PHY100 s Lab: The Relationship Between Liquid Pressure and Depth as it applies to Blood Pressure. Course & Sec:
|
|
|
- Lee Hoover
- 5 years ago
- Views:
Transcription
1 PHY100 s Lab: The Relationship Between Liquid Pressure and Depth as it applies to Blood Pressure Name: Lab Partner: Course & Sec: Date: Disclaimer: The procedures in this lab are not according to proper clinical protocols. This lab is not intended to teach how to take vital signs or instruct on emergency procedures. Materials: Wrist Blood Pressure Monitor, 2 meter sticks, lab jack, barometer Background: Blood pressure is the force exerted by the blood on the walls of the arteries. A blood pressure measurement of the brachial artery is usually expressed as a fraction. The units of the fraction are millimeters (mm) of mercury (Hg), because traditionally, the device used to measure blood pressure (a sphygmomanometer) uses mercury in its gauge. The numerator in this fraction refers to the systolic pressure, which is the peak pressure created when the left ventricle of the heart contracts and forces blood into the aorta. The denominator of the fraction refers to the diastolic pressure, which is the reduced pressure of the blood as it continues to move forward between the contractions of the ventricles. The blood pressure of someone with a systolic pressure of 120 and a diastolic pressure of 80 would be expressed as 120 / 80 mm Hg (16 kpa / 10.5 kpa). The automatic instrument used in this lab to measure blood pressure employs the oscillometric method. The cuff is inflated rapidly so that the artery of the arm is compressed and the passage of the blood is stopped. Then, the cuff is gradually deflated until minute pulsations are perceived by the sensor in the device as the blood starts pumping through the artery. The measurement is the maximum or systolic pressure. A blood pressure measurement of a vessel is affected by several factors, one of the most important of which is the diameter of the blood vessel. Other factors include the elasticity of the vessel, the viscosity of the blood, and the distance between the heart and the place where the blood pressure is measured. We will explore the last of these factors through this lab as we measure blood pressure at the wrist. For a given person, the distance between the wrist and the heart is fixed with regard to the length of that circulation path. However, a change in the orientation of that path will result in a change in the blood pressure measurement as the wrist is held at various heights. Think of a blood vessel as a long fluid filled tube and consider any point on the inner wall of this tube. The pressure (force/area) exerted by the fluid on this point will depend on the magnitude of the pumping force (as provided by the heart muscle), the speed of the fluid at this point, and the weight of the fluid above this point. The pressure exerted by a liquid at any point in the liquid, due to the weight of the liquid above, follows the relationship: Liquid pressure = weight density of the liquid x depth within the liquid P = ρ x g x h in units of N/ m 2 or Pascals (1 N/ m 2 = 1 Pascal). If the tube s orientation is altered, by being flipped vertically or horizontally, then a given point in the tube may experience a change in pressure since the depth in the liquid at that point may have changed. This difference in pressure will depend on the changed depth within the liquid as: P = ρ x g x h, where h is the change in depth, and assume weight density remains constant. 1
2 Experiment: The objective is to quantify the effect by which liquid pressure varies with depth as it relates to blood pressure measurements taken at the wrist height relative to the heart. A total of 3 blood pressure readings will be taken on 2 individuals in your group. For the 1 st individual, measurements will be taken while the person is seated. For the 2 nd individual, measurements will be taken while the person is standing. The height of the wrist wearing the cuff will be varied in each of the three readings for each person. Use of Wrist Blood Pressure monitor: 1. A measurement taken within 30 minutes of eating, smoking, or exercising will not be your true blood pressure. 2. With the palm of your left hand up, put the cuff on your wrist so that the main body is on the same side as your palm. 3. Adjust the cuff, with your palm up, until its edge is positioned ¼ to ½ inches (5 to 10 mm) from the lowest part of your palm. 4. Fasten the cuff around your wrist so that there is no space between the cuff and your wrist. The cuff should fit snugly. Press the surface of the cuff to make sure that it is attached securely. 5. Attach the cuff next to your skin only. Take care that your clothes are not caught by the cuff. Do not push the POWER button before the cuff is completely wound. 6. Do not support your own arm. Support should be provided by a table or another person holding it. If the patient holds his own arm the readings will be higher due to isometric activity. Lightly open your hand. 7. Do not move, chat or strain your arm or hand during the measurement. Do not cross your legs. 8. Breathe deeply 5 or 6 times to relax before the measurement. 9. Press the POWER button. If you hold the button down too long (more than 2 seconds) the power will be turned off. 10. The apparatus automatically starts inflation. Record the reading when the measurement is finished. 11. To stop the measurement for any reason, press the POWER button and the apparatus will stop inflation, discharge air rapidly, and then turn off. 12. Wait at least 5 minutes between measurements. Use the same wrist for repeated measurements. Initial Calculations: 1. The column of mercury fluid in a barometer will reach a vertical height of m (760.0mm) at Standard Atmospheric pressure (measured at sea-level at 0ºC). Use the formula for Liquid Pressure on page 1 to calculate the atmospheric pressure supporting that column of mercury in N/ m 2. This unit is also called Pascals (Pa). Use: density ρ of mercury at 0ºC ~ kg/m 3, g = m /s 2. Std. Atm. Pressure: N/ m 2 or Pa Today s Pressure: 2. Calculate the conversion factor you will need to use throughout this lab to convert from Pascals to mm Hg. To do this, specify how many Pa equal one mm Hg: Std. Atm Pressure = = Std. Height of Hg mm Hg Pa/mm Hg 2
3 Data Set 1: All three measurements taken on Person 1 while seated **Measure to the same black dot on the cuff for every wrist height measurement. A. Seated, wrist resting on lap: B. Seated, wrist at heart level: C. Seated, elbow raised to shoulder height, wrist elevated to head height: Analysis: 1.) Measured change in systolic pressure between positions A and B, P in mm Hg: Change in height, h between positions A and B: Prediction of change in systolic pressure between A and B, P in Pascals, by calculation: Find % difference between measured and predicted P. If result is less than 60%, finish all data collection before troubleshooting. If none of your % differences are less than 20% or any one is greater than 60%, look for errors. Consult instructor as necessary: 3
4 2.) Measured change in systolic pressure between positions A and C, P in mm Hg: Change in height, h between positions A and C: Prediction of change in systolic pressure between A and C, P in Pascals, by calculation: Find % difference between measured and predicted P: Conclusions: In which position was the measured systolic blood pressure the highest and the lowest? For a seated person under normal conditions, is the blood pressure higher in the head or in the feet? Support your answer by relating liquid pressure with depth in the liquid. 4
5 Data Set 2: All three measurements taken on Person 2 while standing Again, refer to Use of Wrist Blood Pressure monitor: on page 2, particularly #6 and #12. D. Standing, wrist down: E. Standing, wrist at heart level: F. Standing, elbow raised to shoulder height, wrist elevated to head height: Analysis: 1.) Measured change in systolic pressure between positions D and E, P in mm Hg: Change in height, h between positions D and E: Prediction of change in systolic pressure between D and E, P in Pascals, by calculation: Find % difference between measured and predicted P: 5
6 2.) Measured change in systolic pressure between positions D and F, P in mm Hg: Change in height, h between positions D and F: Prediction of change in systolic pressure between D and F, P in Pascals, by calculation: Find % difference between measured and predicted P: Conclusions: For Data set 2, in which position was the measured systolic blood pressure the highest and the lowest? If blood pressure is monitored around the ankle of a seated person, how would the measurement compare with one taken at the wrist at heart level? If blood pressure is monitored around the ankle of a person who is lying flat, how would this measurement compare with one taken from the wrist resting alongside the body? 6
Circulation and Respiration: Vital Signs Student Version
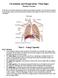 Circulation and Respiration: Vital Signs Student Version In this lab, you will learn about the circulatory and respiratory systems. You will test the capacity of your lungs, measure your blood pressure
Circulation and Respiration: Vital Signs Student Version In this lab, you will learn about the circulatory and respiratory systems. You will test the capacity of your lungs, measure your blood pressure
3.1.3 Weight. Frequency. Weight is obtained at the baseline examination and annually. Equipment
 The excerpt from the SPRINT Manual of Procedures (MOP) below, dated November 1, 2010, includes instructions about blood pressure measurement. Recruitment for the SPRINT trial began on November 8, 2010;
The excerpt from the SPRINT Manual of Procedures (MOP) below, dated November 1, 2010, includes instructions about blood pressure measurement. Recruitment for the SPRINT trial began on November 8, 2010;
CONTENTS SPECIFICATIONS GENERAL INFORMATION RECOMMENDED USE OPERATING PRINCIPLE TIPS ON TAKING YOUR BLOOD PRESSURE 3-4 BATTERY INSTALLATION
 IFU SBPMON107 CONTENTS SPECIFICATIONS GENERAL INFORMATION RECOMMENDED USE OPERATING PRINCIPLE TIPS ON TAKING YOUR BLOOD PRESSURE BATTERY INSTALLATION CORRECT POSITION FOR MEASUREMENT POSITIONING THE CUFF
IFU SBPMON107 CONTENTS SPECIFICATIONS GENERAL INFORMATION RECOMMENDED USE OPERATING PRINCIPLE TIPS ON TAKING YOUR BLOOD PRESSURE BATTERY INSTALLATION CORRECT POSITION FOR MEASUREMENT POSITIONING THE CUFF
Lesson 12: Fluid statics, Continuity equation (Sections ) Chapter 9 Fluids
 Lesson : luid statics, Continuity equation (Sections 9.-9.7) Chapter 9 luids States of Matter - Solid, liquid, gas. luids (liquids and gases) do not hold their shapes. In many cases we can think of liquids
Lesson : luid statics, Continuity equation (Sections 9.-9.7) Chapter 9 luids States of Matter - Solid, liquid, gas. luids (liquids and gases) do not hold their shapes. In many cases we can think of liquids
Gauge Pressure, Absolute Pressure, and Pressure Measurement
 Gauge Pressure, Absolute Pressure, and Pressure Measurement By: OpenStax College Online: This module is copyrig hted by Rice University. It is licensed under the Creative
Gauge Pressure, Absolute Pressure, and Pressure Measurement By: OpenStax College Online: This module is copyrig hted by Rice University. It is licensed under the Creative
Semi-Automatic Blood Pressure Monitor with Memory
 INSTRUCTION MANUAL Semi-Automatic Blood Pressure Monitor with Memory 61-268-001 (Adult size cuff) Please read this instruction manual completely before operating this unit. English Spanish Limited Five
INSTRUCTION MANUAL Semi-Automatic Blood Pressure Monitor with Memory 61-268-001 (Adult size cuff) Please read this instruction manual completely before operating this unit. English Spanish Limited Five
PRESSURE. 7. Fluids 2
 DENSITY Fluids can flow, change shape, split into smaller portions and combine into a larger system One of the best ways to quantify a fluid is in terms of its density The density, ρ, of a material (or
DENSITY Fluids can flow, change shape, split into smaller portions and combine into a larger system One of the best ways to quantify a fluid is in terms of its density The density, ρ, of a material (or
INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR
 INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR Model HEM-412C TABLE OF CONTENTS Introduction 3 Know Your Unit 4 Quick Reference Guide 5 Battery Installation/Replacement 6 How To Apply The Arm
INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR Model HEM-412C TABLE OF CONTENTS Introduction 3 Know Your Unit 4 Quick Reference Guide 5 Battery Installation/Replacement 6 How To Apply The Arm
L 13 Fluid Statics [2] More on fluids. How can a steel boat float. A ship can float in a cup of water! Today s weather
![L 13 Fluid Statics [2] More on fluids. How can a steel boat float. A ship can float in a cup of water! Today s weather L 13 Fluid Statics [2] More on fluids. How can a steel boat float. A ship can float in a cup of water! Today s weather](/thumbs/90/102460926.jpg) L 13 Fluid Statics [2] More on fluids. How can a steel boat float. A ship can float in a cup of water! Today s weather The deeper you go the higher the pressure P Top A hypothetical volume of water inside
L 13 Fluid Statics [2] More on fluids. How can a steel boat float. A ship can float in a cup of water! Today s weather The deeper you go the higher the pressure P Top A hypothetical volume of water inside
Chapter 10 Fluids. Which has a greater density? Ch 10: Problem 5. Ch 10: Problem Phases of Matter Density and Specific Gravity
 Chapter 10 Fluids 10-1 Phases of Matter The three common phases of matter are solid, liquid, and gas. A solid has a definite shape and size. A liquid has a fixed volume but can be any shape. A gas can
Chapter 10 Fluids 10-1 Phases of Matter The three common phases of matter are solid, liquid, and gas. A solid has a definite shape and size. A liquid has a fixed volume but can be any shape. A gas can
Density and Specific Gravity
 Fluids Phases of Matter Matter is anything that has mass and takes up space (volume). The three common phases of matter are solid, liquid, and gas. A solid has a definite shape and size. A liquid has a
Fluids Phases of Matter Matter is anything that has mass and takes up space (volume). The three common phases of matter are solid, liquid, and gas. A solid has a definite shape and size. A liquid has a
Sports Wrist Digital Blood Pressure Monitor Item #
 Instruction Manual Sports Wrist Digital Blood Pressure Monitor Item # 04-885-001 Please read this guidebook completely before operating this unit. Limited Five-Year Warranty The warrantor guarantees that
Instruction Manual Sports Wrist Digital Blood Pressure Monitor Item # 04-885-001 Please read this guidebook completely before operating this unit. Limited Five-Year Warranty The warrantor guarantees that
Chapter 15 Fluids. Copyright 2010 Pearson Education, Inc.
 Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle Fluid Flow and Continuity
Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle Fluid Flow and Continuity
Noninvasive Blood Pressure Measurement. D. J. McMahon rev cewood
 Noninvasive Blood Pressure Measurement D. J. McMahon 141018 rev cewood 2017-10-23 Key Points Noninvasive Blood Pressure Measurement: - ways to measure pressure, direct mechanical measurement: Bourdon Tube
Noninvasive Blood Pressure Measurement D. J. McMahon 141018 rev cewood 2017-10-23 Key Points Noninvasive Blood Pressure Measurement: - ways to measure pressure, direct mechanical measurement: Bourdon Tube
Chapter 13 Fluids. Copyright 2009 Pearson Education, Inc.
 Chapter 13 Fluids Phases of Matter Density and Specific Gravity Pressure in Fluids Atmospheric Pressure and Gauge Pressure Pascal s Principle Units of Chapter 13 Measurement of Pressure; Gauges and the
Chapter 13 Fluids Phases of Matter Density and Specific Gravity Pressure in Fluids Atmospheric Pressure and Gauge Pressure Pascal s Principle Units of Chapter 13 Measurement of Pressure; Gauges and the
Chapter 13 Fluids. Copyright 2009 Pearson Education, Inc.
 Chapter 13 Fluids Phases of Matter Density and Specific Gravity Pressure in Fluids Atmospheric Pressure and Gauge Pressure Pascal s Principle Units of Chapter 13 Measurement of Pressure; Gauges and the
Chapter 13 Fluids Phases of Matter Density and Specific Gravity Pressure in Fluids Atmospheric Pressure and Gauge Pressure Pascal s Principle Units of Chapter 13 Measurement of Pressure; Gauges and the
Old-Exam.Questions-Ch-14 T072 T071
 Old-Exam.Questions-Ch-14 T072 Q23. Water is pumped out of a swimming pool at a speed of 5.0 m/s through a uniform hose of radius 1.0 cm. Find the mass of water pumped out of the pool in one minute. (Density
Old-Exam.Questions-Ch-14 T072 Q23. Water is pumped out of a swimming pool at a speed of 5.0 m/s through a uniform hose of radius 1.0 cm. Find the mass of water pumped out of the pool in one minute. (Density
8. Now plot on the following grid the values of T (K) and V from the table above, and connect the points.
 Charles s Law According to Charles s law, the volume of a fixed mass of gas varies directly with its Kelvin temperature if its pressure is constant. The following table contains Celsius temperature and
Charles s Law According to Charles s law, the volume of a fixed mass of gas varies directly with its Kelvin temperature if its pressure is constant. The following table contains Celsius temperature and
Phys101 Lectures Fluids I. Key points: Pressure and Pascal s Principle Buoyancy and Archimedes Principle. Ref: 10-1,2,3,4,5,6,7.
 Phys101 Lectures 21-22 Fluids I Key points: Pressure and Pascal s Principle Buoyancy and Archimedes Principle Ref: 10-1,2,3,4,5,6,7. Page 1 10-1 Phases of Matter The three common phases of matter are solid,
Phys101 Lectures 21-22 Fluids I Key points: Pressure and Pascal s Principle Buoyancy and Archimedes Principle Ref: 10-1,2,3,4,5,6,7. Page 1 10-1 Phases of Matter The three common phases of matter are solid,
PHYS 101 Previous Exam Problems
 PHYS 101 Previous Exam Problems CHAPTER 14 Fluids Fluids at rest pressure vs. depth Pascal s principle Archimedes s principle Buoynat forces Fluids in motion: Continuity & Bernoulli equations 1. How deep
PHYS 101 Previous Exam Problems CHAPTER 14 Fluids Fluids at rest pressure vs. depth Pascal s principle Archimedes s principle Buoynat forces Fluids in motion: Continuity & Bernoulli equations 1. How deep
Chapter 12. Properties of Gases
 Properties of Gases Each state of matter has its own properties. Gases have unique properties because the distance between the particles of a gas is much greater than the distance between the particles
Properties of Gases Each state of matter has its own properties. Gases have unique properties because the distance between the particles of a gas is much greater than the distance between the particles
Lecture Outline Chapter 15. Physics, 4 th Edition James S. Walker. Copyright 2010 Pearson Education, Inc.
 Lecture Outline Chapter 15 Physics, 4 th Edition James S. Walker Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy
Lecture Outline Chapter 15 Physics, 4 th Edition James S. Walker Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy
Chapter 15 Fluid. Density
 Density Chapter 15 Fluid Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle By Dr. Weining man 1 Units of Chapter 15 Fluid
Density Chapter 15 Fluid Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle By Dr. Weining man 1 Units of Chapter 15 Fluid
Assistant Lecturer Anees Kadhum AL Saadi
 Pressure Variation with Depth Pressure in a static fluid does not change in the horizontal direction as the horizontal forces balance each other out. However, pressure in a static fluid does change with
Pressure Variation with Depth Pressure in a static fluid does not change in the horizontal direction as the horizontal forces balance each other out. However, pressure in a static fluid does change with
 Hydrostatic pressure Consider a tank of fluid which contains a very thin plate of (neutrally buoyant) material with area A. This situation is shown in Figure below. If the plate is in equilibrium (it does
Hydrostatic pressure Consider a tank of fluid which contains a very thin plate of (neutrally buoyant) material with area A. This situation is shown in Figure below. If the plate is in equilibrium (it does
Pressure and Depth. In a static, non-moving fluid
 Pressure and Depth In a static, non-moving fluid Static Fluids Being on the surface of the earth, you can say that we dwell on the bottom of an ocean of air. The pressure we experience is primarily caused
Pressure and Depth In a static, non-moving fluid Static Fluids Being on the surface of the earth, you can say that we dwell on the bottom of an ocean of air. The pressure we experience is primarily caused
Experiment. THE RELATIONSHIP BETWEEN VOLUME AND TEMPERATURE, i.e.,charles Law. By Dale A. Hammond, PhD, Brigham Young University Hawaii
 Experiment THE RELATIONSHIP BETWEEN VOLUME AND TEMPERATURE, i.e.,charles Law By Dale A. Hammond, PhD, Brigham Young University Hawaii The objectives of this experiment are to... LEARNING OBJECTIVES introduce
Experiment THE RELATIONSHIP BETWEEN VOLUME AND TEMPERATURE, i.e.,charles Law By Dale A. Hammond, PhD, Brigham Young University Hawaii The objectives of this experiment are to... LEARNING OBJECTIVES introduce
Fully Fuzzy Auto Digital Wrist Blood Pressure Monitor. tensio control. INSTRUCTION MANUAL Model GP-6220
 Fully Fuzzy Auto Digital Wrist Blood Pressure Monitor tensio control INSTRUCTION MANUAL Model GP-6220 Manufactured by: Geratherm Medical AG Fahrenheitstraße 1 98716 Geschwenda Germany Phone: +49 (0) 36205
Fully Fuzzy Auto Digital Wrist Blood Pressure Monitor tensio control INSTRUCTION MANUAL Model GP-6220 Manufactured by: Geratherm Medical AG Fahrenheitstraße 1 98716 Geschwenda Germany Phone: +49 (0) 36205
Blood Pressure Monitor
 Blood Pressure Monitor egotest Users Instructions Contents of the kit 1 blood pressure instrument with cuff 1 set of instructions for use 1 case Symbols on the blood pressure monitor Symbol 0124 Function/meaning
Blood Pressure Monitor egotest Users Instructions Contents of the kit 1 blood pressure instrument with cuff 1 set of instructions for use 1 case Symbols on the blood pressure monitor Symbol 0124 Function/meaning
Variation of Pressure with Depth in a Fluid *
 OpenStax-CNX module: m42192 1 Variation of Pressure with Depth in a Fluid * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract Dene
OpenStax-CNX module: m42192 1 Variation of Pressure with Depth in a Fluid * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract Dene
Hydrostatics Physics Lab XI
 Hydrostatics Physics Lab XI Objective Students will discover the basic principles of buoyancy in a fluid. Students will also quantitatively demonstrate the variance of pressure with immersion depth in
Hydrostatics Physics Lab XI Objective Students will discover the basic principles of buoyancy in a fluid. Students will also quantitatively demonstrate the variance of pressure with immersion depth in
Comments on Homework. Quiz. Class 3 - Pressure. Atmospheric Pressure. 2. Gauge vs. Absolute Pressure. 1. Definitions. Temperature conversion
 Comments on Homework Quiz Temperature conversion T ( R) = T (K) 1.8 T ( C) = T(K) - 273.15 T ( F) = T( R) - 460 However, difference in temperature is: T ( C) = T (K) T ( F) = T ( R) T ( R) = 1.8 T ( C)
Comments on Homework Quiz Temperature conversion T ( R) = T (K) 1.8 T ( C) = T(K) - 273.15 T ( F) = T( R) - 460 However, difference in temperature is: T ( C) = T (K) T ( F) = T ( R) T ( R) = 1.8 T ( C)
Properties of Fluids SPH4C
 Properties of Fluids SPH4C Fluids Liquids and gases are both fluids: a fluid is any substance that flows and takes the shape of its container. Fluids Liquids and gases are both fluids: a fluid is any substance
Properties of Fluids SPH4C Fluids Liquids and gases are both fluids: a fluid is any substance that flows and takes the shape of its container. Fluids Liquids and gases are both fluids: a fluid is any substance
weight of the book divided by the area of the bottom of the plunger.
 Lab: Boyle s Law Datasheet Name Data: Pressure is defined as force per unit area: P = Force/Area When a book rests on top of the plunger, the pressure it exerts equals the weight of the book divided by
Lab: Boyle s Law Datasheet Name Data: Pressure is defined as force per unit area: P = Force/Area When a book rests on top of the plunger, the pressure it exerts equals the weight of the book divided by
Liquids and Gases. 2/26/2012 Physics 214 Fall
 Liquids and Gases The unit of volume is the meter cubed, m 3, which is a very large volume. Very often we use cm 3 = cc. Other everyday units are gallons, quarts, pints As we know liquids and gases act
Liquids and Gases The unit of volume is the meter cubed, m 3, which is a very large volume. Very often we use cm 3 = cc. Other everyday units are gallons, quarts, pints As we know liquids and gases act
Gases. Chapter 9. Introduction. Chapter Outline
 Chapter 9 Gases 461 Chapter 9 Gases Figure 9.1 The hot air inside these balloons is less dense than the surrounding cool air. This results in a buoyant force that causes the balloons to rise when their
Chapter 9 Gases 461 Chapter 9 Gases Figure 9.1 The hot air inside these balloons is less dense than the surrounding cool air. This results in a buoyant force that causes the balloons to rise when their
Chapter 9 Fluids and Buoyant Force
 Chapter 9 Fluids and Buoyant Force In Physics, liquids and gases are collectively called fluids. 3/0/018 8:56 AM 1 Fluids and Buoyant Force Formula for Mass Density density mass volume m V water 1000 kg
Chapter 9 Fluids and Buoyant Force In Physics, liquids and gases are collectively called fluids. 3/0/018 8:56 AM 1 Fluids and Buoyant Force Formula for Mass Density density mass volume m V water 1000 kg
Gases and Pressure SECTION 11.1
 SECTION 11.1 Gases and In the chapter States of Matter, you read about the kineticmolecular theory of matter. You were also introduced to how this theory explains some of the properties of ideal gases.
SECTION 11.1 Gases and In the chapter States of Matter, you read about the kineticmolecular theory of matter. You were also introduced to how this theory explains some of the properties of ideal gases.
Lab #2: Blood pressure and peripheral circulation
 Lab #2: Blood pressure and peripheral circulation Vertebrates have a closed circulatory system where the blood is always enclosed within blood vessels or the heart. Blood is pumped from the heart (the
Lab #2: Blood pressure and peripheral circulation Vertebrates have a closed circulatory system where the blood is always enclosed within blood vessels or the heart. Blood is pumped from the heart (the
Ch.6. Dr.Rajaa Pressure
 Ch.6. Dr.Rajaa Pressure أ.م.د. رجاء سهيل جنم Pressure: It is the force per unit area in a gas or liquid. (For solid the term pressure is replaced by "stress"). Units: In metric system pressure is measured
Ch.6. Dr.Rajaa Pressure أ.م.د. رجاء سهيل جنم Pressure: It is the force per unit area in a gas or liquid. (For solid the term pressure is replaced by "stress"). Units: In metric system pressure is measured
Comments on Homework. Class 4 - Pressure. Atmospheric Pressure. Gauge vs. Absolute Pressure. 2. Gauge vs. Absolute Pressure. 1.
 Class 4 - Pressure 1. Definitions 2. Gauge Pressure 3. Pressure and Height of Liquid Column (Head) 4. Pressure Measurement and Manometers Please don t forget the special problem for the next HW assignment
Class 4 - Pressure 1. Definitions 2. Gauge Pressure 3. Pressure and Height of Liquid Column (Head) 4. Pressure Measurement and Manometers Please don t forget the special problem for the next HW assignment
Part 1: Inspiratory and expiratory pressures
 BIOEN 327 Autumn 2013 Experimental procedures Throughout these experiments, record in your notebook the purpose of the experiments, the methods you used, and the results. Where possible, make predictions
BIOEN 327 Autumn 2013 Experimental procedures Throughout these experiments, record in your notebook the purpose of the experiments, the methods you used, and the results. Where possible, make predictions
INSTRUCTION MANUAL. Automatic Blood Pressure Monitor with Fit Cuff. =Fit Cuff=!"#$% IA1B. Model
 IA1B INSTRUCTION MANUAL Automatic Blood Pressure Monitor with Fit Cuff =Fit Cuff=!"#$% Model IA1B Contents Introduction... 2 Notes on Safety... 3 Know Your Unit... 5 Quick Reference Guide... 7 Initial
IA1B INSTRUCTION MANUAL Automatic Blood Pressure Monitor with Fit Cuff =Fit Cuff=!"#$% Model IA1B Contents Introduction... 2 Notes on Safety... 3 Know Your Unit... 5 Quick Reference Guide... 7 Initial
PHY131H1S - Class 23. Today: Fluids Pressure Pascal s Law Gauge Pressure Buoyancy, Archimedes Principle. A little pre-class reading quiz
 PHY131H1S - Class 23 Today: Fluids Pressure Pascal s Law Gauge Pressure Buoyancy, Archimedes Principle Archimedes (287-212 BC) was asked to check the amount of silver alloy in the king s crown. The answer
PHY131H1S - Class 23 Today: Fluids Pressure Pascal s Law Gauge Pressure Buoyancy, Archimedes Principle Archimedes (287-212 BC) was asked to check the amount of silver alloy in the king s crown. The answer
CHM Basics of Gases (r14) Charles Taylor 1/9
 CHM 110 - Basics of Gases (r14)- 2014 Charles Taylor 1/9 Introduction The gas phase is noticeably different from the other two phases of matter. Here are some of the more obvious differences. Gases are
CHM 110 - Basics of Gases (r14)- 2014 Charles Taylor 1/9 Introduction The gas phase is noticeably different from the other two phases of matter. Here are some of the more obvious differences. Gases are
PHYS:1200 LECTURE 13 FLUIDS (2)
 1 PHYS:1200 LECTURE 13 FLUIDS (2) Lecture 13 deals with the properties of fluids at rest or fluid statics. We will be discussing mostly liquids and will introduce two important principles of fluid statics:
1 PHYS:1200 LECTURE 13 FLUIDS (2) Lecture 13 deals with the properties of fluids at rest or fluid statics. We will be discussing mostly liquids and will introduce two important principles of fluid statics:
Pressure Measurement
 Pressure Measurement Manometers Sensors, Transducers Ashish J. Modi Lecturer, Dept. of Mech.Engg., Shri S.V.M. inst. Of Technology, Bharuch Pressure Pressure is a force per unit area exerted by a fluid
Pressure Measurement Manometers Sensors, Transducers Ashish J. Modi Lecturer, Dept. of Mech.Engg., Shri S.V.M. inst. Of Technology, Bharuch Pressure Pressure is a force per unit area exerted by a fluid
MANUAL BLOOD PRESSURE MONITOR BPM 168B Contents Blood Pressure Monitor Intended Use What is blood pressure?
 MANUAL BLOOD PRESSURE MONITOR BPM 168B Contents 01... Blood Pressure Monitor Intended Use 02... 1. What is blood pressure? 02... 2. Why is it useful measure blood pressure at home?... A. WHO blood pressure
MANUAL BLOOD PRESSURE MONITOR BPM 168B Contents 01... Blood Pressure Monitor Intended Use 02... 1. What is blood pressure? 02... 2. Why is it useful measure blood pressure at home?... A. WHO blood pressure
SPH 4C Unit 4 Hydraulics and Pneumatic Systems
 SPH 4C Unit 4 Hydraulics and Pneumatic Systems Properties of Fluids and Pressure Learning Goal: I can explain the properties of fluids and identify associated units. Definitions: Fluid: A substance that
SPH 4C Unit 4 Hydraulics and Pneumatic Systems Properties of Fluids and Pressure Learning Goal: I can explain the properties of fluids and identify associated units. Definitions: Fluid: A substance that
Phys101 Lectures Fluids I. Key points: Pressure and Pascal s Principle Buoyancy and Archimedes Principle. Ref: 10-1,2,3,4,5,6,7.
 Phys101 Lectures 24-25 luids I Key points: Pressure and Pascal s Principle Buoyancy and Archimedes Principle Ref: 10-1,2,3,4,5,6,7. Page 1 10-1 Phases of Matter The three common phases of matter are solid,
Phys101 Lectures 24-25 luids I Key points: Pressure and Pascal s Principle Buoyancy and Archimedes Principle Ref: 10-1,2,3,4,5,6,7. Page 1 10-1 Phases of Matter The three common phases of matter are solid,
Chemistry Chapter 12. Characteristics of Gases. Characteristics of Gases 1/31/2012. Gases and Liquids
 Importance of Gases Chemistry Chapter 12 Gases and Liquids Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide, NaN 3. 2 NaN 3 ---> 2 Na + 3 N 2 THREE STATES
Importance of Gases Chemistry Chapter 12 Gases and Liquids Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide, NaN 3. 2 NaN 3 ---> 2 Na + 3 N 2 THREE STATES
Middle East Technical University Department of Mechanical Engineering ME 305 Fluid Mechanics I Fall 2018 Section 4 (Dr.
 Middle East Technical University Department of Mechanical Engineering ME 305 Fluid Mechanics I Fall 2018 Section 4 (Dr. Sert) Study Set 2 Reading Assignments You can find the answers of some of the following
Middle East Technical University Department of Mechanical Engineering ME 305 Fluid Mechanics I Fall 2018 Section 4 (Dr. Sert) Study Set 2 Reading Assignments You can find the answers of some of the following
Talking Wrist Style Blood Pressure Monitor Model INS-LAB-RevB08
 Talking Wrist Style Blood Pressure Monitor Model 1145 1145-INS-LAB-RevB08 Contents Introduction...2 Key Features... 2 Front View... 2 Rear View... 3 LCD Symbols... 3 Safety and Care Instructions... 4 Safety
Talking Wrist Style Blood Pressure Monitor Model 1145 1145-INS-LAB-RevB08 Contents Introduction...2 Key Features... 2 Front View... 2 Rear View... 3 LCD Symbols... 3 Safety and Care Instructions... 4 Safety
Lecture 19 Fluids: density, pressure, Pascal s principle and Buoyancy.
 Lecture 19 Water tower Fluids: density, pressure, Pascal s principle and Buoyancy. Hydraulic press Pascal s vases Barometer What is a fluid? Fluids are substances that flow. substances that take the shape
Lecture 19 Water tower Fluids: density, pressure, Pascal s principle and Buoyancy. Hydraulic press Pascal s vases Barometer What is a fluid? Fluids are substances that flow. substances that take the shape
Gas Laws. Introduction
 Gas Laws Introduction In 1662 Robert Boyle found that, at constant temperature, the pressure of a gas and its volume are inversely proportional such that P x V = constant. This relationship is known as
Gas Laws Introduction In 1662 Robert Boyle found that, at constant temperature, the pressure of a gas and its volume are inversely proportional such that P x V = constant. This relationship is known as
INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR
 TM TM INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR Model HEM-412CREL TABLE OF CONTENTS Introduction 3 Important Safety Notes 4 Know Your Unit 5 Quick Reference Guide 6 A Few Words About Blood
TM TM INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR Model HEM-412CREL TABLE OF CONTENTS Introduction 3 Important Safety Notes 4 Know Your Unit 5 Quick Reference Guide 6 A Few Words About Blood
Hydrostatics. Physics 1425 Lecture 25. Michael Fowler, UVa
 Hydrostatics Physics 1425 Lecture 25 Michael Fowler, UVa Basic Concepts Density Pressure: Pascal s Principle The Crown and the Bathtub Around 250 BC, the king of Syracuse commissioned a new crown,and gave
Hydrostatics Physics 1425 Lecture 25 Michael Fowler, UVa Basic Concepts Density Pressure: Pascal s Principle The Crown and the Bathtub Around 250 BC, the king of Syracuse commissioned a new crown,and gave
Model AUTOMATIC UPPER ARM Blood Pressure Monitor
 AUTOMATIC UPPER ARM Blood Pressure Monitor Model 1130 Real Fuzzy t e c h n o log y Features: Real Fuzzy Technology Automatic 60 sets of memory One-Touch Operation Easy-to-read Display Table of Contents:
AUTOMATIC UPPER ARM Blood Pressure Monitor Model 1130 Real Fuzzy t e c h n o log y Features: Real Fuzzy Technology Automatic 60 sets of memory One-Touch Operation Easy-to-read Display Table of Contents:
Unit 2 Kinetic Theory, Heat, and Thermodynamics: 2.A.1 Problems Temperature and Heat Sections of your book.
 Unit 2 Kinetic Theory, Heat, and Thermodynamics: 2.A.1 Problems Temperature and Heat Sections 10.1 10.2 of your book. Convert the following to Celsius and Kelvin temperatures: 1. 80.0 o F Early E. C.:
Unit 2 Kinetic Theory, Heat, and Thermodynamics: 2.A.1 Problems Temperature and Heat Sections 10.1 10.2 of your book. Convert the following to Celsius and Kelvin temperatures: 1. 80.0 o F Early E. C.:
. In an elevator accelerating upward (A) both the elevator accelerating upward (B) the first is equations are valid
 IIT JEE Achiever 2014 Ist Year Physics-2: Worksheet-1 Date: 2014-06-26 Hydrostatics 1. A liquid can easily change its shape but a solid cannot because (A) the density of a liquid is smaller than that of
IIT JEE Achiever 2014 Ist Year Physics-2: Worksheet-1 Date: 2014-06-26 Hydrostatics 1. A liquid can easily change its shape but a solid cannot because (A) the density of a liquid is smaller than that of
BLOOD PRESSURE SENSOR BT17i USER S GUIDE
 BLOOD PRESSURE SENSOR BT17i USER S GUIDE CENTRE FOR MICROCOMPUTER APPLICATIONS http://www.cma-science.nl Short description The Blood Pressure sensor BT17i allows measuring arterial blood pressure. The
BLOOD PRESSURE SENSOR BT17i USER S GUIDE CENTRE FOR MICROCOMPUTER APPLICATIONS http://www.cma-science.nl Short description The Blood Pressure sensor BT17i allows measuring arterial blood pressure. The
3. A fluid is forced through a pipe of changing cross section as shown. In which section would the pressure of the fluid be a minimum?
 AP Physics Multiple Choice Practice Fluid Mechanics 1. A cork has weight mg and density 5% of water s density. A string is tied around the cork and attached to the bottom of a water-filled container. The
AP Physics Multiple Choice Practice Fluid Mechanics 1. A cork has weight mg and density 5% of water s density. A string is tied around the cork and attached to the bottom of a water-filled container. The
Lecture 20. Static fluids
 Lecture 20 Static fluids Today s Topics: Density Pressure, Depth and Pressure Gauges Pascal s Principle Archimedes Principle Solids and Fluids Solids Maintain their shape. Generally don t flow Limited
Lecture 20 Static fluids Today s Topics: Density Pressure, Depth and Pressure Gauges Pascal s Principle Archimedes Principle Solids and Fluids Solids Maintain their shape. Generally don t flow Limited
Auto Arm Blood Pressure Monitor
 Now you know ys Auto Arm Blood Pressure Monitor Featuring: One-touch operation 85 memories Instruction Manual Table of Contents Important information................... 3 Fuzzy Logic technology.................
Now you know ys Auto Arm Blood Pressure Monitor Featuring: One-touch operation 85 memories Instruction Manual Table of Contents Important information................... 3 Fuzzy Logic technology.................
Oregon Scientific Wrist Blood Pressure Monitor (BPW211)
 Oregon Scientific Wrist Blood Pressure Monitor (BPW211) User Manual TABLE OF CONTENTS Introduction... 2 Key features... 2 Main unit... 2 Plastic storage container... 2 LCD display symbols... 3 Safety and
Oregon Scientific Wrist Blood Pressure Monitor (BPW211) User Manual TABLE OF CONTENTS Introduction... 2 Key features... 2 Main unit... 2 Plastic storage container... 2 LCD display symbols... 3 Safety and
LAB 13: FLUIDS OBJECTIVES
 205 Name Date Partners LAB 13: FLUIDS Fluids are an important part of our body OBJECTIVES OVERVIEW Fluid Properties To learn how some fundamental physical principles apply to fluids. To understand the
205 Name Date Partners LAB 13: FLUIDS Fluids are an important part of our body OBJECTIVES OVERVIEW Fluid Properties To learn how some fundamental physical principles apply to fluids. To understand the
DEVIL PHYSICS THE BADDEST CLASS ON CAMPUS PRE-IB PHYSICS
 DEVIL HYSICS THE BADDEST CLASS ON CAMUS RE-IB HYSICS GIANCOLI LESSON 10-2 TO 10-5 RESSURE IN FLUIDS ATMOSHERIC RESSURE AND GAUGE RESSURE ASCAL S RINCILE MEASUREMENT OF RESSURE: GAUGES AND BAROMETER Objectives
DEVIL HYSICS THE BADDEST CLASS ON CAMUS RE-IB HYSICS GIANCOLI LESSON 10-2 TO 10-5 RESSURE IN FLUIDS ATMOSHERIC RESSURE AND GAUGE RESSURE ASCAL S RINCILE MEASUREMENT OF RESSURE: GAUGES AND BAROMETER Objectives
Instruction Manual. Auto Inflate Blood Pressure Monitor
 TM Auto Inflate Blood Pressure Monitor Instruction Manual Features: Fuzzy Logic Technology Auto memory for up to 90 readings One-Touch Operation Easy-to-read Display Date and Time indications AC Adapter
TM Auto Inflate Blood Pressure Monitor Instruction Manual Features: Fuzzy Logic Technology Auto memory for up to 90 readings One-Touch Operation Easy-to-read Display Date and Time indications AC Adapter
Fluid Mechanics. Liquids and gases have the ability to flow They are called fluids There are a variety of LAWS that fluids obey
 Fluid Mechanics Fluid Mechanics Liquids and gases have the ability to flow They are called fluids There are a variety of LAWS that fluids obey Density Regardless of form (solid, liquid, gas) we can define
Fluid Mechanics Fluid Mechanics Liquids and gases have the ability to flow They are called fluids There are a variety of LAWS that fluids obey Density Regardless of form (solid, liquid, gas) we can define
Notes: Gas Laws (text Ch. 11)
 Name Per. Notes: Gas Laws (text Ch. 11) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to get missing information
Name Per. Notes: Gas Laws (text Ch. 11) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to get missing information
Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes:
 Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
Introduction. Table of Contents. Automatic Wrist Blood Pressure Monitor With Voice-Guided Operation. Model No.: BP5K
 Automatic Wrist Blood Monitor With Voice-Guided Operation Ozeri Customer Service Customer service: 1-877-299-1296 or Email: support@ozeri.com Model No.: BP5K Thank you for choosing an Ozeri Blood Monitor.
Automatic Wrist Blood Monitor With Voice-Guided Operation Ozeri Customer Service Customer service: 1-877-299-1296 or Email: support@ozeri.com Model No.: BP5K Thank you for choosing an Ozeri Blood Monitor.
Gases and Pressure. Main Ideas
 Gases and Pressure Key Terms pressure millimeters of mercury partial pressure newton atmosphere of pressure Dalton s law of partial pressures barometer pascal In the chapter States of Matter, you read
Gases and Pressure Key Terms pressure millimeters of mercury partial pressure newton atmosphere of pressure Dalton s law of partial pressures barometer pascal In the chapter States of Matter, you read
Model UB-328. Wrist Digital Blood Pressure Monitor. Instruction Manual. Manuel d instructions. Manual de Instrucciones. Manuale di Istruzioni
 Wrist Digital Blood Pressure Monitor Model UB-328 Instruction Manual Manuel d instructions Manual de Instrucciones Manuale di Istruzioni WM+PD4000913 ENGLISH Dear Customers Congratulations. You have purchased
Wrist Digital Blood Pressure Monitor Model UB-328 Instruction Manual Manuel d instructions Manual de Instrucciones Manuale di Istruzioni WM+PD4000913 ENGLISH Dear Customers Congratulations. You have purchased
Applications of Bernoulli s principle. Principle states that areas with faster moving fluids will experience less pressure
 Applications of Bernoulli s principle Principle states that areas with faster moving fluids will experience less pressure Artery o When blood flows through narrower regions of arteries, the speed increases
Applications of Bernoulli s principle Principle states that areas with faster moving fluids will experience less pressure Artery o When blood flows through narrower regions of arteries, the speed increases
PHYSICS - CLUTCH CH 17: FLUID MECHANICS.
 !! www.clutchprep.com INTRO TO DENSITY LIQUIDS and GASES are types of. So we use the term to refer generally to both Liquids AND Gases. The DENSITY of a material is a measure of how tight the molecules
!! www.clutchprep.com INTRO TO DENSITY LIQUIDS and GASES are types of. So we use the term to refer generally to both Liquids AND Gases. The DENSITY of a material is a measure of how tight the molecules
TEACHING SCIENCE BY OCEAN INQUIRY Pressure
 TEACHING SCIENCE BY OCEAN INQUIRY Pressure Nine activities are described below. Explanations are provided after the instructions. Goals: The activities below address different aspects of pressure. Activity
TEACHING SCIENCE BY OCEAN INQUIRY Pressure Nine activities are described below. Explanations are provided after the instructions. Goals: The activities below address different aspects of pressure. Activity
Irrigation &Hydraulics Department lb / ft to kg/lit.
 CAIRO UNIVERSITY FLUID MECHANICS Faculty of Engineering nd Year CIVIL ENG. Irrigation &Hydraulics Department 010-011 1. FLUID PROPERTIES 1. Identify the dimensions and units for the following engineering
CAIRO UNIVERSITY FLUID MECHANICS Faculty of Engineering nd Year CIVIL ENG. Irrigation &Hydraulics Department 010-011 1. FLUID PROPERTIES 1. Identify the dimensions and units for the following engineering
Pressure Measurement
 Pressure Measurement Absolute and Gage Pressure P abs = P gage + P atm where P abs = Absolute pressure P abs = Gage pressure P abs = atmospheric pressure A perfect vacuum is the lowest possible pressure.
Pressure Measurement Absolute and Gage Pressure P abs = P gage + P atm where P abs = Absolute pressure P abs = Gage pressure P abs = atmospheric pressure A perfect vacuum is the lowest possible pressure.
mass of container full of air = g mass of container with extra air = g volume of air released = cm 3
 1992 Q32 The air pressure inside the passenger cabin of an airliner is 9 x 10 4 Pa when the airliner is at its cruising height. The pressure of the outside atmosphere at this height is 4 x 10 4 Pa. Calculate
1992 Q32 The air pressure inside the passenger cabin of an airliner is 9 x 10 4 Pa when the airliner is at its cruising height. The pressure of the outside atmosphere at this height is 4 x 10 4 Pa. Calculate
STANDARD OPERATING PROCEDURE ARTSANA MERCURY SPHYGMOMANOMETER
 Page 1 of 8 1. Scope This Standard Operating Procedure (SOP) applies to the staff and students using the Artsana Mercury Sphygmomanometer in the Pharmacy Practice Resource Unit (PPRU) at the Pharmacy Department,
Page 1 of 8 1. Scope This Standard Operating Procedure (SOP) applies to the staff and students using the Artsana Mercury Sphygmomanometer in the Pharmacy Practice Resource Unit (PPRU) at the Pharmacy Department,
Beginning Bed Exercises
 Patient Education Beginning Bed Exercises Perform these exercises while lying on your back in bed. Perform each exercise times. Have someone else help you, if needed. Add cuff weights to your ankles as
Patient Education Beginning Bed Exercises Perform these exercises while lying on your back in bed. Perform each exercise times. Have someone else help you, if needed. Add cuff weights to your ankles as
13.1!"#$#%"&'%()$*+%,+-.$+/*$#
 343%%%%%%%%%5)"./$+%67%%%%%!"#$# 13.1!"#$#%"&'%()$*+%,+-.$+/*$#!"#$%&'($)*!"#$%&'($)+ If you want to understand how gases behave such as why fresh air rushes into your lungs when certain chest muscles
343%%%%%%%%%5)"./$+%67%%%%%!"#$# 13.1!"#$#%"&'%()$*+%,+-.$+/*$#!"#$%&'($)*!"#$%&'($)+ If you want to understand how gases behave such as why fresh air rushes into your lungs when certain chest muscles
LAB 13: FLUIDS OBJECTIVES
 217 Name Date Partners LAB 13: FLUIDS Fluids are an important part of our body OBJECTIVES OVERVIEW Fluid Properties To learn how some fundamental physical principles apply to fluids. To understand the
217 Name Date Partners LAB 13: FLUIDS Fluids are an important part of our body OBJECTIVES OVERVIEW Fluid Properties To learn how some fundamental physical principles apply to fluids. To understand the
Talking Wrist TypeBlood Pressure Monitor. Talking Wrist Type Blood Pressure Monitor Model: BPW810. User Manual
 Talking Wrist TypeBlood Pressure Monitor Talking Wrist Type Blood Pressure Monitor Model: BPW810 User Manual TALKING WRIST TYPE BLOOD PRESSURE MONITOR CONTENTS Model: BPW810 USER MANUAL Deleting the Latest
Talking Wrist TypeBlood Pressure Monitor Talking Wrist Type Blood Pressure Monitor Model: BPW810 User Manual TALKING WRIST TYPE BLOOD PRESSURE MONITOR CONTENTS Model: BPW810 USER MANUAL Deleting the Latest
Objectives: Assisting with Medication, Checking vital Signs
 Assisting with Medication, Checking vital Signs Duty: Assist Client with Personal Hygiene Task : A.12 Remind client to take medication A.17 Check client s temperature A.18 Check client s pulse A.19 Check
Assisting with Medication, Checking vital Signs Duty: Assist Client with Personal Hygiene Task : A.12 Remind client to take medication A.17 Check client s temperature A.18 Check client s pulse A.19 Check
Quiz name: Chapter 13 Test Review - Fluids
 Name: Quiz name: Chapter 13 Test Review - Fluids Date: 1. All fluids are A gases B liquids C gasses or liquids D non-metallic E transparent 2. 1 Pa is A 1 N/m B 1 m/n C 1 kg/(m s) D 1 kg/(m s 2 ) E 1 N/m
Name: Quiz name: Chapter 13 Test Review - Fluids Date: 1. All fluids are A gases B liquids C gasses or liquids D non-metallic E transparent 2. 1 Pa is A 1 N/m B 1 m/n C 1 kg/(m s) D 1 kg/(m s 2 ) E 1 N/m
Chapter 10. Physical Characteristics of Gases
 Chapter 10 Physical Characteristics of Gases Kinetic Molecular Theory An understanding of the behavior of atoms that make up matter Ideal gas: an imaginary gas that perfectly fits all assumptions of the
Chapter 10 Physical Characteristics of Gases Kinetic Molecular Theory An understanding of the behavior of atoms that make up matter Ideal gas: an imaginary gas that perfectly fits all assumptions of the
The Application of Temperature and/or Pressure Correction Factors in Gas Measurement
 The Application of Temperature and/or Pressure Correction Factors in Gas Measurement COMBINED BOYLE S CHARLES GAS LAWS To convert measured volume at metered pressure and temperature to selling volume at
The Application of Temperature and/or Pressure Correction Factors in Gas Measurement COMBINED BOYLE S CHARLES GAS LAWS To convert measured volume at metered pressure and temperature to selling volume at
Operating Instructions Instrucciones de funcionamiento
 Wrist Blood Pressure Monitor Monitor de Presión Arterial de Muñeca Operating Instructions Instrucciones de funcionamiento Model No. EW3003 Modelo No. EW3003 For questions or assistance with your blood
Wrist Blood Pressure Monitor Monitor de Presión Arterial de Muñeca Operating Instructions Instrucciones de funcionamiento Model No. EW3003 Modelo No. EW3003 For questions or assistance with your blood
1. Air is blown through a pipe AB at a rate of 15 litre per minute. The cross-sectional area of broad
 Keshaw Classes IIT/JEE Medical Classes 5-A 11028 / 9, WEA, Sat Nagar, Karol Bagh New Delhi-110005 Mob:9910915514,9953150192 Ph:011-45660510 E-mail : keshawclasses@gmail.com Web:www.keshawclasses.com Solids
Keshaw Classes IIT/JEE Medical Classes 5-A 11028 / 9, WEA, Sat Nagar, Karol Bagh New Delhi-110005 Mob:9910915514,9953150192 Ph:011-45660510 E-mail : keshawclasses@gmail.com Web:www.keshawclasses.com Solids
Chapter 5. Nov 6 1:02 PM
 Chapter 5 Nov 6 1:02 PM Expand to fill their containers Fluid motion (they flow) Have low densities (1/1000 the density of equivalent liquids or solids) Compressible Can Effuse and Diffuse Effuse: The
Chapter 5 Nov 6 1:02 PM Expand to fill their containers Fluid motion (they flow) Have low densities (1/1000 the density of equivalent liquids or solids) Compressible Can Effuse and Diffuse Effuse: The
3 1 PRESSURE. This is illustrated in Fig. 3 3.
 P = 3 psi 66 FLUID MECHANICS 150 pounds A feet = 50 in P = 6 psi P = s W 150 lbf n = = 50 in = 3 psi A feet FIGURE 3 1 The normal stress (or pressure ) on the feet of a chubby person is much greater than
P = 3 psi 66 FLUID MECHANICS 150 pounds A feet = 50 in P = 6 psi P = s W 150 lbf n = = 50 in = 3 psi A feet FIGURE 3 1 The normal stress (or pressure ) on the feet of a chubby person is much greater than
REF CH-302B IDENTIFICATION OF PARTS
 English TM INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR REF CH-302B IDENTIFICATION OF PARTS Exhaust button Air-release system Bulb Systolic blood pressure display section Diastolic blood pressure/pulse
English TM INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR REF CH-302B IDENTIFICATION OF PARTS Exhaust button Air-release system Bulb Systolic blood pressure display section Diastolic blood pressure/pulse
and its weight (in newtons) when located on a planet with an acceleration of gravity equal to 4.0 ft/s 2.
 1.26. A certain object weighs 300 N at the earth's surface. Determine the mass of the object (in kilograms) and its weight (in newtons) when located on a planet with an acceleration of gravity equal to
1.26. A certain object weighs 300 N at the earth's surface. Determine the mass of the object (in kilograms) and its weight (in newtons) when located on a planet with an acceleration of gravity equal to
Exercises The Atmosphere (page 383) 20.2 Atmospheric Pressure (pages )
 Exercises 20.1 The Atmosphere (page 383) 1. The energizes the molecules in Earth s atmosphere. 2. Why is gravity important to Earth s atmosphere? 3. What would happen to Earth s atmosphere without the
Exercises 20.1 The Atmosphere (page 383) 1. The energizes the molecules in Earth s atmosphere. 2. Why is gravity important to Earth s atmosphere? 3. What would happen to Earth s atmosphere without the
DO NOT, under any circumstances, throw this away! This packet MUST be saved for the final exam.
 Name: Period: Unit 2 Packet Energy and States of Matter Unit 2 Packet Contents Sheet (This Paper!) Unit 2 Objectives Notes: Kinetic Molecular Theory of Gases- 3 pgs (with Behavior of Gases Reading, and
Name: Period: Unit 2 Packet Energy and States of Matter Unit 2 Packet Contents Sheet (This Paper!) Unit 2 Objectives Notes: Kinetic Molecular Theory of Gases- 3 pgs (with Behavior of Gases Reading, and
BLOOD PRESSURE SENSOR 0377i
 BLOOD PRESSURE SENSOR 0377i USER S GUIDE CENTRE FOR MICROCOMPUTER APPLICATIONS http://www.cma-science.nl Short description The Blood Pressure sensor 0377i is used to measure arterial blood pressure in
BLOOD PRESSURE SENSOR 0377i USER S GUIDE CENTRE FOR MICROCOMPUTER APPLICATIONS http://www.cma-science.nl Short description The Blood Pressure sensor 0377i is used to measure arterial blood pressure in
Slide 5 / What is the difference between the pressure on the bottom of a pool and the pressure on the water surface? A ρgh B ρg/h C ρ/gh D gh/ρ
 Slide 1 / 47 1 Two substances mercury with a density 13600 kg/m3 and alcohol with a density 800 kg/m3 are selected for an experiment. If the experiment requires equal masses of each liquid, what is the
Slide 1 / 47 1 Two substances mercury with a density 13600 kg/m3 and alcohol with a density 800 kg/m3 are selected for an experiment. If the experiment requires equal masses of each liquid, what is the
LAB 7. ROTATION. 7.1 Problem. 7.2 Equipment. 7.3 Activities
 LAB 7. ROTATION 7.1 Problem How are quantities of rotational motion defined? What sort of influence changes an object s rotation? How do the quantities of rotational motion operate? 7.2 Equipment plumb
LAB 7. ROTATION 7.1 Problem How are quantities of rotational motion defined? What sort of influence changes an object s rotation? How do the quantities of rotational motion operate? 7.2 Equipment plumb
