PN A5. CyTOF 2 Mass Cytometer User Manual
|
|
|
- Aubrey Hunter
- 6 years ago
- Views:
Transcription
1 PN A5 CyTOF 2 Mass Cytometer User Manual
2 Table of Contents PREFACE 2 3 CHAPTER INTRODUCTION TO CyTOF 2 and MASS CYTOMETRY Principles of Mass Cytometry 5 Sample Introduction 7 Ionization 11 Mass Analysis 13 Data Acquisition 19 CHAPTER PREPARING YOUR LABORATORY FOR THE CyTOF 2 MASS CYTOMETER Introduction 22 Instrument Dimensions and Layout 23 Electrical Requirements 26 Gas Requirements 28 Exhaust Requirements 30 Environmental Requirements 33 Materials Required for Operation 34 Summary 35 CHAPTER INSTRUMENT INTERFACE CHAPTER SOFTWARE INTERFACE CHAPTER CyTOF 2 OPERATION Preparation and Start Up 53 Overview of the Software Interface and Fluidic System 62 Daily QC 64 Manual Tuning 74 Bead Sensitivity Test 86 Daily Cleaning 92 Sample Acquisition 94 i
3 Shutdown: Turning Off Plasma 96 Other Features 97 Unexpected Plasma Outages 101 Consumables 107 CHAPTER MAINTENANCE Overview of CyTOF 2 Maintenance and Cleaning 108 Cleaning the Nebulizer after Plasma Shutdown 112 Maintenance of the Spray Chamber and the Torch Assembly 116 Cleaning the Load Coil 121 Removal of the Cones 123 Cleaning of the Cones 127 Reinsertion of the Cones 128 Reassembly of the Torch 129 Installation of Torch Assembly 131 Checking the Torch Alignment 133 Instrument Air Filters 136 Rotary Pumps 136 Unscheduled Maintenance 144 Procedure for Expected Power Outages 148 CHAPTER SAFETY Introduction 150 Safety Alert Conventions General Safety Guidelines 152 Environmental Conditions 153 Electrical Safety 154 Chemical Safety 157 Pressurized Gas Safety 159 Other Hazards 162 References 163 CHAPTER 8 TROUBLESHOOTING ii
4
5 Preface This manual provides: An overview of the CyTOF 2 instrument and technology, Instructions for calibration, operation, data acquisition and maintenance, Safety recommendations for operation of the instrument, Troubleshooting recommendations. This document contains information proprietary and confidential to Fluidigm Corporation and is for customer use in the operation and maintenance of CyTOF equipment or is for vendor use in the specification, fabrication and manufacture of Fluidigm Corporation designed component parts. Any other use, disclosure or reproduction of the information contained herein is strictly forbidden, except as Fluidigm Corporation may authorize in writing. Equipment described in this document may be protected under one or more patents filed in the United States, Canada and other countries. Additional patents are pending. Software described in this document may be furnished under a license agreement. It is against the law to copy the software on any medium, except as specifically allowed in the license agreement. Portions of this document may make reference to other manufacturers products, which may contain parts that are patented and may contain parts whose names are trademarked. Any such usage is intended only to designate those manufacturers products as supplied by Fluidigm Corporation for incorporation into its equipment. Fluidigm Corporation assumes no responsibility or contingent liability for any use to which the purchaser may subject the equipment described herein, or for any adverse circumstances arising therefrom. This is a Class A device and is for use in commercial, industrial or business environments. Warning: This is a Class A product. In a domestic environment this product may cause radio interference, in which case the user may be required to take adequate measures. 2
6 Do not make an unauthorized modifications to your CyTOF 2 system or accompanying computer system. The computer system has been configured to for the use only with the CyTOF 2 system. It is recommended that no modifications or updates to the operating system and drivers be performed. Installation of non-essential software be kept to a minimum. C7-UM-01 Rev 5 3
7 Chapter 1 Introduction to CyTOF 2 and Mass Cytometry The CyTOF 2 mass cytometer analyzes individual cells labeled with stable heavy metal isotopes using state of the art Time of Flight Inductively Coupled Plasma mass spectrometry (TOF ICP MS) technology (Figure 1.1). With over 120 detection channels, the CyTOF 2 has the exquisite ability to simultaneously resolve multiple elemental probes per cell at high acquisition rates without the need for compensation, thereby maximizing the per cell information obtained from a single sample. These attributes provide researchers with an unparalleled ability to generate high resolution phenotypic and functional profiles of cells from normal and diseased states. Figure 1.1 The CyTOF 2 Mass Cytometer. 4
8 Principles of Mass Cytometry Mass cytometry employs elemental tags that have higher molecular weights than those elements that are naturally abundant in biological systems. The CyTOF 2 is specifically designed to measure these high mass elemental tags on a per cell basis. Cells stained with metal conjugated probes in a single cell suspension are introduced into the CyTOF 2. The cells undergo a multi step process within the instrument, resulting in generation of a file that records the identity and amount of each probe on each cell (Figure 1.2). Figure 1.2 Mass Cytometry Workflow. A liquid sample containing cells labeled with heavy metal isotope conjugated probes (A) is introduced into the nebulizer (B) where it is aerosolized. The aerosol droplets are directed into the ICP torch (C) where the cells are vaporized, atomized and ionized. Low mass ions are removed in the RF Quadrupole Ion Guide (D), resulting in a cloud of ions enriched for the probe isotopes. The ion cloud then enters the Time of Flight (TOF) chamber (E) where the probes are separated on the basis of their mass to charge ratio as they accelerate towards the detector. The time resolved detector thus measures a mass spectrum (F) that represents the identity and quantity of each isotopic probe on a per cell basis. Data is generated in.fcs format (G) and analyzed in third party software programs (H). 5
9 A schematic of the instrument is shown in Fig 1.3, divided by color to indicate the major steps of mass cytometry workflow. Each of these steps is described in detail in the following section. Figure 1.3 CyTOF2 schematic. Mass Cytometry workflow is divided into sample introduction (blue), ionization (yellow), mass analysis (green), and data acquisition (red). 6
10 Sample Introduction The sample introduction system de solvates the liquid sample suspension and introduces cells one at a time into the ICP source for ionization (Fig 1.4). The liquid sample is introduced (manually via syringe or automatically via Autosampler) into a nebulizer where it is aerosolized into a heated spray chamber. Within the spray chamber, the high temperature partially vaporizes the aerosol, and argon gas directs the aerosolized cells to the ICP source. These steps are described in detail below. Figure 1.4 Sample Introduction. The liquid sample suspension is syringe injected, then aerosolized by the nebulizer into the spray chamber, which partially vaporizes the aerosol and delivers it to the plasma. Delivery of sample to the nebulizer Liquid cell suspensions are introduced into the instrument manually using a syringe or automatically using an Autosampler. Manual Introduction The manual sample introduction system upstream of the nebulizer is composed of the sample syringe, syringe drive, flow injection valve, dual sample loop system, waste vessel, and carrier fluid vessel (Figure 1.5). First, the initial sample is loaded into a 1 ml syringe and injected through the sample loading port into one 500 L loop of tubing of the dual sample loop system. During this step, the flow injection valve is rotated to open a fluidic pathway from the sample syringe through the sample loop and out to the waste vessel. Thus, any sample in excess of 500 7
11 L is lost to the waste vessel circuit. Once the sample is loaded into the loop, the flow injection valve rotates, opening a fluidic pathway from the syringe drive through the sample loop to the nebulizer. Then the syringe drive pushes carrier fluid through the fluidic circuit, delivering the sample to the nebulizer. The syringe drive controls the volumetric flow rate, and is typically operated at 45 L/min. A couple of special features of the system optimize sample throughput by minimizing time between samples. First, the syringe drive automatically recharges with carrier fluid when it is low by drawing from the carrier fluid vessel, thereby eliminating the need to manually recharge the pump. Secondly, the dual sample loop system allows manual washing of the alternate sample loop during data acquisition from sample in the first loop. Figure 1.5 Schematic of Sample Introduction System upstream of the nebulizer. 8
12 Autosampler If the CyTOF2 is connected to the Autosampler (Fig. 1.6), samples loaded into 96 well plates are automatically introduced into the system, allowing unattended instrument operation and sample data acquisition. The autosampler contains a separate dedicated liquid sampling automation system that is described in detail in the CyTOF Autosampler Manual. Figure 1.6 Image of the AS 5 autosampler. Delivery of de solvated sample aerosol to the ICP source For liquid sample analysis, it is critical to remove as much water as possible from the sample so that it can be efficiently ionized in the plasma. This is achieved first by aerosolizing the sample in the nebulizer followed by delivery of heated aerosol to the plasma by the spray chamber (Fig 1.7) Nebulizer The CyTOF 2 employs a glass concentric nebulizer consisting of an inner capillary that carries the liquid sample and an outer chamber that carries argon gas flow (called nebulizer gas). Both liquid (at 45 ul/min) and gas (at L/min) flows are directed towards the spray chamber through a tapered end (Fig. 1.8). Because the liquid chamber has a small inner diameter, the sample velocity is high and pressure is low within the nebulizer, and as the sample exits the tip, concentric pressure exerted by the exiting nebulizer gas breaks it up into a fine droplet aerosol. 9
13 Figure 1.7 Sample aerosolization and delivery to the ICP torch. Liquid cell suspension is aerosolized by nebulizer gas as it exits the nebulizer. Make up gas carries the aerosol through the heated spray chamber where it is partially de solvated and delivered to the ICP torch. Figure 1.8 Nebulizer. Liquid sample enters from the left and argon Nebulizer gas from the bottom. Sample chamber narrows into a capillary, pulling liquid rapidly to the tip (enlarged, at right, with liquid sample indicated in red) where shear forces exerted by accelerated nebulizer gas break the liquid into aerosol droplets. Spray Chamber The aerosolized sample exits the nebulizer directly into the spray chamber, which is housed within a 200 C heating block. Argon gas (called make up gas) is pumped into the spray chamber (~0.7 L/min), and this high flow of heated gas partially vaporizes the sample to minimize condensation for optimal ionization as it directs the aerosol to the ICP source. 10
14 Ionization The mixture of single cell aerosol droplets and argon that exits the spray chamber is transmitted to the ICP source where it is successively vaporized, atomized and ionized in the plasma for subsequent mass analysis (Fig 1.9). The formation and characteristics of the plasma responsible for the ionization process are described below. Figure 1.9 Electromagnetic energy generated by the RF load coil surrounding the quartz torch sustains argon plasma (orange) that vaporizes, atomizes, and ionizes individual cell aerosols from the spray chamber. The positive ion component of the cell derived plasma cloud enters the ion optics and mass analyzer chambers of the CyTOF2 through the interface. Plasma Torch The plasma is created and maintained within the plasma torch by induction using a radiofrequency generated electromagnetic field. The torch consists of the torch body a fused assembly of two concentric quartz tubes and a quartz sample injector tube that is inserted inside the torch body. When assembled, the torch consists of three concentric chambers. The outermost chamber (between the torch body tubes) contains argon plasma gas flowing at 17 L/min that is ignited to form the plasma. The central chamber (between the inner torch body tube and the sample injector) contains argon auxiliary gas flowing at ~1 L/min that is used to change the position of the base of the plasma relative to the sample injector. The innermost chamber inside the sample injector transmits the argon stream and sample aerosol from the spray chamber directly into the center of the plasma. The torch assembly is mounted inside an induction load coil that is supplied with radio frequency generated current that creates an electromagnetic field. 11
15 Formation of the ICP discharge and ionization of the sample Plasma, the fourth state of matter consisting of charged particles, is formed by collisioninduced ionization of argon gas within an intense electromagnetic field. First, argon plasma gas flows tangentially from the outer chamber of the torch body. RF power supplied to the load coil produces an oscillating current (40 MHz), creating a strong electromagnetic field precisely at the point the plasma gas exits the outer chamber. A high voltage spark strips away free electrons from the exiting argon atoms creating an initial cascade of free electrons. These free electrons accelerate dramatically in the electromagnetic field and collide with sufficient energy to ionize the argon gas into plasma. Temperatures within the plasma typically range from 5,000 to 10,000K. When the aerosolized sample is introduced through the injector into the base of the plasma, the water droplets are rapidly vaporized. The de solvated individual cells are then broken down into a cloud of ground state atoms. Subsequent electron collisions result in ionization of the cell. Thus, the argon ion beam that exits the plasma contains bursts of ion clouds corresponding to individual cells that were introduced into the torch in aerosol form (Fig. 1.10). Figure 1.10 Cross section of the CyTOF 2 plasma torch. 12
16 Mass Analysis The ion beam exiting the plasma contains a heterogeneous mixture of argon ions, endogenous cellular ions, isotopic probe ions, neutral particles, and photons. The beam travels through the interface region into a series of low vacuum chambers that contain ion optics to eliminate unwanted materials and the time of flight mass analyzer to separate the isotopes of interest for downstream quantification and data analysis (Fig 1.11). Figure 1.11 CyTOF2 ion optics. The ion beam leaving the torch enters the low pressure ion optical chamber (green) through the 3 cone interface (red). High mass ions leaving the Quadropole Ion Guide are directed to the Time of Flight chamber (black) where they are separated on the basis of mass to charge ratio and directed to the detector. 13
17 Interface Region In order to analyze the identity and amount of isotopic probes in each cell derived cloud, the high temperature ion beam exiting the plasma at atmospheric pressure (760 Torr) passes through a series of ion focusing and separating chambers. These require low vacuum to eliminate any collisions with gas molecules on the pathway to the detector. To achieve this, the plasma is sampled through an interface region that dramatically reduces the temperature and pressure of the incoming ion clouds (Fig. 1.12). The purpose of the interface region is to efficiently transport ions from the high temperature plasma at atmospheric pressure to the room temperature chambers that house the ion optics at less than 10 3 Torr. The CyTOF2 uses a three cone interface to transport the ion beam into a low pressure vacuum: sampler (1.1 mm diameter orifice), skimmer (1 mm) and reducer (1.2 mm). All three cones are made of nickel, and the interface housing is water cooled to dissipate the significant heat generated by the plasma. The rapidly expanding ion clouds exiting the plasma enter the sampler cone orifice into the sampler skimmer region, which is pumped by a 40 m 3 /h rotary pump to Torr. The ions then pass through the skimmer cone to the skimmer reducer region, which is pumped by the 25 L/s stage of the 3 stage turbo molecular pump to 2 4x10 2 Torr. Finally, the ions pass through the reducer cone which serves not only to reduce the pressure (300 L/s stage of the 3 stage pump de pressurizes the chamber to 3 5x10 4 Torr), but also to focus the ion beam to the downstream ion optics. The ions that emerge from the reducer cone are accelerated and focused by an electrostatic field defined by the potentials of the reducer and a downstream conical lens, and the subsequent highly focused beam is propagated to the ion optics and mass analyzer. 14
18 Figure 1.12 The vacuum interface includes three nickel interface cones: sampler (red), skimmer (blue) and reducer (green). Cells ionized in the plasma (yellow) expand to approximately 2 mm in size before entering the interface through the sampler cone. Once inside the vacuum, the ion clouds no longer expand. Quadrupole Ion Deflector The beam propagating through the reducer contains some non ionized material and photons in addition to ions. If not filtered, neutrals can attach to instrument components resulting in signal drift, and photons that reach the detector are registered erroneously as ions. To eliminate these problems, the ions in the beam are deflected perpendicularly through an electrostatic quadrupole ion deflector. This turns positively charged ions towards the downstream ion optics, while neutrals and photons follow an undisturbed pathway into the turbo molecular pump. RF Quadrupole Ion Guide The pure ion beam leaving the quadrupole ion deflector is dominated by low mass ions that are not of analytical interest (H+, C+, O+, N+, OH+, CO+, O2+, Ar+, ArH+, ArO+) and that are of such 15
19 high abundance that they would quickly damage the detector. To remove these ions, the beam is focused via an Einzel lens and directed into the RF only Quadrupole Ion Guide (Fig. 1.13). The four rods of the quadrupole are supplied with alternating current (AC), with opposing pairs of rods always having the same AC charge that alternates based on the radio frequency setting. Low mass ions (m/z<80) gyrate dramatically and are ejected from the central path of the quadropole, while high mass ions are focused (ie guided) through this pathway. For optimal mass filtration performance, the Ion Guide chamber is pumped by the 400 L/s stage of the 3 stage turbo molecular pump to 2 5 x 10 6 Torr. As a result, a stream of burst events (corresponding to individual cells) that contain only the high molecular weight isotopic probes exits the RF Quadrupole Ion Guide. Figure 1.13 The Quadrupole Ion Guide removes unwanted low molecular weight argon and endogenous cellular ions from the beam that emerges from the quadrupole Ion Deflector, transmitting clouds that contain isotopic probe ions (>80 amu) to the TOF analyzer. Time of Flight Mass Analyzer The burst event ion clouds that exit the Ion Guide consist of a mixture of high molecular weight probes in a randomly distributed array. These ions are then sent to the orthogonal acceleration reflectron Time of Flight (TOF) mass analyzer, which separates the probe ions on the basis of the mass to charge ratio (Fig. 1.14). 16
20 Figure 1.14 Separation of ions in the TOF chamber. Ion clouds are subjected to an electrostatic force that orthogonally accelerates the incoming ions toward the detector. As a result, the ions separate based on their mass/charge ratio, with lighter elements reaching the detector first. The cylindrical beam exiting the Ion Guide first passes through the DC Quadrupole Doublet, which flattens the beam so that it can enter through the rectangular entrance slit into the accelerator chamber of the TOF analyzer (maintained at 10 6 Torr by the TOF turbo molecular pump). At 13 s intervals (frequency of 76.8 khz), a pulse of several hundred volts is applied to the push out plate, accelerating the accumulated packet of ions orthogonally toward the reflector, which redirects the ions toward the detector. The electric fields in the accelerator and reflector are configured to focus ions of into tight time resolved bands regardless of initial position or energy. The relationship between time of ion flight to the detector and their m/z is: in which t0 and A are derived from the mass calibration procedure. Because the isotopes used for probes in mass cytometry have the same charge, each packet of ions resolves into a series of bands, with the lightest probes reaching the detector first and each successively heavier mass reaching the detector at a later time interval. Each time resolved band of ions of mass M is separated from its M+/ 1 neighbor by ns. After the first packet of ions is pushed out and detected, a second pulse pushes out the next packet of ions for detection and the cycle repeats until data acquisition is complete. 17
21 Vacuum system The mass analysis system requires high vacuum to prevent random collisions of ions with gas molecules as they travel to the detector. As described in the various sections above, the CyTOF2 employs a 5 stage differential pumping system to sequentially drop the pressure from 760 Torr outside the interface to 10 6 Torr in the TOF chamber (Table 1.1). The system includes the interface pump for the Sampler Skimmer chambers; a three stage turbo molecular pump for the Skimmer Reducer chamber (stage 1), the Deflector chamber (stage 2) and the Ion Guide chamber (stage 3); and the TOF turbo molecular pump for the TOF chamber. Under standard conditions the 5 stage vacuum system of the CyTOF 2 instrument operates at the five pressure ranges detailed in the table below. Table 1.1 CyTOF2 vacuum system Vacuum Pump Chamber Pressure (Torr) Interface Sampler Skimmer Stage 1 25 L/s Skimmer Reducer 2 4 x 10 2 Turbo molecular, 3 Stage Stage L/s Deflector 3 5 x 10 4 Stage L/s Ion Guide 2 5 x 10 6 Turbo molecular, TOF TOF x
22 Data Acquisition This section describes the process whereby the ions organized by mass in the TOF chamber are detected, converted into digital values and analyzed (Fig. 1.15). Figure 1.15 Detection of ions and data analysis workflow. Detector The ions separated in the TOF chamber are detected using a discrete dynode electron multiplier. When an ion strikes the first dynode of the detector, several secondary electrons are liberated. These electrons strike the next dynode where they generate more electrons. This process is repeated at each dynode, resulting in an electron pulse that is captured by the anode of the detector. The output analog signal is amplified and converted by a dual 8 bit digitizer to digital values at 1 ns sampling intervals. The digitizer trigger delay dictates the first mass channel (i.e. the lowest mass registered) to be recorded per push while the segment length dictates the mass range to be recorded per push. Instruments are set to collect data from at least 120 mass channels (each corresponding to 1 amu), typically starting at mass 88. Dual Count Scale CyTOF resolves multi element samples using time of flight, with ions from each isotope arriving at the detector centered in discrete ns time windows (within each 13 s push) depending on their mass to charge ratio. At very low particle concentrations, the probability of pulse 19
23 signal overlap is negligible, and particle count is most precisely determined by simply counting the number of pulses (i.e. Pulse Count, Fig. 1.16, left). As particle concentration increases, ion pulses begin to arrive at the detector at the same time. In this situation, pulse count underestimates the true ion count, and integrated intensity becomes a more accurate measurement (Fig 1.16, right). The range of data that CyTOF collects requires collection of Dual Data, which means that Pulse Count and Intensity values are collected for every channel. CyTOF plots the entire data range on a single Dual Signal scale, the units of which are actual counts of particles that hit the detector. To achieve this, two things are done. First, a Dual Count Coefficient is applied which converts analog Intensity into actual counts according to the following formula: Counts = Intensity X Dual Count Coefficient Second, a dual switchover threshold is applied, below which Pulse Count is used and above which counts from coefficient converted analog Intensity is used. Using the dual count scale, CyTOF2 quantifies bound particles per cell across a wide range of signal input. Figure 1.16 Impact of analyte concentration on signal measurement. At low analyte concentration (left), pulses do not overlap. Because each pulse delivers a different number of electrons to the anode and therefore different intensity values, it is more precise to count pulses when ion concentration is very low. Here the pulse count is 1. At higher analyte concentrations (right), pulses overlap, and counting pulses will underestimate the true number of particles that hit the detector. Here the pulse count is 8 (if we count discernible peaks) even though 16 ions hit the detector. Thus, at high analyte concentration, it is more accurate to use integrated intensity, and convert this intensity value to counts using a calibration coefficient. Cell Detection and Acquisition Data File format Data for each 13 s push is digitized sequentially and integrated to obtain mass peaks for the channels selected for analysis. The resulting record is processed according to cell event selection criteria set by the user. These criteria include a minimum signal threshold and a range 20
24 for event duration consistent with single cell events. As a result, the data acquired contains the integrated number of total ion counts for each selected analyte on a per cell basis. These data are saved as text (.txt) and flow cytometry standard (.fcs) 3.0 format for data analysis in compatible software programs. 21
25 Chapter 2 Preparing Your Laboratory for the CyTOF 2 Mass Cytometer Introduction This chapter is designed to help you with the preparation for the reception and successful installation of your CyTOF 2 mass cytometer instrument. The CyTOF 2 mass cytometer is shipped to you as a complete system with the exception of the following items which must be obtained prior to installation: electrical power, exhaust vents, and argon gas supply with approved regulator. When preparing the laboratory for instrument installation by a Fluidigm Service Specialist, the following items must be considered: Receiving the instrument System layout Electrical requirements Argon gas requirements Exhaust ventilation Environmental conditions Materials required for maintenance and operation 22
26 Instrument Dimensions and Layout Crate Information The instrument is shipped in a single fully packaged crate. A standard pump truck with minimum rating for 1t/1600lbs is recommended for moving the crate if necessary. Once you have received the crate, store it in a dry place not exposed to weather until the scheduled installation date. Table 2.1 provides the dimensions of the instrument crate. Table 2.1 Dimensions of crated CyTOF 2 instrument. Component Width (cm/in) Height (cm/in) Depth (cm/in) Weight (kg/lb) CyTOF 2 213/84 106/42 157/62 635/1400 CyTOF 2 Information The CyTOF 2 system consists of the main instrument, a refrigerated chiller (Polysciences Cat# 6105PE) and a system computer with workstation as shown in Figure 2.1 below. C D A B Figure 2.1 CyTOF 2 mass cytometer (B) and components including the chiller (A), computer (C) and monitor (D). 23
27 The dimensions of the instrument, chiller and optional autosampler are given in Table 1. Note that the autosampler is designed to rest on the instrument shelf and so does not occupy additional lab space. The system computer may be placed on a separate bench or computer table (not supplied). Table 1.2: Dimensions of CyTOF 2, Chiller and Autosampler Component Width (cm/in) Height (cm/in) Depth (cm/in) Weight (kg/lb) CyTOF 2 97/38 132/52 79/31 285/628 Chiller 38/15 64/25 67/27 81/178 Autosampler i 39/16 24/10 36/14 20/44 It is recommended that the instrument be located near the required electrical and gas supplies. The length of the provided electrical cables is approximately 3.8m or 12.5ft. The CyTOF 2 mass cytometer is on wheels and can be moved for service and regular maintenance if necessary. It is also recommended that you leave a space of at least 30 cm (12 in) behind the instrument to provide adequate clearance for the vent hoses as shown in Figure 1. Allow space (approximately 50 cm /20 in) on the right side of the instrument for access to the circuit breakers. Access for most service procedures is through the front of the instrument. Figure 1.2: Footprint diagram of CyTOF 2 instrument and its accessories. 24
28 The front and rear vents of the chiller must be a minimum of 24 inches (61 cm) away from walls or vertical surfaces so air flow is not restricted. i The Autosampler is optional. When installed, it rests on the instrument shelf and therefore does not take additional lab space 25
29 Electrical Requirements Electrical Specifications Power to the CyTOF 2 instrument is to be delivered from two 30 A single phase V AC, Hz dedicated electrical branch circuits. Table 2.2 details the power specifications of the instrument and its accessories. Table 2.2 CyTOF 2 and accessories Power Consumption Specifications Power Consumption Instrument Maximum Volt Amperes (two circuits) Accessories Chiller (Powered through the instrument) Computer Autosampler (optional) 2 x 4500 VA 2300 VA 1050 VA 100 VA The operating range for the electrical supply is provided in Table 2.3. If the power line is unstable, fluctuates, or is subject to surges, additional control of the incoming power (e.g. surge protection or line conditioning) may be required. Table 2.3 CyTOF 2 Electrical Specifications Electrical Specification Operating Voltage V AC Peak Current (Per circuit) 30A Operating Frequency 50 or 60Hz ±1Hz Maximum Allowable Percent Sag 5 % Maximum allowable Percent Swell 5 % Maximum Supply Voltage Total Distortion 5% Maximum Supply Voltage Distortion by Single Harmonics 3% Phase (single or three) Single or between two of the three phases 26
30 Plug Information Table 2.4 provides the plug information for the instrument and accessories. Table 2.4 Electrical specification for CyTOF 2 instrument and accessories. Accessories Voltage (AC) Current CyTOF 2 2 x V 2 x 30A Chiller Through CyTOF 2 6A Autosampler (optional) V 4.2A Computer V 6A Monitor VAC 6A 60 Hertz Operation Connections The instrument is shipped with two 3.8 meters line cord cables. The installation kit includes two NEMA L6 30R plugs (250 V, 30 A) for use with two 60 Hz single phase outlets. 50 Hertz Operation Connections The instrument is shipped with two 3.8 meters line cord cables. It is up to the service person installing the instrument to wire the cables with IP44 2P+E 32A. The single phase connectors must be supplied by the customer. Connections to a three phase power Connection to a three phase power may be required (by local electrical code). The instrument can be connected to two phases and to the ground wire of the three phase line. The three phase plugs must be supplied by the customer. 27
31 Gas Requirements Argon Specification Ultra High Purity of argon is used as the ICP torch gas with the CyTOF 2 system. The quality criteria for argon are listed in Table 2.5. Table 2.5 Argon Requirements Gas Specification Argon Purity % Impurity Content Oxygen < 5ppm Hydrogen < 1ppm Nitrogen < 20ppm Water < 4ppm Argon gas at 80±1 psi (522±7 kpa) is to be supplied to the CyTOF 2 system from liquid or compressed gas storage tanks at a flow rate of approximately 20 L/min. The choice of liquid argon or compressed gas argon tanks is determined primarily by the availability of each and the usage rate. A regulator able to provide a pressure range of 0 to 100 PSI is required with a ¼ in Swagelok termination. Mechanical pressure regulators are recommended for the argon supply. Note: A liquid cryogenic Argon tank is preferred. Warning! Do not use electronic pressure regulator and auto switching valves as they may affect the plasma stability and may also result in frequent loss of plasma. Warning! It is recommended to install an Oxygen sensor in the room where the operator and gas storage are located. Safe Handling of Gas Cylinders The permanent installation of gas supplies is the responsibility of the user and should conform to local safety and building codes. The following are a list of safety precautions that should be observed when handling argon gas cylinders. 28
32 Fasten all gas cylinders securely to an immovable bulkhead or a permanent wall. When gas cylinders are stored in confined areas, ventilation should be adequate to prevent dangerous accumulations. Move or store gas cylinders only in a vertical position with the valve cap in place. Locate gas cylinders away from heat or ignition sources, including heat lamps. Cylinders have a pressure relief device that will release the contents of the cylinder if the temperature exceeds 52 C (125 F). When storing cylinders external to a building, the cylinders should be stored so that they are protected against temperature extremes (including the direct rays of the sun) and should be stored above ground on a suitable floor. Gas cylinders should be clearly marked to identify the contents and status (e.g. full, empty). Do not attempt to refill gas cylinders. Use only approved mechanical regulators and hose connectors. Left hand thread fittings are used for fuel gas tank connections whereas right hand fittings are used for oxidant and support gas connections. Arrange gas hoses away from foot traffic to avoid damage. Perform periodic gas leak tests by applying a soap solution to all joints and seals. 29
33 Exhaust Requirements The main venting system is required to remove fumes and vapors from the torch housing. Exhaust venting is important for four reasons: It protects laboratory personnel from ozone and hot argon generated in plasma It minimizes the effects of room drafts and the laboratory atmosphere on ICP torch stability. It helps protect the instrument from corrosive vapors which may originate from the samples. It removes dissipated heat which is produced by the ICP torch, ICP power supply and the pump motors. Exhaust Positions The CyTOF 2 instrument has two separate vents, both of which are located at the back of the instrument as show in Figure. The Torch Box Vent exhausts plasma and the vacuum pump system. It removes fumes and vapors from the torch housing and the rough pump exhausts. The torch box vent is 100mm or 4 inches in diameter. The System Vent exhausts heat from the blower that cools the roughing pumps, system power supply and RF generator. The system vent is 150 mm or 6 inches in diameter. Figure 2.3 Instrument rear view drawing with exhaust positions highlighted in red. 30
34 Flow Rates The CyTOF 2 instrument is supplied with 3.6 m (12 ft) of 100 mm (4 in) and 3.6 m (12 ft) of 150 mm (6 in) flexible hoses. A venting system that uses a single inlet duct, having a flow rate of 280 L/sec (600 cfm), should be divided into the two separate 100 mm (4 in) and 150 mm (6 in) ducts equipped with individual dampers. Ensure that there is access to the dampers during installation. Table 2.6 details the exhaust specifications. Table 2.6 Exhaust Specifications. Vent Hose Diam. mm (in) Flow Rate L/s (cfm) Anemometer m/s (ft/min) Vented Outside Lab Power W (BTU/hr) Torch Box 100 (4) 70 +/ 10% (150) 9 (1695) 200 (690) System 150 (6) 210 +/ 10% (450) 11.5 (2250) 2800 (9400) The flow rates as measured with the hoses connected to the ducts will need to be verified and adjusted during installation of the instrument. The static pressure drop caused by the CyTOF 2 system is 1.2 inches H 2 O (200 Pascal). Exhaust System Recommendations The exhaust flow rate at the instrument (the ability to vent the system) is dependent on the blower provided by the customer, the duct length, material and the number of elbows or bends used. If an excessively long duct system or a system with many bends is used, a stronger blower may be necessary to provide sufficient exhaust volume at the instrument. Additional recommendations on the venting system include: The duct casing and venting system should be made of materials suitable for temperatures as high as 70 C (160 F) and be installed to meet local building code requirements. Locate the blower as close to the discharge outlet as possible. All joints on the discharge side should be airtight. Equip the outlet end of the system with a backdraft damper. 31
35 Take the necessary precautions to keep the exhaust outlet away from open windows or inlet vents and to extend it above the roof of the building for proper dispersal of the exhaust. Equip the exhaust end of the system with an exhaust stack to improve the overall efficiency of the system. For best efficiency, make sure the length of the duct that enters into the blower is a straight length at least ten times the duct diameter. An elbow entrance into the blower inlet causes a loss of efficiency. Provide make up air in the same quantity as is exhausted by the system. An airtight laboratory can cause an efficiency loss in the exhaust system. Ensure that the system is drawing properly by placing a piece of cardboard over the mouth of the vent 32
36 Environmental Requirements The CyTOF 2 mass cytometer has been designed for indoor use only. The environment in which the instrument is installed should meet the following conditions: Room Temperature The room temperature should be between 15 and 30 C (59 and 86 F) with a maximum rate of change of 2.8 C (5 F) per hour. Relative Humidity The relative humidity should be between 20 and 80%, noncondensing. Elevation The instrument should not be operated at an elevation greater than 2,000m (6,500ft) above sea level. Use of the instrument at elevations greater than 2,000m is subject to acceptance by local inspection authorities. The instrument should be located in an area that is: Free of smoke and corrosive fumes, Not prone to excessive vibration, Out of direct sunlight, Away from direct sources of heating or cooling. Warning! Do not use the instrument in an area where explosion hazards may exist. 33
37 Materials Required for Operation Table 2.7 provides a list of the materials supplied with the instrument for the installation and operation of the instrument. Table 2.7 Materials supplied for CyTOF 2 instrument installation and operation Description Tuning solution, CyTOF, E Pure, 250 ml Washing Solution, 250 ml EQ 4 Element Beads Normject Syringes 1mL Normject Syringes 3mL Water MilliQ High Quality 18, Deionised water (DIW) of highest grade 18.2MOhm 5mL Round bottom tubes with 35um mesh cell strainer Powder free gloves 50 ml tube 15 ml tube Tuning cells Isopropanol, 100% Kimwipes Supplier Fluidigm Sciences Inc. Fluidigm Sciences Inc. Fluidigm Sciences Inc. Henke Sass Wolf Henke Sass Wolf Milli Q (Millipore) Catalog Number Quantity ~10ml ~10ml mL R per sample 4 units per operation session Constant supply BD Biosciences per sample Fluidigm Sciences Inc ml 34
38 Summary Table 2.8 provides a summary of the requirements for the successful installation of your CyTOF 2 instrument. Table 2.8 Instrument requirement summary Dimensions Width (cm/in) Height (cm/in) Depth (cm/in) Weight (kg/lb) Shipping Crate 213/84 106/42 157/62 635/1400 CyTOF 2 97/38 132/52 79/31 285/628 Chiller 38/15 64/25 67/27 81/178 Electrical Voltage (AC) Current CyTOF 2 2 x V 2 x 30A Computer V 6A Monitor V 6A Gas Purity Pressure Flow Argon ±1PSI 20 L/min Exhaust Hose (mm/in) Flow Rate (L/s & cfm) Anemometer (m/s & ft/min) Vented Outside Lab Power (W & BTU/hr) Torch Box 100/4 70 +/ 10% & & & 690 System 150/ / 10% & & &
39 Chapter 3 Instrument Interface This chapter contains annotated figures of the CyTOF 2 instrument. Status Panel Sample Introduction System Door Handle Front Access Door Figure 3.1 CyTOF 2 Front View. 36
40 DRAIN VESSEL Figure 3.2 Sample Introduction System Schematic. Flow Injection Valve Syringe Pump Drip Tray Drain Vessel Heat Shield Carrier Fluid Reservoir Figure 3.3 Sample Introduction System. 37
41 Make Up Gas Line Nebulizer Port Nebulizer Nebulizer Gas Line Sample Capillary Assembly Figure 3.4 Nebulizer and Connections. Nebulizer Holder Heat Shield Heater Power Cord Heater Ball Joint Clamp Figure 3.5 Heater and Related Parts. 38
42 Make Up Gas Line Spray Chamber Nebulizer Port Spray Chamber Ball Joint Injector Connection Heater Box Lid Figure 3.6 Spray Chamber and Connections. Flow Injection Valve Syringe Pump Figure 3.7 Flow Injection Valve and Syringe Pump. 39
43 Table 3.1 Flow Injection Valve Configuration. Port Number Color Code Function 1 Blue Nebulizer Line 2 & 6 Black Sample Loop 1 3 Brown Waste (Overflow) Line 4 & 8 Grey Sample Loop 2 5 White Upper Syringe Line 7 N/A Luer Injection Port N/A Green Carrier Reservoir Line Torch Assembly Thumb Screws Ball Joint Injector Guide Pins Figure 3.8 Front View of Torch Assembly. 40
44 Plasma Gas Line Auxiliary Gas Line Ignition Pin Plasma Gas Port Injector Injector Holder Injector Sealer Cap Auxiliary Gas Port Torch Body High Voltage Connector Figure 3.9 Rear View of Torch Assembly. RF Fingers Front Shield Load Coil Torch Body Sampler Cone Figure 3.10 Interior View with Front Access Door Open. 41
45 Torch Box High Voltage Connector Guide Pins Figure 3.11 Torch Box. 42
46 Table 3.2 Other CyTOF 2 Parts. Parts Image Location Circuit Breakers and Cords Right Side of Instrument Digital Readout of Vacuum Gauges, Heater Temperature, Make Up Gas and Nebulizer Gas Left Side of Instrument Skimmer/Reducer Cone Behind Sampler Cone 43
47 Table 3.3 CyTOF 2 Glassware. Part Image Nebulizer Spray Chamber Ball Joint Injector Torch Body 44
48 Chapter 4 Software Interface Table 4.1 Main Toolbar (Administrator Mode) Table 4.1 Button Window Function Access chiller and heater controls. Start up and shutdown plasma. Access Analog Controls. Perform manual XY alignment. Check instrument performance and optimize settings. 45
49 Table 4.1 Button Window Function Set syringe speed. Choose a template and set parameters for collecting sample data. Set parameters for analysis, concatenate FCS files and perform normalization. 46
50 Table 4.1 Button Window Function View data in bivariate plots. Perform clustering of data. Convert FCS file to Text format. 47
51 Table 4.1 Button Window Function Software view reflects status of panel on front of CyTOF 2. View settings for RFG power, detector voltage, Make Up and Nebulizer Gas, heater temperature and vacuum. 48
52 Table 4.1 Button Window Function Create and manage user accounts. Launch Cytobank website. Login as administrator or Service. Check software version. 49
53 Table 4.2 Tuning Mode Toolbar Table 4.2 Button Window Function Data Acquisition Settings Choose template and set parameters for Manual Tuning. Mass Graph (Mass Per Reading) View intensity, pulse count or dual count of selected isotopes. 50
54 Table 4.2 Button Window Function TOF Graph (TOF) View selected Time of Flight (TOF) Range or Mass Range. Rerun Run Stop N/A N/A N/A Continue to run sample from current sample loop. Switch valve to change sample loops. Stop viewing data. 51
55 Table 4.3 Syringe Toolbar Toolbar Item Function Status of syringe pump Syringe speed (ml/min) Sample Loop In Use Volume of Syringe Injected/Total Volume Progress Bar Syringe Start (Manual start of syringe) Manual Syringe Stop (Manual stop of syringe) Switch Valve (Manual switch of sample loop valve) Syringe Refresh Button (Empties and refills syringe) 52
56 Chapter 5 CyTOF 2 Operation This chapter describes daily operation of the CyTOF 2 Mass Cytometer, including: Preparation and Start Up Overview of the Software Interface and Fluidic System Daily QC Manual Tuning Bead Sensitivity Test Sample Acquisition Daily Cleaning Shutdown: Turning off Plasma Other Features Consumables Preparation and Startup 1. Check Status Panel lights. Open the CyTOF Software, and locate the Status Panel within the interface on the left. The panel parameter indicator lights should be lit as in the figure below. If the ARGON and AIR lights are not green, turn on the argon supply and check that the exhaust ( AIR ) level is correct. 53
57 2. Close the Status panel and click on. Log into the administrator mode by clicking on Login a. To login for the first time, enter administrator for username and leave password box empty. b. Click Login c. See <User Management> section for instructions on changing personal settings and managing other users 3. Turn on the Heater. a. Click. b. In the Switch Box tab of the Control Panel window, click on Heater> On. c. The Heater module will take ~ 20 minutes to reach 195 to 200 C 54
58 4. While the Heater is warming up, connect the Nebulizer following the steps below. Connecting the Sample Capillary to the Nebulizer Note: Wear gloves to prevent finger oil contamination on the nebulizer glass. 1. Unscrew Swagelok Nut from the connector at the end of the Nebulizer Gas line. Nebulizer Line 2. Remove the Front Ferrule and the black O-ring. Front Ferrule Black O-ring Swagelok Nut 55
59 3. Remove the clean Nebulizer from the Nebulizer soaking container and dry the surface with a Kimwipe (Do not touch tip of Nebulizer with the Kimwipe.). Excess water inside the Nebulizer should also be removed. 4. Put the side arm of the Nebulizer through the Swagelok Nut. Do not shake the Nebulizer. Side Arm Hose clamp Bump + Nebulizer Side Arm 5. Push the O-ring onto the side arm with a tool, such as the Nebulizer cap, pushing it over the hose clamp bump on the Nebulizer side arm. Nebulizer Cap 6. Place the ferrule over the O-ring with smaller orifice facing away from the Nebulizer. 56
60 7. Screw the nut of the union back together. Schematic of the Nebulizer side arm 8. Connect Sample Capillary tube to Nebulizer: a. Loosen the Flangeless Nut on the connector of the Sample Capillary. Flangeless Nut 57
61 b. Insert the sample capillary tubing into the sample inlet end of the nebulizer and push up to tapered portion of the glass capillary inside the nebulizer. Sample Capillary Tubing Tapered portion of the glass capillary 58
62 c. Tighten the Flangeless Nut. Flangeless Nut Sample Inlet of the Nebulizer Tapered Portion of the Glass Capillary Sample Capillary Tubing Flangeless Nut Schematic of sample capillary connected to nebulizer 59
63 Removing Excess Water from the Nebulizer and checking Nebulizer Spray 1. In Control Panel > Analog Control, find Nebulizer Gas. 2. Click Set Actual Current Value. This will start the flow of Nebulizer Gas. 3. Ensure that the Carrier reservoir is filled with deionized water. Once the nebulizer gas has dried all water residue from the nebulizer, click to start the syringe pump. 4. Observe the spray from the nebulizer. It should appear as a fine aerosol that leaves the nebulizer in an even, symmetrical pattern. If not, replace nebulizer. 5. Stop the syringe by clicking. Nebulizer Gas does not need to be stopped if this check is performed prior to starting plasma. 6. Insert the Nebulizer in the Nebulizer Port attached to the spray chamber until it reaches a hard stop. 60
64 Plasma Start 1. The heater should have reached optimal temperature of 200 C by minutes. 2. Check the Status Panel lights and ensure that the Argon light is green. 3. If necessary, fill Carrier reservoir and empty Waste. 4. In Control Panel > Plasma, click on Start Plasma. 5. When plasma starts, the software will give you the following dialog box and the Status panel updates. 61
65 6. Click OK and allow plasma to warm up for minutes. Overview of the Software Interface and Fluidic System This section provides a brief overview of the software interface and fluidic system. Software Interface 62
66 Syringe pump Start Syringe pump Stop Loop switch Reload syringe pump 63
67 Fluidic System The CyTOF 2 utilizes a syringe pump connected to a dual-loop system for sample introduction. Two sample loops (1 and 2) are connected to a single sample line through a flow injection valve. Once plasma has been lit, the syringe pump continuously pushes carrier fluid (DIW) into the active sample loop, as indicated by the software (see red box in figure below). When a new sample is loaded, it will fill the idle loop and be held there until the operator clicks either the Run or Preview button. When either of these buttons is clicked, the valve switches and carrier fluid is pushed through the previously idle loop, and data acquisition of the newlyloaded sample begins. The previously active loop is then idle and available for loading of another sample. Selecting Re-run or Re-preview will not cause valve switching and so sample acquisition will continue to be from the currently active loop. Users can check what loop is in use on the upper right syringe pump status bar as shown below: For optimal signal intensity and resolution, the Syringe Pump speed is set at 45uL/min (0.045 ml/min) and this defines the sample flow rate. The maximum flow rate at which plasma can be sustained is 60uL/min (0.060 ml/min). The syringe pump flow rate can be changed in the Sample Intro window. Daily QC The QC check of the CyTOF2 should be performed every day to ensure performance and data quality. If necessary, users can tune the instrument using the software automatically or manually. Before Starting the Daily QC procedure, check Background 1. Open the Acquisition window. 2. Click on the Control tab. 64
68 3. Click Preview to view background signal for one of the loops. 4. Click Preview again to check the other loop to ensure that the sample introduction system is clean and ready for Tuning. 65
69 Daily QC as part of Automatic tuning (Auto-Tuning) 1. Click on Tuning. 2. In the Profiles Tab, right-click in the table and the following selections will appear. Below is a detailed overview of the Profiles Tab. In the right click menu of the Profiles tab: a. New Calibration: contains new instructions for running a calibration. b. To Set Current Calibration (use results): applies the calibration results from the selected calibration profile. The instrument will be set with the optimum settings found in this profile. 66
70 c. To Set Default Calibration (use parameters for new): sets the calibration instructions in the selected profile as a template. New calibrations created will have the same instructions as this default calibration. d. Run Calibration: starts the calibration. Users need to ensure that the open loop is filled with Tuning solution before clicking Run Calibration as the flow injection valve will switch to acquire the originally open loop. This will only work for calibrations that are new. Completed, stopped, or failed calibrations cannot be run again. e. Re-run Calibration: starts the calibration without switching the flow injection valve, meaning that the acquisition will begin with the same loop that is already being acquired. This should only be used if the user is certain that there is sufficient tuning solution within the current loop. This will only work for calibrations that are new; completed, stopped, or failed calibrations cannot be run again. f. Delete all Non-completed Profiles: cleans up the calibration profile table. g. Reset Current Calibration (unset current profile): reverts to the previous settings of the instrument. h. Reset Default Calibration (unset the active params profile): removes the template settings for new calibrations. A Profile contains instructions for running calibration and results from performing this particular Tuning run. Most of the instructions for running the calibration are factory set, but the selection of the type of calibration to be done is available for administrators in the General Parameters tab: 67
71 Details of each parameter calibration are outlined within their respective tabs: The Results tab shows results of the calibration profile selected. These results are the optimized settings of parameters determined during the selected calibration and are applied to the instrument when the calibration is complete. 3. In the right click menu, select New Calibration. 4. Under General Parameters, check Enable QC Report. Dual Count Calibration is performed by default. 5. Inject 500uL of Tuning solution, and click Run in the Control Tab. 68
72 6. A progress log will appear in the control screen as below. 7. When calibration finishes successfully, click OK. 8. The QC report will be generated in the Results Tab. In the Results Tab, check that: a. The Dual Slopes are within to b. 159 Tb mean dual value is at least 400K. c. 155 Gd Mean dual to 159 Tb mean dual ratio is lower than Note: RSD is relative standard deviation and is equivalent to CV. 69
73 d. The RSDs are lower than 3% for Cs, La, Tb, Tm, and Ir If all of these criteria are met, proceed to Bead Sensitivity Test. Otherwise, proceed to Auto-Tuning: full calibration. Auto-Tuning: Full Calibration 1. In Tuning > Profiles Tab, right-click and select New Calibration. 2. Under General Parameters, ensure proper delay timings are set. Perform Auto-tuning with the following sub calibrations checked: DV Optimization, Dual Pulse Calibration, XY Optimization Gases/Current Optimization and QC report enabled in General Parameters. Mass calibration and Mass Resolution are automatically performed and calculated whenever Auto- Tuning is performed. 70
74 3. Open Detector V parameters and check Detector V1 and Detector V2. These two are to be respectively around +100 and -100 of the current Detector Voltage setting (found in the Analog Controls Tab). Change the settings to the appropriate values. 4. In the Gases/Current Optimization tab, set the following ranges for Gases: set Make-up Gas start to current Make-up gas (can be found in the Analog Controls window) 0.10, Make-up gas end to of start value, and Make-up gas step For instance, if the current Make-up gas is 0.80, set the following: The default values for Nebulizer gas and Current are sufficient for the tuning run. 5. Inject 500 µl of Tuning Solution, and click Run under the Control tab. 71
75 6. After clicking Run, the flow injection valve will switch and the other loop will be available for a new sample. 7. Inject another 500µL of Tuning solution. Once the first loop is finished, the second loop of tuning solution will automatically be acquired for the Auto-Tuning process to continue. 8. A progress log will appear in the control screen as below. 9. When calibration finishes successfully, click OK. In the Results Tab, ensure: a) The Dual Slopes are within b) 159 Tb mean dual value is at least 400K. c) 155 Gd Mean dual to 159 Tb mean dual ratio is lower than 3%. d) The RSDs are lower than 3% for Cs, La, Tb, Tm, and Ir (Note: RSD is relative standard deviation and is equivalent to CV). 72
76 If all of these criteria are met, proceed to Bead Sensitivity Test. If any of these criteria is not met, proceed to Manual Tuning. 10. The profile will be automatically applied and updated in the Analog Controls tab. This can be verified in the Monitor Window: Note: Values in the Monitor window are actual readings. Some of these may not match the Set values exactly (the optimal values displayed). 11. If settings are changed, they can be restored by selecting the Tuning Profile and rightclicking to choose Set Current Calibration (use results). 73
77 This will set any values optimized during the calibration run (i.e. Dual Slope, Detector voltage, Gas Settings, etc.). 12. Record pertinent values in the QC log from the Results tab, including: a. Resolution b. Dual slope values for Cs and Tm c. Mean Dual Count Tb value d. RSD (Dual) values for: Tb, Cs, La, Tm, Ir e. Mean 155Gd Dual counts f. Analog Controls: Detector Voltage, Nebulizer Gas, Makeup Gas, Current Manual Tuning If Auto-Tuning is unsuccessful due to reasons that cannot be resolved by changing appropriate parameters, proceed to perform Manual Tuning as described in the following steps. Mass Calibration 1. Inject Tuning Solution. 2. Select Tuning > Profile, right-click and select New Calibration. 74
78 3. In the General Parameters tab, de-select all tuning parameters except for Dual Calibration (which is selected by default). 5. Select Control tab, click Run. (Note: Running with no parameters selected will activate only Mass Calibration, Mass Resolution and Dual Pulse Calibration.) 75
79 Check Performance before Manual Tuning 1. Ensure that the Syringe Pump speed is set to ml/min in Sample Intro, or by checking the upper right portion of the software interface. 2. Inject 500µL of Tuning Solution into a Sample Loop. 3. In the Data Acquisition Settings window, set the data acquisition parameters as follows: Parameter is Reading by default if empty. Set Pushes/Reading to 204,800. Note: Since there are 76,800 pushes per second, 204,800 pushes equals 2.67 seconds per reading. This is the time required to measure 1 pg of Tb from the 0.5ppb tuning solution at a flow rate of 0.045ml/min. 4. Open the Masses per Reading window and select Dual counts for the Y axis and set max pulse counts to an appropriate value for your instrument. 5. Click Run 6. Wait until the signal stabilizes and observe 159 Tb and Mass 155 ( 155 Gd) Dual count values. a. If 159 Tb dual count levels are comparable to the levels in a well performing operating session (>400K with pushes per reading), AND b. 155 Gd/ 159 Tb ratio is below 3% and comparable to the level from previous days with good performance, begin Auto-Tuning or Manual Tuning. 76
80 c. If the signals are below specification, adjust XY alignment manually (see Manual Tuning > XY Alignment section below), then repeat/begin Auto-Tuning or Manual Tuning. XY Alignment 1. If needed, inject another 500µL of Tuning Solution. 2. Set up the data acquisition parameters in the Data Acquisition Settings window. a. Right click on the Analyte Table on the left to open Templates and select the Tuning Solution Template b. Parameter is Reading by default if empty. c. Set Pushes/Reading to 76,800 to allow 1 reading per second. d. Enter an End Value long enough to complete the alignment. An End Value of 200 (acquisition time of 100 seconds) is usually sufficient. 3. Open the Mass Graph (Masses per Reading) window 4. Select pulse counts for the Y axis and set maximum to 100,000. Adjust max pulse counts to capture Tb and Tm if necessary. Adjust value as needed for signal to be within scale. 77
81 5. Select Control Panel > Devices tab > XY > Setup 6. Align the window such that the Mass Graph Window and the XY Setup window are both visible. Note the Current Position for X and Y before proceeding
82 8. Click Re-run 9. While observing the pulse count signal in the Masses per Reading graph, change X value by steps of 3000 until signal is at its highest. 10. Adjust by smaller steps if necessary. 11. Repeat for the Y value. 12. Once optimal XY coordinates are found, users can choose to return to tuning the instrument with Auto-Tuning and deselecting XY Optimization to run the rest of the optimization. Dual Pulse Calibration and Detector Voltage Optimization 1. Select Tuning > Profile, right-click and select New Calibration. 2. Select DV Optimization in the General Parameters tab. Dual Pulse Calibration is selected by Default. 3. Inject 500µL of Tuning Solution, and click Run in the Control tab. 4. When the run is finished, note the Optimal DV from the Results Tab in Auto-Tuning window. 5. Verify this value is the same as the Detector Voltage in >Analog Controls 79
83 Makeup Gas and Nebulizer Gas Note: Only perform this tuning step if either: 159 Tb Dual Counts are significantly lower than the previous well performing operating session (>400K with pushes per reading), OR 155 Gd/ 159 Tb ratio is at 3% or above. If necessary, tune Makeup and Nebulizer gasses according to the following protocol: 1. In Control Panel >Analog Controls, go to Nebulizer Gas and take note of the current Nebulizer Gas value. Record value in the Gas_Current_X-Y worksheet in the CyTOF2 Manual Tuning Log. Value to record 2. Open the Mass Graph window and Data Acquisition Settings window. Arrange the windows so that both windows are easily accessible. 80
84 3. Select Makeup Gas and enter the parameters as follows: set Make-up Gas start to current Make-up gas value (can be found in the Analog Controls tab) 0.10, Make-up gas end to of start value, and Make-up gas step For instance, if the current Make-up gas is 0.80, set the following: Set Settling Time to 2000 ms, and Pushes/Reading to In the Mass Graph window, select Dual Count for the Y-axis and set the maximum count for the Y-axis to an appropriate value for the instrument. 5. Inject 500µL of tuning solution, and click Run. 6. Select the Makeup Gas value at which 159 Tb Dual Count is at maximum when ratio of Mass 155 Gd/ 159 Tb is <3%. 7. Record this value in the Gas Flow Optimization Log worksheet in a CyTOF QC Log File in the following format: 8. Repeat this process for Makeup Gas for different Nebulizer Gas values from of initial set point up to For example, with an initial set point of 0.15, ramp Makeup Gas at Nebulizer Gas settings of: 0.15, 0.17, 0.19, Fill in the data obtained at each Nebulizer Gas setting for Tb159 and Mass155 dual counts at each Optimal Makeup Gas value. 81
85 Note: If the 159 Tb Dual Count is comparable to the result from the day before, and the ratio of 155 Gd/ 159 Tb is lower than 3% after ramping Makeup Gas at existing Nebulizer Gas, you do not need to ramp again with different Nebulizer Gas settings. Note: It is not necessary to lower Nebulizer Gas for the ramping, because over time the Nebulizer nozzle expands and it is unusual that lower Nebulizer Gas will give higher performance. However, when a new nebulizer is installed, it is advisable to set the nebulizer flow at a lower value (e.g. 0.15) prior to ramping the Makeup Gas. Nebulizer Gas Value (L/min) Optimal Makeup Gas value (L/min) Tb159 Dual count Mass 155 Dual count Mass155/Tb , % , % , % , % 9. Using the table just created, choose the combination of Nebulizer Gas and Makeup Gas where the 159 Tb signal is the highest as long as the 155 Gd/ 159 Tb ratio is below 3%. In this example (see figure above) the optimal combination is: Nebulizer Gas at 0.17 and Makeup Gas at Enter the gas values in Control Panel > Analog Controls and click Set Actual Current Value. 11. If 155 Gd to 159 Tb ratio does not go lower than 3%, try a new Nebulizer. Update these 2 values 82
86 Current Optimization Note: Only perform this tuning step if the 159 Tb Dual Counts are significantly lower than the previous well-performing operating session (>400K with pushes per reading). If necessary, tune Current according to the following protocol: 1. Open the Masses per Reading and Data Acquisition Settings windows. Arrange the windows so that both windows are easily accessible. 2. Select Dual count for the Y-axis and set the maximum count for the Y-axis to an appropriate value for the instrument. 3. Select Current and enter the parameters as shown below: Start Value: 3 End Value: 10 Step Value: 0.5 Settling time: 500 ms Pushes/Reading:
87 4. Inject 500µL of tuning solution, and click Run. Max Tb at current = Choose the Current value at which the Tb Dual Count is at maximum. 6. Enter this Current value in the Analog Controls under Control Panel. Click on Set Actual Current Value Update this value to Current for max Tb sensitivity (e.g. from 4.5 to 7) 84
88 When Manual Tuning is Complete 1. Save a file for 30 readings for the Mass(es) per Reading Graph using the Tuning Solution analyte template with the following parameter settings. Note: To clear the selection for Parameters, click the dropdown menu and press ESC. The 159 Tb dual counts should be >400, Record the following values in the CyTOF2 QC Log: From the Control Panel > Analog Controls Tab: a. Makeup Gas b. Nebulizer Gas c. Current From the Setup>X-Y Setup>Setup a. Current X-Y Values From the Active Auto-Tuning Profile > Results Tab a. Optimal Detector Voltage (DV) 85
89 Bead Sensitivity Test Note: EQ Four Element Calibration Beads (Cat#201078) or CyTOF Calibration Beads (Cat#201073) may be used for the bead sensitivity test. 1. Open, specify in the directory a location to save the file, and set up the experiment details such as Acquisition files and parameters as follows: 2. Right-click in the analyte table on the right to apply a template that contains the correct analytes for the Calibration beads used (F3), or to make a new template with the periodic table and save the template by selecting Create Template From (F7). 3. Use the default settings in the Analysis Parameters Tab. 4. Vortex the beads to ensure that the beads are well-mixed before injecting. 5. Inject 500µL of beads, and click Run in the Control Tab. 6. Once the acquisition finishes, observe the data in a third party FCS file reader. 7. Gate singlet population and doublet population. Add event # of singlets to event # x 2 of doublets. This total should be at least 9,000 events at a flow injection speed of ml/min. If not, rerun beads. 8. Check that the mean of singlet population for 153 Eu is at least If not, rerun beads. Note: You may also use plotviewer for the Bead Sensitivity Test. 1. Open Plotviewer. 86
90 2. From the file menu, open the bead QC FCS file. 87
91 3. From the dropdown menu select Cluster Current View Events from the File menu. The window below will appear. Note: The values above are saved in the software as default. 88
92 4. In the Clustering window select Eu151Di and Eu153Di. In the Number of Clusters box Enter 3. Make the following changes highlighted by red boxes shown below in the Clustering window. For the X-Axis select Eu151Di and for the Y-Axis select Eu153Di from the dropdown. 5. Click Run. a. This creates a cluster file. This is saved with the original FCS file name with _clustered.fcs added. 6. After the clustering is done, the data will appear in a new window(see below). 89
93 Note: By default, the plot will show all clusters (all events). 7. In this case, cluster 0 shows debris, cluster 1 shows doublets/aggregates, and cluster 2 shows singlets. Cluster 0 Cluster 1 Cluster 2 90
94 8. While viewing the singlet cluster, check the cluster events and Eu153 Di mean (m) to see if the minimum bead QC specifications have been met. a. Minimum bead QC specs are: i. Singlet events 9000 ii. Eu153Di(m) 1000 Note: The above bead specifications are the lowest acceptable values. A well maintained instrument which has been tuned is capable of achieving similar bead specifications. If your instrument achieves 20,000 singlet events and suddenly decreases by 50% check the following. Thoroughly mix EQ4 beads (vortex or shake) Clean the sample introduction system (e.g. nebulizer and sample capillary) 91
95 Daily Cleaning Cleaning after Running Tuning Solution 1. Inject 1mL of Washing Solution and click to switch the loop. 2. Wait 2-5 minutes to allow Washing Solution to run through. 3. Repeat steps 1-2 to clean the other loop. 4. Inject 1mL of DIW and click to switch the loop. 5. Wait 2-5 minutes to allow DIW to run through. 6. Repeat steps 4-5 to clean the other loop 7. Check the status by clicking Preview in the Control tab. This will display 10 snapshots of any ion signal traces that are detected. 8. Repeat for the other loop. Cleaning after Running Beads 1. Inject 1mL of DIW and click to switch the loop. 2. Wait 2-5 minutes to allow DIW to run through 3. Click Re-Preview to check for residual beads. 4. Repeat steps 1-3 to clean the other loop. 5. If the beads are persistent in the loops, inject 500uL of Washing Solution and click to switch the loop. 6. Wait 2-5 minutes to allow Washing Solution to run through 7. Repeat steps 5-6 to clean the other loop 8. Flush the Washing Solution out by injecting 1mL of DIW and clicking to switch the loop. 9. Repeat to flush the other loop 10. Wait 2 minutes to allow DIW to run through. 11. Click Preview to check status for both of the loops before proceeding. 92
96 Cleaning Between Samples 1. Inject 1-3mL of DIW and click to switch the loop. 2. Wait 2-5 minutes to allow DIW to run through. 3. Repeat steps 1-2 to clean the other loop. 4. Check background signal using Preview. a. If background signal has returned to baseline, proceed to the next sample. b. If background signal is high, inject 1 ml of Washing Solution and click to switch the loop. c. Inject another 1mL to clean the second loop. d. Wait at least one minute for washing solution to run through. e. Inject 1mL DIW and click to flush through. Repeat for the other loop. f. Check in Preview before proceeding to the next sample. Cleaning Between Experiments or at the End of the Day 9. Inject 1mL of Washing Solution and click to switch the loop. 10. Wait 2-5 minutes to allow Washing Solution to run through. 11. Repeat steps 1-2 to clean the other loop. 12. Inject 1mL of DIW and click to switch the loop. 13. Wait 2-5 minutes to allow Washing Solution to run through. 14. Repeat steps 4-5 to clean the other loop. 15. Click Preview to check both loops. 16. Repeat if background signal has not returned to baseline. 93
97 Sample Acquisition Sample Preparation Please refer to Fluidigm protocols for sample preparation. Before Acquisition It is strongly recommended that users add diluted CyTOF EQ Beads to samples as an internal standard: 1. Vigorously shake or vortex the bottle with EQ Beads. Then dilute the EQ Beads 1/10 in DIW. 2. Add the diluted EQ Beads directly into the vial with the pelleted sample, and mix well. This will be the sample for acquisition. 3. The resulting files can be normalized with the Normalizer Tool in the CyTOF Software. Please see Bead Normalization Tool section for instructions. Set up Acquisition Parameters and Sample Introduction 1. To run samples: open the Acquisition window from in the menu bar. 2. Setup Acquisition Parameters. a. Acquisition time is the duration of the sample acquisition in seconds. When the default syringe speed of ml/min is used, it will take approximately 650 seconds (more accurately 667s) to collect the 500 µl sample loop volume. b. Acquisition delay is typically 40 sec. c. Detector stability delay should be set to 10 sec. 94
98 3. Right-click in the Analyte table (shown below) to apply a template (F3), or to make a new template with the periodic table. 4. Save the template by selecting Create Template From (F7). 5. The Acquisition Templates window will then open with the selected analytes saved. 6. Enter the number of events you wish to collect in Target Cells (Unlimited if 0). If you do not wish to limit the number of events to be acquired and instead run for a specified time, you must enter 0 in the Found Cells Limit box. The setting of 0 is recommended since the events collected are not all cellular events. Use the default settings for other parameters in the Analysis Parameters Tab. Note: Some settings in this tab can also be found in the Analysis tab in the Acquisition window outside of the Acquisition Templates window. It is recommended to make changes in the Acquisition Templates window to ensure that the parameters are consistent across multiple samples in the same experiment. 7. Settings are saved automatically once you navigate away. Click on Select Template to exit this view and return to starting sample acquisitions. 95
99 8. Specify a pathway and filename to save an FCS file. 9. Inject 500 µl of your filtered sample into the injection port and click Run in the Control tab. 10. Once the acquisition finishes, observe the data in Plotviewer, if desired. Increased Sampling Efficiency The CyTOF 2 Dual-Loop fluidics system is composed of a vertical sample loop (Loop 1) and a horizontal sample loop (Loop 2). Loop 2 consistently underperforms compared to Loop 1 due to its horizontal orientation which allows for quicker sedimentation in the loop. For increased sampling efficiency, it is recommended that only Loop 1 be used and the loop should be underfilled with µl of sample. Refer to Technical Note PN A1 Impact of Fluidics on Mass Cytometry Sampling Efficiency for more information. Shutdown: Turning Off Plasma 1. In Control Panel > Plasma > click Stop Plasma 2. Wait until the Plasma Stop Sequence has been completed successfully message appears (see below). The syringe pump, chiller and heater are automatically turned off when the Plasma Stop Sequence is completed. 96
100 3. Remove the sample capillary from the nebulizer. Then remove the nebulizer from the nebulizer port. 4. Disconnect the nebulizer from the gas line. 5. Using the Nebulizer Cleaning Kit, slowly draw 10% Contrad or Decon 90 through the side arm and sample inlet of the nebulizer and soak for 15 min. 6. Rinse the nebulizer 2 to 3 times with DIW (18.2 mω) using the same kit. 7. Leave the nebulizer submerged in a DIW (18.2 mω) bath prior to next use. See Chapter 6: Maintenance, Cleaning the Nebulizer After Plasma Shutdown, for detailed cleaning protocol. Other Features User Management Without logging into the software, User access allows Auto-Tuning and sample acquisition. Logging in as an administrator provides additional access to manual tuning, various settings for tuning and regular optimization of the instrument for consistent performance, as well as access to details for troubleshooting purposes. To log in to the administrator mode for the first time: 1. Open CyTOF Software. 2. Close the Status Panel. 3. Click About in the menu bar. 4. Click Login and enter administrator for user name. 5. Leave password blank and click Login. 97
101 Administrators also have the ability to manage users and other administrators. To manage user settings: 1. Click 2. Under Personal settings, Administrators can edit personal information and change passwords 3. Under User Managements, administrators can create and edit user or administrator accounts for different levels of access. 98
102 FCS File Concatenation Multiple FCS files from the same sample can now be concatenated into one file. Concatenation should be performed before Normalization (see section below). 4. Open FCS Analysis from the menu bar 5. Select the FCS files to be concatenated; Ctrl-select multiple files. 6. Check Concatenate. Note: Only concatenate files that have the same analytes and labels in the channel selection. 7. If normalization is not required to be done but Normalization beads are selected, use the Esc key to clear the selection and uncheck Remove Beads from Results. 8. Click Start to start the FCS concatenation. 9. The concatenated file will appear in the folder specified in Target FCS file, with _0 as a suffix to the file name. Users can also change the name of the concatenated file prior to starting the Concatenation. Bead Normalization Tool This tool normalizes sample intensity data against signal drift using information from EQ beads spiked into the sample. For detailed instructions on the normalization procedure, see the Bead Normalization User Guide (UG13-02). To normalize files: 99
103 1. Open FCS Analysis from the menu bar. 2. Select the FCS file(s) to be normalized and a destination folder. Note: Batch mode is also available. The normalized files will be saved in the same folder with a suffix ( _1 ) appended to the file name. 3. Under Normalization, select the lot of Normalization Beads that were used in the experiment. 100
104 Note: Each lot of beads has a unique bead passport file generated at Fluidigm that contains its expected metal intensities. At present, since there is only one lot of beads (P13H2302), select EQ-P13H2302 from the dropdown. For future lots of beads, load their passports using the appropriate Update button, and then select the loaded passport in the dropdown. 4. Uncheck Remove Beads from Results. 5. Click Start. The normalized data will be written to the destination folder. If normalization and concatenation is both required, it is recommended to concatenate first, and then normalize. This can be accomplished in one step by checking Concatenate as described in the FCS Concatenation section above and selecting the Normalization Beads (unchecking Remove Beads from results ) before starting the FCS analysis. The software will first concatenate the raw data of all selected files, and then normalize. Unexpected Plasma Outages If plasma goes during operation, perform the following steps prior to re-igniting plasma. Ensure Plasma Stop Sequence is Properly Completed 1) Open the Monitor window in the CyTOF software and check the GATE LED light. 101
105 2) If the Gate is still OPEN, click on Stop Plasma in Control Panel > Plasma 3) In Control Panel > General, Check the Auto Plasma Management checkbox and click Set. 102
106 Check if Argon Gas supply is stable 1. Check the Status Panel for the ARGON LED light. If the light is red, check the Argon supply to the instrument. 2. If insufficient Argon supply is the reason for the plasma outage, restart plasma only after the argon supply has been restored. Check for moisture in the Injector If the argon supply is stable, check for any moisture in the injector. Note: The following precautionary measures must be taken before proceeding to step 1 below. Warning! Before opening the CyTOF front access door to the Torch/Cone area, switch OFF the RF generator power using the breaker, located on the right side panel of the CyTOF 2 instrument. Warning! Wait a minimum of 5 minutes after turning off the RF generator power before opening the front access door. Warning! Ensure that the ICP torch and the load coil have sufficiently cooled before performing any maintenance procedures. RFG Circuit Breaker 103
107 1. Remove the syringe that is inserted into the sample introduction port. 2. From the bottom of the Heater Complex, unscrew the blue nut to release the sample line from the heater Sample Line Blue Nut 3. Insert the door handle into the slot in the front access door (see image below). 104
108 Door Handle Front Access Door 4. Remove the tubing from the Carrier fluid Reservoir bottle and Drain Vessel, and place the bottles away from the front access door. 5. Open the front access door by turning the door handle counter-clockwise. 105
109 6. Inspect the areas around the tip of the injector for any droplets or moisture. Injector 7. If moisture is present, remove the Injector and dry the moisture (see Chapter 6: Maintenance, Maintenance of the Spray Chamber and Torch Assembly). 8. Check that the Syringe pump Speed is set correctly to 45 µl/min (0.045mL/min). For more details on troubleshooting plasma problems, please refer to Chapter 8 Troubleshooting. 106
110 Consumables Spare Parts The CyTOF 2 instrument comes with spares of each part listed below. The suggested total number of each spare part to have available is indicated in red. Spare Part Cat# Quantity installed Spares Included Additional Spares Recommended* Nebulizer Torch Body Ball Joint Injector Spray Chamber Sample Capillary Assembly Load Coil Sample Pump Tubing Kit Skimmer-Reducer Assembly Sampler Cone Nebulizer Arm O-rings Pack 5 Pack Nebulizer Arm Ferrule Pack 5 Pack * To order additional parts, visit the Fluidigm online catalog and click on the CyTOF Reagents and Spare Parts. Reagents and Labware Part 1ml and 3ml sterile NormJect luer syringes 5ml Polypropylene Tube with Cell Strainer Cap (35µm) Calcium and Magnesium-free PBS High-grade 18MΩ De-ionized water (DIW) Suggested Supplier Henke Sass Wolf, Chem Glass Life Sciences, Agro Weber Falcon (Cat#352235) Various vendors Millipore Notes Rubber-free Milli-Q Note: It is recommended that glassware and plastics be made of polypropylene or Pyrex to minimize lead contamination. Avoid contact with detergents which may be a source of barium contamination for the CyTOF
111 Chapter 6 Maintenance Overview of CyTOF 2 Maintenance and Cleaning Regular cleaning and maintenance ensures optimal performance of your CyTOF 2 instrument. This section contains three tables (6.1, 6.2 and 6.3) which can be used as quick reference guides for the frequency, reagents and procedures used for cleaning and maintaining various parts of the instrument. Subsequent sections will detail how these procedures are performed. Table 6.1 Quick Reference for Routine Cleaning and Maintenance Part Frequency Performed By Required Reagents Nebulizer Daily Operator 10% Contrad 100 or 10% Decon 90 Type I Ultrapure (18.2 MΩ) Water (DIW) Spray Chamber Torch Body Injector Weekly Operator 10% Contrad 100 or 10% Decon 90 DIW Sampler and Skimmer Reducer Cones Daily Weekly (depending on Instrument usage) Operator 10% Citranox DIW Load Coil Weekly Operator Isopropanol Interface Pump Oil Annually or as needed Field Service HE 100 vacuum oil Engineer/Operator once per year Backing Pump Oil Every 6 months Field Service Engineer HE 100 vacuum oil /Operator Air Filters Annually Field Service Engineer N/A 108
112 Table 6.2 Quick Reference for Cleaning of Glassware Part Frequency Cleaning Solution Procedure Nebulizer Daily 10% Contrad 100 or 10% Decon 90 Type I Ultrapure (18.2 MΩ) Water (DIW) Storage: Soak in DIW (18.2 MΩ) between uses. Cleaning: Draw 10% Contrad or 10% Decon through side arm and sample inlet. For stubborn clogs, soak in diluted Contrad/Decon until clog dissipates Repeat several times with DIW to rinse. Caution: Do not sonicate. Spray Chamber Weekly 10% Contrad 100 or 10% Decon 90 DIW Injector Weekly 10% Contrad 100 or 10% Decon 90 DIW Do not use Citranox. Soak in 10%Contrad/Decon for 15 min. Scrub with brush. Rinse with DIW. Dry thoroughly before installing on instrument. Soak in 10%Contrad/Decon for 1 hour. Rinse with DIW. Dry thoroughly before installing on instrument. Caution: Do not sonicate. Torch Body Weekly 10% Contrad 100 or 10% Decon 90 DIW Soak in 10%Contrad/Decon for 1 hour or overnight if needed. Scrub with brush. Rinse with DIW. Dry thoroughly before installing on instrument. 109
113 Table 6.3 Quick Reference for Cleaning of the Cones and Load Coil Part Frequency Cleaning Solution Procedure Sampler and Weekly 10% Citranox Stack cones in cleaning Skimmer Reducer Type I Ultrapure (18.2 container. MΩ) Water (DIW) Soak and sonicate in 10% Cones Citranox (15 min max) Rinse with DIW (18.2 MΩ) Repeat soak and sonication with DIW. Air dry thoroughly. Load Coil Weekly Isopropanol Clean with load coil core in place. Scrub gently with Scotch Brite Ultra fine Hand Pad moistened with isopropanol. Caution: Do not bend load coil. 110
114 Table 6.4 below summarizes the supplies, tools and cleaning solutions that are required for cleaning and maintenance of the CyTOF 2 instrument. Table 6.4 Supplies Required for Cleaning and Maintenance Supplies Supplier Catalog# Procedures Sonicator Branson Model C Ultrasonic Cleaner (or similar size) Type I Ultrapure (18.2 MΩ) Water (DIW) Contrad 100 or Decon 90 (Decon Labs, 1 gallon): Citranox Liquid Acid Cleaner and Detergent Isopropanol Decon Labs, 1 gallon Alconox Sigma Aldrich Dilute to 10% in DIW x 1 gallon Z ea, 3.7L Dilute to 10% in DIW. Glassware Brushes of Varying Sizes (Restek Nylon Tube brushes and Pipe Cleaners) Scotch Brite Ultra fine Hand Pad 7448 Plastic containers for soaking and rinsing glassware Fisher Scientific M
115 Cleaning the Nebulizer after Plasma Shutdown Timely removal of the nebulizer from the elevated temperature environment of the heater module as well as soaking in DIW (18.2 MΩ) when not in use will help to avoid clogging of the tip. 1. Remove the sample capillary line by loosening the nut that secures the line into the sample inlet. 2. Gently withdraw the sample capillary line from the inlet and set aside. 3. Remove the nebulizer from the nebulizer adapter. 112
116 4. Loosen the nut connecting the nebulizer gas line to the side arm and remove the nebulizer. (The nut does not need to be completely unscrewed in order to remove the nebulizer.) 113
117 5. Retighten the nut to the union, taking care to retain the o ring and ferrule inside. Cleaning with Detergent 1. Label one 5mL round bottom tube and fill with detergent. 2. Lubricate one end of Tubing 1 (large diameter) from the nebulizer cleaning kit. 3. Connect the side arm of the nebulizer to the syringe with Tubing With the nebulizer tip submerged in 10% Contrad (or 10% Decon 90), pull slowly on the syringe plunger, filling the nebulizer body with detergent. Continue until the syringe body is filled with detergent. 5. Detach the tubing from the side arm and discard the liquid from the tubing and syringe by depressing the syringe plunger. Note: The liquid taken up into the syringe should not be pushed back into the nebulizer. 114
118 6. Attach Tubing 2 (small diameter) from the Nebulizer Cleaning Kit and repeat steps 3 5, attaching the tubing to the sample inlet of the nebulizer and fill the sample capillary with detergent. 2. Remove the syringe and tubing from the sample inlet and discard the detergent from the syringe and tubing by depressing the syringe plunger. 3. If the nebulizer is still clogged, soak it in a detergent bath until the clog dissipates. Repeat steps 3 7 for clogs remaining after prolonged soaking. 4. Remove residual detergent from both the nebulizer body and sample capillary by drawing out as much fluid as possible using tubing syringe assemblies 1 and 2, without dipping the nebulizer tip into the detergent tube. 5. Remove the nebulizer from the tubing syringe assembly and pull DIW into each tubing several times, and expel, to rinse detergent from the tubing pieces. Rinsing with DIW 6. Label the other 5 ml tube and fill with DIW. 7. Lubricate the end of the Tubing 1 syringe assembly with DIW. 8. Attach the tubing to the side arm of the nebulizer. 9. Draw DIW through the nebulizer tip via the side arm into the nebulizer body until the syringe body is filled. 10. Detach the tubing from the side arm and discard the liquid from the tubing and syringe by depressing the syringe plunger. 11. Repeat Steps 3 to 5 at least two times. 12. Using the Tubing 2 syringe assembly, repeat Steps 3 to 5, this time attaching the tubing to the sample inlet of the nebulizer, and filling the sample capillary with DIW. 13. Detach the tubing from the sample inlet and discard the liquid from the tubing and syringe by depressing the syringe plunger. 14. Repeat Steps 7 and 8 at least two times. 115
119 15. Re attach the nebulizer for use on the instrument or store until further use by submerging in a DIW bath. Maintenance of the Spray Chamber and the Torch Assembly Warning! Allow heater to cool for at least 30 min after plasma shutdown before attempting disassembly. Removal of the Spray Chamber 1. Slide the heat shield off the heater. 2. Remove the ball joint clamp which secures the spray chamber to the injector. 3. Open the heater lid. 4. Loosen the makeup gas line from the spray chamber sidearm. Nebulizer Port Spray Chamber Heat Shield Make Up Gas Connector 5. Remove the spray chamber from the heater and remove the nebulizer port. 116
120 6. Slide the entire Heater module off the guide pins and then rest on the pins below the drip tray. 7. Remove the ball joint injector by gently pulling and turning until it comes loose from the torch assembly. 117
121 Disassembly of the Torch Body The following precautionary measures must be taken before proceeding to step 1 below and disengaging the torch holder or the torch box. Warning! Before opening the front access door and disengaging the torch box from the vacuum chamber, switch OFF the RF generator power using the breaker, located on the right-side panel of the CyTOF 2 instrument. Warning! Wait a minimum of 5 minutes after turning off the RF generator power before opening the CyTOF access door to Torch/Cone area. Warning! Ensure that the ICP torch and the load coil have sufficiently cooled before performing any maintenance procedures. RFG Circuit Breaker 118
122 1. Loosen the two thumb screws at the front of the torch assembly making sure to loosen in unison. Thumb Screws 2. Slide the torch assembly off the torch box pins to access the torch body. 119
123 3. Carefully grasp the torch with one hand and firmly hold the torch assembly with the other hand. Twist and pull the torch until it is free of the torch holder. 4. Clean the spray chamber, injector, and torch body as detailed in Table Let glassware dry completely before reassembly. 120
124 Cleaning the Load Coil The following procedure outlines the removal and cleaning of the load coil which involves the opening of the front door access of the CyTOF 2 instrument. Warning! Ensure that the ICP torch and the load coil have sufficiently cooled before performing any maintenance procedures. 1. Insert the door handle into the slot in the front access door (see image below). 2. Remove the nebulizer and submerge in a DIW (18.2 mω) bath. 3. Detach the nebulizer line from the heater bulkhead by loosening the blue nut. 121
125 4. Remove the tubing from the carrier fluid reservoir and drain vessel, and place the bottles away from the front access door. 5. Open the front access door by turning the door handle counter clockwise. 6. Install the load coil alignment tool. Load Coil Alignment Tool 7. Remove the front shield by undoing the clips on all four sides and then lifting off. 122
126 Clip Front Shield 8. Using a Scotch Brite ultra fine hand pad moistened with isopropanol, gently rub the surfaces of the load coil to remove any deposits. Be careful not to bend the coils. 9. Remove the load coil alignment tool and visually inspect the coil to look for deposits and/or damage to the coil. 10. Gently clean in between the coils with the hand pad and isopropanol being careful not to bend the coils. 11. Reinstall the front shield. Removal of the Cones Note: The torch assembly should be removed before removing the cones (see Maintenance of the Spray Chamber and Torch Body section above). The cone removal tool contains magnets and pins that allow controlled removal of the sampler and skimmer reducer. Cone Removal Tool Side for Sampler Removal Side for Skimmer Reducer Removal 123
127 Sampler Cone Note: The sampler cone has a lifespan of approximately 500 hours of CyTOF use before it needs to be replaced. 1. The sampler cone face has four holes. The two without threads which lie closer to the sampler orifice are used for removal. Holes without Threads 2. Line up the pins on the cone removal tool with the non threaded holes being careful not to touch the sampler orifice. Note: If it seems too tight to remove, spray isopropanol around the edges of the sampler cone for lubrication to facilitate removal. 124
128 3. Rotate the cone removal tool while pulling forward to release the sampler from the vacuum being careful not to touch the skimmer tip. Note: If the O ring appears expanded or burnt, replace it. 125
129 O ring 4. Remove the sampler cone from the cone removal tool being careful not to come in contact with the sampler orifice. 5. Do not allow the sampler orifice to come into contact with any other surface. Skimmer Reducer Assembly 1. Using the other side of the cone removal tool (two magnets), line up the pins with the two holes of the skimmer. 2. Turn the cone removal tool counter clockwise until the skimmer reducer assembly comes free. 3. Remove the skimmer reducer assembly from the cone removal tool, being careful not to touch the skimmer tip. 4. Do not allow the skimmer/reducer orifice to come into contact with any other surface. 126
130 Cleaning of the Cones During routine maintenance and cleaning; inspect the sampler and skimmer cones, look at the shape of the orifice and look for deposits around the orifices. The cones should be stacked inside the cone cleaning container using the included adaptors as shown in Table 6.4 below. Table 6.4 Adaptors and Cones Step Image Step Image Step 1: Place bottom adapter inside the cone cleaning container (container not shown). Bottom Adaptor Step 2: Place sampler cone on top of bottom adapter. Step 3: Place top adaptor on top of the sampler cone Top Adaptor Step 4: Place the skimmer reducer assembly on top of the top adapter. 127
131 Step Image Step Image Step 5: Stack the cones inside the cleaning container. O ring Screw Note: During all steps of the cleaning process, care should be taken so that nothing comes in contact with the orifices of the cones. 1. Insert the cones and adaptors into the cone cleaning container as described above, adding 10% Citranox at each step. Sonicate for 15 min. Note: To prevent the O ring from coming in contact with the Citranox, do not fill above the level of the screws on the skimmer reducer assembly (see Table 6.4 above). 2. Rinse with DIW. 3. Repeat step 1 twice using DIW. 4. Air dry the cones completely before reinstalling. Note: The concentration of cleaning solutions, sonication times and frequency of cleaning are provided as a guide only and can be modified for the best workflow that suits the user s needs. Reinsertion of the Cones Note: Always install the cones before installing the torch assembly. Skimmer Reducer 1. Place the skimmer reducer assembly on the side of the cone removal tool with two magnets. 2. With a No. 2 pencil, coat the threads of the skimmer reducer assembly with graphite. This allows for easier threading of the skimmer reducer assembly into the interface. 128
132 3. Making sure that the skimmer reducer assembly is seated flush in the interface, begin to turn clockwise. After several turns, turn back a quarter turn to make sure mis threading has not occurred. If there is no resistance while turning back, then continue turning clockwise. 4. Repeat step 3 until the skimmer reducer assembly is firmly seated. Do not over tighten. 5. Detach the cone removal tool from the installed skimmer reducer assembly. Sampler 1. Attach the sampler cone to the side of the cone removal tool with four magnets. 2. Seat the sampler flush in the interface. 3. Make half a turn clockwise while applying gentle forward pressure. 4. Repeat Step 3 until the sampler is seated completely and is flush with the surface of the interface. 5. Detach the cone removal tool from the installed sampler. 6. Press gently along outer edges of sampler to make sure it is seated firmly. Reassembly of the Torch Note: Always install the cones before installing the torch assembly. 1. Install the torch body over the two O rings of the torch holder by pushing and turning. Sprinkle some deionized water to lubricate the O rings if necessary. 2. Turn the torch body so that the gas ports are oriented on top. 3. Connect the auxiliary gas line to the port closest to the torch holder. This port is slightly angled. 4. Connect the plasma gas line (with ignition pin) to the second port. This port is straight. 129
133 5. Ensure that both connections are tight. 6. Install the ball joint injector by pushing and turning until it is fully inserted. 7. If the torch and injector are correctly installed, the injector should be mm from the end of the inner portion of the torch mm distance 130
134 Installation of the Torch Assembly 1. With the CyTOF door closed, slide the torch assembly onto the heater box pins and push flush, making sure to line up the high voltage connector with its port. 2. When installing the torch assembly, ensure that both screws are rotated in unison. 3. Note that the screws have an internal ratcheting system on the black knobs. Over a small range, these knobs are free to rotate without the brass screw being turned. Therefore, when installing/removing the torch assembly, always be sure that the knobs are moving in the same direction as the screw. Carefully preventing the knobs from accidentally rotating in the opposite direction can help ensure synchronized motion of the two screws during installation/removal. 131
135 4. When installing the torch holder, tighten the screws until an audible click is heard. This is to ensure that the torch holder installed properly in its end position. Troubleshooting Installation of the Torch Assembly 1. In the event that the torch holder appears to be stuck on the instrument (i.e. during installation/removal), inspect the relative position of the screw assembly with respect to one another. 2. Open the front door of the instrument to be able to view the screw assembly from the inside. 3. Compare the amount of thread engagement between the two screw assemblies (the brass pieces shown below), then loosen or tighten the corresponding screws to equalize the thread engagement. 132
136 4. Turn the screws in sync to install or remove the torch holder as required. Checking the Torch Alignment It is recommended that you check the torch alignment to determine that the torch is centered in the load coil. It is also important to check the position of the torch relative to the vacuum interface (i.e. the Z alignment). The distance from the torch to the vacuum interface needs to be correct so that the optimal analytical zone of the plasma is sampled. Check Torch Position Relative to Load Coil 1. Open the CyTOF door. 2. Install the torch positioning tool into the end of the torch. 133
137 Torch Positioning Tool 3. Turn the torch positioning tool. It should spin freely. 4. If the tool doesn t spin freely, the torch assembly is likely installed incorrectly. 5. Remove the torch assembly and reinstall following steps 6 and 7 in the section Reassembly of the Torch above. 6. If the torch is still not centered, the load coil may be bent. Inspect the coil to see if the arms installed into the posts are at a 90 o angle to the coils. 7. If not, the load coil may need replacing. Contact support@fluidigm.com for guidance. Check the Z Alignment 1. Gently push the torch positioning tool in as far as it will go. 2. The outer edge of the torch positioning tool should be flush with the edge of the torch. Note: If this is not the case, see Troubleshooting the Z Alignment in the next section. 134
138 Torch positioning tool 4. Ensure that the torch positioning tool is removed before closing the front access door. Troubleshooting the Z Alignment Z Positioning Cap Z Positioning Nut 1. Loosen the black Z positioning cap. 2. Turn the Z positioning nut until the edge of the torch is flush with the outer edge of the torch positioning tool. 3. Retighten the Z positioning cap. 135
139 Instrument Air Filters The CyTOF 2 is equipped with a large and small air filter on the underside of the instrument. These air filters remove particles from the argon gas supply. The filters can be removed by opening the bottom of the instrument and pulling them out. These filters are inspected and changed by the Fluidigm Service Specialist on an annual basis. Rotary Pumps The CyTOF 2 has two rotary pumps, an interface pump and a backing pump. Maintenance of the vacuum pumps includes inspecting the pumps and changing the pump oil. Inspect the pump oil regularly and compare the appearance of the oil with a small sample of new oil. Change the vacuum pump oil if it has an unusual color, is dark, contains particles, or appears dirty or turbid. Typically if the oil is the color of honey then it does not need changing. If it is a darker color then it should be changed immediately. Warning! The interface and backing pumps in the instrument are in close vicinity to areas where high voltages are present. User access to the pumps is not advised. Only Fluidigm trained CyTOF operators may proceed with the following procedure. Disengage the RFG breaker located on the right side of the instrument. Warning! Do not touch electrical wires, contacts, transformers or its components during the oil inspection procedure. These are located in the instrument compartment above the interface pump. There is no need to access this section when servicing the pumps. The following section details the procedure for inspecting and changing the oil for both pumps. 1. Turn the RFG breaker off on the circuit breaker panel on the right side of the instrument (see image below). 2. Turn off the vacuum pumps with the switch on the circuit breaker panel on the right side of the instrument (see image below). 136
140 Vacuum Pump Switch RFG Test Button 3. Remove the nebulizer and place in deionized water. 4. Detach the nebulizer line from the heater bulkhead by loosening the blue nut connecting the nebulizer gas line to the side arm. 5. Remove the tubing from the carrier fluid reservoir and drain vessel, and place the bottles away from the front access door. 6. Insert the handle into the front of the instrument. Open the front access door by approximately 6 inches by turning the door handle counter clockwise. 137
141 Figure 6.1. The door handle inserted into the front of the instrument. The door handle is used to open the front access door. Spring Pin 7. Pull the spring pin to the left to open the bottom compartment. 138
142 8. Open the door on the right front of the instrument to access both pumps. Backing Pump Interface Pump The oil in the backing pump is much slower to age than that of the interface pump, due to a lower load on the backing pump. Please refer to the Rotary Pump Oil Condition Chart at the end of this procedure for determining the state of the oil. 139
143 Replacing Interface Pump Oil Figure 6.2. The interface pump. The oil level in the interface pump should be approximately ¾ full between the Min and Max lines (red boxes) beside the visual inspection window. 1. Open the valve on the interface pump and drain the oil into a tray or container. 140
144 Oil fill port Valve Drain port 2. Consult the Rotary Pump Oil Condition Chart (Figure 6.3 below) to assess the oil state. 3. The oil must be changed before it reaches level 4. If the color is above level 4 this may lead to severe damage to the pump which may require a full service. Note: If the dirty oil is not able to be drained completely, fill 100mL of fresh oil following Steps 4 and 5 below and drain again with the valve and the drain port. Repeat if necessary until all of the dirty oil is flushed out before Step With the Allen key provided, unscrew the top cap for the oil fill port. 5. Refill the oil into the top using the provided funnel. 6. Fill to approximately ¾ full using interface pump sight glass as a guide (see Figure 6.2). 7. Replace the cap. Be careful not to over tighten with the same Allen key to prevent leaking. Note: If replacing backing pump oil, proceed to the next section before closing the front door access and restarting the vacuum pumps. 8. Close right side door and door to bottom compartment. Close front access door. 141
145 9. Start the vacuum pumps and wait for the vacuum level to return to specification. Replacing Backing Pump Oil The oil level should be at least 1/3 full (between the two lines [red boxes above]) on either side of the visual inspection window of the backing pump. 1. Open the top oil cap on the backing pump. 142
146 Top Oil Cap Oil Inspection Window Bottom Oil Cap 2. Open the bottom oil cap and drain the oil into a tray or container. 3. Replace the bottom oil cap. 4. Unscrew the top cap. Pour HE 100 type vacuum oil into the hole at the top using a funnel until the level is 1/2 full in the window. 5. Replace top cap. Be careful not to over tighten to prevent leaking. 6. Close right side door and door to bottom compartment. Close front access door. 7. Start the vacuum pumps and wait for the vacuum level to return to specification. 143
147 Figure 6.3. Rotary pump oil condition chart and oil change recommendations (American Vacuum Society). Unscheduled Maintenance Replacement of Load Coil If the load coil shape is warped or if any deposits or damage exist, the load coil needs to be replaced. Warning! Before disengaging the torch box from the vacuum chamber, switch OFF the RF generator power using the breaker, located on the right side panel of the CyTOF 2 instrument. 144
148 Warning! Wait a minimum of 5 minutes after turning off the RF generator power before opening the CyTOF access door to Torch/Cone area. Warning! Ensure that the ICP torch and the load coil have sufficiently cooled before performing any maintenance procedures. 1. Remove the torch assembly. 2. Remove the nebulizer line, carrier fluid line and drain vessel line (see section above on Rotary Pumps). 3. Open the front access door. 4. Remove the front shield by undoing the clips on all four sides and then lifting off. Clip Front Shield 4. Using a two wrenches, loosen each of the two nuts that hold the load coil in place while simultaneously applying counter force on the two larger nuts using the second wrench. This will to avoid rotating and loosening the threaded coil connection ports. 145
149 Nuts to loosen Nuts to apply counter force 5. Install the new load coil. Make sure that the load coil alignment tool is in place before installing. Zip Ties Load Coil Alignment Tool Note: Carefully remove the zip ties in front of the nuts without damaging the arms of the load coil. 146
150 6. Tighten nuts with the wrench while simultaneously applying counterforce on the larger nuts with a second wrench. Nuts and washers on the posts to avoid turning Nuts to apply counter force Nuts to tighten Load Coil Alignment Tool 7. Remove the load coil alignment tool. 8. Replace the front shield and clip in place. 9. Install the torch assembly. 10. Check that the torch is centered in the load coil (in section Checking the Torch Alignment above). 147
151 Procedure for Expected Power Outages When a power outage is scheduled for the facility, the CyTOF 2 instrument needs to be properly shut down. Follow the steps below to shut down prior to the power outage and restart after power is restored. Note: The following procedures may also be used for shutting down and restarting the instrument for other purposes. CyTOF 2 Shutdown Procedure 1. Ensure that the system is connected to the argon supply for the vacuum chamber to be filled with argon when vented. 2. Press the Vacuum switch OFF on the side panel on the right side of the instrument above the circuit breakers. 3. Wait 10 minutes for the turbo pumps to gradually slow down. At this point, the venting valve opens and the chamber is slowly being filled with argon at a controlled pressure. 4. Shut off the instrument power by switching off the circuit breakers in the following order: AC Outlets, Backing Pump, RF Generator and System. 5. Leave argon supply on during shutdown if you plan to restart the CyTOF 2 immediately. CyTOF 2 Startup Procedure 1. If argon supply is on during the instrument shutdown, proceed to the next step. Otherwise, turn on argon gas supply for 2 hours before restarting the instrument. This will help with achieving the necessary vacuum levels. 2. Switch ON the circuit breakers in the following sequence: System, RF Generator, Backing Pump and AC Outlets. 3. Press the Vacuum ON switch. In the Status Panel on the instrument cover VG1 will turn green, followed by TP1 and TP2 in approximately 6 minutes, and finally VG2 will turn green in approximately 30 minutes. 4. Check in the monitor window to ensure the required vacuum levels are reached: VGauge1a below 1E 6 Torr and VGauge2a within the 1E 4 Torr range before plasma start. See image below. 148
152 Note: When plasma is ignited, the VGAUGE2a vacuum will rise to around 2 4 E 2 Torr as the gate is open. 149
153 ICP-MASS SPECTROMETRY Chapter 7 Safety Introduction This document describes general practices designed to aid you in safely operating the CyTOF 2 and its accessories. This advice is intended to supplement, not supersede, the normal safety codes in your country. The information provided does not cover every safety procedure that should be practiced. Ultimately, maintenance of a safe laboratory environment is the responsibility of the operator and the operator s organization. Please review all manuals supplied with the CyTOF 2 and accessories before you start working with the instrument to prevent personal injury or damage to the instrumentation. Carefully read the safety information in this chapter and in the other manuals supplied. When setting up the instrument or performing analyses or maintenance procedures, strictly follow the instructions provided. Safety Alert Conventions This guide uses specific conventions for presenting information that may require your attention. Refer to the following safety alert conventions. Safety Alerts for Chemicals Fluidigm follows the United Nations Globally Harmonized System of Classification and Labelling of Chemicals (GHS) for communicating chemical hazard information. GHS provides a common means of classifying chemical hazards and a standardized approach to chemical label elements and safety data sheets (SDSs). Key elements include: Pictograms that consist of a symbol on a white background within a red diamondshaped frame. Refer to the individual SDS for the applicable pictograms and warnings pertaining to the chemicals being used. 150
154 Signal words that alert the user to a potential hazard and indicate the severity level. The signal words used for chemical hazards under GHS: DANGER Indicates more severe hazards. WARNING Indicates less severe hazards. Safety Alerts for Instruments For hazards associated with instruments, this guide uses the following indicators: Pictograms that consist of a symbol on a white background within a black triangleshaped frame. Signal words that alert the user to a potential hazard and indicate the severity level. The signal words used for instrument hazards: DANGER Indicates an imminent hazard that will result in severe injury or death if not avoided. WARNING Indicates a potentially hazardous situation that could result in serious injury or death. CAUTION Indicates a potentially hazardous situation that could result in minor or moderate personal injury. IMPORTANT Indicates information necessary for proper use of products or successful outcome of experiments. Symbols The warnings provided in this manual must be observed during operation and maintenance of the CyTOF 2. Symbol Warning Symbol Description General warning symbol. Indicates a hazardous situation, that, if not avoided, could result in death or serious injury. 151
155 Symbol Radio Frequency Radiation Symbol Description Exposure to high frequency radio waves and radiofrequency radiation can result in injuries. Proper safety precautions need to be followed. Hot Surface Symbol Hot surface warning sign, do not touch. Potential for personal injury. Any product, material or substance contained under pressure, including Compressed Gas Hazard compressed gas, dissolved gas or gas liquefied by compression or refrigeration Table 7.1. Hazard Symbols. This table summarizes the hazard symbols that may be observed in this manual as well warning labels on the CyTOF 2 instrument. General Safety Guidelines This section describes some general laboratory safety guidelines. For additional information, we recommend The CRC Handbook of Laboratory Safety (Furr, 1990) and Prudent Practices for Handling Hazardous Chemicals in Laboratories (National Research Council, 1981). Adherence to the following safety precautions should be maintained at all times when setting up, operating, and maintaining the CyTOF 2 instrument. 152
156 ICP-based instruments generate high levels of radio frequency energy within the RF power supply and the torch box. The RF energy is potentially hazardous if allowed to escape. Safety devices and safety interlocks should not be bypassed or disconnected. The power supplies of the CyTOF 2 instrument are capable of generating potentially lethal voltages. Store the removable instrument handle separately from the instrument. No maintenance should be performed by anyone other than a Fluidigm Service Specialist or by the customer's own Fluidigm-trained and appropriately certified maintenance personnel. Do not allow smoking in the work area. Smoking is a source of significant contamination as well as a potential route for ingesting harmful chemicals When installing or moving the instrument contact a Fluidigm Service Specialist for assistance. The total weight of the instrument is 295 kg (650 lbs). Food should not be stored, handled, or consumed in the work area. Environmental Conditions Refer to the Chapter 2: Preparing Your Laboratory for the CyTOF 2 Mass Cytometer guide for the recommended environmental conditions. Laboratory Ventilation Toxic combustion products, metal vapor, and ozone can be generated by the CyTOF instrument, depending upon the type of analysis. Therefore, an efficient ventilation system must be provided for your instrument. When the plasma is on, hot gases are vented through two exhaust vents located at the back of the instrument. Detailed information on exhaust vents are described in the Preparing Your Laboratory for the CyTOF 2 Mass Cytometer guide. Warning! The use of CyTOF instruments without adequate ventilation to outside air may constitute a health hazard. Extreme care should be taken to vent exhaust gases properly. Warning! The CyTOF instrument is designed for analysis of fixed/permealized, non-live cells only. Under normal operation, cells are completely combusted in the ICP. High levels of UV radiation inside the torch box are significantly above the lethal levels for most of single airborne cells. However, in the event of plasma shutdown, the undigested portion of a sample can enter the torch box exhaust gases. Extreme care should be taken to vent exhaust gases properly. 153
157 Electrical Safety The CyTOF series products have been designed to protect the operator from potential electrical hazards. The following section describes recommended electrical safety guidelines. Symbols Title Description Electric Shock Hazard Symbol This sign indicates high electricity, electric shock. Electrical machines and/or equipment in the vicinity. You may suffer severe injuries or even death. The earth-ground symbol represents the any terminal which is intended for connection to an external Earth-Ground Symbol conductor for protection against electric shock or the terminal of a protective earth. Table 7.2. Electrical Hazard Symbols. This table represents the symbols you will see on the CyTOF 2 instrument and its accessories. Water lines should be located away from electrical connections. Condensation and potential leaks may create an unsafe environment in the proximity of electrical connections Warning! If this equipment is used in a manner not specified by Fluidigm Corporation the protection provided by the equipment may be compromised. Warning! Lethal voltages are present at certain areas within the instrument. Installation and internal maintenance of the instrument should be performed only by a Fluidigm Service Specialist or similarly authorized and trained by Fluidigm personal. When the instrument is connected to line power, opening instrument covers is likely to expose live parts. High voltages can still be present even when the power switch is in the off position. Disengage the circuit breakers before performing any service or maintenance on the cones or torch. 154
158 Warning! Before performing maintenance on the cones or torch, switch OFF the RF generator power using the breaker, located at the right rear of the CyTOF2 instrument. Wait at least 5 minutes for residual electrical charge to dissipate. Additional time is required to allow the ICP torch, cones and the load coil to reach room Capacitors inside the instrument may still be charged even if the instrument has been disconnected from all voltage sources. The instrument must be correctly connected to a suitable electrical supply (see Chapter 2: Preparing your Laboratory for the CyTOF 2 Mass Cytometer for further details). For 50 Hz installations, a means of electrically grounding the instrument must be available. The power supply must have a correctly installed protective conductor (earth-ground) and must be installed or checked by a qualified electrician before connecting the instrument. Warning! Any interruption of the protective conductor (earthground) inside or outside the instrument or disconnection of the protective conductor terminal is likely to make the instrument dangerous. Connect the instrument to a correctly installed line power outlet that has a protective conductor connection (earth-ground). Do not operate the instrument with any covers or internal parts removed. Do not attempt to make internal adjustments or replacements except as directed in the user manual. Disconnect the instrument from all voltage sources before opening it for any adjustment, replacement, maintenance, or repair. Warning! The interface and backing pumps in the instrument are in close vicinity to areas where high voltages are present. User access to the pumps is not advised. Only qualified service personnel may proceed with the inspection. 155
159 Warning! Do not touch electrical wires, contacts, transformers or its components during the oil inspection procedure. 240V AC is present on the terminals of the transformer located in a separate section of the instrument above the interface pump. There is no need to access this Use only fuses with the required current rating and of the specified type for replacement. Do not use makeshift fuses or short-circuit the fuse holders. If there are any signs that the instrument is no longer electrically safe for use, make the instrument inoperative and secure it, with a lockout, against any unauthorized or unintentional operation. The electrical safety of the instrument is likely to be compromised if the instrument: o Shows visible damage o Has been subjected to prolonged storage under unfavorable conditions o Has been subjected to severe stress during transportation. Warning! The radio frequency (RF) power supply driving the plasma torch provides up to 1.6 kw. The resulting voltages may cause extensive burns - even death. Under no circumstances should you attempt any physical adjustments of the plasma torch when it is operating. The instrument must be operated with the RF generator in the locked position at all times. Warning! Do not attempt to defeat the safety interlocks. This would place the operator s safety at risk. All interlocks must be engaged before you ignite the plasma. 156
160 Chemical Safety In this section, we have provided some general safety practices that you should observe when working with any chemicals. The responsible individuals must take the necessary precautions to ensure that the surrounding workplace is safe and that instrument operators are not exposed to hazardous levels of toxic substances. When working with any chemicals, refer to the applicable Material Safety Data Sheets (MSDS) provided by the manufacturer or supplier. Symbol Poison Hazard Symbol Corrosive Materials Hazard Description Very hazardous to health when inhaled, swallowed or when they come in contact with the skin. May even lead to death. Hazardous materials, toxic or very toxic materials Potential personal injury hazard. Includes caustic and acid materials that can destroy the skin and eat through metals. Table 7.3. Chemical hazard symbols. This table summarizes the chemical hazard symbols that you may encounter working with CyTOF reagents. When handling any chemical the following safe-handling guidelines should be strictly observed: Use, store, and dispose of chemicals in accordance with the manufacturer's recommendations and regulations applicable to the locality, state/ province, and/or country. When preparing chemical solutions, always work in a fume hood that is suitable for the chemicals you are using. Conduct sample preparation away from the instrument to minimize corrosion and contamination. Clean up spills immediately using the appropriate equipment and supplies and follow the appropriate MSDS guidelines Do not put open containers of solvent near the instrument. Store solvents in an approved cabinet (with the appropriate ventilation) away from the instrument. 157
161 Warning! Some chemicals used with this instrument may be hazardous or may become hazardous after completion of an analysis. Warning! Venting for fumes and disposal of waste must be in accordance with all national, state/provincial and local health and safety regulations and laws. Wear the appropriate personal protective equipment (PPE) at all times while handling chemicals. Use safety glasses (with side shields), goggles, or full-face shields, according to the types of chemicals you will be handling. Warning! Wear suitable protective clothing, including gloves specifically resistant to the chemicals being handled. Warning! Wear protective clothing and gloves. Some reagents are readily absorbed through the skin. Drain Vessel A drain vessel is supplied with the CyTOF 2 instrument. The vessel is made of HDPE and is used to gather the effluent from the Flow Injection Valve of the sample introduction system. For safe operation of your system, you should properly install and maintain the drain vessel and drain tubing. Waste disposal procedures must be in accordance with all national, state/provincial and local health and safety regulations and laws. Drain vessels may contain flammable, acidic, caustic, or organic solutions, cells debris and small amounts of the elements analyzed. 158
162 Warning! It is necessary to follow appropriate waste segregation guidelines in order to prevent effluents from reacting in the drain vessel. Place the drain vessel in an area that is visible to the operator, who can observe the level of collected effluent and empty the vessel when necessary. Check the condition of the drain tubing regularly to monitor deterioration. Organic solvents deteriorate the tubing more quickly than aqueous solutions. When the tubing becomes brittle or cracked, replace it. Empty the drain bottle regularly. Disposal of waste must be in accordance with all national, state/provincial and local health and safety regulations and laws. Pressurized Gas Safety Safe Handling of Gas Cylinders Never place the vessel in an enclosed cabinet. Doing so may result in a build-up of hazardous gases. Do not use a glass drain vessel. A glass drain vessel may break and spill toxic or corrosive liquids. Ar gas used with the CyTOF instrument is normally stored in liquid argon tanks or pressurized containers. Carefully use, store, and handle compressed gases in cylinders. Gas cylinders can be hazardous if they are mishandled. Argon is neither explosive nor combustible. Contact the gas supplier for a MSDS containing detailed information on the potential hazards associated with the gas. Symbol Compressed Gas Hazard Description Any product, material or substance contained under pressure, including compressed gas, dissolved gas or gas liquefied by compression or refrigeration Table 7.4. Compressed gas hazard symbols. 159
163 The following hazards are associated with pressurized containers of argon: Muscle strain Physical injury (i.e., from a bottle falling) Suffocation Warning! If liquid argon is used, the gas cylinder must be fitted with an overpressure regulator, which will vent the cylinder as necessary to prevent it from becoming a safety hazard. Warning! Do not use electronic pressure regulator and auto switching valves as they may affect the plasma stability and may also result in frequent loss of plasma. The following are some general safety practices for the proper identification, storage, and handling of gas cylinders. Legibly mark cylinders to identify their contents. Use the chemical name or commercially accepted name for the gas. In North America, as in most countries, all chemical or gas storage containers must be identified by means of approved labels (i.e., WHMIS labels). Note: See the Preparing Your Laboratory for the CyTOF 2 Mass Cytometer guide for detailed information on the correct storage of gas cylinders. Handling Cylinders Move cylinders with a suitable hand truck after ensuring that the container cap is secured and the cylinder properly fastened to the hand truck. Never roll or drag a compressed gas cylinder. Use a wheel cart. Always use a stand or safety strap while using or storing a cylinder. Replace the protective cap on the valve when the cylinder is not in use. Use only regulators, tubing, and hose connectors specifically approved by an appropriate regulatory agency to be used with the gas in the cylinder. Never lubricate regulators or fittings. 160
164 Do not force caps off with tools. If stuck, contact the supplier. Arrange gas hoses where they will not be damaged or stepped on, and where objects will not be dropped on them. Do not refill gas cylinders. Check the condition of pipes, hoses, and connectors regularly. Perform gas leak tests at all joints and seals of the gas system regularly, using an approved gas leak detection solution. Close all gas cylinder valves tightly at the cylinder when the equipment is turned off. Liquid Argon Handling Carefully inspect argon tanks prior to use Ensure that good ventilation is maintained in the laboratory space that will contain the liquid argon cylinders Warning! It is recommended to install an Oxygen sensor in the room where the operator and gas storage are located. Warning! Liquid argon in the cylinder is maintained at extremely low temperatures. Personal protective equipment including gloves, safety glasses and long sleeved clothing should be worn when operating the cylinder. Cryogenic liquid cylinders contain a vacuum that helps to maintain the integrity of the liquid argon in the cylinder. This vacuum may become compromised if the following symptoms are observed: o The outer vessel shows signs of frosting o The outer vessel sweats in humid conditions o The pressure relief valve opens continuously until the vessel is emptied Never lay or store the cylinder on its side. Cylinders should be stored in a vertical position. Do not roll a cryogenic liquid cylinder. Cylinders may be moved using a cart, overhead crane or hoist. Sample Handling and Preparation Sample preparation for the CyTOF 2 may require the handling of organic or corrosive solutions. MAXPAR reagents that are used with CyTOF instruments are supplied in a solution form. Please refer to the information supplied with MAXPAR reagent Material Safety Data Sheets (MSDS) for safe handling of the reagents. 161
165 Warning! For better control of contamination, dedicate laboratory reagents and consumables to use with CyTOF instrument and MAXPAR reagent only. Other Hazards Protection from Heat Warning! A safety interlock is used to automatically shut off the plasma if the chamber and interface are not fully coupled. Do not defeat the interlock. Do not remove the shield which protects the sample introduction system, the heat shield is designed to protect users from burns from the heater. Hot Surface Temperatures Warning! The torch components, the interface and the sample introduction system components remain hot for some time after the plasma has been shut off. Allow sufficient time for these items to cool to room temperature before you handle them. Protection from Radio Frequency Radiation Warning! Radio Frequency Radiation: The instrument generates high levels of Radio Frequency (RF) energy, which is potentially hazardous if allowed to escape. The instrument is designed to contain the RF energy within the shielded enclosures of the torch compartment and the RF power supply. Safety interlocks prevent you from operating the system without all covers, doors, and shields in place. 162
Agilent 7800 ICP-MS. Specifications and Typical Performance. Fast track metals analysis with Solution-Ready ICP-MS
 Agilent 7800 ICP-MS Specifications and Typical Performance Fast track metals analysis with Solution-Ready ICP-MS The Solution-Ready Agilent 7800 Quadrupole ICP-MS combines proven, robust hardware, auto-optimization
Agilent 7800 ICP-MS Specifications and Typical Performance Fast track metals analysis with Solution-Ready ICP-MS The Solution-Ready Agilent 7800 Quadrupole ICP-MS combines proven, robust hardware, auto-optimization
Helios, a CyTOF System
 PN 400250 A6 Helios, a CyTOF System User Guide SW Version 6.7 Helios, a CyTOF System User Guide 1 For Research Use Only. Not for use in diagnostic procedures. Information in this publication is subject
PN 400250 A6 Helios, a CyTOF System User Guide SW Version 6.7 Helios, a CyTOF System User Guide 1 For Research Use Only. Not for use in diagnostic procedures. Information in this publication is subject
Customer Responsibilities. Important Customer Information Series Q-TOF LC/MS Systems Site Preparation Checklist
 Thank you for purchasing an Agilent system. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation is
Thank you for purchasing an Agilent system. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation is
Agilent ICP-MS Interactive Troubleshooting tool for Low sensitivity
 Agilent ICP-MS Interactive Troubleshooting tool for Low sensitivity Rev. B 2017,Janyary,20 Next > Preconditions 7900, 7800, 7700, or 8800 ICP-MS is used. Sample introduction type is PeriPump. If you are
Agilent ICP-MS Interactive Troubleshooting tool for Low sensitivity Rev. B 2017,Janyary,20 Next > Preconditions 7900, 7800, 7700, or 8800 ICP-MS is used. Sample introduction type is PeriPump. If you are
The HumiSys. RH Generator. Operation. Applications. Designed, built, and supported by InstruQuest Inc.
 The HumiSys RH Generator Designed, built, and supported by InstruQuest Inc. Versatile Relative Humidity Generation and Multi-Sensor System The new HumiSys with single or dual RH probes capabilities is
The HumiSys RH Generator Designed, built, and supported by InstruQuest Inc. Versatile Relative Humidity Generation and Multi-Sensor System The new HumiSys with single or dual RH probes capabilities is
Customer Responsibilities. Important Customer Information. Agilent Infinity II Preparative HPLC System Site Preparation Checklist
 Agilent Site Preparation Infinity II Checklist Preparative HPLC System Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer
Agilent Site Preparation Infinity II Checklist Preparative HPLC System Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer
Customer Responsibilities
 Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
Agilent ICP-MS Interactive Troubleshooting tool for Plasma ignition problem
 Agilent ICP-MS Interactive Troubleshooting tool for Plasma ignition problem Rev. B 2017, January, 20 Next > Preconditions 7900, 7800, 7700, 8800, or 8900 ICP-MS is used. Sample introduction type is PeriPump.
Agilent ICP-MS Interactive Troubleshooting tool for Plasma ignition problem Rev. B 2017, January, 20 Next > Preconditions 7900, 7800, 7700, 8800, or 8900 ICP-MS is used. Sample introduction type is PeriPump.
HumiSys HF High Flow RH Generator
 HumiSys HF High Flow RH Generator Designed, built, and supported by InstruQuest Inc. Versatile Relative Humidity Generation and Multi-Sensor System The HumiSys HF is a high flow version of the previously
HumiSys HF High Flow RH Generator Designed, built, and supported by InstruQuest Inc. Versatile Relative Humidity Generation and Multi-Sensor System The HumiSys HF is a high flow version of the previously
NexION 1000/2000 ICP-MS
 PREPARING YOUR LAB NexION 1000/2000 ICP-MS ICP - Mass Spectrometry Preparation Checklist Environmental conditions Electrical requirements Space requirements Exhaust ventilation Coolant requirements Argon
PREPARING YOUR LAB NexION 1000/2000 ICP-MS ICP - Mass Spectrometry Preparation Checklist Environmental conditions Electrical requirements Space requirements Exhaust ventilation Coolant requirements Argon
6890 GC Site Preparation
 6890 GC Site Preparation (a16011) This document is believed to be accurate and up-to-date. However, Agilent Technologies, Inc. cannot assume responsibility for the use of this material. The information
6890 GC Site Preparation (a16011) This document is believed to be accurate and up-to-date. However, Agilent Technologies, Inc. cannot assume responsibility for the use of this material. The information
Detector Carrier Gas Comments Detector anode purge or reference gas. Electron Capture Nitrogen Maximum sensitivity Nitrogen Argon/Methane
 Gas requirements Gases for packed columns The carrier gas you use depends upon the type of detector and the performance requirements. Table 520-1 lists gas recommendations for packed column use. In general,
Gas requirements Gases for packed columns The carrier gas you use depends upon the type of detector and the performance requirements. Table 520-1 lists gas recommendations for packed column use. In general,
Customer Responsibilities. Important Customer Information. Cary 4000/5000/6000i UV-Vis spectrophotometer Site Preparation Checklist
 Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
Mass Spec will not Autotune
 Mass Spec will not Autotune Applies to 5973A/N MSD What could be the problem? There could be several things that would cause your Mass Spec not to Autotune. The most common, easily corrected Autotune problems
Mass Spec will not Autotune Applies to 5973A/N MSD What could be the problem? There could be several things that would cause your Mass Spec not to Autotune. The most common, easily corrected Autotune problems
Varian 325-MS LC/MS Quadrupole Mass Spectrometer Pre-installation Instructions
 Varian 325-MS LC/MS Quadrupole Mass Spectrometer Pre-installation Instructions Checklist NOTE: Do not unpack the shipping cartons. Check off each checklist box after satisfying each requirement as described
Varian 325-MS LC/MS Quadrupole Mass Spectrometer Pre-installation Instructions Checklist NOTE: Do not unpack the shipping cartons. Check off each checklist box after satisfying each requirement as described
FPG8601 Force Balanced Piston Gauge
 FPG8601 Force Balanced Piston Gauge Reference Level Calibration System for very low pressure Pressure range: 0 to 15 kpa gauge, absolute and absolute differential Standard resolution: 0.010 Pa, high resolution
FPG8601 Force Balanced Piston Gauge Reference Level Calibration System for very low pressure Pressure range: 0 to 15 kpa gauge, absolute and absolute differential Standard resolution: 0.010 Pa, high resolution
Preparing Your Laboratory for the Optima 7300 V and 8300 ICP-OES Spectrometers
 LABORATORY PREPARATION Preparing Your Laboratory for the Optima 7300 V and 8300 ICP-OES Spectrometers TABLE OF CONTENTS Section Subject 1 Suitable Working Area 2 Exhaust Vent 3 Vent Positions 4 Handling
LABORATORY PREPARATION Preparing Your Laboratory for the Optima 7300 V and 8300 ICP-OES Spectrometers TABLE OF CONTENTS Section Subject 1 Suitable Working Area 2 Exhaust Vent 3 Vent Positions 4 Handling
BEST KNOWN METHODS. Transpector XPR3 Gas Analysis System. 1 of 6 DESCRIPTION XPR3 APPLICATIONS PHYSICAL INSTALLATION
 BEST KNOWN METHODS Transpector XPR3 Gas Analysis System DESCRIPTION The Transpector XPR3 is a third-generation, quadrupole-based residual gas analyzer that operates at PVD process pressures and is the
BEST KNOWN METHODS Transpector XPR3 Gas Analysis System DESCRIPTION The Transpector XPR3 is a third-generation, quadrupole-based residual gas analyzer that operates at PVD process pressures and is the
Proudly serving laboratories worldwide since 1979 CALL for Refurbished & Certified Lab Equipment Varian 310
 www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +847.913.0777 for Refurbished & Certified Lab Equipment Varian 310 310 LC/MS Manual System INCLUDES: 310-MS LC/MS triple quadrople
www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +847.913.0777 for Refurbished & Certified Lab Equipment Varian 310 310 LC/MS Manual System INCLUDES: 310-MS LC/MS triple quadrople
Instructions for Assembly, Installation, and Operation of the Gas Addition Kit Accessory with the CEM Discover Systems
 Corporation Issued: 5/09 P/N: 600104 Rev. 2 Instructions for Assembly, Installation, and Operation of the Gas Addition Kit Accessory with the CEM Discover Systems The Gas Addition Accessory permits the
Corporation Issued: 5/09 P/N: 600104 Rev. 2 Instructions for Assembly, Installation, and Operation of the Gas Addition Kit Accessory with the CEM Discover Systems The Gas Addition Accessory permits the
Customer Responsibilities. Important Customer Information. Agilent 6400 Series Triple Quad LC/MS Site Preparation Checklist
 Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
Avio 500 ICP-OES. ICP-Optical Emission Spectroscopy PREPARING YOUR LAB. Preparation Checklist. Suitable Working Area
 PREPARING YOUR LAB Avio 500 ICP-OES ICP-Optical Emission Spectroscopy Preparation Checklist Suitable Working Area Exhaust Vent Vent Positions Handling of Gas Cylinders and Other Suggested Safety Practices
PREPARING YOUR LAB Avio 500 ICP-OES ICP-Optical Emission Spectroscopy Preparation Checklist Suitable Working Area Exhaust Vent Vent Positions Handling of Gas Cylinders and Other Suggested Safety Practices
Customer Responsibilities
 Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
Workhorse-15 Oxygen Generator
 11436 Sorrento Valley Road, San Diego CA 92121 USA 858-558-0202 Fax 858-558-1915 Workhorse-15 Oxygen Generator Model No. 5719, 5727, 5735 for Domestic and International Units Part number 1432 Revision
11436 Sorrento Valley Road, San Diego CA 92121 USA 858-558-0202 Fax 858-558-1915 Workhorse-15 Oxygen Generator Model No. 5719, 5727, 5735 for Domestic and International Units Part number 1432 Revision
Oxford Instruments Plasma Technology. PlasmaPro NGP80. Installation Data Sheet. Original Instructions.
 Oxford Instruments Plasma Technology www.oxford-instruments.com About this data sheet This data sheet provides basic information about the installation of a tool. Floor and wall loadings Table 1 lists
Oxford Instruments Plasma Technology www.oxford-instruments.com About this data sheet This data sheet provides basic information about the installation of a tool. Floor and wall loadings Table 1 lists
OPERATION MANUAL NTF-15
 OPERATION MANUAL NTF-15 Nitrogen Tire Filling Valve Stem Caps (Qty=200) Order P/N 436075 RTI Technologies, Inc 10 Innovation Drive York, PA 17402 800-468-2321 www.rtitech.com 035-81235-00 (Rev B) TABLE
OPERATION MANUAL NTF-15 Nitrogen Tire Filling Valve Stem Caps (Qty=200) Order P/N 436075 RTI Technologies, Inc 10 Innovation Drive York, PA 17402 800-468-2321 www.rtitech.com 035-81235-00 (Rev B) TABLE
UltraLo 1800 Alpha Particle Counter
 XIA LLC UltraLo 1800 Alpha Particle Counter Site Requirements & Planning Version: 0.4 Friday, February 15, 2013 Table of Contents I. Site Preparation Tasks... 3 A. Instrument Overview... 3 B. Installation
XIA LLC UltraLo 1800 Alpha Particle Counter Site Requirements & Planning Version: 0.4 Friday, February 15, 2013 Table of Contents I. Site Preparation Tasks... 3 A. Instrument Overview... 3 B. Installation
BioAerosol Nebulizing Generator. Operation and Maintenance User Manual
 BioAerosol Nebulizing Generator Operation and Maintenance User Manual INTRODUCTION The BANG or BioAerosol Nebulizing Generator is a unique nebulizer for the generation of aqueous aerosols at a low air
BioAerosol Nebulizing Generator Operation and Maintenance User Manual INTRODUCTION The BANG or BioAerosol Nebulizing Generator is a unique nebulizer for the generation of aqueous aerosols at a low air
User guide for operating the Agilent 6420A QqQ mass spectrometer by direct infusion/injection
 User guide for operating the Agilent 6420A QqQ mass spectrometer by direct infusion/injection This user guide does not have the intention to introduce you to any of the theory and/or principles behind
User guide for operating the Agilent 6420A QqQ mass spectrometer by direct infusion/injection This user guide does not have the intention to introduce you to any of the theory and/or principles behind
OPERATION MANUAL NTF-60 Plus
 OPERATION MANUAL NTF-60 Plus Nitrogen Tire Filling Valve Stem Caps (Qty=200) Order P/N 436075 RTI Technologies, Inc 10 Innovation Drive York, PA 17402 800-468-2321 www.rtitech.com 035-81264-00 (Rev A)
OPERATION MANUAL NTF-60 Plus Nitrogen Tire Filling Valve Stem Caps (Qty=200) Order P/N 436075 RTI Technologies, Inc 10 Innovation Drive York, PA 17402 800-468-2321 www.rtitech.com 035-81264-00 (Rev A)
UBEC 1AT. AUTO TANK Fill System Installation, Operation, & Setup Instructions
 Document Number: XE-ATA5PM-R1A UBEC 1AT AUTO TANK Fill System 08899155 Installation, Operation, & Setup Instructions Rev170906-EB-FRC PHYSICAL: 1302 WEST BEARDSLEY AVE ELKHART, IN 46514 WWW.ELKHARTBRASS.COM
Document Number: XE-ATA5PM-R1A UBEC 1AT AUTO TANK Fill System 08899155 Installation, Operation, & Setup Instructions Rev170906-EB-FRC PHYSICAL: 1302 WEST BEARDSLEY AVE ELKHART, IN 46514 WWW.ELKHARTBRASS.COM
UsER manual for Watersens ph -REDOX
 UsER manual for Watersens -REDOX Cl 8 1 2 6 3 3 7 7 4 4 4 4 Parts List 1 Redox Probe 1 x 2 PH Probe 1 x 5 Tube Weight 2 x 6 Connection Valve 1 x chlorine 3 Chlorine and Pumps 2 x 7 Dosing Valve 2 x 5 5
UsER manual for Watersens -REDOX Cl 8 1 2 6 3 3 7 7 4 4 4 4 Parts List 1 Redox Probe 1 x 2 PH Probe 1 x 5 Tube Weight 2 x 6 Connection Valve 1 x chlorine 3 Chlorine and Pumps 2 x 7 Dosing Valve 2 x 5 5
Site Preparation Specification for GCMS system. Dimensions and Weight. Agilent 7820MSD Series G3175A (G7020A), G3176A (G7021A)
 Purpose of Procedure Your site must meet this specification or set of requirements to assure a successful and timely installation of your 7820MSD Series Mass Selective Detector (MSD). This checklist is
Purpose of Procedure Your site must meet this specification or set of requirements to assure a successful and timely installation of your 7820MSD Series Mass Selective Detector (MSD). This checklist is
Columbus Instruments
 0215-003M Portable O 2 /CO 2 /CH 4 Meter User s Manual Columbus Instruments 950 NORTH HAGUE AVENUE TEL:(614) 276-0861 COLUMBUS, OHIO 43204, USA FAX:(614) 276-0529 1 www.colinst.com TOLL FREE 1-800-669-5011
0215-003M Portable O 2 /CO 2 /CH 4 Meter User s Manual Columbus Instruments 950 NORTH HAGUE AVENUE TEL:(614) 276-0861 COLUMBUS, OHIO 43204, USA FAX:(614) 276-0529 1 www.colinst.com TOLL FREE 1-800-669-5011
Information Sheet. About this information sheet. Floor and wall loadings. Attachment of process module to floor. PlasmaPro Estrelas100
 Information Sheet PlasmaPro Estrelas100 About this information sheet This data sheet provides basic information about the installation of a PlasmaPro Estrelas100 tool. Some of the information in this sheet
Information Sheet PlasmaPro Estrelas100 About this information sheet This data sheet provides basic information about the installation of a PlasmaPro Estrelas100 tool. Some of the information in this sheet
The HumiPyc - Model 1 - Gas Pycnometer; Density, Moisture, Permeation Analyzer; RH sensor Calibrator
 The HumiPyc - Model 1 - Gas Pycnometer; Density, Moisture, Permeation Analyzer; RH sensor Calibrator Designed, built, and supported by InstruQuest Inc. Temperature controlled, multi-technique volumetric
The HumiPyc - Model 1 - Gas Pycnometer; Density, Moisture, Permeation Analyzer; RH sensor Calibrator Designed, built, and supported by InstruQuest Inc. Temperature controlled, multi-technique volumetric
SomnoSuite FAQ. Setup. Calibration 4. What are the calibration requirements for the SomnoSuite? Settings
 SomnoSuite FAQ V1.3 January 2015 Setup 1. How do I connect the SomnoSuite to my oxygen source? 2. Is there a way to speed up the downward movement of the pusher block when setting the empty position? 3.
SomnoSuite FAQ V1.3 January 2015 Setup 1. How do I connect the SomnoSuite to my oxygen source? 2. Is there a way to speed up the downward movement of the pusher block when setting the empty position? 3.
Operation Manual - PN A MENSOR MODEL 73 SHOP AIR BOOSTER
 Operation Manual - PN 0017946001 A MENSOR MODEL 73 SHOP AIR BOOSTER Mensor Model 73 Shop Air Booster System (750 psi Version) April 23, 2012 Trademarks / Copyright Mensor is a registered trademark of Mensor
Operation Manual - PN 0017946001 A MENSOR MODEL 73 SHOP AIR BOOSTER Mensor Model 73 Shop Air Booster System (750 psi Version) April 23, 2012 Trademarks / Copyright Mensor is a registered trademark of Mensor
1)! DO NOT PROCEED BEYOND THIS MARK
 Operating Instructions for X-ray Photoelectron Spectrometer: Physical Electronics Model 555 XPS/AES (John H. Thomas, III, Ph.D., Electron Spectroscopy) Sample Insertion: figure 1. Sample insertion rod
Operating Instructions for X-ray Photoelectron Spectrometer: Physical Electronics Model 555 XPS/AES (John H. Thomas, III, Ph.D., Electron Spectroscopy) Sample Insertion: figure 1. Sample insertion rod
Customer Responsibilities
 Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
Technical Information
 Technical Information Installation, Operation, Installation, Operation and Maintenance and Maintenance Manual Manual Balston Model 75700-K728 Nitrogen Generator Figure 1-75700-K728 Overall Dimensions These
Technical Information Installation, Operation, Installation, Operation and Maintenance and Maintenance Manual Manual Balston Model 75700-K728 Nitrogen Generator Figure 1-75700-K728 Overall Dimensions These
METHOD 21 - DETERMINATION OF VOLATILE ORGANIC COMPOUND LEAKS. 1.2 Scope. This method is applicable for the
 1151 METHOD 21 - DETERMINATION OF VOLATILE ORGANIC COMPOUND LEAKS 1.0 Scope and Application. 1.1 Analytes. Analyte Volatile Organic Compounds (VOC) CAS No. No CAS number assigned 1.2 Scope. This method
1151 METHOD 21 - DETERMINATION OF VOLATILE ORGANIC COMPOUND LEAKS 1.0 Scope and Application. 1.1 Analytes. Analyte Volatile Organic Compounds (VOC) CAS No. No CAS number assigned 1.2 Scope. This method
Armfield Distillation Column Operation Guidelines
 Armfield Distillation Column Operation Guidelines 11-2016 R.Cox Safety SAFETY GLASSES ARE REQUIRED WHEN OPERATING THE DISTILLATION COLUMN Wear gloves when mixing alcohol feedstock The column will become
Armfield Distillation Column Operation Guidelines 11-2016 R.Cox Safety SAFETY GLASSES ARE REQUIRED WHEN OPERATING THE DISTILLATION COLUMN Wear gloves when mixing alcohol feedstock The column will become
Vacuum Systems and Cryogenics for Integrated Circuit Fabrication Technology 01
 INAOE. Tonantzintla, Mexico. 2010-06-23. June 23 rd, 2010 Vacuum Systems and Cryogenics for Integrated Circuit Fabrication Technology 01 Joel Molina INAOE Microelectronics Group jmolina@inaoep.mx 1 Vacuum
INAOE. Tonantzintla, Mexico. 2010-06-23. June 23 rd, 2010 Vacuum Systems and Cryogenics for Integrated Circuit Fabrication Technology 01 Joel Molina INAOE Microelectronics Group jmolina@inaoep.mx 1 Vacuum
The Helium Leak Detector
 The Helium Leak Detector Helium Leak Detector Main Components The main components of a helium leak detector are: 1. The analyzed, which enables to separate the tracer gas from other gases inside leak detector.
The Helium Leak Detector Helium Leak Detector Main Components The main components of a helium leak detector are: 1. The analyzed, which enables to separate the tracer gas from other gases inside leak detector.
Technical Specifications of Hydrogen Isotope Handling and Recovery System
 SECTION - C TECHNICAL SPECIFICATIONS OF STORES AND DRAWINGS. Technical Specifications of Hydrogen Isotope Handling and Recovery System INSTITUTE FOR PLASMA RESEARCH GANDHINAGAR, GUJARAT 382428 ANNEXURE-I
SECTION - C TECHNICAL SPECIFICATIONS OF STORES AND DRAWINGS. Technical Specifications of Hydrogen Isotope Handling and Recovery System INSTITUTE FOR PLASMA RESEARCH GANDHINAGAR, GUJARAT 382428 ANNEXURE-I
AB SCIEX QTRAP 4500 System
 MAX cover image size 4.8 inches X 7.8 inches In Body page mode, draw a graphic frame to match this section and then go to the next step. Delete this text from the Front Cover Master page. View>Master Pages,
MAX cover image size 4.8 inches X 7.8 inches In Body page mode, draw a graphic frame to match this section and then go to the next step. Delete this text from the Front Cover Master page. View>Master Pages,
Level MEASUREMENT 1/2016
 Level MEASUREMENT 1/2016 AGENDA 2 A. Introduction B. Float method C. Displacer method D. Hydrostatic pressure method E. Capacitance method G. Ultrasonic method H. Radar method I. Laser method J. Level
Level MEASUREMENT 1/2016 AGENDA 2 A. Introduction B. Float method C. Displacer method D. Hydrostatic pressure method E. Capacitance method G. Ultrasonic method H. Radar method I. Laser method J. Level
TECHNICAL DATA. Trimpac 244a. September 16, 2013
 September 16, 2013 Trimpac 244a 1. DEsCrIpTION DESCRIPTION TRIMPAC Model B-1 and B-1B is a factory-assembled trim package with an electric release module in a metal enclosure. The standard trim normally
September 16, 2013 Trimpac 244a 1. DEsCrIpTION DESCRIPTION TRIMPAC Model B-1 and B-1B is a factory-assembled trim package with an electric release module in a metal enclosure. The standard trim normally
490 Micro GC Site Preparation Checklist
 Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
FTC130 Transmitter. Operating Manual
 Seite 1 von 11 FTC130 Transmitter Fast Thermal Conductivity Analyzer Operating Manual 1.09KD180724CWI1V05 Messkonzept GmbH Seite 2 von 11 1. Intended Use... 3 2. Description... 4 3. Measuring gases and
Seite 1 von 11 FTC130 Transmitter Fast Thermal Conductivity Analyzer Operating Manual 1.09KD180724CWI1V05 Messkonzept GmbH Seite 2 von 11 1. Intended Use... 3 2. Description... 4 3. Measuring gases and
TECHNICAL DATA. Trimpac 251a. Spetember 16, 2013
 Spetember 16, 2013 Trimpac 251a 1. DEsCrIpTION DESCRIPTION TRIMPAC Model B-6 and B-6B is a factory assembled trim package for a double interlocked preaction system with an electric/pneu-lectric release
Spetember 16, 2013 Trimpac 251a 1. DEsCrIpTION DESCRIPTION TRIMPAC Model B-6 and B-6B is a factory assembled trim package for a double interlocked preaction system with an electric/pneu-lectric release
UNITY 2 TM. Air Server Series 2 Operators Manual. Version 1.0. February 2008
 UNITY 2 TM Air Server Series 2 Operators Manual Version 1.0 February 2008 1. Introduction to the Air Server Accessory for UNITY 2...2 1.1. Summary of Operation...2 2. Developing a UNITY 2-Air Server method
UNITY 2 TM Air Server Series 2 Operators Manual Version 1.0 February 2008 1. Introduction to the Air Server Accessory for UNITY 2...2 1.1. Summary of Operation...2 2. Developing a UNITY 2-Air Server method
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/12
 LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
ID-Cube. Installation and Operations Manual For ID-Cube Source and Power Supply. Revision 2 Document #:
 ID-Cube Installation and Operations Manual For ID-Cube Source and Power Supply Revision 2 Document #: 7.1.002 IonSense Inc. 999 Broadway Suite 404 Saugus, MA 01906 Copyright 2014 by IonSense Inc. All rights
ID-Cube Installation and Operations Manual For ID-Cube Source and Power Supply Revision 2 Document #: 7.1.002 IonSense Inc. 999 Broadway Suite 404 Saugus, MA 01906 Copyright 2014 by IonSense Inc. All rights
User s Guide. Vacuum Controller for liquid delivery systems
 Flow Control User s Guide Vacuum Controller for liquid delivery systems Precise Vacuum Control throughout the experiment Flow control Compatible with any perfusion system Ideal for Small Volume Delivery
Flow Control User s Guide Vacuum Controller for liquid delivery systems Precise Vacuum Control throughout the experiment Flow control Compatible with any perfusion system Ideal for Small Volume Delivery
OXY Integral. INTERCON ENTERPRISES INC Tel: Fax: Internet:
 OXY Integral INTERCON ENTERPRISES INC Tel: 800 665 6655 Fax: 604 946 5340 E-Mail: sales@intercononline.com Internet: www.intercononline.com Manual Integral 2006 1 INDEX 2-3 PREFACE 4 INTRODUCTION 5 Principle
OXY Integral INTERCON ENTERPRISES INC Tel: 800 665 6655 Fax: 604 946 5340 E-Mail: sales@intercononline.com Internet: www.intercononline.com Manual Integral 2006 1 INDEX 2-3 PREFACE 4 INTRODUCTION 5 Principle
Pegas 4000 MF Gas Mixer InstructionManual Columbus Instruments
 Pegas 4000 MF Gas Mixer InstructionManual Contents I Table of Contents Foreword Part I Introduction 1 2 1 System overview... 2 2 Specifications... 3 Part II Installation 4 1 Rear panel connections...
Pegas 4000 MF Gas Mixer InstructionManual Contents I Table of Contents Foreword Part I Introduction 1 2 1 System overview... 2 2 Specifications... 3 Part II Installation 4 1 Rear panel connections...
Variable Temperature Operation
 Variable Temperature Operation AVIII 400 MHz Spectrometer November 11, 2012 The CBC NMR Laboratory University of Delaware AVIII 400 User Guide: Variable Temperature Operation 1 INTRODUCTION... 5 1.1 SAFETY...
Variable Temperature Operation AVIII 400 MHz Spectrometer November 11, 2012 The CBC NMR Laboratory University of Delaware AVIII 400 User Guide: Variable Temperature Operation 1 INTRODUCTION... 5 1.1 SAFETY...
Customer Responsibilities
 Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
USER'S GUIDE. Nanoflow Probe. Red Stripe. 3-4mm. ptfe Sleeve. To Injector. ZU1XC Fused Silica TTF115. Fused Silica. ptfe Sleeve
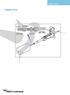 USER'S GUIDE Nanoflow Probe 3-4mm Red Stripe ptfe Sleeve To Injector ZU1XC Fused Silica 20µm 90µm TTF115 ptfe Sleeve Fused Silica 25µm 375µm Micromass UK Limited Floats Road Wythenshawe M23 9LZ Tel: +44
USER'S GUIDE Nanoflow Probe 3-4mm Red Stripe ptfe Sleeve To Injector ZU1XC Fused Silica 20µm 90µm TTF115 ptfe Sleeve Fused Silica 25µm 375µm Micromass UK Limited Floats Road Wythenshawe M23 9LZ Tel: +44
Standard Operating Procedure Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) - Thermo Scientific icap 6300
 Standard Operating Procedure Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) - Thermo Scientific icap 6300 The Thermo Scientific icap 6300 Inductively Coupled Plasma Optical Emission
Standard Operating Procedure Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) - Thermo Scientific icap 6300 The Thermo Scientific icap 6300 Inductively Coupled Plasma Optical Emission
Indian Institute of Technology Kanpur Environmental Engineering and Management Department of Civil Engineering
 Indian Institute of Technology Kanpur Environmental Engineering and Management Department of Civil Engineering Professor Vinod Tare Phones:+91-0512-2597791,7792 Email: vinod@iitk.ac.in Post Office: IIT
Indian Institute of Technology Kanpur Environmental Engineering and Management Department of Civil Engineering Professor Vinod Tare Phones:+91-0512-2597791,7792 Email: vinod@iitk.ac.in Post Office: IIT
This test shall be carried out on all vehicles equipped with open type traction batteries.
 5.4. Determination of hydrogen emissions page 1 RESS-6-15 5.4.1. This test shall be carried out on all vehicles equipped with open type traction batteries. 5.4.2. The test shall be conducted following
5.4. Determination of hydrogen emissions page 1 RESS-6-15 5.4.1. This test shall be carried out on all vehicles equipped with open type traction batteries. 5.4.2. The test shall be conducted following
Superconducting Susceptometer (MPMS-5S) Quantum Design Room 296 (MPMS)
 Superconducting Susceptometer (MPMS-5S) Quantum Design Room 296 (MPMS) Sensitivity: 1x10 11 A m 2 Applied DC fields: 0 T to 5 T Applied AC fields: 0 G to 3 G (zero-to-peak), 0.01 Hz to 1000 Hz Temperatures
Superconducting Susceptometer (MPMS-5S) Quantum Design Room 296 (MPMS) Sensitivity: 1x10 11 A m 2 Applied DC fields: 0 T to 5 T Applied AC fields: 0 G to 3 G (zero-to-peak), 0.01 Hz to 1000 Hz Temperatures
Cover Page for Lab Report Group Portion. Head Losses in Pipes
 Cover Page for Lab Report Group Portion Head Losses in Pipes Prepared by Professor J. M. Cimbala, Penn State University Latest revision: 02 February 2012 Name 1: Name 2: Name 3: [Name 4: ] Date: Section
Cover Page for Lab Report Group Portion Head Losses in Pipes Prepared by Professor J. M. Cimbala, Penn State University Latest revision: 02 February 2012 Name 1: Name 2: Name 3: [Name 4: ] Date: Section
Your local gas generation partner. Precision series Modular gas generation solution for GC.
 Your local gas generation partner Precision series Modular gas generation solution for GC www.peakscientific.com Perform with Precision Specifically designed and engineered for GC laboratory applications,
Your local gas generation partner Precision series Modular gas generation solution for GC www.peakscientific.com Perform with Precision Specifically designed and engineered for GC laboratory applications,
MASS FLOW SYSTEMS MASS FLOW MEASURING, CONTROLLING AND BLENDING SYSTEMS
 MASS FLOW SYSTEMS MASS FLOW MEASURING, CONTROLLING AND BLENDING SYSTEMS Using state-of-the-art measuring and microprocessor technologies, Advanced has assembled a series of systems which can measure mass
MASS FLOW SYSTEMS MASS FLOW MEASURING, CONTROLLING AND BLENDING SYSTEMS Using state-of-the-art measuring and microprocessor technologies, Advanced has assembled a series of systems which can measure mass
Description. Chassis. Cabinet. Long chassis - Type: Dimensions (mm): 2700 x 1200 x 1678
 1 ITV bench is equipped with a INJETVISION vision system whose purpose is to observe and study the jets from high pressure diesel injector in inert atmosphere (nitrogen or CO2) up to 50 bar (± 0.5 bar).
1 ITV bench is equipped with a INJETVISION vision system whose purpose is to observe and study the jets from high pressure diesel injector in inert atmosphere (nitrogen or CO2) up to 50 bar (± 0.5 bar).
Cover Page for Lab Report Group Portion. Pump Performance
 Cover Page for Lab Report Group Portion Pump Performance Prepared by Professor J. M. Cimbala, Penn State University Latest revision: 02 March 2012 Name 1: Name 2: Name 3: [Name 4: ] Date: Section number:
Cover Page for Lab Report Group Portion Pump Performance Prepared by Professor J. M. Cimbala, Penn State University Latest revision: 02 March 2012 Name 1: Name 2: Name 3: [Name 4: ] Date: Section number:
Oxidation Stability of Gasoline and Aviation Fuels
 Oxidation Stability of Gasoline (Induction Period Method) Oxidation Stability of Aviation Fuels (Potential Residue Method) Test Method Provides an indication of the tendency of gasoline and aviation fuels
Oxidation Stability of Gasoline (Induction Period Method) Oxidation Stability of Aviation Fuels (Potential Residue Method) Test Method Provides an indication of the tendency of gasoline and aviation fuels
Welker Sampler. Model GSS-1. Installation, Operation, and Maintenance Manual
 Installation, Operation, and Maintenance Manual Welker Sampler Model GSS-1 The information in this manual has been carefully checked for accuracy and is intended to be used as a guide to operations. Correct
Installation, Operation, and Maintenance Manual Welker Sampler Model GSS-1 The information in this manual has been carefully checked for accuracy and is intended to be used as a guide to operations. Correct
Gases&Technology. Measurement of Impurities in Helium Using the Dielectric Barrier Discharge Helium Ionization Detector. FEATURE.
 Gases&Technology FEATURE Measurement of Impurities in Helium Using the Dielectric Barrier Discharge Helium Ionization Detector. B Y M A T T H E W M O N A G L E Abstract Bulk gases are often delivered to
Gases&Technology FEATURE Measurement of Impurities in Helium Using the Dielectric Barrier Discharge Helium Ionization Detector. B Y M A T T H E W M O N A G L E Abstract Bulk gases are often delivered to
The HumiPyc ( Model 2) - Gas Pycnometer; Density, Moisture, Permeation Analyzer; Filter Integrity Tester; RH sensor Calibrator
 The HumiPyc ( Model 2) - Gas Pycnometer; Density, Moisture, Permeation Analyzer; Filter Integrity Tester; RH sensor Calibrator Designed, built, and supported by InstruQuest Inc. Universal pycnometer, no
The HumiPyc ( Model 2) - Gas Pycnometer; Density, Moisture, Permeation Analyzer; Filter Integrity Tester; RH sensor Calibrator Designed, built, and supported by InstruQuest Inc. Universal pycnometer, no
OEM Manual MODEL 2305 ECONOMICAL DIGITAL SINGLE CYLINDER SCALE
 OEM Manual MODEL 2305 ECONOMICAL DIGITAL SINGLE CYLINDER SCALE 1 These instructions generally describe the installation, operation, and maintenance of subject equipment. The manufacturer reserves the right
OEM Manual MODEL 2305 ECONOMICAL DIGITAL SINGLE CYLINDER SCALE 1 These instructions generally describe the installation, operation, and maintenance of subject equipment. The manufacturer reserves the right
Customer Responsibilities
 Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
INSTALLATION GUIDE Gas Rangetops
 INSTALLATION GUIDE Gas Rangetops Contents Wolf Gas Rangetops........................... 3 Safety Instructions............................ 4 Gas Rangetop Specifications.................... 5 Gas Rangetop
INSTALLATION GUIDE Gas Rangetops Contents Wolf Gas Rangetops........................... 3 Safety Instructions............................ 4 Gas Rangetop Specifications.................... 5 Gas Rangetop
User Manual MFCS TM -EZ
 User Manual MFCS TM -EZ Version 6A_December 2016 1. INTRODUCTION 5 2. GENERAL INFORMATION 6 2.1 General functioning 6 2.2 Different MFCS TM 6 3. PACKAGE CONTENT 8 4. MFCS TM -EZ DESCRIPTION 9 5. CONNECTIONS
User Manual MFCS TM -EZ Version 6A_December 2016 1. INTRODUCTION 5 2. GENERAL INFORMATION 6 2.1 General functioning 6 2.2 Different MFCS TM 6 3. PACKAGE CONTENT 8 4. MFCS TM -EZ DESCRIPTION 9 5. CONNECTIONS
Laboratory Hardware. Custom Gas Chromatography Solutions WASSON - ECE INSTRUMENTATION. Engineered Solutions, Guaranteed Results.
 Laboratory Hardware Custom Gas Chromatography Solutions Engineered Solutions, Guaranteed Results. WASSON - ECE INSTRUMENTATION Laboratory Hardware Wasson-ECE Instrumentation offers hardware-only solutions
Laboratory Hardware Custom Gas Chromatography Solutions Engineered Solutions, Guaranteed Results. WASSON - ECE INSTRUMENTATION Laboratory Hardware Wasson-ECE Instrumentation offers hardware-only solutions
Series Environmental Chambers
 3119-600 Series Environmental Chambers Challenges in Non-Ambient Testing Testing at non-ambient temperatures adds another layer of challenges to your testing laboratory. Ensuring you get accurate and stable
3119-600 Series Environmental Chambers Challenges in Non-Ambient Testing Testing at non-ambient temperatures adds another layer of challenges to your testing laboratory. Ensuring you get accurate and stable
LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary
 ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
Long term stability tests of INO RPC prototypes
 Long term stability tests of INO RPC prototypes While the problem of sudden aging in the glass RPC prototypes is being investigated, a few RPCs of dimension 40 cm 30 cm were fabricated, using glass procured
Long term stability tests of INO RPC prototypes While the problem of sudden aging in the glass RPC prototypes is being investigated, a few RPCs of dimension 40 cm 30 cm were fabricated, using glass procured
Agilent Auxiliary Gas Module AGM 2. User s Guide
 Agilent Auxiliary Gas Module AGM 2 User s Guide Notices Agilent Technologies, Inc. 1992, 1996, 1999, 2010 No part of this manual may be reproduced in any form or by any means (including electronic storage
Agilent Auxiliary Gas Module AGM 2 User s Guide Notices Agilent Technologies, Inc. 1992, 1996, 1999, 2010 No part of this manual may be reproduced in any form or by any means (including electronic storage
MASK INTEGRITY TEST ACCESSORY (MITA) MODEL 8120
 MASK INTEGRITY TEST ACCESSORY (MITA) MODEL 8120 QUICK START GUIDE P/N 6006154, REVISION C MAY 2013 Model 8120 Mask Integrity Tester is patented under U.S. Patent No. 8,312,761. Additional patents are pending.
MASK INTEGRITY TEST ACCESSORY (MITA) MODEL 8120 QUICK START GUIDE P/N 6006154, REVISION C MAY 2013 Model 8120 Mask Integrity Tester is patented under U.S. Patent No. 8,312,761. Additional patents are pending.
Glove Box Installation Manual
 Glove Box Installation Manual 1998 by M. Braun Company File: GB-UNI-INS.DOC! Edition 08-00 by M. Boutin! Subject to be changed without notice Glovebox Installation Your Glove box has been fully assembled,
Glove Box Installation Manual 1998 by M. Braun Company File: GB-UNI-INS.DOC! Edition 08-00 by M. Boutin! Subject to be changed without notice Glovebox Installation Your Glove box has been fully assembled,
ANNEX AMENDMENTS TO THE INTERNATIONAL CODE FOR FIRE SAFETY SYSTEMS (FSS CODE) CHAPTER 15 INERT GAS SYSTEMS
 Annex 3, page 2 ANNEX AMENDMENTS TO THE INTERNATIONAL CODE FOR FIRE SAFETY SYSTEMS (FSS CODE) CHAPTER 15 INERT GAS SYSTEMS The text of existing chapter 15 is replaced by the following: "1 Application This
Annex 3, page 2 ANNEX AMENDMENTS TO THE INTERNATIONAL CODE FOR FIRE SAFETY SYSTEMS (FSS CODE) CHAPTER 15 INERT GAS SYSTEMS The text of existing chapter 15 is replaced by the following: "1 Application This
DD2 Site Preparation Checklist Hardware Site Preparation Specification
 Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
Thank you for purchasing an Agilent instrument. To get you started and to assure a successful and timely installation, please refer to this specification or set of requirements. Correct site preparation
PERFORM Operating Document
 PERFORM Operating Document Use and Maintenance of CO 2 Incubator PC-POD-CA-007-v02 Revision History Version Reason for Revision Date 01 New POD 30-Sep-13 02 Minor revisions for section 2.3, 3.1, 4.3. 14-April-16
PERFORM Operating Document Use and Maintenance of CO 2 Incubator PC-POD-CA-007-v02 Revision History Version Reason for Revision Date 01 New POD 30-Sep-13 02 Minor revisions for section 2.3, 3.1, 4.3. 14-April-16
RDK-408D2 Cold Head. Technical Manual. SHI-APD Cryogenics Inc Vultee Street Allentown, PA U.S.A. Revision A: September 2005
 RDK-408D2 Cold Head Technical Manual SHI-APD Cryogenics Inc. 1833 Vultee Street Allentown, PA 18103-4783 U.S.A. Revision A: September 2005 (Reference SHI Manual: December 18, 2003 266404A CD32ZZ-160A)
RDK-408D2 Cold Head Technical Manual SHI-APD Cryogenics Inc. 1833 Vultee Street Allentown, PA 18103-4783 U.S.A. Revision A: September 2005 (Reference SHI Manual: December 18, 2003 266404A CD32ZZ-160A)
Integral type Differential pressure flowmeter VNT Series
 Integral type Differential pressure flowmeter VNT Series OUTLINE VH series Wafer-Cone differential pressure flowmeter and high precision differential pressure transmitter are integrated into one flowmeter.
Integral type Differential pressure flowmeter VNT Series OUTLINE VH series Wafer-Cone differential pressure flowmeter and high precision differential pressure transmitter are integrated into one flowmeter.
Exercise 2-2. Second-Order Interacting Processes EXERCISE OBJECTIVE DISCUSSION OUTLINE. The actual setup DISCUSSION
 Exercise 2-2 Second-Order Interacting Processes EXERCISE OBJECTIVE Familiarize yourself with second-order interacting processes and experiment with the finer points of controller tuning to gain a deeper
Exercise 2-2 Second-Order Interacting Processes EXERCISE OBJECTIVE Familiarize yourself with second-order interacting processes and experiment with the finer points of controller tuning to gain a deeper
Gas Absorption Draft Standard Operating Procedure
 Gas Absorption Draft Standard Operating Procedure R.C. 9-14 Scope Page 1 Equipment Overview Page 1 Safety Page 2 Operating Procedure Page 4 Equipment Description Page 16 Gas Absorption Experiment Draft
Gas Absorption Draft Standard Operating Procedure R.C. 9-14 Scope Page 1 Equipment Overview Page 1 Safety Page 2 Operating Procedure Page 4 Equipment Description Page 16 Gas Absorption Experiment Draft
Major Design Features
 Major Design Features 3Flex Features Design Benefits Discussion 316 SS fittings and pneumatically actuated, hard-seal valves Provides virtually leak-free gas management with the lowest outgassing rate
Major Design Features 3Flex Features Design Benefits Discussion 316 SS fittings and pneumatically actuated, hard-seal valves Provides virtually leak-free gas management with the lowest outgassing rate
MoveRoll Conveyor Operating and Maintenance Manual
 MoveRoll Conveyor Operating and Maintenance Manual 1. Read this first! This manual contains information for protection of personnel in the roll handling area from possible injury and/or equipment damage.
MoveRoll Conveyor Operating and Maintenance Manual 1. Read this first! This manual contains information for protection of personnel in the roll handling area from possible injury and/or equipment damage.
PURPOSE OF THE POLICY
 Title: Safe Storage, Handling, Use and Disposal Procedures of Compressed Gas Cylinders Effective Date: November 2005 Revision Date: March 1, 2017 Issuing Authority: Responsible Officer: VP, Capital Projects
Title: Safe Storage, Handling, Use and Disposal Procedures of Compressed Gas Cylinders Effective Date: November 2005 Revision Date: March 1, 2017 Issuing Authority: Responsible Officer: VP, Capital Projects
ENSURING AN ACCURATE RESULT IN AN ANALYTICAL INSTRUMENTATION SYSTEM PART 1: UNDERSTANDING AND MEASURING TIME DELAY
 ENSURING AN ACCURATE RESULT IN AN ANALYTICAL INSTRUMENTATION SYSTEM PART 1: UNDERSTANDING AND MEASURING TIME DELAY Process measurements are instantaneous, but analyzer responses never are. From the tap
ENSURING AN ACCURATE RESULT IN AN ANALYTICAL INSTRUMENTATION SYSTEM PART 1: UNDERSTANDING AND MEASURING TIME DELAY Process measurements are instantaneous, but analyzer responses never are. From the tap
MEDICAL EQUIPMENT CATALOG
 THE MOST TRUSTED MEDICAL EQUIPMENT IN NORTH AMERICA MEDICAL EQUIPMENT CATALOG Total Components Including Bulk Oxygen Supply, Back up and Delivery Equipment Designs for High Pressure, Liquid or Bulk Applications
THE MOST TRUSTED MEDICAL EQUIPMENT IN NORTH AMERICA MEDICAL EQUIPMENT CATALOG Total Components Including Bulk Oxygen Supply, Back up and Delivery Equipment Designs for High Pressure, Liquid or Bulk Applications
Markes Thermal Desorption Systems Site Preparation Page 1 of 8
 Thermal Desorption Systems Site Preparation Purpose of site preparation Your site must meet this specification to assure a successful and timely installation of your Agilent thermal desorption equipment.
Thermal Desorption Systems Site Preparation Purpose of site preparation Your site must meet this specification to assure a successful and timely installation of your Agilent thermal desorption equipment.
/ /
 GN 4- kw/5.5-0 hp Dual Output Compressors (Air/Nitrogen) Driving Nitrogen Forward GN: the dual output solution From cargo blanketing in LNG vessels to the inflation of Formula tires, nitrogen is used in
GN 4- kw/5.5-0 hp Dual Output Compressors (Air/Nitrogen) Driving Nitrogen Forward GN: the dual output solution From cargo blanketing in LNG vessels to the inflation of Formula tires, nitrogen is used in

![[Instruments for vacuum measurement, checking and adjustment] 3 [Instruments for vacuum measurement, checking and adjustment] 3](/thumbs/94/121874202.jpg)