small
|
|
|
- Meagan Atkinson
- 5 years ago
- Views:
Transcription
1 Communication Lithium Sulfur Batteries Functional Differentiation of Three Pores for Effective Sulfur Confinement in Li S Battery Qian Wang, Minghui Yang,* Zhen-Bo Wang,* Chao Li, and Da-Ming Gu Shuttle effect of the dissolved intermediates is regarded as the primary cause that leads to fast capacity degradation of Li S battery. Herein, a microporous carbon-coated sulfur composite with novel rambutan shape (R-S@MPC) is synthesized from microporous carbon-coated rambutan-like zinc sulfide (R-ZnS@MPC), via an in situ oxidation process. The R-ZnS is employed as both template and sulfur precursor. The carbon frame of R-S@MPC composite possesses three kinds of pores that are distinctly separated from each other in space and are endowed with the exclusive functions. The central macropore serves as buffer pool to accommodate the dissolved lithium polysulfides (LPSs) and volumetric variation during cycling. The marginal straight-through mesoporous, connected with the central macropore, takes the responsibility of sulfur storage. The micropores, evenly distributed in the outer carbon shell of the as-synthesized R-S@MPC, enable the blockage of LPSs. These pores are expected to perform their respective single function, and collaborate synergistically to suppress the sulfur loss. Therefore, it delivers an outstanding cycling stability, decay rate of 0.013% cycle 1 after 500 cycles at 1 C, when the sulfur loading is kept at 4 mg cm 2. Amid the burgeoning chemical energy storage systems, the lithium sulfur battery has received wide attention for its promising high energy density at low cost. Poor conductivity, volumetric exchange, and shuttle effect, as the primary challenges of Li S batteries, have been intensively investigated in the past decades. [1 5] Especially, the fundamental problem of the shuttle effect that is caused by the intermediates dissolution, leads to significant capacity decay with time. This is considered as the most crucial hindrance to the practical application process of Li S batteries. [6 9] The shuttle problem in category of space gives rise to capacity degradation in time concept. Therefore, aiming at exchanging space for time, researchers have devoted to suppress the capacity degradation, by creating barriers on Dr. Q. Wang, Prof. M. Yang Ningbo Institute of Industrial Technology Chinese Academy of Sciences No Zhongguan West Road, Zhenhai District, Ningbo , China myang@nimte.ac.cn Dr. Q. Wang, Prof. Z.-B. Wang, Dr. C. Li, Prof. D.-M. Gu School of Chemistry and Chemical Engineering Harbin Institute of Technology No. 92 West-Da Zhi Street, Harbin , China wangzhb@hit.edu.cn The ORCID identification number(s) for the author(s) of this article can be found under DOI: /smll the diffusion pathway of the dissolved intermediates. The barriers are mainly in forms of spatial obstacles [10 13] and chemical traps. [14 17] As the initial fortification, the cathode hosts are the first substances that the dissolved intermediates of lithium polysulfides (LPSs) come in contact with, due to the uniform distribution of sulfur into the porous host materials. [18 20] The various nonpolar carbon-based materials, [10 13,18 20] with controllable morphology, dimension, and even pore size distribution, are employed as the host materials to slow down the trend of the LPSs diffusion outwardly, [21 23] by space obstacles and capillary adsorbent. On the other hand, some polar substances, such as polar organic functional groups, [4,9] transition metal, [24] oxides, [25 27] sulfides, [28 30] nitrides, [31,32] etc., are selected to anchor the LPSs by forming chemical bonds, which also achieve good effect of slowing down the outward diffusion. To increase the restrictive effect for LPSs, the same concept is employed to fabricate the interlayers between the cathode and the separator, which enables the creation of second defense on the LPSs path toward the anode metal lithium. [33 37] The third countermeasure is lithium protection, which is meant for the separation of the metal lithium from the shuttled LPSs. This serves as the last defender for this issue. [38 41] Apparently, solving the problem from the origin is the comparatively optimal strategy. Hollow materials, as one of the promising hosts, have been studied a lot for the past few years, due to the presence of porous shell and internal cavity in their structures enable the blockage of LPSs and accommodation of the volumetric expansion, [42 47] respectively. However, in the ordinary porous shells, there are usually four kinds of pores which are: (i) passing pore, (ii) interconnected pore, (iii) dead end pore, and (iv) closed pore, respectively. The diagrammatical representation is shown in Figure 1a. The pores of type (iv) cannot be effectively utilized since there is disconnection with either inside or outside. In the traditional outside-in sulfur loading process, the hollow host can be impregnated with sulfur through the passing pores and interconnected pores, whereas, sulfur prefers to stay in the pores of type (i) and (ii) on the shell, for the capillary adsorption. Thus, the shell additionally shoulders the responsibility of sulfur storage, which should only be in charge of blocking the dissolved LPSs. When the sulfur is loaded into the pores of type (iii), the internal reservation space is wasted, due to their (1 of 8)
2 Figure 1. a) Schematic diagram of sulfur loading and confining of traditional hollow host. b) The preparation route, and c) sulfur confinement mechanism of disconnection with the internal cavity. Moreover, the sulfur stored in the open pores of type (i), (ii), and (iii) on the shell is easier to lose, compared with that loaded in the hollow cavity (Figure 1a). In this context, our research work is focused on the design of a cathode material with a novel hollow structure, in which different pores are assigned to perform their respective duties, and collaborate synergistically to achieve the goal of sulfur confinement. The preparation progress is schematically illustrated in Figure 1b. The ZnS nanorods grew vertically on the surface of the as-prepared ZnS sphere, through a facile solvothermal reaction, to obtain the rambutan-like ZnS (R-ZnS). The R-ZnS, as the sulfur source and template, was coated with the resorcinolformaldehyde (RF) resin and carbonized, to form a microporous carbon coated R-ZnS (R-ZnS@MPC) composite with core shell structure. The R-ZnS was subsequently in situ oxidized to sulfur inside the microporous carbon shell, using the iodine as the oxidation agent. [48,49] Owing to the characteristic adsorption of the iodine, the generated sulfur was oriented deposited on the inwall of the carbon frame, which meant the sulfur was almost loaded in the straight-through mesopores on the edge of the R-S@MPC. The rambutan-like microporous carbon-coated sulfur composite (R-S@MPC) was prepared. The preparation of the R-ZnS and oriented loading of sulfur in the straight-through mesopores were the two highlights in the synthesis process. R-S@MPC had great improvement in the structure, compared with the traditional hollow materials. [43 46] The carbon framework presented a hollow rambutan-shaped micromorphology, when the self-sacrificed template of R-ZnS disappeared. The carbon shell carbonized from the RF resin only possessed micropores distribution. The abundant straight-through holes, distributed on the edge of the R-S@MPC, were originated from the removal of the ZnS nanorod. They belonged to the category of mesopore. Additionally, the straight-through mesopores on the edge were connected with the hollow cavity, due to the special rambutan shape of the R-ZnS. The central hollow cavity belonged to macropore. Thus, the peculiarity of R-S@MPC in the structure was that three kinds of pores coexisted in the frame and were distinctly divided from each other in space. The unique structure led to the functional differentiation of the three kinds of pores. As mentioned earlier, in situ oxidation method guaranteed the generated sulfur to be almost stored in the straight-through mesopores. [48,49] At the first stage of discharge process (Figure 1c), the elemental sulfur was reduced to the LPSs that were subsequently dissolved in the ether-based electrolyte. [7,8] It was difficult for the LPSs to flow out, due to the blockage of the microporous carbon shell. The LPSs could only diffuse into the central macropore, driven by the concentration gradient of LPSs. The macropores, as a buffer pool, undertook the obligation of the LPSs accommodation. In the (2 of 8)
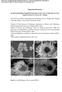
3 second phase of discharge, the LPSs were further reduced to Li 2 S, whose volume increased by 80% compared to the initial elemental sulfur. [9] This volumetric expansion was also accommodated by the hollow macropore. During the charging process, the end product of sulfur was finally re-deposited in the mesopores, following the reverse reaction path. Thereby, in the framework of R-S@MPC, the functions of three pores were precisely divided, central macropore for accommodation, marginal straight-through mesopores for storage, and micropores distributed on the outer carbon shell for blockage. They took their own responsibilities, as well as worked together as a team for effective sulfur confinement, aiming at extending the cycle life. The scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images (Figure 2a,b) display the morphologies of the obtained Rambutan-like ZnS, which has undergone self-assemblage from the ZnS sphere and nanorod (Figure S1, Supporting Information), following the principle of minimum surface energy. The annular with lighter color in Figure 2b is considered to be the ZnS nanorod, for the contrast difference of color. And the thickness of the annular is consistent with the length of the ZnS nanorod (Figure S1b, Supporting Information), suggesting that the ZnS nanorods are standing upright to the ZnS sphere. In addition, the stepwise zoom-in SEM images also exhibit that the ZnS nanorods grow vertically on the surface of the ZnS sphere (Figure S2, Supporting Information). The high-resolution TEM (HRTEM) image (Figure 2c) with the selected area of the R-ZnS exhibits lattice fringes with an interplanar d space of nm, exactly corresponding to that of (002) plane of wurtzite ZnS (JCPDS No ). This is consistent with the strongest diffraction peak of crystal face (002) in the X-ray diffraction (XRD) pattern, as shown in Figure S3 (Supporting Information). The microporous carbon-coated R-ZnS (R-ZnS@MPC) demonstrates the similar configuration with R-ZnS (Figure 2d,e). The only difference, as shown in the HRTEM image of Figure 2f, is that the R-ZnS is observed to be coated outside with a thin layer of carbon that has a uniform thickness of about 3 nm. The R-S@MPC composite obtained from in situ oxidization of the R-ZnS@MPC presents a rambutan appearance with hollow structure, inherited from the self-sacrificed template of ZnS (Figure 2g,h). The local enlarged image in Figure 2i shows intact shell of the one-end-closed holes distributed on the edge of the R-S@MPC composite. This is derived from the disappearance of the ZnS nanorod. These one-end-closed holes can be observed to be connected with the hollow cavity in the SEM image of a cracked sphere (Figure S4, Supporting Information), which indicates they are straight-through pores. More notably, no aggregated sulfur is observed in the TEM images of R-S@MPC (Figure 2h,i), with the fact that the sulfur content reaches up to 75 wt% (Figure 3b). Additionally, the thickness Figure 2. a) SEM, b) TEM, and c) HRTEM images of R-ZnS. d) SEM, e) TEM, and f) HRTEM images of R-ZnS@MPC. g) SEM, h) TEM, and i) HRTEM images of R-S@MPC (3 of 8)
4 NANO MICRO Figure 3. a) The pore size distribution of and R-C. b) TGA measurements of and control sample. c) STEM HAADF, EDX element mappings of d) carbon and e) sulfur of f h) HAADF, element mapping of carbon and sulfur of partial area of the carbon shell. The visual pictures during discharge of i) control sample and j) of the coating carbon layer is investigated to have an effect on the morphology of besides the sulfur content. The decreased thickness of carbon shell leads to the collapse of the structure. While, increased thickness of carbon results in the incomplete reaction during in situ oxidation process (Figure S5, Supporting Information). In the XRD pattern of Figure S6 (Supporting Information), there are no obvious characteristic peaks of sulfur but a wide peak of the amorphous carbon located at (4 of 8)
5 25, also indicating the homogenous distribution of the generated tiny sulfur particles. [50,51] In addition, the N 2 adsorption/desorption measurements of R-S@MPC and R-C are carried out to determine whether the three kinds of pores exist, and how they are distributed. R-C is the carbon frame (Figure S7, Supporting Information), which is obtained by removing the sulfur inside the R-S@MPC under high temperature and low pressure. The steep rises at p/p 0 = 0 in the isothermals (Figure S8, Supporting Information) and the dominate peaks of micropores (pore size 2 nm) in pore size distributions (Figure 3a) reveal the presence of micropores in both R-ZnS@MPC and R-C, indicating the distribution of the micropores on the outer carbon shell. Compared with the R-ZnS@MPC, there is an obvious wide peak of mesopores located at the range of 4 30 nm in the pore size distribution of R-C (Figure 3a), corresponding to the straight-through holes derived from the removal of the ZnS nanorod on the edge of R-ZnS. Combined the test results of the N 2 adsorption/ desorption measurements with the TEM image of the R-S@ MPC (Figure 2i), it was confirmed that R-S@MPC possesses (i) hollow macropore, (ii) the straight-through mesopores connected with the central macropore, and (iii) the micropores distributed on the carbon shell. In Figure S9 (Supporting Information), the micropore volume ( 2 nm) of 0.16 cm 3 g 1 guarantees sulfur blockage effect of microporous carbon shell. It can be calculated that the straight-through mesopore volume (10 50 nm) of 1.61 cm 3 g 1 can provide 1.1 times the volume 75 wt% sulfur needed (based on the sulfur density of 2.07 g cm 3 ), suggesting that the space provided by the mesopores is fully enough to store the sulfur. Similarly, it can be deduced that the internal pore volume can accommodate the generated Li 2 S during discharging, based on the total pore volume of 2.96 cm 3 g 1 and the Li 2 S density of 1.66 g cm 3. In Figure 3c e, the element mappings of R-S@MPC exhibit the similar distribution of sulfur with carbon, as well as the sulfur enrichment on the edge of R-S@MPC. And no obvious signal of Zn can be found in the element mappings of Figure S10 (Supporting Information), confirming that ZnS is completely oxidized to be sulfur in the R-S@MPC composite. We enlarge one tube in the selected area (Figure 3c), as shown in Figure 3f h. It can be clearly observed that the sulfur is mainly distributed inside the straight holes of the carbon tube, indicating the sulfur storage in mesopores. This is consistent with the relative lower surface sulfur atomic concentration of R-S@MPC in the XPS measurement, compared with the control sample that is prepared by melt-diffusion approach (Figure S11, Supporting Information). To investigate the restriction effect of micropores on LPSs, the off-line observable experiment is carried out (Figure 3i,j), simulating the actual discharge process in the battery. The photos of the control sample show that lots of generated LPSs dissolve in the electrolyte in the first few hours, accompanied by the electrolyte color changes into deep yellow gradually (Figure 3i). As discharge continues, part of the dissolved LPSs is further reduced to insoluble Li 2 S 2 or Li 2 S that are deposited on the carbon host, leading to the pale yellow color of the electrolyte. However, part of LPSs cannot be reduced, or eventually move back to the cathode, which causes the irreversible loss of the active sulfur. [27,37] That is why the color cannot disappear completely, at the end of discharge. In contrast, the electrolyte color of R-S@MPC, as shown in Figure 3j, has almost no change, suggesting that LPSs can hardly diffuse into the main electrolyte through the microporous carbon shell, and the dissolved LPSs are all accommodated in the hollow cavity of R-S@MPC. In addition, the thermogravimetric analysis (TGA) is executed, not only to get the sulfur contents of the composites, but also to observe the difficulty level of sulfur loss. In Figure 3b, the weight loss V s temperature of R-S@MPC is about 30 C later than that of the control sample, indicating that the sulfur loss of R-S@MPC is more difficult. This proves that the sulfur confinement within the microporous carbon shell is well established. [49,52,53] In Figure 3, using the following techniques, we verify: (i) the presence and the distribution of the three pores by N 2 adsorption/desorption measurements, (ii) sulfur storage of the marginal straight mesopores by EDX element mappings, and (iii) LPSs blockage of micropores and accommodation of macropores by visualization experiment, which are basically in line with the original material design. As an energy storage material, battery performances are the only criterion for testing it. We fabricate 2032 coin cell, employing R-S@MPC composite as the cathode material, to evaluate the electrochemical performance as shown in Figure 4. In the rate test (Figure 4a,b), the R-S@MPC composite delivers stable reversible capacities under every current rate except the initial 0.2 C, 928 mah g 1 under 0.4 C, 822 mah g 1 under 0.6 C, 733 mah g 1 under 0.8 C, and 657 mah g 1 under 1 C. And when the current reverts to 0.4 C, the discharge capacity gets back to 919 mah g 1, responding to a recovery ratio of 99%. Compared with the control sample, R-S@MPC demonstrates higher rate capacities and superior capacity retention. The discharge voltage plateau at 1 C remains above 1.9 V, even under the high sulfur loading of 3.5 mg cm 2 (Figure 4b). R-S@ MPC and control sample are also investigated under higher discharge current density and low sulfur loading (Figure S12, Supporting Information). R-S@MPC delivers the reversible capacities of 762, 562, and 263 mah g 1, respectively, at 2, 3, and 4 C rate, exhibiting obvious advantages compared with the control sample. However, the rate capacities at 5 C fall significantly. This is attributed to that the microporous carbon shell limited the transmission rate of Li-ion, besides the suppression of the LPSs diffusion, especially under higher current density (5 C means 8.85 ma cm 2 ). The cycling performances are, respectively, measured at 0.2 and 0.5 C, as demonstrated in Figure 4c. R-S@MPC delivers an initial capacity of 1397 mah g 1 at the first cycle (0.1 C) for activation, [54 57] corresponding to an areal capacity of 5.8 mah cm 2. The R-S@MPC composite exhibits satisfactory cycling stability with 100% Coulombic efficiency and a residual specific capacity of 736 mah g 1 after 250 cycles at 0.2 C. It also displays an initial specific capacity of 869 mah g 1 and corresponding capacity retention of 61% after 300 cycles at 0.5 C, which is much higher than that of the control sample. Based on the rate test results, the long-term cycling performance of R-S@MPC under 1 C rate is investigated (Figure 4e and Figure S13, Supporting Information). The initial peak capacity is 470 mah g 1, and 440 mah g 1 remains after 500 cycles. This is corresponded to an ultralow decay rate of 0.013% cycle 1 at the sulfur loading of 4 mg cm 2. Compared with some typical cathode materials (Table S1, Supporting Information), R-S@MPC (5 of 8)
6 Figure 4. a) Rate performance of and control sample at stepwise current rates, and b) charge/discharge voltage profiles in rate test. c) Cycling performance of and control sample at current rate of 0.2 and 0.5 C. d) The EIS of the fresh cell, the cell after 200 and 400 cycles, respectively. e) Cycling capacity and Coulombic efficiency of at 1 C, and the inset photos are the separator and anode lithium metal after 500 cycles. The sulfur loadings are, respectively, 3.5 mg cm 2 (R-S@MPC) and 3.4 mg cm 2 (control sample) in (a), 4.2 mg cm 2 (0.2 C) and 3.2 mg cm 2 (0.5 C) in (c), and 4 mg cm 2 in (e). exhibits higher long-term cycling stability, reflecting the advantages of our work. The extremely low decay rate is ascribed to the functional differentiation and synergistic cooperation of the three pores in carbon frame. At the first stage of discharge, the generated long-chain LPSs gradually dissolve, and quickly transfer to the hollow cavity through the straight-through hole without any obstacles. Owing to the strong electric field force, the LPSs have tendencies to spread out, leading to the LPSs gathering in the straight-through mesopores on the edge of the R-S@MPC. The microporous carbon shell plays its function of sulfur blockage at this time, which buys time for the conversion of LPSs to the insoluble short chain lithium sulfides (Li 2 S/ Li 2 S 2 ). As the reductive reaction continues, abundant active sites provided by the large specific surface area pave way for fast and homogeneous re-deposition of LPSs, which is also reflected by the significantly decreased charge transfer resistance (R ct ) after cycling (Figure 4d). To further substantiate the argument aforementioned, we disassemble the cell after 500 cycles at 1 C. The R-S@MPC composite is characterized by SEM, TEM, and element mapping, as shown in Figure S14 (Supporting Information), verifying the structural integrity after the long-term cycling. Moreover, only slightly corroded metal lithium anode is found, accompanied by the separator without apparent color change, as displayed in the inset of Figure 4e, suggesting that almost no dissolved LPSs diffuse to the bulk electrolyte, neither shuttle to the surface of metal Li. This phenomenon in turn demonstrates the superior sulfur confinement effect of the unique structure, which has great significance to the cycling (6 of 8)
7 stability and the lithium dendrite suppression. The steady concentration and viscosity of the bulk electrolyte ensure fast Li-ion transfer and depressed concentration polarization, which is consistent with the unchanged Warburg resistance between before and after cycling (Figure 4d). And it is accounted as another cause for the splendid cycling stability under high current rate and high loading. In summary, a rambutan-shaped microporous carboncoated sulfur composite with 75 wt% sulfur content has been designed and synthesized, through in situ oxidation of the improved ZnS precursor that is self-assembled by ZnS nanosphere and nanorod. N 2 adsorption/desorption measurement is employed to prove the existence of the hollow macropore, the marginal straight-through mesopores connected with the macropore, and the micropores distributed on the outer carbon shell. The element mappings are applied to confirm the sulfur storage in the straight-through mesopores. And finally, an off-line visualization experiment is adopted to verify the effect of LPSs blockage of micropores and accommodation of macropores. The three pores perform their own single function, and collaborate with each other to suppress the shuttle effect of LPSs. Therefore, a remarkable decay rate of 0.013% cycle 1 is obtained, after 500 cycle at 1 C under sulfur loading of 4 mg cm 2. The strategy applied in this research work demonstrates a new idea to promote the cycling performance of Li S batteries, and provides a facile method to prepare the heterostructured metal sulfides that can also be applied in the field of photocatalysis. Experimental Section Synthesis of R-ZnS Precursor: The preparation schematic diagram is shown in Figure 1. First, the spherical ZnS core was prepared by a solvothermal method as reported previously. [48,49] Element sulfur (Sigma-Aldrich) and Zn(NO 3 ) 2 6H 2 O (Aladdin) were dispersed in glycol to form a uniform suspension, followed by a solvothermal reaction at 150 C. Second, 0.2 g of as-obtained ZnS nanospheres were mixed with 0.4 g zinc diethyl dithiocarbamate (ZDEC) in a mixture of 60 ml 80 wt% hydrazine hydrate and 20 ml deionized water under ultrasonication for 30 min. Then, the solution was transferred to Teflon-lined autoclave, and reacted at 150 C for 48 h. The product of rambutan-like ZnS was collected by centrifugation, and dried at 80 C overnight. Synthesis of R-ZnS@MPC: 0.29 g R-ZnS and g resorcinol were ultrasonically dispersed in 160 ml ethanol. Then, 50 µl of wt% formaldehyde and 4 ml of 28 wt% ammonia aqueous solution were added to the mixture, and stirred for 24 h at 30 C. The solution was sealed in a Teflon-lined autoclave and maintained at 100 C for 24 h. The as-obtained suspension was filtered, washed with deionized water and dried, followed by a calcination process under Ar gas at 750 C for 2 h, at heating rate 2 C min 1. Synthesis of R-S@MPC: 0.4 g of FeSO 4, 0.6 g of Fe 2 (SO 4 ) 3, and 1.8 g of I 2 were dissolved in a mixture of 40 ml of deionized water and 40 ml of ethanol under magnetic stirring. Then, 0.2 g pre-prepared R-ZnS@ MPC was added to the solution, and vigorously stirred for 24 h at 25 C. The solid product of R-S@MPC was collected through filtration, washed with ethanol and dried at 60 C for 24 h. Synthesis of Control Sample: The obtained R-S@MPC was heated in the tube furnace at 500 C and low pressure, under the protection of Ar gas, to obtain the rambutan-shaped hollow carbon (R-C). Then, the R-C was mixed with sulfur, and heated at 155 C for 24 h, under Ar atmosphere, to obtain the S/R-C composite as the control sample. Material Characterization: XRD patterns were recorded on the D/max-RB diffractometer (Rigaku, Japan) with a Cu Kα radiation. TGA measurements were carried out by heating to 500 C at a scanning rate of 5 C min 1 under Ar atmosphere. SEM characterization and EDX analysis were conducted using JSM-7800F. TEM, STEM, HAADF, and EDX element mapping were characterized by FEI Tecnai G 2 F30 field emission scanning transmission electron microscope at 300 kev. A gas adsorption analyzer (ASAP-2020) was used for N 2 adsorption/ desorption measurement, and the specific surface areas and pore-size distribution were calculated using Brunauer Emmett Teller (BET) and density functional theory (DFT) methods, respectively. The XPS spectra were obtained by ESCALAB250 X-ray photoelectron spectrometer using monochromatic Al Kα ( ev) radiation. Electrochemical Measurement: The cathode slurry was prepared by mixing 90 wt% of S-S/MPC, 4 wt% carboxymethylcellulose sodium (CMC), and 6 wt% styrene butadiene rubber (SBR) in deionized water. The electrodes were produced by coating the slurry onto carbon-coated aluminium foil with the sulfur loading of about 3.5 mg cm 2, which were then dried and cut into round disk with a diameter of 12 mm. The 2032 coin cells were fabricated in an Ar-filled glove box and used for the evaluation of the electrochemical performance of the R-S@MPC composite as cathode materials, Li metal served as anode, and Celgard 2325 membrane used as separator. The electrolyte was composed of 1 m lithium bis(trifluoromethanesulfonyl) imide (LiTFSI) in a mixture of 1,3-dioxolane (DOL) and dimethoxymethane (DME) (1:1, v/v) with 5 wt% of LiNO 3 (GuoTaiHuaRong Co., Ltd.). The electrolyte/sulfur ratio of 20 µl mg 1 is adopted. Visualization experiment was performed in a sealed bottle, with R-S@MPC as cathode, and metal lithium as anode. Both electrodes were connected with external circuit, and discharged under 0.1 C-rate (based on the sulfur mass of the cathode). Discharge/ charge and cycling performances were evaluated with a Neware Battery Measurement System (Neware, China) in the voltage range of V (vs Li + /Li). The electrochemical impedance spectroscopy (EIS) of the cells was recorded with a CHI660E electrochemical workstation (ShangHai ChenHua Co., Ltd.) over the frequency range of 100 khz to 100 mhz. Supporting Information Supporting Information is available from the Wiley Online Library or from the author. Acknowledgements This work was supported by the National Natural Science Foundation of China through Grant No M.Y. would like to thank the National Thousand Youth Talents program of China. This research was also financially supported by the National Natural Science Foundation of China (Grant Nos and ), Harbin Technological Achievements Transformation Projects (2016DB4AG023), and HIT Environment and Ecology Innovation Special Funds (Grant No. HSCJ201620). Conflict of Interest The authors declare no conflict of interest. Keywords functional separation of three pores, high cyclic stability under high loading, in situ oxidation preparation, rambutan-shaped sulfur/carbon composites, self-assembled ZnS precursors Received: September 21, 2017 Revised: November 11, 2017 Published online: (7 of 8)
8 [1] L. Xiao, Y. Cao, J. Xiao, B. Schwenzer, M. H. Engelhard, L. V. Saraf, Z. Nie, G. J. Exarhos, J. Liu, Adv. Mater. 2012, 24, [2] Z. Ma, S. Dou, A. Shen, L. Tao, L. Dai, S. Wang, Angew. Chem., Int. Ed. 2015, 54, [3] R. Demir-Cakan, M. Morcrette, Gangulibabu, A. Gueguen, R. Dedryvere, J. M. Tarascon, Energy Environ. Sci. 2013, 6, 176. [4] L. Ji, M. Rao, H. Zheng, L. Zhang, Y. Li, W. Duan, J. Guo, E. J. Cairns, Y. Zhang, J. Am. Chem. Soc. 2011, 133, [5] X. Fang, H. S. Peng, Small 2015, 11, [6] Q. Pang, L. F. Nazar, ACS Nano 2016, 10, [7] X. Liang, Y. Rangom, C. Y. Kwok, Q. Pang, L. F. Nazar, Adv. Mater. 2017, 29, [8] T. Zhao, Y. S. Ye, C. Y. Lao, G. Divitini, P. Coxon, Xi. Y. Peng, X. He, H. K. Kim, K. Xi, C. Ducati, R.J. Chen, Y. J. Liu, S. Ramakrishna, R. V. Kumar, Small 2017, 13, [9] J. Liu, W. Li, L. Duan, X. Li, L. Ji, Z. Geng, K. Huang, L. Lu, L. Zhou, Z. Liu, W. Chen, L. Liu, S. Feng, Y. Zhang, Nano Lett. 2015, 15, [10] J. T. Lee, Y. Zhao, S. Thieme, H. Kim, M. Oschatz, L. Borchardt, A. Magasinski, W. I. Cho, S. Kaskel, G. Yushin, Adv. Mater. 2013, 25, [11] J. L. Shi, C. Tang, H. J. Peng, L. Zhu, X. B. Cheng, J. Q. Huang, W. C. Zhu, Q. Zhang, Small 2015, 11, [12] Z. Lyu, D. Xu, L. Yang, R. Che, R. Feng, J. Zhao, Y. Li, Q. Wu, X. Wang, Z. Hu, Nano Energy 2015, 12, 657. [13] H. Yao, G. Zheng, P.-C. Hsu, D. Kong, J. J. Cha, W. Li, Z. W. Seh, M. T. McDowell, K. Yan, Z. Liang, V. K. Narasimhan, Y. Cui, Nat. Commun. 2014, 5, [14] S. Rehman, T. Y. Tang, Z. S. Ali, X. X. Huang, Y. L. Hou, Small 2017, 13, [15] J. Zhang, H. Hu, Z. Li, X. W. Lou, Angew. Chem., Int. Ed. 2016, 55, [16] Z. W. Seh, W. Li, J. J. Cha, G. Zheng, Y. Yang, M. T. McDowell, P.-C. Hsu, Y. Cui, Nat. Commun. 2013, 4, [17] S. Rehman, S. Guo, Y. Hou, Adv. Mater. 2016, 28, [18] M. Q. Zhao, X.-F. Liu, Q. Zhang, G.-L. Tian, J.-Q. Huang, W. Zhu, F. Wei, ACS Nano 2012, 6, [19] C. Wang, X. Wang, Y. Wang, J. Chen, H. Zhou, Y. Huang, Nano Energy 2015, 11, 678. [20] H. Wang, Y. Yang, Y. Liang, J. T. Robinson, Y. Li, A. Jackson, Y. Cui, H. Dai, Nano Lett. 2011, 11, [21] H. Sohn, M. L. Gordin, T. Xu, S. Chen, D. Lv, J. Song, A. Manivannan, D. Wang, ACS Appl. Mater. Interfaces 2014, 6, [22] H.-J. Peng, J. Liang, L. Zhu, J.-Q. Huang, X.-B. Cheng, X. Guo, W. Ding, W. Zhu, Q. Zhang, ACS Nano 2014, 8, [23] F. Wu, J. T. Lee, N. Nitta, H. Kim, O. Borodin, G. Yushin, Adv. Mater. 2015, 27, 101. [24] T. Chen, B. R. Cheng, G. Y. Zhu, R. P. Chen, Y. Hu, L. B. Ma, H. L. Lv, Y. R. Wang, J. Liang, Z. X. Tie, Z. Jin, J. Liu, Nano Lett. 2017, 17, 437. [25] L. B. Ma, R. P. Chen, G. Y. Zhu, Y. Hu, Y. R. Wang, T. Chen, J. Liu, Z. Jin, ACS Nano 2017, 11, [26] X. Liang, C. Y. Kwok, F. Lodi-Marzano, Q. Pang, M. Cuisinier, H. Huang, C. J. Hart, D. Houtarde, K. Kaup, H. Sommer, T. Brezesinski, J. Janek, L. F. Nazar, Adv. Energy Mater. 2016, 6, [27] G. Zhou, Y. Zhao, C. Zu, A. Manthiram, Nano Energy 2015, 12, 240. [28] H. Wang, Q. Zhang, H. Yao, Z. Liang, H. W. Lee, P. C. Hsu, G. Zheng, Y. Cui, Nano Lett. 2014, 14, [29] T. Y. Lei, Y. M. Xie, X. F. Wang, S. Y. Miao, J. Xiong, C. L. Yan, Small 2017, [30] T. Chen, Z. W. Zhang, B. R. Cheng, R. P. Chen, Y. Hu, L. B. Ma, G. Y. Zhu, J. Liu, Z. Jin, J. Am. Chem. Soc. 2017, 139, [31] D. R. Deng, F. Xue, Y. J. Jia, J. C. Ye, C. D. Bai, M. S. Zheng, Q. F. Dong, ACS Nano 2017, 11, [32] D. R. Deng, T. H. An, Y. J. Li, Q. H. Wu, M. S. Zheng, Q. F. Dong, J. Mater. Chem. A 2016, 4, [33] Y. Zhao, M. Liu, W. Lv, Y. B. He, C. Wang, Q. B. Yun, B. H. Li, F. Y. Kang, Q. H. Yang, Nano Energy 2016, 30, 1. [34] C. Oh, N. Yoon, J. Choi, Y. Choi, S. Ahn, J. K. Lee, J. Mater. Chem. A 2017, 5, [35] Z. A. Ghazi, X. He, A. M. Khattak, N. A. Khan, B. Liang, A. Iqbal, J. X. Wang, H. Sin, L. S. Li, Z. Y. Tang, Adv. Mater. 2017, 29, [36] M. Liu, Q. Li, X. Y. Qin, G. M. Liang, W. J. Han, D. Zhou, Y. B. He, B. H. Li, F. Y. Kang, Small 2017, 13, [37] W. B. Kong, L. J. Yan, Y. F. Luo, D. T. Wang, K. L. Jiang, Q. Q. Li, S. S. Fan, J. P. Wang, Adv. Funct. Mater. 2017, 27, [38] D. C. Lin, Y. Y. Liu, W. Chen, G. M. Zhou, K. Liu, B. Dunn, Y. Cui, Nano Lett. 2017, 17, [39] S. Jin, S. Xin, L. J. Wang, Z. Z. Du, L. N. Cao, J. F. Chen, X. H. Kong, M. Gong, J. L. Lu, Y.W. Zhu, H. X. Ji, R. S. Ruoff, Adv. Mater. 2016, 28, [40] Q. Li, S. Zhu, Y. Y. Lu, Adv. Funct. Mater. 2017, 27, [41] H. K. Jing, L. L. Kong, S. Liu, G. R. Li, X. P. Gao, J. Mater. Chem. A 2015, 3, [42] G. He, S. Evers, X. Liang, M. Cuisinier, A. Garsuch, L. F. Nazar, ACS Nano 2013, 7, [43] Z. Li, Y. Jiang, L. Yuan, Z. Yi, C. Wu, Y. Liu, P. Strasser, Y. Huang, ACS Nano 2014, 8, [44] M. Li, Y. Zhang, X. Wang, W. Ahn, G. Jiang, K. Feng, G. Lui, Z. Chen, Adv. Funct. Mater. 2016, 26, [45] C. Zhang, H. B. Wu, C. Yuan, Z. Guo, X. W. Lou, Angew. Chem., Int. Ed. 2012, 51, [46] S.-H. Chung, C.-H. Chang, A. Manthiram, Energy Environ. Sci. 2016, 9, [47] Z. Li, H. B. Wu, X. W. Lou, Energy Environ. Sci. 2016, 9, [48] Q. Wang, Z. B. Wang, C. Li, D. M. Gu, J. Mater. Chem. A 2017, 5, [49] Q. Wang, Z. B. Wang, M. H. Yang, C. Li, D. M. Gu, J. Mater. Chem. A 2017, 5, [50] Z. Zhang, Q. Li, S. Jiang, K. Zhang, Y. Lai, J. Li, Chem. Eur. J. 2015, 21, [51] R. Chen, T. Zhao, J. Lu, F. Wu, L. Li, J. Chen, G. Tan, Y. Ye, K. Amine, Nano Lett. 2013, 13, [52] D. L. Wang, Y. C. Yu, W. D. Zhou, H. Chen, F. J. DiSalvo, D. A. Muller, H. D. Abruna, Phys. Chem. Chem. Phys. 2013, 15, [53] Y. Zhao, W. L. Wu, J. X. Li, Z. C. Xu, L. H. Guan, Adv. Mater. 2014, 26, [54] F. Pei, T. H. An, J. Zang, X. J. Zhao, X. L. Fang, M. S. Zheng, Q. F. Dong, N. F. Zheng, Adv. Energy Mater. 2016, 6, [55] C. Y. Chen, H. J. Peng, T. Z. Hou, P. Y. Zhai, B. Q. Li, C. Tang, W. C. Zhu, J. Q. Huang, Q. Zhang, Adv. Mater. 2017, 29, [56] T. Chen, L. B. Ma, B. R. Cheng, R. P. Chen, Y. Hu, G. Y. Zhu, Y. R. Wang, J. Liang, Z. X. Tie, J. Liu, Z. Jin, Nano Energy 2017, 38, 239. [57] X. Liang, L. F. Nazar, ACS Nano 2016, 10, (8 of 8)
Fabrication of Novel Lamellar Alternating Nitrogen-Doped
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Fabrication of Novel Lamellar Alternating Nitrogen-Doped Microporous Carbon
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Fabrication of Novel Lamellar Alternating Nitrogen-Doped Microporous Carbon
Supporting Information
 Copyright WILEY VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2016. Supporting Information for Adv. Energy Mater., DOI: 10.1002/aenm.201502539 From Hollow Carbon Spheres to N-Doped Hollow Porous
Copyright WILEY VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2016. Supporting Information for Adv. Energy Mater., DOI: 10.1002/aenm.201502539 From Hollow Carbon Spheres to N-Doped Hollow Porous
Interconnected hierarchical NiCo 2 O 4 microspheres as high performance. electrode material for supercapacitor
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Interconnected hierarchical microspheres as high performance electrode material for
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Interconnected hierarchical microspheres as high performance electrode material for
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) Nanomat Li-S batteries based on
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) Nanomat Li-S batteries based on
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Metal-organic framework-derived CoSe2/(NiCo)Se2
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Metal-organic framework-derived CoSe2/(NiCo)Se2
Supplementary Information. High areal capacity lithium sulfur battery cathode by. site-selective vapor infiltration of hierarchical
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Supplementary Information High areal capacity lithium sulfur battery cathode by site-selective
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Supplementary Information High areal capacity lithium sulfur battery cathode by site-selective
Supporting Information for
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting Information for Core-shell ZnO/ZnFe 2 O 4 @C Mesoporous Nanospheres
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting Information for Core-shell ZnO/ZnFe 2 O 4 @C Mesoporous Nanospheres
Hierachical Nickel-Carbon Structure Templated by Metal-Organic Frameworks for Efficient Overall Water Splitting
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Hierachical Nickel-Carbon Structure
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Hierachical Nickel-Carbon Structure
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Fe 3 O 4 Quantum Dots Decorated MoS 2 Nanosheet
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Fe 3 O 4 Quantum Dots Decorated MoS 2 Nanosheet
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Paragenesis BN/CNTs Hybrid as a Monoclinic Sulfur
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Paragenesis BN/CNTs Hybrid as a Monoclinic Sulfur
Supporting Information. Metal organic framework-derived Fe/C Nanocubes toward efficient microwave absorption
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting Information Metal organic framework-derived Fe/C Nanocubes toward
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting Information Metal organic framework-derived Fe/C Nanocubes toward
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information 3D-composite structure of FeP nanorods supported
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information 3D-composite structure of FeP nanorods supported
Supporting information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry Please do 2018 not adjust margins Supporting information Self-assembled 3D flower-like
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry Please do 2018 not adjust margins Supporting information Self-assembled 3D flower-like
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information High-performance LiTi 2 (PO 4 ) 3
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information High-performance LiTi 2 (PO 4 ) 3
Supporting Information. In-Situ Facile Bubble-Templated Fabrication of New-Type Urchin-Like (Li, Mo)- Doped Lix(Mo0.3V0.7)2O5 for Zn 2+ Storage
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information In-Situ Facile Bubble-Templated Fabrication of New-Type
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information In-Situ Facile Bubble-Templated Fabrication of New-Type
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2019 Supporting Information Bioinspired Pomegranate-like Microflowers Confining
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2019 Supporting Information Bioinspired Pomegranate-like Microflowers Confining
Supplementary Material
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Material Biomass Chitosan Derived Cobalt/Nitrogen Doped Carbon
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Material Biomass Chitosan Derived Cobalt/Nitrogen Doped Carbon
Tunable CoFe-Based Active Sites on 3D Heteroatom Doped. Graphene Aerogel Electrocatalysts via Annealing Gas Regulation for
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Tunable CoFe-Based Active Sites on 3D Heteroatom Doped Graphene Aerogel
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Tunable CoFe-Based Active Sites on 3D Heteroatom Doped Graphene Aerogel
Supplementary Information. O-vacancy Enriched NiO Hexagonal Platelets Fabricated on Ni. Foam as Self-supported Electrode for Extraordinary
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information O-vacancy Enriched NiO Hexagonal Platelets Fabricated
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information O-vacancy Enriched NiO Hexagonal Platelets Fabricated
Supporting Information. Mitigating the P2 O2 phase transition of high-voltage
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2019 Supporting Information Mitigating the P2 O2 phase transition of high-voltage
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2019 Supporting Information Mitigating the P2 O2 phase transition of high-voltage
Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2019 Supplementary Information Mo modulation effect on the hydrogen binding
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2019 Supplementary Information Mo modulation effect on the hydrogen binding
Facile synthesis of N-rich carbon quantum dots by spontaneous. polymerization and incision of solvents as efficient bioimaging probes
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2015 Supporting Information Facile synthesis of N-rich carbon quantum dots by spontaneous polymerization
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2015 Supporting Information Facile synthesis of N-rich carbon quantum dots by spontaneous polymerization
Zhaohuai Li, Qiu He, Xu Xu,* Yan Zhao, Xiaowei Liu, Cheng Zhou, Dong Ai, Lixue Xia, and Liqiang Mai*
 Communication Lithium Sulfur Batteries A 3D Nitrogen-Doped Graphene/TiN Nanowires Composite as a Strong Polysulfide Anchor for Lithium Sulfur Batteries with Enhanced Rate Performance and High Areal Capacity
Communication Lithium Sulfur Batteries A 3D Nitrogen-Doped Graphene/TiN Nanowires Composite as a Strong Polysulfide Anchor for Lithium Sulfur Batteries with Enhanced Rate Performance and High Areal Capacity
noble-metal-free hetero-structural photocatalyst for efficient H 2
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Ni 12 P 5 nanoparticles embedded into porous g-c 3 N 4 nanosheets as a
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Ni 12 P 5 nanoparticles embedded into porous g-c 3 N 4 nanosheets as a
Supporting Information for
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Supporting Information for 3D Hierarchical Ni(PO 3 ) 2 Nanosheet Arrays
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Supporting Information for 3D Hierarchical Ni(PO 3 ) 2 Nanosheet Arrays
Supplementary Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2018 Supplementary Information Core@Shell Structured Co-CoO@NC Nanoparticles Supported on Nitrogen
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2018 Supplementary Information Core@Shell Structured Co-CoO@NC Nanoparticles Supported on Nitrogen
Supplementary Information
 Supplementary Information Fig S1 a top-down SEM image of PS template. b. cross-sectional SEM of PS template (with diameter of 440 nm). Fig. S2 The cross-sectional SEM of the dense Ge film(a) and 3DOM Ge
Supplementary Information Fig S1 a top-down SEM image of PS template. b. cross-sectional SEM of PS template (with diameter of 440 nm). Fig. S2 The cross-sectional SEM of the dense Ge film(a) and 3DOM Ge
Facile fabrication of well-defined polyaniline microtubes derived. from natural kapok fiber for supercapacitor with long-term.
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Facile fabrication of well-defined polyaniline microtubes derived from natural kapok fiber
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Facile fabrication of well-defined polyaniline microtubes derived from natural kapok fiber
Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information Scalable and Ascendant Synthesis of Coated Carbon
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information Scalable and Ascendant Synthesis of Coated Carbon
Ultrathin Co-Fe Hydroxide Nanosheet Arrays for Improved
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2017 Supplementary Information Ultrathin Co-Fe Hydroxide Nanosheet Arrays for Improved Oxygen Evolution
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2017 Supplementary Information Ultrathin Co-Fe Hydroxide Nanosheet Arrays for Improved Oxygen Evolution
Layered polyaniline/graphene film from sandwich-structured polyaniline/graphene/polyaniline nanosheets for high-performance pseudosupercapacitors
 Supporting Information for: Layered polyaniline/graphene film from sandwich-structured polyaniline/graphene/polyaniline nanosheets for high-performance pseudosupercapacitors Zhongqiu Tong a, Yongning Yang
Supporting Information for: Layered polyaniline/graphene film from sandwich-structured polyaniline/graphene/polyaniline nanosheets for high-performance pseudosupercapacitors Zhongqiu Tong a, Yongning Yang
Supporting Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Supporting Information Dual active nitrogen doped hierarchical porous hollow carbon nanospheres
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Supporting Information Dual active nitrogen doped hierarchical porous hollow carbon nanospheres
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2019 Electronic Supplementary Information (ESI) FeP@C Nanoarray Vertically Grown on Graphene Nanosheets:
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2019 Electronic Supplementary Information (ESI) FeP@C Nanoarray Vertically Grown on Graphene Nanosheets:
state asymmetric supercapacitors
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 3D hierarchical CoO@MnO 2 core-shell nanohybrid for high-energy solid state
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 3D hierarchical CoO@MnO 2 core-shell nanohybrid for high-energy solid state
Supporting Information
 Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2016. Supporting Information for Adv. Energy Mater., DOI: 10.1002/aenm.201600322 Integrated Intercalation-Based and Interfacial Sodium
Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2016. Supporting Information for Adv. Energy Mater., DOI: 10.1002/aenm.201600322 Integrated Intercalation-Based and Interfacial Sodium
3D CNTs/Graphene-S-Al 3 Ni 2 Cathodes for High-Sulfur-Loading and Long-Life Lithium Sulfur Batteries
 FULL PAPER Lithium-Sulfur Batteries 3D CNTs/Graphene-S-Al 3 Ni 2 Cathodes for High-Sulfur-Loading and Long-Life Lithium Sulfur Batteries Zeqing Guo, Huagui Nie,* Zhi Yang,* Wuxing Hua, Chunping Ruan, Dan
FULL PAPER Lithium-Sulfur Batteries 3D CNTs/Graphene-S-Al 3 Ni 2 Cathodes for High-Sulfur-Loading and Long-Life Lithium Sulfur Batteries Zeqing Guo, Huagui Nie,* Zhi Yang,* Wuxing Hua, Chunping Ruan, Dan
Supporting Information. High cycling stable supercapacitor through electrochemical. deposition of metal-organic frameworks/polypyrrole positive
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 Supporting Information High cycling stable supercapacitor through electrochemical deposition
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 Supporting Information High cycling stable supercapacitor through electrochemical deposition
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting Information Template-Preparation of Three-Dimensional Molybdenum
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting Information Template-Preparation of Three-Dimensional Molybdenum
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) Hierarchically porous Mo-doped
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) Hierarchically porous Mo-doped
catalytically deposited Cu current collector patterns for high-performance flexible in-plane micro-size energy
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 018 Electronic Supplementary Information (ESI) In-situ growth of Cu(OH) @FeOOH
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 018 Electronic Supplementary Information (ESI) In-situ growth of Cu(OH) @FeOOH
Electronic Supporting Information (ESI)
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Electronic Supporting Information (ESI) Designing a Carbon Nanotubes Interconnected
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Electronic Supporting Information (ESI) Designing a Carbon Nanotubes Interconnected
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Electronic coupling-tunable of cobalt sulfide/carbon
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Electronic coupling-tunable of cobalt sulfide/carbon
Electronic Supplementary Information. Hierarchically porous Fe-N-C nanospindles derived from. porphyrinic coordination network for Oxygen Reduction
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Hierarchically porous Fe-N-C nanospindles
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Hierarchically porous Fe-N-C nanospindles
Nickel Hexacyanoferrate/Carbon Composite as a High-Rate and. Long-Life Cathode Material for Aqueous Hybrid Energy
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Nickel Hexacyanoferrate/Carbon Composite as a High-Rate and Long-Life Cathode Material for Aqueous
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Nickel Hexacyanoferrate/Carbon Composite as a High-Rate and Long-Life Cathode Material for Aqueous
Pingping zhao, Xing Hua, Wei Xu, Wei Luo,* Shengli Chen,* and Gongzhen Cheng
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information Metal-Organic Framework-Derived Hybrid
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information Metal-Organic Framework-Derived Hybrid
Octahedral Pd Nanocages with Porous Shells Converted by Co(OH) 2 Nanocages with Nanosheet surface as Robust Electrocatalysts for Ethanol Oxidation
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Octahedral Pd Nanocages with Porous Shells Converted by Co(OH) 2 Nanocages
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Octahedral Pd Nanocages with Porous Shells Converted by Co(OH) 2 Nanocages
Key Laboratory of Material Chemistry for Energy Conversion and Storage (Ministry
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 Supporting Information for Dramatically enhanced visible-light driven H 2 evolution
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 Supporting Information for Dramatically enhanced visible-light driven H 2 evolution
High performance carbon nanotube based fiber-shaped. supercapacitors using redox additives of polypyrrole and. hydroquinone
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting information High performance carbon nanotube based fiber-shaped
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting information High performance carbon nanotube based fiber-shaped
A reformative oxidation strategy using high concentration nitric acid for. enhancing emission performance of graphene quantum dots
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 A reformative oxidation strategy using high concentration nitric acid for enhancing emission
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 A reformative oxidation strategy using high concentration nitric acid for enhancing emission
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 018 Electronic Supplementary Information Superhydrophilic amorphous Co-B-P nanosheet
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 018 Electronic Supplementary Information Superhydrophilic amorphous Co-B-P nanosheet
Supplementary Information. A synergistic interaction between isolated Au nanoparticles with oxygen vacancies in
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information A synergistic interaction between isolated Au
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Information A synergistic interaction between isolated Au
Supporting Information
 Electronic Supplementary Material (ESI) for Materials Horizons. This journal is The Royal Society of Chemistry 2017 Supporting Information Co/CoP Embedded in Hairy Nitrogen-Doped Carbon Polyhedron as an
Electronic Supplementary Material (ESI) for Materials Horizons. This journal is The Royal Society of Chemistry 2017 Supporting Information Co/CoP Embedded in Hairy Nitrogen-Doped Carbon Polyhedron as an
A Ni 3 N-Co 3 N hybrid nanowire array electrode for high-performance nonenzymatic glucose detection
 Electronic Supplementary Material (ESI) for Analytical Methods. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information A Ni 3 N-Co 3 N hybrid nanowire array electrode
Electronic Supplementary Material (ESI) for Analytical Methods. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information A Ni 3 N-Co 3 N hybrid nanowire array electrode
Journal of Materials Chemistry A. Supporting Information. Cobalt Nickel Boride as an Active Electrocatalyst for Water Splitting
 Electronic Supplementary Material (ESI) for. This journal is The Royal Society of Chemistry Please do 2017 not adjust margins Supporting Information Cobalt Nickel Boride as an Active Electrocatalyst for
Electronic Supplementary Material (ESI) for. This journal is The Royal Society of Chemistry Please do 2017 not adjust margins Supporting Information Cobalt Nickel Boride as an Active Electrocatalyst for
Supporting information
 Electronic Supplementary Material (ESI) for Inorganic Chemistry Frontiers. This journal is the Partner Organisations 2018 Supporting information Cube-Like CuCoO Nanostructures on Reduced Graphene Oxide
Electronic Supplementary Material (ESI) for Inorganic Chemistry Frontiers. This journal is the Partner Organisations 2018 Supporting information Cube-Like CuCoO Nanostructures on Reduced Graphene Oxide
MXene/Graphene Hybrid Fibers for High. Performance Flexible Supercapacitors
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information for MXene/Graphene Hybrid Fibers for High Performance
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information for MXene/Graphene Hybrid Fibers for High Performance
Supporting Information
 Supporting Information A General Strategy to Fabricate P as Highly Efficient Cocatalyst via Photo-Reduction Deposition for Hydrogen Evolution Yuming Dong a, *, Linggang Kong a, Pingping Jiang a, Guangli
Supporting Information A General Strategy to Fabricate P as Highly Efficient Cocatalyst via Photo-Reduction Deposition for Hydrogen Evolution Yuming Dong a, *, Linggang Kong a, Pingping Jiang a, Guangli
Supplementary Information. Indole-Based Conjugated Macromolecule as Redox- Mediated Electrolyte for Ultrahigh Power Supercapacitor
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2017 Supplementary Information Indole-Based Conjugated Macromolecule as Redox-
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2017 Supplementary Information Indole-Based Conjugated Macromolecule as Redox-
Porous and High-strength Graphitic Carbon/SiC Three-Dimensional Electrode for Capacitive Deionization and Fuel Cell Applications
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Porous and High-strength Graphitic Carbon/SiC Three-Dimensional
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Porous and High-strength Graphitic Carbon/SiC Three-Dimensional
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Supporting Information Branched polyethylenimine grafted electrospun polyacrylonitrile
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Supporting Information Branched polyethylenimine grafted electrospun polyacrylonitrile
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Flexible 3D Porous CuO Nanowire Arrays for Enzymeless Glucose
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Flexible 3D Porous CuO Nanowire Arrays for Enzymeless Glucose
high-performance intercalation pseudocapacitive anode for lithiumion capacitors
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2019 A novel calendula-like MnNb 2 O 6 anchored on graphene sheet as high-performance
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2019 A novel calendula-like MnNb 2 O 6 anchored on graphene sheet as high-performance
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information From ZIF-8 Polyhedron to Three-Dimensional Nitrogen
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information From ZIF-8 Polyhedron to Three-Dimensional Nitrogen
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information Experimental Section Materials: Carbon
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information Experimental Section Materials: Carbon
Silver Nanowires Coated on Cotton for Flexible Pressure Sensors. College of Materials Science and Engineering, Key Lab of Guangdong Province for
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2015 Supporting Information Silver Nanowires Coated on Cotton for Flexible Pressure
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2015 Supporting Information Silver Nanowires Coated on Cotton for Flexible Pressure
Supporting Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Supporting Information Phytic acid-derivative transition metal phosphides encapsulated in N,P-codoped
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Supporting Information Phytic acid-derivative transition metal phosphides encapsulated in N,P-codoped
Supporting Information
 Electronic Supplementary Material (ESI) for Sustainable Energy & Fuels. This journal is The Royal Society of Chemistry 2018 Supporting Information Electrospray Synthesis of Si Encapsulated in Graphite/carbon
Electronic Supplementary Material (ESI) for Sustainable Energy & Fuels. This journal is The Royal Society of Chemistry 2018 Supporting Information Electrospray Synthesis of Si Encapsulated in Graphite/carbon
A ligand conformation preorganization approach to construct a. copper-hexacarboxylate framework with a novel topology for
 Electronic Supplementary Material (ESI) for Inorganic Chemistry Frontiers. This journal is the Partner rganisations 2018 A ligand conformation preorganization approach to construct a copper-hexacarboxylate
Electronic Supplementary Material (ESI) for Inorganic Chemistry Frontiers. This journal is the Partner rganisations 2018 A ligand conformation preorganization approach to construct a copper-hexacarboxylate
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry B. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Experimental 2.1 Chemicals and Materials
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry B. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Experimental 2.1 Chemicals and Materials
3D Yolk-Shelled NiGa 2 S 4 Microspheres Confined with Nanosheets for High Performance Supercapacitors
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information (ESI) 3D Yolk-Shelled NiGa 2 S 4 Microspheres
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information (ESI) 3D Yolk-Shelled NiGa 2 S 4 Microspheres
Electronic Supplementary Information for. Highly Stable Mesoporous Silica Nanospheres Embedded with
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 17 Electronic Supplementary Information for Highly Stable Mesoporous Silica Nanospheres Embedded
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 17 Electronic Supplementary Information for Highly Stable Mesoporous Silica Nanospheres Embedded
Nitrogen-Doped Core-Sheath Carbon Nanotube Array for Highly Stretchable Supercapacitor
 www.advancedsciencenews.com Nitrogen-Doped Core-Sheath Carbon Nanotube Array for Highly Stretchable Supercapacitor Zhitao Zhang, Lie Wang, Yiming Li, Yuhang Wang, Jing Zhang, Guozhen Guan, Zhiyong Pan,
www.advancedsciencenews.com Nitrogen-Doped Core-Sheath Carbon Nanotube Array for Highly Stretchable Supercapacitor Zhitao Zhang, Lie Wang, Yiming Li, Yuhang Wang, Jing Zhang, Guozhen Guan, Zhiyong Pan,
Supporting Information. Outstanding hydrogen evolution reaction catalyzed by porous nickel diselenide
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2017 Supporting Information Outstanding hydrogen evolution reaction catalyzed
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2017 Supporting Information Outstanding hydrogen evolution reaction catalyzed
Highly enhanced performance of spongy graphene as oil sorbent
 Supporting Information Highly enhanced performance of spongy graphene as oil sorbent Hengchang Bi, a Xiao Xie, a Kuibo Yin, a Yilong Zhou, a Shu Wan, a Rodney S. Ruoff b and Litao Sun* a a SEU-FEI Nano-Pico
Supporting Information Highly enhanced performance of spongy graphene as oil sorbent Hengchang Bi, a Xiao Xie, a Kuibo Yin, a Yilong Zhou, a Shu Wan, a Rodney S. Ruoff b and Litao Sun* a a SEU-FEI Nano-Pico
Complex Systems and Applications
 Complex Systems and Applications Volume 1 TABLE OF CONTENTS State Feedback and Pole Assignment for A Class of the Second Order Coupled Generalized Distributed Parameter Systems 1 Z. Q. Ge, G. T. Zhu On
Complex Systems and Applications Volume 1 TABLE OF CONTENTS State Feedback and Pole Assignment for A Class of the Second Order Coupled Generalized Distributed Parameter Systems 1 Z. Q. Ge, G. T. Zhu On
Flexible and Printable Paper Based Strain Sensors for Wearable and Large Area Green Electronics
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Supporting Information Flexible and Printable Paper Based Strain Sensors for Wearable and Large
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Supporting Information Flexible and Printable Paper Based Strain Sensors for Wearable and Large
Electronic Supplementary Information (ESI) for Analyst. A Facile Graphene Oxide-Based Fluorescent Nanosensor for in Situ
 Electronic Supplementary Material (ESI) for Analyst. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) for Analyst A Facile Graphene Oxide-Based Fluorescent
Electronic Supplementary Material (ESI) for Analyst. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) for Analyst A Facile Graphene Oxide-Based Fluorescent
Hydrophilic/Hydrophobic Interphase-Mediated. Bubble-Like Stretchable Janus Ultrathin Films. Towards Self-Adaptive and Pneumatic
 1 Supporting Information for Hydrophilic/Hydrophobic Interphase-Mediated Bubble-Like Stretchable Janus Ultrathin Films Towards Self-Adaptive and Pneumatic Multifunctional Electronics Peng Xiao,, Yun Liang,,
1 Supporting Information for Hydrophilic/Hydrophobic Interphase-Mediated Bubble-Like Stretchable Janus Ultrathin Films Towards Self-Adaptive and Pneumatic Multifunctional Electronics Peng Xiao,, Yun Liang,,
Supporting Information. Highly simple and rapid synthesis of ultrathin gold nanowires with
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Highly simple and rapid synthesis of ultrathin gold
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information Highly simple and rapid synthesis of ultrathin gold
Journal of Chemical and Pharmaceutical Research, 2016, 8(6): Research Article. Walking Robot Stability Based on Inverted Pendulum Model
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2016, 8(6):463-467 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Walking Robot Stability Based on Inverted Pendulum
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2016, 8(6):463-467 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Walking Robot Stability Based on Inverted Pendulum
Basketball field goal percentage prediction model research and application based on BP neural network
 ISSN : 0974-7435 Volume 10 Issue 4 BTAIJ, 10(4), 2014 [819-823] Basketball field goal percentage prediction model research and application based on BP neural network Jijun Guo Department of Physical Education,
ISSN : 0974-7435 Volume 10 Issue 4 BTAIJ, 10(4), 2014 [819-823] Basketball field goal percentage prediction model research and application based on BP neural network Jijun Guo Department of Physical Education,
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Cobalt nanoparticles encapsulated in carbon nanotube-grafted
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Cobalt nanoparticles encapsulated in carbon nanotube-grafted
Supporting information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Please note this document replaces the version originally published on
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Please note this document replaces the version originally published on
Recent advances in energy transfer in bulk and nanoscale. luminescent materials: From spectroscopy to applications
 Electronic Supplementary Material (ESI) for Chemical Society Reviews. This journal is The Royal Society of Chemistry 215 Electronic supplementary information Recent advances in energy transfer in ulk and
Electronic Supplementary Material (ESI) for Chemical Society Reviews. This journal is The Royal Society of Chemistry 215 Electronic supplementary information Recent advances in energy transfer in ulk and
Roles of Nitrogen Functionalities in Enhancing the. Excitation-Independent Green-Color Photoluminescence of
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Roles of Nitrogen Functionalities in Enhancing the Excitation-Independent
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Roles of Nitrogen Functionalities in Enhancing the Excitation-Independent
Journal of Chemical and Pharmaceutical Research, 2014, 6(3): Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research 2014 6(3):304-309 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 World men sprint event development status research
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research 2014 6(3):304-309 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 World men sprint event development status research
Curriculum Vitae. Yu (Will) Wang
 Curriculum Vitae Yu (Will) Wang Assistant Research Professor yu.wang3@wsu.edu School of Mechanical and Materials Engineering Washington State University, Pullman, 99164, WA, USA EDUCATION B.S. Sichuan
Curriculum Vitae Yu (Will) Wang Assistant Research Professor yu.wang3@wsu.edu School of Mechanical and Materials Engineering Washington State University, Pullman, 99164, WA, USA EDUCATION B.S. Sichuan
Reactivity Improvement of Ca (OH) 2 Sorbent Using Diatomaceous Earth (DE) From Aceh Province
 Research Inventy: International Journal of Engineering And Science Vol.8, Issue 2 (May 2018), PP -05-10 Issn (e): 2278-4721, Issn (p):2319-6483, www.researchinventy.com Reactivity Improvement of Ca (OH)
Research Inventy: International Journal of Engineering And Science Vol.8, Issue 2 (May 2018), PP -05-10 Issn (e): 2278-4721, Issn (p):2319-6483, www.researchinventy.com Reactivity Improvement of Ca (OH)
Sodium Borohydride Stabilizes Very Active Gold Nanoparticle Catalyst ELECTRONIC SUPPLEMENTARY INFORMATIONS. I. General data..p2
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Sodium Borohydride Stabilizes Very Active Gold Nanoparticle Catalyst Christophe Deraedt, Lionel
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Sodium Borohydride Stabilizes Very Active Gold Nanoparticle Catalyst Christophe Deraedt, Lionel
High performing AgNWs transparent conducting electrodes with 2.5Ω/Sq based upon Roll-to- Roll compatible post processing technique
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2019 High performing AgNWs transparent conducting electrodes with 2.5Ω/Sq based upon Roll-to- Roll
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2019 High performing AgNWs transparent conducting electrodes with 2.5Ω/Sq based upon Roll-to- Roll
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Continuous fabrication of graphene-confined polypyrrole
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Continuous fabrication of graphene-confined polypyrrole
A07 Surfactant Induced Solubilization and Transfer Resistance in Gas-Water and Gas-Oil Systems
 A07 Surfactant Induced Solubilization and Transfer Resistance in Gas-Water and Gas-Oil Systems R Farajzadeh* (TU Delft), A. Banaei (TU Delft), J. Kinkela (TU Delft), T. deloos (TU Delft), S. Rudolph (TU
A07 Surfactant Induced Solubilization and Transfer Resistance in Gas-Water and Gas-Oil Systems R Farajzadeh* (TU Delft), A. Banaei (TU Delft), J. Kinkela (TU Delft), T. deloos (TU Delft), S. Rudolph (TU
The scope of fracture zone measure and high drill gas drainage technology research
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 214, 6(1):45-457 Research Article ISSN : 975-7384 CODEN(USA) : JCPRC5 The scope of fracture zone measure and high drill gas
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 214, 6(1):45-457 Research Article ISSN : 975-7384 CODEN(USA) : JCPRC5 The scope of fracture zone measure and high drill gas
Electronic Supplementary Information for
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information for Al-doping to Synchronously Improve
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information for Al-doping to Synchronously Improve
A Flexible, Lightweight, and Wearable Triboelectric Nanogenerator for Energy Harvesting and Self- Powered Sensing
 FULL PAPER Triboelectric Nanogenerators A Flexible, Lightweight, and Wearable Triboelectric Nanogenerator for Energy Harvesting and Self- Powered Sensing Fan Wu, Congju Li,* Yingying Yin, Ran Cao, Hui
FULL PAPER Triboelectric Nanogenerators A Flexible, Lightweight, and Wearable Triboelectric Nanogenerator for Energy Harvesting and Self- Powered Sensing Fan Wu, Congju Li,* Yingying Yin, Ran Cao, Hui
u = Open Access Reliability Analysis and Optimization of the Ship Ballast Water System Tang Ming 1, Zhu Fa-xin 2,* and Li Yu-le 2 " ) x # m," > 0
 Send Orders for Reprints to reprints@benthamscience.ae 100 The Open Automation and Control Systems Journal, 015, 7, 100-105 Open Access Reliability Analysis and Optimization of the Ship Ballast Water System
Send Orders for Reprints to reprints@benthamscience.ae 100 The Open Automation and Control Systems Journal, 015, 7, 100-105 Open Access Reliability Analysis and Optimization of the Ship Ballast Water System
Hydrophilic Sponges for Leaf-Inspired Continuous Pumping of Liquids
 Communication Elastic Sponges Hydrophilic Sponges for Leaf-Inspired Continuous Pumping of Liquids Tingjiao Zhou, Jinbin Yang, Deyong Zhu, Jieyao Zheng, Stephan Handschuh-Wang, Xiaohu Zhou, Junmin Zhang,
Communication Elastic Sponges Hydrophilic Sponges for Leaf-Inspired Continuous Pumping of Liquids Tingjiao Zhou, Jinbin Yang, Deyong Zhu, Jieyao Zheng, Stephan Handschuh-Wang, Xiaohu Zhou, Junmin Zhang,
HYDROGEN FAST FILLING TO A TYPE IV TANK DEVELOPED FOR MOTORCYCLES
 HYDROGEN FAST FILLING TO A TYPE IV TANK DEVELOPED FOR MOTORCYCLES Yamada, E. 1, Hiraki, W. 2, and Muramatsu, H. 3 1 FC-EV Research Division, Japan Automobile Research Institute, 1328-23 Takaheta, Osaka,
HYDROGEN FAST FILLING TO A TYPE IV TANK DEVELOPED FOR MOTORCYCLES Yamada, E. 1, Hiraki, W. 2, and Muramatsu, H. 3 1 FC-EV Research Division, Japan Automobile Research Institute, 1328-23 Takaheta, Osaka,
Supporting information. A metal-organic framework based multifunctional catalytic platform for organic transformation and environmental remediation
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 Supporting information A metal-organic framework based multifunctional catalytic platform
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 Supporting information A metal-organic framework based multifunctional catalytic platform
Experimental Research on Oxygen-enriched Air Supply System for Gasoline Engine
 Sensors & Transducers 013 by IFSA http://www.sensorsportal.com Experimental Research on Oxygen-enriched Air Supply System for Gasoline Engine 1, Yahong Li, 3 Lixi Zhang, 1 Zelu Sun 1 School of Chemistry
Sensors & Transducers 013 by IFSA http://www.sensorsportal.com Experimental Research on Oxygen-enriched Air Supply System for Gasoline Engine 1, Yahong Li, 3 Lixi Zhang, 1 Zelu Sun 1 School of Chemistry
