Consort MANUAL E4100 E4200 E4300 E4400 E4500
|
|
|
- Rebecca Marsh
- 5 years ago
- Views:
Transcription
1 Consort MANUAL E4100 E4200 E4300 E4400 E4500 November 2011
2 Table of contents Warranty... 1 Safety precautions... 2 General care and maintenance... 3 Environmental conditions... 3 Fitting electrode cables... 4 Vertical gel electrophoresis... 4 Gel preparation... 6 Gel selection... 6 Gel pouring... 8 Gel running Solutions for SDS PAGE gels D electrophoresis Electroblotting Buffer solutions for blotting Sequencing Reagent preparation and gel volumes Assembling the unit Preparation of samples for loading Gel running and ending Solutions for DNA sequencing Denaturing gradient system... 26
3 CONSORT guarantees that the unit you have received has been thoroughly tested and meets its published specification. This unit (excluding all accessories) is warranted against defective material and workmanship for a period of twelve (12) months from the date of shipment ex factory. CONSORT will repair all defective equipment returned during the warranty period without charge, provided the equipment has been used under normal laboratory conditions and in accordance with the operating limitations and maintenance procedures outlined in this instruction manual and when not having been subject to accident, alteration, misuse or abuse. No liability is accepted for loss or damage arising from the incorrect use of this unit. CONSORT s liability is limited to the repair or replacement of the unit or refund of the purchase price, at CONSORT s option. CONSORT is not liable for any consequential damages. CONSORT reserves the right to alter the specification of its products without prior notice. This will enable us to implement developments as soon as they arise. CONSORT products are for research use only. A return authorisation must be obtained from CONSORT before returning any product for warranty repair on a freight prepaid basis. Warranty E4xxx 1
4 Safety precautions When used correctly, these units pose no health risk. However, these units can deliver dangerous levels of electricity and are to be operated only by qualified personnel following the guidelines laid out in this manual. Anyone intending to use this equipment should read the complete manual thoroughly. The unit must never be used without the safety lid correctly in position. The unit should not be used if there is any sign of damage to the external tank or lid. Always isolate electrophoresis units from their power supply before removing the safety cover. Isolate the power supply from the mains first then disconnect the leads. Do not exceed the maximum operating voltage or current. Do not operate the electrophoresis units in metal trays. Acrylamide is a volatile, cumulative neurotoxin and suspected carcinogen. Wear effective protective clothing and follow recommended handling and disposal procedures. Polymerised gels contain some unpolymerised monomer. Handle with gloves only. Following the replacement of a platinum electrode have the unit inspected and approved by your safety officer prior to use. Do not fill the unit with running buffer above the maximum fill lines. Do not move the unit when it is running. Caution: during electrophoresis very low quantities of various gases are produced at the electrodes. The type of gas produced depends on the composition of the buffer employed. To disperse these gases make sure that the apparatus is run in a well ventilated area. 2 E4xxx
5 This apparatus is intended for indoor use only. General care and maintenance Units are best cleaned using warm water and a mild detergent. Water at temperatures above 60 C can cause damage to the unit and components. The tank should be thoroughly rinsed with warm water or distilled water to prevent build up of salts but care should be taken not to damage the enclosed electrode and vigorous cleaning is not necessary or advised. Air drying is preferably before use. The units should only be cleaned with the following: warm water with a mild concentration of soap or other mild detergent (compatible detergents include dish washing liquid, hexane and aliphatic hydrocarbons). The units should not be left to in detergents for more than 30 minutes. The units should never come into contact with the following cleaning agents, these will cause irreversible and accumulative damage: acetone, phenol, chloroform, carbon tetrachloride, methanol, ethanol, isopropyl alcohol alkalis. In case of Rnase Decontamination clean the units with a mild detergent as described above. Wash with 3% hydrogen peroxide (H 2 O 2 ) for 10 minutes. Rinsed with 0.1% DEPC- (diethyl pyrocarbonate) treated distilled water (Caution: DEPC is a suspected carcinogen. Always take the necessary precautions when using.) RNaseZAP (Ambion) can also be used. Please consult the instructions for use with acrylic gel tanks. Environmental conditions This apparatus can be operated safely at an altitude up to 2000 m. The normal operating temperature range is between 4 C and 65 C. Maximum relative humidity 80 % for temperatures up to 31 C decreasing linearly to 50 % relative humidity at 40 C. The apparatus is rated Pollution Degree 2 in accordance with IEC 664. Pollution Degree 2 states that: Normally only non-conductive pollution occurs. Occasionally, however, a temporary conductivity caused by conden sation must be expected. E4xxx 3
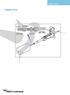
6 Fitting electrode cables 1. Note the position of the lid on the unit. This shows the correct polarity and the correct orientation of the cables, black is negative and red positive. 2. Remove the lid from the unit. Note if the lid is not removed, fitting the cables may result in un-tightening of the gold plug and damage to the electrode. 3. Screw the cables into the tapped holes as fully as possible so that there is no gap between the lid and the leading edge of the cable fitting. 4. Refit the lid. Vertical gel electrophoresis 1. Clean a set of glass plates for each gel first with distilled water and then with 70 % ethanol. One set of glass plates constitutes one notched glass plate and one plain glass plate with bonded spacers. When using a triple glass plate sandwich, two notched glass plates are required, one set of free spacers and a set of plain glass plates with bonded spacers. The plain glass plate is positioned outermost, then a notched glass plate, free spacers and second notched glass plate. Alternatively, accessory notched glass plates with bonded spacers are available. All glass plates, modules and casting base accessories must be completely dry during set up. Wet components are more likely to miss-align and cause leaks. 2. Assemble the glass plates so that the bottom of the glass plates and the spacers are perfectly aligned. For triple plate sandwiches, the free spacers need to be perfectly aligned which is best performed using a small spacer or comb to push the spacers apart. Notched glass plates with bonded spacers do not need manual alignment. Note: the glass plates with bonded spacers have an arrow in the top of the spacers which are slightly longer than the glass plate to indicate the top. 3. The slab gel insert contains pressure bars which impart even pressure onto the glass plates and allow even screw pressure transfer onto the sealing edge of the glass plate, ensuring complete sealing. Ensure that the pressure bars are adequately open for the thickness of spacer used. The bar can be opened by loosening the screws or by sliding the clamps. When using a triple glass plate sandwich, the pressure bars will need to be in the completely open position. 4 E4xxx
7 4. Position the slab gel insert on a flat surface. Do not at this stage insert the slab gel insert into the casting base. 5. Insert the glass plates into the Slab Gel Insert between the pressure bar and the blue gasket and fully tighten the pressure bar screws in the order top then bottom. Fully tighten the screw for the mini vertical and the screws sequentially and in an even manner for the maxi vertical in the order middle two, top then bottom, making sure not to wobble the unit. When only one gel is being run, the dummy plate must be used in the second position and fully tightened. At this stage, check that the bottom edges of the spacers and glass plates are perfectly aligned. 6. Position the slab gel insert in the casting base such that the cam pins have handles pointing downwards and are located in the insert holes. The top of the running module may need to be pushed down very slightly to locate the cam pins. With the cam pin handles facing directly downwards, turn the cam pins fully through 180 or until the insert has tightened onto the silicone mat. It is best to turn the cams in opposite directions to each other. Do not overturn as this will cause the glass plates to push upwards and the assembly will be more likely to leak. The unit is now ready for gel preparation and pouring. Note: always reverse the silicone mat after casting to avoid indentations from persisting. Never leave the casting upstand with glass plates tightened into the casting base for long periods of time as this will also cause indentations in the silicone mat. E4xxx 5
8 Gel preparation 1. It is always advisable to work with pre-made stock solutions which allow added convenience and save time when it comes to gel pouring. The protocol below is given for use of the standard stock solutions advised. This should be adjusted if you are using different stock solutions or gel formulas. 2. Table below shows the volume of agarose solution required to make the desired gel (1 mm thick) for each unit size. Adjustments are needed for 0.75, 1.5 or 2 mm spacers. Model Gel size (cm) Volume (ml) E x10 (one gel + dummy plate) x10 (two gels) 15 10x10 (four gels +triple plates) 30 E x10 (one gel+ dummy plate) 17.5 E4300 E x10 (two gels) 35 20x10 (four gels +triple plates) 70 20x20 (one gel+ dummy plate) 35 20x20 (two gels) 70 20x20 (four gels +triple plates) 140 Gel selection 1. Care should be taken when selecting the pore size of the gel to be used. The pore size or % of gel determines the resolving ability given different sizes of protein. See table below which details and which percentage of gel to use to separate the sizes of proteins indicated. Acrylamide percentage Separating resolution 5 % kda 7.5 % kda 10 % kda 12 % kda 15 % kda 17.5 % kda 2. Prepare gel solutions as per tables below. These give the volumes of solutions from the standard stock solutions. These should be gently mixed avoiding generation of bubbles which will inhibit polymerization by removing free radicals. 6 E4xxx
9 Preparation of the separating gel solution for 10x10 cm gels using 1 mm spacers. Solution ml 5 % 7.5 % 10 % 12 % 15 % 17.5 % distilled water % stock acrylamide x resolving Tris % ammonium persulphate Preparation of the separating gel solution for 20x10 cm gels using 1 mm spacers. Solution ml 5 % 7.5 % 10 % 12 % 15 % 17.5 % distilled water % stock acrylamide x resolving Tris % ammonium persulphate Preparation of the separating gel solution for 20x20 cm gels using 1 mm spacers. Solution ml 5 % 7.5 % 10 % 12 % 15 % 17.5 % distilled water % stock acrylamide x resolving Tris % ammonium persulphate E4xxx 7
10 Gel pouring For gels with stacking layers 1. Insert the comb into the glass plates and mark a point on the glass plates 1 cm below where the comb teeth finish. This indicates where to add the resolving gel to. 2. Add 15 µl of TEMED to the resolving gel solution for 10x10 cm sized gels, 35 µl for 10x20 cm sized gels and 70 µl for 20x20 cm sized gels and mix well but avoid generating air bubbles. 3. Fill the glass plates again avoiding generating any air bubbles. Filling must be performed quickly before the TEMED causes the gel to become too viscous. 4. Overlay the gel extremely carefully with 1 ml of Isobutanol, Isopropanol or distilled water. When using distilled water extra care must be taken to ensure there is no mixing with the gel solution. 5. Let the resolving gel polymerize. Usually this takes around 15 minutes but this can vary due to the freshness of the reagents used. If polymerization is taken a lot longer than this, use fresher stock solutions or add more APS and TEMED. 6. Prepare the stacking gel using table below as a guide. Solution ml E4100 E4200 E4300 E4400 distilled water % stock acrylamide x resolving Tris % ammonium persulphate Carefully mix the stacking gel solution, avoiding generating air bubbles. 8. Pour off the overlay liquid and rinse the gel with distilled water. 9. Add 6.7 µl of TEMED to the stacking gel solution for 10x10 cm gels, 13.4 µl for 10x20 cm gels add 26.8 µl for 20x20 cm gels. Mix well. Use a Pasteur pipette to fill the glass plates up to the top with stacking gel solution. 10. Carefully insert the comb making sure that no air bubbles get trapped under the ends of the comb teeth as these will inhibit sample progression. 11. Allow the stacking gel polymerize for 30 minutes. 8 E4xxx
11 For gels without stacking layers 1. Add 15 µl of TEMED to the resolving gel solution for 10x10 cm sized gels, 35 µl for 10x20 cm sized gels and 70 µl for 20x20 cm sized gels and mix well but avoid generating air bubbles. 2. Fill the glass plates again avoiding generating any air bubbles. Filling must be performed quickly before the TEMED causes the gel to become too viscous. 3. Carefully insert the comb making sure that no air bubbles get trapped under the ends of the comb teeth as these will inhibit sample progression. 4. Let the resolving gel polymerize. Usually this takes around 15 minutes but this can vary due to the freshness of the reagents used. If polymerization is taken a lot longer than this, use fresher stock solutions or add more APS and TEMED. Preparation of denaturated protein samples for loading The instructions given below are for denatured samples. For native samples, please consult a laboratory handbook. 1. Prepare the protein samples for loading. The volume of sample depends on the capacity of the wells. 2. Using a 0.5 ml micro-centrifuge tube or other convenient receptacle, combine the protein sample and 4x sample buffer. It is always advisable to use protein markers in one of the end lanes to indicate sizes of bands. These should be prepared according to the manufacturers instructions. 3. Heat the samples in a water bath or heating block for 2 minutes to denature the samples. 4. Centrifuge the samples in a micro-centrifuge for 20 seconds at rpm. The protein samples are now ready to load. E4xxx 9
12 Loading the samples 1. If desired, fit the cooling pack(s) into the end of the tank. These should be pre-frozen and fitted with the longest side positioned sideways with the end(s) of the tank and pressed into the recess. Or these can be fitted down the front of the tank. Never fit these underneath the module in the bottom of the tank as this will prevent the flow of current through the gel and cause slow runs and over-heating. 2. Transfer the Inner gel module containing cast gels into the main tank in the correct orientation as indicated, [+ve] on the module aligned with [+ve] on the tank, [-ve] on the module aligned with [ ve] on the tank. 3. Fill the outer tank with 1 x reservoir buffer solution. Below table shows the volume of buffer required. Buffer volume ml E4100 E4200 E4300 E4400 Minimum Inner tank is filled to above the wells. Outer tank is filled to just flood the bottom of the glass plates. Cooling potential is at a minimum which may affect resolution. Maximum Inner tank is filled to above the wells. Outer tank is filled to the maximum fill line. Cooling is high offering good resolution of samples. Using the cooling packs Inner tank is filled to above the wells. Cooling packs are inserted behind the gels. Outer tank is filled to the maximum fill line. Cooling is at a maximum Load the samples into the wells using a pipette tip taking care not to damage the wells or induce any air bubbles. 5. Fill any unused wells with 1 x sample buffer. 6. It is a good idea to note the orientation and order the samples were loaded in. This can be done by noting which samples were loaded adjacent to each electrode. 10 E4xxx
13 1. Fit the lid and connect to a power supply. 2. Consult below table for details on recommended power supply voltage settings. 3. Turn the power supply off when the loading dye reaches the bottom of the gel, sooner if your proteins are below 4 kda in size. 4. Remove the gel running module, first emptying the inner buffer into the main tank. Buffer can be re-used but this may affect run quality if continued. 5. Unscrew the glass plates and gently pry apart the glass plates. The gel will usually stick to one of the plates and can be removed by first soaking in buffer and then gently lifting with a spatula. 6. The gel is now ready to be stained with Coomassie or silver stain or the proteins in the gel can be transferred to a membrane by electroblotting for specific band identification and further analysis. Recommended voltage and resultant current for 1 mm thick, 12 % gels. E4100 E4200 E4300 E4400 One gel V V ma ma Two gels V V ma ma Three gels V V ma ma Four gels V V ma ma Gel running E4xxx 11
14 Solutions for SDS PAGE gels Stock 30% acrylamide gel solution 30.0 g acrylamide 0.8 g methylene bisacrylamide add distilled water to a final volume of 100 ml Stock 4 x resolving gel Tris (1.5 M Tris.HCl ph 8.8, 0.4 % SDS) to 110 ml distilled water add 36.4 g of Tris base add 8 ml of 10 % SDS adjust ph to 8.8 with 1 M HCl add distilled water to a final volume of 200 ml Stock 4 x stacking Tris (0.5 M Tris.HCl ph 6.8, 0.4 % SDS) to 110 ml distilled water add g of Tris base add 8 ml of 10 % SDS adjust ph to 6.8 with 1 M HCl add distilled water to a final volume of 200 ml Stock 4 x Tris-glycine tank buffer - SDS 36 g Tris base g glycine add distilled water to a final volume of 3000 ml 1 x Tris-glycine tank buffer - SDS 750 ml of 4 x Tris-glycine reservoir buffer - SDS 30 ml of 10 % SDS add distilled water to a final volume of 3000 ml 10 % AP (ammonium persulphate solution) 0.1 g ammonium persulphate 1 ml distilled water TEMED Stock 4 x sample buffer 4 ml glycerol 2 ml 2-mercaptoethanol 1.2 g SDS 5 ml 4 x stacking Tris 0.03 g bromophenol blue aliquot into 1.5 ml microcentrifuge tubes. Store at -20 C. 12 E4xxx
15 Capillary tube gel pouring There are two methods which can be used for tube gel casting. Method-1 details casting by injection, method-2 details casting by capillary action. Method-1: filling by injection 1. Place the appropriate number of capillary tubes into the Tube Gel Running module, inserting these carefully from the top. 2. Seal the bottom ends of the tubes using NescoFilm. 3. Prepare the following solution: 16 ml distilled water (18 ml for native gels) 2.4 ml glycerol 0.9 ml 4-8 resolyte or other commercially available 40 % ampholyte solution 3.8 ml acrylamide/bis solution 15 µl TEMED 16.2 g urea (omit for native gels) 0.6 ml NP40 (omit for native gels) This will be enough to pour twenty 80 mm capillary tubes or ten 170 mm capillary tubes. This solution should be de-gassed prior to pouring. When ready to pour, add 120 µl of 10 % w/v ammonium persulphate solution. 4. Using a Hamilton or similar syringe, insert the needle into the tube and carefully inject the solution so that the tubes fill from the bottom. Keep filling to 1 cm of the length of the tubes. The tubes can be gently tapped to get rid of air bubbles. 5. Fill the remaining 1 cm gap with water saturated isobutanol. 6. Leave to fully polymerise, which will normally take hours. 7. After polymerisation, remove the water-saturated isobutanol. Tube gels can be used immediately or stored wrapped in a damp paper towel and Nescofilm at 4 C. The Nescofilm at the bottom of the tubes must be removed prior to electrophoresis. 2-D electrophoresis E4xxx 13
16 Method-2: filling by capillary action 1. Place the appropriate number of capillary tubes in a suitable outer receptacle such as a 15 ml falcon tube. 2. The amount of acrylamide required depends on the size of the outer receptacle used. The larger the outer receptacle used, the more acrylamide wastage so the following advised volumes may need to be increased. 3. Prepare the following solution: 32 ml distilled water (18 ml for native gels) 4.8 ml glycerol 1.8 ml 4-8 resolyte or other commercially available 40 % ampholyte solution 7.6 ml acrylamide/bis solution 30 µl TEMED 32.4 g urea (omit for native gels) 1.2 ml NP40 (omit for native gels) This will be enough to pour twenty 80 mm capillary tubes or ten 170 mm capillary tubes. This solution should be de-gassed prior to pouring. When ready to pour, add 240 µl of 10 % w/v ammonium persulphate solution. 4. Allow the tubes to equilibrate for a few moments. 5. Check the height of the acrylamide in the tubes. If the tubes are full so that there is less than a 1 cm non-filled space at the top, remove some of the acrylamide solution from the beaker until the height is 1 cm from the top. If there is a greater than 1 cm space at the top, add more acrylamide solution, so that the solution rises in the tubes until there is a 1 cm space at the top. 6. When the solution has reached to within 1 cm of the top of the tube, stop adding the acrylamide solution. 7. Fill the remaining 1 cm gap with water saturated isobutanol. 8. Leave to fully polymerise, which will normally take hours. 9. After polymerisation, remove the water-saturated isobutanol. Tube gels can be used immediately or stored wrapped in a damp paper towel and Nescofilm at 4 C. The Nescofilm at the bottom of the tubes must be removed prior to electrophoresis. 10. The tubes may contain a residual of acrylamide on the outside and may need cleaning with distilled water before insertion into the tube gel insert. 14 E4xxx
17 1st dimension (IEF) phase tube gel running Buffer and run conditions will vary according to the type of ampholyte used. The following conditions are given as guidelines only and apply when 4-8 Resolyte is the ampholyte used. Other Ampholytes will require different buffer solutions. Please consult manufacturer s instructions. 1. Prepare ca. 500 ml of 10 mm H 3 PO 4 Anode Buffer (1 litre for E4200, 2 litres for E4300) and use this to fill the bottom chamber of the unit so that the bottoms of the capillary tubes are submerged. If less than 10 capillary tubes are to be run, block up the unused tube slots in the internal running module with the blanking plugs provided. For high resolution separations, we recommend filling the lower chamber completely with buffer and using a pre-frozen cooling pack(s). 2. Place the internal running module into the unit and fill the upper buffer reservoir with ±100 ml of 20 mm NaOH cathode buffer (200 ml for E4200, 400 ml for E4300) so that the tops of the capillary tubes are submerged. 3. For the prefocus, load the gels with 10 µl of 1 % ampholyte solution and run for 15 minutes at 200 V, then for 30 minutes at 300 V and then finally 30 minutes at 400 V. The prefocus stage is recommended as it helps set up the ph gradient. 4. Load the tubes with the samples. These should be dissolved in 1 % ampholyte with 20 % glycerol. 5. Replace the safety lid firmly making sure that the electrical connectors form a good contact. 6. Connect the electrophoresis apparatus to the power pack and connect the power pack to the mains supply. Turn all settings to zero before turning on the mains supply. 7. Run at 400 V for 3 hours and then 800 V for 30 minutes. These conditions are for 8 cm tubes. 17 cm tubes need to be run at 400 V for 18 hours and then 800 V for 1 hour. 8. At the end of the run, turn the power supply settings to zero, turn off the mains supply and disconnect the power leads. 9. Remove the internal module and remove the tubes from their slots. The gels can be extracted from the capillary tubes by: inserting a piece of wire with a small plug of cotton wool on the end and using this as a piston to push the gel out. inserting a Gilson tip into the end of the gel and gently squeezing the gel out with air or water. Whichever of these two methods is used, the gels should be handled with care as they are fragile. E4xxx 15
18 2-D size determination phase 1. To prepare the tube gel(s) for the 2-D, size-determining phase, equilibrate them by soaking for 30 minutes in the running buffer to be used for the 2-D phase. 2. Remove the gel(s) from the running buffer pre-soak, and place each lengthways onto the top of a pre-poured slab gel. The slab gel should be cast using a blank or 2-D comb. For details on the casting of slab gels see the previous pages in this manual. 3. Hold the tube gel in place by pouring over it a low % agarose gel containing the tracker dye. 4. Electrophorese as usual for slab gels until the tracker dye has advanced the required distance down the gel. 5. The samples can be visualized using any of the standard staining methods or can be blotted. 16 E4xxx
19 Setting up the blot sandwich 1. Each blot sandwich should be set up as follows: Cassette clamp [-ve] (black) side placed in a tray or other suitable surface. Pre-soaked fibre pad (note two can be used with thin gels). Two pieces of thick filter paper, about mm thick, presoaked in buffer. Gel. Transfer membrane. Usually this requires pre-soaking but consult the manufacturers instructions for the type of membrane you are using. This should be smoothed so that no air bubbles have been trapped. Two pieces of thick filter paper, about mm thick, presoaked in buffer. Pre-soaked fibre pad (note two can be used with thin gels). Cassette clamp [+ve] (red) side slotted into the groove in the bottom of the black cassette. 2. Close the hinge carefully so as to not disturb the sandwich. 3. Fill the tank with buffer solution up to the maximum fill line indicated on the side of each unit. Improved transfer can usually be obtained by using chilled buffer. Electroblotting Buffer volume ml E4100 E4200 E4300 One cassette Two cassettes Three cassettes Four cassettes Each cooling pack will take the place of 100 ml of buffer for E4100 and 500 ml of buffer for E4200 and E4300. E4xxx 17
20 Blot run conditions 1. Insert the cassettes into the slots in the module with the black side of each adjacent to the negative electrode. It is a good idea to note the orientation and order the blot sandwiches were loaded in. This can be done by noting which samples were loaded adjacent to each electrode. 2. Use of a magnetic stirring bar and plate is recommended to mix the buffer to give consistency of transfer. A 4 mm diameter stirring bar should be placed underneath the module, in the centre of the tank. The cooling pack provided, prefrozen, can be inserted at the side or front of the tank for extended blots. Additional cooling packs can be purchased as accessories to further aid cooling. 3. Insert the module, fit the lid and connect to a power supply. 4. Consult below table for details on recommended power supply voltage settings and blot times. Please note voltage and current will vary according to the amount of cassettes, type and temperature of buffer and thickness and percentage of gel. This will also affect quality of transfer so time course of transfer should be performed for your particular samples and conditions. 5. When the blot time is completed, turn the power supply off. 6. Remove the cassettes from the main tank. Buffer can be reused but this may affect run quality if continued. 7. Lift the hinge of each cassette and gently pry apart the blot sandwich and remove the membrane from the gel. 8. The membrane is now ready to be probed. Duration of blotting E4100 E4200 E4300 One hour 100 V 100 V 400 ma 400 ma Three hours 50 V 50 V 200 ma 200 ma 18 E4xxx
21 Towbin Buffer 25 mm Tris, 192 mm glycine, 20 % methanol ph 8.3 Buffer solutions for blotting Towbin Buffer SDS 25 mm Tris 192 mm glycine 20 % methanol ph % (w/v) SDS Bjerrum and Schafer-Nielsen Buffer 48 mm Tris 39 mm glycine 20 % methanol ph 9.2 Dunn Buffer 10 mm NaHCO 3 3 mm NaCO 3 20 % methanol ph 9.9 Do not adjust the ph when making these buffers as this will cause blot over-heating. The ph will vary according to the freshness of the reagents used. E4xxx 19
22 Sequencing Cleaning and preparation of glass plates 1. Better and more consistent results will be obtained if care is taken to ensure that the glass plates are as clean as possible. New glass plates must be cleaned in the same way as used glass plates because these will contain surface debris that may interfere with the gel. 2. First, clean using a neutral detergent and a small brush. Do not use metal wool or other test tube brushes, abrasive cleaning creams or scourers because these can scratch the surface of the glass plates. 3. The glass plates should be washed in the following sequence: distilled water ethanol, acetone, ethanol, distilled water. Thoroughly rinse and dry the glass plates before use. For extra clean plates, these should be wiped with a microscope tissue soaked in chloroform or dichloroethane in a fume hood. 4. To ease separation of the gel from the glass plates once the gel has been run, it is advisable to siliconise the notched glass plate with a tissue soaked in dimethyldichlorosilane. Wipe the plate, including the ears, in a fume hood. Rinse with water and dry with a tissue. 5. The plain glass plate should be siliconised along the outer 1 cm lengths where the spacers will be positioned. This should be periodically repeated when the gels start to stick to the plates. Plates should then be cleaned and siliconised again. 6. The horizontal gel pouring method described in this manual will not work if both plates are siliconised. In that case, use an alternative gel pouring method or do not siliconise the plain glass plate as described above. 7. The above procedures are not necessary every time a gel is poured. 8. After use, first, clean using a neutral detergent and a small brush and wahs with distilled water, ethanol and acetone as described above.
23 Reagent preparation and gel volumes 1. For consistent gels it is advisable to use high quality reagents and where possible deionisine, degass and filtrate acrylamide gel solutions prior to use. 2. Made up acrylamide solutions should be stored in a refrigerator and allowed to reach room temperature prior to pouring. 3. It is always advisable to work using stock solutions which allow added convenience and save time when it comes to gel pouring. Pages 12 list stock solutions for SDS PAGE gels which should be pre-made beforehand. For DNA sequencing see page 18. For native gel formulae and running conditions, please consult a laboratory manual. 4. As a guide, polymerisation conditions should be adjusted to effect polymerisation within about minutes. Test a small volume in a vial prior to pouring the gel. As a rough guide 100 ml of degassed 6% acrylamide gel will set in about 5 minutes at room temperature when gently mixed with 450 µl of freshly prepared 10% (w/v) ammonium persulphate and 200 µl TEMED. The setting time increases to about 10 minutes if the TEMED volume is reduced to 100 µl and to approximately 15 minutes with 75 µl. The amount of catalysts may need to be reduced under warm conditions. 5. Table below shows the volume of agarose solution required to make the desired gel (1 mm thick) for each unit size. Adjustments are needed for 0.75, 1.5 or 2 mm spacers. Model Gel size (cm) Volume (ml) E x Care should be taken when selecting the pore size of the gel to be used. The pore size or % of gel determines the resolving ability given different sizes of protein. See table below which details and which percentage of gel to use to separate the sizes of proteins indicated. Acrylamide percentage Separating resolution 5 % kda 7.5 % kda 10 % kda 12 % kda 15 % kda 17.5 % kda
24 For gel thicknesses thicker than 0.35 mm, first securely tape the bottom of the gel with electrical tape then follow the instructions below. 1. Lay the plain glass plate on a flat surface and arrange the spacers perfectly aligned with the edges of the plate. 2. Carefully place the notched glass plate on top of the plain glass plate and clamp the plates together using bulldog clips arranged along the edge of the glass plates, the pressure clamps of these should be in line with the spacers. 3. Fill a syringe with the required gel mix. Be careful not to agitate or to introduce bubbles into the solution. 4. Position the syringe above one edge of the notch in a vertical position. Steadily eject the gel solution along the notched area moving the syringe spout smoothly from one side of the notch to the other. The gel mix should form a continuous pool along the top of the gel space move down between the glass plates. 5. Be careful not to overfill the notched area. Fill gradually the gel solution should be around half the height of the notches on the notched glass plate. Also ensure not to under fill the notched area as air bubbles are more likely to be introduced between the glass plates. The boundary of the gel should migrate as a straight line. To prevent or expel bubbles, the glass plates can be tapped lightly behind the moving gel boundary to prevent any bubble formation. 6. When the gel boundary reaches the bottom of the glass plates, remove all the surplus gel from the notched area with the syringe. This will ensure that the gel mix doesn t drip from the bottom of the glass plates. 7. Insert the comb. If a square well comb is used, insert the teeth making sure no bubbles are trapped. When using a shark s tooth comb, insert the flat face of the comb at a slight angle to prevent bubbles from being trapped. A few drops of gel mix can be added if necessary. 8. Carefully straighten the shark s tooth comb so that it is parallel to the top of the gel plate and reaches 3-5mm below the notched area. 9. For low percentage and DNA sequencing gels, leave to polymerise completely for at least 90 minutes. Low percentage gels can be left to polymerise overnight. To prevent the ends of the gel from drying out use wet tissues under a nesco film seal.
25 1. Insert the lower buffer chamber into position. 2. Remove the bulldog clips and the bottom tape, if used, from the glass plates. 3. Insert the glass plates behind the clamping bars and tighten the screws. Do not over-tighten the gel plate clamping screws as this may lead to the glass plate breakage and will also make the insertion and removal of combs difficult. 4. Attach the leads to the buffer chambers. 5. Ensure that the buffer drainage tap is in the closed position. 6. Into the upper buffer chamber pour between 400 ml min. and 1000 ml max. of electrophoresis running buffer. 7. Into the bottom buffer chamber pour between 400 ml min. and 1000 ml max. of electrophoresis running buffer. Important: do not fill over the maximum fill lines. 8. Prior to loading samples, flush out the wells with running buffer to clear them of urea and debris. DNA Sequencing Assembling the unit Preparation of samples for loading 1. The volume of sample depends on the capacity of the wells (See Comb specifications page). 2. Heat the sequencing samples in a water bath or heating block at 95 C for 3 minutes, place on ice and centrifuge at rpm for 3 minutes. Return to ice. Denatured protein The instructions given below are for denatured samples. For native samples, please consult a laboratory handbook. 1. Prepare the protein samples for loading. The volume of sample depends on the capacity of the wells. 2. Using a 0.5 ml micro-centrifuge tube or other convenient receptacle, combine the protein sample and 4 x sample buffer. It is always advisable to use protein markers in one of the end lanes to indicate sizes of bands. These should be prepared according to the manufacturers instructions. 3. Heat the samples in a water bath or heating block for 2 minutes to denature the samples. 4. Centrifuge the samples in a micro-centrifuge for 20 seconds at rpm. The protein samples are now ready to load.
26 Gel running and ending Running the gel 1. Remove the square tooth comb before loading the samples. If using a shark s tooth comb, leave this in position as the comb teeth will act as the wells. 2. Load the required volume of sample using a suitable loading tip. If possible avoid taking the sample from the bottom of the tube (particulate materials may cause streaking or smearing). Sample dispersion can be minimized by loading the sample directly onto the bottom of the well and keep it as a thin layer. 3. Fit the safety lid ensuring it is positioned fully down over the electrical connectors. 4. Connect and run the gel at the desired power setting. The leads and electrical connectors are CE safe to 1500 V and users are advised not to exceed this voltage. 5. Typically for DNA sequencing gels, W constant power is advised. For other types of gel please consult a laboratory handbook. 6. Turn the power supply off when the loading dye reaches the bottom of the gel, sooner if your proteins are below 4 Kd in size. Ending the Run 1. Disconnect and turn off the power supply before removing the leads. 2. Remove the safety lid by gripping the edges of the lid and pushing down with your thumbs on the pegs located on the top of the unit. 3. Separate the plates with a strong, thin, broad blade. Do not force the glass plates apart at the notch as this may damage the plates. The gel will usually stick to one of the plates and can be removed by first soaking in buffer and then gently lifting with a spatula. For protein gels, the gel is now ready to be stained with coomassie or silver stain or the proteins in the gel can be transferred to a membrane by electroblotting for specific band identification and further analysis. 24 E4xxx
27 10 x DNA sequencing Buffer g Tris-OH 27.5 g boric acid 7.45 g disodium EDTA add distilled water to a final volume of 1000 ml Acrylamide stock 38% acrylamide 2% bis-acrylamide 8% Gel 40.4 g urea 27.0 ml water 16.8 ml 38/2 acrylamide 8.0 ml 10 x DNA sequencing buffer 6% Gel 40.4 g urea 31.2 ml water 12.6 ml 38/2 acrylamide 8.0 ml 10 x DNA sequencing buffer 5% Gel 40.4 g urea 33.5 ml water 10.5 ml 38/2 acrylamide 8.0 ml 10 x DNA sequencing buffer Solutions for DNA sequencing If necessary mix by heating slightly, degassing is advised. Add 0.7 ml 10% ammonium persulphate, and 25 µl TEMED, and pour gel immediately. E4xxx 25
28 Denaturing gradient system Installation 1. Connect the heating element plug into the socket at the rear of the control unit. 2. Connect the Pt100 temperature sensing probe plug at the rear of the control unit. 3. Ensure the temperature is set to zero. 4. Connect the mains cable socket to the rear of the control unit and connect to an external power source. Do not attempt to apply temperature to the tank without any buffer present! Operation 1. Fill the tank with buffer to the required level. 2. Ensure all the leads are connected in the correct positions as described on page Switch on the control unit and ensure that the red power light illuminates. 4. Set the required temperature on the control unit. 5. The heaters will remain on until the desired temperature has been reached, at which point the unit will switch on and offf to maintain that temperature. 6. To obtain an accurate temperature of the buffer, it may be advisable to attach a temperature strip panel to the front of the tank. Safety considerations 1. Should the sensor develop a fault or become disconnected the heaters will automatically switch off so safeguarding against gel over heating. 2. To replace the fuse isolate on the control unit from the mains supply and open the fuse holder with a screwdriver blade. The holder contains two fuses. Always use the recommended fuse and never replace it with a different one of different rating. 26 E4xxx
29 E4xxx 27
30 ࠀ ࠀ ĀȀ ЀԀ Ԁ Ѐ Ԁကࠀက ကሀȀ Ȁༀ ကᄀ ကሀࠀ Ȁ ࠀ ࠀȀ ЀȀࠀ ĀȀ ЀȀԀ Ȁༀကᄀሀጀ ᔀᘀ ጀကᜀ ᜀԀᔀᔀᤀጀᤀሀᨀᜀ ᄀ ሀက ሀ ᰀሀᘀကሀᴀᔀက ᨀḀἀကጀᜀ Ȁ!""#Ȁ $""#Ȁ %""#Ȁ ""#Ȁ &"" ᔀကᄀࠀကȀ Ԁ Ȁ ࠀက ࠀ က ጀሀԀሀ ကሀ ЀȀԀ Ѐࠀ Ѐ ጀကሀ ကሀȀ ጀᘀ က Ȁ'!"!" * Ȁ+%,$%,ȀȀ (ࠀ Ȁ ࠀԀ*ȀĀ Ѐ () Ѐ Ȁ&""-!.! Ȁ&""-$.! Ȁ'"&&&.$ * Ȁ-/,%%',ȀȀ (ࠀ Ȁ ࠀȀ Ā ᜀ ጀሀἀ0ἀᤀ ᤀᨀ ᰀᴀḀᤀἀἀ ࠀ ᜀ ሀ က ᰀȀ ᘀ ᔀࠀ Ȁ! "#ᤀḀ$ "ἀ$%& &% %Ḁ '( )' %) ᤀḀ$ "ἀ$%& &Ḁ #"!* ( က-ༀȀࠀ Ȁ,Ԁ Ȁ! + ༀ Ѐ ࠀ ĀȀ Ѐ Ԁ ༀጀༀԀༀ ကᄀက ሀ ЀȀကЀጀ Ѐ Ȁ က Ѐ Ȁ ЀЀԀ ᔀ ԀЀ ༀ Ѐༀ ࠀЀ ሀ ࠀሀ ࠀЀༀကЀሀ Ѐ ༀက ကᜀЀༀကༀᘀ Ѐကࠀሀࠀ ༀက ကЀᘀЀ ЀကԀԀက Ԁ Ѐ ࠀༀԀЀሀࠀ Ѐༀᘀᄀက ကᜀЀ Ѐጀᄀကༀ ကကЀᘀЀԀༀȀ ࠀༀԀЀ ကༀᘀ ࠀ Ѐ Ȁ Ѐ ༀ Ѐ Ѐ ࠀȀ ༀ ༀက ᘀЀጀ Ѐࠀ ࠀЀ Ȁ ࠀༀ Ѐ ࠀကༀᘀ Ѐ ᤀЀጀᄀༀԀᘀༀကᨀЀ ԀԀЀሀԀ ကЀ ᤀЀ ሀ ԀԀЀကЀ ᄀ Ѐ ᨀ ЀကᄀሀሀԀ Ѐက ကࠀ Ѐሀ ሀᄀጀԀༀ ЀԀ Ѐᔀ Ԁ
31
32 Consort Parklaan 36 B-2300 Turnhout Belgium Tel: (+32) (0) Fax: (+32) (0) Sales: Support: Information:
EcoCell Vertical Electrophoresis Unit
 Instructions for EcoCell Vertical Electrophoresis Unit Cat. No.: AN 12005 Ringstr. 4 64401 Gross-Bieberau Tel. ++49-6162-809840 Fax ++49-6162-8098420 www.anamed-gele.com Warning These units are capable
Instructions for EcoCell Vertical Electrophoresis Unit Cat. No.: AN 12005 Ringstr. 4 64401 Gross-Bieberau Tel. ++49-6162-809840 Fax ++49-6162-8098420 www.anamed-gele.com Warning These units are capable
Vertical Gel Electrophoresis Systems
 user manual Vertical Gel Electrophoresis Systems Twin Plate Vertical Gel Units Mini Size 10 10 cm Twin Plate Vertical Gel Unit, Cat. No. E4100 Mini Size 10 10 cm Twin Plate Vertical Gel Unit with Casting
user manual Vertical Gel Electrophoresis Systems Twin Plate Vertical Gel Units Mini Size 10 10 cm Twin Plate Vertical Gel Unit, Cat. No. E4100 Mini Size 10 10 cm Twin Plate Vertical Gel Unit with Casting
Topac Inc. 101 Derby Street Hingham MA USA Tel
 Topac Inc 101 Derby Street Hingham MA 02043 USA Tel 781 740 8778 www.topac.com USING THE SCIE-PLAS VERTICAL GEL ELECTROPHORESIS UNITS A. Safety Precautions READ the instructions before using the apparatus.
Topac Inc 101 Derby Street Hingham MA 02043 USA Tel 781 740 8778 www.topac.com USING THE SCIE-PLAS VERTICAL GEL ELECTROPHORESIS UNITS A. Safety Precautions READ the instructions before using the apparatus.
PROTEAN plus Multi-Casting Chamber. Instruction Manual
 PROTEAN plus Multi-Casting Chamber Instruction Manual Catalog Number 165-4160 For Technical Service Call Your Local Bio-Rad Office or in the U.S. Call 1-800-4BIORAD (1-800-424-6723) Table of Contents Section
PROTEAN plus Multi-Casting Chamber Instruction Manual Catalog Number 165-4160 For Technical Service Call Your Local Bio-Rad Office or in the U.S. Call 1-800-4BIORAD (1-800-424-6723) Table of Contents Section
Vertical Dual Slab Gel Systems (DSG Series)
 Vertical Dual Slab Gel Systems (DSG Series) INDEX Ordering Information...3 Kit Contents...3 Introduction...3 Specifications...3 Safety...4 Instructions for Use...4 Running Conditions...6 Maintenance of
Vertical Dual Slab Gel Systems (DSG Series) INDEX Ordering Information...3 Kit Contents...3 Introduction...3 Specifications...3 Safety...4 Instructions for Use...4 Running Conditions...6 Maintenance of
PROTOCOLS. Coomassie Brilliant Blue G (Sigma 98%) ,4 mg. Ethanol 95% ml. Phosphoric acid 85%.(w/v) ml
 PROTOCOLS I. Bradford s method to quantify total protein concentration (Bradford, D, M. M. A rapid and sensitive method for the quantitaton of microgram quantities of protein utilizing the principle of
PROTOCOLS I. Bradford s method to quantify total protein concentration (Bradford, D, M. M. A rapid and sensitive method for the quantitaton of microgram quantities of protein utilizing the principle of
OPERATING MANUAL V16 # V16-2 # V15.17 # V-Series of Vertical Electrophoresis Apparatus. V15.17 Shown
 OPERATING MANUAL V16 #21070010 V16-2 #31071010 V15.17 #21080023 V-Series of Vertical Electrophoresis Apparatus V15.17 Shown V-SERIES APPARATUS OPERATING MANUAL - TABLE OF CONTENTS Before You Begin 1.0
OPERATING MANUAL V16 #21070010 V16-2 #31071010 V15.17 #21080023 V-Series of Vertical Electrophoresis Apparatus V15.17 Shown V-SERIES APPARATUS OPERATING MANUAL - TABLE OF CONTENTS Before You Begin 1.0
Vertical Slab Gel Systems (SG Series)
 INDEX Ordering Information...3 Kit Contents...3 Introduction...4 Specifications...4 General Information...4 Instructions for use...4 Running Conditions...6 Maintenance of Equipment...7 Use and Use Restrictions:
INDEX Ordering Information...3 Kit Contents...3 Introduction...4 Specifications...4 General Information...4 Instructions for use...4 Running Conditions...6 Maintenance of Equipment...7 Use and Use Restrictions:
Digital Melting Point Apparatus
 Digital Melting Point Apparatus Heating Plateau Ramping Start/Stop Plateau set Ramp stop Hold User Guide Version 1.1 Heating Viewing tube Sample Chamber IEC power inlet socket Power on/off Temperature
Digital Melting Point Apparatus Heating Plateau Ramping Start/Stop Plateau set Ramp stop Hold User Guide Version 1.1 Heating Viewing tube Sample Chamber IEC power inlet socket Power on/off Temperature
Sample Concentrator SBH CONC/1 Instructions for use
 Sample Concentrator SBH CONC/1 Instructions for use Thank you for purchasing this piece of Barloworld Scientific equipment. To get the best performance from the equipment, and for your own safety, please
Sample Concentrator SBH CONC/1 Instructions for use Thank you for purchasing this piece of Barloworld Scientific equipment. To get the best performance from the equipment, and for your own safety, please
Unisense In Situ UniAmp Connector System
 Unisense In Situ UniAmp Connector System Overview The Unisense In Situ UniAmp Connector system is designed for easy replacement of sensors. Unisense In Situ UniAmp Connector system is a dedicated system
Unisense In Situ UniAmp Connector System Overview The Unisense In Situ UniAmp Connector system is designed for easy replacement of sensors. Unisense In Situ UniAmp Connector system is a dedicated system
Bante810 Benchtop Dissolved Oxygen Meter Instruction Manual
 Bante810 Benchtop Dissolved Oxygen Meter Instruction Manual BANTE INSTRUMENTS CO., LTD Bante810 Benchtop Dissolved Oxygen Meter 1 Introduction Thank you for selecting the Bante810 benchtop dissolved oxygen
Bante810 Benchtop Dissolved Oxygen Meter Instruction Manual BANTE INSTRUMENTS CO., LTD Bante810 Benchtop Dissolved Oxygen Meter 1 Introduction Thank you for selecting the Bante810 benchtop dissolved oxygen
user manual minive Hoefer minive electrophoresis and electrotransfer unit um SE300-IM/Rev. A0/05-04
 user manual minive Hoefer minive electrophoresis and electrotransfer unit um SE300-IM/Rev. A0/05-04 Page finder Introduction................................ 1 Unpacking.......................... 2 Specifications........................
user manual minive Hoefer minive electrophoresis and electrotransfer unit um SE300-IM/Rev. A0/05-04 Page finder Introduction................................ 1 Unpacking.......................... 2 Specifications........................
NovelBright LED Illuminator
 NovelBright LED Illuminator Instruction Manual version NS01_11 Instruction Manual This LED illuminator is suitable for research use only. It must be used by specialized personnel that know the health risks
NovelBright LED Illuminator Instruction Manual version NS01_11 Instruction Manual This LED illuminator is suitable for research use only. It must be used by specialized personnel that know the health risks
DISSOLVED OXYGEN SENSOR BT34i
 DISSOLVED OXYGEN SENSOR BT34i USER S GUIDE CENTRE FOR MICROCOMPUTER APPLICATIONS http://www.cma-science.nl Short description The CMA Dissolved Oxygen (DO) sensor BT34i measures the concentration of dissolved
DISSOLVED OXYGEN SENSOR BT34i USER S GUIDE CENTRE FOR MICROCOMPUTER APPLICATIONS http://www.cma-science.nl Short description The CMA Dissolved Oxygen (DO) sensor BT34i measures the concentration of dissolved
STEAM STERILIZABLE POLAROGRAPHIC OXYGEN ELECTRODE
 STEAM STERILIZABLE POLAROGRAPHIC OXYGEN ELECTRODE GENERAL INFORMATION This family of dissolved oxygen electrodes is of the steam sterilizable polarographic type, designed to be interchangeable with NBS
STEAM STERILIZABLE POLAROGRAPHIC OXYGEN ELECTRODE GENERAL INFORMATION This family of dissolved oxygen electrodes is of the steam sterilizable polarographic type, designed to be interchangeable with NBS
ROPV R40 E Series User Manual
 HARBIN ROPV INDUSTRY DEVELOPMENT CENTER ROPV R40 E Series User Manual For Use with the Following ROPV Pressure Vessel Models: R40 300E R40 450E Headquarters Tel:(+86)451-82267301 Fax:(+86)451-82267303
HARBIN ROPV INDUSTRY DEVELOPMENT CENTER ROPV R40 E Series User Manual For Use with the Following ROPV Pressure Vessel Models: R40 300E R40 450E Headquarters Tel:(+86)451-82267301 Fax:(+86)451-82267303
For accurate measurements and to prevent damage to the micropipets, follow these important guidelines:
 OXFORD VERSION #2 MEASURE FOR MEASURE MICROPIPETTING AND THE METRIC SYSTEM Concept: Work with DNA and enzymes frequently involves measuring very small volumes, often in the microliter range. A microliter
OXFORD VERSION #2 MEASURE FOR MEASURE MICROPIPETTING AND THE METRIC SYSTEM Concept: Work with DNA and enzymes frequently involves measuring very small volumes, often in the microliter range. A microliter
Instructions for Assembly, Installation, and Operation of the Gas Addition Kit Accessory with the CEM Discover Systems
 Corporation Issued: 5/09 P/N: 600104 Rev. 2 Instructions for Assembly, Installation, and Operation of the Gas Addition Kit Accessory with the CEM Discover Systems The Gas Addition Accessory permits the
Corporation Issued: 5/09 P/N: 600104 Rev. 2 Instructions for Assembly, Installation, and Operation of the Gas Addition Kit Accessory with the CEM Discover Systems The Gas Addition Accessory permits the
HI 2314 HI 2315 HI 23151
 Instruction Manual HI 2314 HI 2315 HI 23151 Multi-Range Conductivity Meters for Laboratories www.hannainst.com Dear Customer, Thank you for choosing a Hanna Instruments product. Please read this instruction
Instruction Manual HI 2314 HI 2315 HI 23151 Multi-Range Conductivity Meters for Laboratories www.hannainst.com Dear Customer, Thank you for choosing a Hanna Instruments product. Please read this instruction
Radnoti Liver Perfusion System
 Radnoti Liver Perfusion System 130003 Offset Offset Gain Gain Radnoti 2006 Description Qty Part # A Base only, for 4-bar stand 1 159950-B4 B Stabilizer Bar only, for 4-Bar stand 2 159950-C4 C Rod 24 Long
Radnoti Liver Perfusion System 130003 Offset Offset Gain Gain Radnoti 2006 Description Qty Part # A Base only, for 4-bar stand 1 159950-B4 B Stabilizer Bar only, for 4-Bar stand 2 159950-C4 C Rod 24 Long
Pressure Injection Cell Operator s Manual
 Pressure Injection Cell Operator s Manual Operator s Manual Pressure Injection Cell CONGRATULATIONS! Congratulations on your purchase of a Next Advance Pressure Injection Cell. Please read this operator
Pressure Injection Cell Operator s Manual Operator s Manual Pressure Injection Cell CONGRATULATIONS! Congratulations on your purchase of a Next Advance Pressure Injection Cell. Please read this operator
EC214 EC215 - EC215R Bench Conductivity Meters
 Instruction Manual EC214 EC215 - EC215R Bench Conductivity Meters http://www.hannainst.com These Instruments are in Compliance with the CE Directives Dear Customer, Thank you for choosing a Hanna Instruments
Instruction Manual EC214 EC215 - EC215R Bench Conductivity Meters http://www.hannainst.com These Instruments are in Compliance with the CE Directives Dear Customer, Thank you for choosing a Hanna Instruments
DOscan10 Pocket Dissolved Oxygen Tester Instruction Manual
 DOscan10 Pocket Dissolved Oxygen Tester Instruction Manual BANTE INSTRUMENTS CO., LTD DOscan10 Pocket Dissolved Oxygen Tester 1 Thank you for selecting the DOscan10 pocket dissolved oxygen tester. This
DOscan10 Pocket Dissolved Oxygen Tester Instruction Manual BANTE INSTRUMENTS CO., LTD DOscan10 Pocket Dissolved Oxygen Tester 1 Thank you for selecting the DOscan10 pocket dissolved oxygen tester. This
STERILIZABLE DISSOLVED OXYGEN SENSORS
 STERILIZABLE DISSOLVED OXYGEN SENSORS USER MANUAL Table of Contents 1. Introduction 2. Operation 3. Calibration 4. Electrode Verification 5. Mounting 6. Maintenance 7. Storage 8. Instructions for Exchange
STERILIZABLE DISSOLVED OXYGEN SENSORS USER MANUAL Table of Contents 1. Introduction 2. Operation 3. Calibration 4. Electrode Verification 5. Mounting 6. Maintenance 7. Storage 8. Instructions for Exchange
Operator s Manual. The Bullet Blender Gold BB24-AU, BB5E-AU
 Operator s Manual The Bullet Blender Gold BB24-AU, BB5E-AU Congratulations! Congratulations on your purchase of a Bullet Blender Gold by Next Advance, Inc., for lysing, disrupting, and homogenizing your
Operator s Manual The Bullet Blender Gold BB24-AU, BB5E-AU Congratulations! Congratulations on your purchase of a Bullet Blender Gold by Next Advance, Inc., for lysing, disrupting, and homogenizing your
The Oxygen Sensor can be connected to all types of einstein Tablets, einstein LabMate, and einstein LabMate +.
 The Oxygen sensor is a maintenance- free galvanic oxygen electrode capable of measuring Oxygen in solutions. With built-in temperature compensation, this sensor provides an easy way for students to receive
The Oxygen sensor is a maintenance- free galvanic oxygen electrode capable of measuring Oxygen in solutions. With built-in temperature compensation, this sensor provides an easy way for students to receive
Techcon Systems TS1258 Pressure Pot
 Techcon Systems TS1258 Pressure Pot User Guide TABLE OF CONTENT 1. SAFETY. 3 1.1 Intended Use 3 1.2 Safety Precaution 3 2. CERTIFICATE OF COMFORMANCE 4 3. FEATURES. 5 4. SPECIFICATIONS 5 5. INSTALLATION
Techcon Systems TS1258 Pressure Pot User Guide TABLE OF CONTENT 1. SAFETY. 3 1.1 Intended Use 3 1.2 Safety Precaution 3 2. CERTIFICATE OF COMFORMANCE 4 3. FEATURES. 5 4. SPECIFICATIONS 5 5. INSTALLATION
Version BA D
 Aurora Dispo osable Cartridge Handling Manual For Aurora cartridge 210 0001 CA D Version 1.00 106 0010 BA D 2011 Boreal Genomics, Inc. All rights reserved. All trademarks are property of their owners.
Aurora Dispo osable Cartridge Handling Manual For Aurora cartridge 210 0001 CA D Version 1.00 106 0010 BA D 2011 Boreal Genomics, Inc. All rights reserved. All trademarks are property of their owners.
Standard Operational Procedure for Capillary Electrophoresis
 Page 1 of 14 Standard Operational Procedure for Capillary Electrophoresis *Please ask an experienced operator for training before you use the CE if you can. A demo is most probably more efficient than
Page 1 of 14 Standard Operational Procedure for Capillary Electrophoresis *Please ask an experienced operator for training before you use the CE if you can. A demo is most probably more efficient than
For accurate measurements and to prevent damage to the micropipets, follow these important guidelines:
 OXFORD 4 SIZES VERSION MEASURE FOR MEASURE MICROPIPETTING AND THE METRIC SYSTEM Concept: Work with DNA and enzymes frequently involves measuring very small volumes, often in the microliter range. A microliter
OXFORD 4 SIZES VERSION MEASURE FOR MEASURE MICROPIPETTING AND THE METRIC SYSTEM Concept: Work with DNA and enzymes frequently involves measuring very small volumes, often in the microliter range. A microliter
Underwater Housing for Canon G7X Mark II
 Underwater Housing for Canon G7X Mark II Product Number 6146.08 Product Registration Please register your product at ikelite.com within 15 days of purchase. Our product registration database is the best
Underwater Housing for Canon G7X Mark II Product Number 6146.08 Product Registration Please register your product at ikelite.com within 15 days of purchase. Our product registration database is the best
Faculty of Biological Sciences Assessment Title SDS-PAGE Gel Electrophoresis preparation of gels Area/Service Protein Production Facility
 Faculty of Biological Sciences Assessment Title SDS-PAGE Gel Electrophoresis preparation of gels Area/Service Protein Production Facility COSHH Assessment Brief Description of Activity, Experiment or Process
Faculty of Biological Sciences Assessment Title SDS-PAGE Gel Electrophoresis preparation of gels Area/Service Protein Production Facility COSHH Assessment Brief Description of Activity, Experiment or Process
Pipettor. User Manual
 Pipettor User Manual Product Code Description Single Chanel Pipettor Variable 550.002.055 Volume 0.5 to 10ul 550.002.060 2 to 20ul 550.002.065 10 to 100ul 550.002.070 20 to 200ul 550.002.075 100 to 100ul
Pipettor User Manual Product Code Description Single Chanel Pipettor Variable 550.002.055 Volume 0.5 to 10ul 550.002.060 2 to 20ul 550.002.065 10 to 100ul 550.002.070 20 to 200ul 550.002.075 100 to 100ul
Warner Instruments, Inc Dixwell Avenue, Hamden, CT (800) / (203) (203) fax
 DH-40i, Rev. 04.03.02 Micro-Incubation Platform For RC-40 Chambers Model DH-40i 1125 Dixwell Avenue, Hamden, CT 06514 (800) 599-4203 / (203) 776-0664 (203) 776-1278 - fax DH-40i, Rev. 04.03.02 Table of
DH-40i, Rev. 04.03.02 Micro-Incubation Platform For RC-40 Chambers Model DH-40i 1125 Dixwell Avenue, Hamden, CT 06514 (800) 599-4203 / (203) 776-0664 (203) 776-1278 - fax DH-40i, Rev. 04.03.02 Table of
Compact Triple Cabinet Outlet Station Model Installation and Operating Instructions
 Compact Triple Cabinet Outlet Station Model 6258-1 Installation and Operating Instructions The Porter Compact Triple Outlet Station (6258-1) provides a quick, safe, and reliable method of connection to
Compact Triple Cabinet Outlet Station Model 6258-1 Installation and Operating Instructions The Porter Compact Triple Outlet Station (6258-1) provides a quick, safe, and reliable method of connection to
Agilent 1290 Infinity Pump Head Maintenance
 Agilent 1290 Infinity Pump Head Maintenance Technical Note Agilent Technologies Notices Agilent Technologies, Inc. 2012-2015, 2016 No part of this manual may be reproduced in any form or by any means (including
Agilent 1290 Infinity Pump Head Maintenance Technical Note Agilent Technologies Notices Agilent Technologies, Inc. 2012-2015, 2016 No part of this manual may be reproduced in any form or by any means (including
Free Chlorine Sensor For additional information, please visit our website at
 Instruction Manual 499ACL01 LIQ_MAN_ABR_499ACL01 April 2014 Free Chlorine Sensor For additional information, please visit our website at www.rosemountanalytical.com. CAUTION Sensor/Process Application
Instruction Manual 499ACL01 LIQ_MAN_ABR_499ACL01 April 2014 Free Chlorine Sensor For additional information, please visit our website at www.rosemountanalytical.com. CAUTION Sensor/Process Application
X-6 STRINGING MACHINE OWNER'S MANUAL. Issue 1 - May Copyright 2004 GAMMA Sports - All Rights Reserved
 X-6 STRINGING MACHINE OWNER'S MANUAL Issue 1 - May 2004 Copyright 2004 GAMMA Sports - All Rights Reserved OWNER'S MANUAL GAMMA X-6 TABLE OF CONTENTS PAGE 1... WARRANTY PAGE 2... FEATURES PAGE 3...ASSEMBLY
X-6 STRINGING MACHINE OWNER'S MANUAL Issue 1 - May 2004 Copyright 2004 GAMMA Sports - All Rights Reserved OWNER'S MANUAL GAMMA X-6 TABLE OF CONTENTS PAGE 1... WARRANTY PAGE 2... FEATURES PAGE 3...ASSEMBLY
Vinmetrica Dissolved Oxygen System User Manual
 Vinmetrica Dissolved Oxygen System User Manual The Vinmetrica dissolved Oxygen system provides a simple, accurate and affordable way to determine oxygen concentrations in wine and other samples. Materials
Vinmetrica Dissolved Oxygen System User Manual The Vinmetrica dissolved Oxygen system provides a simple, accurate and affordable way to determine oxygen concentrations in wine and other samples. Materials
Underwater Housing for Panasonic Lumix DMC-ZS60, DMC-TZ80
 Underwater Housing for Panasonic Lumix DMC-ZS60, DMC-TZ80 Product Number 6170.60 Product Registration Please register your product at ikelite.com within 15 days of purchase. Our product registration database
Underwater Housing for Panasonic Lumix DMC-ZS60, DMC-TZ80 Product Number 6170.60 Product Registration Please register your product at ikelite.com within 15 days of purchase. Our product registration database
INSTRUCTION MANUAL CZ 630/631
 INSTRUCTION MANUAL CZ 630/631 Before handling the air rifle read this manual carefully and observe the following safety instructions. Improper and careless handling of the air rifle could result in unintentional
INSTRUCTION MANUAL CZ 630/631 Before handling the air rifle read this manual carefully and observe the following safety instructions. Improper and careless handling of the air rifle could result in unintentional
Evaluation copy. Interdependence of Plants and Animals. computer OBJECTIVES MATERIALS
 Interdependence of Plants and Animals Computer 14 Plants and animals share many of the same chemicals throughout their lives. In most ecosystems, O 2, CO 2, water, food and nutrients are exchanged between
Interdependence of Plants and Animals Computer 14 Plants and animals share many of the same chemicals throughout their lives. In most ecosystems, O 2, CO 2, water, food and nutrients are exchanged between
WIND CLIPPER KTS ILLUM SCALE INC DEC CLIPPER WIND SYSTEM
 CLIPPER WIND KTS ILLUM SCALE DEC INC CLIPPER WIND SYSTEM TABLE OF CONTENTS INTRODUCTION PRE-TEST OF INSTRUMENT INSTALLING THE MASTHEAD SENSOR UNIT INSTALLING THE DISPLAY NORMAL OPERATION CHANGING THE
CLIPPER WIND KTS ILLUM SCALE DEC INC CLIPPER WIND SYSTEM TABLE OF CONTENTS INTRODUCTION PRE-TEST OF INSTRUMENT INSTALLING THE MASTHEAD SENSOR UNIT INSTALLING THE DISPLAY NORMAL OPERATION CHANGING THE
WXG-4 Manual Polarimeter. Instruction Manual BANTE INSTRUMENTS CO., LTD
 WXG-4 Manual Polarimeter Instruction Manual BANTE INSTRUMENTS CO., LTD WXG-4 Manual Polarimeter 1 Introduction Thank you for selecting the Bante Instrument s WXG-4 manual polarimeter. This manual provides
WXG-4 Manual Polarimeter Instruction Manual BANTE INSTRUMENTS CO., LTD WXG-4 Manual Polarimeter 1 Introduction Thank you for selecting the Bante Instrument s WXG-4 manual polarimeter. This manual provides
Lipodrop 2.0 device coating
 Lipodrop 2.0 device coating Compiled by K.V., K.Ž, J.R AIM: To selectively coat channels in order to make them hydrophobic (pre junction channels) or hydrophilic (post junction channels). REAGENTS USED:
Lipodrop 2.0 device coating Compiled by K.V., K.Ž, J.R AIM: To selectively coat channels in order to make them hydrophobic (pre junction channels) or hydrophilic (post junction channels). REAGENTS USED:
600 / 600FC OWNER'S MANUAL
 PROGRESSION 600 / 600FC OWNER'S MANUAL Issue 2 / Version E - Dec. 10, 1997 Copyright 1997 GAMMA Sports - All Rights Reserved PROGRESSION 600 / 600FC OWNER'S MANUAL TABLE OF CONTENTS PAGE 1... WARRANTY
PROGRESSION 600 / 600FC OWNER'S MANUAL Issue 2 / Version E - Dec. 10, 1997 Copyright 1997 GAMMA Sports - All Rights Reserved PROGRESSION 600 / 600FC OWNER'S MANUAL TABLE OF CONTENTS PAGE 1... WARRANTY
PRESSURE MYOGRAPH - 114P PULSATILE PRESSURE MYOGRAPH - 112PP
 USER GUIDE PRESSURE MYOGRAPH - 114P PULSATILE PRESSURE MYOGRAPH - 112PP User Guide Version 1.1 CONTENTS Chapter 1 - Pressure Myograph Overview 3 1.1 Pressure Myograph 114P Overview. 3 1.2 Pulsation Pressure
USER GUIDE PRESSURE MYOGRAPH - 114P PULSATILE PRESSURE MYOGRAPH - 112PP User Guide Version 1.1 CONTENTS Chapter 1 - Pressure Myograph Overview 3 1.1 Pressure Myograph 114P Overview. 3 1.2 Pulsation Pressure
Compact Triple Cabinet Outlet Station Model B Installation and Operating Instructions
 PORTER Parker Hannifin Corporation Porter Instrument Division 245 Township Line Rd. P.O. Box 907 Hatfield, PA 19440-0907 USA (215) 723-4000 / fax (215) 723-5106 Compact Triple Cabinet Outlet Station Model
PORTER Parker Hannifin Corporation Porter Instrument Division 245 Township Line Rd. P.O. Box 907 Hatfield, PA 19440-0907 USA (215) 723-4000 / fax (215) 723-5106 Compact Triple Cabinet Outlet Station Model
VERTICAL AIR COMPRESSORS
 VERTICAL AIR COMPRESSORS MODEL NO: VE11C150, VE15C150, VE18C150 PART NO: 2226005, 2226000, 2226015 OPERATION & MAINTENANCE INSTRUCTIONS LS0615 INTRODUCTION Thank you for purchasing this CLARKE Vertical
VERTICAL AIR COMPRESSORS MODEL NO: VE11C150, VE15C150, VE18C150 PART NO: 2226005, 2226000, 2226015 OPERATION & MAINTENANCE INSTRUCTIONS LS0615 INTRODUCTION Thank you for purchasing this CLARKE Vertical
Dissolved Oxygen Sensor
 Instruction Manual 499ADO and 499ADO-VP LIQ_MAN_ABR_499ADO May 2014 Dissolved Oxygen Sensor CAUTION For additional information, please visit our website at www.rosemountanalytical.com SENSOR/PROCESS APPLICATION
Instruction Manual 499ADO and 499ADO-VP LIQ_MAN_ABR_499ADO May 2014 Dissolved Oxygen Sensor CAUTION For additional information, please visit our website at www.rosemountanalytical.com SENSOR/PROCESS APPLICATION
Other languages available on our website:
 Other languages available on our website: www.gilson.com. CONTENTS PAGE 1 - INTRODUCTION 2 2 - OPERATION 3 3 - RECOMMENDATIONS 9 4 - SPECIFICATIONS 9 5 - MAINTENANCE 10 6 - DECONTAMINATION 13 7 - SPARE
Other languages available on our website: www.gilson.com. CONTENTS PAGE 1 - INTRODUCTION 2 2 - OPERATION 3 3 - RECOMMENDATIONS 9 4 - SPECIFICATIONS 9 5 - MAINTENANCE 10 6 - DECONTAMINATION 13 7 - SPARE
ATD LB PRESSURE BLASTER INSTRUCTION MANUAL
 ATD-8402 90LB PRESSURE BLASTER INSTRUCTION MANUAL SAVE THESE INSTRUCTIONS SAFETY INSTRUCTIONS FOR SANDBLASTER 1. Before opening the tank release the air pressure on the sand tank. To do this, turn off
ATD-8402 90LB PRESSURE BLASTER INSTRUCTION MANUAL SAVE THESE INSTRUCTIONS SAFETY INSTRUCTIONS FOR SANDBLASTER 1. Before opening the tank release the air pressure on the sand tank. To do this, turn off
Bante820 Portable Dissolved Oxygen Meter Instruction Manual
 Bante820 Portable Dissolved Oxygen Meter Instruction Manual BANTE INSTRUMENTS CO., LTD Bante820 Portable Dissolved Oxygen Meter 1 Introduction Thank you for selecting the Bante820 portable dissolved oxygen
Bante820 Portable Dissolved Oxygen Meter Instruction Manual BANTE INSTRUMENTS CO., LTD Bante820 Portable Dissolved Oxygen Meter 1 Introduction Thank you for selecting the Bante820 portable dissolved oxygen
User's Manual. Heavy Duty Dissolved Oxygen Meter. Model
 User's Manual Heavy Duty Dissolved Oxygen Meter Model 407510 Introduction Congratulations on your purchase of Extech's Heavy Duty Dissolved Oxygen / Temperature Meter which simultaneously displays Dissolved
User's Manual Heavy Duty Dissolved Oxygen Meter Model 407510 Introduction Congratulations on your purchase of Extech's Heavy Duty Dissolved Oxygen / Temperature Meter which simultaneously displays Dissolved
SUMMITTM 400 & 600. Natural Gas Barbecues. Step-By-Step Guide
 SUMMITTM 400 & 600 Natural Gas Barbecues Step-By-Step Guide W E B E R W E B E R W E B E R W E B E R Summit 400 NG Summit 600 NG CANADIAN GAS ASSOCIATION R A P P R O V E D WARNING: Follow all leak check
SUMMITTM 400 & 600 Natural Gas Barbecues Step-By-Step Guide W E B E R W E B E R W E B E R W E B E R Summit 400 NG Summit 600 NG CANADIAN GAS ASSOCIATION R A P P R O V E D WARNING: Follow all leak check
USER GUIDE, VOL.2.0 MYOGRAPH SYSTEM - 112PP/114P
 USER GUIDE, VOL.2.0 MYOGRAPH SYSTEM - 112PP/114P CONTENTS CHAPTER 1 4 1.1 Pressure Myograph 114P overview 4 1.2 Pressure Myograph 112PP overview 5 CHAPTER 2 - SET-UP THE PRESSURE MYOGRAPH 6 2.1 Adjustment
USER GUIDE, VOL.2.0 MYOGRAPH SYSTEM - 112PP/114P CONTENTS CHAPTER 1 4 1.1 Pressure Myograph 114P overview 4 1.2 Pressure Myograph 112PP overview 5 CHAPTER 2 - SET-UP THE PRESSURE MYOGRAPH 6 2.1 Adjustment
INSTRUCTION MANUAL. January 23, 2003, Revision 0
 INSTRUCTION MANUAL Model 810A In-Vitro Test Apparatus for 310B Muscle Lever January 23, 2003, Revision 0 Copyright 2003 Aurora Scientific Inc. Aurora Scientific Inc. 360 Industrial Parkway S., Unit 4 Aurora,
INSTRUCTION MANUAL Model 810A In-Vitro Test Apparatus for 310B Muscle Lever January 23, 2003, Revision 0 Copyright 2003 Aurora Scientific Inc. Aurora Scientific Inc. 360 Industrial Parkway S., Unit 4 Aurora,
METEOR LSR36500 LIGHTING UNDERWATER POOL LIGHT. Operating Instructions
 METEOR LSR36500 UNDERWATER POOL LIGHT Operating Instructions LIGHTING CONTENTS Preface...3 Features...3 Precautions...3 Components and Connectors...4 Installation...5 Controls...7 Appearance...8 Technical
METEOR LSR36500 UNDERWATER POOL LIGHT Operating Instructions LIGHTING CONTENTS Preface...3 Features...3 Precautions...3 Components and Connectors...4 Installation...5 Controls...7 Appearance...8 Technical
GV Standard X-Vent. Setup, Commissioning & Installation Guide
 GV Standard X-Vent Setup, Commissioning & Installation Guide Technical experts in the design, manufacture and supply of precision engineered, architectural rooflights for residential and commercial buildings.
GV Standard X-Vent Setup, Commissioning & Installation Guide Technical experts in the design, manufacture and supply of precision engineered, architectural rooflights for residential and commercial buildings.
! WARNING! IMPORTANT HPA AIR TANK SAFETY INSTRUCTION AND GUIDELINES ! WARNING! IMPORTANT SAFETY INSTRUCTION AND GUIDELINES
 ! WARNING! IMPORTANT SAFETY INSTRUCTION AND GUIDELINES! WARNING! IMPORTANT HPA AIR TANK SAFETY INSTRUCTION AND GUIDELINES This Paintball Marker is NOT A TOY. Misuse can cause serious injury or death. It
! WARNING! IMPORTANT SAFETY INSTRUCTION AND GUIDELINES! WARNING! IMPORTANT HPA AIR TANK SAFETY INSTRUCTION AND GUIDELINES This Paintball Marker is NOT A TOY. Misuse can cause serious injury or death. It
BI-680 Online Dissolved Oxygen Controller Instruction Manual
 BI-680 Online Dissolved Oxygen Controller Instruction Manual BANTE INSTRUMENTS CO., LTD BI-680 Online Dissolved Oxygen Controller 1 Introduction Thank you for selecting the BI-680 online dissolved oxygen
BI-680 Online Dissolved Oxygen Controller Instruction Manual BANTE INSTRUMENTS CO., LTD BI-680 Online Dissolved Oxygen Controller 1 Introduction Thank you for selecting the BI-680 online dissolved oxygen
Med Aire Alternating Pressure Pump and Pad System
 User Manual Med Aire Alternating Pressure Pump and Pad System 14002E 14001E Symbols & Statements NOTE Indicates some tips or some information users should be aware of. CAUTION Indicates correct operating
User Manual Med Aire Alternating Pressure Pump and Pad System 14002E 14001E Symbols & Statements NOTE Indicates some tips or some information users should be aware of. CAUTION Indicates correct operating
TQC Bresle Chloride test 2008
 TQC Bresle Chloride test 2008 SP7310 MANUAL See also : ISO8502-6 ISO8502-9 1 DETERMINATION OF SOLUBLE CONTAMINANTS ON SURFACES IN CONFORMITY WITH ISO 1.1 PREPARATION With this system very low concentrations
TQC Bresle Chloride test 2008 SP7310 MANUAL See also : ISO8502-6 ISO8502-9 1 DETERMINATION OF SOLUBLE CONTAMINANTS ON SURFACES IN CONFORMITY WITH ISO 1.1 PREPARATION With this system very low concentrations
25/04/56. LTC Carmen A. Bell Chief, Laboratory Operations USAMC-AFRIMS
 Pipette Theory and Maintenance LTC Carmen A. Bell Chief, Laboratory Operations USAMC-AFRIMS carmen.bell@afrims.org +6602-696-2725 1 References Pharma Express: http://pharma.financialexpress.com/2008063
Pipette Theory and Maintenance LTC Carmen A. Bell Chief, Laboratory Operations USAMC-AFRIMS carmen.bell@afrims.org +6602-696-2725 1 References Pharma Express: http://pharma.financialexpress.com/2008063
Simple, Robust, Reliable Measures ph Doses either acid (lower) or alkali (raise) Displays ph, Total Dose Count (DCT) and Dose Count per hour (DC/h)
 Simple, Robust, Reliable Measures ph Doses either acid (lower) or alkali (raise) Displays ph, Total Dose Count (DCT) and Dose Count per hour (DC/h) Integrated peristaltic dosing pump When using automatic
Simple, Robust, Reliable Measures ph Doses either acid (lower) or alkali (raise) Displays ph, Total Dose Count (DCT) and Dose Count per hour (DC/h) Integrated peristaltic dosing pump When using automatic
INSTRUCTIONS: CHEMICAL ANALYSIS OF ACTIVE SULFIDES IN OIL-BASED DRILLING FLUID
 OFI Testing Equipment Chemical Analysis of Active Sulfides Instructions Page 1 of 4 INSTRUCTIONS: CHEMICAL ANALYSIS OF ACTIVE SULFIDES IN OIL-BASED DRILLING FLUID DESCRIPTION: 1. The Garrett Gas Train
OFI Testing Equipment Chemical Analysis of Active Sulfides Instructions Page 1 of 4 INSTRUCTIONS: CHEMICAL ANALYSIS OF ACTIVE SULFIDES IN OIL-BASED DRILLING FLUID DESCRIPTION: 1. The Garrett Gas Train
INSTRUCTION MANUAL. Slavia 630/631
 INSTRUCTION MANUAL Slavia 630/631 Before handling the air rifle read this manual carefully and observe the following safety instructions. Improper and careless handling of the air rifle could result in
INSTRUCTION MANUAL Slavia 630/631 Before handling the air rifle read this manual carefully and observe the following safety instructions. Improper and careless handling of the air rifle could result in
Oxygen Sensor with Electrode
 imagine explore learn Oxygen Sensor with Electrode Product Number: ENOXY-A222 Overview The Oxygen sensor is a maintenance-free galvanic oxygen electrode, capable of measuring Oxygen both in the air and
imagine explore learn Oxygen Sensor with Electrode Product Number: ENOXY-A222 Overview The Oxygen sensor is a maintenance-free galvanic oxygen electrode, capable of measuring Oxygen both in the air and
USER'S GUIDE. Nanoflow Probe. Red Stripe. 3-4mm. ptfe Sleeve. To Injector. ZU1XC Fused Silica TTF115. Fused Silica. ptfe Sleeve
 USER'S GUIDE Nanoflow Probe 3-4mm Red Stripe ptfe Sleeve To Injector ZU1XC Fused Silica 20µm 90µm TTF115 ptfe Sleeve Fused Silica 25µm 375µm Micromass UK Limited Floats Road Wythenshawe M23 9LZ Tel: +44
USER'S GUIDE Nanoflow Probe 3-4mm Red Stripe ptfe Sleeve To Injector ZU1XC Fused Silica 20µm 90µm TTF115 ptfe Sleeve Fused Silica 25µm 375µm Micromass UK Limited Floats Road Wythenshawe M23 9LZ Tel: +44
Pectoral Machine. User manual E S S E N T I A L S T R E N G T H
 E L E M E N T and the cable E S S E N T I A L S T R E N G T H User manual 1 and the cable and The identification plate of and manufacturer, affixed on the back panel of the weight stack, gives the following
E L E M E N T and the cable E S S E N T I A L S T R E N G T H User manual 1 and the cable and The identification plate of and manufacturer, affixed on the back panel of the weight stack, gives the following
Thank you for purchasing your new Empire Reloader B Sound-Activated 3-Speed Paintball Hopper!
 Thank you for purchasing your new Empire Reloader B Sound-Activated 3-Speed Paintball Hopper! Should you require any technical assistance on the use of this product, or if your product needs servicing,
Thank you for purchasing your new Empire Reloader B Sound-Activated 3-Speed Paintball Hopper! Should you require any technical assistance on the use of this product, or if your product needs servicing,
200 STRINGING MACHINE
 200 STRINGING MACHINE OWNER S MANUAL Issue 1 - July 2010 Provided by www.gssalliance.com 200 OWNER S MANUAL TABLE OF CONTENTS WARRANTY...PAGE 2 FEATURES...PAGE 3 ASSEMBLY INSTRUCTIONS...PAGE 4 MOUNTING
200 STRINGING MACHINE OWNER S MANUAL Issue 1 - July 2010 Provided by www.gssalliance.com 200 OWNER S MANUAL TABLE OF CONTENTS WARRANTY...PAGE 2 FEATURES...PAGE 3 ASSEMBLY INSTRUCTIONS...PAGE 4 MOUNTING
Calibration Gas Instrument INSTRUCTION MANUAL. Release I. Advanced Calibration Designs, Inc.
 Advanced Calibration Designs, Inc. Calibration Gas Instrument INSTRUCTION MANUAL Release I www.goacd.com Instruction Manual Gas Generator Release I TABLE OF CONTENTS I. General Description Page 2 II. Start-Up
Advanced Calibration Designs, Inc. Calibration Gas Instrument INSTRUCTION MANUAL Release I www.goacd.com Instruction Manual Gas Generator Release I TABLE OF CONTENTS I. General Description Page 2 II. Start-Up
UsER manual for Watersens ph -REDOX
 UsER manual for Watersens -REDOX Cl 8 1 2 6 3 3 7 7 4 4 4 4 Parts List 1 Redox Probe 1 x 2 PH Probe 1 x 5 Tube Weight 2 x 6 Connection Valve 1 x chlorine 3 Chlorine and Pumps 2 x 7 Dosing Valve 2 x 5 5
UsER manual for Watersens -REDOX Cl 8 1 2 6 3 3 7 7 4 4 4 4 Parts List 1 Redox Probe 1 x 2 PH Probe 1 x 5 Tube Weight 2 x 6 Connection Valve 1 x chlorine 3 Chlorine and Pumps 2 x 7 Dosing Valve 2 x 5 5
INSTRUCTION MANUAL. Gas Salamander
 INSTRUCTION MANUAL Gas Salamander This manual contains important information regarding your unit. Please read this manual thoroughly prior to equipment set-up, operation and maintenance. Failure to comply
INSTRUCTION MANUAL Gas Salamander This manual contains important information regarding your unit. Please read this manual thoroughly prior to equipment set-up, operation and maintenance. Failure to comply
OWNER'S MANUAL. Copyright 2003 GAMMA - All Rights Reserved
 OWNER'S MANUAL AL Issue 1 - December 2003 Copyright 2003 GAMMA - All Rights Reserved OWNER'S MANUAL TABLE OF CONTENTS PAGE 1... WARRANTY PAGE 2... ASSEMBLY INSTRUCTIONS PAGE 4... MOUNTING THE RACQUET PAGE
OWNER'S MANUAL AL Issue 1 - December 2003 Copyright 2003 GAMMA - All Rights Reserved OWNER'S MANUAL TABLE OF CONTENTS PAGE 1... WARRANTY PAGE 2... ASSEMBLY INSTRUCTIONS PAGE 4... MOUNTING THE RACQUET PAGE
User Guide. Tripod. flowtech 75 / 100 Tripod. Part No. S S
 User Guide flowtech 75 / 100 Tripod Tripod Part No. S2051-0001 S2052-0001 EN www.sachtler.com TM Copyright 2018 All rights reserved. Original Instructions: English All rights reserved throughout the world.
User Guide flowtech 75 / 100 Tripod Tripod Part No. S2051-0001 S2052-0001 EN www.sachtler.com TM Copyright 2018 All rights reserved. Original Instructions: English All rights reserved throughout the world.
COMPACT TRIPLE WALL OUTLET STATION MODEL INSTALLATION AND OPERATING INSTRUCTIONS
 COMPACT TRIPLE WALL OUTLET STATION MODEL 6255-1 INSTALLATION AND OPERATING INSTRUCTIONS To assure safe operation and conformation to local fire codes, all Porter Outlet Stations are designed to be used
COMPACT TRIPLE WALL OUTLET STATION MODEL 6255-1 INSTALLATION AND OPERATING INSTRUCTIONS To assure safe operation and conformation to local fire codes, all Porter Outlet Stations are designed to be used
Latvin Luxury Shower Panel. Telephone Product Specification. ~ Minimum Working Pressure 1.0 bar ~ Maximum Working Pressure 3.
 Product Specification ~ Minimum Working Pressure 1.0 bar ~ Maximum Working Pressure 3.0 bar Latvin Luxury Shower Panel ~ Fixing Centres 150mm +/- 10mm ~ Outlet size 1/2" Bottom Outlet Always maintain a
Product Specification ~ Minimum Working Pressure 1.0 bar ~ Maximum Working Pressure 3.0 bar Latvin Luxury Shower Panel ~ Fixing Centres 150mm +/- 10mm ~ Outlet size 1/2" Bottom Outlet Always maintain a
RayPette Plus Autoclavable Pipette. User Manual
 RayPette Plus Autoclavable Pipette User Manual CONTENTS 1. YOUR NEW PIPETTE... 1 1.1. Adjustable volume pipettes... 1 1.3 Fully autoclavable... 2 2. UNPACKING... 3 3. INSTALLING THE PIPETTE HOLDER... 4
RayPette Plus Autoclavable Pipette User Manual CONTENTS 1. YOUR NEW PIPETTE... 1 1.1. Adjustable volume pipettes... 1 1.3 Fully autoclavable... 2 2. UNPACKING... 3 3. INSTALLING THE PIPETTE HOLDER... 4
The pipettes cover a volume range from 0.1µl to 10ml.
 CONTENTS 1. YOUR NEW PIPETTE 1 1.1. Adjustable volume pipettes 1 1.2. Fixed volume pipettes 2 1.3 Fully autoclavable 3 2. UNPACKING 3 3. INSTALLING THE PIPETTE HOLDER 4 4. PIPETTE COMPONENTS 5 5. PIPETTE
CONTENTS 1. YOUR NEW PIPETTE 1 1.1. Adjustable volume pipettes 1 1.2. Fixed volume pipettes 2 1.3 Fully autoclavable 3 2. UNPACKING 3 3. INSTALLING THE PIPETTE HOLDER 4 4. PIPETTE COMPONENTS 5 5. PIPETTE
OxyProbe II Instruction Manual
 OxyProbe II Instruction Manual D500 T82 Pg13.5 threaded nut O-Ring Sensor Shaft D600 ESSENTIAL INSTRUCTIONS: READ THIS PAGE BEFORE PROCEEDING! This product has been designed, manufactured, and tested to
OxyProbe II Instruction Manual D500 T82 Pg13.5 threaded nut O-Ring Sensor Shaft D600 ESSENTIAL INSTRUCTIONS: READ THIS PAGE BEFORE PROCEEDING! This product has been designed, manufactured, and tested to
Multi-Probe Sampling System User Guide & Manual
 Multi-Probe Sampling System User Guide & Manual Rev. 06/05/12 Part #22200012 Table of Contents CHAPTER 1: SYSTEM DESCRIPTION... 2 FUNCTION AND THEORY... 3 SYSTEM COMPONENTS... 4 CHAPTER 2: SYSTEM INSTALLATION...
Multi-Probe Sampling System User Guide & Manual Rev. 06/05/12 Part #22200012 Table of Contents CHAPTER 1: SYSTEM DESCRIPTION... 2 FUNCTION AND THEORY... 3 SYSTEM COMPONENTS... 4 CHAPTER 2: SYSTEM INSTALLATION...
SERVICE MANUAL MODEL BA050BMST BREATHING AIR PANEL
 www.modsafe.com SERVICE MANUAL MODEL BA050BMST BREATHING AIR PANEL WARNING: Do not attempt to operate this equipment without first reading and understanding the service manual enclosed with this device.
www.modsafe.com SERVICE MANUAL MODEL BA050BMST BREATHING AIR PANEL WARNING: Do not attempt to operate this equipment without first reading and understanding the service manual enclosed with this device.
Read this first. Zetasizer nano series Self installation and Quick start guide MRK825-02
 ! Read this first Zetasizer nano series Self installation and Quick start guide I N S T R U M E N T S MRK825-02 Zetasizer Nano series Self installation and Quick start guide MAN0383 Issue 1.1 July 2007
! Read this first Zetasizer nano series Self installation and Quick start guide I N S T R U M E N T S MRK825-02 Zetasizer Nano series Self installation and Quick start guide MAN0383 Issue 1.1 July 2007
Paintball Marker. User s Manual. 530 South Springbrook Road Newberg, OR 97132
 Paintball Marker User s Manual 530 South Springbrook Road Newberg, OR 97132 Component Concepts, Inc., 530 South Springbrook Road, Newberg, OR 97132 Phone: (503) 554-8095 Fax: (503) 554-9370 www.phantomonline.com
Paintball Marker User s Manual 530 South Springbrook Road Newberg, OR 97132 Component Concepts, Inc., 530 South Springbrook Road, Newberg, OR 97132 Phone: (503) 554-8095 Fax: (503) 554-9370 www.phantomonline.com
6 & 8 Bubble Panel Installation Manual
 6 & 8 Bubble Panel Installation Manual IMPORTANT RETAIN FOR FUTURE REFERENCE READ CAREFULLY You must use Distilled Water only. Use of any water other than distilled voids the warranty. Please check the
6 & 8 Bubble Panel Installation Manual IMPORTANT RETAIN FOR FUTURE REFERENCE READ CAREFULLY You must use Distilled Water only. Use of any water other than distilled voids the warranty. Please check the
Using the Akta Prime plus October 22, 2012
 Some starting precautions: 1. Vacuum filter all buffers. Removes any large particles/debris that may clog your column De-gases the buffers 2. Clarify lysates first by centrifugation and then filtration
Some starting precautions: 1. Vacuum filter all buffers. Removes any large particles/debris that may clog your column De-gases the buffers 2. Clarify lysates first by centrifugation and then filtration
SERVICE MANUAL MODEL BA100BMST 100 SCFM PORTABLE AIR PANEL
 Product Manual MST Portable Air Filtration System SERVICE MANUAL MODEL BA100BMST 100 SCFM PORTABLE AIR PANEL WARNING: Do not attempt to operate this equipment without first reading and understanding the
Product Manual MST Portable Air Filtration System SERVICE MANUAL MODEL BA100BMST 100 SCFM PORTABLE AIR PANEL WARNING: Do not attempt to operate this equipment without first reading and understanding the
For accurate measurements and to prevent damage to the micropipets, follow these important guidelines:
 ULSTER AND RAININ VERSION MEASURE FOR MEASURE MICROIETTING AND THE METRIC SYSTEM Concept: Work with DNA and enzymes frequently involves measuring very small volumes, often in the microliter range. A microliter
ULSTER AND RAININ VERSION MEASURE FOR MEASURE MICROIETTING AND THE METRIC SYSTEM Concept: Work with DNA and enzymes frequently involves measuring very small volumes, often in the microliter range. A microliter
max 80 cm / 2 6 Trittfrequenzmesser Capteur de Cadence Trapfrequentie Sensor Sensor de Cadencia Sensore di Cadenza Sensor de Cadência Kadencesensor
 1 max 80 cm / 2 6 2a 2b 3 4 5 max 4 mm / 0 16 ENGLISH The Polar Cadence Sensor is designed to measure cadence when cycling. No other use is intended or implied. It is compatible with Polar Cycling Computers
1 max 80 cm / 2 6 2a 2b 3 4 5 max 4 mm / 0 16 ENGLISH The Polar Cadence Sensor is designed to measure cadence when cycling. No other use is intended or implied. It is compatible with Polar Cycling Computers
Linkam Scientific Instruments USER GUIDE TST350. Temperature Controlled Tensile Stress Testing Stage
 Linkam Scientific Instruments TST350 Temperature Controlled Tensile Stress Testing Stage USER GUIDE Contents Before Setting Up Your Equipment... 3 Important Notice... 4 Warranty... 4 Technical Support...
Linkam Scientific Instruments TST350 Temperature Controlled Tensile Stress Testing Stage USER GUIDE Contents Before Setting Up Your Equipment... 3 Important Notice... 4 Warranty... 4 Technical Support...
100L AIR COMPRESSOR MODEL NO: TIGER 16/1010 PART NO: OPERATION & MAINTENANCE INSTRUCTIONS LS01/13
 100L AIR COMPRESSOR MODEL NO: TIGER 16/1010 PART NO: 2244025 OPERATION & MAINTENANCE INSTRUCTIONS LS01/13 INTRODUCTION Thank you for purchasing this product. Before attempting to use this product, please
100L AIR COMPRESSOR MODEL NO: TIGER 16/1010 PART NO: 2244025 OPERATION & MAINTENANCE INSTRUCTIONS LS01/13 INTRODUCTION Thank you for purchasing this product. Before attempting to use this product, please
Tex-414-A, Air Content of Freshly Mixed Concrete by the Volumetric Method
 by the Volumetric Method Contents: Section 1 Overview...2 Section 2 Apparatus...3 Section 3 Sampling Requirements...5 Section 4 Procedures...6 Section 5 Calculation...9 Section 6 Archived Versions...10
by the Volumetric Method Contents: Section 1 Overview...2 Section 2 Apparatus...3 Section 3 Sampling Requirements...5 Section 4 Procedures...6 Section 5 Calculation...9 Section 6 Archived Versions...10
TJF-Q180V Cleaning and Disinfection Checklist
 TJF-Q180V Cleaning and Disinfection Checklist TJF-Q180V Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-Q180V has been performed
TJF-Q180V Cleaning and Disinfection Checklist TJF-Q180V Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-Q180V has been performed
Model VR6 System. Installation, Operation & Maintenance
 Model VR6 System Installation, Operation & Maintenance General: All Archer Instruments chlorination systems are carefully designed and tested for years of safe, accurate field service. All Archer Instruments
Model VR6 System Installation, Operation & Maintenance General: All Archer Instruments chlorination systems are carefully designed and tested for years of safe, accurate field service. All Archer Instruments
User s Guide. CO2 Controller for miniature incubators
 CO2 Control User s Guide CO2 Controller for miniature incubators Precise CO2 Control throughout the experiment Media ph control Compatible with any perfusion system Miniature incubators for any microscope
CO2 Control User s Guide CO2 Controller for miniature incubators Precise CO2 Control throughout the experiment Media ph control Compatible with any perfusion system Miniature incubators for any microscope
Sunrise Fountain Water Wonders Assembly & Mounting Instructions
 Welcome to the Water Wonders family. A few simple steps will ensure that your Sunrise Fountain remains a soothing, enjoyable fountain that brings the sights and sounds of nature to you. Two important considerations
Welcome to the Water Wonders family. A few simple steps will ensure that your Sunrise Fountain remains a soothing, enjoyable fountain that brings the sights and sounds of nature to you. Two important considerations
Contents. Stainless Steel Side Block. 1.1 Separating the Side Block. Stainless Steel Side Block Reassembly of. Assembly from the Helmet Shell
 Separating the Side Block Assembly from the Helmet Shell Contents SSB-1 SSB-3 SSB-5 SSB-5 SSB-7 1.1 Separating the Side Block Assembly from the Helmet Shell 1.2 Side Block Assembly Replacement 1.3 Defogger
Separating the Side Block Assembly from the Helmet Shell Contents SSB-1 SSB-3 SSB-5 SSB-5 SSB-7 1.1 Separating the Side Block Assembly from the Helmet Shell 1.2 Side Block Assembly Replacement 1.3 Defogger
