CHEMISTRY 102D ASSIGNMENTS. WEEK 1 (August 23-27) Introduction, Classification of Matter, Significant Figures, Dimensional Analysis
|
|
|
- Christine Arnold
- 5 years ago
- Views:
Transcription
1 CHEMISTRY 102D ASSIGNMENTS WEEK 1 (August 23-27) Topics: Introduction, Classification of Matter, Significant Figures, Dimensional Analysis Reading: Zumdahl*, Chapter , , 1.9, Appendix A1.1 Learn: The names and symbols of the first 36 elements on the periodic chart and those in the groups (columns) beginning with Li, Be, Cu, Zn, O, F and He. Problems: Zumdahl, Chapter 1: 19, 21, 25, 28, 31, 33, 35, 36, 37(a,b,e,f), 41, 42, 49-52, 73, 75, 77, 79-81, 91, 98, 99 Review Questions: Zumdahl, Chapter 1: 1, 4-7 Topics: Measurements, Units, Atomic Theory, Periodic Table, Nomenclature Reading: Zumdahl, Chapter 1.4, 2.3, Problems: Zumdahl, Chapter 1: Chapter 2: 20, 23-25, 28-30, 33, 47, 50, 51, 53, 57, 59, 61, 63-70, 72, 77, 79, 88, 89, 93-96, 105 Review Questions: Zumdahl, Chapter 2: 1 (a,b), 5-9 *Chemistry by Zumdahl & Zumdahl (Eighth Edition)
2 WEEK 2 (August 30-September 3) Topics: The Mole, Atomic Weight, Molar Mass, Formula Calculations, Percent Composition, Empirical and Molecular Formulas Reading: Zumdahl, Chapter Problems: Zumdahl, Chapter 3: 25, 26 33, 35, 41, 42, 61, 63, 71, 73-76, 80, 83, 84, 87, 88, 119, 121, 131, 135, 137, 138, 141 Review Questions: Zumdahl, Chapter 3: 1-6 Topics: Chemical Reactions, Stoichiometry - Mole and Mass Relations, Limiting Reagents, Percent Yield Reading: Zumdahl, Chapter Problems: Zumdahl, Chapter 3: 29, 30, 89, 90, 95, 96, 100, 105, , 111, , 145, 152 Review Questions: Zumdahl, Chapter 3: 7-10 WEEK 3 (September 6-10) Lecture: (no Monday lecture this week) Topics: Early Experiments to Characterize the Atom; Stoichiometry review Reading: Zumdahl, Chapter 2.4 Problems: Zumdahl, Chapter 2: 21, 22, 42 Review Questions: Zumdahl, Chapter 2: 4 Topics: Solutions: Concentration Units, Electrolytes, Dilution Reading: Zumdahl, Chapter 1.9, 2.8, Problems: Zumdahl, Chapter 1: 73, 75, 77, 79-81, 91 Chapter 2: 79, 80 Chapter 4: 15-16, 24, 29, 31, 33, 34, 37, 39, 100 Review Questions: Zumdahl, Chapter 4: 1-7
3 WEEK 4 (September 13-17) Topics: Solution Stoichiometry, Thermochemistry Reading: Zumdahl, Chapter , Note: The reading assignment in goes into too much detail. Concentrate on the material outlined in the lecture notes Problems: Zumdahl, Chapter 4: 13, 17-19, 43, 45, 47, 49-51, 53, 66*, 67* 20, 56, 57, 61, 63, 74, 75, 77, 93, 94, 97, 103, 109, 125, 128, 129 Chapter 6: 41, 43 Review Questions: Zumdahl, Chapter 4: 8 *For Exercises 4.66 and 4.67, only give the balanced formula equations. Topics: Gases - P, V, T Relationships, Ideal Gas Law, Stoichiometry, Partial Pressures Reading: Zumdahl, Chapter , Appendix A1.3 Problems: Zumdahl, Chapter 5: 23-26, 29, 41, 44, 48, 53, 55, 56, 65, 71, 75-77, 80, 81, 87, 89, 123, 124, 130 Review Questions: Zumdahl, Chapter 5: 1-5 WEEK 5 (September 20-24) Topics: Kinetic Molecular Theory, Real Gases Reading: Zumdahl, Chapter Problems: Zumdahl, Chapter 5: 27, 30-32, 99, , 106 Review Questions: Zumdahl, Chapter 5: 6-10 Topics: Review for Hour Exam I HOUR EXAM I: 7:00 pm THURSDAY, 9/23 (Location to be announced.) Covers all material through Week 4
4 WEEK 6 (September 27-October 1) Topics: Electromagnetic Radiation, Atomic Spectra, the Bohr Model, The Quantum Mechanical Model Reading: Zumdahl, Chapter Problems: Zumdahl, Chapter 7: 20, 23, 32, 41-44, 53, 55, 60, 61, 63, Review Questions: Zumdahl, Chapter 7: 1-3 Topics: Orbitals and Electronic Structure; Periodic Table Reading: Zumdahl, Chapter Problems: Zumdahl, Chapter 7: 26, 33, 68, 72, 74, 77, 78, 81, 83, 85, 87, 92-96, 124, 126 Chapter 8: 44 Handouts Book, p. 50: Problems at the bottom of the page Review Questions: Zumdahl, Chapter 7: 6-8 WEEK 7 (October 4-8) Topics: Periodic Properties Reading: Zumdahl, Chapter , 8.4 Problems: Zumdahl, Chapter 7: 28, 29, 99, 101, 103, 104, , 112, 117, 147, 148, 163 Chapter 8: 21, 39, 49-51, 129 Review Questions: Zumdahl, Chapter 7: 9, 10 Chapter 8: 2 Topics: Introduction to Bonding, Lewis Structures, Nonoctets, Resonance Reading: Zumdahl, Chapter , Problems: Zumdahl, Chapter 8: 15-17, 19, 24, 27, 33-35, 37, 38, 41, 46, 79-89, 92, 93, 133, 136 Handouts Book, p. 57: Complete the Lewis structures for the organic compounds listed on the bottom of this page. Review Questions: Zumdahl, Chapter 8: 1, 5, 6
5 WEEK 8 (October 11-15) Topics: VSEPR, Polarity Reading: Zumdahl, Chapter 8.2, 8.3, 8.13 Problems: Zumdahl, Chapter 8: 20, 26, , , 127, 151 Review Questions: Zumdahl, Chapter 8: 8-10 Topics: Hybrid Orbitals, Delocalization Reading: Zumdahl, Chapter 9.1, 9.5 Problems: Zumdahl, Chapter 9: 6, 9, 11, 12, 16-22, 27, 28, 31, 57, 59, 61, 64, 79 Review Questions: Zumdahl, Chapter 9: 1-4, 10 WEEK 9 (October 18-22) Topics: Intermolecular Forces and Physical Properties Reading: Zumdahl, Chapter , 10.8 (vapor pressure discussion only) Problems: Zumdahl, Chapter 10: 5, 10-14, 21, 25, 33, 35-39, 117, 128 Review Questions: Zumdahl, Chapter 10: 1 Topics: Chemical Equilibrium Reading: Zumdahl, Chapter Problems: Zumdahl, Chapter 13: 11-14, 21, 23, 25, 27, 29, 33, 35, 37, 38, 43, 44 Review Questions: Zumdahl, Chapter 13: 1-5
6 WEEK 10 (October 25-29) Topics: Reaction Quotient, Equilibrium Calculations, LeChatelier's Principle Reading: Zumdahl, Chapter Note: For Section 13.6, concentrate on the pages that discuss treating systems that have small equilibrium constants. Problems: Zumdahl, Chapter 13: 15-17, 19, 20, 28, 39, 41, 47-49, 57, 59, 61, 63, 64, 67-69, 92, 93 Review Questions: Zumdahl, Chapter 13: 6-10 Topics: Review for Hour Exam II HOUR EXAM II: 7:00 pm THURSDAY, 10/28 (Location to be announced.) Covers material through Week 9 WEEK 11 (November 1-5) Topics: Introduction to Acids and Bases, ph Calculations: Strong Acids, Strong Bases Reading: Zumdahl, Chapter , 14.6, Appendix A1.2 Problems: Zumdahl, Chapter 14: 20, 21, 23, 27, 33, 35, 37-39, 41, 43, 44, 47, 51-53, 55, 57, 59, 87, 89, 91, 92, 149, 150 Review Questions: Zumdahl, Chapter 14: 2-4 Topics: Weak Acids, Weak Bases Reading: Zumdahl, Chapter , 14.12, Appendix A1.4 Problems: Zumdahl, Chapter 14: 28, 61-63, 65, 73-75, 77, 85, 95, 96, 101, 102, 156 Review Questions: Zumdahl, Chapter 14: 5, 6
7 WEEK 12 (November 8-12) Topics: Polyprotic Acids, Salts, Acid-Base Properties of Oxides Reading: Zumdahl, Chapter 14.7, 14.8, Problems: Zumdahl, Chapter 14: 25, 30, 32, 104, 107, 111, , 117, , 125, 131, 132, 147, 151, 158, 173 Review Questions: Zumdahl, Chapter 14: 7-9 Topics: Buffers Reading: Zumdahl, Chapter Problems: Zumdahl, Chapter 15: 10, 11, 15, 16, 27-30, 35-37, 41-44, 76, 77 Review Questions: Zumdahl, Chapter 15: 1-4 WEEK 13 (November 15-19) Topics: Titrations Reading: Zumdahl, Chapter 15.4 (with review of Chapter 4.8 on acid-base titrations) Problems: Zumdahl, Chapter 15: 49, 51*, 52*, 53, 55*, 61, 90, 91, 92*, 93, 96 *For Exercise 55, only calculate the ph at 0.0, 10.0, 12.5, 20.0, 25.0 and 30.0 ml of NaOH added, then sketch the titration curve. For Exercises 51, 52, and 92, only calculate the ph at the initial, half-way, and equivalence points, then sketch the titration curves. Review Questions: Zumdahl, Chapter 15: 5-7 Topics: Titrations Reading: Zumdahl, Chapter 15.4 Problems: Zumdahl, Chapter 15: 12, 13, 50, 54*, 57*, 59, 62, 103 *For Exercises and 15.57, only calculate the ph at the initial, half-way and equivalence points, then sketch the titration curves. Review Questions: Zumdahl, Chapter 15: 8 THANKSGIVING BREAK, NOVEMBER 22-26
8 WEEK 14 (November 29-December 3) Topics: Polyprotic Acid Titrations Reading: Zumdahl, Chapter 14.7; Handouts Book, Polyrotic Acid Titrations Problems : Handouts Book, Polyrotic Acid Titrations: Problems 1 and 2, pp Topics: Review for Hour Exam III HOUR EXAM III: 7:00 pm THURSDAY, 12/2 (Location to be announced.) Covers material through Week 13 WEEK 15 (December 6-8) Topics: Oxidation-Reduction Reactions Reading: Zumdahl, Chapter 4.9 Problems: Zumdahl, Chapter 4: 21, 79, 81, 83, 112 Review Questions: Zumdahl, Chapter 4: 9 Topics: Review for Final Quiz Section: (no quiz section Thursday) FINAL EXAM for CHEM 102D: 1:30-4:30 pm Friday, December 17
Chem 110 General Principles of Chemistry
 CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
Chapter 13. Gases. Copyright Cengage Learning. All rights reserved 1
 Chapter 13 Gases Copyright Cengage Learning. All rights reserved 1 Section 13.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage
Chapter 13 Gases Copyright Cengage Learning. All rights reserved 1 Section 13.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage
PURE SUBSTANCE. Nitrogen and gaseous air are pure substances.
 CLASS Third Units PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Air is a mixture of several gases, but it is considered to be a pure substance. Nitrogen and
CLASS Third Units PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Air is a mixture of several gases, but it is considered to be a pure substance. Nitrogen and
Chapter 11. Recall: States of Matter. Properties of Gases. Gases
 Chapter 11 Gases Recall: States of Matter Solids and Liquids: are closely related because in each case the particles are interacting with each other Gases: Properties of Gases Gases can be compressed Gases
Chapter 11 Gases Recall: States of Matter Solids and Liquids: are closely related because in each case the particles are interacting with each other Gases: Properties of Gases Gases can be compressed Gases
VIRGINIA STANDARDS OF LEARNING TEST ITEM SET CHEMISTRY Science Standards of Learning. Released Spring 2015
 VIRGINIA STANDARDS OF LEARNING TEST ITEM SET CHEMISTRY 2010 Science Standards of Learning Released Spring 2015 Property of the Virginia Department of Education Copyright 2015 by the Commonwealth of Virginia,
VIRGINIA STANDARDS OF LEARNING TEST ITEM SET CHEMISTRY 2010 Science Standards of Learning Released Spring 2015 Property of the Virginia Department of Education Copyright 2015 by the Commonwealth of Virginia,
Chapter 10 Gases. Characteristics of Gases. Pressure. The Gas Laws. The Ideal-Gas Equation. Applications of the Ideal-Gas Equation
 Characteristics of Gases Chapter 10 Gases Pressure The Gas Laws The Ideal-Gas Equation Applications of the Ideal-Gas Equation Gas mixtures and partial pressures Kinetic-Molecular Theory Real Gases: Deviations
Characteristics of Gases Chapter 10 Gases Pressure The Gas Laws The Ideal-Gas Equation Applications of the Ideal-Gas Equation Gas mixtures and partial pressures Kinetic-Molecular Theory Real Gases: Deviations
World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases
![World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases](/thumbs/96/127526823.jpg) World of Chemistry Notes for Students [Chapter 3, page ] Chapter 3 Gases ) Sec 3.8 Kinetic Theory of Gases and the Nature of Gases The Kinetic Theory of Matter says that the tiny particles in all forms
World of Chemistry Notes for Students [Chapter 3, page ] Chapter 3 Gases ) Sec 3.8 Kinetic Theory of Gases and the Nature of Gases The Kinetic Theory of Matter says that the tiny particles in all forms
Section 5.1 Pressure. Why study gases? An understanding of real world phenomena. An understanding of how science works.
 Chapter 5 Gases Section 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage Learning. All rights reserved 2 Section 5.1 Pressure
Chapter 5 Gases Section 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. Copyright Cengage Learning. All rights reserved 2 Section 5.1 Pressure
Chemistry 101 Chapter 5 GAS MIXTURES
 GAS MIXTURES Consider mixing equal volumes of 3 different gases, all at the same temperature and pressure in a container of the same size. 1 L He 1 L N 2 1 L O 2 1 L mixture t = 0 0 C t = 0 0 C t = 0 0
GAS MIXTURES Consider mixing equal volumes of 3 different gases, all at the same temperature and pressure in a container of the same size. 1 L He 1 L N 2 1 L O 2 1 L mixture t = 0 0 C t = 0 0 C t = 0 0
NOTES: Behavior of Gases
 NOTES: Behavior of Gases Properties of Gases Gases have weight Gases take up space Gases exert pressure Gases fill their containers Gases are mostly empty space The molecules in a gas are separate, very
NOTES: Behavior of Gases Properties of Gases Gases have weight Gases take up space Gases exert pressure Gases fill their containers Gases are mostly empty space The molecules in a gas are separate, very
To compare one gas to another, it is convenient to define a set of conditions: Standard Temperature and Pressure
 Standard Molar Volume To compare one gas to another, it is convenient to define a set of conditions: Standard Temperature and Pressure At STP, one mole of any gas has a volume of: 22.4 L = (This is a cube
Standard Molar Volume To compare one gas to another, it is convenient to define a set of conditions: Standard Temperature and Pressure At STP, one mole of any gas has a volume of: 22.4 L = (This is a cube
Name Hour. The Behavior of Gases. Practice B
 Name Hour The Behavior of Gases Practice B B 1 Objective 1: Apply Boyle s Law, Charles s Law, and Gay-Lussac s Law to solve problems involving pressure and volume and temperature. 1. A high-altitude balloon
Name Hour The Behavior of Gases Practice B B 1 Objective 1: Apply Boyle s Law, Charles s Law, and Gay-Lussac s Law to solve problems involving pressure and volume and temperature. 1. A high-altitude balloon
Gas Laws. Figure 1: Experimental Set-up with Leveling Bulb. GCC CHM 151LL: Gas Laws GCC, 2019 page 1 of 8
 Gas Laws Introduction Although we cannot see gases, we can observe their behavior and study their properties. This lab will apply several concepts from Ideal Gas Laws. You will use your knowledge of chemical
Gas Laws Introduction Although we cannot see gases, we can observe their behavior and study their properties. This lab will apply several concepts from Ideal Gas Laws. You will use your knowledge of chemical
Behavior of Gases Chapter 12 Assignment & Problem Set
 Behavior of Gases Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Behavior of Gases 2 Study Guide: Things You Must Know Vocabulary (know the definition
Behavior of Gases Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Behavior of Gases 2 Study Guide: Things You Must Know Vocabulary (know the definition
Chemistry HP Unit 6 Gases. Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases
 Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Chemistry HP Unit 6 Gases Learning Targets (Your exam at the end of Unit 6 will assess the following:) 6. Gases 6-1. Define pressure using a mathematical equation. 6-2. Perform calculations involving pressure,
Name Chemistry Pre-AP
 Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
PSI Chemistry: Gases Multiple Choice Review
 PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
PSI Chemistry: Gases Multiple Choice Review Name Kinetic Molecular Theory 1. According to the kinetic-molecular theory, particles of matterare in constant motion (A) have different shapes (B) have different
Practice MC Test unit D (Ch 10) Gas Laws (pg 1 of 10)
 Practice MC Test unit D (Ch 10) Gas Laws (pg 1 of 10) This is practice - Do NOT cheat yourself of finding out what you are capable of doing. Be sure you follow the testing conditions outlined below. DO
Practice MC Test unit D (Ch 10) Gas Laws (pg 1 of 10) This is practice - Do NOT cheat yourself of finding out what you are capable of doing. Be sure you follow the testing conditions outlined below. DO
You should be able to: Describe Equipment Barometer Manometer. 5.1 Pressure Read and outline 5.1 Define Barometer
 A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
Pressure of the atmosphere varies with elevation and weather conditions. Barometer- device used to measure atmospheric pressure.
 Chapter 12 Section 1 Pressure A gas exerts pressure on its surroundings. Blow up a balloon. The gas we are most familiar with is the atmosphere, a mixture of mostly elemental nitrogen and oxygen. Pressure
Chapter 12 Section 1 Pressure A gas exerts pressure on its surroundings. Blow up a balloon. The gas we are most familiar with is the atmosphere, a mixture of mostly elemental nitrogen and oxygen. Pressure
Chemistry 12 Notes on Graphs Involving LeChatelier s Principle
 Chemistry 12 Notes on Graphs Involving LeChatelier s Principle 1. Temperature Changes When a system adjusts due to a temperature change, there are no sudden changes in concentration of any species, so
Chemistry 12 Notes on Graphs Involving LeChatelier s Principle 1. Temperature Changes When a system adjusts due to a temperature change, there are no sudden changes in concentration of any species, so
Unit 10: Gas Laws. Monday Tuesday Wednesday Thursday Friday. 10 Review for Cumulative Retest. 17 Chem Think Gas Laws Tutorial- Computer Lab-
 Unit 10: Gas Laws Name: Monday Tuesday Wednesday Thursday Friday February 8 Stoichiometry Test Review 9 Stoichiometry Test 10 Review for Cumulative Retest 11 Cumulative Re-Test 12 Pressure & Kinetic Theory
Unit 10: Gas Laws Name: Monday Tuesday Wednesday Thursday Friday February 8 Stoichiometry Test Review 9 Stoichiometry Test 10 Review for Cumulative Retest 11 Cumulative Re-Test 12 Pressure & Kinetic Theory
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Test General Chemistry CH116 UMass Boston Summer 2013 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The pressure exerted by a column of
Test General Chemistry CH116 UMass Boston Summer 2013 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The pressure exerted by a column of
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory
 CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
Section 10-1: The Kinetic-Molecular Theory of Matter. 1) How does the word kinetic apply to particles of matter?
 Kinetic-Molecular theory of Matter/Ch10, Gases/Ch11 Column notes: Answer all parts of each question IN YOUR OWN WORDS. Use the text, figures and captions as resources. Section 10-1: The Kinetic-Molecular
Kinetic-Molecular theory of Matter/Ch10, Gases/Ch11 Column notes: Answer all parts of each question IN YOUR OWN WORDS. Use the text, figures and captions as resources. Section 10-1: The Kinetic-Molecular
PROPERTIES OF GASES. [MH5; Ch 5, (only)]
![PROPERTIES OF GASES. [MH5; Ch 5, (only)] PROPERTIES OF GASES. [MH5; Ch 5, (only)]](/thumbs/76/74446950.jpg) PROPERTIES OF GASES [MH5; Ch 5, 5.1-5.5 (only)] FEATURES OF A GAS Molecules in a gas are a long way apart (under normal conditions). Molecules in a gas are in rapid motion in all directions. The forces
PROPERTIES OF GASES [MH5; Ch 5, 5.1-5.5 (only)] FEATURES OF A GAS Molecules in a gas are a long way apart (under normal conditions). Molecules in a gas are in rapid motion in all directions. The forces
Name: Class: Date: SHORT ANSWER Answer the following questions in the space provided.
 CHAPTER 11 REVIEW Gases SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Pressure =. For a constant force, when the surface area is tripled the pressure is (a) doubled. (b)
CHAPTER 11 REVIEW Gases SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Pressure =. For a constant force, when the surface area is tripled the pressure is (a) doubled. (b)
Gases NO CALCULATORS MAY BE USED FOR THESE QUESTIONS
 NO CALCULATORS MAY BE USED FOR THESE QUESTIONS Questions 1-3 refer to the following gases at 0 C and 1 atm. (A) Ar (B) NO 2 (C) Xe (D) H 2 (E) N 2 1. Has an average atomic or molecular speed closest to
NO CALCULATORS MAY BE USED FOR THESE QUESTIONS Questions 1-3 refer to the following gases at 0 C and 1 atm. (A) Ar (B) NO 2 (C) Xe (D) H 2 (E) N 2 1. Has an average atomic or molecular speed closest to
CHE : Organic Chemistry I Fall 2017 Syllabus MWF 8:10-9:10 AM in Hoyt PLH
 CHE 261 04: Organic Chemistry I Fall 2017 Syllabus MWF 8:10-9:10 AM in Hoyt PLH Professor: Dr. Pete Smith Office: Hoyt 215 Email: smithpm@westminster.edu (Best way to contact me.) Phone: 724-946-7299 Office
CHE 261 04: Organic Chemistry I Fall 2017 Syllabus MWF 8:10-9:10 AM in Hoyt PLH Professor: Dr. Pete Smith Office: Hoyt 215 Email: smithpm@westminster.edu (Best way to contact me.) Phone: 724-946-7299 Office
Thermodynamics ERT 206 Properties of Pure Substance HANNA ILYANI ZULHAIMI
 Thermodynamics ERT 206 Properties of Pure Substance HANNA ILYANI ZULHAIMI Outline: Pure Substance Phases of pure substance Phase change process of pure substance Saturation temperature and saturation pressure
Thermodynamics ERT 206 Properties of Pure Substance HANNA ILYANI ZULHAIMI Outline: Pure Substance Phases of pure substance Phase change process of pure substance Saturation temperature and saturation pressure
Example: 25 C = ( ) K = 298 K. Pressure Symbol: p Units: force per area 1Pa (Pascal) = 1 N/m 2
 Chapter 6: Gases 6.1 Measurements on Gases MH5, Chapter 5.1 Let s look at a certain amount of gas, i.e. trapped inside a balloon. To completely describe the state of this gas one has to specify the following
Chapter 6: Gases 6.1 Measurements on Gases MH5, Chapter 5.1 Let s look at a certain amount of gas, i.e. trapped inside a balloon. To completely describe the state of this gas one has to specify the following
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory
 CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases
![Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] D J Weinkauff - Nerinx Hall High School. Chapter 10 Gases](/thumbs/78/77082586.jpg) Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] Chapter 10 Gases We have talked a little about gases in Chapter 3 and we dealt briefly with them in our stoichiometric calculations in
Basic Concepts of Chemistry Notes for Students [Chapter 10, page 1] Chapter 10 Gases We have talked a little about gases in Chapter 3 and we dealt briefly with them in our stoichiometric calculations in
Unit 8: Kinetic Theory Homework Packet (90 points)
 Name: Key Period: By the end of Unit 8, you should be able to: Kinetic Theory Chapter 13-14 4. Define kinetic theory of gases including collisions 5. Define pressure, including atmospheric pressure, vapor
Name: Key Period: By the end of Unit 8, you should be able to: Kinetic Theory Chapter 13-14 4. Define kinetic theory of gases including collisions 5. Define pressure, including atmospheric pressure, vapor
Chemistry 1B Chapter 10 Worksheet - Daley. Name
 Name 1) The National Weather Service routinely supplies atmospheric pressure data to help pilots set their altimeters. The units the NWS uses for atmospheric pressure are inches of mercury. A barometric
Name 1) The National Weather Service routinely supplies atmospheric pressure data to help pilots set their altimeters. The units the NWS uses for atmospheric pressure are inches of mercury. A barometric
Name Unit 9 Notes: Gas Laws Period. Complete throughout unit. Due on test day!
 Name Unit 9 Notes: Gas Laws Period Skills: 1. Gases and Entropy 2. Distinguish between Ideal and Real gases 3. Understand KMT and Avogadro s Law 4. Identify and Solve Boyle s Law Problems 5. Identify and
Name Unit 9 Notes: Gas Laws Period Skills: 1. Gases and Entropy 2. Distinguish between Ideal and Real gases 3. Understand KMT and Avogadro s Law 4. Identify and Solve Boyle s Law Problems 5. Identify and
Honors Chemistry Unit 7 Gas Laws Notes
 Honors Chemistry Unit 7 Gas Laws Notes Kinetic Molecular Theory 1. List the five assumptions: Assumption Description Extra Info 1 Basically means: the particles themselves have compared to the space between
Honors Chemistry Unit 7 Gas Laws Notes Kinetic Molecular Theory 1. List the five assumptions: Assumption Description Extra Info 1 Basically means: the particles themselves have compared to the space between
Ch. 11 Mass transfer principles
 Transport of chemical species in solid, liquid, or gas mixture Transport driven by composition gradient, similar to temperature gradients driving heat transport We will look at two mass transport mechanisms,
Transport of chemical species in solid, liquid, or gas mixture Transport driven by composition gradient, similar to temperature gradients driving heat transport We will look at two mass transport mechanisms,
4.) There are no forces of attraction or repulsion between gas particles. This means that
 KINETIC MOLECULAR (K-M) THEORY OF MATTER NOTES - based on the idea that particles of matter are always in motion - assumptions of the K-M Theory 1.) Gases consist of large numbers of tiny particles that
KINETIC MOLECULAR (K-M) THEORY OF MATTER NOTES - based on the idea that particles of matter are always in motion - assumptions of the K-M Theory 1.) Gases consist of large numbers of tiny particles that
Elements that exist as gases at 25 o C and 1 atmosphere H 2, N 2, O 2, F 2, Cl 2, He, Ne, Ar, Kr, Xe, Rn
 AP Chemistry Chapter 5 Sections 5. 5.9 Note Organizer Pressure, The Gas Laws of Boyle, Charles, and Avogadro, The Ideal Gas Law, Gas Stoichiometry, Dalton s Law of Partial Pressure, The Kinetic olecular
AP Chemistry Chapter 5 Sections 5. 5.9 Note Organizer Pressure, The Gas Laws of Boyle, Charles, and Avogadro, The Ideal Gas Law, Gas Stoichiometry, Dalton s Law of Partial Pressure, The Kinetic olecular
CHM 111 Unit 5 Sample Questions
 Name: Class: Date: As you work these problems, consider and explain: A. What type of question is it? B. How do you know what type of question it is? C. What information are you looking for? D. What information
Name: Class: Date: As you work these problems, consider and explain: A. What type of question is it? B. How do you know what type of question it is? C. What information are you looking for? D. What information
Topic 6: Gases and Colligative Properties
 Topic 6: Gases and Colligative Properties Ideal Gas Equation Boyle noticed an inverse relationship between volume and pressure. Pressure x volume = constant P = a P 1/P Charles found the volume of a gas,
Topic 6: Gases and Colligative Properties Ideal Gas Equation Boyle noticed an inverse relationship between volume and pressure. Pressure x volume = constant P = a P 1/P Charles found the volume of a gas,
1. [Chang7 5.P.013.] Convert 295 mmhg to kpa. kpa Convert 2.0 kpa to mmhg. mmhg
![1. [Chang7 5.P.013.] Convert 295 mmhg to kpa. kpa Convert 2.0 kpa to mmhg. mmhg 1. [Chang7 5.P.013.] Convert 295 mmhg to kpa. kpa Convert 2.0 kpa to mmhg. mmhg](/thumbs/76/74310398.jpg) Score 1. [Chang7 5.P.013.] Convert 295 mmhg to kpa. kpa Convert 2.0 kpa to mmhg. mmhg 2. [Chang7 5.P.019.] The volume of a gas is 5.80 L, measured at 1.00 atm. What is the pressure of the gas in mmhg if
Score 1. [Chang7 5.P.013.] Convert 295 mmhg to kpa. kpa Convert 2.0 kpa to mmhg. mmhg 2. [Chang7 5.P.019.] The volume of a gas is 5.80 L, measured at 1.00 atm. What is the pressure of the gas in mmhg if
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works.
 Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
Chapter 5: Gases 5.1 Pressure Why study gases? An understanding of real world phenomena. An understanding of how science works. A Gas Uniformly fills any container. Easily compressed. Mixes completely
Lecture Handout 5: Gases (Online Text Chapter 6)
 Lecture Handout 5: Gases (Online Text Chapter 6) I. The Structure of a Gas A. Gases are composed of particles that are flying around very fast in their container(s). 1. The particles travel in straight
Lecture Handout 5: Gases (Online Text Chapter 6) I. The Structure of a Gas A. Gases are composed of particles that are flying around very fast in their container(s). 1. The particles travel in straight
Boyle s Law Practice
 Boyle s Law Practice Boyle s Law is an indirect relationship. Most of these problems can be done in your head without showing your work. 1. Herman has 30.0 L of helium gas trapped in a cylinder by a piston.
Boyle s Law Practice Boyle s Law is an indirect relationship. Most of these problems can be done in your head without showing your work. 1. Herman has 30.0 L of helium gas trapped in a cylinder by a piston.
More Practice with Gas Laws KEY
 More Practice with Gas Laws KEY Chemistry Directions: For each question, identify the applicable law and solve for the correct answer using dimensional analysis. Express your answer to the correct number
More Practice with Gas Laws KEY Chemistry Directions: For each question, identify the applicable law and solve for the correct answer using dimensional analysis. Express your answer to the correct number
Chapter 5. Pressure. Atmospheric Pressure. Gases. Force Pressure = Area
 Chapter 5 Gases Water for many homes is supplied by a well The pump removes air from the pipe, decreasing the air pressure in the pipe The pressure then pushes the water up the pipe Pressure Atmospheric
Chapter 5 Gases Water for many homes is supplied by a well The pump removes air from the pipe, decreasing the air pressure in the pipe The pressure then pushes the water up the pipe Pressure Atmospheric
Gas Law Worksheets - WS: Boyle s and Charles Law
 Gas Law Worksheets - WS: Boyle s and Charles Law Boyle s Law states that the volume of a gas varies inversely with its pressure if temperature is held constant. (If one goes up the, other goes down.) We
Gas Law Worksheets - WS: Boyle s and Charles Law Boyle s Law states that the volume of a gas varies inversely with its pressure if temperature is held constant. (If one goes up the, other goes down.) We
Please do not write on this test. Please use the answer sheet. 1) Please choose all conditions that would allow a gas sample to behave ideally.
 AP Chemistry Test (Chapter 5) Please do not write on this test. Please use the answer sheet. Multiple Choice (50%) 1) Please choose all conditions that would allow a gas sample to behave ideally. I) Nonpolar
AP Chemistry Test (Chapter 5) Please do not write on this test. Please use the answer sheet. Multiple Choice (50%) 1) Please choose all conditions that would allow a gas sample to behave ideally. I) Nonpolar
Gas Laws. 1. Gases are said to exert pressure. Provide a molecular-level explanation for this. Copyright Cengage Learning. All rights reserved.
 Chapter 5 Gas Laws Gas Laws 1. Gases are said to exert pressure. Provide a molecular-level explanation for this. 5 2 Gas Laws 2. How does a barometer measure atmospheric pressure? If the atmospheric pressure
Chapter 5 Gas Laws Gas Laws 1. Gases are said to exert pressure. Provide a molecular-level explanation for this. 5 2 Gas Laws 2. How does a barometer measure atmospheric pressure? If the atmospheric pressure
Chemistry Chapter 11 Test Review
 Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
Chapter 13 Gases, Vapors, Liquids, and Solids
 Chapter 13 Gases, Vapors, Liquids, and Solids Property is meaning any measurable characteristic of a substance, such as pressure, volume, or temperature, or a characteristic that can be calculated or deduced,
Chapter 13 Gases, Vapors, Liquids, and Solids Property is meaning any measurable characteristic of a substance, such as pressure, volume, or temperature, or a characteristic that can be calculated or deduced,
Lecture Presentation. Chapter 10. Gases. John D. Bookstaver St. Charles Community College Cottleville, MO Pearson Education, Inc.
 Lecture Presentation Chapter 10 John D. Bookstaver St. Charles Community College Cottleville, MO Characteristics of Unlike liquids and solids, gases Expand to fill their containers. Are highly compressible.
Lecture Presentation Chapter 10 John D. Bookstaver St. Charles Community College Cottleville, MO Characteristics of Unlike liquids and solids, gases Expand to fill their containers. Are highly compressible.
Each gas sample has the same A) density B) mass C) number of molecules D) number of atoms
 1. A real gas behaves most like an ideal gas at A) low pressure and high temperature B) average potential energy of its particles C) ionization energy of its particles D) activation energy of its particles
1. A real gas behaves most like an ideal gas at A) low pressure and high temperature B) average potential energy of its particles C) ionization energy of its particles D) activation energy of its particles
CHEMISTRY - CLUTCH CH.5 - GASES.
 !! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
!! www.clutchprep.com CONCEPT: UNITS OF PRESSURE Pressure is defined as the force exerted per unit of surface area. Pressure = Force Area The SI unit for Pressure is the, which has the units of. The SI
Accelerated Chemistry Study Guide Chapter 13: Gases
 Accelerated Chemistry Study Guide Chapter 13: Gases Terms, definitions, topics Diffusion Kinetic Molecular Theory Atmospheric pressure Barometer Manometer STP Absolute zero Page 1 of 42 Molar volume Partial
Accelerated Chemistry Study Guide Chapter 13: Gases Terms, definitions, topics Diffusion Kinetic Molecular Theory Atmospheric pressure Barometer Manometer STP Absolute zero Page 1 of 42 Molar volume Partial
1. Quantity of a gas (moles) 2. Temperature of the gas. 3. Volume occupied by the gas. 4. Pressure exerted by the gas. PV = nrt
 Experiment 5 Stoichiometry : Gases Determining the Ideal Gas Constant Lab Owl Announcement: Upon completion of this lab log onto OWL. Your fourth Lab Owl assignment, Lab Owl: Exp 5 should appear there.
Experiment 5 Stoichiometry : Gases Determining the Ideal Gas Constant Lab Owl Announcement: Upon completion of this lab log onto OWL. Your fourth Lab Owl assignment, Lab Owl: Exp 5 should appear there.
Final Gas Law Review
 Name: ate: 1 t which temperature is the vapor pressure of ethanol equal to 80 kpa?. 48. 73. 80. 101 4 Gas Molecular Mass (g/mol) 2 4 17 20 The table shown lists four gases and their molecular mass. Which
Name: ate: 1 t which temperature is the vapor pressure of ethanol equal to 80 kpa?. 48. 73. 80. 101 4 Gas Molecular Mass (g/mol) 2 4 17 20 The table shown lists four gases and their molecular mass. Which
Solubility And Temperature Answers
 Temperature Answers Free PDF ebook Download: Temperature Answers Download or Read Online ebook solubility and temperature answers in PDF Format From The Best User Guide Database Using this graph, the solubility
Temperature Answers Free PDF ebook Download: Temperature Answers Download or Read Online ebook solubility and temperature answers in PDF Format From The Best User Guide Database Using this graph, the solubility
Chapter 12. The Gaseous State of Matter
 Chapter 12 The Gaseous State of Matter The air in a hot air balloon expands When it is heated. Some of the air escapes from the top of the balloon, lowering the air density inside the balloon, making the
Chapter 12 The Gaseous State of Matter The air in a hot air balloon expands When it is heated. Some of the air escapes from the top of the balloon, lowering the air density inside the balloon, making the
PETROLEUM ENGINEERING 310 FIRST EXAM. September 22, 2000
 Session: Name: PETROLEUM ENGINEERING 310 FIRST EXAM September 22, 2000 Do all your work on the test paper and the space provided for the answer, do no write on the back. Grading will be based on approach
Session: Name: PETROLEUM ENGINEERING 310 FIRST EXAM September 22, 2000 Do all your work on the test paper and the space provided for the answer, do no write on the back. Grading will be based on approach
Chapter 10: Gases. Characteristics of Gases
 Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
Gas Laws. 2 HCl(aq) + CaCO 3 (s) H 2 O(l) + CO 2 (g) + CaCl 2 (aq) HCl(aq) + NaHCO 3 (s) H 2 O(l) + CO 2 (g) + NaCl(aq)
 Gas Laws Introduction: Although we cannot see gases, we can observe their behavior and study their properties. For example, we can watch a balloon filled with helium gas floating in air and conclude that
Gas Laws Introduction: Although we cannot see gases, we can observe their behavior and study their properties. For example, we can watch a balloon filled with helium gas floating in air and conclude that
Gas Law Review. Honors Chem.
 Gas Law Review Honors Chem. Question 1: KMT 1: What does KMT stand for? 2: Gas particles have no or. 3: Gas particles are not to or by each other. 4: measures the average kinetic energy of gas particles.
Gas Law Review Honors Chem. Question 1: KMT 1: What does KMT stand for? 2: Gas particles have no or. 3: Gas particles are not to or by each other. 4: measures the average kinetic energy of gas particles.
Kinetic-Molecular Theory
 GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
Chemistry 51 Chapter 7 PROPERTIES OF GASES. Gases are the least dense and most mobile of the three phases of matter.
 ROERIES OF GASES Gases are the least dense and most mobile of the three phases of matter. articles of matter in the gas phase are spaced far apart from one another and move rapidly and collide with each
ROERIES OF GASES Gases are the least dense and most mobile of the three phases of matter. articles of matter in the gas phase are spaced far apart from one another and move rapidly and collide with each
Gas Pressure. Pressure is the force exerted per unit area by gas molecules as they strike the surfaces around them.
 Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Unit 9: Gas Laws REGENTS CHEMISTRY
 Name: Unit 9: Gas Laws REGENTS CHEMISTRY 1 Name: Unit 9: Gas Laws The concept of an ideal gas is a model to explain the behavior of gases. A real gas is most like an ideal gas when the real gas is at low
Name: Unit 9: Gas Laws REGENTS CHEMISTRY 1 Name: Unit 9: Gas Laws The concept of an ideal gas is a model to explain the behavior of gases. A real gas is most like an ideal gas when the real gas is at low
UNIT 10 - GASES. Notes & Worksheets - Honors
 Ideal Gas Equation 1 WKSHT 1.) What is the pressure exerted by 2.0 moles of an ideal gas when it occupies a volume of 12.0 L at 373 K? 2.) A flashbulb of volume 2.6 cm 3 contains O 2 gas at a pressure
Ideal Gas Equation 1 WKSHT 1.) What is the pressure exerted by 2.0 moles of an ideal gas when it occupies a volume of 12.0 L at 373 K? 2.) A flashbulb of volume 2.6 cm 3 contains O 2 gas at a pressure
Dalton s Law How is the total pressure of a mixture of gases related to the partial pressures of the component gases?
 Dalton s Law Chapter 4 The Behavior of Gases 4. Properties of Gases 4. The Gas Laws 4. Ideal Gases Dalton s Law How is the total pressure of a mixture of gases related to the partial pressures of the component
Dalton s Law Chapter 4 The Behavior of Gases 4. Properties of Gases 4. The Gas Laws 4. Ideal Gases Dalton s Law How is the total pressure of a mixture of gases related to the partial pressures of the component
A. What are the three states of matter chemists work with?
 Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
Kinetic Molecular Theory Gases. Behavior of gases. Postulate two. Postulate one. Postulate three. Postulate four
 Kinetic Molecular Theory Gases Gas particles are so small that their individual volume can be considered to be negligible Gas particles are in constant motion and the collisions of the particles with the
Kinetic Molecular Theory Gases Gas particles are so small that their individual volume can be considered to be negligible Gas particles are in constant motion and the collisions of the particles with the
Kinetic Molecular Theory imaginary Assumptions of Kinetic Molecular Theory: Problems with KMT:
 AP Chemistry Ms. Ye Name Date Block Kinetic Molecular Theory Explains properties of gases, liquids, and solids in terms of energy using an ideal gas, an imaginary which fits all the assumptions of kinetic
AP Chemistry Ms. Ye Name Date Block Kinetic Molecular Theory Explains properties of gases, liquids, and solids in terms of energy using an ideal gas, an imaginary which fits all the assumptions of kinetic
Question McGraw-Hill Ryerson Limited
 Question 1 Which of the following cannot be explained by considering the empty space between the particles of a gas? A) Gases are more compressible than liquids. B) Gases have lower viscosities than liquids.
Question 1 Which of the following cannot be explained by considering the empty space between the particles of a gas? A) Gases are more compressible than liquids. B) Gases have lower viscosities than liquids.
UNIT 4 IB MATERIAL PARTICLE BEHAVIOR OF MATTER PHASES & ATTRACTIONS
 UNIT 4 IB MATERIAL Name: PARTICLE BEHAVIOR OF MATTER PHASES & ATTRACTIONS ESSENTIALS: Know, Understand, and Be Able To Apply Avogadro s law to calculate reacting volumes of gases. Apply the concept of
UNIT 4 IB MATERIAL Name: PARTICLE BEHAVIOR OF MATTER PHASES & ATTRACTIONS ESSENTIALS: Know, Understand, and Be Able To Apply Avogadro s law to calculate reacting volumes of gases. Apply the concept of
Dr. Rogers Chapter 5 Homework Chem 111 Fall 2003
 Dr. Rogers Chapter 5 Homework Chem 111 Fall 2003 From textbook: 7-33 odd, 37-45 odd, 55, 59, 61 1. Which gaseous molecules (choose one species) effuse slowest? A. SO 2 (g) B. Ar(g) C. NO(g) D. Ne(g) E.
Dr. Rogers Chapter 5 Homework Chem 111 Fall 2003 From textbook: 7-33 odd, 37-45 odd, 55, 59, 61 1. Which gaseous molecules (choose one species) effuse slowest? A. SO 2 (g) B. Ar(g) C. NO(g) D. Ne(g) E.
Chapter 13 Gases and Pressure. Pressure and Force. Pressure is the force per unit area on a surface. Force Area. Pressure =
 Chapter 13 Gas Laws Chapter 13 Gases and Pressure Pressure and Force Pressure is the force per unit area on a surface. Pressure = Force Area Chapter 13 Gases and Pressure Gases in the Atmosphere The atmosphere
Chapter 13 Gas Laws Chapter 13 Gases and Pressure Pressure and Force Pressure is the force per unit area on a surface. Pressure = Force Area Chapter 13 Gases and Pressure Gases in the Atmosphere The atmosphere
Chapter 10. Physical Characteristics of Gases
 Chapter 10 Physical Characteristics of Gases Kinetic Molecular Theory An understanding of the behavior of atoms that make up matter Ideal gas: an imaginary gas that perfectly fits all assumptions of the
Chapter 10 Physical Characteristics of Gases Kinetic Molecular Theory An understanding of the behavior of atoms that make up matter Ideal gas: an imaginary gas that perfectly fits all assumptions of the
Name: Period: Date: CHAPTER 10 NOTES 10.3: The Gas Laws
 Name: Period: Date: 1. Define gas laws: CHAPTER 10 NOTES 10.3: The Gas Laws 2. What units do the following measurements need to be in to describe gases? Boyle s Law a. Temperature b. Volume c. Pressure
Name: Period: Date: 1. Define gas laws: CHAPTER 10 NOTES 10.3: The Gas Laws 2. What units do the following measurements need to be in to describe gases? Boyle s Law a. Temperature b. Volume c. Pressure
Expand to fill their containers, are highly compressible, have extremely low densities.
 Chem150 week6 Handout 1 Gases Characteristics of Gases: Unlike liquids and solids, they Expand to fill their containers, are highly compressible, have extremely low densities. Pressure is the amount of
Chem150 week6 Handout 1 Gases Characteristics of Gases: Unlike liquids and solids, they Expand to fill their containers, are highly compressible, have extremely low densities. Pressure is the amount of
Gilbert Kirss Foster. Chapter 10. Properties of Gases The Air We Breathe
 Gilbert Kirss Foster Chapter 10 Properties of Gases The Air We Breathe Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4
Gilbert Kirss Foster Chapter 10 Properties of Gases The Air We Breathe Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4
PE096: Overview of Gas Processing Technology
 PE096: Overview of Gas Processing Technology PE096 Rev.001 CMCT COURSE OUTLINE Page 1 of 6 Training Description: This course is designed for a broad audience and is participative and interactive, utilizing
PE096: Overview of Gas Processing Technology PE096 Rev.001 CMCT COURSE OUTLINE Page 1 of 6 Training Description: This course is designed for a broad audience and is participative and interactive, utilizing
General Properties of Gases
 GASES Chapter 13 Importance of Gases Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide,, NaN 3. 2 NaN 3 ---> > 2 Na + 3 N 2 THREE STATES OF MATTER General
GASES Chapter 13 Importance of Gases Airbags fill with N 2 gas in an accident. Gas is generated by the decomposition of sodium azide,, NaN 3. 2 NaN 3 ---> > 2 Na + 3 N 2 THREE STATES OF MATTER General
Gas Laws. 2 HCl(aq) + CaCO 3 (s) H 2 O(l) + CO 2 (g) + CaCl 2 (aq) HCl(aq) + NaHCO 3 (s) H 2 O(l) + CO 2 (g) + NaCl(aq)
 Gas Laws Introduction: Although we cannot see gases, we can observe their behavior and study their properties. For example, we can watch a balloon filled with helium gas floating in air and conclude that
Gas Laws Introduction: Although we cannot see gases, we can observe their behavior and study their properties. For example, we can watch a balloon filled with helium gas floating in air and conclude that
Chapter 5. Nov 6 1:02 PM
 Chapter 5 Nov 6 1:02 PM Expand to fill their containers Fluid motion (they flow) Have low densities (1/1000 the density of equivalent liquids or solids) Compressible Can Effuse and Diffuse Effuse: The
Chapter 5 Nov 6 1:02 PM Expand to fill their containers Fluid motion (they flow) Have low densities (1/1000 the density of equivalent liquids or solids) Compressible Can Effuse and Diffuse Effuse: The
Unit 11 Gas Laws Chapters 13 of your textbook
 Unit 11 Gas Laws Chapters 13 of your textbook Early Booklet E.C.: + 2 Unit 11 Hwk. Pts.: / 19 Unit 11 Lab Pts.: / 20 Late, Incomplete, No Work, No Units Fees? Y / N Learning Targets for Unit 11 1.1 I can
Unit 11 Gas Laws Chapters 13 of your textbook Early Booklet E.C.: + 2 Unit 11 Hwk. Pts.: / 19 Unit 11 Lab Pts.: / 20 Late, Incomplete, No Work, No Units Fees? Y / N Learning Targets for Unit 11 1.1 I can
States of Matter. Q 7. Calculate the average of kinetic energy, in joules of the molecules in 8.0 g of methane at 27 o C. (IIT JEE Marks)
 Q 1. States of Matter Calculate density of NH 3 at 30 o C and 5 atm pressure Q 2. (IIT JEE 1978 3 Marks) 3.7 g of a gas at 25 o C occupied the same volume as 0.184g of hydrogen at 17 o C and at the same
Q 1. States of Matter Calculate density of NH 3 at 30 o C and 5 atm pressure Q 2. (IIT JEE 1978 3 Marks) 3.7 g of a gas at 25 o C occupied the same volume as 0.184g of hydrogen at 17 o C and at the same
CHEM 3351 Physical Chemistry I, Fall 2017
 CHEM 3351 Physical Chemistry I, Fall 2017 Problem set 1 Due 9/15/2017 (Friday) 1. An automobile tire was inflated to a pressure of 24 lb in -2 (1.00 atm = 14.7 lb in -2 ) on a winter s day when the temperature
CHEM 3351 Physical Chemistry I, Fall 2017 Problem set 1 Due 9/15/2017 (Friday) 1. An automobile tire was inflated to a pressure of 24 lb in -2 (1.00 atm = 14.7 lb in -2 ) on a winter s day when the temperature
Chemistry 20 Unit 2 Gases FITB Notes. Topic A Characteristics of Gases
 Chemistry 20 Unit 2 Gases FITB Notes General Outcome: Topic A Characteristics of Gases We use technologies that were designed with the knowledge of the visible characteristics ( ) of gases ex. SCUBA equipment,
Chemistry 20 Unit 2 Gases FITB Notes General Outcome: Topic A Characteristics of Gases We use technologies that were designed with the knowledge of the visible characteristics ( ) of gases ex. SCUBA equipment,
Figure Vapor-liquid equilibrium for a binary mixture. The dashed lines show the equilibrium compositions.
 Another way to view this problem is to say that the final volume contains V m 3 of alcohol at 5.93 kpa and 20 C V m 3 of air at 94.07 kpa and 20 C V m 3 of air plus alcohol at 100 kpa and 20 C Thus, the
Another way to view this problem is to say that the final volume contains V m 3 of alcohol at 5.93 kpa and 20 C V m 3 of air at 94.07 kpa and 20 C V m 3 of air plus alcohol at 100 kpa and 20 C Thus, the
Chapter 13: The Behavior of Gases
 Chapter 13: The Behavior of Gases I. First Concepts a. The 3 states of matter most important to us: solids, liquids, and gases. b. Real Gases and Ideal Gases i. Real gases exist, ideal gases do not ii.
Chapter 13: The Behavior of Gases I. First Concepts a. The 3 states of matter most important to us: solids, liquids, and gases. b. Real Gases and Ideal Gases i. Real gases exist, ideal gases do not ii.
GAS LAW WORKSHEET 1 KEY
 377 GAS LAW WORKSHEET 1 KEY 1. A sample of oxygen gas occupies a volume of 436. ml at 1.0 atm. If the temperature is held constant, what would the pressure of this gas be when the gas is compressed to
377 GAS LAW WORKSHEET 1 KEY 1. A sample of oxygen gas occupies a volume of 436. ml at 1.0 atm. If the temperature is held constant, what would the pressure of this gas be when the gas is compressed to
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT.
 Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
2. Convert these pressures to atm: 1 atm! Or to mm Hg, 760 mm Hg! 760 mm Hg! 1 atm. 800 mm Hg 380 mm Hg 0.75 atm 0.25 atm
 Chemistry L 3, Gas laws: Chapter 12: Name! Page 1 pg. 326-355 and Notes: Keep your Forces handout. We will not use kilopascals for pressure on worksheets or tests. Show your work on all worksheets!! Temperature
Chemistry L 3, Gas laws: Chapter 12: Name! Page 1 pg. 326-355 and Notes: Keep your Forces handout. We will not use kilopascals for pressure on worksheets or tests. Show your work on all worksheets!! Temperature
Experiment 12: MOLAR VOLUME OF AN IDEAL GAS
 Experiment 1: MOLAR VOLUME OF AN IDEAL GAS Purpose: Determine the molar volume of a gas at standard temperature and pressure (STP, 0 C and pressure of 1 atm) Performance Goals: Collect and measure the
Experiment 1: MOLAR VOLUME OF AN IDEAL GAS Purpose: Determine the molar volume of a gas at standard temperature and pressure (STP, 0 C and pressure of 1 atm) Performance Goals: Collect and measure the
To play movie you must be in Slide Show Mode CLICK HERE EXERCISE! EXERCISE! To play movie you must be in Slide Show Mode CLICK HERE
 Boyle s Law Boyle s law Pressure and volume are inversely related (constant T, temperature, and n, # of moles of gas). PV k (kis a constant for a given sample of air at a specific temperature) P V P V
Boyle s Law Boyle s law Pressure and volume are inversely related (constant T, temperature, and n, # of moles of gas). PV k (kis a constant for a given sample of air at a specific temperature) P V P V
Boyle s Law VC 09. Experiment 9: Gas Laws. Abstract
 Experiment 9: Gas Laws VC 09 Abstract In this laboratory activity, you will experimentally confirm Boyle s Law, determine absolute zero from Gay-Lussac's Law, and determine the molecular weight of acetone,
Experiment 9: Gas Laws VC 09 Abstract In this laboratory activity, you will experimentally confirm Boyle s Law, determine absolute zero from Gay-Lussac's Law, and determine the molecular weight of acetone,
Chapter 10: Properties of Gases: The Air We Breathe
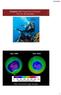 Chapter 10: Properties of Gases: The Air We Breathe Sept, 2006 Sept, 2016 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 The Properties of Gases 10.2 The Kinetic Molecular Theory of Gases* 10.3
Chapter 10: Properties of Gases: The Air We Breathe Sept, 2006 Sept, 2016 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 The Properties of Gases 10.2 The Kinetic Molecular Theory of Gases* 10.3
Multiple Choice (40%)
 AP Chemistry Test (Chapter 5) Please do not write on this test thank you! Multiple Choice (40%) 1) A sealed rigid container is filled with three ideal gases: A, B and C. The partial pressure of each gas
AP Chemistry Test (Chapter 5) Please do not write on this test thank you! Multiple Choice (40%) 1) A sealed rigid container is filled with three ideal gases: A, B and C. The partial pressure of each gas
