Efficiency and Control of a Heat Integrated Distillation Column (HIDiC)
|
|
|
- Calvin George
- 5 years ago
- Views:
Transcription
1 DELFT UNIVERSITY OF TECHNOLOGY, DEPARTMENT OF PROCESS AND ENERGY Efficiency and Control of a Heat Integrated Distillation Column (HIDiC) MSc Thesis Anne Traa 7/25/2010 Graduation Committee: Prof. dr. ir. A.I. Stankiewicz Dr. Sc. Ž. Olujić Prof. ir. J. Grievink Ir. S.A. Tromp Report number: 2420
2
3 Preface During the last seven months I have been working on my final thesis for the master education of Chemical Engineering at the Delft University of Technology. This study is part of the HIDiC-II project at the Process & Energy department. I would like to thank Sander Tromp who as a daily supervisor supported me during this project. I also want to thank Žarko Olujić for his guidance and unlimited knowledge of distillation. Furthermore, I want to thank the people from the workshop who build the experimental setup and helped when there were problems during the experiments. Finally, I would like to thank Anna Sun who worked with me on the CT-HIDiC setup. Together we finalized the setup and performed the first experiments. Delft, 25 July 2010 Anne Traa i
4
5 Abstract Distillation is a very old separation method and one of the most energy consuming separation techniques; it accounts for approximately 40% of the energy consumption by the chemical industry in Western countries. To limit energy consumption, the Heat Integrated Distillation Column (HIDiC) was introduced. A HIDiC is a concept of a distillation column with improved thermodynamic efficiency. In the HIDiC, the rectifying section and stripping section are separated from each other and connected by a compressor. The compressor increases the pressure of the vapour from the top of the stripping section from where it is fed to the bottom of the rectifying section. The rectifying section is operated under higher pressure and temperature than the stripping section and, due to the difference in temperature, heat transfer between the columns is possible. The HIDiC is a very promising concept for reducing energy use in distillation processes, therefore, a setup of a Concentric Tray HIDiC has been built and first experiments are performed with a mixture of ethanol and water. The experimental set-up built at TU Delft Process and Energy department has two separate columns where the rectifying section is placed inside the stripping section. Between the two parts, no mass transfer can take place. In the experiments the column pressure of the rectifying section (inner column) is varied as well as the F-factor of both sections and the effects on the overall column efficiency for both sections is studied and compared with empirical models. For the inner column the efficiency compared well with the empirical model. The column pressure has no effect on the overall column efficiency at constant F-factor and heat transfer seems to also have no effect on the column efficiency. The outer column shows a larger deviation from the model for the overall column efficiency. The outer column is mostly operated in the weeping region and therefore the efficiency increases for increasing F-factor. The pressure drop for both columns is lower than predicted by the model for pressure drop on a sieve tray by Bennett et al; this is due to the low F-factor ranges in which the tests are performed. An increase in the heat received gives for both columns also in increase in the mean column F-factor but for constant F-factor range the efficiency shows no correlation with the amount of heat transferred between the two columns. Another objective for this study is to improve the operability of the experimental setup and one way to improve this is to optimize the controller settings by tuning. Step tests are performed in during operation of the column to determine the characteristics of the specific control loop. All the tuned control loops have first order plus dead time dynamics and SIMC tuning rules are used for determining the controller settings. Simulink is used to model the response of the controlled process and compare it with the uncontrolled process. The controlled process shows faster response to a setpoint change and often less overshoot. The settings for the controllers should be tested in the experimental setup. iii
6
7 Table of Contents Preface Abstract Table of Contents 1 Introduction HIDiC, State of the Art HIDiC-II project TU Delft Research objectives Experimental setup Component description Control structure Column operation Column efficiency Theory Experimental procedure Results and discussion Conclusions Control Theory Experimental procedure Results and discussion Conclusions Recommendations Column efficiency Control The experimental setup Heat panels 6-2 Bibliography Nomenclature Appendices i iii v vii ix xi v
8
9 1 Introduction Distillation is a very old separation method and one of the most energy consuming separation techniques. In distillation high temperature heat is used to heat the mixture in the reboiler and low temperature heat is withdrawn in the condenser at the top of the column. The energy content of the heat is lowered tremendously and therefore the thermodynamic efficiency is even below 10%. (1). Despite the low thermodynamic efficiency distillation is still the most used separation technique in the industry and accounts for approximately 40% of the energy consumption by the chemical industry in Western countries. (2) 1.1 HIDiC, State of the Art The HIDiC concept can best be explained by first discussing three other types of distillation column configurations; the conventional distillation column, the vapour recompression column and the diabatic distillation column. The conventional distillation column is shown in Figure 1.1. The feed is introduced somewhere in the middle of the column. The stage where the feed is entering is called the feed stage and this stage divides the column into two sections; the rectifying section at the top and the stripping section at the bottom. Products are collected at the top and bottom of the column. In the conventional distillation configuration high quality Figure 1.1: Schematic representation of a conventional distillation column. heat with high temperature is introduced in the reboiler where the bottom stream is heated to evaporate a fraction of the bottom liquid stream. In the condenser of the column low quality heat with low temperature is extracted and the condensed stream is the top product. Part of the condensed stream is fed back as a reflux stream to provide a liquid phase for the top stage. The vapour and liquid fractions are in contact with each other on each stage in the distillation column. The concentrations of the components will be in equilibrium with and due to changing composition over the length of the column a temperature profile exists; the top temperature is equal to the top product boiling point and the bottom temperature is equal to the bottom product boiling point. For close boiling mixtures the heat requirement can be very high because large reflux ratios are often needed to obtain certain purity of the products. Two concepts were designed which both can improve the thermodynamic efficiency when compared with the conventional distillation column; the vapour recompression column and the diabatic distillation column shown in Figure 1.2 and Figure 1.3 respectively. Figure 1.2: Vapour Recompression Column Figure 1.3: Diabatic Distillation Column 1-1
10 Introduction The vapour recompression column (VRC), shown in Figure 1.2, has almost the same configuration as the conventional column accept that the condenser at the top of the column is omitted and a compressor is installed instead. The compressor upgrades the temperature and pressure of the top product to such a level that it can be used as a heat source of for the reboiler. (1) The major disadvantage of this type of distillation column is the additional capital investment for the compressor. Also the heat pump efficiency is the highest at lower temperature difference. Therefore this concept is only beneficial for close boiling mixtures where the temperature between top and bottom is not very large and just a small compressor is needed for upgrading the top product temperature and pressure. Another concept for saving energy in distillation columns is the diabatic column shown in Figure 1.3. As is shown in this figure the reboiler and condenser of the conventional distillation column are removed and replaced by a reboiler and condenser which are integrated in the distillation column. By doing this, heat is gradually added in the stripping section and gradually removed in the rectifying section. Therefore the temperature differences for heat transfer are smaller and lower energy losses are the result. This concept is promising although implementation is difficult due to large capital costs and complex tray design. Combining the concepts of vapour recompression and diabatic distillation resulted in another concept for a distillation column with improved thermodynamic efficiency; this is the so-called Heat Integrated Distillation Column (HIDiC). In the HIDiC, the rectifying section and stripping section are separated from each other and connected by a compressor as is shown in Figure 1.4. The compressor increases the pressure of the vapour from the top of the stripping section from where it will be fed to the bottom of the rectifying section. The liquid leaving the bottom of the rectifying section is fed back to the stripping section after the pressure is reduced by, for example, a flash valve. The bottom product is leaving from the bottom of the stripping section and the top product is leaving from the top of the rectifying section. The rectifying section is operated under higher pressure and temperature than the stripping section and due to the heat difference between the two columns heat transfer between the columns is possible. Therefore the rectifying section acts as an internal reboiler for the stripping section and the other way around, the stripping section acts as an internal condenser for the rectifying section, like it was presented for the diabatic distillation column. Several configurations for the stripping and Figure 1.5: Possible configurations for the rectifying section and the stripping section (1). Figure 1.4 Configuration of the Heat Integrated Distillation Column (HIDiC) rectifying section are possible as shown in Figure
11 Introduction 1.2 HIDiC-II project TU Delft For the HIDiC-II project at TU Delft a 0.8 meter diameter HIDiC with concentric column configuration is used. In this case the rectifying section is placed inside the stripping section as is shown in Figure 1.6 and therefore the stripping section has an annular shape. This configuration has the main advantage that the heat from the rectifying section can only be transferred to the stripping section and not to the environment since there is no contact area between the inner column and the environment. The set-up placed at TU Delft, which is referred to as HIDiC, is simply two distillation columns with heat transfer between the columns. The setup has two condensers installed and two reboilers, one for each column. The columns are not connected with each other and no mass transfer takes place between the columns. The ideal HIDiC has no reboiler and also no condenser. A heat exchanger is used to keep the feed at the right conditions and a compressor is installed to connect the two columns. Figure 1.6: TU Delft Concentric Tray HIDiC Figure 1.7: Flow pattern with heat panels. Red = rectifying section, Blue = stripping section The heat transfer area of the inner column is small and therefore there is the possibility to install heat panels inside the setup. Figure 1.7 shows the layout of the installed heat panels. The principle of this design has been proven experimentally by de Rijke (1). When heat panels were installed heat transfer and mass transfer was increased when compared with an annular tray without heat panels. 1.3 Research objectives Previous research was done by de Rijke (1) to study the influence of heat panels on tray hydraulics and the separation efficiency. Furthermore, the effect of the annular tray configuration on efficiency and pressure drop was also studied. It was proven that the annular tray configuration had no effect on the tray efficiency when compared with the conventional cross flow sieve trays. More research was also done to determine the influence of process parameters, column configuration and the degree in which the stripping section and rectifying section are thermally integrated on the energy savings and economic feasibility of the HIDiC compared to conventional VRC. Van het Kaar (3) developed a dynamic model based on the experimental set-up as it is built at the Process and Energy department at TU Delft. In this research also the controllability of a fully functional HIDiC was discussed and optimized by the means of a dynamic model. Following research done by Voorend (4) compared several models for describing the tray efficiency and heat transfer. The models were compared with experimental data from the HIDiC-I project. 1-3
12 Introduction Heat panels were designed with sufficient heat transfer area to be able to demonstrate the operational feasibility of the concentric tray HIDiC. For safety reasons the experimental set-up should first be tested with a mixture of ethanol and water. The next step is to run tests with a mixture of cyclohexane and n-heptane to validate the dynamic models developed in earlier studies. The previous research leads to the following objectives for this report: Finalize the construction, start-up and commissioning of the CT-HIDiC with fully functioning inner and outer column using an ethanol/water system. Generate first experimental data this setup without installed heat panels. Determine the effects of heat exchange and alternative tray design on the tray efficiency, pressure drop and vapour load on the tray. Verify the experimental data with the models for tray efficiency and pressure drop. Optimize the operability of the experimental setup including the control structure. A possible follow-up for the HIDiC-II project is the HIDiC-III project, which should be the last step before implementation in industry. In the HIDiC-III project a compressor will be installed to create a complete HIDiC with the rectifying and stripping section connected with each other. 1-4
13 2 Experimental setup This chapter deals with the equipment and instrumentation setup of the CT- HIDiC at the Process and Energy department at Delft University of Technology. This setup is finished at the end of 2009 and has a height of 8 meters. The setup is designed for a mixture of cyclohexane and n-heptane but currently it is running with a binary mixture of water and ethanol. Figure 2.1 shows a picture of the CT- HIDiC setup at the Process and Energy facility. Following this small introduction the simplified Piping and Instrumentation Diagram is shown in Section 2.1 together with a description of the components in the set-up. The control structure is explained in Section 2.2. Figure 2.1: Left: complete picture of the entire experimental setup. Right: the distillation column on the first floor. 2.1 Component description A simplified Piping and Instrumentation Diagram including the control structure is shown in Figure 2.2. The complete P&ID is shown in Appendix A. In Figure 2.2, the cooling water network, the steam network and the vacuum and nitrogen network are left out. A detailed description of the main components in this P&ID is given below the figure. 2-1
14 Experimental setup Figure 2.2: Simplified process flow diagram for the concentric-tray HIDiC setup at TU Delft Process & Energy department. The setup can be divided into two parts, the inner column with a condenser, reboiler and buffer vessel (indicated in green) and the outer column with a condenser, reboiler and buffer vessel (indicated in blue). The two systems are detached to prevent any mass transfer taking place between the two sections. Heat transfer between the two sections can only take place between the inner and the outer column. The steam is indicated by the red colour and cooling water by the light blue colour The distillation column The inner column (C-200) simulates the rectifying section and is equipped with five sieve trays. The outer column (C-100), simulating the stripping section, has seven sieve trays with annular shape. The inner column is located inside the outer column as is shown in the P&ID in Figure 2.2. The operating pressure of the inner column is between 1 bara and 2.0 bara and the operating pressure of the outer column is between 1 bara and 1.2 bara. 2-2
15 Experimental setup (a) (b) Figure 2.3: Column internals. (a) Tray layout of the HIDiC, (b) Flow profile in a conventional distillation column Figure 2.3.a shows the layout of a sieve tray of the outer column. The downcomer going down to the tray below is shown at the right of the sieve tray and on the left where no sieve holes are placed is the area where the liquid from the downcomer above is falling on the tray. A general flow profile between two trays is shown in Figure 2.3.b. In this figure, the weir height is indicated and the downcomer area. The specifications of both columns are shown in Table 2.1. Table 2.1: Column specifications for the inner (C-200) and outer (C-100) column. Outer column Inner column Number of active trays 7 5 Tray spacing 0.50 m 0.50 m Inner diameter m m Outer diameter (=inner diam. + wall thickness) m m Tray area m m 2 Weir height 50 mm 50 mm Weir length 0.5 m 0.1 m Downcomer area m m 2 Active tray area m m 2 Number of holes Hole diameter m m Hole area m m Condensers Two condensers are installed in the set-up; one for the inner column (E-201) and one for the outer column (E-101). Both condensers are of the shell-and-tube type. The condenser of the inner column has an installed heat transfer area of 7.2 m 2 and the condenser of the outer column has an installed heat transfer area of 40 m 2. For both condensers, cooling water flows through the tubes and the process fluid condenses in the shell. 2-3
16 Experimental setup Reboilers Two reboilers are installed, E-102 for the outer column and E-202 for the inner column. The reboiler for the outer column is a falling film partial evaporator with a heat exchange area of 19.5 m 2. The reboiler of the inner column is a plate heat exchanger with a heat exchange area of 4 m 2. The medium used for heating is steam Buffer vessels The two buffer vessels (T-101 and T-201) are filled with a mixture of water and ethanol. From the buffer vessels the mixture is pumped to the reboiler where it is heated. The vapour and liquid will return to the buffer vessel from where the vapour will flow to the distillation column. The liquid returning from the bottom of the distillation column will flow back to the buffer vessel. T-101 has a volume of 1.06 m 3 and T-201 has a volume of 0.30 m Pumps In total, there are five pumps in the main process streams of the set-up. All pumps are centrifugal pumps and their specifications are shown in Table 2.2. Table 2.2: Pump descriptions and specifications Pump Column Description Maximum Vol. flowrate P-101 Outer Bottom product pump 10 m 3 /h P-102 Outer Boil-up pump 25 m 3 /h P-103 Outer Reflux pump 25 m 3 /h P-201 Inner Reflux pump 2 m 3 /h P-202 Inner Boil-up pump 4 m 3 /h Measurement devices The experimental set-up has many temperature indicators which are all of the Pt100 type. The minimum temperature is -50 C and the maximum temperature is 400 C. Pressure indicators are installed at the top and bottom of each column and in the steam pipelines. The range in which they can measure is shown in Table 2.3. All pressure indicators are electrical except for PI-202, PI-401, PI-402 and PI-501, which are mechanical. Table 2.3: Measuring range of the absolute pressure indicators Min. [bara] Max. [bara] PI PIC PIC PI PI PI PIC PIC PI Differential pressure indicators are installed over each tray in the outer column and the inner and outer column each have a differential pressure indicator installed between the top and the 2-4
17 Experimental setup bottom of the column. All these differential pressure indicators can measure a pressure difference up to 50 mbar. Three mass flow meters are installed in the set-up. One mass flow meter is installed in the reflux stream of the outer column, one in the reflux stream of the inner column and one in the bottom product stream of the inner column. The specifications of the mass flow meters are shown in Table 2.4. Table 2.4: Specifications of the mass flow meters. Min.[kg/h] Max. [kg/h] MFI MFI MFI In total six volumetric flowmeters are installed. They are placed in the incoming cooling water pipelines, the incoming steam pipelines and in the pipeline from the reboiler to the buffer vessel of the inner and outer column. The range in which they can measure is shown in Table 2.5. Table 2.5: Specifications of the volumetric flow meters Min. Max. FI m 3 /h FI l/h FI m 3 /h FI m 3 /h FI m 3 /h FI m 3 /h In the two buffer vessels, the two condensers and the bottom of the outer column level indicators are installed; this is for preventing the pumps to pump when the vessel is empty. 2.2 Control structure In the experimental setup there are 13 control loops which are shown in Table 2.6. The cooling water is used to control the pressure inside the column or the temperature of the reflux stream. During operation the column pressure was controlled instead of the reflux temperature. The steam pressure or the steam flow rate is controlled by the steam control valve and the levels inside the condenser, buffer vessels and the bottom holdup of the outer column are controlled by the pump flow rate. For all the control loops, the default settings for the Proportional action (P), Integral action (I) and Derivative action (D) were set to 10, 1 and 0 respectively but during the first experiments these values were adjusted for better operability. The computer in the control room is equipped with a customized Labview program, made by Carya Automatisering, with which the column can be controlled during operation. The data from the instruments and settings in the program is logged every second during column operation. 2-5
18 Experimental setup Table 2.6: List of control loops present in the experimental setup. Controlled variable Controller Description Manipulated variable Pressure PIC-109 Pressure, outer column CV-301, cooling water PIC-201 Pressure, inner column CV -310, cooling water PIC-403 Steam pressure in the CV -401, steam supply reboiler, outer column PIC-410 Steam pressure in the CV -410, steam supply reboiler, inner column Temperature TI-115 Reflux temperature, outer CV -301, cooling water column TI-202 Reflux temperature, inner CV -310, cooling water column Flow FI-401 Volumetric flowrate of steam CV -401, steam supply in the reboiler, outer column FI-410 Volumetric flowrate of steam CV -410, steam supply in the reboiler, inner column Level LIC-101 Level of bottom holdup, P-101, pump flowrate outer column LIC-102 Level of the buffer vessel, P-102, pump flowrate outer column LIC-103 Level of the condenser, outer P-103, pump flowrate column LIC-201 Level of the condenser, inner P-201, pump flowrate column LIC-202 Level of the buffer vessel, inner column P-202, pump flowrate 2.3 Column operation The inner column and outer column are both filled with a mixture of ethanol and water with an ethanol concentration of less than 20 mol%. When the setup is not running both columns are filled with nitrogen gas to prevent the column from going below atmospheric pressure and therefore prevent air from entering the column. When the column is started up and heated by steam, the nitrogen needs to be removed by opening the vacuum network pipelines at the top of the condensers. During operation the inner column is completely closed but the outer column is under constant nitrogen pressure to prevent a small purge of the process stream to enter the vacuum system which is slightly opened. The pressure in both columns is controlled by the cooling water flowrate. All pumps are controlled by the level indicators of the vessels from which they are pumping. When the level is below 10%, the pump will automatically stop pumping to prevent pump breakdown. The setpoints for the level indicators is set as is shown in Table 2.7. Table 2.7: Setpoints of the level controllers Controller Setpoint LIC % LIC % LIC % LIC % LIC % 2-6
19 Experimental setup The pressure in the steam furnace is 8.5 bara, which is reduced by a reducing valve to approximately 5.5 bara before it enters the setup. After the reducing valve, two control valves are installed which control the amount of steam going to the inner and the outer column. These control valves can be set to pressure control or volumetric flowrate control but for all experiments they were set to pressure control. During operation samples can be taken from the sample points at the reflux streams and bottom product streams of both columns. The sample points are set to purge during operation. 2-7
20
21 3 Column efficiency The overall column efficiency is an important value when one wants to know the performance of a distillation column. The theory about the column efficiency is described in Section 3.1, followed by the experimental procedure and the results obtained by the experiments done on the experimental setup which was described in Chapter Theory Tray efficiency is a measure for the non-ideality of the tray, i.e. higher efficiency means lower nonideality. The number of theoretical trays, N t, is equal to the number of actual trays, N a, multiplied by the overall column efficiency, E oc (Equation 3.1). The non-ideality may lower or enhance the separation; when it enhances the separation the tray efficiency can be more than 100 percent (5). Nt Na Eoc (3.1) The overall column efficiency gives an indication of the overall efficiency of the distillation column and therefore this efficiency is most used by design and operation engineers (6). For determining the overall column efficiency of a total reflux column, the number of theoretical trays can be calculated with the Fenske equation (Equation 3.2). The required top and bottom concentrations (x D and x B ) for component i and j and the relative volatility, α, are the inputs of this equation. N t xd / x B i ln xd / xb j (3.2) ln ij Due to varying relative volatility inside the column, the geometric average from top to bottom is often used: (3.3) avg D B The point efficiency, E og is the efficiency at a single point on the tray. For uniform concentrations on the tray the point efficiency equals the overall tray efficiency which is also known as the Murphree tray efficiency E mv. The average of the overall tray efficiencies of all trays in the column equals the overall column efficiency. Voorend (4) compared four models for the point efficiency, namely: 1. AIChE Bubble tray design manual 2. Chan & Fair 3. Bennett et al 4. Garcia & Fair According to this study the model of Bennett et al (7) gives best results when compared with the experimental data and the model is also favourable due to low complexity. The Bennett equation for the point efficiency on a tray is given by Equation
22 Column efficiency E OG Guhh Fe h L A h 1 exp MG DG (1 e ) G dh Aa 1 m ML DL ( Ah / Aa ) (3.4) The parameters in this equation are described in detail in Appendix B. In the experimental setup complete mixing is assumed due to the relatively small active area. This means that the tray efficiency, E mv, is equal to the point efficiency. The average of the tray efficiencies for each tray is equal to the column efficiency; therefore the average of top and bottom efficiency of the Bennett model is expected to be in agreement with the overall column efficiency of the experimental data. The F-factor is a measure for the gas load in the column and it is given by: FG ug (3.5) G where u G is the superficial gas velocity and ρ G is the gas density. The physical meaning of the F-factor is the dynamic pressure or kinetic energy of the gas flow. The F-factor is used to be able to compare different flow situations at different column pressures. The superficial gas velocity, and therefore the F-factor used in this report, is based on the active area of the tray. The active tray area is defined as: A A 2 A (3.6) a c d where A a is the active area, A c is the column area and A d is the downcomer area. The efficiency profile shows the efficiency of the column as a function of the F-factor as is shown in Figure 3.1. The efficiency profile can roughly be divided into three sections; the weeping region for low F-factors, the entrainment region for high F-factors and the region of stable operation in the middle. Since the efficiency is best controlled in the middle region this is the area where one wants to operate in. Figure 3.1: Schematic representation of an efficiency profile. 3-2
23 Column efficiency The minimum F-factor and the maximum F-factor for stable operation can be calculated. The maximum F-factor is a function of the relative free area, υ, the surface tension of the liquid, σ, and the densities of liquid and gas. The correlation to calculate the maximum F-factor is shown in Equation 3.7. (8) F 2 G,max 2.5 L G Values used for the parameters of this equation are explained in Appendix C. The minimum gas load is proportional to the square root of the hole diameter, d h, and it is also dependent on the relative free area and the densities of gas and liquid. The correlation for the minimum F-factor is shown in Equation 3.8 (8). g 1/4 (3.7) FG,min 0.37 dh g L G / G (3.8) The maximum and minimum F-factors for the TU Delft CT-HIDiC are shown in Table 3.1. Table 3.1: F-factor limits for the mean column F-factor of the inner and outer column. F-factor Outer Inner Minimum Maximum Table 3.1 shows that the inner column should be operated at higher F-factors than the outer column to be in the region of stable operation. Another important design parameter is the pressure drop per tray. The pressure drop per tray can be predicted by the Bennett model for pressure drop on a sieve tray (9). According to Bennett the liquid height on a tray h T is equal to: ht hl hd h (3.9) In this equation h L is the clear liquid height, h D is liquid height equivalent to the dry hole pressure drop and h σ is the liquid height due to surface tension. The parameters in this equation are described in more detail in Appendix B. Multiplying the liquid height on the tray by the density and the gravity constant gives the pressure drop over the tray: Ptray ht g (3.10) L 3.2 Experimental procedure This section explains the experimental procedure followed during the experiments on the setup described in Chapter 2. The data analysis of the data derived during the experiments is done according to the procedure described in Section Experiments The first set of experiments was done without heating the outer column. 3-3
24 Column efficiency The purpose of these experiments is to be able to tell the difference in heat transfer from the inner column to the outer column when the outer column is off and when the outer column is working. Another purpose of this set of experiments is to determine the upper and lower limits for the steam pressure for which the inner column can be operated under constant pressure without cooling water limitations. At a constant column pressure of the inner column tests were done with various steam pressures. The lower and upper limits for the steam pressures tested during these experiments are shown in Table 3.2. This limits the F-factor range which could be tested. Table 3.2: Steam pressure limits for experiments of the inner column without heating the outer column. Steam pressure Column pressure [bara] Lower limit [bara] Upper limit [bara] The second set of experiments was with heating the inner and outer column. The purpose of this set of experiments is to determine the effect of the amount of heat transfer between the inner and outer column on the column efficiency and pressure drop. The pressure of the outer column was set to 1.05 bara and the pressure of the inner column was varied between 1.05 bara and 1.70 bara. At fixed column pressures, the steam pressures of inner and outer column were varied, in order to attain results for a wide F-factor range and range of the amount of heat transferred between the two columns. To prevent any transient effects, the readings of the mass flow meters, the differential pressure indicators and the level indicators of the buffer vessels and condensers were visually inspected until the readings fluctuated along a constant mean; at this point the operation of the column was considered stationary. After at least one hour of stationary operation, samples were taken at the samples points in the setup. The samples were cooled in a water bath and the concentration of ethanol was measured on an Anton Paar DMA 5000 density meter which was calibrated for ethanol/water mixtures Data analysis The number of theoretical stages is calculated by the Fenske equation which is shown in Equation 3.2. This equation is valid for a constant relative volatility (α ij ) between top and bottom. Due to the non-ideal behaviour of water and ethanol the relative volatility is not constant but it is a function of the composition as is shown in Figure 3.2. The trend line in this figure is used in this report to calculate the relative volatility valid only for a certain composition of the ethanol/water mixture. Although the Fenske equation is not valid for the overall column due to the concentration dependency of the relative volatility, it is assumed to be valid across individual trays. It is assumed that the relative volatility changes negligibly from the bottom to the top of a stage and may therefore be considered constant. In Matlab a stage-by stage calculation is performed for the calculation of the number of stages in the column in which the relative volatility of the bottom concentration of the stage is used for the calculation of the top concentration. Appendix D shows the calculation scheme and explanation of the calculations, Appendix E shows the matlab script. 3-4
25 Column efficiency Figure 3.2: Exact values for the relative volatility of ethanol and water as a function of the composition and the 5 th order polynomial trend line with its formula. During operation the values for all measurement devices and control valves were logged. These logs were used for instance for determining the F-factor during one experiment. The F-factor is given by: FG ug (3.11) G The density in this equation is obtained by the ideal gas law: P Mw G RT (3.12) The pressure, P, and temperature, T, in this equation comes from measurements in the experimental setup. The molecular weight, M w, is determined from the samples taken at the top and bottom of the column. R is in this equation the gas constant. The superficial gas velocity in the column is calculated by: u G MFI A G a (3.13) The readings from the mass flow meter (MFI) from experiments are used together with the density of the gas as calculated in Equation 3.12 and the active area of the column from Equation 3.6. Since the column is a total reflux column, the mass flow meter in the reflux stream can be used to describe the mass flow meter of the gas leaving the column at the top. For the bottom of the inner column, the mass flow meter of the bottom is used. For the outer column, no mass flow meter is installed in the bottom product pipeline but instead the volumetric flow meter of the steam together with the steam temperatures, pressure and enthalpies are used to calculate the mass flow of the bottom stream. The pressure at the bottom of the inner column is calculated by adding the differential pressure from dpi-201 to the pressure measured at the top of the column. The mean column F-factor which is used in the results is the average the top and bottom F- factor. The instruments which are used for the calculations of the F-factor for the top and bottom are shown in Table
26 Column efficiency Table 3.3: Instruments used for F-factor calculation. Outer Inner Top Bottom Top Bottom Mass flow [kg/h] MFI-101 FI-401, PIC-403, TI-401, TI-402 MFI-201 MFI-202 Pressure PIC-109 PI-101 PIC-201 dpi-201, PIC-201 Temperature TI-108 TI-110 TI-201 TI-206 Molecular weight SP-101 SP-102 SP-201 SP-202 The values for the instruments are logged every second during operation and, therefore, an average value is used for each instrument for one experiment. As explained in Section 3.2.1, samples were taken after approximately one hour of steady state operation and the time at which the sample is taken is written down. The data of one day of experiments is loaded in Matlab and the average for the instrument readings for each steady state is calculated by taking the average of all values of that instrument for one hour before sampling until sampling time. A timeframe of one hour was taken since it takes about one hour to refresh the volume inside the buffer vessels, condenser and column. With the average values of the instrument readings for the bottom and top, the column averages are calculated and with these values the mean column F-factor is calculated as described in Equation 3.11, 3.12 and Results and discussion Experiments were performed as described in the previous section. This section shows the results for the column performance, the effect of the outer column on inner column efficiency and heat transfer, the effect of heat transfer on efficiency and pressure drop and the effect of pressure on column performance Column performance The performance of the inner and outer column during the experiments is shown in Figure 3.3 and Figure 3.4, respectively. Both figures show the overall column efficiency and the pressure drop per stage as a function of the mean column F-factor for three pressures of the inner column and atmospheric pressure of the outer column. For each experiment, the column pressures were fixed and the steam pressures of both columns were varied as described in Section 3.2. For the inner column, the experimental data compares well with the Bennett model for the efficiency; the experimentally determined efficiency is slightly higher. The model for the pressure drop predicts a higher pressure drop per stage when compared with the experimental data. For low values of the F-factor the model for the pressured drop and the experimental data show higher deviation than for high F-factors. This is due to the fact that the pressure drop model is only valid for high F-factors. The Bennett model for pressure drop assumes that the height of the froth exceeds the weir height, which is not the case at the excessive weeping conditions found at lower F-factors. Therefore, this relation can only be used when the froth height is higher than the weir height. For the outer column the same trend is observed for the pressure drop per stage. As is shown in Equation 3.8 and Table 3.1, the minimum F-factor for preventing operating in the excessive weeping region for the outer column is Therefore, the pressure drop for an F-factor below 0.63 is almost zero. It was observed during operation that, even for a pressure drop of 2 mbar per tray, corresponding to an F-factor of 0.8, weeping occurred. This means that the calculated value for the minimum F-factor is not in agreement with the visual observations but it is in fact higher. The 3-6
27 Column efficiency pressure drop per stage increases while the F-factor is increasing but it does not give the same value as the pressure drop predicted by the model of Bennett et al (9). Figure 3.3: Experimental data for the overall column efficiency and pressure drop per stage of the inner column as a function of the column F-factor for three column pressures. Model lines for the Bennett efficiency and pressure drop are determined for a pressure of 1.05 bar. Figure 3.4: Experimental data for the overall column efficiency and pressure drop per stage of the outer column as a function of the column F-factor for three column pressures. Model lines for the Bennett efficiency and pressure drop are determined for a pressure of 1.05 bar. The efficiency for the outer column also is much lower than predicted by the model of Bennett et al (7). The total column efficiency is the number of theoretical trays calculated by the Fenske equation divided by the number of actual trays. In the outer column there are seven trays installed but the lowest tray at the bottom is not expected to contribute for the separation (i.e. the tray efficiency of this tray approaches zero). When this tray is not included, the overall column 3-7
28 Column efficiency efficiency is higher and more in agreement with the efficiency calculated according to the model of Bennett et al (7). Figure 3.3 and Figure 3.4 show no differences in efficiency and pressure drop for different column pressure at a constant F-factor; it seems that the column pressure has no influence Effect of the outer column on inner column efficiency and heat transfer Figure 3.5 and Figure 3.6 show the heat received by the inner column and the efficiency of the inner column versus the mean F-factor. The two graphs are each for a different column pressure of the inner column. In each figure, two cases are presented: Case 1: Both columns are running, the inner column is at given pressure and the outer column is operated at 1.05 bar Case 2: The inner column is operated at given pressure and the outer column is turned off. The heat received for the given experiments is calculated by Sun (10). Due to uncertainties in the calculations for heat transfer, the exact values cannot be used as such, but the values can only be used to show a trend in heat transfer due to a small offset in the values. Figure 3.5 shows that for an inner column pressure of 1.05 bar, the heat received by the inner column is negative for Case 2, meaning that some heat is lost to the outer column. The opposite is observed for Case 1. It is expected that the operation is adiabatic when both columns are operated at equal column pressures. Figure 3.5 shows that the operation cannot be considered adiabatic for Case 1; this is due to the different composition in the columns and therefore the temperature profile of both columns is not equal. The measurements for both cases are done in different ranges for the F-factor and therefore no conclusions can be made about the effect of the outer column in the inner column performance. It seems that the overall column efficiency of the inner column is higher when more heat is received by the inner column and the efficiency is low when heat is transferred from the inner column to the outer column. However, verification is needed with more experiments in the same F-factor range. Figure 3.5: The heat received by the inner column and the overall column efficiency for two cases: Case 1: inner column = 1.05 bar, outer column = 1.05 bar, Case 2: inner column = 1.05 bar, outer column = off. 3-8
29 Column efficiency Figure 3.6 shows the same graph but for a column pressure of 1.70 bar. In Case 1, more heat is transferred from the inner to the outer column when compared with Case 2. When the outer column is off, the column is filled with nitrogen gas. Phase change gives the highest coefficients for heat transfer and therefore the inner column can transfer more heat to the outer column when it is filled with liquid instead of nitrogen gas. Nitrogen gas can only be heated up to a higher temperature and it cannot undergo any phase change. The liquid and vapour in a running outer column are constantly refreshed and therefore more heat is conducted from inner to outer when the outer column is working when the inner column is at 1.70 bar. Figure 3.6: The heat received by the inner column and the overall column efficiency for two cases: Case 1: inner column = 1.70 bar, outer column = 1.05 bar, Case 2: inner column = 1.70 bar, outer column = off. These two figures show that the differences in the amount of heat transferred between high and low column pressure of the inner column is larger for Case 1 than for Case 2. The heat transfer rate is more affected by the column pressure when the outer column is working. The efficiency shows the same trend but again the measurements are done in different F-factor ranges and more experiments are needed to verify this result in the same F-factor range Effect of heat transfer on efficiency and pressure drop The efficiency and pressure drop per stage of the inner column is shown in Figure 3.7, where it is plotted as a function of the heat received. For this figure, data is used from experiments with inner column pressures of 1.05 bar, 1.40 bar and 1.70 bar and the column pressure of the outer column was 1.05 bar. The graph is plotted for three ranges of the F-factor. When more heat is transferred from the inner to the outer column, the column efficiency of the inner column decreases. For high amounts of heat received in the inner column, the efficiency also decreases. It seems that there is an optimum in efficiency of the inner column with regards to the heat received. Moreover, the heat received shows no effect on the pressure drop for the inner column. 3-9
30 Column efficiency Figure 3.8 shows that the vapour load increases when the heat received increases. When more heat is received from the wall of the inner column the liquid in contact with the wall will evaporate and the vapour load becomes larger. For the same F-factor range the efficiency is not necessarily higher when more heat is received, this can be seen from both Figure 3.7 and Figure 3.8. Figure 3.7: Column efficiency and pressure drop per stage as a function of the heat received in the inner column. The data is plotted for three ranges of the mean column F-factor of the inner column. Figure 3.8: Column efficiency and pressure drop per stage as a function of the heat received in the outer column. The data is plotted for three ranges of the mean column F-factor of the outer column The effect of pressure on column performance In Figure 3.9 data is plotted for different operational pressures of the inner column. As expected a clear correlation is seen between inner column pressure (and therefore temperature) and heat transfer. The efficiency of the inner column is not affected by the amount of heat received in the inner column. There is also no clear correlation between the column pressure and the column efficiency. 3-10
31 Column efficiency Figure 3.9: The heat received and the column efficiency of the inner column versus the F-factor for three column pressures of the inner column Figure 3.10 shows a clear correlation between F-factor and heat transfer. At high amount of heat received the F-factor is higher due to more evaporation in the column. For equal column pressure of the inner and outer column heat is transferred from the outer to the inner column. At higher column pressure of the inner column, the outer column receives more heat. No correlation is shown for the efficiency and the heat received. The only correlation which is shown is the correlation of the efficiency with the F-factor and the correlation with the heat received and the F-factor. Figure 3.10: The heat received and the column efficiency of the outer column versus the F-factor for three column pressures of the inner column 3.4 Conclusions The following conclusions can be drawn from the results shown in Section 3.3: The efficiency of the inner column is in agreement with the Bennett model for the column efficiency between an F-factor range of 0.7 and
32 Column efficiency The efficiency of the outer column is lower than predicted by the Bennett model and it increases for increasing F-factor within an F-factor range between 0.3 and 1.6 this is due to operation in the excessive weeping region. The pressure drop of the inner and outer column is lower than predicted by the Bennett model. This model is not valid for low F-factor ranges because the clear liquid height should be higher than the weir height to be able to apply the model. For higher values of the F- factor the difference between the model and the experimental pressure drop becomes less. The column pressure of the inner column has no effect on the efficiency and pressure drop of the inner and outer column for a constant F-factor. The difference in amount of heat transferred between the columns for different pressures of the inner column is higher when the outer column is turned on than when it is turned off. More heat received does not lead to a higher efficiency and higher pressure drop for the same F-factor range. The mean column F-factor becomes higher when more heat is received. Higher pressure of the inner column leads to higher amount of heat received by the outer column. There is no correlation between the column pressure of the inner column and the column efficiency of the inner and outer column. 3-12
33 4 Control As described in Section 2.2, the HIDiC setup has 13 control loops. By optimizing the control settings the start-up time can be shortened and the process can be maintained at the desired conditions. This chapter presents first the theory about process control which is relevant for this report, Section 4.2 shows the experimental procedure and explains the gproms model which was developed and Section 4.3 discusses the results. 4.1 Theory The dynamic behaviour and steady state response characteristics of a control system should have the following characteristics (10): The system should be stable The effects of disturbances are minimized Rapid and smooth responses to set point changes are obtained Steady state error (offset) is eliminated Excessive control action is avoided The control system is robust, i.e. insensitive to changes in process conditions and to inaccuracies in the process model Normally it is not possible to meet all these criteria and a compromise should be made between performance and robustness of the system. A system has high performance if it has rapid and smooth response to disturbances and set point changes. High robustness means that the process has satisfactory performance for a wide range of process conditions. The choice between a robust system and a high performance system is determined by the settings of the controllers. The controllers installed in the experimental setup are all feedback controllers. The basic modes for feedback control are proportional control (P), integral control (I) and derivative control (D). In this setup all three control modes are used in the so-called PID controller. The P-action in the PID controller is determined by the controller gain, K c, the I-action is determined by the integral time constant, τ I, and the integral of the error. The derivative action (D) is determined by the derivative time constant, τ D, and the derivative of the error. The structure of an ideal PID controller is given in Equation 4.1: OP 1 Kc1 Ds error I s The P-action is given by K c, I-action is given by 1/τ I s and D-action is given by τ D /s. P-action is responsible for the change of the input variable which is directly proportional to the error between the setpoint and the process output. The purpose of I-action is elimination of the offset; this is done by giving a change in the input proportional to the integral of the error. D-action is used to speed up the response or to stabilize the system; this is done by giving a change in the input proportional to the derivative of the error. (4.1) The settings of each PID controller should be tuned; this can be done by several methods (10): 1. Direct synthesis (DS) method 2. Internal Model Control (IMC) method 3. Controller tuning relations 4-1
34 Control 4. Frequency response techniques 5. Computer simulations 6. Online tuning after the control system is installed. The first five methods can be used to determine the controller settings before the control system is installed, for this purpose process models are used. For important control loops, controller settings are often adjusted after the system is installed, this is done by online tuning. Since little is known about the control of the HIDiC column online tuning is preferred to determine the PID settings of the controllers. The step test method is a simple method for online tuning and the least time consuming. Therefore this method is used in this research. The experimental procedure for this method is described in detail in the following section. As will be shown in the results section the dynamics of the control loops in this setup can be described by a first order plus dead time model (FOPDT). The typical response of a FOPDT model to a step test of magnitude M is given in Equation 4.2 and shown in Figure 4.1. KM e Ys () s s1 s (4.2) This response, written in the Laplace domain, has a process gain, K, a time constant, τ and a dead time, θ. The gain of the process is calculated by Equation 4.3 and the time constant, τ is estimated by taking the value for t at which the reponse is 63.2% complete. K M y (4.3) Figure 4.1: First order response for a step input of magnitude M. (10) With a known response of the process the controllers can be tuned. Several tuning relations can be used (11): Ziegler Nichols method Cohen-Coon 4-2
35 Control Åström and Hägglund IMC tuning SIMC tuning The IMC tuning rules are commonly used in industry since it has fast response and good robustness. IMC tuning relations are also useful in processes with a lot of dead time. Simplified IMC (SIMC) tuning relations were proposed by Skogestad (12). These relations have comparable performance but have improved disturbance rejection. The SIMC tuning rules are shown in Table 4.1. These tuning rules were developed for first order and second order processes, the tuning rules for first order processes are obtained by setting τ 2 equal to zero. Table 4.1: Controller tuning rules for G(s) = Ke -θs /(τ 1 s+1)( τ 2 s+1). (12) Conditions K c τ I τ D K K Pressure control The process response time for pressure is normally very small and therefore pressure control is usually done without derivative action and only a PI controller is needed. The integral action normally is very small (τ I is large) Flow rate control Flow control loops are widely used in industry. The response of flow control loops is often fast with almost no time delay. Usually PI controllers are used for flow control; derivative action is normally not used Temperature control Temperature control loops often have a considerable dead time. Due to different process conditions it is difficult to provide guidelines for temperature control. Usually PID controllers are used instead of PI controllers because PID controllers can provide faster response Level control For level control usually a standard P or PI controller is used. Integral control is often used when the offset is important; in some applications a deviation from the setpoint is not a problem like for instance when a level controller is used to prevent a tank from running dry. Then only a P controller is used. Derivative action is normally not used because level measurements are often noisy. When the offset is important the settings for the PI controller are given by: K c 100% (4.4) h 4-3
36 Control 4V I (4.5) KQ c max where V is the volume of the tank, Q max is the maximum volumetric flowrate and Δh is given by: max min h min h h, h h (4.6) sp sp h max is the maximum height, h min is the minimum height and h sp is the setpoint for the height, all are given in percentages of the total height. 4.2 Experimental procedure Two basic methods can be used for controller tuning; online tuning after the controllers are installed and model based tuning. Section describes the procedure involved for online tuning of the controllers and Section describes the Gproms model which can be used for pre determining the PID values based on dynamic simulations Controller tuning As explained in the previous section, step tests are used to determine the process dynamics of a control loop and to define the optimal settings for the PID controllers in the setup. The step test is done by manually adjustment of the manipulated variable. For example, for finding the response of the column pressure subjected to a change of the cooling water control valve, the control mode of the cooling water valve is set to manual and a step change for the cooling water is made. The response of the column pressure is used to determine the process gain, time constant and dead time. It is important to keep all other factors which can influence the controlled variable constant. For the previous example, it is important to have a constant heat input to the column by keeping the steam pressure constant. However, fluctuations in the steam furnace cause fluctuations in the steam supply for the set-up which cannot always be avoided. Step tests were performed for four control loops: CV-310 -> PIC-201 CV-401 -> PIC-403 CV-410 -> PIC-410 CV-401 -> FI-401 The control loops for the cooling water valve of the outer column (CV-301) to the pressure (PIC-109) and temperature (TI-115) of the outer column could not be tuned since the outer column was opened to the atmosphere during the experiments and cooling water had no influence on the pressure. Therefore, no step changes were performed and also the control loop of cooling water and temperature was not investigated. The temperature control loops for both columns were not used during the experiments and therefore both temperature control loops were not tuned The flow indicator of the steam at the side of the inner column (FI-410) gives no accurate reading and therefore this control loop was also not tuned. For level control, no step tests were performed, the tuning is done by applying Equation 4.4, 4.5 and 4.6. The setpoint for the level was determined during experiments, the levels in the condensers and in the bottom of the outer column were set to the lowest value possible to ensure 4-4
37 Control fast refreshment of the liquid volume and to prevent the reflux stream from cooling down too much. For the buffer vessels of the inner and outer column often the liquid level was not controlled but the pumps were set to manual control. Since all liquid pumped from the buffer vessel to the reboiler returns to the buffer vessel, it is not possible to control the liquid level of the vessel by the pumps, once a steady state is reached the level will remain constant. Tuning of the level controllers is done as described in Section Tuning of the other control loops is done according to the SIMC tuning rules. The control structure was tested in Simulink by implementing the controller like shown in Figure 4.2. The disturbance, d, in this control scheme is optional and is not used in Simulink. Figure 4.2: Control scheme of a feedback controller The layout of the process block (Figure 4.3) and the controller block (Figure 4.4) shows how the values for the gain, time constants and delay are implemented in Simulink to test the controlled output. Figure 4.3: The dynamics of the process implemented in Simulink. PV is a constant which is used to set the initial value. Figure 4.4: Implementation of the controller in Simulink Gproms model A Gproms model for dynamic simulation of the experimental setup with cyclohexane and n-heptane has been made by van het Kaar (3). This model is used for dynamic simulations of the HIDiC setup as it is built at the process and energy department. Another purpose for the model is to predetermine the values of the PID controllers inside the set-up. A different model has been made by Voorend (4), this was a simplified version of the first model with the main objective to test different empirical models for tray efficiency, which were compared with results from previous experiments, and to model several heat transfer configurations. 4-5
38 Control In this study, the Gproms models for the tray and heat transfer developed by Voorend are used in the Gproms model of the experimental set-up of van het Kaar. Appendix F shows how both models are combined into a new model. Since the components in the setup have changed from a cyclohexane/n-heptane system to an ethanol/water system a new initialisation of the model is necessary to be able to obtain any results. However, this is beyond the scope of this research and therefore the outcome of the model is not yet verified with the experimental data. 4.3 Results and discussion Settings for the PI(D) controllers are determined by experimental tests and tuning relations. The tuning results are discussed in this section. The results were not validated by experiments and therefore recommendations are done for validating the results and for further improvements Level control The level controllers were tuned with the tuning rules from Equation 4.4, 4.5 and 4.6. The values obtained are shown in Table 4.4. The values used for calculating the controller gain and the integral time constant are shown in Appendix G. Table 4.2: Values for the controller gain and integral time constant for the level controllers. Controlled variable Manipulated variable K c τ I LIC-101 P LIC-102 P Na LIC-103 P LIC-201 P LIC-202 P Na Pressure control The pressure controllers were tuned by first applying a step test to identify the systems response and using the SIMC tuning rules for tuning the controllers. Three pressure control loops were tuned and the results are listed in Table.4.3. Table 4.3: Process gain and time constant and the controller settings obtained by the SIMC tuning rules. Controlled variable Manipulated variable K [-] τ [s] θ [s] K c [-] τ I [s] PIC-201 CV PIC-403 CV PIC-410 CV For the control loop of the cooling water valve settings of the inner column (CV-310) to the pressure of the inner column (PIC-201) a step test was done where the valve opening of the cooling water was changed from 10% opening to 20% opening. The response is shown in red in Figure 4.5. From the figure it looks like the pressure is not yet constant when a step input is done but in fact it is fluctuating around a mean value of 1.06 bar, these fluctuations are due to fluctuations in the steam furnace and cannot be avoided. 4-6
39 Control Figure 4.5: Response of pressure of the inner column (PIC-201) to a change in the cooling water valve opening (CV-310). Besides the experimental values for the step change also the controller settings are shown in Figure 4.5. The purple line (SP) shows the setpoint of the controller. The setpoint is changed from 1.06 bar to 1.02 bar which is the same as the initial and final values for the pressure for a step change in CV-310 from 10% to 20%. For this step input the normal Process Value (PV) is shown as the light blue line in the figure which shows the same trend as the experimental value. The dark blue line (OP) presents the controlled output, it is clear that this value has a faster response than the process value. This shows that in theory the controller settings will be beneficial for this control loop. Another result which is shown in this figure is that the response of the pressure in the column to a setpoint change is slow; when a PI controller is used it still takes 2 minutes for the pressure to come to the new steady state value. This slow response is due to the characteristics of the process. By increase the opening of the cooling water valve the flowrate of cooling water is increased and therefore the cooling rate of the condenser is increased and the column pressure will decrease. The column pressure is not directly related to the cooling water flowrate but there are several steps in between which cause the process to have slow response. The response of steam pressure to a change in the opening of the steam valve is much faster as can be seen in Figure 4.6 and 4.7. Figure 4.6: Response of steam pressure of the outer column (PIC-403) to a change in the steam valve opening (CV-401). 4-7
40 Control In both figures, a step input is done for the steam control valve (CV-401 and CV-410) which is shown by the green line. The response of the experimental value is shown in red and the steady state values for both setpoints were used to determine the process gain and time constant. Figure 4.6 shows a higher value for the experimental value than for the obtained process value; this is again due to the fluctuations of the steam which cannot be seen in this figure. For both Figure 4.6 and 4.7 it is clear that the controlled process has faster response towards a change in setpoint with only small overshoot. Figure 4.7: Response of steam pressure of the outer column (PIC-410) to a change in the steam valve opening (CV-410) Flow rate control In the experimental setup two flow rate control loops are present but only one was tuned due to inaccurate readings of the flow indicator. The control loop which is tuned is the loop from the steam valve opening of the outer column (CV-401) to the steam flowrate of the outer column (FI-401). The results of the controller tuning is shown in Table 4.4. Table 4.4: Process gain and time constant and the controller settings obtained by the SIMC tuning rules. Controlled Manipulated K τ θ K c τ I variable variable [-] [s] [s] [-] [s] FI-401 CV Figure 4.8 shows the response of the experiments to a step input of the steam valve. The response for the flowrate is fast but it has little overshoot before it goes to the steady state value. When the controller is implemented this overshoot becomes less and steady state is reached faster; the response to a step change is faster with controller. 4-8
41 Control Figure 4.8: Response of the steam flowrate of the outer column (FI-401) to a change in the steam valve opening (CV-401) Conclusion and recommendations The figures in this section all show that tuning the controllers should be beneficial for the response of the controlled variables. Although the settings for the controllers have been tuned, the response of the process is not validated by experiments and one must be aware that fine tuning of the controllers should still be done. For implementation, it is recommended to start with the controller settings as described in this section and do more fine-tuning as described by Skogestad (13) to determine the optimal settings for the controllers. These are a few guidelines for tuning as described by Skogestad (13): If the maximum output deviation is too large then the controller gain should be increased If the settling time is too large then the integral time should be reduced If the oscillations are too large and these have a period shorter than the integral time, then the gain should be reduced or the integral time increased If the oscillations are too large and these have a period of more than about three times the integral time, then the product of the controller gain and the integral time should be increased 4-9
42
43 5 Conclusions The experimental facility of the CT-HIDiC is finalized and the first experiments with water and ethanol are performed. The overall column efficiency is determined from experiments by a stage-by-stage Fenske equation. For an F-factor range between 0.7 and 2.0, the efficiency of the inner column corresponds well with the empirical relation developed by Bennett et al. The outer column shows more deviation from the model and the column efficiency determined from experiments shows lower values for the overall column efficiency. The column efficiency of the outer column is increasing, for increasing F-factor from 0.3 to 1.6, due to operation in the weeping region. Visual inspection done during the experiments verifies this result but the calculated minimum F-factor for stable operation suggests that excessive weeping should not occur after an F-factor of 0.63 Pa 0.5. The pressure drop for the inner- and outer column is less than predicted by the model of Bennett et al. This correlation is only valid for higher F-factor ranges where no weeping occurs. For higher F-factors, the difference between the model and the experimental values becomes less. At a constant F-factor, the column pressure of the inner column has no effect on the overall column efficiency and the pressure drop of the inner and outer column, although a higher column pressure of the inner column does lead to higher amounts of heat transferred from inner column to the outer column. Higher amounts of heat transferred from the inner to the outer column lead to higher F- factors of the outer column and in the region in which is operated this leads to higher efficiencies. For higher F-factors of the outer column this is not necessarily the case. For the same column pressure of the inner column, more heat is transferred from the inner column to the outer column when the outer column is working compared to the situation that the outer column is not working. The controllability of the experimental setup is also studied. By applying step changes to the manipulated variables of the control loops, the dynamics of the processes are determined. All processes show first order plus dead time response and therefore SIMC tuning rules can be used for tuning with high performance and good robustness. The control structure with the tuned controller settings is implemented in Simulink to compare the response of the controlled process with the normal process response. The controlled process shows faster response and less overshoot than the normal process. The level controllers are tuned without determining the process characteristics by experiments. The controller settings for all the tuned controllers are shown Table 5.1. Table 5.1: Controller settings Controlled variable Manipulated variable K c τ I LIC-101 P LIC-102 P Na LIC-103 P LIC-201 P LIC-202 P Na PIC-201 CV PIC-403 CV PIC-410 CV FI-401 CV
44
45 6 Recommendations In this section, recommendations are given for the column efficiency and for the control of the HIDiC installation and experiments. 6.1 Column efficiency More information about the column efficiency is needed for a larger range of F-factors and more experiments are necessary to obtain this information. For the inner column, experiments are needed at lower F-factors and higher F-factors to show the weeping region, the region for stable operation and the entrainment region. After the range of F-factors is determined in which the operation is stable, more experiments can be done in this region to determine the effect of heat transfer on column efficiency. For the outer column, more experiments are required in the higher F-factor range because the F-factor range in which there is stable operation is not yet reached. To be able to do experiments in different F-factor ranges, the composition of the process stream has to be changed to cyclohexane/n-heptane because the experimental setup is designed for this system. In the current study, the F-factor range is limited by the condenser capacity and the steam capacity. Cyclohexane and n-heptane both have lower enthalpy of vaporization than water and therefore it is easier to evaporate, thus higher F-factors can be reached. When changing the system from ethanol/water to cyclohexane/n-heptane the previous experiments should be repeated and compared with the empirical models for the overall column efficiency and the pressure drop. 6.2 Control Nine out of 13 control loops have been tuned and experimental tests are needed to test these settings. If the settings don t give a good response more fine-tuning is needed which can be done according to the following rules: If the maximum output deviation is too large then the controller gain should be increased If the settling time is too large then the integral time should be reduced If the oscillations are too large and these have a period shorter than the integral time, then the gain should be reduced or the integral time increased If the oscillations are too large and these have a period of more than about three times the integral time, then the product of the controller gain and the integral time should be increased When changing the components in the experimental setup some controller settings should be revised. For example, the column pressure will have a different relation with the cooling water flow rate but the relation between the steam pressure and the opening of the steam valve will be the same. Besides fine-tuning of the controllers for which the settings are already determined also the control loops which have not been discussed in this report should be tuned. Therefore, more experiments are necessary which can provide more information about the process dynamics of each control loop. Furthermore, a start is made for a dynamic model in Gproms. The model is already finished for a cyclohexane/n-heptane system but if needed the model can be changed to an ethanol/water system by performing a new initialisation of the model. If more experiments will be done with ethanol and water the results of the experiments can be compared with the modelling results and 6-1
46 Recommendations the model can be adjusted. Again, the same thing can be done for cyclohexane/n-heptane and by doing this the flexibility of the model for different components can be studied. 6.3 The experimental setup Besides running more experiments, also some adjustments to the experimental setup are recommended. One important adjustment is in the steam supply. During experiments for this study, the steam entering the setup was constantly fluctuating. Even though the steam reducing valve has been repaired, the steam pressure entering the setup was still fluctuating and these fluctuations cause more fluctuations in the rest of the setup. For this reason it was difficult to determine if the operation was steady state and this also makes tuning of the controllers difficult. Another possibility for improvement, is the condenser of the inner column. This condenser is oversized and therefore the reflux stream of the inner column is sub-cooled when entering the column. This effect might already be avoided when using cyclohexane/n-heptane but, if not, some adjustments to the condenser might be necessary. 6.4 Heat panels After obtaining more results for the concentric tray HIDiC without heat panels, the experiments should be repeated for the same column with heat panels installed. The conditions for the experiments should be the same for experiments with and without heat panels; therefore good documentation of the process conditions is desired. 6-2
47 Bibliography 1. de Rijke, Aris. Development of a Concentric Internally Heat Integrated Distillation Column (HIDiC). Delft : TU Delft, PhD Thesis. 2. Harms, S and Olujic, Z. HIDiC as a retrofit technology. s.l. : Delft University of Technology, Process & Energy department, Leeghwaterstraat 44, Delft. 3. van het Kaar, T. The modeling and control of a Heat Integrated Distillation Colum (HIDiC). Delft : TU Delft, Voorend, J. A Dynamic Model for the Concentric Tray HIDiC Experimental Setup. Delft : TU Delft, Kister, H.Z. Distillation design. s.l. : McGraw-Hill, p Kister, H.Z.. Distillation design. s.l. : McGraw-Hill, pp New Correlation for Sieve-Tray Point Efficiency, Entrainment and Section Efficiency. Bennett, D.L., Watson, D.N., Wiescinski, M. 6, s.l. : AIChE Journal, 1997, Vol Stichlmair, J.G., Fair, J.R. Distillation, principles and practice. s.l. : Wiley VCH, pp New Pressure Drop Correlation for Sieve Tray Distillation Columns. Bennet, D.L., Agrawal, R., Cook, P.J. 3, Allentown PA : AIChE Journal, 1983, Vol. 29, pp Sun, A.R. Internal heat exchange in a concentric tray heat integrated distillation column (HIDiC), Experimental validation of predictive models. Delft : Delft University of Technology, Seborg, D.E., Edgar, T.F., Mellichamp, D.A. Process Dynamics and Control. 2nd. s.l. : John Wiley & Sons, Inc., van der Zalm, G.M. Tuning of PID-Type controllers: Literature overview. s.l. : Eindhoven University of Technology. 13. Probably the best simple PID tuning rules in the world. Skogestad, S. Reno, NV, USA : AIChE annual meeting, November, Perry, R.H., Green, D.W. Perry's Chemical Engineers Handbook. 7th pp. 5-48, Perry, R.H., Green, D.W. Perry's Chemical Engineers Handbook. 7th pp Perry, R.H., Green, D.W. Perry's Chemical Engineers Handbook. 7th pp Rousseau, R.W. Handbook of separation process technology. s.l. : John Wiley & Sons Inc., p X. 18. Surface Tensions of Mixtures at their Boiling Points. Kalbassl, M.A., Biddulph, M.W. 4, s.l. : Journal of Chemical and Engineering Data, 1988, Vol. 33. vii
48
49 Nomenclature a Constant for Bennett pressure drop - A a Active tray area m 2 A c Column area m 2 A d Downcomer area m 2 A h Hole area m 2 b Constant for bubble diameter - C Constant for Bennett tray efficiency - C v Discharge coefficient - D G Vapour diffusivity m 2 s -1 D L Liquid diffusivity m 2 s -1 d bub,max Maximum bubble diameter m d h Hole diameter m E oc Overall column efficiency - E og Point efficiency - E mv Murphree tray efficiency - F G F-factor Pa 0.5 g Gravity constant m s -2 h D Liquid height equivalent to dry hole pressure drop m h Fe Froth height m h L Clear liquid height m h max Maximum relative height % h min Minimum relative height % h sp Relative height of the setpoint % h T Liquid height on tray m h σ Liquid height due to surface tension m k Boltzmann constant m 2 kg s -2 K -1 K Process gain - K c Controller gain - K s Density corrected superficial velocity m s -1 l w Weir length m M Stepsize M w Molecular weight g mol -1 m Slope of the equilibrium line - N a Actual number of trays - N t Theoretical number of trays - P Pressure Pa Q max Maximum volumetric flowrate m 3 s -1 R Gas constant J K -1 mol -1 T Temperature K T 0 Reference temperature K u G Superficial gas velocity m s -1 u h Superficial hole velocity m s -1 x B Mol fraction bottom - V Volume m 3 V b,i Molar volume at normal boiling point m 3 mol -1 V e Molar volume of ethanol m 3 mol -1 V l Volumetric flowrate m 3 s -1 x D Mol fraction distillate - α ij Relative volatility of components i and j - ix
50 Nomenclature α B Relative volatility of the bottom - α D Relative volatility of the distillate - ΔP tray Pressure drop per tray Pa Δh Minimum height difference m Δy Difference in controlled variable output θ Dead time s θ bub Departure contact angle between bubble interphase and tray 0 ε Characteristic Lennard-Jones energy m 2 kg s -2 ε ew Characteristic Lennard-Jones energy of ethanol/water m 2 kg s -2 μ G Viscosity of gas Pa s ρ G Density of gas kg m -3 ρ L Density of liquid kg m -3 ρ MG Molar density of gas mol m -3 ρ ML Molar density of liquid mol m -3 σ Surface tension N/m σ ew Characteristic length ethanol/water Å τ Process time constant s τ D Derivative time constant s τ I Integral time constant s υ Relative free area - υ e Effective froth density - Ω D Collision integral for diffusion - x
51 Appendices Appendix A. Process and Instrumentation Diagram A-1 Appendix B. Point efficiency and pressure drop parameters B-1 B.1. Diffusivity B-1 B.2. Clear liquid height and effective froth height B-2 B.3. Pressure drop calculations B-3 Appendix C. F-factor limits C-1 Appendix D. Efficiency calculations D-1 Appendix E. Matlab script efficiency E-1 Appendix F. Gproms model F-1 Appendix G. Controller tuning G-1 G.1. Level controllers G-1 G.2. Process dynamics from the step test G-1 xi
52
53 Appendix A. Process and Instrumentation Diagram A-1
DISTILLATION POINTS TO REMEMBER
 DISTILLATION POINTS TO REMEMBER 1. Distillation columns carry out physical separation of liquid chemical components from a mixture by a. A combination of transfer of heat energy (to vaporize lighter components)
DISTILLATION POINTS TO REMEMBER 1. Distillation columns carry out physical separation of liquid chemical components from a mixture by a. A combination of transfer of heat energy (to vaporize lighter components)
CHE 306 Stagewise Operations
 CHE 306 Stagewise Operations Fall 2010 Introduction ti to Column Distillation Instructor: Dr. Housam Binous KFUPM, Dhahran 1 Over 90% of separations are done using distillation Over 40 000 distillation
CHE 306 Stagewise Operations Fall 2010 Introduction ti to Column Distillation Instructor: Dr. Housam Binous KFUPM, Dhahran 1 Over 90% of separations are done using distillation Over 40 000 distillation
Liquid -Vapor. Contacting columns
 Liquid -Vapor * Contacting columns Distillation Column Design The design of a distillation column can be divided into the following steps: 1. Specify the degree of separation required: set product specifications.
Liquid -Vapor * Contacting columns Distillation Column Design The design of a distillation column can be divided into the following steps: 1. Specify the degree of separation required: set product specifications.
DISTILLATION PRESSURE CONTROL TROUBLESHOOTING THE HIDDEN PITTFALLS OF OVERDESIGN
 Distillation Absorption 2010 A.B. de Haan, H. Kooijman and A. Górak (Editors) All rights reserved by authors as per DA2010 copyright notice DISTILLATION PRESSURE CONTROL TROUBLESHOOTING THE HIDDEN PITTFALLS
Distillation Absorption 2010 A.B. de Haan, H. Kooijman and A. Górak (Editors) All rights reserved by authors as per DA2010 copyright notice DISTILLATION PRESSURE CONTROL TROUBLESHOOTING THE HIDDEN PITTFALLS
CHEMICAL ENGINEERING LABORATORY CHEG 239W. Control of a Steam-Heated Mixing Tank with a Pneumatic Process Controller
 CHEMICAL ENGINEERING LABORATORY CHEG 239W Control of a Steam-Heated Mixing Tank with a Pneumatic Process Controller Objective The experiment involves tuning a commercial process controller for temperature
CHEMICAL ENGINEERING LABORATORY CHEG 239W Control of a Steam-Heated Mixing Tank with a Pneumatic Process Controller Objective The experiment involves tuning a commercial process controller for temperature
Dynamic Simulation and Control of Vapor Recompression Column
 The 7 th International Chemical Engineering Congress & Exhibition (IChEC 2011) Kish, Iran, 21-24 November, 2011 Dynamic Simulation and Control of Vapor Recompression Column Hojjat Dehghani, Mohammad Ali
The 7 th International Chemical Engineering Congress & Exhibition (IChEC 2011) Kish, Iran, 21-24 November, 2011 Dynamic Simulation and Control of Vapor Recompression Column Hojjat Dehghani, Mohammad Ali
Manual for continuous distillation
 Manual for continuous distillation 1. Week 1: Objectives: Run the column at total reflux. When steady state is reached, take the sample from the top and bottom of the column in order to determine the overall
Manual for continuous distillation 1. Week 1: Objectives: Run the column at total reflux. When steady state is reached, take the sample from the top and bottom of the column in order to determine the overall
DISTILLATION COLUMN PROCESS CONTROL STRATEGIES
 DISTILLATION COLUMN PROCESS CONTROL STRATEGIES Satyajit Deshmukh 1, Salil Rajwade 2, Atharva Pundalik 3, Anil Ranveer 4 ABSTRACT 1,2,3,4 Chemical Engineering Department, Datta Meghe College of Engineering,
DISTILLATION COLUMN PROCESS CONTROL STRATEGIES Satyajit Deshmukh 1, Salil Rajwade 2, Atharva Pundalik 3, Anil Ranveer 4 ABSTRACT 1,2,3,4 Chemical Engineering Department, Datta Meghe College of Engineering,
MODEL PREDICTIVE CONTROL OF INTEGRATED UNIT OPERATIONS CONTROL OF A DIVIDED WALL COLUMN
 MODEL PREDICTIVE CONTROL OF INTEGRATED UNIT OPERATIONS CONTROL OF A DIVIDED WALL COLUMN Prof. Dr. Till Adrian*, Dr. Hartmut Schoenmakers**, Dr. Marco Boll** * Mannheim University of Applied Science, Department
MODEL PREDICTIVE CONTROL OF INTEGRATED UNIT OPERATIONS CONTROL OF A DIVIDED WALL COLUMN Prof. Dr. Till Adrian*, Dr. Hartmut Schoenmakers**, Dr. Marco Boll** * Mannheim University of Applied Science, Department
Distillation Design The McCabe-Thiele Method
 Distillation Design The McCabe-Thiele Method Distiller diagam Introduction Using rigorous tray-by-tray calculations l is time consuming, and is often unnecessary. One quick method of estimation i for number
Distillation Design The McCabe-Thiele Method Distiller diagam Introduction Using rigorous tray-by-tray calculations l is time consuming, and is often unnecessary. One quick method of estimation i for number
PROCESS MONITORING AND CONTROL
 Chemical Engineering Process Design PROCESS MONITORING AND CONTROL Keith Marchildon David Mody Monitoring means Measurement 1. There is no Control without Monitoring 2. Monitoring should not be done without
Chemical Engineering Process Design PROCESS MONITORING AND CONTROL Keith Marchildon David Mody Monitoring means Measurement 1. There is no Control without Monitoring 2. Monitoring should not be done without
Dynamics and Control of Chemical Processes Solution to Lab #8 Shutdown of C4-C5 separation section
 Prof. Davide Manca Politecnico di Milano Dynamics and Control of Chemical Processes Solution to Lab #8 Shutdown of C4-C5 separation section RUN A DISTILLATION COLUMN USING HYSYS/UNISIM IN DYNAMIC MODE
Prof. Davide Manca Politecnico di Milano Dynamics and Control of Chemical Processes Solution to Lab #8 Shutdown of C4-C5 separation section RUN A DISTILLATION COLUMN USING HYSYS/UNISIM IN DYNAMIC MODE
Dynamic Simulation for T-9 Storage Tank (Holding Case)
 Dynamic Simulation for T-9 Storage Tank (Holding Case) CASE 1: 19,642 Kg/Hr (Holding: 52 o C), No Liquid Draw Workshop Description Estimation of vapor flow rate coming out from the T-9 tank for holding
Dynamic Simulation for T-9 Storage Tank (Holding Case) CASE 1: 19,642 Kg/Hr (Holding: 52 o C), No Liquid Draw Workshop Description Estimation of vapor flow rate coming out from the T-9 tank for holding
ERTC PETROCHEMICAL Conference 20 st -22 nd February 2002, Amsterdam, The Netherlands
 Reprint from Presentation at ERTC PETROCHEMICAL Conference 20 st -22 nd February 2002, Amsterdam, The Netherlands and ARTC PETROCHEMICAL Conference 11 th 13 th March 2002, Bangkok, Thailand Sulzer Chemtech,
Reprint from Presentation at ERTC PETROCHEMICAL Conference 20 st -22 nd February 2002, Amsterdam, The Netherlands and ARTC PETROCHEMICAL Conference 11 th 13 th March 2002, Bangkok, Thailand Sulzer Chemtech,
CHE 4115 Chemical Processes Laboratory 2 Experiment 1. Batch Distillation
 CHE 4115 Chemical Processes Laboratory 2 Experiment 1 Batch Distillation BACKGROUND Distillation is one of the most commonly used unit operations in chemical engineering. In general, a distillation operation
CHE 4115 Chemical Processes Laboratory 2 Experiment 1 Batch Distillation BACKGROUND Distillation is one of the most commonly used unit operations in chemical engineering. In general, a distillation operation
Exercise 2-2. Second-Order Interacting Processes EXERCISE OBJECTIVE DISCUSSION OUTLINE. The actual setup DISCUSSION
 Exercise 2-2 Second-Order Interacting Processes EXERCISE OBJECTIVE Familiarize yourself with second-order interacting processes and experiment with the finer points of controller tuning to gain a deeper
Exercise 2-2 Second-Order Interacting Processes EXERCISE OBJECTIVE Familiarize yourself with second-order interacting processes and experiment with the finer points of controller tuning to gain a deeper
Characterizers for control loops
 Characterizers for control loops By: F. G. Shinskey (May 1999) Introduction Commercial controllers such as the PID series (proportional, integral, derivative, and their combinations) are linear devices
Characterizers for control loops By: F. G. Shinskey (May 1999) Introduction Commercial controllers such as the PID series (proportional, integral, derivative, and their combinations) are linear devices
LEAP CO 2 Laboratory CO 2 mixtures test facility
 LEAP CO 2 Laboratory CO 2 mixtures test facility THE PROJECT AIM CO 2 flows made available by several capture techniques are contaminated with impurities and this affects the design and operations of the
LEAP CO 2 Laboratory CO 2 mixtures test facility THE PROJECT AIM CO 2 flows made available by several capture techniques are contaminated with impurities and this affects the design and operations of the
Transient Analyses In Relief Systems
 Transient Analyses In Relief Systems Dirk Deboer, Brady Haneman and Quoc-Khanh Tran Kaiser Engineers Pty Ltd ABSTRACT Analyses of pressure relief systems are concerned with transient process disturbances
Transient Analyses In Relief Systems Dirk Deboer, Brady Haneman and Quoc-Khanh Tran Kaiser Engineers Pty Ltd ABSTRACT Analyses of pressure relief systems are concerned with transient process disturbances
Separation Columns (Distillation)
 Separation Columns (Distillation) 1 Distillation Column Design The design of a distillation column can be divided into the following steps: 1. Specify the degree of separation required: set product specifications.
Separation Columns (Distillation) 1 Distillation Column Design The design of a distillation column can be divided into the following steps: 1. Specify the degree of separation required: set product specifications.
Exercise 5-2. Bubblers EXERCISE OBJECTIVE DISCUSSION OUTLINE. Bubblers DISCUSSION. Learn to measure the level in a vessel using a bubbler.
 Exercise 5-2 Bubblers EXERCISE OBJECTIVE Learn to measure the level in a vessel using a bubbler. DISCUSSION OUTLINE The Discussion of this exercise covers the following points: Bubblers How to measure
Exercise 5-2 Bubblers EXERCISE OBJECTIVE Learn to measure the level in a vessel using a bubbler. DISCUSSION OUTLINE The Discussion of this exercise covers the following points: Bubblers How to measure
Armfield Distillation Column Operation Guidelines
 Armfield Distillation Column Operation Guidelines 11-2016 R.Cox Safety SAFETY GLASSES ARE REQUIRED WHEN OPERATING THE DISTILLATION COLUMN Wear gloves when mixing alcohol feedstock The column will become
Armfield Distillation Column Operation Guidelines 11-2016 R.Cox Safety SAFETY GLASSES ARE REQUIRED WHEN OPERATING THE DISTILLATION COLUMN Wear gloves when mixing alcohol feedstock The column will become
Structure Study of Internal Thermally Coupled Distillation Columns
 2010 The Second China Energy Scientist orum Structure Study of Internal Thermally Coupled istillation Columns Lanyi Sun, Cheng Zhai, Hui Zhang, Qingsong Li State Key Laboratory of Heavy Oil Processing,
2010 The Second China Energy Scientist orum Structure Study of Internal Thermally Coupled istillation Columns Lanyi Sun, Cheng Zhai, Hui Zhang, Qingsong Li State Key Laboratory of Heavy Oil Processing,
LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary
 ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
Multivariable Predictive Control and its Application on the Solvent Dehydration Tower
 Multivariable Predictive Control and its Application on the Solvent Dehydration Tower Yong Gu, Hongye Su, Jian Chu National Key Laboratory of Industrial Control Technology and Institute of Industrial Process
Multivariable Predictive Control and its Application on the Solvent Dehydration Tower Yong Gu, Hongye Su, Jian Chu National Key Laboratory of Industrial Control Technology and Institute of Industrial Process
CHAPTER 2 EXPERIMENTAL SETUP AND PROCEDURE
 22 CHAPTER 2 EXPERIMENTAL SETUP AND PROCEDURE 2.1 EXPERIMENTAL COLUMN All the experiments were carried out in an internal loop airlift fluidized bed and combined loop fluidized bed (an external down comer
22 CHAPTER 2 EXPERIMENTAL SETUP AND PROCEDURE 2.1 EXPERIMENTAL COLUMN All the experiments were carried out in an internal loop airlift fluidized bed and combined loop fluidized bed (an external down comer
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/12
 LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
Generating Calibration Gas Standards
 Technical Note 1001 Metronics Inc. Generating Calibration Gas Standards with Dynacal Permeation Devices Permeation devices provide an excellent method of producing known gas concentrations in the PPM and
Technical Note 1001 Metronics Inc. Generating Calibration Gas Standards with Dynacal Permeation Devices Permeation devices provide an excellent method of producing known gas concentrations in the PPM and
Analysis and Modeling of Vapor Recompressive Distillation Using ASPEN-HYSYS
 Computer Science Journal of Moldova, vol.19, no.2(56), 2011 Analysis and Modeling of Vapor Recompressive Distillation Using ASPEN-HYSYS Cinthujaa C. Sivanantha, Gennaro J. Maffia Abstract HYSYS process
Computer Science Journal of Moldova, vol.19, no.2(56), 2011 Analysis and Modeling of Vapor Recompressive Distillation Using ASPEN-HYSYS Cinthujaa C. Sivanantha, Gennaro J. Maffia Abstract HYSYS process
Energy Saving for Batch Distillation with Mechanical Heat Pumps
 A publication of VOL. 35, 2013 CHEMICAL ENGINEERING TRANSACTIONS Guest Editors: Petar Varbanov, Jiří Klemeš, Panos Seferlis, Athanasios I. Papadopoulos, Spyros Voutetakis Copyright 2013, AIDIC Servizi
A publication of VOL. 35, 2013 CHEMICAL ENGINEERING TRANSACTIONS Guest Editors: Petar Varbanov, Jiří Klemeš, Panos Seferlis, Athanasios I. Papadopoulos, Spyros Voutetakis Copyright 2013, AIDIC Servizi
Gerald D. Anderson. Education Technical Specialist
 Gerald D. Anderson Education Technical Specialist The factors which influence selection of equipment for a liquid level control loop interact significantly. Analyses of these factors and their interactions
Gerald D. Anderson Education Technical Specialist The factors which influence selection of equipment for a liquid level control loop interact significantly. Analyses of these factors and their interactions
A Numerical Study of the Performance of a Heat Exchanger for a Miniature Joule-Thomson Refrigerator
 A Numerical Study of the Performance of a Heat Exchanger for a Miniature Joule-Thomson Refrigerator Yong-Ju Hong 1, Seong-Je Park 1, and Young-Don Choi 2 1 Korea Institute of Machinery & Materials, Daejeon,
A Numerical Study of the Performance of a Heat Exchanger for a Miniature Joule-Thomson Refrigerator Yong-Ju Hong 1, Seong-Je Park 1, and Young-Don Choi 2 1 Korea Institute of Machinery & Materials, Daejeon,
TWO PHASE FLOW METER UTILIZING A SLOTTED PLATE. Acadiana Flow Measurement Society
 TWO PHASE FLOW METER UTILIZING A SLOTTED PLATE Acadiana Flow Measurement Society Gerald L. Morrison Presented by: Mechanical Engineering Department Daniel J. Rudroff 323 Texas A&M University Flowline Meters
TWO PHASE FLOW METER UTILIZING A SLOTTED PLATE Acadiana Flow Measurement Society Gerald L. Morrison Presented by: Mechanical Engineering Department Daniel J. Rudroff 323 Texas A&M University Flowline Meters
Cascade control. Cascade control. 2. Cascade control - details
 Cascade control Statistical Process Control Feedforward and ratio control Cascade control Split range and selective control Control of MIMO processes 1 Structure of discussion: Cascade control Cascade
Cascade control Statistical Process Control Feedforward and ratio control Cascade control Split range and selective control Control of MIMO processes 1 Structure of discussion: Cascade control Cascade
CFD Flow Analysis of a Refrigerant inside Adiabatic Capillary Tube
 CFD Flow Analysis of a Refrigerant inside Adiabatic Capillary Tube Y Raja Kumar ¹, Dr.P Usha sri ² PG Student¹, Associate professor² Department of Mechanical Engineering, University College of Engineering
CFD Flow Analysis of a Refrigerant inside Adiabatic Capillary Tube Y Raja Kumar ¹, Dr.P Usha sri ² PG Student¹, Associate professor² Department of Mechanical Engineering, University College of Engineering
This portion of the piping tutorial covers control valve sizing, control valves, and the use of nodes.
 Piping Tutorial A piping network represents the flow of fluids through several pieces of equipment. If sufficient variables (flow rate and pressure) are specified on the piping network, CHEMCAD calculates
Piping Tutorial A piping network represents the flow of fluids through several pieces of equipment. If sufficient variables (flow rate and pressure) are specified on the piping network, CHEMCAD calculates
Mitos Fluika Pressure and Vacuum Pumps Datasheet
 Unit 1, Anglian Business Park, Orchard Road, Royston, Hertfordshire, SG8 5TW, UK T: +44 (0)1763 242491 F: +44 (0)1763 246125 E: sales@dolomite-microfluidics.com W: www.dolomite-microfluidics.com Dolomite
Unit 1, Anglian Business Park, Orchard Road, Royston, Hertfordshire, SG8 5TW, UK T: +44 (0)1763 242491 F: +44 (0)1763 246125 E: sales@dolomite-microfluidics.com W: www.dolomite-microfluidics.com Dolomite
Instruction Manual. Pipe Friction Training Panel
 Instruction Manual HL 102 Pipe Friction Training Panel 100 90 80 70 60 50 40 30 20 10 HL 102 Instruction Manual This manual must be kept by the unit. Before operating the unit: - Read this manual. - All
Instruction Manual HL 102 Pipe Friction Training Panel 100 90 80 70 60 50 40 30 20 10 HL 102 Instruction Manual This manual must be kept by the unit. Before operating the unit: - Read this manual. - All
Digital Level Control One and Two Loops Proportional and Integral Control Single-Loop and Cascade Control
 Digital Level Control One and Two Loops Proportional and Integral Control Single-Loop and Cascade Control Introduction This experiment offers a look into the broad field of process control. This area of
Digital Level Control One and Two Loops Proportional and Integral Control Single-Loop and Cascade Control Introduction This experiment offers a look into the broad field of process control. This area of
Gas Injection for Hydrodynamic Slug Control
 Proceedings of the IFAC Workshop on Automatic Control in Offshore Oil and Gas Production, Norwegian University of Science and Technology, Trondheim, Norway, May 3 - June, ThB.4 Gas Injection for Hydrodynamic
Proceedings of the IFAC Workshop on Automatic Control in Offshore Oil and Gas Production, Norwegian University of Science and Technology, Trondheim, Norway, May 3 - June, ThB.4 Gas Injection for Hydrodynamic
Removing nitrogen. Nitrogen rejection applications can be divided into two categories
 Removing nitrogen Doug MacKenzie, Ilie Cheta and Darryl Burns, Gas Liquids Engineering, Canada, present a comparative study of four nitrogen removal processes. Nitrogen rejection applications can be divided
Removing nitrogen Doug MacKenzie, Ilie Cheta and Darryl Burns, Gas Liquids Engineering, Canada, present a comparative study of four nitrogen removal processes. Nitrogen rejection applications can be divided
Operating Instructions
 Operating Instructions Preparation for Start-up 1. If the computer system is logged off, log into the computer system. Log into the operator account on the computer. Please ask your TA for username and
Operating Instructions Preparation for Start-up 1. If the computer system is logged off, log into the computer system. Log into the operator account on the computer. Please ask your TA for username and
Inprocess Operator Training Programme
 2016 Inprocess Operator Training Programme ABSORPTION COLUMN These exercises are intended to provide an understanding of absorption columns and the fundamental principles they use to eliminate pollutants
2016 Inprocess Operator Training Programme ABSORPTION COLUMN These exercises are intended to provide an understanding of absorption columns and the fundamental principles they use to eliminate pollutants
Exercise 4-2. Centrifugal Pumps EXERCISE OBJECTIVE DISCUSSION OUTLINE DISCUSSION. Pumps
 Exercise 4-2 Centrifugal Pumps EXERCISE OBJECTIVE Familiarize yourself with the basics of liquid pumps, specifically with the basics of centrifugal pumps. DISCUSSION OUTLINE The Discussion of this exercise
Exercise 4-2 Centrifugal Pumps EXERCISE OBJECTIVE Familiarize yourself with the basics of liquid pumps, specifically with the basics of centrifugal pumps. DISCUSSION OUTLINE The Discussion of this exercise
Process Dynamics, Operations, and Control Lecture Notes - 20
 Lesson 0. Control valves 0.0 Context Controller output is a signal that varies between 0 and 100%. Putting this signal to use requires a final control element, a device that responds to the controller
Lesson 0. Control valves 0.0 Context Controller output is a signal that varies between 0 and 100%. Putting this signal to use requires a final control element, a device that responds to the controller
4.1 Why is the Equilibrium Diameter Important?
 Chapter 4 Equilibrium Calculations 4.1 Why is the Equilibrium Diameter Important? The model described in Chapter 2 provides information on the thermodynamic state of the system and the droplet size distribution.
Chapter 4 Equilibrium Calculations 4.1 Why is the Equilibrium Diameter Important? The model described in Chapter 2 provides information on the thermodynamic state of the system and the droplet size distribution.
NATIONAL UNIVERSITY OF SINGAPORE. EE3302/EE3302E Industrial Control Systems E1: ADVANCED CONTROL SYSTEMS
 NATIONAL UNIVERSITY OF SINGAPORE EE3302/EE3302E Industrial Control Systems E1:. OBJECTIVES: Before embarking on this hands-on session, you should have been introduced to the concepts of cascade and feedforward
NATIONAL UNIVERSITY OF SINGAPORE EE3302/EE3302E Industrial Control Systems E1:. OBJECTIVES: Before embarking on this hands-on session, you should have been introduced to the concepts of cascade and feedforward
2. Determine how the mass transfer rate is affected by gas flow rate and liquid flow rate.
 Goals for Gas Absorption Experiment: 1. Evaluate the performance of packed gas-liquid absorption tower. 2. Determine how the mass transfer rate is affected by gas flow rate and liquid flow rate. 3. Consider
Goals for Gas Absorption Experiment: 1. Evaluate the performance of packed gas-liquid absorption tower. 2. Determine how the mass transfer rate is affected by gas flow rate and liquid flow rate. 3. Consider
OLGA. The Dynamic Three Phase Flow Simulator. Input. Output. Mass transfer Momentum transfer Energy transfer. 9 Conservation equations
 서유택 Flow Assurance The Dynamic Three Phase Flow Simulator 9 Conservation equations Mass (5) Momentum (3) Energy (1) Mass transfer Momentum transfer Energy transfer Input Boundary and initial conditions
서유택 Flow Assurance The Dynamic Three Phase Flow Simulator 9 Conservation equations Mass (5) Momentum (3) Energy (1) Mass transfer Momentum transfer Energy transfer Input Boundary and initial conditions
TKP4550/TKP4551 PROSESS-SYSTEMTEKNIKK (PROCESS SYSTEMS ENGINEERING)
 TKP4550/TKP4551 PROSESS-SYSTEMTEKNIKK (PROCESS SYSTEMS ENGINEERING) Coordinator: Sigurd Skogestad Project proposal from Professor Sigurd Skogestad: sigurd.skogestad@ntnu.no 69. Modelling and optimization
TKP4550/TKP4551 PROSESS-SYSTEMTEKNIKK (PROCESS SYSTEMS ENGINEERING) Coordinator: Sigurd Skogestad Project proposal from Professor Sigurd Skogestad: sigurd.skogestad@ntnu.no 69. Modelling and optimization
S-CO 2 Brayton Recompression Loop Design and Control
 S-CO 2 Brayton Recompression Loop Design and Control 1) Background 2) Recommended Design Features 3) Modeling Strategy IST Model Changes Transient Results Prepared by: Mike Hexemer Advanced Concepts Knolls
S-CO 2 Brayton Recompression Loop Design and Control 1) Background 2) Recommended Design Features 3) Modeling Strategy IST Model Changes Transient Results Prepared by: Mike Hexemer Advanced Concepts Knolls
Simulation and Economic Optimization of Vapor Recompression Configuration for Partial CO2 capture
 Simulation and Economic Optimization of Vapor Recompression Configuration for Partial CO2 capture Lars Erik Øi 1 Erik Sundbø 1 Hassan Ali 1 1 Department of and Process, Energy and Environmental Technology,
Simulation and Economic Optimization of Vapor Recompression Configuration for Partial CO2 capture Lars Erik Øi 1 Erik Sundbø 1 Hassan Ali 1 1 Department of and Process, Energy and Environmental Technology,
Micro Channel Recuperator for a Reverse Brayton Cycle Cryocooler
 Micro Channel Recuperator for a Reverse Brayton Cycle Cryocooler C. Becnel, J. Lagrone, and K. Kelly Mezzo Technologies Baton Rouge, LA USA 70806 ABSTRACT The Missile Defense Agency has supported a research
Micro Channel Recuperator for a Reverse Brayton Cycle Cryocooler C. Becnel, J. Lagrone, and K. Kelly Mezzo Technologies Baton Rouge, LA USA 70806 ABSTRACT The Missile Defense Agency has supported a research
Improving distillation tower operation
 Improving distillation tower operation Measuring differential pressure across long sections of distillation columns has always been challenging, but purpose-built sensor systems provide a solution Fast
Improving distillation tower operation Measuring differential pressure across long sections of distillation columns has always been challenging, but purpose-built sensor systems provide a solution Fast
Experimental Analysis on Vortex Tube Refrigerator Using Different Conical Valve Angles
 International Journal of Engineering Research and Development e-issn: 7-067X, p-issn: 7-00X, www.ijerd.com Volume 3, Issue 4 (August ), PP. 33-39 Experimental Analysis on Vortex Tube Refrigerator Using
International Journal of Engineering Research and Development e-issn: 7-067X, p-issn: 7-00X, www.ijerd.com Volume 3, Issue 4 (August ), PP. 33-39 Experimental Analysis on Vortex Tube Refrigerator Using
UNIVERSITY OF WATERLOO
 UNIVERSITY OF WATERLOO Department of Chemical Engineering ChE 524 Process Control Laboratory Instruction Manual January, 2001 Revised: May, 2009 1 Experiment # 2 - Double Pipe Heat Exchanger Experimental
UNIVERSITY OF WATERLOO Department of Chemical Engineering ChE 524 Process Control Laboratory Instruction Manual January, 2001 Revised: May, 2009 1 Experiment # 2 - Double Pipe Heat Exchanger Experimental
A Depletion Compensated Wet Bath Simulator For Calibrating Evidential Breath Alcohol Analyzers
 A Depletion Compensated Wet Bath Simulator For Calibrating Evidential Breath Alcohol Analyzers Slemeyer, A. University of Applied Sciences of Giessen, Dept. of Electrical Engineering 1 Wiesenstr. 14, D-35390
A Depletion Compensated Wet Bath Simulator For Calibrating Evidential Breath Alcohol Analyzers Slemeyer, A. University of Applied Sciences of Giessen, Dept. of Electrical Engineering 1 Wiesenstr. 14, D-35390
1. Study the performance of a binary distillation column operated in batch mode.
 Goals for batch distillation using the East distillation column: 1. Study the performance of a binary distillation column operated in batch mode. 2. Determine the overall and local efficiency of the column
Goals for batch distillation using the East distillation column: 1. Study the performance of a binary distillation column operated in batch mode. 2. Determine the overall and local efficiency of the column
Chapter 13 Gases, Vapors, Liquids, and Solids
 Chapter 13 Gases, Vapors, Liquids, and Solids Property is meaning any measurable characteristic of a substance, such as pressure, volume, or temperature, or a characteristic that can be calculated or deduced,
Chapter 13 Gases, Vapors, Liquids, and Solids Property is meaning any measurable characteristic of a substance, such as pressure, volume, or temperature, or a characteristic that can be calculated or deduced,
International Journal of Technical Research and Applications e-issn: , Volume 4, Issue 3 (May-June, 2016), PP.
 DESIGN AND ANALYSIS OF FEED CHECK VALVE AS CONTROL VALVE USING CFD SOFTWARE R.Nikhil M.Tech Student Industrial & Production Engineering National Institute of Engineering Mysuru, Karnataka, India -570008
DESIGN AND ANALYSIS OF FEED CHECK VALVE AS CONTROL VALVE USING CFD SOFTWARE R.Nikhil M.Tech Student Industrial & Production Engineering National Institute of Engineering Mysuru, Karnataka, India -570008
Chapter 10 Gases. Characteristics of Gases. Pressure. The Gas Laws. The Ideal-Gas Equation. Applications of the Ideal-Gas Equation
 Characteristics of Gases Chapter 10 Gases Pressure The Gas Laws The Ideal-Gas Equation Applications of the Ideal-Gas Equation Gas mixtures and partial pressures Kinetic-Molecular Theory Real Gases: Deviations
Characteristics of Gases Chapter 10 Gases Pressure The Gas Laws The Ideal-Gas Equation Applications of the Ideal-Gas Equation Gas mixtures and partial pressures Kinetic-Molecular Theory Real Gases: Deviations
Enhancing Thermodynamic Efficiency of Energy Intensive Distillation Columns via Internal Heat Integration *
 . OLUJIÆ et al., Enhancing Thermodynamic Efficiency of Energy Intensive, Chem. Biochem. Eng. Q. 22 (4) 383 392 (2008) 383 Enhancing Thermodynamic Efficiency of Energy Intensive Distillation Columns via
. OLUJIÆ et al., Enhancing Thermodynamic Efficiency of Energy Intensive, Chem. Biochem. Eng. Q. 22 (4) 383 392 (2008) 383 Enhancing Thermodynamic Efficiency of Energy Intensive Distillation Columns via
THE WAY THE VENTURI AND ORIFICES WORK
 Manual M000 rev0 03/00 THE WAY THE VENTURI AND ORIFICES WORK CHAPTER All industrial combustion systems are made up of 3 main parts: ) The mixer which mixes fuel gas with combustion air in the correct ratio
Manual M000 rev0 03/00 THE WAY THE VENTURI AND ORIFICES WORK CHAPTER All industrial combustion systems are made up of 3 main parts: ) The mixer which mixes fuel gas with combustion air in the correct ratio
Level Process Control. Penn State Chemical Engineering
 Level Process Control Penn State Chemical Engineering Revised Spring 2015 1 Table of Contents LEARNING OBJECTIVES... 3 EXPERIMENTAL OBJECTIVES AND OVERVIEW... 3 Pre-lab study... 3 Experiments in the lab...
Level Process Control Penn State Chemical Engineering Revised Spring 2015 1 Table of Contents LEARNING OBJECTIVES... 3 EXPERIMENTAL OBJECTIVES AND OVERVIEW... 3 Pre-lab study... 3 Experiments in the lab...
Pigging as a Flow Assurance Solution Avoiding Slug Catcher Overflow
 Pigging as a Flow Assurance Solution Avoiding Slug Catcher Overflow Aidan O'Donoghue, Pipeline Research Limited, Glasgow, UK This paper sets out to provide an initial method of assessing the bypass requirements
Pigging as a Flow Assurance Solution Avoiding Slug Catcher Overflow Aidan O'Donoghue, Pipeline Research Limited, Glasgow, UK This paper sets out to provide an initial method of assessing the bypass requirements
Chem 110 General Principles of Chemistry
 CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
Aspen HYSYS. Dynamic Modeling Guide
 Aspen HYSYS Dynamic Modeling Guide Version Number: V7.3 March 2011 Copyright (c) 1981-2011 by Aspen Technology, Inc. All rights reserved. Aspen HYSYS and the aspen leaf logo are trademarks or registered
Aspen HYSYS Dynamic Modeling Guide Version Number: V7.3 March 2011 Copyright (c) 1981-2011 by Aspen Technology, Inc. All rights reserved. Aspen HYSYS and the aspen leaf logo are trademarks or registered
An innovative technology for Coriolis metering under entrained gas conditions
 An innovative technology for Coriolis metering under entrained gas conditions Coriolis mass flowmeters are usually only used for single-phase fluids, i.e. either liquids or gases, since it has been found
An innovative technology for Coriolis metering under entrained gas conditions Coriolis mass flowmeters are usually only used for single-phase fluids, i.e. either liquids or gases, since it has been found
Tutorial. BOSfluids. Relief valve
 Tutorial Relief valve The Relief valve tutorial describes the theory and modeling process of a pressure relief valve or safety valve. It covers the algorithm BOSfluids uses to model the valve and a worked
Tutorial Relief valve The Relief valve tutorial describes the theory and modeling process of a pressure relief valve or safety valve. It covers the algorithm BOSfluids uses to model the valve and a worked
Pressure Control. where: p is the pressure F is the normal component of the force A is the area
 Pressure Control First of all, what is pressure, the property we want to control? From Wikipedia, the free encyclopedia. Pressure is the application of force to a surface, and the concentration of that
Pressure Control First of all, what is pressure, the property we want to control? From Wikipedia, the free encyclopedia. Pressure is the application of force to a surface, and the concentration of that
Optimal Operation of Dividing Wall Column using Enhanced Active Vapor Distributor
 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 69, 2018 Guest Editors: Elisabetta Brunazzi, Eva Sorensen Copyright 2018, AIDIC Servizi S.r.l. ISBN 978-88-95608-66-2; ISSN 2283-9216 The Italian
A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 69, 2018 Guest Editors: Elisabetta Brunazzi, Eva Sorensen Copyright 2018, AIDIC Servizi S.r.l. ISBN 978-88-95608-66-2; ISSN 2283-9216 The Italian
Constant pressure air charging cascade control system based on fuzzy PID controller. Jun Zhao & Zheng Zhang
 International Conference on Applied Science and Engineering Innovation (ASEI 2015) Constant pressure air charging cascade control system based on fuzzy PID controller Jun Zhao & Zheng Zhang College of
International Conference on Applied Science and Engineering Innovation (ASEI 2015) Constant pressure air charging cascade control system based on fuzzy PID controller Jun Zhao & Zheng Zhang College of
The Discussion of this exercise covers the following points:
 Exercise 3-2 Orifice Plates EXERCISE OBJECTIVE In this exercise, you will study how differential pressure flowmeters operate. You will describe the relationship between the flow rate and the pressure drop
Exercise 3-2 Orifice Plates EXERCISE OBJECTIVE In this exercise, you will study how differential pressure flowmeters operate. You will describe the relationship between the flow rate and the pressure drop
14 April 2016 Dr. John Hedengren 350 CB Brigham Young University Provo, UT 84606
 14 April 2016 Dr. John Hedengren 350 CB Brigham Young University Provo, UT 84606 Dr. Hedengren: Compressors in gas pipelines are designed to maintain pressure and flow despite many flow disturbances that
14 April 2016 Dr. John Hedengren 350 CB Brigham Young University Provo, UT 84606 Dr. Hedengren: Compressors in gas pipelines are designed to maintain pressure and flow despite many flow disturbances that
CVEN 311 Fluid Dynamics Fall Semester 2011 Dr. Kelly Brumbelow, Texas A&M University. Final Exam
 CVEN 311 Fluid Dynamics Fall Semester 2011 Dr. Kelly Brumbelow, Texas A&M University Final Exam 8 pages, front & back, not including reference sheets; 21 questions An excerpt from the NCEES Fundamentals
CVEN 311 Fluid Dynamics Fall Semester 2011 Dr. Kelly Brumbelow, Texas A&M University Final Exam 8 pages, front & back, not including reference sheets; 21 questions An excerpt from the NCEES Fundamentals
Application Block Library Fan Control Optimization
 Application Block Library Fan Control Optimization About This Document This document gives general description and guidelines for wide range fan operation optimisation. Optimisation of the fan operation
Application Block Library Fan Control Optimization About This Document This document gives general description and guidelines for wide range fan operation optimisation. Optimisation of the fan operation
Title: Choosing the right nitrogen rejection scheme
 Title: Choosing the right nitrogen rejection scheme Authors: Nicolas Chantant, Paul Terrien, Sylvain Gérard (Air Liquide Global E&C Solutions) Abstract: Nitrogen Rejection Units (NRU) are used to extract
Title: Choosing the right nitrogen rejection scheme Authors: Nicolas Chantant, Paul Terrien, Sylvain Gérard (Air Liquide Global E&C Solutions) Abstract: Nitrogen Rejection Units (NRU) are used to extract
Paper 2.2. Operation of Ultrasonic Flow Meters at Conditions Different Than Their Calibration
 Paper 2.2 Operation of Ultrasonic Flow Meters at Conditions Different Than Their Calibration Mr William Freund, Daniel Measurement and Control Mr Klaus Zanker, Daniel Measurement and Control Mr Dale Goodson,
Paper 2.2 Operation of Ultrasonic Flow Meters at Conditions Different Than Their Calibration Mr William Freund, Daniel Measurement and Control Mr Klaus Zanker, Daniel Measurement and Control Mr Dale Goodson,
1 PIPESYS Application
 PIPESYS Application 1-1 1 PIPESYS Application 1.1 Gas Condensate Gathering System In this PIPESYS Application, the performance of a small gascondensate gathering system is modelled. Figure 1.1 shows the
PIPESYS Application 1-1 1 PIPESYS Application 1.1 Gas Condensate Gathering System In this PIPESYS Application, the performance of a small gascondensate gathering system is modelled. Figure 1.1 shows the
Vibration-Free Joule-Thomson Cryocoolers for Distributed Microcooling
 Vibration-Free Joule-Thomson Cryocoolers for Distributed Microcooling W. Chen, M. Zagarola Creare Inc. Hanover, NH, USA ABSTRACT This paper reports on an innovative concept for a space-borne Joule-Thomson
Vibration-Free Joule-Thomson Cryocoolers for Distributed Microcooling W. Chen, M. Zagarola Creare Inc. Hanover, NH, USA ABSTRACT This paper reports on an innovative concept for a space-borne Joule-Thomson
INSTRUMENTS A THERMAL MASS FLOW SENSOR USING A CONSTANT DIFFERENTIAL TEMPERATURE ABOVE THE AMBIENT GAS TEMPERATURE
 TELEDYNE HASTINGS TECHNICAL PAPERS INSTRUMENTS A THERMAL MASS FLOW SENSOR USING A CONSTANT DIFFERENTIAL TEMPERATURE ABOVE THE AMBIENT GAS TEMPERATURE Proceedings of FEDSM 98 1998 ASME Fluids Engineering
TELEDYNE HASTINGS TECHNICAL PAPERS INSTRUMENTS A THERMAL MASS FLOW SENSOR USING A CONSTANT DIFFERENTIAL TEMPERATURE ABOVE THE AMBIENT GAS TEMPERATURE Proceedings of FEDSM 98 1998 ASME Fluids Engineering
Influence of real phase equilibria on the sizing of pressure relief devices
 Influence of real phase equilibria on the sizing of pressure relief devices ACHEMA CONGRESS 2015 15 th June 2015, Frankfurt am Main Stephan Dreisch, Frank Westphal, Monika Christ Motivation / Facts to
Influence of real phase equilibria on the sizing of pressure relief devices ACHEMA CONGRESS 2015 15 th June 2015, Frankfurt am Main Stephan Dreisch, Frank Westphal, Monika Christ Motivation / Facts to
NEW PROGRAM! OIL AND GAS TECHNOLOGY PROGRAM
 NEW PROGRAM! PROGRAM Mechanical Maintenance MODEL H-IRT-1 Industrial Refrigeration Trainer MODEL H-RIG-1C Rigging Systems Trainer MODEL H-IMTS-1 Industrial Maintenance Training System MODEL H-FP-223-14
NEW PROGRAM! PROGRAM Mechanical Maintenance MODEL H-IRT-1 Industrial Refrigeration Trainer MODEL H-RIG-1C Rigging Systems Trainer MODEL H-IMTS-1 Industrial Maintenance Training System MODEL H-FP-223-14
Depropaniser Train VE-107
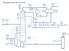 Depropaniser Train 100 -VE-107 PI 1014 PDC HC 1015 Flare 1 48 B = C2 D = ic4 -HA-103 A/B -VA-102 1011 40 39 Cooling water 35 1001 1017 34 33 21 Bottoms from deetaniser 1012 20 18 17 PD 1013 Normally 0
Depropaniser Train 100 -VE-107 PI 1014 PDC HC 1015 Flare 1 48 B = C2 D = ic4 -HA-103 A/B -VA-102 1011 40 39 Cooling water 35 1001 1017 34 33 21 Bottoms from deetaniser 1012 20 18 17 PD 1013 Normally 0
Lab 1c Isentropic Blow-down Process and Discharge Coefficient
 058:080 Experimental Engineering Lab 1c Isentropic Blow-down Process and Discharge Coefficient OBJECTIVES - To study the transient discharge of a rigid pressurized tank; To determine the discharge coefficients
058:080 Experimental Engineering Lab 1c Isentropic Blow-down Process and Discharge Coefficient OBJECTIVES - To study the transient discharge of a rigid pressurized tank; To determine the discharge coefficients
Experimental Verification of Integrated Pressure Suppression Systems in Fusion Reactors at In-Vessel Loss-of -Coolant Events
 Experimental Verification of Integrated Pressure Suppression Systems in Fusion Reactors at In-Vessel Loss-of -Coolant Events K. Takase 1), H. Akimoto 1) 1) Japan Atomic Energy Research Institute (JAERI),
Experimental Verification of Integrated Pressure Suppression Systems in Fusion Reactors at In-Vessel Loss-of -Coolant Events K. Takase 1), H. Akimoto 1) 1) Japan Atomic Energy Research Institute (JAERI),
DISTILLATION CONTROL
 DISTILLATION CONTROL DISTILLATION CONTROL An Engineering Perspective CECIL L. SMITH A JOHN WILEY & SONS, INC., PUBLICATION Copyright 2012 by John Wiley & Sons, Inc. All rights reserved Published by John
DISTILLATION CONTROL DISTILLATION CONTROL An Engineering Perspective CECIL L. SMITH A JOHN WILEY & SONS, INC., PUBLICATION Copyright 2012 by John Wiley & Sons, Inc. All rights reserved Published by John
The capacity of the cargo tank venting system (46 CFR );
 INDUSTRY GUIDELINES FOR DETERMINING THE MAXIMUM LIQUID TRANSFER RATE FOR A TANK VESSEL TRANSFERRING A FLAMMABLE OR COMBUSTIBLE CARGO USING A VAPOR CONTROL SYSTEM This document provides guidance from the
INDUSTRY GUIDELINES FOR DETERMINING THE MAXIMUM LIQUID TRANSFER RATE FOR A TANK VESSEL TRANSFERRING A FLAMMABLE OR COMBUSTIBLE CARGO USING A VAPOR CONTROL SYSTEM This document provides guidance from the
Predicted Dispense Volume vs. Gravimetric Measurement for the MICROLAB 600. November 2010
 Predicted Dispense Volume vs. Gravimetric Measurement for the MICROLAB 600 November 2010 Table of Contents ``Abstract...3 ``Introduction...4 ``Methods & Results...6 ``Data Analysis...9 ``Conclusion...12
Predicted Dispense Volume vs. Gravimetric Measurement for the MICROLAB 600 November 2010 Table of Contents ``Abstract...3 ``Introduction...4 ``Methods & Results...6 ``Data Analysis...9 ``Conclusion...12
How to Combat Process Disturbances and Interactions
 How to Combat Process Disturbances and Interactions Henri A. (Hank) Brittain, Ph.D., Jean-François Dubé, P.Eng. BBA - Top Control Inc. KEYWORDS Decoupling, First-Order Plus DeadTime (FOPDT) Feedforward,
How to Combat Process Disturbances and Interactions Henri A. (Hank) Brittain, Ph.D., Jean-François Dubé, P.Eng. BBA - Top Control Inc. KEYWORDS Decoupling, First-Order Plus DeadTime (FOPDT) Feedforward,
Introductory Lab: Vacuum Methods
 Introductory Lab: Vacuum Methods Experiments in Modern Physics (P451) In this lab you will become familiar with the various components of the lab vacuum system. There are many books on this topic one of
Introductory Lab: Vacuum Methods Experiments in Modern Physics (P451) In this lab you will become familiar with the various components of the lab vacuum system. There are many books on this topic one of
1.2 Example 1: A simple hydraulic system
 Note: It is possible to use more than one fluid in the Hydraulic library. This is important because you can model combined cooling and lubrication systems of a library. The hydraulic library assumes a
Note: It is possible to use more than one fluid in the Hydraulic library. This is important because you can model combined cooling and lubrication systems of a library. The hydraulic library assumes a
Separation of Acetone-Water with Aspen HYSYS V8.0
 Separation of Acetone-Water with Aspen HYSYS V8.0 Liquid-Liquid Extraction with 3-Methylhexane as the Solvent 1. Lesson Objectives Learn how to build an extraction and solvent recovery flowsheet. Learn
Separation of Acetone-Water with Aspen HYSYS V8.0 Liquid-Liquid Extraction with 3-Methylhexane as the Solvent 1. Lesson Objectives Learn how to build an extraction and solvent recovery flowsheet. Learn
CONTROL VALVE WHAT YOU NEED TO LEARN?
 CONTROL VALVE WHAT YOU NEED TO LEARN? i) The control valve characteristics refers to the relationship between the volumetric flowrate F (Y-axis) through the valve AND the valve travel or opening position
CONTROL VALVE WHAT YOU NEED TO LEARN? i) The control valve characteristics refers to the relationship between the volumetric flowrate F (Y-axis) through the valve AND the valve travel or opening position
Experiment Instructions. Circulating Pumps Training Panel
 Experiment Instructions Circulating Pumps Training Panel Experiment Instructions This manual must be kept by the unit. Before operating the unit: - Read this manual. - All participants must be instructed
Experiment Instructions Circulating Pumps Training Panel Experiment Instructions This manual must be kept by the unit. Before operating the unit: - Read this manual. - All participants must be instructed
Heat Pump Connections and Interior Piping
 Job Sheet 3 Heat Pump Connections and Interior Piping OBJECTIVES In this job sheet, you will observe how the presence of air in the ground loop affects the geothermal heat pump performance. You will also
Job Sheet 3 Heat Pump Connections and Interior Piping OBJECTIVES In this job sheet, you will observe how the presence of air in the ground loop affects the geothermal heat pump performance. You will also
PHYS 101 Previous Exam Problems
 PHYS 101 Previous Exam Problems CHAPTER 14 Fluids Fluids at rest pressure vs. depth Pascal s principle Archimedes s principle Buoynat forces Fluids in motion: Continuity & Bernoulli equations 1. How deep
PHYS 101 Previous Exam Problems CHAPTER 14 Fluids Fluids at rest pressure vs. depth Pascal s principle Archimedes s principle Buoynat forces Fluids in motion: Continuity & Bernoulli equations 1. How deep
CTB3365x Introduction to Water Treatment
 CTB3365x Introduction to Water Treatment D4b Aeration Doris van Halem Did you know that there are not just gasses in your glass of sparkling coke, but also in the tap water you drink? Welcome to the water
CTB3365x Introduction to Water Treatment D4b Aeration Doris van Halem Did you know that there are not just gasses in your glass of sparkling coke, but also in the tap water you drink? Welcome to the water
PETROLEUM & GAS PROCESSING TECHNOLOGY (PTT 365) SEPARATION OF PRODUCED FLUID
 PETROLEUM & GAS PROCESSING TECHNOLOGY (PTT 365) SEPARATION OF PRODUCED FLUID Miss Nur Izzati Bte Iberahim Introduction Well effluents flowing from producing wells come out in two phases: vapor and liquid
PETROLEUM & GAS PROCESSING TECHNOLOGY (PTT 365) SEPARATION OF PRODUCED FLUID Miss Nur Izzati Bte Iberahim Introduction Well effluents flowing from producing wells come out in two phases: vapor and liquid
Workshop 302-compressor-anti-surge
 Workshop Objectives Workshop 302-compressor-anti-surge Illustrate how to create a simple anti-surge control on a compressor Workshop Description Flowsheeet: A feed stream at 1 bar with methane, ethane
Workshop Objectives Workshop 302-compressor-anti-surge Illustrate how to create a simple anti-surge control on a compressor Workshop Description Flowsheeet: A feed stream at 1 bar with methane, ethane
