Dynamic Mattress Systems. Instructions for use.
|
|
|
- Elisabeth Freeman
- 5 years ago
- Views:
Transcription
1 Dynamic Mattress Systems Instructions for use
2 Welcome to Sidhil Still making it better... At Sidhil, everything we do is designed around quality. From our modern and efficient manufacturing plant in Halifax, West Yorkshire, we manufacture a range of products for the healthcare market using leading edge production technology and finishing processes. We are the only remaining volume manufacturer of hospital beds in the UK, bringing together innovation in product development, sales, customer service and logistics to provide clear benefits for our customers in terms of flexibility, short production timescales and support from our nationwide network of service and maintenance centres. Excellence in customer service is our key objective. Alongside our focus on innovation, research and product development, we use our UK manufacturing facility to ensure optimum levels of product and spares availability, with unparalleled levels of reliability and performance. Corporate social responsibility is also an issue for Sidhil. We have now received accreditation to ISO 14001, underlining our commitment to maintaining the highest levels of environmental awareness and sustainability across our manufacturing operation. 2
3 CONTENTS 1. INTRODUCTION CONTACT INFORMATION PRODUCT DESCRIPTION Environment Intended Use Features SAFETY Warnings and Cautions Risk Assessment Contraindicators Mattress Load General Warnings TRANSPORT AND STORAGE Storage Transportation Environmental Conditions SYMBOL DEFINITION PARTS IDENTIFICATION INSTALLATION OPERATION Environmental Limits When in Operation Preparing For Use Control Interface Mattress Overview Control Unit Operation Turning On / Off System Setup Alternation Patient Weight and Pressure Safety Functions Function Lock Mute System Functions Therapy Selection Comfort Control Additional Settings Mattress Operation Auto Seat Mode Low Patient Weight CPR Mattress Disconnection and Power Cuts Use of Incontinence Products DECONTAMINATION / CLEANING Control Unit Mattress Alternative Mattress Cover Cleaning Instructions TROUBLESHOOTING MAINTENANCE Disposal of Parts SPECIFICATION ELECTROMAGNETIC COMPATIBILITY (EMC) Requirements According to IEC : WARRANTY
4 1. INTRODUCTION Thank you for purchasing this product. These instructions for use should be read carefully before operating the dynamic mattress and kept for future reference. Please ensure that you understand all instructions, if you have any questions concerning the operation or maintenance of the dynamic mattress please contact your provider. 2. CONTACT INFORMATION For any service, warranty, sales or customer service information on this product please contact your provider or if in doubt contact Sidhil Ltd at the following address. Please quote the control unit and/or mattress serial number on all correspondence. Sidhil Ltd. Sidhil Business Park Holmfield Halifax West Yorkshire HX2 9TN UK Service & Maintenance Tel: +44 (0) Fax: +44 (0) Spares Tel: +44 (0) Fax: +44 (0) Customer Service Tel: +44 (0) Fax: +44 (0) For Service & Support outside the UK & Northern Ireland please contact the local distribution company from where this equipment was purchased. Failure to do so may result in the manufacturer s warranty becoming void. 4
5 3. PRODUCT DESCRIPTION 3.1 Environment Your dynamic mattress system is intended for use in the following environment: A hospital where intensive/acute care is provided and medical supervision is required and monitoring provided. 3.2 Intended Use The Artemis dynamic mattress system is a high quality air support surface suitable for individuals at very high risk, those with up to and including grade 4 pressure ulcers, needing frequent monitoring and positioning. Via the automatic changing pressure in the air cells the mattress provides comfort and pressure relief to patients vulnerable to pressure damage by providing regular periods of pressure reduction to aid blood and lymphatic flow to vulnerable tissue. The mattress system is designed for use on both standard and profiling bed frames. Ideally, patients allocated to this system will have some degree of independent mobility or can be repositioned according to individual needs. For assistance in setting up, using or maintaining your dynamic mattress system or to report unexpected operation refer to the contact details found in section Features Mattress 23 cells: 3 static head cells and 20 alternating cells (including 8 narrow heel cells). Cell on cell construction. 10 minute cycle period. Securing straps. 2 way stretch, vapour permeable and waterproof cover. Automatic patient egress detection (when function is activated on control unit). Cable management routing. Control Unit Provides air supply to mattress. Automatically determines patient weight (±10kg). Automatically sets optimum pressure for patient. Operated via a touch panel with integrated visual display. Alternating, constant low pressure, pulsation and max inflate functions. Customisable functions. Fault indicator displays. 5
6 4. SAFETY 4.1 Warnings and Cautions Warning Caution Warnings in these instructions for use highlight potential hazards that if disregarded could lead to injury or death. Cautions in these instructions for use highlight potential hazards that if disregarded could lead to equipment damage or failure. 4.2 Risk Assessment Bed frames used with the mattress system can vary greatly depending on the specific healthcare setting (i.e. hospitals, nursing homes, home care etc). It is the responsibility of the carer to carry out the necessary risk assessment to ensure the safety of the patient. Before a patient uses the dynamic mattress system a risk assessment must be performed on a patient by patient basis. The risk assessment should include, but is not limited to: Product combinations (bed frame, mattress, side rails etc.). Extent of tissue damage (if any). Entrapment. Patient falls. Small adults (and children). Patients with learning difficulties. Unauthorised people with access to the controls. 4.3 Contraindicators Patient conditions for which the application of pressure relief on an alternating mattress system is a contraindication are as follows: Cervical or skeletal traction Unstable spinal fractures Other contraindications may be relevant which are specific to the patient or care environment. 4.4 Mattress Load Maximum patient weight: 248kg (39 stone) 6
7 4.5 General Warnings Warning The mattress system is to be installed and put into service in accordance with the information provided in these instructions for use. The mattress system is typically not suitable for child use, if it is to be used for child occupancy ensure a risk assessment has been undertaken taking into account the proportions of the child and dimensions of the mattress system. Misused electrical equipment can be hazardous. Exposure of the control unit to any liquid while it is plugged in could result in a severe electrical hazard. The control unit is a precision electronic product. Use care when handling or transporting it. Dropping or other sudden impacts may result in damage to the unit. Do not open the control unit Risk of electrical shock. Repairs and service are to be conducted by suitably trained personnel. If the control unit is not functioning properly, or has been damaged, unplug the unit and take it out of service immediately. Modification of the mattress or control box is not allowed without the permission of Sidhil Ltd A hazard could be introduced. Occupants and users of this equipment must never smoke in close proximity to the control unit, mattress or bedding being used with it - Risk of fire. Accessories that have not been approved or designed for use with the mattress system are not be used. Control unit shall not be used in the presence of flammable gasses or used in oxygen rich environments Risk of explosion / fire. Control unit functions must be locked out when a patient is left unattended. If children, adults with learning difficulties or even pets pose a potential risk of intentional or unintentional tampering with the control unit its suitability for use is to be considered during the initial patient / product risk assessment. The mattress is for single occupancy use. Additional weight could damage the mattress or affect the performance of the mattress system. Minimise articles (e.g. bedding) between the mattress surface and patient, and secure bed sheets loosely so as not to affect mattress functionality. Perform regular patient skin checks Any tissue deterioration may require equipment reallocation and/or a re-assessment of the care being provided. 7
8 5. TRANSPORT AND STORAGE 5.1 Storage Detach the control unit from the mattress. Rotate the CPR dial until it is open. Lay the mattress out flat and position upside down. Ensure there is no air trapped in the cells Note, air will only escape from the CPR outlet and not the mattress connector. Position the control unit on the mattress. Roll from the head end towards the foot end (ensuring the control unit is fully covered). Place into storage bag to protect from dirt, debris, fluids etc. Warning Caution The mattress system is to be decontaminated prior to any storage to avoid risk of cross contamination. Do not fold, crease or stack mattresses and/or control units - damage could be incurred. Do not store whilst inflated - damage could be incurred. 5.2 Transportation Warning Do not remove mattress from bed frame if occupant is still on mattress Risk of falling. If essential that patient is moved whilst staying on mattress the mattress must be re-plugged in immediately once desired location has been reached - Risk of tissue damage due to loss of cell alternation. Caution Do not transport/move whilst inflated (unless safely secured to bed frame) Damage could be incurred. Never drag the mattress - always carry it. 5.3 Environmental Conditions The following conditions should be followed when transporting and storing the dynamic mattress system: Ambient temperature: -25 C to +70 C Humidity: < 93%, non-condensing 8
9 6. SYMBOL DEFINITION The following symbols are found on this bed: (See section 9.3 for interface symbols) Warning Beware of potential hazard Refer to instructions for use - Mandatory Failure to read the instructions for use could introduce a hazard W.E.E.E Label (Waste Electrical and Electronic Equipment) Refer to section 12.1 No smoking near to mattress system or whilst occupying mattress - Risk of fire Type BF Applied Part Applied Part: The parts of the device that come into physical contact with the user/occupant in order for it to carry out its intended function (refer to secion 13 for a description of the applied parts). Type BF: Applied parts which are electrically isolated from earth and other parts of the medical equipment - Complying with specific requirements for protection against electric shock to IEC Mattress The following symbols are found on the mattress: 71 C Machine wash at 71ºC Do not iron Do not bleach Do not dry clean Tumble dry on low heat 9
10 7. PARTS IDENTIFICATION B 6 A C D F E No. Item Description Qty. 1 Air Cell 23 2 Top Cover 1 3 Air Connector 1 4 Mattress Strap 8 5 Base Cover 1 6 CPR dial 1 No. Item Description Qty. A Control Interface 1 B Hook 2 C Mains Cable Port 1 D Pad 2 E Air Connector (attached to mattress) 1 F Air Filter Cover 1 10
11 8. INSTALLATION When specifying a mattress, bedframe and side rail combination a clinical assessment of the patient s needs must be carried out in line with local policy. Warning Caution Refer to the warnings at the end of this section before proceeding with installation. Ensure the mains supply is compatible with the control unit (see section 13 for electrical specification) Avoid placing the mattress system in direct sunlight Direct sunlight could damage the mattress cover. Open all packaging with care. Once removed from the packaging check product for any signs of damage. If damaged do not put into use and contact your provider or Sidhil Ltd. (see section 2). Remove all covers, sheets and the existing mattress from the bed. Position the mattress on top of the bed frame, top cover facing upwards and air hose at the foot of the bed for control unit positioning. Attach to the bed frame by securing the adjustable straps to the moving sections of the bed. For profiling beds, it is essential that straps are secured around the movable sections of the bedframe Damage will be incurred when profiled if secured to fixed parts of frame. Caution To avoid any risk of damage to the mattress ensure there are no sharp objects which may come in contact with it. 11
12 Ensure the CPR dial is rotated to a vertical or horizontal, and closed, position. CPR Open: CPR Closed: Position the control unit by hanging the hooks over the foot board. If there is no foot board place the unit on the floor with the front facing upwards. Ensure the rear of the unit is not obstructed by carpet, rugs etc. It is advisable to place the unit on a firm surface. Attach the air connector to the control unit, ensuring the air hose does not kink or become trapped between parts of the bedframe. Route the mains cable down the length of the mattress using the integral routing sheath. Detach the pop studs from the sheath, insert the cable and reattach all the studs down the full length of the sheath. Plug the mains cable into a suitable mains supply and switch on the control unit (see section 9). The mattress system will start to inflate. Inflation will be complete within 20 minutes. 12
13 Once inflated, ensure the straps that attach the mattress to the bed frame are secure and hold the mattress in place, adjust as necessary. Once the mattress is fully inflated, the bedding can be replaced. Secure sheets loosely enough to ensure they do not interfere with cell alternation. Proceed to section 9 for Operation. Warning Ensure the mattress is used with a compatible side rail and bed frame combination Incorrect combinations can lead to entrapment and/or falls hazards. Ensure the mattress is of the correct type for the patient Incorrect mattress specification could lead to an injury. The mains plug is the disconnect device for the means of isolating the control unit from the mains supply, the plug must be accessible at all times. Ensure the mains cable is plugged into an appropriate power source at all times. To avoid the risk of electric shock, this equipment must only be connected to a supply mains with a protective earth. Ensure the mains cable is not in tension, paying particular attention to when the bed travels up/down. Precautions are to be taken when routing the mains cable around the bed to ensure that it does not become squeezed, trapped or damaged by the bedframe or other ancillary equipment - Risk of electrocution. Any electrical cable that is part of the mattress system or associated ancillary equipment that is found to be damaged must be replaced immediately - Damaged electrical cables can create a risk of electrocution and / or fire. A CE marked extension cable must only be used when it is not possible to reach a wall socket with the equipment mains cable Contact Sidhil Ltd for detail in regards to safe use of extension cables. If an extension cable is used never overload it by plugging in appliances that together will exceed the maximum current rating stated for the extension cable Risk of fire. Block adaptors are not to be used. Ensure multiple socket outlets are not positioned under the bed frame - Liquids that leak onto such a socket could pose an electrical / fire risk. 13
14 Warning Consideration is to be taken in the positioning of the mains cable and air hose to minimise the risk of accidental strangulation resulting from patient, baby or child entanglement Sidhil recommend the use of the mains cable routing sheath that is incorporated down the length of the mattress. Keep away from sources of heat and naked flames (e.g. cigarettes, fireplaces, electric fires, fan heaters, electric blankets etc.) Close proximity could damage the electrical system and / or mattress cover, bedding could catch fire etc. Do not place any objects or items, such as blankets, on or over the control unit - Risk of fire. Avoid placing the mattress system in direct sunlight Direct sunlight could damage the electrical system and / or mattress covers. Avoid placing the mattress system in a moisture rich environment - Prolonged exposure to moisture could damage the electrical system and pose an electrical/fire risk. 14
15 9. OPERATION 9.1. Environmental Limits When in Operation The following conditions should be followed when operating the dynamic mattress system: Ambient temperature: +5 C to +40 C. Humidity: 15-93%, non-condensing. Atmospheric pressure: 700 hpa to 1060 hpa 9.2. Preparing For Use Prior to patient usage of the dynamic mattress system the following must be performed: Ensure the bed and mattress system are at room temperature. Ensure the bed and mattress system have been cleaned and disinfected (see section 10). Ensure the mattress cover has been checked for tears, punctures, abrasion marks etc. and that their are no signs of fluid ingress Control Interface No. Symbol Description 1 Turns system on and off (see 9.5.1). Opens therapy menu 2 Provides 4 different therapy settings (see ). Opens comfort control menu 3 Provides 3 different comfort settings (see ). Selects and initiates the chosen 4 parameter. No. Symbol Description 5 Moves the cursor up / down the selected menu. 6 Mutes the audible signal for a 30 minute period (see ). 7 Returns to main screen. 8 Locks / unlocks interface functions (see ). 9 - Illuminates if power is lost 15
16 9.4. Mattress Overview The zoned mattress offers 3 static head cells, 12 torso cells and 8 heel cells. The cells can be inflated between a range of 13mmHg and 70mmHg, dependent on the function chosen. The heel section has narrower and lower cells. The lower cells offload pressure to protrusions such as the heels and the narrower cells offer a more supportive surface and ensure localised pressure relief for vulnerable areas Control Unit Operation Turning On / Off Hold the button down until an audible signal sounds for a brief period. The screen illuminates. Welcome to Sidhil Artemis Automatic Support Surface Initializing... Please wait until ready The system will be ready for use after an initial inflation period of 20minutes. Once the mattress is fully inflated the panel will instruct the user that the system is ready for use and a short audible signal will sound. Weight(kg): Mode: System is ready to use (12,12) Cycle Time: 10 minutes If the system does not reach the required pressure it will sound a low pressure indication after 20 minutes (see section 11). To turn the system off hold the button down until the screen extinguishes System Setup Once a patient is positioned on the mattress the system will determine the patient s approximate weight and optimum pressure level within a period of 4 minutes. Once weight detection is complete the mattress automatically defaults to alternation mode Alternation The mattress utilises an AB alternation cycle where alternate cells deflate and inflate. As the system is detecting the patient weight the screen shows the changing pressure differential between the A and B cells at that moment in time. In the illustration below A = 34mmHg and B = 33mmHg Weight(kg): Mode: Patient weight detection (34,33) Cycle Time: 10 minutes 16
17 Patient Weight and Pressure When the system is determining the patient weight the control unit is also calculating the optimum pressure level for that weight of patient. The process occurs every 60 minutes or when the control unit detects a significant change in weight. The detection works by increasing the pressure in all cells to maximum and then decreasing them to the lowest possible supportive pressure where the patient weight is at its heaviest (i.e. sacral, legs etc.). The weight guidance indicator is accurate to ±10kg. In the illustration below the weight is approximated to be 100kg with an alternating pressure of 13mmHg. Weight(kg): Mode: Alternating (13,11) Pressure Setting: 13 mmhg Cycle Time: 10 minutes Warning The weight indicator is not to be used for medical purposes, it is to act as information only. If the inclusion of the weight indicator could introduce a hazard it can be hidden, see Once the system has set itself for the patient, re-check it after approximately minutes to ensure the patient is comfortable and that the system is functioning correctly Safety Functions Function Lock The control unit will automatically lockout all functionality 2 minutes after a function change. To unlock the control unit the lock button is pressed for 2 seconds. To reengage the lock the button can either be again pressed for 2 seconds or the user can wait for the automatic lock to re-engage. When pressed the lock indicator illuminates: Weight(kg): Mode: Alternating (13,11) Pressure Setting: 13 mmhg Cycle Time: 10 minutes <LOCK> 17
18 Mute The audio visual signal activates if a fault is detected. To silence the audible signal the mute button is pressed. When the system is muted the screen shows: ****MATTRESS DISCONNECT**** MUTE Please make sure the connector is securely fastened. (note, fault shown is an example only) Re-pressing the mute button reactivates the audible signal. The mute setting will self-cancel after 30 minutes and the audible signal will re-sound. Note: If the power failure indicator activates the mute button will not silence the audible signal. To silence, the system must be turned off by pressing the power button. Warning When silencing the power failure indicator the audible signal will not reactivate after 30 minutes and all lights will extinguish There will be no indication that the system is powered down. Ensure power is returned to the system as soon as possible to resume pressure relief System Functions Therapy Selection When pressed a menu appears offering 4 different settings: 1: Alternating 2: Constant Low Pressure 3: Pulsation 4: Max Inflate Use the up/down cursor key followed by the selection key to initiate the chosen setting. 1) Alternating: Operates an AB cycle where alternate cells deflate and inflate over a defined time period (see section ) causing the pressure over any one part of the body to change regularly, actively encouraging tissue perfusion. 2) Constant low pressure: Reduces the contact pressure by increasing the surface area over which the patient is supported and by contouring to the shape of the body, therefore redistributing pressure away from vulnerable areas. In this setting the system runs in a static mode where the cells are not alternating and are within a pressure range of 10 to 40mmHg (patient weight dependent). When using the constant low pressure setting, to return to alternating it is necessary to manually reselect alternating from the therapy menu, it will not automatically default back. 18
19 3) Pulsation: Creates tissue stimulus by alternately increasing and decreasing the cell pressure of the constant low pressure mode by 20% in each direction. When using the pulsation setting, to return to alternating it is necessary to manually reselect alternating from the therapy menu, it will not automatically default back. 4) Max inflate: Inflates the cells to maximum pressure (40mmHg) to provide a stable, static support surface. The system will automatically revert back to alternation mode after 20 minutes for patient safety Comfort Control When pressed a menu appears offering 3 different settings: 1: Soft/Firm Control a: Firm b: Medium c: Soft Use the up/down cursor key followed by the selection key to initiate the chosen setting. By selecting this function the softness / firmness of the mattress can be manually altered, dependent on the patient s requirements. The pump defaults to medium. Firm = Medium = Soft = + 5mmHg Automated pressure setting 5mmHg Before changing the pressure a clinical judgement is required from frequent monitoring and repositioning of the patient Additional Settings On accessing the comfort control menu ( ) and then pressing the lock ( ) and down arrow ( ) buttons together a hidden menu is accessed: 1: Cycle Time 2: Auto Lock 3: Weight Unit (kg or lbs) Page 1/2 4: Weight Display On/Off 5: Egress Alert On/Off 6: Back to the Main Screen Page 2/3 19
20 1) Cycle time: When pressed a menu appears offering 3 different settings: 1: Cycle Time a: 10 mins b: 15 mins c: 20 mins By selecting this function the cycle time of the alternation sequence can be manually altered, dependent on the patient s requirements. Note, the default cycle time for the system when first turned on is 10 minutes. Use the up/down cursor key followed by the selection key to initiate the chosen setting. 2) Auto lock: When pressed a menu appears offering 2 different settings: 2: Auto Lock a: Enable b: Disable By selecting disable the interface will not automatically lock itself (see section ). Use the up/down cursor key followed by the selection key to initiate the chosen setting. Warning The lock is only to be disabled in an environment where intentional/ unintentional tampering cannot occur Sidhil recommend that the lock is always set to enable regardless of the environment. 3) Weight unit: When pressed a menu appears offering 2 different settings: 3: Weight Unit a: kg b: lbs Use the up/down cursor key followed by the selection key to initiate the chosen unit of measure. 4) Weight display: When pressed a menu appears offering 2 different settings: 4: Weight display a: on b: off Use the up/down cursor key followed by the selection key to show/hide the weight indicator on the main screen. 20
21 5) Egress alert: If activated the pump provides an audio-visual signal if it senses that the occupant has got out of the bed, due to a sudden change in force being exerted on the cells. When selected a menu appears offering 2 different settings: 5: Egress Alert a: on b: off Use the up/down cursor key followed by the selection key to activate/deactivate the egress alert. If active and the occupant gets out of bed an audible signal sounds and a warning screen illuminates for approximately 1 minute followed by a no patient indication: W A R N I N G! NO PATIENT! Can press any key to Cancel. To cancel the audio-visual signal any button on the control interface can be pressed. 21
22 9.6. Mattress Operation Auto Seat Mode The mattress design allows for it to be profiled in an upright position. Pressure will be increased when the patient is sitting in an upright position but depending on patient comfort and clinical judgement the comfort setting may need to be changed. When the backrest travels beyond an angle of 20 the screen on the control interface advises that the backrest has been raised. Weight(kg): Mode: Auto-Seat Pressure Setting: 14 mmhg Cycle Time: 10 minutes Low Patient Weight The mattress accommodates patients whose weight would be considered as particularly low (<40kg). It significantly reduces internal cell pressures to make it suitable for lower weight patients. To activate press the selection button ( ) for 5 seconds - low weight patient illuminates on the screen: Weight(kg): Mode: Alternating (10,11) Setting: Low Weight Patient Cycle Time: 10 minutes To deactivate the low patient weight mode the control unit needs to be switched off and then back on again. While low patient weight mode is selected frequent monitoring and repositioning is advised CPR Rapid deflation of the mattress may be required for emergency treatment or system deflation. The CPR dial is located at the foot end of the mattress. Rotate the CPR dial to the open position, once done the entire system will rapidly deflate. To re-inflate turn the CPR dial to the closed position. Wait for the mattress system to reach optimal pressure prior to a return to normal use. CPR Open: CPR Closed: 22
23 9.8. Mattress Disconnection and Power Cuts If the mattress is to be disconnected from the power supply for an extended period of time and the mattress is to remain inflated or in the event of a mains power failure, carry out the following procedure: Disconnect from the power unit by pressing the tab on the top of the connector (1) and pulling away from the control unit (2) Switch off the control unit (if still operational). Disconnect from the power supply. Note, there is no need for the air connector to be sealed with a cap due to the use of the mattress using non-return valve technology. Warning The mattress will remain inflated for a maximum of 10 hours only Return the system to the mains supply as soon as is practical. Whilst unplugged alternating mode will not be operational Pressure relief will not be provided Use of Incontinence Products Incontinence products such as sheets or pads can be used with the system, however product performance is likely to reduce due to the reduced effectiveness of the alternating pressure distribution. If incontinence products are to be used it is recommended that regular patient skin checks are performed to ensure skin integrity is maintained. 23
24 10. DECONTAMINATION / CLEANING Infection control and routine cleaning must be carried out in accordance with your local Infection control policy or regulatory body. Warning Always disconnect the mattress system and bedframe from the main power supply prior to cleaning. The control unit is rated to IP21 and provides protection from condensation only, do not immerse or soak the control unit Risk of electric shock. Regular cleaning and disinfection of the mattress system will help to prevent the risk of infection to the occupant and/or carer. Prior to transferring the mattress system to another user ensure it has been cleaned and disinfected using the method as detailed below to help prevent the risk of cross infection. Caution If any of the below washing instructions are not followed the product warranty will be invalidated. Do not use solvents, neat bleach, phenolic based cleaning solutions or abrasive products to clean the casing or mattress. Sidhil recommend the use of Tristel Fuse sachets and Tristel Jet gel. Sidhil also recommend the use of Chlor-clean tablets. Follow the product documentation for concentration guidelines and instructions for use Control Unit Check for external damage If damaged take the control unit out of use. All surfaces to be wiped down with a disposable soft cloth moistened with a mild detergent and diluted in warm water (40 C). The control unit is be cleaned by starting with the cleanest parts of it and systematically moving to the dirtiest parts. Extra care should be taken around areas where excess dirt or dust may gather. The cloth should be changed during the cleaning process if it becomes soiled. Wipe down with a clean cloth moistened with clean water to remove detergent residue. If there are blood spillages or bodily fluids present wipe surfaces down with 0.1% Chlorine solution (1,000 ppm). Wipe down with a clean cloth moistened with water. Dry off with a paper towel - Always ensure the cleaned surfaces are allowed to fully dry before putting back into use. 24
25 10.2. Mattress Before attempting to clean the mattress the top cover is to be checked for physical signs of damage that may lead to strike-through (ingress of fluid through cover). This is achieved by unzipping the top cover and looking for signs of staining to the white underside. Any evidence of strike-through (and / or cover damage) will require a new cover to be fitted to the mattress. Warning The cover must not be used if strike-through is evident Risk of cross infection. Caution Frequent or prolonged exposure to higher concentration disinfectant solutions may prematurely age the fabric cover of the mattress. General Cleaning: Wipe down with a clean cloth moistened with a mild detergent and diluted in warm water (40 C). Rinse with cold clean water and a clean cloth and allow to fully dry before use. Decontamination: Mop up any fluid with paper towels. Wipe cover down using cold clean water. Wipe down with a 0.1% Chlorine solution (1,000ppm) in cold water, where necessary a 1% Chlorine solution (10,000ppm) is to be used instead. Rinse with cold clean water and a clean cloth and allow to fully dry before use. Infection control and routine cleaning must be carried out in accordance with local policy Alternative Mattress Cover Cleaning Instructions Alternatively disinfection of the mattress cover may be achieved by laundering as follows: Remove mattress cover. Machine wash at 71 C for not less than 3 minutes or 65 C for not less than 10 minutes. Heavily soiled items should also have a pre-wash/sluice cycle. Allow covers to fully dry before use. (Refer to the Department of Health document CFPP for further detail). 25
26 11. TROUBLESHOOTING Warning The control unit is not to be opened risk of electrocution. Symptoms Indications Actions Power Failure Amber power failure light flashes. Audible signal sounds. Screen extinguishes. Incomplete inflation / Low pressure Audible signal sounds. Screen shows: Warning! ***LOW PRESSURE*** If mains plug, cable or outer casing is visibly damaged turn off at the mains and contact your approved service engineer. 1. Turn off the control unit to silence the alarm and turn off the mains supply (Note, the mute button does not silence the power failure indication). 2. Check the mains cable is fully connected to the control unit and plugged into a wall socket. 3. Switch on at the wall (to ensure the socket is working plug in a fused device that is known to work). 4. Turn on the control unit. If control unit still fails to operate: 5. Turn off the control unit at the wall. 6. Replace the mains plug fuse See section 13 for fuse types. 7. Turn on the control unit. If control unit still fails to operate turn off at the mains and contact your approved service provider. 1. Ensure the mattress air connector is correctly connected to the control unit. 2. Ensure the CPR dial is closed and there is no air leakage. 3. Turn the unit off and then on again to clear the indicator. If a low pressure indicator continues to illuminate: 4. Open the mattress and ensure there is no air leakage within the mattress cells, tubing and connectors. 5. Turn the unit off and then on again to clear the indicator. If a low pressure indicator is still evident turn off at the mains and contact your approved service provider. 26
27 Symptoms Indications Actions High Pressure Audible signal sounds. Screen shows: Warning! ***HIGH PRESSURE*** 1. Ensure the mattress umbilical is not trapped or being squeezed. 2. Open the mattress and ensure none of the air pipes are kinked. 3. Turn the unit off and then on again to clear the indicator. If a high pressure indicator is still evident turn off at the mains and contact your approved service provider. Mattress Disconnection Patient is bottoming out Audible signal sounds. Screen shows: Warning! **MATTRESS DISCONNECT** Please make sure the connector is securely fastened 1. Ensure the mattress air connector is correctly connected to the control unit. 2. Turn the unit off and then on again to clear the indicator. If the indicator is still evident turn off at the mains and contact your approved service provider. 1. Ensure the patient is suited to the maximum rating of the mattress. 2. Ensure the patient is centrally positioned on the mattress. 3. Increase the pressure setting Refer to section
28 12. MAINTENANCE Warning Always disconnect the control unit from the main power supply prior to performing any maintenance procedures (when viable). No modification of this equipment is allowed. The mattress system should be vacated by the patient before any maintenance or inspection takes place. If this is not possible due to the patient s mobility care should be taken for the service engineer not to make contact with the patient when working on electrical items. Only Sidhil approved components specified for the Artemis dynamic system are to be used - if in doubt contact Sidhil Ltd or your local distributor. Only authorised service personnel or Sidhil service engineers should carry out repairs or service activities. For Service & Support outside of the UK & Northern Ireland please contact the local distribution company from where this equipment was purchased. Failure to do so may result in the product warranty becoming void. The mattress system must be serviced once yearly, as a minimum. Sidhil also recommends that the carer performs frequent visual and operational inspections. If there are any signs of damage or the system is not performing as it should withdraw it from service until the system has been repaired and is fit for use again. Sidhil Ltd recommends that the following maintenance procedure is performed every 12 months: Check that the air filter is in good condition and replace or clean as required. Check that all electrical functions operate correctly on the control unit. Check that all audible and visual indicators work appropriately (when plugged in and unplugged from mains supply). Check that the battery is still functional and operates in the event of a power loss. Check that the mattress reaches the required pressures. Check the CPR connection on the mattress. Check the cover for tears, punctures, abrasion marks and split seams. Check for signs of signs of fluid ingress to the underside of the cover. Check that all piping and cells within the mattress are in good condition and that there is no kinking evident. Check the control unit housing is not cracked or damaged, if damaged the control unit must be removed from operation immediately. Check that the mains cable and plug are in good condition, if either is damaged it must be replaced with a complete assembly, the plug must never be re-wired. For more detailed service information, spare parts, circuit diagrams etc. please refer to the service manual. Copies are available from Sidhil Ltd. Contact details can be found in section 2. 28
29 12.1. Disposal of Parts When the electrical system has come to the end of its useful life contact your provider or Sidhil Ltd (see section 2) to arrange for collection, alternatively follow local recycling and W.E.E.E. (Waste Electrical and Electronic Equipment) policies. The control unit used with the mattress system is not to be disposed of in general municipal waste. Some of the electrical components could be harmful to the environment and where viable the components can be recovered and reused/ recycled. The metal and plastic components used in both the mattress and control unit are also to be separated and disposed of following local recycling policy as these can also be recovered and reused/recycled. Warning The mattress system is to be decontaminated before disposal to avoid risk of cross contamination 29
30 13. SPECIFICATION Classification: Electrical shock protection: Class I, Type BF Applied Part: Mattress Liquid ingress protection: IP21 Not AP or APG equipment* Supply Rating: 230V, 50Hz, 25W Fuse Rating: Mains Plug 13A Mains Plug: Type G/BS1363 Mattress Dimensions: (L) 2000mm x (W) 880mm x (D) 200mm Mattress Weight: 11kg Maximum Patient Weight: 248kg (39 stone) No. of Cells: 23 cells which include: 3 static head cells 20 alternating cells (including 8 narrow heel cells) with cell-on-cell function Alternating Therapy: AB pattern Cycle Time: 10, 15 or 20 minutes Pressure Range: Alternating Mode: ±2mmHg Constant Low Pressure Mode: ±2mmHg Pulsation Mode: 80% & 120% of CLP setting Max Inflate: 40 ±2mmHg Control Unit Dimensions: (L) 180mm x (W) 390mm x (D) 14.5mm Control Unit Weight: 4.1kg Cover Material: Dartex Cell Material: TPU Base Material: Nylon fabric with PU coating Transport and Storage Conditions: Ambient Temp: -25 C to +70 C Humidity: < 93%, non-condensing Operational Conditions: Ambient Temp: +5 C to +40 C Humidity 15% - 93%, non-condensing Atmospheric Pressure: 700hPa to 1060hPa Operating Altitude: 2000m Pollution: Degree 2 UV: Intended for indoor use only Noise level: <40dB(A) Expected Service Life: 3 years Safety Standards: IEC : 2005 IEC :2007 IEC : Partial * Not suitable for use in the presence of flammable anaesthetic mixtures with air, oxygen or nitrous oxide. 30
31 14. ELECTROMAGNETIC COMPATIBILITY (EMC) The Artemis control unit has been designed to meet the EMC requirements of IEC : 2007 however it may still be affected by or emit harmful radio frequency (RF) energy. The RF emissions from the mattress control unit are very low and are not likely to cause any interference to nearby electronic equipment, however interference to sensitive equipment is still possible. Likewise if the immunity limits of the control unit are exceeded the system may be seen to operate abnormally. Interference can be received from fixed transmitters (e.g. commercial radio and television towers) and portable / mobile RF communications equipment (e.g. mobile phones). Due to the increasing number of mobile phones and other wireless devices the possibilities of interference to the control unit and other surrounding equipment results in the need for special precautions to be taken regarding EMC. The Artemis is to be installed and put into service according to the information provided within this section to ensure continued and reliable operation. Wireless communications equipment such as wireless network devices, mobile phones, cordless telephones including their base stations and walkie-talkies could all affect the electrical operation of the control unit - separation distances must be considered. If the control unit or any alternative equipment is found to be operating abnormally turn off the piece of equipment that is believed to be causing the interference (if possible) to identify the source of the RF energy. Once identified mitigation measures are to be taken, such as the separation distances being increased and/or the device(s) being re-orientated. As an example a mobile phone typically has a recommended separation distance of at least 3.3m from the control unit. 31
32 14.1. Requirements According to IEC :2007 The Artemis is intended for use in the electromagnetic environment specified below. The customer or the user of the Artemis should ensure that it is used in such an environment. Warning The Artemis control unit should not be used adjacent to or stacked with other equipment where possible, if adjacent or stacked use is necessary the Artemis should be observed to verify normal operation in the configuration in which it is to be used. Guidance and manufacture s declaration electromagnetic emissions Emission test Compliance Electromagnetic environment guidance RF emission CISPR 11 RF emission CISPR 11 Harmonic emissions IEC Voltage fluctuations/ flicker emissions IEC Group 1 Class B Class A Complies The Artemis uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. The Artemis is suitable for use in all establishments, including domestic establishments and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes. 32
33 Guidance and manufacture s declaration electromagnetic immunity Immunity test IEC test level Compliance level Electrostatic discharge (ESD) IEC Electrical fast transient/burst IEC Surge IEC Voltage dips, short interruptions and voltage variations on power supply input lines IEC Power frequency (50/60Hz) magnetic field IEC ±6 kv contact ±8 kv air ±2 kv for power supply lines ± 1 kv line(s) to line(s) ± 2 kv line(s) to earth <5% U T (>95% dip in U T ) for 0.5 cycle 40% U T (60% dip in U T ) for 5 cycles 70% U T (30% dip in U T ) for 25 cycles <5% U T (>95% dip in U T ) for 5 sec ±6 kv contact ±8 kv air ±2kV for power supply lines ±1 kv differential mode ±2 kv common mode <5% U T (>95% dip in U T ) for 0.5 cycle 40% U T (60% dip in U T ) for 5 cycles 70% U T (30% dip in U T ) for 25 cycles <5% U T (>95% dip in U T ) for 5 sec Electromagnetic environment guidance Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%. Mains power quality should be that of a typical commercial or hospital environment. Mains power quality should be that of a typical commercial or hospital environment. Mains power quality should be that of a typical commercial or hospital environment. If the user of the Artemis requires continued operation during power mains interruptions, it is recommended that the Artemis be powered from an uninterruptible power supply or a battery. 3A/m 3A/m Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. NOTE U T is the a.c. mains voltage prior to application of the test level. 33
34 Guidance and manufacture s declaration electromagnetic immunity Immunity test IEC test level Compliance level Electromagnetic environment guidance Portable and mobile RF communications equipment should be used no closer to any part of the Artemis control unit, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter. Recommended separation distance Conducted RF IEC Vrms 150 khz to 80 MHz 3 Vrms d = 1.2 P Radiated RF IEC V/m 80 MHz to 2.5 GHz 3 V/m d = 1.2 P 80 MHz to 800 MHz d = 2.3 P 800 MHz to 2.5 GHz Where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in meters (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey a, should be less than the compliance level in each frequency range b. Interference may occur in the vicinity of equipment marked with the following symbol: NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the Artemis is used exceeds the applicable RF compliance level above, the Artemis should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienteering or relocating the Artemis. b Over the frequency range 150 khz to 80 MHz, field strengths should be less than 3 V/m. 34
35 The Artemis is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the Artemis can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the Artemis as recommended below, according to the maximum output power of the communications equipment. Recommended separation distances between portable and mobile RF communications eqiupment and the Artemis control unit Rated maximum output Separation distance according to frequency of transmitter (m) power of transmitter (W) 150 KHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.5 GHz d= 1.2 P d= 1.2 P d= 2.3 P For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. NOTE 1 NOTE 2 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. 35
36 15. WARRANTY Sidhil Ltd guarantees this product is free from defects in material and workmanship under normal use for 2 years (full parts and labour) from the date of purchase from Sidhil Ltd and its subsidiary companies or its authorised dealers. All implied warranties, including but not limited to those implied warranties of fitness and merchantability, are limited in the total duration of 2 year from date of purchase. Proof of purchase must be presented with any claim. Except as provided herein, Sidhil Ltd, product warranty does not cover damage caused by misuse or abuse, accident, the attachment of any unauthorised accessory, alteration to the product, or any other conditions whatsoever that are beyond the control of Sidhil Ltd. Sidhil Ltd and its subsidiary companies shall have no liability or responsibility to customer or any other person or entity with respect to any liability, loss or damage caused direct or indirectly by use or performance of the product or arising out of any breach of this warranty, including but not limited to any damages resulting from inconvenience, loss of time, property, revenue, or profit or any indirect, special, incidental or consequential damages, even if Sidhil Ltd or their subsidiary companies or authorised dealers has been advised of the possibility of such damages. In the event of a product defect during the warranty period you should contact Sidhil Ltd or their authorised dealer who will at its option unless otherwise provided by law; a) correct the defect by product repair without charge for parts and labour b) replace the product with one of the same or similar design or c) refund the purchase price. All replaced parts and products on which refund is made become the property of Sidhil Ltd. New or reconditioned parts and products may be used in the performance of warranty service. Repaired or replaced parts and products are warranted for the remainder of the original warranty period. You will be charged for repair or replacement of the product made after the expiration of the warranty period. This warranty does not cover; a) damage or failure by or attributes to acts of God, abuse, accident, misuse, improper or abnormal usage, failure to follow instructions, improper installation or maintenance, alterations, lightning or other incidence of excess voltage or current, b) any repairs other than those provided by a Sidhil Ltd authorised technician, c) consumables such as fuses, d) cosmetic damage, e) transportation, shipping or insurance costs or f) costs of product removal, installation setup service adjustment or re-installation. This limited 2 year warranty gives you specific legal rights and you may also have other rights. Sidhil Ltd cannot be held responsible for any injury or incident which relates to the use of this mattress system in conjunction with accessories manufactured by companies other than Sidhil Ltd. All products carry the CE mark in accordance with EC Directive on Medical Devices (93/42/EEC). Sidhil has a policy of continual product improvement and reserves the right to amend specifications covered in these instructions for use. No part of this brochure may be reproduced without the written approval of Sidhil Ltd. 36
37
38
39
40 CONTACT INFORMATION Tel: +44 (0) Fax: +44 (0) Sidhil Business Park, Holmfield, Halifax, HX2 9TN A member of the Siddall & Hilton Ltd. Group of Companies (93/42/EEC) Certificate No. FM14550 INSTRUC/ARTEMIS, 21/07/ Rev.1
(DynaLaL III) Dynamic True Low Air Loss Mattress Replacement System
 (DynaLaL III) User Manual Dynamic True Low Air Loss Mattress Replacement System Alternating + True Low Air Loss Pressure Relief System Manufactured by: Caremed Supply, Inc. Distributed by: Quart Healthcare
(DynaLaL III) User Manual Dynamic True Low Air Loss Mattress Replacement System Alternating + True Low Air Loss Pressure Relief System Manufactured by: Caremed Supply, Inc. Distributed by: Quart Healthcare
Instruction Manual for Configura Cushionair Portable Pump
 Instruction Manual for Configura Cushionair Portable Pump Fitted with battery powered pump, suitable for Configura Portable chairs V E R S I O N O N E M A Y 2 0 1 6 Contents Introduction 3 Set up of Cushionair
Instruction Manual for Configura Cushionair Portable Pump Fitted with battery powered pump, suitable for Configura Portable chairs V E R S I O N O N E M A Y 2 0 1 6 Contents Introduction 3 Set up of Cushionair
Pegasus Aircare Mattress Overlay System Models 2200 and 2201
 Pegasus Aircare Mattress Overlay System Models 2200 and 2201 User Guide LFT11099 Issue 3 PEGASUS AIRCARE Mattress Overlay System Models 2200 and 2201 The PEGASUS AIRCARE Overlay system provides optimum
Pegasus Aircare Mattress Overlay System Models 2200 and 2201 User Guide LFT11099 Issue 3 PEGASUS AIRCARE Mattress Overlay System Models 2200 and 2201 The PEGASUS AIRCARE Overlay system provides optimum
Air Plus User Manual
 Air Plus User Manual Alternating pressure mattress for prophylaxis and treatment of pressure sores Indications for use The Air Plus has been developed as a state of the art pressure relieving system for
Air Plus User Manual Alternating pressure mattress for prophylaxis and treatment of pressure sores Indications for use The Air Plus has been developed as a state of the art pressure relieving system for
Operation Manual. H & R Healthcare 1750 Oak Street Lakewood, NJ Phone: Fax:
 Operation Manual H & R Healthcare 1750 Oak Street Lakewood, NJ 08701 Phone: 800-801-5533 Fax: 732-367-6231 www.handrhealthcare.com Congratulations and thank you for purchasing this Relief Aire Alternating
Operation Manual H & R Healthcare 1750 Oak Street Lakewood, NJ 08701 Phone: 800-801-5533 Fax: 732-367-6231 www.handrhealthcare.com Congratulations and thank you for purchasing this Relief Aire Alternating
Med Aire Alternating Pressure Pump and Pad System
 User Manual Med Aire Alternating Pressure Pump and Pad System 14002E 14001E Symbols & Statements NOTE Indicates some tips or some information users should be aware of. CAUTION Indicates correct operating
User Manual Med Aire Alternating Pressure Pump and Pad System 14002E 14001E Symbols & Statements NOTE Indicates some tips or some information users should be aware of. CAUTION Indicates correct operating
Paradise Pump Series Operator s Manual
 Paradise Pump Series Operator s Manual P/N 12421-000 9/05 Paradise Pump Operator s Manual Important Before using the Paradise Pump Alternating Pressure Relief Systems, please read and understand this
Paradise Pump Series Operator s Manual P/N 12421-000 9/05 Paradise Pump Operator s Manual Important Before using the Paradise Pump Alternating Pressure Relief Systems, please read and understand this
User Manual DIRECTHEALTHCARESERVICES.CO.UK
 User Manual DYNA-FORM AIR PRO-PLUS The Dyna-Form Air Pro-Plus is a pressure relieving mattress suitable for use with patients at VERY HIGH RISK of pressure ulcer damage. Offering high levels of patient
User Manual DYNA-FORM AIR PRO-PLUS The Dyna-Form Air Pro-Plus is a pressure relieving mattress suitable for use with patients at VERY HIGH RISK of pressure ulcer damage. Offering high levels of patient
Diamond 8 Plus. Alternating + Micro Low Air Loss Pressure Relief System. User Manual. Distributed by: Quart Healthcare Inc.
 Diamond 8 Plus Alternating + Micro Low Air Loss Pressure Relief System User Manual Distributed by: Quart Healthcare Inc. www.quarthealthcare.com Warning Connect the Master Control unit to a proper power
Diamond 8 Plus Alternating + Micro Low Air Loss Pressure Relief System User Manual Distributed by: Quart Healthcare Inc. www.quarthealthcare.com Warning Connect the Master Control unit to a proper power
User Manual DIRECTHEALTHCARESERVICES.CO.UK
 User Manual DYNA-FORM MERCURY ADVANCE The Dyna-Form Mercury Advance mattress is a High / Very High Risk dynamic replacement system, combined with the benefits of modern foam technology. Offering high levels
User Manual DYNA-FORM MERCURY ADVANCE The Dyna-Form Mercury Advance mattress is a High / Very High Risk dynamic replacement system, combined with the benefits of modern foam technology. Offering high levels
APH166 Morpheus Air USER MANUAL. Apollo Healthcare Technologies Ltd. Holme Street, Liversedge, West Yorkshire, WF15 6JF.
 APH166 Morpheus Air USER MANUAL Apollo Healthcare Technologies Ltd. Holme Street, Liversedge, West Yorkshire, WF15 6JF. Tel: (01924) 614567 l Fax: (01924) 607480 Issue 1-11/03/2016 IMPORTANT SAFEGUARDS
APH166 Morpheus Air USER MANUAL Apollo Healthcare Technologies Ltd. Holme Street, Liversedge, West Yorkshire, WF15 6JF. Tel: (01924) 614567 l Fax: (01924) 607480 Issue 1-11/03/2016 IMPORTANT SAFEGUARDS
User Manual. 10. Dimensions and Ordering Codes. Integrity Alternating Pressure Mattress System. Alternating Pressure Mattress Systems
 10. Dimensions and Ordering Codes Integrity Alternating Pressure Mattress System Integrity Mattress Specification: Standard Extra Long Extra Narrow HD Dimension approx LxWxH (cm): 196x86x15 220x86x15 216x79x15
10. Dimensions and Ordering Codes Integrity Alternating Pressure Mattress System Integrity Mattress Specification: Standard Extra Long Extra Narrow HD Dimension approx LxWxH (cm): 196x86x15 220x86x15 216x79x15
PLEASE READ CAREFULLY BEFORE INSTALLING OR USING MEGA POOL SAVER MPS 1100
 MPS-1100 User Manual Mega Pool Saver Ltd PLEASE READ CAREFULLY BEFORE INSTALLING OR USING MEGA POOL SAVER MPS 1100 For further up to date instructions on how to install Mega Pool Saver MPS 1100, please
MPS-1100 User Manual Mega Pool Saver Ltd PLEASE READ CAREFULLY BEFORE INSTALLING OR USING MEGA POOL SAVER MPS 1100 For further up to date instructions on how to install Mega Pool Saver MPS 1100, please
RIK Fluid Overlay INSTRUCTIONS FOR USE
 RIK Fluid Overlay INSTRUCTIONS FOR USE P/N KS2XP01-AH Rev B 04/2014...with people in mind EXCEPT FOR THE WARRANTIES EXPRESSLY SET FORTH HEREIN, ALL OTHER WAR- RANTIES, INCLUDING IMPLIED WARRANTIES OF MERCHANTABILITY
RIK Fluid Overlay INSTRUCTIONS FOR USE P/N KS2XP01-AH Rev B 04/2014...with people in mind EXCEPT FOR THE WARRANTIES EXPRESSLY SET FORTH HEREIN, ALL OTHER WAR- RANTIES, INCLUDING IMPLIED WARRANTIES OF MERCHANTABILITY
User Manual DIRECTHEALTHCARESERVICES.CO.UK
 User Manual DYNA-FORM MERCURY ADVANCE The Dyna-Form Mercury Advance is a pressure relieving mattress suitable for use with patients at VERY HIGH RISK of pressure ulcer damage. Offering high levels of patient
User Manual DYNA-FORM MERCURY ADVANCE The Dyna-Form Mercury Advance is a pressure relieving mattress suitable for use with patients at VERY HIGH RISK of pressure ulcer damage. Offering high levels of patient
Alternating Pressure / Low Air Loss Mattress System
 750000 Alternating Pressure / Low Air Loss Mattress System User Manual Important: Do not operate the Mattress System without first reading and understanding this manual! Save this manual for future use.
750000 Alternating Pressure / Low Air Loss Mattress System User Manual Important: Do not operate the Mattress System without first reading and understanding this manual! Save this manual for future use.
User Manual DIRECTHEALTHCARESERVICES.CO.UK
 User Manual DYNA-FORM MERCURY ADVANCE 2 USER MANUAL The Dyna-Form Mercury Advance is a pressure relieving mattress suitable for use with patients at VERY HIGH RISK of pressure ulcer damage. Offering high
User Manual DYNA-FORM MERCURY ADVANCE 2 USER MANUAL The Dyna-Form Mercury Advance is a pressure relieving mattress suitable for use with patients at VERY HIGH RISK of pressure ulcer damage. Offering high
NIMBUS range. with people in mind
 NIMBUS range Advanced Mattress Replacement systems with people in mind OPTIMUM PRESSURE RELIEF AND COMFORT In clinical use for more than 20 years, Nimbus Alternating Mattress Replacement Systems have consistently
NIMBUS range Advanced Mattress Replacement systems with people in mind OPTIMUM PRESSURE RELIEF AND COMFORT In clinical use for more than 20 years, Nimbus Alternating Mattress Replacement Systems have consistently
User manual. PG89 Rev 01 Editi Pressotherapy model. LymphoPress 4
 User manual PG89 Rev 01 Editi 040515 Pressotherapy model LymphoPress 4 I.A.C.E.R. Srl Via S. Pertini 24/A 30030 Martellago (VE) ITALY Tel. 041.5401356 Fax 041.5402684 e-mail: iacer@iacer.it http://www.itechmedicaldivision.com
User manual PG89 Rev 01 Editi 040515 Pressotherapy model LymphoPress 4 I.A.C.E.R. Srl Via S. Pertini 24/A 30030 Martellago (VE) ITALY Tel. 041.5401356 Fax 041.5402684 e-mail: iacer@iacer.it http://www.itechmedicaldivision.com
Actively supporting your health.
 U S E R G U I D E Actively supporting your health www.aeda.com Key Definition of symbols used User guide i Mattress Important information Caution symbol Electrical hazard Aeda a brand actively supporting
U S E R G U I D E Actively supporting your health www.aeda.com Key Definition of symbols used User guide i Mattress Important information Caution symbol Electrical hazard Aeda a brand actively supporting
SELECT SERIES LS300 ALTERNATING PRESSURE / LOW AIR LOSS MATTRESS SYSTEM
 SELECT SERIES LS300 ALTERNATING PRESSURE / LOW AIR LOSS MATTRESS SYSTEM USER MANUAL LS300-INS-LAB-RevB17 Read this manual before operating your Mattress System. Save this manual for future use. The most
SELECT SERIES LS300 ALTERNATING PRESSURE / LOW AIR LOSS MATTRESS SYSTEM USER MANUAL LS300-INS-LAB-RevB17 Read this manual before operating your Mattress System. Save this manual for future use. The most
Digital Melting Point Apparatus
 Digital Melting Point Apparatus Heating Plateau Ramping Start/Stop Plateau set Ramp stop Hold User Guide Version 1.1 Heating Viewing tube Sample Chamber IEC power inlet socket Power on/off Temperature
Digital Melting Point Apparatus Heating Plateau Ramping Start/Stop Plateau set Ramp stop Hold User Guide Version 1.1 Heating Viewing tube Sample Chamber IEC power inlet socket Power on/off Temperature
HoverJack. Air Patient Lift. User Manual. From HoverTech International.
 HoverJack Air Patient Lift From HoverTech International User Manual www.hovermatt.com Table of Contents Symbol References... 02 Intended Use and Precautions... 03 HOVERJACK Introduction... 04 Part Identification
HoverJack Air Patient Lift From HoverTech International User Manual www.hovermatt.com Table of Contents Symbol References... 02 Intended Use and Precautions... 03 HOVERJACK Introduction... 04 Part Identification
ORB-400 BUBBLE/HAZE MACHINE. Item ref: UK User Manual
 ORB-400 BUBBLE/HAZE MACHINE Item ref: 160.462UK User Manual Caution: Please read this manual carefully before operating Damage caused by misuse is not covered by the warranty Introduction Thank you for
ORB-400 BUBBLE/HAZE MACHINE Item ref: 160.462UK User Manual Caution: Please read this manual carefully before operating Damage caused by misuse is not covered by the warranty Introduction Thank you for
MA90 Series Mattresses. MA95 Series Mattresses
 Owner s Operator and Maintenance Manual MA90 Series Mattresses MA90Z Lateral Rotation with Alternating Pressure and On-Demand Low Air Loss System MA90ZB42 Lateral Rotation with Alternating Pressure and
Owner s Operator and Maintenance Manual MA90 Series Mattresses MA90Z Lateral Rotation with Alternating Pressure and On-Demand Low Air Loss System MA90ZB42 Lateral Rotation with Alternating Pressure and
Leglifter. user instructions. Simple solutions for everyday independence
 user instructions Simple solutions for everyday independence The by Mangar International enables people to raise their legs onto the bed independently or with minimal assistance. Power is provided by the
user instructions Simple solutions for everyday independence The by Mangar International enables people to raise their legs onto the bed independently or with minimal assistance. Power is provided by the
USER MANUAL SAVE THESE INSTRUCTIONS. For the most current manual revision, please visit our Website:
 USER MANUAL Model: PM4300 Series SAVE THESE INSTRUCTIONS For the most current manual revision, please visit our Website: www.precisionmedical.com 300 Held Drive Tel: (+001) 610-262-6090 Northampton, PA
USER MANUAL Model: PM4300 Series SAVE THESE INSTRUCTIONS For the most current manual revision, please visit our Website: www.precisionmedical.com 300 Held Drive Tel: (+001) 610-262-6090 Northampton, PA
Contents. Introduction...1 About Breeze... 1 Breeze Overlay... 2 Breeze Mattress Replacement... 2 Breeze Pump... 4 Controls and Indicators...
 English Contents Introduction........................................................1 About Breeze................................................... 1 Breeze Overlay.................................................
English Contents Introduction........................................................1 About Breeze................................................... 1 Breeze Overlay.................................................
DYNAMIC COMPRESSION THERAPY
 PULSE PRESS DYNAMIC COMPRESSION THERAPY MULTITEC 12 User Manual May 2011 0120 CONTENTS:- SAFETY INSTRUCTIONS ELECTROMAGNETIC INTERFERENCE DESCRIPTION OF CONTROLS MODE SELECTION OPERATING INSTRUCTIONS CARE
PULSE PRESS DYNAMIC COMPRESSION THERAPY MULTITEC 12 User Manual May 2011 0120 CONTENTS:- SAFETY INSTRUCTIONS ELECTROMAGNETIC INTERFERENCE DESCRIPTION OF CONTROLS MODE SELECTION OPERATING INSTRUCTIONS CARE
1 METRON MANIPULATION TABLES - WARRANTY STATEMENT
 1 METRON MANIPULATION TABLES - WARRANTY STATEMENT Metron Medical Australia Pty Ltd. will warrant this table, for parts only against defects in manufacture for a period of two (2) years for structural integrity,
1 METRON MANIPULATION TABLES - WARRANTY STATEMENT Metron Medical Australia Pty Ltd. will warrant this table, for parts only against defects in manufacture for a period of two (2) years for structural integrity,
Autom ate d t u r n i n g at yo u r f i n g e r t i ps
 pioneering simplicity Autom ate d t u r n i n g at yo u r f i n g e r t i ps l ateral turning system frontiermedical group Lateral turning system Regular manual turning of patients can be intrusive, particularly
pioneering simplicity Autom ate d t u r n i n g at yo u r f i n g e r t i ps l ateral turning system frontiermedical group Lateral turning system Regular manual turning of patients can be intrusive, particularly
OPERATING INSTRUCTIONS FOR USE GUIDE
 AIROS 8 Sequential Compression Device OPERATING INSTRUCTIONS FOR USE GUIDE www.airosmedical.com 1.866.991.6956 Table of Contents Indications for Use & Contraindications...................... 1 Overview
AIROS 8 Sequential Compression Device OPERATING INSTRUCTIONS FOR USE GUIDE www.airosmedical.com 1.866.991.6956 Table of Contents Indications for Use & Contraindications...................... 1 Overview
SC505 Sof Care Inflator. Operating Instructions and Service Manual
 SC505 Sof Care Inflator Gaymar Industries, Inc. 10 Centre Drive Orchard Park, NY 14127 Toll Free +1 800.828.7341 + 1 716.662.2551 Fax +1 800.993.7890 Outside USA +1 716.662.8636 Outside USA Fax +1 716.662.0730
SC505 Sof Care Inflator Gaymar Industries, Inc. 10 Centre Drive Orchard Park, NY 14127 Toll Free +1 800.828.7341 + 1 716.662.2551 Fax +1 800.993.7890 Outside USA +1 716.662.8636 Outside USA Fax +1 716.662.0730
AT4 Tourniquet System Operating Instructions
 AT4 Tourniquet System Operating Instructions Rx ONLY AT4 Pneumatic Tourniquet Reference No. 40070 AT4 Electronic Tourniquet Reference No. 40080 0473 92410/6/230615 Page 1 92410/6/230615 Page 2 Contents
AT4 Tourniquet System Operating Instructions Rx ONLY AT4 Pneumatic Tourniquet Reference No. 40070 AT4 Electronic Tourniquet Reference No. 40080 0473 92410/6/230615 Page 1 92410/6/230615 Page 2 Contents
VENTI-O 2 plus. O 2 switching valve for: VENTImotion from serial number 4000 VENTIlogic from serial number 4000 VENTImotion 2
 VENTI-O 2 plus O 2 switching valve for: VENTImotion from serial number 4000 VENTIlogic from serial number 4000 VENTImotion 2 Device description and operating instructions Overview VENTI-O 2 plus with therapy
VENTI-O 2 plus O 2 switching valve for: VENTImotion from serial number 4000 VENTIlogic from serial number 4000 VENTImotion 2 Device description and operating instructions Overview VENTI-O 2 plus with therapy
PROGRAMMER OPERATIONS MANUAL
 PROGRAMMER OPERATIONS MANUAL Table of Contents Description 4 Installation 4 Operation 5 Safety Precautions 5 Regulatory & Service Information 6 Important Safety and Usage Information 7 Regulatory Notices
PROGRAMMER OPERATIONS MANUAL Table of Contents Description 4 Installation 4 Operation 5 Safety Precautions 5 Regulatory & Service Information 6 Important Safety and Usage Information 7 Regulatory Notices
Instruction Manual. Low air loss/alternating pressure mattress
 Low air loss/alternating pressure mattress Instruction Manual Drive Medical Design & Manufacturing 99 Seaview Boulevard Port Washington, NY 11050 Toll Free: 877-224-0946 Local: 516-998-4600 www.drivemedical.com
Low air loss/alternating pressure mattress Instruction Manual Drive Medical Design & Manufacturing 99 Seaview Boulevard Port Washington, NY 11050 Toll Free: 877-224-0946 Local: 516-998-4600 www.drivemedical.com
MODEL Maverick Heavy Duty Aerosol Compressor Operator s Manual
 MODEL 50012 Maverick Heavy Duty Aerosol Compressor Operator s Manual This page left blank intentionally CONTENTS Page No. INSPECTION... 1 GENERAL INFORMATION... 1 SAFETY PRECAUTIONS... 2 BASIC OPERATING
MODEL 50012 Maverick Heavy Duty Aerosol Compressor Operator s Manual This page left blank intentionally CONTENTS Page No. INSPECTION... 1 GENERAL INFORMATION... 1 SAFETY PRECAUTIONS... 2 BASIC OPERATING
CircuFlow TM 5150 Series
 CircuFlow TM 5150 Series Sequential Compression Device Operating Instructions www.devonmedicalproducts.com 1.866.446.0092 IFU34.0001 Rev. D 20140313 The Model 5150 - Sequential Compression Device Indications:
CircuFlow TM 5150 Series Sequential Compression Device Operating Instructions www.devonmedicalproducts.com 1.866.446.0092 IFU34.0001 Rev. D 20140313 The Model 5150 - Sequential Compression Device Indications:
USER MANUAL. AltaDyne XT Model A AltaDyne XD Model A Alternating Pressure / Low Air Loss Replacement Mattress System
 AltaDyne XT Model 773400A AltaDyne XD Model 774200A Alternating Pressure / Low Air Loss Replacement Mattress System USER MANUAL Important: Do not operate the Mattress System without first reading and understanding
AltaDyne XT Model 773400A AltaDyne XD Model 774200A Alternating Pressure / Low Air Loss Replacement Mattress System USER MANUAL Important: Do not operate the Mattress System without first reading and understanding
42045 Heavy Duty ADA Base Model Kit: 85/105 PSI (ADA Compressor Only) Heavy Duty ADA Base Model Kit: 110/145 PSI (ADA Compressor Only)
 42045 Heavy Duty ADA Base Model Kit: 85/105 PSI (ADA Compressor Only) 42047 Heavy Duty ADA Base Model Kit: 110/145 PSI (ADA Compressor Only) 45052 Constant Duty ADA Base Model Kit: 85/105 PSI (ADA Compressor
42045 Heavy Duty ADA Base Model Kit: 85/105 PSI (ADA Compressor Only) 42047 Heavy Duty ADA Base Model Kit: 110/145 PSI (ADA Compressor Only) 45052 Constant Duty ADA Base Model Kit: 85/105 PSI (ADA Compressor
Lateral Turning System. User Guide.
 Lateral Turning System User Guide www.frontier-group.co.uk Key This user guide contains important information on correct usage, handling, cleaning and decontamination. Please read carefully prior to use.!
Lateral Turning System User Guide www.frontier-group.co.uk Key This user guide contains important information on correct usage, handling, cleaning and decontamination. Please read carefully prior to use.!
USER MANUAL SAVE THESE INSTRUCTIONS. 300 Held Drive Tel: (+001) Northampton, PA USA Fax: (+001) ISO Certified
 USER MANUAL Model: PM4300 Series (PM4351 Shown) SAVE THESE INSTRUCTIONS Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090 Northampton,
USER MANUAL Model: PM4300 Series (PM4351 Shown) SAVE THESE INSTRUCTIONS Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090 Northampton,
INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR
 INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR Model HEM-412C TABLE OF CONTENTS Introduction 3 Know Your Unit 4 Quick Reference Guide 5 Battery Installation/Replacement 6 How To Apply The Arm
INSTRUCTION MANUAL MANUAL INFLATION BLOOD PRESSURE MONITOR Model HEM-412C TABLE OF CONTENTS Introduction 3 Know Your Unit 4 Quick Reference Guide 5 Battery Installation/Replacement 6 How To Apply The Arm
Operating Instructions Part No
 DIGITAL AUTOMATIC TYRE INFLATOR Operating Instructions Part No. 11.0578 Thank you for selecting this Jamec Pem Automatic Tyre Inflator. Please read this manual before carrying out any installation or service
DIGITAL AUTOMATIC TYRE INFLATOR Operating Instructions Part No. 11.0578 Thank you for selecting this Jamec Pem Automatic Tyre Inflator. Please read this manual before carrying out any installation or service
Calibration Gas Instrument INSTRUCTION MANUAL. Release I. Advanced Calibration Designs, Inc.
 Advanced Calibration Designs, Inc. Calibration Gas Instrument INSTRUCTION MANUAL Release I www.goacd.com Instruction Manual Gas Generator Release I TABLE OF CONTENTS I. General Description Page 2 II. Start-Up
Advanced Calibration Designs, Inc. Calibration Gas Instrument INSTRUCTION MANUAL Release I www.goacd.com Instruction Manual Gas Generator Release I TABLE OF CONTENTS I. General Description Page 2 II. Start-Up
Pressure Automated Calibration Equipment
 GE Measurement & control Pressure Automated Calibration Equipment Safety Instructions and User Guide - K0447 PACE5000 PACE6000 K0447 Issue No. 9 1 10 1 PACE5000 1 2 3 4 5 PACE6000 2 6 7 8 3 4 5 6 7 8 9
GE Measurement & control Pressure Automated Calibration Equipment Safety Instructions and User Guide - K0447 PACE5000 PACE6000 K0447 Issue No. 9 1 10 1 PACE5000 1 2 3 4 5 PACE6000 2 6 7 8 3 4 5 6 7 8 9
SELECT SERIES LS200 ALTERNATING PRESSURE / LOW AIR LOSS MATTRESS SYSTEM
 SELECT SERIES LS200 ALTERNATING PRESSURE / LOW AIR LOSS MATTRESS SYSTEM USER MANUAL LS200-INS-LAB-RevB17 Read this manual before operating your Mattress System. Save this manual for future use. The most
SELECT SERIES LS200 ALTERNATING PRESSURE / LOW AIR LOSS MATTRESS SYSTEM USER MANUAL LS200-INS-LAB-RevB17 Read this manual before operating your Mattress System. Save this manual for future use. The most
PRESSURE REDISTRIBUTION SYSTEM. with people in mind
 ALPHA RESPONSE PRESSURE REDISTRIBUTION SYSTEM with people in mind introduction superior Features For over 0 years the Alpha range of pressure redistributing mattresses has been proven to offer patients,
ALPHA RESPONSE PRESSURE REDISTRIBUTION SYSTEM with people in mind introduction superior Features For over 0 years the Alpha range of pressure redistributing mattresses has been proven to offer patients,
MA60 Series. MA60 Alternating Pressure System. MA65 Alternating Pressure On-Demand Low Air Loss System. Owner s Operator and Maintenance Manual
 Owner s Operator and Maintenance Manual MA60 Series MA60 Alternating Pressure System MA65 Alternating Pressure On-Demand Low Air Loss System DEALER: This manual MUST be given to the user of the product.
Owner s Operator and Maintenance Manual MA60 Series MA60 Alternating Pressure System MA65 Alternating Pressure On-Demand Low Air Loss System DEALER: This manual MUST be given to the user of the product.
Cirrus 5 Oxygen Concentrator Manual
 Cirrus 5 Oxygen Concentrator Manual Important: Make sure you read and understand all of the information JAGZ03-03-3B ver1.0 12/07/2015 contained in this manual before operating your oxygen concentrator!
Cirrus 5 Oxygen Concentrator Manual Important: Make sure you read and understand all of the information JAGZ03-03-3B ver1.0 12/07/2015 contained in this manual before operating your oxygen concentrator!
Owner s Manual & Safety Instructions
 Owner s Manual & Safety Instructions Save This Manual Keep this manual for the safety warnings and precautions, assembly, operating, inspection, maintenance and cleaning procedures. Write the product s
Owner s Manual & Safety Instructions Save This Manual Keep this manual for the safety warnings and precautions, assembly, operating, inspection, maintenance and cleaning procedures. Write the product s
AltaDyne AutoFloat. Alternating Pressure Management Plus. Model # Model # OPERATORS MANUAL
 AltaDyne AutoFloat Alternating Pressure Management Plus Model # 753003 Model # 753004 OPERATORS MANUAL Graham-Field Health Products 2935 Northeast Parkway Atlanta, Georgia 30360 1-800-347-5678 Fax: 1-800-726-0601
AltaDyne AutoFloat Alternating Pressure Management Plus Model # 753003 Model # 753004 OPERATORS MANUAL Graham-Field Health Products 2935 Northeast Parkway Atlanta, Georgia 30360 1-800-347-5678 Fax: 1-800-726-0601
GAS BBQ 4 burner. instructions for. model no: BBQ10
 instructions for GAS BBQ 4 burner model no: BBQ10 Thank you for purchasing a Sealey product. Manufactured to a high standard, this product will, if used according to these instructions, and properly maintained,
instructions for GAS BBQ 4 burner model no: BBQ10 Thank you for purchasing a Sealey product. Manufactured to a high standard, this product will, if used according to these instructions, and properly maintained,
POWERPRESS UNIT. User Manual. Gradient Pneumatic Sequential Compressor. Neomedic
 TIMER (MIN) POWERPRESS UNIT 40 70 40 80 90 10 60 100 MIN MAX PRESSURE (mmhg) User Manual Neomedic 9601 Owens mouth Ave. #8 Chatsworth, CA 91311 Toll Free: 866-990-1168 Tel: 818-998-1023 Fax: 818-998-0277
TIMER (MIN) POWERPRESS UNIT 40 70 40 80 90 10 60 100 MIN MAX PRESSURE (mmhg) User Manual Neomedic 9601 Owens mouth Ave. #8 Chatsworth, CA 91311 Toll Free: 866-990-1168 Tel: 818-998-1023 Fax: 818-998-0277
770000S CLINICAL MAX LOW AIR LOSS WITH ALTERNATING PRESSURE SYSTEM USER MANUAL
 770000S CLINICAL MAX LOW AIR LOSS WITH ALTERNATING PRESSURE SYSTEM USER MANUAL Important: Do not operate the Mattress System without first reading and understanding this manual! Save this manual for future
770000S CLINICAL MAX LOW AIR LOSS WITH ALTERNATING PRESSURE SYSTEM USER MANUAL Important: Do not operate the Mattress System without first reading and understanding this manual! Save this manual for future
NIMBUS 4 NIMBUS PROFESSIONAL
 NIMBUS 4 NIMBUS PROFESSIONAL INSTRUCTIONS FOR USE 0086...with people in mind Contents General Safety....................................................... iii Introduction.........................................................
NIMBUS 4 NIMBUS PROFESSIONAL INSTRUCTIONS FOR USE 0086...with people in mind Contents General Safety....................................................... iii Introduction.........................................................
VERTICAL AIR COMPRESSORS
 VERTICAL AIR COMPRESSORS MODEL NO: VE11C150, VE15C150, VE18C150 PART NO: 2226005, 2226000, 2226015 OPERATION & MAINTENANCE INSTRUCTIONS LS0615 INTRODUCTION Thank you for purchasing this CLARKE Vertical
VERTICAL AIR COMPRESSORS MODEL NO: VE11C150, VE15C150, VE18C150 PART NO: 2226005, 2226000, 2226015 OPERATION & MAINTENANCE INSTRUCTIONS LS0615 INTRODUCTION Thank you for purchasing this CLARKE Vertical
LOGIC RANGE AUTOMATIC PRESSURE
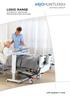 LOGIC RANGE AUTOMATIC PRESSURE REDISTRIBUTING Systems with people in mind Mattress Replacement & Overlay Systems The Auto logic 200 mattress replacement and Auto logic 110 overlay systems are part of the
LOGIC RANGE AUTOMATIC PRESSURE REDISTRIBUTING Systems with people in mind Mattress Replacement & Overlay Systems The Auto logic 200 mattress replacement and Auto logic 110 overlay systems are part of the
U S E R M A N U A L. Model: PM4300 Series SAVE THESE INSTRUCTIONS
 U S E R M A N U A L Model: PM4300 Series (PM4351 Shown) SAVE THESE INSTRUCTIONS CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090
U S E R M A N U A L Model: PM4300 Series (PM4351 Shown) SAVE THESE INSTRUCTIONS CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090
User manual. ZX trolley.
 User manual. ZX trolley. Date July 2011 02 User manual. ZX trolley. ZX trolley. Safe transportation for medical gas cylinders. Important Please read this manual carefully before attempting to use the BOC
User manual. ZX trolley. Date July 2011 02 User manual. ZX trolley. ZX trolley. Safe transportation for medical gas cylinders. Important Please read this manual carefully before attempting to use the BOC
User Manual. PULSE User Manual WARNINGS
 PULSE User Manual PULSE User Manual WARNINGS Table of Contents Warnings 4 Labels 6 Indications for Use 8 Risks and Benefits of the NormaTec PULSE Recovery System 8 Illustrations 9 Operating Instructions
PULSE User Manual PULSE User Manual WARNINGS Table of Contents Warnings 4 Labels 6 Indications for Use 8 Risks and Benefits of the NormaTec PULSE Recovery System 8 Illustrations 9 Operating Instructions
Sensor. Directions For Use
 Nasal Alar SpO2 Sensor Directions For Use Emergo Europe Prinsessegracht 20 2514 AP The Hague The Netherlands Manufactured by: Xhale Assurance, Inc. 3630 SW 47th Ave., Suite 100 Gainesville, FL 32608, USA
Nasal Alar SpO2 Sensor Directions For Use Emergo Europe Prinsessegracht 20 2514 AP The Hague The Netherlands Manufactured by: Xhale Assurance, Inc. 3630 SW 47th Ave., Suite 100 Gainesville, FL 32608, USA
CircuFlow 5200 Series
 CircuFlow 5200 Series Sequential Compression Device Operating Instructions www.devonmedicalproducts.com 1.866.446.0092 IFU04.0001 Rev. F 20140522 Intended Use: The CircuFlow 5200 Series Sequential Compression
CircuFlow 5200 Series Sequential Compression Device Operating Instructions www.devonmedicalproducts.com 1.866.446.0092 IFU04.0001 Rev. F 20140522 Intended Use: The CircuFlow 5200 Series Sequential Compression
12V Mini Air Compressor
 INSTRUCTIONS FOR 12V Mini Air Compressor Stock No.80999 Part No.DA12/250B IMPORTANT: PLEASE READ THESE INSTRUCTIONS CAREFULLY TO ENSURE THE SAFE AND EFFECTIVE USE OF THIS PRODUCT. GENERAL INFORMATION This
INSTRUCTIONS FOR 12V Mini Air Compressor Stock No.80999 Part No.DA12/250B IMPORTANT: PLEASE READ THESE INSTRUCTIONS CAREFULLY TO ENSURE THE SAFE AND EFFECTIVE USE OF THIS PRODUCT. GENERAL INFORMATION This
420C AIR COMPRESSOR KIT PART NO C AIR COMPRESSOR KIT PART NO
 420C AIR COMPRESSOR KIT PART NO. 42042 460C AIR COMPRESSOR KIT PART NO. 46043 420C 460C IMPORTANT: It is essential that you and any other operator of this product read and understand the contents of this
420C AIR COMPRESSOR KIT PART NO. 42042 460C AIR COMPRESSOR KIT PART NO. 46043 420C 460C IMPORTANT: It is essential that you and any other operator of this product read and understand the contents of this
Portable oxygen concentrator
 Table of contents G3 1. Introduction 2 2. Important Safety Rules 3 3. Unpack your Lovego 4 4. User Controls & System Status Indicators 5 5. Quick Start 6 6. How to operate 7 7. How to operate with battery
Table of contents G3 1. Introduction 2 2. Important Safety Rules 3 3. Unpack your Lovego 4 4. User Controls & System Status Indicators 5 5. Quick Start 6 6. How to operate 7 7. How to operate with battery
97C COMPRESSOR KIT 12V PART NO C COMPRESSOR KIT 24V PART NO C COMPRESSOR KIT PART NO
 97C COMPRESSOR KIT 12V PART NO. 00097 97C COMPRESSOR KIT 24V PART NO. 02497 98C COMPRESSOR KIT PART NO. 00098 97C 98C IMPORTANT: It is essential that you and any other operator of this product read and
97C COMPRESSOR KIT 12V PART NO. 00097 97C COMPRESSOR KIT 24V PART NO. 02497 98C COMPRESSOR KIT PART NO. 00098 97C 98C IMPORTANT: It is essential that you and any other operator of this product read and
200 PSI COMPRESSORS - MODEL NUMBERS
 200 PSI COMPRESSORS - MODEL NUMBERS 380C AIR COMPRESSOR KIT PART NO. 38033 480C AIR COMPRESSOR KIT PART NO. 48043 380C 480C IMPORTANT: It is essential that you and any other operator of this product read
200 PSI COMPRESSORS - MODEL NUMBERS 380C AIR COMPRESSOR KIT PART NO. 38033 480C AIR COMPRESSOR KIT PART NO. 48043 380C 480C IMPORTANT: It is essential that you and any other operator of this product read
Regalia Oxygen Concentrator
 Regalia Oxygen Concentrator REGALIA INSTRUCTION MANUAL P/N 5167 Rev B January 2014 Table of Contents Warnings and Cautions... 3 Indications for Use... 4 Introduction... 4 Important Safety Instructions...
Regalia Oxygen Concentrator REGALIA INSTRUCTION MANUAL P/N 5167 Rev B January 2014 Table of Contents Warnings and Cautions... 3 Indications for Use... 4 Introduction... 4 Important Safety Instructions...
FUSION Mattress and Cushion Systems User Guidelines
 ...reducing avoidable harms FUSION Mattress and Cushion Systems User Guidelines Explanation of Label Symbols and Statements Caution Protect from heat and radioactive sources Refer to instructions of use
...reducing avoidable harms FUSION Mattress and Cushion Systems User Guidelines Explanation of Label Symbols and Statements Caution Protect from heat and radioactive sources Refer to instructions of use
MODEL NUMBER: AS18-2 USER GUIDE
 Compressor MODEL NUMBER: AS18-2 USER GUIDE WEBSITE: EMAIL: www.airbrushheaven.co.uk enquiries@airbrushheaven.co.uk 1 YEAR WARRANTY Contents 1. Welcome Section 2. General Information & Safety Instructions
Compressor MODEL NUMBER: AS18-2 USER GUIDE WEBSITE: EMAIL: www.airbrushheaven.co.uk enquiries@airbrushheaven.co.uk 1 YEAR WARRANTY Contents 1. Welcome Section 2. General Information & Safety Instructions
VERTICAL AIR COMPRESSORS
 VERTICAL AIR COMPRESSORS MODEL NO: VE15C150, VE18C150, VE25C150 PART NO: 2226010, 2226020, 2226025 OPERATION & MAINTENANCE INSTRUCTIONS LS0715 INTRODUCTION Thank you for purchasing this CLARKE Vertical
VERTICAL AIR COMPRESSORS MODEL NO: VE15C150, VE18C150, VE25C150 PART NO: 2226010, 2226020, 2226025 OPERATION & MAINTENANCE INSTRUCTIONS LS0715 INTRODUCTION Thank you for purchasing this CLARKE Vertical
100C Air Compressor Kit
 10010 100C Air Compressor (standard mounting bracket, CE Spec) 10014 100C Air Compressor (no leader hose or check valve, CE Spec) 10016 100C Air Compressor (with Omega Bracket, CE Spec) IMPORTANT: It is
10010 100C Air Compressor (standard mounting bracket, CE Spec) 10014 100C Air Compressor (no leader hose or check valve, CE Spec) 10016 100C Air Compressor (with Omega Bracket, CE Spec) IMPORTANT: It is
Key Product Features. Primary Use: Aggressive Treatment of Pressure Ulcers
 AME PressureGuard APM² The PressureGuard APM² enables you to change instantly and easily from alternating pressure to basic lateral rotation with a single, recessed toggle switch. The silent, reliable,
AME PressureGuard APM² The PressureGuard APM² enables you to change instantly and easily from alternating pressure to basic lateral rotation with a single, recessed toggle switch. The silent, reliable,
IMPORTANT SAFETY INSTRUCTIONS
 IMPORTANT SAFETY INSTRUCTIONS CAUTION - To reduce risk of electrical shock: - Do not disassemble. Do not attempt repairs or modifications. Refer to qualified service agencies for all service and repairs.
IMPORTANT SAFETY INSTRUCTIONS CAUTION - To reduce risk of electrical shock: - Do not disassemble. Do not attempt repairs or modifications. Refer to qualified service agencies for all service and repairs.
User Guide Antidecubitus systems EURO BASIC
 User Guide Antidecubitus systems EURO BASIC User Guide Euro Basic Rev 06 del 11/2011 pag. 1 di 16 INDEX Par. Title Page 1 Instructions 3 2 Standards and directives 3 3 Identifying the device 3 4 Description
User Guide Antidecubitus systems EURO BASIC User Guide Euro Basic Rev 06 del 11/2011 pag. 1 di 16 INDEX Par. Title Page 1 Instructions 3 2 Standards and directives 3 3 Identifying the device 3 4 Description
REL-510H WARNING NOTICE 12 TON SINGLE ACTING REMOTE HYDRAULIC CRIMPING HEAD
 OPERATORS ORS GUIDE REL-510H 12 TON SINGLE ACTING REMOTE HYDRAULIC CRIMPING HEAD Compatible with U style and RELIABLE R12 shell type 12 ton compression dies. RELIABLE EQUIPMENT & SERVICE CO., INC. 92 Steamwhistle
OPERATORS ORS GUIDE REL-510H 12 TON SINGLE ACTING REMOTE HYDRAULIC CRIMPING HEAD Compatible with U style and RELIABLE R12 shell type 12 ton compression dies. RELIABLE EQUIPMENT & SERVICE CO., INC. 92 Steamwhistle
General User/ Safety Guide ROYAL/ ROYAL EXTRA REPLACEMENT SYSTEM
 General User/ Safety Guide ROYAL/ ROYAL EXTRA REPLACEMENT SYSTEM ACTIVE MATTRESSES www.harvesthealthcare.co.uk 1 2 CONTENTS WARNINGS & CAUTIONS GENERAL INFORMATION 1 DEFINITION OF THE GROUPS MENTIONED
General User/ Safety Guide ROYAL/ ROYAL EXTRA REPLACEMENT SYSTEM ACTIVE MATTRESSES www.harvesthealthcare.co.uk 1 2 CONTENTS WARNINGS & CAUTIONS GENERAL INFORMATION 1 DEFINITION OF THE GROUPS MENTIONED
D10S/D20S Wall/Post Mount Inflator Quick Start Manual
 PART NUMBER SERIAL NUMBER D10S/D20S Wall/Post Mount Inflator Quick Start Manual Please read and save these instructions. Read carefully before attempting to assemble, install, operate or maintain the product
PART NUMBER SERIAL NUMBER D10S/D20S Wall/Post Mount Inflator Quick Start Manual Please read and save these instructions. Read carefully before attempting to assemble, install, operate or maintain the product
Operating Instructions Part No
 DIGITAL AUTOMATIC TYRE INFLATOR Operating Instructions Part No. 11.0545 Thank you for selecting this Jamec Pem Automatic Tyre Inflator. Please read this manual before carrying out any installation or service
DIGITAL AUTOMATIC TYRE INFLATOR Operating Instructions Part No. 11.0545 Thank you for selecting this Jamec Pem Automatic Tyre Inflator. Please read this manual before carrying out any installation or service
BOC: Living healthcare. Manual. LIV IQ BOC Integrated Valve with digital display portable delivery system for Medical Oxygen. BOC: Living healthcare
 BOC: Living healthcare Manual LIV IQ BOC Integrated Valve with digital display portable delivery system for Medical Oxygen. BOC: Living healthcare 02 Manual LIV IQ Oxygen Manual LIV IQ Oxygen 03 Contents.
BOC: Living healthcare Manual LIV IQ BOC Integrated Valve with digital display portable delivery system for Medical Oxygen. BOC: Living healthcare 02 Manual LIV IQ Oxygen Manual LIV IQ Oxygen 03 Contents.
OPERATOR S MANUAL Ar-Gone Weld Gas Analyzer
 July 2011 OPERATOR S MANUAL Ar-Gone Weld Gas Analyzer WARNING! Before operating this product, read and understand this Operator s Manual. Become familiar with the potential hazards of this unit. Contact
July 2011 OPERATOR S MANUAL Ar-Gone Weld Gas Analyzer WARNING! Before operating this product, read and understand this Operator s Manual. Become familiar with the potential hazards of this unit. Contact
The Univentor 1250 Anaesthesia Unit
 THE UNIVENTOR 1200/1250 ANAESTHESIA UNIT The Univentor 1250 Anaesthesia Unit TABLE OF CONTENTS EDITION 1 Section 1 - WARRANTY & SERVICE 1.1. WARRANTY 2 1.2. DAMAGED SHIPMENTS 2 1.3. SERVICE 2 Section 2
THE UNIVENTOR 1200/1250 ANAESTHESIA UNIT The Univentor 1250 Anaesthesia Unit TABLE OF CONTENTS EDITION 1 Section 1 - WARRANTY & SERVICE 1.1. WARRANTY 2 1.2. DAMAGED SHIPMENTS 2 1.3. SERVICE 2 Section 2
Cadence Sensor W.I.N.D. User Manual
 Cadence Sensor W.I.N.D. User Manual Polar Cadence Sensor W.I.N.D. is designed to measure cadence, i.e. crank revolutions per minute when cycling. No other use is intended or implied. Please follow the
Cadence Sensor W.I.N.D. User Manual Polar Cadence Sensor W.I.N.D. is designed to measure cadence, i.e. crank revolutions per minute when cycling. No other use is intended or implied. Please follow the
450P- RV AUTOMATIC PORTABLE COMPRESSOR EXTREME SERIES
 450P- RV AUTOMATIC PORTABLE COMPRESSOR EXTREME SERIES PART NO. 45053 IMPORTANT: It is essential that you and any other operator of this product read and understand the contents of this manual before installing
450P- RV AUTOMATIC PORTABLE COMPRESSOR EXTREME SERIES PART NO. 45053 IMPORTANT: It is essential that you and any other operator of this product read and understand the contents of this manual before installing
User Manual. This manual MUST be given to the user of the product. BEFORE using this product, read this manual and save for future reference.
 User Manual EN Invacare MA60 Series MA60 Alternating Pressure System MA65 Alternating Pressure On-Demand Low Air Loss System MA65B42 microair Alternating Pressure with Low Air Loss System - Bariatric (42
User Manual EN Invacare MA60 Series MA60 Alternating Pressure System MA65 Alternating Pressure On-Demand Low Air Loss System MA65B42 microair Alternating Pressure with Low Air Loss System - Bariatric (42
Operator s Manual. The Bullet Blender Gold BB24-AU, BB5E-AU
 Operator s Manual The Bullet Blender Gold BB24-AU, BB5E-AU Congratulations! Congratulations on your purchase of a Bullet Blender Gold by Next Advance, Inc., for lysing, disrupting, and homogenizing your
Operator s Manual The Bullet Blender Gold BB24-AU, BB5E-AU Congratulations! Congratulations on your purchase of a Bullet Blender Gold by Next Advance, Inc., for lysing, disrupting, and homogenizing your
Use this key to determine which sections of this Product Manual apply to your job.
 R710 Product Manual Contents Warnings and Important Information 3 Recommended Use 4 Product Information 5 Technical Data 5 Before & During Every Transfer 6 Inspection 6 SoloLift Scale (optional) 6 SoloVest
R710 Product Manual Contents Warnings and Important Information 3 Recommended Use 4 Product Information 5 Technical Data 5 Before & During Every Transfer 6 Inspection 6 SoloLift Scale (optional) 6 SoloVest
200 PSI HIGH-FLOW AIR SOURCE KIT
 200 PSI HIGH-FLOW AIR SOURCE KIT 50% Duty Compressor on 2.0 Gallon Air Tank PART NO. 20008 IMPORTANT: It is essential that you and any other operator of this product read and understand the contents of
200 PSI HIGH-FLOW AIR SOURCE KIT 50% Duty Compressor on 2.0 Gallon Air Tank PART NO. 20008 IMPORTANT: It is essential that you and any other operator of this product read and understand the contents of
NICE1 Cold + Compression Therapy System
 NICE1 Cold + Compression Therapy System Thank you for choosing the NICE1 Cold + Compression Therapy System. The NICE1 uses advanced technology to greatly improve the convenience and efficacy of cold +
NICE1 Cold + Compression Therapy System Thank you for choosing the NICE1 Cold + Compression Therapy System. The NICE1 uses advanced technology to greatly improve the convenience and efficacy of cold +
LIFECOMFORT ACTIVE AIR
 LIFECOMFORT ACTIVE AIR Dynamic Alternating Air es for Pressure Care aspire for... Prevention & Therapy of Pressure Injuries www.aspirecare.com.au LIFECOMFORT ACTIVE AIR MATTRESS RANGE Pressure Injury prevention
LIFECOMFORT ACTIVE AIR Dynamic Alternating Air es for Pressure Care aspire for... Prevention & Therapy of Pressure Injuries www.aspirecare.com.au LIFECOMFORT ACTIVE AIR MATTRESS RANGE Pressure Injury prevention
AKRON CHILDS TILT TABLE
 AKRON CHILDS TILT TABLE Model 8601B USER MANUAL Contents 1. Introduction 2 Warnings, Cautions 2 General Warnings 2 2. Installation 3 3. Operation 4 Wheel Locking 4 Tilting Control 4 Head Section, Foot
AKRON CHILDS TILT TABLE Model 8601B USER MANUAL Contents 1. Introduction 2 Warnings, Cautions 2 General Warnings 2 2. Installation 3 3. Operation 4 Wheel Locking 4 Tilting Control 4 Head Section, Foot
English USER GUIDE User guide
 USER GUIDE English ENGLISH A Elbow E Cushion B Elbow ring 1 Vent (in cushion and elbow) C Headgear 2 Side buttons D Frame 3 Sleeve Intended use The AirFit N30i mask is intended to be used by patients weighing
USER GUIDE English ENGLISH A Elbow E Cushion B Elbow ring 1 Vent (in cushion and elbow) C Headgear 2 Side buttons D Frame 3 Sleeve Intended use The AirFit N30i mask is intended to be used by patients weighing
MODEL NUMBER: PSI AIR SOURCE KIT 200 PSI Compressor on 2.0 Gallon 200 PSI Air Tank
 IMPORTANT SAFETY INSTRUCTIONS CAUTION - To reduce risk of electrical shock or Electrocution: MODEL NUMBER: 20008 200 PSI AIR SOURCE KIT 200 PSI Compressor on 2.0 Gallon 200 PSI Air Tank IMPORTANT: It is
IMPORTANT SAFETY INSTRUCTIONS CAUTION - To reduce risk of electrical shock or Electrocution: MODEL NUMBER: 20008 200 PSI AIR SOURCE KIT 200 PSI Compressor on 2.0 Gallon 200 PSI Air Tank IMPORTANT: It is
Oxygen Dialflow Meter. Instructions for Use
 Oxygen Dialflow Meter Instructions for Use 702-0031.12 December 2017 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in injury to the patient,
Oxygen Dialflow Meter Instructions for Use 702-0031.12 December 2017 1. Symbols Warning! Caution! Indicates a potentially hazardous situation which, if not avoided, could result in injury to the patient,
VGP-4300-G. Travel Pulse. Blood Pressure Monitor for Travel
 VGP-4300-G Travel Pulse Blood Pressure Monitor for Travel Congratulations on your Vitagoods purchase. The Travel Pulse Blood Pressure Monitor, will allow you to measure vital blood pressure parameters
VGP-4300-G Travel Pulse Blood Pressure Monitor for Travel Congratulations on your Vitagoods purchase. The Travel Pulse Blood Pressure Monitor, will allow you to measure vital blood pressure parameters
Vapotherm Q50 Compressor. Instructions for Use
 Vapotherm Q50 Compressor Instructions for Use Contents Quick Start Guide...2 Intended Use...3 Indications, Warnings and Cautions...4 Primary Indications...4 Contraindications...4 Warnings and Cautions...4
Vapotherm Q50 Compressor Instructions for Use Contents Quick Start Guide...2 Intended Use...3 Indications, Warnings and Cautions...4 Primary Indications...4 Contraindications...4 Warnings and Cautions...4
Theatre Control Panel. Instructions for Use. January 2017 ASF-29 (1)
 Theatre Control Panel Instructions for Use January 2017 0197 ASF-29 (1) Contents 1. Instructions For Use 1.1. Introduction 1.2. Explanation of Symbols 1.3. General Safety Instructions Intended Use Operating
Theatre Control Panel Instructions for Use January 2017 0197 ASF-29 (1) Contents 1. Instructions For Use 1.1. Introduction 1.2. Explanation of Symbols 1.3. General Safety Instructions Intended Use Operating
01/18 BATH CHAIRS INSTRUCTIONS FOR USE. Code 7607,7609, 7511,7512
 01/18 BATH CHAIRS INSTRUCTIONS FOR USE Code 7607,7609, 7511,7512 SECTIONS PAGE 1.0 BATH CHAIR 1 2.0 BATH CORNER CHAIR 12 Bath Chairs 1 1.BATH CHAIR INSTRUCTIONS FOR USE Code 7607-7609 Bath Chairs 2 CONTENTS
01/18 BATH CHAIRS INSTRUCTIONS FOR USE Code 7607,7609, 7511,7512 SECTIONS PAGE 1.0 BATH CHAIR 1 2.0 BATH CORNER CHAIR 12 Bath Chairs 1 1.BATH CHAIR INSTRUCTIONS FOR USE Code 7607-7609 Bath Chairs 2 CONTENTS
