75 Inventors: Robert L. Zeller; Sharon D. Fritts, 358
|
|
|
- Paulina Page
- 5 years ago
- Views:
Transcription
1 US595226A United States Patent (19) 11 Patent Number: Zeller et al. (45) Date of Patent: Nov. 16, METHD F RECVERING CHLRINE 3,357,796 12/1967 Howard et al /24 S FRM A GAS MIXTURE 3,399,537 9/196 Honigh / ,599 1/1969 Hildyard / Inventors: Robert L. Zeller; Sharon D. Fritts, Y- a-2 3.E. S. yillnsel et al al. i "se both of Youngstown, David 4,115,529 9/197 Behling /472 Johnson, Williamsville, all of N.Y.; 5,32,17 2- Y-12 4 f1994 Itoh et al /132 Kenneth J. Carlson, Houston, Tex.; 5,5,35 3/1996 Zarchy et al /132 Paul J. rosz, Amherst; Ronald B. Kaplin, Lewiston, both of N.Y. FREIGN PATENT DCUMENTS 73 Assignee: ccidental Chemcial Corporation, of Canada. Niagara Falls, N.Y. THER PUBLICATINS 21 Appl. No.: 9/3,21 Article by A.T. Bozzo et al., Fourth Internati Symposium on Fresh Water From The Sea, Heidelberg, Sep (1973), p. 22 Filed: Jan. 7, Thesis For Degree of Master of Chemical Engineering, (51) Int. Cl."... C1B 7/7 Grad. School, Syracuse University, by Jeffrey R. Kass, 52 U.S. Cl /24 S; 95/16; 95/115; Chlorine Hydrate, ct. 6, 1967, p /132; 423/472; 423/53 Primary Examiner Duane S. Smith 5 Field of Search /24 S, 53, Attorney, Agent, r Firm-Richard D. Fuerle; Anne E. 423/472, 241,475, 24 R; 95/3, 132, Brook, 131, 114, 115, 95, 16, ABSTRACT 56) References Cited U.S. PATENT DCUMENTS Disclosed is a process for Separating chlorine gas from a mixture of gases. The mixture of gases is cooled to a temperature less than C. and is contacted with ice, which E. 3. R al al r 3.3 results in the formation of chlorine hydrate on the ice. The 2.447,34 /194 Balcar /24 S ice is Separated from the mixture of gases and is heated or 2,75,2 6/1956 Hooker et al /233 the operating pressure reduced to release chlorine. 2,75,55 3/1957 Redcay... 95/233 3,254,474 6/1966 Van Dijk /24 S 2 Claims, 3 Drawing Sheets Temperature (C)
2 U.S. Patent Nov. 16, 1999 Sheet 1 of 3 r CN N C) S CD t o & 9 9. I E () H V op N C L w cy). CN v AueMooe euluoluo % vm
3 U.S. Patent Nov. 16, 1999 Sheet 2 of aunssædd (6?sd) 9 Z () eneledue
4 U.S. Patent Nov. 16, 1999 Sheet 3 of 3 Z 9 eunsseld (6?sd) 9 Z () eneledue
5 1 METHD F RECVERING CHLRINE FRM A GAS MIXTURE BACKGRUND F THE INVENTIN This invention relates to a process for Separating chlorine gas from a mixture of gases. In particular, it relates to a process in which a mixture of gases containing chlorine is cooled to less than C. and is contacted with ice, which results in the formation of chlorine hydrate on the ice. To release the chlorine gas from the chlorine hydrate, the ice is Separated from the mixture of gases and is heated and/or the operating pressure on the ice is reduced. In manufacturing chlorine, mixtures of gases that contain chlorine are inevitably produced. While the other gases in the mixture may be harmless, chlorine is toxic and must be removed from the mixture before the mixture can be released to the atmosphere. Many techniques have been used to Separate chlorine from mixtures of gases, the particular technique chosen depending upon the concentration of chlo rine in the mixture and the other gases that are present. ne technique is to pressurize the gas mixture while adding cold water to form Solid chlorine hydrate crystals. The Solid chlorine hydrate is separated from the remaining gases and the pressure on it is relieved and/or it is heated, which releases the chlorine. Liquid water is used in this process because it is believed that chlorine gas is insoluble C. SUMMARY F THE INVENTIN We have discovered that chlorine hydrate will form on the Surface of ice and that the formation of chlorine hydrate on the ice can be used to separate chlorine from a mixture of gases. We have further found that the process of this invention can be used to Separate chlorine from a mixture of gases where the chlorine is at Such low concentrations (i.e.,.5-6 vol %) that liquid water does not readily form chlorine hydrate. BRIEF DESCRIPTIN F THE DRAWING FIG. 1 is a chart where the abscissa is the percentage of chlorine recovered using the process of this invention at temperature given in the ordinate (in C.) and at 69.5 kpa (1 psig) for input gases containing.5 (curve 1), 1 (curve 2), 3 (curve 3), 5 (curve 4), and 1 (curve 5) vol% chlorine. FIG. 2 is a chart showing the relationship between pres Sure (ordinate) in psig and a temperature (abscissa) in C. necessary to achieve 25% chlorine recovery when the input gas contains.1 (curve 1),.2 (curve 2),.1 (curve 3),.2 (curve 4), 1 (curve 5), 2 (curve 6), 1 (curve 7), and 2 (curve ) vol% chlorine. FIG. 3 is a chart showing the relationship between pres Sure (ordinate) in psig and temperature (abscissa) in C. necessary to achieve 75% chlorine recovery when the input gas contains.1 (curve 1),.2 (curve 2),.1 (curve 3),.2 (curve 4), 1 (curve 5), 2 (curve 6), 1 (curve 7), 2 (curve ), and 1 (curve 9) vol% chlorine. The curves shown in FIGS. 1, 2, and 3 were calculated from extrapolated vapor pressure data available in the lit erature. DESCRIPTIN F THE PREFERRED EMBDIMENTS While the method of this invention is applicable to mixtures of gases containing a wide range of chlorine, it is particularly applicable to gases containing about.5 to about 6 vol % chlorine as it is less effective at concentrations below.5 vol% and at over 6 vol% other techniques for Separating chlorine may work better. The process of this invention is particularly preferred for mixtures of gases containing about 3 to about 12 vol% chlorine because other techniques for Separating chlorine from a mixture of gases do not work well in that range. Examples of other common gases that may be in the mixture with chlorine include carbon dioxide, hydrogen, oxygen, nitrogen, and mixtures thereof. These gas mixtures can be the result of chlorine manufacturing processes that include, for example, com pression and liquification. Also, the gas Stream that comes from a chlorine rail car that is being depressurized or purged can contain mixtures of gases that include chlorine. In the first step of the process of this invention, the mixture of gases is cooled to a temperature below C. to keep the water in a Solid (ice) form. A temperature of about -7 to about -1 C. is preferred as that temperature range tends to result in the recovery of more chlorine from the gas mixture. The pressure of the mixture of gases is also important Since a higher pressure will more readily form chlorine hydrate. The amount of chlorine hydrate that forms is also a function of the chlorine content of the mixture of gases. The effect on chlorine recovery of the temperature of the mixture of gases, the pressure on the mixture of gases, and the Volume percent of chlorine in the mixture of gases is given in the accompanying drawings. The drawings Show that temperature and Vol % chlorine in the mixture of gases is very important to achieving a high recovery, while pres sure is less important. Nevertheless, a pressure of about 345 to about 134 kpa (about 5 to about 15 psig) is preferred to enhance chlorine recovery, as at a lower pressure the process is not as economical and higher pressures can present equipment problems. The cold mixture of gases is contacted with ice. This can be accomplished either by forming the ice in Situ by, for example, Spraying water into the frigid mixture of gases, or it can be accomplished by passing the mixture of gases through a bed of ice. The ice is preferably finely divided to present more Surface area to the gases. When the cold mixture of gases contacts the ice, chlorine hydrate forms on the ice. Chlorine hydrate contains varying amounts of water but the general formula for it is usually given as ClinH2, where n is 6 to 1. nce the chlorine hydrate has formed, which occurs immediately after the chlorine contacts the ice, the ice is Separated from the mixture of gases. The pressure on the Separated ice is then relieved or the ice is warmed or both, which results in the release of chlorine from the chlorine hydrate. It is preferable to dry the freed' chlorine, and this can be accomplished, for example, by passing the chlorine through concentrated Sulfuric acid. The water, which con tains Some hypochlorite resulting from the melted ice, can be recycled. The following examples further illustrate this invention. EXAMPLE 1. A thin film of ice crystals was formed in a condenser tube by chilling the tube to -9.3 C. and passing nitrogen gas Saturated with water through it. This ice was chlorinated with 1% Cl at that temperature for several hours at a flow rate of 15.5 ml/min. The chlorine gas remaining in the equipment was purged using cold nitrogen until the gas Stream did not react with a potassium iodide Solution. The nitrogen purge Stream was also monitored quantitatively for
6 3 chlorine with KI Solution so that all of the chlorine gas in the System could be accounted for. The ice was warmed to 2 C. and the water that formed was dissolved in a potassium iodide Solution and titrated with sodium thiosulfate to determine its chlorine content. Using this procedure, a very Small amount of chlorine was found; a repetition of the test produced Similar results. Since a large excess of chlorine was used, the chlorine hydrate yields were based on the amount of ice that was evenly formed along the walls of the condenser tube. The yield obtained during the first run was.7% and the yield obtained during the Second run was.2%. The experimental param eters for both determinations are shown in the following table: Low Temperature Chlorination of Ice Reaction Parameters Parameter Reaction #1 Reaction #2 Saturated N Flow Rate mls/min mlsamin. Condenser Temperature -.7 C. -93 C. Wt. f Ice Formed 4. gms gms. Condenser Tube Area sq. In sq. In. Chlorine Flow Rate 15.5 mls/min mls/min. Chlorine Added gms gms. N Purge Rate mls/min. 493 mls/min. Purge Volume in Equipment 1572 mls 66 mls. Wt. f Water Recovered (Melt) 4. gms gms. Wt. f Chlorine Recovered.146 gms..12 gms. Hydrate Wt. Formed.442 gms..327 gms. Hydrate Yield Based on 1% Ice.74.% 157% Reacting with Cl Since the chlorine recovery was so small, the hydrate formed under these conditions was probably limited to the top Surface layer of the ice crystals. In addition, nitrogen purging likely decomposed much of the chlorine hydrate by Simple Vapor pressure considerations. This example demonstrates that chlorine reacts with ice. EXAMPLE 2 Two additional experiments were completed. In one experiment, 44.9 g of Snow was chlorinated in a cold finger trap at -.6 C., and in the other experiment Some g of crushed ice were chlorinated at C. In the Snow experiment, the recovery yield was only.1%, based on the amount of Snow that was available to react with the excess chlorine. In the other experiment, Several pieces of ice were directly removed from the partially Submerged coldfinger using forceps, placed in a tared weighing bottle, and the contents were reacted with KI Solution. The resulting titration again confirmed a low.2% hydrate yield. Since there were Some concerns that the cold nitrogen purge, used to Sweep out unreacted chlorine gas (234 ml), might also remove Some of the chlorine hydrate, this purge was not employed in a Second Snow chlorination. For the Second Snow experiment, the Snow was carefully arranged in the coldfinger So as to maximize the exposed Surface area to the chlorine gas. In this case, the total contents of the coldfinger (14.5g of chlorinated Snow and unreacted gas) after chlorination at C. were reacted, in Stages, with KI Solution. The resulting Solutions were built up to known volumes before being titrated. After Subtracting for the chlorine gas Volume, the resulting chlorine hydrate yield was calculated to be 11.%. In order to determine if chlorine hydrate had actually formed, a known weight (.37 g) of chlorinated Snow was removed from the coldfinger at C. and added to a flask containing ice water at C. About one half of the crystals sank to the bottom of the flask while the other half floated on the Surface. A control test was also conducted, using the same type of Snow, and all of the Snow crystals floated until they melted. Another weighed Sample (1.6 g) was placed in a stoppered flask while the flask was cooled to 5 C. in a constant temperature bath. Granules having a water white to a slight green tint color were observed to completely melt over a 25 minute period at this temperature. The melting point for chlorine hydrate, Cl:7.27H2, is reported in the literature as C. However, if the chlorine hydrate formation is a Surface reaction, then the Snow crystals can, at 5 C., melt from the inside out, thereby Slowly dissolving the chlorine hydrate in the resulting Snow melt. This example demonstrates that chlorine reacts with ice to form chlorine hydrate. EXAMPLE 3 Two Successful attempts were also completed to form chlorine hydrate in the conventional manner by bubbling chlorine gas (36. ml/min) into cold (+.25 C) water. In each case, a 5 ml gas Washing bottle, Surrounded by an ice bath, was employed as a chlorine hydrate reactor. In the first case, a coarse glass fritted disc was used as the gas disper sion tube and this was later replaced with a glass bulb fitted with Small holes. A magnetic Stirring bar was also used to keep the water mixing during the chlorination. In both runs, the bottom of the gas addition point plugged before all the water (2 ml) had been converted to chlorine hydrate. During the chlorination, most of the chlorine hydrate for mation occurred above the gas addition point and the Subsequent chlorine hydrate resembled a foam that seemed to be carried upward by the gas lift and the stirring action. Some of the chlorine hydrate crystals or slivers did form in the water layer but the majority was in the foam area. This unexpected foam layer occupied a volume of 24 ml (3.5 inchesx2.5 inches) or more than twice the remaining water Volume. The best yield (25.14%) for the chlorine hydrate forma tion was calculated, by the weight gain, based on the amount of chlorine added to the system (21.43 g). The amount of unreacted gas remaining in the dry traps and the amount dissolved in the remaining water volume (117 ml) was taken into account. This example confirms that chlorine reacts with liquid water to form chlorine hydrate under the right conditions. EXAMPLE 4 In an additional Set of experiments, attempts were made to determine the minimum chlorine concentration in a mixed gas Stream that would Sustain chlorine hydrate formation under atmospheric conditions. Chlorine hydrate was again formed by slowly passing pure chlorine through chilled water (.5 C) until the hydrate foam occupied most of the volume in the 5 ml gas scrubber. At this time, the chlorine concentration was reduced to a 5 vol% Cl+95 vol% Nagas mixture. After 42 minutes of using this dilute gas Stream at a feed rate of 13 ml/min, all traces of chlorine hydrate had disappeared. The scrubber water was titrated and found to contain only.113 g/l as NaCl. This indicated that the Stripping action' of the nitrogen combined with the low chlorine concentration would make any chlorine hydrate formation under these conditions improbable. This premise was further enhanced by incrementally increasing the chlorine concentration from 2.6 vol% up to 75 Vol % while lowering the nitrogen content accordingly,
7 S without observing any hydrate formation after 1.4 and 2 hours of gas addition. However, when the chlorine concen tration was increased to 93 vol %, chlorine hydrate was again formed after 23 minutes of gas Sparging at a total flow rate of 452 ml/min at.5 C. nce again the chlorine concentration was diluted down to 2.9 vol% with nitrogen at a combined flow rate of 4 ml/min and the gas was bubbled through the chlorine hydrate and water mixture at.5 C. After 55 minutes, there were no traces of any chlorine hydrate left in the gas Washing bottle. A Small Stream from a commercial electrolytic cell pro ducing chlorine vent gas containing approximately 4 vol% Cl was bubbled through chilled water (1 C.) at atmo Spheric pressure for Several hours, on at least two occasions, without any chlorine hydrate formation being observed. Apparently, the chlorine Solubility was depressed to Such an extent by the inert gases present (nitrogen, oxygen, carbon dioxide, and hydrogen) that the solubility limit for chlorine hydrate was not obtained. The chlorine content of the water under these conditions was found to be 625 ppm, as NaCl. This example demonstrates that chlorine does not react with liquid water to form chlorine hydrate at % CI levels less than 75%. We claim: 1. A process for Separating chlorine gas from a mixture of gases comprising (A) cooling said mixture of gases to less than C.; (B) contacting said mixture of gases with ice, whereby chlorine hydrate forms on Said ice; (C) separating said ice from said mixture of gases; and (D) heating said ice, lowering the pressure on Said ice, or heating Said ice and lowering the pressure on Said ice, to release Said chlorine gas from Said chlorine hydrate. 2. A process according to claim 1 wherein water is Sprayed into Said mixture of gases forming Said ice in situ. 3. A process according to claim 2 wherein Said mixture of gases contains chlorine and gases Selected from the group 4. A process according to claim 2 wherein Said mixture of gases is under a pressure of about 345 to about 134 kpa. 5. A process according to claim 2 including the additional last Step of drying Said chlorine gas. 6. A process according to claim 1 wherein Said mixture of gases is passed through a bed of ice. 7. A process according to claim 6 wherein Said mixture of gases contains chlorine and gases Selected from the group. A process according to claim 6 wherein Said mixture of gases is under a pressure of about 345 to about 134 kpa. 9. A process according to claim 6 including the additional last Step of drying Said chlorine A process for Separating chlorine gas from a mixture of gases containing about.5 to about 6 vol % chlorine comprising (A) cooling said mixture of gases to about -7 to about -1 C.; (B) contacting said cooled mixture of gases at a pressure of about 345 to about 134 kpa with ice, whereby chlorine hydrate forms on Said ice; (C) separating Said ice from Said mixture of gases; and (D) heating said ice, lowering the pressure of said ice, or heating Said ice and lowering the pressure on Said ice, to release chlorine gas from Said chlorine hydrate. 11. A process according to claim 1 wherein water is Sprayed into Said mixture of gases forming Said ice in situ. 12. A process according to claim 11 wherein Said mixture of gases contains chlorine and gases Selected from the group 13. A process according to claim 11 including the addi tional last Step of passing Said chlorine gas through concen trated Sulfuric acid. 14. A process according to claim 1 wherein Said mixture of gases is passed through a bed of ice. 15. A process according to claim 14 wherein Said mixture of gases contains chlorine and gases Selected from the group 16. A process according to claim 14 including the addi tional last Step of passing Said chlorine gas through concen trated Sulfuric acid. 17. A process for Separating chlorine gas from a mixture of gases containing about 3 to about 12 Vol % chlorine and gases selected from the group consisting of carbon dioxide, hydrogen, oxygen, nitrogen, and mixtures thereof, compris Ing (A) cooling said mixture of gases to about -7 to about -1 C.; (B) contacting said mixture of gases at a pressure of about 345 to about 134 kpa with ice, whereby chlorine hydrate forms on Said ice; (C) separating Said ice from Said mixture of gases; and (D) heating said ice, lowering the pressure on said ice, or heating Said ice and lowering the pressure on Said ice, to release chlorine gas from Said chlorine hydrate; and (E) drying said chlorine gas. 1. A process according to claim 17 wherein water is Sprayed into Said mixture of gases forming Said ice in situ. 19. A process according to claim 17 wherein said mixture of gases is passed through a bed of ice. 2. A process according to claim 17 wherein said chlorine gas is dried by passing it through concentrated Sulfuric acid. k k k k k
14 / 44. United States Patent (19) Allam ADM2/A. 11 Patent Number: 4,461,154 45) Date of Patent: Jul. 24, 1984 SAZX A6 AIAW/ 10/ /02 A/?
 United States Patent (19) Allam (54) METHOD AND APPARATUS FOR COMPRESSENG GAS 75 Inventor: Rodney J. Allam, St. Catherines, England 73) Assignee: Air Products and Chemicals, Inc., Allentown, Pa. (21) Appl.
United States Patent (19) Allam (54) METHOD AND APPARATUS FOR COMPRESSENG GAS 75 Inventor: Rodney J. Allam, St. Catherines, England 73) Assignee: Air Products and Chemicals, Inc., Allentown, Pa. (21) Appl.
DURING the course of certain investigations it became
 VOLUMETRIC DETERMINATION OF ETHER OR CYCLOPROPANE, CARBON DIOXIDE, NITROUS OXIDE AND OXYGEN IN ANESTHETIC MIXTURES By F. J. PRIME DURING the course of certain investigations it became necessary to be able
VOLUMETRIC DETERMINATION OF ETHER OR CYCLOPROPANE, CARBON DIOXIDE, NITROUS OXIDE AND OXYGEN IN ANESTHETIC MIXTURES By F. J. PRIME DURING the course of certain investigations it became necessary to be able
Gas Laws. 2 HCl(aq) + CaCO 3 (s) H 2 O(l) + CO 2 (g) + CaCl 2 (aq) HCl(aq) + NaHCO 3 (s) H 2 O(l) + CO 2 (g) + NaCl(aq)
 Gas Laws Introduction: Although we cannot see gases, we can observe their behavior and study their properties. For example, we can watch a balloon filled with helium gas floating in air and conclude that
Gas Laws Introduction: Although we cannot see gases, we can observe their behavior and study their properties. For example, we can watch a balloon filled with helium gas floating in air and conclude that
N3% (12) United States Patent. NNéré. (10) Patent No.: US 7, B2. Rossiter (45) Date of Patent: Nov. 20, 2007
 (12) United States Patent US007298.473B2 (10) Patent o.: US 7,298.473 B2 Rossiter (45) Date of Patent: ov. 20, 2007 (54) SPECTROSCOPY CELL 4,587,835 A 5/1986 Adams 4,674,876 A 6/1987 Rossiter... 356,244
(12) United States Patent US007298.473B2 (10) Patent o.: US 7,298.473 B2 Rossiter (45) Date of Patent: ov. 20, 2007 (54) SPECTROSCOPY CELL 4,587,835 A 5/1986 Adams 4,674,876 A 6/1987 Rossiter... 356,244
CHEM254 #4 The Synthesis of a Tertiary Alcohol Using a Pre-Made Grignard Reagent 1
 CHEM254 #4 The Synthesis of a Tertiary Alcohol Using a Pre-Made Grignard Reagent 1 Background In this project, we will perform a Grignard reaction using a pre-made Grignard reagent. Grignard reagents can
CHEM254 #4 The Synthesis of a Tertiary Alcohol Using a Pre-Made Grignard Reagent 1 Background In this project, we will perform a Grignard reaction using a pre-made Grignard reagent. Grignard reagents can
Determination of R: The Gas-Law Constant
 Determination of R: The Gas-Law Constant PURPOSE: EXPERIMENT 9 To gain a feeling for how well real gases obey the ideal-gas law and to determine the ideal-gas-law constant R. APPARATUS AND CHEMICALS: KClO
Determination of R: The Gas-Law Constant PURPOSE: EXPERIMENT 9 To gain a feeling for how well real gases obey the ideal-gas law and to determine the ideal-gas-law constant R. APPARATUS AND CHEMICALS: KClO
Question McGraw-Hill Ryerson Limited
 Question 1 Which of the following cannot be explained by considering the empty space between the particles of a gas? A) Gases are more compressible than liquids. B) Gases have lower viscosities than liquids.
Question 1 Which of the following cannot be explained by considering the empty space between the particles of a gas? A) Gases are more compressible than liquids. B) Gases have lower viscosities than liquids.
The Determination of the Value for Molar Volume
 Name AP Chemistry The Determination of the Value for Molar Volume Objective Using a chemical reaction that produces a gas, measure the appropriate values to allow a determination of the value for molar
Name AP Chemistry The Determination of the Value for Molar Volume Objective Using a chemical reaction that produces a gas, measure the appropriate values to allow a determination of the value for molar
ExamLearn.ie. The Air & Oxygen
 ExamLearn.ie The Air & Oxygen The Air & Oxygen The air is a mixture of gases, which forms a blanket around the earth. Another name for the air is the atmosphere. *To investigate the percentage of oxygen
ExamLearn.ie The Air & Oxygen The Air & Oxygen The air is a mixture of gases, which forms a blanket around the earth. Another name for the air is the atmosphere. *To investigate the percentage of oxygen
United States Patent (19)
 United States Patent (19) Fujikawa 54 HYDRAULIC CONTROL SYSTEM FOR A ROCK DRILL (75) Inventor: Kozo Fujikawa, Hiroshima, Japan 73) Assignee: Toyo Kogyo Co., Ltd., Hiroshima, Japan 21 Appl. No.: 194,391
United States Patent (19) Fujikawa 54 HYDRAULIC CONTROL SYSTEM FOR A ROCK DRILL (75) Inventor: Kozo Fujikawa, Hiroshima, Japan 73) Assignee: Toyo Kogyo Co., Ltd., Hiroshima, Japan 21 Appl. No.: 194,391
UNIT 10 - GASES. Notes & Worksheets - Honors
 Ideal Gas Equation 1 WKSHT 1.) What is the pressure exerted by 2.0 moles of an ideal gas when it occupies a volume of 12.0 L at 373 K? 2.) A flashbulb of volume 2.6 cm 3 contains O 2 gas at a pressure
Ideal Gas Equation 1 WKSHT 1.) What is the pressure exerted by 2.0 moles of an ideal gas when it occupies a volume of 12.0 L at 373 K? 2.) A flashbulb of volume 2.6 cm 3 contains O 2 gas at a pressure
(12) United States Patent
 (12) United States Patent USOO8352206B2 () Patent No.: Buess (45) Date of Patent: Jan. 8, 2013 (54) METHOD FOR THE SIGNAL (56) References Cited LINEARIZATION OF A GAS SENSOR OUTPUT SIGNAL U.S. PATENT DOCUMENTS
(12) United States Patent USOO8352206B2 () Patent No.: Buess (45) Date of Patent: Jan. 8, 2013 (54) METHOD FOR THE SIGNAL (56) References Cited LINEARIZATION OF A GAS SENSOR OUTPUT SIGNAL U.S. PATENT DOCUMENTS
Gas Laws. 2 HCl(aq) + CaCO 3 (s) H 2 O(l) + CO 2 (g) + CaCl 2 (aq) HCl(aq) + NaHCO 3 (s) H 2 O(l) + CO 2 (g) + NaCl(aq)
 Gas Laws Introduction: Although we cannot see gases, we can observe their behavior and study their properties. For example, we can watch a balloon filled with helium gas floating in air and conclude that
Gas Laws Introduction: Although we cannot see gases, we can observe their behavior and study their properties. For example, we can watch a balloon filled with helium gas floating in air and conclude that
I
 United States Patent [19] Llort et al. 1111111111111111111111111111111111111111111111111111111111111111111111111111111111 I US005833911A ["1 Patent Number: 5,833,911 [451 Date of Patent: Nov. 10, 1998
United States Patent [19] Llort et al. 1111111111111111111111111111111111111111111111111111111111111111111111111111111111 I US005833911A ["1 Patent Number: 5,833,911 [451 Date of Patent: Nov. 10, 1998
(12) United States Patent
 (12) United States Patent WOf USOO6273279B1 (10) Patent No.: (45) Date of Patent: Aug. 14, 2001 (54) GOLF TOWEL HOLDER (76) Inventor: Jerrold M. Wolf, 1036 E. Melody La., Fullerton, CA (US) 92831 (*) Notice:
(12) United States Patent WOf USOO6273279B1 (10) Patent No.: (45) Date of Patent: Aug. 14, 2001 (54) GOLF TOWEL HOLDER (76) Inventor: Jerrold M. Wolf, 1036 E. Melody La., Fullerton, CA (US) 92831 (*) Notice:
Funsheet [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015
![Funsheet [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015 Funsheet [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015](/thumbs/93/112589015.jpg) Funsheet 7.0 7.1 [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015 1. Convert the following pressures. a) 101 kpa =? atm b) 55 Torr =? psi c) 60. mmhg =? bar d) 45 Torr =? kpa e) 5 psi =? atm f) 0.0056 atm =?
Funsheet 7.0 7.1 [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015 1. Convert the following pressures. a) 101 kpa =? atm b) 55 Torr =? psi c) 60. mmhg =? bar d) 45 Torr =? kpa e) 5 psi =? atm f) 0.0056 atm =?
World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases
![World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases](/thumbs/96/127526823.jpg) World of Chemistry Notes for Students [Chapter 3, page ] Chapter 3 Gases ) Sec 3.8 Kinetic Theory of Gases and the Nature of Gases The Kinetic Theory of Matter says that the tiny particles in all forms
World of Chemistry Notes for Students [Chapter 3, page ] Chapter 3 Gases ) Sec 3.8 Kinetic Theory of Gases and the Nature of Gases The Kinetic Theory of Matter says that the tiny particles in all forms
Measuring Carbon Dioxide in Breath
 Measuring Carbon Dioxide in Breath OBJECTIVES 1. Measure the partial pressure of carbon dioxide in your breath 2. Estimate the volume of air you exhale per day 3. Estimate the volume and mass of CO2 you
Measuring Carbon Dioxide in Breath OBJECTIVES 1. Measure the partial pressure of carbon dioxide in your breath 2. Estimate the volume of air you exhale per day 3. Estimate the volume and mass of CO2 you
Chemistry Chapter 11 Test Review
 Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States US 201400.07860A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0007860 A1 LU (43) Pub. Date: Jan. 9, 2014 (54) SMOKELESS PORTABLE ROASTER (57) ABSTRACT (76) Inventor: Chien-Chang
(19) United States US 201400.07860A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0007860 A1 LU (43) Pub. Date: Jan. 9, 2014 (54) SMOKELESS PORTABLE ROASTER (57) ABSTRACT (76) Inventor: Chien-Chang
(12) United States Patent (10) Patent No.: US 8,393,587 B2
 US008393.587B2 (12) United States Patent (10) Patent No.: US 8,393,587 B2 Hoernig (45) Date of Patent: *Mar. 12, 2013 (54) BATH FIXTURE MOUNTING SYSTEM (56) References Cited (75) Inventor: Victor Hoernig,
US008393.587B2 (12) United States Patent (10) Patent No.: US 8,393,587 B2 Hoernig (45) Date of Patent: *Mar. 12, 2013 (54) BATH FIXTURE MOUNTING SYSTEM (56) References Cited (75) Inventor: Victor Hoernig,
(51) Int. Cl... F04B 25/00 about 250 F., as will not allow the partial pressure of the
 USOO58860A United States Patent (19) 11 Patent Number: Cunkelman et al. () Date of Patent: Mar 23, 1999 54) THERMOSTATICALLY CONTROLLED 4,362.462 12/1982 Blotenberg... 417/243 X INTERCOOLER SYSTEM FOR
USOO58860A United States Patent (19) 11 Patent Number: Cunkelman et al. () Date of Patent: Mar 23, 1999 54) THERMOSTATICALLY CONTROLLED 4,362.462 12/1982 Blotenberg... 417/243 X INTERCOOLER SYSTEM FOR
States of Matter Review
 States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
Determination of the Gas-Law Constant (R) using CO2
 Determination of the Gas-Law Constant (R) using CO2 EXPERIMENT 11 Prepared by Edward L. Brown and Miranda Raines, Lee University The student will become familiar with ideal gases and how their properties
Determination of the Gas-Law Constant (R) using CO2 EXPERIMENT 11 Prepared by Edward L. Brown and Miranda Raines, Lee University The student will become familiar with ideal gases and how their properties
United States Patent (19)
 United States Patent (19) Yang USOO58394.71A 11 Patent Number: (45) Date of Patent: 5,839,471 Nov. 24, 1998 54). SEALING MEMBER FOR AWALVE 76 Inventor: Tsai Chen Yang, No. 15-11, Tou Ren Lane, Tou Ren
United States Patent (19) Yang USOO58394.71A 11 Patent Number: (45) Date of Patent: 5,839,471 Nov. 24, 1998 54). SEALING MEMBER FOR AWALVE 76 Inventor: Tsai Chen Yang, No. 15-11, Tou Ren Lane, Tou Ren
PRE LABORATORY ASSIGNMENT: Lab Section Score: /10 READ THE LAB TEXT BEFORE ATTEMPTING THESE PROBLEMS!
 EXPERIMENT # 6 Name: PRE LABORATORY ASSIGNMENT: Lab Section Score: /10 READ THE LAB TEXT BEFORE ATTEMPTING THESE PROBLEMS! 1. Calculate the height of a corresponding column of mercury (in mm) that is at
EXPERIMENT # 6 Name: PRE LABORATORY ASSIGNMENT: Lab Section Score: /10 READ THE LAB TEXT BEFORE ATTEMPTING THESE PROBLEMS! 1. Calculate the height of a corresponding column of mercury (in mm) that is at
Chapter 13 Gases. H. Cannon, C. Clapper and T. Guillot Klein High School. Pressure/Temperature Conversions
 Chapter 13 Gases Pressure/Temperature Conversions Convert the following: 1. 3.50 atm = kpa 2. 123 atm = mmhg 3. 970.0 mmhg = torr 4. 870.0 torr = kpa 5. 250.0 kpa = atm 6. 205.0 mmhg = kpa 7. 12.4 atm
Chapter 13 Gases Pressure/Temperature Conversions Convert the following: 1. 3.50 atm = kpa 2. 123 atm = mmhg 3. 970.0 mmhg = torr 4. 870.0 torr = kpa 5. 250.0 kpa = atm 6. 205.0 mmhg = kpa 7. 12.4 atm
58 Field of Search... 36/29, 28, 3 R, the gaps is to equalize the pressure within the module, so that
 US006009637A United States Patent (19) 11 Patent Number: 6,009,637 Pavone (45) Date of Patent: Jan. 4, 2000 54) HELIUM FOOTWEAR SOLE 5,425,184 6/1995 Lyden et al.... 36/29 5,771,606 6/1998 Litchfield et
US006009637A United States Patent (19) 11 Patent Number: 6,009,637 Pavone (45) Date of Patent: Jan. 4, 2000 54) HELIUM FOOTWEAR SOLE 5,425,184 6/1995 Lyden et al.... 36/29 5,771,606 6/1998 Litchfield et
(12) United States Patent
 USOO7690411 B2 (12) United States Patent Wilson (10) Patent No.: (45) Date of Patent: US 7.690,411 B2 Apr. 6, 2010 (54) TIRE PRESSURE CONTROL SYSTEM (76) Inventor: Seth Wilson, 1218 Puerta Del Sol, San
USOO7690411 B2 (12) United States Patent Wilson (10) Patent No.: (45) Date of Patent: US 7.690,411 B2 Apr. 6, 2010 (54) TIRE PRESSURE CONTROL SYSTEM (76) Inventor: Seth Wilson, 1218 Puerta Del Sol, San
Gases and Pressure. Main Ideas
 Gases and Pressure Key Terms pressure millimeters of mercury partial pressure newton atmosphere of pressure Dalton s law of partial pressures barometer pascal In the chapter States of Matter, you read
Gases and Pressure Key Terms pressure millimeters of mercury partial pressure newton atmosphere of pressure Dalton s law of partial pressures barometer pascal In the chapter States of Matter, you read
USOO A United States Patent (19) 11 Patent Number: 5,893,786 Stevens 45 Date of Patent: Apr. 13, 1999
 III IIII USOO589.3786A United States Patent (19) 11 Patent Number: Stevens 45 Date of Patent: Apr. 13, 1999 54 AUTOMATIC TELESCOPING BOUYANT 5,582,127 12/1996 Willis et al.... 116/210 IDENTIFICATION DEVICE
III IIII USOO589.3786A United States Patent (19) 11 Patent Number: Stevens 45 Date of Patent: Apr. 13, 1999 54 AUTOMATIC TELESCOPING BOUYANT 5,582,127 12/1996 Willis et al.... 116/210 IDENTIFICATION DEVICE
CHM 111 Unit 5 Sample Questions
 Name: Class: Date: As you work these problems, consider and explain: A. What type of question is it? B. How do you know what type of question it is? C. What information are you looking for? D. What information
Name: Class: Date: As you work these problems, consider and explain: A. What type of question is it? B. How do you know what type of question it is? C. What information are you looking for? D. What information
United States Patent (19) Kaneko
 United States Patent (19) Kaneko 54 SHOE SOLE FOR RUNNING SHOES 75 Inventor: Yasunori Kaneko, Osaka, Japan 73 Assignee: Mizuno Corporation, Osaka, Japan 21 Appl. No.: 664,016 22 Filed: Jun. 13, 1996 30
United States Patent (19) Kaneko 54 SHOE SOLE FOR RUNNING SHOES 75 Inventor: Yasunori Kaneko, Osaka, Japan 73 Assignee: Mizuno Corporation, Osaka, Japan 21 Appl. No.: 664,016 22 Filed: Jun. 13, 1996 30
Device for atomizing a liquid
 Page 1 of 6 Device for atomizing a liquid Abstract ( 5 of 5 ) United States Patent 4,504,014 Leuning March 12, 1985 A device (10) for atomizing a liquid is disclosed. The device (10) includes a sprayhead
Page 1 of 6 Device for atomizing a liquid Abstract ( 5 of 5 ) United States Patent 4,504,014 Leuning March 12, 1985 A device (10) for atomizing a liquid is disclosed. The device (10) includes a sprayhead
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT.
 Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
4/26/16. Section 1 Understanding Solutions. Solutions and Suspensions. Solutions and Suspensions. Solutions and Suspensions. Solvents and Solutes
 Section 1 Understanding Solutions On a hot day, you may think that a cool glass of plain water would be refreshing. However, if the glass were actually filled with plain water, it would taste stale. This
Section 1 Understanding Solutions On a hot day, you may think that a cool glass of plain water would be refreshing. However, if the glass were actually filled with plain water, it would taste stale. This
PREPARING GAS SAMPLES IN LARGE PLASTIC SYRINGES
 PREPARING GAS SAMPLES IN LARGE PLASTIC SYRINGES A wide variety of gases can be prepared safely inside a 60 ml plastic syringe. Here you will practice making carbon dioxide, so that you know the technique
PREPARING GAS SAMPLES IN LARGE PLASTIC SYRINGES A wide variety of gases can be prepared safely inside a 60 ml plastic syringe. Here you will practice making carbon dioxide, so that you know the technique
Generating Calibration Gas Standards
 Technical Note 1001 Metronics Inc. Generating Calibration Gas Standards with Dynacal Permeation Devices Permeation devices provide an excellent method of producing known gas concentrations in the PPM and
Technical Note 1001 Metronics Inc. Generating Calibration Gas Standards with Dynacal Permeation Devices Permeation devices provide an excellent method of producing known gas concentrations in the PPM and
(12) United States Patent (10) Patent No.: US 6,426,434 B1
 USOO6426434B1 (12) United States Patent (10) Patent No.: Yoshida et al. () Date of Patent: Jul. 30, 2002 (54) PROCESS FOR THE SYNTHESIS OF UREA JP 62-170 4/1987 JP 63-12 5/1988 (75) Inventors: Kinichi
USOO6426434B1 (12) United States Patent (10) Patent No.: Yoshida et al. () Date of Patent: Jul. 30, 2002 (54) PROCESS FOR THE SYNTHESIS OF UREA JP 62-170 4/1987 JP 63-12 5/1988 (75) Inventors: Kinichi
Problem Solving. Gas Laws
 Skills Worksheet Problem Solving Gas Laws Chemists found that there were relationships among temperature, volume, pressure, and quantity of a gas that could be described mathematically. This chapter deals
Skills Worksheet Problem Solving Gas Laws Chemists found that there were relationships among temperature, volume, pressure, and quantity of a gas that could be described mathematically. This chapter deals
The Determination of the Value for Molar Volume
 Objective The Determination of the Value for Molar Volume Using a chemical reaction that produces a gas, measure the appropriate values to allow a determination of the value for molar volume. Brief Overview
Objective The Determination of the Value for Molar Volume Using a chemical reaction that produces a gas, measure the appropriate values to allow a determination of the value for molar volume. Brief Overview
United States Patent (19)
 United States Patent (19) Oranje (54) DEVICE FOR SEPARATING LIQUIDS AND/OR SOLIDS FROMA HIGH-PRESSURE GAS STREAM 75 inventor: Leendert Oranje, Haren, Netherlands (73) Assignee: N.V. Nederlandse Gasunie,
United States Patent (19) Oranje (54) DEVICE FOR SEPARATING LIQUIDS AND/OR SOLIDS FROMA HIGH-PRESSURE GAS STREAM 75 inventor: Leendert Oranje, Haren, Netherlands (73) Assignee: N.V. Nederlandse Gasunie,
US A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/ A1 Islam et al. (43) Pub. Date: May 22, 2008
 US 20080119574A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0119574 A1 Islam et al. (43) Pub. Date: May 22, 2008 (54) RECOVERY OF ORGANIC COMPOUNDS Related US. Application
US 20080119574A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0119574 A1 Islam et al. (43) Pub. Date: May 22, 2008 (54) RECOVERY OF ORGANIC COMPOUNDS Related US. Application
58) Field of Search... 43/17, 175 provided therein with a green and a red lights, batteries, a
 I USOO5615512A United States Patent (19) 11 Patent Number: Wang 45) Date of Patent: Apr. 1, 1997 54 FLOAT WITH LIGHT INDICATORS 5,351,432 10/1994 Tse... 43/17.5 (76) Inventor: Yi-Chang Wang, No. 43, Chung
I USOO5615512A United States Patent (19) 11 Patent Number: Wang 45) Date of Patent: Apr. 1, 1997 54 FLOAT WITH LIGHT INDICATORS 5,351,432 10/1994 Tse... 43/17.5 (76) Inventor: Yi-Chang Wang, No. 43, Chung
Name Chemistry Pre-AP
 Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
You should be able to: Describe Equipment Barometer Manometer. 5.1 Pressure Read and outline 5.1 Define Barometer
 A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
General Chemistry I Percent Yield of Hydrogen Gas From Magnesium and HCl
 Introduction For chemical reactions involving gases, gas volume measurements provide a convenient means of determining stoichiometric relationships. A gaseous product is collected in a long, thin graduated
Introduction For chemical reactions involving gases, gas volume measurements provide a convenient means of determining stoichiometric relationships. A gaseous product is collected in a long, thin graduated
Fish Farming Water Quality Test Kit
 DOC326.97.00098 Fish Farming Water Quality Test Kit FF-1A (243002) 11/2017, Edition 1 User Manual Table of Contents General information... 3 Safety information... 3 Use of hazard information...3 Product
DOC326.97.00098 Fish Farming Water Quality Test Kit FF-1A (243002) 11/2017, Edition 1 User Manual Table of Contents General information... 3 Safety information... 3 Use of hazard information...3 Product
Boyle s Law Practice
 Boyle s Law Practice Boyle s Law is an indirect relationship. Most of these problems can be done in your head without showing your work. 1. Herman has 30.0 L of helium gas trapped in a cylinder by a piston.
Boyle s Law Practice Boyle s Law is an indirect relationship. Most of these problems can be done in your head without showing your work. 1. Herman has 30.0 L of helium gas trapped in a cylinder by a piston.
Name Hour. The Behavior of Gases. Practice B
 Name Hour The Behavior of Gases Practice B B 1 Objective 1: Apply Boyle s Law, Charles s Law, and Gay-Lussac s Law to solve problems involving pressure and volume and temperature. 1. A high-altitude balloon
Name Hour The Behavior of Gases Practice B B 1 Objective 1: Apply Boyle s Law, Charles s Law, and Gay-Lussac s Law to solve problems involving pressure and volume and temperature. 1. A high-altitude balloon
Please do not write on this test. Please use the answer sheet. 1) Please choose all conditions that would allow a gas sample to behave ideally.
 AP Chemistry Test (Chapter 5) Please do not write on this test. Please use the answer sheet. Multiple Choice (50%) 1) Please choose all conditions that would allow a gas sample to behave ideally. I) Nonpolar
AP Chemistry Test (Chapter 5) Please do not write on this test. Please use the answer sheet. Multiple Choice (50%) 1) Please choose all conditions that would allow a gas sample to behave ideally. I) Nonpolar
Chemistry Honors - Gases
 Name: Class: Date: ID: A Chemistry Honors - Gases Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Why does a can collapse when a vacuum pump removes air
Name: Class: Date: ID: A Chemistry Honors - Gases Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Why does a can collapse when a vacuum pump removes air
(12) United States Patent (10) Patent No.: US 6,880,421 B2. Watanabe et al. (45) Date of Patent: Apr. 19, 2005
 USOO688.0421B2 (12) United States Patent (10) Patent No.: US 6,880,421 B2 Watanabe et al. (45) Date of Patent: Apr. 19, 2005 (54) ROLLED BALL SCREW AND METHOD FOR (56) References Cited ROLLING BALL SCREW
USOO688.0421B2 (12) United States Patent (10) Patent No.: US 6,880,421 B2 Watanabe et al. (45) Date of Patent: Apr. 19, 2005 (54) ROLLED BALL SCREW AND METHOD FOR (56) References Cited ROLLING BALL SCREW
Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases
![Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases](/thumbs/78/77082586.jpg) Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] Chapter 10 Gases We have talked a little about gases in Chapter 3 and we dealt briefly with them in our stoichiometric calculations in
Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] Chapter 10 Gases We have talked a little about gases in Chapter 3 and we dealt briefly with them in our stoichiometric calculations in
United States Patent (19)
 United States Patent (19) Chatigny 54) SAFETY DEVICE FOR FIREARMS 75 Inventor: Raymond E. Chatigny, Westminster, Mass. 73) Assignee: Harrington & Richardson, Inc., Gardner, Mass. 22 Filed: Sept. 16, 1974
United States Patent (19) Chatigny 54) SAFETY DEVICE FOR FIREARMS 75 Inventor: Raymond E. Chatigny, Westminster, Mass. 73) Assignee: Harrington & Richardson, Inc., Gardner, Mass. 22 Filed: Sept. 16, 1974
Gas Laws. Figure 1: Experimental Set-up with Leveling Bulb. GCC CHM 151LL: Gas Laws GCC, 2019 page 1 of 8
 Gas Laws Introduction Although we cannot see gases, we can observe their behavior and study their properties. This lab will apply several concepts from Ideal Gas Laws. You will use your knowledge of chemical
Gas Laws Introduction Although we cannot see gases, we can observe their behavior and study their properties. This lab will apply several concepts from Ideal Gas Laws. You will use your knowledge of chemical
. United States Patent (19) Oonuma et al.
 . United States Patent (19) Oonuma et al. 54) BUFFER DEVICE FOR A ROLLER CHAN AND SPROCKET COUPLING (75) Inventors: Koichiro Oonuma, Shiki; Yoshinori ' Kawashima, Sakado; Toshinori Hanai, Kamifukuoka,
. United States Patent (19) Oonuma et al. 54) BUFFER DEVICE FOR A ROLLER CHAN AND SPROCKET COUPLING (75) Inventors: Koichiro Oonuma, Shiki; Yoshinori ' Kawashima, Sakado; Toshinori Hanai, Kamifukuoka,
Please do not write on this test. Please use the answer sheet.
 AP Chemistry Test (Chapter 5) Multiple Choice (50%) Please do not write on this test. Please use the answer sheet. Form: Snowboard 1) A sample of argon gas is sealed in a rigid metal tank. The temperature
AP Chemistry Test (Chapter 5) Multiple Choice (50%) Please do not write on this test. Please use the answer sheet. Form: Snowboard 1) A sample of argon gas is sealed in a rigid metal tank. The temperature
Completed ALL 2 Warm-up IC Kinetic Molecular Theory Notes. Kinetic Molecular Theory and Pressure Worksheet
 Name: Unit 10- Gas Laws Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 5 Kinetic Molecular Theory Notes IC 1 6 8 Kinetic Molecular Theory and Pressure Worksheet IC 2 9 10 Gas Law
Name: Unit 10- Gas Laws Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 5 Kinetic Molecular Theory Notes IC 1 6 8 Kinetic Molecular Theory and Pressure Worksheet IC 2 9 10 Gas Law
(12) United States Patent (10) Patent No.: US 6,524,267 B1
 USOO6524267B1 (12) United States Patent (10) Patent No.: US 6,524,267 B1 Gremel et al. 45) Date of Patent: Feb. 25, 2003 9 (54) VENOUS FILTER FOR ASSISTED VENOUS (56) References Cited RETUR N U.S. PATENT
USOO6524267B1 (12) United States Patent (10) Patent No.: US 6,524,267 B1 Gremel et al. 45) Date of Patent: Feb. 25, 2003 9 (54) VENOUS FILTER FOR ASSISTED VENOUS (56) References Cited RETUR N U.S. PATENT
Analysis of a KClO3 Mixture and Determination of R
 Experiment 10 Analysis of a KClO3 Mixture and Determination of R Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise.
Experiment 10 Analysis of a KClO3 Mixture and Determination of R Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise.
Accelerated Chemistry Study Guide Chapter 13: Gases
 Accelerated Chemistry Study Guide Chapter 13: Gases Terms, definitions, topics Diffusion Kinetic Molecular Theory Atmospheric pressure Barometer Manometer STP Absolute zero Page 1 of 42 Molar volume Partial
Accelerated Chemistry Study Guide Chapter 13: Gases Terms, definitions, topics Diffusion Kinetic Molecular Theory Atmospheric pressure Barometer Manometer STP Absolute zero Page 1 of 42 Molar volume Partial
Surface Area: the smaller the particles, the the rate of solubility.
 1. What is a solution? 2. What are the parts of a solution? (Ex. Lemonade) a. Solute: Ex. b. Solvent: Ex. 3. What is Solubility? a. Soluble: b. Insoluble: 4. Factors that Affect the Rate of Solubility:
1. What is a solution? 2. What are the parts of a solution? (Ex. Lemonade) a. Solute: Ex. b. Solvent: Ex. 3. What is Solubility? a. Soluble: b. Insoluble: 4. Factors that Affect the Rate of Solubility:
REASONS FOR NATURAL VARIATIONS IN DISSOLVED OXYGEN LEVELS
 Period Date LAB. THE PHYSICAL PROPERTIES OF WATER: DISSOLVED OXYGEN In an aquatic environment, oxygen must be in a solution in a free state (O 2 ) before it is available for use by organisms (bioavailable).
Period Date LAB. THE PHYSICAL PROPERTIES OF WATER: DISSOLVED OXYGEN In an aquatic environment, oxygen must be in a solution in a free state (O 2 ) before it is available for use by organisms (bioavailable).
United States Patent (19) Herro
 United States Patent (19) Herro (54) (76) (22 21 ) 52) (51) 58 (56) ATHLETIC SHOE WITH A DETACHABLE SOLE Inventor: Richard E. Herro, Rte. 5, Mound View Estates, Joliet, Ill. 60436 Filed: Jan. 21, 1976
United States Patent (19) Herro (54) (76) (22 21 ) 52) (51) 58 (56) ATHLETIC SHOE WITH A DETACHABLE SOLE Inventor: Richard E. Herro, Rte. 5, Mound View Estates, Joliet, Ill. 60436 Filed: Jan. 21, 1976
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States US 20090005197A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0005197 A1 Mayer (43) Pub. Date: Jan. 1, 2009 (54) HOCKEY STICK HAVING AN ANGLED (52) U.S. Cl.... 473/560;
(19) United States US 20090005197A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0005197 A1 Mayer (43) Pub. Date: Jan. 1, 2009 (54) HOCKEY STICK HAVING AN ANGLED (52) U.S. Cl.... 473/560;
Blade guard for rotary lawn mowers
 Iowa State University Patents Iowa State University Research Foundation, Inc. 7-26-1977 Blade guard for rotary lawn mowers Wesley F. Buchele Iowa State University William I. Baldwin Iowa State University
Iowa State University Patents Iowa State University Research Foundation, Inc. 7-26-1977 Blade guard for rotary lawn mowers Wesley F. Buchele Iowa State University William I. Baldwin Iowa State University
Date: Period: Gas Laws Worksheet #1 - Boyle s, Charles, Gay-Lussac s, and Combined Gas Law
 Name: Date: Period: Gas Laws Worksheet #1 - Boyle s, Charles, Gay-Lussac s, and Combined Gas Law Boyle s Law: V1P1 = V2P2 1. A gas sample contained in a cylinder equipped with a moveable piston occupied
Name: Date: Period: Gas Laws Worksheet #1 - Boyle s, Charles, Gay-Lussac s, and Combined Gas Law Boyle s Law: V1P1 = V2P2 1. A gas sample contained in a cylinder equipped with a moveable piston occupied
(12) United States Patent (10) Patent No.: US 6,368,227 B1
 USOO6368227B1 (12) United States Patent (10) Patent No.: US 6,368,227 B1 Olson (45) Date of Patent: Apr. 9, 2002 (54) METHOD OF SWINGING ON A SWING 5,413.298 A * 5/1995 Perreault... 248/228 (76) Inventor:
USOO6368227B1 (12) United States Patent (10) Patent No.: US 6,368,227 B1 Olson (45) Date of Patent: Apr. 9, 2002 (54) METHOD OF SWINGING ON A SWING 5,413.298 A * 5/1995 Perreault... 248/228 (76) Inventor:
United States Patent (19) Widecrantz et al.
 United States Patent (19) Widecrantz et al. 54 76 22) 21 (52) (51) 58 56 ONE WAY WALVE PRESSURE PUMP TURBINE GENERATOR STATION Inventors: Kaj Widecrantz, P.O. Box 72; William R. Gatton, P.O. Box 222, both
United States Patent (19) Widecrantz et al. 54 76 22) 21 (52) (51) 58 56 ONE WAY WALVE PRESSURE PUMP TURBINE GENERATOR STATION Inventors: Kaj Widecrantz, P.O. Box 72; William R. Gatton, P.O. Box 222, both
CHEMISTRY - CLUTCH CH.5 - GASES.
 !! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
!! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
 US 2013 0186486A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0186486A1 Ding (43) Pub. Date: Jul. 25, 2013 (54) SYSTEM FOR AND METHOD OF (52) U.S. Cl. MONITORING FLOW
US 2013 0186486A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0186486A1 Ding (43) Pub. Date: Jul. 25, 2013 (54) SYSTEM FOR AND METHOD OF (52) U.S. Cl. MONITORING FLOW
PREPARATION AND PROPERTIES OF ATMOSPHERIC GASES I: Carbon Dioxide and Oxygen
 PREPARATION AND PROPERTIES OF ATMOSPHERIC GASES I: Carbon Dioxide and Oxygen.... :O = C = O:.... :O = O: INTRODUCTION The atmosphere consists predominantly of three gases -- nitrogen (N 2 ) 78%, oxygen
PREPARATION AND PROPERTIES OF ATMOSPHERIC GASES I: Carbon Dioxide and Oxygen.... :O = C = O:.... :O = O: INTRODUCTION The atmosphere consists predominantly of three gases -- nitrogen (N 2 ) 78%, oxygen
States of Matter. Q 7. Calculate the average of kinetic energy, in joules of the molecules in 8.0 g of methane at 27 o C. (IIT JEE Marks)
 Q 1. States of Matter Calculate density of NH 3 at 30 o C and 5 atm pressure Q 2. (IIT JEE 1978 3 Marks) 3.7 g of a gas at 25 o C occupied the same volume as 0.184g of hydrogen at 17 o C and at the same
Q 1. States of Matter Calculate density of NH 3 at 30 o C and 5 atm pressure Q 2. (IIT JEE 1978 3 Marks) 3.7 g of a gas at 25 o C occupied the same volume as 0.184g of hydrogen at 17 o C and at the same
A SIMPLE TITRATION METHOD FOR DETERMINING THE SPECIFIC GRAVITY ON ONE DROP OF URINE
 J. clin. Path. (1951), 4, 491. A SIMPLE TITRATION METHOD FOR DETERMINING THE SPECIFIC GRAVITY ON ONE DROP OF URINE BY From the Pathological Laboratory, the Peace Memorial Hospital, Watford (RECEIVED FOR
J. clin. Path. (1951), 4, 491. A SIMPLE TITRATION METHOD FOR DETERMINING THE SPECIFIC GRAVITY ON ONE DROP OF URINE BY From the Pathological Laboratory, the Peace Memorial Hospital, Watford (RECEIVED FOR
Figure Vapor-liquid equilibrium for a binary mixture. The dashed lines show the equilibrium compositions.
 Another way to view this problem is to say that the final volume contains V m 3 of alcohol at 5.93 kpa and 20 C V m 3 of air at 94.07 kpa and 20 C V m 3 of air plus alcohol at 100 kpa and 20 C Thus, the
Another way to view this problem is to say that the final volume contains V m 3 of alcohol at 5.93 kpa and 20 C V m 3 of air at 94.07 kpa and 20 C V m 3 of air plus alcohol at 100 kpa and 20 C Thus, the
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 US 2004O126242A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0126242 A1 Howard et al. (43) Pub. Date: Jul. 1, 2004 (54) BOAT PROPELLER AND GUARD DEVICE (52) U.S. Cl....
US 2004O126242A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0126242 A1 Howard et al. (43) Pub. Date: Jul. 1, 2004 (54) BOAT PROPELLER AND GUARD DEVICE (52) U.S. Cl....
United States Patent (19)
 United States Patent (19) Crump 11 Patent Number: Date of Patent: Apr. 3, 1990 54 ADJUSTABLE HEIGHT WHEELCHAIR RAMP WITHSUPPORTING LEGS 76 Inventor: 21 22 (51) (52 58 (56) Robert Crump, 333 Guthrie Rd.,
United States Patent (19) Crump 11 Patent Number: Date of Patent: Apr. 3, 1990 54 ADJUSTABLE HEIGHT WHEELCHAIR RAMP WITHSUPPORTING LEGS 76 Inventor: 21 22 (51) (52 58 (56) Robert Crump, 333 Guthrie Rd.,
acrolein, acetaldehyde and acetone( cm -1 ); methanol (1306 cm -1 ); ethylene (949 cm -1 ); and isoprene (893 cm -1 ).
 acrolein, acetaldehyde and acetone(1550 1800 cm -1 ); methanol (1306 cm -1 ); ethylene (949 cm -1 ); and isoprene (893 cm -1 ). 5 Figure 4a 6 Figure 4b Figure 4c 7 Figure 5 Questions in Student Handout
acrolein, acetaldehyde and acetone(1550 1800 cm -1 ); methanol (1306 cm -1 ); ethylene (949 cm -1 ); and isoprene (893 cm -1 ). 5 Figure 4a 6 Figure 4b Figure 4c 7 Figure 5 Questions in Student Handout
Each gas sample has the same A) density B) mass C) number of molecules D) number of atoms
 1. A real gas behaves most like an ideal gas at A) low pressure and high temperature B) average potential energy of its particles C) ionization energy of its particles D) activation energy of its particles
1. A real gas behaves most like an ideal gas at A) low pressure and high temperature B) average potential energy of its particles C) ionization energy of its particles D) activation energy of its particles
Chemistry 1B Chapter 10 Worksheet - Daley. Name
 Name 1) The National Weather Service routinely supplies atmospheric pressure data to help pilots set their altimeters. The units the NWS uses for atmospheric pressure are inches of mercury. A barometric
Name 1) The National Weather Service routinely supplies atmospheric pressure data to help pilots set their altimeters. The units the NWS uses for atmospheric pressure are inches of mercury. A barometric
United States Patent (19)
 United States Patent (19) Hepworth et al. 54 CARRYING CASE FOR FLY FISHING ROD AND REEL 76 Inventors: Allen Hepworth; Gordon Smith, both of Port Moody; Walter Johb, Surrey, all of Canada (21) Appl. No.:
United States Patent (19) Hepworth et al. 54 CARRYING CASE FOR FLY FISHING ROD AND REEL 76 Inventors: Allen Hepworth; Gordon Smith, both of Port Moody; Walter Johb, Surrey, all of Canada (21) Appl. No.:
Chemistry HP Unit 6 Gases. Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases
 Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Wang 45 Date of Patent: Sep. 23, 1997
 US005669536A United States Patent (19) 11 Patent Number: Wang 45 Date of Patent: Sep. 23, 1997 54 DEVICE FOR LOCATING SHACKLE LOCK 5,127,562 7/1992 Zane et al...... 224/935 ON BICYCLE FRAME 5,386,961 2/1995
US005669536A United States Patent (19) 11 Patent Number: Wang 45 Date of Patent: Sep. 23, 1997 54 DEVICE FOR LOCATING SHACKLE LOCK 5,127,562 7/1992 Zane et al...... 224/935 ON BICYCLE FRAME 5,386,961 2/1995
Illllllllllllllllllllllllllllllllllllllllllllllllllllllll l lll l l ll l
 Illllllllllllllllllllllllllllllllllllllllllllllllllllllll l lll l l ll l USOO562018OA United States Patent [191 [11] Patent Number: 5,620,180 Hong [45] Date of Patent: Apr. 15, 1997 [54] GRIP OF BADMINTON
Illllllllllllllllllllllllllllllllllllllllllllllllllllllll l lll l l ll l USOO562018OA United States Patent [191 [11] Patent Number: 5,620,180 Hong [45] Date of Patent: Apr. 15, 1997 [54] GRIP OF BADMINTON
(12) United States Patent (10) Patent No.: US 7,730,548 B1
 USOO77548B1 (12) United States Patent () Patent No.: US 7,7,548 B1 McCraney (45) Date of Patent: Jun. 8, 20 (54) BALLISTICSVEST PAD COVER 5,127,5 A 7, 1992 Sacks 5,471,906 A 12/1995 Bachner, Jr. et al.
USOO77548B1 (12) United States Patent () Patent No.: US 7,7,548 B1 McCraney (45) Date of Patent: Jun. 8, 20 (54) BALLISTICSVEST PAD COVER 5,127,5 A 7, 1992 Sacks 5,471,906 A 12/1995 Bachner, Jr. et al.
The grade 6 English science unit, Gases, meets the academic content standards set in the Korean curriculum, which state students should:
 This area deals with the properties of gases as small collections of particles. Different kinds of intangible and invisible gases attract students curiosity and promote their use of reasoning skills. Students
This area deals with the properties of gases as small collections of particles. Different kinds of intangible and invisible gases attract students curiosity and promote their use of reasoning skills. Students
United States Patent (19) Salandre
 United States Patent (19) Salandre 11) Patent Number: 45) Date of Patent: Jun. 19, 1990 (54 HANDGUN HOLSTER AND RETENTION APPARATUS 76 Inventor: Stephen M. Salandre, P.O. Box 130, Taos, N. Mex. 87571 (21)
United States Patent (19) Salandre 11) Patent Number: 45) Date of Patent: Jun. 19, 1990 (54 HANDGUN HOLSTER AND RETENTION APPARATUS 76 Inventor: Stephen M. Salandre, P.O. Box 130, Taos, N. Mex. 87571 (21)
The Ideal Gas Constant
 Chem 2115 Experiment # 8 The Ideal Gas Constant OBJECTIVE: This experiment is designed to provide experience in gas handling methods and experimental insight into the relationships between pressure, volume,
Chem 2115 Experiment # 8 The Ideal Gas Constant OBJECTIVE: This experiment is designed to provide experience in gas handling methods and experimental insight into the relationships between pressure, volume,
Multiple Choice (40%)
 AP Chemistry Test (Chapter 5) Please do not write on this test thank you! Multiple Choice (40%) 1) A sealed rigid container is filled with three ideal gases: A, B and C. The partial pressure of each gas
AP Chemistry Test (Chapter 5) Please do not write on this test thank you! Multiple Choice (40%) 1) A sealed rigid container is filled with three ideal gases: A, B and C. The partial pressure of each gas
United States Patent (19)
 United States Patent (19) Ledbetter 54 GRIPPING ASSEMBLY FOR USE WITH CABLE EXERCISINGEQUIPMENT (76) Inventor: Daniel R. Ledbetter, 231 E. 18th St.-hC, Costa Mesa, Calif. 92627 (21) Appl. No.: 38,050 (22
United States Patent (19) Ledbetter 54 GRIPPING ASSEMBLY FOR USE WITH CABLE EXERCISINGEQUIPMENT (76) Inventor: Daniel R. Ledbetter, 231 E. 18th St.-hC, Costa Mesa, Calif. 92627 (21) Appl. No.: 38,050 (22
The Decomposition of Potassium Chlorate
 The Decomposition of Potassium Chlorate Small quantities of molecular oxygen (O 2 ) can be obtained from the thermal decomposition of certain oxides, peroxides, and salts of oxoacids. Some examples of
The Decomposition of Potassium Chlorate Small quantities of molecular oxygen (O 2 ) can be obtained from the thermal decomposition of certain oxides, peroxides, and salts of oxoacids. Some examples of
TEPZZ 7 Z67A_T EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION
 (19) TEPZZ 7 Z67A_T (11) EP 2 711 067 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 26.03.14 Bulletin 14/13 (21) Application number: 12188.1 (1) Int Cl.: B01D 3/60 (06.01) B01D 3/92 (06.01)
(19) TEPZZ 7 Z67A_T (11) EP 2 711 067 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 26.03.14 Bulletin 14/13 (21) Application number: 12188.1 (1) Int Cl.: B01D 3/60 (06.01) B01D 3/92 (06.01)
AP Biology 12 Cellular Respiration Lab
 AP Biology 12 Cellular Respiration Lab Background: Each individual cell is responsible for the energy exchanges necessary to sustain its ordered structure. Cells accomplish this task by breaking down nutrient
AP Biology 12 Cellular Respiration Lab Background: Each individual cell is responsible for the energy exchanges necessary to sustain its ordered structure. Cells accomplish this task by breaking down nutrient
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
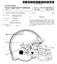 US 20110214225A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0214225A1 Norris (43) Pub. Date: Sep. 8, 2011 (54) LATERAL VISION FOOTBALL HELMET (52) U.S. Cl.... 2A425 (76)
US 20110214225A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0214225A1 Norris (43) Pub. Date: Sep. 8, 2011 (54) LATERAL VISION FOOTBALL HELMET (52) U.S. Cl.... 2A425 (76)
A. What are the three states of matter chemists work with?
 Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
United States Patent 19 Carver
 United States Patent 19 Carver 11) 4,290,300 45) Sep. 22, 1981 54 76 21 22 (51) (52) (58) SUCROSE DENSITY GRADIENT SYSTEM Inventor: Appl. No.: 952,283 Joseph Carver, 31 West Blvd., East Rockaway, N.Y.
United States Patent 19 Carver 11) 4,290,300 45) Sep. 22, 1981 54 76 21 22 (51) (52) (58) SUCROSE DENSITY GRADIENT SYSTEM Inventor: Appl. No.: 952,283 Joseph Carver, 31 West Blvd., East Rockaway, N.Y.
(12) United States Patent (10) Patent No.: US 6,641,487 B1
 USOO6641487B1 (12) United States Patent (10) Patent No.: US 6,641,487 B1 Hamburger (45) Date of Patent: Nov. 4, 2003 (54) ADJUSTABLY WEIGHTED GOLF CLUB 4,872,684. A 10/1989 Dippel PUTTER HEAD WITH REMOVABLE
USOO6641487B1 (12) United States Patent (10) Patent No.: US 6,641,487 B1 Hamburger (45) Date of Patent: Nov. 4, 2003 (54) ADJUSTABLY WEIGHTED GOLF CLUB 4,872,684. A 10/1989 Dippel PUTTER HEAD WITH REMOVABLE
(12) United States Patent (10) Patent No.: US 6,456,197 B1
 USOO6456197B1 (12) United States Patent (10) Patent No.: Lauritsen et al. (45) Date of Patent: Sep. 24, 2002 (54) OIL-IN-WATER DETECTOR BUOY (56) References Cited ARRANGEMENT U.S. PATENT DOCUMENTS (75)
USOO6456197B1 (12) United States Patent (10) Patent No.: Lauritsen et al. (45) Date of Patent: Sep. 24, 2002 (54) OIL-IN-WATER DETECTOR BUOY (56) References Cited ARRANGEMENT U.S. PATENT DOCUMENTS (75)
CTB3365x Introduction to Water Treatment
 CTB3365x Introduction to Water Treatment D4b Aeration Doris van Halem Did you know that there are not just gasses in your glass of sparkling coke, but also in the tap water you drink? Welcome to the water
CTB3365x Introduction to Water Treatment D4b Aeration Doris van Halem Did you know that there are not just gasses in your glass of sparkling coke, but also in the tap water you drink? Welcome to the water
