Gundersen, 1939), however, it seems unlikely that the limbal circulation. Columbia, Missouri 65201, U.S.A. (Received 25 March 1976)
|
|
|
- Julius Harvey
- 5 years ago
- Views:
Transcription
1 J. Phyaiol. (1977), 270, pp With 2 text-figure8 Printed in Great Britain DIFFUSION OF OXYGEN AT THE ENDOTHELIAL SURFACE OF THE RABBIT CORNEA BY R. E. BARR, M. HENNESSEY* AND V. G. MURPHYt From the Department of Ophthalmology, University of Missouri, Columbia, Missouri 65201, U.S.A. (Received 25 March 1976) SUMMARY 1. An in vitro investigation was made to determine the oxygen tension level required at the endothelial surface of rabbit cornea to produce a net oxygen flux into the cornea across this surface when the epithelial surface was exposed to air. 2. The experimental design was based on a mathematical model which showed that the direction of oxygen flux measured in an agar layer adjacent to the endothelium was the same as the direction of oxygen flux across the endothelial surface. 3. From micro-electrode measurements of oxygen tension in the agar layer, it was found that an oxygen tension greater than 102 mmhg at the endothelial surface was required to cause a net flux of oxygen into the cornea. 4. Comparing this result to the in vivo situation, it was concluded that all layers of the rabbit cornea receive oxygen from the atmosphere under open eye conditions. INTRODUCTION Under open-eye conditions there are three possible sources of oxygen for the cornea: the atmosphere, the aqueous humor and the limbal circulation. In view of the work of Gruber (1894) and others (Bullot, 1904; Gundersen, 1939), however, it seems unlikely that the limbal circulation plays a significant role. Moreover, it seems to be generally agreed (Langham, 1952; Hill & Fatt, 1963; Fatt & Bieber, 1968; Barr & Silver, 1973; Barr & Roetman, 1974, for examples) that the anterior and central regions of the cornea derive their oxygen from the atmosphere, presumably by passive diffusion down a concentration gradient. Only the source of oxygen for the posterior region of the cornea, principally the endothelium, remains * Present address: New Jersey Medical School, Newark, New Jersey. t Present address: Department of Chemical Engineering, Iowa State University, Ames, Iowa. 1-2
2 2 R. E. BARR, M. HENNESSEY AND V. G. MURPHY in question. Some studies (Riley, 1969; Fatt, Freeman & Lin, 1974) seem to indicate that this tissue is supplied by the anterior uveal circulation via the aqueous humor. However, recent in vivo investigations (Barr & Roetman, 1974) have shown that, at least in anaesthetized rabbits, there is a flow of oxygen from the endothelium into the aqueous. In the present study a novel in vitro preparation was employed to determine how the direction of oxygen transport at the posterior endothelial surface varied as a function of the boundary condition at that surface. The results were then compared with what is known about the normal in vivo state and reasonable conclusions drawn concerning the source of oxygen for the posterior region of the cornea under normal open eye conditions. Theory In order to appreciate the experimental design and protocol, an understanding of the mathematical model that was the basis for the experiments is necessary. Therefore a brief description of the model will be given here. P=Pa Cornea x=/, X=O Agar P=P ~ ~ ~ X4 Pb Fig. 1. Diagram of the model upon which the calculations were based. The boundary conditions Pa and pb are oxygen pressures at the surfaces x = 1, and x = -lo, respectively. Consider a model composed of two planar sheets, as shown in Fig. 1. One sheet represents the cornea and the second a layer of agar. The corneal endothelium lies on the agar. From Fick's laws (Crank, 1967), the differential equations describing oxygen diffusion through these layers are d2pl/dx2-q1/dlkl = 0 (11 > x > 0), (1) d2po/dx2 = 0 (0 > x > -lo)' (2) where P. Q. D and k are oxygen tension, oxygen consumption, oxygen diffusivity and oxygen solubility, respectively, and the subscripts 0 and 1 refer to the agar and corneal layers, respectively. These equations may be
3 OXYGEN DIFFUSION INTO CORNEA solved using the boundary conditions P1 = Pa at x = 11, PI = PO and Dlkl(dP1/dx) = Doko(dPo/dx) at x = 0, and PO = Pb at x= -l to yield the following expression for the oxygen tension gradient in the agar layer: dp0/dx = [(Pa - Pb) - Q1J12/2Dlk1]/[10 + (D0k0/D3Lk1)l1]. (3) Recalling that flux is proportional to the negative of the gradient, we see that, if dpo/dx is greater than zero, oxygen diffusion in the agar is in the negative x-direction, i.e. from the cornea into the agar. Likewise, if dpo/dx is less than zero, the opposite is true. Examination of the right side of eqn. (3) reveals that the denominator is always positive; therefore, the sign of the gradient, and hence, the direction of the flux, is solely determined by the quantity [(Pa-Pb)-Q1J2/2D1k1]. Since DlkL(dP1/dx) = Doko(dPo/dx) at x = 0, the sign of dp1/dx must be the same as that of dpo/dx. Hence, the direction of oxygen flux at the endothelial surface is the same as the direction of flux in the agar. Suppose now that the agar layer were not present, as under in vivo conditions, and that the oxygen tension at the posterior endothelial surface (x = 0) were Pb. Under these conditions, the gradient at the surface x = 0 is given by (dp/dx)x=o = [(Pa-Pb)-Q1112/2D1k1]/11. (4) Since 1l must be positive, the sign of the gradient and hence, the direction of the oxygen flux, is again determined by the quantity [(Pa - Pb) - Q,112/2Dlkl]. Thus, for any given Pa and Pb, oxygen flux at the posterior endothelial surface in vivo will have the same direction as the flux observed in the agar layer of the in vitro situation described in Fig. 1. It can also be shown that this conclusion holds when the cornea is simulated by a three-layered model, epithelium-stroma-endothelium, each having (different) characteristic values for Q, D and k. 3 METHODS An in vitro system involving an excised cornea was developed in which oxygen fluxes either into or out of the endothelial surface could be determined. The experimental apparatus employed is diagrammed in Fig. 2. Corneas with a scleral rim were carefully extracted from eyes of New Zealand White rabbits (2-3 kg) terminated by an overdose of Nembutal or air embolism. The procedure employed to extricate the cornea and scleral rim was such that no corneal wrinkling occurred in a high percentage of the experiments. The cornea was placed on a preset, premeasured, sterile agar mould and its thickness measured using an electrode mounted to a micromanipulator. The cornea and agar mould were then mounted in the chamber as shown in Fig. 2. The upper section was filled with a Krebs bicarbonate Ringer solution adjusted to ph of 7-4. The liquid height in the column produced a pressure of about 12 mmhg at the cornea. A pointed oxygen micro-electrode, described in more detail in Barr & Silver (1973), mounted on a long glass shaft attached to a
4 4 R. E. BARR, M. HENNESSEY AND V. G. MURPHY micromanipulator was inserted down the column and into the agar mould. The Ringer solution was equilibrated at various oxygen tensions by bubbling gases with known oxygen partial pressures through the solution. The oxygen partial pressure in the equilibrating gas was continuously monitored with a Beckman M735 oxygen analyzer placed in the gas line. Bubbling was continued until the microelectrode current reached a steady-state value, which usually required min. /Ag-gCI -Reference electrode. Fluid level * t J Bathing solution Pb. B-Rubber gasket * 0~1-Agar '- Cornea and sclera Pa a~~~~~~~p Fig. 2. Diagram of the main part of the experimental apparatus. This vessel was surrounded by a thermostatically controlled water bath. The electrode shaft at the top was attached to a micromanipulator. This indicated that the Ringer solution had equilibrated at the new oxygen tension level and that a new steady-state oxygen gradient through the agar had been established. The entire chamber was surrounded by a water-jacket maintained at 360 C, and the section below the cornea was continuously flushed with air that had passed through a water-trap at room temperature and then through a tube coiled in the 36 C water-jacket. Thus the corneal epithelium was exposed to air about 300 C with a relative humidity of about %. After the Ringer and cornea-agar system had equilibrated for a given oxygen tension in the gas permeating the Ringer, the gas was shut off and the
5 OXYGEN DIFFUSION INTO CORNEA 5 micro-electrode was moved down through the agar mould for a distance of about mm in 0-4 mm steps. At each step the electrode current was recorded. The direction of movement was then reversed and the set of measurements repeated in reverse order. In this way the oxygen gradient through the agar mould was determined and any drift in electrode current could be taken into account. Depending upon the direction of the observed gradient, the oxygen tension of the equilibrating gas was either increased or decreased until the value producing a zero gradient in the agar was determined (or at least closely bracketed). For most experiments, a minimum of five gradient determinations were made. When the initial choice of Pb yielded a zero gradient through the agar, two more values of Pb' one above and one below the initial value, were also included in the experiment to ensure that the system was functioning properly. Following an experimental run, the cornea and agar mould were removed from the test cell and corneal thickness remeasured. At this time a visual examination of the cornea was also made. All experiments were completed within 2 hr following excision of the cornea. RESULTS The results of the experiments, for Pa = 155 mmhg, are shown in Table 1. Fewer data points are reported for Pb at zero net flux because it was not always possible to experimentally obtain this condition. In all cases, however, values of Pb that closely bracketed this condition (i.e values that produced slightly positive and slightly negative gradients) were determined. TABLE 1. Values of Pb producing different directions of net oxygen flux at the corneal surface. Each value is the mean + 1 S.D. deviation (number of corneas contributing to the data) Oxygen flux direction Out of cornea Zero net flux Into cornea Solution Po2 (Pb in mmhg) 95-9 ± 7-1 (35) ± 5-5 (28) (35) Corneal thickness measurements indicated an average central thickness of mm. The average increase in corneal thickness during an experiment was mm. In separate experiments, the accuracy of the thickness measuring technique was determined to be mm. Visual examination of the corneas following experiments showed no corneal clouding had developed. No significant differences were seen in results obtained from animals terminated by air embolism or by an overdose of Nembutal. DISCUSSION Anytime the cornea is manipulated during an experimental procedure, the question of possible corneal damage arises. In an experiment investigating corneal metabolism, this question is paramount. There are two aspects to consider. (1) Is the corneal metabolism during the experiment
6 6 R. E. BARR, M. HENNESSEY AND V. G. MURPHY the same as normal in vivo corneal metabolism and (2) does this metabolism change during the experiment? There was no way the first question could be quantitatively answered for the present experiments. There was, however, quantitative evidence that corneal metabolism did not change significantly during the course of the experiments. The procedure used to find a zero oxygen gradient in the agar required sequential changes in Pb such that its value was first above, then below, then above, then below, etc., the zero gradient value of Pb by ever decreasing amounts. Had corneal metabolism been changing, the zero gradient value of Pb would have changed causing changes in the direction of observed gradients for some of the Pb values near the zero gradient Pb. This did not occur for the data reported. In the present study it was experimentally determined that, with the epithelium exposed to air (Pa = 155 mmhg), the oxygen tension at the posterior endothelial surface (Pb) had to exceed mmhg before there was a flux of oxygen into the cornea at that surface. This is consistent with the results of Freeman (1972). In this earlier work, measurements of oxygen tension at the endothelial surface of a whole cornea mounted such that the epithelium was in contact with air and the endothelium with an oxygen impermeable barrier yielded values of and mmhg in two sets of experiments. These are not significantly different from the mmhg reported here. Assuming that corneal Q and Dk under in vivo conditions are similar to what they were in the in vitro experiments, it then follows that the cornea cannot receive oxygen from the aqueous humor unless the oxygen tension in the latter is above mmhg. Since this value is equal to or greater than the oxygen tension in arterial blood (Prince, 1964), it must be concluded that under open-eye conditions all layers of the rabbit cornea receive their oxygen from the atmosphere. This is consistent with the earlier work of Barr & Roetman (1974) in which a steep oxygen gradient (sloping away from the endothelium) was found in the anterior chamber of anaesthetized rabbits. For human eyes, the situation may not be so obvious. If one assumes that the human cornea has the same overall oxygen consumption (Q) and oxygen permeability (Dk) as the rabbit cornea, then manipulation of eqn. (4) using 11 = 0 5 mmhg yields a value of Pb = 79 mmhg for the zero flux condition. Kleifeld & Neumann (1959) measured an oxygen tension of 52-6 mmhg in human aqueous humor, and Thiel (1967) obtained a value of 59.7 mmhg. Since these are about 30 % lower than the zero flux value computed above, one would expect that under open eye conditions oxygen would diffuse from the endothelium to the aqueous humor in humans as well as rabbits. However, in a recent computer study
7 OXYGEN DIFFUSION INTO CORNEA 7 (Fatt et al. 1974) an opposite flux was calculated using an assumed Pb of 55 mmhg and earlier (Freeman, 1972; Freeman & Fatt, 1972) experimentally determined values of Q and Dk for the three principal layers of the cornea. At present, it cannot be determined whether this discrepancy is due to differences in the over-all Q and Dk values for human vs. rabbit corneas or to inaccuracies in the extremely difficult measurements required to determine Q and Dk for the individual layers. Eqn. (4) above can be used to determine a value of Q/Dk for the whole cornea. For the zero flux condition at 360 C, Pa- Pb was found to be 52-5 mmhg; therefore, using the average corneal thickness of 418,um, one obtains Q/Dk = 6*0 x 104 mmhg/cm2. Analysis of the data from Freeman (1972) and Freeman & Fatt (1972) gives an over-all Q/Dk of 9-6 x 104 mmhg/cm2 at 230 C. This value would probably increase for a temperature of C, since Q approximately doubles for every 100 C increase and Dk only increases at a rate of about 1-2 % per 0 C. A still higher estimate for Q/Dk at 370 C, about 97 x 104 mmhg/cm2, can be obtained from the data of Langham (1952) and Heald & Langham (1956). This is an order of magnitude greater than the other two values and appears to be primarily due to a difference in values for DM. For example, Heald & Langham (1956) report a stromal Dk of 0 68 x (ml. 02.cm2)/(ml. tissue.mmhg.sec) at 370C; whereas, Freeman & Fatt (1972) give 30 x (ml. 02 cm2)/(ml. tissue. mmhg. sec) at 230 C. Assuming a rise of 1% per 0 C, the latter value would be about 3-5 x (ml. 02. cm2)/ (ml. tissue. mmhg. sec) at 370 C. Since, in the present study, the surface temperature at the epithelium was 300 C, the Q of the epithelium was probably somewhat lower than it would be at 360 C. This would partially explain the low Q/Dk value obtained; however, a corrected value would still be considerably below the other values presented. Further explanation of these disparities is not possible at this time. Finally, it is worth noting that the experimental technique employed in this study made use of uncalibrated oxygen micro-electrodes. The approach taken was such that it was only necessary to determine the direction of the oxygen gradient in the agar layer produced by a given oxygen tension in the Ringer. Thus, since the latter was known from the oxygen partial pressure in the equilibrating gas, the micro-electrode measurements were only used to determine the direction (rather than the magnitude) of the resulting gradient. This eliminated many of the difficulties inherent in experiments requiring stable, calibrated microelectrodes.
8 8 R. E. BARR, M. HENNESSEY AND V. G. MURPHY REFERENCES BARR, R. E. & ROETMAN, E. L. (1974). Oxygen gradients in the anterior chamber of rabbits. Invest. Ophthal. 13, BARR, R. E. & SILVER, I. A. (1973). Effects of corneal environment on oxygen tension in the anterior chambers of rabbits. Invest. Ophthal. 12, BULLOT, G. (1904). On the action of oxygen at low and high pressure upon the corneal endothelium. J. Physiol. 31, CRANK, J. (1967). The Mathematics of Diffusion, 1st edn., pp London: Oxford Clarendon Press. FArT, I. & BIEBER, M. T. (1968). The steady-state distribution of oxygen and carbon dioxide in the in vivo cornea. I. The open eye in air and the closed eye. Expl Eye Re8. 7, FATr, I., FREEMAN, R. D. & LIN, D. (1974). Oxygen tension distributions in the cornea: A re-examination. Expl Eye Re8. 18, FREEMAN, R. D. (1972). Oxygen consumption by the component layers of the cornea. J. Physiol. 225, FREEMAN, R. D. & FATT, I. (1972). Oxygen permeability of the limiting layers of the cornea. Biophys. J. 12, GRUBER, R. (1894). tvber Rostablagerung in der Hornhaut. Albrecht v. Graefes Arch. Ophthal. 40, GUNDERSEN, T. (1939). Vascular obliteration for various types of keratitis: Its significance regarding nutrition of corneal epithelium. Albrecht v. Graefes Arch. Ophthal. 21, HEALD, D. & LANGHAM, M. E. (1956). Permeability of the cornea and the bloodaqueous barrier to oxygen. Br. J. Ophthal. 40, HILL, R. M. & FATT, I. (1963). Oxygen uptake from a reservoir of limited volume by the human cornea in vivo. Science, N.Y. 142, KLEIFELD, VON 0. & NEUMANN, H. G. (1959). Der Sauerstoffgehalt des menschlichen Kammerwassers. Klin. Mbl. Augenheilk. 135, LANGHAM, M. (1952). Utilization of oxygen by the component layers of the living cornea. J. Physiol. 117, PRINCE, J. H. (1964). The Rabbit in Eye Research, p Springfield, Illinois: Charles C. Thomas. RILEY, M. V. (1969). Glucose and oxygen utilization by the rabbit cornea. Expl Eye Res. 8, THIEL, H. J. (1967). Die gleichzeitige Bestimmung von ph, PCO2 and P02 im Kammerwasser des Menschen. Albrecht v. Graefes Arch. Ophthal. 174,
Oxygen tension under a contact lens. Kenneth A. Poise and Marianne Decker
 Oxygen tension under a contact lens Kenneth A. Poise and Marianne Decker Cornea! thickness changes were measured on human subjects who wore gel lenses that varied in center thickness. Using these measurements
Oxygen tension under a contact lens Kenneth A. Poise and Marianne Decker Cornea! thickness changes were measured on human subjects who wore gel lenses that varied in center thickness. Using these measurements
H.ydrophilic soft contact lenses made
 Permeability of hydrophilic contact lenses Dennis R. Morrison and Henry F. Edelhauser Oxygen permeability and water diffusion characteristics were determined for several types of hydroxyethyl-methacrylate
Permeability of hydrophilic contact lenses Dennis R. Morrison and Henry F. Edelhauser Oxygen permeability and water diffusion characteristics were determined for several types of hydroxyethyl-methacrylate
supply cannot provide metabolites for the entire tissue. Therefore, the endothelium).
 J. Physiol. (1972), 225, pp. 15-32 15 With 5 text-ftgurew Printed in Great Britain OXYGEN CONSUMPTION BY THE COMPONENT LAYERS OF THE CORNEA BY RALPH D. FREEMAN From the Group in Biophy8ic8, School of Optometry,
J. Physiol. (1972), 225, pp. 15-32 15 With 5 text-ftgurew Printed in Great Britain OXYGEN CONSUMPTION BY THE COMPONENT LAYERS OF THE CORNEA BY RALPH D. FREEMAN From the Group in Biophy8ic8, School of Optometry,
CHAPTER 3 RESULTS AND DISCUSSION
 CHAPTER 3 RESULTS AND DISCUSSION The research included the development and verification of the earlier apparatus, and the collection and validation of moisture diffusion data under both isothermal and
CHAPTER 3 RESULTS AND DISCUSSION The research included the development and verification of the earlier apparatus, and the collection and validation of moisture diffusion data under both isothermal and
BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY
 BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY Dr.R.Selvakumar.M.D.D.A.DNB Professor of Anaesthesiology, K.A.P.Viswanatham Govt medical college, Trichy. The current practice of Anaesthesiology demands knowledge
BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY Dr.R.Selvakumar.M.D.D.A.DNB Professor of Anaesthesiology, K.A.P.Viswanatham Govt medical college, Trichy. The current practice of Anaesthesiology demands knowledge
Section Two Diffusion of gases
 Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Table of Contents. By Adam Hollingworth
 By Adam Hollingworth Table of Contents Oxygen Cascade... 2 Diffusion... 2 Laws of Diffusion... 2 Diffusion & Perfusion Limitations... 3 Oxygen Uptake Along Pulmon Capillary... 4 Measurement of Diffusing
By Adam Hollingworth Table of Contents Oxygen Cascade... 2 Diffusion... 2 Laws of Diffusion... 2 Diffusion & Perfusion Limitations... 3 Oxygen Uptake Along Pulmon Capillary... 4 Measurement of Diffusing
Collin County Community College. Lung Physiology
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
RESPIRATORY GAS EXCHANGE
 RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
PICU Resident Self-Study Tutorial The Basic Physics of Oxygen Transport. I was told that there would be no math!
 Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
(a) (i) Describe how a large difference in oxygen concentration is maintained between a fish gill and the surrounding water.
 1. Answers should be written in continuous prose. Credit will be given for biological accuracy, the organisation and presentation of information and the way in which an answer is expressed. Fick s law
1. Answers should be written in continuous prose. Credit will be given for biological accuracy, the organisation and presentation of information and the way in which an answer is expressed. Fick s law
Lung Volumes and Capacities
 Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Life 23 - Respiration in Air Raven & Johnson Ch. 53 (part)
 1 Life 23 - Respiration in Air Raven & Johnson Ch. 53 (part) Objectives 1: Compare the properties of air and water as media for respiration, and the consequences for the evolution of respiratory systems
1 Life 23 - Respiration in Air Raven & Johnson Ch. 53 (part) Objectives 1: Compare the properties of air and water as media for respiration, and the consequences for the evolution of respiratory systems
THE literature on this subject, which was reviewed recently (CAMPBELL, doses of amytal, and in addition received A.C.E. mixture during the
 -~~ -v GAS TENSIONS IN THE MUCOUS MEMBRANE OF THE STOMACH AND SMALL INTESTINE. By J. ARGYLL CAMPBELL. From the National Institute for Medical Research, Hampstead. (With six figures in the text.) (Received
-~~ -v GAS TENSIONS IN THE MUCOUS MEMBRANE OF THE STOMACH AND SMALL INTESTINE. By J. ARGYLL CAMPBELL. From the National Institute for Medical Research, Hampstead. (With six figures in the text.) (Received
Aerobic reoxidation in marine sediments
 Aerobic reoxidation in marine sediments This exercise consists of three small experiments\demonstrations that should be run in parallel. Read the whole description and divide the work between the members
Aerobic reoxidation in marine sediments This exercise consists of three small experiments\demonstrations that should be run in parallel. Read the whole description and divide the work between the members
Respiratory System. Part 2
 Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
ALVEOLAR - BLOOD GAS EXCHANGE 1
 ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
Retinal vascular response to breathing increased carbon dioxide and oxygen concentrations. Regina Frayser and John B. Hickam
 Retinal vascular response to breathing increased carbon dioxide and oxygen concentrations Regina Frayser and John B. Hickam The retina has a high rate of oxygen consumption, and the retinal vessels are
Retinal vascular response to breathing increased carbon dioxide and oxygen concentrations Regina Frayser and John B. Hickam The retina has a high rate of oxygen consumption, and the retinal vessels are
EMERGENCY MEDICINE AND THE LABORATORY. Ehsan Bolvardi(MD)
 EMERGENCY MEDICINE AND THE LABORATORY Ehsan Bolvardi(MD) bolvardie@mums.ac.ir Objective ED and laboratory Inappropriate use of laboratory ABG sampling ABG sampling error Introduction The laboratory is
EMERGENCY MEDICINE AND THE LABORATORY Ehsan Bolvardi(MD) bolvardie@mums.ac.ir Objective ED and laboratory Inappropriate use of laboratory ABG sampling ABG sampling error Introduction The laboratory is
found that stretching increased the oxygen usage within limits but
 v 1 -. 1 qa,.x.- U I di i - -10 1. 1 4 INFLUENCE OF RESTING LENGTH ON THE OXYGEN USE OF PLAIN MUSCLE. By J. CHRISTODoss DAVID. From the Department of Pharmacology, Edinburgh University. (Received for publication
v 1 -. 1 qa,.x.- U I di i - -10 1. 1 4 INFLUENCE OF RESTING LENGTH ON THE OXYGEN USE OF PLAIN MUSCLE. By J. CHRISTODoss DAVID. From the Department of Pharmacology, Edinburgh University. (Received for publication
I Physical Principles of Gas Exchange
 Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
O2100C Oxygen Measurement Module Technical Use Notes do not use other wall adapters with the O2100Cmodule. 10% / V 5% / V 2% / V 1% / V 10% / V
 O2100C Oxygen Measurement Module The O2100C module measures the partial pressure of O2 and thus the module output is proportional to the pressure in the sample cell. Gas sampled must be free of liquids
O2100C Oxygen Measurement Module The O2100C module measures the partial pressure of O2 and thus the module output is proportional to the pressure in the sample cell. Gas sampled must be free of liquids
LAB 06 Organismal Respiration
 LAB 06 Organismal Respiration Objectives: To learn how a respirometer can be used to determine a respiration rate. Identify and explain the effect of seed germination on cell respiration. To design and
LAB 06 Organismal Respiration Objectives: To learn how a respirometer can be used to determine a respiration rate. Identify and explain the effect of seed germination on cell respiration. To design and
Experiment. THE RELATIONSHIP BETWEEN VOLUME AND TEMPERATURE, i.e.,charles Law. By Dale A. Hammond, PhD, Brigham Young University Hawaii
 Experiment THE RELATIONSHIP BETWEEN VOLUME AND TEMPERATURE, i.e.,charles Law By Dale A. Hammond, PhD, Brigham Young University Hawaii The objectives of this experiment are to... LEARNING OBJECTIVES introduce
Experiment THE RELATIONSHIP BETWEEN VOLUME AND TEMPERATURE, i.e.,charles Law By Dale A. Hammond, PhD, Brigham Young University Hawaii The objectives of this experiment are to... LEARNING OBJECTIVES introduce
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/12
 LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
partial pressure is to be applied to the dissociation curve of fully oxygenated
 6I2. I27. I THE DETERMINATION OF THE CARBON DIOXIDE CONTENT OF THE MIXED VENOUS BLOOD. Part I. The effect of oxygenation and the critical oxygen tension. BY M. C. G. ISRAELS (Platt Physiological Scholar)
6I2. I27. I THE DETERMINATION OF THE CARBON DIOXIDE CONTENT OF THE MIXED VENOUS BLOOD. Part I. The effect of oxygenation and the critical oxygen tension. BY M. C. G. ISRAELS (Platt Physiological Scholar)
Fall 2004 Homework Problem Set 9 Due Wednesday, November 24, at start of class
 0.30 Fall 004 Homework Problem Set 9 Due Wednesday, November 4, at start of class Part A. Consider an iron surface which serves as a catalyst for the production of ammonia from nitrogen and hydrogen. The
0.30 Fall 004 Homework Problem Set 9 Due Wednesday, November 4, at start of class Part A. Consider an iron surface which serves as a catalyst for the production of ammonia from nitrogen and hydrogen. The
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1
 AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
Physiology Unit 4 RESPIRATORY PHYSIOLOGY
 Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
2. State the volume of air remaining in the lungs after a normal breathing.
 CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
 Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Oxygen supply to the retina from the retinal and choroidal circulations at normal and increased arterial oxygen tensions
 Oxygen supply to the retina from the retinal and choroidal circulations at normal and increased arterial oxygen tensions C. T. ollery, C. J. Bulpitt, and Eva M. Kohner uring oxygen breathing small retinal
Oxygen supply to the retina from the retinal and choroidal circulations at normal and increased arterial oxygen tensions C. T. ollery, C. J. Bulpitt, and Eva M. Kohner uring oxygen breathing small retinal
Gas Laws. Introduction
 Gas Laws Introduction In 1662 Robert Boyle found that, at constant temperature, the pressure of a gas and its volume are inversely proportional such that P x V = constant. This relationship is known as
Gas Laws Introduction In 1662 Robert Boyle found that, at constant temperature, the pressure of a gas and its volume are inversely proportional such that P x V = constant. This relationship is known as
GASEOUS EXCHANGE 17 JULY 2013
 GASEOUS EXCHANGE 17 JULY 2013 Lesson Description In this lesson we: Discuss what is gaseous exchange? Consider requirements of an efficient gaseous exchange surface. Look at diversity in gas exchange systems.
GASEOUS EXCHANGE 17 JULY 2013 Lesson Description In this lesson we: Discuss what is gaseous exchange? Consider requirements of an efficient gaseous exchange surface. Look at diversity in gas exchange systems.
Oxygen convulsions are believed by many workers to be caused by an accumulation
 272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
AP Biology Lab - Cell Respiration
 AP Biology Lab - Cell Respiration This investigation uses respirometry techniques to calculate the rate of oxygen consumption (cellular respiration) in germinating pea seeds. The effect of temperature
AP Biology Lab - Cell Respiration This investigation uses respirometry techniques to calculate the rate of oxygen consumption (cellular respiration) in germinating pea seeds. The effect of temperature
CHAPTER 3: The respiratory system
 CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
By: Aseel Jamil Al-twaijer. Lec : physical principles of gas exchange
 By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
Ch. 11 Mass transfer principles
 Transport of chemical species in solid, liquid, or gas mixture Transport driven by composition gradient, similar to temperature gradients driving heat transport We will look at two mass transport mechanisms,
Transport of chemical species in solid, liquid, or gas mixture Transport driven by composition gradient, similar to temperature gradients driving heat transport We will look at two mass transport mechanisms,
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation
 Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary
 ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D.
 Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Gerald D. Anderson. Education Technical Specialist
 Gerald D. Anderson Education Technical Specialist The factors which influence selection of equipment for a liquid level control loop interact significantly. Analyses of these factors and their interactions
Gerald D. Anderson Education Technical Specialist The factors which influence selection of equipment for a liquid level control loop interact significantly. Analyses of these factors and their interactions
RESPIRATORY REGULATION DURING EXERCISE
 RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
Lecture 8 questions and answers The Biological Pump
 Lecture 8 questions and answers The Biological Pump (1) How long would it take a particle of about 2micron in size and a density of 1.5 g/cm 3 to get to the bottom of the sea (4000m)? How do particles
Lecture 8 questions and answers The Biological Pump (1) How long would it take a particle of about 2micron in size and a density of 1.5 g/cm 3 to get to the bottom of the sea (4000m)? How do particles
Problem Solving. Gas Laws
 Skills Worksheet Problem Solving Gas Laws Chemists found that there were relationships among temperature, volume, pressure, and quantity of a gas that could be described mathematically. This chapter deals
Skills Worksheet Problem Solving Gas Laws Chemists found that there were relationships among temperature, volume, pressure, and quantity of a gas that could be described mathematically. This chapter deals
Gas Exchange in Animals. Uptake of O2 from environment and discharge of CO2. Respiratory medium! water for aquatic animals, air for terrestial
 Gas Exchange in Animals Uptake of O2 from environment and discharge of CO2 Respiratory medium! water for aquatic animals, air for terrestial Respiratory surface! skin, gills, lungs Circulatory System O2/CO2
Gas Exchange in Animals Uptake of O2 from environment and discharge of CO2 Respiratory medium! water for aquatic animals, air for terrestial Respiratory surface! skin, gills, lungs Circulatory System O2/CO2
Section Three Gas transport
 Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
The Variation of Muscle Oxygen Consumption With Velocity of Shortening
 The Variation of Muscle Oxygen Consumption With Velocity of Shortening R.J. BASKIN From the Department of Zoology, University of California, Davis ABSTRACT Total oxygen consumption following contraction
The Variation of Muscle Oxygen Consumption With Velocity of Shortening R.J. BASKIN From the Department of Zoology, University of California, Davis ABSTRACT Total oxygen consumption following contraction
Infiltration and Air Pressure Build-up
 1 Introduction Infiltration and Air Pressure Build-up This air flow example illustrates how the build-up of air pressure in advance of a wetting front can inhibit water movement. The example is based on
1 Introduction Infiltration and Air Pressure Build-up This air flow example illustrates how the build-up of air pressure in advance of a wetting front can inhibit water movement. The example is based on
Then the partial pressure of oxygen is x 760 = 160 mm Hg
 1 AP Biology March 2008 Respiration Chapter 42 Gas exchange occurs across specialized respiratory surfaces. 1) Gas exchange: the uptake of molecular oxygen (O2) from the environment and the discharge of
1 AP Biology March 2008 Respiration Chapter 42 Gas exchange occurs across specialized respiratory surfaces. 1) Gas exchange: the uptake of molecular oxygen (O2) from the environment and the discharge of
The physiological functions of respiration and circulation. Mechanics. exercise 7. Respiratory Volumes. Objectives
 exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
STEAM STERILIZABLE POLAROGRAPHIC OXYGEN ELECTRODE
 STEAM STERILIZABLE POLAROGRAPHIC OXYGEN ELECTRODE GENERAL INFORMATION This family of dissolved oxygen electrodes is of the steam sterilizable polarographic type, designed to be interchangeable with NBS
STEAM STERILIZABLE POLAROGRAPHIC OXYGEN ELECTRODE GENERAL INFORMATION This family of dissolved oxygen electrodes is of the steam sterilizable polarographic type, designed to be interchangeable with NBS
C 6 H 12 O 6 + 6O 2 6H CO kilocalories of energy/mole of glucose
 Objectives Before doing this lab you should understand respiration, dormancy, and germination. After doing this lab you should be able to relate gas production to respiration rate. Introduction Aerobic
Objectives Before doing this lab you should understand respiration, dormancy, and germination. After doing this lab you should be able to relate gas production to respiration rate. Introduction Aerobic
CHAPTER 3: The cardio-respiratory system
 : The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
: The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
The Physiologic Basis of DLCO testing. Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan
 The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
Gilbert Kirss Foster. Chapter 10. Properties of Gases The Air We Breathe
 Gilbert Kirss Foster Chapter 10 Properties of Gases The Air We Breathe Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4
Gilbert Kirss Foster Chapter 10 Properties of Gases The Air We Breathe Chapter Outline 10.1 The Properties of Gases 10.2 Effusion and the Kinetic Molecular Theory of Gases 10.3 Atmospheric Pressure 10.4
Pulmonary Circulation
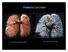 Pulmonary Circulation resin cast of pulmonary arteries resin cast of pulmonary veins Blood Flow to the Lungs Pulmonary Circulation Systemic Circulation Blood supply to the conducting zone provided by the
Pulmonary Circulation resin cast of pulmonary arteries resin cast of pulmonary veins Blood Flow to the Lungs Pulmonary Circulation Systemic Circulation Blood supply to the conducting zone provided by the
Inquiry Investigation: Factors Affecting Photosynthesis
 Inquiry Investigation: Factors Affecting Photosynthesis Background Photosynthesis fuels ecosystems and replenishes the Earth's atmosphere with oxygen. Like all enzyme-driven reactions, the rate of photosynthesis
Inquiry Investigation: Factors Affecting Photosynthesis Background Photosynthesis fuels ecosystems and replenishes the Earth's atmosphere with oxygen. Like all enzyme-driven reactions, the rate of photosynthesis
To derive from experiment the relationships between Pressure (P), Volume (V), Temperature (T), and Water Solubility of gases.
 PROPERTIES OF GASES: PRESSURE, VOLUME, TEMPERATURE, & SOLUBILITY RELATIONSHIPS PURPOSE: To derive from experiment the relationships between Pressure (P), Volume (V), Temperature (T), and Water Solubility
PROPERTIES OF GASES: PRESSURE, VOLUME, TEMPERATURE, & SOLUBILITY RELATIONSHIPS PURPOSE: To derive from experiment the relationships between Pressure (P), Volume (V), Temperature (T), and Water Solubility
Life 24 - Blood and Circulation Raven & Johnson Ch 52 & 53 (parts)
 1 Life 24 - Blood and Circulation Raven & Johnson Ch 52 & 53 (parts) Objectives 1: Understand the importance of oxygen carrier molecules in respiration 2: Describe the characteristics and locations of
1 Life 24 - Blood and Circulation Raven & Johnson Ch 52 & 53 (parts) Objectives 1: Understand the importance of oxygen carrier molecules in respiration 2: Describe the characteristics and locations of
Dissolved Oxygen Meter. ino2. Instruction Manual. Serial No..
 Innovative Instruments, Inc. http://www.2in.com Dissolved Oxygen Meter ino2 Instruction Manual Serial No.. Innovative Instruments, Inc. 8533 Queen Brooks Ct. Tampa, FL 33637. USA Phone: 813-727-0676. Fax:
Innovative Instruments, Inc. http://www.2in.com Dissolved Oxygen Meter ino2 Instruction Manual Serial No.. Innovative Instruments, Inc. 8533 Queen Brooks Ct. Tampa, FL 33637. USA Phone: 813-727-0676. Fax:
αo 2 : solubility coefficient of O 2
 Version 2006 Dr. Puntarica Suwanprathes 1) Fick s law of diffusion 2) facts which limit gas transfer 3) diffusion capacity gas volume gaseous phase dissolved gas exert pressure*** Solubility of Gas C =P.
Version 2006 Dr. Puntarica Suwanprathes 1) Fick s law of diffusion 2) facts which limit gas transfer 3) diffusion capacity gas volume gaseous phase dissolved gas exert pressure*** Solubility of Gas C =P.
APPENDIX. working blood volume was also rather large; Evans, Grande, and. equilibrated to the new mixture is partially dependent upon the rate
 612.172-5 APPENDIX A SIMPLIFIED HEART OXYGENATOR CIRCUIT FOR BLOOD- FED HEARTS. By J. YULE BOG-UE and R. A. GREGORY.' SINCE 1934 studies on the carbohydrate metabolism of the blood-fed heart without lungs
612.172-5 APPENDIX A SIMPLIFIED HEART OXYGENATOR CIRCUIT FOR BLOOD- FED HEARTS. By J. YULE BOG-UE and R. A. GREGORY.' SINCE 1934 studies on the carbohydrate metabolism of the blood-fed heart without lungs
ISE-730 Oxygen Electrode and DO2-100 Currentto-Voltage
 Technical Note ISE-730 and DO2-100 Overview In 1954, Dr. Leland Clark invented the first membrane-covered electrode designed to measure the concentration of oxygen in blood, or solution. This electrode
Technical Note ISE-730 and DO2-100 Overview In 1954, Dr. Leland Clark invented the first membrane-covered electrode designed to measure the concentration of oxygen in blood, or solution. This electrode
States of Matter Review
 States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
States of Matter Review May 13 8:16 PM Physical States of Matter (Phases) Solid Liquid Melting Gas Condensation Freezing Evaporation Deposition Sublimation Sep 13 6:04 PM 1 May 13 8:11 PM Gases Chapter
The Gas Laws: Boyle's Law and Charles Law
 Exercise 6 Page 1 Illinois Central College CHEMISTRY 130 Name The Gas Laws: Boyle's Law and Charles Law Objective The simple laws governing the properties of gases can be readily demonstrated experimentally.
Exercise 6 Page 1 Illinois Central College CHEMISTRY 130 Name The Gas Laws: Boyle's Law and Charles Law Objective The simple laws governing the properties of gases can be readily demonstrated experimentally.
(fig. 3) must be at the same temperature as the water in this chamber CALORIMETRIC STUDIES OF THE EXTREMITIES
 CALORIMETRIC STUDIES OF THE EXTREMITIES II. EXPERIMENTAL APPARATUS AND PROCEDURES' By ROY KEGERREIS (Received for publication July 1, 1926) The calorimeter used in these experiments is a modification of
CALORIMETRIC STUDIES OF THE EXTREMITIES II. EXPERIMENTAL APPARATUS AND PROCEDURES' By ROY KEGERREIS (Received for publication July 1, 1926) The calorimeter used in these experiments is a modification of
TECHNICAL BULLETIN. TriControls. Control Materials for Liquid QC and Calibration Verification
 i-stat TECHNICAL BULLETIN Tris Materials for Liquid QC and OVERVIEW As part of the READi initiative (Responds, Enhances, And Delivers innovations), Abbott Point of Care (APOC) has released a new set of
i-stat TECHNICAL BULLETIN Tris Materials for Liquid QC and OVERVIEW As part of the READi initiative (Responds, Enhances, And Delivers innovations), Abbott Point of Care (APOC) has released a new set of
Biology Unit 2, Structure of Life, Lab Activity 2-3
 Biology Unit 2, Structure of Life, Lab Activity 2-3 Cellular respiration is the release of energy from organic compounds by metabolic chemical oxidation in the mitochondria within each cell. Cellular respiration
Biology Unit 2, Structure of Life, Lab Activity 2-3 Cellular respiration is the release of energy from organic compounds by metabolic chemical oxidation in the mitochondria within each cell. Cellular respiration
Gas volume and pressure are indirectly proportional.
 Section 2 The Gas Laws Key Terms Boyle s law Charles s law combined gas law absolute zero Gay-Lussac s law Scientists have been studying physical properties of gases for hundreds of years In 1662, Robert
Section 2 The Gas Laws Key Terms Boyle s law Charles s law combined gas law absolute zero Gay-Lussac s law Scientists have been studying physical properties of gases for hundreds of years In 1662, Robert
Applied Physics Topics 2
 Applied Physics Topics 2 Dr Andrey Varvinskiy Consultant Anaesthetist Torbay Hospital, UK EDAIC Paper B Lead and Examiner TOPICS 2 Gas Laws Other Laws: Dalton, Avogadro Critical temperature Critical pressure
Applied Physics Topics 2 Dr Andrey Varvinskiy Consultant Anaesthetist Torbay Hospital, UK EDAIC Paper B Lead and Examiner TOPICS 2 Gas Laws Other Laws: Dalton, Avogadro Critical temperature Critical pressure
Alveolus and Respiratory Membrane
 Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
11/22/ (4) Harmonization: <846> SPECIFIC SURFACE AREA
 BRIEFING 846 Specific Surface Area, USP 27 page 2385. The European Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the Specific Surface Area General Chapter, as part
BRIEFING 846 Specific Surface Area, USP 27 page 2385. The European Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the Specific Surface Area General Chapter, as part
OXYGEN CONSUMPTION AND TEMPERATURE IN THE AQUATIC ENVIRONMENT
 OXYGEN CONSUMPTION AND TEMPERATURE IN THE AQUATIC ENVIRONMENT BACKGROUND READING Animal Physiology by Hill, Wyse & Anderson, 2004: pp. 130 139 & 198 201. PRE-LAB (Due at the start of the lab) ** In your
OXYGEN CONSUMPTION AND TEMPERATURE IN THE AQUATIC ENVIRONMENT BACKGROUND READING Animal Physiology by Hill, Wyse & Anderson, 2004: pp. 130 139 & 198 201. PRE-LAB (Due at the start of the lab) ** In your
Respiratory Lecture Test Questions Set 3
 Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
UNIT 15 WATER HAMMER AND SURGE TANKS
 UNT 15 WATER HAMMER AND SURGE TANKS Structure 15.1 ntroduction Objectives 15.2 Water Hammer 15.2.1 Expression for Rise in Pressure 15.3 Rapid Acceleration of Flow 15.4 Surge Tanks 15.5 Summary 15.6 Keywords
UNT 15 WATER HAMMER AND SURGE TANKS Structure 15.1 ntroduction Objectives 15.2 Water Hammer 15.2.1 Expression for Rise in Pressure 15.3 Rapid Acceleration of Flow 15.4 Surge Tanks 15.5 Summary 15.6 Keywords
Topic 13: Gas Exchange Ch. 42. Gas Exchange pp Gas Exchange. Gas Exchange in Fish pp Gas Exchange in Fish
 Topic 13: Gas Exchange Ch. 42 Fig. 42.24 Gas Exchange pp.979-989 Gas exchange involves the uptake of oxygen and the discharge of carbon dioxide (i.e. respiration or breathing). It is necessary for cellular
Topic 13: Gas Exchange Ch. 42 Fig. 42.24 Gas Exchange pp.979-989 Gas exchange involves the uptake of oxygen and the discharge of carbon dioxide (i.e. respiration or breathing). It is necessary for cellular
The relationship of pressure and aqueous outflow in enucleated human eyes*
 The relationship of pressure and aqueous outflow in enucleated human eyes* Bruce A. Ellingsen and W. Morton Grant The influence of intraocular pressure on resistance to aqueous outflow was evaluated in
The relationship of pressure and aqueous outflow in enucleated human eyes* Bruce A. Ellingsen and W. Morton Grant The influence of intraocular pressure on resistance to aqueous outflow was evaluated in
AIRFLOW GENERATION IN A TUNNEL USING A SACCARDO VENTILATION SYSTEM AGAINST THE BUOYANCY EFFECT PRODUCED BY A FIRE
 - 247 - AIRFLOW GENERATION IN A TUNNEL USING A SACCARDO VENTILATION SYSTEM AGAINST THE BUOYANCY EFFECT PRODUCED BY A FIRE J D Castro a, C W Pope a and R D Matthews b a Mott MacDonald Ltd, St Anne House,
- 247 - AIRFLOW GENERATION IN A TUNNEL USING A SACCARDO VENTILATION SYSTEM AGAINST THE BUOYANCY EFFECT PRODUCED BY A FIRE J D Castro a, C W Pope a and R D Matthews b a Mott MacDonald Ltd, St Anne House,
CHAPTER 31 IDEAL GAS LAWS
 CHAPTER 31 IDEAL GAS LAWS EXERCISE 144, Page 317 1. The pressure of a mass of gas is increased from 150 kpa to 750 kpa at constant temperature. Determine the final volume of the gas, if its initial volume
CHAPTER 31 IDEAL GAS LAWS EXERCISE 144, Page 317 1. The pressure of a mass of gas is increased from 150 kpa to 750 kpa at constant temperature. Determine the final volume of the gas, if its initial volume
Specifications...Easy CLIA Classification: Sample Type: Blood gas analyzer operation has never been simpler Sample Size: Measured Parameters PCO2
 medicacorp.com easy inside and out With EasyBloodGas, Medica has redefined blood gas analyzer design The sophistication of traditional blood gas analyzers has been packaged in a new compact format with
medicacorp.com easy inside and out With EasyBloodGas, Medica has redefined blood gas analyzer design The sophistication of traditional blood gas analyzers has been packaged in a new compact format with
Procedure 1: Volume vs. Pressure 1.) Using the lap tops, go to the Physics Education Technology from the University of Colorado at:
 Deriving the Gas Laws Background The gaseous state of matter consists of particles (gas molecules like oxygen, nitrogen, and carbon dioxide) which, according to the kinetic theory of gases, are in constant
Deriving the Gas Laws Background The gaseous state of matter consists of particles (gas molecules like oxygen, nitrogen, and carbon dioxide) which, according to the kinetic theory of gases, are in constant
Appendix 2. Basic physical properties applied to the respiratory system
 Appendix 2. Basic physical properties applied to the respiratory system Fluid is a general definition of a state of matter characterized by a weak intermolecular connection (Van der Waal's cohesive forces),
Appendix 2. Basic physical properties applied to the respiratory system Fluid is a general definition of a state of matter characterized by a weak intermolecular connection (Van der Waal's cohesive forces),
Comparative Study of Oxygen Permeation Through Polymers and Gas Barrier Films
 Comparative Study of Oxygen Permeation Through Polymers and Gas Barrier Films H. Nörenberg and C. Deng, University of Oxford, Department of Materials, United Kindgom; H.-J. Kosmella, GKSS Geesthacht/Teltow,
Comparative Study of Oxygen Permeation Through Polymers and Gas Barrier Films H. Nörenberg and C. Deng, University of Oxford, Department of Materials, United Kindgom; H.-J. Kosmella, GKSS Geesthacht/Teltow,
AP Lab 11.3 Archimedes Principle
 ame School Date AP Lab 11.3 Archimedes Principle Explore the Apparatus We ll use the Buoyancy Apparatus in this lab activity. Before starting this activity check to see if there is an introductory video
ame School Date AP Lab 11.3 Archimedes Principle Explore the Apparatus We ll use the Buoyancy Apparatus in this lab activity. Before starting this activity check to see if there is an introductory video
Generating Calibration Gas Standards
 Technical Note 1001 Metronics Inc. Generating Calibration Gas Standards with Dynacal Permeation Devices Permeation devices provide an excellent method of producing known gas concentrations in the PPM and
Technical Note 1001 Metronics Inc. Generating Calibration Gas Standards with Dynacal Permeation Devices Permeation devices provide an excellent method of producing known gas concentrations in the PPM and
Comparison of oxygen flux in hydrogel and silicone hydrogel contact lenses. By: Jonathan Cohan Eun Ae Cho Megan Connors
 Comparison of oxygen flux in hydrogel and silicone hydrogel contact lenses By: Jonathan Cohan Eun Ae Cho Megan Connors BEE 453: Computer-Aided Engineering: Applications to Biomedical Processes Professor
Comparison of oxygen flux in hydrogel and silicone hydrogel contact lenses By: Jonathan Cohan Eun Ae Cho Megan Connors BEE 453: Computer-Aided Engineering: Applications to Biomedical Processes Professor
Name. Student I.D.. Section:. Use g = 10 m/s 2
 Prince Sultan University Department of Mathematics & Physics SCI 101- General Sciences Second Exam Second Semester, Term 142 Wednesday 22/4/2015 Examination Time : 60 minutes Name. Student I.D.. Section:.
Prince Sultan University Department of Mathematics & Physics SCI 101- General Sciences Second Exam Second Semester, Term 142 Wednesday 22/4/2015 Examination Time : 60 minutes Name. Student I.D.. Section:.
Homeostasis and Negative Feedback Concepts and Breathing Experiments 1
 Homeostasis and Negative Feedback Concepts and Breathing Experiments 1 I. Homeostasis and Negative Feedback Homeostasis refers to the maintenance of relatively constant internal conditions. For example,
Homeostasis and Negative Feedback Concepts and Breathing Experiments 1 I. Homeostasis and Negative Feedback Homeostasis refers to the maintenance of relatively constant internal conditions. For example,
FRANZ CELL TEST OF NANO GLUTATHIONE WITH HPLC ANALYSIS
 FRANZ CELL TEST OF NANO GLUTATHIONE WITH HPLC ANALYSIS Analysis Conducted BY CC RESEARCH (P) Ltd., For Nanoceutical Solutions Inc. USA 2950 Thousand Oaks drive, Suite 3, San Antonio, Texas EXECUTIVE SUMMARY
FRANZ CELL TEST OF NANO GLUTATHIONE WITH HPLC ANALYSIS Analysis Conducted BY CC RESEARCH (P) Ltd., For Nanoceutical Solutions Inc. USA 2950 Thousand Oaks drive, Suite 3, San Antonio, Texas EXECUTIVE SUMMARY
[2] Explain how the alveoli create a surface for efficient gaseous exchange
![[2] Explain how the alveoli create a surface for efficient gaseous exchange [2] Explain how the alveoli create a surface for efficient gaseous exchange](/thumbs/89/98827571.jpg) 1 (a) (i) Name the two types of epithelial tissue found in the lungs and airways.... [2] (ii) The epithelial cells in the lungs are arranged into structures called alveoli. Explain how the alveoli create
1 (a) (i) Name the two types of epithelial tissue found in the lungs and airways.... [2] (ii) The epithelial cells in the lungs are arranged into structures called alveoli. Explain how the alveoli create
IN experimental plant physiology the problem of the control of
 [ 119 ] THE CONTROL OF ATMOSPHERIC HUMIDITY IN A CLOSED SYSTEM BY B. D. BOLAS From the Department of Plant Physiology and Pathology, Imperial College of Science and Technology, London (With 4 figures in
[ 119 ] THE CONTROL OF ATMOSPHERIC HUMIDITY IN A CLOSED SYSTEM BY B. D. BOLAS From the Department of Plant Physiology and Pathology, Imperial College of Science and Technology, London (With 4 figures in
THE COLLEGE OF AERONAUTICS CRANFIELD
 THE COLLEGE OF AERONAUTICS CRANFIELD AERODYNAMIC CHARACTERISTICS OF A 40 SWEPT BACK WING OF ASPECT RATIO 4.5 by P. S. BARNA NOTE NO. 65 MAY, 1957 CRANFIELD A preliminary report on the aerodynamic characteristics
THE COLLEGE OF AERONAUTICS CRANFIELD AERODYNAMIC CHARACTERISTICS OF A 40 SWEPT BACK WING OF ASPECT RATIO 4.5 by P. S. BARNA NOTE NO. 65 MAY, 1957 CRANFIELD A preliminary report on the aerodynamic characteristics
Making Measurements Leaks
 Check the seal around leaves and stems When the gaskets are compressed around thicker leaves and stems, small gaps will be created. At high flow rates, this may not be a problem, but at lower rates, be
Check the seal around leaves and stems When the gaskets are compressed around thicker leaves and stems, small gaps will be created. At high flow rates, this may not be a problem, but at lower rates, be
Pulmonary Circulation Linda Costanzo Ph.D.
 Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
BREATHING AND EXCHANGE OF GASES
 96 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 17 BREATHING AND EXCHANGE OF GASES MULTIPLE CHOICE QUESTIONS 1. Respiration in insects is called direct because a. The cell exchange O 2 directly with the air in the
96 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 17 BREATHING AND EXCHANGE OF GASES MULTIPLE CHOICE QUESTIONS 1. Respiration in insects is called direct because a. The cell exchange O 2 directly with the air in the
AP Biology. Investigation 6: Cellular Respiration. Investigation 6: Cellular Respiration. Investigation 6: Cellular Respiration
 AP Biology Learning Objectives Investigation 6: Cellular Respiration To learn how a respirometer system can be used to measure respiration rates in plant seeds or small invertebrates, such as insects or
AP Biology Learning Objectives Investigation 6: Cellular Respiration To learn how a respirometer system can be used to measure respiration rates in plant seeds or small invertebrates, such as insects or
CHM Basics of Gases (r14) Charles Taylor 1/9
 CHM 110 - Basics of Gases (r14)- 2014 Charles Taylor 1/9 Introduction The gas phase is noticeably different from the other two phases of matter. Here are some of the more obvious differences. Gases are
CHM 110 - Basics of Gases (r14)- 2014 Charles Taylor 1/9 Introduction The gas phase is noticeably different from the other two phases of matter. Here are some of the more obvious differences. Gases are
(A) The partial pressure in the lungs is higher than in the blood, and oxygen diffuses out of the lungs passively.
 DAT Biology - Problem Drill 12: The Respiratory System Question No. 1 of 10 1. Which statement about the partial pressure of oxygen inside the lungs is correct? Question #01 (A) The partial pressure in
DAT Biology - Problem Drill 12: The Respiratory System Question No. 1 of 10 1. Which statement about the partial pressure of oxygen inside the lungs is correct? Question #01 (A) The partial pressure in
