Aspects of the marine ecology of Atlantic salmon (Salmo salar L.)
|
|
|
- Sabina French
- 5 years ago
- Views:
Transcription
1 Aspects of the marine ecology of Atlantic salmon (Salmo salar L.) Jan Arge Jacobsen Dr. scient. thesis Department of Fisheries and Marine Biology University of Bergen, Norway 2000
2
3 Aspects of the marine ecology of Atlantic salmon (Salmo salar L.) Jan Arge Jacobsen Thesis submitted in partial fulfilment of the requirements for the degree of Dr. scient. Department of Fisheries and Marine Biology University of Bergen, Norway 2000 ISBN
4 "All that we do is touched with ocean, yet we remain on the shore of what we know." from THE LIVING SEA MacGillivray Freeman Films
5 PREFACE AND ACKNOWLEDGEMENTS My interest in fish biology stems from childhood when my parents gave me the book "Havfisk" by Bent J. Muus 1 and illustrated by Preben Dahlstrøm, as a Christmas present in This book made a considerable impression on me, and it contained an ingenious "labyrinth" of the animal evolution that stimulated my curiosity about the development of our world. The work presented here is the result of on-going research on Atlantic salmon at 'Fiskirannsóknarstovan' and a recent joint Nordic project on salmon in the Faroese area, supported by the Faroese government, 'Felagið Laksaskip', the Norwegian Directorate for Nature Management, the Nordic Council of Ministers (project no ), and Norwegian Institute for Nature Research. My gratitude especially to 'Nordisk forskerutdanningsakademi' for a personal grant in 1996/1997, and to 'Granskingarráð Føroya', 'Vísindagrunnir Føroya Sparikassa' and 'Minningargrunnur Dánjal Niclasen' for financial support during the last part of my stay in Bergen. I wish to express me deepest gratitude for the close co-operation with my supervisor Dr. Lars Petter Hansen. His valuable help and support through these years is greatly appreciated. I am thankful for all the help and support received from Prof. Gunnar Nævdal during my stay at Department of Fisheries and Marine Biology. Furthermore, I acknowledge my co-authors Eilif Gaard, Roar A. Lund and Niall O'Maoileidigh for fruitful co-operation, as well as the support received from Dr. Sigurd Stefansson. I would also like to thank Hjalti í Jákupsstovu for his support and patience during my work with the thesis, and thanks are also due to the staff at 'Fiskirannsóknarstovan'. The help I received from those who collected the samples on-board and analysed the material is greatly appreciated: the late Jón Jákup Høisted, Rógvi Mouritsen, Arnold Hendriksen, Súni Lamhauge, Eydna Poulsen, Anna Johansen, Marit Pedersen, Ólavur Olsen, Høgni Debess, Gunnel Østborg and Berit Larsen. Thanks also to the skippers and crew onboard the salmon long-liners Polarlaks and Hvítiklettur. The staff and fellow students at the Department of Fisheries and Marine Biology are gratefully acknowledged for their encouragement and help during my stay there. Finally I would like to thank the staff at 'Føroyska Viðskiftastovan' in Copenhagen for their kind hospitality, and the members of the ICES Working Group on Atlantic salmon for stimulating discussions. I am deeply indebted to my wife Anna and our two children Hilmar and Konni for their love and enduring support thank you all! Bergen February 2000 Jan Arge Jacobsen 1 Muus, B.J. and Dahlstrøm, P Havfisk og fiskeri i Nordvesteuropa. 2. udgave. GEC Gads Forlag, København. v
6 Aspects of the marine ecology of Atlantic salmon ABSTRACT The present thesis focuses on the oceanic life-history of Atlantic salmon (Salmo salar L.) in the Northeast Atlantic Ocean. The main aims were to study the spatial and temporal stock structure of salmon, their feeding habits in the high seas, and possible interactions of escaped farmed salmon with wild salmon. The productive frontal areas north of the Faroes Islands and in the Norwegian Sea are important feeding grounds for salmon, and a fishery for salmon has developed in these areas. The fish was sampled by floating long-lines during autumn (November December) and winter (January March), and a total of about 25,000 salmon have been examined during this study. Salmon that had escaped from fish farms were found intermingled with wild salmon in the high seas. The proportion of escaped fish in the fishery was low until 1988, when it increased and reached a peak around 1990 and decreased again in recent years. It is concluded that if farmed components in catches are not accounted for, catches of wild salmon will be overestimated resulting in erroneous stock assessments of wild salmon. Fish originating from the entire salmon distribution area may occur at Faroes in parts of their sea phase. Most of the wild salmon tagged at Faroes were recaptured in Norway, but significant number of returns were observed in Scotland and Russia as well. The incidence of salmon originating from other countries around the Northeast Atlantic and Canada was low. Most of the fish farm escapees originated from Norwegian fish farms. It is suggest that significant numbers of salmon caught in the Faroes area during autumn originate from southern European countries and that fish from northern regions appear to be more abundant in the winter. Recaptures in the Faroese fishery during autumn and winter of salmon tagged as smolts in different countries support this. The salmon feed mainly on hyperiid amphipods, euphausiids, shrimps, lanternfishes, pearlsides and barracudinas, and less on larger pelagic fish and squid. However, if available they tend to select larger prey and prefer fish to crustaceans. Escaped farmed salmon were feeding and growing as efficiently as wild salmon, indicating that those fish that survived until capture were completely adapted to feed in the marine environment. It is still an open question whether food is a limiting factor for growth and survival in the sea. However, the wide variety of food in different areas and periods suggests that salmon abundance is unlikely to be very sensitive to annual changes in the availability of any particular prey. The sea lice (Lepeophtheirus salmonis) were found to infest salmon in the open ocean. Practically all fish were infested with on average 30 lice per salmon. Most lice were adult ovigerous females. Escaped farmed salmon had significantly higher load of lice than wild salmon. vi
7 Aspects of the marine ecology of Atlantic salmon CONTENTS Preface and acknowledgements... v Abstract... vi Contents... 1 List of papers... 3 Glossary... 4 Introduction... 5 Objectives and aims... 8 The high seas fishery for salmon... 9 The offshore fishery at West Greenland... 9 The high seas fishery for salmon in the Norwegian Sea... 9 Fishing gear and methods used in the Faroese high seas fishery Summary of results Synoptic discussion Temporal and spatial stock structures of wild and escaped farmed salmon Geographic origin Temporal and spatial stock structures Migration and dispersal Management considerations Diet and feeding habits of wild and escaped farmed salmon in the sea The diet of salmon Comparison of the marine feeding habits from other areas and life stages Is salmon an opportunistic feeder? Is food a limiting factor for growth and survival of salmon? Possible interactions between fish farm escapees and wild salmon in the ocean Conclusions and perspectives References Papers I - V attached. 1
8 Aspects of the marine ecology of Atlantic salmon
9 Aspects of the marine ecology of Atlantic salmon LIST OF PAPERS This thesis is based on the following papers: I. Hansen, L.P., Jacobsen, J.A. and Lund, R.A The incidence of escaped farmed Atlantic salmon, Salmo salar L., in the Faroese fishery and estimates of catches of wild salmon. ICES Journal of Marine Science 56: II. III. IV. Jacobsen, J.A., Lund, R.A., Hansen, L.P. and O'Maoileidigh, N Seasonal differences in origin of Atlantic salmon (Salmo salar L.) in the Norwegian Sea based on estimates from age structures and tag recaptures. (Submitted for publication) Hansen, L.P. and Jacobsen, J.A Origin, migration and growth of wild and escaped farmed Atlantic salmon, Salmo salar L., in oceanic areas north of the Faroe Islands. (Submitted for publication) Jacobsen, J.A. and Hansen, L.P Feeding habits of wild and escaped farmed Atlantic salmon, Salmo salar L., in the Northeast Atlantic. (Submitted for publication) V. Jacobsen, J.A. and Gaard, E Open-ocean infestation by salmon lice (Lepeophtheirus salmonis): comparison of wild and escaped farmed Atlantic salmon (Salmo salar L.). ICES Journal of Marine Science, 54:
10 Aspects of the marine ecology of Atlantic salmon GLOSSARY Wild salmon. Salmon that have spent their whole life cycle in the wild and were the result of natural spawning. Farmed or escaped salmon. Salmon of farmed origin escaped at various stages from captivity or from smolt rearing farms. Smolt 1. Salmon when it is ready to leave fresh water and enter the sea phase of its life, usually in spring or early summer (May/June). Post-smolt 1. Salmon the first few (2-4) months after sea-entry as smolt during spring. 1SW salmon 1. One sea-winter salmon, i.e. salmon in its first winter in the sea, defined to be the period from autumn the year of sea-entry to the autumn the year after. 2SW salmon 1. Two sea-winter salmon, i.e. salmon in its second winter in the sea, defined to be the period from the second autumn to the third autumn after sea-entry. 3SW salmon 1. Three sea-winter salmon, i.e. salmon in its third winter in the sea, defined to be the period from the third autumn to the fourth autumn after sea-entry. 2+SW salmon or MSW salmon 1. Multi-sea-winter salmon, i.e. salmon older than 1SW. Grilse. Salmon returning to spawn as 1SW salmon. Forklength and total length. Forklength is length from snout to cleavage of the fork in the tail fin. Total length is length from snout to tip of tail fin. EEZ. Exclusive Economic Zone. CPUE. Catch per unit effort, e.g. number of salmon caught per 1000 hooks fished per day. Discards. Salmon below 60 cm total length (approximately 57 cm forklength) obligatory to release at sea when caught. TAC. Total allowable catch. SST. Sea-surface temperature. ICES. The International Council for the Exploration of the Sea. NASCO. The North Atlantic Salmon Conservation Organisation. 1 See Allan & Ritter (1977) for a detailed salmonid terminology. 4
11 INTRODUCTION Atlantic salmon (Salmo salar L.) are distributed widely in the North Atlantic Ocean and have been harvested for many years by anglers in freshwater, by commercial fishermen in fjords and coastal areas as well as at the feeding areas in the high seas. The oceanic phase of salmon begins when they leave freshwater as newly transformed smolts during spring and commence their seaward migration to their oceanic feeding areas. The salmon spend normally one to three years, occasionally up to five years feeding in the open ocean, before returning "home" to their natal river to spawn (Mills, 1989). The freshwater phases of Atlantic salmon's life history have been extensively studied. In contrast much less is known about their life in sea, especially in the oceanic phase (e.g. Reddin, 1988; Shearer, 1992; Friedland & Reddin, 1993; Mills, 1993; Hansen & Quinn, 1998). The oceanic phase of salmon has frequently been referred to as a 'black box' in recognition of our lack of understanding and knowledge of this important life stage (Figure 1). However, it is surprising considering the importance of the high seas fisheries for salmon that there is a scarcity of information on life history in the sea. The occurrence of escaped farmed salmon in coastal (e.g. Lund et al., 1991; Youngson & Verspoor, 1998) and oceanic areas (Hansen et al., 1993a) has only underlined the need for further data on their biology to address possible interactions with wild salmon. GROWTH AND SURVIVAL PREDATION AND COMPETITION STOCK STRUCTURE, MIGRATION AND DISTRIBUTION FOOD AND FOOD AVAILABILITY FISHERY BLACK BOX PARASITES AND DISEASES ESCAPED FARMED FISH ENVIRONMENT: TEMPERATURE AND CURRENTS Figure 1. The lack of knowledge of the marine phase of wild Atlantic salmon has often been symbolised as a 'black box'. Here exemplified as various factors and their interactions affecting the salmons' life in the sea. 5
12 Introduction Smolt tagging programmes in many countries and adult tagging experiments at sea during since 1960s have provided information of the composition of country of origin of salmon in the North Atlantic. The general pattern is that salmon from the Northwest Atlantic is mainly confined to the western area, while salmon from the Northeast Atlantic is found both in the eastern and western part of the Atlantic (e.g. Parrish & Horsted, 1980; Jákupsstovu, 1988; Reddin, 1988). The analyses show that salmon in the fishery off West Greenland are split fairly even between North America and Europe (Reddin, 1988; Reddin & Friedland, 1999), however with temporal and spatial variations (Lear & Sandeman, 1980; Parrish & Horsted, 1980). Canada probably accounts for most of the North American component and Scotland (and the southern European regions) accounts for most of the European fish (Ruggles & Ritter, 1980; Swain, 1980; Jensen, 1980a). In the Northeast Atlantic the results show that in the northern Norwegian Sea salmon from Norway is most dominant with salmon from UK, Sweden and Russia also present (L.P. Hansen, NINA, Norway, unpublished data), while recaptures in the central and southern Norwegian Sea indicate higher proportions of salmon from the southern European regions present (Jákupsstovu, 1988; ICES, 1996). However, in retrospect the results from the previous major sea tagging experiment conducted in the sea around the Faroe Islands in (Jákupsstovu, 1988) indicated an overweight of recaptures from the southern European regions as compared with later estimates of fish tagged as smolts and recaptured in the Faroese fishery (ICES, 1996). Thus a more representative distribution of the origin of the salmon stocks in the Norwegian Sea was justified (ICES, 1984; 1996). For the management of salmon stocks, it is of great importance to identify precisely the country of origin of the stocks fished on the high seas, and to obtain better knowledge of their temporal and spatial population structure. In this way the fishery could target the effort towards less threatened stock complexes in the sea, by e.g. temporal and spatial restrictions in the fishery. The food and feeding habits of salmon in the Northeast Atlantic are only poorly known (see Hislop & Shelton, 1993 for a review). In general salmon probably spend most of its time pelagically in the ocean close to the sea surface (Templeman, 1967; Reddin, 1985; Dutil & Coutu, 1988) preying on different pelagic animals such as fish, crustaceans and squids, and has been suggested to feed opportunistically (Hansen & Pethon, 1985; Reddin, 1988; Hislop & Shelton, 1993). However, there is no information available to compare the distribution of food organisms available with what salmon really eat. The studies so far in the Norwegian Sea mainly give a qualitative picture of the importance of the prey species for salmon, and some suffer from low number of stomachs examined (Struthers, 1970; 1971; Thurow, 1973; Hislop & Youngson, 1984; Hansen & Pethon, 6
13 Introduction 1985). A more detailed study of the feeding habits of salmon in the sea was warranted. Such studies could shed light on their trophic position in the pelagic ecosystem, i.e. link their feeding preferences to growth and condition at sea; compare differences in feeding with respect to size or age; evaluate possible changes in feeding habits with changes in the environment (temperature) and season; and assess the likelihood that food might be a limiting factor for growth and survival in the sea. Furthermore, the occurrence of escaped farmed salmon among the wild fish had to be addressed with respect to their possible interactions, such as food competition and transmission of parasites. Escaped farmed salmon have been observed in wide areas of the north Atlantic (Lund et al., 1991; Gausen & Moen, 1991; Webb & Youngson, 1992; Carr et al., 1997; Stokesbury & Lacroix, 1997; Youngson et al., 1997), as well as in the high seas salmon fisheries in the Norwegian Sea (Hansen et al., 1993a). Several detrimental effects on wild stocks have been suggested from the escapees, of which interbreeding and transmissions of parasites and diseases in coastal areas were considered the most severe (e.g. Hindar et al., 1991; Håstein & Lindstad, 1991; McVicar, 1997; Sægrov et al., 1997; Bakke & Harris, 1998). Of the harmful parasites in the sea, particularly sea lice (Lepeophtheirus salmonis) have caused great controversy, especially in the coastal areas of the significant fish farming production countries such as Norway, Scotland and Ireland, where large runs of wild salmon also occur (Hutchinson, 1997). Sea louse is a naturally occurring parasite on salmon, but there are few studies of their natural occurrence and levels of infection on salmon in undisturbed coastal areas (but see Youngson et al., 1997). In the offshore areas of the North Atlantic only few studies of infection on wild salmon have been reported (Pippy, 1969; Wootten et al., 1982; Berland, 1993; Holst et al., 1993), and none from the central Norwegian Sea. There is thus a paucity of information on lice infestations of wild salmon during their high seas feeding phase. The number of wild salmon have declined demonstrative throughout their native range for at least two decades (e.g. Parrish et al., 1998), and this has intensified the research on salmon to find possible clues to the decline in the stocks (ICES, 1999b). The current status of the rivers in both North America and Europe show a clear geographical pattern, with most populations in the southern portions of the salmon range in severe conditions or extirpated; in the north the populations are stable; and at intermediate latitudes, populations are declining (Figs 1 and 2: Parrish et al., 1998). Many of these declines or extirpations could be attributed to the construction of mainstream dams, pollution (including acid rain), and total dewatering of streams. Along with overfishing and recently, changing ocean conditions, and intensive aquaculture (Parrish et al., 1998). 7
14 Introduction ICES has during many years estimated the exploitation rates of various homewater stocks in the Faroese high seas fishery (e.g. ICES, 1984; 1996). In the assessment of salmon stocks at the sea, it is evident that catch records from fisheries exploiting both wild fish and farm escapees will confound the assessment of stock status of wild fish, and it is therefore of great importance to identify the proportion of farmed fish and adjust catch records accordingly. One important feeding area for salmon in the Northeast Atlantic is the central and southern Norwegian Sea, i.e. the area to the north of the Faroe Islands (Jákupsstovu, 1988), where a high seas fishery for salmon has developed. This area also hosts other pelagic fish species of large biomasses such as herring (Clupea harengus), blue whiting (Micromesistius poutassou) and Atlantic mackerel (Scomber scombrus) (ICES, 1999a; 1999c). Objectives and aims The present thesis focuses on the oceanic life-history of Atlantic salmon based on material from the Norwegian Sea. The main aims were to study the spatial and temporal stock structure of salmon and their feeding habits in the high seas. Further, possible interactions of escaped farmed salmon intermingled with the wild salmon in the high seas has been investigated. Thus, the aims were to: estimate the proportions of escaped farmed salmon in the Faroese high seas fishery (Paper I), study the migration, dispersal and origin of wild and farmed salmon (Paper II and III), describe qualitatively and quantitatively the feeding habits of wild and escaped farmed salmon (Paper IV), examine whether salmon are selective feeders and assess whether food is a limiting factor for growth and survival in the sea (Paper IV), estimate the sea lice infestation in the ocean and compare the level of infection of wild and escaped farmed salmon (Paper V), and assess the likelihood that fish farm escapees might transfer increased number of salmon lice from the coastal areas to the wild salmon in the ocean (Paper V). 8
15 THE HIGH SEAS FISHERY FOR SALMON Since the discussion in the present thesis is focused on the ecology of salmon from the high seas, some basic background is provided of the major sea fisheries for salmon, i.e. the Norwegian Sea fishery and the fishery off West Greenland. This overview emphasises the Faroese fishery for salmon in the North Atlantic, beginning with a brief description of the fishery off West Greenland and continuing with a more detailed account of the Norwegian Sea fishery, including the fishery north of the Faroe Islands. Atlantic salmon have been exploited throughout the whole geographical range of the species in the North Atlantic, with catches fluctuating between 6,000 and 12,000 tonnes through 1960 and 1990, but decreased considerably during the 1990s to the present level of approximately 3,000 tonnes, including 1,000 tonnes unreported catches (Figure 2). The high seas fishery has gradually diminished in recent years and today practically all salmon are taken in coastal and inshore areas and in rivers, except a small amount (<50 tonnes) taken at West Greenland and north of the Faroes (ICES, 1999b). The offshore fishery at West Greenland In the late 1950s an autumn salmon fishery developed gradually off the west coast of Greenland. Until 1965 only Greenlandic vessels took part in the fishery using fixed gillnets. During the period from 1965 to 1975 vessels from the Faroes, Norway and Denmark participated in the fishery introducing drift nets (Christensen & Lear, 1980; Jensen, 1988). The catches ranged from 1,000 to 2,700 tonnes during this period and increased partly due to the switch from multifilament to monofilament nylon in the drift nets permitting fishing also during daylight (Jensen, 1988). From 1976 foreign vessels were not allowed to fish for salmon at Greenland and the fishery became quota regulated. The fishery off West Greenland remained high until late 1980s, and has dropped considerably in recent years, and is below 50 tonnes today (Figure 2). The salmon caught during autumn in the West Greenland area consisted of mainly 1SW non-maturing salmon (90-99%), destined to return as MSW salmon to spawn (ICES, 1984), i.e. the salmon will spend at least one more year in the sea before return. The high seas fishery for salmon in the Norwegian Sea In the mid 1960s a high seas fishery in the northern Norwegian Sea commenced (Christensen & Lear, 1980; ICES, 1984) followed by the development of a fishery around the Faroe Islands in late 1960s (Jákupsstovu, 1988). 9
16 The high seas fishery for salmon Greenland NWA NEA Unreported catches Catch (t round weight) Figure 2. Total catches of salmon in homewaters and on the high seas in the North Atlantic since 1960 divided by area: the Northwest Atlantic (excluding Greenland), at Greenland (West Greenland), and in the Northeast Atlantic. Estimates of unreported catches available from 1987 are also shown (ICES, 1999b). The fishery in the northern Norwegian Sea (Figure 3) was initiated by Danish vessels in 1967 using long-lines, and they were subsequently joined by Norwegian vessels and to a smaller extent vessels from Sweden, Faroes, Finland, and Federal Republic of Germany. The catches rose to approximately 1,000 tonnes in 1969 and 1970 and levelled off at about 500 tonnes up to 1975 (Figure 4). However, as a consequence of a national ban on salmon long-lining by Norway in 1975, the fishery was again carried on primarily by Danish vessels with catches between 200 and 500 tonnes up to 1984, when the fishery in the northern Norwegian Sea ceased. Simultaneously as the northern Norwegian Sea fishery commenced, a small long-line fishery was developed by Faroese fishermen in the coastal waters around the isles, following successful experimental long-line cruises around the Faroes in April 1968 and 1969 by R/V Jens Chr. Svabo (Jákupsstovu, 1988). Initially, the area fished was relatively close and around the isles with annual catches ranging between 20 and 40 tonnes consisting of mainly one sea-winter (1SW) fish (60-90%). However, in 1979, two years after the establishment of the 200 nm Exclusive Economic Zone (EEZ) around the Faroes, the fishery increased substantially and peaked in 1981 with a catch of 1,025 tonnes (Figure 4) and the fishery had extended northwards off the Faroes with practically no fishery south of the isles, resulting in significantly higher proportions of 2SW salmon (80%) in the catches (Jákupsstovu, 1988). 10
17 The high seas fishery for salmon 75 N Jan Mayen Winter/spring Norwegian Sea Iceland Winter Autumn 1000 m Faroe Islands Norway 60 N Scotland 0 Figure 3. Approximate high seas fishing areas for salmon in the Northeast Atlantic since The large area in the Norwegian Sea indicates multinational fishing areas prior to The fishery in the northern part of the Norwegian Sea was carried out during winter and spring. In recent years the fishery was confined inside the Faroese 200 nm fishing limit, with the autumn fishery usually being carried out closer to the isles than the winter fishery. After the formation of the North Atlantic Salmon Conservation Organisation (NASCO) in 1984, a quota was put on the Faroese fishery and it has been regulated since then. The regulation consisted of a TAC (total allowable catch) and various effort limitations, e.g. total number of hooks allowed, number of vessels per year and restricted fishing periods. The catches decreased from 630 tonnes in 1984 to around 300 tonnes in From 1991 and onwards the salmon boat owners organisation, "Felagið Laksaskip", has agreed on a buy-out compensation for the quota from various parties around the North Atlantic. The 11
18 The high seas fishery for salmon Faroese Government, however, has continued to supervise sampling inside the EEZ to monitor the salmon present in the Faroese area and to obtain biological samples (e.g. size, age and weight distributions, recaptures of tagged fish), and to update time series of catch, catch per unit effort (CPUE), and of the proportion of escaped farmed fish in the area. The annual catches between 5-30 tonnes since 1991 was due to this research activity (Figure 4). In the Norwegian Sea between 80 and 90% of the salmon caught were sexually maturing and destined to return to freshwater spawn the following autumn, irrespective of sea age (Youngson & McLay, 1985; Jákupsstovu, 1988). Faroes Denmark Norway Other countries 1,600 1,400 1,200 1, Catch (t round weight) Quota buy-out in 1991 Figure 4. Catches of Atlantic salmon in the Norwegian Sea since 1966, showing the rise and fall of this high seas fishery (ICES, 1984; 1999b). After 1984 only the Faroese fished for salmon in the high seas. An unknown amount of unreported catches were presumably taken in the area north of the 200 nm fishing limit in the early 1990s. The Faroese quota has been purchased since Fishing gear and methods used in the Faroese high seas fishery The Faroese fishery for salmon is carried out by wooden boats (20-50 GRT) and by larger steel vessels up to 290 GRT. One of the largest salmon long-liner, M/S Hvítiklettur, is shown in Figure 5. The fishery in the early days began in late winter and early spring north of the Faroes and continued until June/July as far north as Jan Mayen (Figure 3). However, during the 1980s the fishing period gradually started earlier in the year, and at present the fishery begins in November and continues until the end of March, with a break of two weeks during Christmas. The trips lasted from 9 days (maximum for vessels with no freezing capacity) to one month for the largest vessels. The floating long-line used is 12
19 The high seas fishery for salmon essentially a replicate of the gear used by Danish fishermen in the Baltic (Figure 6). The number of hooks set during a fishing day varies between 1500 and 2500 hooks, and the total length of the line in the sea thus ranged from 25 to 45 km. The whole length of line is referred to as a set. A radio-transmitting "Dhan" buoy is attached at intervals to the line. The snood is weighted down approximately at its midpoint by a barrel lead to separate it from the main line (Figure 6). The long-line is usually set before dusk and the hooks are baited during setting with sprat (12 cm average length). Hauling starts approximately at noon and is completed between 5 and 10 hours later, depending on weather conditions and possible complications, e.g. breaking of the line, which frequently occurs. In those cases the remaining line can be found by picking up the radio transmissions from one of the Dhan buoys. Figure 5. The Faroese long-liner M/S Hvítiklettur, one of the largest salmon fishing vessels in the fleet (105 feet, 279 GRT) and the one used in the research fishing during the project (Photo by Sigbjørg Justesen). In the commercial fishery salmon below 60 cm total length (discards) were not permitted to land and were discarded if caught. Catches tend to be best in rough weather (Jan Arge Jacobsen, unpublished data), and this tendency has also been reported in the Northwest Atlantic (Christensen & Lear, 1980; Reddin & Friedland, 1993) and in the Baltic (Thurow, 1974). Further details on the fishing methods, gear and vessels can be found elsewhere (Mills & Smart, 1982). 13
20 The high seas fishery for salmon Long-line vessel Radio Dhan buoy Plastic floats Swivel Ulstron line Barrel lead 18.5m 4.5m Snood Bait (sprat) Figure 6. Schematic drawing of a salmon long-line as used in the Faroese high seas fishery. This thesis is based on samplings from the Faroese high seas fishery, either during the commercial salmon fishery or during a directed research fishery with a hired salmon longliner north of the Faroes. 14
21 SUMMARY OF RESULTS Fishing periods: autumn is defined as November-December and winter as February-March. Paper I. Trends in the incidence of farmed Atlantic salmon in the Norwegian Sea The proportion of Atlantic salmon escaped from fish farms and caught in the Faroese salmon fishery (1980/ /1996) was estimated using scale analysis. The estimated proportion of farmed salmon in the fishery was relatively low from 1980/1981 to 1986/1987, but increased considerably thereafter, and reached a peak in the 1989/1990 fishing season when more than 40% of the catch was estimated to be of farmed origin. Later, the proportion declined, and in recent seasons the proportions of farmed salmon were estimated to be around 20%. The development in the proportion of escaped farmed salmon in the fisheries is thus relatively consistent with the increase in overall production of farmed salmon in the Northeast Atlantic, which increased considerably in 1988 and 1989, with Norway (74%) and Scotland (17%) accounting for the majority of the production. The exception being the years after 1992 where the proportion in the fisheries levelled off while the aquaculture production has increased considerably, totalling 410 thousand tonnes in 1995, which was 115 times the nominal catch of salmon in the North Atlantic. The estimated proportions of fish farm escapees were used to split the Faroese catch into wild and farmed components. It is concluded that if farmed components in salmon catches are not accounted for, catches of wild salmon will be overestimated and assessments of fisheries and stocks of wild salmon confounded. Furthermore, the increase observed in catch per unit of effort (CPUE) in the 1980s and early 1990s might have been caused by an increasing abundance of farmed salmon. Paper II. Seasonal differences in origin of Atlantic salmon in the Norwegian Sea based on estimates from age structures and tag recaptures To test if the population structure of salmon at the feeding areas in the Norwegian Sea north of the Faroes is stable throughout autumn and winter, river and sea age distribution was estimated from 2,350 scale samples obtained in the autumn and winter during four fishing periods 1991/ /1995. In addition, the origin of recaptures of salmon tagged as smolts in different European countries was compared between the two seasons. The fish classified as being of farmed origin from scale characteristics was excluded from the analyses. Age compositions in samples from the four fishing periods showed consistent patterns. The average smolt age (±SE) was significantly lower in the autumn than in the 15
22 Summary of results winter (2.5 ± 0.04 and 2.7 ± 0.03, range 1-5) as was average sea age (1.9 ± 0.03 and 2.2 ± 0.02, range 1-6). As salmon from southern European countries tend to smolt at an earlier age and produce more one-sea winter salmon than in northern Europe, we suggest that a significant proportion of the salmon caught in the Faroes area during autumn originate from southern European countries and that fish from northern regions appear to be more abundant in the winter. Recaptures in the Faroese fishery during autumn and winter of salmon tagged as smolts in different countries support this. Paper III. Origin, migration and dispersal of wild and farmed Atlantic salmon, Salmo salar L., in oceanic areas north of the Faroe Islands The distribution, migration, origin and growth of wild and escaped farmed Atlantic salmon was examined in the area north of the Faroe Islands. Between November 1992 and March 1995 a total of 5,448 salmon (3,811 wild and 1,637 fish farm escapees) were individually tagged and released back into the sea. A total of 106 fish (87 wild and 19 farmed) have been reported recaptured. The recapture rate of wild salmon (2.3 %) was significantly higher than that of farmed salmon (1.2 %). Recoveries of wild salmon were reported from homewater in nine north Atlantic countries, and in a number of different rivers throughout the distribution range of Atlantic salmon included Russia, Iceland, Spain and Canada. Most tags were recovered in Norway, but significant number of returns were observed in Scotland and Russia as well. Fish tagged in the autumn tended to return to areas closer to the Faroes than fish tagged in the winter. This strongly suggests that salmon originating from most areas of the distribution range are at some life stage present in this area, but in variable proportions at different times. Most of the salmon returned home to spawn the next autumn, and the fish that stayed for another year originated from northern areas of Europe. All recoveries of farmed salmon were done in Norway except one at the west coast of Sweden, suggesting that they mainly had escaped from Norwegian fish farms. Assessment of the proportion of wild salmon from different countries present in this area revealed that 40% of the fish were of Norwegian origin, and Scotland and Russia accounted for about 20% each. Four tags of wild fish were reported from Canada (7%), all in the same year when they were tagged. This demonstrates that adult Atlantic salmon can cross the north Atlantic ocean in less than 6 months. Estimated migration speed of wild fish and farmed fish returning to Norway and their specific growth rate was not different. Paper IV. Feeding habits of wild and escaped farmed of Atlantic salmon, Salmo salar L., in the Northeast Atlantic 16
23 Summary of results The stomach contents of 2992 wild and 863 escaped farmed Atlantic salmon caught during autumn and winter in the Northeast Atlantic during three fishing periods 1992/ /1995 were analysed. The salmon fed mainly on hyperiid amphipods, euphausiids, shrimps, lanternfishes, pearlsides and barracudinas, and less on larger pelagic fish and squid. Crustaceans accounted for 95% in number (30% by weight) and fish for 5% in number (66% by weight). There was no difference in condition factor, number and weight proportions of prey, or in diet overlap between wild and farmed salmon, which suggests that farmed salmon that survive until they were captured are completely adapted to feed in the marine environment. The proportion of stomachs containing food was significantly lower during autumn (53%) than during winter (78%). The ambient sea-surface temperature is ca 7 C in autumn compared to ca 3 C in winter; therefore temperature dependent evacuation rate could explain the apparent lower stomach content during the autumn. The significantly higher condition of 3+SW salmon compared to the smaller 1 and 2SW fish, irrespective of season, might be a reflection of higher tolerance to low temperatures, greater forage potential or prey capture success. Although salmon show patterns of opportunistic feeding behaviour, there was also evidence of selective foraging. Generally fish were preferred over crustaceans, and amphipods were chosen over euphausiids. Large salmon tended to be more piscivory than smaller fish. Paper V. Open ocean infestation by salmon lice (Lepeophtheirus salmonis): comparison of wild and escaped farmed Atlantic salmon The infestation of salmon lice (L. salmonis) was investigated on 128 salmon caught on long-line in the Norwegian Sea between during November March The overall prevalence was 99%, the mean intensity and abundance was 30 lice per salmon. Most lice were adults (90%), with 2/3 being adult ovigerous females. These adult lice are estimated to be at least 3 months old at the prevailing sea-surface temperatures in the area: 7 C in November and 3 C in March. The prevalence and abundance of lice on one sea winter salmon were significantly higher on escaped farmed fish than on the wild salmon. However, no difference in abundance was observed between two sea winter wild and farmed salmon. The average number of lice per surface area of fish (density) was significantly higher in two sea winter salmon than in one sea winter salmon, indicating an accumulation of lice on the salmon in the oceanic phase (i.e. infestation). The prevalence of Caligus elongatus was 6% with an abundance of 1 lice per salmon, however, with one farmed salmon carrying 94% of all C. elongatus observed. 17
24 SYNOPTIC DISCUSSION The discussion is divided into three sections, 1) temporal and spatial stock structure of wild and escaped farmed salmon in the ocean, 2) feeding habits of wild and escaped farmed fish in the sea with emphasis on whether salmon is an opportunistic feeder, and 3) possible interactions between fish farm escapees and wild salmon on the high seas. In the discussion I frequently refer to the autumn (November-December) and winter (February-March) fishing periods and fishing areas in the Norwegian Sea (Figure 3, page 11). Temporal and spatial stock structures of wild and escaped farmed salmon In the Norwegian Sea north of the Faroes it is evident that many salmon populations are present (Paper II and III). Fish are of different sea ages, river ages (Paper II), and large numbers of fish that had escaped from fish farms were also present (Paper I). It is also evident that salmon migrate and disperse in the sea, apparently not randomly, but rather in relation to environmental factors (currents and SST), food availability, predation risk, competition, and temporal and spatial constraints due to their origin as smolt as well as to their homing migration (Paper II, III and IV). In order that the population characteristics of the wild stocks should not be confounded, it is necessary to account for the proportion of escaped farmed fish in the various analyses presented (Paper I-V). Why do salmon migrate to oceanic feeding areas before returning to freshwater again? The anadromy of salmon has been considered an evolutionary response to low productivity in freshwater, resulting in fish migrating towards more productive marine habitats in temperate and high latitudes (Gross et al., 1988). However, with variations and exceptions (e.g. McDowall, 1988; Fleming & Gross, 1990). Thus, in order to maximise its lifetime reproductive rate, salmon have to balance the various cost and risks of freshwater and marine migration to reach favourable oceanic feeding grounds that can support rapid growth in habitats with acceptable environmental conditions. Furthermore, in the marine phase, the residence have to be adjusted according to the growth rate in the sea, the maturation schedule and the distance home. Geographic origin The tagging results positioned Norway as the main contributor of salmon to the southern Norwegian Sea, with significant contributions also from Scotland and Russia (Paper III). Only minor contributions were estimated from the other European countries and Canada. The majority of the salmon (91%) were recovered in home waters the same year as they 18
25 Synoptic discussion were tagged. Most of the fish farm escapees originate from Norway (Paper III), which is in line with the fact that Norway accounts for about two thirds of the total aquaculture production of farmed Atlantic salmon in the Northeast Atlantic in recent years (ICES, 1999b). High proportions of fish farm escapees are also observed in Norwegian homewater fisheries (Lund et al., 1991; 1996), and tagged farmed salmon released on the Norwegian coast have been recaptured in the Faroese fishery (Hansen et al., 1987). It has been shown that the farmed fish home to the general areas of escapement from deliberate releases of tagged farmed fish in Norway (Hansen & Jonsson, 1991). It seems unclear whether farmed fish escaping from cages in Scotland, Faroes and Ireland also contribute to the Faroese fishery. Webb and Youngson (1992) found that about 20% of the catches on the west coast of Scotland were escapees, while they have not been detected in catches on the east coast (Youngson et al., 1997). Thus, it cannot be ruled out that some of the Scottish escapees might migrate further north into the Norwegian Sea. In the following discussion the recaptures from the countries around the Northeast Atlantic were grouped by two main geographical regions, i.e. a northern European group (Norway, Russia, Sweden, Denmark, Iceland and Faroe Islands) and a southern European group (Ireland, Scotland, England/Wales, Northern-Ireland, France and Spain) to reveal possible spatial differences in proportions of recaptures. The recaptures in the Northwest Atlantic included Canada and West Greenland, and escaped farmed fish were excluded. Previous sea tagging experiments in the Norwegian Sea have shown that in the northern parts of the area salmon from Norway are most dominant with salmon from UK, Sweden and Russia also present (unpublished data, L.P. Hansen, Norway). The previous major sea tagging at Faroes in (Jákupsstovu, 1988), indicates a much higher proportion of salmon from the southern regions compared with the other tagging results (Table 1). The 90 recaptures, of which 90% were reported in homewaters the same year as they were tagged, indicated that salmon from Scotland and Norway, and to a lesser extent Ireland, were the main contributors to the stock around Faroes, with only a few tags recovered in England/Wales, Sweden, Russia, and off West Greenland (Table 1). The present sea tagging results (Paper III) give a higher proportion of salmon from the northern region compared to the previous tagging at Faroes (Table 1). The main reason for the difference seems to be that the previous tagging was conducted relatively close to the isles, and even south of the isles, resulting in mainly 1SW salmon (85%) being tagged, while in the present tagging mainly 2SW salmon (80%) were tagged (Paper III). The higher proportion of 2+SW in the commercial catches in the 1980s were due a northward shift of the fishing areas, resulting in larger salmon (ICES, 1984; Jákupsstovu, 1988). 19
26 20 Table 1. Recapture proportions (%) of wild Atlantic salmon from various tagging programmes in the North Atlantic since 1960 by country of origin. The countries were grouped into southern and northern European countries for comparisons, as well as in the Northwest Atlantic and Northeast Atlantic. The results from the present tagging experiment is shown as raw recapture numbers c to be comparable to the other results, and as adjusted recaptures d, i.e. corrected for tag reporting rates and exploitation rates in homewaters (Paper III). No. re Northern group Southern group NWA Region NEA Region N. Atlantic Tagging area captured NO SW RU DK FO IC IR SC EN/WA NI FR SP CA W GR N EU S EU NWA NEA Homewaters a Faroes b Norwegian Sea c 87 g Norwegian Sea d 87 g Average NEA e West Greenland f a smolt tagging (Paper II), b previous sea tagging (Jákupsstovu, 1988), c present sea tagging (Paper III), d present sea tagging adjusted (corrected) for tag reporting rates and exploitation rates in homewaters (Paper III), e Average percentage recapture rates in the Norwegian Sea based on the three data series: Homewaters (Paper II), Faroes (Jákupsstovu, 1988) and Norwegian Sea corrected (Paper III). f previous sea tagging at West Greenland, excluding recaptures in the fishery during tagging (Jensen, 1980a). g In total 106 fish were recaptured, but 19 were of farmed origin and were excluded from the table. Synoptic discussion
27 Synoptic discussion It is clear from Table 1 that the recaptures of salmon tagged as smolts in homewaters (Paper II) obviously reflect only those countries that tag smolts. For example Russia practically does not tag smolts, which is also evident in their absence in the recaptures from the homewater tagging programme (Table 1). However, their presence became evident in the recent sea tagging experiment (Table 1) (Paper III). It should be noted that only the wild component was used in the recapture proportions in the present tagging experiment, and that in the period practically no fish farm escapees were in the sea (aquaculture were in its infancy in the early 1970s). In the Northwest Atlantic approximately 2/3 of the salmon present were estimated to originate from Northeast Atlantic and the remainder from Canada (Table 1). However, more recent analysis of scale characteristics have revealed a more even split between North American and European salmon present in the West Greenland area (Reddin et al., 1988), but with proportions of North American salmon varying from 34% in 1971 to 75% in 1990 (Reddin & Friedland, 1999). Scotland, England and Wales seem to be main countries of origin of the European component in the West Greenland area (Swain, 1980). It should be noted that probably very few salmon from the northern European regions migrate to West Greenland, although single recaptures from Norway and Sweden have been made in this area (Swain, 1980). Temporal and spatial stock structures In the Norwegian Sea there is evidence for a seasonal change in stock complexes entering and departing the feeding areas north of the Faroes (Paper II). Particularly salmon from areas in southern and mid part of Europe are observed in higher numbers in the autumn than in winter, when fish from northern areas were more abundant (Paper II and III). There may be several explanations for the apparent change in the stock composition of salmon from autumn to winter. Smolts from different areas move into the ocean at different times. Fish from southern Europe may leave their home rivers as early as beginning of April (e.g. Baglinière, 1976), whereas smolts from northernmost Norway go to sea in late June or in the beginning of July (e.g. Hvidsten et al., 1995). Advection and active movement would displace salmon post-smolts from different regions differently in the ocean during their first months at sea (Paper II). Such considerations have been made for salmon in the West Greenland area (Jensen, 1980a; Reddin & Shearer, 1987; Reddin et al., 1988) and in the Gulf of St. Lawrence (Reddin & Short, 1991; Friedland et al., 1999). Due to the earlier smolt runs in the south and the great influence of the northward flowing North Atlantic Current in the Norwegian Sea (Hansen et al., 1998), the majority of the 21
28 Synoptic discussion north European post-smolt might be located further to the north in November December than those from the southern countries. Thus the northern group is expected to be less represented in the autumn catches compared to later in the fishing season (Paper II). The recapture of relatively large southern European post-smolts north of Scotland and in the Norwegian Sea in June and July (Shelton et al., 1997; Holm et al., 2000), at a time when the majority of the e.g. Norwegian smolts just have left their rivers (Hvidsten et al., 1995) and are smaller, indicate that there are sequential/temporal and spatial differences in the salmon stocks in the sea during the early period after seaward migration (Holm et al., 1998). This is also suggested for salmon in the West Greenland area (Reddin & Burfitt, 1980; Reddin et al., 1988) and in the Gulf of St. Lawrence (Reddin & Short, 1991). It should therefore not be surprising to find that these fish tend to keep their segregation into November, maybe more "diluted" at this later stage, when they recruit the fishery in the Faroese area. However, when they have entered the area north of the Faroes late in the year, no sign of schooling behaviour could be detected (Jákupsstovu, 1988). Some segregation, however, may be persistent during their feeding phase, as indicated further below. A simple calculation of fish density in the feeding area support the view of solitary predatory behaviour during the marine feeding phase. In Paper I, the average CPUE (number of salmon per 1000 hooks per day) of 55 salmon (range 30-80) during the period 1981/ /1995, is equivalent to an average of 55/18 = 3 salmon per kilometre longline fished (range ), since there are 18 m between the hooks (see Figure 6, page 14). Furthermore, the fish are generally not clustered on the line when caught (Jákupsstovu, 1988). Depending on assumptions of the distance the baited long-line attract salmon (e.g. 1-2 km), the density in the sea would range from 1 to 3 salmon per square kilometre. Hansen (1984) found the density to be about one salmon per square kilometre calculated from a diffusion model, and Thurow (1973) calculated an average density of 1.5 salmon per square kilometre off northern Norway. Thus the density of salmon in the oceanic feeding areas is low compared to other major pelagic stocks (herring, mackerel and blue whiting) feeding in the area from late winter (Vilhjálmsson et al., 1997; Holst et al., 1998; ICES, 1999a; 1999c). It was hypothesised (Paper II) that the spatial segregation was the result of a tradeoff between food availability and thermal limitations, with the smaller salmon requiring higher ambient temperature. Thus, significant parts of the 1SW stocks might be confined to the southern and warmer side of the subarctic front in the southern Norwegian Sea during winter. This front is located in east-west direction approximately between the autumn and winter feeding areas and turns northward in a north-eastern direction into the eastern part of 22
29 Synoptic discussion the winter feeding area (Hansen, 1985). Such a distribution would lead to a shift in the smolt age distribution and in the proportions of country of origin from the autumn area to the winter area, in the ways observed in Paper II. However, the suggestion of temporal segregation relies on the assumption that the movement of the fishery reflects a migration of salmon from the autumn to the winter feeding areas. Which might be a reasonable assumption, because fishing fleets usually concentrate in areas with highest CPUE (e.g. Healey et al., 1990). However, since the division between the warmer and the colder side of the front coincide with the autumn and winter sampling areas that were not fished synoptically, we cannot verify our suggestions from the present data. Although the spatial changes in the shift of the centre of seasonal distribution involved are not great (60 nm), the relatively large drop in SST from autumn to winter might indicate that two different water masses were exploited (Paper II). Thus, if the above assumption on temporal segregation does not hold, the results could indicate a stock segregation in the feeding areas north of the Faroes throughout the seasons. An indirect support for the alternative suggestion was given in Paper II, and was based on the earlier sea tagging experiment in the Faroese area (Jákupsstovu, 1988). This tagging was performed during winter in the area south of the present winter feeding area (i.e. closer to the Faroes), where mainly 1SW fish was caught and tagged as opposed to the present sea tagging (Paper III). The tag returns from the previous tagging experiment indicated a more southern origin of the fish in this area, as compared to the results from the present sea tagging (see Table 1). Thus, the observed stock segregation, at least in the winter feeding areas, might be the result of environmental conditions and the geographic origin of the fish. Salmon have been observed to feed heavily on crustaceans, especially on the hyperiid amphipod Themisto spp. as well as on mesopelagic fish species in these feeding areas (Paper IV). Although the abundance of prey does not seem to be limiting, salmon have to compete with other major pelagic fish species, i.e. blue whiting, herring and mackerel, which are present in large numbers from early spring (ICES, 1999a; 1999c). The longer photoperiod to the north and novel feeding areas were suggested to be the main driving forces for horizontal migrations in these pelagic fish and furthermore the largest fish would benefit most energetically from such migrations (Nøttestad et al., 1999). The abundance of T. libellula, which is a relatively large and important crustacean prey for salmon (Paper IV), is very high in the water mass on the colder side of the front (Dalpadado et al., 1998). Since neither herring, mackerel nor blue whiting enter the colder areas during their feeding migrations (Vilhjálmsson et al., 1997; Holst et al., 1998; ICES, 1999a), it is possible that large salmon might find an area with less competition during late winter (Paper II). Thus, 23
30 Synoptic discussion competition might also play a role to segregate the various age groups of salmon feeding in the sea. Others have found thermally dependent segregation of salmon in the sea. Jákupsstovu (1988) found that the proportions of 1SW salmon compared to MSW salmon were higher in areas with SST above 4 C while the proportions MSW were higher in the colder areas with less than < 4 C. Reddin and Shearer (1987) found that the low temperature (< 4 C) seemed to limit salmon distribution and alter their migration routes in the Northwest Atlantic, and Reddin (1988) also found a more restricted distribution of the 1SW salmon compared to the wide distribution of MSW fish in the Northwest Atlantic. At West Greenland in mid 1980s, lower SST were associated with higher mean sea ages in the catches, mainly because a decline in the proportions of 1SW fish (ICES, 1985). In Paper IV it was found that the condition factor of large (3+SW) salmon was significantly higher than of 1 and 2SW fish, and furthermore, that the condition of the large fish was independent on season, while the condition decreased from autumn to winter for the smaller fish. This indirectly support the suggested trade-off between food and thermal limitations with lower profitability for the small salmon during the winter period. It is well known that the MSW stock components from many southern areas utilise other main feeding areas in addition to the Norwegian Sea. For example the West Greenland area is a major feeding area for MSW stocks from Scotland, England/Wales and Ireland (Swain, 1980; Jensen, 1980a; 1980b). The Irminger Sea and East Greenland areas probably host many European stocks (Jensen, 1967; Jensen & Lear, 1980; Horsted, 1988; Scarnecchia, 1989). The recaptures of salmon tagged as 1SW fish in the Faroese area and recaptured in the Irminger Sea the following year (only 2 recoveries), and conversely a salmon tagged in Irminger Sea in spring and recovered in the Faroese area the following autumn (ICES, 1984), indicate that some stocks, probably the MSW components of those stocks, might have a two-stage or even a three-stage migration and feeding cycle in the ocean: They might use the southern Norwegian Sea as a pathway to, from or during both ways to the feeding areas in the western Atlantic. Such migrants might explain parts of the observed temporal and spatial changes in the age structure and recovery proportions in the areas north of the Faroes. Turrell and Shelton (1993) gave a scenario of a hypothesised 'life-trajectory' of a British salmon migrating to West Greenland via Faroes, south Iceland and south Greenland, and they found such migration routes to fit spatially within the 4-8 C isotherms and temporally into the availability to the Faroese fishery in the Norwegian Sea and to the West Greenland fishery the following autumn. This conform to the multi-stage migration suggestion above. 24
31 Synoptic discussion In conclusion, there seem to be substantial evidence for a succession of stock complexes entering and departing the feeding areas in the Norwegian Sea. Partly due to differences in timing of smolt runs from the various geographical regions in the Northeast Atlantic as well as temporal and spatial constraints for the homeward migration due to maturation, and partly due to a segregation of salmon in a trade-off between food availability and thermal limitations, probably intensified by higher competition from other major pelagic stocks on the warmer side of the front. Migration and dispersal In the Norwegian Sea between 80 and 90% of the salmon caught during autumn and winter are maturing during the year and destined to return to freshwater the forthcoming autumn, irrespective of sea age (Youngson & McLay, 1985; Jákupsstovu, 1988; Paper III). The distance home from the feeding areas north of the Faroes to the home countries is shortest for Norwegian salmon (600 km) and longest for the Canadian salmon (4550 km). Average values of distance, speed and growth rates by country are shown in Table 2, which is an extract of the recapture details in Paper III. The migration speed was estimated from the distance home divided by the time from early March until recapture the same year (Paper III). However, for several reasons the estimates of migration speed are minimum estimates. The distance is the shortest strait line in the sea from tagging to recapture, and might furthermore be biased if the time of departure from the feeding area is dependent on the distance home. It is also probable that the fish continue to feed in the area at least until May before the onset of anorexia (Stead et al., 1999) as most of the recaptures were made in July (90% recovered from June September). Therefore it is difficult to limit the period to that used for active homeward migration. Furthermore, fish slow down in coastal waters and fjords (Hansen et al., 1993b) and do not necessarily ascend the river immediately upon return to coastal waters (Jonsson et al., 1990). The results from Table 2 indicate that the migration speed was apparently higher for fish from distant areas. However, if fish from distant areas leave the feeding area earlier than those from nearby areas, then their true average migration speed might be the same. Average swimming speed of one body lengths per second (BL/s) of homing salmon were reported in the Pacific (Hartt, 1966; Quinn, 1988; 1990; Ogura & Ishida, 1995), which is considered the optimal long-distance migration speed (Weihs, 1973; Ware, 1978). By adopting a swimming speed 1 BL/s as the optimal homing migration speed (Weihs, 1973), the journey home as well as the average number of days feeding in the area prior to homeward migration (last two columns in Table 2) could be estimated. These estimates 25
32 Synoptic discussion show that on average the fish could feed for four more months before the onset of homeward migration, of course varying according to the different countries involved (Table 2). The estimated minimum duration of the active homeward journey would be 10 days to Iceland to over two months to Canada from the feeding areas. The growth in length ranged from nil to a maximum of 34 cm for a Canadian 2SW salmon, and the average daily growth in length of cm, or 0.19% gain in body weight per day (G) (Paper III). There was no significant differences in growth rates among salmon from different regions, neither between wild and farmed salmon nor between fish that stayed for another year at sea and those that left the area within 12 months (Paper III). The average growth rate in the present work corresponds to the growth rates of salmon tagged in West Greenland and recaptured in North America (0.03 cm per day) and in Europe (0.05 cm per day) (Jensen, 1980a). A growth of about 500 g (from 3.3 to 3.8 kg) in three months was reported for 2SW salmon in the area north of the Faroes from February to end of April 1983 (ICES, 1984). This correspond to an average length increase of 3.4 cm, using the length-weight regression of 2SW salmon in Paper III (w = l 3.052, n= 645, r 2 = 0.78, p < 0.001). Of fish recaptured relatively close to the tagging site there was no apparent difference in the distribution of salmon that was tagged in the autumn or in the winter (Paper III). This does not seem to be the case of fish that were recaptured relatively far away from the tagging sites. They were all tagged in the winter. This may suggest that salmon from distant areas are not present at the Faroes during autumn, and corresponds to the suggestion in Paper II that the northern group arrived at the feeding areas later in the season. This might also be true for the distant western group. The wild salmon that stayed for another year in the ocean were all 3SW fish upon return belonging to the northern region (Norway, Russia and Sweden) (Paper III). Fish that were tagged in the winter seem to survive better than fish tagged in the autumn. There may be several explanations for this. First the size distribution of the fish that were tagged in the autumn were smaller than of those tagged in the winter, and smaller fish may thus be more vulnerable to handling and tagging stress (Fowler & Stobo, 1999) and subsequent predation. Second, these fish will spend on average three months longer in the sea than fish tagged in the winter, and may be exposed to different marine mortality factors for a longer period of time. Furthermore, based on smolt age and sea age distribution of salmon, Paper II suggested that fish originating from stocks in southern Europe were more abundant in the autumn than in winter. If this holds true, the possibility cannot be ruled out that the apparent differences in survival observed may reflect the trends observed in last decade that mortality of salmon stocks from southern Europe has increased 26
33 Synoptic discussion more than for salmon from intermediate and northern regions in the Atlantic (Parrish et al., 1998; ICES, 1999b). The low recapture rate of wild salmon (2.3%) in the present sea tagging experiment (Paper III) compared to the previous sea tagging (4.6%) (Jákupsstovu, 1988) might further indicate an overall decrease in marine survival in recent years. In the Baltic McKinnel and Karlström (1999) reported that the most recent smolt tagging releases have had the lowest recapture rates in 40 years, which also indicate poorer conditions for salmon in recent years in the Baltic. Table 2. Average migration distance, migration speed and growth rate (G, specific growth rate) by type of fish (wild and farmed) and country of origin. Data from Paper III. N is number recaptured and the entries are average values. The rightmost two columns show the number of days left for feeding prior to homeward migration and the number of days to travel home with one BL/s. Distance Speed a Speed a G Days b Days b Country N km km/d BL/s % w/d feeding travelling Wild salmon Norway Scotland Ireland Russia Sweden Canada Denmark Spain Iceland England Total wild: Farmed salmon Norway Sweden Total farmed: Grand total: a Migration speed in km per day and body lengths per s (BL/s) from March the first in the year of recapture. b Number of days corresponding to a swimming speed of 1 BL/s during homing migration (Weihs, 1973). Only three of 19 farmed salmon were recovered from the autumn tagging (Paper III), which apparently is in contrast to the suggested poorer survival rates of salmon from the southern European region above, because the farmed fish apparently comes from Norway (Paper I and III). However, the survival (return rate) of fish farm escapees have been shown to depend heavily on the time of escape, i.e. the highest survival is observed if escaping in spring (Hansen & Jonsson, 1989; Hansen & Jonsson, 1991). Thus, this may help to explain the lower survival of farmed salmon. 27
34 Synoptic discussion During tagging many (32%) of the fish were released with the hook left in, to avoid excessive handling of the fish and if the removal was considered to damage the fish. Of the fish recovered, the return rates of fish with the hook left in when tagged was significantly higher than of fish with no hook when released (Paper III), which might indicate that for some of the fish the removal of the hook was lethal. However, there was a tendency that growth rate was lower in the releases with the hook left in (Paper III). However, long-line caught salmon at West Greenland have been reported to be in "excellent condition for tagging" (Munro & Swain, 1980). Management considerations Below is shown an example of practical use of the stock structure estimates from the previous sections on the Faroese high seas fishery. The knowledge of the temporal and spatial composition of the salmon stocks present in the Faroese area enable an admittedly coarse assessment of the expected exploitation rates on the various stock complexes fished in the long-line fishery for Atlantic salmon north of the Faroes. From Paper III approximately 65% of the salmon in the Faroese fishery were estimated to originate from countries belonging to the northern European region and approximately 28% were estimated to originate from the southern European region. These figures add up to 93%, which is the sum of the northern and the southern stock complexes, the remaining 7% were estimated to originate from Canada (Table 1), discounting farmed salmon (representing 20% on average, Paper I). From recoveries of microtags and external tags in the same fishery (Paper II), the approximate sea age distribution for the northern group was 12% 1SW and 88% 2+SW salmon, respectively and for the southern group 70% 1SW and 30% 2+SW salmon, respectively. In the Northwest Atlantic only 2SW were recovered. Thus, the Faroese fishery exploits mainly the 2+SW northern stock complex (65% of 88% = 57%), secondly the 1SW southern stock complex (28% of 71% = 22%), thirdly the 2+SW southern stock complex (28% of 30% = 8%), and fourthly the 1SW northern stock complex (65% of 12% = 8%), and 7% from the Northwest Atlantic (Table 3). If the average recapture rates of country of origin based on the three data series described in Table 1 were used, i.e. 57% from the north, 40% from the south and 3% from Northwest Atlantic, the result would be as shown in second part of Table 3. 28
35 Synoptic discussion Table 3. Approximate proportions (%) of various stock components and age groups exploited by the Faroese fishery, as inferred from tag recaptures in the North Atlantic. Sea age Sea age Region 1SW 2+SW Total a 1SW 2+SW Total b Northern Southern NWA Total a Proportion by region from Paper III, and b average from Table 1. This opens up the possibility for a rational management of the fishery. If for example certain stock components and age groups were considered threatened, the entries in Table 3 would indicate which components were fished and also where restrictions might have a desired effect. However, for an effective management, more detailed information about the temporal and spatial stock distributions is required. Diet and feeding habits of wild and escaped farmed salmon in the sea This section compares the diet of wild and escaped farmed salmon, among sea ages, between seasons, and from various geographic regions and life stages. Then it was examined whether salmon are opportunistic feeders, and finally I assess the likelihood of food being a limiting factor for salmon in the sea. The diet of salmon Important food for salmon are crustaceans (amphipods, euphausiids, pelagic shrimps), mesopelagic fish (pearlside, lanternfishes, barracudinas), larger fish (blue whiting, herring, capelin, and mackerel), and juvenile squid (Paper IV). Crustacean prey dominated in number while fish dominated in weight. Relatively few larger pelagic fish such as herring, blue whiting and mackerel were taken. Wild and farmed salmon seemed not to differ in these figures (Paper IV). During autumn half of the stomachs contained food while ca 80% contained food during winter and this difference was consistent in the three fishing periods (Paper IV). Thus, there seemed to be a lower feeding rate in the autumn season compared to the winter season. Lower feeding in autumn have been reported for salmon in the Labrador Sea (Lear, 1980) and in the Baltic (Christensen, 1961; Thurow, 1966). However, the higher condition factor of salmon in the autumn than in winter seems to contradict the apparent lower feeding intensity during autumn (Paper IV). The explanation to the apparent ambiguity 29
36 Synoptic discussion might be found in the fact that the gastric evacuation rate is highly positively correlated with temperature (Dos Santos & Jobling, 1991). The ambient temperature decreases from 7 C in autumn to 3 C in winter, and would result in a lower rate of emptying of the stomachs during winter. This would give the impression that during winter fewer stomachs were empty, and that the non-empty stomachs on average contained more food. There was a shift in the prey species composition with age or size of the salmon. The smaller (1SW) salmon had mainly eaten amphipods, lanternfishes and some barracudinas, while the larger 3+SW salmon had mainly eaten barracudinas, lanternfishes and some larger fish. The 2SW salmon had eaten the various prey groups in intermediate proportions compared to the 1 and 3+SW salmon (Paper IV). The larger fish prey was thus preferred with increasing age of the salmon to the smaller mesopelagic fish and crustaceans. The shift in food composition was the same for wild and escaped farmed salmon (Paper IV). Size selective feeding was reported for salmon in the Labrador Sea and on the Newfoundland shelf during spring, where the proportion of mesopelagic shrimps increased with fish size on behalf of amphipods and lanternfishes (Lear, 1980). There was also a tendency that the proportion of capelin increased with fish size at West Greenland, again on behalf of amphipods and of euphausiids (Lear, 1980). There were some indications that escaped farmed salmon fed more actively or was more eager to take the bait than wild salmon, since farmed salmon contained a higher proportion of bait than did wild salmon (Paper IV). However, the biological significance of this observation is not clear, but might indicate that farmed fish have greater appetite during winter. The proportion of farmed fish that contained food was also higher than that of wild fish, supporting the previous statement. Therefore, it might be speculated that the feeding behaviour of escaped farmed salmon differ from that of wild salmon due to their farmed prehistory (i.e. their genetic alteration from wild strains). For example behavioural differences could result in different hooking probability of the two groups, or alternatively farmed salmon have been selected for fast and efficient growth that may have resulted in a higher demand for energy (Thodesen et al., 1999), and thus have made farmed salmon a more aggressive feeder. Thus, wild and farmed salmon differed in very few if any aspects of feeding. No difference was observed in diet overlap, nor in frequency, number or weight proportions of prey between wild and farmed salmon (Paper IV). The indication of a more eagerly feeding behaviour, and the higher proportion of farmed fish containing food compared to wild fish, strongly suggests that farmed salmon that survive until they were captured, are completely adapted to feed in the marine environment. 30
37 Synoptic discussion Comparison of the marine feeding habits from other areas and life stages A summary of published accounts on the food of Atlantic salmon is presented in Table 4 as the number of occurrences (%) of each prey type for various geographic areas in the North Atlantic, including the results from the present study (Paper IV). Table 4 is similar to Hislop and Shelton's (1993) Table 5.1, but with percentage of occurrences instead of the number of occurrences. Similarly the corresponding weight percentages of prey is given in Table 5, along with the new data from the Norwegian Sea (Paper IV). In the tables, immature salmon is termed as 'adults' when they are found in the feeding areas in the open sea and as 'homing adults' when they are maturing and have initiated their homeward migration and are found on the continental shelves and in coastal areas. There seem to be systematic differences in diet composition at different life stages of salmon as well as geographical differences. The general picture is that the feeding intensity decreases towards the spawning time, and salmon usually become anorexic the last part of their oceanic stage (e.g. Kadri et al., 1997; Stead et al., 1999). This is reflected in the high percentage of empty stomachs in samples of maturing salmon in coastal and estuarine areas, as compared to immature salmon in oceanic areas (Table 4). The food of adult salmon in coastal areas returning to spawn (e.g. Blair, 1965; Grønvik & Klemetsen, 1987; Hislop & Webb, 1992), where fish are totally dominant as prey (se the two first columns in Table 4 and 5). However, with one notable exception (included in the data from the Canadian Shelf, Table 4 and 5), where amphipods were the main food (Neilson & Gillis, 1979). The prey composition in the oceanic phase seems to be more varied, and includes capelin, sandeel, herring, barracudinas, lanternfishes, crustaceans and squid in varying proportions (Table 4 and 5). There seems, however to be a systematic difference in the oceanic food composition between the Northwest and Northeast Atlantic areas, and also within the regions. In the Northeast Atlantic fish account for approximately 66% in weight, whereas in the Northwest Atlantic fish constitute around 82% in weight, with mainly crustaceans contributing the rest (Table 5). Thus, there might be a higher reliance of fish as prey in the Northwest Atlantic as compared to the Northeast Atlantic. Hislop and Shelton (1993) proposed that the crustaceans were a much less important prey than fish in the North Atlantic. The present data was not available to them, but it emphasise the importance of crustacean prey in the oceanic areas in the Northeast Atlantic, contributing 30% in weight (Table 5). To assess the importance of various prey for salmon, it is necessary to account for the duration of the various oceanic life stages. Three life stages (feeding phases) might be considered, i.e. the post-smolt, open ocean, and homing phase. Thus, a phase duration food composition principle could be used in such assessments (although 31
38 Synoptic discussion not attempted here). I would, however, stress the importance of the hyperiid amphipods as prey for salmon beside various mesopelagic fish in the oceanic areas in the Northwest Atlantic (Table 4 and 5). Furthermore, especially in the autumn salmon seemed to rely on amphipods as food (Paper IV). Differences in fish prey in the offshore areas within the Northwest Atlantic region are also evident (Table 4 and 5). Salmon off West Greenland mainly rely on capelin and sandeel as food while salmon in the Labrador Sea and Davis Strait areas feed on mesopelagic fish, which is more comparable to the fish prey types in the Northeast Atlantic (Table 5). This regional difference could be due to differences in availability and abundance of the various prey types. Jensen (1967) noted a similar contrast in the food content of salmon from the Irminger Sea and off West Greenland. If the feeding habits differ between the Northwest and Northeast Atlantic, and even within the western Atlantic, it may affect the marine survival of salmon in the various areas differently. The high variance in the abundance of the short-lived capelin stock in the Northwest Atlantic (e.g. Methven & Piatt, 1989; Frank et al., 1996), might have profound influences on the oceanic survival of salmon feeding in those areas. Thus it could be speculated that the ocean survival will fluctuate more in the Northwest Atlantic than in the Northeast Atlantic, due to the apparent higher reliance on capelin and sandeel as food in the Northwest Atlantic (off West Greenland) compared to a more "balanced" diet in the Northeast Atlantic (Table 4 and 5). There seem to be evidence for a greater decline in the salmon stocks (particularly MSW stocks) in the Northwest Atlantic and in mid and southern Europe, than of stocks from the northern European region (e.g. Parrish et al., 1998; ICES, 1999b). Large proportions of these MSW stocks feed in the Northwest Atlantic area and off West Greenland during some part of their oceanic phase (e.g. Shearer, 1992; Mills, 1993). Thus, I speculate that one reason for the decline might be deteriorating feeding conditions in the Northwest Atlantic during last decade, due to the harsh environmental conditions there since 1990 (DFO, 1998). However, with some improvement in the environmental conditions the last couple of years (DFO, 1998). 32
39 Synoptic discussion Table 4. Food of salmon in the North Atlantic. Frequency of occurrence (%) of prey in each major geographic area. From Table 5.1 in Hislop and Shelton (1993) and references therein, and from Paper IV. A + means less than 1%. Life stage and period a Homing adults Homing adults Adults winter Adults autumn Adults winter Adults winter Adults winter Area Canadian shelf British Isles Labrador Sea/David Strait West Greenland Norwegian Sea Faroes 1983 Faroes Paper IV No. examined No. empty or with only bait No. with food Percentage with food (1) FISH Clupeoids Clupea harengus Sprattus sprattus 15 Alosa pseudoharengus + Clupeidae 1 Capelin Mallotus villosus Fry (mostly Mallotus villosus) 4 Sandeel Ammodyteidae Lantern fishes Benthosema glaciale Notoscopelus kroeyeri Lampanyctus macdonaldi 1 + Lampanyctus crocodilus + Myctophum punctatum + Protomyctophum arcticum + Hierops arctica + Myctophidae Barracudinas Paralepis c. borealis + Notolepis rissoi kroyeri + + Paralepidae Pearlside Maurolicus muelleri Other fish Unidentified fish (2) INVERTEBRATES Annelids Insects Crustaceans Copepoda + + Amphipoda Isopoda 3 Euphausiids Other crustaceans Unidentified crustaceans 2 19 Mollusca Spiratella helicina 1 Squid Gonatus fabricii Unidentified/other squid Unidentified invertebrates (3) UNIDENTIFIED REMAINS
40 Synoptic discussion Table 5. Food of preadult and adult Atlantic salmon in the North Atlantic. Percentage composition by weight in the diet in each major geographic area. From Table 5.2 in (Hislop & Shelton, 1993) and references therein. Old Faroese data recalculated from Hislop and Youngson (1984) and new Faroese data from Paper IV. A + means less than 1%. Life stage and period Area Homing adults Canadian shelf Homing adults British Isles Adults winter Labrador Sea/David Strait Adults autumn West Greenland Adults winter Faroes 1983 a Adults winter Faroes Paper IV No. examined No. empty or with only bait No. with food Percentage with food (a) Total stomach content Fish Crustaceans Molluscs + Squid Annelids Insects + Unidentified (b) Principal fish prey Clupeoids Capelin Sandeel Lantern fishes Barracudinas Pearlside 1 16 Others (c) Principal crustacean prey Amphipods Euphausiids Other a The weight percentages from the Faroese area in 1983 (Hislop & Youngson, 1984) have been recalculated in order to conform to the species list in the present stomach data (Paper IV). Is salmon an opportunistic feeder? Several authors have suggested that Atlantic salmon are opportunistic feeders (Hansen & Pethon, 1985; Reddin, 1988; Pearcy, 1992; Hislop & Shelton, 1993; Sturlaugsson, 1994). However, in the Atlantic there is no information that compares the diet of salmon with the potential prey available. Although information from literature on plankton and micronekton distributions in the Northeast Atlantic (e.g. Dunbar, 1964; Johnson & Kitchell, 1996; Dalpadado et al., 1998) might give a clue to the potential prey of salmon, the nonoverlapping temporal and spatial nature of the data prevents any conclusion on prey selection. Furthermore, the vertical distribution of salmon in the sea is poorly known and have been mainly inferred from catching (Templeman, 1968; Reddin, 1985; Reddin & 34
41 Synoptic discussion Shearer, 1987; Dutil & Coutu, 1988). However, salmon is known to undertake deep dives, deeper than 150 m (Jákupsstovu, 1988) for shorter or longer periods. Thus, so far the feeding behaviour and forage strategy of Atlantic salmon has only been inferred from indirect measures such as stomach analysis. In the present investigations (Paper IV) corresponding plankton samples (MIK net) from the upper 50 m were taken on 13 fishing locations to compare the diet of salmon to available food. Even if most of the species occurred in both data sets, their relative proportion differed greatly (see Table 5 in Paper IV). The results suggest that fish were preferred over crustaceans, and amphipods were preferred over euphausiids. Furthermore, of the three euphausiids present the larger Meganyctiphanes norvegica was preferred over the smaller Thysanoessa inermis and Th. longicaudata by salmon. The euphausiids ingested were larger than those from the habitat samples (Paper IV). The preference of fish to crustaceans, and of the larger M. norvegica over Thysanoessa spp. can be explained by size selective feeding of salmon. However, the preference of amphipods to euphausiids seems more subtle, as their sizes are comparable in the plankton samples, although in the stomachs the euphausiids were larger than the amphipods. The energetic content of euphausiids and amphipods might be different as well as their visual contrast in the sea, in particular the large and heavily pigmented compound eye of amphipods may make them more conspicuous (Zaret & Kerfoot, 1975), or their swimming and predatory escape behaviour might be different. It further seems that although there are numerous Thysanoessa spp. available in the upper 50 m from the plankton data, this genus is hardly preyed upon by salmon. In the Pacific, Peterson et al. (1982) also found a preference for amphipods to similar sized stages of a copepod of juvenile coho (Oncorhynchus kisutch) and chinook salmon (Oncorhynchus tshawytscha) off the Oregon coast, and Brodeur et al. (1992) estimated that the consumption of euphausiids as percentage of total biomass was quite low (0.01% d -1 ) by coho and chinook, although the abundance of euphausiids from plankton tows were high. Holst et al. (1996) found a tendency of size-selective feeding of salmon post-smolts of the hyperiid amphipods (Themisto spp.), as indicated by a positive relation between prey size in the stomachs and post-smolts size. This corresponds with the findings of a weak, but significant correlation between length of salmon and length of Themisto libellula prey (Paper IV). Furthermore, Holst et al. (1996) noted that given that O-group fish were available in the area as observed from the trawl catches and that amphipods also were available from parallel plankton sampling, the post-smolts fed mainly on O-group fish, indicating a selective feeding strategy. Selective feeding was also reported by Lear (1972) 35
42 Synoptic discussion in the Northwest Atlantic, where salmon preferred herring to capelin, and the large salmon contained significantly more herring than small salmon. Salmon did not feed on Sagitta spp., although it has been reported as food for salmon in the Pacific (Brodeur & Pearcy, 1990; Tadokoro et al., 1996). It might be speculated that Sagitta spp. either is too transparent, unpalatable, or is a low energy prey. At least two year classes (1 and 2+ group) of pearlside (Maurolicus müelleri) were present in the salmon stomachs, however, only the smaller 1 group was present in the plankton samples (Paper IV), apparently indicating size selective feeding. Alternatively, the larger 2+ group of pearlside might avoid the plankton sampler or being distributed below the sampling depth of 50 m. The pearlside has been reported in some areas to be separated into two vertical layers in the sea during winter, with the older individuals occupying the lower layer (Goodson et al., 1995) and are reported to be most numerous below 200 m depth in the Norwegian Sea (Dalpadado et al., 1998). Thus salmon probably also feed deeper than 50 m. Salmon are generally found to inhabit the upper surface layers (Templeman, 1967; Reddin, 1985; Dutil & Coutu, 1988) most of the time with occasional deep ascents for shorter or longer time periods, deeper that 150 m (Jákupsstovu, 1988). Pacific salmon also occasionally feed deeper than m during day (Pearcy et al., 1988). In summary, although salmon show patterns of opportunistic feeding behaviour, there was also evidence of selective foraging. Generally fish were preferred over crustaceans, and amphipods were chosen over euphausiids. Furthermore, large salmon tended to be more piscivory than smaller fish. Such a feeding behaviour of salmon might influence the dispersal on the feeding areas. Although temperature constraints are indicated to be a strong decisive factor for oceanic distribution of salmon (Reddin, 1985). In Paper II, it was suggested that the spatial segregation of various stock complexes and age groups salmon in the feeding area was the result of a trade-off between food availability, age specific thermal limitations (or preferences), and possibly competition from other large pelagic stocks feeding in the area. Is food a limiting factor for growth and survival of salmon? Survival in the marine environment is a complex process, and Anderson (1988) summarised four major hypotheses that were used to explain marine mortality of fishes: starvation, predation, physical dispersal, and disease. The effect of temperature and size on development, mortality and survival rates of the early pelagic life stages of marine fishes was reviewed by Pepin (1991). Conditions that determine growth rate during larval phase, 36
43 Synoptic discussion such as food availability and temperature, are thought ultimately to determine survival (Ware, 1975; Anderson, 1988; Pepin, 1991). It is likely that the growth-survival/mortality hypothesis put forward for other pelagic marine fishes (Anderson, 1988) also apply to the early marine phase of Atlantic salmon. There is, however, limited knowledge of the factors influencing the growth and survival of post-smolts (Friedland & Reddin, 1993; Friedland et al., 1998), and it remains to be demonstrated that survival of salmon is a direct function of growth, mediated through size-dependent predation. Neither are the indirect effects of climate, such as food availability evident. It has been shown that ocean climate conditions may affect marine survival of salmon (e.g. Friedland et al., 1993; Friedland et al., 1998). Presently there is no clear single factor that can be identified that cause mortality of salmon in the marine environment. Mortality is probably a combination of different factors as well as synergism between them. Traditionally it is assumed that the major mortality in salmon occur at an early phase of the post-smolt phase (Holtby et al., 1990; Hansen & Quinn, 1998; Friedland, 1998). Growth in salmonids is correlated with water temperature (Brett, 1979), and Friedland et al. (1998) also observed that enhanced growth was associated with years having favourable temperature conditions, which in turn resulted in higher survival (return) rate to the rivers. The suggested relationship between growth of salmon and temperature does not help to explain whether the reduced growth rates of Atlantic salmon in recent years (ICES, 1999b) were due to a reduced abundance of food of high quality, or a result of lower ocean temperatures. The slower growth rates may result in a higher predation pressure (Ware, 1975; Folkvord & Hunter, 1986; Pepin et al., 1987). The biomass of salmon in the ocean is very low compared with other fish species such as herring, mackerel and blue whiting. Furthermore, several studies suggest that marine mortality is density independent (Chadwick, 1988; Chadwick & Claytor, 1990; Crozier & Kennedy, 1993; Jonsson et al., 1998). It is also suggested that salmon is an opportunistic feeder (Paper IV), and therefore should not be dependent on one particular prey. Food is therefore expected to be a limiting factor only in extreme cases. An external and man made effect on food availability is the potential effect of the industrial fisheries (reduction fishery) mainly operating in coastal areas and on continental shelves, which is obviously the removal of potential prey for salmon. A change in age and size structure of the prey populations indirectly affects the salmon by changing the predator-prey relationship. However, the likely effect might be less than anticipated due to the presumed opportunistic forage behaviour of salmon, and the relatively low number of salmon in the sea, provided that the removal is less than the biomass needed as prey. 37
44 Synoptic discussion In summary, no conclusive statement can be made whether food is limiting for growth and marine survival, and it is suggested that only in extreme cases would food be limiting for salmon in the high seas. Furthermore, since the effect of temperature on survival is not clear, it is an open question whether salmon (in large number) might be lost due to lethal (low) temperatures in the sea. Possible interactions between fish farm escapees and wild salmon in the ocean In the following section possible interactions between fish farm escapees and wild salmon on the high seas are examined. This is approached by first giving an overview of suggested interactions and then a discussion of the various aspects covered by the papers in the present thesis. Escaped farmed salmon have been observed in most areas of the north Atlantic where wild salmon are also found (Lund et al., 1991; Gausen & Moen, 1991; Webb & Youngson, 1992; Carr et al., 1997; Stokesbury & Lacroix, 1997; Youngson et al., 1997), including the high seas fisheries in the Norwegian Sea (Hansen et al., 1993a; Paper I). Several detrimental effects on wild stocks have been suggested from the fish farm escapees (Hindar et al., 1991; Webb et al., 1991; Gross, 1998; Youngson & Verspoor, 1998; Paper I, IV and V), which include: interbreeding and redd destruction, predation on outgoing smolts by escapees, food and space competition in coastal and oceanic areas, and source of diseases and parasites and possible transfer of increased levels to wild salmon. Furthermore, incorrect catch statistics would confound assessment of wild salmon. Interbreeding and transmissions of parasites (especially Gyrodactylus salaris) and diseases in coastal areas were considered the most severe (e.g. Hindar et al., 1991; Håstein & Lindstad, 1991; Crozier & Kennedy, 1993; McVicar, 1997; Sægrov et al., 1997; Bakke & Harris, 1998). Of the harmful parasites in the sea, particularly sea lice (Lepeophtheirus salmonis) have caused great controversy, especially in the coastal areas of the significant fish farming production countries such as Norway, Scotland and Ireland, where large runs of wild salmon also occur (Hutchinson, 1997). The effect of fish farming on the survival of salmon in the sea has been discussed extensively in recent years (McVicar, 1997), with the nearly exponential increase in fish farming industry since late 1980s (ICES, 1999b). However, the extent and severity of fish farming to ocean mortality of wild salmon is poorly known (McVicar, 1997; Bakke & 38
45 Synoptic discussion Harris, 1998). Gausen and Moen (1991) reported that high proportions of escaped farmed salmon (>20%) were found only in rivers having fish farms situated closer than 20 km from river outlet. However, it is difficult to draw conclusions on the severity of lice infestations on wild salmon on the coast in relation to numbers and proximity of the fish farms in an area, as no conclusive evidence is provided yet (McVicar, 1997). Experiments with smolts kept in tanks show that salmon smolts suffer high stress with a load higher than about 10 preadult and adult salmon lice (Nolan et al., 1999), which might render the fish more susceptible to secondary infections, as well as displaying depressed growth rates resulting in either direct or indirect mortality due to higher vulnerability to predators of the infected smolts. A burden of 30 copepodid lice on smolts becomes lethal when they transform into preadult stages on the smolt (Grimnes & Jakobsen, 1996). Thus, sea lice is an important pathogen responsible for high losses of seafarmed Atlantic salmon (Brandal & Egidius, 1979; Grimnes & Jakobsen, 1996). However, in wild stocks in coastal areas, sea lice are rarely reported to cause severe pathology (White, 1940; Nagasawa, 1987; Johnson et al., 1996). In the Northeast Atlantic there is a paucity of information on salmon lice infestations of wild salmon during their oceanic phase. In the offshore areas of the North Atlantic only four reports of infection on wild salmon have previously been presented (Pippy, 1969; Wootten et al., 1982; Berland, 1993; Holst et al., 1993), and they indicated a level of infestation of 8 20 lice per fish. However, these estimates are considered to suffer from a downward bias due to sampling methods. Nagasawa (1985) showed that Pacific salmon caught by long-line carried over four times as many lice as salmon from comparable gillnet catches and Holst et al. (1993) found an inverse correlation between scale losses and prevalence of lice on post-smolts from trawl catches, suggesting loss of lice during capture by these gears as a result of skin abrasion. The present estimate of an abundance of 30 lice per salmon (99.2% prevalence) is considered less biased due to the present sampling method with long-line (Paper V). It is thus suggested that a level of about 30 lice on a salmon (>40 cm) is not considered harmful for the survival of the fish in the oceanic feeding phase. The levels of lice were denser on 1SW escaped farmed salmon than on 1SW wild salmon (Paper III). It is thus likely that these escapees carry higher load of lice from coastal areas to the high seas than wild salmon. Furthermore, the presence of both chalimus and pre-adult (juvenile) stages of lice on the salmon throughout the seasons and the increasing abundance and density of lice with sea age of wild salmon indicate that infestation occurs in the open sea (Paper V). Thus it is possible that escaped farmed salmon transfer increasing numbers of sea lice to wild salmon in the ocean. Unfortunately, no historic data exist to compare lice levels of salmon on the high seas, and furthermore it 39
46 Synoptic discussion is not clear which and how the mechanisms might operate in high seas transmissions and infestations. It is suggested that most of the fish farm escapees in the sea during autumn and winter have escaped from Norwegian fish farms (Paper I and III). Our results demonstrate that of the 1SW salmon the fish farm escapees had a significantly higher load of lice than the wild salmon (Paper V), and it might be speculated that the escapees caught in the high seas were the survivors, with the most heavily infected fish lost either directly due to predation or indirectly due to subsequent diseases. For example the observation of four heavily infected 1SW escaped farmed salmon with more than 100 adult lice, and one with nearly 300 lice, caught in an area far north of the traditional autumn fishing grounds (Paper V), were obviously in the wrong place at the wrong time, with lice levels that were considered lethal (Grimnes & Jakobsen, 1996). Furthermore, these four fish had a higher than average number of juvenile lice stages attached, indicating that they probably had escaped fairly recently. There were no differences in the feeding habits between wild and escaped farmed salmon, and it was suggested in Paper IV that the escapees feed as efficiently as wild salmon and that they were completely adapted to the marine environment. Farmed salmon were also observed feeding in Scottish waters (Hislop & Webb, 1992). Thus, the farmed salmon might compete for food with wild salmon in the high seas, although it is suggested that food does not seem to be a limiting factor for survival of salmon in the sea. Generally the ocean survival seems to be lower for hatchery reared and farmed salmon (Jonsson et al., 1991; Paper III) than in wild fish and their reproductive success is inferior to wild salmon (Jonsson et al., 1990; Lura & Sægrov, 1991; Fleming et al., 1996). Thus it seems as reared salmon in general have lover fitness than wild. ICES has during many years estimated the exploitation rates of various homewater stocks in the Faroese high seas fishery (e.g. ICES, 1984; 1996). In the assessment of salmon stocks at the sea, it is important to estimate the farmed and ranched component. Unless they are accounted for, the presence of a high proportion of such fish will result in an overestimate of the catches of wild salmon resulting in the size and status of the wild stocks being concealed. Had escaped fish not been accounted for, the assessments of the Faroese fishery would have been impaired, particularly from the 1988/1989 fishing period to the 1991/1992 fishing period, when the farmed proportion was very high. An example from the present thesis (Paper I) would be the statistically significant increasing trend in the overall CPUE observed in the Faroese fishery during the period 1980/ /1993 (r s = 0.81, p< 0.001), which usually is interpreted as an increase in stock abundance. However, the apparent increasing abundance in the area could in fact be explained by an 40
47 Synoptic discussion increasing abundance of farmed salmon in the area, rather than of wild fish. The adjusted CPUE of wild salmon only for the same period (Paper I) showed no trend with time (r s = 0.2, p> 0.05). The results from the present thesis show that (a low) infestation of salmon lice occur in the open ocean and further indicate that escaped farmed salmon may carry a greater number of lice to the sea due to it higher load of parasite compared to wild salmon. The presence of farmed fish in catches would confound stock assessments of wild salmon if not discounted for, and they may possibly affect wild salmon by competing for food on the high seas. Furthermore, there is an indication from tagging that the escapees do not "home" as precisely as do wild salmon and that ocean survival of farmed salmon might be lower than for wild salmon. 41
48 CONCLUSIONS AND PERSPECTIVES This thesis provide new information on various aspects of the marine ecology of Atlantic salmon in the ocean. Further, I have examined possible interactions between wild and escaped farmed salmon in the high seas. This knowledge contribute to improved understanding of the general biology of salmon in the oceanic phase, and may help to develop reliable assessment models of wild salmon. It is shown that farmed Atlantic salmon that escape from fish farms disperse in the ocean and are found intermingled with the wild salmon. Furthermore, I suggest that farmed salmon may simply be lost at sea (unable to home), experience higher mortality, or are subjected to lower catchability in homewaters because they entered homewaters at different times than wild salmon. Most of the fish farm escapees originate from Norwegian fish farms. It is shown that wild salmon from large parts of their natural distribution range are present in the Faroes area during some stage of their oceanic feeding phase. Norwegian salmon are most abundant, but relative high number of salmon from Scotland and Russia are also present. Even fish of Canadian origin are found in the area. Apparently there is a change in stock composition of wild salmon from autumn to winter, with an increasing proportion of fish from more northern areas present during winter. This is presumably related to geographic origin of the fish, timing of migration, food availability, competition and sea-surface temperature. Escaped farmed fish feed and grow as effectively as the wild salmon in the sea, suggesting that the fish farm escapees that survived until captured have adapted to the marine environment. Evidence for both opportunistic and selective forage strategies of salmon have been provided, and food does not appear to be limiting. It was documented that lice infestations occur in the high seas, however at a much lower levels than in coastal areas. There is a potential for transfer of lice from escaped farmed to wild salmon in the ocean, since the escaped farmed salmon had higher load of lice than wild salmon the first winter at sea. Further research should emphasise the temporal and spatial aspects of distribution and migration in relation to biological and short- and long-term oceanographic and environmental factors. The large numbers of escaped farmed salmon in the sea should be further addressed, as there is still limited knowledge of their behaviour and ecology. The biomass of salmon in the ocean is very small compared with the biomass of other pelagic marine species (e.g. herring, mackerel, blue whiting and capelin) and there is a need to examine whether these species interact with Atlantic salmon. 42
49 Conclusions and perspectives The effect of predation on salmon survival and abundance in the marine phase is virtually unknown, and is in many cases dominated by anecdotal evidence. Noting that predation on salmon is extremely difficult to document in the ocean, dedicated investigations on this subject are certainly needed. In the present thesis I have focused on salmon of 'catchable' size, i.e. approximately a half year after ocean migration. However, recent work suggest that the post-smolt stage or early sea phase is very important in determining the survival of salmon in the sea, and therefore intensified study of this life-stage is encouraged. Further, the recent development of archival tags provide great opportunities to study details of behaviour of salmon in the sea. Thus, I have tried to peer into the 'black box' and expose some of its contents, but still there are large areas in darkness. Much shall be learned from dedicated future research on the marine behaviour and ecology of Atlantic salmon. 43
50 REFERENCES Allan, I. R. H., and Ritter, J. A Salmonid terminology. J. Cons. int. Explor. Mer., 37 (3): Anderson, J. T A review of size dependent survival during pre-recruit stages of fishes in relation to recruitment. J. Northw. Atl. Fish. Sci., 8: Baglinière, J.-L Etude des populations de sammon Atlantique (Salmo salar L., 1766) en Bretagne-Basse-Normandic. II -Activite de devalaison des smolts sur l'elle. Ann. Hydrobiol., 7: Bakke, T. A., and Harris, P. D Diseases and parasites in wild Atlantic salmon (Salmo salar) populations. Can. J. Fish. Aquat. Sci., 55 (Supplement 1): Berland, B Salmon lice on wild salmon (Salmo salar L.) in western Norway. In Pathogens of wild and farmed fish: Sea lice, pp Ed. by G.A. Boxshall and D. Defaye. Ellis Horwood, Chichester, West Sussex, England. 378 pp. Blair, A. A Bay of Islands and Humber River Atlantic salmon investigations. J. Fish. Res. Bd Can., 22: Brandal, P. O., and Egidius, E Treatment of salmon lice (Lepeophtheirus salmonis Krøyer, 1938) with Neguvon-description of method and equipment. Aquaculture, 18: Brett, J. R Environmental factors and growth. Fish Physiol., 8: Brodeur, R. D., Francis, R. C., and Pearcy, W. G Food consumption of juvenile coho (Oncorhynchus kisutch) and chinook salmon (O. tshawytscha) on the continental shelf off Washington and Oregon. Can. J. Fish. Aquat. Sci., 49 (8): Brodeur, R. D., and Pearcy, W. G Trophic relations of juvenile Pacific salmon off the Oregon and Washington coast. Fish. Bull. (U. S. ), 88: Carr, J. W., Anderson, J. M., Whoriskey, F. G., and Dilworth, T The occurrence and spawning of cultured Atlantic salmon (Salmo salar) in a Canadian river. ICES J. mar. Sci., 54 (6): Chadwick, E. M. P Relationship between Atlantic salmon smolts and adults in Canadian rivers. In Atlantic Salmon: Planning for the future, pp Ed. by D. Mills and D. Piggins. Croom Helm, London. 587 pp. Chadwick, E. M. P., and Claytor, R. R Predictability in a small commercial Atlantic salmon fishery in western Newfoundland. Fisheries Research, 10: Christensen, O Preliminary results of an investigation on the food of Baltic salmon. ICES CM 1961/93, 6 pp. Christensen, O., and Lear, W. H Distribution and abundance of Atlantic salmon at West Greenland. Rapp. P. -v. Réun. Cons. int. Explor. Mer, 176: Crozier, W. W., and Kennedy, G. J. A Marine survival of wild and hatchery-reared Atlantic salmon (Salmo salar L.) from the River Bush, Northern Ireland. In Salmon in the sea and new enhancement strategies, pp Ed. by D. Mills. Fishing News Books, Oxford. 424 pp. Dalpadado, P., Ellertsen, B., Melle, W., and Skjoldal, H. R Summer distribution patterns and biomass estimates of macrozooplankton and micronekton in the Nordic Seas. Sarsia, 83: DFO State of the Ocean: Northwest Atlantic. DFO Science Stock Status Report G0-01, (1998):
51 References Dos Santos, J., and Jobling, M Factors affecting gastric evacuation in cod, Gadus morhua L., fed single-meals of natural prey. J. Fish Biol., 38 (5): Dunbar, M. J Serial Atlas of the marine environment. American Geographical Society, New York, (Folio 6): plates. Dutil, J. D., and Coutu, J. M Early marine life of Atlantic salmon, Salmo salar, postsmolts in the northern Gulf of St. Lawrence. Fish. Bull. (U. S. ), 86 (2): Fleming, I. A., and Gross, M. R Latitudinal clines - a trade-off between egg number and size in Pacific salmon. Ecology, 71 (1): Fleming, I. A., Jonsson, B., Gross, M. R., and Lamberg, A An experimental study of the reproductive behaviour and success of farmed and wild Atlantic salmon (Salmo salar). Journal of Applied Ecology, 33 (4): Folkvord, A., and Hunter, J. R Size-specific vulnerability of northern anchovy Engraulis mordax larvae to predation by fishes. Fish. Bull. (U. S. ), 84: Fowler, G. M., and Stobo, W. T Effects of release parameters on recovery rates of tagged groundfish species. Can. J. Fish. Aquat. Sci., 56 (10): Frank, K. T., Carscadden, J., and Simon, J. E Recent excursions of capelin (Mallotus villosus) to the Scotian Shelf and Flemish Cap during anomalous hydrographic conditions. Can. J. Fish. Aquat. Sci., 53: Friedland, K. D Ocean climate influences on critical Atlantic salmon (Salmo salar) life history events. Can. J. Fish. Aquat. Sci., 55 (Supplement 1): Friedland, K. D., Hansen, L. P., and Dunkley, D. A Marine temperatures experienced by postsmolts and the survival of Atlantic salmon, Salmo salar L., in the North Sea area. Fish. Oceanogr., 7 (1): Friedland, K. D., Hansen, L. P., Dunkley, D. A., and MacLean, J. C Linkage between ocean climate, post-smolt growth, and survival of Atlantic salmon (Salmo salar L.) in the North Sea area. ICES J. mar. Sci., (In press) Friedland, K. D., and Reddin, D. G Marine survival of Atlantic salmon from indices of postsmolt growth and sea temperature. In Salmon in the sea and new enhancement strategies, pp Ed. by D. Mills. Fishing News Books, Oxford. 424 pp. Friedland, K. D., Reddin, D. G., and Kocik, J. F Marine survival of North American and European Atlantic salmon: effects of growth and environment. ICES J. mar. Sci., 50: Gausen, D., and Moen, V Large-scale escapes of farmed Atlantic salmon (Salmo salar ) into Norwegian rivers threaten natural populations. Can. J. Fish. Aquat. Sci., 48 (3): Goodson, M. S., Giske, J., and Rosland, R Growth and ovarian development of Maurolicus muelleri during spring. Mar. Biol., 124: Grimnes, A., and Jakobsen, P. J The physiological effects of salmon lice infection on postsmolt of Atlantic salmon. J. Fish Biol., 48 (6): Gross, M. R One species with two biologies: Atlantic salmon (Salmo salar) in the wild and in aquaculture. Can. J. Fish. Aquat. Sci., 55 (Supplement 1): Gross, M. R., Coleman, R. M., and McDowall, R. M Aquatic productivity and the evolution of diadromous fish migration. Science, 239 (4845): Grønvik, S., and Klemetsen, A Marine food and diet overlap of co-occurring Arctic charr Salvelinus alpinus (L.), brown trout Salmo trutta L. and Atlantic salmon S. salar L. off Senja, N. Norway. Polar Biol., 7 (3):
52 References Hansen, B Assessment of a salmon stock based on long-line catch data. ICES CM 1984/M: 6, 12 pp. Hansen, B The circulation of the northern part of the Northeast Atlantic. Rit Fiskideildar, 9: Hansen, B., Østerhus, S., Dooley, H. D., Gould, W. J., and Rickards, L. J North Atlantic - Norwegian Sea exchanges. ICES Coop.Res.Rep. 1998/255, 82 pp. Hansen, L. P., Døving, K. B., and Jonsson, B Migration of farmed adult Atlantic salmon with and without olfactory sense, released on the Norwegian coast. J. Fish Biol., 30 (6): Hansen, L. P., Jacobsen, J. A., and Lund, R. A. 1993a. High numbers of farmed Atlantic salmon, Salmo salar L., observed in oceanic waters north of the Faroe Islands. Aquacult. Fish. Manage., 24 (6): Hansen, L. P., and Jonsson, B Salmon ranching experiments in the River Imsa: Effect of timing of Atlantic Salmon (Salmo salar) smolt migration on survival to adults. Aquaculture, 82: Hansen, L. P., and Jonsson, B The effect of timing of Atlantic salmon smolt and post-smolt release on the distribution of adult return. Aquaculture, 98: Hansen, L. P., Jonsson, N., and Jonsson, B. 1993b. Oceanic migration in homing Atlantic salmon. Anim. Behav., 45 (4): Hansen, L. P., and Pethon, P The food of Atlantic salmon, Salmo salar L., caught by long-line in northern Norwegian waters. J. Fish Biol., 26: Hansen, L. P., and Quinn, T. P The marine phase of the Atlantic salmon (Salmo salar) life cycle, with comparisons to Pacific salmon. Can. J. Fish. Aquat. Sci., 55 (Supplement 1): Hartt, A. C Migrations of salmon in the North Pacific ocean and Bering Sea as determined by seining and tagging. Int. North Pac. Fish. Comm.,Bull., 19: Healey, M. C., Thomson, R. E., and Morris, J. F. T Distribution of commercial troll fishing vessels off southwest Vancouver island in relation to fishing success and oceanic water properties and circulation. Can. J. Fish. Aquat. Sci., 47 (10): Hindar, K., Ryman, N., and Utter, F Genetic effects of cultured fish on natural fish populations. Can. J. Fish. Aquat. Sci., 48 (5): Hislop, J. R. G., and Shelton, R. G. J Marine predators and prey of Atlantic salmon (Salmo salar L.). In Salmon in the sea and new enhancement strategies, pp Ed. by D. Mills. Fishing News Books, Oxford. 424 pp. Hislop, J. R. G., and Webb, J. H Escaped farmed Atlantic salmon (Salmo salar L.) feeding in Scottish coastal waters. Aquacult. Fish. Manage., 23: Hislop, J. R. G., and Youngson, A. F A note on the stomach contents of salmon caught by longline north of the Faroe Islands in March, ICES CM 1984/M: 17, 4 pp. Holm, M., Holst, J. C., and Hansen, L. P Spatial and temporal distribution of Atlantic salmon post-smolts in the Norwegian Sea and adjacent areas: Origin of fish, age structure and relation to hydrographical conditions in the sea. ICES CM 1998/N: 15, 18 pp. Holm, M., Holst, J. C., and Hansen, L. P Spatial and temporal distribution of Atlantic salmon (Salmo salar L.) post-smolts in the Norwegian Sea and adjacent areas. ICES J. mar. Sci., In press Holst, J. C., Arrhenius, F., Hammer, C., Håkansson, N., Jacobsen, J. A., Krysov, A., Melle, W., and Vilhjálmsson, H Report on surveys of the distribution, abundance and migrations of the Norwegian spring-spawning herring, other pelagic fish and the environment of the Norwegian Sea and adjacent waters in late winter, spring and summer of ICES CM 1998/D: 3 46
53 References Holst, J. C., Hansen, L. P., and Holm, M Observations of abundance, stock composition, body size and food of postsmolts of Atlantic salmon in the NE Atlantic during summer. ICES CM 1996/M: 4, 15 pp. Holst, J. C., Nilsen, F., Hodneland, K., and Nylund, A Observations of the biology and parasites of postsmolt Atlantic salmon, Salmo salar, from the Norwegian Sea. J. Fish Biol., 42 (6): Holtby, L. B., Andersen, B. C., and Kadowaki, R. K Importance of smolt size and early ocean growth to interannual variability in marine survival of coho salmon (Oncorhynchus kisutch). Can. J. Fish. Aquat. Sci., 47 (11): Horsted, S. A Future investigations on the ocean life of salmon. In Atlantic Salmon: Planning for the future, pp Ed. by D. Mills and D. Piggins. Croom Helm, London. 587 pp. Hutchinson, P (Editor) Interactions between salmon culture and wild stocks of Atlantic salmon: The scientific and management issues. ICES J. mar. Sci., 54 (6): Hvidsten, N. A., Jensen, A. J., Vivås, H., Bakke, Ø., and Heggberget, T. G Downstream migration of Atlantic salmon smolts in relation to water flow, water temperature, moon phase and social interactions. Nord. J. Freshw. Res., 70: Håstein, T., and Lindstad, T Diseases in wild and cultured salmon: possible interaction. Aquaculture, 98 (1-3): ICES Report of the meeting of the Working Group on North Atlantic Salmon. ICES CM 1984/Assess: 16, 54 pp. ICES Report of meeting of the Working Group on Atlantic salmon. ICES CM, Assess (11) ICES Report of the Working Group on North Atlantic salmon. ICES CM 1996/Assess: 11, 227 pp. ICES 1999a. Report of the Northern pelagic and blue whiting fisheries Working Group. ICES CM 1999a/ACFM: 18, 238 pp. ICES 1999b. Report of the Working Group on North Atlantic salmon. ICES CM 1999b/ACFM: 14, 288 pp. ICES 1999c. Report of the Working Group on the assessment of mackerel, horse mackerel, sardine and anchovy. ICES CM 1999c/ACFM: 6, 468 pp. Jensen, J. M Atlantic salmon caught in the Irminger Sea. J. Fish. Res. Bd Can., 24 (12): Jensen, J. M. 1980a. Recaptures from international tagging experiments at West Greenland. Rapp. P. -v. Réun. Cons. int. Explor. Mer, 176: Jensen, J. M. 1980b. Recaptures of salmon at west Greenland tagged as smolts outside Greenland waters. Rapp. P. -v. Réun. Cons. int. Explor. Mer, 176: Jensen, J. M Exploitation and migration of salmon on the high seas, in relation to Greenland. In Atlantic Salmon: Planning for the future, pp Ed. by D. Mills and D. Piggins. Croom Helm, London. 587 pp. Jensen, J. M., and Lear, W. H Atlantic salmon caught in the Irminger Sea and at West Greenland. J. Northw. Atl. Fish. Sci., 1: Johnson, S. C., Blaylock, R. B., Elphick, J., and Hyatt, K. D Disease caused by the salmon louse Lepeophtheirus salmonis (Copepoda: Caligidae) in wild sockeye salmon (Oncorhynchus nerka) stocks of Alberni Inlet, British Columbia. Can. J. Fish. Aquat. Sci., 53: Johnson, T. B., and Kitchell, J. F Long-term changes in zooplanktivorous fish community composition: implications for food webs. Can. J. Fish. Aquat. Sci., 53:
54 References Jonsson, B., Jonsson, N., and Hansen, L. P Does juvenile experience affect migration and spawning of adult Atlantic salmon? Behav. Ecol. Sociobiol., 26 (4): Jonsson, B., Jonsson, N., and Hansen, L. P Differences in life history and migratory behaviour between wild and hatchery-reared Atlantic salmon in nature. Aquaculture, 98: Jonsson, N., Jonsson, B., and Hansen, L. P Partial segregation in the timing of migration of Atlantic salmon of different ages. Anim. Behav., 40 (2): Jonsson, N., Jonsson, B., and Hansen, L. P The relative role of density-dependent and densityindependent survival in the life cycle of Atlantic salmon Salmo salar. J. Anim. Ecol., 67 (5): Jákupsstovu, S. H. í Exploitation and migration of salmon in Faroese waters. In Atlantic Salmon: Planning for the future, pp Ed. by D. Mills and D. Piggins. Croom Helm, London. 587 pp. Kadri, S., Thorpe, J. E., and Metcalfe, N. B Anorexia in one-sea-winter Atlantic salmon (Salmo salar) during summer, associated with sexual maturation. Aquaculture, 151 (1-4): Lear, W. H Food and feeding of Atlantic salmon in coastal and over oceanic depths. Res. Bull. int. Comm. Northw. Atlant. Fish., 9: Lear, W. H Food of Atlantic salmon in the West Greenland-Labrador Sea area. Rapp. P. -v. Réun. Cons. int. Explor. Mer, 176: Lear, W. H., and Sandeman, E. J Use of scale characters and discriminant functions for identifying continental origin of Atlantic salmon. Rapp. P. -v. Réun. Cons. int. Explor. Mer, 176: Lund, R. A., Økland, F., and Hansen, L. P Farmed Atlantic salmon (Salmo salar) in fisheries and rivers in Norway. Aquaculture, 98: Lund, R. A., Østborg, G. M., and Hansen, L. P Rømt oppdrettslaks i sjø og elvefisket i årene NINA Oppdragsmelding, 411: Lura, H., and Sægrov, H Documentation of successful spawning of escaped farmed female Atlantic salmon, Salmo salar, in Norwegian rivers. Aquaculture, 98 (1-3): McDowall, R. M Diadromy in Fishes. Chroom Helm, London. 308 pp. McKinnel, S., and Karlström, Ö Spatial and temporal covariation in the recruitment and abundance of Atlantic salmon populations in the Baltic Sea. ICES J. mar. Sci., 56: McVicar, A. H Disease and parasite implications of the coexistence of wild and cultured Atlantic salmon populations. ICES J. mar. Sci., 54 (6): Methven, D. A., and Piatt, J. F Seasonal and annual variation in the diet of Atlantic cod (Gadus morhua) in relation to the abundance of capelin (Mallotus villosus) off eastern Newfoumdland, Canada. J. Cons. int. Explor. Mer., 45: Mills, D Ecology and Management of Atlantic Salmon. Chapman and Hall, London. 351 pp. Mills, D Salmon in the sea and new enhancement strategies. Fishing News Books, Oxford. 424 pp. Mills, D., and Smart, N Report on a visit to the Faroes. Atlantic Salmon Trust, Pitlochry. 52 pp. Munro, W. R., and Swain, A Age, weight and length distributions and sex ratio of salmon caught off West Greenland. Rapp. P. -v. Réun. Cons. int. Explor. Mer, 176:
55 References Nagasawa, K Comparison of the infection levels of Lepeophtheirus salmonis (Copepoda) on chum salmon captured by two methods. Jap. J. Ichthyol., 32 (3): Nagasawa, K Prevalence and abundance of Lepeophtheirus salmonis (Copepoda: Caligidae) on high-seas salmon and trout in the North Pacific Ocean. Nippon Suisan Gakkaishi (Bull. Jap. Soc. Sci. Fish. ), 53 (12): Neilson, J. D., and Gillis, D. J A note on the stomach contents of adult Atlantic salmon (Salmo salar, Linnaeus) from Port Burwell, Northwest Territories. Can. J. Zool., 57 (7): Nolan, D. T., Reilly, P., and Wendelaar Bonga, S. E Infection with low numbers of the sea louse Lepeophtheirus salmonis induces stress-related effects in postsmolt Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci., 56: Nøttestad, L., Giske, J., Holst, J. C., and Huse, G A length-based hypothesis for feeding migrations in pelagic fish. Can. J. Fish. Aquat. Sci., 56 (Suppl. 1): Ogura, M., and Ishida, Y Homing behaviour and vertical movements of four species of Pacific salmon (Oncorhynchus nerka spp.) in the central Bering Sea. Can. J. Fish. Aquat. Sci., 52: Parrish, B. B., and Horsted, S. A (Editors). ICES/ICNAF joint investigations on North Atlantic salmon. Rapp. P. -v. Réun. Cons. int. Explor. Mer, 176: Parrish, D. L., Behnke, R. J., Gephard, S. R., McCormick, S. D., and Reeves, G. H Why aren't there more Atlantic salmon (Salmo salar)? Can. J. Fish. Aquat. Sci., 51 (Supplement 1): Pearcy, W. G Ocean ecology of North Pacific salmonids. Washington Sea Grant Program, University of Washington, Seattle. 179 pp. Pearcy, W. G., Brodeur, R. D., Shenker, J. M., Smoker, W. W., and Endo, Y Food habits of Pacific salmon and steelhead trout, midwater trawl catches and oceanographic conditions in the Gulf of Alaska, Bulletin of the Ocean Research Institute, University of Tokyo, 26 (2): Pepin, P The effect of temperature and size on development, mortality and survival rates of the pelagic early life stages of marine fishes. Can. J. Fish. Aquat. Sci., 48: Pepin, P., Spearre, S. Jr., and Koslow, J. A Predation on larval fish by Atlantic mackerel, Scomber scombrus, with comparison of predation by zooplankton. Can. J. Fish. Aquat. Sci., 44: Peterson, W. T., Brodeur, R. D., and Pearcy, W. G Food habits of juvenile salmon in the Oregon coastal zone, June Fish. Bull. (U. S. ), 80 (4): Pippy, J. H. C Preliminary report on parasites as biological tags in Atlantic salmon (Salmo salar). I. Investigations 1966 to Fish. Res. Bd Can. Techn. Rep, 134: Quinn, T. P Estimated swimming speeds of migrating adult sockeye salmon. Can. J. Zool., 66 (10): Quinn, T. P Current controversies in the study of salmon homing. Ethology Ecology and Evolution, 2 (1): Reddin, D. G Atlantic salmon (Salmo salar) on and east of the Grand Bank. J. Northw. Atl. Fish. Sci., 6: Reddin, D. G Ocean life of Atlantic salmon (Salmo salar L.) in the Northwest Atlantic. In Atlantic salmon: Planning for the future, pp Ed. by D. Mills and D. Piggins. Timber Press, Portland, Oregon. 578 pp. Reddin, D. G., and Burfitt, R. F The stock composition of Atlantic salmon off West Greenland in ICES CM 1980/M: 25, 8 pp. 49
56 References Reddin, D. G., and Friedland, K. D Marine environmental factors influencing the movement and survival of Atlantic salmon. In Salmon in the sea and new enhancement strategies, pp Ed. by D. Mills. Fishing News Books, Oxford. 424 pp. Reddin, D. G., and Friedland, K. D A history of identification to continent of origin of Atlantic salmon (Salmo salar L.) at west Greenland, Fisheries Research, 43 (1-3): Reddin, D. G., and Shearer, W. M Sea-Surface temperature and distribution of Atlantic salmon in the Northwest Atlantic ocean. American Fisheries Society Symposium, 1: Reddin, D. G., and Short, P. B Postsmolt Atlantic salmon (Salmo salar) in the Labrador Sea. Can. J. Fish. Aquat. Sci., 48: 2-6. Reddin, D. G., Stansbury, D. E., and Short, P. B Continent of origin of Atlantic salmon (Salmo salar L) at west Greenland. J. Cons. int. Explor. Mer., 44 (2): Ruggles, C. P., and Ritter, J. A Review of North American smolt tagging to assess the Atlantic salmon fishery off West Greenland. Rapp. P. -v. Réun. Cons. int. Explor. Mer, 176: Scarnecchia, D. L Effects of oceanic variations and the West Greenland fishery on age at maturity of Icelandic west coast stocks of Icelandic salmon (Salmo salar). Can. J. Fish. Aquat. Sci., 46: Shearer, W. M The Atlantic Salmon: Natural History, Exploitation and Future Management. Fishing News Books, Oxford. 244 pp. Shelton, R. G. J., Turrell, W. R., MacDonald, A., McLaren, I. S., and Nicoll, N. T Records of post-smolt Atlantic salmon, Salmo salar L., in the Faroe-Shetland Channel in June Fisheries Research, (31): Stead, S. M., Houlihan, D. F., McLay, H. A., and Johnstone, R Food consumption and growth in maturing Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci., 56 (11): Stokesbury, M. J., and Lacroix, G. L High incidence of hatchery origin Atlantic salmon in the smolt output of a Canadian river. ICES J. mar. Sci., 54: Struthers, G A report on a salmon long lining cruise off the Faroes during April, Freshwater Fisheries Laboratory, Pitlochry, Rep., (54 FW 70): 1-8. Struthers, G A report on the 1971 salmon long lining cruise off the Faroes. Freshwater Fisheries Laboratory, Pitlochry, Rep., (33 FW 71): 1-6. Sturlaugsson, J Food of ranched Atlantic salmon ( Salmo salar L.) postsmolts in coastal waters, W-Iceland. Nord. J. Freshw. Res., 69: Swain, A Tagging of salmon smolts in European rivers with special reference to recaptures off West Greenland in 1972 and earlier years. Rapp. P. -v. Réun. Cons. int. Explor. Mer, 176: Sægrov, H., Hindar, K., Kålås, S., and Lura, H Escaped farmed Atlantic salmon replaces the original salmon stock in the River Vosso, western Norway. ICES J. mar. Sci., 54: Tadokoro, K., Ishida, Y., Davis, N. D., Ueyanagi, S., and Sugimoto, T Change in chum salmon (Oncorhynchus keta) stomach contents associated with fluctuation of pink salmon (O. gorbuscha) abundance in the central Subarctic Pacific and Bering Sea. Fish. Oceanogr., 5 (2): Templeman, W Atlantic salmon from the Labrador Sea and off West Greenland, taken during A. T. Cameron cruise, July-August Res. Bull. int. Comm. Northw. Atlant. Fish., 4: Templeman, W Distribution and characteristics of Atlantic salmon over oceanic depths and on the bank and shelf slope areas off Newfoundland, March-May, Res. Bull. int. Comm. Northw. Atlant. Fish., 5: Thodesen, J., Grisdale-Helland, B., Helland, S., and Gjerde, B Feed intake, growth and feed utilization of offspring from wild and selected Atlantic salmon. Aquaculture, 180:
57 References Thurow, F Beträge zur Biologie und Bestandskunde des Atlantischen Lachses (Salmo salar L.) in der Ostsee. Ber. Dt. Wiss. Komm. Meeresforsch., 18: Thurow, F Research vessel fishing on salmon off Norway. Arch. Fisch Wiss., 24: Thurow, F Variations of catch rates and local movements of salmon in the Baltic Sea. Ber. Dt. Wiss. Komm. Meeresforsch., 23: Turrell, W. R., and Shelton, R. G. J Climatic change in the north-eastern Atlantic and its impacts on salmon stocks. In Salmon in the sea and new enhancement strategies, pp Ed. by D. Mills. Fishing News Books, Oxford. 424 pp. Vilhjálmsson, H., Misund, O. A., Arrhenius, F., Holst, J. C., Gislason, A., Gudmundsdottir, A., Jacobsen, J. A., Krysov, A., Malmberg, S. A., and Reid, D Report on surveys of the distribution, abundance and migrations of the Norwegian spring-spawning herring, other pelagic fish and the environment of the Norwegian Sea and adjacent waters in late winter, spring and summer of ICES CM 1997/Y: 04, 20 pp. Ware, D. M Relation between egg size, growth and natural mortality of larval fish. J. Fish. Res. Bd Can., 32: Ware, D. M Bioenergetics of pelagic fish: theoretical change in swimming speed and ration with body size. J. Fish. Res. Bd Can., 35 (2): Webb, J. H., Hay, D. W., Cunningham, E. P., and Youngson, A. F The spawning behaviour of escaped farmed and wild adult Atlantic salmon (Salmo salar L.) in a northern Scottish river. Aquaculture, 98 (1-3): Webb, J. H., and Youngson, A. F Reared Atlantic salmon, Salmo salar L., in the catches of a salmon fishery on the western coast of Scotland. Aquacult. Fish. Manage., 23 (3): Weihs, D Optimal fish cruising speed. Nature, 245: White, H. C "Sea lice" (Lepeophtheirus salmonis) and death of salmon. J. Fish. Res. Bd Can., 5: Wootten, R., Smith, J. W., and Needham, E. A Aspects of the biology of the parasitic copepods Lepeophtheirus salmonis and Caligus elongatus on farmed salmonids and their treatments. Proc. R. Soc. Edinb. (B), 81: Youngson, A. F., and McLay, H. A Changes in steroid hormone levels in blood serum as an early predictor of approaching maturity in Atlantic salmon (Salmo salar L.). ICES CM 1985/M: 17 Youngson, A. F., and Verspoor, E Interactions between wild and introduced Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci., Supplement 1 (55): Youngson, A. F., Webb, J. H., MacLean, J. C., and Whyte, B. M Frequency of occurrence of reared Atlantic salmon in Scottish salmon fisheries. ICES J. mar. Sci., 54 (6): Zaret, T. M., and Kerfoot, W. C Fish predation on Bosmina longirostris: Body-size selection versus visibility selection. Ecology, 56:
58 Aspects of the marine ecology of Atlantic salmon
59 Aspects of the marine ecology of Atlantic salmon PAPER I The incidence of escaped farmed Atlantic salmon, Salmo salar L., in the Faroese fishery and estimates of catches of wild salmon.
60
61
62
63
64
65
66
67 Aspects of the marine ecology of Atlantic salmon
68 Aspects of the marine ecology of Atlantic salmon PAPER II Seasonal differences in origin of Atlantic salmon (Salmo salar L.) in the Norwegian Sea based on estimates from age structures and tag recaptures.
69 Seasonal differences in the origin of Atlantic salmon (Salmo salar L.) in the Norwegian Sea based on estimates from age structures and tag recaptures 1 Jan A Jacobsen a,*, Roar A Lund b, Lars P Hansen c and Niall O'Maoileidigh d a Fisheries Laboratory of the Faroes, P.O.Box 3051, FR-110 Tórshavn, Faroe Islands. b Norwegian Institute for Nature Research, Tungasletta 2, N-7005 Trondheim, Norway. c Norwegian Institute for Nature Research, Dronningensgate 13, N-0105 Oslo, Norway. d Fisheries Research Centre, Abbotstown, Castleneck, 15 Dublin, Ireland Abstract To test if the population structure of Atlantic salmon (Salmo salar L.) at the feeding areas in the Norwegian Sea north of the Faroes is stable throughout autumn (November December) and winter (February March), river and sea age distribution was estimated from 2,350 scale samples obtained in the autumn and winter during four consecutive fishing periods 1991/ /1995. In addition, we compared the origin of recaptures of salmon tagged as smolts in different European countries between the two seasons. The fish were classified as being of wild or fish farm origin from scale characteristics, and farmed salmon were excluded from the analyses. Age compositions in samples from the four fishing periods showed consistent patterns. The average smolt age (±SE) was significantly lower in the autumn than in the winter (2.5 ± 0.04 and 2.7 ± 0.03, range 1-5) as was average sea age (1.9 ± 0.03 and 2.2 ± 0.02, range 1-6). As salmon from southern European countries tend to smolt at an earlier age and produce more one-sea winter salmon than in northern Europe, we suggest that a significant proportion of the salmon caught in the Faroes area during autumn originate from southern European countries and that fish from northern regions appear to be more abundant in the winter. Recaptures in the Faroese fishery during autumn and winter of salmon tagged as smolts in different countries support this. Keywords: Escaped farmed salmon; high seas fishery; country of origin; recaptures; Salmo salar; sea age; smolt age; tagging; wild salmon * Corresponding author. Tel.: ; fax: ; address: janarge@frs.fo (J.A. Jacobsen).
70 2 1. Introduction Atlantic salmon (Salmo salar L.) are widely distributed in the Northeast Atlantic ocean, and one important feeding area for salmon is the central and southern Norwegian Sea, i.e. the area north off the Faroe Islands (Jákupsstovu, 1988). This area is characterised by a front that separates the warmer Atlantic water from south-west, and the colder and less saline Arctic water from north-west (Hansen, 1985). Salmon are typically distributed in or close to the frontal areas (Jákupsstovu, 1988). A high seas fishery for salmon developed in this area in the 1960s, and Faroese long-liners have exploited this resource since late 1970s (Jákupsstovu, 1988). From recaptures of externally tagged salmon in the Faroes fishery in the early 1980 s, there is direct evidence that salmon of different smolt year classes originating from different rivers in Norway, Scotland and Sweden are caught together during the same time period in the same statistical rectangle (Hansen, 1993). Subsequent tagging programmes using external and coded wire tags (CWT) showed that salmon from Ireland, England and Wales (and to a lesser extent Iceland, France and Spain), were also caught together in these areas during the fishing season (ICES, 1996). The catches consist mainly of fish spending their second winter in the ocean (2SW salmon; ca 80% of the catch), but some 1SW and 3+SW salmon are also caught (ICES, 1996). Reports from fishermen and from landing statistics suggest that fish caught during autumn are smaller than fish landed during the winter period. It is not clear whether this difference could be accounted for by growth, i.e. by the longer feeding period of fish that were sampled in the winter period, or whether this reflects changes in the population structure. Smolt and sea age of salmon differ between salmon populations from different regions of Europe, tending to be lower in salmon stocks from the southern regions such as Spain, France, UK and Ireland than in northern regions like Norway and Russia (Templeman, 1967; Power, 1969; Baglinière, 1976; Munro and Swain, 1980; Metcalfe and Thorpe, 1990; ICES, 1996). We tested the hypothesis that the population structure of Atlantic salmon at Faroes is stable throughout the autumn and winter by examining the temporal and spatial differences in smolt and sea age in salmon during their feeding period north of the Faroes. Furthermore, we compared the origin of salmon tagged as smolts in different European countries that were recaptured in this area in the autumn and winter.
71 3 2. Materials and methods Scale samples of 2,350 Atlantic salmon were collected north of the Faroe Islands during autumn (November December) and winter (February March, a few samples were also taken in April 1992) in four consecutive fishing periods 1991/ /1995 (Fig. 1). The fish were sampled randomly from the long-lines during haul-back, and scale samples were collected from the dorso-lateral area on the left side of the fish as recommended by Shearer (1992). Fork lengths and total weights of the fish were recorded. The sea-surface temperature (SST) was measured four times a day, before and after setting, and before and after hauling of the long-line. There was a gradual shift in the fishing areas in a north-eastern direction from the beginning of the fishing period (early autumn) to the end of the period (late winter), this was accompanied by a shift in the ambient SST (±SD) at the fishing locations from 7.7ºC ± 1.1ºC (n = 29) in the autumn to 3.3ºC ± 0.8ºC (n = 27) in the winter. A salmon which has spent its first winter in the sea is termed a one sea-winter (1SW) salmon. Salmon spending two, three or more winters at sea are termed two sea-winter (2SW) salmon, three sea-winter (3SW) salmon etc. When 3SW and older salmon have been pooled the term 3+SW is used. In order to interpret the results it should be noted that the sea age of the fish is defined as changing in September and not the beginning of the year, i.e. a salmon is assumed to be the same sea age throughout the autumn and winter seasons. Since the late 1980's, the expanding fish farm industry in the Northeast Atlantic has resulted in the presence of fish farm escapees in the fishing areas (Hansen et al., 1993; 1999). These escapees were identified using scale analysis (Lund and Hansen, 1991). The number of scale samples collected in the different fishing periods is shown in Table 1. Of the 2,350 fish examined, 678 (29%) were judged to be of farmed origin and 1525 (65%) were identified as wild salmon. Approximately 6% of the fish could not be classified to either group. The farmed and the unclassified fish were excluded from the analysis. It was possible to estimate sea age from 1520 (99.7% of the number of scales available) fish and smolt age from 1335 (87.5%) wild fish. The reason for the relatively low number of samples with readable smolt age was due to the fact that a number of scales had incomplete annual zones at the freshwater stage. During the sampling period all fish caught were screened for external Carlin tags and internal coded wire tags and 296 tagged salmon were recaptured. These tagged fish
72 4 were released as smolts (during spring and summer) and originated from several countries. The recaptures were not weighted by the number of tagged fish released from each country. Instead, the proportions of recoveries were used for comparison between the autumn and winter seasons. Chi-square tests were used to analyse differences in smolt and sea age distributions between autumn and winter and t-tests were used to compare mean lengths between seasons. 3. Results The smolt age of wild salmon varied between 1 and 5 years, and mean smolt ages during the four fishing periods varied between 2.3 and 2.6 years in the autumn samples and between 2.7 and 2.8 years in the winter samples (Table 2). The smolt age distributions were significantly different between autumn and winter, being higher in the winter samples in all fishing periods (χ 2 tests, df = 3 in all cases, 1991/1992: p = 0.02, 1992/1993: p < 0.001, 1993/1994: p = 0.001, 1994/1995: p < 0.001). The sea age of wild salmon varied between 1 and 6 years, and mean sea age during the four fishing periods varied between 1.8 and 2.0 years in the autumn samples and between 2.1 and 2.4 years in the winter samples (Table 3). The sea age distributions were significantly different between the autumn and winter samples in all fishing periods (χ 2 tests, df = 2 in all cases, p < for all four periods). The size of the wild fish (total material) ranged from 38 to 118 cm, and the 1SW, 2SW and 3+SW age groups can be identified as three successive peaks in the length distributions (Fig. 2). The mean size at sea age (±SE) was significantly lower in the autumn samples (45 ± 0.4, 67 ± 0.2, 83 ± 1.2) than in the winter samples (48 ± 0.4, 72 ± 0.2, 88 ± 0.5) for age 1, 2 and 3SW, respectively (t-tests, p < for all ages, pooled material, Fig. 3). This was consistent in all four fishing periods. The tagged salmon recovered from the Faroese fishery in 1991 to 1995 were mainly tagged as smolts in Norway and Ireland (Table 4). This is not surprising as these countries account for most of the tagged smolts in the east Atlantic. To test possible spatial and temporal differences in population structures and hence in recaptures, the origin of the tag recaptures were grouped by two main geographical regions, i.e. a northern European group (Norway, Sweden, Iceland and Faroe Islands) and a southern European group (Ireland, Scotland, England/Wales, Northern-Ireland, France and
73 5 Spain). The proportions of salmon recaptured (Table 4) from the northern and the southern countries were significantly different between seasons (χ 2 = 54.0, df = 1, p < 0.001). The recapture proportions of the northern and southern groups were similar during autumn (53.5 vs. 46.5%) whereas during winter the proportion of salmon from the southern European region was much lower (9.9%) than that from the northern region (90.1%) (Fig. 4). To examine this difference more closely, we compared tag recaptures of salmon originating from the northern and southern group analysed by sea age (Table 5). There were higher proportions of 1SW fish of northern origin recaptured in winter than of southern origin, which is opposite to the situation in the autumn (χ 2 = 17.5, df = 1, p < 0.001). For 2+SW fish the same holds true with 2/3 of the northern fish recaptured in winter when only 1/3 of the southern fish were recaptured (χ 2 = 13.7, df = 1, p < 0.001). Thus, for all ages, the proportions of salmon recovered from the northern region were lower during autumn (34%) than during winter (66%) while the proportion of fish from the southern region seem to be much lower during winter (85% in autumn and 15% in winter) (χ 2 = 54.0, df = 1, p < 0.001). There were no significant seasonal differences in the recovery proportions between 1 and 2+SW salmon within each region (p = and for northern and southern group, respectively). In total for both seasons, 87% of the fish from the northern region were recaptured as 2+SW and 13% as 1SW, and from the southern region the great majority of the fish was recovered as 1SW fish (74%) whereas 26% were recovered as 2+SW (Table 5). The difference in the proportion of 1 and 2+SW fish of northern and southern origin was highly significant (χ 2 = 93.0, df = 1, p < 0.001). These results indicate a structural change from autumn to winter in both the total proportions of fish available as well as in the age distributions from the two fishing areas. 4. Discussion Based on the data in the present paper we reject the null hypothesis that there are no temporal or spatial differences in population structure of Atlantic salmon during their feeding period north of the Faroes. Alternatively, there appears to be evidence for a change in stock complexes entering and departing these areas. Particularly salmon from
74 6 areas in southern and mid part of Europe are observed in higher proportions in the autumn (November December) than in winter (February March) when fish from northern areas were more abundant. We observed that salmon caught in the area north of the Faroes had lower smolt and sea ages during autumn than in the winter period. Because salmon from southern parts of Europe generally are of lower smolt ages as well as lower sea ages than from northern areas we suggest that our observations are at least partly due to a change in proportions of the salmon stocks present in the Faroes area with time, with the higher proportion during the autumn period originating from southern European countries while relatively more salmon from northern European countries inhabit the Faroese area during the winter period. The proportions of total tag recaptures of fish from the northern and southern region were not different in the autumn, whereas the proportion of fish from the southern region where considerably reduced during winter. For one-sea winter fish, salmon from the southern European countries predominated in the autumn, whereas in the winter, a higher proportion of fish from the northern countries were taken. Among the 2+SW fish, the predominant component was of northern origin, during both autumn and winter. This, together with the observed changes in river and sea age composition suggests a change in the stock composition in the area. Some of this difference, however, may be accounted for by the fact that the Faroes salmon fishery moves somewhat towards north-east during winter. The centre of distribution of the samples is shifted about 60 nm to the north-east from autumn to winter. It might be suspected that the newcomers (1SW) from the northern areas, which have spent less time in the sea for growth than the southern European cohorts, are too small to be taken effectively by the long-lines in the autumn season. The size selectivity of the salmon long-line is not known, but generally for long-lines it has been suggested that bait size is the most important size selection factor followed by hook size in catching cod and haddock by long-lines (Løkkeborg, 1994). We used whole sprat (approximately 12 cm in total length) as baits, which might reduce the catch efficiency for smaller salmon. However, fish as small as 38 cm total length were taken by these long-lines. For the comparison between the autumn and winter seasons we would not expect any significant difference in catch efficiency of MSW fish due to their relatively large size (the mean length of 2SW fish increased by 3-5 cm from autumn to winter). However, for 1SW fish, there may be a threshold size where the catchability increases. If this is affecting the sampling, then the proportion of 1SW fish from the northern regions
75 7 should have increased as the season progressed as the fish grew in length. If this was the only temporal change in stock structure in the area, this should have led to a decrease in the mean sea age during winter, but we observed an increase during this period. Between 80 and 90% of the salmon present in the area north of the Faroes are estimated to become sexually mature during the year and spawn in the autumn, irrespective of age (Youngson and McLay, 1985; Jákupsstovu, 1988; Hansen and Jacobsen, 2000). The timing when these fish leave the Faroese area could explain the observed decline of 1SW fish of southern origin from autumn to winter. If so, then the southern component should depart rather early. However, evidence points to the opposite, e.g. for the River Dee, Scotland, the MSW salmon both return to and ascend the river earlier than the later returning grilse (Hawkins, 1987), and in Norway the MSW salmon approaches the coastal areas earlier than the 1SW salmon on their journey home (Jonsson et al., 1990). The grilse component of the Irish stock arrive back to the home coast sometime in early summer (from early May onwards). These fish arrive back to the coast later than the "spring" salmon which are generally large 2SW or more rarely 3SW fish and appear off the Irish coast from February to May. However, it has been suggested that only a relatively small number of salmon from Ireland are present at Faroes during the time of the fishery (Browne et al., 1994). Spatial difference in post-smolt distribution were reported by Holm et al. (1998) who found large numbers of post-smolts with river age 1 and 2 far north in the Norwegian Sea during summer, suggesting that a large part of those originated in more southern areas of Europe, whereas the abundance of smolts with high river ages was low in the same area. Spatial changes in smolt age distributions have also been observed in other areas. Analyses of scale samples from a large multinational offshore research fishing at West Greenland in 1972, revealed a significant change from lower smolt ages in the north (Disco area) to higher smolt ages in the south, and in the Labrador area the smolt ages were significantly higher than at West Greenland (Munro and Swain, 1980). They suggested that the fish taken at Labrador originated "from rivers of the more northerly latitudes." (Munro and Swain, 1980). Reddin and Short (1991) also noted that the river ages of post-smolt salmon caught in the Labrador sea could be used to infer their origin due to the relationship of river age and latitude. Jensen and Lear (1980) found that only after discriminating between European and North American salmon in the Irminger Sea, a difference in smolt age became evident, with mean smolt age of European salmon
76 8 being significantly lower (1.9, range ) than for North American salmon (3.4, range 2 4). There may be several explanations for the apparent change in the stock composition of salmon from autumn to winter. Smolts from different areas move into the ocean at different times; fish from southern Europe may leave their home rivers as early as beginning of April (e.g. Baglinière, 1976), whereas smolts from northernmost Norway go to sea in late June or in the beginning of July (e.g. Hvidsten et al., 1995). This different timing and location would probably result in a different distribution of postsmolts from different stock complexes. The recapture of relatively large southern European post-smolts north of Scotland and in the Norwegian Sea in June and July (Shelton et al., 1997; Holm et al., 2000), at a time when the majority of the e.g. Norwegian smolts just have left their rivers (Hvidsten et al., 1995) and are smaller, indicate that there are sequential/temporal and spatial differences in the salmon stocks in the sea during the early period after seaward migration. Holst et al. (1996) noted that post-smolts of river age 3 and especially 4+ seemed to be missing from pelagic trawl catches during summer in the Norwegian Sea and suggested that the northern post-smolt were outside their sampling area. Jákupsstovu (1988) found that the proportions of 1SW salmon tended to be higher in areas with sea surface temperatures (SST) above 4 C, compared to MSW fish which showed a higher abundance in the colder areas (< 4 C). Reddin and Shearer (1987) found that low SST (< 4 C) seemed to limit salmon distribution and alter their migration routes in the Northwest Atlantic, and Reddin (1988) also found a more restricted distribution of 1SW salmon compared to the wide distribution of MSW fish in the Northwest Atlantic. Thus, significant parts of the 1SW stocks might be confined to the warmer side of the subarctic front in the southern Norwegian Sea during winter. This front is located in an east-west direction approximately between the autumn and winter sampling areas (Fig. 1), and turns northward in a north-eastern direction into the eastern part of the winter area (Hansen, 1985). However, during summer and autumn the upper 50 m are heated, resulting in the higher autumn temperatures in the area. The suggestion of a temporal segregation relies on the assumption that the movement of the fishery (on average 60 nm) reflects a migration of salmon from the autumn to the winter feeding areas, and that the largest salmon migrate farthest into the colder winter area. Fishing fleets usually concentrate in areas with highest catch per unit effort (e.g. Healey et al., 1990). However, since the division between the warmer and the colder
77 9 side of the front coincide with the autumn and winter sampling areas that were not fished synoptically, we cannot verify these suggestions from the present data. The relatively large drop in SST from autumn to winter might indicate that two different water masses were exploited. Thus, if the above assumption on temporal segregation does not hold, our results could indicate a general stock segregation in the feeding areas north of the Faroes throughout the seasons. An indirect support for the alternative suggestion can be found from the age structure and origin of salmon tagged at sea during the winters of in the Faroese area. Jákupsstovu (1988) noted that the high proportion of 1SW (85%) fish available for tagging, was the result of the relatively southern area fished during that period (i.e. south of the present winter feeding area), as opposed to further north where 2SW fish were more abundant. Furthermore, salmon from the southern European countries dominated in the returns from this tagging experiment (Jákupsstovu, 1988), as opposed to the observation in the present data, where approximately 90% of the recaptures originated from northern European countries during winter (Table 4). Thus, the observed stock segregation, at least in the winter feeding areas, is apparently related to environmental conditions and the geographic origin of the fish. A word of caution is warranted here, since the recapture rates from tagging of adult fish at sea are not directly comparable to recaptures of salmon tagged as smolt in homewaters. This is because the estimated recovery proportions from the various regions might be biased due to unequal mortality after tagging, different recapture probabilities and the fact that not all countries tag salmon. Salmon have been observed to feed heavily on crustaceans, especially on the hyperiid amphipod Themisto spp. as well as on mesopelagic fish species in these feeding areas (Jacobsen and Hansen, 2000). Although the abundance of prey does not seem to be limiting, salmon have to compete with other major pelagic fish species, i.e. blue whiting, herring and mackerel, which are present in large numbers from early spring (ICES, 1999a; 1999b). The abundance of T. libellula, which is a relatively large and important crustacean prey for salmon (Jacobsen and Hansen, 2000), is very high in the water mass on the colder side of the front (Dalpadado et al., 1998). Since neither herring, mackerel nor blue whiting enter the colder areas during their feeding migrations (Vilhjálmsson et al., 1997; Holst et al., 1998; ICES, 1999a), it is possible that large salmon enter an area with few competitors. It could therefore be speculated that spatial
78 segregation of salmon is a result of a trade-off between food availability, competition and thermal limitations. This prediction that could be tested in future investigations. 10 Acknowledgements Thanks to G.M. Østborg at the Norwegian Institute for Nature Research, Trondheim, Norway, who read the scales. We thank the skipper and crew on board Hvítiklettur and Polarlaks and the staff at the Faroese Fishery Laboratory for carrying out the sampling. The Faroese Government, the Nordic Council of Ministers, the Norwegian Research Council and the Directorate for Nature Management in Norway provided financial support. References Baglinière, J.-L Etude des populations de sammon Atlantique (Salmo salar L., 1766) en Bretagne-Basse-Normandic. II -Activite de devalaison des smolts sur l'elle. Ann. Hydrobiol., 7, Browne, J., O'Maoileidigh, N., McDermott, T., Cullen, A., Bond, N., McEvoy, B High seas and homewater exploitation of an Irish reared salmon stock. ICES CM 1994/M:10 Dalpadado, P., Ellertsen, B., Melle, W., Skjoldal, H.R Summer distribution patterns and biomass estimates of macrozooplankton and micronekton in the Nordic Seas. Sarsia, 83, Hansen, B The circulation of the northern part of the Northeast Atlantic. Rit Fiskideildar, 9, Hansen, L.P Movement and migration of salmon at sea. In: D. Mills (Ed.), Salmon in the sea and new enhancement strategies, Fishing News Books, Oxford, pp Hansen, L.P., Jacobsen, J.A Origin, migration and growth of wild and farmed Atlantic salmon, Salmo salar L., in oceanic areas north of the Faroe Islands. Submitted Hansen, L.P., Jacobsen, J.A., Lund, R.A High numbers of farmed Atlantic salmon, Salmo salar L., observed in oceanic waters north of the Faroe Islands. Aquacult. Fish. Manage., 24 (6),
79 11 Hansen, L.P., Jacobsen, J.A., Lund, R.A The incidence of escaped farmed Atlantic salmon, Salmo salar L., in the Faroese fishery and estimates of catches of wild salmon. ICES J. mar. Sci., 56 (2), Hawkins, A.D The return migration of adult Atlantic salmon to the Aberdeenshire Dee, Scotland. American Fisheries Society Symposium, 1, Healey, M.C., Thomson, R.E., Morris, J.F.T Distribution of commercial troll fishing vessels off southwest Vancouver island in relation to fishing success and oceanic water properties and circulation. Can. J. Fish. Aquat. Sci., 47 (10), Holm, M., Holst, J.C., Hansen, L.P Spatial and temporal distribution of Atlantic salmon post-smolts in the Norwegian Sea and adjacent areas: Origin of fish, age structure and relation to hydrographical conditions in the sea. ICES CM 1998/N:15, 18 pp. Holm, M., Holst, J.C., Hansen, L.P Spatial and temporal distribution of Atlantic salmon (Salmo salar L.) post-smolts in the Norwegian Sea and adjacent areas. ICES J. mar. Sci. In press Holst, J.C., Arrhenius, F., Hammer, C., Håkansson, N., Jacobsen, J.A., Krysov, A., Melle, W., Vilhjálmsson, H Report on surveys of the distribution, abundance and migrations of the Norwegian spring-spawning herring, other pelagic fish and the environment of the Norwegian Sea and adjacent waters in late winter, spring and summer of ICES CM 1998/D:3 Holst, J.C., Hansen, L.P., Holm, M Observations of abundance, stock composition, body size and food of postsmolts of Atlantic salmon in the NE Atlantic during summer. ICES CM 1996/M:4, 15 pp. Hvidsten, N.A., Jensen, A.J., Vivås, H., Bakke, Ø., Heggberget, T.G Downstream migration of Atlantic salmon smolts in relation to water flow, water temperature, moon phase and social interactions. Nord. J. Freshw. Res., 70, ICES Report of the Working Group on North Atlantic salmon. ICES CM 1996/Assess:11, 227 pp. ICES 1999a. Report of the Northern pelagic and blue whiting fisheries Working Group. ICES CM 1999a/ACFM:18, 238 pp. ICES 1999b. Report of the Working Group on the assessment of mackerel, horse mackerel, sardine and anchovy. ICES CM 1999b/ACFM:6, 468 pp. Jacobsen, J.A., Hansen, L.P Feeding habits of wild and escaped farmed of Atlantic salmon, Salmo salar L., in the Northeast Atlantic. Submitted Jensen, J.M., Lear, W.H Atlantic salmon caught in the Irminger Sea and at West Greenland. J. Northw. Atl. Fish. Sci., 1, Jonsson, N., Jonsson, B., Hansen, L.P Partial segregation in the timing of migration of Atlantic salmon of different ages. Anim. Behav., 40 (2),
80 12 Jákupsstovu, S.H.í Exploitation and migration of salmon in Faroese waters. In: D. Mills, D. Piggins (Eds.), Atlantic Salmon: Planning for the future, Croom Helm, London, pp Lund, R.A., Hansen, L.P Identification of reared and wild Atlantic salmon, Salmo salar L., using scale characters. Aquacult. Fish. Manage., 22, Løkkeborg, S Fish behaviour and longlining. In: A. Fernö, S. Olsen (Eds.), Marine fish behaviour in capture and abundance estimation, Fishing News Books, Oxford, pp Metcalfe, N.B., Thorpe, J.E Determinants of geographical variation in the age of seaward migrating salmon, Salmo salar. J. Anim. Ecol., 59 (1), Munro, W.R., Swain, A Age, weight and length distributions and sex ratio of salmon caught off West Greenland. Rapp. P. -v. Réun. Cons. int. Explor. Mer, 176, Power, G The salmon of Ungava Bay. Arct. Inst. N. Am. Tech. Pap., 22, Reddin, D.G Ocean life of Atlantic salmon (Salmo salar L.) in the Northwest Atlantic. In: D. Mills, D. Piggins (Eds.), Atlantic salmon: Planning for the future, Timber Press, Portland, Oregon, pp Reddin, D.G., Shearer, W.M Sea-Surface temperature and distribution of Atlantic salmon in the Northwest Atlantic ocean. American Fisheries Society Symposium, 1, Reddin, D.G., Short, P.B Postsmolt Atlantic salmon (Salmo salar) in the Labrador Sea. Can. J. Fish. Aquat. Sci., 48, 2-6. Shearer, W.M Atlantic salmon scale reading guidelines. ICES Coop. Res. Rep., 188, Shelton, R.G.J., Turrell, W.R., MacDonald, A., McLaren, I.S., Nicoll, N.T Records of post-smolt Atlantic salmon, Salmo salar L., in the Faroe-Shetland Channel in June Fisheries Research (31), Templeman, W Atlantic salmon from the Labrador Sea and off West Greenland, taken during A. T. Cameron cruise, July-August Res. Bull. int. Comm. Northw. Atlant. Fish., 4, Vilhjálmsson, H., Misund, O.A., Arrhenius, F., Holst, J.C., Gislason, A., Gudmundsdottir, A., Jacobsen, J.A., Krysov, A., Malmberg, S.A., Reid, D Report on surveys of the distribution, abundance and migrations of the Norwegian spring-spawning herring, other pelagic fish and the environment of the Norwegian Sea and adjacent waters in late winter, spring and summer of ICES CM 1997/Y:04, 20 pp. Youngson, A.F., McLay, H.A Changes in steroid hormone levels in blood serum as an early predictor of approaching maturity in Atlantic salmon (Salmo salar L.). ICES CM 1985/M:17
81 13 Table 1 Number of scale samples collected north of the Faroes by fishing period (1991/ /1995) and by autumn (November December) and winter (February March) periods. The scale samples are split into wild, farmed and unknown type. Fishing Wild salmon Farmed salmon Unknown type Period Autumn Winter Autumn Winter Autumn Winter Total 1991/ / / / Total Table 2 Smolt age distribution of wild salmon in numbers (% in brackets) by season (autumn: November December and winter: February March) for each fishing period (1991/ /1995). Total by season in bold. Age group 4+ includes smolt age 4 and higher in samples. Mean smolt age is given for each period, SE is standard error and N is number of samples. Smolt age Season Mean SE N Autumn (8) 70 (39) 68 (38) 27 (15) Autumn (12) 21 (53) 12 (30) 2 (5) Autumn (16) 74 (36) 68 (34) 29 (14) Autumn (6) 57 (50) 31 (27) 20 (17) Total autumn 58(11) 222 (41) 179 (33) 78 a (15) Winter (2) 105 (41) 144 (51) 39 (6) Winter (0) 34 (33) 57 (55) 12 (12) Winter (4) 86 (37) 99 (44) 40 (15) Winter (1) 66 (41) 67 (41) 29 (18) Total winter 20 (3) 291 (36) 367 (46) 120 a (15) a Samples including eight individuals of smolt age 5.
82 14 Table 3 Sea age (SW) distribution of wild salmon in numbers (% in brackets) by season (autumn: November December and winter: February March) for each fishing period (1991/ /1995). Total by season in bold. Age group 3+ includes sea age 3 and higher in samples. Mean sea age is given for each period, SE is standard error and N is number of samples. Sea age Season 1SW 2SW 3+SW Mean SE N Autumn (10) 159 (86) 14 (4) Autumn (31) 41 (63) 4 (6) Autumn (12) 212 (73) 22 (15) Autumn (19) 93 (78) 3 (3) Total autumn 78 (12) 505 (81) 43 a (7) Winter (1) 252 (80) 64 (19) Winter (1) 75 (60) 49 (39) Winter (23) 140 (48) 75 (29) Winter (3) 137 (80) 29 (17) Total winter 73 (8) 604 (68) 217 b (24) a Samples including five individuals of sea age 4 and 5. b Samples including 32 individuals of sea age 4, 5, and 6.
83 15 Table 4 Number of tag (external and internal) recaptures 1991/1992 to 1994/1995, by season and country of origin. The countries of origin are grouped into southern and northern European regions (in bold) for comparisons. Column percentages in brackets. Country/region Autumn (%) Winter (%) Total (%) Norway 49 (38.6) 99 (81.8) 148 (59.7) Sweden 6 (4.7) 8 (6.6) 14 (5.6) Faroes 1 (0.8) 2 (1.7) 3 (1.2) Iceland 3 (2.4) - 3 (1.2) Northern region: 59 (46.5) 109 (90.1) 168 (67.7) Ireland 51 (40.2) 8 (6.6) 59 (23.5) Scotland 6 (4.7) 1 (0.8) 7 (2.8) England/Wales 7 (5.5) 3 (2.5) 11 (4.0) N. Ireland 2 (1.6) - 2 (0.8) France 1 (0.8) - 1 (0.4) Spain 1 (0.8) - 1 (0.4) Southern region: 68 (53.5) 12 (9.9) 80 (32.3) Total 127 (100) 121 (100) 248 (100) Table 5 Tag recaptures by season (autumn and winter) and sea age (1 and 2+SW) of smolts tagged in northern and southern European region from the period 1991/ /1995. Percentages in brackets: column percentages in the main body of the table and row percentages in the bottom row. Northern region Southern region Season 1SW 2+SW Total 1SW 2+SW Total Grand total Autumn 9 (43) 50 (34) 59 (34) 52 (88) 16 (76) 68 (85) 126 (51) Winter 12 (57) 97 (66) 109 (66) 7 (12) 5 (24) 12 (15) 121 (49) Total 21 (13) 147 (87) 168 (68) 59 (74) 21 (26) 80 (32) 247 (100)
84 16 Winter fishery 65 N Iceland 63 N Autumn fishery 500 m 1000 m Faroe Islands 61 N 10 W 5 W Shetland 0 E Fig. 1. Fishing locations and main areas fished during the sampling programme at Faroes were 2350 salmon were sampled for scale analyses during four fishing periods 1991/ /1995. In some locations a star represent several fishing operations (i.e. long-line sets). The autumn fishery (November December) is located closer to the isles and more westerly than the winter fishery (February March), which is located further to the north-east. There is some overlap between autumn and winter fishing stations, not shown on the map.
85 Autumn, n= 693 Winter, n= 979 1SW 2SW 3+SW Proportion (%) Forklength (cm) Fig. 2. Length frequency (%) distribution (forklength) of wild salmon caught during autumn (November December) and winter (February March) north off the Faroes in four fishing periods 1991/ /1995. The 1SW, 2SW and 3+SW age groups can be identified as 3 successive peaks in the length distribution with modes around cm, cm and cm during autumn and winter, respectively.
86 Autumn Winter Average forklength (cm) SW 2SW 3SW Sea age Fig. 3. Average forklength (±SE) by sea age (SW) of wild salmon caught during autumn (November December) and winter (February March) north of the Faroes in four fishing periods 1991/ / Southern Northern 80 Proportion (%) Autumn Season Winter Fig. 4. Tag recapture proportions (%) by season and geographic regions, i.e. the southern and northern European countries for comparisons. Data from recaptures in the Norwegian Sea
87 Aspects of the marine ecology of Atlantic salmon
88 Aspects of the marine ecology of Atlantic salmon PAPER III Origin, migration and growth of wild and escaped farmed Atlantic salmon, Salmo salar L., in oceanic areas north of the Faroe Islands.
89 1 Origin, migration and growth of wild and escaped farmed Atlantic salmon, Salmo salar L., in oceanic areas north of the Faroe Islands LARS P. HANSEN 1* & JAN ARGE JACOBSEN 2 1 Norwegian Institute for Nature Research, Dronningensgt. 13, P.O. Box 736, Sentrum, N-0105 Oslo, Norway 2 Fisheries Laboratory of the Faroes, P.O. Box 3051, FO-110 Tórshavn, Faroe Islands Abstract: We examined the distribution, migration, origin and growth of wild and escaped farmed Atlantic salmon, Salmo salar L., in the north east Atlantic ocean north of the Faroe Islands. Between November 1992 and March 1995 a total of 5,448 salmon (3,811 wild and 1,637 fish farm escapees) were caught by floating long-lines, individually tagged and released back into the sea. A total of 106 fish (87 wild and 19 farmed) have been reported recaptured. The recapture rate of wild salmon (2.3 %) was significantly higher than that of farmed salmon (1.2 %). Recoveries of wild salmon were reported from homewater in nine north Atlantic countries, and in a number of different rivers throughout the distribution range of Atlantic salmon included Russia, Iceland, Spain and Canada. Most tags were recovered in Norway, but significant number of returns were observed in Scotland and Russia as well. No fish were recaptured at Faroes. Fish tagged in the autumn tended to return to areas closer to the tagging site than fish tagged in the winter. This strongly suggests that salmon originating from most areas of the distribution range are at some life stage present in this area, but in variable proportions at different times. Most of the salmon returned home to spawn the next autumn, and the fish that stayed for another year originated from northern areas of Europe. All recoveries of farmed salmon were done in Norway except one at the west coast of Sweden, suggesting that they mainly had escaped from Norwegian fish farms. Assessment of the proportion of wild salmon from different countries present in this area revealed that 40% of the fish were of Norwegian origin, and Scotland and Russia accounted for about 20% each. Four tags of wild fish were reported from Canada, all in the same year when they were tagged. This demonstrates that adult Atlantic salmon can cross the north Atlantic ocean in less than 6 months. Estimated migration speed of wild fish and farmed fish returning to Norway and their specific growth rate was not different. Key words: migration, growth, tagging, ocean, farmed salmon, wild salmon Running head: Migration of wild and escaped farmed salmon *Author to whom correspondence should be addressed: Tel.: ; fax: , l.p.hansen@ninaosl.ninaniku.no
90 Migration of wild and escaped farmed salmon 2 INTRODUCTION Atlantic salmon are distributed over large areas in the Northeast Atlantic, but there is still relatively little information on the distribution and migration patterns of this species in the ocean (e.g. Hansen & Quinn, 1998). Inside the Faroes Exclusive Economic Zone (EEZ) salmon have been exploited for a relatively long period of time (Jákupsstovu, 1988). This fishery exploits mainly two-sea-winter (2SW) fish, although some 1 and 3SW fish are also caught. Recaptures at Faroes of salmon tagged as smolts in different countries have revealed that salmon from many countries are present in the area, but no quantitative analysis of stock distribution has been carried out. Most of these recaptures, however, originate from hatcheries. From recaptures of tagged salmon in the Faroes fishery in the 1980s there is direct evidence that salmon of different smolt year classes originating from different rivers in Norway, Scotland and Sweden are caught side by side during the same time period in the same area (Hansen, 1993). A tagging experiment at sea around the Faroe Islands was carried out during the period 1969 to 1976 (Jákupsstovu, 1988). Most of the salmon tagged was small (1SW), and may thus not reflect the composition of stocks in the area. Most recaptures of the tagged fish were reported from Scotland and Norway, and there were also several fish recovered from Ireland. In recent years large numbers of farmed salmon have been observed at Faroes (Hansen et al., 1993a), accounting for a significant proportion of the Faroese salmon catch (Hansen et al., 1999). There is direct evidence that farmed salmon escaping from net pens in Norway enter this area (Hansen et al., 1987), but there is no information on the movement, survival and growth of these fish in the ocean. The main goal of this paper is to examine the origin, migration and growth of wild and escaped farmed salmon utilising the Faroese area during parts of their oceanic feeding phase. MATERIAL AND METHODS As a part of a salmon research project in the north east Atlantic ocean at Faroes from 1992 to 1995, wild and farmed Atlantic salmon were tagged and released back into the sea. The salmon were caught north of the Faroe Islands using commercial floating long-
91 Migration of wild and escaped farmed salmon 3 lines that were baited with sprats. The lines were usually set early in the morning, hauling started approximately at noon and was completed between 5 and 10 hours later. The average number of hooks in each set was about The fishing took place between November and March during the three fishing periods 1992/93, 1993/94 and 1994/95. The area fished during the autumn part (November-December) was closer to the Faroes than the area fished in the winter (February-March) (Fig. 1). Immediately after capture the salmon that were judged to have a fair probability of survival were individually tagged with numbered Lea tags. The fish were measured (fork length) and a few scales were removed from the dorso-lateral area as recommended by Shearer, (1992). If possible, the hook was removed, but in cases where removal could seriously damage the fish, the hook was left in the fish. The salmon were kept in a tank with a continuos inflow of sea water onboard the vessel for some time allowing the fish to recover after handling and tagging. If the fish appeared fit by visual inspection, it was released. All fish were determined to be of wild or farmed origin by examining whether they showed external characters like e.g. fin erosion which is common on reared salmon (Lund et al., 1989), and by analysis of scales (Lund & Hansen, 1991). In total 3811 wild and 1637 farmed salmon were tagged and released (Table I), and Fig. 2 shows the length distribution in the autumn and winter of both groups of fish. It can be observed that the length distribution of wild and farmed salmon is similar. The sea age of wild salmon was estimated by splitting the length frequencies into sea age cohorts (see e.g. ICES, 1996), i.e. fish less than 57 cm forklength were taken to be one sea winter (1SW), fish between 57 and 82 cm forklength were taken to be 2SW, and fish larger than 82 cm were 3SW. Among the wild salmon 2SW fish are the dominating sea age group as it accounts for more than 80% of the wild fish tagged. It should be noted that the incidence of 1SW fish is more pronounced in the autumn than in the winter, whereas 3 SW fish occurred in a higher proportion in the winter. Tags were reported from commercial fishermen operating in home waters, and from anglers in rivers, and they were asked to submit information about date, time and site of recapture, the size of the fish and the gear used for catching the fish. The distance between tagging and recapture site (migration distance) was estimated using the shortest distance (straight line between the two sites), and are thus minimum distances. To estimate the origin of wild salmon in this area we corrected the observed number of recaptures by country with their respective mean exploitation rates in
92 Migration of wild and escaped farmed salmon 4 homewaters plus and minus 10% error (ICES, 1996). The recaptures from each country were also adjusted for their respective homewater tag reporting rates (as provided by the ICES North Atlantic Salmon Working Group members, 1997). Furthermore, error estimates due to small number of recaptures were introduced using the binomial distribution model. 'At Risk' simulations were used to estimate error bounds (95% confidence limits) on the estimated proportion of fish returning to different countries. Farmed salmon were not included in this analysis. Weight (g) of individual fish was estimated from length-weight regression (loglog regression) of fish that were not tagged (W = L , r 2 = 0.92, n = 3643, p < 0.001). Specific growth rate G (percent gain in weight per day) was calculated (lnw r - lnw t )100/D, where W r and W t is weight at recapture and tagging, respectively, and D is number of days in liberty. RESULTS RECAPTURE RATES The overall reported recapture-rate of the number of salmon tagged was small, only 106 fish were recovered which is 1.9% of the number of salmon tagged. Of wild fish 87 individuals (2.3% of the number tagged) were recaptured, whereas 19 (1.2%) of the fish identified as fish farm escapees were recovered (Table II). The recapture rates of fish of farmed origin was significantly lower than for wild fish (χ 2 = 6.8, df= 1, p= 0.009). For both wild and farmed salmon, the recapture rates were lower of fish tagged in the autumn than in winter (χ 2 = 7.3, df= 1, p= 0.007). Tags were reported from many countries in the North Atlantic (Table III), both from marine fisheries and in freshwater. No tags were recovered from the research fishery at Faroes nor from West Greenland. The recapture rate of wild salmon increased significantly with sea age at tagging (Fig. 4) (G= 15.4, df= 2, p< 0.001). However, the power of the test might be questioned, as only two 1SW fish were recovered, but the recapture rate of 3SW was significantly higher than of 2SW fish (χ 2 = 10.6, df= 1, p= 0.001). The recapture rates of fish with the hook left in when released was significantly higher than of fish with no hook when released (χ 2 = 7.5, df= 1, p= 0.006), which might indicate that for some of the fish the removal of the hook prior to release was lethal.
93 Migration of wild and escaped farmed salmon 5 GEOGRAPHICAL DISTRIBUTION OF RECAPTURES In wild fish there was an apparent difference in the geographical distribution of recaptures of fish tagged and released in the autumn (Fig. 5) and the winter (Fig. 6). Fish tagged in the autumn were recaptured relatively close to the site of release, in south Norway, Scotland, Ireland and Iceland, whereas fish tagged in the winter were also recaptured in more distant areas such as Russia, north Norway, Sweden, Denmark and Spain and Canada. This suggests that fish from distant areas are more abundant in the Faroese area in the winter than in the autumn. Tag returns were scattered over large areas of Norway and Scotland suggesting that fish from large areas of these countries were present in the same areas at Faroes. Tag recaptures were also reported from major salmon rivers in these countries such as River Numedalslågen, R. Drammenselv, R. Gaula, and R. Namsen in Norway; R. Spey, R. Brora, R. Tay, R. North Esk, and R. Dee in Scotland. It is interesting to note that four tags were reported from Canada, one tagged in March 1993 and recaptured in River Miramichi in September 1993, three tagged in February/March 1995 and of those two subsequently recaptured in the Miramichi in September 1995 and one in Kouchibouguac River (close to Miramichi) in October This demonstrates that salmon are able to cross the north Atlantic ocean in 6 months or less. Of the 19 fish farm escapees recaptured, 18 were recovered from Norway, and one from the west coast of Sweden (Table IV). The geographical position of the recaptures demonstrates that the fish were distributed over large areas of coastal Norway (Fig. 7). ORIGIN OF SALMON Overall estimates of the proportion of wild salmon originating from different countries in the research fishery during these three fishing periods are presented in Table 5 together with the assumptions and approximations made. It is not surprising that Norway accounts for the major proportion (40%), whereas the mean estimated proportion of salmon from Scotland and Russia is close to 20 %. The estimates indicate that there are only a relatively small number of fish from other countries in the area. No similar analysis was carried out for the farmed fish, but the great majority appear to be of Norwegian origin.
94 Migration of wild and escaped farmed salmon 6 SEXUAL MATURITY Of the wild fish tagged and released, 7 individuals of a total of 87 fish recaptured in home waters (8.0 %), stayed for an additional year in the sea before returning to home waters (Table III). This is due to the fact that these fish grew cm in length until they were recaptured more than one year after they were tagged. Five of these fish originated from Norway, one from Sweden and one from Russia. They were all tagged as 2SW fish, and thus they returned home as 3SW fish. The recaptures from mid and southern part of Europe returned home during the same season they were released. Of the farmed fish 2 of a total of 19 fish recaptured (10.5%) stayed for an additional year in the sea. MIGRATION DISTANCE AND SPEED The bulk of the fish were recaptured between 3 and 11 months after release, ranging from 48 days for a Scottish 2SW wild salmon (tagged 17 March and recaptured 1 May 1993) to nearly two years of a Norwegian 2SW wild salmon (Fig 8). Two escaped farmed fish stayed for 1½ year at sea before returning to Norway. The shortest journey home was made by a salmon homing to the west coast of Norway (600 km), and the longest migration distance was to Canada (4500 km). Estimated migration speed (shortest distance travelled/number of days from tagging till recapture) was highly variable between individual fish, and the estimated speed was higher in fish that were tagged in the winter than fish tagged in the autumn (Fig 9). The estimated migration speed was approximately 5 km/day for Norwegian, Scottish and Irish fish tagged in the autumn, whereas fish of the same origin tagged in the winter were estimated to move with a speed of 7-10 km/day. Fish from more distant waters like Russia and Canada tagged in the winter moved km/day. There was no difference in estimated migration speed of farmed and wild salmon returning to Norway. GROWTH The specific growth rate (G, % weight gain per day) was highly variable among individual fish. The overall mean G was 0.19, ranging from 0 to The G of wild salmon recaptured during autumn (0.17, n= 19) and winter (0.20, n= 64) was not different (t-test, df= 81, p= 0.472). Neither was there any difference between wild salmon recaptured less than one year (G= 0.19, n= 76) and after two years (G= 0.14, n=
95 Migration of wild and escaped farmed salmon 7 7) at sea (t-test, df= 81, p= 0.403), nor between wild (G= 0.19, n= 83) and farmed (G= 0.19, n= 19) salmon (t-test, df= 100, p= 0.995). DISCUSSION Fish that were tagged in the winter seem to survive better than fish tagged in the autumn. There may be several explanations for this. Firstly, the fish that were tagged in the autumn were smaller than those tagged in the winter, and may thus be more vulnerable to handling and tagging stress (Fowler & Stobo, 1999) and subsequent size selective predation. Secondly, these fish will spend longer time in the sea than fish tagged in the winter, and may thus be exposed to marine mortality factors for a longer period of time. Furthermore, based on smolt age and sea age distribution of salmon at Faroes, Jacobsen et al. (2000) suggested that fish originating from rivers in mid and southern Europe were more abundant in the Faroese area in the autumn than in winter. If this holds true, we cannot rule out the possibility that the apparent differences in survival reported in the present paper may reflect a trend observed in last decade, that marine mortality rates of salmon stocks from southern Europe has increased more quickly than for salmon from northern regions in the Atlantic (Parrish et al., 1998; ICES, 1999). The relatively low recapture rate of wild salmon (2.3%) in the present sea tagging experiment compared with the previous sea tagging project in the Faroese area (4.6%) (Jákupsstovu, 1988) might further indicate a decrease in marine survival. However, in recent years significant reductions in fishing efforts have been made both at Faroes as well as in home waters which have resulted in a decline of exploitation rates (ICES 1999). Atlantic salmon home with high precision to the rivers they left as smolts (e.g. Thorpe, 1988; Hansen et al., 1993b). Thus it is highly reasonable to conclude that salmon tagged at Faroes and subsequently recaptured in freshwater are most likely to have returned home. It is also reasonable to assume that salmon caught in coastal areas in homewater have returned to their country of origin. The estimated proportions of salmon from different countries in the research fishery show that Norwegian salmon stocks account for the major part of the stock complex, although there are also significant numbers of Scottish, Russian and Irish
96 Migration of wild and escaped farmed salmon 8 salmon. Because 1SW fish accounts for most of Irish salmon runs, it is likely that the proportion of Irish salmon north of the Faroes is underestimated. The reason for this is the apparent high handling and tagging mortality of the 1 SW salmon released. This is further supported by the observed presence of many microtagged 1 SW salmon of Irish origin reported from the Faroes fishery during autumn (Jacobsen et al., 2000). All in all, the geographical distribution of tag returns to homewaters, strongly suggests that fish from most areas of the distribution range of Atlantic salmon at some life stage are present in the Norwegian Sea area north of the Faroe Islands. This does not mean that all stocks are systematically abundant in the area, but pass through occasionally, or that components of stocks use the area for feeding. This is supported by the fact that tagged wild fish were recovered in Canada, Spain and eastern part of European Russia, as well as in all major salmon producing countries in Europe. Over 90 % of the fish recaptured were reported from home waters the same season as they were tagged, and relatively few fish stayed in the ocean for another year. This shows that a large proportion of the salmon in the area was indeed sexually maturing the next autumn. This figure is an overestimate as fish that stay in the ocean for another year are subjected to a higher natural mortality than fish that returned home one year earlier. Indirect estimates of the incidence of maturity of salmon caught in the Faroes fishery in 1982 and 1983 suggested that 80% of the MSW fish were maturing, (ICES, 1984), which corresponds to the figure in the present paper. It was interesting to note that fish that stayed for another year in the ocean were of northern origin (Norway, Sweden and Russia). The material is relatively small, but it could be speculated if fish from the northern countries mainly stay in the north east Atlantic during the marine phase, whereas fish from mid and southern Europe stay in the area for a limited time arriving from other oceanic areas. As support for this, it has been shown that fish from south and mid Europe are much more abundant at Greenland than fish from the northern countries (Jensen, 1980b). The distribution of salmon at Faroes has earlier been assessed from sampling the fishery for a number of years. Some of the results have been reported by Jákupsstovu (1988), who also reported on a tagging program during the period 1969 to 1976 where in total 1,946 salmon caught on long line were tagged and released back into the sea. A total of 90 recaptures were reported, 33 in Scotland, 31 in Norway and 15 in Ireland suggesting that salmon from these countries were most abundant around Faroes, followed by England/Wales (5), Sweden (2), Russia (1), and 3 from West Greenland.
97 Migration of wild and escaped farmed salmon 9 These results are somewhat different than those in the present study, and the main reason for this may be that the previous tagging was conducted relatively close to the Faroes, and even south of the islands, and the fact that the great majority of the salmon tagged was 1SW fish (85% of the total number tagged), whereas the present tagging experiment was conducted further north and mainly 2SW salmon (80%) where tagged. The size distribution of salmon in the area north of the Faroes generally increases with latitude (Jákupsstovu, 1988; ICES, 1996). Among the fish tagged during (Jákupsstovu, 1988) it is worth noting that some fish in the area may have been on their way westwards, as they were reported from West Greenland later the same year. Conversely salmon tagged at West Greenland have been reported in the area north of the Faroes the following year (ICES, 1984). From this it may be suggested that salmon of European origin may move through the Faroese area on their way to the feeding areas in the west Atlantic as well as on their way home. The fact that there were no tag returns in Greenland from the present experiment is probably caused by the very significant reduction in the fishing effort in this area in recent years (ICES, 1996). It is well known that MSW fish of European origin are present at both west and east Greenland (Jensen, 1967; Swain, 1980; Jensen & Lear, 1980; Jensen, 1980a; 1980b; Horsted, 1988; Scarnecchia, 1989). The abundance of farmed fish in the ocean has been relatively high in recent years (Hansen et al., 1999). In several countries a large salmon farming industry has developed, particularly in Norway and Scotland, which account for most of the total production (540,000 tonnes in 1998) in the North Atlantic (ICES, 1999). Escapees of farmed fish have been observed in several areas in the Northeast Atlantic, and contribute to a relatively large extent to salmon fisheries in Norway and Faroes. Hansen and Jonsson (1991) showed that reared salmon kept in saltwater, tagged and released into a Norwegian fjord every month throughout a year, tended to return to the geographical area of release and enter nearby rivers to spawn, except when released in late winter when they tended to stray farther away from the release site. When the fish were released in late summer and autumn, their survival was poor (Hansen & Jonsson, 1989). These observations have recently been supported by sequential releases of individually tagged large farmed salmon from two fish farms in Norway (L.P. Hansen personal observations). Thus this may help to explain the observed lower recapture rate of farmed salmon in the present study. Alternatively, it has been observed that escaped farmed salmon enter fjords and freshwater later in the season than wild fish (Lund et al.,
98 Migration of wild and escaped farmed salmon ) which may result in a lower exploitation rate on farmed than wild fish, and thus lower number of tags recovered if a large proportion of the farmed fish enter home waters after the fishing season has closed. The observation that most of the tagged farmed salmon in the present experiment were recaptured in Norway, suggest that these fish escaped from Norwegian fish farms. The estimates of migration speed are minimum estimates, and are of course highly dependent on when the fish decide to go home, how long time they have been in home waters before capture. Furthermore, the estimated distance travelled is based on the shortest distance between site of release and site of recapture which is definitely not the actual migration route. Apparently the estimated migration speed of fish tagged in the autumn was lower than for those tagged in the winter. However, Atlantic salmon tagged off Norway travelled on average only between 9 and 14 km/d (Hansen et al., 1993b), which compares to our estimates of salmon tagged in the winter, either indicating a systematic difference between the areas or more likely the inherent problems in such analyses to limit the period used for active homeward migration. Probably the main reason for the difference between fish tagged in the autumn and winter is due to the possibility that salmon return home later in the winter. Furthermore, salmon slow down in coastal waters and fjords (Hansen et al., 1993b) and do not ascend the freshwater immediately upon return to coastal water (Jonsson et al., 1990). Thus swimming speed of salmon caught in freshwater are therefore further underestimated. The specific growth rate was similar for wild and farmed salmon. This is supported by observations of feeding habits of salmon in this area where no difference in number or weight proportions of different prey between wild and farmed salmon was observed (Jacobsen & Hansen, 2000). Furthermore, there was no difference in condition factor between the two groups. This strongly suggests that fish farm escapees that survive until capture in the Faroes area have adapted to the marine environment. We sincerely thank the crews on Hvítiklettur and Polarlaks, and the staff at Faroes Fisheries Laboratory for carrying out the sampling. We are also much indebted to L. Fløystad, G.M. Østborg, R.A. Lund and B. Larsen for excellent assistance in the laboratory. The Faroese Government, the Nordic Council of Ministers, the Norwegian Research Council and the Directorate for Nature Management in Norway provided financial support. We are grateful to members of ICES North Atlantic Salmon Working Group for fruitful discussions and
99 Migration of wild and escaped farmed salmon 11 information about homewater exploitation rates and tag reporting rates of salmon. We also appreciate useful help from Ted Potter in the analysis of origin of salmon. References Fowler, G. M. & Stobo, W. T. (1999). Effects of release parameters on recovery rates of tagged groundfish species. Canadian Journal of Fisheries and Aquatic Sciences 56, Hansen, L.P. (1993). Movement and migration of salmon at sea. In Salmon in the sea and new enhancement strategies (Mills, D., ed.). pp Fishing News Books. Hansen, L. P., Døving, K. B. & Jonsson, B. (1987). Migration of farmed adult Atlantic salmon with and without olfactory sense, released on the Norwegian coast. Journal of Fish Biology 30, Hansen, L. P., Jacobsen, J. A. & Lund, R. A. (1993a). High numbers of farmed Atlantic salmon, Salmo salar L., observed in oceanic waters north of the Faroe Islands. Aquaculture and Fisheries Management 24, Hansen, L. P., Jacobsen, J. A. & Lund, R. A. (1999). The incidence of escaped farmed Atlantic salmon, Salmo salar L., in the Faroese fishery and estimates of catches of wild salmon. ICES Journal of Marine Science 56, Hansen, L. P. & Jonsson, B. (1989). Salmon ranching experiments in the River Imsa: Effect of timing of Atlantic salmon (Salmo salar) smolt migration on survival to adults. Aquaculture 82, Hansen, L. P. & Jonsson, B. (1991). Effect of smolt age on migratory behaviour of Baltic salmon, Salmo salar L., transplanted to the East Atlantic. Aquaculture and Fisheries Management 22, Hansen, L. P., Jonsson, N. & Jonsson, B. (1993b). Oceanic migration in homing Atlantic salmon. Animal Behaviour 45, Hansen, L. P. & Quinn, T. P. (1998). The marine phase of the Atlantic salmon (Salmo salar) life cycle, with comparisons to Pacific salmon. Canadian Journal of Fisheries and Aquatic Sciences 55 (Supplement 1), Horsted, S. A. (1988). Future investigations on the ocean life of salmon. In Atlantic Salmon: Planning for the future (Mills, D. & Piggins, D., eds), pp London: Croom Helm. ICES (1984). Report of the meeting of the Working Group on North Atlantic Salmon. ICES CM 1984/Assess: 16, 54 pp. ICES (1996). Report of the Working Group on North Atlantic salmon. ICES CM 1996/Assess: 11, 227 pp. ICES (1999). Report of the working group on north Atlantic salmon. I.C.E.S. C.M. 1999/ACFM:14, 293 pp. Jacobsen, J.A. & Hansen, L.P. (2000). Feeding habits of wild and escaped farmed Atlantic salmon, Salmo salar L., in the Northeast Atlantic. Submitted for publication. Jacobsen, J. A., Lund, R. A., Hansen, L. P. & Ó Maoiléidigh, N. (2000). Seasonal differences in the origin of Atlantic salmon (Salmo salar L.) in the Norwegian Sea based on estimates from age structures and tag recaptures. Submitted for publication Jensen, J. M. (1967). Atlantic salmon caught in the Irminger Sea. Journal of the Fisheries Research Board of Canada 24,
100 Migration of wild and escaped farmed salmon 12 Jensen, J. M. (1980a). Recaptures from international tagging experiments at West Greenland. Rapports et Procès-verbaux des Réunions du Conseil International pour l'exploration de la Mer 176, Jensen, J. M. (1980b). Recaptures of salmon at west Greenland tagged as smolts outside Greenland waters. Rapports et Procès-verbaux des Réunions du Conseil International pour l'exploration de la Mer 176, Jensen, J. M. & Lear, W. H. (1980). Atlantic salmon caught in the Irminger Sea and at West Greenland. Journal of Northwest Atlantic Fisheries Science 1, Jonsson, N., Jonsson, B. & Hansen, L. P. (1990). Partial segregation in the timing of migration of Atlantic salmon of different ages. Animal Behaviour 40, Jákupsstovu, S. H. í. (1988). Exploitation and migration of salmon in Faroese waters. In Atlantic Salmon: Planning for the future (Mills, D. & Piggins, D., eds), pp London: Croom Helm. Lund, R. A. & Hansen, L. P. (1991). Identification of reared and wild Atlantic salmon, Salmo salar L., using scale characters. Aquaculture and Fisheries Management 22, Lund, R. A., Hansen, L. P. & Järvi, T. (1989). Identification of reared and wild salmon by external morphology, size of fins and scale characteristics. NINA Forskningsrapport (1), (In Norwegian with English abstract.) Lund, R. A., Økland, F. & Hansen, L. P. (1991). Farmed Atlantic salmon (Salmo salar) in fisheries and rivers in Norway. Aquaculture 98, Parrish, D. L., Behnke, R. J., Gephard, S. R., McCormick, S. D. & Reeves, G. H. (1998). Why aren't there more Atlantic salmon (Salmo salar)? Canadian Journal of Fisheries and Aquatic Sciences 51 (Supplement 1), Scarnecchia, D. L. (1989). Effects of oceanic variations and the West Greenland fishery on age at maturity of Icelandic west coast stocks of Icelandic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 46, Shearer, W. M Atlantic salmon scale reading guidelines. ICES Cooperative Research Report (188), Swain, A. (1980). Tagging of salmon smolts in European rivers with special reference to recaptures off West Greenland in 1972 and earlier years. Rapports et Procèsverbaux des Réunions du Conseil International pour l'exploration de la Mer 176, Thorpe, J. E. (1988). Salmon migration. Science Progress (Oxford) 72,
101 Migration of wild and escaped farmed salmon 13 Table I. Number of wild and escaped farmed salmon tagged and released by month in Faroese waters November 1992 to March Month No. wild salmon No. farmed salmon Total November December March November December February March November December February March Total Table II. Reported recapture rates of salmon in number (% in brackets) tagged at Faroes in the autumn (November-December) and winter (February-March). Season Wild Farmed Total Autumn (1.6) 1 (0.4) 12 (1.3) Winter (2.7) 8 (1.0) 44 (2.1) Autumn (1.3) 1 (1.1) 3 (1.3) Winter (2.4) 2 (2.4) 7 (2.4) Autumn (1.3) 1 (0.7) 8 (1.2) Winter (3.0) 6 (2.4) 30 (2.8) Total 87 (2.3) 19 (1.2) 106 (1.9)
102 Migration of wild and escaped farmed salmon 14 Table III. Recaptures in number of wild salmon in different countries tagged in the Norwegian Sea, north of the Faroes during the 1992/1993, 1993/1994 and 1994/1995 fishing periods. Country Tagged 1992/1993 Tagged 1993/1994 Tagged 1994/1995 Total Rec.93 Rec.94 Rec.94 Rec.95 Rec.95 Rec.96 No % Norway Scotland Ireland Russia Sweden Canada Denmark England Iceland Spain Total Table IV. Number of escaped farmed salmon recaptured by country. The fish were tagged at Faroes during the 1992/93, 1993/94 and 1994/95 fishing periods. Country Tagged 1992/1993 Tagged 1993/1994 Tagged 1994/1995 Total Rec.93 Rec.94 Rec.94 Rec.95 Rec.95 Rec.96 No % Norway Sweden Total
103 Migration of wild and escaped farmed salmon 15 Table V. Results of 'At Risk' simulation to estimate proportion (%) of fish tagged at Faroes returning to different countries. Confidence limits (95%) were applied based on 1000 simulations. Recoveries were adjusted for homewater exploitation rates and tag reporting rates as provided by the North Atlantic Salmon Working Group members, Country No. Tag reporting rate Exploitation rate Estimated Simulation recapt. min max min max recapt. '-5% Mean(%) '+95% Norway Scotland Russia Canada Ireland Denmark England Sweden Spain Iceland Total
104 Migration of wild and escaped farmed salmon N Iceland 1000 m 500 m Winter fishing 63 N 500 m 1000 m Autumn fishing Faroe Islands 61 N 10 W 5 W Shetland 0 E Fig. 1. Areas of tagging north of the Faroes. The autumn fishery is located closer to the isles and as the season progresses the fishery moves in a north-eastern direction farther into the Norwegian Sea.
105 Migration of wild and escaped farmed salmon 17 % a) Tagged salmon in the autumn 1992 b) Tagged salmon in the winter 1993 Wild, n= 673 Farmed, n= 274 % Wild, n= 1311 Farmed, n= Forklength (cm) Forklength (cm) % c) Tagged salmon in the autumn 1993 d) Tagged salmon in the winter Wild, n= 228 Farmed, n= 91 % 12 Wild, n= Farmed, n= Forklength (cm) Forklength (cm) % e) Tagged salmon in the autumn 1994 f) Tagged salmon in the winter Wild, n= 541 Farmed, n= 142 % 12 Wild, n= Farmed, n= Forklength (cm) Forklength (cm) Fig. 2. Forklength distributions of wild and farmed salmon tagged north of the Faroes during three fishing periods 1992/ /95. The fishing period is divided into an autumn (November-December) and winter (February-March) season.
106 Migration of wild and escaped farmed salmon Autumn (Nov-Dec) Winter (Feb-Mar) Proportion (%) SW 2SW 3+SW Sea age Fig. 3. Estimated sea age distribution of wild Atlantic salmon tagged north of the Faroes by season. Total number of observations is 3811 fish. Recapture rate (%) SW 2SW 3+SW Sea age Fig. 4. Recapture rates by sea age of wild salmon tagged and released north of the Faroes during 1992/ /1995. The figures show the number of observations.
107 Migration of wild and escaped farmed salmon W 30 W 0 75 N 30 E 60 N 45 N Tagging area Fig 5. Geographical distribution of wild salmon tagged and released north of the Faroe Islands in the autumn and recaptured in home waters. The lines indicate the shortest direct migration route home. 60 W 30 W 0 75 N 30 E 60 N 45 N Tagging area Fig. 6. Geographical distribution of wild salmon tagged and released north of the Faroe Islands in the winter and recaptured in home waters. The lines indicate the shortest direct migration route home.
108 Migration of wild and escaped farmed salmon W 30 W 0 75 N 30 E 60 N 45 N Tagging area Fig. 7. Geographical distribution of escaped farmed salmon tagged and released north of the Faroe Islands in the autumn and recaptured in home waters. The lines indicate the shortest direct migration route home Minimum distance (km) Time (days) Fig. 8. Minimum distances travelled versus days in liberty. Wild salmon is shown as open circles and escaped farmed salmon as filled squares.
109 Migration of wild and escaped farmed salmon Farmed salmon Autumn Wild salmon Winter Speed (km per day) NO SW NO SC IR RU CA SW DE EN SP IC Country Fig. 9. Estimated migration speed (km per day) of farmed and wild salmon by country of origin. Speed was calculated as the average speed from tagging in the Northeast Atlantic to recapture in the home countries. Confidence intervals (95%) are indicated where number of recoveries were greater than two fish. Growth rate (% gain in wt per day) Autumn Winter Farmed Wild salmon salmon NO SW NO SC IR RU CA SW DE EN SP IC Country Fig. 10. Estimated specific growth rates (G, % gain in weight per day) of farmed and wild salmon by country of origin. Confidence intervals (95%) are indicated where number of recoveries were greater than two fish.
110 Aspects of the marine ecology of Atlantic salmon
111 Aspects of the marine ecology of Atlantic salmon PAPER IV Feeding habits of wild and escaped farmed Atlantic salmon, Salmo salar L., in the Northeast Atlantic.
112 1 Feeding habits of wild and escaped farmed Atlantic salmon, Salmo salar L., in the Northeast Atlantic Jan Arge Jacobsen and Lars Petter Hansen Abstract: The stomach contents of 2992 wild and 863 escaped farmed Atlantic salmon caught on floating long-lines in a Faroese research fishery in the late autumn (November December) and winter (February March) feeding areas in the Northeast Atlantic (63 66 N and 1-10 W) during three consecutive fishing periods 1992/ /1995 were analysed. The salmon fed mainly on hyperiid amphipods, euphausiids, shrimps, lanternfishes, pearlsides and barracudinas, and less on larger pelagic fish and squid. Crustaceans accounted for 95% in number (30% by weight) and fish for 5% in number (66%) by weight. There was no difference in condition factor, number and weight proportions of prey, or diet overlap between wild and farmed salmon, which suggests that farmed salmon that survive until they were captured are completely adapted to feed in the marine environment. The proportion of stomachs containing food was significantly lower during autumn (53%) than during winter (78%). The ambient sea-surface temperature is ca 7 C in autumn compared to ca 3 C in winter; therefore temperature dependent evacuation rate could explain the apparent lower stomach content during the autumn. The significantly higher condition of 3+SW salmon compared to the smaller 1 and 2SW fish, irrespective of season, might be a reflection of higher tolerance to low temperatures, greater forage potential or prey capture success. Although salmon show patterns of opportunistic feeding behaviour, there was also evidence of selective foraging. Generally fish were preferred over crustaceans, and amphipods were chosen over euphausiids. Large salmon tended to be more piscivory than smaller fish. J.A. Jacobsen 1. Fiskirannsóknarstovan, Nóatún, P.O. Box 3051, FO-110 Tórshavn, Faroe Islands. L.P. Hansen. Norwegian Institute for Nature Research, P.O. Box 736, Sentrum, N-0105 Oslo, Norway 1 Author to whom all correspondence should be addressed. janarge@frs.fo
113 Jacobsen & Hansen 2 Introduction The abundance of Atlantic salmon (Salmo salar L.) has generally decreased during the last two decades (Parrish et al. 1998; ICES 1999). There are a number of reasons for this, included degradation of freshwater habitat suitable for salmon, pollution, effects of parasites and diseases, and perhaps overfishing (Parrish et al. 1998; see Mather 1998). Furthermore, it has been observed that surface temperatures in the ocean have declined, and effects of this has been suggested to increase post-smolt mortality (Friedland et al. 1993; Reddin and Friedland 1993; Friedland et al. 1998). Although more information about Atlantic salmon in the marine phase has been gained in recent years (e.g. Mills 1993), still little is known about the food and feeding habits of salmon in the Northeast Atlantic (see Hislop and Shelton [1993] for a review). The few studies so far in the Faroese area and the Norwegian Sea mainly give a qualitative assessment of the importance of the prey species for salmon (Struthers 1970; Struthers 1971; Thurow 1973; Hislop and Youngson 1984; Hansen and Pethon 1985). Salmon probably spend most of its time pelagically in the ocean close to the sea surface (Templeman 1967; Reddin 1985; Dutil and Coutu 1988) preying on different pelagic animals such as fish, crustaceans and squids. The seaward migration of young salmon post-smolts occurs in spring. The salmon spend normally one to three years, occasionally up to five years in the open ocean. One important feeding area for salmon in the Northeast Atlantic during the autumn and winter months is in the central and southern Norwegian Sea, i.e. the area to the north of the Faroe Islands. This area is characterised by a front that separates the warmer Atlantic water from south-west and the colder and less saline Arctic water from northwest (Hansen 1985). Salmon are typically distributed in or close to the frontal areas (Jákupsstovu 1988). Due to the large fish farming effort in the Northeast Atlantic, significant numbers of fish farm escapees have been observed mingled with the wild salmon in oceanic waters north of the Faroes (Hansen et al. 1993; 1999). The fate of the escaped salmon is poorly known, and we do not know how well escaped farmed salmon adapt to the marine environment. Several authors have suggested that Atlantic salmon are opportunistic feeders (Hansen and Pethon 1985; Reddin 1988; Pearcy 1992; Hislop and Shelton 1993;
114 Jacobsen & Hansen 3 Sturlaugsson 1994). However, in the Atlantic there is no information that compares the diet of salmon with the potential prey available. Although information from literature on plankton and micronekton distributions in the Northeast Atlantic (e.g. Dunbar 1964; Dalpadado et al. 1998) might give a clue to the potential prey of salmon, the nonoverlapping temporal and spatial nature of the data prevents any conclusion on prey selection. So far the feeding behaviour and forage strategy of Atlantic salmon has only been inferred from indirect measures such as stomach analysis. In this paper we tested the hypothesis that Atlantic salmon that escape from fish farms adapt to the marine environment as do wild fish. This was done by comparing the qualitative and quantitative feeding habits of wild and escaped farmed salmon during autumn and winter in the sea north of the Faroes. Furthermore, we tested if there were differences in stomach contents among fish of different sea ages, and between seasons. Finally we tested whether Atlantic salmon are selective or opportunistic feeders by comparing stomach contents with available prey. Materials and methods Sampling Atlantic salmon were caught in an experimental fishery with floating long-lines in the Norwegian Sea (north of the Faroes) between N and 1 10 W (Fig. 1). Sampling took place during the autumn (November-December) and winter (February- March) fishery in the three fishing periods 1992/1993, 1993/1994 and 1994/1995. The experimental fishery followed the general pattern of the commercial salmon fisheries, which usually starts in November in the north-western area relatively close to the Faroes, and as the season progresses the fishery moves gradually in an north-eastern direction (Fig. 1). Normally there is a period of one to two months (late December to mid February) separating the autumn and winter fishery when fishing is difficult due to bad weather. The long-lines (2000 hooks baited with sprat) were set early in the morning before dusk. Hauling started approximately at noon and was completed between 5 and 10 hours later, depending on the weather conditions and complications, such as breaking of the line. Usually the first 50 salmon caught from each set were sampled for stomach
115 Jacobsen & Hansen 4 analysis. The sea-surface temperature (SST) was measured four times a day, before and after setting and hauling of the long-line, respectively. There was no indication of that salmon regurgitates after capture. This cannot be completely verified, but neither the scientific staff onboard the research vessel nor the salmon fishermen noted any remains of stomach content on deck or in the water when hauling the line. From catches in a total of 106 fishing sets 3855 stomachs were collected (Table 1), and they were removed and frozen immediately after capture. Fork lengths and gutted weights were measured and a scale sample was collected from each fish. On 13 fishing locations concurrent plankton samples were obtained (Fig. 1, encircled points) using a modified Isaackid midwater trawl (MIK), which is a framed 2 m diameter plankton net with 2.5 mm meshes in the foremost 11 m and 0.5 mm in the hindmost 2 m. The net was towed with a speed of 2.5 knots for 10 minutes at three depths, 5 m, 25 m, and 50 m, respectively. This sampling was carried out in the morning (about and h a.m.) just after the setting of long-lines were completed, except on the first station when the sample was taken at h. Wild and farmed salmon and sea age Scales were used to identify if the fish was of wild or farmed origin (Lund and Hansen 1991). Salmon during its first winter in sea is termed one sea-winter (1SW) salmon. Salmon during its second winter in sea is termed two sea-winter (2SW) salmon, and similarly for 3SW and older salmon (Fig. 2). Scale samples from a total of 1112 (37%) wild and 369 (43%) farmed salmon were available. When no scale samples were available the fish was determined to be of wild or farmed origin by examining the presence of external characters like fin erosion which is common on reared salmon (Lund et al. 1989). The sea age of those fish was estimated from the material with sea age calculated from scales using the following simple discrimination algorithm. A length (length-split) was chosen from the known sample that separated the 1 and 2SW and 2 and 3+SW (3SW and older) wild salmon most efficiently, i.e. with lowest misclassification rate (Fig. 2). The estimated length-split between 1 and 2SW fish was 57 cm and between 2 and 3+SW fish 81 cm. The total misclassification was 6% (67 out of 1112 wild salmon). For farmed fish, however, the same rules could not be used with same efficiency due to considerable overlap in length at various sea ages (Fig. 2). It was therefore decided to use the length splits obtained from the wild salmon. In doing so, the
116 Jacobsen & Hansen 5 number of 1SW fish was underestimated and the number of 2 and 3+SW salmon was overestimated (Fig. 2) with 22.5% misclassification (83 out of 369 farmed salmon). The relatively large discrepancy in the sea age of the escaped farmed fish is mainly due to the variable size at time of escape from sea cages at the coast and the fact that reared salmon is larger at-age than wild salmon due to the high growth rate under rearing conditions. Furthermore, the age of the farmed fish tend to be overestimated due to marks or checks in the scales being erroneously interpreted as winter bands (Lund et al. 1989). The decision to use the same length splits for both wild and farmed salmon, however, facilitates comparisons between different sea age groups in the analyses. Stomach analysis The identifiable stomach content was separated on species, measured and weighted individually if possible, or divided into length groups, which were weighted and counted. The remaining food items were grouped by the degree of digestion to genus, family or taxon, leaving the most digested material impossible to identify either to fish remains, crustacea remains or organic remains. For quantitative analyses of the feeding habits of salmon, it is important that the number and corresponding weight of each prey species or prey group is available, to enable calculations of relative abundances. In 9% of the cases only weights or lengths of prey were recorded, in those cases were corresponding mean weights or mean lengths, respectively, used of prey from less digested stomachs in the same set. In the numerical analyses (i.e. diet overlap) stomachs containing remains were excluded, and judging from the frequency of the unidentified matter (2-13%, mean 6% of number and 5-33%, mean 16% of weight) in the various categories in the analyses, no one data set was likely to be more affected than the other. It is thus assumed that the total prey species distribution in the stomachs containing remains is similar to those stomachs containing only identifiable material. For statistical analyses the data were grouped by several categories: season (autumn and winter), type of fish (wild and farmed) and sea age (1, 2, and 3+SW salmon). We further performed analyses by year (actually by fishing period lasting from November to March) where data permitted such analyses, and unless otherwise stated all three years were pooled. Sprats that were obviously baits were observed in nearly half of the stomachs, and some salmon had taken more than one sprat. Furthermore, bird feathers and different
117 Jacobsen & Hansen 6 inorganic material, such as nylon gut, sheets of plastic, dry paint etc. were also present in a few stomachs. Stomachs that contained bait, feathers or inorganic material only were considered as being empty. Condition The condition factor (K) for each category to be compared was calculated as K = 10 5 w/l 3, w is gutted weight in kg and l is forklength in cm. Data were log transformed prior to ANOVA to normalise the data. Feeding intensity and diet overlap The feeding intensity was examined by use of the proportion of empty stomachs to the total number of stomachs examined, as well as by the ratio of the average stomach content of prey (g) to body weight (g 10 3 ), the data were log-transformed to ensure normality. Relative abundance of each species was studied by use of three frequently used measures: (1) the percentage frequency of occurrence (%F), based on the number of stomachs in which each food item occurred in relation to the total number of stomachs sampled (including empty stomachs), (2) the percentage in number (%N) of each prey item in all stomachs in a sample, and (3) the weight percentages (%W) of each prey item in all stomachs in a sample. The intraspecific diet overlap between wild and farmed salmon, among sea ages of salmon, and between seasons was investigated using the simplified Morisita overlap index (Horn 1966) between predator j and k, C H = 2 p ij p ik /( p 2 ij + p 2 ik ), where p ij and p ik are the proportions (number or weight) of prey species i in the stomach contents of the predator j and k, respectively. The diet overlap was estimated for several pairs of predator groups, dependent on the problems addressed, i.e. between wild and farmed salmon, between sea ages, between autumn and winter samples. To assess the degree of diet overlap, the prey species (i) were grouped into 10 ecological meaningful prey groups based on taxonomic adherence and size: 1 = amphipods, 2 = euphausiids, 3 = shrimps, 4 = other crustaceans, 5 = silverside, 6 = lanternfishes, 7 = other small fish, 8 = barracudinas, 9 = other large fish, and 10 = squids. The overlap indices are critically dependent on the taxonomic resolution of the prey species groups (Krebs 1989; Hansson et al. 1996). High degree of pooling gives a high index and vice versa. Because of this sensitivity, we do not present statistical
118 Jacobsen & Hansen 7 significance of the results, but use them for descriptive purposes and give a qualitative discussion of the results. However, Zaret and Rand (1971) considered values larger than 0.6 biologically significant, Langeton (1982) classified the values of the percentage overlap index as low (<30%), medium (30-60%), or high (>60%), and Pearcy et al. (1988) considered values larger than 75% as high. These classifications where, however, not based on statistical considerations. Prey availability and prey selection The simplified Morisita overlap index was used to compare the MIK plankton samples and the salmon stomach samples. However, due to avoidance problems of large fish such as herring, barracudinas and the larger capelin (>50 mm) from the MIK plankton sampler, these groups were excluded from the comparative analysis, as well as jellyfish and remains of fish and crustaceans, that could not be enumerated. Results Of the 3855 stomachs collected, 1176 (31%) were empty or contained only bait, unidentified or inorganic material. On average only half of the stomachs sampled in the autumn (53%) contained food while during winter 78% contained food. This difference was consistent in the three fishing periods. The frequency of farmed fish containing food was significantly higher than in wild fish in winter samples: farmed 85% and wild 76% (χ 2 = 18.74, df = 1, p < 0.001), but were marginally non-significant in the autumn samples: farmed 57% and wild 51% (p = 0.057). There was no conclusive trend with age in the proportion of empty stomachs. The dietary importance of different food by seasons of wild and farmed salmon is shown in Tables 2 and 3. The most important crustaceans were amphipods (Themisto libellula, T. compressa, T. abyssorum and Eusirus holmi), shrimps (Hymenodora glacialis) and krill (Meganyctiphanes norvegica and Thysanoessa inermis). The most important fishes were pearlside (Maurolicus muelleri), lanternfishes (Benthosema glaciale and Notoscopelus kroeyeri), barracudinas (Notolepis rissoi kroyeri, Paralepis coregonoides borealis), blue whiting (Micromesistius poutassou), herring (Clupea harengus), capelin (Mallotus villosus), and mackerel (Scomber scombrus). Apparently
119 Jacobsen & Hansen 8 there was no difference in the prey species composition between wild and farmed salmon. The abundance of the various prey species differed between seasons, but were also highly dependent on whether frequency of occurrence, number or weight percentages were calculated. The occurrence and numerical representations favour the importance of numerous small and frequently occurring prey (e.g. crustaceans) whereas the weight representation favour the larger and often infrequent prey such as fish. Crustaceans including Themisto spp., euphausiids and pelagic shrimps accounted for 95% of the food in number, but by weight to about 30%. By weight 66% of the stomach content was fish, particularly mesopelagic fish such as lanternfishes, pearlsides, barracudinas, and silverside, corresponding to only 5% in number. Some larger pelagic fish such as herring, blue whiting and mackerel were also present (0.1% by number and 13% by weight). Fish and crustacean prey accounted for 96% of the weight of all prey taken, the remainder was equally divided by unidentified organic remains and squid. These figures were similar for wild and farmed salmon (Tables 2 and 3). Condition The multiple ANOVA of K-factor on season (autumn and winter), type (wild or farmed) and sea age (1, 2, and 3+SW) resulted in season and sea age being highly significant, with no difference in condition between wild and escaped farmed salmon during the feeding phase in the sea (Table 4). The significant difference between the age groups was due to a large increase in the K-factor from the smaller 1 and 2SW salmon (which were not significantly different) to the larger 3+SW fish (by comparison of the overlap of the 95% confidence intervals, Fig. 3). Both 1 and 2SW salmon had significantly higher K-factor in autumn than in winter, but the condition of 3+SW salmon did not depend on season (Fig. 3). Food content From a regression analysis with log transformed data of the non-empty stomachs the average food content, s (g), was found to be proportional to the length, l (cm), of the fish, s = l (r 2 = 0.58, p < 0.001, Fig. 4). The value of the allometric exponent (2.238) suggests that neither the weight nor length in third power would have standardised the data with respect to size of the fish. The resulting exponent would be negative and produce a hyperbolic decreasing trend with fish size. In our case since the
120 Jacobsen & Hansen 9 weight (g) of the salmon is proportional to the length of the fish as w = l 3.22 (r 2 = 0.95, p < 0.001), the stomach content/body weight ratio as a function of fish length would be s/w = l , or approximately inversely related to fish length. Thus all quantitative of comparisons stomach content were restricted to length or age groups to avoid ontogenetic dependencies. Quantitative differences in stomach contents were observed between autumn and winter. A separate ANOVA by sea age of the logarithm of the stomach content/body weight ratio, ln(s/w), on season and type of salmon (Fig. 5), showed no difference between wild and farmed salmon, but significantly higher ratio in the winter than in the autumn for age group 1 (1.966 vs , F 1,276 = 26.0, p < 0.001) and 2 (1.053 vs , F 1,1913 = 162.3, p < 0.001), but was marginally insignificant for 3+SW salmon (0.416 vs , F 1,478 = 3.5, p = 0.060). It seems that the feeding rate of the larger 3+SW salmon is less dependent on season than for the smaller salmon. The increase in the average food content per salmon from autumn to winter was mainly due to a relative increase of mesopelagic fish (lanternfishes, silverside and barracudinas) in the diet (Table 2 and 3). Bait Of the 3855 salmon caught and analysed, sprats that were obviously baits were observed in 1554 (40%) stomachs, 1164 (30%) wild and 390 (10%) farmed salmon. Of these 114 (3%) salmon, 78 (2%) wild and 36 (1%) farmed, had taken more than one sprat, and even a few salmon had taken up to four baits prior to capture. We anticipated that the frequency of fish that contained bait versus no bait when caught would be independent of season, type of fish and size or age of fish. However, there were significantly higher proportion of salmon that contained bait during winter (43%) than during autumn (35%) (χ 2 = 27.3, df = 1, p < 0.001), as well as significantly higher proportion of farmed salmon (45%) containing bait compared to wild salmon (39%) (χ 2 = 11.0, df = 1, p < 0.001). There were also significant differences in the proportions that contained bait versus no bait among sea ages of salmon (χ 2 = 30.0, df = 2, p < 0.001), both during autumn and winter and for wild and farmed salmon. Bonferroni adjusted posteriori tests showed that the proportion of the smaller 1SW (22%) salmon containing bait was significantly lower than for the larger 2 (41%) and 3+SW (52%) salmon, irrespective of season and type of fish (χ 2 = 77.2, df = 1, p < 0.001). The 2 and 3+SW salmon were not significantly different (average 43%).
121 Jacobsen & Hansen 10 The proportion of salmon that had taken two or more baits prior to capture versus those containing only one bait was significantly higher during winter (9%) than during the autumn (3%) (χ 2 = 17.4, df = 1, p < 0.001), and this difference was highly significant for wild salmon: winter (2%) and autumn (8%) (χ 2 = 15.1, df = 1, p < 0.001), although the same tendency was observed for farmed salmon, the results were non-significant: winter (5%) and autumn (11%) (p = 0.064). No differences were observed among sea ages of salmon in this respect. Prey type versus sea-surface temperature Seasonal differences in temperature regimes were observed between the autumn and wintering feeding areas (Fig. 1), with a shift in the ambient sea-surface temperature (SST±SD) at the fishing locations (sets) from 7ºC ± 1.6ºC (n = 48) in the autumn to 3ºC ± 1.3ºC (n = 70) in the winter. The species composition of Themisto libellula and T. compressa f. compressa (the 'short-legged' form of T. compressa [Schneppenheim & Weigmann-Haas 1986], a phenotype developing in warmer water than the 'long-legged' form [Sheader 1975]), two of the three hyperiid amphipods of the genus Themisto observed as prey, showed an inverse relationship of abundance (number and weight) in the stomachs of both wild (Table 2) and farmed salmon (Table 3). When T. compressa f. compressa is present in large numbers, then the number of T. libellula is low and vice versa. Linear regressions of the natural logarithm of prey weight percentages of T. libellula and T. c. f. compressa, respectively on ambient SST (Fig. 6), showed that the abundance of T. libellula was significantly positively related to ambient SST (F 1,63 = 14.8, r 2 = 0.196, p < 0.001) and the abundance of T. c. f. compressa were significantly negatively related to ambient SST (F 1,43 = 21.6, r 2 = 0.315, p < 0.001). Thus, of the amphipods T. libellula dominated in the stomachs of salmon caught in the colder areas or periods while T. c. f. compressa dominated in salmon from the warmer areas. Diet overlap There was no difference in the food content between wild and farmed salmon, the simplified Morisita overlap index was 0.995, indicating a very high degree of overlap in choice of prey species or prey species groups. The high overlap was also evident between seasons (autumn, and winter, 0.991) and among sea age groups 1, 2 and
122 Jacobsen & Hansen 11 3+SW (0.876, 0.984, and 0.981, respectively). Thus we pooled wild and farmed salmon in the further analyses. The seasonal index was 0.584, indicating a moderately low overlap in the stomach content between autumn and winter. The seasonal overlap within each age group was 0.706, 0.527, and for sea age 1, 2 and 3+SW, respectively, indicating that the differences between seasons was mainly due to 2SW salmon, which also is the main age group caught (72%). The seasonal differences in prey (Fig. 7) show that mainly amphipods and the group "Other large fish" were eaten during autumn, while the contribution to the weight percentages were more evenly distributed by lanternfishes, barracudinas, silverside, "Other large fish" and amphipods during winter. A closer examination of the seasonal differences of the large fish prey, i.e. the groups barracudinas and "Other large fish" show that mainly blue whiting, Micromesistius poutassou, and to a lesser extent herring, Clupea harengus, mackerel, Scomber scombrus, and Paralepidae (Table 2 and 3) were eaten during autumn, while mainly Paralepidae and herring, and some fry of capelin, Mallotus villosus were eaten during winter (Fig. 8). Although the contribution in weight is high of the larger fish prey, only 136 salmon (3.5%) had taken those prey. The 1 and 2SW salmon had a relatively high diet overlap (0.764). However, the moderate diet overlap between 2 and 3+SW salmon (0.648) and the low overlap between 1 and 3+SW (0.359) indicated a shift in prey composition among age (size) groups of salmon. The smaller 1SW salmon had mainly eaten amphipods and lanternfishes, and some barracudinas, while the larger 3+SW salmon had mainly eaten large fish, barracudinas and lanternfishes, and the 2SW salmon had eaten the various prey groups in intermediate proportions compared to the 1SW and 3+SW salmon (Fig. 9). The larger fish prey was thus preferred with increasing age (size) of the predator to smaller mesopelagic fish and crustaceans. The age distribution of salmon containing large prey (3% 1SW, 66% 2SW, and 31% 3+SW) was significantly larger than for the remaining fish (11% 1SW, 72% 2SW, and 17% 3+SW) (χ 2, df = 2, p < 0.001), the difference between each proportion within each age was significant (χ 2 tests adjusted for multiple comparisons). The reported age specific changes towards fish with increasing age were evident both in the autumn and winter season.
123 Jacobsen & Hansen 12 Prey size to fish size The relationships between average prey size and fish size for Themisto libellula, Meganyctiphanes norvegica, Hymenodora glacialis and Maurolicus muelleri were examined. Generally the average prey size in the stomachs did not depend on fish size, except for T. libellula, where a significant positive relationship was observed (r 2 = 0.039, df = 426, p < 0.001). The prey length data of T. libellula were transformed to natural logarithms to assure that they were normally distributed. However, the regression had a very low explanatory value (4%). Prey availability and prey selection In total 319 non-empty salmon stomachs were observed at the 13 fishing stations where corresponding MIK plankton samples were available. The abundance of prey species and their weight percentages show that the plankton tows generally included the same species as found in the stomachs, i.e. the hyperiid amphipods, euphausiids, shrimps, lanternfishes and pearlsides (Table 5). However, Sagitta spp. and a few individuals of the small copepod, Calanus finmarchius, that were observed in the plankton samples, were absent from the stomachs. On the other hand, large fish such as herring, barracudinas and the larger capelin (>50 mm) found in the salmon stomachs were not caught with the net probably due to avoidance. The data set for calculating overlap index was thus limited by excluding larger fish as well as jellyfish and remains of fish and crustaceans that could not be enumerated (Table 5). Although most of the species occurred in both data sets, their relative proportion differed greatly resulting in a low overlap index (0.3). Atlantic salmon appeared to select small pelagic fish to crustaceans, and furthermore to prefer amphipods (Themisto spp.) to euphausiids, although the availability of the euphausiids was significantly higher as indicated from the plankton samples (Table 5). On average the salmon had taken twice as much amphipods than euphausiids while the plankton sampler caught 13 times more euphausiids than amphipods. There were also great variations and low overlap between the two prey groups at the individual sampling stations, but the preference of amphipods to euphausiids was also evident at sampling station level (Fig. 10). Of the euphausiids, it seems as salmon prefer the larger Meganyctiphanes norvegica to the smaller Thysanoessa longicaudata and T. inermis, suggesting size selective feeding (Fig. 10). To further explore possible selective feeding behaviour by salmon, the percentage length distribution of six species or groups were studied in more detail (Fig. 11a-f). The
124 Jacobsen & Hansen 13 pearlside, Maurolicus müelleri (Fig. 11a); the amphipods Themisto libellula (Fig. 11b), T. compressa, including both forms, i.e. T. c. f compressa and T. c. f bispinosa (Fig. 11d), and T. abyssorum (Fig. 11e); the Euphausiids Meganyctiphanes norvegica (Fig. 11e), and Thysanoessa spp., including both Thysanoessa inermis and Thysanoessa longicaudata (Fig. 11a). These groups were chosen due to available length distributions in both the plankton and the stomach samples. For four of the six groups the size distribution of the prey eaten by salmon was larger than that of the animals caught in the corresponding plankton samples. This was especially true for the M. müelleri, where the 1 and 2+ groups were eaten by salmon but only the 1 groups was caught in the plankton net (Fig. 11a). Of the amphipods there was an almost perfect overlap between the samples, i.e. Themisto libellula and T. compressa, respectively (Fig. 11b-c), but the salmon had taken exclusively larger T. abyssorum than were caught in the environment (Fig. 11d). Similarly the euphausiids eaten were larger than those caught in the plankton net (Fig. 11e-f). Discussion Stomach contents Oceanic waters north of the Faroes and in the southern Norwegian Sea are important feeding areas for salmon in the Northeast Atlantic during winter (Jákupsstovu 1988), and in October/November the first 1SW salmon appear in the long-line catches north off the isles. However, salmon in their second winter at sea (2SW) are the dominating component in this fishery. The results of the present study revealed that crustaceans including Themisto spp., euphausiids and pelagic shrimps accounted for more than 95% of the food in number, but by weight 66% of the stomach content was fish, particularly lantern fishes, pearlsides, barracudinas, and silversides. Some larger pelagic fish such as herring, blue whiting and mackerel were also present (0.1% by number and 13% by weight). Hislop and Shelton (1993) proposed that the crustaceans were a much less important prey than fish in the North Atlantic. However, the extensive material studied here places more emphasis on crustacean prey in the Northeast Atlantic, particularly the hyperiid amphipods and to a lesser extent euphausiids as the average prey in salmon beside mesopelagic fishes. Especially in the autumn salmon seemed to rely on amphipods as
125 Jacobsen & Hansen 14 food. This is in contrast to the food of adult fish in coastal areas returning to spawn (Blair 1965; Grønvik and Klemetsen 1987; Hislop and Webb 1992), where fish totally dominate, however with one notable exception where crustaceans were the main food (Neilson and Gillis 1979). Condition It appears that the condition factor (K-factor) for salmon in the sea is relatively high, especially for 3+SW salmon, and is comparable to salmon in e.g. West Greenland waters (Lear 1972; Munro and Swain 1980), but higher than those observed by Dwyer and Piper (1987). The higher condition during autumn than in winter is consistent with the observations made by Dwyer and Piper (1987), although the level of the predicted K-factors around 0.82 and 0.85 with ambient temperatures of 3 and 7 C, respectively (Dwyer and Piper 1987), were lower than our estimates of 0.9 and 0.94 respectively, for the same temperature ranges. The significantly higher condition of 3+SW salmon than of the smaller salmon, irrespective of season, might be a reflection of higher tolerance (due to its size) to low temperatures, greater potential to forage over a wide area and improved capture success of various prey available. In addition, the feeding rate (stomach content/body weight ratio) of the larger 3+SW salmon is more independent of season than for the smaller salmon. Since about 90% of the salmon present in the area north of the Faroes were estimated to mature, irrespective of age (ICES 1984; Youngson and McLay 1985; Jákupsstovu 1988), most of the fish will undertake the homeward migration sometime during winter of spring. Thorpe (1994) and Kadri et al. (1995; 1997) indicated that in maturing salmon a certain threshold level of energy reserves have to be achieved at a certain date for the fish to mature. Because salmon feed less during homing migration, we suggest that the large fish have stored sufficient energy earlier in the season than younger fish, due to the observation that older individuals home earlier in the season than younger fish (Hawkins 1987; Jonsson et al. 1990). Feeding intensity On the assumption that the proportion of empty stomachs is indicative of the intensity of feeding (Rae 1967; Bowman and Bowman 1980), then salmon feed more intensively in winter than in autumn, when nearly half of the stomachs were empty. Of the stomachs containing food, the feeding rate in weight increased from autumn to
126 Jacobsen & Hansen 15 winter for the 1 and 2SW fish, but not for 3+SW salmon. The increase was mainly due to higher proportions of fish in the diet in winter, particularly of mesopelagic fish. The apparent low feeding rate in late autumn could be an indication of reduced food availability in the sea during this period. In comparison salmon sampled in the Labrador Sea (Lear 1980) had less food in their stomachs in the autumn than in the spring (3.1 g and 5.7 g food per kg of salmon, respectively) and were feeding less actively (28% and 8% empty stomachs, respectively). Lower feeding rates during winter were also observed in the Baltic (Christensen 1961; Thurow 1966). The higher condition factor of salmon in the autumn than winter seems to contradict the statement above on lower food availability during autumn. The explanation to the apparent ambiguity might be found in the fact that the gastric evacuation rate is highly dependent on temperature (Dos Santos and Jobling 1991). The lower rate of emptying of the stomach during winter caused by lower ambient temperature would give the impression that fewer stomachs were empty and, that the non-empty stomachs on average would contain more food due to slow digestion. We observed a significantly lower proportion of the salmon containing bait during autumn than in winter, and similarly a significantly lower proportion of salmon that had taken two or more baits prior to capture compared to only one bait during autumn than in winter. However, the biological significance of these observations is not clear, but might indicate that the fish have greater appetite during the winter. The observation that a higher proportion of escaped farmed fish containing bait than wild salmon is difficult to explain. The probability that salmon take the bait depends on whether the bait and hook was approached and swallowed in one bout or the capture was a result of hooking without swallowing. If the feeding behaviour of the escaped farmed salmon was different, e.g. due to its farmed prehistory, it might result in different behaviour when approaching the bait. Alternatively farmed salmon have been selected for fast and efficient growth. This may have resulted in a higher demand for energy (Thodesen et al. 1999) and thus made farmed salmon a more aggressive feeder. If the escaped farmed fish behave different compared to wild salmon in the sea, either having problems to adapt to the wild or being more eager to take food due to their farmed prehistory, it could be expected that the condition factor of two groups were different. However, no difference in condition was observed between wild and escaped farmed salmon during the feeding phase in the sea. Neither were any differences observed in frequency, number or weight proportions of prey between wild and farmed
127 Jacobsen & Hansen 16 salmon, nor in diet overlap. Furthermore, a higher proportion of farmed fish contained food than wild fish. This strongly suggests that fish farm escapees that survived until capture have adapted to feed in the oceanic environment. Farmed salmon caught in Scottish coastal waters have also been observed to feed on natural prey (Hislop and Webb 1992). Reiriz et al. (1998) found that naive hatcheryreared juvenile Atlantic salmon parr exposed to natural prey had a fast learning response towards novel prey so it matched the efficiency of wild-caught fish. The fast learning response would allow fish to maintain a high foraging efficiency when faced with frequent changes in the availability of different prey types (Fisher and Pearcy 1996; Orr and Bowering 1997; Cortés 1997; Andersen 1999). Prey availability and stomach content The comparative material from the salmon stomachs and the plankton samples might contain several potential sources of bias, including avoidance from the towed net sampler, limiting sampling range (0-50 m) and uncertainty in the interpretation of gut samples on how accurately they represent relative abundance of prey as consumed (Kohler and Ney 1982). The validity of the plankton samples as to be representative of prey availability could not be addressed. However, all but one net sample was taken in the morning between 07 and 08 h, after setting of the line and before hauling, thus minimising possible diurnal variations in the material and further ensuring that the plankton samples were obtained while the long-line was fishing. However, not necessarily when the salmon was feeding. Although the large fish species such as herring, barracudinas and the larger capelin (>50 mm) were excluded prior to the comparative analysis, the resulting overlap index was low (0.3), which might indicate at least a partly selective feeding strategy of salmon. Though possible, we tend to rule out that avoidance from the net could have caused the observed differences in the proportions between the net and the stomachs. Judging from the fact that pearlside up to 40 mm and capelin fry (40-50 mm) were caught in the plankton net, the avoidance of crustaceans (mainly < 30 mm) from the net should be negligible. We further assume no difference in avoidance behaviour between amphipods and euphausiids of similar size. Other researchers have reported avoidance of large zooplankton and fish (> 45 mm) from the MIK plankton net sampler and similar sampling devices (Munk 1988; Dalpadado et al. 1998).
128 Jacobsen & Hansen 17 Even if most of the species occurred in both data sets, their relative proportion differed greatly. Our results suggest that fish were preferred over crustaceans, of crustaceans the amphipods were preferred over euphausiids, and of the three species of euphausiids the larger Meganyctiphanes norvegica was preferred over the smaller Thysanoessa inermis and Th. longicaudata. Furthermore, within each of the three euphausiids species, the prey ingested was larger than from the habitat samples. The preference of fish to crustaceans, and of the larger M. norvegica over Thysanoessa spp. can be explained by size selective feeding of salmon. However, the preference of amphipods to euphausiids seems more subtle, as their sizes are comparable in the plankton samples, although in the stomachs the euphausiids were larger than the amphipods. The energetic content of euphausiids and amphipods might be different as well as their visual contrast in the sea, in particular the large and heavily pigmented compound eye of amphipods may make them more conspicuous (Zaret and Kerfoot 1975), or their swimming and predatory escape behaviour might be different. It further seems that although there are numerous Thysanoessa spp. available in the upper 50 m from the plankton data, this genus is hardly preyed upon by salmon. In the Pacific, Peterson et al. (1982) also found a preference for amphipods to similar sized stages of a copepod of juvenile coho (Oncorhynchus kisutch) and chinook salmon (Oncorhynchus tshawytscha) off the Oregon coast, and Brodeur et al. (1992) estimated that the consumption of euphausiids as percentage of total biomass was quite low (0.01% d -1 ) by coho and chinook, although the abundance of euphausiids from plankton tows were high. Holst et al. (1996) found a tendency of size-selective feeding of salmon post-smolts of the hyperiid amphipods (Themisto spp.), as indicated by a positive relation between prey size in the stomachs and post-smolts size. This corresponds with our findings of a weak, but significant correlation between length of salmon and length of Themisto libellula prey. Furthermore, Holst et al. (1996) noted that given that O-group fish were available in the area as observed from the trawl catches and that amphipods also were available from parallel plankton sampling, the post-smolts fed mainly on O-group fish, indicating a selective feeding strategy. Selective feeding was also reported by Lear (1972) in the Northwest Atlantic, where salmon preferred herring to capelin, and the large salmon contained significantly more herring than small salmon.
129 Jacobsen & Hansen 18 Salmon did not feed on Sagitta spp., although it has been reported as food for salmon in the Pacific (Brodeur and Pearcy 1990; Tadokoro et al. 1996). It might be speculated that Sagitta spp. either is too transparent, unpalatable, or is a low energy prey. At least two year classes (1 and 2+ group) of pearlside (Maurolicus müelleri) were present in the salmon stomachs, however, only the smaller 1 group was present in the plankton samples, apparently indicating size selective feeding. Alternatively, the larger 2+ group of pearlside might avoid the plankton sampler or being distributed below the sampling depth of 50 m. The pearlside has been reported in some areas to be separated into two vertical layers in the sea during winter, with the older individuals occupying the lower layer (Goodson et al. 1995) and are reported to be most numerous below 200 m depth in the Norwegian Sea (Dalpadado et al. 1998). Thus salmon probably also feed deeper than 50 m depth. Salmon are generally found to inhabit the upper surface layers (Templeman 1967; Reddin 1985; Dutil and Coutu 1988) most of the time with occasional deep ascents for shorter or longer time periods, deeper that 150 m (Jákupsstovu 1988). Pacific salmon also occasionally feed deeper than m during day (Pearcy et al. 1988). A shift in prey composition among age (size) groups of salmon was also indicated, the smaller 1SW salmon had taken higher proportions of amphipods compared to the larger 2+SW fish, while the proportion of fish prey, particularly barracudinas and other large fish increased with age, indicating a higher degree of piscivory with age. The tendency that the warmwater phenotype, Themisto compressa f. compressa (Sheader 1975) was more abundant in salmon stomachs then the T. libellula (considered an arctic or subarctic species) in warmer water and vice versa in colder water may lend support to the hypothesis that salmon are opportunistic feeder in the sense of a capability to feed on whatever prey organisms were available. However, the relatively low overlap between salmon diet and the available prey in our study, and the preference of amphipods to euphausiids and of the larger Meganyctiphanes norvegica to the smaller Thysanoessa spp. (both euphausiids), seems to render salmon as a selective feeder. We conclude that crustaceans and particularly the hyperiid amphipods of the genus Themisto, euphausiids and mesopelagic shrimps are important sources of food for salmon in the autumn period and equally important becomes the different mesopelagic fish as lantern fishes, pearlsides and barracudinas during the late winter period. The occasional presence of larger fish in the stomachs, such as herring, blue whiting and
130 Jacobsen & Hansen 19 mackerel is not considered as a main source of food for salmon in the sea. We were not able to assess whether food is a limiting factor for salmon in the sea. We suggest that salmon feed opportunistically, however, selecting the larger size range of available prey. Finally we conclude that escaped farmed salmon that survive until capture adapt well to the "wild" life in the ocean. Acknowledgements Sincere thanks to the skipper and crew on the salmon longliner M/S Hvítiklettur and to S. Lamhauge, E. Poulsen, R. Mourtisen and A. Hendriksen for their spirit and enthusiasm in data collection and analysis of the stomach material. The help from the staff at the Fishery Laboratory of the Faroes is very much appreciated. The scales were kindly read by G. Østborg, Norwegian Institute for Nature Research. The research was funded jointly by the Nordic Council of Ministers, the Norwegian Directorate for Nature Management and the Faroese Home government. The first author was in receipt of a personal grant from "Granskingarráð Føroya", "Vísindagrunnir Føroya Sparikassa" and "Minningargrunnur Dánjal Niclasen" during autumn References Andersen, N.G The effects of predator size, temperature, and prey characteristics on gastric evacuation in whiting. J. Fish Biol. 54: Blair, A.A Bay of Islands and Humber River Atlantic salmon investigations. J. Fish. Res. Bd Can. 22: Bowman, R.E., and Bowman, E.W Diurnal variation in the feeding intensity and catchability of silver hake (Merluccius bilinearis). Can. J. Fish. Aquat. Sci. 37: Brodeur, R.D., Francis, R.C., and Pearcy, W.G Food consumption of juvenile coho (Oncorhynchus kisutch) and chinook salmon (O. tshawytscha) on the continental shelf off Washington and Oregon. Can. J. Fish. Aquat. Sci. 49 (8): Brodeur, R.D., and Pearcy, W.G Trophic relations of juvenile Pacific salmon off the Oregon and Washington coast. Fish. Bull. (U. S. ) 88: Christensen, O Preliminary results of an investigation on the food of Baltic salmon. ICES CM No. 93, 6 pp. Cortés, E A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Can. J. Fish. Aquat. Sci. 54: Dalpadado, P., Ellertsen, B., Melle, W., and Skjoldal, H.R Summer distribution patterns and biomass estimates of macrozooplankton and micronekton in the Nordic Seas. Sarsia 83: Dos Santos, J., and Jobling, M Factors affecting gastric evacuation in cod, Gadus morhua L., fed single-meals of natural prey. J. Fish Biol. 38 (5):
131 Jacobsen & Hansen 20 Dunbar, M.J Serial Atlas of the marine environment. American Geographical Society, New York (Folio 6): 1-2. Dutil, J.D., and Coutu, J.M Early marine life of Atlantic salmon, Salmo salar, postsmolts in the northern Gulf of St. Lawrence. Fish. Bull. (U. S. ) 86 (2): Dwyer, W.P., and Piper, R.G Atlantic salmon growth efficiency as affected by temperature. The Progressive Fish-Culturist 49: Fisher, J.P., and Pearcy, W.G Dietary overlap of juvenile fall- and spring-run chinook salmon, Oncorhynchus tshawytscha, in Coos Bay, Oregon. Fish. Bull. (U. S. ) 95: Friedland, K.D., Hansen, L.P., and Dunkley, D.A Marine temperatures experienced by postsmolts and the survival of Atlantic salmon, Salmo salar L., in the North Sea area. Fish. Oceanogr. 7 (1): Friedland, K.D., Reddin, D.G., and Kocik, J.F Marine survival of North American and European Atlantic salmon: effects of growth and environment. ICES J. mar. Sci. 50: Goodson, M.S., Giske, J., and Rosland, R Growth and ovarian development of Maurolicus muelleri during spring. Mar. Biol. 124: Grønvik, S., and Klemetsen, A Marine food and diet overlap of co-occurring Arctic charr Salvelinus alpinus (L.), brown trout Salmo trutta L. and Atlantic salmon S. salar L. off Senja, N. Norway. Polar Biol. 7 (3): Hansen, B The circulation of the northern part of the Northeast Atlantic. Rit Fiskideildar 9: Hansen, L.P., Jacobsen, J.A., and Lund, R.A High numbers of farmed Atlantic salmon, Salmo salar L., observed in oceanic waters north of the Faroe Islands. Aquacult. Fish. Manage. 24 (6): Hansen, L.P., Jacobsen, J.A., and Lund, R.A The incidence of escaped farmed Atlantic salmon, Salmo salar L., in the Faroese fishery and estimates of catches of wild salmon. ICES J. mar. Sci. 56 (2): Hansen, L.P., and Pethon, P The food of Atlantic salmon, Salmo salar L., caught by long-line in northern Norwegian waters. J. Fish Biol. 26: Hansson, S., Rudstam, L.G., Kitchell, J.F., Hildén, M., Johnson, B.L., and Peppard, P.E Predation rates by North Sea cod (Gadus morhua) - predictions from models on gastric evacuation and bioenergetics. ICES J. mar. Sci. 53: Hawkins, A.D The return migration of adult Atlantic salmon to the Aberdeenshire Dee, Scotland. American Fisheries Society Symposium 1: Hislop, J.R.G., and Shelton, R.G.J Marine predators and prey of Atlantic salmon (Salmo salar L.). In Salmon in the sea and new enhancement strategies. Edited by D. Mills. Fishing News Books, Oxford. pp Hislop, J.R.G., and Webb, J.H Escaped farmed Atlantic salmon (Salmo salar L.) feeding in Scottish coastal waters. Aquacult. Fish. Manage. 23: Hislop, J.R.G., and Youngson, A.F A note on the stomach contents of salmon caught by longline north of the Faroe Islands in March, ICES CM No. M17, 4 pp. Holst, J.C., Hansen, L.P., and Holm, M Observations of abundance, stock composition, body size and food of postsmolts of Atlantic salmon in the NE Atlantic during summer. ICES CM No. M4, 15 pp. Horn, H.S Measurement of "overlap" in comparative ecological studies. American Naturalist 100: ICES Report of the meeting of the Working Group on North Atlantic Salmon. ICES CM No. Assess16, 54 pp. ICES Report of the Working Group on North Atlantic salmon. ICES CM No. ACFM14, 288 pp. Jonsson, N., Jonsson, B., and Hansen, L.P Partial segregation in the timing of migration of Atlantic salmon of different ages. Anim. Behav. 40 (2):
132 Jacobsen & Hansen 21 Jákupsstovu, S.H.í Exploitation and migration of salmon in Faroese waters. In Atlantic Salmon: Planning for the future. Edited by D. Mills and D. Piggins. Croom Helm, London. pp Kadri, S., Metcalfe, N.B., Huntingford, F.A., and Thorpe, J.E What controls the onset of anorexia in maturing adult female Atlantic salmon? Funct. Ecol. 9 (5): Kadri, S., Thorpe, J.E., and Metcalfe, N.B Anorexia in one-sea-winter Atlantic salmon (Salmo salar) during summer, associated with sexual maturation. Aquaculture 151 (1-4): Kohler, C.C., and Ney, J.J A comparison of methods for quantitative analysis of feeding selection of fishes. Environ. Biol. Fish. 7 (4): Krebs, C.J Ecological Methodology. Harper Collins Publishers, New York. Langeton, R.W Diet overlap. Fish. Bull. (U. S. ) 80: Lear, W.H Food and feeding of Atlantic salmon in coastal and over oceanic depths. Res. Bull. int. Comm. Northw. Atlant. Fish. 9: Lear, W.H Food of Atlantic salmon in the West Greenland-Labrador Sea area. Rapp. P. - v. Réun. Cons. int. Explor. Mer 176: Lund, R.A., and Hansen, L.P Identification of reared and wild Atlantic salmon, Salmo salar L., using scale characters. Aquacult. Fish. Manage. 22: Lund, R.A., Hansen, L.P., and Järvi, T Identification of reared and wild salmon by external morphology, size of fins and scale characteristics. NINA Forskningsrapport (1): Mather, M.E The role of context-specific predation in understanding patterns exhibited by anadromous salmon. Can. J. Fish. Aquat. Sci. 55 (Supplement 1): Mills, D Salmon in the sea and new enhancement strategies. Fishing News Books, Oxford. Munk, P Catching large herring larvae: gear applicability and larval distribution. J. Cons. int. Explor. Mer. 45: Munro, W.R., and Swain, A Age, weight and length distributions and sex ratio of salmon caught off West Greenland. Rapp. P. -v. Réun. Cons. int. Explor. Mer 176: Neilson, J.D., and Gillis, D.J A note on the stomach contents of adult Atlantic salmon (Salmo salar, Linnaeus) from Port Burwell, Northwest Territories. Can. J. Zool. 57 (7): Orr, D.C., and Bowering, W.R A multivariate analysis of food and feeding trends among Greenland halibut (Reinhardtitus hippoglossoides) sampled in Davis Strait, during ICES J. mar. Sci. 54 (5): Parrish, D.L., Behnke, R.J., Gephard, S.R., McCormick, S.D., and Reeves, G.H Why aren't there more Atlantic salmon (Salmo salar)? Can. J. Fish. Aquat. Sci. 51 (Supplement 1): Pearcy, W.G Ocean ecology of North Pacific salmonids. Washington Sea Grant Program, University of Washington, Seattle. Pearcy, W.G., Brodeur, R.D., Shenker, J.M., Smoker, W.W., and Endo, Y Food habits of Pacific salmon and steelhead trout, midwater trawl catches and oceanographic conditions in the Gulf of Alaska, Bulletin of the Ocean Research Institute, University of Tokyo 26 (2): Peterson, W.T., Brodeur, R.D., and Pearcy, W.G Food habits of juvenile salmon in the Oregon coastal zone, June Fish. Bull. (U. S. ) 80 (4): Rae, B.B The food of cod on Faroese grounds. Mar. Res. Scot. 6: Reddin, D.G Atlantic salmon (Salmo salar) on and east of the Grand Bank. J. Northw. Atl. Fish. Sci. 6: Reddin, D.G Ocean life of Atlantic salmon (Salmo salar L.) in the Northwest Atlantic. In Atlantic salmon: Planning for the future. Edited by D. Mills and D. Piggins. Timber Press, Portland, Oregon. pp Reddin, D.G., and Friedland, K.D Marine environmental factors influencing the movement and survival of Atlantic salmon. In Salmon in the sea and new enhancement strategies. Edited by D. Mills. Fishing News Books, Oxford. pp
133 Jacobsen & Hansen 22 Reiriz, L., Nicieza, A.G., and Braña, F Prey selection by experienced and naive juvenile Atlantic salmon. J. Fish Biol. 53 (1): Schneppenheim, R., and Weigmann-Haas, R Morphological and Electrophoretic Studies of the Genus Themisto (Amphipoda: Hyperiidea) from the South and North Atlantic. Polar Biol. 6: Sheader, M Factors influencing change in the phenotype of the planktonic amphipod Parathemisto gauduchaudii (Guérin). J. Mar. Biol. Ass. U. K. 55: Struthers, G A report on a salmon long lining cruise off the Faroes during April, Freshwater Fisheries Laboratory, Pitlochry, Rep. (54 FW 70): 1-8. Struthers, G A report on the 1971 salmon long lining cruise off the Faroes. Freshwater Fisheries Laboratory, Pitlochry, Rep. (33 FW 71): 1-6. Sturlaugsson, J Food of ranched Atlantic salmon ( Salmo salar L.) postsmolts in coastal waters, W-Iceland. Nord. J. Freshw. Res. 69: Tadokoro, K., Ishida, Y., Davis, N.D., Ueyanagi, S., and Sugimoto, T Change in chum salmon (Oncorhynchus keta) stomach contents associated with fluctuation of pink salmon (O. gorbuscha) abundance in the central Subarctic Pacific and Bering Sea. Fish. Oceanogr. 5 (2): Templeman, W Atlantic salmon from the Labrador Sea and off West Greenland, taken during A. T. Cameron cruise, July-August Res. Bull. int. Comm. Northw. Atlant. Fish. 4: Thodesen, J., Grisdale-Helland, B., Helland, S., and Gjerde, B Feed intake, growth and feed utilization of offspring from wild and selected Atlantic salmon. Aquaculture 180: Thorpe, J.E Salmonid flexibility - responses to environmental extremes. Transactions of the American Fisheries Society 123 (4): Thurow, F Beträge zur Biologie und Bestandskunde des Atlantischen Lachses (Salmo salar L.) in der Ostsee. Ber. Dt. Wiss. Komm. Meeresforsch. 18: Thurow, F Research vessel fishing on salmon off Norway. Arch. Fisch Wiss. 24: Youngson, A.F., and McLay, H.A Changes in steroid hormone levels in blood serum as an early predictor of approaching maturity in Atlantic salmon (Salmo salar L.). ICES CM No. M17 Zaret, T.M., and Kerfoot, W.C Fish predation on Bosmina longirostris: Body-size selection versus visibility selection. Ecology 56: Zaret, T.M., and Rand, A.S Competition in tropical stream fishes: support for the competitive exclusion principle. Ecology 52:
134 Jacobsen & Hansen 23 Table 1. The salmon stomachs collected from 106 fishing sets in the Faroese research fishery during three fishing periods 1992/ /1995. The number of salmon stomachs sampled by season, by type of fish (wild or farmed), and by sea age (1, 2 and 3+ sea winters) are shown. Sea age 3+ includes some fish older that 3SW. Fishing Sea age Period Season Type 1SW 2SW 3+SW Total 1992 Autumn Farmed Wild Winter Farmed Wild Subtotal: Autumn Farmed Wild Winter Farmed Wild Subtotal: Autumn Farmed Wild Winter Farmed Wild Subtotal: Grand total:
135 Jacobsen & Hansen 24 Table 2. Diet of wild salmon: frequency of occurrence (%F, including empty stomachs), number (%N), and weight percentages (%W) of prey items in salmon by autumn (A, November- December), winter (W, February-March) and in total. The entries 0 means < 0.1%. Data were collected from 2992 wild salmon stomachs of which 961 (32%) were empty. %F %N %W Prey groups A W Total A W Total A W Total Crustaceans: Hyperiid amphipods: Themisto spp Themisto libellula Themisto compressa Themisto abyssorum Euphausiids: Euphausiidae Meganyctiphanes norvegica Thysanoessa inermis Thysanoessa longicaudata Shrimps: Hymenodora glacialis Sergestes arcticus Pasiphea tarda Other crustaceans: Paraeuchaeta norvegica Gammaridea Aristias tumidus Eusirus holmi Crustacea remains: Fishes: Silversides: Maurolicus muelleri Baracudinas: Paralepidae Notolepis rissoi kroyeri Paralepis coregonoides borealis Lanternfishes: Myctophidae Lampanyctus crocodilus Notoscopelus kroeyeri Myctophum punctatum Benthosema glaciale Other fish: Ammodyteidae Mallotus villosus Fry (mostly Mallotus villosus) Clupea harengus Micromesistius poutassou Onogadus argentatus Lycenchelys sp Scomber scombrus Belone belone Gasterosteus aculeatus Fish remains: Squid: Gonatidae Gonatus fabricii Remains organic: Birds and bird remains:
136 Jacobsen & Hansen 25 Table 3. Diet of farmed salmon: frequency of occurrence (%F, including empty stomachs), number (%N), and weight percentages (%W) of prey items in salmon by autumn (A, November- December), winter (W, February-March) and in total. The entries 0 means < 0.1%. Data were collected from 863 farmed salmon stomachs of which 215 (25%) were empty. %F %N %W Prey groups A W Total A W Total A W Total Crustaceans: Hyperiid amphipods: Themisto spp Themisto libellula Themisto compressa Themisto abyssorum Euphausiids: Euphausiidae Meganyctiphanes norvegica Thysanoessa inermis Thysanoessa longicaudata Shrimps: Hymenodora glacialis Sergestes arcticus Pasiphea tarda Other crustaceans: Paraeuchaeta norvegica Gammaridea Aristias tumidus Eusirus holmi Crustacea remains: Fishes: Silversides: Maurolicus muelleri Baracudinas: Paralepidae Notolepis rissoi kroyeri Paralepis coregonoides borealis Lanternfishes: Myctophidae Lampanyctus crocodilus Notoscopelus kroeyeri Myctophum punctatum Benthosema glaciale Other fish: Ammodyteidae Mallotus villosus Fry (mostly Mallotus villosus) Clupea harengus Micromesistius poutassou Onogadus argentatus Lycenchelys sp Scomber scombrus Belone belone Gasterosteus aculeatus Fish remains: Squid: Gonatidae Gonatus fabricii Remains organic: Birds and bird remains:
137 Jacobsen & Hansen 26 Table 4. ANOVA of K-factor (K = 10 5 w/l 3 ) for 3855 salmon grouped by season (autumn or winter), type (wild or farmed) and sea age (1, 2 or 3+SW). *** = 0.1% significance level. Source of variation Sum of Squares df Mean- Square F-ratio P Sign. Season *** Type Sea age *** Season * Type Season * Sea age Type * Sea age Error
138 Jacobsen & Hansen 27 Table 5. Total wet-weight percentages of different prey groups in the salmon stomachs and in the MIK plankton samples from 13 corresponding fishing locations. Table entries marked with a dash ( ), means that prey species/group were not found in the sample. Total wet-weights (g) of all samples are given at the bottom row in parenthesis. Remains of a crustacea and fish were found in the stomach content due to advanced digestion, while in the MIK samples the material was fresh and identifiable to species or family. To calculate the simplified Morisita overlap index between the prey species in the stomachs and in the MIK samples, the data set was reduced by excluding large fish that avoided the plankton net, and remains and jellyfish that could not be enumerated, i.e. the entries marked with a star (*) in the two rightmost columns. Species/group Category Stomachs weight % a MIK samples weight % b Stomachs Weight % MIK samples weight % Scyphosoa Jellyfish 2.17 * * Tomopteris sp. Polychaeta Bivalvia Mussels Sagitta sp. Chaetognatha Lepeophtheirus salmonis c Crustaceans Calanus finmarchicus Crustaceans Aristias tumidus Crustaceans Eusirus holmi Crustaceans Pareuchaeta norvegica Crustaceans Themisto spp. Crustaceans Ephausiidae Crustaceans Hymenodora glacialis Crustaceans Crustacea remains Crustaceans 5.62 * * Gonatus fabricii Squid Maurolicus müelleri Fish -small Myctophidae Fish -small Mallotus villosus d Fish -small Mallotus villosus d Fish -large 0.44 * * Paralepidae Fish -large 9.45 * * Clupea harengus Fish -large * * Fish remains Fish * * Total wet-weight (g) (1719) (837) (1006) (819) a Total weight % in 319 non-empty salmon stomachs (of 481 sampled) from 13 fishing locations. b Total weight % in the MIK plankton samples taken on the 13 fishing locations as above. c A few salmon lice have been found in salmon stomachs from other samples in the same area. d Only capelin fry (< 50 mm) was caught in the MIK samples while both fry and adult specimens were found in the salmon stomachs.
139 Jacobsen & Hansen 28 Fig. 1. Fishing locations where 3855 stomachs of Atlantic salmon were sampled during the autumn (November-December) and winter (February-March) fishery in 1992/1993, 1993/1994, and 1994/1995 north off the Faroes. Plankton samples were taken on 15 locations in 1994 and 1995 (MIK plankton net). The main fisheries take place in the autumn closer to the Faroes than the in the winter season (inside the stippled area). The 500 and 1000 m depth contours are shown. 65 N Iceland 1992/93 season 1993/94 season 1994/95 season MIK plankton net 1000 m 500 m Winter fishing 63 N 500 m 1000 m Autumn fishing Faroe Islands 61 N 10 W 5 W Shetland 0 E
140 Jacobsen & Hansen 29 Fig. 2. Size distribution (forklength) by sea age of wild salmon (upper panel) and escaped farmed salmon (lower panel) during 1992/ /95 fishing seasons north of the Faroes (autumn and winter samples pooled). Sea age was determined by scale readings of 1112 wild and 369 farmed salmon, respectively. Sample percentages of total number of each type are shown in brackets. 8 6 Wild, N= 1112 (37%) 1SW 2SW 3+SW % Farmed, N= 369 (43%) % Forklength (cm)
141 Jacobsen & Hansen 30 Fig. 3. K-factor (K = w10 5 /l 3 ) in 3855 salmon grouped by season (autumn or winter) and sea age (1, 2 or 3+SW). Wild and farmed salmon were pooled due to no difference in K-factor. Confidence intervals (95%) are indicated around the estimates and a star (*) under a pair of observations indicate significant differences between seasons. K-factor Autumn Winter 0.80 * * 1SW 2SW 3+SW Sea age Fig. 4. Regression of forklength on average stomach food content per cm group (log-log regression with 95% confidence bounds on the regression line). Data were obtained from 2679 non-empty salmon stomachs sampled during autumn and winter 1992/1993, 1993/1994, and 1994/1995 north off the Faroes. 4 ln (avg. stomach content) ln (forklength)
142 Jacobsen & Hansen 31 Fig. 5. Average stomach content of prey (g) 10 3 / body weight (g) ratio (s10 3 /w) by sea age 1, 2 and 3+SW, respectively, grouped by season (autumn or winter). Wild and farmed salmon were pooled as there were no differences between them. Confidence intervals (95%) are indicated around the estimates and a star (*) indicate significant differences between seasons. ln(s 10 3 /w ) Autumn Winter * * 1SW 2SW 3+SW Sea age Fig. 6. Percentage weight distribution of two hyperiid amphipods Themisto libellula and T. compressa f. compressa, respectively, in the salmon stomachs grouped by SST (sea-surface temperature) from the period The 95% confidence intervals on the regression lines are indicated. 6 T. compressa f. c T. libellula 4 ln(weight of prey) SST ( C)
143 Jacobsen & Hansen 32 Fig. 7. Diet of salmon by weight proportions between autumn and winter, for the 10 prey groups defined and used in the calculation of diet overlap. Mean prey length (mm) is indicated below each prey group. Squid were not measured, but were juveniles. 0.6 Autumn Winter 0.5 Weight proportion Amphipods Euphausiids Shrimps Other crustaceans Silverside Lanternfishes Other small fish Barracudinas Other large fish Mean length: Squids Fig. 8. Weight proportion of large fish prey taken by salmon by season (autumn and winter). Mean prey length (mm) is indicated below each prey group. Only one Belone belone was measured Autumn Winter Weight proportion Micromesistius poutassou Scomber scombrus Belone belone Mallotus villosus Clupea harengus Paralepidae Mean length:
144 Jacobsen & Hansen 33 Fig. 9. Diet of 1, 2, and 3+SW salmon by weight proportions in the 10 prey groups defined and used in the calculation of diet overlap. The smaller 1SW salmon had taken higher proportions of crustaceans (amphipods) while the proportion of fish, particularly barracudinas and other large fish increased with sea age. Mean prey length (mm) is indicated below each prey group. Squid were not measured, but were juveniles SW 2SW 3+SW 0.4 Weight proportion Amphipods Euphausiids Shrimps Other crustaceans Silverside Lanternfishes Other small fish Barracudinas Other large fish Mean length: Squids
145 Jacobsen & Hansen 34 Fig. 10. Relative weight distribution (%) of two Themisto spp. species and three euphausiids species from MIK plankton net samples, 0-50 m depth (upper panel) and from corresponding salmon stomachs samples (lower panel), at 9 of the 13 fishing locations with detailed species segregation of euphausiids and amphipods in both samples. Simplified Morisita overlap indices are shown on top of upper panel, a star indicates high overlap. Number of stomachs are labelled on top of lower panel. Themisto libellula Meganyctiphanes norvegica Thysanoessa longicaudata Themisto compressa Thysanoessa inermis %W from MIK plankton net 100% 80% 60% 40% 20% 0% * %W from salmon stomachs 100% 80% 60% 40% 20% 0% Fishing date (location)
146 Jacobsen & Hansen 35 Fig. 11. Length distribution of various prey from corresponding stomach samples and MIK plankton net samples (0-50 m depth), at 9 of the 13 fishing locations with detailed species segregation of euphausiids and amphipods in both samples: a) Maurolicus müelleri, b) Themisto libellula, c) Themisto compressa, i.e. T. c. f compressa and T. c. f bispinosa, d) Themisto abyssorum, e) Meganyctiphanes norvegica, and f) Thysanoessa spp., i.e. both Th. inermis and Th. longicaudata. a) Maurolicus müelleri Stomachs, n= 50 MIK, n= Length (mm) b) Themisto libellula Stomachs, n= 1418 MIK, n= Length (mm) 30 Thysanoessa inermis Stomachs, n= 675 MIK, n= Themisto abyssorum Stomachs, n= 22 MIK, n= c) Length (mm) d) Length (mm) e) Meganyctiphanes norvegicus Stomachs, n= 216 MIK, n= Length (mm) f) Thysanoessa inermis Stomachs, n= 16 MIK, n= Length (mm)
147 Aspects of the marine ecology of Atlantic salmon
148 Aspects of the marine ecology of Atlantic salmon PAPER V Open-ocean infestation by salmon lice (Lepeophtheirus salmonis): comparison of wild and escaped farmed Atlantic salmon (Salmo salar L.).
149
150
151
152
153
154
155
Origin and migration of wild and escaped farmed Atlantic salmon, Salmo salar L., tagged and released north of the Faroe Islands
 Not to be cited without prior reference to the authors International Council for the Exploration of the Sea CM 1997/AA:5 Origin and migration of wild and escaped farmed Atlantic salmon, Salmo salar L.,
Not to be cited without prior reference to the authors International Council for the Exploration of the Sea CM 1997/AA:5 Origin and migration of wild and escaped farmed Atlantic salmon, Salmo salar L.,
REPORT OF ICES ADVISORY COMMITTEE NORTH ATLANTIC SALMON STOCKS. NORTH ATLANTIC SALMON CONSERVATION ORGANIZATION NEAC Area
 REPORT OF ICES ADVISORY COMMITTEE ON NORTH ATLANTIC SALMON STOCKS TO NORTH ATLANTIC SALMON CONSERVATION ORGANIZATION NEAC Area CNL(14)8 Advice generated by ICES in response to terms of reference from NASCO
REPORT OF ICES ADVISORY COMMITTEE ON NORTH ATLANTIC SALMON STOCKS TO NORTH ATLANTIC SALMON CONSERVATION ORGANIZATION NEAC Area CNL(14)8 Advice generated by ICES in response to terms of reference from NASCO
10.4 Advice May 2014
 10.4 Advice May 2014 ECOREGION STOCK North Atlantic Atlantic salmon at West Greenland Advice for 2014 The previous advice provided by ICES (2012) indicated that there were no mixed-stock fishery catch
10.4 Advice May 2014 ECOREGION STOCK North Atlantic Atlantic salmon at West Greenland Advice for 2014 The previous advice provided by ICES (2012) indicated that there were no mixed-stock fishery catch
North-East Atlantic Commission NEA(18)10. Presentation of the ICES Advice for the North-East Atlantic stocks to the Commission
 North-East Atlantic Commission NEA(18)10 Presentation of the ICES Advice for the North-East Atlantic stocks to the Commission sal.27.neac Atlantic salmon from Northeast Atlantic Terms of Reference 2.
North-East Atlantic Commission NEA(18)10 Presentation of the ICES Advice for the North-East Atlantic stocks to the Commission sal.27.neac Atlantic salmon from Northeast Atlantic Terms of Reference 2.
9.4.5 Advice September Widely distributed and migratory stocks Herring in the Northeast Atlantic (Norwegian spring-spawning herring)
 9.4.5 Advice September 212 ECOREGION STOCK Widely distributed and migratory stocks Herring in the Northeast Atlantic (Norwegian spring-spawning herring) Advice for 213 ICES advises on the basis of the
9.4.5 Advice September 212 ECOREGION STOCK Widely distributed and migratory stocks Herring in the Northeast Atlantic (Norwegian spring-spawning herring) Advice for 213 ICES advises on the basis of the
The Spatial and Temporal Distribution of Salmon and the Pelagic Fisheries in the North- East Atlantic: A Potential for By-catch?
 The Spatial and Temporal Distribution of Salmon and the Pelagic Fisheries in the North- East Atlantic: A Potential for By-catch? Marianne Holm 1, Árni Ísaksson 2, Jan Arge Jacobsen 3, Lars Petter Hansen
The Spatial and Temporal Distribution of Salmon and the Pelagic Fisheries in the North- East Atlantic: A Potential for By-catch? Marianne Holm 1, Árni Ísaksson 2, Jan Arge Jacobsen 3, Lars Petter Hansen
4.9.5 Norwegian spring-spawning herring
 4.9.5 Norwegian springspawning herring State of the stock Spawning biomass in relation to precautionary limits Acceptable Fishing mortality in relation to precautionary limits Acceptable Fishing mortality
4.9.5 Norwegian springspawning herring State of the stock Spawning biomass in relation to precautionary limits Acceptable Fishing mortality in relation to precautionary limits Acceptable Fishing mortality
ATLANTIC SALMON NEWFOUNDLAND AND LABRADOR, SALMON FISHING AREAS 1-14B. The Fisheries. Newfoundland Region Stock Status Report D2-01
 Fisheries Pêches and Oceans et Océans DFO Science Newfoundland Region Stock Status Report D2-01 ATLANTIC SALMON NEWFOUNDLAND AND LABRADOR, SALMON FISHING AREAS 1-14B Background There are 15 Atlantic salmon
Fisheries Pêches and Oceans et Océans DFO Science Newfoundland Region Stock Status Report D2-01 ATLANTIC SALMON NEWFOUNDLAND AND LABRADOR, SALMON FISHING AREAS 1-14B Background There are 15 Atlantic salmon
Map Showing NAFO Management Units
 Map Showing NAFO Management Units Biology Are 6 species of seals in Atlantic Canadian waters, all of which occur in Newfoundland Two Arctic Species (Ringed, Bearded) Two temperate (Grey, Harbour) Two migratory
Map Showing NAFO Management Units Biology Are 6 species of seals in Atlantic Canadian waters, all of which occur in Newfoundland Two Arctic Species (Ringed, Bearded) Two temperate (Grey, Harbour) Two migratory
Northeast Atlantic Mackerel, Handlines
 Northeast Atlantic Mackerel, Handlines Northeast Atlantic Mackerel, Handlines Content last updated 3rd Apr 2017 Stock: Mackerel (Scomber scombrus) in subareas 1 7 and 14, and in divisions 8.a e and 9.a
Northeast Atlantic Mackerel, Handlines Northeast Atlantic Mackerel, Handlines Content last updated 3rd Apr 2017 Stock: Mackerel (Scomber scombrus) in subareas 1 7 and 14, and in divisions 8.a e and 9.a
10.3 Advice May 2014
 1.3 Advice May 214 ECOREGION STOCK North Atlantic Atlantic salmon from North America Advice for 214 Because the NASCO Framework of Indicators of North American stocks for 213 (run in January 214) did not
1.3 Advice May 214 ECOREGION STOCK North Atlantic Atlantic salmon from North America Advice for 214 Because the NASCO Framework of Indicators of North American stocks for 213 (run in January 214) did not
Migration and survival of farmed Atlantic salmon (Salmo salar L.) released from two Norwegian fish farms
 ICES Journal of Marine Science, 63: 1211e1217 (2006) doi:10.1016/j.icesjms.2006.04.022 Migration and survival of farmed Atlantic salmon (Salmo salar L.) released from two Norwegian fish farms Lars P. Hansen
ICES Journal of Marine Science, 63: 1211e1217 (2006) doi:10.1016/j.icesjms.2006.04.022 Migration and survival of farmed Atlantic salmon (Salmo salar L.) released from two Norwegian fish farms Lars P. Hansen
Monitoring of sea trout post-smolts, 2012
 Monitoring of sea trout post-smolts, 2012 A report to the West Sutherland Fisheries Trust, Report No. WSFT2/13 January 2013 Shona Marshall Fisheries Biologist West Sutherland Fisheries Trust Gardeners
Monitoring of sea trout post-smolts, 2012 A report to the West Sutherland Fisheries Trust, Report No. WSFT2/13 January 2013 Shona Marshall Fisheries Biologist West Sutherland Fisheries Trust Gardeners
International cooperation on the conservation & restoration of wild Atlantic salmon the work of NASCO
 International cooperation on the conservation & restoration of wild Atlantic salmon the work of NASCO Malcolm Windsor and Peter Hutchinson Photo courtesy of Gilbert van Ryckevorsel The Convention entered
International cooperation on the conservation & restoration of wild Atlantic salmon the work of NASCO Malcolm Windsor and Peter Hutchinson Photo courtesy of Gilbert van Ryckevorsel The Convention entered
CNL(09)16 Summary of Annual Reports on Implementation Plans
 Agenda item 6. For Decision CNL(9)6 Summary of Annual Reports on Implementation Plans Background CNL(9)6 Summary of Annual Reports on Implementation Plans. The Council s Guidelines for the Preparation
Agenda item 6. For Decision CNL(9)6 Summary of Annual Reports on Implementation Plans Background CNL(9)6 Summary of Annual Reports on Implementation Plans. The Council s Guidelines for the Preparation
ASSESSMENT OF THE WEST COAST OF NEWFOUNDLAND (DIVISION 4R) HERRING STOCKS IN 2011
 Canadian Science Advisory Secretariat Science Advisory Report 212/24 ASSESSMENT OF THE WEST COAST OF NEWFOUNDLAND (DIVISION 4R) HERRING STOCKS IN 211 Context Figure 1. Map of unit areas of NAFO Division
Canadian Science Advisory Secretariat Science Advisory Report 212/24 ASSESSMENT OF THE WEST COAST OF NEWFOUNDLAND (DIVISION 4R) HERRING STOCKS IN 211 Context Figure 1. Map of unit areas of NAFO Division
Council CNL(18)21. Annual Progress Report on Actions Taken Under the Implementation Plan for the Calendar Year 2017
 Agenda item 7.1 For information Council CNL(18)21 Annual Progress Report on Actions Taken Under the Implementation Plan for the Calendar Year 2017 Denmark (in respect of the Faroe Islands and Greenland)
Agenda item 7.1 For information Council CNL(18)21 Annual Progress Report on Actions Taken Under the Implementation Plan for the Calendar Year 2017 Denmark (in respect of the Faroe Islands and Greenland)
Spurdog (Squalus acanthias) in the Northeast Atlantic
 ICES Advice on fishing opportunities, catch, and effort Northeast Atlantic Published 11 October 2016 9.3.17 Spurdog (Squalus acanthias) in the Northeast Atlantic ICES stock advice ICES advises that when
ICES Advice on fishing opportunities, catch, and effort Northeast Atlantic Published 11 October 2016 9.3.17 Spurdog (Squalus acanthias) in the Northeast Atlantic ICES stock advice ICES advises that when
Environment, Climate Change and Land Reform Committee. Environmental impacts of salmon farming. Written submission from Fisheries Management Scotland
 Environment, Climate Change and Land Reform Committee Environmental impacts of salmon farming Written submission from Fisheries Management Scotland Fisheries Management Scotland are the representative
Environment, Climate Change and Land Reform Committee Environmental impacts of salmon farming Written submission from Fisheries Management Scotland Fisheries Management Scotland are the representative
Stock characteristics, fisheries and management of Greenland halibut (Reinhardtius hippoglossoides (Walbaum)) in the Northeast Arctic
 The 10 th Norwegian Russian Symposium Stock characteristics, fisheries and management of Greenland halibut (Reinhardtius hippoglossoides (Walbaum)) in the Northeast Arctic by Kjell H. Nedreaas (IMR) &
The 10 th Norwegian Russian Symposium Stock characteristics, fisheries and management of Greenland halibut (Reinhardtius hippoglossoides (Walbaum)) in the Northeast Arctic by Kjell H. Nedreaas (IMR) &
Where do the northern Atlantic salmon feed during their sea residence in the Norwegian, Greenland or Barents Sea?
 Where do the northern Atlantic salmon feed during their sea residence in the Norwegian, Greenland or Barents Sea? Martin-A. Svenning Norwegian institute for nature research Department of Arctic Ecology
Where do the northern Atlantic salmon feed during their sea residence in the Norwegian, Greenland or Barents Sea? Martin-A. Svenning Norwegian institute for nature research Department of Arctic Ecology
Mackerel (Scomber scombrus) in subareas 1 8 and 14, and in Division 9.a (the Northeast Atlantic and adjacent waters)
 ICES Advice on fishing opportunities, catch, and effort Ecoregions in the Northeast Atlantic and Arctic Ocean Published 29 September 2017 DOI: 10.17895/ices.pub.3023 Mackerel (Scomber scombrus) in subareas
ICES Advice on fishing opportunities, catch, and effort Ecoregions in the Northeast Atlantic and Arctic Ocean Published 29 September 2017 DOI: 10.17895/ices.pub.3023 Mackerel (Scomber scombrus) in subareas
Challenges and opportunities in managing Atlantic salmon - the international aspects
 Challenges and opportunities in managing Atlantic salmon - the international aspects Peter Hutchinson, Secretary of NASCO The Convention Scientific advice Salmon fisheries Research on salmon at sea Implications
Challenges and opportunities in managing Atlantic salmon - the international aspects Peter Hutchinson, Secretary of NASCO The Convention Scientific advice Salmon fisheries Research on salmon at sea Implications
Council CNL(17)33. Annual Progress Report on Actions Taken Under the Implementation Plan for the Calendar Year EU - Denmark
 Agenda item 6.3 For information Council CNL(17)33 Annual Progress Report on Actions Taken Under the Implementation Plan for the Calendar Year 2016 EU - Denmark CNL(17)33 Annual Progress Report on Actions
Agenda item 6.3 For information Council CNL(17)33 Annual Progress Report on Actions Taken Under the Implementation Plan for the Calendar Year 2016 EU - Denmark CNL(17)33 Annual Progress Report on Actions
Blue whiting (Micromesistius poutassou) in subareas 1 9, 12, and 14 (Northeast Atlantic and adjacent waters)
 ICES Advice on fishing opportunities, catch, and effort Ecoregions of the Northeast Atlantic and Arctic Ocean Published 28 September 2018 nea https://doi.org/10.17895/ices.pub.4536 Blue whiting (Micromesistius
ICES Advice on fishing opportunities, catch, and effort Ecoregions of the Northeast Atlantic and Arctic Ocean Published 28 September 2018 nea https://doi.org/10.17895/ices.pub.4536 Blue whiting (Micromesistius
Trends in Scottish Fish Stocks 2018
 Port Arthur, Scalloway, Shetland, ZE1 UN, Scotland, UK Tel: +44 ()1595 772 Fax: +44 ()1595 7721 Email: info@nafc.uhi.ac.uk Web: www.nafc.ac.uk Trends in Scottish Fish Stocks 218 Ian R. Napier 14 th September
Port Arthur, Scalloway, Shetland, ZE1 UN, Scotland, UK Tel: +44 ()1595 772 Fax: +44 ()1595 7721 Email: info@nafc.uhi.ac.uk Web: www.nafc.ac.uk Trends in Scottish Fish Stocks 218 Ian R. Napier 14 th September
Trends in Scottish Fish Stocks 2017
 Port Arthur, Scalloway, Shetland, ZE1 0UN, Scotland, UK Tel: +44 (0)1595 772000 Fax: +44 (0)1595 772001 Email: info@nafc.uhi.ac.uk Web: www.nafc.ac.uk Trends in Scottish Fish Stocks 2017 Ian R. Napier
Port Arthur, Scalloway, Shetland, ZE1 0UN, Scotland, UK Tel: +44 (0)1595 772000 Fax: +44 (0)1595 772001 Email: info@nafc.uhi.ac.uk Web: www.nafc.ac.uk Trends in Scottish Fish Stocks 2017 Ian R. Napier
North East Atlantic Fisheries Baltic Sprat Whitepaper March 2011
 North East Atlantic Fisheries Baltic Sprat Whitepaper March 2011 1. Introduction Sprat is a clupeid fish found widely in the North East Atlantic area. Sprat is a relatively short-lived species. The stock
North East Atlantic Fisheries Baltic Sprat Whitepaper March 2011 1. Introduction Sprat is a clupeid fish found widely in the North East Atlantic area. Sprat is a relatively short-lived species. The stock
COUNTRIES THAT CONTRAVENED SCIENTIFIC ADVICE BY HARVESTING MIXED POPULATIONS OF NORTH AMERICAN SALMON IN 2017
 SCIENTIFIC ADVICE The International Council for the Exploration of the Sea (ICES) advises: There is no surplus of multi-sea-winter salmon for a fishery at West Greenland for 2018, 2019, and 2020. ICES
SCIENTIFIC ADVICE The International Council for the Exploration of the Sea (ICES) advises: There is no surplus of multi-sea-winter salmon for a fishery at West Greenland for 2018, 2019, and 2020. ICES
North Labrador Arctic Charr
 Fisheries and Oceans Pêches et Océans Canada Canada DFO Science Newfoundland Region Stock Status Report D2-07(2001) substantive in some years. The north Labrador area is composed of various stock complexes
Fisheries and Oceans Pêches et Océans Canada Canada DFO Science Newfoundland Region Stock Status Report D2-07(2001) substantive in some years. The north Labrador area is composed of various stock complexes
Blue whiting (Micromesistius poutassou) in subareas 1 9, 12, and 14 (Northeast Atlantic and adjacent waters)
 ICES Advice on fishing opportunities, catch, and effort Ecoregions of the Northeast Atlantic and Arctic Ocean Published 29 September 2017 DOI: 10.17895/ices.pub.3030 Blue whiting (Micromesistius poutassou)
ICES Advice on fishing opportunities, catch, and effort Ecoregions of the Northeast Atlantic and Arctic Ocean Published 29 September 2017 DOI: 10.17895/ices.pub.3030 Blue whiting (Micromesistius poutassou)
Fast Tracking the Development of Environmental- Friendly Fishing Methods
 Irish Presidency of the Council of Fisheries Ministers of the European Union Ministerial & Stakeholders Conference Fast Tracking the Development of Environmental- Friendly Fishing Methods Norwegian efforts
Irish Presidency of the Council of Fisheries Ministers of the European Union Ministerial & Stakeholders Conference Fast Tracking the Development of Environmental- Friendly Fishing Methods Norwegian efforts
9.4.5 Advice October Widely Distributed and Migratory Stocks Herring in the Northeast Atlantic (Norwegian spring-spawning herring)
 9.4.5 Advice October 21 ECOREGION STOCK Widely Distributed and Migratory Stocks Herring in the Northeast Atlantic (Norwegian spring-spawning herring) Advice for 211 Management Objective (s) Landings in
9.4.5 Advice October 21 ECOREGION STOCK Widely Distributed and Migratory Stocks Herring in the Northeast Atlantic (Norwegian spring-spawning herring) Advice for 211 Management Objective (s) Landings in
Cod in the Northern Gulf of St. Lawrence
 DFO Sciences Stock Status Report A4-1 (1998) 52. 51. 5. Latitude 49. 48. 47. 4S 4R 3Pn Background Cod in the northern Gulf of (Divisions 3Pn, 4RS) undertake distant annual migrations. In winter, the fish
DFO Sciences Stock Status Report A4-1 (1998) 52. 51. 5. Latitude 49. 48. 47. 4S 4R 3Pn Background Cod in the northern Gulf of (Divisions 3Pn, 4RS) undertake distant annual migrations. In winter, the fish
FISHERIES CO-OPERATION ICELAND AND NORWAY WITH. Presented by Philip Rodgers ERINSHORE ECONOMICS
 FISHERIES CO-OPERATION WITH ICELAND AND NORWAY Presented by Philip Rodgers 17/12/2013 Fisheries Cooperation with Norway and Iceland 1 Objective To consider the current situation in the fishery for highly
FISHERIES CO-OPERATION WITH ICELAND AND NORWAY Presented by Philip Rodgers 17/12/2013 Fisheries Cooperation with Norway and Iceland 1 Objective To consider the current situation in the fishery for highly
Final report on fish diet and stomach analyses
 SALSEA-MERGE FP7-ENV-2007-1 Grant Agreement No 212529 Work Package 4 Deliverable - D 4.3 Final report on fish diet and stomach analyses (Month 40) D 4.3 - Final report on fish diet and stomach analyses
SALSEA-MERGE FP7-ENV-2007-1 Grant Agreement No 212529 Work Package 4 Deliverable - D 4.3 Final report on fish diet and stomach analyses (Month 40) D 4.3 - Final report on fish diet and stomach analyses
Advice October 2013
 5.4.21.3 Advice October 213 ECOREGION Celtic Sea and West of Scotland STOCK Nephrops on Porcupine Bank (FU 16) Advice for 214 ICES advises on the basis of the MSY approach that catches from FU 16 in 214
5.4.21.3 Advice October 213 ECOREGION Celtic Sea and West of Scotland STOCK Nephrops on Porcupine Bank (FU 16) Advice for 214 ICES advises on the basis of the MSY approach that catches from FU 16 in 214
2.3.1 Advice May Capelin in Subareas V and XIV and Division IIa west of 5 W (Iceland East Greenland Jan Mayen area).
 2.3.1 Advice May 2014 ECOREGION Iceland and East Greenland STOCK Capelin in Subareas V and XIV and Division IIa west of 5 W (Iceland East Greenland Jan Mayen area) Advice for 2014/2015 ICES advises on
2.3.1 Advice May 2014 ECOREGION Iceland and East Greenland STOCK Capelin in Subareas V and XIV and Division IIa west of 5 W (Iceland East Greenland Jan Mayen area) Advice for 2014/2015 ICES advises on
Advice June 2014
 5.3.23 Advice June 2014 ECOREGION STOCK Celtic Sea and West of Scotland Plaice in Division VIIa (Irish Sea) Advice for 2015 Based on ICES approach to data-limited stocks, ICES advises that catches should
5.3.23 Advice June 2014 ECOREGION STOCK Celtic Sea and West of Scotland Plaice in Division VIIa (Irish Sea) Advice for 2015 Based on ICES approach to data-limited stocks, ICES advises that catches should
Stocking success of Scottish Atlantic salmon in two Spanish rivers
 Journal of Fish Biology (1997) 51, 1265 1269 Stocking success of Scottish Atlantic salmon in two Spanish rivers E. VERSPOOR* AND C. GARCIA DE LEÁNIZ *Marine Laboratory, P.O. Box 101, Aberdeen AB11 9DB,
Journal of Fish Biology (1997) 51, 1265 1269 Stocking success of Scottish Atlantic salmon in two Spanish rivers E. VERSPOOR* AND C. GARCIA DE LEÁNIZ *Marine Laboratory, P.O. Box 101, Aberdeen AB11 9DB,
Council CNL(14)45 The management approach to salmon fisheries in Norway (Tabled by Norway)
 Agenda Item 6.2 Agenda Item 6.2 For Information Council CNL(14)45 The management approach to salmon fisheries in Norway (Tabled by Norway) 1983 1985 1987 1989 1991 1993 1995 1997 1999 2001 2003 2005 2007
Agenda Item 6.2 Agenda Item 6.2 For Information Council CNL(14)45 The management approach to salmon fisheries in Norway (Tabled by Norway) 1983 1985 1987 1989 1991 1993 1995 1997 1999 2001 2003 2005 2007
COUNTRIES THAT CONTRAVENE SCIENTIFIC ADVICE BY HARVESTING MIXED-POPULATIONS OF NORTH AMERICAN SALMON
 SCIENTIFIC ADVICE A Backgrounder from Atlantic Salmon Federation P. O. Box 5200, St. Andrews, NB E5B 3S8 P. O. Box 807, Calais, ME USA 04619 0807 Tel: (506) 529 4581 www.asf.ca State of North American
SCIENTIFIC ADVICE A Backgrounder from Atlantic Salmon Federation P. O. Box 5200, St. Andrews, NB E5B 3S8 P. O. Box 807, Calais, ME USA 04619 0807 Tel: (506) 529 4581 www.asf.ca State of North American
Policy on the Management of Sea Lice
 Suite 3/11 King James VI Business Centre Friarton Road Perth PH2 8DG Tel: 01738 472032 Policy on the Management of Sea Lice August 2010 www.atlanticsalmontrust.org 2 Atlantic Salmon Trust Policy on the
Suite 3/11 King James VI Business Centre Friarton Road Perth PH2 8DG Tel: 01738 472032 Policy on the Management of Sea Lice August 2010 www.atlanticsalmontrust.org 2 Atlantic Salmon Trust Policy on the
Report of the Working Group on Widely Distributed
 ICES WGWIDE REPORT 2017 ICES ADVISORY COMMITTEE ICES CM 2017/ACOM:23 Report of the Working Group on Widely Distributed Stocks (WGWIDE) 30 August -5 September 2017 ICES Headquarters, Copenhagen, Denmark
ICES WGWIDE REPORT 2017 ICES ADVISORY COMMITTEE ICES CM 2017/ACOM:23 Report of the Working Group on Widely Distributed Stocks (WGWIDE) 30 August -5 September 2017 ICES Headquarters, Copenhagen, Denmark
Factors influencing production
 Fisheries Reading: Miller Ch. 15 Supplementary: Levinton, Ch. 18 Krkošek et al. Epizootics of wild fish induced by farm fish. Proceedings of the National Academy of Sciences (2006) vol. 103 (42) pp. 15506
Fisheries Reading: Miller Ch. 15 Supplementary: Levinton, Ch. 18 Krkošek et al. Epizootics of wild fish induced by farm fish. Proceedings of the National Academy of Sciences (2006) vol. 103 (42) pp. 15506
FISHING ACTIVITY: SEABED TRAWLING
 FISHING ACTIVITY: SEABED TRAWLING Environmental Snapshot March 2010 Key points In 2008, 68 large (>28 m) fishing vessels conducted 38,648 seabed trawls covering 85,222 km 2. Since 2005, the number of trawls
FISHING ACTIVITY: SEABED TRAWLING Environmental Snapshot March 2010 Key points In 2008, 68 large (>28 m) fishing vessels conducted 38,648 seabed trawls covering 85,222 km 2. Since 2005, the number of trawls
The fishery for jack mackerel in the Eastern Central Pacific by European trawlers in 2008 and 2009
 Eighth International Meeting: SWG: Jack Mackerel Sub-Group SP-08-SWG-JM-01 The fishery for jack mackerel in the Eastern Central Pacific by European trawlers in 2008 and 2009 Ad Corten Corten Marine Research
Eighth International Meeting: SWG: Jack Mackerel Sub-Group SP-08-SWG-JM-01 The fishery for jack mackerel in the Eastern Central Pacific by European trawlers in 2008 and 2009 Ad Corten Corten Marine Research
5. purse seines 3 000
 Sea Bass Q and A Latest News What have the 28 Member States decided on 2 July? The EU has today taken another step to protect sea bass. The 28 EU member states agreed to the Commission's proposal to increase
Sea Bass Q and A Latest News What have the 28 Member States decided on 2 July? The EU has today taken another step to protect sea bass. The 28 EU member states agreed to the Commission's proposal to increase
Herring in the North Sea, Exploitation and Conservation. Presentation by Dr Beatriz A. Roel
 Herring in the North Sea, Exploitation and Conservation Presentation by Dr Beatriz A. Roel Geographic Distribution of Atlantic and Pacific herring Herring reproduction Herring use external fertilization
Herring in the North Sea, Exploitation and Conservation Presentation by Dr Beatriz A. Roel Geographic Distribution of Atlantic and Pacific herring Herring reproduction Herring use external fertilization
3.4.3 Advice June Barents Sea and Norwegian Sea Cod in Subareas I and II (Norwegian coastal waters cod)
 3.4.3 Advice June 2013 ECOREGION STOCK Barents Sea and Norwegian Sea Cod in Subareas I and II (Norwegian coastal waters cod) Advice for 2014 ICES advises on the basis of the Norwegian rebuilding plan,
3.4.3 Advice June 2013 ECOREGION STOCK Barents Sea and Norwegian Sea Cod in Subareas I and II (Norwegian coastal waters cod) Advice for 2014 ICES advises on the basis of the Norwegian rebuilding plan,
The Hague, Kingdom of the Netherlands October 2016 SC The European Union Annual report
 4 th Meeting of the Scientific Committee The Hague, Kingdom of the Netherlands 1-1 October 216 SC-4-1 The European Union Annual report Ad Corten Consultant Dutch Ministry of Economic Affairs 1 National
4 th Meeting of the Scientific Committee The Hague, Kingdom of the Netherlands 1-1 October 216 SC-4-1 The European Union Annual report Ad Corten Consultant Dutch Ministry of Economic Affairs 1 National
Sprat (Sprattus sprattus) in subdivisions (Baltic Sea)
 ICES Advice on fishing opportunities, catch, and effort Baltic Sea Ecoregion Published 31 May 2016 8.3.18 Sprat (Sprattus sprattus) in subdivisions 22 32 (Baltic Sea) ICES stock advice ICES advises that
ICES Advice on fishing opportunities, catch, and effort Baltic Sea Ecoregion Published 31 May 2016 8.3.18 Sprat (Sprattus sprattus) in subdivisions 22 32 (Baltic Sea) ICES stock advice ICES advises that
ASMFC Stock Assessment Overview: Red Drum
 Introduction This document presents a summary of the 217 stock assessments for red drum. These assessments were initially conducted through the Southeast Data, Assessment and Review (SEDAR) process using
Introduction This document presents a summary of the 217 stock assessments for red drum. These assessments were initially conducted through the Southeast Data, Assessment and Review (SEDAR) process using
Report on a Salmon Survey in the Waterford Estuary in 2010
 Report on a Salmon Survey in the Waterford Estuary in 2010 Dr. P. Gargan Inland Fisheries Ireland June 2011 1 Contents 1. Introduction 3 2. Proposal for a Survey in Waterford estuary in 2010 3 3. Survey
Report on a Salmon Survey in the Waterford Estuary in 2010 Dr. P. Gargan Inland Fisheries Ireland June 2011 1 Contents 1. Introduction 3 2. Proposal for a Survey in Waterford estuary in 2010 3 3. Survey
ICES advice on fishing opportunities
 ICES Advice on fishing opportunities, catch, and effort Northeast Atlantic and Arctic Ocean Published 22 October 2018 Version 2: 25 October 2018 https://doi.org/10.17895/ices.pub.4568 Herring (Clupea harengus)
ICES Advice on fishing opportunities, catch, and effort Northeast Atlantic and Arctic Ocean Published 22 October 2018 Version 2: 25 October 2018 https://doi.org/10.17895/ices.pub.4568 Herring (Clupea harengus)
NINA Aquatic Research Station, Ims
 NINA Aquatic Research Station, Ims NINA Aquatic Research Station, Ims NINA Aquatic Research Station, Ims NINA The Norwegian Institute for Nature Research (NINA) is Norway s leading institute for applied
NINA Aquatic Research Station, Ims NINA Aquatic Research Station, Ims NINA Aquatic Research Station, Ims NINA The Norwegian Institute for Nature Research (NINA) is Norway s leading institute for applied
Table 1 Reported 2016 catches by fisheries. Licence type Reported consumption type Reported 2016 catch (t) Licensed
 ICES Advice on fishing opportunities, catch, and effort West of Greenland and Greenland Sea Ecoregions sal.2127.wgc Published 5 May 2017 DOI: 10.17895/ices.pub.3220 Atlantic salmon at West Greenland Summary
ICES Advice on fishing opportunities, catch, and effort West of Greenland and Greenland Sea Ecoregions sal.2127.wgc Published 5 May 2017 DOI: 10.17895/ices.pub.3220 Atlantic salmon at West Greenland Summary
SA2 + Div. 3K Redfish
 Fisheries and Oceans Pêches et Océans Canada Canada DFO Science Stock Status Report A2-15(21) entered the fishery in 1975 and averaged about 16,5 t from 1978-1986. The steady reduction in catches from
Fisheries and Oceans Pêches et Océans Canada Canada DFO Science Stock Status Report A2-15(21) entered the fishery in 1975 and averaged about 16,5 t from 1978-1986. The steady reduction in catches from
REPORT OF ICES ADVISORY COMMITTEE NORTH ATLANTIC SALMON STOCKS. NORTH ATLANTIC SALMON CONSERVATION ORGANIZATION June 5 to 8, 2012
 REPORT OF ICES ADVISORY COMMITTEE ON NORTH ATLANTIC SALMON STOCKS TO NORTH ATLANTIC SALMON CONSERVATION ORGANIZATION June 5 to 8, 2012 Manuela Azevedo & Hans Lassen CNL(12)8 Advice generated by ICES in
REPORT OF ICES ADVISORY COMMITTEE ON NORTH ATLANTIC SALMON STOCKS TO NORTH ATLANTIC SALMON CONSERVATION ORGANIZATION June 5 to 8, 2012 Manuela Azevedo & Hans Lassen CNL(12)8 Advice generated by ICES in
Haddock, Iceland, ICES Va, Danish Seine
 Haddock, Iceland, ICES Va, Danish Seine Haddock, Iceland, ICES Va, Danish Seine Content last updated 2nd Aug 2017 Stock: Haddock in the Iceland grounds (ICES Division Va) Management: Iceland Overview Haddock
Haddock, Iceland, ICES Va, Danish Seine Haddock, Iceland, ICES Va, Danish Seine Content last updated 2nd Aug 2017 Stock: Haddock in the Iceland grounds (ICES Division Va) Management: Iceland Overview Haddock
SMALL BOAT TUNA LONGLINE FISHERY NORTH-WEST COAST OF SRI LANKA R. Maldeniya
 SMALL BOAT TUNA LONGLINE FISHERY NORTH-WEST COAST OF SRI LANKA R. Maldeniya National Aquatic Resources Agency Crow Island, Colombo 5 Sri Lanka INTRODUCTION Studies made by Sivasubramanium (97) and Maldeniya
SMALL BOAT TUNA LONGLINE FISHERY NORTH-WEST COAST OF SRI LANKA R. Maldeniya National Aquatic Resources Agency Crow Island, Colombo 5 Sri Lanka INTRODUCTION Studies made by Sivasubramanium (97) and Maldeniya
ASMFC Stock Assessment Overview: Red Drum
 Purpose The purpose of this document is to improve the understanding and transparency of the Commission s stock assessment process and results. It is the first of several that will be developed throughout
Purpose The purpose of this document is to improve the understanding and transparency of the Commission s stock assessment process and results. It is the first of several that will be developed throughout
Advice May Herring in Subdivisions and 32 (excluding Gulf of Riga herring)
 8.3.10 Advice May 2014 ECOREGION STOCK Baltic Sea Herring in Subdivisions 25 29 and 32 (excluding Gulf of Riga herring) Advice for 2015 ICES advises on the basis of the MSY approach that catches in 2015
8.3.10 Advice May 2014 ECOREGION STOCK Baltic Sea Herring in Subdivisions 25 29 and 32 (excluding Gulf of Riga herring) Advice for 2015 ICES advises on the basis of the MSY approach that catches in 2015
A Combined Recruitment Index for Demersal Juvenile Cod in NAFO Divisions 3K and 3L
 NAFO Sci. Coun. Studies, 29: 23 29 A Combined Recruitment Index for Demersal Juvenile Cod in NAFO Divisions 3K and 3L David C. Schneider Ocean Sciences Centre, Memorial University St. John's, Newfoundland,
NAFO Sci. Coun. Studies, 29: 23 29 A Combined Recruitment Index for Demersal Juvenile Cod in NAFO Divisions 3K and 3L David C. Schneider Ocean Sciences Centre, Memorial University St. John's, Newfoundland,
Arctic Frontiers, Tromsø, January 24 th Thorbjørn Thorvik, Senior adviser. The Norwegian Directorate of Fisheries.
 Sustainable harvesting at lower trophic levels: The Norwegian management plan for Calanus finmarchicus and the framework for utilizing mesopelagic species Thorbjørn Thorvik, Senior adviser. The Norwegian
Sustainable harvesting at lower trophic levels: The Norwegian management plan for Calanus finmarchicus and the framework for utilizing mesopelagic species Thorbjørn Thorvik, Senior adviser. The Norwegian
North-East Atlantic Commission NEA(18)05. Mixed-Stock Fisheries. (Tabled by the European Union)
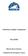 North-East Atlantic Commission NEA(18)05 Mixed-Stock Fisheries (Tabled by the European Union) NEA(18)05 Mixed-Stock Fisheries (Tabled by the European Union) 1) Brief description of existing MSFs EU-Finland
North-East Atlantic Commission NEA(18)05 Mixed-Stock Fisheries (Tabled by the European Union) NEA(18)05 Mixed-Stock Fisheries (Tabled by the European Union) 1) Brief description of existing MSFs EU-Finland
Advice June 2012
 2.4.1 Advice June 212 ECOREGION STOCK Iceland and East Greenland Beaked redfish (Sebastes mentella) in Subareas V, XII, and XIV and NAFO Subareas 1+2 (Deep pelagic stock > 5 m) Advice for 213 The advice
2.4.1 Advice June 212 ECOREGION STOCK Iceland and East Greenland Beaked redfish (Sebastes mentella) in Subareas V, XII, and XIV and NAFO Subareas 1+2 (Deep pelagic stock > 5 m) Advice for 213 The advice
A. Bjordal and A. B. Skar
 International Council for the Exploration of the Sea Demersal Fish Committee - C.M. 1992/G:35 Ref. F Tagging of saithe (Pollachius virens L.) at a Norwegian fish farm: preliminary results on migration
International Council for the Exploration of the Sea Demersal Fish Committee - C.M. 1992/G:35 Ref. F Tagging of saithe (Pollachius virens L.) at a Norwegian fish farm: preliminary results on migration
The Emerging View of New England Cod Stock Structure
 Cod Population Structure and New England Fisheries Symposium: Furthering our understanding by integrating knowledge gained through science and fishing Putting it All Together: The Emerging View of New
Cod Population Structure and New England Fisheries Symposium: Furthering our understanding by integrating knowledge gained through science and fishing Putting it All Together: The Emerging View of New
Sustainable Fisheries for Future Generations The Fisheries White Paper
 Sustainable Fisheries for Future Generations The Fisheries White Paper Key messages The Fisheries White Paper Sustainable Fisheries for Future Generations charts the course for a sustainable and profitable
Sustainable Fisheries for Future Generations The Fisheries White Paper Key messages The Fisheries White Paper Sustainable Fisheries for Future Generations charts the course for a sustainable and profitable
ASSESSMENT OF THE WEST COAST OF NEWFOUNDLAND (DIVISION 4R) HERRING STOCKS IN 2013
 Canadian Science Advisory Secretariat Quebec Region Science Advisory Report 214/56 ASSESSMENT OF THE WEST COAST OF NEWFOUNDLAND (DIVISION 4R) HERRING STOCKS IN 213 Quebec 4Ra 4S 4R 4Rc 4Rb U.S.A. (Maine)
Canadian Science Advisory Secretariat Quebec Region Science Advisory Report 214/56 ASSESSMENT OF THE WEST COAST OF NEWFOUNDLAND (DIVISION 4R) HERRING STOCKS IN 213 Quebec 4Ra 4S 4R 4Rc 4Rb U.S.A. (Maine)
Fish Conservation and Management
 Fish Conservation and Management CONS 486 Applied life history: individual growth, population growth, competition, predation Ross Chapter 3 Applied life history Age and size at maturity Growth Competition
Fish Conservation and Management CONS 486 Applied life history: individual growth, population growth, competition, predation Ross Chapter 3 Applied life history Age and size at maturity Growth Competition
West Coast Rock Lobster. Description of sector. History of the fishery: Catch history
 West Coast Rock Lobster Description of sector History of the fishery: The commercial harvesting of West Coast rock lobster commenced in the late 1800s, and peaked in the early 1950s, yielding an annual
West Coast Rock Lobster Description of sector History of the fishery: The commercial harvesting of West Coast rock lobster commenced in the late 1800s, and peaked in the early 1950s, yielding an annual
SUMMARY OF ICES 2009 ADVICE FOR PELAGIC SPECIES incl Blue whiting, capelin, herring, Norway pout, sandeel and sprat
 SUMMARY OF ICES ADVICE FOR PELAGIC SPECIES incl Blue whiting, capelin, herring, Norway pout, BLUE WHITING Blue whiting combined stock Sub-areas I-IX, XII and XIV Status of stock in October 543,043 Due
SUMMARY OF ICES ADVICE FOR PELAGIC SPECIES incl Blue whiting, capelin, herring, Norway pout, BLUE WHITING Blue whiting combined stock Sub-areas I-IX, XII and XIV Status of stock in October 543,043 Due
STOCK STATUS OF SOUTHERN BLUEFIN TUNA
 7 th Expert Consultation on Indian Ocean Tunas, Victoria, Seychelles, 9-14 November, 1998 STOCK STATUS OF SOUTHERN BLUEFIN TUNA Tsuji, S. 1 Introduction The Commission for the Conservation of Southern
7 th Expert Consultation on Indian Ocean Tunas, Victoria, Seychelles, 9-14 November, 1998 STOCK STATUS OF SOUTHERN BLUEFIN TUNA Tsuji, S. 1 Introduction The Commission for the Conservation of Southern
Advice June 2012
 5.4.29 Advice June 2012 ECOREGION STOCK Celtic Sea and West of Scotland + North Sea Anglerfish (Lophius piscatorius and L. budegassa) in Division IIIa, and Subareas IV and VI Advice for 2013 Based on the
5.4.29 Advice June 2012 ECOREGION STOCK Celtic Sea and West of Scotland + North Sea Anglerfish (Lophius piscatorius and L. budegassa) in Division IIIa, and Subareas IV and VI Advice for 2013 Based on the
Why has the cod stock recovered in the North Sea?
 Why has the cod stock recovered in the North Sea? Summary The expansion of European fisheries during the 1970s and 1980s resulted in high fishing pressure on stocks of cod, haddock, whiting and saithe
Why has the cod stock recovered in the North Sea? Summary The expansion of European fisheries during the 1970s and 1980s resulted in high fishing pressure on stocks of cod, haddock, whiting and saithe
Beaked redfish (Sebastes mentella) in subareas 1 and 2 (Northeast Arctic)
 ICES Advice on fishing opportunities, catch, and effort Arctic Ocean, Barents Sea, Faroes, Greenland Sea, Published 13 June 2017 Icelandic Waters, and Norwegian Sea Ecoregions DOI: 10.17895/ices.pub.3212
ICES Advice on fishing opportunities, catch, and effort Arctic Ocean, Barents Sea, Faroes, Greenland Sea, Published 13 June 2017 Icelandic Waters, and Norwegian Sea Ecoregions DOI: 10.17895/ices.pub.3212
Council CNL(14)21. Annual Progress Report on Actions Taken Under Implementation Plans for the Calendar Year EU Denmark
 Agenda Item 6.1 For Information Council CNL(14)21 Annual Progress Report on Actions Taken Under Implementation Plans for the Calendar Year 2013 EU Denmark CNL(14)21 Annual Progress Report on Actions taken
Agenda Item 6.1 For Information Council CNL(14)21 Annual Progress Report on Actions Taken Under Implementation Plans for the Calendar Year 2013 EU Denmark CNL(14)21 Annual Progress Report on Actions taken
Hatcheries: Role in Restoration and Enhancement of Salmon Populations
 Hatcheries: Role in Restoration and Enhancement of Salmon Populations Hatcheries play a large role in the management, ecology, and evolution of Pacific salmon. Why were/are they built? What are the assumptions
Hatcheries: Role in Restoration and Enhancement of Salmon Populations Hatcheries play a large role in the management, ecology, and evolution of Pacific salmon. Why were/are they built? What are the assumptions
COMMISSIO STAFF WORKI G PAPER. Executive Summary of the Impact Assessment. Accompanying the document
 EUROPEAN COMMISSION Brussels, 12.8.2011 SEC(2011) 986 final COMMISSIO STAFF WORKI G PAPER Executive Summary of the Impact Assessment Accompanying the document Proposal for a Regulation of the European
EUROPEAN COMMISSION Brussels, 12.8.2011 SEC(2011) 986 final COMMISSIO STAFF WORKI G PAPER Executive Summary of the Impact Assessment Accompanying the document Proposal for a Regulation of the European
IYS(18)06_EU UK (Northern Ireland) Report on Planned Actions to Implement the International Year of the Salmon (IYS) Initiative
 IYS(18)06_EU UK (Northern Ireland) Report on Planned Actions to Implement the International Year of the Salmon (IYS) Initiative The primary purpose of this IYS reporting template is for Parties / jurisdictions
IYS(18)06_EU UK (Northern Ireland) Report on Planned Actions to Implement the International Year of the Salmon (IYS) Initiative The primary purpose of this IYS reporting template is for Parties / jurisdictions
Atlantic cod, Norwegian Coastal cod, Gillnet
 Atlantic cod, Norwegian Coastal cod, Gillnet Content last updated 1st Aug 2017 Stock: Norwegian Coastal cod Management: Norway Atlantic cod, Norwegian Coastal cod, Gillnet Overview Atlantic cod, Gadus
Atlantic cod, Norwegian Coastal cod, Gillnet Content last updated 1st Aug 2017 Stock: Norwegian Coastal cod Management: Norway Atlantic cod, Norwegian Coastal cod, Gillnet Overview Atlantic cod, Gadus
Burr Postsmolt ID Series
 Regional and temporal variation in marine growth of Atlantic salmon(salmo salar, L.) from North-East Atlantic populations links to marine survival and oceanographic conditions Niall Ó Maoiléidigh, Arne
Regional and temporal variation in marine growth of Atlantic salmon(salmo salar, L.) from North-East Atlantic populations links to marine survival and oceanographic conditions Niall Ó Maoiléidigh, Arne
Advice June Sole in Division IIIa and Subdivisions (Skagerrak, Kattegat, and the Belts)
 6.3.26 Advice June 2014 ECOREGION STOCK North Sea Sole in Division IIIa and Subdivisions 22 24 (Skagerrak, Kattegat, and the Belts) Advice for 2015 ICES advises on the basis of the MSY approach that catches
6.3.26 Advice June 2014 ECOREGION STOCK North Sea Sole in Division IIIa and Subdivisions 22 24 (Skagerrak, Kattegat, and the Belts) Advice for 2015 ICES advises on the basis of the MSY approach that catches
ICES WGNAS REPORT Report of the Working Group on North Atlantic Salmon (WGNAS) 30 March 8 April Copenhagen, Denmark
 ICES WGNAS REPORT 2016 ICES ADVISORY COMMITTEE ICES CM 2016/ACOM:10 Report of the Working Group on North Atlantic Salmon (WGNAS) 30 March 8 April 2016 Copenhagen, Denmark International Council for the
ICES WGNAS REPORT 2016 ICES ADVISORY COMMITTEE ICES CM 2016/ACOM:10 Report of the Working Group on North Atlantic Salmon (WGNAS) 30 March 8 April 2016 Copenhagen, Denmark International Council for the
Not to be cited without permission Working paper 2002/22
 Not to be cited without permission Working paper 2002/22 International Council for the Exploration of the Sea North Atlantic Salmon Working Group Occurrence of tagged Icelandic salmon in the salmon fisheries
Not to be cited without permission Working paper 2002/22 International Council for the Exploration of the Sea North Atlantic Salmon Working Group Occurrence of tagged Icelandic salmon in the salmon fisheries
Council CNL(11)35. Annual Report on Actions Taken Under Implementation Plans. EU - France
 Agenda Item 6.1 For Information Council CNL(11)35 Annual Report on Actions Taken Under Implementation Plans EU - France Annual Report on actions taken under Implementation Plans for the Calendar Year
Agenda Item 6.1 For Information Council CNL(11)35 Annual Report on Actions Taken Under Implementation Plans EU - France Annual Report on actions taken under Implementation Plans for the Calendar Year
ASMFC Stock Assessment Overview: Atlantic Menhaden
 Introduction This document presents a summary of the 217 Stock Assessment Update for Atlantic menhaden. The assessment is an update to the 215 Benchmark Stock Assessment that was peer reviewed by an independent
Introduction This document presents a summary of the 217 Stock Assessment Update for Atlantic menhaden. The assessment is an update to the 215 Benchmark Stock Assessment that was peer reviewed by an independent
Cod (Gadus morhua) in subareas 1 and 2 (Northeast Arctic)
 ICES Advice on fishing opportunities, catch, and effort Arctic Ocean, Barents Sea, Faroes, Greenland Sea, Published 13 June 2018 Icelandic Waters, and Norwegian Sea ecoregions https://doi.org/10.17895/ices.pub.4412
ICES Advice on fishing opportunities, catch, and effort Arctic Ocean, Barents Sea, Faroes, Greenland Sea, Published 13 June 2018 Icelandic Waters, and Norwegian Sea ecoregions https://doi.org/10.17895/ices.pub.4412
Beaked redfish (Sebastes mentella) in subareas 1 and 2 (Northeast Arctic)
 ICES Advice on fishing opportunities, catch, and effort Arctic Ocean, Barents Sea, Faroes, Greenland Sea, Published 28 September 2018 Icelandic Waters, and Norwegian Sea Ecoregions DOI: 10.17895/ices.pub.4538
ICES Advice on fishing opportunities, catch, and effort Arctic Ocean, Barents Sea, Faroes, Greenland Sea, Published 28 September 2018 Icelandic Waters, and Norwegian Sea Ecoregions DOI: 10.17895/ices.pub.4538
Feeding ecology of herring and mackerel in Icelandic waters
 Workshop on the Future Potential of Zooplankton and Mesopelagic Species October 1st - 2nd 2018, Reykjavík, Iceland Feeding ecology of herring and mackerel in Icelandic waters and other aspects of relevance
Workshop on the Future Potential of Zooplankton and Mesopelagic Species October 1st - 2nd 2018, Reykjavík, Iceland Feeding ecology of herring and mackerel in Icelandic waters and other aspects of relevance
Fishing mortality in relation to highest yield. Fishing mortality in relation to agreed target
 3.4 Stock summaries 3.4. Northeast Arctic cod State of the stock Spawning biomass in relation to precautionary limits Full reproductive capacity Fishing mortality in relation to precautionary limits/management
3.4 Stock summaries 3.4. Northeast Arctic cod State of the stock Spawning biomass in relation to precautionary limits Full reproductive capacity Fishing mortality in relation to precautionary limits/management
Freshwater Fisheries in Iceland
 1 Freshwater Fisheries in Iceland Árni Ísaksson Directorate of Freshwater Fisheries Introduction This paper gives a brief overview of the freshwater and salmonid resources in Iceland. There is a growing
1 Freshwater Fisheries in Iceland Árni Ísaksson Directorate of Freshwater Fisheries Introduction This paper gives a brief overview of the freshwater and salmonid resources in Iceland. There is a growing
Advice October 2014
 5.3.21.3 Advice October 2014 ECOREGION Celtic Sea and West of Scotland STOCK Nephrops on Porcupine Bank (FU 16) Advice for 2015 ICES advises on the basis of the MSY approach that catches from FU 16 in
5.3.21.3 Advice October 2014 ECOREGION Celtic Sea and West of Scotland STOCK Nephrops on Porcupine Bank (FU 16) Advice for 2015 ICES advises on the basis of the MSY approach that catches from FU 16 in
Council CNL(18)20. Salmon farming: NGOs demand that Governments honour the Williamsburg Resolution commitments. (Tabled by the NGOs)
 Agenda item 7.1 For information Council CNL(18)20 Salmon farming: NGOs demand that Governments honour the Williamsburg Resolution commitments (Tabled by the NGOs) CNL(18)20 Salmon farming: NGOs demand
Agenda item 7.1 For information Council CNL(18)20 Salmon farming: NGOs demand that Governments honour the Williamsburg Resolution commitments (Tabled by the NGOs) CNL(18)20 Salmon farming: NGOs demand
West Coast of Newfoundland Atlantic Herring (Division 4R)
 DFO Science Stock Status Report B-1 5 59 5 57 QUEBEC LOWER NORTH SHORE 51 Meters Ra STRAIT OF BELLE NEW FEROLLE ST. JOHN'S BAY POINT RICHE West Coast of Newfoundland Atlantic Herring (Division R) 5 BEAUGE
DFO Science Stock Status Report B-1 5 59 5 57 QUEBEC LOWER NORTH SHORE 51 Meters Ra STRAIT OF BELLE NEW FEROLLE ST. JOHN'S BAY POINT RICHE West Coast of Newfoundland Atlantic Herring (Division R) 5 BEAUGE
BSAC recommendations for the fishery in the Baltic Sea in 2018
 Copenhagen 7 th July 2017 BSAC recommendations for the fishery in the Baltic Sea in 2018 The BSAC recommends setting the catch levels for the Baltic stocks in 2018 at the values indicated in the table
Copenhagen 7 th July 2017 BSAC recommendations for the fishery in the Baltic Sea in 2018 The BSAC recommends setting the catch levels for the Baltic stocks in 2018 at the values indicated in the table
Eastern and South Shore Nova Scotia Lobster LFAs The Fishery. DFO Atlantic Fisheries Stock Status Report 96/117E.
 Maritimes Region DFO Atlantic Fisheries Stock Status Report 96/117E Eastern and South Shore Nova Scotia Lobster LFAs 31-33 Background Lobsters first entering the fishery in LFAs 31, 32, and 33 are probably
Maritimes Region DFO Atlantic Fisheries Stock Status Report 96/117E Eastern and South Shore Nova Scotia Lobster LFAs 31-33 Background Lobsters first entering the fishery in LFAs 31, 32, and 33 are probably
IP(08)03. Fisheries Management Focus Area Report. European Union - Finland
 IP(08)03 Fisheries Management Focus Area Report European Union - Finland Focus Area Report on Management of Salmon Fisheries EU - FINLAND 31 March 2008 1. Introduction In Finland, there are two rivers
IP(08)03 Fisheries Management Focus Area Report European Union - Finland Focus Area Report on Management of Salmon Fisheries EU - FINLAND 31 March 2008 1. Introduction In Finland, there are two rivers
