2,658,073. PURIFICATION OF PARA-AMINOSALICYEic. ACES ANDITFSALKALIMETAL SALTS Marrine A. Terpstra, Kirkwood. Mo. assignor. tor
|
|
|
- Kellie Reynolds
- 5 years ago
- Views:
Transcription
1 Patented Nov. 3, 193 2,68,073 UNITED STATES This invention relates to para-aminosalicylic acid and its alkali metal salts; more Specifically, this invention relates to an improvement in the process for the purification of crude para-amino salicylic acid and its alkali metal Salts, whereby substantially pure para-aminosalicylic acid and its alkali metal salts, substantially free from colored bodies, may be obtained. Para-aminosalicylic acid and its alkali metal salts have found wide spread utility as a pharmaceutical, particularly in the treatinent of tuberculosis. In such an application, exceptional purity is of primary importance. Heretofore Substantially pure para-aminosalicylic acid or its alkali metal salts has been most difficult to ob- PATENT OFFICE 2,68,073 PURIFICATION OF PARA-AMINOSALICYEic ACES ANDITFSALKALIMETAL SALTS Marrine A. Terpstra, Kirkwood. Mo. assignor. tor Monsanto Chernical Company, St. Louis, Mo, a corporation of Delaware No Drawing. Application March 18, 190, Seria. No. 0,4 12 Cairis; (C.260s-9) 2. assay of the finished material, as determined by tiltra-violet absorption techniques, was significant ly less than 100%. Thus, according to the purifi cation or refining techniques: heretofore used, para-aminosalicylic acid of a pharmaceutical grade was exceptionally difficult to obtain. It is an object of this invention, therefore, to provide an iniproved purification process for Crude: para-aiminosalicylic acid and its alkali metal salts. It is a furthier object of this invention to pro vide an improvement in the process for the puri fication of para-aminosalicylic acid, and its alkali metal SaltS whereby Substantially pure para aminosalicylic acid or its alkali metal salts are tain on a commercial Scale. obtained, Substantially free from colored bodies. Various processes are utilized for the produc- Further' objects will become apparent from a tion of para-aminiosalicylic acid and its alkali description of the novel process of this inven metal salts. The most frequently used process g tion. is the carboxylation of meta-annincphenol. In 20 Most unexpectedly it has been found that if a all of these processes a crude para-aminosalicylic crude alkali metal Salt of para-aminosalicylic acid or a crude alkali retail salt of para-araino- acid is dissolved in water or if a crude para salicyclic acid is obtained. Which contains a. COEl- allinosalicylic acid is converted to its alkali metal siderable quantity of highly colored side reaction Salt and dissolved in Water, there exists a prefer products. Heretofore purification of these crude ential solubility of the contaminants present in reaction products has been most frequently at- the Crude: material in the mother liquor as: comi tempted through the solution of the crude para- pared to the Solubility of the alkali metal salt of aninosalicylic acid as its alkali retail salt in an para-aminosalicylic acid in the mother liquor. aqueous medium, treatment of such a. Solution In view of this unexpected phenomenon, a sub with activated carbon, removal of the activated 30 stantially pure alkali metal Salt of para-amino carbon and the filterable impurities, and precipi- Salicylic acid may be isolated from the impurities tation of para-aminosalicylic acid from such a contained in the crude by crystallization of the solution by the addition of a mineral acid. In alkali metal Salt of para-arninosalicylic acid other words, according to the methods hereto- from an aqueous solution of a crude alkali metal fore used, crude para-aminosalicylie acid was 3 Salt of para-aminosalicylic acid. According to purified by the treatment of its alkali metal Salt the novel grocess of this invention, therefore, in an aqueous medium followed by acidification crude para-aminosalicylic acidor its alkali metal and precipitation therefrom of para-amino- Salt is purified by dissolving:the crude as its alkali Salicylic acid. metal salt in water, followed by crystallization such a process of purification has not proved 40 therefona of the alkali prietal salt of para-amino to be a satisfactory commercial process for the Salicylic acid. The alkali metal salt of para purification of crude para-aminosalicylic acid or aminosalicylic acid thus obtained may be re its alkali metal salts. In order to obtain a para- Covered by filtration or centrification, washed aminosalicylic acid by this process, substantially free from mother liquor and dried. The alkali free from colored bodies and other impurities, it 4 metal Salt of para-aminosalicylic acid thus, ob was found that repeated reprecipitations from tained may be used as such since it is exception the aqueous solution was necessary. Such re- ally pure and essentially free from colored bodies, peated reprecipitations were exceptionally costly, or it may be converted to para-aminosalicylic acid and due to the loss of product which necessarily by acidification. The para-arniiaosalicylie: acid accompanies each such unit operation, the yield 0 thus obtained is substantially pure and essentially of the finished product was significantly de- free from colored bodies. The alkali metal salt creased. It was further found that even if on of para-afinosalicylic acid obtained according to repeated reprecipitations of crude para-amino- this process: may be further purified, if desired, salicylic acid fron airi acteous mediting a product by redissolving. in water, treating with activated with a substantially good color was obtained, the carbon if further improvement.in-color is desired,
2 3 followed by crystallization of the alkali metal salt of para-aminosalicylic acid therefrom Ol' acidification and precipitation of para-amino salicylic acid therefrom. Precipitation as the acid at this stage is not deleterious as the in purities contained in the crude material have al ready been separated therefrom in the previous crystallization procedure. The following examples are illustrative of the novel process of this invention: - O Eacample I 0 g. of crude para-aminosalicylic acid, ob tained by the carboxylation of meta-amino phenol, was added to a solution of 13 g. of Sodium hydroxide contained in 9 g. of water while main taining a temperature of approximately C. When all of the para-aminosalicylic acid had been added, the batch was heated to C. to effect complete solution and the ph of the : solution then adjusted to about 8. to about 9.0 by the addition of sodium hydroxide or crude para-aminosalicylic acid. The batch was then slowly cooled to about 0 C. thereby crystallizing sodium para-aminosalicylate dihydrate. The slurry of sodium para-salicylate was fil tered and the filter cake washed with a Small amount of water at approximately 0 C. The sodium para-aminosalicylate was then dis solved in 100 ml. of water and treated with ap proximately 2. g. of activated carbon. The car bon was removed by filtration. The filtrate was then acidified with concentrated hydrochloric acid to a ph of 3.0 to 3. thereby precipitating para-aminosalicylic acid. The slurry of para 3. aminosalicylic acid was cooled to 10 C. and filtered. The cake of para-aminosalicylic acid was washed with cold water until essentially free from chlorides. The filter cake was then dried under reduced pressure at 40-0 C. 40 The precipitation of para-aminosalicylic acid, filtration of the precipitate and the Washing of the precipitate described above, was carried out in an atmosphere of carbon dioxide. Para-aminosalicylic acid thus obtained is prac tically White in color and assays approximately 100% by ultraviolet light absorption analytical techniques. Eacample II 0 0 parts by weight of crude para-aminosalicylic acid was added to a water solution containing 13 parts by weight of sodium hydroxide and 9 parts by weight of water while maintaining the ten perature at approximately C. When all of the para-aminosalicylic acid had been added, the batch was heated to C. to effect complete solution. The ph of the solution was then ad justed to 8. to 9.0 by the addition of either So dium hydroxide or crude para-aminosalicylic 60 acid. 23 parts by weight of Sodium chloride was then added to the aqueous solution of sodium para-aminosalicylate in order to completely Saturate the water present With Sodium chloride. The batch was slowly cooled to 0 C. The 6 slurry of sodium para-aminosalicylate thus formed was then filtered. The filter cake was washed free from mother liquor with cold (0 C.) saturated Sodium chloride solution. The sodium para-aminosalicylate cake thus obtained was redissolved in 22 parts by weight of water and 4. parts by weight of activated carbon added in 1. parts by weight increments with 1 minutes agitation after each addition. The car bon was then removed by filtration, Under an 70 4 atmosphere of carbon dioxide, the filtrate was then acidified with concentrated hydrochloric acid to a ph of 3.0 to 3. in order to precipitate para-aminosalicylic acid. The slurry was cooled to 10 C. and filtered. The filter cake of para aminosalicylic acid thus obtained was washed with cold water (10 C.) until essentially free of chlorides. The wet para-aminosalicylic acid was then dried under reduced pressure at 40-0 C. An excellent yield of substantially pure para aminosalicylic acid was obtained having the foll lowing properties: Assay (ultraviolet absorp- substantially 100% tion). Ash % Heavy metals (p.p.m.) n-aminophenol % Color: Lumetron photoelectric colorimeter using g. Sample in 30 ml. Of a 10% NaHCO3 Water Solution contained in 20 mm. cell. Filter Absorbancy 660 mpi mpi mpi mpi Eacample III The procedure described in Example II was repeated utilizing acetic acid, in place of con centrated hydrochloric acid, as the acidification agent for the precipitation of para-aminosali cylic acid. An excellent yield of substantially pure para-aminosalicylic acid was obtained hav ing properties substantially identical to those ex hibited by the para-aminosalicylic acid obtained in Example II. Eacample IV The procedure described in Example II was repeated utilizing sulfuric acid, in place of con centrated hydrochloric acid, as the acidification agent for the precipitation of para-aminosali cylic acid. An excellent yield of substantially pure para-aminosalicylic acid was obtained hav ing properties substantially identical to those ex hibited by the para-aminosalicylic acid obtained in Example II. Eacample V 0 parts by Weight of crude para-aminosali cylic acid was added to a solution of sodium hy droxide containing 13 parts by weight of sodium hydroxide and 60 parts by weight of water while maintaining the temperature at approximately C. When all of the para-aminosalicylic acid had been added, the batch was heated to C. to effect complete solution. The ph of the Solution was then adjusted to 8. to 9.0 and 23 parts by weight of sodium chloride added. The batch was cooled to 0 C., crystallizing so dium para-aminosalicylate. The sodium para aminosalicylate was then filtered and washed free of mother liquor. The wet sodium para aminosalicylate was then dried under reduced pressure at 40-0 C. Sodium para-aminosali cylate, free from colored bodies, was thus ob tained. In contrast to the exceptionally pure para aminosalicylic acid obtained by the procedure described in Example II wherein the alkali metal Salt of para-aminosalicylic acid was isolated from the highly colored bodies contained in the
3 S crude material by recrystallization of the alkali metal salt from an aqueous solution, is the rela tively poor quality of para-aminosalicylic acid obtained according to the methods of purifica tion heretofore used as exemplified by the fol lowing example: Eacample VI 0 parts by weight of a crude para-aminosali cylic acid was slurried with approximately 70 parts by weight of water. To this slurry was then added approximately 3 parts by Weight of an aqueous. 2% sodium hydroxide solution while maintaining the temperature below 2 C. The ph of the solution was adjusted to about 7.0 to parts by weight of activated carbon. WaS then added in 3 parts by weight increments with 1-20 minutes agitation after each addition. The activated carbon was then removed by filtra tion and para-aminosalicylic acid precipitated from the aqueous medium by acidification to a ph of 3.0 with hydrochloric acid. The slurry of para-aminosalicylic acid thus obtained was filtered. The filter cake Of para aminosalicylic acid was redissolved and reprecipi tated as para-aminosalicylic acid a second and third time in accordance with the procedure set forth for the first precipitation of para-amino Salicylic acid. The filter cake of para-aminosalicylic acid : thus obtained was dried under reduced pressure at a temperature of about 40-0 C. The para aminosalicylic acid thus obtained had the foll lowing properties: Assay (ultraviolet ab Sorption) approximately 98% Ash % Heavy metals (p. p. m.) mi-aminophenol % Color: Lumetron photoelectric colorimeter using g. sample in 30 ml, of a 10% NaHCO3 Water solution contained in a 20 mm, cell. Fitor Absorbancy 660 mpi mpi a-- 1,204 1 m.pl mpi ,036 Comparing the physical properties of para aminosalicylic acid obtained according to the novel process of this invention as Set forth in Example II with the properties of para-amino salicylic acid obtained according to the process as heretofore carried out, as described in Ex ample VI, the outstanding utility of the novel process of this invention is clearly evident. Ac cording to the process heretofore used, para aminosalicylic assaying approximately 98% was obtained in contrast to para-aminosalicylic acid assaying substantially 100% as obtained ac cording to the novel process of this invention. More striking is the difference in color between the materials obtained by these two processes as determined on a Lumetron photoelectric colorimeter. It may be said that the novel proce ess of this invention results in a most significant improvement in the color of the finished product as compared to the product obtained according to the prior art process for the purification of crude para-aminosalicylic acid, The procedural aspects of the novel process of this invention as set forth in Examples. I to. W 2,68,078 6 are subject to many variations. For example, in the formation of the alkali metal salt of crude para-aminosalicylic acid in the first step of the process, alkaline materials other than Sodiuli hydroxide may be utilized. The alkali metal car bonates, bicarbonates and hydroxides, such as sodium carbonate, sodium bicarbonate, potassiumi carbonate, potassium bicarbonate and potassiu?ii hydroxide, exemplify other materials which may be utilized in this step. The concentration of the alkali metal salt of crude para-aminosalicylic acid in the aqueous medium set forth in Examples I to W is also Sub ject to wide variations. However, excessively dilute solutions of the alkali metal salt of para aminosalicylic acid would cause excessive losses of the product in the mother liquor, and highly concentrated solutions would be difficult to trans fer on a plant. Scale. Solubility of the alkali metal salt of para aminosalicylic acid in the mother liquor may be significantly decreased without deleteriously af fecting the solubility of the innipurities in the mother liquor by saturating the mother liquor with sodium chloride. Utilizing such a proce dure necessarily affords a better yield of final product because of the ability to effect a more complete crystallization of the alkali metal Salt of para-aminosalicylic acid from a Saturated brine solution rather than a water solution. How ever, the use of a saturated brine solution, is not required in order to obtain a finished product having exceptional purity. The temperature maintained during the solu tion of the alkali metal salt of para-amino salicylic acid and its subsequent crystallization may also be varied over a wide range. For prac tical reasons, elevated temperatures in the range of from about C. are preferred during the Solution of the crude alkali metal Salt of para-aminosalicylic acid in Water. During the crystallization of the alkali metal salt of para aminosalicylic acid from the aqueous solution, lower temperatures are preferred, such as within the range of from about -10 C. to about --20 C. The lower the temperature utilized at this stage of the process, the more complete will be the crystallization of the alkali metal salt. of para aminosalicylic acid from the aqueous medium. The minimum temperature which may be uti lized in this process is governed solely by the Solidification point of the aqueous medium itself. It is preferred that the precipitated alkali metal Salt of para-aminosalicylic acid be Washed With a Saturated brine solution rather than Water. Again, this preference is not significant from the Standpoint of the quality of the finished product but rather from an economical stand point in that by Washing with a brine solution rather than water, a significantly smaller quan tity of the alkali metal salt of para-amino Salicylic acid is lost by solution in the Washing recilinn. The redissolving of the alkali metal salt. of para-aminosalicylic acid and subsequent treat ment. With activated carbon as set forth in Ex ample II is advantageous if an unusually high quality product is desired. If this additional purification step is utilized, any of the well known inert decolorizing agents may be utilized. Typi cal of Such agents are fuller's earth, attapulgus earth, diatomaceous earth, activated carbon; etc. The para-aminosalicylic acid may be precipitated from the aqueous medium by acidification. With any acid having an ionization constant higher
4 7 than the ionization constant of para-aminosali cylic acid. Typical of such acids which may be utilized are the mineral acids, such as hydro chloric acid, sulfuric acid, etc., and organic acids, such as acetic acid. Because of the instability of para-aminosalicylic acid at elevated tempera tures in the presence of air, it is preferred that this latter purification step be carried out under an inert atmosphere, Such as nitrogen or carbon dioxide. The ph ranges employed in the various steps of the process for the purification of crude para aminosalicylic acid may also be varied to a con siderable extent. In the preparation of the al kali metal salt of the crude para-aminosalicylic acid, the ph may be maintained in the range of about 7. to about It has been found, however, that when the ph in this step of the process is maintained within the range of about 8. to 9.0, a more effective removal of the col ored bodies from the crude para-aminosalicylic acid is possible. During the precipitation of para-aminosalicylic acid in the Second purifica tion step, if performed, it is preferred that a ph in the range of from about 2. to about 3. is recommended for the precipitation of the para aminosalicylic acid. A lower ph is conducive to the formation of the acid salt. A somewhat higher ph may be employed but a loss in yield will result due to the incomplete precipitation of the para-aminosalicylic acid. The finished product, whether it be the alkali metal salt of para-aminosalicylic acid of para aminosalicylic acid, is preferably dried under re duced pressure in Order to minimize decomposi tion. The products may, however, be air dried at moderate temperatures, such as at 3-4 C. What is claimed is: 1. In a process for the purification of crude water and while maintaining the ph of the solu tion so formed in the range of from about 8. to about 9.0 crystallizing therefrom the alkali metal salt of para-aminosalicylic acid by cooling said water solution. 2. In a process for the purification of Crude water, saturating said water Solution of the al kali metal salt of crude para-aminosalicylic acid with sodium chloride, and while maintaining the ph of the solution so formed in the range of from the alkali metal salt of para-aminosalicylic acid by cooling said Water Solution. 3. In a process for the purification of crude salts, the steps comprising Solubilizing an alkali water at a temperature in the range of from about C. and while maintaining the ph of the solution so formed in the range of from about 8. to about 9.0 crystallizing therefrom the alkali metal salt of para-aminosalicylic acid by cooling said water solution to a temperature in the range from about -10 C. to about 20 C. 4. In a process for the purification of crude salts, the steps comprising Solubilizing an alkali water at a temperature in the range of from about C., saturating said Water Solution of the alkali metal salt of crude para-amino salicylic acid with sodium chloride, and while maintaining the ph of the solution so formed in the range of from about 8. to about 9.0 Crystallizing therefrom the alkali metal Salt of para-aminosalicylic acid by cooling said water solution to a temperature in the range from about -10 C. to about 20 C.. The process as described in claim 3 wherein the alkali metal Salt of para-aminosalicylic acid is sodium para-aminosalicylate. 6. The process as described in claim 4 wherein the alkali metal salt of para-aminosalicylic acid is sodium para-aminosalicylate. 7. In a process for the purification of crude para-aminosalicylic acid and its alkali metal Salts, the steps comprising dissolving an alkali metal Salt of crude para-aminosalicylic acid in Water, Saturating said water Solution of the al kali metal salt of crude para-aminosalicylic acid With sodium chloride, and while maintaining the ph of the solution so formed in the range of from the alkali metal salt of para-aminosalicylic acid by cooling Said Water solution, recovering and redissolving the alkali metal Salt of para aminosalicylic acid in Water, treating the water solution so formed with an inert decolorizing agent, and acidifying and precipitating there from para-aminosalicylic acid. 8. In a process for the purification of crude para-aminosalicyclic acid and its alkali metal Salts, the Steps comprising dissolving an alkali metal Salt of crude para-aminosalicyclic acid in Water, Saturating Said water Solution of the alkali metal Salt of crude para-aminosalicyclic acid with Sodium chloride, and while maintaining the ph of the Solution. So formed in the range of from the alkali metal Salt of para-aminosali cylic acid by cooling Said water solution, recover ing and redissolving the alkali metal Salt of para aminosalicyclic acid in water, treating the water Solution. So formed with an inert decolorizing agent, and acidifying the water solution of the alkali metal Salt of para-aminosalicyclic acid to a ph in the range of from about 2. to about 3. by the addition of a mineral acid and precipitat ing therefrom para-aminosalicyclic acid. 9. The process as described in claim 8 wherein the mineral acid is hydrochloric acid. 10. The process as described in claim 8 where in the mineral acid is Sulfuric acid. i1. The process as described in claim 8 wherein the alkali metal Salt of para-aminosalicyclic acid is Sodium pala-aminosalicylate and wherein the mineral acid is hydrochloric acid. 2. In a process for the purification of crude para-aminosalicyclic acid and its alkali metal metal Salt of crude para-aminosalicyclic acid in Water, Saturating said water solution of the alkali metal Salt of Crude para-aminosalicyclic acid with sodium chloride, and while maintaining the ph of the Solution so formed in the range of from the alkali metal salt of para-aminosalicyclic acid by cooling said water solution, recovering and redissolving the alkali metal Salt of para aminosalicyclic acid in water, treating the water solution so formed with an inert decolorizing agent, and acidifying the water solution of the alkali metal salt of para-aminosalicyclic acid to a ph in the range from about 2. to about 3. by
5 9 10 the addition of acetic acid, and precipitating Number Name Date therefron para-aminosalicyclic acid. 2,80,19 Rosdahl Dec. 2, 191. MARRINE A.ERPSTRA. FOREIGN PATENTS References Cited in the file of this patent Number Country Date UNITED STATES PATENTS 13,76 Germany July 2, 1904 Number Name Date OTHER REFERENCES 427,6 Gnehm et al May 13, 1890 MacArdle, "Solvents in Syn. Org. Chem.' (Wan 63,993 Welter July 14, 1896 O Nostrand), pp (192). 1973,74. Marshall Sept. 11, 1934 Gatterman, Organic Chem.' (Macmillan), pp. 2,40,104 Doub Feb. 6, (1896). 2,40,78 Hultquist Feb. 6, 191 Rosdahl, Svensk. Kem. Tid, vol. 60, p. 13 (Jan. 2,48,27 Goldberg et al Apr. 10, ).
United States Patent (19)
 United States Patent (19) Pederson, Jr. (54) TREATMENT OF WHEY (75) Inventor: Harold T. Pederson, Jr., Livermore, Calif, (73) Assignee: Patent Technology, Inc., San Francisco, Calif. (21) Appl. No.: 907,669
United States Patent (19) Pederson, Jr. (54) TREATMENT OF WHEY (75) Inventor: Harold T. Pederson, Jr., Livermore, Calif, (73) Assignee: Patent Technology, Inc., San Francisco, Calif. (21) Appl. No.: 907,669
14 / 44. United States Patent (19) Allam ADM2/A. 11 Patent Number: 4,461,154 45) Date of Patent: Jul. 24, 1984 SAZX A6 AIAW/ 10/ /02 A/?
 United States Patent (19) Allam (54) METHOD AND APPARATUS FOR COMPRESSENG GAS 75 Inventor: Rodney J. Allam, St. Catherines, England 73) Assignee: Air Products and Chemicals, Inc., Allentown, Pa. (21) Appl.
United States Patent (19) Allam (54) METHOD AND APPARATUS FOR COMPRESSENG GAS 75 Inventor: Rodney J. Allam, St. Catherines, England 73) Assignee: Air Products and Chemicals, Inc., Allentown, Pa. (21) Appl.
Aug. 14, A4i/2 (2//y. D. COHN 2,758,416 KNEE JOINT STRUCTURE FOR WALKING DOLLS Filed Aug. 24, A772A/Ay INVENTOR.
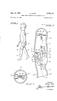 Aug. 14, 196 D. COHN KNEE JOINT STRUCTURE FOR WALKING DOLLS Filed Aug. 24, 19 2 Sheets-Sheet INVENTOR. A4i/2 (2//y A772A/Ay Aug. 14, 196 D. COHN KNEE JOINT STRUCTURE FOR WALKING DOLLS Filed Aug. 24, 19
Aug. 14, 196 D. COHN KNEE JOINT STRUCTURE FOR WALKING DOLLS Filed Aug. 24, 19 2 Sheets-Sheet INVENTOR. A4i/2 (2//y A772A/Ay Aug. 14, 196 D. COHN KNEE JOINT STRUCTURE FOR WALKING DOLLS Filed Aug. 24, 19
(12) United States Patent (10) Patent No.: US 6,426,434 B1
 USOO6426434B1 (12) United States Patent (10) Patent No.: Yoshida et al. () Date of Patent: Jul. 30, 2002 (54) PROCESS FOR THE SYNTHESIS OF UREA JP 62-170 4/1987 JP 63-12 5/1988 (75) Inventors: Kinichi
USOO6426434B1 (12) United States Patent (10) Patent No.: Yoshida et al. () Date of Patent: Jul. 30, 2002 (54) PROCESS FOR THE SYNTHESIS OF UREA JP 62-170 4/1987 JP 63-12 5/1988 (75) Inventors: Kinichi
Environmental Engineering-I By Prof S S JAHAGIRDAR
 L- 28 Disinfection Part- I Environmental Engineering-I By Prof S S JAHAGIRDAR Contents Disinfection techniques- Ozonation, u/v radiation. Chemistry of chlorination, chlorine demand curve. Types of chlorination,
L- 28 Disinfection Part- I Environmental Engineering-I By Prof S S JAHAGIRDAR Contents Disinfection techniques- Ozonation, u/v radiation. Chemistry of chlorination, chlorine demand curve. Types of chlorination,
United States Patent (19) Herro
 United States Patent (19) Herro (54) (76) (22 21 ) 52) (51) 58 (56) ATHLETIC SHOE WITH A DETACHABLE SOLE Inventor: Richard E. Herro, Rte. 5, Mound View Estates, Joliet, Ill. 60436 Filed: Jan. 21, 1976
United States Patent (19) Herro (54) (76) (22 21 ) 52) (51) 58 (56) ATHLETIC SHOE WITH A DETACHABLE SOLE Inventor: Richard E. Herro, Rte. 5, Mound View Estates, Joliet, Ill. 60436 Filed: Jan. 21, 1976
Feb. 27, 1973 R. L. CRNDORFF, JR 3,717,943 MUD RESISTANT ELASTOMERS INVENTOR. Roy L.ORNDORFF, JR. ATTY.
 Feb. 27, 1973 R. L. CRNDORFF, JR Filed Aug. 23. l97 2. Sheets-Sheet l INVENTOR Roy L.ORNDORFF, JR. ATTY. Feb. 27, 1973 R. L. ORNDORFF, JR Filed Aug. 23. l97) 2 Sheets-Sheet 2-32 2M 223 %2-Z ZZ 222s INVENTOR
Feb. 27, 1973 R. L. CRNDORFF, JR Filed Aug. 23. l97 2. Sheets-Sheet l INVENTOR Roy L.ORNDORFF, JR. ATTY. Feb. 27, 1973 R. L. ORNDORFF, JR Filed Aug. 23. l97) 2 Sheets-Sheet 2-32 2M 223 %2-Z ZZ 222s INVENTOR
United States Patent (19)
 United States Patent (19) Fujikawa 54 HYDRAULIC CONTROL SYSTEM FOR A ROCK DRILL (75) Inventor: Kozo Fujikawa, Hiroshima, Japan 73) Assignee: Toyo Kogyo Co., Ltd., Hiroshima, Japan 21 Appl. No.: 194,391
United States Patent (19) Fujikawa 54 HYDRAULIC CONTROL SYSTEM FOR A ROCK DRILL (75) Inventor: Kozo Fujikawa, Hiroshima, Japan 73) Assignee: Toyo Kogyo Co., Ltd., Hiroshima, Japan 21 Appl. No.: 194,391
(ii) 4,314,077. United States Patent ( Feb. 2, the inerts and the solution of urea coming from the (54) METHOD FOR THE PRODUCTION OF
 United States Patent (19 Zardi et al. (54) METHOD FOR THE PRODUCTION OF UREA AND PURIFICATION OF WATER 75 Inventors: Umberto Zardi, San Donato Milanese; Vincenzo Lagana', Milan, both of Italy 73) Assignee:
United States Patent (19 Zardi et al. (54) METHOD FOR THE PRODUCTION OF UREA AND PURIFICATION OF WATER 75 Inventors: Umberto Zardi, San Donato Milanese; Vincenzo Lagana', Milan, both of Italy 73) Assignee:
N3% (12) United States Patent. NNéré. (10) Patent No.: US 7, B2. Rossiter (45) Date of Patent: Nov. 20, 2007
 (12) United States Patent US007298.473B2 (10) Patent o.: US 7,298.473 B2 Rossiter (45) Date of Patent: ov. 20, 2007 (54) SPECTROSCOPY CELL 4,587,835 A 5/1986 Adams 4,674,876 A 6/1987 Rossiter... 356,244
(12) United States Patent US007298.473B2 (10) Patent o.: US 7,298.473 B2 Rossiter (45) Date of Patent: ov. 20, 2007 (54) SPECTROSCOPY CELL 4,587,835 A 5/1986 Adams 4,674,876 A 6/1987 Rossiter... 356,244
W I L D W E L L C O N T R O L FLUIDS
 FLUIDS Fluids Learning Objectives You will learn about different fluids that can be used in well control. You will become familiar with the characteristics and limitations of fluids. You will learn general
FLUIDS Fluids Learning Objectives You will learn about different fluids that can be used in well control. You will become familiar with the characteristics and limitations of fluids. You will learn general
Pool Water Chemistry Guide
 Pool Water Chemistry Guide water test The water chemistry information in this guide will help you keep your pool water clean and clear, swimmer safe, and plaster friendly. the clear choice in pool care
Pool Water Chemistry Guide water test The water chemistry information in this guide will help you keep your pool water clean and clear, swimmer safe, and plaster friendly. the clear choice in pool care
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0176367 A1 PENNINGTON et al. US 201701.76367A1 (43) Pub. Date: Jun. 22, 2017 (54) (71) (72) (21) (22) (60) APPARATUS TO MEASURE
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0176367 A1 PENNINGTON et al. US 201701.76367A1 (43) Pub. Date: Jun. 22, 2017 (54) (71) (72) (21) (22) (60) APPARATUS TO MEASURE
CHEM254 #4 The Synthesis of a Tertiary Alcohol Using a Pre-Made Grignard Reagent 1
 CHEM254 #4 The Synthesis of a Tertiary Alcohol Using a Pre-Made Grignard Reagent 1 Background In this project, we will perform a Grignard reaction using a pre-made Grignard reagent. Grignard reagents can
CHEM254 #4 The Synthesis of a Tertiary Alcohol Using a Pre-Made Grignard Reagent 1 Background In this project, we will perform a Grignard reaction using a pre-made Grignard reagent. Grignard reagents can
Milk Protein Products: Casein & Whey
 Milk Protein Products: Casein & Whey Introduction Currently, various types of casein and of whey protein are widely used in food processing, due to: Provide foods with a specific nutritive value Infant
Milk Protein Products: Casein & Whey Introduction Currently, various types of casein and of whey protein are widely used in food processing, due to: Provide foods with a specific nutritive value Infant
(12) United States Patent (10) Patent No.: US 6,641,487 B1
 USOO6641487B1 (12) United States Patent (10) Patent No.: US 6,641,487 B1 Hamburger (45) Date of Patent: Nov. 4, 2003 (54) ADJUSTABLY WEIGHTED GOLF CLUB 4,872,684. A 10/1989 Dippel PUTTER HEAD WITH REMOVABLE
USOO6641487B1 (12) United States Patent (10) Patent No.: US 6,641,487 B1 Hamburger (45) Date of Patent: Nov. 4, 2003 (54) ADJUSTABLY WEIGHTED GOLF CLUB 4,872,684. A 10/1989 Dippel PUTTER HEAD WITH REMOVABLE
April 27, ,582,836 A. C. LINK. METALLIC GOLF CLUB HEAD Filed July 17, % 17evera/or. Atóerz C. Zarz/C, ly /%22.6% to
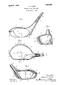 April 27, 1926. 1,582,836 A. C. LINK METALLIC GOLF CLUB HEAD Filed July 17, 1925-7% / 17evera/or Atóerz C. Zarz/C, ly /%22.6% to 90 25 Patented Apr. 27, 1926. 1,582,836 UNITED STATES PATENT OFFICE. ALBERT
April 27, 1926. 1,582,836 A. C. LINK METALLIC GOLF CLUB HEAD Filed July 17, 1925-7% / 17evera/or Atóerz C. Zarz/C, ly /%22.6% to 90 25 Patented Apr. 27, 1926. 1,582,836 UNITED STATES PATENT OFFICE. ALBERT
Slide 1. Slide 2. Slide 3 What do YOU know about oxygen? Dissolved Oxygen. Oxygen. I can t breath in the water but the fish can!
 Slide 1 Dissolved Oxygen I can t breath in the water but the fish can! Slide 2 Oxygen Oxygen is one of the fundamental resources required by life forms on Earth. Aquatic ecosystems have a wide assortment
Slide 1 Dissolved Oxygen I can t breath in the water but the fish can! Slide 2 Oxygen Oxygen is one of the fundamental resources required by life forms on Earth. Aquatic ecosystems have a wide assortment
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 US 2004O126242A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0126242 A1 Howard et al. (43) Pub. Date: Jul. 1, 2004 (54) BOAT PROPELLER AND GUARD DEVICE (52) U.S. Cl....
US 2004O126242A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0126242 A1 Howard et al. (43) Pub. Date: Jul. 1, 2004 (54) BOAT PROPELLER AND GUARD DEVICE (52) U.S. Cl....
United States Patent (19) Condo et al.
 United States Patent (19) Condo et al. 54 BOXING TRAINING APPARATUS 75 Inventors: Girolamao Condo; Luigi Trocola, both of Eislingen, Fed. Rep. of Germany 73) Assignee: Petra Condo, Eislingen, Fed. Rep.
United States Patent (19) Condo et al. 54 BOXING TRAINING APPARATUS 75 Inventors: Girolamao Condo; Luigi Trocola, both of Eislingen, Fed. Rep. of Germany 73) Assignee: Petra Condo, Eislingen, Fed. Rep.
FOR LESS! The Secret to a Sparkling Pool and Patio. Enjoy a crystal clear pool with ARM & HAMMER Baking Soda
 The Secret to a Sparkling Pool and Patio FOR LESS! Enjoy a crystal clear pool with ARM & HAMMER Baking Soda ARM & HAMMER Pool Care and Outdoor Cleaning Guide 1 Pool Care: Made Easy Crystal Clear Opening
The Secret to a Sparkling Pool and Patio FOR LESS! Enjoy a crystal clear pool with ARM & HAMMER Baking Soda ARM & HAMMER Pool Care and Outdoor Cleaning Guide 1 Pool Care: Made Easy Crystal Clear Opening
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States US 20090005197A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0005197 A1 Mayer (43) Pub. Date: Jan. 1, 2009 (54) HOCKEY STICK HAVING AN ANGLED (52) U.S. Cl.... 473/560;
(19) United States US 20090005197A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0005197 A1 Mayer (43) Pub. Date: Jan. 1, 2009 (54) HOCKEY STICK HAVING AN ANGLED (52) U.S. Cl.... 473/560;
Water Treatment: Fundamentals and Practical Implications of Bubble Formation
 Water Treatment: Fundamentals and Practical Implications of Bubble Formation Paolo Scardina Thesis submitted to the Faculty of the Virginia Polytechnic Institute and State University In partial fulfillment
Water Treatment: Fundamentals and Practical Implications of Bubble Formation Paolo Scardina Thesis submitted to the Faculty of the Virginia Polytechnic Institute and State University In partial fulfillment
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 US 2015O129357A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0129357 A1 ROth (43) Pub. Date: May 14, 2015 (54) GUIDED TYPE FALL ARRESTER - BODY (52) U.S. Cl. CONTROL SYSTEM
US 2015O129357A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0129357 A1 ROth (43) Pub. Date: May 14, 2015 (54) GUIDED TYPE FALL ARRESTER - BODY (52) U.S. Cl. CONTROL SYSTEM
Pharmaceutical Grade Nitrogen & Gardner Denver / CompAir PSA Nitrogen Gas Generation Systems
 Pharmaceutical Grade Nitrogen & Gardner Denver / CompAir PSA Nitrogen Gas Generation Systems The purpose of this document is to clarify the specification for pharmaceutical grade nitrogen gas and confirm
Pharmaceutical Grade Nitrogen & Gardner Denver / CompAir PSA Nitrogen Gas Generation Systems The purpose of this document is to clarify the specification for pharmaceutical grade nitrogen gas and confirm
United States Patent (19) Kaneko
 United States Patent (19) Kaneko 54 SHOE SOLE FOR RUNNING SHOES 75 Inventor: Yasunori Kaneko, Osaka, Japan 73 Assignee: Mizuno Corporation, Osaka, Japan 21 Appl. No.: 664,016 22 Filed: Jun. 13, 1996 30
United States Patent (19) Kaneko 54 SHOE SOLE FOR RUNNING SHOES 75 Inventor: Yasunori Kaneko, Osaka, Japan 73 Assignee: Mizuno Corporation, Osaka, Japan 21 Appl. No.: 664,016 22 Filed: Jun. 13, 1996 30
Feb. 24, 1953 H., R. WILSON 2,629,609 WHEELED GOLF BAG
 Feb. 24, 193 H., R. WILSON WHEELED GOLF BAG Filed Aug. 6, 192 2. SHEETS-SHEET l Feb. 24, 193 Filed Aug. 6, 19l H. R. WSON WHEELED GOLF BAG 2 SHEETS-SHEET 2 y? c/eve? %ary 22 72 o 2/ (totaggy Zsola Patented
Feb. 24, 193 H., R. WILSON WHEELED GOLF BAG Filed Aug. 6, 192 2. SHEETS-SHEET l Feb. 24, 193 Filed Aug. 6, 19l H. R. WSON WHEELED GOLF BAG 2 SHEETS-SHEET 2 y? c/eve? %ary 22 72 o 2/ (totaggy Zsola Patented
4/26/16. Section 1 Understanding Solutions. Solutions and Suspensions. Solutions and Suspensions. Solutions and Suspensions. Solvents and Solutes
 Section 1 Understanding Solutions On a hot day, you may think that a cool glass of plain water would be refreshing. However, if the glass were actually filled with plain water, it would taste stale. This
Section 1 Understanding Solutions On a hot day, you may think that a cool glass of plain water would be refreshing. However, if the glass were actually filled with plain water, it would taste stale. This
im,?har. In 4. Inventor: Wesley D. Burgess, His Attorney. years, is a Re fig. 4. o-6 o title?: 15! / 3.
 April 18, 1950 W. D. BURGESS 2,504,642 METHOD FOR PUNCH PRESS SET UPS Filed July 23, 1945 3. Sheets-Sheet fig2.?t-o 2 fig1. -7 -- A " ' im,?har. In 4. o-6 o title?: 15! / 3. years, is a Re fig. 4. 23-e
April 18, 1950 W. D. BURGESS 2,504,642 METHOD FOR PUNCH PRESS SET UPS Filed July 23, 1945 3. Sheets-Sheet fig2.?t-o 2 fig1. -7 -- A " ' im,?har. In 4. o-6 o title?: 15! / 3. years, is a Re fig. 4. 23-e
52 U.S. C /232; 423/210; 55/16 55/16. (56) References Cited U.S. PATENT DOCUMENTS 3,624,983 12/1971 Ward et al..., 55/16
 United States Patent (19) Ward, III (54 SEPARATION OF HYDROGEN SULFIDE FROM GAS MIXTURE INCLUDING CARBON DOXDE (75) Inventor: William J. Ward, III, Schenectady, N.Y. 73) Assignee: General Electric Company,
United States Patent (19) Ward, III (54 SEPARATION OF HYDROGEN SULFIDE FROM GAS MIXTURE INCLUDING CARBON DOXDE (75) Inventor: William J. Ward, III, Schenectady, N.Y. 73) Assignee: General Electric Company,
ARM & HAMMER Baking Soda
 Pool Care: Made Easy Crystal Clear Opening ARM & HAMMER Baking Soda 1 A Safe and Natural Start ARM & HAMMER Baking Soda is the quick, safe and natural way to open your pool for the season. Use our conveniently
Pool Care: Made Easy Crystal Clear Opening ARM & HAMMER Baking Soda 1 A Safe and Natural Start ARM & HAMMER Baking Soda is the quick, safe and natural way to open your pool for the season. Use our conveniently
HHHHHHHHHHHHIII. United States Patent (19) (45) Date of Patent: Aug. 23, ) Patent Number: 5,
 United States Patent (19) Schaefer et al. 54 METHOD AND APPARATUS FOR THE COATING OF SUBSTRATES 75) Inventors: Christian Schaefer, Hanau am Main; Klaus Hartig, Ronneburg, both of 73) Assignee: 21 Appl.
United States Patent (19) Schaefer et al. 54 METHOD AND APPARATUS FOR THE COATING OF SUBSTRATES 75) Inventors: Christian Schaefer, Hanau am Main; Klaus Hartig, Ronneburg, both of 73) Assignee: 21 Appl.
product ospa lithium focus
 lithium White granules Lithium Hypochlorite 500kg 1 kg 10 kg Instantly soluble. Stable at higher temperatures. Convenient packaging sizes. Contains no calcium. No pre-dissolving necessary. Won't cloud
lithium White granules Lithium Hypochlorite 500kg 1 kg 10 kg Instantly soluble. Stable at higher temperatures. Convenient packaging sizes. Contains no calcium. No pre-dissolving necessary. Won't cloud
Jan. 12, 1965 E. F. RES 3,165,112 WALKER OR WALKER AID. Filed July 20, 196l. INVENTOR. El-Mear F Rt as. 62. (Pavel. A440/wey
 Jan. 12, 1965 E. F. RES WALKER OR WALKER AID Filed July, 196l. BY INVENTOR. El-Mear F Rt as 62. (Pavel A4/wey United States Patent Office 1 WALKER ORWALKERAID d Eimer Manufacturing F. Ries, Cincinnati,
Jan. 12, 1965 E. F. RES WALKER OR WALKER AID Filed July, 196l. BY INVENTOR. El-Mear F Rt as 62. (Pavel A4/wey United States Patent Office 1 WALKER ORWALKERAID d Eimer Manufacturing F. Ries, Cincinnati,
Dissolved Oxygen Guide
 Educat i onser i es Di ssol vedoxygengui de Dissolved Oxygen Guide Introduction Dissolved oxygen probes provide a convenient approach to essentially direct measurement of molecular oxygen. The membrane
Educat i onser i es Di ssol vedoxygengui de Dissolved Oxygen Guide Introduction Dissolved oxygen probes provide a convenient approach to essentially direct measurement of molecular oxygen. The membrane
DURING the course of certain investigations it became
 VOLUMETRIC DETERMINATION OF ETHER OR CYCLOPROPANE, CARBON DIOXIDE, NITROUS OXIDE AND OXYGEN IN ANESTHETIC MIXTURES By F. J. PRIME DURING the course of certain investigations it became necessary to be able
VOLUMETRIC DETERMINATION OF ETHER OR CYCLOPROPANE, CARBON DIOXIDE, NITROUS OXIDE AND OXYGEN IN ANESTHETIC MIXTURES By F. J. PRIME DURING the course of certain investigations it became necessary to be able
Water Balance Indexes Revised September 2017
 Water Balance Indexes Revised September 2017 Brought to you by the APSP Recreational Water Quality Committee (RWQC) I. INTRODUCTION There are several water balance indexes that are being used as tools
Water Balance Indexes Revised September 2017 Brought to you by the APSP Recreational Water Quality Committee (RWQC) I. INTRODUCTION There are several water balance indexes that are being used as tools
exercising facility (14), when the arms of the person are to
 USOO5906563A United States Patent (19) 11 Patent Number: 5,906,563 Pittari (45) Date of Patent: May 25, 1999 54) DUAL EXERCISE BIKE 5,284.462 2/1994 Olschansky et al.... 482/64 5,342,262 8/1994 Hansen......
USOO5906563A United States Patent (19) 11 Patent Number: 5,906,563 Pittari (45) Date of Patent: May 25, 1999 54) DUAL EXERCISE BIKE 5,284.462 2/1994 Olschansky et al.... 482/64 5,342,262 8/1994 Hansen......
3,367,102 WIRE ROPE AND METHOD OF MA KING SAME. 5 Sheets-Sheet, l INVENTOR. a22a-ee ad a 12aaaa 4-, % Az-Zava-Vay-1s
 Feb. 6, 1968 A, F. MEGER WIRE ROPE AND METHOD OF MA KING SAME Fied Jan. ll, l966 5 Sheets-Sheet, l BY INVENTOR. a22a-ee ad a 12aaaa 4-, 2-4.21%-24. 44 Az-Zava-Vay-1s Feb. 6, 1968 A. F. MEGER WRE ROPE AND
Feb. 6, 1968 A, F. MEGER WIRE ROPE AND METHOD OF MA KING SAME Fied Jan. ll, l966 5 Sheets-Sheet, l BY INVENTOR. a22a-ee ad a 12aaaa 4-, 2-4.21%-24. 44 Az-Zava-Vay-1s Feb. 6, 1968 A. F. MEGER WRE ROPE AND
ByJosea/ / /a/e-aestra. CAar/es a o/6ers/aw. Nov. 7, 1967 C. F. OLDERSHAW ETAL 3,351,542. ELECTROLYTIC CHLORINATION AND ph CONTROL.
 Nov. 7, 1967 C. F. OLDERSHAW ETAL 3,351,542 ELECTROLYTIC CHLORINATION AND ph CONTROL OF SWIMMING POOL WATER Filed Nov. 14, 1966 3 Sheets-Sheet l Z3 INVENTORS. CAar/es a o/6ers/aw ByJosea/ / /a/e-aestra
Nov. 7, 1967 C. F. OLDERSHAW ETAL 3,351,542 ELECTROLYTIC CHLORINATION AND ph CONTROL OF SWIMMING POOL WATER Filed Nov. 14, 1966 3 Sheets-Sheet l Z3 INVENTORS. CAar/es a o/6ers/aw ByJosea/ / /a/e-aestra
Why The Dog Died (A Lesson in Fundamental Pool Water Chemistry)
 Why The Dog Died (A Lesson in Fundamental Pool Water Chemistry) I told YOU we should have gotten another Pool Guy! Presented by A Passion for Healthy Water and Aquatic Education www.anotherperfectpoolnews.com
Why The Dog Died (A Lesson in Fundamental Pool Water Chemistry) I told YOU we should have gotten another Pool Guy! Presented by A Passion for Healthy Water and Aquatic Education www.anotherperfectpoolnews.com
(12) United States Patent (10) Patent No.: US 6,311,857 B1
 USOO6311857B1 (12) United States Patent (10) Patent No.: US 6,311,857 B1 Al-Darraii (45) Date of Patent: Nov. 6, 2001 (54) STAND USING HOCKEY STICK SUPPORTS 5,848,716 12/1998 Waranius... 211/85.7 X 6,073,783
USOO6311857B1 (12) United States Patent (10) Patent No.: US 6,311,857 B1 Al-Darraii (45) Date of Patent: Nov. 6, 2001 (54) STAND USING HOCKEY STICK SUPPORTS 5,848,716 12/1998 Waranius... 211/85.7 X 6,073,783
SAFETY DATA SHEET. Section 1: Product and Company Identity
 SAFETY DATA SHEET Section 1: Product and Company Identity Name: Gendex Process Chemistry - Developer Product Number: 1.0069274 & 0.8223045 (296-49,296-40) Product Use: Photographic Developer Manufacturer:
SAFETY DATA SHEET Section 1: Product and Company Identity Name: Gendex Process Chemistry - Developer Product Number: 1.0069274 & 0.8223045 (296-49,296-40) Product Use: Photographic Developer Manufacturer:
Oct. 7, 1952 H. A. KUEHNEL 2,612,717 FISH LURE INVENTOR. A/2A/AY A. A1/EHWEL. BY ; oema Y
 Oct. 7, 192 H. A. KUEHNEL 2,612,717 FISH LURE Filed March 7, 1949 2 SHEETS-SHEET l INVENTOR. A/2A/AY A. A1/EHWEL BY ; 2 2-477 oema Y Oct. 7, 192 H, A, KUEHNEL 2,612,717 FSH LURE Filed March 7, 1949 2.
Oct. 7, 192 H. A. KUEHNEL 2,612,717 FISH LURE Filed March 7, 1949 2 SHEETS-SHEET l INVENTOR. A/2A/AY A. A1/EHWEL BY ; 2 2-477 oema Y Oct. 7, 192 H, A, KUEHNEL 2,612,717 FSH LURE Filed March 7, 1949 2.
. United States Patent (19) Oonuma et al.
 . United States Patent (19) Oonuma et al. 54) BUFFER DEVICE FOR A ROLLER CHAN AND SPROCKET COUPLING (75) Inventors: Koichiro Oonuma, Shiki; Yoshinori ' Kawashima, Sakado; Toshinori Hanai, Kamifukuoka,
. United States Patent (19) Oonuma et al. 54) BUFFER DEVICE FOR A ROLLER CHAN AND SPROCKET COUPLING (75) Inventors: Koichiro Oonuma, Shiki; Yoshinori ' Kawashima, Sakado; Toshinori Hanai, Kamifukuoka,
Countrywide Pools Northam
 Countrywide Pools Northam CountrywidePools Looking After Your New Pool... 3 Table of Contents Sanitising Your Pool... 4 Chlorine... 4 Pool Water Testing... 5 Daily Testing... 5 Weekly Testing... 5 Pool
Countrywide Pools Northam CountrywidePools Looking After Your New Pool... 3 Table of Contents Sanitising Your Pool... 4 Chlorine... 4 Pool Water Testing... 5 Daily Testing... 5 Weekly Testing... 5 Pool
United States Patent (19)
 United States Patent (19) Oranje (54) DEVICE FOR SEPARATING LIQUIDS AND/OR SOLIDS FROMA HIGH-PRESSURE GAS STREAM 75 inventor: Leendert Oranje, Haren, Netherlands (73) Assignee: N.V. Nederlandse Gasunie,
United States Patent (19) Oranje (54) DEVICE FOR SEPARATING LIQUIDS AND/OR SOLIDS FROMA HIGH-PRESSURE GAS STREAM 75 inventor: Leendert Oranje, Haren, Netherlands (73) Assignee: N.V. Nederlandse Gasunie,
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States US 20060049223A1. (12) Patent Application Publication (10) Pub. No.: US 2006/0049223 A1 Mora et al. (43) Pub. Date: Mar. 9, 2006 (54) (76) (21) (22) (60) SCORECARD HOLDER FOR GOLF Inventors:
(19) United States US 20060049223A1. (12) Patent Application Publication (10) Pub. No.: US 2006/0049223 A1 Mora et al. (43) Pub. Date: Mar. 9, 2006 (54) (76) (21) (22) (60) SCORECARD HOLDER FOR GOLF Inventors:
United States Patent (19)
 United States Patent (19) Crump 11 Patent Number: Date of Patent: Apr. 3, 1990 54 ADJUSTABLE HEIGHT WHEELCHAIR RAMP WITHSUPPORTING LEGS 76 Inventor: 21 22 (51) (52 58 (56) Robert Crump, 333 Guthrie Rd.,
United States Patent (19) Crump 11 Patent Number: Date of Patent: Apr. 3, 1990 54 ADJUSTABLE HEIGHT WHEELCHAIR RAMP WITHSUPPORTING LEGS 76 Inventor: 21 22 (51) (52 58 (56) Robert Crump, 333 Guthrie Rd.,
United States Patent (19) Salandre
 United States Patent (19) Salandre 11) Patent Number: 45) Date of Patent: Jun. 19, 1990 (54 HANDGUN HOLSTER AND RETENTION APPARATUS 76 Inventor: Stephen M. Salandre, P.O. Box 130, Taos, N. Mex. 87571 (21)
United States Patent (19) Salandre 11) Patent Number: 45) Date of Patent: Jun. 19, 1990 (54 HANDGUN HOLSTER AND RETENTION APPARATUS 76 Inventor: Stephen M. Salandre, P.O. Box 130, Taos, N. Mex. 87571 (21)
COMBINED SCIENCE 0653/6 CO-ORDINATED SCIENCES 0654/6
 Centre Number Candidate Number Candidate Name International General Certificate of Secondary Education UNIVERSITY OF CAMBRIDGE LOCAL EXAMINATIONS SYNDICATE COMBINED SCIENCE 0653/6 CO-ORDINATED SCIENCES
Centre Number Candidate Number Candidate Name International General Certificate of Secondary Education UNIVERSITY OF CAMBRIDGE LOCAL EXAMINATIONS SYNDICATE COMBINED SCIENCE 0653/6 CO-ORDINATED SCIENCES
R. GUII, PROCESS OF BOTTLING BEER, APPLICATION FILED APR, 5, 1907, Patented Nov. 2, , SHEETS-SHEET 1. Z27, Z?a a.
 938,577. R. GUII, PROCESS OF BOTTLING BEER, APPLICATION FILED APR, 5, 1907, Z27, Z?a 236 2.42 a.2) Patented Nov. 2, 1909. 3 SHEETS-SHEET 1. 46é 938,577. R, GULL. PROCESS OF BOTTLING BEER, APPLICATION FILED
938,577. R. GUII, PROCESS OF BOTTLING BEER, APPLICATION FILED APR, 5, 1907, Z27, Z?a 236 2.42 a.2) Patented Nov. 2, 1909. 3 SHEETS-SHEET 1. 46é 938,577. R, GULL. PROCESS OF BOTTLING BEER, APPLICATION FILED
Solubility And Temperature Answers
 Temperature Answers Free PDF ebook Download: Temperature Answers Download or Read Online ebook solubility and temperature answers in PDF Format From The Best User Guide Database Using this graph, the solubility
Temperature Answers Free PDF ebook Download: Temperature Answers Download or Read Online ebook solubility and temperature answers in PDF Format From The Best User Guide Database Using this graph, the solubility
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States US 20090235422A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0235422 A1 Lueking (43) Pub. Date: (54) APPARATUS AND METHODS FOR HOLDING Publication Classification SHN GUARDS
(19) United States US 20090235422A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0235422 A1 Lueking (43) Pub. Date: (54) APPARATUS AND METHODS FOR HOLDING Publication Classification SHN GUARDS
March 13, Filed July l, T. A. FOX 3,024,679 SKIN PASS MILLS AND METHOD OF ROLLING 3 Sheets-Sheet. NWeNTOr Thomas A.
 March 13, 1962 Filed July l, 1957 T. A. FOX 3,024,679 SKIN PASS MILLS AND METHOD OF ROLLING 3 Sheets-Sheet Sh NWeNTOr Thomas A. Fox March 13, 1962 T. A. FOX 3,024,679 SKIN PASS MILLS AND METHOD OF ROLLING
March 13, 1962 Filed July l, 1957 T. A. FOX 3,024,679 SKIN PASS MILLS AND METHOD OF ROLLING 3 Sheets-Sheet Sh NWeNTOr Thomas A. Fox March 13, 1962 T. A. FOX 3,024,679 SKIN PASS MILLS AND METHOD OF ROLLING
11 May, 2017 Moderated by Bryan Crist Agilent Technologies
 11 May, 2017 Moderated by Bryan Crist Agilent Technologies 1 Welcome to the DDG Online Meeting This is our 26 th session Second meeting in 2017; now in our 7 th year of consecutive meetings Worldwide users
11 May, 2017 Moderated by Bryan Crist Agilent Technologies 1 Welcome to the DDG Online Meeting This is our 26 th session Second meeting in 2017; now in our 7 th year of consecutive meetings Worldwide users
United States Patent (19) Häberle
 United States Patent (19) Häberle 11 Patent Number: 45 Date of Patent: 4,856,119 Aug. 15, 1989 (54) HELMET WITH THREE-POINT CHIN STRAP 75 Inventor: Hermann Hiberle, Ulm-Unterweiler, Fed. Rep. of Germany
United States Patent (19) Häberle 11 Patent Number: 45 Date of Patent: 4,856,119 Aug. 15, 1989 (54) HELMET WITH THREE-POINT CHIN STRAP 75 Inventor: Hermann Hiberle, Ulm-Unterweiler, Fed. Rep. of Germany
(12) United States Patent
 (12) United States Patent WOf USOO6273279B1 (10) Patent No.: (45) Date of Patent: Aug. 14, 2001 (54) GOLF TOWEL HOLDER (76) Inventor: Jerrold M. Wolf, 1036 E. Melody La., Fullerton, CA (US) 92831 (*) Notice:
(12) United States Patent WOf USOO6273279B1 (10) Patent No.: (45) Date of Patent: Aug. 14, 2001 (54) GOLF TOWEL HOLDER (76) Inventor: Jerrold M. Wolf, 1036 E. Melody La., Fullerton, CA (US) 92831 (*) Notice:
Egg...","...7. Primary Examiner-Dean Kramer
 USOO5513884A United States Patent 19 11 Patent Number: Bucher 45) Date of Patent: May 7, 1996 54) GOLF BALL RETRIEVING DEVICE 3,520,569 7/1970 Anderson... 2.94/19.2 3,659,891 5/1972 Pettenon et al....
USOO5513884A United States Patent 19 11 Patent Number: Bucher 45) Date of Patent: May 7, 1996 54) GOLF BALL RETRIEVING DEVICE 3,520,569 7/1970 Anderson... 2.94/19.2 3,659,891 5/1972 Pettenon et al....
Feb. 6, 1968 T. D. GASS 3,367,062
 Feb. 6, 1968 T. D. GASS LURE LADDER FOR A FISHING T ACKLE BOX Filed April 7, 1966 3. Sheets-Sheet l INVENTOR. 7A7AOAO4a A. 62/.456 1772aMafy-s. Feb. 6, 1968 Filed April 7, 1966 T. D. GASS LURE LA))ER FOR
Feb. 6, 1968 T. D. GASS LURE LADDER FOR A FISHING T ACKLE BOX Filed April 7, 1966 3. Sheets-Sheet l INVENTOR. 7A7AOAO4a A. 62/.456 1772aMafy-s. Feb. 6, 1968 Filed April 7, 1966 T. D. GASS LURE LA))ER FOR
Name: SoluDent 30 Concentrate Developer Product Number: , Product Use: Photographic Developer
 SAFETY DATA SHEET Section 1: Product and Company Identity Name: SoluDent 30 Concentrate Developer Product Number: 240-79,240-76 Product Use: Photographic Developer Distributed By: Henry Schein Telephone:
SAFETY DATA SHEET Section 1: Product and Company Identity Name: SoluDent 30 Concentrate Developer Product Number: 240-79,240-76 Product Use: Photographic Developer Distributed By: Henry Schein Telephone:
Nov. 27, H. N. BARNUM 1,982,491
 Nov. 27, 1934. H. N. BARNUM 1,982,491 TOY BASKET BALL GAME Filed March 2, 1933. 2. Sheets-Sheet l Nov. 27, 1934. H. N. BARNUM 1,982,491 TOY BASKET BALL GAME Filed March 2, 1933 2. Sheets-Sheet 2 NWeNor
Nov. 27, 1934. H. N. BARNUM 1,982,491 TOY BASKET BALL GAME Filed March 2, 1933. 2. Sheets-Sheet l Nov. 27, 1934. H. N. BARNUM 1,982,491 TOY BASKET BALL GAME Filed March 2, 1933 2. Sheets-Sheet 2 NWeNor
United States Patent (19) Miettinen
 United States Patent (19) Miettinen (54) (75) (73) 21 22 (51) (52) (58) (56) BOWLING BALL INCLUDING A MEANS FOR DISPLACING THE CENTER OF GRAVITY Inventor: Seppo I. Miettinen, Helsinki, Finland Assignee:
United States Patent (19) Miettinen (54) (75) (73) 21 22 (51) (52) (58) (56) BOWLING BALL INCLUDING A MEANS FOR DISPLACING THE CENTER OF GRAVITY Inventor: Seppo I. Miettinen, Helsinki, Finland Assignee:
April 17, ,666, Sheets-Sheet. B. N. WALIS AIRSHIP Filed May 29, 1925
 April 17, 1928. B. N. WALIS AIRSHIP Filed May 29, 1925 2 Sheets-Sheet April 17, 1928. B, N, WALIS AIRSHIP Filed May 29, 1925 fm/aav7oa a 21. Maeela a 77OAAWAYS 0 Patented Apr. 17, 1928. UNITED STATES PATENT
April 17, 1928. B. N. WALIS AIRSHIP Filed May 29, 1925 2 Sheets-Sheet April 17, 1928. B, N, WALIS AIRSHIP Filed May 29, 1925 fm/aav7oa a 21. Maeela a 77OAAWAYS 0 Patented Apr. 17, 1928. UNITED STATES PATENT
Aug. 5, 1952 T. G. BENNETT 2,605,917 HOISTING AND DUMPING APPARATUS FOR OYSTER DREDGES OR THE LIKE. Sne for Cattorneys
 Aug. 5, 1952 T. G. BENNETT 2,605,917 HOISTING AND DUMPING APPARATUS FOR OYSTER DREDGES OR THE LIKE Filed July 26, 1950 3 Sheets-Sheet Sne for 42-4-42-42 Cattorneys Aug. 5, 1952 T, G, BENNETT 2,605,917
Aug. 5, 1952 T. G. BENNETT 2,605,917 HOISTING AND DUMPING APPARATUS FOR OYSTER DREDGES OR THE LIKE Filed July 26, 1950 3 Sheets-Sheet Sne for 42-4-42-42 Cattorneys Aug. 5, 1952 T, G, BENNETT 2,605,917
United States Patent (19)
 United States Patent (19) Segerson et al. 11) E 45 Reissued Re. 30,877 Mar. 9, 1982 54) MECHANISM FOR RELEASABLY ATTACHING AN OBJECT TO A KITE 76 Inventors: James M. Segerson, 6871 Meadowbrook, Olive Branch,
United States Patent (19) Segerson et al. 11) E 45 Reissued Re. 30,877 Mar. 9, 1982 54) MECHANISM FOR RELEASABLY ATTACHING AN OBJECT TO A KITE 76 Inventors: James M. Segerson, 6871 Meadowbrook, Olive Branch,
Surface Area: the smaller the particles, the the rate of solubility.
 1. What is a solution? 2. What are the parts of a solution? (Ex. Lemonade) a. Solute: Ex. b. Solvent: Ex. 3. What is Solubility? a. Soluble: b. Insoluble: 4. Factors that Affect the Rate of Solubility:
1. What is a solution? 2. What are the parts of a solution? (Ex. Lemonade) a. Solute: Ex. b. Solvent: Ex. 3. What is Solubility? a. Soluble: b. Insoluble: 4. Factors that Affect the Rate of Solubility:
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States US 20160355422A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0355422 A1 PARK et al. (43) Pub. Date: (54) BALLAST WATER TREATMENT SYSTEM Publication Classification BY USING
(19) United States US 20160355422A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0355422 A1 PARK et al. (43) Pub. Date: (54) BALLAST WATER TREATMENT SYSTEM Publication Classification BY USING
United States Patent (19) Colas
 United States Patent (19) Colas 4 PROCESS FOR THE PRESERVATION OF RAM SEMEN BY FREEZING (7) Inventor: Guy Colas, Tours, France 73) Assignee: Institut National de la Recherche Agronomique, Paris, France
United States Patent (19) Colas 4 PROCESS FOR THE PRESERVATION OF RAM SEMEN BY FREEZING (7) Inventor: Guy Colas, Tours, France 73) Assignee: Institut National de la Recherche Agronomique, Paris, France
Worksheet: Solubility
 1. According to your Reference Tables, which substance forms an unsaturated solution when 80 grams of the substance is dissolved in 100 grams of H 2 O at 10 C? (A) KI (B) KNO 3 (C) NaNO 3 (D) NaCl 2. The
1. According to your Reference Tables, which substance forms an unsaturated solution when 80 grams of the substance is dissolved in 100 grams of H 2 O at 10 C? (A) KI (B) KNO 3 (C) NaNO 3 (D) NaCl 2. The
7%averzazz az72eways. AZafaa 77/owa soy. /v/a/y7 Oa. July 7, 1964 W. A. THOMPSON 3,139,732 BOAT STABILIZING AND LIFTING DEVICE.
 July 7, 1964 W. A. THOMPSON Filed May 5, 1961 BOAT STABILIZING AND LIFTING DEVICE 2 Sheets-Sheet /v/a/y7 Oa AZafaa 77/owa soy 7%averzazz az72eways July 7, 1964 Filed May 5, l96l W. A. THOMPSON BOAT STABILIZING
July 7, 1964 W. A. THOMPSON Filed May 5, 1961 BOAT STABILIZING AND LIFTING DEVICE 2 Sheets-Sheet /v/a/y7 Oa AZafaa 77/owa soy 7%averzazz az72eways July 7, 1964 Filed May 5, l96l W. A. THOMPSON BOAT STABILIZING
4,886,274 Dec. 12, 1989
 United States Patent [191 Park [11] Patent Number: [45] Date of Patent; 4,886,274 Dec. 12, 1989 [54] PORTABLE PRACI ICING PUII ING GREEN [76] Inventor: Young Go Park, Apartment #KA-305 Dong-A Apartments,
United States Patent [191 Park [11] Patent Number: [45] Date of Patent; 4,886,274 Dec. 12, 1989 [54] PORTABLE PRACI ICING PUII ING GREEN [76] Inventor: Young Go Park, Apartment #KA-305 Dong-A Apartments,
(51) Int. Cl... F04B 25/00 about 250 F., as will not allow the partial pressure of the
 USOO58860A United States Patent (19) 11 Patent Number: Cunkelman et al. () Date of Patent: Mar 23, 1999 54) THERMOSTATICALLY CONTROLLED 4,362.462 12/1982 Blotenberg... 417/243 X INTERCOOLER SYSTEM FOR
USOO58860A United States Patent (19) 11 Patent Number: Cunkelman et al. () Date of Patent: Mar 23, 1999 54) THERMOSTATICALLY CONTROLLED 4,362.462 12/1982 Blotenberg... 417/243 X INTERCOOLER SYSTEM FOR
United States Patent (19) HOath
 United States Patent (19) HOath 54) TRACER GAS LEAK DETECTION WITH GROSS LEAK DETECTION BY MEASURING DIFFERENTIAL PRESSURE 75 Inventor: Stephen D. Hoath, Eastbourne, England 73 Assignee: The BOC Group
United States Patent (19) HOath 54) TRACER GAS LEAK DETECTION WITH GROSS LEAK DETECTION BY MEASURING DIFFERENTIAL PRESSURE 75 Inventor: Stephen D. Hoath, Eastbourne, England 73 Assignee: The BOC Group
Feb. 21, ,972,697. MOLECULAR BEAM APPARATUS OF THE MASER TyPE S. A. JOHNSON ETAL. Filed June 26, 1958 AEG/747OA NVENORS AORNEY
 Feb. 21, 1961 S. A. JOHNSON ETAL MOLECULAR BEAM APPARATUS OF THE MASER TyPE Filed June 26, 1958 2,972,697 AEG/747OA S R NVENORS 4? AORNEY United States Patent Office 2,972,697 Patented Feb. 21, 1961 2,972,697
Feb. 21, 1961 S. A. JOHNSON ETAL MOLECULAR BEAM APPARATUS OF THE MASER TyPE Filed June 26, 1958 2,972,697 AEG/747OA S R NVENORS 4? AORNEY United States Patent Office 2,972,697 Patented Feb. 21, 1961 2,972,697
TEPZZ 7 Z67A_T EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION
 (19) TEPZZ 7 Z67A_T (11) EP 2 711 067 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 26.03.14 Bulletin 14/13 (21) Application number: 12188.1 (1) Int Cl.: B01D 3/60 (06.01) B01D 3/92 (06.01)
(19) TEPZZ 7 Z67A_T (11) EP 2 711 067 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 26.03.14 Bulletin 14/13 (21) Application number: 12188.1 (1) Int Cl.: B01D 3/60 (06.01) B01D 3/92 (06.01)
US A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/ A1 Islam et al. (43) Pub. Date: May 22, 2008
 US 20080119574A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0119574 A1 Islam et al. (43) Pub. Date: May 22, 2008 (54) RECOVERY OF ORGANIC COMPOUNDS Related US. Application
US 20080119574A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0119574 A1 Islam et al. (43) Pub. Date: May 22, 2008 (54) RECOVERY OF ORGANIC COMPOUNDS Related US. Application
NIANGUA SNAPSHOT ISSUE Nutrients 2 Suspended Sediment 3 Salinity 4 Summary 5
 Niangua River Watershed Snapshot Volume 14 Number 2 The 125-mile long Niangua River meanders northward from the town of Marshfield on I-44 to the Lake of the Ozarks. Its watershed covers more than 658,000
Niangua River Watershed Snapshot Volume 14 Number 2 The 125-mile long Niangua River meanders northward from the town of Marshfield on I-44 to the Lake of the Ozarks. Its watershed covers more than 658,000
Getting the most out of milk. Alfa Laval Foodec decanter centrifuges for casein and lactose processing
 Getting the most out of milk Alfa Laval s for casein and lactose Hygiene makes the difference Casein and lactose are two important milk by-products with considerable market potential if they can be processed
Getting the most out of milk Alfa Laval s for casein and lactose Hygiene makes the difference Casein and lactose are two important milk by-products with considerable market potential if they can be processed
For Establishing and Maintaining Your Spa using PROtech Spa Chemicals
 Spa Care For Establishing and Maintaining Your Spa using PROtech Spa Chemicals QUICK START Add water up to the level recommended by the manufacturer of your spa and let the pump circulate for at least
Spa Care For Establishing and Maintaining Your Spa using PROtech Spa Chemicals QUICK START Add water up to the level recommended by the manufacturer of your spa and let the pump circulate for at least
3 Solubility and Concentration
 CHAPTER 8 SECTION Solutions 3 Solubility and Concentration KEY IDEAS As you read this section, keep these questions in mind: What is solubility? What happens when you add more solute to a saturated solution?
CHAPTER 8 SECTION Solutions 3 Solubility and Concentration KEY IDEAS As you read this section, keep these questions in mind: What is solubility? What happens when you add more solute to a saturated solution?
United States Patent 19
 USOO5595174A 11 Patent Number: 5,595,174 Gwaltney 45 Date of Patent: Jan. 21, 1997 United States Patent 19 (54) NASALADAPTOR, MASK, AND METHOD 4,478,215 10/1984 Hanlon... 128/2013 4,614,186 971986 John...
USOO5595174A 11 Patent Number: 5,595,174 Gwaltney 45 Date of Patent: Jan. 21, 1997 United States Patent 19 (54) NASALADAPTOR, MASK, AND METHOD 4,478,215 10/1984 Hanlon... 128/2013 4,614,186 971986 John...
(12) United States Patent (10) Patent No.: US 6,253,575 B1. Chludzinski (45) Date of Patent: Jul. 3, 2001
 USOO6253575B1 (12) United States Patent (10) Patent No.: US 6,253,575 B1 Chludzinski (45) Date of Patent: Jul. 3, 2001 (54) HELIUM RECYCLING FOR OPTICAL FIBER 4,863,501 9/1989 Mansfield... 65/416 MANUFACTURING
USOO6253575B1 (12) United States Patent (10) Patent No.: US 6,253,575 B1 Chludzinski (45) Date of Patent: Jul. 3, 2001 (54) HELIUM RECYCLING FOR OPTICAL FIBER 4,863,501 9/1989 Mansfield... 65/416 MANUFACTURING
Well Testing Plan. New York Marginal Well Study. Introduction. I. Tests to be Conducted. II. Testing Procedures
 Well Testing Plan New York Marginal Well Study Introduction The Independent Oil and Gas Association of New York, Inc. (IOGANY) has been awarded funding for a Marginal Well Testing Program from the New
Well Testing Plan New York Marginal Well Study Introduction The Independent Oil and Gas Association of New York, Inc. (IOGANY) has been awarded funding for a Marginal Well Testing Program from the New
Svensk läkemedelsstandard
 Svensk läkemedelsstandard 2018.2 Nitrogen Postadress/Postal address: P.O. Box 26, SE-751 03 Uppsala, SWEDEN Besöksadress/Visiting address: Dag Hammarskjölds väg 42, Uppsala Telefon/Phone: +46 (0)18 17
Svensk läkemedelsstandard 2018.2 Nitrogen Postadress/Postal address: P.O. Box 26, SE-751 03 Uppsala, SWEDEN Besöksadress/Visiting address: Dag Hammarskjölds väg 42, Uppsala Telefon/Phone: +46 (0)18 17
Device for atomizing a liquid
 Page 1 of 6 Device for atomizing a liquid Abstract ( 5 of 5 ) United States Patent 4,504,014 Leuning March 12, 1985 A device (10) for atomizing a liquid is disclosed. The device (10) includes a sprayhead
Page 1 of 6 Device for atomizing a liquid Abstract ( 5 of 5 ) United States Patent 4,504,014 Leuning March 12, 1985 A device (10) for atomizing a liquid is disclosed. The device (10) includes a sprayhead
3,505,188. April 7, 1970 PEI-TA PAN ELECTROLYC FOTATION METHOD AND APPARATUS. Filed June 8, Sheets-Sheet. ?aezaezzzzzzzzzz N S SSSSSSSSS T.I.
 April 7, 19 PEI-TA PAN ELECTROLYC FOTATION METHOD AND APPARATUS Filed June 8, 1965 2. Sheets-Sheet N Z2 22 MY A. S?aezaezzzzzzzzzz SM SSSSSSSSS T.I. April 7, 19 PEI-TA PAN ELECTROLYTIC FLOTATION METHOD
April 7, 19 PEI-TA PAN ELECTROLYC FOTATION METHOD AND APPARATUS Filed June 8, 1965 2. Sheets-Sheet N Z2 22 MY A. S?aezaezzzzzzzzzz SM SSSSSSSSS T.I. April 7, 19 PEI-TA PAN ELECTROLYTIC FLOTATION METHOD
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States US 20080072365A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0072365A1 Alberto (43) Pub. Date: Mar. 27, 2008 (54) SPACE-SAVING SCUBA DIVING MASK (75) Inventor: Carlos
(19) United States US 20080072365A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0072365A1 Alberto (43) Pub. Date: Mar. 27, 2008 (54) SPACE-SAVING SCUBA DIVING MASK (75) Inventor: Carlos
Potter Nitrogen Generators
 Potter Nitrogen Generators What is nitrogen? Nitrogen the inert gas Earth s atmosphere the air we breathe is composed of nitrogen, oxygen, and water vapor. While this atmosphere is great for life, it is
Potter Nitrogen Generators What is nitrogen? Nitrogen the inert gas Earth s atmosphere the air we breathe is composed of nitrogen, oxygen, and water vapor. While this atmosphere is great for life, it is
United States Patent (19)
 United States Patent (19) Connolly 54 METHOD OF PRODUCING MILK PROTEIN ISOLATES AND MILK PROTEIN/VEGETABLE PROTEIN ISOLATES AND COMPOSITIONS OF SAME (75) Inventor: Philip B. Connolly, Sebastopol, Calif.
United States Patent (19) Connolly 54 METHOD OF PRODUCING MILK PROTEIN ISOLATES AND MILK PROTEIN/VEGETABLE PROTEIN ISOLATES AND COMPOSITIONS OF SAME (75) Inventor: Philip B. Connolly, Sebastopol, Calif.
User Manual. MagnaMaster and ChloroMaster Electrolytic Chlorinator.
 User Manual MagnaMaster and ChloroMaster Electrolytic Chlorinator www.directpoolsupplies.com.au Contents Introducing the MagnaMaster and ChloroMaster 1 How it Works 1 Installation Guide 1 Initial Pool
User Manual MagnaMaster and ChloroMaster Electrolytic Chlorinator www.directpoolsupplies.com.au Contents Introducing the MagnaMaster and ChloroMaster 1 How it Works 1 Installation Guide 1 Initial Pool
Ingredient CAS Number Weight % ACGIH TLV PEL STEL Hydrochloric acid % %
 MSDS Document Page 1 of 5 Product 1. Chemical Product and Company Identification Trade Name of this Product Manufacturer Haviland Products Company 421 Ann Street N.W. Grand Rapids, MI 49504 Phone Number
MSDS Document Page 1 of 5 Product 1. Chemical Product and Company Identification Trade Name of this Product Manufacturer Haviland Products Company 421 Ann Street N.W. Grand Rapids, MI 49504 Phone Number
(12) United States Patent (10) Patent No.: US 7,730,548 B1
 USOO77548B1 (12) United States Patent () Patent No.: US 7,7,548 B1 McCraney (45) Date of Patent: Jun. 8, 20 (54) BALLISTICSVEST PAD COVER 5,127,5 A 7, 1992 Sacks 5,471,906 A 12/1995 Bachner, Jr. et al.
USOO77548B1 (12) United States Patent () Patent No.: US 7,7,548 B1 McCraney (45) Date of Patent: Jun. 8, 20 (54) BALLISTICSVEST PAD COVER 5,127,5 A 7, 1992 Sacks 5,471,906 A 12/1995 Bachner, Jr. et al.
United States Patent (19) Mills
 United States Patent (19) Mills 54 75 73 21 22 51 (52) 58 56 PRESSURE ROLLER FUSER WITH COPY WRNKLE CONTROL Inventor: Assignee: Appl. No.: 170,629 Borden H. Mills, Webster, N.Y. Eastman Kodak Company,
United States Patent (19) Mills 54 75 73 21 22 51 (52) 58 56 PRESSURE ROLLER FUSER WITH COPY WRNKLE CONTROL Inventor: Assignee: Appl. No.: 170,629 Borden H. Mills, Webster, N.Y. Eastman Kodak Company,
MM2. Fig. l. .oœua. Dec. 17, 1957 H. E. MUELLER HEINZ E. MUELLER. ATT RNEYsY PROPELLANT DISPLACEMENT GAS GENERATORS
 Dec. 17, 1957 H. E. MUELLER 25816 419 PROPELLANT DISPLACEMENT GAS GENERATORS Filed March 7, 1952 2 Sheets-Sheet 1 Fig. l. IIIIIIIIIIIIIIIIIIIIIIIIIIII. O MM2 HEINZ E. MUELLER. BY.oœua ATT RNEYsY Dec. 17,
Dec. 17, 1957 H. E. MUELLER 25816 419 PROPELLANT DISPLACEMENT GAS GENERATORS Filed March 7, 1952 2 Sheets-Sheet 1 Fig. l. IIIIIIIIIIIIIIIIIIIIIIIIIIII. O MM2 HEINZ E. MUELLER. BY.oœua ATT RNEYsY Dec. 17,
(12) United States Patent
 USOO7690411 B2 (12) United States Patent Wilson (10) Patent No.: (45) Date of Patent: US 7.690,411 B2 Apr. 6, 2010 (54) TIRE PRESSURE CONTROL SYSTEM (76) Inventor: Seth Wilson, 1218 Puerta Del Sol, San
USOO7690411 B2 (12) United States Patent Wilson (10) Patent No.: (45) Date of Patent: US 7.690,411 B2 Apr. 6, 2010 (54) TIRE PRESSURE CONTROL SYSTEM (76) Inventor: Seth Wilson, 1218 Puerta Del Sol, San
CHAPTER 5 : THE AIR AROUND US
 CHAPTER 5 : THE AIR AROUND US The Composition of Air Air is a mixture consist of Nitrogen 78% Oxygen 21% Carbon dioxide 0.03% Inert gases 0.97% Water vapour Microorganism Dust The percentage of the constituents
CHAPTER 5 : THE AIR AROUND US The Composition of Air Air is a mixture consist of Nitrogen 78% Oxygen 21% Carbon dioxide 0.03% Inert gases 0.97% Water vapour Microorganism Dust The percentage of the constituents
Svensk läkemedelsstandard
 Svensk läkemedelsstandard 2013.2 Air, Medicinal Postadress/Postal address: P.O. Box 26, SE-751 03 Uppsala, SWEDEN Besöksadress/Visiting address: Dag Hammarskjölds väg 42, Uppsala Telefon/Phone: +46 (0)18
Svensk läkemedelsstandard 2013.2 Air, Medicinal Postadress/Postal address: P.O. Box 26, SE-751 03 Uppsala, SWEDEN Besöksadress/Visiting address: Dag Hammarskjölds väg 42, Uppsala Telefon/Phone: +46 (0)18
DISSOLVING AND SOLUBILITY
 DISSOLVING AND SOLUBILITY QUICK REVIEW: Homogeneous mixtures: a solution of substances in which no settling occurs (looks like one thing). A solution occurs when the particles of the components slip in
DISSOLVING AND SOLUBILITY QUICK REVIEW: Homogeneous mixtures: a solution of substances in which no settling occurs (looks like one thing). A solution occurs when the particles of the components slip in
800.Keen.Gas
 C 2 H 2 ACETYLENE Acetylene is a colorless and tasteless gas with a garlic-like odor. It is flammable and can be an asphyxiant. In order to prevent explosive decomposition, acetylene is supplied in cylinders
C 2 H 2 ACETYLENE Acetylene is a colorless and tasteless gas with a garlic-like odor. It is flammable and can be an asphyxiant. In order to prevent explosive decomposition, acetylene is supplied in cylinders
