during the apnceic pause, when the H2CO3 concentration of the blood is investigators, and the results of these researches have been most thoroughly
|
|
|
- Mervin Walton
- 6 years ago
- Views:
Transcription
1 CHANGES IN THE HYDROGEN ION CONCENTRATION OF THE BLOOD PRODUCED BY PULMONARY VENTILATION. By T. H. MILROY (Department of Physiology, Queen's University, Belfast). (Received for publication 16th February 1914.) DURING rapid ventilation of the lungs with air, the carbonic acid content of the blood is lowered, and if, after a sufficient interval, the ventilation be stopped, the respiratory movements cease until the alveolar carbonic acid pressure and therefore the carbonic acid concentration of the blood have risen again to the stimulating level for the respiratory centre. That the carbonic acid plays the predominant part in exciting the centre to action has been shown by many experiments. The withdrawal of carbonic acid from the blood during excessive ventilation must lead to a diminution in the hydrogen ion concentration, (H+), from the decrease in the concentration of H2CO3, unless there be during this period an increase in the acid products of incomplete combustion of the material used by the tissues during their activity. So also during the apnceic pause, when the H2CO3 concentration of the blood is rising there must be an increase in the (H+) unless there be an increased alkali transport from tissues to blood during this period. The means which the organism possesses of maintaining a constancy in the reaction of the blood and tissue fluids have been very carefully studied by many investigators, and the results of these researches have been most thoroughly analysed by Henderson (1). This paper will deal in the main with the quantitative changes in the (H+) of the blood produced by excessive pulmonary ventilation with air or other gas inixture, and will also refer to the subsequent changes in ionic concentration during the pause and after the respirations have resumed their normal character. It is necessary to refer in some detail to the method which has been employed to determine the (H+) of the blood, namely, the electrometric method, as the recent workers in this subject have directed attention to certain inaccuracies which are difficult to avoid in the case of blood estimations. One must, however, omit very many important points in connection with the rnethod, as a full description would occupy too much space, and the method as applied to blood has already been explained by many others, especially within recent years by Soirensen (2) and Hasselbalch (3). There are certain difficulties attached to the use of the hydrogen
2 142 Milroy electrode in the case of such a fluid as blood. In the first place, one cannot saturate this fluid with hydrogen in the usual way by passing a stream of the gas through the blood, as by this procedure the carbonic acid would be removed from the blood, with a resultant lowering of the (H+). In the second place, owing to the rich oxygen content of the blood, the platinum hydrogen plate undergoes partial depolarisation, and so the readings cannot be constant until the blood is completely reduced. And in the third place, as hydrogen cannot be passed through the blood, and as the fluid requires to be saturated with the gas at atmnospheric pressure, a certain amount of time must elapse in the case of a hydrogen electrode with a stationary hydrogen atmosphere before such a viscous fluid as blood can be saturated. To prevent an alteration in the (H+) from a withdrawal of carbonic acid, Hober passed through the blood hydrogen with a percentage of carbonic acid approximating to that existing in the blood. This in many respects rather inconvenient method has not been employed to any great extent. Hasselbalch (5) then introduced another method, using an electrode which enables one to remove the first specimen of the blood from the electrode after gaseous equilibrium has been established between the blood and the stationary hydrogen atmosphere, and to replace this with a fresh specimen of the same blood while still retaining the original gas in the electrode. It is true that the partial pressure of the hydrogen is too low, as it is mixed with carbonic acid; but this introduces a very small error, as the measured potential varies only as the logarithm of the hydrogen pressure. Michaelis and Davidoff (6) and many others referred to by Sorensen adopted the method of the hydrogen electrode, with stationary hydrogen atmosphere and minimal contact between platinum plate and blood, without, however, replacing the first specimen of blood by a second or third one, regarding the lowering of (1H+) from the loss of carbonic acid from the blood as being too small to introduce anything beyond an inappreciable fall in (H+). Michaelis first directed attention to the advantage of ininimal contact between plate and blood in order to obtain rapidly constant readings of the potential. Hasselbalch and his co-workers adopted this plan of minimal contact. Now, as regards the preliminary depolarisation of the plate, this is bound to occur when a hydrogen plate is brought into contact with a fluid so rich in' oxygen as arterial blood. In Hasselbalch and Lundsgaard's earlier papers sufficient attention was not directed to the depolarisation as a factor leading to inconstant readings. All who have used the electrode must have been troubled by this error, and Konikoff (7) has recently drawn attention to it. At the outset of my experiments, I was so much troubled by this depolarisation that for the last year I have made use of blood plasma instead of the entire blood. The long periods referred to by Hasselbalch as necessary before constant readinigs can be obtained were undoubtedly due mainly to the very slow reduction of the blood. The
3 Changes in the Hydrogen Ion Concentration of the Blood >ip changes in the readings noticeable on shaking the electrode after a constant reading has been previously arrived at are due to a renewed depolarisation with a consequent fall in potential followed by a rise as the hydrogen film again forms on the plate surface. Constant readings are more quickly attained by minimal contact between the plate and the blood owing to better reduction of the superficial film of blood and the less general depolarisation of the plate surface. Hasselbalch's method of replacing the first by a second or third specimen of blood leads naturally to renewed depolarisation, although the final readings may be and probably are correct. It is necessary, however, to have complete reduction of the blood in order to obtain absolutely constant readings. As regards the difficulties entailed in the saturation of a viscous fluid like blood with hydrogen, these can be removed by frequent shaking of the electrode. The method suggested by Wilke (8), namely, the use of a platinum capillary electrode with a high internal pressure of hydrogen, does not appear satisfactory unless one knows the actual pressure of the hydrogen on the outer side of the capillary. Very briefly reference may now be made to the method which has been adopted in carrying out the electrometric measurements. In the first place, as regards the charging of the electrode (one of the Hasselbalch type) the hydrogen, which was obtained from the action of aluminium amalgam (9) on water, was passed for about an hour through the usual series of liquids to render it oxygen-free and saturated with water vapour, passing it finally through a specimen of blood plasma into the empty electrode. While the hydrogen was still passing through, blood plasma was quickly introduced into the electrode until the plate was approximately half immersed in the fluid; the electrode was now closed and carefully sealed with melted paraffin at any place where there might be a tendency to leakage. It was then shaken gently for ten minutes, allowed to stand for about five minutes, and finally, before connecting up with the calomel electrode through the strong KCI solution (3 5 N) in the contact dish, the tube used for dipping into the latter was opened with the end submlerged in a small quantity of the blood plasma. The hydrogen electrode and the calomel electrode were now connected up through the contact fluid and an estimation of the e.m.f. carried out in the usual way. The calomel electrode was of the form employed by Pauli, and was prepared as described by Loomis and Acree (10). The estimations were all made by the usual zero method, using a calibrated potentiometer, accumulator, standard Weston cell ( volt), and an Ayrton-Mather galvanometer of the d'arsonval type. Accurate details as regards the measurement of the e.m.f. of the electrode system are given in Soirensen's article already referred to. The hydrogen and calomel electrodes were frequently subjected to controls as described by Loomiis and Acree. As regards the method of obtaining the blood, it was taken from the
4 144 Milroy carotid artery with as little agitation as possible, into a centrifuge tube containing two or three milligrammes of hirudin dissolved in a very small quantity of saline. The tube when filled was closed by a paraffined cork and put into the centrifuge. The plasma after separation was taken from the tube, every precaution being taken to prevent loss of carbonic acid, and introduced into the electrode. The after treatment of the electrode has been already described. As soon as a constant reading of the e.m.f. had been obtained, the electrode was removed from the contact dish, the connecting tube closed, and the electrode again shaken and allowed to stand for a short time before the second reading was taken. This was repeated until the readings were absolutely constant. This was the case usually after the third shaking. In some cases Hasselbalch's procedure was adopted introducing a second specimen of plasma while retaining the gas mixture, but this was found to be unnecessary, as the differences between the two final readings were so small and a larger amount of blood was required to be taken than was advisable, three or four specimens being required in each ventilation experiment, as will be afterwards described. Before each specimen of plasmna was introduced the electrode was charged afresh with hydrogen, and when the estimations in a series were completed the plasma was washed well out of the electrode with conductivity water and the latter then saturated with hydrogen. The platinum plates were thus kept ready for further estimations. It was found necessary to replatinise the plates for a short time, more frequently than is necessary when standard solutions are used. The estimations were in most cases made at 15 C. and corrected for 180, in a few cases also at 380, but the former were more satisfactory. An air thermostat of the Hasselbalch type did not prove very satisfactory for estimations at 38, as it was difficult to maintain a constant temperature in the electrode system. In cases where it is advisable to replace the blood plasma used in the first case by a second specimen of the same, the electrode used by Konikoff is very useful, although I have mainly used the Hasselbalch type. The slight fall in (H+) due to a loss of CO2 from blood to hydrogen atmosphere may, in my opinion, be disregarded, and so by the use of one specimen only a continual depolarisation of the plate by the fresh oxygenholding fluid is avoided. My aim was to investigate the (H+) changes produced by a ventilation period and subsequent thereto. Therefore a specimen of blood was taken just before ventilation, a second one just at the close of a ventilation period, a third one at the close of the pause, and in many cases a fourth one after breathing had resumed its normal character. In some cases one or other of the samples was omitted in order to see whether the effects differed with variations in the loss of blood. The same electrode system was used for each sample in the series, the hydrogen electrode being charged afresh before the introduction of the new specimen.
5 Changes in the Hydrogen Ion Concentration of the Blood 145 The effect of variations in the composition of the gas mixture used for ventilation was also studied. It is advisable before giving the experimental results to refer to the calculation of (H+) from the e.m.f. The hydrogen ion concentration of the blood was calculated from the e.m.f. in the usual way, making use of Nernst's well-known formula: 7rp= r log CO, where 7r = e.m.f. of the unknown fluid in the electrode system, 7ro = e.m.f. of a hydrogen electrode when the (H+) of the fluid (CO = 1) is normal, and C and CO= the respective H+ concentrations of the two fluids. 7ro may be taken as *3377 volt at 180 C. Therefore 7p= log!. p C may therefore be calculated when ;r is measured. Or if p be put in place of C (10--P), the results may be stated in terms of Sorensen's negative hydrogen ion exponent written by him ph+. Thus 7rp- '3377 P P0577 As one is dealing with solutions much below normal ionic concentration, PH+ is a negative exponent, so that a rise in ph+ signifies a fall in the hydrogen ionic concentration (H+). The results of the estimations in the various experiments will be given for convenience both as (H+) and as p1+. The animals were anasthetised with ether, and pulmonary ventilation was carried out by connecting the trachea tube with the tubes of the Meyer's pump. In the smaller animals 100 c.c. of air or oxygen, as the case might be, were driven in at a rate of 67 times per minute for varying periods, and the examination of the blood made in the way and at the times above mentioned. The first experiments refer to ventilation with air, and finally with oxygen, as the addition of the latter produced a longer apnceic pause. I. Cat, kgm. 15 min. ventilation with air, followed by 2 min. 15 sec. ventilation with oxygen. The apnoeic pause was 3 min. 10 sec. in duration. Specimens of blood taken. (H+) x 1O-7N. a. Before ventilation b. After ventilation c. At end of aprcea d. 10 min. later.. *3890 7'41
6 146 Milroy II. Cat, kgm. Air Apnoea 2 inin. 50 sec. veintilation 5 min. Oxygen 2 min. a. b. C. d. Specimeens of blood taken. Before ventilation After ventilation End of apncea. 5 niii. later (H+) x 10- N. *4169 * * III. Cat, kgm. 16 Apnoea 2 min. 45 sec. Specimens of blood taken. min. air. 2 min. 10 sec. oxygen. (H+) x 10- N. a. b. C. d. Before velntilation After ventilation Encd of apnca. 6 min. later * ' IV. Cat, kgn. 9 min. air. 2 min.oxygen. Apncea 2 min. Specimens of blood taken. (H+)x10 N. a. Before ventilation b. After ventilation c. End of apncea d. 9 min. later V. Cat, kgm. 10 min. air. 1 lain. 25 sec. oxygen. nearly 4 min. Apnoea a. b. C. d. Specimens of blood taken. Before ventilation After ventilation End of apncea. 7 min. later (H+) x *47 ~~~~ VI. Dog, kgm. 15 min. air (200 c.c. per stroke of pump 67 times per mninute) and oxygen finally for 1 min. Apnoeic pause 3 min. 25 sec. Specimens of blood taken. (H+) x 10- N. PH+ a..... b. c. After ventilation End of apncea '60
7 Changes in the Hydrogen Ion Concentration of the Blood VII. Cat, kgm. 30 min. air. 3 min. oxygen. Apncea 1 min. 3 sec. 147 Specinmens of blood taken. (H+) x 10-7 N. PH+ a.... b. After ventilation c. End of apnoea. *1820 * As the results obtained by ventilation with air were of the same character as those just referred to when the air period was followed by a short one with oxygen, the former will now be given before consideration of the nature of the effects produced by the latter. VIII. Cat, kgm. Air ventilation 15 min. Apnceic pause 1 min. 30 sec. Specimens of blo xod taken. (H+) x 19- N. a. Before ventilation b. After ventilation c. End of apncea. d. 15 min. later *3236 *1445 *2399 * IX. Cat, kgm. Ventilation with air for 21 min., one sample of blood being taken after 6 min. ventilation and a second after 21 min. ventilation. Apnceic pause 3 min., but sample of blood was taken shortly before the close of the pause. Specimens of blood taken. (H+) x 10-7 N. a. Before ventilation b. After 6 min. ventilation bl. After 21 min. ventilation.. * c. Shortly before end of apncea * X. Dog, kgin. 200 c.c. air pumped per minute for 15 min. Apncea 2 min. in at a rate of 67 Specimens of blood taken. (H+) x 10-7 N. a. Before ventilation b. After ventilation c. End of apncea _~~~~
8 148 Milroy XI. Catj kgm. Air 30 min. Apneea 1I min. Specimens of blood taken. (H+) x 10-7 N. P a. Before ventilation. * b. After ventilation c. End of apncea XII. Cat, kgm. Air 10 min. Apnoea 1 min. 30 sec. Specimens of blood taken. (H+) x 10-7 N. PH+ a. Before ventilation 0. * b. After ventilation c. End of apncea d. 6 nmin. later In this case, when sample d was taken the breathing was more forcible than at the beginning before ventilation. In all the experiments referred to, ventilation, whether with air alone or with air and oxygen, has led to a fall in (II+), and in all those cases an apnceic pause has followed this fall in hydrogen ion concentration. In certain comparatively rare cases no pause follows the excessive pulmonary ventilation, and in one case where this occurred I examined the blood and found that the hydrogen ion concentration had not fallen at the close of the ventilation period. This is of interest as perhaps shedding light upon the absence of an apnoeic pause after excessive respiration, noticed by Boothby (11) in his own case. XIII. Cat, kgm. Air ventilation 9 min. No pause followed, but samples of blood were taken at 1 min. 45 sec. after stoppage of pump, and again 10 min. later. Control specimens were taken shortly after the first ones, passing the second specimen in after the reading for the first one had been taken. Specimnens of blood taken. (H+) x 10 -' N. PH+ 1st 2nd 1st 2nd specimen. specimen. specimen. specimen. a. Before ventilation.. * b. After ventilation... *3715 * d. 1 min. 45 sec. later while breathing was going on.... *2884 *3467 7, d1. 10 min. later *
9 Changes in the Hydrogen Ion Concentration of the Blood 149 It is evident that ventilation has not produced the usual effect upon the (H+), and the slight differences in (H+) at different periods after the ventilation, viz. between *35 x 1O-7 and *28 x 1O-7, are such as might occur under normal conditions in the blood from changes in the amplitude of the respirations. This departure from the usual effect produced by ventilation may have been due to some circulatory disturbance such as Boothby refers to. On the other hand, the ventilation may have led to the usual lowering in H2CO3 concentration, and accompanying this there may have been a rise in (H+) due to the appearance of acid products of incomplete oxidation. In the air and oxygen experiments there is produced by the ventilation as a rule a fall in (H+) of from *14 to *17 x 1O-7, followed by a rise during the pause, which usually reaches, before breathing again starts, the level before ventilation, or may reach a distinctly higher one, as in Experiment IX., when the blood was slightly on the acid side of the neutral point. Experiments VIII.-XI. were carried out in order to see whether any difference in the results was observable if one or other of the blood samples in the series was omitted, thus leading to a diminished loss of blood. It is evident that in these cases also the results are of the samie character as those obtained when a complete series was taken. In the air ventilation experiments there is a fall in the (H+) of froin *13 to *18 x 1O-7. Just at the close of the ventilation the (H+) lies between *10 and *19 x 10-7 in the various experiments. At the end of the pause there is a rise in (H+), the final value of which varies according to the original reaction of the blood. The hydrogen ion concentration at the end of the pause may be taken as varying from *20 to *35 x It very rarely exceeds the original (H+), before ventilation. The (H+) at the end of a pause produced by air and oxygen ventilation may rise, on the other hand, distinctly above the original value, and is rarely more than slightly below it. In all probability, therefore, oxygen raises the threshold of excitation of the respiratory centre for the hydrogen ion stimulus. Brief reference will now be made to the effects produced by air ventilation followed by a short period of ventilation with air to which carbonic acid has been added. It is well known that the addition of carbonic acid to the air used for ventilation prevents the apnoeic pause, when the CO2 content of the gas mixture is at or above alveolar CO pressure. It is interesting, therefore, to determine whether the fall in the (H+) produced by ordinary air ventilation is prevented, as one would expect, by giving at the conclusion of a period of air ventilation a gas mixture rich in carbonic acid. [XIV.
10 150 Milroy XIV. Dog, kgm. Air ventilation (200 c.c. at usual rate) for 12 min., then 1 min. with 12 per cent. carbonic acid in air. Very forcible breathing started immediately on stoppage of the pump. Samples of blood were taken before ventilation and at different times after stoppage of the pump. Specimens of blood taken. (H+) x 10- N. PH a. Before ventilation b. Just at close of ventilation * c. 2 min. later d. 31 min. later XV. Cat, kgm. Air ventilation for 12 min., followed by 2 min. 15 sec. ventilation with 12 per cent. carbonic acid in oxygen. On stoppage of the pump there was an inappreciable pause, and the breathing was much less forcible than after the carbonic acid air ventilation. Specimens of blood taken. (H+) x 10 N. Before ventilation.. * At close of ventilation min. later min. later. * In both cases the finial ventilation with a 12 per cent. CO2 gas mixture produced a rise in (H+) instead of the fall observed in all cases of ventilation with air or air and oxygen. The respirations, which started immediately on stoppage of the pump, at least in the case of the air-co2 mixture, were forcible and led to a rapid fall in the (H+) from the removal of the excess of carbonic acid in the blood. In the case of the oxygen-carbonic acid ventilation there was a very short apncea, followed by respirations which were less forcible than in the preceding case, and therefore the excess of CO2 was not so quickly removed, with the result that the fall in (H+) was less rapid. The oxygen had evidently led to a decrease in the excitability of the centre, as in both cases the ventilation led to the same rise in (H+), namely, about 12 x The forcible breathing which usually follows inhalation of gas mixtures rich in CO2 is often interrupted by periods when the respirations are less in amplitude, these being evidently due to the overaction of the respiratory centre leading to an excessive washing out of the carbonic acid and so to a lowering of the (H+). In conclusion, I may refer briefly to certain recent investigations dealing with differences in the (H+) of the blood due to variations in the H2C53 concentration in the same. Michaelis and Davidoff, in the paper
11 Changes in the Hydrogen Ion Concentration of the Blood 151 already referred to, were able to recognise a difference in the hydrogen ion concentration of arterial and venous blood in the rabbit. Carotid artery. Jugular vein. PH at 180 P at 18 a. b. a. b. 7, So that during ordinary respiratory exchange the (H+) of venous blood falls in becomiing arterialised to the extent of from -05 to *08 x They obtained rather more alkaline values for human venous blood than Hasselbalch and Lundsgaard, but Hasselbalch, in his most recent paper, gives values more nearly resembling those of Michaelis and Davidoff. The experiments of Hasselbalch and Lundsgaard, and Lundsgaard (13) alone, on the (H+) of blood in gaseous equilibrium with gas mixtures containing CO2 at different partial pressures are of very great interest and importance. The following results were obtained for ox blood at 38.50, for the corpuscles and for the serum of the same: Defibrinated ox blood. Blood corpuscles. Mm. C0O H+ Mill. C02. PH Serum. Mm. C02. PH , In the first set there was a rise in (H+) from *281 8 x 10-7 to *4898 x 10-7 when the CO2 pressure was raised from 20 to 40 mimi. With a further rise in CO2 pressure the ionic rise became less marked. This rise of approximately *2 x 10-7 produced by increased H2CO3 concentration is similar to VOL. VIII., NOS. 2 AND
12 152 Milroy the fall produced by air or air and oxygen ventilation brought about by the removal of CO2 and so decreased H2CO3 concentration. Again, in Hdber's experiments (loc. cit.), where various hydrogen-co2 mixtures were passed through the blood, the results show the same rise in (H+) with increasing H2CO3 concentration. Although the blood specimens were from different animals and consequently were scarcely uniform, the general effect is quite definite. C02 per cent. (H+) X 10-7 N. - in hydrogen. 0 * ,6 ' '44 * '18 * '81 * '15 *49 7'31 4'26 * '51 *79 7' '89 7o05 Thus a rise in the C02 percentage from 1'6 to 6 per cent. leads to a rise in the (H+) of the blood from *31 to *79 x These experiments of Hasselbalch, Lundsgaard, and Hober deal with the changes which altered H2CO3 concentration produces in the blood in vitro. In the living animal such changes might not be observable owing to rapid replacement of C02 by the tissues or from some other cause. Hasselbalch (14) studied the changes in the reaction of the rabbit's blood during natural pulmonary ventilation, especially as regards their relationship to the alveolar carbonic acid pressure. He found that with increased natural ventilation theph+ rose (i.e. (H+) fell) and the alveolar carbonic acid pressure fell. In his first experiment with an alveolar ventilation of 945 c.c. per minute, the P.+ was 7'42, while with a ventilation of 1139 c.c. per minute it was found to be 7'46. He also found that a rise in the percentage of carbonic acid in the gas mixture inhaled led to a rise in the hydrogen ion concentration of the blood. For the evidence which this author has brought forward that the degree of ventilation seems to be dependent upon the hydrogen ionic concentration rather than the alveolar carbonic acid pressure, his paper should be consulted. The papers which I have referred to in the course of this contribution to the subject give the full literature on the subject of the reaction of the blood, and so I have omitted reference to many papers of great importance. As to the effect produced by an increased H2C003 concentration of the blood on the ratios of the various salts of the alkalies in the blood to one another and the influence of the protein content on the carbonic acid transport, attention may be directed to the extremely interesting article by Henderson already referred to.
13 Changes in the Hydrogen Ion Concentration of the Blood 153 I may now shortly summarise my results:- 1. Pulmonary ventilation with air or air and oxygen produces a rapid fall in hydrogen ion concentration, this apparently reaching its lowest level after very short periods of ventilation. 2. This fall in (H+) is always associated with cessation of breathing when the artificial ventilation ceases. 3. During the apnceic pause the (H+) rapidly rises approximately to its old level prior to ventilation, but may be below or, more rarely, slightly above that point.. 4. The effects produced on the (H+) by the subsequent breathing depend upon the amplitude and frequency of the respirations, hyperpncea leading to a fall and diminished breathing to a rise in (H+). 5. Ventilation with gas mixtures rich in carbonic acid, even when given only for short periods after the main ventilation with air, lead to a rise in (H+) instead of a fall, and no apnceic pause follows the period of artificial ventilation. 6. It is probable that in cases where air ventilation is not followed by an apnceic pause the (H+) has not fallen as a result of the ventilation. 7. The extremely short duration of all those (H+) changes in the blood, and their close relationship to simultaneously occurring and easily recognisable variations in the H2C03 concentration, make it extremely likely that these are causally related to one another, and that the activity of the respiratory centre is very largely dependent upon the hydrogen ion concentration of the blood. 8. The excitability of the centre as gauged by its threshold of excitation by (H+) is evidently lowered by iniereased oxygen absorption. LITERATURE. (1) HENDERSON, Ergebnisse der Physiologie, 1909, p (2) S6RENSEN, Ergebnisse der Physiologie, 1912, p. 393 (giving the literature up to this date). (3) HASS-ELBALCH, Biochem. Zeitschr., 1913, Bd. xlix. p (4) HOBER, Arch. f. d. ges. Physiologie, L903, Ed. xcix. p (5) HASSELBALCH, Biochem. Zeitschr., 1911, Ed. xxx. p (6) MICHAELIs and DAVIDOFF, Biochem. Zeitschr., 1912, Bd. xlvi. p (7) KONIKOFF, Biochem. Zeitschr., 1913, Bd. li. p (8) WILKE, Zeitsch. f. Elektrochemie, 1913, Bd. mix. p (9) WISLICENUS, Journ. f. prakt. Chem., 1896, Bd. liv. p. 54. (10) LooMIs and ACREE, Amer. Chem. Journ., 1911, vol. xlvi. p (11) BOOTHBY, Journ. Physiol., 1912, vol. xlv. p (12) HASSEILBALCH and LUNDSGAARD, Biochem. Zeitschr., 1912, Bd. xxxviii. p. 77. (13) LUNDSGAARD, Biochem. Zeitschr., 1912, Bd. xli. p (14) HASSEILBALCH, Skand. Arch., 1912, Bd. xxvii.-xxviii. p. 13.
The physiological functions of respiration and circulation. Mechanics. exercise 7. Respiratory Volumes. Objectives
 exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
Oxygen convulsions are believed by many workers to be caused by an accumulation
 272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
(Received 16 January 1946)
 186 J. Physiol. (I946) I05, I86-I90 6I2.2I5.9 THE ABSORPTION OF FLUIDS FROM THE LUNGS BY F. C. COURTICE AND P. J. PHIPPS From the Experimental Station, Porton and the Laboratory of Physiology, Oxford (Received
186 J. Physiol. (I946) I05, I86-I90 6I2.2I5.9 THE ABSORPTION OF FLUIDS FROM THE LUNGS BY F. C. COURTICE AND P. J. PHIPPS From the Experimental Station, Porton and the Laboratory of Physiology, Oxford (Received
The over-ventilated cat shows a similar adjustment to diminished. being over-ventilated, and he considered that on that account there was
 6I2.235:6I2.26I THE SOURCE OF COa EXPIRED AND THE SITE OF ITS RETENTION. BY LAURENCE IRVING, J. K. W. FERGUSON AND F. B. PLEWES. (From the Department of Physiology, University of Toronto.) AFTER evisceration
6I2.235:6I2.26I THE SOURCE OF COa EXPIRED AND THE SITE OF ITS RETENTION. BY LAURENCE IRVING, J. K. W. FERGUSON AND F. B. PLEWES. (From the Department of Physiology, University of Toronto.) AFTER evisceration
2. State the volume of air remaining in the lungs after a normal breathing.
 CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
(From the Physiological Laboratory, Groningen, Holland.)
 6I2. I27.3 THE CARRIAGE OF CARBON DIOXIDE BY BLOOD. BY M. N. J. DIRKEN AND H. W. MOOK. (From the Physiological Laboratory, Groningen, Holland.) INTRODUCTION. THE opinion held by B ohr, B ayliss and others
6I2. I27.3 THE CARRIAGE OF CARBON DIOXIDE BY BLOOD. BY M. N. J. DIRKEN AND H. W. MOOK. (From the Physiological Laboratory, Groningen, Holland.) INTRODUCTION. THE opinion held by B ohr, B ayliss and others
(From the Zoological Laboratory, University of Pennsylvania, Philadelphia.)
 A SIMPLE MICRO VESSEL WITH ELECTRODE FOR DETER- MINING THE HYDROGEN ION CONCENTRATION OF SMALL AMOUNTS OF FLUID. BY JOSEPtt HALL BODINE A~rD DAVID E. FINK. (From the Zoological Laboratory, University of
A SIMPLE MICRO VESSEL WITH ELECTRODE FOR DETER- MINING THE HYDROGEN ION CONCENTRATION OF SMALL AMOUNTS OF FLUID. BY JOSEPtt HALL BODINE A~rD DAVID E. FINK. (From the Zoological Laboratory, University of
Douglas and Haldane(2) has shown that the oxygen determinations. since it forms the basis of the "Coefficient of Utilisation" (Krrogh) and
 THE MEASUREMENT OF THE OXYGEN CONTENT OF THE MIXED VENOUS BLOOD, AND OF THE VOLUME OF BLOOD CIRCULATING PER MINUTE. BY J. BARCROFT, F. J. W. ROUGHTON AND R. SHOJI. (From the Physiological Laboratory, Cambridge.)
THE MEASUREMENT OF THE OXYGEN CONTENT OF THE MIXED VENOUS BLOOD, AND OF THE VOLUME OF BLOOD CIRCULATING PER MINUTE. BY J. BARCROFT, F. J. W. ROUGHTON AND R. SHOJI. (From the Physiological Laboratory, Cambridge.)
Section Three Gas transport
 Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
partial pressure is to be applied to the dissociation curve of fully oxygenated
 6I2. I27. I THE DETERMINATION OF THE CARBON DIOXIDE CONTENT OF THE MIXED VENOUS BLOOD. Part I. The effect of oxygenation and the critical oxygen tension. BY M. C. G. ISRAELS (Platt Physiological Scholar)
6I2. I27. I THE DETERMINATION OF THE CARBON DIOXIDE CONTENT OF THE MIXED VENOUS BLOOD. Part I. The effect of oxygenation and the critical oxygen tension. BY M. C. G. ISRAELS (Platt Physiological Scholar)
THE literature on this subject, which was reviewed recently (CAMPBELL, doses of amytal, and in addition received A.C.E. mixture during the
 -~~ -v GAS TENSIONS IN THE MUCOUS MEMBRANE OF THE STOMACH AND SMALL INTESTINE. By J. ARGYLL CAMPBELL. From the National Institute for Medical Research, Hampstead. (With six figures in the text.) (Received
-~~ -v GAS TENSIONS IN THE MUCOUS MEMBRANE OF THE STOMACH AND SMALL INTESTINE. By J. ARGYLL CAMPBELL. From the National Institute for Medical Research, Hampstead. (With six figures in the text.) (Received
J. Physiol. (I941) I00, I98-21I 6I :6I2.825
 198 J. Physiol. (I941) I00, I9821I 6I2.22.02:6I2.825 THE EFFECT OF OXYGEN LACK ON THE CEREBRAL CIRCULATION BY F. C. COURTICE From the Departments of Physiology and of Surgery, Oxford (Received 24 March
198 J. Physiol. (I941) I00, I9821I 6I2.22.02:6I2.825 THE EFFECT OF OXYGEN LACK ON THE CEREBRAL CIRCULATION BY F. C. COURTICE From the Departments of Physiology and of Surgery, Oxford (Received 24 March
(fig. 3) must be at the same temperature as the water in this chamber CALORIMETRIC STUDIES OF THE EXTREMITIES
 CALORIMETRIC STUDIES OF THE EXTREMITIES II. EXPERIMENTAL APPARATUS AND PROCEDURES' By ROY KEGERREIS (Received for publication July 1, 1926) The calorimeter used in these experiments is a modification of
CALORIMETRIC STUDIES OF THE EXTREMITIES II. EXPERIMENTAL APPARATUS AND PROCEDURES' By ROY KEGERREIS (Received for publication July 1, 1926) The calorimeter used in these experiments is a modification of
Essential Skills Course Acute Care Module. Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook
 Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
 Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
CHAPTER 3: The respiratory system
 CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
RESPIRATORY REGULATION DURING EXERCISE
 RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
Human Biology Respiratory System
 Human Biology Respiratory System Respiratory System Responsible for process of breathing Works in cooperation with Circulatory system Three types: 1. Internal Respiration 2. External Respiration 3. Cellular
Human Biology Respiratory System Respiratory System Responsible for process of breathing Works in cooperation with Circulatory system Three types: 1. Internal Respiration 2. External Respiration 3. Cellular
Using such a method, Morawitz and Siebeck (1) found that the. composition of the alveolar air or of the blood. Unless the obstruc- 483
 THE EFFECT OF SOME PATHOLOGICAL CONDITIONS UPON DYSPNEA DURING EXERCISE I. ARTIFICIAL STENOSIS BY A. W. HEWLETT, J. K. LEWIS AND ANNA FRANKLIN (From the Department of Medicine, Stanford Medical School)
THE EFFECT OF SOME PATHOLOGICAL CONDITIONS UPON DYSPNEA DURING EXERCISE I. ARTIFICIAL STENOSIS BY A. W. HEWLETT, J. K. LEWIS AND ANNA FRANKLIN (From the Department of Medicine, Stanford Medical School)
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D.
 Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
I Physical Principles of Gas Exchange
 Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Prem?ous researches. The previous work on C02 partial pressure in
 THE CARBON DIOXIDE PARTIAL PRESSURE IN BODY CAVITIES AND TISSUE SPACES UNDER VARIOUS CONDITIONS. BY J. ARGYLL CAMPBELL. (From the Department of Applied Physiology, National Institute for Medical Research,
THE CARBON DIOXIDE PARTIAL PRESSURE IN BODY CAVITIES AND TISSUE SPACES UNDER VARIOUS CONDITIONS. BY J. ARGYLL CAMPBELL. (From the Department of Applied Physiology, National Institute for Medical Research,
Gas Exchange ACTIVITY OVERVIEW SUMMARY KEY CONCEPTS AND PROCESS SKILLS KEY VOCABULARY. Teacher s Guide B-75 L A B O R ATO R Y
 Gas Exchange 17 40- to 2 50-minute sessions ACTIVITY OVERVIEW L A B O R ATO R Y SUMMARY This activity explores the role of the respiratory system in the regulation of gases in the blood. Students investigate
Gas Exchange 17 40- to 2 50-minute sessions ACTIVITY OVERVIEW L A B O R ATO R Y SUMMARY This activity explores the role of the respiratory system in the regulation of gases in the blood. Students investigate
Section Two Diffusion of gases
 Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation
 Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
Respiratory Lecture Test Questions Set 3
 Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
transients' of large amplitude can be imposed on the arterial, cardiac and Since both coughing and the Valsalva manoeuvre raise intrathoracic pressure
 351 J. Physiol. (I953) I22, 35I-357 EFFECTS OF COUGHING ON INTRATHORACIC PRESSURE, ARTERIAL PRESSURE AND PERIPHERAL BLOOD FLOW BY E. P. SHARPEY-SCHAFER From the Department of Medicine, St Thomas's Hospital
351 J. Physiol. (I953) I22, 35I-357 EFFECTS OF COUGHING ON INTRATHORACIC PRESSURE, ARTERIAL PRESSURE AND PERIPHERAL BLOOD FLOW BY E. P. SHARPEY-SCHAFER From the Department of Medicine, St Thomas's Hospital
Recitation question # 05
 Recitation and Lab # 05 The goal of this recitations / labs is to review material related to the CV and respiratory lectures for the second test of this course. Info required to answer this recitation
Recitation and Lab # 05 The goal of this recitations / labs is to review material related to the CV and respiratory lectures for the second test of this course. Info required to answer this recitation
Some major points on the Effects of Hypoxia
 Some major points on the Effects of Hypoxia Source: Kings College London http://www.kcl.ac.uk/teares/gktvc/vc/dental/year1/lectures/rbmsmajorpoints/effectsofhypoxia.htm Cells obtain their energy from oxygen.
Some major points on the Effects of Hypoxia Source: Kings College London http://www.kcl.ac.uk/teares/gktvc/vc/dental/year1/lectures/rbmsmajorpoints/effectsofhypoxia.htm Cells obtain their energy from oxygen.
6I unstable compound with phosphoric acid, which disappears during fatigue. (From the Department of Physiology, The University, Sheffield.
 6I2.744 ON THE CREATINE AND PHOSPHORUS CONTENT OF MUSCLE. BY MARION BROWN1 AND C. G. IMRIE. (From the Department of Physiology, The University, Sheffield.) IT is well known that the concentration of creatine
6I2.744 ON THE CREATINE AND PHOSPHORUS CONTENT OF MUSCLE. BY MARION BROWN1 AND C. G. IMRIE. (From the Department of Physiology, The University, Sheffield.) IT is well known that the concentration of creatine
CHAPTER 3: The cardio-respiratory system
 : The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
: The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
6I2.2I6:6I alveolar pressure. It follows that the evident alteration in the respiratory rhythm is an alteration in amplitude.
 6I2.2I6:6I2.223.11 SOME EFFECTS OF CARBONIC ACID ON THE CHARACTER OF HUMAN RESPIRATION. BY J. BARCROFT AND R. MARGARIA' (Turin). (From the Physiological Laboratory, Cambridge.) THE following facts concerning
6I2.2I6:6I2.223.11 SOME EFFECTS OF CARBONIC ACID ON THE CHARACTER OF HUMAN RESPIRATION. BY J. BARCROFT AND R. MARGARIA' (Turin). (From the Physiological Laboratory, Cambridge.) THE following facts concerning
Lung Volumes and Capacities
 Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing
 Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
PROBLEM SET 7. Assigned: April 1, 2004 Due: April 9, 2004
 Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
I. Gas Exchange Respiratory Surfaces Respiratory Surface:
 I. Gas Exchange Respiratory Surfaces Respiratory Surface: Common characteristics of respiratory surfaces: a) Moist: allows for the RAPID diffusion of dissolved gasses across its surface. Whereas the respiratory
I. Gas Exchange Respiratory Surfaces Respiratory Surface: Common characteristics of respiratory surfaces: a) Moist: allows for the RAPID diffusion of dissolved gasses across its surface. Whereas the respiratory
Lesson 9.1: The Importance of an Organ Delivery System
 Lesson 9.1: The Importance of an Organ Delivery System Animals require a continuous supply of oxygen (O 2 ) for cellular respiration, and they must expel carbon dioxide (CO 2 ), the waste product of this
Lesson 9.1: The Importance of an Organ Delivery System Animals require a continuous supply of oxygen (O 2 ) for cellular respiration, and they must expel carbon dioxide (CO 2 ), the waste product of this
Respiration - Human 1
 Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
Respiration. The resspiratory system
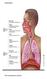 Respiration The resspiratory system The Alveoli The lungs have about 300 million alveoli, with a total crosssec onal area of 50 70 m2.. Each alveolar sac is surrounded by blood capillaries. The walls of
Respiration The resspiratory system The Alveoli The lungs have about 300 million alveoli, with a total crosssec onal area of 50 70 m2.. Each alveolar sac is surrounded by blood capillaries. The walls of
BREATHING AND EXCHANGE OF GASES
 96 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 17 BREATHING AND EXCHANGE OF GASES MULTIPLE CHOICE QUESTIONS 1. Respiration in insects is called direct because a. The cell exchange O 2 directly with the air in the
96 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 17 BREATHING AND EXCHANGE OF GASES MULTIPLE CHOICE QUESTIONS 1. Respiration in insects is called direct because a. The cell exchange O 2 directly with the air in the
IV. FROM AQUATIC TO ATMOSPHERIC BREATHING: THE TRACHEA & THE LUNG
 GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
Oxygen and Carbon dioxide Transport. Dr. Laila Al-Dokhi
 Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
RESPIRATION OF MUSCLE. By W. M. FLETCHER, M.A., M.B., Fellow of Trinity College, Cambrtidge. (Three Figures
 THE INFLUENCE OF OXYGEN UPON THE SURVIVAL RESPIRATION OF MUSCLE. By W. M. FLETCHER, M.A., M.B., Fellow of Trinity College, Cambrtidge. (Three Figures in Text.) (From the Physiologial Laboratory, Cambridge.)
THE INFLUENCE OF OXYGEN UPON THE SURVIVAL RESPIRATION OF MUSCLE. By W. M. FLETCHER, M.A., M.B., Fellow of Trinity College, Cambrtidge. (Three Figures in Text.) (From the Physiologial Laboratory, Cambridge.)
APPENDIX. working blood volume was also rather large; Evans, Grande, and. equilibrated to the new mixture is partially dependent upon the rate
 612.172-5 APPENDIX A SIMPLIFIED HEART OXYGENATOR CIRCUIT FOR BLOOD- FED HEARTS. By J. YULE BOG-UE and R. A. GREGORY.' SINCE 1934 studies on the carbohydrate metabolism of the blood-fed heart without lungs
612.172-5 APPENDIX A SIMPLIFIED HEART OXYGENATOR CIRCUIT FOR BLOOD- FED HEARTS. By J. YULE BOG-UE and R. A. GREGORY.' SINCE 1934 studies on the carbohydrate metabolism of the blood-fed heart without lungs
throughout. The constant-flow respiration was administered through a intravenously at appropriate intervals (in addition to the general
 414 6I2.22I:6I2.2I5.5 GASEOUS INTERCHANGES THROUGH THE VISCERAL PLEURA OF THE CAT. By M. KREMER, A. T. WILSON AND SAMSON WRIGHT. (Department of Physiology, Middlesex Hospital Medical School.) (Received
414 6I2.22I:6I2.2I5.5 GASEOUS INTERCHANGES THROUGH THE VISCERAL PLEURA OF THE CAT. By M. KREMER, A. T. WILSON AND SAMSON WRIGHT. (Department of Physiology, Middlesex Hospital Medical School.) (Received
DURING the course of certain investigations it became
 VOLUMETRIC DETERMINATION OF ETHER OR CYCLOPROPANE, CARBON DIOXIDE, NITROUS OXIDE AND OXYGEN IN ANESTHETIC MIXTURES By F. J. PRIME DURING the course of certain investigations it became necessary to be able
VOLUMETRIC DETERMINATION OF ETHER OR CYCLOPROPANE, CARBON DIOXIDE, NITROUS OXIDE AND OXYGEN IN ANESTHETIC MIXTURES By F. J. PRIME DURING the course of certain investigations it became necessary to be able
Physiology Unit 4 RESPIRATORY PHYSIOLOGY
 Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
P215 Respiratory System, Part 2
 P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
plethysmographic methods that when the subject was pinched on the upper
 24 J. Physiol. (I95I) II2, 24-2I 6I2.I5.6II.976 THE DECREASE IN HAND BLOOD FLOW FOLLOWING INFLATION OF AN ARTERIAL OCCLUSION CUFF ON THE OPPOSITE ARM BY IAN C. RODDIE From the Department of Physiology,
24 J. Physiol. (I95I) II2, 24-2I 6I2.I5.6II.976 THE DECREASE IN HAND BLOOD FLOW FOLLOWING INFLATION OF AN ARTERIAL OCCLUSION CUFF ON THE OPPOSITE ARM BY IAN C. RODDIE From the Department of Physiology,
GAS CYLINDERS RULES, 2004
 GAS CYLINDERS RULES, 2004 In exercise of the powers conferred by sections 5 and 7 of the Explosives Act, 1884 (4 of 1884) and in suppression of the Gas Cylinders Rules, 1981, except in respect things done
GAS CYLINDERS RULES, 2004 In exercise of the powers conferred by sections 5 and 7 of the Explosives Act, 1884 (4 of 1884) and in suppression of the Gas Cylinders Rules, 1981, except in respect things done
Alveolus and Respiratory Membrane
 Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Respiration. Figure 22: Schematic representation of the respiratory system
 Respiration One of the seven characteristics of something which is living is respiration. Strictly speaking, respiration is the process that takes place at cellular level and is one of three different
Respiration One of the seven characteristics of something which is living is respiration. Strictly speaking, respiration is the process that takes place at cellular level and is one of three different
Department of Biology Work Sheet Respiratory system,9 class
 I. Name the following : Department of Biology Work Sheet Respiratory system,9 class 1. A muscular sheet separating the thoracic and abdominal cavities. 2. A respiratory tube supported by cartilaginous
I. Name the following : Department of Biology Work Sheet Respiratory system,9 class 1. A muscular sheet separating the thoracic and abdominal cavities. 2. A respiratory tube supported by cartilaginous
Circulatory And Respiration
 Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Gas Exchange in Animals. Uptake of O2 from environment and discharge of CO2. Respiratory medium! water for aquatic animals, air for terrestial
 Gas Exchange in Animals Uptake of O2 from environment and discharge of CO2 Respiratory medium! water for aquatic animals, air for terrestial Respiratory surface! skin, gills, lungs Circulatory System O2/CO2
Gas Exchange in Animals Uptake of O2 from environment and discharge of CO2 Respiratory medium! water for aquatic animals, air for terrestial Respiratory surface! skin, gills, lungs Circulatory System O2/CO2
HCO - 3 H 2 CO 3 CO 2 + H H H + Breathing rate is regulated by blood ph and C02. CO2 and Bicarbonate act as a ph Buffer in the blood
 Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
Respiratory System Physiology. Dr. Vedat Evren
 Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
VOLUNTARY BREATHHOLDING. I. PULMONARY GAS
 VOLUNTARY BREATHHOLDING. I. PULMONARY GAS EXCHANGE DURING BREATHHOLDING'1 By CHARLES D. STEVENS, EUGENE B. FERRIS, JOSEPH P. WEBB, GEORGE L. ENGEL, AND MYRTLE LOGAN (From the Departments of Internal Medicine
VOLUNTARY BREATHHOLDING. I. PULMONARY GAS EXCHANGE DURING BREATHHOLDING'1 By CHARLES D. STEVENS, EUGENE B. FERRIS, JOSEPH P. WEBB, GEORGE L. ENGEL, AND MYRTLE LOGAN (From the Departments of Internal Medicine
BREATHING AND EXCHANGE OF GASES
 96 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 17 BREATHING AND EXCHANGE OF GASES MULTIPLE CHOICE QUESTIONS 1. Respiration in insects is called direct because a. The tissues exchange O 2 directly with the air in
96 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 17 BREATHING AND EXCHANGE OF GASES MULTIPLE CHOICE QUESTIONS 1. Respiration in insects is called direct because a. The tissues exchange O 2 directly with the air in
PICU Resident Self-Study Tutorial The Basic Physics of Oxygen Transport. I was told that there would be no math!
 Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
that, as a means of progression, walking is suitable for lower speeds
 2 6I2 744.22 ENERGY EXPENDITURE IN WALKING AND RUNNING. BY M. OGASAWARA. (From the Department of Industrial Physiology, London School of Hygiene and Tropical Medicine.) (Received February 28, 1934.) IT
2 6I2 744.22 ENERGY EXPENDITURE IN WALKING AND RUNNING. BY M. OGASAWARA. (From the Department of Industrial Physiology, London School of Hygiene and Tropical Medicine.) (Received February 28, 1934.) IT
Structures of the Respiratory System include:
 Respiratory System Structures of the Respiratory System include: ü Oral Cavity ü Nasal Cavity ü Pharynx ü Epiglottis ü Larynx ü Trachea ü Diaphragm ü Lung ü Bronchus ü Bronchioles ü Alveolus ü Pulmonary
Respiratory System Structures of the Respiratory System include: ü Oral Cavity ü Nasal Cavity ü Pharynx ü Epiglottis ü Larynx ü Trachea ü Diaphragm ü Lung ü Bronchus ü Bronchioles ü Alveolus ü Pulmonary
transfer, in part to difference of opinion regarding the mechanics of
 6I2.235 ON THE PARTIAL PRESSURES OF OXYGEN AND CARBON DIOXIDE IN ARTERIAL BLOOD AND ALVEOLAR AIR. By A. V. BOCK, D. B. DILL, H. T. EDWARDS, L. J. HENDERSON AND J. H. TALBOTT. (From the Fatigue Laboratory,
6I2.235 ON THE PARTIAL PRESSURES OF OXYGEN AND CARBON DIOXIDE IN ARTERIAL BLOOD AND ALVEOLAR AIR. By A. V. BOCK, D. B. DILL, H. T. EDWARDS, L. J. HENDERSON AND J. H. TALBOTT. (From the Fatigue Laboratory,
Chapter 11: Respiratory System Review Assignment
 Name: Date: Mark: / 45 Chapter 11: Respiratory System Review Assignment Multiple Choice = 45 Marks Identify the choice that best completes the statement or answers the question. 1. Which of the following
Name: Date: Mark: / 45 Chapter 11: Respiratory System Review Assignment Multiple Choice = 45 Marks Identify the choice that best completes the statement or answers the question. 1. Which of the following
Exploring the relationship between Heart Rate (HR) and Ventilation Rate (R) in humans.
 Exploring the relationship between Heart Rate (HR) and Ventilation Rate (R) in humans. The Research Question In this investigation I will be considering the following general research question: Does increased
Exploring the relationship between Heart Rate (HR) and Ventilation Rate (R) in humans. The Research Question In this investigation I will be considering the following general research question: Does increased
Respiratory System. Part 2
 Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Human gas exchange. Question Paper. Save My Exams! The Home of Revision. Cambridge International Examinations. 56 minutes. Time Allowed: Score: /46
 Human gas exchange Question Paper Level Subject Exam oard Topic Sub Topic ooklet O Level iology ambridge International Examinations Respiration Human gas exchange Question Paper Time llowed: 56 minutes
Human gas exchange Question Paper Level Subject Exam oard Topic Sub Topic ooklet O Level iology ambridge International Examinations Respiration Human gas exchange Question Paper Time llowed: 56 minutes
Breathing: The normal rate is about 14 to 20 times a minute. Taking in of air is called Inspiration and the forcing out of air is called Expiration.
 Biology 12 Respiration Divisions of Respiration Breathing: entrance and exit of air into and out of the lungs External Respiration: exchange of gases(o2 and CO2) between air (in alveoli) and blood Internal
Biology 12 Respiration Divisions of Respiration Breathing: entrance and exit of air into and out of the lungs External Respiration: exchange of gases(o2 and CO2) between air (in alveoli) and blood Internal
2.1.1 List the principal structures of the
 physiology 2.1.1 List the principal structures of the The principle structures of the respiratory are: Nose/Mouth used for inhalation of oxygen-rich air and expelling carbon dioxide rich air Pharynx -
physiology 2.1.1 List the principal structures of the The principle structures of the respiratory are: Nose/Mouth used for inhalation of oxygen-rich air and expelling carbon dioxide rich air Pharynx -
Monday, ! Today: Respiratory system! 5/20/14! Transport of Blood! What we ve been covering! Circulatory system! Parts of blood! Heart! tubing!
 Monday, 5.19.14! What we ve been covering! Circulatory system! Parts of blood! Heart! tubing! Transport of Blood! What is transported! Nutrients! Oxygen! Carbon Dioxide! Hormones! Antibodies! What it is/does!
Monday, 5.19.14! What we ve been covering! Circulatory system! Parts of blood! Heart! tubing! Transport of Blood! What is transported! Nutrients! Oxygen! Carbon Dioxide! Hormones! Antibodies! What it is/does!
Collin County Community College. Lung Physiology
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Levels of CO2 in Arterial Blood of Carp under Carbon Dioxide Anesthesia
 J. Nutr. Sci. Vitaminol., 28, 35-39, 1982 Levels of CO2 in Arterial Blood of Carp under Carbon Dioxide Anesthesia Hisateru MITSUDA, Saburo UENO, Hiroshi MIZUNO, Tadashi UEDA, Hiromi FUJIKAWA, Tomoko NOHARA,
J. Nutr. Sci. Vitaminol., 28, 35-39, 1982 Levels of CO2 in Arterial Blood of Carp under Carbon Dioxide Anesthesia Hisateru MITSUDA, Saburo UENO, Hiroshi MIZUNO, Tadashi UEDA, Hiromi FUJIKAWA, Tomoko NOHARA,
alveoli Chapter 42. Gas Exchange elephant seals gills AP Biology
 alveoli Chapter 42. Gas Exchange gills elephant seals Gas exchange O 2 & CO 2 exchange exchange between environment & cells provides O 2 for aerobic cellular respiration need moist membrane need high
alveoli Chapter 42. Gas Exchange gills elephant seals Gas exchange O 2 & CO 2 exchange exchange between environment & cells provides O 2 for aerobic cellular respiration need moist membrane need high
Chapter 4: Ventilation Test Bank MULTIPLE CHOICE
 Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
AIIMS, New Delhi. Dr. K. K. Deepak, Prof. & HOD, Physiology AIIMS, New Delhi Dr. Geetanjali Bade, Asst. Professor AIIMS, New Delhi
 Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
208 W. 5 th Street, P.O. Box 507 Benton, KY (270) Expanded PVC Sheet, Polyvinyl Chloride Sheet, Foamed PVC
 Section I Manufacturer: Emergency Phone Number: Trade Name: Synonym: Product Identification USA, Inc. 208 W. 5 th Street, P.O. Box 507 Benton, KY 42025 (270) 527-4200 1-800-424-9300 Chemtrec To be used
Section I Manufacturer: Emergency Phone Number: Trade Name: Synonym: Product Identification USA, Inc. 208 W. 5 th Street, P.O. Box 507 Benton, KY 42025 (270) 527-4200 1-800-424-9300 Chemtrec To be used
BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY
 BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY Dr.R.Selvakumar.M.D.D.A.DNB Professor of Anaesthesiology, K.A.P.Viswanatham Govt medical college, Trichy. The current practice of Anaesthesiology demands knowledge
BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY Dr.R.Selvakumar.M.D.D.A.DNB Professor of Anaesthesiology, K.A.P.Viswanatham Govt medical college, Trichy. The current practice of Anaesthesiology demands knowledge
2803/01 Transport. January Mark Scheme
 Transport ADVICE TO EXAMINERS ON THE ANNOTATION OF SCRIPTS 1. Please ensure that you use the final version of the. You are advised to destroy all draft versions. 2. Please mark all post-standardisation
Transport ADVICE TO EXAMINERS ON THE ANNOTATION OF SCRIPTS 1. Please ensure that you use the final version of the. You are advised to destroy all draft versions. 2. Please mark all post-standardisation
SUBCUTANEOUS GAS EQUILIBRATION IN
 Tho'ax (1960), 15, 37. SUBCUTANEOUS GAS EQUILIBRATION IN CLINICAL PRACTICE BY From the Brook General Hospital, Shooters Hill, London When surgical emphysema is deliberately induced by injecting air under
Tho'ax (1960), 15, 37. SUBCUTANEOUS GAS EQUILIBRATION IN CLINICAL PRACTICE BY From the Brook General Hospital, Shooters Hill, London When surgical emphysema is deliberately induced by injecting air under
Gaseous exchange. Grade 11
 z Gaseous exchange Grade 11 z Terminology 1. Breathing 2. Gaseous exchange 3. Diffusion 4. Spongy mesophyll cells 5. Tracheae 6. Gills 7. Alveoli 8. Larynx 9. Diaphragm 10. Endothelium 1. Pleura 2. Squamous
z Gaseous exchange Grade 11 z Terminology 1. Breathing 2. Gaseous exchange 3. Diffusion 4. Spongy mesophyll cells 5. Tracheae 6. Gills 7. Alveoli 8. Larynx 9. Diaphragm 10. Endothelium 1. Pleura 2. Squamous
Safety Data Sheet(SDS)
 Product Name: BMS 309403 Revision Date: 8/30/2018 Safety Data Sheet(SDS) I. PRODUCT AND COMPANY IDENTIFICATION Product Name: BMS 309403 Product Catalog Number: B7794 CAS Number: 300657-03-8 Company: APExBIO
Product Name: BMS 309403 Revision Date: 8/30/2018 Safety Data Sheet(SDS) I. PRODUCT AND COMPANY IDENTIFICATION Product Name: BMS 309403 Product Catalog Number: B7794 CAS Number: 300657-03-8 Company: APExBIO
 2803/01 Transport January 2004 Mark Scheme ADVICE TO EXAMINERS ON THE ANNOTATION OF SCRIPTS 1. Please ensure that you use the final version of the Mark Scheme. You are advised to destroy all draft versions.
2803/01 Transport January 2004 Mark Scheme ADVICE TO EXAMINERS ON THE ANNOTATION OF SCRIPTS 1. Please ensure that you use the final version of the Mark Scheme. You are advised to destroy all draft versions.
PROBLEM SET 9. SOLUTIONS April 23, 2004
 Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Gas exchange. Tissue cells CO2 CO 2 O 2. Pulmonary capillary. Tissue capillaries
 Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
GASEOUS EXCHANGE 17 JULY 2013
 GASEOUS EXCHANGE 17 JULY 2013 Lesson Description In this lesson we: Discuss what is gaseous exchange? Consider requirements of an efficient gaseous exchange surface. Look at diversity in gas exchange systems.
GASEOUS EXCHANGE 17 JULY 2013 Lesson Description In this lesson we: Discuss what is gaseous exchange? Consider requirements of an efficient gaseous exchange surface. Look at diversity in gas exchange systems.
Respiratory Physiology Gaseous Exchange
 Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
AP Biology. Chapter 42. Gas Exchange. Optimizing gas exchange. Gas exchange. Gas exchange in many forms. Evolution of gas exchange structures
 alveoli Chapter 42. Gas Exchange gills elephant seals Gas exchange & C exchange exchange between environment & cells provides for aerobic cellular respiration need moist membrane need high surface area
alveoli Chapter 42. Gas Exchange gills elephant seals Gas exchange & C exchange exchange between environment & cells provides for aerobic cellular respiration need moist membrane need high surface area
What does the % represent on the beakers?
 DISSOLVED OXYGEN VIDEO FAQs What does the % represent on the beakers? What are the glass tubes to beakers for? How is the temperature being kept the same (at 5 o then 35 o )? What is salinity in parts
DISSOLVED OXYGEN VIDEO FAQs What does the % represent on the beakers? What are the glass tubes to beakers for? How is the temperature being kept the same (at 5 o then 35 o )? What is salinity in parts
Pulmonary Circulation Linda Costanzo Ph.D.
 Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
UNIFYING CONCEPTS OF ANIMAL CIRCULATION
 UNIFYING CONCEPTS OF ANIMAL CIRCULATION Every organism must exchange materials with its environment, relying upon diffusion, the spontaneous movement of molecules from an area of higher concentration to
UNIFYING CONCEPTS OF ANIMAL CIRCULATION Every organism must exchange materials with its environment, relying upon diffusion, the spontaneous movement of molecules from an area of higher concentration to
These two respiratory media (air & water) impose rather different constraints on oxygen uptake:
 Topic 19: OXYGEN UPTAKE AND TRANSPORT (lectures 29-30) OBJECTIVES: 1. Be able to compare air vs. water as a respiratory medium with respect to oxygen content, diffusion coefficient, viscosity and water
Topic 19: OXYGEN UPTAKE AND TRANSPORT (lectures 29-30) OBJECTIVES: 1. Be able to compare air vs. water as a respiratory medium with respect to oxygen content, diffusion coefficient, viscosity and water
Retinal vascular response to breathing increased carbon dioxide and oxygen concentrations. Regina Frayser and John B. Hickam
 Retinal vascular response to breathing increased carbon dioxide and oxygen concentrations Regina Frayser and John B. Hickam The retina has a high rate of oxygen consumption, and the retinal vessels are
Retinal vascular response to breathing increased carbon dioxide and oxygen concentrations Regina Frayser and John B. Hickam The retina has a high rate of oxygen consumption, and the retinal vessels are
Gases and Respiration. Respiration Overview I
 Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
Department of Biology Work Sheet Respiratory system
 Department of Biology Work Sheet Respiratory system 1. Name the following : i. A muscular sheet separating the thoracic and abdominal cavities. ii. A respiratory tube supported by cartilaginous rings.
Department of Biology Work Sheet Respiratory system 1. Name the following : i. A muscular sheet separating the thoracic and abdominal cavities. ii. A respiratory tube supported by cartilaginous rings.
Evaluation of Hydropath Clearwell Technology On Carbonate Brine Scaling Using Tube Blocking Method
 Flow Assurance R-4-319 November 24 Evaluation of Hydropath Clearwell Technology On Carbonate Brine Scaling Using Tube Blocking Method Final Report Authors: S. Brown & J. Rohan Work By: J. Rohan Westport
Flow Assurance R-4-319 November 24 Evaluation of Hydropath Clearwell Technology On Carbonate Brine Scaling Using Tube Blocking Method Final Report Authors: S. Brown & J. Rohan Work By: J. Rohan Westport
by the treated lung may only be about one-half the value originally by Atwell, Hickam, Pryor and Page [1951], Peters and Roos [1952],
![by the treated lung may only be about one-half the value originally by Atwell, Hickam, Pryor and Page [1951], Peters and Roos [1952], by the treated lung may only be about one-half the value originally by Atwell, Hickam, Pryor and Page [1951], Peters and Roos [1952],](/thumbs/88/117182097.jpg) THE DEVELOPMENT OF AN INCREASED PULMONARY VASCULAR RESISTANCE BY LOCAL HYPOXIA. By H. HEEMSTRA. From the Physiological Institute, University of Groningen, Netherlands. (Received for publication 2nd December
THE DEVELOPMENT OF AN INCREASED PULMONARY VASCULAR RESISTANCE BY LOCAL HYPOXIA. By H. HEEMSTRA. From the Physiological Institute, University of Groningen, Netherlands. (Received for publication 2nd December
Chapter 7. Coal Cargoes. Properties and Characteristics 7.1
 Chapter 7 Coal Cargoes This chapter, and all of the chapters in Part 2, should be read in conjunction with the booklet Carrying Solid Bulk Cargoes Safely (Reference 16), published by Lloyd s Register/UK
Chapter 7 Coal Cargoes This chapter, and all of the chapters in Part 2, should be read in conjunction with the booklet Carrying Solid Bulk Cargoes Safely (Reference 16), published by Lloyd s Register/UK
BIOLOGY 12: UNIT J - CHAPTER 15 - REVIEW WORKSHEET RESPIRATORY SYSTEM
 BIOLOGY 12: UNIT J - CHAPTER 15 - REVIEW WORKSHEET RESPIRATORY SYSTEM A. CHAPTER REVIEW 1. Define the four components of respiration. 2. What happens to the air as it moves along the air passages? What
BIOLOGY 12: UNIT J - CHAPTER 15 - REVIEW WORKSHEET RESPIRATORY SYSTEM A. CHAPTER REVIEW 1. Define the four components of respiration. 2. What happens to the air as it moves along the air passages? What
Respiratory physiology II.
 Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
Experiment B-3 Respiration
 1 Experiment B-3 Respiration Objectives To study the diffusion process of oxygen and carbon dioxide between the alveoli and pulmonary capillaries. To determine the percentage of oxygen in exhaled air while
1 Experiment B-3 Respiration Objectives To study the diffusion process of oxygen and carbon dioxide between the alveoli and pulmonary capillaries. To determine the percentage of oxygen in exhaled air while
