Cardiovascular and Anthropometric Measures
|
|
|
- Jason Andrews
- 5 years ago
- Views:
Transcription
1 2009 Add Health Wave IV Documentation Report Cardiovascular and Anthropometric Measures Report prepared by Pamela Entzel Eric A. Whitsel Andrea Richardson Joyce Tabor Suzanne Hallquist Jon Hussey Carolyn T. Halpern Kathleen Mullan Harris Add Health is supported by grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Further information may be obtained by contacting Add Health at Carolina Population Center University of North Carolina at Chapel Hill 123 Franklin Street, Room 403-C Chapel Hill, NC
2 Table of Contents Section Page 1. Introduction General Overview of Data Collection Blood Pressure Cuff Size Cardiovascular Measures Height Weight Waist Circumference References Appendix A: Microlife Blood Pressure Monitor Calibration Form and Protocol
3 1. Introduction During the Wave II and III in-home interviews, Add Health collected measures of respondent height and weight. To better understand the social, behavioral and biological linkages in health trajectories as the Add Health cohort ages through adulthood, the Wave IV study design included an expanded set of anthropometric measures as well as several new measures of cardiovascular health. The Wave IV cardiovascular and anthropometric measures were collected in the following order: Blood pressure cuff size Systolic blood pressure (SBP, mmhg) Diastolic blood pressure (DBP, mmhg) Pulse rate (PR, beats/min) Height (cm) Weight (kg) Waist circumference (cm) In addition, the Add Health Wave IV data set includes the following constructed measures, derived from those listed above: Blood pressure classification 1 Pulse pressure (mmhg) Mean arterial pressure (mmhg) Body mass index (BMI, kg/m 2 ) BMI classification 2 This document summarizes the rationale, measurement, equipment, protocol and data cleaning procedures for each of the cardiovascular and anthropometric measures collected at Wave IV. It also documents how constructed variables were derived from the cardiovascular and anthropometric measures collected in the field. Documentation of other Wave IV biological measures, including metabolic, inflammatory, immune and genetic measures, will be provided in separate reports. 2. General Overview of Data Collection A Blaise computer-assisted interview (CAI) program guided trained and certified field interviewers (FIs) through collection of all cardiovascular and anthropometric measures. Help screens with step-by-step measurement instructions were accessible within the program. Each FI also carried a Job Aids Booklet that served as a quick reference guide to study protocols. Respondents were free to decline any or all measurements while participating in other components of the interview. However, if a respondent s blood pressure cuff size could not be measured for any reason, no blood pressure reading was taken. If the respondent was incarcerated at the time of the interview and policies or practices at the correctional facility prevented collection of a particular measurement (e.g., the facility would not permit use of a tape 2
4 measure to measure waist circumference), the respondent could chose to participate in collection of the other measures. In the Wave IV data set, any measures that are missing due to unique circumstances at correctional facilities are coded as legitimate skips. As explained below, FIs provided respondents with written documentation of their systolic and diastolic blood pressures at the time of the interview. However, particular care was taken to prevent respondents from becoming embarrassed or self-conscious about their anthropometric measures. FIs, for example, were trained to remain courteous and professional at all times, never calling attention to or commenting on a respondent s height, weight or waist circumference. FIs were also trained not to share anthropometric measures with respondents unless they specifically requested them. Some measurement protocols were revised in the period between the Wave IV Pretest (conducted in 2007) and the Main Study (conducted in ). Where the Pretest and Main Study data collection protocols differed significantly, this report documents the key differences between them. Pretest cases in the Wave IV data set are flagged for identification. 3. Blood Pressure Cuff Size 3.1 Rationale Arm circumference was measured to guide selection of an appropriately-sized blood pressure cuff for the cardiovascular measures. 3.2 Measurement The field interviewer measured the circumference of the arm on which the blood pressure reading would be taken. For each measurement, the FI recorded the arm to be measured (right or left) and the cuff to be used. Cuff sizes were recorded as Adult, Large adult, or Large adult, but arm exceeds large adult size cuff. 3.3 Equipment A 16-inch, low-stretch, fiberglass tailor s tape (Exhibit 1) marked with (1) a 13-inch threshold and (2) the words Adult and Large printed in permanent ink to the left and right of the threshold. Exhibit 1. Tailor s tape used to determine blood pressure cuff size. 3.4 Protocol Main study 3
5 FIs used the respondent s right arm for the arm circumference and blood pressure measurements, unless one of the following contraindications was present: open sores, wounds, gauze dressings or rashes; casts, splints or shunts; intravenous (IV) catheters or other attached medical devices; swelling, withering or paralysis; or arm on same side as prior mastectomy. All female respondents were asked specifically whether they had had a mastectomy and, if so, on which side. If there were contraindications to using the right arm for measurement, the left arm was used. If there were contraindications on both arms, no arm circumference or blood pressure measurements were taken. To measure arm circumference accurately, FIs asked respondents to remove bulky outer garments (e.g., sweaters or jackets) and, if applicable, push up their shirt sleeves to expose the upper arm. FIs also instructed respondents to relax their shoulders and allow their arm to be measured while hanging loosely at their side. FIs wrapped the 16-inch tailor s tape around the respondent s upper arm, midway between the shoulder and elbow. If the measured circumference was < 13 inches, FIs recorded the cuff size as Adult. If the circumference was inches, FIs recorded it as Large adult. If the respondent s arm was > 16 inches meaning that the two ends of the tailor s tape did not meet when wrapped around the arm FIs recorded it as Large adult, but arm exceeds large adult size cuff. For respondents in the latter category, FIs were trained to proceed with the blood pressure reading, if possible, using the Large adult cuff Pretest variations During the Pretest, FIs used a SECA 200 English-increment circumference tape measure to measure arm circumference to the nearest 1/4 inch. The FI keyed this measurement into the CAI instrument, which was programmed to calculate and display the appropriate cuff size. If the measured arm circumference was < 12 inches, the Adult cuff was used. If the circumference was 12 inches, the Large adult cuff was used. 3.5 Constructed variables No constructed variables were derived from the arm circumference measure. 3.6 Data cleaning For all cases, cuff size skip logic was checked for consistency. For example, if a respondent refused the arm circumference measurement, no cuff size should have been recorded. 4
6 Because FIs were instructed to record arm circumference during the Pretest and cuff size during the Main Study, the cuff size variable was computed for Pretest respondents. Also, due to protocol changes, the Large adult cuff was used in the Pretest, whereas the Adult cuff was used in the Main Study, to measure blood pressure of respondents with an arm circumference of inches. Pretest respondents whose arm measurements were between 12 and inches are flagged in the data file. 4. Cardiovascular Measures 4.1 Rationale Blood pressure and pulse rate were measured because of their established relationship with cardiovascular disease morbidity and mortality. 4.2 Measurement Trained and certified field interviewers measured respondents resting, seated systolic and diastolic blood pressures (mmhg) and pulse rate (beats/minute). Three serial measurements were performed at 30-second intervals. 4.3 Equipment Factory calibrated, Microlife BP3MC1-PC-IB oscillometric blood pressure monitor (MicroLife USA, Inc.; Dunedin, FL) recommended for clinical and home use by the British Hypertension Society (Exhibit 2). Accessories: Adult cuff (S101, for arm circumferences: in) and Large adult cuff (S102, for arm circumferences: in). Monitor specifications: Weight: 735 g (with batteries) Size: 160 (W) x 140 (L) x 98 (H) mm Storage Temperature: 20 C-50 C (-4 F -122 F) Humidity: 15%-90% relative humidity maximum Operation Temperature: 10 C-40 C (50 F-104 F) Display: Liquid crystal display (LCD) Measuring Method: Oscillometric Pressure Sensor: Capacitive Measuring Range: mmhg (SBP and DBP); 40 to 200 beats/min (PR) Cuff Pressure Display Range: mmhg Measuring Resolution: 1 mmhg Accuracy: ±3 mmhg (SBP & DBP); ±5 % (PR) Power Source: [1] 4 AA batteries, 1.5V [2] AC Adapter 6V DC 600 ma (4.5-6 V DC) 5
7 Exhibit 2. Microlife blood pressure monitor, AC adapter and cuff 4.4 Protocol Main Study As discussed in the cuff size protocol above, all blood pressure measurements were taken on the right arm, absent contraindications. Respondents were asked to remove bulky outer garments and to push up their sleeve to expose the arm to be measured. If the respondent had left his or her seat for any reason during the five minutes prior to the blood pressure measurement, the FI had the respondent rest in the seated position for five minutes before proceeding with the reading. All respondents were asked to sit still and silently with both feet on the floor and legs uncrossed during the entire series of blood pressure measurements. FIs were trained to position the face of the Microlife monitor away from the respondent, outside of his or her view. For each respondent, the FI administered three separate readings at 30 second intervals. A timer programmed into the CAI instrument guaranteed the duration of the inter-reading interval. A visual cue and audible chime alerted the FI when the 30-second interval had ended and the next reading was to begin. During each interval, FIs instructed respondents to raise their elbow to the level of the shoulder and hold the arm upward at a 90 degree angle for five seconds. Immediately following each of the three readings, FIs keyed SBP, DBP and PR into the CAI instrument. The first set of readings was keyed once. To ensure accuracy of the results reported to respondents, the second and third SBP and DBP were keyed twice and discrepant entries identified in real time. If the Microlife blood pressure monitor delivered an error message in place of a reading, interviewers recorded the result as equipment malfunction. To resolve the problem generating an error message, FIs referred to the Job Aids Booklet, which included a list of 6
8 possible error codes with recommended courses of action. If three errors were encountered during the course of the blood pressure measurements, the CAI instrument prompted the field interviewer to discontinue use of the monitor and contact his or her field supervisor. Any monitors or cuffs that were known to be malfunctioning were withdrawn from the field. Following collection of the three blood pressure readings and all anthropometric measures, the CAI instrument guided the FI through completion of a blood pressure results form, which the FI then gave to the respondent. On this form the FI recorded the date of the reading, the average of the second and third SBP, the average of the second and third DBP, and an appropriate recommendation for follow-up with a health care provider, as calculated and displayed by the CAI instrument, which was programmed to take risk factors into consideration (see below). If either the second or third reading was not obtained, the respondent was given the result of the single available reading. If both the second and third readings were missing, no results were reported to the respondent. Recommendations as to when to follow up with a health care provider (i.e., 2 years, 1 year, 2 months, 1 month, 1 week, or Today ) were based on guidelines of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC7) 1 and took the following factors into account: Average SBP and DBP; Calculated BMI; Reported pregnancy status; Reported current smoking status; and Reported history of high blood cholesterol, diabetes, heart disease, chronic kidney disease, and/or stroke. When average systolic and diastolic blood pressures did not fall within the same follow-up category, the earlier of the two follow-up periods was recommended. When JNC7 guidelines recommended that the respondent follow up with a health care provider on the day of the reading (average SBP > 179 with risk factor present, or average DBP > 109 with risk factor present), the CAI instrument prompted the FI to offer to call a health care provider, friend or relative with whom the respondent could discuss the results. Additionally, the blood pressure results form included general information on risk factors for heart disease and contact information for the American Heart Association. To help ensure data quality and accuracy, pressures generated by a factory-calibrated pressure meter (Netech DigiMano, Model 2000; Netech Corporation; Farmingdale, NY) over a range of mmhg were compared with pressures measured by all MicroLife monitor / cuff pairs returned from the field after the Main Study was complete. See Appendix A for Microlife Blood Pressure Monitor Calibration Form and Protocol Pretest variations During the Pretest, the blood pressure results form was completed and given to the respondent at the end of the interview, rather than after the anthropometric measures. 7
9 4.5 Constructed variables Systolic and diastolic blood pressures and pulse rate Systolic blood pressure, diastolic blood pressure and pulse rate were constructed as the average of measures 2 and 3. When either the second or third measure was missing, the other single measure was used. In cases where both measures 2 and 3 were missing, the first measure was used. The Wave IV data set includes a variable to indicate the number of measures from which SBP, DBP and PR were constructed Blood pressure classification Blood pressure classification was constructed for all respondents based on guidelines from the Seventh Report of the Joint National Committee on Prevention Detection, Evaluation, and Treatment of High Blood Pressure (JNC7). 1 JNC7 Categories are defined as follows: JNC7 Category SBP (mmhg) DBP (mmhg) Normal <120 and <80 Pre-hypertension or Hypertension, Stage or Hypertension, Stage or Pulse pressure The single pulse pressure measure was constructed as the average of the difference between systolic and diastolic blood pressures using measures 2 and 3. When either the second or third measure was missing, the difference between systolic and diastolic blood pressure for the other single measure was used. In the cases when both measures 2 and 3 were missing, the difference between the first systolic and diastolic blood pressures was used. The formula is: ((SBP2 - DBP2) + (SBP3 - DBP3))/ Mean arterial pressure Mean arterial pressure was conventionally approximated as the weighted sum of systolic and diastolic blood pressure at the second and third readings, where the weights for SBP (1/3) and DBP (2/3) reflect the typical contributions of ventricular systole and diastole to the duration of the cardiac cycle. When either the second or third measure was missing, the other single measure was used. In cases where both measures 2 and 3 were missing, the first measure was used. The formula is: (((SBP2 + (2*DBP2))/3) + ((SBP3 + (2*DBP3))/3))/2 8
10 4.6 Data cleaning Skip logic was evaluated for all measures. Distributions of manually keyed systolic and diastolic blood pressures also were examined for digit preference of FIs, values exceeding the Microlifespecified ranges of measurement, and outliers. 5. Height 5.1 Rationale Height was measured to enable computation of body mass index (see below), an independent predictor of cardiovascular disease risk factors, morbidity and mortality, as well as a primary tool used in characterizing the epidemiology of obesity in the U.S. 5.2 Measurement Height was measured to the nearest 0.5 cm for all respondents who were capable of maintaining a standing position without assistance. 5.3 Equipment Carpenter s square, steel tape measure (1 mm graduation; 7.5 m maximum), and pre-printed, adherent Post-it note (Exhibit 3). Exhibit 3. Height measurement equipment 9
11 5.4 Protocol Main study To maintain data quality, field interviewers were trained to measure height against a smooth wall in an area without rugs or carpeting, if possible. The FI asked the respondent to remove his or her shoes and any hat, hair ornaments or other accessories that could affect the measurement. If the respondent refused or was unable to remove his or her shoes or interfering accessories, the FI was trained to measure the height of those items separately and record the results in the CAI instrument. The respondent was instructed to take a deep breath and stand as tall as possible against the wall, with their feet flat on the floor, both heels together and toes pointed slightly apart. The FI checked to be sure that the respondent s weight was evenly distributed and that their head, shoulder blades, buttocks and heels touched the wall, to the extent possible. The FI also aligned the respondent s head in the Frankfurt position (Exhibit 4), with the horizontal line from the ear canal to the lower border of the orbit of the eye parallel to the floor and perpendicular to the wall. Height was measured at the end of the respondent s normal exhalation. Exhibit 4. Head in Frankfurt position with carpenter s square 10
12 To take the measurement, the FI rested the carpenter s square firmly on top of the respondent s head so that the sides of the square that form a right angle were flush with the wall and resting on the respondent. The FI then placed the top edge of a Post-it note at the bottom edge of the square, marking the respondent s height, and asked the respondent to step away from the wall. Next, the FI used the tape measure to measure the distance from the floor to the top of the Post-it note, asking the respondent to hold the bottom end of the tape in place, if necessary. The FI measured height to the nearest 0.5 cm and printed the measurement on the Post-it note, in the space marked Height, before removing the Post-it note from the wall. The FI used the same Post-it note to temporarily record the weight and waist circumference before entering all three measurements into the computer Pretest variations During the Pretest, height was measured to the nearest 1/4 inch, using a steel tape measure with 1/16 inch graduations. The measurement was recorded in whole feet and whole and quarter inches Constructed variables Measured height was used in the construction of body mass index (see section 6.5). 5.6 Data cleaning The skip logic and distribution of manually keyed height were checked for inconsistencies, digit preference of FIs and outliers. If the respondent wore shoes, hair ornaments, etc. during the height measurement, the measured height of these items was subtracted from the respondent s height. Pretest height measurements recorded in feet and inches were converted to centimeters. 6. Weight 6.1 Rationale Weight was measured to enable computation of body mass index (see below), an independent predictor of cardiovascular disease risk factors, morbidity and mortality, as well as a primary tool used in characterizing the epidemiology of obesity in the U.S. 6.2 Measurement Weight was measured to the nearest 0.1 kg for all respondents who were capable of standing unassisted. 6.3 Equipment Health-o-meter 844KL High Capacity Digital Bathroom Scale (Jarden Corporation; Rye, NY) (Exhibit 5). Specifications: 11
13 200 kg / 440 lbs maximum capacity 0.1 kg / 0.1 lb graduations 12 5/8 x 12 5/8 platform 4.5 lbs Digital display 4-point load cell Lithium battery No moving parts Automatic shut-off after 30 seconds of inactivity Low battery warning display ( Lo ) Exhibit 5. Health-o-meter 844KL digital scale 6.4 Protocol Main study During FI training, each FI set the graduation switch on the bottom of his or her scale to kg and placed tape over the switch to maintain the metric setting. FIs were also trained to check the switch in the field before each measurement to ensure that weight results were recorded in kilograms. To measure a respondent s weight, the field interviewer placed the scale on a hard, flat surface, avoiding rugs and carpeting, if possible. The FI asked the respondent to remove their shoes and any change, wallets or keys from their pockets. Respondents were not asked to remove any 12
14 clothing. The FI instructed the respondent to stand on the scale with their weight evenly distributed, looking straight ahead. The FI recorded the weight to the nearest 0.1 kg on the preprinted Post-it note for later entry into the computer. If the respondent weighed over 200 kilograms, the scale displayed the message OL. In these cases, for data entry purposes, the FI recorded the weight as 201. To ensure the quality and accuracy of the weight measurements, field interviewers were required to perform a weekly calibration of the digital scale and submit the results to their supervisors. For every week of active data collection, each FI measured and reported the weight of (1) himself/herself, (2) the Add Health laptop computer, and (3) himself/herself holding the laptop computer. If the summed weights of the FI and the computer differed from the measured weight of the FI holding the computer by more than 1 kg, the field interviewer immediately notified his or her field supervisor of the discrepancy. FIs were also instructed to immediately report any problems with or damage to the scales. Any scales with suspected problems were withdrawn from the field and replaced Pretest During the Pretest, weight was measured to the nearest 0.2 lb using a Tanita HD351 digital bathroom scale with a maximum capacity of 440 lbs (200 kg) and graduations of either 0.2 lb or 0.1 kg. For Pretest respondents whose weight exceeded 440 lbs, a value of 441 was keyed into the computer. The Tanita scale was not available in the quantities needed for the main study, necessitating the switch to the Health-o-meter 844KL. 6.5 Constructed variables Body mass index (BMI) was calculated according to the metric imperial formula: BMI (kg/m 2 ) = weight (kg) / height (m 2 ) In addition, all respondents were assigned a BMI classification according to the categorization scheme recommended by the National Institutes of Health Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults 2 : 1 = underweight ( <18.5 kg/m 2 ) 2 = normal ( <25 kg/m 2 ) 3 = overweight (25 - <30 kg/m 2 ) 4 = obese I (30 - <35 kg/m 2 ) 5 = obese II (35 - <40 kg/m 2 ) 6 = obese III ( 40 kg/m 2 ) 6.6 Data cleaning The skip logic and distribution of manually keyed weight were checked for inconsistencies, digit preference of FIs, and outliers. Outlying differences between self-reported and measured weights were also examined in the context of respondent gender, height, waist and Wave III 13
15 weights, when available. If measured weight was inconsistent with sex, self-reported weight, waist and height measures, the weight variable was assigned the code Waist Circumference 7.1 Rationale Waist circumference is positively correlated with abdominal fat content. Waist circumference was measured because a disproportionate excess of abdominal relative to total body fat is an independent predictor of cardiovascular disease risk factors, morbidity and mortality. It too is a primary tool used in characterizing the epidemiology of obesity in the U.S. 7.2 Measurement Waist circumference was measured to the nearest 0.5 cm at the superior border of the iliac crest for all respondents capable of standing unassisted, including pregnant women. 7.3 Equipment SECA 200 metric-increment circumference tape measure (Seca Corp., North America East; Hanover, MD) (Exhibit 6). Specifications: 200 cm maximum range 1 mm graduations 2-sided cm scaling 90 x 25 x 65 mm 50 g Fiberglass tape Plastic case Automatic roll-up End-peg positioned 7.4 Protocol Exhibit 6. SECA 200 tape measure Main study FIs asked respondents to remove bulky outer garments and stand relaxed, breathing normally, with their weight evenly distributed. To locate the iliac crest, FIs placed their hands on the abdomen at the bottom of the rib cage and gently palpated downward until encountering the left and right superior borders of the pelvis. To avoid surprise and put the respondent at ease, FIs were trained to demonstrate the examination on themselves before touching respondents. 14
16 Once the upper left and right borders of the iliac crest were located, FIs asked respondents to mark the locations with their own hands so that FIs could measure the waist. FIs wrapped the SECA tape around the waist at the level of the superior iliac crest, making sure that the tape was not twisted and remained parallel to the floor (Exhibit 7). When FIs could not easily reach around the respondent, they were allowed to ask the respondent to do so and then hand the tape measure back to the FI for adjustment. The protocol also allowed FIs to walk around the respondent with the tape, if needed. The measurement was taken at the end of the respondent s normal exhalation and recorded on a Post-it note for subsequent entry in the CAI instrument. Any broken or malfunctioning SECA tapes were reported and replaced immediately throughout data collection. Exhibit 7. Tape measure placement Pretest During the Pretest, waist was measured to the nearest 1/4 inch with a SECA English-increment circumference measuring tape (1/8 inch graduation). 7.5 Constructed variables No constructed variables were derived from the waist circumference measurement. 7.6 Data cleaning The skip logic and distribution of manually keyed waist measurements were checked for inconsistencies, digit preference of FIs, and outliers. Pretest waist measurements recorded in whole and partial inches were converted to centimeters. 8. References 1 Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and 15
17 Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42(6): National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. Obes Res 1998;6(S2):51S-209S. 16
18 Appendix A: Microlife Blood Pressure Monitor Calibration Form and Protocol Tech ID: FI Cuff: FI Monitor: Test Date: Cuff: ADULT or LARGE ADULT Visual Check TUBE HAS CRACKING?... Y N NO MATCHING TUBING TUBE HAS HOLES?... Y N NO MATCHING TUBING CUFF HAS WORN OUTER CLOTH OR VELCRO?... Y N NO MATCHING CUFF TUBE LEAKS?... Y N NO MATCHING TUBING CUFF HAS LEAKAGE OF CUFF BLADDER?... Y N NO MATCHING CUFF COMMENTS: Calibration Check with Pressure-Vacuum Meter Observed pressure values on the Digimano Pressure-Vacuum Meter and the Microlife from 280 to 20 (± 2) mmhg in approximate decrements of 20 (± 2) mmhg. MEASUREMENT NUMBER DIGIMANO MICROLIFE 1 (280)... mmhg... mmhg 2 (260)... mmhg... mmhg 3 (240)... mmhg... mmhg 4 (220)... mmhg... mmhg 5 (200)... mmhg... mmhg 6 (180)... mmhg... mmhg 7 (160)... mmhg... mmhg 8 (140)... mmhg... mmhg 9 (120)... mmhg... mmhg 10 (100)... mmhg... mmhg 11 (80)... mmhg... mmhg 12 (60)... mmhg... mmhg 13 (40)... mmhg... mmhg 14 (20)... mmhg... mmhg 17
19 Equipment Required for Blood Pressure Monitor Calibration DigiMano Digital Pressure/Vacuum Meter, Netech Model 2000 (range: mmhg). Blood Pressure Calibration Kit (syringe; silicone tubing; T adapter; Luer-lock; Velcro tape; valve cap) Microlife 3MC1-PC_IB Oscillometric Blood Pressure Monitor ADULT and LARGE ADULT blood pressure cuffs Testing Protocol The following sequence of steps details the Microlife accuracy testing protocol. 1. Plug in and connect the AC adapter to the Microlife. 2. Seal the pressure release hole on the back of the Microlife with Velcro tape. 3. Record Tech ID, Field Interviewer number on the cuff package (FI Cuff), Field Interviewer number on the monitor (FI Monitor), and date on the form. The FI cuff number is found on the Ziplock bag and the FI Monitor number is on the back of the monitor. Use the code of 999 if the cuff does not have a FI number. 4. Circle ADULT on the form to indicate that the ADULT cuff is being evaluated. 5. Inspect the ADULT cuff tubing for holes or cracks which would allow air to leak out. Cracking is commonly found around the connection points to the sphygmomanometer and cuff. Answer questions on form about holes and cracking. 6. Inspect the ADULT cuff for signs of wear-and-tear to the outer cloth casing and Velcro fabric. Record your evaluation on the form. 7. Attach ADULT cuff to ADULT PVC pipe and place the pipe vertically on the table. Tightly connect the ADULT cuff tubing to the Microlife. The bottom edge of the cuff should rest 1 inch (black mark) above the end of the PVC pipe. The small white arrow (Artery Mark) on the cuff should be pointing down. You should be able to fit 2 fingers between the pipe and the cuff. 8. Connect the black L adapter on the ADULT cuff to the Microlife. 9. Turn on the Microlife to inflate the ADULT cuff to determine if the bladder within the ADULT cuff is leak-proof. Answer the questions on the form about ADULT cuff damage, air leakage, and tube leakage. 10. Disconnect the ADULT cuff tubing from the Microlife. 11. Remove the black connector from the ADULT cuff tubing and put aside. 12. Holding in the black lead screw on the syringe, screw and pull the grey plunger past the 60 cc mark (to the end of the syringe). Connect the white silicone tubing to the syringe by rotating it to engage the Luer Lock. 13. Snugly connect the black ADULT cuff tubing to the Y adapter on the white silicone tubing. 14. Connect the white silicone tubing to the Microlife with the black L adapter. 15. Turn on the DigiMano. Check for the low battery light and change the battery if necessary. 16. Ensure that the red light below mmhg on the DigiMano is flashing, indicating that the correct measurement (millimeters of mercury) is selected. 17. Zero the pressure vacuum meter by pressing the zero button on the front of the DigiMano. The display should read Pick up the syringe and then press the ON/OFF START button on the Microlife to start the reading. 18
20 19. When the Microlife stops inflating the cuff, while watching the DigiMano, adjust the pressure in the syringe until the *DigiMano* displays a stable value near 280 mmhg (+/- 2 mmhg). If stability is a problem, examine the calibration system, listen for air leaks, and reseal the pressure release hole on the back of the Microlife with the Velcro tape. 20. Slowly release the pressure in 20 mmhg decrements according to the readings on the *DigiMano*. Coarse adjust the pressure by holding in the black lead screw and pulling or pushing the grey plunger, as needed. Fine adjust the pressure by rotating the grey plunger. Carefully record on the form the pressure readings from the DigiMano and Microlife at 20 mmhg decrements, i.e. from 280, to 260,, to 60, and finally to 20 mmhg. 21. Disconnect the ADULT cuff from the white tubing. Place the black L adapter back in the cuff tubing. Put the ADULT cuff in the Ziplock bag. 22. Disconnect the syringe from the white silicone tubing by rotating it to disengage the Luer Lock. 23. Remove the LARGE ADULT cuff from the Ziplock bag. The LARGE ADULT cuff has a valve that needs to be sealed before taking the measurements. Seal this valve by replacing the stem and the short black tubing with a black plastic cap. 24. Using another form, record Tech ID, Field Interviewer number on the cuff package (FI Cuff), Field Interviewer number on the monitor (FI Monitor), and date on the form. The FI cuff number is found on the Ziplock bag and the FI Monitor number is on the back of the monitor. Use the code of 999 if the cuff does not have a FI number. 25. Circle LARGE ADULT on the form to indicate that the LARGE ADULT cuff is being evaluated. 26. Inspect the LARGE ADULT cuff tubing for holes or cracks which would allow air to leak out. Cracking is commonly found around the connection points to the sphygmomanometer and cuff. Answer questions on form about holes and cracking. 27. Inspect the LARGE ADULT cuff for signs of wear and tear to the outer cloth casing and Velcro fabric. Record your evaluation on the form. 28. Attach LARGE ADULT cuff to LARGE ADULT PVC pipe and place the pipe vertically on the table. Tightly connect the LARGE ADULT cuff tubing to the Microlife. The bottom edge of the cuff should rest 1 inch (black mark) above the end of the PVC pipe. The small white arrow (Artery Mark) on the cuff should be pointing down. You should be able to fit 2 fingers between the pipe and the cuff. 29. Connect the black L adapter on the LARGE ADULT cuff to the Microlife. 30. Turn on the Microlife to inflate the LARGE ADULT cuff to determine if the bladder within the LARGE ADULT cuff is leak proof. Answer the questions on the form about LARGE ADULT cuff damage, air leakage, and tube leakage. 31. Disconnect the LARGE ADULT cuff tubing from the Microlife. 32. Remove the black connector from the LARGE ADULT cuff tubing and put aside. 33. Holding in the black lead screw on the syringe, twist and pull the grey plunger past the 60 cc mark (to the end of the syringe). Connect the white silicone tubing to the syringe by rotating it to engage the Luer Lock. 34. Snugly connect the LARGE ADULT cuff black tubing to the Y adapter on the white silicone tubing. 35. Connect the white silicone tubing to the Microlife with the black L adapter. 36. Turn on the DigiMano. Check for the low battery light and change the battery if necessary. 37. Ensure that the red light below mmhg on the DigiMano is flashing, indicating that the correct measurement (millimeters of mercury) is selected. 19
21 38. Zero the pressure vacuum meter by pressing the zero button on the front of the DigiMano. The display should read Holding the syringe, press the ON/OFF START button on the Microlife to start the reading. 40. When the Microlife stops inflating the cuff, while watching the DigiMano, adjust the pressure in the syringe until the *DigiMano* displays a stable value near 280 mmhg (+/- 2 mmhg). If stability is a problem, examine the calibration system, listen for air leaks, and reseal the pressure release hole on the back of the Microlife with the Velcro tape. 41. Slowly release the pressure in 20 mmhg decrements according to the readings on the *DigiMano*. Coarse adjust the pressure by holding in the black lead screw and pulling or pushing the grey plunger, as needed. Fine adjust the pressure by rotating the grey plunger. Carefully record on the form the pressure readings from the DigiMano and Microlife at 20 mmhg decrements, i.e. from 280, to 260,, to 60, and finally to 40 mmhg. 42. Disconnect the syringe from the white silicone tubing by rotating it to disengage the Luer Lock. 43. Disconnect the LARGE ADULT cuff from the white tubing. Place the black L adapter back in the cuff tubing. 44. Remove the black plastic cap from the valve on the LARGE ADULT cuff and replace it with the stem and the short black tubing. 45. Put the ADULT cuff in the Ziplock bag. 46. Remove the Velcro tape from the back of the monitor. 47. Return the Microlife to the bag, attach the cuffs, and put them in the box with the tested monitors. NOTE: If pressure is lost during the calibration process, do the following: 1. Disconnect the syringe from the white silicone tubing by rotating it to disengage the Luer Lock. 2. Holding in the black lead screw on the syringe, twist and pull the grey plunger past the 60 cc mark (to the end of the syringe). Connect the white silicone tubing to the syringe by rotating it to engage the Luer Lock. 3. Ensure that the red light below mmhg on the DigiMano is flashing, indicating that the correct measurement (millimeters of mercury) is selected. 4. Zero the pressure vacuum meter by pressing the zero button on the front of the DigiMano. The display should read Holding the syringe, press the ON/OFF START button on the Microlife to start the reading. 6. Adjust the DigiMono to next pressure on the list. Continue taking the measurements. 20
3.1.3 Weight. Frequency. Weight is obtained at the baseline examination and annually. Equipment
 The excerpt from the SPRINT Manual of Procedures (MOP) below, dated November 1, 2010, includes instructions about blood pressure measurement. Recruitment for the SPRINT trial began on November 8, 2010;
The excerpt from the SPRINT Manual of Procedures (MOP) below, dated November 1, 2010, includes instructions about blood pressure measurement. Recruitment for the SPRINT trial began on November 8, 2010;
CONTENTS SPECIFICATIONS GENERAL INFORMATION RECOMMENDED USE OPERATING PRINCIPLE TIPS ON TAKING YOUR BLOOD PRESSURE 3-4 BATTERY INSTALLATION
 IFU SBPMON107 CONTENTS SPECIFICATIONS GENERAL INFORMATION RECOMMENDED USE OPERATING PRINCIPLE TIPS ON TAKING YOUR BLOOD PRESSURE BATTERY INSTALLATION CORRECT POSITION FOR MEASUREMENT POSITIONING THE CUFF
IFU SBPMON107 CONTENTS SPECIFICATIONS GENERAL INFORMATION RECOMMENDED USE OPERATING PRINCIPLE TIPS ON TAKING YOUR BLOOD PRESSURE BATTERY INSTALLATION CORRECT POSITION FOR MEASUREMENT POSITIONING THE CUFF
INSTRUCTION MANUAL. Automatic Blood Pressure Monitor with Fit Cuff. =Fit Cuff=!"#$% IA1B. Model
 IA1B INSTRUCTION MANUAL Automatic Blood Pressure Monitor with Fit Cuff =Fit Cuff=!"#$% Model IA1B Contents Introduction... 2 Notes on Safety... 3 Know Your Unit... 5 Quick Reference Guide... 7 Initial
IA1B INSTRUCTION MANUAL Automatic Blood Pressure Monitor with Fit Cuff =Fit Cuff=!"#$% Model IA1B Contents Introduction... 2 Notes on Safety... 3 Know Your Unit... 5 Quick Reference Guide... 7 Initial
Semi-Automatic Blood Pressure Monitor with Memory
 INSTRUCTION MANUAL Semi-Automatic Blood Pressure Monitor with Memory 61-268-001 (Adult size cuff) Please read this instruction manual completely before operating this unit. English Spanish Limited Five
INSTRUCTION MANUAL Semi-Automatic Blood Pressure Monitor with Memory 61-268-001 (Adult size cuff) Please read this instruction manual completely before operating this unit. English Spanish Limited Five
Program B-4 Evaluating Physical Development and Nutrition
 Program B-4 Evaluating Physical Development and Nutrition 31 32 I. The Importance of Body Measurement Body measurements are one way we measure a student's physical development. We can evaluate health,
Program B-4 Evaluating Physical Development and Nutrition 31 32 I. The Importance of Body Measurement Body measurements are one way we measure a student's physical development. We can evaluate health,
Instruction Manual. Auto Inflate Blood Pressure Monitor
 TM Auto Inflate Blood Pressure Monitor Instruction Manual Features: Fuzzy Logic Technology Auto memory for up to 90 readings One-Touch Operation Easy-to-read Display Date and Time indications AC Adapter
TM Auto Inflate Blood Pressure Monitor Instruction Manual Features: Fuzzy Logic Technology Auto memory for up to 90 readings One-Touch Operation Easy-to-read Display Date and Time indications AC Adapter
MANUAL BLOOD PRESSURE MONITOR BPM 168B Contents Blood Pressure Monitor Intended Use What is blood pressure?
 MANUAL BLOOD PRESSURE MONITOR BPM 168B Contents 01... Blood Pressure Monitor Intended Use 02... 1. What is blood pressure? 02... 2. Why is it useful measure blood pressure at home?... A. WHO blood pressure
MANUAL BLOOD PRESSURE MONITOR BPM 168B Contents 01... Blood Pressure Monitor Intended Use 02... 1. What is blood pressure? 02... 2. Why is it useful measure blood pressure at home?... A. WHO blood pressure
Model AUTOMATIC UPPER ARM Blood Pressure Monitor
 AUTOMATIC UPPER ARM Blood Pressure Monitor Model 1130 Real Fuzzy t e c h n o log y Features: Real Fuzzy Technology Automatic 60 sets of memory One-Touch Operation Easy-to-read Display Table of Contents:
AUTOMATIC UPPER ARM Blood Pressure Monitor Model 1130 Real Fuzzy t e c h n o log y Features: Real Fuzzy Technology Automatic 60 sets of memory One-Touch Operation Easy-to-read Display Table of Contents:
INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR
 INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR Model HEM-412C TABLE OF CONTENTS Introduction 3 Know Your Unit 4 Quick Reference Guide 5 Battery Installation/Replacement 6 How To Apply The Arm
INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR Model HEM-412C TABLE OF CONTENTS Introduction 3 Know Your Unit 4 Quick Reference Guide 5 Battery Installation/Replacement 6 How To Apply The Arm
Model UB-328. Wrist Digital Blood Pressure Monitor. Instruction Manual. Manuel d instructions. Manual de Instrucciones. Manuale di Istruzioni
 Wrist Digital Blood Pressure Monitor Model UB-328 Instruction Manual Manuel d instructions Manual de Instrucciones Manuale di Istruzioni WM+PD4000913 ENGLISH Dear Customers Congratulations. You have purchased
Wrist Digital Blood Pressure Monitor Model UB-328 Instruction Manual Manuel d instructions Manual de Instrucciones Manuale di Istruzioni WM+PD4000913 ENGLISH Dear Customers Congratulations. You have purchased
Blood Pressure Monitor
 EXTRA LARGE ARM Blood Pressure Monitor Trilingual Instruction Guide MODEL UA-789 Please read this important information before using your monitor. Please remember that only a medical practitioner is qualified
EXTRA LARGE ARM Blood Pressure Monitor Trilingual Instruction Guide MODEL UA-789 Please read this important information before using your monitor. Please remember that only a medical practitioner is qualified
Digital Blood Pressure Monitor. Model UA-787
 Digital Blood Pressure Monitor Model UA-787 Instruction Manual Manuel d instructions Manual de Instrucciones Manuale di Istruzioni UA-787EX-C WM:PD4000287 Preliminary Remarks This device conforms to the
Digital Blood Pressure Monitor Model UA-787 Instruction Manual Manuel d instructions Manual de Instrucciones Manuale di Istruzioni UA-787EX-C WM:PD4000287 Preliminary Remarks This device conforms to the
Sports Wrist Digital Blood Pressure Monitor Item #
 Instruction Manual Sports Wrist Digital Blood Pressure Monitor Item # 04-885-001 Please read this guidebook completely before operating this unit. Limited Five-Year Warranty The warrantor guarantees that
Instruction Manual Sports Wrist Digital Blood Pressure Monitor Item # 04-885-001 Please read this guidebook completely before operating this unit. Limited Five-Year Warranty The warrantor guarantees that
MEDICARE 60. Semi-automatic upper arm blood pressure monitor. Instruction manual
 MEDICARE 60 Semi-automatic upper arm blood pressure monitor Instruction manual closing date: april 2002 by ibp innovative business promotion gmbh, Germany table of contents 1 introduction... 2 2 preliminary
MEDICARE 60 Semi-automatic upper arm blood pressure monitor Instruction manual closing date: april 2002 by ibp innovative business promotion gmbh, Germany table of contents 1 introduction... 2 2 preliminary
TD-3140 Blood Pressure Monitor. Owner s Manual
 TD-3140 Blood Pressure Monitor Owner s Manual Dear TD-3140 System Owner: Thank you for choosing TD-3140 Blood Pressure Monitoring System. This manual provides important information to help you operate
TD-3140 Blood Pressure Monitor Owner s Manual Dear TD-3140 System Owner: Thank you for choosing TD-3140 Blood Pressure Monitoring System. This manual provides important information to help you operate
TD-3140 Blood Pressure Monitor. Owner s Manual
 TD-3140 Blood Pressure Monitor Owner s Manual Dear TD-3140 System Owner: Thank you for choosing TD-3140 Blood Pressure Monitoring System. This manual provides important information to help you operate
TD-3140 Blood Pressure Monitor Owner s Manual Dear TD-3140 System Owner: Thank you for choosing TD-3140 Blood Pressure Monitoring System. This manual provides important information to help you operate
Troubleshooting Guide: 640 Pediatric Exam Table with Midmark Scale
 Troubleshooting Guide: 640 Pediatric Exam Table with Midmark Scale Contents Description Refer To: Scale Troubleshooting Chart Troubleshooting Error Codes Error Messages Adjustments / Repair Procedures
Troubleshooting Guide: 640 Pediatric Exam Table with Midmark Scale Contents Description Refer To: Scale Troubleshooting Chart Troubleshooting Error Codes Error Messages Adjustments / Repair Procedures
The RICH LIFE Project: Blood Pressure Training Certification Checklists
 December 2016 to September 2020 The RICH LIFE Project: Blood Pressure Training Certification Checklists Reducing Inequities in Care of Hypertension: Lifestyle Improvement for Everyone PRINCIPAL INVESTIGATORS:
December 2016 to September 2020 The RICH LIFE Project: Blood Pressure Training Certification Checklists Reducing Inequities in Care of Hypertension: Lifestyle Improvement for Everyone PRINCIPAL INVESTIGATORS:
Introduction. Table of Contents. Automatic Wrist Blood Pressure Monitor With Voice-Guided Operation. Model No.: BP5K
 Automatic Wrist Blood Monitor With Voice-Guided Operation Ozeri Customer Service Customer service: 1-877-299-1296 or Email: support@ozeri.com Model No.: BP5K Thank you for choosing an Ozeri Blood Monitor.
Automatic Wrist Blood Monitor With Voice-Guided Operation Ozeri Customer Service Customer service: 1-877-299-1296 or Email: support@ozeri.com Model No.: BP5K Thank you for choosing an Ozeri Blood Monitor.
HOME BLOOD PRESSURE KIT INSTRUCTIONS TRILINGUAL INSTRUCTION GUIDE MODEL UA-100
 HOME BLOOD PRESSURE KIT INSTRUCTIONS TRILINGUAL INSTRUCTION GUIDE MODEL UA-100 IMPORTANT INFORMATION Please read this important information before using your monitor. Please remember that only a medical
HOME BLOOD PRESSURE KIT INSTRUCTIONS TRILINGUAL INSTRUCTION GUIDE MODEL UA-100 IMPORTANT INFORMATION Please read this important information before using your monitor. Please remember that only a medical
Blood Pressure Monitoring: Arterial Line
 Approved by: Blood Pressure Monitoring: Arterial Line Gail Cameron Senior Director, Operations, Maternal, Neonatal & Child Health Programs Dr. Ensenat Medical Director, Neonatology Neonatal Nursery Policy
Approved by: Blood Pressure Monitoring: Arterial Line Gail Cameron Senior Director, Operations, Maternal, Neonatal & Child Health Programs Dr. Ensenat Medical Director, Neonatology Neonatal Nursery Policy
DuPont Personal Protection Instruction Manual for Universal Pressure Test Kit No
 DuPont Personal Protection Instruction Manual for Universal Pressure Test Kit No. 990810 Effective: October 2006 BE SURE TO READ AND UNDERSTAND THESE INSTRUCTIONS BEFORE ATTEMPTING TO PERFORM AN INFLATION
DuPont Personal Protection Instruction Manual for Universal Pressure Test Kit No. 990810 Effective: October 2006 BE SURE TO READ AND UNDERSTAND THESE INSTRUCTIONS BEFORE ATTEMPTING TO PERFORM AN INFLATION
Digital Blood Pressure Monitor for the Upper Arm INSTRUCTION MANUAL
 Digital Blood Pressure Monitor for the Upper Arm INSTRUCTION MANUAL GT-6630 Contents Information You Should Know Before Operating the Unit... 25 About the Unit... 28 Function Descriptions... 28 Explanation
Digital Blood Pressure Monitor for the Upper Arm INSTRUCTION MANUAL GT-6630 Contents Information You Should Know Before Operating the Unit... 25 About the Unit... 28 Function Descriptions... 28 Explanation
The Univentor 1250 Anaesthesia Unit
 THE UNIVENTOR 1200/1250 ANAESTHESIA UNIT The Univentor 1250 Anaesthesia Unit TABLE OF CONTENTS EDITION 1 Section 1 - WARRANTY & SERVICE 1.1. WARRANTY 2 1.2. DAMAGED SHIPMENTS 2 1.3. SERVICE 2 Section 2
THE UNIVENTOR 1200/1250 ANAESTHESIA UNIT The Univentor 1250 Anaesthesia Unit TABLE OF CONTENTS EDITION 1 Section 1 - WARRANTY & SERVICE 1.1. WARRANTY 2 1.2. DAMAGED SHIPMENTS 2 1.3. SERVICE 2 Section 2
WELCOME TO THE REVOLUTION
 USER GUIDE WELCOME TO THE REVOLUTION THANK YOU FOR CHOOSING THE GCQUAD We listened to what you wanted - and created the most accurate, versatile and game-enhancing ball and club analysis solution available
USER GUIDE WELCOME TO THE REVOLUTION THANK YOU FOR CHOOSING THE GCQUAD We listened to what you wanted - and created the most accurate, versatile and game-enhancing ball and club analysis solution available
PHY100 s Lab: The Relationship Between Liquid Pressure and Depth as it applies to Blood Pressure. Course & Sec:
 PHY100 s Lab: The Relationship Between Liquid Pressure and Depth as it applies to Blood Pressure Name: Lab Partner: Course & Sec: Date: Disclaimer: The procedures in this lab are not according to proper
PHY100 s Lab: The Relationship Between Liquid Pressure and Depth as it applies to Blood Pressure Name: Lab Partner: Course & Sec: Date: Disclaimer: The procedures in this lab are not according to proper
Physical Measures ID: PHYSICAL MEASURES & BIOMARKERS
 Physical Measures ID: PHYSICAL MEASURES & BIOMARKERS 0 IWER: Text in bold and italics is to be read to the respondent. Normal text is interviewer instructions or questions that are to be answered by you.
Physical Measures ID: PHYSICAL MEASURES & BIOMARKERS 0 IWER: Text in bold and italics is to be read to the respondent. Normal text is interviewer instructions or questions that are to be answered by you.
Operating Instructions Part No
 DIGITAL AUTOMATIC TYRE INFLATOR Operating Instructions Part No. 11.0545 Thank you for selecting this Jamec Pem Automatic Tyre Inflator. Please read this manual before carrying out any installation or service
DIGITAL AUTOMATIC TYRE INFLATOR Operating Instructions Part No. 11.0545 Thank you for selecting this Jamec Pem Automatic Tyre Inflator. Please read this manual before carrying out any installation or service
Tourniquet Systems Pressure Infusion
 Tourniquet Systems Pressure Infusion VBM Medizintechnik GmbH VBM 1 VBM 2 Summary Tourniquets and Accessories Digital Tourniquets... Compressed Air Tourniquets... Electronic Tourniquets... Application Tourniquets...
Tourniquet Systems Pressure Infusion VBM Medizintechnik GmbH VBM 1 VBM 2 Summary Tourniquets and Accessories Digital Tourniquets... Compressed Air Tourniquets... Electronic Tourniquets... Application Tourniquets...
Installation Instructions
 Model: ACT-4100 ACT-4100-3 ACT-4100-6 SUN ELECTRIC CORPORATION Page: 1 of 5 Installation Instructions INSTALLATION MUST BE PERFORMED BY QUALIFIED SUN PERSONNEL ONLY INSTALLATION OVERVIEW: The Installation
Model: ACT-4100 ACT-4100-3 ACT-4100-6 SUN ELECTRIC CORPORATION Page: 1 of 5 Installation Instructions INSTALLATION MUST BE PERFORMED BY QUALIFIED SUN PERSONNEL ONLY INSTALLATION OVERVIEW: The Installation
Research nurses trained in the method are responsible for recording all anthropometric measurements from all subjects.
 Anthropometric measures No: 007D 1. Introduction Anthropometric measurements are widely used to predict body density or fat content. A variety of different procedures can be done. For this project waist/hip
Anthropometric measures No: 007D 1. Introduction Anthropometric measurements are widely used to predict body density or fat content. A variety of different procedures can be done. For this project waist/hip
Auto Arm Blood Pressure Monitor
 Now you know ys Auto Arm Blood Pressure Monitor Featuring: One-touch operation 85 memories Instruction Manual Table of Contents Important information................... 3 Fuzzy Logic technology.................
Now you know ys Auto Arm Blood Pressure Monitor Featuring: One-touch operation 85 memories Instruction Manual Table of Contents Important information................... 3 Fuzzy Logic technology.................
Installation, Operation and Maintenance Manual for Back Pressure Regulator
 Installation, Operation and Maintenance Manual for Back Pressure Regulator Model 8860 2009 Groth Corporation IOM-8860 Rev. B 12541 Ref. ID: 95565 Page 2 of 13 Table of Contents I. INTRODUCTION 3 II. DESIGN
Installation, Operation and Maintenance Manual for Back Pressure Regulator Model 8860 2009 Groth Corporation IOM-8860 Rev. B 12541 Ref. ID: 95565 Page 2 of 13 Table of Contents I. INTRODUCTION 3 II. DESIGN
American Weigh Scales, Inc. BLADE Series D i g i t a l P o c k e t S c a l e BLADE-550. User Manual BLADE-400 BLADE-1000
 American Weigh Scales, Inc. BLADE Series D i g i t a l P o c k e t S c a l e BLADE-50 BLADE-400 BLADE-550 BLADE-1000 User Manual Blade Series Manual Thank you for purchasing the American Weigh BLADE-Series
American Weigh Scales, Inc. BLADE Series D i g i t a l P o c k e t S c a l e BLADE-50 BLADE-400 BLADE-550 BLADE-1000 User Manual Blade Series Manual Thank you for purchasing the American Weigh BLADE-Series
Digital Blood Pressure Monitor. Model UA-705. Instruction Manual. Manuel d instructions. Manual de Instrucciones. Manuale di Istruzioni 1WMPD B
 Digital Blood Pressure Monitor Model UA-705 Instruction Manual Manuel d instructions Manual de Instrucciones Manuale di Istruzioni 1WMPD4000988B ENGLISH Contents Dear Customers... 2 Preliminary Remarks...
Digital Blood Pressure Monitor Model UA-705 Instruction Manual Manuel d instructions Manual de Instrucciones Manuale di Istruzioni 1WMPD4000988B ENGLISH Contents Dear Customers... 2 Preliminary Remarks...
REF CH-302B IDENTIFICATION OF PARTS
 English TM INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR REF CH-302B IDENTIFICATION OF PARTS Exhaust button Air-release system Bulb Systolic blood pressure display section Diastolic blood pressure/pulse
English TM INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR REF CH-302B IDENTIFICATION OF PARTS Exhaust button Air-release system Bulb Systolic blood pressure display section Diastolic blood pressure/pulse
Digital Display large color coded LED display is easy to read precise measuring shows actual cuff pressure
 Pressure Regulation microprocessor controlled fast cuff inflation and defl ation regulator knob allows very precise regulation limits pressure to max. 600 mmhg Digital Display large color coded LED display
Pressure Regulation microprocessor controlled fast cuff inflation and defl ation regulator knob allows very precise regulation limits pressure to max. 600 mmhg Digital Display large color coded LED display
Oregon Scientific Wrist Blood Pressure Monitor (BPW211)
 Oregon Scientific Wrist Blood Pressure Monitor (BPW211) User Manual TABLE OF CONTENTS Introduction... 2 Key features... 2 Main unit... 2 Plastic storage container... 2 LCD display symbols... 3 Safety and
Oregon Scientific Wrist Blood Pressure Monitor (BPW211) User Manual TABLE OF CONTENTS Introduction... 2 Key features... 2 Main unit... 2 Plastic storage container... 2 LCD display symbols... 3 Safety and
Installation Instructions
 SUN ELECTRIC CORPORATION Model: MRC-450 MRC-450-3 MRC-450-6 MRC-450-GR Page: 1 of 5 Installation Instructions INSTALLATION MUST BE PERFORMED BY QUALIFIED SUN PERSONNEL ONLY INSTALLATION OVERVIEW: The Installation
SUN ELECTRIC CORPORATION Model: MRC-450 MRC-450-3 MRC-450-6 MRC-450-GR Page: 1 of 5 Installation Instructions INSTALLATION MUST BE PERFORMED BY QUALIFIED SUN PERSONNEL ONLY INSTALLATION OVERVIEW: The Installation
Pegas 4000 MF Gas Mixer InstructionManual Columbus Instruments
 Pegas 4000 MF Gas Mixer InstructionManual Contents I Table of Contents Foreword Part I Introduction 1 2 1 System overview... 2 2 Specifications... 3 Part II Installation 4 1 Rear panel connections...
Pegas 4000 MF Gas Mixer InstructionManual Contents I Table of Contents Foreword Part I Introduction 1 2 1 System overview... 2 2 Specifications... 3 Part II Installation 4 1 Rear panel connections...
Validation of a Step Test in Children Ages 7-11
 Validation of a Step Test in Children Ages 7-11 Emilene Clark Aurora Fry Colleen Smith Tonya Thomas Primary Advisor: Jim Farris, PT, PhD April 20, 2011 Background Exercise Testing Maximal Testing Sub-maximal
Validation of a Step Test in Children Ages 7-11 Emilene Clark Aurora Fry Colleen Smith Tonya Thomas Primary Advisor: Jim Farris, PT, PhD April 20, 2011 Background Exercise Testing Maximal Testing Sub-maximal
Review Supplement Info Waist-to hip Ratio, Gurth Measurements & Skin Fold
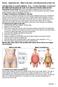 THIS MATERIAL IS A SUPPLEMENTAL TOOL. IT IS NOT INTENDED TO REPLACE INFORMATION PROVIDED IN YOUR TEXT AND/OR STUDENT HAND-BOOKS. Assessing Percent Body Fat: While hydrostatic weighing and the Bod Pod are
THIS MATERIAL IS A SUPPLEMENTAL TOOL. IT IS NOT INTENDED TO REPLACE INFORMATION PROVIDED IN YOUR TEXT AND/OR STUDENT HAND-BOOKS. Assessing Percent Body Fat: While hydrostatic weighing and the Bod Pod are
Jackson Heart Study Protocol. Manual 3. Blood Pressure. Visit 2. Version 2.0. September 9, For Copies, Please Contact:
 Jackson Heart Study Protocol Manual 3 Blood Pressure Visit 2 Version 2.0 September 9, 2005 For Copies, Please Contact: Jackson Heart Study Coordinating Center Jackson Medical Mall 350 W. Woodrow Wilson
Jackson Heart Study Protocol Manual 3 Blood Pressure Visit 2 Version 2.0 September 9, 2005 For Copies, Please Contact: Jackson Heart Study Coordinating Center Jackson Medical Mall 350 W. Woodrow Wilson
English Español Português Italiano Deutch Français INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR MODEL CH-656C. - Eng 1 -
 English Español Português Italiano Deutch Français INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR MODEL CH-656C M - Eng 1 - CLEANING METHOD OF CUFF After cleaning the cuff with a neutral detergent,
English Español Português Italiano Deutch Français INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR MODEL CH-656C M - Eng 1 - CLEANING METHOD OF CUFF After cleaning the cuff with a neutral detergent,
Operating Instructions Part No
 DIGITAL AUTOMATIC TYRE INFLATOR Operating Instructions Part No. 11.0578 Thank you for selecting this Jamec Pem Automatic Tyre Inflator. Please read this manual before carrying out any installation or service
DIGITAL AUTOMATIC TYRE INFLATOR Operating Instructions Part No. 11.0578 Thank you for selecting this Jamec Pem Automatic Tyre Inflator. Please read this manual before carrying out any installation or service
Pressure Dump Valve Service Kit for Series 2300 Units
 Instruction Sheet Pressure Dump Valve Service Kit for Series 00 Units. Overview The Nordson pressure dump valve is used to relieve hydraulic pressure instantly in Series 00 applicator tanks when the unit
Instruction Sheet Pressure Dump Valve Service Kit for Series 00 Units. Overview The Nordson pressure dump valve is used to relieve hydraulic pressure instantly in Series 00 applicator tanks when the unit
Installation Instructions
 Model: ACT-4500 ACT-4500-3 ACT-4500-6 SUN ELECTRIC CORPORATION Page: 1 of 5 Installation Instructions INSTALLATION MUST BE PERFORMED BY QUALIFIED SUN PERSONNEL ONLY INSTALLATION OVERVIEW: The Installation
Model: ACT-4500 ACT-4500-3 ACT-4500-6 SUN ELECTRIC CORPORATION Page: 1 of 5 Installation Instructions INSTALLATION MUST BE PERFORMED BY QUALIFIED SUN PERSONNEL ONLY INSTALLATION OVERVIEW: The Installation
OXY Integral. INTERCON ENTERPRISES INC Tel: Fax: Internet:
 OXY Integral INTERCON ENTERPRISES INC Tel: 800 665 6655 Fax: 604 946 5340 E-Mail: sales@intercononline.com Internet: www.intercononline.com Manual Integral 2006 1 INDEX 2-3 PREFACE 4 INTRODUCTION 5 Principle
OXY Integral INTERCON ENTERPRISES INC Tel: 800 665 6655 Fax: 604 946 5340 E-Mail: sales@intercononline.com Internet: www.intercononline.com Manual Integral 2006 1 INDEX 2-3 PREFACE 4 INTRODUCTION 5 Principle
Aneroid Sphygmomanometer. Use, Care, & Maintenance
 Aneroid Sphygmomanometer Use, Care, & Maintenance 1 Device Description and Intended Use An aneroid sphygmomanometer is used by professional healthcare providers and individuals trained in the auscultatory
Aneroid Sphygmomanometer Use, Care, & Maintenance 1 Device Description and Intended Use An aneroid sphygmomanometer is used by professional healthcare providers and individuals trained in the auscultatory
Contents. Safety Notice. Wrist Type
 Wrist Type Contents 1 Safety Notice 2 Safety Notice... 02 Unit Illustration... 05 Important Testing Guidelines... 07 Quick Start... 08 Unit Operation... 10 Battery Installation... 10 Thank you for purchasing
Wrist Type Contents 1 Safety Notice 2 Safety Notice... 02 Unit Illustration... 05 Important Testing Guidelines... 07 Quick Start... 08 Unit Operation... 10 Battery Installation... 10 Thank you for purchasing
Blood Pressure Monitor
 QUICK RESPONSE Blood Pressure Monitor Trilingual Instruction Guide MODEL UA-787 English Français Español THIS MONITOR IS CLINICALLY VALIDATED This LifeSource blood pressure monitor has undergone and passed
QUICK RESPONSE Blood Pressure Monitor Trilingual Instruction Guide MODEL UA-787 English Français Español THIS MONITOR IS CLINICALLY VALIDATED This LifeSource blood pressure monitor has undergone and passed
Circulation and Respiration: Vital Signs Student Version
 Circulation and Respiration: Vital Signs Student Version In this lab, you will learn about the circulatory and respiratory systems. You will test the capacity of your lungs, measure your blood pressure
Circulation and Respiration: Vital Signs Student Version In this lab, you will learn about the circulatory and respiratory systems. You will test the capacity of your lungs, measure your blood pressure
Chair exercises Sally Ann Belward, Falls Clinical Lead Physiotherapist
 Chair exercises Sally Ann Belward, Falls Clinical Lead Physiotherapist Exercise safety Exercise should be comfortable and fun Ensure participants are sat on a sturdy chair, have comfortable clothing and
Chair exercises Sally Ann Belward, Falls Clinical Lead Physiotherapist Exercise safety Exercise should be comfortable and fun Ensure participants are sat on a sturdy chair, have comfortable clothing and
MODEL CH-432B INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR. - Eng 1 - English Español Português Deutch. Italiano Français CH-432B
 English Español Português Deutch INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR MODEL CH-432B CH-432B POWER START Italiano Français - Eng 1 - CLEANING METHOD OF CUFF After cleaning the cuff with
English Español Português Deutch INSTRUCTION MANUAL FOR DIGITAL BLOOD PRESSURE MONITOR MODEL CH-432B CH-432B POWER START Italiano Français - Eng 1 - CLEANING METHOD OF CUFF After cleaning the cuff with
OPERATOR S MANUAL Ar-Gone Weld Gas Analyzer
 July 2011 OPERATOR S MANUAL Ar-Gone Weld Gas Analyzer WARNING! Before operating this product, read and understand this Operator s Manual. Become familiar with the potential hazards of this unit. Contact
July 2011 OPERATOR S MANUAL Ar-Gone Weld Gas Analyzer WARNING! Before operating this product, read and understand this Operator s Manual. Become familiar with the potential hazards of this unit. Contact
Standard. Abdominal Aortic Aneurysm (AAA) Repair Trainer. Part No: 60610
 User Guide Abdominal Aortic Aneurysm (AAA) Repair Trainer Part No: 6060 Standard Part No: 065-088 Issue, June 005 04 Limbs & Things For more Vascular training products visit limbsandthings.com Limbs &
User Guide Abdominal Aortic Aneurysm (AAA) Repair Trainer Part No: 6060 Standard Part No: 065-088 Issue, June 005 04 Limbs & Things For more Vascular training products visit limbsandthings.com Limbs &
Superconducting Susceptometer (MPMS-5S) Quantum Design Room 296 (MPMS)
 Superconducting Susceptometer (MPMS-5S) Quantum Design Room 296 (MPMS) Sensitivity: 1x10 11 A m 2 Applied DC fields: 0 T to 5 T Applied AC fields: 0 G to 3 G (zero-to-peak), 0.01 Hz to 1000 Hz Temperatures
Superconducting Susceptometer (MPMS-5S) Quantum Design Room 296 (MPMS) Sensitivity: 1x10 11 A m 2 Applied DC fields: 0 T to 5 T Applied AC fields: 0 G to 3 G (zero-to-peak), 0.01 Hz to 1000 Hz Temperatures
DIAGNOSTIX TM 922 MERCURIAL BLOOD PRESSURE INSTRUMENT. Use, Care, & Maintenance
 DIAGNOSTIX TM 922 MERCURIAL BLOOD PRESSURE INSTRUMENT Use, Care, & Maintenance 1 A Special Thank You... Thank you for choosing an ADC blood pressure instrument. We re proud of the care and quality that
DIAGNOSTIX TM 922 MERCURIAL BLOOD PRESSURE INSTRUMENT Use, Care, & Maintenance 1 A Special Thank You... Thank you for choosing an ADC blood pressure instrument. We re proud of the care and quality that
Blood Pressure Monitor
 Blood Pressure Monitor egotest Users Instructions Contents of the kit 1 blood pressure instrument with cuff 1 set of instructions for use 1 case Symbols on the blood pressure monitor Symbol 0124 Function/meaning
Blood Pressure Monitor egotest Users Instructions Contents of the kit 1 blood pressure instrument with cuff 1 set of instructions for use 1 case Symbols on the blood pressure monitor Symbol 0124 Function/meaning
Ab Plank with Straight Leg Raise
 Ab Plank with Straight Leg Raise Position yourself face up with your knees bent at 90 degrees, feet flat on the floor. Your hands should be directly under your shoulders facing forward. While in this position
Ab Plank with Straight Leg Raise Position yourself face up with your knees bent at 90 degrees, feet flat on the floor. Your hands should be directly under your shoulders facing forward. While in this position
Abdominal Aortic Aneurysm (AAA) Repair Trainer User Guide
 Abdominal Aortic Aneurysm (AAA) Repair Trainer User Guide Designed and manufactured by Limbs & Things Limited, Sussex Street, St. Philips, Bristol, BS 0RA, UK. Telephone: +44 (0)7 3 0500 Fax: +44 (0)7
Abdominal Aortic Aneurysm (AAA) Repair Trainer User Guide Designed and manufactured by Limbs & Things Limited, Sussex Street, St. Philips, Bristol, BS 0RA, UK. Telephone: +44 (0)7 3 0500 Fax: +44 (0)7
Nita Newborn Model 1800 User Manual
 Nita Newborn Model 1800 User Manual 308 South Sequoia Parkway, Canby, Oregon 97013 USA ph. 503.651.5050 fax 503.651.5052 email info@vatainc.com www.vatainc.com Meet Nita Newborn TM Nita Newborn is a unique
Nita Newborn Model 1800 User Manual 308 South Sequoia Parkway, Canby, Oregon 97013 USA ph. 503.651.5050 fax 503.651.5052 email info@vatainc.com www.vatainc.com Meet Nita Newborn TM Nita Newborn is a unique
THE UNIVENTOR 400 ANAESTHESIA UNIT
 THE UNIVENTOR 400 ANAESTHESIA UNIT TABLE OF CONTENTS EDITION 3 Section 1 - WARRANTY & SERVICE 1.1. WARRANTY 2 1.2. DAMAGED SHIPMENTS 2 1.3. SERVICE 2 Section 2 - INTRODUCTION 2.1. INTRODUCTION 3 Section
THE UNIVENTOR 400 ANAESTHESIA UNIT TABLE OF CONTENTS EDITION 3 Section 1 - WARRANTY & SERVICE 1.1. WARRANTY 2 1.2. DAMAGED SHIPMENTS 2 1.3. SERVICE 2 Section 2 - INTRODUCTION 2.1. INTRODUCTION 3 Section
Module No GETTING ACQUAINTED GENERAL GUIDE TIMEKEEPING
 Module No. 2196 2196-1 GETTING ACQUAINTED Congratulations upon your selection of this CASIO Pressure Monitor Watch (BP-1B, Module No. 2196). To get the most out of your purchase, be sure to carefully read
Module No. 2196 2196-1 GETTING ACQUAINTED Congratulations upon your selection of this CASIO Pressure Monitor Watch (BP-1B, Module No. 2196). To get the most out of your purchase, be sure to carefully read
INSTRUCTOR RESOURCES
 Gases: Dalton s Law INSTRUCTOR RESOURCES By Dale A. Hammond, PhD LEARNING OBJECTIVES introduce the concept of ideal gases. experimentally determine the relationship between pressure and amount of gas,
Gases: Dalton s Law INSTRUCTOR RESOURCES By Dale A. Hammond, PhD LEARNING OBJECTIVES introduce the concept of ideal gases. experimentally determine the relationship between pressure and amount of gas,
INSTRUCTION MANUAL. January 23, 2003, Revision 0
 INSTRUCTION MANUAL Model 810A In-Vitro Test Apparatus for 310B Muscle Lever January 23, 2003, Revision 0 Copyright 2003 Aurora Scientific Inc. Aurora Scientific Inc. 360 Industrial Parkway S., Unit 4 Aurora,
INSTRUCTION MANUAL Model 810A In-Vitro Test Apparatus for 310B Muscle Lever January 23, 2003, Revision 0 Copyright 2003 Aurora Scientific Inc. Aurora Scientific Inc. 360 Industrial Parkway S., Unit 4 Aurora,
TECHNICAL CHARACTERISTIC CT 071
 MADE IN FRANCE Regulatory information CE Marking Index of classification Notified body CE 0459 Im G-MED PHTALATES LATEX Sphygmomanometer hand-held type with aneroid membrane, very light. This precision
MADE IN FRANCE Regulatory information CE Marking Index of classification Notified body CE 0459 Im G-MED PHTALATES LATEX Sphygmomanometer hand-held type with aneroid membrane, very light. This precision
How Do I Install My Garden Master Bucket Garden?
 How Do I Install My Garden Master Bucket Garden? 1. On the arrival of your Bucket Garden open the bucket and remove the 4 screws from the outside of the bucket rim and pull the 2 buckets apart. Check the
How Do I Install My Garden Master Bucket Garden? 1. On the arrival of your Bucket Garden open the bucket and remove the 4 screws from the outside of the bucket rim and pull the 2 buckets apart. Check the
INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR
 TM TM INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR Model HEM-412CREL TABLE OF CONTENTS Introduction 3 Important Safety Notes 4 Know Your Unit 5 Quick Reference Guide 6 A Few Words About Blood
TM TM INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR Model HEM-412CREL TABLE OF CONTENTS Introduction 3 Important Safety Notes 4 Know Your Unit 5 Quick Reference Guide 6 A Few Words About Blood
A Liter a Lung Measuring Lung Capacity
 A Liter a Lung Measuring Lung Capacity OBJECTIVE In this investigation, students will compare the actual and expected vital capacities of their classmates. LEVEL Middle Grades Life Science CONNECTIONS
A Liter a Lung Measuring Lung Capacity OBJECTIVE In this investigation, students will compare the actual and expected vital capacities of their classmates. LEVEL Middle Grades Life Science CONNECTIONS
Columbus Instruments
 0215-003M Portable O 2 /CO 2 /CH 4 Meter User s Manual Columbus Instruments 950 NORTH HAGUE AVENUE TEL:(614) 276-0861 COLUMBUS, OHIO 43204, USA FAX:(614) 276-0529 1 www.colinst.com TOLL FREE 1-800-669-5011
0215-003M Portable O 2 /CO 2 /CH 4 Meter User s Manual Columbus Instruments 950 NORTH HAGUE AVENUE TEL:(614) 276-0861 COLUMBUS, OHIO 43204, USA FAX:(614) 276-0529 1 www.colinst.com TOLL FREE 1-800-669-5011
LESSON ASSIGNMENT. Perform Cardiopulmonary Resuscitation on a Child or Infant. After completing this lesson, you should be able to:
 LESSON ASSIGNMENT LESSON 6 Perform Cardiopulmonary Resuscitation on a Child or Infant. TEXT ASSIGNMENT Paragraphs 6-1 through 6-7. LESSON OBJECTIVES After completing this lesson, you should be able to:
LESSON ASSIGNMENT LESSON 6 Perform Cardiopulmonary Resuscitation on a Child or Infant. TEXT ASSIGNMENT Paragraphs 6-1 through 6-7. LESSON OBJECTIVES After completing this lesson, you should be able to:
5 series Blood Pressure Monitor
 INSTRUCTION MANUAL 5 series Blood Pressure Monitor Model BP742 ENGLISH ESPAÑOL table TABLE of OF contents CONTENTS Before using the Monitor Introduction... 3 Safety Information...4 Operating the Device...4
INSTRUCTION MANUAL 5 series Blood Pressure Monitor Model BP742 ENGLISH ESPAÑOL table TABLE of OF contents CONTENTS Before using the Monitor Introduction... 3 Safety Information...4 Operating the Device...4
Operating Instructions Instrucciones de funcionamiento
 Wrist Blood Pressure Monitor Monitor de Presión Arterial de Muñeca Operating Instructions Instrucciones de funcionamiento Model No. EW3003 Modelo No. EW3003 For questions or assistance with your blood
Wrist Blood Pressure Monitor Monitor de Presión Arterial de Muñeca Operating Instructions Instrucciones de funcionamiento Model No. EW3003 Modelo No. EW3003 For questions or assistance with your blood
Non-Invasive Blood Pressure (NIBP)
 Non-Invasive Blood Pressure (NIBP) October 2007 9650-1214-01 Rev. G The issue date or revision level for this operation guide is shown on the front cover. ZOLL and E Series are registered trademarks of
Non-Invasive Blood Pressure (NIBP) October 2007 9650-1214-01 Rev. G The issue date or revision level for this operation guide is shown on the front cover. ZOLL and E Series are registered trademarks of
GEN-1e CO2 Generator OVERVIEW INSTALLATION PROPANE GENERATORS ONLY NATURAL GAS GENERATORS ONLY
 GEN-1e CO2 Generator Custom Automated Products offers economical and safe carbon dioxide generators. They produce CO2 by burning either propane or natural gas. We have designed our CO2 generator to allow
GEN-1e CO2 Generator Custom Automated Products offers economical and safe carbon dioxide generators. They produce CO2 by burning either propane or natural gas. We have designed our CO2 generator to allow
POWERPRESS UNIT. User Manual. Gradient Pneumatic Sequential Compressor. Neomedic
 TIMER (MIN) POWERPRESS UNIT 40 70 40 80 90 10 60 100 MIN MAX PRESSURE (mmhg) User Manual Neomedic 9601 Owens mouth Ave. #8 Chatsworth, CA 91311 Toll Free: 866-990-1168 Tel: 818-998-1023 Fax: 818-998-0277
TIMER (MIN) POWERPRESS UNIT 40 70 40 80 90 10 60 100 MIN MAX PRESSURE (mmhg) User Manual Neomedic 9601 Owens mouth Ave. #8 Chatsworth, CA 91311 Toll Free: 866-990-1168 Tel: 818-998-1023 Fax: 818-998-0277
User Manual. Hopkins IMPACT Digital Wrist BP RELATED ITEMS. Item #526014
 RELATED ITEMS User Manual Hopkins IMPACT Digital Wrist BP Hopkins Flex Temp Thermometer #579420 Hopkins Wave t m Pulse Oximeter #594029 Item #526014 Hopkins IMPACT Digital Wrist BP 6850 Southbelt Dr Caledonia,
RELATED ITEMS User Manual Hopkins IMPACT Digital Wrist BP Hopkins Flex Temp Thermometer #579420 Hopkins Wave t m Pulse Oximeter #594029 Item #526014 Hopkins IMPACT Digital Wrist BP 6850 Southbelt Dr Caledonia,
Fully Fuzzy Auto Digital Wrist Blood Pressure Monitor. tensio control. INSTRUCTION MANUAL Model GP-6220
 Fully Fuzzy Auto Digital Wrist Blood Pressure Monitor tensio control INSTRUCTION MANUAL Model GP-6220 Manufactured by: Geratherm Medical AG Fahrenheitstraße 1 98716 Geschwenda Germany Phone: +49 (0) 36205
Fully Fuzzy Auto Digital Wrist Blood Pressure Monitor tensio control INSTRUCTION MANUAL Model GP-6220 Manufactured by: Geratherm Medical AG Fahrenheitstraße 1 98716 Geschwenda Germany Phone: +49 (0) 36205
6833_INSTRUCTIONS MANUAL DIGITAL WIRST BLOOD PRESSURE
 6833_INSTRUCTIONS MANUAL DIGITAL WIRST BLOOD PRESSURE PURCHASE ACKNOWLEDGEMENT JOCCA thanks you for the trust placed in the purchase of our product and we are certain that you will always be satisfied
6833_INSTRUCTIONS MANUAL DIGITAL WIRST BLOOD PRESSURE PURCHASE ACKNOWLEDGEMENT JOCCA thanks you for the trust placed in the purchase of our product and we are certain that you will always be satisfied
Standard. Bag & Stand Advanced Venepuncture Arm. Light. Part No: Brown. Part No: Black. Part No: 00442
 Setup Guide Bag & Stand Advanced Standard Light. Part No: 00440 Brown. Part No: 0044 Black. Part No: 0044 Part No: 065-0 Issue, May 0 0 Limbs & Things For more skills training products visit limbsandthings.com
Setup Guide Bag & Stand Advanced Standard Light. Part No: 00440 Brown. Part No: 0044 Black. Part No: 0044 Part No: 065-0 Issue, May 0 0 Limbs & Things For more skills training products visit limbsandthings.com
Use this key to determine which sections of this Product Manual apply to your job.
 R710 Product Manual Contents Warnings and Important Information 3 Recommended Use 4 Product Information 5 Technical Data 5 Before & During Every Transfer 6 Inspection 6 SoloLift Scale (optional) 6 SoloVest
R710 Product Manual Contents Warnings and Important Information 3 Recommended Use 4 Product Information 5 Technical Data 5 Before & During Every Transfer 6 Inspection 6 SoloLift Scale (optional) 6 SoloVest
Techcon Systems TS1258 Pressure Pot
 Techcon Systems TS1258 Pressure Pot User Guide TABLE OF CONTENT 1. SAFETY. 3 1.1 Intended Use 3 1.2 Safety Precaution 3 2. CERTIFICATE OF COMFORMANCE 4 3. FEATURES. 5 4. SPECIFICATIONS 5 5. INSTALLATION
Techcon Systems TS1258 Pressure Pot User Guide TABLE OF CONTENT 1. SAFETY. 3 1.1 Intended Use 3 1.2 Safety Precaution 3 2. CERTIFICATE OF COMFORMANCE 4 3. FEATURES. 5 4. SPECIFICATIONS 5 5. INSTALLATION
Anti-flood device Model B-1
 December 4, 2009 Dry Systems 123a 1. DESCRIPTION The Anti-flood Device is required when Viking accelerators are installed on dry systems according to Viking Model E-1 Accelerator Trim Charts. In the SET
December 4, 2009 Dry Systems 123a 1. DESCRIPTION The Anti-flood Device is required when Viking accelerators are installed on dry systems according to Viking Model E-1 Accelerator Trim Charts. In the SET
Digital Ear Thermometer
 Adtemp TM Digital Ear Thermometer 421 Digital Ear Thermometer Instruction Manual PLEASE NOTE: THIS MEDICAL INSTRUMENT MUST BE USED ACCORDING TO INSTRUCTIONS TO ENSURE ACCURATE READINGS. Questions? Call
Adtemp TM Digital Ear Thermometer 421 Digital Ear Thermometer Instruction Manual PLEASE NOTE: THIS MEDICAL INSTRUMENT MUST BE USED ACCORDING TO INSTRUCTIONS TO ENSURE ACCURATE READINGS. Questions? Call
Talking Wrist TypeBlood Pressure Monitor. Talking Wrist Type Blood Pressure Monitor Model: BPW810. User Manual
 Talking Wrist TypeBlood Pressure Monitor Talking Wrist Type Blood Pressure Monitor Model: BPW810 User Manual TALKING WRIST TYPE BLOOD PRESSURE MONITOR CONTENTS Model: BPW810 USER MANUAL Deleting the Latest
Talking Wrist TypeBlood Pressure Monitor Talking Wrist Type Blood Pressure Monitor Model: BPW810 User Manual TALKING WRIST TYPE BLOOD PRESSURE MONITOR CONTENTS Model: BPW810 USER MANUAL Deleting the Latest
boso Germany Do you? Premium quality essgeräte für die Selbstmessung I use equipment of BOSCH + SOHN to measure blood pressure.
 I use equipment of BOSCH + SOHN to measure blood pressure. Do you? If you self-test, trust the brand that 77% of all German doctors use. essgeräte für die Selbstmessung Premium quality Premium quality
I use equipment of BOSCH + SOHN to measure blood pressure. Do you? If you self-test, trust the brand that 77% of all German doctors use. essgeräte für die Selbstmessung Premium quality Premium quality
QUICK START GUIDE TO DRINKING WATER MONITORING
 APPENDIX F QUICK START GUIDE TO DRINKING WATER MONITORING Introduction: This Quick Start guide is designed to allow users who are already familiar with the basic use of YSI 6-series sondes for surface
APPENDIX F QUICK START GUIDE TO DRINKING WATER MONITORING Introduction: This Quick Start guide is designed to allow users who are already familiar with the basic use of YSI 6-series sondes for surface
NAVIGATOR PROP BUILDING INSTRUCTIONS & PHOTOS
 NAVIGATOR PROP BUILDING INSTRUCTIONS & PHOTOS Science under the ice Ice sheet At regional competitions the ice is simulated by 8 ft x 4 ft ½-inch foam sheeting (Home Depot part #703990 [in store only],
NAVIGATOR PROP BUILDING INSTRUCTIONS & PHOTOS Science under the ice Ice sheet At regional competitions the ice is simulated by 8 ft x 4 ft ½-inch foam sheeting (Home Depot part #703990 [in store only],
Technical Service Bulletin June 2015 TSB122.06
 Technical Service Bulletin June 2015 TSB122.06 Element Loading Guidelines This bulletin provides general information and guidelines for installing Hydranautics elements in order to provide optimally reliable
Technical Service Bulletin June 2015 TSB122.06 Element Loading Guidelines This bulletin provides general information and guidelines for installing Hydranautics elements in order to provide optimally reliable
2 Field Practices & Measurements
 2 Field Practices & Measurements Objectives: 1. Become familiar with surveying equipment 2. Care of equipment 3. Student responsibilities 4. Field notes 5. Significant figures 6. Tolerances 1. Become familiar
2 Field Practices & Measurements Objectives: 1. Become familiar with surveying equipment 2. Care of equipment 3. Student responsibilities 4. Field notes 5. Significant figures 6. Tolerances 1. Become familiar
VISCOMETER, OFI, 8-SPEED, MODEL 800 PART No
 OFI Testing Equipment Viscometer, 8 Speed, Model 800 Instructions Part # 130-10 Page 1 of 4 VISCOMETER, OFI, 8-SPEED, MODEL 800 PART No. 130-10 The model 800 eight speed electronic viscometer is designed
OFI Testing Equipment Viscometer, 8 Speed, Model 800 Instructions Part # 130-10 Page 1 of 4 VISCOMETER, OFI, 8-SPEED, MODEL 800 PART No. 130-10 The model 800 eight speed electronic viscometer is designed
USE, CARE & MAINTENANCE DIAGNOSTIX TM 972 MERCURIAL BLOOD PRESSURE INSTRUMENT
 USE, CARE & MAINTENANCE DIAGNOSTIX TM 972 MERCURIAL BLOOD PRESSURE INSTRUMENT 1 A Special Thank You... Thank you for choosing an ADC blood pressure instrument. We re proud of the care and quality that
USE, CARE & MAINTENANCE DIAGNOSTIX TM 972 MERCURIAL BLOOD PRESSURE INSTRUMENT 1 A Special Thank You... Thank you for choosing an ADC blood pressure instrument. We re proud of the care and quality that
Talking Wrist Style Blood Pressure Monitor Model INS-LAB-RevB08
 Talking Wrist Style Blood Pressure Monitor Model 1145 1145-INS-LAB-RevB08 Contents Introduction...2 Key Features... 2 Front View... 2 Rear View... 3 LCD Symbols... 3 Safety and Care Instructions... 4 Safety
Talking Wrist Style Blood Pressure Monitor Model 1145 1145-INS-LAB-RevB08 Contents Introduction...2 Key Features... 2 Front View... 2 Rear View... 3 LCD Symbols... 3 Safety and Care Instructions... 4 Safety
Digital Tourniquets. Product Details. For electricity VAC, 50/60 Hz
 Digital Tourniquets For electricity 100-240 VAC, 50/60 Hz Product Details Digital Display Large color coded LED display is easy to read Precise monitoring shows actual cuff pressure Precision Pressure
Digital Tourniquets For electricity 100-240 VAC, 50/60 Hz Product Details Digital Display Large color coded LED display is easy to read Precise monitoring shows actual cuff pressure Precision Pressure
Warranty The device shall have a 6-year warranty at minimum
 Bid Specifications Defibrillator The AED must have a high-resolution liquid crystal display with capacitive touch panel. The AED must have an ON/OFF button. The AED must have a SHOCK button that illuminates
Bid Specifications Defibrillator The AED must have a high-resolution liquid crystal display with capacitive touch panel. The AED must have an ON/OFF button. The AED must have a SHOCK button that illuminates
Garment Styles Design Choices Modifications Measuring Guide
 Male style openings Horizontal fly opening: A standard opening and will provide some pressure over the genital area Diagonal fly opening Standard design is lining If more pressure is required over the
Male style openings Horizontal fly opening: A standard opening and will provide some pressure over the genital area Diagonal fly opening Standard design is lining If more pressure is required over the
Model 130M Pneumatic Controller
 Instruction MI 017-450 May 1978 Model 130M Pneumatic Controller Installation and Operation Manual Control Unit Controller Model 130M Controller is a pneumatic, shelf-mounted instrument with a separate
Instruction MI 017-450 May 1978 Model 130M Pneumatic Controller Installation and Operation Manual Control Unit Controller Model 130M Controller is a pneumatic, shelf-mounted instrument with a separate
Mirage Vista Nasal Mask
 English 60 50 40 30 20 10 0 4 6 10 12 14 16 1 20 ENGLISH Mirage Vista Nasal Mask Rx Only The Mirage Vista Nasal Mask is an accessory to a non-continuous ventilator (respirator) intended for multipatient
English 60 50 40 30 20 10 0 4 6 10 12 14 16 1 20 ENGLISH Mirage Vista Nasal Mask Rx Only The Mirage Vista Nasal Mask is an accessory to a non-continuous ventilator (respirator) intended for multipatient
Cover Page for Lab Report Group Portion. Head Losses in Pipes
 Cover Page for Lab Report Group Portion Head Losses in Pipes Prepared by Professor J. M. Cimbala, Penn State University Latest revision: 02 February 2012 Name 1: Name 2: Name 3: [Name 4: ] Date: Section
Cover Page for Lab Report Group Portion Head Losses in Pipes Prepared by Professor J. M. Cimbala, Penn State University Latest revision: 02 February 2012 Name 1: Name 2: Name 3: [Name 4: ] Date: Section
