ULTRASOUND-INDUCED POLYMERISATIONS IN HIGH- PRESSURE FLUIDS
|
|
|
- Mabel Shelton
- 5 years ago
- Views:
Transcription
1 ULTRASOUND-INDUCED POLYMERISATIONS IN HIGH- PRESSURE FLUIDS Kuijpers M.W.A.*, Kemmere M.F. and Keurentjes J.T.F. Process Development Group, Department of Chemical Engineering and Chemistry Eindhoven University of Technology P.O. Box 513, 5600 MB, Eindhoven, The Netherlands Fax: +31 (0) Ultrasound-induced cavitation is known to enhance chemical reactions as well as mass transfer at ambient pressures. Ultrasound is rarely studied at higher pressures, since a high static pressure hampers the growth of cavities. However, in the case of compressed gases such as liquid carbon dioxide, propane and ammonia, the static pressure is counteracted by the higher vapour pressure, which enables cavitation. With the use of a dynamic bubble model, the possibility of cavitation and the resulting hot-spot formation upon bubble collapse have been predicted. These simulations show that the implosions of cavities in high-pressure fluids generate temperatures (+585 K) at which radicals can be formed. To validate this, radical formation and polymerisation experiments have been performed in CO 2 -expanded methyl methacrylate systems. The radical formation rate in this fluid is approximately 1.5*10 14 s -1. Cavitation-induced polymerisations in these systems have resulted in high-molecular weight polymers. INTRODUCTION Sonochemistry comprises all chemical and physical effects that are induced by ultrasound. Most of these effects are caused by cavitations, i.e. the collapse of microscopic bubbles in a liquid. The chemical effects of ultrasound include the formation of radicals and the enhancement of reaction rates at ambient pressures [1]. The enhanced dissolution of a solid reactant or catalyst caused by renewal of the liquid at the solid-liquid interface illustrates a mechanical effect induced by ultrasound. In ordinary solvents, cavitation does not occur at elevated pressures [2]. As a result, sonochemical studies have been limited to atmospheric conditions. In the case of high-pressure fluids such as liquid carbon dioxide, ethylene and propane, the static pressure is counteracted by the higher vapour pressure, resulting in cavitation and radical formation [3]. Especially the application of liquid carbon dioxide is interesting since it is regarded as an environmentally friendly compound. Additionally, carbon dioxide is non-toxic, non-flammable and naturally abundant. In this work cavitation effects have been studied at elevated pressure in liquid carbon dioxide and CO 2 -expanded methyl methacrylate (MMA). The maximum temperature and bubble wall velocity during collapse have been calculated using a dynamic bubble model for liquid CO 2. To validate these calculations, high-pressure cavitation experiments have been performed in these fluids. In addition, radical formation experiments and polymerisation experiments have been performed to prove the hot-spot formation in CO 2 -expanded MMA. CAVITATION BUBBLE DYNAMICS Cavities, i.e. microscopic bubbles in the liquid, are generated when the negative pressure during the rarefaction phase of the sound wave is sufficiently large to disrupt the liquid. In water, the implosions of these cavities generate temperatures and pressures of approximately 5000 K and 200 bar, respectively, due to compression of the gas-phase inside the cavity [4,5].
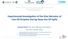
2 To initiate the growth of a cavitation bubble a minimum acoustic pressure has to be applied. This critical pressure is described by the Blake threshold pressure [6]: 4 2 σ PB = P0 Pv + σ (1) 3 3 σ 3 P0 + 2 Pv R0 R 0 Equation 1 assumes that the external pressure (P 0 ), the vapour pressure (P v ), the surface tension (σ) and the equilibrium radius of the bubble (R 0 ) determine the required negative pressure in the liquid to produce an explosive growth of a cavity. During pressurisation of a liquid the Blake threshold pressure increases, which implies that higher acoustic pressures are needed to produce cavitations. Obviously, no cavitation occurs when the Blake pressure exceeds the maximum acoustic pressure that can be produced. Depending on the ultrasound equipment, cavitation in water stops at a hydrostatic pressure in the order of 30 bar. Since cavitation requires a phase boundary, no cavitation is possible at temperatures higher than the critical temperature, due to the absence of the liquid-gas interface. d 3* κ 4 η R d 2 3 d 1 2 σ R0 2 σ R R + R = + P + 0 P P dt P0 P ( t) (2) 2 v v ac d t 2 dt ρ R0 R R R The Blake threshold describes the onset of growth of the cavitation bubble, as it is based on a static equilibrium between the bubble and the liquid. After the explosive growth the bubble is no longer static, which requires a dynamic model in order to calculate the motion of the bubble radius (R). In this study, the Kyuchi-Yasui model [7] is used to describe the dynamic movement of the bubble. The model is based on the Rayleigh-Plesset equation (Equation 2), which includes the surface tension, the vapour pressure, the liquid viscosity (η), the polytropic index of the gas phase (κ) and the acoustic pressure (P ac ). The temperature, vapour and gas pressure inside the bubble are derived from the Rayleigh-Plesset equation in combination with a mass and energy balance over the bubble. EXPERIMENTAL Cavitation in liquid-carbon dioxide and the radical formation experiments were studied in a jacketed 175 ml high-pressure vessel, equipped with quartz windows. A schematic drawing of this vessel is given in Figure 1. Sonification of the liquid was performed using 20 khz ultrasound, which was produced by a Sonics and Materials VC-750 ultrasonic generator. A titanium ½ inch full wave probe was used to couple the piezoelectric transducer to the liquid. The probe was connected to the vessel at its nodes. The temperature inside the vessel was controlled by a heating/cooling bath. A camera in front of the quartz windows was used to observe cavitation events in the high-pressure vessel. Moreover, the concentration of the radical scavenger was measured by UV-vis spectroscopy through these windows. The absorption decrease of the active radical scavenger (1,1-diphenyl-2-picrylhydrazyl, DPPH) was used to determine the radical formation rate [8] in CO 2 -expanded MMA. The absorption coefficients for the active and inactive radical-scavenger are 8*10 3 and 2*10 3 L/(mol cm), respectively. The CO 2 -MMA system with a high molar fraction of CO 2, required an additional argon head pressure to avoid boiling and to allow for cavitation. The composition and density of the MMA-CO 2 system is modelled with the Peng-Robinson equation of state and the Panagiotopoulos and Reid mixing rule [9]. The polymerisation experiments were performed in a 1.8 L high-pressure reaction calorimeter RC1e (Mettler-Toledo GmbH, HP60 reactor,
3 Switzerland). A detailed description of this equipment is given by Varela de la Rosa et al. [10] Figure 1. Schematic drawing of the high-pressure vessel (1) attachment points for ultrasound horn (2) cooling/heating jacket, (3) quartz windows, (4) ultrasound horn. BUBBLE DYNAMICS The possibility of ultrasound-induced cavitation and in situ radical formation in liquid carbon dioxide has been studied. To assign the determining physical properties of high-pressure fluids, the growth and the implosion of a cavity in water and in liquid carbon dioxide have been compared. Since the Blake threshold pressure of liquid carbon dioxide at 58.2 bar equals the threshold pressure of water at 1 bar and 20 C, 58.2 bar has been used in the calculations. In Figure 2 the calculated Blake threshold pressure of carbon dioxide and water at 58.2 bar is given as a function of temperature. The threshold curves are calculated using Equation 1, in which the vapour pressure is a determining parameter, next to the hydrostatic pressure and the surface tension. For water the Blake threshold is only determined by the static pressure and Figure 2. Calculated Blake threshold pressure and vapour pressure for water and CO 2 at 58.2 bar as a function of temperature. Figure 3. Calculated radius of a cavitation bubble as a function of time in water and carbon dioxide.
4 the surface tension of the liquid. As the vapour pressure of water is low does not change significantly with increasing temperature, the threshold pressure of water is virtually constant. In most liquids a high static pressure hampers the growth of nuclei as a result of the minor influence of the vapour pressure. In the case of carbon dioxide, which condenses at a substantially higher pressure, the vapour pressure does have a significant influence as can be seen in Figure 2. As a result of this, the Blake threshold is reduced, which enables cavitations in liquid carbon dioxide at higher pressures. The dynamic movement of the bubble wall has been calculated using the Kyuchi-Yasui model. Initially, a small cavity with a radius of 10-5 m is present, which consists of argon and the corresponding vapour of the liquid phase. In the simulations, an ultrasound wave with an acoustic pressure of 10 bar and a frequency of 20 khz is imposed on this bubble. The variation of the radius of the bubble is shown in Figure 3. As the acoustic pressure for water at 58.2 bar is lower than the Blake threshold pressure almost no bubble movement is observed and no cavitation can occur. In contrast to the situation in water, carbon dioxide at 58.2 bar allows for cavitation and the bubble exhibits a similar movement compared to water at 1 bar. Moreover, the maximum temperatures (585 K) and maximum bubble wall velocities (777 m/s) reached during collapse in liquid CO 2 are in the same order of magnitude as compared to water at ambient pressure (722 K and 840 m/s). CAVITATION AND RADICAL FORMATION Experiments in the high-pressure vessel showed that the required threshold pressure could easily be exceeded at a relatively low acoustic pressure for liquid CO 2 (10 C, 75 bar), proving the influence of the vapour pressure in the Blake Threshold calculations and the dynamic simulations. The occurrence of cavitation has been visually observed. Extinction (-) Time Wave lenght (nm) Figure 4. UV-Vis Spectra of DPPH in time for CO 2 -MMA ( ) under sonification, at 20 C. Concentration DPPH (10-5 mol/m 3 ) =0.27, 0 MPa argon =0.27, 0.7 MPa argon =0.64, 0.5 MPa argon Time (10 3 s) Figure 5. Concentration of DPPH in time as determined by UV-Vis analysis at 520 nm, for three different systems at 20 C. To show that bubble collapse in liquid CO 2 is sufficiently strong to induce radical formation, measurements were done in CO 2 -methyl methacrylate (MMA) systems at high pressure. These UV-vis measurements show a decrease in the absorption of the radical scavenger during sonification (Figure 4), meaning that radicals are produced. The radical formation rate is
5 approximately 1.5*10 14 s -1, for which no noticeable differences between the three systems have been observed (Figure 5). POLYMERISATION In ultrasound-induced bulk polymerisations at ambient pressures a strong viscosity increase occurs which hinders cavitation, and hence radical formation. This limits the conversion to low values [11]. Since high-pressure carbon dioxide acts as an anti-solvent for polymers, the viscosity will not increase. The gyration radius of a polymer is smaller when an anti-solvent is present. Additionally, a low solvent viscosity will be maintained even at high conversions, as the polymer will precipitate from the solution. In polymerisation reactions, high-pressure CO 2 acts as an anti-solvent, thus enabling a viscosity reduction that leads to high conversion. The polymerisations performed in CO 2 -expanded MMA at 293K and 6 bar showed a minor viscosity increase up to a conversion of 5%. A similar experiment performed in pure MMA showed a decrease of the overall heat-transfer coefficient (U), which is a measure of the liquid viscosity. A lower U implies a higher liquid viscosity. The polymerisations resulted in polymethyl methacrylate with a molecular weight of approximately 10 5 g/mol (no CO 2 ) and 10 7 g/mol (6 bar CO 2 ). CONCLUSION Ultrasound has been applied to create cavitation in high-pressure liquid carbon dioxide (10 C, 75 bar) in which the threshold pressure for cavitation can be exceeded at a relatively low acoustic intensity. The hydrostatic pressure is counteracted by the high vapor pressure of carbon dioxide, as explained by the Blake Threshold model. Using a dynamic bubble model, the formation of hot spots upon bubble collapse is predicted. This hot-spot formation has been experimentally confirmed by radical formation and polymerization experiments. The radical formation rate is approximately 1.5*10 14 s -1 in CO 2 -expanded MMA at 20 C. The ultrasoundinduced precipitation polymerisations resulted in polymethyl methacrylate with a molecular weight of approximately g/mol. Due to the anti-solvent behavior of CO 2 only a slight viscosity increase is found during polymerization up to 5%. REFERENCES [1] THOMPSON, L.H., DORAISWAMY, L.K., Ind. Eng. Chem. Res., Vol. 38, 1999, p [2] BERLAN, J., TRABELSI, F., DELMAS, H., WILHELM, A.M., PETRIGNANI, J.F., Ultrason. Sonochem., Vol. 1, 1994, p. S97 [3] KUIJPERS, M.W.A., VAN ECK, D., KEMMERE, M.F., KEURENTJES, J.T.F., Science, Vol. 298, 2002, p [4] SUSLICK, K.S., Sci. Am., Vol. 260, 1989, p. 80 [5] LUCHE, J.-L., Synthetic Organic Sonochemistry, Plenum, New York, 1998 [6] LEIGHTON, T.J., The Acoustic Bubble, Academic Press, London, 1998 [7] YASUI, K., J. Acoust. Soc. Am., Vol. 98, 1995, p [8] KUIJPERS, M.W.A., KEMMERE, M.F., KEURENTJES, J.T.F., Ultrasonics, Vol. 40, 2002, p. 675 [9] KEMMERE, M.F., CLEVEN, M.H.W., VAN SCHILT, M.A., KEURENTJES, J.T.F., Chem. Eng. Sci., Vol. 57, 2002, 3929 [10] VARELA DE LA ROSA, L., SUDOL, E.D., EL-AASSER, M.S., KLEIN, A., J. Polym. Sci., Vol. 34, 1996, p. 461 [11] PRICE, G.J., Ultrason. Sonochem., Vol. 3, 1996, p. S229
MODELING OF THERMAL BEHAVIOR INSIDE A BUBBLE
 CAV2001:sessionB6.002 1 MODEING OF THERMA BEHAVIOR INSIDE A BUBBE Boonchai ERTNUWAT *, Kazuyasu SUGIYAMA ** and Yoichiro MATSUMOTO *** *, ***Dept. of Mechanical Engineering, The University of Tokyo, Tokyo,
CAV2001:sessionB6.002 1 MODEING OF THERMA BEHAVIOR INSIDE A BUBBE Boonchai ERTNUWAT *, Kazuyasu SUGIYAMA ** and Yoichiro MATSUMOTO *** *, ***Dept. of Mechanical Engineering, The University of Tokyo, Tokyo,
Effect of gases on radical production rates during single-bubble cavitation
 Effect of gases on radical production rates during single-bubble cavitation Shin-ichi Hatanaka Citation: Proc. Mtgs. Acoust. 19, 4596 (213); View online: https://doi.org/1.1121/1.4799389 View Table of
Effect of gases on radical production rates during single-bubble cavitation Shin-ichi Hatanaka Citation: Proc. Mtgs. Acoust. 19, 4596 (213); View online: https://doi.org/1.1121/1.4799389 View Table of
Flammability Assessments of Sonication Process in Organic Mixture
 1393 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 56, 2017 Guest Editors: Jiří Jaromír Klemeš, Peng Yen Liew, Wai Shin Ho, Jeng Shiun Lim Copyright 2017, AIDIC Servizi S.r.l., ISBN 978-88-95608-47-1;
1393 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 56, 2017 Guest Editors: Jiří Jaromír Klemeš, Peng Yen Liew, Wai Shin Ho, Jeng Shiun Lim Copyright 2017, AIDIC Servizi S.r.l., ISBN 978-88-95608-47-1;
Mechanics of collapsing cavitation bubbles
 1 Mechanics of collapsing cavitation bubbles Leen van Wijngaarden University of Twente, Physics of Fluids group,and J.M.Burgers Centre for Fluid Dynamics, P.O.Box 217, 7500AE Enschede, The Netherlands
1 Mechanics of collapsing cavitation bubbles Leen van Wijngaarden University of Twente, Physics of Fluids group,and J.M.Burgers Centre for Fluid Dynamics, P.O.Box 217, 7500AE Enschede, The Netherlands
Under pressure pushing down
 Under pressure pushing down on me When Dalton was conducting his studies, which led him to the atomic-molecular theory of matter, he also included studies of the behaviour of gases. These led him to propose,
Under pressure pushing down on me When Dalton was conducting his studies, which led him to the atomic-molecular theory of matter, he also included studies of the behaviour of gases. These led him to propose,
Trapped Bubble Dynamics in Cryogenic Fluids. Department of Physics and Astronomy, University of California, Los Angeles, CA 90095, USA
 Trapped Bubble Dynamics in Cryogenic Fluids O. Baghdassarian, H. Cho y,e.varoquaux Λ, and G. A. Williams Department of Physics and Astronomy, University of California, Los Angeles, CA 90095, USA Initial
Trapped Bubble Dynamics in Cryogenic Fluids O. Baghdassarian, H. Cho y,e.varoquaux Λ, and G. A. Williams Department of Physics and Astronomy, University of California, Los Angeles, CA 90095, USA Initial
5 cm. Flash 20 khz. Camera
 BUBBLE SIZE DISTRIBUTIONS AND STRUCTURES IN ACOUSTIC CAVITATION METTIN R., LUTHER S., LAUTERBORN W. Drittes Physikalisches Institut, Universitat Gottingen Burgerstr. 42-44, D-37073 Gottingen (Germany)
BUBBLE SIZE DISTRIBUTIONS AND STRUCTURES IN ACOUSTIC CAVITATION METTIN R., LUTHER S., LAUTERBORN W. Drittes Physikalisches Institut, Universitat Gottingen Burgerstr. 42-44, D-37073 Gottingen (Germany)
Chem 110 General Principles of Chemistry
 CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
1. A pure substance has a specific volume of 0.08 L/mol at a pressure of 3 atm and 298 K. The substance is most likely:
 Name: September 19, 2014 EXAM 1 P a g e 1 1. A pure substance has a specific volume of 0.08 L/mol at a pressure of 3 atm and 298 K. The substance is most likely: a. Liquid b. Gas c. Supercritical Fluid
Name: September 19, 2014 EXAM 1 P a g e 1 1. A pure substance has a specific volume of 0.08 L/mol at a pressure of 3 atm and 298 K. The substance is most likely: a. Liquid b. Gas c. Supercritical Fluid
Chemistry 1B Chapter 10 Worksheet - Daley. Name
 Name 1) The National Weather Service routinely supplies atmospheric pressure data to help pilots set their altimeters. The units the NWS uses for atmospheric pressure are inches of mercury. A barometric
Name 1) The National Weather Service routinely supplies atmospheric pressure data to help pilots set their altimeters. The units the NWS uses for atmospheric pressure are inches of mercury. A barometric
Paper 2.2. Operation of Ultrasonic Flow Meters at Conditions Different Than Their Calibration
 Paper 2.2 Operation of Ultrasonic Flow Meters at Conditions Different Than Their Calibration Mr William Freund, Daniel Measurement and Control Mr Klaus Zanker, Daniel Measurement and Control Mr Dale Goodson,
Paper 2.2 Operation of Ultrasonic Flow Meters at Conditions Different Than Their Calibration Mr William Freund, Daniel Measurement and Control Mr Klaus Zanker, Daniel Measurement and Control Mr Dale Goodson,
University of Twente
 University of Twente Internship Report Parameterstudy on a computational method for the prediction of noise production caused by sheet cavitation on a ship propeller Author: R.M. van Dijk Supervisors:
University of Twente Internship Report Parameterstudy on a computational method for the prediction of noise production caused by sheet cavitation on a ship propeller Author: R.M. van Dijk Supervisors:
Figure Vapor-liquid equilibrium for a binary mixture. The dashed lines show the equilibrium compositions.
 Another way to view this problem is to say that the final volume contains V m 3 of alcohol at 5.93 kpa and 20 C V m 3 of air at 94.07 kpa and 20 C V m 3 of air plus alcohol at 100 kpa and 20 C Thus, the
Another way to view this problem is to say that the final volume contains V m 3 of alcohol at 5.93 kpa and 20 C V m 3 of air at 94.07 kpa and 20 C V m 3 of air plus alcohol at 100 kpa and 20 C Thus, the
MODELING AND SIMULATION OF VALVE COEFFICIENTS AND CAVITATION CHARACTERISTICS IN A BALL VALVE
 Proceedings of the 37 th International & 4 th National Conference on Fluid Mechanics and Fluid Power FMFP2010 December 16-18, 2010, IIT Madras, Chennai, India FMFP2010 341 MODELING AND SIMULATION OF VALVE
Proceedings of the 37 th International & 4 th National Conference on Fluid Mechanics and Fluid Power FMFP2010 December 16-18, 2010, IIT Madras, Chennai, India FMFP2010 341 MODELING AND SIMULATION OF VALVE
LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary
 ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
LEAP CO 2 Laboratory CO 2 mixtures test facility
 LEAP CO 2 Laboratory CO 2 mixtures test facility THE PROJECT AIM CO 2 flows made available by several capture techniques are contaminated with impurities and this affects the design and operations of the
LEAP CO 2 Laboratory CO 2 mixtures test facility THE PROJECT AIM CO 2 flows made available by several capture techniques are contaminated with impurities and this affects the design and operations of the
A Model of Gas Bubble Growth by Comsol Multiphysics
 Excerpt from the Proceedings of the COMSO Conference 21 Paris A Model of Gas Bubble Growth by Comsol Multiphysics B.Chinè *1,2 and M. Monno 1,3 1 aboratorio MUSP, Macchine Utensili e Sistemi di Produzione,
Excerpt from the Proceedings of the COMSO Conference 21 Paris A Model of Gas Bubble Growth by Comsol Multiphysics B.Chinè *1,2 and M. Monno 1,3 1 aboratorio MUSP, Macchine Utensili e Sistemi di Produzione,
A Computational Assessment of Gas Jets in a Bubbly Co-Flow 1
 A Computational Assessment of Gas Jets in a Bubbly Co-Flow 1 Melissa Fronzeo*, 1 Michael Kinzel 1 The Pennsylvania State University, University Park, PA, USA Abstract In this effort, Computational Fluid
A Computational Assessment of Gas Jets in a Bubbly Co-Flow 1 Melissa Fronzeo*, 1 Michael Kinzel 1 The Pennsylvania State University, University Park, PA, USA Abstract In this effort, Computational Fluid
Numerical Simulations of a Train of Air Bubbles Rising Through Stagnant Water
 Numerical Simulations of a Train of Air Bubbles Rising Through Stagnant Water Hong Xu, Chokri Guetari ANSYS INC. Abstract Transient numerical simulations of the rise of a train of gas bubbles in a liquid
Numerical Simulations of a Train of Air Bubbles Rising Through Stagnant Water Hong Xu, Chokri Guetari ANSYS INC. Abstract Transient numerical simulations of the rise of a train of gas bubbles in a liquid
Kinetic-Molecular Theory
 GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
PHYSICALLY REALISTIC MODELS OF CATASTROPHIC BUBBLE COLLAPSES
 CAV2001:sessionB6.001 1 PHYSICALLY REALISTIC MODELS OF CATASTROPHIC BUBBLE COLLAPSES Brian D. Storey 1,HaoLin 2,&Andrew J. Szeri 2 1. Franklin W. Olin College of Engineering, Needham, MA 02492-1245, USA.
CAV2001:sessionB6.001 1 PHYSICALLY REALISTIC MODELS OF CATASTROPHIC BUBBLE COLLAPSES Brian D. Storey 1,HaoLin 2,&Andrew J. Szeri 2 1. Franklin W. Olin College of Engineering, Needham, MA 02492-1245, USA.
Thermodynamics ERT 206 Properties of Pure Substance HANNA ILYANI ZULHAIMI
 Thermodynamics ERT 206 Properties of Pure Substance HANNA ILYANI ZULHAIMI Outline: Pure Substance Phases of pure substance Phase change process of pure substance Saturation temperature and saturation pressure
Thermodynamics ERT 206 Properties of Pure Substance HANNA ILYANI ZULHAIMI Outline: Pure Substance Phases of pure substance Phase change process of pure substance Saturation temperature and saturation pressure
Tutorial. BOSfluids. Relief valve
 Tutorial Relief valve The Relief valve tutorial describes the theory and modeling process of a pressure relief valve or safety valve. It covers the algorithm BOSfluids uses to model the valve and a worked
Tutorial Relief valve The Relief valve tutorial describes the theory and modeling process of a pressure relief valve or safety valve. It covers the algorithm BOSfluids uses to model the valve and a worked
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Test General Chemistry CH116 UMass Boston Summer 2013 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The pressure exerted by a column of
Test General Chemistry CH116 UMass Boston Summer 2013 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The pressure exerted by a column of
The Discussion of this exercise covers the following points: Pumps Basic operation of a liquid pump Types of liquid pumps The centrifugal pump.
 Exercise 2-3 Centrifugal Pumps EXERCISE OBJECTIVE In this exercise, you will become familiar with the operation of a centrifugal pump and read its performance chart. You will also observe the effect that
Exercise 2-3 Centrifugal Pumps EXERCISE OBJECTIVE In this exercise, you will become familiar with the operation of a centrifugal pump and read its performance chart. You will also observe the effect that
CHEM 355 EXPERIMENT 7. Viscosity of gases: Estimation of molecular diameter
 CHEM 355 EXPERIMENT 7 Viscosity of gases: Estimation of molecular diameter Expressed most simply, the viscosity of a fluid (liquid or gas) relates to its resistance to flow. The viscosity of a gas is determined
CHEM 355 EXPERIMENT 7 Viscosity of gases: Estimation of molecular diameter Expressed most simply, the viscosity of a fluid (liquid or gas) relates to its resistance to flow. The viscosity of a gas is determined
PROPERTIES OF GASES. [MH5; Ch 5, (only)]
![PROPERTIES OF GASES. [MH5; Ch 5, (only)] PROPERTIES OF GASES. [MH5; Ch 5, (only)]](/thumbs/76/74446950.jpg) PROPERTIES OF GASES [MH5; Ch 5, 5.1-5.5 (only)] FEATURES OF A GAS Molecules in a gas are a long way apart (under normal conditions). Molecules in a gas are in rapid motion in all directions. The forces
PROPERTIES OF GASES [MH5; Ch 5, 5.1-5.5 (only)] FEATURES OF A GAS Molecules in a gas are a long way apart (under normal conditions). Molecules in a gas are in rapid motion in all directions. The forces
Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops
 C h e m i s t r y 1 2 C h a p t e r 11 G a s e s P a g e 1 Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops Gas Properties: Gases have high kinetic energy low
C h e m i s t r y 1 2 C h a p t e r 11 G a s e s P a g e 1 Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops Gas Properties: Gases have high kinetic energy low
Wind Regimes 1. 1 Wind Regimes
 Wind Regimes 1 1 Wind Regimes The proper design of a wind turbine for a site requires an accurate characterization of the wind at the site where it will operate. This requires an understanding of the sources
Wind Regimes 1 1 Wind Regimes The proper design of a wind turbine for a site requires an accurate characterization of the wind at the site where it will operate. This requires an understanding of the sources
FLOW VISUALIZATION STUDY ON TWO-PHASE CRYOGENIC FLOW
 Paper ID ILASS08-3-2 ILASS08-A127 ILASS 2008 Sep. 8-10, 2008, Como Lake, Italy FLOW VISUALIZATION STUDY ON TWO-PHASE CRYOGENIC FLOW Maria Grazia De Giorgi *, Antonio Ficarella, Maria Giovanna Rodio *,
Paper ID ILASS08-3-2 ILASS08-A127 ILASS 2008 Sep. 8-10, 2008, Como Lake, Italy FLOW VISUALIZATION STUDY ON TWO-PHASE CRYOGENIC FLOW Maria Grazia De Giorgi *, Antonio Ficarella, Maria Giovanna Rodio *,
Assumptions 1 At specified conditions, air behaves as an ideal gas. 2 The volume of the tire remains constant.
 PTT 04/ Applied Fluid Mechanics Sem, Session015/016 ASSIGNMENT 1 CHAPTER AND CHAPTER 1. The air in an automobile tire with a volume of 0.0740 m is at 0 C and 140 kpa. Determine the amount of air that must
PTT 04/ Applied Fluid Mechanics Sem, Session015/016 ASSIGNMENT 1 CHAPTER AND CHAPTER 1. The air in an automobile tire with a volume of 0.0740 m is at 0 C and 140 kpa. Determine the amount of air that must
You should be able to: Describe Equipment Barometer Manometer. 5.1 Pressure Read and outline 5.1 Define Barometer
 A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
Chemistry A Molecular Approach. Fourth Edition. Chapter 5. Gases. Copyright 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
 Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Workshop 1: Bubbly Flow in a Rectangular Bubble Column. Multiphase Flow Modeling In ANSYS CFX Release ANSYS, Inc. WS1-1 Release 14.
 Workshop 1: Bubbly Flow in a Rectangular Bubble Column 14. 5 Release Multiphase Flow Modeling In ANSYS CFX 2013 ANSYS, Inc. WS1-1 Release 14.5 Introduction This workshop models the dispersion of air bubbles
Workshop 1: Bubbly Flow in a Rectangular Bubble Column 14. 5 Release Multiphase Flow Modeling In ANSYS CFX 2013 ANSYS, Inc. WS1-1 Release 14.5 Introduction This workshop models the dispersion of air bubbles
1.2 Example 1: A simple hydraulic system
 Note: It is possible to use more than one fluid in the Hydraulic library. This is important because you can model combined cooling and lubrication systems of a library. The hydraulic library assumes a
Note: It is possible to use more than one fluid in the Hydraulic library. This is important because you can model combined cooling and lubrication systems of a library. The hydraulic library assumes a
Use a Controlled Vibration to Mixing and Separation of a Gas Bubbles and a Liquid Under Reduced and Microgravity Conditions
 ng & Process Technology rijournal of Chemical Enginee Research Article Article Journal of Chemical Engineering & Process Technology Shoikhedbrod, J Chem Eng Process Technol 2016, 7:4 DOI: 10.4172/2157-7048.1000305
ng & Process Technology rijournal of Chemical Enginee Research Article Article Journal of Chemical Engineering & Process Technology Shoikhedbrod, J Chem Eng Process Technol 2016, 7:4 DOI: 10.4172/2157-7048.1000305
PURE SUBSTANCE. Nitrogen and gaseous air are pure substances.
 CLASS Third Units PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Air is a mixture of several gases, but it is considered to be a pure substance. Nitrogen and
CLASS Third Units PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Air is a mixture of several gases, but it is considered to be a pure substance. Nitrogen and
Physical explosion analysis in heat exchanger network design
 IOP Conference Series: Earth and Environmental Science PAPER OPEN ACCESS Physical explosion analysis in heat exchanger network design To cite this article: M Pasha et al 2016 IOP Conf. Ser.: Earth Environ.
IOP Conference Series: Earth and Environmental Science PAPER OPEN ACCESS Physical explosion analysis in heat exchanger network design To cite this article: M Pasha et al 2016 IOP Conf. Ser.: Earth Environ.
Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases
![Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases](/thumbs/78/77082586.jpg) Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] Chapter 10 Gases We have talked a little about gases in Chapter 3 and we dealt briefly with them in our stoichiometric calculations in
Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] Chapter 10 Gases We have talked a little about gases in Chapter 3 and we dealt briefly with them in our stoichiometric calculations in
PSI Chemistry: Gases Multiple Choice Review
 PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
Transverse waves cause particles to vibrate perpendicularly to the direction of the wave's motion (e.g. waves on a string, ripples on a pond).
 Waves Introduction A vibration must be the source of a wave. Waves in turn also cause vibrations. They are intrinsically connected. Waves transmit energy. There are different ways in which waves can be
Waves Introduction A vibration must be the source of a wave. Waves in turn also cause vibrations. They are intrinsically connected. Waves transmit energy. There are different ways in which waves can be
Proceedings of Meetings on Acoustics
 Proceedings of Meetings on Acoustics Volume 9, 2010 http://acousticalsociety.org/ 159th Meeting Acoustical Society of America/NOISE-CON 2010 Baltimore, Maryland 19-23 April 2010 Session 1pBB: Biomedical
Proceedings of Meetings on Acoustics Volume 9, 2010 http://acousticalsociety.org/ 159th Meeting Acoustical Society of America/NOISE-CON 2010 Baltimore, Maryland 19-23 April 2010 Session 1pBB: Biomedical
Chapter 15 Fluids. Copyright 2010 Pearson Education, Inc.
 Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle Fluid Flow and Continuity
Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle Fluid Flow and Continuity
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/12
 LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
CALCULATING THE SPEED OF SOUND IN NATURAL GAS USING AGA REPORT NO Walnut Lake Rd th Street Houston TX Garner, IA 50438
 CALCULATING THE SPEED OF SOUND IN NATURAL GAS USING AGA REPORT NO. 10 Jerry Paul Smith Joel Clancy JPS Measurement Consultants, Inc Colorado Engineering Experiment Station, Inc (CEESI) 13002 Walnut Lake
CALCULATING THE SPEED OF SOUND IN NATURAL GAS USING AGA REPORT NO. 10 Jerry Paul Smith Joel Clancy JPS Measurement Consultants, Inc Colorado Engineering Experiment Station, Inc (CEESI) 13002 Walnut Lake
Laboratory studies of water column separation
 IOP Conference Series: Materials Science and Engineering OPEN ACCESS Laboratory studies of water column separation To cite this article: R Autrique and E Rodal 2013 IOP Conf. Ser.: Mater. Sci. Eng. 52
IOP Conference Series: Materials Science and Engineering OPEN ACCESS Laboratory studies of water column separation To cite this article: R Autrique and E Rodal 2013 IOP Conf. Ser.: Mater. Sci. Eng. 52
Injector Dynamics Assumptions and their Impact on Predicting Cavitation and Performance
 Injector Dynamics Assumptions and their Impact on Predicting Cavitation and Performance Frank Husmeier, Cummins Fuel Systems Presented by Laz Foley, ANSYS Outline Overview Computational Domain and Boundary
Injector Dynamics Assumptions and their Impact on Predicting Cavitation and Performance Frank Husmeier, Cummins Fuel Systems Presented by Laz Foley, ANSYS Outline Overview Computational Domain and Boundary
Lecture Outline Chapter 15. Physics, 4 th Edition James S. Walker. Copyright 2010 Pearson Education, Inc.
 Lecture Outline Chapter 15 Physics, 4 th Edition James S. Walker Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy
Lecture Outline Chapter 15 Physics, 4 th Edition James S. Walker Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy
A. What are the three states of matter chemists work with?
 Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
Bubble-Based Resonance-Doppler Sensor for Liquid Characterization
 Bubble-Based Resonance-Doppler Sensor for Liquid Characterization Naveen N. Sinha Los Alamos High School Los Alamos, New Mexico 87544 dipensin@msn.com (55) 672-1916 Abstract I have developed a novel acoustic
Bubble-Based Resonance-Doppler Sensor for Liquid Characterization Naveen N. Sinha Los Alamos High School Los Alamos, New Mexico 87544 dipensin@msn.com (55) 672-1916 Abstract I have developed a novel acoustic
Ch. 11 Mass transfer principles
 Transport of chemical species in solid, liquid, or gas mixture Transport driven by composition gradient, similar to temperature gradients driving heat transport We will look at two mass transport mechanisms,
Transport of chemical species in solid, liquid, or gas mixture Transport driven by composition gradient, similar to temperature gradients driving heat transport We will look at two mass transport mechanisms,
Theoretical Solution 1, 9 th Asian Physics Olympiad (Mongolia)
 Solutions: S1. The condition of the survival and growth for AB appeared in the water volume at height h < H is the competiveness of the pressures acting inside and outside (atmospheric, hydrostatic and
Solutions: S1. The condition of the survival and growth for AB appeared in the water volume at height h < H is the competiveness of the pressures acting inside and outside (atmospheric, hydrostatic and
B outflow. Outflow. B1 Introduction. introduction
 B outflow introduction B1 Introduction The subject of this chapter is the release, or better the incidental release of hazardous materials. It is obvious that this topic is much broader than just a chapter.
B outflow introduction B1 Introduction The subject of this chapter is the release, or better the incidental release of hazardous materials. It is obvious that this topic is much broader than just a chapter.
Energy and mass transfer in gas-liquid reactors.
 Energy and mass transfer in gas-liquid reactors. John M Smith School of Engineering (D2) University of Surrey, Guildford GU2 7XH, UK j.smith@surrey.ac.uk 1 Energy and mass transfer in gas-liquid reactors.
Energy and mass transfer in gas-liquid reactors. John M Smith School of Engineering (D2) University of Surrey, Guildford GU2 7XH, UK j.smith@surrey.ac.uk 1 Energy and mass transfer in gas-liquid reactors.
States of Matter Review
 States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
Air Bubble Defects in Dispensing Nanoimprint Lithography
 Air Bubble Defects in Dispensing Nanoimprint Lithography Abstract We report a theoretical study and dynamic simulation to understand the dynamic behavior of the air bubble defects in Dispensing Nanoimprint
Air Bubble Defects in Dispensing Nanoimprint Lithography Abstract We report a theoretical study and dynamic simulation to understand the dynamic behavior of the air bubble defects in Dispensing Nanoimprint
An underwater explosion is an explosion where the point of detonation is below the surface of the water.
 Underwater Explosion 1 Introduction An underwater explosion is an explosion where the point of detonation is below the surface of the water. Underwater explosion are categorized in accordance with their
Underwater Explosion 1 Introduction An underwater explosion is an explosion where the point of detonation is below the surface of the water. Underwater explosion are categorized in accordance with their
Thin Film Slot Coating in a Rarefied Low Viscosity Gas
 www.bradford.ac.uk Thin Film Slot Coating in a Rarefied Low Viscosity Gas H. Benkreira & J.B. Ikin* School of Engineering, Design & Technology University of Bradford-UK *Multicoat Ltd., 21 Turnfield Road,
www.bradford.ac.uk Thin Film Slot Coating in a Rarefied Low Viscosity Gas H. Benkreira & J.B. Ikin* School of Engineering, Design & Technology University of Bradford-UK *Multicoat Ltd., 21 Turnfield Road,
Evaluation of Cavitation in a Liquid-Liquid Ejector
 ILASS Americas, 24 th Annual Conference on Liquid Atomization and Spray Systems, San Antonio, TX, May 2012 Evaluation of Cavitation in a Liquid-Liquid Ejector Carsten Mehring * Central Engineering, Parker
ILASS Americas, 24 th Annual Conference on Liquid Atomization and Spray Systems, San Antonio, TX, May 2012 Evaluation of Cavitation in a Liquid-Liquid Ejector Carsten Mehring * Central Engineering, Parker
THE EFFECT OF GAS DIFFUSION ON BUBBLE DYNAMICS
 Cav03-GS-2-004 Fifth International Symposium on Cavitation (cav2003) Osaka, Japan, November 1-4, 2003 THE EFFECT OF GAS DIFFUSION ON BUBBLE DYNAMICS B. Brunn/Darmstadt University of Technology Chair of
Cav03-GS-2-004 Fifth International Symposium on Cavitation (cav2003) Osaka, Japan, November 1-4, 2003 THE EFFECT OF GAS DIFFUSION ON BUBBLE DYNAMICS B. Brunn/Darmstadt University of Technology Chair of
TUTORIAL. NPSHA for those who hate that stuffy word. by Jacques Chaurette p. eng. copyright 2006
 TUTORIAL NPSHA for those who hate that stuffy word by Jacques Chaurette p. eng. www.lightmypump.com copyright 2006 page.2 NPSHA for those who hate that stuffy word This article follows the same approach
TUTORIAL NPSHA for those who hate that stuffy word by Jacques Chaurette p. eng. www.lightmypump.com copyright 2006 page.2 NPSHA for those who hate that stuffy word This article follows the same approach
8.1 Properties of Gases. Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases.
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8. Chapter 8
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases. Chapter 8. Chapter 8. Gases Properties of Gases. We are surrounded by gases, but we are often
 Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
Chemistry HP Unit 6 Gases. Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases
 Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Pressure Measurement
 Pressure Measurement Manometers Sensors, Transducers Ashish J. Modi Lecturer, Dept. of Mech.Engg., Shri S.V.M. inst. Of Technology, Bharuch Pressure Pressure is a force per unit area exerted by a fluid
Pressure Measurement Manometers Sensors, Transducers Ashish J. Modi Lecturer, Dept. of Mech.Engg., Shri S.V.M. inst. Of Technology, Bharuch Pressure Pressure is a force per unit area exerted by a fluid
Honors Chemistry Unit 7 Gas Laws Notes
 Honors Chemistry Unit 7 Gas Laws Notes Kinetic Molecular Theory 1. List the five assumptions: Assumption Description Extra Info 1 Basically means: the particles themselves have compared to the space between
Honors Chemistry Unit 7 Gas Laws Notes Kinetic Molecular Theory 1. List the five assumptions: Assumption Description Extra Info 1 Basically means: the particles themselves have compared to the space between
Kinetic Molecular Theory
 Kinetic Molecular Theory Name Period Unit 7 HW 1 Worksheet (Goals 1 & 2) 1. Describe how gases, liquids, and solids compare using the following table. Volume (definite or indefinite) Molecular Motion (high,
Kinetic Molecular Theory Name Period Unit 7 HW 1 Worksheet (Goals 1 & 2) 1. Describe how gases, liquids, and solids compare using the following table. Volume (definite or indefinite) Molecular Motion (high,
1 PIPESYS Application
 PIPESYS Application 1-1 1 PIPESYS Application 1.1 Gas Condensate Gathering System In this PIPESYS Application, the performance of a small gascondensate gathering system is modelled. Figure 1.1 shows the
PIPESYS Application 1-1 1 PIPESYS Application 1.1 Gas Condensate Gathering System In this PIPESYS Application, the performance of a small gascondensate gathering system is modelled. Figure 1.1 shows the
Effects of non-condensable gas on cavitating flow over a cylinder
 Effects of non-condensable gas on cavitating flow over a cylinder Abstract Filipe Brandao; Mrugank Bhatt; Krishnan Mahesh* University of Minnesota, Minneapolis, MN, USA The effect of non-condensable gas
Effects of non-condensable gas on cavitating flow over a cylinder Abstract Filipe Brandao; Mrugank Bhatt; Krishnan Mahesh* University of Minnesota, Minneapolis, MN, USA The effect of non-condensable gas
Performance Overview calibration Laboratory EP Instruments Messtechnik und Kalibrierung GmbH
 Performance Overview calibration Laboratory EP Instruments Messtechnik und Kalibrierung GmbH 1 D-K-15143-01-00 Services around the measurement technology in Germanys most accurate DAkkS laboratory*. Whether
Performance Overview calibration Laboratory EP Instruments Messtechnik und Kalibrierung GmbH 1 D-K-15143-01-00 Services around the measurement technology in Germanys most accurate DAkkS laboratory*. Whether
Laser-Induced Bubbles in Glycerol-Water Mixtures
 Laser-Induced Bubbles in Glycerol-Water Mixtures Allison R. McCarn, Erin M. Englert, and Gary A. Williams Department of Physics and Astronomy, University of California, Los Angeles, California 90095, USA
Laser-Induced Bubbles in Glycerol-Water Mixtures Allison R. McCarn, Erin M. Englert, and Gary A. Williams Department of Physics and Astronomy, University of California, Los Angeles, California 90095, USA
CCC Annual Report. UIUC, August 14, Gas Flow Through Upper Tundish Nozzle Refractory and Bubble Size Evolution Inside SEN
 CCC Annual Report UIUC, August 14, 213 Gas Flow Through Upper Tundish Nozzle Refractory and Bubble Size Evolution Inside SEN Rui Liu and Seong-Mook Cho Department of Mechanical Science & Engineering University
CCC Annual Report UIUC, August 14, 213 Gas Flow Through Upper Tundish Nozzle Refractory and Bubble Size Evolution Inside SEN Rui Liu and Seong-Mook Cho Department of Mechanical Science & Engineering University
Gas Vapor Injection on Refrigerant Cycle Using Piston Technology
 Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 2012 Gas Vapor Injection on Refrigerant Cycle Using Piston Technology Sophie
Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 2012 Gas Vapor Injection on Refrigerant Cycle Using Piston Technology Sophie
Bubble-bubble interactions and wall pressures/temperatures produced by the collapse of a bubble pair near a rigid surface
 Bubble-bubble interactions and wall pressures/temperatures produced by the collapse of a bubble pair near a rigid surface Abstract Shahaboddin A. Beig*; Eric Johnsen; Mechanical Engineering Department,
Bubble-bubble interactions and wall pressures/temperatures produced by the collapse of a bubble pair near a rigid surface Abstract Shahaboddin A. Beig*; Eric Johnsen; Mechanical Engineering Department,
Numerical prediction and experimental verification of cavitation of Globe type Control Valves
 Proceedings of the 7 th International Symposium on Cavitation CAV2009 Paper No. 22 August 17-22, 2009, Ann Arbor, Michigan, USA Numerical prediction and experimental verification of cavitation of Globe
Proceedings of the 7 th International Symposium on Cavitation CAV2009 Paper No. 22 August 17-22, 2009, Ann Arbor, Michigan, USA Numerical prediction and experimental verification of cavitation of Globe
Chapter 14-Gases. Dr. Walker
 Chapter 14-Gases Dr. Walker State of Matter Gases are one of the four states of matter along with solids, liquids, and plasma Conversion to Gases From liquids Evaporation Example: Boiling water From solids
Chapter 14-Gases Dr. Walker State of Matter Gases are one of the four states of matter along with solids, liquids, and plasma Conversion to Gases From liquids Evaporation Example: Boiling water From solids
CHEMISTRY - CLUTCH CH.5 - GASES.
 !! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
!! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
Kyoung-Su Im, Z.-C Zhang, and Grant Cook, Jr. LSTC, Livermore, CA USA. 06/14/2016, Dearborn, MI
 Kyoung-Su Im, Z.-C Zhang, and Grant Cook, Jr. LSTC, Livermore, CA 94551 USA 06/14/2016, Dearborn, MI New Inflator Models! Inflator models In LS-DYNA: Pyrotechnic inflator (PI) model. Hybrid cold flow model
Kyoung-Su Im, Z.-C Zhang, and Grant Cook, Jr. LSTC, Livermore, CA 94551 USA 06/14/2016, Dearborn, MI New Inflator Models! Inflator models In LS-DYNA: Pyrotechnic inflator (PI) model. Hybrid cold flow model
Name /74. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch 11 Gases STUDY GUIDE Accelerated Chemistry SCANTRON Name /74 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements
Ch 11 Gases STUDY GUIDE Accelerated Chemistry SCANTRON Name /74 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements
Visual Observation of Nucleate Boiling and Sliding Phenomena of Boiling Bubbles on a Horizontal Tube Heater
 Proceedings of the 2 nd World Congress on Mechanical, Chemical, and Material Engineering (MCM'16) Budapest, Hungary August 22 23, 216 Paper No. HTFF 146 DOI:.11159/htff16.146 Visual Observation of Nucleate
Proceedings of the 2 nd World Congress on Mechanical, Chemical, and Material Engineering (MCM'16) Budapest, Hungary August 22 23, 216 Paper No. HTFF 146 DOI:.11159/htff16.146 Visual Observation of Nucleate
Chapter 15 Fluid. Density
 Density Chapter 15 Fluid Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle By Dr. Weining man 1 Units of Chapter 15 Fluid
Density Chapter 15 Fluid Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle By Dr. Weining man 1 Units of Chapter 15 Fluid
Gases. Unit 10. How do gases behave?
 Gases Unit 10 How do gases behave? Gases are perhaps the most mysterious of all of the phases of matter. For the most part gases are invisible to us, and it was once believed that in the air there is no
Gases Unit 10 How do gases behave? Gases are perhaps the most mysterious of all of the phases of matter. For the most part gases are invisible to us, and it was once believed that in the air there is no
Chapter 2: Pure Substances a) Phase Change, Property Tables and Diagrams
 Chapter 2: Pure Substances a) Phase Change, Property Tables and Diagrams In this chapter we consider the property values and relationships of a pure substance (such as water) which can exist in three phases
Chapter 2: Pure Substances a) Phase Change, Property Tables and Diagrams In this chapter we consider the property values and relationships of a pure substance (such as water) which can exist in three phases
UNIT 10 - GASES. Notes & Worksheets - Honors
 Ideal Gas Equation 1 WKSHT 1.) What is the pressure exerted by 2.0 moles of an ideal gas when it occupies a volume of 12.0 L at 373 K? 2.) A flashbulb of volume 2.6 cm 3 contains O 2 gas at a pressure
Ideal Gas Equation 1 WKSHT 1.) What is the pressure exerted by 2.0 moles of an ideal gas when it occupies a volume of 12.0 L at 373 K? 2.) A flashbulb of volume 2.6 cm 3 contains O 2 gas at a pressure
Micro Channel Recuperator for a Reverse Brayton Cycle Cryocooler
 Micro Channel Recuperator for a Reverse Brayton Cycle Cryocooler C. Becnel, J. Lagrone, and K. Kelly Mezzo Technologies Baton Rouge, LA USA 70806 ABSTRACT The Missile Defense Agency has supported a research
Micro Channel Recuperator for a Reverse Brayton Cycle Cryocooler C. Becnel, J. Lagrone, and K. Kelly Mezzo Technologies Baton Rouge, LA USA 70806 ABSTRACT The Missile Defense Agency has supported a research
Vapour pressure of liquids SURFACE TENSION
 Vapour pressure of liquids A liquid in a closed container is subjected to partial vapour pressure due to the escaping molecules from the surface; it reaches a stage of equilibrium when this pressure reaches
Vapour pressure of liquids A liquid in a closed container is subjected to partial vapour pressure due to the escaping molecules from the surface; it reaches a stage of equilibrium when this pressure reaches
4. Using the kinetic molecular theory, explain why a gas can be easily compressed, while a liquid and a solid cannot?
 Name Period HW 1 Worksheet (Goals 1-4) - Kinetic Molecular Theory 1. Describe how gases, liquids, and solids compare using the following table. Solids Liquids Gases Volume (definite or indefinite) Molecular
Name Period HW 1 Worksheet (Goals 1-4) - Kinetic Molecular Theory 1. Describe how gases, liquids, and solids compare using the following table. Solids Liquids Gases Volume (definite or indefinite) Molecular
CTB3365x Introduction to Water Treatment
 CTB3365x Introduction to Water Treatment D4b Aeration Doris van Halem Did you know that there are not just gasses in your glass of sparkling coke, but also in the tap water you drink? Welcome to the water
CTB3365x Introduction to Water Treatment D4b Aeration Doris van Halem Did you know that there are not just gasses in your glass of sparkling coke, but also in the tap water you drink? Welcome to the water
Dr. Rogers Chapter 5 Homework Chem 111 Fall 2003
 Dr. Rogers Chapter 5 Homework Chem 111 Fall 2003 From textbook: 7-33 odd, 37-45 odd, 55, 59, 61 1. Which gaseous molecules (choose one species) effuse slowest? A. SO 2 (g) B. Ar(g) C. NO(g) D. Ne(g) E.
Dr. Rogers Chapter 5 Homework Chem 111 Fall 2003 From textbook: 7-33 odd, 37-45 odd, 55, 59, 61 1. Which gaseous molecules (choose one species) effuse slowest? A. SO 2 (g) B. Ar(g) C. NO(g) D. Ne(g) E.
Judith Herzfeld 1996,1998. These exercises are provided here for classroom and study use only. All other uses are copyright protected.
 Judith Herzfeld 1996,1998 These exercises are provided here for classroom and study use only. All other uses are copyright protected. 2.7-110 A yardstick laid on a table with a few inches hanging over
Judith Herzfeld 1996,1998 These exercises are provided here for classroom and study use only. All other uses are copyright protected. 2.7-110 A yardstick laid on a table with a few inches hanging over
A07 Surfactant Induced Solubilization and Transfer Resistance in Gas-Water and Gas-Oil Systems
 A07 Surfactant Induced Solubilization and Transfer Resistance in Gas-Water and Gas-Oil Systems R Farajzadeh* (TU Delft), A. Banaei (TU Delft), J. Kinkela (TU Delft), T. deloos (TU Delft), S. Rudolph (TU
A07 Surfactant Induced Solubilization and Transfer Resistance in Gas-Water and Gas-Oil Systems R Farajzadeh* (TU Delft), A. Banaei (TU Delft), J. Kinkela (TU Delft), T. deloos (TU Delft), S. Rudolph (TU
The Characteristics of Cavitation Bubbles Induced by the Secondary Shock Wave in an HM-3 Lithotripter and Its Effect on Stone Comminution
 The Characteristics of Cavitation Bubbles Induced by the Secondary Shock Wave in an HM-3 Lithotripter and Its Effect on Stone Comminution Yufeng Zhou, Jun Qin, and Pei Zhong Department of Mechanical Engineering
The Characteristics of Cavitation Bubbles Induced by the Secondary Shock Wave in an HM-3 Lithotripter and Its Effect on Stone Comminution Yufeng Zhou, Jun Qin, and Pei Zhong Department of Mechanical Engineering
Name Chemistry Pre-AP
 Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Experimental Investigation of the Rise Behavior of Live-Oil Droplets during Deep-Sea Oil Spills
 Marine Technology Society (MTS) / Gulf of Mexico Research Initiative (GoMRI) Advancing Oil Spill Research Part 2 Webinar, March 13, 2018 Experimental Investigation of the Rise Behavior of Live-Oil Droplets
Marine Technology Society (MTS) / Gulf of Mexico Research Initiative (GoMRI) Advancing Oil Spill Research Part 2 Webinar, March 13, 2018 Experimental Investigation of the Rise Behavior of Live-Oil Droplets
CFD Modelling of LPG dispersion
 CHAM Limited Pioneering CFD Software for Education & Industry CFD Modelling of LPG dispersion Demonstration example The following describes the numerical analysis of the dispersion of gas release from
CHAM Limited Pioneering CFD Software for Education & Industry CFD Modelling of LPG dispersion Demonstration example The following describes the numerical analysis of the dispersion of gas release from
Gas Pressure. Pressure is the force exerted per unit area by gas molecules as they strike the surfaces around them.
 Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Generating Calibration Gas Standards
 Technical Note 1001 Metronics Inc. Generating Calibration Gas Standards with Dynacal Permeation Devices Permeation devices provide an excellent method of producing known gas concentrations in the PPM and
Technical Note 1001 Metronics Inc. Generating Calibration Gas Standards with Dynacal Permeation Devices Permeation devices provide an excellent method of producing known gas concentrations in the PPM and
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT.
 Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
Figure 1: Two possible mechanisms of cavitation erosion.
 High speed observation of damage created by a collapse of a single cavitation bubble 1 Matevž Dular*, 1 Žan Pirc, 1 Tomaž Požar, 1 Rok Petkovšek 1 Faculty of Mechanical Engineering, University of Ljubljana,
High speed observation of damage created by a collapse of a single cavitation bubble 1 Matevž Dular*, 1 Žan Pirc, 1 Tomaž Požar, 1 Rok Petkovšek 1 Faculty of Mechanical Engineering, University of Ljubljana,
