AEROSTATS OPEN THE VISTA
|
|
|
- Laureen Cunningham
- 6 years ago
- Views:
Transcription
1 1 AEROSTATS OPEN THE VISTA Human space exploration began in a subtle manner, with the use of aerostats balloons, as we more commonly call them today. These quiet, fragile creations, fully at the mercy of the winds, played an important role in the conquest of space. The balloon was an invention of an earlier time, and within the period of a few months in 1783, two forms of buoyancy were used to lift the first humans into space. A New Invention: The Balloon In 1766 Englishman Henry Cavendish discovered the properties of a gas that he called phlogiston, which was one-fourteenth as light as air. When enclosed in a lightweight container, it satisfied the principles that Archimedes had defined regarding buoyancy; it floated. When it was recognized that this colorless, odorless, and nontoxic element produced water when it was burned, it received the Greek name hydrogen for water-former. Because of its most dangerous property, most people referred to it as inflammable air. In France, starting in June 1783, Joseph-Michel Montgolfier and his brother, Jacques-Étienne Montgolfier, experimented with the ability to fly small silk sacks filled with hydrogen. But, as the silk allowed the hydrogen to quickly escape because of its porous nature, they moved on to using smoke. The particulates in the smoke tended to seal the envelope and retain the hot gas. These sacks, too, defied gravity supported by the buoyant properties of rising hot air. Floating bags of hot air had been noted in both the Chinese and Indian cultures in past centuries. However, when the smoke cooled, it also descended. The Montgolfier brothers filled inverted paper bags with a light cloth fabric with the smoke from a large hay fire. They did not actually understand the physics involved and believed that some properties of the smoke, 3
2 4 The History of Human Space Flight The Archimedes Principle Archimedes (c. 287 BC c. 212 BC), the Greek mathematician and philosopher, is credited with being the first to define the principles of buoyancy in his treatise On Floating Bodies. He writes: An object immersed in a fluid is buoyed up by a force equal to the weight it displaces. Thus, a container (balloon) of a substance lighter than air (air being a fluid) will rise until its weight is equal to the volume of air it displaces. not the hot air, provided the lifting action. Over a period of several months, they experimented with ever-larger bags (a product of their family s business). Their success generated not only notoriety but also an enthusiasm for ballooning that was caught by many and the science of aerostats was born. It is from this word that the term aeronaut was also coined. In an effort to validate scientifically the work of the Montgolfiers, the Academy of Sciences in Paris authorized French inventor, scientist, and mathematician Jacques Alexandre Cesar Charles to duplicate their work. However, Charles believed they had continued to use hydrogen and thus the validation effort actually expanded on this original path to lighter-thanair flight and a friendly rivalry. Adding to the rapid advance in the art of ballooning was the invention of a rubber coating for silk (by the brothers Jean and Noël Robert), that allowed Charles envelope to retain the hydrogen. The inflammable air was generated by pouring sulfuric acid over iron filings. This produced hydrogen gas, which filled the envelope of his balloon that he aptly named the Globe. America s Ben Franklin (then U.S. ambassador to France) was one of those notables who watched the ascent of these as-yet unmanned aerostats with great interest. In Versailles, the Montgolfiers next spectacular balloon was Aérostat Réveillon, named for Jean-Baptiste Réveillon the manufacturer who produced the beautifully adorned paper from which Jacques-Étienne fashioned the envelopes. This colorful 40 ft. tall balloon was sent aloft in September The passengers aboard this pioneering flight were a duck, a sheep, and a rooster. Each animal had a scientific purpose, with the sheep representing a close approximation of the human physiology. There was concern about possible negative effects of a flight into the upper atmosphere thus the caution.
3 Aerostats Open the Vista 5 Competition developed between the brothers and Jacques Charles to send aloft a human. Several tethered tests were made with Jacques-Étienne on board to gain experience and confidence in the balloon. Techniques for feeding the central fire basket to supply the smoke (hot air) were also developed. King Louis XVI had become an ardent supporter of these experiments and suggested that two prisoners be the first aeronauts because of the risk. However, the brothers prevailed, citing the high probability of success and the fame the first fliers would acquire. Thus, Jean-François Pilâtre de Rozier, a French chemistry and physics teacher, and François Laurent le Vieux d Arlandes made the first human free balloon flight in November 1783 in a Montgolfier balloon. Launched near Paris in the presence of the king and Marie Antoinette, their twenty-five-minute flight traveled more than 5 mi. while attaining an altitude of about 3,000 ft. The 75 ft. tall balloon had a volume of 56,000 cu. ft., which compares with a typical modern-day four-passenger hot air balloon. While the event is not celebrated to the extent of the first powered heavier-than-air flight by the Wright brothers, Orville and Wilber Wright, in 1903, it was the first major milestone for humans to break away from the gravity well that constrains us to the planet. With the ability to seal the envelope, hydrogen became the buoyant gas of choice. The use of hot air faded for two hundred years until the use of propane burners brought a resurgence to the sport of hot air ballooning in the 1970s. From this point forward, the space above Earth could now be explored and its attributes measured. However, most of the flights for the next hundred years were made to set records and exploit the moneymaking potential of balloon exhibition flights rather than to advance science. A month following the first human ascension, in December 1783, Jacques Charles and Nicolas-Louis Robert launched their balloon in Paris. The 13,000 cu. ft. hydrogen-filled balloon used sand ballast to control altitude. They ascended to a height of about 1,800 ft. and landed after a two-hour flight covering 16 mi. Sand was the ballast of choice, being easily dropped without hurting anyone below, as the new aeronauts learned their craft by trial and error. De Rozier died when another balloon he was piloting crashed during an attempt to fly across the English Channel. He and his companion, Pierre Romain, became the first known fatalities in the conquest of space. The first balloon flight in America took place a decade later, in January
4 Ballast and Buoyancy Gas balloons use ballast to control buoyancy during flight. Sand or water is carried aloft at launch and released in controlled measures by the pilot to adjust the balloon s altitude by reducing the gross weight of the balloon, which will then rise to a new pressure altitude. To descend, lifting gas is released by a valve atop the envelope. The balloon will remain at its equilibrium altitude until there is another dynamic change in the lift equation. The descent to the ground is a critical operation as the trade-off between the valving of lifting gas and the descent rate is controlled by releasing the remaining ballast. Hot air balloons simply allow the envelope to cool to descend, and heat the air to stop the decent or to re-ascend. Jean-François Pilâtre de Rozier and François Laurent le Vieux d Arlandes made the first free balloon flight using hot air as a lifting medium in November Courtesy of Bibliothèque Nationale, Paris.
5 Aerostats Open the Vista , when the noted French balloonist Jean-Pierre Blanchard ascended from Philadelphia, Pennsylvania, and landed in Deptford Township in New Jersey. Witnessing the ascent that day were President George Washington and future presidents John Adams, Thomas Jefferson, James Madison, and James Monroe. Ascending to New Heights Although the emphasis of these early flights was to entertain by setting records rather than to advance true science, the almost tragic by-product of many stunts revealed the lack of knowledge of this new high-altitude environment. By 1804 several balloonists had topped 20,000 ft. The 1862 ascent of Henry Coxwell and James Glaisher of England claimed 29,000 ft., and the pair narrowly avoided death from hypoxia and freezing. The year 1875 saw the deaths of Théodore Sivel and Joseph Croce-Spinelli in their record attempt to 28,000 ft. A third member of the crew of the balloon named Zenith, Gaston Tissandier, barely survived. There was a lot to learn about traveling in this region high above Earth. Arthur Berson, a Polish meteorologist and pioneer of aerology, and Reinhard Süring, a German meteorologist, set a record of 35,424 ft. in 1901 (although the actual height was doubted by many authorities of the time). This represents the altitude at which current jetliners routinely operate and is about one-tenth the distance to the Kármán line. The world had to wait more than twenty-five years before that record would be surpassed. In 1896 French meteorologist Léon P. Tisserenc de Bort undertook a significant investigation of the upper atmosphere by sending several unmanned balloons to 50,000 ft. In the days before electronic telemetry, Tisserenc de Bort s effort relied on mechanical recording devices, which revealed significant changes in the character of the regions of the atmosphere through which the balloon passed. He termed the areas closest to Earth (within 6 8 mi.) the troposphere and the area above 40,000 ft. the stratosphere, where he perceived there was virtually no weather phenomena. By the late 1920s supercharged engines allowed airplanes to achieve brief flights of up to 30,000 ft., and heavy clothing and oxygen were now recognized as minimal equipment. Lacking pressure-breathing apparatus, U.S. Army captain Hawthorne C. Gray passed out at 27,000 ft. during his 1927 flight, and the balloon descended on its own with the pilot regaining consciousness in time to avoid a catastrophic landing. The sand ballast alone weighed two tons.
6 8 The History of Human Space Flight Oxygen Systems As aircraft achieved higher altitudes, these early pilots recognized that they were not getting enough oxygen into their blood, and they were suffering from hypoxia. Although oxygen levels at all altitudes are constant (about 20 percent of the gases in the atmosphere, with nitrogen making up about 78 percent), simply providing higher percentages of oxygen when flying above 12,000 ft. was not enough. As the altitudes went beyond 25,000 ft., the lack of atmospheric pressure, mandatory for the oxygen to enter the hemoglobin of the blood, required pressure breathing. With discoveries such as this, science was now beginning to play a more significant role in enabling these higher flights. In his second attempt two months later he reached 42,240 ft. but ran out of ballast and had to parachute from the rapidly descending gondola at 8,000 ft. Because he did not land with his aircraft, as Fédération aéronautique internationale (FAI) rules required, his record was not recognized. A similar situation confronted the first Soviet cosmonaut thirty-five years later. By this time the goal of simply establishing a new record, as appealing as that may have been to the participants (and for national pride), could no longer be the sole justification for flights. Science had to be the fundamental rationale, especially when government sponsorship was involved the U.S. Army, in Gray s situation. Mechanically driven instruments had to be perfected that would just like the crew function reliably in the low pressures and temperatures experienced. On his third and fatal flight, despite extensive preparation, Gray scribbled a final hypoxic note at 40,000 ft. that described the deep blue sky and brilliant sun. He also indicated that he had depleted his ballast. With his radio antenna broken (while discarding an empty oxygen tank), there was no communication, and his fate was not known until the following day when his lifeless body was found in the remains of the gondola. The cause of death was apparently not from the hard landing but more likely due to hypoxia or heart failure. The recording instruments indicated 44,000 ft. Capt. H. C. Gray, the first human ever to exceed 40,000 ft., is interred in Arlington National Cemetery.
7 Aerostats Open the Vista 9 Despite the advanced planning and courage of men like Gray, the technology of the time had not kept pace. Effective life-support systems as well as more advanced envelopes and ballast were needed, along with more accurate and reliable scientific instruments. Auguste Piccard: 1931 Flight Swiss physics professor Auguste Piccard profoundly changed the character of exploration of the upper atmosphere the fringe of space. In retrospect of his accomplishments, he wrote: The sport of the scientist consist of utilizing all that he knows of foreseeing the dangers, of studying every detail with profound attention, in always using the admirable instrument of mathematical analysis where ever it could shed its magic light upon his work (quoted in Ryan 1995, 40). Piccard was an early ballooning enthusiast who, with his twin brother, Jean, had flown from Zurich into France in Both had served in the Swiss Army lighter-than-air service in He recognized the need to provide a safer life-support system for high-altitude flight and in 1931 had fashioned a hermetically sealed (airtight) crew capsule the precursor of the true spacecraft. The spherical, 7 ft. diameter cabin had eight small double-pane glass portholes and could accommodate two observers and a few hundred pounds of scientific instrumentation. Piccard s first record flight attempt took place in May 1931, from Augsburg, Germany, using a 500,000 cu. ft. hydrogen balloon. Ascending rapidly, Pressurized Environments By sealing the capsule, the occupants were maintained at the ambient pressure of the launch environment sea-level pressure being 14.7 psi (760 mm) and the standard percentage of oxygen would suffice. This also allowed greater warmth as higher-pressure air can more effectively maintain heat. A small Dewar of liquid oxygen provided for the replacement of that vital life-sustaining gas. It was periodically poured out on the floor of the gondola and, due to its extremely low temperature (-297 degrees), it immediately evaporated into the cabin s atmosphere. To scrub the encapsulated air of the exhaled carbon dioxide, Piccard adapted a recent discovery of chemically removing the gas.
Introduction Hot Air Ballooning
 Introduction Hot Air Ballooning Did the Wright Brothers get it wrong? Balloon is the way to fly Sir Arthur C. Clarke Enjoy the High-Life; view Sri Lanka's Capital and exotic centre of the south from the
Introduction Hot Air Ballooning Did the Wright Brothers get it wrong? Balloon is the way to fly Sir Arthur C. Clarke Enjoy the High-Life; view Sri Lanka's Capital and exotic centre of the south from the
Level: DRA: Genre: Strategy: Skill: Word Count: Online Leveled Books HOUGHTON MIFFLIN
 HOUGHTON MIFFLIN by Rufus Albermarle ILLUSTRATION CREDIT: McEntee Art and Design PHOTOGRAPHY CREDITS: Cover (balloon) PhotoLink. (sky) PhotoDisc. 1 Shutterstock. 2 PhotoLink. 2 3 PhotoDisc. 4 Hans Weisenhoffer.
HOUGHTON MIFFLIN by Rufus Albermarle ILLUSTRATION CREDIT: McEntee Art and Design PHOTOGRAPHY CREDITS: Cover (balloon) PhotoLink. (sky) PhotoDisc. 1 Shutterstock. 2 PhotoLink. 2 3 PhotoDisc. 4 Hans Weisenhoffer.
Ballooning. With RE/MAX
 Ballooning With RE/MAX From Annonay to Albuquerque 2 Basic Design of a Sport Balloon 3 How Does It Fly? 5 Common Questions About Hot Air Ballooning 6 Balloon Language 8 Copyright 2009 RE/MAX International,
Ballooning With RE/MAX From Annonay to Albuquerque 2 Basic Design of a Sport Balloon 3 How Does It Fly? 5 Common Questions About Hot Air Ballooning 6 Balloon Language 8 Copyright 2009 RE/MAX International,
From and
 From http://www.school-for-champions.com/science/fluidpressure.htm and http://www.school-forchampions.com/science/fluidfloating.htm by Ron Kurtus, School for Champions Pressure in Fluids by Ron Kurtus
From http://www.school-for-champions.com/science/fluidpressure.htm and http://www.school-forchampions.com/science/fluidfloating.htm by Ron Kurtus, School for Champions Pressure in Fluids by Ron Kurtus
10.4 Buoyancy is a force
 Chapter 10.4 Learning Goals Define buoyancy. Explain the relationship between density and buoyancy. Discuss applications of Archimedes principle. 10.4 Buoyancy is a force Buoyancy is a measure of the upward
Chapter 10.4 Learning Goals Define buoyancy. Explain the relationship between density and buoyancy. Discuss applications of Archimedes principle. 10.4 Buoyancy is a force Buoyancy is a measure of the upward
Up Up and Away on Beautiful Balloons: Scaling up from Party Favors to Scientific Payloads
 Up Up and Away on Beautiful Balloons: Scaling up from Party Favors to Scientific Payloads How many helium filled balloons would it take to lift you? To lift a big telescope? Take a guess for each and record
Up Up and Away on Beautiful Balloons: Scaling up from Party Favors to Scientific Payloads How many helium filled balloons would it take to lift you? To lift a big telescope? Take a guess for each and record
What are some properties of fluids? Why does a lake freeze from the top downward?
 Fluid Mechanics > A fluid is any substance that capable of flowing, which includes liquids, gases and powdered solids. Therefore fluids have some similar mechanical properties. ex; Both can not support
Fluid Mechanics > A fluid is any substance that capable of flowing, which includes liquids, gases and powdered solids. Therefore fluids have some similar mechanical properties. ex; Both can not support
To be fair, Hegel was the originator of the philosophical method known as the dialectic he presented a thesis, an antithesis and felt that reality
 To be fair, Hegel was the originator of the philosophical method known as the dialectic he presented a thesis, an antithesis and felt that reality was somewhere in between, a synthesis if you will. So
To be fair, Hegel was the originator of the philosophical method known as the dialectic he presented a thesis, an antithesis and felt that reality was somewhere in between, a synthesis if you will. So
Technical Knowledge and Aerostatics (Balloon) This syllabus presupposes a knowledge and understanding already attained at PPL level.
 Subject No 29 Technical Knowledge and Aerostatics (Balloon) Note: This syllabus is primarily based on a balloon designed above 120000 cubic feet maximum. Each subject has been given a subject number and
Subject No 29 Technical Knowledge and Aerostatics (Balloon) Note: This syllabus is primarily based on a balloon designed above 120000 cubic feet maximum. Each subject has been given a subject number and
Chapter Overview. Discovering Flight The Early Days of Flight. Chapter 1, Lesson 1
 Discovering Flight Chapter Overview Discovering Flight The Early Days of Flight Lesson Overview How humans tried to fly in ancient times Key aviation devices created during ancient times Why machines do
Discovering Flight Chapter Overview Discovering Flight The Early Days of Flight Lesson Overview How humans tried to fly in ancient times Key aviation devices created during ancient times Why machines do
INTRODUCTION TO FLIGHT (REVIEW, AEROSPACE DIMENSIONS, MODULE 1)
 INTRODUCTION TO FLIGHT (REVIEW, AEROSPACE DIMENSIONS, MODULE 1) CAPTAIN. JERRY PAINTER AEROSPACE EDUCATION OFFICER COMPOSITE SQUADRON 316, (CIVIL AIR PATROL) CASA GRANDE, ARIZONA IMPORTANT TERMS-THE LANGUAGE
INTRODUCTION TO FLIGHT (REVIEW, AEROSPACE DIMENSIONS, MODULE 1) CAPTAIN. JERRY PAINTER AEROSPACE EDUCATION OFFICER COMPOSITE SQUADRON 316, (CIVIL AIR PATROL) CASA GRANDE, ARIZONA IMPORTANT TERMS-THE LANGUAGE
Wasn t it your. It s All About Dreams. by Glen Moyer
 It s All About Dreams by Glen Moyer Wasn t it your fantasy? To grab hold to a bunch of toy balloons and fly silently off into space? With that single inquiry, cluster balloonist, pilot and BFA member Jonathanan
It s All About Dreams by Glen Moyer Wasn t it your fantasy? To grab hold to a bunch of toy balloons and fly silently off into space? With that single inquiry, cluster balloonist, pilot and BFA member Jonathanan
Bill Wagenborg Chemistry Lesson Plan March Lesson Title: Understanding Charles Law. Grade Level: 8 Time Frame: 1-2 (45 Minute) Classes
 Bill Wagenborg Chemistry Lesson Plan March 2007 Lesson Title: Understanding Charles Law Grade Level: 8 Time Frame: 1-2 (45 Minute) Classes Goals/Objectives: The Students Will Be Able To: - follow a sequenced
Bill Wagenborg Chemistry Lesson Plan March 2007 Lesson Title: Understanding Charles Law Grade Level: 8 Time Frame: 1-2 (45 Minute) Classes Goals/Objectives: The Students Will Be Able To: - follow a sequenced
Specific gravity: Everything you ever wanted to know about volume, pressure and more
 Specific gravity: Everything you ever wanted to know about volume, pressure and more Specific Gravity Part I: What is specific gravity? Grandpa, I kind of understand what gravity is, but what is specific
Specific gravity: Everything you ever wanted to know about volume, pressure and more Specific Gravity Part I: What is specific gravity? Grandpa, I kind of understand what gravity is, but what is specific
Properties of Fluids. How do ships float?
 How do ships float? Despite their weight ships are able to float. This is because a greater force pushing up on the ship opposes the weight or force of the ship pushing down. How do ships float? This supporting
How do ships float? Despite their weight ships are able to float. This is because a greater force pushing up on the ship opposes the weight or force of the ship pushing down. How do ships float? This supporting
Unit 1 Lesson 5 Fluids and Pressure. Copyright Houghton Mifflin Harcourt Publishing Company
 Feel the Pressure! What are fluids? A fluid is any material that can flow and that takes the shape of its container. A fluid can flow because its particles easily move past each other. Liquids and gases,
Feel the Pressure! What are fluids? A fluid is any material that can flow and that takes the shape of its container. A fluid can flow because its particles easily move past each other. Liquids and gases,
6.9B verify through investigations that thermal energy moves in a predictable pattern from warmer to cooler 6.5B recognize that a limited number of
 6.9B verify through investigations that thermal energy moves in a predictable pattern from warmer to cooler 6.5B recognize that a limited number of elements comprise the largest portion of oceans and atmosphere
6.9B verify through investigations that thermal energy moves in a predictable pattern from warmer to cooler 6.5B recognize that a limited number of elements comprise the largest portion of oceans and atmosphere
PHYSICS. Light: Sound:
 PHYSICS Light: The speed of light changes as it passes through different things such as air, glass and water. This affects the way we see things underwater with a diving mask. As the light passes through
PHYSICS Light: The speed of light changes as it passes through different things such as air, glass and water. This affects the way we see things underwater with a diving mask. As the light passes through
Your web browser (Safari 7) is out of date. For more security, comfort and the best experience on this site: Update your browser Ignore
 Your web browser (Safari 7) is out of date. For more security, comfort and the best experience on this site: Update your browser Ignore BATHYSCAPHE For the complete encyclopedic entry with media resources,
Your web browser (Safari 7) is out of date. For more security, comfort and the best experience on this site: Update your browser Ignore BATHYSCAPHE For the complete encyclopedic entry with media resources,
5.0 Neutral Buoyancy Test
 5.0 Neutral Buoyancy Test Montgolfier balloons use solar energy to heat the air inside the balloon. The balloon used for this project is made out of a lightweight, black material that absorbs the solar
5.0 Neutral Buoyancy Test Montgolfier balloons use solar energy to heat the air inside the balloon. The balloon used for this project is made out of a lightweight, black material that absorbs the solar
Gases and Pressure. Main Ideas
 Gases and Pressure Key Terms pressure millimeters of mercury partial pressure newton atmosphere of pressure Dalton s law of partial pressures barometer pascal In the chapter States of Matter, you read
Gases and Pressure Key Terms pressure millimeters of mercury partial pressure newton atmosphere of pressure Dalton s law of partial pressures barometer pascal In the chapter States of Matter, you read
DIVING PHYSICS EXAMPLE QUESTIONS
 DIVING PHYSICS EXAMPLE QUESTIONS PLEASE NOTE: 1 bar = 10 Meter in Salt water 1 bar = 10.2 Meter in Fresh water. Will be GIVEN to you for calculations. 10m in Salt water = 1 bar 10m in Fresh water = 0.98
DIVING PHYSICS EXAMPLE QUESTIONS PLEASE NOTE: 1 bar = 10 Meter in Salt water 1 bar = 10.2 Meter in Fresh water. Will be GIVEN to you for calculations. 10m in Salt water = 1 bar 10m in Fresh water = 0.98
Lesson 1: Introduction to Learning Aviation Science. by: Alex Stackhouse
 Lesson 1: Introduction to Learning Aviation Science by: Alex Stackhouse Important Info. Scientists believe that the brain recalls new information more efficiently if the new information is associated in
Lesson 1: Introduction to Learning Aviation Science by: Alex Stackhouse Important Info. Scientists believe that the brain recalls new information more efficiently if the new information is associated in
At 6:23 hours on 2 June, Captain Kittinger's Man-High (I) flight began, from Fleming Field, South Saint Paul, Minnesota.
 Source: stratocat.com.ar Data of the stratospheric balloon launched on 6/2/1957 from Fleming Field Airport, South St. Paul, Minnesota, US for MANHIGH I - (Capt. Joseph W. Kittinger II) Details of the balloon
Source: stratocat.com.ar Data of the stratospheric balloon launched on 6/2/1957 from Fleming Field Airport, South St. Paul, Minnesota, US for MANHIGH I - (Capt. Joseph W. Kittinger II) Details of the balloon
Chapter 10: Properties of Gases: The Air We Breathe
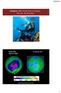 Chapter 10: Properties of Gases: The Air We Breathe South Pole Sept 24, 2006 15 February 2017 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 10.2 10.3 The Properties of Gases Effusion and the Kinetic
Chapter 10: Properties of Gases: The Air We Breathe South Pole Sept 24, 2006 15 February 2017 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 10.2 10.3 The Properties of Gases Effusion and the Kinetic
Related Careers: Aircraft Instrument Repairer Aircraft Designer Aircraft Engineer Aircraft Electronics Specialist Aircraft Mechanic Pilot US Military
 Airplane Design and Flight Fascination with Flight Objective: 1. You will be able to define the basic terms related to airplane flight. 2. You will test fly your airplane and make adjustments to improve
Airplane Design and Flight Fascination with Flight Objective: 1. You will be able to define the basic terms related to airplane flight. 2. You will test fly your airplane and make adjustments to improve
Conceptual Physics Matter Liquids Gases
 Conceptual Physics Matter Liquids Gases Lana Sheridan De Anza College July 25, 2017 Last time atomic structure forms of matter solids density elasticity liquids & pressure Overview liquids pressure surface
Conceptual Physics Matter Liquids Gases Lana Sheridan De Anza College July 25, 2017 Last time atomic structure forms of matter solids density elasticity liquids & pressure Overview liquids pressure surface
REPUBLIC OF KENYA MINISTRY OF TRANSPORT AND COMMUNICATIONS CIVIL AIRCRAFT ACCIDENT REPORT TRANSWORLD SAFARIS KENYA LIMITED
 REPUBLIC OF KENYA MINISTRY OF TRANSPORT AND COMMUNICATIONS CIVIL AIRCRAFT ACCIDENT REPORT CAV/ACC /6/00 OPERATOR: AIRCRAFT: REGISTRATION: PLACE: TRANSWORLD SAFARIS KENYA LIMITED LINDSTRAND LBL 260A BALLOON
REPUBLIC OF KENYA MINISTRY OF TRANSPORT AND COMMUNICATIONS CIVIL AIRCRAFT ACCIDENT REPORT CAV/ACC /6/00 OPERATOR: AIRCRAFT: REGISTRATION: PLACE: TRANSWORLD SAFARIS KENYA LIMITED LINDSTRAND LBL 260A BALLOON
DEMONSTRATION 2.1 PROPERTIES OF CO 2. Chapter 2: Gases
 DEMONSTRATION 2.1 Chapter 2: Gases PROPERTIES OF CO 2 This demonstration has two aims: firstly, to show that carbon dioxide gas is denser than air; secondly, to show that carbon dioxide will not support
DEMONSTRATION 2.1 Chapter 2: Gases PROPERTIES OF CO 2 This demonstration has two aims: firstly, to show that carbon dioxide gas is denser than air; secondly, to show that carbon dioxide will not support
Name Class Date. (pp ) Write the letter of the correct answer in the space provided.
 Skills Worksheet Directed Reading A Section: Buoyancy and Density (pp. 412 419) 1. What is the upward force that fluids exert on all matter called? a. pascal force b. atmospheric pressure c. buoyant force
Skills Worksheet Directed Reading A Section: Buoyancy and Density (pp. 412 419) 1. What is the upward force that fluids exert on all matter called? a. pascal force b. atmospheric pressure c. buoyant force
In the liquid phase, molecules can flow freely from position to position by sliding over one another. A liquid takes the shape of its container.
 In the liquid phase, molecules can flow freely from position to position by sliding over one another. A liquid takes the shape of its container. In the liquid phase, molecules can flow freely from position
In the liquid phase, molecules can flow freely from position to position by sliding over one another. A liquid takes the shape of its container. In the liquid phase, molecules can flow freely from position
2016 Released Items: Grade 3 Research Simulation Task
 2016 Released Items: Grade 3 Research Simulation Task The Research Simulation Task requires students to analyze an informational topic through several articles or multimedia stimuli. Students read and
2016 Released Items: Grade 3 Research Simulation Task The Research Simulation Task requires students to analyze an informational topic through several articles or multimedia stimuli. Students read and
In the liquid phase, molecules can flow freely from position. another. A liquid takes the shape of its container. 19.
 In the liquid phase, molecules can flow freely from position to position by sliding over one another. A liquid takes the shape of its container. In the liquid phase, molecules can flow freely from position
In the liquid phase, molecules can flow freely from position to position by sliding over one another. A liquid takes the shape of its container. In the liquid phase, molecules can flow freely from position
Grade 8 Science: Unit 2-Fluids Chapter 9: Force, Pressure Area
 Grade 8 Science: Unit 2-Fluids Chapter 9: Force, Pressure Area Key Terms: hydraulic systems, incompressible, mass, neutral buoyancy, pascal, pneumatic systems, pressure, unbalanced forces, weight, Archimedes
Grade 8 Science: Unit 2-Fluids Chapter 9: Force, Pressure Area Key Terms: hydraulic systems, incompressible, mass, neutral buoyancy, pascal, pneumatic systems, pressure, unbalanced forces, weight, Archimedes
Our Air- Why We Should Care!
 Our Air- Why We Should Care! We live on earth, but we live in its atmosphere- a mixture of gases we call air. Just as water surrounds aquatic life, air surrounds us. People seldom think about the sea of
Our Air- Why We Should Care! We live on earth, but we live in its atmosphere- a mixture of gases we call air. Just as water surrounds aquatic life, air surrounds us. People seldom think about the sea of
Fluid Mechanics. Liquids and gases have the ability to flow They are called fluids There are a variety of LAWS that fluids obey
 Fluid Mechanics Fluid Mechanics Liquids and gases have the ability to flow They are called fluids There are a variety of LAWS that fluids obey Density Regardless of form (solid, liquid, gas) we can define
Fluid Mechanics Fluid Mechanics Liquids and gases have the ability to flow They are called fluids There are a variety of LAWS that fluids obey Density Regardless of form (solid, liquid, gas) we can define
Why do things float? Climate and Global Change. Introduction
 Why do things float? Introduction Archimedes of Syracuse (ca. 287-212 B.C.), a physical scientist, is credited with understanding two basic principles: When describing the mechanical advantage gained by
Why do things float? Introduction Archimedes of Syracuse (ca. 287-212 B.C.), a physical scientist, is credited with understanding two basic principles: When describing the mechanical advantage gained by
Elizabethtown College Department of Physics and Engineering PHY 103
 Elizabethtown College Department of Physics and Engineering PHY 103 Lab 10 Buoyancy and Lawn Chair Balloon Flight Introduction In this lab we will review the principle of buoyancy and apply the concept
Elizabethtown College Department of Physics and Engineering PHY 103 Lab 10 Buoyancy and Lawn Chair Balloon Flight Introduction In this lab we will review the principle of buoyancy and apply the concept
Adventure on a Hot Air Balloon
 Adventure on a Hot Air Balloon Adventure on a Hot Air Balloon The wind is starting to blow stronger, and when you re riding in a basket under a hot air balloon, just 400 feet above ground, that s not necessarily
Adventure on a Hot Air Balloon Adventure on a Hot Air Balloon The wind is starting to blow stronger, and when you re riding in a basket under a hot air balloon, just 400 feet above ground, that s not necessarily
The History of Kites
 The History of Kites The flying of kites began 3000 years ago in China. Ancient Chinese people would use bamboo and silk, two products readily available, to make the kites. They were often used during
The History of Kites The flying of kites began 3000 years ago in China. Ancient Chinese people would use bamboo and silk, two products readily available, to make the kites. They were often used during
Call for Ideas for Balloon Experiments within the HEMERA project
 Call for Ideas for Balloon Experiments within the HEMERA project HEMERA is a balloon infrastructure project, funded by the European Commission within its programme Horizon 2020. The project is coordinated
Call for Ideas for Balloon Experiments within the HEMERA project HEMERA is a balloon infrastructure project, funded by the European Commission within its programme Horizon 2020. The project is coordinated
What do we know about air? What have we observed?
 Air and Flight---Properties of Air Air: - we know it exists, - it s all around us, - we see moving trees, - it fills our lungs, - it has substance but can t be seen Air: - colourless, odourless and tasteless,
Air and Flight---Properties of Air Air: - we know it exists, - it s all around us, - we see moving trees, - it fills our lungs, - it has substance but can t be seen Air: - colourless, odourless and tasteless,
Buoyancy and Density. Buoyant Force and Fluid Pressure. Key Concept Buoyant force and density affect whether an object will float or sink in a fluid.
 2 Buoyancy and Density Key Concept Buoyant force and density affect whether an object will float or sink in a fluid. What You Will Learn All fluids exert an upward buoyant force on objects in the fluid.
2 Buoyancy and Density Key Concept Buoyant force and density affect whether an object will float or sink in a fluid. What You Will Learn All fluids exert an upward buoyant force on objects in the fluid.
8.1 Properties of Gases. Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases.
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8. Chapter 8
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases. Chapter 8. Chapter 8. Gases Properties of Gases. We are surrounded by gases, but we are often
 Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
Time Machine (1915): When chemicals became weapons in WWI
 Time Machine (1915): When chemicals became weapons in WWI By Scientific American, adapted by Newsela staff on 05.10.16 Word Count 937 Level 1030L Three models of respirators to prevent ingesting poisonous
Time Machine (1915): When chemicals became weapons in WWI By Scientific American, adapted by Newsela staff on 05.10.16 Word Count 937 Level 1030L Three models of respirators to prevent ingesting poisonous
Gases and Pressure SECTION 11.1
 SECTION 11.1 Gases and In the chapter States of Matter, you read about the kineticmolecular theory of matter. You were also introduced to how this theory explains some of the properties of ideal gases.
SECTION 11.1 Gases and In the chapter States of Matter, you read about the kineticmolecular theory of matter. You were also introduced to how this theory explains some of the properties of ideal gases.
PHYS:1200 LECTURE 13 FLUIDS (2)
 1 PHYS:1200 LECTURE 13 FLUIDS (2) Lecture 13 deals with the properties of fluids at rest or fluid statics. We will be discussing mostly liquids and will introduce two important principles of fluid statics:
1 PHYS:1200 LECTURE 13 FLUIDS (2) Lecture 13 deals with the properties of fluids at rest or fluid statics. We will be discussing mostly liquids and will introduce two important principles of fluid statics:
Phys101 Lectures Fluids I. Key points: Pressure and Pascal s Principle Buoyancy and Archimedes Principle. Ref: 10-1,2,3,4,5,6,7.
 Phys101 Lectures 21-22 Fluids I Key points: Pressure and Pascal s Principle Buoyancy and Archimedes Principle Ref: 10-1,2,3,4,5,6,7. Page 1 10-1 Phases of Matter The three common phases of matter are solid,
Phys101 Lectures 21-22 Fluids I Key points: Pressure and Pascal s Principle Buoyancy and Archimedes Principle Ref: 10-1,2,3,4,5,6,7. Page 1 10-1 Phases of Matter The three common phases of matter are solid,
Phys101 Lectures Fluids I. Key points: Pressure and Pascal s Principle Buoyancy and Archimedes Principle. Ref: 10-1,2,3,4,5,6,7.
 Phys101 Lectures 24-25 luids I Key points: Pressure and Pascal s Principle Buoyancy and Archimedes Principle Ref: 10-1,2,3,4,5,6,7. Page 1 10-1 Phases of Matter The three common phases of matter are solid,
Phys101 Lectures 24-25 luids I Key points: Pressure and Pascal s Principle Buoyancy and Archimedes Principle Ref: 10-1,2,3,4,5,6,7. Page 1 10-1 Phases of Matter The three common phases of matter are solid,
Chapter 13 Temperature, Kinetic Theory, and the Gas Laws 497
 Chapter 13 Temperature, Kinetic Theory, and the Gas Laws 497 Figure 13.25 This photograph of Apollo 17 Commander Eugene Cernan driving the lunar rover on the Moon in 1972 looks as though it was taken at
Chapter 13 Temperature, Kinetic Theory, and the Gas Laws 497 Figure 13.25 This photograph of Apollo 17 Commander Eugene Cernan driving the lunar rover on the Moon in 1972 looks as though it was taken at
102 Hawthorne C. Gray
 The Library of America Story of the Week Reprinted from Into the Blue: American Writers on Aviation and Spaceflight (Library of America, 2011), pages 102 06. 102 Hawthorne C. Gray First published in the
The Library of America Story of the Week Reprinted from Into the Blue: American Writers on Aviation and Spaceflight (Library of America, 2011), pages 102 06. 102 Hawthorne C. Gray First published in the
Exercises The Atmosphere (page 383) 20.2 Atmospheric Pressure (pages )
 Exercises 20.1 The Atmosphere (page 383) 1. The energizes the molecules in Earth s atmosphere. 2. Why is gravity important to Earth s atmosphere? 3. What would happen to Earth s atmosphere without the
Exercises 20.1 The Atmosphere (page 383) 1. The energizes the molecules in Earth s atmosphere. 2. Why is gravity important to Earth s atmosphere? 3. What would happen to Earth s atmosphere without the
Fluids: Floating & Flying. Student Leaning Objectives 2/16/2016. Distinguish between force and pressure. Recall factors that allow floating
 Fluids: Floating & Flying (Chapter 3) Student Leaning Objectives Distinguish between force and pressure Recall factors that allow floating Differentiate between cohesion and adhesion Analyze Pascal s principle
Fluids: Floating & Flying (Chapter 3) Student Leaning Objectives Distinguish between force and pressure Recall factors that allow floating Differentiate between cohesion and adhesion Analyze Pascal s principle
Created by Glenn Gibson Air and Aerodynamics Flight Note Pack
 Air and Aerodynamics Flight Note Pack Essential Questions of Aerodynamics The students should be able to answer the following questions: 1. Why does air exert pressure on objects in our atmosphere? 2.
Air and Aerodynamics Flight Note Pack Essential Questions of Aerodynamics The students should be able to answer the following questions: 1. Why does air exert pressure on objects in our atmosphere? 2.
Vacuum P=0. h=76 cm A B C. Barometer
 Recap: Pressure Pressure = Force per unit area (P = F /A; units: Pascals) Density of object = mass / volume (ρ = m /V; units: kg / m 3 ) Pascal s Law:Pressure is transmitted equally in all directions throughout
Recap: Pressure Pressure = Force per unit area (P = F /A; units: Pascals) Density of object = mass / volume (ρ = m /V; units: kg / m 3 ) Pascal s Law:Pressure is transmitted equally in all directions throughout
Navy Fighter Pilots Tolerate Higher-G Maneuvers Thanks to Oxygen Regulator Bellows
 Navy Fighter Pilots Tolerate Higher-G Maneuvers Thanks to Oxygen Regulator Bellows Low Spring-Rate Sensor Applies Positive Pressure Breathing for G with Quick, Consistent Response Produced by: Mission
Navy Fighter Pilots Tolerate Higher-G Maneuvers Thanks to Oxygen Regulator Bellows Low Spring-Rate Sensor Applies Positive Pressure Breathing for G with Quick, Consistent Response Produced by: Mission
SUBSTATION MAINTENANCE ELECTRICAL OPERATING PROCEDURE
 Page 1 of 6 A. PURPOSE AND SCOPE This procedure establishes safe working practices for handling sulfur hexafluoride (SF 6 ) gas and release minimization practices. This document also specifies methods
Page 1 of 6 A. PURPOSE AND SCOPE This procedure establishes safe working practices for handling sulfur hexafluoride (SF 6 ) gas and release minimization practices. This document also specifies methods
The Quarter Pounder A Vehicle to Launch Quarter Pound Payloads to Low Earth Orbit
 The Quarter Pounder A Vehicle to Launch Quarter Pound Payloads to Low Earth Orbit Ed LeBouthillier 1 Contents Introduction... 3 Requirements... 4 Orbital Altitudes... 4 Orbital Velocities... 4 Summary...4
The Quarter Pounder A Vehicle to Launch Quarter Pound Payloads to Low Earth Orbit Ed LeBouthillier 1 Contents Introduction... 3 Requirements... 4 Orbital Altitudes... 4 Orbital Velocities... 4 Summary...4
DUQUESNE UNIVERSITY. Liquid Nitrogen Safety
 DUQUESNE UNIVERSITY Liquid Nitrogen Safety Prepared by: Environmental Health and Safety Department TABLE OF CONTENTS Page Introduction 1 Handling Liquid Nitrogen 1 First Aid Notice 5 Introduction The safe
DUQUESNE UNIVERSITY Liquid Nitrogen Safety Prepared by: Environmental Health and Safety Department TABLE OF CONTENTS Page Introduction 1 Handling Liquid Nitrogen 1 First Aid Notice 5 Introduction The safe
Additional Information
 Buoyancy Additional Information Any object, fully or partially immersed in a fluid, is buoyed up by a force equal to the weight of the fluid displaced by the object. Archimedes of Syracuse Archimedes principle
Buoyancy Additional Information Any object, fully or partially immersed in a fluid, is buoyed up by a force equal to the weight of the fluid displaced by the object. Archimedes of Syracuse Archimedes principle
More About Solids, Liquids and Gases ASSIGNMENT
 More About Solids, Liquids and Gases ASSIGNMENT 1. Fill in the blank spaces by choosing the correct words from the list given below: List : water, density, altitudes, lateral, intermolecular, force, cohesion,
More About Solids, Liquids and Gases ASSIGNMENT 1. Fill in the blank spaces by choosing the correct words from the list given below: List : water, density, altitudes, lateral, intermolecular, force, cohesion,
Fluids. How do fluids exert pressure? What causes objects to float? What happens when pressure in a fluid changes? What affects the speed of a fluid?
 CHAPTER 3 SECTION 3 States of Matter Fluids KEY IDEAS As you read this section, keep these questions in mind: How do fluids exert pressure? What causes objects to float? What happens when pressure in a
CHAPTER 3 SECTION 3 States of Matter Fluids KEY IDEAS As you read this section, keep these questions in mind: How do fluids exert pressure? What causes objects to float? What happens when pressure in a
The grade 6 English science unit, Gases, meets the academic content standards set in the Korean curriculum, which state students should:
 This area deals with the properties of gases as small collections of particles. Different kinds of intangible and invisible gases attract students curiosity and promote their use of reasoning skills. Students
This area deals with the properties of gases as small collections of particles. Different kinds of intangible and invisible gases attract students curiosity and promote their use of reasoning skills. Students
Chapter 1. The Science of Marine Biology - Why is it important? Marine Biology. The scientific study of the organisms that live in the sea
 Chapter 1 The Science of Marine Biology - Why is it important? Marine Biology Marine Biology - The scientific study of the organisms that live in the sea 1 Practical Reasons to study marine biology Vast
Chapter 1 The Science of Marine Biology - Why is it important? Marine Biology Marine Biology - The scientific study of the organisms that live in the sea 1 Practical Reasons to study marine biology Vast
Unit 7. Pressure in fluids
 -- Unit 7. Pressure in fluids Index 1.- Pressure...2 2.- Fluids...2 3.- Pressure in fluids...3 4.- Pascal's principle...5 5.- Archimedes principle...6 6.- Atmospheric pressure...7 6.1.- Torricelli and
-- Unit 7. Pressure in fluids Index 1.- Pressure...2 2.- Fluids...2 3.- Pressure in fluids...3 4.- Pascal's principle...5 5.- Archimedes principle...6 6.- Atmospheric pressure...7 6.1.- Torricelli and
LESSON 2: SUBMARINE BUOYANCY INVESTIGATION
 LESSON 2: SUBMARINE BUOYANCY INVESTIGATION Lesson overview This lesson encourages students to investigate hands-on the property of neutral buoyancy, and to discuss its importance in terms of submarines.
LESSON 2: SUBMARINE BUOYANCY INVESTIGATION Lesson overview This lesson encourages students to investigate hands-on the property of neutral buoyancy, and to discuss its importance in terms of submarines.
Notes Chapter 3. Buoyancy
 Notes Chapter 3 Buoyancy Pressure in a Fluid 3.2 Pressure and the Buoyant Forces Liquids and gases are fluids materials that can flow and have no definite shape. Objects in a fluid experience a buoyant
Notes Chapter 3 Buoyancy Pressure in a Fluid 3.2 Pressure and the Buoyant Forces Liquids and gases are fluids materials that can flow and have no definite shape. Objects in a fluid experience a buoyant
Science 8 Chapter 9 Section 1
 Science 8 Chapter 9 Section 1 Forces and Buoyancy (pp. 334-347) Forces Force: anything that causes a change in the motion of an object; a push or pull on an object balanced forces: the condition in which
Science 8 Chapter 9 Section 1 Forces and Buoyancy (pp. 334-347) Forces Force: anything that causes a change in the motion of an object; a push or pull on an object balanced forces: the condition in which
1/21/2014. Diving Physics. Introduction
 Diving Physics Topics in this Chapter Introduction Principles of Pressure Properties of Water Temperature Archimedes Principle Charles Law & Guy-Lussac s Law Combined & Ideal Gas Laws Dalton s Law Henry
Diving Physics Topics in this Chapter Introduction Principles of Pressure Properties of Water Temperature Archimedes Principle Charles Law & Guy-Lussac s Law Combined & Ideal Gas Laws Dalton s Law Henry
SCIENCE and TECHNOLOGY CYCLE 3 MCCAIG ELEMENTARY
 NAME SCIENCE and TECHNOLOGY CYCLE 3 MCCAIG ELEMENTARY Air: - colourless, odourless and tasteless, Air and Flight--- Properties of Air - a gas made mainly of nitrogen (78%), oxygen (21%) and small amounts
NAME SCIENCE and TECHNOLOGY CYCLE 3 MCCAIG ELEMENTARY Air: - colourless, odourless and tasteless, Air and Flight--- Properties of Air - a gas made mainly of nitrogen (78%), oxygen (21%) and small amounts
Chapter 9. Forces and Fluids
 Chapter 9 Forces and Fluids Key Terms hydraulic systems incompressible mass neutral buoyancy pascal pneumatic systems pressure unbalanced forces weight Archimedes principle average density balanced forces
Chapter 9 Forces and Fluids Key Terms hydraulic systems incompressible mass neutral buoyancy pascal pneumatic systems pressure unbalanced forces weight Archimedes principle average density balanced forces
Fluids. James H Dann, Ph.D. Say Thanks to the Authors Click (No sign in required)
 Fluids James H Dann, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive content, visit
Fluids James H Dann, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive content, visit
ConcepTest PowerPoints
 ConcepTest PowerPoints Chapter 10 Physics: Principles with Applications, 6 th edition Giancoli 2005 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for
ConcepTest PowerPoints Chapter 10 Physics: Principles with Applications, 6 th edition Giancoli 2005 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for
Post-Show FLIGHT. After the Show. Traveling Science Shows
 Traveling Science Shows Post-Show FLIGHT After the Show We recently presented a flight show at your school, and thought you and your students might like to continue investigating this topic. The following
Traveling Science Shows Post-Show FLIGHT After the Show We recently presented a flight show at your school, and thought you and your students might like to continue investigating this topic. The following
Chapter Five: Density and Buoyancy
 Chapter Five: Density and Buoyancy 5.1 Density 5.2 Buoyancy 5.3 Heat Affects Density and Buoyancy 5.1 Mass and Weight Mass is the amount of matter in an object. Weight is a measure of the pulling force
Chapter Five: Density and Buoyancy 5.1 Density 5.2 Buoyancy 5.3 Heat Affects Density and Buoyancy 5.1 Mass and Weight Mass is the amount of matter in an object. Weight is a measure of the pulling force
Conceptual Physics Fundamentals
 Conceptual Physics Fundamentals Chapter 7: FLUID MECHANICS This lecture will help you understand: Density Pressure Pressure in a Liquid Buoyancy in a Liquid Pressure in a Gas Atmospheric Pressure Pascal
Conceptual Physics Fundamentals Chapter 7: FLUID MECHANICS This lecture will help you understand: Density Pressure Pressure in a Liquid Buoyancy in a Liquid Pressure in a Gas Atmospheric Pressure Pascal
Chapter 10: Properties of Gases: The Air We Breathe
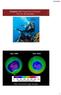 Chapter 10: Properties of Gases: The Air We Breathe Sept, 2006 Sept, 2016 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 The Properties of Gases 10.2 The Kinetic Molecular Theory of Gases* 10.3
Chapter 10: Properties of Gases: The Air We Breathe Sept, 2006 Sept, 2016 http://ozonewatch.gsfc.nasa.gov 1 Chapter Outline 10.1 The Properties of Gases 10.2 The Kinetic Molecular Theory of Gases* 10.3
etcadvancedpilottraining.com Upset Prevention and Recovery Training (UPRT) Altitude Awareness Training Situational Awareness (SA)
 ADVANCED PILOT TRAINING Upset Prevention and Recovery Training (UPRT) Altitude Awareness Training Situational Awareness (SA) Spatial Disorientation (SD) etcadvancedpilottraining.com ETC s NASTAR Center
ADVANCED PILOT TRAINING Upset Prevention and Recovery Training (UPRT) Altitude Awareness Training Situational Awareness (SA) Spatial Disorientation (SD) etcadvancedpilottraining.com ETC s NASTAR Center
Ocean Exploration. Copyright 2012 LessonSnips
 Ocean Exploration Approximately 71 percent of the surface of the earth is covered by the ocean yet very little of the ocean has been explored. Once sailing ships capable of traversing the ocean were built,
Ocean Exploration Approximately 71 percent of the surface of the earth is covered by the ocean yet very little of the ocean has been explored. Once sailing ships capable of traversing the ocean were built,
Concept of Fluid. Density. Pressure: Pressure in a Fluid. Pascal s principle. Buoyancy. Archimede s Principle. Forces on submerged surfaces
 FLUID MECHANICS The fluid essential to all life has a beauty of its own. It also helps support the weight of this swimmer. (credit: Terren, Wikimedia Commons) Concept of Fluid Density Pressure: Pressure
FLUID MECHANICS The fluid essential to all life has a beauty of its own. It also helps support the weight of this swimmer. (credit: Terren, Wikimedia Commons) Concept of Fluid Density Pressure: Pressure
BUOYANCY, FLOATATION AND STABILITY
 BUOYANCY, FLOATATION AND STABILITY Archimedes Principle When a stationary body is completely submerged in a fluid, or floating so that it is only partially submerged, the resultant fluid force acting on
BUOYANCY, FLOATATION AND STABILITY Archimedes Principle When a stationary body is completely submerged in a fluid, or floating so that it is only partially submerged, the resultant fluid force acting on
Flight. Mysteries. Mysteries of Flight A Reading A Z Level U Benchmark Book Word Count: 1,324 BENCHMARK U.
 Mysteries of Flight A Reading A Z Level U Benchmark Book Word Count: 1,324 BENCHMARK U Mysteries of Flight Written by Lisa Trumbauer Visit www.readinga-z.com for thousands of books and materials. www.readinga-z.com
Mysteries of Flight A Reading A Z Level U Benchmark Book Word Count: 1,324 BENCHMARK U Mysteries of Flight Written by Lisa Trumbauer Visit www.readinga-z.com for thousands of books and materials. www.readinga-z.com
Exploration Series. HOT AIR BALLOON Interactive Physics Simulation Page 01
 HOT AIR BALLOON ------- Interactive Physics Simulation ------- Page 01 How do you control a hot air balloon? A hot air balloon floats because atmospheric pressure is greatest closer to the ground. The
HOT AIR BALLOON ------- Interactive Physics Simulation ------- Page 01 How do you control a hot air balloon? A hot air balloon floats because atmospheric pressure is greatest closer to the ground. The
1 MS Earth s Atmosphere
 CHAPTER 1 MS Earth s Atmosphere Chapter Outline 1.1 THE ATMOSPHERE 1.2 ENERGY IN THE ATMOSPHERE 1.3 LAYERS OF THE ATMOSPHERE 1.4 AIR MOVEMENT 1.5 REFERENCES Did you ever see such an awesome sight? This
CHAPTER 1 MS Earth s Atmosphere Chapter Outline 1.1 THE ATMOSPHERE 1.2 ENERGY IN THE ATMOSPHERE 1.3 LAYERS OF THE ATMOSPHERE 1.4 AIR MOVEMENT 1.5 REFERENCES Did you ever see such an awesome sight? This
Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes:
 Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
HW #10 posted, due Thursday, Dec 2, 11:59 p.m. (last HW that contributes to the final grade)
 HW #10 posted, due Thursday, Dec 2, 11:59 p.m. (last HW that contributes to the final grade) Last Lecture Class: States/Phases of Matter, Deformation of Solids, Density, Pressure Today: Pressure vs. Depth,
HW #10 posted, due Thursday, Dec 2, 11:59 p.m. (last HW that contributes to the final grade) Last Lecture Class: States/Phases of Matter, Deformation of Solids, Density, Pressure Today: Pressure vs. Depth,
-i- -iv- THIS PAGE INTENTIONALLY BLANK KAVANAGH MODEL
 -iv- -i- KAVANAGH MODEL C-56, C-65, C-77, D-77, D-84, D-90, D-105 E-120, E-140, E-160, E-180, E-200, E-210, E-240, E-260, E-300 THIS PAGE INTENTIONALLY BLANK HOT AIR BALLOON AUSTRALIAN APPROVED FLIGHT
-iv- -i- KAVANAGH MODEL C-56, C-65, C-77, D-77, D-84, D-90, D-105 E-120, E-140, E-160, E-180, E-200, E-210, E-240, E-260, E-300 THIS PAGE INTENTIONALLY BLANK HOT AIR BALLOON AUSTRALIAN APPROVED FLIGHT
L-14 Fluids [3] Fluids in Motion Fluid Dynamics Hydrodynamics Aerodynamics
![L-14 Fluids [3] Fluids in Motion Fluid Dynamics Hydrodynamics Aerodynamics L-14 Fluids [3] Fluids in Motion Fluid Dynamics Hydrodynamics Aerodynamics](/thumbs/90/104328217.jpg) L-14 Fluids [3] Fluids in Motion Fluid Dynamics Hydrodynamics Aerodynamics Archimedes Principle F B W A buoyant force F B equal to the weight of displaced water is exerted on a submerged object. Will it
L-14 Fluids [3] Fluids in Motion Fluid Dynamics Hydrodynamics Aerodynamics Archimedes Principle F B W A buoyant force F B equal to the weight of displaced water is exerted on a submerged object. Will it
CHAPTER 16 %UHDWKLQJ*DV0L[LQJ3URFHGXUHV
 CHAPTER 16 %UHDWKLQJ*DV0L[LQJ3URFHGXUHV 16-1 INTRODUCTION 16-1.1 Purpose. The purpose of this chapter is to familiarize divers with the techniques used to mix divers breathing gas. 16-1.2 Scope. This chapter
CHAPTER 16 %UHDWKLQJ*DV0L[LQJ3URFHGXUHV 16-1 INTRODUCTION 16-1.1 Purpose. The purpose of this chapter is to familiarize divers with the techniques used to mix divers breathing gas. 16-1.2 Scope. This chapter
BRIGHT SPARKS FEBRUARY HOLIDAY Winchester Science Centre
 BRIGHT SPARKS World of gases From climate change to cooking dinner, the chemistry of gases is part of everyday life. Join us for a day, jam packed full of science tricks, messy experiments, fun games,
BRIGHT SPARKS World of gases From climate change to cooking dinner, the chemistry of gases is part of everyday life. Join us for a day, jam packed full of science tricks, messy experiments, fun games,
KEY CONCEPT Earth s atmosphere supports life. Living things need food, water, and air Matter can be solid, liquid, or gas
 KEY CONCEPT Earth s atmosphere supports life. BEFORE, you learned Living things need food, water, and air Matter can be solid, liquid, or gas NOW, you will learn Why the atmosphere is important to living
KEY CONCEPT Earth s atmosphere supports life. BEFORE, you learned Living things need food, water, and air Matter can be solid, liquid, or gas NOW, you will learn Why the atmosphere is important to living
ATMOSPHERE AND PHYSIOLOGICAL REQUIREMENTS
 OXYGEN ATMOSPHERE AND PHYSIOLOGICAL REQUIREMENTS Air at sea level contains about 21% of oxygen at a pressure of 14.7 psi Air above sea level contains the same percentage of oxygen but at a reduced pressure;
OXYGEN ATMOSPHERE AND PHYSIOLOGICAL REQUIREMENTS Air at sea level contains about 21% of oxygen at a pressure of 14.7 psi Air above sea level contains the same percentage of oxygen but at a reduced pressure;
THE SEPARATION OF FUSELAGE NOSE SECTION AND FUSELAGE MIDSECTION OF COMMERCIAL PLANE
 THE SEPARATION OF FUSELAGE NOSE SECTION AND FUSELAGE MIDSECTION OF COMMERCIAL PLANE Faculty of Mechanical Engineering. Smt. Kashibai Navale College of engineering Pune-41, India E-mail: akshaymahale59@gmail.com
THE SEPARATION OF FUSELAGE NOSE SECTION AND FUSELAGE MIDSECTION OF COMMERCIAL PLANE Faculty of Mechanical Engineering. Smt. Kashibai Navale College of engineering Pune-41, India E-mail: akshaymahale59@gmail.com
It s a Gas - Natural Gas
 Lesson Plan - Page 1 Topic Natural gas Source Oil and Natural Gas, pages 20-21, 22-23 Objective Students will learn that natural gas is a substance formed over millions of years from decaying ocean plants
Lesson Plan - Page 1 Topic Natural gas Source Oil and Natural Gas, pages 20-21, 22-23 Objective Students will learn that natural gas is a substance formed over millions of years from decaying ocean plants
FIRST GRADE ATMOSPHERE
 FIRST GRADE ATMOSPHERE 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF FIRST GRADE WATER WEEK 1. PRE: Investigating the water cycle. LAB: Experiencing surface tension. POST: Discovering how
FIRST GRADE ATMOSPHERE 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF FIRST GRADE WATER WEEK 1. PRE: Investigating the water cycle. LAB: Experiencing surface tension. POST: Discovering how
Page 1. Balance of Gravity Energy More compressed at sea level than at higher altitudes Moon has no atmosphere
 Earth s Atmosphere Gases and Plasmas Balance of Gravity Energy More compressed at sea level than at higher altitudes Moon has no atmosphere Magdeburg Hemispheres Weight of Air mass of air that would occupy
Earth s Atmosphere Gases and Plasmas Balance of Gravity Energy More compressed at sea level than at higher altitudes Moon has no atmosphere Magdeburg Hemispheres Weight of Air mass of air that would occupy
IT S A GAS
 IT S A GAS IT S A GAS The Nature of Gases Gases have some interesting characteristics that have fascinated scientists for 300 years. The first gas to be studied was air & it was a long time before it was
IT S A GAS IT S A GAS The Nature of Gases Gases have some interesting characteristics that have fascinated scientists for 300 years. The first gas to be studied was air & it was a long time before it was
Gas Laws. Essential Learning Outcomes: 1. Change can be measured. 2. Changes can occur within a substance that alters its identity.
 Gas Laws Gas Laws: Gases and pressures affect our lives every day. From the weather we experience to the air we breathe, it all has to do with gases and pressures. Why do we have wind? Why do we have the
Gas Laws Gas Laws: Gases and pressures affect our lives every day. From the weather we experience to the air we breathe, it all has to do with gases and pressures. Why do we have wind? Why do we have the
