S8 Lightweight System
|
|
|
- Molly Greene
- 6 years ago
- Views:
Transcription
1 S8 Lightweight System POSITIVE AIRWAY PRESSURE DEVICE User Guide English ResMed Ltd (Manufacturer) 1 Elizabeth Macarthur Drive Bella Vista NSW 2153 Australia ResMed Corp (US Designated Agent) Danielson Street Poway CA USA ResMed (UK) Ltd (EU Authorized Representative) 96 Milton Park Abingdon Oxfordshire OX14 4RY UK ResMed Offices Australia, Austria, Belgium, Brazil, China, Finland, France, Germany, Greece, Hong Kong, India, Ireland, Italy, Japan, Malaysia, Mexico, Netherlands, New Zealand, Norway, Portugal, Singapore, South Africa, Spain, Sweden, Switzerland, UK, USA (see for contact details). Protected by patents: US , US , US Other patents pending. Protected by design registrations: AU , AU , AU , AU , AU , AU , AU , CN , CN , CN , CN , CN , CN , CN , EP , HK , JP , JP , JP , JP , JP , JP , NZ , NZ , NZ , NZ , NZ , NZ , NZ , US D544598, US D553228, US D557406, US D557407, US D560795, US D561891, US D Others pending. S8, S8 Lightweight, ResScan, ResControl, ResLink and ResTraxx are trademarks of ResMed Ltd and Lightweight and HumidAire are registered in U.S. Patent and Trademark Office ResMed Ltd /
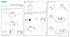
2 B INTEGRATED HUMIDIFIER D SETTING UP THE S8 Component of /2 S8 Lightweight System ILLUSTRATIONS A SYSTEM COMPONENTS Carry handle HumidAire 3i 2008 ResMed Ltd. 1 2 LCD screen Keypad Air outlet C OTHER ACCESSORIES Connector plug 1 3 m Module Air filter cover DC input Air inlet 2 AC input 52 cm 2 m ResMed Ltd ResMed Ltd ResMed Ltd.
3 E HUMIDAIRE 3i and S8 LIGHTWEIGHT H USING THE DATA CARD 2008 ResMed Ltd ResMed Ltd. F BLANK MODULE Data communications port I USING A HUMIDAIRE G 1 2 DATA CARD MODULE 2008 ResMed Ltd ResMed Ltd ResMed Ltd. Note: Some products not available in all regions
4 CONTENTS INTRODUCTION YOUR RESPONSIBILITY 1 MEDICAL INFORMATION ABOUT THE S8 LIGHTWEIGHT 1 CONTRAINDICATIONS 1 WARNINGS 2 CAUTIONS 3 ADVERSE EFFECTS 3 THE S8 LIGHTWEIGHT SYSTEM S8 LIGHTWEIGHT 5 MASKS 5 HUMIDIFIERS 6 RESSCAN DATA CARD MODULE 6 ACCESSORIES 6 HOW TO USE THE S8 LIGHTWEIGHT SET UP THE S8 LIGHTWEIGHT 7 ASSEMBLE THE MASK 7 START TREATMENT 7 STOP TREATMENT 8 HOW TO ATTACH A HUMIDIFIER ATTACH A HUMIDAIRE 3I HUMIDIFIER 9 ATTACH A HUMIDAIRE HUMIDIFIER 9 HOW TO USE THE KEYPAD HOW TO CHANGE THE SETTINGS ON YOUR S8 LIGHTWEIGHT 12 S8 MODULES USING A BLANK MODULE 15 USING A RESSCAN DATA CARD MODULE 15 CLEANING AND MAINTENANCE DAILY CLEANING 17 WEEKLY CLEANING 17 PERIODIC CLEANING 17 REPLACING THE AIR FILTER 18 SERVICING 18 FREQUENTLY ASKED QUESTIONS TROUBLESHOOTING SYSTEM SPECIFICATIONS CONTENTS
5 S8 LIGHTWEIGHT 23 ACCESSORIES 23 INDEX
6 INTRODUCTION Thank you for choosing the S8 LIGHTWEIGHT flow generator. The S8 LIGHTWEIGHT is a compact, all-in-one package with an inbuilt power supply and the option of integrated data management and humidification. This user manual contains the information you need for the correct use of your S8 LIGHTWEIGHT. INTRODUCTION YOUR RESPONSIBILITY The owner or user of this system shall have sole responsibility and liability for any injury to persons or damage to property resulting from: operation which is not in accordance with the operating instructions supplied maintenance or modifications carried out unless in accordance with authorised instructions and by authorised persons. Please read this manual carefully before use. This manual contains special terms and icons that appear in the margins to draw your attention to specific and important information. Warning alerts you to possible injury. Caution explains special measures for the safe and effective use of the device. Note is an informative or helpful note. MEDICAL INFORMATION ABOUT THE S8 LIGHTWEIGHT The S8 LIGHTWEIGHT continuous positive airway pressure (CPAP) system is indicated for the treatment of obstructive sleep apnoea (OSA) in adult patients. The S8 LIGHTWEIGHT CPAP system is intended for home and hospital use. CONTRAINDICATIONS The S8 LIGHTWEIGHT is not a life support device and may stop operating during power failure or certain fault conditions. It should not be used by patients who are dependent on continuous therapy. Positive airway pressure therapy may be contraindicated in some patients with the following pre-existing conditions: severe bullous lung disease pneumothorax pathologically low blood pressure dehydration cerebrospinal fluid leak, recent cranial surgery, or trauma. 1
7 WARNINGS Read the entire manual before using the S8 LIGHTWEIGHT. Advice contained in this manual should not supersede instructions given by the prescribing physician. A patient should not connect a device to the data communication port unless instructed to do so by their health care provider or physician. Only ResMed products are designed to be connected to the data communication port. Connecting other devices could result in injury, or damage to the S8 LIGHTWEIGHT device. The S8 LIGHTWEIGHT should only be used with masks (and connectors) * recommended by ResMed, or by a physician or respiratory therapist. A mask should not be used unless the S8 LIGHTWEIGHT device is turned on and operating properly. The vent hole or holes associated with the mask should never be blocked. Explanation: The S8 LIGHTWEIGHT is intended to be used with special masks (or connectors) * which have vent holes to allow continuous flow of air out of the mask. When the device is turned on and functioning properly, new air from the device flushes the exhaled air out through the mask vent holes. However, when the device is not operating, insufficient fresh air will be provided through the mask, and the exhaled air may be rebreathed. Rebreathing of exhaled air for longer than several minutes can, in some circumstances, lead to suffocation. This applies to most models of CPAP devices. In the event of power failure or machine malfunction, remove the mask. The S8 LIGHTWEIGHT can be set to deliver pressures up to 20 cm H 2 O. In the unlikely event of certain fault conditions, pressures up to 30 cm H 2 O are possible. If oxygen is used with this device, the oxygen flow must be turned off when the device is not operating. If oxygen has been left on, turn off the flow generator, then wait 30 minutes before turning on the flow generatory again. Explanation: When the CPAP device is not in operation and the oxygen flow is left on, oxygen delivered into the air delivery tubing may accumulate within the CPAP machine enclosure and create a risk of fire. This applies to most types of CPAP machines. Oxygen supports combustion. Oxygen should not be used while you are smoking or in the presence of an open flame. Always ensure airflow is being generated by the device before the oxygen supply is turned on. Always turn the oxygen supply off before stopping the airflow from the device. Note: At a fixed rate of supplemental oxygen flow, the inhaled oxygen concentration will vary, depending on where the oxygen is introduced, the pressure settings, patient breathing pattern, mask selection and leak rate. * Ports may be incorporated into the mask or in connectors that are near the mask. 2
8 Do not use the S8 LIGHTWEIGHT if there are obvious external defects or unexplained changes in performance. Do not open the S8 LIGHTWEIGHT case. There are no user serviceable parts inside. Repairs and internal servicing should only be performed by an authorised service agent. Explosion hazard do not use in the vicinity of flammable anaesthetics. CAUTIONS At low pressures, the flow through the exhalation ports of your mask may not clear all exhaled gas from the tubing. Some rebreathing may occur. The airflow for breathing produced by this device can be as much as 6 C higher than the temperature of the room. Caution should be exercised if the room temperature is warmer than 32 C. When AC mains power ( V AC) is not available, always use a ResMed DC-12 converter. (The DC-12 converter is available as an optional accessory. It is not supplied with all models.) If supplied, the Data Card module is designed for use with specific ResMed devices. Only use the Data Card module (if supplied) as specified in this manual. Note: The above are general warnings and cautions. Specific warnings, cautions and notes appear with the relevant instructions in the manual. MEDICAL INFORMATION ADVERSE EFFECTS Patients should report unusual chest pain, severe headache or increased breathlessness to their prescribing physician. An acute upper respiratory tract infection may require temporary discontinuation of treatment. The following side effects may arise during the course of therapy with the CPAP device: drying of the nose, mouth or throat nosebleed bloating ear or sinus discomfort eye irritation skin rashes chest discomfort. 3
9 4
10 THE S8 LIGHTWEIGHT SYSTEM The S8 LIGHTWEIGHT device is part of a system that consists of six elements: S8 LIGHTWEIGHT flow generator Mask Humidifier Data management products Premium noise guard (front cap) Accessories. THE S8 LIGHTWEIGHT SYSTEM S8 LIGHTWEIGHT MASKS Please refer to the illustrations in section A of the illustration sheet. FRONT VIEW (A-1) Shows the keypad and LCD screen, and the air outlet. REAR VIEW (A-2) Shows where the module attaches, and the location of the air filter and power sockets. POWER CORD (A-3) Power cord to attach the S8 LIGHTWEIGHT to the AC (mains) power. CARRY BAG (A-4) The S8 LIGHTWEIGHT carry bag contains two pouches: one for storing the S8 device, the other for storing the HUMIDAIRE 3i. When storing the HUMIDAIRE 3i, always put it in the larger, mesh pouch. AIR TUBING The 2 m air tubing (A-5) connects the S8 LIGHTWEIGHT to your mask. You will need medium 52 cm air tubing (see C-3) to connect the S8 LIGHTWEIGHT to the HUMIDAIRE humidifier. You will also need a ResMed mask system (supplied separately). The ResMed mask systems that are recommended for use with the S8 LIGHTWEIGHT are: NASAL MASKS MODULAR NASAL MASK MIRAGE VISTA NASAL MASK ULTRA MIRAGE NASAL MASK MIRAGE NASAL MASK MIRAGE ACTIVA NASAL MASK PAPILLON. NASAL PILLOWS SYSTEMS MIRAGE SWIFT NASAL PILLOWS SYSTEM. 5
11 ! FULL FACE MASKS MIRAGE FULL FACE MASK MIRAGE FULL FACE MASK SERIES 2 ULTRA MIRAGE FULL FACE MASK. WARNING ResMed mask systems are compatible for use with the S8 Lightweight. Please refer to Warnings on page 2. HUMIDIFIERS A humidifier may be required if you are experiencing dryness of the nose, throat or mouth. The S8 LIGHTWEIGHT is compatible for use with: HUMIDAIRE 3i integrated heated humidifier (Figure B on the illustrations sheet) HUMIDAIRE heated humidifier (Figure I on the illustrations sheet). RESSCAN DATA CARD MODULE The RESSCAN Data Card module and RESSCAN Data Card (C-1) may be used with the S8 LIGHTWEIGHT either to help your clinician to monitor your treatment or to provide you with updates to your device settings. See Using a ResScan Data Card Module on page 15 for information about how to use the Data Card module and Data Card. ACCESSORIES Please refer to the illustrations in section C of the illustration sheet. Other accessories for use with the S8 LIGHTWEIGHT: Premium noise guard (front cap): standard with S8 LIGHTWEIGHT (C-2) Air tubing 3 m (C-3) Medium air tubing 52 cm for HUMIDAIRE connection (C-4) DC-12 converter (C-5) Blank module (C-6). Note: ResMed regularly releases new products. Please check our website at < 6
12 HOW TO USE THE S8 LIGHTWEIGHT Please refer to the illustrations in section D of the illustration sheet. SET UP THE S8 LIGHTWEIGHT 1 Place the S8 LIGHTWEIGHT on a table near the head of your bed.! CAUTION Be careful not to place the device where it can be bumped or where someone is likely to trip over the power cord. Note: You can also place your S8 Lightweight on the floor beside or underneath your bed. Ensure that the area is free from dust and clear of bedding, clothes or any other objects that could block the air inlet. HOW TO USE THE S8 LIGHTWEIGHT 2 Connect the power cord to the socket at the rear of the flow generator (D-1). Plug the other end of the power cord into the power outlet. WARNING! Make sure the power cord and plug are in good condition and the equipment is not damaged. 3 Connect one end of the air tubing firmly onto the air outlet (D-2). WARNING! Only ResMed air tubing should be used with your flow generator. A different type of air tubing may alter the pressure you actually receive, reducing the effectiveness of your treatment. Blocking the hose while in operation could lead to overheating of the device. In the clinical environment any personal computer that is used with your CPAP system must be at least 1.5 m away from, or at least 2.5 m above, the patient. It must also comply with the relevant test standard. For personal computers the international standard is IEC or equivalent. ASSEMBLE THE MASK 1 Assemble your mask system according to the mask user instructions. 2 Connect your mask system to the free end of the air tubing (D-3). The S8 LIGHTWEIGHT is now ready for use (D-4). START TREATMENT 1 Make sure the power is on. The product name is displayed briefly on the LCD screen, then the standby (Ramp) screen appears. The key and LCD backlights also turn on. 2 Lie down and arrange the air tubing so that it is free to move if you turn in your sleep. 7
13 ! CAUTION Do not leave long lengths of air tubing around the top of your bed. They could twist around your head or neck while you are sleeping. Make sure the area around the flow generator is dry and clean. It should also be clear of bedding, clothes and other potential blockages. 3 Alter the ramp time if required (see How to Change the Ramp Time on page 13). 4 To start treatment, press the Start/Stop key. 5 Fit your mask as described in the mask user instructions. WARNING! A mask should not be used unless the S8 Lightweight device is turned on and operating properly. 6 When ramping begins the LCD will display the word "RAMPING", with the current pressure indicated as dashes. Each dash is approximately equivalent to 2 cm H 2 O. For example, 6 dashes represents approximately 12 cm H 2 O pressure. STOP TREATMENT To stop treatment at any time, remove your mask and press the Start/Stop key. 8
14 HOW TO ATTACH A HUMIDIFIER ATTACH A HUMIDAIRE 3i HUMIDIFIER Please refer to the illustration in section E of the illustration sheet, and your HumidAire 3i manual. The HUMIDAIRE 3i attaches to the front of the S8 LIGHTWEIGHT to provide heated humidification. No other accessories are required for its use. Notes: a. Make sure the water chamber of the HumidAire 3i is empty before you attach or detach the humidifier. b. Remove the connector plug on the S8 Lightweight (see section A of the illustrations sheet) before you attach the HumidAire 3i. Reattach the connector plug firmly after you detach the HumidAire 3i. HOW TO ATTACH A HUMIDIFIER STORING THE HUMIDAIRE 3i Store the HUMIDAIRE 3i in the larger, mesh-top pouch of the S8 LIGHTWEIGHT carry bag when not in use. ATTACH A HUMIDAIRE HUMIDIFIER Please refer to the illustrations in section I of the illustration sheet. To connect your S8 LIGHTWEIGHT to a HUMIDAIRE humidifier you need medium sized (52 cm) air tubing. 1 Fill the HUMIDAIRE water chamber and place inside the HUMIDAIRE. Connect the medium (52 cm) and long (2 m) air tubing (I-1), and close the humidifier lid. 2 Place the S8 LIGHTWEIGHT on top of the HUMIDAIRE (I-2) and connect the free end of the medium air tubing to the air outlet (I-3). Note: To avoid water spilling into the S8 LIGHTWEIGHT, do not put it underneath the humidifier. 3 Assemble your mask system and connect it to the free end of the long air tubing (I-4). 4 Plug the HUMIDAIRE power cord into a power outlet. Connect the S8 LIGHTWEIGHT power cord to the socket at the rear of the S8 LIGHTWEIGHT and plug the other end into a power outlet. Please refer to the HumidAire User s Manual for further details. WARNING! Make sure that the power cord and plug are in good condition and the equipment is not damaged. The S8 LIGHTWEIGHT is now ready for use with the HUMIDAIRE. 9
15 10
16 HOW TO USE THE KEYPAD The control panel of the S8 LIGHTWEIGHT includes an LCD screen and keypad for navigating through the menus and delivering treatment. HOW TO USE THE KEYPAD LCD screen Up key Left key Right key Down key Start/Stop key LCD SCREEN The LCD screen displays the menus and treatment screens. KEYPAD KEYS The S8 LIGHTWEIGHT keypad has the following keys: Key Start/Stop Function Starts or stops treatment. Up Down Left Right Allows you to increase settings options and scroll through the S8 LIGHTWEIGHT menu. Allows you to decrease settings options and scroll through the S8 LIGHTWEIGHT menu. Performs the function indicated by the guiding text displayed above it on the LCD screen. Guiding text includes menu, change and apply. Performs the function indicated by the guiding text displayed above it on the LCD screen. Guiding text includes exit and cancel. BACKLIGHTS The keypad and LCD are equipped with backlights that come on when the device is powered. The LCD backlight turns off after two minutes of inactivity, and comes back 11
17 on when you press a key. Once the Start/Stop key has been pressed to start treatment, the LCD backlight stays on for two minutes. The keypad backlight is always on while the S8 LIGHTWEIGHT is on. HOW TO CHANGE THE SETTINGS ON YOUR S8 LIGHTWEIGHT You can change settings (such as Ramp Time, Altitude) on the S8 LIGHTWEIGHT by using the keypad and LCD. When the S8 LIGHTWEIGHT is in standby mode you can display a series of screens (a menu) on the LCD screen in order to view and change the settings for a particular function. S8 LIGHTWEIGHT S8 PATIENT CLASSIC MENU S8 RAMP CLASSIC Menu 10min ALT: 0-610m Change Exit USED HRS: 960 Exit USAGE: 120/180 Exit LANG: Change ENGLISH Exit SW: SX Exit Use the menu screens to change the ramp time, altitude compensation and language. You can also view details about the usage and software version of your S8 LIGHTWEIGHT. HOW TO ENTER THE MENU SCREENS When the S8 LIGHTWEIGHT standby screen is displayed you will see the word Menu in the left-hand corner. To select the S8 LIGHTWEIGHT menu, press the Left key. HOW TO SCROLL THROUGH THE MENU When you are in the menu, scroll through items by pressing the Up or Down keys. HOW TO CHANGE A SETTING OPTION When you have scrolled to the item you require: 1. Press the Left key (Change). 2. Press the Up or Down keys until you see the setting option that you require. 3. Press the Left key (Apply) to select the setting option. If you wish to exit without changing the option, press the Right key (Cancel). 12
18 HOW TO EXIT OUT OF A MENU Press the Right key (Exit). This will take you back to the Patient Menu screen. HOW TO EXIT OUT OF AN ITEM WITHIN THE MENU Press the Right key (Exit). HOW TO CHANGE THE RAMP TIME During ramp time, the pressure increases from a low pressure to the prescribed treatment pressure. You can set the ramp time in 5-minute intervals, from 0 minutes (OFF) up to a maximum ramp time set by your clinician. The standby screen displays the current ramp time in the right-hand corner. To change the ramp time, press the Up or Down key until the desired ramp time appears. Once a ramp time is selected, begin therapy by pressing the Start/Stop key. When ramping begins the LCD will display the word "RAMPING", with the current pressure indicated as dashes. Each dash is approximately equivalent to 2 cm H 2 O. For example, six dashes represents approximately 12 cm H 2 O pressure. HOW TO USE THE KEYPAD HOW TO CHANGE THE ALTITUDE SETTING If you move or travel with your S8 LIGHTWEIGHT to areas of different altitude, you will need to adjust the altitude compensation setting. Changes in altitude will affect the pressure delivered by the S8 LIGHTWEIGHT. You can use the Altitude setting (ALT) to specify the altitude range at which you are using your S8 LIGHTWEIGHT. Select the S8 LIGHTWEIGHT menu by pressing the Left key in the standby (RAMP) screen. Scroll to ALT and select Change (Left key). Press the Up or Down keys until you see the setting option that you require. There are four altitude settings to choose from: m m m m. For example, if you are at an altitude of 947 m, your altitude compensation setting would be m, as 947 m falls within that range. Press the Left key (Apply) to select the correct setting. HOW TO CHANGE THE LANGUAGE SETTING To display menus and messages in a different language, select the S8 LIGHTWEIGHT menu by pressing the Left key in the standby (Ramp) screen. Scroll to LANG and select Change (Left key). Press the Up or Down keys until you see the language that you require. OTHER VIEWABLE INFORMATION You can also view the total number of hours of treatment (USED HRS); the number of sessions that the S8 LIGHTWEIGHT was used in a usage period (USAGE); and the current software version installed on your unit (SW). Access to all of these screens is provided via the menu. See How to enter the menu screens on page
19 MESSAGES ON THE S8 LIGHTWEIGHT LCD Your clinician may have set your S8 LIGHTWEIGHT to remind you about important events, such as when to replace your mask, when to insert your Data Card (if your device is Data Card enabled) and so on. The reminder message is displayed on the LCD and is visible if the device is not delivering therapy. The backlight on the LCD flashes when a message is displayed. Your clinician can set any of the following reminders on your LCD: Message Description Action INSERT CARD REPLACE MASK CALL PROVIDER REPLACE FILTER SERVICE DUE May appear if your device is Data Card enabled. May appear when your mask is due for replacement. May appear as a reminder to contact your clinician; for example to discuss how your therapy is going. May appear as a reminder to replace the air filter on your device. May appear as a reminder to return your device for service. Insert your Data Card and follow any instructions that your clinician has given you. When you have done this, the message will disappear from the LCD. Pressing the Left (Ok) key will also remove the message. Press the Left (Ok) key to remove the message from your LCD, and replace your mask with a new one. Press the Left (Ok) key to remove the message from your LCD, and contact your clinician. Press the Left (Ok) key to remove the message from your LCD, and replace the air filter. Press the Left (Ok) key to remove the message from your LCD, and contact your clinician. 14
20 S8 MODULES Note: Do not remove modules while power is connected to the S8 Lightweight. Your S8 LIGHTWEIGHT will have one of two modules fitted to the back: a blank module or a RESSCAN Data Card module. USING A BLANK MODULE Please refer to the illustrations in section F of the illustration sheet. The blank module is a protective cover on the S8 LIGHTWEIGHT. At times, you may wish to remove or attach this module, for example to use the serial adapter. To remove the blank module, pull it off the back of the S8 LIGHTWEIGHT to show the data communications port (F-1). To attach the blank module, push the blank module onto the data communications port, until the module clicks into place (F-2). Note: To protect the data communications port, always ensure there is a module attached to the device. S8 MODULES USING A RESSCAN DATA CARD MODULE Please refer to the illustrations in section G of the illustration sheet. The RESSCAN Data Card module is an accessory to the S8 LIGHTWEIGHT. Use a RESSCAN Data Card with the Data Card module to collect data and update settings in the S8 LIGHTWEIGHT. The module and card give your clinician convenient access to data about your treatment, and can provide you with new device settings from your clinician without you having to leave home. ATTACHING THE MODULE To attach the Data Card module, push it onto the data communications port at the back of your S8 LIGHTWEIGHT until it clicks into place (G-1). REMOVING THE MODULE To remove the Data Card module, pull it off the back of the S8 LIGHTWEIGHT (G-2). Note: To protect the data communications port, always ensure there is a module attached to the device. USING THE DATA CARD TO COLLECT DATA Please refer to the illustrations in section H of the illustration sheet. If your clinician needs to review your treatment, they will ask you to use the Data Card to copy data from your S8 LIGHTWEIGHT, and to return the card to them. The Data Card will be provided in a convenient mailback envelope. Data that is copied to a Data Card is still stored and available on the S8 LIGHTWEIGHT. It takes approximately five seconds to copy data onto the Data Card. 1 INSERT THE DATA CARD Switch on the S8 LIGHTWEIGHT and wait until you see the standby (Ramp) screen. 15
21 Hold the Data Card with the arrow facing up and insert it into the slot in the Data Card module (H-1). Push the card in smoothly until it stops moving (H-2) and messages start appearing on the LCD. 2 COPY DATA ONTO THE DATA CARD Data copying starts automatically when the Data Card is inserted into the Data Card module. The Card Inserted Please Wait message is displayed on the S8 LIGHTWEIGHT LCD while data is being copied. Copying takes approximately five seconds. The Copy Complete Remove Card message is displayed on the LCD when copying has finished. Remove the Data Card from the Data Card module. 3 REMOVE THE DATA CARD Grip the end of the Data Card and pull it out of the Data Card module (H-3). 4 STORE THE DATA CARD Your Data Card should be stored in its mailback envelope when not in use. 5 MAIL THE DATA CARD TO YOUR CLINICIAN A bubble mailer envelope is provided to ensure safe transportation of the Data Card. When mailing the Data Card to your clinician: 1. Insert the Data Card into the pocket provided on the mailback envelope. Fold up the mailback envelope. 2. Place the mailback envelope containing the Data Card in the bubble mailer envelope and seal it. 3. Make sure that your clinician s name and address details are on the bubble mailer envelope, and mail it back immediately. USING THE DATA CARD TO UPDATE SETTINGS If your clinician has provided a Data Card with new device settings: With the device in standby (Ramp) mode, insert the Data Card into the slot on the Data Card module. Updating will start automatically. The Card Inserted Please Wait message is displayed on the LCD while updating is in progress. Updating takes approximately five seconds. The Settings Success Remove Card message is displayed on the LCD if the settings were updated successfully. Note: This message only appears once. If you re-insert the Data Card after you have updated your settings, this message is not displayed. Remove the Data Card from the Data Card module. Store the Data Card in its mailback envelope when not in use. WARNING If your clinician has told you to use this Data Card to update the settings on your device and the Settings Success message does not appear, contact your clinician immediately.! 16
22 CLEANING AND MAINTENANCE You should regularly carry out the cleaning and maintenance described in this section. Refer to your mask manual for detailed instructions. DAILY CLEANING Mask Clean the mask according to the instructions supplied with the mask. Air tubing Disconnect the air tubing from the S8 LIGHTWEIGHT (and humidifier, if used) and hang the tubing and mask in a clean, dry place until next use. Humidifier If you are using a humidifier, clean it according to the instructions in the humidifier user s manual. CLEANING AND MAINTENANCE WEEKLY CLEANING 1. Remove the air tubing from the S8 LIGHTWEIGHT and the mask. 2. Wash the air tubing in warm water using mild detergent. Rinse thoroughly, hang, and allow to dry. 3. Before next use, assemble the mask and headgear according to the user instructions. 4. Reconnect the air tubing to the air outlet and mask. PERIODIC CLEANING 1. Clean the exterior of the S8 LIGHTWEIGHT with a damp cloth and mild detergent. 2. Check the air filter for blockage or holes (see Replacing the Air Filter on page 18). WARNING! Beware of electric shock. Do not immerse the unit or power cord in water. Always unplug the unit before cleaning and be sure it is dry before plugging back in. The mask system and air tubing are subject to normal wear and tear. Inspect them regularly for damage. 17
23 REPLACING THE AIR FILTER To replace the air filter: 1. Remove the air filter cover at the back of the S8 LIGHTWEIGHT. Air filter cover 2. Remove and discard the old air filter. 3. Insert a new filter with the blue tinted side facing out from the device. 4. Replace the air filter cover.! Inspect the air filter every month to check if it is blocked by dirt or contains holes. With normal use of an S8 LIGHTWEIGHT, the air filter needs to be replaced every six months (or more often if your device is in a dusty environment). WARNING Do not wash the air filter. The air filter is not washable or reusable. The air filter cover protects the device in the event of accidental liquid spillage onto the device. Ensure the air filter and air filter cover are fitted at all times. SERVICING CAUTION! Do not attempt to open the S8 Lightweight case. There are no user serviceable parts inside. Inspection and repair should only be performed by an authorised agent. Under no circumstances should you attempt to service or repair the flow generator yourself. This device is intended to provide safe and reliable operation when operated and maintained in accordance with the instructions provided by ResMed. To ensure that your device continues to provide optimum performance it is recommended that this product (S8 Lightweight) be inspected by an authorised ResMed Service Centre five years from the date of purchase. Applicable ResMed warranty details are provided with the device at the time of original supply. Of course, as with all electrical devices, if any irregularity becomes apparent, you should exercise caution and have the device inspected by an Authorised ResMed Service Centre. If you feel that your flow generator is not performing properly, see Troubleshooting on page
24 FREQUENTLY ASKED QUESTIONS HOW WILL IT FEEL WHEN I AM BREATHING WITH THE S8 LIGHTWEIGHT? When you first use your S8 LIGHTWEIGHT, you may feel uncomfortable breathing against the airflow. This is normal. You will continue to breathe normally while you sleep. Take time to adjust to this new sensation. WHAT DO I DO WHEN I WANT TO GET UP IN THE NIGHT? If you need to get up during the night, remove your mask and stop therapy. Always remember to restart therapy and put your mask on when you return to bed. WHAT DO I DO IF I FEEL AIR LEAKING OUT OF THE MASK? The flow generator delivers the most effective treatment when the mask is well fitted and comfortable. Treatment can be affected by leaks, so it is important to eliminate any leaks that may arise. For example, try repositioning the mask and adjusting the straps. If you are using a nasal mask, try to keep your mouth closed during treatment. Air leaks from your mouth can decrease the effectiveness of your treatment. If mouth leaks are a problem, a full face mask or chin strap may help. Contact your clinician or equipment supplier for further details. If you are using a full face mask and you have problems trying to get a comfortable mask fit, contact your sleep clinic or equipment supplier. You may benefit from a different size or style of mask. ANYTHING SPECIAL I SHOULD DO BEFORE PUTTING THE MASK ON? Before wearing your mask, wash your face to remove excess facial oils. This will allow a better fit and prolong the life of the mask cushion. Do not use moisturiser when wearing a mask. MY MOUTH FEELS DRY DURING TREATMENT WHAT CAN I DO? You may experience dryness of the nose, mouth and/or throat during the course of treatment, especially during winter. In many cases, a humidifier may resolve this discomfort. Contact your clinician for advice. MY NOSE IS IRRITATED DURING TREATMENT WHAT CAN I DO? You may experience sneezing and/or a runny or blocked nose during the first few weeks of treatment. In many cases, nasal irritation can be resolved with a humidifier. Consult your clinician or equipment supplier for advice. CAN I TRAVEL WITH THE S8 LIGHTWEIGHT? International use Your S8 LIGHTWEIGHT flow generator has an internal power supply that enables it to operate in other countries. It will operate on power supplies of V and 50/60 Hz. No special adjustment is necessary, but you may need a plug adapter for the power outlet. FREQUENTLY ASKED QUESTIONS 19
25 Altitude changes If you are moving or travelling to a higher or lower altitude, see How to change the Altitude setting on page 13. On an aircraft Please consult the medical services department of your carrier if you intend to use your S8 LIGHTWEIGHT on an aircraft. The S8 LIGHTWEIGHT complies with US FCC Part 15, Class B requirements. Note: Do not use your S8 Lightweight while the aircraft is taking off or landing.! CAN I USE MY S8 LIGHTWEIGHT IF MAINS (AC) POWER IS NOT AVAILABLE? A DC-12 converter is available to connect the S8 LIGHTWEIGHT to a 12V or 24V DC power source. Contact your clinician for details. CAUTION The device should not be connected to both AC and DC power sources simultaneously. 20
26 TROUBLESHOOTING If there is a problem, try the following suggestions. If the problem cannot be solved, contact your equipment supplier or ResMed. Do not attempt to open the device. Problem Possible Cause Solution TROUBLESHOOTING No display. Power not connected. Ensure the appropriate power cord is connected to your S8 Lightweight and the power switch (if available) is on. Insufficient air delivered from S8 Lightweight. Excessive air delivered from the S8 Lightweight. Display error message: Exxxx Call Service (where xxxx defines an error) or MOTOR FAULT Call Service or UPLOAD LANGUAGES Call Service or DATA LOST Call Service Ramp Time is in use. Air filter is dirty. Air tubing is kinked or punctured. Air tubing not connected properly. Mask and headgear not positioned correctly. Cap(s) missing from access port(s) on mask. Altitude compensation setting is incorrect. Pressure required for treatment may have changed. Altitude compensation setting is incorrect. Component failure. Wait for air pressure to build up or change ramp time. Replace air filter. Straighten or replace tubing. Check air tubing. Adjust position of mask and headgear. Replace cap(s). Adjust altitude compensation setting to the correct altitude range. See your clinician to adjust the pressure. Adjust altitude compensation setting to the correct altitude range. Call service. 21
27 Problem Possible Cause Solution The following message is displayed on the LCD after you try to update settings using the Data Card: Card Error Remove Card The following message is displayed on the LCD after you try to copy data to a Data Card: Card Error Remove Card The following message is displayed on the LCD after you try to update the settings using the Data Card: Settings Invalid Remove Card The following message is displayed on the LCD after you try to update the settings using the Data Card: Settings Error Remove Card The following message is NOT displayed on the LCD after you try to update the settings using the Data Card: Settings Success Remove Card The Data Card is not inserted correctly. The Data Card is only partly inserted. You may have removed the Data Card before settings were copied to the S8 Lightweight. The card is not a ResScan Data Card. The Data Card is not inserted correctly. The card is not a ResScan Data Card. The identification details on the Data Card do not match the details on your device. There is a data error on the Data Card. The settings were not updated. Ensure that the Data Card is inserted with the arrow facing up and pointing towards the device. Ensure that the device is switched on and in standby mode before you insert the Data Card. Ensure that the Data Card is inserted into the slot on the Data Card module as far as it will go. Reinsert the Data Card and wait for the Settings Success Remove Card message to appear on the LCD. Only use a ResScan Data Card. Ensure that the Data Card is inserted with the arrow facing up and pointing toward the device. Use a ResScan Data Card, as specified in this manual. Contact your clinician immediately. Contact your clinician immediately. Contact your clinician immediately. 22
28 SYSTEM SPECIFICATIONS S8 LIGHTWEIGHT Performance: Operating pressure range: 4 to 20 cm H 2 O Dimensions (H x W x D): 112 mm x 164 mm x 145 mm Weight: 1.3 kg Power Supply Input range for S8 LIGHTWEIGHT with HUMIDAIRE 3i: V, 50/60Hz; 110V, 400Hz; 2.5A < 140VA (110W) (maximum power consumption). Instantaneous peak power consumption <340VA. Refer to the DC-12 converter instructions for DC ratings. Only use the ResMed DC-12 converter for DC input. Actual power consumption will vary, depending on factors such as the use of accessories, height above sea level and ambient temperature. Power consumption values for typical treatment conditions (using a breathing machine set to 0.5 L and 15 breaths/min with 2 m air tubing and an ULTRA MIRAGE mask) are given in the following table. SYSTEM SPECIFICATIONS Device Pressure (cm H 2 O) Power (VA) Power (W) Power outlet for HumidAire 3i Outlet range: V, 50/60Hz; 110V, 400Hz; 2.0A Air Filter: Two-layered, powder-bonded, polyester non-woven fibre Air Tubing: Flexible plastic, 2 m Air Outlet: The 22 mm conical outlet complies with EN IEC Classifications: Class II (double insulation), Type CF Noise Level: Radiated sound pressure is measured at <30dBA at 10 cm H 2 O and 1 m according to ISO ACCESSORIES S8 LIGHTWEIGHT DATA CARD Dimensions (L x W x D): mm x mm x 0.85 mm 23
29 DATA CARD MODULE Dimensions (H x W x D): 49.7 mm x 42.2 mm x 12 mm ALL DEVICES (WHERE APPLICABLE) Housing Construction: Flame retardant engineering thermoplastic Environmental Conditions Operating Temperature: +5 C to +40 C Operating Humidity: 10% 95% non-condensing Storage and Transport Temperature: -20 C to +60 C Storage and Transport Humidity: 10% 95% non-condensing Operating Altitude: sea level to 2591 m Electromagnetic Compatibility Product complies with all applicable electromagnetic compatibility requirements (EMC) according to IEC , for residential, commercial, and light industry environments. For further details, see Guidance and Manufacturer s Declaration - Electromagnetic Emissions and Immunity on page 25. Note: The manufacturer reserves the right to change these specifications without notice. SYMBOLS WHICH APPEAR ON THE DEVICE Attention, consult accompanying documents Type CF equipment Class II equipment Drip proof Dangerous voltage Start/Stop 24
30 GUIDANCE AND MANUFACTURER S DECLARATION - ELECTROMAGNETIC EMISSIONS AND IMMUNITY Guidance and manufacturer s declaration electromagnetic emissions The S8 series of devices is intended for use in the electromagnetic environment specified below. The customer or the user of the S8 device should assure that the device is used in such an environment. Emissions test Compliance Electromagnetic environment guidance SYSTEM SPECIFICATIONS RF emissions CISPR11 Group 1 The S8 device uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. RF emissions CISPR 11 with serial adapter RF emissions CISPR 11 with USB adapter Harmonic Emissions IEC Voltage Fluctuations/Flicker Emissions IEC Class B* Class B Class A Complies The S8 device is suitable for use in all establishments, including domestic establishments and those directly connected to the public low-voltage network that supplies buildings used for domestic purposes. * Class B for all system configurations except when a PC is attached to the S8 device via a serial adapter, in which case the system is Class A compliant. Class A is suitable for use in all establishments other than domestic and those directly connected to a low voltage power supply network which supplies buildings used for domestic purposes. Medical Electrical Equipment needs special precautions regarding EMC and needs to be installed and put into service according to EMC information provided in this document. Warnings: The S8 device should not be used adjacent to or stacked with other equipment. If adjacent or stacked use is necessary, the S8 device should be observed to verify normal operation in the configuration in which it will be used. The use of accessories (eg humidifiers) other than those specified in this manual is not recommended. They may result in increased emissions or decreased immunity of the S8 device. 25
31 Guidance and manufacturer s declaration electromagnetic immunity The S8 series of devices is intended for use in the electromagnetic environment specified below. The customer or the user of the S8 device should assure that the device is used in such an environment. Immunity test IEC test level Compliance level Electromagnetic environment guidance Electrostatic discharge (ESD) IEC ±6 kv contact ±8 kv air ±6 kv contact ±8 kv air Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%. Electrical fast transient/burst IEC ±2 kv for power supply lines ±1 kv for input/ output lines ±2 kv Not Applicable Mains power quality should be that of a typical commercial or hospital environment. Surge IEC ±1 kv differential mode ±2 kv common mode ±1 kv differential mode ±2 kv common mode Mains power quality should be that of a typical commercial or hospital environment. Voltage dips, short interruptions and voltage variations on power supply input lines. IEC <5% Ut (>95% dip in Ut) for 0.5 cycle 40% Ut (60% dip in Ut) for 5 cycles 70% Ut (30% dip in Ut) for 25 cycles <5% Ut (>95%dip in Ut) for 5 sec < 12V (>95% dip in 240V) for 0.5 cycle 96V (60% dip in 240V) for 5 cycles 168V (30% dip in 240V) for 25 cycles <12V (>95%dip in 240V) for 5 sec Mains power quality should be that of a typical commercial or hospital environment. If the user of the S8 device requires continued operation during power mains interruptions, it is recommended that the S8 device be powered from an uninterruptible power source. Power frequency (50/60 Hz) magnetic field IEC A/m 3 A/m Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. NOTE: Ut is the AC mains voltage prior to application of the test level. 26
32 Guidance and manufacturer s declaration electromagnetic immunity The S8 series of devices is intended for use in the electromagnetic environment specified below. The customer or the user of the S8 device should assure that the device is used in such an environment. Immunity test IEC test level Compliance level Electromagnetic environment guidance SYSTEM SPECIFICATIONS Conducted RF IEC Radiated RF IEC Vrms 150 khz to 80 MHz 10 V/m 80 MHz to 2.5 GHz 3 Vrms d = 1.17 P Portable and mobile RF communications equipment should be used no closer to any part of the S8 device, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter. Recommended separation distance 10 V/m d = 0.35 P 80 MHz to 800 MHz d = 0.70 P 800 MHz to 2.5 GHz where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in meters (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey, a should be less than the compliance level in each frequency range. b Interference may occur in the vicinity of equipment marked with the following symbol: NOTE 1: At 80 MHz and 800MHz, the higher frequency range applies. NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the S8 device is used exceeds the applicable RF compliance level above, the S8 device should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the S8 device. b Over the frequency range 150 khz to 80 MHz, field strengths should be less than 10 V/m. 27
33 Recommended separation distances between portable and mobile RF communications equipment and the S8 series of devices The S8 series of devices is intended for use in an environment in which radiated RF disturbances are controlled. The customer or the user of the S8 device can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the S8 device as recommended below, according to the maximum output power of the communications equipment. Separation distance according to frequency of transmitter m Rated maximum output power of transmitter W 150kHz to 80MHz d = 1.17 P 80 MHz to 800 MHz d = 0.35 P 800MHz to 2.5 GHz d = 0.7 P For transmitters rated at a maximum output power not listed above, the recommended separation distance d in metres (m) can be determined using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. 28
34 INDEX A accessories 6 air filter 5, 21 replacing 18 air pressure 21 air tubing 5 cleaning 17 connect to air outlet 7 kinked 21 positioning 7 aircraft 20 airflow breathing with 19 altitude setting 13, 20, 21 B backlight LCD and keypad 11 C carry bag 5 cleaning daily cleaning 17 periodic 17 contraindications 1 CPAP 1 D Data Card 15 copying data 16 data card 22 inserting 15 mailback envelope 16 removing 16 Data Card module 15 DC-12 converter 20 down key 11 dryness mouth, nose or throat 19 E error message 21 F frequently asked questions 19 H headgear 21 HumidAire attaching 9 HumidAire 3i attaching 9 storage 5 humidifier 19 cleaning 17 humidifiers 6 I international use 19 K keypad 5, 11 keypad keys functions 11 L language setting 13 LCD 5, 7, 11 error messages 22 messages 14 left key 11 M mailback envelope 16 mask 19, 21 air leak 19 attaching 7 cleaning 17 removing 19 mask leak 19 mask port 21 masks 5 medical information 1 menu screens 12 access 12 change 12 exit 13 scrolling 12 module attaching 5 blank 15 Data Card 15 modules 15 blank 15 INDEX 29
35 N nasal irritation 19 P power cord 5, 21 connecting 7 power sockets 5 power sources 20 power supply 23 R ramp time 8, 13, 21 reminder replace mask 14 reminders 14 ResScan Data Card module 6 right key 11 S S8 Lightweight dimensions 23, 24 servicing 18 settings 12 set-up 7 start treatment 8 stop treatment 8 travelling with 19 setting up 7 software version number 13 standby screen 7 start/stop key 11 SW 13 system specifications 23 T troubleshooting 21 U up key 11 Usage 13 use on aircraft 20 Used hours 13 W weekly cleaning 17 30
S8 Lightweight II. User Guide. English POSITIVE AIRWAY PRESSURE DEVICE
 S8 Lightweight II POSITIVE AIRWAY PRESSURE DEVICE User Guide English ResMed Ltd (Manufacturer) 1 Elizabeth Macarthur Drive Bella Vista NSW 2153 Australia ResMed Corp (US Designated Agent) 14040 Danielson
S8 Lightweight II POSITIVE AIRWAY PRESSURE DEVICE User Guide English ResMed Ltd (Manufacturer) 1 Elizabeth Macarthur Drive Bella Vista NSW 2153 Australia ResMed Corp (US Designated Agent) 14040 Danielson
S8 AutoScore II System User Guide. English
 S8 AutoScore II System User Guide English ResMed Ltd (Manufacturer) 1 Elizabeth Macarthur Drive Bella Vista NSW 2153 Australia ResMed Corp (US Designated Agent) 14040 Danielson Street Poway CA 92064-6857
S8 AutoScore II System User Guide English ResMed Ltd (Manufacturer) 1 Elizabeth Macarthur Drive Bella Vista NSW 2153 Australia ResMed Corp (US Designated Agent) 14040 Danielson Street Poway CA 92064-6857
S8 Series II. S8 AutoSet Spirit II S8 AutoScore II. User Guide. English POSITIVE AIRWAY PRESSURE DEVICES
 S8 Series II S8 AutoSet Spirit II S8 AutoScore II POSITIVE AIRWAY PRESSURE DEVICES User Guide English ResMed Ltd (Manufacturer) 1 Elizabeth Macarthur Drive Bella Vista NSW 2153 Australia ResMed Corp (US
S8 Series II S8 AutoSet Spirit II S8 AutoScore II POSITIVE AIRWAY PRESSURE DEVICES User Guide English ResMed Ltd (Manufacturer) 1 Elizabeth Macarthur Drive Bella Vista NSW 2153 Australia ResMed Corp (US
VPAP IV VPAP IV ST. User Guide. English. Positive Airway pressure device
 VPAP IV VPAP IV ST Positive Airway pressure device User Guide English English VPAP IV VPAP IV ST POSITIVE AIRWAY PRESSURE DEVICE User Guide English Manufacturer: ResMed Ltd 1 Elizabeth Macarthur Drive
VPAP IV VPAP IV ST Positive Airway pressure device User Guide English English VPAP IV VPAP IV ST POSITIVE AIRWAY PRESSURE DEVICE User Guide English Manufacturer: ResMed Ltd 1 Elizabeth Macarthur Drive
USER S MANUAL. Reorder number: /
 RESMED USER S MANUAL Reorder number: 308167/1 04 05 Manufactured by: ResMed Ltd 97 Waterloo Road North Ryde NSW 2113 Australia Tel: +61 (2) 9886 5000 or 1 800 658 189 (toll free) Fax: +61 (2) 9878 0120
RESMED USER S MANUAL Reorder number: 308167/1 04 05 Manufactured by: ResMed Ltd 97 Waterloo Road North Ryde NSW 2113 Australia Tel: +61 (2) 9886 5000 or 1 800 658 189 (toll free) Fax: +61 (2) 9878 0120
S8 Auto 25. User Guide. English. Positive Airway pressure device
 S8 Auto 25 Positive Airway pressure device User Guide S8 Auto 25 POSITIVE AIRWAY PRESSURE DEVICE User Guide ResMed Ltd (Manufacturer) 1 Elizabeth Macarthur Drive Bella Vista NSW 2153 Australia ResMed Corp
S8 Auto 25 Positive Airway pressure device User Guide S8 Auto 25 POSITIVE AIRWAY PRESSURE DEVICE User Guide ResMed Ltd (Manufacturer) 1 Elizabeth Macarthur Drive Bella Vista NSW 2153 Australia ResMed Corp
Mirage Micro. User Guide. English. nasal mask
 Mirage Micro nasal mask User Guide English Mirage Micro nasal mask Thank you for choosing the Mirage Micro. Intended Use Rx Only The Mirage Micro channels airflow noninvasively to a patient from a positive
Mirage Micro nasal mask User Guide English Mirage Micro nasal mask Thank you for choosing the Mirage Micro. Intended Use Rx Only The Mirage Micro channels airflow noninvasively to a patient from a positive
S8 AutoSet Spirit System
 B INTEGRATED HUMIDIFIER D SETTING UP THE S8 Component of 338193/1 S8 AutoSet Spirit System ILLUSTRATIONS A SYSTEM COMPONENTS Carry handle HumidAire 3i 2005 ResMed Ltd. 1 2 LCD screen Keypad Air outlet
B INTEGRATED HUMIDIFIER D SETTING UP THE S8 Component of 338193/1 S8 AutoSet Spirit System ILLUSTRATIONS A SYSTEM COMPONENTS Carry handle HumidAire 3i 2005 ResMed Ltd. 1 2 LCD screen Keypad Air outlet
Please read the entire Information and Welcome Guides before using your device.
 S9 Auto 25 POSITIVE AIRWAY PRESSURE DEVICE Information Guide Please read the entire Information and Welcome Guides before using your device. Indications for use The S9 Auto 25 is indicated for the treatment
S9 Auto 25 POSITIVE AIRWAY PRESSURE DEVICE Information Guide Please read the entire Information and Welcome Guides before using your device. Indications for use The S9 Auto 25 is indicated for the treatment
CPAP System. User Manual. NOTE: The C-Flex mark is used under license.
 CPAP System User Manual NOTE: The C-Flex mark is used under license. Table of Contents Symbol Key... 2 Intended Use & Contraindications... 3 Specifications... 4 Warnings... 5 Cautions... 6 Unpacking the
CPAP System User Manual NOTE: The C-Flex mark is used under license. Table of Contents Symbol Key... 2 Intended Use & Contraindications... 3 Specifications... 4 Warnings... 5 Cautions... 6 Unpacking the
VPAP III ST-A User Manual. English
 VPAP III ST-A User Manual English B HUMIDIFIERS VPAP III ST-A Illustrations Component of 248602/1 A SYSTEM COMPONENTS 1 HumidAire 2i 2 HumidAire 2iC LCD screen Handle Control panel Removable front cover
VPAP III ST-A User Manual English B HUMIDIFIERS VPAP III ST-A Illustrations Component of 248602/1 A SYSTEM COMPONENTS 1 HumidAire 2i 2 HumidAire 2iC LCD screen Handle Control panel Removable front cover
English Please read the entire Information and Welcome Guides before using your device.
 VPAP ST with ivaps NONINVASIVE VENTILATOR Information Guide English Please read the entire Information and Welcome Guides before using your device. Indications for use The VPAP ST is indicated to provide
VPAP ST with ivaps NONINVASIVE VENTILATOR Information Guide English Please read the entire Information and Welcome Guides before using your device. Indications for use The VPAP ST is indicated to provide
Mirage Vista Nasal Mask
 English 60 50 40 30 20 10 0 4 6 10 12 14 16 1 20 ENGLISH Mirage Vista Nasal Mask Rx Only The Mirage Vista Nasal Mask is an accessory to a non-continuous ventilator (respirator) intended for multipatient
English 60 50 40 30 20 10 0 4 6 10 12 14 16 1 20 ENGLISH Mirage Vista Nasal Mask Rx Only The Mirage Vista Nasal Mask is an accessory to a non-continuous ventilator (respirator) intended for multipatient
English Please read the entire Information and Welcome Guides before using your device.
 VPAP Adapt POSITIVE AIRWAY PRESSURE DEVICE Information Guide English Please read the entire Information and Welcome Guides before using your device. Indications for use The VPAP Adapt is indicated to stabilise
VPAP Adapt POSITIVE AIRWAY PRESSURE DEVICE Information Guide English Please read the entire Information and Welcome Guides before using your device. Indications for use The VPAP Adapt is indicated to stabilise
VPAP IV ST POSITIVE AIRWAY PRESSURE DEVICE
 VPAP IV ST POSITIVE AIRWAY PRESSURE DEVICE Introducing the latest addition to the ResMed VPAP range the total solution in high performance bilevel therapy VPAP IV ST - Comfortable, Compact and Qu Comprehensive
VPAP IV ST POSITIVE AIRWAY PRESSURE DEVICE Introducing the latest addition to the ResMed VPAP range the total solution in high performance bilevel therapy VPAP IV ST - Comfortable, Compact and Qu Comprehensive
Swift LT NASAL PILLOWS SYSTEM. User Guide English
 Swift LT NASAL PILLOWS SYSTEM User Guide English Swift LT NASAL PILLOWS SYSTEM Thank you for choosing the Swift LT. Intended Use Rx Only The Swift LT channels airflow noninvasively to a patient from a
Swift LT NASAL PILLOWS SYSTEM User Guide English Swift LT NASAL PILLOWS SYSTEM Thank you for choosing the Swift LT. Intended Use Rx Only The Swift LT channels airflow noninvasively to a patient from a
pro pro 2 AND User Manual
 pro 2 AND This BiPAP system is covered by one or more of the following patents: US Patent Nos. 5,148,802; 5,313,937; 5,433,193; 5,632,269; 5,803,065; 6,029,664; 6,305,374; 6,539,940; 5,239,995; Re 35,295;
pro 2 AND This BiPAP system is covered by one or more of the following patents: US Patent Nos. 5,148,802; 5,313,937; 5,433,193; 5,632,269; 5,803,065; 6,029,664; 6,305,374; 6,539,940; 5,239,995; Re 35,295;
English. ivolve Full Face Mask. User Manual
 ivolve Full Face Mask User Manual 0123 Table of Contents ivolve Full Face Mask... 1 Intended Use... 1 Before Using the Mask... 2 Parts of the ivolve Full Face Mask... 4 Getting the Right Cushion Size...
ivolve Full Face Mask User Manual 0123 Table of Contents ivolve Full Face Mask... 1 Intended Use... 1 Before Using the Mask... 2 Parts of the ivolve Full Face Mask... 4 Getting the Right Cushion Size...
Instructions for Use. OptiLife Nasal Lab Mask
 Instructions for Use OptiLife Nasal Lab Mask Intended Use The OptiLife Nasal Mask is intended to provide an interface for application of CPAP or bi-level therapy to patients. The mask is for multi-patient
Instructions for Use OptiLife Nasal Lab Mask Intended Use The OptiLife Nasal Mask is intended to provide an interface for application of CPAP or bi-level therapy to patients. The mask is for multi-patient
RESmart 25T/30T BPAP System. User Manual
 RESmart 25T/30T BPAP System User Manual 0123 Table of Contents 1. Introduction... 2 1.1 System Contents... 2 1.2 Intended Use... 2 1.3 Warnings, Cautions and Contraindications... 4 1.3.1 Warnings... 4
RESmart 25T/30T BPAP System User Manual 0123 Table of Contents 1. Introduction... 2 1.1 System Contents... 2 1.2 Intended Use... 2 1.3 Warnings, Cautions and Contraindications... 4 1.3.1 Warnings... 4
Mirage Quattro FULL FACE MASK
 Mirage Quattro FULL FACE MASK User Guide English 61856/3 07 03 MIRAGE QUATTRO USER ENG ROW 6 1 8 5 6 Global leaders in sleep and respiratory medicine www.resmed.com ResMed Ltd (Manufacturer) 1 Elizabeth
Mirage Quattro FULL FACE MASK User Guide English 61856/3 07 03 MIRAGE QUATTRO USER ENG ROW 6 1 8 5 6 Global leaders in sleep and respiratory medicine www.resmed.com ResMed Ltd (Manufacturer) 1 Elizabeth
C-1 C-7. C-6 Short air tubing Upper Velcro straps. C-a C-5 C-2 C-4 C-3 B-1 B-9 B-10 B-2 B-7 B-8 B-3 B-6 D-1 D-2 D-13 D-3 D-12 D-10 D-11 D-4 D-5 D-8
 A Forehead support Forehead pads C Mask Frame Mask Elbow Cushion clip Mask Cushion C-7 C-1 Vent cover Ports cap Cuff ActiveCell C-6 Short air tubing Upper Velcro straps Swivel Lower Velcro straps Headgear
A Forehead support Forehead pads C Mask Frame Mask Elbow Cushion clip Mask Cushion C-7 C-1 Vent cover Ports cap Cuff ActiveCell C-6 Short air tubing Upper Velcro straps Swivel Lower Velcro straps Headgear
REMstar. M Series JR 1/22/08 EN-DOM
 REMstar M Series 1050727 JR 1/22/08 EN-DOM Im p o r t a n t! Fill in the information below when you receive the REMstar M Series system. Serial No.: (located on the bottom of the device) System Prescribed
REMstar M Series 1050727 JR 1/22/08 EN-DOM Im p o r t a n t! Fill in the information below when you receive the REMstar M Series system. Serial No.: (located on the bottom of the device) System Prescribed
Cirrus 5 Oxygen Concentrator Manual
 Cirrus 5 Oxygen Concentrator Manual Important: Make sure you read and understand all of the information JAGZ03-03-3B ver1.0 12/07/2015 contained in this manual before operating your oxygen concentrator!
Cirrus 5 Oxygen Concentrator Manual Important: Make sure you read and understand all of the information JAGZ03-03-3B ver1.0 12/07/2015 contained in this manual before operating your oxygen concentrator!
User guide User guide English
 User guide English ENGLISH Welcome The AirSense 10 AutoSet and AirSense 10 Elite are ResMed's premium auto-adjusting pressure and Continuous Positive Airway Pressure (CPAP) devices. WARNING Read this entire
User guide English ENGLISH Welcome The AirSense 10 AutoSet and AirSense 10 Elite are ResMed's premium auto-adjusting pressure and Continuous Positive Airway Pressure (CPAP) devices. WARNING Read this entire
Mirage FX. User Guide. English NASAL MASK
 Mirage FX NASAL MASK User Guide English Did you know? Replacing your mask and supplies is one of the most important steps in maintaining effective therapy. Replace when... The cushion is no longer clear
Mirage FX NASAL MASK User Guide English Did you know? Replacing your mask and supplies is one of the most important steps in maintaining effective therapy. Replace when... The cushion is no longer clear
Mirage Micro. User Guide. English. nasal mask
 Mirage Micro nasal mask User Guide English Mirage Micro nasal mask Thank you for choosing the Mirage Micro. Intended Use Rx Only The Mirage Micro channels airflow noninvasively to a patient from a positive
Mirage Micro nasal mask User Guide English Mirage Micro nasal mask Thank you for choosing the Mirage Micro. Intended Use Rx Only The Mirage Micro channels airflow noninvasively to a patient from a positive
Mirage Liberty. User Guide 61850/ APAC ENG MIRAGE LIBERTY USER r2 cover.qxp 3/04/2007 4:39 PM Page 1. English FULL FACE MASK
 61850r2 cover.qxp 3/04/2007 4:39 PM Page 1 Mirage Liberty FULL FACE MASK User Guide English 61850/2 07 04 MIRAGE LIBERTY USER APAC ENG 6 1 8 5 0 Global leaders in sleep and respiratory medicine www.resmed.com
61850r2 cover.qxp 3/04/2007 4:39 PM Page 1 Mirage Liberty FULL FACE MASK User Guide English 61850/2 07 04 MIRAGE LIBERTY USER APAC ENG 6 1 8 5 0 Global leaders in sleep and respiratory medicine www.resmed.com
PROGRAMMER OPERATIONS MANUAL
 PROGRAMMER OPERATIONS MANUAL Table of Contents Description 4 Installation 4 Operation 5 Safety Precautions 5 Regulatory & Service Information 6 Important Safety and Usage Information 7 Regulatory Notices
PROGRAMMER OPERATIONS MANUAL Table of Contents Description 4 Installation 4 Operation 5 Safety Precautions 5 Regulatory & Service Information 6 Important Safety and Usage Information 7 Regulatory Notices
User guide User guide English
 User guide English ENGLISH Welcome The AirCurve 10 VAuto and AirCurve 10 S are bilevel positive airway pressure devices. WARNING Read this entire guide before using the device. Use the device according
User guide English ENGLISH Welcome The AirCurve 10 VAuto and AirCurve 10 S are bilevel positive airway pressure devices. WARNING Read this entire guide before using the device. Use the device according
A M Respironics Deutschland M u r r y s v i l l e, PA Gewerbestrasse 17 w w w. r e s p i r o n i c s. c o m
 1001 Murry Ridge Lane Respironics Deutschland M u r r y s v i l l e, PA 1 5 6 6 8 Gewerbestrasse 17 w w w. r e s p i r o n i c s. c o m 8221 Herrsching, German y 1020121 A M1040442 9/12/06 E N - U S, F
1001 Murry Ridge Lane Respironics Deutschland M u r r y s v i l l e, PA 1 5 6 6 8 Gewerbestrasse 17 w w w. r e s p i r o n i c s. c o m 8221 Herrsching, German y 1020121 A M1040442 9/12/06 E N - U S, F
English USER GUIDE User guide
 USER GUIDE English ENGLISH A Elbow E Cushion B Elbow ring 1 Vent (in cushion and elbow) C Headgear 2 Side buttons D Frame 3 Sleeve Intended use The AirFit N30i mask is intended to be used by patients weighing
USER GUIDE English ENGLISH A Elbow E Cushion B Elbow ring 1 Vent (in cushion and elbow) C Headgear 2 Side buttons D Frame 3 Sleeve Intended use The AirFit N30i mask is intended to be used by patients weighing
EasyFit Lite Nasal Mask, Silicone. Nasal Mask. EasyFit Lite Nasal Mask. Device Description and Instructions for Use. Caution:
 EasyFit Lite Nasal Mask EasyFit Lite Nasal Mask, Silicone Nasal Mask Device Description and Instructions for Use Caution: Federal Law (U.S.A.) restricts this device to sale by or on the order of a physician.
EasyFit Lite Nasal Mask EasyFit Lite Nasal Mask, Silicone Nasal Mask Device Description and Instructions for Use Caution: Federal Law (U.S.A.) restricts this device to sale by or on the order of a physician.
USER MANUAL SAVE THESE INSTRUCTIONS. For the most current manual revision, please visit our Website:
 USER MANUAL Model: PM4300 Series SAVE THESE INSTRUCTIONS For the most current manual revision, please visit our Website: www.precisionmedical.com 300 Held Drive Tel: (+001) 610-262-6090 Northampton, PA
USER MANUAL Model: PM4300 Series SAVE THESE INSTRUCTIONS For the most current manual revision, please visit our Website: www.precisionmedical.com 300 Held Drive Tel: (+001) 610-262-6090 Northampton, PA
Model 1700 Patient Operating Instructions. Model 1700
 1700-00C.QXD 10/21/99 8:38 AM Page 1 The Respironics Tranquility Bilevel is a medical device. It has been prescribed by a physician to assist breathing. Compact Compact Bilevel Bilevel System System Model
1700-00C.QXD 10/21/99 8:38 AM Page 1 The Respironics Tranquility Bilevel is a medical device. It has been prescribed by a physician to assist breathing. Compact Compact Bilevel Bilevel System System Model
REMstar. M Series with SmartCard JR 3/05/2009 EN-DOM
 REMstar M Series with SmartCard 1058374 JR 3/05/2009 EN-DOM Im p o r t a n t! Fill in the information below when you receive the REMstar M Series system. Serial No.: (located on the bottom of the device)
REMstar M Series with SmartCard 1058374 JR 3/05/2009 EN-DOM Im p o r t a n t! Fill in the information below when you receive the REMstar M Series system. Serial No.: (located on the bottom of the device)
Oxygen Dialflow Meter. Instructions for Use
 Oxygen Dialflow Meter Instructions for Use 702-0031.9 May 2014 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in personal injury to the user
Oxygen Dialflow Meter Instructions for Use 702-0031.9 May 2014 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in personal injury to the user
My CoughAssist. A patient guide to CoughAssist T70. Please visit
 Philips Healthcare is part of Royal Philips Electronics Europe, Middle East, Africa +49 7031 463 2254 How to reach us www.philips.com/healthcare healthcare@philips.com Latin America +55 11 2125 0744 Asia
Philips Healthcare is part of Royal Philips Electronics Europe, Middle East, Africa +49 7031 463 2254 How to reach us www.philips.com/healthcare healthcare@philips.com Latin America +55 11 2125 0744 Asia
Sensor. Directions For Use
 Nasal Alar SpO2 Sensor Directions For Use Emergo Europe Prinsessegracht 20 2514 AP The Hague The Netherlands Manufactured by: Xhale Assurance, Inc. 3630 SW 47th Ave., Suite 100 Gainesville, FL 32608, USA
Nasal Alar SpO2 Sensor Directions For Use Emergo Europe Prinsessegracht 20 2514 AP The Hague The Netherlands Manufactured by: Xhale Assurance, Inc. 3630 SW 47th Ave., Suite 100 Gainesville, FL 32608, USA
(DynaLaL III) Dynamic True Low Air Loss Mattress Replacement System
 (DynaLaL III) User Manual Dynamic True Low Air Loss Mattress Replacement System Alternating + True Low Air Loss Pressure Relief System Manufactured by: Caremed Supply, Inc. Distributed by: Quart Healthcare
(DynaLaL III) User Manual Dynamic True Low Air Loss Mattress Replacement System Alternating + True Low Air Loss Pressure Relief System Manufactured by: Caremed Supply, Inc. Distributed by: Quart Healthcare
BiPAP Auto. M Series JR 1/22/08 EN-DOM
 BiPAP Auto M Series 1050651 JR 1/22/08 EN-DOM Im p o r t a n t! Fill in the information below when you receive the BiPAP Auto M Series system. Serial No.: (located on the bottom of the device) System Prescribed
BiPAP Auto M Series 1050651 JR 1/22/08 EN-DOM Im p o r t a n t! Fill in the information below when you receive the BiPAP Auto M Series system. Serial No.: (located on the bottom of the device) System Prescribed
REMstar Pro. M Series JR 1/22/08 EN-DOM
 REMstar Pro M Series 1050656 JR 1/22/08 EN-DOM Im p o r t a n t! Fill in the information below when you receive the REMstar Pro M Series system. Serial No.: (located on the bottom of the device) System
REMstar Pro M Series 1050656 JR 1/22/08 EN-DOM Im p o r t a n t! Fill in the information below when you receive the REMstar Pro M Series system. Serial No.: (located on the bottom of the device) System
User manual. PG89 Rev 01 Editi Pressotherapy model. LymphoPress 4
 User manual PG89 Rev 01 Editi 040515 Pressotherapy model LymphoPress 4 I.A.C.E.R. Srl Via S. Pertini 24/A 30030 Martellago (VE) ITALY Tel. 041.5401356 Fax 041.5402684 e-mail: iacer@iacer.it http://www.itechmedicaldivision.com
User manual PG89 Rev 01 Editi 040515 Pressotherapy model LymphoPress 4 I.A.C.E.R. Srl Via S. Pertini 24/A 30030 Martellago (VE) ITALY Tel. 041.5401356 Fax 041.5402684 e-mail: iacer@iacer.it http://www.itechmedicaldivision.com
Owner s Manual. Model No.: 9S Item No Please read the instruction manual before use
 Owner s Manual Model No.: 9S-005720 Item No. 7541 Please read the instruction manual before use Contents Important Safeguards... 1 1. Introduction... 3 2. Product Description...5 3. Installation...6 4.
Owner s Manual Model No.: 9S-005720 Item No. 7541 Please read the instruction manual before use Contents Important Safeguards... 1 1. Introduction... 3 2. Product Description...5 3. Installation...6 4.
U S E R M A N U A L. Model: PM4300 Series SAVE THESE INSTRUCTIONS
 U S E R M A N U A L Model: PM4300 Series (PM4351 Shown) SAVE THESE INSTRUCTIONS CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090
U S E R M A N U A L Model: PM4300 Series (PM4351 Shown) SAVE THESE INSTRUCTIONS CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090
USER MANUAL SAVE THESE INSTRUCTIONS. 300 Held Drive Tel: (+001) Northampton, PA USA Fax: (+001) ISO Certified
 USER MANUAL Model: PM4300 Series (PM4351 Shown) SAVE THESE INSTRUCTIONS Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090 Northampton,
USER MANUAL Model: PM4300 Series (PM4351 Shown) SAVE THESE INSTRUCTIONS Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090 Northampton,
REF R00 JH 02/04/11 USER MANUAL
 REF 1082564 1082563 R00 JH 02/04/11 USER MANUAL UltraFill User Manual Table of Contents Introduction...3 How the System Works...7 Troubleshooting Guide... 19 Cleaning and Maintenance... 22 Transporting
REF 1082564 1082563 R00 JH 02/04/11 USER MANUAL UltraFill User Manual Table of Contents Introduction...3 How the System Works...7 Troubleshooting Guide... 19 Cleaning and Maintenance... 22 Transporting
Micro Dial-Flowmeter. Instructions for Use
 Micro Dial-Flowmeter Instructions for Use 702-0082.8 May 2014 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in personal injury to the user
Micro Dial-Flowmeter Instructions for Use 702-0082.8 May 2014 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in personal injury to the user
Device overview. 6 Breathing circuit connection. Note: When the remote control foot pedal is connected, the manual switch is disabled.
 CoughAssist E70 Quick start guide 1 2 3 4 5 Device overview Starting therapy Modifying therapy settings Monitoring view and icons 6 7 Suggested guidelines for therapy Device menu Mask fitting 1 Device
CoughAssist E70 Quick start guide 1 2 3 4 5 Device overview Starting therapy Modifying therapy settings Monitoring view and icons 6 7 Suggested guidelines for therapy Device menu Mask fitting 1 Device
English USER GUIDE User guide
 USER GUIDE English ENGLISH A QuietAir / standard elbow 3 Side buttons B Frame 4 Magnetic clips C Cushion 5 Upper headgear strap D Headgear 6 Lower headgear strap 1 Vent 7 Top headgear strap 2 Anti-Asphyxia
USER GUIDE English ENGLISH A QuietAir / standard elbow 3 Side buttons B Frame 4 Magnetic clips C Cushion 5 Upper headgear strap D Headgear 6 Lower headgear strap 1 Vent 7 Top headgear strap 2 Anti-Asphyxia
Table of Contents. isleep 20 clinician s manual
 Table of Contents 1 Intended Use of the isleep 20... 3 1.1 What is the isleep 20?... 3 1.2 Indications for Use... 4 1.3 Contraindications... 4 1.4 About this Manual... 5 2 Safety Information... 7 2.1 General
Table of Contents 1 Intended Use of the isleep 20... 3 1.1 What is the isleep 20?... 3 1.2 Indications for Use... 4 1.3 Contraindications... 4 1.4 About this Manual... 5 2 Safety Information... 7 2.1 General
Pressure Regulator. Instructions for Use
 Pressure Regulator Instructions for Use 702-0083.9 December 2017 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in injury to the patient, the
Pressure Regulator Instructions for Use 702-0083.9 December 2017 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in injury to the patient, the
Oxygen Dialflow Meter. Instructions for Use
 Oxygen Dialflow Meter Instructions for Use 702-0031.12 December 2017 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in injury to the patient,
Oxygen Dialflow Meter Instructions for Use 702-0031.12 December 2017 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in injury to the patient,
AUTOVENT 4000 VENTILATOR
 OVERVIEW AUTOVENT 4000 Only properly trained and approved Escambia County Bureau of Public Safety Paramedics are to use the AutoVent 4000 ventilator manufactured by LSP to transport patients already on
OVERVIEW AUTOVENT 4000 Only properly trained and approved Escambia County Bureau of Public Safety Paramedics are to use the AutoVent 4000 ventilator manufactured by LSP to transport patients already on
Mirage Micro for Kids. nasal mask
 Mirage Micro for Kids nasal mask Mirage Micro for Kids A B C D Removal / Enlèvement / Cómo quitarse la mascarilla / Remoção Disassembly / Démontage / Desmontaje / Desmontagem 1 2 3 4 5 6 7 8 Reassembly
Mirage Micro for Kids nasal mask Mirage Micro for Kids A B C D Removal / Enlèvement / Cómo quitarse la mascarilla / Remoção Disassembly / Démontage / Desmontaje / Desmontagem 1 2 3 4 5 6 7 8 Reassembly
SC505 Sof Care Inflator. Operating Instructions and Service Manual
 SC505 Sof Care Inflator Gaymar Industries, Inc. 10 Centre Drive Orchard Park, NY 14127 Toll Free +1 800.828.7341 + 1 716.662.2551 Fax +1 800.993.7890 Outside USA +1 716.662.8636 Outside USA Fax +1 716.662.0730
SC505 Sof Care Inflator Gaymar Industries, Inc. 10 Centre Drive Orchard Park, NY 14127 Toll Free +1 800.828.7341 + 1 716.662.2551 Fax +1 800.993.7890 Outside USA +1 716.662.8636 Outside USA Fax +1 716.662.0730
Table of Contents. Doc. No En S-1 BREAS MEDICAL 1
 PV 10 User Manual Table of Contents Table of Contents 1 Intended Use of the PV 10...3 1.1 What is the PV 10?...4 1.2 Indications for Use...4 1.3 Contraindications...5 1.4 About this Manual...6 2 Important
PV 10 User Manual Table of Contents Table of Contents 1 Intended Use of the PV 10...3 1.1 What is the PV 10?...4 1.2 Indications for Use...4 1.3 Contraindications...5 1.4 About this Manual...6 2 Important
Regalia Oxygen Concentrator
 Regalia Oxygen Concentrator REGALIA INSTRUCTION MANUAL P/N 5167 Rev B January 2014 Table of Contents Warnings and Cautions... 3 Indications for Use... 4 Introduction... 4 Important Safety Instructions...
Regalia Oxygen Concentrator REGALIA INSTRUCTION MANUAL P/N 5167 Rev B January 2014 Table of Contents Warnings and Cautions... 3 Indications for Use... 4 Introduction... 4 Important Safety Instructions...
MAGICFX CONFETTIPISTOL USER AND INSTALLATION MANUAL
 English MAGICFX CONFETTIPISTOL USER AND INSTALLATION MANUAL CONFETTI FX / STREAMER FX Manual trigger PART01129 rev 01-00 DISCLAIMER WARNING Read this manual carefully before installing and/or using this
English MAGICFX CONFETTIPISTOL USER AND INSTALLATION MANUAL CONFETTI FX / STREAMER FX Manual trigger PART01129 rev 01-00 DISCLAIMER WARNING Read this manual carefully before installing and/or using this
Contents Warnings, Cautions, and Notes Contents of the Package Introduction to the Harmony S/T A Tour of the Harmony S/T...
 S/T Patient Guide 1004221 JR 3/15/01 Contents 1 Warnings, Cautions, and Notes... 3 Warnings... 3 Cautions... 4 Temperatures... 4 Intended Use/Indications for Use... 5 Contraindications... 5 1 Contents
S/T Patient Guide 1004221 JR 3/15/01 Contents 1 Warnings, Cautions, and Notes... 3 Warnings... 3 Cautions... 4 Temperatures... 4 Intended Use/Indications for Use... 5 Contraindications... 5 1 Contents
Content
 Content Medical Disclaimer Intended Use Additional Information on Blood Pressure Precautions Parts Indicator & Classification of Blood Pressure Setting up your Blood Pressure Monitor Loading Batteries
Content Medical Disclaimer Intended Use Additional Information on Blood Pressure Precautions Parts Indicator & Classification of Blood Pressure Setting up your Blood Pressure Monitor Loading Batteries
Med Aire Alternating Pressure Pump and Pad System
 User Manual Med Aire Alternating Pressure Pump and Pad System 14002E 14001E Symbols & Statements NOTE Indicates some tips or some information users should be aware of. CAUTION Indicates correct operating
User Manual Med Aire Alternating Pressure Pump and Pad System 14002E 14001E Symbols & Statements NOTE Indicates some tips or some information users should be aware of. CAUTION Indicates correct operating
HoverJack. Air Patient Lift. User Manual. From HoverTech International.
 HoverJack Air Patient Lift From HoverTech International User Manual www.hovermatt.com Table of Contents Symbol References... 02 Intended Use and Precautions... 03 HOVERJACK Introduction... 04 Part Identification
HoverJack Air Patient Lift From HoverTech International User Manual www.hovermatt.com Table of Contents Symbol References... 02 Intended Use and Precautions... 03 HOVERJACK Introduction... 04 Part Identification
Dialflow Regulator. Instructions for Use
 Dialflow Regulator Instructions for Use 702-0030.11 May 2014 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in personal injury to the user
Dialflow Regulator Instructions for Use 702-0030.11 May 2014 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in personal injury to the user
Digital Melting Point Apparatus
 Digital Melting Point Apparatus Heating Plateau Ramping Start/Stop Plateau set Ramp stop Hold User Guide Version 1.1 Heating Viewing tube Sample Chamber IEC power inlet socket Power on/off Temperature
Digital Melting Point Apparatus Heating Plateau Ramping Start/Stop Plateau set Ramp stop Hold User Guide Version 1.1 Heating Viewing tube Sample Chamber IEC power inlet socket Power on/off Temperature
Instruction Manual for Configura Cushionair Portable Pump
 Instruction Manual for Configura Cushionair Portable Pump Fitted with battery powered pump, suitable for Configura Portable chairs V E R S I O N O N E M A Y 2 0 1 6 Contents Introduction 3 Set up of Cushionair
Instruction Manual for Configura Cushionair Portable Pump Fitted with battery powered pump, suitable for Configura Portable chairs V E R S I O N O N E M A Y 2 0 1 6 Contents Introduction 3 Set up of Cushionair
EasyLife INSTRUCTIONS FOR USE. Respironics Inc Murry Ridge Lane Murrysville, PA USA JDH 3/25/10
 Respironics Inc. 1001 Murry Ridge Lane Murrysville, PA 15668 USA 1067787 JDH 3/25/10 INSTRUCTIONS FOR USE 2010 Koninklijke Philips Electronics N.V. All rights reserved. EASYLife 1 Table of Contents 2 Package
Respironics Inc. 1001 Murry Ridge Lane Murrysville, PA 15668 USA 1067787 JDH 3/25/10 INSTRUCTIONS FOR USE 2010 Koninklijke Philips Electronics N.V. All rights reserved. EASYLife 1 Table of Contents 2 Package
Vapotherm Q50 Compressor. Instructions for Use
 Vapotherm Q50 Compressor Instructions for Use Contents Quick Start Guide...2 Intended Use...3 Indications, Warnings and Cautions...4 Primary Indications...4 Contraindications...4 Warnings and Cautions...4
Vapotherm Q50 Compressor Instructions for Use Contents Quick Start Guide...2 Intended Use...3 Indications, Warnings and Cautions...4 Primary Indications...4 Contraindications...4 Warnings and Cautions...4
Rev. B. Operating Instructions
 780745-00 Rev. B Operating Instructions 780745-00 Rev. B TABLE OF CONTENTS PAGE Warnings, Cautions, and Notes..............................4-7 Definition of Symbols......................................
780745-00 Rev. B Operating Instructions 780745-00 Rev. B TABLE OF CONTENTS PAGE Warnings, Cautions, and Notes..............................4-7 Definition of Symbols......................................
User Manual DIRECTHEALTHCARESERVICES.CO.UK
 User Manual DYNA-FORM AIR PRO-PLUS The Dyna-Form Air Pro-Plus is a pressure relieving mattress suitable for use with patients at VERY HIGH RISK of pressure ulcer damage. Offering high levels of patient
User Manual DYNA-FORM AIR PRO-PLUS The Dyna-Form Air Pro-Plus is a pressure relieving mattress suitable for use with patients at VERY HIGH RISK of pressure ulcer damage. Offering high levels of patient
GoodKnight 420S. Patient Manual. Ref. : M-139DFU02-30 Revision B
 GoodKnight 420S Patient Manual Ref. : M-139DFU02-30 Revision B Revision B List of patient manual revisions GoodKnight 420S The pages below are included in the American version of the GoodKnight 420S patient
GoodKnight 420S Patient Manual Ref. : M-139DFU02-30 Revision B Revision B List of patient manual revisions GoodKnight 420S The pages below are included in the American version of the GoodKnight 420S patient
MASK INTEGRITY TEST ACCESSORY (MITA) MODEL 8120
 MASK INTEGRITY TEST ACCESSORY (MITA) MODEL 8120 QUICK START GUIDE P/N 6006154, REVISION C MAY 2013 Model 8120 Mask Integrity Tester is patented under U.S. Patent No. 8,312,761. Additional patents are pending.
MASK INTEGRITY TEST ACCESSORY (MITA) MODEL 8120 QUICK START GUIDE P/N 6006154, REVISION C MAY 2013 Model 8120 Mask Integrity Tester is patented under U.S. Patent No. 8,312,761. Additional patents are pending.
DYNAMIC COMPRESSION THERAPY
 PULSE PRESS DYNAMIC COMPRESSION THERAPY MULTITEC 12 User Manual May 2011 0120 CONTENTS:- SAFETY INSTRUCTIONS ELECTROMAGNETIC INTERFERENCE DESCRIPTION OF CONTROLS MODE SELECTION OPERATING INSTRUCTIONS CARE
PULSE PRESS DYNAMIC COMPRESSION THERAPY MULTITEC 12 User Manual May 2011 0120 CONTENTS:- SAFETY INSTRUCTIONS ELECTROMAGNETIC INTERFERENCE DESCRIPTION OF CONTROLS MODE SELECTION OPERATING INSTRUCTIONS CARE
Owner s Manual & Safety Instructions
 Owner s Manual & Safety Instructions Save This Manual Keep this manual for the safety warnings and precautions, assembly, operating, inspection, maintenance and cleaning procedures. Write the product s
Owner s Manual & Safety Instructions Save This Manual Keep this manual for the safety warnings and precautions, assembly, operating, inspection, maintenance and cleaning procedures. Write the product s
DRAFT U S E R M A N U A L CAUTION. Model: 19MFA1001 Series. Federal (USA) law restricts this device to sale by or on the order of a physician.
 U S E R M A N U A L Model: 19MFA1001 Series SAVE THESE INSTRUCTIONS Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090 Northampton,
U S E R M A N U A L Model: 19MFA1001 Series SAVE THESE INSTRUCTIONS Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090 Northampton,
Astral in AirView: Improving patient care through connectivity. ResMed.com
 Astral in AirView: Improving patient care through connectivity ResMed.com Using Astral in AirView via the ResMed Connectivity Module (RCM) Astral is ResMed s portable, invasive and non-invasive life support
Astral in AirView: Improving patient care through connectivity ResMed.com Using Astral in AirView via the ResMed Connectivity Module (RCM) Astral is ResMed s portable, invasive and non-invasive life support
VENTI-O 2 plus. O 2 switching valve for: VENTImotion from serial number 4000 VENTIlogic from serial number 4000 VENTImotion 2
 VENTI-O 2 plus O 2 switching valve for: VENTImotion from serial number 4000 VENTIlogic from serial number 4000 VENTImotion 2 Device description and operating instructions Overview VENTI-O 2 plus with therapy
VENTI-O 2 plus O 2 switching valve for: VENTImotion from serial number 4000 VENTIlogic from serial number 4000 VENTImotion 2 Device description and operating instructions Overview VENTI-O 2 plus with therapy
Operating Instructions Part No
 DIGITAL AUTOMATIC TYRE INFLATOR Operating Instructions Part No. 11.0545 Thank you for selecting this Jamec Pem Automatic Tyre Inflator. Please read this manual before carrying out any installation or service
DIGITAL AUTOMATIC TYRE INFLATOR Operating Instructions Part No. 11.0545 Thank you for selecting this Jamec Pem Automatic Tyre Inflator. Please read this manual before carrying out any installation or service
NICE1 Cold + Compression Therapy System
 NICE1 Cold + Compression Therapy System Thank you for choosing the NICE1 Cold + Compression Therapy System. The NICE1 uses advanced technology to greatly improve the convenience and efficacy of cold +
NICE1 Cold + Compression Therapy System Thank you for choosing the NICE1 Cold + Compression Therapy System. The NICE1 uses advanced technology to greatly improve the convenience and efficacy of cold +
Paradise Pump Series Operator s Manual
 Paradise Pump Series Operator s Manual P/N 12421-000 9/05 Paradise Pump Operator s Manual Important Before using the Paradise Pump Alternating Pressure Relief Systems, please read and understand this
Paradise Pump Series Operator s Manual P/N 12421-000 9/05 Paradise Pump Operator s Manual Important Before using the Paradise Pump Alternating Pressure Relief Systems, please read and understand this
Mirage Quattro FULL FACE MASK
 Mirage Quattro FULL FACE MASK User Guide English 618250/3 2017-12 MIRAGE QUATTRO USER APAC - ENG 618250 Global leaders in sleep and respiratory medicine www.resmed.com Manufacturer: ResMed Ltd 1 Elizabeth
Mirage Quattro FULL FACE MASK User Guide English 618250/3 2017-12 MIRAGE QUATTRO USER APAC - ENG 618250 Global leaders in sleep and respiratory medicine www.resmed.com Manufacturer: ResMed Ltd 1 Elizabeth
Table of Contents. Operating Instructions. Resource v.2 Conserving Regulator
 Operating Instructions Table of Contents Resource v.2 Conserving Regulator Safety Information Device Precautions Introduction Product Features Product Specifications Feature Illustrations Set Up Usage
Operating Instructions Table of Contents Resource v.2 Conserving Regulator Safety Information Device Precautions Introduction Product Features Product Specifications Feature Illustrations Set Up Usage
POWERPRESS UNIT. User Manual. Gradient Pneumatic Sequential Compressor. Neomedic
 TIMER (MIN) POWERPRESS UNIT 40 70 40 80 90 10 60 100 MIN MAX PRESSURE (mmhg) User Manual Neomedic 9601 Owens mouth Ave. #8 Chatsworth, CA 91311 Toll Free: 866-990-1168 Tel: 818-998-1023 Fax: 818-998-0277
TIMER (MIN) POWERPRESS UNIT 40 70 40 80 90 10 60 100 MIN MAX PRESSURE (mmhg) User Manual Neomedic 9601 Owens mouth Ave. #8 Chatsworth, CA 91311 Toll Free: 866-990-1168 Tel: 818-998-1023 Fax: 818-998-0277
Home Oxygen Therapy Patient Handbook
 Home Oxygen Therapy Patient Handbook Office Hours 8:30 a.m. to 5:00 p.m. Monday-Friday Emergency Answering Service 24 hours a day, 7 days a week Birmingham Office: (205) 981-2333 5025 Highway 280 Suite
Home Oxygen Therapy Patient Handbook Office Hours 8:30 a.m. to 5:00 p.m. Monday-Friday Emergency Answering Service 24 hours a day, 7 days a week Birmingham Office: (205) 981-2333 5025 Highway 280 Suite
My BiPAP ventilator. My helpful guide
 My BiPAP ventilator My helpful guide Inhale. Exhale. And everything in between. How does it work? Brief Overview The lungs are vital organs that bring fresh oxygen into the body and remove carbon dioxide
My BiPAP ventilator My helpful guide Inhale. Exhale. And everything in between. How does it work? Brief Overview The lungs are vital organs that bring fresh oxygen into the body and remove carbon dioxide
CircuFlow TM 5150 Series
 CircuFlow TM 5150 Series Sequential Compression Device Operating Instructions www.devonmedicalproducts.com 1.866.446.0092 IFU34.0001 Rev. D 20140313 The Model 5150 - Sequential Compression Device Indications:
CircuFlow TM 5150 Series Sequential Compression Device Operating Instructions www.devonmedicalproducts.com 1.866.446.0092 IFU34.0001 Rev. D 20140313 The Model 5150 - Sequential Compression Device Indications:
Comfortmax Thermax Hot/Cold Water Circulation System. Instructions for Use
 Comfortmax Thermax Hot/Cold Water Circulation System Instructions for Use THIS DEVICE CAN BE COLD ENOUGH TO CAUSE SERIOUS INJURY. SERIOUS ADVERSE REACTIONS AND SAFETY HAZARDS MAY OCCUR WHEN USING THIS
Comfortmax Thermax Hot/Cold Water Circulation System Instructions for Use THIS DEVICE CAN BE COLD ENOUGH TO CAUSE SERIOUS INJURY. SERIOUS ADVERSE REACTIONS AND SAFETY HAZARDS MAY OCCUR WHEN USING THIS
Astral in AirView: Improving patient care through connectivity. ResMed.com
 Astral in AirView: Improving patient care through connectivity ResMed.com This guide will assist you with: Setting up the ResMed Connectivity Module for Astral 2 Troubleshooting the ResMed Connectivity
Astral in AirView: Improving patient care through connectivity ResMed.com This guide will assist you with: Setting up the ResMed Connectivity Module for Astral 2 Troubleshooting the ResMed Connectivity
Intermittent Pneumatic Compression System
 User Manual Intermittent Pneumatic Compression System To ensure safe use of this device, it is essential that you read and fully understand the information contained within this manual. Mechanical prophylaxis
User Manual Intermittent Pneumatic Compression System To ensure safe use of this device, it is essential that you read and fully understand the information contained within this manual. Mechanical prophylaxis
OPERATING INSTRUCTIONS FOR USE GUIDE
 AIROS 8 Sequential Compression Device OPERATING INSTRUCTIONS FOR USE GUIDE www.airosmedical.com 1.866.991.6956 Table of Contents Indications for Use & Contraindications...................... 1 Overview
AIROS 8 Sequential Compression Device OPERATING INSTRUCTIONS FOR USE GUIDE www.airosmedical.com 1.866.991.6956 Table of Contents Indications for Use & Contraindications...................... 1 Overview
2010 Koninklijke Philips Electronics N.V. All rights reserved. INSTRUCTIONS FOR USE
 1067728 JDH 03/30/2010 ComfortLite 2 2010 Koninklijke Philips Electronics N.V. All rights reserved. INSTRUCTIONS FOR USE ComfortLite 2 Nasal Mask 1 Table of Contents 3 Package Contents 4 Intended Use 5
1067728 JDH 03/30/2010 ComfortLite 2 2010 Koninklijke Philips Electronics N.V. All rights reserved. INSTRUCTIONS FOR USE ComfortLite 2 Nasal Mask 1 Table of Contents 3 Package Contents 4 Intended Use 5
Manual: Biphasic Positive Airway Pressure (BiPAP) Ventilation
 RCH@Home Manual: Biphasic Positive Airway Pressure (BiPAP) Ventilation 1. Commonly used terms... 2 1.1 Inspiration... 2 1.2 Expiration... 2 1.3 Breath rate (bpm)... 2 1.4 Ventilation... 2 1.5 Biphasic
RCH@Home Manual: Biphasic Positive Airway Pressure (BiPAP) Ventilation 1. Commonly used terms... 2 1.1 Inspiration... 2 1.2 Expiration... 2 1.3 Breath rate (bpm)... 2 1.4 Ventilation... 2 1.5 Biphasic
Oxygen Concentrator Instruction
 WARNING-Read instruction before operating this equipment Oxygen Concentrator Instruction K5BW WARNING Oxygen therapy can be hazardous in certain conditions. Seeking medical advice before using an oxygen
WARNING-Read instruction before operating this equipment Oxygen Concentrator Instruction K5BW WARNING Oxygen therapy can be hazardous in certain conditions. Seeking medical advice before using an oxygen
STAGE, STADIUM & X-TREME SHOT.
 USER AND INSTALLATION MANUAL ENGLISH STAGE, STADIUM & X-TREME SHOT. CONFETTI & STREAMER FX PART01339 rev 01-00 DISCLAIMER WARNING Read this manual carefully before installing and/or using this product.
USER AND INSTALLATION MANUAL ENGLISH STAGE, STADIUM & X-TREME SHOT. CONFETTI & STREAMER FX PART01339 rev 01-00 DISCLAIMER WARNING Read this manual carefully before installing and/or using this product.
VGP-4300-G. Travel Pulse. Blood Pressure Monitor for Travel
 VGP-4300-G Travel Pulse Blood Pressure Monitor for Travel Congratulations on your Vitagoods purchase. The Travel Pulse Blood Pressure Monitor, will allow you to measure vital blood pressure parameters
VGP-4300-G Travel Pulse Blood Pressure Monitor for Travel Congratulations on your Vitagoods purchase. The Travel Pulse Blood Pressure Monitor, will allow you to measure vital blood pressure parameters
OC4000 USER'S GUIDE I O OXYGEN CONCENTRATOR FOR VETERINARY USE ONLY. by Supera Anesthesia Innovations GLOSSARY OF SYMBOLS. : ON (power switched on)
 by Supera Anesthesia Innovations USER'S GUIDE OC4000 OXYGEN CONCENTRATOR FOR VETERINARY USE ONLY I O : ON (power switched on) : OFF (power switched off) : Class II protection : Do not expose to open flames
by Supera Anesthesia Innovations USER'S GUIDE OC4000 OXYGEN CONCENTRATOR FOR VETERINARY USE ONLY I O : ON (power switched on) : OFF (power switched off) : Class II protection : Do not expose to open flames
patient education program 4156 South 52 nd Street, Omaha, NE ChildrensOmaha.org/HomeHealthcare
 patient education program 4156 South 52 nd Street, Omaha, NE 68117 800-747-7334 402-734-6741 ChildrensOmaha.org/HomeHealthcare We know children CHILDREN S HOME HEALTHCARE Trilogy Ventilator Instructions
patient education program 4156 South 52 nd Street, Omaha, NE 68117 800-747-7334 402-734-6741 ChildrensOmaha.org/HomeHealthcare We know children CHILDREN S HOME HEALTHCARE Trilogy Ventilator Instructions
Pressure Regulators. Instructions for Use
 Pressure Regulators Instructions for Use 702-0032.11 May 2014 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in personal injury to the user
Pressure Regulators Instructions for Use 702-0032.11 May 2014 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in personal injury to the user
RPS900W Redundant Power Supply. Installation Guide.
 RPS900W Redundant Power Supply Installation Guide www.edge-core.com Installation Guide RPS900W Redundant Power Supply Single DC Output Port with Dual Output Voltages RPS900W E10013-CS-R01 1500000081A
RPS900W Redundant Power Supply Installation Guide www.edge-core.com Installation Guide RPS900W Redundant Power Supply Single DC Output Port with Dual Output Voltages RPS900W E10013-CS-R01 1500000081A
Domiciliary Oxygen Switch. Instructions for Use
 Domiciliary Oxygen Switch Instructions for Use 702-0041.11 July 2017 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in personal injury to the
Domiciliary Oxygen Switch Instructions for Use 702-0041.11 July 2017 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in personal injury to the
