Effects of Dutasteride on Prostate Carcinoma Primary Cultures: A Comparative Study With Finasteride and MK386
|
|
|
- Alexina Cole
- 6 years ago
- Views:
Transcription
1 Effects of Dutasteride on Prostate Carcinoma Primary Cultures: A Comparative Study With Finasteride and MK386 Claudio Festuccia,* Giovanni Luca Gravina,* Paola Muzi, Roberto Pomante, Adriano Angelucci, Carlo Vicentini and Mauro Bologna From the Departments of Experimental Medicine (CF, PM, AA, MB), Surgery (GLG, CV) and Basic and Applied Biology (MB), University of L Aquila, L Aquila and Pathology Department, G. Mazzini Hospital (RP), Teramo, Italy Purpose: The profound decrease in serum dihydrotestosterone observed with the dual -reductase 5 inhibitor dutasteride makes it an attractive agent for prostate cancer therapy. To our knowledge we compared for the first time the antitumor effe of dutasteride with that of the specific -reductase-1 5 inhibitor MK386 and the specific -reductase-2 5 inhibitor finasteride in human prostate primary cultures. Materials and Methods: Biochemical markers of the cellular response to -reductase 5 inhibitors were evaluated in primary cultures of prostate epithelial cancer cells from 54 patients with prostate carcinoma. Results: In our cohort of 54 patients prostate cancer cell growth decreased with dutasteride in 42 (about 78%), whereas i 21 (39%) it decreased with finasteride or MK386 alone. We observed a relationship between the levels -reductase of 5 enzymes in cell culture extracts and those revealed by immunohistochemistry in sections of samples from which we established primary cultures. Finasteride effects depended on -reductase-2 5 levels and they were higher when the reductase-1:2 ratio was low. However, dutasteride effects were related -reductase-1 to 5 and 2 levels, and were not influenced 5 by the 5 -reductase-1:2 ratio. Conversely the effects of MK386 were related -reductase-1 to 5 levels and they were higher when the 5 -reductase-1:2 ratio was high. Conclusions: Our data may provide a rationale for the use of a dual -reductase 5 inhibitor rather than a mono specific inhibitor for the prevention or treatment of early prostate cancer. This finding appears to confirm some preliminary clinica results and it could be due to the simultaneous presence of each -reductase 5 isoenzyme in prostate tumor cells. Key Words: prostate; prostatic neoplasms; cholestenone 5 alpha-reductase; receptors, androgen; dutasteride Dihydrotestosterone is the major intracellular growth nant type in benign prostate tissue, expression of the type 1 factor for normal and neoplastic prostatic epithelial enzyme seems to be increased in localized and more advanced prostate cancers. 2 4 cells due to its high affinity binding to AR. The intracellular DHT concentration determines the prostatic cell Finasteride, which is specific for SDRA2, was the first content via its ability to regulate the proportion of ligandsrd5a inhibitor tested as monotherapy for metastatic pros- cancer. 5 The clinical efficacy of finasteride monotherapy occupied AR. After a critical threshold of AR is occupied bytate DHT signal transduction pathways controlling the growth in metastatic cases has been modest. The reason for the and functional activities of the prostatic epithelium are ac-limitetivated. Thus, the intracellular DHT concentration is para- is a potent (ie IC nmol/l), time dependent, effectiveness of finasteride may be that, although it irreversible mount and regulated by the supply of testosterone and otherinhibitor of human SRD5A2, it is not as potent (ie IC precursors from the systemic circulation, and the complex nmol/l) and not an irreversible inhibitor of the human interplay between intracellular prostatic enzymes of andro- SRD5A1 isoform. gen metabolism, particularly the SRD5A family of reductive The SRD5A inhibitor dutasteride is identical to finasteride except in position 17. This modification shifts the enzymes that irreversibly convert testosterone into DHT. The SRD5A family includes 2 isoforms, of which each is serum half-life and increases the inhibition potency of dutasteride as a reversible SRD5A1 inhibitor and a time depen- 1 encoded by a distinct gene. SRD5A1 is expressed widely and it is the major isoform expressed in many tissues. 6,7 dent, irreversible SRD5A2 inhibitor. Due to its long serum SRD5A2, which is more restrictive in its expression, is the half-life and low IC major isoform expressed by male sex accessory tissues. Although the type 2 enzyme has been identified as the 50 for SRD5A1 and SRD5A2 dutasteride may effectively be used in clinical settings as a dual SRD5A domi- Submitted for publication September 24, Study received institutional review board approval. Supported by a grant from GlaxoSmithKline. * Equal study contribution. Correspondence: Department of Experimental Medicine, Via Vetoio, Coppito-2, L Aquila, Italy (telephone: ; FAX: ; maurobo@univaq.it). inhibitor. We determined whether dutasteride would decrease cell proliferation and induce apoptosis in a series of prostate epithelial cancer specimens in primary culture. We also compared the effects of dutasteride with those of finasteride and the SRD5A1 specific inhibitor MK386 which are effective in prostate cancer cell lines and primary cultures, as previously demonstrated. 8,9 Dutasteride is also able to in /08/ /0 367 Vol. 180, , July 2008 THE JOURNAL OF UROLOGY Printed in U.S.A. Copyright 2008 by AMERICAN UROLOGICAL ASSOCIATION DOI: /j.juro
2 368 EFFECTS OF DUTASTERIDE ON PROSTATE CANCER PRIMARY CULTURES duce cell growth inhibition and apoptosis in prostate cancer cells and primary cultures. 10,11 MATERIALS AND METHODS Patient Selection From December 2005 to December 2006 men with histologically proven prostate cancer were recruited at the urology clinic of our institution. Patients were eligible if they had localized histologically confirmed adenocarcinoma of the prostate and did not undergo previous anti-hormonal or radiation therapy, chemotherapy or investigational agents. Our institutional review board approved the protocol and written informed consent was obtained from all human subjects. Eligible patients formed a cohort of 54 men. All tumors were graded according to the Gleason system. Histopathological tumor staging was assessed according to the TNM 2002 system. Immunohistochemical Analysis Type I and II 5 -reductase expression was evaluated in 4 m tissue sections cut from blocks selected for the presence of representative tumor tissue. AR antibody (clone N20, Santa Cruz Biotechnology, Santa Cruz, California), and type I and II 5 -reductase antibody were used. Scoring Methods All cases were histopathologically and independently interpreted by 2 investigators (RP and MB) without discrepancies in all interpretations. For statistic evaluations IR was classified as 0 no staining; 1 faint, 2 moderate and 3 strong. We also considered the percent of prostate cancer cells expressing IR as a score of 1 less than 10%, 2 10% to 50% and 3 greater than 50% of cells with IR. The IHC score was derived from the sum of the previous 2 parameters. An IHC score of greater than 4 was considered high expression. Primary Cultures Samples were obtained from radical prostatectomy and used to establish primary tumor cultures. After surgery a wedgeshaped specimen of fresh prostate was removed. The parts of tissue adjacent to those used for primary cultures underwent pathological examination to confirm prostatic origin, diagnosis and the absence of other diseases. Primary cultures were grown and characterized according to a previously described method. 12 Apoptosis Apoptosis was quantified as the percent of cells with hypodiploid DNA, as assessed using a HT titer TACS assay kit (Trevigen, Gaithersburg, Maryland), which is a colorimetric quantitative assay for detecting apoptosis. Western Blot Analysis Total protein was isolated from cell extracts in RIPA buffer and protein content was assayed using a DC protein assay kit (Bio-Rad ). Protein (50 g) was run on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted. Blots were blocked with 1% bovine serum albumin/tris buffered saline and 0.1% Tween-20 for 1 hour at room temperature. Subsequently they were incubated in SDRA1, SDRA2, prostate specific antigen, AR or CK8/CK18 primary antibody. Blots were developed using an enhanced chemiluminescence substrate system (Amersham Biosciences, Newcastle-upon-Tyne, United Kingdom) for detecting horseradish peroxidase. Statistical Analysis Statistical analysis was performed using SPSS 11.0 software with p 0.05 considered significant. All statistical tests were 2-tailed. Differences in categorical variables were compared with the chi-square test. Continuous variables were analyzed using the Kruskal-Wallis test, followed by post hoc analysis (Tukey HSD) to compare the growth inhibition of primary tumor cultures after treatments. RESULTS SDR5 Levels in Prostate Cancer Tissues Table 1 lists patient clinical and pathological characteristics. The expression of SDR5A1 and was analyzed in tissue sections derived from 54 prostate tissue samples from radical prostatectomies used to establish primary cultures. Table 2 shows IHC results. We observed that 85% of samples (46 of 54) expressed at least 1 isoenzyme at high levels, that is 41% (22 of 54) showed high levels of SDR5A1, 32% (17 of 54) showed increased expression and 13% (7 of 54) had simultaneously high levels of the 2 SDR5 isoenzymes. We did not find statistically significant differences between SDR5A isoenzyme expression in our patient cohort (p 0.423). We observed also that the levels of SDR5A1 and did not significantly correlate with clinical and pathological parameters (data not shown). However, we noted a significant correlation between Gleason score and the percent of prostate cancers with high SDR5A1 and low expression (p 0.005, table 2). Pharmacological Treatment For pharmacological treatments primary cultures and cell 4 lines were seeded at a density of 2 cells 10 per dish in 50 mm Petri dishes. Cells were left to attach and grow in 5% fetal calf serum Dulbecco s modified Eagle s medium for 24 hours. All other cells were treated with finasteride, dutasteride and MK386 (5 and 10 M), always in the presence of testosterone (10 12 M). Cells trypsinized and resuspended in 20 ml saline were counted every 48 hours by a hemocytometer (LabRecyclers, Gaithersburg, Maryland) with 5 independent counts performed per dish. All experiments were done in triplicate. TABLE 1. Demographic and pathological characteristics in 54 study patients Variables Mean SD age 65 9 Mean SD prostate specific antigen at diagnosis (ng/ml) No. prostatectomy Gleason score (%): 6 or Less 30/54 (56) 7 18/54 (33) /54 (11) No. pathological stage (%): pt2c 38 (70) pt3a 16 (30)
3 EFFECTS OF DUTASTERIDE ON PROSTATE CANCER PRIMARY CULTURES 369 TABLE 2. Expression of SDR5A1 and 2 in prostatectomy specimens and prostate cancer primary tumor cultures No./Total No. (%) High SDR5A1 High High SDR5A1/Neg/Low High SDR5A1/Neg/Low Neg/Low SDR5A1/High Prostatectomy high expression 22/54 (41) 17/54 (32) 7/54 (13) 15/54 (28) 10/54 (19) p Value (chi-square test) Prostatectomy Gleason grade: /30 (33) 7/30 (23) 4/30 (13) 6/30 (20) 7/30 (23) 7 7/18 (39) 8/18 (44) 2/18 (11) 4/18 (22) 2/18 (11) /6 (83) 2/6 (33) 1/6 (17) 5/6 (83) 1/6 (17) p Value (chi-square test) Prostate Ca high expression 36/54 (67) 26/54 (48) 12/54 (22) 24/54 (44) 14/54 (26) p Value (chi-square test) SDR5 Levels in Prostate Cancer Tissue Primary Cultures Characterization of primary epithelial cancer cell cultures was done as previously described (see figure). 12 We also assessed the expression of SDR5A1 and SDRA2 by densitometric analysis of bands generated by Western blot analysis (part B of figure). Globally we observed that 67% of cultures (36 of 54) were SDR5A1 positive, whereas 48% (26 of 54) were positive (p 0.080). Only 4% of cultures (2 of 54) were doubly negative. Of the cases 44% (24 of 54) showed only SDR5A1 and 26% (14 of 54) showed only expression. Table 2 shows statistical analyses. Comparative Analysis of Dutasteride, Finasteride and MK386 Biological Effects When comparing drugs at the same doses (1 and at 5 M), we observed that dutasteride cell growth inhibition was higher with respect to finasteride and to MK386 (tables 3 to 5). Finasteride growth inhibition was higher compared to that of MK386 at 1 and 5 M. All previous comparisons were statistically highly significant. Using 1-way ANOVA we observed no statistically significant differences between cell growth inhibition and Gleason score for all pharmacological treatments. Apoptosis was measured at 72 hours of treatment. It was induced in 82% of primary cultures (44 of 54) treated with 5 M dutasteride. Finasteride (5 M) was able to induce apoptosis in 69% of primary cultures (37 of 54). Apoptosis was induced in 74% of primary cultures (40 of 54) treated with 1 M dutasteride. Instead, finasteride (1 M) was able to induce apoptosis in 57.4% of primary cultures (31 of 54). The difference between apoptosis induced by dutasteride and finasteride was statistically significant (tables 6 to 8). Comparisons between finasteride and MK386, and between dutasteride and MK386 also revealed a significant difference at the 2 drug concentrations. In addition, in this case apoptosis induced by the dual inhibition of SDR5A1/2 enzymes with dutasteride was similar to the expected inhibition of finasteride plus MK386. Comparisons Between Drug Effects, and SDR5A1 and 2 Expression We also performed correlation analysis between the effects of single drugs and the levels of SDR5 enzymes in primary TABLE 3. SRD5A inhibitor effects on tumor growth inhibition Mean SE % Inhibition* Prostate epithelial cells characterization in culture. A, light microscope shows isolated primary prostate epithelial cell culture from representative patient sample with characteristic cobblestone morphology associated with epithelial cell cultures. There was no morphological evidence of contaminating fibroblast/stromal cells. Reduced from 100. B, primary prostate cancer epithelial cells expressed cytokeratin 18 (K18), SDR5A1 (SR1) and SDRA2 (SR2) on Western blot * Vs primary tumor culture controls.
4 370 EFFECTS OF DUTASTERIDE ON PROSTATE CANCER PRIMARY CULTURES TABLE 4. SRD5A inhibitor effects on tumor growth inhibition cultures. We considered the percent of growth inhibition (decrease vs control) and the apoptotic rate measured at 5 M. Values were plotted vs the densitometric values of SDR5A enzymes. In this analysis we made certain observations. 1) The efficacy of finasteride significantly depended on levels present in primary cultures for cell growth inhibition and apoptosis (r 0.547, p 0.01 and r 0.836, p 0.001, respectively). 2) The efficacy of MK386 significantly depended on SDR5A1 levels present in primary cultures for cell growth inhibition and apoptosis (r 0.631, p 0.01 and r 0.756, p 0.005, respectively). 3) Dutasteride efficacy highly correlated with SDR5A1 expression (r 0.788, p and r 0.844, p 0.001) and with expression (r 0.624, p 0.01 and r 0.788, p 0.001) for cell growth inhibition and apoptosis, respectively. When considering the ratio between SDRA1 and 2, finasteride was highly effective when this ratio was low for cell growth inhibition and apoptosis (r 0.835, p 0.01 and r 0.638, p 0.05, respectively), whereas dutasteride effects did not show any significant influence in relation to this ratio. DISCUSSION Mean SE % Tumor Growth* 1 M finasteride vs 1 M dutasteride vs M finasteride vs 1 M MK vs M finasteride vs 5 M dutasteride vs M finasteride vs 5 M MK vs M dutasteride vs 1 M MK vs M dutasteride vs 5 M MK vs For all comparisons p * Vs primary tumor culture controls. During prostatic carcinogenesis molecular changes can determine a gain of function in the AR axis from a paracrine to an autocrine pathway, in which occupancy of AR by DHT in the nuclei of malignant cells directly controls the autocrine production of growth factors for the survival and proliferation of these malignant cells. Data from the Prostate Cancer Prevention Trial, in which the SRD5A2 specific inhibitor finasteride decreased the relative risk of biopsy proven prostate carcinoma by approximately 25%, 13 provide credible evidence of the effectiveness of such treatment in men with localized prostate cancer. Enhanced expression of the SRD5A1 isoform by prostate cancer cells can explain why previous clinical trials of finasteride for metastatic prostate TABLE 6. SRD5A inhibitor effects on apoptosis Mean SE % Apoptosis* * Apoptosis (absolute value of apoptosis cell percent in treated primary cultures) (absolute value of apoptotic cell percent in untreated primary cultures). cancer treatment have had limited success when used as monotherapy, 5 or when combined with antiandrogens. Dutasteride is a dual SDR5A inhibitor. This drug is being studied to decrease the risk of prostate cancer in men at risk in the Reduction by Dutasteride of Prostate Cancer Events trial, 14 of which the results will be available in In addition, the Reduction by Dutasteride of Clinical Progression Events in Expectant Management trial is being done to assess the effect of dutasteride on decreasing disease progression in men with low risk, localized prostate cancer. 15 The underlying study hypothesis is that by decreasing androgenic stimulation to prostate cancer tissue dutasteride will inhibit the growth of such cancers, thereby decreasing disease progression and eliminating the need for or extending the time to the implementation of more aggressive therapy. Dutasteride may have higher chemopreventive effects than finasteride by lowering intraprostatic DHT concentrations more effectively. 16 The primary end point of our study was the comparison of the effects of dutasteride, finasteride and the specific SDR5A1 inhibitor MK386 using primary cultures from prostate cancer tissue samples. The study of prostate cancer biology is made difficult by the lack of appropriate in vitro and in vivo models. The most used cancer cell lines, which have been established from human metastatic lesions, do not accurately recapitulate the biological behavior of primary tumors compared to primary cultures generated from clinical prostate cancer specimens. A procedure to propagate human prostatic epithelial cells in vitro for a limited number of cell generations has been developed by and optimized for prostate cancer cells from primary tumors by our research group. 12 Previously we have reported that finasteride effects were higher compared to MK386 effects. 9 In the current study to our knowledge we report for the first time that dutasteride is significantly more effective than MK386 or TABLE 5. SRD5A inhibitor effects on tumor growth inhibition vs prostatectomy Gleason grade Inhibition Eta Correlation p Value TABLE 7. SRD5A inhibitor effects on apoptosis Mean SE % Apoptosis* 1 M finasteride vs 1 M dutasteride vs M finasteride vs 1 M MK vs M dutasteride vs 1 M MK vs M finasteride vs 5 M dutasteride vs M finasteride vs 5 M MK vs M dutasteride vs 5 M MK vs For all comparisons p * Apoptosis (absolute value of apoptosis cell percent in treated primary cultures) (absolute value of apoptotic cell percent in untreated primary cultures).
5 EFFECTS OF DUTASTERIDE ON PROSTATE CANCER PRIMARY CULTURES 371 TABLE 8. SRD5A inhibitor effects on apoptosis vs prostatectomy Gleason grade Apoptosis Eta Correlation p Value finasteride in vitro. This result confirms the clinical data. It may be due to the simultaneous presence of the 2 SDR5A isoenzymes in prostate tumor cells. We observed that 72 hours of treatment with dutasteride was able to induce apoptosis in almost all primary cultures. This finding is different from those of McCrohan et al, who analyzed apoptosis at 24 hours of culture, 11 and similar to those of Lazier et al, who analyzed dutasteride effects at 9 days of incubation. 10 McCrohan et al justified the choice of 24 hours by indicating that long-term treatment (9 days) could mask dutasteride effects due to cell overgrowth and cell death due to confluency. 11 However, we think that shortterm treatment (24 hours) can induce just the initial phases of apoptosis due to the time requirements of hormonal inhibition and, thus, underestimate the effect on apoptosis. Therefore, we analyzed pro-apoptotic effects at 72 hours, when these effects appear to be maximal. In addition, the method which we used for apoptosis assay was a colorimetric kit, which is able to discriminate apoptosis from post-apoptotic necrosis. Moreover, McCrohan et al invoked the increased percent of a basal phenotype in primary cultures as a potential factor for their negative results in low grade tumors. In fact, basal cells would not require DHT for survival. 17 This seems not to be the case in our cultures because they had an androgen sensitive phenotype. Additionally, the heterogeneous response in terms of efficacy observed in our cultures after all treatments could have been due to different expression in SDR5A isoenzymes as well as in phosphoinositide 30-kinase/Akt activity, which is increased after the down modulation of PTEN (phosphatase and tensin homologue deleted from chromosome 10), as documented in about 30% of primary prostate cancers. 18 In this regard increased Akt activity seems to be associated with a decreased proapoptotic effect. 19 When analyzing the expression of SDR5A1 and 2, we observed a direct relationship between the levels of these enzymes in tissue cell culture extracts and those observed by IHC in tissue sections of samples from which we established primary cultures. This suggests that primary cultures maintain the biological properties of primary tumors and, therefore, they represent a good study model for evaluating drug efficacy. Additionally, we did not find statistically significant differences between SDR5A1 and 2 expression in our cohort in culture or in tissue sections. This is in agreement with the results of Nakamura et al, 20 although we found a lower percent of high SDR5A isoenzyme expression by IHC. This can be explained by considering differences in the clinical and pathological characteristics of patients, and in the scoring method. An interesting result is that finasteride effects depended on in vitro levels and they were higher when the SDR5A1:2 ratio was low, whereas dutasteride effects were related to SDR5A1 and 2 levels but were not influenced by the SDR5A1:2 ratio. Conversely the effects of MK386 were related to SDRA5A1 levels and they were higher when the SDR5A1:2 ratio was high. Moreover, the magnitude of cell growth inhibition and apoptosis induced by dutasteride was similar to the inhibition achieved by the combination of finasteride and MK386. In fact, in our patient cohort about 77.8% of tumors (42 of 54), which highly expressed either or both isoforms, could be effectively growth inhibited with dutasteride, whereas 38.9% (21 of 54), which highly expressed the SDRA5A2 isoform, could be growth inhibited with finasteride alone and 38.9% (21 of 54), which highly expressed the SDRA5A1 isoform, could be growth inhibited with MK386 alone (each p 0.043). CONCLUSIONS Our preclinical study suggests that a higher proportion of patients with prostate cancer could probably be treated advantageously with dutasteride compared to finasteride or MK386. We believe that this result may be due to the high expression of either or both of the SDR5A isoenzymes in prostate tumors. Nevertheless, clinical studies are needed to substantiate these preclinical results. ACKNOWLEDGMENTS Types I and II 5 -reductase antibody was provided by Lynn Thomas, Dalhousie University, Halifax, Nova Scotia, Canada. Finasteride and MK386 were provided by Dr. Luigi Carratelli, MSD Italy, Rome, Italy. Dutasteride was provided by Dr. Roger Rittmaster, GlaxoSmithKline, Research Triangle Park, North Carolina. Abbreviations and Acronyms AR androgen receptor DHT dihydrotestosterone IC 50 concentration inhibiting 50% response IHC immunohistochemistry IR immunoreactivity SDR5A steroid 5 -reductase SRD5A1 type I SRD5A isoform SRD5A2 type II SRD5A isoform REFERENCES 1. Russell DW and Wilson JD: Steroid 5 -reductase: two genes/ two enzymes. Annu Rev Biochem 1994; 63: Thomas LN, Lazier CB, Gupta R, Norman RW, Troyer DA, O Brien SP et al: Differential alterations in 5 alpha-reductase type 1 and type 2 levels during development and progression of prostate cancer. Prostate 2005; 63: Thomas LN, Douglas RC, Vessey JP, Gupta R, Fontaine D, Norman RW et al: 5alpha-reductase type 1 immunostaining is enhanced in some prostate cancers compared with benign prostatic hyperplasia epithelium. J Urol 2003; 170: Titus MA, Gregory CW, Ford OH III, Schell MJ, Maygarden SJ and Mohler JL: Steroid 5 -reductase isozymes I and II in recurrent prostate cancer. Clin Cancer Res 2005; 11: 4365.
6 372 EFFECTS OF DUTASTERIDE ON PROSTATE CANCER PRIMARY CULTURES 5. Thompson IM, Pauler Ankerst D, Chi C, Goodman PJ, Tangen CM, Lippman SM et al: Prediction of prostate cancer for patients receiving finasteride: results from the prostate cancer prevention trial. J Clin Oncol 2007; 25: Xu Y, Dalrymple SL, Becker RE, Denmeade SR and Isaacs JT: Pharmacologic basis for the enhanced efficacy of dutasteride against prostatic cancers. Clin Cancer Res 2006; 12: Moss ML, Kuzmic P, Stuart JD, Tian G, Peranteau AG, Frye SV et al: Inhibition of human steroid 5alpha reductases type I and II by 6-aza-steroids: structural determinants of one-step vs two-step mechanism. Biochemistry 1996; 35: Bologna M, Muzi P, Biordi L, Festuccia C and Vicentini C: Finasteride dose-dependently reduces the proliferation rate of the LnCap human prostatic cancer cell line in vitro. Urology 1995; 45: Festuccia C, Angelucci A, Gravina GL, Muzi P, Vicentini C and Bologna M: Effects of 5 alpha reductase inhibitors on androgen-dependent human prostatic carcinoma cells. J Cancer Res Clin Oncol 2005; 131: Lazier CB, Thomas LN, Douglas RC, Vessey JP and Rittmaster RS: Dutasteride, the dual 5alpha-reductase inhibitor, inhibits androgen action and promotes cell death in the LNCaP prostate cancer cell line. Prostate 2004; 58: McCrohan MA, Morrissey C, O Keane C, Mulligan N, Watson C, Smith J et al: Effects of the dual 5 alpha-reductase inhibitor dutasteride on apoptosis in primary cultures of prostate cancer epithelial cells and cell lines. Cancer 2006; 106: Festuccia C, Angelucci A, Gravina GL, Muzi P, Miano R, Vicentini C et al: Epithelial and prostatic marker expression in short-term primary cultures of human prostate tissue samples. Int J Oncol 2005; 26: Thorpe JF, Jain S, Marczylo TH, Gescher AJ, Steward WP and Mellon JK: A review of phase III clinical trials of prostate cancer chemoprevention. Ann R Coll Surg Engl 2007; 89: Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG et al: The influence of finasteride on the development of prostate cancer. N Engl J Med 2003; 349: Fleshner N, Gomella LG, Cookson MS, Finelli A, Evans A, Taneja S et al: Delay in the progression of low-risk prostate cancer: rationale and design of the Reduction by Dutasteride of Clinical Progression Events in Expectant Management (REDEEM) trial. Contemp Clin Trials 2007; 28: Gleave M, Qian J, Andreou C, Pommerville P, Chin J, Casey R et al: The effects of the dual 5a-reductase inhibitor dutasteride on localized prostate cancer results from a 4 month pre-radical prostatectomy study. Prostate 2006; 66: Heer R, Robson CN, Shenton BK and Leung HY: The role of androgen in determining differentiation and regulation of androgen receptor expression in the human prostatic epithelium transient amplifying population. J Cell Physiol 2007; 212: Yoshimoto M, Cutz JC, Nuin PA, Joshua AM, Bayani J, Evans AJ et al: Interphase FISH analysis of PTEN in histologic sections shows genomic deletions in 68% of primary prostate cancer and 23% of high-grade prostatic intra-epithelial neoplasias. Cancer Genet Cytogenet 2006; 169: Bertram J, Peacock JW, Fazli L, Mui AL, Chung SW, Cox ME et al: Loss of PTEN is associated with progression to androgen independence. Prostate 2006; 66: Nakamura Y, Suzuki T, Nakabayashi M, Endoh M, Sakamoto K, Mikami Y et al: In situ androgen producing enzymes in human prostate cancer. Endocr Relat Cancer 2005; 12: 101.
Clinical impact of type I and type II 5 alpha-reductase inhibition in prostatic disease: review and update 아주대학교김선일
 Clinical impact of type I and type II 5 alpha-reductase inhibition in prostatic disease: review and update 아주대학교김선일 Development of 5ARI Discovery of 5AR type I and type II Pharmacodynamic and pharmacokinetic
Clinical impact of type I and type II 5 alpha-reductase inhibition in prostatic disease: review and update 아주대학교김선일 Development of 5ARI Discovery of 5AR type I and type II Pharmacodynamic and pharmacokinetic
The effect of dutasteride on intraprostatic dihydrotestosterone concentrations in men with benign prostatic hyperplasia
 ORIGINAL ARTICLE (27) 1, 149 154 & 27 Nature Publishing Group All rights reserved 1365-7852/7 $3. www.nature.com/pcan The effect of dutasteride on intraprostatic dihydrotestosterone concentrations in men
ORIGINAL ARTICLE (27) 1, 149 154 & 27 Nature Publishing Group All rights reserved 1365-7852/7 $3. www.nature.com/pcan The effect of dutasteride on intraprostatic dihydrotestosterone concentrations in men
STUDY OF THE EFFECT OF AN EXTRACT OF Serenoa repens on the production of the 5-α reductasa enzyme
 STUDY OF THE EFFECT OF AN EXTRACT OF Serenoa repens on the production of the 5-α reductasa enzyme 1) ROLE OF 5-α-ALFA-REDUCTASA 5-α reductasas (5-α-R) are a family of enzymes involved in steroid metabolism.
STUDY OF THE EFFECT OF AN EXTRACT OF Serenoa repens on the production of the 5-α reductasa enzyme 1) ROLE OF 5-α-ALFA-REDUCTASA 5-α reductasas (5-α-R) are a family of enzymes involved in steroid metabolism.
Biochemical Applications of Computational Chemistry
 Biochemical Applications of Computational Chemistry 1 Chemistry Today: A Different View Old Way New Way Correlate Structural Design Interpret Desired Properties Synthesize Build Measure Simulate Compounds
Biochemical Applications of Computational Chemistry 1 Chemistry Today: A Different View Old Way New Way Correlate Structural Design Interpret Desired Properties Synthesize Build Measure Simulate Compounds
Reproductive DHT Analyte Information
 Reproductive DHT Analyte Information - 1 - DHT Introduction Dihydrotestosterone (DHT) together with other important steroid hormones such as testosterone, androstenedione (ASD) and dehydroepiandrosterone
Reproductive DHT Analyte Information - 1 - DHT Introduction Dihydrotestosterone (DHT) together with other important steroid hormones such as testosterone, androstenedione (ASD) and dehydroepiandrosterone
It is estimated that 24% to 90% of US men older than the age of
 REPORTS Finasteride Versus Dutasteride: A Real-world Economic Evaluation Thomas C. Fenter, MD; M. Chris Runken, PharmD; Libby Black, PharmD; Michael Eaddy, PharmD, PhD It is estimated that 24% to 90% of
REPORTS Finasteride Versus Dutasteride: A Real-world Economic Evaluation Thomas C. Fenter, MD; M. Chris Runken, PharmD; Libby Black, PharmD; Michael Eaddy, PharmD, PhD It is estimated that 24% to 90% of
EFFECTS OF REVIVOGEN SCALP THERAPY ON TESTOSTERONE METABOLISM IN RECONSTRUCTED HUMAN EPIDERMIS
 IN VITRO BIOLOGICAL TESTING BIOalternatives The state-of-the-art laboratory Proposal n : AD070315C-2 Study n : AD070315B EFFECTS OF REVIVOGEN SCALP THERAPY ON TESTOSTERONE METABOLISM IN RECONSTRUCTED HUMAN
IN VITRO BIOLOGICAL TESTING BIOalternatives The state-of-the-art laboratory Proposal n : AD070315C-2 Study n : AD070315B EFFECTS OF REVIVOGEN SCALP THERAPY ON TESTOSTERONE METABOLISM IN RECONSTRUCTED HUMAN
Natural Hair Transplant Medical Center, Inc Dove Street, Suite #250, Newport Beach, CA Phone
 Natural Hair Transplant Medical Center, Inc. 1000 Dove Street, Suite #250, Newport Beach, CA 92660 Phone-949-622-6969 Finasteride (PROPECIA ) Acknowledgement Finasteride is an oral medication, manufactured
Natural Hair Transplant Medical Center, Inc. 1000 Dove Street, Suite #250, Newport Beach, CA 92660 Phone-949-622-6969 Finasteride (PROPECIA ) Acknowledgement Finasteride is an oral medication, manufactured
The Effect of Finasteride and Dutasteride on the Growth of WPE1-NA22 Prostate Cancer Xenografts in Nude Mice
 The Effect of Finasteride and Dutasteride on the Growth of WPE1-NA22 Prostate Cancer Xenografts in Nude Mice Alexander B. Opoku-Acheampong, Michelle K. Nelsen, Dave Unis, Brian L. Lindshield* Department
The Effect of Finasteride and Dutasteride on the Growth of WPE1-NA22 Prostate Cancer Xenografts in Nude Mice Alexander B. Opoku-Acheampong, Michelle K. Nelsen, Dave Unis, Brian L. Lindshield* Department
WHY THE USE OF EITHER 5ALPHA REDUCTASE (5AR) INHIBITORS DUTASTERIDE/AVODART (MY CHOICE) OR FINASTERIDE/PROSCAR IN ANDROGEN DEPRIVATION THERAPY?
 WHY THE USE OF EITHER 5ALPHA REDUCTASE (5AR) INHIBITORS DUTASTERIDE/AVODART (MY CHOICE) OR FINASTERIDE/PROSCAR IN ANDROGEN DEPRIVATION THERAPY? by Charles (Chuck) Maack, Prostate Cancer Activist/Mentor
WHY THE USE OF EITHER 5ALPHA REDUCTASE (5AR) INHIBITORS DUTASTERIDE/AVODART (MY CHOICE) OR FINASTERIDE/PROSCAR IN ANDROGEN DEPRIVATION THERAPY? by Charles (Chuck) Maack, Prostate Cancer Activist/Mentor
PHYSICIANS CIRCULAR FINASTERIDE PROSCAR. Tablet 5-Alpha Reductase Inhibitor
 PHYSICIANS CIRCULAR FINASTERIDE PROSCAR Tablet 5-Alpha Reductase Inhibitor FINASTERIDE (PROSCAR) a synthetic 4-azasteroid compound, is a specific inhibitor of Type II 5α-reductase, an intracellular enzyme
PHYSICIANS CIRCULAR FINASTERIDE PROSCAR Tablet 5-Alpha Reductase Inhibitor FINASTERIDE (PROSCAR) a synthetic 4-azasteroid compound, is a specific inhibitor of Type II 5α-reductase, an intracellular enzyme
M0BCore Safety Profile. Pharmaceutical form(s)/strength: 5 mg SE/H/PSUR/0002/006 Date of FAR:
 M0BCore Safety Profile Active substance: Finasteride Pharmaceutical form(s)/strength: 5 mg P-RMS: SE/H/PSUR/0002/006 Date of FAR: 16.05.2014 4.3 Contraindications Finasteride is not indicated for use in
M0BCore Safety Profile Active substance: Finasteride Pharmaceutical form(s)/strength: 5 mg P-RMS: SE/H/PSUR/0002/006 Date of FAR: 16.05.2014 4.3 Contraindications Finasteride is not indicated for use in
Dutasteride and Metformin Reduce the Growth of LNCaP Cells and Alter the SREBP-1 Pathway
 Send Orders of Reprints at reprints@benthamscience.net 1 The Open Prostate Cancer Journal, 213, 6, 1-15 Open Access Dutasteride and Metformin Reduce the Growth of LNCaP Cells and Alter the SREBP-1 Pathway
Send Orders of Reprints at reprints@benthamscience.net 1 The Open Prostate Cancer Journal, 213, 6, 1-15 Open Access Dutasteride and Metformin Reduce the Growth of LNCaP Cells and Alter the SREBP-1 Pathway
FINCAR Tablets (Finasteride)
 Published on: 10 Jul 2014 FINCAR Tablets (Finasteride) Composition FINCAR Tablets Each film-coated tablet contains: Finasteride USP - 5 mg Dosage Form Tablet Pharmacology Pharmacodynamics Mechanism of
Published on: 10 Jul 2014 FINCAR Tablets (Finasteride) Composition FINCAR Tablets Each film-coated tablet contains: Finasteride USP - 5 mg Dosage Form Tablet Pharmacology Pharmacodynamics Mechanism of
EFFECTS OF CLEAROGEN ACNE LOTION ON TESTOSTERONE METABOLISM IN RECONSTRUCTED HUMAN EPIDERMIS
 IN VITRO BIOLOGICAL TESTING BIOalternatives The state-of-the-art laboratory Proposal n : AD070315C-2 Study n : AD070315A EFFECTS OF CLEAROGEN ACNE LOTION ON TESTOSTERONE METABOLISM IN RECONSTRUCTED HUMAN
IN VITRO BIOLOGICAL TESTING BIOalternatives The state-of-the-art laboratory Proposal n : AD070315C-2 Study n : AD070315A EFFECTS OF CLEAROGEN ACNE LOTION ON TESTOSTERONE METABOLISM IN RECONSTRUCTED HUMAN
Skin metabolism of steroid hormones as endogenous compounds?
 Skin metabolism of steroid hormones as endogenous compounds? Van Luu-The Department of Molecular Medicine Laval University Québec, Canada This work has been supported by L Oréal Research Steroid hormones
Skin metabolism of steroid hormones as endogenous compounds? Van Luu-The Department of Molecular Medicine Laval University Québec, Canada This work has been supported by L Oréal Research Steroid hormones
Male pattern baldness is the most common type of balding among males. It affects roughly, 50% of men by the age of 50,
 Dihydrotestosterone (DHT) Male pattern baldness is the most common type of balding among males. It affects roughly, 30% of men by the age of 30, 50% of men by the age of 50, 57% of men by the age of 60.
Dihydrotestosterone (DHT) Male pattern baldness is the most common type of balding among males. It affects roughly, 30% of men by the age of 30, 50% of men by the age of 50, 57% of men by the age of 60.
Update on the use of dutasteride in the management of benign prostatic hypertrophy
 REVIEW Update on the use of dutasteride in the management of benign prostatic hypertrophy Joe Miller Thomas Tarter Division of Urology, Southern Illinois University School of Medicine, Springfield, IL,
REVIEW Update on the use of dutasteride in the management of benign prostatic hypertrophy Joe Miller Thomas Tarter Division of Urology, Southern Illinois University School of Medicine, Springfield, IL,
Figure 2. RESULTS DATA ANALYSIS
 ANDROGEN PARAMETERS IN HIRSUTE AND NORMAL FEMALE PATIENTS: IS THERE A ROLE FOR THE FREE ANDROGEN INDEX (FAI)? Castracane VD 1, Childress E 1, Tawwater B 1, Vankrieken L 2, El Shami AS 2 ( 1 Department
ANDROGEN PARAMETERS IN HIRSUTE AND NORMAL FEMALE PATIENTS: IS THERE A ROLE FOR THE FREE ANDROGEN INDEX (FAI)? Castracane VD 1, Childress E 1, Tawwater B 1, Vankrieken L 2, El Shami AS 2 ( 1 Department
Comparison of Clinical Efficacy of Finasteride and Dutasteride as 5-alpha Reductase Inhibitor
 대한남성과학회지 : 제 30 권제 1 호 2012 년 4 월 Korean J Androl. Vol. 30, No. 1, April 2012 http://dx.doi.org/10.5534/kja.2012.30.1.45 Comparison of Clinical Efficacy of Finasteride and Dutasteride as 5-alpha Reductase
대한남성과학회지 : 제 30 권제 1 호 2012 년 4 월 Korean J Androl. Vol. 30, No. 1, April 2012 http://dx.doi.org/10.5534/kja.2012.30.1.45 Comparison of Clinical Efficacy of Finasteride and Dutasteride as 5-alpha Reductase
WADA Technical Document TD2014EAAS. Endogenous Anabolic Androgenic Steroids Measurement and Reporting
 Endogenous Anabolic Androgenic Steroids Measurement and Reporting 1.0 Introduction The purpose of this Technical is to harmonize the approaches to the measurement and reporting of endogenous anabolic androgenic
Endogenous Anabolic Androgenic Steroids Measurement and Reporting 1.0 Introduction The purpose of this Technical is to harmonize the approaches to the measurement and reporting of endogenous anabolic androgenic
Castration resistant prostate cancer-what is it? and what do we do about it? Urology Postgraduate Course February 13, 2009
 Castration resistant prostate cancer-what is it? and what do we do about it? Urology Postgraduate Course February 13, 2009 Charles J Ryan, MD Associate Professor of Clinical Medicine Helen Diller Family
Castration resistant prostate cancer-what is it? and what do we do about it? Urology Postgraduate Course February 13, 2009 Charles J Ryan, MD Associate Professor of Clinical Medicine Helen Diller Family
Effect of Dutasteride on the Risk of Prostate Cancer
 The new england journal of medicine original article Effect of on the Risk of Prostate Cancer Gerald L. Andriole, M.D., David G. Bostwick, M.D., Otis W. Brawley, M.D., Leonard G. Gomella, M.D., Michael
The new england journal of medicine original article Effect of on the Risk of Prostate Cancer Gerald L. Andriole, M.D., David G. Bostwick, M.D., Otis W. Brawley, M.D., Leonard G. Gomella, M.D., Michael
FINASTERIDE (PROPECIA, PROSCAR) SIDE EFFECT & CONSENT FORM
 750 West Broadway Street Suite 905 Vancouver, BC M5Z 1K1 FAX: (604) 648-9003 vancouveroffice@donovanmedical.com FINASTERIDE (PROPECIA, PROSCAR) SIDE EFFECT & CONSENT FORM What is finasteride? Finasteride
750 West Broadway Street Suite 905 Vancouver, BC M5Z 1K1 FAX: (604) 648-9003 vancouveroffice@donovanmedical.com FINASTERIDE (PROPECIA, PROSCAR) SIDE EFFECT & CONSENT FORM What is finasteride? Finasteride
Prostate enlargement, also known as benign prostatic hyperplasia
 REPORTS Differences in Alpha Blocker Usage Among Enlarged Prostate Patients Receiving Combination Therapy with 5ARIs Michael Naslund, MD, MBA; Libby Black, PharmD; Michael Eaddy, PharmD, PhD; LaKeasha
REPORTS Differences in Alpha Blocker Usage Among Enlarged Prostate Patients Receiving Combination Therapy with 5ARIs Michael Naslund, MD, MBA; Libby Black, PharmD; Michael Eaddy, PharmD, PhD; LaKeasha
ANNEX 3 TO THE DRAFT REPORT OF THE OECD VALIDATION OF THE RAT HERSHBERGER BIOASSAY: PHASE 2
 ANNEX 3 TO THE DRAFT REPORT OF THE OECD VALIDATION OF THE RAT HERSHBERGER BIOASSAY: PHASE 2 Hershberger Assay Interlaboratory Study: Statistical analysis of phase 2 data from 16 laboratories in the multi-chemical
ANNEX 3 TO THE DRAFT REPORT OF THE OECD VALIDATION OF THE RAT HERSHBERGER BIOASSAY: PHASE 2 Hershberger Assay Interlaboratory Study: Statistical analysis of phase 2 data from 16 laboratories in the multi-chemical
Elements for a Public Summary. Overview of disease epidemiology
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Benign prostatic hyperplasia (BPH) (an increase in size of the prostate that is not cancerous) is the most prevalent of all diseases
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Benign prostatic hyperplasia (BPH) (an increase in size of the prostate that is not cancerous) is the most prevalent of all diseases
DUPROST Capsules (Dutasteride)
 Published on: 10 Jul 2014 DUPROST Capsules (Dutasteride) Composition Each soft gelatin capsule contains: Dutasteride... 0.5 mg Dosage Form Capsule Pharmacology Pharmacodynamics Mechanism of Action Dutasteride
Published on: 10 Jul 2014 DUPROST Capsules (Dutasteride) Composition Each soft gelatin capsule contains: Dutasteride... 0.5 mg Dosage Form Capsule Pharmacology Pharmacodynamics Mechanism of Action Dutasteride
Summary. Introduction. Dow Stough, MD
 Original Contribution Blackwell Publishing ORIGINAL Inc CONTRIBUTION Dutasteride improves male pattern hair loss in a randomized study in identical twins Dow Stough, MD The Dermatology Clinic, Hot Springs,
Original Contribution Blackwell Publishing ORIGINAL Inc CONTRIBUTION Dutasteride improves male pattern hair loss in a randomized study in identical twins Dow Stough, MD The Dermatology Clinic, Hot Springs,
PRODUCT INFORMATION TESTOVIRON DEPOT. (testosterone enanthate)
 PRODUCT INFORMATION TESTOVIRON DEPOT (testosterone enanthate) NAME OF THE MEDICINE Testosterone enanthate is designated chemically as 17 beta-heptanoyloxy-4-androstene-3- one. The empirical formula of
PRODUCT INFORMATION TESTOVIRON DEPOT (testosterone enanthate) NAME OF THE MEDICINE Testosterone enanthate is designated chemically as 17 beta-heptanoyloxy-4-androstene-3- one. The empirical formula of
1001 West Broadway, Vancouver, BC V6H 4B1. Topical Finasteride
 1001 West Broadway, Vancouver, BC V6H 4B1 Topical Finasteride 1 Topical finasteride is a solution containing the drug finasteride typically sold under the brand names Propecia and Proscar. The Finasteride
1001 West Broadway, Vancouver, BC V6H 4B1 Topical Finasteride 1 Topical finasteride is a solution containing the drug finasteride typically sold under the brand names Propecia and Proscar. The Finasteride
Efficacy and Safety of Long-TermTreatment with the Dual 5a-Reductase Inhibitor Dutasteride in Men with Symptomatic Benign Prostatic Hyperplasia
 European Urology European Urology 46 (2004) 488 495 Efficacy and Safety of Long-TermTreatment with the Dual 5a-Reductase Inhibitor Dutasteride in Men with Symptomatic Benign Prostatic Hyperplasia Frans
European Urology European Urology 46 (2004) 488 495 Efficacy and Safety of Long-TermTreatment with the Dual 5a-Reductase Inhibitor Dutasteride in Men with Symptomatic Benign Prostatic Hyperplasia Frans
Androgenes and Antiandrogenes
 Androgenes and Antiandrogenes Androgens The androgens are a group of steroids that have anabolic and/or masculinizing effects in both males and females. Testosterone [tess-toss-terone], the most important
Androgenes and Antiandrogenes Androgens The androgens are a group of steroids that have anabolic and/or masculinizing effects in both males and females. Testosterone [tess-toss-terone], the most important
Pharmacogenetic analysis of human steroid 5α reductase type II: comparison of finasteride and dutasteride
 617 Pharmacogenetic analysis of human steroid 5α reductase type II: comparison of finasteride and dutasteride Nick Makridakis 1 and Juergen K V Reichardt 1,2 1 Department of Biochemistry and Molecular
617 Pharmacogenetic analysis of human steroid 5α reductase type II: comparison of finasteride and dutasteride Nick Makridakis 1 and Juergen K V Reichardt 1,2 1 Department of Biochemistry and Molecular
Current status of 5α-reductase inhibitors in the management of lower urinary tract symptoms and BPH
 World J Urol (2010) 28:9 15 DOI 10.1007/s00345-009-0493-y TOPIC PAPER Current status of 5α-reductase inhibitors in the management of lower urinary tract symptoms and BPH Stavros Gravas Matthias Oelke Received:
World J Urol (2010) 28:9 15 DOI 10.1007/s00345-009-0493-y TOPIC PAPER Current status of 5α-reductase inhibitors in the management of lower urinary tract symptoms and BPH Stavros Gravas Matthias Oelke Received:
Pre-Lab. 1. What do people mean when they say teenagers have raging hormones?
 Name: Period: Date: You ve got MALE! Hormonal Control of Male Reproductive Processes This simulation explores how sex determination works. You will learn about the impact of testosterone concentration
Name: Period: Date: You ve got MALE! Hormonal Control of Male Reproductive Processes This simulation explores how sex determination works. You will learn about the impact of testosterone concentration
MALE PATTERN BALDNESS
 MALE PATTERN BALDNESS Male-pattern hair loss (MPHL), also known as androgenic alopecia and male pattern baldness, is hair loss that occurs due to an underlying susceptibility of hair follicles to shrinkage
MALE PATTERN BALDNESS Male-pattern hair loss (MPHL), also known as androgenic alopecia and male pattern baldness, is hair loss that occurs due to an underlying susceptibility of hair follicles to shrinkage
Associate Professor Geoff Braatvedt
 Associate Professor Geoff Braatvedt Endocrinologist Diabetologist and Physician Green Lane and Auckland City Hospitals Auckland 14:00-14:55 WS #145: Approach to Low Testosterone Values 15:05-16:00 WS #157:
Associate Professor Geoff Braatvedt Endocrinologist Diabetologist and Physician Green Lane and Auckland City Hospitals Auckland 14:00-14:55 WS #145: Approach to Low Testosterone Values 15:05-16:00 WS #157:
Updates on Anti-doping and TUE Management in Paralympic Sport
 International Paralympic Committee Updates on Anti-doping and TUE Management in Paralympic Sport Matthew Fedoruk, Ph.D. March 15, 2018 PyeongChang 2018 IPC Medical / Sports Science Committee Workshops
International Paralympic Committee Updates on Anti-doping and TUE Management in Paralympic Sport Matthew Fedoruk, Ph.D. March 15, 2018 PyeongChang 2018 IPC Medical / Sports Science Committee Workshops
EFFECTS OF METHYLHEXANAMINE (DMAA) ON C2C12 AND 3T3 STEM CELLS. Cameron Franz Pittsburgh Central Catholic High School Grade 11
 EFFECTS OF METHYLHEXANAMINE (DMAA) ON C2C12 AND 3T3 STEM CELLS Cameron Franz Pittsburgh Central Catholic High School Grade 11 Tissue Engineering TE is the development and manipulation of artificial implants,
EFFECTS OF METHYLHEXANAMINE (DMAA) ON C2C12 AND 3T3 STEM CELLS Cameron Franz Pittsburgh Central Catholic High School Grade 11 Tissue Engineering TE is the development and manipulation of artificial implants,
Analysis of Variance. Copyright 2014 Pearson Education, Inc.
 Analysis of Variance 12-1 Learning Outcomes Outcome 1. Understand the basic logic of analysis of variance. Outcome 2. Perform a hypothesis test for a single-factor design using analysis of variance manually
Analysis of Variance 12-1 Learning Outcomes Outcome 1. Understand the basic logic of analysis of variance. Outcome 2. Perform a hypothesis test for a single-factor design using analysis of variance manually
Effect of dutasteride 0.5 mg/d in men with androgenetic alopecia recalcitrant to finasteride
 Report Effect of dutasteride 0.5 mg/d in men with androgenetic alopecia recalcitrant to finasteride Jae Yoon Jung 1, MD, Je Ho Yeon 1, MD, Jee Woong Choi 1, MD, Soon Hyo Kwon 1, MD, Beom Joon Kim 2, MD,
Report Effect of dutasteride 0.5 mg/d in men with androgenetic alopecia recalcitrant to finasteride Jae Yoon Jung 1, MD, Je Ho Yeon 1, MD, Jee Woong Choi 1, MD, Soon Hyo Kwon 1, MD, Beom Joon Kim 2, MD,
Development of a Clinical Research Method for the Measurement of Testosterone. Dihydrotestosterone
 Development of a Clinical Research Method for the Measurement of Testosterone and Dihydrotestosterone Martijn van Faassen, Maarten van den Noort and Ido Kima University Medical Center, Groningen, Netherlands
Development of a Clinical Research Method for the Measurement of Testosterone and Dihydrotestosterone Martijn van Faassen, Maarten van den Noort and Ido Kima University Medical Center, Groningen, Netherlands
Sue Marty, Ph.D., D.A.B.T. The Dow Chemical Company TERA Endocrine Workshop April 23, 2013
 Sue Marty, Ph.D., D.A.B.T. The Dow Chemical Company TERA Endocrine Workshop April 23, 2013 Agenda Value of WoE/MoA determinations in EDSP Information used to examine potential endocrine activity and/or
Sue Marty, Ph.D., D.A.B.T. The Dow Chemical Company TERA Endocrine Workshop April 23, 2013 Agenda Value of WoE/MoA determinations in EDSP Information used to examine potential endocrine activity and/or
The ICL Insider. Lab Testing: Testosterone. In This Issue. The Debate
 The ICL Insider February 2017 Volume 2, Issue 2 Lab Testing: Testosterone In This Issue Testosterone shashtilak@iclabs.ca Next Issue: Expected Release April 2017 The Debate Aging is accompanied by various
The ICL Insider February 2017 Volume 2, Issue 2 Lab Testing: Testosterone In This Issue Testosterone shashtilak@iclabs.ca Next Issue: Expected Release April 2017 The Debate Aging is accompanied by various
biosensis Human Soluble Tumor Necrosis Factor receptor type II (stnfrii) ELISA Kit Protocol
 biosensis Human Soluble Tumor Necrosis Factor receptor type II (stnfrii) ELISA Kit Protocol Catalog No: BEK-2103-2P For quantitative detection of human soluble TNFRII (stnfrii) in human cell culture supernatants,
biosensis Human Soluble Tumor Necrosis Factor receptor type II (stnfrii) ELISA Kit Protocol Catalog No: BEK-2103-2P For quantitative detection of human soluble TNFRII (stnfrii) in human cell culture supernatants,
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 AVODART SOFT CAPSULES 0.5 MG 1. NAME OF THE MEDICINAL PRODUCT AVODART 0.5 mg capsules, soft. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 0.5 mg dutasteride. For excipients, see 6.1.
AVODART SOFT CAPSULES 0.5 MG 1. NAME OF THE MEDICINAL PRODUCT AVODART 0.5 mg capsules, soft. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 0.5 mg dutasteride. For excipients, see 6.1.
CHEMILUMINESCENCE ENZYME IMMUNOASSAY (CLIA) TESTOSTERONE. Testosterone. Cat #
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
Use of biotechnology to improve muscle growth in aquaculture species: Preliminary results on the use of myostatin in tilapia
 Nov. 12, 2011 HAAA Workshop Use of biotechnology to improve muscle growth in aquaculture species: Preliminary results on the use of myostatin in tilapia Yong Soo Kim, PhD Department of Human Nutrition,
Nov. 12, 2011 HAAA Workshop Use of biotechnology to improve muscle growth in aquaculture species: Preliminary results on the use of myostatin in tilapia Yong Soo Kim, PhD Department of Human Nutrition,
F7 (Human) Chromogenic Activity Assay Kit
 F7 (Human) Chromogenic Activity Assay Kit Catalog Number KA0971 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the
F7 (Human) Chromogenic Activity Assay Kit Catalog Number KA0971 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the
TESTOFEN HUMAN CLINICAL TRIAL GENCOR PACIFIC, INC. Copyright 2006 by Gencor Pacific, Inc.
 GENCOR PACIFIC, INC. 920 E. Orangethorpe Avenue, Suite B, Anaheim, CA 92801 Ph: 714.870.8723 714.870.8724 efax: 732.875.0306 drjit@gencorpacific.com gita@gencorpacific.com www.gencorpacific.com TESTOFEN
GENCOR PACIFIC, INC. 920 E. Orangethorpe Avenue, Suite B, Anaheim, CA 92801 Ph: 714.870.8723 714.870.8724 efax: 732.875.0306 drjit@gencorpacific.com gita@gencorpacific.com www.gencorpacific.com TESTOFEN
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 AVODART SOFT CAPSULES 0.5 MG 1. NAME OF THE MEDICINAL PRODUCT Avodart 0.5 mg capsules, soft. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 0.5 mg dutasteride. For excipients, see 6.1.
AVODART SOFT CAPSULES 0.5 MG 1. NAME OF THE MEDICINAL PRODUCT Avodart 0.5 mg capsules, soft. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 0.5 mg dutasteride. For excipients, see 6.1.
Enlarged prostate (EP), also known as benign prostatic
 REPORTS A Large Retrospective Analysis of Acute Urinary Retention and Prostate-related Surgery in BPH Patients Treated with 5-alpha Reductase Inhibitors: Dutasteride Versus Finasteride Muta M. Issa, MD,
REPORTS A Large Retrospective Analysis of Acute Urinary Retention and Prostate-related Surgery in BPH Patients Treated with 5-alpha Reductase Inhibitors: Dutasteride Versus Finasteride Muta M. Issa, MD,
Hepatitis B surface. antigen prevalence. HepatitisB_whocdscsrlyo2002_2.pdf. Accessed 20 October, 2010.) Intermediate 2 7% High 8% Low <2%
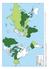 Hepatitis B surface antigen prevalence High 8% Intermediate 2 7% Low
Hepatitis B surface antigen prevalence High 8% Intermediate 2 7% Low
biosensis Rat Fibronectin ELISA Kit Protocol
 biosensis Rat Fibronectin ELISA Kit Protocol Catalog No: BEK-2017-2P For quantitative detection of rat Fibronectin in cell culture supernatants, serum, and citrate, heparin, or EDTA plasma samples only
biosensis Rat Fibronectin ELISA Kit Protocol Catalog No: BEK-2017-2P For quantitative detection of rat Fibronectin in cell culture supernatants, serum, and citrate, heparin, or EDTA plasma samples only
MSD 96-Well MULTI-ARRAY and MULTI-SPOT Human Granulocyte Colony Stimulating Factor (hg-csf) Ultrasensitive Assay
 MSD 96-Well MULTI-ARRAY and MULTI-SPOT Human Granulocyte Colony Stimulating Factor (hg-csf) Ultrasensitive Assay Summary This assay measures Human Granulocyte Colony Stimulating Factor (G-CSF) in a 96-well
MSD 96-Well MULTI-ARRAY and MULTI-SPOT Human Granulocyte Colony Stimulating Factor (hg-csf) Ultrasensitive Assay Summary This assay measures Human Granulocyte Colony Stimulating Factor (G-CSF) in a 96-well
TESTOFEN. Anabolic & Androgenic Activity GENCOR PACIFIC, INC. Fenugreek Extract standardized for FENUSIDE TM. Copyright 2005 by Gencor Pacific, Inc.
 GENCOR PACIFIC, INC. 920E. Orangethorpe Avenue, Suite B, Anaheim, CA. 92801 Ph: 714.870.8723 714.870.8724 efax: 732.875.0306 drjit@gencorpacific.com manu@gencorpacific.com www.gencorpacific.com TESTOFEN
GENCOR PACIFIC, INC. 920E. Orangethorpe Avenue, Suite B, Anaheim, CA. 92801 Ph: 714.870.8723 714.870.8724 efax: 732.875.0306 drjit@gencorpacific.com manu@gencorpacific.com www.gencorpacific.com TESTOFEN
Preventive and Therapeutic Efficacy of Finasteride and Dutasteride in TRAMP Mice
 Preventive and Therapeutic Efficacy of Finasteride and Dutasteride in TRAMP Mice Alexander B. Opoku-Acheampong 1, Dave Unis 1,2, Jamie N. Henningson 3, Amanda P. Beck 4, Brian L. Lindshield 1* 1 Department
Preventive and Therapeutic Efficacy of Finasteride and Dutasteride in TRAMP Mice Alexander B. Opoku-Acheampong 1, Dave Unis 1,2, Jamie N. Henningson 3, Amanda P. Beck 4, Brian L. Lindshield 1* 1 Department
STUDY PERFORMANCE REPORT
 STUDY PERFORMANCE REPORT State: Michigan Project No.: F-80-R-7 Study No.: 230654 Title: Evaluation of brown trout and steelhead competitive interactions in Hunt Creek, Michigan. Period Covered: October
STUDY PERFORMANCE REPORT State: Michigan Project No.: F-80-R-7 Study No.: 230654 Title: Evaluation of brown trout and steelhead competitive interactions in Hunt Creek, Michigan. Period Covered: October
HUMAN IL6 KITS PROTOCOL
 HUMAN IL6 KITS PROTOCOL Part # 62HIL06PEG & 62HIL06PEH Test size: 500 tests (62HIL06PEG), 10,000 tests (62HIL06PEH) - assay volume: 20 µl Revision: 04 (Jan. 2018) Store at: -60 C or below This product
HUMAN IL6 KITS PROTOCOL Part # 62HIL06PEG & 62HIL06PEH Test size: 500 tests (62HIL06PEG), 10,000 tests (62HIL06PEH) - assay volume: 20 µl Revision: 04 (Jan. 2018) Store at: -60 C or below This product
SUMMARY OF PRODUCT CHARACTERISTICS
 1. NAME OF THE MEDICINAL PRODUCT Avodart 0.5 mg soft capsules. SUMMARY OF PRODUCT CHARACTERISTICS 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 0.5 mg dutasteride. Excipient with known
1. NAME OF THE MEDICINAL PRODUCT Avodart 0.5 mg soft capsules. SUMMARY OF PRODUCT CHARACTERISTICS 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 0.5 mg dutasteride. Excipient with known
Clinical Significance of 5 -Reductase Activity
 Clinical Significance of 5 -Reductase Activity By Jonathan Wright, MD and Barry Wheeler, ND 5 -Reductase (5AR) is the enzyme that catalyzes the conversion of (among others): Testosterone 5 -DHT Androstenedione
Clinical Significance of 5 -Reductase Activity By Jonathan Wright, MD and Barry Wheeler, ND 5 -Reductase (5AR) is the enzyme that catalyzes the conversion of (among others): Testosterone 5 -DHT Androstenedione
biosensis Human IGF-II, Insulin-like growth factor II, Somatomedin-A ELISA Kit Protocol
 biosensis Human IGF-II, Insulin-like growth factor II, Somatomedin-A ELISA Kit Protocol Catalog No: BEK-2029-1P For quantitative detection of human IGF-II in cell culture supernatants, cell lysates, tissue
biosensis Human IGF-II, Insulin-like growth factor II, Somatomedin-A ELISA Kit Protocol Catalog No: BEK-2029-1P For quantitative detection of human IGF-II in cell culture supernatants, cell lysates, tissue
The Science of. NUTRICULA Longevity Journal
 32 December, 2011 The Science of 33 NUTRICULA Longevity Journal As men age, there is often a decline in libido and sexual function. This decline frequently interferes in intimacy within romantic relationships,
32 December, 2011 The Science of 33 NUTRICULA Longevity Journal As men age, there is often a decline in libido and sexual function. This decline frequently interferes in intimacy within romantic relationships,
National Institute for Public Health and the Environment Annual CRL workshop 22 October Update on natural Hormone studies
 2008 Annual CRL workshop 22 October 2008 Update on natural Hormone studies Natural hormone studies: update Possible approaches - C12/C13 ratio: can result in proof of abuse - Determination of intact esters
2008 Annual CRL workshop 22 October 2008 Update on natural Hormone studies Natural hormone studies: update Possible approaches - C12/C13 ratio: can result in proof of abuse - Determination of intact esters
biosensis Human TNFα/Cachectin/TNFSF2 ELISA Kit Protocol
 biosensis Human TNFα/Cachectin/TNFSF2 ELISA Kit Protocol Catalog No: BEK-2100-1P For quantitative detection of human TNFα in cell culture supernatants, serum, and heparin, EDTA or citrate treated plasma
biosensis Human TNFα/Cachectin/TNFSF2 ELISA Kit Protocol Catalog No: BEK-2100-1P For quantitative detection of human TNFα in cell culture supernatants, serum, and heparin, EDTA or citrate treated plasma
Safety and Tolerability of the Dual 5a-Reductase Inhibitor Dutasteride in thetreatment of Benign Prostatic Hyperplasia
 European Urology European Urology 44 (2003) 82 88 Safety and Tolerability of the Dual 5a-Reductase Inhibitor in thetreatment of Benign Prostatic Hyperplasia Gerald L. Andriole a,*,1, Roger Kirby b a Division
European Urology European Urology 44 (2003) 82 88 Safety and Tolerability of the Dual 5a-Reductase Inhibitor in thetreatment of Benign Prostatic Hyperplasia Gerald L. Andriole a,*,1, Roger Kirby b a Division
TRT and LUTS. Mick Jagger, 70 years. PRISM IV Sept 2014
 TRT and LUTS Mick Jagger, 70 years PRISM IV Sept 2014 Combination of LUTS and LOH In ageing male : -LUTS increases -LOH increases So: the combination is a common clinical scenario TRT and LUTS Startingpoint
TRT and LUTS Mick Jagger, 70 years PRISM IV Sept 2014 Combination of LUTS and LOH In ageing male : -LUTS increases -LOH increases So: the combination is a common clinical scenario TRT and LUTS Startingpoint
This is an English translation of the original Chinese instruction leaflet generated by Google Translate. No amendments were made.
 Approved Date: December 26, 2006 Revision Date: September 12, 2012 Modify Date: December 1, 2013 Finasteride tablets instructions Please read the instructions carefully and use under the guidance of a
Approved Date: December 26, 2006 Revision Date: September 12, 2012 Modify Date: December 1, 2013 Finasteride tablets instructions Please read the instructions carefully and use under the guidance of a
biosensis Mouse Brain-derived neurotrophic factor (BDNF) ELISA Kit Protocol
 biosensis Mouse Brain-derived neurotrophic factor (BDNF) ELISA Kit Protocol Catalog No: BEK-2003-2P For quantitative detection of mouse BDNF in cell culture supernatants, cell lysates, serum, and citrate,
biosensis Mouse Brain-derived neurotrophic factor (BDNF) ELISA Kit Protocol Catalog No: BEK-2003-2P For quantitative detection of mouse BDNF in cell culture supernatants, cell lysates, serum, and citrate,
TF (Human) Chromogenic Activity Assay Kit
 TF (Human) Chromogenic Activity Assay Kit Catalog Number KA0975 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the
TF (Human) Chromogenic Activity Assay Kit Catalog Number KA0975 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Deratos 0.5 mg capsules, soft 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 0.5 mg dutasteride. For the full list
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Deratos 0.5 mg capsules, soft 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 0.5 mg dutasteride. For the full list
PRODUCT INFORMATION PRIMOTESTON DEPOT. (testosterone enantate)
 PRODUCT INFORMATION PRIMOTESTON DEPOT (testosterone enantate) NAME OF THE MEDICINE Testosterone enantate is designated chemically as 17 beta-heptanoyloxy-4-androstene-3- one. The empirical formula of testosterone
PRODUCT INFORMATION PRIMOTESTON DEPOT (testosterone enantate) NAME OF THE MEDICINE Testosterone enantate is designated chemically as 17 beta-heptanoyloxy-4-androstene-3- one. The empirical formula of testosterone
Maximum Hair Minimum Loss
 Hi and welcome to your free hair loss information report. Written by Spex, a fellow hair- loss sufferer, UK Veteran of 10 years. I m Spex and within the next few emails you will gain access and insight
Hi and welcome to your free hair loss information report. Written by Spex, a fellow hair- loss sufferer, UK Veteran of 10 years. I m Spex and within the next few emails you will gain access and insight
Advanced Animal Science TEKS/LINKS Student Objectives One Credit
 First Six Weeks Career/Safety/Work Habits AAS 1(A) The student will identify career development and entrepreneurship opportunities in the field of animal systems. AAS 1(B) The student will apply competencies
First Six Weeks Career/Safety/Work Habits AAS 1(A) The student will identify career development and entrepreneurship opportunities in the field of animal systems. AAS 1(B) The student will apply competencies
FULL PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AVODART safely and effectively. See full prescribing information for AVODART. AVODART (dutasteride)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AVODART safely and effectively. See full prescribing information for AVODART. AVODART (dutasteride)
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Dutasteride 0.5 mg Capsules, Soft 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 0.5 mg dutasteride. For the full list
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Dutasteride 0.5 mg Capsules, Soft 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 0.5 mg dutasteride. For the full list
THE INFLUENCE OF SLOW RECOVERY INSOLE ON PLANTAR PRESSURE AND CONTACT AREA DURING WALKING
 March 12, 2015 5:39:44pm WSPC/170-JMMB 1540005 ISSN: 0219-51942nd Reading Journal of Mechanics in Medicine and Biology Vol. 15, No. 2 (2015) 1540005 (6 pages) c World Scientific Publishing Company DOI:
March 12, 2015 5:39:44pm WSPC/170-JMMB 1540005 ISSN: 0219-51942nd Reading Journal of Mechanics in Medicine and Biology Vol. 15, No. 2 (2015) 1540005 (6 pages) c World Scientific Publishing Company DOI:
biosensis Human Lipocalin-2/NGAL ELISA Kit Protocol
 biosensis Human Lipocalin-2/NGAL ELISA Kit Protocol Catalog No: BEK-2141-2P For quantitative detection of human Lipocalin-2 in cell culture supernatants, serum, and heparin treated plasma, saliva, and
biosensis Human Lipocalin-2/NGAL ELISA Kit Protocol Catalog No: BEK-2141-2P For quantitative detection of human Lipocalin-2 in cell culture supernatants, serum, and heparin treated plasma, saliva, and
CHEMILUMINESCENCE IMMUNOASSAY (CLIA) Testosterone
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
Efficacy and tolerability of the dual 5a-reductase inhibitor, dutasteride, in the treatment of benign prostatic hyperplasia in African-American men
 (2006) 9, 432 438 & 2006 Nature Publishing Group All rights reserved 1365-7852/06 $30.00 ORIGINAL ARTICLE www.nature.com/pcan Efficacy and tolerability of the dual 5a-reductase inhibitor, dutasteride,
(2006) 9, 432 438 & 2006 Nature Publishing Group All rights reserved 1365-7852/06 $30.00 ORIGINAL ARTICLE www.nature.com/pcan Efficacy and tolerability of the dual 5a-reductase inhibitor, dutasteride,
Use of Performance Enhancing Substances Good Chemistry Gone Bad. Evan M. Klass, M.D., F.A.C.P.
 Use of Performance Enhancing Substances 2017 Good Chemistry Gone Bad Evan M. Klass, M.D., F.A.C.P. Doping the use of banned athletic performance-enhancing drugs by competitors Performance enhancing substances
Use of Performance Enhancing Substances 2017 Good Chemistry Gone Bad Evan M. Klass, M.D., F.A.C.P. Doping the use of banned athletic performance-enhancing drugs by competitors Performance enhancing substances
Relationship between Aerobic Training and Testosterone Levels in Male Athletes
 Relationship between Aerobic Training and Testosterone Levels in Male Athletes Siu Yuen Ng Biology 493 13 th December, 2010 Abstract Salivary testosterone levels of 11 athletes and 15 non athletes were
Relationship between Aerobic Training and Testosterone Levels in Male Athletes Siu Yuen Ng Biology 493 13 th December, 2010 Abstract Salivary testosterone levels of 11 athletes and 15 non athletes were
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
biosensis Mouse Vascular endothelial growth factor A/VEGF-A/VEGF-164/VEGF-1/VEGF- 120/VEGF-2 ELISA Kit Protocol
 biosensis Mouse Vascular endothelial growth factor A/VEGF-A/VEGF-164/VEGF-1/VEGF- 120/VEGF-2 ELISA Kit Protocol Catalog No: BEK-2110-1P For quantitative detection of mouse VEGF-A (VEGF164&VEGF120) in mouse
biosensis Mouse Vascular endothelial growth factor A/VEGF-A/VEGF-164/VEGF-1/VEGF- 120/VEGF-2 ELISA Kit Protocol Catalog No: BEK-2110-1P For quantitative detection of mouse VEGF-A (VEGF164&VEGF120) in mouse
european urology 55 (2009)
 european urology 55 (2009) 461 471 available at www.sciencedirect.com journal homepage: www.europeanurology.com Benign Prostatic Hyperplasia The Influence of Baseline Parameters on Changes in International
european urology 55 (2009) 461 471 available at www.sciencedirect.com journal homepage: www.europeanurology.com Benign Prostatic Hyperplasia The Influence of Baseline Parameters on Changes in International
biosensis Mouse Interleukin-1 beta (IL-1β) ELISA Kit Protocol
 biosensis Mouse Interleukin-1 beta (IL-1β) ELISA Kit Protocol Catalog Number: BEK-2151-1P For quantitative detection of mouse IL-1β in cell culture supernatant, cell lysates, and serum and hepain or EDTA
biosensis Mouse Interleukin-1 beta (IL-1β) ELISA Kit Protocol Catalog Number: BEK-2151-1P For quantitative detection of mouse IL-1β in cell culture supernatant, cell lysates, and serum and hepain or EDTA
Issues. What is a low testosterone? Who needs testosterone therapy? Benefits/adverse effects of testosterone replacement Treatment options
 Male Hypogonadism Jauch Symposium Waterloo, IA May 17, 2013 Janet A. Schlechte, M.D. Disclosure of Financial Relationships Janet A. Schlechte, M.D. has no relationships with any proprietary entity producing
Male Hypogonadism Jauch Symposium Waterloo, IA May 17, 2013 Janet A. Schlechte, M.D. Disclosure of Financial Relationships Janet A. Schlechte, M.D. has no relationships with any proprietary entity producing
Product Monograph. Sandoz Dutasteride
 Product Monograph Pr Sandoz Dutasteride Dutasteride Capsules of 0.5 mg Type I and II 5 Alpha-reductase Inhibitor Sandoz Canada Inc. 145 Jules-Léger Boucherville, QC, Canada J4B 7K8 Date of Preparation:
Product Monograph Pr Sandoz Dutasteride Dutasteride Capsules of 0.5 mg Type I and II 5 Alpha-reductase Inhibitor Sandoz Canada Inc. 145 Jules-Léger Boucherville, QC, Canada J4B 7K8 Date of Preparation:
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Finasterid Actavis 5 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 5 mg finasteride.
SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Finasterid Actavis 5 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 5 mg finasteride.
biosensis Rat Interleukin-1 beta, IL-1β ELISA Kit Protocol
 biosensis Rat Interleukin-1 beta, IL-1β ELISA Kit Protocol For the quantitative detection of rat IL-1β in cell culture supernatants, serum, heparin or EDTA treated plasma samples, and cell homogenates
biosensis Rat Interleukin-1 beta, IL-1β ELISA Kit Protocol For the quantitative detection of rat IL-1β in cell culture supernatants, serum, heparin or EDTA treated plasma samples, and cell homogenates
The capsules are opaque, yellow, oblong soft gelatin capsules filled with an oily and yellowish liquid, without printing.
 1. NAAM VAN HET GENEESMIDDEL Dutasteride 0,5 mg Teva, zachte capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 0.5 mg of dutasteride. Excipient with known effect: lecithin (may
1. NAAM VAN HET GENEESMIDDEL Dutasteride 0,5 mg Teva, zachte capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 0.5 mg of dutasteride. Excipient with known effect: lecithin (may
DOSAGE FORMS AND STRENGTHS
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use finasteride tablets USP safely and effectively. See full prescribing information for finasteride
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use finasteride tablets USP safely and effectively. See full prescribing information for finasteride
DUTASTERIDE capsules, for oral use Initial U.S. Approval: 2001
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Dutasteride capsules safely and effectively. See full prescribing information for Dutasteride capsules.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Dutasteride capsules safely and effectively. See full prescribing information for Dutasteride capsules.
WADA Technical Document TD2016EAAS. Endogenous Anabolic Androgenic Steroids Measurement and Reporting
 Endogenous Anabolic Androgenic Steroids Measurement and Reporting Introduction The purpose of this Technical Document (TD) is to harmonize the approaches to the measurement and reporting of Endogenous
Endogenous Anabolic Androgenic Steroids Measurement and Reporting Introduction The purpose of this Technical Document (TD) is to harmonize the approaches to the measurement and reporting of Endogenous
biosensis Rat IGF-1/Somatomedin/Insulin-like growth factor ELISA Kit Protocol
 biosensis Rat IGF-1/Somatomedin/Insulin-like growth factor ELISA Kit Protocol Catalog No: BEK-2150-1P For quantitative detection of rat IGF-1 in cell culture supernatants, cell and tissue homogenates,
biosensis Rat IGF-1/Somatomedin/Insulin-like growth factor ELISA Kit Protocol Catalog No: BEK-2150-1P For quantitative detection of rat IGF-1 in cell culture supernatants, cell and tissue homogenates,
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Finpros 5 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 5 mg finasteride. Excipient
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Finpros 5 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 5 mg finasteride. Excipient
EUROPEAN UROLOGY 57 (2010)
 EUROPEAN UROLOGY 57 (2010) 123 131 available at www.sciencedirect.com journal homepage: www.europeanurology.com Benign Prostatic Hyperplasia The Effects of Combination Therapy with Dutasteride and Tamsulosin
EUROPEAN UROLOGY 57 (2010) 123 131 available at www.sciencedirect.com journal homepage: www.europeanurology.com Benign Prostatic Hyperplasia The Effects of Combination Therapy with Dutasteride and Tamsulosin
imágenes en el Hospital Clinic
 Experiencias en telepatología y digitalización masiva de Centre de Diagnòstic Biomèdic imágenes en el Hospital Clinic Jaume Ordi M.D., Ph.D. Department of Pathology Hospital Clínic Barcelona jordi@clinic.ub.es
Experiencias en telepatología y digitalización masiva de Centre de Diagnòstic Biomèdic imágenes en el Hospital Clinic Jaume Ordi M.D., Ph.D. Department of Pathology Hospital Clínic Barcelona jordi@clinic.ub.es
Dutasteride Avodart Softgel Capsule
 Dutasteride Avodart Softgel Capsule PRODUCT DESCRIPTION Dutasteride (Avodart ) is available as dull yellow, opaque, oblong soft gelatin capsules containing a clear, colourless to slightly yellow liquid.
Dutasteride Avodart Softgel Capsule PRODUCT DESCRIPTION Dutasteride (Avodart ) is available as dull yellow, opaque, oblong soft gelatin capsules containing a clear, colourless to slightly yellow liquid.
