What does the % represent on the beakers?
|
|
|
- David Powers
- 6 years ago
- Views:
Transcription
1 DISSOLVED OXYGEN VIDEO FAQs What does the % represent on the beakers? What are the glass tubes to beakers for? How is the temperature being kept the same (at 5 o then 35 o )? What is salinity in parts per thousand actually mean? What does D.O. in parts per million actually mean? If this is a cause/effect relationship, what s cause & what s effect? What (physically) is the reason that temperature allows so much more DO in a beaker of same volume H 2 O when temp is lower? What is the reason that dissolved oxygen declines as salinity increases? How does the DO meter actually work? What is being recorded/sensors sensing what? What months or season are fish most likely to die as temperature increases and DO decreases? If fish can filter/use D.O. in seawater, what about the salt? What type(s) of graphics are appropriate for this type of data? What does the % represent on the beakers? The symbol on the beakers was not the percent symbol (%) but rather the similar looking symbol for parts-per-thousand ( ) or ppt. See PARTS PER Hands-on Demo on the Kilroy Academy Resources Page under Salinity Math. What are the glass tubes to beakers for? Page 1 of 5
2 The flexible tubing to the beakers is aquarium airline attached to air diffusers (airstones) in order to bubble air and deliver oxygen to the water. The goal is to maximize the oxygen available to see how much the water can hold at different temperatures and different salinities. How is the temperature being kept the same (at 5 o then 35 o )? The beakers are placed in water baths. The cold water bath contains ice cubes, which naturally maintains a constant temperature (until all the ice melts). The warm water bath has an aquarium heater, which contains a thermostat to maintain a constant temperature (in an air-conditioned cool environment). What is salinity in parts per thousand actually mean? Salinity is the measure as the amount of salt (by weight) dissolved in a known weight of freshwater (or volume when using metric units). Seawater that is 35 ppt (or 35 ) is equal to 35 grams of NaCl dissolved into 1,000 grams (or 1 kilogram) of fresh water and in the metric system of measure, 1 kilrogram = 1 liter in volume*. *Note: One kilogram and one liter are used interchangeably but this is only true at 4 0 C. At 35 0 C 1 kg is about 1006 ml of water. What does D.O. in parts per million (ppm) actually mean? Parts per million is also measured in terms of weight of gas in water. A dissolved oxygen measurement of 8 ppm means 8 milligrams of the gaseous solute oxygen is dissolved in 1,000 grams of fresh water. If this is a cause/effect relationship, what s cause & what s effect? This demonstration is an example of the relationship of two causes (temperature and salinity) on a single effect (dissolved oxygen). An increase in either cause, temperature or salinity, results in a decline in DO (effect). Therefore, the highest saturation of dissolved oxygen is at the lowest temperature in freshwater, and the lowest saturation of dissolved oxygen is in the warmest, highest saline water. What (physically) is the reason that temperature allows so much more DO in a beaker of same volume H 2 O when temp is lower? The solubility of gases in liquids is describe by Henry s Law At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid." The partial pressure of a gas is a measure of Page 2 of 5
3 thermodynamic activity of the gas's molecules. Gases dissolve, diffuse, and react according to their partial pressures, and not according to their concentrations in gas mixtures or liquids. In understanding the effects of temperature on the solubility of gases, it is first important to remember that temperature is a measure of the average kinetic energy. As temperature increases, kinetic energy increases. The greater kinetic energy results in greater molecular motion of the gas particles. As a result, the gas particles dissolved in the liquid are more likely to escape to the gas phase and the existing gas particles are less likely to be dissolved. What is the reason that dissolved oxygen declines as salinity increases? Dissolved salts, such as sodium chloride (NaCl), or other solutes such as sugar, occupy space in water that would otherwise be available for oxygen molecules (O2) to dissolve in. Thus, as the amount of salts dissolved in water increases, DO decreases. How does the DO meter actually work? What is being recorded/sensors sensing what? The typical Dissolved Oxygen meter has a membrane, electrodes, and an electrolyte, which is a substance that ionizes when dissolved in water. Oxygen which has passed through the membrane is reduced (which means gains electrons) by the working electrode. The working electrode uses a noble metal such as platinum, and the opposite electrode uses a different nobel metal such as silver. For the electrolyte, a potassium chloride solution is used, and the membrane is made out of Teflon. Voltage is applied between the two electrodes so that the threshold diffusion current for oxygen is generated there. The oxygen which has passed through the membrane is reduced with the working electrode. A reduction current in proportion to the dissolved oxygen is generated, and then the dissolved oxygen is measured. The current which has flowed in proportion to the concentration of the dissolved oxygen is processed with the current amplifier, and then the concentration of the dissolved oxygen is measured and displayed Cole-Parmer Instrument Company, LLC Note: A YSI Model 55 Handheld Oxygen System was utilized during the hands-on demonstration. For more information about this meter, visit Page 3 of 5
4 What months or season are fish most likely to die as temperature increases and DO decreases? Fish kills typically occur in the summer months when water temperatures are higher and oxygen solubility (DO) is lowest. However, in Florida this is only part of the picture. The warm summer months coincide with the rainy season, resulting in more runoff and more nutrient input to our coastal waters. The warm temperatures, nutrient enrichment, and long, intense daylight periods result in phytoplankton blooms. During the day the algae photosynthesizes resulting in dissolved oxygen saturation, but during the dark evenings the algae respires, consuming O2 and producing CO2. Just prior to dawn, the O2 concentration is at its lowest (often approaching 0) and results in fish mortality. (Typically, catfish farmers will begin the aeration paddles in their ponds around 3:00 am.) In addition, as the algae dies, sinks to the bottom, and begins bacterial decomposition the respiring bacteria create a Biological Oxygen Demand that also contributes to a decline in dissolved O2 concentrations. If fish can filter/use D.O. in seawater, what about the salt? Most fish exchange gases using gills on either side of the pharynx (throat). Gills are tissues that consist of fabric-like structures called filaments. Each filament contains a capillary network that provides a large surface area for exchanging oxygen and carbon dioxide. Fish exchange gases by pulling oxygen-rich water through their mouths and pumping it over their gills. The blood in the gills contains hemoglobin, the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates. Hemoglobin in the blood carries oxygen from the respiratory organs (lungs or gills) to the rest of the body (i.e. the tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power the functions of the organism in the process called metabolism. What type(s) of graphics are appropriate for this type of data? A 3-axis graph is most appropriate: x-axis salinity, y-axis temperature, and z-axis dissolved oxygen. A 3-dimensional graph may be generated on Excel. Please view the supplemental resources that accompany the Dissolved Oxygen video to access a blank graph. REFERENCES Page 4 of 5
5 ACKNOWLEDGEMENTS Thank you to Sebastian River High School Teacher Holly Hoier, LeRoy Creswell and Dr. Edie Widder for creating this resource. Special thanks to Indian River Impact 100 for funding Kilroy Academy. Made possible with funding provided by. Copyright 2015 Ocean Research & Conservation Association, Inc. Page 5 of 5
Dissolved Oxygen Guide
 Educat i onser i es Di ssol vedoxygengui de Dissolved Oxygen Guide Introduction Dissolved oxygen probes provide a convenient approach to essentially direct measurement of molecular oxygen. The membrane
Educat i onser i es Di ssol vedoxygengui de Dissolved Oxygen Guide Introduction Dissolved oxygen probes provide a convenient approach to essentially direct measurement of molecular oxygen. The membrane
Experiment C-6 Gas Solubility
 1 Experiment C-6 Gas Solubility Objectives To measure dissolved oxygen in water by using an oxygen sensor. To learn about physical factors that influence oxygen solubility in water. To examine the dissolved
1 Experiment C-6 Gas Solubility Objectives To measure dissolved oxygen in water by using an oxygen sensor. To learn about physical factors that influence oxygen solubility in water. To examine the dissolved
Solutions and Units of Concentration. May 25, 2015
 Solutions and Units of Concentration May 25, 2015 What is Solubility? Solubility: the maximum amount of solute that will dissolve in a certain amount of solvent at a given temperature Example: grams of
Solutions and Units of Concentration May 25, 2015 What is Solubility? Solubility: the maximum amount of solute that will dissolve in a certain amount of solvent at a given temperature Example: grams of
4/26/16. Section 1 Understanding Solutions. Solutions and Suspensions. Solutions and Suspensions. Solutions and Suspensions. Solvents and Solutes
 Section 1 Understanding Solutions On a hot day, you may think that a cool glass of plain water would be refreshing. However, if the glass were actually filled with plain water, it would taste stale. This
Section 1 Understanding Solutions On a hot day, you may think that a cool glass of plain water would be refreshing. However, if the glass were actually filled with plain water, it would taste stale. This
Chapter 5 TEST: Gases
 Chapter 5 TEST: Gases 1) Gases generally have A) low density B) high density C) closely packed particles D) no increase in volume when temperature is increased E) no decrease in volume when pressure is
Chapter 5 TEST: Gases 1) Gases generally have A) low density B) high density C) closely packed particles D) no increase in volume when temperature is increased E) no decrease in volume when pressure is
Salinity and Dissolved Oxygen (D.O.) Lecture 2
 Salinity and Dissolved Oxygen (D.O.) Lecture 2 Salinity defn Salinity is the saltiness or dissolved salt content of a body of water. fresh water (or sweet water) is less than 0.05%. brackish water is 0.03
Salinity and Dissolved Oxygen (D.O.) Lecture 2 Salinity defn Salinity is the saltiness or dissolved salt content of a body of water. fresh water (or sweet water) is less than 0.05%. brackish water is 0.03
(a) (i) Describe how a large difference in oxygen concentration is maintained between a fish gill and the surrounding water.
 1. Answers should be written in continuous prose. Credit will be given for biological accuracy, the organisation and presentation of information and the way in which an answer is expressed. Fick s law
1. Answers should be written in continuous prose. Credit will be given for biological accuracy, the organisation and presentation of information and the way in which an answer is expressed. Fick s law
Dissolved Oxygen and measurement possibilities. Berno Lüpkes, 15 th March 2017
 Dissolved Oxygen and measurement possibilities Berno Lüpkes, 15 th March 2017 Content 1. Introduction to Dissolved Oxygen 2. Amperometric measurement principle 3. Optical measurement principle 4. Optical
Dissolved Oxygen and measurement possibilities Berno Lüpkes, 15 th March 2017 Content 1. Introduction to Dissolved Oxygen 2. Amperometric measurement principle 3. Optical measurement principle 4. Optical
Then the partial pressure of oxygen is. b) Gases will diffuse down a pressure gradient across a respiratory surface if it is: i) permeable ii) moist
 1 AP Biology March 2008 Respiration Chapter 42 Gas exchange occurs across specialized respiratory surfaces. 1) Gas exchange: Relies on the diffusion of gases down pressure gradients. At sea level, atmosphere
1 AP Biology March 2008 Respiration Chapter 42 Gas exchange occurs across specialized respiratory surfaces. 1) Gas exchange: Relies on the diffusion of gases down pressure gradients. At sea level, atmosphere
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT.
 Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
Honors Chemistry - Problem Set Chapter 13 Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. Atmospheric pressure is 760 mm Hg. 2. The SI unit of pressure is
3 Solubility and Concentration
 CHAPTER 8 SECTION Solutions 3 Solubility and Concentration KEY IDEAS As you read this section, keep these questions in mind: What is solubility? What happens when you add more solute to a saturated solution?
CHAPTER 8 SECTION Solutions 3 Solubility and Concentration KEY IDEAS As you read this section, keep these questions in mind: What is solubility? What happens when you add more solute to a saturated solution?
Deep Water Currents Lab
 Deep Water Currents Lab Background: Anyone visiting the seashore is struck by the constant motion of water traveling on the surface of the ocean in the form of waves. But beneath the ocean's surface, water
Deep Water Currents Lab Background: Anyone visiting the seashore is struck by the constant motion of water traveling on the surface of the ocean in the form of waves. But beneath the ocean's surface, water
REASONS FOR NATURAL VARIATIONS IN DISSOLVED OXYGEN LEVELS
 Period Date LAB. THE PHYSICAL PROPERTIES OF WATER: DISSOLVED OXYGEN In an aquatic environment, oxygen must be in a solution in a free state (O 2 ) before it is available for use by organisms (bioavailable).
Period Date LAB. THE PHYSICAL PROPERTIES OF WATER: DISSOLVED OXYGEN In an aquatic environment, oxygen must be in a solution in a free state (O 2 ) before it is available for use by organisms (bioavailable).
Introduction to ChemSense
 Introduction to ChemSense As you may have seen, the ChemSense software allows you to create drawings and animation of chemical phenomena (and non-chemical phenomena for some of you). The software also
Introduction to ChemSense As you may have seen, the ChemSense software allows you to create drawings and animation of chemical phenomena (and non-chemical phenomena for some of you). The software also
Comparing Respiratory Systems
 Comparing Respiratory Systems Respiration Respiration is a process involving the movement of oxygen gas into cells and carbon dioxide out of cells, (This better called BREATHING ) in order to facilitate
Comparing Respiratory Systems Respiration Respiration is a process involving the movement of oxygen gas into cells and carbon dioxide out of cells, (This better called BREATHING ) in order to facilitate
These two respiratory media (air & water) impose rather different constraints on oxygen uptake:
 Topic 19: OXYGEN UPTAKE AND TRANSPORT (lectures 29-30) OBJECTIVES: 1. Be able to compare air vs. water as a respiratory medium with respect to oxygen content, diffusion coefficient, viscosity and water
Topic 19: OXYGEN UPTAKE AND TRANSPORT (lectures 29-30) OBJECTIVES: 1. Be able to compare air vs. water as a respiratory medium with respect to oxygen content, diffusion coefficient, viscosity and water
DISSOLVED OXYGEN SENSOR BT34i
 DISSOLVED OXYGEN SENSOR BT34i USER S GUIDE CENTRE FOR MICROCOMPUTER APPLICATIONS http://www.cma-science.nl Short description The CMA Dissolved Oxygen (DO) sensor BT34i measures the concentration of dissolved
DISSOLVED OXYGEN SENSOR BT34i USER S GUIDE CENTRE FOR MICROCOMPUTER APPLICATIONS http://www.cma-science.nl Short description The CMA Dissolved Oxygen (DO) sensor BT34i measures the concentration of dissolved
Title: Solubility of Gas A Daily Experience. Subject: Chemistry. Grade Level: 10 th 12 th
 Title: Solubility of Gas A Daily Experience Subject: Chemistry Grade Level: 10 th 12 th Rational or Purpose: This lesson brings an everyday life experience to students knowledge on solubility of gas in
Title: Solubility of Gas A Daily Experience Subject: Chemistry Grade Level: 10 th 12 th Rational or Purpose: This lesson brings an everyday life experience to students knowledge on solubility of gas in
Gas Exchange Respiratory Systems
 alveoli gills Gas Exchange Respiratory Systems elephant seals 2008-2009 Why do we need a respiratory system? respiration for respiration Need O 2 in for aerobic cellular respiration make ATP Need CO 2
alveoli gills Gas Exchange Respiratory Systems elephant seals 2008-2009 Why do we need a respiratory system? respiration for respiration Need O 2 in for aerobic cellular respiration make ATP Need CO 2
Noxious Fumes and Gases
 Noxious Fumes and Gases The tissues of the body require oxygen 0 2 for normal metabolic processes (ie. the oxidation of food to produce energy). They must also eliminate CO, which is the waste product
Noxious Fumes and Gases The tissues of the body require oxygen 0 2 for normal metabolic processes (ie. the oxidation of food to produce energy). They must also eliminate CO, which is the waste product
Introduction The Smart Q Oxygen Adaptor enables an Oxygen electrode to be connected to an EasySense unit.
 The Oxygen Adaptor & Electrode Arbor Scientific Oxygen Adapter (Product No PC-3130) Comes with Oxygen Electrode (PC-3131) 3 membrane modules 50 ml electrolyte Polishing paper for cathode Oxygen Electrode
The Oxygen Adaptor & Electrode Arbor Scientific Oxygen Adapter (Product No PC-3130) Comes with Oxygen Electrode (PC-3131) 3 membrane modules 50 ml electrolyte Polishing paper for cathode Oxygen Electrode
(ml/l), (mg/l) or (ppm)
 Oxygen Optode What is this sensor? This sensor is used to detect dissolved oxygen and oxygen saturation levels in a given body of water i. Oxygen sensors detect Dissolved Oxygen, often abbreviated to DO.
Oxygen Optode What is this sensor? This sensor is used to detect dissolved oxygen and oxygen saturation levels in a given body of water i. Oxygen sensors detect Dissolved Oxygen, often abbreviated to DO.
alveoli Chapter 42. Gas Exchange elephant seals gills AP Biology
 alveoli Chapter 42. Gas Exchange gills elephant seals Gas exchange O 2 & CO 2 exchange exchange between environment & cells provides O 2 for aerobic cellular respiration need moist membrane need high
alveoli Chapter 42. Gas Exchange gills elephant seals Gas exchange O 2 & CO 2 exchange exchange between environment & cells provides O 2 for aerobic cellular respiration need moist membrane need high
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory
 CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
Worksheet: Solubility
 1. According to your Reference Tables, which substance forms an unsaturated solution when 80 grams of the substance is dissolved in 100 grams of H 2 O at 10 C? (A) KI (B) KNO 3 (C) NaNO 3 (D) NaCl 2. The
1. According to your Reference Tables, which substance forms an unsaturated solution when 80 grams of the substance is dissolved in 100 grams of H 2 O at 10 C? (A) KI (B) KNO 3 (C) NaNO 3 (D) NaCl 2. The
PRODUCT SHEET. Order probe only as RXPROBE02
 SS69L DISSOLVED OXYGEN PROBE TRANSDUCER Order probe only as RXPROBE02 Order interface only as BSL-TCI16 SS69L Components The SS69L transducer measures dissolved oxygen. The SS69L includes a dissolved oxygen
SS69L DISSOLVED OXYGEN PROBE TRANSDUCER Order probe only as RXPROBE02 Order interface only as BSL-TCI16 SS69L Components The SS69L transducer measures dissolved oxygen. The SS69L includes a dissolved oxygen
SS32L Dissolved O 2 Probe
 BIOPAC WWW.biopac.com Systems, Inc. Application Note PH-185 SS32L Dissolved O 2 Probe SS32L Dissolved O 2 Probe BIOPAC Software SS32L Specifications BSL PRO v. 3.6.6 BIOPAC Hardware SS32L Dissolved O 2
BIOPAC WWW.biopac.com Systems, Inc. Application Note PH-185 SS32L Dissolved O 2 Probe SS32L Dissolved O 2 Probe BIOPAC Software SS32L Specifications BSL PRO v. 3.6.6 BIOPAC Hardware SS32L Dissolved O 2
Section Two Diffusion of gases
 Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
respiratory cycle. point in the volumes: 500 milliliters. for men. expiration, up to 1200 milliliters extra makes breathing Respiratory
 10 II. RESPIRATORY VOLUMES, CAPACITIES & PULMONARY FUNCTION TESTS Respiratory volume is the term used for various volumes of air moved by or associated with the lungs at a given point in the respiratory
10 II. RESPIRATORY VOLUMES, CAPACITIES & PULMONARY FUNCTION TESTS Respiratory volume is the term used for various volumes of air moved by or associated with the lungs at a given point in the respiratory
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing
 Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY
 BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY Dr.R.Selvakumar.M.D.D.A.DNB Professor of Anaesthesiology, K.A.P.Viswanatham Govt medical college, Trichy. The current practice of Anaesthesiology demands knowledge
BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY Dr.R.Selvakumar.M.D.D.A.DNB Professor of Anaesthesiology, K.A.P.Viswanatham Govt medical college, Trichy. The current practice of Anaesthesiology demands knowledge
IASbaba ILP: Value Add Material - Matter MATTER. Iasbaba.com Page 1
 MATTER Iasbaba.com Page 1 LIQUID A. VAPOUR PRESSURE: Pressure of vapour in a closed container Changes majorly with temperature Has an exponential relationship with temperature Increases with increase in
MATTER Iasbaba.com Page 1 LIQUID A. VAPOUR PRESSURE: Pressure of vapour in a closed container Changes majorly with temperature Has an exponential relationship with temperature Increases with increase in
AP Biology. Chapter 42. Gas Exchange. Optimizing gas exchange. Gas exchange. Gas exchange in many forms. Evolution of gas exchange structures
 alveoli Chapter 42. Gas Exchange gills elephant seals Gas exchange & C exchange exchange between environment & cells provides for aerobic cellular respiration need moist membrane need high surface area
alveoli Chapter 42. Gas Exchange gills elephant seals Gas exchange & C exchange exchange between environment & cells provides for aerobic cellular respiration need moist membrane need high surface area
AP Biology. Gas Exchange Respiratory Systems. Gas exchange. Why do we need a respiratory system? Optimizing gas exchange. Gas exchange in many forms
 alveoli Gas Exchange Respiratory Systems gills elephant seals 2008-2009 Why do we need a respiratory system? Need O 2 in food respiration for respiration for aerobic cellular respiration make ATP Need
alveoli Gas Exchange Respiratory Systems gills elephant seals 2008-2009 Why do we need a respiratory system? Need O 2 in food respiration for respiration for aerobic cellular respiration make ATP Need
12mm Dissolved Oxygen Sensor Care and Use Instructions
 12mm Dissolved Oxygen Sensor Care and Use Instructions How Dissolved Oxygen Sensors Work Your Dissolved Oxygen (DO) Probe is a galvanic electrochemistry device; i.e. it does not require power from your
12mm Dissolved Oxygen Sensor Care and Use Instructions How Dissolved Oxygen Sensors Work Your Dissolved Oxygen (DO) Probe is a galvanic electrochemistry device; i.e. it does not require power from your
Animal Systems: The Respiratory System
 Animal Systems: The Respiratory System Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems The Digestive The Circulatory
Animal Systems: The Respiratory System Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems The Digestive The Circulatory
maximum grams of solute that will dissolve in 100 g of solvent at a given temperature Varies with temperature Sugar is more soluble than salt
 maximum grams of solute that will dissolve in 100 g of solvent at a given temperature Varies with temperature Sugar is more soluble than salt Solids are more soluble at... high temperatures. Gases are
maximum grams of solute that will dissolve in 100 g of solvent at a given temperature Varies with temperature Sugar is more soluble than salt Solids are more soluble at... high temperatures. Gases are
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1
 AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
The diagram shows an alveolus next to a blood capillary in a lung. (a) (i) Draw a ring around the correct answer to complete the sentence. diffusion.
 BREATHING / GAS EXCHANGE. NAME. Q.Gas exchange takes place in the lungs. The diagram shows an alveolus next to a blood capillary in a lung. The arrows show the movement of two gases, A and B. (a) (i) Draw
BREATHING / GAS EXCHANGE. NAME. Q.Gas exchange takes place in the lungs. The diagram shows an alveolus next to a blood capillary in a lung. The arrows show the movement of two gases, A and B. (a) (i) Draw
DISSOLVING AND SOLUBILITY
 DISSOLVING AND SOLUBILITY QUICK REVIEW: Homogeneous mixtures: a solution of substances in which no settling occurs (looks like one thing). A solution occurs when the particles of the components slip in
DISSOLVING AND SOLUBILITY QUICK REVIEW: Homogeneous mixtures: a solution of substances in which no settling occurs (looks like one thing). A solution occurs when the particles of the components slip in
Gas Exchange & Circulation
 Why is gas exchange important? Gas Exchange & Circulation Read Ch. 42 start with 42.5: Gas Exchange in Animals Respiration: C 6 H 12 O 6 + O 2! Energy + CO 2 + H 2 O Photosynthesis: Energy + CO 2 + H 2
Why is gas exchange important? Gas Exchange & Circulation Read Ch. 42 start with 42.5: Gas Exchange in Animals Respiration: C 6 H 12 O 6 + O 2! Energy + CO 2 + H 2 O Photosynthesis: Energy + CO 2 + H 2
States of Matter Review
 States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
UNIT 2 Chapter 3. Elodea and Photosynthesis. The Origins of Life. Learning Outcomes: Chapter 3 Lab/Activity #2. Introduction: Safety Issues:
 The Origins of Life UNIT 2 Chapter 3 Name: Section: Date: Chapter 3 Lab/Activity #2 Elodea and Photosynthesis Introduction: Photosynthetic organisms (cyanobacteria) first evolved about 3.5 billion years
The Origins of Life UNIT 2 Chapter 3 Name: Section: Date: Chapter 3 Lab/Activity #2 Elodea and Photosynthesis Introduction: Photosynthetic organisms (cyanobacteria) first evolved about 3.5 billion years
Bante810 Benchtop Dissolved Oxygen Meter Instruction Manual
 Bante810 Benchtop Dissolved Oxygen Meter Instruction Manual BANTE INSTRUMENTS CO., LTD Bante810 Benchtop Dissolved Oxygen Meter 1 Introduction Thank you for selecting the Bante810 benchtop dissolved oxygen
Bante810 Benchtop Dissolved Oxygen Meter Instruction Manual BANTE INSTRUMENTS CO., LTD Bante810 Benchtop Dissolved Oxygen Meter 1 Introduction Thank you for selecting the Bante810 benchtop dissolved oxygen
Applied Physics Topics 2
 Applied Physics Topics 2 Dr Andrey Varvinskiy Consultant Anaesthetist Torbay Hospital, UK EDAIC Paper B Lead and Examiner TOPICS 2 Gas Laws Other Laws: Dalton, Avogadro Critical temperature Critical pressure
Applied Physics Topics 2 Dr Andrey Varvinskiy Consultant Anaesthetist Torbay Hospital, UK EDAIC Paper B Lead and Examiner TOPICS 2 Gas Laws Other Laws: Dalton, Avogadro Critical temperature Critical pressure
CTB3365x Introduction to Water Treatment
 CTB3365x Introduction to Water Treatment D4b Aeration Doris van Halem Did you know that there are not just gasses in your glass of sparkling coke, but also in the tap water you drink? Welcome to the water
CTB3365x Introduction to Water Treatment D4b Aeration Doris van Halem Did you know that there are not just gasses in your glass of sparkling coke, but also in the tap water you drink? Welcome to the water
Then the partial pressure of oxygen is x 760 = 160 mm Hg
 1 AP Biology March 2008 Respiration Chapter 42 Gas exchange occurs across specialized respiratory surfaces. 1) Gas exchange: the uptake of molecular oxygen (O2) from the environment and the discharge of
1 AP Biology March 2008 Respiration Chapter 42 Gas exchange occurs across specialized respiratory surfaces. 1) Gas exchange: the uptake of molecular oxygen (O2) from the environment and the discharge of
To compare one gas to another, it is convenient to define a set of conditions: Standard Temperature and Pressure
 Standard Molar Volume To compare one gas to another, it is convenient to define a set of conditions: Standard Temperature and Pressure At STP, one mole of any gas has a volume of: 22.4 L = (This is a cube
Standard Molar Volume To compare one gas to another, it is convenient to define a set of conditions: Standard Temperature and Pressure At STP, one mole of any gas has a volume of: 22.4 L = (This is a cube
Pop Quiz. What produces mucus, HCl and pepsinogen in the stomach? List a water soluable vitamin What is a ruminant stomach?
 Pop Quiz What produces mucus, HCl and pepsinogen in the stomach? List a water soluable vitamin What is a ruminant stomach? Respiratory System Review Cellular respiration: obtain glucose and oxygen, get
Pop Quiz What produces mucus, HCl and pepsinogen in the stomach? List a water soluable vitamin What is a ruminant stomach? Respiratory System Review Cellular respiration: obtain glucose and oxygen, get
 Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Structures of the Respiratory System include:
 Respiratory System Structures of the Respiratory System include: ü Oral Cavity ü Nasal Cavity ü Pharynx ü Epiglottis ü Larynx ü Trachea ü Diaphragm ü Lung ü Bronchus ü Bronchioles ü Alveolus ü Pulmonary
Respiratory System Structures of the Respiratory System include: ü Oral Cavity ü Nasal Cavity ü Pharynx ü Epiglottis ü Larynx ü Trachea ü Diaphragm ü Lung ü Bronchus ü Bronchioles ü Alveolus ü Pulmonary
Multiple Choice (40%)
 AP Chemistry Test (Chapter 5) Please do not write on this test thank you! Multiple Choice (40%) 1) A sealed rigid container is filled with three ideal gases: A, B and C. The partial pressure of each gas
AP Chemistry Test (Chapter 5) Please do not write on this test thank you! Multiple Choice (40%) 1) A sealed rigid container is filled with three ideal gases: A, B and C. The partial pressure of each gas
Oxygen and Carbon dioxide Transport. Dr. Laila Al-Dokhi
 Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
RESPIRATORY REGULATION DURING EXERCISE
 RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
Seawater. Earth is an Ocean Planet
 Seawater Earth is an Ocean Planet Topics Origin of the Ocean and Atmosphere Hydrologic Cycle Biogeochemical Cycle Seawater Salinity Variations in Seawater Chemistry Carbonic Acid System Topics Origin of
Seawater Earth is an Ocean Planet Topics Origin of the Ocean and Atmosphere Hydrologic Cycle Biogeochemical Cycle Seawater Salinity Variations in Seawater Chemistry Carbonic Acid System Topics Origin of
Respiratory Lecture Test Questions Set 3
 Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
CTB3365x Introduction to Water Treatment
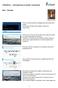 CTB3365x Introduction to Water Treatment W4e Aeration Merle de Kreuk There is one last thing of the biological part that needs some design. As you know, nitrifyers need oxygen, and also the heterotrophs,
CTB3365x Introduction to Water Treatment W4e Aeration Merle de Kreuk There is one last thing of the biological part that needs some design. As you know, nitrifyers need oxygen, and also the heterotrophs,
Chapter 3. Solids, Liquids, and Gases
 Chapter 3 Solids, Liquids, and Gases Section 1: States of Matter Learning Objectives: Describe the characteristics of a solid Describe the characteristics of a liquid Describe the characteristics of a
Chapter 3 Solids, Liquids, and Gases Section 1: States of Matter Learning Objectives: Describe the characteristics of a solid Describe the characteristics of a liquid Describe the characteristics of a
Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases.
 Chapter 8 Gases Practice Problems Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases. Summary: In a gas, particles are so far
Chapter 8 Gases Practice Problems Section 8.1 Properties of Gases Goal: Describe the kinetic molecular theory of gases and the units of measurement used for gases. Summary: In a gas, particles are so far
Judith Herzfeld 1996,1998. These exercises are provided here for classroom and study use only. All other uses are copyright protected.
 Judith Herzfeld 1996,1998 These exercises are provided here for classroom and study use only. All other uses are copyright protected. 2.7-110 A yardstick laid on a table with a few inches hanging over
Judith Herzfeld 1996,1998 These exercises are provided here for classroom and study use only. All other uses are copyright protected. 2.7-110 A yardstick laid on a table with a few inches hanging over
Topic 6: Gases and Colligative Properties
 Topic 6: Gases and Colligative Properties Ideal Gas Equation Boyle noticed an inverse relationship between volume and pressure. Pressure x volume = constant P = a P 1/P Charles found the volume of a gas,
Topic 6: Gases and Colligative Properties Ideal Gas Equation Boyle noticed an inverse relationship between volume and pressure. Pressure x volume = constant P = a P 1/P Charles found the volume of a gas,
Liquid Thin Film Technology
 Liquid Thin Film Technology New innovative way of enabling gas transfer in/out of liquid with extremely high power efficiency US Patent No. US 8,292,271 B2 and US 7,494,534 B2 3/15/2015 Riverforest Corporation
Liquid Thin Film Technology New innovative way of enabling gas transfer in/out of liquid with extremely high power efficiency US Patent No. US 8,292,271 B2 and US 7,494,534 B2 3/15/2015 Riverforest Corporation
Chemistry Chapter 11 Test Review
 Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
Chemistry Chapter 11 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Pressure is the force per unit a. volume. c. length. b. surface area.
Introduction to Biological Science - BIOL Gas Exchange
 Gas Exchange A. Influence of concentration gradient on gas diffusion rate 1. You have two tubes of permeable membrane. a. Add an arrow to illustrate concurrent fluid flow in Tubes A and B. Tube A Tube
Gas Exchange A. Influence of concentration gradient on gas diffusion rate 1. You have two tubes of permeable membrane. a. Add an arrow to illustrate concurrent fluid flow in Tubes A and B. Tube A Tube
The reactants for this process are and. The products for this process are and. The gases for this process are and.
 REVIEW All organisms (plants and animals) need energyto carry out their life processes. The chemical reaction which provides this energy is called. In larger animals it is often called respiration because
REVIEW All organisms (plants and animals) need energyto carry out their life processes. The chemical reaction which provides this energy is called. In larger animals it is often called respiration because
Preliminary Biology Assessment Task #1. Part 1 is to be completed and handed in before the start of period 1 on Friday 13/05/2016.
 Preliminary Biology Assessment Task #1 Assessment Overview: There are THREE (3) parts to this assessment. Part 1: Research and planning; To be done in own time. Part 1 is to be completed and handed in
Preliminary Biology Assessment Task #1 Assessment Overview: There are THREE (3) parts to this assessment. Part 1: Research and planning; To be done in own time. Part 1 is to be completed and handed in
MiSP Solubility L2 Teacher Guide. Introduction
 MiSP Solubility L2 Teacher Guide Introduction In this unit students will learn about solubility. Students should already be familiar with the basic chemistry concepts. They should know that some substances
MiSP Solubility L2 Teacher Guide Introduction In this unit students will learn about solubility. Students should already be familiar with the basic chemistry concepts. They should know that some substances
Systems of distribution
 Systems of distribution Outline Distribution of respiratory gases, and in blood Respiratory systems - transport of oxygen to tissues - radically different designs in mammals, birds, insects Vertebrate
Systems of distribution Outline Distribution of respiratory gases, and in blood Respiratory systems - transport of oxygen to tissues - radically different designs in mammals, birds, insects Vertebrate
Lab 5- Cellular Respiration
 Lab 5- Cellular Respiration Background: Many cellular processes require energy. Aerobic cellular respiration supplies energy by the oxidation of glucose. This is a complex process involving a number of
Lab 5- Cellular Respiration Background: Many cellular processes require energy. Aerobic cellular respiration supplies energy by the oxidation of glucose. This is a complex process involving a number of
Each gas sample has the same A) density B) mass C) number of molecules D) number of atoms
 1. A real gas behaves most like an ideal gas at A) low pressure and high temperature B) average potential energy of its particles C) ionization energy of its particles D) activation energy of its particles
1. A real gas behaves most like an ideal gas at A) low pressure and high temperature B) average potential energy of its particles C) ionization energy of its particles D) activation energy of its particles
Gina M. Barrier, Director Northwest Outreach Office The Science House North Carolina State University
 Gina M. Barrier, Director Northwest Outreach Office The Science House North Carolina State University www.science-house.org Outreach program of the College of Sciences, NC State University Established
Gina M. Barrier, Director Northwest Outreach Office The Science House North Carolina State University www.science-house.org Outreach program of the College of Sciences, NC State University Established
Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes:
 Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
Name: Unit 9 Packet: Gas Laws Introduction to Gas Laws Notes: Block: In chemistry, the relationships between gas physical properties are described as gas laws. Some of these properties are pressure, volume,
OXYGEN CONSUMPTION AND TEMPERATURE IN THE AQUATIC ENVIRONMENT
 OXYGEN CONSUMPTION AND TEMPERATURE IN THE AQUATIC ENVIRONMENT BACKGROUND READING Animal Physiology by Hill, Wyse & Anderson, 2004: pp. 130 139 & 198 201. PRE-LAB (Due at the start of the lab) ** In your
OXYGEN CONSUMPTION AND TEMPERATURE IN THE AQUATIC ENVIRONMENT BACKGROUND READING Animal Physiology by Hill, Wyse & Anderson, 2004: pp. 130 139 & 198 201. PRE-LAB (Due at the start of the lab) ** In your
B14 gas exchange note, reading and questions.notebook April 13, 2011
 Review All organisms (plants and animals) need energy to carry out their life processes. The chemi reaction which provides this energy is called. In larger animals it is sometimes called respiration because
Review All organisms (plants and animals) need energy to carry out their life processes. The chemi reaction which provides this energy is called. In larger animals it is sometimes called respiration because
Capillaries - Teacher s Guide (Human Biology)
 Capillaries - Teacher s Guide (Human Biology) The Program in Human Biology, Stanford Uni- versity, (HumBio) CK12 Editor Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required)
Capillaries - Teacher s Guide (Human Biology) The Program in Human Biology, Stanford Uni- versity, (HumBio) CK12 Editor Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required)
Completed ALL 2 Warm-up IC Kinetic Molecular Theory Notes. Kinetic Molecular Theory and Pressure Worksheet
 Name: Unit 10- Gas Laws Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 5 Kinetic Molecular Theory Notes IC 1 6 8 Kinetic Molecular Theory and Pressure Worksheet IC 2 9 10 Gas Law
Name: Unit 10- Gas Laws Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 5 Kinetic Molecular Theory Notes IC 1 6 8 Kinetic Molecular Theory and Pressure Worksheet IC 2 9 10 Gas Law
GASEOUS EXCHANGE IN PLANTS & ANIMALS 30 JULY 2014
 GASEOUS EXCHANGE IN PLANTS & ANIMALS 30 JULY 2014 In this lesson, we: Lesson Description Define gaseous exchange o o Look at the requirements for efficient gaseous exchange Study gaseous exchange in various
GASEOUS EXCHANGE IN PLANTS & ANIMALS 30 JULY 2014 In this lesson, we: Lesson Description Define gaseous exchange o o Look at the requirements for efficient gaseous exchange Study gaseous exchange in various
P215 Respiratory System, Part 2
 P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
Studying Carbon Dioxide
 Activity 3 Studying Carbon Dioxide GOALS In this activity you will: Generate CO 2 by various methods, then collect and characterize it. Explore how the volume of a gas varies with temperature. Compare
Activity 3 Studying Carbon Dioxide GOALS In this activity you will: Generate CO 2 by various methods, then collect and characterize it. Explore how the volume of a gas varies with temperature. Compare
Dissolved Oxygen Meter. ino2. Instruction Manual. Serial No..
 Innovative Instruments, Inc. http://www.2in.com Dissolved Oxygen Meter ino2 Instruction Manual Serial No.. Innovative Instruments, Inc. 8533 Queen Brooks Ct. Tampa, FL 33637. USA Phone: 813-727-0676. Fax:
Innovative Instruments, Inc. http://www.2in.com Dissolved Oxygen Meter ino2 Instruction Manual Serial No.. Innovative Instruments, Inc. 8533 Queen Brooks Ct. Tampa, FL 33637. USA Phone: 813-727-0676. Fax:
Kinetic Molecular Theory
 Kinetic Molecular Theory Name Period Unit 7 HW 1 Worksheet (Goals 1 & 2) 1. Describe how gases, liquids, and solids compare using the following table. Volume (definite or indefinite) Molecular Motion (high,
Kinetic Molecular Theory Name Period Unit 7 HW 1 Worksheet (Goals 1 & 2) 1. Describe how gases, liquids, and solids compare using the following table. Volume (definite or indefinite) Molecular Motion (high,
Temp in Kelvin = (Temp in O C) Temp in o C = (Temp in Kelvin) Perform the following conversions:
 Study: *Questions About Gases WS and More Questions About Gases WS *Go back over your notes taken from the book and in class. *Use your book: Chapter 3 PG 66-97 The Kelvin temperature of a gas (or any
Study: *Questions About Gases WS and More Questions About Gases WS *Go back over your notes taken from the book and in class. *Use your book: Chapter 3 PG 66-97 The Kelvin temperature of a gas (or any
Bioreactors Prof G. K. Suraishkumar Department of Biotechnology Indian Institute of Technology, Madras. Lecture - 15 Bioreactor env.par.
 Bioreactors Prof G. K. Suraishkumar Department of Biotechnology Indian Institute of Technology, Madras Lecture - 15 Bioreactor env.par. (DO) Welcome to lecture 15 NPTEL online certification course on Bioreactors.
Bioreactors Prof G. K. Suraishkumar Department of Biotechnology Indian Institute of Technology, Madras Lecture - 15 Bioreactor env.par. (DO) Welcome to lecture 15 NPTEL online certification course on Bioreactors.
PICU Resident Self-Study Tutorial The Basic Physics of Oxygen Transport. I was told that there would be no math!
 Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
AP Biology 12 Cellular Respiration Lab
 AP Biology 12 Cellular Respiration Lab Background: Each individual cell is responsible for the energy exchanges necessary to sustain its ordered structure. Cells accomplish this task by breaking down nutrient
AP Biology 12 Cellular Respiration Lab Background: Each individual cell is responsible for the energy exchanges necessary to sustain its ordered structure. Cells accomplish this task by breaking down nutrient
COMBINED SCIENCE 0653/6 CO-ORDINATED SCIENCES 0654/6
 Centre Number Candidate Number Candidate Name International General Certificate of Secondary Education UNIVERSITY OF CAMBRIDGE LOCAL EXAMINATIONS SYNDICATE COMBINED SCIENCE 0653/6 CO-ORDINATED SCIENCES
Centre Number Candidate Number Candidate Name International General Certificate of Secondary Education UNIVERSITY OF CAMBRIDGE LOCAL EXAMINATIONS SYNDICATE COMBINED SCIENCE 0653/6 CO-ORDINATED SCIENCES
Unit 14 Gas Laws Funsheets
 Name: Period: Unit 14 Gas Laws Funsheets Part A: Vocabulary and Concepts- Answer the following questions. Refer to your notes and the PowerPoint for help. 1. List 5 different common uses for gases: a.
Name: Period: Unit 14 Gas Laws Funsheets Part A: Vocabulary and Concepts- Answer the following questions. Refer to your notes and the PowerPoint for help. 1. List 5 different common uses for gases: a.
Please do not write on this test. Please use the answer sheet. 1) Please choose all conditions that would allow a gas sample to behave ideally.
 AP Chemistry Test (Chapter 5) Please do not write on this test. Please use the answer sheet. Multiple Choice (50%) 1) Please choose all conditions that would allow a gas sample to behave ideally. I) Nonpolar
AP Chemistry Test (Chapter 5) Please do not write on this test. Please use the answer sheet. Multiple Choice (50%) 1) Please choose all conditions that would allow a gas sample to behave ideally. I) Nonpolar
Mix and Flow of Matter Unit Test. For each of the following hazardous products match the correct WHMIS symbol
 /40 Student Name Class Section 1.1 WHMIS For each of the following hazardous products match the correct WHMIS symbol 1 Flammable A. 2 Corrosive B. 3 Dangerously Reactive C. Section 1.2 The Many Uses of
/40 Student Name Class Section 1.1 WHMIS For each of the following hazardous products match the correct WHMIS symbol 1 Flammable A. 2 Corrosive B. 3 Dangerously Reactive C. Section 1.2 The Many Uses of
Name Chemistry Pre-AP
 Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Name Chemistry Pre-AP Notes: Gas Laws and Gas Stoichiometry Period Part 1: The Nature of Gases and The Gas Laws I. Nature of Gases A. Kinetic-Molecular Theory The - theory was developed to account for
Transport in cells. Specification coverage
 3 Transport in cells T F A R What does smelling your best friend s deodorant have in common with making a cup of tea? One thing is that they both involve the movement of particles. Particles spread out
3 Transport in cells T F A R What does smelling your best friend s deodorant have in common with making a cup of tea? One thing is that they both involve the movement of particles. Particles spread out
Overview. Learning Objectives
 0 Overview This module: Reviews the selection of a GLOBE hydrology site Reviews the water sampling technique used in GLOBE hydrology protocols Provides a step by step introduction of the protocol method
0 Overview This module: Reviews the selection of a GLOBE hydrology site Reviews the water sampling technique used in GLOBE hydrology protocols Provides a step by step introduction of the protocol method
Unit 8: Kinetic Theory Homework Packet (90 points)
 Name: Key Period: By the end of Unit 8, you should be able to: Kinetic Theory Chapter 13-14 4. Define kinetic theory of gases including collisions 5. Define pressure, including atmospheric pressure, vapor
Name: Key Period: By the end of Unit 8, you should be able to: Kinetic Theory Chapter 13-14 4. Define kinetic theory of gases including collisions 5. Define pressure, including atmospheric pressure, vapor
AP Biology Lab - Cell Respiration
 AP Biology Lab - Cell Respiration This investigation uses respirometry techniques to calculate the rate of oxygen consumption (cellular respiration) in germinating pea seeds. The effect of temperature
AP Biology Lab - Cell Respiration This investigation uses respirometry techniques to calculate the rate of oxygen consumption (cellular respiration) in germinating pea seeds. The effect of temperature
Alveolus and Respiratory Membrane
 Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Name Gas Law Date. Version 3
 Name Gas Law Date 1. A real gas behaves least like an ideal gas under the conditions of 1) low temperature and high pressure 2) high temperature and low pressure 3) high temperature and high pressure 4)
Name Gas Law Date 1. A real gas behaves least like an ideal gas under the conditions of 1) low temperature and high pressure 2) high temperature and low pressure 3) high temperature and high pressure 4)
Funsheet [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015
![Funsheet [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015 Funsheet [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015](/thumbs/93/112589015.jpg) Funsheet 7.0 7.1 [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015 1. Convert the following pressures. a) 101 kpa =? atm b) 55 Torr =? psi c) 60. mmhg =? bar d) 45 Torr =? kpa e) 5 psi =? atm f) 0.0056 atm =?
Funsheet 7.0 7.1 [WHAT IS PRESSURE AND TEMPERATURE] Gu 2015 1. Convert the following pressures. a) 101 kpa =? atm b) 55 Torr =? psi c) 60. mmhg =? bar d) 45 Torr =? kpa e) 5 psi =? atm f) 0.0056 atm =?
You should be able to: Describe Equipment Barometer Manometer. 5.1 Pressure Read and outline 5.1 Define Barometer
 A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
Respiration. Chapter 33
 Respiration Chapter 33 Learning Objectives: Understand the basis of gas exchange and factors that influence diffusion of gases in and out of tissues Compare and contrast different respiratory systems among
Respiration Chapter 33 Learning Objectives: Understand the basis of gas exchange and factors that influence diffusion of gases in and out of tissues Compare and contrast different respiratory systems among
Solubility Unit. Solubility Unit Teacher Guide L1-3. Introduction:
 Solubility Unit Introduction: In this unit the students will learn about solubility. Students should already be familiar with the basic chemistry concepts. They should know that some substances are soluble
Solubility Unit Introduction: In this unit the students will learn about solubility. Students should already be familiar with the basic chemistry concepts. They should know that some substances are soluble
