(PCO2 -B). (3) V, pco2 relation. Smyth, Semple and Gelfand, 1958; Lerche, Katsaros, Lerche and Loeschcke,
|
|
|
- Violet Parrish
- 5 years ago
- Views:
Transcription
1 THE EFFECT OF MAINTAINED AMMONIUM CHLORIDE ACIDOSIS ON THE RELATION BETWEEN PULMONARY VENTILATION AND ALVEOLAR OXYGEN AND CARBON DIOXIDE IN MAN. By D. J. C. CUNNINGHAM, D. G. SHAW, S. LAHIRI and B. B. LLOYD. From the University Laboratory of Physiology, Oxford. (Received for publication 2nd June 1961) Five men ingested about 12 g. NH4C1 daily for about a week. Acidaemia was assessed by measuring plasma (HCO3) and alveolar pco,. Before, during and after the period of ingestion the subjects were given various gas mixtures to breathe, and pulmonary ventilation (V) and the alveolar gas pressures (pco,, PO2) were measured in the steady state. The effect of acidaemia on the parameters of the equation V =D(1 +o C)(PCo2 - B) was examined. Only B, which is related to the "CO,-threshold", was consistently changed, being reduced in acidcemia. THE relation between alveolar carbon dioxide pressure (pco2, mm. Hg) and pulmonary ventilation (V, 1./min., B.T.P.S.) may be expressed, over a certain range, by the equation V=S(pCO2 -B).* (1 ) B, the intercept obtained by producing the V, pco2 line to the pco2 axis, is the pco2 of Nielsen's [1936] "apncea point", and is related to the various "CO2-thresholds " in the literature, while S may be termed the "CO2- sensitivity". Nielsen found that in chronic NH4C1 acidosis B was reduced, while S, measured at approximately normal alveolar oxygen pressure (PO2' mm. Hg), remained substantially unchanged. Later workers [Lambertsen, Smyth, Semple and Gelfand, 1958; Lerche, Katsaros, Lerche and Loeschcke, 1960] have obtained similar results, and Lerche et al. have found no difference between the effects of NH4C1, CaCl2 and acetazolamide ("Diamox") on the V, pco2 relation. Lloyd, Jukes and Cunningham [1958] found that the effect of hypoxia could be described by the equation S =D(1 -A ) (2) B being largely unaffected, and it is convenient to combine equations (1) and (2) to give A V=D(1 (PCO2 -B). (3) 323
2 324 Cunningham, Shaw, Lahiri and Lloyd The physiological significance of A and C is still obscure; D is the value of S when there is no hypoxic stimulus to respiration. We have exposed five subjects to various combinations of hypercapnia and hypoxia before, during and after NH4Cl acidosis maintained for some days, and have obtained values of the parameters B, C, D and A. METHODS The subjects were five healthy males (Table I). Blood was drawn and the respiratory parameters determined on most mornings of an day period on subjects 19, 65 and 66, and on alternate mornings, Sundays excluded, of a similar period on subjects 67 and 68. During the middle 5-8 days the subjects took entericcoated capsules of NH4Cl (10-20 g./24-hr. period) throughout the dav, the exact doses being shown in Table I. Subjects 66, 67 and 68 took placebo tablets of entericcoated NaCl when not taking NH4C1, and subject 65 took them during the recovery period after NH4C1. No subject was told what gas mixture he was receiving, and only subject 19 knew what tablets he was taking. All subjects were asked to lead a quiet and orderly life and to avoid excessive exercise during the period of investigation. They were allowed a cup of tea without sugar on rising but were otherwise fasting when they came to the laboratory for experiments in the morning. The apparatus for supplying the subject with any mixture of 02, CO2 and N2, and for measuring V and the alveolar gas pressures has been described [Cunningham, Cormack, O'Riordan, Jukes and Lloyd, 1957]. Appropriate checks were made on the end-tidal sampling. Estimations of pco2 were made with the flowbridge analyser and, with those of P02, in a development of the Haldane apparatus [Lloyd, 1958]. The approximate composition of the inspired gas mixture for a given ventilation and alveolar gas composition was calculated according to equation (1) of Lloyd et al. [ Determination of Respiratory Responses to C02 and Hypoxia.-In a typical experiment on subject 65, he reclined in an adjustable dental chair, and was given four gas mixtures in succession calculated to keep V constant at 20 1./min. and alveolar P02 at 125, 70, 57 and 47 mm.; alveolar pco2 was not allowed to fall below the resting level. On each mixture several observations of V and pco2 were made to ensure that a steady state had been reached: this took up to 20 min. for the first and about 8 min. for subsequent mixtures. End-tidal samples were then collected for immediate or subsequent analysis. The subject was then given seven gas mixtures calculated to keep V between 50 and 60 1./min. and alveolar P02 at 650, 120, 70, 60, 50, 45 and 40 mm., in that order. The aim was to keep V constant by progressive substitution of one respiratory stimulus (hypoxia) for the other (hypercapnia). The subject was usually unaware of changes in the gas mixture at any one level of V. On changing from low to high V there was a preliminary period on a mixture of CO2 and air during which no end-tidal samples were collected. The experiments on subject 19 were similar, but partly exploratory, and the order of gas mixtures varied from experiment to experiment: the linearity of the V, pco2 line at constant P02 was checked in two experiments (19, 13 and 19, 14 in fig. 1). The results on subject 65 adequately confirmed the validity of equation (3), and so the parameters for subjects 66, 67 and 68 were determined by three measurements of PO2' PCO2 and V at high V, and duplicate measurements of one set of values at low V, in each experiment. Sets of measurements were made in the following sequence: first preliminary period: 20 min., V ca /min., P02 ca. 70 mm.; first measurement period: 20 min., V ca /min., P02 ca. 70 mm.; second, third and fourth measurement periods: 20 min. each, V ca /min., P02 ca. 40, 70 and 650 mm.; then gradual lowering of CO2 and O2 until second preliminary period: 20 min., air breathed; fifth measurement period: a repetition of the first measurement period.
3 Ammonium Chloride and Pulmonary Ventilation The two preliminary periods, at high and low V, were found to reduce the differences between the results from the first and fifth measurement periods at low V. Determinations on Blood.-At the beginning of each experiment except the first three on subject 19 the left hand and wrist were immersed in water at C. for a quarter of an hour. 20 ml. blood was drawn from a superficial forearm vein without stasis into a glass syringe with heparin solution in its dead space while the subject was breathing 2 per cent CO2 in air through valves: end-tidal pco2, measured before and during venepuncture, fluctuated little. Most of the blood was transferred to ice-cold centrifuge tubes under paraffin, while some was used for determination of 02 saturation and capacity. 02 saturation and capacity, and plasma C02 content of the " arterialized " venous samples were measured in a modified Haldane blood-gas apparatus [Shaw, 1959] by the method of Courtice and Douglas [1947], the water bath being as usual at room temperature. The arteriovenous (AV) difference for the C02 content of whole blood, obtained from the AV 02 difference by assuming an arterial saturation of 97-5 per cent and a tissue R.Q. of 0-8, was assumed to be distributed equally between the cells and plasma [Dill, Edwards and Consolazio, 1937], and was subtracted from the venous plasma C02 content to give the arterial plasma C02 content. Van Slyke's value, 0-51, for the Bunsen coefficient for C02 in plasma at 38 C. was assumed for the calculation of the arterial plasma [HCO], the arterial pco2 being taken to equal the measured end-tidal pco2. The arterialized venous saturation was above 90 per cent (this corresponds to an AV [HCO] difference of less than 0-6 m.equiv./l.) for forty-two bloods out of forty-six; for three it lay between 86 and 90 per cent, and for the other (experiment 19, 6) it was 35 per cent. There was venous stasis on this occasion and the R.Q. was assumed to be 0 5. This procedure gave an arterial plasma pco2, [HCO3] point for each experiment. Each experiment also yielded a value of B (equation (1)) and the corresponding value, [HCO3IB, for the arterial plasma at this pco2 was obtained by means of small extrapolations based on the nomogram of Dill et al. [1937] for normal young men. 325 RESULTS The daily dosage of NH4Cl and NaCl, the results on blood, and the values of the parameters B, D, A and C are given in Table I. The respiratory results of all experiments are shown in fig. 1, in which V is plotted against pco2, PO2 being given in the legend. Fig. 2 is an enlarged picture of two typical experiments on subject 65, when normal and acidotic, and the P02's are written in against the V, pco2 points. As was to be expected from our previous work, the points at high V in a single experiment are spread out in the direction of the pco2 axis; the spread is greatest at low P02 and diminishes as normal P02 is approached, but is still apparent above it. The decrease in V and/or rise in pco2 on raising P02 to 650 mm. is a consistent finding at high V (in eighteen out of twenty-two experiments in this series) and shows little tendency to change with time, unlike the short-lived effect of high P02 under resting conditions [cf. Dejours, Labrousse, Raynaud, Girard and Teillac, 1958]. The' spread when V is 20 1./min. is much smaller, and the V, pco2 lines at various po2's form a fan. Acidosis had little effect on the shape of the fan, but displaced it bodily to the left; i.e. all pco2's were lower. B of equation (1) was in the first place determined at each P02 by drawing isoxic" lines based on two points at high and low V. The PO2'S of such a
4 326 Cunningham, Shaw, Lahiri and Lloyd TABLE I.-AGE, WEIGHT, HEIGHT AND MONTH OF EXPERIMENT; DAILY DOSAGE OF NH4C1 OR NaCl; MEASURE- MENTS ON ARTERIALIZED VENOUS BLOOD; DERIVED VALUES FOR ARTERIAL PLASMA; ALVEOLAR pco2 DURING VENEPUNCTURE; RESPIRATORY PARAMETERS B, D, A AND C. Experi. g./day a HbO2 Date ment I 02 capacity per m NaCi NH4C cent Derived arterial mm. 1./min./ Hg plasma m.equiv./l. mm. Hg pco2 mm. Hg P02 PC02 [HCOZ] [HCO, ]B Alveolar B D A C Subject 19, 38 years, 182 cm., 91 kg., December a (41-1) Subject 65, 22 years, 190 cm., 72 kg., January Subject 66, 19 years, 184 cm., 84 kg., March Subject 67, 23 years, 191 cm., 85 kg., April
5 Ammonium Chloride and Pulmonary Ventilation TABLE Experi. g./day ~ HbO2 I.-(continued) Derived arterial 1./min. / mm. Hg Date me0nt m - 02 capacity plasma m.equiv./l. mm. HgpCO2 mm. Hg P02 I enacthc ml./l00 ml. per pco2 -~----- NaCl NH4Cl cent [HCO3] [HCO,]B Alveolar B D A C Subject 68, 23 years, 185 cm., 83 kg., April pair of points seldom agreed exactly and the position of each line 327 was fixed by a standard iterative procedure. In subject 65, B was scarcely affected by hypoxia (figs. 1 and 4), the mnean decrease for all experiments being 0-5 mm. pco2 as P02 fell from 120 to 45 mm.; hypoxia thus exerted its effect almost entirely upon S of equation (1), and it was legitimate in this subject to determine a mean value of B for each experiment and to draw V, pco2 lines at the various PO2'S through it and the points at high V (fig. 1). Values of B for subject 19 showed a positive correlation with P02 (fig. 4), unlike the results on subject 65 and on the eight subjects of Lloyd et al. [1958]. In the twelve satisfactory experiments on subject 19, B fell by 1.6 mm. pco2 as P02 fell from 120 to 50 mm., and this relation was used to obtain for each experiment a smoothed set of B's for the PO2's at high V, and a corresponding set of S's (fig. 1). The values of B given for this subject in Table I and fig. 5 are for a P02 of ca. 120 mm. B for the curtailed experiments on the other three subjects was obtained by joining the high point at about 70 mm. pog to the mean of the two low points at about the same P02 and obtaining the intercept on the pco2 axis. Sometimes the line was drawn slightly to one side of the high point in accordance with the difference between the low and high P02'S, though these differences were less than 5 mm. in all but three experiments. A value of S for each of the high points was then obtained by joining them to the intercept B. S is plotted against P02 in fig. 3 for the experiments shown in fig. 2: A, C and D of equation (3) may be obtained from such plots by graphic iteration [Lloyd et at., 1958] or, as has been done here, by fitting a rectangular hyperbola by the method of Hey and Hey [1960] with the help of a suitably programmed computer (e.g. Mercury, Ferranti Ltd.). The curve shown in fig. 3 is that for the experiment before acidosis, and points of the experiment during acidosis lie close to it. When only three pairs of values of S and P02 are available they yield three simultaneous equations by substitution in equation (2) from which values of A, C and D can be calculated explicitly.
6 328 t I Cunningham, ~Shaw, tt 1 *.* Lahtirl -- :1 an( cl T I Lloydt b5.8,j't /i/ 30 / 2* 4 f/k - 3E 33Q -> 4-7K 50 K II4 30I307 S 1/1/ o 1/11/ W" 8 taax/ u X 30 L " Alveolar pccqmm Hq FIG. 1
7 Ammonium Chloride and Pulmonary Ventilation 329 FIG. 1.-The relation between V, pco2 and po9 in normal and acidotic subjects. Each small rectangle refers to one or two experiments on a subject, experiment numbers being given at top left for closed circles * (usually in acidosis) and bottom right for crosses x. The lines have been drawn (with one or two exceptions) through the points at high V and the appropriate vallue of B on the pco2 axis. The values of P02 at a given ventilation usually increase from left to right, as pco2 increases: for subject 65, experiment 65, 1 they are, from left to right at high V (above 30 l./min.)-41, 49, 54, 61, 116, 643; at low V 47, 67, 59, 122. Subject 65 PO2 P0 2 38, 43, 51, 59, 70, 119, 653; 52, 9 61, 69, , 49, 60, 69, 80, 118, 651; 10 52, 62, 71, , 49, 60, 68, 79, 118, 648; 11 51, 60, 67, , 50, 60, 68, 79, 118, 645; 12 47, 59, 67, , 46, 53, 61, 70, 121, 650; 13 50, 62, 70, , 49, 57, 63, 123, 655; 49, 62, , 46, 53, 62, 73, 119, 650; 49, 62, 70, Subject 19 PO. 44, 50, 55, 60, 67, 83, 117; 53, 9 60, 74, , 50, 54, 58, 64, 80, 118; , 49, 54, 58, 67, 81, 116; 50, 11 59, 73, , 51, 54, 58, 69, 117, 630; 52, 12 60, 73, , 49, 53, 58, 69, 117, 630; 13 51, , 45, 52, 58, 70, 116, 650; 50, 14 58, 73, 123 P02 40, 45, 51, 59, 70, 117, 650; 60, , 45, 52, 59, 70, 119, 648; 60 (V20), 68 (V21), , 45, 52, 60, 69, 118, 648; 60, 69, , 47, 53, 58, 73, 122, 652; 61, 70, , 48, 55, 63, 72, 122, 656; 63, 70, , 44, 52, 59, 68, 117, 645; 68, 60, , 49, 48, 48, 50, 48, PO2 47, 50, 55, 79, 630; 51, 59 46, 57, 69, 116, 630; 50, 58, 71, , 47, 53, 57, 68, 115, 627; 50, 57, 72, , , 66, 109, 625; 53, 48, 60, 74, , 48, 50, 55, 66 (V53), 117 (V35), 630, 123; 109, , 47, 51 (V44), 48 (V51), 56 (V46), 67 (V46), 620 (V47), 121 (V42); 49, 63, 47, , 67, 668; 42, 66, 660; 44, 68, 662; 43, 68, 661; 42, 67, 660; 41, 66, 648; 41, 66, 645; 41, 67, 650; PO2 P02 Subject 66 71, , , (V20), 70 (T22) 9 70 (V21), 71 (V22) 10 Subject 67 P02 67, , , , 68, 665; 44, 70, 667; 42, 68, 665; 44, 67, 657; 46, 69, 657; 40, 69, 665; 40, 68, 660; 40, 68, 660; PO2 74, 72 71, 74 80, 70 73, 71 72, 70 PO. 72, 71 71, 70 72, PO2 44, 69, 650; 69, 72 40, 67, 645; 70, 68 43, 70, 660; 72, 71 Subject Where there is ambiguity the figure for V is given in brackets. PO. 42, 70, 665; 74, 72 41, 68, 650; 79, 71 41, 69, 655; 69, 69
8 330 Cunningham, Shaw, Lahiri and Lloyd B.T. P. S. Expt. b5,10 Expt. 65, b5o 59 X b x X X x *,126)0 6 0\67 Aleoa/mCm.m.Hg AlveolarpCOx mm. Hg. FIG. 2.-The effect of acidosis on the VI PCO2, P02 relation. Enlarged picture of experiments 65, 10 and 65, 4 from fig. 1. Crosses x -xperiment before, closed circles 0-experiment during acidosis. Numbers near points indicate P02. The fan Of 'VI pco2 lines is displaced 7 mm. pco2 to the left during acidosis, but is otherwise very similar (see fig. 3). u20 0- E C 0 E E.1 Expts x :AD: X-, D I D bs b50 Alveola r po2, mm Hq. FIG. 3.-The effect of acidosis on the S, P02 relation (equation 2). The slope S of the V, pco2 lines from fig. 2 is plotted against alveolar P02. Crosses x -experiment before, closed circles *- experiment during acidosis. The hyperbola of equation (2) has been fitted to the crosses x. The proximity of the closed circles * to the hyperbola indicates that acidosis has little effect on parameters A, C and D (cf. Table I).
9 Ammonium Chloride and Pulmonary Ventilation 331 The extent to which the four parameters varied from day to day and the effects of ammonium chloride are shown in Table I. Only B varied consistently with the degree of acidosis, and fig. 5 shows the plots of B against (HCO3)B for the five subjects. The absolute values are of some interest. B was close to the resting pco2. C, the critical PO2 at which S approaches infinity, lay around 40 mm. in subject 19 and usually between 20 and 35 mm. in the other, younger, 2 Subject 19 o B - B; * pco2,mm. -2 Subject 6s ; : %0 40 bo 80 1oo 120 Alveolar poz mm. Hg. FIG. 4.-The effect of hypoxia on B (equation 1) for two subjects. For each experiment the mean B has been subtracted from the individual B at each P02, and the differences, B - B, are plotted against the P02's for all experiments on one subject. Hypoxia is associated with a fall in B in subject 19. subjects. D, the limiting value of S when hypoxic stimulation is absent, was between 2.5 and 7 l./min./mm. pco2. AD is the area constant of the hyperbola relating S to PO2 (fig. 3); A, which like C has the dimensions of P02, was more variable and usually lower than C. DiscUSSION We have avoided the sampling of arterial blood because in general blood gas analysis lacks the precision of gas analysis and because arterial puncture for each experiment over the long experimental period seemed unwarranted. The point of the " arterialized " venous blood sample drawn was to characterize the plasma [HCO-], pco2 relations on any one day, and not to determine a particular set of values for its own sake in relation to V. Our work has been based on alveolar gas pressures and we have given reasons in earlier papers for supposing that these are close to arterial gas pressures for CO2 and that the alveolar-arterial PO2 difference is small and
10 332 Cunningham, Shaw, Lahiri and Lloyd nearly constant in healthy young subjects breathing at /min., with scarcely elevated metabolisms. No doubt if accurate arterial gas pressures were available slightly different values for the parameters would emerge, but it is doubtful if the general form of the relations would change, or if our general conclusions would be affected. These experiments throw no light on the a' r5 0.94,r 66 / 67 r:0.97 E E 00 30, / u 30 r 0:.93,e r: Arterial plasma [HC0f8m-equiv/l. FIG. 5.-The correlation between B (equation 1) and [HCO3]B (arterial plasma bicarbonate when pco2=b) in dietary acidlemia. Closed circles *-before and during ingestion of NH4Cl; open circles o after ingestion. Subject numbers are at top left, correlation coefficients at bottom right. The broken lines in the diagram for subject 68 approximately represent the pco,, [HCO ] dissociation curve for true plasma during and after acidosis. question as to which pco2, arterial or venous (jugular or mixed), is an "effective stimulus ", but it is worth noting that if the interaction between hypoxia and CO2 or acid is peripheral [Joels and Neil, 1961], arterial levels are still of importance. In the plots of B against [HCO3]B (fig. 5) the points obtained during the transitions between the normal and acid states lie on substantially the same lines whatever the direction of change, and this absence of hysteresis is consistent with the view that in our experiments there were no long-term adjustments in the mechanism by which CO2 and acid affect V.
11 Ammonium Chloride and Pulmonary Ventilation The exact determination of B is difficult, but it is critically important for assessing the other parameters. Its value is highly dependent on the low-v determinations which, like the resting V and pco2, are subject to minute-tominute, hour-to-hour and day-to-day fluctuations which are greater than the errors of measurement, and due to causes which are certainly not fully known to us: postural variations in our experiments were small. In the period before acid was taken variations in B appear as scatter mainly across the B, [HCO3]B lines shown in fig. 5 and cannot therefore on our evidence be attributed to day-to-day changes in acid-base balance. The evidence for regarding B as being little affected by hypoxia is provided by the experiments of Lloyd et al. [1958] on eight subjects, of Loescheke and Gertz [1958] on five subjects and by the present data on subject 65. The direct correlation of B and P02 in subject 19 (fig. 4), who was some 15 years older than seven of the eight subjects of Lloyd et al. [1958] is exceptional. The effect varied with the plan of the experinients and may be attributed in part to a phenomenon recently noted in this laboratory by which, contrary to expectations, the inhalation of C02-rich mixtures may give rise to a " metabolic " as well as a respiratory acidaemia. Random effects of this phenomenon were largelv avoided by the standardized form of the experiments on subjects In our early experiments [Lloyd et al., 1958] we determined B at each P02 with greater precision than in the present work and at that time we saw a tendency for it to increase slightly in hypoxia. If the direct effect of hypoxia is, as we think, confined to S in equation (1), we would expect an accompanying small increase in B because of the shift towards alkalinity as heemoglobin becomes progressively less oxygenated at constant pco2. This secondary effect would be equivalent to that of ca. 2 mm. pco2 in the most severe hypoxia we have studied. The related acid-base effect that springs from changes in haemoglobin saturation as pco2 changes at constant P02 in a C02-inhalation experiment is smaller. The respiratory parameters other than B show little response to acid; the observation that the response to hypoxia, expressed by A and C, is largely unchanged in dietary acidosis appears to be a new one. The absence of a material change in D in acidosis is relevant to the problem as to which of the factors in what might be termed the Henderson-Hasselbalch complex (pco2, ph, [HCO-], [OH-] etc.) are "direct" stimuli. This problem can also be attacked by examining the correlations between various pairs of variables selected from B, phb, [HCO3]B, [OH-]B, etc. B and [HCO3]B, which are the pair closest to the original measurements, show a high degree of correlation (fig. 5), but at this stage this cannot be taken to mean that pco2 and arterial plasma [HCO-] are necessarily the "direct " factors affecting respiration. The lines drawn in fig. 5 are the reduced major axes, and are quite distinct from pco2, [HCO3] dissociation curves, the approximate slopes of which for normal and acid bloods are shown by the broken lines in the diagram for subject 68. The shift between these two broken lines represents the metabolic acideemia following the ingestion of NH4C1 by this subject. 333
12 334 Cunningham, Shaw, Lahiri and Lloyd ACKNOWLEDGMENTS D. G. S. and S. L. are grateful to Professor E. G. T. Liddell, F.R.S., for laboratory facilities and, respectively, to the Medical Research Council for a Scholarship and to the Government of West Bengal for an Overseas State Scholarship. We thank Mr. T. J. Meadows, Mr. E. Aldsworth and Mr. J. R. Griffiths for skilled assistance, and subjects 65, 66, 67 and 68 for cheerful participation. REFERENCES COURTICE, F. C. and DOUGLAS, C. G. (1947). "The ferricyanide method of blood-gas analysis", J. Physiol. 105, CUNNINGHAM, D. J. C., CORMACK, R. S., O'RIORDAN, J. L. H., JUKES, M. G. M. and LLOYD, B. B. (1957). "An arrangement for studying the respiratory effects in man of various factors", Quart. J. exp. Physiol. 42, DEJOURS, P., LABROUSSE, Y., RAYNAUD, J., GIRARD, F. and TEILLAC, A. ( 1958). "Stimulus oxygene de la ventilation au repos et au cours de l'exercice musculaire, 'a basse altitude (50 m), chez l'homme", Rev. franc. d'etudes clin. et biol. 3, DILL, D. B., EDWARDS, H. T. and CONSOLAZIO, W. V. (1937). "Blood as a physicochemical system. XI", J. biol. Chem. 118, HEY, E. N. and HEY, M. H. (1960). "The statistical estimation of a rectangular hyperbola", Biometrics 16, JOELS, N. and NEIL, E. (1961). "The influence of anoxia and hypercapnia, separately and in combination, on chemoreceptor impulse discharge", J. Physiol. 155, 45-46P. LAMBERTSEN, C. J., SMYTH, M., SEMPLE, S. J. G. and GELFAND, R. (1958). "Respiratory effects in normal men of blood ph change at 'constant' arterial and internal jugular venous Pco2", Fed. Proc. 17, 92. LERCHE, D., KATSAROS, B., LERCHE, G. and LOESCHCKE, H. H. (1960). 'Vergleich der Wirkung verschiedener Acidosen (NH4C1, CaCl, Acetazolamid) auf die Lungenbeliiftung beim Menschen ", Pflug. Arch. ges. Physiol. 270, LLOYD, B. B. (1958). "A development of Haldane's gas analysis apparatus", J. Physiol. 143, 5-6P. LLOYD, B. B., JUKES, M. G. M. and CUNNINGHAM, D. J. C. (1958). "The relation between alveolar oxygen pressure and the respiratory response to carbon dioxide in man", Quart. J. exp. Physiol. 43, LOESCHCKE, H. H. and GERTZ, K. H. (1958). "Einfluss des 0,-Druckes in der Einatmungsluft auf die Atemtatigkeit des Menschen, gepriift unter Konstanthaltung des alveolaren CO2-Druckes", Pfliig. Arch. ges. Physiol. 267, NIELSEN, M. (1936). "Untersuchungen fiber die Atemregulation beim Menschen", Skand. Arch. Physiol. 74, Suppl. 10, SHAW, D. G. (1959). "Some factors affecting respiration in man", B.Sc. thesis. Oxford University.
PACo2 and to CO2 combined with hypoxia were measured in duplicate with an
 THE REPEATABILITY OF VENTILATORY RESPONSES TO EXCESS OF CARBON DIOXIDE AND LACK OF OXYGEN. By J. L. ANDERTON, E. A. HARRIS and K. B. SLAWSON. From the Department of Therapeutics, University of Edinurgh.
THE REPEATABILITY OF VENTILATORY RESPONSES TO EXCESS OF CARBON DIOXIDE AND LACK OF OXYGEN. By J. L. ANDERTON, E. A. HARRIS and K. B. SLAWSON. From the Department of Therapeutics, University of Edinurgh.
J. Physiol. (I941) I00, I98-21I 6I :6I2.825
 198 J. Physiol. (I941) I00, I9821I 6I2.22.02:6I2.825 THE EFFECT OF OXYGEN LACK ON THE CEREBRAL CIRCULATION BY F. C. COURTICE From the Departments of Physiology and of Surgery, Oxford (Received 24 March
198 J. Physiol. (I941) I00, I9821I 6I2.22.02:6I2.825 THE EFFECT OF OXYGEN LACK ON THE CEREBRAL CIRCULATION BY F. C. COURTICE From the Departments of Physiology and of Surgery, Oxford (Received 24 March
Section Three Gas transport
 Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
RESPIRATORY REGULATION DURING EXERCISE
 RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
Respiratory physiology II.
 Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
Physiology Unit 4 RESPIRATORY PHYSIOLOGY
 Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
EFFECT OF ETHAMIVAN (VANILLIC ACID DIETHYLAMIDE) ON THE RESPIRATORY RESPONSE OF HEALTHY YOUNG MEN TO CARBON -DIOXIDE, IN THE ABSENCE OF HYPOXIA
 Brit. J. Pharmacol. (1962), 19, 142-152. EFFECT OF ETHAMIVAN (VANILLIC ACID DIETHYLAMIDE) ON THE RESPIRATORY RESPONSE OF HEALTHY YOUNG MEN TO CARBON -DIOIDE, IN THE ABSENCE OF HYPOIA BY J. L. ANDERTON,
Brit. J. Pharmacol. (1962), 19, 142-152. EFFECT OF ETHAMIVAN (VANILLIC ACID DIETHYLAMIDE) ON THE RESPIRATORY RESPONSE OF HEALTHY YOUNG MEN TO CARBON -DIOIDE, IN THE ABSENCE OF HYPOIA BY J. L. ANDERTON,
PCO2 has been shown to increase with the severity of exercise (Nielsen, 1936).
 554 J. Physiol. (I955) I29, 554-563 THE ROLE OF BODY TEMPERATURE IN CONTROLLING VENTILATION DURING EXERCISE IN ONE NORMAL SUBJECT BREATHING OXYGEN By J. E. COTES From the Pneumoconiosis Research Unit of
554 J. Physiol. (I955) I29, 554-563 THE ROLE OF BODY TEMPERATURE IN CONTROLLING VENTILATION DURING EXERCISE IN ONE NORMAL SUBJECT BREATHING OXYGEN By J. E. COTES From the Pneumoconiosis Research Unit of
Douglas and Haldane(2) has shown that the oxygen determinations. since it forms the basis of the "Coefficient of Utilisation" (Krrogh) and
 THE MEASUREMENT OF THE OXYGEN CONTENT OF THE MIXED VENOUS BLOOD, AND OF THE VOLUME OF BLOOD CIRCULATING PER MINUTE. BY J. BARCROFT, F. J. W. ROUGHTON AND R. SHOJI. (From the Physiological Laboratory, Cambridge.)
THE MEASUREMENT OF THE OXYGEN CONTENT OF THE MIXED VENOUS BLOOD, AND OF THE VOLUME OF BLOOD CIRCULATING PER MINUTE. BY J. BARCROFT, F. J. W. ROUGHTON AND R. SHOJI. (From the Physiological Laboratory, Cambridge.)
technique which have led to increased precision
 THE ACCURACY OF DIRECT DETERMINATIONS OF OXYGEN AND CARBON DIOXIDE TENSIONS IN HUMAN BLOOD IN VITRO 1 By GILES F. FILLEY, ESTHER GAY, AND GEORGE W. WRIGHT (From the Department of Physiology, the Edwtvard
THE ACCURACY OF DIRECT DETERMINATIONS OF OXYGEN AND CARBON DIOXIDE TENSIONS IN HUMAN BLOOD IN VITRO 1 By GILES F. FILLEY, ESTHER GAY, AND GEORGE W. WRIGHT (From the Department of Physiology, the Edwtvard
plethysmographic methods that when the subject was pinched on the upper
 24 J. Physiol. (I95I) II2, 24-2I 6I2.I5.6II.976 THE DECREASE IN HAND BLOOD FLOW FOLLOWING INFLATION OF AN ARTERIAL OCCLUSION CUFF ON THE OPPOSITE ARM BY IAN C. RODDIE From the Department of Physiology,
24 J. Physiol. (I95I) II2, 24-2I 6I2.I5.6II.976 THE DECREASE IN HAND BLOOD FLOW FOLLOWING INFLATION OF AN ARTERIAL OCCLUSION CUFF ON THE OPPOSITE ARM BY IAN C. RODDIE From the Department of Physiology,
transfer, in part to difference of opinion regarding the mechanics of
 6I2.235 ON THE PARTIAL PRESSURES OF OXYGEN AND CARBON DIOXIDE IN ARTERIAL BLOOD AND ALVEOLAR AIR. By A. V. BOCK, D. B. DILL, H. T. EDWARDS, L. J. HENDERSON AND J. H. TALBOTT. (From the Fatigue Laboratory,
6I2.235 ON THE PARTIAL PRESSURES OF OXYGEN AND CARBON DIOXIDE IN ARTERIAL BLOOD AND ALVEOLAR AIR. By A. V. BOCK, D. B. DILL, H. T. EDWARDS, L. J. HENDERSON AND J. H. TALBOTT. (From the Fatigue Laboratory,
CHAPTER 3: The respiratory system
 CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
- How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have
 - How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have the highest blood flow of all organs, which makes them
- How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have the highest blood flow of all organs, which makes them
Oxygen and Carbon dioxide Transport. Dr. Laila Al-Dokhi
 Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
 Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
2. State the volume of air remaining in the lungs after a normal breathing.
 CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
PICU Resident Self-Study Tutorial The Basic Physics of Oxygen Transport. I was told that there would be no math!
 Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
The over-ventilated cat shows a similar adjustment to diminished. being over-ventilated, and he considered that on that account there was
 6I2.235:6I2.26I THE SOURCE OF COa EXPIRED AND THE SITE OF ITS RETENTION. BY LAURENCE IRVING, J. K. W. FERGUSON AND F. B. PLEWES. (From the Department of Physiology, University of Toronto.) AFTER evisceration
6I2.235:6I2.26I THE SOURCE OF COa EXPIRED AND THE SITE OF ITS RETENTION. BY LAURENCE IRVING, J. K. W. FERGUSON AND F. B. PLEWES. (From the Department of Physiology, University of Toronto.) AFTER evisceration
PULMONARY VENTILATION OF MAN AT ALTITUDE
 PULMONARY VENTILATION OF MAN AT ALTITUDE By S. LAHIRI. From tile Himalayan Scientific & Mountaineering Expedition 1960-6/ and Presidency College, Calcutta. Although the physiological changes associated
PULMONARY VENTILATION OF MAN AT ALTITUDE By S. LAHIRI. From tile Himalayan Scientific & Mountaineering Expedition 1960-6/ and Presidency College, Calcutta. Although the physiological changes associated
Some major points on the Effects of Hypoxia
 Some major points on the Effects of Hypoxia Source: Kings College London http://www.kcl.ac.uk/teares/gktvc/vc/dental/year1/lectures/rbmsmajorpoints/effectsofhypoxia.htm Cells obtain their energy from oxygen.
Some major points on the Effects of Hypoxia Source: Kings College London http://www.kcl.ac.uk/teares/gktvc/vc/dental/year1/lectures/rbmsmajorpoints/effectsofhypoxia.htm Cells obtain their energy from oxygen.
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing
 Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
PCo2 is independent of the duration of the breath hold. These results suggest that the
 Q. Ji exp. Phy8iol. (1971) 56, 92-100 MECHANICAL AND CHEMICAL CONTROL OF BREATH HOLDING. By G. R. KELMAN and K. T. WANN. From the Department of Physiology, University of Aberdeen, Aberdeen, Scotland. (Received
Q. Ji exp. Phy8iol. (1971) 56, 92-100 MECHANICAL AND CHEMICAL CONTROL OF BREATH HOLDING. By G. R. KELMAN and K. T. WANN. From the Department of Physiology, University of Aberdeen, Aberdeen, Scotland. (Received
Circulatory And Respiration
 Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
I Physical Principles of Gas Exchange
 Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
CHAPTER 3: The cardio-respiratory system
 : The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
: The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
Respiration (revised 2006) Pulmonary Mechanics
 Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
Chapter 4: Ventilation Test Bank MULTIPLE CHOICE
 Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
HCO - 3 H 2 CO 3 CO 2 + H H H + Breathing rate is regulated by blood ph and C02. CO2 and Bicarbonate act as a ph Buffer in the blood
 Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
Oxygen convulsions are believed by many workers to be caused by an accumulation
 272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
partial pressure is to be applied to the dissociation curve of fully oxygenated
 6I2. I27. I THE DETERMINATION OF THE CARBON DIOXIDE CONTENT OF THE MIXED VENOUS BLOOD. Part I. The effect of oxygenation and the critical oxygen tension. BY M. C. G. ISRAELS (Platt Physiological Scholar)
6I2. I27. I THE DETERMINATION OF THE CARBON DIOXIDE CONTENT OF THE MIXED VENOUS BLOOD. Part I. The effect of oxygenation and the critical oxygen tension. BY M. C. G. ISRAELS (Platt Physiological Scholar)
ALVEOLAR - BLOOD GAS EXCHANGE 1
 ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
Chapter 13 The Respiratory System
 Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Retinal vascular response to breathing increased carbon dioxide and oxygen concentrations. Regina Frayser and John B. Hickam
 Retinal vascular response to breathing increased carbon dioxide and oxygen concentrations Regina Frayser and John B. Hickam The retina has a high rate of oxygen consumption, and the retinal vessels are
Retinal vascular response to breathing increased carbon dioxide and oxygen concentrations Regina Frayser and John B. Hickam The retina has a high rate of oxygen consumption, and the retinal vessels are
Exam Key. NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: % of grade in class
 NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: 100 20% of grade in class 1) An arterial blood sample for a patient at sea level is obtained, and the following physiological values
NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: 100 20% of grade in class 1) An arterial blood sample for a patient at sea level is obtained, and the following physiological values
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation
 Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
Respiratory Lecture Test Questions Set 3
 Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
Respiratory System Study Guide, Chapter 16
 Part I. Clinical Applications Name: Respiratory System Study Guide, Chapter 16 Lab Day/Time: 1. A person with ketoacidosis may hyperventilate. Explain why this occurs, and explain why this hyperventilation
Part I. Clinical Applications Name: Respiratory System Study Guide, Chapter 16 Lab Day/Time: 1. A person with ketoacidosis may hyperventilate. Explain why this occurs, and explain why this hyperventilation
(Received for publication: 17 February 1960) Hypoxia has been shown to produce an increased pulmonary arterial
 J. Phy8iol. (196), 153, pp. 413-422 413 With 4 text-figures Printed in Great Britain CHANGES IN ph OF THE PERFUSATE DURING HYPOXIA IN ISOLATED PERFUSED CAT LUNGS BY HELEN N. DUKE, THE LATE ESTHER M. KILLICK
J. Phy8iol. (196), 153, pp. 413-422 413 With 4 text-figures Printed in Great Britain CHANGES IN ph OF THE PERFUSATE DURING HYPOXIA IN ISOLATED PERFUSED CAT LUNGS BY HELEN N. DUKE, THE LATE ESTHER M. KILLICK
that, as a means of progression, walking is suitable for lower speeds
 2 6I2 744.22 ENERGY EXPENDITURE IN WALKING AND RUNNING. BY M. OGASAWARA. (From the Department of Industrial Physiology, London School of Hygiene and Tropical Medicine.) (Received February 28, 1934.) IT
2 6I2 744.22 ENERGY EXPENDITURE IN WALKING AND RUNNING. BY M. OGASAWARA. (From the Department of Industrial Physiology, London School of Hygiene and Tropical Medicine.) (Received February 28, 1934.) IT
Essential Skills Course Acute Care Module. Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook
 Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
transients' of large amplitude can be imposed on the arterial, cardiac and Since both coughing and the Valsalva manoeuvre raise intrathoracic pressure
 351 J. Physiol. (I953) I22, 35I-357 EFFECTS OF COUGHING ON INTRATHORACIC PRESSURE, ARTERIAL PRESSURE AND PERIPHERAL BLOOD FLOW BY E. P. SHARPEY-SCHAFER From the Department of Medicine, St Thomas's Hospital
351 J. Physiol. (I953) I22, 35I-357 EFFECTS OF COUGHING ON INTRATHORACIC PRESSURE, ARTERIAL PRESSURE AND PERIPHERAL BLOOD FLOW BY E. P. SHARPEY-SCHAFER From the Department of Medicine, St Thomas's Hospital
Respiration - Human 1
 Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
APPENDIX. working blood volume was also rather large; Evans, Grande, and. equilibrated to the new mixture is partially dependent upon the rate
 612.172-5 APPENDIX A SIMPLIFIED HEART OXYGENATOR CIRCUIT FOR BLOOD- FED HEARTS. By J. YULE BOG-UE and R. A. GREGORY.' SINCE 1934 studies on the carbohydrate metabolism of the blood-fed heart without lungs
612.172-5 APPENDIX A SIMPLIFIED HEART OXYGENATOR CIRCUIT FOR BLOOD- FED HEARTS. By J. YULE BOG-UE and R. A. GREGORY.' SINCE 1934 studies on the carbohydrate metabolism of the blood-fed heart without lungs
NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: % of grade in class
 NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: 100 20% of grade in class 1) An arterial blood sample for a patient at sea level is obtained, and the following physiological values
NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: 100 20% of grade in class 1) An arterial blood sample for a patient at sea level is obtained, and the following physiological values
By S. GODFREY and E. J. M. CAMPBELL. From the Department of
 Q. Jl exp. Physiol. (1969) 54, 117-128 MECHANICAL AND CHEMICAL CONTROL OF BREATH HOLDING. By S. GODFREY and E. J. M. CAMPBELL. From the Department of Medicine, Royal Postgraduate Medical School, DuCane
Q. Jl exp. Physiol. (1969) 54, 117-128 MECHANICAL AND CHEMICAL CONTROL OF BREATH HOLDING. By S. GODFREY and E. J. M. CAMPBELL. From the Department of Medicine, Royal Postgraduate Medical School, DuCane
Lung Volumes and Capacities
 Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
administration of 5% carbon dioxide to an infant has any chemical effect in
 264 J. Physiol. (I953) I22, 264-273 THE EFFECT OF INHALATION OF CARBON DIOXIDE IN AIR ON THE RESPIRATION OF THE FULL-TERM AND PREMATURE INFANT BY K. W. CROSS, J. M. D. HOOPER AND T. E. OPP1R From the Physiology
264 J. Physiol. (I953) I22, 264-273 THE EFFECT OF INHALATION OF CARBON DIOXIDE IN AIR ON THE RESPIRATION OF THE FULL-TERM AND PREMATURE INFANT BY K. W. CROSS, J. M. D. HOOPER AND T. E. OPP1R From the Physiology
IV. FROM AQUATIC TO ATMOSPHERIC BREATHING: THE TRACHEA & THE LUNG
 GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
Masaji Mochizuki ABSTRACT. ]p(deox). The Haldane effects of [CO2] and [HCO3. ] were obtained by subtracting [CO2]p(ox) from [CO2]p(deox) and [HCO3
![Masaji Mochizuki ABSTRACT. ]p(deox). The Haldane effects of [CO2] and [HCO3. ] were obtained by subtracting [CO2]p(ox) from [CO2]p(deox) and [HCO3 Masaji Mochizuki ABSTRACT. ]p(deox). The Haldane effects of [CO2] and [HCO3. ] were obtained by subtracting [CO2]p(ox) from [CO2]p(deox) and [HCO3](/thumbs/76/73452816.jpg) Yamagata Med J 2006242)51-58 in vivo Masaji Mochizuki Emeritus Professor of Yamagata University, Yamagata, Japan Geriatric Respiratory Research Center, Nishimaruyama Hospital, Chuo-Ku, Sapporo, Japan Accepted
Yamagata Med J 2006242)51-58 in vivo Masaji Mochizuki Emeritus Professor of Yamagata University, Yamagata, Japan Geriatric Respiratory Research Center, Nishimaruyama Hospital, Chuo-Ku, Sapporo, Japan Accepted
RESPIRATORY GAS EXCHANGE
 RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
P215 Respiratory System, Part 2
 P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
6I2.2I6:6I alveolar pressure. It follows that the evident alteration in the respiratory rhythm is an alteration in amplitude.
 6I2.2I6:6I2.223.11 SOME EFFECTS OF CARBONIC ACID ON THE CHARACTER OF HUMAN RESPIRATION. BY J. BARCROFT AND R. MARGARIA' (Turin). (From the Physiological Laboratory, Cambridge.) THE following facts concerning
6I2.2I6:6I2.223.11 SOME EFFECTS OF CARBONIC ACID ON THE CHARACTER OF HUMAN RESPIRATION. BY J. BARCROFT AND R. MARGARIA' (Turin). (From the Physiological Laboratory, Cambridge.) THE following facts concerning
CHAPTER 6. Oxygen Transport. Copyright 2008 Thomson Delmar Learning
 CHAPTER 6 Oxygen Transport Normal Blood Gas Value Ranges Table 6-1 OXYGEN TRANSPORT Oxygen Dissolved in the Blood Plasma Dissolve means that the gas maintains its precise molecular structure About.003
CHAPTER 6 Oxygen Transport Normal Blood Gas Value Ranges Table 6-1 OXYGEN TRANSPORT Oxygen Dissolved in the Blood Plasma Dissolve means that the gas maintains its precise molecular structure About.003
Recitation question # 05
 Recitation and Lab # 05 The goal of this recitations / labs is to review material related to the CV and respiratory lectures for the second test of this course. Info required to answer this recitation
Recitation and Lab # 05 The goal of this recitations / labs is to review material related to the CV and respiratory lectures for the second test of this course. Info required to answer this recitation
THE literature on this subject, which was reviewed recently (CAMPBELL, doses of amytal, and in addition received A.C.E. mixture during the
 -~~ -v GAS TENSIONS IN THE MUCOUS MEMBRANE OF THE STOMACH AND SMALL INTESTINE. By J. ARGYLL CAMPBELL. From the National Institute for Medical Research, Hampstead. (With six figures in the text.) (Received
-~~ -v GAS TENSIONS IN THE MUCOUS MEMBRANE OF THE STOMACH AND SMALL INTESTINE. By J. ARGYLL CAMPBELL. From the National Institute for Medical Research, Hampstead. (With six figures in the text.) (Received
Collin County Community College. Lung Physiology
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
PCO2 levels apparently differed by less than 5 mm Hg. Fowler [1954] and. Godfrey and Campbell [1969] have shown that it is possible to resume a
![PCO2 levels apparently differed by less than 5 mm Hg. Fowler [1954] and. Godfrey and Campbell [1969] have shown that it is possible to resume a PCO2 levels apparently differed by less than 5 mm Hg. Fowler [1954] and. Godfrey and Campbell [1969] have shown that it is possible to resume a](/thumbs/85/91672390.jpg) Q. Ji exp. Physiol. (1969) 54, 129-140 THE INFLUENCE OF LUNG SHRINKAGE ON BREATH HOLDING TIME. By S. GODFREY, R. H. T. EDWARDS and D. A. WARRELL. From the Department of Medicine, Royal Postgraduate Medical
Q. Ji exp. Physiol. (1969) 54, 129-140 THE INFLUENCE OF LUNG SHRINKAGE ON BREATH HOLDING TIME. By S. GODFREY, R. H. T. EDWARDS and D. A. WARRELL. From the Department of Medicine, Royal Postgraduate Medical
RESPIRATORY MUSCLES IN HEALTH AND EMPHYSEMA *
 THE OXYGEN CONSUMPTION AND EFFICIENCY OF THE RESPIRATORY MUSCLES IN HEALTH AND EMPHYSEMA * BY REUBEN M. CHERNIACK t (From The Winnipeg General Hospital and the Departments of Medicine and Physiology and
THE OXYGEN CONSUMPTION AND EFFICIENCY OF THE RESPIRATORY MUSCLES IN HEALTH AND EMPHYSEMA * BY REUBEN M. CHERNIACK t (From The Winnipeg General Hospital and the Departments of Medicine and Physiology and
For more information about how to cite these materials visit
 Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Louis D Alecy, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Respiratory Physiology. Adeyomoye O.I
 Respiratory Physiology By Adeyomoye O.I Outline Introduction Hypoxia Dyspnea Control of breathing Ventilation/perfusion ratios Respiratory/barometric changes in exercise Intra-pulmonary & intra-pleural
Respiratory Physiology By Adeyomoye O.I Outline Introduction Hypoxia Dyspnea Control of breathing Ventilation/perfusion ratios Respiratory/barometric changes in exercise Intra-pulmonary & intra-pleural
Section Two Diffusion of gases
 Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Chapter 23. Gas Exchange and Transportation
 Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity other minor gases argon,
Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity other minor gases argon,
BREATH-BY-BREATH METHOD
 BREATH-BY-BREATH METHOD COR-MAN-0000-005-IN / EN Issue A, Rev. 2 2013-07 INNOISION ApS Skovvænge DK-5620 Glamsbjerg Denmark Tel.: +45 65 95 91 00 Fax: +45 65 95 78 00 info@innovision.dk www.innovision.dk
BREATH-BY-BREATH METHOD COR-MAN-0000-005-IN / EN Issue A, Rev. 2 2013-07 INNOISION ApS Skovvænge DK-5620 Glamsbjerg Denmark Tel.: +45 65 95 91 00 Fax: +45 65 95 78 00 info@innovision.dk www.innovision.dk
Respiration. The resspiratory system
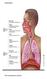 Respiration The resspiratory system The Alveoli The lungs have about 300 million alveoli, with a total crosssec onal area of 50 70 m2.. Each alveolar sac is surrounded by blood capillaries. The walls of
Respiration The resspiratory system The Alveoli The lungs have about 300 million alveoli, with a total crosssec onal area of 50 70 m2.. Each alveolar sac is surrounded by blood capillaries. The walls of
Pulmonary Circulation Linda Costanzo Ph.D.
 Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
Respiratory System Physiology. Dr. Vedat Evren
 Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Baltimore, Md.) shown as points in Figure For comparison a. viduals who had lived at altitude for relatively ex-
 3 S. ~~~~~~~~~~~~~~~~~1.2 ADAPTIVE VALUE OF RESPIRATORY ADJUSTMENTS TO SHUNT HYPOXIA AND TO ALTITUDE HYPOXIAI By GEORGE HUSSON2 AND A. B. OTIS-8 (From the Department of Surgery, The Johns Hopkins University
3 S. ~~~~~~~~~~~~~~~~~1.2 ADAPTIVE VALUE OF RESPIRATORY ADJUSTMENTS TO SHUNT HYPOXIA AND TO ALTITUDE HYPOXIAI By GEORGE HUSSON2 AND A. B. OTIS-8 (From the Department of Surgery, The Johns Hopkins University
Chapter 23. Gas Exchange and Transportation
 Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity and minor gases argon,
Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity and minor gases argon,
(Received 16 January 1946)
 186 J. Physiol. (I946) I05, I86-I90 6I2.2I5.9 THE ABSORPTION OF FLUIDS FROM THE LUNGS BY F. C. COURTICE AND P. J. PHIPPS From the Experimental Station, Porton and the Laboratory of Physiology, Oxford (Received
186 J. Physiol. (I946) I05, I86-I90 6I2.2I5.9 THE ABSORPTION OF FLUIDS FROM THE LUNGS BY F. C. COURTICE AND P. J. PHIPPS From the Experimental Station, Porton and the Laboratory of Physiology, Oxford (Received
AIIMS, New Delhi. Dr. K. K. Deepak, Prof. & HOD, Physiology AIIMS, New Delhi Dr. Geetanjali Bade, Asst. Professor AIIMS, New Delhi
 Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
LAB 7 HUMAN RESPIRATORY LAB. Complete the charts on pgs. 67 and 68 and read directions for using BIOPAC
 66 LAB 7 HUMAN RESPIRATORY LAB Assignments: Due before lab: Quiz: Three Respiratory Interactive Physiology Animations pages 69 73. Complete the charts on pgs. 67 and 68 and read directions for using BIOPAC
66 LAB 7 HUMAN RESPIRATORY LAB Assignments: Due before lab: Quiz: Three Respiratory Interactive Physiology Animations pages 69 73. Complete the charts on pgs. 67 and 68 and read directions for using BIOPAC
Gases and Respiration. Respiration Overview I
 Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
Respiratory System. Part 2
 Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
[see Margaria, 1929]. The quantitative relationship, however, between. is reached, further increments in oxygen pressure are accompanied
![[see Margaria, 1929]. The quantitative relationship, however, between. is reached, further increments in oxygen pressure are accompanied [see Margaria, 1929]. The quantitative relationship, however, between. is reached, further increments in oxygen pressure are accompanied](/thumbs/93/111758192.jpg) 6I2.OI4.464 ADAPTATIONS OF THE ORGANISM TO CHANGES IN OXYGEN PRESSURE. BY D. B. DILL, H. T. EDWARDS, A. FOLLING, S. A. OBERG, A. M. PAPPENHEIMER, JR. AND J. H. TALBOTT. (From the Fatigue Laboratory, Morgan
6I2.OI4.464 ADAPTATIONS OF THE ORGANISM TO CHANGES IN OXYGEN PRESSURE. BY D. B. DILL, H. T. EDWARDS, A. FOLLING, S. A. OBERG, A. M. PAPPENHEIMER, JR. AND J. H. TALBOTT. (From the Fatigue Laboratory, Morgan
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D.
 Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
SUBCUTANEOUS GAS EQUILIBRATION IN
 Tho'ax (1960), 15, 37. SUBCUTANEOUS GAS EQUILIBRATION IN CLINICAL PRACTICE BY From the Brook General Hospital, Shooters Hill, London When surgical emphysema is deliberately induced by injecting air under
Tho'ax (1960), 15, 37. SUBCUTANEOUS GAS EQUILIBRATION IN CLINICAL PRACTICE BY From the Brook General Hospital, Shooters Hill, London When surgical emphysema is deliberately induced by injecting air under
VOLUNTARY BREATHHOLDING. I. PULMONARY GAS
 VOLUNTARY BREATHHOLDING. I. PULMONARY GAS EXCHANGE DURING BREATHHOLDING'1 By CHARLES D. STEVENS, EUGENE B. FERRIS, JOSEPH P. WEBB, GEORGE L. ENGEL, AND MYRTLE LOGAN (From the Departments of Internal Medicine
VOLUNTARY BREATHHOLDING. I. PULMONARY GAS EXCHANGE DURING BREATHHOLDING'1 By CHARLES D. STEVENS, EUGENE B. FERRIS, JOSEPH P. WEBB, GEORGE L. ENGEL, AND MYRTLE LOGAN (From the Departments of Internal Medicine
RESPIRATION III SEMESTER BOTANY MODULE II
 III SEMESTER BOTANY MODULE II RESPIRATION Lung Capacities and Volumes Tidal volume (TV) air that moves into and out of the lungs with each breath (approximately 500 ml) Inspiratory reserve volume (IRV)
III SEMESTER BOTANY MODULE II RESPIRATION Lung Capacities and Volumes Tidal volume (TV) air that moves into and out of the lungs with each breath (approximately 500 ml) Inspiratory reserve volume (IRV)
Equation 1: F spring = kx. Where F is the force of the spring, k is the spring constant and x is the displacement of the spring. Equation 2: F = mg
 1 Introduction Relationship between Spring Constant and Length of Bungee Cord In this experiment, we aimed to model the behavior of the bungee cord that will be used in the Bungee Challenge. Specifically,
1 Introduction Relationship between Spring Constant and Length of Bungee Cord In this experiment, we aimed to model the behavior of the bungee cord that will be used in the Bungee Challenge. Specifically,
RESPIRATORY PHYSIOLOGY. Anaesthesiology Block 18 (GNK 586) Prof Pierre Fourie
 RESPIRATORY PHYSIOLOGY Anaesthesiology Block 18 (GNK 586) Prof Pierre Fourie Outline Ventilation Diffusion Perfusion Ventilation-Perfusion relationship Work of breathing Control of Ventilation 2 This image
RESPIRATORY PHYSIOLOGY Anaesthesiology Block 18 (GNK 586) Prof Pierre Fourie Outline Ventilation Diffusion Perfusion Ventilation-Perfusion relationship Work of breathing Control of Ventilation 2 This image
Rodney Shandukani 14/03/2012
 Rodney Shandukani 14/03/2012 OXYGEN THERAPY Aerobic metabolism accounts for 90% of Oxygen consumption by tissues. generates ATP by oxidative phosphorylation. Oxygen cascade: Oxygen exerts a partial pressure,
Rodney Shandukani 14/03/2012 OXYGEN THERAPY Aerobic metabolism accounts for 90% of Oxygen consumption by tissues. generates ATP by oxidative phosphorylation. Oxygen cascade: Oxygen exerts a partial pressure,
Hypoxia Following Rapid Decompression to 18,288 m (60,000 ft) Attributable to Alveolar Hypoventilation
 Hypoxia Following Rapid Decompression to 18,288 m (60,000 ft) Attributable to Alveolar Hypoventilation Desmond M Connolly PhD QinetiQ Aircrew Systems Senior Medical Officer Timothy J D Oyly BSc Amanda
Hypoxia Following Rapid Decompression to 18,288 m (60,000 ft) Attributable to Alveolar Hypoventilation Desmond M Connolly PhD QinetiQ Aircrew Systems Senior Medical Officer Timothy J D Oyly BSc Amanda
Capnography in the Veterinary Technician Toolbox. Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA
 Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
EMERGENCY MEDICINE AND THE LABORATORY. Ehsan Bolvardi(MD)
 EMERGENCY MEDICINE AND THE LABORATORY Ehsan Bolvardi(MD) bolvardie@mums.ac.ir Objective ED and laboratory Inappropriate use of laboratory ABG sampling ABG sampling error Introduction The laboratory is
EMERGENCY MEDICINE AND THE LABORATORY Ehsan Bolvardi(MD) bolvardie@mums.ac.ir Objective ED and laboratory Inappropriate use of laboratory ABG sampling ABG sampling error Introduction The laboratory is
Chapter 13 The Respiratory System
 VI edit Pag 451-499 Chapter 13 The Respiratory System V edit. Pag 459-509 Tissue cell Alveoli of lungs Atmosphere 1 External respiration Ventilation or gas exchange between the atmosphere and air sacs
VI edit Pag 451-499 Chapter 13 The Respiratory System V edit. Pag 459-509 Tissue cell Alveoli of lungs Atmosphere 1 External respiration Ventilation or gas exchange between the atmosphere and air sacs
Using such a method, Morawitz and Siebeck (1) found that the. composition of the alveolar air or of the blood. Unless the obstruc- 483
 THE EFFECT OF SOME PATHOLOGICAL CONDITIONS UPON DYSPNEA DURING EXERCISE I. ARTIFICIAL STENOSIS BY A. W. HEWLETT, J. K. LEWIS AND ANNA FRANKLIN (From the Department of Medicine, Stanford Medical School)
THE EFFECT OF SOME PATHOLOGICAL CONDITIONS UPON DYSPNEA DURING EXERCISE I. ARTIFICIAL STENOSIS BY A. W. HEWLETT, J. K. LEWIS AND ANNA FRANKLIN (From the Department of Medicine, Stanford Medical School)
The physiological functions of respiration and circulation. Mechanics. exercise 7. Respiratory Volumes. Objectives
 exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
Alveolus and Respiratory Membrane
 Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Chapter 22 The Respiratory System
 Chapter 22 The Respiratory System 1 Respiration Pulmonary ventilation (breathing): movement of air into and out of the lungs External respiration: O 2 and CO 2 exchange between the lungs and the blood
Chapter 22 The Respiratory System 1 Respiration Pulmonary ventilation (breathing): movement of air into and out of the lungs External respiration: O 2 and CO 2 exchange between the lungs and the blood
Observations of the Properties of the Human Respiratory System. April Ramos Dela Fuente. Bill Keenen; Tommy Kham; Grace Park
 P a g e 1 Observations of the Properties of the Human Respiratory System April Ramos Dela Fuente Bill Keenen; Tommy Kham; Grace Park NPB 101L - Section 06 - Ailsa Dalgliesh 11/25/14 P a g e 2 INTRODUCTION
P a g e 1 Observations of the Properties of the Human Respiratory System April Ramos Dela Fuente Bill Keenen; Tommy Kham; Grace Park NPB 101L - Section 06 - Ailsa Dalgliesh 11/25/14 P a g e 2 INTRODUCTION
Gas Laws. Introduction
 Gas Laws Introduction In 1662 Robert Boyle found that, at constant temperature, the pressure of a gas and its volume are inversely proportional such that P x V = constant. This relationship is known as
Gas Laws Introduction In 1662 Robert Boyle found that, at constant temperature, the pressure of a gas and its volume are inversely proportional such that P x V = constant. This relationship is known as
Hutchinson(l) in 1846 to that volume of air which can be expelled
 THE INFLUENCE OF POSTURE ON THE VOLUME OF THE RESERVE AIR. BY WILLIAM H. WILSON. (From the Physiological Department of the Faculty of Medicine, Cairo.) THE purpose of this communication is to draw attention
THE INFLUENCE OF POSTURE ON THE VOLUME OF THE RESERVE AIR. BY WILLIAM H. WILSON. (From the Physiological Department of the Faculty of Medicine, Cairo.) THE purpose of this communication is to draw attention
Students measure the change in pressure by varying the volume of trapped air in a syringe while:
 How Does a Trapped Gas Behave? Teacher Information Objective Students investigate the effect of changes in the volume of a confined gas on pressure at constant temperature. Using the pressure sensor, students
How Does a Trapped Gas Behave? Teacher Information Objective Students investigate the effect of changes in the volume of a confined gas on pressure at constant temperature. Using the pressure sensor, students
Department of Biology Work Sheet Respiratory system,9 class
 I. Name the following : Department of Biology Work Sheet Respiratory system,9 class 1. A muscular sheet separating the thoracic and abdominal cavities. 2. A respiratory tube supported by cartilaginous
I. Name the following : Department of Biology Work Sheet Respiratory system,9 class 1. A muscular sheet separating the thoracic and abdominal cavities. 2. A respiratory tube supported by cartilaginous
GAS EXCHANGE & PHYSIOLOGY
 GAS EXCHANGE & PHYSIOLOGY Atmospheric Pressure Intra-Alveolar Pressure Inspiration 760 mm HG at Sea Level (= 1 atm) Pressure due to gases (N2, O2, CO2, Misc.) Pressure inside the alveolus (air sac) Phrenic
GAS EXCHANGE & PHYSIOLOGY Atmospheric Pressure Intra-Alveolar Pressure Inspiration 760 mm HG at Sea Level (= 1 atm) Pressure due to gases (N2, O2, CO2, Misc.) Pressure inside the alveolus (air sac) Phrenic
Pulmonary Circulation
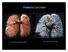 Pulmonary Circulation resin cast of pulmonary arteries resin cast of pulmonary veins Blood Flow to the Lungs Pulmonary Circulation Systemic Circulation Blood supply to the conducting zone provided by the
Pulmonary Circulation resin cast of pulmonary arteries resin cast of pulmonary veins Blood Flow to the Lungs Pulmonary Circulation Systemic Circulation Blood supply to the conducting zone provided by the
HUMAN Sample Experiment High Altitude Simulation (a one variable experiment) (version 5/04/06)
 HUMAN Sample Experiment High Altitude Simulation (a one variable experiment) (version 5/04/06) Students wish to simulate an ascent to the summit of Mount Mckinley (12,500 ft.), perhaps to compare the simulated
HUMAN Sample Experiment High Altitude Simulation (a one variable experiment) (version 5/04/06) Students wish to simulate an ascent to the summit of Mount Mckinley (12,500 ft.), perhaps to compare the simulated
UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT
 UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION
UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION
exchange of carbon dioxide and of oxygen between the blood and the air in
 M. M. HENRY WILLIAMS, JR.*Cardiorespiratory Laboratory, Grasslands WILLIAMS, JR.* Hospital, Valhalla, New York SOME APPLICATIONS OF PULMONARY PHYSIOLOGY TO CLINICAL MEDICINE During the past ten years a
M. M. HENRY WILLIAMS, JR.*Cardiorespiratory Laboratory, Grasslands WILLIAMS, JR.* Hospital, Valhalla, New York SOME APPLICATIONS OF PULMONARY PHYSIOLOGY TO CLINICAL MEDICINE During the past ten years a
