Improved technique for estimating pleural pressure from esophageal balloons.
|
|
|
- Oscar Reynolds
- 5 years ago
- Views:
Transcription
1 Improved technique for estimating pleural pressure from esophageal balloons J. MILIC-EMILI, J. MEAD, J. M. TURNER, AND E. M. GLAUSER2 Department of Physiology, Haruard School of Public Health, Boston, Massachusetts MILIC-EMILI, J., J. MEAD, J. M. TURNER, AND E. M. GLAUSER. Improved technique for estimating pleural pressure from esophageal balloons. J. Appl. Physiol. I g(2) : 27-2 I I. I g64.-esophageal pressure has been measured in eight healthy men during breath holding (glottis open) at various fixed lung volumes with a rubber balloon (length: IO cm; perimeter: 3. cm; wall thickness: ca..6 mm) containing various volumes of air. The tip of the balloon was placed at a constant distance of 4 cm from the nares. Esophageal pressure was found to increase with balloon volume at all lung volumes but not uniformly, the effect of balloon volume being greatest at both extremes of the vital capacity. As a result, lung volume-pressure curves obtained from balloons containing volumes of gas such as are commonly used are distorted. One can avoid these distortions by repeating measurements at different balloon volumes and extrapolating esophageal pressure to zero balloon volume, or by making measurements at very small balloon volumes. A close approximation of the extrapolated pressures was obtained with the present balloon containing.2 ml air. The esophageal pressures at or near zero balloon volume probably reflect closely local absolute pleural pressures over the full vital capacity range in some subjects and above 2% of the vital capacity in most subjects. lung volume-pressure curves M EASUREMENTS OF VOLUME-PRESSURE relationships of human lungs are usually based on indirect determination of pleural pressure obtained from the esophagus. The most widely used method for measuring esophageal pressure employs air-containing latex balloons sealed over catheters which transmit balloon pressure to manometers (I, 4, g, I 3). The pressure within the balloon placed in the esophagus will be the same as local pleural pressure only if the pressure difference across all intervening structures (balloon wall, esophageal wall, and various mediastinal structures) is zero. Over a certain Received for publication I 3 August I This study was supported in part by National Institutes of Health Grants HTS-379, E-I I 7, and H-339, and Institute of General Medical Sciences. Grant GM Present address: Woman s Medical College of Pennsylvania, Philadelphia, Pa. range of balloon volumes there is only a negligible pressure drop across the wall of the balloon itself. Within this range, however, when the balloon is placed in the esophagus balloon pressure increases with balloon volume, presumably as a result of distension and displacement of the esophageal wall and surrounding structures (9, I 3). As a result, the pressure in the esophageal balloon tends to be more positive than pleural pressure, a fact that has been generally recognized. Since the magnitude of this difference was unknown, little use has been made of esophageal pressure as a measure of absolute pleural pressure. On the other hand, it has been thought that variations in pleural pressure would be reliably recorded from such balloons, the assumption being that the influence of balloon volume per se on pressure does not change with lung volume. The present study shows that this is not the case. An effect of balloon volume on the recorded pressure was found which increased at both extremes of the vital capacity. As a result, volumepressure curves for lungs based on esophageal pressures are distorted at these lung volumes. These distortions can be avoided if one repeats measurements at various balloon volumes and extrapolates esophageal pressures to zero balloon volume or, more practically, can be rendered negligible by making measurements at smaller balloon volumes than have commonly been used in the past. In addition, the results suggest that esophageal pressures at or near zero balloon volume probably reflect closely local absolute pleural pressures. MATERIALS AND METHODS Esophageal pressure was measured in eight healthy men during breath holding (glottis open) at various fixed lung volumes with a balloon containing various volumes of air. The physical characteristics and vital capacities of the subjects are given in Table I. All measurements were made with the subjects in the upright posture. Lung volumes were measured with a g-liter Collins spirometer. The esophageal balloon was made of rubber and had the following dimensions: length, IO cm; perim-
2 28 MILIC-EMIL& MEAD, TURNER, AND GLAUSER eter, 3. cm; wall thickness, ca..6 mm. The balloon was sealed over the end of a polyethylene catheter (i.d., o. 14 cm; o.d., o. r g cm; length, IOO cm), which had a number of spirally arranged holes in the part covered by the ba.lloon. The volume-pressure characteristics of the balloon used are given in Fig. 2. The catheter was connected to a pressure transducer (Sanborn model 268B). Pressure was recorded on a direct-writing oscillograph (Sanborn Poly-Viso). The volume displacement coefficient of the catheter-manometer unit was about.2 ml/cm H2. As a result of the small volume. + IO I I. pes. cm H 9!. TABLE I. Physical characteristics and vital cahacities (IX> of subjects -2 Subject JT FS DL JS ME NF JM RK Am, yr Weight, kg Height, cm WE? I I = I I -1 SEC- I FIG. I. Esophageal pressure during lung deflation in subject ME. Lung volumes, expressed as per cent of the vital capacity, are indicated on the tracing. Balloon volume: 2 ml. displacement coefficient of the catheter-manometer unit used, changes in balloon volume resulting from pressure changes were small. The balloon was introduced through the nose into the esophagus and placed at a distance of 4 cm from the balloon tip to the nares. Prior to each measurement the balloon was emptied by having the subject perform a Valsalva maneuver while the catheter was open to the atmosphere. The balloon was then connected to a syringe and 8 ml air were introduced in order to distend the walls of the balloon evenly. Following this, air was withdrawn into the syringe until the desired amount (.2- ml) remained in the balloon, and the catheter was connected to the manometer. All measurements were made at balloon volumes where there was a negligible pressure drop across the balloon wall. Changes in balloon volume, resulting from pressure variations in the closed balloon-catheter-manometer system, were taken into account. To obtain consistent results it was found necessary to standardize the procedure as follows. Before each measurement the subject performed three full inspirations. At the end of the third full inspiration, FIG. 2. Broken line: pressure-volume relationship of balloon in air. Solid lines : esophageal pressure-balloon volume curves obtained at different lung volumes, expressed as per cent of the vital capacity. As a result of the small volume displacement coefficient of the catheter-manometer system used, changes in balloon volume due to compression or expansion of gas were negligible. Dotted line : esophageal pressure-balloon volume relationship for a system with a volume displacement coefficient of. ml/cm HZO, the balloon containing 2 ml of gas at atmospheric pressure. The pressures at the various lung volumes that would be obtained with such a system are indicated by the intercepts of the dotted line with the iso-lung volume curves. he exhaled stepwise to fixed lung volumes until residual volume was attained. Each lung volume was maintained (glottis open) long enough to measure the static esophageal pressure (in general for 2-4 set). During the period of breath holding esophageal pressure did not change appreciably, except at total lung capacity where it decreased markedly with time (). At this lung volume only the most negative values of esophageal pressure were taken into consideration. All data were obtained during lung deflation and were repeated at least two times. L4 typical record is shown in Fig. I. RESULTS Figure 2 illustrates the relationship between esopha.geal pressure (Pes) and balloon volume (Ves) obtained at various lung volumes in subject ME. At a constant lung volume esophageal pressure increased linearly with balloon volume. The change in esophageal pressure resulting from a unit change in balloon volume, i.e., APes/AVes, varied at different lung volumes, being greatest at the extremes of the vital capacity. Figure 3 shows corresponding lung volume-esophageal pressure curves. In five of the other subjects studied, the relationship between Pes and Ves at any given lung volume was also found to be approximately linear. Their values of Apes/
3 ESOPHAGEAL 8 PRESSURE I v 1. I. I. % vc Ves, ml 4 (2) 2 FIG. 3. Solid lines: lung volume-esophageal pressure curves at different balloon volumes from data in Fig. 2. Broken line: lung volume-pressure curve based on esophageal pressure extrapolated to zero balloon volume, i.e., the intercepts on the ordinate of the esophageal pressure-balloon volume curves in Fig. 2. Dotted line: lung volume-esophageal pressure curve for a catheter-manometer system with a volume displacement coefficient of. ml/cm Hz with 2 ml of air introduced into the balloon at atmospheric pressure APes/AVes, cmh&l/mi FIG. 4. Change in esophageal pressure per unit change in balloon volume (A Pes/A Ves) at various fixed lung volumes for the six subjects in whom the relationship between esophageal pressure and balloon volume was linear. DISCUSS1 ON Our results confirm the previous finding that esophageal pressure increases with balloon volume (9, I 3). In addition we have found that the effect of balloon volume on esophageal pressure is greatest at both extremes of the vital capacity, particularly so at the high lung volumes. As a result, with increasing balloon volume, lung volume-pressure curves based on balloon pressures are displaced in the direction of more positive pressures, but not uniformly, being displaced more at high and low than at intermediate lung volumes. Curves based on pressures obtained by extrapolation to zero balloon volume probablv reflect more closely the actual volume-pressure behavior of lungs. A close approximation of extrapolated curves was obtained with the present balloon containing a volume of.2 ml. The results of the present investigation raise the question whether the pressures at zero balloon volume correspond to absolute pleural pressures. The results obtained at low lung volumes are interesting in this connection. In four of our subjects esophageal pressures at zero balloon volume were still slightly subatmospheric at residual volume. Since at residual volume the lungs are not completely collapsed, negativity of pleural pressure +I t 1 I I 1 1 t pes, cm Hz JM IO AVes obtained at different lung volumes are shown in Fig. 4 together with the results obtained on the subject in Fig. 2. In all these subjects APes/AVes increased as total lung capacity or residual volumes were approached. In the other two subjects studied (JM and RK), the relationship between balloon volume and esophageal pressure was approximately linear only at balloon volumes greater than about 2 ml (Fig. ). In both subjects the changes in esophageal pressure resulting from changes in balloon volume tended again to be greatest at the extremes of the vital capacity. The lung volume-pressure curves based on esoph.ageal pressures extrapolated to zero balloon volume for the eight subjects studied are given. in Fig. 6. FIG.. fixed lung linear. Esophageal volumes I in pressure-balloon volume curves at various a subject in whom this relationship was not at this lung volume may be assumed, and the pressures observed in these individuals are reasonable. In the other four subjects, at lung volumes lower than about 2 % of the vital capacity, esophageal pressures at zero balloon volumes increased disproportionately and became supra-atmospheric. It seems likely that in these instances esophageal pressures deviated from pleural pressures as a result of compression of the esophagus and
4 21 MILIC-EMILI, MEAD, TURNER, AND G-LAUSER balloon by mediastinal structures. It should be noted that at lung volumes higher than about 2 % of the vital capacity the behavior of esophageal pressure at zero balloon volume was, in general, similar for all subjects studied. We have tentatively concluded that esophageal pressure at or near zero balloon volume probably approximates closely local absolute pleural pressure in all of our subjects when lung volume is above about 2oY of the vital capacity. In some subjects it probably approximates local pleural pressure at all lung volumes. In the past, esophageal pressure has commonly been measured at relatively high balloon volumes. For the most part measurements have been made in the middle range of lung volumes where the effect of balloon volume is comparatively small. The pressures recorded, however, were presumably more positive than pleural pressures. In a few studies esophageal pressures were also measured at the extremes of the vital capacity, where balloon volume may produce considerable distortions. Accordingly, it is not surprising that the pressures obtained in these studies at total lung capacity and at residual volume were, in general, appreciably more positive than the pressures obtained in the present investigation by extrapolation to zero balloon volume, particularly so when relatively large balloon volumes were used. Published results are surnmarized in Table 2. In the previous studies balloons of various dimensions IO 8 2 t1 -IO ;7 FIG. 6. Lung volume-pressure curves based on esophageal pressure extrapolated to zero balloon volume for the eight subjects. have been used; the shorter and narrower the balloon, the larger are the changes in pressure produced by a given balloon volume and, hence, the larger the distortions (I 3). An additional source of error is introduced by catheter-manometer systems with large volume displacement coefficients (I 2, I 3), as illustrated in Figs. 2 and 3. Finally, errors may also be introduced if the balloon extends into the upper third of the esophagus (I I )* The dimensions of the balloon in the present study represent an attempt to minimize the effect of balloon volume while avoiding the upper part of the esophagus. Accordingly, the balloon had a larger perimeter than in most previous studies. Its length was, however, limited to I o cm, since with longer balloons it is difficult to avoid the upper third of the esophagus unless part of the bal I FIG. 7. Esophageal pressure-balloon volume curves at 4 and 8oY of the vital capacity. Solid circles: experimental results from Fig.. Solid lines- esophageal pressure-balloon volume curves calculated, using values for constants indicated in the figure, according - to the following equation: -. Pes = Peso < d2 X Esp )( Grad X Ves where Peso is the esophageal pressure at zero balloon volume at the uppermost point of the IO-cm long balloon, Esp is the specific elastance of the esophagus (i.e., the ratio of pressure change to balloon volume for a unit length of the esophagus), and Grad is the pressure gradient along the esophagus (i.e., the change in pressure at zero balloon volume per unit variation in level along the esophagus). Peso and Grad were obtained as described elsewhere (I I ) ; Esp was obtained from pressure-volume measurements made with a short balloon (length, 2 cm; perimeter, 3. cm; wall thickness,.6 mm) as previously described (I 3). TABLE 2. Comparison of esophageal pressures obtained at residual volume (RV) and total lung capacity (TLC) by p revious workers with present results Investigators No. of Subj. Age, yr Balloon Vol., ml Pes at RV, cm H2D Pes at TLC, cm H2 Mead et al. (IO) Slagter and Heemstra (14) Frank et al. (3) Knowles et al. (7) Car et al. (2) Hyatt (6) Macklem and Becklake (8) Macklem and Becklake (unpubl. results) Present results I2 II 4 1 I2 8 (32-7 ).-1 (19-22 > 2 (+18 to +36) 28 (22-47) I 33 (28-4 ) 2 +8 (19-4 > I 49 o- 3 (26-36 > I-2 3 (24-3 >. (-2 to -33) -3 (-21 to -4) (-1 to -27) -3o* -3 (-3 to -34) -33 (-23 to -4) 34 (27-43 ) +.8 (-2 to +g)* -38 (-29 to -6) Values are means, with ranges in parentheses. * Consistent values of Pes at RV were obtained only in six subjects.
5 ESOPHAGEAL PRESSURE 211 loon extends into the stomach. In addition, the reduced wall thickness of the present balloon together with the small volume displacement coefficient of the cathetermanometer unit made it possible to obtain accurate measurements of esophageal pressure at smaller balloon volumes than in the past. In agreement with the results of previous studies (9, 13) the relationship between esophageal pressure and balloon volume was, in general, approximately linear. In two of our subjects, however, this was not true at low balloon volumes. We felt that this might be explained by nonuniformity of esophageal pressure over the segment of esophagus encompassing the balloon. If a balloon containing a small volume of gas is placed in a region of the esophagus where pressure is not uniform from point to point, the gas will be displaced to the more negative points, and the remainder of the balloon will be collapsed. With increasing balloon volume the pressure inside the balloon increases as a result of distention and displacement of the esophageal wall and surrounding structures. Accordingly, the segment of balloon containing gas must increase progressively until it comprises the whole length of the balloon. Since the longer the length of the air-containing segment the smaller the strain on the esophageal wall and its surrounding structures induced by a given change in balloon volume, the slope APes/AVes will decrease with increasing balloon volume until the balloon is distended along its entire length. Changes in esophageal pressure resulting from any further increase in balloon volume will reflect the volume-pressure relationship of the segment of esopha.gus REFERENCES I. BUYTENDIJK, H. J. Oesophagusdruck en longelasticiteit. Groningen : Oppenheim, I gag. 2. CARO, C. G., j. BUTLER, AND A. B. DUBOIS. J. C&z. Invest. 39: 73, *9* 3. FRANK, N. R., J. MEAD, AND B. G. FERRIS. J. Clin. Invest. 36: 168, FRY, D. L., W. W. STEAD, R. V. EBERT, R. I. LUBIN, AND H. S. WELLS. J. Lab. Clin. Med. 4 : 664, I 92.. HUGHES, R., A. J. MAY, AND J. G. WIDDICOMBE. J. Physiol., London 146: 8, HYATT, R. E. Am. Rev. Resfirat. Diseases 83 : 676, KNOWLES, J. H., S. K. HONG, AND H. RAHN. J. AfipZ. Physiol. 14: 2, 99 encompassing the entire balloon, which appears to be linear within our experimental limits. Since the topography of esophageal pressure had been measured in three of our subjects (I I), it was possible to test this hypothesis. In line with our expectations, in subjects A4E and NF, who exhibited a linear relationship between esophageal pressure and balloon volume, the esophageal pressures at zero balloon volume were found to be relatively uniform in the segment of the esophagus encompassing the present balloon. This was not the case for subject JM, one of the two subjects with nonlinear Pes versus Ves curves. Since in this subject in the region of the esophagus occupied by the present balloon a) esophageal pressure at zero balloon volume increased approximately linearly with descent along the esophagus (I I) (Fig. 4), and b) the mechanical properties of the esophagus, as expressed by the ratio of pressure change to balloon volume for a unit length of the esophagus or specific elastance of the esophagus (13), have been shown to be uniform in the segment of esophagus concerned, the relationship between Pes and Ves can be predicted. Figure 7 shows the predicted relationship between Pes and Ves at 4 and 8% of the vital capacity together with the experimental data for subject JA4. It can be seen that the experimental results are in good agreement with the predicted values, lending further support to the hypothesis that nonuniformity of esophageal pressure accounted for the instances of nonlinearity in the relationship between esophageal pressure and balloon volume IO. II MACKLEN, P. T., AND M. R. BECKLAKE. Am. Rev. Resfirat. Diseases 87 : 47, MEAD, J., M. B. MCILROY, N. J. SELVERSTONE, AND B. C. KRIETE. J. Appl. Physiol. 7 : 491, I 9. MEAD, J., I. LINDGREN, AND E. A. GAENSLER. J. Clin. Invest. 34: 1, 19. MILIC-EMILI, J., J. MEAD, AND J. M. TURNER. J. A@Z. Physiol. I g : 2 I 2, I 964. MILIC-EMILI, J., AND J. M. PETIT. J. ApfZ. Physiol. 14 : 3,rgsg. PETIT, J. M., AND J. MILIC-EMILI. J. A$@. Physiol. 13 : 481, 198. SLAGTER, B., AND H. HEEMSTRA. Acta Physiol. Pharmacol. iveerz. 4: =
STATIC BIOMECHANICS OF THE MAMMALIAN RESPIRATORY SYSTEM 1
 STATIC BIOMECHANICS OF THE MAMMALIAN RESPIRATORY SYSTEM 1 Summary: We begin a consideration of breathing in mammals. We will start with the definitions of a series of sub- volumes of the total volume of
STATIC BIOMECHANICS OF THE MAMMALIAN RESPIRATORY SYSTEM 1 Summary: We begin a consideration of breathing in mammals. We will start with the definitions of a series of sub- volumes of the total volume of
RESPIRATORY MUSCLES IN HEALTH AND EMPHYSEMA *
 THE OXYGEN CONSUMPTION AND EFFICIENCY OF THE RESPIRATORY MUSCLES IN HEALTH AND EMPHYSEMA * BY REUBEN M. CHERNIACK t (From The Winnipeg General Hospital and the Departments of Medicine and Physiology and
THE OXYGEN CONSUMPTION AND EFFICIENCY OF THE RESPIRATORY MUSCLES IN HEALTH AND EMPHYSEMA * BY REUBEN M. CHERNIACK t (From The Winnipeg General Hospital and the Departments of Medicine and Physiology and
Equation 1: F spring = kx. Where F is the force of the spring, k is the spring constant and x is the displacement of the spring. Equation 2: F = mg
 1 Introduction Relationship between Spring Constant and Length of Bungee Cord In this experiment, we aimed to model the behavior of the bungee cord that will be used in the Bungee Challenge. Specifically,
1 Introduction Relationship between Spring Constant and Length of Bungee Cord In this experiment, we aimed to model the behavior of the bungee cord that will be used in the Bungee Challenge. Specifically,
Pulmonary Function I (modified by C. S. Tritt, April 10, 2006) Volumes and Capacities
 I. Introduction Pulmonary Function I (modified by C. S. Tritt, April 10, 2006) Volumes and Capacities The volume of air a person inhales (inspires) and exhales (expires) can be measured with a spirometer
I. Introduction Pulmonary Function I (modified by C. S. Tritt, April 10, 2006) Volumes and Capacities The volume of air a person inhales (inspires) and exhales (expires) can be measured with a spirometer
Gas Laws. Introduction
 Gas Laws Introduction In 1662 Robert Boyle found that, at constant temperature, the pressure of a gas and its volume are inversely proportional such that P x V = constant. This relationship is known as
Gas Laws Introduction In 1662 Robert Boyle found that, at constant temperature, the pressure of a gas and its volume are inversely proportional such that P x V = constant. This relationship is known as
Gas Pressure. Pressure is the force exerted per unit area by gas molecules as they strike the surfaces around them.
 Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Measuring Lung Capacity
 Name Class Date Chapter 37 Circulatory and Respiratory Systems Measuring Lung Capacity Introduction The amount of air that you move in and out of your lungs depends on how quickly you are breathing. The
Name Class Date Chapter 37 Circulatory and Respiratory Systems Measuring Lung Capacity Introduction The amount of air that you move in and out of your lungs depends on how quickly you are breathing. The
CHM Basics of Gases (r14) Charles Taylor 1/9
 CHM 110 - Basics of Gases (r14)- 2014 Charles Taylor 1/9 Introduction The gas phase is noticeably different from the other two phases of matter. Here are some of the more obvious differences. Gases are
CHM 110 - Basics of Gases (r14)- 2014 Charles Taylor 1/9 Introduction The gas phase is noticeably different from the other two phases of matter. Here are some of the more obvious differences. Gases are
changes in lung volumes occurring during forward RESPIRATORY MECHANICS DURING FORWARD ACCELERATION * during negative pressure breathing (4-7).
 RESPIRATORY MECHANICS DURING FORWARD ACCELERATION * By JOHN F. WATSON, NEIL S. CHERNIACK AND FRED W. ZECHMAN t (From the Acceleration Section, Biophysics Branch, Biomedical Laboratory, Aerospace Medical
RESPIRATORY MECHANICS DURING FORWARD ACCELERATION * By JOHN F. WATSON, NEIL S. CHERNIACK AND FRED W. ZECHMAN t (From the Acceleration Section, Biophysics Branch, Biomedical Laboratory, Aerospace Medical
Cornell Institute for. Biology Teachers. Respirometry Part I: Lung Volumes and Capacities. Lab issue/rev. date: 12/12/96. Title:
 Cornell Institute for Biology Teachers Copyright Cornell Institute for Biology Teachers, 1999. This work may be copied by the original recipient from CIBT to provide copies for users working under the
Cornell Institute for Biology Teachers Copyright Cornell Institute for Biology Teachers, 1999. This work may be copied by the original recipient from CIBT to provide copies for users working under the
Two interconnected rubber balloons as a demonstration showing the effect of surface tension
 Two interconnected rubber balloons as a demonstration showing the effect of surface tension Abstract Science One 28-9 CHEN, Chieh-Shan The two interconnected rubber balloons system is a demonstration widely
Two interconnected rubber balloons as a demonstration showing the effect of surface tension Abstract Science One 28-9 CHEN, Chieh-Shan The two interconnected rubber balloons system is a demonstration widely
April KHALED MOUSA BACHA. Physiology #2. Dr. Nayef AL-Gharaibeh. Pulmonary volumes & capacities
 25 th April Physiology #2 Pulmonary volumes & capacities Dr. Nayef AL-Gharaibeh KHALED MOUSA BACHA We will start this lecture by explaining an important concept from the previous one: Intrapleural pressure
25 th April Physiology #2 Pulmonary volumes & capacities Dr. Nayef AL-Gharaibeh KHALED MOUSA BACHA We will start this lecture by explaining an important concept from the previous one: Intrapleural pressure
THE COLLEGE OF AERONAUTICS CRANFIELD
 THE COLLEGE OF AERONAUTICS CRANFIELD AERODYNAMIC CHARACTERISTICS OF A 40 SWEPT BACK WING OF ASPECT RATIO 4.5 by P. S. BARNA NOTE NO. 65 MAY, 1957 CRANFIELD A preliminary report on the aerodynamic characteristics
THE COLLEGE OF AERONAUTICS CRANFIELD AERODYNAMIC CHARACTERISTICS OF A 40 SWEPT BACK WING OF ASPECT RATIO 4.5 by P. S. BARNA NOTE NO. 65 MAY, 1957 CRANFIELD A preliminary report on the aerodynamic characteristics
For more information about how to cite these materials visit
 Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Restrictive and Obstructive Airway Diseases
 iworx Physiology Lab Experiment Experiment HS-8-1 Restrictive and Obstructive Airway Diseases Note: The lab presented here is intended for evaluation purposes only. iworx users should refer to the User
iworx Physiology Lab Experiment Experiment HS-8-1 Restrictive and Obstructive Airway Diseases Note: The lab presented here is intended for evaluation purposes only. iworx users should refer to the User
THE PHYSICAL PROPERTIES OF NORMAL LUNGS
 Thorax (1952), 7, 285. THE PHYSICAL PROPERTIES OF NORMAL LUNGS REMOVED AFTER DEATH BY M. B. McILROY From the Medical Professorial Unit, St. Bartholomew's Hospital, London (RECEIVED FOR PUBLICATION OCTOBER
Thorax (1952), 7, 285. THE PHYSICAL PROPERTIES OF NORMAL LUNGS REMOVED AFTER DEATH BY M. B. McILROY From the Medical Professorial Unit, St. Bartholomew's Hospital, London (RECEIVED FOR PUBLICATION OCTOBER
(Received 9 September 1940)
 257 J. Physiol. (I 94I) 99, 257-264 6I2.2II A METHOD OF RECORDING THE RESPIRATION BY J. H. GADDUM From the College of the Pharmaceutical Society, 17 Bloomsbury Square, London, W.C. 2 (Received 9 September
257 J. Physiol. (I 94I) 99, 257-264 6I2.2II A METHOD OF RECORDING THE RESPIRATION BY J. H. GADDUM From the College of the Pharmaceutical Society, 17 Bloomsbury Square, London, W.C. 2 (Received 9 September
Part 1: Inspiratory and expiratory pressures
 BIOEN 327 Autumn 2013 Experimental procedures Throughout these experiments, record in your notebook the purpose of the experiments, the methods you used, and the results. Where possible, make predictions
BIOEN 327 Autumn 2013 Experimental procedures Throughout these experiments, record in your notebook the purpose of the experiments, the methods you used, and the results. Where possible, make predictions
Pressure Measurement
 Pressure Measurement Absolute and Gage Pressure P abs = P gage + P atm where P abs = Absolute pressure P abs = Gage pressure P abs = atmospheric pressure A perfect vacuum is the lowest possible pressure.
Pressure Measurement Absolute and Gage Pressure P abs = P gage + P atm where P abs = Absolute pressure P abs = Gage pressure P abs = atmospheric pressure A perfect vacuum is the lowest possible pressure.
Applying Hooke s Law to Multiple Bungee Cords. Introduction
 Applying Hooke s Law to Multiple Bungee Cords Introduction Hooke s Law declares that the force exerted on a spring is proportional to the amount of stretch or compression on the spring, is always directed
Applying Hooke s Law to Multiple Bungee Cords Introduction Hooke s Law declares that the force exerted on a spring is proportional to the amount of stretch or compression on the spring, is always directed
Gilbert Kirss Foster. Chapter 10. Properties of Gases The Air We Breathe
 Gilbert Kirss Foster Chapter 10 Properties of Gases The Air We Breathe Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4
Gilbert Kirss Foster Chapter 10 Properties of Gases The Air We Breathe Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4
Gas volume and pressure are indirectly proportional.
 Section 2 The Gas Laws Key Terms Boyle s law Charles s law combined gas law absolute zero Gay-Lussac s law Scientists have been studying physical properties of gases for hundreds of years In 1662, Robert
Section 2 The Gas Laws Key Terms Boyle s law Charles s law combined gas law absolute zero Gay-Lussac s law Scientists have been studying physical properties of gases for hundreds of years In 1662, Robert
Physiology of the Respiratory System
 Biology 212: Anatomy and Physiology II Physiology of the Respiratory System References: Saladin, KS: Anatomy and Physiology, The Unity of Form and Function 8 th (2018). Required reading before beginning
Biology 212: Anatomy and Physiology II Physiology of the Respiratory System References: Saladin, KS: Anatomy and Physiology, The Unity of Form and Function 8 th (2018). Required reading before beginning
Physiology - lecture 3
 Physiology - lecture 3 Residual Volume (RV):the amount of gas remaining in the lung at the end of a maximal exhalation Tidal Volume (TV):the volume of gas inhaled and exhaled during one respiratory cycle.
Physiology - lecture 3 Residual Volume (RV):the amount of gas remaining in the lung at the end of a maximal exhalation Tidal Volume (TV):the volume of gas inhaled and exhaled during one respiratory cycle.
LAB 7 HUMAN RESPIRATORY LAB. Complete the charts on pgs. 67 and 68 and read directions for using BIOPAC
 66 LAB 7 HUMAN RESPIRATORY LAB Assignments: Due before lab: Quiz: Three Respiratory Interactive Physiology Animations pages 69 73. Complete the charts on pgs. 67 and 68 and read directions for using BIOPAC
66 LAB 7 HUMAN RESPIRATORY LAB Assignments: Due before lab: Quiz: Three Respiratory Interactive Physiology Animations pages 69 73. Complete the charts on pgs. 67 and 68 and read directions for using BIOPAC
RESPIRATORY PHYSIOLOGY, PHYSICS AND PATHOLOGY IN RELATION TO ANAESTHESIA AND INTENSIVE CARE
 Course n : Course 3 Title: RESPIRATORY PHYSIOLOGY, PHYSICS AND PATHOLOGY IN RELATION TO ANAESTHESIA AND INTENSIVE CARE Sub-category: Intensive Care for Respiratory Distress Topic: Pulmonary Function and
Course n : Course 3 Title: RESPIRATORY PHYSIOLOGY, PHYSICS AND PATHOLOGY IN RELATION TO ANAESTHESIA AND INTENSIVE CARE Sub-category: Intensive Care for Respiratory Distress Topic: Pulmonary Function and
Activity 2: Examining the Effect of Changing Airway Resistance on Respiratory Volumes
 1 BGYC34 PhysioEx Lab 7 Respiratory Systems Mechanics Marking Scheme Part 1 Complete PhysioEx lab #7. Hand-in all of the pages associated with the lab. Note that there are 5 activities to be completed.
1 BGYC34 PhysioEx Lab 7 Respiratory Systems Mechanics Marking Scheme Part 1 Complete PhysioEx lab #7. Hand-in all of the pages associated with the lab. Note that there are 5 activities to be completed.
The Variation of Muscle Oxygen Consumption With Velocity of Shortening
 The Variation of Muscle Oxygen Consumption With Velocity of Shortening R.J. BASKIN From the Department of Zoology, University of California, Davis ABSTRACT Total oxygen consumption following contraction
The Variation of Muscle Oxygen Consumption With Velocity of Shortening R.J. BASKIN From the Department of Zoology, University of California, Davis ABSTRACT Total oxygen consumption following contraction
Lab Report Outline the Bones of the Story
 Lab Report Outline the Bones of the Story In this course, you are asked to write only the outline of a lab report. A good lab report provides a complete record of your experiment, and even in outline form
Lab Report Outline the Bones of the Story In this course, you are asked to write only the outline of a lab report. A good lab report provides a complete record of your experiment, and even in outline form
Stikstofuitwas en Heliumdilutie. Eric Derom Universitair Ziekenhuis Gent
 Stikstofuitwas en Heliumdilutie Eric Derom Universitair Ziekenhuis Gent Background Overview Helium-dilution technique Principle of Technique Equipment Procedure and Calculations Quality Control Nitrogen
Stikstofuitwas en Heliumdilutie Eric Derom Universitair Ziekenhuis Gent Background Overview Helium-dilution technique Principle of Technique Equipment Procedure and Calculations Quality Control Nitrogen
Characterizers for control loops
 Characterizers for control loops By: F. G. Shinskey (May 1999) Introduction Commercial controllers such as the PID series (proportional, integral, derivative, and their combinations) are linear devices
Characterizers for control loops By: F. G. Shinskey (May 1999) Introduction Commercial controllers such as the PID series (proportional, integral, derivative, and their combinations) are linear devices
COURSE NUMBER: ME 321 Fluid Mechanics I Fluid statics. Course teacher Dr. M. Mahbubur Razzaque Professor Department of Mechanical Engineering BUET
 COURSE NUMBER: ME 321 Fluid Mechanics I Fluid statics Course teacher Dr. M. Mahbubur Razzaque Professor Department of Mechanical Engineering BUET 1 Fluid statics Fluid statics is the study of fluids in
COURSE NUMBER: ME 321 Fluid Mechanics I Fluid statics Course teacher Dr. M. Mahbubur Razzaque Professor Department of Mechanical Engineering BUET 1 Fluid statics Fluid statics is the study of fluids in
World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases
![World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases World of Chemistry Notes for Students [Chapter 13, page 1] Chapter 13 Gases](/thumbs/96/127526823.jpg) World of Chemistry Notes for Students [Chapter 3, page ] Chapter 3 Gases ) Sec 3.8 Kinetic Theory of Gases and the Nature of Gases The Kinetic Theory of Matter says that the tiny particles in all forms
World of Chemistry Notes for Students [Chapter 3, page ] Chapter 3 Gases ) Sec 3.8 Kinetic Theory of Gases and the Nature of Gases The Kinetic Theory of Matter says that the tiny particles in all forms
ALVEOLAR - BLOOD GAS EXCHANGE 1
 ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
CHAPTER 3 RESULTS AND DISCUSSION
 CHAPTER 3 RESULTS AND DISCUSSION The research included the development and verification of the earlier apparatus, and the collection and validation of moisture diffusion data under both isothermal and
CHAPTER 3 RESULTS AND DISCUSSION The research included the development and verification of the earlier apparatus, and the collection and validation of moisture diffusion data under both isothermal and
The purpose of this experiment is to find this acceleration for a puck moving on an inclined air table.
 Experiment : Motion in an Inclined Plane PURPOSE The purpose of this experiment is to find this acceleration for a puck moving on an inclined air table. GENERAL In Experiment-1 you were concerned with
Experiment : Motion in an Inclined Plane PURPOSE The purpose of this experiment is to find this acceleration for a puck moving on an inclined air table. GENERAL In Experiment-1 you were concerned with
Fashionable, don t you think?
 Fashionable, don t you think? 1. Passageway 2. Structure 3. Passageway 4. What is the name of the structure labeled with # 9 in the model at left? 5. What is the name of the structure labeled with # 11
Fashionable, don t you think? 1. Passageway 2. Structure 3. Passageway 4. What is the name of the structure labeled with # 9 in the model at left? 5. What is the name of the structure labeled with # 11
You Are Really Full of Hot Air!
 You Are Really Full of Hot Air! Student Information Page 5B Activity Introduction: You re just full of hot air! How many times has someone said this to you when they didn t quite believe what you were
You Are Really Full of Hot Air! Student Information Page 5B Activity Introduction: You re just full of hot air! How many times has someone said this to you when they didn t quite believe what you were
APRV: Moving beyond ARDSnet
 APRV: Moving beyond ARDSnet Matthew Lissauer, MD Associate Professor of Surgery Medical Director, Surgical Critical Care Rutgers, The State University of New Jersey What is APRV? APRV is different from
APRV: Moving beyond ARDSnet Matthew Lissauer, MD Associate Professor of Surgery Medical Director, Surgical Critical Care Rutgers, The State University of New Jersey What is APRV? APRV is different from
Lab #12:Boyle s Law, Dec. 20, 2016 Pressure-Volume Relationship in Gases
 Chemistry Unit 6:States of Matter & Basic Gas Laws Name Lab Partner Lab #12:Boyle s Law, Dec. 20, 2016 Pressure-Volume Relationship in Gases Purpose: The primary objective of this experiment is to determine
Chemistry Unit 6:States of Matter & Basic Gas Laws Name Lab Partner Lab #12:Boyle s Law, Dec. 20, 2016 Pressure-Volume Relationship in Gases Purpose: The primary objective of this experiment is to determine
A Liter a Lung Measuring Lung Capacity
 A Liter a Lung Measuring Lung Capacity OBJECTIVE In this investigation, students will compare the actual and expected vital capacities of their classmates. LEVEL Middle Grades Life Science CONNECTIONS
A Liter a Lung Measuring Lung Capacity OBJECTIVE In this investigation, students will compare the actual and expected vital capacities of their classmates. LEVEL Middle Grades Life Science CONNECTIONS
WIND-INDUCED LOADS OVER DOUBLE CANTILEVER BRIDGES UNDER CONSTRUCTION
 WIND-INDUCED LOADS OVER DOUBLE CANTILEVER BRIDGES UNDER CONSTRUCTION S. Pindado, J. Meseguer, J. M. Perales, A. Sanz-Andres and A. Martinez Key words: Wind loads, bridge construction, yawing moment. Abstract.
WIND-INDUCED LOADS OVER DOUBLE CANTILEVER BRIDGES UNDER CONSTRUCTION S. Pindado, J. Meseguer, J. M. Perales, A. Sanz-Andres and A. Martinez Key words: Wind loads, bridge construction, yawing moment. Abstract.
Respiration Lab Instructions
 Respiration Lab Instructions This laboratory investigation can be performed in any order. Be sure to read all instructions for each section before performing the experiment. PART 1 STUDENT WET SPIROMETER
Respiration Lab Instructions This laboratory investigation can be performed in any order. Be sure to read all instructions for each section before performing the experiment. PART 1 STUDENT WET SPIROMETER
PHYS 101 Previous Exam Problems
 PHYS 101 Previous Exam Problems CHAPTER 14 Fluids Fluids at rest pressure vs. depth Pascal s principle Archimedes s principle Buoynat forces Fluids in motion: Continuity & Bernoulli equations 1. How deep
PHYS 101 Previous Exam Problems CHAPTER 14 Fluids Fluids at rest pressure vs. depth Pascal s principle Archimedes s principle Buoynat forces Fluids in motion: Continuity & Bernoulli equations 1. How deep
The physiological functions of respiration and circulation. Mechanics. exercise 7. Respiratory Volumes. Objectives
 exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
CHAPTER 3: The cardio-respiratory system
 : The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
: The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
Pulmonary Circulation
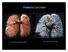 Pulmonary Circulation resin cast of pulmonary arteries resin cast of pulmonary veins Blood Flow to the Lungs Pulmonary Circulation Systemic Circulation Blood supply to the conducting zone provided by the
Pulmonary Circulation resin cast of pulmonary arteries resin cast of pulmonary veins Blood Flow to the Lungs Pulmonary Circulation Systemic Circulation Blood supply to the conducting zone provided by the
Students measure the change in pressure by varying the volume of trapped air in a syringe while:
 How Does a Trapped Gas Behave? Teacher Information Objective Students investigate the effect of changes in the volume of a confined gas on pressure at constant temperature. Using the pressure sensor, students
How Does a Trapped Gas Behave? Teacher Information Objective Students investigate the effect of changes in the volume of a confined gas on pressure at constant temperature. Using the pressure sensor, students
Boyle s Law VC 09. Experiment 9: Gas Laws. Abstract
 Experiment 9: Gas Laws VC 09 Abstract In this laboratory activity, you will experimentally confirm Boyle s Law, determine absolute zero from Gay-Lussac's Law, and determine the molecular weight of acetone,
Experiment 9: Gas Laws VC 09 Abstract In this laboratory activity, you will experimentally confirm Boyle s Law, determine absolute zero from Gay-Lussac's Law, and determine the molecular weight of acetone,
iworx Sample Lab Experiment HE-5: Resting Metabolic Rate (RMR)
 Experiment HE-5: Resting Metabolic Rate (RMR) Before Starting 1. Read the procedures for the experiment completely before beginning the experiment. Have a good understanding of how to perform the experiment
Experiment HE-5: Resting Metabolic Rate (RMR) Before Starting 1. Read the procedures for the experiment completely before beginning the experiment. Have a good understanding of how to perform the experiment
9A Gas volume and pressure are indirectly proportional.
 The Gas Laws Key Terms Boyle s law Charles s law combined gas law absolute zero Gay-Lussac s law Scientists have been studying physical properties of gases for hundreds of years In 1662, Robert Boyle discovered
The Gas Laws Key Terms Boyle s law Charles s law combined gas law absolute zero Gay-Lussac s law Scientists have been studying physical properties of gases for hundreds of years In 1662, Robert Boyle discovered
Bottom End-Closure Design Optimization of DOT-39 Non-Refillable Refrigerant Cylinders
 Y. Kisioglu a) Department of Mechanical Education, Kocaeli University, Izmit, Kocaeli, 41100, Turkey J. R. Brevick Industrial and Systems Engineering, The Ohio State University, Columbus, Ohio G. L. Kinzel
Y. Kisioglu a) Department of Mechanical Education, Kocaeli University, Izmit, Kocaeli, 41100, Turkey J. R. Brevick Industrial and Systems Engineering, The Ohio State University, Columbus, Ohio G. L. Kinzel
Chemistry A Molecular Approach. Fourth Edition. Chapter 5. Gases. Copyright 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
 Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
States of Matter. The Behavior of Gases
 States of Matter The Behavior of Gases What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement
States of Matter The Behavior of Gases What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement
STUDIES ON CROP FUNCTION IN THE COCKROACH (PERIPLANETA AMERICANA L.)
 J. Exp. Biol. (1964), 41. 513-524 513 With 10 text-figures Printed in Great Britain STUDIES ON CROP FUNCTION IN THE COCKROACH (PERIPLANETA AMERICANA L.) III. PRESSURE CHANGES DURING FEEDING AND CROP-EMPTYING
J. Exp. Biol. (1964), 41. 513-524 513 With 10 text-figures Printed in Great Britain STUDIES ON CROP FUNCTION IN THE COCKROACH (PERIPLANETA AMERICANA L.) III. PRESSURE CHANGES DURING FEEDING AND CROP-EMPTYING
The effect of external compression of the eye on intraocular pressure
 -ff r r JL. The effect of external compression of the eye on intraocular pressure I. Its variations with magnitude of compression and with age Mansour F. Armaly and Adrian H. Halasa i $ V' y. A The effect
-ff r r JL. The effect of external compression of the eye on intraocular pressure I. Its variations with magnitude of compression and with age Mansour F. Armaly and Adrian H. Halasa i $ V' y. A The effect
CONTROL VALVE WHAT YOU NEED TO LEARN?
 CONTROL VALVE WHAT YOU NEED TO LEARN? i) The control valve characteristics refers to the relationship between the volumetric flowrate F (Y-axis) through the valve AND the valve travel or opening position
CONTROL VALVE WHAT YOU NEED TO LEARN? i) The control valve characteristics refers to the relationship between the volumetric flowrate F (Y-axis) through the valve AND the valve travel or opening position
plethysmographic methods that when the subject was pinched on the upper
 24 J. Physiol. (I95I) II2, 24-2I 6I2.I5.6II.976 THE DECREASE IN HAND BLOOD FLOW FOLLOWING INFLATION OF AN ARTERIAL OCCLUSION CUFF ON THE OPPOSITE ARM BY IAN C. RODDIE From the Department of Physiology,
24 J. Physiol. (I95I) II2, 24-2I 6I2.I5.6II.976 THE DECREASE IN HAND BLOOD FLOW FOLLOWING INFLATION OF AN ARTERIAL OCCLUSION CUFF ON THE OPPOSITE ARM BY IAN C. RODDIE From the Department of Physiology,
LAB 3: RESPIRATORY MECHANICS
 BIOEN 327 Autumn 2012 LAB 3: RESPIRATORY MECHANICS Pressures throughout the cardiovascular system are important for the health of the body and of the system itself. Today we explore pulmonary pressures
BIOEN 327 Autumn 2012 LAB 3: RESPIRATORY MECHANICS Pressures throughout the cardiovascular system are important for the health of the body and of the system itself. Today we explore pulmonary pressures
Prof AH Basson, Pr Eng March Departement Meganiese en Megatroniese Ingenieurswese. Department of Mechanical and Mechatronic Engineering
 An evaluation of the effectiveness of the Sinapi Chest Drain's flutter valve in comparison to a water seal as found in an UWD to resolve pneumo- or hemothorax Prof AH Basson, Pr Eng March 2010 Departement
An evaluation of the effectiveness of the Sinapi Chest Drain's flutter valve in comparison to a water seal as found in an UWD to resolve pneumo- or hemothorax Prof AH Basson, Pr Eng March 2010 Departement
Unit 8: Gases and States of Matter
 Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
Unit 8: Gases and States of Matter Gases Particles that have no definite shape or volume. They adapt to the shape and volume of their container. Ideal gases are imaginary gases that comply with all the
3 1 PRESSURE. This is illustrated in Fig. 3 3.
 P = 3 psi 66 FLUID MECHANICS 150 pounds A feet = 50 in P = 6 psi P = s W 150 lbf n = = 50 in = 3 psi A feet FIGURE 3 1 The normal stress (or pressure ) on the feet of a chubby person is much greater than
P = 3 psi 66 FLUID MECHANICS 150 pounds A feet = 50 in P = 6 psi P = s W 150 lbf n = = 50 in = 3 psi A feet FIGURE 3 1 The normal stress (or pressure ) on the feet of a chubby person is much greater than
Lab 3. The Respiratory System (designed by Heather E. M. Liwanag with T.M. Williams)
 Name Lab Partners Lab 3. The Respiratory System (designed by Heather E. M. Liwanag with T.M. Williams) Part 1. Lung Volumes and Capacities Objectives 1. Obtain graphical representation of lung capacities
Name Lab Partners Lab 3. The Respiratory System (designed by Heather E. M. Liwanag with T.M. Williams) Part 1. Lung Volumes and Capacities Objectives 1. Obtain graphical representation of lung capacities
CHAPTER 3: The respiratory system
 CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
Collin County Community College. Lung Physiology
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Respiratory Pulmonary Ventilation
 Respiratory Pulmonary Ventilation Pulmonary Ventilation Pulmonary ventilation is the act of breathing and the first step in the respiratory process. Pulmonary ventilation brings in air with a new supply
Respiratory Pulmonary Ventilation Pulmonary Ventilation Pulmonary ventilation is the act of breathing and the first step in the respiratory process. Pulmonary ventilation brings in air with a new supply
RESPIRATORY PHYSIOLOGY RELEVANT TO HFOV
 RESPIRATORY PHYSIOLOGY RELEVANT TO Physiology of CMV 1 What is? Ventilation using a relatively high continuous distending pressure at the airway opening, around which an oscillatory wave is generated to
RESPIRATORY PHYSIOLOGY RELEVANT TO Physiology of CMV 1 What is? Ventilation using a relatively high continuous distending pressure at the airway opening, around which an oscillatory wave is generated to
PCo2 is independent of the duration of the breath hold. These results suggest that the
 Q. Ji exp. Phy8iol. (1971) 56, 92-100 MECHANICAL AND CHEMICAL CONTROL OF BREATH HOLDING. By G. R. KELMAN and K. T. WANN. From the Department of Physiology, University of Aberdeen, Aberdeen, Scotland. (Received
Q. Ji exp. Phy8iol. (1971) 56, 92-100 MECHANICAL AND CHEMICAL CONTROL OF BREATH HOLDING. By G. R. KELMAN and K. T. WANN. From the Department of Physiology, University of Aberdeen, Aberdeen, Scotland. (Received
weight of the book divided by the area of the bottom of the plunger.
 Lab: Boyle s Law Datasheet Name Data: Pressure is defined as force per unit area: P = Force/Area When a book rests on top of the plunger, the pressure it exerts equals the weight of the book divided by
Lab: Boyle s Law Datasheet Name Data: Pressure is defined as force per unit area: P = Force/Area When a book rests on top of the plunger, the pressure it exerts equals the weight of the book divided by
BASIC QUANTITIES OF GASES
 BASIC QUANTITIES OF GASES PRESSURE (P): Definition: 1 atm = 101325 Pa = 1,01325 bar (1 bar = 10 5 Pa) 1 atm = cmhg = mmhg (Torr) Manometer: Barometer: VOLUME (V): - - - Unit: 1 NUMBER OF MOLES (n): Avogadro
BASIC QUANTITIES OF GASES PRESSURE (P): Definition: 1 atm = 101325 Pa = 1,01325 bar (1 bar = 10 5 Pa) 1 atm = cmhg = mmhg (Torr) Manometer: Barometer: VOLUME (V): - - - Unit: 1 NUMBER OF MOLES (n): Avogadro
SHOT ON GOAL. Name: Football scoring a goal and trigonometry Ian Edwards Luther College Teachers Teaching with Technology
 SHOT ON GOAL Name: Football scoring a goal and trigonometry 2006 Ian Edwards Luther College Teachers Teaching with Technology Shot on Goal Trigonometry page 2 THE TASKS You are an assistant coach with
SHOT ON GOAL Name: Football scoring a goal and trigonometry 2006 Ian Edwards Luther College Teachers Teaching with Technology Shot on Goal Trigonometry page 2 THE TASKS You are an assistant coach with
STIFFNESS INVESTIGATION OF PNEUMATIC CYLINDERS. A. Czmerk, A. Bojtos ABSTRACT
 59 th ILMENAU SCIENTIFIC COLLOQUIUM Technische Universität Ilmenau, 11 15 September 2017 URN: urn:nbn:de:gbv:ilm1-2017iwk-148:6 STIFFNESS INVESTIGATION OF PNEUMATIC CYLINDERS A. Czmerk, A. Bojtos Budapest
59 th ILMENAU SCIENTIFIC COLLOQUIUM Technische Universität Ilmenau, 11 15 September 2017 URN: urn:nbn:de:gbv:ilm1-2017iwk-148:6 STIFFNESS INVESTIGATION OF PNEUMATIC CYLINDERS A. Czmerk, A. Bojtos Budapest
The Gas Laws: Boyle's Law and Charles Law
 Exercise 6 Page 1 Illinois Central College CHEMISTRY 130 Name The Gas Laws: Boyle's Law and Charles Law Objective The simple laws governing the properties of gases can be readily demonstrated experimentally.
Exercise 6 Page 1 Illinois Central College CHEMISTRY 130 Name The Gas Laws: Boyle's Law and Charles Law Objective The simple laws governing the properties of gases can be readily demonstrated experimentally.
INSTRUCTOR RESOURCES
 Gases: Dalton s Law INSTRUCTOR RESOURCES By Dale A. Hammond, PhD LEARNING OBJECTIVES introduce the concept of ideal gases. experimentally determine the relationship between pressure and amount of gas,
Gases: Dalton s Law INSTRUCTOR RESOURCES By Dale A. Hammond, PhD LEARNING OBJECTIVES introduce the concept of ideal gases. experimentally determine the relationship between pressure and amount of gas,
PCO2 levels apparently differed by less than 5 mm Hg. Fowler [1954] and. Godfrey and Campbell [1969] have shown that it is possible to resume a
![PCO2 levels apparently differed by less than 5 mm Hg. Fowler [1954] and. Godfrey and Campbell [1969] have shown that it is possible to resume a PCO2 levels apparently differed by less than 5 mm Hg. Fowler [1954] and. Godfrey and Campbell [1969] have shown that it is possible to resume a](/thumbs/85/91672390.jpg) Q. Ji exp. Physiol. (1969) 54, 129-140 THE INFLUENCE OF LUNG SHRINKAGE ON BREATH HOLDING TIME. By S. GODFREY, R. H. T. EDWARDS and D. A. WARRELL. From the Department of Medicine, Royal Postgraduate Medical
Q. Ji exp. Physiol. (1969) 54, 129-140 THE INFLUENCE OF LUNG SHRINKAGE ON BREATH HOLDING TIME. By S. GODFREY, R. H. T. EDWARDS and D. A. WARRELL. From the Department of Medicine, Royal Postgraduate Medical
Aerodynamic behavior of a discus
 Available online at www.sciencedirect.com Procedia Engineering 34 (2012 ) 92 97 9 th Conference of the International Sports Engineering Association (ISEA) Aerodynamic behavior of a discus Kazuya Seo a*,
Available online at www.sciencedirect.com Procedia Engineering 34 (2012 ) 92 97 9 th Conference of the International Sports Engineering Association (ISEA) Aerodynamic behavior of a discus Kazuya Seo a*,
IN experimental plant physiology the problem of the control of
 [ 119 ] THE CONTROL OF ATMOSPHERIC HUMIDITY IN A CLOSED SYSTEM BY B. D. BOLAS From the Department of Plant Physiology and Pathology, Imperial College of Science and Technology, London (With 4 figures in
[ 119 ] THE CONTROL OF ATMOSPHERIC HUMIDITY IN A CLOSED SYSTEM BY B. D. BOLAS From the Department of Plant Physiology and Pathology, Imperial College of Science and Technology, London (With 4 figures in
Homeostasis and Negative Feedback Concepts and Breathing Experiments 1
 Homeostasis and Negative Feedback Concepts and Breathing Experiments 1 I. Homeostasis and Negative Feedback Homeostasis refers to the maintenance of relatively constant internal conditions. For example,
Homeostasis and Negative Feedback Concepts and Breathing Experiments 1 I. Homeostasis and Negative Feedback Homeostasis refers to the maintenance of relatively constant internal conditions. For example,
Influence of Driving Pressure on Raised-volume Forced Expiration in Infants
 Influence of Driving Pressure on Raised-volume Forced Expiration in Infants MARK J. HAYDEN, PETER D. SLY, SUNALENE G. DEVADASON, LYLE C. GURRIN, JOHANNES H. WILDHABER, and PETER N. LESOUËF Department of
Influence of Driving Pressure on Raised-volume Forced Expiration in Infants MARK J. HAYDEN, PETER D. SLY, SUNALENE G. DEVADASON, LYLE C. GURRIN, JOHANNES H. WILDHABER, and PETER N. LESOUËF Department of
The tensile capacity of suction caissons in sand under rapid loading
 Frontiers in Offshore Geotechnics: ISFOG 25 Gourvenec & Cassidy (eds) 25 Taylor & Francis Group, London, ISBN 415 3963 X The tensile capacity of suction caissons in sand under rapid loading Guy T. Houlsby,
Frontiers in Offshore Geotechnics: ISFOG 25 Gourvenec & Cassidy (eds) 25 Taylor & Francis Group, London, ISBN 415 3963 X The tensile capacity of suction caissons in sand under rapid loading Guy T. Houlsby,
WAVE PRESSURE DISTRIBUTION ON PERMEABLE VERTICAL WALLS
 Abstract WAVE PRESSURE DISTRIBUTION ON PERMEABLE VERTICAL WALLS Hendrik Bergmann, Hocine Oumeraci The pressure distribution at permeable vertical walls is investigated within a comprehensive large-scale
Abstract WAVE PRESSURE DISTRIBUTION ON PERMEABLE VERTICAL WALLS Hendrik Bergmann, Hocine Oumeraci The pressure distribution at permeable vertical walls is investigated within a comprehensive large-scale
Is lung capacity affected by smoking, sport, height or gender. Table of contents
 Sample project This Maths Studies project has been graded by a moderator. As you read through it, you will see comments from the moderator in boxes like this: At the end of the sample project is a summary
Sample project This Maths Studies project has been graded by a moderator. As you read through it, you will see comments from the moderator in boxes like this: At the end of the sample project is a summary
CORESTA RECOMMENDED METHOD N 6
 CORESTA RECOMMENDED METHOD N 6 DETERMINATION OF VENTILATION DEFINITIONS AND MEASUREMENT PRINCIPLES (2015 Revision September 2016) 1. SCOPE This CORESTA Recommended Method specifies a method for the determination
CORESTA RECOMMENDED METHOD N 6 DETERMINATION OF VENTILATION DEFINITIONS AND MEASUREMENT PRINCIPLES (2015 Revision September 2016) 1. SCOPE This CORESTA Recommended Method specifies a method for the determination
A VALID APPROACH TO CORRECT CAPILLARY PRESSURE CURVES- A CASE STUDY OF BEREA AND TIGHT GAS SANDS
 SCA2009-4 /6 A VALID APPROACH TO CORRECT CAPILLARY PRESSURE CURVES- A CASE STUDY OF BEREA AND TIGHT GAS SANDS Gbenga M. Funmilayo, Shameem Siddiqui: Texas Tech University, Lubbock USA This paper was prepared
SCA2009-4 /6 A VALID APPROACH TO CORRECT CAPILLARY PRESSURE CURVES- A CASE STUDY OF BEREA AND TIGHT GAS SANDS Gbenga M. Funmilayo, Shameem Siddiqui: Texas Tech University, Lubbock USA This paper was prepared
Injector Dynamics Assumptions and their Impact on Predicting Cavitation and Performance
 Injector Dynamics Assumptions and their Impact on Predicting Cavitation and Performance Frank Husmeier, Cummins Fuel Systems Presented by Laz Foley, ANSYS Outline Overview Computational Domain and Boundary
Injector Dynamics Assumptions and their Impact on Predicting Cavitation and Performance Frank Husmeier, Cummins Fuel Systems Presented by Laz Foley, ANSYS Outline Overview Computational Domain and Boundary
Pressure of the atmosphere varies with elevation and weather conditions. Barometer- device used to measure atmospheric pressure.
 Chapter 12 Section 1 Pressure A gas exerts pressure on its surroundings. Blow up a balloon. The gas we are most familiar with is the atmosphere, a mixture of mostly elemental nitrogen and oxygen. Pressure
Chapter 12 Section 1 Pressure A gas exerts pressure on its surroundings. Blow up a balloon. The gas we are most familiar with is the atmosphere, a mixture of mostly elemental nitrogen and oxygen. Pressure
a) List and define all assumptions for multiple OLS regression. These are all listed in section 6.5
 Prof. C. M. Dalton ECN 209A Spring 2015 Practice Problems (After HW1, HW2, before HW3) CORRECTED VERSION Question 1. Draw and describe a relationship with heteroskedastic errors. Support your claim with
Prof. C. M. Dalton ECN 209A Spring 2015 Practice Problems (After HW1, HW2, before HW3) CORRECTED VERSION Question 1. Draw and describe a relationship with heteroskedastic errors. Support your claim with
Investigating the effects of interchanging components used to perform ripple assessments on calibrated vector network analysers
 Abstract Investigating the effects of interchanging components used to perform ripple assessments on calibrated vector network analysers Andrew G Morgan and Nick M Ridler Centre for Electromagnetic and
Abstract Investigating the effects of interchanging components used to perform ripple assessments on calibrated vector network analysers Andrew G Morgan and Nick M Ridler Centre for Electromagnetic and
Introduction. Respiration. Chapter 10. Objectives. Objectives. The Respiratory System
 Introduction Respiration Chapter 10 The Respiratory System Provides a means of gas exchange between the environment and the body Plays a role in the regulation of acidbase balance during exercise Objectives
Introduction Respiration Chapter 10 The Respiratory System Provides a means of gas exchange between the environment and the body Plays a role in the regulation of acidbase balance during exercise Objectives
PROBLEM SET 8. SOLUTIONS April 15, 2004
 Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Gas Laws. Essential Learning Outcomes: 1. Change can be measured. 2. Changes can occur within a substance that alters its identity.
 Gas Laws Gas Laws: Gases and pressures affect our lives every day. From the weather we experience to the air we breathe, it all has to do with gases and pressures. Why do we have wind? Why do we have the
Gas Laws Gas Laws: Gases and pressures affect our lives every day. From the weather we experience to the air we breathe, it all has to do with gases and pressures. Why do we have wind? Why do we have the
CHAPTER 5: VACUUM TEST WITH VERTICAL DRAINS
 CHAPTER 5: VACUUM TEST WITH VERTICAL DRAINS 5.1 Introduction Using surcharging as the sole soil consolidation mean can take a long time to reach the desired soil settlement. Soil consolidation using prefabricated
CHAPTER 5: VACUUM TEST WITH VERTICAL DRAINS 5.1 Introduction Using surcharging as the sole soil consolidation mean can take a long time to reach the desired soil settlement. Soil consolidation using prefabricated
SEALANT BEHAVIOR OF GASKETED-SEGMENTAL CONCRETE TUNNEL LINING
 4 th International Conference on Earthquake Geotechnical Engineering June 25-28, 2007 Paper No.1636 SEALANT BEHAVIOR OF GASKETED-SEGMENTAL CONCRETE TUNNEL LINING Faisal SHALABI 1, Edward CORDING 2, and
4 th International Conference on Earthquake Geotechnical Engineering June 25-28, 2007 Paper No.1636 SEALANT BEHAVIOR OF GASKETED-SEGMENTAL CONCRETE TUNNEL LINING Faisal SHALABI 1, Edward CORDING 2, and
y ) s x x )(y i (x i r = 1 n 1 s y Statistics Lecture 7 Exploring Data , y 2 ,y n (x 1 ),,(x n ),(x 2 ,y 1 How two variables vary together
 Statistics 111 - Lecture 7 Exploring Data Numerical Summaries for Relationships between Variables Administrative Notes Homework 1 due in recitation: Friday, Feb. 5 Homework 2 now posted on course website:
Statistics 111 - Lecture 7 Exploring Data Numerical Summaries for Relationships between Variables Administrative Notes Homework 1 due in recitation: Friday, Feb. 5 Homework 2 now posted on course website:
Elastic Properties of the Centrilobular Emphysematous Space
 Elastic Properties of the Centrilobular Emphysematous Space J. C. HOGG, S. J. NEPSZY, P. T. MACKLEM, and W. M. THURLBECK From the Department of Pathology, McGill University, and the Joint Cardiorespiratory
Elastic Properties of the Centrilobular Emphysematous Space J. C. HOGG, S. J. NEPSZY, P. T. MACKLEM, and W. M. THURLBECK From the Department of Pathology, McGill University, and the Joint Cardiorespiratory
Experiment 11: The Ideal Gas Law
 Experiment 11: The Ideal Gas Law The behavior of an ideal gas is described by its equation of state, PV = nrt. You will look at two special cases of this. Part 1: Determination of Absolute Zero. You will
Experiment 11: The Ideal Gas Law The behavior of an ideal gas is described by its equation of state, PV = nrt. You will look at two special cases of this. Part 1: Determination of Absolute Zero. You will
Process Dynamics, Operations, and Control Lecture Notes - 20
 Lesson 0. Control valves 0.0 Context Controller output is a signal that varies between 0 and 100%. Putting this signal to use requires a final control element, a device that responds to the controller
Lesson 0. Control valves 0.0 Context Controller output is a signal that varies between 0 and 100%. Putting this signal to use requires a final control element, a device that responds to the controller
Aerodynamic Analysis of a Symmetric Aerofoil
 214 IJEDR Volume 2, Issue 4 ISSN: 2321-9939 Aerodynamic Analysis of a Symmetric Aerofoil Narayan U Rathod Department of Mechanical Engineering, BMS college of Engineering, Bangalore, India Abstract - The
214 IJEDR Volume 2, Issue 4 ISSN: 2321-9939 Aerodynamic Analysis of a Symmetric Aerofoil Narayan U Rathod Department of Mechanical Engineering, BMS college of Engineering, Bangalore, India Abstract - The
Analysis of Backward Falls Caused by Accelerated Floor Movements Using a Dummy
 Original Article Analysis of Backward Falls Caused by Accelerated Floor Movements Using a Dummy Hisao NAGATA 1 * and Hisato OHNO 2 1 National Institute of Occupational Safety and Health, 1 4 6 Umezono,
Original Article Analysis of Backward Falls Caused by Accelerated Floor Movements Using a Dummy Hisao NAGATA 1 * and Hisato OHNO 2 1 National Institute of Occupational Safety and Health, 1 4 6 Umezono,
DURING the course of certain investigations it became
 VOLUMETRIC DETERMINATION OF ETHER OR CYCLOPROPANE, CARBON DIOXIDE, NITROUS OXIDE AND OXYGEN IN ANESTHETIC MIXTURES By F. J. PRIME DURING the course of certain investigations it became necessary to be able
VOLUMETRIC DETERMINATION OF ETHER OR CYCLOPROPANE, CARBON DIOXIDE, NITROUS OXIDE AND OXYGEN IN ANESTHETIC MIXTURES By F. J. PRIME DURING the course of certain investigations it became necessary to be able
