Surface Tension of the Alveoli. Aslı AYKAÇ, PhD. NEU Faculty of Medicine Department of Biophysics
|
|
|
- Andrea Carter
- 5 years ago
- Views:
Transcription
1 Surface Tension of the Alveoli Aslı AYKAÇ, PhD. NEU Faculty of Medicine Department of Biophysics
2 Learning Objectives Define surface tension and use Laplace s law to understand how the pressure inside a bubble is related to the radius and surface tension State how the surfactant reduces surface tension and stabilizes the size of the alveoli
3 Surface Tension. Cohesion and Adhesion Tendency of the lungs to CONTRACT as a result of surface tension Cohesion and Adhesion Liquids strong attractive forces between indivual molecules COHESIVE FORCES forces between LIKE molecules ADHESIVE FORCES forces between UNLIKE molecules cohesion adhesion adhesion cohesion
4 Examples 1. capillary action 2. two pieces of microscope slides with water between them
5 Surface Tension Enhancement of the intermolecular attractive forces at the surface. Strong cohesive forces at the surface.
6 Surface Tension A molecule that moves outside the surface of a liquid tends to be pulled back in by the attractive forces of its neighbours. An interior molecule is pulled about the same in all directions by its neighbours and thus has no net force on it on the average.
7 Surface Tension: Definition The inward molecular attraction forces which must be overcome to increase the surface area. ST is the energy required to increase the surface area of a liquid by a unit amount. It requires work to move molecules to the surface against the net inward cohesive force that is perpendicular to the surface. Due to ST, the surface of a liquid tends to behave like a stretched elastic membrane. The surface molecules will be more ordered and resistant to molecular disruption
8 Surface Tension Examples: Walking on Water Small insects such as the water strider can walk on water because their weight is not enough to penetrate the surface. When an object is on the surface of the fluid, the surface under tension will behave like an elastic membrane. There will be a small depression on the surface of the water. The vertical components of the forces by the molecules on the object will balance out the weight of the object. Depressing the surface under the foot of the insect gives an upward component to the surface-tension forces, which supports the insect on the surface of water. groups.physics.umn.edu R Nave
9 Surface Tension Examples: Don't touch the tent! Common tent materials are somewhat rainproof in that the surface tension of water will bridge the pores in the finely woven material. But if you touch the tent material with your finger, you break the surface tension and the rain will drip through.
10 Surface Tension Examples: Floating a Needle If carefully placed on the surface, a small needle can be made to float on the surface of water even though it is several times as dense as water. If the surface is agitated to break up the surface tension, then needle will quickly sink. At the bottom of the pot you can see the sunken strip of tissue paper.
11 Surface Tension Examples: Raindrops Surface tension makes the surface contract to its smallest possible area smallest surface area for a given volume SPHERE
12 Surface Tension: Measurement Anologous to PRESSURE but P of a fluid exerts an OUTWARD FORCE and tends to EXPAND a volume ST of a fluid exerts an INWARD FORCE and tends to SHRINK a surface The surface tension strength of a liquid is defined as the FORCE PER UNIT LENGTH that the surface exerts on any line in the surface. = F / l Units: Newton/meter This force lies on the surface and is PERPENDICULAR to the line
13 Surface Tension of Various Liquids LIQUID SURFACE TENSION ( N/m, 20 o C) water 72.8 X10-3 blood plasma 50 X 10-3 lung surfactant 1 X 10-3 benzene 28.8 X10-3 mercury 464 X 10-3 ST depends on the molecular properties of the liquid ---- intrinsic property
14 Pressure in a Bubble Surface tension tends to shrink a bubble, but it is resisted by the pressure (P i ) inside the bubble which is greater than the pressure outside (P o ) the bubble. This pressure difference results in an OUTWARD FORCE on the bubble that equals the INWARD FORCE of surface tension. P t = transmural pressure P i - P o = 2 r
15 Pressure in a Bubble P difference is proportional to ST. In other words, a larger P is needed to form a bubble in a liquid with a large ST. P difference is INVERSELY proportional to the RADIUS of the bubble. Means that the P difference is greater in a small bubble than a large one. Example : harder to blow up a balloon first
16 Surfactants ( Surface Active Agents) Some chemicals change the adhesive and cohesive forces in a liquid. SURFACTANT : Any substance that reduces the surface tension of a liquid
17 Surfactant Molecules in Water exper2/exper2.htm
18 Surface Tension of the Alveoli Surfactant decreases surface tension which: increases pulmonary compliance (reducing the effort needed to expand the lungs) reduces tendency for alveoli to collapse stabilizes the size of the alveoli prevents edema ( movement of fluid into alveoli)
19 Surface Tension of the Alveoli During inhalation r= 0.5 X 10-4 to 1.0 X 10-4 m mucous tissue fluid lining the alveoli Pi= -3mm Hg normally has a ST of N/m Po= - 4 mm Hg
20 Surface Tension of the Alveoli with this ST: P t = P i - P o = 2 = 2 ( N/m) r 0.5 X 10-4 m = 2 X 10 3 N/m 2 = 2000 Pa = 15 mmhg Since P i = - 3 mm Hg, then P o = -18 mm Hg
21 Surface Tension of the Alveoli However, P o is only -4 mm Hg so that the actual pressure difference, P i - P o = 1 mm Hg only,or 15 fold less than that would be required to expand the alveolus with a ST of 0.05 N/m. The walls of the alveoli secrete a SURFACTANT which reduces the surface tension.: Lipoprotein mixture secreted by special cells, type II granular pneumocytes: dipalmitoyl phosphatidylcholine
22 Surface Tension of the Alveoli The surfactant is very important in minimizing the effect of ST in causing collapse of the lungs. Some newborns, especially premature ones do not secrete enough of this surfactant: HYALINE MEMBRANE DISEASE (RESPIRATORY DISTRESS SYNDROME).
23 Surface Tension of the Alveoli There is a FIXED AMOUNT of surfactant in each alveolus and its ability to reduce ST depends on its CONCENTRATION. a. Alveolus deflated conc. of surfactant per unit area is high ST is low therefore alveolus expands without difficulty (inhaling). b. Alveolus expands conc. of surfactant per unit area is low ST is high therefore this helps collapse of the alveolus and expel air (exhaling).
24 Surfactant Stabilizes the Size of the Alveoli Second function of the surfactant: to prevent the small alveoli from collapsing and discharging their contents into larger alveoli. LAPLACE's LAW: For a given wall tension, the internal pressure rises as the radius of the alveolus decreases P= r As the diameter becomes less, the pressure required to keep the alveolus from collapsing further becomes proportionately GREATER.
25 Pressure inside a Bubble The presure inside a soap bubble depends on its radius. When the valve between the two bubbles is closed, the pressure is greater in the smaller bubble (P= 4 /R). When the valve is opened, the smaller bubble empties into the larger, leaving a spherical cap with the same radius of curvature as the new large bubble.
26 Instability of the Alveoli: Without Surfactant Air displaced from the smaller bubble to the larger one: collapse of the alveolus. The reason why most alveoli do not collapse is because as the alveolar radius decreases, the ST is also reduced. due to unique properties of the surfactant: not constant
27 Instability of the Alveoli: With Surfactant. Laplace s Law The larger alveoli contract more (high ST) The smaller alveoli contract less (low ST) Alveolus larger conc of surfactant ST contract more Alveolus smaller conc of surfactant ST contract less As applied to the grape-like alveolus, where only the inner wall has a liquid surface exposed to gas, the formula is P = 2T/r.
28 Laplace s Law
4/18/12 MECHANISM OF RESPIRATION. Every Breath You Take. Fun Facts
 Objectives MECHANISM OF RESPIRATION Dr Badri Paudel Explain how the intrapulmonary and intrapleural pressures vary during ventilation and relate these pressure changes to Boyle s law. Define the terms
Objectives MECHANISM OF RESPIRATION Dr Badri Paudel Explain how the intrapulmonary and intrapleural pressures vary during ventilation and relate these pressure changes to Boyle s law. Define the terms
RSPT 1060 OBJECTIVES OBJECTIVES OBJECTIVES EQUATION OF MOTION. MODULE C Applied Physics Lesson #1 - Mechanics. Ventilation vs.
 RSPT 1060 MODULE C Applied Physics Lesson #1 - Mechanics OBJECTIVES At the end of this module, the student should be able to define the terms and abbreviations used in the module. draw & explain the equation
RSPT 1060 MODULE C Applied Physics Lesson #1 - Mechanics OBJECTIVES At the end of this module, the student should be able to define the terms and abbreviations used in the module. draw & explain the equation
(A) The partial pressure in the lungs is higher than in the blood, and oxygen diffuses out of the lungs passively.
 DAT Biology - Problem Drill 12: The Respiratory System Question No. 1 of 10 1. Which statement about the partial pressure of oxygen inside the lungs is correct? Question #01 (A) The partial pressure in
DAT Biology - Problem Drill 12: The Respiratory System Question No. 1 of 10 1. Which statement about the partial pressure of oxygen inside the lungs is correct? Question #01 (A) The partial pressure in
Respiratory System. Part 2
 Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Physiology - lecture 3
 Physiology - lecture 3 Residual Volume (RV):the amount of gas remaining in the lung at the end of a maximal exhalation Tidal Volume (TV):the volume of gas inhaled and exhaled during one respiratory cycle.
Physiology - lecture 3 Residual Volume (RV):the amount of gas remaining in the lung at the end of a maximal exhalation Tidal Volume (TV):the volume of gas inhaled and exhaled during one respiratory cycle.
Chapter 4 Surface Tension
 Chapter 4 Surface Tension Contents. Introduction 4.1 Surface tension, Angle of Contact and capillary Rise Method 4.2 Rise of Liquid in a Conical Capillary Tube 4.3 Energy Required to Raise a Liquid in
Chapter 4 Surface Tension Contents. Introduction 4.1 Surface tension, Angle of Contact and capillary Rise Method 4.2 Rise of Liquid in a Conical Capillary Tube 4.3 Energy Required to Raise a Liquid in
Collin County Community College. Lung Physiology
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
VISUAL PHYSICS School of Physics University of Sydney Australia. Why can a steel needle float but a larger piece of steel sink?
 VISUAL PYSIS School of Physics University of Sydney Australia SURFAE TENSION Why can a steel needle float but a larger piece of steel sink?? Ducks have drowned in farmyard ponds into which washing water
VISUAL PYSIS School of Physics University of Sydney Australia SURFAE TENSION Why can a steel needle float but a larger piece of steel sink?? Ducks have drowned in farmyard ponds into which washing water
Respiratory Pulmonary Ventilation
 Respiratory Pulmonary Ventilation Pulmonary Ventilation Pulmonary ventilation is the act of breathing and the first step in the respiratory process. Pulmonary ventilation brings in air with a new supply
Respiratory Pulmonary Ventilation Pulmonary Ventilation Pulmonary ventilation is the act of breathing and the first step in the respiratory process. Pulmonary ventilation brings in air with a new supply
Surface Tension Teacher Handout
 Module Overview Surface tension is the result of forces between molecules in a liquid, called cohesive forces. At the surface of a liquid, the molecules do not have liquid molecules all around them. Therefore
Module Overview Surface tension is the result of forces between molecules in a liquid, called cohesive forces. At the surface of a liquid, the molecules do not have liquid molecules all around them. Therefore
Physics 221, March 1. Key Concepts: Density and pressure Buoyancy Pumps and siphons Surface tension
 Physics 221, March 1 Key Concepts: Density and pressure Buoyancy Pumps and siphons Surface tension Fluids: Liquids Incompressible Gases Compressible Definitions Particle density: Density: Pressure: ρ particle
Physics 221, March 1 Key Concepts: Density and pressure Buoyancy Pumps and siphons Surface tension Fluids: Liquids Incompressible Gases Compressible Definitions Particle density: Density: Pressure: ρ particle
Surface Tension Student Handout
 Background Surface tension is the result of forces between molecules in a liquid, called cohesive forces. At the surface of a liquid, the molecules do not have liquid molecules all around them. Therefore
Background Surface tension is the result of forces between molecules in a liquid, called cohesive forces. At the surface of a liquid, the molecules do not have liquid molecules all around them. Therefore
Vapour pressure of liquids SURFACE TENSION
 Vapour pressure of liquids A liquid in a closed container is subjected to partial vapour pressure due to the escaping molecules from the surface; it reaches a stage of equilibrium when this pressure reaches
Vapour pressure of liquids A liquid in a closed container is subjected to partial vapour pressure due to the escaping molecules from the surface; it reaches a stage of equilibrium when this pressure reaches
I Physical Principles of Gas Exchange
 Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Chapter 15 Fluid. Density
 Density Chapter 15 Fluid Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle By Dr. Weining man 1 Units of Chapter 15 Fluid
Density Chapter 15 Fluid Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy Applications of Archimedes Principle By Dr. Weining man 1 Units of Chapter 15 Fluid
Figure 5.1: molecular interaction
 Chapter 5 Surface tension 5.1 Definition If a line element be imagined on the surface of a liquid in any direction, the surface tension of the liquid, at a given temperature, is defined as the force per
Chapter 5 Surface tension 5.1 Definition If a line element be imagined on the surface of a liquid in any direction, the surface tension of the liquid, at a given temperature, is defined as the force per
Lecture Outline Chapter 15. Physics, 4 th Edition James S. Walker. Copyright 2010 Pearson Education, Inc.
 Lecture Outline Chapter 15 Physics, 4 th Edition James S. Walker Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy
Lecture Outline Chapter 15 Physics, 4 th Edition James S. Walker Chapter 15 Fluids Density Units of Chapter 15 Pressure Static Equilibrium in Fluids: Pressure and Depth Archimedes Principle and Buoyancy
More About Solids, Liquids and Gases ASSIGNMENT
 More About Solids, Liquids and Gases ASSIGNMENT 1. Fill in the blank spaces by choosing the correct words from the list given below: List : water, density, altitudes, lateral, intermolecular, force, cohesion,
More About Solids, Liquids and Gases ASSIGNMENT 1. Fill in the blank spaces by choosing the correct words from the list given below: List : water, density, altitudes, lateral, intermolecular, force, cohesion,
Section Two Diffusion of gases
 Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Chapter 13. liquids. gases. 1) Fluids exert pressure. a) because they're made up of matter and therefore forces are applied to them
 \ Chapter 13 Fluids 1) Fluids exert pressure a) because they're made up of matter and therefore forces are applied to them liquids gases b) they are made of matter in constant motion colliding with other
\ Chapter 13 Fluids 1) Fluids exert pressure a) because they're made up of matter and therefore forces are applied to them liquids gases b) they are made of matter in constant motion colliding with other
Lung Volumes and Ventilation
 Respiratory System ssrisuma@rics.bwh.harvard.edu Lung Volumes and Ventilation Minute ventilation Volume of an inspired or expired air per minute = tidal volume (V T ) x respiratory rate Dead space ventilation
Respiratory System ssrisuma@rics.bwh.harvard.edu Lung Volumes and Ventilation Minute ventilation Volume of an inspired or expired air per minute = tidal volume (V T ) x respiratory rate Dead space ventilation
The physiological functions of respiration and circulation. Mechanics. exercise 7. Respiratory Volumes. Objectives
 exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
Respiratory System Physiology. Dr. Vedat Evren
 Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 11 Respiratory System 2 Pulmonary Ventilation Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session plan o Pulmonary Ventilation
BIOH122 Human Biological Science 2 Session 11 Respiratory System 2 Pulmonary Ventilation Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session plan o Pulmonary Ventilation
Airway: the tubes through which air flows between atmosphere and alveoli. Upper airway. Lower airway
 Respiration Yu Yanqin ( 虞燕琴 ), PhD Dept. of fph Physiology Zhejiang University, School of Medicine Respiration Definition: the bodily processes involved in exchange of oxygen (O 2 ) and carbon dioxide
Respiration Yu Yanqin ( 虞燕琴 ), PhD Dept. of fph Physiology Zhejiang University, School of Medicine Respiration Definition: the bodily processes involved in exchange of oxygen (O 2 ) and carbon dioxide
The Physiologic Basis of DLCO testing. Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan
 The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
AP Physics B Ch 10 Fluids. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Name: Period: Date: AP Physics B Ch 10 Fluids 1) The three common phases of matter are A) solid, liquid, and vapor. B) solid, plasma, and gas. C) condensate, plasma, and gas. D) solid, liquid, and gas.
Name: Period: Date: AP Physics B Ch 10 Fluids 1) The three common phases of matter are A) solid, liquid, and vapor. B) solid, plasma, and gas. C) condensate, plasma, and gas. D) solid, liquid, and gas.
L 15 Fluids [4] The Venturi Meter. Bernoulli s principle WIND. Basic principles of fluid motion. Why does a roof blow off in high winds?
![L 15 Fluids [4] The Venturi Meter. Bernoulli s principle WIND. Basic principles of fluid motion. Why does a roof blow off in high winds? L 15 Fluids [4] The Venturi Meter. Bernoulli s principle WIND. Basic principles of fluid motion. Why does a roof blow off in high winds?](/thumbs/72/68053820.jpg) Basic principles of fluid motion L 15 Fluids [4] >Fluid flow and Bernoulli s s principle >Airplanes and curveballs >viscosity (real fluids) v 1 Continuity equation: v A = constant A 1 A 2 v 2 Bernoulli
Basic principles of fluid motion L 15 Fluids [4] >Fluid flow and Bernoulli s s principle >Airplanes and curveballs >viscosity (real fluids) v 1 Continuity equation: v A = constant A 1 A 2 v 2 Bernoulli
Extreme Science. Surface Tension
 Extreme Science Surface Tension Lesson We've all seen bubbles, or wondered why raindrops look like they do when traveling down a window. Well the answer is a simple one, and a complicated one, all at the
Extreme Science Surface Tension Lesson We've all seen bubbles, or wondered why raindrops look like they do when traveling down a window. Well the answer is a simple one, and a complicated one, all at the
Surface Tension- Worksheet
 Surface Tension- Worksheet A-Surface tension phenomenon 1. After watching the video, explain how can insects walk on the surface of the water? 2. Using the glass beaker, water and paper clip in front of
Surface Tension- Worksheet A-Surface tension phenomenon 1. After watching the video, explain how can insects walk on the surface of the water? 2. Using the glass beaker, water and paper clip in front of
THE MECHANICS of RESPIRATION. Introduction
 THE MECHANICS of RESPIRATION Dr. James Duffin Departments of Physiology and Anaesthesia General Learning Objectives: 1. How is air moved into and out of the lungs? 2. What mechanical factors affect the
THE MECHANICS of RESPIRATION Dr. James Duffin Departments of Physiology and Anaesthesia General Learning Objectives: 1. How is air moved into and out of the lungs? 2. What mechanical factors affect the
Two interconnected rubber balloons as a demonstration showing the effect of surface tension
 Two interconnected rubber balloons as a demonstration showing the effect of surface tension Abstract Science One 28-9 CHEN, Chieh-Shan The two interconnected rubber balloons system is a demonstration widely
Two interconnected rubber balloons as a demonstration showing the effect of surface tension Abstract Science One 28-9 CHEN, Chieh-Shan The two interconnected rubber balloons system is a demonstration widely
Conceptual Physics Matter Liquids Gases
 Conceptual Physics Matter Liquids Gases Lana Sheridan De Anza College July 25, 2017 Last time atomic structure forms of matter solids density elasticity liquids & pressure Overview liquids pressure surface
Conceptual Physics Matter Liquids Gases Lana Sheridan De Anza College July 25, 2017 Last time atomic structure forms of matter solids density elasticity liquids & pressure Overview liquids pressure surface
Respiratory Lecture Test Questions Set 1
 Respiratory Lecture Test Questions Set 1 1. The term "respiration" in its most complete meaning is: a. breathing b. oxygen transport c. carbon dioxide transport d. cellular energy production e. all of
Respiratory Lecture Test Questions Set 1 1. The term "respiration" in its most complete meaning is: a. breathing b. oxygen transport c. carbon dioxide transport d. cellular energy production e. all of
Density. Chapters 12-14: Phases of Matter. Example: Density. Conceptual Check. Springs 2/27/12. Mass Density vs. Weight Density
 Chapters 12-14: Phases of Matter Density Sequence of increasing molecule motion (and kinetic energy) Solid Liquid Gas The densities of most liquids and solids vary slightly with changes in temperature
Chapters 12-14: Phases of Matter Density Sequence of increasing molecule motion (and kinetic energy) Solid Liquid Gas The densities of most liquids and solids vary slightly with changes in temperature
Chapter 17 Mechanics of Breathing
 1 Chapter 17 Mechanics of Breathing Running Problem COPD: Chronic Obstructive Pulmonary Disease (impaired air exchanged) - Chronic Bronchitis: (Blue Bloaters) Bluish tinge of skin and tendency to be overweight
1 Chapter 17 Mechanics of Breathing Running Problem COPD: Chronic Obstructive Pulmonary Disease (impaired air exchanged) - Chronic Bronchitis: (Blue Bloaters) Bluish tinge of skin and tendency to be overweight
Lung Volumes and Capacities
 Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Name period date assigned date due date returned
 Name period date assigned date due date returned procedure 1. Take one balloon and stretch it out 2. Take one deep breath and blow into the balloon until you cannot breath out anymore. Do Not Take A Second
Name period date assigned date due date returned procedure 1. Take one balloon and stretch it out 2. Take one deep breath and blow into the balloon until you cannot breath out anymore. Do Not Take A Second
For more information about how to cite these materials visit
 Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
RESPIRATORY GAS EXCHANGE
 RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
Chapter 42 Part III The Respiratory System
 Biology 120 J. Greg Doheny Chapter 42 Part III The Respiratory System Notes: In this section we will discuss the breathing system, also known as the respiratory system. This should not be confused with
Biology 120 J. Greg Doheny Chapter 42 Part III The Respiratory System Notes: In this section we will discuss the breathing system, also known as the respiratory system. This should not be confused with
GAS EXCHANGE & PHYSIOLOGY
 GAS EXCHANGE & PHYSIOLOGY Atmospheric Pressure Intra-Alveolar Pressure Inspiration 760 mm HG at Sea Level (= 1 atm) Pressure due to gases (N2, O2, CO2, Misc.) Pressure inside the alveolus (air sac) Phrenic
GAS EXCHANGE & PHYSIOLOGY Atmospheric Pressure Intra-Alveolar Pressure Inspiration 760 mm HG at Sea Level (= 1 atm) Pressure due to gases (N2, O2, CO2, Misc.) Pressure inside the alveolus (air sac) Phrenic
. In an elevator accelerating upward (A) both the elevator accelerating upward (B) the first is equations are valid
 IIT JEE Achiever 2014 Ist Year Physics-2: Worksheet-1 Date: 2014-06-26 Hydrostatics 1. A liquid can easily change its shape but a solid cannot because (A) the density of a liquid is smaller than that of
IIT JEE Achiever 2014 Ist Year Physics-2: Worksheet-1 Date: 2014-06-26 Hydrostatics 1. A liquid can easily change its shape but a solid cannot because (A) the density of a liquid is smaller than that of
(Slide 1) Lecture Notes: Respiratory System
 (Slide 1) Lecture Notes: Respiratory System I. (Slide 2) The Respiratory Tract A) Major structures and regions of the respiratory Tract/Route INTO body 1) nose 2) nasal cavity 3) pharynx 4) glottis 5)
(Slide 1) Lecture Notes: Respiratory System I. (Slide 2) The Respiratory Tract A) Major structures and regions of the respiratory Tract/Route INTO body 1) nose 2) nasal cavity 3) pharynx 4) glottis 5)
Respiration. The resspiratory system
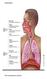 Respiration The resspiratory system The Alveoli The lungs have about 300 million alveoli, with a total crosssec onal area of 50 70 m2.. Each alveolar sac is surrounded by blood capillaries. The walls of
Respiration The resspiratory system The Alveoli The lungs have about 300 million alveoli, with a total crosssec onal area of 50 70 m2.. Each alveolar sac is surrounded by blood capillaries. The walls of
Chapter 13 Fluids. Copyright 2009 Pearson Education, Inc.
 Chapter 13 Fluids Phases of Matter Density and Specific Gravity Pressure in Fluids Atmospheric Pressure and Gauge Pressure Pascal s Principle Units of Chapter 13 Measurement of Pressure; Gauges and the
Chapter 13 Fluids Phases of Matter Density and Specific Gravity Pressure in Fluids Atmospheric Pressure and Gauge Pressure Pascal s Principle Units of Chapter 13 Measurement of Pressure; Gauges and the
Circulatory And Respiration
 Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Respiration - Human 1
 Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
Physiology Unit 4 RESPIRATORY PHYSIOLOGY
 Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
HMP 210: MEDICAL PHYSIOLOGY III. Dr Lee Ngugi Kigera
 HMP 210: MEDICAL PHYSIOLOGY III Dr Lee Ngugi Kigera HMP 200: RESPIRATORY PHYSIOLOGY AND MECHANICS OF RESPIRATION HMP 201: TRANSPORT OF GASES AND RESPIRATORY CONTROL Reference books Review of Medical Physiology
HMP 210: MEDICAL PHYSIOLOGY III Dr Lee Ngugi Kigera HMP 200: RESPIRATORY PHYSIOLOGY AND MECHANICS OF RESPIRATION HMP 201: TRANSPORT OF GASES AND RESPIRATORY CONTROL Reference books Review of Medical Physiology
I. Gas Exchange Respiratory Surfaces Respiratory Surface:
 I. Gas Exchange Respiratory Surfaces Respiratory Surface: Common characteristics of respiratory surfaces: a) Moist: allows for the RAPID diffusion of dissolved gasses across its surface. Whereas the respiratory
I. Gas Exchange Respiratory Surfaces Respiratory Surface: Common characteristics of respiratory surfaces: a) Moist: allows for the RAPID diffusion of dissolved gasses across its surface. Whereas the respiratory
Respiratory Physiology Gaseous Exchange
 Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
Chapter 13 The Respiratory System
 Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Mechanics of ventilation: static lung mechanics
 Mechanics of ventilation: static lung mechanics Specific learning objectives: Elastic properties of lung and chest wall Compliance: measure of elastic properties of lung An isolated lung tends to contract
Mechanics of ventilation: static lung mechanics Specific learning objectives: Elastic properties of lung and chest wall Compliance: measure of elastic properties of lung An isolated lung tends to contract
A deliberation on the surface tension theory
 A deliberation on the surface tension theory Hamid V. Ansari Department of Physics, Isfahan University, Isfahan, IRAN Personal address: No. 16, Salman-Farsi Lane, Zeinabieh Street, Isfahan, Postal Code
A deliberation on the surface tension theory Hamid V. Ansari Department of Physics, Isfahan University, Isfahan, IRAN Personal address: No. 16, Salman-Farsi Lane, Zeinabieh Street, Isfahan, Postal Code
αo 2 : solubility coefficient of O 2
 Version 2006 Dr. Puntarica Suwanprathes 1) Fick s law of diffusion 2) facts which limit gas transfer 3) diffusion capacity gas volume gaseous phase dissolved gas exert pressure*** Solubility of Gas C =P.
Version 2006 Dr. Puntarica Suwanprathes 1) Fick s law of diffusion 2) facts which limit gas transfer 3) diffusion capacity gas volume gaseous phase dissolved gas exert pressure*** Solubility of Gas C =P.
Hypothetical Cause of Flat Chest Kitten Syndrome
 Hypothetical Cause of Flat Chest Kitten Syndrome Ray Wigley (MD) What this paper sets out to do is expand on a theory as to the probable cause of FCKS. It suggest that FCKS is related to a condition called
Hypothetical Cause of Flat Chest Kitten Syndrome Ray Wigley (MD) What this paper sets out to do is expand on a theory as to the probable cause of FCKS. It suggest that FCKS is related to a condition called
PROBLEM SET 8. SOLUTIONS April 15, 2004
 Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Physiology (3) Pulmonary Function Test:
 Pulmonary Function Test: Today we will continue with the pulmonary function test, and the question is: why do we do pulmonary function tests for patients? Can pulmonary function tests tell us what type
Pulmonary Function Test: Today we will continue with the pulmonary function test, and the question is: why do we do pulmonary function tests for patients? Can pulmonary function tests tell us what type
Unit 8: Gases and States of Matter
 Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D.
 Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
1. Label a diagram of the respiratory system. Objective sheet 3 Notes
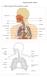 1. Label a diagram of the respiratory system Objective sheet 3 Notes 2. Functions of the respiratory structures Name Description Function Nasal Cavity Trachea Bronchi (Singular Bronchus) Bronchioles Lungs
1. Label a diagram of the respiratory system Objective sheet 3 Notes 2. Functions of the respiratory structures Name Description Function Nasal Cavity Trachea Bronchi (Singular Bronchus) Bronchioles Lungs
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation
 Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
By: Aseel Jamil Al-twaijer. Lec : physical principles of gas exchange
 By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
GASEOUS EXCHANGE 17 JULY 2013
 GASEOUS EXCHANGE 17 JULY 2013 Lesson Description In this lesson we: Discuss what is gaseous exchange? Consider requirements of an efficient gaseous exchange surface. Look at diversity in gas exchange systems.
GASEOUS EXCHANGE 17 JULY 2013 Lesson Description In this lesson we: Discuss what is gaseous exchange? Consider requirements of an efficient gaseous exchange surface. Look at diversity in gas exchange systems.
PHYS 101 Previous Exam Problems
 PHYS 101 Previous Exam Problems CHAPTER 14 Fluids Fluids at rest pressure vs. depth Pascal s principle Archimedes s principle Buoynat forces Fluids in motion: Continuity & Bernoulli equations 1. How deep
PHYS 101 Previous Exam Problems CHAPTER 14 Fluids Fluids at rest pressure vs. depth Pascal s principle Archimedes s principle Buoynat forces Fluids in motion: Continuity & Bernoulli equations 1. How deep
Animal Systems: The Respiratory System
 Animal Systems: The Respiratory System Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems The Digestive The Circulatory
Animal Systems: The Respiratory System Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems The Digestive The Circulatory
Monday, ! Today: Respiratory system! 5/20/14! Transport of Blood! What we ve been covering! Circulatory system! Parts of blood! Heart! tubing!
 Monday, 5.19.14! What we ve been covering! Circulatory system! Parts of blood! Heart! tubing! Transport of Blood! What is transported! Nutrients! Oxygen! Carbon Dioxide! Hormones! Antibodies! What it is/does!
Monday, 5.19.14! What we ve been covering! Circulatory system! Parts of blood! Heart! tubing! Transport of Blood! What is transported! Nutrients! Oxygen! Carbon Dioxide! Hormones! Antibodies! What it is/does!
PHY131H1S - Class 23. Today: Fluids Pressure Pascal s Law Gauge Pressure Buoyancy, Archimedes Principle. A little pre-class reading quiz
 PHY131H1S - Class 23 Today: Fluids Pressure Pascal s Law Gauge Pressure Buoyancy, Archimedes Principle Archimedes (287-212 BC) was asked to check the amount of silver alloy in the king s crown. The answer
PHY131H1S - Class 23 Today: Fluids Pressure Pascal s Law Gauge Pressure Buoyancy, Archimedes Principle Archimedes (287-212 BC) was asked to check the amount of silver alloy in the king s crown. The answer
Please pick up your midterms from front of class
 Please pick up your midterms from front of class Average: 26.7/40 ~ 67 % Top grade: 39/40 = 97.5% Test % score distribution: Make sure you go through your test and the solutions carefully to understand
Please pick up your midterms from front of class Average: 26.7/40 ~ 67 % Top grade: 39/40 = 97.5% Test % score distribution: Make sure you go through your test and the solutions carefully to understand
Test Bank for Pilbeams Mechanical Ventilation Physiological and Clinical Applications 6th Edition by Cairo
 Test Bank for Pilbeams Mechanical Ventilation Physiological and Clinical Applications 6th Edition by Cairo Link full download: http://testbankair.com/download/test-bank-for-pilbeams-mechanicalventilation-physiological-and-clinical-applications-6th-edition-by-cairo/
Test Bank for Pilbeams Mechanical Ventilation Physiological and Clinical Applications 6th Edition by Cairo Link full download: http://testbankair.com/download/test-bank-for-pilbeams-mechanicalventilation-physiological-and-clinical-applications-6th-edition-by-cairo/
Chapter 14-Gases. Dr. Walker
 Chapter 14-Gases Dr. Walker State of Matter Gases are one of the four states of matter along with solids, liquids, and plasma Conversion to Gases From liquids Evaporation Example: Boiling water From solids
Chapter 14-Gases Dr. Walker State of Matter Gases are one of the four states of matter along with solids, liquids, and plasma Conversion to Gases From liquids Evaporation Example: Boiling water From solids
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The concept of pressure involves both 1) A) force and area. B) force and volume. C) area
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The concept of pressure involves both 1) A) force and area. B) force and volume. C) area
PARTS AND STRUCTURE OF THE RESPIRATORY SYSTEM
 PARTS AND STRUCTURE OF THE RESPIRATORY SYSTEM Parts of the Respiratory System The RS can be divided into two parts: 1. Respiratory Tract, (path that air follows). Nasal passage Pharynx Larynx Trachea Bronchi,
PARTS AND STRUCTURE OF THE RESPIRATORY SYSTEM Parts of the Respiratory System The RS can be divided into two parts: 1. Respiratory Tract, (path that air follows). Nasal passage Pharynx Larynx Trachea Bronchi,
25/4/2016. Physiology #01 Respiratory system Nayef Garaibeh Rawan Alwaten
 25/4/2016 Physiology #01 Respiratory system Nayef Garaibeh Rawan Alwaten Respiratory System Introduction: - We breath while we are sleeping, talking, working and resting. - Respiratory diseases are abundant
25/4/2016 Physiology #01 Respiratory system Nayef Garaibeh Rawan Alwaten Respiratory System Introduction: - We breath while we are sleeping, talking, working and resting. - Respiratory diseases are abundant
Gas exchange. Tissue cells CO2 CO 2 O 2. Pulmonary capillary. Tissue capillaries
 Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
Section Three Gas transport
 Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
The Respiratory System Part I. Dr. Adelina Vlad
 The Respiratory System Part I Dr. Adelina Vlad The Respiratory Process Breathing automatic, rhythmic and centrally-regulated mechanical process by which the atmospheric gas moves into and out of the lungs
The Respiratory System Part I Dr. Adelina Vlad The Respiratory Process Breathing automatic, rhythmic and centrally-regulated mechanical process by which the atmospheric gas moves into and out of the lungs
Chapter 12. Properties of Gases
 Properties of Gases Each state of matter has its own properties. Gases have unique properties because the distance between the particles of a gas is much greater than the distance between the particles
Properties of Gases Each state of matter has its own properties. Gases have unique properties because the distance between the particles of a gas is much greater than the distance between the particles
Mini-Labs. 7. Movie Fog 8. Jet Power 9. Sauce Pan 10. Magnetic Bubbles 11. Aquarium Magic 12. Hockey Puck 13. Carbon dioxide balloons
 Mini-Labs (13) Mini-Labs 1. Magic Raisins 2. Mysterious Balloons 3. Candle Power 4. Super-cooled Liquid 5. Singing Tongs 6. Film Canister 7. Movie Fog 8. Jet Power 9. Sauce Pan 10. Magnetic Bubbles 11.
Mini-Labs (13) Mini-Labs 1. Magic Raisins 2. Mysterious Balloons 3. Candle Power 4. Super-cooled Liquid 5. Singing Tongs 6. Film Canister 7. Movie Fog 8. Jet Power 9. Sauce Pan 10. Magnetic Bubbles 11.
Part II. Under Construction Station Instructions. Lab Station A - Blue Print: There is O 2 Here!
 Lab Station A - Blue Print: There is O 2 Here! Description: In this lab, you will consider the problem: What happened to the oxygen in the air we breathed in? Air that enters the body upon inhalation contains
Lab Station A - Blue Print: There is O 2 Here! Description: In this lab, you will consider the problem: What happened to the oxygen in the air we breathed in? Air that enters the body upon inhalation contains
Respiratory Lecture Test Questions Set 3
 Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
Then the partial pressure of oxygen is x 760 = 160 mm Hg
 1 AP Biology March 2008 Respiration Chapter 42 Gas exchange occurs across specialized respiratory surfaces. 1) Gas exchange: the uptake of molecular oxygen (O2) from the environment and the discharge of
1 AP Biology March 2008 Respiration Chapter 42 Gas exchange occurs across specialized respiratory surfaces. 1) Gas exchange: the uptake of molecular oxygen (O2) from the environment and the discharge of
Chapter 1: Respiration
 Chapter 1: Respiration Respiration Human Breathing Mechanism Transport of oxygen Importance of a healthy respiratory system Respiratory system lungs inhalation exhalation Diffusion of oxygen by blood Transport
Chapter 1: Respiration Respiration Human Breathing Mechanism Transport of oxygen Importance of a healthy respiratory system Respiratory system lungs inhalation exhalation Diffusion of oxygen by blood Transport
Respiratory System. Prepared by: Dorota Marczuk-Krynicka, MD, PhD
 Respiratory System Prepared by: Dorota Marczuk-Krynicka, MD, PhD Lungs: Ventilation Perfusion Gas Exchange - Diffusion 1. Airways and Airway Resistance (AWR) 2. Mechanics of Breathing and Lung (Elastic)
Respiratory System Prepared by: Dorota Marczuk-Krynicka, MD, PhD Lungs: Ventilation Perfusion Gas Exchange - Diffusion 1. Airways and Airway Resistance (AWR) 2. Mechanics of Breathing and Lung (Elastic)
2/28/18. Respiratory System. 1 Copyright 2016 by Elsevier Inc. All rights reserved. Introduction. Anatomy. Physiology. Respiratory System
 Introduction Respiratory System Chapter 28 Respiration: We inhale air, extract oxygen from it, exhale air Cardiovascular and respiratory systems work together Failure of either system: - Disruption of
Introduction Respiratory System Chapter 28 Respiration: We inhale air, extract oxygen from it, exhale air Cardiovascular and respiratory systems work together Failure of either system: - Disruption of
Chapter 13 The Respiratory System
 VI edit Pag 451-499 Chapter 13 The Respiratory System V edit. Pag 459-509 Tissue cell Alveoli of lungs Atmosphere 1 External respiration Ventilation or gas exchange between the atmosphere and air sacs
VI edit Pag 451-499 Chapter 13 The Respiratory System V edit. Pag 459-509 Tissue cell Alveoli of lungs Atmosphere 1 External respiration Ventilation or gas exchange between the atmosphere and air sacs
UNIT 9 - RESPIRATORY SYSTEM LECTURE NOTES
 UNIT 9 - RESPIRATORY SYSTEM LECTURE NOTES 9.01 GENERAL FUNCTIONS OF THE RESPIRATORY SYSTEM A. Brings oxygenated air to the alveoli B. Removes air containing carbon dioxide C. Filters, warms, and humidifies
UNIT 9 - RESPIRATORY SYSTEM LECTURE NOTES 9.01 GENERAL FUNCTIONS OF THE RESPIRATORY SYSTEM A. Brings oxygenated air to the alveoli B. Removes air containing carbon dioxide C. Filters, warms, and humidifies
Gas exchange and gas transfer
 Gas exchange and gas transfer Color index: Red: important Green: doctor s notes Grey: extra information Pink: found only in female s slides Blue: found only in male s slides Yellow: numbers Physiology
Gas exchange and gas transfer Color index: Red: important Green: doctor s notes Grey: extra information Pink: found only in female s slides Blue: found only in male s slides Yellow: numbers Physiology
Notes Chapter 3. Buoyancy
 Notes Chapter 3 Buoyancy Pressure in a Fluid 3.2 Pressure and the Buoyant Forces Liquids and gases are fluids materials that can flow and have no definite shape. Objects in a fluid experience a buoyant
Notes Chapter 3 Buoyancy Pressure in a Fluid 3.2 Pressure and the Buoyant Forces Liquids and gases are fluids materials that can flow and have no definite shape. Objects in a fluid experience a buoyant
Gas Exchange in Animals. Uptake of O2 from environment and discharge of CO2. Respiratory medium! water for aquatic animals, air for terrestial
 Gas Exchange in Animals Uptake of O2 from environment and discharge of CO2 Respiratory medium! water for aquatic animals, air for terrestial Respiratory surface! skin, gills, lungs Circulatory System O2/CO2
Gas Exchange in Animals Uptake of O2 from environment and discharge of CO2 Respiratory medium! water for aquatic animals, air for terrestial Respiratory surface! skin, gills, lungs Circulatory System O2/CO2
Essential Skills Course Acute Care Module. Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook
 Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
Yanal. Jumana Jihad. Jamil Nazzal. 0 P a g e
 2 Yanal Jumana Jihad Jamil Nazzal 0 P a g e note: this sheet was written and corrected according to the records from section 2 so you may find differences in the arrangement of topics from the records
2 Yanal Jumana Jihad Jamil Nazzal 0 P a g e note: this sheet was written and corrected according to the records from section 2 so you may find differences in the arrangement of topics from the records
Respiration. The ins and outs
 Respiration The ins and outs Functions 1. To bring O 2 into the body and transfer it to the blood stream 2. To remove CO 2 Circulation and respiration work together to achieve these functions Why Do We
Respiration The ins and outs Functions 1. To bring O 2 into the body and transfer it to the blood stream 2. To remove CO 2 Circulation and respiration work together to achieve these functions Why Do We
Experiment B-3 Respiration
 1 Experiment B-3 Respiration Objectives To study the diffusion process of oxygen and carbon dioxide between the alveoli and pulmonary capillaries. To determine the percentage of oxygen in exhaled air while
1 Experiment B-3 Respiration Objectives To study the diffusion process of oxygen and carbon dioxide between the alveoli and pulmonary capillaries. To determine the percentage of oxygen in exhaled air while
Old-Exam.Questions-Ch-14 T072 T071
 Old-Exam.Questions-Ch-14 T072 Q23. Water is pumped out of a swimming pool at a speed of 5.0 m/s through a uniform hose of radius 1.0 cm. Find the mass of water pumped out of the pool in one minute. (Density
Old-Exam.Questions-Ch-14 T072 Q23. Water is pumped out of a swimming pool at a speed of 5.0 m/s through a uniform hose of radius 1.0 cm. Find the mass of water pumped out of the pool in one minute. (Density
8.1 Properties of Gases. Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases.
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8. Chapter 8
 Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases Chapter 8 Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle s Law) 8.3 Temperature and Volume (Charles Law) 8.4 Temperature and Pressure (Guy-Lussac s Law) 8.5 The Combined Gas Low
Gases. Chapter 8. Chapter 8. Gases Properties of Gases. We are surrounded by gases, but we are often
 Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
Gases Chapter 8 8.1 Properties of Gases Goal: Describe the Kinetic Molecular Theory of Gases and the units of measurement used for gases. Chapter 8 8.1 - Properties of Gases 8.2 Pressure and Volume (Boyle
General, Organic & Biological Chemistry, 5e (Timberlake) Chapter 8 Gases. 8.1 Multiple-Choice Questions
 Instant download and all chapters Test Bank General, Organic, and Biological Chemistry Structures of Life 5th Edition Timberlake https://testbanklab.com/download/test-bank-general-organic-biological-chemistry-structureslife-5th-edition-timberlake/
Instant download and all chapters Test Bank General, Organic, and Biological Chemistry Structures of Life 5th Edition Timberlake https://testbanklab.com/download/test-bank-general-organic-biological-chemistry-structureslife-5th-edition-timberlake/
