Stopped-flow Kinetics
|
|
|
- Rodger Cunningham
- 5 years ago
- Views:
Transcription
1 Introduction Stopped-flow Kinetics A number of conventional kinetic techniques measure reactions on a time scale of minutes or hours. The most straightforward way to measure the rate of chemical reactions is to mix two reagent solutions and observe the subsequent change. This operation is best carried out in the observation cell of a spectrophotometer if a change in the UV-visible spectrum is produced. There are two factors that limit the minimum half-life of reactions that can be studied in this way: the speed of mixing and the speed of observation. The fastest way to fill the observation cell is by the stopped-flow technique. The figure on page IX-7 shows a schematic diagram of a stopped-flow apparatus that fits easily into a spectrophotometer. To perform a run, two drive syringes are filled with reagent from reservoirs and the contents are briskly expelled into the flow circuit using the drive plate. A small volume (250 µl) of each reagent is displaced through the mixer to fill the observation cell. The flow is stopped when the syringe's plunger hits the mechanical stop. The reaction initiated by mixing proceeds in the observation cell and the change in absorbance is monitored by the spectrophotometer. The minimum observable half-life is determined by the spectrophotometer electronics. The freshly mixed solution is only about 20 ms old, but not all commercial spectrophotometers can follow absorbance change in the millisecond time range. Half-lives of down to about 0.5 s can be measured using stopped-flow in Cary 219 spectrophotometer (using the chart recorder). For shorter half-lives we monitor the signal change using an older Beckman spectrophotometer which has been modified for a faster time response. A Macintosh computer is used to collect and analyze the data, giving a system response time of about 0.05 seconds. Try to verify this number in your experiments. The reaction that you will be studying is that between ferric and thiocyanate ions in perchloric acid solution 1 : k f Fe 3+ (aq) + SCN (aq) > FeSCN 2+ (aq) (IX.1) < k r where k f and k r are the rate constants for the forward and reverse reactions. The kinetic analysis is given in Shoemaker et al. 2. The instructor will demonstrate the use of the stopped-flow apparatus. Since the apparatus is fragile and expensive, exercise great care in carrying out the operations. The base and support brackets are made of Acetal, which is attacked by most acids and bases, including perchloric acid. Any spills should be cleaned up immediately. The rate measurements should be carried out at several temperatures in the range 5 to 30 C, starting at the lowest temperature. For each temperature, the rate constant k f is calculated from the average value of three tracings of rise of absorbance with time. Also determine the energy of activation for the reaction.
2 Procedure Two solutions are provided: M Fe(NO 3 ) 3, 0.14M NaClO 4, 0.20M HClO 4 (color coded red) M NaSCN, 0.14M NaClO 4, 0.20M HClO 4 (color coded green) Please be careful not to contaminate or dilute these solutions. Waste solutions are handled with glassware that is color coded pink. Data Collection: 0. Turn on the constant-temperature circulator bath and set the temperature to 5 C, if it is not already on. On the RTE-100, hold down "display" while adjusting course and fine knobs to the desired temperature. It may take min for the temperature to begin dropping. 1. Read page IX-8 describing the operation on the SFA-11. Practice operation of the apparatus with distilled, deionized water and be sure to remove all air bubbles from the viewing region of the cuvette. Remove the waste solution periodically with a Pasteur pipet and dispose of the waste in the appropriately labeled flask. This is important when using the perchloric acidbased reagents because the apparatus has a slow leak, and perchloric acid reacts with the base material. 2. Close the shutter on the Beckman spectrophotometer and turn the instrument on. Adjust the wavelength to 460nm. What color is 460nm? 3. With the shutter on the spectrophotometer closed, adjust the dark current for a 0% transmittance reading. 4. Insert the stopped-flow apparatus cuvette into the cuvette holder of the spectrophotometer, secure it with black masking tape, and cover the sample-holder portion of the spectrophotometer with black cloth. Adjust the slits on the spectrophotometer to read 100% transmittance. 5. Close the shutter and check the 0% transmittance (infinite absorbance) reading. Open the shutter and check the 100% transmittance (zero absorbance) reading. 6. Load the Fe 3+ and SCN sample solutions into the appropriate syringes. Inject samples until the waste solution is a dark orange. Watch the meter for a change in the absorbance. When all of the water has been flushed, the absorbance should be about 0.5 or 0.4. When the (transparent) reagents are injected, the absorbance should drop to a low value (<0.2), then return to the maximum value. Your objective is to measure the "decay-time" for this process. 7. When you are satisfied with the measurements that you see on the meter, turn the meter off and plug in the coaxial cable that is attached to the Macintosh to the meter box.
3 8. To collect data on the Macintosh, you will use a data collection routine "ABS_scope_5.1" written by Bryce Babcock in the LabVIEW programming environment. An upgrade is scheduled for the academic year, so your details may vary. Open the program and examine the options available. The default settings are probably: 2ms = Time Base (ΔT/Ch) 2048 = # Samples Cont(inuous run) = off The only parameters that you will need to change are the number of samples and the time base. At higher temperatures, the decay rate will be faster, and you will want to decrease the time base. Resist the temptation to open menus, click on buttons, and do all of the things that you normally do with a new Macintosh program. You run the risk of altering the data collection routine. In particular, do not Open..., or Save from the File menu, as this will open or save a new data collection program (not a data file). If you have changed several parameters, but want to get back to the original settings, under OPERATE choose "Reinitialize All to Default."
4 9. To start a run, click on the blue "RUN" button. Immediately after clicking, push in the plunger of the stopped-flow apparatus to start the reaction. This synchronization will take some practice. 10. Wait a minute or so for the program to process the data. The run button will blink yellow briefly. Eventually, you will see a plot of your data appear on the screen. 11. If you like the data, click on the blue SAVE button. The save dialog is rather strange. (It looks like an open-file procedure). The first time create a folder for your data by selecting "new," entering a folder name, and clicking on "folder." Then save the file by clicking "new," entering the file name, and clicking on "file." It will save you a great deal of time during data analysis if you record in your lab notebook the time over which the decay is exponential. A! A The first 100 or so data points are worthless, because the reaction has not yet started. The final 100 or so data points are also worthless because the reaction is essentially complete. The range of exponential decay return to A is still exponential at long times, but the resolution of the data collection is not high enough to pick up the small differences between A and A t. The "art" of data analysis will come later when you must decide exactly where to truncate the data for your fit. A range where the absorbance lies between 10 and 90 % of its maximum range is reasonable. You should also note the approximate value of A, the infinite time absorbance. 12. Collect two or three data scans at each of four or five temperatures in the range 5 to 30 C. (The data below room temperature will probably be more reliable.) For temperatures above 5 C, the reaction will be faster and you can use 1 ms for the time base (ΔT/Ch) with 2048 samples. You may want to make even further adjustments, such as reducing the number of samples, as you continue to raise the temperature. 13. When you are finished, quit the LabVIEW routine by clicking on Quit in the File menu. Please also unplug the coaxial cable from the meter-box, turn off the spectrophotometer, turn off the circulator bath, and rinse the stopped-flow apparatus thoroughly with water. time
5 Data Processing 1. The data files that you have saved are in text-file format so that a word processor, a spreadsheet or a plotting program may read it. You may choose any analysis program, such as Excel, Kaleidagraph, DeltaGraph, or Igor. The first line lists the file name including path where it was saved plus the date and time. The second line lists the number of samples collected (2048 in most cases), the number of channels collected, and the gain. The third line lists 4 triggering parameters followed by a very important number: The sample time interval. This tells you how many milliseconds between each absorbance reading. Following these data collection parameters are the actual absorbance reading, all in one column. 2. Open the analysis program and open the data file of interest. (NOTE: if you are using Kaleidagraph and some data columns disappear, 1. set-up importing "text" but then say cancel just before starting, 2. then import normally.) 3. You will need to generate a time column for your data. You can use the series function in Excel to step up from 0 in increments of the sample time interval. 4. Scroll to the very end of the file and record the maximum absorbance reading from column one. Column two contains the time in milliseconds. If the run did not go to completion, it may be safer to use the initial (pre-injection) value for A. 5. Delete or mask the leading and ending series of data points, so that you have a set of points where exponential decay is observed. (Masking in Kaleidagraph tells it to ignore these certain points.) The range where data points are exponential is somewhat arbitrary, and is best chosen by examining a plot. Hopefully, you recorded the proper range when you collected the data. 6. Generate a new data column of the natural log of your final absorbance minus the absorbance at any given time, ln (A A t ). 7. Prepare a graph of ln(a A t ) versus time, and find the slope of this line using a linear-leastsquares approach. The slope of this line is equal to ( [Fe 3+ ] + 1 K eq )k f, where k f is the rate constant for the reaction Fe 3+ + SCN FeSCN 2+. Use the equilibrium constant, K eq = (146±5)M 1 at 25 C. Note that you will have to adjust the equilibrium constant for the temperature that you used. 8. Alternatively, instead of step 6 and 7 you can directly plot A t vs. t, and use an exponential fitting function to the data. The fit routine will adjust the initial and final value of the absorption automatically. Instead of the slope of the linear fit use the exponential decay rate. There are advantages to either approach. 9. The Arrhenius equation, k f = A exp(-e a / RT) may be used to calculate the activation energy, E a, for the reaction. Prepare a plot of ln(k f ) versus (1/T).
6 ====================== Kaleidagraph ======================= "File" "Import" "text" {default setting of tab-delineated is good} You can rename the columns (such as "Time" and "Absorbance") by double clicking on the current column names, "A", "B",...(Kaleidagraph may make the first data point the column headers--you can easily afford to lose the first data point, so just write over it with your column names) To do the data manipulation, you need to add more columns in the data section, under "Data" choose the appropriate command. Then, "Windows" "Formula Entry" "c3=c2-c1" or "c4=c2^2." Next, make a graph From the menu at the top, choose "Gallery," "Linear," and then "Scatter" or "Line." Pick the appropriate X and Y axes. If you want to find the best-fit line, choose "Curve Fit" and "Linear." If Kaleidagraph does not automatically display the equation, choose "Plot" and "Display Equation." Be sure to look at the line it gives you to make sure the line is reasonable. Once you have your graph, you can make all kinds of changes--double click on the title or axes text and play with the font, etc. Also, you might find more points you want to delete. If you make several plots, the previous ones are hidden. To see them, choose "Windows" and "Show Plot." ====================== Excel 4.0 ======================= Open one of your data files. If you don't know exactly which range of data you want to keep and what to throw out, you can make a quick graph and throw out at least those points that are clearly not going to be good (all of the initial stuff and much of the end) In cell c1, type "=-a1" {return}. Now copy this to the rest of the column. Either use cut and paste, or highlight the appropriate cells and use apple-d to "fill down." Making a graph Highlight the numbers you've entered. Select button second from right at top toolbar. (The one that's a combination bar graph, line, and scatter plot) Go to a free area of your worksheet and highlight a region where you want to place your graph. Answer the questions (try x-y scatter first, format 1 or 2) Play with options. Once you've made a graph of the data in the form where it should be linear, you might identify more data points at either end to delete. Then, to get the slope of the numbers. Go to a blank cell. From the top menu items select "Formula," "Paste Function," "Statistical," "Slope" "OK"
7 Where it asks for "Known-Y's" highlight the y-data with the mouse. Then with the mouse go back to the formula, highlight the words "Known-x's" and then go to the spreadsheet and highlight your x-data. Press enter Find the intercept similarly. Lastly, to get this line onto our graph you calculate the y-points from the slope and intercept and your x-data, put these into a new column, and graph your y data and the calculated y-line against the x-points. References 1. J. F. Below, Jr., R. E. Connick, and C. P. Coppel, J. Am. Chem. Soc. 80, 2961 (1958). 2. D.P. Shoemaker, C.W. Garland, J.I. Steinfeld, and J.W. Nibler, Experiments in Physical Chemistry, 4th ed. (McGraw-Hill Book Co., New York, 1981). The third or fifth edition of this text is acceptable. Copies are available on the CHEM 302 reserve shelf or from the chemistry office. Feb-91 JT; Jan-92-epl, Jan-97 BK; April-99 BK From the screen click the Start button first and second the arrow. To save the data the red light on the top of the menu should be on or running. After saving the data press the red button to stop the run. Remember that the saving is always as a new file.
8 Fig: 1: Schematic of Stopped Flow Apparatus
9 Operation of the SFA-11 There are two positions for the three-way taps. In position 1 the operating lever points horizontally towards the syringe. In position 2 the lever points vertically downwards. 1. Half - fill the open syringes, with their reagent solutions. 2. Turn all taps to 1. Push and pull rapidly each drive syringe piston so as to fill the drive syringes and expel air bubbles. Pull the drive syringe pistons out as far as possible. Push the stopping syringe piston in as far as possible. 3. Turn all taps to 2. Push firmly on the drive plate. The best way to do this is to squeeze the drive plate and the stopping block firmly together, using the thumbs. and first fingers of both hands, one on each side. 4. Turn all taps to 1. Push the stopping syringe in as far as possible, and pull the drive syringe pistons out as far as possible. 5. Repeat 3 and 4 a few t times with the taps set at 1, push and pull the waste syringe piston to and fro, so as to expel all the air bubbles. 6. To prepare the unit for a run, now that all air bubbles have been expelled, turn all taps to 1, fill the drive syringes and empty the waste syringe. Refill the reservoir syringes as required to prevent further air being drawn in. 7. To perform a run, turn the taps to 2, start the data-capture system, and squeeze the drive plate and stopping block firmly together. The spectrophotometer signal may vary slightly with how hard this squeeze is. For reproducibility adopt the same procedure for each run.. If the run is complete in less that a minute or so, keep squeezing until the reaction is complete. If the reaction is slower, release the pressure as soon as the flow stops. SPECTROPHOTOMETER REPONSE TIME. A useful check on the spectrophotometer and data-capture system response time may be obtained by observing the initial part of the run. At the start of the push the old, reacted, solution is swept out of the sample cell in about 20 milliseconds, and there is a rapid absorbance change as the new, unreacted, solution comes in. The observed time for this process will be the response time for the spectrophotometer and data-capture system, which is normally much greater than 20 milliseconds.
Vapor Pressure of Liquids
 Vapor Pressure of Liquids In this experiment, you will investigate the relationship between the vapor pressure of a liquid and its temperature. When a liquid is added to the Erlenmeyer flask shown in Figure
Vapor Pressure of Liquids In this experiment, you will investigate the relationship between the vapor pressure of a liquid and its temperature. When a liquid is added to the Erlenmeyer flask shown in Figure
Vapor Pressure of Liquids
 Experiment 10 In this experiment, you will investigate the relationship between the vapor pressure of a liquid and its temperature. When a liquid is added to the Erlenmeyer flask shown in Figure 1, it
Experiment 10 In this experiment, you will investigate the relationship between the vapor pressure of a liquid and its temperature. When a liquid is added to the Erlenmeyer flask shown in Figure 1, it
SOP for use of the SX19 Stopped-Flow Instrument
 SOP for use of the SX19 Stopped-Flow Instrument The main components of the stopped flow system are: Always book the system before use. Starting up and preparing the SX19 for use: 1. Turn on the compressed
SOP for use of the SX19 Stopped-Flow Instrument The main components of the stopped flow system are: Always book the system before use. Starting up and preparing the SX19 for use: 1. Turn on the compressed
Evaluation copy. Vapor Pressure of Liquids. computer OBJECTIVES MATERIALS
 Vapor Pressure of Liquids Computer 10 In this experiment, you will investigate the relationship between the vapor pressure of a liquid and its temperature. When a liquid is added to the Erlenmeyer flask
Vapor Pressure of Liquids Computer 10 In this experiment, you will investigate the relationship between the vapor pressure of a liquid and its temperature. When a liquid is added to the Erlenmeyer flask
Boyle s Law: Pressure-Volume Relationship in Gases
 Boyle s Law: Pressure-Volume Relationship in Gases The primary objective of this experiment is to determine the relationship between the pressure and volume of a confined gas. The gas we will use is air,
Boyle s Law: Pressure-Volume Relationship in Gases The primary objective of this experiment is to determine the relationship between the pressure and volume of a confined gas. The gas we will use is air,
Boyle s Law: Pressure-Volume. Relationship in Gases
 Boyle s Law: Pressure-Volume Relationship in Gases The primary objective of this experiment is to determine the relationship between the pressure and volume of a confined gas. The gas we use will be air,
Boyle s Law: Pressure-Volume Relationship in Gases The primary objective of this experiment is to determine the relationship between the pressure and volume of a confined gas. The gas we use will be air,
Boyle s Law: Pressure-Volume Relationship in Gases. PRELAB QUESTIONS (Answer on your own notebook paper)
 Boyle s Law: Pressure-Volume Relationship in Gases Experiment 18 GRADE LEVEL INDICATORS Construct, interpret and apply physical and conceptual models that represent or explain systems, objects, events
Boyle s Law: Pressure-Volume Relationship in Gases Experiment 18 GRADE LEVEL INDICATORS Construct, interpret and apply physical and conceptual models that represent or explain systems, objects, events
Quantitative Analysis of Hydrocarbons by Gas Chromatography
 Quantitative Analysis of Hydrocarbons by Gas Chromatography Introduction Gas-liquid chromatography (GLC) accomplishes a separation by partitioning solutes between a mobile gas phase and a stationary liquid
Quantitative Analysis of Hydrocarbons by Gas Chromatography Introduction Gas-liquid chromatography (GLC) accomplishes a separation by partitioning solutes between a mobile gas phase and a stationary liquid
Boyle s Law. Pressure-Volume Relationship in Gases. Figure 1
 Boyle s Law Pressure-Volume Relationship in Gases The primary objective of this experiment is to determine the relationship between the pressure and volume of a confined gas. The gas we use will be air,
Boyle s Law Pressure-Volume Relationship in Gases The primary objective of this experiment is to determine the relationship between the pressure and volume of a confined gas. The gas we use will be air,
AKTA pure 25 New Owner s Intro
 AKTA pure 25 New Owner s Intro The exercise below will give a quick demonstration of how easy and intuitive the AKTA pure 25 will be for you in demonstrating downstream processing to your students. Steps
AKTA pure 25 New Owner s Intro The exercise below will give a quick demonstration of how easy and intuitive the AKTA pure 25 will be for you in demonstrating downstream processing to your students. Steps
Boyle s Law: Pressure-Volume Relationship in Gases
 Boyle s Law: Pressure-Volume Relationship in Gases Computer 6 The primary objective of this experiment is to determine the relationship between the pressure and volume of a confined gas. The gas we use
Boyle s Law: Pressure-Volume Relationship in Gases Computer 6 The primary objective of this experiment is to determine the relationship between the pressure and volume of a confined gas. The gas we use
Boyle s Law: Pressure-Volume Relationship in Gases
 Boyle s Law: Pressure-Volume Relationship in Gases Experiment The primary objective of this experiment is to determine the relationship between the pressure and volume of a confined gas. The gas we use
Boyle s Law: Pressure-Volume Relationship in Gases Experiment The primary objective of this experiment is to determine the relationship between the pressure and volume of a confined gas. The gas we use
Experiment 11: The Ideal Gas Law
 Experiment 11: The Ideal Gas Law The behavior of an ideal gas is described by its equation of state, PV = nrt. You will look at two special cases of this. Part 1: Determination of Absolute Zero. You will
Experiment 11: The Ideal Gas Law The behavior of an ideal gas is described by its equation of state, PV = nrt. You will look at two special cases of this. Part 1: Determination of Absolute Zero. You will
EXPERIMENT 12 BEHAVIOR OF GASES
 EXPERIMENT 12 BEHAVIOR OF GASES INTRODUCTION A large number of substances of considerable chemical interest are gases. For example, CO 2 is currently in the news because it is thought to be partly responsible
EXPERIMENT 12 BEHAVIOR OF GASES INTRODUCTION A large number of substances of considerable chemical interest are gases. For example, CO 2 is currently in the news because it is thought to be partly responsible
The Gas Laws: Boyle's Law and Charles Law
 Exercise 6 Page 1 Illinois Central College CHEMISTRY 130 Name The Gas Laws: Boyle's Law and Charles Law Objective The simple laws governing the properties of gases can be readily demonstrated experimentally.
Exercise 6 Page 1 Illinois Central College CHEMISTRY 130 Name The Gas Laws: Boyle's Law and Charles Law Objective The simple laws governing the properties of gases can be readily demonstrated experimentally.
Vapor Pressure of Liquids
 Vapor Pressure of Liquids Calculator 10 In this experiment, you will investigate the relationship between the vapor pressure of a liquid and its temperature. When a liquid is added to the Erlenmeyer flask
Vapor Pressure of Liquids Calculator 10 In this experiment, you will investigate the relationship between the vapor pressure of a liquid and its temperature. When a liquid is added to the Erlenmeyer flask
INTRODUCTION TO THE SPECTROPHOTOMETER AND PIPETTING SKILLS
 INTRODUCTION TO THE SPECTROPHOTOMETER AND PIPETTING SKILLS Section A: Intro to the spectrophotometer A commonly used instrument in the analysis of cellular extracts is the Spectrophotometer. Today you
INTRODUCTION TO THE SPECTROPHOTOMETER AND PIPETTING SKILLS Section A: Intro to the spectrophotometer A commonly used instrument in the analysis of cellular extracts is the Spectrophotometer. Today you
Lab 1. Adiabatic and reversible compression of a gas
 Lab 1. Adiabatic and reversible compression of a gas Introduction The initial and final states of an adiabatic and reversible volume change of an ideal gas can be determined by the First Law of Thermodynamics
Lab 1. Adiabatic and reversible compression of a gas Introduction The initial and final states of an adiabatic and reversible volume change of an ideal gas can be determined by the First Law of Thermodynamics
Transpiration. DataQuest OBJECTIVES MATERIALS
 Transpiration DataQuest 13 Water is transported in plants, from the roots to the leaves, following a decreasing water potential gradient. Transpiration, or loss of water from the leaves, helps to create
Transpiration DataQuest 13 Water is transported in plants, from the roots to the leaves, following a decreasing water potential gradient. Transpiration, or loss of water from the leaves, helps to create
EXPERIMENT 12 GAS LAWS ( BOYLE S AND GAY-LUSSAC S LAW)
 EXPERIMENT 12 GAS LAWS ( BOYLE S AND GAY-LUSSAC S LAW) INTRODUCTION: In order to specify fully the condition of a gas it is necessary to know its pressure, volume, and temperature. This quantities are
EXPERIMENT 12 GAS LAWS ( BOYLE S AND GAY-LUSSAC S LAW) INTRODUCTION: In order to specify fully the condition of a gas it is necessary to know its pressure, volume, and temperature. This quantities are
Any laboratory is equipped with specific tools, equipment,
 Use of Laboratory Equipment and Supplies 3 When you have completed this exercise, you will be able to: 1. Use a balance. 2. Use pipettes and graduated cylinders to measure the volume of liquids. 3. Use
Use of Laboratory Equipment and Supplies 3 When you have completed this exercise, you will be able to: 1. Use a balance. 2. Use pipettes and graduated cylinders to measure the volume of liquids. 3. Use
Name Student Activity
 Open the TI-Nspire document Boyles_Law.tns. In this activity, you will use a Gas Pressure Sensor to measure the pressure of an air sample inside a syringe. Using graphs, you will apply your results to
Open the TI-Nspire document Boyles_Law.tns. In this activity, you will use a Gas Pressure Sensor to measure the pressure of an air sample inside a syringe. Using graphs, you will apply your results to
VOLUMETRIC TECHNIQUES
 REVISED 10/14 CHEMISTRY 1101L VOLUMETRIC TECHNIQUES Volume measurements are important in many experimental procedures. Sometimes volume measurements must be exact; other times they can be approximate.
REVISED 10/14 CHEMISTRY 1101L VOLUMETRIC TECHNIQUES Volume measurements are important in many experimental procedures. Sometimes volume measurements must be exact; other times they can be approximate.
UNITY 2 TM. Air Server Series 2 Operators Manual. Version 1.0. February 2008
 UNITY 2 TM Air Server Series 2 Operators Manual Version 1.0 February 2008 1. Introduction to the Air Server Accessory for UNITY 2...2 1.1. Summary of Operation...2 2. Developing a UNITY 2-Air Server method
UNITY 2 TM Air Server Series 2 Operators Manual Version 1.0 February 2008 1. Introduction to the Air Server Accessory for UNITY 2...2 1.1. Summary of Operation...2 2. Developing a UNITY 2-Air Server method
The Decomposition of Hydrogen Peroxide
 The Decomposition of Hydrogen Peroxide Calculator 12 The decomposition of hydrogen peroxide in aqueous solution proceeds very slowly. A bottle of 3% hydrogen peroxide sitting on a grocery store shelf is
The Decomposition of Hydrogen Peroxide Calculator 12 The decomposition of hydrogen peroxide in aqueous solution proceeds very slowly. A bottle of 3% hydrogen peroxide sitting on a grocery store shelf is
User s Booklet for the Wyatt minidawn Light Scattering Instrumentation
 User s Booklet for the Wyatt minidawn Light Scattering Instrumentation The Wyatt minidawn Light Scattering instrument is able to measure the weight average molecular weight of a synthetic polymer or a
User s Booklet for the Wyatt minidawn Light Scattering Instrumentation The Wyatt minidawn Light Scattering instrument is able to measure the weight average molecular weight of a synthetic polymer or a
In addition to reading this assignment, also read Appendices A and B.
 1 Kinematics I Introduction In addition to reading this assignment, also read Appendices A and B. We will be using a motion detector to track the positions of objects with time in several lab exercises
1 Kinematics I Introduction In addition to reading this assignment, also read Appendices A and B. We will be using a motion detector to track the positions of objects with time in several lab exercises
Vapor Pressure of Liquids
 Vapor Pressure of Liquids Experiment 10 In this experiment, you will investigate the relationship between the vapor pressure of a liquid and its temperature. When a liquid is added to the Erlenmeyer flask
Vapor Pressure of Liquids Experiment 10 In this experiment, you will investigate the relationship between the vapor pressure of a liquid and its temperature. When a liquid is added to the Erlenmeyer flask
Pipetting and Determining Protein Concentration
 Pipetting and Determining Protein Concentration Background Information: When performing experiments in Cell Biology, it is often necessary to use very small volumes of reagents sometimes because the reagents
Pipetting and Determining Protein Concentration Background Information: When performing experiments in Cell Biology, it is often necessary to use very small volumes of reagents sometimes because the reagents
Determination of Zn using Atomic Absorption with Multiple Standard Additions
 1. Purpose Determination of Zn using Atomic Absorption with Multiple Standard Additions This procedure will determine the concentration of zinc at the parts-per-million level using flame atomic absorption
1. Purpose Determination of Zn using Atomic Absorption with Multiple Standard Additions This procedure will determine the concentration of zinc at the parts-per-million level using flame atomic absorption
The Ideal Gas Constant
 Chem 2115 Experiment # 8 The Ideal Gas Constant OBJECTIVE: This experiment is designed to provide experience in gas handling methods and experimental insight into the relationships between pressure, volume,
Chem 2115 Experiment # 8 The Ideal Gas Constant OBJECTIVE: This experiment is designed to provide experience in gas handling methods and experimental insight into the relationships between pressure, volume,
Manual for continuous distillation
 Manual for continuous distillation 1. Week 1: Objectives: Run the column at total reflux. When steady state is reached, take the sample from the top and bottom of the column in order to determine the overall
Manual for continuous distillation 1. Week 1: Objectives: Run the column at total reflux. When steady state is reached, take the sample from the top and bottom of the column in order to determine the overall
Combination Analysis Tutorial
 Combination Analysis Tutorial 3-1 Combination Analysis Tutorial It is inherent in the Swedge analysis (when the Block Shape = Wedge), that tetrahedral wedges can only be formed by the intersection of 2
Combination Analysis Tutorial 3-1 Combination Analysis Tutorial It is inherent in the Swedge analysis (when the Block Shape = Wedge), that tetrahedral wedges can only be formed by the intersection of 2
JASCO 810 CD SPECTROPLOARIMETER STANDARD OPERATING PROCEDURE
 JASCO 810 CD SPECTROPLOARIMETER STANDARD OPERATING PROCEDURE Purpose of this Instrument: This instrument is for measuring differences in the absorption of lefthanded polarized light versus right-handed
JASCO 810 CD SPECTROPLOARIMETER STANDARD OPERATING PROCEDURE Purpose of this Instrument: This instrument is for measuring differences in the absorption of lefthanded polarized light versus right-handed
Cover Page for Lab Report Group Portion. Head Losses in Pipes
 Cover Page for Lab Report Group Portion Head Losses in Pipes Prepared by Professor J. M. Cimbala, Penn State University Latest revision: 02 February 2012 Name 1: Name 2: Name 3: [Name 4: ] Date: Section
Cover Page for Lab Report Group Portion Head Losses in Pipes Prepared by Professor J. M. Cimbala, Penn State University Latest revision: 02 February 2012 Name 1: Name 2: Name 3: [Name 4: ] Date: Section
Using the Akta Prime plus October 22, 2012
 Some starting precautions: 1. Vacuum filter all buffers. Removes any large particles/debris that may clog your column De-gases the buffers 2. Clarify lysates first by centrifugation and then filtration
Some starting precautions: 1. Vacuum filter all buffers. Removes any large particles/debris that may clog your column De-gases the buffers 2. Clarify lysates first by centrifugation and then filtration
TEMPERATURE S RELATIONSHIP TO GAS & VAPOR PRESSURE
 TEMPERATURE S RELATIONSHIP TO GAS & VAPOR PRESSURE Adapted from "Chemistry with Computers" Vernier Software, Portland OR, 1997 ELECTRONIC LABORATORY NOTEBOOK (ELN) INSTRUCTIONS Read the directions and
TEMPERATURE S RELATIONSHIP TO GAS & VAPOR PRESSURE Adapted from "Chemistry with Computers" Vernier Software, Portland OR, 1997 ELECTRONIC LABORATORY NOTEBOOK (ELN) INSTRUCTIONS Read the directions and
Biology Unit 2, Structure of Life, Lab Activity 2-3
 Biology Unit 2, Structure of Life, Lab Activity 2-3 Cellular respiration is the release of energy from organic compounds by metabolic chemical oxidation in the mitochondria within each cell. Cellular respiration
Biology Unit 2, Structure of Life, Lab Activity 2-3 Cellular respiration is the release of energy from organic compounds by metabolic chemical oxidation in the mitochondria within each cell. Cellular respiration
Determination of Zn using Atomic Absorption with Multiple Standard Additions
 Determination of Zn using Atomic Absorption with Multiple Standard Additions 1. Purpose This procedure will determine the concentration of zinc at the parts-per-million level using flame atomic absorption
Determination of Zn using Atomic Absorption with Multiple Standard Additions 1. Purpose This procedure will determine the concentration of zinc at the parts-per-million level using flame atomic absorption
EXPERIMENT XI. Careful!! Improper handling of the vacuum line may result in the release of SO 2 which is an irritating and suffocating gas.
 Chem 366-3 Page XI - 1 EXPERIMENT XI INFRARED SPECTRUM OF SO2 (S&G, 5th ed. Expt 36, 6th ed. Expt. 35) 1. Pre-Lab preparation. The description of this experiment has disappeared from the more recent editions
Chem 366-3 Page XI - 1 EXPERIMENT XI INFRARED SPECTRUM OF SO2 (S&G, 5th ed. Expt 36, 6th ed. Expt. 35) 1. Pre-Lab preparation. The description of this experiment has disappeared from the more recent editions
Introduction. Objectives. Hazards. Procedure
 Experiment: Exploring Gases Note to Students: Check with your instructor to see which parts of this lab (Parts A, B, or C) you will complete. Introduction Gases are made up of molecules that are in constant
Experiment: Exploring Gases Note to Students: Check with your instructor to see which parts of this lab (Parts A, B, or C) you will complete. Introduction Gases are made up of molecules that are in constant
Ball Toss. Vernier Motion Detector
 Experiment 6 When a juggler tosses a ball straight upward, the ball slows down until it reaches the top of its path. The ball then speeds up on its way back down. A graph of its velocity vs. time would
Experiment 6 When a juggler tosses a ball straight upward, the ball slows down until it reaches the top of its path. The ball then speeds up on its way back down. A graph of its velocity vs. time would
CHEMICAL ENGINEERING LABORATORY CHEG 239W. Control of a Steam-Heated Mixing Tank with a Pneumatic Process Controller
 CHEMICAL ENGINEERING LABORATORY CHEG 239W Control of a Steam-Heated Mixing Tank with a Pneumatic Process Controller Objective The experiment involves tuning a commercial process controller for temperature
CHEMICAL ENGINEERING LABORATORY CHEG 239W Control of a Steam-Heated Mixing Tank with a Pneumatic Process Controller Objective The experiment involves tuning a commercial process controller for temperature
Lab #12:Boyle s Law, Dec. 20, 2016 Pressure-Volume Relationship in Gases
 Chemistry Unit 6:States of Matter & Basic Gas Laws Name Lab Partner Lab #12:Boyle s Law, Dec. 20, 2016 Pressure-Volume Relationship in Gases Purpose: The primary objective of this experiment is to determine
Chemistry Unit 6:States of Matter & Basic Gas Laws Name Lab Partner Lab #12:Boyle s Law, Dec. 20, 2016 Pressure-Volume Relationship in Gases Purpose: The primary objective of this experiment is to determine
1. Determining Solution Concentration
 In this exercise you will determine the concentration of salt solutions by measuring samples with known concentration and making a calibration curve. You will review units of concentration, and how to
In this exercise you will determine the concentration of salt solutions by measuring samples with known concentration and making a calibration curve. You will review units of concentration, and how to
Biochemistry Laboratory I CHEM 4401 Pipetting & Scales
 Biochemistry Laboratory I CHEM 4401 Pipetting & Scales Pipettors are one of the most basic, and most used, instruments in the biochemistry laboratory. pipettors are used to transfer small volumes of liquids,
Biochemistry Laboratory I CHEM 4401 Pipetting & Scales Pipettors are one of the most basic, and most used, instruments in the biochemistry laboratory. pipettors are used to transfer small volumes of liquids,
PREPARING GAS SAMPLES IN LARGE PLASTIC SYRINGES
 PREPARING GAS SAMPLES IN LARGE PLASTIC SYRINGES A wide variety of gases can be prepared safely inside a 60 ml plastic syringe. Here you will practice making carbon dioxide, so that you know the technique
PREPARING GAS SAMPLES IN LARGE PLASTIC SYRINGES A wide variety of gases can be prepared safely inside a 60 ml plastic syringe. Here you will practice making carbon dioxide, so that you know the technique
CHE 4115 Chemical Processes Laboratory 2 Experiment 1. Batch Distillation
 CHE 4115 Chemical Processes Laboratory 2 Experiment 1 Batch Distillation BACKGROUND Distillation is one of the most commonly used unit operations in chemical engineering. In general, a distillation operation
CHE 4115 Chemical Processes Laboratory 2 Experiment 1 Batch Distillation BACKGROUND Distillation is one of the most commonly used unit operations in chemical engineering. In general, a distillation operation
Boyle s Law VC 09. Experiment 9: Gas Laws. Abstract
 Experiment 9: Gas Laws VC 09 Abstract In this laboratory activity, you will experimentally confirm Boyle s Law, determine absolute zero from Gay-Lussac's Law, and determine the molecular weight of acetone,
Experiment 9: Gas Laws VC 09 Abstract In this laboratory activity, you will experimentally confirm Boyle s Law, determine absolute zero from Gay-Lussac's Law, and determine the molecular weight of acetone,
Physics Experiment 17 Ideal Gas Law Qualitative Study
 Physics 210 17-1 Experiment 17 Ideal Gas Law Qualitative Study Note 1: Parts of this lab involve using a laptop computer and the PASCO ScienceWorkshop Interface to collect data. The lab also involves use
Physics 210 17-1 Experiment 17 Ideal Gas Law Qualitative Study Note 1: Parts of this lab involve using a laptop computer and the PASCO ScienceWorkshop Interface to collect data. The lab also involves use
Describing a journey made by an object is very boring if you just use words. As with much of science, graphs are more revealing.
 Distance vs. Time Describing a journey made by an object is very boring if you just use words. As with much of science, graphs are more revealing. Plotting distance against time can tell you a lot about
Distance vs. Time Describing a journey made by an object is very boring if you just use words. As with much of science, graphs are more revealing. Plotting distance against time can tell you a lot about
Experiment 8 GAS LAWS
 Experiment 8 GAS LAWS FV 6/25/2017 MATERIALS: Amontons Law apparatus, Boyle s Law apparatus, Avogadro s Corollary apparatus, four beakers (2 L), warm-water bath, ice, barometer, digital thermometer, air
Experiment 8 GAS LAWS FV 6/25/2017 MATERIALS: Amontons Law apparatus, Boyle s Law apparatus, Avogadro s Corollary apparatus, four beakers (2 L), warm-water bath, ice, barometer, digital thermometer, air
Lab 4: Transpiration
 Lab 4: Transpiration Water is transported in plants, from the roots to the leaves, following a decreasing water potential gradient. Transpiration, or loss of water from the leaves, helps to create a lower
Lab 4: Transpiration Water is transported in plants, from the roots to the leaves, following a decreasing water potential gradient. Transpiration, or loss of water from the leaves, helps to create a lower
Armfield Distillation Column Operation Guidelines
 Armfield Distillation Column Operation Guidelines 11-2016 R.Cox Safety SAFETY GLASSES ARE REQUIRED WHEN OPERATING THE DISTILLATION COLUMN Wear gloves when mixing alcohol feedstock The column will become
Armfield Distillation Column Operation Guidelines 11-2016 R.Cox Safety SAFETY GLASSES ARE REQUIRED WHEN OPERATING THE DISTILLATION COLUMN Wear gloves when mixing alcohol feedstock The column will become
Fun with Gas Laws. Prepared by Vance O. Kennedy and Ross S. Nord, Eastern Michigan University PURPOSE
 Experiment 2 Fun with Gas Laws Prepared by Vance O. Kennedy and Ross S. Nord, Eastern Michigan University PURPOSE The purpose of this laboratory experience is to explore the gas law relationships between
Experiment 2 Fun with Gas Laws Prepared by Vance O. Kennedy and Ross S. Nord, Eastern Michigan University PURPOSE The purpose of this laboratory experience is to explore the gas law relationships between
Cover Page for Lab Report Group Portion. Boundary Layer Measurements
 Cover Page for Lab Report Group Portion Boundary Layer Measurements Prepared by Professor J. M. Cimbala, Penn State University Latest revision: 30 March 2012 Name 1: Name 2: Name 3: [Name 4: ] Date: Section
Cover Page for Lab Report Group Portion Boundary Layer Measurements Prepared by Professor J. M. Cimbala, Penn State University Latest revision: 30 March 2012 Name 1: Name 2: Name 3: [Name 4: ] Date: Section
Cover Page for Lab Report Group Portion. Flow Visualization in a Water Channel
 Cover Page for Lab Report Group Portion Flow Visualization in a Water Channel Prepared by Professor J. M. Cimbala, Penn State University Latest revision: 08 September 2017 Name 1: Name 2: Name 3: [Name
Cover Page for Lab Report Group Portion Flow Visualization in a Water Channel Prepared by Professor J. M. Cimbala, Penn State University Latest revision: 08 September 2017 Name 1: Name 2: Name 3: [Name
CHM 100 / Introductory Laboratory Experiment (r10) 1/11
 CHM 100 / 110 - Introductory Laboratory Experiment (r10) 1/11 Purpose This introductory exercise will familiarize you with a few of the measurements we make in the chemistry laboratory and the level of
CHM 100 / 110 - Introductory Laboratory Experiment (r10) 1/11 Purpose This introductory exercise will familiarize you with a few of the measurements we make in the chemistry laboratory and the level of
Exploring the Properties of Gases. Evaluation copy. 10 cm in diameter and 25 cm high)
 Exploring the Properties of Gases Computer 30 The purpose of this investigation is to conduct a series of experiments, each of which illustrates a different gas law. You will be given a list of equipment
Exploring the Properties of Gases Computer 30 The purpose of this investigation is to conduct a series of experiments, each of which illustrates a different gas law. You will be given a list of equipment
Technical Procedure for General Laboratory Equipment
 Technical Procedure for General Laboratory Equipment 1.0 Purpose - This procedure specifies the required elements for the use of general laboratory equipment. 2.0 Scope - This procedure applies to all
Technical Procedure for General Laboratory Equipment 1.0 Purpose - This procedure specifies the required elements for the use of general laboratory equipment. 2.0 Scope - This procedure applies to all
Vapor Sorption Analyzer
 Vapor Sorption Analyzer Quick Start Guide 1. Getting Started Place the AquaLab VSA on a flat surface. Use the bubble level(figure 1: Adjustment and bubble level) and use the adjustable feet to level the
Vapor Sorption Analyzer Quick Start Guide 1. Getting Started Place the AquaLab VSA on a flat surface. Use the bubble level(figure 1: Adjustment and bubble level) and use the adjustable feet to level the
Exploring the Properties of Gases
 Computer 30 The purpose of this investigation is to conduct a series of experiments, each of which illustrates a different gas law. You will be given a list of equipment and materials and some general
Computer 30 The purpose of this investigation is to conduct a series of experiments, each of which illustrates a different gas law. You will be given a list of equipment and materials and some general
SOLUBILITY OF A SOLID IN WATER
 1516L Experiment 2 SOLUBILITY OF A SOLID IN WATER Objectives In this experiment you will determine the solubility of potassium nitrate (KNO 3 ) in water at various temperatures. You will prepare a plot
1516L Experiment 2 SOLUBILITY OF A SOLID IN WATER Objectives In this experiment you will determine the solubility of potassium nitrate (KNO 3 ) in water at various temperatures. You will prepare a plot
BASIC Z-STACK AND TIME SERIES SCAN ON THE ZEISS LIGHTSHEET Z. 1
 BASIC Z-STACK AND TIME SERIES SCAN ON THE ZEISS LIGHTSHEET Z. 1 The front door of the main body of the instrument may be open when you arrive. Take the sample chamber and slide it into position with the
BASIC Z-STACK AND TIME SERIES SCAN ON THE ZEISS LIGHTSHEET Z. 1 The front door of the main body of the instrument may be open when you arrive. Take the sample chamber and slide it into position with the
CHM Introductory Laboratory Experiment (r17sd) 1/13
 CHM 110 - Introductory Laboratory Experiment (r17sd) 1/13 Purpose This introductory exercise will familiarize you with a few of the measurements we make in the chemistry laboratory and the level of uncertainty
CHM 110 - Introductory Laboratory Experiment (r17sd) 1/13 Purpose This introductory exercise will familiarize you with a few of the measurements we make in the chemistry laboratory and the level of uncertainty
Tutorial 2 Time-Dependent Consolidation. Staging Groundwater Time-dependent consolidation Point query Line query Graph Query
 Tutorial 2 Time-Dependent Consolidation Staging Groundwater Time-dependent consolidation Point query Line query Graph Query Model Set-up For this tutorial we will start with the model from Tutorial 1 Quick
Tutorial 2 Time-Dependent Consolidation Staging Groundwater Time-dependent consolidation Point query Line query Graph Query Model Set-up For this tutorial we will start with the model from Tutorial 1 Quick
PRESSURE-TEMPERATURE RELATIONSHIP IN GASES
 PRESSURE-TEMPERATURE RELATIONSHIP IN GASES LAB PS2.PALM INTRODUCTION Gases are made up of molecules that are in constant motion and exert pressure when they collide with the walls of their container. The
PRESSURE-TEMPERATURE RELATIONSHIP IN GASES LAB PS2.PALM INTRODUCTION Gases are made up of molecules that are in constant motion and exert pressure when they collide with the walls of their container. The
NanoSight NS300. NanoSight NS300. Operation instructions. Laser Spectroscopy Labs, UCI
 NanoSight NS300 Operation instructions Injection/flushing brief overview: 1. Do not exceed flow of 1 ml per 20 seconds. 2. Inject two 1 ml syringes with nano-pure or DI water. 3. If the water does not
NanoSight NS300 Operation instructions Injection/flushing brief overview: 1. Do not exceed flow of 1 ml per 20 seconds. 2. Inject two 1 ml syringes with nano-pure or DI water. 3. If the water does not
Aerobic Respiration. Evaluation copy
 Aerobic Respiration Computer 17 Aerobic cellular respiration is the process of converting the chemical energy of organic molecules into a form immediately usable by organisms. Glucose may be oxidized completely
Aerobic Respiration Computer 17 Aerobic cellular respiration is the process of converting the chemical energy of organic molecules into a form immediately usable by organisms. Glucose may be oxidized completely
Target Density Lab SCIENTIFIC. Density Inquiry Lab Activities. Introduction. Concepts. Materials. Safety Precautions. Preparation
 Target Density Lab Density Inquiry Lab Activities SCIENTIFIC Introduction The concept of density is reinforced as students measure the volume and mass of an unknown liquid in a graduated cylinder, graph
Target Density Lab Density Inquiry Lab Activities SCIENTIFIC Introduction The concept of density is reinforced as students measure the volume and mass of an unknown liquid in a graduated cylinder, graph
Olympus Production Contour Plot
 Olympus Production Contour Plot Overview An alternative to the Scale Scenario calculation is the Contour Diagram. The conditions defined in the Scale Scenario are specific to production locations (wellhead,
Olympus Production Contour Plot Overview An alternative to the Scale Scenario calculation is the Contour Diagram. The conditions defined in the Scale Scenario are specific to production locations (wellhead,
29 Pressure, Temperature relationship of a gas
 Chemistry Sensors: Loggers: Gas Pressure, Temperature Any EASYSENSE Logging time: EasyLog Teacher s notes 29 Pressure, Temperature relationship of a gas Read The ideal gas laws tell us that if we keep
Chemistry Sensors: Loggers: Gas Pressure, Temperature Any EASYSENSE Logging time: EasyLog Teacher s notes 29 Pressure, Temperature relationship of a gas Read The ideal gas laws tell us that if we keep
SOP: Buck Scientific BLC-20P HPLC Operation
 Page 1 of 11 Approvals Preparer: John Buford Reviewer: Tim Kull Reviewer: Dr. Margaret Bryans Date: 16OCT13 Date: 30OCT13 Date: 31OCT13 1. Purpose 1.1. Basic operation of the Buck Scientific BLC-20P isocratic
Page 1 of 11 Approvals Preparer: John Buford Reviewer: Tim Kull Reviewer: Dr. Margaret Bryans Date: 16OCT13 Date: 30OCT13 Date: 31OCT13 1. Purpose 1.1. Basic operation of the Buck Scientific BLC-20P isocratic
Tension Cracks. Topics Covered. Tension crack boundaries Tension crack depth Query slice data Thrust line Sensitivity analysis.
 Tension Cracks 16-1 Tension Cracks In slope stability analyses with cohesive soils, tension forces may be observed in the upper part of the slope. In general, soils cannot support tension so the results
Tension Cracks 16-1 Tension Cracks In slope stability analyses with cohesive soils, tension forces may be observed in the upper part of the slope. In general, soils cannot support tension so the results
Exploring the Properties of Gases
 Exploring the Properties of Gases LabQuest 30 The purpose of this investigation is to conduct a series of experiments, each of which illustrates a different gas law. You will be given a list of equipment
Exploring the Properties of Gases LabQuest 30 The purpose of this investigation is to conduct a series of experiments, each of which illustrates a different gas law. You will be given a list of equipment
WMS 8.4 Tutorial Hydraulics and Floodplain Modeling HY-8 Modeling Wizard Learn how to model a culvert using HY-8 and WMS
 v. 8.4 WMS 8.4 Tutorial Hydraulics and Floodplain Modeling HY-8 Modeling Wizard Learn how to model a culvert using HY-8 and WMS Objectives Define a conceptual schematic of the roadway, invert, and downstream
v. 8.4 WMS 8.4 Tutorial Hydraulics and Floodplain Modeling HY-8 Modeling Wizard Learn how to model a culvert using HY-8 and WMS Objectives Define a conceptual schematic of the roadway, invert, and downstream
FOURIER TRANSFORM INFRARED SPECTROSCOPY
 Purpose and Scope: This document describes the procedures and policies for using the MSE Bruker FTIR Spectrometer. The scope of this document is to establish user procedures. Instrument maintenance and
Purpose and Scope: This document describes the procedures and policies for using the MSE Bruker FTIR Spectrometer. The scope of this document is to establish user procedures. Instrument maintenance and
BASIC LABORATORY TECHNIQUES (Revised )
 BASIC LABORATORY TECHNIQUES (Revised 1-2-16) (See Appendix II: Summary for making Spreadsheets and Graphs with Excel and Appendix III parts C, C1 and C2: Significant figures, scientific notation and rounding)
BASIC LABORATORY TECHNIQUES (Revised 1-2-16) (See Appendix II: Summary for making Spreadsheets and Graphs with Excel and Appendix III parts C, C1 and C2: Significant figures, scientific notation and rounding)
Vapor Pressure of Liquids
 Vapor Pressure of Liquids In this experiment, you will investigate the relationship between the vapor pressure of a liquid and its temperature. When a liquid is added to the Erlenmeyer flask shown in Figure
Vapor Pressure of Liquids In this experiment, you will investigate the relationship between the vapor pressure of a liquid and its temperature. When a liquid is added to the Erlenmeyer flask shown in Figure
CENTER PIVOT EVALUATION AND DESIGN
 CENTER PIVOT EVALUATION AND DESIGN Dale F. Heermann Agricultural Engineer USDA-ARS 2150 Centre Avenue, Building D, Suite 320 Fort Collins, CO 80526 Voice -970-492-7410 Fax - 970-492-7408 Email - dale.heermann@ars.usda.gov
CENTER PIVOT EVALUATION AND DESIGN Dale F. Heermann Agricultural Engineer USDA-ARS 2150 Centre Avenue, Building D, Suite 320 Fort Collins, CO 80526 Voice -970-492-7410 Fax - 970-492-7408 Email - dale.heermann@ars.usda.gov
Heat Engine. Reading: Appropriate sections for first, second law of thermodynamics, and PV diagrams.
 Heat Engine Equipment: Capstone, 2 large glass beakers (one for ice water, the other for boiling water), temperature sensor, pressure sensor, rotary motion sensor, meter stick, calipers, set of weights,
Heat Engine Equipment: Capstone, 2 large glass beakers (one for ice water, the other for boiling water), temperature sensor, pressure sensor, rotary motion sensor, meter stick, calipers, set of weights,
Cover Page for Lab Report Group Portion. Lift on a Wing
 Cover Page for Lab Report Group Portion Lift on a Wing Prepared by Professor J. M. Cimbala, Penn State University Latest revision: 17 January 2017 Name 1: Name 2: Name 3: [Name 4: ] Date: Section number:
Cover Page for Lab Report Group Portion Lift on a Wing Prepared by Professor J. M. Cimbala, Penn State University Latest revision: 17 January 2017 Name 1: Name 2: Name 3: [Name 4: ] Date: Section number:
Experiment P18: Buoyant Force (Force Sensor)
 PASCO scientific Physics Lab Manual: P18-1 Experiment P18: (Force Sensor) Concept Time SW Interface Macintosh file Windows file Newton's Laws 45 m 300/500/700 P18 P18_BUOY.SWS EQUIPMENT NEEDED CONSUMABLES
PASCO scientific Physics Lab Manual: P18-1 Experiment P18: (Force Sensor) Concept Time SW Interface Macintosh file Windows file Newton's Laws 45 m 300/500/700 P18 P18_BUOY.SWS EQUIPMENT NEEDED CONSUMABLES
16. Studio ScaleChem Calculations
 16. Studio ScaleChem Calculations Calculations Overview Calculations: Adding a new brine sample Studio ScaleChem can be used to calculate scaling at one or more user specified temperatures and pressures.
16. Studio ScaleChem Calculations Calculations Overview Calculations: Adding a new brine sample Studio ScaleChem can be used to calculate scaling at one or more user specified temperatures and pressures.
Read this first. Zetasizer nano series Self installation and Quick start guide MRK825-02
 ! Read this first Zetasizer nano series Self installation and Quick start guide I N S T R U M E N T S MRK825-02 Zetasizer Nano series Self installation and Quick start guide MAN0383 Issue 1.1 July 2007
! Read this first Zetasizer nano series Self installation and Quick start guide I N S T R U M E N T S MRK825-02 Zetasizer Nano series Self installation and Quick start guide MAN0383 Issue 1.1 July 2007
Chapter 7 Single Point Calculations
 Chapter 7 Single Point Calculations Objectives By this point we have learned a great deal about the thermodynamics of the OLI Software and the internal workings of the simulation engine. We have also learned
Chapter 7 Single Point Calculations Objectives By this point we have learned a great deal about the thermodynamics of the OLI Software and the internal workings of the simulation engine. We have also learned
Chapter : Linear Motion 2
 Text: Chapter 2.5-2.9 Think and Explain: 4-8 Think and Solve: 2-4 Chapter 2.5-2.9: Linear Motion 2 NAME: Vocabulary: constant acceleration, acceleration due to gravity, free fall Equations: s = d t v =
Text: Chapter 2.5-2.9 Think and Explain: 4-8 Think and Solve: 2-4 Chapter 2.5-2.9: Linear Motion 2 NAME: Vocabulary: constant acceleration, acceleration due to gravity, free fall Equations: s = d t v =
FISH 415 LIMNOLOGY UI Moscow
 FISH 415 LIMNOLOGY UI Moscow Sampling Equipment Purpose: - to familiarize you with limnological sampling equipment - to use some of the equipment to obtain profiles of temperature, dissolved oxygen, conductivity
FISH 415 LIMNOLOGY UI Moscow Sampling Equipment Purpose: - to familiarize you with limnological sampling equipment - to use some of the equipment to obtain profiles of temperature, dissolved oxygen, conductivity
BEFORE YOU OPEN ANY FILES:
 Dive Analysis Lab * Make sure to download all the data files for the lab onto your computer. * Bring your computer to lab. * Bring a blank disk or memory stick to class to save your work and files. The
Dive Analysis Lab * Make sure to download all the data files for the lab onto your computer. * Bring your computer to lab. * Bring a blank disk or memory stick to class to save your work and files. The
SOLUBILITY OF A SOLID IN WATER
 1516L Experiment 1 SOLUBILITY OF A SOLID IN WATER Objectives In this experiment you will determine the solubility of potassium nitrate (KNO 3 ) in water at various temperatures. You will prepare a plot
1516L Experiment 1 SOLUBILITY OF A SOLID IN WATER Objectives In this experiment you will determine the solubility of potassium nitrate (KNO 3 ) in water at various temperatures. You will prepare a plot
1)! DO NOT PROCEED BEYOND THIS MARK
 Operating Instructions for X-ray Photoelectron Spectrometer: Physical Electronics Model 555 XPS/AES (John H. Thomas, III, Ph.D., Electron Spectroscopy) Sample Insertion: figure 1. Sample insertion rod
Operating Instructions for X-ray Photoelectron Spectrometer: Physical Electronics Model 555 XPS/AES (John H. Thomas, III, Ph.D., Electron Spectroscopy) Sample Insertion: figure 1. Sample insertion rod
For accurate measurements and to prevent damage to the micropipets, follow these important guidelines:
 OXFORD VERSION #2 MEASURE FOR MEASURE MICROPIPETTING AND THE METRIC SYSTEM Concept: Work with DNA and enzymes frequently involves measuring very small volumes, often in the microliter range. A microliter
OXFORD VERSION #2 MEASURE FOR MEASURE MICROPIPETTING AND THE METRIC SYSTEM Concept: Work with DNA and enzymes frequently involves measuring very small volumes, often in the microliter range. A microliter
LABORATORY INVESTIGATION
 LABORATORY INVESTIGATION MEASURING THE RATE OF PHOTOSYNTHESIS Light and Photosynthesis About 2.5-3 billion years ago a new chemical process, photosynthesis, was evolved by a unicellular life form. This
LABORATORY INVESTIGATION MEASURING THE RATE OF PHOTOSYNTHESIS Light and Photosynthesis About 2.5-3 billion years ago a new chemical process, photosynthesis, was evolved by a unicellular life form. This
Determination of Sodium using Atomic Emission
 Determination of Sodium using Atomic Emission 1. Purpose The purpose of this procedure is to determine the concentration of sodium ion in parts per million in an unknown sample. 2. Background Atomic emission
Determination of Sodium using Atomic Emission 1. Purpose The purpose of this procedure is to determine the concentration of sodium ion in parts per million in an unknown sample. 2. Background Atomic emission
MoLE Gas Laws Activities
 MoLE Gas Laws Activities To begin this assignment you must be able to log on to the Internet using Internet Explorer (Microsoft) 4.5 or higher. If you do not have the current version of the browser, go
MoLE Gas Laws Activities To begin this assignment you must be able to log on to the Internet using Internet Explorer (Microsoft) 4.5 or higher. If you do not have the current version of the browser, go
EXPERIMENT 1 BASIC LABORATORY TECHNIQUES AND TREATMENT OF DATA MEASUREMENTS
 EXPERIMENT 1 BASIC LABORATORY TECHNIQUES AND TREATMENT OF DATA MEASUREMENTS Introduction In the following experiment you will be required to use a Bunsen burner, balance, a pipet, graduated cylinder, flask,
EXPERIMENT 1 BASIC LABORATORY TECHNIQUES AND TREATMENT OF DATA MEASUREMENTS Introduction In the following experiment you will be required to use a Bunsen burner, balance, a pipet, graduated cylinder, flask,
QFM-4000 User s Manual Version 2.0
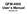 QFM-4000 User s Manual Version 2.0 Table of contents 1. INTRODUCTION AND SPECIFICATIONS... 4 1.1. General description... 4 1.2. The mechanical design... 4 1.3. Intelligent power supply... 4 1.4. Microcomputer
QFM-4000 User s Manual Version 2.0 Table of contents 1. INTRODUCTION AND SPECIFICATIONS... 4 1.1. General description... 4 1.2. The mechanical design... 4 1.3. Intelligent power supply... 4 1.4. Microcomputer
BASIC LABORATORY TECHNIQUES (Revised )
 BASIC LABORATORY TECHNIQUES (Revised 1-6-13) A. WEIGHING The determination of the quantity of matter in a sample is most directly determined by measuring its mass. The process by which we determine the
BASIC LABORATORY TECHNIQUES (Revised 1-6-13) A. WEIGHING The determination of the quantity of matter in a sample is most directly determined by measuring its mass. The process by which we determine the
To Logon On to your tee sheet, start by opening your browser. (NOTE: Internet Explorer V. 6.0 or greater is required.)
 1. Log-On To Logon On to your tee sheet, start by opening your browser. (NOTE: Internet Explorer V. 6.0 or greater is required.) (NOTE: Logon ID s must be 7 characters or more and passwords are case sensitive.)
1. Log-On To Logon On to your tee sheet, start by opening your browser. (NOTE: Internet Explorer V. 6.0 or greater is required.) (NOTE: Logon ID s must be 7 characters or more and passwords are case sensitive.)
Exercise 3. Power Versus Wind Speed EXERCISE OBJECTIVE DISCUSSION OUTLINE. Air density DISCUSSION
 Exercise 3 Power Versus Wind Speed EXERCISE OBJECTIVE When you have completed this exercise, you will know how to calculate the power contained in the wind, and how wind power varies with wind speed. You
Exercise 3 Power Versus Wind Speed EXERCISE OBJECTIVE When you have completed this exercise, you will know how to calculate the power contained in the wind, and how wind power varies with wind speed. You
