WARNING: All medications should be closely monitored to detect any alterations in the effective concentration due to the ultrafiltration process.
|
|
|
- Cecil Nash
- 5 years ago
- Views:
Transcription
1 HEMOBAG BSD2000 Product Code: HBBSD2000 Caution: Federal (USA) law restricts this device to sale by or on the order of a physician Product Description The patented HEMOBAG autotransfusion device is a blood storage reservoir for blood that can be circulated, hemoconcentrated and transfused back to the same patient as a form of autologous whole blood management and conservation. It is designed for salvaging anticoagulated whole blood from cardiopulmonary bypass circuits and other extracorporeal circuits and reservoirs, and can be hemoconcentrated by ultrafiltration. Indications for Use: (FOR SINGLE USE ONLY, DO NOT RESTERILIZE) The HEMOBAG Blood Salvage Device is a blood storage reservoir for blood that can be circulated and hemoconcentrated. It is designed for use in salvaging anticoagulated whole blood from extracorporeal circuits and reservoirs. When used in connection with the TS3 Tubing Set and a commercially available hemoconcentrator, the HEMOBAG serves as a hemoconcentrating reservoir for use in a closed circuit recovery loop for the removal of excess plasma water, low molecular weight solutes and platelet inhibitors. This patented processing method concentrates the diluted anticoagulated whole blood within the closed circulatory recovery loop of the TS3 Tubing Set and the HEMOBAG blood salvage device producing a richly concentrated, anticoagulated, autologous whole blood product for autotransfusion that is quickly made available for Infusion. The HEMOBAG is designed only for use with TS3 Tubing set and any commercially available hemoconcentrator with universal dialysis connectors or ¼ outlet posts. Contraindications The HEMOBAG is designed and sold for use only as indicated. The responsibility for the use of this device belongs solely to the prescribing physician. WARNING: Protamine is specifically contraindicated for use within this device. Other medications (i.e., anticoagulants) should be monitored to ensure Activated Clotting Times are satisfactory for anticoagulation. The responsibility for levels and adjustments of medications in the circulatory path of these products, HEMOBAG, TS3 Tubing Set, and any associated hemoconcentrator is that of the prescribing physician. WARNING: All medications should be closely monitored to detect any alterations in the effective concentration due to the ultrafiltration process. General Warnings Read all instructions and warnings before use. Specific warnings related to the set up and use of this device or system are in Bold print in the various sections of these instructions. Specific attention must be devoted to understanding these warnings. Use of this product is limited by law (USA) to supervision by a licensed medical practitioner. Product must be set up and utilized by personnel trained in the use of extracorporeal and cardiopulmonary circuits, devices and reservoirs. All gas bubbles have the potential of becoming gaseous emboli, and therefore must be dealt with carefully. Remove all gaseous bubbles from the HEMOBAG before hemoconcentrating and gravity infusing the blood. Product is Sterile and Non-Pyrogenic if package is sealed and undamaged. Do not use if the package is damaged or opened as this may lead to harm to the patient due to contamination. Connector covers should remain on device until time of connection. Single Use Only! Reuse of the product may cause harm to the patient due to contamination. Do not Re-sterilize. Removed wording and inserted wording nest page about Avoid Temperatures above 50 etc Autologous blood is a very precious substance; therefore, once the HEMOBAG has been filled it must be handled with care (Autologous whole blood is considered to be the most precious substance on the earth). Dispose of product in accordance with OSHA Regulations for Bloodborne Pathogens. Page 1 of 5 DFU HBBSD /2017
2 Avoid contact with halogenated hydrocarbon based anesthetic agents as they attack plastics. Do not store blood in the HEMOBAG for extended periods of time. It is recommended to following the AABB Guidance for Standards for Perioperative Autologous Blood Collection and Administration when using this device. Note: When returning heparinized concentrated whole blood back to the patient, it may be necessary to administer additional Protamine to the patient to reverse the effects of the heparin. General Precaution Avoid exposure to temperatures above 50 C, or below 0 C. Store in a clean, dry location. Instructions for Use Filling the HEMOBAG (Step by Step) Schematics of Product - Figure #1. The numbers on the schematic correspond with the numbers noted below in the instructions for use. Precaution: Use aseptic technique when making all connections. Verify package has not been damaged or opened. Do not use if package is damaged or open. Open the HEMOBAG onto a sterile field using aseptic techniques. Once the package has been opened, protect all connections by placing leash caps and plugs in or on the appropriate connectors. Assure all other caps and clamps of the HEMOBAG are closed. Open the top Arterial Infusion Port 2H by sliding the large white Slide Clamp or Master Clip 3H and removing the Green Cap 4H. The HEMOBAG is ready to be filled by way of the sterile field after the Arterial or forward flow line is removed from the patient and free (make sure that all slide clamps are securely closed). Before pumping the blood into the HEMOBAG, drain all blood-filled lines (i.e., venous line, cardioplegia lines, manifold and sampling lines, etc.) back to the extracorporeal circuit s reservoir. Attach the arterial or forward flow line (or drainage port) of the extracorporeal circuit to the ¼ to 3/8 stepped Universal Arterial Infusion Connector 1H (Port with the Green Cap) at the top of the HEMOBAG. Blood from the extracorporeal circuit or reservoir is flushed (or drained) into the HEMOBAG using crystalloid solution to displace the blood in the extracorporeal circuit. The crystalloid solution is kept in the extracorporeal circuit in order to keep it primed and ready for use. The HEMOBAG will hold a maximum of 2000 milliliters of fluid from any extracorporeal circuit. WARNING: DO NOT OVERFILL the HEMOBAG. Maximum volume of HEMOBAG is 2000 milliliters of fluid. Graduation markings on the HEMOBAG represent approximate volumes and should not be used to measure the exact volume of fluid. When the HEMOBAG has been filled (2000 ml Maximum) and the extracorporeal circuit has been primed with crystalloid solution, clamp off the arterial or forward flow line with a tubing clamp and close the HEMOBAG using the Master Clip 3H. Detach the fill line from the Arterial Infusion Port 1H of the HEMOBAG. Purge the air from the HEMOBAG, close the Master Clip 3H tightly and firmly place the Green Cap 4H onto the Arterial Infusion Port. Wipe the exterior of the bag clean if needed. The HEMOBAG is now a closed, sterile reservoir of blood that can be carefully connected to the closed hemoconcentrating recovery loop consisting of the HEMOBAG, the TS3 Tubing Set, a hemoconcentrator, a ¼ inch recirculating pump that is not in use at the time and a pressure transducer. Instructions for Use withts3 Tubing Set (Step by Step) Hang the HEMOBAG using the Master Clip 3H at the top of the bag carefully. A QC sample may be drawn at this time from the needle-less sampling port for baseline lab values Note: Before connecting the HEMOBAG to the TS3 Tubing Set, ensure the TS3 Tubing Set recovery loop and hemoconcentrator have been set up, primed and all air has been removed in accordance with the TS3 Directions for Use. Note: Before connecting the HEMOBAG to the TS3 Tubing Set, verify the TS3 s recovery loop Inlet Line (Blue pinch Clamp 8T) is going into a roller or positive displacement pump and the forward flow from the pump is connected to a hemoconcentrator such that the hemoconcentrator s outlet flow tubing (Red Pinch Clamp 3T) will go back into the HEMOBAG creating the closed hemoconcentrating recovery loop. (make sure that there is at least 2 inches of ¼ line between the roller head and the Y before the hemoconcentrator) Helpful Tip: The male luer on the inflow line of the standard loop (9T now with a white cover cap) before the Y at the bottom of the hemoconcentrator should be connected to a pressure transducer to observe hemoconcentrating pressures. Page 2 of 5 DFU HBBSD /2017
3 Remove the Yellow Dust Cap 18H from the Needle-less Sampling Port 14H on the side of the of the HEMOBAG. Connect the Male Connector 7H (Blue capped connector) of the HEMOBAG to the Female Inlet Line connector 7T (Blue Pinch Clamp) of the TS3 Tubing going to the roller pump. Connect the Male Outlet Line Connector 6T of the TS3 Tubing Set from the hemoconcentrator (Red pinch Clamp3T) to the Female connector 10H (Red Cap Plug 11H) of the HEMOBAG. You are now ready to circulate the fluid in the closed hemoconcentrating Recovery Loop. WARNING: Verify that the Yellow Dust Cap 18H has been removed from the Needle-less Sampling Port in order to prevent blood from leaking out of the sampling port. At this point, the HEMOBAG and TS3 Tubing Set should look like this: Open the slide clamps on the Inlet 12H and Outlet 6H Ports of the HEMOBAG and open the Red 3T and Blue 8T Pinch Clamps of the TS3 Tubing Set Recovery loop. Allow the small amounts of air from the connections to rise to the top of the HEMOBAG. Slowly start the roller pump circulating the flow of blood from the HEMOBAG male connector 7H (Blue Cap 8H) through the roller pump, to the hemoconcentrator, and then from the hemoconcentrator back to the female connector 10H (Red Cap Plug 11H) of the HEMOBAG completing the closed hemoconcentration Recovery Loop. WARNING: Verify there is no build up of pressure in the TS3 Tubing Set by squeezing the tubing between your fingers distal to the roller pump and proximal to the hemoconcentrator at the start of circulation and periodically throughout ultrafiltration process. For convenience the inflow line male luer of the standard loop distal to the roller pump and proximal to the hemoconcentrator should be connected to a pressure transducer to observe hemoconcentrating pressures. It also necessary to agitate the Page 3 of 5 DFU HBBSD /2017
4 A P P RO XIM A TE VO LUM E (M ILLI LITE RS ) ANTICOAGULATED BLOOD ONLY D O NOT PRE S S URI ZE m l m ax OR HEMOBAG U S P A TE NT /2 1 /3 1 /4 Hemobag by squeezing the bag from both sides at least once every minute or so to insure optimal mixing occurs. This will lessen the hemoconcentrating pressures in the Recovery loop as the blood becomes more concentrated. WARNING: When using the HEMOBAG and the TS3 Tubing Set with a commercial hemoconcentrator, DO NOT exceed the recommended flow rates, pressure, and maximum hematocrit levels in the Directions for Use of the hemoconcentrator when concentrating blood. When the blood in the closed hemoconcentrating Recovery Loop and HEMOBAG have reached a satisfactory level of fluid reduction (not to exceed a HCT of 50%), stop the roller pump, A QC sample may be drawn at this time from the needle-less sampling port. Now close the HEMOBAG using Slide Clamp (Blue) 6H Securely. Flush the TS3 Tubing Set by chasing a small amount of crystalloid fluid through the Needle-less Sampling port luer 14H on the side of the HEMOBAG outlet port (a 60cc syringe may be used for this step and disconnected) do this by starting the roller pump again, pump the blood in the TS3 Tubing Set Recovery loop and hemoconcentrator back through Inlet (Red Cap Plug 11H) Port 13H into the HEMOBAG. When crystalloid fluid or air is seen exiting the hemoconcentrator, stop the roller pump and close the HEMOBAG using the Slide Clamp (Red) 12H Securely. Close the Red pinch clamp 3T and the Blue Pinch clamp 8T before disconnecting; All Clamps are closed securely now. Disconnect the HEMOBAG from the closed hemoconcentration Recovery Loop and Cap the (Red Plug Cap 11H), and the (Blue Cap 8H) of the HEMOBAG, then reconnect the 2 ends (Male Connector 6T and Female Connector 7T) of the TS3 Tubing Set to close the Recovery Loop. Apply the completed Patient Information Label to the side of the HEMOBAG. The HEMOBAG is now ready to be handed off for rapid autologous whole blood gravity infusion through the large bore IV line infusion port 9H. Note: Ensure the HEMOBAG is free of any air or bubbles before beginning nonpressurized gravity infusion. Note: For Jehovah s Witness Patients, retrograde prime an I.V. pressure Line and 3 way stopcock to the Inlet port luer (17H) of the HEMOBAG and the patient prior to filling the HEMOBAG from the Arterial or forward flow line. (See Note: When utilizing the HEMOBAG with a system outside of the traditional CPB extracorporeal circuit, be sure to have the closed TS3 tubing set and hemoconcentrator Set Up and Primed before connecting the HEMOBAG and the tubing sets Recovery Loop. (Refer to the TS3 Package Insert for Instructions) 1H U niversal 1/4" - 3/8" C onnec tor 13H 2H Arterial Infusion Port 12H Red Slide Clamp 17H Male Luer 11H Inlet P ort Female Connector Red Plug 10H Female Connector 9H IV Line Transfusion Port B af fle 16H 7H M ale Connector 3H Master Clip W hite S lide Clam p Blue Slide Clamp 5H 6H 8H M ale Connector Blue Cap 4H Green Cap Transfusion Port W hite S lide Clam p 15H 14H N eedless S am pling M ale Luer 18H Yellow Vented Cap FIGURE 1 Page 4 of 5 DFU HBBSD /2017
5 WARRANTY WARRANTY- LIMITATION OF LIABILITY: GLOBAL BLOOD RESOURCES LLC (GBR) MAKES NO EXPRESS OR IMPLIED WARRANTIES OF ANY KIND INCLUDING ANY WARRANTY OF MERCHANTABILITY OR FITNESS FOR PARTICULAR PURPOSE. GBR S SOLE OBLIGATION AND PURCHASER S EXCLUSIVE REMEDY IN THE EVENT THAT THE PRODUCT CANNOT BE CONFIGURED OR DOES NOT PERFORM IN ACCORDANCE WITH THE INSTRUCTIONS CONTAINED HEREIN, SHALL BE, TO REPLACE THE PRODUCT UPON RETURN OF THE ORIGINALLY PURCHASED PRODUCT. GBR SHALL NOT BE LIABLE IN TORT, CONTRACT OR ANY OTHER ACTION FOR SPECIAL, PROXIMATE, INDIRECT, INCIDENTAL OR CONSEQUENTIAL DAMAGES ARISING FROM THE USE OR MISUSE OF THIS PRODUCT. LIMITED PATENT LICENSE: this product and the process of salvaging blood as described in the instructions are covered by one or more U.S. Patent No. 5,928,178. 6,398,751. 7,033,334 and and additional U.S. and foreign patents pending. Any authorized purchaser of this product acquires an implied license under these patents to use the product only one time for performing the method only one time, and acquires no other right or license under the patents. Manufactured For: Global Blood Resources, LLC Emergo Europe P.O. Box 383 Somers, CT USA Prinsessegracht 20 Tel (800) AP The Hague Fax (860) The Netherlands Manufactured By: Robling Medical, Incorporated 90 Weathers Street Youngsville, NC USA Page 5 of 5 DFU HBBSD /2017
1.060 Set-Up and Priming of the Bypass Circuit. Perfusion Technology Department. Perfusionists
 TITLE/DESCRIPTION: DEPARTMENT: PERSONNEL: 1.060 Set-Up and Priming of the Bypass Circuit Perfusion Technology Department Perfusionists EFFECTIVE DATE: 8/97 REVISED: 09/07, 04/09, 9/17 PURPOSE: The function
TITLE/DESCRIPTION: DEPARTMENT: PERSONNEL: 1.060 Set-Up and Priming of the Bypass Circuit Perfusion Technology Department Perfusionists EFFECTIVE DATE: 8/97 REVISED: 09/07, 04/09, 9/17 PURPOSE: The function
Heart/Lung Perfusion Packs INSTRUCTIONS FOR USE
 Heart/Lung Perfusion Packs INSTRUCTIONS FOR USE DESCRIPTION A Heart/Lung Perfusion Pack is either a customized tubing pack built to user specifications or a stock tubing pack, designed for use during surgery
Heart/Lung Perfusion Packs INSTRUCTIONS FOR USE DESCRIPTION A Heart/Lung Perfusion Pack is either a customized tubing pack built to user specifications or a stock tubing pack, designed for use during surgery
AQUADEX FLEXFLOW SYSTEM QUICK REFERENCE GUIDE
 AQUADEX FLEXFLOW SYSTEM QUICK REFERENCE GUIDE AQUADEX FLEXFLOW SYSTEM DISCLAIMER The quick reference guide is not intended to replace CHF Solutions, Inc. Aquadex FlexFlow Direction For Use (DFU). Always
AQUADEX FLEXFLOW SYSTEM QUICK REFERENCE GUIDE AQUADEX FLEXFLOW SYSTEM DISCLAIMER The quick reference guide is not intended to replace CHF Solutions, Inc. Aquadex FlexFlow Direction For Use (DFU). Always
Contacts. Quick Start Guide
 Contacts Clinical Support Specialist: Phone: Cell Phone: Email: Fresenius Renal Technologies A division of Fresenius Medical Care North America 920 Winter Street Waltham, MA 02451 Technical Service Customer
Contacts Clinical Support Specialist: Phone: Cell Phone: Email: Fresenius Renal Technologies A division of Fresenius Medical Care North America 920 Winter Street Waltham, MA 02451 Technical Service Customer
Alfred ECMO Circuit Priming and Storage Guide
 Alfred ECMO Circuit Priming and Storage Guide 1 March 2012 Check List Page 2. Assembly, Priming and Storage Page 3. Prepare ECMO trolley and Circuit immediately before use Page 6. Connection to Patient
Alfred ECMO Circuit Priming and Storage Guide 1 March 2012 Check List Page 2. Assembly, Priming and Storage Page 3. Prepare ECMO trolley and Circuit immediately before use Page 6. Connection to Patient
Indications for Use: Caution: Note:
 IV 1 2 IV This reference is to be used in conjunction with the Crit-Line IV Monitor User s Guide (P/N CL80050002). Refer to the User s Guide for a complete description of alerts, warnings, cautions, and
IV 1 2 IV This reference is to be used in conjunction with the Crit-Line IV Monitor User s Guide (P/N CL80050002). Refer to the User s Guide for a complete description of alerts, warnings, cautions, and
USER MANUAL FLOW SELECTOR PM1000 SAVE THESE INSTRUCTIONS. Federal (USA) law restricts this device to sale by or on the order of a physician.
 USER MANUAL FLOW SELECTOR PM1000 SAVE THESE INSTRUCTIONS CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. RECEIVING / INSPECTION Remove the Precision Medical,
USER MANUAL FLOW SELECTOR PM1000 SAVE THESE INSTRUCTIONS CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. RECEIVING / INSPECTION Remove the Precision Medical,
The NAMIC Dream Kit. Single Operator Control. Reduce Contrast Waste. Less Force to Inject. Compensator * Manifold. How the Compensator Manifold Works:
 Dream Kit The NAMIC Dream Kit Single Operator Control. Reduce Contrast Waste. Less Force to Inject. For more than 40 years, NAMIC * Fluid Management products have been providing cardiologists, technicians
Dream Kit The NAMIC Dream Kit Single Operator Control. Reduce Contrast Waste. Less Force to Inject. For more than 40 years, NAMIC * Fluid Management products have been providing cardiologists, technicians
HEALTH SERVICES CODE A.6 CATEGORY: RN - SNP
 NURSING PROCEDURE TITLE ARTERIAL BLOOD SAMPLING (NEONATAL) A. Umbilical Catheter Blood Collection B. Radial Arterial Line CATEGORY: RN - SNP PURPOSE To obtain blood samples for laboratory analysis NURSING
NURSING PROCEDURE TITLE ARTERIAL BLOOD SAMPLING (NEONATAL) A. Umbilical Catheter Blood Collection B. Radial Arterial Line CATEGORY: RN - SNP PURPOSE To obtain blood samples for laboratory analysis NURSING
Blood Pressure Monitoring: Arterial Line
 Approved by: Blood Pressure Monitoring: Arterial Line Gail Cameron Senior Director, Operations, Maternal, Neonatal & Child Health Programs Dr. Ensenat Medical Director, Neonatology Neonatal Nursery Policy
Approved by: Blood Pressure Monitoring: Arterial Line Gail Cameron Senior Director, Operations, Maternal, Neonatal & Child Health Programs Dr. Ensenat Medical Director, Neonatology Neonatal Nursery Policy
Instruction Manual LIMITED 1 YEAR WARRANTY. Hydraulic Punch Driver Read this material before using this product.
 Instruction Manual Hydraulic Punch Driver 902-483 LIMITED 1 YEAR WARRANTY We make every effort to assure that its products meet high quality and durability standards, and warrant to the original purchaser
Instruction Manual Hydraulic Punch Driver 902-483 LIMITED 1 YEAR WARRANTY We make every effort to assure that its products meet high quality and durability standards, and warrant to the original purchaser
Parts identification:
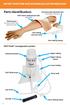 ARTERY PUNCTURE AND INTRAMUSCULAR TRAINING ARM Parts identification: FAST-derm replacement skin (removable) Intramuscular injection pad (removable for cleaning) Training arm (includes one FAST-derm replacement
ARTERY PUNCTURE AND INTRAMUSCULAR TRAINING ARM Parts identification: FAST-derm replacement skin (removable) Intramuscular injection pad (removable for cleaning) Training arm (includes one FAST-derm replacement
GETZ EQUIPMENT INNOVATORS PART NO.: 9G59554 MODEL: MS 36 SC-R HYDROSTATIC TEST PUMP
 GETZ EQUIPMENT INNOVATORS PART NO.: 9G59554 MODEL: MS 36 SC-R HYDROSTATIC TEST PUMP LIMITED WARRANTY Getz Equipment Innovators warrants its products, and component parts of any product manufactured by
GETZ EQUIPMENT INNOVATORS PART NO.: 9G59554 MODEL: MS 36 SC-R HYDROSTATIC TEST PUMP LIMITED WARRANTY Getz Equipment Innovators warrants its products, and component parts of any product manufactured by
KEOFITT ASEPTIC SAMPLING BAG (PAT. PEND.) Item no , & User Manual
 KEOFITT ASEPTIC SAMPLING BAG (PAT. PEND.) Item no. 120500, 121000 & 122000 User Manual S O M E P E O P L E W O U L D N T G A M B L E W I T H T H E I R S A M P L E T M INTRODUCTION: MANUFACTURER: Keofitt
KEOFITT ASEPTIC SAMPLING BAG (PAT. PEND.) Item no. 120500, 121000 & 122000 User Manual S O M E P E O P L E W O U L D N T G A M B L E W I T H T H E I R S A M P L E T M INTRODUCTION: MANUFACTURER: Keofitt
Keofitt Aseptic Sampling Bag (Pat. pend.) Item no , & User Manual
 Keofitt Aseptic Sampling Bag (Pat. pend.) Item no. 120500, 121000 & 122000 User Manual s o m e p e o p l e w o u l d n t g a m B l e w i t h t h e i r s a m p l e t m Introduction: Manufacturer: Keofitt
Keofitt Aseptic Sampling Bag (Pat. pend.) Item no. 120500, 121000 & 122000 User Manual s o m e p e o p l e w o u l d n t g a m B l e w i t h t h e i r s a m p l e t m Introduction: Manufacturer: Keofitt
453 Series Steam Heated Vaporizing Regulator
 ADI 0453A Certified ISO 9001:2000 453 Series Steam Heated Vaporizing Regulator INSTALLATION AND OPERATION INSTRUCTIONS Before Installing or Operating, Read and Comply with These Instructions Controls Corporation
ADI 0453A Certified ISO 9001:2000 453 Series Steam Heated Vaporizing Regulator INSTALLATION AND OPERATION INSTRUCTIONS Before Installing or Operating, Read and Comply with These Instructions Controls Corporation
C O L O G Y. New. g u O N. l o t a C a. The high security connection
 O N g u e C O L O G Y New l o t a C a The high security connection Preparation devices for medications/solutions Bag qimospike B 7218.01 qimospike B is an IV fluid bag access spike fitted with qimo. Aspiration
O N g u e C O L O G Y New l o t a C a The high security connection Preparation devices for medications/solutions Bag qimospike B 7218.01 qimospike B is an IV fluid bag access spike fitted with qimo. Aspiration
U S E R M A N U A L AIR FLOWMETER. MODELS: 1MFA2001 (shown) 1MFA9001 CAUTION. ISO Certified
 U S E R M A N U A L AIR FLOWMETER MODELS: 1MFA2001 (shown) 1MFA9001 ISO 13485 Certified Authorized Your EU local Representative: distributor: EMERGO EUROPE, INC. Molenstraat 15 2513866 BH -624 The -3952
U S E R M A N U A L AIR FLOWMETER MODELS: 1MFA2001 (shown) 1MFA9001 ISO 13485 Certified Authorized Your EU local Representative: distributor: EMERGO EUROPE, INC. Molenstraat 15 2513866 BH -624 The -3952
MANITOBA RENAL PROGRAM
 MANITOBA RENAL PROGRAM SUBJECT Fresenius 5008 Preparation for Hemodialysis using the ONLINEplus System SECTION CODE 30.10.02 30.10 Hemodialysis: Equipment and Procedures AUTHORIZATION Professional Advisory
MANITOBA RENAL PROGRAM SUBJECT Fresenius 5008 Preparation for Hemodialysis using the ONLINEplus System SECTION CODE 30.10.02 30.10 Hemodialysis: Equipment and Procedures AUTHORIZATION Professional Advisory
P5523. Users Manual. Liquid to Gas Separator. Test Equipment Depot Washington Street Melrose, MA TestEquipmentDepot.
 Test Equipment Depot - 800.517.8431-99 Washington Street Melrose, MA 02176 TestEquipmentDepot.com P5523 Liquid to Gas Separator Users Manual PN 3952355 November 2010 2010 Fluke Corporation. All rights
Test Equipment Depot - 800.517.8431-99 Washington Street Melrose, MA 02176 TestEquipmentDepot.com P5523 Liquid to Gas Separator Users Manual PN 3952355 November 2010 2010 Fluke Corporation. All rights
U S E R M A N U A L CAUTION. SAVE THESE INSTRUCTIONS Federal (USA) law restricts this device to sale by or on the order of a physician.
 U S E R M A N U A L 1600 SERIES OXYGEN REGULATOR 168715G (Shown) SAVE THESE INSTRUCTIONS Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090
U S E R M A N U A L 1600 SERIES OXYGEN REGULATOR 168715G (Shown) SAVE THESE INSTRUCTIONS Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090
PUMP WITH FIXED FLOW RATE. Instructions for Use
 PUMP WITH FIXED FLOW RATE Instructions for Use ON-Q* Pump with Fixed Flow Rate Instructions For Use Important Information Please read the entire document before operating the ON-Q* device. Follow all
PUMP WITH FIXED FLOW RATE Instructions for Use ON-Q* Pump with Fixed Flow Rate Instructions For Use Important Information Please read the entire document before operating the ON-Q* device. Follow all
DRAFT U S E R M A N U A L CAUTION. Model: 19MFA1001 Series. Federal (USA) law restricts this device to sale by or on the order of a physician.
 U S E R M A N U A L Model: 19MFA1001 Series SAVE THESE INSTRUCTIONS Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090 Northampton,
U S E R M A N U A L Model: 19MFA1001 Series SAVE THESE INSTRUCTIONS Federal (USA) law restricts this device to sale by or on the order of a physician. 300 Held Drive Tel: (+001) 610-262-6090 Northampton,
SDS -Series 4C-D201/-D202 Supplemental Drying System User s Manual
 SDS -Series 4C-D201/-D202 Supplemental Drying System User s Manual 8 Executive Drive P.O. Box 2105 Toms River, NJ 08754 (732) 244-0010 (800) 337-3762 fax (732) 244-8140 www.permapure.com info@permapure.com
SDS -Series 4C-D201/-D202 Supplemental Drying System User s Manual 8 Executive Drive P.O. Box 2105 Toms River, NJ 08754 (732) 244-0010 (800) 337-3762 fax (732) 244-8140 www.permapure.com info@permapure.com
ACRYLIC FLOW METERS 0-30, 0-70 & LPM
 ACRYLIC FLOW METERS 0-30, 0-70 & 0-120 LPM ASSEMBLY INSTRUCTIONS & INSTRUCTIONS FOR USE R219P86 (0-120LPM) R219P87 (0-70LPM) R219P88 (0-30 LPM) R138P11 Rev. D TABLE OF CONTENTS: 1.0 PRODUCT OVERVIEW...1
ACRYLIC FLOW METERS 0-30, 0-70 & 0-120 LPM ASSEMBLY INSTRUCTIONS & INSTRUCTIONS FOR USE R219P86 (0-120LPM) R219P87 (0-70LPM) R219P88 (0-30 LPM) R138P11 Rev. D TABLE OF CONTENTS: 1.0 PRODUCT OVERVIEW...1
1020 Industrial Drive, Orlinda, TN fax
 Operation Manual Ultrafiltration for High Purity Distribution K-A-HPTUF Series 615-654-4441 sales@specialtyh2o.com 615-654-4449 fax TABLE OF CONTENTS Section 1 GENERAL 1.1 Warnings and Cautions... 1 1.2
Operation Manual Ultrafiltration for High Purity Distribution K-A-HPTUF Series 615-654-4441 sales@specialtyh2o.com 615-654-4449 fax TABLE OF CONTENTS Section 1 GENERAL 1.1 Warnings and Cautions... 1 1.2
PEDIATRIC INJECTABLE TRAINING ARM SIMULATOR LF00958U INSTRUCTION MANUAL
 PEDIATRIC INJECTABLE TRAINING ARM SIMULATOR LF00958U INSTRUCTION MANUAL DO NOT REMOVE FILM FROM TUBING! THIS PRODUCT CONTAINS DRY NATURAL RUBBER! Products by NASCO About the Simulator The Life/form Pediatric
PEDIATRIC INJECTABLE TRAINING ARM SIMULATOR LF00958U INSTRUCTION MANUAL DO NOT REMOVE FILM FROM TUBING! THIS PRODUCT CONTAINS DRY NATURAL RUBBER! Products by NASCO About the Simulator The Life/form Pediatric
FLOWMETER MODELS: 1MFA, 4MFA, 6MFA and 8MFA Series SAVE THESE INSTRUCTIONS
 USER MANUAL FLOWMETER MODELS: 1MFA, 4MFA, 6MFA and 8MFA Series 1MFA3001 (Shown) 8MFA1001 (Shown) SAVE THESE INSTRUCTIONS Federal (USA) law restricts this device to sale by or on the order of a physician.
USER MANUAL FLOWMETER MODELS: 1MFA, 4MFA, 6MFA and 8MFA Series 1MFA3001 (Shown) 8MFA1001 (Shown) SAVE THESE INSTRUCTIONS Federal (USA) law restricts this device to sale by or on the order of a physician.
Endo-Flush Order # ZUTR30004 OPERATION MANUAL. Zutron Medical, LLC W 98 th St #40-27 Lenexa, KS Phone Fax
 OPERATION MANUAL Zutron Medical, LLC 17501 W 98 th St #40-27 Lenexa, KS 66219 Phone 877-343-5873 Fax 913-967-5944 ZUT-Lab-004-30004 REV. 03312017 Table of Contents 2 Introduction 1. Intended Use 2. Labels,
OPERATION MANUAL Zutron Medical, LLC 17501 W 98 th St #40-27 Lenexa, KS 66219 Phone 877-343-5873 Fax 913-967-5944 ZUT-Lab-004-30004 REV. 03312017 Table of Contents 2 Introduction 1. Intended Use 2. Labels,
Yoke Block Instruction Manual
 Yoke Block Instruction Manual ! WARNING IMPORTANT: READ MANUAL COMPLETELY BEFORE OPERATING THIS DEVICE This manual contains instructions on periodically required checks to be performed by the user. These
Yoke Block Instruction Manual ! WARNING IMPORTANT: READ MANUAL COMPLETELY BEFORE OPERATING THIS DEVICE This manual contains instructions on periodically required checks to be performed by the user. These
INFUSION SYSTEM (110V) (220V) USER S MANUAL
 INFUSION SYSTEM 20-6000-00 (110V) 20-6005-00 (220V) USER S MANUAL 8000 South Kolb Road Tucson, AZ 85756 Phone 800.528.1597 Fax 520.885.1189 www.wellsgrp.com email: sales@wellsgrp.com GENERAL DESCRIPTION
INFUSION SYSTEM 20-6000-00 (110V) 20-6005-00 (220V) USER S MANUAL 8000 South Kolb Road Tucson, AZ 85756 Phone 800.528.1597 Fax 520.885.1189 www.wellsgrp.com email: sales@wellsgrp.com GENERAL DESCRIPTION
Adult All Integrated Mini Bypass System
 Adult All Integrated Mini Bypass System Clinical Flexibility without Sacrificing Patient Safety Imagine a new, innovative, completely closed system that includes a venous bubble trap, centrifugal pump,
Adult All Integrated Mini Bypass System Clinical Flexibility without Sacrificing Patient Safety Imagine a new, innovative, completely closed system that includes a venous bubble trap, centrifugal pump,
Integrity Testing LifeASSURE BNA045 and BNA065 Series Filter Cartridges
 Integrity Testing LifeASSURE BNA045 and BNA065 Series Filter Cartridges SAFETY INFORMATION Read, understand, and follow all safety information contained in these instructions and the instructions provided
Integrity Testing LifeASSURE BNA045 and BNA065 Series Filter Cartridges SAFETY INFORMATION Read, understand, and follow all safety information contained in these instructions and the instructions provided
COLLECTION OF BLOOD. Types of patients
 COLLECTION OF BLOOD Capillary Sampling The specimen of choice for laboratory analysis is generally a venous specimen. For blood gas analysis, the specimen of choice originates from an arterial draw. When
COLLECTION OF BLOOD Capillary Sampling The specimen of choice for laboratory analysis is generally a venous specimen. For blood gas analysis, the specimen of choice originates from an arterial draw. When
DIAGNOSTIC CARDIOLOGY
 DIAGNOSTIC CARDIOLOGY For Your Procedural Needs Manufactured to the Highest Standards, Customised to Your Specific Needs MEDLINE PROCEDURE PACKS The items contained within this brochure are perfect for
DIAGNOSTIC CARDIOLOGY For Your Procedural Needs Manufactured to the Highest Standards, Customised to Your Specific Needs MEDLINE PROCEDURE PACKS The items contained within this brochure are perfect for
Quiz for Module 2: Aquarius Training (Rev. 1) Lesson 1: Introduction to Aquarius
 Quiz for Module 2: Aquarius Training (Rev. 1) Lesson 1: Introduction to Aquarius page 1 of 2 Name: Date: Score: Instructor: Location: Please circle the correct answers: 1. Select the answer(s) that represent
Quiz for Module 2: Aquarius Training (Rev. 1) Lesson 1: Introduction to Aquarius page 1 of 2 Name: Date: Score: Instructor: Location: Please circle the correct answers: 1. Select the answer(s) that represent
INSTRUCTIONS FOR MODELS SG3897 AND SG3898 CROSS PURGE ASSEMBLIES
 INSTRUCTIONS FOR MODELS SG3897 AND SG3898 CROSS PURGE ASSEMBLIES THIS BOOKLET CONTAINS PROPRIETARY INFORMATION OF ADVANCED SPECIALTY GAS EQUIPMENT CORP. AND IS PROVIDED TO THE PURCHASER SOLELY FOR USE
INSTRUCTIONS FOR MODELS SG3897 AND SG3898 CROSS PURGE ASSEMBLIES THIS BOOKLET CONTAINS PROPRIETARY INFORMATION OF ADVANCED SPECIALTY GAS EQUIPMENT CORP. AND IS PROVIDED TO THE PURCHASER SOLELY FOR USE
Assisting with Insertion. Care of Intraspinal Catheters
 Guidelines included: Assisting with an Insertion Care of Various types of Intraspinal s Care of the Intraspinal Infusion Monitoring Removal of the Short Term Non Assisting with Insertion INR should be
Guidelines included: Assisting with an Insertion Care of Various types of Intraspinal s Care of the Intraspinal Infusion Monitoring Removal of the Short Term Non Assisting with Insertion INR should be
DONOR TM Pre-Evacuated Post-Operative Autologous Blood Reinfusion System
 Providing Value To Life DONOR TM Pre-Evacuated Post-Operative Autologous Blood Reinfusion System your partner in blood management Specifications Pre-evacuated postoperative autologous blood reinfusion
Providing Value To Life DONOR TM Pre-Evacuated Post-Operative Autologous Blood Reinfusion System your partner in blood management Specifications Pre-evacuated postoperative autologous blood reinfusion
ASAHI Neurovascular Guide Wire
 ASAHI Neurovascular Guide Wire AMK-DT244 Ver.1.00 / 11TS073 Legal manufacturer Consult instructions for use Do not use if package is damaged RX ONLY Caution: Federal (USA) Law restricts this device to
ASAHI Neurovascular Guide Wire AMK-DT244 Ver.1.00 / 11TS073 Legal manufacturer Consult instructions for use Do not use if package is damaged RX ONLY Caution: Federal (USA) Law restricts this device to
Haemodialysis. Bloodlines, Tubing Systems, Accessories Product Range. Product name / Headline Subline
 Haemodialysis Bloodlines, Tubing Systems, Accessories Product Range Product name / Headline Subline Ensuring safety with the authentic Fresenius Medical Care Original Bloodlines e Com ac G u ibility at
Haemodialysis Bloodlines, Tubing Systems, Accessories Product Range Product name / Headline Subline Ensuring safety with the authentic Fresenius Medical Care Original Bloodlines e Com ac G u ibility at
BlenderBuddy 2. Operating Manual & Instructions For Use ENGLISH R233M01 REV. A
 BlenderBuddy 2 Operating Manual & Instructions For Use ENGLISH R233M01 REV. A Maxtec 2305 South 1070 West Salt Lake City, Utah 84119 USA TEL (800) 748.5355 FAX (801) 973.6090 www.maxtec.com E-mail: sales@maxtec.com
BlenderBuddy 2 Operating Manual & Instructions For Use ENGLISH R233M01 REV. A Maxtec 2305 South 1070 West Salt Lake City, Utah 84119 USA TEL (800) 748.5355 FAX (801) 973.6090 www.maxtec.com E-mail: sales@maxtec.com
Bloodlines, Tubing Systems, Accessories
 NEPHROXA Your Partner In Renal Care Haemodialysis Bloodlines, Tubing Systems, Accessories Product Range 2010/2011 5008 Therapy System the innovation Enabling the application of ONLINE Haemodiafi ltration
NEPHROXA Your Partner In Renal Care Haemodialysis Bloodlines, Tubing Systems, Accessories Product Range 2010/2011 5008 Therapy System the innovation Enabling the application of ONLINE Haemodiafi ltration
FlashGARD HP Reverse Osmosis Filtration System Model Number FSTMO75 Part Number
 3M Water Filtration Products FlashGARD HP Reverse Osmosis Filtration System Model Number FSTMO75 Part Number 56123-06 Installer: Please leave this manual with owner/operator. End User: Please retain for
3M Water Filtration Products FlashGARD HP Reverse Osmosis Filtration System Model Number FSTMO75 Part Number 56123-06 Installer: Please leave this manual with owner/operator. End User: Please retain for
Assembly and Installation Procedures
 Assembly and Installation Procedures for Pall Supracap 100 Capsules 1. Introduction The following procedures must be followed for the installation of Pall Supracap 100 Capsules. The instructions contained
Assembly and Installation Procedures for Pall Supracap 100 Capsules 1. Introduction The following procedures must be followed for the installation of Pall Supracap 100 Capsules. The instructions contained
NB/NBR NITROGEN BOOSTER FOR AVIATION SERVICE
 NB/NBR NITROGEN BOOSTER FOR AVIATION SERVICE INSTALLATION, OPERATION & MAINTENANCE MANUAL INTERFACE DEVICES, INC. 230 Depot Road, Milford, CT 06460 Ph: (203) 878-4648, Fx: (203) 882-0885, E-mail: info@interfacedevices.com
NB/NBR NITROGEN BOOSTER FOR AVIATION SERVICE INSTALLATION, OPERATION & MAINTENANCE MANUAL INTERFACE DEVICES, INC. 230 Depot Road, Milford, CT 06460 Ph: (203) 878-4648, Fx: (203) 882-0885, E-mail: info@interfacedevices.com
Infant IV Leg LF03636U Instruction Manual
 Infant IV Leg LF03636U Instruction Manual Products by Nasco About the Simulator The Life/form Infant IV Leg Simulator is an exciting training aid for practicing and demonstrating intravenous puncture
Infant IV Leg LF03636U Instruction Manual Products by Nasco About the Simulator The Life/form Infant IV Leg Simulator is an exciting training aid for practicing and demonstrating intravenous puncture
USER MANUAL. AIR-OXYGEN BLENDER (DISS and NIST Connections) Model No. PM5200 Series PM5300 Series (shown) SAVE THESE INSTRUCTIONS
 USER MANUAL (DISS and NIST Connections) Model No. PM5200 Series PM5300 Series (shown) SAVE THESE INSTRUCTIONS CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician.
USER MANUAL (DISS and NIST Connections) Model No. PM5200 Series PM5300 Series (shown) SAVE THESE INSTRUCTIONS CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician.
MyoSure Setup Simplified
 MyoSure Setup Simplified This is designed as a quick reference guide only. This guide is not a substitute for the Operator s Manual and/or Instructions for Use (IFU). MyoSure Setup with Aquilex MyoSure
MyoSure Setup Simplified This is designed as a quick reference guide only. This guide is not a substitute for the Operator s Manual and/or Instructions for Use (IFU). MyoSure Setup with Aquilex MyoSure
Summary of Cardiac Anesthesia Technician Responsibilities
 Summary of Cardiac Anesthesia Technician Responsibilities Division of Cardiac Anesthesia, BIDMC Before Bringing Patient into Room 1) Check to see that drawsheet is folded and that the 5 ECG leads are properly
Summary of Cardiac Anesthesia Technician Responsibilities Division of Cardiac Anesthesia, BIDMC Before Bringing Patient into Room 1) Check to see that drawsheet is folded and that the 5 ECG leads are properly
PRE-TRANSFUSION GUIDELINES
 PRE-TRANSFUSION GUIDELINES TACTICAL COMBAT CASUALTY CARE PATIENT STABILIZATION REQUIREMENTS HEMORRHAGE Bleeding is CONTROLLED by use of pressure dressing, tourniquet or fibrin bandage AIRWAY Secured and
PRE-TRANSFUSION GUIDELINES TACTICAL COMBAT CASUALTY CARE PATIENT STABILIZATION REQUIREMENTS HEMORRHAGE Bleeding is CONTROLLED by use of pressure dressing, tourniquet or fibrin bandage AIRWAY Secured and
DFB FLOW METERS (DESIGNED FOR BLENDERS, DUAL TAPER)
 DFB FLOW METERS (DESIGNED FOR BLENDERS, DUAL TAPER) 0-3, 0-15, 0-30 & 0-70 LPM ASSEMBLY INSTRUCTIONS & INSTRUCTIONS FOR USE R219P87-400 (0-70 LPM) R219P88-400 (0-30 LPM) R219P79-400 (0-15 LPM) R219P99-400
DFB FLOW METERS (DESIGNED FOR BLENDERS, DUAL TAPER) 0-3, 0-15, 0-30 & 0-70 LPM ASSEMBLY INSTRUCTIONS & INSTRUCTIONS FOR USE R219P87-400 (0-70 LPM) R219P88-400 (0-30 LPM) R219P79-400 (0-15 LPM) R219P99-400
OPERATOR S MANUAL. P/N GB Rev. 008
 ltm OPERATOR S MANUAL P/N 4533708GB Rev. 008 s ii Copyright Copyright Level 1 H-1200 Fast Flow Fluid Warmer Part Number: 4533708GB Rev. 008 (2010-02) This revision supercedes all previous revisions. Under
ltm OPERATOR S MANUAL P/N 4533708GB Rev. 008 s ii Copyright Copyright Level 1 H-1200 Fast Flow Fluid Warmer Part Number: 4533708GB Rev. 008 (2010-02) This revision supercedes all previous revisions. Under
NEXUS TKO. Specialty Neonatal/Pediatric. Safety Infusion Sets. Blood Reflux Protection 24/7! Anti-Reflux Technology Provides
 Specialty Neonatal/Pediatric Safety Infusion Sets Anti-Reflux Technology Provides Blood Reflux Protection 24/7! NEXUS TKO TECHNOLOGY THAT NEVER SLEEPS! Nexus TKO Neonatal Safety Catheter Nexus Tri-Seal
Specialty Neonatal/Pediatric Safety Infusion Sets Anti-Reflux Technology Provides Blood Reflux Protection 24/7! NEXUS TKO TECHNOLOGY THAT NEVER SLEEPS! Nexus TKO Neonatal Safety Catheter Nexus Tri-Seal
AQUARIUS Continuous Renal Replacement Therapy with Regional Citrate Anticoagulation (RCA) Competency
 Demonstrates an understanding of why RCA has been prescribed Explain the anticoagulation protocol Demonstrate an understanding of potential complications associated with CRRT. Explain potential indications
Demonstrates an understanding of why RCA has been prescribed Explain the anticoagulation protocol Demonstrate an understanding of potential complications associated with CRRT. Explain potential indications
RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS
 RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS TRUST THE POWER OF PREVENTION WITH CINRYZE Whether your patients are new to treatment or are continuing their journey, remind
RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS TRUST THE POWER OF PREVENTION WITH CINRYZE Whether your patients are new to treatment or are continuing their journey, remind
New. The high security connection
 L ON Cata C lo gu e O O G Y New The high security connection Preparation devices for medications/solutions Bag qimospike B 7218.01 qimospike B is an IV fluid bag access spike fitted with qimo. Aspiration
L ON Cata C lo gu e O O G Y New The high security connection Preparation devices for medications/solutions Bag qimospike B 7218.01 qimospike B is an IV fluid bag access spike fitted with qimo. Aspiration
Corning HYPERStack Cell Culture Vessel Closed System
 Corning HYPERStack Cell Culture Vessel Closed System User Guide Corning HYPERStack Vessel Components Liquid Handling Section F E B M D G B K J I A Liquid handling manifold B 3/8" I.D. liquid handling tubing
Corning HYPERStack Cell Culture Vessel Closed System User Guide Corning HYPERStack Vessel Components Liquid Handling Section F E B M D G B K J I A Liquid handling manifold B 3/8" I.D. liquid handling tubing
Sterile Visual Flow Indicator
 Sterile Visual Flow Indicator Sanitary Process Connection Installation / Operation / Maintenance Manual P.O. Box 1116 Twinsburg, OH 44087 Phone: 330/405-3040 Fax: 330/405-3070 E-mail: view@ljstar.com Web
Sterile Visual Flow Indicator Sanitary Process Connection Installation / Operation / Maintenance Manual P.O. Box 1116 Twinsburg, OH 44087 Phone: 330/405-3040 Fax: 330/405-3070 E-mail: view@ljstar.com Web
ALLEGRO INDUSTRIES SERIES 9910 (LP) CONTINUOUS FLOW, SUPPLIED AIR HOOD RESPIRATORS P/N 9910 P/N 9910R P/N 9910D P/N 9910DS P/N 9912D
 U S E R M A N U A L ALLEGRO INDUSTRIES SERIES 9910 (LP) CONTINUOUS FLOW, SUPPLIED AIR HOOD RESPIRATORS P/N 9910 P/N 9910R P/N 9910D P/N 9910DS P/N 9912D Read and understand all instructions before using
U S E R M A N U A L ALLEGRO INDUSTRIES SERIES 9910 (LP) CONTINUOUS FLOW, SUPPLIED AIR HOOD RESPIRATORS P/N 9910 P/N 9910R P/N 9910D P/N 9910DS P/N 9912D Read and understand all instructions before using
A complete family of Pediatric Perfusion Systems
 LEADING THE FIELD OF PEDIATRIC PERFUSION FOR OVER 25 YEARS A complete family of Pediatric Perfusion Systems Perfusion systems designed for the broadest range of pediatric patients Small, sensitive neonatal
LEADING THE FIELD OF PEDIATRIC PERFUSION FOR OVER 25 YEARS A complete family of Pediatric Perfusion Systems Perfusion systems designed for the broadest range of pediatric patients Small, sensitive neonatal
3M Liqui-Cel EXF-4x13 and 4x28 Series Membrane Contactors
 Membrane Contactors 3M Liqui-Cel EXF-4x13 and 4x28 Series Membrane Contactors Assembly and Disassembly Instructions 3M.com/Liqui-Cel TABLE OF CONTENTS I. Safety and Warning 3 II. Assembly Parts 4 III.
Membrane Contactors 3M Liqui-Cel EXF-4x13 and 4x28 Series Membrane Contactors Assembly and Disassembly Instructions 3M.com/Liqui-Cel TABLE OF CONTENTS I. Safety and Warning 3 II. Assembly Parts 4 III.
IVIG Educational Presentation
 IVIG Educational Presentation Administration of IVIG direct from the Bottle Note: This is not the procedure please refer to the procedure in epops 16/06/2017 IVIG Administion from a bottle 1 Change in
IVIG Educational Presentation Administration of IVIG direct from the Bottle Note: This is not the procedure please refer to the procedure in epops 16/06/2017 IVIG Administion from a bottle 1 Change in
Patients Guide for Self Administering Intravenous Antibiotics General points
 Patients Guide for Self Administering Intravenous Antibiotics General points An intravenous line is an open route for infection therefore you need to prevent this by washing your hands before any procedure
Patients Guide for Self Administering Intravenous Antibiotics General points An intravenous line is an open route for infection therefore you need to prevent this by washing your hands before any procedure
WEATHERIZING INSTRUCTIONS
 FOR PNEUMATIC MASTS The Will-Burt Company 169 S. Main Street Orrville, OH 44667 www.willburt.com TP-4744301-C, 2017 The Will-Burt Company Pneumatic Mast Warranty Will-Burt warrants its pneumatic masts
FOR PNEUMATIC MASTS The Will-Burt Company 169 S. Main Street Orrville, OH 44667 www.willburt.com TP-4744301-C, 2017 The Will-Burt Company Pneumatic Mast Warranty Will-Burt warrants its pneumatic masts
Considerations in Circuit Miniaturization
 Considerations in Circuit Miniaturization The AmSECT 40th International Conference Pediatric Track Edward Darling, C.C.P. Upstate Medical University Syracuse, NY Objectives Investigate the long held dogma
Considerations in Circuit Miniaturization The AmSECT 40th International Conference Pediatric Track Edward Darling, C.C.P. Upstate Medical University Syracuse, NY Objectives Investigate the long held dogma
Newborn Nursing Skills and ALS Simulator LF01400U Instruction Manual
 Newborn Nursing Skills and ALS Simulator LF01400U Instruction Manual Products by Nasco About the Simulator Meeting your neonatal resuscitation program course curriculum, the Life/form Newborn Nursing Skills
Newborn Nursing Skills and ALS Simulator LF01400U Instruction Manual Products by Nasco About the Simulator Meeting your neonatal resuscitation program course curriculum, the Life/form Newborn Nursing Skills
Digital Vacuum Regulator
 Temperature Control for Research and Industry Digital Vacuum Regulator User s Manual Model 300 INDEX SECTION PAGE 1. QUICK OPERATING INSTRUCTIONS........................... 3 Safety Notices.................................................
Temperature Control for Research and Industry Digital Vacuum Regulator User s Manual Model 300 INDEX SECTION PAGE 1. QUICK OPERATING INSTRUCTIONS........................... 3 Safety Notices.................................................
4 and 8 Station PDS Control Installation & Operators Manual
 4 and 8 Station PDS Control Installation & Operators Manual 1402-15 11/2000 June 2003 MW1402C Chore-Time Warranty 4 and 8 Station PDS Control Chore-Time Warranty Chore-Time Equipment ( Chore-Time ) warrants
4 and 8 Station PDS Control Installation & Operators Manual 1402-15 11/2000 June 2003 MW1402C Chore-Time Warranty 4 and 8 Station PDS Control Chore-Time Warranty Chore-Time Equipment ( Chore-Time ) warrants
Manual Solvent Purification System. Innovative Technology, Inc.
 Innovative Technology, Inc. 2 New Pasture Road, Newburyport, MA 01950-4054 USA Email: Info@gloveboxes.com TEL: +1 978 462 4415 FAX: +1 978 462 3338 Manual Solvent Purification System Copyright Innovative
Innovative Technology, Inc. 2 New Pasture Road, Newburyport, MA 01950-4054 USA Email: Info@gloveboxes.com TEL: +1 978 462 4415 FAX: +1 978 462 3338 Manual Solvent Purification System Copyright Innovative
SSFU SUPER SPRAYFAST UNIVERSAL ADHESIVE APPLICATOR
 S S F U SSFU SUPER SPRAYFAST UNIVERSAL ADHESIVE APPLICATOR MACHINERY DIVISION OWNER S MANUAL UNIT INSTRUCTIONS Please follow all SSFU Safety Instructions. Contact your Duro Dyne Tech Service if you have
S S F U SSFU SUPER SPRAYFAST UNIVERSAL ADHESIVE APPLICATOR MACHINERY DIVISION OWNER S MANUAL UNIT INSTRUCTIONS Please follow all SSFU Safety Instructions. Contact your Duro Dyne Tech Service if you have
PreludeSYNC. Radial Compression Device INSTRUCTIONS FOR USE
 PreludeSYNC Radial Compression Device INSTRUCTIONS FOR USE PreludeSYNC Radial Compression Device English INSTRUCTIONS FOR USE PRODUCT DESCRIPTION The Prelude Sync Radial Compression Device is a sterile,
PreludeSYNC Radial Compression Device INSTRUCTIONS FOR USE PreludeSYNC Radial Compression Device English INSTRUCTIONS FOR USE PRODUCT DESCRIPTION The Prelude Sync Radial Compression Device is a sterile,
Aseptic Techniques. Techniques for Sterile Compounding. Pharmacy Technician Training Systems Passassured, LLC
 Aseptic Techniques Techniques for Sterile Compounding Pharmacy Technician Training Systems Passassured, LLC Aseptic Techniques, Tech for Sterile Compounding PassAssured's Pharmacy Technician Training Program
Aseptic Techniques Techniques for Sterile Compounding Pharmacy Technician Training Systems Passassured, LLC Aseptic Techniques, Tech for Sterile Compounding PassAssured's Pharmacy Technician Training Program
Skills & Scenarios 81
 Skills & Scenarios 81 Psychomotor Skills Checklist Skill Lab Clinical Date Initial Date Initial 1. Change I.V. tubing 2. Change I.V solution 3. Regulate I.V. flow rate 4. Flush peripheral lock 5. Change
Skills & Scenarios 81 Psychomotor Skills Checklist Skill Lab Clinical Date Initial Date Initial 1. Change I.V. tubing 2. Change I.V solution 3. Regulate I.V. flow rate 4. Flush peripheral lock 5. Change
Research Grade Blood Pressure Transducer User's Manual
 Research Grade Blood Pressure Transducer User's Manual Research Grade Blood Pressure Transducer, 110 VAC/60 Hz MA1 60-3002 Research Grade Blood Pressure Transducer, 220 VAC/50 Hz MA1 60-3003 WEEE/RoHS
Research Grade Blood Pressure Transducer User's Manual Research Grade Blood Pressure Transducer, 110 VAC/60 Hz MA1 60-3002 Research Grade Blood Pressure Transducer, 220 VAC/50 Hz MA1 60-3003 WEEE/RoHS
RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS. Hereditary Angioedema
 RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS Hereditary Angioedema TRUST PROVEN PREVENTION WITH CINRYZE Whether your patients are new to treatment or are continuing their
RECONSTITUTION AND ADMINISTRATION INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS Hereditary Angioedema TRUST PROVEN PREVENTION WITH CINRYZE Whether your patients are new to treatment or are continuing their
GETZ EQUIPMENT INNOVATORS RECOVERY SYSTEM CLEAN AGENT FE-36 / HALOTRON 1 PART NO. 4G59751 OPERATIONS MANUAL
 GETZ EQUIPMENT INNOVATORS CLEAN AGENT FE-36 / HALOTRON 1 PART NO. 4G59751 OPERATIONS MANUAL GETZ EQUIPMENT INNOVATORS PEKIN, ILLINOIS, U.S.A. PHONE: (888) 747-4389 FAX: (309) 495-0625 Limited Warranty
GETZ EQUIPMENT INNOVATORS CLEAN AGENT FE-36 / HALOTRON 1 PART NO. 4G59751 OPERATIONS MANUAL GETZ EQUIPMENT INNOVATORS PEKIN, ILLINOIS, U.S.A. PHONE: (888) 747-4389 FAX: (309) 495-0625 Limited Warranty
Instructions For Use. English 1 of 16
 Instructions For Use Introduction.......................................... 2 Indications for Use................................... 2 Contraindications.................................... 2 Caution...............................................
Instructions For Use Introduction.......................................... 2 Indications for Use................................... 2 Contraindications.................................... 2 Caution...............................................
INSTALLATION. and INSTRUCTION MANUAL. for QUALITY AIR BREATHING SYSTEMS. Model 50-P-Mini Portable Systems Outfitted with ABM-725 Monitor
 INSTALLATION and INSTRUCTION MANUAL for QUALITY AIR BREATHING SYSTEMS Model 50-P-Mini Portable Systems Outfitted with ABM-725 Monitor M A R T E C H S E R V I C E S C O M P A N Y P.O. Box 7079 OFFICE: (507)
INSTALLATION and INSTRUCTION MANUAL for QUALITY AIR BREATHING SYSTEMS Model 50-P-Mini Portable Systems Outfitted with ABM-725 Monitor M A R T E C H S E R V I C E S C O M P A N Y P.O. Box 7079 OFFICE: (507)
Shared Haemodialysis Care Handbook. Name Hospital number: Shared HD Care Nurse: Date: Machine type: Dialysis Unit:
 Shared Haemodialysis Care Handbook Name Hospital number: Shared HD Care Nurse: Machine type: Dialysis Unit: Contents Functions of the kidney/principles of Haemodialysis... 2 Hand washing for dialysis...
Shared Haemodialysis Care Handbook Name Hospital number: Shared HD Care Nurse: Machine type: Dialysis Unit: Contents Functions of the kidney/principles of Haemodialysis... 2 Hand washing for dialysis...
HemoCue Hb Procedure Template
 HemoCue Hb 201 + Procedure Template PURPOSE The HemoCue Hb 201 + System is used for the quantitative determination of hemoglobin in blood using a specially designed analyzer, HemoCue Hb 201 +, and specially
HemoCue Hb 201 + Procedure Template PURPOSE The HemoCue Hb 201 + System is used for the quantitative determination of hemoglobin in blood using a specially designed analyzer, HemoCue Hb 201 +, and specially
Fingertip Pulse Oximeter
 Instruction Manual Fingertip Pulse Oximeter Item # 40-810-000 Item # 40-811-000 Item # 40-812-000 Item # 40-813-000 Please read this guidebook completely before operating this unit. Limited Two-Year Warranty
Instruction Manual Fingertip Pulse Oximeter Item # 40-810-000 Item # 40-811-000 Item # 40-812-000 Item # 40-813-000 Please read this guidebook completely before operating this unit. Limited Two-Year Warranty
AQUARIUS Continuous Renal Replacement Therapy Competency
 Preparation Explain the procedure to the patient and significant others Prepare the patient. Prepare the environment. Ensure a safe environment for the patient and staff Gather equipment required to Setup
Preparation Explain the procedure to the patient and significant others Prepare the patient. Prepare the environment. Ensure a safe environment for the patient and staff Gather equipment required to Setup
APCO ASU-SCAV & ASU-CAV SINGLE BODY COMBINATION AIR VALVES
 APCO ASU-SCAV & ASU-CAV SINGLE BODY COMBINATION AIR VALVES Instruction D12039 December 2015 Instructions These instructions provide installation, operation and maintenance information for the. They are
APCO ASU-SCAV & ASU-CAV SINGLE BODY COMBINATION AIR VALVES Instruction D12039 December 2015 Instructions These instructions provide installation, operation and maintenance information for the. They are
Instructions and User Guide. Ultrasound Musculoskeletal Training Model BPMSK-1000 Series. April 26, 2016 Rev 1.0
 Instructions and User Guide Ultrasound Musculoskeletal Training Model BPMSK-1000 Series April 26, 2016 Rev 1.0 1 Overview 1 Giving you the confidence only experience can offer Congratulations on the purchase
Instructions and User Guide Ultrasound Musculoskeletal Training Model BPMSK-1000 Series April 26, 2016 Rev 1.0 1 Overview 1 Giving you the confidence only experience can offer Congratulations on the purchase
(12) United States Patent (10) Patent No.: US 6,524,267 B1
 USOO6524267B1 (12) United States Patent (10) Patent No.: US 6,524,267 B1 Gremel et al. 45) Date of Patent: Feb. 25, 2003 9 (54) VENOUS FILTER FOR ASSISTED VENOUS (56) References Cited RETUR N U.S. PATENT
USOO6524267B1 (12) United States Patent (10) Patent No.: US 6,524,267 B1 Gremel et al. 45) Date of Patent: Feb. 25, 2003 9 (54) VENOUS FILTER FOR ASSISTED VENOUS (56) References Cited RETUR N U.S. PATENT
CRRT with Prismaflex LEADS TO More Flexibility, Ease of Use and Safety
 CRRT with Prismaflex LEADS TO More Flexibility, Ease of Use and Safety Leading the way Science is changing and so are your requirements For many years, Gambro has focused its efforts on development of
CRRT with Prismaflex LEADS TO More Flexibility, Ease of Use and Safety Leading the way Science is changing and so are your requirements For many years, Gambro has focused its efforts on development of
Accu-Tab PowerBase 3012AT by Axiall Corporation
 Accu-Tab PowerBase 3012AT by Axiall Corporation Installation and Operating Instructions DANGER: DO NOT MIX CHEMICALS! The PowerBase 3012AT chlorinator system is designed for use with Axiall Corp. approved
Accu-Tab PowerBase 3012AT by Axiall Corporation Installation and Operating Instructions DANGER: DO NOT MIX CHEMICALS! The PowerBase 3012AT chlorinator system is designed for use with Axiall Corp. approved
6 & 8 Bubble Panel Installation Manual
 6 & 8 Bubble Panel Installation Manual IMPORTANT RETAIN FOR FUTURE REFERENCE READ CAREFULLY You must use Distilled Water only. Use of any water other than distilled voids the warranty. Please check the
6 & 8 Bubble Panel Installation Manual IMPORTANT RETAIN FOR FUTURE REFERENCE READ CAREFULLY You must use Distilled Water only. Use of any water other than distilled voids the warranty. Please check the
Using the Akta Prime plus October 22, 2012
 Some starting precautions: 1. Vacuum filter all buffers. Removes any large particles/debris that may clog your column De-gases the buffers 2. Clarify lysates first by centrifugation and then filtration
Some starting precautions: 1. Vacuum filter all buffers. Removes any large particles/debris that may clog your column De-gases the buffers 2. Clarify lysates first by centrifugation and then filtration
B. Assemble the Method-25 sample train with a heated probe/filter.
 6/30/2015 VOC Reporting, Inc. Method 25 Heated Controller Operating Instructions. pg. 1 of 5 INSTRUCTIONS FOR EPA METHOD-25 FIELD SAMPLE COLLECTION USING A VRi HEATED PROBE AND FILTER ASSEMBLY Introduction:
6/30/2015 VOC Reporting, Inc. Method 25 Heated Controller Operating Instructions. pg. 1 of 5 INSTRUCTIONS FOR EPA METHOD-25 FIELD SAMPLE COLLECTION USING A VRi HEATED PROBE AND FILTER ASSEMBLY Introduction:
Standard. Abdominal Aortic Aneurysm (AAA) Repair Trainer. Part No: 60610
 User Guide Abdominal Aortic Aneurysm (AAA) Repair Trainer Part No: 6060 Standard Part No: 065-088 Issue, June 005 04 Limbs & Things For more Vascular training products visit limbsandthings.com Limbs &
User Guide Abdominal Aortic Aneurysm (AAA) Repair Trainer Part No: 6060 Standard Part No: 065-088 Issue, June 005 04 Limbs & Things For more Vascular training products visit limbsandthings.com Limbs &
Concentrate Distribution System (Stand Mounted) A-CDS-70-X & A-CDS-TANK-X-ST Industrial Drive, Orlinda, TN 37141
 0 Operation Manual Concentrate Distribution System (Stand Mounted) A-CDS-70-X & A-CDS-TANK-X-ST 615-654-4441 sales@specialtyh2o.com 615-654-4449 fax TABLE OF CONTENTS Section 1 GENERAL 1.1 Warnings and
0 Operation Manual Concentrate Distribution System (Stand Mounted) A-CDS-70-X & A-CDS-TANK-X-ST 615-654-4441 sales@specialtyh2o.com 615-654-4449 fax TABLE OF CONTENTS Section 1 GENERAL 1.1 Warnings and
Optimized Perfusion Management and Patient Outcome
 Optimized Perfusion Management and Patient Outcome GEORGE JUSTISON CCP MANAGER PERFUSION SERVICES UNIVERSITY OF COLORADO HOSPITAL University of Colorado Hospital Main hospital in University of Colorado
Optimized Perfusion Management and Patient Outcome GEORGE JUSTISON CCP MANAGER PERFUSION SERVICES UNIVERSITY OF COLORADO HOSPITAL University of Colorado Hospital Main hospital in University of Colorado
Advanced IV Hand LF01139U & LF01146U Instruction Manual
 Advanced IV Hand LF01139U & LF01146U Instruction Manual WARNING: This product contains dry natural rubber. Do not remove film from tubing! Products by Nasco List of Components 3 cc syringe with needle
Advanced IV Hand LF01139U & LF01146U Instruction Manual WARNING: This product contains dry natural rubber. Do not remove film from tubing! Products by Nasco List of Components 3 cc syringe with needle
Troubleshooting Guide Aquarius
 Low Access Pressure Access pressure has dropped below the lower alarm limit of 0 to - 250mmHg Blood pump is off Blood flow is too low Turn blood pump on Check blood flow rate. Consider increasing the blood
Low Access Pressure Access pressure has dropped below the lower alarm limit of 0 to - 250mmHg Blood pump is off Blood flow is too low Turn blood pump on Check blood flow rate. Consider increasing the blood
Arterial and Venous Patient Training Arm S
 Arterial and Venous Patient Training Arm S402.100 Gaumard Simulators for Health Care Education Arterial and Venous Patient Training Arm is an interactive educational system developed to assist a certified
Arterial and Venous Patient Training Arm S402.100 Gaumard Simulators for Health Care Education Arterial and Venous Patient Training Arm is an interactive educational system developed to assist a certified
Skills & Scenarios 81
 Skills & Scenarios 81 Psychomotor Skills Checklist Skill Lab Clinical Date Initial Date Initial 1. Change I.V. tubing 2. Change I.V solution 3. Regulate I.V. flow rate 4. Flush peripheral lock 5. Change
Skills & Scenarios 81 Psychomotor Skills Checklist Skill Lab Clinical Date Initial Date Initial 1. Change I.V. tubing 2. Change I.V solution 3. Regulate I.V. flow rate 4. Flush peripheral lock 5. Change
Table Top and Convertible Anesthesia Machine Models: M3000 and M3100 (SHOWN WITH OPTIONAL EQUIPMENT)
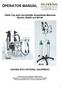 OPERATOR MANUAL Table Top and Convertible Anesthesia Machine Models: M3000 and M3100 M3000 M3100 (SHOWN WITH OPTIONAL EQUIPMENT) 12330 SE Hwy 212 Clackamas, Oregon 97015 (503) 822-1157 Local and International
OPERATOR MANUAL Table Top and Convertible Anesthesia Machine Models: M3000 and M3100 M3000 M3100 (SHOWN WITH OPTIONAL EQUIPMENT) 12330 SE Hwy 212 Clackamas, Oregon 97015 (503) 822-1157 Local and International
Abdominal Aortic Aneurysm (AAA) Repair Trainer User Guide
 Abdominal Aortic Aneurysm (AAA) Repair Trainer User Guide Designed and manufactured by Limbs & Things Limited, Sussex Street, St. Philips, Bristol, BS 0RA, UK. Telephone: +44 (0)7 3 0500 Fax: +44 (0)7
Abdominal Aortic Aneurysm (AAA) Repair Trainer User Guide Designed and manufactured by Limbs & Things Limited, Sussex Street, St. Philips, Bristol, BS 0RA, UK. Telephone: +44 (0)7 3 0500 Fax: +44 (0)7
