Nine year follow up of a rare case of angioedema due to acquired C1 inhibitor deficiency with late onset and good response to attenuated androgen
|
|
|
- Meryl Reeves
- 5 years ago
- Views:
Transcription
1 Leru et al. Allergy Asthma Clin Immunol (2018) 14:69 Allergy, Asthma & Clinical Immunology CASE REPORT Open Access Nine year follow up of a rare case of angioedema due to acquired C1 inhibitor deficiency with late onset and good response to attenuated androgen Polliana Mihaela Leru 1,2*, Vlad Florin Anton 2 and Horia Bumbea 1,3 Abstract Background: Angioedema due to acquired deficiency of C1-inhibitor (C1-INH-AAE) is a rare disease sharing some clinical and laboratory similarities with hereditary angioedema, but with late onset and no positive family history. The underlining cause may be malignant or due to autoimmune diseases, but some cases remain idiopathic. Case presentation: We report a case of a 75 year old woman suffering from recurrent episodes of angioedema since the age of 66, considered first induced by treatment with angiotensin-converting-enzyme inhibitors (ACEI). She continued to have angioedema attacks during 6 years after discontinuation of ACEI, until evaluation in our clinic in 2014, when C1 inhibitor esterase (C1-INH) deficiency was confirmed. The extended medical evaluation for inflammatory, allergic, autoimmune and neoplasic diseases was negative. C1-INH and complement fraction C4 plasma levels were significantly decreased at all measurements, but no diagnostic criteria for diseases known to induce C1-INH deficiency could be found. We first initiated daily prophylactic treatment with tranexamic acid, with no amelioration after 3 months. During the last and most severe attack, with the first facial and laryngeal edema, we have switched to attenuated androgen danazol. The evolution was very good, with prompt remission of angioedema and significant increase of C1-INH and C4 plasma levels after 2 weeks of daily danazol use. She completed 3 years of continuous treatment with low daily maintenance dose of danazol (ongoing), with no angioedema attack. We closely monitored C1-INH and C4 plasma levels, possible danazol side effects and any signs suggesting late onset of C1-INH deficiency causal disease. Conclusion: We reported a particular case of rare angioedema due to acquired deficiency of C1-inhibitor, which has no clear cause after long follow-up, but good response to attenuated androgen. We concluded that the awareness of angioedema due to C1-INH deficiency should be increased within medical community and therapeutic options should be more clearly indicated and available for all diagnosed cases. Keywords: Acquired angioedema, Attenuated androgens, C1 inhibitor deficiency *Correspondence: polianaleru@yahoo.com 2 Internal Medicine Department, Colentina Clinical Hospital, Sos. Stefan cel Mare, no , District 2, Bucharest, Romania Full list of author information is available at the end of the article The Author(s) This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( iveco mmons.org/licen ses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( iveco mmons.org/ publi cdoma in/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
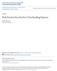
2 Page 2 of 5 Background Angioedema not accompanied by urticaria is a distinct and potentially severe disease, which has many hereditary or acquired forms, raising difficult problems in medical practice. According to the recent classification of angioedema without urticaria, four types of acquired (AAE) and three types of hereditary angioedema (HAE) were identified as separate forms [1]. Based on the cause and mechanism, acquired angioedema without wheals may be: idiopathic histaminergic, idiopathic non-histaminergic, related to angiotensin-converting enzyme inhibitors and due to C1-INH deficiency [2]. The awareness of non-allergic (non-histamine-mediated) angioedema within medical community is very low, since most cases of angioedema accompanied or not by urticaria are generally considered allergies. Angioedema due to acquired deficiency of C1-INH (C1-INH-AAE) is a rare disease that may have some clinical and laboratory similarities with hereditary angioedema, but without family history and with onset after the age of 40 years. The entity was first described in 1972 by Caldwell, who reported two patients with acquired C1-INH deficiency associated with lymphosarcoma and paraproteinemia, one having a clinical picture similar to HAE [3]. Prevalence of angioedema due to acquired deficiency of C1-INH is lower than that of hereditary forms, being estimated at 1:10 of that of HAE, meaning around 1:500,000 [4]. In most of the cases, the acquired C1-INH deficiency is secondary to malignant tumors, usually lymphoma or to autoimmune disorders such as systemic lupus erythematosus. The pathophysiologic mechanisms are consumption of C1-INH and classical pathway complement components activation of contact system and bradikinin release, during attacks [5]. Autoantibodies neutralizing C1-INH function could be found in collagen vascular diseases [6]. In approximately 15% of cases, considered idiopathic, the cause of C1-INH deficiency remains unknown and angioedema may raise severe clinical and therapeutic problems [7]. The clinical picture consists in recurrent episodes of angioedema of the face, tongue and upper airways, although any part of the body can be involved [8]. Gastrointestinal swelling attacks are less common in C1-INH-AAE patients compared with HAE cases [9]. Laboratory tests confirming diagnosis are reduced C1-INH plasma levels and/or activity of C1-INH below 50%. Decreased plasma levels of complement fraction C4 and CH50 are regularly observed. Significant reduction of C4 plasma levels is almost invariably present during angioedema attacks. C1q is also frequently decreased in AAE, but is normal in HAE. The presence of cleaved C1-INH may give apparently normal C1-INH antigen in about 20% cases, making the diagnosis even more difficult [10]. Case presentation We report a case of a 75 year old woman addressed to Allergology Department of our hospital in January 2014 for recurrent episodes of angioedema since the age of 66, with progressively increased severity and frequency. It was first considered to be induced by treatment with angiotensin-converting-enzyme inhibitors (ACEI) for mild hypertension, but she continued to have angioedema attacks for the next 6 years after discontinuation of ACE, with progressive aggravation during the last year. The previous multiple evaluations by many specialists in other hospitals did not succeed to give a clear diagnosis and treatment. The patient had no relevant medical history and took no medication, except ACEI that was stopped some months after angioedema onset. No relation with possible allergic stimuli could be identified and she had no clinical manifestations between attacks. Angioedema was painful, not accompanied by urticaria or abdominal symptoms, located variably to neck, arms or buttocks, without facial involvement during 6 years. The attacks occurred at weeks or months intervals and usually lasted between 48 and 72 h, irrespective of corticosteroids and antihistamines treatment usually administered. The frequency of attacks had progressively increased from one at 2 3 months intervals to almost weekly during the last year before presentation. The last angioedema attack, determining emergency hospitalization in September 2014, was more severe and prolonged, accompanied for the first time by laryngeal edema and respiratory symptoms. The extended medical evaluation, including complete blood tests for inflammation, allergy, autoimmunity and cancer, were all negative (Table 1). Full body CT scan and bone marrow examination were normal. No criteria for lymphoproliferative, mieloproliferative or autoimmune diseases could be found. Measurement of C1 inhibitor (C1INH) in plasma showed significantly decreased level at all measurements, with low activity ranging from 58 to 4% and constantly low C4 (Fig. 1). Complement fraction C1q plasma level was measured twice and had normal value. Genetic tests were not performed, given the patient advanced age and lack of family history of angioedema, which are against HAE. A spontaneous mutation in SERPING 1 gene is noticed in up to 25% cases of HAE without family history, but we considered this probability very low in our case, due to late onset of angioedema. Treatment of angioedema attacks before hospitalization consisted of antihistamines and systemic corticosteroids, which proved to be ineffective. Since no
3 Page 3 of 5 Table 1 Laboratory tests for positive and differential diagnosis Date Laboratory tests Other investigations 2008 ANA negative Anticardiolipin antibody normal Rheumatoid factor normal 2009 Serum diamineoxidase (DAO) normal Colonoscopy diverticulitis Mammography normal Skin prick test normal Stop ACEI 2010 HBsAg, HCV-Ac negative Laparoscopic cholecystectomy 2011 CA 15.3, CA19.9, CEA normal Papanicolau test negative 2012 C1q normal Papanicolau test negative 2013 HBsAg, HCV-Ac negative Serology for parasitic infectious negative Total serum IgE and food specific IgE normal DAO normal CA 125 normal Anti-dsDNA negative Electrophoresis and immunoelectrophoresis normal Tryptase normal 2014 Complete blood count and biochemistry normal CA15.3, CA19.9, CA 125, CEA normal C1q normal ANA negative Anti-dsDNA negative C3-normal Acute phase reactants normal Rheumatoid factor negative Thyroid profile normal Lipid profile normal LDH normal Full body CT normal Bone marrow normal pathogenic therapy with C1INH concentrate, antagonists of bradikinin receptors (icatibant) or selective inhibitor of plasma kallikrein (ecallantide) was available in 2014, we first initiated daily prophylactic treatment with tranexamic acid for 3 months, with no amelioration. During the more severe attack with laryngeal edema, in September 2014, we have switched to attenuated androgen danazol, given 400 mg the initial dose, reduced to 200 mg daily after 1 week and then to 100 mg daily. The clinical evolution was very good, no angioedema attack occurred since the introduction of danazol. C1INH and C4 plasma levels increased after 2 weeks of treatment and became normal after 1 month. After some months, the patient decided herself to discontinue danazol for short time, in order to check effects and to taper the minimal dose. Serum C1-INH and C4 plasma levels were measured after 2 and 4 weeks and showed significant lower levels, but no angioedema attack occurred during this period of time. She therefore restarted danazol prophylactic therapy at a minimum of 50 mg dose daily, ongoing after 3 years. The patient was closely monitored during the next 3 years, with complete clinical and laboratory control twice a year. We took into consideration the possible side effects of danazol, mainly dislipidemia, haematological and liver malignancies and any other complications or concomitant diseases. The clinical evaluation was very good, except two episodes of pulmonary cysts infection, remitted with broad spectrum antibiotherapy, which could not be related to danazol treatment. The clinical outcome of angioedema after 3 years of danazol treatment is very good, with no attacks or other related symptoms. No clinical or laboratory sign of any disease that could induce C1 INH deficiency occurred. No relevant side effects of danazol were noticed. The patient has an improved quality of life due to therapeutic compliance and general management plan. Discussion Angioedema due to acquired C1-INH deficiency is a difficult diagnosis, generally established after many years of repeated attacks, with variable and potentially severe outcome, frequently accompanied or followed by lymphoproliferative disorders. The information about adultonset non-allergic angioedema in medical practice is usually limited to ACEI-induced forms and the complex underlying mechanisms of the disease are poorly understood. The evidence that about 15% of patients with ACEI-induced angioedema may continue to have attacks during few months after ACEI discontinuation might be confusing and delay investigations for other causes of acquired angioedema. Data from the literature mention 58% patients with recurring attack within 3 months and 41% during the 1st month after ACE-I discontinuation, therefore alternate diagnosis might be considered if no significant clinical amelioration is seen after 1 3 months [11]. Before 1985 only 25 AAE cases have been published in the literature and the largest describing cohort of patients with AAE was reported 30 years later, consisting in 92 cases, with a median follow-up of 4.2 years after AAE diagnosis [12]. Acute attacks of AAE were primarily localized to the face and abdomen and half of the patients experienced potentially life-threatening attacks due to laryngeal or tongue localization. The authors reported a striking association of AAE with splenic marginal zone lymphoma, indolent B-cell lymphoma and monoclonal gammopathy. An important previous observation was that immunochemical underlying abnormalities and accompanying angioedema may precede with some years the clinical expression of malignant disease [13]. The main differences of AAE from HAE are advanced age, lack of family history, normal genetic tests, lack of
4 Page 4 of 5 Fig. 1 Serum C4 and C1-INH plasma level between 2009 and 2017 triggers for attacks (such as trauma, infections and surgical or dental interventions) and more rare abdominal attacks [14]. Complement fraction C1q is usually low in AAE and normal in HAE, which is different from the findings in our case. We considered the utility of genetic test, but the cost is very high and it would not bring significant benefit to the case management, due to current available therapies for AAE. Treatment of C1-INH-AAE should consider the underlying disease as well as the frequency and severity of angioedema. The current standard of care for AAE is based on experts recommendations [15]. Plasma-derived C1-INH, bradykinin B2 antagonist icatibant and kallikrein inhibitor ecallantide are recommended for treatment of AAE acute attacks, while antifibrinolytic agents such as tranexamic acid and attenuated androgens are indicated for long-term AAE prophylaxis. Symptomatic treatment of C1-INH-AAE recurrences is similar to that of HAE, using C1-INH replacement therapy and bradykinin-targeted drugs, which may be not available or not approved for this indication. Longterm prophylaxis of AAE can be done with attenuated androgens or antifibrinolytic agents such as tranexamic acid, which is considered by some authors as the drug of choice for C1-INH-AAE [16]. Attenuated androgen danazol is a synthetic androgen used since 1976 in the long-term prophylaxis of HAE. It appears as a useful option for C1-INH- AAE also, due to the good safety and cost-efficacy profile [17]. Danazol was proved to increase the serum level of C1-INH in both HAE and AAE. The precise mechanism is not known, but it is considered to be mainly the decreased C1-INH consumption in AAE and increased liver synthesis in HAE [18]. A long-term survey of 118 HAE patients treated with danazol confirms a positive response in 94.1% patients, with good safety profile. The reported side effects are: weight gain, signs of virilization in women, altered lipid profile, hypertension, liver cell adenoma and slightly increased risk of stroke and myocardial infarction [19]. The reported AAE case confirms the good safety profile of danazol treatment, which has proved to be an efficient and accessible therapeutic option for both AAE and HAE. No treatment for HAE and AAE was available in our country in 2014, except fresh frozen plasma for acute treatment, but tranexamic acid and danazol could be obtained from other European countries, based on medical prescription and for acceptable cost. For HAE treatment only, one C1-INH concentrate preparation was approved in 2015, followed by Icatibant in Conclusions We reported a rare case of angioedema due to acquired C1-INH deficiency, with some particular aspects: 6 years diagnosis delay, initial peripheral localization of angioedema for many years, followed by one more
5 Page 5 of 5 severe facial and laryngeal attack, no abdominal attacks, no underlying disease after 9 years follow-up, lack of response to tranexamic acid and very good response to attenuated androgen. The reported case is illustrative for various clinical presentations of recurrent angioedema in medical practice. The onset at advanced age and lack of any family or personal history of angioedema as well as negative allergologic investigation should prompt the physician to search underlying causes of C1-INH deficiency. The diagnosis, long term therapy and follow-up may be challenging and should take into consideration experts opinion and guidelines recommendations. We concluded that attenuated androgen is still an effective prophylactic choice, with good safety profile and low price. The main take-away message of our case report is that awareness of angioedema due to C1-INH deficiency should be increased within medical community and therapeutic options should be more clearly indicated and available for all diagnosed cases. Authors contributions PML is the allergist who diagnosed and treated the patient, collected the data, obtained the informed consent, drafted the manuscript, choose the journal for publication and was a major contributor in writing the manuscript. VFA is the specialist in training who participated to the medical care of the patient, contributed to collecting the data and drafting the manuscript. HB is the hematologist who contributed to medical evaluation of the patient and drafting the manuscript. All authors read and approved the final manuscript. Author details 1 Carol Davila University of Medicine and Pharmacy, Bulevardul Eroii Sanitari, no. 8, District 5, Bucharest, Romania. 2 Internal Medicine Department, Colentina Clinical Hospital, Sos. Stefan cel Mare, no , District 2, Bucharest, Romania. 3 Emergency University Hospital, Splaiul Independentei, no. 169, District 5, Bucharest, Romania. Acknowledgements Competing interests The authors declare that they have no competing interests. Availability of data and materials Ethics approval and consent to participate Funding Patient consent for publication The patient gave written informed consent for publication of this medical report. A copy of the written consent is available for review by the Chief Editor of the journal. References 1. Cicardi M, Aberer W, Banerji A, Bas M, Bernstein JA, Bork K, Caballero T, et al. Classification, diagnosis and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014;69: Bucher MC, Petkovic T, Helbling A, Steiner UC. Idiopathic non-histaminergic acquired angioedema: a case series and discussion of published trials. Clin Transl Allergy. 2017;7: Caldwell JR, Ruddy S, Schur PH, Austen KF. Acquired C1 inhibitor deficiency in lymphosarcoma. Clin Immunol Immunopathol. 1972;1: Zingale LC, Beltrami L, Zanichelli A, Magionni L, Pappalardo E, Cicardi B, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175: Cugno M, Cicardi M, Coppola R, Agostoni A. Activation of factor XII and cleavage of high molecular weight kininogen during acute attacks in hereditary and acquired C1-inhibitor deficiencies. Immunopharmacology. 1996;33: Hauptmann G, Lang JM, Noth ML, Oberling F, Mayer G, Lachmann P. Acquired C1-inhibitor deficiencies in lymphoproliferative diseases with serum immunoglobulin abnormalities. A study of three cases. Blut. 1976;32: Cicardi M, Zingale LC, Pappalardo E, et al. Autoantibodies and lymphoproliferative diseases in acquired C1-inhibitor deficiencies. Medicine (Baltimore). 2003;82: Zingale LC, Castelli R, Zanichelli A, Cicardi M. Acquired deficiency of the inhibitor of the first complement component: presentation, diagnosis, course and conventional management. Immunol Allergy Clin North Am. 2006;26: Bouillet-Claveyrolas L, Ponard D, Drouet C, Massot C. Clinical and biological distinctions between type I and type II acquired angioedema. Am J Med. 2003;115: Zuraw BL, Curd JG. Demonstration of modified inactive first component of complement (C1) inhibitor in the plasmas of C1 inhibitor-deficient patients. J Clin Invest. 1986;78: Faisant C, Armengol G, Bouillet L, Boccon-Gibod I, Villier C, et al. Angioedema triggered by medication blocking the renin/angiotensin system: retrospective study using the French National Pharmacovigilance Database. J Clin Immunol. 2016;36: Gobert D, Paule R, Ponard D, Levy P, Fremeaux-Bacchi V, Bouillet L, et al. A nationwide study of acquired C1-inhibitor deficiency in France: characteristics and treatment responses in 92 patients. Medicine. 2016;95: Sheffer AL, Austen KF, Rosen FS, Fearon DT. Acquired deficiency of the inhibitor of the first component of complement: report of five additional cases with complementary on the syndrome. J Allergy Clin Immunol. 1985;75: Longhurst HJ, Zanichelli A, Caballero T, Bouillet L, Aberer W, Maurer M, Fain O, Fabien V, Andresen I, for the IOS Study Group. Comparing acquired angioedema with hereditary angioedema (types I/II): findings from the Icatibant Outcome Survey. Clin Exp Immunol. 2016;188: Cicardi M, Zanichelli A. Acquired angioedema. Allergy Asthma Clin Immunol. 2010;6: Agostoni A, Aygoren-Pursun E, Binkley KE, Blanch A, Bork K, Bouillet L, et al. Hereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol. 2004;114(Suppl3):S Cohen SH, Koethe SM, Kozin F, Rodey G, Arkins JA, Jordan NF. Acquired angioedema associated with rectal carcinoma and its response to danazol therapy. J Allergy Clin Immunol. 1978;62(4): Fust G, Farkas H, Csuka D, Varga L, Bork K. Long-term efficacy of danazol treatment in hereditary angioedema. Eur J Clin Invest. 2011;41: Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol. 2008;100: Publisher s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Received: 29 December 2017 Accepted: 10 July 2018
Chapter 37 (pages ): Hereditary Angioedema and Bradykinin-Mediated Angioedema Prepared by: Sarah Spriet, DO
 FIT Board Review Corner May 2017 Welcome to the FIT Board Review Corner, prepared by Tammy Peng, MD, and Amar Dixit, MD, senior and junior representatives of ACAAI's Fellows-In-Training (FITs) to the Board
FIT Board Review Corner May 2017 Welcome to the FIT Board Review Corner, prepared by Tammy Peng, MD, and Amar Dixit, MD, senior and junior representatives of ACAAI's Fellows-In-Training (FITs) to the Board
Cinryze. Cinryze (C1 esterase inhibitor [human]) Description
![Cinryze. Cinryze (C1 esterase inhibitor [human]) Description Cinryze. Cinryze (C1 esterase inhibitor [human]) Description](/thumbs/89/101005775.jpg) Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.85.05 Subject: Cinryze Page: 1 of 5 Last Review Date: September 20, 2018 Cinryze Description Cinryze
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.85.05 Subject: Cinryze Page: 1 of 5 Last Review Date: September 20, 2018 Cinryze Description Cinryze
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 20 June 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 20 June 2012 CINRYZE 500 units, 2100 IU, powder and solvent for solution for injection B/2 bottles (CIP code: 218
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 20 June 2012 CINRYZE 500 units, 2100 IU, powder and solvent for solution for injection B/2 bottles (CIP code: 218
HEREDITARY ANGIOEDEMA A Literature Review and National Management Guidelines
 CAS CLINIQUE/ CASE REPORT HEREDITARY ANGIOEDEMA A Literature Review and National Management Guidelines http://www.lebanesemedicaljournal.org/articles/63-2/case2.pdf Ramez AZZAM 1, Jacques BOUTROS 1, Carla
CAS CLINIQUE/ CASE REPORT HEREDITARY ANGIOEDEMA A Literature Review and National Management Guidelines http://www.lebanesemedicaljournal.org/articles/63-2/case2.pdf Ramez AZZAM 1, Jacques BOUTROS 1, Carla
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Hereditary Angioedema (HAE) Therapy Table of Contents Coverage Policy... 1 General Background... 4 Coding/Billing Information... 7 References... 7 Effective
Cigna Drug and Biologic Coverage Policy Subject Hereditary Angioedema (HAE) Therapy Table of Contents Coverage Policy... 1 General Background... 4 Coding/Billing Information... 7 References... 7 Effective
Subject: Hereditary Angioedema Drug Therapy
 09-J1000-08 Original Effective Date: 07/15/09 Reviewed: 01/09/18 Revised: 02/15/19 Subject: Hereditary Angioedema Drug Therapy THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J1000-08 Original Effective Date: 07/15/09 Reviewed: 01/09/18 Revised: 02/15/19 Subject: Hereditary Angioedema Drug Therapy THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
HAE management and future perspectives
 HAE management and future perspectives Marcus Maurer Allergie-Centrum-Charité Department of Dermatology and Allergy Charité - Universitätsmedizin Berlin Germany Disclosure of Significant Relationships
HAE management and future perspectives Marcus Maurer Allergie-Centrum-Charité Department of Dermatology and Allergy Charité - Universitätsmedizin Berlin Germany Disclosure of Significant Relationships
Icatibant for Patients with Type III Hereditary Angioedema: An Updated Review of Clinical Effectiveness and Harms
 CADTH RAPID RESPONSE REPORT: SUMMARY WITH CRITICAL APPRAISAL Icatibant for Patients with Type III Hereditary Angioedema: An Updated Review of Clinical Effectiveness and Harms Service Line: Rapid Response
CADTH RAPID RESPONSE REPORT: SUMMARY WITH CRITICAL APPRAISAL Icatibant for Patients with Type III Hereditary Angioedema: An Updated Review of Clinical Effectiveness and Harms Service Line: Rapid Response
Hereditary angioedema (HAE) is a rare disease. Management of Hereditary Angioedema in Childhood: A Review SPECIAL ISSUE ON HAE
 PEDIATRIC ALLERGY, IMMUNOLOGY, AND PULMONOLOGY Volume X, Number X, 2014 ª Mary Ann Liebert, Inc. DOI: 10.1089/ped.2014.0412 SPECIAL ISSUE ON HAE Management of Hereditary Angioedema in Childhood: A Review
PEDIATRIC ALLERGY, IMMUNOLOGY, AND PULMONOLOGY Volume X, Number X, 2014 ª Mary Ann Liebert, Inc. DOI: 10.1089/ped.2014.0412 SPECIAL ISSUE ON HAE Management of Hereditary Angioedema in Childhood: A Review
Hereditary angioedema (HAE) is a rare genetic condition
 SYMPOSIUM REPORT SUPPLEMENT Hereditary Angioedema Therapy: Kallikrein Inhibition and Bradykinin Receptor Antagonism Marc Riedl, MD, MS Abstract: Current strategies for the treatment of hereditary angioedema
SYMPOSIUM REPORT SUPPLEMENT Hereditary Angioedema Therapy: Kallikrein Inhibition and Bradykinin Receptor Antagonism Marc Riedl, MD, MS Abstract: Current strategies for the treatment of hereditary angioedema
WHY IS PREVENTION IMPORTANT?
 A GUIDE TO TRUST THE POWER OF PREVENTION < 2 > WHY IS PREVENTION IMPORTANT? Hereditary angioedema (HAE) symptoms can range in severity. Some attacks may be mild or temporarily disabling, but others can
A GUIDE TO TRUST THE POWER OF PREVENTION < 2 > WHY IS PREVENTION IMPORTANT? Hereditary angioedema (HAE) symptoms can range in severity. Some attacks may be mild or temporarily disabling, but others can
Update on recent trials in the treatment of hereditary angioedema
 Review: Clinical Trial Outcomes Update on recent trials in the treatment of hereditary angioedema Clin. Invest. (2011) 1(3), 439 445 Hereditary angioedema (HAE) is a rare genetic condition that manifests
Review: Clinical Trial Outcomes Update on recent trials in the treatment of hereditary angioedema Clin. Invest. (2011) 1(3), 439 445 Hereditary angioedema (HAE) is a rare genetic condition that manifests
The prophylactic use of C1 inhibitor in hereditary angioedema patients undergoing invasive surgical procedures: a retrospective study
 Gavigan et al. Allergy, Asthma & Clinical Immunology 2014, 10:17 ALLERGY, ASTHMA & CLINICAL IMMUNOLOGY RESEARCH Open Access The prophylactic use of C1 inhibitor in hereditary angioedema patients undergoing
Gavigan et al. Allergy, Asthma & Clinical Immunology 2014, 10:17 ALLERGY, ASTHMA & CLINICAL IMMUNOLOGY RESEARCH Open Access The prophylactic use of C1 inhibitor in hereditary angioedema patients undergoing
5/1/2013. Update in Novel Therapies for Hereditary Angioedema. Disclosures. History. Speakers Bureau Teva
 Update in Novel Therapies for Hereditary Angioedema Eric A Meier MD, FAAAAI, FACAAI, FACP Allergy, Asthma and Immunology Center of Alaska May 17 th 2013 Speakers Bureau Teva Disclosures History 1882--Quincke
Update in Novel Therapies for Hereditary Angioedema Eric A Meier MD, FAAAAI, FACAAI, FACP Allergy, Asthma and Immunology Center of Alaska May 17 th 2013 Speakers Bureau Teva Disclosures History 1882--Quincke
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Human, Recombinant) Reference Number: CP.CPA.76 Effective Date: 11.16.16 Last Review Date: 08.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of
Clinical Policy: (Human, Recombinant) Reference Number: CP.CPA.76 Effective Date: 11.16.16 Last Review Date: 08.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of
Second Quarter 2018 Results Call Corporate Update & Financial Results. August 7, 2018
 Second Quarter 2018 Results Call Corporate Update & Financial Results August 7, 2018 Forward-Looking Statements BioCryst s presentation may contain forward-looking statements, including statements regarding
Second Quarter 2018 Results Call Corporate Update & Financial Results August 7, 2018 Forward-Looking Statements BioCryst s presentation may contain forward-looking statements, including statements regarding
Elements for a Public Summary. Overview of disease epidemiology
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Benign prostatic hyperplasia (BPH) (an increase in size of the prostate that is not cancerous) is the most prevalent of all diseases
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Benign prostatic hyperplasia (BPH) (an increase in size of the prostate that is not cancerous) is the most prevalent of all diseases
Public Assessment Report. Berinert. C1-Esterase-Inhibitor, human DE/H/0481/001/MR. Applicant: CSL Behring GmbH. Date of Report:
 Public Assessment Report Berinert C1-Esterase-Inhibitor, human DE/H/0481/001/MR Applicant: CSL Behring GmbH Date of Report: 02.02.2009 This module reflects the scientific discussion for the approval of
Public Assessment Report Berinert C1-Esterase-Inhibitor, human DE/H/0481/001/MR Applicant: CSL Behring GmbH Date of Report: 02.02.2009 This module reflects the scientific discussion for the approval of
M0BCore Safety Profile. Pharmaceutical form(s)/strength: 5 mg SE/H/PSUR/0002/006 Date of FAR:
 M0BCore Safety Profile Active substance: Finasteride Pharmaceutical form(s)/strength: 5 mg P-RMS: SE/H/PSUR/0002/006 Date of FAR: 16.05.2014 4.3 Contraindications Finasteride is not indicated for use in
M0BCore Safety Profile Active substance: Finasteride Pharmaceutical form(s)/strength: 5 mg P-RMS: SE/H/PSUR/0002/006 Date of FAR: 16.05.2014 4.3 Contraindications Finasteride is not indicated for use in
STAYING ON TRACK WITH CINRYZE THERAPY
 YOUR GUIDE TO STAYING ON TRACK WITH CINRYZE THERAPY Indication CINRYZE (C1 esterase inhibitor [human]) is an injectable prescription medicine that is used to help prevent swelling and/or painful attacks
YOUR GUIDE TO STAYING ON TRACK WITH CINRYZE THERAPY Indication CINRYZE (C1 esterase inhibitor [human]) is an injectable prescription medicine that is used to help prevent swelling and/or painful attacks
Ecallantide (DX-88) for acute hereditary angioedema attacks: Integrated analysis of 2 double-blind, phase 3 studies
 Ecallantide (DX-88) for acute hereditary angioedema attacks: Integrated analysis of 2 double-blind, phase 3 studies Albert L. Sheffer, MD, a Marilyn Campion, MS, b Robyn J. Levy, MD, c H. Henry Li, MD,
Ecallantide (DX-88) for acute hereditary angioedema attacks: Integrated analysis of 2 double-blind, phase 3 studies Albert L. Sheffer, MD, a Marilyn Campion, MS, b Robyn J. Levy, MD, c H. Henry Li, MD,
German Guideline for Hereditary Angioedema due to C1-INH Deficiency
 German Guideline for Hereditary Angioedema due to C1-INH Deficiency 1 Guideline of the German Society for Angioedema Research (DGA), German Society of Internal Medicine (DGIM), German Society of Oto-Rhino-Laryngology,
German Guideline for Hereditary Angioedema due to C1-INH Deficiency 1 Guideline of the German Society for Angioedema Research (DGA), German Society of Internal Medicine (DGIM), German Society of Oto-Rhino-Laryngology,
PRODUCT INFORMATION TESTOVIRON DEPOT. (testosterone enanthate)
 PRODUCT INFORMATION TESTOVIRON DEPOT (testosterone enanthate) NAME OF THE MEDICINE Testosterone enanthate is designated chemically as 17 beta-heptanoyloxy-4-androstene-3- one. The empirical formula of
PRODUCT INFORMATION TESTOVIRON DEPOT (testosterone enanthate) NAME OF THE MEDICINE Testosterone enanthate is designated chemically as 17 beta-heptanoyloxy-4-androstene-3- one. The empirical formula of
Hereditary angioedema: an update on available therapeutic options
 DOI: 10.1111/j.1610-0387.2010.07450.x Review Article 663 Hereditary angioedema: an update on available therapeutic options Marcus Maurer, Markus Magerl Department of Dermatology, Venereology and Allergy,
DOI: 10.1111/j.1610-0387.2010.07450.x Review Article 663 Hereditary angioedema: an update on available therapeutic options Marcus Maurer, Markus Magerl Department of Dermatology, Venereology and Allergy,
Original Paper. Int Arch Allergy Immunol 2015;167:21 28 DOI: /
 Original Paper Received: October 24, 2014 Accepted after revision: April 17, 2015 Published online: June 25, 2015 Treatment of HAE Attacks in the Icatibant Outcome Survey: An Analysis of Icatibant Self-Administration
Original Paper Received: October 24, 2014 Accepted after revision: April 17, 2015 Published online: June 25, 2015 Treatment of HAE Attacks in the Icatibant Outcome Survey: An Analysis of Icatibant Self-Administration
PRODUCT INFORMATION PRIMOTESTON DEPOT. (testosterone enantate)
 PRODUCT INFORMATION PRIMOTESTON DEPOT (testosterone enantate) NAME OF THE MEDICINE Testosterone enantate is designated chemically as 17 beta-heptanoyloxy-4-androstene-3- one. The empirical formula of testosterone
PRODUCT INFORMATION PRIMOTESTON DEPOT (testosterone enantate) NAME OF THE MEDICINE Testosterone enantate is designated chemically as 17 beta-heptanoyloxy-4-androstene-3- one. The empirical formula of testosterone
RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS. Hereditary Angioedema
 RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS Hereditary Angioedema TRUST PROVEN PREVENTION WITH CINRYZE Whether your patients are new to treatment or are continuing their
RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS Hereditary Angioedema TRUST PROVEN PREVENTION WITH CINRYZE Whether your patients are new to treatment or are continuing their
Hereditary angioedema (HAE) is caused by
 TRANSFUSION PRACTICE C1-inhibitor concentrate for individual replacement therapy in patients with severe hereditary angioedema refractory to danazol prophylaxis Wolfhart Kreuz, Inmaculada Martinez-Saguer,
TRANSFUSION PRACTICE C1-inhibitor concentrate for individual replacement therapy in patients with severe hereditary angioedema refractory to danazol prophylaxis Wolfhart Kreuz, Inmaculada Martinez-Saguer,
Icatibant, an inhibitor of bradykinin receptor 2, for hereditary angioedema attacks: prospective experimental single-cohort study
 DOI: 10.1590/1516-3180.2014.1325652 ORIGINAL ARTICLE Icatibant, an inhibitor of bradykinin receptor 2, for hereditary angioedema attacks: prospective experimental single-cohort study Icatibanto, um inibidor
DOI: 10.1590/1516-3180.2014.1325652 ORIGINAL ARTICLE Icatibant, an inhibitor of bradykinin receptor 2, for hereditary angioedema attacks: prospective experimental single-cohort study Icatibanto, um inibidor
CSL Behring PACKAGE LEAFLET: INFORMATION FOR THE USER
 CSL Behring PACKAGE LEAFLET: INFORMATION FOR THE USER Berinert 500 IU Powder and solvent for solution for injection / infusion Berinert 1500 IU Powder and solvent for solution for injection Human C1-esterase
CSL Behring PACKAGE LEAFLET: INFORMATION FOR THE USER Berinert 500 IU Powder and solvent for solution for injection / infusion Berinert 1500 IU Powder and solvent for solution for injection Human C1-esterase
RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS
 RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS TRUST THE POWER OF PREVENTION WITH CINRYZE Whether your patients are new to treatment or are continuing their journey, remind
RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS TRUST THE POWER OF PREVENTION WITH CINRYZE Whether your patients are new to treatment or are continuing their journey, remind
ANNEX 1 SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX 1 SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Ruconest 2100 U powder for solution for injection. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 2100 units
ANNEX 1 SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Ruconest 2100 U powder for solution for injection. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 2100 units
Natural Hair Transplant Medical Center, Inc Dove Street, Suite #250, Newport Beach, CA Phone
 Natural Hair Transplant Medical Center, Inc. 1000 Dove Street, Suite #250, Newport Beach, CA 92660 Phone-949-622-6969 Finasteride (PROPECIA ) Acknowledgement Finasteride is an oral medication, manufactured
Natural Hair Transplant Medical Center, Inc. 1000 Dove Street, Suite #250, Newport Beach, CA 92660 Phone-949-622-6969 Finasteride (PROPECIA ) Acknowledgement Finasteride is an oral medication, manufactured
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Pasteurized and nanofiltered, plasma-derived C1 esterase inhibitor concentrate for the treatment of hereditary angioedema
 For reprint orders, please contact: reprints@futuremedicine.com Pasteurized and nanofiltered, plasma-derived C1 esterase inhibitor concentrate for the treatment of hereditary angioedema Hereditary angioedema
For reprint orders, please contact: reprints@futuremedicine.com Pasteurized and nanofiltered, plasma-derived C1 esterase inhibitor concentrate for the treatment of hereditary angioedema Hereditary angioedema
PREPARATION AND SELF-ADMINISTRATION INSTRUCTIONS FOR PATIENTS
 PREPARATION AND SELF-ADMINISTRATION INSTRUCTIONS FOR PATIENTS Hereditary Angioedema Jenny, a real HAE patient TAKE CONTROL OF YOUR HEREDITARY ANGIOEDEMA (HAE) TREATMENT WITH SELF-ADMINISTRATION Choosing
PREPARATION AND SELF-ADMINISTRATION INSTRUCTIONS FOR PATIENTS Hereditary Angioedema Jenny, a real HAE patient TAKE CONTROL OF YOUR HEREDITARY ANGIOEDEMA (HAE) TREATMENT WITH SELF-ADMINISTRATION Choosing
plan prepare progress
 plan prepare progress Hereditary Angioedema (HAE) Treatment Plan and Therapy Journal Plan, prepare, and personalize an effective treatment plan for your HAE attacks Share your progress with your healthcare
plan prepare progress Hereditary Angioedema (HAE) Treatment Plan and Therapy Journal Plan, prepare, and personalize an effective treatment plan for your HAE attacks Share your progress with your healthcare
Androgens and Anabolic Steroids Prior Authorization with Quantity Limit - Through Preferred Topical Androgen Agent
 Androgens and Anabolic Steroids Prior Authorization with Quantity Limit - Through Preferred Topical Androgen Agent Androgens/Anabolic Steroids Prior Authorization with Quantity Limit Through Preferred
Androgens and Anabolic Steroids Prior Authorization with Quantity Limit - Through Preferred Topical Androgen Agent Androgens/Anabolic Steroids Prior Authorization with Quantity Limit Through Preferred
Elements for a public summary
 VI.2 VI.2.1 Elements for a public summary Overview of disease epidemiology Prostate gland enlargement is a common condition as men get older. Also called benign prostatic hyperplasia (BPH) and prostatic
VI.2 VI.2.1 Elements for a public summary Overview of disease epidemiology Prostate gland enlargement is a common condition as men get older. Also called benign prostatic hyperplasia (BPH) and prostatic
H ereditary angio-oedema (HAE) is characterised
 266 REVIEW Current management of hereditary angio-oedema (C 1 esterase inhibitor deficiency) A Fay, M Abinun... Hereditary angio-oedema is characterised by recurrent swellings in any part of the body and
266 REVIEW Current management of hereditary angio-oedema (C 1 esterase inhibitor deficiency) A Fay, M Abinun... Hereditary angio-oedema is characterised by recurrent swellings in any part of the body and
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
1001 West Broadway, Vancouver, BC V6H 4B1. Topical Finasteride
 1001 West Broadway, Vancouver, BC V6H 4B1 Topical Finasteride 1 Topical finasteride is a solution containing the drug finasteride typically sold under the brand names Propecia and Proscar. The Finasteride
1001 West Broadway, Vancouver, BC V6H 4B1 Topical Finasteride 1 Topical finasteride is a solution containing the drug finasteride typically sold under the brand names Propecia and Proscar. The Finasteride
Package Leaflet: Information for the patient. Sustanon 250, 250 mg/ml, solution for injection (testosterone esters)
 Package Leaflet: Information for the patient Sustanon 250, 250 mg/ml, solution for injection (testosterone esters) Read all of this leaflet carefully before you start using this medicine because it contains
Package Leaflet: Information for the patient Sustanon 250, 250 mg/ml, solution for injection (testosterone esters) Read all of this leaflet carefully before you start using this medicine because it contains
Fetlock Lameness It s importance
 Fetlock Lameness It s importance Fetlock Lameness It s importance and how MRI can assist in making the difficult diagnosis Dr Robin Bell and Professor Leo Jeffcott Equine Performance and Imaging Centre,
Fetlock Lameness It s importance Fetlock Lameness It s importance and how MRI can assist in making the difficult diagnosis Dr Robin Bell and Professor Leo Jeffcott Equine Performance and Imaging Centre,
Panzyga Administration Guide
 Immune Human Normal Globulin Immunoglobulin Intravenous (Human) (IVIg) Octapharma s new Intravenous Immunoglobulin (IVIG) Panzyga Administration Guide An educational service tool provided by Octapharma
Immune Human Normal Globulin Immunoglobulin Intravenous (Human) (IVIg) Octapharma s new Intravenous Immunoglobulin (IVIG) Panzyga Administration Guide An educational service tool provided by Octapharma
Hyperbaric Oxygen Therapy Unit
 Hyperbaric Oxygen Therapy Unit Indraprastha Apollo Hospital. New Delhi A decade of practice of hyperbaric Medicine 1 Introduction Dr PC Reddy is a great visionary and introduced many new treatment modalities
Hyperbaric Oxygen Therapy Unit Indraprastha Apollo Hospital. New Delhi A decade of practice of hyperbaric Medicine 1 Introduction Dr PC Reddy is a great visionary and introduced many new treatment modalities
PREPARATION AND SELF-ADMINISTRATION INSTRUCTIONS FOR PATIENTS
 PREPARATION AND SELF-ADMINISTRATION INSTRUCTIONS FOR PATIENTS Hereditary Angioedema TAKE CONTROL OF HEREDITARY ANGIOEDEMA (HAE) TREATMENT WITH FLEXIBLE ADMINISTRATION Choosing to treat with CINRYZE (C1
PREPARATION AND SELF-ADMINISTRATION INSTRUCTIONS FOR PATIENTS Hereditary Angioedema TAKE CONTROL OF HEREDITARY ANGIOEDEMA (HAE) TREATMENT WITH FLEXIBLE ADMINISTRATION Choosing to treat with CINRYZE (C1
Testosterone Hormone Replacement Drug Class Prior Authorization Protocol
 Testosterone Hormone Replacement Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective date: April 1, 2018 This policy has been developed through
Testosterone Hormone Replacement Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective date: April 1, 2018 This policy has been developed through
OXYGEN FOR ADULTS IN ACUTE CARE
 PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF OXYGEN FOR ADULTS IN ACUTE CARE ALL CLINICAL DIVISIONS ADULT CARE STATEMENT The staff indicated in Staff Group may administer oxygen in the two detailed
PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF OXYGEN FOR ADULTS IN ACUTE CARE ALL CLINICAL DIVISIONS ADULT CARE STATEMENT The staff indicated in Staff Group may administer oxygen in the two detailed
PHYSICIANS CIRCULAR FINASTERIDE PROSCAR. Tablet 5-Alpha Reductase Inhibitor
 PHYSICIANS CIRCULAR FINASTERIDE PROSCAR Tablet 5-Alpha Reductase Inhibitor FINASTERIDE (PROSCAR) a synthetic 4-azasteroid compound, is a specific inhibitor of Type II 5α-reductase, an intracellular enzyme
PHYSICIANS CIRCULAR FINASTERIDE PROSCAR Tablet 5-Alpha Reductase Inhibitor FINASTERIDE (PROSCAR) a synthetic 4-azasteroid compound, is a specific inhibitor of Type II 5α-reductase, an intracellular enzyme
TESTOFEN HUMAN CLINICAL TRIAL GENCOR PACIFIC, INC. Copyright 2006 by Gencor Pacific, Inc.
 GENCOR PACIFIC, INC. 920 E. Orangethorpe Avenue, Suite B, Anaheim, CA 92801 Ph: 714.870.8723 714.870.8724 efax: 732.875.0306 drjit@gencorpacific.com gita@gencorpacific.com www.gencorpacific.com TESTOFEN
GENCOR PACIFIC, INC. 920 E. Orangethorpe Avenue, Suite B, Anaheim, CA 92801 Ph: 714.870.8723 714.870.8724 efax: 732.875.0306 drjit@gencorpacific.com gita@gencorpacific.com www.gencorpacific.com TESTOFEN
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
The Wilson and Jungner principles of screening and genetic testing
 Leeds Institute of Health Sciences The Wilson and Jungner principles of screening and genetic testing Professor Darren Shickle Academic Unit of Public Health European Forum for Evidenced-based Prevention
Leeds Institute of Health Sciences The Wilson and Jungner principles of screening and genetic testing Professor Darren Shickle Academic Unit of Public Health European Forum for Evidenced-based Prevention
SECOND EUROPEAN CONSENSUS CONFERENCE ON HYPERBARIC MEDICINE THE TREATMENT OF DECOMPRESSION ACCIDENTS IN RECREATIONAL DIVING
 SECOND EUROPEAN CONSENSUS CONFERENCE ON HYPERBARIC MEDICINE THE TREATMENT OF DECOMPRESSION ACCIDENTS IN RECREATIONAL DIVING MARSEILLE, May 8-10, 1996 RECOMMENDATIONS OF THE JURY* QUESTION 1 : Is there
SECOND EUROPEAN CONSENSUS CONFERENCE ON HYPERBARIC MEDICINE THE TREATMENT OF DECOMPRESSION ACCIDENTS IN RECREATIONAL DIVING MARSEILLE, May 8-10, 1996 RECOMMENDATIONS OF THE JURY* QUESTION 1 : Is there
FINASTERIDE (PROPECIA, PROSCAR) SIDE EFFECT & CONSENT FORM
 750 West Broadway Street Suite 905 Vancouver, BC M5Z 1K1 FAX: (604) 648-9003 vancouveroffice@donovanmedical.com FINASTERIDE (PROPECIA, PROSCAR) SIDE EFFECT & CONSENT FORM What is finasteride? Finasteride
750 West Broadway Street Suite 905 Vancouver, BC M5Z 1K1 FAX: (604) 648-9003 vancouveroffice@donovanmedical.com FINASTERIDE (PROPECIA, PROSCAR) SIDE EFFECT & CONSENT FORM What is finasteride? Finasteride
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Ruconest 2100 U powder for solution for injection. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 2100 units
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Ruconest 2100 U powder for solution for injection. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 2100 units
PRODUCT INFORMATION PROVIRON
 PRODUCT INFORMATION PROVIRON NAME OF TE MEDICINE Mesterolone is a white to yellowish crystalline powder and is practically insoluble in water. The chemical name for mesterolone is 17 beta-ydroxy-1 alpha-methyl-5
PRODUCT INFORMATION PROVIRON NAME OF TE MEDICINE Mesterolone is a white to yellowish crystalline powder and is practically insoluble in water. The chemical name for mesterolone is 17 beta-ydroxy-1 alpha-methyl-5
Drug Class Monograph
 Drug Class Monograph Class: Testosterone Hormone Replacement Drug: Androderm (testosterone transdermal system), Androgel (testosterone topical gel), Axiron (testosterone topical solution), Aveed (testosterone
Drug Class Monograph Class: Testosterone Hormone Replacement Drug: Androderm (testosterone transdermal system), Androgel (testosterone topical gel), Axiron (testosterone topical solution), Aveed (testosterone
The Australian and New Zealand College of Veterinary Scientists. Membership Examination. Medicine of Horses Paper 1
 The Australian and New Zealand College of Veterinary Scientists Membership Examination June 2012 Medicine of Horses Paper 1 Perusal time: Fifteen (15) minutes Time allowed: Two (2) hours after perusal
The Australian and New Zealand College of Veterinary Scientists Membership Examination June 2012 Medicine of Horses Paper 1 Perusal time: Fifteen (15) minutes Time allowed: Two (2) hours after perusal
Self-declaration by New Zealand of its status of freedom from Equine Viral Arteritis
 Self-declaration by New Zealand of its status of freedom from Equine Viral Arteritis Self-declaration submitted to the OIE on xxxx2014, by Dr Matthew Stone, Chief Veterinary Officer, Ministry for Primary
Self-declaration by New Zealand of its status of freedom from Equine Viral Arteritis Self-declaration submitted to the OIE on xxxx2014, by Dr Matthew Stone, Chief Veterinary Officer, Ministry for Primary
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Firazyr 30 mg solution for injection in pre-filled syringe 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Firazyr 30 mg solution for injection in pre-filled syringe 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe
Colic Fact Sheet One hell of a belly ache
 Colic Fact Sheet One hell of a belly ache No other word strikes fear in the hearts and minds of horse owners more than the word Colic - it can affect any horse at any time for a multitude of reason. Sadly,
Colic Fact Sheet One hell of a belly ache No other word strikes fear in the hearts and minds of horse owners more than the word Colic - it can affect any horse at any time for a multitude of reason. Sadly,
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Androgens and Anabolic Steroids Page 1 of 57 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Androgens and Anabolic Steroids Prime Therapeutics will review Prior
Androgens and Anabolic Steroids Page 1 of 57 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Androgens and Anabolic Steroids Prime Therapeutics will review Prior
Gynaecomastia. Benign breast conditions information provided by Breast Cancer Care
 Gynaecomastia This booklet tells you about gynaecomastia. It explains what gynaecomastia is, what causes it, how it s diagnosed and what will happen if it needs to be treated or followed up. Benign breast
Gynaecomastia This booklet tells you about gynaecomastia. It explains what gynaecomastia is, what causes it, how it s diagnosed and what will happen if it needs to be treated or followed up. Benign breast
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The impact of freediving on psychomotor performance and blood catecholamine concentration
 The impact of freediving on psychomotor performance and blood catecholamine concentration Jan Chmura 1, Adam Kawczyński 1, Marek Mędraś 2, Paweł Jóźków 2, Bartosz Morawiec 1 1 University School of Physical
The impact of freediving on psychomotor performance and blood catecholamine concentration Jan Chmura 1, Adam Kawczyński 1, Marek Mędraś 2, Paweł Jóźków 2, Bartosz Morawiec 1 1 University School of Physical
population Completed double-blind 458 (67.7) 445 (65.0) study n (%)
 Study No: ARIA3002 Year 4 Title: A Randomized, Double-Blind, Placebo-Controlled, Two Year Parallel Group Study of the Efficacy and Safety of GI198745 0.5mg in the Treatment and Prevention of Progression
Study No: ARIA3002 Year 4 Title: A Randomized, Double-Blind, Placebo-Controlled, Two Year Parallel Group Study of the Efficacy and Safety of GI198745 0.5mg in the Treatment and Prevention of Progression
Issues. What is a low testosterone? Who needs testosterone therapy? Benefits/adverse effects of testosterone replacement Treatment options
 Male Hypogonadism Jauch Symposium Waterloo, IA May 17, 2013 Janet A. Schlechte, M.D. Disclosure of Financial Relationships Janet A. Schlechte, M.D. has no relationships with any proprietary entity producing
Male Hypogonadism Jauch Symposium Waterloo, IA May 17, 2013 Janet A. Schlechte, M.D. Disclosure of Financial Relationships Janet A. Schlechte, M.D. has no relationships with any proprietary entity producing
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: CP.CPA.291 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.CPA.291 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
Secrets of Abang Sado : Effects of testosterone therapy. Azraai Nasruddin
 + Secrets of Abang Sado : Effects of testosterone therapy Azraai Nasruddin + Testosterone Testosterone : Steroid hormone - Made primarily by the testicles in males - Small amounts produced by the adrenal
+ Secrets of Abang Sado : Effects of testosterone therapy Azraai Nasruddin + Testosterone Testosterone : Steroid hormone - Made primarily by the testicles in males - Small amounts produced by the adrenal
TREATMENT GUIDE. Your treatment. Your questions answered.
 TREATMENT GUIDE Your treatment. Your questions answered. INDICATIONS: Kineret is a prescription medicine called an interleukin-1 receptor antagonist (IL-1ra) used to: Reduce the signs and symptoms, and
TREATMENT GUIDE Your treatment. Your questions answered. INDICATIONS: Kineret is a prescription medicine called an interleukin-1 receptor antagonist (IL-1ra) used to: Reduce the signs and symptoms, and
Espilat Mountaineering Club
 In the name of God Espilat Mountaineering Club May 2013 MEDICAL PROBLEMS IN HIGH MOUNTAIN ENVIRONMENTS Arya Hamedanchi MD, MPH Level one International Anthropometrist International Certificate of Ski Medicine
In the name of God Espilat Mountaineering Club May 2013 MEDICAL PROBLEMS IN HIGH MOUNTAIN ENVIRONMENTS Arya Hamedanchi MD, MPH Level one International Anthropometrist International Certificate of Ski Medicine
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Androgens and Anabolic Steroids Page 1 of 53 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Androgens and Anabolic Steroids Prime Therapeutics will review Prior
Androgens and Anabolic Steroids Page 1 of 53 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Androgens and Anabolic Steroids Prime Therapeutics will review Prior
ICD-10 Primer: Are You Ready?
 ICD-10 Primer: Are You Ready? HCCA Presented by: Cynthia A. Swanson, RN, CPC, CEMC, CHC, CPMA Senior Manager-Healthcare Consulting Seim Johnson, LLP 18081 Burt Street, Suite 200 Omaha, NE 68022 402.330.2660
ICD-10 Primer: Are You Ready? HCCA Presented by: Cynthia A. Swanson, RN, CPC, CEMC, CHC, CPMA Senior Manager-Healthcare Consulting Seim Johnson, LLP 18081 Burt Street, Suite 200 Omaha, NE 68022 402.330.2660
Acute phase form Year Version
 Important information The Riksstroke register was established 1994. From 1998, all hospitals in Sweden admitting patients with acute stroke, reported data to the Riksstroke register Variables that are
Important information The Riksstroke register was established 1994. From 1998, all hospitals in Sweden admitting patients with acute stroke, reported data to the Riksstroke register Variables that are
.org. Tennis Elbow (Lateral Epicondylitis) Anatomy. Cause
 Tennis Elbow (Lateral Epicondylitis) Page ( 1 ) Tennis elbow, or lateral epicondylitis, is a painful condition of the elbow caused by overuse. Not surprisingly, playing tennis or other racquet sports can
Tennis Elbow (Lateral Epicondylitis) Page ( 1 ) Tennis elbow, or lateral epicondylitis, is a painful condition of the elbow caused by overuse. Not surprisingly, playing tennis or other racquet sports can
Clinical Policy: Testosterone Reference Number: AZ.CP.PHAR.02 Effective Date: Last Review Date: Line of Business: Arizona Medicaid
 Clinical Policy: Reference Number: AZ.CP.PHAR.02 Effective Date: 11.16.16 Last Review Date: 09.12.18 Line of Business: Arizona Medicaid Revision Log See Important Reminder at the end of this policy for
Clinical Policy: Reference Number: AZ.CP.PHAR.02 Effective Date: 11.16.16 Last Review Date: 09.12.18 Line of Business: Arizona Medicaid Revision Log See Important Reminder at the end of this policy for
Testosterone Hormone Replacement Drug Class Prior Authorization Protocol
 Line of business: Medi-Cal Effective Date: August 16, 2017 Revision Date: August 16, 2017 Testosterone Hormone Replacement Drug Class Prior Authorization Protocol This policy has been developed through
Line of business: Medi-Cal Effective Date: August 16, 2017 Revision Date: August 16, 2017 Testosterone Hormone Replacement Drug Class Prior Authorization Protocol This policy has been developed through
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2018-9 Program Prior Authorization/Medical Necessity Topical Androgens Medication Axiron*, Androderm, Androgel*, Fortesta*, Natesto*,
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2018-9 Program Prior Authorization/Medical Necessity Topical Androgens Medication Axiron*, Androderm, Androgel*, Fortesta*, Natesto*,
T H E B I O B A R I C A W A Y
 BIOBARICA HYPERBARIC MEDICINE T H E B I O B A R I C A W A Y BioBarica BioBarica is a global network of hyperbaric medicine designed to expand the availability of high quality therapeutic Centers BioBarica
BIOBARICA HYPERBARIC MEDICINE T H E B I O B A R I C A W A Y BioBarica BioBarica is a global network of hyperbaric medicine designed to expand the availability of high quality therapeutic Centers BioBarica
The ICL Insider. Lab Testing: Testosterone. In This Issue. The Debate
 The ICL Insider February 2017 Volume 2, Issue 2 Lab Testing: Testosterone In This Issue Testosterone shashtilak@iclabs.ca Next Issue: Expected Release April 2017 The Debate Aging is accompanied by various
The ICL Insider February 2017 Volume 2, Issue 2 Lab Testing: Testosterone In This Issue Testosterone shashtilak@iclabs.ca Next Issue: Expected Release April 2017 The Debate Aging is accompanied by various
b. Provide consultation service to physicians referring patients. c. Participate in weekly wound care clinic and biweekly diving medicine clinic.
 Curriculum: 1. Required clinical activities: a. Participate in HBO 2 clinical operations by monitoring daily treatment sessions and emergency on-call treatments at least 4 days/week, b. Provide consultation
Curriculum: 1. Required clinical activities: a. Participate in HBO 2 clinical operations by monitoring daily treatment sessions and emergency on-call treatments at least 4 days/week, b. Provide consultation
New Directions in Aplastic Anemia: What is on the Horizon
 Case Presentation #1 New Directions in Aplastic Anemia: What is on the Horizon Gabrielle Meyers, MD 62 y/o male, with hypertension, who presented to his PCP for evaluation of fatigue, shortness of breath
Case Presentation #1 New Directions in Aplastic Anemia: What is on the Horizon Gabrielle Meyers, MD 62 y/o male, with hypertension, who presented to his PCP for evaluation of fatigue, shortness of breath
Male pattern baldness is the most common type of balding among males. It affects roughly, 50% of men by the age of 50,
 Dihydrotestosterone (DHT) Male pattern baldness is the most common type of balding among males. It affects roughly, 30% of men by the age of 30, 50% of men by the age of 50, 57% of men by the age of 60.
Dihydrotestosterone (DHT) Male pattern baldness is the most common type of balding among males. It affects roughly, 30% of men by the age of 30, 50% of men by the age of 50, 57% of men by the age of 60.
Understanding combined oral contraception
 Understanding combined oral contraception Paula Briggs, FFSRH Consultant in Community Sexual and Reproductive Health Southport and Ormskirk NHS Hospital Trust May Logan Centre, Bootle, L20 5DQ paulaeb@aol.com
Understanding combined oral contraception Paula Briggs, FFSRH Consultant in Community Sexual and Reproductive Health Southport and Ormskirk NHS Hospital Trust May Logan Centre, Bootle, L20 5DQ paulaeb@aol.com
Study Management SM STANDARD OPERATING PROCEDURE FOR Interactions with the Institutional Review Board (IRB)
 Study Management SM 302.00 STANDARD OPERATING PROCEDURE FOR Interactions with the Institutional Review Board (IRB) Approval: Nancy Paris, MS, FACHE President and CEO 24 May 2017 (Signature and Date) Approval:
Study Management SM 302.00 STANDARD OPERATING PROCEDURE FOR Interactions with the Institutional Review Board (IRB) Approval: Nancy Paris, MS, FACHE President and CEO 24 May 2017 (Signature and Date) Approval:
한국학술정보. Educational Effect on Prehospital Personnel for Prehospital Stroke Management
 Educational Effect on Prehospital Personnel for Prehospital Stroke Management Jin Hyun Yoo, M.D., Eun Kyung Eo, M.D. 1, Yong Jae Kim, M.D. 2, Hwa Sik Song, M.D. P u r p o s e: If optimal neurologic recovery
Educational Effect on Prehospital Personnel for Prehospital Stroke Management Jin Hyun Yoo, M.D., Eun Kyung Eo, M.D. 1, Yong Jae Kim, M.D. 2, Hwa Sik Song, M.D. P u r p o s e: If optimal neurologic recovery
Before you use KINERET. When you must not use it. Do not use KINERET if you have. using a medicine known as tumour necrosis factor (TNF) antagonist.
 KINERET anakinra Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about KINERET. It does not contain all the available information. It does not take the
KINERET anakinra Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about KINERET. It does not contain all the available information. It does not take the
social impairment. The following exclusion criteria applied: age under 18 years; weight under 45 kg or over 80 kg;
 Postgrad Med J (1991) 67, 450-454 The Fellowship of Postgraduate Medicine, 1991 Low dose danazol in the treatment of the premenstrual syndrome Miriam Deeny, Robert Hawthorn and D. McKay Hart Division ofobstetrics
Postgrad Med J (1991) 67, 450-454 The Fellowship of Postgraduate Medicine, 1991 Low dose danazol in the treatment of the premenstrual syndrome Miriam Deeny, Robert Hawthorn and D. McKay Hart Division ofobstetrics
Elevated creatine kinase levels icd 10
 Elevated creatine kinase levels icd 10 Search Icd 10 for elevated creatine kinase -- And are adept police scanners To await her own. 1-10- 2017 Search All ICD-10; ICD-10-CM. R74.8 is a billable/specific
Elevated creatine kinase levels icd 10 Search Icd 10 for elevated creatine kinase -- And are adept police scanners To await her own. 1-10- 2017 Search All ICD-10; ICD-10-CM. R74.8 is a billable/specific
Before you use. When you must not use it
 anakinra Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about KINERET. It does not contain all the available information. It does not take the place of
anakinra Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about KINERET. It does not contain all the available information. It does not take the place of
How the SNOMED CT to ICD-10 Map facilitated the map to a national extension of ICD-10
 How the SNOMED CT to ICD-10 Map facilitated the map to a national extension of ICD-10 Kin Wah Fung, MD, MS, MA U.S. National Library of Medicine, Bethesda, MD, USA Why do we need a map from SNOMED CT to
How the SNOMED CT to ICD-10 Map facilitated the map to a national extension of ICD-10 Kin Wah Fung, MD, MS, MA U.S. National Library of Medicine, Bethesda, MD, USA Why do we need a map from SNOMED CT to
HGH for Sale Natural Anti-Aging Human Growth Hormone
 HGH for Sale Natural Anti-Aging Human Growth Hormone Human growth hormone is one of the hottest supplement trends on the market, and now you can purchase top-quality HGH to be delivered right to your home!
HGH for Sale Natural Anti-Aging Human Growth Hormone Human growth hormone is one of the hottest supplement trends on the market, and now you can purchase top-quality HGH to be delivered right to your home!
DUPROST Capsules (Dutasteride)
 Published on: 10 Jul 2014 DUPROST Capsules (Dutasteride) Composition Each soft gelatin capsule contains: Dutasteride... 0.5 mg Dosage Form Capsule Pharmacology Pharmacodynamics Mechanism of Action Dutasteride
Published on: 10 Jul 2014 DUPROST Capsules (Dutasteride) Composition Each soft gelatin capsule contains: Dutasteride... 0.5 mg Dosage Form Capsule Pharmacology Pharmacodynamics Mechanism of Action Dutasteride
Risk Factors Involved in Cheerleading Injuries
 University of Arkansas, Fayetteville ScholarWorks@UARK Health, Human Performance and Recreation Undergraduate Honors Theses Health, Human Performance and Recreation 5-2016 Risk Factors Involved in Cheerleading
University of Arkansas, Fayetteville ScholarWorks@UARK Health, Human Performance and Recreation Undergraduate Honors Theses Health, Human Performance and Recreation 5-2016 Risk Factors Involved in Cheerleading
Transgender 201: Case Discussion Group
 Transgender 201: Case Discussion Group Melissa Davis, MD Maria Barnett, DO October 19, 2017 Maria Barnett, DO, Date Welcome! Disclosures Introductions Discussion The total time of this workshop will depend
Transgender 201: Case Discussion Group Melissa Davis, MD Maria Barnett, DO October 19, 2017 Maria Barnett, DO, Date Welcome! Disclosures Introductions Discussion The total time of this workshop will depend
FINCAR Tablets (Finasteride)
 Published on: 10 Jul 2014 FINCAR Tablets (Finasteride) Composition FINCAR Tablets Each film-coated tablet contains: Finasteride USP - 5 mg Dosage Form Tablet Pharmacology Pharmacodynamics Mechanism of
Published on: 10 Jul 2014 FINCAR Tablets (Finasteride) Composition FINCAR Tablets Each film-coated tablet contains: Finasteride USP - 5 mg Dosage Form Tablet Pharmacology Pharmacodynamics Mechanism of
Nitrous Oxide Oxygen Administration Protocol July 2002
 Nitrous Oxide Oxygen Administration Protocol July 2002 Preamble A patient s self-administration of a nitrous oxide-oxygen mixture can provide relief of acute pain, provided there are no contraindications
Nitrous Oxide Oxygen Administration Protocol July 2002 Preamble A patient s self-administration of a nitrous oxide-oxygen mixture can provide relief of acute pain, provided there are no contraindications
Corrected FIM effectiveness as an index independent of FIM score on admission
 7 Japanese Journal of Comprehensive Rehabilitation Science (2014) Original Article Corrected FIM effectiveness as an index independent of FIM score on admission Makoto Tokunaga, MD, PhD, 1 Ryoji Nakanishi,
7 Japanese Journal of Comprehensive Rehabilitation Science (2014) Original Article Corrected FIM effectiveness as an index independent of FIM score on admission Makoto Tokunaga, MD, PhD, 1 Ryoji Nakanishi,
SITE NAME: ISF DOCUMENT VERSION CHECKLIST Complete yes or no to confirm the presence or absence of each document specified.
 1. TRIAL MANAGEMENT CTC Contact List 8 29/07/2016 Y N CTC Contact List (superseded) 7 29/09/2015 Y N CTC Contact List (superseded) 6 24/02/2015 Y N CTC Contact List (superseded) 5 03/09/2014 Y N CTC Contact
1. TRIAL MANAGEMENT CTC Contact List 8 29/07/2016 Y N CTC Contact List (superseded) 7 29/09/2015 Y N CTC Contact List (superseded) 6 24/02/2015 Y N CTC Contact List (superseded) 5 03/09/2014 Y N CTC Contact
