272 AWMEM7OAS. 224%-77%ale.24. June 11, 1963 M. L. KASBOHM ETAL 3,093,697 PROCESS FOR PRODUCING ACETYLENE HARTLEY C.
|
|
|
- Randolf Price
- 5 years ago
- Views:
Transcription
1 June 11, 1963 M. L. KASBOHM ETAL 3,093,697 PROCESS FOR PRODUCING ACETYLENE Filed May 27, Sheets-Sheet l AWMEM7OAS MARTIN L, KASBOHM HARRY J. PORTZER HARTLEY C. DELLINGER PAUL D, FRANSON IRWING A. COHEN 224%-77%ale.24 a 77OAPWA
2 June 11, 1963 M. L. KASBOHM ETAL 3,093,697 PROCESS FOR PRODUCING ACETYLENE Filed May 27, l960 5 Sheets-Sheet 2 2-roarrard t %22%29a EE 223 A1 /WMEW7OAPS MARTIN L. KASBOHM HARRY J. PORTZER HART LEY C. DELLINGER PAUL. D. FRANSON RVNG. A. COHEN a 77OAPWA
3 June 11, 1963 M. L. KASBOHM ETAL 3,093,697 PROCESS FOR PRODUCING ACETYLENE Filed May 27, Sheets-Sheet 3 st CONVERSION OF METHANE TO ACETYLENE FOR VARIOUS HYDROGEN DILUTION RATIOS O MOLAR RATIO (H2/CH4 /W/EW7OARS MARTN L. KASBOHM HARRY J. PORTZER 2A HARTLEY C. DELLINGER PAUL. D. FRANSON 24. IRVNG A. COHEN G^ Z26, 27.%deeme--
4 June 11, 1963 M. L. KASBOHM ETAL 3,093,697 PROCESS FOR PRODUCING ACETYLENE Filed May 27, Sheets-Sheet 4 N E a. Ed a As as a usu wo : m 3. a is is a 2 to O p (es C M S V i //W/EW7OAPS MARTEN L. KASBOHM HARRY J. PORTZR HARTEY C. DELLENGER PAUL D. FRANSON RVNG A. COHN A1 Z24/7,%22ze2. a 77OAPWA
5 June 11, 1963 Filed May 27, 1960 quâa se9 S r M. L. KASBOHM ETAL PROCESS FOR PRODUCING ACETYLENE S / 3,093,697 5 Sheets-Sheet 5 quæa se //WWEW7OAPS MARTIN L. KASBOHM HARRY J. PORTZER
6 United States Patent Office Aragvwww.swituarvaeuiwaraitskaskwakaradkshawawatrasawa crowaves 3,093,697 PROCESS FOR PRODUCING ACETYLENE Martin L. Kasbohm and Harry J. Portzer, Buffalo, Hartley C. Dellinger, Tonawanda, and Irving A. Cohen, Buf falo, N.Y., and Paul O. Franson, St. Albans, W. Va., as signors to Union Carbide Corporation, a corporation of New York Filed May 27, 1950, Ser. No. 32,251 This invention 19 relates Claims. to (CI. an improved ) process for the production and recovery of acetylene and, more partic ularly, to an improved process for producing acetylene by the pyrolysis of a suitable hydrocarbon stock and Subsequent separation and recovering the acetylene so produced by means of a relatively low pressure-low tem perature system. Regenerative hydrocarbon cracking processes for pro duction of acetylene, wherein a refractory material is heated by products of a combustion reaction and, then, a suitable hydrocarbon stock in gaseous or vaporous form, is exposed to the hot refractory, have been known for many years. Thus far none of these prior art processes has achieved widespread commercial use, the main dis advantage being uneconomically low conversions of hy drocarbons to acetylene. Similarly known have been acetylene recovery systems operating at relatively high pressures, such as up to 4 to 13 atmospheres or higher, in order to raise the partial pressure of the acetylene in the product gas stream and thus attempt to render the recovery system more efficient. This has required a considerable investment in compres sors. Even when low separation temperatures in the order of -50 C. (-58. F.) have been used, high pressures have still been employed to maintain higher acetylene partial pressures. High pressures were partic uarly thought to be necessary when the acetylene content of the product gas stream was less than about 10 volume percent. It is, accordingly, an object of the present invention to produce a valuable acetylene-containing off-gas, the term "off-gas' defining that gas which results from and is formed by pyrolysis of a gas (hereafter called "in gas') being or containing a suitable hydrocarbon stock, and recovering the acetylene thus produced by an efficient low pressure-low temperature system. It is another object of the invention to provide an improved process for producing an off-gas containing acetylene from a suitable hydrocarbon stock in-gas, in which a relatively high conversion to acetylene is realized, and to economically recover acetylene from the off-gas stream wherein the volume percent of acetylene is rela tively low. Still another object is to provide an improved integrated process for producing acetylene-containing gases from methane and the like wherein the overall conversion of carbon in methane to carbon in acetylene is increased, and wherein plant investment costs in the recovery sys tem are relatively small because of the relatively low pressure and other features of the recovery system. These and other objects and advantages of this inven tion will be apparent from the following description and accompanying drawings in which: FIG. 1 is a schematic view of a two-section regenera tive furnace suitable for performing the pyrolysis of hy drocarbon stock; FIG. 2 is a schematic view of a three-section regenera tive furnace also suitable for performing the pyrolysis of hydrocarbon stock; FIG. 3 is a schematic view of a pair of the three section regenerative furnaces of FIG. 2 connected to operate in alternate sequence, so that while one unit is on a reaction stroke, the other unit is on a regenerative stroke; O ,093,697 faterated June 1, FIG. 4 is a graph showing the conversion of methane to acetylene for various hydrogen dilution ratios obtain able by the present process; FiG. 5 is a diagrammatic representation or flow dia gram of one embodiment of the acetylene recovery sys temportion of this invention; FIG. 6 is a diagrammatic representation of a second emodiment of the acetylene recovery system of this invention. Increased conversion of suitable hydrocarbon stocks 70 to acetylene is aided when the acetylene produced has a low partial pressure. it was also known to obtain this low partial pressure by diluting the hydrocarbon stock with materials such as steam and hydrogen. Steam appears to be the preferred diluent in prior regenerative cracking processes, and the prior art has indicated that the presence of hydrogen is harmful since it appeared to inhibit the cracking reaction. In striking contrast, the present process requires hydrogen diluent, and em ploys hydrogen dilution limits which unexpectedly permit the obtainance of relatively high acetylene conversion ratios making this particular pyrolysis system commer cially attractive. The use of hydrogen diluent in the preferred dilution ration herein described results in a much lower percentage of carbon oxides in the off-gas. This in turn simplifies the subsequent acetylene separa tion and recovery step by using a relatively low pressure low temperature system rather than a high pressure one. According to the present invention, a process of pro ducing acetylene from an in-gas containing a suitable hydrocarbon stock is provided in which in-gas containing hydrogen and the hydrocarbon stock in a molar ratio of between about 1 to 8 moles of hydrogen for each carbon atom of the hydrocarbon molecule is passed through a heat transfer zone which has been heated to a Sutiable reaction tempearture. The in-gas is reacted in Such Zone so as to convert at least a portion thereof to acetylene, and the resulting acetylene-containing off-gas is dis charged from the heated reaction zone. The hot off-gas is then progressively cooled to a relatively low tempera ture to remove condensable impurities therefrom. The reaction contact time, which is the period during which the hydrocarbon is at reaction temperature, is preferably between about 0.01 to 0.05 second for maximum yields of acetylene when the optimum reaction temperature for the particular feed stock is used. Conversion or reaction times shorter than this range result in incomplete crack ing while reaction times longer than such range result in the decomposition of acetylene. Some higher acetylene impurities are removed in a sep arate step, while the purified acetylene gas stream is con tacted with a solvent which selectively absorbs acetylene and thereby separates the acetylene from less soluble im purities contained therein. Acetylene is then recovered from the solvent. The cooling may be conveniently carried out by passing the acetylene gas stream in counter current heat exchange through one or more self-cleaning heat exchange zones. Such heat exchange zones may be provided, for example, by reversing heat exchangers or regenerative heat exchangers. Pebble bed regenerative heat exchangers are preferred. Preferentially, the process of this invention employs the steps of pyrolyzing in a two-stroke' cycle the hydrocar bon stock by employing a ratio of hydrogen moles for each carbon atom of the hydrocarbon molecule of between 2 and 4 when at least most of the hydrocarbon in the in-gas contains less than about 7 carbon atoms, and of between about 3 and 6 when at least most of the hydrocarbon in the in-gas contains at least about 8 carbon atoms; cooling the off-gas streams, containing acetylene and other ma terials, to room temperature, preferably in a creeping pebble bed regenerator, to remove carbon and tars; pass
7 3,098, ing the cooled gases through a blower to increase the pres sure to slightly above atmospheric; cooling the gases to a A packed pebble bed can also be used if desired with the relatively low temperature, preferably in another creeping voids comprising the aforementioned passages. The process of the present invention is initiated by in pebble bed regenerator, to remove condensable materials; passing the cold gases through an adsorption trap to re troducing an oxidant gas, such as air, in conduit 15 and moval final amounts of condensable materials and some passing such gas through control valve 16 therein to sec higher acetylenes; passing the cleaned, acetylene-contain ond regenerative mass passages 14 for flow therethrough ing gases through a cold solvent adsorption system to dis to a combustion chamber 19 separating the two regenera solve acetylene; passing the solution through a stripping tive masses 11 and 12. During flow through passages 14, the air stream is preheated by abstracting heat stored in column to remove materials less soluble than acetylene; 10 second mass 12 during countercurrent flow of the hot off then separating the acetylene from the solvent. It should be understood that various operating cycles may be employed in the pyrolysis of hydrocarbon stock. The following description pertains primarily to a "two stroke' cycle which is a preferred embodiment of this invention. The in-gas is directed through passages of a heated first regenerative mass as a reaction stroke in which at least part of such gas is converted to acetylene. The acetylene-containing reacted gas is discharged there from as a valuable off-gas and quenched by flow through passages of a second relatively cool regenerative mass to cool such gas to a temperature at which relatively little acetylene decomposition occurs. The second mass is simultaneously reheated by such cooling of the off-gas. The first and second regenerative masses are then conven iently returned to proper operating conditions by first terminating the flow of in-gas. A stream of oxidant gas is then introduced into the outlet of the second regenera tive mass in a direction countercurrent to the previous flow of off-gas. As the oxidant flows through the passages of the second regenerative mass, it is heated and the mass is simultaneously cooled. A combustible fuel is provided and burned with at least part of the preheated oxidant gas to form a hot combustion gas mixture, the relative quanti ties of combustible fuel and oxidant gas being such that an excess of unreacted oxidant gas remains after the combus tion. The hot combustion gas mixture and unreacted oxidant gas are then directed through passages of the cooled first regenerative mass so as to reheat such mass for subsequent reaction therein of countercurrently flowing in-gas. The first mass is further regenerated by burning off carbon deposited in the passages during the previous reaction stroke, the burnoff being effected by the unre acted oxidant gas. To obtain continuous acetylene production, further preferred embodiment of the invention utilizes a pair of units. The first unit contains the first and second regener ative masses while the second unit contains third and fourth regenerative masses. The fluid flows between the first and second regenerative masses, and the third and fourth regenerative masses are periodically reversed so that the third and fourth masses are placed on reaction stroke with the in-gas flowing through the passages of the fourth mass for reaction therein and the resulting off-gas flowing through the third mass passages for quenching therein. During this period, the first and second masses are on regenerative stroke with the oxidant gas flowing through the second regenerative mass passages for pre heating therein, and the hot combustion gas mixture and unreacted oxidant gas flowing through the first regenera tive mass passages for heating and regeneration of such a SS. Referring now specifically to FIG. 1, the regenerative furnace indicated generally at 10 comprises two regener ative masses 11 and 12 constructed and arranged in a Series relationship. The first and second masses 11 and 12 each contain a set of passages 13 and 14, respectively, extending therethrough from end to end. The construc tion of such furnaces will be well understood by those skilled in the art, and any refractory materials that can withstand the reaction conditions may be used. Although We prefer to use alumina molded with the passages in place, other materials and arrangements such as stacked tiles of silicon carbide and the like would also be suitable gas from the previous in-gas stroke, as will be described later in more detail. A stream of combustible fuel such as hydrogen, methane, ethane, or a mixture thereof, is provided in conduit 17 and directed through control valve 18 into combustion chamber 19 for mixing with the pre heated air stream. The gas mixture is spontaneously ignited in chamber 19 due to the high temperature of the oxidant. However, a convenient ignition source such as pilot flame 36 may be used if necessary or desirable. The hot combustion gas mixture formed in chamber 19 is passed therefrom through first regenerative mass passages 13 so as to reheat mass 11 to a suitable reaction temperature for Subsequent processing of the in-gas to be cracked. The cooled combustion gas mixture is dis charged from the end of passages 13 into conduit and control valve 21 therein for venting to the atmosphere or further processing as desired. The preferred in-gas reaction temperature depends to a certain extent on the type of hydrocarbon stock available. For example, a reaction temperature of about 2700 F. to 2800 F. is preferred for methane, whereas lower temperatures are most suitable for higher molecular weight hydrocarbons. An additional preferred feature of the aforedescribed regenerative stroke is removal of the carbon deposited in the first regenerative mass passages 13 during crack ing of the hydrocarbon stock containing in-gas. This is accomplished by providing the oxidant gas in a quan tity which is in excess of that required to burn the com bustible fuel in chamber 19, so that the hot combustion gas entering first regenerative mass passages 13 contains a portion of unreacted oxidant. The latter oxidizes and burns out the carbon from such passages, and the carbon oxides-containing gas is discharged therefrom as part of the cooled combustion gas mixture. The flow of oxidant gas must be such that proper heat balances are obtained in both regenerative masses 11 and 12 of the furnace to provide efficient reaction and quenching temperatures. A suitable hydrocarbon feed stock is then introduced through conduit 22 and control valve 23, and is mixed with a hydrogen-containing gas stream which is intro duced through branch conduit 24 and control valve 25 therein. The resulting in-gas mixture is directed through conduit 26 and control valve 26a therein into passages 13 of the preheated first regenerative mass 11 for crack ing therein. As previously discussed, the flow ratio be tween the hydrocarbon feed and the hydrogen containing gas is a critical part of the invention, and the preferred ratio varies depending on the hydrocarbon feed supplied. It therefore is desirable to provide a method for monitor ing and adjusting the ratio at will. To this end, analyzer and proportional flow controller 27 is supplied to moni tor the hydrogen-hydrocarbon ratio in conduit 26, tap conduit 28 providing the necessary communicating means. Signals are then sent from analyzer-controller 27 through conduit 29 to valve 25 for automatic control thereof. During the start-up and initial operating period, it is necessary to supply at least most of the hydrogen re quired to form the in-gas as fresh hydrogen gas through conduit 24 and automatic control valve 25 as previously described. However, as soon as normal operating con ditions are attained, hydrogen will be available from the acetylene separation system in which product acetylene is separated from the off-gas. When this condition is realized, hydrogen gas is preferably recycled from the
8 5 acetylene separation system through branch conduit 30 and control valve 31 therein to conduit 26 so as to pro vide at least part of the makeup hydrogen required for the in-gas. The quantity of recycle hydrogen is regulated to provide the desired hydrogen-hydrocarbon feed ratio, as previously described, and such regulation is prefer ably effected by means of analyzer and proportional flow controller 27 which, for example, may control the set ting of automatic valve 31 by intercommunicating con duit 32. It is to be understood that if hydrogen-contain ing gas is to be simultaneously supplied to conduit 26 through conduits 24 and 30, it may be desirable to pro vide a second analyzer and proportional flow controller similar to controller 27. Such controllers would be con Structed and operated in a manner well known to those skilled in the art. Returning now to the in-gas in conduit 26, such gas is passed into first regenerative passages 13 which have previously been purged of deposited carbon and heated to the desired reaction temperature. The in-gas is par tially converted to acetylene in such passages. One ad vantage of the present process is that the reaction step is most efficiently performed at pressures of less than 2 atmospheres, and preferably at a pressure of 1 to 2 at mospheres. In the prior art regenerative cracking proc esses it was necessary to operate at subatmospheric pres Sures to improve conversions, but the acetylene conver sion efficiency of the present invention is sufficiently high so that reduced pressure operation is usually not necessary, thus effecting a substantial saving in equip ment costs. The only possible advantage of operating at a Subatmospheric pressure in the present process is to increase the concentration of acetylene in the off-gas and thus facilitate easier acetylene separation or recovery. This advantage must be weighed economically against the additional cost of equipment to produce the reduced pressure. Since the acetylene will in turn decompose if main tained for a relatively long period at the reaction tem perature, the in-gas contact time in the hot passages 13 is preferably closely regulated between about 0.01 and 0.05 second when the optimum reaction temperature for the particular feed stock is used. This range has been found to provide an optimum balance between maximum hydro carbon Stock conversion and minimum acetylene decom position. Such control may be accomplished, for exam ple, by appropriate adjustment of the in-gas flow rate. During the acetylene conversion, elemental carbon is de posited in Such passages. The resulting acetylene-con taining reacted gas is discharged from the opposite end of partially cooled passages 13 into combustion chamber 19 as off-gas, and hence into the communicating end of relatively cool second regenerative mass passages 14 for rapid quenching therein to below 1000 F. In this man ner, acetylene decomposition is further minimized. The cooled off-gas is discharged from the partially rewarmed passages 14 into conduit 33 for flow through control valve 34 therein and is then passed to the acetylene sep aration and recovery system indicated generally as item 35. The recycle stream consists mainly of hydrogen but also contains unreacted hydrocarbon stock, especially when methane is used as the feed stock. The recycle stream is returned to furnace 10 through conduits 30 and 26, as previously described. When the hydrocarbon feed stock contains a substantial quantity of methane, the re cycling of unreacted methane to the in-gas stream in com bination with the preferred hydrogen moles to carbon atom ratio of between about 2 and 4 has enabled the present process to provide the highest known conversions of methane to acetylene. This is a decided commercial advantage since methane is a readily available raw ma terial. FIG. 2 illustrates a three-section regenerative furnace which constitutes the preferred means of performing the 8,093, pyrolysis of the acetylene-containing hydrocarbon, Three regenerative masses 111, 1.12a and 112b are provided with passages 113, 114a and 114b, respectively, and the combustible fuel stream in conduit 117 is passed through branch conduits 117a and 1.7b to combustion chambers 119a and 119b, respectively. Combustion chamber 119a Separates regenerative mass 11i from one end of mass 112a, and combustion chamber 119b separates the op posite end of regenerative mass 2a from mass 112b. During the regenerative stroke, oxidant gas is introduced through conduit 115 into warm passages 114b for pre heating, and is then mixed with fuel in combustion cham bers 119a and 119b for burning therein to form a hot combustion gas mixture. The hot combustion gas, which preferably contains a portion of unreacted oxidant, then flows through passages 14a and 113 so as to simultane ously reheat regenerative masses 111 and 112a to the de sired reaction temperature and remove the previously de posited carbon. During the reaction stroke, in-gas flows through conduit 126 into passages 113 and 114.a for par tial conversion to acetylene therein, the resulting off-gas passing through passages 114b for quenching. The cooled off-gas is discharged from the furnace 10 through iconduit 133 and control valve 34 therein for passage to the acetylene recovery system (not shown). One important advantage of the FIG. 2 three-section furnace as compared to the two-section furnace of FIG. 1, is that complete combustion of the oxidant and fuel on the regenerating portion of the cycle can take place over a greater passage length and thus avoid the forma tion of excessive hot spots in the reaction zone which re duce the overall efficiency of the process. The furnaces illustrated in FIGS. 1 and 2 operate on an intermittent basis; that is, first on reaction stroke and then on regenerative stroke. Consequently, such furnaces do not provide a continuous supply, of acetylene-contain ing off-gas, which may be desirable when the subsequent recovery step is also performed on alternate strokes basis. FIG. 3 illustrates a system providing a continuous supply of off-gas, in which two furnaces 210a and 210b are piped in parallel so that when one furnace is on the reaction stroke the other furnace is on the regenerative stroke. Each furnace as illustrated has three sections and operates in substantially the same manner as the furnace illustrated in FIG. 2. It is to be understood, however, that each furnace may be provided with any other desired num ber of sections, for example, two sections as shown in the FIG. 1 embodiment. Referring more specifically to FIG. 3, at the illustrated moment in the operating sequence, furnace 210a is proc essing in-gas and furnace 250b is being regenerated. The in-gas is introduced in conduit 226 and passed through reversing valve 223a to furnace 210a for consecutive flow through passages 213a, combustion chamber 219a, pas Sages 214a, combustion chamber 219b, and passages 314a. The resulting cooled acetylene-containing off-gas is dis charged from passages 354a through conduit 2 and re versing valve 234a therein to conduit 233 for passage to the acetylene recovery system (not shown). Simulta neously, an oxidant stream is introduced through conduit 215 for flow through reversing valve 256b to furnace 210b and through passages 314b where it is preheated. Combustion fuel gas is introduced through conduit 217 and reversing valve 258b to combustion chambers 3i. 9a and 319b, where it mixes with preheated oxidant and burns. The resulting hot combustion gases flow through passages 214b and 2.13b where they transfer their heat to the regenerative masses 212b and 2.11b, respectively. The resulting cooled combustion gas mixture is discharged from furnace 210b through reversing valve 221b and con duit 2 for release to the atmosphere or further treat ment as desired. At periodic intervals, for example, when the tempera ture in the cracking sections of the furnaces drops below
9 3,098,697 7 effective cracking level, the flows between furnaces 210a and 210b are reversed. Thus, the in-gas flows to fur nace 210b through conduit 226 and reversing valve 223b, and emerges from passages 34b through conduit 2 and reversing valve 234b therein. During this period, the oxidant gas flows through conduit 215 and reversing Valve 216a therein to furnace a. The cooled com bustion gas mixture formed therein is discharged from passages 213a through conduit 2 and reversing valve 22a therein. 0. FIG. 4 is a graph illustrating the results of a substan tial number of tests in which methane was partially con verted to acetylene by the pyrolytic cracking step of the present invention, using the apparatus shown by FIG. 1. Various hydrogen dilution ratios were used, and the 5 graph clearly shows the criticality of such ratios with respect to acetylene conversion and concentration in the off-gas. It is significant to note that the acetylene con version percentage passes through a peak betwen molar ratios of 2 and 4, which is the preferred ratio range for hydrocarbon stocks containing less than about seven car bon atoms, Referring more specifically to FIG. 5, the acetylene-con taining product off-gas is introduced from conduit 33 of FIG. 1 into the recovery apparatus through line 60. This gas stream, coming from the preceding hydrocarbon pyro lytic cracking process, is cooled to about room temperature by heat exchange with a process gas stream, such as air, in creeping pebble bed regenerative exchanger 61. The creeping pebble bed regenerators are operated in a pair with one exchanger 62 being cooled by exchange with process gas from line 63 while the other exchanger 61 is cooling the off-gas stream. These regenerators also Serve to clean carbon and tars from the off-gas stream. Regenerators 6 and 62 are called "hot' regenerators since they operate at room temperature and above. The construction and operation of the creeping pebble bed regenerators preferably employed in the process of this invention are described in more detail in copending application Serial No. 776,549, filed November 26, 1958, now U.S. Patent No. 3,092,836. The hot regenerator is preferably operated in such fashion that the temperature of the pebbles at the hot end (gas inlet) is high enough to burn off or vaporize carbonaceous materials which have been deposited on the pebbles. There is also a slow moving of the pebbles through the regenerator from the cold end to the warm end. In this fashion material deposited on the pebbles in the cooler region of the regenerator is slowly moved toward the warm end where it will be either vaporized and removed by the purge gas stream or burned off to provide self-cleaning operation. A separate burner could alternatively be used to burn off pebble deposits. The cold end (gas outlet) is kept as cool as possible to lower power requirements of a subsequent blower 65 and to condense as much as possible of the contaminants from the gas stream. This desired cold end condition is ob tained by using sufficient process gas or air on the re generative cycle so that the outlet temperature of the off-gas is approximately the same as the inlet tempera ture of the process gas or air. The warm purge gas from the hot regenerator in line 64 may then be used in any desired way or discarded. If air is used for heat exchange, the resulting warm process air could be used in the combustion cycle of the preceding regenerative cracking furnace to maintain proper heat balance in the furnace or as oxidant for a partial oxidation burner. While the slow movement of pebbles in the hot regen erator from cold to warm end is preferable in most cases, situations where the quantity of condensed materials is sufficiently large to disrupt pebble flow may best be handled by flow of pebbles in the opposite direction from warm to cold end. In this situation the pebbles would not be internally self-cleaned, but the outlet gas will still be clean due to condensation of most contaminants on the cold pebbles. The pebbles may then be cleaned ex ternally to the regenerator by treatment in a furnace to vaporize or burn off carbonaceous deposits. While the preferred process modification is to use the creeping bed regenerators to cool the acetylene-contain ing off-gas stream to about room temperature, it should be understood that other means, such as water towers or other types of heat exchangers, could also be used to achieve the same result and still be within the limits of our invention. The cooled off-gas stream from the pebble bed regen erator at about room temperature and atmospheric pres sure is then compressed to a pressure less than about 3 atmospheres absolute in blower 65 and passed prefer ably into a set of creeping pebble-bed cold regenerators 66 and 67 operated cyclically in a pair, where the off-gas stream is cooled to about -1 C. to -160 C. These regenerators are called "cold regenerators because they operate below room temperature. These cold regenera tors are similar in construction and operation to the hot regenerators described hereinabove. The use of Such re generators with their low cost heat exchange surface per mits a high thermal efficiency in the recovery of refrigera tion and provides a self-cleaning heat exchange System for continuous operation. The minimum operating tem perature is determined by the point at which acetylene begins to condense. This point would be determined by the pressure used in this stage. The optimum cold end temperature is determined by an economic balance be tween equipment investment and operating costs. In these cold regenerators heat exchange is obtained be tween the off-gas stream and vent gas from the Subsequent separation system. This cooling of the gas also removes heat of compression introduced by blower 65. These regenerators can further clean the gas stream by freezing out additional condensable material, such as diacetylene, if this is desired. In one embodiment of the present process, the tem perature of the off-gases at the outlet of the cold regen erator is kept in the range of -150 F. to -160 F., at 10 pounds per square inch gauge pressure to freeze out substantially all of the diacetylene. Since the tempera ture of the cold gas entering the heat exchanger is fixed by the operating conditions of the separation cycle, the control of the outlet temperature of the gases from the cold regenerator is accomplished by varying the rate of pebble flow from the cool end to the warm end of the regenerator. With respect to the total heat exchange, the flow of pebbles adds to the flow of cold vent gas to give a control over the overall flow of cold heat exchange material. When a subsequent step, such as an adsorbent bed, is used to remove substantially all of the higher acetylene, such as diacetylene, the cold regenerators can be operated at -1 F. to -130 F. at the outlet end. While the preferred process modification is to use the creeping bed regenerators to cool the gas stream from room temperature to a relatively cold temperature, it should be understood that other types of heat exchangers could be used and still be within the scope of this inven tion. If reversing heat exchangers were used, pressures up to about 3 atmospheres absolute might be necessary in order to obtain self-cleaning' action. In this case the minimum operation temperature would be about -150 F. in order to prevent condensation of acetylene. FIG. 5 shows passage of acetylene-containing off-gas (intake stroke) through regenerator 61, blower 65 and regenerator 66 while regenerators 62 and 67 are being purged with process gas or air and recycled vent gas from the acetlyene adsorber 70. It is understood that the in take and purge strokes are periodically reversed so that the acetylene-containing gas passes through regenerator 62, blower 65 and regenerator 67, while regenerators 61 and 66 are being purged. Means for providing this alter nation of intake and purge strokes is well known to those skilled in the art.
10 3,098,697 Ror final cleanup, the off-gas stream may be passed through a cold adsorbent trap 68 maintained at a tempera ture below about 32 F. to remove the last traces of di acetylene and some other higher acetylenes, such as meth yl acetylene and vinyl acetylene. Some olefins such as 5 allene are also removed. The trap is preferably main tained at a temperature below - F. and specifically at a temperature near that of the cold regenerator outlet temperature. Low temperatures are used in order to in crease adsorptive capacity and also to prevent polymeriza O tion of the adsorbed impurities. Silica gel is a convenient adsorbent, but other known adsorbents such as activated alumina or activated charcoal can be used as well. A companion adsorbent trap 69 may also be used for con tinuous operation. One trap is regenerated by means of 5 a cold vent gas stream, while the other is adsorbing. In the preferred form of this invention the adsorbent trap is designed to remove all the higher acetylenes, especially diacetylene. This bed can be reused since the adsorbed higher acetylenes do not polymerize and foul the adsorb ent under the low temperature adsorption conditions used. The cleaned gas from the cold adsorbent trap 68 then passes through linee 96 to the main absorber column 70 where cold solvent at a temperature below about -1 F. and a pressure of less than about 2 atmospheres abso 25 lute enters through line 76 and removes the acetylene and other soluble constituents. Typical operation is at about -139 F. and 6 p.s.i.g. Efficient liquid-gas contact in the absorber column is obtained by means of packing or trays. The acetylene is absorbed in the main section of 30 the column 70 and goes out with the main solvent stream 71. The stripped vent gas stream from the top of the column 70 is recycled through line 72 to regenerator 67 to pick up material adsorbed and condensed on the peb bles during the intake stroke and to recover the refrigera 35 tion in the stream. The vent gas in line 72 recycled to re generator 67 is then divided inside the regenerator in order to maintain proper internal heat balance. The main portion of the gas passes through regenerator 67 and leaves through line 86. The remaining portion leaves re generator 67 through line 87, passes through blower 38 and heat exchanger 92, and forms the cooling purge gas for adsorbent bed 69. The resulting purge gas returns to the main vent gas stream 86 through line 89. Proper heat balance conditions are maintained by line 91 and 43 heat exchanger 92. An optional heat exchanger 73 may need to be interposed in line 72 to help balance the heat capacity of the gas streams in regenerators 66 and 67. This recycled gas in line 86 may then be added to the hy drocarbon stock prior to the cracking process if so de 50 sired. Heat of absorption is removed from the main absorber column 70 by means of a refrigeration coil 74. Acetone is the preferred solvent for this absorption step since it has high selectivity for acetylene at these low tempera 55 tures, although other solvents such as ammonia, dimethyl formamide, N-methyl-2-pyroidone and 3-butyrolactone may be used. The main solvent stream 71 containing the acetylene in solution exchanges heat in heat exchanger 75 with the 60 stripped solvent stream returning from the solvent storage container 77 through pump 98, line 99 and line 76. The acetylene-rich solvent stream 71 is then preferably fed to a combination vent column and acetylene stripper 78. For purpose of clarity, this stripper 78 may be divided 65 into three sections. In the upper section, between the feed point of line 71 and the top of the column, a small stream of solvent 94 is employed to scrub acetylene from the vent gases rising through the stripper, thus providing a vent gas stream rich in components less soluble than 70 acetylene, such as carbon dioxide, hydrogen, methane and carbon monoxide. This vent gas stream is taken off overhead through line 79. In the middle section of the stripper 78, between the feed point of line 71 and the acetylene discharge line 80, the descending solvent stream 75 O is stripped of components less soluble than acetylene by a stream of gaseous acetylene (not shown) formed in the bottom section of the stripper. In the bottom section of the stripper 78, below the acetylene discharge line 80, the descending solvent stream is stripped of acetylene by sol vent vapor (not shown) generated by boiling the stripped Solvent at the bottom of the column with a steam-heated heat exchanger (also not shown). The overall opera tion in column 78 is temperature-driven. The vent gas is obtained by a combination of temperature increase and purging by acetylene gas which, in turn, is obtained by purging of solvent vapor. The solvent vapor is also ob tained by a temperature increase. The small stream of solvent 94 introduced in the upper Section of the column is obtained from solvent storage 77 through pump 98 and line 99 and constitutes a portion of the Stripped solvent stream, the rest of it being diverted through line 76. The vent gas taken off overhead through line 79 may be recycled to the main gas stream to recover its acetylene content or it may be vented if desired, Alternatively, two separate columns, a vent column and a stripper column, may be employed. The combined vent column and acetylene stripper, described herein above, is, however, preferred. An acetylene stream having a purity of about 99.5 percent is taken off as a sidestream from the vent column and acetylene stripper 78 through line 8G and forms the desired product stream. The stripped solvent from the bottom of this column is then returned to the solvent Storage 77 through line 95 for reuse. It is understood that various engineering variations could be used to strip the acetylene from the main solvent stream leaving absorber 73. The above-detailed process is the one presently pre ferred. - In another embodiment of the invention, shown in FiG. 6, the adsorbent bed 168 may be designed to remove only diacetylene and not other higher acetylenes such as methyl acetylene. The methyl acetylene, which is gen erally more soluble in appropriate solvents than is acety lene, is then selectively absorbed in the bottom section 181 of the absorber 170 and is taken off in a bottom stream through line 82. This stream 82 containing methyl acetylene, solvent and a small amount of acety lene is purged of substantially all of its acetylene content in an acetylene recovery column 183. A portion of the vent gas from absorber 275 in line 190 is used to purge this column, and then this gas returns with its acetylene con tent to absorber 70 through line 84. The solvent and methyl acetylene from the bottom of column 283 are then passed through line 28 to a stripper column 185 for sep aration. The resulting stripped solvent is returned to sol vent storage 77 through line 2. This relatively low pressure, low temperature separa tion system reduces the large investment required in the usual separation systems for recovery of low concentra tions of acetylene from cracked gases. It eliminates the large number of compressors required by prior art re covery methods which operated at higher pressures since it only needs a relatively low pressure compressor to force the cracked gases through the separation cycle into the preferred form of the invention. The use of low tem perature solvent absorption at a relatively low pressure requires smaller amounts of solvent due to increased sol ubility of acetylene at the low temperature. The use of creeping bed regenerators provides a novel and conven ient method for cleaning the cracked gas stream while also permitting low pressure, low temperature processing without excessive heat exchange costs. This improved process is especially useful for removing acetylene present in gas streams in low concentrations. The adsorption bed for removal of higher acetylenes is novel and preferred in that it eliminates the necessity for separate solvent towers to recover these impurities. Such towers are large and expensive since they must han dle the entire acetylene-containing gas stream as well as
11 the solvent. Low temperature adsorption is unique in that the beds can be resued since the adsorbed higher acetylenes do not polymerize at the operating conditions. The preferential removal of higher acetylenes by adsorp tion was also unexpected and has proved to be a useful feature of this invention. What is claimed is: 1. A process of producing acetylene from an in-gas the steps of passing an in-gas containing hydrogen and said hydrocarbon stock in a molar ratio of between about 1 to 8 moles of hydrogen for each carbon atom of the hydrocarbon molecule through a heat transfer zone which has been heated to a suitable Teaction temperature to form a heated reaction Zone; reacting Said in-gas in said heated reaction zone so as to convert at least part of said in-gas to acetylene; discharging the acetylene-con taining off-gas from said heated reaction Zone; cooling said off-gas to a temperature below about -1 F. at a pressure below about 3 atmospheres absolute to remove condensable materials; contacting the cold gas stream with an adsorbent at a temperature below 32 F. to remove final amounts of condensable materials and at least Some amounts of higher acetylenes and discharging the resultant gas stream from the adsorbent at a temperature below 32 F. So as to substantially prevent polymerization of the hydrocarbon impurities adsorbed therein; contacting the resultant gas stream at a temperature below about -1 F. and a pressure below about 2 atmospheres ab solute with a solvent wherein said acetylene is selectively dissolved to separate said acetylene from said non-con densable materials; and recovering said acetylene from said solvent. 2. A process for producing acetylene from an in-gas the steps of passing an in-gas containing hydrogen and said hydrocarbon stock in a molar ratio of between about 1 to 8 moles of hydrogen for each carbon atom of the hydrocarbon molecule through a heat transfer Zone which has been heated to a suitable reaction temperature to form a heated reaction zone; reacting said in-gas in said heated reaction zone for a period of between about 0.01 and 0.05 second and at a pressure of between about 1 and 2 atmospheres absolute so as to convert at least part of such in-gas to acetylene; discharging the acetylene containing off-gas from said heated reaction Zone; pro gressively cooling said off-gas stream to a temperature of about -1 F. to -160 F. under pressures of less than about 3 atmospheres absolute to remove a substantial por tion of the condensable materials contained therein; con tacting the cold gas stream with an adsorbent at a tem perature below 32 F. to remove final amounts of con densable materials and at least some amounts of higher acetylenes and discharging the resultant gas stream from the adsorbent at a temperature below 32 F. So as to substantially prevent polymerization of the hydrocarbon impurities adsorbed therein; contacting the resultant par tially purified gas stream at a temperature below about -1 F. and a pressure of less than about 2 atmospheres absolute with a solvent wherein the acetylene is selectively dissolved to separate said acetylene from Some of the less soluble impurities and from most of the substantially in soluble non-condensable materials in the gas stream; vent ing the remaining reductively less soluble impurities from the solvent; and recovering the acetylene from said Sol went. 3. A process of producing acetylene from an in-gas the steps of passing an in-gas containing hydrogen and said hydrocarbon stock in a molar ratio of between about 2 to 6 moles of hydrogen for each carbon atom of the hydrocarbon molecule through a heat transfer Zone which has been heated to a suitable reaction temperature to form a heated reaction zone; reacting said in-gas in Said heated reaction zone for a period of between about 0.01 and 3,093, second and at a pressure of between about 1 and 2 atmospheres absolute so as to convert at least part of such in-gas to acetylene; discharging the acetylene-containing off-gas from said heated reaction zone; progressively cool ing said off-gas stream to a temperature of about -1 F. to -160 F. under pressures of less than about 3 at mospheres absolute to remove a substantial portion of the condensable materials contained therein; contacting the cold gas stream with an adsorbent at a temperature below F. to remove final amounts of condensable materials and at least some amounts of higher acetylenes and dis charging the resultant gas stream from the adsorbent at a temperature below 32 F. so as to substantially prevent polymerization of the hydrocarbon impurities adsorbed 5 therein; contacting the resultant partially purified gas stream at a temeprature below about -1 F. and a pressure of less than about 2 atmospheres absolute with a solvent wherein the acetylene is selectively dissolved to separate said acetylene from some of the less soluble im purities and from most of the substantially insoluble non condensable materials in the gas stream; venting the re maining reductively less solubie impurities from the Sol vent; and recovering the acetylene from said solvent. 4. A process of producing acetylene from an in-gas 25 containing a suitable hydrocarbon stock which com prises the steps of passing an in-gas containing hy drogen and said hydrocarbon stock in a molar ratio of between about 1 to 8 moles of hydrogen for each carbon atom of the hydrocarbon molecule through a heat transfer 30 zone which has been heated to a suitable reaction tem perature to form a heated reaction Zone; reacting said in-gas in Said heated reaction Zone for a period of be tween about 0.01 and 0.05 second and at a pressure of between about 1 and 2 atmospheres absolute so as to 35 convert at least part of such in-gas to acetylene; dis charging the acetylene-containing off-gas from said heat ed reaction zone; separating from said off-gas stream at least part of the remaining hydrogen-containing gas and recycling said hydrogen-containing gas to said heated re action zone so as to provide at least part of the hydro gen of said in-gas; progressively cooling said off-gas stream to a temperature of about -1 F. to -160 F. under pressures of less than about 3 atmospheres absolute to remove a substantial portion of the condensable mate rials contained therein; contacting the cold gas stream with an adsorbent at a temperature below 32 F. to re move final amounts of condensable materials and at least some amounts of higher acetylenes and discharging the 50 resultant gas stream from the adsorbent at a temperature below 32 F. so as to substantially prevent polymeriza tion of the hydrocarbon impurities adsorbed therein; con tacting the resultant partially purified gas stream at a temperature below about -1 F. and a pressure of less 55 than about 2 atmospheres absolute with a solvent where in the acetylene is selectively dissolved to separate said acetylene from some of the less soluble impurities and from most of the substantially insoluble non-condensable materials in the gas stream; venting the remaining reduc 60 tively less soluble impurities from the solvent; and re covering the acetylene from said solvent. 5. A process of producing acetylene from an in-gas the steps of passing an in-gas containing hydrogen and said hydrocarbon stock in a molar ratio of between about 2 to 6 moles of hydrogen for each carbon atom of the hydrocarbon molecule through a heat transfer zone which has been heated to a suitable reaction temperature to form a heated reaction Zone; reacting said in-gas in said heated reaction zone for a period of between about 0.01 and 0.05 second and at a pressure of between about 1 and 2 atmospheres absolute so as to convert at least part of such in-gas to acetylene; discharging the acetylene-con taining off-gas from said heated reaction zone; cooling said off-gas in a first creeping pebble bed regenerative 75
12 13 heat exchanger to about room temperature at a pressure below about 3 atmospheres absolute in heat exchange with material selected from the class consisting of proc ess gas and process air to remove a portion of the con densable materials contained therein; further cooling said off-gas in a second creeping pebble-bed regenerative heat exchanger to about -1 F. to -130 F. at a pressure below about 3 atmospheres absolute in heat exchange with a cold vent gas stream to remove additional amounts of condensable materials; treating the acetylene-con taining gas stream with an adsorbent at a temperature below - F. and substantially the same as the outlet temperature of said second creeping pebble bed regenera tor to remove final amounts of condensable materials and substantially all of the higher acetylenes and discharging the resultant gas stream from the adsorbent at a tempera ture below 32 F. so as to substantially prevent polym erization of the hydrocarbon impurities adsorbed there in; contacting the resultant purified gas stream with a sol vent at a temperature below about -1 F. and a pres Sure of less than about 2 atmospheres absolute, said sol vent selectively absorbing acetylene and thereby sepa rating the acetylene from some of the less soluble impuri ties and from most of the non-condensables; recycling said less soluble and non-condensable materials as said cold vent gas stream for heat exchange in said second creeping pebble bed regenerative heat exchanger; venting the re maining less soluble impurities from the solvent; and re covering the acetylene from the solvent. 6. The process in accordance with claim 5, wherein the absorbent is silica gel and the solvent is acetone. 7. A process of producing acetylene from an in-gas the steps of directing a hot combustion gas mixture through passages of a first regenerative mass to heat such mass to a suitable reaction temperature; directing through said passages of the heated first regenerative mass an in-gas containing hydrogen and said hydrocarbon stock in a molar ratio of between about 1 to 8 moles of hydro gen for each carbon atom of the hydrocarbon molecule and reacting said in-gas for a period of between about 0.01 and 0.05 second and at a pressure of between about 1 and 2 atmospheres absolute in contact with such first heated regenerative mass so as to convert at least part of such in-gas to acetylene; discharging the acetylene-con taining reacted gas from the first regenerative mass pas sages as said valuable off-gas and directing Such hot gas through passages of a second relatively cool regen erative mass for quenching the off-gas to a lower tempera ture at which relatively little acetylene decomposition occurs; discharging the quenching off-gas from said pas Sages in the warmed second regenerative mass, and re covering the heat abstracted by said second regenerative mass from the hot off-gas by providing an oxidant gas and directing such oxidant gas through the second re generative mass passages; providing a combustible fuel and burning such fuel with the heated oxidant gas so as to form said hot combustion gas mixture; discharging the acetylene-containing off-gas from said heated reac tion zone; progressively cooling said off-gas stream to a temperature of about -1 F. to -160-F, under pres Sures of less than about 3 atmospheres absolute to re move a substantial portion of the condensable materials therefrom; contacting the cold gas stream with an ad Sorbent at a temperature below 32 F. to remove final amounts of condensable materials and at least some amounts of higher acetylenes and discharging the result ant gas stream from the adsorbent at a temperature be low 32 F. so as to substantially prevent polymerization of the hydrocarbon impurities adsorbed therein; contact ing the resultant partially purified gas stream at a tem perature below about -1 F. and a pressure of less than about 2 atmospheres absolute with a solvent which selectively dissolves acetylene to separate the acetylene 8,098,697 5 O from some of the relatively less soluble impurities and from most of the substantially insoluble non-condensable materials in the gas stream; venting the remaining re ductively less soluble impurities from the solvent; and recovering the acetylene from the solvent. 8. A process of producing acetylene from an in-gas the steps of directing a hot combustion gas mixture through passages of a first regenerative mass to heat such mass to a suitable reaction temperature; directing through said passages of the heated first regenerative mass an in gas containing hydrogen and said hydrocarbon stock in a molar ratio of between about 1 to 8 moles of hydrogen for each carbon atom of the hydrocarbon molecule and reacting said in-gas for a period of between about 0.01 and 0.05 second and at a pressure of between about 1. and 2 atmospheres absolute in contact with such first heated regenerative mass so as to convert at least part of such in-gas to acetylene; discharging the acetylene containing reacted gas from the first regenerative mass passages as said Valuable off-gas and directing such hot gas through passages of a second relatively cool regen erative mass for quenching the off-gas to a lower tem perature at which relatively little acetylene decomposi tion occurs; discharging the quenching off-gas from said passages in the warmed second regenerative mass, and recovering the heat abstracted by said second regenera tive mass from the hot off-gas by providing an oxidant gas and directing such oxidant gas through the second regenerative mass passages; providing a combustible fuel and burning such fuel with the heated oxidant gas so as to form said hot combustion gas mixture; discharging the acetylene-containing off-gas from said heated reac tion Zone; cooling said off-gas in a first creeping pebble bed regenerative heat exchanger to about room tempera ture in heat exchange with material selected from the class consisting of process gas and process air to remove a portion of the condensable materials contained there in; further cooling said off-gas in a second creeping peb bie bed regenerative heat exchanger to about -1 F. to -130 F. in heat exchange with a cold vent gas stream to remove additional amounts of condensable materials; treating the acetylene-containing gas stream with an ad Sorbent at a temperature below - F. and substan tially the same as the outlet temperature of said second creeping pebble bed regenerator to remove final amounts of condensable materials and substantially all of the higher acetylene and discharging the resultant gas stream from the adsorbent at a temperature below 32 F. so as to substantially prevent polymerization of the hydrocar bon impurities adsorbed therein; contacting the resultant purified gas stream with a solvent at a temperature below about -1' F. and a pressure of less than about 2 at mospheres absolute, said solvent selectively absorbing acetylene and thereby separating the acetylene from some of the less soluble impurities and from most of the non condensables; recycling said less soluble and non-con densable materials as said cold vent gas stream for heat exchange in said second creeping pebble bed regenerative heat exchanger; venting the remaining less soluble im purities from the solvent; and recovering the acetylene from the solvent. 9. A process of producing acetylene from an in-gas the steps of directing a hot combustion gas mixture through passages of a first regenerative mass to heat such mass to a Suitable reaction temperature; directing through Said passages of the heated first regenerative mass an in-gas containing hydrogen and said hydrocarbon stock in a molar ratio of between about 2 to 6 moles of hy drogen for each carbon atom of the hydrocarbon mole cule and reacting said in-gas for a period of between about 0.01 and 0.05 second and at a pressure of between about 1 and 2 atmospheres absolute in contact with such
13 15 first heated regenerative mass so as to convert at least part of Such in-gas to acetylene; discharging the acetylene containing reacted gas from the first regenerative mass passages as Said valuable off-gas and directing such hot gas through passages of a second relatively cool regen erative mass for quenching the off-gas to a lower tem perature at which relatively little acetylene decomposi tion occurs; discharging the quenching off-gas from said passages in the Warmed second regenerative mass, and recovering the heat abstracted by said second regenera tive mass from the hot off-gas by providing an oxidant gas and directing such oxidant gas through the second regenerative mass passages; providing a combustible fuel and burning such fuel with the heated oxidant gas so as to form said hot combustion gas mixture; discharging the acetylene-containing off-gas from said heated reaction Zone; cooling said off-gas in a first creeping pebble bed regenerative heat exchanger to about room temperature at a pressure below about 3 atmospheres absolute in heat exchange with material selected from the class con sisting of process gas and process air to remove a por tion of the condensable materials contained therein; fur ther cooling said off-gas in a second creeping pebble bed regenerative heat exchanger to about -1 F. to -130' F. at a pressure below about 3 atmospheres ab solute in heat exchange with a cold vent gas stream to remove additional amounts of condensable materials; treating the acetylene-containing gas stream with an ad Sorbent at a temperature below - F. and substan tially the same as the outlet temperature of said second creeping pebble bed regenerator to remove final amounts of condensable materials and substantially all of the higher acetylenes and discharging the resultant gas stream from the adsorbent at a temperature below 32 F. so as to substantially prevent polymerization of the hydro carbon impurities adsorbed therein; contacting the re Sultant purified gas stream with a solvent at a tem perature below about -1 F. and a pressure of less than about 2 atmospheres absolute, said solvent selec tively absorbing acetylene and thereby separating the acetylene from some of the less soluble impurities and from most of the non-condensables; recycling said less soluble and non-condensable materials as said cold vent gas stream for heat exchange in said second creeping pebble bed regenerative heat exchanger; venting the re maining less soluble impurities from the solvent; and recovering the acetylene from the solvent. 10. The process in accordance with claim 9 wherein the absorbent is silica gel and the solvent is acetone. 11. A process of producing acetylene from an in-gas the steps of directing a hot combustion gas mixture through passages of a first regenerative mass to heat such mass to a suitable reaction temperature; directing through said passages of the heated first regenerative mass an in gas containing hydrogen and said hydrocarbon stock in a molar ratio of between about 1 to 8 moles of hydrogen for each carbon atom of the hydrocarbon molecule and reacting said in-gas for a period of between about 0.01 and 0.05 second and at a pressure of between about 1 and 2 atmospheres absolute in contact with such first heated regnerative mass so as to convert at least part of Such in-gas to acetylene; discharging the acetylene-containing reacted gas from the first regenerative mass passages as said valuable off-gas and directing such hot gas through passages of a second relatively cool regenerative mass for quenching the off-gas to a lower temperature at which rela tively little acetylene decomposition occurs; discharging the quenching off-gas from said passages in the warmed second regenerative mass, and recovering the heat ab stracted by said second regenerative mass from the hot off-gas by providing an oxidant gas and directing Such oxidant gas through the second regenerative mass pas sages: providing a combustible fuel and burning such fuel with the heated oxidant gas so as to form said hot com 3,093, bustion gas mixture; discharging the acetylene-contain ing off-gas from said heated reaction zone; separating from said off-gas stream at least part of the remaining hydro gen-containing gas and recycling said hydrogen-contain ing gas to said heat reaction zone so as to provide at least part of the hydrogen of said in-gas; progressively cooling said off-gas Stream to a temperature of about -1 F. to -160 F. under pressures of less than about 3 atmos pheres absolute to remove a substantial portion of the condensable materials contained therein; contacting the cold gas stream with an adsorbent at a temperature below 32 F. to remove final amounts of condensable materials and at least some amounts of higher acetylenes and dis charging the resultant gas stream from the adsorbent at a temperature below 32 F. so as to substantially prevent polymerization of the hydrocarbon impurities adsorbed therein; contacting the resultant partially purified gas Stream at a temperature below about -1 F. and a pressure of less than about 2 atmospheres absolute with a solvent wherein the acetylene is selectively dissolved to separate said acetylene from some of the less soluble im purities and from most of the substantially insoluble non condensable materials in the gas stream; venting the re maining reductively less soluble impurities from the sol vent; and recovering the acetylene from said solvent. 12. A process of producing acetylene from an in-gas the steps of directing a hot combustion gas mixture through passages of a first regenerative mass to heat such mass to a suitable reaction temperature; directing through said passages of the heated first regenerative mass an in gas containing hydrogen and said hydrocarbon stock in a molar ratio of between about 1 to 8 moles of hydrogen for each carbon atom of the hydrocarbon molecule and reacting said in-gas for a period of between about 0.01 and 0.05 second and at a pressure of between about 1 and 2 atmospheres absolute in contact with such first heated regenerative mass so as to convert at least part of such in-gas to acetylene; discharging the acetylene-con taining reacted gas from from the first regenerative mass passages as said valuable off-gas and directing Such hot gas through passages of a second relatively cool regenera tive mass for quenching the off-gas to a lower temperature at which relatively little acetylene decomposition occurs; discharging the quenching off-gas from Said passages in the warmed second regenerative mass, and recovering the heat abstracted by said second regenerative mass from the hot off-gas by providing an oxidant gas and directing such oxidant gas through the second regenerative mass pas sages; providing a combustible fuel and burning such fuel with the heated oxidant gas so as to form said hot con bustion gas mixture; discharging the acetylene-containing off-gas from said heated reaction zone; separating from said off-gas stream at least part of the remaining hydro gen-containing gas and recycling said hydrogen-contain ing gas to said heat reaction zone so as to provide at least part of the hydrogen of said in-gas; cooling said off-gas in a first creeping pebble bed regenerative heat exchanger to about room temperature in heat exchange with material selected from the class consisting of process gas and process air to remove a portion of the condensable materials contained therein; further cooling said off-gas in a second creeping pebble bed regenerative heat exchanger to about -1 F. to -130 F. in heat exchange with a cold vent gas stream to remove additional amounts of condensable materials: treating the acetylene-containing gas stream with an adsorbent at a temperature below - F. and substantially the same as the outlet tempera ture of said second creeping pebble bed regenerator to remove final amounts of condensable materials and sub stantially all of the higher acetylenes and discharging the resultant gas stream from the adsorbent at a tempera ture below 32 F. so as to substantially prevent polymeri zation of the hydrocarbon impurities adsorbed therein; contacting the resultant purified gas stream with a solvent at a temperature below about -1 F. and a pressure
14 irrs, of less than about 2 atmospheres absolute, said solvent se lectively absorbing acetylene and thereby separating the acetylene from some of the less soluble impurities and from most of the non-condensables; recycling said less soluble and non-condensable materials as said cold vent gas stream for heat exchange in said second creeping peb ble bed regenerative heat exchanger; venting the remain ing less soluble impurities from the solvent; and recover ing the acetylene from the solvent. 13. The process in accordance with claim 12 wherein the absorbent is silica gel and the solvent is acetone. 14. A process of producing acetylene from an in-gas containing a suitable hydrocarbon stock, which comprises the steps of directing through passages of a heated first regenerative mass as a reaction stroke, an in-gas contain ing hydrogen and said hydrocarbon stock in a molar ratio of between about 1 to 8 moles of hydrogen for each carbon atom of the hydrocarbon molecule and reacting said in-gas in contact with such first heated regenerative mass so as to convert at least part of such in-gas to acetylene; discharging the acetylene-containing reacted gas from the first regenerative mass passages as said valuable off-gas and quenching such hot gas by flow through passages of a second relatively cool regenerative mass to cool such off-gas to a lower temperature at which relatively little acetylene decomposition occurs and simul taneously heat the second regenerative mass; discharging the quenched off-gas from said passages in the heated sec ond regenerative mass; as a simultaneous regenerative Stroke, providing an oxidant gas and directing such ox idant gas through passages of a heated third regenerative mass So as to preheat such oxidant and simultaneously recool such third mass for subsequent countercurrent pas Sage of hot off-gas therethrough; providing a combustible fuel and burning such fuel with part of the preheated oxidant gas to form a hot combustion gas mixture, the relative quantities of combustible fuel and oxidant gas being Such that an excess of unreacted oxidant gas re mains after such combustion; directing the hot combus tion gas mixture and unreacted oxidant gas through pas Sages of a cooled fourth regenerative mass so as to heat such mass for subsequent reaction of countercurrently flowing in-gas therein, and simultaneously regenerate the mass by burning off carbon deposited in said passages during the previous reaction stroke, the burnoff being effected by said unreacted oxidant gas; discharging the combustion gas mixture and the carbon oxides-containing reduced gas from the heated and regenerated fourth mass; and periodically reversing the fluid flows between the first and second regenerative masses, and the third and fourth regenerative masses so that the third and fourth masses are on reaction stroke with said in-gas flowing through the fourth regenerative mass passages for reaction therein and the resulting off-gas flowing through the third regenerative mass passages for quenching therein, and the first and second masses are on regenerative stroke with said oxidant gas flowing through the second regenerative mass passages for preheating therein, and said hot con bustion gas mixture and unreacted oxidant gas flowing through the first regenerative mass passages for heating and regeneration of such mass; progressively cooling said off-gas stream to a temperature of about -1' F. to -160 F. under pressures of less than about 3 atmos pheres absolute to remove a substantial portion of the condensable materials therefrom; contacting the cold gas stream with an adsorbent at a temperature below 32 F. to remove final amounts of condensable materials and at least some amounts of higher acetylenes and discharg ing the resultant gas stream from the adsorbent at a tem perature below 32 F. so as to substantially prevent polymerization of the hydrocarbon impurities adsorbed therein; contacting the resultant partially purified gas stream at a temperature below about -1 F. and a pressure of less than about 2 atmospheres absolute with a solvent which selectively dissolves acetylene to separate 8,098, the acetylene from some of the relatively less soluble im purities and from most of the substantially insoluble non-condensable materials in the gas stream; venting the remaining reductively less soluble impurities from the Sol vent; and recovering the acetylene from the solvent. 15. A process of producing acetylene from an in-gas containing a suitable hydrocarbon stock, which comprises the steps of directing through passages of a heated first regenerative mass as a reaction stroke, an in-gas contain ing hydrogen and said hydrocarbon stock in a molar ratio of between about 2 to 6 moles of hydrogen for each car bon atom of the hydrocarbon molecule and reacting said in-gas for a period of between 0.01 and 0.05 second and at a pressure of between about 1 and 2 atmospheres in contact with such first heated regenerative mass so as to convert at least part of such in-gas to acetylene; dis charging the acetylene-containing reacted gas from the first regenerative mass passages as said valuable off-gas and quenching such hot gas by flow through passages of a second relatively cool regenerative mass to cool such off-gas to a lower temperature at which relatively little acetylene decomposition occurs and simultaneously heat the second regenerative mass; discharging the quenched off-gas from said passages in the heated second regen erative mass; as a simultaneous regenerative stroke, pro viding an oxidant gas and directing such oxidant gas through passages of a heated third regenerative mass so as to preheat such oxidant and simultaneously recool such third mass for subsequent countercurrent passage of hot off-gas therethrough; providing a combustible fuel and burning such fuel with part of the preheated oxidant gas to form a hot combustion gas mixture, the relative quantities of combustible fuel and oxidant gas being such that an excess of unreacted oxidant gas remains after such combustion; directing the hot combustion gas mixture and unreacted oxidant gas through passages of a cooled fourth regenerative mass so as to heat for a period of between 0.01 and 0.05 second and at a pressure of between about 1 and 2 atmospheres absolute such mass for subsequent reaction of countercurrently flowing in-gas therein, and simultaneously regenerate the mass by burning off carbon deposited in said passages during the previous reaction stroke, the burnoff being effected by said unreacted oxidant gas; discharging the combustion gas mixture and the car bon oxides-containing reduced gas from the heated and regenerated fourth mass; and periodically reversing the fluid flows between the first and second regenerative masses, and the third and fourth regenerative masses so that the third and fourth masses are on reaction stroke with said in-gas flowing through the fourth regenerative mass passages for reaction therein and the resulting off gas flowing through the third regenerative mass passages for quenching therein, and the first and second masses are on regenerative stroke with said oxidant gas flow ing through the second regenerative mass passages for preheating therein, and said hot combustion gas mixture and unreacted oxidant gas flowing through the first re generative mass passages for heating and regeneration of such mass; progressively cooling said off-gas stream to a temperature of about -1' F. to -160 F. under pressures of less than about 3 atmospheres absolute to remove a substantial portion of the condensable ma terials contained therein; contacting the cold gas stream with an adsorbent at a temperature below 32 F. to remove final amounts of condensable materials and at least Some amounts of higher acetylenes and discharg ing the resultant gas Stream from the adsorbent at a tem perature below 32 F. so as to substantially prevent polymerization of the hydrocarbon impurities adsorbed therein; contacting the resultant partially purified gas stream at a temperature below about -1 F. and a pressure of less than about 2 atmospheres absolute with a solvent wherein the acetylene is selectively dissolved to separate said acetylene from some of the less soluble impurities and from most of the substantially insoluble
15 9 non-condensable materials in the gas stream; venting the remaining reductively less soluble impurities from the solvent; and recovering the acetylene from said solvent. 16. A process of producing acetylene from an in-gas containing a suitable hydrocarbon stock, which comprises the steps of directing through passages of a heated first regenerative mass as a reaction stroke, an. in-gas contain ing hydrogen and said hydrocarbon stock in a molar ratio of between about 2 to 6 moles of hydrogen for each car bon atom of the hydrocarbon molecule and reacting said in-gas for a period uf between 0.01 and 0.05 second and at a pressure of between about 1 and 2 atmospheres in contact with such first heated regenerative mass so as to convert at least part of such in-gas to acetylene; dis charging the acetylene-containing reacted gas from the first regenerative mass passages as said valuable off-gas and quenching such hot gas by flow through passages of a second relatively cool regenerative mass to cool such off-gas to a lower temperature at which relatively little acetylene decomposition occurs and simultaneously heat the second regenerative mass; discharging the quenched off-gas from said passages in the heated second regenera tive mass; as a simultaneous regenerative stroke, provid ing an oxidant gas and directing such oxidant gas through passages of a heated third regenerative mass so as to pre heat Such oxidant and simultaneously recool such third mass for Subsequent countercurrent passage of hot off gas therethrough; providing a combustible fuel and burn ing such fuel with part of the preheated oxidant gas to form a hot combustion gas mixture, the relative quantities of combustible fuel and oxidant gas being such that an excess of unreacted oxidant gas remains after such com bustion; directing the hot combustion gas mixture and unreacted oxidant gas through passages of a cooled fourth regenerative mass so as to heat for a period of between 0.01 and 0.05 second and at a pressure of between about 1 and 2 atmospheres absolute such mass for subsequent reaction of countercurrently flowing in-gas therein, and simultaneously regenerate the mass by burning off carbon deposited in said passages during the previous reaction stroke, the burnoff being effected by said unreacted oxidant gas; discharging the combustion gas mixture and the carbon oxides-containing reduced gas from the heated and regenerated fourth mass; and periodically reversing the fluid flows between the first and second regenerative masses, and the third and fourth regenerative masses so that the third and fourth masses are on reaction stroke with said in-gas flowing through the fourth regenerative mass passages for reaction therein and the resulting off gas flowing through the third regenerative mass passages for quenching therein, and the first and second asses are on regenerative stroke with said oxidant gas flowing through the second regenerative mass passages for pre heating therein, and said hot combustion gas mixture and unreacted oxidant gas flowing through the first regenera tive mass passages for heating and regeneration of such mass: cooling said off-gas in a first Creeping pebble bed regenerative heat exchanger to about room temperature at a pressure below about 3 atmospheres absolute in heat exchange with material selected from the class consist ing of process gas and process air to remove a portion of the condensable materials contained therein; further cool ing said off-gas in a second creeping pebble bed regen erative heat exchanger to about -1 F. to -130 F. at a pressure below about 3 atmospheres absolute in heat exchange with a cold vent gas stream to remove additional amounts of condensable materials; treating the acetylene containing gas stream with an adsorbent at a temperature below - F. and Substantially the same as the outlet temperature of said second creeping pebble bed Iegenera to to remove final amounts of condensable materials and substantially all of the higher acetylenes and discharging the resultant gas stream from the adsorbent at a tempera ture below 32 F. so as to Substantially prevent polym erization of the hydrocarbon impurities adsorbed therein; 3,093,69? contacting the resultant purified gas stream with a solvent at a temperature below about -1 F. and a pressure of less than about 2 atmospheres absolute, said solvent selectively absorbing acetylene and thereby separating the acetylene from some of the less soluble impurities and from most of the noncondensables; recycling said less Soluble and non-condensable materials as said cold vent gas stream for heat exchange in said second creeping pebble bed regenerative heat exchanger; venting the re maining less soluble impurities from the solvent; and recovering the acetylene from the solvent. 17. The process in accordance with claim 16 wherein the absorbent is silica gel and the solvent is acetone. 18. A process of producing acetylene from an in-gas containing a suitable methane stock, which comprises the steps of directing through passages of a heated first re generative mass as a reaction stroke, an in-gas contain ing hydrogen and said methane stock in a molar ratio of hydrogen to methane of about 1 to 8 and reacting said in-gas for a period of between 0.01 and 0.05 second and at a pressure of between about 1 and 2 atmospheres in contact with Such first heated regenerative mass so as to convert at least part of such in-gas to acetylene; dis charging the acetylene-containing reacted gas from the first regenerative mass passages as said valuable off-gas and quenching Such hot gas by flow through passages of a second relatively cool regenerative mass to cool such off-gas to a lower temperature at which relatively little acetylene decomposition occurs and simultaneously heat the second regenerative mass; discharging the quenched off-gas from said passages in the heated second regenera tive mass; as a simultaneous regenerative stroke, provid ing an oxidant gas and directing such oxidant gas through passages of a heated third regenerative mass so as to pre heat such oxidant and simultaneously cool such third mass for Subsequent countercurrent passage of hot off gas therethrough; providing a combustible fuel and burn ing such fuel with part of the preheated oxidant gas to form a hot combustion gas mixture, the relative quan tities of combustible fuel and oxidant gas being such that an excess of unreacted oxidant gas remains after such combustion; directing the hot combustion gas mixture and unreacted oxidant gas through passages of a cooled fourth regenerative mass so as to heat such mass for Subsequent reaction of countercurrently flowing in-gas therein, and simultaneously regenerate the mass by burning off carbon deposited in said passages during the previous reaction stroke, the burnoff being effected by Said unreacted ox idant gas; discharging the combustion gas mixture and the carbon oxides-containing reduced gas from the heated and regenerated fourth mass; and periodically reversing the fluid flows between the first and second regenerative asses, and the third and fourth regenerative masses so that the third and fourth masses are on reaction stroke With said in-gas flowing through the fourth regenerative mass passages for reaction therein and the resulting off gas flowing through the third regenerative mass passages for quenching therein, and the first and second lasses are On regenerative stroke with said oxidant gas flowing through the second regenerative mass passages for pre heating therein, and said hot combustion gas mixture and unreacted oxidant gas flowing through the first regenera tive mass passages for heating and regeneration of such mass; cooling said off-gas in a first Creeping pebble bed regenerative heat exchanger to about room temperature at pressure below about 3 atmospheres absolute in hea exchange with material selected from the class consisting of process gas and process air to remove a portion of the condensable materials contained therein; further cooling said off-gas in a second creeping pebble bed regenerative heat exchanger to about -1 F. to -130 F. at a Pressure below about 3 atmospheres absolute in heat ex change with a cold vent gas stream to remove additional amounts of condensable materials; treating the acetylene Sontaining gas stream with an adsorbent at a temperature below - F. and Substantially the same as the outlet
United States Patent (19) 11) 4,217,759
 United States Patent (19) 11) 4,217,759 Shen Oy --- 45 45 Aug. 2 19, 9 1980 54). CRYOGENIC PROCESS FOR SEPARATING 3,508,413 4/1970 Pryor... 62/24 SYNTHESS GAS 3,553,972 1/1971 Markbreiter et al.... 62/23
United States Patent (19) 11) 4,217,759 Shen Oy --- 45 45 Aug. 2 19, 9 1980 54). CRYOGENIC PROCESS FOR SEPARATING 3,508,413 4/1970 Pryor... 62/24 SYNTHESS GAS 3,553,972 1/1971 Markbreiter et al.... 62/23
14 / 44. United States Patent (19) Allam ADM2/A. 11 Patent Number: 4,461,154 45) Date of Patent: Jul. 24, 1984 SAZX A6 AIAW/ 10/ /02 A/?
 United States Patent (19) Allam (54) METHOD AND APPARATUS FOR COMPRESSENG GAS 75 Inventor: Rodney J. Allam, St. Catherines, England 73) Assignee: Air Products and Chemicals, Inc., Allentown, Pa. (21) Appl.
United States Patent (19) Allam (54) METHOD AND APPARATUS FOR COMPRESSENG GAS 75 Inventor: Rodney J. Allam, St. Catherines, England 73) Assignee: Air Products and Chemicals, Inc., Allentown, Pa. (21) Appl.
(51) Int. Cl... F04B 25/00 about 250 F., as will not allow the partial pressure of the
 USOO58860A United States Patent (19) 11 Patent Number: Cunkelman et al. () Date of Patent: Mar 23, 1999 54) THERMOSTATICALLY CONTROLLED 4,362.462 12/1982 Blotenberg... 417/243 X INTERCOOLER SYSTEM FOR
USOO58860A United States Patent (19) 11 Patent Number: Cunkelman et al. () Date of Patent: Mar 23, 1999 54) THERMOSTATICALLY CONTROLLED 4,362.462 12/1982 Blotenberg... 417/243 X INTERCOOLER SYSTEM FOR
J. s. scoggin ETAL SEPARATION OF SOLID POLYMERS AND LIQUID DILUENT 3,152,872. Oct. 13, rowers. Filed March 6, 196 INVENTORS
 Oct. 13, 1964 J. s. scoggin ETAL SEPARATION OF SOLID POLYMERS AND LIQUID DILUENT Filed March 6, 196 3,152,872 ------------ INVENTORS SCOG GIN MBLE BY???????.????????????????? 47 rowers United States Patent
Oct. 13, 1964 J. s. scoggin ETAL SEPARATION OF SOLID POLYMERS AND LIQUID DILUENT Filed March 6, 196 3,152,872 ------------ INVENTORS SCOG GIN MBLE BY???????.????????????????? 47 rowers United States Patent
United States Patent (19)
 United States Patent (19) Fujikawa 54 HYDRAULIC CONTROL SYSTEM FOR A ROCK DRILL (75) Inventor: Kozo Fujikawa, Hiroshima, Japan 73) Assignee: Toyo Kogyo Co., Ltd., Hiroshima, Japan 21 Appl. No.: 194,391
United States Patent (19) Fujikawa 54 HYDRAULIC CONTROL SYSTEM FOR A ROCK DRILL (75) Inventor: Kozo Fujikawa, Hiroshima, Japan 73) Assignee: Toyo Kogyo Co., Ltd., Hiroshima, Japan 21 Appl. No.: 194,391
11 5,617,741 McNeil et al. (45) Date of Patent: Apr. 8, Antwerpen, Belgium /1989 United Kingdom.
 United States Patent (19) US00561.7741A Patent Number: 11 McNeil et al. () Date of Patent: Apr. 8, 1997 (54) DUAL COLUMN PROCESS TO REMOVE 5,7,505 11/1993 Butts... 62/927 X NITROGEN FROM NATURAL GAS FOREIGN
United States Patent (19) US00561.7741A Patent Number: 11 McNeil et al. () Date of Patent: Apr. 8, 1997 (54) DUAL COLUMN PROCESS TO REMOVE 5,7,505 11/1993 Butts... 62/927 X NITROGEN FROM NATURAL GAS FOREIGN
US A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/ A1 Islam et al. (43) Pub. Date: May 22, 2008
 US 20080119574A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0119574 A1 Islam et al. (43) Pub. Date: May 22, 2008 (54) RECOVERY OF ORGANIC COMPOUNDS Related US. Application
US 20080119574A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0119574 A1 Islam et al. (43) Pub. Date: May 22, 2008 (54) RECOVERY OF ORGANIC COMPOUNDS Related US. Application
Linn... 95/240 X McIntire et al... 95/240 X. Avery... 55/58. gas. Zacher et al / y. 2d Y 3. O5 CD-H24O. At ll
 United States Patent (19) Howard et al. (54) (75) (73) (21) 22) (51) (52) 58 56) RECOVERY OF HYDROCARBONS FROM POLYALKENE PRODUCT PURGE GAS Inventors: Lee J. Howard, Allentown; Howard C. Rowles, Center
United States Patent (19) Howard et al. (54) (75) (73) (21) 22) (51) (52) 58 56) RECOVERY OF HYDROCARBONS FROM POLYALKENE PRODUCT PURGE GAS Inventors: Lee J. Howard, Allentown; Howard C. Rowles, Center
(12) United States Patent (10) Patent No.: US 6,426,434 B1
 USOO6426434B1 (12) United States Patent (10) Patent No.: Yoshida et al. () Date of Patent: Jul. 30, 2002 (54) PROCESS FOR THE SYNTHESIS OF UREA JP 62-170 4/1987 JP 63-12 5/1988 (75) Inventors: Kinichi
USOO6426434B1 (12) United States Patent (10) Patent No.: Yoshida et al. () Date of Patent: Jul. 30, 2002 (54) PROCESS FOR THE SYNTHESIS OF UREA JP 62-170 4/1987 JP 63-12 5/1988 (75) Inventors: Kinichi
FlashCO2, CO2 at 23 $/ton
 FlashCO2, CO2 at 23 $/ton A cost effective solution of capturing CO2 from Steam Methane Reforming (SMR) Hydrogen production plants by the FlashCO2 process Introduction to a cost effective solution Driven
FlashCO2, CO2 at 23 $/ton A cost effective solution of capturing CO2 from Steam Methane Reforming (SMR) Hydrogen production plants by the FlashCO2 process Introduction to a cost effective solution Driven
United States Patent (19) Johnson
 United States Patent (19) Johnson 54). SILVER RECVERY FRM PHTGRAPHIC WASTES 76 Inventor: Edward B. Johnson, 44 34th St. North, Arlington, Va. 227 (22 Filed: Dec. 29, 1971 21 Appl. No.: 213,564 Related
United States Patent (19) Johnson 54). SILVER RECVERY FRM PHTGRAPHIC WASTES 76 Inventor: Edward B. Johnson, 44 34th St. North, Arlington, Va. 227 (22 Filed: Dec. 29, 1971 21 Appl. No.: 213,564 Related
52 U.S. C /232; 423/210; 55/16 55/16. (56) References Cited U.S. PATENT DOCUMENTS 3,624,983 12/1971 Ward et al..., 55/16
 United States Patent (19) Ward, III (54 SEPARATION OF HYDROGEN SULFIDE FROM GAS MIXTURE INCLUDING CARBON DOXDE (75) Inventor: William J. Ward, III, Schenectady, N.Y. 73) Assignee: General Electric Company,
United States Patent (19) Ward, III (54 SEPARATION OF HYDROGEN SULFIDE FROM GAS MIXTURE INCLUDING CARBON DOXDE (75) Inventor: William J. Ward, III, Schenectady, N.Y. 73) Assignee: General Electric Company,
7%averzazz az72eways. AZafaa 77/owa soy. /v/a/y7 Oa. July 7, 1964 W. A. THOMPSON 3,139,732 BOAT STABILIZING AND LIFTING DEVICE.
 July 7, 1964 W. A. THOMPSON Filed May 5, 1961 BOAT STABILIZING AND LIFTING DEVICE 2 Sheets-Sheet /v/a/y7 Oa AZafaa 77/owa soy 7%averzazz az72eways July 7, 1964 Filed May 5, l96l W. A. THOMPSON BOAT STABILIZING
July 7, 1964 W. A. THOMPSON Filed May 5, 1961 BOAT STABILIZING AND LIFTING DEVICE 2 Sheets-Sheet /v/a/y7 Oa AZafaa 77/owa soy 7%averzazz az72eways July 7, 1964 Filed May 5, l96l W. A. THOMPSON BOAT STABILIZING
April 17, ,666, Sheets-Sheet. B. N. WALIS AIRSHIP Filed May 29, 1925
 April 17, 1928. B. N. WALIS AIRSHIP Filed May 29, 1925 2 Sheets-Sheet April 17, 1928. B, N, WALIS AIRSHIP Filed May 29, 1925 fm/aav7oa a 21. Maeela a 77OAAWAYS 0 Patented Apr. 17, 1928. UNITED STATES PATENT
April 17, 1928. B. N. WALIS AIRSHIP Filed May 29, 1925 2 Sheets-Sheet April 17, 1928. B, N, WALIS AIRSHIP Filed May 29, 1925 fm/aav7oa a 21. Maeela a 77OAAWAYS 0 Patented Apr. 17, 1928. UNITED STATES PATENT
Removing nitrogen. Nitrogen rejection applications can be divided into two categories
 Removing nitrogen Doug MacKenzie, Ilie Cheta and Darryl Burns, Gas Liquids Engineering, Canada, present a comparative study of four nitrogen removal processes. Nitrogen rejection applications can be divided
Removing nitrogen Doug MacKenzie, Ilie Cheta and Darryl Burns, Gas Liquids Engineering, Canada, present a comparative study of four nitrogen removal processes. Nitrogen rejection applications can be divided
United States Patent: 4,311,561. ( 42 of 76 ) United States Patent 4,311,561 Hastings January 19, Apparatus for extracting bitumen from tar sand
 United States Patent: 4,311,561 ( 42 of 76 ) United States Patent 4,311,561 Hastings January 19, 1982 Apparatus for extracting bitumen from tar sand Abstract A method and apparatus for extracting bitumen
United States Patent: 4,311,561 ( 42 of 76 ) United States Patent 4,311,561 Hastings January 19, 1982 Apparatus for extracting bitumen from tar sand Abstract A method and apparatus for extracting bitumen
MM2. Fig. l. .oœua. Dec. 17, 1957 H. E. MUELLER HEINZ E. MUELLER. ATT RNEYsY PROPELLANT DISPLACEMENT GAS GENERATORS
 Dec. 17, 1957 H. E. MUELLER 25816 419 PROPELLANT DISPLACEMENT GAS GENERATORS Filed March 7, 1952 2 Sheets-Sheet 1 Fig. l. IIIIIIIIIIIIIIIIIIIIIIIIIIII. O MM2 HEINZ E. MUELLER. BY.oœua ATT RNEYsY Dec. 17,
Dec. 17, 1957 H. E. MUELLER 25816 419 PROPELLANT DISPLACEMENT GAS GENERATORS Filed March 7, 1952 2 Sheets-Sheet 1 Fig. l. IIIIIIIIIIIIIIIIIIIIIIIIIIII. O MM2 HEINZ E. MUELLER. BY.oœua ATT RNEYsY Dec. 17,
Feb. 21, ,972,697. MOLECULAR BEAM APPARATUS OF THE MASER TyPE S. A. JOHNSON ETAL. Filed June 26, 1958 AEG/747OA NVENORS AORNEY
 Feb. 21, 1961 S. A. JOHNSON ETAL MOLECULAR BEAM APPARATUS OF THE MASER TyPE Filed June 26, 1958 2,972,697 AEG/747OA S R NVENORS 4? AORNEY United States Patent Office 2,972,697 Patented Feb. 21, 1961 2,972,697
Feb. 21, 1961 S. A. JOHNSON ETAL MOLECULAR BEAM APPARATUS OF THE MASER TyPE Filed June 26, 1958 2,972,697 AEG/747OA S R NVENORS 4? AORNEY United States Patent Office 2,972,697 Patented Feb. 21, 1961 2,972,697
United States Patent (19)
 United States Patent (19) Oranje (54) DEVICE FOR SEPARATING LIQUIDS AND/OR SOLIDS FROMA HIGH-PRESSURE GAS STREAM 75 inventor: Leendert Oranje, Haren, Netherlands (73) Assignee: N.V. Nederlandse Gasunie,
United States Patent (19) Oranje (54) DEVICE FOR SEPARATING LIQUIDS AND/OR SOLIDS FROMA HIGH-PRESSURE GAS STREAM 75 inventor: Leendert Oranje, Haren, Netherlands (73) Assignee: N.V. Nederlandse Gasunie,
Egg...","...7. Primary Examiner-Dean Kramer
 USOO5513884A United States Patent 19 11 Patent Number: Bucher 45) Date of Patent: May 7, 1996 54) GOLF BALL RETRIEVING DEVICE 3,520,569 7/1970 Anderson... 2.94/19.2 3,659,891 5/1972 Pettenon et al....
USOO5513884A United States Patent 19 11 Patent Number: Bucher 45) Date of Patent: May 7, 1996 54) GOLF BALL RETRIEVING DEVICE 3,520,569 7/1970 Anderson... 2.94/19.2 3,659,891 5/1972 Pettenon et al....
United States Patent (19) Shihara et al.
 United States Patent (19) Shihara et al. 54 HYDRAULIC CONTROL SYSTEM FOR A ROCK DRILL 75) Inventors: Takeo Shihara; Toshihiro Niihara; Hidemi Iwata; Noboru Ishii, all of Hiroshima, Japan 73 Assignee: Toyo
United States Patent (19) Shihara et al. 54 HYDRAULIC CONTROL SYSTEM FOR A ROCK DRILL 75) Inventors: Takeo Shihara; Toshihiro Niihara; Hidemi Iwata; Noboru Ishii, all of Hiroshima, Japan 73 Assignee: Toyo
Name Chemistry Pre-AP
 Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Protective Atmospheres, Measurement Technologies and Troubleshooting Tools
 Protective Atmospheres, Measurement Technologies and Troubleshooting Tools Furnace atmospheres are critical to meet metallurgical specifications defined by control processes. The makeup of a furnace s
Protective Atmospheres, Measurement Technologies and Troubleshooting Tools Furnace atmospheres are critical to meet metallurgical specifications defined by control processes. The makeup of a furnace s
N3% (12) United States Patent. NNéré. (10) Patent No.: US 7, B2. Rossiter (45) Date of Patent: Nov. 20, 2007
 (12) United States Patent US007298.473B2 (10) Patent o.: US 7,298.473 B2 Rossiter (45) Date of Patent: ov. 20, 2007 (54) SPECTROSCOPY CELL 4,587,835 A 5/1986 Adams 4,674,876 A 6/1987 Rossiter... 356,244
(12) United States Patent US007298.473B2 (10) Patent o.: US 7,298.473 B2 Rossiter (45) Date of Patent: ov. 20, 2007 (54) SPECTROSCOPY CELL 4,587,835 A 5/1986 Adams 4,674,876 A 6/1987 Rossiter... 356,244
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States US 201400.07860A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0007860 A1 LU (43) Pub. Date: Jan. 9, 2014 (54) SMOKELESS PORTABLE ROASTER (57) ABSTRACT (76) Inventor: Chien-Chang
(19) United States US 201400.07860A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0007860 A1 LU (43) Pub. Date: Jan. 9, 2014 (54) SMOKELESS PORTABLE ROASTER (57) ABSTRACT (76) Inventor: Chien-Chang
XDF BURNERS DUAL FUEL EXCESS AIR BURNER FEATURES DESCRIPTION EXCESS AIR OPERATION
 DUAL FUEL EXCESS AIR BURNER MODEL: 3610, 3651 Revision: 0 FEATURES Burns all fuel gases or light oils Nozzle mix design for on ratio control or excess air 350% excess air all sizes on gas or oil Turndown
DUAL FUEL EXCESS AIR BURNER MODEL: 3610, 3651 Revision: 0 FEATURES Burns all fuel gases or light oils Nozzle mix design for on ratio control or excess air 350% excess air all sizes on gas or oil Turndown
United States Patent (19)
 United States Patent (19) Crump 11 Patent Number: Date of Patent: Apr. 3, 1990 54 ADJUSTABLE HEIGHT WHEELCHAIR RAMP WITHSUPPORTING LEGS 76 Inventor: 21 22 (51) (52 58 (56) Robert Crump, 333 Guthrie Rd.,
United States Patent (19) Crump 11 Patent Number: Date of Patent: Apr. 3, 1990 54 ADJUSTABLE HEIGHT WHEELCHAIR RAMP WITHSUPPORTING LEGS 76 Inventor: 21 22 (51) (52 58 (56) Robert Crump, 333 Guthrie Rd.,
Chemistry HP Unit 6 Gases. Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases
 Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Digester Processes. 1. Raw Sludge Pumping System
 Digester Processes 1. Raw Sludge Pumping System Removes accumulated sludge from the primary clarifiers, pumped through 1 of 2 pipes either 150 or 200mm in diameter (Fig. 1.1). Fig 1.1 Pipes feeding Digesters
Digester Processes 1. Raw Sludge Pumping System Removes accumulated sludge from the primary clarifiers, pumped through 1 of 2 pipes either 150 or 200mm in diameter (Fig. 1.1). Fig 1.1 Pipes feeding Digesters
FUNDAMENTALS OF PRESSURE REGULATORS ROBERT BENNETT MANAGER OF TRAINING ELSTER AMERICAN METER
 FUNDAMENTALS OF PRESSURE REGULATORS ROBERT BENNETT MANAGER OF TRAINING ELSTER AMERICAN METER SUPPLY = DEMAND FUNCTION OF A REGULATOR A regulator may be defined as a "mechanism for controlling or governing
FUNDAMENTALS OF PRESSURE REGULATORS ROBERT BENNETT MANAGER OF TRAINING ELSTER AMERICAN METER SUPPLY = DEMAND FUNCTION OF A REGULATOR A regulator may be defined as a "mechanism for controlling or governing
A NEW PROCESS FOR IMPROVED LIQUEFACTION EFFICIENCY
 WHITE PAPER A NEW PROCESS FOR IMPROVED LIQUEFACTION EFFICIENCY Author(s): Adam Jones and Grant Johnson, Costain Natural Resources First published: GPAE, September 2014 www.costain.com A New Process for
WHITE PAPER A NEW PROCESS FOR IMPROVED LIQUEFACTION EFFICIENCY Author(s): Adam Jones and Grant Johnson, Costain Natural Resources First published: GPAE, September 2014 www.costain.com A New Process for
You should be able to: Describe Equipment Barometer Manometer. 5.1 Pressure Read and outline 5.1 Define Barometer
 A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
United States Patent (19) Widecrantz et al.
 United States Patent (19) Widecrantz et al. 54 76 22) 21 (52) (51) 58 56 ONE WAY WALVE PRESSURE PUMP TURBINE GENERATOR STATION Inventors: Kaj Widecrantz, P.O. Box 72; William R. Gatton, P.O. Box 222, both
United States Patent (19) Widecrantz et al. 54 76 22) 21 (52) (51) 58 56 ONE WAY WALVE PRESSURE PUMP TURBINE GENERATOR STATION Inventors: Kaj Widecrantz, P.O. Box 72; William R. Gatton, P.O. Box 222, both
Aug. 25, 1970 J. A. W. KAEDING ET AL 3,525,437
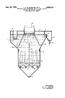 Aug., 1970 J. A. W. KAEDING ET AL APPARATUS FOR SEPARATING SOLIDS FROM LIQUIDS AND FOR THECKENING SLUDGES Filed April 18, 1968 NVENTORS J. A. W. KAEONG W.A. GRUNERT United States Patent Office Patented
Aug., 1970 J. A. W. KAEDING ET AL APPARATUS FOR SEPARATING SOLIDS FROM LIQUIDS AND FOR THECKENING SLUDGES Filed April 18, 1968 NVENTORS J. A. W. KAEONG W.A. GRUNERT United States Patent Office Patented
Optimizing Effective Absorption during Wet Natural Gas Dehydration by Tri Ethylene Glycol
 IOSR Journal of Applied Chemistry (IOSRJAC) ISSN : 2278-5736 Volume 2, Issue 2 (Sep-Oct. 212), PP 1-6 Optimizing Effective Absorption during Wet Natural Gas Dehydration by Tri Ethylene Glycol Khan, Mohd
IOSR Journal of Applied Chemistry (IOSRJAC) ISSN : 2278-5736 Volume 2, Issue 2 (Sep-Oct. 212), PP 1-6 Optimizing Effective Absorption during Wet Natural Gas Dehydration by Tri Ethylene Glycol Khan, Mohd
NITROGEN GENERATION FOR INDUSTRIAL APPLICATIONS
 TRIDENT NOTES: NUMBER 5 DECEMBER 2017 NITROGEN GENERATION FOR INDUSTRIAL APPLICATIONS Industry requires nitrogen Dozens of gases are used by industry. First among these in terms of quantity consumed is
TRIDENT NOTES: NUMBER 5 DECEMBER 2017 NITROGEN GENERATION FOR INDUSTRIAL APPLICATIONS Industry requires nitrogen Dozens of gases are used by industry. First among these in terms of quantity consumed is
PTRT 2470: Petroleum Data Management 3 - Facilities Test 4 (Spring 2017)
 Use scantron to answer all questions PTRT 2470: Petroleum Data Management 3 - Facilities Test 4 (Spring 2017) 1. The term dehydration of natural gas means A. addition of water vapor B. removal of water
Use scantron to answer all questions PTRT 2470: Petroleum Data Management 3 - Facilities Test 4 (Spring 2017) 1. The term dehydration of natural gas means A. addition of water vapor B. removal of water
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States US 20090005197A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0005197 A1 Mayer (43) Pub. Date: Jan. 1, 2009 (54) HOCKEY STICK HAVING AN ANGLED (52) U.S. Cl.... 473/560;
(19) United States US 20090005197A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0005197 A1 Mayer (43) Pub. Date: Jan. 1, 2009 (54) HOCKEY STICK HAVING AN ANGLED (52) U.S. Cl.... 473/560;
Another convenient term is gauge pressure, which is a pressure measured above barometric pressure.
 VACUUM Theory and Applications Vacuum may be defined as the complete emptiness of a given volume. It is impossible to obtain a perfect vacuum, but it is possible to obtain a level of vacuum, defined as
VACUUM Theory and Applications Vacuum may be defined as the complete emptiness of a given volume. It is impossible to obtain a perfect vacuum, but it is possible to obtain a level of vacuum, defined as
 www.ompressedairsystems.com ultra-high purity nitrogen generators nitrogen purity: 95% to 99.999% www.ompressedairsystems.com ultra-high purity nitrogen generators nitrogen purity: 95% to 99.999% Leading
www.ompressedairsystems.com ultra-high purity nitrogen generators nitrogen purity: 95% to 99.999% www.ompressedairsystems.com ultra-high purity nitrogen generators nitrogen purity: 95% to 99.999% Leading
TEPZZ 7 Z67A_T EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION
 (19) TEPZZ 7 Z67A_T (11) EP 2 711 067 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 26.03.14 Bulletin 14/13 (21) Application number: 12188.1 (1) Int Cl.: B01D 3/60 (06.01) B01D 3/92 (06.01)
(19) TEPZZ 7 Z67A_T (11) EP 2 711 067 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 26.03.14 Bulletin 14/13 (21) Application number: 12188.1 (1) Int Cl.: B01D 3/60 (06.01) B01D 3/92 (06.01)
A leat A2. ByWC7252.7%ZW. July 17, J. B. FOWLER ET AL 1,966,791 METHOD OF AND APPARATUS FOR HANDLING HEATING SYSTEM RETURNS
 July 17, 1934. J. B. FWLER ET AL 1,966,791 METHD F AND APPARATUS FR HANDLING HEATING SYSTEM RETURNS Filed April 4, 1932 2 Sheets-Sheet s VENTRS u% saa/74. A2 aa (202aea/62a-v ByWC722.7%ZW 1-41. 3. A leat
July 17, 1934. J. B. FWLER ET AL 1,966,791 METHD F AND APPARATUS FR HANDLING HEATING SYSTEM RETURNS Filed April 4, 1932 2 Sheets-Sheet s VENTRS u% saa/74. A2 aa (202aea/62a-v ByWC722.7%ZW 1-41. 3. A leat
High Efficiency SO2 Scrubber Design to Reduce Caustic Consumption
 High Efficiency SO2 Scrubber Design to Reduce Caustic Consumption Paper # 35 Andrew C. Bartocci Envitech, Inc. 2924 Emerson Street- Suite 320 San Diego, CA 92106 Ph: 619-223-9925, ext. 203 ABSTRACT An
High Efficiency SO2 Scrubber Design to Reduce Caustic Consumption Paper # 35 Andrew C. Bartocci Envitech, Inc. 2924 Emerson Street- Suite 320 San Diego, CA 92106 Ph: 619-223-9925, ext. 203 ABSTRACT An
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 US 2004O126242A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0126242 A1 Howard et al. (43) Pub. Date: Jul. 1, 2004 (54) BOAT PROPELLER AND GUARD DEVICE (52) U.S. Cl....
US 2004O126242A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0126242 A1 Howard et al. (43) Pub. Date: Jul. 1, 2004 (54) BOAT PROPELLER AND GUARD DEVICE (52) U.S. Cl....
Energy Recovery Through Incineration in Paint Drying Process
 Energy Recovery Through Incineration in Paint Drying Process KLAUS H. HEMSATH ARVIND C. THEKDI FRANK J. VEREECKE Midland-Ross Corporation Toledo, Ohio The conventional oven-incinerator system in paint
Energy Recovery Through Incineration in Paint Drying Process KLAUS H. HEMSATH ARVIND C. THEKDI FRANK J. VEREECKE Midland-Ross Corporation Toledo, Ohio The conventional oven-incinerator system in paint
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 US 2007.0125537A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0125537 A1 Lokhandwala et al. (43) Pub. Date: (54) NATURAL. GAS TREATMENT PROCESS FOR Publication Classification
US 2007.0125537A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0125537 A1 Lokhandwala et al. (43) Pub. Date: (54) NATURAL. GAS TREATMENT PROCESS FOR Publication Classification
Simplicity in VRU by using a Beam Gas Compressor
 Simplicity in VRU by using a Beam Gas Compressor By Charlie D. McCoy and Mark Lancaster Abstract: Vapor Recovery Units are often expensive, complicated to operate and unable to deal with High H2S and liquids.
Simplicity in VRU by using a Beam Gas Compressor By Charlie D. McCoy and Mark Lancaster Abstract: Vapor Recovery Units are often expensive, complicated to operate and unable to deal with High H2S and liquids.
(12) United States Patent (10) Patent No.: US 6,524,267 B1
 USOO6524267B1 (12) United States Patent (10) Patent No.: US 6,524,267 B1 Gremel et al. 45) Date of Patent: Feb. 25, 2003 9 (54) VENOUS FILTER FOR ASSISTED VENOUS (56) References Cited RETUR N U.S. PATENT
USOO6524267B1 (12) United States Patent (10) Patent No.: US 6,524,267 B1 Gremel et al. 45) Date of Patent: Feb. 25, 2003 9 (54) VENOUS FILTER FOR ASSISTED VENOUS (56) References Cited RETUR N U.S. PATENT
(12) United States Patent
 (12) United States Patent USOO8352206B2 () Patent No.: Buess (45) Date of Patent: Jan. 8, 2013 (54) METHOD FOR THE SIGNAL (56) References Cited LINEARIZATION OF A GAS SENSOR OUTPUT SIGNAL U.S. PATENT DOCUMENTS
(12) United States Patent USOO8352206B2 () Patent No.: Buess (45) Date of Patent: Jan. 8, 2013 (54) METHOD FOR THE SIGNAL (56) References Cited LINEARIZATION OF A GAS SENSOR OUTPUT SIGNAL U.S. PATENT DOCUMENTS
(ii) 4,314,077. United States Patent ( Feb. 2, the inerts and the solution of urea coming from the (54) METHOD FOR THE PRODUCTION OF
 United States Patent (19 Zardi et al. (54) METHOD FOR THE PRODUCTION OF UREA AND PURIFICATION OF WATER 75 Inventors: Umberto Zardi, San Donato Milanese; Vincenzo Lagana', Milan, both of Italy 73) Assignee:
United States Patent (19 Zardi et al. (54) METHOD FOR THE PRODUCTION OF UREA AND PURIFICATION OF WATER 75 Inventors: Umberto Zardi, San Donato Milanese; Vincenzo Lagana', Milan, both of Italy 73) Assignee:
Feb. 6, 1968 T. D. GASS 3,367,062
 Feb. 6, 1968 T. D. GASS LURE LADDER FOR A FISHING T ACKLE BOX Filed April 7, 1966 3. Sheets-Sheet l INVENTOR. 7A7AOAO4a A. 62/.456 1772aMafy-s. Feb. 6, 1968 Filed April 7, 1966 T. D. GASS LURE LA))ER FOR
Feb. 6, 1968 T. D. GASS LURE LADDER FOR A FISHING T ACKLE BOX Filed April 7, 1966 3. Sheets-Sheet l INVENTOR. 7A7AOAO4a A. 62/.456 1772aMafy-s. Feb. 6, 1968 Filed April 7, 1966 T. D. GASS LURE LA))ER FOR
(12) United States Patent (10) Patent No.: US 7,516,566 B2
 US007516566B2 (12) United States Patent () Patent No.: Yu (45) Date of Patent: Apr. 14, 2009 (54) STEAM IRON HAVING A LIGHTWEIGHT (56) References Cited (75) (73) (*) (21) (22) (86) (87) (65) (30) Foreign
US007516566B2 (12) United States Patent () Patent No.: Yu (45) Date of Patent: Apr. 14, 2009 (54) STEAM IRON HAVING A LIGHTWEIGHT (56) References Cited (75) (73) (*) (21) (22) (86) (87) (65) (30) Foreign
United States Patent (19) Vidlinic
 United States Patent (19) Vidlinic (54) 76 (1) ) 51 (5) (58) 56) GAME FOR PRACTICNG SOCCER SKILLS Inventor: Blagoje Vidinic, 1B Rene Hirschler, 67000 Strasbourg, France Appl. No.: 6,967 Filed: May 14,
United States Patent (19) Vidlinic (54) 76 (1) ) 51 (5) (58) 56) GAME FOR PRACTICNG SOCCER SKILLS Inventor: Blagoje Vidinic, 1B Rene Hirschler, 67000 Strasbourg, France Appl. No.: 6,967 Filed: May 14,
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 US 2004O112455A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0112455A1 Nelson (43) Pub. Date: (54) PRESSURE CONTROLLED METHOD FOR (52) U.S. Cl.... 141/2 DISPENSINGA CARBONATED
US 2004O112455A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0112455A1 Nelson (43) Pub. Date: (54) PRESSURE CONTROLLED METHOD FOR (52) U.S. Cl.... 141/2 DISPENSINGA CARBONATED
Waste to energy conversion Dr. Prasenjit Mondal Department of Chemical Engineering Indian Institute of Technology, Roorkee. Lecture 25 Gas Cleanup 2
 Waste to energy conversion Dr. Prasenjit Mondal Department of Chemical Engineering Indian Institute of Technology, Roorkee Lecture 25 Gas Cleanup 2 Hi friends. Now we will start discussion on the second
Waste to energy conversion Dr. Prasenjit Mondal Department of Chemical Engineering Indian Institute of Technology, Roorkee Lecture 25 Gas Cleanup 2 Hi friends. Now we will start discussion on the second
(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
 US 2013 0186486A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0186486A1 Ding (43) Pub. Date: Jul. 25, 2013 (54) SYSTEM FOR AND METHOD OF (52) U.S. Cl. MONITORING FLOW
US 2013 0186486A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0186486A1 Ding (43) Pub. Date: Jul. 25, 2013 (54) SYSTEM FOR AND METHOD OF (52) U.S. Cl. MONITORING FLOW
DEHYDRATION OF ACID GAS PRIOR TO INJECTION Eugene W. Grynia, John J. Carroll, Peter J. Griffin, Gas Liquids Engineering, Calgary, Canada
 DEHYDRATION OF ACID GAS PRIOR TO INJECTION Eugene W. Grynia, John J. Carroll, Peter J. Griffin, Gas Liquids Engineering, Calgary, Canada Acid gas is a mixture of hydrogen sulfide and carbon dioxide, with
DEHYDRATION OF ACID GAS PRIOR TO INJECTION Eugene W. Grynia, John J. Carroll, Peter J. Griffin, Gas Liquids Engineering, Calgary, Canada Acid gas is a mixture of hydrogen sulfide and carbon dioxide, with
OBSOLETE DESIGN DATA FOAM MAKER APPLICATION
 Foam 410 a 1. DESCRIPTION Foam makers are in-line foam dis - charge devices. Foam makers are utilized in hazards where enhanced foam qualities are needed to protect a hazard. Foam makers are gen er - ally
Foam 410 a 1. DESCRIPTION Foam makers are in-line foam dis - charge devices. Foam makers are utilized in hazards where enhanced foam qualities are needed to protect a hazard. Foam makers are gen er - ally
THE WAY THE VENTURI AND ORIFICES WORK
 Manual M000 rev0 03/00 THE WAY THE VENTURI AND ORIFICES WORK CHAPTER All industrial combustion systems are made up of 3 main parts: ) The mixer which mixes fuel gas with combustion air in the correct ratio
Manual M000 rev0 03/00 THE WAY THE VENTURI AND ORIFICES WORK CHAPTER All industrial combustion systems are made up of 3 main parts: ) The mixer which mixes fuel gas with combustion air in the correct ratio
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 US 2015O129357A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0129357 A1 ROth (43) Pub. Date: May 14, 2015 (54) GUIDED TYPE FALL ARRESTER - BODY (52) U.S. Cl. CONTROL SYSTEM
US 2015O129357A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0129357 A1 ROth (43) Pub. Date: May 14, 2015 (54) GUIDED TYPE FALL ARRESTER - BODY (52) U.S. Cl. CONTROL SYSTEM
*EP A2* EP A2 (19) (11) EP A2 (12) EUROPEAN PATENT APPLICATION. (43) Date of publication: Bulletin 2003/34
 (19) Europäisches Patentamt European Patent Office Office européen des brevets *EP00133679A2* (11) EP 1 336 79 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 20.08.2003 Bulletin 2003/34
(19) Europäisches Patentamt European Patent Office Office européen des brevets *EP00133679A2* (11) EP 1 336 79 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 20.08.2003 Bulletin 2003/34
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States US 20060049223A1. (12) Patent Application Publication (10) Pub. No.: US 2006/0049223 A1 Mora et al. (43) Pub. Date: Mar. 9, 2006 (54) (76) (21) (22) (60) SCORECARD HOLDER FOR GOLF Inventors:
(19) United States US 20060049223A1. (12) Patent Application Publication (10) Pub. No.: US 2006/0049223 A1 Mora et al. (43) Pub. Date: Mar. 9, 2006 (54) (76) (21) (22) (60) SCORECARD HOLDER FOR GOLF Inventors:
Kinetic-Molecular Theory
 GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
INTEROFFICE MEMORANDUM MODIFICATIONS TO DEW POINT CONTROL PROCESS
 INTEROFFICE MEMORANDUM TO: FROM: SUBJECT: NEW ENGINEER I.M. ANOLDGUY MODIFICATIONS TO DEW POINT CONTROL PROCESS DATE: JANUARY 10, 2017 We are looking to make modifications to our initial design for a DPC
INTEROFFICE MEMORANDUM TO: FROM: SUBJECT: NEW ENGINEER I.M. ANOLDGUY MODIFICATIONS TO DEW POINT CONTROL PROCESS DATE: JANUARY 10, 2017 We are looking to make modifications to our initial design for a DPC
Focus on VOC Emissions Reduction Using an Oxygen Based Inerting Control System For Inert Gas Blanketing of Chemical Process Vessels
 Inerting Control Systems by NTRON PROCESS ANALYZER DIVISION of NEUTRONICS INC. EXTON. PA. 19341 RJN Rev. A June 2001 Focus on VOC Emissions Reduction Using an Oxygen Based Inerting Control System For Inert
Inerting Control Systems by NTRON PROCESS ANALYZER DIVISION of NEUTRONICS INC. EXTON. PA. 19341 RJN Rev. A June 2001 Focus on VOC Emissions Reduction Using an Oxygen Based Inerting Control System For Inert
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 US 2011 0315140A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/031514.0 A1 Shuman (43) Pub. Date: Dec. 29, 2011 (54) PORTABLE OXYGEN CONCENTRATOR (52) U.S. Cl.... 128/204.23;
US 2011 0315140A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/031514.0 A1 Shuman (43) Pub. Date: Dec. 29, 2011 (54) PORTABLE OXYGEN CONCENTRATOR (52) U.S. Cl.... 128/204.23;
Inerting System Design for Medium Speed Vertical Spindle Coal Pulverizers TABLE OF CONTENTS
 Inerting System Design for Medium Speed Vertical Spindle Coal Pulverizers The PRB Coal Users Group plans to develop a Design Guide for Mill Inerting as an aid to users when designing a mill inerting system.
Inerting System Design for Medium Speed Vertical Spindle Coal Pulverizers The PRB Coal Users Group plans to develop a Design Guide for Mill Inerting as an aid to users when designing a mill inerting system.
AP TOPIC 6: Gases. Revised August General properties and kinetic theory
 AP OPIC 6: Gases General properties and kinetic theory Gases are made up of particles that have (relatively) large amounts of energy. A gas has no definite shape or volume and will expand to fill as much
AP OPIC 6: Gases General properties and kinetic theory Gases are made up of particles that have (relatively) large amounts of energy. A gas has no definite shape or volume and will expand to fill as much
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 (19) United States US 2005.0065403A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0065403 A1 Takase et al. (43) Pub. Date: Mar. 24, 2005 (54) ENDOSCOPE FOR STERILIZING BUILT-IN ELONGATED
(19) United States US 2005.0065403A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0065403 A1 Takase et al. (43) Pub. Date: Mar. 24, 2005 (54) ENDOSCOPE FOR STERILIZING BUILT-IN ELONGATED
Nov. 6, 1962 P. T. JOHNSON 3,061,954 CONTROL MECHANISM FOR SCRAPER BOWL AND APRON FEU. is stics E. E. H. H. E. INVENTOR. ATTORNEY...
 Nov. 6, 1962 P. T. JOHNSON 3,061,954 CONTROL MECHANISM FOR SCRAPER BOWL AND APRON Filed April 7, 196l. 2 Sheets-Sheet S FEU st is stics E. E. H. H. E. INVENTOR. BY ATTORNEY... Nov. 6, 1962 P. T. JOHNSON
Nov. 6, 1962 P. T. JOHNSON 3,061,954 CONTROL MECHANISM FOR SCRAPER BOWL AND APRON Filed April 7, 196l. 2 Sheets-Sheet S FEU st is stics E. E. H. H. E. INVENTOR. BY ATTORNEY... Nov. 6, 1962 P. T. JOHNSON
Describe Flammable Gas Measurement
 Training Module Describe Flammable 100% 90% C 80% No Combustion 70% 60% 50% 40% 30% 20% UEL B Combustion No Combustion 10% 0% LEL A Human Development HDC Human Development All rights reserved. No part
Training Module Describe Flammable 100% 90% C 80% No Combustion 70% 60% 50% 40% 30% 20% UEL B Combustion No Combustion 10% 0% LEL A Human Development HDC Human Development All rights reserved. No part
United States Patent (19) Kaneko
 United States Patent (19) Kaneko 54 SHOE SOLE FOR RUNNING SHOES 75 Inventor: Yasunori Kaneko, Osaka, Japan 73 Assignee: Mizuno Corporation, Osaka, Japan 21 Appl. No.: 664,016 22 Filed: Jun. 13, 1996 30
United States Patent (19) Kaneko 54 SHOE SOLE FOR RUNNING SHOES 75 Inventor: Yasunori Kaneko, Osaka, Japan 73 Assignee: Mizuno Corporation, Osaka, Japan 21 Appl. No.: 664,016 22 Filed: Jun. 13, 1996 30
Example: 25 C = ( ) K = 298 K. Pressure Symbol: p Units: force per area 1Pa (Pascal) = 1 N/m 2
 Chapter 6: Gases 6.1 Measurements on Gases MH5, Chapter 5.1 Let s look at a certain amount of gas, i.e. trapped inside a balloon. To completely describe the state of this gas one has to specify the following
Chapter 6: Gases 6.1 Measurements on Gases MH5, Chapter 5.1 Let s look at a certain amount of gas, i.e. trapped inside a balloon. To completely describe the state of this gas one has to specify the following
Inventor Laurent Bissonnette NOTICE
 Serial No. 8.0 Filing Date 1 June 9 Inventor Laurent Bissonnette NOTICE The above identified patent application is available for licensing. Requests for information should be addressed to: OFFICE OF NAVAL
Serial No. 8.0 Filing Date 1 June 9 Inventor Laurent Bissonnette NOTICE The above identified patent application is available for licensing. Requests for information should be addressed to: OFFICE OF NAVAL
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0176367 A1 PENNINGTON et al. US 201701.76367A1 (43) Pub. Date: Jun. 22, 2017 (54) (71) (72) (21) (22) (60) APPARATUS TO MEASURE
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0176367 A1 PENNINGTON et al. US 201701.76367A1 (43) Pub. Date: Jun. 22, 2017 (54) (71) (72) (21) (22) (60) APPARATUS TO MEASURE
Air Eliminators and Combination Air Eliminators Strainers
 Description Air Eliminators and Combination Air Eliminator Strainers are designed to provide separation, elimination and prevention of air in piping systems for a variety of installations and conditions.
Description Air Eliminators and Combination Air Eliminator Strainers are designed to provide separation, elimination and prevention of air in piping systems for a variety of installations and conditions.
Experimental Analysis on Vortex Tube Refrigerator Using Different Conical Valve Angles
 International Journal of Engineering Research and Development e-issn: 7-067X, p-issn: 7-00X, www.ijerd.com Volume 3, Issue 4 (August ), PP. 33-39 Experimental Analysis on Vortex Tube Refrigerator Using
International Journal of Engineering Research and Development e-issn: 7-067X, p-issn: 7-00X, www.ijerd.com Volume 3, Issue 4 (August ), PP. 33-39 Experimental Analysis on Vortex Tube Refrigerator Using
Diagnosing EVAP systems By: Bernie Thompson
 Diagnosing EVAP systems By: Bernie Thompson Perhaps one of the toughest jobs in the automotive field is diagnosing small leaks, especially A/C and Fuel Vapor (EVAP) leaks. Finding these leaks can be time
Diagnosing EVAP systems By: Bernie Thompson Perhaps one of the toughest jobs in the automotive field is diagnosing small leaks, especially A/C and Fuel Vapor (EVAP) leaks. Finding these leaks can be time
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States US 20090235422A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0235422 A1 Lueking (43) Pub. Date: (54) APPARATUS AND METHODS FOR HOLDING Publication Classification SHN GUARDS
(19) United States US 20090235422A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0235422 A1 Lueking (43) Pub. Date: (54) APPARATUS AND METHODS FOR HOLDING Publication Classification SHN GUARDS
Compressed Gases Storage and Handling Safety Policy. Individual Unit Function: Safety Procedure No.: SOP-0103 Page: 1 of 6
 Procedure No.: SOP-0103 Page: 1 of 6 MIDGA Reviewed: 07/09/18 Effective: 1/14/98 Supersedes: Original Preparer: Owner: Approver: Safety Safety Safety 1. PURPOSE The purpose of this procedure is to establish
Procedure No.: SOP-0103 Page: 1 of 6 MIDGA Reviewed: 07/09/18 Effective: 1/14/98 Supersedes: Original Preparer: Owner: Approver: Safety Safety Safety 1. PURPOSE The purpose of this procedure is to establish
(12) United States Patent
 US008807568B1 (12) United States Patent Ruder (10) Patent No.: (45) Date of Patent: Aug. 19, 2014 (54) BALL GAME (71) Applicant: Christofer Joseph Ruder, Chicago, IL (US) (72) Inventor: Christofer Joseph
US008807568B1 (12) United States Patent Ruder (10) Patent No.: (45) Date of Patent: Aug. 19, 2014 (54) BALL GAME (71) Applicant: Christofer Joseph Ruder, Chicago, IL (US) (72) Inventor: Christofer Joseph
March 13, Filed July l, T. A. FOX 3,024,679 SKIN PASS MILLS AND METHOD OF ROLLING 3 Sheets-Sheet. NWeNTOr Thomas A.
 March 13, 1962 Filed July l, 1957 T. A. FOX 3,024,679 SKIN PASS MILLS AND METHOD OF ROLLING 3 Sheets-Sheet Sh NWeNTOr Thomas A. Fox March 13, 1962 T. A. FOX 3,024,679 SKIN PASS MILLS AND METHOD OF ROLLING
March 13, 1962 Filed July l, 1957 T. A. FOX 3,024,679 SKIN PASS MILLS AND METHOD OF ROLLING 3 Sheets-Sheet Sh NWeNTOr Thomas A. Fox March 13, 1962 T. A. FOX 3,024,679 SKIN PASS MILLS AND METHOD OF ROLLING
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0066794 A1 Durfee US 2008 OO66794A1 (43) Pub. Date: Mar. 20, 2008 (54) (76) (21) (22) (60) AUTOMATIC HUNTING BLIND Inventor:
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0066794 A1 Durfee US 2008 OO66794A1 (43) Pub. Date: Mar. 20, 2008 (54) (76) (21) (22) (60) AUTOMATIC HUNTING BLIND Inventor:
22 Filed: Jul. 15, 1993 (5ll Int. Cl... F25, 3/02 52 U.S. C... 62/25; 62/39; 62/4
 United tates Patent (19) Agrawal et al. U534164.6A 11 Patent Number: Date of Patent: Aug. 30, 1994 (54) TRIPLE CLUMN DITILLATIN YTEM FR XYGEN AND PREURIZED NITRGEN PRDUCTIN 75 Inventors: Rakesh Agrawal,
United tates Patent (19) Agrawal et al. U534164.6A 11 Patent Number: Date of Patent: Aug. 30, 1994 (54) TRIPLE CLUMN DITILLATIN YTEM FR XYGEN AND PREURIZED NITRGEN PRDUCTIN 75 Inventors: Rakesh Agrawal,
Fundamentals of Compressed Air Systems. Pre-Workshop Assignment
 Page 1 In order to ensure that the Compressed Air Challenge Fundamentals of Compressed Air Systems Training is most useful to you, it will be important for you to bring information about your plant s compressed
Page 1 In order to ensure that the Compressed Air Challenge Fundamentals of Compressed Air Systems Training is most useful to you, it will be important for you to bring information about your plant s compressed
Natural Gas Desulfurization Process By MEA Amine: The preferable Engineering Design Procedure
 Natural Gas Desulfurization Process By MEA Amine: The preferable Engineering Design Procedure Ribwar Abdulrahman 1, Ibtisam Kamal 2, Jagar Ali 3 1 Faculty of Engineering, KoyaUniversity, Kurdistan region,
Natural Gas Desulfurization Process By MEA Amine: The preferable Engineering Design Procedure Ribwar Abdulrahman 1, Ibtisam Kamal 2, Jagar Ali 3 1 Faculty of Engineering, KoyaUniversity, Kurdistan region,
Jan. 12, 1965 E. F. RES 3,165,112 WALKER OR WALKER AID. Filed July 20, 196l. INVENTOR. El-Mear F Rt as. 62. (Pavel. A440/wey
 Jan. 12, 1965 E. F. RES WALKER OR WALKER AID Filed July, 196l. BY INVENTOR. El-Mear F Rt as 62. (Pavel A4/wey United States Patent Office 1 WALKER ORWALKERAID d Eimer Manufacturing F. Ries, Cincinnati,
Jan. 12, 1965 E. F. RES WALKER OR WALKER AID Filed July, 196l. BY INVENTOR. El-Mear F Rt as 62. (Pavel A4/wey United States Patent Office 1 WALKER ORWALKERAID d Eimer Manufacturing F. Ries, Cincinnati,
Ball Beating Lubrication in Refrigetation Compressors
 Purdue University Purdue e-pubs International Compressor Engineering Conference School of Mechanical Engineering 1996 Ball Beating Lubrication in Refrigetation Compressors B. Jacobson SKF Engineering &
Purdue University Purdue e-pubs International Compressor Engineering Conference School of Mechanical Engineering 1996 Ball Beating Lubrication in Refrigetation Compressors B. Jacobson SKF Engineering &
ANNEX AMENDMENTS TO THE INTERNATIONAL CODE FOR FIRE SAFETY SYSTEMS (FSS CODE) CHAPTER 15 INERT GAS SYSTEMS
 Annex 3, page 2 ANNEX AMENDMENTS TO THE INTERNATIONAL CODE FOR FIRE SAFETY SYSTEMS (FSS CODE) CHAPTER 15 INERT GAS SYSTEMS The text of existing chapter 15 is replaced by the following: "1 Application This
Annex 3, page 2 ANNEX AMENDMENTS TO THE INTERNATIONAL CODE FOR FIRE SAFETY SYSTEMS (FSS CODE) CHAPTER 15 INERT GAS SYSTEMS The text of existing chapter 15 is replaced by the following: "1 Application This
2/4% May 9, 1967 W. P. MOORE ETAL 3,318,887. Charles B. R. Fitz-WilliamJr. William P. Moore. Filed July 17, 1964 CYANURIC ACID PRODUCTION ATTORNEY
 May 9, 1967 W. P. MOORE ETAL 3,318,887 CYANURIC ACID PRODUCTION Filed July 17, 1964 NVENTORS William P. Moore Charles B. R. Fitz-WilliamJr. 2/4% ATTORNEY United States Patent Office 3,318,887 Patented
May 9, 1967 W. P. MOORE ETAL 3,318,887 CYANURIC ACID PRODUCTION Filed July 17, 1964 NVENTORS William P. Moore Charles B. R. Fitz-WilliamJr. 2/4% ATTORNEY United States Patent Office 3,318,887 Patented
United States Patent (19) Salandre
 United States Patent (19) Salandre 11) Patent Number: 45) Date of Patent: Jun. 19, 1990 (54 HANDGUN HOLSTER AND RETENTION APPARATUS 76 Inventor: Stephen M. Salandre, P.O. Box 130, Taos, N. Mex. 87571 (21)
United States Patent (19) Salandre 11) Patent Number: 45) Date of Patent: Jun. 19, 1990 (54 HANDGUN HOLSTER AND RETENTION APPARATUS 76 Inventor: Stephen M. Salandre, P.O. Box 130, Taos, N. Mex. 87571 (21)
United States Patent (19)
 United States Patent (19) Schmidtke 54 (75) (73) (21) 22 (86) (87) (51) (52) (58) (56) METHOD AND DEVICE FOR DISSOLVNG GAS, ESPECIALLY CARBON DIOXIDE, IN LIQUID FUEL AND FOR DISTRIBUTING THE FUEL IN A
United States Patent (19) Schmidtke 54 (75) (73) (21) 22 (86) (87) (51) (52) (58) (56) METHOD AND DEVICE FOR DISSOLVNG GAS, ESPECIALLY CARBON DIOXIDE, IN LIQUID FUEL AND FOR DISTRIBUTING THE FUEL IN A
Inprocess Operator Training Programme
 2016 Inprocess Operator Training Programme ABSORPTION COLUMN These exercises are intended to provide an understanding of absorption columns and the fundamental principles they use to eliminate pollutants
2016 Inprocess Operator Training Programme ABSORPTION COLUMN These exercises are intended to provide an understanding of absorption columns and the fundamental principles they use to eliminate pollutants
Feb. 27, 1973 R. L. CRNDORFF, JR 3,717,943 MUD RESISTANT ELASTOMERS INVENTOR. Roy L.ORNDORFF, JR. ATTY.
 Feb. 27, 1973 R. L. CRNDORFF, JR Filed Aug. 23. l97 2. Sheets-Sheet l INVENTOR Roy L.ORNDORFF, JR. ATTY. Feb. 27, 1973 R. L. ORNDORFF, JR Filed Aug. 23. l97) 2 Sheets-Sheet 2-32 2M 223 %2-Z ZZ 222s INVENTOR
Feb. 27, 1973 R. L. CRNDORFF, JR Filed Aug. 23. l97 2. Sheets-Sheet l INVENTOR Roy L.ORNDORFF, JR. ATTY. Feb. 27, 1973 R. L. ORNDORFF, JR Filed Aug. 23. l97) 2 Sheets-Sheet 2-32 2M 223 %2-Z ZZ 222s INVENTOR
States of Matter. Q 7. Calculate the average of kinetic energy, in joules of the molecules in 8.0 g of methane at 27 o C. (IIT JEE Marks)
 Q 1. States of Matter Calculate density of NH 3 at 30 o C and 5 atm pressure Q 2. (IIT JEE 1978 3 Marks) 3.7 g of a gas at 25 o C occupied the same volume as 0.184g of hydrogen at 17 o C and at the same
Q 1. States of Matter Calculate density of NH 3 at 30 o C and 5 atm pressure Q 2. (IIT JEE 1978 3 Marks) 3.7 g of a gas at 25 o C occupied the same volume as 0.184g of hydrogen at 17 o C and at the same
UNIT 10 - GASES. Notes & Worksheets - Honors
 Ideal Gas Equation 1 WKSHT 1.) What is the pressure exerted by 2.0 moles of an ideal gas when it occupies a volume of 12.0 L at 373 K? 2.) A flashbulb of volume 2.6 cm 3 contains O 2 gas at a pressure
Ideal Gas Equation 1 WKSHT 1.) What is the pressure exerted by 2.0 moles of an ideal gas when it occupies a volume of 12.0 L at 373 K? 2.) A flashbulb of volume 2.6 cm 3 contains O 2 gas at a pressure
33S z (12) United States Patent US 8,828, 150 B2. Sep. 9, (45) Date of Patent: (10) Patent No.: Foerster et al.
 USOO8828OB2 (12) United States Patent Foerster et al. () Patent No.: () Date of Patent: Sep. 9, 2014 (54) METHOD FOR CARBURIZING WORKPIECES AND ITS APPLICATION (75) Inventors: Lothar Foerster, Stuttgart
USOO8828OB2 (12) United States Patent Foerster et al. () Patent No.: () Date of Patent: Sep. 9, 2014 (54) METHOD FOR CARBURIZING WORKPIECES AND ITS APPLICATION (75) Inventors: Lothar Foerster, Stuttgart
Instructor s t Background
 Teknologi Pemrosesan Gas (TKK 564) Instructor: Dr. Istadi (http://tekim.undip.ac.id/staf/istadi ) Email: il iistadi@undip.ac.id di di id Instructor s t Background BEng. (1995): Universitas Diponegoro Meng.
Teknologi Pemrosesan Gas (TKK 564) Instructor: Dr. Istadi (http://tekim.undip.ac.id/staf/istadi ) Email: il iistadi@undip.ac.id di di id Instructor s t Background BEng. (1995): Universitas Diponegoro Meng.
BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY
 BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY Dr.R.Selvakumar.M.D.D.A.DNB Professor of Anaesthesiology, K.A.P.Viswanatham Govt medical college, Trichy. The current practice of Anaesthesiology demands knowledge
BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY Dr.R.Selvakumar.M.D.D.A.DNB Professor of Anaesthesiology, K.A.P.Viswanatham Govt medical college, Trichy. The current practice of Anaesthesiology demands knowledge
maintaining storage tank dissolved oxygen levels utilizing gas transfer membranes
 Water Technologies & Solutions technical paper maintaining storage tank dissolved oxygen levels utilizing gas transfer membranes Authors: W.E. Haas, SUEZ, J. Helmrich, Florida Power and Light and J.E.
Water Technologies & Solutions technical paper maintaining storage tank dissolved oxygen levels utilizing gas transfer membranes Authors: W.E. Haas, SUEZ, J. Helmrich, Florida Power and Light and J.E.
