Disclosure OXYGEN BASICS. Objec ves 8/15/14 BUT IN REALITY IT WILL SUFFICE IF YOU TAKE HOME 3 KEY MESSAGES
|
|
|
- Luke Lewis
- 5 years ago
- Views:
Transcription
1 Disclosure OXYGEN BASICS UTHSCSA No other financial or commercial affiliaons Jesus R. Guajardo, MD, MHPE, PhD Objecves This is a entry level lecture about some key points about the clinical use of oxygen Appropriate for MS, residents, RTs, and health care personnel who provide oxygen to end of this talk, the listener should be able to Define Respiraon Describe the transport of O2 in the human body List several variables that may affect the amount of oxygen available for respiraon To get a general understanding of the variables, not to learn all the formulas and lile details (that will happen during the pulmo rotaon) BUT IN REALITY IT WILL SUFFICE IF YOU TAKE HOME 3 KEY MESSAGES FOCUSING SCENARIOS FIRST ONE 1
2 Normal ABG ph: ( ) pco2: 40 mmhg (35-45 mmhg) po2: 100 mmhg ( mmhg) HCO3: 24 meq/l (21-28 meq/l) B.E. - 2 to +2 meq/l Now Imagine You arrive to the intensive care unit and have a paent with narcoc overdose recently placed on a venlator with good breaths and you are giving FiO2 100% O2, sats are at 100% ABG is ph: 7.40 CO2: 40 mmhg PaO2: 250 mmhg HCO3: 26 R these values OK? Make a Wish SECOND SCENARIO You have a paent with a bad lung condion (CF, fibrosis, cancer, etc) and the paent is on the ward on 4 lpm O2 NC and 90%. Stable for the last few weeks. Final wish is go to Hawaii and bite the dust there. Expected demise in 2-3 months. Has plane ckets to Hawaii for next week (SAàHouàIsland). Make a Wish Any recommendaons for the trip? a. Bring a souvenir? b. Call the airline to make sure she takes a commercial flight- approved oxygen device? c. Galveston not good enough? d. Exchange the plane ckets for cruise ship ckets? THIRD SCENARIO 2
3 JOY RIDE JOY RIDE You and your friends are going for a balloon ride. You want to go as high as you can. For this, you prepare with an oxygen tank and a non- rebreather mask (also with good clothes and just in case, a parachute) Is this enough for your oxygen requirements? Oxygen Tubing FOURTH SCENARIO You have a paent at home with a large oxygen tank. He is takes 3 lpm per NC He likes to piggyback the oxygen tubing, so he can walk all around the house without dragging the tank. He has 300 feet of tubing Is that bad?? Know this Guy? FIFTH SCENARIO 3
4 Oxygen We all seen these hook ups for oxygen. When you turn the knob What comes out: LAST SCENARIO a. 100 % O2 at the dialed lpm? b. 21% O2 that needs to go through a concentrator? c. O2 % depends on the device aached at the end of hose d. O2 % depends on the number dialed e. Compressed air that needs to be brought up to 100% O2 Areas to be Reviewed LETS START WITH BASIC CONCEPTS Atmosphere The Hook- ups No definite boundary with outer space Most of it (75%) contained on the first miles For praccal purposes, all over the world: 21% O2 & 79% N2. So, room air (RA) = 21% O2. If one is breathing RA, then FiO2 = 21%. 4
5 The Hook- ups What comes out when you turn open the knob of the green hook up (O2)? 100% O2 at the number of lpm indicated by the lile ball Clinical Applicaon Easy Queson: How much oxygen is being given? Easy answer: As many lpm as the valve indicates And for these devices? the % dialed the # lpm dialed Not as easy Queson: What s the FiO2 for these paents? The answer requires some math, a table or chart, experience, or amazing guessing. Let s take a very quick view. Pt s Wt % O2 Flow O2 2 lpm Pt wt = 70 kg TV = 500 cc RR = 12 I:E 1:2 1 s ins, 2 s exh 1 s 500 cc inhaled NC 2lpm=2kcc60s =2k/60à ~35cc/sec TV 500 cc 21%O2=105cc 79%N2=395cc 105cc O2TV + 35cc O2NC 140 cc of O2 Out of 500 = 28% ~ 0.28 Assumpons Simple Chart for Adults when using NC: O2 2 lpm Pt wt = 2 kg TV = 15 cc RR = 40 I:E 1:2 0.5 s ins, 1 s exh 0.5 s 15cc inhaled NC 2lpm=2kcc60s =2k/60à ~35cc/sec (17.5 per 0.5 sec) TV 15 cc 21%O2=3.15cc 79%N2=11.85cc 3.15cc O2TV cc O2NC 21 cc of O2 Out of 15 = high % ~ High Flow Lpm Charts are a lile more complex for Infants Oxygen Devices COMMON OXYGEN DEVICES 5
6 Short Summary O2 concentraon in the atmosphere is 21% O2 at room air (RA) is 21% FiO2 is the inspired fracon of O2 in the RA, FiO2 = RA = 21% For adults and as a general rule, NC increases 3% FiO2 for each lpm, but, for the pediatric populaon this is not the case There are many oxygen delivery devices; keep in mind the characteriscs of the most common ones O2 Transport We have reviewed O2 %, RA, FiO2, and oxygen delivery devices Now lets review other terms: atmospheric pressure, PAO2, PaO2, and hemoglobin dissociaon curve Atmospheric Pressure Commercial Plane Traveling Atmospheric Pressure Atmospheric pressure hp://usatoday30.usatoday.com/news/naon/ oxygen.htm 1 mol of a gas occupy 22.4 liters at STP (Standard Temp = 0 C Pressure: 760 mmhg) Oxygen atomic weight: ~16. O2=32 Daltons 1 mol of O2 = 32 gm. 1 kg = 700 liters gm per liter of O2 (at 760 and O⁰ C) Ideal gas equaon by Emile Clapeyron (1834) combining Boyle s and Charles laws V=nRT/P How do we use these values clinically? At body temperature ( = 311 K), one liter of O2 will have Alt (m) kpa mmhg Atm Moles Gms Rel% Sea Level Denver Cabin Pressure Mexico City MachuPicchu Mt Olympus Mt Everest Mars (Planet) Humidificaon H20 50 mmhg Alveolar Equaon Lungs [O2] Atm Sea level ~ 760 mmhg Room Air 21% 02 (160 mmhg) 79% Nitrogen (600 mmhg) 760 mmhg at mouth minus 50 when humidified mes the FiO2 à 150 Minus CO2 à 100 mmhg How much O2 in the alveoli? PAO2= (FiO2 * (AtmPress H20) ) - PCO2/RQ PAO2= 100 mmhg (80-100) or ~13% (down from 21%) CO2 coming out at mmhg PA The alveolar gas equaon helps esmate the PAO2 and from there we can esmate the arterial pressure as it should be within 5-10 mmhg at RA 100% FiO2)? Formula: (FiO2 * (AtmPress H20) ) minus PCO2/RQ Normal: ( 0.21 * ( ) ) minus 40 /0.8 à ~ 100 mmhg Then the PaO2 should be mmhg at sea level (760 mmhg) And how is this related to hemoglobin saturaon? Pa 6
7 Sea Level = ~ 100 mmhg (0.21*(763-50)) (40/.08) Denver = ~ 72 mmhg (0.21*(630-50)) (40/.08) Mt Everest < 30 mmhg (0.21*(253-50)) (40/.08) On 100% FiO2 here in SA > 550 mmhg (1.00*(764-50)) (40/.08) Alveolar Pressures Keep in mind 30 mmhgà 50% & 60 mmhgà90% Worked Case Make a Wish: Hawaii Scenario: Pt on 4 lpm O2 NC with 90% sats Assumpons: TV 500 cc in 1 s Insp. FiO2? 4 lpm is 4000/60 is ~ 67 cc/sec. 500cc is 105 cc of O2 (21%) +395 ccn2. 105cc (21%) + 67cc 4 lpm)= cc O2 of 500 is around 35% O2. FiO2=0.35 Alveolar Equaon PA= [FiO2(AP- H2O)] (CO2/RQ) PA= 0.35 (760-50) 40/0.8 à ~ 200 mmhg Assumpon from the Alveolar Equaon PA= 0.35 (760-50) 40/0.8 à ~ 200 mmhg Assumpon from the Hbg dissociaon 90% there should be ~ 60 mmhg PaO2 There seems to be an A- a gradient of 140 mmhg (200-60). Therefore we need to keep the same PA while the atmospheric pressure is reduced in the plane (lets assume 550 (to be safe, as 565 mmhg is the minimum by FAA). Alveolar Equaon: PA= [FiO2(AP- H2O)] (CO2/RQ) 200= FiO2 (550-50) 40/0.8 à Solving for FiO2 à = 0.5 So the paent needs, at least 50% O2 50% O2? How? Tough! You will need several big tanks. NC won t do it A Paral rebreather mask will do Try to get that by the FAA and United, American, etc. Good Luck!!!! Length of Tubing Remember the Hagen- Poiseuille (pwaa- zuh- ee) Law/ Equaon ΔP= (8nlV)/(πr4) ΔP à Pressure difference n à Viscosity l à length of pipe V à flow rate r à radius of pipe Hagen- Poiseuille ΔP= (8nlV)/(πr4) Then length maers But for praccal purposes, if the tubing is less than 100, things should be OK 7
8 100% O2? ANOTHER CASE You arrive to the intensive care unit and have a paent with narcoc overdose recently placed on a venlator with good breaths and you are giving FiO2 100% O2, sats are at 100% ABG: ph: 7.40 CO2: 40 PaO2: 250 mmhg HCO3: 26 Is this OK? 100% O2 According to the alveolar gas equaon, somebody on 100% O2 should have a PaO2 of à (1*(763-50) ) - (40/0.8) à ~660 mmhg Above 550 should be OK And the PA should closely match the Pa ( mmhg difference OK at high O2 concentraons) If your paent s PaO2 is 250, then there is severe lung disease This the main use of the alveolar equaon To predict & assess pts in different clinical condions During illness And during acvity, such as climbing or space acvies Predicons 1875 Gaston Tissandier, Joseph Croce- Spinelli and Théodore Sive. Set to break the altude record 8,600 mts ( ~270 mmhg) Joe and Theo died Gaston with mental damage What was the predicted PaO2 and sats at that altude? PA= (0.21*(270-50)) (40/0.8) PA = Neg #! Compensaon by hypervenlaon PA= (0.21*(270-50)) (7/0.8) PA= on the 30s! Sats: ~ 50% The Jump Mt Everest 2012 Felix Baumgartner 38k mts (24 miles) Broke speed of sound Suit pressurized at 3.5 psi 3.5 psi = 180 mmhg Alveolar Equaon (1*(180-50) ) 50 ) = PA= 80 Sats on the high 90s. That s good for decision making and acvity 8
9 8,850 m (29 k ) 250 mm Hg PA= FiO2*(Atm- H20) (CO2/RQ) PA= 0.21 * (250 50) (40/0.8) PA= VERY LOW Compensaon: Hypervenlaon with respiratory alkalosis PA= 0.21 * (250 50) (7/0.8) PA=42 8 = 34 mmhg Mt Everest Short Summary Atmospheric pressure, along with FiO2 are two important components for hemoglobin saturaon PaO2 should be close to PAO2, if not, there is a problem somewhere (lungs? Heart? Other?) 30 mmhg à50% and 60 mmhg à 90% Paents with chronic lung disease who need oxygen (and others) should be assessed before traveling on planes or to high altude cies Bear in mind the variables of the alveolar equaon: atm pressure, FiO2, and CO2 Respiratory Variables CO 2 O 2 1 GASES (CO2 & O2) NEURO Control 2 LUNG ANATOMY VENTILATION MECHANICS 3 DIFFUSION INTO BLOOD Alveolar/Capillary Finally, and very briefly HEMOGLOBIN 4 GAS TRANSPORT IN BLOOD Hgb and Plasma CO 2 O 2 5 CELLULAR DIFFUSION OUTSIDE VIDEO SOURCE Hemoglobin 2α + 2β subunits Haldane: Tissues: CO2 pickup facilitates O2 release Lungs: O2 loading increases CO2 release Bohr: Tissues: O2 release facilitates H+ pickup Lungs: O2 pickup facilitates H+ release 2,3- DPG Hgb releases O2 from the 2β subunits first The, 2,3- DPG at ssues binds the 2β DPG binding changes the shape of Hgb This allosteric change promotes the release of O2 by the 2β subunits W/o DPG, Hbg at 50% efficiency (like myoglobin) Live high, train low High: + DPG, mitochondria, EPO, Hct Low: longer peak levels when training hps:// Oxygen Transport from Lungs to Cells 10/2013 hp://faculty.etsu.edu/currie/hemoglobin.htm 9
10 Hyperbaric Chamber 98% of O2 carried by Hgb (%Sat*1.34*Hgb) + (PaO2 *.003) Oxygen Content (CaO2) Arterial (97-100% sats) 100 * 1.34 * 15mg/dl = ml/dl 100 mmhg * = 0.3 ml/dl Venous CvO2 (60-80% sats) 80 * 1.34 * 15mg/dl = ml/dl 45 mmhg * = 0.14 ml/dl Oxygen Consumpon (VO2) Q * (CaO2- CvO2) à CaO2- CvO2à~ 5ml/dl * Cardiac Output The amount of oxygen needed could be supported in a hyperbaric chamber (2-6 atm) just with the oxygen that is diluted. No hgb needed! At 6 atm * 760 = 4,560 mmhg 4,560 * = ml/dl Short Summary Hgb is the major carried of O2 Allosteric effects: Haldane, Bohr, and 2,3 DPG Hyperbaric chambers can provide a great amount of dissolved O2 due to the increased pressure. At 3 atm * 760 = 2,280 mmhg 2,280 * = 7.07 ml/dl Acid- Base Nomograms FINALLY ABG Interpretaon Nomogram Use these lile graphs to figure out if your paent is in : Chronic or Acute Respiratory or Metabolic Acidosis or Alkalosis Final Summary AND THAT S IT! Now to the final summary 21% of O2 in the atmosphere But pressure varies and it affects the real amount of oxygen available The alveolar equaon is an important formula to develop clinical predicons There are many oxygen delivery devices, familiarize with them. Hemoglobin is affected by allosteric changes: Haldane, Bohr, and 2,3 DPG You may use an ABG nomogram while you get used to interpret gases 10
11 Take Home Points The larger the dal breath, the lower the FiO2 provided by NC. One lpm is not the same in a neonate, a child or an adult. If you have two paents on 100% FiO2 and the saturaon of both is 100%, that doesn t mean that the lungs are OK. Pa of 100 vs Pa of 500 mmhg Keep in mind the atmospheric pressure when taking decisions about O2 in planes or going to other cies. Use the alveolar equaon PAO2= (FiO2 * (AtmPress H20) ) - PCO2/RQ Thanks!! Oxygen Basics Jesus R. Guajardo, MD, MHPE, PhD 11
PICU Resident Self-Study Tutorial The Basic Physics of Oxygen Transport. I was told that there would be no math!
 Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
I Physical Principles of Gas Exchange
 Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
RC-178 a/a ratio. Better. PaO2 ACM than. a/a= PAO2. guessing!! Copyrights All rights reserved Louis M. Sinopoli
 RC-178 a/a ratio Better a/a= PaO2 ACM than PAO2 guessing!! 1 A relative RC-178 a/a ratio way to judge the lungs ability to transport O2. Determine new FIO2 to achieve PaO 2 amount that got through the:
RC-178 a/a ratio Better a/a= PaO2 ACM than PAO2 guessing!! 1 A relative RC-178 a/a ratio way to judge the lungs ability to transport O2. Determine new FIO2 to achieve PaO 2 amount that got through the:
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D.
 Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Alveolus and Respiratory Membrane
 Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Physiology Unit 4 RESPIRATORY PHYSIOLOGY
 Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
Respiratory physiology II.
 Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
Circulatory And Respiration
 Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Fysiologie van de ademhaling - gasuitwisseling
 What you will learn in this lecture... Lessenreeks co s 014-015 Fysiologie van de ademhaling - gasuitwisseling Professor Dr. Steffen Rex Department of Anesthesiology University Hospitals Leuven Department
What you will learn in this lecture... Lessenreeks co s 014-015 Fysiologie van de ademhaling - gasuitwisseling Professor Dr. Steffen Rex Department of Anesthesiology University Hospitals Leuven Department
Respiratory System Study Guide, Chapter 16
 Part I. Clinical Applications Name: Respiratory System Study Guide, Chapter 16 Lab Day/Time: 1. A person with ketoacidosis may hyperventilate. Explain why this occurs, and explain why this hyperventilation
Part I. Clinical Applications Name: Respiratory System Study Guide, Chapter 16 Lab Day/Time: 1. A person with ketoacidosis may hyperventilate. Explain why this occurs, and explain why this hyperventilation
Section Three Gas transport
 Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
Blood gas adventures at various altitudes. Friedrich Luft Experimental and Clinical Research Center, Berlin-Buch
 Blood gas adventures at various altitudes Friedrich Luft Experimental and Clinical Research Center, Berlin-Buch Mount Everest 8848 M Any point in bird watching here? Respiration is gas exchange: the process
Blood gas adventures at various altitudes Friedrich Luft Experimental and Clinical Research Center, Berlin-Buch Mount Everest 8848 M Any point in bird watching here? Respiration is gas exchange: the process
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1
 AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
Respiratory system & exercise. Dr. Rehab F Gwada
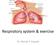 Respiratory system & exercise Dr. Rehab F Gwada Objectives of lecture Outline the major anatomical components & important functions of the respiratory system. Describe the mechanics of ventilation. List
Respiratory system & exercise Dr. Rehab F Gwada Objectives of lecture Outline the major anatomical components & important functions of the respiratory system. Describe the mechanics of ventilation. List
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing
 Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation
 Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
For more information about how to cite these materials visit
 Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Oxygen and Carbon dioxide Transport. Dr. Laila Al-Dokhi
 Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
Gases and Respiration. Respiration Overview I
 Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
HCO - 3 H 2 CO 3 CO 2 + H H H + Breathing rate is regulated by blood ph and C02. CO2 and Bicarbonate act as a ph Buffer in the blood
 Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
P215 Respiratory System, Part 2
 P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
Essential Skills Course Acute Care Module. Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook
 Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
After having worked out this lecture, you will be able to describe the oxygen cascade and to calculate the inspiratory and the alveolar partial
 1 After having worked out this lecture, you will be able to describe the oxygen cascade and to calculate the inspiratory and the alveolar partial pressure of oxygen. You will have understood the process
1 After having worked out this lecture, you will be able to describe the oxygen cascade and to calculate the inspiratory and the alveolar partial pressure of oxygen. You will have understood the process
Table of Contents. By Adam Hollingworth
 By Adam Hollingworth Table of Contents Oxygen Cascade... 2 Diffusion... 2 Laws of Diffusion... 2 Diffusion & Perfusion Limitations... 3 Oxygen Uptake Along Pulmon Capillary... 4 Measurement of Diffusing
By Adam Hollingworth Table of Contents Oxygen Cascade... 2 Diffusion... 2 Laws of Diffusion... 2 Diffusion & Perfusion Limitations... 3 Oxygen Uptake Along Pulmon Capillary... 4 Measurement of Diffusing
Respiratory System Physiology. Dr. Vedat Evren
 Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
CHAPTER 3: The cardio-respiratory system
 : The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
: The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
Gas Exchange in Animals. Uptake of O2 from environment and discharge of CO2. Respiratory medium! water for aquatic animals, air for terrestial
 Gas Exchange in Animals Uptake of O2 from environment and discharge of CO2 Respiratory medium! water for aquatic animals, air for terrestial Respiratory surface! skin, gills, lungs Circulatory System O2/CO2
Gas Exchange in Animals Uptake of O2 from environment and discharge of CO2 Respiratory medium! water for aquatic animals, air for terrestial Respiratory surface! skin, gills, lungs Circulatory System O2/CO2
Unit 14 Gas Laws Funsheets
 Name: Period: Unit 14 Gas Laws Funsheets Part A: Vocabulary and Concepts- Answer the following questions. Refer to your notes and the PowerPoint for help. 1. List 5 different common uses for gases: a.
Name: Period: Unit 14 Gas Laws Funsheets Part A: Vocabulary and Concepts- Answer the following questions. Refer to your notes and the PowerPoint for help. 1. List 5 different common uses for gases: a.
Respiration (revised 2006) Pulmonary Mechanics
 Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
PHTY 300 Wk 1 Lectures
 PHTY 300 Wk 1 Lectures Arterial Blood Gas Components The test provides information on - Acid base balance - Oxygenation - Hemoglobin levels - Electrolyte blood glucose, lactate, renal function When initially
PHTY 300 Wk 1 Lectures Arterial Blood Gas Components The test provides information on - Acid base balance - Oxygenation - Hemoglobin levels - Electrolyte blood glucose, lactate, renal function When initially
Respiration - Human 1
 Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
Section Two Diffusion of gases
 Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
RESPIRATORY GAS EXCHANGE
 RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
CHAPTER 3: The respiratory system
 CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
By: Aseel Jamil Al-twaijer. Lec : physical principles of gas exchange
 By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
Rodney Shandukani 14/03/2012
 Rodney Shandukani 14/03/2012 OXYGEN THERAPY Aerobic metabolism accounts for 90% of Oxygen consumption by tissues. generates ATP by oxidative phosphorylation. Oxygen cascade: Oxygen exerts a partial pressure,
Rodney Shandukani 14/03/2012 OXYGEN THERAPY Aerobic metabolism accounts for 90% of Oxygen consumption by tissues. generates ATP by oxidative phosphorylation. Oxygen cascade: Oxygen exerts a partial pressure,
Gas exchange. Tissue cells CO2 CO 2 O 2. Pulmonary capillary. Tissue capillaries
 Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
Yanal. Jumana Jihad. Jamil Nazzal. 0 P a g e
 2 Yanal Jumana Jihad Jamil Nazzal 0 P a g e note: this sheet was written and corrected according to the records from section 2 so you may find differences in the arrangement of topics from the records
2 Yanal Jumana Jihad Jamil Nazzal 0 P a g e note: this sheet was written and corrected according to the records from section 2 so you may find differences in the arrangement of topics from the records
Funsheet [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015
![Funsheet [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015 Funsheet [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015](/thumbs/93/112589015.jpg) Funsheet 7.0 7.1 [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015 1. Convert the following pressures. a) 101 kpa =? atm b) 55 Torr =? psi c) 60. mmhg =? bar d) 45 Torr =? kpa e) 5 psi =? atm f) 0.0056 atm =?
Funsheet 7.0 7.1 [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015 1. Convert the following pressures. a) 101 kpa =? atm b) 55 Torr =? psi c) 60. mmhg =? bar d) 45 Torr =? kpa e) 5 psi =? atm f) 0.0056 atm =?
Respiratory Lecture Test Questions Set 3
 Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
Lecture 8: Heme/Non Heme Iron Proteins and O 2 Management II. Plus a bit of catalysis in Oxygen processes
 Lecture 8: Heme/Non Heme Iron Proteins and O 2 Management II Plus a bit of catalysis in Oxygen processes Hemoglobin Key Properties Ubiquitous O2 transport protein A globular soluble protein, 2X2 chains
Lecture 8: Heme/Non Heme Iron Proteins and O 2 Management II Plus a bit of catalysis in Oxygen processes Hemoglobin Key Properties Ubiquitous O2 transport protein A globular soluble protein, 2X2 chains
660 mm Hg (normal, 100 mm Hg, room air) Paco, (arterial Pc02) 36 mm Hg (normal, 40 mm Hg) % saturation 50% (normal, 95%-100%)
 148 PHYSIOLOGY CASES AND PROBLEMS Case 26 Carbon Monoxide Poisoning Herman Neiswander is a 65-year-old retired landscape architect in northern Wisconsin. One cold January morning, he decided to warm his
148 PHYSIOLOGY CASES AND PROBLEMS Case 26 Carbon Monoxide Poisoning Herman Neiswander is a 65-year-old retired landscape architect in northern Wisconsin. One cold January morning, he decided to warm his
More Practice with Gas Laws KEY
 More Practice with Gas Laws KEY Chemistry Directions: For each question, identify the applicable law and solve for the correct answer using dimensional analysis. Express your answer to the correct number
More Practice with Gas Laws KEY Chemistry Directions: For each question, identify the applicable law and solve for the correct answer using dimensional analysis. Express your answer to the correct number
You should be able to: Describe Equipment Barometer Manometer. 5.1 Pressure Read and outline 5.1 Define Barometer
 A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases.
 Chapter 8 Gases Practice Problems Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases. Summary: In a gas, particles are so far
Chapter 8 Gases Practice Problems Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases. Summary: In a gas, particles are so far
CHAPTER 6. Oxygen Transport. Copyright 2008 Thomson Delmar Learning
 CHAPTER 6 Oxygen Transport Normal Blood Gas Value Ranges Table 6-1 OXYGEN TRANSPORT Oxygen Dissolved in the Blood Plasma Dissolve means that the gas maintains its precise molecular structure About.003
CHAPTER 6 Oxygen Transport Normal Blood Gas Value Ranges Table 6-1 OXYGEN TRANSPORT Oxygen Dissolved in the Blood Plasma Dissolve means that the gas maintains its precise molecular structure About.003
GAS EXCHANGE & PHYSIOLOGY
 GAS EXCHANGE & PHYSIOLOGY Atmospheric Pressure Intra-Alveolar Pressure Inspiration 760 mm HG at Sea Level (= 1 atm) Pressure due to gases (N2, O2, CO2, Misc.) Pressure inside the alveolus (air sac) Phrenic
GAS EXCHANGE & PHYSIOLOGY Atmospheric Pressure Intra-Alveolar Pressure Inspiration 760 mm HG at Sea Level (= 1 atm) Pressure due to gases (N2, O2, CO2, Misc.) Pressure inside the alveolus (air sac) Phrenic
Respiratory System. Part 2
 Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Exam Key. NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: % of grade in class
 NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: 100 20% of grade in class 1) An arterial blood sample for a patient at sea level is obtained, and the following physiological values
NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: 100 20% of grade in class 1) An arterial blood sample for a patient at sea level is obtained, and the following physiological values
birth: a transition better guidelines better outcomes the birth experience a challenging transition the fountains of life: 2/8/2018
 better guidelines better outcomes neonatal resuscitation Anne G. Wlodaver, MD neonatology OU medical center the birth experience a challenging transition birth requires major and sudden transitions some
better guidelines better outcomes neonatal resuscitation Anne G. Wlodaver, MD neonatology OU medical center the birth experience a challenging transition birth requires major and sudden transitions some
Chapter 23. Gas Exchange and Transportation
 Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity and minor gases argon,
Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity and minor gases argon,
Oxygen, Carbon Dioxide Respiration Gas Transport Chapter 21-23
 nd Lecture Fri 06 Mar 009 Vertebrate Physiology ECOL 437 (MCB/VetSci 437) Univ. of Arizona, spring 009 Kevin Bonine & Kevin Oh Oxygen, Carbon Dioxide Respiration Gas Transport Chapter 1-3 1 Housekeeping,
nd Lecture Fri 06 Mar 009 Vertebrate Physiology ECOL 437 (MCB/VetSci 437) Univ. of Arizona, spring 009 Kevin Bonine & Kevin Oh Oxygen, Carbon Dioxide Respiration Gas Transport Chapter 1-3 1 Housekeeping,
NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: % of grade in class
 NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: 100 20% of grade in class 1) An arterial blood sample for a patient at sea level is obtained, and the following physiological values
NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: 100 20% of grade in class 1) An arterial blood sample for a patient at sea level is obtained, and the following physiological values
Respiratory Medicine. A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics. Alveolar Gas Equation. See online here
 Respiratory Medicine A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics See online here Alveolar gas equation helps to calculate the partial pressure of oxygen in alveoli and A-a gradient is the
Respiratory Medicine A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics See online here Alveolar gas equation helps to calculate the partial pressure of oxygen in alveoli and A-a gradient is the
Chapter 23. Gas Exchange and Transportation
 Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity other minor gases argon,
Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity other minor gases argon,
OXYGEN THERAPY. (Non-invasive O2 therapy in patient >8yrs)
 OXYGEN THERAPY (Non-invasive O2 therapy in patient >8yrs) Learning aims Indications and precautions for O2 therapy Targets of therapy Standard notation O2 delivery devices Taps, tanks and tubing Notation
OXYGEN THERAPY (Non-invasive O2 therapy in patient >8yrs) Learning aims Indications and precautions for O2 therapy Targets of therapy Standard notation O2 delivery devices Taps, tanks and tubing Notation
Gas Law Worksheets - WS: Boyle s and Charles Law
 Gas Law Worksheets - WS: Boyle s and Charles Law Boyle s Law states that the volume of a gas varies inversely with its pressure if temperature is held constant. (If one goes up the, other goes down.) We
Gas Law Worksheets - WS: Boyle s and Charles Law Boyle s Law states that the volume of a gas varies inversely with its pressure if temperature is held constant. (If one goes up the, other goes down.) We
AIIMS, New Delhi. Dr. K. K. Deepak, Prof. & HOD, Physiology AIIMS, New Delhi Dr. Geetanjali Bade, Asst. Professor AIIMS, New Delhi
 Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
AP Biology. Gas Exchange Respiratory Systems. Gas exchange. Why do we need a respiratory system? Optimizing gas exchange. Gas exchange in many forms
 alveoli Gas Exchange Respiratory Systems gills elephant seals 2008-2009 Why do we need a respiratory system? Need O 2 in food respiration for respiration for aerobic cellular respiration make ATP Need
alveoli Gas Exchange Respiratory Systems gills elephant seals 2008-2009 Why do we need a respiratory system? Need O 2 in food respiration for respiration for aerobic cellular respiration make ATP Need
These two respiratory media (air & water) impose rather different constraints on oxygen uptake:
 Topic 19: OXYGEN UPTAKE AND TRANSPORT (lectures 29-30) OBJECTIVES: 1. Be able to compare air vs. water as a respiratory medium with respect to oxygen content, diffusion coefficient, viscosity and water
Topic 19: OXYGEN UPTAKE AND TRANSPORT (lectures 29-30) OBJECTIVES: 1. Be able to compare air vs. water as a respiratory medium with respect to oxygen content, diffusion coefficient, viscosity and water
Kinetic Molecular Theory imaginary Assumptions of Kinetic Molecular Theory: Problems with KMT:
 AP Chemistry Ms. Ye Name Date Block Kinetic Molecular Theory Explains properties of gases, liquids, and solids in terms of energy using an ideal gas, an imaginary which fits all the assumptions of kinetic
AP Chemistry Ms. Ye Name Date Block Kinetic Molecular Theory Explains properties of gases, liquids, and solids in terms of energy using an ideal gas, an imaginary which fits all the assumptions of kinetic
Collin County Community College. Lung Physiology
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
respiratory cycle. point in the volumes: 500 milliliters. for men. expiration, up to 1200 milliliters extra makes breathing Respiratory
 10 II. RESPIRATORY VOLUMES, CAPACITIES & PULMONARY FUNCTION TESTS Respiratory volume is the term used for various volumes of air moved by or associated with the lungs at a given point in the respiratory
10 II. RESPIRATORY VOLUMES, CAPACITIES & PULMONARY FUNCTION TESTS Respiratory volume is the term used for various volumes of air moved by or associated with the lungs at a given point in the respiratory
Characteristics of Gases
 Lecture 25-27 Gases Characteristics of Gases Unlike liquids and solids, gases Expand to fill their containers. Are highly compressible. Have extremely low densi@es. Pressure of a gas Force per unit area
Lecture 25-27 Gases Characteristics of Gases Unlike liquids and solids, gases Expand to fill their containers. Are highly compressible. Have extremely low densi@es. Pressure of a gas Force per unit area
Gas Exchange Respiratory Systems
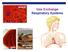 alveoli gills Gas Exchange Respiratory Systems elephant seals 2008-2009 Why do we need a respiratory system? respiration for respiration Need O 2 in for aerobic cellular respiration make ATP Need CO 2
alveoli gills Gas Exchange Respiratory Systems elephant seals 2008-2009 Why do we need a respiratory system? respiration for respiration Need O 2 in for aerobic cellular respiration make ATP Need CO 2
RESPIRATORY REGULATION DURING EXERCISE
 RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
Chapter 13 The Respiratory System
 Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Notes: Gas Laws (text Ch. 11)
 Name Per. Notes: Gas Laws (text Ch. 11) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to get missing information
Name Per. Notes: Gas Laws (text Ch. 11) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to get missing information
Under ideal conditions, the rates at which different gases diffuse (spread out) are proportional to their molar masses.
 Chemistry Ms. Ye Name Date Block Graham s Law of Diffusion- Under ideal conditions, the rates at which different gases diffuse (spread out) are proportional to their molar masses. In other words, gas molecules
Chemistry Ms. Ye Name Date Block Graham s Law of Diffusion- Under ideal conditions, the rates at which different gases diffuse (spread out) are proportional to their molar masses. In other words, gas molecules
Unit 8: Gases and States of Matter
 Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
Ch 16: Respiratory System
 Ch 16: Respiratory System SLOs: Explain how intrapulmonary pressures change during breathing Explain surface tension and the role of surfactant in respiratory physiology. Compare and contrast compliance
Ch 16: Respiratory System SLOs: Explain how intrapulmonary pressures change during breathing Explain surface tension and the role of surfactant in respiratory physiology. Compare and contrast compliance
Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops
 C h e m i s t r y 1 2 C h a p t e r 11 G a s e s P a g e 1 Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops Gas Properties: Gases have high kinetic energy low
C h e m i s t r y 1 2 C h a p t e r 11 G a s e s P a g e 1 Chapter 11: Gases: Homework: Read Chapter 11. Keep up with MasteringChemistry and workshops Gas Properties: Gases have high kinetic energy low
Lung Volumes and Capacities
 Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Introduction. Respiration. Chapter 10. Objectives. Objectives. The Respiratory System
 Introduction Respiration Chapter 10 The Respiratory System Provides a means of gas exchange between the environment and the body Plays a role in the regulation of acidbase balance during exercise Objectives
Introduction Respiration Chapter 10 The Respiratory System Provides a means of gas exchange between the environment and the body Plays a role in the regulation of acidbase balance during exercise Objectives
Vienna, Austria May 2005 MONITORING GAS EXCHANGE: FROM THEORY TO CLINICAL APPLICATION
 EUROANESTHESIA 2005 Vienna, Austria 28-31 May 2005 MONITORING GAS EXCHANGE: FROM THEORY TO CLINICAL APPLICATION 5RC2 OLA STENQVIST Department of Anaesthesia and Intensive Care Sahlgrenska University Hospital
EUROANESTHESIA 2005 Vienna, Austria 28-31 May 2005 MONITORING GAS EXCHANGE: FROM THEORY TO CLINICAL APPLICATION 5RC2 OLA STENQVIST Department of Anaesthesia and Intensive Care Sahlgrenska University Hospital
Body tissue INTERSTITIAL FLUID Capillary Net fluid movement out Net fluid movement in Direction of blood flow Blood pressure
 Capillary Func4on exchange of substances between blood and inters44al fluid across thin endothelial capillary walls difference between blood pressure and osmo4c pressure Drives fluids out of capillaries
Capillary Func4on exchange of substances between blood and inters44al fluid across thin endothelial capillary walls difference between blood pressure and osmo4c pressure Drives fluids out of capillaries
The physiological functions of respiration and circulation. Mechanics. exercise 7. Respiratory Volumes. Objectives
 exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
alveoli Chapter 42. Gas Exchange elephant seals gills AP Biology
 alveoli Chapter 42. Gas Exchange gills elephant seals Gas exchange O 2 & CO 2 exchange exchange between environment & cells provides O 2 for aerobic cellular respiration need moist membrane need high
alveoli Chapter 42. Gas Exchange gills elephant seals Gas exchange O 2 & CO 2 exchange exchange between environment & cells provides O 2 for aerobic cellular respiration need moist membrane need high
Chemistry Chapter 12. Characteristics of Gases. Characteristics of Gases 1/31/2012. Gases and Liquids
 Importance of Gases Chemistry Chapter 12 Gases and Liquids Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide, NaN 3. 2 NaN 3 ---> 2 Na + 3 N 2 THREE STATES
Importance of Gases Chemistry Chapter 12 Gases and Liquids Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide, NaN 3. 2 NaN 3 ---> 2 Na + 3 N 2 THREE STATES
Lung Volumes and Ventilation
 Respiratory System ssrisuma@rics.bwh.harvard.edu Lung Volumes and Ventilation Minute ventilation Volume of an inspired or expired air per minute = tidal volume (V T ) x respiratory rate Dead space ventilation
Respiratory System ssrisuma@rics.bwh.harvard.edu Lung Volumes and Ventilation Minute ventilation Volume of an inspired or expired air per minute = tidal volume (V T ) x respiratory rate Dead space ventilation
The Physiologic Basis of DLCO testing. Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan
 The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
Capnography in the Veterinary Technician Toolbox. Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA
 Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
Section 01: The Pulmonary System
 Section 01: The Pulmonary System Chapter 12 Pulmonary Structure and Function Chapter 13 Gas Exchange and Transport Chapter 14 Dynamics of Pulmonary Ventilation HPHE 6710 Exercise Physiology II Dr. Cheatham
Section 01: The Pulmonary System Chapter 12 Pulmonary Structure and Function Chapter 13 Gas Exchange and Transport Chapter 14 Dynamics of Pulmonary Ventilation HPHE 6710 Exercise Physiology II Dr. Cheatham
- How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have
 - How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have the highest blood flow of all organs, which makes them
- How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have the highest blood flow of all organs, which makes them
Chapter 4: Ventilation Test Bank MULTIPLE CHOICE
 Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
8.1 Properties of Gases. Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases.
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8. Chapter 8
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases. Chapter 8. Chapter 8. Gases Properties of Gases. We are surrounded by gases, but we are often
 Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
Clinical Skills. Administering Oxygen
 Clinical Skills Administering Oxygen Updated July 2017 Clare Cann Original 2012 Carole Loveridge, Lecturer in Women`s Health Aims and Objectives Aims and Objectives The aim of this module is to facilitate
Clinical Skills Administering Oxygen Updated July 2017 Clare Cann Original 2012 Carole Loveridge, Lecturer in Women`s Health Aims and Objectives Aims and Objectives The aim of this module is to facilitate
 Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
AP Biology. Chapter 42. Gas Exchange. Optimizing gas exchange. Gas exchange. Gas exchange in many forms. Evolution of gas exchange structures
 alveoli Chapter 42. Gas Exchange gills elephant seals Gas exchange & C exchange exchange between environment & cells provides for aerobic cellular respiration need moist membrane need high surface area
alveoli Chapter 42. Gas Exchange gills elephant seals Gas exchange & C exchange exchange between environment & cells provides for aerobic cellular respiration need moist membrane need high surface area
Gas Laws V 1 V 2 T 1. Gas Laws.notebook. May 05, T = k P 1 V 1 = P 2 V 2. = 70 kpa. P. V = k. k = 1 atm = kpa
 Gas Laws At constant temperature, all gases behave the same when compressed As increasing pressure is applied to a gas in a closed container, the volume of the gas decreases he product of pressure and
Gas Laws At constant temperature, all gases behave the same when compressed As increasing pressure is applied to a gas in a closed container, the volume of the gas decreases he product of pressure and
Humidity Therapy. Terms to know:
 RC-170 Humidity Therapy Terms to know: Humidity: Water in a vapor state of matter. Absolute Humidity (mg/liter) actual amount of water in a vapor form. Relative Humidity (% up to 100) absolute compared
RC-170 Humidity Therapy Terms to know: Humidity: Water in a vapor state of matter. Absolute Humidity (mg/liter) actual amount of water in a vapor form. Relative Humidity (% up to 100) absolute compared
Chapter 5. Pressure. Atmospheric Pressure. Gases. Force Pressure = Area
 Chapter 5 Gases Water for many homes is supplied by a well The pump removes air from the pipe, decreasing the air pressure in the pipe The pressure then pushes the water up the pipe Pressure Atmospheric
Chapter 5 Gases Water for many homes is supplied by a well The pump removes air from the pipe, decreasing the air pressure in the pipe The pressure then pushes the water up the pipe Pressure Atmospheric
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works.
 Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
For more information about how to cite these materials visit
 Author(s): John G. Younger, M.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/
Author(s): John G. Younger, M.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/
Respiratory Physiology Gaseous Exchange
 Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
Recitation question # 05
 Recitation and Lab # 05 The goal of this recitations / labs is to review material related to the CV and respiratory lectures for the second test of this course. Info required to answer this recitation
Recitation and Lab # 05 The goal of this recitations / labs is to review material related to the CV and respiratory lectures for the second test of this course. Info required to answer this recitation
Example 5.1 Converting between Pressure Units
 Example 5.1 Converting between Pressure Units For Practice 5.1 Your local weather report announces that the barometric pressure is 30.44 in Hg. Convert this pressure to psi. For More Practice 5.1 Convert
Example 5.1 Converting between Pressure Units For Practice 5.1 Your local weather report announces that the barometric pressure is 30.44 in Hg. Convert this pressure to psi. For More Practice 5.1 Convert
Tter person TERM PAPER. Date: 30/4/ MEDICAL VENTILATOR
 Tter person TERM PAPER BM 600 Nuruddin Bahar Date: 30/4/2012 09002040 MEDICAL VENTILATOR It is an artificial ventilating device which is used to mechanically move breathable air in and out of lungs at
Tter person TERM PAPER BM 600 Nuruddin Bahar Date: 30/4/2012 09002040 MEDICAL VENTILATOR It is an artificial ventilating device which is used to mechanically move breathable air in and out of lungs at
