Diffusing Capacity and Pulmonary Capillary Blood Flow at Hyperbaric Pressures *
|
|
|
- Cory Daniels
- 5 years ago
- Views:
Transcription
1 Journal of Clinical Investigation Vol. 44, No. 1, 1965 Diffusing Capacity and Pulmonary Capillary Blood Flow at Hyperbaric Pressures * JEAN R. NAIRN,t GORDON G. POWERt R. W. HYDE, R. E. FORSTER, C. J. LAMBERTSEN, AND J. DICKSON (From the Department of Physiology, Graduate School of Medicine, and the Department of Pharmacology, School of Medicine, University of Pennsylvania, Philadelphia, Pa.) The pulmonary diffusing capacity (DLCO) has been observed to fall when the alveolar oxygen tension rises from 8 to 6 mm Hg (1, 2). By using the single breath technique in a hyperbaric pressure chamber, we have been able to extend the range of oxygen tensions studied up to 3,2 mm Hg. Also we have measured in these experiments the effect of exposure of the pulmonary capillary bed to a partial pressure of oxygen of approximately 2,6 mm Hg for a period of 1 minutes. In addition, prompted by the suggestions of J. S. Haldane (3), who postulated that inert molecules such as nitrogen may impede the mean free path of carbon monoxide molecules to the alveolar membrane and decrease the rate of uptake of carbon monoxide, we have studied the effect of large partial pressures of nitrogen on DLCO. Methods In a hyperbaric chamber operating up to 4.8 atmospheres absolute pressure, a modification of the Krogh single breath technique (1) was used to measure the diffusing capacity for carbon monoxide (IDLco). Simultaneously pulmonary capillary blood flow (Qc) was measured by adding acetylene to the inspired mixture (4, 5). Neon instead of helium was used as the inert tracer gas (6). At sea level, the volume of the pulmonary parenchymal * Submitted for publication March 26, 1965; accepted June 1, This work was supported by a grant from the Life Insurance Medical Research Fund and by grant He from the National Institutes of Health. t Isaac Ott Research Fellow and Fellow of the William McCann Research Trust. t Postdoctoral fellow of the National Institutes of Health. Daland Fellow of the American Philosophical Society. Address requests for reprints to Dr. R. W. Hyde, Dept. of Physiology, University of Pennsylvania, Graduate Division of the School of Medicine, Philadelphia, Pa tissue in milliliters (VT) was measured by plotting the acetylene disappearance with different times of breath holding in each subject (4). The concentrations of neon, carbon monoxide, and acetylene were approximately constant in the inspired mixture, being.5%,.4%, and.5%, respectively, with a balance of oxygen and nitrogen. Four separate inspired mixtures were employed in which the oxygen concentrations were 6%, 21%, 6%, and 98%o. By breathing different concentrations of oxygen for a few breaths followed by a breath of one of the inspired mixtures described, the alveolar oxygen tension of the subject during the breath holding period could be varied at will. Neon, carbon monoxide, acetylene, and carbon dioxide concentrations were measured on a gas chromatograph (6) 1 that has an error of ± 1%o. The oxygen concentration of the gases was measured on a mass spectrometer 2 that has an accuracy of within 1%o. The residual volume in each subject was measured by the closed circuit helium technique (7). The pressure in the chamber in pounds per square inch was indicated on a calibrated Bourdon tube recording gauge outside the chamber that had a resolution of within i pound per square inch (5 mm Hg). The plasma CO tension was estimated by having the subject rebreathe oxygen and hold his breath for 2 minutes and then measuring the carbon monoxide concentration in the expired sample (2, 8). This procedure was repeated at intervals throughout the experiment and the equilibrated capillary CO tension for each estimation of DLco calculated by interpolation to the prevailing oxygen tension. The apparatus used was similar to that previously described (5), mylar bags 3 and copper and tygon tubing only being incorporated, as acetylene is absorbed by rubber. The partial pressure of nitrogen (PN2) in the alveoli during the breath-holding period was estimated thus: PN= PB - (PH2 + Po + PCo2), where PB is the total barometric pressure, PH2o is the water vapor pressure 1 We found that the values of the neon/co ratio drifted by 2% throughout the day of each experiment. We have therefore incorporated a correction factor to compensate for the drift on the basis of hourly sampling of the standard mixture. The neon/c2h2 ratio remained constant throughout the day. 2 Model A, Consolidated Engineering Co., Pasadena, Calif. 3 Supplied by Vac-Pac, Baltimore, Md.
2 1592 NAIRN, POWER, HYDE, FORSTER, LAMBERTSEN, AND DICKSON TABLE I Physical characteristics of experimental subjects Surface Subject Sex Age Height Weight area years inches pounds m2 REF M RWH M GGP M GWK M JRN F at body temperature, and Po2 and Pco2 are partial pressures of oxygen and carbon dioxide, respectively, in alveolar gas. The mean intracapillary oxygen tension at which the breath-holding maneuver was performed was calculated from the expired alveolar oxygen tension by assuming a diffusing capacity for oxygen of 2 ml O per (mm Hg X minutes) (9). The alveolar oxygen tension present during the 5 to 1 minutes before the breath-holding procedure was calculated from the alveolar air equation (1). For convenience, this oxygen tension will be called hereafter the "preparatory level" to distinguish it from the mean in- TABLE II Diffusing capacity (DLco) and pulmonary capillary blood flow (cc) in two subjects in group A tracapillary oxygen tension at which the breath-holding maneuver was performed. The subjects studied included four males and one female who were symptomless and whose chests radiologically showed no active pulmonary disease or other abnormality. Their physical characteristics are shown in Table I. All were experienced in respiratory maneuvers. The following experimental procedure was performed in each subject. At sea level, two control measurements, one at approximately 6 mm Hg and another at approximately 12 mm Hg mean intracapillary oxygen tension, were performed in the chamber with the doors open. The chamber was then sealed and the air compressed to 3.5 atmospheres absolute pressure. At least four breathholding maneuvers were performed at this pressure at mean intracapillary oxygen tensions from 2 to 2,4 mm Hg. Between each maneuver a gas mixture containing 6% oxygen in a balance of nitrogen was inspired for 5 to 8 minutes through a tight fitting mask so that at 3.5 atmospheres the preparatory level oxygen tension was approximately equal to that of breathing air at sea level (11 mm Hg). After these measurements at 3.5 atmospheres the subjects were divided into groups A and B and the procedure continued as follows. In each of two subjects (RWH and GGP) in group A three further breath-hold- "Preparatory Mean level" intracapilalveolar oxygen lary oxygen PN2 in Subject Condition* tension tension alveolit DLco Qc mm Hg mm Hg mm Hg ml CO/ L/min (min Xmm Hg) RWH atmos , pheres 116 1, ,23 1, , ,579 2, ,579 1, , , GGP atmos- 1, pheres 1, ,142 1, , ,593 2, ,593 1, , , * Measurements listed in order performed. t PN2= partial pressure of nitrogen.
3 DLCO AND Qc AT HYPERBARIC PRESSURES 1593 TABLE III Diffusing capacity and pulmonary capillary blood flow in three subjects in group B "Preparatory Mean level" intracapil- Nitrogen alveolar oxygen lary oxygen tension in. Subject Condition* tension tension alveoli DLCO Qc REF 4.8 atmospheres 3.5 atmospheres mm Hg mm Hg ,95 3,115 2,257 1,87 1,75 1, mm Hg ,491 2, ml CO/ Limin (min Xmm Hg) JRN 3.5 atmospheres 4.8 atmospheres ,175 1,865 1,555 1, ,195 3, ,54 1,383 2, GWK 3.5 atmospheres 4.8 atmospheres ,25 1,85 1,545 1, ,965 2, ,51 1,378 2, * Measurements listed in the order performed. ing maneuvers were performed at 3.5 atmospheres, varying the mean intracapillary oxygen tension at the time of breath holding from 6 to 2,4 mm Hg. However, approximately 1% oxygen was administered through a mouthpiece for a preliminary 1 minutes before each run, maintaining a preparatory level oxygen tension of approximately 2,6 mm Hg for this time, and not 11 mm Hg as in the experiments above. With three subjects (REF, JRN, and GWK) in group B, the pressure was increased to 4.8 atmospheres, where duplicate measurements were made at a mean intracapillary oxygen tension of 3, mm Hg. Between measurements a mixture of 6% oxygen in nitrogen was inspired through a mask for 5 to 8 minutes. Graduated decompression took between 3 and 4 hours during which time the subject was reclining and intermittently sleeping in an easy chair. The final controls were performed on return to sea level. Estimations of plasma CO tension as described above were made between every second breath-holding maneuver. At each barometric pressure the highest oxygen tension runs were performed first.
4 1594 NAIRN, POWER, HYDE, FORSTER, LAMBERTSEN, AND DICKSON O x x z I- u ORWH *GWK X GGP AJRN o REF diffusing capacity was reduced on the average to 15 %o of the value measured at normal alveolar oxygen tension (approximately 11 mm Hg). 1/DLco increased approximately in a linear relation with the mean intracapillary oxygen tension (see Figure 2). The average correlation coefficient between 1/DLco and mean intracapillary oxygen tension in the five subjects was.986. Figure 3 shows 1/DLco measured after a preceding preparatory level of 116 mm alveolar oxygen tension and again after a preparatory level of approximately 2,58 mm oxygen tension in one subject (RWH). The mean intracapillary oxygen tension at which DLCO was measured is shown on the abscissa. The straight, dashed line is the re-.14r SUBJECT-RWH A 16 FIG. 1. EFFECT OF MEAN INTRACAPILLARY OXYGEN TENSION ON PULMONARY DIFFUSING CAPACITY (DLco) IN FIVE SUBJECTS. The lines show the trend in each subject. Results The results are shown in Tables II and III. The pulmonary diffusing capacity (DLCO) in milliliters CO per (minute X millimeters Hg) decreased in all subjects with rising mean intracapillary oxygen tension (Figure 1). In the three subjects in group B (JRN, REF, and GWK) where duplicate measurements were made at 4.8 atmospheres and the mean intracapillary oxygen tension was between 2,9 mm Hg and 3,2 mm Hg, the.2 SUBJECT-GWK A/ DLC, MIN X MM HG ML CO *I, X SEA LEVEL. 35 ATMOS *5 X A 46 ATMOS MEAN INTRACAPILLARY OXYGEN TENSION NHNN O iwō 61 ' FIG. 2. THE RELATIONSHIP BETWEEN DLco AND MEAN OXYGEN TENSION AT SEA LEVEL AND AT INTRACAPILLARY 3.5 AND 4.8 ATMOSPHERES PRESSURE IN SUBJECT GWK. The straight line is the regression based on all the points. r = DLICO.6s MlNxMM NG ML CO , A ". OXYGEN PREPARATORY LEVEL 116 MM -.1 A OXYGEN PREPARATORY LEVEL 258 MM MEAN INTRACAPILLARY OXYGEN TENSION MM HG 5' I ' 25' FIG. 3. THE EFFECT OF DIFFERENT PREPARATORY LEVELS OF OXYGEN ON DLco. The straight line is the regression based on the lower preparatory level points. The higher preparatory level points lie within 2 SE of this line. gression line based on the measurements made after a preparatory level at the lower oxygen tension. The higher preparatory level points lie within 2 SE of this line. In the other subject who performed these maneuvers (GGP), there was also no consistent difference observed between the values of DLCo measured after different preparatory levels of 2 tension. The partial pressure of nitrogen (PN2) present at the time of breath holding varied from 3 mm to 2,4 mm Hg. Figure 4 shows the values of 1/DLco plotted against the mean intracapillary oxygen tension with the PN2 values indicated as numbers. The presence of large partial pressures of nitrogen did not affect the value of 1/DLCO in a consistent fashion in any of the subjects studied. The control values of DLCO after the whole procedure consistently fell in all five subjects in com-
5 I Ao Lco M111MM HG ML CO 5223 SUBJECT-RWH 2 55k. *@2342 / > 369 DLco AND Qc AT HYPERBARIC PRESSURES MEAN INTRACAPILLARY OXYGEN TENSION MM HCG So' ' FIG. 4. EFFECT OF INDEPENDENT VARIATION OF THE PARTIAL PRESSURE OF NITROGEN (PNz) ON DLco. The figures beside the points are values of PN2 present at the time of breath holding in millimeters Hg. The straight line is a regression line relating DLco and the intracapillary oxygen tension. parison with those measured before. The average fall from the initial value was 14.4% with normal alveolar oxygen tensions (12 mm Hg) and 13.% with oxygen tensions of approximately 57 mm Hg. The pulmonary capillary blood flow (Qc) fell from the mean value of 7.9 L per minute at sea level to 5.8 L per minute at 3.5 atmospheres, which represents a 27% drop from the initial value. In two subjects (RWH and REF) Qc returned after the whole procedure to the original sea level value. The final average control value of Qc in the other three subjects (GWK, JRN, and GGP) was 66%o, 82%, and 75%, respectively, of their initial sea level control. Of the two subjects in group A, the average Qc of subject GGP dropped from 7. L per minute to 5.1 L per minute after approximately 1% oxygen had been inspired for 1 minutes at 3.5 atmospheres, but Qc increased slightly in RWH (Figure 5). There was no obvious difference in Qc measured at 3.5 atmospheres and 4.8 atmospheres in the three subjects in group B. No correlation was seen between Qc and the mean intracapillary oxygen tension during breath holding. Discussion These experiments showed that the diffusing capacity for carbon monoxide decreases progressively with rising oxygen tension from 1 to 3,2 mm Hg. The mechanism for this decrease is thought to be as follows. The rate of removal of carbon monoxide from the alveolar gas is limited, in part, by the rate of formation of carboxyhemoglobin in the red cell which, in turn, is proportional to the concentration of unsaturated hemoglobin molecules present. As a consequence of the rising Po2 in the red cell, fewer unsaturated hemoglobin molecules are available for the formation of carboxyhemoglobin. These data show that even in the presence of a mean intracapillary oxygen tension of approximately 3, mm Hg, there is a measurable diffusing capacity for carbon monoxide, which can only mean there is still unsaturated hemoglobin present. This is not so surprising when it is considered that although there may be only a minute amount of unsaturated hemoglobin present at any instant, it is the net result of extremely rapid association with and dissociation from oxygen. In the above experiments oxygen may have altered the actual properties of the pulmonary mem- z K Ia Ca -i 2-a aso -i 4 4 K ḴA I a_ So * ~~~~~~~~ * *~ ;O I 1595 RWH * GGP REF GWK *JRN A T N S p H E R E S IL II '2 '3 64 TIME IN HOURS FIG. 5. EFFECT OF DIFFERENT BAROMETRIC PRESSURE ON THE AVERAGE VALUES OF CAPILLARY BLOOD FLOW IN FIVE SUBJECTS. The dots are measurements made after a preparatory level of approximately 11 mm 2 tension, and the stars after a preparatory level of 2,58 mm 2 tension. The abscissa is only an approximate time schedule and applies in all subjects with the exception of REF, where measurements at 4.8 atmospheres were performed before those at 3.5 atmospheres. r
6 1596 NAIRN, POWER, HYDE, FORSTER, LAMBERTSEN, AND DICKSON TABLE IV Calculated values of Vc and DM above and below mean intracapillary oxygen tensions of 6 mm Hg* Vc DM Alveolar Alveolar Alveolar Alveolar Po2 Po2 Po2 Po2 <6 >6 <6 >6 Subject mm Hgt mm HgJ mm Hgt mm HgJ ml ml CO/(min Xmm Hg) RWH GWK REF GGP JRN * Vc = blood volume in the pulmonary capillaries; DM = diffusing qapacity of the membrane. Using measured 9 relationship to oxygen tension. t Using assumed a relationship to oxygen tension. has been measured only at 2 tensions less than 6 mm Hg, and values for higher oxygen tensions had to be obtained by extrapolation from the known data. brane and vascular bed thereby affecting the diffusing capacity, DLco. But, as previously pointed out (11), this seems unlikely since changes in the membrane and vascular bed would have to occur within the several seconds required for the measurement of Dico by the single breath technique. In the preparatory level experiments we tested for possible changes in the pulmonary diffusing surface by having subjects breathe oxygen for 1 minutes at an alveolar oxygen tension of approximately 2,6 mm Hg. We attempted to separate possible effects on the pulmonary diffusing surface from changes in DLco resulting from altered reaction kinetics within the red cell, which are influenced by the oxygen tension at the particular moment, by quickly (within 5 to 8 seconds) changing the alveolar oxygen tension and then measuring DLco. We found that DLCO was affected only by the oxygen tension in the alveoli at the time of breath holding and not by the preparatory level and have concluded, therefore, that the diffusing surface and gas exchange vessels are not affected by breathing oxygen for 1 minutes with an alveolar partial pressure of approximately 2,6 mm Hg. Other investigators have reported pulmonary edema in animals (12) and a lowering of DLco in human subjects (13) who breathed oxygen for longer periods at sea level, changes that infer injury to the pulmonary membrane and vascular bed. Possibly in our experiments a longer exposure to oxygen would have produced a decrease in diffusing capacity, but we voluntarily restricted our experiments to 1 minutes of oxygen breathing at 3.5 atmospheres in order to reduce the likelihood of oxygen toxicity and seizures. The concept of large numbers of nitrogen molecules impeding the diffusion of carbon monoxide molecules in the gas phase and thereby producing gradients within the alveolar air has been discussed previously (11). On theoretical grounds the nitrogen pressures in our experiments were not high enough to have impaired diffusion significantly. However, if this were an important facet and had affected the rate of gas diffusion within the alveolar air, a decrease in DLCo would have been found at increased atmospheric pressure when there were more nitrogen molecules present. In our experiments a variation of nitrogen partial pressures from 3 to 2,4 mm apparently did not affect DLco, results which support the theory that diffusion within the alveolar gas itself is not a significant limitation to the passage of gas into the blood. In these experiments a nearly linear relationship has been shown to exist between 1/DLco and the mean intracapillary oxygen tension at the time of breath holding in all subjects. We have applied the equation (14), 1/Drco = (1/DM) + (1/OVc), [1] to calculate the value of DM, the diffusing capacity of the membrane in milliliters CO per (minute X millimeters Hg), and Vc, the volume of the blood in milliliters in the pulmonary capillaries in all subjects using the measured values for (14), the rate at which the red cells in 1. ml of pulmonary capillary blood will absorb CO in milliliters per minute per millimeter Hg plasma CO tension. If the ratio of permeability of the red cell membrane to that of the red cell interior is assumed to be 2.5, is related to the intracorpuscular oxygen tension as follows (5): 1/9 = Po2, [2] where Po2 is the intracorpuscular oxygen tension in atmospheres. Equation 2 has been used to calculate the value of 1/6 from the measured mean intracapillary oxygen tension in all our experiments, and with this value, DM and Vc have been calculated from Equation 1. As Equation 2 is based on measurements of oxygen tensions only up to 6 mm Hg, the calculated values of DmI and Vc from
7 DLco AND Qc AT HYPERBARIC PRESSURES 1597 oxygen tensions above 6 mm Hg have been kept separate from those measured at sea level (i.e., at oxygen tensions below 6 mm Hg). The results of these calculated values are shown in Table IV. When Vc calculated from values of obtained by extrapolation to higher oxygen tensions are compared with Vc calculated from experimental values of at oxygen tensions below 6 mm Hg, four of five subjects show similar results. Three of the five subjects also have comparable DM values. If the error of the method incurred at very high oxygen tensions, as explained in detail below, is taken into account, these results probably mean that 1/ bears the same relationship to the mean intracapillary oxygen tension up to 3, mm Hg as defined up to 6 mm Hg tension by Equation 2. In the measurement of DLoo at high oxygen partial pressures, the ratio of the initial alveolar CO concentration to the final alveolar CO concentration (FAC(OO/FACOO) approaches unity, and in this event any error is greatly magnified. The equilibrated plasma CO tension becomes critical as well. For example, if a subject has a DLCO of 3 ml of CO per (minute X millimeters Hg), an alveolar volume of 5, ml standard temperature and pressure, dry, and a breath-holding time of 1 seconds, a 5 % error in the measured expired alveolar concentration of carbon monoxide will introduce a 7% error in the calculated DrLco. However, if the DLco is 5 ml of CO per (minute X millimeters Hg) with the same alveolar volume and breathholding time, a 5 o error in the measurement of the expired alveolar concentration will be magnified to a 32%o error in the calculated DLco. This magnification of errors at high oxygen tension may explain, in part, the wide variation in some of the measurements of DM and Vc at oxygen tensions over 6 mm Hg (see Table IV). The average control DLCo decreased 14%o in all subjects after the day's experiment in the chamber when compared to initial control values. To elucidate the matter further, two subjects, GGP and GWK, who had previously shown an average fall in DLCO of 15% and 25%o, respectively, were tested again. The subjects were seated in the chamber in easy chairs, but were not exposed to hyperbaric pressures or given any supplemental oxygen. Five measurements of Dr-co and Qc were made in each subject at 5-minute intervals in the morning and another five in the afternoon approximately 4 to 5 hours later. The results are shown in Table V, and we see that DLco dropped in much the same way as in the original experiments; the significance levels are shown. Since these subjects breathed only room air at sea level, this fall in DLC cannot be due to oxygen inhalation or to increased barometric pressure per se, but rather seems related to the subjects' prolonged inactivity, (15) and to changes in Qc (5). This is an interesting finding that will require further investigation. We are aware of reports that during oxygen breathing at 2. to 3.5 atmospheres, normal men showed an unexplained alveolar-arterial oxygen tension difference indirectly measured to be as high as 1 mm Hg at 2. atmospheres (16), 4 mm Hg at 3. atmospheres (17), and 55 mm Hg at 3.5 atmospheres (18). On the other hand, no large alveolar-arterial oxygen tension difference was found by Rennie and Pappenheimer (19) in dogs breathing oxygen at 2 atmospheres ambient pressure. In recent applications of the oxygen electrode to the measurement of arterial Po2 in hyperoxygenated subjects, large alveolar-arterial gradients were again reported (2). This possible increase in the alveolar-arterial oxygen gradient does not necessarily relate to the depression of DLCo by oxygen observed in the present study. Even though the uptake of oxygen in the lungs normally appears to be partially limited by the rate at which oxygen can react with intracellular, unsaturated hemoglobin (21), this does not in any way imply that complete equilibration between alveolar and capillary oxygen tension would not be expected theoretically at the end of the capillary no matter how great the absolute alveolar oxygen tension. For example, the mixed venous blood entering the pulmonary capillary bed would have a relatively low Po2 and therefore a normal Dr.o2, leading to nearly complete saturation of all the hemoglobin before the blood Po2 could rise over 15 mm Hg. Once the hemoglobin is fully saturated, further increase in oxygen content in the blood is only in the form of physically dissolved gas, and the only limitation to further transfer is the diffusing capacity of the pulmonary membrane (22). In these circumstances, the reaction rate for oxygen with hemoglobin is of no further concern. For reasons previously given the pulmonary membrane does not appear to be affected by short
8 1598 NAIRN, POWER, HYDE, FORSTER, LAMBERTSEN, AND DICKSON TABLE V DLco and Qc before and after a 4- to 5-hour period sitting at sea level Mean intracapillary DLCO Qc oxygen tension Subject Before After Before After Before After ml CO/(min Xmm Hg) L/min mm Hg GGP 39.9 ± ± ± p <.5 NS at.5 level GWK 34.1 ± i = p <.1 NS at.1 level periods of high Po2, and, at an alveolar Po2 of 2, mm Hg, equilibration of physically dissolved oxygen can be calculated to be 99%o complete in less than.3 second, assuming a pulmonary capillary blood volume of 1 ml and a diffusing capacity for oxygen of the pulmonary membrane of 4 ml per minute per mm Hg (23, 24). The fact that the plot of 1/DLco against Po2 (Figure 4) remains linear at the extremely high alveolar oxygen tensions implies that the mean capillary Po2 continues to rise linearly as alveolar Po2 rises. Thus, since neither diffusion limitation, gross shunting of blood across the lungs, nor other mechanisms to interfere with the movement of oxygen have been identified as a consequence of short periods of oxygen breathing at high pressures, the authors are not now prepared to explain the apparent gross interference with oxygen uptake. Summary The pulmonary diffusing capacity (DLco) and capillary blood flow (Qc) were calculated from the disappearance of low concentrations of carbon monoxide and acetylene from the alveolar gas during breath holding. Such measurements were performed in five normal subjects in a hyperbaric pressure chamber at sea level and at 3.5 and 4.8 atmospheres absolute. DLco was found to decrease progressively with rising oxygen tension. 1/Drco increased linearly with rising mean intracapillary oxygen tension from 11 to 3,2 mm Hg (average r =.986). DLCo was not changed significantly by independent variation of nitrogen partial pressure from 3 to 2,4 mm Hg at constant alveolar oxygen tension. The pulmonary diffusing surface did not measurably alter with exposure to an alveolar oxygen tension of 2,4 mm Hg for 1 minutes before the measurement of DLCO. Control values at sea level of DLco and Qc were done before and after the above studies. An average 14% decrease in DLCO was found accompanied by a drop in Qc. These changes were thought to result from the subjects' prolonged inactivity in the chamber during decompression rather than to be due to oxygen inhalation or to increased barometric pressure per se. Acknowledgments The authors wish to express their appreciation to Mrs. Mary Friedmann, Mr. Denis Umidi, and Mr. George Kriebel for their assistance. References 1. Ogilvie, C. M., R. E. Forster, W. S. Blakemore, and J. W. Morton. A standardized breath holding technique for the clinical measurement of the diffusing capacity of the lung for carbon monoxide. J. clin. Invest. 1957, 36, Forster, R. E., F. J. W. Roughton, L. Cander, W. A. Briscoe, and F. Kreuzer. Apparent pulmonary diffusing capacity for CO at varying alveolar 2 tensions. J. appl. Physiol. 1957, 11, Haldane, J. S. Respiration. New Haven, Yale University Press, 1922, p Cander, L., and R. E. Forster. Determination of pulmonary parenchymal tissue volume and pulmonary capillary blood flow in man. J. appl. Physiol. 1959, 14, Johnson, R. L., Jr., W. S. Spicer, J. M. Bishop, and R. E. Forster. Pulmonary capillary blood volume, flow and diffusing capacity during exercise. J. appl. Physiol. 196, 15, Lawson, W. H., Jr., H. N. Duke, R. W. Hyde, and R. E. Forster. Relation of pulmonary arterial and venous pressure to diffusing capacity. J. appl. Physiol. 1964, 19, 381.
9 Dico AND Qc AT HYPERBARIC PRESSURES Bates, D. V., and R. V. Christie. Intrapulmonary mixing of helium in health and emphysema. Clin. Sci. 195, 9, Forster, R. E., W. S. Fowler, D. V. Bates, and B. Van Lingen. The absorption of carbon monoxide by the lungs during breathholding. J. clin. Invest. 1954, 33, McNeill, R. S., J. Rankin, and R. E. Forster. The diffusing capacity of the pulmonary membrane and the pulmonary capillary blood volume in cardiopulmonary disease. Clin. Sci. 1958, 17, Comroe, J. H., Jr., R. E. Forster, A. B. DuBois, W. A. Briscoe, and H. Carlsen. The Lung. Chicago, Year Book, Forster, R. E. Exchange of gases between alveolar air and pulmonary capillary blood: pulmonary diffusing capacity. Physiol. Rev. 1957, 37, Comroe, J. H., Jr., R. D. Dripps, P. R. Dumke, and M. Deming. Oxygen toxicity; the effect of inhalation of high concentrations of oxygen for twenty-four hours on normal men at sea level and at simulated altitude of -18, feet. J. Amer. med. Ass. 1945, 128, Ernsting, J. The effect of breathing high concentrations of oxygen upon the diffusing capacity of the lung in man. J. Physiol. (Lond.) 1961, 155, 51P. 14. F. J. W. Roughton, and R. E. Forster. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J. appl. Physiol. 1957, 11, Young, W. A., D. B. Shaw, and D. V. Bates. Effect of low concentrations of ozone on pulmonary function in man. J. appl. Physiol. 1964, 19, Lambertsen, C. J., S. G. Owen, H. Wendel, M. W. Stroud, A. A. Lurie, W. Lochner, and G. F. Clark. Respiratory and cerebral circulatory control during exercise at.21 and 2. atmospheres inspired po2. J. appl. Physiol. 1959, 14, Lambertsen, C. J., R. H. Krough, D. V. Cooper, G. L. Emmel, H. H. Loeschcke, and C. F. Schmidt. Comparison of relationship of respiratory minute volume to pco2 and ph of arterial and internal jugular blood in normal man during hyperventilation produced by low concentrations of CO at 1 atmosphere and by O at 3. atmospheres. J. appl. Physiol. 1953, 5, Lambertsen, C. J., J. H. Ewing, R. H. Kough, R. Gould, and M. W. Stroud, 3rd. Oxygen toxicity. Arterial and internal jugular blood gas composition in man during inhalation of air, 1% 2 and 2% CO, in 2 at 3.5 atmospheres ambient pressure. J. appl. Physiol. 1955, 8, Rennie, D. W., and J. R. Pappenheimer. Arterial oxygen pressure in dogs breathing oxygen at 2.5 atmospheres pressure. Proc. Soc. exp. Biol. (N. Y.) 1958, 99, Saltzman, Herbert. Circulation, in press. 21. Staub, N. C., J. M. Bishop, and R. E. Forster. Importance of diffusion and chemical reaction rates in O uptake in the lung. J. appl. Physiol. 1962, 17, Fenn, W. O., and H. Rahn, section eds. Respiration, Handbook of Physiology. Washington, D. C., American Physiological Society, 1964, section 3, vol. 1, p Staub, N. C. Alveolar-arterial oxygen tension gradient due to diffusion. J. appl. Physiol. 1962, 18, Hyde, R. W., and R. E. Forster. Determination of the pulmonary diffusing capacity for 2 (DLo2) by a breathholding technique. Physiologist 1963, 6, 26. SPECIAL NOTICE TO SUBSCRIBERS Post Offices will no longer forward the Journal when you move. Please notify The Journal of Clinical Investigation, Business Office, 1 Stoughton Street, Boston, Mass. 2118, at once when you have a change of address, and do not omit the Zip Code number.
Section Two Diffusion of gases
 Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
I Physical Principles of Gas Exchange
 Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
RESPIRATORY GAS EXCHANGE
 RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D.
 Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
The Physiologic Basis of DLCO testing. Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan
 The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
Lung Volumes and Capacities
 Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Oxygen convulsions are believed by many workers to be caused by an accumulation
 272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT
 UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION
UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION
Respiratory physiology II.
 Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
Respiration (revised 2006) Pulmonary Mechanics
 Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
660 mm Hg (normal, 100 mm Hg, room air) Paco, (arterial Pc02) 36 mm Hg (normal, 40 mm Hg) % saturation 50% (normal, 95%-100%)
 148 PHYSIOLOGY CASES AND PROBLEMS Case 26 Carbon Monoxide Poisoning Herman Neiswander is a 65-year-old retired landscape architect in northern Wisconsin. One cold January morning, he decided to warm his
148 PHYSIOLOGY CASES AND PROBLEMS Case 26 Carbon Monoxide Poisoning Herman Neiswander is a 65-year-old retired landscape architect in northern Wisconsin. One cold January morning, he decided to warm his
RESPIRATORY REGULATION DURING EXERCISE
 RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation
 Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
The physiological functions of respiration and circulation. Mechanics. exercise 7. Respiratory Volumes. Objectives
 exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
AIIMS, New Delhi. Dr. K. K. Deepak, Prof. & HOD, Physiology AIIMS, New Delhi Dr. Geetanjali Bade, Asst. Professor AIIMS, New Delhi
 Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing
 Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
P215 Respiratory System, Part 2
 P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
Table of Contents. By Adam Hollingworth
 By Adam Hollingworth Table of Contents Oxygen Cascade... 2 Diffusion... 2 Laws of Diffusion... 2 Diffusion & Perfusion Limitations... 3 Oxygen Uptake Along Pulmon Capillary... 4 Measurement of Diffusing
By Adam Hollingworth Table of Contents Oxygen Cascade... 2 Diffusion... 2 Laws of Diffusion... 2 Diffusion & Perfusion Limitations... 3 Oxygen Uptake Along Pulmon Capillary... 4 Measurement of Diffusing
PICU Resident Self-Study Tutorial The Basic Physics of Oxygen Transport. I was told that there would be no math!
 Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
 Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Douglas and Haldane(2) has shown that the oxygen determinations. since it forms the basis of the "Coefficient of Utilisation" (Krrogh) and
 THE MEASUREMENT OF THE OXYGEN CONTENT OF THE MIXED VENOUS BLOOD, AND OF THE VOLUME OF BLOOD CIRCULATING PER MINUTE. BY J. BARCROFT, F. J. W. ROUGHTON AND R. SHOJI. (From the Physiological Laboratory, Cambridge.)
THE MEASUREMENT OF THE OXYGEN CONTENT OF THE MIXED VENOUS BLOOD, AND OF THE VOLUME OF BLOOD CIRCULATING PER MINUTE. BY J. BARCROFT, F. J. W. ROUGHTON AND R. SHOJI. (From the Physiological Laboratory, Cambridge.)
Respiratory System Physiology. Dr. Vedat Evren
 Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Measurement of 02 Diffusing Capacity of the Lungs with a Stable 02 Isotope*
 Journal of Clinical Investigation Vol. 45, No. 7, 1966 Measurement of 02 Diffusing Capacity of the Lungs with a Stable 02 Isotope* R. W. HYDEI R. E. FORSTER, G. G. POWER,t J. NAIRN, AND R. RYNES (From
Journal of Clinical Investigation Vol. 45, No. 7, 1966 Measurement of 02 Diffusing Capacity of the Lungs with a Stable 02 Isotope* R. W. HYDEI R. E. FORSTER, G. G. POWER,t J. NAIRN, AND R. RYNES (From
CHAPTER 6. Oxygen Transport. Copyright 2008 Thomson Delmar Learning
 CHAPTER 6 Oxygen Transport Normal Blood Gas Value Ranges Table 6-1 OXYGEN TRANSPORT Oxygen Dissolved in the Blood Plasma Dissolve means that the gas maintains its precise molecular structure About.003
CHAPTER 6 Oxygen Transport Normal Blood Gas Value Ranges Table 6-1 OXYGEN TRANSPORT Oxygen Dissolved in the Blood Plasma Dissolve means that the gas maintains its precise molecular structure About.003
By: Aseel Jamil Al-twaijer. Lec : physical principles of gas exchange
 By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
Section Three Gas transport
 Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
Chapter 4: Ventilation Test Bank MULTIPLE CHOICE
 Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
Collin County Community College. Lung Physiology
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
CHAPTER 3: The respiratory system
 CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
Pulmonary Circulation Linda Costanzo Ph.D.
 Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
CHAPTER 3: The cardio-respiratory system
 : The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
: The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
2. State the volume of air remaining in the lungs after a normal breathing.
 CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
respiratory cycle. point in the volumes: 500 milliliters. for men. expiration, up to 1200 milliliters extra makes breathing Respiratory
 10 II. RESPIRATORY VOLUMES, CAPACITIES & PULMONARY FUNCTION TESTS Respiratory volume is the term used for various volumes of air moved by or associated with the lungs at a given point in the respiratory
10 II. RESPIRATORY VOLUMES, CAPACITIES & PULMONARY FUNCTION TESTS Respiratory volume is the term used for various volumes of air moved by or associated with the lungs at a given point in the respiratory
IV. FROM AQUATIC TO ATMOSPHERIC BREATHING: THE TRACHEA & THE LUNG
 GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
02 (Do2) and three using CO (Dco) namely, the
 A MODIFICATION OF THE KROGH CARBON MONOXIDE BREATH HOLDING TECHNIQUE FOR ESTIMATING THE DIFFUSING CAPACITY OF THE LUNG; A COMPARISON WITH THREE OTHER METHODS 1 By R. E. FORSTER, J. E. COHN,2 W. A. BRISCOE,8
A MODIFICATION OF THE KROGH CARBON MONOXIDE BREATH HOLDING TECHNIQUE FOR ESTIMATING THE DIFFUSING CAPACITY OF THE LUNG; A COMPARISON WITH THREE OTHER METHODS 1 By R. E. FORSTER, J. E. COHN,2 W. A. BRISCOE,8
Respiration - Human 1
 Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
PCO2 levels apparently differed by less than 5 mm Hg. Fowler [1954] and. Godfrey and Campbell [1969] have shown that it is possible to resume a
![PCO2 levels apparently differed by less than 5 mm Hg. Fowler [1954] and. Godfrey and Campbell [1969] have shown that it is possible to resume a PCO2 levels apparently differed by less than 5 mm Hg. Fowler [1954] and. Godfrey and Campbell [1969] have shown that it is possible to resume a](/thumbs/85/91672390.jpg) Q. Ji exp. Physiol. (1969) 54, 129-140 THE INFLUENCE OF LUNG SHRINKAGE ON BREATH HOLDING TIME. By S. GODFREY, R. H. T. EDWARDS and D. A. WARRELL. From the Department of Medicine, Royal Postgraduate Medical
Q. Ji exp. Physiol. (1969) 54, 129-140 THE INFLUENCE OF LUNG SHRINKAGE ON BREATH HOLDING TIME. By S. GODFREY, R. H. T. EDWARDS and D. A. WARRELL. From the Department of Medicine, Royal Postgraduate Medical
Circulatory And Respiration
 Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
exchange of carbon dioxide and of oxygen between the blood and the air in
 M. M. HENRY WILLIAMS, JR.*Cardiorespiratory Laboratory, Grasslands WILLIAMS, JR.* Hospital, Valhalla, New York SOME APPLICATIONS OF PULMONARY PHYSIOLOGY TO CLINICAL MEDICINE During the past ten years a
M. M. HENRY WILLIAMS, JR.*Cardiorespiratory Laboratory, Grasslands WILLIAMS, JR.* Hospital, Valhalla, New York SOME APPLICATIONS OF PULMONARY PHYSIOLOGY TO CLINICAL MEDICINE During the past ten years a
Diffusion. Dr. Gyanendra Agrawal Senior Resident Deptt. of Pulmonary Medicine PGIMER, Chandigarh
 Diffusion Dr. Gyanendra Agrawal Senior Resident Deptt. of Pulmonary Medicine PGIMER, Chandigarh Diffusion Primary function of lung gas exchange Movement of gas across the blood gas interface is by simple
Diffusion Dr. Gyanendra Agrawal Senior Resident Deptt. of Pulmonary Medicine PGIMER, Chandigarh Diffusion Primary function of lung gas exchange Movement of gas across the blood gas interface is by simple
Respiratory Physiology Gaseous Exchange
 Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
Retinal vascular response to breathing increased carbon dioxide and oxygen concentrations. Regina Frayser and John B. Hickam
 Retinal vascular response to breathing increased carbon dioxide and oxygen concentrations Regina Frayser and John B. Hickam The retina has a high rate of oxygen consumption, and the retinal vessels are
Retinal vascular response to breathing increased carbon dioxide and oxygen concentrations Regina Frayser and John B. Hickam The retina has a high rate of oxygen consumption, and the retinal vessels are
Respiratory Lecture Test Questions Set 3
 Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
αo 2 : solubility coefficient of O 2
 Version 2006 Dr. Puntarica Suwanprathes 1) Fick s law of diffusion 2) facts which limit gas transfer 3) diffusion capacity gas volume gaseous phase dissolved gas exert pressure*** Solubility of Gas C =P.
Version 2006 Dr. Puntarica Suwanprathes 1) Fick s law of diffusion 2) facts which limit gas transfer 3) diffusion capacity gas volume gaseous phase dissolved gas exert pressure*** Solubility of Gas C =P.
logarithmic plot of the concentration of expired alveolar carbon monoxide against time of breathholding
 Thorax 1983;38:1-9 Editorial The single-breath carbon monoxide transfer test 25 years on: a reappraisal 1-Physiological considerations The breath-holding or single-breath technique for the measurement
Thorax 1983;38:1-9 Editorial The single-breath carbon monoxide transfer test 25 years on: a reappraisal 1-Physiological considerations The breath-holding or single-breath technique for the measurement
VOLUNTARY BREATHHOLDING. I. PULMONARY GAS
 VOLUNTARY BREATHHOLDING. I. PULMONARY GAS EXCHANGE DURING BREATHHOLDING'1 By CHARLES D. STEVENS, EUGENE B. FERRIS, JOSEPH P. WEBB, GEORGE L. ENGEL, AND MYRTLE LOGAN (From the Departments of Internal Medicine
VOLUNTARY BREATHHOLDING. I. PULMONARY GAS EXCHANGE DURING BREATHHOLDING'1 By CHARLES D. STEVENS, EUGENE B. FERRIS, JOSEPH P. WEBB, GEORGE L. ENGEL, AND MYRTLE LOGAN (From the Departments of Internal Medicine
ALVEOLAR - BLOOD GAS EXCHANGE 1
 ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
Gas Exchange ACTIVITY OVERVIEW SUMMARY KEY CONCEPTS AND PROCESS SKILLS KEY VOCABULARY. Teacher s Guide B-75 L A B O R ATO R Y
 Gas Exchange 17 40- to 2 50-minute sessions ACTIVITY OVERVIEW L A B O R ATO R Y SUMMARY This activity explores the role of the respiratory system in the regulation of gases in the blood. Students investigate
Gas Exchange 17 40- to 2 50-minute sessions ACTIVITY OVERVIEW L A B O R ATO R Y SUMMARY This activity explores the role of the respiratory system in the regulation of gases in the blood. Students investigate
Gas exchange. Tissue cells CO2 CO 2 O 2. Pulmonary capillary. Tissue capillaries
 Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
Oxygen and Carbon dioxide Transport. Dr. Laila Al-Dokhi
 Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
technique which have led to increased precision
 THE ACCURACY OF DIRECT DETERMINATIONS OF OXYGEN AND CARBON DIOXIDE TENSIONS IN HUMAN BLOOD IN VITRO 1 By GILES F. FILLEY, ESTHER GAY, AND GEORGE W. WRIGHT (From the Department of Physiology, the Edwtvard
THE ACCURACY OF DIRECT DETERMINATIONS OF OXYGEN AND CARBON DIOXIDE TENSIONS IN HUMAN BLOOD IN VITRO 1 By GILES F. FILLEY, ESTHER GAY, AND GEORGE W. WRIGHT (From the Department of Physiology, the Edwtvard
Respiratory System. Part 2
 Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1
 AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
AN OVERVIEW OF RESPIRATION AND AN INTRODUCTION TO DIFFUSION AND SOLUBILITY OF GASES 1 Summary: This set of notes gives an overview of respiration and then follows the overview with a detailed discussion
Essential Skills Course Acute Care Module. Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook
 Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
Respiratory System Study Guide, Chapter 16
 Part I. Clinical Applications Name: Respiratory System Study Guide, Chapter 16 Lab Day/Time: 1. A person with ketoacidosis may hyperventilate. Explain why this occurs, and explain why this hyperventilation
Part I. Clinical Applications Name: Respiratory System Study Guide, Chapter 16 Lab Day/Time: 1. A person with ketoacidosis may hyperventilate. Explain why this occurs, and explain why this hyperventilation
BREATHING AND EXCHANGE OF GASES
 96 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 17 BREATHING AND EXCHANGE OF GASES MULTIPLE CHOICE QUESTIONS 1. Respiration in insects is called direct because a. The cell exchange O 2 directly with the air in the
96 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 17 BREATHING AND EXCHANGE OF GASES MULTIPLE CHOICE QUESTIONS 1. Respiration in insects is called direct because a. The cell exchange O 2 directly with the air in the
Yanal. Jumana Jihad. Jamil Nazzal. 0 P a g e
 2 Yanal Jumana Jihad Jamil Nazzal 0 P a g e note: this sheet was written and corrected according to the records from section 2 so you may find differences in the arrangement of topics from the records
2 Yanal Jumana Jihad Jamil Nazzal 0 P a g e note: this sheet was written and corrected according to the records from section 2 so you may find differences in the arrangement of topics from the records
Respiratory Medicine. A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics. Alveolar Gas Equation. See online here
 Respiratory Medicine A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics See online here Alveolar gas equation helps to calculate the partial pressure of oxygen in alveoli and A-a gradient is the
Respiratory Medicine A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics See online here Alveolar gas equation helps to calculate the partial pressure of oxygen in alveoli and A-a gradient is the
Gas Pressure. Pressure is the force exerted per unit area by gas molecules as they strike the surfaces around them.
 Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Pco2 *20times = 0.6, 2.4, so the co2 carried in the arterial blood in dissolved form is more than the o2 because of its solubility.
 Physiology, sheet #9 Oxygen, is first dissolved in the plasma and the cytosol of the rbc, we have around blood constitutes 7% of our body weight, oxygen, in the capillaries is present in the rbc s and
Physiology, sheet #9 Oxygen, is first dissolved in the plasma and the cytosol of the rbc, we have around blood constitutes 7% of our body weight, oxygen, in the capillaries is present in the rbc s and
Effect of Lung Inflation on Pulmonary Diffusing Capacity at Rest and Exercise *
 Journal of Clinical Investigation Vol. 45, No. 4, 1966 Effect of Lung Inflation on Pulmonary Diffusing Capacity at Rest and Exercise * JOHN M. MILLER AND ROBERT L. JOHNSON, JR.t (From the Cardiopulmonary
Journal of Clinical Investigation Vol. 45, No. 4, 1966 Effect of Lung Inflation on Pulmonary Diffusing Capacity at Rest and Exercise * JOHN M. MILLER AND ROBERT L. JOHNSON, JR.t (From the Cardiopulmonary
PROBLEM SET 9. SOLUTIONS April 23, 2004
 Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Respiratory System. Prepared by: Dorota Marczuk-Krynicka, MD, PhD
 Respiratory System Prepared by: Dorota Marczuk-Krynicka, MD, PhD Lungs: Ventilation Perfusion Gas Exchange - Diffusion 1. Airways and Airway Resistance (AWR) 2. Mechanics of Breathing and Lung (Elastic)
Respiratory System Prepared by: Dorota Marczuk-Krynicka, MD, PhD Lungs: Ventilation Perfusion Gas Exchange - Diffusion 1. Airways and Airway Resistance (AWR) 2. Mechanics of Breathing and Lung (Elastic)
Respiratory Physiology. Adeyomoye O.I
 Respiratory Physiology By Adeyomoye O.I Outline Introduction Hypoxia Dyspnea Control of breathing Ventilation/perfusion ratios Respiratory/barometric changes in exercise Intra-pulmonary & intra-pleural
Respiratory Physiology By Adeyomoye O.I Outline Introduction Hypoxia Dyspnea Control of breathing Ventilation/perfusion ratios Respiratory/barometric changes in exercise Intra-pulmonary & intra-pleural
BREATHING AND EXCHANGE OF GASES
 96 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 17 BREATHING AND EXCHANGE OF GASES MULTIPLE CHOICE QUESTIONS 1. Respiration in insects is called direct because a. The tissues exchange O 2 directly with the air in
96 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 17 BREATHING AND EXCHANGE OF GASES MULTIPLE CHOICE QUESTIONS 1. Respiration in insects is called direct because a. The tissues exchange O 2 directly with the air in
CAPACITY OF THE LUNGS FOR CARBON MONOXIDE.
 A COMPARISON OF METHODS OF MEASURING THE DIFFUSING CAPACITY OF THE LUNGS FOR CARBON MONOXIDE. INVESTIGATION BY FRACTIONAL ANALYSIS OF THE ALVEOLAR AIR BY R. MARSHALL 1 (From the Medical Unit, St. Bartholomew's
A COMPARISON OF METHODS OF MEASURING THE DIFFUSING CAPACITY OF THE LUNGS FOR CARBON MONOXIDE. INVESTIGATION BY FRACTIONAL ANALYSIS OF THE ALVEOLAR AIR BY R. MARSHALL 1 (From the Medical Unit, St. Bartholomew's
Chapter 13 The Respiratory System
 Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
VENTILATION AND PERFUSION IN HEALTH AND DISEASE. Dr.HARIPRASAD VS
 VENTILATION AND PERFUSION IN HEALTH AND DISEASE Dr.HARIPRASAD VS Ventilation Total ventilation - total rate of air flow in and out of the lung during normal tidal breathing. Alveolar ventilation -represents
VENTILATION AND PERFUSION IN HEALTH AND DISEASE Dr.HARIPRASAD VS Ventilation Total ventilation - total rate of air flow in and out of the lung during normal tidal breathing. Alveolar ventilation -represents
Measurement of cardiac output by Alveolar gas exchange - Inert gas rebreathing
 Measurement of cardiac output by Alveolar gas exchange - Inert gas rebreathing Carlo Capelli SS.MM. Università degli Studi di Verona Soluble Inert gas They dissolve and do not form bonds with haemoglobyn
Measurement of cardiac output by Alveolar gas exchange - Inert gas rebreathing Carlo Capelli SS.MM. Università degli Studi di Verona Soluble Inert gas They dissolve and do not form bonds with haemoglobyn
Alveolus and Respiratory Membrane
 Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
J. Physiol. (I941) I00, I98-21I 6I :6I2.825
 198 J. Physiol. (I941) I00, I9821I 6I2.22.02:6I2.825 THE EFFECT OF OXYGEN LACK ON THE CEREBRAL CIRCULATION BY F. C. COURTICE From the Departments of Physiology and of Surgery, Oxford (Received 24 March
198 J. Physiol. (I941) I00, I9821I 6I2.22.02:6I2.825 THE EFFECT OF OXYGEN LACK ON THE CEREBRAL CIRCULATION BY F. C. COURTICE From the Departments of Physiology and of Surgery, Oxford (Received 24 March
HCO - 3 H 2 CO 3 CO 2 + H H H + Breathing rate is regulated by blood ph and C02. CO2 and Bicarbonate act as a ph Buffer in the blood
 Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
RESPIRATORY MUSCLES IN HEALTH AND EMPHYSEMA *
 THE OXYGEN CONSUMPTION AND EFFICIENCY OF THE RESPIRATORY MUSCLES IN HEALTH AND EMPHYSEMA * BY REUBEN M. CHERNIACK t (From The Winnipeg General Hospital and the Departments of Medicine and Physiology and
THE OXYGEN CONSUMPTION AND EFFICIENCY OF THE RESPIRATORY MUSCLES IN HEALTH AND EMPHYSEMA * BY REUBEN M. CHERNIACK t (From The Winnipeg General Hospital and the Departments of Medicine and Physiology and
(a) (i) Describe how a large difference in oxygen concentration is maintained between a fish gill and the surrounding water.
 1. Answers should be written in continuous prose. Credit will be given for biological accuracy, the organisation and presentation of information and the way in which an answer is expressed. Fick s law
1. Answers should be written in continuous prose. Credit will be given for biological accuracy, the organisation and presentation of information and the way in which an answer is expressed. Fick s law
Public Assessment Report Scientific discussion. Lung test gas CO (He) AGA, 0.28%, inhalation gas, compressed (carbon monoxide, helium) SE/H/1154/01/MR
 Public Assessment Report Scientific discussion Lung test gas CO (He) AGA, 0.28%, inhalation gas, compressed (carbon monoxide, helium) SE/H/1154/01/MR This module reflects the scientific discussion for
Public Assessment Report Scientific discussion Lung test gas CO (He) AGA, 0.28%, inhalation gas, compressed (carbon monoxide, helium) SE/H/1154/01/MR This module reflects the scientific discussion for
San Francisco) throughout the lung and, therefore, uneven ventilation-blood
 Journal of Clhnical Investsgasson Vol. 41, No. 3, 1962 THE CAUSE OF ARTERIAL HYPOXEMIA AT REST IN PATIENTS WITH "ALVEOLAR-CAPILLARY BLOCK SYNDROME"* By T. N. FINLEYj E. W. SWENSON t AND J. H. COMROE, JR.
Journal of Clhnical Investsgasson Vol. 41, No. 3, 1962 THE CAUSE OF ARTERIAL HYPOXEMIA AT REST IN PATIENTS WITH "ALVEOLAR-CAPILLARY BLOCK SYNDROME"* By T. N. FINLEYj E. W. SWENSON t AND J. H. COMROE, JR.
Capnography in the Veterinary Technician Toolbox. Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA
 Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
Gases and Respiration. Respiration Overview I
 Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
Gas Exchange in Animals. Uptake of O2 from environment and discharge of CO2. Respiratory medium! water for aquatic animals, air for terrestial
 Gas Exchange in Animals Uptake of O2 from environment and discharge of CO2 Respiratory medium! water for aquatic animals, air for terrestial Respiratory surface! skin, gills, lungs Circulatory System O2/CO2
Gas Exchange in Animals Uptake of O2 from environment and discharge of CO2 Respiratory medium! water for aquatic animals, air for terrestial Respiratory surface! skin, gills, lungs Circulatory System O2/CO2
(Received 16 January 1946)
 186 J. Physiol. (I946) I05, I86-I90 6I2.2I5.9 THE ABSORPTION OF FLUIDS FROM THE LUNGS BY F. C. COURTICE AND P. J. PHIPPS From the Experimental Station, Porton and the Laboratory of Physiology, Oxford (Received
186 J. Physiol. (I946) I05, I86-I90 6I2.2I5.9 THE ABSORPTION OF FLUIDS FROM THE LUNGS BY F. C. COURTICE AND P. J. PHIPPS From the Experimental Station, Porton and the Laboratory of Physiology, Oxford (Received
Chapter 23. Gas Exchange and Transportation
 Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity and minor gases argon,
Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity and minor gases argon,
Chapter 23. Gas Exchange and Transportation
 Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity other minor gases argon,
Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity other minor gases argon,
partial pressure is to be applied to the dissociation curve of fully oxygenated
 6I2. I27. I THE DETERMINATION OF THE CARBON DIOXIDE CONTENT OF THE MIXED VENOUS BLOOD. Part I. The effect of oxygenation and the critical oxygen tension. BY M. C. G. ISRAELS (Platt Physiological Scholar)
6I2. I27. I THE DETERMINATION OF THE CARBON DIOXIDE CONTENT OF THE MIXED VENOUS BLOOD. Part I. The effect of oxygenation and the critical oxygen tension. BY M. C. G. ISRAELS (Platt Physiological Scholar)
SERIAL DETERMINATION OF CARDIAC OUTPUT DURING PROLONGED WORK BY A CO2-REBREATHING TECHNIQUE
 J. Human Ergol., 4: 35-41,1975 SERIAL DETERMINATION OF CARDIAC OUTPUT DURING PROLONGED WORK BY A CO2-REBREATHING TECHNIQUE Brian A. WILSON and R. T. HERMISTON* Department of Human Kinetics, University
J. Human Ergol., 4: 35-41,1975 SERIAL DETERMINATION OF CARDIAC OUTPUT DURING PROLONGED WORK BY A CO2-REBREATHING TECHNIQUE Brian A. WILSON and R. T. HERMISTON* Department of Human Kinetics, University
THE literature on this subject, which was reviewed recently (CAMPBELL, doses of amytal, and in addition received A.C.E. mixture during the
 -~~ -v GAS TENSIONS IN THE MUCOUS MEMBRANE OF THE STOMACH AND SMALL INTESTINE. By J. ARGYLL CAMPBELL. From the National Institute for Medical Research, Hampstead. (With six figures in the text.) (Received
-~~ -v GAS TENSIONS IN THE MUCOUS MEMBRANE OF THE STOMACH AND SMALL INTESTINE. By J. ARGYLL CAMPBELL. From the National Institute for Medical Research, Hampstead. (With six figures in the text.) (Received
Diffusing Capacity: 2017 ATS/ERS Standards for single-breath carbon uptake in the lung. Susan Blonshine RRT, RPFT, FAARC, AE-C
 Diffusing Capacity: 2017 ATS/ERS Standards for single-breath carbon uptake in the lung Susan Blonshine RRT, RPFT, FAARC, AE-C Joint ATS/ERS Taskforce Recent literature reviewed Surveyed current technical
Diffusing Capacity: 2017 ATS/ERS Standards for single-breath carbon uptake in the lung Susan Blonshine RRT, RPFT, FAARC, AE-C Joint ATS/ERS Taskforce Recent literature reviewed Surveyed current technical
Stikstofuitwas en Heliumdilutie. Eric Derom Universitair Ziekenhuis Gent
 Stikstofuitwas en Heliumdilutie Eric Derom Universitair Ziekenhuis Gent Background Overview Helium-dilution technique Principle of Technique Equipment Procedure and Calculations Quality Control Nitrogen
Stikstofuitwas en Heliumdilutie Eric Derom Universitair Ziekenhuis Gent Background Overview Helium-dilution technique Principle of Technique Equipment Procedure and Calculations Quality Control Nitrogen
Respiration. The resspiratory system
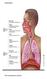 Respiration The resspiratory system The Alveoli The lungs have about 300 million alveoli, with a total crosssec onal area of 50 70 m2.. Each alveolar sac is surrounded by blood capillaries. The walls of
Respiration The resspiratory system The Alveoli The lungs have about 300 million alveoli, with a total crosssec onal area of 50 70 m2.. Each alveolar sac is surrounded by blood capillaries. The walls of
Physiology Unit 4 RESPIRATORY PHYSIOLOGY
 Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
Non-Invasive Estimation of the Effective Pulmonary Blood Flow and Gas Exchange from Respiratory Analysis
 Non-Invasive Estimation of the Effective Pulmonary Blood Flow and Gas Exchange from Respiratory Analysis P. Christensen Introduction Monitoring of the effective pulmonary blood flow is important in the
Non-Invasive Estimation of the Effective Pulmonary Blood Flow and Gas Exchange from Respiratory Analysis P. Christensen Introduction Monitoring of the effective pulmonary blood flow is important in the
RESPIRATION III SEMESTER BOTANY MODULE II
 III SEMESTER BOTANY MODULE II RESPIRATION Lung Capacities and Volumes Tidal volume (TV) air that moves into and out of the lungs with each breath (approximately 500 ml) Inspiratory reserve volume (IRV)
III SEMESTER BOTANY MODULE II RESPIRATION Lung Capacities and Volumes Tidal volume (TV) air that moves into and out of the lungs with each breath (approximately 500 ml) Inspiratory reserve volume (IRV)
Respiratory Pulmonary Ventilation
 Respiratory Pulmonary Ventilation Pulmonary Ventilation Pulmonary ventilation is the act of breathing and the first step in the respiratory process. Pulmonary ventilation brings in air with a new supply
Respiratory Pulmonary Ventilation Pulmonary Ventilation Pulmonary ventilation is the act of breathing and the first step in the respiratory process. Pulmonary ventilation brings in air with a new supply
BREATH-BY-BREATH METHOD
 BREATH-BY-BREATH METHOD COR-MAN-0000-005-IN / EN Issue A, Rev. 2 2013-07 INNOISION ApS Skovvænge DK-5620 Glamsbjerg Denmark Tel.: +45 65 95 91 00 Fax: +45 65 95 78 00 info@innovision.dk www.innovision.dk
BREATH-BY-BREATH METHOD COR-MAN-0000-005-IN / EN Issue A, Rev. 2 2013-07 INNOISION ApS Skovvænge DK-5620 Glamsbjerg Denmark Tel.: +45 65 95 91 00 Fax: +45 65 95 78 00 info@innovision.dk www.innovision.dk
transfer, in part to difference of opinion regarding the mechanics of
 6I2.235 ON THE PARTIAL PRESSURES OF OXYGEN AND CARBON DIOXIDE IN ARTERIAL BLOOD AND ALVEOLAR AIR. By A. V. BOCK, D. B. DILL, H. T. EDWARDS, L. J. HENDERSON AND J. H. TALBOTT. (From the Fatigue Laboratory,
6I2.235 ON THE PARTIAL PRESSURES OF OXYGEN AND CARBON DIOXIDE IN ARTERIAL BLOOD AND ALVEOLAR AIR. By A. V. BOCK, D. B. DILL, H. T. EDWARDS, L. J. HENDERSON AND J. H. TALBOTT. (From the Fatigue Laboratory,
Determination of Distribution of Diffusing Capacity in
 Journal of Clinical Investigation Vol. 46, No. 3, 1967 Determination of Distribution of Diffusing Capacity in Relation to Blood Flow in the Human Lung * RICHARD W. HYDE,t RICHARD RYNES,t GORDON G. POWER,
Journal of Clinical Investigation Vol. 46, No. 3, 1967 Determination of Distribution of Diffusing Capacity in Relation to Blood Flow in the Human Lung * RICHARD W. HYDE,t RICHARD RYNES,t GORDON G. POWER,
Chemistry A Molecular Approach. Fourth Edition. Chapter 5. Gases. Copyright 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
 Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Chemistry A Molecular Approach Fourth Edition Chapter 5 Gases Supersonic Skydiving and the Risk of Decompression Gas Gases are composed of particles that are moving around very fast in their container(s).
Respiration. Figure 22: Schematic representation of the respiratory system
 Respiration One of the seven characteristics of something which is living is respiration. Strictly speaking, respiration is the process that takes place at cellular level and is one of three different
Respiration One of the seven characteristics of something which is living is respiration. Strictly speaking, respiration is the process that takes place at cellular level and is one of three different
1) Kety and others have attempted to predict
 BY J. W. SEVERINGHAUS 2 (From the Department of Anesthesia, Hospital of the University of Pennsylvania, and Harrison Department of Surgical Research, University of Pennsylvania, Philadelphia, Pa.) (Submitted
BY J. W. SEVERINGHAUS 2 (From the Department of Anesthesia, Hospital of the University of Pennsylvania, and Harrison Department of Surgical Research, University of Pennsylvania, Philadelphia, Pa.) (Submitted
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lung test gas, CO/C 2 H 2 /CH 4 AGA 0.3%, 0.3%, 0.3% medicinal gas, compressed 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Carbon monoxide
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lung test gas, CO/C 2 H 2 /CH 4 AGA 0.3%, 0.3%, 0.3% medicinal gas, compressed 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Carbon monoxide
Kinetic-Molecular Theory
 GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
