Preparation and Gas Separation Properties of Asymmetric Polysulfone Membranes by a Dual Bath Method
|
|
|
- Brittany Dickerson
- 5 years ago
- Views:
Transcription
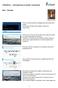
1 Korean J. Chem. Eng., 17(2), (2000) Preparation and Gas Separation Properties of Asymmetric Polysulfone Membranes by a Dual Bath Method Wan-Jin Lee, Deuk-San Kim and Jin-Hwan Kim* Faculty of Applied Chemistry, College of Engineering, Chonnam National University, Kwangju , Korea *Department of Chemical Engineering, Chonnam National University, Kwangju , Korea (Received 7 December 1998 accepted 10 January 2000) Abstract Defect-free skinned asymmetric gas separation membranes were prepared by a dual bath coagulation method that is a wet/wet phase inversion technique. The membranes were cast from a polysulfone/n,n-dimethylacetamide solution. In two sequent nonsolvent baths, the first bath using iso-propanol (IPA) leads to the formation of a dense skin top layer and the second bath using water makes the actual polymer precipitation. The top skin layer thickness was governed by changing the immersion time of the first IPA bath. We suggest that the growth rate of the skin layer is to be determined by a diffusion process. Key words: Defect-free Asymmetric Membrane, Dual Bath Method, Wet/Wet Phase Inversion Technique INTRODUCTION The method for preparing asymmetric membranes except for thin film composite process coating on the membrane surface is by liquid-liquid phase separation process. In both asymmetric and composite membranes the hydrodynamic resistance is largely determined by the thin dense top layer. This top layer must avoid defects because a few defects can significantly reduce the selectivity without having much influence on the flux. It is very difficult to make an ultrathin and defect-free top layer from a glassy polymer by enhancing flux. However, two phase inversion methods such as the dual method [van't Hof et al., 1992] and the evaporation method [Pinnau and Koros, 1991] can be used to prepare a defect-free asymmetric membrane. These processes include dry phase separation, dry/wet phase separation, and wet/ wet phase separation. A dry/wet phase separation process can be used to form ultrathin and defect-free asymmetric membranes by using several glassy polymers [Pesek and Koros, 1993; van t Hof et al., 1992]. The evaporation process is dry step requiring loss of a volatile solvent from a casting solution containing a less volatile nonsolvent component. The selective loss of the volatile solvent makes unstable the top skin layer region of the nascent membrane. Interfacial dry phase separation can be observed by the almost instantaneous onset of turbidity in this top skin layer region. The nascent membrane is then immersed in a nonsolvent coagulant that is a wet-phase separation step. In this quench step, the bulk of the membrane structure is formed and the remaining solvents and nonsolvents are extracted. A wet/wet phase separation process is that membranes are formed by contacting polymer solution with two nonsolvent baths in series. The first bath is used to obtain a concentrated layer of polymer at the interface. This step makes ultrathin skin top layer similar to the evaporation step of dry/wet phase separation process. To whom correspondence should be addressed. wjlee@chonnam.chonnam.ac.kr On the other hand, the second bath is responsible for the actual coagulation to be precipitated. The choice of nonsolvents for the bath strongly depends upon the type of solvent to be dissolved in the polymer solution. At the first bath, a ultrathin interfacial layer according to a high polymer concentration is formed. This step is the delayed liquid-liquid phase separation process to make a dense skin top layer. Although this results in a densified top layer, it usually contains defects and has no gas separation properties. Therefore this step requires a precise technique to make an ultrathin and defect-free skin top layer. The polymer concentration in the sublayer as supporter has hardly changed when the phase separation process starts, and a relatively open substructure like sponge-type structure is formed. At the second bath, the substructure is made as a precipitation process. This step is an instantaneous phase separation process. It is very hard to make asymmetric membrane having defectfree top layer by a wet/wet method to use a glassy polymer such as polysulfone. Also, because of difficult preparation of asymmetric membrane, the composite membrane coating on the membrane surface is mainly used. It is not easy find articles of asymmetric polysulfone membrane prepared by a wet/wet method. The objective of this study is to prepare asymmetric polysulfone membrane with defect-free top layer by a wet/wet membrane. The gas separation properties of asymmetric polysulfone membranes were made by wet/wet phase inversion processes. It is demonstrated that optimized wet/wet phase inversion processes generate ultrathin and defect-free skinned asymmetric membranes. The structure of asymmetric polysulfone membranes made by dual bath method has been illustrated from scanning electron microscopy (SEM). The effect of gas permeability and selectivity with immersion time of IPA first bath, temperature and pressure was examined. EXPERIMENTAL 1. Materials Polysulfone (Udel P-3500) polymer was kindly supplied by 143
2 144 W.-J. Lee et al. Fig. 2. Experimental apparatus for permeability measurement. 1. Gas bombe 10. Flower meter 2. On-off valve 11. Three-way valve 3. Filter 12. Tee 4. MFC 13. Vacuum pump 5. Readout 14. Gas chromatography 6. Check valve 15. Recorder 7. Pressure gauge 16. Flask 8. Cell 17. Digital balance 9. Temperature reader Fig. 1. Preparation of an asymmetric polysulfone membrane. Sunkyung Co. Ltd. in Korea. Polysulfone is an amorphous hydrophobic polymer which has a glass transition temperature of 185 o C. N,N-dimethylacetamide (DMAc), purchased from Aldrich Co., was used as the casting solvent and its boiling temperature is o C. Also, iso-propanol and deionized water were used as nonsolvents. 2. Preparation of Membranes Fig. 1 shows the procedure of the preparation of asymmetric membrane. Membranes were made from 26 wt% polysulfone and 74 wt% DMAc. The polysulfone which is the polymer was dried in an oven (150 o C) for more than 3 days to remove absorbed water vapor before use. The polymer was then completely dissolved in DMAc at 80 o C for 12 hrs under nitrogen. It was degassed from casting solution with ultra cleaner. The membranes were cast on glass plates to a thickness of 250 µm at the conditions of room temperature and relative humidity 50%. Immediately after casting, the membranes were immersed in the first coagulation bath of iso-propanol in the range of 10 to 90 seconds, and then the membranes were placed into a second bath of deionized water for more than 1 day for the solvent to come out. After being dried for 12 hrs the membranes were dried for 3 days in vacuum oven of 100 o C. 3. Gas Permeability Measurements The material of the permeability cell is stainless steel and the effective membrane area is cm 2. The schematic diagram of the apparatus of gas permeability measurement is given in Fig. 2. Pure gas such as oxygen and nitrogen to be permeated was fed into upstream side, while downstream side was filled with the same gas at atmosphere. Permeability measurements for oxygen and nitrogen were made by the variable volume method. The experiment March, 2000 was carried out with the upstream pressure in the range of 2-10 bar. The gas flux, J, can be expressed as P/l, and P/l is determined by the following equation: P 1 --= l ( qt P) A P= D S θ= l 2 ( 6D) (3) where P/l represents the gas flow to be permeated, q/t is the volumetric flow rate of gas permeation, l is skin layer thickness of membrane, P is the pressure difference on the high and low pressure sides of the membrane and A is the effective membrane area. The gas permeation fluxes are reported in gas permeation units. P, D and S are permeability, diffusivity and solubility, respectively. Also, θ represents time lag. RESULTS AND DISCUSSION 1. Morphology of Asymmetric Polysulfone Membranes The membrane prepared by the dual bath system depends on the two types of phase separation processes in polymer-solventnonsolvent system. Membranes are formed by contacting a polymer solution with two successive nonsolvent baths. One is the delayed phase separation process, the other is instantaneous phase separation process. In this process, because the diffusional process starts at the interface between cast film and first bath, the change in composition of polymer occurs in the outermost region, upper part, of the film. Hence, the first IPA bath is used to obtained a concentrated layer of polymer at the interface. In case of immersing to a second water bath, the polymer concentration in the sublayer as supporter cannot be significantly changed. Therefore, this step gives an open and porous structure like sponge-type. Finally, the polymer is actually precipitated. Table 1 shows the nd selectivity for asymmetric polysulfone membrane with immersion (1) (2)
3 Gas Separation Properties of Polysulfone Membranes by a Dual Bath Method 145 Table 1. Permeance and selectivity of asymmetric polysulfone membranes Immersion time P (bar) T ( o C) 10s s s s s s a 10 6 cm 3 (STP)/cmHg sec cm ( /N 2 ) ( /N 2 ) ( /N 2 ) ( /N 2 ) ( /N 2 ) time, temperature and pressure. Fig. 3(a) represents an SEM photograph for the structure of the membrane immersed only in a water bath for 24 hrs without immersion into an IPA bath. In this case, the membrane has no distinctive skin layer, the oxygen permeance of the membrane was cm 3 (STP)/cmHg-sec-cm 2 at 4.0 bar and the selectivity for /N 2 was 0.95, indicating that gas transport was predominantly determined by pore flow. This result demonstrates that the skin layers of the membranes made by wet phase inversion were highly defective. The reason is that the liquid-liquid phase separation is instantaneous since solvent DMAc present in polymer solution rapidly contacts with water which is very polar. As shown in Fig. 3(a) the cross-section of a membrane consists of a sponge-like substructure containing the macrovoids. Fig. 3(b) to (g) represent SEM photographs of the cross-sectional view of the membranes immersed in the second water bath for 24 hrs after being immersed in the first IPA bath up to 10, 30, 50, 60, 70 and 80 sec. These results are the structures of membranes with increasing immersion time. The skin layer thickness increased up to about 2, 5, 7, 11, 12 and 13 µm according as immersion times increased up to 10, 30, 50, 60, 70 and 80 sec. Hence, the skin layer thickness is governed by changing the immersion time of the first IPA bath. Fig. 3(b) to (d) represent the cross-section of a membrane after 10, 30 and 50 sec diffusional processes at the film/bath interface. It is interesting to note that the sublayer (bottom layer) obtained by instantaneous phase separation shows the structure such as the type of sponge containing microvoids. However, the top region represents the ultrathin skin layer supported by highly porous substructure. This result indicates that the structure of the skin layer is a microphase separation process, which is a delayed phase separation process. The oxygen permeabilities of the membranes were , and cm 3 (STP)/cmHg-sec-cm 2 at 4.0 bar and 25 o C, and the selectivities for /N 2 were, 1.55 and 1.78, respectively. The fact that the oxygen selectivities were nearly 1.0 suggested the small microvoids will exist to the skin Korean J. Chem. Eng.(Vol. 17, No. 2)
4 146 W.-J. Lee et al. Fig. 4. Effect of immersion time in IPA on the gas separation characteristics of polysulfone at 25 o C, 4 bar (Note: permeability of oxygen and nitrogen for dense polysulfone membrane is and cm 3 (STP)cm/cmHg-seccm 2 ; selectivity is 6.2). Fig. 3. SEM photographs of the cross sectional views: (a) immersed only in a water bath, (b)-(g) immersed in second water bath for 24 hrs after immersed into first IPA bath. layer. These phenomena might be due to the fact that the particles of the nodules can not develop in the skin layer. This might be caused by variations in the residence time in the first bath. Membranes immersed to the first IPA bath during 60, 70 and 80 sec as shown in Fig. 3(e) to (g) showed a substantial increase in oxygen selectivities. In particular, a selectivity of oxygen of a membrane immersed to the first IPA bath during 80 sec is up to 6.0. But the oxygen permeability of a membrane is decreased up to 10 6 cm 3 (STP)/cmHg-sec-cm 2 at 4.0 bar. In this study, in case of immersing to first IPA bath during 90 sec or more, the oxygen permeability showed very low number, that is, cm 3 (STP)/cmHg-sec-cm 2, and the selectivity is 6.2 [Pinnau and Koros, 1991]. It might be due to the fact that the skin layer of a membrane is very thick and dense. As shown in Fig. 3 membranes with higher oxygen selectivities can be prepared by the dual bath method, and we can confirm that the gas flux strongly depends upon the combination of nonsolvents. By combining the IPA of March, 2000 the first bath and water of the second bath, the highest flux can be obtained. The high flux is due to the fact that residual IPA is rapidly removed from the film surface. The reason is the low density of IPA and the poor miscibility of IPA and water. The relatively short contact time with the first nonsolvent results in an ultrathin top layer and a good flux. But it must preceded the dual bath procedure to be avoided defects in membranes. 2. Effect of Gas Permeability and with Immersion Time Fig. 4(a) and (b) show the effects of oxygen and nitrogen permeabilities and selectivity with immersion times of 0, 10, 30, 50, 60, 70 and 80 sec in IPA bath at 25 o C, 4 bar. The oxygen permeability decreases and oxygen selectivity increases with increasing immersion time. The reason is that the resistance of gas permeance increases with an increase of the thickness of skin layer. Particularly, when the immersion time was over 60 sec, the selectivity was more than It is assumed that it is the very definite pore structure which excludes the larger nitrogen molecule to a greater extent than the smaller oxygen molecule. Hence, separation is determined by the selective diffusion of oxygen to nitrogen rather than specific interaction. 3. Effect of Gas Permeability and with Temperature Fig. 5(a) and (b) indicate the effect of oxygen permeability and
5 Gas Separation Properties of Polysulfone Membranes by a Dual Bath Method 147 Fig. 5. Effect of temperature on the gas separation characteristics of polysulfone after immersed to IPA during (b) 10 sec, (c) 30 sec, (d) 50 sec, (e) 60 sec, (f) 70 sec and (g) 80 sec. selectivity with increasing temperature in immersion time. With increasing temperature in the range of o C, considered here, the oxygen permeability of the membrane increases gradually, while the selectivity of the membrane decreases slightly. The increase of oxygen permeability might be due to the increase of diffusion by movement of gas molecules with increasing temperature. Particularly, the large increase of oxygen permeability in the range of 10, 30 and 50 sec of immersion time seems that micropores or defects within the skin layer of the polymer are formed. In the case that the immersion time is 60, 70, 80 sec of immersion time, the segment movement of polymer is slow with increasing the skin layer. These results indicate that free volume within the skin layer is decreased with increasing immersion time. The decrease of free volume means an increase of intermolecular and intramolecular polymer chain packing density. 4. Effect of Oxygen Permeability and with Pressure Fig. 6(a) and (b) represent the effect of oxygen permeability and selectivity with increasing pressure (2-10 bar) of upstream Fig. 6. Effect of pressure on the gas separation characteristics of polysulfone after being immersed to IPA during (b) 10 sec, (c) 30 sec, (d) 50 sec, (e) 60 sec, (f) 70 sec and (g) 80 sec. side in immersion time. The oxygen permeability with increased pressure is nearly constant. As shown by this result, this mechanism is governed by diffusion process. The reason is that the oxygen permeability only depends on the change of temperature, but not the change of pressure. 5. Effect of Flux with Permeating Time Table 2 shows gas separation properties of polysulfone membranes with immersion time (60, 70 and 80 sec). The gas separation properties of Table 2 were calculated from Eqs. (1)-(3). The skin layer thickness, l, is approximately measured from SEM photographs. Also, Fig. 7 indicates the cumulative gas flux to be permeated with immersion time. As mentioned above, oxygen permeability increased with increasing temperature, and was nearly constant with increasing pressure. Hence, we can suggest following Henry's law that permeability (P) is equal to diffusivity (D) times solubility (S). By extrapolating from flux vs. permeating time, we can find out time lag (θ). The time lags were approximately 0, 0, 0, 180, 300 and 420 sec with immersion time (10, 30, 50, 60, 70 and 80 sec). The time lag increased as immersion Table 2. Coefficients of diffusivity and solubility of asymmetric polysulfone membranes Time (sec) l (µm) θ (min) q/t (cm 3 /min) P (bar) P [cm 3 (STP)/cm 2 cmhg sec] D (cm 2 /sec) S (cm 3 /cm 3 cmhg) Korean J. Chem. Eng.(Vol. 17, No. 2)
6 148 W.-J. Lee et al. REFERENCES Fig. 7. Effect of flux with permeating time at 25 o C, 4 bar. time increased. This means that the gas flux is inversely proportional to the skin layer thickness. After steady state, the relationship between gas flux and permeating time shows to be linear. We suggest that the growth rate of the skin layer is to be determined by a diffusion process. CONCLUSIONS A wet/wet phase separation process in combination with two series nonsolvent bath was used to prepare ultrathin and defectfree asymmetric polysulfone membranes for gas separations. The skin layer thickness was determined by changing the immersion time of the first IPA bath. The skin layer thickness increased from about 2 µm to about 13 µm as immersion times increased from 10 sec to 80 sec. The time lag increased as immersion time increased. Hence, the growth rate of the skin layer is to be governed by a diffusional process. The oxygen permeability decreased as the skin layer increased. A selectivity of oxygen of a membrane immersed to first IPA bath during 80 sec obtained up to about 6.0. Krantz, W. B., Ray R. J., Sani, R. L. and Gleason, K. J., Theoretical Study of the Transport Processes Occurring During the Evaporation Step in Asymmetric Membrane Casting, J. Membrane Sci., 29, 11 (1986). Lai, J. Y., Chen, S. H., Lee, M. H. and Shyu, S. S., Preparation of Polycarbonate/Metal Salt Gas Separation Membrane, J. Applied Polymer Sci., 47, 1513 (1993). Pesek, S. C. and Koros, W. J., Aqueous Quenched Asymmetric Polysulfone Membranes Prepared by Dry/Wet Phase Separation, J. Membrane Sci., 81, 71 (1993). Pinnau, I. and Koros, W. J., Structures and Gas Separation Properties of Asymmetric Polysulfone Membranes Made by Dry, Wet, and Dry/Wet Phase Inversion, J. Applied Polymer Sci., 43, 1491 (1991). Sada, E., Kumazawa, H., Xu, P. and Inoue, H., Transport of a Gas through Asymmetric Polysulfone Membranes with Deposited Plasma-polymerizes Thin Layer, J. Applied Polymer Sci., 41, 2427 (1990). Schell, W. J., Commercial Applications for Gas Permeation Membrane Systems, J. Membrane Sci., 22, 217 (1985). Suzuki, H., Tanaka, K., Kita, H. and Okamoto, K., Preparation of Composite Hollow Fiber Membranes of Poly(ethylene oxide)-containing Polymide and their C /N 2 Separation Properties, J. Membrane Sci., 146, 31 (1997). van't Hof, J. A., Reuvers, A. J., Boom, R. M., Rolevink, H. H. M. and Smolders, C. A., Preparation of Asymmetric Gas Separation Membranes with High by a Dual-bath Coagulation Method, J. Membrane Sci., 70, 17 (1992). Yamasaki, A., Tyagi, R. K., Fouda, A. E., Matsuura, T. and Jonasson, K., Effect of Gelation Conditions on Gas Separation Performance for Asymmetric Polysulfone Membranes, J. Membrane Sci., 123, 89 (1997). March, 2000
Separation of N 2 /SF 6 binary mixtures using polyethersulfone (PESf) hollow fiber membrane
 Korean J. Chem. Eng., 9(8), 1081-1085 (01) DOI: 10.1007/s11814-011-094-z INVITED REVIEW PAPER Separation of binary mixtures using polyethersulfone (PESf) hollow fiber membrane Dae-Hoon Kim*, Yong-Hae Ko**,
Korean J. Chem. Eng., 9(8), 1081-1085 (01) DOI: 10.1007/s11814-011-094-z INVITED REVIEW PAPER Separation of binary mixtures using polyethersulfone (PESf) hollow fiber membrane Dae-Hoon Kim*, Yong-Hae Ko**,
how to meet today s dissolved oxygen specifications with degasification membranes
 Water Technologies & Solutions technical paper how to meet today s dissolved oxygen specifications with degasification membranes Author: Fred Wiesler, Membrana Charlotte, Ionics Note: SUEZ purchased Ionics
Water Technologies & Solutions technical paper how to meet today s dissolved oxygen specifications with degasification membranes Author: Fred Wiesler, Membrana Charlotte, Ionics Note: SUEZ purchased Ionics
A07 Surfactant Induced Solubilization and Transfer Resistance in Gas-Water and Gas-Oil Systems
 A07 Surfactant Induced Solubilization and Transfer Resistance in Gas-Water and Gas-Oil Systems R Farajzadeh* (TU Delft), A. Banaei (TU Delft), J. Kinkela (TU Delft), T. deloos (TU Delft), S. Rudolph (TU
A07 Surfactant Induced Solubilization and Transfer Resistance in Gas-Water and Gas-Oil Systems R Farajzadeh* (TU Delft), A. Banaei (TU Delft), J. Kinkela (TU Delft), T. deloos (TU Delft), S. Rudolph (TU
Air entrainment in Dip coating under vacuum
 Air entrainment in Dip coating under vacuum M.I. Khan, R. Patel, H. Benkreira, IRC, School of Engineering, Design and Technology, University of Bradford, BD7 1DP, Abstract Air entrainment studies in dip
Air entrainment in Dip coating under vacuum M.I. Khan, R. Patel, H. Benkreira, IRC, School of Engineering, Design and Technology, University of Bradford, BD7 1DP, Abstract Air entrainment studies in dip
Dissolved Oxygen Guide
 Educat i onser i es Di ssol vedoxygengui de Dissolved Oxygen Guide Introduction Dissolved oxygen probes provide a convenient approach to essentially direct measurement of molecular oxygen. The membrane
Educat i onser i es Di ssol vedoxygengui de Dissolved Oxygen Guide Introduction Dissolved oxygen probes provide a convenient approach to essentially direct measurement of molecular oxygen. The membrane
Flow behavior of wakes in a three-phase slurry bubble column with viscous liquid medium
 Korean J. Chem. Eng., 28(3), 974-979 (2011) DOI: 10.1007/s11814-010-0403-4 INVITED REVIEW PAPER Flow behavior of wakes in a three-phase slurry bubble column with viscous liquid medium Dae Ho Lim*, Ji Hwa
Korean J. Chem. Eng., 28(3), 974-979 (2011) DOI: 10.1007/s11814-010-0403-4 INVITED REVIEW PAPER Flow behavior of wakes in a three-phase slurry bubble column with viscous liquid medium Dae Ho Lim*, Ji Hwa
A variable-pressure constant-volume method was employed to determine the gas permeation
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 S.1. Gas permeation experiments A variable-pressure constant-volume method was employed to
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 S.1. Gas permeation experiments A variable-pressure constant-volume method was employed to
Generating Calibration Gas Standards
 Technical Note 1001 Metronics Inc. Generating Calibration Gas Standards with Dynacal Permeation Devices Permeation devices provide an excellent method of producing known gas concentrations in the PPM and
Technical Note 1001 Metronics Inc. Generating Calibration Gas Standards with Dynacal Permeation Devices Permeation devices provide an excellent method of producing known gas concentrations in the PPM and
Comparative Study of Oxygen Permeation Through Polymers and Gas Barrier Films
 Comparative Study of Oxygen Permeation Through Polymers and Gas Barrier Films H. Nörenberg and C. Deng, University of Oxford, Department of Materials, United Kindgom; H.-J. Kosmella, GKSS Geesthacht/Teltow,
Comparative Study of Oxygen Permeation Through Polymers and Gas Barrier Films H. Nörenberg and C. Deng, University of Oxford, Department of Materials, United Kindgom; H.-J. Kosmella, GKSS Geesthacht/Teltow,
Automatic Permeability Testing: The Challenges and Solutions Author: Alyce Hartvigsen, PBI-Dansensor A/S, Ringsted, Denmark
 Automatic Permeability Testing: The Challenges and Solutions Author: Alyce Hartvigsen, PBI-Dansensor A/S, Ringsted, Denmark In recent years, numerous product developments and major changes in the distribution
Automatic Permeability Testing: The Challenges and Solutions Author: Alyce Hartvigsen, PBI-Dansensor A/S, Ringsted, Denmark In recent years, numerous product developments and major changes in the distribution
Plasma Sources and Feedback Control in Pretreatment Web Coating Applications
 Plasma Sources and Feedback Control in Pretreatment Web Coating Applications Joseph Brindley, Benoit Daniel, Victor Bellido-Gonzalez, Dermot Monaghan Gencoa Ltd., Physics Rd, L24 9HP Liverpool, UK (+44)
Plasma Sources and Feedback Control in Pretreatment Web Coating Applications Joseph Brindley, Benoit Daniel, Victor Bellido-Gonzalez, Dermot Monaghan Gencoa Ltd., Physics Rd, L24 9HP Liverpool, UK (+44)
CTB3365x Introduction to Water Treatment
 CTB3365x Introduction to Water Treatment D4b Aeration Doris van Halem Did you know that there are not just gasses in your glass of sparkling coke, but also in the tap water you drink? Welcome to the water
CTB3365x Introduction to Water Treatment D4b Aeration Doris van Halem Did you know that there are not just gasses in your glass of sparkling coke, but also in the tap water you drink? Welcome to the water
Multiple effects of operating variables on the bubble properties in three-phase slurry bubble columns
 Korean J. Chem. Eng., 26(2), 587-591 (2009) SHORT COMMUNICATION Multiple effects of operating variables on the bubble properties in three-phase slurry bubble columns Ik Sang Shin*, Sung Mo Son*, Uk Yeong
Korean J. Chem. Eng., 26(2), 587-591 (2009) SHORT COMMUNICATION Multiple effects of operating variables on the bubble properties in three-phase slurry bubble columns Ik Sang Shin*, Sung Mo Son*, Uk Yeong
Introduction of Vacuum Science & Technology. Diffusion pumps used on the Calutron mass spectrometers during the Manhattan Project.
 Introduction of Vacuum Science & Technology Diffusion pumps used on the Calutron mass spectrometers during the Manhattan Project. 1 What is a vacuum? 760 mm Hg Vacuum ATM A vacuum is defined as less than
Introduction of Vacuum Science & Technology Diffusion pumps used on the Calutron mass spectrometers during the Manhattan Project. 1 What is a vacuum? 760 mm Hg Vacuum ATM A vacuum is defined as less than
Measurements of k L a. Steady-State Mass Balance Method:
 Measurements of k a Steady-State Mass Balance Method: In theory, the K a in an apparatus that is operating continuously under steady-state conditions could be evaluated from the flow rates and the concentrations
Measurements of k a Steady-State Mass Balance Method: In theory, the K a in an apparatus that is operating continuously under steady-state conditions could be evaluated from the flow rates and the concentrations
Development of High-speed Gas Dissolution Device
 Development of High-speed Gas Dissolution Device Yoichi Nakano*, Atsushi Suehiro**, Tetsuhiko Fujisato***, Jun Ma**** Kesayoshi Hadano****, Masayuki Fukagawa***** *Ube National College of Technology, Tokiwadai
Development of High-speed Gas Dissolution Device Yoichi Nakano*, Atsushi Suehiro**, Tetsuhiko Fujisato***, Jun Ma**** Kesayoshi Hadano****, Masayuki Fukagawa***** *Ube National College of Technology, Tokiwadai
Ch. 11 Mass transfer principles
 Transport of chemical species in solid, liquid, or gas mixture Transport driven by composition gradient, similar to temperature gradients driving heat transport We will look at two mass transport mechanisms,
Transport of chemical species in solid, liquid, or gas mixture Transport driven by composition gradient, similar to temperature gradients driving heat transport We will look at two mass transport mechanisms,
IASbaba ILP: Value Add Material - Matter MATTER. Iasbaba.com Page 1
 MATTER Iasbaba.com Page 1 LIQUID A. VAPOUR PRESSURE: Pressure of vapour in a closed container Changes majorly with temperature Has an exponential relationship with temperature Increases with increase in
MATTER Iasbaba.com Page 1 LIQUID A. VAPOUR PRESSURE: Pressure of vapour in a closed container Changes majorly with temperature Has an exponential relationship with temperature Increases with increase in
Experimental Research on Oxygen-enriched Air Supply System for Gasoline Engine
 Sensors & Transducers 013 by IFSA http://www.sensorsportal.com Experimental Research on Oxygen-enriched Air Supply System for Gasoline Engine 1, Yahong Li, 3 Lixi Zhang, 1 Zelu Sun 1 School of Chemistry
Sensors & Transducers 013 by IFSA http://www.sensorsportal.com Experimental Research on Oxygen-enriched Air Supply System for Gasoline Engine 1, Yahong Li, 3 Lixi Zhang, 1 Zelu Sun 1 School of Chemistry
Concentration and diafiltration of viral antigens with SmartFlow TFF
 The SmartFlow filter Concentration and diafiltration of viral antigens WORKS from NCSRT is intended for concentrating viral antigens from both clarified and non-clarified sources. The subsequent diafiltration
The SmartFlow filter Concentration and diafiltration of viral antigens WORKS from NCSRT is intended for concentrating viral antigens from both clarified and non-clarified sources. The subsequent diafiltration
Two interconnected rubber balloons as a demonstration showing the effect of surface tension
 Two interconnected rubber balloons as a demonstration showing the effect of surface tension Abstract Science One 28-9 CHEN, Chieh-Shan The two interconnected rubber balloons system is a demonstration widely
Two interconnected rubber balloons as a demonstration showing the effect of surface tension Abstract Science One 28-9 CHEN, Chieh-Shan The two interconnected rubber balloons system is a demonstration widely
DEVICES FOR FIELD DETERMINATION OF WATER VAPOR IN NATURAL GAS Betsy Murphy MNM Enterprises 801 N. Riverside Drive Fort Worth, Texas 76111
 INTRODUCTION Water vapor in natural gas has more than a substantial effect on the quality of the gas stream. Without quality measurement of water vapor the gas is basically not saleable. Contracts are
INTRODUCTION Water vapor in natural gas has more than a substantial effect on the quality of the gas stream. Without quality measurement of water vapor the gas is basically not saleable. Contracts are
PHASE EQUILIBRIA: HOW TO CALCULATE OXYGEN SOLUBILITY IN CELL CULTURE MEDIUM
 BE.360J/10.449J SUPPLEMENTARY HANDOUT FALL 2002 PHASE EQUILIBRIA: HOW TO CALCULATE OXYGEN SOLUBILITY IN CELL CULTURE MEDIUM 1. Phase Equilibria Introduction A collection of molecules is in equilibrium
BE.360J/10.449J SUPPLEMENTARY HANDOUT FALL 2002 PHASE EQUILIBRIA: HOW TO CALCULATE OXYGEN SOLUBILITY IN CELL CULTURE MEDIUM 1. Phase Equilibria Introduction A collection of molecules is in equilibrium
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/12
 LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
CHAPTER 3 RESULTS AND DISCUSSION
 CHAPTER 3 RESULTS AND DISCUSSION The research included the development and verification of the earlier apparatus, and the collection and validation of moisture diffusion data under both isothermal and
CHAPTER 3 RESULTS AND DISCUSSION The research included the development and verification of the earlier apparatus, and the collection and validation of moisture diffusion data under both isothermal and
CTB3365x Introduction to Water Treatment
 CTB3365x Introduction to Water Treatment W4e Aeration Merle de Kreuk There is one last thing of the biological part that needs some design. As you know, nitrifyers need oxygen, and also the heterotrophs,
CTB3365x Introduction to Water Treatment W4e Aeration Merle de Kreuk There is one last thing of the biological part that needs some design. As you know, nitrifyers need oxygen, and also the heterotrophs,
CRYSTALLIZATION FOULING IN PACKED COLUMNS
 CRYSTALLIZATION FOULING IN PACKED COLUMNS D. Großerichter and J. Stichlmair Lehrstuhl für Fluidverfahrenstechnik, Technische Universität München, Munich, Germany ABSTRACT Fouling due to crystallization
CRYSTALLIZATION FOULING IN PACKED COLUMNS D. Großerichter and J. Stichlmair Lehrstuhl für Fluidverfahrenstechnik, Technische Universität München, Munich, Germany ABSTRACT Fouling due to crystallization
Plasma Cleaner. Yamato Scientific America. Contents. Innovating Science for Over 125 Years. Gas Plasma Dry Cleaner PDC200/210/510 PDC610G.
 Yamato Scientific America Innovating Science for Over 125 Years Plasma Cleaner Contents Gas Plasma Dry Cleaner PDC200/210/510 PDC610G Gas Plasma Reactor 145 146 147 149 144 Gas Plasma Dry Cleaner Plasma
Yamato Scientific America Innovating Science for Over 125 Years Plasma Cleaner Contents Gas Plasma Dry Cleaner PDC200/210/510 PDC610G Gas Plasma Reactor 145 146 147 149 144 Gas Plasma Dry Cleaner Plasma
Flow in a shock tube
 Flow in a shock tube April 30, 05 Summary In the lab the shock Mach number as well as the Mach number downstream the moving shock are determined for different pressure ratios between the high and low pressure
Flow in a shock tube April 30, 05 Summary In the lab the shock Mach number as well as the Mach number downstream the moving shock are determined for different pressure ratios between the high and low pressure
Recovery of helium from rejected gas streams in Natural gas processing [1, 2, 3].
![Recovery of helium from rejected gas streams in Natural gas processing [1, 2, 3]. Recovery of helium from rejected gas streams in Natural gas processing [1, 2, 3].](/thumbs/89/97531732.jpg) Gas Separation by Membranes Membrane technology in gas separation and purification has grown exponentially since they were first introduced about 30 years ago. Compared to other conventional gas separation
Gas Separation by Membranes Membrane technology in gas separation and purification has grown exponentially since they were first introduced about 30 years ago. Compared to other conventional gas separation
Thin Film Slot Coating in a Rarefied Low Viscosity Gas
 www.bradford.ac.uk Thin Film Slot Coating in a Rarefied Low Viscosity Gas H. Benkreira & J.B. Ikin* School of Engineering, Design & Technology University of Bradford-UK *Multicoat Ltd., 21 Turnfield Road,
www.bradford.ac.uk Thin Film Slot Coating in a Rarefied Low Viscosity Gas H. Benkreira & J.B. Ikin* School of Engineering, Design & Technology University of Bradford-UK *Multicoat Ltd., 21 Turnfield Road,
The SPI Sputter Coater Handbook
 The SPI Sputter Coater Handbook Coating of Specimens SPI-Module Sputter Coater with Etch Mode 1. Mount the specimens onto the SEM stub. Keep in mind that many adhesives have high vapor pressure solvents
The SPI Sputter Coater Handbook Coating of Specimens SPI-Module Sputter Coater with Etch Mode 1. Mount the specimens onto the SEM stub. Keep in mind that many adhesives have high vapor pressure solvents
Reactivity Improvement of Ca (OH) 2 Sorbent Using Diatomaceous Earth (DE) From Aceh Province
 Research Inventy: International Journal of Engineering And Science Vol.8, Issue 2 (May 2018), PP -05-10 Issn (e): 2278-4721, Issn (p):2319-6483, www.researchinventy.com Reactivity Improvement of Ca (OH)
Research Inventy: International Journal of Engineering And Science Vol.8, Issue 2 (May 2018), PP -05-10 Issn (e): 2278-4721, Issn (p):2319-6483, www.researchinventy.com Reactivity Improvement of Ca (OH)
Winery Trials on Gas Control Using 3M Liqui-Cel. 3M Separation & Purification Sciences Erwin Ona, Senior Applications Engr
 Winery Trials on Gas Control Using 3M Liqui-Cel 3M Separation & Purification Sciences Erwin Ona, Senior Applications Engr 3M Liqui-Cel hollow fiber technology 200-220 µm 300 µm X40, X50 30 nanometer Effective
Winery Trials on Gas Control Using 3M Liqui-Cel 3M Separation & Purification Sciences Erwin Ona, Senior Applications Engr 3M Liqui-Cel hollow fiber technology 200-220 µm 300 µm X40, X50 30 nanometer Effective
Meteorology. Circle the letter that corresponds to the correct answer
 Chapter 4 Worksheet 3 Meteorology Name: Circle the letter that corresponds to the correct answer 1) Natural convection and turbulence are most likely to occur when: a) temperature decreases rapidly with
Chapter 4 Worksheet 3 Meteorology Name: Circle the letter that corresponds to the correct answer 1) Natural convection and turbulence are most likely to occur when: a) temperature decreases rapidly with
Membrane modules for nitrogen and oxygen generator systems. Technology Overview ENGINEERING YOUR SUCCESS.
 Membrane modules for nitrogen and oxygen generator systems Technology Overview ENGINEERING YOUR SUCCESS. Parker modules the heart of OEM tailor-made nitrogen generators OEM (Original Equipment Manufacturer)
Membrane modules for nitrogen and oxygen generator systems Technology Overview ENGINEERING YOUR SUCCESS. Parker modules the heart of OEM tailor-made nitrogen generators OEM (Original Equipment Manufacturer)
How To Use Getters and Getter Pumps
 A Journal of Practical and Useful Vacuum Technology From By Phil Danielson How To Use Getters and Getter Pumps Gettering is a vacuum pumping technology that has been with us, in many forms, for almost
A Journal of Practical and Useful Vacuum Technology From By Phil Danielson How To Use Getters and Getter Pumps Gettering is a vacuum pumping technology that has been with us, in many forms, for almost
Carbon dioxide absorption in a membrane contactor
 FellesLab Aug-Nov 2018 p. 1 of 5 Carbon dioide absorption in a membrane contactor Mahdi Ahmadi Department of Chemical Engineering, NTNU 13. August 2018 Abstract The objective of this laboratory eperiment
FellesLab Aug-Nov 2018 p. 1 of 5 Carbon dioide absorption in a membrane contactor Mahdi Ahmadi Department of Chemical Engineering, NTNU 13. August 2018 Abstract The objective of this laboratory eperiment
Fast method for Ginseng Analyses using Agilent Poroshell 120 Columns Scaled from a Traditional Method
 Fast method for Ginseng Analyses using Agilent Poroshell Columns Scaled from a Traditional Method Application Note Pharmaceutical Author Rongjie Fu Agilent Technologies (Shanghai) Co. Ltd. 4 Ying Lun Road
Fast method for Ginseng Analyses using Agilent Poroshell Columns Scaled from a Traditional Method Application Note Pharmaceutical Author Rongjie Fu Agilent Technologies (Shanghai) Co. Ltd. 4 Ying Lun Road
Supplementary Information:
 Supplementary Information: UV-responsive nano-sponge for oil absorption and desorption Do Hyun Kim 1,2, Min Chan Jung 3, So-Hye Cho 2, Sang Hoon Kim 2, Ho-Young Kim 3, Heon Ju Lee 2, Kyu Hwan Oh 1, and
Supplementary Information: UV-responsive nano-sponge for oil absorption and desorption Do Hyun Kim 1,2, Min Chan Jung 3, So-Hye Cho 2, Sang Hoon Kim 2, Ho-Young Kim 3, Heon Ju Lee 2, Kyu Hwan Oh 1, and
Visual Observation of Nucleate Boiling and Sliding Phenomena of Boiling Bubbles on a Horizontal Tube Heater
 Proceedings of the 2 nd World Congress on Mechanical, Chemical, and Material Engineering (MCM'16) Budapest, Hungary August 22 23, 216 Paper No. HTFF 146 DOI:.11159/htff16.146 Visual Observation of Nucleate
Proceedings of the 2 nd World Congress on Mechanical, Chemical, and Material Engineering (MCM'16) Budapest, Hungary August 22 23, 216 Paper No. HTFF 146 DOI:.11159/htff16.146 Visual Observation of Nucleate
Accurate Measurement of Steam Flow Properties
 Accurate Measurement of Steam Flow Properties Kewen Li and Roland N. Horne Stanford Geothermal Program, Stanford University (Proceedings of 1999 GRC Annual Meeting on October 17-20, Reno, California, USA)
Accurate Measurement of Steam Flow Properties Kewen Li and Roland N. Horne Stanford Geothermal Program, Stanford University (Proceedings of 1999 GRC Annual Meeting on October 17-20, Reno, California, USA)
The Polyethylene Casing as Diffusion Barrier for Polyurethane Insulated District Heating Pipes
 Polyethylene Casing as Diffusion Barrier for Polyurethane Insulated District Heating Pipes The Polyethylene Casing as Diffusion Barrier for Polyurethane Insulated District Heating Pipes Maria Olsson and
Polyethylene Casing as Diffusion Barrier for Polyurethane Insulated District Heating Pipes The Polyethylene Casing as Diffusion Barrier for Polyurethane Insulated District Heating Pipes Maria Olsson and
H. Yasuda* ant William Stone, Jr. Polymer Division, Eye Research Cedars-Sinai Medical Center Los Angeles, California
 CLEARING HO US E SFOR FEDERAL SCIENTIPIC AND TECHNICAL INFORMATION Hta r d c o ;, xv'i f ci e - _ PERMEABILITY OF POLYMER MMBRANES TO DISSOLVED OXYGEN CA-_, H. Yasuda* ant William Stone, Jr. Polymer Division,
CLEARING HO US E SFOR FEDERAL SCIENTIPIC AND TECHNICAL INFORMATION Hta r d c o ;, xv'i f ci e - _ PERMEABILITY OF POLYMER MMBRANES TO DISSOLVED OXYGEN CA-_, H. Yasuda* ant William Stone, Jr. Polymer Division,
Experimental Studies on the Instabilities of Viscous Fingering in a Hele-Shaw Cell
 Korean J. Chem. Eng., 17(2), 169-173 (2000) Experimental Studies on the Instabilities of Viscous Fingering in a Hele-Shaw Cell Chung Gi Baig, Young Ho Chun*, Eun Su Cho* and Chang Kyun Choi School of Chemical
Korean J. Chem. Eng., 17(2), 169-173 (2000) Experimental Studies on the Instabilities of Viscous Fingering in a Hele-Shaw Cell Chung Gi Baig, Young Ho Chun*, Eun Su Cho* and Chang Kyun Choi School of Chemical
CHAPTER 5: VACUUM TEST WITH VERTICAL DRAINS
 CHAPTER 5: VACUUM TEST WITH VERTICAL DRAINS 5.1 Introduction Using surcharging as the sole soil consolidation mean can take a long time to reach the desired soil settlement. Soil consolidation using prefabricated
CHAPTER 5: VACUUM TEST WITH VERTICAL DRAINS 5.1 Introduction Using surcharging as the sole soil consolidation mean can take a long time to reach the desired soil settlement. Soil consolidation using prefabricated
LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary
 ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
DRINKING WATER - LAB EXPERIMENTS LAB EXPERIMENTS. Nanofiltration
 DRINKING WATER - LAB EXPERIMENTS LAB EXPERIMENTS Nanofiltration nanofiltration lab experiments Framework This module explains the lab experiment on nanofiltration. Contents This module has the following
DRINKING WATER - LAB EXPERIMENTS LAB EXPERIMENTS Nanofiltration nanofiltration lab experiments Framework This module explains the lab experiment on nanofiltration. Contents This module has the following
IN-PLACE DENSITY WAQTC FOP AASHTO T 209 (16)
 THEORETICAL MAXIMUM SPECIFIC GRAVITY (Gmm) AND DENSITY OF HOT MIX ASPHALT (HMA) PAVING MIXTURES FOP FOR AASHTO T 209 Scope This procedure covers the determination of the maximum specific gravity (Gmm)
THEORETICAL MAXIMUM SPECIFIC GRAVITY (Gmm) AND DENSITY OF HOT MIX ASPHALT (HMA) PAVING MIXTURES FOP FOR AASHTO T 209 Scope This procedure covers the determination of the maximum specific gravity (Gmm)
Lab 3 Introduction to Quantitative Analysis: Pumps and Measurements of Flow
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641, Spring 2008 Lab 3 Introduction to Quantitative Analysis: Pumps and Measurements of Flow Purpose of Lab 3: 1) To gain a
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641, Spring 2008 Lab 3 Introduction to Quantitative Analysis: Pumps and Measurements of Flow Purpose of Lab 3: 1) To gain a
REPRODUCIBILITY IN OPTICAL THIN FILM PROCESSING PART 1, THE VACUUM AND PUMPING
 ABSTRACT REPRODUCIBILITY IN OPTICAL THIN FILM PROCESSING PART 1, THE VACUUM AND PUMPING Ronald R. Willey Willey Optical, Consultants, 13039 Cedar Street, Charlevoix, MI 49720, USA Ph 231-237-9392, ron@willeyoptical.com
ABSTRACT REPRODUCIBILITY IN OPTICAL THIN FILM PROCESSING PART 1, THE VACUUM AND PUMPING Ronald R. Willey Willey Optical, Consultants, 13039 Cedar Street, Charlevoix, MI 49720, USA Ph 231-237-9392, ron@willeyoptical.com
To compare one gas to another, it is convenient to define a set of conditions: Standard Temperature and Pressure
 Standard Molar Volume To compare one gas to another, it is convenient to define a set of conditions: Standard Temperature and Pressure At STP, one mole of any gas has a volume of: 22.4 L = (This is a cube
Standard Molar Volume To compare one gas to another, it is convenient to define a set of conditions: Standard Temperature and Pressure At STP, one mole of any gas has a volume of: 22.4 L = (This is a cube
Improvements in the Reliability, Costs and Processing of WLP/RDL Circuits
 Improvements in the Reliability, Costs and Processing of WLP/RDL Circuits Bill Moffat, Chief Executive Officer John Das, Ph.D., Process Engineer Wesley Lau, Senior Sales Engineer Kenneth Sautter, Senior
Improvements in the Reliability, Costs and Processing of WLP/RDL Circuits Bill Moffat, Chief Executive Officer John Das, Ph.D., Process Engineer Wesley Lau, Senior Sales Engineer Kenneth Sautter, Senior
VISUAL PHYSICS School of Physics University of Sydney Australia. Why can a steel needle float but a larger piece of steel sink?
 VISUAL PYSIS School of Physics University of Sydney Australia SURFAE TENSION Why can a steel needle float but a larger piece of steel sink?? Ducks have drowned in farmyard ponds into which washing water
VISUAL PYSIS School of Physics University of Sydney Australia SURFAE TENSION Why can a steel needle float but a larger piece of steel sink?? Ducks have drowned in farmyard ponds into which washing water
FOUP material influence on HF contamination during queue-time
 Solid State Phenomena Online: 2014-09-26 ISSN: 1662-9779, Vol. 219, pp 251-255 doi:10.4028/www.scientific.net/ssp.219.251 2015 Trans Tech Publications, Switzerland FOUP material influence on HF contamination
Solid State Phenomena Online: 2014-09-26 ISSN: 1662-9779, Vol. 219, pp 251-255 doi:10.4028/www.scientific.net/ssp.219.251 2015 Trans Tech Publications, Switzerland FOUP material influence on HF contamination
Characterizing a Cu/Mn Alloy for Extracting Oxygen from Inert Gas. Streams
 Characterizing a Cu/Mn Alloy for Extracting Oxygen from Inert Gas Streams Grace Lenhard Prattsburgh Central School LLE advisors: Walter Shmayda and Matthew Sharpe Laboratory for Laser Energetics University
Characterizing a Cu/Mn Alloy for Extracting Oxygen from Inert Gas Streams Grace Lenhard Prattsburgh Central School LLE advisors: Walter Shmayda and Matthew Sharpe Laboratory for Laser Energetics University
A Journal of Practical and Useful Vacuum Technology. By Phil Danielson
 A Journal of Practical and Useful Vacuum Technology From By Phil Danielson Thermal Conductivity Gauges Thermal conductivity pressure gauges are extremely common in vacuum technology, but an understanding
A Journal of Practical and Useful Vacuum Technology From By Phil Danielson Thermal Conductivity Gauges Thermal conductivity pressure gauges are extremely common in vacuum technology, but an understanding
Figure Vapor-liquid equilibrium for a binary mixture. The dashed lines show the equilibrium compositions.
 Another way to view this problem is to say that the final volume contains V m 3 of alcohol at 5.93 kpa and 20 C V m 3 of air at 94.07 kpa and 20 C V m 3 of air plus alcohol at 100 kpa and 20 C Thus, the
Another way to view this problem is to say that the final volume contains V m 3 of alcohol at 5.93 kpa and 20 C V m 3 of air at 94.07 kpa and 20 C V m 3 of air plus alcohol at 100 kpa and 20 C Thus, the
Introduction IP codes are used in electrical product catalogs, etc., to indicate waterproofness and so on.
 Introduction IP codes are used in electrical product catalogs, etc., to indicate waterproofness and so on. For the purposes of this document, IP code refers to the degree of protection (IP code) provided
Introduction IP codes are used in electrical product catalogs, etc., to indicate waterproofness and so on. For the purposes of this document, IP code refers to the degree of protection (IP code) provided
Both physical and chemical effects come into play either separately or in combination.
 A Journal of Practical and Useful Vacuum Technology By Phil Danielson WHY CREATE A VACUUM? From Creating a vacuum has become a fairly common and often routine undertaking, but the commonness of the activity
A Journal of Practical and Useful Vacuum Technology By Phil Danielson WHY CREATE A VACUUM? From Creating a vacuum has become a fairly common and often routine undertaking, but the commonness of the activity
Bioreactors Prof G. K. Suraishkumar Department of Biotechnology Indian Institute of Technology, Madras. Lecture - 15 Bioreactor env.par.
 Bioreactors Prof G. K. Suraishkumar Department of Biotechnology Indian Institute of Technology, Madras Lecture - 15 Bioreactor env.par. (DO) Welcome to lecture 15 NPTEL online certification course on Bioreactors.
Bioreactors Prof G. K. Suraishkumar Department of Biotechnology Indian Institute of Technology, Madras Lecture - 15 Bioreactor env.par. (DO) Welcome to lecture 15 NPTEL online certification course on Bioreactors.
CHE 4115 Chemical Processes Laboratory 2 Experiment 1. Batch Distillation
 CHE 4115 Chemical Processes Laboratory 2 Experiment 1 Batch Distillation BACKGROUND Distillation is one of the most commonly used unit operations in chemical engineering. In general, a distillation operation
CHE 4115 Chemical Processes Laboratory 2 Experiment 1 Batch Distillation BACKGROUND Distillation is one of the most commonly used unit operations in chemical engineering. In general, a distillation operation
9/28/2017 OBJ: SWBAT determine the solubility of 10 compounds. 1. What makes Magic Sand hydrophobic?
 9/28/2017 OBJ: SWBAT determine the solubility of 10 compounds 1. What makes Magic Sand hydrophobic? Solutions Pure Substances & Mixtures o What's the matter? o Pure Substances have a definite set of physical
9/28/2017 OBJ: SWBAT determine the solubility of 10 compounds 1. What makes Magic Sand hydrophobic? Solutions Pure Substances & Mixtures o What's the matter? o Pure Substances have a definite set of physical
KATEDRA MATERIÁLOVÉHO INŽENÝRSTVÍ A CHEMIE. 123MEAN water vapor transport
 KATEDRA MATERIÁLOVÉHO INŽENÝRSTVÍ A CHEMIE 123MEAN water vapor transport Water vapor transport : Thermal insulation properties of porous building materials dramatically decrease because of water vapor
KATEDRA MATERIÁLOVÉHO INŽENÝRSTVÍ A CHEMIE 123MEAN water vapor transport Water vapor transport : Thermal insulation properties of porous building materials dramatically decrease because of water vapor
Chapter 13 Temperature, Kinetic Theory, and the Gas Laws 497
 Chapter 13 Temperature, Kinetic Theory, and the Gas Laws 497 Figure 13.25 This photograph of Apollo 17 Commander Eugene Cernan driving the lunar rover on the Moon in 1972 looks as though it was taken at
Chapter 13 Temperature, Kinetic Theory, and the Gas Laws 497 Figure 13.25 This photograph of Apollo 17 Commander Eugene Cernan driving the lunar rover on the Moon in 1972 looks as though it was taken at
Abrasive wear of UHMWPE yarns against ceramic pins
 Abrasive wear of UHMWPE yarns against ceramic pins Juan Pu Ph.D student Mechanical Engineering, UC Berkeley Outline 1. Introduction 2. Experimental setup 3. Surface characterization of UHMWPE fibers and
Abrasive wear of UHMWPE yarns against ceramic pins Juan Pu Ph.D student Mechanical Engineering, UC Berkeley Outline 1. Introduction 2. Experimental setup 3. Surface characterization of UHMWPE fibers and
Quantification of Contamination Using Quartz Crystal Thickness Monitors
 Quantification of Contamination Using Quartz Crystal Thickness Monitors Christopher G. Morgan, Mark M. Gleason and Ronald Vane XEI Scientific, Inc. 1755 E. Bayshore Rd., Suite 17 Redwood City, CA 9463
Quantification of Contamination Using Quartz Crystal Thickness Monitors Christopher G. Morgan, Mark M. Gleason and Ronald Vane XEI Scientific, Inc. 1755 E. Bayshore Rd., Suite 17 Redwood City, CA 9463
Fall 2004 Homework Problem Set 9 Due Wednesday, November 24, at start of class
 0.30 Fall 004 Homework Problem Set 9 Due Wednesday, November 4, at start of class Part A. Consider an iron surface which serves as a catalyst for the production of ammonia from nitrogen and hydrogen. The
0.30 Fall 004 Homework Problem Set 9 Due Wednesday, November 4, at start of class Part A. Consider an iron surface which serves as a catalyst for the production of ammonia from nitrogen and hydrogen. The
Isotopes and Gases. Isotopes as constraints to air sea gas exchange, gas exchange time scales, the various time scales of carbon (isotope) exchange
 Isotopes and Gases Isotopes as constraints to air sea gas exchange, gas exchange time scales, the various time scales of carbon (isotope) exchange Isotope effects in gas solution and molecular diffusion
Isotopes and Gases Isotopes as constraints to air sea gas exchange, gas exchange time scales, the various time scales of carbon (isotope) exchange Isotope effects in gas solution and molecular diffusion
Outcomes: Operationally define vapour pressure
 Vapour Pressure Outcomes: Operationally define vapour pressure Vapour Pressure: Recall that in order for a liquid to VAPORIZE, it must overcome the FORCE of the ATMOSPHERIC PRESSURE. Vapour Pressure (P
Vapour Pressure Outcomes: Operationally define vapour pressure Vapour Pressure: Recall that in order for a liquid to VAPORIZE, it must overcome the FORCE of the ATMOSPHERIC PRESSURE. Vapour Pressure (P
Noble-Gas -Infused Neoprene Closed-Cell Foams Achieving Ultra-Low Thermal Conductivity Fabrics
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2018 Supplemental Information: Noble-Gas -Infused Neoprene Closed-Cell Foams Achieving Ultra-Low
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2018 Supplemental Information: Noble-Gas -Infused Neoprene Closed-Cell Foams Achieving Ultra-Low
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT.
 Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1
 AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
A. What are the three states of matter chemists work with?
 Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
FRANZ CELL TEST OF NANO GLUTATHIONE WITH HPLC ANALYSIS
 FRANZ CELL TEST OF NANO GLUTATHIONE WITH HPLC ANALYSIS Analysis Conducted BY CC RESEARCH (P) Ltd., For Nanoceutical Solutions Inc. USA 2950 Thousand Oaks drive, Suite 3, San Antonio, Texas EXECUTIVE SUMMARY
FRANZ CELL TEST OF NANO GLUTATHIONE WITH HPLC ANALYSIS Analysis Conducted BY CC RESEARCH (P) Ltd., For Nanoceutical Solutions Inc. USA 2950 Thousand Oaks drive, Suite 3, San Antonio, Texas EXECUTIVE SUMMARY
Technical Service Bulletin July 2014 TSB
 Technical Service Bulletin July 2014 TSB 105.11 REVERSE OSMOSIS AND NANOFILTRATION MEMBRANE ELEMENT PRECAUTIONS PERMEATE VALVE OPERATION The membrane element shall not, at any time, be exposed to permeate
Technical Service Bulletin July 2014 TSB 105.11 REVERSE OSMOSIS AND NANOFILTRATION MEMBRANE ELEMENT PRECAUTIONS PERMEATE VALVE OPERATION The membrane element shall not, at any time, be exposed to permeate
MEDICAL GASES Manufacture, Storage, Transport & Delivery B.KIRUTHIGA LECTURER DEPT OF PHARMACEUTICAL CHEMISTRY
 MEDICAL GASES Manufacture, Storage, Transport & Delivery B.KIRUTHIGA LECTURER DEPT OF PHARMACEUTICAL CHEMISTRY Composition of the Air We breathe earth s atmosphere composed of: Nitrogen (78%) Oxygen (21%)
MEDICAL GASES Manufacture, Storage, Transport & Delivery B.KIRUTHIGA LECTURER DEPT OF PHARMACEUTICAL CHEMISTRY Composition of the Air We breathe earth s atmosphere composed of: Nitrogen (78%) Oxygen (21%)
CO2 absorption at elevated pressures using a hollow fiber membrane contactor Dindore, V.Y.; Brilman, D.W.F.; Feron, P.H.M.
 University of Groningen CO2 absorption at elevated pressures using a hollow fiber membrane contactor Dindore, V.Y.; Brilman, D.W.F.; Feron, P.H.M.; Versteeg, Geert Published in: Journal of Membrane Science
University of Groningen CO2 absorption at elevated pressures using a hollow fiber membrane contactor Dindore, V.Y.; Brilman, D.W.F.; Feron, P.H.M.; Versteeg, Geert Published in: Journal of Membrane Science
FAST EVAPORATION AT NORMAL PRESSURE
 FAST EVAPORATION AT NORMAL PRESSURE FAST EVAPORATION BY BLOWING-DOWN... The VAPORNADO Plus blow-down system is an innovative and efficient instrument for the evaporation of samples and fractions at normal
FAST EVAPORATION AT NORMAL PRESSURE FAST EVAPORATION BY BLOWING-DOWN... The VAPORNADO Plus blow-down system is an innovative and efficient instrument for the evaporation of samples and fractions at normal
Phase Changes * OpenStax
 OpenStax-CNX module: m42218 1 Phase Changes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract Interpret a phase diagram. State Dalton's
OpenStax-CNX module: m42218 1 Phase Changes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract Interpret a phase diagram. State Dalton's
Chemistry A Molecular Approach. Fourth Edition. Chapter 5. Gases. Copyright 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
 Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Passive Gas-Liquid Separation Using Hydrophobic Porous Polymer Membranes: A Study on the Effect of Operating Pressure on Membrane Area Requirement
 UNF Digital Commons UNF Theses and Dissertations Student Scholarship 2012 Passive Gas-Liquid Separation Using Hydrophobic Porous Polymer Membranes: A Study on the Effect of Operating Pressure on Membrane
UNF Digital Commons UNF Theses and Dissertations Student Scholarship 2012 Passive Gas-Liquid Separation Using Hydrophobic Porous Polymer Membranes: A Study on the Effect of Operating Pressure on Membrane
METHOD OF TEST FOR THEORETICAL MAXIMUM RELATIVE DENSITY OF BITUMINOUS PAVING MIXTURES LS-264 R27 ASTM D2041/D2041M
 3. APPARATUS 3.1 BEAKERS OR VESSELS: Stainless steel vessel, 4000 ml capacity, no spout... 3.2 BALANCE: 4000 g capacity with an accuracy of ± 0.1 g... 3.3 RIFFLE SPLITTER: For splitting hot mix samples.
3. APPARATUS 3.1 BEAKERS OR VESSELS: Stainless steel vessel, 4000 ml capacity, no spout... 3.2 BALANCE: 4000 g capacity with an accuracy of ± 0.1 g... 3.3 RIFFLE SPLITTER: For splitting hot mix samples.
Gas Pressure. Pressure is the force exerted per unit area by gas molecules as they strike the surfaces around them.
 Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Predicted Dispense Volume vs. Gravimetric Measurement for the MICROLAB 600. November 2010
 Predicted Dispense Volume vs. Gravimetric Measurement for the MICROLAB 600 November 2010 Table of Contents ``Abstract...3 ``Introduction...4 ``Methods & Results...6 ``Data Analysis...9 ``Conclusion...12
Predicted Dispense Volume vs. Gravimetric Measurement for the MICROLAB 600 November 2010 Table of Contents ``Abstract...3 ``Introduction...4 ``Methods & Results...6 ``Data Analysis...9 ``Conclusion...12
Experimental and modelling study of the solubility of CO 2 in various CaCl 2 solutions at different temperatures and pressures
 Pet.Sci.(014)11:569-577 DOI 10.1007/s118-014-0373-1 569 and modelling study of the solubility of in various CaCl s at different temperatures and pressures Alireza Bastami 1, Mohammad Allahgholi 1 and Peyman
Pet.Sci.(014)11:569-577 DOI 10.1007/s118-014-0373-1 569 and modelling study of the solubility of in various CaCl s at different temperatures and pressures Alireza Bastami 1, Mohammad Allahgholi 1 and Peyman
ASPHALT WAQTC FOP AASHTO T 209 (12)
 THEORETICL MXIMUM SPECIFIC GRVITY (G mm ) ND DENSITY OF HOT MIX SPHLT (HM) PVING MIXTURES FOP FOR SHTO T 209 Scope This procedure covers the determination of the maximum specific gravity (G mm ) of uncompacted
THEORETICL MXIMUM SPECIFIC GRVITY (G mm ) ND DENSITY OF HOT MIX SPHLT (HM) PVING MIXTURES FOP FOR SHTO T 209 Scope This procedure covers the determination of the maximum specific gravity (G mm ) of uncompacted
GRADE 6: Materials 1. UNIT 6M.1 7 hours. Solubility. Resources. About this unit. Previous learning. Expectations. Key vocabulary and technical terms
 GRADE 6: Materials 1 Solubility UNIT 6M.1 7 hours About this unit This is the first of four units on materials in Grade 6. This unit builds on the study of the properties of water in Unit 5M.1. Unit 7M.1
GRADE 6: Materials 1 Solubility UNIT 6M.1 7 hours About this unit This is the first of four units on materials in Grade 6. This unit builds on the study of the properties of water in Unit 5M.1. Unit 7M.1
The temperature gradient plate consists of a high precision chromium measuring plate, with equispaced temperature sensors beneath the surface.
 Temperature Gradient Plate MFFT 10 / MFFT 20 ISO 2115 ASTM D 2354 The new designed temperature gradient plate for measuring the Minimum Film Forming Temperature, MFFT The minimum film forming temperature
Temperature Gradient Plate MFFT 10 / MFFT 20 ISO 2115 ASTM D 2354 The new designed temperature gradient plate for measuring the Minimum Film Forming Temperature, MFFT The minimum film forming temperature
Dissolved Oxygen Meter. ino2. Instruction Manual. Serial No..
 Innovative Instruments, Inc. http://www.2in.com Dissolved Oxygen Meter ino2 Instruction Manual Serial No.. Innovative Instruments, Inc. 8533 Queen Brooks Ct. Tampa, FL 33637. USA Phone: 813-727-0676. Fax:
Innovative Instruments, Inc. http://www.2in.com Dissolved Oxygen Meter ino2 Instruction Manual Serial No.. Innovative Instruments, Inc. 8533 Queen Brooks Ct. Tampa, FL 33637. USA Phone: 813-727-0676. Fax:
The Origin and Time Dependence of the Amount and Composition of Non- Constituent Gases Present in Crystal Growth Systems
 The Origin and Time Dependence of the Amount and Composition of Non- Constituent Gases Present in Crystal Growth Systems Witold Palosz A Desorption of inert gases from, and their diffusion through the
The Origin and Time Dependence of the Amount and Composition of Non- Constituent Gases Present in Crystal Growth Systems Witold Palosz A Desorption of inert gases from, and their diffusion through the
H o w t o U s e a R e g u l a t o r t o R e d u c e T i m e D e l a y i n a n A n a l y t i c a l S y s t e m
 H o w t o U s e a R e g u l a t o r t o R e d u c e T i m e D e l a y i n a n A n a l y t i c a l S y s t e m By Doug Nordstrom and Mike Adkins Swagelok Company Process measurements are instantaneous but
H o w t o U s e a R e g u l a t o r t o R e d u c e T i m e D e l a y i n a n A n a l y t i c a l S y s t e m By Doug Nordstrom and Mike Adkins Swagelok Company Process measurements are instantaneous but
Chemistry 20 Unit 2 Gases FITB Notes. Topic A Characteristics of Gases
 Chemistry 20 Unit 2 Gases FITB Notes General Outcome: Topic A Characteristics of Gases We use technologies that were designed with the knowledge of the visible characteristics ( ) of gases ex. SCUBA equipment,
Chemistry 20 Unit 2 Gases FITB Notes General Outcome: Topic A Characteristics of Gases We use technologies that were designed with the knowledge of the visible characteristics ( ) of gases ex. SCUBA equipment,
Detecting Hydrogen in Helium Streams. Samuel Goodman. Pittsford Mendon High School. Rochester, New York. Advisor: Dr.
 1 Detecting Hydrogen in Helium Streams Samuel Goodman Pittsford Mendon High School Rochester, New York Advisor: Dr. Walter Shmayda Laboratory for Laser Energetics University of Rochester Rochester, New
1 Detecting Hydrogen in Helium Streams Samuel Goodman Pittsford Mendon High School Rochester, New York Advisor: Dr. Walter Shmayda Laboratory for Laser Energetics University of Rochester Rochester, New
arxiv: v1 [physics.ins-det] 16 Aug 2014
![arxiv: v1 [physics.ins-det] 16 Aug 2014 arxiv: v1 [physics.ins-det] 16 Aug 2014](/thumbs/90/101993511.jpg) Preprint typeset in JINST style - HYPER VERSION RPC Gap Production and Performance for CMS RE4 Upgrade arxiv:1408.3720v1 [physics.ins-det] 16 Aug 2014 Sung Keun Park, Min Ho Kang and Kyong Sei Lee Korea
Preprint typeset in JINST style - HYPER VERSION RPC Gap Production and Performance for CMS RE4 Upgrade arxiv:1408.3720v1 [physics.ins-det] 16 Aug 2014 Sung Keun Park, Min Ho Kang and Kyong Sei Lee Korea
Prediction of the long-term insulating capacity of cyclopentane-blown polyurethane foam
 Prediction of the long-term insulating capacity of cyclopentane-blown polyurethane foam Camilla Persson, Licentiate of Engineering Johan Claesson, Professor camilla.persson@chalmers.se johan.claesson@chalmers.se
Prediction of the long-term insulating capacity of cyclopentane-blown polyurethane foam Camilla Persson, Licentiate of Engineering Johan Claesson, Professor camilla.persson@chalmers.se johan.claesson@chalmers.se
THERMODYNAMICS OF A GAS PHASE REACTION: DISSOCIATION OF N 2 O 4
 THERMODYNAMICS OF A GAS PHASE REACTION: DISSOCIATION OF N 2 O 4 OBJECTIVES 1. To measure the equilibrium constant, enthalpy, entropy, and Gibbs free energy change of the reaction N2O4(g) = 2 NO2(g). 2.
THERMODYNAMICS OF A GAS PHASE REACTION: DISSOCIATION OF N 2 O 4 OBJECTIVES 1. To measure the equilibrium constant, enthalpy, entropy, and Gibbs free energy change of the reaction N2O4(g) = 2 NO2(g). 2.
LEAP CO 2 Laboratory CO 2 mixtures test facility
 LEAP CO 2 Laboratory CO 2 mixtures test facility THE PROJECT AIM CO 2 flows made available by several capture techniques are contaminated with impurities and this affects the design and operations of the
LEAP CO 2 Laboratory CO 2 mixtures test facility THE PROJECT AIM CO 2 flows made available by several capture techniques are contaminated with impurities and this affects the design and operations of the
Experiment 8: Minor Losses
 Experiment 8: Minor Losses Purpose: To determine the loss factors for flow through a range of pipe fittings including bends, a contraction, an enlargement and a gate-valve. Introduction: Energy losses
Experiment 8: Minor Losses Purpose: To determine the loss factors for flow through a range of pipe fittings including bends, a contraction, an enlargement and a gate-valve. Introduction: Energy losses
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Metal-organic framework-derived CoSe2/(NiCo)Se2
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Metal-organic framework-derived CoSe2/(NiCo)Se2
