NORMAL CARDIAC OXYGEN CONSUMPTION
|
|
|
- Camron Blake
- 5 years ago
- Views:
Transcription
1 A THEORETCAL PREDCTON OF THE NORMAL CARDAC OXYGEN CONSUMPTON THEODORE A. WLSON From the Department of Aeronautics and Engineering Mechanics, University of Minnesota, Minneapolis, Minnesota ABsTRAcr A model is described from which the entropy production associated with the process of transporting oxygen and carbon dioxide between the lungs and the muscles of the body can be calculated. The two entropy sources which are assumed to be the dominant ones for this process are the entropy production associated with the metabolism of the heart and the entropy production associated with the diffusion of oxygen and carbon dioxide into and out of the blood. The hypothesis that the observed blood flow is the one for which a given amount of oxygen and carbon dioxide is transported between the lungs and the muscles with minimum total entropy production is used to predict the value of the slope of the cardiac oxygen consumption vs. blood flow curve. At a blood flow of 15 liters/min, the predicted value of the slope of this curve is 1.2 ml/liter. NTRODUCTON There are many biologists who feel that the evolutionary process has resulted in an arrangement of biological systems and a method of operation of biological systems that could be described as a design. One hypothesis for describing the design is this: The parameters of the system have values for which the system can accomplish a given function with minimum entropy production. This hypothesis is motivated by the idea expressed by several physicists over the past century [Boltmann (1886) and Schrodinger (1945), for example] that entropy is the critical thermodynamic quantity in maintaining both the function and the structure of the organism. This hypothesis has been tested as a description of the observed values of the following three parameters: the total ventilation as a function of the total oxygen consumption (Wilson, 1964), the capillary spacing in muscle tissue as a function of oxygen consumption in the muscle (Wilson, 1966), and the diameter of the tubes of the bronchial tree as a function of the location of the tube (Wilson, 1967). n each of these cases a model is postulated from which the total entropy production can be calculated as a function of the value of the parameter being investigated. The value of the parameter for which the total entropy production is minimum is calculated and 585
2 this value is found to agree with the observed values. The success of the hypothesis in describing the values of physiological parameters in these cases perhaps justifies the use of the hypothesis to predict the value of a physiological parameter that has not been established experimentally. n the following analysis, the principle is applied to the problem of describing the blood flow to the skeletal musculature. t is assumed that the circulating blood must supply oxygen to the muscles at a given rate and that the magnitude of the blood flow is chosen so that this function is accomplished with minimum entropy production. There are two entropy sources which depend on the magnitude of the blood flow and which are assumed to be the dominant entropy sources for this problem: the metabolism of the heart and the diffusion of oxygen and carbon dioxide into and out of the blood. The entropy production due to the metabolism of the heart increases with increasing cardiac output while the entropy production in the diffusion process decreases with increasing blood flow. There is a particular blood flow for which the total entropy production is a minimum. n order to carry out this analysis and obtain a hypothetical optimum blood flow which can be compared with the observed flow, the experimentally determined value of the heart metabolism must be known as a function of cardiac output. These data are not available and the principle will be used to predict the slope of the heart metabolism vs. cardiac output curves for a normal adult. The predicted cardiac metabolism is the one required in order that the hypothetical and the observed blood flow agree. ANALYSS A schematic diagram of the process is shown in Fig. 1. n the steady state, the entropy content of the material inside the dashed lines is constant, and the total entropy production inside the box must equal the net entropy flux out of the box. The entropy production due to the metabolism of the heart is balanced by the entropy flux associated with the flux of heat out of the system. The entropy flux associated with the metabolites supplying the heart is neglected. The entropy production due to diffusion is balanced by a net entropy flux out of the box associated with the oxygen and carbon dioxide fluxes. The entropy of the oxygen entering the muscle is greater than the entropy of the oxygen being supplied by the lung and the entropy of the carbon dioxide entering the lung is greater than the entropy of the carbon dioxide being removed from the muscle. The total entropy production or the net entropy flux from the system S is a function of the blood flow Q and the observed blood flow is assumed to be the one for which ds/dq =. The entropy production due to the metabolism of the heart is approximately equal to the energy released divided by the absolute temperature since all of the energy is eventually degraded into heat within the body. The entropy production in the gas transport can be evaluated as a function of Q by calculating the concentration of the oxygen and carbon dioxide in the muscle tissue as a function 586 BOPHYSCAL JOURNAL VOLUME
3 of Q since the entropy of the gases in the lung is fixed, independent of Q. Assuming that the dissolved gases have the properties of an ideal solution, the specific entropy s of the dissolved gases is a function of the temperature T which is independent of Q and of the logarithm of the concentration in the tissues which depends on Q LUNG P 2 AND P 2 GVEN s = K(T)-R lnc. (1) 11 FGuRE 1 A schematic diagram showing the system for which an entropy balance is made. The part of the system inside the dashed line is assumed to be in a steady state so that the entropy production in this part of the system is balanced by a net entropy flux across the dashed-line boundary. MUSCLE Po2 AND F 2 DEPEND ON Then ds/dq is given by equation (2) - ds HdVH,o, _ R dc2 + RMdQR dq-t dq VMO2C2dQ VC2 ' C C2 dq where the symbols are as follows: S total entropy production in cal/degree mi, Q blood flow rate in liters/min, V.2 im,o2 cardiac oxygen consumption in ml/min, oxygen consumption of the muscles in liters/min, (2) THEoDoRE A. WLSON Cardiac Oxygen Consumption 587
4 FM,CO2 Co2 Cc2 H= carbon dioxide production of the muscles in liters/min, oxygen concentration in the muscle tissue carbon dioxide concentration in the muscle tissue. 5 cal/ml, the energy released per milliliter of oxygen consumed by the heart, 3K.9 cal/degree liter STP, the universal gas constant 7 - e 6 E 2 QU e C) m co ' VOL x 2 'i' OXYGEN TENSON PO2 N mm HG FGURE 2 A diagram showing the oxygen and carbon dioxide content in liters STP per liter of blood for blood in equilibrium with the oxygen and carbon dioxide tension shown on the abscissa and ordinate. The assumed arterial tensions are marked by a cross. The locus of possible venous tension for an (RQ) of.85 is marked by a dashed line. 11 The last two terms in equation (2) have different signs because the fluxes of oxygen and carbon dioxide are in opposite directions. n order for S to be a minimum at the observed value of Q, ds/dq must be ero at the observed value of Q. The predicted value of d1'h,o2/dq is obtained by setting the right-hand side of equation (2) equal to ero and evaluating the last two terms at the observed value of Q corresponding to any given J'M,O2 _H_2 dq = 5.4 [JMo2 dco2 _ - C2 M,CO2 dcco2l Q1B8 dq CCO2 dqj (3) 588 BoPHYsCAL JOURNAL VOLUME
5 The rest of the analysis consists of graphical evaluation of the right-hand side of equation (3) using the blood chemistry characteristics and a model for the distribution of the circulating blood. 32 VENOUS OXYGEN 58 TENSON N MUSCLE w - U) 5O O 52 a 1-7 < X VENOUS CARBON DOXDE RATO OF BLOOD SUPPLYTOOXYGENCONSUMPTENSON N MUSCLE RATO OF BLOOD SUPPLY TO OXYGEN CONSUMPTON OMM FGURE 3 The venous oxygen tension and venous carbon dioxide tension is plotted against the parameter, blood flow to the muscles divided by oxygen consumption of the muscles. Points on these curves correspond to points along the locus shown in Fig. 2. The respiratory quotient (RQ) is assumed to be.85 VM,CO2 =. 85 VM,O2 * (4) The partial pressure of oxygen and carbon dioxide in the lung and the corresponding tensions in the arterial blood are assumed to be 1 and 4 mm Hg, respectively. The locus of possible venous gas tensions for (RQ) =.85 is shown in Fig. 2. The particular venous tension on this locus is determined by the value of QM/ VM,2 where QM is the blood flow to the muscles. The venous oxygen and carbon dioxide tensions Pv,o2 and PV.C2 are plotted as a function of QM/ VM,2 in Fig. 3. THEODORE A. WLSON Cardiac Oxygen Consumption 589
6 The concentration of oxygen and carbon dioxide in the muscle is assumed to be in equilibrium with the venous oxygen and carbon dioxide tensions. This is a very good approximation in the case of carbon dioxide which has a large diffusion coefficient. The diffusion of oxygen through the tissues outside of the capillaries is slow enough that the oxygen concentration varies from point to point. The average oxygen concentration is nearly equal to the one assumed here (Wilson, 1966). f it is 18 BLOOD FLOW VS. TOTAL OXYGEN CONSUMPTON L._ 9 -Jo 1 _ 8 BLOOD FLOW TO THE MUSCLES * BLOOD FLOW TO THE ORGANS AND OTHER TSSUES TOTAL OXYGEN CONSUMPTON %2 N liters/mn FGURE 4 The assumed distribution of the total blood flow between the muscles and the organs and other tissues as a function of the total oxygen consumption. further assumed that the concentration of the dissolved gas is linearly proportional to the tension with which it is in equilibrium, then the concentration appearing in equation (3) can be replaced by the venous tensions dco2-1 dpv,2 C2 dq PV12 dq 1 dcco2-1 dpvc2 Cco2 dq PV,Co2 dq t can be seen from Fig. 3 that for a fixed value of 1'MF,2, the value of dpv,o2/dq (5) 59 BOPHYSCAL JOURNAL VOLUME
7 is positive and dpv,co2/dq is negative. The entropy production due to diffusion therefore decreases with increasing blood flow. This result is consistent with the idea that the diffusion of oxygen takes place across a drop in the chemical potential between the lung and the venous blood, for example, and that the magnitude of the chemical potential difference decreases with increasing blood flow. An assumption concerning the distribution of the total blood flow between the muscles and the organs and other tissues is needed to complete the model. n Fig. 4, the total blood flow is shown as a function of the total oxygen consumption. This curve is a composite of the experimentally determined curves for four normal adults a. Uf) x w - W E ~ ZO.8- CALCULATED SLOPE OF CARDAC OXYGEN CONSUMPTON CURVE d1vh /do v S. LJ a- (f-j 'a > C).61- Q4- TOTAL BLOOD FLOW - -j TOTAL BLOOD FLOW N liter/mn FGURE 5 The calculated value of dvh,o2/dq in milliliters per liter as a function of total blood flow. (Bock, Van Caulert, Dill, Foiling, and Hurxthal, 1928). At rest, the total blood flow is 7 liters/min. At rest, 17 % of the total blood flow is used to supply the muscles and 83 % of the total blood flow is used to supply the organs and other tissues. t is assumed that the oxygen consumption of the organs and other tissues and the blood supply to the organs and other tissues is constant, independent of total oxygen consumption, at.22 liters/min oxygen consumption and 5.8 liters/min blood flow. The blood supply to certain organs decreases with increasing load of exercise while the supply to other organs increases. Certainly, the most important contribution to the increase in the total blood flow with increasing oxygen consumption during exercise is the increase in the blood supply to the muscles. The blood supply to the THEODORE A. WLSON Cardiac Oxygen Consumption 591
8 muscles is assumed to increase from 1.2 liters/min at rest to 13.5 liters/min at a total oxygen-consumption level of 2. liters/min. Now the right-hand side of equation (3) can be evaluated graphically using Figs. 2, 3, and 4. For example, at a total oxygen consumption of 1. liters/min, the oxygen consumption of the muscle is.78 liters/min, the total blood flow is 12.2 liters/min, the blood flow to the muscles is 6.4 liters/min, and QM/ VM,O, = 8.2. From Fig. 2, it can be seen that Pv,o2 = 21.5 mm Hg. Now the same figure can be used to evaluate VM,O2(dPv, o2/dq) by taking F~M,2 as fixed and measuring the slope of the E -5 D CARDAC OXYGEN - U) 4 CONSUMPTON _- o > 3 A--- ~~x p b P~~_--X, U) B-Xi 2_ < 2. MECHANCAL WORK o 1 c ~~~~~~OUTPUT w at a TOTAL BLOOD FLOW N liters/mn FGURE 6 Curves showing cardiac oxygen consumption as a function of total blood flow. P, the value predicted from the hypothesis. A, B, and C, values inferred from various experimental data. X, two points for the human heart in vivo. The oxygen consumption equivalent of the mechanical work output of the heart is also shown for comparison. curve for an imagined variation of Q about its observed value. The value at this point is Similarly, VM,O2dP,, - 15 PV.2 dq VM,CO2 dpv.c2-3 PV.C2 dq 592 BOPHYSCAL JOURNAL VOLUME
9 The predicted value of (dt>'11,2/dq) at Q = 12.2 is (d1'1h,2/dq) =.97 ml/liter The value of dj,2/dq calculated in this way for the range of Q is shown in Fig. 5. This curve can be integrated to give i'h,2 except for the constant of integration, the value of 1H,O, at rest. This value is taken to be 25 ml/min and the resulting curve of cardiac oxygen consumption vs. cardiac output is given in Fig. 6. The oxygen equivalent of the mechanical work done by the heart is also shown in Fig. 6. The predicted efficiency varies from 16 % at rest to 45 % at 1t2 = 2. liters/min. AVALABLE EXPERMENTAL EVDENCE Some indications of the value of the normal cardiac oxygen consumption which can be inferred from the available data on cardiac oxygen consumption are also shown in Fig. 6. Kat and Feinberg (1958) have reviewed the data from experiments on isolated hearts. The best relation between the observed value of cardiac oxygen consumption per 1 g of heart weight and the parameters of heart rate, blood pressure, and cardiac output for three sets of experiments are given below: PH,2 = 6.38 (i4.33) +.12 (-.3) (BP) +.1 (4.1)Q V11C2 = (i3.33) (i.74) (HR) X (BP) VH1,2 X (-4.5)Q = 6.58 (-2.88) +.6 (i.34) (HR) X (BP) X lo-, +. (-.2)Q n these relations, BP is mean arterial blood pressure in millimeters of Hg and HR is heart rate in beats per minute. The curves labeled A, B, and C in Fig. 6 are obtained from these relations by using the representation of the physiological values of blood pressure and heart rate given below. These relations represent the data reported by Bock et al. (1928) (BP) = Q (HR) = Q. The heart weight was taken as 2 g in calculating the curves shown on the figure. This is a small heart weight but the experiments were done with smaller hearts and the scaling would not be expected to be linear in heart weight. The uncertainty in the coefficients of relations A, B, and C and the differences between the curves obtained from these relations are at least in part due to the fact that each of the independent variables in these relations were varied independently over wide ranges in (A) (B) (C) THEODORE A. WLSON Cardiac Oxygen Consumption 593
10 the experiments and many of the data are for the heart operating under conditions much different from the normal physiological conditions. Lombardo, Rose, Taeschler, Tuluy, and Bing (1953) have reported measurements of the oxygen consumption of the left ventrical of the human heart in vivo. Only two points for a single normal subject are given. These are shown by the two crosses in Fig. 6. The only feature of this experimental information that is significant for comparison with the results of the analysis in this paper is the slope of the curve of Fig. 6 since the placement of the predicted curve is not obtained from the analysis. The available experimental data do not seem to allow a conclusive comparison. Perhaps it indicates a slightly higher slope than the predicted one. DSCUSSON t is probably worth mentioning again that the application of the minimum-entropyproduction design hypothesis has been reversed in this analysis. The point of view normally assumed in applying the hypothesis would be to take the cardiac-oxygenconsumption characteristics and the blood chemistry characteristics as given and to assume the blood flow to be free to take any value. The hypothesis states that the observed flow is the one for which the total entropy production is minimum. However, since the cardiac oxygen consumption has not been measured over the whole range of cardiac output under normal conditions, the calculation was inverted in order to find the cardiac oxygen consumption characteristics which must exist in order for the hypothetical blood flow to agree with the observed flow. The quantity directly calculated is the slope of the cardiac oxygen consumption curve dvh,o,/dq. This slope is found as a function of only one variable, the blood flow rate. The assumptions used in the model have a smaller effect on the calculation at higher blood flow. The critical test of the hypothesis is that the value of dvh,o,/dq should be approximately equal to 1.2 ml/liter for a value of Q near 15 liters/min. This work was supported by NH grant HE Received for publication 24 March REFERENCES BOCK, A. V., C. VAN CAULERT, D. B. DLL, A. FOLLNG, and L. M. HURXrHAL J. Physiol. 66:136. BOLTZMANN, L Address to the Kaiserliche Academie Der Wissenschaften. KATZ, L. N., and H. FENBERG Circulation Res. 6:656. LOMBARDO, T. A., L. ROSE, M. TAESCHLER, S. TuLuY, and R. J. BNG Circulation 7:71. SCHRODNGER, E What s Life? The MacMillan Company, New York. WLSON, T. A Experienitia 2:333. WLSON, T. A J. Theoret. Biol. 11:436. WLSON, T. A Nature 213: BoPHYSCAL JOURNAL VOLUME
Lung Volumes and Capacities
 Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
I Physical Principles of Gas Exchange
 Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Section Three Gas transport
 Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
Collin County Community College. Lung Physiology
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
CHAPTER 6. Oxygen Transport. Copyright 2008 Thomson Delmar Learning
 CHAPTER 6 Oxygen Transport Normal Blood Gas Value Ranges Table 6-1 OXYGEN TRANSPORT Oxygen Dissolved in the Blood Plasma Dissolve means that the gas maintains its precise molecular structure About.003
CHAPTER 6 Oxygen Transport Normal Blood Gas Value Ranges Table 6-1 OXYGEN TRANSPORT Oxygen Dissolved in the Blood Plasma Dissolve means that the gas maintains its precise molecular structure About.003
PICU Resident Self-Study Tutorial The Basic Physics of Oxygen Transport. I was told that there would be no math!
 Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1
 AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
Douglas and Haldane(2) has shown that the oxygen determinations. since it forms the basis of the "Coefficient of Utilisation" (Krrogh) and
 THE MEASUREMENT OF THE OXYGEN CONTENT OF THE MIXED VENOUS BLOOD, AND OF THE VOLUME OF BLOOD CIRCULATING PER MINUTE. BY J. BARCROFT, F. J. W. ROUGHTON AND R. SHOJI. (From the Physiological Laboratory, Cambridge.)
THE MEASUREMENT OF THE OXYGEN CONTENT OF THE MIXED VENOUS BLOOD, AND OF THE VOLUME OF BLOOD CIRCULATING PER MINUTE. BY J. BARCROFT, F. J. W. ROUGHTON AND R. SHOJI. (From the Physiological Laboratory, Cambridge.)
ALVEOLAR - BLOOD GAS EXCHANGE 1
 ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
transfer, in part to difference of opinion regarding the mechanics of
 6I2.235 ON THE PARTIAL PRESSURES OF OXYGEN AND CARBON DIOXIDE IN ARTERIAL BLOOD AND ALVEOLAR AIR. By A. V. BOCK, D. B. DILL, H. T. EDWARDS, L. J. HENDERSON AND J. H. TALBOTT. (From the Fatigue Laboratory,
6I2.235 ON THE PARTIAL PRESSURES OF OXYGEN AND CARBON DIOXIDE IN ARTERIAL BLOOD AND ALVEOLAR AIR. By A. V. BOCK, D. B. DILL, H. T. EDWARDS, L. J. HENDERSON AND J. H. TALBOTT. (From the Fatigue Laboratory,
UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT
 UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION
UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION
RESPIRATORY REGULATION DURING EXERCISE
 RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
The physiological functions of respiration and circulation. Mechanics. exercise 7. Respiratory Volumes. Objectives
 exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
Chapter 4: Ventilation Test Bank MULTIPLE CHOICE
 Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
P215 Respiratory System, Part 2
 P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
IV. FROM AQUATIC TO ATMOSPHERIC BREATHING: THE TRACHEA & THE LUNG
 GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
By: Aseel Jamil Al-twaijer. Lec : physical principles of gas exchange
 By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
RESPIRATORY GAS EXCHANGE
 RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
The Gas Laws: Boyle's Law and Charles Law
 Exercise 6 Page 1 Illinois Central College CHEMISTRY 130 Name The Gas Laws: Boyle's Law and Charles Law Objective The simple laws governing the properties of gases can be readily demonstrated experimentally.
Exercise 6 Page 1 Illinois Central College CHEMISTRY 130 Name The Gas Laws: Boyle's Law and Charles Law Objective The simple laws governing the properties of gases can be readily demonstrated experimentally.
Chemistry 12 Notes on Graphs Involving LeChatelier s Principle
 Chemistry 12 Notes on Graphs Involving LeChatelier s Principle 1. Temperature Changes When a system adjusts due to a temperature change, there are no sudden changes in concentration of any species, so
Chemistry 12 Notes on Graphs Involving LeChatelier s Principle 1. Temperature Changes When a system adjusts due to a temperature change, there are no sudden changes in concentration of any species, so
The Variation of Muscle Oxygen Consumption With Velocity of Shortening
 The Variation of Muscle Oxygen Consumption With Velocity of Shortening R.J. BASKIN From the Department of Zoology, University of California, Davis ABSTRACT Total oxygen consumption following contraction
The Variation of Muscle Oxygen Consumption With Velocity of Shortening R.J. BASKIN From the Department of Zoology, University of California, Davis ABSTRACT Total oxygen consumption following contraction
Section Two Diffusion of gases
 Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Respiration. Figure 22: Schematic representation of the respiratory system
 Respiration One of the seven characteristics of something which is living is respiration. Strictly speaking, respiration is the process that takes place at cellular level and is one of three different
Respiration One of the seven characteristics of something which is living is respiration. Strictly speaking, respiration is the process that takes place at cellular level and is one of three different
partial pressure is to be applied to the dissociation curve of fully oxygenated
 6I2. I27. I THE DETERMINATION OF THE CARBON DIOXIDE CONTENT OF THE MIXED VENOUS BLOOD. Part I. The effect of oxygenation and the critical oxygen tension. BY M. C. G. ISRAELS (Platt Physiological Scholar)
6I2. I27. I THE DETERMINATION OF THE CARBON DIOXIDE CONTENT OF THE MIXED VENOUS BLOOD. Part I. The effect of oxygenation and the critical oxygen tension. BY M. C. G. ISRAELS (Platt Physiological Scholar)
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D.
 Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Respiratory Medicine. A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics. Alveolar Gas Equation. See online here
 Respiratory Medicine A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics See online here Alveolar gas equation helps to calculate the partial pressure of oxygen in alveoli and A-a gradient is the
Respiratory Medicine A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics See online here Alveolar gas equation helps to calculate the partial pressure of oxygen in alveoli and A-a gradient is the
12. Laboratory testing
 12. Laboratory testing The performance lab of a Sports Medical Center offers various tests. In this paper we elaborate the testing of the aerobic system of a runner on a treadmill. To test the aerobic
12. Laboratory testing The performance lab of a Sports Medical Center offers various tests. In this paper we elaborate the testing of the aerobic system of a runner on a treadmill. To test the aerobic
iworx Sample Lab Experiment HE-5: Resting Metabolic Rate (RMR)
 Experiment HE-5: Resting Metabolic Rate (RMR) Before Starting 1. Read the procedures for the experiment completely before beginning the experiment. Have a good understanding of how to perform the experiment
Experiment HE-5: Resting Metabolic Rate (RMR) Before Starting 1. Read the procedures for the experiment completely before beginning the experiment. Have a good understanding of how to perform the experiment
CHAPTER 3: The respiratory system
 CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
Chapter 10 Gases. Characteristics of Gases. Pressure. The Gas Laws. The Ideal-Gas Equation. Applications of the Ideal-Gas Equation
 Characteristics of Gases Chapter 10 Gases Pressure The Gas Laws The Ideal-Gas Equation Applications of the Ideal-Gas Equation Gas mixtures and partial pressures Kinetic-Molecular Theory Real Gases: Deviations
Characteristics of Gases Chapter 10 Gases Pressure The Gas Laws The Ideal-Gas Equation Applications of the Ideal-Gas Equation Gas mixtures and partial pressures Kinetic-Molecular Theory Real Gases: Deviations
Chapter 10: Gases. Characteristics of Gases
 Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
Chapter 10: Gases Learning Outcomes: Calculate pressure and convert between pressure units with an emphasis on torr and atmospheres. Calculate P, V, n, or T using the ideal-gas equation. Explain how the
A Nomogram Of Performances In Endurance Running Based On Logarithmic Model Of Péronnet-Thibault
 American Journal of Engineering Research (AJER) e-issn: 2320-0847 p-issn : 2320-0936 Volume-6, Issue-9, pp-78-85 www.ajer.org Research Paper Open Access A Nomogram Of Performances In Endurance Running
American Journal of Engineering Research (AJER) e-issn: 2320-0847 p-issn : 2320-0936 Volume-6, Issue-9, pp-78-85 www.ajer.org Research Paper Open Access A Nomogram Of Performances In Endurance Running
Oxygen and Carbon dioxide Transport. Dr. Laila Al-Dokhi
 Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
CHAPTER 3: The cardio-respiratory system
 : The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
: The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
HCO - 3 H 2 CO 3 CO 2 + H H H + Breathing rate is regulated by blood ph and C02. CO2 and Bicarbonate act as a ph Buffer in the blood
 Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation
 Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing
 Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
DECOMPRESSION THEORY - NEO-HALDANE MODELS
 DECOMPRESSION THEORY - NEO-HALDANE MODELS This section describes the Haldane or neo-haldane decompression theories. On each dive the divers body takes up inert gasses, like Nitrogen. After the dive the
DECOMPRESSION THEORY - NEO-HALDANE MODELS This section describes the Haldane or neo-haldane decompression theories. On each dive the divers body takes up inert gasses, like Nitrogen. After the dive the
Kinetic Molecular Theory imaginary Assumptions of Kinetic Molecular Theory: Problems with KMT:
 AP Chemistry Ms. Ye Name Date Block Kinetic Molecular Theory Explains properties of gases, liquids, and solids in terms of energy using an ideal gas, an imaginary which fits all the assumptions of kinetic
AP Chemistry Ms. Ye Name Date Block Kinetic Molecular Theory Explains properties of gases, liquids, and solids in terms of energy using an ideal gas, an imaginary which fits all the assumptions of kinetic
Gases and Respiration. Respiration Overview I
 Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
2. State the volume of air remaining in the lungs after a normal breathing.
 CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
Modeling Gas Dynamics in California Sea Lions
 DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Modeling Gas Dynamics in California Sea Lions Andreas Fahlman Department of Life Sciences Texas A&M University-Corpus Christi
DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Modeling Gas Dynamics in California Sea Lions Andreas Fahlman Department of Life Sciences Texas A&M University-Corpus Christi
Pulmonary Circulation Linda Costanzo Ph.D.
 Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
The equation describes anaerobic respiration in muscle cells. How can you tell from the equation that this process is anaerobic?
 Respiration. Name.. Q.Anaerobic respiration happens in muscle cells and yeast cells. The equation describes anaerobic respiration in muscle cells. glucose lactic acid (a) How can you tell from the equation
Respiration. Name.. Q.Anaerobic respiration happens in muscle cells and yeast cells. The equation describes anaerobic respiration in muscle cells. glucose lactic acid (a) How can you tell from the equation
You should be able to: Describe Equipment Barometer Manometer. 5.1 Pressure Read and outline 5.1 Define Barometer
 A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
A P CHEMISTRY - Unit 5: Gases Unit 5: Gases Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great
Table of Contents. By Adam Hollingworth
 By Adam Hollingworth Table of Contents Oxygen Cascade... 2 Diffusion... 2 Laws of Diffusion... 2 Diffusion & Perfusion Limitations... 3 Oxygen Uptake Along Pulmon Capillary... 4 Measurement of Diffusing
By Adam Hollingworth Table of Contents Oxygen Cascade... 2 Diffusion... 2 Laws of Diffusion... 2 Diffusion & Perfusion Limitations... 3 Oxygen Uptake Along Pulmon Capillary... 4 Measurement of Diffusing
Chemistry A Molecular Approach. Fourth Edition. Chapter 5. Gases. Copyright 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
 Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
For more information about how to cite these materials visit
 Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Chem 110 General Principles of Chemistry
 CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
CHEM110 Worksheet - Gases Chem 110 General Principles of Chemistry Chapter 9 Gases (pages 337-373) In this chapter we - first contrast gases with liquids and solids and then discuss gas pressure. - review
SIMULATION OF THE HUMAN LUNG. Noah D. Syroid, Volker E. Boehm, and Dwayne R. Westenskow
 SMULATON OF THE HUMAN LUNG Noah D. Syroid, Volker E. Boehm, and Dwayne R. Westenskow Abstract A human lung simulator was implemented using a model based on the Fick principle. The simulator is designed
SMULATON OF THE HUMAN LUNG Noah D. Syroid, Volker E. Boehm, and Dwayne R. Westenskow Abstract A human lung simulator was implemented using a model based on the Fick principle. The simulator is designed
Fall 2004 Homework Problem Set 9 Due Wednesday, November 24, at start of class
 0.30 Fall 004 Homework Problem Set 9 Due Wednesday, November 4, at start of class Part A. Consider an iron surface which serves as a catalyst for the production of ammonia from nitrogen and hydrogen. The
0.30 Fall 004 Homework Problem Set 9 Due Wednesday, November 4, at start of class Part A. Consider an iron surface which serves as a catalyst for the production of ammonia from nitrogen and hydrogen. The
Aerospace Physiology. Lecture #11 Oct. 7, 2014 Cardiopulmonary physiology. Musculoskeletal Vestibular Neurological Environmental Effects MARYLAND
 Lecture #11 Oct. 7, 2014 Cardiopulmonary physiology Respiratory Cardiovascular Musculoskeletal Vestibular Neurological Environmental Effects 1 2014 David L. Akin - All rights reserved http://spacecraft.ssl.umd.edu
Lecture #11 Oct. 7, 2014 Cardiopulmonary physiology Respiratory Cardiovascular Musculoskeletal Vestibular Neurological Environmental Effects 1 2014 David L. Akin - All rights reserved http://spacecraft.ssl.umd.edu
660 mm Hg (normal, 100 mm Hg, room air) Paco, (arterial Pc02) 36 mm Hg (normal, 40 mm Hg) % saturation 50% (normal, 95%-100%)
 148 PHYSIOLOGY CASES AND PROBLEMS Case 26 Carbon Monoxide Poisoning Herman Neiswander is a 65-year-old retired landscape architect in northern Wisconsin. One cold January morning, he decided to warm his
148 PHYSIOLOGY CASES AND PROBLEMS Case 26 Carbon Monoxide Poisoning Herman Neiswander is a 65-year-old retired landscape architect in northern Wisconsin. One cold January morning, he decided to warm his
Respiratory System Physiology. Dr. Vedat Evren
 Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Pco2 *20times = 0.6, 2.4, so the co2 carried in the arterial blood in dissolved form is more than the o2 because of its solubility.
 Physiology, sheet #9 Oxygen, is first dissolved in the plasma and the cytosol of the rbc, we have around blood constitutes 7% of our body weight, oxygen, in the capillaries is present in the rbc s and
Physiology, sheet #9 Oxygen, is first dissolved in the plasma and the cytosol of the rbc, we have around blood constitutes 7% of our body weight, oxygen, in the capillaries is present in the rbc s and
Introductory Lab: Vacuum Methods
 Introductory Lab: Vacuum Methods Experiments in Modern Physics (P451) In this lab you will become familiar with the various components of the lab vacuum system. There are many books on this topic one of
Introductory Lab: Vacuum Methods Experiments in Modern Physics (P451) In this lab you will become familiar with the various components of the lab vacuum system. There are many books on this topic one of
Gas volume and pressure are indirectly proportional.
 Section 2 The Gas Laws Key Terms Boyle s law Charles s law combined gas law absolute zero Gay-Lussac s law Scientists have been studying physical properties of gases for hundreds of years In 1662, Robert
Section 2 The Gas Laws Key Terms Boyle s law Charles s law combined gas law absolute zero Gay-Lussac s law Scientists have been studying physical properties of gases for hundreds of years In 1662, Robert
1) Kety and others have attempted to predict
 BY J. W. SEVERINGHAUS 2 (From the Department of Anesthesia, Hospital of the University of Pennsylvania, and Harrison Department of Surgical Research, University of Pennsylvania, Philadelphia, Pa.) (Submitted
BY J. W. SEVERINGHAUS 2 (From the Department of Anesthesia, Hospital of the University of Pennsylvania, and Harrison Department of Surgical Research, University of Pennsylvania, Philadelphia, Pa.) (Submitted
Respiratory physiology II.
 Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
Respiratory System. Part 2
 Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Lab #2: Blood pressure and peripheral circulation
 Lab #2: Blood pressure and peripheral circulation Vertebrates have a closed circulatory system where the blood is always enclosed within blood vessels or the heart. Blood is pumped from the heart (the
Lab #2: Blood pressure and peripheral circulation Vertebrates have a closed circulatory system where the blood is always enclosed within blood vessels or the heart. Blood is pumped from the heart (the
Gas Pressure. Pressure is the force exerted per unit area by gas molecules as they strike the surfaces around them.
 Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Gas Exchange ACTIVITY OVERVIEW SUMMARY KEY CONCEPTS AND PROCESS SKILLS KEY VOCABULARY. Teacher s Guide B-75 L A B O R ATO R Y
 Gas Exchange 17 40- to 2 50-minute sessions ACTIVITY OVERVIEW L A B O R ATO R Y SUMMARY This activity explores the role of the respiratory system in the regulation of gases in the blood. Students investigate
Gas Exchange 17 40- to 2 50-minute sessions ACTIVITY OVERVIEW L A B O R ATO R Y SUMMARY This activity explores the role of the respiratory system in the regulation of gases in the blood. Students investigate
Study of an Oxygenation Process in Capillary. in the Presence of Magnetic Field
 Int. Journal of Math. Analysis, Vol. 4,, no. 35, 697-76 Study of an Oxygenation Process in Capillary in the Presence of Magnetic Field ekha Bali* and Usha Awasthi ** Harcourt Butler Technological Institute,
Int. Journal of Math. Analysis, Vol. 4,, no. 35, 697-76 Study of an Oxygenation Process in Capillary in the Presence of Magnetic Field ekha Bali* and Usha Awasthi ** Harcourt Butler Technological Institute,
PHASE EQUILIBRIA: HOW TO CALCULATE OXYGEN SOLUBILITY IN CELL CULTURE MEDIUM
 BE.360J/10.449J SUPPLEMENTARY HANDOUT FALL 2002 PHASE EQUILIBRIA: HOW TO CALCULATE OXYGEN SOLUBILITY IN CELL CULTURE MEDIUM 1. Phase Equilibria Introduction A collection of molecules is in equilibrium
BE.360J/10.449J SUPPLEMENTARY HANDOUT FALL 2002 PHASE EQUILIBRIA: HOW TO CALCULATE OXYGEN SOLUBILITY IN CELL CULTURE MEDIUM 1. Phase Equilibria Introduction A collection of molecules is in equilibrium
Pulmonary Circulation
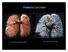 Pulmonary Circulation resin cast of pulmonary arteries resin cast of pulmonary veins Blood Flow to the Lungs Pulmonary Circulation Systemic Circulation Blood supply to the conducting zone provided by the
Pulmonary Circulation resin cast of pulmonary arteries resin cast of pulmonary veins Blood Flow to the Lungs Pulmonary Circulation Systemic Circulation Blood supply to the conducting zone provided by the
A CONCEPT OF MEAN ALVEOLAR AIR AND THE VENTI- LATION -BLOODFLOW RELATIONSHIPS DURING PULMONARY GAS EXCHANGE
 A CONCEPT OF MEAN ALVEOLAR AIR AND THE VENTI- LATION -BLOODFLOW RELATIONSHIPS DURING PULMONARY GAS EXCHANGE HERMANN RAHN From the Department of Physiology and Vital Economics, University @ Rochester, School
A CONCEPT OF MEAN ALVEOLAR AIR AND THE VENTI- LATION -BLOODFLOW RELATIONSHIPS DURING PULMONARY GAS EXCHANGE HERMANN RAHN From the Department of Physiology and Vital Economics, University @ Rochester, School
GASEOUS EXCHANGE 17 JULY 2013
 GASEOUS EXCHANGE 17 JULY 2013 Lesson Description In this lesson we: Discuss what is gaseous exchange? Consider requirements of an efficient gaseous exchange surface. Look at diversity in gas exchange systems.
GASEOUS EXCHANGE 17 JULY 2013 Lesson Description In this lesson we: Discuss what is gaseous exchange? Consider requirements of an efficient gaseous exchange surface. Look at diversity in gas exchange systems.
that, as a means of progression, walking is suitable for lower speeds
 2 6I2 744.22 ENERGY EXPENDITURE IN WALKING AND RUNNING. BY M. OGASAWARA. (From the Department of Industrial Physiology, London School of Hygiene and Tropical Medicine.) (Received February 28, 1934.) IT
2 6I2 744.22 ENERGY EXPENDITURE IN WALKING AND RUNNING. BY M. OGASAWARA. (From the Department of Industrial Physiology, London School of Hygiene and Tropical Medicine.) (Received February 28, 1934.) IT
Homeostasis and Negative Feedback Concepts and Breathing Experiments 1
 Homeostasis and Negative Feedback Concepts and Breathing Experiments 1 I. Homeostasis and Negative Feedback Homeostasis refers to the maintenance of relatively constant internal conditions. For example,
Homeostasis and Negative Feedback Concepts and Breathing Experiments 1 I. Homeostasis and Negative Feedback Homeostasis refers to the maintenance of relatively constant internal conditions. For example,
Figure Vapor-liquid equilibrium for a binary mixture. The dashed lines show the equilibrium compositions.
 Another way to view this problem is to say that the final volume contains V m 3 of alcohol at 5.93 kpa and 20 C V m 3 of air at 94.07 kpa and 20 C V m 3 of air plus alcohol at 100 kpa and 20 C Thus, the
Another way to view this problem is to say that the final volume contains V m 3 of alcohol at 5.93 kpa and 20 C V m 3 of air at 94.07 kpa and 20 C V m 3 of air plus alcohol at 100 kpa and 20 C Thus, the
Oxygen convulsions are believed by many workers to be caused by an accumulation
 272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
Transport of Oxygen and Carbon Dioxide in Blood and Tissue Fluids
 C H A P T E R 4 Transport of Oxygen and Carbon Dioxide in Blood and Tissue Fluids Once oxygen has diffused from the alveoli into the pulmonary blood, it is transported to the peripheral tissue capillaries
C H A P T E R 4 Transport of Oxygen and Carbon Dioxide in Blood and Tissue Fluids Once oxygen has diffused from the alveoli into the pulmonary blood, it is transported to the peripheral tissue capillaries
Introduction. Respiration. Chapter 10. Objectives. Objectives. The Respiratory System
 Introduction Respiration Chapter 10 The Respiratory System Provides a means of gas exchange between the environment and the body Plays a role in the regulation of acidbase balance during exercise Objectives
Introduction Respiration Chapter 10 The Respiratory System Provides a means of gas exchange between the environment and the body Plays a role in the regulation of acidbase balance during exercise Objectives
Applied Physics Topics 2
 Applied Physics Topics 2 Dr Andrey Varvinskiy Consultant Anaesthetist Torbay Hospital, UK EDAIC Paper B Lead and Examiner TOPICS 2 Gas Laws Other Laws: Dalton, Avogadro Critical temperature Critical pressure
Applied Physics Topics 2 Dr Andrey Varvinskiy Consultant Anaesthetist Torbay Hospital, UK EDAIC Paper B Lead and Examiner TOPICS 2 Gas Laws Other Laws: Dalton, Avogadro Critical temperature Critical pressure
Measurement of cardiac output by Alveolar gas exchange - CO 2 -O 2 based methods
 Measurement of cardiac output by Alveolar gas exchange - CO 2 -O 2 based methods Carlo Capelli SS.MM. Università degli Studi di Verona Why CO? V O 2 = CO * (C a C v )O 2 It s a determinat of V O 2 It dictates/modulates
Measurement of cardiac output by Alveolar gas exchange - CO 2 -O 2 based methods Carlo Capelli SS.MM. Università degli Studi di Verona Why CO? V O 2 = CO * (C a C v )O 2 It s a determinat of V O 2 It dictates/modulates
Chapter 3 Atmospheric Thermodynamics
 Chapter 3 Atmospheric Thermodynamics Spring 2017 Partial Pressure and Dalton Dalton's law of partial pressure: total pressure exerted by a mixture of gases which do not interact chemically is equal to
Chapter 3 Atmospheric Thermodynamics Spring 2017 Partial Pressure and Dalton Dalton's law of partial pressure: total pressure exerted by a mixture of gases which do not interact chemically is equal to
PROPERTIES OF GASES. [MH5; Ch 5, (only)]
![PROPERTIES OF GASES. [MH5; Ch 5, (only)] PROPERTIES OF GASES. [MH5; Ch 5, (only)]](/thumbs/76/74446950.jpg) PROPERTIES OF GASES [MH5; Ch 5, 5.1-5.5 (only)] FEATURES OF A GAS Molecules in a gas are a long way apart (under normal conditions). Molecules in a gas are in rapid motion in all directions. The forces
PROPERTIES OF GASES [MH5; Ch 5, 5.1-5.5 (only)] FEATURES OF A GAS Molecules in a gas are a long way apart (under normal conditions). Molecules in a gas are in rapid motion in all directions. The forces
1. What function relating the variables best describes this situation? 3. How high was the balloon 5 minutes before it was sighted?
 Hot-Air Balloon At the West Texas Balloon Festival, a hot-air balloon is sighted at an altitude of 800 feet and appears to be descending at a steady rate of 20 feet per minute. Spectators are wondering
Hot-Air Balloon At the West Texas Balloon Festival, a hot-air balloon is sighted at an altitude of 800 feet and appears to be descending at a steady rate of 20 feet per minute. Spectators are wondering
LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary
 ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
CTB3365x Introduction to Water Treatment
 CTB3365x Introduction to Water Treatment D4b Aeration Doris van Halem Did you know that there are not just gasses in your glass of sparkling coke, but also in the tap water you drink? Welcome to the water
CTB3365x Introduction to Water Treatment D4b Aeration Doris van Halem Did you know that there are not just gasses in your glass of sparkling coke, but also in the tap water you drink? Welcome to the water
iworx Sample Lab Experiment HE-5: Resting Metabolic Rate (RMR)
 Experiment HE-5: Resting Metabolic Rate (RMR) Before Starting 1. Read the procedures for the experiment completely before beginning the experiment. Have a good understanding of how to perform the experiment
Experiment HE-5: Resting Metabolic Rate (RMR) Before Starting 1. Read the procedures for the experiment completely before beginning the experiment. Have a good understanding of how to perform the experiment
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory
 CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
CP Chapter 13/14 Notes The Property of Gases Kinetic Molecular Theory Kinetic Molecular Theory of Gases The word kinetic refers to. Kinetic energy is the an object has because of its motion. Kinetic Molecular
Systems of distribution
 Systems of distribution Outline Distribution of respiratory gases, and in blood Respiratory systems - transport of oxygen to tissues - radically different designs in mammals, birds, insects Vertebrate
Systems of distribution Outline Distribution of respiratory gases, and in blood Respiratory systems - transport of oxygen to tissues - radically different designs in mammals, birds, insects Vertebrate
LABORATORY EXERCISE 1 CONTROL VALVE CHARACTERISTICS
 Date: Name: LABORATORY EXERCISE 1 CONTROL VALVE CHARACTERISTICS OBJECTIVE: To demonstrate the relation between valve stem position and the fluid flow through a control valve, for both linear and equal
Date: Name: LABORATORY EXERCISE 1 CONTROL VALVE CHARACTERISTICS OBJECTIVE: To demonstrate the relation between valve stem position and the fluid flow through a control valve, for both linear and equal
LAB 7 HUMAN RESPIRATORY LAB. Complete the charts on pgs. 67 and 68 and read directions for using BIOPAC
 66 LAB 7 HUMAN RESPIRATORY LAB Assignments: Due before lab: Quiz: Three Respiratory Interactive Physiology Animations pages 69 73. Complete the charts on pgs. 67 and 68 and read directions for using BIOPAC
66 LAB 7 HUMAN RESPIRATORY LAB Assignments: Due before lab: Quiz: Three Respiratory Interactive Physiology Animations pages 69 73. Complete the charts on pgs. 67 and 68 and read directions for using BIOPAC
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/12
 LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
Capnography in the Veterinary Technician Toolbox. Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA
 Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
Chapter 9 Gases: Their Properties and Behavior
 Chapter 9 Gases: Their Properties and Behavior 國防醫學院生化學科王明芳老師 2011-11-15 & 2011-11-22 Chapter 9/1 Gases and Gas Pressure Gas mixtures are homogeneous and compressible. Air-the mixture of gases. Molecular
Chapter 9 Gases: Their Properties and Behavior 國防醫學院生化學科王明芳老師 2011-11-15 & 2011-11-22 Chapter 9/1 Gases and Gas Pressure Gas mixtures are homogeneous and compressible. Air-the mixture of gases. Molecular
Recitation question # 05
 Recitation and Lab # 05 The goal of this recitations / labs is to review material related to the CV and respiratory lectures for the second test of this course. Info required to answer this recitation
Recitation and Lab # 05 The goal of this recitations / labs is to review material related to the CV and respiratory lectures for the second test of this course. Info required to answer this recitation
Respiration - Human 1
 Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
Respiration (revised 2006) Pulmonary Mechanics
 Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
Characterizers for control loops
 Characterizers for control loops By: F. G. Shinskey (May 1999) Introduction Commercial controllers such as the PID series (proportional, integral, derivative, and their combinations) are linear devices
Characterizers for control loops By: F. G. Shinskey (May 1999) Introduction Commercial controllers such as the PID series (proportional, integral, derivative, and their combinations) are linear devices
Human gas exchange. Question Paper. Save My Exams! The Home of Revision. Cambridge International Examinations. 56 minutes. Time Allowed: Score: /46
 Human gas exchange Question Paper Level Subject Exam oard Topic Sub Topic ooklet O Level iology ambridge International Examinations Respiration Human gas exchange Question Paper Time llowed: 56 minutes
Human gas exchange Question Paper Level Subject Exam oard Topic Sub Topic ooklet O Level iology ambridge International Examinations Respiration Human gas exchange Question Paper Time llowed: 56 minutes
(a) (i) Describe how a large difference in oxygen concentration is maintained between a fish gill and the surrounding water.
 1. Answers should be written in continuous prose. Credit will be given for biological accuracy, the organisation and presentation of information and the way in which an answer is expressed. Fick s law
1. Answers should be written in continuous prose. Credit will be given for biological accuracy, the organisation and presentation of information and the way in which an answer is expressed. Fick s law
Circulatory And Respiration
 Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
- How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have
 - How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have the highest blood flow of all organs, which makes them
- How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have the highest blood flow of all organs, which makes them
Modeling Diffusion Rates of a Gas in an Enclosed Space
 Modeling Diffusion Rates of a Gas in an Enclosed Space By: Chirag Kulkarni, Haoran Fei, Henry Friedlander Abstract: This research attempts to identify the relationship between pressure of a certain gas
Modeling Diffusion Rates of a Gas in an Enclosed Space By: Chirag Kulkarni, Haoran Fei, Henry Friedlander Abstract: This research attempts to identify the relationship between pressure of a certain gas
PURE SUBSTANCE. Nitrogen and gaseous air are pure substances.
 CLASS Third Units PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Air is a mixture of several gases, but it is considered to be a pure substance. Nitrogen and
CLASS Third Units PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Air is a mixture of several gases, but it is considered to be a pure substance. Nitrogen and
exchange of carbon dioxide and of oxygen between the blood and the air in
 M. M. HENRY WILLIAMS, JR.*Cardiorespiratory Laboratory, Grasslands WILLIAMS, JR.* Hospital, Valhalla, New York SOME APPLICATIONS OF PULMONARY PHYSIOLOGY TO CLINICAL MEDICINE During the past ten years a
M. M. HENRY WILLIAMS, JR.*Cardiorespiratory Laboratory, Grasslands WILLIAMS, JR.* Hospital, Valhalla, New York SOME APPLICATIONS OF PULMONARY PHYSIOLOGY TO CLINICAL MEDICINE During the past ten years a
