg = ½ mu2rms urms = the root-mean-squared speed of the molecules; m = molecular mass.
8 Effusion Relative Rates of Effusion: ( Rate )gas 1 = ( Rate )gas 2 M2 M1 where M is the molar mass
9 Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4 The Gas Laws 10.5 The Combined Gas Law 10.6 Ideal Gases and the Ideal Gas Law 10.7 Densities of Gases 10.8 Gases in Chemical Reactions 10.9 Mixtures of Gases Solubility of Gases and Henry s Law Gas Diffusion: Molecules Moving Rapidly Real Gases
10 The Gas Phase The Atmosphere: Layer of gases 50 km thick Composition is fairly consistent Properties vary with location» Pressure, density
11 Pressure Pressure: Force/unit area (P = F/A) Atmospheric pressure = pressure exerted due to gravity acting on air above Earth s surface Units of Pressure: SI units: newton/meter2 = 1 pascal (Pa) 1 standard atmosphere (1 atm) = 101,325 Pa 1 atm = 760 mmhg = 760 torr
12 Measurement of Pressure Barometer: measures atmospheric pressure Height of Hg column based on balance of forces: gravity (pulls Hg down). atmospheric pressure (pushes Hg up into evacuated tube)
13 Elevation and Atmospheric Pressure 0.35 atm 0.62 atm 0.83 atm Sea level
14 Relationship between Pressure Units
15 Measuring Pressure: Manometer Closed systems: Δh of Hg column is a direct measure of gas pressure (b) Open systems: Δh is negative if Pgas < Patm (d) Δh is positive if Pgas > Patm (e)
16 Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4 The Gas Laws 10.5 The Combined Gas Law 10.6 Ideal Gases and the Ideal Gas Law 10.7 Densities of Gases 10.8 Gases in Chemical Reactions 10.9 Mixtures of Gases Solubility of Gases and Henry s Law Gas Diffusion: Molecules Moving Rapidly Real Gases
17 Boyle s Law Gases are compressible Pressure as Volume Boyle s Law: P 1/V (T and n fixed) or, P V = constant or, P1V1 = P2V2 Decreasing volume increases number of collisions/area; P (KMT Postulates #3 & 4)
18 Practice: Boyle s Law A balloon is filled with carbon dioxide to a pressure of 1.85 atm and has a volume of 1.54 L. If temperature remains constant, what is the final volume when the pressure is increased to 2.50 atm? Collect and Organize: Analyze: Solve: Think about It:
19 Charles s Law Charles s Law: V T (P, n constant) V1 V2 or, = T1 T2 Volume of a gas extrapolates to zero at absolute zero (0 K = 273 C). Kinetic energy as T ; force of collisions increases and gas expands to maintain constant P (KMT Post. #3, 4 & 5).
20 Avogadro s Law Volume is directly proportional to the number of moles of gas, V n (T, P constant) or, V1 V2 n1 n2 V1 V2 or, n1 n2 Increasing n increases the number of collisions, gas expands to keep pressure constant (KMT Post. #3 & 4).
21 Amonton s Law P T (n, V constant) P = constant T P = constant T Increasing T will increase force of collisions if volume is kept constant; P will increase (KMT Post. #3, 4 & 5). P =constant T
22 Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4 The Gas Laws 10.5 The Combined Gas Law 10.6 Ideal Gases and the Ideal Gas Law 10.7 Densities of Gases 10.8 Gases in Chemical Reactions 10.9 Mixtures of Gases Solubility of Gases and Henry s Law Gas Diffusion: Molecules Moving Rapidly Real Gases
23 Combined Gas Law Boyle s Law: P V = constant Charles s Law: V/T = constant Avogadro s Law: V/n = constant Combining the gas laws: P V constant n T If n is constant, then PV/T = constant, and
24 Practice: Combined Gas Law A sample of oxygen gas is at atm and occupies a volume of 10.0 L at 0 C. What is the pressure of the gas if it is at 15.0 L at 25 C? Collect and Organize: Analyze: Solve: Think about It:
25 Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4 The Gas Laws 10.5 The Combined Gas Law 10.6 Ideal Gases and the Ideal Gas Law 10.7 Densities of Gases 10.8 Gases in Chemical Reactions 10.9 Mixtures of Gases Solubility of Gases and Henry s Law Gas Diffusion: Molecules Moving Rapidly Real Gases
26 Ideal Gas Law P V Combined Gas Law: constant R n T This rearranges to: PV = nrt R = universal gas constant = L atm K 1 mol 1 P = pressure (in atm) V = volume (in liters) n = moles T = temperature (in kelvin)
27 Universal Gas Constant Value of universal gas constant depends on the units Using SI units of V and P, L atm R = mol K Using 1 atm = kpa to convert to atm L atm R = mol K
28 Practice: Ideal Gas Law Calculate the moles of gas contained in a 4.0 L container at STP. Collect and Organize: Analyze: Solve: Think about It:
29 Practice: Ideal Gas Law Calculate the pressure of 4.0 mol of methane gas in a 12.3 L container at 25 C. Collect and Organize: Analyze: Solve: Think about It:
30 Practice: Ideal Gas Law An experiment shows that a g sample of an unknown gas occupies 127 ml at 98 C and 754 torr pressure. Calculate the molar mass of the gas. (Hint: M = g/n) Collect and Organize: Analyze: Solve: Think about It:
31 Reference Points for Gases Standard Temperature and Pressure (STP): P = 1 atmosphere; T = 0 C (273 K) Molar Volume: For 1 mol of an ideal gas at STP (calculated from the ideal gas law): V = 22.4 L
32 Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4 The Gas Laws 10.5 The Combined Gas Law 10.6 Ideal Gases and the Ideal Gas Law 10.7 Densities of Gases 10.8 Gases in Chemical Reactions 10.9 Mixtures of Gases Solubility of Gases and Henry s Law Gas Diffusion: Molecules Moving Rapidly Real Gases
33 Densities of Gases Can be calculated from molar mass (M) and molar volume (V/n). From Ideal Gas Law: PV = nrt Density: m PM d= = V RT m PM d= = V RT When P in atm, T in kelvin, d = g/l
34 Buoyancy: Densities of Gases Buoyancy depends on differences in gas densities. Depends on: 1. Molar Masses He(g) = g/l* N2(g) = 1.19 g/l* 2. Temperature Charles s Law: density as Temp. * At 15 C and 1 atm
35 Practice: Densities of Gases When HNO3(aq) and NaHCO3(aq) are mixed together, a reaction takes place in which a gas is one of the products. The gas has a d = 1.83 g/l at 1.00 atm and 23 C. What is the molar mass and identity of the gas? Collect and Organize: Analyze: Solve: Think about It:
36 Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4 The Gas Laws 10.5 The Combined Gas Law 10.6 Ideal Gases and the Ideal Gas Law 10.7 Densities of Gases 10.8 Gases in Chemical Reactions 10.9 Mixtures of Gases Solubility of Gases and Henry s Law Gas Diffusion: Molecules Moving Rapidly Real Gases
37 Stoichiometric Calculations Using Gases Stoichiometric Calculations: Depend on mole/mole ratios of reactants and/or products Moles of gas can be calculated from ideal gas law if P, V, and T are known PV n RT
38 Practice: Gas Stoichiometry Automobile air bags inflate during a crash or sudden stop by the rapid generation of N2(g) from sodium azide: 2NaN3(s) 2Na(s) + 3N2(g) How many grams of sodium azide are needed to produce sufficient N2(g) to fill a cm bag to a pressure of 1.20 atm at 15 C? Collect and Organize: Analyze: Solve: Think about It:
39 Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4 The Gas Laws 10.5 The Combined Gas Law 10.6 Ideal Gases and the Ideal Gas Law 10.7 Densities of Gases 10.8 Gases in Chemical Reactions 10.9 Mixtures of Gases Solubility of Gases and Henry s Law Gas Diffusion: Molecules Moving Rapidly Real Gases
40 Dalton s Law of Partial Pressures For a mixture of gases in a container: Ptotal = P1 + P2 + P Total pressure depends only on total number moles of gas, not on their identities (KMT Post. #2).
41 Mole Fraction & Partial Pressure Mole Fraction: Ratio of the # of moles of a given component in a mixture to the total # of moles in a mixture: n1 n1 x1 = = ntotal n1 + n2 + n Mole Fraction in Terms of Pressure: When V and T are constant, P n
42 Mole Fraction & Partial Pressure (cont d) Since P n P1 x1 = Ptotal And: P1 x1 = Ptotal Then P1 x1 = Ptotal
43 Practice: Mole Fraction At 25 C, a 1.0 L flask contains mol of oxygen, mg of nitrogen, and molecules of carbon dioxide. Calculate the partial pressure and mole fraction of each gas. Collect and Organize: Analyze: Solve: Think about It:
44 Collecting a Gas over Water 2KClO3(s) 2KCl(s) + 3O2(g) Gases collected: O2(g) and H2O(g) Ptotal = PO2 + PH 2O Ptotal = PO2 + PHO2
45 Practice: Partial Pressure of Water A sample of KClO3 is heated and decomposes to produce O2 gas. The gas is collected by water displacement at 25 C. The total volume of the collected gas is 329 ml at a pressure of 744 torr. How many moles of oxygen are formed? Collect and Organize: Analyze: Solve: Think about It:
46 Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4 The Gas Laws 10.5 The Combined Gas Law 10.6 Ideal Gases and the Ideal Gas Law 10.7 Densities of Gases 10.8 Gases in Chemical Reactions 10.9 Mixtures of Gases Solubility of Gases and Henry s Law Gas Diffusion: Molecules Moving Rapidly Real Gases
47 Solubility of Gases Solubility of gases depends on T and P Solubility as Pressure Solubility as Temperature
48 Henry s Law Henry s Law: The higher the partial pressure of the gas above a liquid, the more soluble Cgas Pgas Cgas = kh Pgas
49 Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4 The Gas Laws 10.5 The Combined Gas Law 10.6 Ideal Gases and the Ideal Gas Law 10.7 Densities of Gases 10.8 Gases in Chemical Reactions 10.9 Mixtures of Gases Solubility of Gases and Henry s Law Gas Diffusion: Molecules Moving Rapidly Real Gases
50 Diffusion and Effusion Graham s Law: (Distance)gas 1 M1 = Rate of Effusion and Diffusion (Distance)gas 2 M2 Distance )gas 1 ( Relative Rates of Effusion: = ( Distance )gas 2 ( Distance )gas 1 M1 Diffusion (Distance): = ( Distance )gas 2 M 2 M1 M2
51 Practice: Graham s Law List the following gases, which are at the same temperature, in the order of increasing rates of diffusion: He, Kr, NO, O2 Collect and Organize: Analyze: Solve: Think about It:
52 Practice: Graham s Law Calculate the molar mass of a gas if equal volumes of oxygen gas and the unknown gas take 3.25 and 4.60 min, respectively, to effuse through a small hole at constant pressure and temperature. Collect and Organize: Analyze: Solve: Think about It:
53 Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4 The Gas Laws 10.5 The Combined Gas Law 10.6 Ideal Gases and the Ideal Gas Law 10.7 Densities of Gases 10.8 Gases in Chemical Reactions 10.9 Mixtures of Gases Solubility of Gases and Henry s Law Gas Diffusion: Molecules Moving Rapidly Real Gases
54 Real Gases Ideal gas behavior must be corrected when at high pressure (smaller volume) and low temperature (attractive forces become important).
55 Ideal vs. Real Gases Assumptions of Kinetic Molecular Theory: #1: Vgas is negligible compared to Vcontainer. #5: Gas molecules act independently (i.e., don t interact with each other). Valid at STP, but not at higher pressures: Volume occupied by gas molecules is not negligible. Attractive forces between gas molecules are significant.
56 Deviations from Ideal Behavior Vgas Vcontainer Attraction between gas molecules.
57 Real Gases Corrections to Ideal Gas Law: van der Waals Equation n a P V nb n R T 2 V 2 corrected pressure corrected volume Pideal Videal
58 ChemTours: Chapter 10 Click here to launch t he ChemTours website 58
59 This concludes the Lecture PowerPoint presentation for Chapter 10 GILBERT KIRSS FOSTER 59
Chapter 6 10/14/13. Gas Law. Volume change with temperature and pressure.
 Chapter 6 10/14/13 Gas Law 1. Properties of a Gas a. Neither definite shape nor volume i. Uniformly fills any container i Exerts pressure on surroundings Volume change with temperature and pressure. b.
Chapter 6 10/14/13 Gas Law 1. Properties of a Gas a. Neither definite shape nor volume i. Uniformly fills any container i Exerts pressure on surroundings Volume change with temperature and pressure. b.
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works.
 Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
You should be able to: Describe Equipment Barometer Manometer. 5.1 Pressure Read and outline 5.1 Define Barometer
 A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
Chapter 12. The Gaseous State of Matter
 Chapter 12 The Gaseous State of Matter The air in a hot air balloon expands When it is heated. Some of the air escapes from the top of the balloon, lowering the air density inside the balloon, making the
Chapter 12 The Gaseous State of Matter The air in a hot air balloon expands When it is heated. Some of the air escapes from the top of the balloon, lowering the air density inside the balloon, making the
GASES. Unit #8. AP Chemistry
 GASES Unit #8 AP Chemistry I. Characteristics of Gases A. Gas Characteristics: 1. Fills its container a. no definite shape b. no definite vol. 2. Easily mixes w/ other gases 3. Exerts pressure on its surroundings
GASES Unit #8 AP Chemistry I. Characteristics of Gases A. Gas Characteristics: 1. Fills its container a. no definite shape b. no definite vol. 2. Easily mixes w/ other gases 3. Exerts pressure on its surroundings
Chapter 10: Gases. Characteristics of Gases
 Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
Elements that exist as gases at 25 o C and 1 atmosphere H 2, N 2, O 2, F 2, Cl 2, He, Ne, Ar, Kr, Xe, Rn
 AP Chemistry Chapter 5 Sections 5. 5.9 Note Organizer Pressure, The Gas Laws of Boyle, Charles, and Avogadro, The Ideal Gas Law, Gas Stoichiometry, Dalton s Law of Partial Pressure, The Kinetic olecular
AP Chemistry Chapter 5 Sections 5. 5.9 Note Organizer Pressure, The Gas Laws of Boyle, Charles, and Avogadro, The Ideal Gas Law, Gas Stoichiometry, Dalton s Law of Partial Pressure, The Kinetic olecular
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory
 CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
Chapter 9 Gases: Their Properties and Behavior
 Chapter 9 Gases: Their Properties and Behavior 國防醫學院生化學科王明芳老師 2011-11-15 & 2011-11-22 Chapter 9/1 Gases and Gas Pressure Gas mixtures are homogeneous and compressible. Air-the mixture of gases. Molecular
Chapter 9 Gases: Their Properties and Behavior 國防醫學院生化學科王明芳老師 2011-11-15 & 2011-11-22 Chapter 9/1 Gases and Gas Pressure Gas mixtures are homogeneous and compressible. Air-the mixture of gases. Molecular
Unit 8: Gases and States of Matter
 Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
Section 5.1 Pressure. Why study gases? An understanding of real world phenomena. An understanding of how science works.
 Chapter 5 Gases Section 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage Learning. All rights reserved 2 Section 5.1 Pressure
Chapter 5 Gases Section 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage Learning. All rights reserved 2 Section 5.1 Pressure
Lecture Presentation. Chapter 10. Gases. John D. Bookstaver St. Charles Community College Cottleville, MO Pearson Education, Inc.
 Lecture Presentation Chapter 10 John D. Bookstaver St. Charles Community College Cottleville, MO Characteristics of Unlike liquids and solids, gases Expand to fill their containers. Are highly compressible.
Lecture Presentation Chapter 10 John D. Bookstaver St. Charles Community College Cottleville, MO Characteristics of Unlike liquids and solids, gases Expand to fill their containers. Are highly compressible.
Gas Pressure. Pressure is the force exerted per unit area by gas molecules as they strike the surfaces around them.
 Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory
 CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
Kinetic Molecular Theory imaginary Assumptions of Kinetic Molecular Theory: Problems with KMT:
 AP Chemistry Ms. Ye Name Date Block Kinetic Molecular Theory Explains properties of gases, liquids, and solids in terms of energy using an ideal gas, an imaginary which fits all the assumptions of kinetic
AP Chemistry Ms. Ye Name Date Block Kinetic Molecular Theory Explains properties of gases, liquids, and solids in terms of energy using an ideal gas, an imaginary which fits all the assumptions of kinetic
Chapter 10 Gases. Characteristics of Gases. Pressure. The Gas Laws. The Ideal-Gas Equation. Applications of the Ideal-Gas Equation
 Characteristics of Gases Chapter 10 Gases Pressure The Gas Laws The Ideal-Gas Equation Applications of the Ideal-Gas Equation Gas mixtures and partial pressures Kinetic-Molecular Theory Real Gases: Deviations
Characteristics of Gases Chapter 10 Gases Pressure The Gas Laws The Ideal-Gas Equation Applications of the Ideal-Gas Equation Gas mixtures and partial pressures Kinetic-Molecular Theory Real Gases: Deviations
Chapter 5. Pressure. Atmospheric Pressure. Gases. Force Pressure = Area
 Chapter 5 Gases Water for many homes is supplied by a well The pump removes air from the pipe, decreasing the air pressure in the pipe The pressure then pushes the water up the pipe Pressure Atmospheric
Chapter 5 Gases Water for many homes is supplied by a well The pump removes air from the pipe, decreasing the air pressure in the pipe The pressure then pushes the water up the pipe Pressure Atmospheric
Chapter 13 Gases and Pressure. Pressure and Force. Pressure is the force per unit area on a surface. Force Area. Pressure =
 Chapter 13 Gas Laws Chapter 13 Gases and Pressure Pressure and Force Pressure is the force per unit area on a surface. Pressure = Force Area Chapter 13 Gases and Pressure Gases in the Atmosphere The atmosphere
Chapter 13 Gas Laws Chapter 13 Gases and Pressure Pressure and Force Pressure is the force per unit area on a surface. Pressure = Force Area Chapter 13 Gases and Pressure Gases in the Atmosphere The atmosphere
The Kinetic-Molecular Theory of Gases based on the idea that particles are always in motion
 The Kinetic-Molecular Theory of Gases based on the idea that particles are always in motion Five assumptions: 1. Most of the volume occupied dby a gas is empty space 2. Collisions between gas particles
The Kinetic-Molecular Theory of Gases based on the idea that particles are always in motion Five assumptions: 1. Most of the volume occupied dby a gas is empty space 2. Collisions between gas particles
Gas Law Worksheets - WS: Boyle s and Charles Law
 Gas Law Worksheets - WS: Boyle s and Charles Law Boyle s Law states that the volume of a gas varies inversely with its pressure if temperature is held constant. (If one goes up the, other goes down.) We
Gas Law Worksheets - WS: Boyle s and Charles Law Boyle s Law states that the volume of a gas varies inversely with its pressure if temperature is held constant. (If one goes up the, other goes down.) We
Chapter 10: Properties of Gases: The Air We Breathe
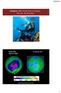 Chapter 10: Properties of Gases: The Air We Breathe South Pole Sept 24, 2006 15 February 2017 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 10.2 10.3 The Properties of Gases Effusion and the Kinetic
Chapter 10: Properties of Gases: The Air We Breathe South Pole Sept 24, 2006 15 February 2017 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 10.2 10.3 The Properties of Gases Effusion and the Kinetic
Chapter 5. Nov 6 1:02 PM
 Chapter 5 Nov 6 1:02 PM Expand to fill their containers Fluid motion (they flow) Have low densities (1/1000 the density of equivalent liquids or solids) Compressible Can Effuse and Diffuse Effuse: The
Chapter 5 Nov 6 1:02 PM Expand to fill their containers Fluid motion (they flow) Have low densities (1/1000 the density of equivalent liquids or solids) Compressible Can Effuse and Diffuse Effuse: The
Chem 110 General Principles of Chemistry
 CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes:
 Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
NOTES: Behavior of Gases
 NOTES: Behavior of Gases Properties of Gases Gases have weight Gases take up space Gases exert pressure Gases fill their containers Gases are mostly empty space The molecules in a gas are separate, very
NOTES: Behavior of Gases Properties of Gases Gases have weight Gases take up space Gases exert pressure Gases fill their containers Gases are mostly empty space The molecules in a gas are separate, very
Chapter 13. Gases. Copyright Cengage Learning. All rights reserved 1
 Chapter 13 Gases Copyright Cengage Learning. All rights reserved 1 Section 13.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage
Chapter 13 Gases Copyright Cengage Learning. All rights reserved 1 Section 13.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage
Gases. Chapter 5: Gas Laws Demonstration. September 10, Chapter 5 Gasses.notebook. Dec 18 10:23 AM. Jan 1 4:11 PM. Crushing 55 gallon drum
 Chapter 5: Gases Dec 18 10:23 AM Gas Laws Demonstration Crushing 55 gallon drum Egg in a bottle Student in a bag Boiling Water Charles gas Law Water in a flask Ballon in a bottle Jan 1 4:11 PM 1 5.1 Pressure
Chapter 5: Gases Dec 18 10:23 AM Gas Laws Demonstration Crushing 55 gallon drum Egg in a bottle Student in a bag Boiling Water Charles gas Law Water in a flask Ballon in a bottle Jan 1 4:11 PM 1 5.1 Pressure
Expand to fill their containers, are highly compressible, have extremely low densities.
 Chem150 week6 Handout 1 Gases Characteristics of Gases: Unlike liquids and solids, they Expand to fill their containers, are highly compressible, have extremely low densities. Pressure is the amount of
Chem150 week6 Handout 1 Gases Characteristics of Gases: Unlike liquids and solids, they Expand to fill their containers, are highly compressible, have extremely low densities. Pressure is the amount of
Chemistry 101 Chapter 5 GAS MIXTURES
 GAS MIXTURES Consider mixing equal volumes of 3 different gases, all at the same temperature and pressure in a container of the same size. 1 L He 1 L N 2 1 L O 2 1 L mixture t = 0 0 C t = 0 0 C t = 0 0
GAS MIXTURES Consider mixing equal volumes of 3 different gases, all at the same temperature and pressure in a container of the same size. 1 L He 1 L N 2 1 L O 2 1 L mixture t = 0 0 C t = 0 0 C t = 0 0
Pressure of the atmosphere varies with elevation and weather conditions. Barometer- device used to measure atmospheric pressure.
 Chapter 12 Section 1 Pressure A gas exerts pressure on its surroundings. Blow up a balloon. The gas we are most familiar with is the atmosphere, a mixture of mostly elemental nitrogen and oxygen. Pressure
Chapter 12 Section 1 Pressure A gas exerts pressure on its surroundings. Blow up a balloon. The gas we are most familiar with is the atmosphere, a mixture of mostly elemental nitrogen and oxygen. Pressure
Chemistry A Molecular Approach. Fourth Edition. Chapter 5. Gases. Copyright 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
 Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
4.) There are no forces of attraction or repulsion between gas particles. This means that
 KINETIC MOLECULAR (K-M) THEORY OF MATTER NOTES - based on the idea that particles of matter are always in motion - assumptions of the K-M Theory 1.) Gases consist of large numbers of tiny particles that
KINETIC MOLECULAR (K-M) THEORY OF MATTER NOTES - based on the idea that particles of matter are always in motion - assumptions of the K-M Theory 1.) Gases consist of large numbers of tiny particles that
States of Matter Review
 States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
Honors Chemistry Unit 7 Gas Laws Notes
 Honors Chemistry Unit 7 Gas Laws Notes Kinetic Molecular Theory 1. List the five assumptions: Assumption Description Extra Info 1 Basically means: the particles themselves have compared to the space between
Honors Chemistry Unit 7 Gas Laws Notes Kinetic Molecular Theory 1. List the five assumptions: Assumption Description Extra Info 1 Basically means: the particles themselves have compared to the space between
Chapter 10: Properties of Gases: The Air We Breathe
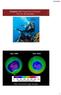 Chapter 10: Properties of Gases: The Air We Breathe Sept, 2006 Sept, 2016 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 The Properties of Gases 10.2 The Kinetic Molecular Theory of Gases* 10.3
Chapter 10: Properties of Gases: The Air We Breathe Sept, 2006 Sept, 2016 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 The Properties of Gases 10.2 The Kinetic Molecular Theory of Gases* 10.3
C h e m i s t r y 1 A : C h a p t e r 5 P a g e 1
 C h e m i s t r y 1 A : C h a p t e r 5 P a g e 1 Chapter 5: Gases Homework: Read Chapter 5. Work out sample/practice exercises Keep up with MasteringChemistry assignments Gas Properties: Ideal Gas: Gases
C h e m i s t r y 1 A : C h a p t e r 5 P a g e 1 Chapter 5: Gases Homework: Read Chapter 5. Work out sample/practice exercises Keep up with MasteringChemistry assignments Gas Properties: Ideal Gas: Gases
Chapter 11. Recall: States of Matter. Properties of Gases. Gases
 Chapter 11 Gases Recall: States of Matter Solids and Liquids: are closely related because in each case the particles are interacting with each other Gases: Properties of Gases Gases can be compressed Gases
Chapter 11 Gases Recall: States of Matter Solids and Liquids: are closely related because in each case the particles are interacting with each other Gases: Properties of Gases Gases can be compressed Gases
Kinetic-Molecular Theory
 GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
AP TOPIC 6: Gases. Revised August General properties and kinetic theory
 AP OPIC 6: Gases General properties and kinetic theory Gases are made up of particles that have (relatively) large amounts of energy. A gas has no definite shape or volume and will expand to fill as much
AP OPIC 6: Gases General properties and kinetic theory Gases are made up of particles that have (relatively) large amounts of energy. A gas has no definite shape or volume and will expand to fill as much
Kinetic Molecular Theory Gases. Behavior of gases. Postulate two. Postulate one. Postulate three. Postulate four
 Kinetic Molecular Theory Gases Gas particles are so small that their individual volume can be considered to be negligible Gas particles are in constant motion and the collisions of the particles with the
Kinetic Molecular Theory Gases Gas particles are so small that their individual volume can be considered to be negligible Gas particles are in constant motion and the collisions of the particles with the
Kinetic-Molecular Theory of Matter
 Gases Properties of Gases Gas Pressure Gases What gases are important for each of the following: O 2, CO 2 and/or He? A. B. C. D. 1 2 Gases What gases are important for each of the following: O 2, CO 2
Gases Properties of Gases Gas Pressure Gases What gases are important for each of the following: O 2, CO 2 and/or He? A. B. C. D. 1 2 Gases What gases are important for each of the following: O 2, CO 2
temperature and pressure unchanging
 Gas Laws Review I. Variables Used to Describe a Gas A. Pressure (P) kpa, atm, mmhg (torr) -Pressure=force exerted per unit area (force/area) -Generated by collisions within container walls (more collisions=more
Gas Laws Review I. Variables Used to Describe a Gas A. Pressure (P) kpa, atm, mmhg (torr) -Pressure=force exerted per unit area (force/area) -Generated by collisions within container walls (more collisions=more
PSI Chemistry: Gases Multiple Choice Review
 PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
Chemistry Chapter 12. Characteristics of Gases. Characteristics of Gases 1/31/2012. Gases and Liquids
 Importance of Gases Chemistry Chapter 12 Gases and Liquids Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide, NaN 3. 2 NaN 3 ---> 2 Na + 3 N 2 THREE STATES
Importance of Gases Chemistry Chapter 12 Gases and Liquids Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide, NaN 3. 2 NaN 3 ---> 2 Na + 3 N 2 THREE STATES
Section 8: Gases. The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC (c).
 Section 8: Gases The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 8.01 Simple Gas Laws Chemistry (9)(A) 8.02 Ideal Gas Law Chemistry
Section 8: Gases The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 8.01 Simple Gas Laws Chemistry (9)(A) 8.02 Ideal Gas Law Chemistry
Name Chemistry Pre-AP
 Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
CHEMISTRY - CLUTCH CH.5 - GASES.
 !! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
!! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
2. Calculate the ratio of diffusion rates for carbon monoxide (CO) and carbon dioxide (CO2). υa = MB = 44 = 1.25
 Gas laws worksheet (2-08) (modified 3/17) Answer key Graham s Law 1. Calculate the ratio of effusion rates for nitrogen (N2) and neon (Ne). υa = MB = 20 = 0.845 υb MA 28 2. Calculate the ratio of diffusion
Gas laws worksheet (2-08) (modified 3/17) Answer key Graham s Law 1. Calculate the ratio of effusion rates for nitrogen (N2) and neon (Ne). υa = MB = 20 = 0.845 υb MA 28 2. Calculate the ratio of diffusion
To play movie you must be in Slide Show Mode CLICK HERE EXERCISE! EXERCISE! To play movie you must be in Slide Show Mode CLICK HERE
 Boyle s Law Boyle s law Pressure and volume are inversely related (constant T, temperature, and n, # of moles of gas). PV k (kis a constant for a given sample of air at a specific temperature) P V P V
Boyle s Law Boyle s law Pressure and volume are inversely related (constant T, temperature, and n, # of moles of gas). PV k (kis a constant for a given sample of air at a specific temperature) P V P V
A. What are the three states of matter chemists work with?
 Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops
 C h e m i s t r y 1 2 C h a p t e r 11 G a s e s P a g e 1 Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops Gas Properties: Gases have high kinetic energy low
C h e m i s t r y 1 2 C h a p t e r 11 G a s e s P a g e 1 Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops Gas Properties: Gases have high kinetic energy low
Chemistry HP Unit 6 Gases. Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases
 Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Unit 8: Kinetic Theory Homework Packet (90 points)
 Name: Key Period: By the end of Unit 8, you should be able to: Kinetic Theory Chapter 13-14 4. Define kinetic theory of gases including collisions 5. Define pressure, including atmospheric pressure, vapor
Name: Key Period: By the end of Unit 8, you should be able to: Kinetic Theory Chapter 13-14 4. Define kinetic theory of gases including collisions 5. Define pressure, including atmospheric pressure, vapor
General Properties of Gases
 GASES Chapter 13 Importance of Gases Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide,, NaN 3. 2 NaN 3 ---> > 2 Na + 3 N 2 THREE STATES OF MATTER General
GASES Chapter 13 Importance of Gases Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide,, NaN 3. 2 NaN 3 ---> > 2 Na + 3 N 2 THREE STATES OF MATTER General
Chemistry: It s a gas
 Chemistry: It s a gas Part IV Molar mass of a gas Density of a gas Dalton s Law: high altitudes and scuba diving Kinetic Molecular Theory: fast gases are hot! I have a chemistry test on Friday, there is
Chemistry: It s a gas Part IV Molar mass of a gas Density of a gas Dalton s Law: high altitudes and scuba diving Kinetic Molecular Theory: fast gases are hot! I have a chemistry test on Friday, there is
Behavior of Gases Chapter 12 Assignment & Problem Set
 Behavior of Gases Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Behavior of Gases 2 Study Guide: Things You Must Know Vocabulary (know the definition
Behavior of Gases Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Behavior of Gases 2 Study Guide: Things You Must Know Vocabulary (know the definition
World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases
![World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases](/thumbs/96/127526823.jpg) World of Chemistry Notes for Students [Chapter 3, page ] Chapter 3 Gases ) Sec 3.8 Kinetic Theory of Gases and the Nature of Gases The Kinetic Theory of Matter says that the tiny particles in all forms
World of Chemistry Notes for Students [Chapter 3, page ] Chapter 3 Gases ) Sec 3.8 Kinetic Theory of Gases and the Nature of Gases The Kinetic Theory of Matter says that the tiny particles in all forms
Chapter 14-Gases. Dr. Walker
 Chapter 14-Gases Dr. Walker State of Matter Gases are one of the four states of matter along with solids, liquids, and plasma Conversion to Gases From liquids Evaporation Example: Boiling water From solids
Chapter 14-Gases Dr. Walker State of Matter Gases are one of the four states of matter along with solids, liquids, and plasma Conversion to Gases From liquids Evaporation Example: Boiling water From solids
Chapter 5 Gases. AP CHEMISTRY Chapter 5 Scotch Plains-Fanwood High School Page 1
 Chapter 5 Gases Kinetic Theory All matter is composed of tiny particles that are in continuous, random motion. Gas Pressure = Force Demo: Test tube/h2o beaker Area Demo: Can AP CHEMISTRY Chapter 5 Scotch
Chapter 5 Gases Kinetic Theory All matter is composed of tiny particles that are in continuous, random motion. Gas Pressure = Force Demo: Test tube/h2o beaker Area Demo: Can AP CHEMISTRY Chapter 5 Scotch
Section 10-1: The Kinetic-Molecular Theory of Matter. 1) How does the word kinetic apply to particles of matter?
 Kinetic-Molecular theory of Matter/Ch10, Gases/Ch11 Column notes: Answer all parts of each question IN YOUR OWN WORDS. Use the text, figures and captions as resources. Section 10-1: The Kinetic-Molecular
Kinetic-Molecular theory of Matter/Ch10, Gases/Ch11 Column notes: Answer all parts of each question IN YOUR OWN WORDS. Use the text, figures and captions as resources. Section 10-1: The Kinetic-Molecular
Name Unit 9 Notes: Gas Laws Period. Complete throughout unit. Due on test day!
 Name Unit 9 Notes: Gas Laws Period Skills: 1. Gases and Entropy 2. Distinguish between Ideal and Real gases 3. Understand KMT and Avogadro s Law 4. Identify and Solve Boyle s Law Problems 5. Identify and
Name Unit 9 Notes: Gas Laws Period Skills: 1. Gases and Entropy 2. Distinguish between Ideal and Real gases 3. Understand KMT and Avogadro s Law 4. Identify and Solve Boyle s Law Problems 5. Identify and
Behavior of Gases. Gases are mostly The molecules in a gas are separate, very small and very
 Properties of Gases Gases have Gases Gases exert Gases fill their containers Behavior of Gases Gases are mostly The molecules in a gas are separate, very small and very Kinetic Theory of Matter: Gas molecules
Properties of Gases Gases have Gases Gases exert Gases fill their containers Behavior of Gases Gases are mostly The molecules in a gas are separate, very small and very Kinetic Theory of Matter: Gas molecules
Worksheet 1.7: Gas Laws. Charles Law. Guy-Lassac's Law. Standard Conditions. Abbreviations. Conversions. Gas Law s Equation Symbols
 Name Block Worksheet 1.7: Gas Laws Boyle s Law Charles Law Guy-Lassac's Law Combined Gas Law For a given mass of gas at constant temperature, the volume of a gas varies inversely with pressure PV = k The
Name Block Worksheet 1.7: Gas Laws Boyle s Law Charles Law Guy-Lassac's Law Combined Gas Law For a given mass of gas at constant temperature, the volume of a gas varies inversely with pressure PV = k The
Gases. Edward Wen, PhD
 Gases Edward Wen, PhD Properties of Gases expand to completely fill their container take the shape of their container low density much less than solid or liquid state compressible when pressure is changed.
Gases Edward Wen, PhD Properties of Gases expand to completely fill their container take the shape of their container low density much less than solid or liquid state compressible when pressure is changed.
Lecture Handout 5: Gases (Online Text Chapter 6)
 Lecture Handout 5: Gases (Online Text Chapter 6) I. The Structure of a Gas A. Gases are composed of particles that are flying around very fast in their container(s). 1. The particles travel in straight
Lecture Handout 5: Gases (Online Text Chapter 6) I. The Structure of a Gas A. Gases are composed of particles that are flying around very fast in their container(s). 1. The particles travel in straight
Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases
![Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases](/thumbs/78/77082586.jpg) Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] Chapter 10 Gases We have talked a little about gases in Chapter 3 and we dealt briefly with them in our stoichiometric calculations in
Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] Chapter 10 Gases We have talked a little about gases in Chapter 3 and we dealt briefly with them in our stoichiometric calculations in
Simple Gas Laws. To facilitate comparison of gases, the following standards are used: STP: O C (273 K) and kpa. SATP: 25 C (298 K) and 101.
 Simple Gas Laws To facilitate comparison of gases, the following standards are used: STP: O C (273 K) and 101.3 kpa If assuming 1 mol, V = 22.4L SATP: 25 C (298 K) and 101.3 kpa If assuming 1 mol, V =
Simple Gas Laws To facilitate comparison of gases, the following standards are used: STP: O C (273 K) and 101.3 kpa If assuming 1 mol, V = 22.4L SATP: 25 C (298 K) and 101.3 kpa If assuming 1 mol, V =
Boyle s Law Practice
 Boyle s Law Practice Boyle s Law is an indirect relationship. Most of these problems can be done in your head without showing your work. 1. Herman has 30.0 L of helium gas trapped in a cylinder by a piston.
Boyle s Law Practice Boyle s Law is an indirect relationship. Most of these problems can be done in your head without showing your work. 1. Herman has 30.0 L of helium gas trapped in a cylinder by a piston.
Lab Dates. CRHS Academic Chemistry Unit 11 Gas Laws Notes
 Name Period CRHS Academic Chemistry Unit 11 Gas Laws Notes Quiz Date Lab Dates Exam Date Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Name Period CRHS Academic Chemistry Unit 11 Gas Laws Notes Quiz Date Lab Dates Exam Date Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Chemistry Chapter 11 Test Review
 Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases.
 Chapter 8 Gases Practice Problems Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases. Summary: In a gas, particles are so far
Chapter 8 Gases Practice Problems Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases. Summary: In a gas, particles are so far
Substances that are liquids or solids under ordinary conditions may also exist as gases. These are often referred to as vapors. Properties of Gases
 Common Student Misconceptions Students need to be told to always use temperature in Kelvin in gas problems. Students should always use units in gas-law problems to keep track of required conversions. Due
Common Student Misconceptions Students need to be told to always use temperature in Kelvin in gas problems. Students should always use units in gas-law problems to keep track of required conversions. Due
CHAPTER 14. The Behavior of Gases Properties of Gases. Factors Affecting Gas Pressure
 CHAPTER 14 The Behavior of Gases 14.1 Properties of Gases Compressibility:the volume of matter decreasing under pressure. Gases are easily compressed due to the large amount of space between gas particles.
CHAPTER 14 The Behavior of Gases 14.1 Properties of Gases Compressibility:the volume of matter decreasing under pressure. Gases are easily compressed due to the large amount of space between gas particles.
Practice MC Test unit D (Ch 10) Gas Laws (pg 1 of 10)
 Practice MC Test unit D (Ch 10) Gas Laws (pg 1 of 10) This is practice - Do NOT cheat yourself of finding out what you are capable of doing. Be sure you follow the testing conditions outlined below. DO
Practice MC Test unit D (Ch 10) Gas Laws (pg 1 of 10) This is practice - Do NOT cheat yourself of finding out what you are capable of doing. Be sure you follow the testing conditions outlined below. DO
General Chemistry II CHEM 116
 General Chemistry II CHEM 116 Professor Evans (first two weeks) Professor Satyamurti (last four weeks) Syllabus; 2 exams (22.5 % each) and a final (45 %), lecture attendance 5 %, discussion attendance
General Chemistry II CHEM 116 Professor Evans (first two weeks) Professor Satyamurti (last four weeks) Syllabus; 2 exams (22.5 % each) and a final (45 %), lecture attendance 5 %, discussion attendance
of Gases Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of General Properties
 BEHAVIOR OF GASES Chapter 12 1 Importance of Gases 2 Hot Air Balloons How Do They Work? 3 Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide,, NaN 3. 2 NaN
BEHAVIOR OF GASES Chapter 12 1 Importance of Gases 2 Hot Air Balloons How Do They Work? 3 Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide,, NaN 3. 2 NaN
Gases Chapter 11 (and 10)
 Gases Chapter 11 (and 10) Warm up 1. What is a gas? 2. What is pressure? 3. What units are used to measure pressure? Properties of Gas Expansion: indefinite shape and volume Fluidity: particle move pass
Gases Chapter 11 (and 10) Warm up 1. What is a gas? 2. What is pressure? 3. What units are used to measure pressure? Properties of Gas Expansion: indefinite shape and volume Fluidity: particle move pass
Accelerated Chemistry Study Guide Chapter 13: Gases
 Accelerated Chemistry Study Guide Chapter 13: Gases Terms, definitions, topics Diffusion Kinetic Molecular Theory Atmospheric pressure Barometer Manometer STP Absolute zero Page 1 of 42 Molar volume Partial
Accelerated Chemistry Study Guide Chapter 13: Gases Terms, definitions, topics Diffusion Kinetic Molecular Theory Atmospheric pressure Barometer Manometer STP Absolute zero Page 1 of 42 Molar volume Partial
Name /74. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch 11 Gases STUDY GUIDE Accelerated Chemistry SCANTRON Name /74 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements
Ch 11 Gases STUDY GUIDE Accelerated Chemistry SCANTRON Name /74 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements
THE GAS STATE. Unit 4. CHAPTER KEY TERMS HOME WORK 9.1 Kinetic Molecular Theory States of Matter Solid, Liquid, gas.
 Unit 4 THE GAS STATE CHAPTER KEY TERMS HOME WORK 9. Kinetic Molecular Theory States of Matter Solid, Liquid, gas Page 4 # to 4 9. Boyles Law P α /V PV = Constant P V = P V Pressure Atmospheric Pressure
Unit 4 THE GAS STATE CHAPTER KEY TERMS HOME WORK 9. Kinetic Molecular Theory States of Matter Solid, Liquid, gas Page 4 # to 4 9. Boyles Law P α /V PV = Constant P V = P V Pressure Atmospheric Pressure
B. As the gas particles move and strike a surface, they push on that surface 1. If we could measure the total amount of force exerted by gas
 Chapter 5: Gases I. The Structure of a Gas A. Gases are composed of particles that are flying around very fast in their container(s). 1. The particles travel in straight lines until they encounter either
Chapter 5: Gases I. The Structure of a Gas A. Gases are composed of particles that are flying around very fast in their container(s). 1. The particles travel in straight lines until they encounter either
Chemistry 20 Unit 2 Gases FITB Notes. Topic A Characteristics of Gases
 Chemistry 20 Unit 2 Gases FITB Notes General Outcome: Topic A Characteristics of Gases We use technologies that were designed with the knowledge of the visible characteristics ( ) of gases ex. SCUBA equipment,
Chemistry 20 Unit 2 Gases FITB Notes General Outcome: Topic A Characteristics of Gases We use technologies that were designed with the knowledge of the visible characteristics ( ) of gases ex. SCUBA equipment,
Student Worksheet for Chemical Gas Laws
 Student Worksheet for Chemical Attempt to work the following practice problems after working through the sample problems in the videos. Answers are given on the last page(s). Relevant Equations Boyle s
Student Worksheet for Chemical Attempt to work the following practice problems after working through the sample problems in the videos. Answers are given on the last page(s). Relevant Equations Boyle s
Chemistry 51 Chapter 7 PROPERTIES OF GASES. Gases are the least dense and most mobile of the three phases of matter.
 ROERIES OF GASES Gases are the least dense and most mobile of the three phases of matter. articles of matter in the gas phase are spaced far apart from one another and move rapidly and collide with each
ROERIES OF GASES Gases are the least dense and most mobile of the three phases of matter. articles of matter in the gas phase are spaced far apart from one another and move rapidly and collide with each
Chapter 10. Physical Characteristics of Gases
 Chapter 10 Physical Characteristics of Gases Kinetic Molecular Theory An understanding of the behavior of atoms that make up matter Ideal gas: an imaginary gas that perfectly fits all assumptions of the
Chapter 10 Physical Characteristics of Gases Kinetic Molecular Theory An understanding of the behavior of atoms that make up matter Ideal gas: an imaginary gas that perfectly fits all assumptions of the
4. Using the kinetic molecular theory, explain why a gas can be easily compressed, while a liquid and a solid cannot?
 Name Period HW 1 Worksheet (Goals 1-4) - Kinetic Molecular Theory 1. Describe how gases, liquids, and solids compare using the following table. Solids Liquids Gases Volume (definite or indefinite) Molecular
Name Period HW 1 Worksheet (Goals 1-4) - Kinetic Molecular Theory 1. Describe how gases, liquids, and solids compare using the following table. Solids Liquids Gases Volume (definite or indefinite) Molecular
Chemistry Chapter 10 Test
 Chemistry Chapter 10 Test True/False Indicate whether the sentence or statement is true or false. 1. KMT stands for Kinetic Mole Theory. 2. One of the assumptions in the KMT is that the particles are spread
Chemistry Chapter 10 Test True/False Indicate whether the sentence or statement is true or false. 1. KMT stands for Kinetic Mole Theory. 2. One of the assumptions in the KMT is that the particles are spread
Ch. 14 The Behavior of Gases
 Ch. 14 The Behavior of Gases 14.1 PROPERTIES OF GASES Compressibility Compressibility: a measure of how much the volume of matter decreases under pressure Gases are easily compressed because of the spaces
Ch. 14 The Behavior of Gases 14.1 PROPERTIES OF GASES Compressibility Compressibility: a measure of how much the volume of matter decreases under pressure Gases are easily compressed because of the spaces
AP* Chemistry GASES mm Hg = torr =1.00 atm = kpa 10 5 Pa
 THE PROPERTIES OF GASES Only 4 quantities are needed to define the state of a gas: a) the quantity of the gas, n (in moles) b) the temperature of the gas, T (in KELVINS) c) the volume of the gas, V (in
THE PROPERTIES OF GASES Only 4 quantities are needed to define the state of a gas: a) the quantity of the gas, n (in moles) b) the temperature of the gas, T (in KELVINS) c) the volume of the gas, V (in
Chapter 13: The Behavior of Gases
 Chapter 13: The Behavior of Gases I. First Concepts a. The 3 states of matter most important to us: solids, liquids, and gases. b. Real Gases and Ideal Gases i. Real gases exist, ideal gases do not ii.
Chapter 13: The Behavior of Gases I. First Concepts a. The 3 states of matter most important to us: solids, liquids, and gases. b. Real Gases and Ideal Gases i. Real gases exist, ideal gases do not ii.
Dr. Rogers Chapter 5 Homework Chem 111 Fall 2003
 Dr. Rogers Chapter 5 Homework Chem 111 Fall 2003 From textbook: 7-33 odd, 37-45 odd, 55, 59, 61 1. Which gaseous molecules (choose one species) effuse slowest? A. SO 2 (g) B. Ar(g) C. NO(g) D. Ne(g) E.
Dr. Rogers Chapter 5 Homework Chem 111 Fall 2003 From textbook: 7-33 odd, 37-45 odd, 55, 59, 61 1. Which gaseous molecules (choose one species) effuse slowest? A. SO 2 (g) B. Ar(g) C. NO(g) D. Ne(g) E.
8. Now plot on the following grid the values of T (K) and V from the table above, and connect the points.
 Charles s Law According to Charles s law, the volume of a fixed mass of gas varies directly with its Kelvin temperature if its pressure is constant. The following table contains Celsius temperature and
Charles s Law According to Charles s law, the volume of a fixed mass of gas varies directly with its Kelvin temperature if its pressure is constant. The following table contains Celsius temperature and
8.1 Properties of Gases. Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases.
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8. Chapter 8
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases. Chapter 8. Chapter 8. Gases Properties of Gases. We are surrounded by gases, but we are often
 Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
Multiple Choice (40%)
 AP Chemistry Test (Chapter 5) Please do not write on this test thank you! Multiple Choice (40%) 1) A sealed rigid container is filled with three ideal gases: A, B and C. The partial pressure of each gas
AP Chemistry Test (Chapter 5) Please do not write on this test thank you! Multiple Choice (40%) 1) A sealed rigid container is filled with three ideal gases: A, B and C. The partial pressure of each gas
Gas Laws For CHM1020
 Gas Laws For CHM1020 PROPERTIES OF GASES 1. Variable shape and volume (same shape and volume as container) 2. Expand uniformly (as container increases in volume, gas expands and distributes uniformly in
Gas Laws For CHM1020 PROPERTIES OF GASES 1. Variable shape and volume (same shape and volume as container) 2. Expand uniformly (as container increases in volume, gas expands and distributes uniformly in
Notes: Gas Laws (text Ch. 11)
 Name Per. Notes: Gas Laws (text Ch. 11) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to get missing information
Name Per. Notes: Gas Laws (text Ch. 11) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to get missing information
(for tutoring, homework help, or help with online classes)
 www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. Which statement is inconsistent with the kinetic theory of an ideal gas? 1. The forces of repulsion between gas molecules
www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. Which statement is inconsistent with the kinetic theory of an ideal gas? 1. The forces of repulsion between gas molecules
Name: Class: Date: SHORT ANSWER Answer the following questions in the space provided.
 CHAPTER 11 REVIEW Gases SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Pressure =. For a constant force, when the surface area is tripled the pressure is (a) doubled. (b)
CHAPTER 11 REVIEW Gases SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Pressure =. For a constant force, when the surface area is tripled the pressure is (a) doubled. (b)
