Hongyun Yang 2, Donald R. Cahela 1 and Bruce J. Tatarchuk 1
|
|
|
- Claire Little
- 5 years ago
- Views:
Transcription
1 A Study of Kinetic Effects Using Microfibrous ous Entrapped ZnO Sorbents for H 2 S Removal Hongyun Yang 2, Donald R. ahela and Bruce J. Tatarchuk. enter for Microfibrous Materials Manufacturing Department of hemical Engineering Auburn University, AL, IntraMicron Inc., 368 Industry Dr., Auburn, AL, November 7, 27 AIhE 27 Annual Meeting, Salt Lake ity, Utah
2 Objectives To establish a mathematic model for adsorption/reaction processes using both packed beds and microfibrous entrapped sorbents. To investigate the effects due to using microfibrous media. AIhE 27 Annual Meeting, Salt Lake ity, Utah 2
3 Outline Microfibrous Entrapped Sorbents/atalysts Model Evaluation Particle size effects The relationship between lumped shape factor (K) and apparent reaction rate constant (k a ) The relationship between K and challenge concentration ( A ) The relationship between K and sorebent bed capacity density ( c ) Effects of microfibrous media and void omposite Bed (Packed Bed+Polisher) onclusions Acknowledgements AIhE 27 Annual Meeting, Salt Lake ity, Utah 3
4 Microfibrous Entrapped Sorbents/atalysts t t oncept: Properties: Unique form factors and thermal integration High contacting efficiency High intrabed thermal conductivity Enhanced mass and heat transfer Ease of regeneration High voidage (i.e., 75% for glass fiber entrapped sorbents) Microfibrous Entrapped Micro-catalyst/sorbent particles AIhE 27 Annual Meeting, Salt Lake ity, Utah 4
5 Glass Fiber Entrapped Sorbents SiO 2 particle glass fiber 2m Properties of Glass Fiber Entrapped Sorbent (GFE) omponent Wt.% Vol.% ZnO 2 N.A. SiO Fiber 22 3 Void N.A. 75 Glass fiber entrapped SiO 2 particles AIhE 27 Annual Meeting, Salt Lake ity, Utah 5
6 Bed Service Time Models General Equation t=a +B Term Yoon s Model Wheeler Model Mecklenburg Model A W e F W W s F c W s AZn' F c B W e kf W k s c v An' dg ( ) a c.4 ( ) D a.67 W s F Yoon s model b b ln ln A b A ln b A A ln A K t K=? Yoon, Y.H., Nelson, J.H., (984). Application of Gas Adsorption Kinetics: I. A Theoretical Model for Respirator artridge Service Life. American Industrial Hygiene Association Journal, 45, AIhE 27 Annual Meeting, Salt Lake ity, Utah 6
7 Modified Amundson Model For a second order heterogeneous reaction Amundson model A A k 2c zt exp k t exp 2 A U Modified Amundson model A ln k A 2 A A ln K t A t k 2 k a c c defined as capacity density, is actually an effective ZnO molar density. Apparent reaction rate Gas reactant A K k a c Solid reactant Amundson, N.R., (948). A note on the mathematics of adsorption in beds. Journal of Physical olloid hemistry, 52, AIhE 27 Annual Meeting, Salt Lake ity, Utah 7
8 Mass Transfer orrelation for Apparent Reaction Rate k k a c 6( ) d p D AB kc Sh 3 d Sh J d Re Sc p J d.24 Re.39 Re" Re ( ) k a d p U.6 Sc 3 D d AB 2 p K.6.39 d pu DAB 3 A Sc 2 d p c 7.44 a f a p External surface area based average For Packed Bed d d d s f f p p d su DAB 3 k a Sc d s d p K d U D d sd p c For Microfibrous Media.39 s 3 AB A Sc AIhE 27 Annual Meeting, Salt Lake ity, Utah 8
9 Model Evaluation Breakthrough curve Ln( /-)~t 6 4 H2S / Ln(/-) = -.257t R 2 = ln(/- -) Time (min) A commercial ZnO sorbent (6-8 mesh, g) was tested t with challenge gas concentration ti of 2 vol. % H 2 S in H 2 and flow rate of ml/min STP, at 4 º. AIhE 27 Annual Meeting, Salt Lake ity, Utah 9
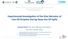
10 Particle Size Effects H2S / ZnO.5 mm ZnO 4-6 mesh ZnO 6-8 mesh ZnO 8- mesh ZnO -4 mesh ZnO/SiO2 GFES Time (min) Breakthrough curves of the commercial ZnO sorbent particles (.2 g,.8 g ZnO) with different sizes, and these of ZnO/SiO2 sorbent and glass fiber entrapped ZnO/SiO2 sorbent (GFES). Tested with challenge gas of 2 vol. % H 2 S in H 2 at a face velocity of.2 cm/s at 4 º. AIhE 27 Annual Meeting, Salt Lake ity, Utah
11 Lumped K vs. k a H2S / m:v..6 2m:2v m:4v.4.4 8m:8v GFES.2.. K (s - ) K K =.7-4 k a R 2 =.996 A ka c A Apparent rate constant k a (s - ) Time (min) Breakthrough curves of ZnO/SiO 2 sorbent at different face velocities (m=.g, v=.24 cm/s). Tested with challenge gas of 2 vol. % H 2 S in H 2 at 4 º. AIhE 27 Annual Meeting, Salt Lake ity, Utah c 4
12 Lumped K vs. / H2S K =2.6 K 2 =.2 K 3 =.67 =2ppmv =98 ppmv =495 ppmv K (s - ) K = 8843 A 2 R 2 =.9985.E+.E-7 2.E-7 3.E-7 4.E-7 H 2S initial concentration (mol/cm 3 ) K k a Time (min) c k A a c In each experiment,.8 g ZnO/SiO 2 was tested at face velocity of 5. cm/s at 4º. AIhE 27 Annual Meeting, Salt Lake ity, Utah 2
13 Lumped K vs. c H2S / ZnO wt% 7% 3% 9% 5% Time (min) Breakthrough curves of ZnO/SiO 2 sorbents at various ZnO loadings and the same amount of ZnO of.34 g. Sorbents tested with challenge gas of 2 vol. % H 2 S in H 2 at a face velocity of.2 cm/s at 4 º. AIhE 27 Annual Meeting, Salt Lake ity, Utah 3
14 Lumped K vs. c ont d c / H2S sorbent only M sorbent mixed with part inert M2 sorbent mixed with 2 part inert M3 sorbent mixed with 2 part inert M4 sorbent mixed with 2 part inert Time (min) Breakthrough curves of ZnO/SiO 2 sorbents (.2g ZnO/SiO 2 with ZnO loading at 7 wt.%) diluted by various amount of SiO 2 particles. Tested with challenge gas of 2 vol. % H 2 S in H 2 at a face velocity of.2 cm/s at 4 º. AIhE 27 Annual Meeting, Salt Lake ity, Utah 4
15 Lumped K vs. c -ont d c K= /c R 2 =.999 ka A =.95-5 A K k A a c K (s - ) K= /c R 2 = B / c (cm 3 /mol) Tested at a face velocity of.2 cm/s at 4 with 2 vol. % H 2 S -H 2. (A) packed kdbd beds of fzo/sio ZnO/SiO 2 sorbents (-2 m) ) with various ZnO Oloadings (B) diluted packed beds of ZnO/SiO 2 sorbents (-2 m). AIhE 27 Annual Meeting, Salt Lake ity, Utah 5
16 Effects of Void and Microfibrous Media Packed bed Packed bed Microfibrous (void iddiluted) d) entrapped sorbents* c (mol/cm ) d p (m) d s (m) k c /k cp k a /k ap.6.22 K/K p.67.3 K/K p (experimental) -.32 *ontains 3 vol.% 8um glass fiber AIhE 27 Annual Meeting, Salt Lake ity, Utah 6
17 Kinetics in omposite Bed H2S /.9 A-Polisher -2 m ZnO/SiO 2 in microfibrous media SiO 2 24 vol.% and 53 w t. % ZnO 2 w t.% of composite Thickness: 4.4mm B+A Packed Bed +Polisher B+A B-Packed Bed Performance of Polishing Sorbent and Packed Bed + Polishing ppm H 2S in H 2 2, 4 o, 8.5 cm/s..3.2 Area=Area2 G72-E, 3/6" extraduate.7 9 w t.% ZnO Bed thickness: 2.2 cm BedID:24cm ID:2.4. Packed Bed 56 % omposite Bed 35 % BT ppm BT 94 vs. 29 min. Time (min.) AIhE 27 Annual Meeting, Salt Lake ity, Utah 7
18 Kinetics in omposite Bed K (s - ) τ (min.) z c (cm) Test initial final initial final ( ppmv) (. ppmv)* Polishing layer Packed bed omposite bed AIhE 27 Annual Meeting, Salt Lake ity, Utah 8
19 onclusions A modified damundson model successfully characterized dthe adsorption process taking place in fixed bed reactors; Lumped K is introduced to describe the breakthrough curves. K is a function of apparent reaction rate, challenge gas concentration, and sorbent capacity density; High voidage decreased the apparent reaction rate; the micronsized fibers reduce the characteristic dimension. The low capacity density increase the lumped K; Microfibrous entrapped sorbents have low capacity but high sorbent utilization. omposite bed (a packed bed followed by microfibrous entrapped sorbent polisher) synergistically combines the high volume loading of packed beds and the overall contacting efficiency of small particulates. AIhE 27 Annual Meeting, Salt Lake ity, Utah 9
20 Acknowledgements This work was supported by the US Army under a U.S. Army contract at Auburn University (ARMY-W56HZV ) administered i d through h the US Army Tank- Automotive Research, Development and Engineering enter (TARDE). AIhE 27 Annual Meeting, Salt Lake ity, Utah 2
21 Thank you for your attention AIhE 27 Annual Meeting, Salt Lake ity, Utah 2
Kyoung-Su Im, Z.-C Zhang, and Grant Cook, Jr. LSTC, Livermore, CA USA. 06/14/2016, Dearborn, MI
 Kyoung-Su Im, Z.-C Zhang, and Grant Cook, Jr. LSTC, Livermore, CA 94551 USA 06/14/2016, Dearborn, MI New Inflator Models! Inflator models In LS-DYNA: Pyrotechnic inflator (PI) model. Hybrid cold flow model
Kyoung-Su Im, Z.-C Zhang, and Grant Cook, Jr. LSTC, Livermore, CA 94551 USA 06/14/2016, Dearborn, MI New Inflator Models! Inflator models In LS-DYNA: Pyrotechnic inflator (PI) model. Hybrid cold flow model
Chemistry 51 Chapter 7 PROPERTIES OF GASES. Gases are the least dense and most mobile of the three phases of matter.
 ROERIES OF GASES Gases are the least dense and most mobile of the three phases of matter. articles of matter in the gas phase are spaced far apart from one another and move rapidly and collide with each
ROERIES OF GASES Gases are the least dense and most mobile of the three phases of matter. articles of matter in the gas phase are spaced far apart from one another and move rapidly and collide with each
Figure 1 Schematic of opposing air bearing concept
 Theoretical Analysis of Opposing Air Bearing Concept This concept utilizes air bearings to constrain five degrees of freedom of the optic as shown in the figure below. Three pairs of inherently compensated
Theoretical Analysis of Opposing Air Bearing Concept This concept utilizes air bearings to constrain five degrees of freedom of the optic as shown in the figure below. Three pairs of inherently compensated
Chapter 11. Recall: States of Matter. Properties of Gases. Gases
 Chapter 11 Gases Recall: States of Matter Solids and Liquids: are closely related because in each case the particles are interacting with each other Gases: Properties of Gases Gases can be compressed Gases
Chapter 11 Gases Recall: States of Matter Solids and Liquids: are closely related because in each case the particles are interacting with each other Gases: Properties of Gases Gases can be compressed Gases
Chapter 5 TEST: Gases
 Chapter 5 TEST: Gases 1) Gases generally have A) low density B) high density C) closely packed particles D) no increase in volume when temperature is increased E) no decrease in volume when pressure is
Chapter 5 TEST: Gases 1) Gases generally have A) low density B) high density C) closely packed particles D) no increase in volume when temperature is increased E) no decrease in volume when pressure is
You should be able to: Describe Equipment Barometer Manometer. 5.1 Pressure Read and outline 5.1 Define Barometer
 A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
Name Chemistry Pre-AP
 Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Gilbert Kirss Foster. Chapter 10. Properties of Gases The Air We Breathe
 Gilbert Kirss Foster Chapter 10 Properties of Gases The Air We Breathe Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4
Gilbert Kirss Foster Chapter 10 Properties of Gases The Air We Breathe Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4
MoLE Gas Laws Activities
 MoLE Gas Laws Activities To begin this assignment you must be able to log on to the Internet using Internet Explorer (Microsoft) 4.5 or higher. If you do not have the current version of the browser, go
MoLE Gas Laws Activities To begin this assignment you must be able to log on to the Internet using Internet Explorer (Microsoft) 4.5 or higher. If you do not have the current version of the browser, go
1. A pure substance has a specific volume of 0.08 L/mol at a pressure of 3 atm and 298 K. The substance is most likely:
 Name: September 19, 2014 EXAM 1 P a g e 1 1. A pure substance has a specific volume of 0.08 L/mol at a pressure of 3 atm and 298 K. The substance is most likely: a. Liquid b. Gas c. Supercritical Fluid
Name: September 19, 2014 EXAM 1 P a g e 1 1. A pure substance has a specific volume of 0.08 L/mol at a pressure of 3 atm and 298 K. The substance is most likely: a. Liquid b. Gas c. Supercritical Fluid
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works.
 Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
GASES. Unit #8. AP Chemistry
 GASES Unit #8 AP Chemistry I. Characteristics of Gases A. Gas Characteristics: 1. Fills its container a. no definite shape b. no definite vol. 2. Easily mixes w/ other gases 3. Exerts pressure on its surroundings
GASES Unit #8 AP Chemistry I. Characteristics of Gases A. Gas Characteristics: 1. Fills its container a. no definite shape b. no definite vol. 2. Easily mixes w/ other gases 3. Exerts pressure on its surroundings
SCH3U7 Quantitative Chemistry
 SCH3U7 Quantitative Chemistry So far, we have looked at solids and liquids (solutions) Today we will look at gases and the laws that govern their behaviour in chemical reactions 4 Factors Affecting Gases
SCH3U7 Quantitative Chemistry So far, we have looked at solids and liquids (solutions) Today we will look at gases and the laws that govern their behaviour in chemical reactions 4 Factors Affecting Gases
CHEMISTRY - CLUTCH CH.5 - GASES.
 !! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
!! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
Topic 6: Gases and Colligative Properties
 Topic 6: Gases and Colligative Properties Ideal Gas Equation Boyle noticed an inverse relationship between volume and pressure. Pressure x volume = constant P = a P 1/P Charles found the volume of a gas,
Topic 6: Gases and Colligative Properties Ideal Gas Equation Boyle noticed an inverse relationship between volume and pressure. Pressure x volume = constant P = a P 1/P Charles found the volume of a gas,
Reactivity Improvement of Ca (OH) 2 Sorbent Using Diatomaceous Earth (DE) From Aceh Province
 Research Inventy: International Journal of Engineering And Science Vol.8, Issue 2 (May 2018), PP -05-10 Issn (e): 2278-4721, Issn (p):2319-6483, www.researchinventy.com Reactivity Improvement of Ca (OH)
Research Inventy: International Journal of Engineering And Science Vol.8, Issue 2 (May 2018), PP -05-10 Issn (e): 2278-4721, Issn (p):2319-6483, www.researchinventy.com Reactivity Improvement of Ca (OH)
Completed ALL 2 Warm-up IC Kinetic Molecular Theory Notes. Kinetic Molecular Theory and Pressure Worksheet
 Name: Unit 10- Gas Laws Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 5 Kinetic Molecular Theory Notes IC 1 6 8 Kinetic Molecular Theory and Pressure Worksheet IC 2 9 10 Gas Law
Name: Unit 10- Gas Laws Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 5 Kinetic Molecular Theory Notes IC 1 6 8 Kinetic Molecular Theory and Pressure Worksheet IC 2 9 10 Gas Law
Chapter 6 10/14/13. Gas Law. Volume change with temperature and pressure.
 Chapter 6 10/14/13 Gas Law 1. Properties of a Gas a. Neither definite shape nor volume i. Uniformly fills any container i Exerts pressure on surroundings Volume change with temperature and pressure. b.
Chapter 6 10/14/13 Gas Law 1. Properties of a Gas a. Neither definite shape nor volume i. Uniformly fills any container i Exerts pressure on surroundings Volume change with temperature and pressure. b.
Characterizing a Cu/Mn Alloy for Extracting Oxygen from Inert Gas. Streams
 Characterizing a Cu/Mn Alloy for Extracting Oxygen from Inert Gas Streams Grace Lenhard Prattsburgh Central School LLE advisors: Walter Shmayda and Matthew Sharpe Laboratory for Laser Energetics University
Characterizing a Cu/Mn Alloy for Extracting Oxygen from Inert Gas Streams Grace Lenhard Prattsburgh Central School LLE advisors: Walter Shmayda and Matthew Sharpe Laboratory for Laser Energetics University
Modeling Approaches to Increase the Efficiency of Clear-Point- Based Solubility Characterization
 Modeling Approaches to Increase the Efficiency of Clear-Point- Based Solubility Characterization Paul Larsen, Dallin Whitaker Crop Protection Product Design & Process R&D OCTOBER 4, 2018 TECHNOBIS CRYSTALLI
Modeling Approaches to Increase the Efficiency of Clear-Point- Based Solubility Characterization Paul Larsen, Dallin Whitaker Crop Protection Product Design & Process R&D OCTOBER 4, 2018 TECHNOBIS CRYSTALLI
Chapter 9 Gases: Their Properties and Behavior
 Chapter 9 Gases: Their Properties and Behavior 國防醫學院生化學科王明芳老師 2011-11-15 & 2011-11-22 Chapter 9/1 Gases and Gas Pressure Gas mixtures are homogeneous and compressible. Air-the mixture of gases. Molecular
Chapter 9 Gases: Their Properties and Behavior 國防醫學院生化學科王明芳老師 2011-11-15 & 2011-11-22 Chapter 9/1 Gases and Gas Pressure Gas mixtures are homogeneous and compressible. Air-the mixture of gases. Molecular
Figure Vapor-liquid equilibrium for a binary mixture. The dashed lines show the equilibrium compositions.
 Another way to view this problem is to say that the final volume contains V m 3 of alcohol at 5.93 kpa and 20 C V m 3 of air at 94.07 kpa and 20 C V m 3 of air plus alcohol at 100 kpa and 20 C Thus, the
Another way to view this problem is to say that the final volume contains V m 3 of alcohol at 5.93 kpa and 20 C V m 3 of air at 94.07 kpa and 20 C V m 3 of air plus alcohol at 100 kpa and 20 C Thus, the
Chapter 13. Gases. Copyright Cengage Learning. All rights reserved 1
 Chapter 13 Gases Copyright Cengage Learning. All rights reserved 1 Section 13.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage
Chapter 13 Gases Copyright Cengage Learning. All rights reserved 1 Section 13.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory
 CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
CHEM 3351 Physical Chemistry I, Fall 2017
 CHEM 3351 Physical Chemistry I, Fall 2017 Problem set 1 Due 9/15/2017 (Friday) 1. An automobile tire was inflated to a pressure of 24 lb in -2 (1.00 atm = 14.7 lb in -2 ) on a winter s day when the temperature
CHEM 3351 Physical Chemistry I, Fall 2017 Problem set 1 Due 9/15/2017 (Friday) 1. An automobile tire was inflated to a pressure of 24 lb in -2 (1.00 atm = 14.7 lb in -2 ) on a winter s day when the temperature
Chemistry A Molecular Approach. Fourth Edition. Chapter 5. Gases. Copyright 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
 Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
START UP MODELING OF KAIST MICRO MODULAR REACTOR COMPRESSOR USING BETA LINE METHOD WITH GAMMA+ CODE
 The 6th International Supercritical CO2 Power Cycles Symposium March 27-29, 2018, Pittsburgh, Pennsylvania START UP MODELING OF KAIST MICRO MODULAR REACTOR COMPRESSOR USING BETA LINE METHOD WITH GAMMA+
The 6th International Supercritical CO2 Power Cycles Symposium March 27-29, 2018, Pittsburgh, Pennsylvania START UP MODELING OF KAIST MICRO MODULAR REACTOR COMPRESSOR USING BETA LINE METHOD WITH GAMMA+
[2] After a certain time, the temperature of the water has decreased to below room temperature.
![[2] After a certain time, the temperature of the water has decreased to below room temperature. [2] After a certain time, the temperature of the water has decreased to below room temperature.](/thumbs/88/115891089.jpg) 1 (a) Explain, in terms of molecules, why it is possible to compress a gas, but not a liquid. (b) Two containers made of insulating material contain the same volume of water at room temperature. The containers
1 (a) Explain, in terms of molecules, why it is possible to compress a gas, but not a liquid. (b) Two containers made of insulating material contain the same volume of water at room temperature. The containers
Chapter 5. Nov 6 1:02 PM
 Chapter 5 Nov 6 1:02 PM Expand to fill their containers Fluid motion (they flow) Have low densities (1/1000 the density of equivalent liquids or solids) Compressible Can Effuse and Diffuse Effuse: The
Chapter 5 Nov 6 1:02 PM Expand to fill their containers Fluid motion (they flow) Have low densities (1/1000 the density of equivalent liquids or solids) Compressible Can Effuse and Diffuse Effuse: The
Honors Chemistry Unit 7 Gas Laws Notes
 Honors Chemistry Unit 7 Gas Laws Notes Kinetic Molecular Theory 1. List the five assumptions: Assumption Description Extra Info 1 Basically means: the particles themselves have compared to the space between
Honors Chemistry Unit 7 Gas Laws Notes Kinetic Molecular Theory 1. List the five assumptions: Assumption Description Extra Info 1 Basically means: the particles themselves have compared to the space between
Please do not write on this test. Please use the answer sheet. 1) Please choose all conditions that would allow a gas sample to behave ideally.
 AP Chemistry Test (Chapter 5) Please do not write on this test. Please use the answer sheet. Multiple Choice (50%) 1) Please choose all conditions that would allow a gas sample to behave ideally. I) Nonpolar
AP Chemistry Test (Chapter 5) Please do not write on this test. Please use the answer sheet. Multiple Choice (50%) 1) Please choose all conditions that would allow a gas sample to behave ideally. I) Nonpolar
Name Unit 9 Notes: Gas Laws Period. Complete throughout unit. Due on test day!
 Name Unit 9 Notes: Gas Laws Period Skills: 1. Gases and Entropy 2. Distinguish between Ideal and Real gases 3. Understand KMT and Avogadro s Law 4. Identify and Solve Boyle s Law Problems 5. Identify and
Name Unit 9 Notes: Gas Laws Period Skills: 1. Gases and Entropy 2. Distinguish between Ideal and Real gases 3. Understand KMT and Avogadro s Law 4. Identify and Solve Boyle s Law Problems 5. Identify and
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certifi cate of Education Advanced Level
 *0337350796* UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certifi cate of Education Advanced Level CHEMISTRY 9701/53 Paper 5 Planning, Analysis and Evaluation October/November 2012 1 hour
*0337350796* UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certifi cate of Education Advanced Level CHEMISTRY 9701/53 Paper 5 Planning, Analysis and Evaluation October/November 2012 1 hour
Ch. 11 Mass transfer principles
 Transport of chemical species in solid, liquid, or gas mixture Transport driven by composition gradient, similar to temperature gradients driving heat transport We will look at two mass transport mechanisms,
Transport of chemical species in solid, liquid, or gas mixture Transport driven by composition gradient, similar to temperature gradients driving heat transport We will look at two mass transport mechanisms,
CHEMISTRY 102D ASSIGNMENTS. WEEK 1 (August 23-27) Introduction, Classification of Matter, Significant Figures, Dimensional Analysis
 CHEMISTRY 102D ASSIGNMENTS WEEK 1 (August 23-27) Topics: Introduction, Classification of Matter, Significant Figures, Dimensional Analysis Reading: Zumdahl*, Chapter 1.1-1.3, 1.5-1.7, 1.9, Appendix A1.1
CHEMISTRY 102D ASSIGNMENTS WEEK 1 (August 23-27) Topics: Introduction, Classification of Matter, Significant Figures, Dimensional Analysis Reading: Zumdahl*, Chapter 1.1-1.3, 1.5-1.7, 1.9, Appendix A1.1
CHAPTER 14. The Behavior of Gases Properties of Gases. Factors Affecting Gas Pressure
 CHAPTER 14 The Behavior of Gases 14.1 Properties of Gases Compressibility:the volume of matter decreasing under pressure. Gases are easily compressed due to the large amount of space between gas particles.
CHAPTER 14 The Behavior of Gases 14.1 Properties of Gases Compressibility:the volume of matter decreasing under pressure. Gases are easily compressed due to the large amount of space between gas particles.
ANSWERS TO QUESTIONS IN THE NOTES AUTUMN 2018
 ANSWERS TO QUESTIONS IN THE NOTES AUTUMN 2018 Section 1.2 Example. The discharge in a channel with bottom width 3 m is 12 m 3 s 1. If Manning s n is 0.013 m -1/3 s and the streamwise slope is 1 in 200,
ANSWERS TO QUESTIONS IN THE NOTES AUTUMN 2018 Section 1.2 Example. The discharge in a channel with bottom width 3 m is 12 m 3 s 1. If Manning s n is 0.013 m -1/3 s and the streamwise slope is 1 in 200,
A. What are the three states of matter chemists work with?
 Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
Generating Calibration Gas Standards
 Technical Note 1001 Metronics Inc. Generating Calibration Gas Standards with Dynacal Permeation Devices Permeation devices provide an excellent method of producing known gas concentrations in the PPM and
Technical Note 1001 Metronics Inc. Generating Calibration Gas Standards with Dynacal Permeation Devices Permeation devices provide an excellent method of producing known gas concentrations in the PPM and
Chemistry Chapter 11 Test Review
 Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
MEASUREMENTS OF UNDERWATER EXPLOSION PERFORMANCES BY PRESSURE GAUGE USING FLUOROPOLYMER
 MEASUREMENTS OF UNDERWATER EXPLOSION PERFORMANCES BY PRESSURE GAUGE USING FLUOROPOLYMER Kenji MURATA, Katsuhiko TAKAHASHI, Yukio KATO* NOF Corporation 61-1 Kitakomatsudani, Taketoyo-cho, Chita-gun, Aichi
MEASUREMENTS OF UNDERWATER EXPLOSION PERFORMANCES BY PRESSURE GAUGE USING FLUOROPOLYMER Kenji MURATA, Katsuhiko TAKAHASHI, Yukio KATO* NOF Corporation 61-1 Kitakomatsudani, Taketoyo-cho, Chita-gun, Aichi
Chapter 10: Gases. Characteristics of Gases
 Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
Chem 110 General Principles of Chemistry
 CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
Kinetic-Molecular Theory of Matter
 Gases Properties of Gases Gas Pressure Gases What gases are important for each of the following: O 2, CO 2 and/or He? A. B. C. D. 1 2 Gases What gases are important for each of the following: O 2, CO 2
Gases Properties of Gases Gas Pressure Gases What gases are important for each of the following: O 2, CO 2 and/or He? A. B. C. D. 1 2 Gases What gases are important for each of the following: O 2, CO 2
THE UNIVERSITY OF TRINIDAD & TOBAGO FINAL EXAMINATION APRIL 2014 PLEASE READ ALL INSTRUCTIONS CAREFULLY BEFORE YOU BEGIN THIS EXAMINATION
 THE UNIVERSITY OF TRINIDAD & TOBAGO FINAL EXAMINATION APRIL 2014 ourse ode and Title: MATERIAL BALANE Programme: NETD HEMIAL ENGINEERING Date and Time: Tuesday 22 nd April 2014, 9.00 a.m. 12.00 noon Duration:
THE UNIVERSITY OF TRINIDAD & TOBAGO FINAL EXAMINATION APRIL 2014 ourse ode and Title: MATERIAL BALANE Programme: NETD HEMIAL ENGINEERING Date and Time: Tuesday 22 nd April 2014, 9.00 a.m. 12.00 noon Duration:
Chapter 12. The Gaseous State of Matter
 Chapter 12 The Gaseous State of Matter The air in a hot air balloon expands When it is heated. Some of the air escapes from the top of the balloon, lowering the air density inside the balloon, making the
Chapter 12 The Gaseous State of Matter The air in a hot air balloon expands When it is heated. Some of the air escapes from the top of the balloon, lowering the air density inside the balloon, making the
INTERACTION BETWEEN WIND-DRIVEN AND BUOYANCY-DRIVEN NATURAL VENTILATION Bo Wang, Foster and Partners, London, UK
 INTERACTION BETWEEN WIND-DRIVEN AND BUOYANCY-DRIVEN NATURAL VENTILATION Bo Wang, Foster and Partners, London, UK ABSTRACT Ventilation stacks are becoming increasingly common in the design of naturally
INTERACTION BETWEEN WIND-DRIVEN AND BUOYANCY-DRIVEN NATURAL VENTILATION Bo Wang, Foster and Partners, London, UK ABSTRACT Ventilation stacks are becoming increasingly common in the design of naturally
EXPERIMENTAL ANALYSIS OF FLOW OVER SYMMETRICAL AEROFOIL Mayank Pawar 1, Zankhan Sonara 2 1,2
 EXPERIMENTAL ANALYSIS OF FLOW OVER SYMMETRICAL AEROFOIL Mayank Pawar 1, Zankhan Sonara 2 1,2 Assistant Professor,Chandubhai S. Patel Institute of Technology, CHARUSAT, Changa, Gujarat, India Abstract The
EXPERIMENTAL ANALYSIS OF FLOW OVER SYMMETRICAL AEROFOIL Mayank Pawar 1, Zankhan Sonara 2 1,2 Assistant Professor,Chandubhai S. Patel Institute of Technology, CHARUSAT, Changa, Gujarat, India Abstract The
Example: 25 C = ( ) K = 298 K. Pressure Symbol: p Units: force per area 1Pa (Pascal) = 1 N/m 2
 Chapter 6: Gases 6.1 Measurements on Gases MH5, Chapter 5.1 Let s look at a certain amount of gas, i.e. trapped inside a balloon. To completely describe the state of this gas one has to specify the following
Chapter 6: Gases 6.1 Measurements on Gases MH5, Chapter 5.1 Let s look at a certain amount of gas, i.e. trapped inside a balloon. To completely describe the state of this gas one has to specify the following
A) It cannot be predicted. B) It is squared. C) It is doubled. D) It is halved. E) It does not change.
 AP Chemistry Test (Chapter 5) Class Set Multiple Choice (50%) 1) A sample of argon gas is sealed in a container. The volume of the container is doubled at a constant temperature. What happens to the pressure
AP Chemistry Test (Chapter 5) Class Set Multiple Choice (50%) 1) A sample of argon gas is sealed in a container. The volume of the container is doubled at a constant temperature. What happens to the pressure
Please do not write on this test. Please use the answer sheet.
 AP Chemistry Test (Chapter 5) Multiple Choice (50%) Please do not write on this test. Please use the answer sheet. Form: Snowboard 1) A sample of argon gas is sealed in a rigid metal tank. The temperature
AP Chemistry Test (Chapter 5) Multiple Choice (50%) Please do not write on this test. Please use the answer sheet. Form: Snowboard 1) A sample of argon gas is sealed in a rigid metal tank. The temperature
PSI Chemistry: Gases Multiple Choice Review
 PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
Fall 2004 Homework Problem Set 9 Due Wednesday, November 24, at start of class
 0.30 Fall 004 Homework Problem Set 9 Due Wednesday, November 4, at start of class Part A. Consider an iron surface which serves as a catalyst for the production of ammonia from nitrogen and hydrogen. The
0.30 Fall 004 Homework Problem Set 9 Due Wednesday, November 4, at start of class Part A. Consider an iron surface which serves as a catalyst for the production of ammonia from nitrogen and hydrogen. The
Gases. Unit 10. How do gases behave?
 Gases Unit 10 How do gases behave? Gases are perhaps the most mysterious of all of the phases of matter. For the most part gases are invisible to us, and it was once believed that in the air there is no
Gases Unit 10 How do gases behave? Gases are perhaps the most mysterious of all of the phases of matter. For the most part gases are invisible to us, and it was once believed that in the air there is no
Chapter 13 Gases and Pressure. Pressure and Force. Pressure is the force per unit area on a surface. Force Area. Pressure =
 Chapter 13 Gas Laws Chapter 13 Gases and Pressure Pressure and Force Pressure is the force per unit area on a surface. Pressure = Force Area Chapter 13 Gases and Pressure Gases in the Atmosphere The atmosphere
Chapter 13 Gas Laws Chapter 13 Gases and Pressure Pressure and Force Pressure is the force per unit area on a surface. Pressure = Force Area Chapter 13 Gases and Pressure Gases in the Atmosphere The atmosphere
CHEMISTRY 11 AP GAS LAW CALCULATIONS WORKSHEET
 CHEMISTRY 11 AP GAS LAW CALCULATIONS WORKSHEET 1) Consider the following identical containers each filled with 1 mole of helium gas. Container one is rigid and cannot expand. The piston in container two
CHEMISTRY 11 AP GAS LAW CALCULATIONS WORKSHEET 1) Consider the following identical containers each filled with 1 mole of helium gas. Container one is rigid and cannot expand. The piston in container two
EXPERIMENT 8 BUOYANT FORCES
 EXPERIMENT 8 BUOYANT FORCES INTRODUCTION: The purpose of this experiment is to determine buoyant forces on submerged solid objects, and to investigate the dependence of buoyant forces on volumes and masses
EXPERIMENT 8 BUOYANT FORCES INTRODUCTION: The purpose of this experiment is to determine buoyant forces on submerged solid objects, and to investigate the dependence of buoyant forces on volumes and masses
Elements that exist as gases at 25 o C and 1 atmosphere H 2, N 2, O 2, F 2, Cl 2, He, Ne, Ar, Kr, Xe, Rn
 AP Chemistry Chapter 5 Sections 5. 5.9 Note Organizer Pressure, The Gas Laws of Boyle, Charles, and Avogadro, The Ideal Gas Law, Gas Stoichiometry, Dalton s Law of Partial Pressure, The Kinetic olecular
AP Chemistry Chapter 5 Sections 5. 5.9 Note Organizer Pressure, The Gas Laws of Boyle, Charles, and Avogadro, The Ideal Gas Law, Gas Stoichiometry, Dalton s Law of Partial Pressure, The Kinetic olecular
Prevention Of Accidents Caused By Rotating Transit Bus Wheels By James M. Green, P.E., DEE
 Prevention Of Accidents Caused By Rotating Transit Bus Wheels By James M. Green, P.E., DEE Introduction The accident statistics for injuries caused by pedestrians or cyclists being injured, or killed,
Prevention Of Accidents Caused By Rotating Transit Bus Wheels By James M. Green, P.E., DEE Introduction The accident statistics for injuries caused by pedestrians or cyclists being injured, or killed,
AIR EJECTOR WITH A DIFFUSER THAT INCLUDES BOUNDARY LAYER SUCTION
 Engineering MECHANICS, Vol. 20, 2013, No. 3/4, p. 213 220 213 AIR EJECTOR WITH A DIFFUSER THAT INCLUDES BOUNDARY LAYER SUCTION Václav Dvořák* The article deals with axial-symmetric subsonic air-to-air
Engineering MECHANICS, Vol. 20, 2013, No. 3/4, p. 213 220 213 AIR EJECTOR WITH A DIFFUSER THAT INCLUDES BOUNDARY LAYER SUCTION Václav Dvořák* The article deals with axial-symmetric subsonic air-to-air
Chapter 10 Gases. Characteristics of Gases. Pressure. The Gas Laws. The Ideal-Gas Equation. Applications of the Ideal-Gas Equation
 Characteristics of Gases Chapter 10 Gases Pressure The Gas Laws The Ideal-Gas Equation Applications of the Ideal-Gas Equation Gas mixtures and partial pressures Kinetic-Molecular Theory Real Gases: Deviations
Characteristics of Gases Chapter 10 Gases Pressure The Gas Laws The Ideal-Gas Equation Applications of the Ideal-Gas Equation Gas mixtures and partial pressures Kinetic-Molecular Theory Real Gases: Deviations
Chemistry 20 Unit 2 Gases FITB Notes. Topic A Characteristics of Gases
 Chemistry 20 Unit 2 Gases FITB Notes General Outcome: Topic A Characteristics of Gases We use technologies that were designed with the knowledge of the visible characteristics ( ) of gases ex. SCUBA equipment,
Chemistry 20 Unit 2 Gases FITB Notes General Outcome: Topic A Characteristics of Gases We use technologies that were designed with the knowledge of the visible characteristics ( ) of gases ex. SCUBA equipment,
CFD AND EXPERIMENTAL STUDY OF AERODYNAMIC DEGRADATION OF ICED AIRFOILS
 Colloquium FLUID DYNAMICS 2008 Institute of Thermomechanics AS CR, v.v.i., Prague, October 22-24, 2008 p.1 CFD AND EXPERIMENTAL STUDY OF AERODYNAMIC DEGRADATION OF ICED AIRFOILS Vladimír Horák 1, Dalibor
Colloquium FLUID DYNAMICS 2008 Institute of Thermomechanics AS CR, v.v.i., Prague, October 22-24, 2008 p.1 CFD AND EXPERIMENTAL STUDY OF AERODYNAMIC DEGRADATION OF ICED AIRFOILS Vladimír Horák 1, Dalibor
AP TOPIC 6: Gases. Revised August General properties and kinetic theory
 AP OPIC 6: Gases General properties and kinetic theory Gases are made up of particles that have (relatively) large amounts of energy. A gas has no definite shape or volume and will expand to fill as much
AP OPIC 6: Gases General properties and kinetic theory Gases are made up of particles that have (relatively) large amounts of energy. A gas has no definite shape or volume and will expand to fill as much
Lecture Presentation. Chapter 10. Gases. John D. Bookstaver St. Charles Community College Cottleville, MO Pearson Education, Inc.
 Lecture Presentation Chapter 10 John D. Bookstaver St. Charles Community College Cottleville, MO Characteristics of Unlike liquids and solids, gases Expand to fill their containers. Are highly compressible.
Lecture Presentation Chapter 10 John D. Bookstaver St. Charles Community College Cottleville, MO Characteristics of Unlike liquids and solids, gases Expand to fill their containers. Are highly compressible.
Additional Reading General, Organic and Biological Chemistry, by Timberlake, chapter 8.
 Gas Laws EXPERIMENTAL TASK Determine the mathematical relationship between the volume of a gas sample and its absolute temperature, using experimental data; and to determine the mathematical relationship
Gas Laws EXPERIMENTAL TASK Determine the mathematical relationship between the volume of a gas sample and its absolute temperature, using experimental data; and to determine the mathematical relationship
Section 5.1 Pressure. Why study gases? An understanding of real world phenomena. An understanding of how science works.
 Chapter 5 Gases Section 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage Learning. All rights reserved 2 Section 5.1 Pressure
Chapter 5 Gases Section 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage Learning. All rights reserved 2 Section 5.1 Pressure
DEVIL PHYSICS THE BADDEST CLASS ON CAMPUS AP PHYSICS
 DEVIL PHYSICS THE BADDEST CLASS ON CAMPUS AP PHYSICS LSN 11-7: WAVE MOTION LSN 11-8: TYPES OF WAVES; LONGITUDINAL AND TRANSVERSE LSN 11-9: ENERGY TRANSPORTED BY WAVES Physics of Waves Questions From Reading
DEVIL PHYSICS THE BADDEST CLASS ON CAMPUS AP PHYSICS LSN 11-7: WAVE MOTION LSN 11-8: TYPES OF WAVES; LONGITUDINAL AND TRANSVERSE LSN 11-9: ENERGY TRANSPORTED BY WAVES Physics of Waves Questions From Reading
BODY FORM INFLUENCES ON THE DRAG EXPERIENCED BY JUNIOR SWIMMERS. Australia, Perth, Australia
 1 BODY FORM INFLUENCES ON THE DRAG EXPERIENCED BY JUNIOR SWIMMERS Andrew Lyttle 1, Nat Benjanuvatra 2, Brian A Blanksby 2, Bruce C Elliott 2 1 Western Australian Institute of Sport, Perth, Australia 2
1 BODY FORM INFLUENCES ON THE DRAG EXPERIENCED BY JUNIOR SWIMMERS Andrew Lyttle 1, Nat Benjanuvatra 2, Brian A Blanksby 2, Bruce C Elliott 2 1 Western Australian Institute of Sport, Perth, Australia 2
Kinetic Molecular Theory imaginary Assumptions of Kinetic Molecular Theory: Problems with KMT:
 AP Chemistry Ms. Ye Name Date Block Kinetic Molecular Theory Explains properties of gases, liquids, and solids in terms of energy using an ideal gas, an imaginary which fits all the assumptions of kinetic
AP Chemistry Ms. Ye Name Date Block Kinetic Molecular Theory Explains properties of gases, liquids, and solids in terms of energy using an ideal gas, an imaginary which fits all the assumptions of kinetic
Boyle s Law VC 09. Experiment 9: Gas Laws. Abstract
 Experiment 9: Gas Laws VC 09 Abstract In this laboratory activity, you will experimentally confirm Boyle s Law, determine absolute zero from Gay-Lussac's Law, and determine the molecular weight of acetone,
Experiment 9: Gas Laws VC 09 Abstract In this laboratory activity, you will experimentally confirm Boyle s Law, determine absolute zero from Gay-Lussac's Law, and determine the molecular weight of acetone,
Chapter 5. Pressure. Atmospheric Pressure. Gases. Force Pressure = Area
 Chapter 5 Gases Water for many homes is supplied by a well The pump removes air from the pipe, decreasing the air pressure in the pipe The pressure then pushes the water up the pipe Pressure Atmospheric
Chapter 5 Gases Water for many homes is supplied by a well The pump removes air from the pipe, decreasing the air pressure in the pipe The pressure then pushes the water up the pipe Pressure Atmospheric
SIMULATION OF ENTRAPMENTS IN LCM PROCESSES
 Douai, FRANCE - July 6 SIMULATION OF ENTRAPMENTS IN LCM PROCESSES René Arbter, Paolo Ermanni Centre of Structure Technologies ETH Zurich, Leonhardstr. 7, 89 Zurich, Switzerland: rarbter@ethz.ch ABSTRACT:
Douai, FRANCE - July 6 SIMULATION OF ENTRAPMENTS IN LCM PROCESSES René Arbter, Paolo Ermanni Centre of Structure Technologies ETH Zurich, Leonhardstr. 7, 89 Zurich, Switzerland: rarbter@ethz.ch ABSTRACT:
Measurements of k L a. Steady-State Mass Balance Method:
 Measurements of k a Steady-State Mass Balance Method: In theory, the K a in an apparatus that is operating continuously under steady-state conditions could be evaluated from the flow rates and the concentrations
Measurements of k a Steady-State Mass Balance Method: In theory, the K a in an apparatus that is operating continuously under steady-state conditions could be evaluated from the flow rates and the concentrations
Micro Channel Recuperator for a Reverse Brayton Cycle Cryocooler
 Micro Channel Recuperator for a Reverse Brayton Cycle Cryocooler C. Becnel, J. Lagrone, and K. Kelly Mezzo Technologies Baton Rouge, LA USA 70806 ABSTRACT The Missile Defense Agency has supported a research
Micro Channel Recuperator for a Reverse Brayton Cycle Cryocooler C. Becnel, J. Lagrone, and K. Kelly Mezzo Technologies Baton Rouge, LA USA 70806 ABSTRACT The Missile Defense Agency has supported a research
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory
 CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
Kinetic Molecular Theory Gases. Behavior of gases. Postulate two. Postulate one. Postulate three. Postulate four
 Kinetic Molecular Theory Gases Gas particles are so small that their individual volume can be considered to be negligible Gas particles are in constant motion and the collisions of the particles with the
Kinetic Molecular Theory Gases Gas particles are so small that their individual volume can be considered to be negligible Gas particles are in constant motion and the collisions of the particles with the
LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary
 ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
Investigation on 3-D Wing of commercial Aeroplane with Aerofoil NACA 2415 Using CFD Fluent
 Investigation on 3-D of commercial Aeroplane with Aerofoil NACA 2415 Using CFD Fluent Rohit Jain 1, Mr. Sandeep Jain 2, Mr. Lokesh Bajpai 3 1PG Student, 2 Associate Professor, 3 Professor & Head 1 2 3
Investigation on 3-D of commercial Aeroplane with Aerofoil NACA 2415 Using CFD Fluent Rohit Jain 1, Mr. Sandeep Jain 2, Mr. Lokesh Bajpai 3 1PG Student, 2 Associate Professor, 3 Professor & Head 1 2 3
Experimental Investigation of the Rise Behavior of Live-Oil Droplets during Deep-Sea Oil Spills
 Marine Technology Society (MTS) / Gulf of Mexico Research Initiative (GoMRI) Advancing Oil Spill Research Part 2 Webinar, March 13, 2018 Experimental Investigation of the Rise Behavior of Live-Oil Droplets
Marine Technology Society (MTS) / Gulf of Mexico Research Initiative (GoMRI) Advancing Oil Spill Research Part 2 Webinar, March 13, 2018 Experimental Investigation of the Rise Behavior of Live-Oil Droplets
Lecture 8 questions and answers The Biological Pump
 Lecture 8 questions and answers The Biological Pump (1) How long would it take a particle of about 2micron in size and a density of 1.5 g/cm 3 to get to the bottom of the sea (4000m)? How do particles
Lecture 8 questions and answers The Biological Pump (1) How long would it take a particle of about 2micron in size and a density of 1.5 g/cm 3 to get to the bottom of the sea (4000m)? How do particles
OPTIMIZATION OF RECUPERATER FIN GEOMETRY FOR MICRO GAS TURBINE
 OPTIMIZATION OF RECUPERATER FIN GEOMETRY FOR MICRO GAS TURBINE S.Ramamurthy 1 and Bharat Makwana 2 1 Scientist,National Aerospace Laboratories, Bangalore, ramamurthy_srm@yahoo.com 2 Engineer,INOX Private
OPTIMIZATION OF RECUPERATER FIN GEOMETRY FOR MICRO GAS TURBINE S.Ramamurthy 1 and Bharat Makwana 2 1 Scientist,National Aerospace Laboratories, Bangalore, ramamurthy_srm@yahoo.com 2 Engineer,INOX Private
ON THE DEGASSING KINETICS IN A LADLE EQUIPPED WITH A ROTATING IMPELLER ASSISTED THROUGH PHYSICAL MODELING
 ON THE DEGASSING KINETICS IN A LADLE EQUIPPED WITH A ROTATING IMPELLER ASSISTED THROUGH PHYSICAL MODELING Marco RAMÍREZ-ARGÁEZ a, Ivan TORRES a, Eudoxio RAMOS a, Carlos GONZÁLEZ a, José CAMACHO b a Facultad
ON THE DEGASSING KINETICS IN A LADLE EQUIPPED WITH A ROTATING IMPELLER ASSISTED THROUGH PHYSICAL MODELING Marco RAMÍREZ-ARGÁEZ a, Ivan TORRES a, Eudoxio RAMOS a, Carlos GONZÁLEZ a, José CAMACHO b a Facultad
Gas Law Review. Honors Chem.
 Gas Law Review Honors Chem. Question 1: KMT 1: What does KMT stand for? 2: Gas particles have no or. 3: Gas particles are not to or by each other. 4: measures the average kinetic energy of gas particles.
Gas Law Review Honors Chem. Question 1: KMT 1: What does KMT stand for? 2: Gas particles have no or. 3: Gas particles are not to or by each other. 4: measures the average kinetic energy of gas particles.
Simple Gas Laws. To facilitate comparison of gases, the following standards are used: STP: O C (273 K) and kpa. SATP: 25 C (298 K) and 101.
 Simple Gas Laws To facilitate comparison of gases, the following standards are used: STP: O C (273 K) and 101.3 kpa If assuming 1 mol, V = 22.4L SATP: 25 C (298 K) and 101.3 kpa If assuming 1 mol, V =
Simple Gas Laws To facilitate comparison of gases, the following standards are used: STP: O C (273 K) and 101.3 kpa If assuming 1 mol, V = 22.4L SATP: 25 C (298 K) and 101.3 kpa If assuming 1 mol, V =
Chapter 5 Gases. AP CHEMISTRY Chapter 5 Scotch Plains-Fanwood High School Page 1
 Chapter 5 Gases Kinetic Theory All matter is composed of tiny particles that are in continuous, random motion. Gas Pressure = Force Demo: Test tube/h2o beaker Area Demo: Can AP CHEMISTRY Chapter 5 Scotch
Chapter 5 Gases Kinetic Theory All matter is composed of tiny particles that are in continuous, random motion. Gas Pressure = Force Demo: Test tube/h2o beaker Area Demo: Can AP CHEMISTRY Chapter 5 Scotch
Kinetic-Molecular Theory
 GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
Gases NO CALCULATORS MAY BE USED FOR THESE QUESTIONS
 NO CALCULATORS MAY BE USED FOR THESE QUESTIONS Questions 1-3 refer to the following gases at 0 C and 1 atm. (A) Ar (B) NO 2 (C) Xe (D) H 2 (E) N 2 1. Has an average atomic or molecular speed closest to
NO CALCULATORS MAY BE USED FOR THESE QUESTIONS Questions 1-3 refer to the following gases at 0 C and 1 atm. (A) Ar (B) NO 2 (C) Xe (D) H 2 (E) N 2 1. Has an average atomic or molecular speed closest to
Chemistry HP Unit 6 Gases. Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases
 Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Gas Law Worksheets - WS: Boyle s and Charles Law
 Gas Law Worksheets - WS: Boyle s and Charles Law Boyle s Law states that the volume of a gas varies inversely with its pressure if temperature is held constant. (If one goes up the, other goes down.) We
Gas Law Worksheets - WS: Boyle s and Charles Law Boyle s Law states that the volume of a gas varies inversely with its pressure if temperature is held constant. (If one goes up the, other goes down.) We
POLYCULTURE OF GRASS CARP AND NILE TILAPIA WITH NAPIER GRASS AS THE SOLE NUTRIENT INPUT IN THE SUBTROPICAL CLIMATE OF NEPAL
 POLYCULTURE OF GRASS CARP AND NILE TILAPIA WITH NAPIER GRASS AS THE SOLE NUTRIENT INPUT IN THE SUBTROPICAL CLIMATE OF NEPAL Narayan P. Pandit, Madhav K. Shrestha* (IAAS, Nepal) Yang Yi (AIT, Thailand)
POLYCULTURE OF GRASS CARP AND NILE TILAPIA WITH NAPIER GRASS AS THE SOLE NUTRIENT INPUT IN THE SUBTROPICAL CLIMATE OF NEPAL Narayan P. Pandit, Madhav K. Shrestha* (IAAS, Nepal) Yang Yi (AIT, Thailand)
Gas Pressure. Pressure is the force exerted per unit area by gas molecules as they strike the surfaces around them.
 Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
To compare one gas to another, it is convenient to define a set of conditions: Standard Temperature and Pressure
 Standard Molar Volume To compare one gas to another, it is convenient to define a set of conditions: Standard Temperature and Pressure At STP, one mole of any gas has a volume of: 22.4 L = (This is a cube
Standard Molar Volume To compare one gas to another, it is convenient to define a set of conditions: Standard Temperature and Pressure At STP, one mole of any gas has a volume of: 22.4 L = (This is a cube
4.) There are no forces of attraction or repulsion between gas particles. This means that
 KINETIC MOLECULAR (K-M) THEORY OF MATTER NOTES - based on the idea that particles of matter are always in motion - assumptions of the K-M Theory 1.) Gases consist of large numbers of tiny particles that
KINETIC MOLECULAR (K-M) THEORY OF MATTER NOTES - based on the idea that particles of matter are always in motion - assumptions of the K-M Theory 1.) Gases consist of large numbers of tiny particles that
Additional Information
 Buoyancy Additional Information Any object, fully or partially immersed in a fluid, is buoyed up by a force equal to the weight of the fluid displaced by the object. Archimedes of Syracuse Archimedes principle
Buoyancy Additional Information Any object, fully or partially immersed in a fluid, is buoyed up by a force equal to the weight of the fluid displaced by the object. Archimedes of Syracuse Archimedes principle
Chemistry 1B Chapter 10 Worksheet - Daley. Name
 Name 1) The National Weather Service routinely supplies atmospheric pressure data to help pilots set their altimeters. The units the NWS uses for atmospheric pressure are inches of mercury. A barometric
Name 1) The National Weather Service routinely supplies atmospheric pressure data to help pilots set their altimeters. The units the NWS uses for atmospheric pressure are inches of mercury. A barometric
COMPARATIVE ANALYSIS OF THE FLOODING TIME MAIN ENGINE SHIP TYPE 888 DEFINED FROM CALCULATIONS AND MODEL RESEARCH
 Journal of KONES Powertrain and Transport, Vol. 17, No. 4 1 COMPARATIVE ANALYSIS OF THE FLOODING TIME MAIN ENGINE SHIP TYPE 888 DEFINED FROM CALCULATIONS AND MODEL RESEARCH Waldemar Mironiuk Naval University
Journal of KONES Powertrain and Transport, Vol. 17, No. 4 1 COMPARATIVE ANALYSIS OF THE FLOODING TIME MAIN ENGINE SHIP TYPE 888 DEFINED FROM CALCULATIONS AND MODEL RESEARCH Waldemar Mironiuk Naval University
Understanding Hydrated Lime Properties In Acid Gas Control
 Understanding Hydrated Lime Properties In Acid Gas Control Mike Tate McIlvaine Company Hot Topic Hour June 21, 2012 Understanding Sorbent Properties Material Handling Chemistry Reactivity Effect on Particulate
Understanding Hydrated Lime Properties In Acid Gas Control Mike Tate McIlvaine Company Hot Topic Hour June 21, 2012 Understanding Sorbent Properties Material Handling Chemistry Reactivity Effect on Particulate
11 Properties of Gases
 South asadena Honors Chemistry Name 11 roperties of Gases eriod Date S A I O N 1 E M E R A U R E Standard emperature is: 0 C or 273 K Convert: 26.0 C 299 K 400 K _127 C 100 K 173 C 135 C _408_ K -127 C
South asadena Honors Chemistry Name 11 roperties of Gases eriod Date S A I O N 1 E M E R A U R E Standard emperature is: 0 C or 273 K Convert: 26.0 C 299 K 400 K _127 C 100 K 173 C 135 C _408_ K -127 C
Multiple Choice (40%)
 AP Chemistry Test (Chapter 5) Please do not write on this test thank you! Multiple Choice (40%) 1) A sealed rigid container is filled with three ideal gases: A, B and C. The partial pressure of each gas
AP Chemistry Test (Chapter 5) Please do not write on this test thank you! Multiple Choice (40%) 1) A sealed rigid container is filled with three ideal gases: A, B and C. The partial pressure of each gas
