After having worked out this lecture, you will be able to describe the oxygen cascade and to calculate the inspiratory and the alveolar partial
|
|
|
- Josephine Johnston
- 5 years ago
- Views:
Transcription
1 1
2 After having worked out this lecture, you will be able to describe the oxygen cascade and to calculate the inspiratory and the alveolar partial pressure of oxygen. You will have understood the process of oxygen diffusion and the importance of intra- and extrapulmonary shunts. Moreover, you will have come acquainted with the principles and concepts of oxygen transport. Furthermore, you will be able to calculate oxygen content, delivery and consumption. After all, this will have led to a profound understanding of the available strategies that are commonly used to treat disturbances in the oxygen cascade. 2
3 Our life is critically dependent upon the continuous availability of substantial amounts of oxygen as: 1) Under normal conditions, a normal adult consumes 3-4 ml oxygen / kg / min (i.e., 300 ml/min for a body weight of 75kg). 2) Our body does not possess relevant stores for oxygen. When breathing air, approximately only 1550 ml of oxygen reserves are available. After having saturated a patient with 100% oxygen, the oxygen reserves are three times higher, but are still not sufficient to allow the tolerance of longer periods of oxygen shortage. 3
4 1) Most of the vital organs are not able to tolerate hypoxia for longer time periods. 4
5 The purpose of the cardio-pulmonary system is to extract oxygen from the inhaled air and to deliver it to the mitochondria of the cells. Several organs are involved in this vital task: the lungs, the blood, the heart and the vessels. 5
6 During the way of the oxygen from atmosphere to mitochondria, the oxygen tension is constantly declining, from 159 mmhg in the atmosphere (at sea level) to 4-23mmHg (within the mitochondria). The decline of the oxygen tension is described by the term oxygen cascade and results from dilution, extraction and/or other losses. 6
7 The first determinant of oxygen uptake into the lungs is the inspiratory partial pressure of oxygen, PiO 2. 7
8 Inhaled oxygen is a gas that exerts a partial pressure (PiO 2 ), which is according to Dalton s law - determined by the fraction of inspired oxygen (Fi gas, or FiO 2 ) and the prevailing environmental/atmospheric/barometric pressure (i.e., the pressure that is exerted by all gases being present in a gas mixture (P total, or P B ). At sea level, the atmospheric pressure is 760mmHg, and the concentration of oxygen in inhaled air is 21% (20.94% to be exact). This results in a PiO 2 of 760 x 0.21 = 159 mmhg. In contrast, if pure oxygen is inhaled, this results in a PiO 2 of 760 x 1.0 = 760 mmhg. Of note, these calculations are made for dry oxygen at 0 C (STPD = standard temperature pressure dry conditions). 8
9 The first decline in oxygen tension occurs due to the dilution of inspired by water vapor. Within the upper airways, the inspired air is humified with water vapor. Hence, the barometric or total pressure of the inhaled gas mixture is now exerted by all inhaled gases present in this mixture PLUS water vapor). Hence, the gases now exert a total pressure that is equal to the barometric pressure reduced by the partial pressure of saturated water vapour (P b -P H2O ). The PiO 2 can therefore be re-calculated as: (760-47) x = 149mmHg. These calculation is made for water-saturated oxygen at 37 C (BTPD = body temperature pressure dry conditions). 9
10 The dependency of the PiO 2 on the concentration of inhaled oxygen gives us an excellent and easily available opportunity to increase PiO 2 by increasing FiO 2. This can be achieved in a stepwise manner by using nasal probes, oxygen masks, oxygen masks with reservoirs or as a last step mechanical ventilation by means of an endotracheal tube. Only this last measure allows the delivery of a true FiO 2 of 100%. 10
11 As already described, the PiO 2 is not only dependent upon the FiO 2, but also on the barometric pressure (P B ). Of note, the barometric pressure steadily decreases with increasing altitude, whereas the concentration of oxygen (i.e., FiO 2 ) is always constant. 11
12 The importance of P B is illustrated in this table. At sea level, the P B is 760mmHg which results in a PiO 2 of 149mmHg (with a FiO 2 of 21%). At the approximate altitude of the summit of the Mount Everest, P B is only 247mmHg. This results in a PiO 2 of only 42mmHg and equals the PiO 2 that would be achieved at sea level if the FiO 2 would be as low as 5.9%. In contrast, to achieve a sea level of PiO 2 at the Mount Everest, one would have to breathe a FiO 2 of 74.5% in order to compensate for the low P B. This is the reason why climbers usually use oxygen masks in these altitudes. At an altitude of m, P B equals the partial pressure that is exerted by water vapour at body temperature. At this point (called the Armstrong-limit), the blood begins to boil. Therefore, astronauts (and also Felix Baumgartner for his supersonic jump from an altitude of 38km) have to wear space suits. 12
13 The above-described theoretical considerations are strikingly illustrated by this investigation in Mount Everest climbers. Their blood gas values(documented at an altitude of 8400m) are depicted in this table. 14
14 The uptake of oxygen into the alveoli represents the next step in the oxygen cascade. 15
15 Within the alveoli, the inhaled air mixes with carbon dioxide. Hence, the alveolar partial pressure of oxygen (PAO 2 ) equals the PiO 2 that is however reduced by the alveolar partial pressure of CO 2 (PACO 2 ) PACO 2 can be estimated by the arterial partial pressure of CO 2 (PaCO 2 ), which can be measured with a blood gas analyzer. PAO 2 is the calculated from the ALVEOLAR AIR EQUATION: PAO 2 = PIO 2 PaCO 2 /RQ. RQ is the respiratory quotient, which represents the amount of carbon dioxide produced for any given amount of oxygen metabolized. The RQ is dependent upon the carbon content of food (1.0 for pure carbohydrates metabolization, 0,7 for pure fat metabolization). With the assumption that the RQ is 0.8, the PAO2 will then be 149 (40/0.9) = 105mmHg. This corresponds to a fraction of alveolar oxygen of 14%. For scientific reasons, the PAO 2 has to be determined with a more complex formula that however requires the determination of exhaled oxygen and exhaled carbon dioxide. The alveolar air equation illustrates why hypoventilation (with an increase in the PACO 2 ) causes hypoxia and why hyperventilation (with a decrease in PACO 2 ) is a compensatory response to alveolar hypoxia in high altitude. 16
16 PAO 2 is also dependent upon oxygen consumption (VO 2 ). With a high oxygen consumption, it is difficult to build up a high PAO 2 (as the oxygen is immediately utilized). That means that the alveolar ventilation has to increase when the PAO 2 is to be maintained in the presence of a high VO 2. If the alveolar ventilation is not compensatorily increased, then any increase in VO 2 will lead to a decrease in PAO 2. 17
17 The next barrier oxygen has to overcome is the transport of oxygen from the alveolus into the alveolar capillary. This is achieved by passive diffusion. 18
18 The diffusion of oxygen is affected by several barriers. First, in order to get into contact with the alveolar capillaries, oxygen has to reach the borders of the gas space within the alveolus. However, as all gases are distributed uniformly within the alveolus, this theoretical concern does not significantly impair oxygen diffusion. The next barrier oxygen has to pass is the alveolar lining fluid which is very thin in healthy conditions in order not to impair gas exchange. Also the subsequent tissue barrier is very thin, consisting of the alveolar epithelium, the interstitial space and the capillary endothelium. Then, oxygen has to pass the plasma layer to finally diffuse into the erythrocytes where oxygen will be taken up by hemoglobin. 19
19 The diffusion of oxygen into the alveolar capillary can be quantitatively described by Fick s law. Fick's law postulates that the flux of a given substance (Δn/Δt) goes from regions of high concentration to regions of low concentration, with a magnitude that is proportional to the concentration gradient (Δc). In the case of oxygen, this corresponds with the gradient between PAO 2 and PcapO 2. Moreover, the flux is proportional to the diffusion coefficient (D) (that is specific for a given substance) and to the area (A) that is available for diffusion. In contrast, the flux is indirectly proportional to the distance the particles have to move from one side of the barrier to the other side (χ, wall thickness). Several pathological conditions severly affect diffusion of oxygen across the alveolar-capillary membrane: The destruction of alveoli in emphysema reduces the area of the alveolarcapillary membrane. Alveolar hypoxia decreases the concentration gradient. Both pulmonary edema and fibrosis increase wall thickness. 20
20 The diffusion capacity can be estimated by approximative formulas, both for men and women. The diffusion capacity is dependent amongst others - upon lung volume, posture, age, sex and race. 21
21 Although the diffusion barriers are minimal in healthy conditions, there is a considerable difference between the PO2 in the alveoli and the PO 2 in the arterial blood: the alveolar-arterial PO 2 difference (ΔAaPO 2 ). It has already been elaborated that PAO 2 is approximately 105mmHg. As a rule of thumb, PaO 2 equals *age. This results in an ΔAaPO 2 of 15-35mmHg. 22
22 Part of the ΔAaPO 2 can be explained with small ventilation perfusion abnormalities. 23
23 Admixture of venous blood to arterial blood is another contributor to the ΔAaPO 2. Venous admixture can be caused by anatomical/extrapulmonary shunts, e.g. Thebesian veins, bronchial veins or shunts owing to congenital heart disease. Venous admixture can also be caused by intrapulmonary shunts, e.g. due to atelectasis, pneumonia or ARDS. In these cases, parts of the lungs are still perfused, but not ventilated anymore. The V/Q quotient is < 1. 24
24 The shunt fraction (Qs/Qt) represents the fraction of the shunt flow (Qs) with respect to the total blood flow (Qt). The shunt fraction can be calculated with the formula that is elaborated on this slide. For the calculation of the shunt fraction, one has to determine the oxygen content of the arterial blood (CaO 2 ), that of the mixed venous blood (CvO 2 ) and that of the pulmonary capillary blood (CcO 2 ). CaO 2 is calculated from a blood gas analysis of arterial blood. CvO 2 is calculated from a blood gas analysis of mixed venous, i.e. pulmonary arterial, blood (obtained from a pulmonary arterial catheter). CcO 2 is calculated from a blood gas analysis of pulmonary capillary blood (obtained from a pulmonary arterial catheter in wedge position and with inflated wedge balloon). For the calculation of oxygen content, see below. 25
25 The venous admixture has a significant impact on oxygenation. Of note, the relationship between arterial oxygen content and PaO 2 follows a sigmoidal shape. This means that already minor changes in arterial oxygen content result in significant changes in PaO 2. In contrast, the relationship between arterial carbon dioxide content and PaCO 2 is nearly linear and much steeper. That means that even major changes in CaCO 2 are associated with only minimal effects on PaCO 2. In summary, venous admixture immediately results in arterial hypoxia whereas PaCO 2 is virtually not affected. Venous admixture reduces the arterial O2 content and increases the arterial CO2 content. Because PaO2 is usually on the flat part of the haemoglobin dissociation curve, => small reduction in O2 content leads to large drop in PaO2 => increased A-a gradient Because CO2 dissociation curve is usually steep and more linear, 26
26 The dependency of PaO 2 upon CaO 2 also explains that with increasing shunt fractions, it becomes impossible to treat hypoxia with added inspired oxygen. This is demontrated by the iso-shunt diagram that illustrates the dependency of PaO 2 on FiO 2 for different shunt fractions. 27
27 The last step of the oxygen cascade consists of the transport of oxygen to the cells. 28
28 Within the blood, oxygen is transported in two ways: 1) Dissolved in plasma (as phsyical solution) 2) Chemically bound to hemoglobin within the erythrocytes. The amount of oxygen that is dissolved in plasma can be quantified with the law of Henry. This law states: "At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid." Hence, the concentration of a dissolved gas equals the product of the partial pressure exerted by this gas and the solulibility coefficient of this specific gas. For oxygen, this co-efficient is as low as ml / ml blood / mmhg. With a PO 2 of 100 mmhg, only 0.3 ml oxygen are dissolved per 100ml blood. Even with a FiO 2 of 1, only 1.9ml oxygen are dissolved under normobaric conditions. Hence, transport of oxygen by solution is negligible. The law of Henry also explains why the relationship between oxygen content and 29
29 Most of the oxygen is transported within the erythrocytes by chemical binding to hemoglobin. Hemoglobin is a complex molecule consisting of two α- and two β-subunits. The three-dimensional structure of hemoglobin is best described as a pair of identical αβ dimers (α 1 β 1 and α 2 β 2 ) that associate to form the hemoglobin tetramer. Each of the subunits contains a heme group that consists of a porphyrin ring with an iron atom in the center. 30
30 Oxygen is bound to the iron atom within the heme group. The binding of oxygen to an iron atom results in conformational changes of the hemoglobin molecule which facilitate the binding of oxygen to the iron atom of the second hemoglobin subunit. Of note, hemoglobin containing one oxygen molecule binds oxygen 3 times as strongly as does fully deoxygenated hemoglobin. The binding of oxygen to the iron atom in the second subunit then facilitates the binding of oxygen to the third subunit etc. The remaining open fourth binding site has an affinity for oxygen more than 20- fold as great as that of fully deoxygenated hemoglobin binding its first oxygen. This binding behaviour of hemoglobin is called cooperative binding. 31
31 As outlined before, binding of the 1 st O 2 molecule increases the affinity of haemoglobin for oxygen, making it easier for the next oxygen molecule to bind. This binding behaviour results in a sigmoidal shape of the oxyhemoglobin dissociation curve, which describes the relationship between partial pressure of oxygen and the saturation of hemoglobin with oxygen. The sigmoidal shape is associated with significant advantages for the oxygen transport: Arterial blood operates in the upper flat part of the dissociation curve. In this part of the curve, (i.e., in regions with high PaO 2 ), hemoglobin has a high affinity for oxygen. Hence, decreases in PaO 2 are tolerated over a relatively wide range without significant decreases in saturation. Moreover, a maximal saturation is already achieved at normal PaO 2. Last, oxygen uptake in the lungs facilitated. In contrast, blood within the capillary regions operates in the steep part of the curve. In this part of the curve, already minor changes in PaO 2 result in significant decreases of oxygen saturation. Hence, in the presence of a low PaO 2 (which is typical for the capillary bed), hemoglobin has a low affinity for oxygen. This facilitates oxygen delivery to the peripheral tissues. 32
32 Interestingly, the position of the hemoglobin dissociation curve can be shifted either to the right or the left. The position of the hemoglobin dissociation curve can be quantified with the P50-value. The P50 is the PaO 2 with which a 50%-saturation of hemoglobin can be achieved. Under normal conditions, the P50 is 27mmHg. A right shift of the hemoglobin dissociation curve means that the P50 is higher than 27mmHg. In other words, a higher PaO 2 is needed to achieve a 50% saturation. This is the consequence of a lower affinity of hemoglobin for oxygen which means that the release of oxygen from the hemoglobin molecule is facilitated. In contrast, a left shift of the hemoglobin dissociation curve means that the P50 is lower than 27mmHg. In other words, a a 50% saturation is already achieved with a lower PaO 2. This is the consequence of a higher affinity of hemoglobin for oxygen which means that on the one hand, the release of oxygen from the hemoglobin molecule is impaired. On the other hand, the binding of oxygen to hemoglobin within the lungs is facilitated. 33
33 The position of the hemoglobin dissociation curve can be shifted by several variables. A right shift is caused by a decrease in ph, an increase in pco 2, an increase in temperature, and an increase in 2,3- diphosphoglycerate. A left shift is caused by an increase in ph, a decrease in pco 2, a decrease in temperature, and a decrease in 2,3- diphosphoglycerate. 34
34 The Bohr effect is a physiological phenomenon first described in 1904 by the Danish physiologist Christian Bohr, stating that hemoglobin's oxygen binding affinity is inversely related both to acidity and to the concentration of carbon dioxide. Within the lungs, there is a high ph and low CO 2 -tensions. This results in a high affinity of hemoglobin for oxygen and hence facilitates oxygen uptake by hemoglobin. Within the metabolically active peripheral tissues however, there is a relatively low ph and high CO 2 -tensions. This results in a low affinity of hemoglobin for oxygen and hence facilitates oxygen release from hemoglobin. 35
35 36
36 Of note, hemoglobin does not only transport oxygen, but also carbon dioxide. The Haldane effect is a property of hemoglobin first described by John Scott Haldane, stating that the affinity of hemoglobin for carbon dioxide is inversely related to the oxygen concentration. In other words, deoxygenation of the hemoglobin increases its ability to carry carbon dioxide. In contrast, oxygenated blood has a reduced capacity for carbon dioxide. Within the oxygen-rich capillaries of the lung, the Haldane effect facilitates the release of carbon dioxide from hemoglobin to plasma. Within oxygen-poor capillaries of the peripheral tissues however, the Haldane effect results in a high affinity of hemoglobin for carbon dioxide and thus promotes the uptake of carbon dioxide by hemoglobin and the removal of carbon dioxide from the peripheral tissues. 37
37 Given the absence of mitochondria within erythrocytes, red-cell metabolism depends solely on glycolysis, and 2,3- bisphosphoglycerate is a normal metabolic intermediate. Normally, 1,3-bisphosphoglycerate is converted to 3- phosphoglycerate, producing one ATP molecule. 1,3-Bisphosphoglycerate can also be converted to 2,3-bisphosphoglycerate by bisphosphoglycerate synthase through a minor pathway without producing ATP. This pathway is called Rapoport-Luebering-shun t. In most cells, the concentration of 2,3-bisphosphoglycerate is very low, because of potent feedback inhibition of bisphosphoglycerate synthase. However, in red cells, 2,3-bis-phosphoglycerate becomes sequestered by binding to deoxyhemoglobin; without the normal inhibition, 2,3-bisphosphoglycerate can therefore accumulate in high concentrations. The binding of 2,3-bisphosphoglycerate to hemoglobin changes the conformation of the hemoglobin molecules and reduces the affinity of hemoglobin for oxygen. The binding of 2,3-bisphosphoglycerate also lowers the intracellular ph and further enhances the Bohr effect. The P 50 increases directly with the 2,3-bisphosphoglycerate concentration, which increases whenever the availability of oxygen is diminished (as in hypoxia or anemia) or the flux through glycolysis is stimulated (as in alkalosis). Conversely, the 2,3-bisphosphoglycerate concentration is reduced in aging red cells and under conditions of hyperoxia or inhibition of glycolysis (by acidosis or hypophosphatemia). Other organophosphates and anions, such as chloride, also compete with 2,3-bisphosphoglycerate for binding sites on hemoglobin. Hence, their presence can reduce the regulatory effect of 2,3-bisphosphoglycerate on oxygen affinity. In normal red cells, 2,3-DPG seems to be of minor significance. In contrast, the influence of 2,3-DPG becomes relevant during blood transfusion. Packed red blood cells have to be stored under hypothermic conditions which leads to the inhibition of glycolysis within the erythrocytes. As a consequence, 2,3-DPG concentrations are reduced for up to 48 hours after transfusion. This can result in a significant left shift of the hemoglobin dissociation curve. 38
38 Of note, different hemoglobin molecules are characterized by different dissociation curves. In comparison to adult hemoglobin, fetal hemoglobin shows a characteristic leftward shift that facilitates oxygen uptake within the placenta where oxygen tensions are low. Also myoglobin shows a significant left shift. This guarantees that oxygen within the muscles is only released during exercise, when muscle oxygen tension drops. Carboxyhemoglobin has the steepest and most left-shifted dissociation curve. This results in an extremely high affinity of hemoglobin for carbon monoxide and explains why oxygen is easily displaced from hemoglobin in the presence of carbon monoxide. 39
39 Oxygen saturation of hemoglobin can be measured either non-invasively (by pulse-oximetry) or invasively, by analysing arterial blood. Pulse oxymeters usually operate with two wavelengths so that only the concentrations of oxygenated and de-oxygenated hemoglobin can be measured. The resulting peripheral oxygen saturation is % In contrast, blood gas analysers operate with multiple wavelengths so that not only the concentrations of oxgenated and de-oxygenated hemoglobin can be measured, but also the concentrations of methemoglobin, carboxyhemoglobin and sulfhemoglobin. Hence, the resulting arterial oxygen saturation is usually lower than the peripheral oxygen saturation and reaches 96-98%. 40
40 The oxygen binding capacity of hemoglobin is represented by Hüfner s constant. Hüfner s constant describes how much oxygen (in ml) can be transported by 1g of hemoglobin. The exact value for this constant is still controversial and differs between authors. In theory, each gram of Hb can bind 1.39 ml of oxygen. However, in practice, the presence of abnormal forms of Hb, such as carboxyhaemoglobin and methemoglobin, reduces the oxygen binding capacity of Hb to 1.34 or 1.31 ml/g. In any case, this demonstrates that oxygen transport by chemical binding to hemoglobin is very effective. You will remember that in 100 ml blood, only 0.3 ml of oxygen are physically dissolved. 41
41 With all the above information, one can now easily calculate oxygen content of the blood. The oxygen content is the sum of dissolved and chemically bound oxygen. Within the arterial blood, the calculation with the given variables results in an arterial oxygen content of 20 ml/dl. Within mixed venous blood, oxygen content is 15 ml/dl. This results in an arteriovenous oxygen difference of 5 ml/dl and in an oxygen extraction rate of 25%. 42
42 As you can easily see from the formulas for the calculation of oxygen content, the hemoglobin concentration has a huge impact on oxygen content. As depicted on the left figure, the arterial oxygen content decreases dramatically in anemia. This is further illustrated by the table at the left bottom. The critical dependency of oxygen content upon the hemoglobin concentration is also the reason why endurance athletes regularly perform trainings at high altitude (to increase endogenous erythropoeitin levels) and why doping with erythropoeitin is so effective. 43
43 Given the influences of various factors on oxygen status, it is of utmost importance to distinguish between different pathologic conditions. Hypoxia describes a condition in which PaO 2 is too low. Hypoxygenation describes the condition of inadequate saturation of hemoglobin with oxygen. Hypoxemia is a condition of inadequately low arterial oxygen content. Ischemia depicts a situation in which there is no oxygen supply due to an interruption of blood flow. Of note, hypoxemia can be either hypoxic of origin (i.e., due to a low PaO 2 or a low SaO 2 ), anemic (due to a low hemoglobin concentration) or toxic (due to the presence of abnormal hemoglobins). It is of clinical relevance that the tolerance for the different hypoxemias differ. In fact, an anemic hypoxemia is tolerated best as in anemia, 2,3-DPG concentrations rise and the hemoglobin dissociation curve is shifted to the right. 44
44 As a matter of fact, oxygenated blood has to be transported to the peripheral tissues in order to be able to provide an adequate oxygen supply. This can be quantified by calculating oxygen delivery which represents the product of cardiac output and arterial oxygen content. Given the reference values for cardiac output and oxygen content, it becomes evident that 1000 ml oxygen are transported per minute within the arterial blood. Of note, 10 is a correction factor that allows the conversion of metric units. Accordingly, oxygen consumption can be calculated as the product of arteriovenous oxygen difference and cardiac output. Oxygen consumption is usually 250 ml per minute. Hence, under normal conditions, only 25% of the delivered oxygen are extracted from the arterial blood ( oxygen extraction rate ). 45
45 As outlined above, oxygen extraction rate under normal conditions is very low. Hence, there is a considerable reserve for increasing the extraction rate. In fact, in conditions of an inadequately low oxygen delivery, the tissues respond with an increase in oxygen extraction. This is the only way to ensure oxygenation as oxygen consumption - at least initially remains constant. The increase in oxygen extraction is reflected by a decrease in the oxygen content of the blood returning to the heart. This underlines the importance of central venous or mixed venous oxygen saturation in the estimation of the adequacy of oxygen delivery. 46
46 The very last step of the oxygen cascade is represented by the diffusion of oxygen from the capillaries to the cells. Also this process obeys to Fick s law (see above) and can be affected by several factors that are depicted on the slide. In particular, the diffusion distance is of major relevance. This explains why critically ill patients with a capillary leak and resulting interstitial edema are particularly prone to tissue hypoxia. 47
47 In summary, this figure comprehensively illustrates the oxygen cascade. 48
48 Understanding the major steps of the oxygen cascade helps us to target our therapeutic strategies that aim to prevent tissue hypoxia. In any case, a sufficient oxygen saturation has to be maintained, e.g. by administering oxygen, providing airway maintenance and ventilating the patient. In order to maximise oxygen delivery, cardiac output and hemoglobin concentration have to be optimized. Unfortunately, no therapeutic strategies are available at the moment that could specifically help to optimize oxygen delivery within the microcirculation or the oxygen uptake by the mitochondria. Of note, oxygen release from hemoglobin in the peripheral tissues is affected by the position of the hemoglobin dissociation curve which should receive more attention in our management strategies. 49
49 50
50 51
51 52
Fysiologie van de ademhaling - gasuitwisseling
 What you will learn in this lecture... Lessenreeks co s 014-015 Fysiologie van de ademhaling - gasuitwisseling Professor Dr. Steffen Rex Department of Anesthesiology University Hospitals Leuven Department
What you will learn in this lecture... Lessenreeks co s 014-015 Fysiologie van de ademhaling - gasuitwisseling Professor Dr. Steffen Rex Department of Anesthesiology University Hospitals Leuven Department
CHAPTER 6. Oxygen Transport. Copyright 2008 Thomson Delmar Learning
 CHAPTER 6 Oxygen Transport Normal Blood Gas Value Ranges Table 6-1 OXYGEN TRANSPORT Oxygen Dissolved in the Blood Plasma Dissolve means that the gas maintains its precise molecular structure About.003
CHAPTER 6 Oxygen Transport Normal Blood Gas Value Ranges Table 6-1 OXYGEN TRANSPORT Oxygen Dissolved in the Blood Plasma Dissolve means that the gas maintains its precise molecular structure About.003
Respiratory physiology II.
 Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
PICU Resident Self-Study Tutorial The Basic Physics of Oxygen Transport. I was told that there would be no math!
 Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
Oxygen and Carbon dioxide Transport. Dr. Laila Al-Dokhi
 Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
RESPIRATORY GAS EXCHANGE
 RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
Section Three Gas transport
 Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
Respiration (revised 2006) Pulmonary Mechanics
 Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
Rodney Shandukani 14/03/2012
 Rodney Shandukani 14/03/2012 OXYGEN THERAPY Aerobic metabolism accounts for 90% of Oxygen consumption by tissues. generates ATP by oxidative phosphorylation. Oxygen cascade: Oxygen exerts a partial pressure,
Rodney Shandukani 14/03/2012 OXYGEN THERAPY Aerobic metabolism accounts for 90% of Oxygen consumption by tissues. generates ATP by oxidative phosphorylation. Oxygen cascade: Oxygen exerts a partial pressure,
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation
 Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
Gas exchange. Tissue cells CO2 CO 2 O 2. Pulmonary capillary. Tissue capillaries
 Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
I Physical Principles of Gas Exchange
 Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Lung Volumes and Capacities
 Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing
 Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D.
 Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
The Physiologic Basis of DLCO testing. Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan
 The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
Gases and Respiration. Respiration Overview I
 Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
HCO - 3 H 2 CO 3 CO 2 + H H H + Breathing rate is regulated by blood ph and C02. CO2 and Bicarbonate act as a ph Buffer in the blood
 Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
Respiratory Medicine. A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics. Alveolar Gas Equation. See online here
 Respiratory Medicine A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics See online here Alveolar gas equation helps to calculate the partial pressure of oxygen in alveoli and A-a gradient is the
Respiratory Medicine A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics See online here Alveolar gas equation helps to calculate the partial pressure of oxygen in alveoli and A-a gradient is the
Table of Contents. By Adam Hollingworth
 By Adam Hollingworth Table of Contents Oxygen Cascade... 2 Diffusion... 2 Laws of Diffusion... 2 Diffusion & Perfusion Limitations... 3 Oxygen Uptake Along Pulmon Capillary... 4 Measurement of Diffusing
By Adam Hollingworth Table of Contents Oxygen Cascade... 2 Diffusion... 2 Laws of Diffusion... 2 Diffusion & Perfusion Limitations... 3 Oxygen Uptake Along Pulmon Capillary... 4 Measurement of Diffusing
Section Two Diffusion of gases
 Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Alveolus and Respiratory Membrane
 Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Respiratory System Physiology. Dr. Vedat Evren
 Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Physiology Unit 4 RESPIRATORY PHYSIOLOGY
 Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
Blood gas adventures at various altitudes. Friedrich Luft Experimental and Clinical Research Center, Berlin-Buch
 Blood gas adventures at various altitudes Friedrich Luft Experimental and Clinical Research Center, Berlin-Buch Mount Everest 8848 M Any point in bird watching here? Respiration is gas exchange: the process
Blood gas adventures at various altitudes Friedrich Luft Experimental and Clinical Research Center, Berlin-Buch Mount Everest 8848 M Any point in bird watching here? Respiration is gas exchange: the process
By: Aseel Jamil Al-twaijer. Lec : physical principles of gas exchange
 By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
660 mm Hg (normal, 100 mm Hg, room air) Paco, (arterial Pc02) 36 mm Hg (normal, 40 mm Hg) % saturation 50% (normal, 95%-100%)
 148 PHYSIOLOGY CASES AND PROBLEMS Case 26 Carbon Monoxide Poisoning Herman Neiswander is a 65-year-old retired landscape architect in northern Wisconsin. One cold January morning, he decided to warm his
148 PHYSIOLOGY CASES AND PROBLEMS Case 26 Carbon Monoxide Poisoning Herman Neiswander is a 65-year-old retired landscape architect in northern Wisconsin. One cold January morning, he decided to warm his
RESPIRATORY REGULATION DURING EXERCISE
 RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
Chapter 13 The Respiratory System
 Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Respiration - Human 1
 Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
P215 Respiratory System, Part 2
 P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
VENTILATION AND PERFUSION IN HEALTH AND DISEASE. Dr.HARIPRASAD VS
 VENTILATION AND PERFUSION IN HEALTH AND DISEASE Dr.HARIPRASAD VS Ventilation Total ventilation - total rate of air flow in and out of the lung during normal tidal breathing. Alveolar ventilation -represents
VENTILATION AND PERFUSION IN HEALTH AND DISEASE Dr.HARIPRASAD VS Ventilation Total ventilation - total rate of air flow in and out of the lung during normal tidal breathing. Alveolar ventilation -represents
Respiratory System Study Guide, Chapter 16
 Part I. Clinical Applications Name: Respiratory System Study Guide, Chapter 16 Lab Day/Time: 1. A person with ketoacidosis may hyperventilate. Explain why this occurs, and explain why this hyperventilation
Part I. Clinical Applications Name: Respiratory System Study Guide, Chapter 16 Lab Day/Time: 1. A person with ketoacidosis may hyperventilate. Explain why this occurs, and explain why this hyperventilation
Pco2 *20times = 0.6, 2.4, so the co2 carried in the arterial blood in dissolved form is more than the o2 because of its solubility.
 Physiology, sheet #9 Oxygen, is first dissolved in the plasma and the cytosol of the rbc, we have around blood constitutes 7% of our body weight, oxygen, in the capillaries is present in the rbc s and
Physiology, sheet #9 Oxygen, is first dissolved in the plasma and the cytosol of the rbc, we have around blood constitutes 7% of our body weight, oxygen, in the capillaries is present in the rbc s and
Collin County Community College. Lung Physiology
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
AIIMS, New Delhi. Dr. K. K. Deepak, Prof. & HOD, Physiology AIIMS, New Delhi Dr. Geetanjali Bade, Asst. Professor AIIMS, New Delhi
 Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT
 UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION
UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION
Essential Skills Course Acute Care Module. Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook
 Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
GAS EXCHANGE & PHYSIOLOGY
 GAS EXCHANGE & PHYSIOLOGY Atmospheric Pressure Intra-Alveolar Pressure Inspiration 760 mm HG at Sea Level (= 1 atm) Pressure due to gases (N2, O2, CO2, Misc.) Pressure inside the alveolus (air sac) Phrenic
GAS EXCHANGE & PHYSIOLOGY Atmospheric Pressure Intra-Alveolar Pressure Inspiration 760 mm HG at Sea Level (= 1 atm) Pressure due to gases (N2, O2, CO2, Misc.) Pressure inside the alveolus (air sac) Phrenic
 Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
respiratory cycle. point in the volumes: 500 milliliters. for men. expiration, up to 1200 milliliters extra makes breathing Respiratory
 10 II. RESPIRATORY VOLUMES, CAPACITIES & PULMONARY FUNCTION TESTS Respiratory volume is the term used for various volumes of air moved by or associated with the lungs at a given point in the respiratory
10 II. RESPIRATORY VOLUMES, CAPACITIES & PULMONARY FUNCTION TESTS Respiratory volume is the term used for various volumes of air moved by or associated with the lungs at a given point in the respiratory
- How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have
 - How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have the highest blood flow of all organs, which makes them
- How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have the highest blood flow of all organs, which makes them
Respiratory Physiology. Adeyomoye O.I
 Respiratory Physiology By Adeyomoye O.I Outline Introduction Hypoxia Dyspnea Control of breathing Ventilation/perfusion ratios Respiratory/barometric changes in exercise Intra-pulmonary & intra-pleural
Respiratory Physiology By Adeyomoye O.I Outline Introduction Hypoxia Dyspnea Control of breathing Ventilation/perfusion ratios Respiratory/barometric changes in exercise Intra-pulmonary & intra-pleural
Chapter 13 The Respiratory System
 VI edit Pag 451-499 Chapter 13 The Respiratory System V edit. Pag 459-509 Tissue cell Alveoli of lungs Atmosphere 1 External respiration Ventilation or gas exchange between the atmosphere and air sacs
VI edit Pag 451-499 Chapter 13 The Respiratory System V edit. Pag 459-509 Tissue cell Alveoli of lungs Atmosphere 1 External respiration Ventilation or gas exchange between the atmosphere and air sacs
CHAPTER 3: The respiratory system
 CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
Respiratory System. Prepared by: Dorota Marczuk-Krynicka, MD, PhD
 Respiratory System Prepared by: Dorota Marczuk-Krynicka, MD, PhD Lungs: Ventilation Perfusion Gas Exchange - Diffusion 1. Airways and Airway Resistance (AWR) 2. Mechanics of Breathing and Lung (Elastic)
Respiratory System Prepared by: Dorota Marczuk-Krynicka, MD, PhD Lungs: Ventilation Perfusion Gas Exchange - Diffusion 1. Airways and Airway Resistance (AWR) 2. Mechanics of Breathing and Lung (Elastic)
Chapter 23. Gas Exchange and Transportation
 Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity and minor gases argon,
Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity and minor gases argon,
CHAPTER 3: The cardio-respiratory system
 : The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
: The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
Respiratory Lecture Test Questions Set 3
 Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
Chapter 23. Gas Exchange and Transportation
 Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity other minor gases argon,
Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity other minor gases argon,
Some major points on the Effects of Hypoxia
 Some major points on the Effects of Hypoxia Source: Kings College London http://www.kcl.ac.uk/teares/gktvc/vc/dental/year1/lectures/rbmsmajorpoints/effectsofhypoxia.htm Cells obtain their energy from oxygen.
Some major points on the Effects of Hypoxia Source: Kings College London http://www.kcl.ac.uk/teares/gktvc/vc/dental/year1/lectures/rbmsmajorpoints/effectsofhypoxia.htm Cells obtain their energy from oxygen.
Lung Volumes and Ventilation
 Respiratory System ssrisuma@rics.bwh.harvard.edu Lung Volumes and Ventilation Minute ventilation Volume of an inspired or expired air per minute = tidal volume (V T ) x respiratory rate Dead space ventilation
Respiratory System ssrisuma@rics.bwh.harvard.edu Lung Volumes and Ventilation Minute ventilation Volume of an inspired or expired air per minute = tidal volume (V T ) x respiratory rate Dead space ventilation
82 Respiratory Tract NOTES
 82 Respiratory Tract NOTES RESPIRATORY TRACT The respiratory tract conducts air to the lungs where gaseous exchange occurs. It is separated into air-conducting and respiratory (where gas exchange occurs)
82 Respiratory Tract NOTES RESPIRATORY TRACT The respiratory tract conducts air to the lungs where gaseous exchange occurs. It is separated into air-conducting and respiratory (where gas exchange occurs)
Respiratory System. Part 2
 Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
PROBLEM SET 9. SOLUTIONS April 23, 2004
 Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
For more information about how to cite these materials visit
 Author(s): John G. Younger, M.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/
Author(s): John G. Younger, M.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/
ALVEOLAR - BLOOD GAS EXCHANGE 1
 ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
RESPIRATORY PHYSIOLOGY. Anaesthesiology Block 18 (GNK 586) Prof Pierre Fourie
 RESPIRATORY PHYSIOLOGY Anaesthesiology Block 18 (GNK 586) Prof Pierre Fourie Outline Ventilation Diffusion Perfusion Ventilation-Perfusion relationship Work of breathing Control of Ventilation 2 This image
RESPIRATORY PHYSIOLOGY Anaesthesiology Block 18 (GNK 586) Prof Pierre Fourie Outline Ventilation Diffusion Perfusion Ventilation-Perfusion relationship Work of breathing Control of Ventilation 2 This image
Respiratory Anatomy and Physiology. Respiratory Anatomy. Function of the Respiratory System
 Respiratory Anatomy and Physiology Michaela Dixon Clinical Development Nurse PICU BRHFC Respiratory Anatomy Function of the Respiratory System - In conjunction with the cardiovascular system, to supply
Respiratory Anatomy and Physiology Michaela Dixon Clinical Development Nurse PICU BRHFC Respiratory Anatomy Function of the Respiratory System - In conjunction with the cardiovascular system, to supply
Yanal. Jumana Jihad. Jamil Nazzal. 0 P a g e
 2 Yanal Jumana Jihad Jamil Nazzal 0 P a g e note: this sheet was written and corrected according to the records from section 2 so you may find differences in the arrangement of topics from the records
2 Yanal Jumana Jihad Jamil Nazzal 0 P a g e note: this sheet was written and corrected according to the records from section 2 so you may find differences in the arrangement of topics from the records
2. State the volume of air remaining in the lungs after a normal breathing.
 CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
RESPIRATORY MONITORING AND OXIMETRY
 RESPIRATORY MONITORING AND OXIMETRY EE 471 F2016 Prof. Yasser Mostafa Kadah Introduction Respiratory monitoring includes measurement, evaluation, and monitoring of parameters of respiratory system, First
RESPIRATORY MONITORING AND OXIMETRY EE 471 F2016 Prof. Yasser Mostafa Kadah Introduction Respiratory monitoring includes measurement, evaluation, and monitoring of parameters of respiratory system, First
Circulatory And Respiration
 Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Respiratory system & exercise. Dr. Rehab F Gwada
 Respiratory system & exercise Dr. Rehab F Gwada Objectives of lecture Outline the major anatomical components & important functions of the respiratory system. Describe the mechanics of ventilation. List
Respiratory system & exercise Dr. Rehab F Gwada Objectives of lecture Outline the major anatomical components & important functions of the respiratory system. Describe the mechanics of ventilation. List
Pulmonary Circulation Linda Costanzo Ph.D.
 Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
IV. FROM AQUATIC TO ATMOSPHERIC BREATHING: THE TRACHEA & THE LUNG
 GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
The physiological basis of pulmonary gas exchange: implications for clinical interpretation of arterial blood gases
 ERJ Express. Published on October 16, 214 as doi: 1.1183/931936.39214 REVIEW IN PRESS CORRECTED PROOF The physiological basis of pulmonary gas exchange: implications for clinical interpretation of arterial
ERJ Express. Published on October 16, 214 as doi: 1.1183/931936.39214 REVIEW IN PRESS CORRECTED PROOF The physiological basis of pulmonary gas exchange: implications for clinical interpretation of arterial
For more information about how to cite these materials visit
 Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
OXYGEN PHYSIOLOGY AND PULSE OXIMETRY
 Louis Al-Saleem 5/4/13 OXYGEN PHYSIOLOGY AND PULSE OXIMETRY A very experienced senior resuscitation nurse approached me at work recently, and asked if there was any circulating academic evidence about
Louis Al-Saleem 5/4/13 OXYGEN PHYSIOLOGY AND PULSE OXIMETRY A very experienced senior resuscitation nurse approached me at work recently, and asked if there was any circulating academic evidence about
Recitation question # 05
 Recitation and Lab # 05 The goal of this recitations / labs is to review material related to the CV and respiratory lectures for the second test of this course. Info required to answer this recitation
Recitation and Lab # 05 The goal of this recitations / labs is to review material related to the CV and respiratory lectures for the second test of this course. Info required to answer this recitation
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1
 AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
Capnography in the Veterinary Technician Toolbox. Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA
 Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
αo 2 : solubility coefficient of O 2
 Version 2006 Dr. Puntarica Suwanprathes 1) Fick s law of diffusion 2) facts which limit gas transfer 3) diffusion capacity gas volume gaseous phase dissolved gas exert pressure*** Solubility of Gas C =P.
Version 2006 Dr. Puntarica Suwanprathes 1) Fick s law of diffusion 2) facts which limit gas transfer 3) diffusion capacity gas volume gaseous phase dissolved gas exert pressure*** Solubility of Gas C =P.
4. For external respiration to occur effectively, you need three parameters. They are:
 Self Assessment Module D Name: ANSWER KEY 1. Hypoxia should be assumed whenever the PaO 2 is below 45 mm Hg. 2. Name some clinical conditions that will result in hyperventilation (respiratory alkalosis).
Self Assessment Module D Name: ANSWER KEY 1. Hypoxia should be assumed whenever the PaO 2 is below 45 mm Hg. 2. Name some clinical conditions that will result in hyperventilation (respiratory alkalosis).
Lecture 8: Heme/Non Heme Iron Proteins and O 2 Management II. Plus a bit of catalysis in Oxygen processes
 Lecture 8: Heme/Non Heme Iron Proteins and O 2 Management II Plus a bit of catalysis in Oxygen processes Hemoglobin Key Properties Ubiquitous O2 transport protein A globular soluble protein, 2X2 chains
Lecture 8: Heme/Non Heme Iron Proteins and O 2 Management II Plus a bit of catalysis in Oxygen processes Hemoglobin Key Properties Ubiquitous O2 transport protein A globular soluble protein, 2X2 chains
Section 01: The Pulmonary System
 Section 01: The Pulmonary System Chapter 12 Pulmonary Structure and Function Chapter 13 Gas Exchange and Transport Chapter 14 Dynamics of Pulmonary Ventilation HPHE 6710 Exercise Physiology II Dr. Cheatham
Section 01: The Pulmonary System Chapter 12 Pulmonary Structure and Function Chapter 13 Gas Exchange and Transport Chapter 14 Dynamics of Pulmonary Ventilation HPHE 6710 Exercise Physiology II Dr. Cheatham
Transport of Oxygen and Carbon Dioxide in Blood and Tissue Fluids
 C H A P T E R 4 Transport of Oxygen and Carbon Dioxide in Blood and Tissue Fluids Once oxygen has diffused from the alveoli into the pulmonary blood, it is transported to the peripheral tissue capillaries
C H A P T E R 4 Transport of Oxygen and Carbon Dioxide in Blood and Tissue Fluids Once oxygen has diffused from the alveoli into the pulmonary blood, it is transported to the peripheral tissue capillaries
RESPIRATION III SEMESTER BOTANY MODULE II
 III SEMESTER BOTANY MODULE II RESPIRATION Lung Capacities and Volumes Tidal volume (TV) air that moves into and out of the lungs with each breath (approximately 500 ml) Inspiratory reserve volume (IRV)
III SEMESTER BOTANY MODULE II RESPIRATION Lung Capacities and Volumes Tidal volume (TV) air that moves into and out of the lungs with each breath (approximately 500 ml) Inspiratory reserve volume (IRV)
Oxygen, Carbon Dioxide Respiration Gas Transport Chapter 21-23
 nd Lecture Fri 06 Mar 009 Vertebrate Physiology ECOL 437 (MCB/VetSci 437) Univ. of Arizona, spring 009 Kevin Bonine & Kevin Oh Oxygen, Carbon Dioxide Respiration Gas Transport Chapter 1-3 1 Housekeeping,
nd Lecture Fri 06 Mar 009 Vertebrate Physiology ECOL 437 (MCB/VetSci 437) Univ. of Arizona, spring 009 Kevin Bonine & Kevin Oh Oxygen, Carbon Dioxide Respiration Gas Transport Chapter 1-3 1 Housekeeping,
Office. Hypoxia. Or this. Or even this. Hypoxia E-1. COL Brian W. Smalley DO, MSPH, CPE
 Hypoxia Office COL Brian W. Smalley DO, MSPH, CPE Or this Or even this Hypoxia State of oxygen deficiency in the blood cells and tissues sufficient to cause impairment of function 4 Types Hypoxic Hypemic
Hypoxia Office COL Brian W. Smalley DO, MSPH, CPE Or this Or even this Hypoxia State of oxygen deficiency in the blood cells and tissues sufficient to cause impairment of function 4 Types Hypoxic Hypemic
Respiratory Physiology Gaseous Exchange
 Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
Life 24 - Blood and Circulation Raven & Johnson Ch 52 & 53 (parts)
 1 Life 24 - Blood and Circulation Raven & Johnson Ch 52 & 53 (parts) Objectives 1: Understand the importance of oxygen carrier molecules in respiration 2: Describe the characteristics and locations of
1 Life 24 - Blood and Circulation Raven & Johnson Ch 52 & 53 (parts) Objectives 1: Understand the importance of oxygen carrier molecules in respiration 2: Describe the characteristics and locations of
Gas exchange and ventilation perfusion relationships in the lung
 ERJ Express. Published on July 28, 214 as doi: 1.1183/931936.3714 REVIEW IN PRESS CORRECTED PROOF Gas exchange and ventilation perfusion relationships in the lung Johan Petersson 1,2 and Robb W. Glenny
ERJ Express. Published on July 28, 214 as doi: 1.1183/931936.3714 REVIEW IN PRESS CORRECTED PROOF Gas exchange and ventilation perfusion relationships in the lung Johan Petersson 1,2 and Robb W. Glenny
Chapter 4: Ventilation Test Bank MULTIPLE CHOICE
 Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
Deborah Dewaay MD Division of General Internal Medicine and Geriatrics Hospital Medicine Acknowledgment: Antine Stenbit MD
 Deborah Dewaay MD Division of General Internal Medicine and Geriatrics Hospital Medicine 2013 Acknowledgment: Antine Stenbit MD Objectives Knowledge: Understand the difference between hypoxia and hypoxemia
Deborah Dewaay MD Division of General Internal Medicine and Geriatrics Hospital Medicine 2013 Acknowledgment: Antine Stenbit MD Objectives Knowledge: Understand the difference between hypoxia and hypoxemia
Gas Exchange Respiratory Systems
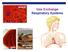 alveoli gills Gas Exchange Respiratory Systems elephant seals 2008-2009 Why do we need a respiratory system? respiration for respiration Need O 2 in for aerobic cellular respiration make ATP Need CO 2
alveoli gills Gas Exchange Respiratory Systems elephant seals 2008-2009 Why do we need a respiratory system? respiration for respiration Need O 2 in for aerobic cellular respiration make ATP Need CO 2
AP Biology. Gas Exchange Respiratory Systems. Gas exchange. Why do we need a respiratory system? Optimizing gas exchange. Gas exchange in many forms
 alveoli Gas Exchange Respiratory Systems gills elephant seals 2008-2009 Why do we need a respiratory system? Need O 2 in food respiration for respiration for aerobic cellular respiration make ATP Need
alveoli Gas Exchange Respiratory Systems gills elephant seals 2008-2009 Why do we need a respiratory system? Need O 2 in food respiration for respiration for aerobic cellular respiration make ATP Need
Respiratory Physiology
 chapter 4 Respiratory Physiology I. LUNG VOLUMES AND CAPACITIES A. Lung volumes (Figure 4-1) 1. Tidal volume (TV) is the volume inspired or expired with each normal breath. 2. Inspiratory reserve volume
chapter 4 Respiratory Physiology I. LUNG VOLUMES AND CAPACITIES A. Lung volumes (Figure 4-1) 1. Tidal volume (TV) is the volume inspired or expired with each normal breath. 2. Inspiratory reserve volume
Vienna, Austria May 2005 MONITORING GAS EXCHANGE: FROM THEORY TO CLINICAL APPLICATION
 EUROANESTHESIA 2005 Vienna, Austria 28-31 May 2005 MONITORING GAS EXCHANGE: FROM THEORY TO CLINICAL APPLICATION 5RC2 OLA STENQVIST Department of Anaesthesia and Intensive Care Sahlgrenska University Hospital
EUROANESTHESIA 2005 Vienna, Austria 28-31 May 2005 MONITORING GAS EXCHANGE: FROM THEORY TO CLINICAL APPLICATION 5RC2 OLA STENQVIST Department of Anaesthesia and Intensive Care Sahlgrenska University Hospital
Chapter 16 Respiration. Respiration. Steps in Respiration. Functions of the respiratory system
 Chapter 16 Respiration Functions of the respiratory system Respiration The term respiration includes 3 separate functions: Ventilation: Breathing. Gas exchange: Occurs between air and blood in the lungs.
Chapter 16 Respiration Functions of the respiratory system Respiration The term respiration includes 3 separate functions: Ventilation: Breathing. Gas exchange: Occurs between air and blood in the lungs.
I. Gas Exchange Respiratory Surfaces Respiratory Surface:
 I. Gas Exchange Respiratory Surfaces Respiratory Surface: Common characteristics of respiratory surfaces: a) Moist: allows for the RAPID diffusion of dissolved gasses across its surface. Whereas the respiratory
I. Gas Exchange Respiratory Surfaces Respiratory Surface: Common characteristics of respiratory surfaces: a) Moist: allows for the RAPID diffusion of dissolved gasses across its surface. Whereas the respiratory
Introduction. Respiration. Chapter 10. Objectives. Objectives. The Respiratory System
 Introduction Respiration Chapter 10 The Respiratory System Provides a means of gas exchange between the environment and the body Plays a role in the regulation of acidbase balance during exercise Objectives
Introduction Respiration Chapter 10 The Respiratory System Provides a means of gas exchange between the environment and the body Plays a role in the regulation of acidbase balance during exercise Objectives
Ch 16: Respiratory System
 Ch 16: Respiratory System SLOs: Explain how intrapulmonary pressures change during breathing Explain surface tension and the role of surfactant in respiratory physiology. Compare and contrast compliance
Ch 16: Respiratory System SLOs: Explain how intrapulmonary pressures change during breathing Explain surface tension and the role of surfactant in respiratory physiology. Compare and contrast compliance
Animal Physiology Prof. Mainak Das Department of Biological Sciences and Bioengineering Indian Institute of Technology, Kanpur. Module - 01 Lecture 28
 Animal Physiology Prof. Mainak Das Department of Biological Sciences and Bioengineering Indian Institute of Technology, Kanpur Module - 01 Lecture 28 Welcome back, so we are in to the Animal Physiology
Animal Physiology Prof. Mainak Das Department of Biological Sciences and Bioengineering Indian Institute of Technology, Kanpur Module - 01 Lecture 28 Welcome back, so we are in to the Animal Physiology
How and why Eurotrol s CueSee Hypoxic works
 How and why Eurotrol s CueSee Hypoxic works Thank you for taking the time to learn some of the advantages and principles behind Eurotrol s CueSee Hypoxic for the validation of low po2 blood gas measurements.
How and why Eurotrol s CueSee Hypoxic works Thank you for taking the time to learn some of the advantages and principles behind Eurotrol s CueSee Hypoxic for the validation of low po2 blood gas measurements.
Ebtihal Al-Remawi. Afnan Ali. Yanal. 1 P a g e
 #1 Ebtihal Al-Remawi Afnan Ali Yanal 1 P a g e 1/15 *before we start: if you are watching the video and have no time for some laughs go to minute (5:28 to 7:02) then go to minute (10:14). I will be adding
#1 Ebtihal Al-Remawi Afnan Ali Yanal 1 P a g e 1/15 *before we start: if you are watching the video and have no time for some laughs go to minute (5:28 to 7:02) then go to minute (10:14). I will be adding
These two respiratory media (air & water) impose rather different constraints on oxygen uptake:
 Topic 19: OXYGEN UPTAKE AND TRANSPORT (lectures 29-30) OBJECTIVES: 1. Be able to compare air vs. water as a respiratory medium with respect to oxygen content, diffusion coefficient, viscosity and water
Topic 19: OXYGEN UPTAKE AND TRANSPORT (lectures 29-30) OBJECTIVES: 1. Be able to compare air vs. water as a respiratory medium with respect to oxygen content, diffusion coefficient, viscosity and water
Breathing oxygenates the blood to allow food to be respired
 Chapter 6 Breathing oxygenates the blood to allow food to be respired This chapter covers: the structure of the human gas exchange system the mechanism of breathing gas exchange in the alveoli the concept
Chapter 6 Breathing oxygenates the blood to allow food to be respired This chapter covers: the structure of the human gas exchange system the mechanism of breathing gas exchange in the alveoli the concept
Chapter 22 The Respiratory System
 Chapter 22 The Respiratory System 1 Respiration Pulmonary ventilation (breathing): movement of air into and out of the lungs External respiration: O 2 and CO 2 exchange between the lungs and the blood
Chapter 22 The Respiratory System 1 Respiration Pulmonary ventilation (breathing): movement of air into and out of the lungs External respiration: O 2 and CO 2 exchange between the lungs and the blood
Lesson 9.1: The Importance of an Organ Delivery System
 Lesson 9.1: The Importance of an Organ Delivery System Animals require a continuous supply of oxygen (O 2 ) for cellular respiration, and they must expel carbon dioxide (CO 2 ), the waste product of this
Lesson 9.1: The Importance of an Organ Delivery System Animals require a continuous supply of oxygen (O 2 ) for cellular respiration, and they must expel carbon dioxide (CO 2 ), the waste product of this
