HELIUM IN THE TREATMENT OF ASTHMA
|
|
|
- Laura Baldwin
- 5 years ago
- Views:
Transcription
1 Thorax (1946), 1, HELIUM IN THE TREATMENT OF ASTHMA BY From the Medical Unit, St. Thomas's Hospital, London INTRODUCTION The substitution of helium for nitrogen in the inspired air has been advocated for two entirely different reasons. First, Sayers and Yant (1926) suggested that owing to the low solubility of helium its use in diving and other operations involving respiration under high pressure might reduce the incidence of caisson disease. Secondly, Barach (1934) suggested that the lesser mass of the helium molecule might make a helium-oxygen mixture easier to breathe than air in conditions of obstructed respiration and in asthma. In support of this latter possibility Barach (1936) showed that in tracheotomized dogs, in which an obstruction had been inserted in the airway, the substitution of helium for nitrogen in the inspired air considerably reduced the negative intrapleural pressure on inspiration, for example, from to cm. of water. At the same time what he defined as the 'pulmonary pressure,' that is, the pressure difference set up in the trachea on respiration, was also reduced, for example, from 3.56 to 2.4 cm. of water. A similar experiment with men, in which the subjects breathed through a hole I in. diameter, also showed a reduction of per cent in the 'pulmonary pressure' with helium. Barach concluded from these results that the use of helium-oxygen mixtures resulted in a saving of pulmonary effort, and that consequently there were grounds for thinking that relief might be obtained in cases of asthma, in which the increased pulmonary effort is an outstanding symptom. Unfortunately asthma is a very variable condition, many cases having a large psychological element and others being complicated by a concomitant bronchitis. Consequently it is impossible to reach a final conclusion on-the results of a small number of cases. However, in view of the considerable theoretical interest attached to the use of helium, it is worth while reviewing even a small experience. RESULTS In order to test Barach's hypothesis six cases of asthma admitted consecutively to St. Thomas's Hospital were treated by respiration of a mixture containing 80 per cent helium- and 20 per cent oxygen, and their progress was followed. The gas was given through a B.L.B. oro-nasal mask. The type used was the original one described by Lovelace (1938), with a spring expiratory valve and three
2 HELIUM IN TREATMENT OF ASTHMA adjustable ports. The valve was unscrewed as far as possible so that any effect of expiring against a positive pressure (Barach and Swenson, 1939) should be minimal. The ports were kept closed and the rate of flow used was 8-11 litres a minute. This rate, which was more than enough to prevent the bag collapsing, was chosen to ensure that expired air was not collected in the bag; for Card, Forest Smith, Griffiths, McSwiney and Savage (1940) showed that though a maximal concentration of oxygen (approximately 90 per cent) could be obtained in the alveoli with 6-7 litres per min. there was, with this model, an appreciable collection of carbon dioxide in the bag (approximately 1 per cent) at rates less than 8.1 litres per minute. To ensure that the patients' condition was steady they were first observed for minutes resting quietly in bed, screened-off from the rest of the ward. The mask was then placed in position and oxygen administered for a further period of minutes. The oxygen was then switched off and administration of the helium mixture begun. The cylinders containing oxygen and the helium mixture were kept behind the head of the bed and were connected to the mask through a Y-piece. It was hoped that by this means the change from oxygen to the helium mixture might go unnoticed by the patient, so that any psychological effect could be eliminated. At the same time, any effect produced by the helium mixture could be compared with that produced by oxygen alone. After minutes, in those patients whose clinical condition permitted it, oxygen was again switched on in place of the helium mixture, and finally all treatment was stopped. A brief analysis of the history and clinical condition of the patients before treatment was started is given in Table I. TABLE I SHOWING THE CLINICAL DETAILS OF THE PATIENTS BEFORE TREATMENT Duration Severity Reaction to Case Sex Age Length Frequency of Usual duration of of adrenaline of history attacks of attacks present present in present attack attack attack 1 M mnths. most nights j-3 hrs. 2 hrs. moderate not given about 2 a.m. 2 M mnths. one a month 4 days 3 days severe poor 3 F yrs. most nights never quite free worse last moderate not given 2 dasys 4 M yrs. 2 or 3 a week 4 hrs. 2 hrs. moderate not given 5 M yrs. one a week - 2 wks. moderate nasal spray 6 ineffective 6 if 36 2 yrs. most nights j hr. 12 hrs. mild poor The effect of treatment was observed by repeated measurements of the blood pressure, pulse and respiration rates, by the presence or absence of cyanosis, and by a clinical estimate of the patient's distress. Measurements of the chest expansion were made in the first two cases, but were abandoned, as they were difficult 31
3 32 to make accurately and they did not alter appreciably when there was- a marked change in the other measurements. The observations made half an hour after the beginning of the control, oxygen, and helium periods are tabled below for comparison (Table II). This period was chosen because it generally took about half an hour for the patient's condition to become steady after changing the treatment. Observations made 10 minutes after the start of helium therapy are also included, because they seemed sufficiently constant to be significant. TABLE IX SHOWING THE EFFECT OF 1 00% OXYGEN AND A 80% HELIUM AND 20% OXYGEN MIXTURE ON THE BLOOD PRESSURE, PULSE AND RESPIRATION RATES OF 6 ASTHMATICS. i hr. after i hr. after 10 mins. after j hr. after Case start of start of start of start of After control period oxygen helium helium Treatment Blood pressure (mm. Hg) 1 155* t 225 -t t Pulse.rate per min * t 118 -_ Respiration-rate per min. 1 24* t t * Observed after 40 minutes. t Observed after 20 minutes. : Not observed owing to patient's distress. The full observations made on Cases 1, 2 and 4 are shown graphically in Figs. 1, 2, and 3. In Case 2 (Fig. 2) the patient's distress became so severe on switching over to the helium mixture that the mask had to be removed and the treatment stopped. In Case 5 the result was almost identical, the helium having to be stopped after 13 minutes. In this patient the psychological factor was certainly of considerable importance, as was illustrated by his bad response to pure oxygen; unfortunately he appeared to have got the idea that he was being experimented on.
4 HELIUM IN TREATMENT OF ASTHMA 33 In Cases 3 and 6 very little effect at all was noticed, save for a slight improvement with oxygen. These were the two mildest cases in the series. In summary the observations showed- (1) A slight general improvement with oxygen. (2) A temporary aggravation of the distress in all cases on changing from oxygen to the helium mixture, which became maximal in from 10 to 15 minutes, and in two cases was so severe that the treatment had to be abandoned. P R !I * % N N \ * (--He+O02 I v- (2 0 I ,A\ \ \O-- N a.'._ O--0S a- \-\ ~ No, x-x B.P. TIME IN MINUTES 1-2 MILD.-. P. DISTRESS 3 MODERATE O---o- R. 4-5 SEVERE GRAPH SHOWING THE EFFECT OF HELIUM ANO OXYGEN AND OF OXYGEN ON CASE 1. FIG. 1. (3) Those patients who were able to tolerate the helium-oxygen mixture improved slowly after the initial period of exacerbation, until after half an hour there was a definite improvement. (4) The degree of improvement with the helium, when it occurred, was not notably different from that obtained with 100 per cent oxygen. (5) Cyanosis was present initially in Cases 1, 2 and 3. It cleared with oxygen, but returned with the helium mixture. (6) The patient's subjective distress was closely correlated with the objective changes in the blood pressure, pulse, and respiration rates. / / P.,, R.
5 34 (7) Pulsus paradoxus, which was recognizable in all cases with the aid of the sphygmomanometer, was unaffected by treatment. The finding of pulsus paradoxus in asthma was unexpected but was quite definite. The decrease in systolic pressure during inspiration varied from 10 to 40 mm. of mercury in five cases, whilst in the other (Case 2) no pressure at all was recordable in late inspiration. In this case intermission of the pulse was also recognizable clinically. I have not been able to trace any other reference to the association of pulsus paradoxus with asthma, though it is well recognized to occur with laryngeal obstruction and other conditions resulting in severe dyspnoea. P. 120 B.P CHe + o A/ 11'120 BP R. / \ P \ /,n4 a TIME IN MINUTES GRAPH SHOWING THE EFFECT OF HELIUM AND OXYGEN AND OF OXYGEN ON CASE 2. FIG. 2. Whilst it is conceivable that an inspiratory reduction in systolic pressure could be the result of an increased negative intrapleural pressure dilating the lung capillaries and thus temporarily reducing the inflow to the left ventricle, it seems more likely that the effect was a local one produced by deformation of the subclavian artery as described by Falconer and McQueen (1915). According to them, such an effect is produced occasionally in normal people during forced inspiration, when it can be eliminated by bringing the shoulders forward. DISCUSSION Barach's original assumption (1934) was that the light helium molecule would be easier to breathe than the heavier nitrogen molecule, because in moving a gas " work is in general proportional to the density " of the gas moved. He also
6 HELIUM IN TREATMENT OF ASTHMA believed that the smaller helium molecule would appreciably facilitate the flow of gases by increasing the rate of diffusion. This thesis has been criticized by Dean and Visscher (1941) on the grounds that it ignores the factor of gas viscosity. In his later works Barach has considered this aspect of the problem, and his present views are stated fully in his book on inhalational therapy (1945). His point is that his results (1936), which demonstrate decreased pulmonary effort on respiring helium, when the gases were 140 P. 120 R if 1P -O-, 100~~~~~~~~~~~~~~~~0 4-.He ''j 20 80P0 * / TIME IN KNUTlLLES. GRAPH SHOWING THE EFFECT OF HELIUM AND OXYGEN AND OF OXYGEN ON CASE 4. FIG. 3. breathed through a partially obstructed orifice (see above), cannot be explained on the assumption that the work done in moving the respiratory gases is proportional to their viscosity-for the viscosity of helium is greater than that of air. He therefore concludes that the main gas movement must be by diffusion or effusion; in which case he states that the rate of flow obeys Graham's law for effusion, that is, it is inversely proportional to the square root of the molecular weight. In fact the physical processes involved are very much more complicated. The rate of diffusion of a gas is, under normal conditions of temperature and pressure, slow in comparison with the rate of flow produced by pressure differences across an orifice. Were it not, there would be no need for respiratory movements, for oxygen would simply diffuse into the lungs and carbon dioxide diffuse out without 35
7 36 any need for effort on the part of the animal at all. The only circumstance in which diffusion becomes the most important method of flow through an orifice is when the diameter of the orifice is small in comparison with the mean free path of the gas (Taylor, 1931); as the mean free paths of helium and nitrogen (at N.T.P.) are of the order of and mm. respectively, while the diameter of the smallest tubes in the lung is of the order of 0.1 mm., it hardly seems likely that diffusion can play an important part in the flow of the respiratory gases. Moreover, when the diameter of the orifice is small in comparison with the mean free path of the gas, each constituent gas in a mixture behaves independently of the other gases, so that the substitution of helium for nitrogen would have no effect on the flow of oxygen or CO2. On the other hand, conditions in the alveoli are different. Here there is no question of flow through an orifice, and the movement of gases from the centre of the alveolus to the lung membrane is largely by means of diffusion of one gas into another. The laws describing this process are very complicated (Taylor, 1931), but there is no doubt that in these circumstances the substitution of helium for nitrogen would increase the rate of diffusion of both oxygen and carbon dioxide, and it is possible that an improvement resulting from the use of helium might be due to this factor. The alternative process of effusion mentioned by Barach is unlikely to play any part in the movement of gases in the respiratory passages. Effusion takes place when a gas passes through a tube whose diameter is small in comparison with the mean free path of the gas, as, for example, when a gas passes through a porous plate of gypsum, and once again the motion of one constituent in a mixture is independent of the others (Taylor, 1931). Under the conditions which occur in the respiratory passages the methods of gas flow are either 'streamline' or 'turbulent.' These have been thoroughly examined by Dean and Visscher (1941). According to them, so long as gases move with streamline flow the advantage is all with air as compared with helium mixtures; for then the pressure required to move a gas is proportional to the rate of flow of the gas and its viscosity, and the viscosity of an 80 per cent helium and 20 per cent oxygen mixture is 1.11 compared with 1.00 for air. However, as the rate of flow is increased a velocity is reached, the critical velocity, at which the flow becomes turbulent. When the flow is turbulent the pressure required to move a gas is proportional to the square of the velocity of the gas and to its density. Now an 80 per cent helium and 20 per cent oxygen mixture has a density of 0.33 against 1.00 for air, and so has a considerable advantage over air; moreover, the mixture has the further advantage that its critical velocity is higher and that under conditions in which air is flowing turbulently a helium-oxygen mixture may still be flowing according to Poiseuille's law of streamline flow. Dean and Visscher have shown that under normal conditions air probably flows in the respiratory tract in a streamline manner, and that the ideal method of producing turbulent flow in the respiratory tract is to introduce an obstruction into
8 HELIUM IN TREATMENT OF ASTHMA the largest passages. Experiments on dogs showed that in the absence of obstruction there was no reduction in respiratory effort on substituting helium for nitrogen, but that when an obstruction was placed in series with the trachea a considerable reduction was effected. On the other hand, after large doses of pilocarpine, which may be presumed to have constricted the peripheral bronchioles and simulated as nearly as possible the conditions of asthma, helium effected only a very slight reduction in the work required for ventilation. In fact, there was a 10 per cent reduction in the ' viscous' work, but the 'elastic' work (required to distend the lungs), which had been markedly raised by the pilocarpine and formed the greater part of the work done, was unaltered. Dean and Visscher concluded that helium and oxygen mixtures cause no reduction in respiratory effort during normal respiration, that they are unlikely to produce more than a minimal reduction, if any, in uncomplicated asthma, but that they may produce a considerable reduction in respiratory effort when the larger passages are obstructed. As a rough guide helium may be expected to be beneficial when respiration is very noisy, that is, when the air flow is turbulent. Reports on the value of helium-oxygen mixtures in asthma leave the verdict non proven. Several papers claim that helium and oxygen is effective, particularly in cases of status asthmaticus and otherwise intractable asthma (Maytum, 1939; London, 1940 ; Barach and Cromwell, 1940). Barach (1935) reported four cases in detail in two of which relief was obtained immediately, and Maytum, Prickman and Boothby (1935) also reported three which " almost immediately improved both subjectively and objectively." It is interesting that in one of Barach's four cases (Case 3) there was the same difficulty in making the patient tolerate the mask that I found in two of my cases (Cases 2 and 5). According to his description " at first she struggled against the mask but after 15 min. of per cent helium she was relaxed and accepted treatment willingly." It is difficult to attribute this resistance entirely to the discomfort produced by the mask as oxygen had been previously well tolerated in my second case, and all my cases showed some objective signs of deterioration on changing from pure oxygen to the helium-oxygen mixture. The difficulty in assessing these reports is that even the most severe asthma is largely conditioned by mental influences, and it is impossible to evaluate how far these imiay have played a part in the improvement. In no instance is any mention made whether the patient knew a fresh treatment was being given or not. However, in the majority of cases, treatment has had to be continued over a long period before lasting relief has been obtained. In his recent book Barach (1945) has reported 76 out of 84 cases of severe asthma were " decisively benefited" by helium-oxygen therapy, but treatment had to be continued for from one to five days and ancillary methods, namely, intravenous aminophyllin, were also employed. This need for a long period of treatment was commented on by Piness (1939), who stated that so far as he could see from the literature, patients treated 37
9 38 with helium and oxygen did not obtain quicker relief than from other methods of treatment. Whilst it is true that after ten years no outright condemnation of the method has been published, according to Visscher (1941) " in uncomplicated asthma it has not been conspicuously successful"; my results lend support to this conclusion. Possibly the presence or absence of bronchitis in association with the asthma accounts for the different experiences. SUMMARY AND CONCLUSIONS (1) Six cases of mild to severe asthma were treated by respiration through a B.L.B. mask of an 80 per cent helium and 20 per cent oxygen mixture. (2) Two patients were unable to tolerate the treatment. In the rest some improvement was noted comparable to that obtained from 100 per cent oxygen. (3) Physical considerations indicate that helium is unlikely to be of great value in asthma, where the obstruction is in the smallest passages and where it is probable that at least part of the increased respiratory effort is due to changes in tissue elasticity. (4) Where a respiratory obstruction exists in the larger passages, for example, laryngeal obstruction, there are good theoretical grounds, supported by experimental evidence, for expecting relief with helium. ACKNOWLEDGEMENTS My thanks are due to Sir Maurice Cassidy and Prof. 0. L. V. S. de Wesselow for permission to treat their patients, and to Prof. B. A. McSwiney for his encouragement, to which the initiation of this work is due, and for his continued help and advice. REFERENCES Barach, A. L. (1934). Proc. Soc. exp. Biol., 32, 462. Barach, A. L. (1935). Ann. intern. Med., 9, 739. Barach, A. L. (1936). J. cdin. Invest., 15, 47. Barach, A. L. (1945). Principles and Practices of Inhalational Therapy, pp. 88 et seq. Barach, A. L., and Cromwell, H. A. (1940). M. Clin. N. Amer., 24, 621. Barach, A. L., and Swenson, P. C. (1939). Arch. intern. Med., 63, 946. Card, W. I., Forest Smith, J., Griffiths, W. S., McSwiney, B. A., and Savage, B. (1940). Lancet, 1, 398. Dean, R. B., and Visscher, M. B. (1941). Amer. J. Physiol., 134, 450. Falconer, A. W., and McQueen (1915). Quart. J. Med., 8, 38. London, M. (1940). Ohio St. med. J., 36, 945. Lovelace, W. R. (1938). Proc. Mayo Clin., 13, 646. Maytum, C. K. (1939). J. Allergy, 10, 264. Maytum, C. K., Prickman, L. E., and Boothby, W. M. (1935). Proc. Mayo Clin., 10, 788. Piness, G. (1939). J. Allergy, 10, 271. Sayers, R. R., and Yant, W. P. (1926). Anesth. & Analges., 5, 127. Taylor, H. S. (1931). Physical Chemistry, 1, pp. 172 et seq. Visscher, M. B. (1941). Proc. int. Assemb. post-grad. Ass. N. Amer., p. 320.
Respiratory Pulmonary Ventilation
 Respiratory Pulmonary Ventilation Pulmonary Ventilation Pulmonary ventilation is the act of breathing and the first step in the respiratory process. Pulmonary ventilation brings in air with a new supply
Respiratory Pulmonary Ventilation Pulmonary Ventilation Pulmonary ventilation is the act of breathing and the first step in the respiratory process. Pulmonary ventilation brings in air with a new supply
breathing oxygen-rich gas mixtures" was made in 1911 by Benedict the London Hospital.
 THE; EFFECT OF INHALATION OF OXYGEN ON THE RATE OF THE PULSE IN HEALTH. BY JOHN PARKINSON, M.D., M.R.C.P., Medical Registrar to the London Hospital. NONE of the early workers on the effect of oxygen on
THE; EFFECT OF INHALATION OF OXYGEN ON THE RATE OF THE PULSE IN HEALTH. BY JOHN PARKINSON, M.D., M.R.C.P., Medical Registrar to the London Hospital. NONE of the early workers on the effect of oxygen on
Collin County Community College. Lung Physiology
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Lung Volumes and Capacities
 Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Introduction. Respiration. Chapter 10. Objectives. Objectives. The Respiratory System
 Introduction Respiration Chapter 10 The Respiratory System Provides a means of gas exchange between the environment and the body Plays a role in the regulation of acidbase balance during exercise Objectives
Introduction Respiration Chapter 10 The Respiratory System Provides a means of gas exchange between the environment and the body Plays a role in the regulation of acidbase balance during exercise Objectives
Respiratory Physiology Gaseous Exchange
 Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
Sign up to receive ATOTW weekly -
 THE PHYSICS OF FLOW ANAESTHESIA TUTORIAL OF THE WEEK 84 9TH APRIL 2008 Paul Clements, SpR in Anaesthetics, Hope Hospital, Salford, UK. Carl Gwinnutt, Consultant Anaesthetist, Hope Hospital, Salford, UK.
THE PHYSICS OF FLOW ANAESTHESIA TUTORIAL OF THE WEEK 84 9TH APRIL 2008 Paul Clements, SpR in Anaesthetics, Hope Hospital, Salford, UK. Carl Gwinnutt, Consultant Anaesthetist, Hope Hospital, Salford, UK.
throughout. The constant-flow respiration was administered through a intravenously at appropriate intervals (in addition to the general
 414 6I2.22I:6I2.2I5.5 GASEOUS INTERCHANGES THROUGH THE VISCERAL PLEURA OF THE CAT. By M. KREMER, A. T. WILSON AND SAMSON WRIGHT. (Department of Physiology, Middlesex Hospital Medical School.) (Received
414 6I2.22I:6I2.2I5.5 GASEOUS INTERCHANGES THROUGH THE VISCERAL PLEURA OF THE CAT. By M. KREMER, A. T. WILSON AND SAMSON WRIGHT. (Department of Physiology, Middlesex Hospital Medical School.) (Received
The physiological functions of respiration and circulation. Mechanics. exercise 7. Respiratory Volumes. Objectives
 exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
RESPIRATORY MUSCLES IN HEALTH AND EMPHYSEMA *
 THE OXYGEN CONSUMPTION AND EFFICIENCY OF THE RESPIRATORY MUSCLES IN HEALTH AND EMPHYSEMA * BY REUBEN M. CHERNIACK t (From The Winnipeg General Hospital and the Departments of Medicine and Physiology and
THE OXYGEN CONSUMPTION AND EFFICIENCY OF THE RESPIRATORY MUSCLES IN HEALTH AND EMPHYSEMA * BY REUBEN M. CHERNIACK t (From The Winnipeg General Hospital and the Departments of Medicine and Physiology and
Respiratory System Physiology. Dr. Vedat Evren
 Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Respiratory Physiology. Adeyomoye O.I
 Respiratory Physiology By Adeyomoye O.I Outline Introduction Hypoxia Dyspnea Control of breathing Ventilation/perfusion ratios Respiratory/barometric changes in exercise Intra-pulmonary & intra-pleural
Respiratory Physiology By Adeyomoye O.I Outline Introduction Hypoxia Dyspnea Control of breathing Ventilation/perfusion ratios Respiratory/barometric changes in exercise Intra-pulmonary & intra-pleural
Chapter 4: Ventilation Test Bank MULTIPLE CHOICE
 Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
Chapter 37: Pulmonary Ventilation. Chad & Angela
 Chapter 37: Pulmonary Ventilation Chad & Angela Respiratory Structures Basic Structures of Respiration Nasal/Oral Cavities Larynx Trachea Bronchi Secondary Bronchi Bronchioles Alveoli Mechanics of Ventilation
Chapter 37: Pulmonary Ventilation Chad & Angela Respiratory Structures Basic Structures of Respiration Nasal/Oral Cavities Larynx Trachea Bronchi Secondary Bronchi Bronchioles Alveoli Mechanics of Ventilation
Human gas exchange. Question Paper. Save My Exams! The Home of Revision. Cambridge International Examinations. 56 minutes. Time Allowed: Score: /46
 Human gas exchange Question Paper Level Subject Exam oard Topic Sub Topic ooklet O Level iology ambridge International Examinations Respiration Human gas exchange Question Paper Time llowed: 56 minutes
Human gas exchange Question Paper Level Subject Exam oard Topic Sub Topic ooklet O Level iology ambridge International Examinations Respiration Human gas exchange Question Paper Time llowed: 56 minutes
CHAPTER 3: The respiratory system
 CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
Clinical Skills. Administering Oxygen
 Clinical Skills Administering Oxygen Updated July 2017 Clare Cann Original 2012 Carole Loveridge, Lecturer in Women`s Health Aims and Objectives Aims and Objectives The aim of this module is to facilitate
Clinical Skills Administering Oxygen Updated July 2017 Clare Cann Original 2012 Carole Loveridge, Lecturer in Women`s Health Aims and Objectives Aims and Objectives The aim of this module is to facilitate
Test Bank for Pilbeams Mechanical Ventilation Physiological and Clinical Applications 6th Edition by Cairo
 Test Bank for Pilbeams Mechanical Ventilation Physiological and Clinical Applications 6th Edition by Cairo Link full download: http://testbankair.com/download/test-bank-for-pilbeams-mechanicalventilation-physiological-and-clinical-applications-6th-edition-by-cairo/
Test Bank for Pilbeams Mechanical Ventilation Physiological and Clinical Applications 6th Edition by Cairo Link full download: http://testbankair.com/download/test-bank-for-pilbeams-mechanicalventilation-physiological-and-clinical-applications-6th-edition-by-cairo/
Oxygen convulsions are believed by many workers to be caused by an accumulation
 272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
You should be able to: Describe Equipment Barometer Manometer. 5.1 Pressure Read and outline 5.1 Define Barometer
 A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
Respiratory System. Part 2
 Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Department of Biology Work Sheet Respiratory system,9 class
 I. Name the following : Department of Biology Work Sheet Respiratory system,9 class 1. A muscular sheet separating the thoracic and abdominal cavities. 2. A respiratory tube supported by cartilaginous
I. Name the following : Department of Biology Work Sheet Respiratory system,9 class 1. A muscular sheet separating the thoracic and abdominal cavities. 2. A respiratory tube supported by cartilaginous
I Physical Principles of Gas Exchange
 Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
4/18/12 MECHANISM OF RESPIRATION. Every Breath You Take. Fun Facts
 Objectives MECHANISM OF RESPIRATION Dr Badri Paudel Explain how the intrapulmonary and intrapleural pressures vary during ventilation and relate these pressure changes to Boyle s law. Define the terms
Objectives MECHANISM OF RESPIRATION Dr Badri Paudel Explain how the intrapulmonary and intrapleural pressures vary during ventilation and relate these pressure changes to Boyle s law. Define the terms
Section Two Diffusion of gases
 Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Gas exchange. Tissue cells CO2 CO 2 O 2. Pulmonary capillary. Tissue capillaries
 Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
CHAPTER 3: The cardio-respiratory system
 : The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
: The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
(Received 16 January 1946)
 186 J. Physiol. (I946) I05, I86-I90 6I2.2I5.9 THE ABSORPTION OF FLUIDS FROM THE LUNGS BY F. C. COURTICE AND P. J. PHIPPS From the Experimental Station, Porton and the Laboratory of Physiology, Oxford (Received
186 J. Physiol. (I946) I05, I86-I90 6I2.2I5.9 THE ABSORPTION OF FLUIDS FROM THE LUNGS BY F. C. COURTICE AND P. J. PHIPPS From the Experimental Station, Porton and the Laboratory of Physiology, Oxford (Received
Lab 3. The Respiratory System (designed by Heather E. M. Liwanag with T.M. Williams)
 Name Lab Partners Lab 3. The Respiratory System (designed by Heather E. M. Liwanag with T.M. Williams) Part 1. Lung Volumes and Capacities Objectives 1. Obtain graphical representation of lung capacities
Name Lab Partners Lab 3. The Respiratory System (designed by Heather E. M. Liwanag with T.M. Williams) Part 1. Lung Volumes and Capacities Objectives 1. Obtain graphical representation of lung capacities
RSPT 1060 OBJECTIVES OBJECTIVES OBJECTIVES EQUATION OF MOTION. MODULE C Applied Physics Lesson #1 - Mechanics. Ventilation vs.
 RSPT 1060 MODULE C Applied Physics Lesson #1 - Mechanics OBJECTIVES At the end of this module, the student should be able to define the terms and abbreviations used in the module. draw & explain the equation
RSPT 1060 MODULE C Applied Physics Lesson #1 - Mechanics OBJECTIVES At the end of this module, the student should be able to define the terms and abbreviations used in the module. draw & explain the equation
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 11 Respiratory System 2 Pulmonary Ventilation Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session plan o Pulmonary Ventilation
BIOH122 Human Biological Science 2 Session 11 Respiratory System 2 Pulmonary Ventilation Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session plan o Pulmonary Ventilation
GASEOUS EXCHANGE IN HUMANS 06 AUGUST 2014
 GASEOUS EXCHANGE IN HUMANS 06 AUGUST 2014 In this lesson we: Lesson Description Look at gaseous exchange in humans in terms of o Ventilation o Inspiration o Expiration o Transport of gases o Homeostatic
GASEOUS EXCHANGE IN HUMANS 06 AUGUST 2014 In this lesson we: Lesson Description Look at gaseous exchange in humans in terms of o Ventilation o Inspiration o Expiration o Transport of gases o Homeostatic
Gas Pressure. Pressure is the force exerted per unit area by gas molecules as they strike the surfaces around them.
 Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Alveolus and Respiratory Membrane
 Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
RESPIRATORY REGULATION DURING EXERCISE
 RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
respiratory cycle. point in the volumes: 500 milliliters. for men. expiration, up to 1200 milliliters extra makes breathing Respiratory
 10 II. RESPIRATORY VOLUMES, CAPACITIES & PULMONARY FUNCTION TESTS Respiratory volume is the term used for various volumes of air moved by or associated with the lungs at a given point in the respiratory
10 II. RESPIRATORY VOLUMES, CAPACITIES & PULMONARY FUNCTION TESTS Respiratory volume is the term used for various volumes of air moved by or associated with the lungs at a given point in the respiratory
BIOLOGY 12: UNIT J - CHAPTER 15 - REVIEW WORKSHEET RESPIRATORY SYSTEM
 BIOLOGY 12: UNIT J - CHAPTER 15 - REVIEW WORKSHEET RESPIRATORY SYSTEM A. CHAPTER REVIEW 1. Define the four components of respiration. 2. What happens to the air as it moves along the air passages? What
BIOLOGY 12: UNIT J - CHAPTER 15 - REVIEW WORKSHEET RESPIRATORY SYSTEM A. CHAPTER REVIEW 1. Define the four components of respiration. 2. What happens to the air as it moves along the air passages? What
UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT
 UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION
UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION
CARBON DIOXIDE ELIMINATION FROM SEMICLOSED SYSTEMS
 Brit. J. Anaesth. (1956), 28, 196 CARBON DIOXIDE ELIMINATION FROM SEMICLOSED SYSTEMS BY RUSSELL M. DAVIES, I. R. VERNER Queen Victoria Hospital, East Grinstead AND A. BRACKEN Research and Development Centre,
Brit. J. Anaesth. (1956), 28, 196 CARBON DIOXIDE ELIMINATION FROM SEMICLOSED SYSTEMS BY RUSSELL M. DAVIES, I. R. VERNER Queen Victoria Hospital, East Grinstead AND A. BRACKEN Research and Development Centre,
transients' of large amplitude can be imposed on the arterial, cardiac and Since both coughing and the Valsalva manoeuvre raise intrathoracic pressure
 351 J. Physiol. (I953) I22, 35I-357 EFFECTS OF COUGHING ON INTRATHORACIC PRESSURE, ARTERIAL PRESSURE AND PERIPHERAL BLOOD FLOW BY E. P. SHARPEY-SCHAFER From the Department of Medicine, St Thomas's Hospital
351 J. Physiol. (I953) I22, 35I-357 EFFECTS OF COUGHING ON INTRATHORACIC PRESSURE, ARTERIAL PRESSURE AND PERIPHERAL BLOOD FLOW BY E. P. SHARPEY-SCHAFER From the Department of Medicine, St Thomas's Hospital
Dalton s Law How is the total pressure of a mixture of gases related to the partial pressures of the component gases?
 Dalton s Law Chapter 4 The Behavior of Gases 4. Properties of Gases 4. The Gas Laws 4. Ideal Gases Dalton s Law How is the total pressure of a mixture of gases related to the partial pressures of the component
Dalton s Law Chapter 4 The Behavior of Gases 4. Properties of Gases 4. The Gas Laws 4. Ideal Gases Dalton s Law How is the total pressure of a mixture of gases related to the partial pressures of the component
Chemistry A Molecular Approach. Fourth Edition. Chapter 5. Gases. Copyright 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
 Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
 Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
THE PHYSICAL PROPERTIES OF NORMAL LUNGS
 Thorax (1952), 7, 285. THE PHYSICAL PROPERTIES OF NORMAL LUNGS REMOVED AFTER DEATH BY M. B. McILROY From the Medical Professorial Unit, St. Bartholomew's Hospital, London (RECEIVED FOR PUBLICATION OCTOBER
Thorax (1952), 7, 285. THE PHYSICAL PROPERTIES OF NORMAL LUNGS REMOVED AFTER DEATH BY M. B. McILROY From the Medical Professorial Unit, St. Bartholomew's Hospital, London (RECEIVED FOR PUBLICATION OCTOBER
PARTS AND STRUCTURE OF THE RESPIRATORY SYSTEM
 PARTS AND STRUCTURE OF THE RESPIRATORY SYSTEM Parts of the Respiratory System The RS can be divided into two parts: 1. Respiratory Tract, (path that air follows). Nasal passage Pharynx Larynx Trachea Bronchi,
PARTS AND STRUCTURE OF THE RESPIRATORY SYSTEM Parts of the Respiratory System The RS can be divided into two parts: 1. Respiratory Tract, (path that air follows). Nasal passage Pharynx Larynx Trachea Bronchi,
For more information about how to cite these materials visit
 Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
RESPIRATORY PHYSIOLOGY RELEVANT TO HFOV
 RESPIRATORY PHYSIOLOGY RELEVANT TO Physiology of CMV 1 What is? Ventilation using a relatively high continuous distending pressure at the airway opening, around which an oscillatory wave is generated to
RESPIRATORY PHYSIOLOGY RELEVANT TO Physiology of CMV 1 What is? Ventilation using a relatively high continuous distending pressure at the airway opening, around which an oscillatory wave is generated to
Respiration. The ins and outs
 Respiration The ins and outs Functions 1. To bring O 2 into the body and transfer it to the blood stream 2. To remove CO 2 Circulation and respiration work together to achieve these functions Why Do We
Respiration The ins and outs Functions 1. To bring O 2 into the body and transfer it to the blood stream 2. To remove CO 2 Circulation and respiration work together to achieve these functions Why Do We
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/12
 LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
Respiratory Physiology 2
 Respiratory Physiology 2 Session Objectives. What you will cover Gaseous Exchange Control of Breathing Rate Your objectives are State the function of support structures and epithelia of the bronchial tree
Respiratory Physiology 2 Session Objectives. What you will cover Gaseous Exchange Control of Breathing Rate Your objectives are State the function of support structures and epithelia of the bronchial tree
CHEM 355 EXPERIMENT 7. Viscosity of gases: Estimation of molecular diameter
 CHEM 355 EXPERIMENT 7 Viscosity of gases: Estimation of molecular diameter Expressed most simply, the viscosity of a fluid (liquid or gas) relates to its resistance to flow. The viscosity of a gas is determined
CHEM 355 EXPERIMENT 7 Viscosity of gases: Estimation of molecular diameter Expressed most simply, the viscosity of a fluid (liquid or gas) relates to its resistance to flow. The viscosity of a gas is determined
Exploring the relationship between Heart Rate (HR) and Ventilation Rate (R) in humans.
 Exploring the relationship between Heart Rate (HR) and Ventilation Rate (R) in humans. The Research Question In this investigation I will be considering the following general research question: Does increased
Exploring the relationship between Heart Rate (HR) and Ventilation Rate (R) in humans. The Research Question In this investigation I will be considering the following general research question: Does increased
Lectures on Medical Biophysics Department of Biophysics, Medical Faculty, Masaryk University in Brno. Biophysics of breathing.
 Lectures on Medical Biophysics Department of Biophysics, Medical Faculty, Masaryk University in Brno Biophysics of breathing. Spirometry 1 Lecture outline Mechanisms of gas exchange between organism and
Lectures on Medical Biophysics Department of Biophysics, Medical Faculty, Masaryk University in Brno Biophysics of breathing. Spirometry 1 Lecture outline Mechanisms of gas exchange between organism and
Respiration. The resspiratory system
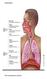 Respiration The resspiratory system The Alveoli The lungs have about 300 million alveoli, with a total crosssec onal area of 50 70 m2.. Each alveolar sac is surrounded by blood capillaries. The walls of
Respiration The resspiratory system The Alveoli The lungs have about 300 million alveoli, with a total crosssec onal area of 50 70 m2.. Each alveolar sac is surrounded by blood capillaries. The walls of
Using the Lifebox oximeter in the neonatal unit. Tutorial 1 the basics
 Using the Lifebox oximeter in the neonatal unit Tutorial 1 the basics Lifebox 2014. 2011. All rights reserved The Lifebox Pulse Oximeter In this tutorial you will learn about: The function of a pulse oximeter
Using the Lifebox oximeter in the neonatal unit Tutorial 1 the basics Lifebox 2014. 2011. All rights reserved The Lifebox Pulse Oximeter In this tutorial you will learn about: The function of a pulse oximeter
The Physiologic Basis of DLCO testing. Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan
 The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
For more information about how to cite these materials visit
 Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Douglas and Haldane(2) has shown that the oxygen determinations. since it forms the basis of the "Coefficient of Utilisation" (Krrogh) and
 THE MEASUREMENT OF THE OXYGEN CONTENT OF THE MIXED VENOUS BLOOD, AND OF THE VOLUME OF BLOOD CIRCULATING PER MINUTE. BY J. BARCROFT, F. J. W. ROUGHTON AND R. SHOJI. (From the Physiological Laboratory, Cambridge.)
THE MEASUREMENT OF THE OXYGEN CONTENT OF THE MIXED VENOUS BLOOD, AND OF THE VOLUME OF BLOOD CIRCULATING PER MINUTE. BY J. BARCROFT, F. J. W. ROUGHTON AND R. SHOJI. (From the Physiological Laboratory, Cambridge.)
Respiration. Figure 22: Schematic representation of the respiratory system
 Respiration One of the seven characteristics of something which is living is respiration. Strictly speaking, respiration is the process that takes place at cellular level and is one of three different
Respiration One of the seven characteristics of something which is living is respiration. Strictly speaking, respiration is the process that takes place at cellular level and is one of three different
6I2.2I6:6I alveolar pressure. It follows that the evident alteration in the respiratory rhythm is an alteration in amplitude.
 6I2.2I6:6I2.223.11 SOME EFFECTS OF CARBONIC ACID ON THE CHARACTER OF HUMAN RESPIRATION. BY J. BARCROFT AND R. MARGARIA' (Turin). (From the Physiological Laboratory, Cambridge.) THE following facts concerning
6I2.2I6:6I2.223.11 SOME EFFECTS OF CARBONIC ACID ON THE CHARACTER OF HUMAN RESPIRATION. BY J. BARCROFT AND R. MARGARIA' (Turin). (From the Physiological Laboratory, Cambridge.) THE following facts concerning
Monday, ! Today: Respiratory system! 5/20/14! Transport of Blood! What we ve been covering! Circulatory system! Parts of blood! Heart! tubing!
 Monday, 5.19.14! What we ve been covering! Circulatory system! Parts of blood! Heart! tubing! Transport of Blood! What is transported! Nutrients! Oxygen! Carbon Dioxide! Hormones! Antibodies! What it is/does!
Monday, 5.19.14! What we ve been covering! Circulatory system! Parts of blood! Heart! tubing! Transport of Blood! What is transported! Nutrients! Oxygen! Carbon Dioxide! Hormones! Antibodies! What it is/does!
Mechanical Ventilation
 Mechanical Ventilation Chapter 4 Mechanical Ventilation Equipment When providing mechanical ventilation for pediatric casualties, it is important to select the appropriately sized bag-valve mask or endotracheal
Mechanical Ventilation Chapter 4 Mechanical Ventilation Equipment When providing mechanical ventilation for pediatric casualties, it is important to select the appropriately sized bag-valve mask or endotracheal
Airway: the tubes through which air flows between atmosphere and alveoli. Upper airway. Lower airway
 Respiration Yu Yanqin ( 虞燕琴 ), PhD Dept. of fph Physiology Zhejiang University, School of Medicine Respiration Definition: the bodily processes involved in exchange of oxygen (O 2 ) and carbon dioxide
Respiration Yu Yanqin ( 虞燕琴 ), PhD Dept. of fph Physiology Zhejiang University, School of Medicine Respiration Definition: the bodily processes involved in exchange of oxygen (O 2 ) and carbon dioxide
Physiology Unit 4 RESPIRATORY PHYSIOLOGY
 Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
1.2 The structure and functions of the cardio-respiratory system Learning objectives
 1.2 The structure and functions of the cardio-respiratory system Learning objectives To understand the functions of the circulatory system. To be able to identify the differences between veins, arteries
1.2 The structure and functions of the cardio-respiratory system Learning objectives To understand the functions of the circulatory system. To be able to identify the differences between veins, arteries
(Received 9 September 1940)
 257 J. Physiol. (I 94I) 99, 257-264 6I2.2II A METHOD OF RECORDING THE RESPIRATION BY J. H. GADDUM From the College of the Pharmaceutical Society, 17 Bloomsbury Square, London, W.C. 2 (Received 9 September
257 J. Physiol. (I 94I) 99, 257-264 6I2.2II A METHOD OF RECORDING THE RESPIRATION BY J. H. GADDUM From the College of the Pharmaceutical Society, 17 Bloomsbury Square, London, W.C. 2 (Received 9 September
Experiment B-3 Respiration
 1 Experiment B-3 Respiration Objectives To study the diffusion process of oxygen and carbon dioxide between the alveoli and pulmonary capillaries. To determine the percentage of oxygen in exhaled air while
1 Experiment B-3 Respiration Objectives To study the diffusion process of oxygen and carbon dioxide between the alveoli and pulmonary capillaries. To determine the percentage of oxygen in exhaled air while
MEDICAL EQUIPMENT IV MECHANICAL VENTILATORS. Prof. Yasser Mostafa Kadah
 MEDICAL EQUIPMENT IV - 2013 MECHANICAL VENTILATORS Prof. Yasser Mostafa Kadah Mechanical Ventilator A ventilator is a machine, a system of related elements designed to alter, transmit, and direct energy
MEDICAL EQUIPMENT IV - 2013 MECHANICAL VENTILATORS Prof. Yasser Mostafa Kadah Mechanical Ventilator A ventilator is a machine, a system of related elements designed to alter, transmit, and direct energy
1. Label a diagram of the respiratory system. Objective sheet 3 Notes
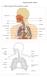 1. Label a diagram of the respiratory system Objective sheet 3 Notes 2. Functions of the respiratory structures Name Description Function Nasal Cavity Trachea Bronchi (Singular Bronchus) Bronchioles Lungs
1. Label a diagram of the respiratory system Objective sheet 3 Notes 2. Functions of the respiratory structures Name Description Function Nasal Cavity Trachea Bronchi (Singular Bronchus) Bronchioles Lungs
Table of Contents. By Adam Hollingworth
 By Adam Hollingworth Table of Contents Oxygen Cascade... 2 Diffusion... 2 Laws of Diffusion... 2 Diffusion & Perfusion Limitations... 3 Oxygen Uptake Along Pulmon Capillary... 4 Measurement of Diffusing
By Adam Hollingworth Table of Contents Oxygen Cascade... 2 Diffusion... 2 Laws of Diffusion... 2 Diffusion & Perfusion Limitations... 3 Oxygen Uptake Along Pulmon Capillary... 4 Measurement of Diffusing
Initiation and Management of Airway Pressure Release Ventilation (APRV)
 Initiation and Management of Airway Pressure Release Ventilation (APRV) Eric Kriner RRT Pulmonary Critical Care Clinical Specialist Pulmonary Services Department Medstar Washington Hospital Center Disclosures
Initiation and Management of Airway Pressure Release Ventilation (APRV) Eric Kriner RRT Pulmonary Critical Care Clinical Specialist Pulmonary Services Department Medstar Washington Hospital Center Disclosures
2. State the volume of air remaining in the lungs after a normal breathing.
 CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
Structures of the Respiratory System include:
 Respiratory System Structures of the Respiratory System include: ü Oral Cavity ü Nasal Cavity ü Pharynx ü Epiglottis ü Larynx ü Trachea ü Diaphragm ü Lung ü Bronchus ü Bronchioles ü Alveolus ü Pulmonary
Respiratory System Structures of the Respiratory System include: ü Oral Cavity ü Nasal Cavity ü Pharynx ü Epiglottis ü Larynx ü Trachea ü Diaphragm ü Lung ü Bronchus ü Bronchioles ü Alveolus ü Pulmonary
VENTILATORS PURPOSE OBJECTIVES
 VENTILATORS PURPOSE To familiarize and acquaint the transfer Paramedic with the skills and knowledge necessary to adequately maintain a ventilator in the interfacility transfer environment. COGNITIVE OBJECTIVES
VENTILATORS PURPOSE To familiarize and acquaint the transfer Paramedic with the skills and knowledge necessary to adequately maintain a ventilator in the interfacility transfer environment. COGNITIVE OBJECTIVES
Capnography in the Veterinary Technician Toolbox. Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA
 Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
Let s talk about Capnography
 Let s talk about Capnography This is one of a series of articles by Keith Simpson BVSc MRCVS MIET (Electronics) discussing the practical aspects of some common monitoring techniques. Capnometry is the
Let s talk about Capnography This is one of a series of articles by Keith Simpson BVSc MRCVS MIET (Electronics) discussing the practical aspects of some common monitoring techniques. Capnometry is the
AP TOPIC 6: Gases. Revised August General properties and kinetic theory
 AP OPIC 6: Gases General properties and kinetic theory Gases are made up of particles that have (relatively) large amounts of energy. A gas has no definite shape or volume and will expand to fill as much
AP OPIC 6: Gases General properties and kinetic theory Gases are made up of particles that have (relatively) large amounts of energy. A gas has no definite shape or volume and will expand to fill as much
G622. APPLIED SCIENCE Monitoring the Activity of the Human Body ADVANCED SUBSIDIARY GCE. Thursday 27 May 2010 Afternoon. Duration: 1 hour 30 minutes
 ADVANCED SUBSIDIARY GCE APPLIED SCIENCE Monitoring the Activity of the Human Body G622 *OCE/17533* Candidates answer on the Question Paper OCR Supplied Materials: None Other Materials Required: Electronic
ADVANCED SUBSIDIARY GCE APPLIED SCIENCE Monitoring the Activity of the Human Body G622 *OCE/17533* Candidates answer on the Question Paper OCR Supplied Materials: None Other Materials Required: Electronic
TV = Tidal volume (500ml) IRV = Inspiratory reserve volume (3,000 ml)
 By: Amin alajlouni Lec: 2nd record Date: 29/10/2017 First of all, this is my first sheet so excuse any mistakes I might make and let's start: As we said before in our last lecture about lung capacities
By: Amin alajlouni Lec: 2nd record Date: 29/10/2017 First of all, this is my first sheet so excuse any mistakes I might make and let's start: As we said before in our last lecture about lung capacities
PROBLEM SET 9. SOLUTIONS April 23, 2004
 Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
PROBLEM SET 8. SOLUTIONS April 15, 2004
 Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Section 8: Gases. The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC (c).
 Section 8: Gases The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 8.01 Simple Gas Laws Chemistry (9)(A) 8.02 Ideal Gas Law Chemistry
Section 8: Gases The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 8.01 Simple Gas Laws Chemistry (9)(A) 8.02 Ideal Gas Law Chemistry
Glucose + Oxygen Carbon Dioxide + Water + Energy C6H12O CO2 + 6H20 + energy
 Cell Respiration - A food oxidization process in body cells to produce energy - Occurs (happens) in animal cells and plant cells Notes : Metabolic Reactions in Gaseous Exchange CELL RESPIRATION Photosynthesis
Cell Respiration - A food oxidization process in body cells to produce energy - Occurs (happens) in animal cells and plant cells Notes : Metabolic Reactions in Gaseous Exchange CELL RESPIRATION Photosynthesis
Figure 1. A schematic diagram of the human respiratory system.
 Introduction to Respiration In this experiment, you will investigate various aspects of normal breathing, hyperventilation, rebreathing the effect of changing airway resistance and ways in which to measure
Introduction to Respiration In this experiment, you will investigate various aspects of normal breathing, hyperventilation, rebreathing the effect of changing airway resistance and ways in which to measure
Standards and guidelines for care and management of patients requiring oxygen therapy.
 PURPOSE Standards and guidelines for care and management of patients requiring oxygen therapy. STANDARDS Ongoing management of oxygen therapy requires a prescriber s order. The order must specify oxygen
PURPOSE Standards and guidelines for care and management of patients requiring oxygen therapy. STANDARDS Ongoing management of oxygen therapy requires a prescriber s order. The order must specify oxygen
RESPIRATORY PHYSIOLOGY. Anaesthesiology Block 18 (GNK 586) Prof Pierre Fourie
 RESPIRATORY PHYSIOLOGY Anaesthesiology Block 18 (GNK 586) Prof Pierre Fourie Outline Ventilation Diffusion Perfusion Ventilation-Perfusion relationship Work of breathing Control of Ventilation 2 This image
RESPIRATORY PHYSIOLOGY Anaesthesiology Block 18 (GNK 586) Prof Pierre Fourie Outline Ventilation Diffusion Perfusion Ventilation-Perfusion relationship Work of breathing Control of Ventilation 2 This image
Overview: Curriculum Goals: Materials: Explore:
 Overview: Students will study air quality. They will examine the ways that wildfire smoke affects the air. They will recognise why people are evacuated when air becomes smoke filled. Curriculum Goals:
Overview: Students will study air quality. They will examine the ways that wildfire smoke affects the air. They will recognise why people are evacuated when air becomes smoke filled. Curriculum Goals:
Boyle s Law Practice
 Boyle s Law Practice Boyle s Law is an indirect relationship. Most of these problems can be done in your head without showing your work. 1. Herman has 30.0 L of helium gas trapped in a cylinder by a piston.
Boyle s Law Practice Boyle s Law is an indirect relationship. Most of these problems can be done in your head without showing your work. 1. Herman has 30.0 L of helium gas trapped in a cylinder by a piston.
Worksheet 12 - Partial Pressures and the Kinetic Molecular Theory of Gases
 Worksheet 12 - Partial Pressures and the Kinetic olecular Theory of Gases Dalton's Law of Partial Pressures states that the sums of the pressures of each gas in the mixture add to give the total pressure
Worksheet 12 - Partial Pressures and the Kinetic olecular Theory of Gases Dalton's Law of Partial Pressures states that the sums of the pressures of each gas in the mixture add to give the total pressure
Gases. Name: Class: Date: Matching
 Name: Class: Date: Gases Matching Match each item with the correct statement below. a. Boyle's law d. Graham's law b. Charles's law e. Gay-Lussac's law c. Dalton's law f. ideal gas law 1. For a given mass
Name: Class: Date: Gases Matching Match each item with the correct statement below. a. Boyle's law d. Graham's law b. Charles's law e. Gay-Lussac's law c. Dalton's law f. ideal gas law 1. For a given mass
Chapter 13 The Respiratory System
 Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Chapter 9 Airway Respirations Metabolism Oxygen Requirements Respiratory Anatomy Respiratory Anatomy Respiratory Anatomy Diaphragm
 1 Chapter 9 Airway 2 Respirations Every cell of the body requires to survive Oxygen must come in and carbon must go out 3 Metabolism Metabolism--Process where the body s cells convert food to Adequate
1 Chapter 9 Airway 2 Respirations Every cell of the body requires to survive Oxygen must come in and carbon must go out 3 Metabolism Metabolism--Process where the body s cells convert food to Adequate
EMS INTER-FACILITY TRANSPORT WITH MECHANICAL VENTILATOR COURSE OBJECTIVES
 GENERAL PROVISIONS: EMS INTER-FACILITY TRANSPORT WITH MECHANICAL VENTILATOR COURSE OBJECTIVES Individuals providing Inter-facility transport with Mechanical Ventilator must have successfully completed
GENERAL PROVISIONS: EMS INTER-FACILITY TRANSPORT WITH MECHANICAL VENTILATOR COURSE OBJECTIVES Individuals providing Inter-facility transport with Mechanical Ventilator must have successfully completed
Respiratory System. Prepared by: Dorota Marczuk-Krynicka, MD, PhD
 Respiratory System Prepared by: Dorota Marczuk-Krynicka, MD, PhD Lungs: Ventilation Perfusion Gas Exchange - Diffusion 1. Airways and Airway Resistance (AWR) 2. Mechanics of Breathing and Lung (Elastic)
Respiratory System Prepared by: Dorota Marczuk-Krynicka, MD, PhD Lungs: Ventilation Perfusion Gas Exchange - Diffusion 1. Airways and Airway Resistance (AWR) 2. Mechanics of Breathing and Lung (Elastic)
Breathing Process: Inhalation
 Airway Chapter 6 Breathing Process: Inhalation Active part of breathing Diaphragm and intercostal muscles contract, allowing the lungs to expand. The decrease in pressure allows lungs to fill with air.
Airway Chapter 6 Breathing Process: Inhalation Active part of breathing Diaphragm and intercostal muscles contract, allowing the lungs to expand. The decrease in pressure allows lungs to fill with air.
Animal Physiology Prof. Mainak Das Department of Biological Sciences and Bioengineering Indian Institute of Technology, Kanpur. Module - 01 Lecture 28
 Animal Physiology Prof. Mainak Das Department of Biological Sciences and Bioengineering Indian Institute of Technology, Kanpur Module - 01 Lecture 28 Welcome back, so we are in to the Animal Physiology
Animal Physiology Prof. Mainak Das Department of Biological Sciences and Bioengineering Indian Institute of Technology, Kanpur Module - 01 Lecture 28 Welcome back, so we are in to the Animal Physiology
Name period date assigned date due date returned
 Name period date assigned date due date returned procedure 1. Take one balloon and stretch it out 2. Take one deep breath and blow into the balloon until you cannot breath out anymore. Do Not Take A Second
Name period date assigned date due date returned procedure 1. Take one balloon and stretch it out 2. Take one deep breath and blow into the balloon until you cannot breath out anymore. Do Not Take A Second
REVISION: GASEOUS EXCHANGE 24 SEPTEMBER 2014 Lesson Description
 REVISION: GASEOUS EXCHANGE 24 SEPTEMBER 2014 Lesson Description In this lesson, we revise: Gaseous Exchange in Plants & Animals Gaseous Exchange in Humans Excretion in Humans Focus on the Kidney Gaseous
REVISION: GASEOUS EXCHANGE 24 SEPTEMBER 2014 Lesson Description In this lesson, we revise: Gaseous Exchange in Plants & Animals Gaseous Exchange in Humans Excretion in Humans Focus on the Kidney Gaseous
A. What are the three states of matter chemists work with?
 Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
Chapter 10 and 12 The Behavior of Gases Chapter 10 The States of Matter A. What are the three states of matter chemists work with? Section 10.1 Pg 267 B. We will explain the behavior of gases using the
GASEOUS EXCHANGE 17 JULY 2013
 GASEOUS EXCHANGE 17 JULY 2013 Lesson Description In this lesson we: Discuss what is gaseous exchange? Consider requirements of an efficient gaseous exchange surface. Look at diversity in gas exchange systems.
GASEOUS EXCHANGE 17 JULY 2013 Lesson Description In this lesson we: Discuss what is gaseous exchange? Consider requirements of an efficient gaseous exchange surface. Look at diversity in gas exchange systems.
