FUSION Mattress and Cushion Systems User Guidelines
|
|
|
- Cody McGee
- 6 years ago
- Views:
Transcription
1 ...reducing avoidable harms FUSION Mattress and Cushion Systems User Guidelines
2 Explanation of Label Symbols and Statements Caution Protect from heat and radioactive sources Refer to instructions of use / booklet Temperature limitation Medical Devices Directive 93/42/EEC Humidity limitation Atmospheric pressure limitation North America ETL listed Class II Equipment (Double Insulated) Do not dispose of with the normal household waste (please refer to www. talleygroup.com for further details) Manufacturer WARNING CAUTION Suitable for connection to type BF applied parts This is a statement that alerts the user to the possibility of serious injury or other adverse reactions with the use or misuse of the device This is a statement that alerts the user to the possibility of a problem with the system associated with its use or misuse Date of Manufacture Fragile, handle with care Keep dry IP21 Operating Instructions IP: Ingress Protection 2: Protection against fingers or other object not greater than 80mm in length and 12mm in diameter 1: Protection from vertically dripping water 1
3 Contents Page EXPLANATION OF LABELS SYMBOLS AND STATEMENTS 1 INTRODUCTION 2 CAUTIONS AND WARNINGS INFORMATION 3 INSTALLATION AND USER GUIDELINES 5 CARE AND MAINTENANCE 8 FAULT FINDING 10 SPECIFICATIONS 12 EMI/EMC STATEMENT AND MANUFACTURER S DECLARATION 14 Introduction Thank you for choosing to use a Talley pressure area care product from the FUSION range, effective for the prevention and management of pressure ulcers. The FUSION HYBRID mattress combines the comfort and pressure reducing properties of high performance foam with the clinically proven pressure relieving benefits of a 1-in-2 active alternating air pressure cycle, all in the same mattress. Used alone, the mattress provides comfort and pressure care for patients at elevated risk of pressure damage. Once the power unit is connected, the mattress provides all the benefits associated with an active 1-in-2 cell cycle alternating air pressure mattress. The versatile FUSION HYBRID is suitable for patients at all risk levels, providing the ability to step up or down according to the level of patient care required throughout their treatment. Benefitting from the same features as the mattress, the FUSION HYBRID cushion can be used with or without the power unit depending on the user s requirement and offers the same pressure reducing/pressure relieving qualities for when the user is seated out of bed. The FUSION RESPONSE mattress replacement system combines responsive air flow distribution with high performance foam to provide a support surface that offers reactive therapy by distributing the patient s weight over the surface area, reducing average interface pressures and providing high levels of comfort. 2
4 Cautions and Warnings CONTRAINDICATIONS FOR USE Alternating pressure therapy should not be used for patients with unstable fractures, gross oedema, burns or an intolerance to motion. There are no special skills required to operate the system. The medical professional is responsible for applying his/her best medical judgment when using this system. Select correct setting for therapy required. Care should be taken not to accidently change pressures once set as the efficiency of the therapy may be reduced. This could also be caused by pets, pests or children. The electricity supply is of the type indicated on the power unit Check the mains lead is free from damage and is positioned so as not to cause an obstruction, or injury, e.g. strangulation. Ensure the mains lead or pump cannot become trapped or crushed, e.g. via raising or lowering of bed or bed rails or any other moving object. The power unit must only be used with a suitable approved cord and plug set as supplied by Talley. The system is not used in the presence of flammable anaesthetics Suitable for continuous use. Not suitable for sterilisation. Do not position the power unit to make it difficult to disconnect the power supply, plug or mattress. Do not place device on or near a heat source. Do not use with hot water bottles or electric blankets. The materials used in the manufacture of all components of the system comply with the required fire safety regulations. Talley advice against smoking whilst the system is in use, to prevent the accidental secondary ignition of associated items which may be flammable, such as bed linen. Do not allow sharp objects to penetrate the mattress/cushion material. Do not modify the mattress, cushion or power unit in any way. Do not store in damp conditions. Not for use in an oxygen enriched environment. Not for use in an outdoor environment. 3
5 Intended for home healthcare use and professional healthcare facility environments where operators with medical training are continually available when patients are present. The power unit is intended to be hung over the bed s footboard. Wireless equipment such as mobile phones should be kept at least 10 feet or 3.3 meters away from the equipment. Do not connect to any other medical device or equipment. Risk of fire if incorrect fuse used. The mattress, cushion and pump should be cleaned between patient use, please refer Care and Maintenance section for all warnings and cautions. Mattress/cushion must be properly set up as directed. Check periodically to ensure patient support and comfort, adjusting the Comfort Control setting if desired. All hoses must be free of kinks, twists, properly connected and positioned so as not to cause an obstruction or injury. In order for the alternating air pressure mattress/cushion to operate effectively, please avoid placing objects on the surface that may obstruct the movement of air between the cells. For the same reason, please discourage people from sitting on the edge or on the end of the mattress whilst it is in use. Do not use abrasive cleaners, phenol disinfectants, solvents or alcohol-based cleansers, e.g. Dettol, Phenicol, Hibiscrub, Clearsol, Stericol, Hycoline, as these may destroy the cover materials*. Do not place heavy objects on the surface of foam mattresses/cushions when not in use. It should be noted that the use of a cushion will increase the patient s seated height by approximately 5cm, and care should be taken to ensure the patient s comfort and security regarding height of foot and arm rests. The system is used as part of a pressure ulcer prevention program, not solely relied upon for this purpose. The above warnings, cautions and any safety considerations should be observed on a routine and regular basis, not only upon installation. 4
6 Installation and User Guidelines INSTALLING MATTRESSES 1. All mattresses: Remove any existing mattress from the bed frame (the FUSION mattresses are intended to completely replace the bed mattress). 2. Place the mattress on the bed frame/bed base. Ensure the printed cover is uppermost and the mattress hose can exit at the foot end on the right hand side, if applicable. The FUSION HYBRID and FUSION RESPONSE mattresses are now ready to use as a static foam mattress. 3. Powered mattress only: If using the FUSION HYBRID as a powered mattress, suspend the power unit from the foot board of the bed having first adjusted the hanger brackets as instructed at the rear of the unit. Alternatively the power unit may be placed on the floor. 4. The mattress air supply hose can be found at the foot end of the mattress under the mattress cover skirt. Attach the mattress air supply hose to the power unit by matching up the button on the mattress hose connector with the alignment notch on the power unit connection port and pushing together (Fig. 1). Ensure that this has been correctly clicked into place otherwise a leak may occur. 6. Plug the smaller end of the power lead into the left hand side of the power unit, and the other end into mains outlet in wall. click Fig. 1 Ensure that the mains lead and tubing cannot become trapped in the bed frame (the mattress includes cable management loops for routing of the mains lead). 7. Switch the power on at the outlet, and at the side of power unit adjacent to the power lead entry. 8. The system will display STARTING, then INITIALISING whilst the mattress inflates (this will take approximately 2 minutes). Note: It is important that during the INITIALISING phase the mattress connector is not disconnected from the power unit. If this is done, the power unit must be switched off, MUTE button pressed when the sounder is heard, the mattress connector re-engaged, and the power unit restarted. If UNCALIBRATED is displayed when switched on, the system will continue to operate but should be recalibrated as soon as possible. 9. Allow the mattress to inflate before positioning the patient on the mattress. If, however, the patient is already in-situ, having been using the foam mattress without power, the mattress will still inflate and there is no need to disturb the patient. 10. Once inflated the system will automatically default to ACTIVE mode. 5
7 The comfort setting can be adjusted using the up and down arrow buttons (see COMFORT CONTROL on page 6). NB. The power unit will automatically lock 2 minutes after last button operation when running to prevent inadvertent operation of button functions (except MUTE), as indicated by on the display screen. Press and hold the MUTE/UNLOCK button until power unit beeps if further button operation is needed (i.e. change of therapy mode or comfort setting). 11. Place the carry bag and user manual in a safe place for future use. INSTALLING CUSHION The FUSION HYBRID cushion can be used with or without the power unit depending on the requirements of the user. Place the cushion on a chair, ensuring that it is placed the correct way up with the BACK labelling facing the back of the chair. The cushion features a non-slip base for security in the chair. If choosing to use the cushion with the power unit, follow the connection instructions described in points 4. to 11. above (the cushion will take approx. 30 seconds to inflate). It should be noted that the use of a cushion will increase the patient s seated height by approximately 5cm, and care should be taken to ensure the patient s comfort and security regarding height of foot and arm rests. OPERATION BUTTONS The operation buttons on the face of the power unit provide the following functions. COMFORT CONTROL Air pressure is regulated within each of the cells throughout the cycle so that support, posture and therapy are constantly maintained at optimum levels, in response to patient weight, movement and position. Equalisation of cell pressure automatically takes place at each stage of the 1-in-2 cycle, again to ensure precise pressure and therapy is provided. The automatic default comfort setting is MEDIUM. However, if the patient prefers a firmer or softer mattress, increase or decrease the comfort control setting accordingly using the UP and DOWN arrow buttons (SOFT/MEDIUM/FIRM). The comfort setting is shown on the display screen. Check periodically to ensure patient support and comfort. DATA Pressing the DATA button at any time switches the display into DATA mode. Use the up and down arrow buttons to scroll through the product data and user information set. Pressing the DATA button again returns the display to the previous mode. NB. Used for accessing information only, does not affect mode of operation.
8 MUTE/UNLOCK Press to silence the sounder and to clear the message from the display screen. The power unit will automatically lock 2 minutes after the last button operation when running to prevent the inadvertent operation of button functions (except MUTE), as indicated by on the display screen. Press and hold the MUTE/UNLOCK button until the power unit beeps if further button operation is needed (i.e. comfort setting). The power unit will lock again 2 minutes after the last button operation. NB. After power failure/switching the power off, pressing MUTE cancels the system s previous settings. When power returns the default setting of ACTIVE mode, MEDIUM comfort setting is invoked. (Note that previous settings are automatically cancelled if the duration between switch off and switch on is greater than 12 seconds. If power returns before a period of 12 seconds has passed and the MUTE button has not been pressed, the system will return to the previous mode of operation.) MAXIMUM USER WEIGHT GUIDELINES FUSION HYBRID mattress: 250kg (39 stone) max. FUSION RESPONSE mattress: 250kg (39 stone) max. FUSION HYBRID cushion: 150kg (24 stone) max. CPR FACILITY (Fig. 2) For rapid deflation of the powered mattress, press the button on the mattress hose connector and pull the mattress hose away from the power unit to disconnect. The FUSION mattresses can be used on profiling bed frames, slatted frames, in-filled frames and divans. Back rests or pillows for support should be placed beneath the mattress to allow uninterrupted body contact with the mattress surface. Fig. 2 When a bottom sheet is added to the mattress, ensure that it is left loose to allow the mattress surface to conform with the patient s body as much as possible. Avoid using fitted sheets. The use of incontinence sheets / excessive bedding beneath the patient may reduce the pressure reducing/relieving effect of the mattress. To remove air from the mattress when dismantling the system, use the CPR facility as described above. Care should be taken when raising and lowering bed safety side rails in order to avoid possible interference with the mattress air supply hose, if applicable. 7 A gap of 2.5cm on either side of the mattress should not be exceeded when side rails are deployed.
9 Care and Maintenance COVER Always keep the mattress/cushion cover as clean as is practicable. The material is waterproof and vapour permeable. Inspect top cover for signs of damage or wear which could result in the contamination of the interior, e.g. tears, holes, damage to seams or zips, underside staining, etc. The frequency of these checks should be at each decontamination process, i.e. between patients or patient occupancy (or weekly for longer term patients). Care should be taken to avoid puncturing cover with objects such as needles, scalpels, pat slides, acrylic nails, etc. The cover may be removed and cleaned in accordance with The Revised Healthcare Cleaning Manual June 2009 subject to the following action: Following the use of a detergent and or disinfectant solution the mattress cover should be rinsed with clean water using a clean cloth and allowed to dry. Frequent or prolonged exposure to high concentrations of aggressive disinfectant solutions will reduce the useful life of the mattress/cushion cover. Where high concentration disinfectants e.g. > 10,000ppm chlorine releasing agent (e.g. Haztab or bleach) or combined cleaning/chlorine releasing agent (e.g. Chlorcleam, Actichlor) and detergent solutions are used to remove blood or other body fluids, mattresses/cushions should be thoroughly rinsed with clean water to remove any residues. This will help prevent any possible long term compatibility issues associated with disinfectant residues*. Alternatively disinfection may be achieved by laundering cover at temperatures not exceeding 65 0 C for 10 minutes or 73 0 C for 3 minutes which may include a chlorine rinse. Do not use abrasive cleaners, phenol disinfectants, solvents or alcohol-based cleansers, e.g. Dettol, Phenicol, Hibiscrub, Clearsol, Stericol, Hycoline, as these may destroy the cover materials*. Do not iron. Ensure that the mattress/cushion cover is thoroughly dried before remaking the bed or placing in storage. INTERIOR COMPONENTS Check air cells and mattress/cushion interior for signs of damage or contamination, e.g. staining or evidence of fluid ingress. The frequency of these checks should be at each decontamination process, i.e. between patients or patient occupancy (or weekly for longer term patients). Care should be taken to avoid puncturing air cells with objects such as needles, scalpels, pat slides, acrylic nails, etc. The individual cells can also be wiped clean with a mild antiseptic solution*. All cells are replaceable and can be obtained easily from Talley. Do not immerse the mattress/cushion in water or wet the foam components. 8
10 POWER UNIT Always disconnect the power unit from the electricity supply before carrying out maintenance, repairs, servicing or cleaning. Check all electrical connections and power lead for signs of excessive wear. The power unit can be wiped down with detergent or disinfectant solution or wipe*. Do not use solvents. Unsuitable for sterilisation. Dispose of the pump / mattress / cushion in accordance with the local regulations including WEEE requirements. * In line with the MHRA Medical Device Alert (MDA/2013/019), Talley advises customers to use ph neutral, high level disinfectant cleaning products to sanitise reusable medical devices to prevent damage to materials and the degradation of plastic surfaces after prolonged use. The use of inappropriate cleaning and detergent materials on medical equipment could damage surfaces and may compromise the ability to decontaminate medical devices adequately or may interfere with device function. Talley recommends the use of TECcare CONTROL antimicrobial wipes and fluid to clean and decontaminate all products it supplies to health and social care facilities. TECcare CONTROL products provide class leading broad spectrum, high level disinfection with an exceptional safety profile. Being ph neutral TECcare CONTROL can be universally used on all hard and soft surfaces without any detrimental effect. TECcare CONTROL is CE marked for cleaning medical equipment. SERVICING Once the initial guarantee period expires, Talley recommend that all power units should be serviced annually or as indicated by the hours to service display. The unit contains no user serviceable parts and should only be serviced by either Talley or an authorised dealer. Talley will make available on request service manuals, component parts lists and other information necessary for Talley, an authorised dealer or a competent electrical engineer to repair or service the system. Talley s standard terms and conditions apply to all sales. A copy is available on request. For service, maintenance and any questions regarding this, or any other product, please contact Talley. It is the customer s responsibility to ensure the following prior to collection: the system is cleaned of any obvious contaminants. contamination status is documented. assistance is given to Talley personnel to bag the equipment if the mattress has been in a known or suspected infectious environment. 9 TRANSPORT AND STORAGE Handle with care. Please report instances of damage or impact to Talley Service Department. Transport 25 C without relative humidity control; and +70 C at a relative humidity up to 93 %, non-condensing. An atmospheric pressure range of 700 hpa to hpa. Suitable for all standard modes of transport when in the correct packaging.
11 OPERATIONAL CONDITIONS A temperature range of +5 C to +40 C; A relative humidity range of 15% to 93%, non-condensing; and Operational Atmospheric Pressure: 700 hpa to 1060 hpa Suitable for pollution degree 2 Operational altitude m IP Rating: IP21 pump only TRANSPORTATION OF MATTRESS SYSTEM The mattress should be transported flat and not rolled. Mattresses should not be stored more than 6 high as this can potentially be a safety hazard when handling. To protect the covers, mattresses should be packed in minimum 200 micron clear polythene. MANUFACTURER S GUARANTEE The FUSION power units and mattress are covered by a 24 month manufacturer s guarantee. The intended design life is 5 years if fully serviced. Fault Finding The power unit can be reset by pressing the MUTE button once. This also silences the sounder and clears the message from the display screen. All systems have a fault log that records the last 5 faults via the DATA display mode. If problems reoccur contact Talley. AC FAIL fault indicates a mains power failure, a sounder will be heard if power is interrupted, e.g. power unit switched off, power cut, disconnection of mains lead. Press MUTE or re-connect to power supply. ROTOR SYSTEM fault indicates the automatic sequential cycle has stopped or there is a fault in the system. Switch power off, press MUTE button, then switch power on again. If the fault re-occurs, contact Talley. LOW PRESSURE fault indicates pressure has fallen below the minimum allowable levels. Check that the hose is connected to the power unit correctly. Check that the internal cells are connected and that no cell is punctured. Press the MUTE button to clear the message and to silence the sounder. If the fault re-occurs, contact Talley. EMI fault indicates that the unit detects the pressure sensor amplifier is adversely affected by external RF fields. This will clear when interference ceases. OTHER FAULTS PUMP OR TRIAC fault - indicates a pump control failure or an open pump coil fault. Should this occur, contact Talley. 10
12 UNCALIBRATED - contact Talley for recalibration. If you have any queries relating to this system please contact Talley or your local authorised dealer. LOW PRESSURE FAULT ROTOR SYSTEM FAULT PUMP or TRIAC FAULT AC FAIL FAULT Check mattress air supply hose is correctly attached to power unit (i.e. mattress hose connector is correctly aligned with power unit connector and has clicked into place). Reset power unit by pressing MUTE button. If fault persists, power unit requires inspection. Reset power unit by pressing MUTE button. If fault persists, power unit requires inspection. Indicates mains power failure. Silence sounder by pressing MUTE button. Reconnect to power supply to continue using mattress. Check air cells and tubing are intact. MATTRESS NOT INFLATING (no sounder) UNCALIBRATED FAULT EMI FAULT Reset power unit by pressing MUTE button. Check that mains power is connected, indicated by illuminated LCD display, and allow at least 2 minutes for mattress to inflate. The system will continue to operate but contact Talley for recalibration as soon as possible. Caused by external RF fields. Fault will clear when interference ceases. If fault persists, system requires inspection. Reset power unit by pressing MUTE button. 11
13 Specifications POWER UNIT (Medical Device Classification: Class IIa) Model Ref.: Type 19 Construction: ABS Plastic Dimensions: 335mm/13.2 x 233mm/9.2 x 165mm/6.5 Weight: 3.4 kg / 7.5 lbs Mains Cable: 5 metres / 16.5 For the USA:- only a hospital grade attachment plug with a 15A NEMAP 5-15 configuration and 18AWG hospital grade flexible cord is to be used, as supplied by Talley. Electricity Supply: 230V ~ 50Hz (CE marked) 120V ~ 60Hz (cetlus listed) 230V ~ 60Hz Rated Input: 9.4 VA Fuse Rating: T500mA 250V HRC (ceramic) 5 x 20 mm IP Rating: IP21 Cycle Time: 8 minutes (continuous) FUSION HYBRID MATTRESS (Medical Device Classification: Class IIa) Construction: FOAM FRAME: Combustion modified polyether foam RX33/190 HEAD SECTION: Combustion modified polyether foam RX39/120 CELLS: PU film encasing combustion modified polyether foam RX39/120 inner cells COVER: PU coated stretch 240g/m 2 Polyamide (with PU coated stretch 215g/m 2 Polyamide non-slip base) Number of Cells: 18 (10 torso cells + 8 heel zone cells + cut foam head section insert) Mode of Operation: Reactive static foam / Active 1-in-2 cell cycle Dimensions*: 1970mm x 880mm x 170mm Weight: 11.9kg FUSION HYBRID CUSHION (Medical Device Classification: Class IIa) Construction: CELLS: PU film encasing combustion modified polyether foam RX39/120 inner cells COVER: PU coated stretch 240g/m 2 Polyamide (with PU coated stretch 215g/m 2 Polyamide non-slip base) Number of Cells: 7 Mode of Operation: Reactive static foam / Active 1-in-2 cell cycle Dimensions*: 430mm x 430mm x 65mm Weight: 1.1kg (*UK standard size - approx.max. top surface, inflated + /- 15mm) 12
14 FUSION RESPONSE MATTRESS (Medical Device Classification: Class I) Construction: FOAM FRAME: Combustion modified polyether foam RX33/190 HEAD SECTION: Combustion modified polyether foam RX39/120 CELLS: PU film encasing combustion modified polyether foam RX39/120 inner cells COVER: PU coated stretch 240g/m 2 Polyamide (with PU coated stretch 215g/m 2 Polyamide non-slip base) Number of Cells: 18 (10 torso cells + 8 heel zone cells + cut foam head section insert) Mode of Operation: Reactive static foam with Responsive Airflow Distribution Dimensions*: 1970mm x 880mm x 170mm Weight: 11.9kg Talley manufacture products to comply with National and International safety standards and are certified to ISO9001, ISO13485 and Directive 93/42/EEC. Every care has been taken to ensure that the information contained in this manual was correct at the time of going to press. However, Talley reserve the right to modify the specification of any product without prior notice in line with a policy of continual product development. Our standard terms and conditions apply. 13
15 EMI/EMC Statement and Manufacturer s Declaration This equipment has been tested and found to comply with the limits of EN These limits are designed to provide reasonable protection against harmful interference in both a medical and residential environment. This equipment generates, uses and can radiate radio frequency energy and, if not used in accordance with manufacturer s instructions, may cause harmful interference to radio communications. However, there is no guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference to radio or television reception or other equipment, which can be determined by turning the equipment off and on, the user is encouraged to try to correct the interference by one of the following measures: Reorient or relocate the receiving antenna. Increase the separation between the equipment. Connect the equipment to an outlet on a circuit different from that to which the receiver or equipment was connected. The equipment having been tested to operate within the limits of electromagnetic compatibility. (Immunity to interference from nearby sources radiating radio frequency energy). Sources exceeding these limits may give rise to operation faults. Where possible the system will sense the interference and if it is of short duration transparently take countermeasures whilst operating near normally, or failing this will issue a warning and take measures for the continued safely of the user. Further increased levels of energy may cause the system to stop operating, continuously generate random faults or continuous resets. Try to ascertain the source of the interference by turning nearby or suspect equipment off, and see if the interference effects stop. In any such event the user is encouraged to try to correct the interference by one of the following measures: Have the interfering equipment repaired or replaced. Reorient or relocate the interfering equipment. Increase the separation between the equipment and the possible source of the interference. Connect the equipment to an outlet on a circuit different from that to which the interfering equipment was connected. Information regarding Electro Magnetic Compatibility (EMC) according to IEC With the increased number of electronic devices such as PC s and mobile telephones, medical devices in use may be susceptible to electromagnetic interference from other devices. The EMC (Electro Magnetic Compatibility) standard IEC defines the levels of immunity to these electromagnetic interferences. From the other hand, medical devices must not interfere with other devices. IEC also defines the maximum levels of emissions for these medical devices. The FUSION conforms to this IEC standard for immunity and emission. Nevertheless, special precautions need to be observed: The FUSION needs to be installed and put into service according to the EMC information below. The FUSION is intended for use in the electromagnetic environment specified in the tables below. The user of the FUSION should assure that it is used in such environment. In general, although the FUSION complies too the EMC standards, it can be affected by portable and mobile RF communications equipment (such as mobile telephones). The FUSION should not be used adjacent to or stacked with other equipment. In case adjacent or stacked use is necessary, the FUSION should be observed to verify normal operation. Guidance and Manufacturer s Declaration: Electromagnetic Emissions (IEC ) Emissions Test Compliance Electromagnetic environment - guidance RF emissions CISPR 11 Class B The FUSION systems are suitable for use in all establishments, Harmonics emissions Class A including domestic establishments and those directly connected to the public low-voltage pump supply network that supplies buildings Voltage fluctuations / flicker emissions Complies used for domestic purposes. 14
16 Guidance and Manufacturer s Declaration: Electromagnetic Immunity (IEC ) Immunity Test IEC Test Level Compliance Level Electromagnetic Environment - Guidance Electrostatic discharge (ESD) IEC Electrical fast transient/burst IEC Surge IEC Voltage dips, short interruptions and voltage variations on mains supply IEC Mains frequency (50/60Hz) magnetic field IEC Immunity Test ± 6kV contact ± 8kV air ± 2 kv For mains supply lines ± 1kV For input/output lines ± 6kV contact ± 8kV air ± 2 kv For mains supply lines ± 1kV For input/output lines ± 1kV line(s) to line ± 1kV line(s) to line Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%. Mains supply quality for the mains adapter should be that of a typical commercial and/or hospital environment. Mains supply quality for the mains adapter should be that of a typical commercial and/or hospital environment. <5%Ur (>95%Ur) for 0.5 cycle 40%Ur (60% dip in Ur) for 5 cycles <5%Ur (>95%Ur) for 0.5 cycle 40%Ur (60% dip in Ur) for 5 cycles Mains power quality should be that of a typical commercial and/or hospital environment. If the user of the FUSION requires continued operation during 70%Ur (30% dip in Ur) for 25 cycles 70%Ur (30% dip in Ur) for 25 cycles power mains interruption, it is recommended that the >5%Ur (>95% dip in Ur) for 5 secs >5%Ur (>95% dip in Ur) for 5 secs FUSION be powered from an uninterruptible power supply or battery. 3 A/m 3 A/m Mains frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial and/or hospital environment. Note: Ur is the A.C. mains voltage prior to application of the test level. Guidance and Manufacturer s Declaration: Electromagnetic Immunity (IEC ) IEC Compliance Electromagnetic Environment - Guidance Test Level Level 3 V rms Conducted RF 150 khz ~ 80 MHz IEC V/m Radiated RF 80 MHz ~ IEC GHz 3 V rms 3 V/m Portable and mobile RF communications equipment should be used no closer to any part of the FUSION including cables, than the recommended separation distance calculated from the equation appropriate to the frequency of the transmitter. Recommend separation distance d = 1.2 P d = 1.2 P 80 MHz to 800 MHz d = 2.3 P 800 MHz to 2.5 GHz where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in meters (m). Field strengths from fixed RF transmitters as determined by an electromagnetic site survey, a should be less than the compliance level in each frequency range. b Interference may occur in the vicinity of equipment marked with the following symbol: Note1: At 80 MHz and 800 MHz, the higher frequency range applies. Note2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects, and people. a Field strengths from fixed transmitters, such as base stations for radio (cellular/ cordless) telephones and land mobile radio, AM and FM radio broadcast, and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the FUSION is used exceeds the applicable RF compliance level above, the FUSION should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the FUSION. b Over the frequency range 150 khz to 80MHz, field strengths should be less than 3 V/m. Recommended Separation Distance Between Portable and Mobile RF Communications Equipment and the FUSION The FUSION is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The user of the FUSION can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the FUSION as recommended below, according to the maximum output power of the communications equipment. Output Power of Transmitter in Separation distance according to frequency of transmitter in Meters (m) Watts (W) 150 khz to 80 MHz d = 1.2 P 80 MHz to 800 MHz d = 1.2 P 800 MHz to 2.5GHz d = 2.3 P For transmitters rated at a maximum output power not listed above, the recommended separation distance d in Meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in Watts (W) according to the transmitter manufacturer. Note: At 80MHz and 800MHz, the separation distance for the higher frequency range applies. Note: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects, and people. This medical device is compliant with: IEC rd edition Medical electrical equipment safety and essential performance IEC Home healthcare environment USER MANUAL PART NUMBER /5 Talley Group Limited Premier Way, Abbey Park Industrial Estate, Romsey, Hampshire, SO51 9DQ England TEL: +44(0) FAX: +44(0) sales@talleygroup.com 02/2017
User Manual DIRECTHEALTHCARESERVICES.CO.UK
 User Manual DYNA-FORM MERCURY ADVANCE The Dyna-Form Mercury Advance is a pressure relieving mattress suitable for use with patients at VERY HIGH RISK of pressure ulcer damage. Offering high levels of patient
User Manual DYNA-FORM MERCURY ADVANCE The Dyna-Form Mercury Advance is a pressure relieving mattress suitable for use with patients at VERY HIGH RISK of pressure ulcer damage. Offering high levels of patient
User Manual DIRECTHEALTHCARESERVICES.CO.UK
 User Manual DYNA-FORM MERCURY ADVANCE The Dyna-Form Mercury Advance mattress is a High / Very High Risk dynamic replacement system, combined with the benefits of modern foam technology. Offering high levels
User Manual DYNA-FORM MERCURY ADVANCE The Dyna-Form Mercury Advance mattress is a High / Very High Risk dynamic replacement system, combined with the benefits of modern foam technology. Offering high levels
User Manual DIRECTHEALTHCARESERVICES.CO.UK
 User Manual DYNA-FORM MERCURY ADVANCE 2 USER MANUAL The Dyna-Form Mercury Advance is a pressure relieving mattress suitable for use with patients at VERY HIGH RISK of pressure ulcer damage. Offering high
User Manual DYNA-FORM MERCURY ADVANCE 2 USER MANUAL The Dyna-Form Mercury Advance is a pressure relieving mattress suitable for use with patients at VERY HIGH RISK of pressure ulcer damage. Offering high
(DynaLaL III) Dynamic True Low Air Loss Mattress Replacement System
 (DynaLaL III) User Manual Dynamic True Low Air Loss Mattress Replacement System Alternating + True Low Air Loss Pressure Relief System Manufactured by: Caremed Supply, Inc. Distributed by: Quart Healthcare
(DynaLaL III) User Manual Dynamic True Low Air Loss Mattress Replacement System Alternating + True Low Air Loss Pressure Relief System Manufactured by: Caremed Supply, Inc. Distributed by: Quart Healthcare
User Manual DIRECTHEALTHCARESERVICES.CO.UK
 User Manual DYNA-FORM AIR PRO-PLUS The Dyna-Form Air Pro-Plus is a pressure relieving mattress suitable for use with patients at VERY HIGH RISK of pressure ulcer damage. Offering high levels of patient
User Manual DYNA-FORM AIR PRO-PLUS The Dyna-Form Air Pro-Plus is a pressure relieving mattress suitable for use with patients at VERY HIGH RISK of pressure ulcer damage. Offering high levels of patient
SALISBURY ACTIVE OVERLAY SYSTEM
 General User/ Safety Guide SALISBURY ACTIVE OVERLAY SYSTEM ACTIVE MATTRESSES www.harvesthealthcare.co.uk 1 2 CONTENTS WARNINGS & CAUTIONS GENERAL INFORMATION 1 DEFINITION OF THE GROUPS MENTIONED 2 NON-COMPLIANT
General User/ Safety Guide SALISBURY ACTIVE OVERLAY SYSTEM ACTIVE MATTRESSES www.harvesthealthcare.co.uk 1 2 CONTENTS WARNINGS & CAUTIONS GENERAL INFORMATION 1 DEFINITION OF THE GROUPS MENTIONED 2 NON-COMPLIANT
General User/ Safety Guide ROYAL/ ROYAL EXTRA REPLACEMENT SYSTEM
 General User/ Safety Guide ROYAL/ ROYAL EXTRA REPLACEMENT SYSTEM ACTIVE MATTRESSES www.harvesthealthcare.co.uk 1 2 CONTENTS WARNINGS & CAUTIONS GENERAL INFORMATION 1 DEFINITION OF THE GROUPS MENTIONED
General User/ Safety Guide ROYAL/ ROYAL EXTRA REPLACEMENT SYSTEM ACTIVE MATTRESSES www.harvesthealthcare.co.uk 1 2 CONTENTS WARNINGS & CAUTIONS GENERAL INFORMATION 1 DEFINITION OF THE GROUPS MENTIONED
Instruction Manual for Configura Cushionair Portable Pump
 Instruction Manual for Configura Cushionair Portable Pump Fitted with battery powered pump, suitable for Configura Portable chairs V E R S I O N O N E M A Y 2 0 1 6 Contents Introduction 3 Set up of Cushionair
Instruction Manual for Configura Cushionair Portable Pump Fitted with battery powered pump, suitable for Configura Portable chairs V E R S I O N O N E M A Y 2 0 1 6 Contents Introduction 3 Set up of Cushionair
General User/ Safety Guide ROYAL/ ROYAL EXTRA REPLACEMENT SYSTEM
 General User/ Safety Guide ROYAL/ ROYAL EXTRA REPLACEMENT SYSTEM ACTIVE MATTRESSES www.harvesthealthcare.co.uk E sales@harvesthealthcare.co.uk www.harvesthealthcare.co.uk 1 CONTENTS WARNINGS & CAUTIONS
General User/ Safety Guide ROYAL/ ROYAL EXTRA REPLACEMENT SYSTEM ACTIVE MATTRESSES www.harvesthealthcare.co.uk E sales@harvesthealthcare.co.uk www.harvesthealthcare.co.uk 1 CONTENTS WARNINGS & CAUTIONS
SUPREME 2 OVERLAY SYSTEM
 General User/ Safety Guide SUPREME 2 OVERLAY SYSTEM ACTIVE MATTRESSES www.harvesthealthcare.co.uk E sales@harvesthealthcare.co.uk www.harvesthealthcare.co.uk 1 CONTENTS WARNINGS & CAUTIONS GENERAL INFORMATION
General User/ Safety Guide SUPREME 2 OVERLAY SYSTEM ACTIVE MATTRESSES www.harvesthealthcare.co.uk E sales@harvesthealthcare.co.uk www.harvesthealthcare.co.uk 1 CONTENTS WARNINGS & CAUTIONS GENERAL INFORMATION
Paradise Pump Series Operator s Manual
 Paradise Pump Series Operator s Manual P/N 12421-000 9/05 Paradise Pump Operator s Manual Important Before using the Paradise Pump Alternating Pressure Relief Systems, please read and understand this
Paradise Pump Series Operator s Manual P/N 12421-000 9/05 Paradise Pump Operator s Manual Important Before using the Paradise Pump Alternating Pressure Relief Systems, please read and understand this
User Manual. 10. Dimensions and Ordering Codes. Integrity Alternating Pressure Mattress System. Alternating Pressure Mattress Systems
 10. Dimensions and Ordering Codes Integrity Alternating Pressure Mattress System Integrity Mattress Specification: Standard Extra Long Extra Narrow HD Dimension approx LxWxH (cm): 196x86x15 220x86x15 216x79x15
10. Dimensions and Ordering Codes Integrity Alternating Pressure Mattress System Integrity Mattress Specification: Standard Extra Long Extra Narrow HD Dimension approx LxWxH (cm): 196x86x15 220x86x15 216x79x15
Pegasus Aircare Mattress Overlay System Models 2200 and 2201
 Pegasus Aircare Mattress Overlay System Models 2200 and 2201 User Guide LFT11099 Issue 3 PEGASUS AIRCARE Mattress Overlay System Models 2200 and 2201 The PEGASUS AIRCARE Overlay system provides optimum
Pegasus Aircare Mattress Overlay System Models 2200 and 2201 User Guide LFT11099 Issue 3 PEGASUS AIRCARE Mattress Overlay System Models 2200 and 2201 The PEGASUS AIRCARE Overlay system provides optimum
Med Aire Alternating Pressure Pump and Pad System
 User Manual Med Aire Alternating Pressure Pump and Pad System 14002E 14001E Symbols & Statements NOTE Indicates some tips or some information users should be aware of. CAUTION Indicates correct operating
User Manual Med Aire Alternating Pressure Pump and Pad System 14002E 14001E Symbols & Statements NOTE Indicates some tips or some information users should be aware of. CAUTION Indicates correct operating
Air Plus User Manual
 Air Plus User Manual Alternating pressure mattress for prophylaxis and treatment of pressure sores Indications for use The Air Plus has been developed as a state of the art pressure relieving system for
Air Plus User Manual Alternating pressure mattress for prophylaxis and treatment of pressure sores Indications for use The Air Plus has been developed as a state of the art pressure relieving system for
PROGRAMMER OPERATIONS MANUAL
 PROGRAMMER OPERATIONS MANUAL Table of Contents Description 4 Installation 4 Operation 5 Safety Precautions 5 Regulatory & Service Information 6 Important Safety and Usage Information 7 Regulatory Notices
PROGRAMMER OPERATIONS MANUAL Table of Contents Description 4 Installation 4 Operation 5 Safety Precautions 5 Regulatory & Service Information 6 Important Safety and Usage Information 7 Regulatory Notices
APH166 Morpheus Air USER MANUAL. Apollo Healthcare Technologies Ltd. Holme Street, Liversedge, West Yorkshire, WF15 6JF.
 APH166 Morpheus Air USER MANUAL Apollo Healthcare Technologies Ltd. Holme Street, Liversedge, West Yorkshire, WF15 6JF. Tel: (01924) 614567 l Fax: (01924) 607480 Issue 1-11/03/2016 IMPORTANT SAFEGUARDS
APH166 Morpheus Air USER MANUAL Apollo Healthcare Technologies Ltd. Holme Street, Liversedge, West Yorkshire, WF15 6JF. Tel: (01924) 614567 l Fax: (01924) 607480 Issue 1-11/03/2016 IMPORTANT SAFEGUARDS
User manual. PG89 Rev 01 Editi Pressotherapy model. LymphoPress 4
 User manual PG89 Rev 01 Editi 040515 Pressotherapy model LymphoPress 4 I.A.C.E.R. Srl Via S. Pertini 24/A 30030 Martellago (VE) ITALY Tel. 041.5401356 Fax 041.5402684 e-mail: iacer@iacer.it http://www.itechmedicaldivision.com
User manual PG89 Rev 01 Editi 040515 Pressotherapy model LymphoPress 4 I.A.C.E.R. Srl Via S. Pertini 24/A 30030 Martellago (VE) ITALY Tel. 041.5401356 Fax 041.5402684 e-mail: iacer@iacer.it http://www.itechmedicaldivision.com
Cirrus 5 Oxygen Concentrator Manual
 Cirrus 5 Oxygen Concentrator Manual Important: Make sure you read and understand all of the information JAGZ03-03-3B ver1.0 12/07/2015 contained in this manual before operating your oxygen concentrator!
Cirrus 5 Oxygen Concentrator Manual Important: Make sure you read and understand all of the information JAGZ03-03-3B ver1.0 12/07/2015 contained in this manual before operating your oxygen concentrator!
Alternating Pressure / Low Air Loss Mattress System
 750000 Alternating Pressure / Low Air Loss Mattress System User Manual Important: Do not operate the Mattress System without first reading and understanding this manual! Save this manual for future use.
750000 Alternating Pressure / Low Air Loss Mattress System User Manual Important: Do not operate the Mattress System without first reading and understanding this manual! Save this manual for future use.
Actively supporting your health.
 U S E R G U I D E Actively supporting your health www.aeda.com Key Definition of symbols used User guide i Mattress Important information Caution symbol Electrical hazard Aeda a brand actively supporting
U S E R G U I D E Actively supporting your health www.aeda.com Key Definition of symbols used User guide i Mattress Important information Caution symbol Electrical hazard Aeda a brand actively supporting
Instruction Manual. Low air loss/alternating pressure mattress
 Low air loss/alternating pressure mattress Instruction Manual Drive Medical Design & Manufacturing 99 Seaview Boulevard Port Washington, NY 11050 Toll Free: 877-224-0946 Local: 516-998-4600 www.drivemedical.com
Low air loss/alternating pressure mattress Instruction Manual Drive Medical Design & Manufacturing 99 Seaview Boulevard Port Washington, NY 11050 Toll Free: 877-224-0946 Local: 516-998-4600 www.drivemedical.com
Diamond 8 Plus. Alternating + Micro Low Air Loss Pressure Relief System. User Manual. Distributed by: Quart Healthcare Inc.
 Diamond 8 Plus Alternating + Micro Low Air Loss Pressure Relief System User Manual Distributed by: Quart Healthcare Inc. www.quarthealthcare.com Warning Connect the Master Control unit to a proper power
Diamond 8 Plus Alternating + Micro Low Air Loss Pressure Relief System User Manual Distributed by: Quart Healthcare Inc. www.quarthealthcare.com Warning Connect the Master Control unit to a proper power
HoverJack. Air Patient Lift. User Manual. From HoverTech International.
 HoverJack Air Patient Lift From HoverTech International User Manual www.hovermatt.com Table of Contents Symbol References... 02 Intended Use and Precautions... 03 HOVERJACK Introduction... 04 Part Identification
HoverJack Air Patient Lift From HoverTech International User Manual www.hovermatt.com Table of Contents Symbol References... 02 Intended Use and Precautions... 03 HOVERJACK Introduction... 04 Part Identification
POWERPRESS UNIT. User Manual. Gradient Pneumatic Sequential Compressor. Neomedic
 TIMER (MIN) POWERPRESS UNIT 40 70 40 80 90 10 60 100 MIN MAX PRESSURE (mmhg) User Manual Neomedic 9601 Owens mouth Ave. #8 Chatsworth, CA 91311 Toll Free: 866-990-1168 Tel: 818-998-1023 Fax: 818-998-0277
TIMER (MIN) POWERPRESS UNIT 40 70 40 80 90 10 60 100 MIN MAX PRESSURE (mmhg) User Manual Neomedic 9601 Owens mouth Ave. #8 Chatsworth, CA 91311 Toll Free: 866-990-1168 Tel: 818-998-1023 Fax: 818-998-0277
Operation Manual. H & R Healthcare 1750 Oak Street Lakewood, NJ Phone: Fax:
 Operation Manual H & R Healthcare 1750 Oak Street Lakewood, NJ 08701 Phone: 800-801-5533 Fax: 732-367-6231 www.handrhealthcare.com Congratulations and thank you for purchasing this Relief Aire Alternating
Operation Manual H & R Healthcare 1750 Oak Street Lakewood, NJ 08701 Phone: 800-801-5533 Fax: 732-367-6231 www.handrhealthcare.com Congratulations and thank you for purchasing this Relief Aire Alternating
Sensor. Directions For Use
 Nasal Alar SpO2 Sensor Directions For Use Emergo Europe Prinsessegracht 20 2514 AP The Hague The Netherlands Manufactured by: Xhale Assurance, Inc. 3630 SW 47th Ave., Suite 100 Gainesville, FL 32608, USA
Nasal Alar SpO2 Sensor Directions For Use Emergo Europe Prinsessegracht 20 2514 AP The Hague The Netherlands Manufactured by: Xhale Assurance, Inc. 3630 SW 47th Ave., Suite 100 Gainesville, FL 32608, USA
SELECT SERIES LS300 ALTERNATING PRESSURE / LOW AIR LOSS MATTRESS SYSTEM
 SELECT SERIES LS300 ALTERNATING PRESSURE / LOW AIR LOSS MATTRESS SYSTEM USER MANUAL LS300-INS-LAB-RevB17 Read this manual before operating your Mattress System. Save this manual for future use. The most
SELECT SERIES LS300 ALTERNATING PRESSURE / LOW AIR LOSS MATTRESS SYSTEM USER MANUAL LS300-INS-LAB-RevB17 Read this manual before operating your Mattress System. Save this manual for future use. The most
Owner s Manual & Safety Instructions
 Owner s Manual & Safety Instructions Save This Manual Keep this manual for the safety warnings and precautions, assembly, operating, inspection, maintenance and cleaning procedures. Write the product s
Owner s Manual & Safety Instructions Save This Manual Keep this manual for the safety warnings and precautions, assembly, operating, inspection, maintenance and cleaning procedures. Write the product s
DYNAMIC COMPRESSION THERAPY
 PULSE PRESS DYNAMIC COMPRESSION THERAPY MULTITEC 12 User Manual May 2011 0120 CONTENTS:- SAFETY INSTRUCTIONS ELECTROMAGNETIC INTERFERENCE DESCRIPTION OF CONTROLS MODE SELECTION OPERATING INSTRUCTIONS CARE
PULSE PRESS DYNAMIC COMPRESSION THERAPY MULTITEC 12 User Manual May 2011 0120 CONTENTS:- SAFETY INSTRUCTIONS ELECTROMAGNETIC INTERFERENCE DESCRIPTION OF CONTROLS MODE SELECTION OPERATING INSTRUCTIONS CARE
Contents. Introduction...1 About Breeze... 1 Breeze Overlay... 2 Breeze Mattress Replacement... 2 Breeze Pump... 4 Controls and Indicators...
 English Contents Introduction........................................................1 About Breeze................................................... 1 Breeze Overlay.................................................
English Contents Introduction........................................................1 About Breeze................................................... 1 Breeze Overlay.................................................
AT4 Tourniquet System Operating Instructions
 AT4 Tourniquet System Operating Instructions Rx ONLY AT4 Pneumatic Tourniquet Reference No. 40070 AT4 Electronic Tourniquet Reference No. 40080 0473 92410/6/230615 Page 1 92410/6/230615 Page 2 Contents
AT4 Tourniquet System Operating Instructions Rx ONLY AT4 Pneumatic Tourniquet Reference No. 40070 AT4 Electronic Tourniquet Reference No. 40080 0473 92410/6/230615 Page 1 92410/6/230615 Page 2 Contents
NICE1 Cold + Compression Therapy System
 NICE1 Cold + Compression Therapy System Thank you for choosing the NICE1 Cold + Compression Therapy System. The NICE1 uses advanced technology to greatly improve the convenience and efficacy of cold +
NICE1 Cold + Compression Therapy System Thank you for choosing the NICE1 Cold + Compression Therapy System. The NICE1 uses advanced technology to greatly improve the convenience and efficacy of cold +
CircuFlow TM 5150 Series
 CircuFlow TM 5150 Series Sequential Compression Device Operating Instructions www.devonmedicalproducts.com 1.866.446.0092 IFU34.0001 Rev. D 20140313 The Model 5150 - Sequential Compression Device Indications:
CircuFlow TM 5150 Series Sequential Compression Device Operating Instructions www.devonmedicalproducts.com 1.866.446.0092 IFU34.0001 Rev. D 20140313 The Model 5150 - Sequential Compression Device Indications:
SC505 Sof Care Inflator. Operating Instructions and Service Manual
 SC505 Sof Care Inflator Gaymar Industries, Inc. 10 Centre Drive Orchard Park, NY 14127 Toll Free +1 800.828.7341 + 1 716.662.2551 Fax +1 800.993.7890 Outside USA +1 716.662.8636 Outside USA Fax +1 716.662.0730
SC505 Sof Care Inflator Gaymar Industries, Inc. 10 Centre Drive Orchard Park, NY 14127 Toll Free +1 800.828.7341 + 1 716.662.2551 Fax +1 800.993.7890 Outside USA +1 716.662.8636 Outside USA Fax +1 716.662.0730
Dynamic Mattress Systems. Instructions for use.
 Dynamic Mattress Systems Instructions for use www.sidhil.com Welcome to Sidhil Still making it better... At Sidhil, everything we do is designed around quality. From our modern and efficient manufacturing
Dynamic Mattress Systems Instructions for use www.sidhil.com Welcome to Sidhil Still making it better... At Sidhil, everything we do is designed around quality. From our modern and efficient manufacturing
UK RBF Bath buddy instruction 16pp 21/9/07 09:07 Page 1. Bath-Buddy / Bathmate Instruction Book
 UK RBF Bath buddy instruction 16pp 21/9/07 09:07 Page 1 Bath-Buddy / Bathmate Instruction Book UK RBF Bath buddy instruction 16pp 21/9/07 09:07 Page 2 Thank you for purchasing a Bath-Buddy/Bathmate portable
UK RBF Bath buddy instruction 16pp 21/9/07 09:07 Page 1 Bath-Buddy / Bathmate Instruction Book UK RBF Bath buddy instruction 16pp 21/9/07 09:07 Page 2 Thank you for purchasing a Bath-Buddy/Bathmate portable
Rigel 601 CHECKBOX. Instruction Manual. 348A551 Issue 2.0. April Seaward Electronic Ltd. Issue 2.0
 Rigel 601 CHECKBOX Instruction Manual 348A551 Issue 2.0 April 2006 2006 Seaward Electronic Ltd. Issue 2.0 Limited Warranty & Limitation of Liability Rigel Medical guarantees this product for a period of
Rigel 601 CHECKBOX Instruction Manual 348A551 Issue 2.0 April 2006 2006 Seaward Electronic Ltd. Issue 2.0 Limited Warranty & Limitation of Liability Rigel Medical guarantees this product for a period of
VGP-4300-G. Travel Pulse. Blood Pressure Monitor for Travel
 VGP-4300-G Travel Pulse Blood Pressure Monitor for Travel Congratulations on your Vitagoods purchase. The Travel Pulse Blood Pressure Monitor, will allow you to measure vital blood pressure parameters
VGP-4300-G Travel Pulse Blood Pressure Monitor for Travel Congratulations on your Vitagoods purchase. The Travel Pulse Blood Pressure Monitor, will allow you to measure vital blood pressure parameters
Key Product Features. Primary Use: Aggressive Treatment of Pressure Ulcers
 AME PressureGuard APM² The PressureGuard APM² enables you to change instantly and easily from alternating pressure to basic lateral rotation with a single, recessed toggle switch. The silent, reliable,
AME PressureGuard APM² The PressureGuard APM² enables you to change instantly and easily from alternating pressure to basic lateral rotation with a single, recessed toggle switch. The silent, reliable,
Digital Melting Point Apparatus
 Digital Melting Point Apparatus Heating Plateau Ramping Start/Stop Plateau set Ramp stop Hold User Guide Version 1.1 Heating Viewing tube Sample Chamber IEC power inlet socket Power on/off Temperature
Digital Melting Point Apparatus Heating Plateau Ramping Start/Stop Plateau set Ramp stop Hold User Guide Version 1.1 Heating Viewing tube Sample Chamber IEC power inlet socket Power on/off Temperature
OPERATING INSTRUCTIONS FOR USE GUIDE
 AIROS 8 Sequential Compression Device OPERATING INSTRUCTIONS FOR USE GUIDE www.airosmedical.com 1.866.991.6956 Table of Contents Indications for Use & Contraindications...................... 1 Overview
AIROS 8 Sequential Compression Device OPERATING INSTRUCTIONS FOR USE GUIDE www.airosmedical.com 1.866.991.6956 Table of Contents Indications for Use & Contraindications...................... 1 Overview
AltaDyne AutoFloat. Alternating Pressure Management Plus. Model # Model # OPERATORS MANUAL
 AltaDyne AutoFloat Alternating Pressure Management Plus Model # 753003 Model # 753004 OPERATORS MANUAL Graham-Field Health Products 2935 Northeast Parkway Atlanta, Georgia 30360 1-800-347-5678 Fax: 1-800-726-0601
AltaDyne AutoFloat Alternating Pressure Management Plus Model # 753003 Model # 753004 OPERATORS MANUAL Graham-Field Health Products 2935 Northeast Parkway Atlanta, Georgia 30360 1-800-347-5678 Fax: 1-800-726-0601
1 METRON MANIPULATION TABLES - WARRANTY STATEMENT
 1 METRON MANIPULATION TABLES - WARRANTY STATEMENT Metron Medical Australia Pty Ltd. will warrant this table, for parts only against defects in manufacture for a period of two (2) years for structural integrity,
1 METRON MANIPULATION TABLES - WARRANTY STATEMENT Metron Medical Australia Pty Ltd. will warrant this table, for parts only against defects in manufacture for a period of two (2) years for structural integrity,
Regalia Oxygen Concentrator
 Regalia Oxygen Concentrator REGALIA INSTRUCTION MANUAL P/N 5167 Rev B January 2014 Table of Contents Warnings and Cautions... 3 Indications for Use... 4 Introduction... 4 Important Safety Instructions...
Regalia Oxygen Concentrator REGALIA INSTRUCTION MANUAL P/N 5167 Rev B January 2014 Table of Contents Warnings and Cautions... 3 Indications for Use... 4 Introduction... 4 Important Safety Instructions...
Content
 Content Medical Disclaimer Intended Use Additional Information on Blood Pressure Precautions Parts Indicator & Classification of Blood Pressure Setting up your Blood Pressure Monitor Loading Batteries
Content Medical Disclaimer Intended Use Additional Information on Blood Pressure Precautions Parts Indicator & Classification of Blood Pressure Setting up your Blood Pressure Monitor Loading Batteries
USER MANUAL. AltaDyne XT Model A AltaDyne XD Model A Alternating Pressure / Low Air Loss Replacement Mattress System
 AltaDyne XT Model 773400A AltaDyne XD Model 774200A Alternating Pressure / Low Air Loss Replacement Mattress System USER MANUAL Important: Do not operate the Mattress System without first reading and understanding
AltaDyne XT Model 773400A AltaDyne XD Model 774200A Alternating Pressure / Low Air Loss Replacement Mattress System USER MANUAL Important: Do not operate the Mattress System without first reading and understanding
Operator s Manual. Dynamic Low-Air-Loss Therapy with Alternating Pressure
 Dynamic Low-Air-Loss Therapy with Alternating Pressure REF C2500 Control Unit REF M2500 Series Mattress REF Aire Select Safety Mattress Operator s Manual Table Of Contents Section Description Page 1.0
Dynamic Low-Air-Loss Therapy with Alternating Pressure REF C2500 Control Unit REF M2500 Series Mattress REF Aire Select Safety Mattress Operator s Manual Table Of Contents Section Description Page 1.0
User Manual KANMED WarmCloud Art no OT /9
 User Manual KANMED WarmCloud Art no OT-600-070/9 2013-05-17 0413 Caution Incorrect use of heating equipment can cause serious injury. Read this manual carefully. Manufactured by: KANMED AB www.kanmed.se
User Manual KANMED WarmCloud Art no OT-600-070/9 2013-05-17 0413 Caution Incorrect use of heating equipment can cause serious injury. Read this manual carefully. Manufactured by: KANMED AB www.kanmed.se
CircuFlow 5200 Series
 CircuFlow 5200 Series Sequential Compression Device Operating Instructions www.devonmedicalproducts.com 1.866.446.0092 IFU04.0001 Rev. F 20140522 Intended Use: The CircuFlow 5200 Series Sequential Compression
CircuFlow 5200 Series Sequential Compression Device Operating Instructions www.devonmedicalproducts.com 1.866.446.0092 IFU04.0001 Rev. F 20140522 Intended Use: The CircuFlow 5200 Series Sequential Compression
Pressure Automated Calibration Equipment
 GE Measurement & control Pressure Automated Calibration Equipment Safety Instructions and User Guide - K0447 PACE5000 PACE6000 K0447 Issue No. 9 1 10 1 PACE5000 1 2 3 4 5 PACE6000 2 6 7 8 3 4 5 6 7 8 9
GE Measurement & control Pressure Automated Calibration Equipment Safety Instructions and User Guide - K0447 PACE5000 PACE6000 K0447 Issue No. 9 1 10 1 PACE5000 1 2 3 4 5 PACE6000 2 6 7 8 3 4 5 6 7 8 9
LOGIC RANGE AUTOMATIC PRESSURE
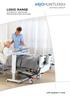 LOGIC RANGE AUTOMATIC PRESSURE REDISTRIBUTING Systems with people in mind Mattress Replacement & Overlay Systems The Auto logic 200 mattress replacement and Auto logic 110 overlay systems are part of the
LOGIC RANGE AUTOMATIC PRESSURE REDISTRIBUTING Systems with people in mind Mattress Replacement & Overlay Systems The Auto logic 200 mattress replacement and Auto logic 110 overlay systems are part of the
Operating Instructions Part No
 DIGITAL AUTOMATIC TYRE INFLATOR Operating Instructions Part No. 11.0545 Thank you for selecting this Jamec Pem Automatic Tyre Inflator. Please read this manual before carrying out any installation or service
DIGITAL AUTOMATIC TYRE INFLATOR Operating Instructions Part No. 11.0545 Thank you for selecting this Jamec Pem Automatic Tyre Inflator. Please read this manual before carrying out any installation or service
Bath Buddy / Bathmate Instruction Book
 Bath Buddy / Bathmate Instruction Book Thank you for purchasing the Bath Buddy / Bathmate portable bath lift. This simple bathing aid is designed The Bath Buddy/Bathmate is all that s needed to gently
Bath Buddy / Bathmate Instruction Book Thank you for purchasing the Bath Buddy / Bathmate portable bath lift. This simple bathing aid is designed The Bath Buddy/Bathmate is all that s needed to gently
Oxygen Dialflow Meter. Instructions for Use
 Oxygen Dialflow Meter Instructions for Use 702-0031.9 May 2014 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in personal injury to the user
Oxygen Dialflow Meter Instructions for Use 702-0031.9 May 2014 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in personal injury to the user
12V Mini Air Compressor
 INSTRUCTIONS FOR 12V Mini Air Compressor Stock No.80999 Part No.DA12/250B IMPORTANT: PLEASE READ THESE INSTRUCTIONS CAREFULLY TO ENSURE THE SAFE AND EFFECTIVE USE OF THIS PRODUCT. GENERAL INFORMATION This
INSTRUCTIONS FOR 12V Mini Air Compressor Stock No.80999 Part No.DA12/250B IMPORTANT: PLEASE READ THESE INSTRUCTIONS CAREFULLY TO ENSURE THE SAFE AND EFFECTIVE USE OF THIS PRODUCT. GENERAL INFORMATION This
AUTOMATIC TIRE INFLATOR # MW-60, MW-60-4WAY & MW-64HP
 USER MANUEL AUTOMATIC TIRE INFLATOR # MW-60, MW-60-4WAY & MW-64HP TIRE EQUIPMENT MANUFACTURER 1.866.409.RACK WWW.MARTINSINDUSTRIES.COM info@martinsindustries.com PARTS Verify that the following components
USER MANUEL AUTOMATIC TIRE INFLATOR # MW-60, MW-60-4WAY & MW-64HP TIRE EQUIPMENT MANUFACTURER 1.866.409.RACK WWW.MARTINSINDUSTRIES.COM info@martinsindustries.com PARTS Verify that the following components
NIMBUS 4 NIMBUS PROFESSIONAL
 NIMBUS 4 NIMBUS PROFESSIONAL INSTRUCTIONS FOR USE 0086...with people in mind Contents General Safety....................................................... iii Introduction.........................................................
NIMBUS 4 NIMBUS PROFESSIONAL INSTRUCTIONS FOR USE 0086...with people in mind Contents General Safety....................................................... iii Introduction.........................................................
RIK Fluid Overlay INSTRUCTIONS FOR USE
 RIK Fluid Overlay INSTRUCTIONS FOR USE P/N KS2XP01-AH Rev B 04/2014...with people in mind EXCEPT FOR THE WARRANTIES EXPRESSLY SET FORTH HEREIN, ALL OTHER WAR- RANTIES, INCLUDING IMPLIED WARRANTIES OF MERCHANTABILITY
RIK Fluid Overlay INSTRUCTIONS FOR USE P/N KS2XP01-AH Rev B 04/2014...with people in mind EXCEPT FOR THE WARRANTIES EXPRESSLY SET FORTH HEREIN, ALL OTHER WAR- RANTIES, INCLUDING IMPLIED WARRANTIES OF MERCHANTABILITY
AKRON CHILDS TILT TABLE
 AKRON CHILDS TILT TABLE Model 8601B USER MANUAL Contents 1. Introduction 2 Warnings, Cautions 2 General Warnings 2 2. Installation 3 3. Operation 4 Wheel Locking 4 Tilting Control 4 Head Section, Foot
AKRON CHILDS TILT TABLE Model 8601B USER MANUAL Contents 1. Introduction 2 Warnings, Cautions 2 General Warnings 2 2. Installation 3 3. Operation 4 Wheel Locking 4 Tilting Control 4 Head Section, Foot
Intermittent Pneumatic Compression System
 User Manual Intermittent Pneumatic Compression System To ensure safe use of this device, it is essential that you read and fully understand the information contained within this manual. Mechanical prophylaxis
User Manual Intermittent Pneumatic Compression System To ensure safe use of this device, it is essential that you read and fully understand the information contained within this manual. Mechanical prophylaxis
User Guide Antidecubitus systems EURO BASIC
 User Guide Antidecubitus systems EURO BASIC User Guide Euro Basic Rev 06 del 11/2011 pag. 1 di 16 INDEX Par. Title Page 1 Instructions 3 2 Standards and directives 3 3 Identifying the device 3 4 Description
User Guide Antidecubitus systems EURO BASIC User Guide Euro Basic Rev 06 del 11/2011 pag. 1 di 16 INDEX Par. Title Page 1 Instructions 3 2 Standards and directives 3 3 Identifying the device 3 4 Description
SkillGuide. User Guide. English
 SkillGuide User Guide English SkillGuide SkillGuide is a feedback device designed to provide real-time and summative feedback on CPR performance. www.laerdal.com Items included SkillGuide and User Guide.
SkillGuide User Guide English SkillGuide SkillGuide is a feedback device designed to provide real-time and summative feedback on CPR performance. www.laerdal.com Items included SkillGuide and User Guide.
CircuFlow TM 5208 Series
 CircuFlow TM 5208 Series Sequential Compression Device Operating Instructions www.devonmedicalproducts.com 1.866.446.0092 IFU35.0001 Rev. E 20140313 Intended Use: The CircuFlow 5208 Series Sequential Compression
CircuFlow TM 5208 Series Sequential Compression Device Operating Instructions www.devonmedicalproducts.com 1.866.446.0092 IFU35.0001 Rev. E 20140313 Intended Use: The CircuFlow 5208 Series Sequential Compression
Oxygen Dialflow Meter. Instructions for Use
 Oxygen Dialflow Meter Instructions for Use 702-0031.12 December 2017 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in injury to the patient,
Oxygen Dialflow Meter Instructions for Use 702-0031.12 December 2017 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in injury to the patient,
PRESSURE REDISTRIBUTION SYSTEM. with people in mind
 ALPHA RESPONSE PRESSURE REDISTRIBUTION SYSTEM with people in mind introduction superior Features For over 0 years the Alpha range of pressure redistributing mattresses has been proven to offer patients,
ALPHA RESPONSE PRESSURE REDISTRIBUTION SYSTEM with people in mind introduction superior Features For over 0 years the Alpha range of pressure redistributing mattresses has been proven to offer patients,
VERTICAL AIR COMPRESSORS
 VERTICAL AIR COMPRESSORS MODEL NO: VE15C150, VE18C150, VE25C150 PART NO: 2226010, 2226020, 2226025 OPERATION & MAINTENANCE INSTRUCTIONS LS0715 INTRODUCTION Thank you for purchasing this CLARKE Vertical
VERTICAL AIR COMPRESSORS MODEL NO: VE15C150, VE18C150, VE25C150 PART NO: 2226010, 2226020, 2226025 OPERATION & MAINTENANCE INSTRUCTIONS LS0715 INTRODUCTION Thank you for purchasing this CLARKE Vertical
User Manual. PULSE User Manual WARNINGS
 PULSE User Manual PULSE User Manual WARNINGS Table of Contents Warnings 4 Labels 6 Indications for Use 8 Risks and Benefits of the NormaTec PULSE Recovery System 8 Illustrations 9 Operating Instructions
PULSE User Manual PULSE User Manual WARNINGS Table of Contents Warnings 4 Labels 6 Indications for Use 8 Risks and Benefits of the NormaTec PULSE Recovery System 8 Illustrations 9 Operating Instructions
Resusci Anne Simulator
 EN Resusci Anne Simulator Important Product Information www.laerdal.com Cautions and Warnings A Caution identifies conditions, hazards, or unsafe practices that can result in minor personal injury or damage
EN Resusci Anne Simulator Important Product Information www.laerdal.com Cautions and Warnings A Caution identifies conditions, hazards, or unsafe practices that can result in minor personal injury or damage
Use this key to determine which sections of this Product Manual apply to your job.
 R710 Product Manual Contents Warnings and Important Information 3 Recommended Use 4 Product Information 5 Technical Data 5 Before & During Every Transfer 6 Inspection 6 SoloLift Scale (optional) 6 SoloVest
R710 Product Manual Contents Warnings and Important Information 3 Recommended Use 4 Product Information 5 Technical Data 5 Before & During Every Transfer 6 Inspection 6 SoloLift Scale (optional) 6 SoloVest
ORB-400 BUBBLE/HAZE MACHINE. Item ref: UK User Manual
 ORB-400 BUBBLE/HAZE MACHINE Item ref: 160.462UK User Manual Caution: Please read this manual carefully before operating Damage caused by misuse is not covered by the warranty Introduction Thank you for
ORB-400 BUBBLE/HAZE MACHINE Item ref: 160.462UK User Manual Caution: Please read this manual carefully before operating Damage caused by misuse is not covered by the warranty Introduction Thank you for
USER MANUAL SAVE THESE INSTRUCTIONS. For the most current manual revision, please visit our Website:
 USER MANUAL Model: PM4300 Series SAVE THESE INSTRUCTIONS For the most current manual revision, please visit our Website: www.precisionmedical.com 300 Held Drive Tel: (+001) 610-262-6090 Northampton, PA
USER MANUAL Model: PM4300 Series SAVE THESE INSTRUCTIONS For the most current manual revision, please visit our Website: www.precisionmedical.com 300 Held Drive Tel: (+001) 610-262-6090 Northampton, PA
Operating Instructions Part No
 DIGITAL AUTOMATIC TYRE INFLATOR Operating Instructions Part No. 11.0578 Thank you for selecting this Jamec Pem Automatic Tyre Inflator. Please read this manual before carrying out any installation or service
DIGITAL AUTOMATIC TYRE INFLATOR Operating Instructions Part No. 11.0578 Thank you for selecting this Jamec Pem Automatic Tyre Inflator. Please read this manual before carrying out any installation or service
VERTICAL AIR COMPRESSORS
 VERTICAL AIR COMPRESSORS MODEL NO: VE11C150, VE15C150, VE18C150 PART NO: 2226005, 2226000, 2226015 OPERATION & MAINTENANCE INSTRUCTIONS LS0615 INTRODUCTION Thank you for purchasing this CLARKE Vertical
VERTICAL AIR COMPRESSORS MODEL NO: VE11C150, VE15C150, VE18C150 PART NO: 2226005, 2226000, 2226015 OPERATION & MAINTENANCE INSTRUCTIONS LS0615 INTRODUCTION Thank you for purchasing this CLARKE Vertical
REF R00 JH 02/04/11 USER MANUAL
 REF 1082564 1082563 R00 JH 02/04/11 USER MANUAL UltraFill User Manual Table of Contents Introduction...3 How the System Works...7 Troubleshooting Guide... 19 Cleaning and Maintenance... 22 Transporting
REF 1082564 1082563 R00 JH 02/04/11 USER MANUAL UltraFill User Manual Table of Contents Introduction...3 How the System Works...7 Troubleshooting Guide... 19 Cleaning and Maintenance... 22 Transporting
Inflation System Directions for Use: Obalon EzFill Dispenser TM & Obalon EzFill Can TM
 Inflation System Directions for Use: Obalon EzFill Dispenser TM & Obalon EzFill Can TM Caution: Federal (USA) law restricts this device to sale by or on the order of a physician Rx Only Page 1 OBALON INFLATION
Inflation System Directions for Use: Obalon EzFill Dispenser TM & Obalon EzFill Can TM Caution: Federal (USA) law restricts this device to sale by or on the order of a physician Rx Only Page 1 OBALON INFLATION
USER MANUAL. SAVE THESE INSTRUCTIONS (For latest revision, go to
 USER MANUAL Model: PM5950 SAVE THESE INSTRUCTIONS (For latest revision, go to www.precisionmedical.com) Federal (USA) law restricts this device to sale by or on the order of a physician. Tel: (+001) 610-262-6090
USER MANUAL Model: PM5950 SAVE THESE INSTRUCTIONS (For latest revision, go to www.precisionmedical.com) Federal (USA) law restricts this device to sale by or on the order of a physician. Tel: (+001) 610-262-6090
MA90 Series Mattresses. MA95 Series Mattresses
 Owner s Operator and Maintenance Manual MA90 Series Mattresses MA90Z Lateral Rotation with Alternating Pressure and On-Demand Low Air Loss System MA90ZB42 Lateral Rotation with Alternating Pressure and
Owner s Operator and Maintenance Manual MA90 Series Mattresses MA90Z Lateral Rotation with Alternating Pressure and On-Demand Low Air Loss System MA90ZB42 Lateral Rotation with Alternating Pressure and
PLEASE READ CAREFULLY BEFORE INSTALLING OR USING MEGA POOL SAVER MPS 1100
 MPS-1100 User Manual Mega Pool Saver Ltd PLEASE READ CAREFULLY BEFORE INSTALLING OR USING MEGA POOL SAVER MPS 1100 For further up to date instructions on how to install Mega Pool Saver MPS 1100, please
MPS-1100 User Manual Mega Pool Saver Ltd PLEASE READ CAREFULLY BEFORE INSTALLING OR USING MEGA POOL SAVER MPS 1100 For further up to date instructions on how to install Mega Pool Saver MPS 1100, please
Vapotherm Q50 Compressor. Instructions for Use
 Vapotherm Q50 Compressor Instructions for Use Contents Quick Start Guide...2 Intended Use...3 Indications, Warnings and Cautions...4 Primary Indications...4 Contraindications...4 Warnings and Cautions...4
Vapotherm Q50 Compressor Instructions for Use Contents Quick Start Guide...2 Intended Use...3 Indications, Warnings and Cautions...4 Primary Indications...4 Contraindications...4 Warnings and Cautions...4
BOC: Living healthcare. Manual. LIV IQ BOC Integrated Valve with digital display portable delivery system for Medical Oxygen. BOC: Living healthcare
 BOC: Living healthcare Manual LIV IQ BOC Integrated Valve with digital display portable delivery system for Medical Oxygen. BOC: Living healthcare 02 Manual LIV IQ Oxygen Manual LIV IQ Oxygen 03 Contents.
BOC: Living healthcare Manual LIV IQ BOC Integrated Valve with digital display portable delivery system for Medical Oxygen. BOC: Living healthcare 02 Manual LIV IQ Oxygen Manual LIV IQ Oxygen 03 Contents.
Lateral Turning System. User Guide.
 Lateral Turning System User Guide www.frontier-group.co.uk Key This user guide contains important information on correct usage, handling, cleaning and decontamination. Please read carefully prior to use.!
Lateral Turning System User Guide www.frontier-group.co.uk Key This user guide contains important information on correct usage, handling, cleaning and decontamination. Please read carefully prior to use.!
WARNING: EXPLOSION HAZARD
 Section 1 Safety 1.1 Instructions for the Safe Operation and Use of the Pulse Oximeter Do not attempt to service the Pulse Oximeter yourself. Only qualified service personnel should attempt any necessary
Section 1 Safety 1.1 Instructions for the Safe Operation and Use of the Pulse Oximeter Do not attempt to service the Pulse Oximeter yourself. Only qualified service personnel should attempt any necessary
OPERATOR S MANUAL Ar-Gone Weld Gas Analyzer
 July 2011 OPERATOR S MANUAL Ar-Gone Weld Gas Analyzer WARNING! Before operating this product, read and understand this Operator s Manual. Become familiar with the potential hazards of this unit. Contact
July 2011 OPERATOR S MANUAL Ar-Gone Weld Gas Analyzer WARNING! Before operating this product, read and understand this Operator s Manual. Become familiar with the potential hazards of this unit. Contact
Endo-Flush Order # ZUTR30004 OPERATION MANUAL. Zutron Medical, LLC W 98 th St #40-27 Lenexa, KS Phone Fax
 OPERATION MANUAL Zutron Medical, LLC 17501 W 98 th St #40-27 Lenexa, KS 66219 Phone 877-343-5873 Fax 913-967-5944 ZUT-Lab-004-30004 REV. 03312017 Table of Contents 2 Introduction 1. Intended Use 2. Labels,
OPERATION MANUAL Zutron Medical, LLC 17501 W 98 th St #40-27 Lenexa, KS 66219 Phone 877-343-5873 Fax 913-967-5944 ZUT-Lab-004-30004 REV. 03312017 Table of Contents 2 Introduction 1. Intended Use 2. Labels,
McMurdo S20 & Kannad Marine R10 AIS MOB Devices
 SERVICE BULLETIN Issue No: MG018 Date: 22 nd January 2016 McMurdo S20 & Kannad Marine R10 AIS MOB Devices Battery change & maintenance instructions INTRODUCTION 1.1 Scope: This document provides the instructions
SERVICE BULLETIN Issue No: MG018 Date: 22 nd January 2016 McMurdo S20 & Kannad Marine R10 AIS MOB Devices Battery change & maintenance instructions INTRODUCTION 1.1 Scope: This document provides the instructions
Gas density monitor With integrated transmitter Model GDM-100-TI
 SF 6 gas solutions Gas density monitor With integrated transmitter Model GDM-100-TI grid Products WIKA data sheet SP 60.05 for further approvals see page 5 Applications Gas density monitoring of closed
SF 6 gas solutions Gas density monitor With integrated transmitter Model GDM-100-TI grid Products WIKA data sheet SP 60.05 for further approvals see page 5 Applications Gas density monitoring of closed
12V MINI AIR COMPRESSOR
 12V MINI AIR COMPRESSOR STOCK No.65958 PART No.DA12/250A INSTRUCTIONS IMPORTANT: PLEASE READ THESE INSTRUCTIONS CAREFULLY TO ENSURE THE SAFE AND EFFECTIVE USE OF THIS TOOL. 08/2001 GENERAL INFORMATION
12V MINI AIR COMPRESSOR STOCK No.65958 PART No.DA12/250A INSTRUCTIONS IMPORTANT: PLEASE READ THESE INSTRUCTIONS CAREFULLY TO ENSURE THE SAFE AND EFFECTIVE USE OF THIS TOOL. 08/2001 GENERAL INFORMATION
U S E R M A N U A L. Model: PM4300 Series SAVE THESE INSTRUCTIONS
 U S E R M A N U A L Model: PM4300 Series (PM4351 Shown) SAVE THESE INSTRUCTIONS CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090
U S E R M A N U A L Model: PM4300 Series (PM4351 Shown) SAVE THESE INSTRUCTIONS CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090
Sensor for Air Bubble Detection at Liquid Filled Tubes. SONOCHECK Type ABD06.xx. Operating Manual
 Sensor for Air Bubble Detection at Liquid Filled Tubes SONOCHECK Type ABD06.xx Operating Manual Manufacturer: Model: Type: SONOTEC Ultraschallsensorik Halle GmbH Air Bubble Detector ABD06.xx SONOTEC Ultraschallsensorik
Sensor for Air Bubble Detection at Liquid Filled Tubes SONOCHECK Type ABD06.xx Operating Manual Manufacturer: Model: Type: SONOTEC Ultraschallsensorik Halle GmbH Air Bubble Detector ABD06.xx SONOTEC Ultraschallsensorik
User Manual. Wrist Blood Pressure Monitor. Wrist Type
 Version:1.0 User Manual Wrist Blood Pressure Monitor EBP-017 Wrist Type EASY AT HOME MEDICAL,LLC Any questions,please call toll-free : 1-855-822-6999 M-F 9 a.m.-5 p.m. CST E-MAIL:service@healthcare-manager.com
Version:1.0 User Manual Wrist Blood Pressure Monitor EBP-017 Wrist Type EASY AT HOME MEDICAL,LLC Any questions,please call toll-free : 1-855-822-6999 M-F 9 a.m.-5 p.m. CST E-MAIL:service@healthcare-manager.com
OXY Integral. INTERCON ENTERPRISES INC Tel: Fax: Internet:
 OXY Integral INTERCON ENTERPRISES INC Tel: 800 665 6655 Fax: 604 946 5340 E-Mail: sales@intercononline.com Internet: www.intercononline.com Manual Integral 2006 1 INDEX 2-3 PREFACE 4 INTRODUCTION 5 Principle
OXY Integral INTERCON ENTERPRISES INC Tel: 800 665 6655 Fax: 604 946 5340 E-Mail: sales@intercononline.com Internet: www.intercononline.com Manual Integral 2006 1 INDEX 2-3 PREFACE 4 INTRODUCTION 5 Principle
USER MANUAL. The Vest Airway Clearance System, Model 104 From Hill-Rom USR150 REV 3
 USER MANUAL The Vest Airway Clearance System, Model 104 From Hill-Rom USR150 REV 3 2007 by Hill-Rom Services, Inc. ALL RIGHTS RESERVED. Manufactured by: HILL-ROM 4349 CORPORATE ROAD CHARLESTON, SC 29405
USER MANUAL The Vest Airway Clearance System, Model 104 From Hill-Rom USR150 REV 3 2007 by Hill-Rom Services, Inc. ALL RIGHTS RESERVED. Manufactured by: HILL-ROM 4349 CORPORATE ROAD CHARLESTON, SC 29405
Micro Dial-Flowmeter. Instructions for Use
 Micro Dial-Flowmeter Instructions for Use 702-0082.8 May 2014 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in personal injury to the user
Micro Dial-Flowmeter Instructions for Use 702-0082.8 May 2014 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in personal injury to the user
BLOOD PRESSURE MONITOR
 E BLOOD PRESSURE MONITOR Instructions and Guarantee BPA-9300 GB SAFETY INFORMATION The below signs might be in the user manual, labeling or other components. They are the requirement of standard and using.
E BLOOD PRESSURE MONITOR Instructions and Guarantee BPA-9300 GB SAFETY INFORMATION The below signs might be in the user manual, labeling or other components. They are the requirement of standard and using.
OPERATOR S MANUAL. Pediatric/Infant cap-one Mask YG-232T/YG-242T A. General. Safety Information WARNING WARNING WARNING. Components WARNING
 OPERATOR S MANUAL 0614-905493A First Edition: 12 Dec 2011 Second Edition: 12 Feb 2015 Printed: Pediatric/Infant cap-one Mask YG-232T/YG-242T Symbol Description Attention, consult operator s manual Date
OPERATOR S MANUAL 0614-905493A First Edition: 12 Dec 2011 Second Edition: 12 Feb 2015 Printed: Pediatric/Infant cap-one Mask YG-232T/YG-242T Symbol Description Attention, consult operator s manual Date
3 GALLON, OILLESS PANCAKE COMPRESSOR INSTRUCTIONS. Item #31289
 3 GALLON, OILLESS PANCAKE COMPRESSOR INSTRUCTIONS Item #31289 The EASTWOOD 3 GALLON, OILLESS PANCAKE COMPRESSOR, with an Integral Air Regulator, efficiently supplies all compressed air requirements for
3 GALLON, OILLESS PANCAKE COMPRESSOR INSTRUCTIONS Item #31289 The EASTWOOD 3 GALLON, OILLESS PANCAKE COMPRESSOR, with an Integral Air Regulator, efficiently supplies all compressed air requirements for
Manual for HoverApps Devices
 For Product Demonstrations & Training Videos, please visit www.youtube.com/user/hovermatt Manual for HoverApps Devices HoverTech Wedge HoverTech Roller Air Supply Table of Contents HT-Wedge... HT-Wedge
For Product Demonstrations & Training Videos, please visit www.youtube.com/user/hovermatt Manual for HoverApps Devices HoverTech Wedge HoverTech Roller Air Supply Table of Contents HT-Wedge... HT-Wedge
77-I0PN230 Rev. A. Model 1030 User Manual
 77I0PN230 Rev. A TABLE OF CONTENTS FUNCTION...2 TREATMENT...3 INDICATIONS FOR USE...4 CONTRAINDICATIONS...4 CLEANING...4 MAINTENANCE...4 CLASSIFICATION & SYMBOLS...4 DISINFECTION / STERILIZATION...4 SAFETY
77I0PN230 Rev. A TABLE OF CONTENTS FUNCTION...2 TREATMENT...3 INDICATIONS FOR USE...4 CONTRAINDICATIONS...4 CLEANING...4 MAINTENANCE...4 CLASSIFICATION & SYMBOLS...4 DISINFECTION / STERILIZATION...4 SAFETY
Theatre Control Panel. Instructions for Use. January 2017 ASF-29 (1)
 Theatre Control Panel Instructions for Use January 2017 0197 ASF-29 (1) Contents 1. Instructions For Use 1.1. Introduction 1.2. Explanation of Symbols 1.3. General Safety Instructions Intended Use Operating
Theatre Control Panel Instructions for Use January 2017 0197 ASF-29 (1) Contents 1. Instructions For Use 1.1. Introduction 1.2. Explanation of Symbols 1.3. General Safety Instructions Intended Use Operating
