Table Animation 17 Observe the behavior of a mixture of nonreacting gases. Section Resources
|
|
|
- Charity Gibbs
- 5 years ago
- Views:
Transcription
1 Gases: Mixtures and Movements 1 FOCUS Objectives Relate the total pressure of a mixture of gases to the partial pressures of the component gases Explain how the molar mass of a gas affects the rate at which the gas diffuses and effuses. Guide for Reading Build Vocabulary L2 Imagine a Picture As students learn about effusion and diffusion, have them visualize each process at the microscopic level. Then have them describe what is happening. Reading Strategy L2 Monitor Your Understanding If students are having difficulty reading Graham s Law, have them think about how they are reading and how they might deal with the difficulty. 2 INSTRUCT Guide for Reading Key Concepts How is the total pressure of a mixture of gases related to the partial pressures of the component gases? How does the molar mass of a gas affect the rate at which the gas effuses or diffuses? Vocabulary partial pressure Dalton s law of partial pressures diffusion effusion Graham s law of effusion Reading Strategy Previewing Before you read, look at the equation on this page that is highlighted in yellow. In your own words, describe the relationship that the equation is expressing. The top of Mount Everest is more than 29,000 feet above sea level. A list of gear for an expedition to Mount Everest includes climbing equipment such as an ice axe and a climbing harness. It includes ski goggles and a down parka with a hood. All the items on the list are important, but none is as important as the compressed-gas cylinders of oxygen. In this section, you will find out why a supply of oxygen is essential at higher altitudes. Dalton s Law Gas pressure results from collisions of particles in a gas with an object. If the number of particles increases in a given volume, more collisions occur. If the average kinetic energy of the particles increases, more collisions occur. In both cases, the pressure increases. Gas pressure depends only on the number of particles in a given volume and on their average kinetic energy. Particles in a mixture of gases at the same temperature have the same average kinetic energy. So the kind of particle is not important. Table 14.1 shows the composition of dry air, air that does not contain any water vapor. The contribution each gas in a mixture makes to the total pressure is called the partial pressure exerted by that gas. In dry air, the partial pressure of nitrogen is kpa. In a mixture of gases, the total pressure is the sum of the partial pressures of the gases. P total P 1 P 2 P 3 This equation is a mathematical expression of a law proposed by Dalton. Dalton s law of partial pressures states that, at constant volume and temperature, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the component gases. Have students study the photograph and read the text that opens the section. Ask students to try to explain why high-altitude climbers carry a supply of oxygen. (Students may recall that atmospheric pressure decreases with altitude. They may conclude that the drop in pressure is related to a decrease in the concentration of the gases in air, including oxygen.) Dalton s Law Discuss Assess students knowledge about gases by asking them to compare the number of particles in two identical, sealed containers of propane and helium gas at the same temperature L2 and pressure. (Equal volumes of gases at the same temperature and pressure contain equal numbers of particles.) Animation 17 Observe the behavior of a mixture of nonreacting gases. 432 Chapter 14 Section Resources Table 14.1 Print Guided Reading and Study Workbook, Section 14.4 Core Teaching Resources, Section 14.4 Review Transparencies, T158 T159 Laboratory Manual, Lab 25 Small-Scale Chemistry Laboratory Manual, Lab 21 Composition of Dry Air Component Volume (%) Partial pressure (kpa) Nitrogen Oxygen Carbon dioxide Argon and others Total Technology Interactive Textbook with ChemASAP, Animation 17, 18; Problem-Solving 14.32; Assessment 14.4 Go Online, Section Chapter 14
2 100 kpa Container A 250 kpa Container B 200 kpa Container C 550 kpa Container T Look at Figure Containers A, B, C, and T (for total) have the same volume and are at the same temperature. The gases in containers A, B, and C are combined in container T. Because the volumes are identical, each gas in the mixture exerts the pressure it exerted before the gases were mixed in container T. So the pressure in container T (550 kpa) is the sum of the pressures in containers A, B, and C ( kpa). If the percent composition of a mixture of gases does not change, the fraction of the pressure exerted by a gas does not change as the total pressure changes. This fact is important for people who must operate at high altitudes. For example, at the top of Mount Everest, the total atmospheric pressure is kpa. This is about one-third of its value at sea level. The partial pressure of oxygen is also reduced by one third, to 7.06 kpa. The partial pressure of oxygen must be kpa or higher to support respiration in humans. The climber in Figure needs an oxygen mask and a cylinder of compressed oxygen to survive. Figure Three gases are combined in container T. The pressure that each gas exerts is independent of the pressure exerted by the other two gases. The pressure in container T is the sum of the pressures in containers A, B, and C. Interpreting Diagrams What is the relationship between the number of particles in containers A and C and the partial pressures of the gases in A and C? CLASS Activity i Model Partial Pressure L2 Purpose Students make a model that is analogous to Dalton s law. Materials balance, 4 marbles, 2 buttons, 3 pennies, 4 nickels Procedure Have students find the total mass of the marbles and record it in a data table. Have them repeat this process for the buttons and the pennies. Then, have them add all the marbles, buttons, and pennies together and find the overall mass. Ask, How does the total mass of the items compare to the sum of the masses of the individual items? (The sum of the individual masses should equal the total mass. ) If the nickels are added to the mixture, how could you find the mass of the nickels? (The mass of the nickels could be calculated by finding a new total mass and subtracting from it the masses of the marbles, the buttons, and the pennies.) Expected Outcome Students should recognize that mass is being used to represent pressure, and that the contribution of each type of item to the total mass is independent of the other types of items in the mixture. FYI You may want to compare the use of a compressed gas at high altitudes and the use of a compressed gas during deep underwater dives. Figure This climber is using a tank of compressed gas to supplement the supply of oxygen available at high altitudes. 433 Facts and Figures Relative Partial Pressure The relative partial pressure exerted by a gas in a mixture of gases does not vary with temperature, pressure, or volume of the mixture. The ratio of n 1 /n (the relative amount of the gas in the mixture) remains the same. Because the value of n 1 /n remains constant, the relative partial pressure (P 1 /P) exerted by the gas remains the same. Answers to... Figure The ratio of the particles in Containers C and A (2:1) is equal to the ratio of their partial pressures. The Behavior of Gases 433
3 Section 14.4 (continued) Sample Problem 14.6 Answers kpa kpa kpa = 93.4 kpa or kpa kpa 6.6 kpa 23.0 kpa = 3.3 kpa Practice Problems Plus L2 The pressure in an automobile tire filled with air is kpa. The P O2 = 51.3 kpa, P CO2 = 0.10 kpa, and P others = 2.3 kpa. What is the P N2? (191.3 kpa) Use Visuals Have students examine the visual on this page and read the caption. Ask, How does the amount of oxygen in the tank of compressed air compare to the amount of oxygen in the air you normally breathe? (Air is usually about 21% oxygen, so the air in the tanks contains about the same percentage of oxygen.) Firefighters carry tanks of compressed air. The tanks contain from 19.5% to 23.5% oxygen by volume. Problem Solving Solve Problem 32 with the help of an interactive guided tutorial. SAMPLE PROBLEM 14.6 Using Dalton s Law of Partial Pressures Air contains oxygen, nitrogen, carbon dioxide, and trace amounts of other gases. What is the partial pressure of oxygen 1P O2 2 at kpa of total pressure if the partial pressures of nitrogen, carbon dioxide, and other gases are kpa, kpa, and 0.94 kpa, respectively? Analyze List the knowns and the unknown. Knowns P N kpa P CO kpa P others 0.94 kpa P total kpa Practice Problems Unknown P O2? kpa Use Dalton s law of partial pressures 1P total P O2 + P N2 + P CO2 + P others 2 to calculate the unknown value 1P O2 2. Calculate Solve for the unknown. Rearrange Dalton s law to isolate P O2. Substitute the values for the partial pressures and solve the equation. P O2 P total - 1P N2 + P CO2 + P others kpa kpa kpa kpa kpa Evaluate Does the result make sense? The partial pressure of oxygen must be smaller than that of nitrogen because P total is only kpa. The other partial pressures are small, so an answer of kpa seems reasonable. 31. Determine the total pressure of a gas mixture that contains oxygen, nitrogen, and helium. The partial pressures are: P O kpa, P N kpa, and P He 26.7 kpa. 32. A gas mixture containing oxygen, nitrogen, and carbon dioxide has a total pressure of 32.9 kpa. If 6.6 kpa and P N2 P O kpa, what is? P CO2 434 Chapter Chapter 14
4 Graham s Law Suppose you open a perfume bottle in one corner of a room. At some point a person standing in the opposite corner will be able to smell the perfume. Molecules in the perfume evaporate and diffuse, or spread out, through the air in the room. Diffusion is the tendency of molecules to move toward areas of lower concentration until the concentration is uniform throughout. In Figure 14.18A, bromine vapor is diffusing through the air in a graduated cylinder. The bromine vapor in the bottom of the cylinder has started to move upward toward the area where there is a lower concentration of bromine. In Figure 14.18B, the bromine has diffused almost to the top of the cylinder. If the process is allowed to continue, the bromine vapor will spill out of the cylinder. There is another process that involves the movement of molecules in a gas. This process is called effusion. During effusion, a gas escapes through a tiny hole in its container. With effusion and diffusion, the type of particle is important. Gases of lower molar mass diffuse and effuse faster than gases of higher molar mass. Thomas Graham s Contribution i The Scottish chemist Thomas Graham studied rates of effusion during the 1840s. From his observations, he proposed a law. Graham s law of effusion states that the rate of effusion of a gas is inversely proportional to the square root of the gas s molar mass. This law can also be applied to the diffusion of gases. Graham s law makes sense if you know how the mass, velocity, and kinetic energy of a moving object are related. The expression that relates the mass (m) and the velocity (v) of an object to its kinetic energy (KE) is KE 1/2mv 2. For the kinetic energy to be constant, any increase in mass must be balanced by a decrease in velocity. For example, a ball with a mass of 2 g must travel at 5 m/s to have the same kinetic energy as a ball with a mass of 1 g traveling at 7 m/s. There is an important principle here. If two objects with different masses have the same kinetic energy, the lighter object must move faster. a b Word Origins Diffusion and effusion come from the Latin fundere meaning to pour. They differ only in their prefixes. The prefix dismeans apart. The prefix exmeans out. How do these prefixes help to contrast what happens to a gas during diffusion and effusion? For: Links on Diffusion/Effusion Visit: Web Code: cdn-1144 Figure The diffusion of one substance through another is a relatively slow process. a Bromine vapor is diffusing upward through the air in a graduated cylinder. b After several hours, bromine vapors are near the top of the cylinder. Predicting What will happen as the bromine continues to diffuse? Graham s Law Word Origins During diffusion, the particles move apart. During effusion, the particles move out of a tiny hole. (Students may have trouble with the scientific definitions of effusion and diffusion because of the multiple, often overlapping, meanings of these terms in common usage. For example, in many dictionaries one definition of effuse is to spread out; diffuse; radiate. ) Use Visuals Figure Have students study the photograph. Explain that diffusion is a relatively slow process whose rate is inversely proportional to the square root of the molar mass of the gas. CLASS Activity i L2 Effusion Purpose Students compare effusion rates of helium and air. Materials 2 round, identical balloons; helium; metric tape measure. Safety Wear safety goggles when handling balloons. Procedure Fill two identical, round non-latex balloons with equal volumes of helium and air. Have volunteers measure the circumference of the balloons. One day later, measure the circumferences again. Expected Outcome Helium effused from the balloon at a faster rate. Section 14.4 Gases: Mixtures and Movements 435 Differentiated Instruction Less Proficient Readers Have students work in groups to rank the relative rates of diffusion at a constant temperature for carbon dioxide, helium, and nitrogen. (helium > nitrogen > carbon dioxide) Download a worksheet on Diffusion/Effusion for students to complete, and find additional teacher support from NSTA SciLinks. Answers to... Figure The bromine will spill out of the container. The Behavior of Gases 435
5 Section 14.4 (continued) 3 ASSESS Evaluate Understanding di L2 Tell students that the partial pressures of oxygen and hydrogen gases in a container are both 100 kpa. Ask, In which sample are there more molecules? In which sample do the molecules have greater average kinetic energy? (Both gases have the same number of molecules and same average kinetic energy.) Ask, About how much faster does helium diffuse compared to oxygen? (Helium diffuses almost three times faster than oxygen.) Reteach Remind students that diffusion is a general term that applies to molecules moving away from a region of high concentration. Effusion is a specific example of diffusion in which molecules pass through a narrow opening. Graham s law applies to both. Elements Handbook Both tables report data for dry air. Both show that air is mainly nitrogen and oxygen. Table 14.1 uses percents and includes a compound. The table on R4 uses ppm and provides specific data for more elements. If your class subscribes to the Interactive Textbook, use it to review key concepts in Section with ChemASAP Figure The character balloons used in parades are filled with helium gas so that they will float. Animation 18 Observe the processes of gas effusion and diffusion. 436 Chapter Section Assessment 33. Key Concept In a mixture of gases, how is the total pressure determined? 34. Key Concept What is the effect of molar mass on rates of diffusion and effusion? 35. How is the partial pressure of a gas in a mixture calculated? 36. What distinguishes effusion from diffusion? How are these processes similar? 37. How can you compare the rates of effusion of two gases in a mixture? 38. Explain why the rates of diffusion of nitrogen gas and carbon monoxide are almost identical at the same temperature. Comparing Effusion Rates The balloon in Figure is used in holiday parades. It is filled with helium so that it will float above the crowd along the parade route. There is a drawback to using helium in a balloon. Both helium atoms and the molecules in air can pass freely through the tiny pores in a balloon. But a helium-filled balloon will deflate sooner than an air-filled balloon. Kinetic theory can explain this difference. If the balloons are at the same temperature, the particles in each balloon have the same average kinetic energy. But helium atoms are less massive than oxygen or nitrogen molecules. So the molecules in air move more slowly than helium atoms with the same kinetic energy. Because the rate of effusion is related only to a particle s speed, Graham s law can be written as follows for two gases, A and B. Rate A Rate B B molar mass B molar mass A The rates of effusion of two gases are inversely proportional to the square roots of their molar masses. You can use the expression to compare the rates of effusion of nitrogen (molar mass 28.0 g) and helium (molar mass 4.0 g). Helium effuses (and diffuses) nearly three times faster than nitrogen at the same temperature. Rate He 28.0 g Rate N2 B 4.0 g H db k Table 14.1 on page 432 and the Elements in the Atmosphere table on page R4 provide data on the composition of air. Look at the data included in each table. Identify two ways in which the tables are similar. Describe at least three differences. Assessment 14.4 Test yourself on the concepts in Section Chapter Total pressure is equal to the sum of the partial pressures of the components. 34. Gases with lower molar masses diffuse and effuse faster than gases with higher molar masses. 35. Subtract the partial pressures of the other gases from the total pressure. 36. During effusion, a gas escapes through a tiny hole in its container. In both cases, the rate depends on the molar mass. Section 14.4 Assessment 37. The rate of effusion of two gases in a mixture is inversely proportional to the square roots of their molar masses. 38. Carbon monoxide and nitrogen have almost identical molar masses when the masses are rounded to two significant figures (28 g).
6 Small-Scale LAB Diffusion Purpose To infer diffusion of a gas by observing color changes during chemical reactions. Materials clear plastic cup or Petri dish reaction surface dropper bottles containing bromthymol blue, hydrochloric acid, and sodium hydrogen sulfite ruler cotton swab NaOH, NH 4Cl, KI, and NaNO 2 (optional) Procedure 1. Use the plastic cup or Petri dish to draw the large circle shown below on a sheet of paper. Analyze and Conclude 1. The drops near the center change immediately. As the gas diffuses, all the drops change color. The color change begins at the outer edge of each drop. center yellow Small drops are BTB Center mixing is HCl + NaHSO 3 2. Place a reaction surface over the grid and add small drops of bromthymol blue (BTB) in the pattern shown by the small circles. Make sure the drops do not touch one another. 3. Mix one drop each of hydrochloric acid (HCl) and sodium hydrogen sulfite (NaHSO 3 ) in the center of the pattern. 4. Place the cup or Petri dish over the grid and observe what happens. 5. If you plan to do You re The Chemist Activity 1, don t dispose of your materials yet. Analyze Using your experimental data, record the answers to the following questions below your data table. 1. Describe in detail the changes you observed in the drops of BTB over time. Draw pictures to illustrate the changes. green 2. Draw a series of pictures showing how one of the BTB drops might look over time if you could view the drop from the side. 3. The BTB changed even though you added nothing to it. If the mixture in the center circle produced a gas, would this explain the change in the drops of BTB? Use kinetic theory to explain your answer. 4. Translate the following word equation into a balanced chemical equation: Sodium hydrogen sulfite reacts with hydrochloric acid to produce sulfur dioxide gas, water, and sodium chloride. You re The Chemist The following small-scale activities allow you to develop your own procedures and analyze the results. 1. Analyze It! Carefully absorb the center mixture of the original experiment onto a cotton swab and replace it with one drop of NaOH and one drop of NH 4 Cl. Describe what happens and explain in terms of kinetic theory. Ammonium chloride reacts with sodium hydroxide to produce ammonia gas, water, and sodium chloride. Write and balance a chemical equation to describe this reaction. 2. Design It! Design an experiment to observe the effect of the size of the BTB drops on the rate at which they change. Explain your results in terms of kinetic theory. 3. Analyze It! Repeat the original experiment, using KI in place of BTB and mixing sodium nitrite (NaNO 2 ) with hydrochloric acid at the center. Record your results. Write and balance an equation for the reaction. NaNO 2 reacts with HCl to produce nitrogen monoxide gas, water, sodium nitrate, and sodium chloride. 2.. green Small-Scale Lab 437 yellow 3. The particles of gas produced are in motion. As the particles diffuse from the center, they collide and react with molecules of BTB. 4. NaHSO 3 + HCl SO 2 + H 2 O + NaCl Small-Scale LAB Diffusion L2 Objective After completing this activity, students will be able to: infer diffusion of a gas by observing color changes during reactions. Skills Focus Inferring, Drawing Conclusions Prep Time 30 minutes Class Time 40 minutes Advance Preparation Solution Preparation 1.0M HCl 82 ml of 12M in 1.0 L 0.04% BTB 100 mg in 16.0 ml 0.01M NaOH, dilute to 250 ml 0.5M NaOH 20.0 g in 1.0 L 1.0M NH 4 Cl 13.4 g in 250 ml 0.4M NaNO g in 250 ml 0.1M Kl 4.2 g in 250 ml 1.0M NaHSO 3 26 g in 250 ml Safety Always slowly add acid to water. Teaching Tips Before class, add a drop of BTB to the paper to be sure the paper is not acidic. The BTB should remain blue. Expected Outcome The drops of BTB turn yellow, starting with those closest to the center. You re the Chemist! 1. As ammonia diffuses, BTB changes from yellow to blue. NH 4 Cl + NaOH NH 3 + H 2 O + NaCl 2. Vary the size of the BTB drops from pin-heads to puddles. Tiny drops are better able to detect small quantities of gases. 3. The KI turned orange in the same manner as the BTB turned yellow. 3NaNO 2 + 2HCl 2NO + H 2 O + NaNO 3 + 2NaCl For Enrichment L3 Have students design an experiment that shows the effect of temperature on rate of diffusion. The drops change color more quickly at higher temperatures. The Behavior of Gases 437
Dalton s Law How is the total pressure of a mixture of gases related to the partial pressures of the component gases?
 Dalton s Law Chapter 4 The Behavior of Gases 4. Properties of Gases 4. The Gas Laws 4. Ideal Gases Dalton s Law How is the total pressure of a mixture of gases related to the partial pressures of the component
Dalton s Law Chapter 4 The Behavior of Gases 4. Properties of Gases 4. The Gas Laws 4. Ideal Gases Dalton s Law How is the total pressure of a mixture of gases related to the partial pressures of the component
4.) There are no forces of attraction or repulsion between gas particles. This means that
 KINETIC MOLECULAR (K-M) THEORY OF MATTER NOTES - based on the idea that particles of matter are always in motion - assumptions of the K-M Theory 1.) Gases consist of large numbers of tiny particles that
KINETIC MOLECULAR (K-M) THEORY OF MATTER NOTES - based on the idea that particles of matter are always in motion - assumptions of the K-M Theory 1.) Gases consist of large numbers of tiny particles that
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT.
 Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
Guide for Reading. Vocabulary compressibility
 14.1 Properties of Gases Connecting to Your World In organized soccer, there are rules about equipment. For international competitions, the ball s mass must be not more than 450 grams and not less than
14.1 Properties of Gases Connecting to Your World In organized soccer, there are rules about equipment. For international competitions, the ball s mass must be not more than 450 grams and not less than
THE BEHAVIOR OF GASES
 14 THE BEHAVIOR OF GASES SECTION 14.1 PROPERTIES OF GASES (pages 413 417) This section uses kinetic theory to explain the properties of gases. This section also explains how gas pressure is affected by
14 THE BEHAVIOR OF GASES SECTION 14.1 PROPERTIES OF GASES (pages 413 417) This section uses kinetic theory to explain the properties of gases. This section also explains how gas pressure is affected by
Chapter 5 TEST: Gases
 Chapter 5 TEST: Gases 1) Gases generally have A) low density B) high density C) closely packed particles D) no increase in volume when temperature is increased E) no decrease in volume when pressure is
Chapter 5 TEST: Gases 1) Gases generally have A) low density B) high density C) closely packed particles D) no increase in volume when temperature is increased E) no decrease in volume when pressure is
Worksheet 12 - Partial Pressures and the Kinetic Molecular Theory of Gases
 Worksheet 12 - Partial Pressures and the Kinetic olecular Theory of Gases Dalton's Law of Partial Pressures states that the sums of the pressures of each gas in the mixture add to give the total pressure
Worksheet 12 - Partial Pressures and the Kinetic olecular Theory of Gases Dalton's Law of Partial Pressures states that the sums of the pressures of each gas in the mixture add to give the total pressure
You should be able to: Describe Equipment Barometer Manometer. 5.1 Pressure Read and outline 5.1 Define Barometer
 A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
substitution Rearrangement solving for n/v Convert n to MASS (m) by multiplying BOTH sides by molar mass (M) d = m/v
 3.8.12 A 2.07 L cylinder contains 2.88 mol of Helium gas at 22 C. What the pressure in atmospheres of the gas in the cylinder? How could we find the density of this gas? HW solve the density of the above
3.8.12 A 2.07 L cylinder contains 2.88 mol of Helium gas at 22 C. What the pressure in atmospheres of the gas in the cylinder? How could we find the density of this gas? HW solve the density of the above
Name: Chapter 13: Gases
 Name: Chapter 13: Gases Gases and gas behavior is one of the most important and most fun things to learn during your year in chemistry. Here are all of the gas notes and worksheets in two packets. We will
Name: Chapter 13: Gases Gases and gas behavior is one of the most important and most fun things to learn during your year in chemistry. Here are all of the gas notes and worksheets in two packets. We will
Chemistry 101 Chapter 5 GAS MIXTURES
 GAS MIXTURES Consider mixing equal volumes of 3 different gases, all at the same temperature and pressure in a container of the same size. 1 L He 1 L N 2 1 L O 2 1 L mixture t = 0 0 C t = 0 0 C t = 0 0
GAS MIXTURES Consider mixing equal volumes of 3 different gases, all at the same temperature and pressure in a container of the same size. 1 L He 1 L N 2 1 L O 2 1 L mixture t = 0 0 C t = 0 0 C t = 0 0
Chemistry Chapter 11 Test Review
 Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
CHEMISTRY - CLUTCH CH.5 - GASES.
 !! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
!! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
Name Class Date. What are some properties of gases? How do changes of pressure, temperature, or volume affect a gas?
 CHAPTER 3 States of Matter 4 Behavior of Gases SECTION KEY IDEAS As you read this section, keep these questions in mind: What are some properties of gases? How do changes of pressure, temperature, or volume
CHAPTER 3 States of Matter 4 Behavior of Gases SECTION KEY IDEAS As you read this section, keep these questions in mind: What are some properties of gases? How do changes of pressure, temperature, or volume
Name Chemistry Pre-AP
 Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Gas Law Worksheets - WS: Boyle s and Charles Law
 Gas Law Worksheets - WS: Boyle s and Charles Law Boyle s Law states that the volume of a gas varies inversely with its pressure if temperature is held constant. (If one goes up the, other goes down.) We
Gas Law Worksheets - WS: Boyle s and Charles Law Boyle s Law states that the volume of a gas varies inversely with its pressure if temperature is held constant. (If one goes up the, other goes down.) We
Kinetic Molecular Theory
 Kinetic Molecular Theory Name Period Unit 7 HW 1 Worksheet (Goals 1 & 2) 1. Describe how gases, liquids, and solids compare using the following table. Volume (definite or indefinite) Molecular Motion (high,
Kinetic Molecular Theory Name Period Unit 7 HW 1 Worksheet (Goals 1 & 2) 1. Describe how gases, liquids, and solids compare using the following table. Volume (definite or indefinite) Molecular Motion (high,
World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases
![World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases](/thumbs/96/127526823.jpg) World of Chemistry Notes for Students [Chapter 3, page ] Chapter 3 Gases ) Sec 3.8 Kinetic Theory of Gases and the Nature of Gases The Kinetic Theory of Matter says that the tiny particles in all forms
World of Chemistry Notes for Students [Chapter 3, page ] Chapter 3 Gases ) Sec 3.8 Kinetic Theory of Gases and the Nature of Gases The Kinetic Theory of Matter says that the tiny particles in all forms
Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases.
 Chapter 8 Gases Practice Problems Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases. Summary: In a gas, particles are so far
Chapter 8 Gases Practice Problems Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases. Summary: In a gas, particles are so far
Completed ALL 2 Warm-up IC Kinetic Molecular Theory Notes. Kinetic Molecular Theory and Pressure Worksheet
 Name: Unit 10- Gas Laws Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 5 Kinetic Molecular Theory Notes IC 1 6 8 Kinetic Molecular Theory and Pressure Worksheet IC 2 9 10 Gas Law
Name: Unit 10- Gas Laws Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 5 Kinetic Molecular Theory Notes IC 1 6 8 Kinetic Molecular Theory and Pressure Worksheet IC 2 9 10 Gas Law
A. What are the three states of matter chemists work with?
 Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
Ch. 14 The Behavior of Gases
 Ch. 14 The Behavior of Gases 14.1 PROPERTIES OF GASES Compressibility Compressibility: a measure of how much the volume of matter decreases under pressure Gases are easily compressed because of the spaces
Ch. 14 The Behavior of Gases 14.1 PROPERTIES OF GASES Compressibility Compressibility: a measure of how much the volume of matter decreases under pressure Gases are easily compressed because of the spaces
States of Matter Review
 States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
Gas Pressure. Pressure is the force exerted per unit area by gas molecules as they strike the surfaces around them.
 Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
The Behavior of gases. Section 14.1: Properties of Gases
 The Behavior of gases Section 14.1: Properties of Gases Why do soccer balls explode if you over pump them? What is meant by the term compressibility? Compressibility is a measure of how much the volume
The Behavior of gases Section 14.1: Properties of Gases Why do soccer balls explode if you over pump them? What is meant by the term compressibility? Compressibility is a measure of how much the volume
Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes:
 Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases
![Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases](/thumbs/78/77082586.jpg) Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] Chapter 10 Gases We have talked a little about gases in Chapter 3 and we dealt briefly with them in our stoichiometric calculations in
Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] Chapter 10 Gases We have talked a little about gases in Chapter 3 and we dealt briefly with them in our stoichiometric calculations in
Unit 8: Kinetic Theory Homework Packet (90 points)
 Name: Key Period: By the end of Unit 8, you should be able to: Kinetic Theory Chapter 13-14 4. Define kinetic theory of gases including collisions 5. Define pressure, including atmospheric pressure, vapor
Name: Key Period: By the end of Unit 8, you should be able to: Kinetic Theory Chapter 13-14 4. Define kinetic theory of gases including collisions 5. Define pressure, including atmospheric pressure, vapor
Problem Solving. Gas Laws
 Skills Worksheet Problem Solving Gas Laws Chemists found that there were relationships among temperature, volume, pressure, and quantity of a gas that could be described mathematically. This chapter deals
Skills Worksheet Problem Solving Gas Laws Chemists found that there were relationships among temperature, volume, pressure, and quantity of a gas that could be described mathematically. This chapter deals
Kinetic-Molecular Theory
 GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
Section 10-1: The Kinetic-Molecular Theory of Matter. 1) How does the word kinetic apply to particles of matter?
 Kinetic-Molecular theory of Matter/Ch10, Gases/Ch11 Column notes: Answer all parts of each question IN YOUR OWN WORDS. Use the text, figures and captions as resources. Section 10-1: The Kinetic-Molecular
Kinetic-Molecular theory of Matter/Ch10, Gases/Ch11 Column notes: Answer all parts of each question IN YOUR OWN WORDS. Use the text, figures and captions as resources. Section 10-1: The Kinetic-Molecular
8.1 Properties of Gases. Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases.
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8. Chapter 8
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases. Chapter 8. Chapter 8. Gases Properties of Gases. We are surrounded by gases, but we are often
 Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
Unit 8: Gases and States of Matter
 Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
Kinetic Molecular Theory Gases. Behavior of gases. Postulate two. Postulate one. Postulate three. Postulate four
 Kinetic Molecular Theory Gases Gas particles are so small that their individual volume can be considered to be negligible Gas particles are in constant motion and the collisions of the particles with the
Kinetic Molecular Theory Gases Gas particles are so small that their individual volume can be considered to be negligible Gas particles are in constant motion and the collisions of the particles with the
Name: Class: Date: SHORT ANSWER Answer the following questions in the space provided.
 CHAPTER 11 REVIEW Gases SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Pressure =. For a constant force, when the surface area is tripled the pressure is (a) doubled. (b)
CHAPTER 11 REVIEW Gases SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Pressure =. For a constant force, when the surface area is tripled the pressure is (a) doubled. (b)
Boyle s Law Practice
 Boyle s Law Practice Boyle s Law is an indirect relationship. Most of these problems can be done in your head without showing your work. 1. Herman has 30.0 L of helium gas trapped in a cylinder by a piston.
Boyle s Law Practice Boyle s Law is an indirect relationship. Most of these problems can be done in your head without showing your work. 1. Herman has 30.0 L of helium gas trapped in a cylinder by a piston.
Accelerated Chemistry Study Guide Chapter 13: Gases
 Accelerated Chemistry Study Guide Chapter 13: Gases Terms, definitions, topics Diffusion Kinetic Molecular Theory Atmospheric pressure Barometer Manometer STP Absolute zero Page 1 of 42 Molar volume Partial
Accelerated Chemistry Study Guide Chapter 13: Gases Terms, definitions, topics Diffusion Kinetic Molecular Theory Atmospheric pressure Barometer Manometer STP Absolute zero Page 1 of 42 Molar volume Partial
Chapter 9 Gases: Their Properties and Behavior
 Chapter 9 Gases: Their Properties and Behavior 國防醫學院生化學科王明芳老師 2011-11-15 & 2011-11-22 Chapter 9/1 Gases and Gas Pressure Gas mixtures are homogeneous and compressible. Air-the mixture of gases. Molecular
Chapter 9 Gases: Their Properties and Behavior 國防醫學院生化學科王明芳老師 2011-11-15 & 2011-11-22 Chapter 9/1 Gases and Gas Pressure Gas mixtures are homogeneous and compressible. Air-the mixture of gases. Molecular
4. Using the kinetic molecular theory, explain why a gas can be easily compressed, while a liquid and a solid cannot?
 Name Period HW 1 Worksheet (Goals 1-4) - Kinetic Molecular Theory 1. Describe how gases, liquids, and solids compare using the following table. Solids Liquids Gases Volume (definite or indefinite) Molecular
Name Period HW 1 Worksheet (Goals 1-4) - Kinetic Molecular Theory 1. Describe how gases, liquids, and solids compare using the following table. Solids Liquids Gases Volume (definite or indefinite) Molecular
Section 8: Gases. The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC (c).
 Section 8: Gases The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 8.01 Simple Gas Laws Chemistry (9)(A) 8.02 Ideal Gas Law Chemistry
Section 8: Gases The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 8.01 Simple Gas Laws Chemistry (9)(A) 8.02 Ideal Gas Law Chemistry
Chemistry 1B Chapter 10 Worksheet - Daley. Name
 Name 1) The National Weather Service routinely supplies atmospheric pressure data to help pilots set their altimeters. The units the NWS uses for atmospheric pressure are inches of mercury. A barometric
Name 1) The National Weather Service routinely supplies atmospheric pressure data to help pilots set their altimeters. The units the NWS uses for atmospheric pressure are inches of mercury. A barometric
Unit 10: Gas Laws. Monday Tuesday Wednesday Thursday Friday. 10 Review for Cumulative Retest. 17 Chem Think Gas Laws Tutorial- Computer Lab-
 Unit 10: Gas Laws Name: Monday Tuesday Wednesday Thursday Friday February 8 Stoichiometry Test Review 9 Stoichiometry Test 10 Review for Cumulative Retest 11 Cumulative Re-Test 12 Pressure & Kinetic Theory
Unit 10: Gas Laws Name: Monday Tuesday Wednesday Thursday Friday February 8 Stoichiometry Test Review 9 Stoichiometry Test 10 Review for Cumulative Retest 11 Cumulative Re-Test 12 Pressure & Kinetic Theory
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works.
 Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
Unit 9: Gas Laws REGENTS CHEMISTRY
 Name: Unit 9: Gas Laws REGENTS CHEMISTRY 1 Name: Unit 9: Gas Laws The concept of an ideal gas is a model to explain the behavior of gases. A real gas is most like an ideal gas when the real gas is at low
Name: Unit 9: Gas Laws REGENTS CHEMISTRY 1 Name: Unit 9: Gas Laws The concept of an ideal gas is a model to explain the behavior of gases. A real gas is most like an ideal gas when the real gas is at low
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory
 CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
2. Calculate the ratio of diffusion rates for carbon monoxide (CO) and carbon dioxide (CO2). υa = MB = 44 = 1.25
 Gas laws worksheet (2-08) (modified 3/17) Answer key Graham s Law 1. Calculate the ratio of effusion rates for nitrogen (N2) and neon (Ne). υa = MB = 20 = 0.845 υb MA 28 2. Calculate the ratio of diffusion
Gas laws worksheet (2-08) (modified 3/17) Answer key Graham s Law 1. Calculate the ratio of effusion rates for nitrogen (N2) and neon (Ne). υa = MB = 20 = 0.845 υb MA 28 2. Calculate the ratio of diffusion
PSI Chemistry: Gases Multiple Choice Review
 PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
Kinetic Molecular Theory imaginary Assumptions of Kinetic Molecular Theory: Problems with KMT:
 AP Chemistry Ms. Ye Name Date Block Kinetic Molecular Theory Explains properties of gases, liquids, and solids in terms of energy using an ideal gas, an imaginary which fits all the assumptions of kinetic
AP Chemistry Ms. Ye Name Date Block Kinetic Molecular Theory Explains properties of gases, liquids, and solids in terms of energy using an ideal gas, an imaginary which fits all the assumptions of kinetic
Chem 110 General Principles of Chemistry
 CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory
 CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
Name Hour. The Behavior of Gases. Practice B
 Name Hour The Behavior of Gases Practice B B 1 Objective 1: Apply Boyle s Law, Charles s Law, and Gay-Lussac s Law to solve problems involving pressure and volume and temperature. 1. A high-altitude balloon
Name Hour The Behavior of Gases Practice B B 1 Objective 1: Apply Boyle s Law, Charles s Law, and Gay-Lussac s Law to solve problems involving pressure and volume and temperature. 1. A high-altitude balloon
Gases. Name: Class: Date: Matching
 Name: Class: Date: Gases Matching Match each item with the correct statement below. a. Boyle's law d. Graham's law b. Charles's law e. Gay-Lussac's law c. Dalton's law f. ideal gas law 1. For a given mass
Name: Class: Date: Gases Matching Match each item with the correct statement below. a. Boyle's law d. Graham's law b. Charles's law e. Gay-Lussac's law c. Dalton's law f. ideal gas law 1. For a given mass
Chapter 12. The Gaseous State of Matter
 Chapter 12 The Gaseous State of Matter The air in a hot air balloon expands When it is heated. Some of the air escapes from the top of the balloon, lowering the air density inside the balloon, making the
Chapter 12 The Gaseous State of Matter The air in a hot air balloon expands When it is heated. Some of the air escapes from the top of the balloon, lowering the air density inside the balloon, making the
Name Unit 9 Notes: Gas Laws Period. Complete throughout unit. Due on test day!
 Name Unit 9 Notes: Gas Laws Period Skills: 1. Gases and Entropy 2. Distinguish between Ideal and Real gases 3. Understand KMT and Avogadro s Law 4. Identify and Solve Boyle s Law Problems 5. Identify and
Name Unit 9 Notes: Gas Laws Period Skills: 1. Gases and Entropy 2. Distinguish between Ideal and Real gases 3. Understand KMT and Avogadro s Law 4. Identify and Solve Boyle s Law Problems 5. Identify and
Chapter 5. Nov 6 1:02 PM
 Chapter 5 Nov 6 1:02 PM Expand to fill their containers Fluid motion (they flow) Have low densities (1/1000 the density of equivalent liquids or solids) Compressible Can Effuse and Diffuse Effuse: The
Chapter 5 Nov 6 1:02 PM Expand to fill their containers Fluid motion (they flow) Have low densities (1/1000 the density of equivalent liquids or solids) Compressible Can Effuse and Diffuse Effuse: The
Gases and Pressure. Main Ideas
 Gases and Pressure Key Terms pressure millimeters of mercury partial pressure newton atmosphere of pressure Dalton s law of partial pressures barometer pascal In the chapter States of Matter, you read
Gases and Pressure Key Terms pressure millimeters of mercury partial pressure newton atmosphere of pressure Dalton s law of partial pressures barometer pascal In the chapter States of Matter, you read
Under ideal conditions, the rates at which different gases diffuse (spread out) are proportional to their molar masses.
 Chemistry Ms. Ye Name Date Block Graham s Law of Diffusion- Under ideal conditions, the rates at which different gases diffuse (spread out) are proportional to their molar masses. In other words, gas molecules
Chemistry Ms. Ye Name Date Block Graham s Law of Diffusion- Under ideal conditions, the rates at which different gases diffuse (spread out) are proportional to their molar masses. In other words, gas molecules
Chemistry HP Unit 6 Gases. Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases
 Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
To convert to millimeters of mercury, we derive a unit factor related to the equivalent relationship 29.9 in. Hg = 760 mm Hg.
 Example Exercise 11.1 Gas Pressure Conversion Meteorologists state that a falling barometer indicates an approaching storm. Given a barometric pressure of 27.5 in. Hg, express the pressure in each of the
Example Exercise 11.1 Gas Pressure Conversion Meteorologists state that a falling barometer indicates an approaching storm. Given a barometric pressure of 27.5 in. Hg, express the pressure in each of the
Honors Chemistry Unit 7 Gas Laws Notes
 Honors Chemistry Unit 7 Gas Laws Notes Kinetic Molecular Theory 1. List the five assumptions: Assumption Description Extra Info 1 Basically means: the particles themselves have compared to the space between
Honors Chemistry Unit 7 Gas Laws Notes Kinetic Molecular Theory 1. List the five assumptions: Assumption Description Extra Info 1 Basically means: the particles themselves have compared to the space between
Section 5.1 Pressure. Why study gases? An understanding of real world phenomena. An understanding of how science works.
 Chapter 5 Gases Section 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage Learning. All rights reserved 2 Section 5.1 Pressure
Chapter 5 Gases Section 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage Learning. All rights reserved 2 Section 5.1 Pressure
Kinetic-Molecular Theory of Matter
 Gases Properties of Gases Gas Pressure Gases What gases are important for each of the following: O 2, CO 2 and/or He? A. B. C. D. 1 2 Gases What gases are important for each of the following: O 2, CO 2
Gases Properties of Gases Gas Pressure Gases What gases are important for each of the following: O 2, CO 2 and/or He? A. B. C. D. 1 2 Gases What gases are important for each of the following: O 2, CO 2
Gas Laws Packet Ideal Gas Law Worksheet PV = nrt
 Gas Laws Packet Ideal Gas Law Worksheet PV = nrt Use the ideal gas law, PV-nRT, and the universal gas constant R = 0.0821 L*atm to solve the following problems: K*mol If pressure is needed in kpa then
Gas Laws Packet Ideal Gas Law Worksheet PV = nrt Use the ideal gas law, PV-nRT, and the universal gas constant R = 0.0821 L*atm to solve the following problems: K*mol If pressure is needed in kpa then
Chapter 10: Gases. Characteristics of Gases
 Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
Expand to fill their containers, are highly compressible, have extremely low densities.
 Chem150 week6 Handout 1 Gases Characteristics of Gases: Unlike liquids and solids, they Expand to fill their containers, are highly compressible, have extremely low densities. Pressure is the amount of
Chem150 week6 Handout 1 Gases Characteristics of Gases: Unlike liquids and solids, they Expand to fill their containers, are highly compressible, have extremely low densities. Pressure is the amount of
UNIT 10 - GASES. Notes & Worksheets - Honors
 Ideal Gas Equation 1 WKSHT 1.) What is the pressure exerted by 2.0 moles of an ideal gas when it occupies a volume of 12.0 L at 373 K? 2.) A flashbulb of volume 2.6 cm 3 contains O 2 gas at a pressure
Ideal Gas Equation 1 WKSHT 1.) What is the pressure exerted by 2.0 moles of an ideal gas when it occupies a volume of 12.0 L at 373 K? 2.) A flashbulb of volume 2.6 cm 3 contains O 2 gas at a pressure
AP TOPIC 6: Gases. Revised August General properties and kinetic theory
 AP OPIC 6: Gases General properties and kinetic theory Gases are made up of particles that have (relatively) large amounts of energy. A gas has no definite shape or volume and will expand to fill as much
AP OPIC 6: Gases General properties and kinetic theory Gases are made up of particles that have (relatively) large amounts of energy. A gas has no definite shape or volume and will expand to fill as much
Chapter 5. Pressure. Atmospheric Pressure. Gases. Force Pressure = Area
 Chapter 5 Gases Water for many homes is supplied by a well The pump removes air from the pipe, decreasing the air pressure in the pipe The pressure then pushes the water up the pipe Pressure Atmospheric
Chapter 5 Gases Water for many homes is supplied by a well The pump removes air from the pipe, decreasing the air pressure in the pipe The pressure then pushes the water up the pipe Pressure Atmospheric
Behavior of Gases. Gases are mostly The molecules in a gas are separate, very small and very
 Properties of Gases Gases have Gases Gases exert Gases fill their containers Behavior of Gases Gases are mostly The molecules in a gas are separate, very small and very Kinetic Theory of Matter: Gas molecules
Properties of Gases Gases have Gases Gases exert Gases fill their containers Behavior of Gases Gases are mostly The molecules in a gas are separate, very small and very Kinetic Theory of Matter: Gas molecules
Chemistry A Molecular Approach. Fourth Edition. Chapter 5. Gases. Copyright 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
 Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Chapter 10: Properties of Gases: The Air We Breathe
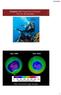 Chapter 10: Properties of Gases: The Air We Breathe Sept, 2006 Sept, 2016 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 The Properties of Gases 10.2 The Kinetic Molecular Theory of Gases* 10.3
Chapter 10: Properties of Gases: The Air We Breathe Sept, 2006 Sept, 2016 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 The Properties of Gases 10.2 The Kinetic Molecular Theory of Gases* 10.3
Chapter 13: The Behavior of Gases
 Chapter 13: The Behavior of Gases I. First Concepts a. The 3 states of matter most important to us: solids, liquids, and gases. b. Real Gases and Ideal Gases i. Real gases exist, ideal gases do not ii.
Chapter 13: The Behavior of Gases I. First Concepts a. The 3 states of matter most important to us: solids, liquids, and gases. b. Real Gases and Ideal Gases i. Real gases exist, ideal gases do not ii.
Chapter 10: Properties of Gases: The Air We Breathe
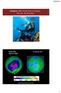 Chapter 10: Properties of Gases: The Air We Breathe South Pole Sept 24, 2006 15 February 2017 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 10.2 10.3 The Properties of Gases Effusion and the Kinetic
Chapter 10: Properties of Gases: The Air We Breathe South Pole Sept 24, 2006 15 February 2017 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 10.2 10.3 The Properties of Gases Effusion and the Kinetic
Name /74. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch 11 Gases STUDY GUIDE Accelerated Chemistry SCANTRON Name /74 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements
Ch 11 Gases STUDY GUIDE Accelerated Chemistry SCANTRON Name /74 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements
Chapter 1, Lesson 5: Air, It s Really There
 Chapter 1, Lesson 5: Air, It s Really There Key Concepts In a gas, the particles (atoms and molecules) have weak attractions for one another. They are able to move freely past each other with little interaction
Chapter 1, Lesson 5: Air, It s Really There Key Concepts In a gas, the particles (atoms and molecules) have weak attractions for one another. They are able to move freely past each other with little interaction
Chemistry Honors - Gases
 Name: Class: Date: ID: A Chemistry Honors - Gases Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Why does a can collapse when a vacuum pump removes air
Name: Class: Date: ID: A Chemistry Honors - Gases Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Why does a can collapse when a vacuum pump removes air
States of Matter. Q 7. Calculate the average of kinetic energy, in joules of the molecules in 8.0 g of methane at 27 o C. (IIT JEE Marks)
 Q 1. States of Matter Calculate density of NH 3 at 30 o C and 5 atm pressure Q 2. (IIT JEE 1978 3 Marks) 3.7 g of a gas at 25 o C occupied the same volume as 0.184g of hydrogen at 17 o C and at the same
Q 1. States of Matter Calculate density of NH 3 at 30 o C and 5 atm pressure Q 2. (IIT JEE 1978 3 Marks) 3.7 g of a gas at 25 o C occupied the same volume as 0.184g of hydrogen at 17 o C and at the same
Temperature Temperature
 Temperature Temperature is a measure of how hot or cold an object is compared to another object. indicates that heat flows from the object with a higher temperature to the object with a lower temperature.
Temperature Temperature is a measure of how hot or cold an object is compared to another object. indicates that heat flows from the object with a higher temperature to the object with a lower temperature.
Gas Laws. Essential Learning Outcomes: 1. Change can be measured. 2. Changes can occur within a substance that alters its identity.
 Gas Laws Gas Laws: Gases and pressures affect our lives every day. From the weather we experience to the air we breathe, it all has to do with gases and pressures. Why do we have wind? Why do we have the
Gas Laws Gas Laws: Gases and pressures affect our lives every day. From the weather we experience to the air we breathe, it all has to do with gases and pressures. Why do we have wind? Why do we have the
Chapter 13. Gases. Copyright Cengage Learning. All rights reserved 1
 Chapter 13 Gases Copyright Cengage Learning. All rights reserved 1 Section 13.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage
Chapter 13 Gases Copyright Cengage Learning. All rights reserved 1 Section 13.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage
Gases and Pressure SECTION 11.1
 SECTION 11.1 Gases and In the chapter States of Matter, you read about the kineticmolecular theory of matter. You were also introduced to how this theory explains some of the properties of ideal gases.
SECTION 11.1 Gases and In the chapter States of Matter, you read about the kineticmolecular theory of matter. You were also introduced to how this theory explains some of the properties of ideal gases.
temperature and pressure unchanging
 Gas Laws Review I. Variables Used to Describe a Gas A. Pressure (P) kpa, atm, mmhg (torr) -Pressure=force exerted per unit area (force/area) -Generated by collisions within container walls (more collisions=more
Gas Laws Review I. Variables Used to Describe a Gas A. Pressure (P) kpa, atm, mmhg (torr) -Pressure=force exerted per unit area (force/area) -Generated by collisions within container walls (more collisions=more
8. Now plot on the following grid the values of T (K) and V from the table above, and connect the points.
 Charles s Law According to Charles s law, the volume of a fixed mass of gas varies directly with its Kelvin temperature if its pressure is constant. The following table contains Celsius temperature and
Charles s Law According to Charles s law, the volume of a fixed mass of gas varies directly with its Kelvin temperature if its pressure is constant. The following table contains Celsius temperature and
(for tutoring, homework help, or help with online classes)
 www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. Which statement is inconsistent with the kinetic theory of an ideal gas? 1. The forces of repulsion between gas molecules
www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. Which statement is inconsistent with the kinetic theory of an ideal gas? 1. The forces of repulsion between gas molecules
Chemistry: It s a gas
 Chemistry: It s a gas Part IV Molar mass of a gas Density of a gas Dalton s Law: high altitudes and scuba diving Kinetic Molecular Theory: fast gases are hot! I have a chemistry test on Friday, there is
Chemistry: It s a gas Part IV Molar mass of a gas Density of a gas Dalton s Law: high altitudes and scuba diving Kinetic Molecular Theory: fast gases are hot! I have a chemistry test on Friday, there is
Chapter 14-Gases. Dr. Walker
 Chapter 14-Gases Dr. Walker State of Matter Gases are one of the four states of matter along with solids, liquids, and plasma Conversion to Gases From liquids Evaporation Example: Boiling water From solids
Chapter 14-Gases Dr. Walker State of Matter Gases are one of the four states of matter along with solids, liquids, and plasma Conversion to Gases From liquids Evaporation Example: Boiling water From solids
GASES. Unit #8. AP Chemistry
 GASES Unit #8 AP Chemistry I. Characteristics of Gases A. Gas Characteristics: 1. Fills its container a. no definite shape b. no definite vol. 2. Easily mixes w/ other gases 3. Exerts pressure on its surroundings
GASES Unit #8 AP Chemistry I. Characteristics of Gases A. Gas Characteristics: 1. Fills its container a. no definite shape b. no definite vol. 2. Easily mixes w/ other gases 3. Exerts pressure on its surroundings
Practice MC Test unit D (Ch 10) Gas Laws (pg 1 of 10)
 Practice MC Test unit D (Ch 10) Gas Laws (pg 1 of 10) This is practice - Do NOT cheat yourself of finding out what you are capable of doing. Be sure you follow the testing conditions outlined below. DO
Practice MC Test unit D (Ch 10) Gas Laws (pg 1 of 10) This is practice - Do NOT cheat yourself of finding out what you are capable of doing. Be sure you follow the testing conditions outlined below. DO
Date: Period: Gas Laws Worksheet #1 - Boyle s, Charles, Gay-Lussac s, and Combined Gas Law
 Name: Date: Period: Gas Laws Worksheet #1 - Boyle s, Charles, Gay-Lussac s, and Combined Gas Law Boyle s Law: V1P1 = V2P2 1. A gas sample contained in a cylinder equipped with a moveable piston occupied
Name: Date: Period: Gas Laws Worksheet #1 - Boyle s, Charles, Gay-Lussac s, and Combined Gas Law Boyle s Law: V1P1 = V2P2 1. A gas sample contained in a cylinder equipped with a moveable piston occupied
Gas volume and pressure are indirectly proportional.
 Section 2 The Gas Laws Key Terms Boyle s law Charles s law combined gas law absolute zero Gay-Lussac s law Scientists have been studying physical properties of gases for hundreds of years In 1662, Robert
Section 2 The Gas Laws Key Terms Boyle s law Charles s law combined gas law absolute zero Gay-Lussac s law Scientists have been studying physical properties of gases for hundreds of years In 1662, Robert
Chemistry 51 Chapter 7 PROPERTIES OF GASES. Gases are the least dense and most mobile of the three phases of matter.
 ROERIES OF GASES Gases are the least dense and most mobile of the three phases of matter. articles of matter in the gas phase are spaced far apart from one another and move rapidly and collide with each
ROERIES OF GASES Gases are the least dense and most mobile of the three phases of matter. articles of matter in the gas phase are spaced far apart from one another and move rapidly and collide with each
Chemistry Chapter 10 Test
 Chemistry Chapter 10 Test True/False Indicate whether the sentence or statement is true or false. 1. KMT stands for Kinetic Mole Theory. 2. One of the assumptions in the KMT is that the particles are spread
Chemistry Chapter 10 Test True/False Indicate whether the sentence or statement is true or false. 1. KMT stands for Kinetic Mole Theory. 2. One of the assumptions in the KMT is that the particles are spread
Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops
 C h e m i s t r y 1 2 C h a p t e r 11 G a s e s P a g e 1 Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops Gas Properties: Gases have high kinetic energy low
C h e m i s t r y 1 2 C h a p t e r 11 G a s e s P a g e 1 Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops Gas Properties: Gases have high kinetic energy low
UNIT 4 IB MATERIAL PARTICLE BEHAVIOR OF MATTER PHASES & ATTRACTIONS
 UNIT 4 IB MATERIAL Name: PARTICLE BEHAVIOR OF MATTER PHASES & ATTRACTIONS ESSENTIALS: Know, Understand, and Be Able To Apply Avogadro s law to calculate reacting volumes of gases. Apply the concept of
UNIT 4 IB MATERIAL Name: PARTICLE BEHAVIOR OF MATTER PHASES & ATTRACTIONS ESSENTIALS: Know, Understand, and Be Able To Apply Avogadro s law to calculate reacting volumes of gases. Apply the concept of
Example: 25 C = ( ) K = 298 K. Pressure Symbol: p Units: force per area 1Pa (Pascal) = 1 N/m 2
 Chapter 6: Gases 6.1 Measurements on Gases MH5, Chapter 5.1 Let s look at a certain amount of gas, i.e. trapped inside a balloon. To completely describe the state of this gas one has to specify the following
Chapter 6: Gases 6.1 Measurements on Gases MH5, Chapter 5.1 Let s look at a certain amount of gas, i.e. trapped inside a balloon. To completely describe the state of this gas one has to specify the following
Chapter 13 Gases and Pressure. Pressure and Force. Pressure is the force per unit area on a surface. Force Area. Pressure =
 Chapter 13 Gas Laws Chapter 13 Gases and Pressure Pressure and Force Pressure is the force per unit area on a surface. Pressure = Force Area Chapter 13 Gases and Pressure Gases in the Atmosphere The atmosphere
Chapter 13 Gas Laws Chapter 13 Gases and Pressure Pressure and Force Pressure is the force per unit area on a surface. Pressure = Force Area Chapter 13 Gases and Pressure Gases in the Atmosphere The atmosphere
Gas Laws Chapter 14. Complete the following pressure conversion. Be sure to show how units cancel.
 Gas Laws Chapter 14 Complete the following pressure conversion. Be sure to show how units cancel. 1 atm = 760 mm Hg = 760 torr = 101.3 kpa = 14.7 psi = 1.013 bar 1. The air pressure for a certain tire
Gas Laws Chapter 14 Complete the following pressure conversion. Be sure to show how units cancel. 1 atm = 760 mm Hg = 760 torr = 101.3 kpa = 14.7 psi = 1.013 bar 1. The air pressure for a certain tire
Unit 14 Gas Laws Funsheets
 Name: Period: Unit 14 Gas Laws Funsheets Part A: Vocabulary and Concepts- Answer the following questions. Refer to your notes and the PowerPoint for help. 1. List 5 different common uses for gases: a.
Name: Period: Unit 14 Gas Laws Funsheets Part A: Vocabulary and Concepts- Answer the following questions. Refer to your notes and the PowerPoint for help. 1. List 5 different common uses for gases: a.
