Issuance of Four Section 10(a)(1)(A) Permits for Spring Chinook Salmon Hatchery Programs in the Methow Subbasin
|
|
|
- Branden Sanders
- 5 years ago
- Views:
Transcription
1 Endangered Species Act (ESA) Section 7(a)(2) Biological Opinion and Magnuson-Stevens Fishery Conservation and Management Act Essential Fish Habitat (EFH) Consultation Issuance of Four Section 10(a)(1)(A) Permits for Spring Chinook Salmon Hatchery Programs in the Methow Subbasin NMFS Consultation Number: WCR Action Agencies: Program Operators: National Marine Fisheries Service U.S. Fish and Wildlife Service U.S. Bureau of Reclamation Public Utility District No. 1 of Douglas County Public Utility District No. 2 of Grant County Public Utility District No. 1 of Chelan County Washington Department of Fish and Wildlife U.S. Fish and Wildlife Service Confederated Tribes and Bands of the Yakama Nation Affected Species and Determinations: ESA-Listed Species Status Is Action Likely to Adversely Affect Species or Critical Habitat? Upper Columbia River steelhead (Oncorhynchus mykiss) Is Action Likely To Jeopardize the Species? Threatened Yes No No Is Action Likely To Destroy or Adversely Modify Critical Habitat? Upper Columbia River spring Chinook salmon (O. tshawytscha) Endangered Yes No No Fishery Management Plan That Describes EFH in the Project Area Does Action Have an Adverse Effect on EFH? Are EFH Conservation Recommendations Provided? Pacific Coast salmon Yes Yes Consultation Conducted By: The National Marine Fisheries Service, West Coast Region Issued By: Barry A. Thom Regional Administrator Date: 10/13/2016 i
2 This page intentionally left blank. ii
3 TABLE OF CONTENTS 1. INTRODUCTION Background Consultation History Proposed Action Methow Hatchery spring Chinook salmon program WNFH spring Chinook salmon program Action Area Interrelated and Interdependent Actions ENDANGERED SPECIES ACT: BIOLOGICAL OPINION AND INCIDENTAL TAKE STATEMENT Introduction to the Biological Opinion Range-wide Status of the Species and Critical Habitat Status of Listed Species Life History and Status of UCR Chinook Salmon Life History and Status of UCR Steelhead Status of Critical Habitat Critical Habitat for Upper Columbia River Spring Chinook Salmon Critical Habitat for Upper Columbia River Steelhead Climate Change Environmental Baseline Description of Area and Effects on the Landscape and Habitat Methow Subbasin Entiat River Basin Recent habitat restoration activities Carlton Complex fire Artificial Propagation History of hatcheries in the Upper Columbia Spring Chinook salmon Summer Chinook salmon Steelhead Coho salmon Harvest Other Effects on ESA Protected Species and on Designated Critical Habitat Factors Considered When Analyzing Hatchery Effects iii
4 Factor 1. The hatchery program does or does not remove fish from the natural population and use them for hatchery broodstock Factor 2. Hatchery fish and the progeny of naturally spawning hatchery fish on spawning grounds and encounters with natural-origin and hatchery fish at adult collection facilities Genetic effects Ecological effects Adult Collection Facilities Factor 3. Hatchery fish and the progeny of naturally spawning hatchery fish in juvenile rearing areas Competition Predation Disease Acclimation Factor 4. Hatchery fish and the progeny of naturally spawning hatchery fish in the migration corridor, in the estuary, and in the ocean Factor 5. Research, monitoring, and evaluation that exists because of the hatchery program Observing/Harassing Capturing/handling Fin clipping and tagging Factor 6. Construction, operation, and maintenance, of facilities that exist because of the hatchery program Factor 7. Fisheries that exist because of the hatchery program Effects of the Proposed Action Factor 1. The hatchery program does or does not remove fish from the natural population and use them for hatchery broodstock Factor 2. Hatchery fish and the progeny of naturally spawning hatchery fish on spawning grounds and encounters with natural-origin and hatchery fish at adult collection facilities Genetic interactions between hatchery- and natural-origin adults Ecological interactions between hatchery- and natural-origin adults Encounter of listed species at adult collection facilities Factor 3. Hatchery fish and the progeny of naturally spawning hatchery fish in juvenile rearing areas Impacts from released hatchery fish Impacts from progeny of naturally spawning hatchery fish Factor 4. Hatchery fish and the progeny of naturally spawning hatchery fish in the migration corridor, estuary, and ocean Factor 5. Research, monitoring, and evaluation that exists because of the hatchery program 76 iv
5 Factor 6. Construction, operation, and maintenance of facilities that exist because of the hatchery programs Factor 7. Fisheries that exist because of the hatchery program Effects of the Action on Critical Habitat Cumulative Effects Integration and Synthesis UCR Spring Chinook Salmon UCR Steelhead Critical Habitat Climate Change Conclusion Incidental Take Statement Amount or Extent of Take Hatchery fish and the progeny of naturally spawning hatchery fish on spawning grounds and encounters with natural-origin and hatchery fish at adult collection facilities (Factor 2) Hatchery fish and the progeny of naturally spawning hatchery fish in juvenile rearing areas (Factor 3) RM&E that exists because of the hatchery program (Factor 5) Effect of the Take Reasonable and Prudent Measures Terms and Conditions Conservation Recommendations Reinitiation of Consultation MAGNUSON-STEVENS FISHERY CONSERVATION AND MANAGEMENT ACT ESSENTIAL FISH HABITAT CONSULTATION Essential Fish Habitat Affected by the Project Adverse Effects on Essential Fish Habitat Essential Fish Habitat Conservation Recommendations Statutory Response Requirement Supplemental Consultation DATA QUALITY ACT DOCUMENTATION AND PRE-DISSEMINATION REVIEW Utility Integrity Objectivity REFERENCES Tables v
6 Table 1. Total Methow Subbasin broodstock collection necessary to meet production targets for the Methow Hatchery spring Chinook conservation program Table 2. Methow Hatchery spring Chinook salmon release schedule Table 3. Expected adult returns for the Methow program, based on historical (brood years ) smoltadult survival rates (SAR; DPUD and WDFW 2010a, Tables 1-11,12,13) Table 4. Water use associated with facilities operated as part of the Proposed Action Table 5. Target partial phos for WNFH returns based on natural run size Table 6. Stream reaches of the Entiat and Methow Subbasins included in the action area. Stream reach information on use by anadromous fish is from (RTT 2013) Table 7. ESA- listed species considered in this consultation, their listing status, critical habitation designation, and protective regulations Table 8. Risk levels and viability ratings for natural-origin UCR spring Chinook salmon populations from (NWFSC 2015) Table 9. Estimates of the percent natural-origin spawners for UCR spring Chinook salmon populations (NWFSC 2015) Table 10. Risk levels and viability ratings for natural-origin UCR steelhead populations from (NWFSC 2015) Table 11. Estimates of the percent natural-origin spawners for UCR steelhead populations (NWFSC 2015) Table 12. PCEs of critical habitat designated for ESA-listed salmon and steelhead considered in this opinion Table 13. An overview of the range of effects on natural population viability parameters from the two categories of hatchery programs Table 14. Estimated contribution of Carson stock to Methow program broodstock (modified from Table 1 of WDFW 2003) Table 15. Proportionate natural influence (PNI) as a function of varying pnob and phos Table 16. Maximum allowable proportion of returning hatchery-origin fish to be removed, after broodstock collection, to achieve specified levels of pnob without mining natural-origin fish (refer to text for explanation) Table 17. Total phosphorous imported by adult returns from the proposed hatchery programs based on the equation in Scheuerell et al. (2005) Table 18. Number of adult (unless otherwise noted) spring Chinook salmon and steelhead handled and mortalities resulting from handling at each of the adult collection facilities. Numbers are an average over the most recent 6-10 years with the range in parentheses (Frady 2016; Humling 2016c) Table 19. Fish numbers, lengths, and residence, for hatchery juvenile spring Chinook salmon released by the programs Table 20. Mean fork length (mm) and percentage of population (shown in parentheses) for natural-origin Methow spring Chinook salmon and steelhead juveniles potentially involved in ecological interactions. 74 Table 21. Juvenile encounter and mortality data for the Twisp and Methow River smolt traps, electrofishing and angling combined in Table 22. Water use associated with facilities operated as part of the Proposed Action Table 23. UCR spring Chinook salmon and steelhead handling and mortality associated with adult collection for the Proposed Action Figures Figure 1. Methow Subbasin, and location of hatchery facilities discussed in this opinion Figure 2. Gene-flow management regime for the PUD spring Chinook salmon program Figure 3. Upper Columbia River Spring Chinook Salmon ESU (ICTRT 2008) vi
7 Figure 4. Matrix used to assess population status across VSP parameters for UCR spring Chinook salmon. Percentages for abundance and productivity scores represent the probability of extinction in a 100-year time period (NWFSC 2015) Figure 5. Upper Columbia River steelhead DPS (ICTRT 2008) Figure 6. Matrix used to assess population status across VSP parameters for UCR steelhead. Percentages for abundance and productivity scores represent the probability of extinction in a 100-year time period (NWFSC 2015) (ICTRT 2007b) Figure 7. Perimeter map of Carlton Complex fire ( accessed August 27, 2014) Figure 8. Annual spring Chinook salmon releases into the Methow Subbasin, Figure 9. Releases of summer/fall Chinook salmon from the Methow (Carlton) acclimation pond (Snow et al. 2014) Figure 10. Total summer steelhead smolt releases into the Methow Subbasin (Snow et al. 2014; USFWS 2012c) Figure 11. Mean summer steelhead smolt releases by location (DART, (Snow et al. 2014; USFWS 2012c) Figure 12. Annual coho salmon smolt releases into the Methow Subbasin (Kamphaus 2013). 37 Figure 13. ICTRT (2007b) risk criteria associated with spawner composition for viability assessment of exogenous spawners on maintaining natural patterns of gene flow. Exogenous fish are considered to be all fish hatchery origin, and non-normative strays of natural origin Figure 14. Relative proportions of types of matings as a function of proportion of hatchery-origin fish on the spawning grounds (phos) Figure 15. Stepping stone relationship proposed for WNFH and Methow spring Chinook salmon programs (USFWS 2012c) Figure 16. Methow Subbasin spring Chinook salmon spawners (all ages) Dashed and solid lines represent hatchery- and natural-origin spawners, respectively Figure 17. phos among spring Chinook salmon in the Methow Subbasin, (Snow et al. 2014). Dashed line is projection on what phos would have been had no natural-origin fish been taken for broodstock, and the solid line is for realized phos Figure 18. Proportionate contribution of the Methow and WNFH spring Chinook salmon hatchery programs to Entiat spring Chinook salmon escapement (Cooper 2014a; Snow 2014) Figure 19. Distribution of hatchery- and natural-origin spring Chinook salmon spawners in the Methow (upper), Chewuch (middle), and Twisp Rivers (lower) (Snow et al. 2013; CH. 5 Tables 6-8). WNFH and Methow Hatchery are on the Methow River at about Rkm 82 (all distances are in river kilometers from the mouth of the Methow River) Figure 20. Water routing for Methow and Winthrop National Fish Hatcheries (Humling 2016b).79 vii
8 Acronyms and Abbreviations BKD BPA BOR CBFWA CCT CFR CFS CIG DPUD CHART CPUD CWT DO DPS DPUD EFH EIS ELISA EPA ESA ESU FCRPS FERC FPA FR GPUD HCP HCP HC HETT HGMP HOR HPA HSRG ICTRT IHOT ISAB ITP Bacterial Kidney Disease Bonneville Power Administration Bureau of Reclamation Columbia Basin Fish and Wildlife Authority Confederated Colville Tribes Code of Federal Regulations Cubic feet per second Climate Impacts Group Public Utility District No. 1 of Douglas County Critical Habitat Analytical Review Team Public Utility District No. 1 of Chelan County coded-wire tag (or tagged) dissolved oxygen distinct population segment Public Utility District No. 1 of Douglas County essential fish habitat Environmental Impact Statement Enzyme-Linked Immunosorbent Assay U.S. Environmental Protection Agency Endangered Species Act evolutionarily significant unit Federal Columbia River Power System Federal Energy Regulatory Commission Federal Power Act Federal Register Public Utility District No. 2 of Grant County Habitat Conservation Plan Habitat Conservation Plan Hatchery Committee Hatchery Evaluation Technical Team Hatchery and Genetic Management Plan hatchery-origin return Hydraulic Project Approval Hatchery Scientific Review Group Interior Columbia Technical Review Team Integrated Hatchery Operations Team Independent Scientific Advisory Board Incidental Take Permit ITS JFP LCR MaSA MCR MiSa MPG MSA NMFS NNI NOAA NMFS NOR NOS NPCC NPDES NRC NWIFC PCE PFMC phob phos PIT PNI pnob PUD PRCC RM&E RRS RTT SCA UCR UCSRB USFWS WDFW WNFH YN Incidental Take Statement Joint Fisheries Parties Lower Columbia River Major Spawning Area Middle Columbia River Minor Spawning Area Major Population Group Magnuson-Stevens Fishery Conservation Act National Marine Fisheries Service No Net Impact National Oceanic and Atmospheric Administration National Marine Fisheries Service Natural-origin recruit Natural-origin spawner Northwest Power Conservation Council National Pollutant Discharge Elimination System National Research Council Northwest Indian Fisheries Commission Primary constituent element Pacific Fishery Management Council Proportion of hatchery-origin broodstock Proportion of hatchery-origin spawners Passive Integrated Transponder (tag) Proportionate natural influence Proportion of natural-origin broodstock Public Utility District Priest Rapids Coordinating Committee Research, monitoring and evaluation Relative reproductive success Recovery Technical Team Supplemental Comprehensive Analysis Upper Columbia River Upper Columbia Salmon Recovery Board United States Fish and Wildlife Service Washington Department of Fish and Wildlife Winthrop National Fish Hatchery Yakama Nation (Confederated Tribes and Bands of the Yakama Nation) viii
9 1. INTRODUCTION This introduction section provides information relevant to the other sections of this document, and is incorporated by reference into Sections 2 and 3. The Proposed Actions are: (1) the approval of four section 10(a)(1)(A) permits by NMFS for the continued operation of two hatchery programs (Methow Hatchery and WNFH), which are intended to enhance UCR spring Chinook salmon that are listed as an endangered species under the ESA and; (2) the funding of the WNFH spring Chinook salmon program by the BOR and operation of the WNFH by the USFWS. The permits will allow operation of related hatchery programs that will collect listed adult spring Chinook salmon and release their progeny into the Methow Subbasin of Washington, where they may affect naturally produced listed UCR spring Chinook salmon and steelhead. The programs, as well as research, monitoring and evaluation associated with them is described in the application documents, which consist of three hatchery and genetic management plans (CPUD 2015; DPUD and WDFW 2010; USFWS 2012b), supplemental information (DPUD and WDFW 2012; USFWS 2012a), and a management framework developed in consultation with the applicants and the Yakama Nation (Busack 2013). The permits will also authorize RM&E associated with the hatchery programs. The proposed action also includes funding of the WNFH spring Chinook salmon program by the BOR and operation of the WNFH by the USFWS. A description of effects, benefits, and risks of the three programs is provided in Section of this biological opinion. Both the Methow Hatchery and WNFH spring Chinook salmon programs are intended to increase the abundance of Methow spring Chinook salmon pursuant to goals identified in recovery plans. The Methow program is funded by the GPUD, DPUD, and CPUD as mitigation for dam losses and operates as a supplementation program to increase the number of fish on the spawning grounds. The WNFH program is funded by the BOR as mitigation for fishery losses due to the construction and operation of Grand Coulee Dam and currently serves as a safety-net program, acting as backup for the Methow Hatchery program. Both programs have been operating for some time, but the Proposed Action includes program modifications to reduce hatchery impacts on listed fish Background NMFS prepared the biological opinion and incidental take statement (ITS) portions of this document in accordance with section 7(b) of the ESA of 1973, as amended (16 U.S.C. 1531, et seq.), and implementing regulations at 50 CFR 402. The opinion documents consultation by NMFS, as the Action Agency, on the actions proposed by the program operators and funders. NMFS also completed an Essential Fish Habitat (EFH) consultation. It was prepared in accordance with section 305(b)(2) of the Magnuson-Stevens Fishery Conservation and Management Act (MSA) (16 U.S.C. 1801, et seq.) and implementing regulations at 50 CFR 600. The opinion, ITS, and EFH conservation recommendations are in compliance with section 515 of the Treasury and General Government Appropriations Act of 2001 (Public Law ) ( Data Quality Act ) and underwent pre-dissemination review. The document will be available through NMFS Public Consultation Tracking System. A complete record of this consultation is on file at the Sustainable Fisheries Division (SFD) in Portland, Oregon. 1
10 1.2. Consultation History The first hatchery consultations in the Columbia Basin followed the first listings of Columbia Basin salmon under the ESA. Snake River sockeye salmon were listed as an endangered species on November 20, 1991, Snake River spring/summer Chinook salmon and Snake River fall Chinook salmon were listed as threatened species on April 22, 1992, and the first hatchery consultation and opinion was completed on April 7, 1994 (NMFS 1994). The 1994 opinion was superseded by Endangered Species Act Section 7 Biological Opinion on Hatchery Operations in the Columbia River Basin, Consultation Number 383 completed on April 5, 1995 (NMFS 1995b). This opinion determined that hatchery actions jeopardize listed Snake River salmon and required implementation of reasonable and prudent alternatives (RPAs) to avoid jeopardy. A new opinion was completed on March 29, 1999, after UCR steelhead were listed under the ESA (62 FR 43937, August 18, 1997) and following the expiration of the previous opinion on December 31, 1998 (NMFS 1999). That opinion concluded that Federal and non-federal hatchery programs jeopardize Lower Columbia River (LCR) steelhead and Snake River steelhead protected under the ESA and described RPAs necessary to avoid jeopardy. Those measures and conditions included restricting the use of non-endemic steelhead for hatchery broodstock and limiting stray rates of non-endemic salmon and steelhead to less than 5 percent of the annual natural population in the receiving stream. Soon after, NMFS reinitiated consultation when LCR Chinook salmon, UCR spring Chinook salmon, Upper Willamette Chinook salmon, Upper Willamette steelhead, Columbia River chum salmon, and Middle Columbia steelhead were added to the list of endangered and threatened species (Smith 1999). Between 1991 and the summer of 1999, the number of distinct groups of Columbia Basin salmon and steelhead listed under the ESA increased from 3 to 12, and this prompted NMFS to reassess its approach to hatchery consultations. In July 1999, NMFS announced that it intended to conduct five consultations and issue five opinions instead of writing one biological opinion on all hatchery programs in the Columbia River Basin (Smith 1999). Opinions would be issued for hatchery programs in the (1) Upper Willamette, (2) MCR), (3) LCR, (4) Snake River, and (5) UCR, with the UCR NMFS first priority (Smith 1999). Between August 2002 and October 2003, NMFS completed consultations under the ESA for approximately twenty hatchery programs in the UCR. For the MCR, NMFS completed a draft opinion and distributed it to hatchery operators and to funding agencies for review on January 4, 2001, but completion of consultation was put on hold pending several important basin-wide review and planning processes. The increase in ESA listings during the mid to late 1990s triggered a period of investigation, planning, and reporting across multiple jurisdictions and this served to complicate, at least from a resources and scheduling standpoint, hatchery consultations. A review of Federal funded hatchery programs ordered by Congress was underway at about the same time that the 2000 Federal Columbia River Power System (FCRPS) opinion was issued by NMFS (NMFS 2000a). The Northwest Power and Conservation Council (Council) was asked to develop a set of coordinated policies to guide the future use of artificial propagation, and RPA 169 of the FCRPS opinion called for the completion of NMFS-approved hatchery operating plans (i.e., HGMPs) by the end of The RPA required the Action Agencies to facilitate this process, first by assisting in the development of HGMPs, and then by helping to implement identified hatchery reforms (NMFS 2000a). Also at this time, a new U.S. v. Oregon Columbia River Fisheries Management Plan (CRFMP), which included goals for hatchery management, was under negotiation and new information and science on the status and recovery goals for salmon and steelhead was emerging from Technical Recovery Teams (TRTs). Work on HGMPs under the FCRPS opinion was undertaken in cooperation with the Council s Artificial Production Review and Evaluation process, with CRFMP negotiations, and with ESA recovery planning (Foster 2004; Jones Jr. 2002). 2
11 HGMPs were submitted to NMFS under RPA 169; however, many were incomplete and, therefore, were not found to be sufficient 1 for ESA consultation. ESA consultations and an opinion were completed in 2007 for nine hatchery programs that produce a substantial proportion of the total number of salmon and steelhead released into the Columbia River annually. These programs are located in the LCR and MCR and are operated by the FWS and by the Washington Department of Fish and Wildlife (WDFW). NMFS opinion (NMFS 2007) determined that operation of the programs would not jeopardize salmon and steelhead protected under the ESA. On May 5, 2008, NMFS published a Supplemental Comprehensive Analysis (SCA) (NMFS 2008d) and an opinion and RPAs for the FCRPS to avoid jeopardizing ESA-listed salmon and steelhead in the Columbia Basin (NMFS 2008b). The SCA environmental baseline included the past effects of hatchery operations in the Columbia River Basin. Where hatchery consultations have expired or where hatchery operations have yet to undergo ESA section 7consultation, the effects of future operations cannot be included in the baseline. In some instances, effects are ongoing (e.g., returning adults from past hatchery practices) and included in this analysis despite the fact that future operations cannot be included in the baseline. The Proposed Action does not encompass hatchery operations per se, and therefore no incidental take coverage is offered through this biological opinion to hatcheries operating in the region. Instead, we expect the operators of each hatchery to address its obligations under the ESA in separate consultations, as required (see NMFS 2008d, p. 5-40). Because it was aware of the scope and complexity of ESA consultations facing the co-managers and hatchery operators, NMFS offered substantial advice and guidance to help with the consultations. In September 2008, NMFS announced its intent to conduct a series of ESA consultations and that from a scientific perspective, it is advisable to review all hatchery programs (i.e., Federal and non-federal) in the UCR affecting ESA-listed salmon and steelhead concurrently (Walton 2008). In November 2008, NMFS expressed again, the need for reevaluation of UCR hatchery programs and provided a framework for ensuring that these hatchery programs are in compliance with the Federal Endangered Species Act (Jones Jr. 2008). NMFS also promised to share key considerations in analyzing HGMPs and provided those materials to interested parties in February 2009 (Jones Jr. 2009). On April 28, 2010 (Walton 2010), NMFS issued a letter to co-managers, hatchery operators, and hatchery funding agencies that described how NMFS has been working with co-managers throughout the Northwest on the development and submittal of fishery and hatchery plans in compliance with the Federal Endangered Species Act (ESA). NMFS stated, In order to facilitate the evaluation of hatchery and fishery plans, we want to clarify the process, including consistency with U.S. v. Oregon, habitat conservation plans and other agreements. With respect to Development of Hatchery and Harvest Plans for Submittal under the ESA, NMFS clarified: The development of fishery and hatchery plans for review under the ESA should consider existing agreements and be based on best available science; any applicable multiparty agreements should be considered, and the submittal package should explicitly reference how such agreements were considered. In the Columbia River, for example, the U.S. v. Oregon agreement is the starting place for developing hatchery and harvest plans for ESA review." 1 Sufficient means that an HGMP meets the criteria listed at 50 CFR (b)(5)(i), which include (1) the purpose of the hatchery program is described in meaningful and measureable terms, (2) available scientific and commercial information and data are included, (3) the Proposed Action, including any research, monitoring, and evaluation, is clearly described both spatially and temporally, (4) application materials provide an analysis of effects on ESA-listed species, and (5) preliminary review suggests that the program has addressed criteria for issuance of ESA authorization such that public review of the application materials would be meaningful. 3
12 The consultation history for the two Methow Subbasin programs is extensive. Both of these programs have operated under section 10(a)(1)(A) permits. The Methow program has operated under permit 1196 (NMFS 2002c; NMFS 2004a), initially issued in 2002 and amended in NMFS requested a new HGMP in 2008, and DPUD submitted a new HGMP (DPUD and WDFW 2010) on March 12, 2010 (Bickford and Bartlett 2010). The WNFH program operated under section 10(1)(a)(1)(A) permit 1300 (NMFS 2002b), also issued in 2002, and which expired in USFWS submitted a new HGMP to initiate consultation in 2009 (Gale 2009). Extensive discussion took place between NMFS and the fishery managers regarding spring Chinook management in the Methow Subbasin, focused on concerns about the large number of hatchery fish on the spawning grounds, an issue which had been highlighted both by the FCRPS Biological Opinion Supplementary Comprehensive Analysis (SCA) (NMFS 2008d), USFWS Hatchery Review Team (USFWS 2009b), and the Hatchery Scientific Review Group (HSRG 2009). As an outgrowth of this discussion, in 2012 NMFS pressed WDFW, USFWS, and the PUDs to develop methods to reduce the cumulative proportion of hatchery-origin fish on the spawning grounds (phos) to under 25 percent. This lead to a modeling effort evaluating methods for phos reductions from both the Methow and WNFH programs with written summaries (DPUD and WDFW 2012; USFWS 2012a) which became supplemental information for the HGMPs. DPUD, GPUD, and WDFW submitted this supporting information in late 2012 (Bickford et al. 2012) to accompany the 2010 HGMP for the Methow program. At the same time USFWS submitted (Gale 2012) a revised HGMP and supplemental information for the WNFH program (USFWS 2012a; USFWS 2012b). NMFS declared application packages for both programs sufficient for consultation in March 2013 (Jones Jr. 2013a; Jones Jr. 2013b). The modelling of methods to reduce phos involved no tribal biologists, and tribes participated only in the later stages of the review process. Regrettably, the responsibility for this lies completely with NMFS. YN staff voiced concerns that the plans NMFS had declared sufficient were in some areas inconsistent with the U.S v. Oregon production agreement. Another series of discussions began to achieve agreement between YN and NMFS, primarily on the subject of adult management, but also on other details of the programs that were unclear, such as minimum escapement standards. The discussion led to the development by YN Fisheries Resource Management staff of a proposal for management of Methow Subbasin spring Chinook salmon (Yakama Nation 2013). Results of the discussions were summarized in a management framework document (Busack 2013). The HGMPs and supporting materials for both programs were made available for public comment upon publication of a notice of availability in the Federal Register on December 10, 2013 (December 10, FR 74116). The public comment period expired on January 9, NMFS received no comments. Since the comment period, CPUD submitted a separate HGMP to NMFS for review in August NMFS made the HGMP available for public comment on April 18, No comments were received. During this time, NMFS and the applicants reached agreement on the gene-flow management regime detailed later in this document (Section ) 1.3. Proposed Action Action means all activities, of any kind, authorized, funded, or carried out, in whole or in part, by the action agency or action agencies (50 CFR ). Interrelated actions are those that are part of a larger action and depend on the larger action for their justification. Interdependent actions are those that have no independent utility apart from the action under consideration (50 CFR ). See Section 1.5 for a more detailed discussion of interrelated and interdependent actions. 4
13 NMFS describes a hatchery program as a group of fish that have a separate purpose and that may have independent spawning, rearing, marking and release strategies (NMFS 2008b). The operation and management of every hatchery program is unique in time, and specific to an identifiable stock and its native habitat (Flagg et al. 2004). The Proposed Actions are: (1) the approval of four section 10(a)(1)(A) permits by NMFS for the continued operation of two hatchery programs, which are intended to enhance UCR spring Chinook salmon that are listed as an endangered species under the ESA and; (2) the funding of the WNFH spring Chinook salmon program by the BOR and operation of the WNFH by the USFWS. The proposed permits authorize the operation of hatchery programs for UCR spring Chinook salmon conducted at Methow Hatchery (permits 18925, 20462, and 20533) and Winthrop National Fish Hatchery (permit 18927) in the Methow Subbasin. All activities necessary for broodstock collection, rearing, acclimation, and RM&E at sites and facilities affiliated with these programs are also authorized by the permits. Fisheries are not part of the Proposed Action. The effects of the issuance of the four permits are fully described in the summary of the underlying activities presented below. The hatchery programs described in this document use ESA-listed UCR spring Chinook salmon as broodstock. The programs are funded as mitigation for losses of salmon caused by construction and/or operation of Grand Coulee, Wanapum, Wells, Rock Island, Rocky Reach, and Priest Rapids Dams. The hatchery production from these programs is intended to be consistent with the Upper Columbia ESA Recovery Plan for the Upper Columbia (Upper Columbia Salmon Recovery Board 2007) and with the U.S. v. Oregon Management Agreement as modified in January 2009 (U.S. v. Oregon 2009). Both programs are described individually in detail below. Descriptions include the purpose and goals as stated by the operators, history, facilities involved, broodstock collection activities, juvenile release strategies, and marking protocols. The HGMPs contain a considerable amount of detail on fish cultural methods beyond that presented in this section. Research, monitoring, and evaluation activities are also described. Unless otherwise indicated, all information in section 1.3 is from the Methow Hatchery (hereafter, Methow) spring Chinook salmon program HGMP (DPUD and WDFW 2010), the Winthrop NFH spring Chinook salmon program (hereafter WNFH) HGMP (USFWS 2012b), supporting documents discussing adult management in the Methow and WNFH programs (DPUD and WDFW 2012; USFWS 2012a), and a NMFS management framework document (Busack 2013) Methow Hatchery spring Chinook salmon program The goal of the Methow program, as stated by the applicants, is the rebuilding of naturally reproducing populations of Methow River spring Chinook in their native habitats using locally adapted broodstock, while maintaining genetic and ecologic integrity, and supporting harvest where and when consistent with recovery objectives (DPUD and WDFW 2010a). The program was developed as mitigation for passage losses caused by Upper Columbia dams owned and operated by public utility districts in Washington State. More specifically, the stated purpose of the program is to meet the HCP NNI passage-loss mitigation goals established in the Wells HCP in a manner consistent with overall HCP objectives of rebuilding natural populations. In addition to providing mitigation for passage losses at Wells Dam, the Methow Hatchery spring Chinook program also provides NNI mitigation for the Rocky Reach and Rock Island HCPs and for the Priest Rapids Salmon and Steelhead Agreement (SSA). With respect to Douglas PUD, the purpose of this hatchery program is to satisfy the hatchery compensation terms of the Wells HCP, which was executed pursuant to Section 10 of the ESA as a vehicle to permit Douglas PUD to carry out its functions in a manner consistent with the ESA. (DPUD and WDFW 2010a). The Methow program is a conservation program intended to rebuild the natural population using a fully integrated broodstock-collection protocol and consists of two components, Twisp River and Methow/Chewuch Rivers that will be managed with distinct strategies. Differences in the proposed management of these two components (as described below) is necessitated by the following: (1) dissimilarity in the proportion of natural- 5
14 and hatchery-origin recruits (NORs and HORs, respectively) represented in the spawning escapements to the Twisp River versus the remainder of the major spawning areas (MaSAs) within the Methow Subbasin, and (2) the opportunity to control phos in the Twisp via the Twisp weir, an option unavailable within other MaSAs in the Methow Subbasin. The division of habitat into MaSAs and MiSAs (minor spawning aggregates) is useful for better understanding the spatial structure and habitat requirements of the listed species. Fish residence in the MaSas and MiSAs that comprise a population also helps maintain the genetic diversity of the population through local adaptation to a particular spawning area. 6
15 Figure 1. Methow Subbasin, and location of hatchery facilities discussed in this opinion. Proposed hatchery broodstock collection Broodstock origin and number: There are two spring Chinook components operated at the Methow Hatchery: Twisp Conservation and Methow Conservation. The Twisp Conservation component will collect up to 20 Twisp stock natural-origin (preferred) or hatchery-origin broodstock. The Methow component will collect up to 7
16 106 Methow-Chewuch (composite) stock natural-origin (preferred) or hatchery-origin broodstock. These numbers include a 10 percent over-collection, to account for mortality events in the hatchery (Table 1). Table 1. Total Methow Subbasin broodstock collection necessary to meet production targets for the Methow Hatchery spring Chinook conservation program. Component Collection Location Method Number a Methow Conservation Wells Dam, Methow Hatchery, WNFH Fishway trap, weir 106 Twisp Conservation Wells Dam, Twisp Weir, Methow Hatchery, WNFH, Methow Subbasin 8 Fishway trap, weir, hook and line, seine 20 Total 126 a All values based on a current, mean age-4 fecundity of 4,000, an egg-to-smolt survival of 0.90, a 10 percent over-collection allowance for BKD management, a 1:1 male:female ratio, and 95 percent pre-spawn adult survival. Broodstock selection: The maximum extraction rate of natural-origin fish collected for broodstock will not exceed 33 percent of the NORs to the Methow Subbasin or to the Twisp River. In years when natural-origin run size allows a 100 percent natural-origin broodstock (pnob of 1.0), the actual extraction rate will be lower than 33 percent of the NORs. Subject to that constraint, the Twisp component will be managed for a minimum pnob of 0.5, and the Methow component will maximize pnob to the extent possible within the constraints of the sliding scale (see below). Collection of adult broodstock for supplementation programs shall be random, and representative of the run-at-large with respect to migration timing, age structure, morphology, and sex ratio. Method and location for collecting broodstock: The Methow program will collect both natural- and hatcheryorigin broodstock at the Twisp weir, Methow Hatchery and WNFH outfalls, and Wells Dam (Twisp-origin adults collected at Wells Dam are genetically differentiated from Methow/Chewuch spring Chinook). The proposed program may also collect broodstock by other methods such as angling or seining in the Methow Subbasin. Methow Hatchery-origin adults that voluntarily enter the Winthrop National Fish Hatchery (WNFH) will be transferred to Methow Hatchery until the broodstock-collection goals for the Methow Hatchery program have been satisfied, after which WNFH will retain Methow Hatchery-origin adults for use in their segregated program or for removal to control phos. Additionally, Methow Hatchery-origin returns to the Methow Hatchery that exceed broodstock and escapement needs will be provided to the WNFH for their use as broodstock, until their broodstock needs are met. Natural-origin spring Chinook are retained from Wells Dam and the Twisp weir as necessary to achieve the goals of the Twisp program so long as the aggregate collection at Wells Dam and Twisp River weir does not exceed 33 percent of the estimated Twisp River NORs past Wells Dam. Broodstock collection and spawning protocols are developed annually, approved by the HCP Hatchery Committees, and submitted to NMFS to allow for consideration of annual variation in run sizes, ages, and origins (natural and hatchery). All hatchery and natural-origin fish collected at Methow Hatchery, Twisp Weir, and Winthrop NFH for broodstock will be held at the Methow Hatchery. Fish collected at Wells Dam may be held temporarily at Wells Hatchery before being moved to Methow Hatchery. Duration of broodstock collection: Trapping at Wells Dam generally occurs at the east and west ladder traps beginning in early May, or when the first spring Chinook salmon are observed passing Wells Dam, and
17 continues through about the third week of June. Trapping may at the Wells Dam fishway traps at the discretion of the Wells HCP coordinating Committee. Trapping may occur up to 7 days per week and up to 24-hours per day at the Twisp Weir, Methow Hatchery and Wells Hatchery volunteer traps. To meet Methow Hatchery broodstock collection needs for hatchery-origin Methow composite and Twisp River stocks, hatchery fish are generally collected at Methow Hatchery, Winthrop NFH, and the Twisp River weir beginning in May, or at such time as spring Chinook arrive at those locations, and continuing through September. Natural-origin spring Chinook are retained from the Twisp weir as necessary to bolster the Twisp program production so long as the aggregate collection at Wells Dam and Twisp River weir does not exceed 33 percent of the estimated Twisp River NORs past Wells Dam. Encounters, sorting, and handling, with adult and juvenile ESA listed fish: ESA-listed fish will be handled and sorted prior to handling and sorting non-listed species. Traps will be checked and all fish removed at least daily. Non-target fish will be released back to the river unharmed. Trapping will cease at water temperatures exceeding 21 C. The following procedures will be employed to minimize potential adverse impacts on spring Chinook associated with broodstock-collection activities: o All species will be held for a minimal duration in the traps (less than 24 hours) o Traps and holding areas will be locked or secured against tampering or vandalism o All natural-origin spring Chinook salmon in excess of broodstock goals will be released upstream immediately without harm o Spring Chinook salmon will be transferred using water-to-water techniques o Hook-and-line collections (if any) will be conducted under strict oversight by staff Proposed mating protocols All males and females collected for broodstock will be examined weekly during the spawning season to determine ripeness, and all fish will be spawned when ripe. Spawning activities for ESA-listed spring Chinook retained from the Methow Subbasin will normally occur from mid-august to mid-september. In-situ stock separation of ESA-listed WNFH and Methow Hatchery-origin and natural-origin spring Chinook salmon, and out-of basin stray fish is accomplished through scale sample, DNA sample, and/or coded-wire tag (CWT) analysis; only natural- and Methow Hatchery origin adults will be spawned. Only natural x natural and hatchery x natural parental crosses will be made (no hatchery x hatchery crosses) for the Twisp component; though not preferred, some hatchery x hatchery crosses may be necessary for the Methow/Chewuch component in years with very low escapement. Males may be live-spawned on the first spawning day as necessary to make up for a naturally occurring low male-to-female ratio. However, inclusion of jack Chinook salmon in the run-at-large broodstock collections helps to alleviate occasional shortages in adult males. Spawning protocols reflect the need to maintain genetic diversity of the separate spring Chinook populations. A 2 x 2 factorial mating scheme is employed. Thus, when two females are spawned with two males, four separate genetic crosses result: female 1 x male 1; female 1 x male 2; female 2 x male 1; female 2 x male 2. In some cases, not enough females, males, or fish of the necessary stock/origin are available on an individual spawn day, and a standard one-male-to-one-female strategy is employed. After fertilization, the eggs are combined and incubated as individual female lots. 9
18 Proposed protocols for each release group (annually) Life stage released: All fish released will be yearling smolts at approximately 15 fish per pound or as determined by the HCP Hatchery Committee. Acclimation and duration of acclimation: The Twisp Conservation component will be acclimated in Twisp Pond (Table 2). The pond will be shared with summer steelhead yearling smolts from Wells Hatchery as well as coho salmon from WNFH. Spring Chinook salmon will be introduced to the pond in March, as conditions allow (typically on or about March 15). Acclimation time will range from approximately days. A portion of the Methow Conservation component will be acclimated in Pond 13 located at Rkm 84.5 at the Methow Hatchery. Spring Chinook salmon will be introduced to the pond after marking, typically in May or June of the year prior to release. The pond may be shared with summer steelhead yearling smolts from Wells Hatchery. Acclimation will range from approximately days, or longer if fish are introduced to the pond earlier. Other acclimation ponds for the Methow Conservation component include Goat Wall in the Methow River and Chewuch Pond in the Chewuch River. Fish will be transferred from Methow Hatchery to these acclimation ponds between February and March. Acclimation time will range from approximately days. The extent to which fish can be held in the acclimation ponds is dependent on river and water conditions. Fish may need to be released earlier in the event of high discharge. Volitional release: Fish are provided a volitional release for approximately 20 days; the remaining fish are forced out. Marking: All Methow Hatchery smolts will be marked according to a coordinated marking scheme for spring Chinook salmon releases above Wells Dam (determined by the HCP Hatchery Committee) to distinguish specific hatchery crosses and release locations, and to facilitate removal of hatchery-origin fish. Currently, all Methow Hatchery spring Chinook salmon receive a CWT with no external mark; a portion are PIT-tagged. Maximum number released: The number of fish to be released from each Methow Hatchery spring Chinook component is presented in Table 2. Release numbers may fluctuate +/- 10 percent. Table 2. Methow Hatchery spring Chinook salmon release schedule. Age Proposed Release Size (fpp) Class Number Date Location (Rkm) Yearling 109, April-May Methow Hatchery (84.5) Yearling 29, April-May Twisp Acclimation Pond (11) Yearling 25, April-May Goat Wall Acclimation Pond (112) Yearling 60, April-May Chewuch Acclimation Pond (12.9) Release location(s): Fish are currently released from Methow Hatchery, and the Twisp and Chewuch Acclimation Ponds. Fish are also proposed for release from Goat Wall Acclimation Pond (Table 2). Future acclimation facilities within the Methow Subbasin may be developed by others and may receive releases of spring Chinook salmon from the Methow Hatchery spring Chinook program at the discretion of the HCP Hatchery Committees (and the PRCC HSC, when applicable). 10
19 Time of release: Releases from the acclimation ponds occur from April through May 25. Release dates may vary depending on environmental conditions. Fish health certification: A fish-health specialist will conduct a standard fish-health monitoring (monthly checks of salmon and steelhead) with intensified efforts to monitor presence of specific pathogens that are known to occur in the donor populations. Significant fish mortality attributed to an unknown cause(s) will be sampled for histopathological study. Fish-health maintenance strategies are described in IHOT (1995). Incidence of viral pathogens in salmon and steelhead broodstock will be determined by sampling fish at spawning in accordance with the Salmonid Disease Control Policy of the Fisheries Co-Managers of Washington State (NWIFC and WDFW 2006). Populations of particular concern may be sampled at the 100-percent level and may require segregation of eggs/progeny in early incubation or rearing, and/or culling. Specifically, incidence of Renibacterium salmoninarum (Rs, causative agent of BKD) in salmon broodstock will be controlled by hatchery staff who will segregate eggs/progeny based on levels of Rs antigen. This segregation protects progeny with negative or low Rs levels from the potential transmission of Rs bacteria from progeny with high Rs levels. Progeny of any segregation study will also be tested; at a minimum, each segregation group would be sampled at release. Necropsy-based condition assessments (based on organosomatic indices) will be used to assess condition of hatchery-reared salmon smolts at release, and natural-origin salmon during outmigration. If needed, condition assessments will be performed at other key times during hatchery rearing. Proposed adult management Anticipated number or range in adult hatchery fish returns originating from this program: Table 3. Expected adult returns for the Methow program, based on historical (brood years ) smolt-adult survival rates (SAR; DPUD and WDFW 2010a, Tables 1-11,12,13). Component Mean Percent SAR (Range) Expected Range of Adult Returns Methow 0.31 ( ) Twisp 0.24 ( ) Removal of hatchery-origin adults and the anticipated number of natural-origin fish encountered: Between 1996 and 2008, estimated escapement of Methow Subbasin natural-origin adults ranged from 19 to 1832, with a mean of 355. Fish in excess of conservation need will be removed at one or more of the following: Wells Hatchery, Wells Dam, Methow Hatchery volunteer channel, WNFH volunteer channel, and Twisp weir. Appropriate uses for hatchery fish that are removed: Broodstock for the WNFH program, food banks, instream nutrient enhancement. Are hatchery fish intended to spawn naturally (Y/N): Yes. Performance standard: Management of returning adults from the Methow program will occur based on the natural-origin fish run size measured at Wells Dam. When the natural run is below 300 fish, the program will manage to a PUD partial phos (calculated as HOSPUD/(HOSPUD + HOSWNFH + NOS)) based on the naturalorigin run size (PUD PHOS = x + 0.8), where y = phos and x = natural run size (Figure 2; left). This partial phos scale is based on achieving a total of 500 spawners for the Methow Subbasin. In addition, when the natural-origin run is less than 100 fish, no natural-origin fish are to be collected for broodstock. When the natural run is greater than 300, the program will be managed to achieve a PUD PNI target (PNI = 0.8(1 ee ( xx) ); Figure 2; right). 11
20 PUD Partial phos PUD PNI Natural Run Figure 2. Gene-flow management regime for the PUD spring Chinook salmon program. Proposed research, monitoring, and evaluation Adult sampling, purpose, methodology, location, and the number of fish handled: Adult spring Chinook are sampled at Wells Dam, Methow Hatchery, and the Twisp Weir. Adult spring Chinook are sampled for stock assessment, to estimate run timing, to collect biological data and samples, and to tag fish (PIT tag, other tags or marks as appropriate). At Wells Dam and Twisp Weir, stock assessment is performed during broodstock collection. Collected fish are sampled for length, sex, scales, origin (using hatchery marks or tags), and all released fish are PIT-tagged. At Wells Dam, suspected natural origin fish targeted for broodstock are tissue sampled (fin-clip) for DNA analysis and determination of stock origin. Tissue samples are processed weekly, and fish of Twisp and Methow Composite origin are retained for broodstock as necessary; fish of other stocks are released. In addition to broodstock collection, sampling at Wells Dam may occur up to three days per week (16-hours per day) to provide a more representative stock assessment of the run at large. No more than 10 percent of the run will be handled for stock assessment activities at Wells Dam. Juvenile sampling, purpose, methodology, location, and number of fish handled: Juvenile fish may be sampled in streams to estimate population size, survival, growth, spatial and temporal distribution, and to collect biological data and samples. Juveniles may be captured by rotary screw trap, hook-and-line, electrofishing, seining, snerding, weirs, or other capture methods as appropriate. Juveniles may be detected or observed using telemetry, PIT tags, and visual observation (e.g., snorkeling). Juveniles may be sampled throughout the Methow Subbasin and in the Columbia River. Juvenile fish traps are generally operated to achieve a sample efficiency of 4 to 20 percent of the total run. Mortality is expected to be less than 2 percent of the target species. Proposed operation and maintenance of hatchery facilities Natural Run Water source(s) and quantity for hatchery facilities: Water withdrawal for use in hatcheries is monitored through the Washington State Department of Ecology and the Washington State chapter Revised Code of Washington (RCW) water code. Methow Hatchery has both groundwater and surface water. The facility has six wells capable of producing the full groundwater right of 10 cubic feet per second (cfs). Groundwater temperatures are steady at 8.9 C year round. Methow Hatchery also has senior uninterruptible rights to 7 cfs of surface water and 18 cfs of junior interruptible water rights. The 7 cfs surface-water right is held by USFWS, but granted to Douglas PUD by USFWS under the terms of a Memorandum of Understanding in exchange for improvements to the intake structure of the Foghorn Ditch plus improvements to the ladder at Foghorn Dam. All surface water is diverted from Foghorn Irrigation Ditch. During low flow periods, the Methow hatchery would likely use well water. A number of acclimation sites are proposed with the Methow Subbasin that will operate from February 1 to May 31. Water rights for each of the acclimation sites is detailed in Table 4. Water for Twisp Pond is diverted 12
21 from the Twisp Valley Power and Irrigation Company ditch. Water for the Chewuch Pond is diverted from the Chewuch Canal Company irrigation ditch. Water for Goat Wall Pond is from Gate Creek, a tributary of the Methow River. Table 4. Water use associated with facilities operated as part of the Proposed Action. Hatchery Facility Total Facility Water (cfs) Surface Water (cfs) Groundwater (cfs) Surface Water Source/Discharge Location Water Diversion Distance (Rkm) Methow Hatchery Methow River 0.91 Twisp Pond Twisp River 0.2 Chewuch River Chewuch River 0.05 Goat Wall Pond Gate Creek 0.1 Water diversions meet NMFS screen criteria: Water intakes into artificial propagation facilities have been screened in compliance with NMFS screening criteria (NMFS 1995a; NMFS 1996). Permanent or temporary barriers to juvenile or adult fish passage: Not applicable. Instream structures: Not applicable. Streambank armoring or alterations: Not applicable. Pollutant discharge and location(s): Wells and Methow hatcheries, operated by WDFW, monitor their discharge in accordance with the National Pollutant Discharge Elimination System (NPDES) permit. This permit is administered by the Washington State Department of Ecology under agreement with the EPA. The permit was renewed effective August 1, 2010 and will expire August 1, Hatchery wastewater discharge is monitored monthly at each of the steelhead production facilities in the Upper Columbia Basin. Facilities are exempted from sampling during any month where fish on hand fall below 20,000 pounds, and feed used falls below 5,000 pounds. An exception to this is offline settling basin discharges, which are to be monitored once per month when ponds are in use and discharging to receiving waters WNFH spring Chinook salmon program The goal of this program is to compensate for lost fish production due to the construction of Grand Coulee Dam through the rearing and release of juvenile spring Chinook salmon, such that sufficient numbers of returning adults are available for harvest as well as providing sufficient broodstock for production. Additionally, the program functions as a safety-net for the Methow program by genetically linking the two programs by using excess returns to the Methow program for WNFH broodstock. The location of WNFH is shown in Figure 1. Approximately 1/3 of the spring Chinook salmon production at WNFH is transferred to the Chief Joseph Hatchery Okanogan spring Chinook salmon program. Proposed hatchery broodstock collection Broodstock origin and number: Methow River composite stock spring Chinook salmon are collected annually with a goal of 400 hatchery-origin adults. Broodstock selection: Adult hatchery-origin adults of either WNFH or Methow Hatchery origin will be used. A stepping stone model of broodstock management will be used to ensure that the WNFH safety net program will not appreciably differentiate from the conservation program at Methow Hatchery by maximizing the number of Methow Hatchery-origin adults for broodstock. 13
22 Method and location for collecting broodstock: Hatchery adults will be collected as volunteers to WNFH or transferred after collection by WDFW. Duration of collection: The hatchery operates the ladder and adult collection facilities from mid-may into September to ensure collection from the full spectrum of the run. Encounters, sorting, and handling, with ESA and non-esa listed fish, adults, and juveniles: Encounters with natural origin spring Chinook adults while operating the WNFH ladder and trap are rare. Occasionally, listed natural-origin spring Chinook adults are captured (< 1 per year on average). In these cases, the individuals will either be transferred to WDFW for use as broodstock at Methow FH or immediately returned to the natural environment. All listed hatchery-origin spring Chinook adults collected will be retained for broodstock and adult management (i.e., phos) purposes. In cases of low natural- and hatchery-origin return years hatchery adults collected at Winthrop may be transferred to Methow FH for use as broodstock (i.e., safety net purposes). Any non-target natural-origin adults or juveniles that volunteer to the WNFH hatchery ladder will be returned unharmed to the natural environment, residual ESA listed hatchery origin juveniles (steelhead and spring Chinook salmon) captured in the adult pond will be retained to reduce ecological impacts on natural origin juveniles. Proposed mating protocols Fish will be collected from the entire spectrum of the run and mated randomly in 1x1 individual matings The inclusion of jacks is limited to < 10 percent unless required to meet broodstock requirements (i.e., due to low return) Backup males will be used only in cases where a problem is noted with the milt (e.g., blood, water, etc.) Proposed protocols for each release group (annually) Life stage: Yearling smolt Size at release: fish per pound Acclimation and duration of acclimation: Not applicable-on station release. Volitional Release: Not currently employed. External mark(s): On station releases are 100 percent adipose fin clipped, subject to exceptions during extreme low return years as prescribed in U.S. v Oregon Agreement. Internal marks/tags: 100 percent coded wire tag. A proportion of the on station release are also PIT tagged (>5,000) to monitor juvenile survival. Maximum number released: 400,000 Release location: On station in the Methow River Time of release: April to coincide with increasing flows and initiation of bypass operations at downstream hydro-facilities. 14
23 Fish health certification: Sixty fish from all juvenile lots are sampled and tested for reportable bacterial and viral pathogens with methods that meet or exceed all national, international, IHOT or co-manager requirements. Monthly monitoring of juveniles for parasites, gill, internal organ, and overall condition continues until release. Proposed adult management Anticipated number or range in adult hatchery fish returns originating from this program: Over BY s 1996 through 2006, smolt-adult survival ranged from to percent, with a mean of percent (USFWS 2012b). Returns from a release of 400,000 smolts should therefore return between 388 and 2752 fish to the basin, with a mean of Removal of hatchery-origin adults and the anticipated number of natural-origin fish encountered: Fish in excess of conservation needs will be removed at one or more of the following: Methow Hatchery volunteer channel, WNFH volunteer channel, Twisp weir, and at other locations appropriate to effectively manage phos. Appropriate uses for hatchery fish that are removed: Tribes, Food banks, in-stream nutrient enhancement. Are hatchery fish intended to spawn naturally (Y/N): No. Performance standard for phos (proportion of naturally spawning fish that are of hatchery-origin): Management of returning adults from the Winthrop program will target a partial phos (calculated as HOSWNFH/(HOSPUD + HOSWNFH + NOS)), which will vary based on run natural run size. Table 5. Target partial phos for WNFH returns based on natural run size. Natural Run Proposed Research, Monitoring, and Evaluation 15 WNFH phos > Adult sampling, purpose, methodology, location, and the number of fish handled: Up to 100 percent of the adults returning to the facility will be handled and interrogated for external marks, tags and other identifying information (e.g., sex, size, scales) to meet M&E objectives. Fish will be anesthetized with either chemical anesthetic (MS-222) or using carbon dioxide to allow handling. All adult handling will occur within the existing infrastructure in the spawning building at Winthrop NFH and will be centered on spawning, broodstock collection, and adult management activities. Juvenile sampling, purpose, methodology, location and number of fish handled: Juvenile fish will be handled during on station rearing for collection of standard hatchery rearing and fish health data to meet a number of M&E objectives including marking and tagging objectives. Up to 100 percent of the ESA listed spring Chinook juveniles being reared at WNFH will be handled on several occasions to maintain the integrity of the rearing conditions and ensure the high quality of the fish being released. Proposed operation and maintenance of hatchery facilities Water source(s) and quantity for hatchery facilities: WNFH withdraws approximately 75 percent (up to 50 cfs) of its water supply from the Methow River and 25 percent (16.7 cfs) from ground water supply. The
24 Foghorn Diversion dam is about 3.7 Rkm upstream of WNFH and diverts water from the Methow River into the Foghorn Irrigation Ditch. This is the source of Methow River water for WNFH. From the intake of the hatchery to the return point of the water in the Methow River is 1.44 Rkm. Water diversions meet NMFS screen criteria: The intake has a trash rack at the ditch leading to the screen chamber. Directly below the hatchery intake and screen chamber, a new NOAA fisheries compliant screen was constructed and installed in 2000 to prevent natural-origin fish from entering the Foghorn Irrigation Ditch. Bypass pipes lead fish away from both screens to the main bypass channel (Spring Creek/outfall channel), which leads back to the Methow River. Permanent or temporary barriers to juvenile or adult fish passage: None Instream structures: None Streambank armoring or alterations: None planned or applicable. Pollutant discharge and location(s): WNFH complies with the current National Pollutant Discharge Elimination System (NPDES) permit standards (WAG ). Discharge occurs below the hatchery ladder into Spring Creek/outfall channel. All cleaning effluent is discharged to a settling facility before discharge into Spring Creek/outfall channel Action Area The Action Area means all areas to be affected directly or indirectly by the Federal action and not merely the immediate area involved in the action (50 CFR ). The action area resulting from this analysis includes all reaches of tributaries of the Methow and Entiat Rivers accessible to anadromous fish downstream to their confluences with the Columbia River. The action area also includes Wells Dam on the Columbia River, where some broodstock fish may be trapped and surplus returning adult fish removed. The Proposed Action takes place in the Methow Subbasin. The Entiat Subbasin is included in the action area because fish from the Methow programs stray into it; therefore, only the effect of Methow Hatchery and WNFH fish straying into the Entiat population will be considered in the Entiat Subbasin. Table 6. Stream reaches of the Entiat and Methow Subbasins included in the action area. Stream reach information on use by anadromous fish is from (RTT 2013). Watershed (subbasin) Entiat Methow Assessment Unit (or primary sub-watershed) 16 River miles Secondary and tertiary subwatersheds Fish Use 1 Upper-middle Entiat Middle Entiat Roaring, Stormy, Mud MaSA for spring Chinook and Creeks steelhead. Lower Entiat 0-16 MaSA for spring Chinook and steelhead. Mad River 0 -? Tillicum MaSA for spring Chinook and steelhead. Upper Methow Goat, Little Boulder MaSA for spring Chinook and Creeks steelhead. Upper-Middle Methow Hancock, Wolf MaSA for spring Chinook and steelhead. Middle Methow MaSA for steelhead and summer Chinook. Lower Methow MiSA for steelhead.
25 Watershed (subbasin) Chewuch Twisp Assessment Unit (or primary sub-watershed) Early Winters Creek 0 -? River miles Lost River Wolf Creek 0-? Beaver Creek 0-10 Gold Creek Libby Creek Upper Chewuch Lower Chewuch 0-20 Upper Twisp Lower Twisp MaSA: Major Spawning Area. MiSA: Minor Spawning Area. Secondary and tertiary subwatersheds Frazier, Lightning, Blue Buck, and South Fork Beaver Creeks South Fork, North Fork, Crater, Foggy Dew Creeks North Fork Libby, South Fork Libby Creeks Thirtymile, Andrews, Lake Creeks Twentymile, Eightmile, Boulder, Cub Creeks Reynolds, South, North Creeks Little Bridge, Poorman, Buttermilk Creeks Fish Use 1 MaSA for spring Chinook and steelhead. MaSA for spring Chinook and steelhead. MaSA for steelhead. MiSA for steelhead. MiSA for steelhead. MaSA for spring Chinook and steelhead. MaSA for spring Chinook and steelhead. MaSA for spring Chinook and steelhead. MaSA for spring Chinook and steelhead. The operation of hatchery facilities has the potential to affect ESA-listed salmon and steelhead in streams adjacent to hatchery facilities through the diversion of surface water or the maintenance of instream structures (e.g., water intake and discharge structures) and the release of effluent. Under the Proposed Action, a number of hatchery facilities will be used, depending on the program, to spawn, incubate, and rear salmon for the Methow spring Chinook salmon program. These facilities are the Methow Fish Hatchery, WNFH, Twisp Acclimation Pond, Chewuch Acclimation Pond, Wells Hatchery, and Wells Dam (trap broodstock). Available knowledge and techniques are insufficient to discern the role and contribution of the Proposed Action to density dependent interactions affecting salmon and steelhead growth and survival in the mainstem Columbia River, the Columbia River estuary, and the Pacific Ocean (NMFS 2009a). Scientific literature generally concludes that the influence of density dependent interactions on growth and survival is likely immeasurably small. While there is evidence that hatchery production, on a scale many times larger than the Proposed Action, can impact salmon survival at sea, the degree of impact or level of influence is not yet understood or predictable. NMFS will monitor emerging science and information and will reinitiate section 7 consultation in the event that new information reveals effects of the action that may affect listed species or critical habitat in a manner or to an extent not considered in this consultation (50 CFR ) Interrelated and Interdependent Actions Fisheries are not part of this Proposed Action. No tributary fisheries exist that target hatchery-origin returns from these programs. There are existing mainstem Columbia River and ocean fisheries that may catch fish from these programs. However, these mixed fisheries would exist with or without these programs, and have previously been evaluated in a separate biological opinion (NMFS 2008c). The impacts of fisheries in the action area on these programs and, in particular, on ESA-listed salmonids returning to the action area for this opinion are included in the environmental baseline for past fisheries and cumulative effects for future fisheries 17
26 that have not undergone ESA consultation. Accordingly, NMFS has not identified any interrelated or interdependent actions associated with this Proposed Action. 2. ENDANGERED SPECIES ACT: BIOLOGICAL OPINION AND INCIDENTAL TAKE STATEMENT The ESA establishes a national program for conserving threatened and endangered species of fish, wildlife, plants, and the habitat upon which they depend. As required by section 7(a)(2) of the ESA, Federal agencies must ensure that their actions are not likely to jeopardize the continued existence of endangered or threatened species, or adversely modify or destroy their designated critical habitat. Per the requirements of the ESA, Federal action agencies consult with NMFS and section 7(b)(3) requires that, at the conclusion of consultation, NMFS provides an opinion stating how the agency s actions would affect listed species and their critical habitat. If incidental take is expected, section 7(b)(4) requires NMFS to provide an incidental take statement (ITS) that specifies the impact of any incidental taking and includes non-discretionary reasonable and prudent measures and terms and conditions to minimize such impacts Introduction to the Biological Opinion This biological opinion includes both a jeopardy analysis and an adverse modification analysis. The jeopardy analysis relies upon the regulatory definition of to jeopardize the continued existence of a listed species, which is to engage in an action that would be expected, directly or indirectly, to reduce appreciably the likelihood of both the survival and recovery of a listed species in the wild by reducing the reproduction, numbers, or distribution of that species (50 CFR ). Therefore, the jeopardy analysis considers both survival and recovery of the species. For its critical habitat analysis, this biological opinion relies on the definition of destruction or adverse modification, which is a direct or indirect alteration that appreciably diminishes the value of critical habitat for the conservation of a listed species. Such alterations may include, but are not limited to, those that alter the physical or biological features essential to the conservation of a species or that preclude or significantly delay development of such features (81 FR 7214, February 11, 2016). We use the following approach to determine whether a proposed action is likely to jeopardize listed species or destroy or adversely modify critical habitat: Range-wide status of the species and critical habitat This section describes the status of species and critical habitat that are the subject of this opinion. The status review starts with a description of the general life history characteristics and the population structure of the ESU/DPS, including the strata or major population groups (MPG) where they occur. NMFS has developed specific guidance for analyzing the status of salmon and steelhead populations in a viable salmonid populations (VSP) paper (McElhany et al. 2000). The VSP approach considers four attributes, the abundance, productivity, spatial structure, and diversity of each population (natural-origin fish only), as part of the overall review of a species status. For salmon and steelhead protected under the ESA, the VSP criteria therefore encompass the species reproduction, numbers, or distribution (50 CFR ). In describing the range-wide status of listed species, NMFS reviews available information on the VSP parameters including abundance, productivity trends (information on trends, supplements the assessment of abundance and productivity parameters), spatial structure, and diversity. We also summarize available estimates of extinction risk that are used to characterize the viability of the populations and ESU/DPS, and the limiting factors and threats. To source this information, NMFS relies on viability assessments and criteria in technical recovery team documents, ESA Status Review updates, and recovery plans. We determine the status of critical habitat by 18
27 examining its physical and biological features (also called primary constituent elements or PCEs). Status of the species and critical habitat are discussed in Section 2.2. Description of the environmental baseline The environmental baseline includes the past and present impacts of Federal, state, or private actions and other human activities in the action area on ESA-listed species. It includes the anticipated impacts of proposed Federal projects that have already undergone formal or early section 7 consultation and the impacts of state or private actions that are contemporaneous with the consultation in process. The environmental baseline is discussed in Section 2.3 of this opinion. Cumulative effects Cumulative effects, as defined in NMFS implementing regulations (50 CFR ), are the effects of future state or private activities, not involving Federal activities, that are reasonably certain to occur within the action area. Future Federal actions that are unrelated to the Proposed Action are not considered because they require separate section 7 consultation. Cumulative effects are considered in Section 2.5 of this opinion. Integration and synthesis Integration and synthesis of information from previous sections occurs in Section 2.6 of this opinion. In this step, NMFS analyzes the expected effects of the Proposed Action (Section 2.4) on the status of ESA protected populations in the Action Area under the environmental baseline (Section 2.3) and to cumulative effects (Section 2.5). Impacts on individuals within the affected populations are analyzed to determine their effects on the VSP parameters for the affected populations, and these are combined with the overall status of the stratum/mpg to determine the effects on the ESA-listed species (ESU/DPS). The result will then be used to formulate the agency s opinion as to whether the hatchery action is likely to: (1) result in appreciable reductions in the likelihood of both survival and recovery of the species in the wild by reducing its numbers, reproduction, or distribution; or (2) reduce the value of designated or proposed critical habitat. Jeopardy and adverse modification Based on the Integration and Synthesis analysis in Section 2.6, the opinion determines whether the Proposed Action is likely to jeopardize ESA protected species or destroy or adversely modify designated critical habitat in Section 2.7. Reasonable and prudent alternative(s) to the Proposed Action If NMFS determines that the action under consultation is likely to jeopardize the continued existence of listed species or destroy or adversely modify designated critical habitat, NMFS must identify an RPA or RPAs to the Proposed Action Range-wide Status of the Species and Critical Habitat This opinion examines the status of each species (as defined below) that would be affected by the Proposed Action. The species and the designated critical habitat that are likely to be affected by the Proposed Action, and any existing protective regulations, are described in Table 7. The status is determined by the level of extinction risk that the listed species face, based on parameters considered in documents such as recovery plans, status reviews, and listing decisions. The species status section helps to inform the description of the species current reproduction, numbers, or distribution as described in 50 CFR The opinion also examines the 19
28 condition of critical habitat throughout the designated area, evaluates the conservation value of the various watersheds and coastal and marine environments that make up the designated area, and discusses the current function of the essential physical and biological features that help to form that conservation value. One factor affecting the rangewide status of UCR spring Chinook salmon and steelhead, and aquatic habitat at large, is climate change. The current effects of climate change in the action area are discussed in Section 2.2.3, Climate Change, below. Table 7. ESA- listed species considered in this consultation, their listing status, critical habitation designation, and protective regulations. Species Listing Status Critical Habitat Chinook salmon (O. tshawytscha) Upper Columbia River spring-run Endangered 70 FR 37160; June 28, FR 52630; Sept 2, 2005 Protective Regulations 70 FR 37160; June 28, 2005 Steelhead (O. mykiss) Upper Columbia Threatened 74 FR 42605; August 24, FR 52630; Sept 2, FR 37160; June 28, 2005 Species Definition: The ESA of 1973, as amended, 16 U.S.C et seq. defines species to include any distinct population segment (DPS) of any species of vertebrate fish or wildlife which interbreeds when mature. To identify DPSs of salmon species, NMFS follows the Policy on Applying the Definition of Species under the ESA to Pacific Salmon (56 FR 58612). Under this policy, a group of Pacific salmon is considered a distinct population, and hence a species under the ESA if it represents an evolutionarily significant unit (ESU) of the biological species. The group must satisfy two criteria to be considered an ESU: (1) It must be substantially reproductively isolated from other con-specific population units; and (2) It must represent an important component in the evolutionary legacy of the species. For example, UCR Chinook salmon constitutes an ESU of the taxonomic species O. tshawytscha and therefore is considered a species under the ESA. To identify DPSs of steelhead, NMFS applies the joint FWS-NMFS DPS policy (61 FR 4722). Under this policy, a DPS of steelhead must be discrete from other populations, and it must be significant to its taxon. For example, the UCR steelhead constitute a DPS of the taxonomic species O. mykiss and is considered a species under the ESA Status of Listed Species A population is a group of individuals of a single species inhabiting a specific area. For Pacific salmon and steelhead, NMFS commonly uses four parameters to assess the viability of the populations that, together, constitute the species: spatial structure, diversity, abundance, and productivity (McElhany et al. 2000). These VSP parameters therefore encompass the species reproduction, numbers, or distribution as described in 50 CFR When these parameters are collectively at appropriate levels, they maintain a population s capacity to adapt to various environmental conditions and allow it to sustain itself in the natural environment. These 20
29 attributes are influenced by survival, behavior, and experiences throughout a species entire life cycle, and these characteristics, in turn, are influenced by habitat and other environmental conditions. Abundance generally refers to the number of naturally-produced adults (i.e., the progeny of naturallyspawning parents) in the natural environment (e.g., on spawning grounds). Productivity, as applied to viability factors, refers to the number of individuals produced during a fish s entire life cycle; i.e., the number of naturally-spawning adults produced per their naturally spawning parental pair. When the number of progeny replaces or exceeds the number of parents, a population is stable or increasing. When the number of progeny fail to replace the number of parents, the population is declining. McElhany et al. (2000) use the terms population growth rate and productivity interchangeably when referring to reproduction over the entire life cycle. They also refer to trend in abundance, which is the manifestation of long-term population growth rate. Spatial structure refers both to the spatial distributions of individuals in the population and the processes that generate that distribution. A population s spatial structure depends fundamentally on quality and spatial configuration of habitat and the dynamics and dispersal characteristics of individuals in the population. Diversity refers to the distribution of traits within and among populations. These range in scale from DNA sequence variation at single genes to complex life history traits (McElhany et al. 2000). For species with multiple populations, once the biological status of a species populations has been determined, NMFS assesses the status of the entire species using criteria for groups of populations, as described in recovery plans and guidance documents from technical recovery teams. Considerations for species viability include having multiple populations that are viable, ensuring that populations with unique life histories and phenotypes are viable, and that some viable populations are both widespread, to avoid concurrent extinctions from mass catastrophes, and spatially close, to allow functioning as metapopulations (McElhany et al. 2000) Life History and Status of UCR Chinook Salmon Chinook salmon (Oncorhynchus tshawytscha) have a wide variety of life history patterns that include: variation in age at seaward migration; length of freshwater, estuarine, and oceanic residence; ocean distribution; ocean migratory patterns; and age and season of spawning migration. Two distinct races of Chinook salmon are generally recognized: stream-type and ocean-type (Healey 1991; Myers et al. 1998). ESA-listed UCR spring Chinook salmon are stream-type. Stream-type Chinook salmon spend 2 to 3 years in coastal ocean waters, and enter freshwater in February through April. Spring Chinook salmon also spawn and rear high in the watershed and reside in freshwater for a year. The historical UCR Spring Chinook Salmon ESU comprises three major population groups (MPGs) and eight populations; however, the ESU is currently limited to one MPG and three extant populations. Approximately half of the area that originally produced spring Chinook salmon in this ESU is blocked by dams. What remains of the ESU includes all naturally spawned fish upstream of Rock Island Dam and downstream of Chief Joseph Dam in Washington State, excluding the Okanogan River (64 FR 14208, March 24, 1999) (Figure 3). The ESU originally included six artificial propagation programs: the Twisp, Chewuch, Methow Composite, Winthrop NFH, Chiwawa, and White River hatchery programs (79 FR 20802, April 14, 2014). Currently, the three Methow Subbasin programs (Twisp, Chewuch, Methow Composite) are considered a single program, with two components: Twisp and Methow (the previous Chewuch and Methow programs combined). Furthermore, a Nason Creek program began in the Wenatchee Subbasin (Grant County PUD et al. 2009b), while the White River releases were discontinued after 2015 (Grant County PUD et al. 2009a). 21
30 Figure 3. Upper Columbia River Spring Chinook Salmon ESU (ICTRT 2008). Risk for Abundance/Productivity Risk for Spatial Structure / Diversity Very Low Low Moderate High Very Low (<1%) Low (1-5%) Moderate (6-25%) High (>25%) High High High Highly Viable Highly Viable Viable Maintained Viable Viable Viable Maintained Maintained Maintained Maintained High High: Wenatchee Entiat Methow Figure 4. Matrix used to assess population status across VSP parameters for UCR spring Chinook salmon. Percentages for abundance and productivity scores represent the probability of extinction in a 100-year time period (NWFSC 2015). Abundance, Productivity, Spatial Structure, and Diversity For the most recent period ( ), abundance has increased for all three populations, but productivity for all three populations remains below replacement (Table 8). For spatial structure and diversity, there is a consistent and substantial decline in the proportion of natural-origin fish on the spawning grounds for all three populations. Natural-origin fish now make up fewer than fifty percent of the spawners for all three populations (Table 9). Although increases in natural-origin abundance relative to the extremely low levels observed during the mid- 1990s are encouraging, overall productivity has decreased to extremely low levels for the two largest populations (Wenatchee and Methow). The predominance of hatchery fish on the spawning grounds, 22
31 particularly for the Wenatchee and Methow populations, is an increasing risk, and populations that rely on hatchery spawners are not viable (McElhany et al. 2000). Based on the combined ratings for abundance/productivity and spatial structure/diversity, all three extant populations and the ESU remain at high risk of extinction (Figure 4; Table 8). Table 8. Risk levels and viability ratings for natural-origin UCR spring Chinook salmon populations from (NWFSC 2015). Population Minimum Abundance Threshold Abundance and Productivity (A/P) Spawning Abundance Productivity A/P Risk Spatial Structure and Diversity (SS/D) Natural Processes Risk Diversity Risk SS/D Risk Ove Ri Wenatchee River ( ) 0.60 High Low High High Hig Entiat River (78-354) 0.94 High Moderate High High Hig Methow River ( ) 0.46 High Low High High Hig Table 9. Estimates of the percent natural-origin spawners for UCR spring Chinook salmon populations (NWFSC 2015). Population % Natural-origin (5-year average) 1995 to to to Wenatchee River Entiat River Methow River Many factors affect the abundance, productivity, spatial structure, and diversity of the UCR Spring Chinook Salmon ESU. Factors limiting the ESU s survival and recovery include: past management practices such as the Grand Coulee Fish Maintenance Project survival through the FCRPS degradation and loss of estuarine areas that help the fish survive the transition between fresh and marine waters spawning and rearing areas that have lost deep pools, cover, side-channel refuge areas, and high quality spawning gravels interbreeding and competition with hatchery fish that far outnumber fish from natural populations Life History and Status of UCR Steelhead Steelhead (O. mykiss) occur as two basic anadromous run types based on the level of sexual maturity at the time of river entry and the duration of the spawning migration (Burgner et al. 1992). The stream-maturing type (inland), or summer steelhead, enters freshwater in a sexually immature condition and requires several months 23
32 in freshwater to mature and spawn. The ocean-maturing type (coastal), or winter steelhead, enters freshwater with well-developed gonads and spawns shortly after river entry (Barnhart 1986). UCR steelhead are summer steelhead, returning to freshwater between May and October, and require up to 1 year in freshwater to mature before spawning (Chapman et al. 1994). Spawning occurs between January and June. In general, summer steelhead prefer smaller, higher-gradient streams relative to other Pacific salmon, and they spawn farther upstream than winter steelhead (Behnke and American Fisheries Society 1992; Withler 1966). Progeny typically reside in freshwater for two years before migrating to the ocean, but freshwater residence can vary from 1-7 years (Peven et al. 1994). For UCR steelhead, marine residence is typically one year, although the proportion of two-year ocean fish can be substantial in some years. They migrate directly offshore during their first summer rather than migrating nearer to the coast as do salmon. During fall and winter, juveniles move southward and eastward (Hartt and Dell 1986). The UCR Steelhead DPS includes all naturally spawned steelhead populations below natural and man-made impassable barriers in streams in the Columbia River Basin upstream of the Yakima River, Washington to the U.S. Canada border. The UCR Steelhead DPS also includes six artificial propagation programs: the Wenatchee River, Wells Hatchery (in the Methow and Okanogan rivers), Winthrop NFH, Omak Creek, and the Ringold steelhead hatchery programs. The UCR Steelhead DPS consisted of three MPGs before the construction of Grand Coulee Dam, but it is currently limited to one MPG with four extant populations: Wenatchee, Methow, Okanogan, and Entiat. A fifth population in the Crab Creek drainage is believed to be functionally extinct. What remains of the DPS includes all naturally spawned populations in all tributaries accessible to steelhead upstream from the Yakima River in Washington State, to the U.S. Canada border (Figure 5). Figure 5. Upper Columbia River steelhead DPS (ICTRT 2008). Abundance, Productivity, Spatial Structure, and Diversity Status of the species is determined based on the abundance, productivity, spatial structure, and diversity of its constituent natural populations. Best available information indicates that the UCR Steelhead DPS is at high risk and remains at threatened status. The ESA Recovery Plan (Upper Columbia Salmon Recovery Board 2007) 24
33 calls for improvement in each of the four extant steelhead populations (no more than a 5 percent risk of extinction in 100 years) and for a level of spatial structure and diversity that restores the distribution of natural populations to previously occupied areas and allows natural patterns of genetic and phenotypic diversity to be expressed. This corresponds to a threshold of at least viable status for each of the three natural populations, which falls into the category of high risk (Figure 6). Risk for Abundance / Productivity Risk for Spatial Structure / Diversity Very Low Low Moderate High Very Low (<1%) Highly Viable Highly Viable Viable Maintained Low (1-5%) Viable Viable Viable Maintained: Wenatchee Moderate Maintained Maintained Maintained High (6-25%) High (>25%) High High High High: Entiat Methow Okanogan Figure 6. Matrix used to assess population status across VSP parameters for UCR steelhead. Percentages for abundance and productivity scores represent the probability of extinction in a 100-year time period (NWFSC 2015) (ICTRT 2007b). For the period, abundance has increased for natural-origin spawners in each of the four extant populations (Table 10). However, natural-origin returns remain well below target levels for three of the four populations. Productivity remained the same for three of the four populations and decreased for the Entiat population relative to the last review (Ford 2011). For spatial structure and diversity, hatchery origin returns continue to constitute a high fraction (Table 11) of total spawners in natural spawning areas for the DPS as a whole (NWFSC 2015). The predominance of hatchery fish on the spawning grounds is an increasing risk, and populations that rely solely on hatchery spawners are not viable over the long-term (McElhany et al. 2000). Based on the combined ratings for abundance/productivity and spatial structure/diversity, three of the four extant populations and the DPS remain at high risk of extinction. Table 10. Risk levels and viability ratings for natural-origin UCR steelhead populations from (NWFSC 2015). Population Minimum Abundance Threshold Abundance and Productivity (A/P) Spawning Abundance Productivity A/P Risk Spatial Structure and Diversity (SS/D) Natural Processes Risk Diversity Risk SS/D Risk Ove Ri Wenatchee River ( ) Low Low High High Manta Entiat River (59-310) High Moderate High High Hig Methow River ( ) High Low High High Hig Okanogan River ( ) High High High High Hig Table 11. Estimates of the percent natural-origin spawners for UCR steelhead populations (NWFSC 25
34 2015). Population % Natural-origin (5-year average) 1995 to to to Wenatchee River Entiat River Methow River Okanogan River Many factors affect the abundance, productivity, spatial structure, and diversity of the UCR Steelhead DPS. Factors limiting the DPS s survival and recovery include: past management practices such as the Grand Coulee Fish Maintenance Project survival through the FCRPS degradation and loss of estuarine areas that help the fish survive the transition between fresh and marine waters spawning and rearing areas that have lost deep pools, cover, side-channel refuge areas, and high quality spawning gravels predation by native and non-native species harvest interbreeding and competition with hatchery fish that far outnumber fish from natural populations Status of Critical Habitat In this section, we examine the range-wide status of designated critical habitat for the affected salmonid species. For UCR spring Chinook salmon and UCR steelhead, critical habitat was designated in 70 FR (September 2, 2005). UCR spring Chinook salmon critical habitat includes all Columbia River estuarine areas and river reaches proceeding upstream to Chief Joseph Dam, as well as specific stream reaches in the following subbasins: Chief Joseph, Methow, Upper Columbia/Entiat and Wenatchee. UCR steelhead critical habitat includes river reaches proceeding upstream to Chief Joseph Dam, as well as specific stream reaches in the following subbasins: Columbia River/Lynch Coulee, Chief Joseph, Okanogan, Salmon, Methow, Similkameen, Chewuch, Twisp, Entiat, Wenatchee, Chiwawa, Nason, and Icicle. UCR steelhead and spring Chinook salmon have overlapping ranges, because of similar life history characteristics. The status of critical habitat is based primarily on a watershed-level analysis of conservation value that focused on the presence of ESA-listed species and physical features that are essential to the species conservation. The NMFS organized information at the 5th field hydrologic unit code (HUC) watershed scale because it corresponds to the spatial distribution and site fidelity scales of salmon and steelhead populations(mcelhany et al. 2000). The analysis for the 2005 designations of salmon and steelhead species was completed by Critical Habitat Analytical Review Teams (CHARTs) that focused on large geographical areas corresponding approximately to recovery domains (NMFS 2005b). Each watershed was ranked using a conservation value attributed to the quantity of stream habitat with primary constituent elements (PCE), the present condition of those PCEs, the likelihood of achieving PCE potential (either naturally or through active restoration), support for rare or important genetic or life history characteristics, support for abundant populations, and support for spawning and rearing populations. In some cases, our understanding of these interim conservation values has 26
35 been further refined by the work of technical recovery teams and other recovery planning efforts that have better explained the habitat attributes, ecological interactions, and population characteristics important to each species. NMFS reviews the status of designated critical habitat affected by the Proposed Action by examining the condition and trends of PCEs throughout the designated area. These PCEs vary slightly for some species, due to biological and administrative reasons, but all consist of site types and site attributes associated with life history events (Table 12). Table 12. PCEs of critical habitat designated for ESA-listed salmon and steelhead considered in this opinion. Primary Constituent Elements Site Type Freshwater spawning Freshwater rearing Freshwater migration Estuarine areas Nearshore marine areas Offshore marine areas Site Attribute Substrate Water quality Water quantity Floodplain connectivity Forage Natural cover Water quality Water quantity Free of artificial obstruction Natural cover Water quality Water quantity Forage Free of artificial obstruction Natural cover Salinity Water quality Water quantity Forage Free of artificial obstruction Natural cover Water quantity Water quality Forage Water quality Species Life History Event Adult spawning Embryo incubation Alevin growth and development Fry emergence from gravel Fry/parr/smolt growth and development Adult sexual maturation Adult upstream migration and holding Kelt (steelhead) seaward migration Fry/parr/smolt growth, development, and seaward migration Adult sexual maturation and reverse smoltification Adult upstream migration and holding Kelt (steelhead) seaward migration Fry/parr/smolt growth, development, and seaward migration Adult growth and sexual maturation Adult spawning migration Nearshore juvenile rearing Adult growth and sexual maturation Adult spawning migration Subadult rearing Habitat quality in tributary streams in the Interior Columbia Recovery Domain range from excellent in wilderness and road-less areas, to poor in areas subject to heavy agricultural and urban development (NMFS 2009b; Wissmar et al. 1994). Critical habitat throughout much of the Interior Columbia Recovery Domain has been degraded by intense agriculture, alteration of stream morphology (i.e., channel modifications and diking), riparian vegetation disturbance, wetland draining and conversion, livestock grazing, dredging, road construction and maintenance, logging, mining, and urbanization. Reduced summer stream flows, impaired water quality, and reduction of habitat complexity are common problems for critical habitat in developed areas. Currently, state water law over-allocates water in many stream reaches designated as critical habitat in the Interior Columbia Recovery Domain, with more allocated water rights than existing stream-flow conditions can support. Withdrawal of water, particularly during low-flow periods that commonly overlap with agricultural 27
36 withdrawals, often increases summer stream temperatures, blocks fish migration, strands fish, and alters sediment transport (Spence et al. 1996). Reduced tributary stream flow has been identified as a major limiting factor for all listed salmon and steelhead species in this area (NMFS 2011a) Despite these degraded habitat conditions, the HUCs that have been identified as critical habitat for these species are largely ranked as having high conservation value. Conservation value reflects several factors: (1) how important the area is for various life history stages, (2) how necessary the area is to access other vital areas of habitat, and (3) the relative importance of the populations the area supports relative to the overall viability of the ESU or DPS Critical Habitat for Upper Columbia River Spring Chinook Salmon The UCR Spring Chinook Salmon ESU s range consists of 31 watersheds. The CHART assigned five watersheds a medium rating, and 26 received a high rating of conservation value to the ESU (NMFS 2005a). Many factors, both human-caused and natural, have contributed to the decline of UCR spring Chinook salmon critical habitat. Upper Columbia River spring Chinook salmon habitat has been altered through activities such as urban development, logging, grazing, power generation, and agriculture, resulting in the loss of important spawning and rearing habitat and the loss or degradation of migration corridors. The following are the major factors limiting the conservation value of UCR spring Chinook salmon critical habitat: 1. Mortality during operation of the Columbia River hydropower system (freshwater migration corridors without obstructions) 2. Degraded tributary riparian condition and loss of in-channel large wood (freshwater rearing sites with natural cover such as shade, submerged and overhanging large wood, log jams and beaver dams to form and maintain physical conditions that support juvenile growth and development) 3. Altered tributary floodplain and channel morphology (freshwater spawning sites with water quantity and quality conditions and substrate supporting spawning, incubation, and larval development; freshwater rearing sites with floodplain connectivity to form and maintain physical habitat conditions that support juvenile growth and development) 4. Reduced tributary stream flow and altered passage (freshwater spawning sites with water quantity conditions supporting spawning, incubation, and larval development; freshwater rearing sites with water quantity to form and maintain physical habitat conditions that support juvenile growth and development) 5. Decreased capacity of critical habitat to support successful spawning, rearing, and migration due to climate change (increasing temperature and peak flows and decreasing base flows) 6. Inaccessible suitable habitats in the Okanogan Subbasin that were historically available Critical Habitat for Upper Columbia River Steelhead The UCR Steelhead DPS s range includes 42 watersheds. The CHART assigned low, medium, and high conservation value ratings to three, eight, and 31 watersheds, respectively (NMFS 2005a). Many factors, both human-caused and natural, have contributed to the decline of UCR steelhead and the conservation value of essential features and PCEs of designated critical habitat over the past century. Steelhead habitat has been altered through activities such as urban development, logging, grazing, power generation, and agriculture. These habitat alterations have resulted in the loss of important spawning and rearing habitat and the 28
37 loss or degradation of migration corridors. The following are the major factors limiting the conservation value of critical habitat for UCR steelhead in addition to factors 1 through 5 above for spring Chinook salmon: 1. Excessive sediment in tributaries (spawning sites with substrate to support egg incubation and larval growth and development; juvenile migration corridors and rearing sites with forage to support juvenile growth and development) 2. Degraded tributary water quality (spawning sites with water quality to support egg incubation and larval growth and development; juvenile rearing sites and migration corridors with water quality supporting juvenile growth and development) 3. Substantial truncation of their range in the Okanogan Subbasin Climate Change Climate change has negative implications for designated critical habitats in the Pacific Northwest (ISAB 2007; Scheuerell and Williams 2005; Zabel et al. 2006). Average annual Northwest air temperatures have increased by approximately 1ºC since 1900, or about 50 percent more than the global average over the same period (ISAB 2007). The latest climate models project a warming of 0.1 ºC to 0.6 ºC per decade over the next century. According to the Independent Scientific Advisory Board (ISAB), these effects pose the following impacts over the next 40 years: Warmer air temperatures will result in diminished snowpacks and a shift to more winter/spring rain and runoff, rather than snow that is stored until the spring/summer melt season. With a smaller snowpack, these watersheds will see their runoff diminished earlier in the season, resulting in lower streamflows in the June through September period. River flows in general and peak river flows are likely to increase during the winter due to more precipitation falling as rain rather than snow. Water temperatures are expected to rise, especially during the summer months when lower streamflows co-occur with warmer air temperatures. These changes will not be spatially homogeneous across the entire Pacific Northwest. Low-lying areas are likely to be more affected. Climate change may have long-term effects that include, but are not limited to, depletion of cold water habitat, variation in quality and quantity of tributary rearing habitat, alterations to migration patterns, accelerated embryo development, premature emergence of fry, and increased competition among species (ISAB 2007). To mitigate for the effects of climate change on listed salmonids, the ISAB (2007) recommends planning now for future climate conditions by implementing protective tributary, mainstem, and estuarine habitat measures, as well as protective hydropower mitigation measures. In particular, the ISAB (2007) suggests increased summer flow augmentation from cool/cold storage reservoirs to reduce water temperatures or to create cool water refugia in mainstem reservoirs and the estuary. The ISAB (2007) also calls for the protection and restoration of riparian buffers, wetlands, and floodplains. Although there were no specific recommendations for hatchery programs, the ISAB recommends managing to accommodate uncertainty (ISAB 2007). Hatchery programs could help with this uncertainty by serving as reserves for ESA-listed species. These will be most effective if hatchery programs make an effort to propagate populations with large phenotypic and genetic diversity to provide the greatest potential for adaptation Environmental Baseline 29
38 In the Environmental Baseline section, NMFS describes what is affecting ESA-listed species and designated critical habitat in the action area before including any effects resulting from the Proposed Action. The environmental baseline includes the past and present impacts of all Federal, state, or private actions and other human activities in the action area and the anticipated impacts of all proposed federal projects in the action area that have already undergone formal or early section 7 consultation (50 CFR ). The effects of future actions over which the Federal agency has discretionary involvement or control will be analyzed as effects of the action. Wide varieties of human activities have affected Upper Columbia spring Chinook salmon, steelhead, and PCEs in the action area. These activities, more recently, include reclamation actions that are having beneficial effects Description of Area and Effects on the Landscape and Habitat Material in this section is primarily taken from (RTT 2013). We have limited our discussion here to the Entiat and Methow Subbasins, which are the only two subbasins included in the action area Methow Subbasin The Methow Subbasin is located in north-central Washington and lies entirely within Okanogan County. The subbasin consists of about 1,167,764 acres. About 89 percent of the subbasin is in public ownership. The remaining 11 percent is privately owned and is primarily within the valley bottoms. The subbasin consists of fourteen primary subwatersheds: Early Winters Creek, Upper Methow River, Lost River, Wolf Creek, Middle Methow River, Upper and Lower Chewuch River, Upper and Lower Twisp River, Beaver Creek, Gold Creek, Libby Creek, and the Lower Methow River. Many factors, including mining, grazing, water diversions and timber harvest, have historically contributed to habitat degradation in the Methow Subbasin. Although beaver trapping began in the early 1800s, and no doubt had an effect on riparian conditions, mining was probably the first major activity affecting riparian and stream conditions. Mining began in the Methow Subbasin in the 1870s (Mullan et al. 1992). After the advent of mining was a period of intense livestock grazing. Grazing pressure was highest from the late 1800s to the 1930s, with subsequent reductions as allotment systems replaced the open range. Water diversion began in the mid-1880s, reducing stream flow and in some cases, may have come close to completely drying up the river, undoubtedly affecting adult migration and rearing capacity (Mullan et al. 1992). Timber harvest began in the 1920s, and up until 1955, selective harvest or high grading was the primary harvest method. Since then, partial cutting and clear-cutting have been the predominant practices, with the 1980s being the period of most intense harvest. The Methow Subbasin currently has a high proportion of pristine habitat in the upper portions of major tributaries. The primary habitat conditions in the Methow Subbasin that limit abundance, productivity, spatial structure, and diversity of spring Chinook salmon are mostly found in the middle and lower mainstem and lower portions of major tributaries that have been affected by state highways, county roads, and housing and agricultural development that have diminished the overall function of the stream channel and floodplain. In Cub, Boulder, Eightmile, and Falls Creeks (all in the Chewuch Subbasin), and in the Goat, Beaver, Libby, and Gold Creek upper Methow Subbasin) drainages, impacts also extend into the upper reaches of the drainages. These impacts are mostly the result of past timber harvest operations, road building and placement, and grazing (Andonaegui 2000). All of these anthropogenic alterations have impaired instream complexity, wood and gravel recruitment, floodwater retention, and water quality. In some portions of the watershed, human alterations to the environment are exacerbating naturally limiting conditions by reducing habitat quality and quantity. Additionally, late summer and winter instream flow conditions often reduce migration, spawning, and rearing habitat for native salmonids. This problem is partly 30
39 natural (a result of watershed-specific weather and geomorphic conditions) but is exacerbated by irrigation withdrawals in late summer Entiat River Basin The Entiat Subbasin encompasses 268,000 acres. The area is nearly 42 miles long and varies in width from five to fourteen miles. Approximately 224,000 acres of the subbasin is in public ownership, primarily the U.S. Forest Service, the Bureau of Land Management, and WDFW. In the lower valley, about 1,300 acres of orchard land are primarily classified as prime agricultural land. The lower miles, more than 70 percent of the length accessible to salmon and steelhead, is privately owned. The historical pattern of land use in the Entiat Subbasin follows a familiar pattern in the Pacific Northwest. Beaver trapping began in the early 1800s, and no doubt had an effect on riparian conditions; however, mining was probably the first major activity affecting riparian and stream conditions. Water diversion began in the mid- 1880s, affecting stream flow and in some cases, may have come close to completely drying up the river, affecting adult migration and rearing capacity (Mullan et al. 1992). Timber harvest began in the 1920s, and up until 1955, selective harvest or high grading was the primary harvest method. Since then, partial cutting and clear-cutting have been the predominant practices with the 1980s being the period of most intense harvest. These factors have reduced habitat diversity, connectivity, water quantity and quality, and riparian function in many assessment units within the basin. However, some of the assessment units contain headwater areas that are in relatively pristine condition and serve as strongholds for listed species and species of concern. The primary habitat conditions in the Entiat Subbasin that currently limit salmon and steelhead include reduced stream channel configuration and complexity from logging, and flood control measures that straightened and removed large woody debris from the channel. These historical and ongoing activities have led to a condition with low instream habitat diversity including few pools, lack of large wood accumulations, and disconnected side channels, wetlands, and floodplains. The result is a reduction in resting and rearing areas for both adult and juvenile salmon throughout the Entiat River Recent habitat restoration activities Since the mid- to late 1990s, various organizations have been coordinating, developing, and implementing habitat restoration projects in all of the subbasins within the Upper Columbia Region. The focus of these projects has been to reduce the effects of ecological concerns (formerly known as limiting factors) that impact environmental factors that influence productivity and abundance of salmonids. In a recent analysis of the implementation of restoration projects, (UCSRB 2014) determined that to date (early 2014), 278 projects have been completed, which has restored 22 miles of stream, 11 miles of off-channel habitat, and 127 acres of riparian forest. Ninety-three fish passage barriers have been removed that opened up 282 miles of habitat. Over 3,300 acres of habitat and 47 miles of stream bank have been protected. These projects have undoubtedly changed the environmental baseline and will continue to do so in the future Carlton Complex fire On July 14, 2014, lightning strikes started the Carlton Complex fire, the largest fire ever recorded in Washington. The fire burned approximately 253,377 acres, extending from the Columbia upstream nearly to Winthrop (Figure 7). The large amount of new barren hillsides will result in higher runoff rates generally, and higher impacts from rain and snow events. Massive mudslides have occurred in the basin as a result of the fire (G. Mackey, DPUD, personal communication, August 2014). The impact on salmonids still remains to be seen, but it is inconceivable that this fire will not have a serious impact on salmonid productivity for the next few 31
40 years. Climate models have predicted that climate change has contributed to the frequency and magnitude of fires in the Pacific Northwest (Rogers et al. 2011). Figure 7. Perimeter map of Carlton Complex fire ( accessed August 27, 2014) Artificial Propagation Another important aspect of the environmental baseline is hatchery effects effects from hatchery programs located in the action area. The relevance of older (pre 1970s) programs to the current status of fish in these basins is questionable, except for some potential legacy effects discussed below. Of more concern are immediate effects, such as handling, predation, competition, and disease. Therefore, this opinion will focus more on effects of relatively recent programs. Currently, there are spring Chinook salmon, summer Chinook salmon, coho salmon, and steelhead programs in the Methow Subbasin. In the Entiat Subbasin, steelhead releases ceased in 1999, and the long-term spring Chinook salmon program transitioned to a summer Chinook salmon program in Release numbers of all species except coho salmon have recently been revised to reflect the terms of mitigation agreements that allow adjustment based on no-net-impact (NNI) analyses of 32
41 dam mortalities(chelan County Public Utility District 2002a; Chelan County Public Utility District 2002b; Douglas County Public Utility District 2002) History of hatcheries in the Upper Columbia Hatcheries in the Upper Columbia Basin began their operations as early as the 1890s. The first hatcheries that released spring Chinook began operation in 1899 on the Wenatchee River (Chiwaukum Creek) and near the confluence of the Twisp River on the Methow River. These hatcheries, operated by WDFW, were built to replenish salmon runs (primarily Chinook and coho) that had virtually been eliminated by the 1890s (Gilbert and Everman 1895). The Leavenworth National Fish Hatchery Complex was constructed between 1938 and The Complex consists of three large hatchery facilities, Leavenworth National Fish Hatchery (LNFH), Entiat National Fish Hatchery (ENFH), and Winthrop National Fish Hatchery (WNFH), which are operated by the USFWS. They were constructed as mitigation facilities to compensate for the loss of spawning and rearing habitat caused by the construction of Grand Coulee Dam. The facility planned for the Okanogan River was never constructed. These programs were authorized as part of the Grand Coulee Fish Maintenance Project (GCFMP) 3 on April 3, 1937, and reauthorized by the Mitchell Act (52 Stat. 345) on May 11, In 1989, new artificial propagation programs funded by Chelan PUD began as mitigation for Rock Island Dam, including the Chiwawa River spring Chinook program. In 1991, Douglas PUD began funding artificial propagation programs of spring Chinook salmon in the Methow Subbasin as mitigation for Wells Dam. In 2002, HCP agreements among CPUD, DPUD, NMFS, USFWS, WDFW, the Colville Tribes, and the Yakama Nation formalized funding and actions for continued operation of the hatchery programs initiated in the 1960s and the relatively newer programs started in 1989 and Recently, the GPUD has begun spring Chinook programs in the Wenatchee River, and the Colville Tribes operate a (non-listed) spring Chinook salmon program at Chief Joseph Hatchery. In 2014, the Colville Tribe also received listed spring Chinook salmon from WNFH for release into the Okanogan Subbasin Spring Chinook salmon The Entiat National Fish Hatchery released spring Chinook from 1976 to the 2007 (last release). Monitoring showed that nearly half of the naturally spawning population was made up of returning hatchery fish, which were primarily Carson stock ancestry. Ford et al. (2004) found that the genetic similarity between Entiat River wild and Entiat NFH spring Chinook samples suggests that Entiat NFH salmon have successfully spawned and introgressed into or replaced the natural Entiat River population. Because of these findings and continued 2 The concept of NNI as defined by the HCPs requires 100 percent survival of plan species passing the hydroelectric projects of the agreements. NNI consists of two components: (1) 91 percent Combined Adult and Juvenile Project Survival achieved by project improvement measures implemented within the geographic area of the Project and (2) 9 percent compensation for Unavoidable Project Mortality provided through hatchery and tributary programs, with 7 percent compensation provided through hatchery programs and 2 percent compensation provided through tributary programs. The 7 percent hatchery component is recalculated every 10 years and is based on juvenile survival through the projects and the abundance of adults returning past the projects. 3 Historically, artificial propagation efforts have also had a significant impact on spring-run populations, either through hatchery-based enhancement or the extensive trapping and transportation activities associated with the GCFMP. Because spring-run Chinook salmon populations were at severely depressed levels at the time of the GCFMP, naturally spawning populations in this ESU were founded by the same GCFMP homogenized stock. 33
42 concerns that the Entiat NFH fish were a potential threat to the natural-origin spring Chinook salmon population in the Entiat River basin, the spring Chinook program was discontinued in The WNFH cultured and released mixed-origin Carson-stock 4 spring Chinook salmon for decades in the Methow Subbasin (ODFW and CTUIR 2010). The WNFH began rearing spring Chinook salmon in 1974 using Carson stock. After the UCR Spring Chinook salmon ESU was listed under the ESA in 1999, the WNFH spring Chinook salmon program switched to the Methow composite stock (see description below) in Currently, the program collects broodstock for rearing of 600,000 smolts per year; 400,000 are released into the Methow River and the remaining 200,000 are transferred to Chief Joseph Hatchery for use in a reintroduction program in the Okanogan River operated by the Confederated Tribes of the Colville Reservation. Between 1994 and 2014, the average number of spring Chinook salmon released from WNFH is just under 623,000, and has ranged from approximately 15,000 to 1.2 million (Figure 8). A state-run, PUD-funded spring Chinook salmon program began at the Methow Hatchery in 1992, and produced up to 550,000 smolts annually. The program has since been reduced to 223,764 fish per year because of NNI recalculations. Originally, the program s intent was to use only natural-origin fish and to keep separate populations for the Twisp, Upper Methow and Chewuch rivers. However, keeping the separate populations was difficult because of low numbers of adults returning and the presence of out-of-basin stray hatchery fish returning to the Methow Subbasin. Because of these difficulties, the co-managers decided to combine the Chewuch and Methow stocks into the Methow Composite stock beginning in The number of spring Chinook salmon released into the Methow Subbasin from the Methow hatchery between has averaged approximately 332,000 fish and ranged from approximately 29,000 to 612,000 (Figure 8). The total releases of spring Chinook salmon from both the Methow and Winthrop hatcheries has ranged from approximately 44,000 to 1.4 million fish (Figure 8). Number Smolts Released Methow Hatchery Winthrop NFH Total Release Year 4 The Carson stock originated from fish trapped from the spring Chinook salmon run passing Bonneville Dam. Genetic data indicate that the Carson stock was derived from a mixture of upper Columbia and Snake River populations. The first returns to Carson NFH occurred in This hatchery stock of spring Chinook salmon has been maintained since. Carson-origin spring Chinook salmon eggs, fry, and fingerlings have been transferred to many localities within the Northwest including Alaska. In addition to Carson NFH, this stock is currently being propagated at several hatcheries, including Leavenworth and Little White Salmon NFHs, Ringold Springs, and Umatilla. 34
43 Figure 8. Annual spring Chinook salmon releases into the Methow Subbasin, (Cooper 2014b; Snow 2014) Summer Chinook salmon Recently, the Entiat NFH transitioned from propagating spring Chinook salmon to summer Chinook salmon (NMFS 2013; USFWS 2009a), with a goal of releasing 400,000 fish annually. Summer/fall Chinook salmon programs also exist in the Methow Subbasin. These programs originated with the HCP settlement agreements (with the exception of the Carlton program) and have been operated under permit 1347 (NMFS 2003b). The Methow program originally released 400,000 fish but has been resized to release 200,000 fish. Releases of summer/fall Chinook in the Methow River have averaged approximately 382,000, ranging from approximately 205,000 to 541,000 fish. Number Smolts Released 600, , , , , ,000 0 Release Year Figure 9. Releases of summer/fall Chinook salmon from the Methow (Carlton) acclimation pond (Snow et al. 2014) Steelhead Methow (Carlton) Mean The Wells steelhead program began in Until 1998, steelhead released into all UCR subbasins were produced at Wells Hatchery from broodstock collected at Wells Dam and Priest Rapids Dam. From 1964 to 1983, steelhead broodstock were obtained at Priest Rapids Dam, which were propagated at Chelan Fish Hatchery. From 1984 through 1995, broodstock for steelhead production throughout the entire UCR was derived from Wells Dam and fish hatchery. WDFW initiated changes in mitigation hatchery steelhead production in 1996, which re-directed artificial production programs toward development of locally adapted broodstocks and improvement in the perceived fitness of the Wells Fish Hatchery population. Some production was moved to Eastbank Hatchery with the creation of the Wenatchee program (using broodstock collected in the Wenatchee Subbasin). This program was authorized to release up to 400,000 fish annually, and was recently resized by the HCP Hatchery Committee to 247,300 fish in Up until 1999, 40,000 hatchery steelhead were released (as part of the Rock Island Settlement Agreement) into the Entiat River. The HCP developed a Biological Assessment and Management Plan (NMFS et al. 1998), and recommended that one population within the Upper Columbia (referred to then as the mid-columbia) region be 35
44 a reference population that had no hatchery fish released. This suggestion allowed for a comparison in trends of abundance and other indicators between the reference population and other populations where hatchery fish were (are) released, for an estimate of hatchery program s effectiveness. This recommendation was adopted for the Entiat River in 1999, and hatchery steelhead have not been released in the basin since then. Wells stock continues to be released by Wells Hatchery, but the Wells Hatchery Committee continues to explore options for development of a locally adapted broodstock program. WNFH is using an endemic Methow stock for conservation purposes. Historically, the two hatcheries have been authorized to release up to 448,000 steelhead into the Methow Subbasin, but this has been reduced to 348,000 because of the NNI recalculation. Releases in the Methow River (including a small number of fish released into the Columbia River) have averaged nearly 413,000 fish from 1992 to 2013, ranging from approximately 260,000 to 711,000. Releases from WNFH have averaged approximately 113,000, ranging from about 99,000 to 150,000 fish between 1999 and The Wells program has released fish into the Methow, Twisp, Chewuch and Columbia Rivers (Figure 10). Returning fish from both programs interbreed with natural-origin fish and the percentage of hatchery-origin fish on the spawning grounds is very high (~76 percent; NWFSC 2015). Figure 10. Total summer steelhead smolt releases into the Methow Subbasin (Snow et al. 2014; USFWS 2012c). Number Smolts Released Number Smolts Released , , , , , , , , Methow River (WNFH) Coho salmon Methow Total Mean Methow River (Wells) Twisp River Release Release Year Location Chewuch River Columbia River Methow Basin Total Figure 11. Mean summer steelhead smolt releases by location (DART, (Snow et al. 2014; USFWS 2012c). Substantial natural coho salmon production has not existed in the Upper Columbia Region for many decades (YN 2012). Sizable releases of hatchery coho salmon occurred sporadically since 1942 (Mullan 1984). However, in the mid-1990s, the Yakama Nation began a pilot program to reintroduce coho into the Wenatchee and Methow Subbasins (mid-columbia coho reintroduction program (MCCRP)). That program was recently granted full implementation status, and annual releases into the Methow Subbasin have averaged about 332,000 and ranged from approximately 74,000 to 538,000 (excluding 1999, when no fish were released). The previous goal in the Methow River was to release 500,000 fish, and now with full implementation, the goal for the next phase of the project has risen to 1.0 million. 36
45 Number Smolts Released 600, , , , , ,000 0 Methow Total Mean Release Year Figure 12. Annual coho salmon smolt releases into the Methow Subbasin (Kamphaus 2013) Harvest The final important aspect of the environmental baseline is harvest effects effects of fisheries located inside the Entiat and Methow Subbasins. Spring Chinook salmon are not harvested in these subbasins, although selective fisheries for adult management purposes (similar to that done in the Wenatchee Basin (WDFW 2010)) is a future possibility. Mark-selective steelhead fisheries operate in both subbasins under permit 1395 (NMFS 2003a). Mainstem Columbia River fisheries targeting Chinook salmon and steelhead produced by these programs have been evaluated and authorized under a separate opinion under the United States v. Oregon Management Agreement (NMFS 2008c) Other Congress established the Pacific Coastal Salmon Recovery Fund (PCSRF) to help protect and recover salmon and steelhead populations and their habitats (NMFS 2006). The states of Washington, Oregon, California, Idaho, and Alaska, and the Pacific Coastal and Columbia River Tribes receive PCSRF appropriations from NMFS each year. The fund supplements existing state, tribal, and local programs to foster development of Federal-state-tribal-local partnerships in salmon and steelhead recovery. The PCSRF has made substantial progress in achieving program goals, as indicated in annual Reports to Congress, workshops, and independent reviews. Information relevant to the environmental baseline is also discussed in detail in Chapter 5 of the Supplemental Comprehensive Analysis (SCA), which in turn cross-references back to the related 2008 FCRPS biological opinion (NMFS 2008b; NMFS 2008d). Chapter 5 of the SCA, and related portions of the FCRPS Opinion, provide an analysis of the effects of past and ongoing human and natural factors on the current status of the species, their habitats and ecosystems, within the entire Columbia River Basin. In addition, chapter 5 of the SCA, and related portions of the FCRPS Opinion, evaluate the effects of those ongoing actions on designated critical habitat with that same area. Those portions of chapter 5 of the SCA, and environmental baseline section of the FCRPS Opinion, that deal with effects in the action area (as described in Section 1.4 above) are hereby incorporated here by reference. In addition, the environmental baseline for this opinion includes the impacts of the Proposed Action described in the FCRPS and Reclamation biological opinions Effects on ESA Protected Species and on Designated Critical Habitat This section describes the effects of the Proposed Action, independent of the environmental baseline and cumulative effects. The methodology and best scientific information NMFS utilizes for analyzing hatchery 37
46 effects is summarized first in Section 2.4.1, and application of the methodology and analysis of the Proposed Action itself follows in Section Under the ESA, effects of the action means the direct and indirect effects of an action on the species or critical habitat, together with the effects of other activities that are interrelated or interdependent with that action, that will be added to the environmental baseline (50 CFR ). Indirect effects are those that are caused by the proposed action and are later in time, but still are reasonably certain to occur. Effects of the Proposed Action that are expected to occur later in time (i.e., after the 10-year timeframe of the Proposed Action) are included in the analysis in this opinion to the extent they can be meaningfully evaluated. A more comprehensive analysis of whether the Proposed Action is likely to appreciably reduce the likelihood of survival and recovery of ESA protected species or result in the destruction or adverse modification of their designated critical habitat is discussed in Section Factors Considered When Analyzing Hatchery Effects NMFS has substantial experience with hatchery programs and has developed and published a series of guidance documents for designing and evaluating hatchery programs following best available science 5 (Hard et al. 1992; Jones Jr. 2006; McElhany et al. 2000; NMFS 2004b; NMFS 2005c; NMFS 2008a; NMFS 2011c). A key factor in analyzing a hatchery program for its effects, positive and negative, on the status of salmon and steelhead is to consider the genetic resources that reside in the program. Such genetic resources can represent the ecological and genetic diversity of a species. Hatchery programs with a level of genetic divergence relative to the local natural population(s) that is no more than what occurs within the ESU are considered part of the ESU and will be included in any listing of the ESU (NMFS 2005c). NMFS monitors hatchery practices for whether they promote the conservation of genetic resources included in a salmon ESU or steelhead DPS and updates the status of genetic resources residing in hatchery programs every five years. Jones Jr. (2015) provides the most recent update of the relatedness of Pacific Northwest hatchery programs to salmon ESUs and steelhead DPSs listed under the ESA. Generally speaking, hatchery programs that are reproductively connected or integrated with a natural population, if one still exists, contain genetic resources that represent the ecological and genetic diversity of a species and are included in an ESU or steelhead DPS. When a hatchery program actively maintains distinctions or promotes differentiation between hatchery fish and fish from a native population, NMFS refers to the program as isolated (also sometimes referred to as a segregated program). Generally speaking, isolated hatchery programs have a level of genetic divergence, relative to the local natural population(s), that is more than what occurs within the ESU and are not considered part of an ESU or steelhead DPS. They promote domestication or selection in the hatchery over selection in the wild and select for and culture a stock of fish with different phenotypes (e.g., different ocean migrations and spatial and temporal spawning distribution) compared to the native population (extant in the wild, in a hatchery, or both). For Pacific salmon, NMFS evaluates extinction processes and effects of the Proposed Action beginning at the population scale (McElhany et al. 2000). NMFS defines population performance measures in terms of natural-origin fish and four key parameters or attributes (abundance, productivity, spatial structure, and diversity), then relates effects of the Proposed Action at the population scale to the MPG level, and ultimately to the survival and recovery of an entire ESU or DPS. A Proposed Action is analyzed for effects, positive and negative, on the attributes that define population viability, including abundance, productivity, spatial structure, and diversity. Because of the potential for circumventing the high rates of early mortality typically experienced in the wild, artificial propagation may be useful in the recovery of listed salmon species. However, artificial propagation entails risks as well as opportunities for salmon conservation (Hard et al. 1992). The effects of a hatchery program on the status of an ESU or steelhead DPS will depend on which of the four key attributes are currently limiting the ESU, and how the hatchery fish within the ESU affect each of the attributes (NMFS 2005c). The presence of hatchery fish 5 These documents are available upon request from NMFS SFD in Portland, Oregon. 38
47 within the ESU can positively affect the overall status of the ESU by increasing the number of natural spawners, by serving as a source population for repopulating unoccupied habitat and increasing spatial distribution, and by conserving genetic resources. Conversely, a hatchery program managed without adequate consideration can affect a listing determination by reducing adaptive genetic diversity of the ESU, and by reducing the reproductive fitness and productivity of the ESU (NMFS 2005c). NMFS also analyzes and takes into account the effects of hatchery facilities (e.g., weirs and water diversions) on each VSP attribute and on designated critical habitat. NMFS analysis of the Proposed Action is in terms of effects the Proposed Action would be expected to have on ESA-listed species and on designated critical habitat, based on the best scientific information available. This analysis allows for quantification (wherever possible) of the various factors of hatchery operation to be applied to each applicable life-stage of the listed species at the population level (described in Section 2.4.2), which in turn allows the combination of all such effects with other effects accruing to the species to determine the likelihood of posing jeopardy to the species as a whole (described in Section 2.6). The effects, positive and negative, for the two categories of hatchery programs are summarized in Table 13. Generally speaking, effects range from beneficial to negative when programs use local fish 6 for hatchery broodstock, and from negligible to negative when programs do not use local fish for broodstock 7. Hatchery programs can benefit population viability, but only if they use genetic resources that represent the ecological and genetic diversity of the target or affected natural population(s). When hatchery programs use genetic resources that do not represent the ecological and genetic diversity of the target or affected natural population(s), NMFS is particularly interested in how effective the program will be at isolating hatchery fish and at avoiding co-occurrence and effects that potentially disadvantage fish from natural populations. NMFS applies available scientific information, identifies the types of circumstances and conditions that are unique to individual hatchery programs, then refines the range in effects for a specific hatchery program. Information that NMFS needs to analyze the effects of a hatchery program on ESA-listed species must be included in an HGMP. Draft HGMPs are reviewed by NMFS for their sufficiency before formal review and analysis of the Proposed Action can begin. Analysis of an HGMP or Proposed Action for its effects on ESA-listed species and on designated critical habitat depends on seven factors. These factors are: (1) the hatchery program does or does not remove fish from the natural population and use them for hatchery broodstock, (2) hatchery fish and the progeny of naturally spawning hatchery fish on spawning grounds and encounters with natural-origin and hatchery fish at adult collection facilities, (3) hatchery fish and the progeny of naturally spawning hatchery fish in juvenile rearing areas, (4) hatchery fish and the progeny of naturally spawning hatchery fish in the migration corridor, estuary, and ocean, (5) RM&E that exists because of the hatchery program, (6) operation, maintenance, and construction of hatchery facilities that exist because of the hatchery program, and (7) fisheries that exist because of the hatchery program, including terminal fisheries intended to reduce the escapement of hatchery-origin fish to spawning grounds. The analysis assigns an effect for each factor from the following categories: (1) positive or beneficial effect on population viability, 6 The term local fish is defined to mean fish with a level of genetic divergence relative to the local natural population(s) that is no more than what occurs within the ESU or steelhead DPS (70 FR 37215, June 28, 2005). 7 Exceptions include restoring extirpated populations and gene banks. 39
48 (2) negligible effect on population viability, and (3) negative effect on population viability. The effects of hatchery fish on ESU/DPS status will depend on which of the four VSP criteria are currently limiting the ESU/DPS and how the hatchery program affects each of the criteria (NMFS 2005c). The category of effect assigned to a factor is based on an analysis of each factor weighed against each affected population s current risk level for abundance, productivity, spatial structure, and diversity, the role or importance of the affected natural population(s) in ESU or steelhead DPS recovery, the target viability for the affected natural population(s), and the environmental baseline including the factors currently limiting population viability. Table 13. An overview of the range of effects on natural population viability parameters from the two 40
49 categories of hatchery programs. Natural population viability parameter Productivity Diversity Abundance Spatial Structure Hatchery broodstock originate from the local population and are included in the ESU or DPS Positive to negative effect Hatcheries are unlikely to benefit productivity except in cases where the natural population s small size is, in itself, a predominant factor limiting population growth (i.e., productivity) (NMFS 2004c). Positive to negative effect Hatcheries can temporarily support natural populations that might otherwise be extirpated or suffer severe bottlenecks and have the potential to increase the effective size of small natural populations. On the other hand, broodstock collection that homogenizes population structure is a threat to population diversity. Positive to negative effect Hatchery-origin fish can positively affect the status of an ESU by contributing to the abundance of the natural populations in the ESU (70 FR 37204, June 28, 2005, at 37215). Increased abundance can also increase density dependent effects. Positive to negative effect Hatcheries can accelerate re-colonization and increase population spatial structure, but only in conjunction with remediation of the factor(s) that limited spatial structure in the first place. Any benefits to spatial structure over the long term depend on the degree to which the hatchery stock(s) add to (rather than replace) natural populations (70 FR 37204, June 28, 2005 at 37213). Hatchery broodstock originate from a non-local population or from fish that are not included in the same ESU or DPS Negligible to negative effect Productivity is dependent on differences between hatchery fish and the local natural population (i.e., the more distant the origin of the hatchery fish, the greater the threat), the duration and strength of selection in the hatchery, and the level of isolation achieved by the hatchery program (i.e., the greater the isolation, the closer to a negligible effect). Negligible to negative effect Diversity is dependent on the differences between hatchery fish and the local natural population (i.e., the more distant the origin of the hatchery fish, the greater the threat) and the level of isolation achieved by the hatchery program (i.e., the greater the isolation, the closer to a negligible effect). Negligible to negative effect Abundance is dependent on the level of isolation achieved by the hatchery program (i.e., the greater the isolation, the closer to a negligible effect), handling, RM&E, and facility operation, maintenance and construction effects. Negligible to negative effect Spatial structure is dependent on facility operation, maintenance, and construction effects and the level of isolation achieved by the hatchery program (i.e., the greater the isolation, the closer to a negligible effect) Factor 1. The hatchery program does or does not remove fish from the natural population and use them for hatchery broodstock This factor considers the risk to a natural population from the removal of natural-origin fish for hatchery broodstock. The level of effect for this factor ranges from neutral or negligible to negative. A primary consideration in analyzing and assigning effects for broodstock collection is the origin and number of fish collected. The analysis considers whether broodstock are of local origin and the biological pros and cons of using ESA-listed fish (natural or hatchery-origin) for hatchery broodstock. It considers the maximum number of 41
50 fish proposed for collection and the proportion of the donor population tapped to provide hatchery broodstock. Mining a natural population to supply hatchery broodstock can reduce population abundance and spatial structure. Also considered here is whether the program backfills with fish from outside the local or immediate area. The physical process of collecting hatchery broodstock and the effect of the process on ESA-listed species is considered under Factor Factor 2. Hatchery fish and the progeny of naturally spawning hatchery fish on spawning grounds and encounters with natural-origin and hatchery fish at adult collection facilities NMFS also analyzes the effects of hatchery fish and the progeny of naturally spawning hatchery fish on the spawning grounds. The level of effect for this factor ranges from positive to negative. There are two aspects to this part of the analysis: genetic effects and ecological effects. NMFS generally views genetic effects as detrimental because we believe that artificial breeding and rearing is likely to result in some degree of genetic change and fitness reduction in hatchery fish and in the progeny of naturally spawning hatchery fish relative to desired levels of diversity and productivity for natural populations based on the weight of available scientific information at this time. Hatchery fish can thus pose a risk to diversity and to natural population rebuilding and recovery when they interbreed with fish from natural populations. However, NMFS recognizes that beneficial effects exist as well, and that the risks just mentioned may be outweighed under circumstances where demographic or short-term extinction risk to the population is greater than risks to population diversity and productivity. Conservation hatchery programs may accelerate recovery of a target population by increasing abundance faster than may occur naturally (Waples 1999). Hatchery programs can also be used to create genetic reserves for a population to prevent the loss of its unique traits due to catastrophes (Ford 2011). NMFS also recognizes there is considerable debate regarding genetic risk. The extent and duration of genetic change and fitness loss and the short- and long-term implications and consequences for different species (i.e., for species with multiple life-history types and species subjected to different hatchery practices and protocols) remain unclear and should be the subject of further scientific investigation. As a result, NMFS believes that hatchery intervention is a legitimate and useful tool to alleviate short-term extinction risk, but otherwise managers should seek to limit interactions between hatchery and natural-origin fish and implement hatchery practices that harmonize conservation with the implementation of treaty Indian fishing rights and other applicable laws and policies (NMFS 2011d) Genetic effects Hatchery fish can have a variety of genetic effects on natural population productivity and diversity when they interbreed with natural-origin fish. Although there is biological interdependence between them, NMFS considers three major areas of genetic effects of hatchery programs: within-population diversity, outbreeding effects, and hatchery-induced selection. As we have stated above, in most cases, the effects are viewed as risks, but in small populations these effects can sometimes be beneficial, reducing extinction risks. First, within-population genetic diversity is a general term for the quantity, variety, and combinations of genetic material in a population (Busack and Currens 1995). Within-population diversity is gained through mutations or gene flow from other populations (described below under outbreeding effects) and is lost primarily due to genetic drift, a random loss of diversity due to population size. The rate of loss is determined by the population s effective population size (Ne), which can be considerably smaller than its census size. For a population to maintain genetic diversity reasonably well, the effective size should be in the hundreds (e.g., Lande 1987), and diversity loss can be severe if Ne drops to a few dozen. 42
51 Hatchery programs, simply by virtue of creating more fish, can increase Ne. In very small populations, this increase can be a benefit, making selection more effective and reducing other small-population risks (e.g., (e.g., Lacy 1987; Whitlock 2000; Willi et al. 2006). Conservation hatchery programs can thus serve to protect genetic diversity; several programs, such as the Snake River sockeye salmon program, are important genetic reserves. However, hatchery programs can also directly depress Ne by two principal methods. One is by the simple removal of fish from the population so that they can be used in the hatchery broodstock. If a substantial portion of the population is taken into a hatchery, the hatchery becomes responsible for that portion of the effective size, and if the operation fails, the effective size of the population will be reduced (Waples and Do 1994). Two is when Ne is reduced considerably below the census number of broodstock by using a skewed sex ratio, spawning males multiple times (Busack 2007), and by pooling gametes. Pooling semen is especially problematic because when semen of several males is mixed and applied to eggs, a large portion of the eggs may be fertilized by a single male (Gharrett and Shirley 1985; Withler 1988). An extreme form of Ne reduction is the Ryman-Laikre effect (Ryman et al. 1995; Ryman and Laikre 1991), when Ne is reduced through the return to the spawning grounds of large numbers of hatchery fish from very few parents. On the other hand, factorial mating schemes, in which fish are systematically mated multiple times, can be used to increase Ne (Busack and Knudsen 2007; Fiumera et al. 2004). Inbreeding depression, another Ne-related phenomenon, is caused by the mating of closely related individuals (e.g., siblings, half-siblings, cousins). The smaller the population, the more likely spawners will be related. Related individuals are likely to contain similar genetic material, and the resulting offspring may then have reduced survival because they are less variable genetically or have double doses of deleterious mutations. The lowered fitness of fish due to inbreeding depression accentuates the genetic risk problem, helping to push a small population toward extinction. Outbreeding effects, the second major area of genetic effects of hatchery programs, are caused by gene flow from other populations. Gene flow occurs naturally among salmon and steelhead populations, a process referred to as straying (Quinn 1993; Quinn 1997). Natural straying serves a valuable function in preserving diversity that would otherwise be lost through genetic drift and in re-colonizing vacant habitat, and straying is considered a risk only when it occurs at unnatural levels or from unnatural sources. Hatchery programs can result in straying outside natural patterns for two reasons. First, hatchery fish may exhibit reduced homing fidelity relative to natural-origin fish (Goodman 2005; Grant 1997; Jonsson et al. 2003; Quinn 1997), resulting in unnatural levels of gene flow into recipient populations, either in terms of sources or rates. Second, even if hatchery fish home at the same level of fidelity as natural-origin fish, their higher abundance can cause unnatural straying levels into recipient populations. One goal for hatchery programs should be to ensure that hatchery practices do not lead to higher rates of genetic exchange with fish from natural populations than would occur naturally (Ryman 1991). Rearing and release practices and ancestral origin of the hatchery fish can all play a role in straying (Quinn 1997). Gene flow from other populations can have two effects. It can increase genetic diversity (e.g., Ayllon et al. 2006), which can be a benefit in small populations, but it can also alter established allele frequencies (and coadapted gene complexes) and reduce the population s level of adaptation, a phenomenon called outbreeding depression (Edmands 2007; McClelland and Naish 2007). In general, the greater the geographic separation between the source or origin of hatchery fish and the recipient natural population, the greater the genetic difference between the two populations (ICTRT 2007b), and the greater potential for outbreeding depression. For this reason, NMFS advises hatchery action agencies to develop locally derived hatchery broodstock. Additionally, unusual rates of straying into other populations within or beyond the population s MPG, salmon ESU, or a steelhead DPS can have an homogenizing effect, decreasing intra-population genetic variability (e.g.(vasemagi et al. 2005), and increasing risk to population diversity, one of the four attributes measured to 43
52 determine population viability. Reduction of within-population and among-population diversity can reduce adaptive potential. The proportion of hatchery fish (phos) 8 among natural spawners is often used as a surrogate measure of gene flow. Appropriate cautions and qualifications should be considered when using this proportion to analyze outbreeding effects. Adult salmon may wander on their return migration, entering and then leaving tributary streams before spawning (Pastor 2004). These dip-in fish may be detected and counted as strays, but may eventually spawn in other areas, resulting in an overestimate of the number of strays that potentially interbreed with the natural population (Keefer et al. 2008). Caution must also be taken in assuming that strays contribute genetically in proportion to their abundance. Several studies demonstrate little genetic impact from straying despite a considerable presence of strays in the spawning population (Blankenship et al. 2007; Saisa et al. 2003). The causative factors for poorer breeding success of strays are likely similar to those identified as responsible for reduced productivity of hatchery-origin fish in general, e.g., differences in run and spawn timing, spawning in less productive habitats, and reduced survival of their progeny (Leider et al. 1990; Reisenbichler and McIntyre 1977; Williamson et al. 2010). Hatchery-influenced selection (often called domestication), the third major area of genetic effects of hatchery programs, occurs when selection pressures imposed by hatchery spawning and rearing differ greatly from those imposed by the natural environment and causes genetic change that is passed on to natural populations through interbreeding with hatchery-origin fish. These differing selection pressures can be a result of differences in environments or a consequence of protocols and practices used by a hatchery program. Hatchery-influenced selection can range from relaxation of selection that would normally occur in nature, to selection for different characteristics in the hatchery and natural environments, to intentional selection for desired characteristics (Waples 1999). Genetic change and fitness reduction resulting from hatchery-influenced selection depends on: (1) the difference in selection pressures; (2) the exposure or amount of time the fish spends in the hatchery environment; and (3) the duration of hatchery program operation (i.e., the number of generations that fish are propagated by the program). For an individual, the amount of time a fish spend in the hatchery mostly equates to fish culture. For a population, exposure is determined by the proportion of natural-origin fish in the hatchery broodstock, the proportion of natural spawners consisting of hatchery-origin fish (Ford 2002; Lynch and O'Hely 2001), and the number of years the exposure takes place. In assessing risk or determining impact, all three factors must be considered. Strong selective fish culture with low hatchery-wild interbreeding can pose less risk than relatively weaker selective fish culture with high levels of interbreeding. Most of the empirical evidence of fitness depression due to hatchery-influenced selection comes from studies of species that are reared in the hatchery environment for an extended period one to two years prior to release (Berejikian and Ford 2004). Exposure time in the hatchery for fall and summer Chinook salmon and Chum salmon is much shorter, just a few months. One especially well-publicized steelhead study (Araki et al. 2007; Araki et al. 2008), showed dramatic fitness declines in the progeny of naturally spawning Hood River hatchery steelhead. Researchers and managers alike have wondered if these results could be considered a potential outcome applicable to all salmonid species, life-history types, and hatchery rearing strategies, but researchers have not reached a definitive conclusion. Besides the Hood River steelhead work, a number of studies are available on the relative reproductive success (RRS) of hatchery- and natural-origin fish (e.g., Berntson et al. 2011; Ford et al. 2012; Hess et al. 2012; 8 It is important to reiterate that as NMFS analyzes them, outbreeding effects are a risk only when the hatchery fish are from a different population than the naturally produced fish. If they are from the same population, then the risk is from hatchery-influenced selection. 44
53 Theriault et al. 2011). All have shown that, generally, hatchery-origin fish have lower reproductive success; however, the differences have not always been statistically significant and, in some years in some studies, the opposite was true. Lowered reproductive success of hatchery-origin fish in these studies is typically considered evidence of hatchery-influenced selection. Although RRS may be a result of hatchery-influenced selection, studies must be carried out for multiple generations to unambiguously detect a genetic effect. To date, only the Hood River steelhead (Araki et al. 2007; Christie et al. 2011) and Wenatchee spring Chinook salmon (Ford et al. 2012) RRS studies have reported multiple-generation effects. Critical information for analysis of hatchery-induced selection includes the number, location, and timing of naturally spawning hatchery fish, the estimated level of gene flow between hatchery-origin and natural-origin fish, the origin of the hatchery stock (the more distant the origin compared to the affected natural population, the greater the threat), the level and intensity of hatchery selection and the number of years the operation has been run in this way. Efforts to control and evaluate the risk of hatchery-influenced selection are currently largely focused on gene flow between natural-origin and hatchery-origin fish 9. The Interior Columbia Technical Recovery Team (ICTRT) developed guidelines based on the proportion of spawners in the wild consisting of hatchery-origin fish (phos) (Figure 13). More recently, the Hatchery Scientific Review Group (HSRG) developed gene-flow guidelines based on mathematical models developed by (Ford 2002) and by(lynch and O'Hely 2001). Guidelines for isolated programs are based on phos, but guidelines for integrated programs are based also on a metric called proportionate natural influence (PNI), which is a function of phos and the proportion of natural-origin fish in the broodstock (pnob) 10. PNI is, in theory, a reflection of the relative strength of selection in the hatchery and natural environments; a PNI value greater than 0.5 indicates dominance of natural selective forces. The HSRG guidelines vary according to type of program and conservation importance of the population. When the underlying natural population is of high conservation importance, the guidelines are a phos of no greater than 5 percent for isolated programs. For integrated programs, the guidelines are a phos no greater than 30 percent and PNI of at least 67 percent for integrated programs (HSRG 2009). Higher levels of hatchery influence are acceptable, however, when a population is at high risk or very high risk of extinction due to low abundance and the hatchery program is being used to conserve the population and reduce extinction risk in the short-term. (HSRG 2004)offered additional guidance regarding isolated programs, stating that risk increases dramatically as the level of divergence increases, especially if the hatchery stock has been selected directly or indirectly for characteristics that differ from the natural population. The HSRG recently produced an update report (HSRG 2014) that stated that the guidelines for isolated programs may not provide as much protection from fitness loss as the corresponding guidelines for integrated programs. 9 Gene flow between natural-origin and hatchery-origin fish is often interpreted as meaning actual matings between natural-origin and hatchery-origin fish. In some contexts, it can mean that. However, in this document, unless otherwise specified, gene flow means contributing to the same progeny population. For example, hatchery-origin spawners in the wild will either spawn with other hatcheryorigin fish or with natural-origin fish. Natural-origin spawners in the wild will either spawn with other natural-origin fish or with hatchery-origin fish. But all these matings, to the extent they are successful, will generate the next generation of natural-origin fish. In other words, all will contribute to the natural-origin gene pool. 10 PNI is computed as pnob/(pnob+phos). This statistic is really an approximation of the true proportionate natural influence, but operationally the distinction is unimportant. 45
54 Figure 13. ICTRT (2007b) risk criteria associated with spawner composition for viability assessment of exogenous spawners on maintaining natural patterns of gene flow. Exogenous fish are considered to be all fish hatchery origin, and non-normative strays of natural origin. Another HSRG team recently reviewed California hatchery programs and developed guidelines that differed considerably from those developed by the earlier group (California HSRG 2012). The California HSRG felt that truly isolated programs in which no hatchery-origin returnees interact genetically with natural populations were impossible in California, and was generally unsupportive of the concept. However, if programs were to be managed as isolated, they recommend a phos of less than 5 percent. They rejected development of overall phos guidelines for integrated programs because the optimal phos will depend upon multiple factors, such as the amount of spawning by natural-origin fish in areas integrated with the hatchery, the value of pnob, the importance of the integrated population to the larger stock, the fitness differences between hatchery- and natural-origin fish, and societal values, such as angling opportunity. They recommended that program-specific plans be developed with corresponding population-specific targets and thresholds for phos, pnob, and PNI that reflect these factors. However, they did state that PNI should exceed 50 percent in most cases, although in supplementation or reintroduction programs the acceptable phos could be much higher than 5 percent, even approaching 100 percent at times. They also recommended for conservation programs that pnob approach 100 percent, but pnob levels should not be so high they pose demographic risk to the natural population. Discussions involving phos can be problematic due to variation in its definition. Most commonly, the term phos refers to the proportion of the total natural spawning population consisting of hatchery fish, and the term has been used in this way in all NMFS documents. However, the HSRG has defined phos inconsistently in its Columbia Basin system report, equating it with the proportion of the natural spawning population that is made up of hatchery fish in the Conclusion, Principles and Recommendations section (HSRG 2009), but with the proportion of effective hatchery origin spawners in their gene-flow criteria. In addition, in their Analytical 46
55 Methods and Information Sources section (appendix C in HSRG 2009) they introduce a new term, effective phos (phoseff) defined as the effective proportion of hatchery fish in the naturally spawning population. This confusion was cleared up in the 2014 update document, where it is clearly stated that the metric of interest is effective phos (HSRG 2014). The HSRG recognized that hatchery fish spawning naturally may on average produce fewer adult progeny than natural-origin spawners, as described above. To account for this difference the HSRG defined effective phos as: phoseff = RRS * phoscensus where phoscensus is the proportion of the naturally spawning population that is composed of hatchery-origin adults (HSRG 2014). In the 2014 report, the HSRG explicitly addressed the differences between census phos and effective phos, by defining PNI as: PNI = pnob (pnob + phoseff) NMFS feels that adjustment of census phos by RRS should be done very cautiously, not nearly as freely as the HSRG document would suggest because the Ford (2002) model, which is the foundation of the HSRG geneflow guidelines, implicitly includes a genetic component of RRS. In that model, hatchery fish are expected to have RRS < 1 (compared to natural fish) due to selection in the hatchery. A component of reduced RRS of hatchery fish is therefore already incorporated in the model and by extension the calculation of PNI. Therefore reducing phos values by multiplying by RRS will result in underestimating the relevant phos and therefore overestimating PNI. Such adjustments would be particularly inappropriate for hatchery programs with low pnob, as these programs may well have a substantial reduction in RRS due to genetic factors already incorporated in the model. In some cases, adjusting phos downward may be appropriate, however, particularly if there is strong evidence of a non-genetic component to RRS. Wenatchee spring Chinook salmon (Williamson et al. 2010) is an example case with potentially justified adjustment by RRS, where the spatial distribution of natural-origin and hatcheryorigin spawners differs, and the hatchery-origin fish tend to spawn in poorer habitat. However, even in a situation like the Wenatchee spring Chinook salmon, it is unclear how much of an adjustment would be appropriate. By the same logic, it might also be appropriate to adjust pnob in some circumstances. For example, if hatchery juveniles produced from natural-origin broodstock tend to mature early and residualize (due to non-genetic effects of rearing), as has been documented in some spring Chinook salmon and steelhead programs, the effective pnob might be much lower than the census pnob. It is also important to recognize that PNI is only an approximation of relative trait value, based on a model that is itself very simplistic. To the degree that PNI fails to capture important biological information, it would be better to work to include this biological information in the underlying models rather than make ad hoc adjustments to a statistic that was only intended to be rough guideline to managers. We look forward to seeing this issue further clarified in the near future. In the meantime, except for cases in which an adjustment for RRS has strong justification, NMFS feels that census phos, rather than effective phos, is the appropriate metric to use for genetic risk evaluation. Additional perspective on phos that is independent of HSRG modelling is provided by a simple analysis of the expected proportions of mating types. Figure 14 shows the expected proportion of mating types in a mixed population of natural-origin (N) and hatchery-origin (H) fish as a function of the census phos, assuming that N 47
56 and H adults mate randomly 11. For example, at a census phos level of 10 percent, 81 percent of the matings will be NxN, 18 percent will be NxH, and 1 percent will be HxH. This diagram can also be interpreted as probability of parentage of naturally produced progeny, assuming random mating and equal reproductive success of all mating types. Under this interpretation, progeny produced by a parental group with a phos level of 10 percent will have an 81 percent chance of having two natural-origin parents, etc. Random mating assumes that the natural-origin and hatchery-origin spawners overlap completely spatially and temporally. As overlap decreases, the proportion of NxH matings decreases; with no overlap, the proportion of NxN matings is 1 minus phos and the proportion of HxH matings equals phos. RRS does not affect the mating type proportions directly but changes their effective proportions. Overlap and RRS can be related. For example, in the Wenatchee River, hatchery spring Chinook salmon tend to spawn lower in the system than natural-origin fish, and this accounts for a considerable amount of their lowered reproductive success (Williamson et al. 2010). In that particular situation the hatchery-origin fish were spawning in inferior habitat. Figure 14. Relative proportions of types of matings as a function of proportion of hatchery-origin fish on the spawning grounds (phos) Ecological effects Ecological effects for this factor (i.e., hatchery fish and the progeny of naturally spawning hatchery fish on the spawning grounds) refer to effects from competition for spawning sites and redd superimposition, contributions to marine-derived nutrients, and the removal of fine sediments from spawning gravels. Ecological effects on the spawning grounds may be positive or negative. To the extent that hatcheries contribute added fish to the ecosystem, there can be positive effects. For example, when anadromous salmonids return to spawn, hatcheryorigin and natural-origin alike, they transport marine-derived nutrients stored in their bodies to freshwater and terrestrial ecosystems. Their carcasses provide a direct food source for juvenile salmonids and other fish, aquatic invertebrates, and terrestrial animals, and their decomposition supplies nutrients that may increase 11 These computations are purely theoretical, based on a simple mathematical binomial expansion ((a+b) 2 =a 2 + 2ab + b 2 ). 48
57 primary and secondary production (Gresh et al. 2000; Kline et al. 1990; Larkin and Slaney 1996; Murota 2003; Piorkowski 1995; Quamme and Slaney 2003; Wipfli et al. 2003). As a result, the growth and survival of juvenile salmonids may increase (Bell 2001; Bilton et al. 1982; Bradford et al. 2000; Brakensiek 2002; Hager and Noble 1976; Hartman and Scrivener 1990; Holtby 1988; Johnston et al. 1990; Larkin and Slaney 1996; Quinn and Peterson 1996; Ward and Slaney 1988). Additionally, studies have demonstrated that perturbation of spawning gravels by spawning salmonids loosens cemented (compacted) gravel areas used by spawning salmon (e.g., (Montgomery et al. 1996). The act of spawning also coarsens gravel in spawning reaches, removing fine material that blocks interstitial gravel flow and reduces the survival of incubating eggs in egg pockets of redds. The added spawner density resulting from hatchery-origin fish spawning in the wild can have negative consequences at times. In particular, the potential exists for hatchery-derived fish to superimpose or destroy the eggs and embryos of ESA-listed species when there is spatial overlap between hatchery and natural spawners. Redd superimposition has been shown to be a cause of egg loss in pink salmon and other species (e.g., Fukushima et al. 1998) Adult Collection Facilities The analysis also considers the effects from encounters with natural-origin fish that are incidental to broodstock collection. Here, NMFS analyzes effects from sorting, holding, and handling natural-origin fish in the course of broodstock collection. Some programs collect their broodstock from fish voluntarily entering the hatchery, typically into a ladder and holding pond, while others sort through the run at large, usually at a weir, ladder, or sampling facility. Generally speaking, the more a hatchery program accesses the run at large for hatchery broodstock that is, the more fish that are handled or delayed during migration the greater the negative effect on natural-origin and hatchery-origin fish that are intended to spawn naturally and on ESA-listed species. The information NMFS uses for this analysis includes a description of the facilities, practices, and protocols for collecting broodstock, the environmental conditions under which broodstock collection is conducted, and the encounter rate for ESA-listed fish. NMFS also analyzes the effects of structures, either temporary or permanent, that are used to collect hatchery broodstock, and remove hatchery fish from the river or stream and prevent them from spawning naturally, on juvenile and adult fish from encounters with these structures. NMFS determines through the analysis, for example, whether the spatial structure, productivity, or abundance of a natural population is affected when fish encounter a structure used for broodstock collection, usually a weir or ladder Factor 3. Hatchery fish and the progeny of naturally spawning hatchery fish in juvenile rearing areas NMFS also analyzes the potential for competition and predation when the progeny of naturally spawning hatchery fish and hatchery releases share juvenile rearing areas. The level of effect for this factor ranges from neutral or negligible to negative Competition Generally speaking, competition and a corresponding reduction in productivity and survival may result from direct or indirect interactions. Direct interactions occur when hatchery-origin fish interfere with the accessibility to limited resources by natural-origin fish, and indirect interactions occur when the utilization of a limited resource by hatchery fish reduces the amount available for fish from the natural population (SIWG 1984). Natural-origin fish may be competitively displaced by hatchery fish early in life, especially when hatchery fish 49
58 are more numerous, are of equal or greater size, take up residency before naturally produced fry emerge from redds, and residualize. Hatchery fish might alter natural-origin salmon behavioral patterns and habitat use, making natural-origin fish more susceptible to predators (Hillman and Mullan 1989; Steward and Bjornn 1990). Hatchery-origin fish may also alter natural-origin salmonid migratory responses or movement patterns, leading to a decrease in foraging success by the natural-origin fish (Hillman and Mullan 1989; Steward and Bjornn 1990). Actual impacts on natural-origin fish would thus depend on the degree of dietary overlap, food availability, size-related differences in prey selection, foraging tactics, and differences in microhabitat use (Steward and Bjornn 1990). Specific hazards associated with competitive impacts of hatchery salmonids on listed natural-origin salmonids may include competition for food and rearing sites (NMFS 2012a). In an assessment of the potential ecological impacts of hatchery fish production on naturally produced salmonids, the Species Interaction Work Group (SIWG 1984) concluded that naturally produced coho and Chinook salmon and steelhead are all potentially at high risk due to competition (both interspecific and intraspecific) from hatchery fish of any of these three species. In contrast, the risk to naturally produced pink, chum, and sockeye salmon due to competition from hatchery salmon and steelhead was judged to be low. Several factors influence the risk of competition posed by hatchery releases: whether competition is intra- or interspecific; the duration of freshwater co-occurrence of hatchery and natural-origin fish; relative body sizes of the two groups; prior residence of shared habitat; environmentally induced developmental differences; and density in shared habitat (Tatara and Berejikian 2012). Intraspecific competition would be expected to be greater than interspecific, and competition would be expected to increase with prolonged freshwater cooccurrence. Hatchery smolts are commonly larger than natural-origin fish, and larger fish usually are superior competitors. However, natural-origin fish have the competitive advantage of prior residence when defending territories and resources in shared natural freshwater habitat. Tatara and Berejikian (2012) further reported that hatchery-influenced developmental differences from co-occurring natural-origin fish are variable and can favor both hatchery- and natural-origin fish. They concluded that of all factors, fish density of the composite population in relation to habitat carrying capacity likely exerts the greatest influence. En masse hatchery salmon smolt releases may cause displacement of rearing natural-origin juvenile salmonids from occupied stream areas, leading to abandonment of advantageous feeding stations, or premature outmigration by natural-origin juvenile salmonids. Pearsons et al. (1994) reported small-scale displacement of juvenile naturally produced rainbow trout from stream sections by hatchery steelhead. Small-scale displacements and agonistic interactions observed between hatchery steelhead and natural-origin juvenile trout were most likely a result of size differences and not something inherently different about hatchery fish. A proportion of the smolts released from a hatchery may not migrate to the ocean but rather reside for a period of time in the vicinity of the release point. These non-migratory smolts (residuals) may directly compete for food and space with natural-origin juvenile salmonids of similar age. Although this behavior has been studied and observed, most frequently in the case of hatchery steelhead, residualism has been reported as a potential issue for hatchery coho and Chinook salmon as well. Adverse impacts of residual hatchery Chinook and coho salmon on natural-origin salmonids can occur, especially given that the number of smolts per release is generally higher; however, the issue of residualism for these species has not been as widely investigated compared to steelhead. Therefore, for all species, monitoring of natural stream areas in the vicinity of hatchery release points may be necessary to determine the potential effects of hatchery smolt residualism on naturalorigin juvenile salmonids. 50
59 The risk of adverse competitive interactions between hatchery- and natural-origin fish can be minimized by: Releasing hatchery smolts that are physiologically ready to migrate. Hatchery fish released as smolts emigrate seaward soon after liberation, minimizing the potential for competition with juvenile naturally produced fish in freshwater (California HSRG 2012; Steward and Bjornn 1990) Operating hatcheries such that hatchery fish are reared to a size sufficient to ensure that smoltification occurs in nearly the entire population Releasing hatchery smolts in lower river areas, below areas used for stream-rearing by naturally produced juveniles Monitoring the incidence of non-migratory smolts (residuals) after release and adjusting rearing strategies, release location, and release timing if substantial competition with naturally rearing juveniles is determined likely Critical to analyzing competition risk is information on the quality and quantity of spawning and rearing habitat in the action area, 12 including the distribution of spawning and rearing habitat by quality and best estimates for spawning and rearing habitat capacity. Additional important information includes the abundance, distribution, and timing for naturally spawning hatchery fish and natural-origin fish; the timing of emergence; the distribution and estimated abundance for progeny from both hatchery and natural-origin natural spawners; the abundance, size, distribution, and timing for juvenile hatchery fish in the action area; and the size of hatchery fish relative to co-occurring natural-origin fish Predation Another potential ecological effect of hatchery releases is predation. Salmon and steelhead are piscivorous and can prey on other salmon and steelhead. Predation, either direct (consumption by hatchery fish) or indirect (increases in predation by other predator species due to enhanced attraction), can result from hatchery fish released into the wild. Considered here is predation by hatchery-origin fish, the progeny of naturally spawning hatchery fish, and avian and other predators attracted to the area by an abundance of hatchery fish. Hatchery fish originating from egg boxes and fish planted as non-migrant fry or fingerlings can prey upon fish from the local natural population during juvenile rearing. Hatchery fish released at a later stage, so they are more likely to emigrate quickly to the ocean, can prey on fry and fingerlings that are encountered during the downstream migration. Some of these hatchery fish do not emigrate and instead take up residence in the stream (residuals) where they can prey on stream-rearing juveniles over a more prolonged period, as discussed above. The progeny of naturally spawning hatchery fish also can prey on fish from a natural population and pose a threat. In general, the threat from predation is greatest when natural populations of salmon and steelhead are at low abundance, when spatial structure is already reduced, when habitat, particularly refuge habitat, is limited, and when environmental conditions favor high visibility. (SIWG 1984) rated most risks associated with predation as unknown because there was relatively little documentation in the literature of predation interactions in either freshwater or marine areas at the time. More studies are now available, but they are still too sparse to allow many generalizations to be made about risk. Newly released hatchery-origin yearling salmon and steelhead may prey on juvenile fall Chinook and steelhead and other juvenile salmon in the freshwater and marine environments (Hargreaves and LeBrasseur 1986; Hawkins and Tipping 1999; Pearsons and Fritts 1999). Low predation rates have been reported for released steelhead juveniles (Hawkins and Tipping 1999; Naman and Sharpe 2012). Hatchery steelhead release timing and protocols used widely in the Pacific Northwest were shown to be associated with negligible predation by migrating hatchery steelhead on fall Chinook fry, which had already emigrated or had grown large enough to 12 Action area means all areas to be affected directly or indirectly by the action in which the effects of the action can be meaningfully detected and evaluated. 51
60 reduce or eliminate their susceptibility to predation when hatchery steelhead entered the rivers (Sharpe et al. 2008). Hawkins (1998) documented hatchery spring Chinook salmon yearling predation on naturally produced fall Chinook salmon juveniles in the Lewis River. Predation on smaller Chinook salmon was found to be much higher in naturally produced smolts (coho salmon and cutthroat, predominately) than their hatchery counterparts. Predation may be greatest when large numbers of hatchery smolts encounter newly emerged fry or fingerlings, or when hatchery fish are large relative to naturally produced fish (SIWG 1984). Due to their location in the stream or river, size, and time of emergence, newly emerged salmonid fry are likely to be the most vulnerable to predation. Their vulnerability is believed to be greatest immediately upon emergence from the gravel and then their vulnerability decreases as they move into shallow, shoreline areas (USFWS 1994). Emigration out of important rearing areas and foraging inefficiency of newly released hatchery smolts may reduce the degree of predation on salmonid fry (USFWS 1994). Some reports suggest that hatchery fish can prey on fish that are up to 1/2 their length (HSRG 2004; Pearsons and Fritts 1999), but other studies have concluded that salmonid predators prey on fish 1/3 or less their length (Beauchamp 1990; Cannamela 1992; CBFWA 1996; Hillman and Mullan 1989; Horner 1978). Hatchery fish may also be less efficient predators as compared to their natural-origin conspecifics, reducing the potential for predation impacts (Bachman 1984; Olla et al. 1998; Sosiak et al. 1979). There are several steps that hatchery programs can implement to reduce or avoid the threat of predation: Releasing all hatchery fish as actively migrating smolts through volitional release practices so that the fish migrate quickly seaward, limiting the duration of interaction with any co-occurring natural-origin fish downstream of the release site. Ensuring that a high proportion of the population have physiologically achieved full smolt status. Juvenile salmon tend to migrate seaward rapidly when fully smolted, limiting the duration of interaction between hatchery fish and naturally produced fish present within, and downstream of, release areas. Releasing hatchery smolts in lower river areas near river mouths and below upstream areas used for stream-rearing young-of-the-year naturally produced salmon fry, thereby reducing the likelihood for interaction between the hatchery and naturally produced fish. Operating hatchery programs and releases to minimize the potential for residualism Disease The release of hatchery fish and hatchery effluent into juvenile rearing areas can lead to transmission of pathogens, contact with chemicals or altering of environmental parameters (e.g., dissolved oxygen) that can result in disease outbreaks. Fish diseases can be subdivided into two main categories: infectious and noninfectious. Infectious diseases are those caused by pathogens such as viruses, bacteria, and parasites. Noninfectious diseases are those that cannot be transmitted between fish and are typically caused by genetic or environmental factors (e.g., low dissolved oxygen). Pathogens can also be categorized as exotic or endemic. For our purposes, exotic pathogens are those that have no history of occurrence within state boundaries. For example, Oncorhynchus masou virus (OMV) would be considered an exotic pathogen if identified anywhere in Washington state. Endemic pathogens are native to a state, but may not be present in all watersheds. In natural fish populations, the risk of disease associated with hatchery programs may increase through a variety of mechanisms (Naish et al. 2008), including: Introduction of exotic pathogens Introduction of endemic pathogens to a new watershed 52
61 Intentional release of infected fish or fish carcasses Continual pathogen reservoir Pathogen amplification The transmission of pathogens between hatchery and natural fish can occur indirectly through hatchery water influent/effluent or directly via contact with infected fish. Within a hatchery, the likelihood of transmission leading to an epizootic (i.e., disease outbreak) is increased compared to the natural environment because hatchery fish are reared at higher densities and closer proximity than would naturally occur. During an epizootic, hatchery fish can shed relatively large amounts of pathogen into the hatchery effluent and ultimately, the environment, amplifying pathogen numbers. However, few, if any, examples of hatcheries contributing to an increase in disease in natural populations have been reported (Naish et al. 2008; Steward and Bjornn 1990). This lack of reporting is because both hatchery and natural-origin salmon and trout are susceptible to the same pathogens (Noakes et al. 2000), which are often endemic and ubiquitous (e.g., Renibacterium salmoninarum, the cause of Bacterial Kidney Disease). Adherence to a number of state, federal, and tribal fish health policies limits the disease risks associated with hatchery programs (IHOT 1995; NWIFC and WDFW 2006; ODFW 2003; USFWS 2004). Specifically, the policies govern the transfer of fish, eggs, carcasses, and water to prevent the spread of exotic and endemic reportable pathogens. For all pathogens, both reportable and non-reportable, pathogen spread and amplification are minimized through regular monitoring (typically monthly) removing mortalities, and disinfecting all eggs. Vaccines may provide additional protection from certain pathogens when available (e.g., Vibrio anguillarum). If a pathogen is determined to be the cause of fish mortality, treatments (e.g., antibiotics) will be used to limit further pathogen transmission and amplification. Some pathogens, such as infectious hematopoietic necrosis virus (IHNV), have no known treatment. Thus, if an epizootic occurs for those pathogens, the only way to control pathogen amplification is to cull infected individuals or terminate all susceptible fish. In addition, current hatchery operations often rear hatchery fish on a timeline that mimics their natural life history, which limits the presence of fish susceptible to pathogen infection and prevents hatchery fish from becoming a pathogen reservoir when no natural fish hosts are present. In addition to the state, federal and tribal fish health policies, disease risks can be further minimized by preventing pathogens from entering the hatchery facility through the treatment of incoming water (e.g., by using ozone) or by leaving the hatchery through hatchery effluent (Naish et al. 2008). Although preventing the exposure of fish to any pathogens prior to their release into the natural environment may make the hatchery fish more susceptible to infection after release into the natural environment, reduced fish densities in the natural environment compared to hatcheries likely reduces the risk of fish encountering pathogens at infectious levels (Naish et al. 2008). Treating the hatchery effluent would also minimize amplification, but would not reduce disease outbreaks within the hatchery itself caused by pathogens present in the incoming water supply. Another challenge with treating hatchery effluent is the lack of reliable, standardized guidelines for testing or a consistent practice of controlling pathogens in effluent (LaPatra 2003). However, hatchery facilities located near marine waters likely limit freshwater pathogen amplification downstream of the hatchery without human intervention because the pathogens are killed before transmission to fish when the effluent mixes with saltwater. Noninfectious diseases are those that cannot be transmitted between fish and are typically caused by genetic or environmental factors (e.g., low dissolved oxygen). Hatchery facilities routinely use a variety of chemicals for treatment and sanitation purposes. Chlorine levels in the hatchery effluent, specifically, are monitored with a National Pollutant Discharge Elimination System (NPDES) permit administered by the Environmental Protection Agency. Other chemicals are discharged in accordance with manufacturer instructions. The NPDES permit also requires monitoring of settleable and unsettleable solids, temperature, and dissolved oxygen in the hatchery effluent on a regular basis to ensure compliance with environmental standards and to prevent fish mortality. In contrast to infectious diseases, which typically are manifest by a limited number of life stages and 53
62 over a protracted time period, non-infectious diseases caused by environmental factors typically affect all life stages of fish indiscriminately and over a relatively short period of time. One group of non-infectious diseases that are expected to occur rarely in current hatchery operations are those caused by nutritional deficiencies because of the vast literature available on successful rearing of salmon and trout in aquaculture Acclimation One factor the can affect hatchery fish distribution and the potential to spatially overlap with natural-origin spawners, and thus the potential for genetic and ecological impacts, is the acclimation (the process of allowing fish to adjust to the environment in which they will be released) of hatchery juveniles before release. Acclimation of hatchery juvenile before release increases the probability that hatchery adults will home back to the release location, reducing their potential to stray into natural spawning areas. Acclimating fish for a period of time also allows them to recover from the stress caused by the transportation of the fish to the release location and by handling. (Dittman and Quinn 2008) provide an extensive literature review and introduction to homing of Pacific salmon. They note that, as early as the 19 th century, marking studies had shown that salmonids would home to the stream, or even the specific reach, where they originated. The ability to home to their home or natal stream is thought to be due to odors to which the juvenile salmonids were exposed while living in the stream (olfactory imprinting) and migrating from it years earlier (Dittman and Quinn 2008; Keefer and Caudill 2013). Fisheries managers use this innate ability of salmon and steelhead to home to specific streams by using acclimation ponds to support the reintroduction of species into newly accessible habitat or into areas where they have been extirpated (Dunnigan 2000; Quinn 1997; YKFP 2008). (Dittman and Quinn 2008) reference numerous experiments that indicated that a critical period for olfactory imprinting is during the parr-smolt transformation, which is the period when the salmonids go through changes in physiology, morphology, and behavior in preparation for transitioning from fresh water to the ocean (Beckman et al. 2000; Hoar 1976). Salmon species with more complex life histories (e.g., sockeye salmon) may imprint at multiple times from emergence to early migration (Dittman et al. 2010). Imprinting to a particular location, be it the hatchery, or an acclimation pond, through the acclimation and release of hatchery salmon and steelhead is employed by fisheries managers with the goal that the hatchery fish released from these locations will return to that particular site and not stray into other areas (Bentzen et al. 2001; Fulton and Pearson 1981; Hard and Heard 1999; Kostow 2009; Quinn 1997; Westley et al. 2013). However, this strategy may result in varying levels of success in regards to the proportion of the returning fish that stray outside of their natal stream. (e.g., (Clarke et al. 2011; Kenaston et al. 2001). Having hatchery salmon and steelhead home to a particular location is one measure that can be taken to reduce the proportion of hatchery fish in the naturally spawning population. By having the hatchery fish home to a particular location, those fish can be removed (e.g., through fisheries, use of a weir) or they can be isolated from primary spawning areas. Factors that can affect the success of homing include: The timing of the acclimation, such that a majority of the hatchery juveniles are going through the parrsmolt transformation during acclimation A water source unique enough to attract returning adults Whether or not the hatchery fish can access the stream reach where they were released Whether or not the water quantity and quality is such that returning hatchery fish will hold in that area before removal and/or their harvest in fisheries. 54
63 Factor 4. Hatchery fish and the progeny of naturally spawning hatchery fish in the migration corridor, in the estuary, and in the ocean Migrating hatchery fish can also potentially affect natural-origin fish through competition, predation and pathogen transmission resulting in disease. Based on a review of the scientific literature, NMFS conclusion is that the influence of density-dependent interactions, such as competition, predation and disease discussed in Factor 3, on the growth and survival of salmon and steelhead is likely small compared with the effects of largescale and regional environmental conditions. While there is evidence that large-scale hatchery production can effect salmon survival at sea, the degree of effect or level of influence is not yet well understood or predictable. The same thing is true for mainstem rivers and estuaries. NMFS will watch for new research to discern and to measure the frequency, the intensity, and any effects resulting from density-dependent interactions between hatchery and natural-origin fish. In the meantime, NMFS will monitor emerging science and information and will consider re-initiation of section 7 consultation in the event that new information reveals effects of the action that may affect listed species or critical habitat in a manner or to an extent not considered in this consultation (50 CFR ) Factor 5. Research, monitoring, and evaluation that exists because of the hatchery program NMFS also analyzes proposed RM&E for its effects on listed species and on designated critical habitat. The level of effect for this factor ranges from positive to negative. Generally speaking, negative effects on the fish from RM&E are weighed against the value or benefit of new information, particularly information that tests key assumptions and that reduces uncertainty. RM&E actions can cause harmful changes in behavior and reduced survival; such actions include, but are not limited to: Observation during surveying Collecting and handling (purposeful or inadvertent) Holding the fish in captivity, sampling (e.g., the removal of scales and tissues) Tagging and fin-clipping, and observing the fish (in-water or from the bank) Observing/Harassing For some parts of the proposed studies, listed fish would be observed in-water (e.g., by snorkel surveys, wading surveys, or observation from the banks). Direct observation is the least disruptive method for determining a species presence/absence and estimating their relative numbers. Its effects are also generally the shortest-lived and least harmful of the research activities discussed in this section because a cautious observer can effectively obtain data while only slightly disrupting fishes behavior. Fry and juveniles frightened by the turbulence and sound created by observers are likely to seek temporary refuge in deeper water, or behind/under rocks or vegetation. In extreme cases, some individuals may leave a particular pool or habitat type and then return when observers leave the area. At times, the research involves observing adult fish, which are more sensitive to disturbance. These avoidance behaviors are expected to be in the range of normal predator and disturbance behaviors. Redds may be visually inspected, but would not be walked on Capturing/handling Any physical handling or psychological disturbance is known to be stressful to fish (Sharpe et al. 1998). Primary contributing factors to stress and death from handling are excessive doses of anesthetic, differences in water temperatures (between the river and holding vessel), dissolved oxygen conditions, the amount of time fish are held out of the water, and physical trauma. Stress increases rapidly if the water temperature exceeds 18ºC or dissolved oxygen is below saturation. Fish transferred to holding tanks can experience trauma if care is not taken in the transfer process, and fish can experience stress and injury from overcrowding in traps if the traps 55
64 are not emptied regularly. Decreased survival can result from high stress levels because stress can be immediately debilitating, and may also increase the potential for vulnerability to subsequent challenges (Sharpe et al. 1998). Debris buildup at traps can also kill or injure fish if the traps are not monitored and cleared regularly Fin clipping and tagging Many studies have examined the effects of fin clips on fish growth, survival, and behavior. The results of these studies are somewhat varied, but fin clips do not generally alter fish growth (Brynildson and Brynildson 1967; Gjerde and Refstie 1988). Mortality among fin-clipped fish is variable, but can be as high as 80 percent (Nicola and Cordone 1973). In some cases, though, no significant difference in mortality was found between clipped and un-clipped fish (Gjerde and Refstie 1988; Vincent-Lang 1993). The mortality rate typically depends on which fin is clipped. Recovery rates are generally higher for adipose- and pelvic-fin-clipped fish than for those that have clipped pectoral, dorsal, or anal fins (Nicola and Cordone 1973), probably because the adipose and pelvic fins are not as important as other fins for movement or balance (McNeil and Crossman 1979). However, some work has shown that fish without an adipose fin may have a more difficult time swimming through turbulent water (Buckland-Nicks et al. 2011; Reimchen and Temple 2003). In addition to fin clipping, PIT tags and CWTs are included in the Proposed Action. PIT tags are inserted into the body cavity of the fish just in front of the pelvic girdle. The tagging procedure requires that the fish be captured and extensively handled, so it is critical that researchers ensure that the operations take place in the safest possible manner. Tagging needs to take place where there is cold water of high quality, a carefully controlled environment for administering anesthesia, sanitary conditions, quality control checking, and a recovery holding tank. Most studies have concluded that PIT tags generally have very little effect on growth, mortality, or behavior. Early studies of PIT tags showed no long-term effect on growth or survival (Prentice et al. 1987; Prentice and Park 1984; Rondorf and Miller 1994). In a study between the tailraces of Lower Granite and McNary Dams (225 km), (Hockersmith et al. 2000) concluded that the performance of yearling Chinook salmon was not adversely affected by orally or surgically implanted sham radio tags or PIT tags. However, (Knudsen et al. 2009) found that, over several brood years, PIT tag induced smolt-adult mortality in Yakima River spring Chinook salmon averaged 10.3 percent and was at times as high as 33.3 percent. Coded-wire tags are made of magnetized, stainless-steel wire and are injected into the nasal cartilage of a salmon and thus cause little direct tissue damage (Bergman et al. 1968; Bordner et al. 1990). The conditions under which CWTs should be inserted are similar to those required for PIT tags. A major advantage to using CWTs is that they have a negligible effect on the biological condition or response of tagged salmon (Vander Haegen et al. 2005); however, if the tag is placed too deeply in the snout of a fish, it may kill the fish, reduce its growth, or damage olfactory tissue (Fletcher et al. 1987; Peltz and Miller 1990). This latter effect can create problems for species like salmon because they use olfactory clues to guide their spawning migrations (Morrison and Zajac 1987). Mortality from tagging is both acute (occurring during or soon after tagging) and delayed (occurring long after the fish have been released into the environment). Acute mortality is caused by trauma induced during capture, tagging, and release it can be reduced by handling fish as gently as possible. Delayed mortality occurs if the tag or the tagging procedure harms the animal. Tags may cause wounds that do not heal properly, may make swimming more difficult, or may make tagged animals more vulnerable to predation (Howe and Hoyt 1982; Matthews and Reavis 1990; Moring 1990). Tagging may also reduce fish growth by increasing the energetic costs of swimming and maintaining balance. 56
65 NMFS has developed general guidelines to reduce impacts when collecting listed adult and juvenile salmonids (NMFS 2000b; NMFS 2008a) that have been incorporated as terms and conditions into section 7 opinions and section 10 permits for research and enhancement. Additional monitoring principles for supplementation programs have been developed by the (AHSWG 2008). The effects of these actions should not be confused with handling effects analyzed under broodstock collection. In addition, NMFS also considers the overall effectiveness of the RM&E program. There are five factors that NMFS takes into account when it assesses the beneficial and negative effects of hatchery RM&E: (1) the status of the affected species and effects of the proposed RM&E on the species and on designated critical habitat, (2) critical uncertainties concerning effects on the species, (3) performance monitoring and determining the effectiveness of the hatchery program at achieving its goals and objectives, (4) identifying and quantifying collateral effects, and (5) tracking compliance of the hatchery program with the terms and conditions for implementing the program. After assessing the proposed hatchery RM&E and before it makes any recommendations to the action agency(s) NMFS considers the benefit or usefulness of new or additional information, whether the desired information is available from another source, the effects on ESA-listed species, and cost. Hatchery actions also must be assessed for masking effects. For these purposes, masking is when hatchery fish included in the Proposed Action mix with and are not identifiable from other fish. The effect of masking is that it undermines and confuses RM&E and status and trends monitoring. Both adult and juvenile hatchery fish can have masking effects. When presented with a proposed hatchery action, NMFS analyzes the nature and level of uncertainties caused by masking and whether and to what extent listed salmon and steelhead are at increased risk. The analysis also takes into account the role of the affected salmon and steelhead population(s) in recovery and whether unidentifiable hatchery fish compromise important RM&E Factor 6. Construction, operation, and maintenance, of facilities that exist because of the hatchery program The construction/installation, operation, and maintenance of hatchery facilities can alter fish behavior and can injure or kill eggs, juveniles, and adults. These actions can also degrade habitat function and reduce or block access to spawning and rearing habitats altogether. Here, NMFS analyzes changes to: riparian habitat, channel morphology, habitat complexity, in-stream substrates, and water quantity and quality attributable to operation, maintenance, and construction activities. NMFS also confirms whether water diversions and fish passage facilities are constructed and operated consistent with NMFS criteria. The level of effect for this factor ranges from neutral or negligible to negative Factor 7. Fisheries that exist because of the hatchery program There are two aspects of fisheries that are potentially relevant to NMFS analysis of the Proposed Action in a section 7 consultation. One is where there are fisheries that exist because of the HGMP that describes the Proposed Action (i.e., the fishery is an interrelated and interdependent action), and listed species are inadvertently and incidentally taken in those fisheries. The other is when fisheries are used as a tool to prevent the hatchery fish associated with the HGMP, including hatchery fish included in an ESA-listed salmon ESU or steelhead DPS, from spawning naturally. The level of effect for this factor ranges from neutral or negligible to negative. Many hatchery programs are capable of producing more fish than are immediately useful in the conservation and recovery of an ESU and can play an important role in fulfilling trust and treaty obligations with regard to harvest of some Pacific salmon and steelhead populations. For ESUs listed as threatened, NMFS will, where appropriate, exercise its authority under section 4(d) of the ESA to allow the harvest of listed hatchery fish that 57
66 are surplus to the conservation and recovery needs of the ESU, in accordance with approved harvest plans (NMFS 2005c). In any event, fisheries must be strictly regulated based on the take, including catch and release effects, of ESA-listed species Effects of the Proposed Action Analysis of the Proposed Action identified that within the action area, ESA-listed species are likely to be negatively affected, and take will occur from five of the seven factors described in Section 2.4.1: the removal of natural-origin adults for broodstock; hatchery fish and the progeny of naturally spawning hatchery fish on spawning grounds and encounters with natural-origin and hatchery fish at adult collection facilities; hatchery fish and progeny of naturally spawning hatchery fish in juvenile rearing areas (i.e., competition, and predation); RM&E that exists because of the hatchery program; and operation and maintenance of the hatchery facilities. In addition to the seven factors discussed in detail, we also evaluate the Proposed Action for consistency with the Upper Columbia Salmon and Steelhead Recovery Plan (Upper Columbia Salmon Recovery Board 2007). Objectives for hatchery production in the Upper Columbia stated in the plan, are presented verbatim below: Short-Term Objectives 1. Continue to use artificial production to maintain critically depressed populations in a manner that is consistent with recovery and avoids extinction. 2. Use artificial production to seed unused, accessible habitats. 3. Use artificial production to provide for tribal and non-tribal fishery obligations as consistent with recovery criteria. 4. Use harvest or other methods to reduce the proportion of hatchery-produced fish in naturally spawning populations. 5. To the extent possible, use local broodstocks in hatchery programs. 6. To the extent possible, integrate federal, state, and tribal-operated hatchery programs that use locally derived stocks. 7. Reduce the amount of in-basin straying from current hatchery programs. Long-Term Objectives 1. Phase out the use of out-of-basin stock in the federal programs at Leavenworth and Entiat National Fish Hatcheries if continued research indicates that the programs threaten recovery of listed fish and those threats cannot be minimized through operational or other changes. 2. Help develop ongoing hatchery programs that are consistent with recovery. 3. Provide for tribal and non-tribal fishery obligations. 4. Use harvest or other methods to reduce the proportion of hatchery-produced fish in naturally spawning populations. 5. Manage hatcheries to achieve sufficient natural productivity and diversity to de-list populations and to avert re-listing of populations Factor 1. The hatchery program does or does not remove fish from the natural population and use them for hatchery broodstock Negative effect on spring Chinook salmon; not applicable for steelhead: The Methow program will be the conservation program for Methow spring Chinook salmon, taking both natural-origin and hatchery-origin fish as broodstock. The Methow program will collect up to 33 percent of the natural-origin run for broodstock, except when numbers are below 100. In the case of return numbers below 100, no natural-origin broodstock will be collected, to limit the risks of natural-origin fish not being able to locate mates when spawning naturally. The removal of up to 33 percent of the natural-origin run (when 100) is acceptable because when the natural 58
67 run size exceeds 300 fish, a minimum PNI of 0.5 can be achieved while removing up to 33 percent naturalorigin fish for broodstock. When run size is between 100 and 300 natural-origin fish, NMFS believes that removal of 33 percent of the natural-fish for brood is still warranted to ensure the supplementation program remains closely linked with the natural population, thus balancing the risk of removing too many natural-origin fish with the risk of propagating hatchery-fish that are substantially diverged from the natural population. In addition, a 33 percent limit also allows a majority of the natural-origin fish to spawn in the wild. A more thorough discussion of the effects of removing natural-origin fish for broodstock is addressed in Section , Genetic effects, specifically in the discussion of gene flow management. The WNFH Chinook program will use hatchery-origin fish exclusively, a considerable proportion of them being Methow program returnees. No steelhead are intentionally collected for the proposed action Factor 2. Hatchery fish and the progeny of naturally spawning hatchery fish on spawning grounds and encounters with natural-origin and hatchery fish at adult collection facilities Small negative effect on spring Chinook salmon; negligible effect on steelhead: The Methow spring Chinook salmon population needs hatchery support to aid in recovery, and the Proposed Action will provide such support with much less genetic and ecological risk than has been the pattern in the environmental baseline. The Methow program supports conservation of diversity among subpopulations. Chinook salmon and steelhead spawning seasons do not overlap temporally, so no interactions are expected on spawning grounds. Many Chinook salmon beyond broodstock needs will be captured during broodstock collection, and steelhead may on occasion be trapped during this time as well. Captured fish may experience handling stress and migration interference Genetic interactions between hatchery- and natural-origin adults Genetic interactions due to hatchery operations are a considerable concern in the UCR Spring Chinook Salmon ESU. The Supplemental Comprehensive Analysis of the FCRPS biological opinion section 8.6 (NMFS 2008d) cited straying hatchery fish, compositing fish for broodstock, low proportion of natural-origin fish in some broodstocks, and a high proportion of hatchery fish on the spawning grounds as factors contributing to high diversity risk in the ESU. In the Methow specifically, the SCA concluded that the Methow program had reduced extinction risk while it has imposed hybridization and the loss of genetic variation. Furthermore, while the SCA considered discontinuation of the Carson stock an improvement, it noted that both the NFH [WNFH] and PUD [Methow] programs still rely on a high percentage of hatchery-origin fish for broodstock, and they use a composite stock (i.e., a combination of Methow and Chewuch River fish. This practice homogenizes Methow Chinook, breaking down genetic differentiation and posing a continued risk to the natural population. The SCA s hatchery appendix concludes that both the WNFH and Methow programs are not among the top five factors limiting productivity, and both slow the trend toward extinction, despite the large proportion of hatchery-origin fish. It is also important to keep in mind that overall hatchery production of spring Chinook in Methow Subbasin has been reduced from the 1.15 million maximum in 2008 to current levels of 624,000. The two concerns just mentioned touch on two of the three general areas of genetic risk we discussed in Section : outbreeding effects and hatchery-influenced selection. In this section, we will analyze effects of the Proposed Action on the Methow spring Chinook salmon population in these areas, as well as diversity. In addition to these three issues, we will also analyze the effects of Methow spring Chinook salmon production on Entiat spring Chinook salmon. 59
68 Diversity and outbreeding effects In terms of conservation of genetic diversity overall, the Proposed Action is likely a benefit to the Methow spring Chinook salmon population. Although the natural-origin population size is considerably less than decades ago, it has remained in the hundreds annually, likely because of the hatchery programs. A longstanding guideline for conservation of genetic diversity is that although short-term dips to smaller sizes can be sustained without serious loss of genetic diversity, the effective population size should be 500 or more over the long term (Section ). Assuming a generation time of four years, which is reasonable for Methow spring Chinook salmon, an average of 125 effective spawners/year is required. Methow spring Chinook salmon have been examined genetically multiple times; data on effective number of spawners (Nb ) is summarized in Table 1 of Small et al. (2007) by tributary, year, and production type (e.g., Chewuch 2005 hatchery). Estimates can be combined to yield total population Nb estimates. Adding samples for years when enough samples from enough areas were available indicates that the 125 threshold has been met on multiple occasions, but only if hatchery samples were included, implying that the hatcheries are providing a conservation benefit for diversity. It should be noted, however, that no information was available beyond 2006, and a complete set of samples including WNFH was available for A new comprehensive sampling and analysis is recommended. Compositing Chewuch and Methow Subpopulations Another aspect of diversity to be considered is subpopulation structure. When the Methow program began, WDFW recognized the Twisp, Methow, and Chewuch spring Chinook salmon subpopulations as distinct populations based on genetic data (Marshall et al. 1995). Fish culture from broodstock collection onward was done on a tributary-specific basis. A NMFS-led committee later concluded that the spring Chinook salmon in the Methow Subbasin should be considered a single population for ESA purposes (Ford et al. 2001), and in 1998 the Methow and Chewuch fish were combined to form the Methow-Composite stock (NMFS et al. 1998). The Twisp subpopulation was not included in the compositing process, and continues to be managed as a separate unit. The consequence of combining the Methow and Chewuch production is unknown. It is possible that some level of outbreeding depression has occurred, because the differentiation between the subpopulations almost certainly arose after the Grand Coulee Fish Maintenance Program combined the Upper Columbia spring Chinook populations in the 1940s (Utter et al. 1995). In addition, genetic data indicate that the natural-origin fish in the Chewuch and Methow have become more similar since compositing occurred (Small et al. 2007), although they are still genetically distinct. The Proposed Action continues the compositing of Methow and Chewuch, but also continues the separate culture of the Twisp subpopulation, the most differentiated subpopulation (Ford et al. 2001). In addition, the Twisp subcomponent will be managed for lower levels of hatchery influence than the composite component. Natural-origin Wenatchee spring Chinook salmon display quite high fidelity to natal areas within the basin (Ford et al. 2015), so it is reasonable to assume that Methow spring Chinook salmon would as well because both populations are within the same ESU. Given the opportunity, subpopulation differentiation should develop again, and in the long term, this would be desirable to aid recovery of the Methow spring Chinook salmon population. Currently, the demographic challenges facing the population make increasing subpopulation differentiation less of a concern. Carson Stock Influence The Carson stock was cultured in the WNFH program for over 20 years until 2000, and was incorporated into the Methow program from the beginning. The first broodstock collections for the Methow component of the Methow program incorporated fish with hatchery scale patterns, assumed to be Carson fish from the WNFH 60
69 program (WDFW 2003). Moreover, it is likely that Carson fish had interbred with natural-origin Methow fish to a noticeable degree by that time. Incorporation of fish with Carson fish ancestry into Methow program broodstock has continued (Table 14). Early genetic collections of Methow fish showed similarity with the WNFH collections and differences with early Chewuch collections (Small et al. 2007). Genetically, the current situation is that the Twisp hatchery- and natural-origin collections form a group distinct from the Methow- Chewuch-WNFH group, reflecting the effects of both the compositing event and gene flow from the Carson stock (Small et al. 2007). Table 14. Estimated contribution of Carson stock to Methow program broodstock (modified from Table 1 of WDFW 2003). Brood Year Stock Number of % Carson Broodstock Ancestry 1992 Chewuch Twisp Methow 96 > Chewuch Twisp Methow Chewuch Twisp Methow Methow Chewuch Twisp Methow Chewuch Twisp Methow Composite Twisp Methow Composite Twisp Methow Composite Twisp Methow Composite Twisp Methow Composite Twisp Methow Composite Twisp 28 0 The Carson-stock influence has also possibly resulted in some level of outbreeding depression in addition to contributing to decreased diversity. Outbreeding depression in fishes appears to be an extremely unpredictable phenomenon, dependent on a number of factors (McClelland and Naish 2007). In this case, the effect may have been moderated due to the long history of the Carson stock in the basin, and thus time to achieve some local level of adaptation. Relationship between WNFH and Methow programs The Proposed Action does, as per numerous recommendations by review panels (USFWS 2007), tightly link the WNFH and Methow production genetically, which will reduce the likelihood of genetic divergence of the WNFH stock. This will occur by using Methow program returnees for up to 100 percent of the WNFH 61
70 broodstock, making the WNFH program a safety net component of the Methow program, and will reduce the impacts of WNFH fish spawning in the wild, because they will be more closely related to the natural-origin population. The relationship between the two programs is diagrammed in Figure 15. Figure 15. Stepping stone relationship proposed for WNFH and Methow spring Chinook salmon programs (USFWS 2012c) Hatchery-influenced selection effects The two recent regional hatchery review processes (HSRG 2009; USFWS 2007) have commented on the apparently large level of hatchery influence on natural spring Chinook salmon production in the Methow Subbasin. The HSRG analysis of Methow spring Chinook salmon is of great interest because its solution for the Methow provides substantial context for discussion of the Proposed Action, though the review offered no substantive recommendations for reducing the potential genetic influence of hatcheries. The HSRG designated all three UCR spring Chinook salmon populations (Wenatchee, Methow, and Entiat) as primary consistent with the ICTRT s recovery scenario (ICTRT 2007a) for the ESU, which requires that all three extant populations reach viability. The HSRG concluded that the Wenatchee and Entiat should be managed as primary, which would include imposition of phos and PNI guidelines (Section ). However, because of habitat limitations and lack of structures within the Methow Subbasin (with the exception of the Twisp weir) for controlling the number of hatchery-origin fish on the spawning grounds, the HSRG concluded that the population should be managed as stabilizing, which involves no criteria for phos or PNI. Thus, although the Methow program results in a high proportion of hatchery-origin fish on the spawning grounds, it was considered necessary as a gene bank. As previously mentioned in the Environmental Baseline (Section ), the percentage of natural-origin fish on the spawning grounds in the Methow Subbasin has declined dramatically since hatchery releases began in 1976, and this decline makes the potential for hatchery-influenced selection high. The phos for the Methow program started increasing substantially when the program began operation in the early 1990s. This increase is in part due to the success of the program in returning fish to the Methow Subbasin, but the increase was heightened by a considerable drop in natural production at the same time, which has persisted to the present day (Figure 16). This decline was a general phenomenon that occurred in the Entiat and Wenatchee Basins as well, 62
71 and began before Methow program fish returned to the basin; so, it does not appear to have been caused by the hatchery programs. Methow Subbasin spring Chinook Spawners (all ages) 10,000 9,000 8,000 7,000 6,000 5,000 4,000 3,000 2,000 1, Return Year Figure 16. Methow Subbasin spring Chinook salmon spawners (all ages) Dashed and solid lines represent hatchery- and natural-origin spawners, respectively 13. Another possible contributor to an increase in phos is use of natural-origin fish in the broodstock; selectively removing natural-origin fish without a corresponding removal of hatchery fish increases phos. Figure 17 compares realized phos to what phos would have been by replacing natural-origin fish removed for broodstock with hatchery-origin fish ( no pnob phos), so that the total number of spawners remains the same. In the early years of the program, this was impossible because the returns of hatchery fish were not large enough, which accounts for the large differences between realized phos and no pnob phos in those years. Since the year 2000, removal of natural-origin fish for broodstock has had little impact on phos. Realized phos since 2000 has averaged 78 percent, and no pnob phos has averaged 75 percent. Two additional aspects of phos need to be considered. First, the gross number of fish on the spawning grounds may not represent reproductive output. The spawning distributions of natural-origin and hatchery-origin fish differ in the Methow (Snow et al. 2014), with a heavy concentration of hatchery-origin fish near the hatcheries (Figure 19). The spawners near the hatcheries are nearly 100 percent hatchery fish, and spawning in these reaches have in some years accounted for more than 20 percent of the redds in the subbasin. If fish spawning in these areas have greatly lowered reproductive success relative to fish spawning elsewhere, as in the Wenatchee Subbasin (Williamson et al. 2010), then phos may somewhat over-represent the genetic influence of hatchery fish. Second, the phos statistic can be deceptive in terms of inferences one might make. A decrease from a phos of 75 percent to 40 percent, for example, assuming that levels of natural-origin fish are not changed, will decrease the spawning population of hatchery-origin fish about 75 percent. The proportion of NxN matings, assuming complete overlap in spawning distribution, should increase nearly four-fold (Figure 14). If the difference in spawner distributions currently observed continues, the increase in NxN matings should be even greater. Our 13 There are serious issues with historical estimates prior to 2000 of hatchery fish on the spawning grounds. In most years, it appears data from carcasses, if collected, was only used to estimate ages not origin, the only exception is Origins based on scales was required because only a small fraction of fish released from WNFH were adipose fin clipped. When data and an approach to estimate hatchery fish on the spawning grounds between 1981 and 1999 is complete, the applicants will submit it to NOAA for review before updating the current time series (A. Murdoch, WDFW, personal communication, September 14, 2016). 63
72 conclusion is that, because PNI is used as a metric, it is still acceptable for hatchery influence to be large when natural-origin returns are low. However, the increase in NxN matings may provide an added benefit in increasing opportunities for adaptation. Thus, the Proposed Action likely provides a net genetic benefit to the population. phos among Methow spring Chinook salmon (all ages) Return Year Figure 17. phos among spring Chinook salmon in the Methow Subbasin, (Snow et al. 2014). Dashed line is projection on what phos would have been had no natural-origin fish been taken for broodstock, and the solid line is for realized phos 13. Gene-flow management regime The Proposed Action includes two important elements that should considerably reduce the genetic risk of hatchery-induced selection from large numbers of hatchery fish on the spawning grounds. First, the overall goal for spring Chinook releases into the Methow Subbasin will be reduced. Previously, as many as 1.15 million spring Chinook salmon smolts could be released into the basin. Under the proposed action, the total allowable release will now be 624,000. Although this is a 46 percent decrease in goal, the effect may be far less. Releases in averaged 864,000, approximately 25 percent below the goal of 1.15 million. Thus, the decrease in released fish will be about 27 percent, which, without any other measures taken to reduce the abundance of hatchery-origin spawners, should result in a phos reduction from 75 percent to 69 percent 14. Second, and more important, the gene-flow management regimes for both programs (Figure 2 and Table 5) will target a minimum overall basin PNI of 0.5 based on the standards of phos and/or PNI for each program. This regime was developed over a considerable period of time. Given the complexity of the situation two large hatchery programs and no obvious simple method of controlling the number of hatchery fish on the spawning grounds the initial emphasis was on phos restriction. NMFS challenged the applicants to develop adult management methods capable of decreasing phos from present levels to as low as 25 percent. Using a combination of WNFH and Methow hatchery outfall channels, Wells hatchery volunteer channel, Twisp weir, possibly Foghorn Dam, and (as a last resort) Wells Dam for the modeling, the applicants concluded that a level as low as 28 percent was possible (DPUD and WDFW 2012). This analysis included removal of fish from both the WNFH and Methow programs, but did not include the 61,000 fish from the CPUD portion of the program, which could result in an 11 percent increase, to slightly over 30 percent. This work formed the basis for a 2013 management framework (Busack 2013). In 2014, Mackey (2014) developed a PNI-based alternative to the 2013 framework. However, in Mackey s scheme, numerical targets for PNI, phos, or pnob were absent when 14 Often it is assumed that a hatchery production decrease of X percent translates to a phos reduction of X percent, but this is incorrect. Although the hatchery proportion is being reduced, so is the entire hatchery-origin + natural-origin production, and this must be accounted for in the calculation
73 the natural-origin returns were less than 300 fish, and the regime did not consider the WNFH program. The revised scheme (Table 5, Figure 2) addresses both concerns. The target is now 500 total spawners when the natural-origin run is below 300 and to halt the collection of natural-origin broodstock when the run is less than 100. The difference in both cases will be made up with hatchery-origin spawners. In the revised framework that is part of the Proposed Action, the WNFH program was allocated a partial phos value of 20 percent (calculated as HOSWNFH/ Total HOS+ NOS) up to a natural-origin return of 500 fish. Then the target decreases to 15 percent and it decreases again to 10 percent when natural-origin run size exceeds 900 fish. NMFS overall basin goal, which includes both programs, is a PNI of 0.5 when the natural-origin run exceeds 300 fish. Appropriate inclusion of the WNFH production required development of a new approach to PNI calculations. Applicants argued that because the WNFH production was genetically linked to the Methow production through use of Methow returnees as broodstock, this needed to be considered in calculation of PNI. In response, (Busack 2015) extended the (Ford 2002) model to three populations and developed a spreadsheet tool (Busack 2016) to estimate PNI in the population, considering the effects of both hatchery programs and their linkage. In analyzing the risks and benefits of the gene-flow management regime, it is useful to consider the Methow Subbasin phos and PNI goals with respect to HSRG gene-flow guidelines (Section ). Although NMFS does not consider HSRG gene-flow guidelines as standards that must be met in all cases for sufficient reduction of the risk of hatchery-induced selection for ESA purposes, NMFS considers them adequate for reducing risk. In other words, an integrated program does not necessarily have to be managed for a PNI of at least 0.67 with phos no greater than 0.3 to put a population on a trajectory of viability for diversity. But NMFS accepts provisionally that meeting those guidelines is one way to manage for viability in most cases. At the same time, NMFS recognizes that, in the short term, demographic concerns must take priority over gene-flow guidelines. In a case like the Methow, where low abundance and productivity increases extinction risk, strict adherence to genetic-only guidelines such as those of the HSRG would not be expected. The gene-flow guidelines in the Proposed Action represent an important step toward meeting a PNI level that is consistent with population viability, and are a huge improvement over the previous levels of gene flow. An additional benefit of reducing phos per the guidelines is that, with decreases in the proportion of hatchery fish on spawning grounds, the magnitude of ecological interactions between natural- and hatchery-origin fish also decreases. At the same time, the allowances made for increasing gene flow from hatchery-origin fish when natural-origin returns are lower provides a balance between genetic and demographic risks. The key to being able to implement the gene-flow guidelines is the ability to remove surplus hatchery-origin fish without a major weir. Removal of surplus hatchery-origin fish to limit hatchery influence was first attempted for both of the programs in the Methow Subbasin in The applicants were able to achieve an overall PNI for the Methow Subbasin of 0.5 using the 3-population model (Busack 2015) by removing approximately 7,800 of the 10,000 fish that returned (WDFW 2015). In applying this new management regime to the 2015 return, the overall basin PNI goal would have been met and WNFH would have been able to limit their partial phos to about 16 percent, close to their partial phos target of 15 percent for the natural-origin run size, which was 692. However, the Methow program would not have met the PUD PNI target of This could have been achieved by either removing more hatchery fish, or increasing the pnob (Table 15). However, even with a pnob of 1, the PUD PNI would still fall short of the target of Using the 3-population model, a PUD phos of 0.3 would also be needed to achieve the target PUD PNI. This target would have required the removal of 491 additional fish from the Methow program. While this may have been difficult to achieve in 2015, two important caveats should be noted. First, the ladder and trap for the Methow hatchery was not running at maximum capacity in 2015, which likely limited the fish removed at this facility. Second, the decrease in juvenile releases from 550,000 to 224,000 had not been realized. This would have reduced the number of Methow program fish by about 60 percent. 65
74 Table 15. Proportionate natural influence (PNI) as a function of varying pnob and phos. pnob phos During the consultation, discussions arose about the possibility that, under gene-flow regimes that couple high pnob and aggressive removal of hatchery returnees to decrease phos, hatchery-origin returns to the spawning grounds could fall below one recruit per natural-origin broodstock fish, effectively providing less of a return than if the fish had been allowed to spawn naturally. This aspect of supplementation has not, to the best of our knowledge, been analyzed elsewhere. Based on a preliminary analysis, this mining of natural-origin fish will not occur as long as the remaining number of hatchery-origin fish on the spawning grounds exceeds the number of fish (natural-origin and hatchery-origin combined) used as broodstock 15. Therefore, this will cap the allowable removal rate of HORs returning from the PUD program. The maximum allowable removal rate (rem) of hatchery-origin fish (post broodstock collection) is a function of pnob and the hatchery recruitment rate (HRR) (defined as the number of returnees per broodstock fish back to the basin before any are taken for broodstock or removed for adult management). Mining will not occur as long as: rem < 1/(HRR+pNOB-1) In putting this into context with Methow program return abundance from , mining is expected to occur rarely. In 2006, the adult return from the Methow Hatchery program was 435 adults (Snow et al. 2014). Even if broodstock for that year required all hatchery-origin fish, 305 ( ) would remain. Thus, mining is expected to be a rare event as hatchery-origin returns, at least in the last 10 years, have not led to a mining situation, because removal rates are based on the HRR and pnob. The maximum removal rates for a range of pnob and HRR values, necessary for avoiding mining, are summarized in Table 16. The equation and resulting values in the table require some explanation. Note first that the only reference to natural-origin fish in the equation is pnob. The only limitation on the ability to achieve a specified pnob level is the number of returning natural-origin fish and cap on the proportion that are taken. The table assumes that the specified pnob is achievable, and then presents the maximum permissible rate of removal of hatchery fish; i.e., for a given pnob, the removal rate that would provide the lowest phos value without mining. For example, consider the situation in which a pnob of 1.0 is desired and the HRR is 3. According to the table, the maximum removal rate of hatchery-origin fish in such a situation would be 67 percent; lower removal rates are perfectly acceptable, but of course will result in higher phos values. The tabulated values at first glance can seem counter-intuitive. For example, it seems reasonable that, if 67 percent removal is acceptable for a pnob of 1.0, then a higher value would be acceptable for a pnob of 0.9, but this is not the case. The reason is that at a pnob of 0.9, for example, because some hatchery-origin fish need to be collected for broodstock, fewer are available for removal, so the acceptable removal rate is reduced (to
75 percent in this case), under the same HRR. Thus, we anticipate that the maximum removal rates for hatchery fish will not exceed those depicted in Table 16 for a given combination of variables, to avoid a potential mining situation. Table 16. Maximum allowable proportion of returning hatchery-origin fish to be removed, after broodstock collection, to achieve specified levels of pnob without mining natural-origin fish (refer to text for explanation). Adult returns/broodstock fish pnob Another feasibility consideration is that, under the new gene-flow management regime, phos levels decrease as natural production levels increase, if this increase in natural production does not correspond with an increase in hatchery survival (as a result, for example, of improved migratory conditions). Conversely, if returns of hatchery fish increase as well, the higher PUD PNI levels may be difficult to achieve without imposing more aggressive adult management measures, such as increased removal of hatchery adults or implementation of a selective fishery in the Methow Subbasin. Realistically, we do not anticipate an increase in natural- and hatchery-origin returns that would require more aggressive hatchery-origin fish removal than what was considered here without substantial improvements in migratory, spawning and rearing habitat that are needed to accompany an increase in smolt to adult survival Methow Subbasin spring Chinook salmon straying into Entiat Subbasin Historical CWT recoveries(cooper 2014a; Snow 2014)were examined for both the WNFH and Methow programs to determine the straying of fish from both programs into the Entiat Subbasin. As is the case with most hatchery programs, recoveries were widespread, with no patterns emerging. However, fish from both programs have been recovered in several years in the Entiat Subbasin. Figure 18 presents estimated contributions of all out-of-basin hatchery programs for which tags have been recovered to the Entiat escapement for The WNFH and Methow programs have both averaged 2 percent contribution to the Entiat population. We expect this number to be reduced by about 40 percent to 1.2 percent once the returns from the program reductions (from 1.06 million released in 2012 to 624,000; Figure 8) under the proposed action are realized starting in 2018, assuming that reductions in juvenile releases equates to a similar reduction in adult returns. Although the Methow Subbasin hatchery programs are common contributors to Entiat escapement, their contribution levels are well below the ICTRT recommendation of up to 10 percent for spawners within the same MPG, which equates to a moderate risk. Therefore, we do not believe these programs pose a threat at this time. Straying of fish from the Proposed Action into other basins of the upper Columbia also occurs. (Snow et al. 2014) found that the proportion of the Wenatchee Basin population (based on the Chiwawa River) composed of fish from the Methow program was an average of 0.47 percent from In addition, fish from the Methow program were only found in two of the years during this period, 2006 (2 fish) and 2010 (6 fish). Likewise, fish from the WNFH program were only found (identified via CWT) in the Wenatchee Basin in 3 years out of the 15 years data are available ( ) with number ranging from 1 to 4 annually. Although 67
76 low numbers of fish from each program have been identified in the Okanogan Subbasin, 3 fish from the WNFH program and 19 fish from the Methow program (Snow et al. 2014), there is no extant spring Chinook salmon population in that area, so there is no risk of genetic introgression. However, in 2014, a non-essential experimental population of spring Chinook salmon in the Okanogan Subbasin was designated under the ESA 10(j). Thus, future effects of straying of fish from the Proposed Action into the Okanogan Subbasin could be a concern, but the relatively low level of straying into the Okanogan Subbasin, and the fact that the stock is being established with the Methow-Chewuch Composite stock at WNFH, suggests that the effects of straying on natural-origin spring Chinook salmon in the Okanogan Subbasin would be negligible. In addition, the levels are expected to decline in the future as a result of the program reductions, decreasing the risk of outbreeding effects and loss of genetic diversity across all populations within the ESU. Proportionate Contribution Total strays WNFH Methow Figure 18. Proportionate contribution of the Methow and WNFH spring Chinook salmon hatchery programs to Entiat spring Chinook salmon escapement (Cooper 2014a; Snow 2014) Ecological interactions between hatchery- and natural-origin adults Methow spring Chinook salmon Release Year In its review of the WNFH program, the HRT (USFWS 2007) commented on the ecological risks posed to the Methow spring Chinook salmon population, such as spawning site competition and redd superimposition from large numbers of hatchery fish on the spawning grounds. The HRT specifically pointed out risk from oversizing the supplementation program based on the production capacity of the hatchery rather than the biological needs of the naturally-spawning population and capacity of the habitat, and also stated that forcing all surplus hatchery-origin spring Chinook to spawn naturally in an uncontrolled manner can potentially overwhelm the reproductive output of natural-origin fish. They also stated that [l]arge numbers of hatchery-origin spring Chinook spawning naturally poses an indirect ecological risk to the natural population of spring Chinook by not considering competition for food and space from other fish and aquatic species that now occupy habitat and niches formerly occupied by previously-abundant salmonids. It is not clear that the abundance of hatchery fish on the spawning grounds presents as much of an apparent risk of competition and redd superimposition as the numbers would suggest because the spawning distribution of hatchery fish seems to differ from that of natural-origin fish, at least in the Methow. Figure 18 shows the distribution of female carcasses, by production type, in the Methow, Chewuch, and Twisp in In the 68
77 Methow, an obvious difference between the distributions of hatchery-origin and natural-origin fish exists, with spawners in the hatchery outfalls and the area just above the hatcheries (Rkm 82-86) being almost entirely hatchery-origin fish. These areas are thought by the operators to be low-quality habitat, chosen by the hatcheryorigin spawners because they were acclimated to the hatchery sites. If so, the potential for competition, including redd superimposition, is greatly reduced. Possibly, the reproductive success of hatchery-origin spawners is reduced relative to fish spawning in other areas because of such site selection, similar to the situation observed in Wenatchee spring Chinook salmon (Ford and Williamson 2009). The spawning distribution pattern is not as clear in the other streams, but hatchery-origin fish do appear more concentrated in the lower reaches of both the Twisp and Chewuch, making it more likely for competition and redd superimposition to occur there. Under the Proposed Action, the proportion of hatchery-origin fish on the spawning grounds will be reduced (see Section ), which will reduce overall spawner density (assuming the level of natural production remains the same), likely leading to a decrease in the risk of spawning site competition and redd superimposition. If the large numbers of hatchery-origin spawners have had a negative impact on natural spawners, we would expect natural-origin fish to recolonize areas previously occupied by hatchery-origin fish (i.e. Rkm above the hatcheries). Any benefits of the large number of hatchery spawners, such as gravel conditioning (Montgomery et al. 1996) and marine-derived nutrient delivery (Cederholm et al. 1999; Scheuerell et al. 2005), will also be diminished. Based on the anticipated returns from both programs after programs reductions are realized, about 42.0 kg of phosphorous is contributed to the Methow Subbasin annually by returning adults produced by the hatchery programs. However, the numbers in Table 17 are likely an overestimation of nutrient contribution, because this assumes that all returning fish spawn naturally. Under the new gene-flow management regime, a number of returning hatchery-origin fish will be removed prior to spawning naturally, which will limit the contribution of marine-derived nutrients. Table 17. Total phosphorous imported by adult returns from the proposed hatchery programs based on the equation in Scheuerell et al. (2005). Program Number of Adults (A t) 1 Adult mass (kg) Concentration of phosphorous (kg/adult) Methow Methow-Twisp WNFH Data from Section 1.3 Phosphorous imported (kg/year) 69
78 Number of female carcasses HOR NOR Number of female carcasses Number of female carcasses HOR NOR HOR NOR River Kilometer (Rkm) Figure 19. Distribution of hatchery- and natural-origin spring Chinook salmon spawners in the Methow (upper), Chewuch (middle), and Twisp Rivers (lower) (Snow et al. 2013; CH. 5 Tables 6-8). WNFH and Methow Hatchery are on the Methow River at about Rkm 82 (all distances are in river kilometers from the mouth of the Methow River). 70
79 Steelhead in the Methow Subbasin The Proposed Action should have a negligible impact on steelhead in terms of direct competitive interactions, as the spawning times of the two species do not overlap. In the Methow Subbasin, the number of spring Chinook salmon hatchery-origin spawners will be reduced over present levels, so ecological benefits (i.e., nutrient cycling) accruing from them will be reduced, resulting in a small negative effect. As mentioned above, it is not clear how important these benefits are. Should natural production of spring Chinook salmon in the Methow increase because of the Proposed Action, the net effect may be positive Encounter of listed species at adult collection facilities Hatchery programs that collect returning adults for broodstock or sampling may impact both target and nontarget species through the physical activities of trapping, removal, handling, sampling, tagging, and transport. Trapping includes taking volunteers returning to the hatchery, using a weir, and using a fish ladder-trap combination associated with a dam to collect fish. The operation of adult collection facilities poses a potential threat to ESA-listed spring Chinook salmon and steelhead through handling related stress, mortality, and delay. The threat will be minimized by processing and collecting or passing fish within 24 hours of initial trapping. Methow Hatchery and WNFH Ladders Both Methow and Winthrop Hatcheries collect adults that volunteer into the ladder and trap at the outfall of the hatchery facility. In general, this type of collection minimizes the impact on natural-origin fish because they do not typically volunteer into hatchery outfalls when homing. Because there is no weir or passage barrier associated with the ladders, migration and spawning distribution is not impeded for fish that pass the facility entrance. Those that do not volunteer into the ladder can move freely upstream. Natural-origin fish that enter the Methow or WNFH ladder and trap will likely be held for broodstock, and therefore not experience the potential effects of fish that would be passed to spawn in the wild. Very few natural-origin fish of either species are encountered during spring Chinook salmon broodstock collection at these two facilities (Table 18), and we do not anticipate these encounters to change in the future. Wells Dam Wells Dam is equipped with two fish ladders (on the east and west ends of the dam), which provide passage above the dam. Both east- and west-side ladders have a trap for sampling adults and/or collecting broodstock. Most fish will be sampled and passed, though some Chinook salmon will be held for broodstock and transferred to Methow for spawning. During Chinook salmon broodstock collection, and prior to steelhead broodstock collection, steelhead may be encountered in the trap, but these numbers are low (Table 18). Twisp River Weir The Twisp River weir is a permanent weir at Rkm 10 with trap boxes installed annually to collect broodstock and to control hatchery-origin spawners above the weir. The weir panels are lowered to the river bottom when the weir is not in use. More listed steelhead are encountered here than at the other collection facilities, but mortalities are still low (Table 18). Stranding of steelhead and spring Chinook salmon on the weir pickets during upstream and downstream movement may also occur in addition to those effects described above. However, the weir is capable of submerging the pickets to allow stranded fish to swim off. If the weir cannot adequately address kelt migration or spring Chinook fallback, trapping will cease and the weir panels will be lowered (pending appropriate flow conditions). Weir technology has improved greatly over the previous couple of decades and the technology is now widely and effectively applied throughout the Pacific Northwest (NMFS 2010; NMFS 2011d); the Twisp River weir is operated in a manner consistent with this current standard application, and would continue to be so operated. 71
80 Weir operation may also affect spawning distribution due to delay (see Section ). A shift in the spawning distribution relative to what was observed prior to the installation of the weir may indicate that the weir is affecting the natural spawning distribution of spring Chinook salmon in the Twisp River. Likewise, delay in the upstream migration of spring Chinook salmon and steelhead due to the installation and operation of in-river weirs and traps may affect the date of first spawning and the peak spawning date. In 2009, PIT tag arrays were installed in the lower Twisp River to help monitor migration patterns of both adult and juvenile Chinook salmon in the Twisp River. Beginning in 2010, WDFW began monitoring spawning distribution using the Twisp River weir. (Snow et al. 2014) observed no significant differences in the distribution of hatchery and natural-origin carcasses or spawn timing of either Chinook or steelhead (females) within the Twisp River. This finding indicates that there is no effect on the distribution of listed fish from the Twisp River weir, and we do not anticipate this to change in the future. Table 18. Number of adult (unless otherwise noted) spring Chinook salmon and steelhead handled and mortalities resulting from handling at each of the adult collection facilities. Numbers are an average over the most recent 6-10 years with the range in parentheses (Frady 2016; Humling 2016c). Facility Number of Spring Chinook Salmon Handled/Mortalities 1 Number of Steelhead Handled/Mortalities Natural-origin Hatchery-origin Natural-origin Hatchery-origin Wells 111 (24-185)/0 (0-1) 909 ( )/ 1 (0-2)/0 2 (0-6)/0 Dam 1 (0-3) Twisp 43 (2-104)/0 114 (0-204)/0 15 (5-32) 2 /0 29 (15-52) 1 /0 (0-1) Weir Methow 0 (0-1)/0 49 (0-266)/0 2 (0-9)/0 1 (0-3)/0 Hatchery WNFH 0 (0-2)/ 0 (0-2) 2293 ( )/ 37 (6-109) < 10/ 2 (juveniles) 40 (19-54)/ 35 (16-43) juveniles 3 1 Mortalities of spring Chinook do not include those used for broodstock. 2 A portion of these fish may have been recaptured and handled a second time. 3 These are mostly precocial males that are euthanized. In summary, the facilities involved in these programs are all operated in a manner that NMFS considers low risk, resulting in an overall small negative effect and NMFS expects this effect of the Proposed Action to remain small into the future Factor 3. Hatchery fish and the progeny of naturally spawning hatchery fish in juvenile rearing areas Small negative effect on spring Chinook salmon; negligible effect on steelhead: The Methow spring Chinook salmon population needs hatchery support, and the Proposed Action will provide such support with much less genetic and ecological risk, as discussed below, than has been the pattern in the environmental baseline by releasing fewer fish. Effects on steelhead are negligible due to the later emergence timing of steelhead and habitat segregation between the two species. 72
81 Impacts from released hatchery fish Competition and Disease Recently, a multiagency technical team called HETT 16 completed a large-scale effort (Mackey et al. 2014) to model the ecological impacts (predation, disease, competition) of all Upper Columbia salmon and steelhead hatchery programs on various non-target taxa of concern (NTTOC), including juvenile spring Chinook salmon and steelhead using what is called the PCD Risk model (Busack et al. 2005; Pearsons and Busack 2012). PCD Risk is an individual-based model that simulates predation, competition, and disease impacts on naturally produced salmonids caused by hatchery smolts in fresh water as they move downstream. Although we could not use the results of the NTTOC simulations because there were some bugs in the program code and target species were not included (i.e., Methow River hatchery spring Chinook salmon effects on natural-origin spring Chinook salmon in the Methow River), it was clear that strong inferences about the potential for ecological interactions could be made from input data provided to us by the HETT researchers. Thus, we have used those input data to inform a modified model developed by NMFS staff 17 to analyze the potential for ecological interactions between the released spring Chinook salmon and the listed natural-origin juvenile salmon and steelhead in the Methow Subbasin. In the case of hatchery fish survival, we chose to assume 100 percent of the fish survived as opposed to the 97 percent in the HETT database, because the modified model, at this point, cannot allocate this small amount of mortality over multiple days. However, the 100 percent assumption is also a precautionary assumption because it would lead to a larger effect. The HETT researchers used triangular distributions for nearly all variables. Unless otherwise specified, we used only the modal (most likely) values. For this Opinion, we calculated a 10 percent overage for the numbers of fish released at each site. This is because in some years, given the uncertainties in producing fish up to a 10 percent overage in production and release is being proposed to account for any fish that may be lost during rearing (Table 19). Although only 25,000 fish are to be released at Goat Well currently, we used up to 34,000 in the model in the event larger releases are considered in the future because this is the capacity of that acclimation site. Table 19. Fish numbers, lengths, and residence, for hatchery juvenile spring Chinook salmon released by the programs. Release Site Maximum number of Length fish (mm) Residence (days) Twisp Pond 32, Methow Hatchery 152, Chewuch Pond 66, Goat Wall Acclimation Site 34, WNFH 440, Although the PCD Risk program also deals with disease, this portion of the model is not nearly as well developed as the material on competition and predation. Therefore, we have not utilized the NTTOC modelling information in dealing with disease. Analysis of potential disease effects is in Section Hatchery Evaluation Technical Team for the HCP Wells Hatchery Committee, HCP Rocky Reach Hatchery Committee, HCP Rock Island Hatchery Committee, and the Priest Rapids Hatchery Sub-Committee 17 C. Busack is revising the model code in Fortran. The percent residuals and disease parameters have been silenced in the program, until it can be determined how best to use these data, given the uncertainty with disease effects and the new information emerging on precocial maturation of males in hatchery spring Chinook salmon. 73
82 Table 20. Mean fork length (mm) and percentage of population (shown in parentheses) for natural-origin Methow spring Chinook salmon and steelhead juveniles potentially involved in ecological interactions. Spring Chinook Salmon Summer Steelhead Subbasin Subyearling Yearling Age 1 Age 2 Age 3 Methow 38 (97) 100 (3) (60) 170 (34) 179 (6) Chewuch 38 (97) 96 (3) 105 (60) 161 (34) 178 (6) Twisp 38 (88) 96 (12) 105 (60) 161 (34) 178 (6) The results of our modeling demonstrate that about 2.1 percent of the Methow spring Chinook salmon population will be lost to predation by hatchery spring Chinook salmon released into the Methow Subbasin annually. Competition among hatchery and natural-origin spring Chinook salmon is unlikely to occur (0 percent in the model output). This is likely because the majority of spring Chinook salmon present in the Methow Subbasin are subyearlings (Table 20). Hatchery spring Chinook salmon releases are likely to result in competition that eventually leads to death of 0.4 percent of the steelhead population. The percent of the steelhead population affected by predation is likely to be zero percent annually. These modeled estimates are likely explained by the fact that steelhead do not emerge until after hatchery releases occur. Thus, age-0 steelhead are not vulnerable to predation and/or competition with hatchery releases. In addition, steelhead juveniles occupy different habitat than spring Chinook juveniles; habitat segregation, the percentage of hatchery-origin population that are excluded from competitive interactions because they occupy different habitats, between hatchery spring Chinook salmon and steelhead was 60 percent (HETT 2014). Based on these results, NMFS concludes that the effects of competition and predation by hatchery spring Chinook salmon are low. However, two important things need to be considered and monitored. The first is that the residence time of hatchery fish within the system has a large influence on the model outcome. The faster hatchery fish move out of the Methow Subbasin. the lower the potential for competition and predation. Thus, we expect that 90 percent of the hatchery fish will have left the Methow Subbasin and have passed Wells Dam on the mainstem Columbia River within 14 days of release. Data from Snow (2016) demonstrates that 90 percent of the hatchery spring Chinook salmon from the Methow Subbasin pass Rocky Reach Juvenile Bypass in about 15.9 days, which is 67 Rkm below Wells Dam. The second consideration is that the model at this point does not incorporate residualism. Recent work has shown that spring Chinook salmon hatchery programs can have high rates of precocious maturation in males (Beckman and Larsen 2005; Harstad et al. 2014; Larsen et al. 2004; Larsen et al. 2013). Precocious males may forgo ocean migration, thus increasing their potential for competition and predation on natural-origin salmon and steelhead in juvenile rearing areas. Harstad et al found that for the WNFH spring Chinook salmon program, an average of 22.5 (ranging from 11 to 37.4 from ) percent of the males matured precociously. Thus, if we expect that half of the program production is male, then potentially up to 11 percent of production could residualize on average per year. Sampling for precocious males was conducted in 2016 for both programs; we would need to continue sampling, using a 300 fish sample for each program (Harstad et al. 2014) for an additional four years before using this value in the model. Although ecological interactions can be minimized by limiting residence times and keeping residualism rates low, we recognize that in practice this is difficult to accomplish. This is because larger fish tend to outmigrate 74
83 faster, but also are more likely to become precocious and residualize (Pearsons et al. 2013). Thus, both metrics will need to be considered before making any modifications to program release strategies Disease Some major diseases identified in salmonids from the Upper Columbia River are Bacterial Kidney Disease (BKD) and Infectious Hematopoietic Necrosis (IHN). Both are caused by pathogens the first, a bacterium (Renibacterium salmoninarum), and the second, IHN virus. Over the last five years ( ), no major disease outbreaks have occurred in spring Chinook salmon from the Methow Hatchery or WNFH programs. Historically, BKD was a problem at WNFH, but since the culling of high and moderate ELISA profiles, decreasing rearing densities, and the use of a chiller unit to delay emergence, outbreaks are rare (Humling 2016a). The last detection of IHN occurred in September of 2010 at the Methow hatchery, but it is regularly detected in spring Chinook salmon at WNFH. However, this likely poses a low risk to the natural-origin fish in the area, because no observable mortality has occurred with the strain of IHNV identified from infected fish in any of the three fish species reared at WNFH (steelhead, and coho and spring Chinook salmon). In 2015, several mortality events occurred at the Methow Hatchery associated with some opportunistic infections and a malfunction with the marking machine. These resulted in a loss of 3.3 percent of the 217,280 Methow-Chewuch fish being reared in the hatchery. In January of 2016, fry from one Twisp female showed signs of developmental issues and died soon after ponding, representing about 17 percent of the fish from the Twisp component. Thus, the effects of disease on ESA-listed fish are negligible only a small percentage of fish are lost during rearing and no major pathogens have been detected over the last five years. Because no substantial changes in fish health protocols have been proposed, NMFS expects this level of effect to continue into the future Impacts from progeny of naturally spawning hatchery fish Methow spring Chinook salmon Naturally spawning hatchery-origin spring Chinook salmon are likely to be less efficient at reproduction than their natural-origin counterparts (Christie et al. 2014), but the progeny of hatchery-origin spawners are likely to make up a sizable portion of the juvenile fish population. This is actually a desired result of the supplementation program, and the only expected effect of this added production is a density dependent response of decreasing growth and potential exceedance of habitat capacity. The Proposed Action will reduce the number of hatcheryorigin fish spawning in nature compared to the Environmental Baseline, leading to the reduction of hatcheryorigin progeny. There is no evidence of density dependence under the current programs, but the Proposed Action will make these effects even less likely Methow steelhead Because spring Chinook salmon historically coexisted in substantial numbers with steelhead, it follows that there must have been adequate passage and habitat to allow both species to be productive and abundant. It does not follow automatically, however, that the historical situation can be restored under present-day conditions. Habitat and passage conditions have changed considerably over time to the point that both species are so depleted that they are listed under the ESA. The NTTOC modelers (HETT 2014) assumed 60 percent habitat partitioning between spring Chinook salmon and steelhead. At present, because of the likely habitat partitioning between the two species, the spring Chinook salmon supplementation effort in the Methow poses little risk to steelhead. But ecological impacts may increase in the 75
84 future if the spring Chinook salmon populations grow. Should the situation arise where spring Chinook salmon natural production is limiting steelhead natural production in the Methow Subbasin, recovery planners would have to prioritize one species over another. NMFS concludes that the monitoring efforts should detect negative impacts before they reach problematic levels, and we have included language in the ITS (Section 2.8.4) to ensure that monitoring takes place Factor 4. Hatchery fish and the progeny of naturally spawning hatchery fish in the migration corridor, estuary, and ocean Negligible effect: Best available information does not indicate that the release of hatchery fish from the Methow spring Chinook salmon program would exacerbate density-dependent effects on ESA-listed species in the mainstem Columbia River, in the estuary, or in the Pacific Ocean. NMFS has been investigating this factor for some time. The Proposed Recovery Plan for Snake River Salmon (NMFS 1995b) described the issue in this manner. There is intense debate over the issues of carrying capacity and density-dependent effects on natural populations of salmon. However, there is little definitive information available to directly address the effects of ecological factors on survival and growth in natural populations of Pacific salmon. Thus, many of the ecological consequences of releasing hatchery fish into the wild are poorly defined. The proposed recovery plan called on hatchery operators and funding entities to limit annual releases of anadromous fishes from Columbia Basin hatcheries and, in fact, releases have declined substantially. Hatchery releases for the entire Columbia River Basin now vary between 130 and 145 million fish annually compared to a previous annual production of approximately 200 million fish back in the late 1990s. More recently, NMFS reviewed the literature for new and emerging scientific information over the role and the consequences of density-dependent interactions. At full production, hatchery releases from the two programs analyzed in this biological opinion will constitute less than one percent of the total hatchery production and less than 0.05 percent of all juvenile salmonids in the Columbia basin. The SCA for the FCRPS opinion (NMFS 2008d) and the September 2009 FCRPS Adaptive Management Implementation Plan (NMFS 2009a) both concluded that available knowledge and research abilities are insufficient to discern any important role or contribution of hatchery fish in density-dependent interactions affecting salmon and steelhead growth and survival in the mainstem Columbia River, the Columbia River estuary, and in the Pacific Ocean. From the scientific literature, the general conclusion is that the influence of density-dependent interactions on growth and survival is likely small compared with the effects of large scale and regional environmental conditions. Although there is evidence that hatchery production, on a scale many times larger than the Proposed Action, can impact salmon survival in the migration corridor, estuary, and ocean, the degree of impact or level of influence is not yet understood or predictable. Regardless, hatchery production on the scale considered in this opinion is very unlikely to substantially affect salmon survival or recovery in these life stages Factor 5. Research, monitoring, and evaluation that exists because of the hatchery program Small negative effect: The proposed RM&E directly related to fish culture uses well-established (e.g., (AHSWG 2008)) methods and protocols. For the programs included in this proposed action, the egg-to-smolt survival is approximately 86 percent (CPUD 2015; DPUD and WDFW 2010; USFWS 2012b). In addition, fish health monitoring requires the capture and euthanizing of small sample groups (10-15 fish) from each release group. These survival rates pose no risk to the population because it greatly exceeds the survival in a wild population (e.g., egg-to-smolt was 7 percent for Chinook salmon (Bradford 1995)). 76
85 For some parts of the proposed studies, listed fish would be observed in-water (e.g., by snorkel surveys, wading surveys, or observation from the banks). However, any avoidance behaviors are expected to be in the range of normal predator and disturbance behaviors. Redds may be visually inspected, but would not be walked on. Sampling for juvenile spring Chinook salmon occurs in the Methow Subbasin for assessment of hatchery program effects on the listed population. There are two rotary screw traps operated for sampling of juveniles to assess juvenile outmigration timing and survival. One trap is located in the Twisp River at Rkm 2 and the other is in the Methow River at Rkm 18. The traps typically operate from late February to December, although traps may be inoperable during periods of high or low water flow or when temperatures exceed 18 C. Electrofishing and angling for juvenile spring Chinook salmon also occurs throughout the Methow subbasin to assess survival from the parr-to-smolt and smolt-to-adult life stages. Collection usually takes place from June through November. Because sampling occurs for a majority of the year and juvenile spring Chinook salmon and steelhead are both present in the system during this time, the encounters with steelhead are considered incidental to spring Chinook sampling, and are analyzed here, but will also be considered in a future opinion on the Methow steelhead programs. Juvenile sampling is anticipated to encounter about percent of natural-origin and 6.7 percent of the hatchery-origin spring Chinook salmon, and about percent natural-origin and 1.9 percent of the hatchery-origin steelhead. Although it is difficult to assess the sublethal effects of capturing and tagging (e.g., interference with swimming making fish more vulnerable to predation), if all the fish captured, handled and tagged suffered from sublethal effects, the impact to the natural-origin population would be less than 5 percent. Mortality resulting from trapping and tagging was less than one percent for spring Chinook salmon and steelhead in 2013 (Table 21). When fish were held overnight after capturing and tagging occurred, no additional mortality occurred, suggesting that lethal effects of capturing and tagging are immediate (Ben Goodman and Charles Snow, WDFW, personal communication, June 29, 2016). Over the past four years, PIT tagging of natural-origin spring Chinook salmon in the Methow Subbasin has increased from 171 fish in 2010 to 4,479 fish in NMFS anticipates the number of fish captured, handled and tagged to continue to increase to better understand juvenile emigration and survival of all life stages. Because of this increase and the annual variability in fish productivity, we assume that up to 20 percent of the spring Chinook juvenile population and 10 percent of the juvenile steelhead population may be captured, handled, and tagged each year. Table 21. Juvenile encounter and mortality data for the Twisp and Methow River smolt traps, electrofishing and angling combined in Species Spring Chinook Salmon (natural) Spring Chinook salmon (hatchery) Summer Steelhead (natural) Summer Steelhead (hatchery) Estimated Number % Mortalities Fish Population Size 1 Tagged Encountered Encountered Encountered (%) (234570) (3.3) (0.59) (0.35) (220340) (3.8) (0.53) (0.22) 1 The natural-origin population estimates are from (HETT 2014). The mean value is displayed with the minimum value in parentheses. Hatchery estimates total the release numbers of both programs for both species. The proposed research activities will have no measurable effects on the listed salmonids habitat. 77
86 Factor 6. Construction, operation, and maintenance of facilities that exist because of the hatchery programs Small negative effect: NMFS also evaluated the construction, operation, and the maintenance of hatchery facilities associated with the hatchery programs in the Methow Subbasin and concluded that this factor has a small negative effect on ESA-listed species. In this section, we evaluate how the facilities themselves affect the fish and designated critical habitat. Although no construction is included in the proposed Action, water intake structures and water withdrawal present another set of potential effects on listed salmonids. Each facility with intakes, pumps, and screens has the potential to impact fish, as does the release of effluent. Below, we consider these potential impacts for each facility. Unless groundwater withdrawals have been shown to have a direct connection to surface water levels, it is unlikely that groundwater withdrawals impact any ESA-listed salmonids. NMFS is not aware of any effects of groundwater withdrawals at any of the facilities. 78
87 Figure 20. Water routing for Methow and Winthrop National Fish Hatcheries (Humling 2016b). Table 22. Water use associated with facilities operated as part of the Proposed Action. Facility Surface Water Rights (cfs) Surface Water Source Average Flow (cfs, ) 1 Lowest Monthly Flow (cfs, ) 1 Average % of River Flow (Max) WNFH 50 Methow River (48.5) Methow via Foghorn 25 Hatchery irrigation Ditch (24.2) Twisp River Pond 6 Twisp River (18.3) Chewuch River Pond 6 Chewuch River (12.3) Gate Creek and Goat Wall Pond ) Cold Creek Data Source: (USGS 2014); gauges ; ; The acclimation sites only operate from February to May; only these months were considered when calculating annual flow and identifying lowest monthly flow. The Methow hatchery operates year round and thus the full 12 months was used to calculate annual flow. Values for Gate and Cold Creeks are estimated (M. Humling, USFWS, personal communication). 2 Because the gauge for the Methow River is just below the confluence with the Chewuch River, the flow from the Chewuch River is subtracted from flow in the Methow River at Winthrop, Washington. None of the facilities included in this Proposed Action de-water stream reaches. These facilities do typically divert small proportions of water from the river (Table 22) that are likely imperceptible to salmon and steelhead rearing or migrating through the project area. However, water is already diverted from the Methow River by the 79
Hatchery Scientific Review Group Review and Recommendations
 Hatchery Scientific Review Group Review and Recommendations Lochsa River Spring Chinook Population and Related Hatchery Programs January 31, 2009 Lochsa River Spring Chinook Population Report Page - 1
Hatchery Scientific Review Group Review and Recommendations Lochsa River Spring Chinook Population and Related Hatchery Programs January 31, 2009 Lochsa River Spring Chinook Population Report Page - 1
LEAVENWORTH COMPLEX HATCHERY PROGRAMS. Presentation to the Independent Science Advisory Board
 LEAVENWORTH COMPLEX HATCHERY PROGRAMS Presentation to the Independent Science Advisory Board FACILIT Y LOCATION AND PURPOSE Leavenworth NFH Spring Chinook (unlisted) and YN Coho production Entiat NFH Summer
LEAVENWORTH COMPLEX HATCHERY PROGRAMS Presentation to the Independent Science Advisory Board FACILIT Y LOCATION AND PURPOSE Leavenworth NFH Spring Chinook (unlisted) and YN Coho production Entiat NFH Summer
Hatchery Scientific Review Group Review and Recommendations
 Hatchery Scientific Review Group Review and Recommendations Walla Walla River Summer Steelhead Population and Related Hatchery Programs January 31, 2009 Note: Spawning reaches likely vary from those depicted.
Hatchery Scientific Review Group Review and Recommendations Walla Walla River Summer Steelhead Population and Related Hatchery Programs January 31, 2009 Note: Spawning reaches likely vary from those depicted.
Strategies for mitigating ecological effects of hatchery programs
 Strategies for mitigating ecological effects of hatchery programs Some case studies from the Pacific Northwest Kathryn Kostow Oregon Department of Fish and Wildlife Ecological risks occur when the presence
Strategies for mitigating ecological effects of hatchery programs Some case studies from the Pacific Northwest Kathryn Kostow Oregon Department of Fish and Wildlife Ecological risks occur when the presence
Hatchery Scientific Review Group Review and Recommendations
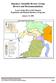 Hatchery Scientific Review Group Review and Recommendations Lower Snake River Fall Chinook Population and Related Hatchery Programs January 31, 2009 Lower Snake River Fall Chinook Population Report Page
Hatchery Scientific Review Group Review and Recommendations Lower Snake River Fall Chinook Population and Related Hatchery Programs January 31, 2009 Lower Snake River Fall Chinook Population Report Page
September 4, Update on Columbia basin Salmon and Steelhead Recovery Planning
 Bill Bradbury Chair Oregon Henry Lorenzen Oregon W. Bill Booth Idaho James A. Yost Idaho Jennifer Anders Vice Chair Montana Pat Smith Montana Tom Karier Washington Phil Rockefeller Washington September
Bill Bradbury Chair Oregon Henry Lorenzen Oregon W. Bill Booth Idaho James A. Yost Idaho Jennifer Anders Vice Chair Montana Pat Smith Montana Tom Karier Washington Phil Rockefeller Washington September
107 FERC 61,282 UNITED STATES OF AMERICA FEDERAL ENERGY REGULATORY COMMISSION
 107 FERC 61,282 UNITED STATES OF AMERICA FEDERAL ENERGY REGULATORY COMMISSION Before Commissioners: Pat Wood, III, Chairman; Nora Mead Brownell, and Joseph T. Kelliher. Public Utility District No. 1 of
107 FERC 61,282 UNITED STATES OF AMERICA FEDERAL ENERGY REGULATORY COMMISSION Before Commissioners: Pat Wood, III, Chairman; Nora Mead Brownell, and Joseph T. Kelliher. Public Utility District No. 1 of
Honorable Kimberly D. Bose July 2, 2013 Secretary Federal Energy Regulatory Commission 888 1st Street N.E. Washington, D.C
 Via Electronic Filing Honorable Kimberly D. Bose July 2, 2013 Secretary Federal Energy Regulatory Commission 888 1st Street N.E. Washington, D.C. 20426 Subject: Wells Hydroelectric Project No. 2149 Wells
Via Electronic Filing Honorable Kimberly D. Bose July 2, 2013 Secretary Federal Energy Regulatory Commission 888 1st Street N.E. Washington, D.C. 20426 Subject: Wells Hydroelectric Project No. 2149 Wells
Hatchery Scientific Review Group Review and Recommendations
 Hatchery Scientific Review Group Review and Recommendations Willamette McKenzie Spring Chinook Salmon Population and Related Hatchery Programs January 31, 2009 Columbia River Hatchery Reform Project -
Hatchery Scientific Review Group Review and Recommendations Willamette McKenzie Spring Chinook Salmon Population and Related Hatchery Programs January 31, 2009 Columbia River Hatchery Reform Project -
145 FERC 62,070 UNITED STATES OF AMERICA FEDERAL ENERGY REGULATORY COMMISSION
 145 FERC 62,070 UNITED STATES OF AMERICA FEDERAL ENERGY REGULATORY COMMISSION Public Utility District No. 1 of Douglas County, Washington Project No. 2149-163 ORDER APPROVING BULL TROUT STRANDING, ENTRAPMENT,
145 FERC 62,070 UNITED STATES OF AMERICA FEDERAL ENERGY REGULATORY COMMISSION Public Utility District No. 1 of Douglas County, Washington Project No. 2149-163 ORDER APPROVING BULL TROUT STRANDING, ENTRAPMENT,
Attachment 1. Agenda Item Summary BACKGROUND
 Attachment 1 Agenda Item Summary BACKGROUND Spring Chinook Salmon: Prior to the late 1970s, non-treaty spring Chinook fisheries in the mainstem Columbia River occurred from February through May and harvested
Attachment 1 Agenda Item Summary BACKGROUND Spring Chinook Salmon: Prior to the late 1970s, non-treaty spring Chinook fisheries in the mainstem Columbia River occurred from February through May and harvested
Subject: Wells Hydroelectric Project FERC Project No Bull Trout Management Plan and Incidental Take Annual Report
 Ms. Jessica Gonzales April 15, 2015 Wenatchee Office Lead Central Washington Field Office U. S. Fish and Wildlife Service 215 Melody Lane, Suite 119 Wenatchee, WA 98801-5933 Subject: Wells Hydroelectric
Ms. Jessica Gonzales April 15, 2015 Wenatchee Office Lead Central Washington Field Office U. S. Fish and Wildlife Service 215 Melody Lane, Suite 119 Wenatchee, WA 98801-5933 Subject: Wells Hydroelectric
Grande Ronde Basin Spring Chinook Salmon Captive Broodstock Program: F 1 Generation
 Grande Ronde Basin Spring Chinook Salmon Captive Broodstock Program: F 1 Generation Tim Hoffnagle, Rich Carmichael, Joseph Feldhaus, Deb Eddy, Nick Albrecht and Sally Gee Oregon Department of Fish and
Grande Ronde Basin Spring Chinook Salmon Captive Broodstock Program: F 1 Generation Tim Hoffnagle, Rich Carmichael, Joseph Feldhaus, Deb Eddy, Nick Albrecht and Sally Gee Oregon Department of Fish and
Appendix C - Guidance for Integrating EFH Consultations with Endangered Species Act Section 7 Consultations
 Appendix C - Guidance for Integrating EFH Consultations with Endangered Species Act Section 7 Consultations C.1 Guidance for Integrating Magnuson-Stevens Fishery Conservation and Management Act EFH Consultations
Appendix C - Guidance for Integrating EFH Consultations with Endangered Species Act Section 7 Consultations C.1 Guidance for Integrating Magnuson-Stevens Fishery Conservation and Management Act EFH Consultations
Subject: Wells Hydroelectric Project FERC Project No Annual Report Anadromous Fish Agreement and Habitat Conservation Plan
 April 7, 2010 Honorable Kimberly D. Bose Secretary Federal Energy Regulatory Commission 888 1st Street N.E. Washington, D.C. 20426 Subject: Wells Hydroelectric Project FERC Project No. 2149 Annual Report
April 7, 2010 Honorable Kimberly D. Bose Secretary Federal Energy Regulatory Commission 888 1st Street N.E. Washington, D.C. 20426 Subject: Wells Hydroelectric Project FERC Project No. 2149 Annual Report
Incidental Take Permits for HCP Plan Species
 Incidental Take Permits for HCP Plan Species Permit No. 1391 Permit for Incidental Take of Endangered or Threatened Species during the Operation and Maintenance of the Wells Hydroelectric Project (2003)
Incidental Take Permits for HCP Plan Species Permit No. 1391 Permit for Incidental Take of Endangered or Threatened Species during the Operation and Maintenance of the Wells Hydroelectric Project (2003)
Summary of HSRG Findings for Chum Populations in the Lower Columbia River and Gorge
 Summary of HSRG Findings for Chum Populations in the Lower Columbia River and Gorge The Congressionally-established Hatchery and Scientific Review Group (HSRG) developed a foundation of salmon hatchery
Summary of HSRG Findings for Chum Populations in the Lower Columbia River and Gorge The Congressionally-established Hatchery and Scientific Review Group (HSRG) developed a foundation of salmon hatchery
Steve Hemstrom Sr. Fisheries Biologist Chelan PUD Natural Resources Desk: Cell:
 From: To: Cc: Subject: Date: Attachments: Hemstrom, Steven "Lewis, Stephen" Sokolowski, Rosana 2014 Rocky Reach Bull Trout Report Wednesday, June 18, 2014 3:01:07 PM Final 2014 Rocky Reach Bull Trout Observations
From: To: Cc: Subject: Date: Attachments: Hemstrom, Steven "Lewis, Stephen" Sokolowski, Rosana 2014 Rocky Reach Bull Trout Report Wednesday, June 18, 2014 3:01:07 PM Final 2014 Rocky Reach Bull Trout Observations
Kirt Hughes Washington Department of Fish and Wildlife Region 6 - Fish Program Manager
 Kirt Hughes Region 6 - Fish Program Manager Habitat Hatcheries Harvest Clean Water Act Shorelines Harvest Hydro Habitat Forest Practices Non-regulatory Programs Water Supply & Conservation Growth Management
Kirt Hughes Region 6 - Fish Program Manager Habitat Hatcheries Harvest Clean Water Act Shorelines Harvest Hydro Habitat Forest Practices Non-regulatory Programs Water Supply & Conservation Growth Management
ESCA. Endangered Species Conservation Act of 1969 Changed in 1973 to ESA Amended several times
 ESCA Endangered Species Conservation Act of 1969 Changed in 1973 to ESA Amended several times International Efforts http://www.cites.org/ Convention on International Trade in Endangered Species of Wild
ESCA Endangered Species Conservation Act of 1969 Changed in 1973 to ESA Amended several times International Efforts http://www.cites.org/ Convention on International Trade in Endangered Species of Wild
THE CONFEDERATED TRIBES OF THE WARM SPRINGS RESERVATION OF OREGON
 THE CONFEDERATED TRIBES OF THE WARM SPRINGS RESERVATION OF OREGON To: Branch of Natural Resources P.0. Box C, Warm Springs, Oregon 97761 Phone (541) 553-2002/2003 Fax (541) 553-1994 The Independent Science
THE CONFEDERATED TRIBES OF THE WARM SPRINGS RESERVATION OF OREGON To: Branch of Natural Resources P.0. Box C, Warm Springs, Oregon 97761 Phone (541) 553-2002/2003 Fax (541) 553-1994 The Independent Science
The following language describing the performance standards was taken from the Reasonable and Prudent Alternative Table of Actions in the 2008 BIOP:
 FISH PASSAGE CENTER 1827 NE 44 th Ave., Suite 240, Portland, OR 97213 Phone: (503) 230-4099 Fax: (503) 230-7559 http://www.fpc.org/ e-mail us at fpcstaff@fpc.org MEMORANDUM TO: FROM: Michele DeHart Margaret
FISH PASSAGE CENTER 1827 NE 44 th Ave., Suite 240, Portland, OR 97213 Phone: (503) 230-4099 Fax: (503) 230-7559 http://www.fpc.org/ e-mail us at fpcstaff@fpc.org MEMORANDUM TO: FROM: Michele DeHart Margaret
Backgrounder and Frequently Asked Questions
 Backgrounder and Frequently Asked Questions Who Sent the Letter? The 60-day Notice of Intent to File Suit was sent by Conservation Angler, Wild Fish Conservancy, Snake River Waterkeeper, Friends of the
Backgrounder and Frequently Asked Questions Who Sent the Letter? The 60-day Notice of Intent to File Suit was sent by Conservation Angler, Wild Fish Conservancy, Snake River Waterkeeper, Friends of the
Comparative Survival Study
 Comparative Survival Study SARs and Productivity Presenter: Charlie Petrosky CRSO Workshop September 21, 2017 1 Smolt to Adult Survival Rate (SAR) Goals PATH (1998); NMFS 2000 BiOp: 2% SAR met interim
Comparative Survival Study SARs and Productivity Presenter: Charlie Petrosky CRSO Workshop September 21, 2017 1 Smolt to Adult Survival Rate (SAR) Goals PATH (1998); NMFS 2000 BiOp: 2% SAR met interim
January 13, Mr. William C. Maslen Manager, Fish and Wildlife Division Bonneville Power Administration P.O. Box 3621 Portland, Oregon 97208
 W. Bill Booth Chair Idaho James A. Yost Idaho Tom Karier Washington Dick Wallace Washington Bruce A. Measure Vice-Chair Montana Rhonda Whiting Montana Melinda S. Eden Oregon Joan M. Dukes Oregon January
W. Bill Booth Chair Idaho James A. Yost Idaho Tom Karier Washington Dick Wallace Washington Bruce A. Measure Vice-Chair Montana Rhonda Whiting Montana Melinda S. Eden Oregon Joan M. Dukes Oregon January
Implementing Hatchery Reform in the State of Idaho
 Implementing atchery Reform in the State of Idaho Paul Kline Idaho Department of Fish and Game Washington/British Columbia Chapter Meeting American Fisheries Society March 20, 2018 1 Idaho s atchery Programs
Implementing atchery Reform in the State of Idaho Paul Kline Idaho Department of Fish and Game Washington/British Columbia Chapter Meeting American Fisheries Society March 20, 2018 1 Idaho s atchery Programs
LOWER SNAKE RIVER COMPENSATION PLAN: Oregon Spring Chinook Salmon Evaluation Studies 2006 Annual Progress Report
 LOWER SNAKE RIVER COMPENSATION PLAN: Oregon Spring Chinook Salmon Evaluation Studies 2006 Annual Progress Report Oregon Department of Fish and Wildlife Fish Research and Development, NE Region Fred R.
LOWER SNAKE RIVER COMPENSATION PLAN: Oregon Spring Chinook Salmon Evaluation Studies 2006 Annual Progress Report Oregon Department of Fish and Wildlife Fish Research and Development, NE Region Fred R.
Spilling Water at Hydroelectric Projects in the Columbia and Snake Rivers How Does It Benefit Salmon?
 Spilling Water at Hydroelectric Projects in the Columbia and Snake Rivers How Does It Benefit Salmon? Hydropower development in the Columbia and Snake rivers has left its mark on salmonid populations,
Spilling Water at Hydroelectric Projects in the Columbia and Snake Rivers How Does It Benefit Salmon? Hydropower development in the Columbia and Snake rivers has left its mark on salmonid populations,
LOWER SNAKE RIVER COMPENSATION PLAN: Oregon Spring Chinook Salmon Evaluation Studies 2007 Annual Progress Report
 LOWER SNAKE RIVER COMPENSATION PLAN: Oregon Spring Chinook Salmon Evaluation Studies 2007 Annual Progress Report Oregon Department of Fish and Wildlife Fish Research and Development, NE Region Joseph W.
LOWER SNAKE RIVER COMPENSATION PLAN: Oregon Spring Chinook Salmon Evaluation Studies 2007 Annual Progress Report Oregon Department of Fish and Wildlife Fish Research and Development, NE Region Joseph W.
June 3, 2014 MEMORANDUM. Council Members. Stacy Horton, Policy Analyst, Washington. SUBJECT: Final 2012 Hatchery Fin Clip Report
 Bill Bradbury Chair Oregon Henry Lorenzen Oregon W. Bill Booth Idaho James A. Yost Idaho Jennifer Anders Vice Chair Montana Pat Smith Montana Tom Karier Washington Phil Rockefeller Washington June 3, 2014
Bill Bradbury Chair Oregon Henry Lorenzen Oregon W. Bill Booth Idaho James A. Yost Idaho Jennifer Anders Vice Chair Montana Pat Smith Montana Tom Karier Washington Phil Rockefeller Washington June 3, 2014
MEMORANDUM. Ron Boyce, ODFW Bob Heinith, CRITFC. Michele DeHart. DATE: November 30, Operations
 FISH PASSAGE CENTER 1827 NE 44 th Ave., Suite 240, Portland, OR 97213 Phone: (503) 230-4099 Fax: (503) 230-7559 http://www.fpc.org/ e-mail us at fpcstaff@fpc.org MEMORANDUM TO: Ron Boyce, ODFW Bob Heinith,
FISH PASSAGE CENTER 1827 NE 44 th Ave., Suite 240, Portland, OR 97213 Phone: (503) 230-4099 Fax: (503) 230-7559 http://www.fpc.org/ e-mail us at fpcstaff@fpc.org MEMORANDUM TO: Ron Boyce, ODFW Bob Heinith,
10/29/ :08 AM. Mountain Whitefish, Mussels (freshwater) and Eulachon (candlefish)(smelt) The current Program makes no mention of these species
 Staff summary of Issues and Recommendations Species Specifics (other than Salmon, Steelhead and wildlife) *Preliminary draft, please refer to full recommendations for complete review 10/29/2013 10:08 AM
Staff summary of Issues and Recommendations Species Specifics (other than Salmon, Steelhead and wildlife) *Preliminary draft, please refer to full recommendations for complete review 10/29/2013 10:08 AM
FINAL HATCHERY AND GENETIC MANAGEMENT PLAN (HGMP)
 FINAL HATCHERY AND GENETIC MANAGEMENT PLAN (HGMP) Hatchery Program: Sandy Hatchery Spring Chinook Species or Hatchery Stock: Sandy River Spring Chinook (Stock 11) Agency/Operator: Oregon Department of
FINAL HATCHERY AND GENETIC MANAGEMENT PLAN (HGMP) Hatchery Program: Sandy Hatchery Spring Chinook Species or Hatchery Stock: Sandy River Spring Chinook (Stock 11) Agency/Operator: Oregon Department of
Shasta Dam Fish Passage Evaluation. Public Stakeholder Webinar
 Shasta Dam Fish Passage Evaluation Public Stakeholder Webinar 9-24-2015 Today s Topics 1. Introduction Craig Moyle (MWH) 2. Project Overview John Hannon (Reclamation) 3. Sacramento River Conditions and
Shasta Dam Fish Passage Evaluation Public Stakeholder Webinar 9-24-2015 Today s Topics 1. Introduction Craig Moyle (MWH) 2. Project Overview John Hannon (Reclamation) 3. Sacramento River Conditions and
TESTIMONY OF THE COLUMBIA RIVER TREATY TRIBES BEFORE PACIFIC FISHERIES MANAGEMENT COUNCIL April 12, 2010 Portland, OR
 Agenda Item H.1.f Supplemental Tribal Report 2 April 2010 TESTIMONY OF THE COLUMBIA RIVER TREATY TRIBES BEFORE PACIFIC FISHERIES MANAGEMENT COUNCIL April 12, 2010 Portland, OR Good day Mr. Chairman and
Agenda Item H.1.f Supplemental Tribal Report 2 April 2010 TESTIMONY OF THE COLUMBIA RIVER TREATY TRIBES BEFORE PACIFIC FISHERIES MANAGEMENT COUNCIL April 12, 2010 Portland, OR Good day Mr. Chairman and
MEMORANDUM. Joan Dukes, NPCC. Michele DeHart. DATE: August 5, Data Request
 FISH PASSAGE CENTER 1827 NE 44 th Ave., Suite 240, Portland, OR 97213 Phone: (503) 230-4099 Fax: (503) 230-7559 http://www.fpc.org/ e-mail us at fpcstaff@fpc.org MEMORANDUM TO: Joan Dukes, NPCC FROM: Michele
FISH PASSAGE CENTER 1827 NE 44 th Ave., Suite 240, Portland, OR 97213 Phone: (503) 230-4099 Fax: (503) 230-7559 http://www.fpc.org/ e-mail us at fpcstaff@fpc.org MEMORANDUM TO: Joan Dukes, NPCC FROM: Michele
Appendix D HATCHERY AND GENETIC MANAGEMENT PLAN (HGMP) Washington Department Fish and Wildlife
 Appendix D HATCHERY AND GENETIC MANAGEMENT PLAN (HGMP) Washington Department Fish and Wildlife SECTION 1. GENERAL PROGRAM DESCRIPTION 1.1) Name of Program Upper Columbia Summer Chinook Salmon Mitigation
Appendix D HATCHERY AND GENETIC MANAGEMENT PLAN (HGMP) Washington Department Fish and Wildlife SECTION 1. GENERAL PROGRAM DESCRIPTION 1.1) Name of Program Upper Columbia Summer Chinook Salmon Mitigation
2013 WHITE SALMON CHINOOK SALMON VSP MONITORING. Jeremy Wilson Washington Department of Fish and Wildlife
 2013 WHITE SALMON CHINOOK SALMON VSP MONITORING Jeremy Wilson Washington Department of Fish and Wildlife Study Area Outline History of WDFW Chinook Monitoring in WS 2013 Objectives 2013 Study Design 2013
2013 WHITE SALMON CHINOOK SALMON VSP MONITORING Jeremy Wilson Washington Department of Fish and Wildlife Study Area Outline History of WDFW Chinook Monitoring in WS 2013 Objectives 2013 Study Design 2013
Fall Chinook Work Group
 Tuesday, Grant PUD (Bureau of Reclamation Building) Ephrata, WA Technical members Paul Wagner, NMFS Jeff Fryer, CRITFC Holly Harwood, BPA Steve Hemstrom, CPUD Bill Tweit, WDFW Patrick McGuire, WDOE Peter
Tuesday, Grant PUD (Bureau of Reclamation Building) Ephrata, WA Technical members Paul Wagner, NMFS Jeff Fryer, CRITFC Holly Harwood, BPA Steve Hemstrom, CPUD Bill Tweit, WDFW Patrick McGuire, WDOE Peter
MEMORANDUM. July 2, Council members. Tony Grover, Fish and Wildlife Division Director SUBJECT:
 Bill Bradbury Chair Oregon Henry Lorenzen Oregon W. Bill Booth Idaho James A. Yost Idaho Jennifer Anders Vice Chair Montana Pat Smith Montana Tom Karier Washington Phil Rockefeller Washington July 2, 2013
Bill Bradbury Chair Oregon Henry Lorenzen Oregon W. Bill Booth Idaho James A. Yost Idaho Jennifer Anders Vice Chair Montana Pat Smith Montana Tom Karier Washington Phil Rockefeller Washington July 2, 2013
ESTIMATED RETURNS AND HARVEST OF COLUMBIA RIVER FALL CHINOOK 2000 TO BY JOHN McKERN FISH PASSAGE SOLUTIONS
 ESTIMATED RETURNS AND HARVEST OF COLUMBIA RIVER FALL CHINOOK 2000 TO 2007 BY JOHN McKERN FISH PASSAGE SOLUTIONS ESTIMATED RETURNS AND HARVEST OF COLUMBIA RIVER FALL CHINOOK 2000 TO 2007 This analysis of
ESTIMATED RETURNS AND HARVEST OF COLUMBIA RIVER FALL CHINOOK 2000 TO 2007 BY JOHN McKERN FISH PASSAGE SOLUTIONS ESTIMATED RETURNS AND HARVEST OF COLUMBIA RIVER FALL CHINOOK 2000 TO 2007 This analysis of
Endangered Species Act and FERC Hydroelectric Projects. Jeff Murphy & Julie Crocker NHA New England Meeting November 16, 2010
 Endangered Species Act and FERC Hydroelectric Projects Jeff Murphy & Julie Crocker NHA New England Meeting November 16, 2010 Shortnose Sturgeon Federally listed as endangered in 1967 Listed under the sole
Endangered Species Act and FERC Hydroelectric Projects Jeff Murphy & Julie Crocker NHA New England Meeting November 16, 2010 Shortnose Sturgeon Federally listed as endangered in 1967 Listed under the sole
Yakima/Klickitat Fisheries Project
 Yakima/Klickitat Fisheries Project Lower Yakima River Supplementation and Research Project Operations and Maintenance Annual Report 2002-2003 March 2004 DOE/BP-00006677-1 This Document should be cited
Yakima/Klickitat Fisheries Project Lower Yakima River Supplementation and Research Project Operations and Maintenance Annual Report 2002-2003 March 2004 DOE/BP-00006677-1 This Document should be cited
Update on Columbia Basin Partnership Task Force
 Update on Columbia Basin Partnership Task Force June 25, 2018 Marla Harrison Port of Portland M A F A C C B P T A S K F O R C E Overview of Today s Presentation: Background on Columbia Basin & why we need
Update on Columbia Basin Partnership Task Force June 25, 2018 Marla Harrison Port of Portland M A F A C C B P T A S K F O R C E Overview of Today s Presentation: Background on Columbia Basin & why we need
January 4, Addresses water quality within the Council program.
 Phil Rockefeller Chair Washington Tom Karier Washington Henry Lorenzen Oregon Bill Bradbury Oregon W. Bill Booth Vice Chair Idaho James Yost Idaho Pat Smith Montana Jennifer Anders Montana January 4, 2016
Phil Rockefeller Chair Washington Tom Karier Washington Henry Lorenzen Oregon Bill Bradbury Oregon W. Bill Booth Vice Chair Idaho James Yost Idaho Pat Smith Montana Jennifer Anders Montana January 4, 2016
Brian Missildine Natural Resource Scientist Hatchery Evaluation and Assessment Team Lead Washington-British Columbia Annual General Meeting Kelowna,
 Brian Missildine Natural Resource Scientist Hatchery Evaluation and Assessment Team Lead Washington-British Columbia Annual General Meeting Kelowna, BC March 19-22 REFORMER REFORMED WA Dept. of Fish and
Brian Missildine Natural Resource Scientist Hatchery Evaluation and Assessment Team Lead Washington-British Columbia Annual General Meeting Kelowna, BC March 19-22 REFORMER REFORMED WA Dept. of Fish and
Yakima River Basin Coho Reintroduction Feasibility Study
 Yakima River Basin Coho Reintroduction Feasibility Study Yakima Klickitat Fisheries Project Goals and Mission The purposes of the YKFP are to: enhance existing stocks of anadromous fish in the Yakima and
Yakima River Basin Coho Reintroduction Feasibility Study Yakima Klickitat Fisheries Project Goals and Mission The purposes of the YKFP are to: enhance existing stocks of anadromous fish in the Yakima and
Transition to a locally adapted steelhead stock at Winthrop NFH
 Transition to a locally adapted steelhead stock at Winthrop NFH Northwest Fisheries Science Center Chris Tatara, Bill Gale*, Penny Swanson, Don Larsen, Matt Cooper*, Chris Pasley*, and Barry Berejikian
Transition to a locally adapted steelhead stock at Winthrop NFH Northwest Fisheries Science Center Chris Tatara, Bill Gale*, Penny Swanson, Don Larsen, Matt Cooper*, Chris Pasley*, and Barry Berejikian
THE ENDANGERED SPECIES ACT
 ENVIRONMENTAL ASSESSMENT OF A NATIONAL MARINE FISHERIES SERVICE ACTION OF ISSUING A PERMIT (#1196) TO THE WASHINGTON DEPARTMENT OF FISH AND WILDLIFE UNDER SECTION 10(a)(1)(A) OF THE ENDANGERED SPECIES
ENVIRONMENTAL ASSESSMENT OF A NATIONAL MARINE FISHERIES SERVICE ACTION OF ISSUING A PERMIT (#1196) TO THE WASHINGTON DEPARTMENT OF FISH AND WILDLIFE UNDER SECTION 10(a)(1)(A) OF THE ENDANGERED SPECIES
Conditions affecting the 2011 and 2012 Fall Chinook Adult Returns to Spring Creek National Fish Hatchery.
 FISH PASSAGE CENTER 1827 NE 44 th Ave., Suite 240, Portland, OR 97213 Phone: (503) 230-4099 Fax: (503) 230-7559 http://www.fpc.org/ e-mail us at fpcstaff@fpc.org MEMORANDUM TO: Liz Hamilton, NSIA FROM:
FISH PASSAGE CENTER 1827 NE 44 th Ave., Suite 240, Portland, OR 97213 Phone: (503) 230-4099 Fax: (503) 230-7559 http://www.fpc.org/ e-mail us at fpcstaff@fpc.org MEMORANDUM TO: Liz Hamilton, NSIA FROM:
Perspectives of a State Director Selective fisheries as a tool in fisheries management and salmon recovery
 Perspectives of a State Director Selective fisheries as a tool in fisheries management and salmon recovery Jeffrey P. Koenings, PhD. Washington Department of Fish and Wildlife American Fisheries Society
Perspectives of a State Director Selective fisheries as a tool in fisheries management and salmon recovery Jeffrey P. Koenings, PhD. Washington Department of Fish and Wildlife American Fisheries Society
Press Release New Bilateral Agreement May 22, 2008
 Informational Report 3 June 2008 Press Release New Bilateral Agreement May 22, 2008 The Pacific Salmon Commission is pleased to announce that it has recommended a new bilateral agreement for the conservation
Informational Report 3 June 2008 Press Release New Bilateral Agreement May 22, 2008 The Pacific Salmon Commission is pleased to announce that it has recommended a new bilateral agreement for the conservation
Hatcheries: Role in Restoration and Enhancement of Salmon Populations
 Hatcheries: Role in Restoration and Enhancement of Salmon Populations Hatcheries play a large role in the management, ecology, and evolution of Pacific salmon. Why were/are they built? What are the assumptions
Hatcheries: Role in Restoration and Enhancement of Salmon Populations Hatcheries play a large role in the management, ecology, and evolution of Pacific salmon. Why were/are they built? What are the assumptions
 Informational Report 1 USFWS Mass Marking Update April 2005 Update on USFWS 2005 Mass Marking Initiative Background Under Section 138 of FY 2003 Omnibus Appropriations Act (PL 108-7), Congress directed
Informational Report 1 USFWS Mass Marking Update April 2005 Update on USFWS 2005 Mass Marking Initiative Background Under Section 138 of FY 2003 Omnibus Appropriations Act (PL 108-7), Congress directed
Okanagan Sockeye Reintroduction
 Okanagan Sockeye Reintroduction Backgrounder Elders accounts, and other forms of Traditional Ecological Knowledge (TEK) tell us that many species of salmon once came into the Okanagan Valley and tell us
Okanagan Sockeye Reintroduction Backgrounder Elders accounts, and other forms of Traditional Ecological Knowledge (TEK) tell us that many species of salmon once came into the Okanagan Valley and tell us
Hatchery Reform and our Pacific Region National Fish Hatcheries. Presented by Doug Olson
 Hatchery Reform and our Pacific Region National Fish Hatcheries Presented by Doug Olson What is Hatchery Reform? Hatchery reform is actually quite simple in principle: its managing hatcheries as a type
Hatchery Reform and our Pacific Region National Fish Hatcheries Presented by Doug Olson What is Hatchery Reform? Hatchery reform is actually quite simple in principle: its managing hatcheries as a type
Case 6:17-cv MC Document 1 Filed 05/22/17 Page 1 of 12
 Case 6:17-cv-00801-MC Document 1 Filed 05/22/17 Page 1 of 12 Peter M.K. Frost (OSB #911843) Western Environmental Law Center 1216 Lincoln Street Eugene, Oregon 97401 Tel: 541-359-3238 Email: frost@westernlaw.org
Case 6:17-cv-00801-MC Document 1 Filed 05/22/17 Page 1 of 12 Peter M.K. Frost (OSB #911843) Western Environmental Law Center 1216 Lincoln Street Eugene, Oregon 97401 Tel: 541-359-3238 Email: frost@westernlaw.org
LOOKINGGLASS HATCHERY
 LOOKINGGLASS HATCHERY PROGRAM MANAGEMENT PLAN 2018 Lookingglass Hatchery And Imnaha Satellite Facility INTRODUCTION Lookingglass Hatchery is located along Lookingglass Creek, a tributary to the Grande
LOOKINGGLASS HATCHERY PROGRAM MANAGEMENT PLAN 2018 Lookingglass Hatchery And Imnaha Satellite Facility INTRODUCTION Lookingglass Hatchery is located along Lookingglass Creek, a tributary to the Grande
29.0 CALIFORNIA CENTRAL VALLEY STEELHEAD ESU
 29.0 CALIFORNIA CENTRAL VALLEY STEELHEAD ESU 29.1 BACKGROUND 29.1.1 Description of the ESU The California Central Valley Steelhead (CCVS) ESU includes all naturally spawned populations of steelhead (and
29.0 CALIFORNIA CENTRAL VALLEY STEELHEAD ESU 29.1 BACKGROUND 29.1.1 Description of the ESU The California Central Valley Steelhead (CCVS) ESU includes all naturally spawned populations of steelhead (and
Preliminary Summary of Out-of-Basin Steelhead Strays in the John Day River Basin
 Preliminary Summary of Out-of-Basin Steelhead Strays in the John Day River Basin Prepared by: James R. Ruzycki and Richard W. Carmichael Oregon Department of Fish and Wildlife La Grande, Oregon Introduction
Preliminary Summary of Out-of-Basin Steelhead Strays in the John Day River Basin Prepared by: James R. Ruzycki and Richard W. Carmichael Oregon Department of Fish and Wildlife La Grande, Oregon Introduction
Comparative Survival Study
 Agenda Item C.1.a Supplemental PPT Presentation June 2012 Comparative Survival Study Habitat Committee meeting Pacific Fishery Management Council June 12, 2012 Comparative Survival Study Initiated in 1996
Agenda Item C.1.a Supplemental PPT Presentation June 2012 Comparative Survival Study Habitat Committee meeting Pacific Fishery Management Council June 12, 2012 Comparative Survival Study Initiated in 1996
Hatchery Information for Subbasin Planning
 Appendix E Wenatchee Subbasin Plan Hatchery Information for Subbasin Planning Chuck Peven, editor; PCI Stephen H. Smith; SHS Fisheries Consulting, Inc. Dave Carie; USFWS Mike Tonseth; WDFW Andrew Murdoch;
Appendix E Wenatchee Subbasin Plan Hatchery Information for Subbasin Planning Chuck Peven, editor; PCI Stephen H. Smith; SHS Fisheries Consulting, Inc. Dave Carie; USFWS Mike Tonseth; WDFW Andrew Murdoch;
Oregon Department of Fish and Wildlife: Inland Fisheries - Hatchery Management
 Oregon Department of Fish and Wildlife: Inland Fisheries - Hatchery Management Primary Outcome Area: Economy & Jobs Secondary Outcome Area: Healthy Environments Program Contact: Ed Bowles, 503-947-6206
Oregon Department of Fish and Wildlife: Inland Fisheries - Hatchery Management Primary Outcome Area: Economy & Jobs Secondary Outcome Area: Healthy Environments Program Contact: Ed Bowles, 503-947-6206
Old Document: Sandy River Coho Final HGMP 78 pages (1.18 MB) 12/10/2013 3:11:23 PM
 Summary 12/10/2013 3:11:56 PM Differences exist between documents. New Document: sandy_co_hgmp_11-6-13_final 76 pages (841 KB) 12/10/2013 3:11:23 PM Used to display results. Old Document: Sandy River Coho
Summary 12/10/2013 3:11:56 PM Differences exist between documents. New Document: sandy_co_hgmp_11-6-13_final 76 pages (841 KB) 12/10/2013 3:11:23 PM Used to display results. Old Document: Sandy River Coho
F INAL MEMORANDUM ACTION ITEM SUMMARY. Date: December 17, To: Wells, Rocky Reach, and Rock Island HCPs Hatchery Committees.
 720 Olive Way, Suite 1900 Seattle, Washington 98101 Phone 206.287.9130 Fax 206.287.9131 www.anchorqea.com F INAL MEMORANDUM To: From: Cc: Re: Wells, Rocky Reach, and Rock Island HCPs Hatchery Committees
720 Olive Way, Suite 1900 Seattle, Washington 98101 Phone 206.287.9130 Fax 206.287.9131 www.anchorqea.com F INAL MEMORANDUM To: From: Cc: Re: Wells, Rocky Reach, and Rock Island HCPs Hatchery Committees
EXHIBIT ARWA-700 TESTIMONY OF PAUL BRATOVICH
 EXHIBIT ARWA-700 TESTIMONY OF PAUL BRATOVICH 1. I am a fisheries biologist employed by the firm of HDR, Inc. I hold a Bachelor of Science degree in Fisheries from the University of Washington, located
EXHIBIT ARWA-700 TESTIMONY OF PAUL BRATOVICH 1. I am a fisheries biologist employed by the firm of HDR, Inc. I hold a Bachelor of Science degree in Fisheries from the University of Washington, located
Coho. Oregon Native Fish Status Report 13
 12 Coho Coho salmon are widespread in small, low gradient streams of the coast and lower Columbia. They enter freshwater and spawn after fall rains raise river levels, typically from September through
12 Coho Coho salmon are widespread in small, low gradient streams of the coast and lower Columbia. They enter freshwater and spawn after fall rains raise river levels, typically from September through
Klickitat Spring Chinook Production Program Klickitat Hatchery Complex
 Klickitat Spring Chinook Production Program Klickitat Hatchery Complex 1 This page is intentionally blank. 2 HATCHERY AND GENETIC MANAGEMENT PLAN (HGMP) Final Draft Hatchery Program Klickitat Spring Chinook
Klickitat Spring Chinook Production Program Klickitat Hatchery Complex 1 This page is intentionally blank. 2 HATCHERY AND GENETIC MANAGEMENT PLAN (HGMP) Final Draft Hatchery Program Klickitat Spring Chinook
Cowlitz River Fisheries and Hatchery Management Plan (FHMP)
 Cowlitz River Fisheries and Hatchery Management Plan (FHMP) Final August 2004 Prepared by Tacoma Power Table of Contents Executive Summary... ix 1.0 Introduction...1 2.0 Settlement Principles and License
Cowlitz River Fisheries and Hatchery Management Plan (FHMP) Final August 2004 Prepared by Tacoma Power Table of Contents Executive Summary... ix 1.0 Introduction...1 2.0 Settlement Principles and License
May 28, SUBJECT: Management Recommendations from ISRP/ISAB s Tagging Report #2009-1
 W. Bill Booth Chair Idaho James A. Yost Idaho Tom Karier Washington Dick Wallace Washington Bruce A. Measure Vice-Chair Montana Rhonda Whiting Montana Melinda S. Eden Oregon Joan M. Dukes Oregon May 28,
W. Bill Booth Chair Idaho James A. Yost Idaho Tom Karier Washington Dick Wallace Washington Bruce A. Measure Vice-Chair Montana Rhonda Whiting Montana Melinda S. Eden Oregon Joan M. Dukes Oregon May 28,
Klickitat Complex - Fall Chinook
 Klickitat Complex - Fall Chinook Updated November 2013 1 This page is intentionally blank. Updated November 2013 2 HATCHERY AND GENETIC MANAGEMENT PLAN (HGMP) Final DRAFT Hatchery Program Klickitat Complex
Klickitat Complex - Fall Chinook Updated November 2013 1 This page is intentionally blank. Updated November 2013 2 HATCHERY AND GENETIC MANAGEMENT PLAN (HGMP) Final DRAFT Hatchery Program Klickitat Complex
Past, Present and Future Activities Being Conducted in the Klamath River Basin Related to the Protection and Recovery of Fish and Their Habitat
 Past, Present and Future Activities Being Conducted in the Klamath River Basin Related to the Protection and Recovery of Fish and Their Habitat National Marine Fisheries Service March 2003 The Department
Past, Present and Future Activities Being Conducted in the Klamath River Basin Related to the Protection and Recovery of Fish and Their Habitat National Marine Fisheries Service March 2003 The Department
Survival Testing at Rocky Reach and Rock Island Dams
 FISH PASSAGE CENTER 1827 NE 44 th Ave., Suite 240, Portland, OR 97213 Phone: (503) 230-4099 Fax: (503) 230-7559 http://www.fpc.org/ e-mail us at fpcstaff@fpc.org MEMORANDUM TO: FROM: Michele DeHart Erin
FISH PASSAGE CENTER 1827 NE 44 th Ave., Suite 240, Portland, OR 97213 Phone: (503) 230-4099 Fax: (503) 230-7559 http://www.fpc.org/ e-mail us at fpcstaff@fpc.org MEMORANDUM TO: FROM: Michele DeHart Erin
***Please Note*** April 3, Dear advisory committee members:
 April 3, 29 Dear advisory committee members: The fifth meeting of the CHF advisory committee will be held April 13 in Grants Pass from 6:-8:3 PM, and the purpose of this document is to help committee members
April 3, 29 Dear advisory committee members: The fifth meeting of the CHF advisory committee will be held April 13 in Grants Pass from 6:-8:3 PM, and the purpose of this document is to help committee members
Management Strategies for Columbia River Recreational and Commercial Fisheries: 2013 and Beyond
 Management Strategies for Columbia River Recreational and Commercial Fisheries: 2013 and Beyond Recommendation of the Columbia River Fishery Management Workgroup to the Fish and Wildlife Commissions of
Management Strategies for Columbia River Recreational and Commercial Fisheries: 2013 and Beyond Recommendation of the Columbia River Fishery Management Workgroup to the Fish and Wildlife Commissions of
Memorandum FINAL. Action Item Summary
 720 Olive Way, Suite 1900 Seattle, Washington 98101 206.287.9130 Memorandum To: Wells, Rocky Reach, and Rock Island HCPs Hatchery Committees From: Tracy Hillman, Chairman cc: Sarah Montgomery, Anchor QEA,
720 Olive Way, Suite 1900 Seattle, Washington 98101 206.287.9130 Memorandum To: Wells, Rocky Reach, and Rock Island HCPs Hatchery Committees From: Tracy Hillman, Chairman cc: Sarah Montgomery, Anchor QEA,
Sockeye Reintroduction program. April 12, 2014 BCWF AGA Howie Wright
 Sockeye Reintroduction program April 12, 2014 BCWF AGA Howie Wright 8 Communities 1. Lower Similkameen Indian Band 2. Upper Similkameen Indian Band 3. Osoyoos Indian Band 4. Penticton Indian Band 5. Westbank
Sockeye Reintroduction program April 12, 2014 BCWF AGA Howie Wright 8 Communities 1. Lower Similkameen Indian Band 2. Upper Similkameen Indian Band 3. Osoyoos Indian Band 4. Penticton Indian Band 5. Westbank
Fisheries Off West Coast States; Coastal Pelagic Species Fisheries; Annual. AGENCY: National Marine Fisheries Service (NMFS), National Oceanic and
 This document is scheduled to be published in the Federal Register on 06/30/2017 and available online at https://federalregister.gov/d/2017-13685, and on FDsys.gov Billing Code 3510-22-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 06/30/2017 and available online at https://federalregister.gov/d/2017-13685, and on FDsys.gov Billing Code 3510-22-P DEPARTMENT OF
Confederated Tribes and Bands of the Yakama Nation. Klickitat Hatchery Complex - Coho
 Klickitat Hatchery Complex - Coho HATCHERY AND GENETIC MANAGEMENT PLAN (HGMP) Final Draft Hatchery Program Klickitat Hatchery Complex - Coho Species or Hatchery Stock Oncorhynchus kisutch Type N Coho Salmon
Klickitat Hatchery Complex - Coho HATCHERY AND GENETIC MANAGEMENT PLAN (HGMP) Final Draft Hatchery Program Klickitat Hatchery Complex - Coho Species or Hatchery Stock Oncorhynchus kisutch Type N Coho Salmon
Project Proposal FY 2007 Funding (Funding available through December 31, 2009)
 Project Proposal FY 2007 Funding (Funding available through December 31, 2009) Project Name: Clearwater River Coho Salmon Production Project V PCSRF Fiscal Year: Fiscal Year 2008, October 1, 2007 thru
Project Proposal FY 2007 Funding (Funding available through December 31, 2009) Project Name: Clearwater River Coho Salmon Production Project V PCSRF Fiscal Year: Fiscal Year 2008, October 1, 2007 thru
Joint Columbia River Management Staff
 2017 JOINT STAFF REPORT: STOCK STATUS AND FISHERIES FOR SPRING CHINOOK, SUMMER CHINOOK, SOCKEYE, STEELHEAD, AND OTHER SPECIES Joint Columbia River Management Staff Oregon Department of Fish & Wildlife
2017 JOINT STAFF REPORT: STOCK STATUS AND FISHERIES FOR SPRING CHINOOK, SUMMER CHINOOK, SOCKEYE, STEELHEAD, AND OTHER SPECIES Joint Columbia River Management Staff Oregon Department of Fish & Wildlife
OREGON AND WASHINGTON DEPARTMENTS OF FISH AND WILDLIFE JOINT STAFF REPORT - WINTER FACT SHEET NO.
 OREGON AND WASHINGTON DEPARTMENTS OF FISH AND WILDLIFE JOINT STAFF REPORT - WINTER FACT SHEET NO. 3a Columbia River Compact/Joint State Hearing February 21, 2018 Fisheries under consideration: Mainstem
OREGON AND WASHINGTON DEPARTMENTS OF FISH AND WILDLIFE JOINT STAFF REPORT - WINTER FACT SHEET NO. 3a Columbia River Compact/Joint State Hearing February 21, 2018 Fisheries under consideration: Mainstem
Steelhead Kelt Reconditioning and Reproductive Success Studies in the Columbia River Basin
 Steelhead Kelt Reconditioning and Reproductive Success Studies in the Columbia River Basin Hatch, 1 D.R., D.E. Fast 2, W.J. Bosch 2, J.W. Blodgett 2, J.L.J. Trammell 2, A.L. Pierce, 1,3 S.R. Everett 4,
Steelhead Kelt Reconditioning and Reproductive Success Studies in the Columbia River Basin Hatch, 1 D.R., D.E. Fast 2, W.J. Bosch 2, J.W. Blodgett 2, J.L.J. Trammell 2, A.L. Pierce, 1,3 S.R. Everett 4,
APPENDIX D: LEWIS RIVER HATCHERY REVIEW
 APPENDIX D: LEWIS RIVER HATCHERY REVIEW JANUARY 14, 2004 Prepared for PacifiCorp and Cowlitz PUD Prepared by S.P. Cramer & Associates, Inc. 600 NW Fariss Gresham, Oregon 97030 www.spcramer.com D-1 TABLE
APPENDIX D: LEWIS RIVER HATCHERY REVIEW JANUARY 14, 2004 Prepared for PacifiCorp and Cowlitz PUD Prepared by S.P. Cramer & Associates, Inc. 600 NW Fariss Gresham, Oregon 97030 www.spcramer.com D-1 TABLE
HATCHERY AND GENETIC MANAGEMENT PLAN (HGMP)
 HATCHERY AND GENETIC MANAGEMENT PLAN (HGMP) Hatchery Program: SAFE Spring Chinook Species or Hatchery Stock: Spring Chinook stocks 022 and 024 (Willamette Stocks) Agency/Operator: Oregon Department of
HATCHERY AND GENETIC MANAGEMENT PLAN (HGMP) Hatchery Program: SAFE Spring Chinook Species or Hatchery Stock: Spring Chinook stocks 022 and 024 (Willamette Stocks) Agency/Operator: Oregon Department of
Staff, Organizations Directly Affected (including but not limited to):
 3-2-02.01 Steelhead Stream Classification Effective Date: December 13, 2005 This Policy Replaces: None. Staff, Organizations Directly Affected (including but not limited to): Ministry of Water, Land and
3-2-02.01 Steelhead Stream Classification Effective Date: December 13, 2005 This Policy Replaces: None. Staff, Organizations Directly Affected (including but not limited to): Ministry of Water, Land and
[FWS R5 ES 2015 N021; FXES FF05E00000] Endangered and Threatened Wildlife and Plants; Draft Recovery Plan for the Gulf
![[FWS R5 ES 2015 N021; FXES FF05E00000] Endangered and Threatened Wildlife and Plants; Draft Recovery Plan for the Gulf [FWS R5 ES 2015 N021; FXES FF05E00000] Endangered and Threatened Wildlife and Plants; Draft Recovery Plan for the Gulf](/thumbs/96/127515577.jpg) This document is scheduled to be published in the Federal Register on 03/31/2016 and available online at http://federalregister.gov/a/2016-07227, and on FDsys.gov Billing Code 4333 15 DEPARTMENT OF THE
This document is scheduled to be published in the Federal Register on 03/31/2016 and available online at http://federalregister.gov/a/2016-07227, and on FDsys.gov Billing Code 4333 15 DEPARTMENT OF THE
May 31, IDFG hatchery /supplementation polices and activities
 Joan M. Dukes Chair Oregon Bruce A. Measure Montana James A. Yost Idaho W. Bill Booth Idaho Rhonda Whiting Vice-Chair Montana Bill Bradbury Oregon Tom Karier Washington Phil Rockefeller Washington May
Joan M. Dukes Chair Oregon Bruce A. Measure Montana James A. Yost Idaho W. Bill Booth Idaho Rhonda Whiting Vice-Chair Montana Bill Bradbury Oregon Tom Karier Washington Phil Rockefeller Washington May
Throughout the Pacific Northwest, salmon and steelhead have been listed under the Endangered Species Act because their existence is either threatened
 Throughout the Pacific Northwest, salmon and steelhead have been listed under the Endangered Species Act because their existence is either threatened or endangered. The Upper Willamette River Basin s spring
Throughout the Pacific Northwest, salmon and steelhead have been listed under the Endangered Species Act because their existence is either threatened or endangered. The Upper Willamette River Basin s spring
The Blue Heron Slough Conservation Bank
 1 The Blue Heron Slough Conservation Bank CONSERVATION BANKING July 19-23, 2010 CASE STUDY SERIES The Blue Heron Slough Conservation Bank (Washington) I. OVERVIEW & BACKGROUND: Location: Snohomish River
1 The Blue Heron Slough Conservation Bank CONSERVATION BANKING July 19-23, 2010 CASE STUDY SERIES The Blue Heron Slough Conservation Bank (Washington) I. OVERVIEW & BACKGROUND: Location: Snohomish River
Master Plan for the Snake River Sockeye Program: Springfield Hatchery
 Independent Scientific Review Panel for the Northwest Power & Conservation Council 851 SW 6 th Avenue, Suite 1100 Portland, Oregon 97204 www.nwcouncil.org/fw/isrp Review of the Master Plan for the Snake
Independent Scientific Review Panel for the Northwest Power & Conservation Council 851 SW 6 th Avenue, Suite 1100 Portland, Oregon 97204 www.nwcouncil.org/fw/isrp Review of the Master Plan for the Snake
COLUMBIA RIVER SALMON AND STEELHEAD HARVEST 1980 TO by John McKern for The Columbia-Snake River Irrigators Association
 COLUMBIA RIVER SALMON AND STEELHEAD HARVEST 198 TO 26 by John McKern for The Columbia-Snake River Irrigators Association COLUMBIA RIVER SALMON AND STEELHEAD HARVEST 198 THROUGH 26 By John McKern FISH PASSAGE
COLUMBIA RIVER SALMON AND STEELHEAD HARVEST 198 TO 26 by John McKern for The Columbia-Snake River Irrigators Association COLUMBIA RIVER SALMON AND STEELHEAD HARVEST 198 THROUGH 26 By John McKern FISH PASSAGE
OREGON AND WASHINGTON DEPARTMENTS OF FISH AND WILDLIFE JOINT STAFF REPORT SUMMER FACT SHEET NO. 1 June 10, 2010
 OREGON AND WASHINGTON DEPARTMENTS OF FISH AND WILDLIFE JOINT STAFF REPORT SUMMER FACT SHEET NO. 1 June 10, 2010 Fisheries under consideration: Non-Indian commercial salmon STOCK STATUS Upper Columbia Summer
OREGON AND WASHINGTON DEPARTMENTS OF FISH AND WILDLIFE JOINT STAFF REPORT SUMMER FACT SHEET NO. 1 June 10, 2010 Fisheries under consideration: Non-Indian commercial salmon STOCK STATUS Upper Columbia Summer
Recreational Sturgeon Commercial Shad MANAGEMENT GUIDELINES
 OREGON AND WASHINGTON DEPARTMENTS OF FISH AND WILDLIFE JOINT STAFF REPORT - SUMMER FACT SHEET NO. 2 Columbia River Compact/Joint State Hearing June 28, 2005 Fisheries under consideration: Recreational
OREGON AND WASHINGTON DEPARTMENTS OF FISH AND WILDLIFE JOINT STAFF REPORT - SUMMER FACT SHEET NO. 2 Columbia River Compact/Joint State Hearing June 28, 2005 Fisheries under consideration: Recreational
Joint Columbia River Management Staff
 2009 JOINT STAFF REPORT: STOCK STATUS AND FISHERIES FOR SPRING CHINOOK, SUMMER CHINOOK, SOCKEYE, STEELHEAD, AND OTHER SPECIES, AND MISCELLANEOUS REGULATIONS Joint Columbia River Management Staff Oregon
2009 JOINT STAFF REPORT: STOCK STATUS AND FISHERIES FOR SPRING CHINOOK, SUMMER CHINOOK, SOCKEYE, STEELHEAD, AND OTHER SPECIES, AND MISCELLANEOUS REGULATIONS Joint Columbia River Management Staff Oregon
3. The qualification raised by the ISRP is addressed in #2 above and in the work area submittal and review by the ISRP as addressed in #1.
 Please find attached a response from The Confederated Tribes of the Warm Springs Reservation of Oregon (CTWSRO) for Project # 2008-301-00, Habitat Restoration Planning, Design and Implementation within
Please find attached a response from The Confederated Tribes of the Warm Springs Reservation of Oregon (CTWSRO) for Project # 2008-301-00, Habitat Restoration Planning, Design and Implementation within
Wild Steelhead Coalition Richard Burge Conservation VP September 11, 2006
 Wild Steelhead Coalition Richard Burge Conservation VP September 11, 2006 The following are talking points for the WDFW SEPA Scoping Meetings regarding the preparation of the State-wide and the Puget Sound
Wild Steelhead Coalition Richard Burge Conservation VP September 11, 2006 The following are talking points for the WDFW SEPA Scoping Meetings regarding the preparation of the State-wide and the Puget Sound
June 14, Sincerely,
 Joan M. Dukes Chair Oregon Bruce A. Measure Montana James A. Yost Idaho W. Bill Booth Idaho Rhonda Whiting Vice-Chair Montana Bill Bradbury Oregon Tom Karier Washington Phil Rockefeller Washington June
Joan M. Dukes Chair Oregon Bruce A. Measure Montana James A. Yost Idaho W. Bill Booth Idaho Rhonda Whiting Vice-Chair Montana Bill Bradbury Oregon Tom Karier Washington Phil Rockefeller Washington June
State of Washington Dept. of Fish and Wildlife invites applications for the position of: Permanent Fisheries Biologist 4 *
 State of Washington Dept. of Fish and Wildlife invites applications for the position of: Permanent Fisheries Biologist 4 *00593-15 SALARY: $3,819.00 - $5,010.00 Monthly OPENING DATE: 01/21/15 CLOSING DATE:
State of Washington Dept. of Fish and Wildlife invites applications for the position of: Permanent Fisheries Biologist 4 *00593-15 SALARY: $3,819.00 - $5,010.00 Monthly OPENING DATE: 01/21/15 CLOSING DATE:
Nez Perce Treaty of 1855
 2007 Nez Perce Tribal Steelhead Fishery Proposal A Harvest Recovery Strategy Presented by Joseph Oatman NPT Harvest Biologist FISH 510 -- Advanced Fish Management Nez Perce Treaty of 1855 The exclusive
2007 Nez Perce Tribal Steelhead Fishery Proposal A Harvest Recovery Strategy Presented by Joseph Oatman NPT Harvest Biologist FISH 510 -- Advanced Fish Management Nez Perce Treaty of 1855 The exclusive
