Drexel-SDP GK-12 LESSON
|
|
|
- Doreen Waters
- 5 years ago
- Views:
Transcription
1 Lesson: Pressure Drexel-SDP GK-1 LESSON Subject Area(s) Data Analysis & Probability, Measurement, Number & Operations, Physical Science, Science and Technology Associated Unit Vital Mechanics Lesson Title Pressure Many different forms of pressure used in a single activity. Grade Level 7 (6-1) Lesson # of 3 Lesson Dependency Time Required 0 minutes Summary Various types and applications of pressure are covered in the accompanying Powerpoint slides. This lesson is designed to enumerate the implications of pressure and how it is used. The associated activity relates blood pressure waveforms with sugar consumption, but the theme can is versatile and can be extended to other applications of pressure hydraulics and sound, for example.
2 Engineering Connection Pressure governs many biological and environmental processes, and engineers in particular have used the concepts to their advantage for many years. Similar to the previous lesson, Collisions, the energy of particles on the molecular level can be changed dramatically with pressure (gas laws). On a larger scale, pressure influences particle movement due to gravity (water tower), chemical reactions (engine pistons), and even weather patterns (areas of high and low pressure). Earthquakes, tsunamis, and sound are all caused by pressure waves that propagate through solids, liquids, and gases, respectively. The good news is that, besides acts of nature, we can control pressure and use it to generate electricity (hydroelectricity, wind power), water and septic systems, and create mechanical motion (engines). Among these uses, pressure occurs naturally in our body s circulatory system. When we have our blood pressure taken, the doctor or nurse reads our systolic and diastolic pressure as a ratio, like 10 over 80. This is measure of the pressure in the cuff (around our arm) necessary to close our brachial artery. Under normal conditions, our vasculature is a closed system and responds to stimuli just as temperature has an effect on an ideal gas in a container. Keywords pressure, gas law, heart rate, exercise, hydrostatic, hydraulic, pulmonary, cardiovascular Educational Standards PA Science: o Unifying themes o 3..7.B Apply process knowledge to make and interpret observations PA Math: o.1.8.d Apply ratio and proportion to mathematical problem situations involving distance, rate, time, and similar triangles o.4.5.b Use models, number facts, properties, and relationships to check and verify predictions and explain reasoning o.5.8.b Verify and interpret results using precise mathematical language, notation and representations, including numerical tables and equations, simple algebraic equations and formulas, charts, graphs, and diagrams o.7.8.d Compare and contrast results from observations and mathematical models o.8.8.b Discover, describe, and generalize patterns, including linear, exponential, and simple quadratic relationships
3 Pre-Requisite Knowledge Learning Objectives After this lesson, students should be able to: Identify applications that rely on pressure Discuss the effects of temperature on pressure Describe the effect of negative pressure (vacuum) on liquids and solids with trapped air spaces Predict the outcome of pressure effects on a material Introduction / Motivation [refer to Powerpoint slides] Ask students what they think of when they hear the word pressure. (Not necessarily how they would define pressure ). How have they heard it used what contexts? (Peer pressure, tire pressure, water pressure, etc.). How have they used pressure already today? (Shower, brush teeth, tire pressure in bus and car wheels, other ordinary events). Describe engineering definition of pressure and give examples of real-world applications (from Engineering Connection). Show slides on example applications (water tower, post-mix fountain soda dispenser, hydraulics, SCUBA and nose/ear airspaces under hydrostatic pressure, cardiovascular system) and ask students to point out where pressure exists. Several media clips can be played or demonstrated boiling water at room temperature under a vacuum, hard-boiled egg in the Erlenmeyer flask, candle in the inverted Erlenmeyer flask, quenching aluminum can with steam in ice-cold water bath. Other demonstrations can be done with a laboratory vacuum (consumer models and shop-vacs don t have the pull). Puffed marshmallows or marshmallow Peeps under vacuum will explode if the pressure drops quickly enough. Otherwise, marshmallows will enlarge as the relative pressure between air pockets and the chamber increases, after which they collapse then the air is sucked out of the pockets. Balloons under vacuum may enlarge or burst. Vocabulary / Definitions Word Definition Pressure (Engineering): Force per unit area (stress), acting perpendicular to a surface. Negative pressure. Vacuum Atmospheric pressure is 14.7 psi (pounds per square inch) The amount of pressure necessary to close a blood vessel (usually the brachial Blood Pressure artery), measured by a sphygmomamometer. Lesson Background & Concepts for Teachers Pressure is transferred in different ways, so there are several methods to analyze pressure changes. Let s start with the Ideal Gas Law definition from Chemistry: PV = nrt
4 where P is pressure, V is volume, T is temperature, and n is the number of moles of the gas (a measure of quantity) and R is the universal gas constant. Assuming that our initial amount of gas and the universal gas constant do not change, the relation simplifies to PV = T. This can be re-written to show a a change between two states, PV 1 1 T = P V. 1 T Consider the egg in the flask and candle in the flask clips. The egg in the flask demonstration involved heated air T 1 due to the match, and for the short time duration V 1 =V. After the match went out, the air temperature decreased (T ). Rearranging the last equation, P TV1 T = = P T V 1 1 T1 Since the pressure ratio is equal to the temperature ratio, it is clear that the temperature drop created the pressure drop that caused the egg to be pulled into the bottle. Likewise, the candle in the flask clip shows a candle burning for an extended amount of time in an inverted flask. Here the end result is a reduction in pressure that pulls fluid up the neck of the flask. If we use the same equations and logic, the candle heats the air (T >T 1 ), and consequently P <P 1 (pressure increases) if no volume effects are considered. In order to get the condition of P >P 1 (a reduction in pressure), the volume decrease (V 1 <V ) must be greater than temperature increase. So while the candle is burning the oxygen, it is decreasing the amount of gas volume at a rate greater than it is increasing the temperature, resulting in the pressure drop that pulls fluid up the neck of the flask. Another common usage of pressure besides gases applies to fluids. Cheap pressure gauges (pitot tubes) act on the basis of the hydrostatic pressure of the fluid. Concealed air pockets in submarines and on SCUBA divers are subject to hydrostatic pressures of water, which scales with density and height of a vertical water column, kg kg m kg 3 ( 9.81 m p = ρgh = 1000 )( l m) = 9810 l = 9810 l. 3 m s N kg m kg By the definition of pressure, 1 = 1 = 1 so the units check out. Please note that m pressure is always compressive, so it would carry a negative sign in an equation. One great example of a structure designed to withstand hydrostatic pressure at great depths is the Hoover Dam. While the top span is 144 feet long and 45 feet wide, the base of the dam is 660 feet wide ( football fields) extending into Lake Mead. More information on the engineering of the Hoover Dam can be found here. Associated Activities Amp d Up
5 Lesson Closure After the associated activity, Pump d Up, mention that heart rate is a physiological health factor if it is abnormal, it may be a sign of a health condition. At the same time, even if a heart rate is in the normal range, exposure to abnormal amounts of exercise might cause health complications. This is why gym equipment such as ellipticals, treadmills, and stationary bikes feature a heart rate monitor. Every person has a target heart rate for cardiovascular training a point that exercises the heart without causing too much stress (demand). A maximum heart rate is figured as HR target = 0 age and a target heart rate for exercise is factored as 50-85% of the maximum heart rate (AHA). Overuse injuries are the most common for athletes, which is why it is important to exercise in moderation and train or condition our bodies before participating in athletically demanding events. Failure to use a training regimen may not only cause cardiovascular complications, but it could also fatigue our joints and subject our bones, muscles, and ligaments to abnormal loadings (shin splints). Additional Multimedia Support vital_mechanics lesson_pressure_multimedia.zip Owner Drexel University GK-1 Program Contributors John C. Fitzpatrick, Mechanical Engineering and Mechanics, Drexel University Copyright Copyright 008 Drexel University GK-1 Program. Reproduction permission is granted for nonprofit educational use.
Section 5.1 Pressure. Why study gases? An understanding of real world phenomena. An understanding of how science works.
 Chapter 5 Gases Section 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage Learning. All rights reserved 2 Section 5.1 Pressure
Chapter 5 Gases Section 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage Learning. All rights reserved 2 Section 5.1 Pressure
Judith Herzfeld 1996,1998. These exercises are provided here for classroom and study use only. All other uses are copyright protected.
 Judith Herzfeld 1996,1998 These exercises are provided here for classroom and study use only. All other uses are copyright protected. 2.7-110 A yardstick laid on a table with a few inches hanging over
Judith Herzfeld 1996,1998 These exercises are provided here for classroom and study use only. All other uses are copyright protected. 2.7-110 A yardstick laid on a table with a few inches hanging over
Pressure of the atmosphere varies with elevation and weather conditions. Barometer- device used to measure atmospheric pressure.
 Chapter 12 Section 1 Pressure A gas exerts pressure on its surroundings. Blow up a balloon. The gas we are most familiar with is the atmosphere, a mixture of mostly elemental nitrogen and oxygen. Pressure
Chapter 12 Section 1 Pressure A gas exerts pressure on its surroundings. Blow up a balloon. The gas we are most familiar with is the atmosphere, a mixture of mostly elemental nitrogen and oxygen. Pressure
DO NOT, under any circumstances, throw this away! This packet MUST be saved for the final exam.
 Name: Period: Unit 2 Packet Energy and States of Matter Unit 2 Packet Contents Sheet (This Paper!) Unit 2 Objectives Notes: Kinetic Molecular Theory of Gases- 3 pgs (with Behavior of Gases Reading, and
Name: Period: Unit 2 Packet Energy and States of Matter Unit 2 Packet Contents Sheet (This Paper!) Unit 2 Objectives Notes: Kinetic Molecular Theory of Gases- 3 pgs (with Behavior of Gases Reading, and
Chapter 13. Gases. Copyright Cengage Learning. All rights reserved 1
 Chapter 13 Gases Copyright Cengage Learning. All rights reserved 1 Section 13.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage
Chapter 13 Gases Copyright Cengage Learning. All rights reserved 1 Section 13.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage
Lesson 12: Fluid statics, Continuity equation (Sections ) Chapter 9 Fluids
 Lesson : luid statics, Continuity equation (Sections 9.-9.7) Chapter 9 luids States of Matter - Solid, liquid, gas. luids (liquids and gases) do not hold their shapes. In many cases we can think of liquids
Lesson : luid statics, Continuity equation (Sections 9.-9.7) Chapter 9 luids States of Matter - Solid, liquid, gas. luids (liquids and gases) do not hold their shapes. In many cases we can think of liquids
Gases. Unit 10. How do gases behave?
 Gases Unit 10 How do gases behave? Gases are perhaps the most mysterious of all of the phases of matter. For the most part gases are invisible to us, and it was once believed that in the air there is no
Gases Unit 10 How do gases behave? Gases are perhaps the most mysterious of all of the phases of matter. For the most part gases are invisible to us, and it was once believed that in the air there is no
Practice Packet Unit 8: Gases
 Regents Chemistry: Mr. Palermo Practice Packet Unit 8: Gases Vocabulary: Lesson 1: Lesson 2: Lesson 3: Study Guide: 1 Vocabulary For each word, provide a short but specific definition from YOUR OWN BRAIN!
Regents Chemistry: Mr. Palermo Practice Packet Unit 8: Gases Vocabulary: Lesson 1: Lesson 2: Lesson 3: Study Guide: 1 Vocabulary For each word, provide a short but specific definition from YOUR OWN BRAIN!
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works.
 Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
Chemistry Chapter 12. Characteristics of Gases. Characteristics of Gases 1/31/2012. Gases and Liquids
 Importance of Gases Chemistry Chapter 12 Gases and Liquids Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide, NaN 3. 2 NaN 3 ---> 2 Na + 3 N 2 THREE STATES
Importance of Gases Chemistry Chapter 12 Gases and Liquids Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide, NaN 3. 2 NaN 3 ---> 2 Na + 3 N 2 THREE STATES
Name Class Date. What are some properties of gases? How do changes of pressure, temperature, or volume affect a gas?
 CHAPTER 3 States of Matter 4 Behavior of Gases SECTION KEY IDEAS As you read this section, keep these questions in mind: What are some properties of gases? How do changes of pressure, temperature, or volume
CHAPTER 3 States of Matter 4 Behavior of Gases SECTION KEY IDEAS As you read this section, keep these questions in mind: What are some properties of gases? How do changes of pressure, temperature, or volume
Gases and Pressure. Main Ideas
 Gases and Pressure Key Terms pressure millimeters of mercury partial pressure newton atmosphere of pressure Dalton s law of partial pressures barometer pascal In the chapter States of Matter, you read
Gases and Pressure Key Terms pressure millimeters of mercury partial pressure newton atmosphere of pressure Dalton s law of partial pressures barometer pascal In the chapter States of Matter, you read
Name /74. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch 11 Gases STUDY GUIDE Accelerated Chemistry SCANTRON Name /74 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements
Ch 11 Gases STUDY GUIDE Accelerated Chemistry SCANTRON Name /74 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements
3 1 PRESSURE. This is illustrated in Fig. 3 3.
 P = 3 psi 66 FLUID MECHANICS 150 pounds A feet = 50 in P = 6 psi P = s W 150 lbf n = = 50 in = 3 psi A feet FIGURE 3 1 The normal stress (or pressure ) on the feet of a chubby person is much greater than
P = 3 psi 66 FLUID MECHANICS 150 pounds A feet = 50 in P = 6 psi P = s W 150 lbf n = = 50 in = 3 psi A feet FIGURE 3 1 The normal stress (or pressure ) on the feet of a chubby person is much greater than
Chapter 10: Gases. Characteristics of Gases
 Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
Gases and Pressure SECTION 11.1
 SECTION 11.1 Gases and In the chapter States of Matter, you read about the kineticmolecular theory of matter. You were also introduced to how this theory explains some of the properties of ideal gases.
SECTION 11.1 Gases and In the chapter States of Matter, you read about the kineticmolecular theory of matter. You were also introduced to how this theory explains some of the properties of ideal gases.
Practice Packet Unit 8: Gases
 Name: Regents Chemistry: Practice Packet Unit 8: Gases www.chempride.weebly.com Vocabulary: Absolute Zero: Avogadro s Hypothesis: (Normal) Boiling Point: Direct Relationship: Evaporating: Gas: Ideal Gas:
Name: Regents Chemistry: Practice Packet Unit 8: Gases www.chempride.weebly.com Vocabulary: Absolute Zero: Avogadro s Hypothesis: (Normal) Boiling Point: Direct Relationship: Evaporating: Gas: Ideal Gas:
MS.RAJA ELGADY/PRESSURE PAPER 3
 1- (a) A water tank has a rectangular base of dimensions 1.5m by 1.2m and contains 1440 kg of water. Calculate (i) the weight of the water, weight =...... [1] (ii) the pressure exerted by the water on
1- (a) A water tank has a rectangular base of dimensions 1.5m by 1.2m and contains 1440 kg of water. Calculate (i) the weight of the water, weight =...... [1] (ii) the pressure exerted by the water on
Unit 9: Gas Laws REGENTS CHEMISTRY
 Name: Unit 9: Gas Laws REGENTS CHEMISTRY 1 Name: Unit 9: Gas Laws The concept of an ideal gas is a model to explain the behavior of gases. A real gas is most like an ideal gas when the real gas is at low
Name: Unit 9: Gas Laws REGENTS CHEMISTRY 1 Name: Unit 9: Gas Laws The concept of an ideal gas is a model to explain the behavior of gases. A real gas is most like an ideal gas when the real gas is at low
More Practice with Gas Laws KEY
 More Practice with Gas Laws KEY Chemistry Directions: For each question, identify the applicable law and solve for the correct answer using dimensional analysis. Express your answer to the correct number
More Practice with Gas Laws KEY Chemistry Directions: For each question, identify the applicable law and solve for the correct answer using dimensional analysis. Express your answer to the correct number
Gas volume and pressure are indirectly proportional.
 Section 2 The Gas Laws Key Terms Boyle s law Charles s law combined gas law absolute zero Gay-Lussac s law Scientists have been studying physical properties of gases for hundreds of years In 1662, Robert
Section 2 The Gas Laws Key Terms Boyle s law Charles s law combined gas law absolute zero Gay-Lussac s law Scientists have been studying physical properties of gases for hundreds of years In 1662, Robert
Activity Template. Drexel-SDP GK-12 ACTIVITY
 Activity Template Subject Area(s): Physical Science Associated Unit: Astronomy Associated Lesson: Waves Activity Title: Waves on a Slinky Grade Level 6 (6-8) Activity Dependency: None Time Required: 30
Activity Template Subject Area(s): Physical Science Associated Unit: Astronomy Associated Lesson: Waves Activity Title: Waves on a Slinky Grade Level 6 (6-8) Activity Dependency: None Time Required: 30
Additional Reading General, Organic and Biological Chemistry, by Timberlake, chapter 8.
 Gas Laws EXPERIMENTAL TASK Determine the mathematical relationship between the volume of a gas sample and its absolute temperature, using experimental data; and to determine the mathematical relationship
Gas Laws EXPERIMENTAL TASK Determine the mathematical relationship between the volume of a gas sample and its absolute temperature, using experimental data; and to determine the mathematical relationship
Introduction to Gas Laws Lab NAME: DATE: PERIOD:
 Introduction to Gas Laws Lab NAME: DATE: PERIOD: Background: In a gas, particles are spread far apart; therefore a gas takes up more volume than a solid or a liquid. For example, water in the form of steam
Introduction to Gas Laws Lab NAME: DATE: PERIOD: Background: In a gas, particles are spread far apart; therefore a gas takes up more volume than a solid or a liquid. For example, water in the form of steam
Pressure is defined as force per unit area. Any fluid can exert a force
 Physics Notes Chapter 9 Fluid Mechanics Fluids Fluids are materials that flow, which include both liquids and gases. Liquids have a definite volume but gases do not. In our analysis of fluids it is necessary
Physics Notes Chapter 9 Fluid Mechanics Fluids Fluids are materials that flow, which include both liquids and gases. Liquids have a definite volume but gases do not. In our analysis of fluids it is necessary
Name: Class: Date: SHORT ANSWER Answer the following questions in the space provided.
 CHAPTER 11 REVIEW Gases SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Pressure =. For a constant force, when the surface area is tripled the pressure is (a) doubled. (b)
CHAPTER 11 REVIEW Gases SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Pressure =. For a constant force, when the surface area is tripled the pressure is (a) doubled. (b)
To derive from experiment the relationships between Pressure (P), Volume (V), Temperature (T), and Water Solubility of gases.
 PROPERTIES OF GASES: PRESSURE, VOLUME, TEMPERATURE, & SOLUBILITY RELATIONSHIPS PURPOSE: To derive from experiment the relationships between Pressure (P), Volume (V), Temperature (T), and Water Solubility
PROPERTIES OF GASES: PRESSURE, VOLUME, TEMPERATURE, & SOLUBILITY RELATIONSHIPS PURPOSE: To derive from experiment the relationships between Pressure (P), Volume (V), Temperature (T), and Water Solubility
Temp in Kelvin = (Temp in O C) Temp in o C = (Temp in Kelvin) Perform the following conversions:
 Study: *Questions About Gases WS and More Questions About Gases WS *Go back over your notes taken from the book and in class. *Use your book: Chapter 3 PG 66-97 The Kelvin temperature of a gas (or any
Study: *Questions About Gases WS and More Questions About Gases WS *Go back over your notes taken from the book and in class. *Use your book: Chapter 3 PG 66-97 The Kelvin temperature of a gas (or any
Pressure Control. where: p is the pressure F is the normal component of the force A is the area
 Pressure Control First of all, what is pressure, the property we want to control? From Wikipedia, the free encyclopedia. Pressure is the application of force to a surface, and the concentration of that
Pressure Control First of all, what is pressure, the property we want to control? From Wikipedia, the free encyclopedia. Pressure is the application of force to a surface, and the concentration of that
End of Chapter Exercises
 End of Chapter Exercises Exercises 1 12 are conceptual questions that are designed to see if you have understood the main concepts of the chapter. 1. While on an airplane, you take a drink from your water
End of Chapter Exercises Exercises 1 12 are conceptual questions that are designed to see if you have understood the main concepts of the chapter. 1. While on an airplane, you take a drink from your water
Chapter 11 The Behavior of Gases
 Chapter 11 The Behavior of Gases 1 Section 11.1 The Properties of Gases Objectives: Explain why gases are easier to compress than solids or liquids are. Describe the three factors that affect gas pressure
Chapter 11 The Behavior of Gases 1 Section 11.1 The Properties of Gases Objectives: Explain why gases are easier to compress than solids or liquids are. Describe the three factors that affect gas pressure
PRESSURE-TEMPERATURE RELATIONSHIP IN GASES
 PRESSURE-TEMPERATURE RELATIONSHIP IN GASES LAB PS2.PALM INTRODUCTION Gases are made up of molecules that are in constant motion and exert pressure when they collide with the walls of their container. The
PRESSURE-TEMPERATURE RELATIONSHIP IN GASES LAB PS2.PALM INTRODUCTION Gases are made up of molecules that are in constant motion and exert pressure when they collide with the walls of their container. The
Experiment #12. Gas Laws.
 Goal To observe gas laws in the laboratory. Experiment #12. Gas Laws. Introduction All ideal gases, regardless of molar mass or chemical properties, follow the same gas laws under most conditions. Gas
Goal To observe gas laws in the laboratory. Experiment #12. Gas Laws. Introduction All ideal gases, regardless of molar mass or chemical properties, follow the same gas laws under most conditions. Gas
CHM111 Lab Gas Laws Grading Rubric
 Name Team Name CHM111 Lab Gas Laws Grading Rubric Criteria Points possible Points earned Lab Performance Printed lab handout and rubric was brought to lab 3 Safety and proper waste disposal procedures
Name Team Name CHM111 Lab Gas Laws Grading Rubric Criteria Points possible Points earned Lab Performance Printed lab handout and rubric was brought to lab 3 Safety and proper waste disposal procedures
Title: Solubility of Gas A Daily Experience. Subject: Chemistry. Grade Level: 10 th 12 th
 Title: Solubility of Gas A Daily Experience Subject: Chemistry Grade Level: 10 th 12 th Rational or Purpose: This lesson brings an everyday life experience to students knowledge on solubility of gas in
Title: Solubility of Gas A Daily Experience Subject: Chemistry Grade Level: 10 th 12 th Rational or Purpose: This lesson brings an everyday life experience to students knowledge on solubility of gas in
Drexel-SDP GK-12 ACTIVITY
 Drexel-SDP GK-12 ACTIVITY Subject Area(s): Astronomy, Gravity, Friction, Momentum Associated Unit: None Lesson Title: Cars, Gravity, Momentum and Friction Header: Microsoft Word drawn image of a car consisting
Drexel-SDP GK-12 ACTIVITY Subject Area(s): Astronomy, Gravity, Friction, Momentum Associated Unit: None Lesson Title: Cars, Gravity, Momentum and Friction Header: Microsoft Word drawn image of a car consisting
Chapter 14-Gases. Dr. Walker
 Chapter 14-Gases Dr. Walker State of Matter Gases are one of the four states of matter along with solids, liquids, and plasma Conversion to Gases From liquids Evaporation Example: Boiling water From solids
Chapter 14-Gases Dr. Walker State of Matter Gases are one of the four states of matter along with solids, liquids, and plasma Conversion to Gases From liquids Evaporation Example: Boiling water From solids
Gas Laws. Essential Learning Outcomes: 1. Change can be measured. 2. Changes can occur within a substance that alters its identity.
 Gas Laws Gas Laws: Gases and pressures affect our lives every day. From the weather we experience to the air we breathe, it all has to do with gases and pressures. Why do we have wind? Why do we have the
Gas Laws Gas Laws: Gases and pressures affect our lives every day. From the weather we experience to the air we breathe, it all has to do with gases and pressures. Why do we have wind? Why do we have the
EXPERIMENT 8 Ideal Gas Law: Molecular Weight of a Vapor
 EXPERIMENT 8 Ideal Gas Law: Molecular Weight of a Vapor Purpose: In this experiment you will use the ideal gas law to calculate the molecular weight of a volatile liquid compound by measuring the mass,
EXPERIMENT 8 Ideal Gas Law: Molecular Weight of a Vapor Purpose: In this experiment you will use the ideal gas law to calculate the molecular weight of a volatile liquid compound by measuring the mass,
Gases. Edward Wen, PhD
 Gases Edward Wen, PhD Properties of Gases expand to completely fill their container take the shape of their container low density much less than solid or liquid state compressible when pressure is changed.
Gases Edward Wen, PhD Properties of Gases expand to completely fill their container take the shape of their container low density much less than solid or liquid state compressible when pressure is changed.
The Kinetic-Molecular Theory of Gases based on the idea that particles are always in motion
 The Kinetic-Molecular Theory of Gases based on the idea that particles are always in motion Five assumptions: 1. Most of the volume occupied dby a gas is empty space 2. Collisions between gas particles
The Kinetic-Molecular Theory of Gases based on the idea that particles are always in motion Five assumptions: 1. Most of the volume occupied dby a gas is empty space 2. Collisions between gas particles
You should be able to: Describe Equipment Barometer Manometer. 5.1 Pressure Read and outline 5.1 Define Barometer
 A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
Unit 14 Gas Laws Funsheets
 Name: Period: Unit 14 Gas Laws Funsheets Part A: Vocabulary and Concepts- Answer the following questions. Refer to your notes and the PowerPoint for help. 1. List 5 different common uses for gases: a.
Name: Period: Unit 14 Gas Laws Funsheets Part A: Vocabulary and Concepts- Answer the following questions. Refer to your notes and the PowerPoint for help. 1. List 5 different common uses for gases: a.
Chapter 10 Gases. Characteristics of Gases. Pressure. The Gas Laws. The Ideal-Gas Equation. Applications of the Ideal-Gas Equation
 Characteristics of Gases Chapter 10 Gases Pressure The Gas Laws The Ideal-Gas Equation Applications of the Ideal-Gas Equation Gas mixtures and partial pressures Kinetic-Molecular Theory Real Gases: Deviations
Characteristics of Gases Chapter 10 Gases Pressure The Gas Laws The Ideal-Gas Equation Applications of the Ideal-Gas Equation Gas mixtures and partial pressures Kinetic-Molecular Theory Real Gases: Deviations
What is Boyle s law and how can it be demonstrated?
 Name: Relationship Between Gas Variables Gas Laws Simulation Introduction: Scientists in the late 1800 s noted relationships between various variables related to gases (pressure, volume, temperature),
Name: Relationship Between Gas Variables Gas Laws Simulation Introduction: Scientists in the late 1800 s noted relationships between various variables related to gases (pressure, volume, temperature),
CHEMISTRY - CLUTCH CH.5 - GASES.
 !! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
!! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
States of Matter Review
 States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
11.1 Dumas Method - Pre-Lab Questions
 11.1 Dumas Method - Pre-Lab Questions Name: Instructor: Date: Section/Group: Show all work for full credit. 1. If a 275-mL gas container has pressure of 732.6 mm Hg at -28 C, how many moles of gas are
11.1 Dumas Method - Pre-Lab Questions Name: Instructor: Date: Section/Group: Show all work for full credit. 1. If a 275-mL gas container has pressure of 732.6 mm Hg at -28 C, how many moles of gas are
Example: 25 C = ( ) K = 298 K. Pressure Symbol: p Units: force per area 1Pa (Pascal) = 1 N/m 2
 Chapter 6: Gases 6.1 Measurements on Gases MH5, Chapter 5.1 Let s look at a certain amount of gas, i.e. trapped inside a balloon. To completely describe the state of this gas one has to specify the following
Chapter 6: Gases 6.1 Measurements on Gases MH5, Chapter 5.1 Let s look at a certain amount of gas, i.e. trapped inside a balloon. To completely describe the state of this gas one has to specify the following
Chapter 13 Fluids. Copyright 2009 Pearson Education, Inc.
 Chapter 13 Fluids Phases of Matter Density and Specific Gravity Pressure in Fluids Atmospheric Pressure and Gauge Pressure Pascal s Principle Units of Chapter 13 Measurement of Pressure; Gauges and the
Chapter 13 Fluids Phases of Matter Density and Specific Gravity Pressure in Fluids Atmospheric Pressure and Gauge Pressure Pascal s Principle Units of Chapter 13 Measurement of Pressure; Gauges and the
Page 1. Question 17.1a Nitrogen and Oxygen I. Question 17.1b Nitrogen and Oxygen II. Question 17.2a Ideal Gas Law I
 ConcepTest Clicker Questions Chapter 17 Question 17.1a Nitrogen and Oxygen I Which has more molecules a mole of nitrogen (N 2 ) gas or a mole of oxygen (O 2 ) gas? Physics, 4 th Edition James S. Walker
ConcepTest Clicker Questions Chapter 17 Question 17.1a Nitrogen and Oxygen I Which has more molecules a mole of nitrogen (N 2 ) gas or a mole of oxygen (O 2 ) gas? Physics, 4 th Edition James S. Walker
Procedure 1: Volume vs. Pressure 1.) Using the lap tops, go to the Physics Education Technology from the University of Colorado at:
 Deriving the Gas Laws Background The gaseous state of matter consists of particles (gas molecules like oxygen, nitrogen, and carbon dioxide) which, according to the kinetic theory of gases, are in constant
Deriving the Gas Laws Background The gaseous state of matter consists of particles (gas molecules like oxygen, nitrogen, and carbon dioxide) which, according to the kinetic theory of gases, are in constant
CHEM1901/3 Worksheet 8: The Ideal Gas Law: PV = nrt
 CHEM1901/3 Worksheet 8: The Ideal Gas Law: PV = nrt The Ideal Gas Law Model 1: The Gas Laws T (K) Kelvin or absolute temperature = T ( C) + 273. T(K) is always 0 K Boyle s Law (1660). The volume of a gas
CHEM1901/3 Worksheet 8: The Ideal Gas Law: PV = nrt The Ideal Gas Law Model 1: The Gas Laws T (K) Kelvin or absolute temperature = T ( C) + 273. T(K) is always 0 K Boyle s Law (1660). The volume of a gas
Chemistry HP Unit 6 Gases. Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases
 Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Name Chemistry Pre-AP
 Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Chapter 13: The Behavior of Gases
 Chapter 13: The Behavior of Gases I. First Concepts a. The 3 states of matter most important to us: solids, liquids, and gases. b. Real Gases and Ideal Gases i. Real gases exist, ideal gases do not ii.
Chapter 13: The Behavior of Gases I. First Concepts a. The 3 states of matter most important to us: solids, liquids, and gases. b. Real Gases and Ideal Gases i. Real gases exist, ideal gases do not ii.
Chemistry A Molecular Approach. Fourth Edition. Chapter 5. Gases. Copyright 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
 Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Kinetic-Molecular Theory
 GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
To convert to millimeters of mercury, we derive a unit factor related to the equivalent relationship 29.9 in. Hg = 760 mm Hg.
 Example Exercise 11.1 Gas Pressure Conversion Meteorologists state that a falling barometer indicates an approaching storm. Given a barometric pressure of 27.5 in. Hg, express the pressure in each of the
Example Exercise 11.1 Gas Pressure Conversion Meteorologists state that a falling barometer indicates an approaching storm. Given a barometric pressure of 27.5 in. Hg, express the pressure in each of the
PHY100 s Lab: The Relationship Between Liquid Pressure and Depth as it applies to Blood Pressure. Course & Sec:
 PHY100 s Lab: The Relationship Between Liquid Pressure and Depth as it applies to Blood Pressure Name: Lab Partner: Course & Sec: Date: Disclaimer: The procedures in this lab are not according to proper
PHY100 s Lab: The Relationship Between Liquid Pressure and Depth as it applies to Blood Pressure Name: Lab Partner: Course & Sec: Date: Disclaimer: The procedures in this lab are not according to proper
Introduction. Objectives. Hazards. Procedure
 Experiment: Exploring Gases Note to Students: Check with your instructor to see which parts of this lab (Parts A, B, or C) you will complete. Introduction Gases are made up of molecules that are in constant
Experiment: Exploring Gases Note to Students: Check with your instructor to see which parts of this lab (Parts A, B, or C) you will complete. Introduction Gases are made up of molecules that are in constant
SCH3U7 Quantitative Chemistry
 SCH3U7 Quantitative Chemistry So far, we have looked at solids and liquids (solutions) Today we will look at gases and the laws that govern their behaviour in chemical reactions 4 Factors Affecting Gases
SCH3U7 Quantitative Chemistry So far, we have looked at solids and liquids (solutions) Today we will look at gases and the laws that govern their behaviour in chemical reactions 4 Factors Affecting Gases
8.1 Properties of Gases. Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases.
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8. Chapter 8
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases. Chapter 8. Chapter 8. Gases Properties of Gases. We are surrounded by gases, but we are often
 Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
Guide for Reading. Vocabulary compressibility
 14.1 Properties of Gases Connecting to Your World In organized soccer, there are rules about equipment. For international competitions, the ball s mass must be not more than 450 grams and not less than
14.1 Properties of Gases Connecting to Your World In organized soccer, there are rules about equipment. For international competitions, the ball s mass must be not more than 450 grams and not less than
I. Introduction. Lesson title: How does pressure effect a scuba diver at different depths?
 I. Introduction Lesson title: How does pressure effect a scuba diver at different depths? Grade level audience: Regents Chemistry 11th Grade Lesson overview: Students have been introduced to the definition
I. Introduction Lesson title: How does pressure effect a scuba diver at different depths? Grade level audience: Regents Chemistry 11th Grade Lesson overview: Students have been introduced to the definition
Comments on Homework. Class 4 - Pressure. Atmospheric Pressure. Gauge vs. Absolute Pressure. 2. Gauge vs. Absolute Pressure. 1.
 Class 4 - Pressure 1. Definitions 2. Gauge Pressure 3. Pressure and Height of Liquid Column (Head) 4. Pressure Measurement and Manometers Please don t forget the special problem for the next HW assignment
Class 4 - Pressure 1. Definitions 2. Gauge Pressure 3. Pressure and Height of Liquid Column (Head) 4. Pressure Measurement and Manometers Please don t forget the special problem for the next HW assignment
Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases
![Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases](/thumbs/78/77082586.jpg) Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] Chapter 10 Gases We have talked a little about gases in Chapter 3 and we dealt briefly with them in our stoichiometric calculations in
Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] Chapter 10 Gases We have talked a little about gases in Chapter 3 and we dealt briefly with them in our stoichiometric calculations in
Chemistry 20 Unit 2 Gases FITB Notes. Topic A Characteristics of Gases
 Chemistry 20 Unit 2 Gases FITB Notes General Outcome: Topic A Characteristics of Gases We use technologies that were designed with the knowledge of the visible characteristics ( ) of gases ex. SCUBA equipment,
Chemistry 20 Unit 2 Gases FITB Notes General Outcome: Topic A Characteristics of Gases We use technologies that were designed with the knowledge of the visible characteristics ( ) of gases ex. SCUBA equipment,
PHYS:1200 LECTURE 13 FLUIDS (2)
 1 PHYS:1200 LECTURE 13 FLUIDS (2) Lecture 13 deals with the properties of fluids at rest or fluid statics. We will be discussing mostly liquids and will introduce two important principles of fluid statics:
1 PHYS:1200 LECTURE 13 FLUIDS (2) Lecture 13 deals with the properties of fluids at rest or fluid statics. We will be discussing mostly liquids and will introduce two important principles of fluid statics:
Liquids and Gases. 2/26/2012 Physics 214 Fall
 Liquids and Gases The unit of volume is the meter cubed, m 3, which is a very large volume. Very often we use cm 3 = cc. Other everyday units are gallons, quarts, pints As we know liquids and gases act
Liquids and Gases The unit of volume is the meter cubed, m 3, which is a very large volume. Very often we use cm 3 = cc. Other everyday units are gallons, quarts, pints As we know liquids and gases act
Chapter 5. Pressure. Atmospheric Pressure. Gases. Force Pressure = Area
 Chapter 5 Gases Water for many homes is supplied by a well The pump removes air from the pipe, decreasing the air pressure in the pipe The pressure then pushes the water up the pipe Pressure Atmospheric
Chapter 5 Gases Water for many homes is supplied by a well The pump removes air from the pipe, decreasing the air pressure in the pipe The pressure then pushes the water up the pipe Pressure Atmospheric
End of Chapter Exercises
 End of Chapter Exercises Exercises 1 12 are conceptual questions that are designed to see if you have understood the main concepts of the chapter. 1. While on an airplane, you take a drink from your water
End of Chapter Exercises Exercises 1 12 are conceptual questions that are designed to see if you have understood the main concepts of the chapter. 1. While on an airplane, you take a drink from your water
AP B Fluids Practice Problems. Multiple Choice. Slide 2 / 43. Slide 1 / 43. Slide 4 / 43. Slide 3 / 43. Slide 6 / 43. Slide 5 / 43
 Slide 1 / 43 Slide 2 / 43 P Fluids Practice Problems Multiple hoice Slide 3 / 43 1 Two substances mercury with a density 13600 kg/m 3 and alcohol with a density 0.8 kg/m 3 are selected for an experiment.
Slide 1 / 43 Slide 2 / 43 P Fluids Practice Problems Multiple hoice Slide 3 / 43 1 Two substances mercury with a density 13600 kg/m 3 and alcohol with a density 0.8 kg/m 3 are selected for an experiment.
Air exerts a on the walls of its container. Air Pressure is due to the of the molecules in the air as they
 Lesson 1,2: What is Air Pressure? Air exerts a on the walls of its container. This is often called Pressure. Air Pressure is due to the of the molecules in the air as they collide with the walls. Air has.
Lesson 1,2: What is Air Pressure? Air exerts a on the walls of its container. This is often called Pressure. Air Pressure is due to the of the molecules in the air as they collide with the walls. Air has.
Properties of Fluids SPH4C
 Properties of Fluids SPH4C Fluids Liquids and gases are both fluids: a fluid is any substance that flows and takes the shape of its container. Fluids Liquids and gases are both fluids: a fluid is any substance
Properties of Fluids SPH4C Fluids Liquids and gases are both fluids: a fluid is any substance that flows and takes the shape of its container. Fluids Liquids and gases are both fluids: a fluid is any substance
Chapter 5. Nov 6 1:02 PM
 Chapter 5 Nov 6 1:02 PM Expand to fill their containers Fluid motion (they flow) Have low densities (1/1000 the density of equivalent liquids or solids) Compressible Can Effuse and Diffuse Effuse: The
Chapter 5 Nov 6 1:02 PM Expand to fill their containers Fluid motion (they flow) Have low densities (1/1000 the density of equivalent liquids or solids) Compressible Can Effuse and Diffuse Effuse: The
Variation of Pressure with Depth in a Fluid *
 OpenStax-CNX module: m42192 1 Variation of Pressure with Depth in a Fluid * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract Dene
OpenStax-CNX module: m42192 1 Variation of Pressure with Depth in a Fluid * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract Dene
Chem 110 General Principles of Chemistry
 CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
PSI Chemistry: Gases Multiple Choice Review
 PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
13.1!"#$#%"&'%()$*+%,+-.$+/*$#
 343%%%%%%%%%5)"./$+%67%%%%%!"#$# 13.1!"#$#%"&'%()$*+%,+-.$+/*$#!"#$%&'($)*!"#$%&'($)+ If you want to understand how gases behave such as why fresh air rushes into your lungs when certain chest muscles
343%%%%%%%%%5)"./$+%67%%%%%!"#$# 13.1!"#$#%"&'%()$*+%,+-.$+/*$#!"#$%&'($)*!"#$%&'($)+ If you want to understand how gases behave such as why fresh air rushes into your lungs when certain chest muscles
Gauge Pressure, Absolute Pressure, and Pressure Measurement
 Gauge Pressure, Absolute Pressure, and Pressure Measurement By: OpenStax College Online: This module is copyrig hted by Rice University. It is licensed under the Creative
Gauge Pressure, Absolute Pressure, and Pressure Measurement By: OpenStax College Online: This module is copyrig hted by Rice University. It is licensed under the Creative
Name: Chapter 13: Gases
 Name: Chapter 13: Gases Gases and gas behavior is one of the most important and most fun things to learn during your year in chemistry. Here are all of the gas notes and worksheets in two packets. We will
Name: Chapter 13: Gases Gases and gas behavior is one of the most important and most fun things to learn during your year in chemistry. Here are all of the gas notes and worksheets in two packets. We will
Pulmonary Circulation
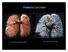 Pulmonary Circulation resin cast of pulmonary arteries resin cast of pulmonary veins Blood Flow to the Lungs Pulmonary Circulation Systemic Circulation Blood supply to the conducting zone provided by the
Pulmonary Circulation resin cast of pulmonary arteries resin cast of pulmonary veins Blood Flow to the Lungs Pulmonary Circulation Systemic Circulation Blood supply to the conducting zone provided by the
UNIT 2 FLUIDS PHYS:1200 LECTURE 12 FLUIDS (1)
 1 UNIT 2 FLUIDS PHYS:1200 LECTURE 12 FLUIDS (1) Lecture 12 is the first lecture on the new topic of fluids. Thus far we have been discussing the physics of ideal solid objects that do not change their
1 UNIT 2 FLUIDS PHYS:1200 LECTURE 12 FLUIDS (1) Lecture 12 is the first lecture on the new topic of fluids. Thus far we have been discussing the physics of ideal solid objects that do not change their
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *8269683414* PHYSICS 0625/31 Paper 3 Extended October/November 2014 1 hour 15 minutes Candidates
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *8269683414* PHYSICS 0625/31 Paper 3 Extended October/November 2014 1 hour 15 minutes Candidates
World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases
![World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases](/thumbs/96/127526823.jpg) World of Chemistry Notes for Students [Chapter 3, page ] Chapter 3 Gases ) Sec 3.8 Kinetic Theory of Gases and the Nature of Gases The Kinetic Theory of Matter says that the tiny particles in all forms
World of Chemistry Notes for Students [Chapter 3, page ] Chapter 3 Gases ) Sec 3.8 Kinetic Theory of Gases and the Nature of Gases The Kinetic Theory of Matter says that the tiny particles in all forms
2. Calculate the ratio of diffusion rates for carbon monoxide (CO) and carbon dioxide (CO2). υa = MB = 44 = 1.25
 Gas laws worksheet (2-08) (modified 3/17) Answer key Graham s Law 1. Calculate the ratio of effusion rates for nitrogen (N2) and neon (Ne). υa = MB = 20 = 0.845 υb MA 28 2. Calculate the ratio of diffusion
Gas laws worksheet (2-08) (modified 3/17) Answer key Graham s Law 1. Calculate the ratio of effusion rates for nitrogen (N2) and neon (Ne). υa = MB = 20 = 0.845 υb MA 28 2. Calculate the ratio of diffusion
Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases.
 Chapter 8 Gases Practice Problems Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases. Summary: In a gas, particles are so far
Chapter 8 Gases Practice Problems Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases. Summary: In a gas, particles are so far
Section 3: Fluids. States of Matter Section 3. Preview Key Ideas Bellringer Pressure
 Section 3: Fluids Preview Key Ideas Bellringer Pressure Buoyant Force Comparing Weight and Buoyant Force Pascal s Principle Math Skills Fluids in Motion Key Ideas How do fluids exert pressure? What force
Section 3: Fluids Preview Key Ideas Bellringer Pressure Buoyant Force Comparing Weight and Buoyant Force Pascal s Principle Math Skills Fluids in Motion Key Ideas How do fluids exert pressure? What force
Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops
 C h e m i s t r y 1 2 C h a p t e r 11 G a s e s P a g e 1 Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops Gas Properties: Gases have high kinetic energy low
C h e m i s t r y 1 2 C h a p t e r 11 G a s e s P a g e 1 Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops Gas Properties: Gases have high kinetic energy low
Science of Diving: How Science Helps Navy Divers Stay Safe
 Science of Diving: How Science Helps Navy Divers Stay Safe Science Topic: Physiology and Physics Grades: 9 th 12 th Essential Questions: What conditions lead to perils facing divers including the bends,
Science of Diving: How Science Helps Navy Divers Stay Safe Science Topic: Physiology and Physics Grades: 9 th 12 th Essential Questions: What conditions lead to perils facing divers including the bends,
Comments on Homework. Quiz. Class 3 - Pressure. Atmospheric Pressure. 2. Gauge vs. Absolute Pressure. 1. Definitions. Temperature conversion
 Comments on Homework Quiz Temperature conversion T ( R) = T (K) 1.8 T ( C) = T(K) - 273.15 T ( F) = T( R) - 460 However, difference in temperature is: T ( C) = T (K) T ( F) = T ( R) T ( R) = 1.8 T ( C)
Comments on Homework Quiz Temperature conversion T ( R) = T (K) 1.8 T ( C) = T(K) - 273.15 T ( F) = T( R) - 460 However, difference in temperature is: T ( C) = T (K) T ( F) = T ( R) T ( R) = 1.8 T ( C)
Introductory Physics PHYS101
 Introductory Physics PHYS101 Dr Richard H. Cyburt Office Hours Assistant Professor of Physics My office: 402c in the Science Building My phone: (304) 384-6006 My email: rcyburt@concord.edu TRF 9:30-11:00am
Introductory Physics PHYS101 Dr Richard H. Cyburt Office Hours Assistant Professor of Physics My office: 402c in the Science Building My phone: (304) 384-6006 My email: rcyburt@concord.edu TRF 9:30-11:00am
A) It cannot be predicted. B) It is squared. C) It is doubled. D) It is halved. E) It does not change.
 AP Chemistry Test (Chapter 5) Class Set Multiple Choice (50%) 1) A sample of argon gas is sealed in a container. The volume of the container is doubled at a constant temperature. What happens to the pressure
AP Chemistry Test (Chapter 5) Class Set Multiple Choice (50%) 1) A sample of argon gas is sealed in a container. The volume of the container is doubled at a constant temperature. What happens to the pressure
Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes:
 Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
Gases Chapter 11 (and 10)
 Gases Chapter 11 (and 10) Warm up 1. What is a gas? 2. What is pressure? 3. What units are used to measure pressure? Properties of Gas Expansion: indefinite shape and volume Fluidity: particle move pass
Gases Chapter 11 (and 10) Warm up 1. What is a gas? 2. What is pressure? 3. What units are used to measure pressure? Properties of Gas Expansion: indefinite shape and volume Fluidity: particle move pass
IT S A GAS
 IT S A GAS IT S A GAS The Nature of Gases Gases have some interesting characteristics that have fascinated scientists for 300 years. The first gas to be studied was air & it was a long time before it was
IT S A GAS IT S A GAS The Nature of Gases Gases have some interesting characteristics that have fascinated scientists for 300 years. The first gas to be studied was air & it was a long time before it was
1 Fluids and Pressure
 CHAPTER 3 1 Fluids and Pressure SECTION Forces in Fluids BEFORE YOU READ After you read this section, you should be able to answer these questions: What are fluids? What is atmospheric pressure? What is
CHAPTER 3 1 Fluids and Pressure SECTION Forces in Fluids BEFORE YOU READ After you read this section, you should be able to answer these questions: What are fluids? What is atmospheric pressure? What is
