hemoglobin of the blood with carbon monoxide.are too well known to
|
|
|
- Oscar Henry
- 5 years ago
- Views:
Transcription
1 THE ELIMINATION OF CARBON MONOXIDE FROM THE BLOOD A THEORETICAL AND ExPERIMENTAL STUIDY By WILLIAM C. STADIE AND KIRBY A. MARTIN (From the Department of Internal Medicine, Yale University School of Medicine and the Medical Service of the New Haven Hospital) (Received for publication July 11, 1925) The symptoms attending the partial or complete saturation of the hemoglobin of the blood with carbon monoxide.are too well known to require detailed comment. With as little as 40 per cent of the hemoglobin combined with carbon monoxide there may ensue deep coma, marked central nervous system symptoms with ankle clonus, positive Babinski and Kernig signs and often death. Occasionally the blood becomes free of carbon monoxide, the coma terminates, but the patient subsequently sinks into coma again and dies, probably as a result of central nervous system damage. The exact explanation of this train of symptoms is not entirely clear.- Presumably the effects of carbon monoxide asphyxia are those of anoxemia. For, as is well known, anoxemia long continued results in irremedial damage to nervous tissue. This anoxemia is not due primarily to a reduction of the total oxygen of the blood as is commonly supposed, but rather to a marked lowering of the partial pressure at which oxygen is available for tissue metabolism. As will be shown subsequently this effect is the result of a marked alteration in the form of oxygen dissociation curve of blood by carbon monoxide. In the light of this alteration the desperate condition of a subject whose blood is half saturated with CO as contrasted with the comparatively comfortable condition of one who as in anemia cases, has merely lost half his hemoglobin is understandable. That the effects of carbon monoxide are entirely those of anoxemia is at present an assumption based upon the fact that its toxic effects are supposedly nil. This will be discussed below. 77
2 78 ELIMINATION OF CARBON MONOXIDE Carbon monoxide, as contrasted with oxygen, has a peculiarly great affinity for hemoglobin. A very small concentration of carbon monoxide in the alveolar air will keep the blood highly saturated with CO. CO at a few millimeters of partial pressure has the same combining capacity as oxygen at a partial pressure of several hundred millimeters. This peculiarity of CO is as yet unexplained, but it enables a small concentration of carbon monoxide in the alveolar air to keep the blood highly saturated with CO and so lead to dangerous asphyxiation. Conversely the recovery of asphyxiated subjects is slow. The elimination of CO from the blood depends upon the reversal of the reaction. Hb + CO. HbCO Under the ordinary circumstances of recovery the conditions favor the establishment of an equilibrium toward the right. The blood gives up a small amount of CO to the alveolar air. But this small amount is sufficient to reestablish equilibrium at a point close to the original saturation. Part of the CO is removed from the alveolar air at each respiration it is true but as a rule the respirations are depressed. A kind of vicious cycle is set up. The two factors, great affinity of CO for hemoglobin and subnormal respiration, lengthen the time of recovery to hours. Unless the CO be rapidly eliminated in a subject with over 60 per cent of saturation, death usually occurs.' The inhalation of CO2 with pure oxygen effects this rapid elimination. First used by Henderson (1921) its use in many hospitals and particularly in mine rescue stations has demonstrated its value. Henderson and subsequently Yant and Sayre (1922) showed in experimental animals and subjects that the addition of CO2 to the air or oxygen inhaled shortened the time of elimination of CO frequently to as brief a time as one-half hour. But these investigators attributed the action of CO2 to the increased pulmonary ventilation which it I It has been erroneously supposed by some that the velocity of reaction of CO with Hb is a factor; i.e., reaction (1) goes with great speed toward the right and slowly toward the left. Hartridge (1922) has shown that the reaction is practically instantaneous in either direction. If the conditions are properly selected the reaction in vivo can be made to go with great rapidity in either direction as the subsequent protocols will show.
3 WILLIAM C. STADIE AND KIRBY A. MARTIN 79 caused. This increased ventilation washed out the CO from the alveolar air rapidly, hastening the elimination from the blood by lowering the alveolar CO pressure to a minimum. But increased ventilation may not be the sole or even the important factor operating when CO2 was inhaled by asphyxiated animals. The increased hydrogen ion concentration of the blood during CO2 inhalation must be taken into account and this was not considered by either Henderson or Yant and Sayre. Theoretical considerations of the equilibrium relations of oxyhemoglobin reduced hemoglobin, carbon monoxide hemoglobin, oxygen carbon monoxide and hydrogen ion concentration, supported by experimental evidence, have enabled us to predict that an increase of the acidity of the blood by any agent, ventilatory rate remaining constant, would result in an increased rate of elimination of CO from the blood. Experimental rates of elimination under these conditions were found to be quite in accord with the prediction. In addition, the relations developed showed that the primary effect of carbon monoxide was a profound alteration of the oxygen dissociation curve, which, rather than the mere loss of functioning hemoglobin, was the cause of the anoxemia. EQUILIBRIUUM BETWEEN Hb, HbO2, HbCO, 02, CO AND H+. THIEORY Two assumptions are made to develop an expression for the effect of CO on the combination of oxygen with hemoglobin. 1. Hemoglobin combines with a mixture of 02 and CO as if they were one substance due regard being paid to the relative affiity of the two gases. I.e., a partial pressure Pco of CO is equivalent to a partial pressure (K'co,o. Pco) of oxygen, K'co/o being a constant unaltered for a given blood by any condition save temperature. It expresses the relative affinity or saturating capacity of CO for hemoglobin as compared to oxygen. At 38 C. in human blood it has a value of 300 =L 50 as experimentally determined by Barcroft, Douglass and many others. In other words, a gas mixture containing Po, MMm. of 02 and Pco mm. of CO would be equivalent to [Gas] = (Po2 + K'coioPco) mm. of02in its combining value toward hemoglobin. Hence for the reaction. (Hb)n + ngas = (Hb Gas). we have by mass action [Hb Gas]n (1) 1 - [Hbln [PO2 + K'coo COcO] -
4 80 80ELIMINATION OF CARBON MONOXIDE Here [HbGas] represents the fraction of total hemoglobin combined with 02 and CO. 2. The ratio of HbO2 to HbCO in any mixture of hemoglobin is proportional to the partial pressure of the two gases in equilibrium with it. I.e., [HbCO] K_010 PcO (2) These assumptions were likewise employed by Hill (1921) in the development of an hypothesis which postulated the dissociation of hemoglobin into a non-protein fraction combining with oxygen or carbon monoxide and a protein fraction not capable of combination with either. Equation 2 is known to be true for the case where the hemoglobin is completely saturated with gas. But it is as yet undetermined whether this relation holds true when the hemoglobin is not completely saturated, i.e., when there is still some reduced hemoglobin present. However, the validity of this relation for partially saturated hemoglobin is here assumed. The identity of Kco/o and K'coio remains to be proved. Since [Gas] = Po, + K'co/o Pco and [HbGas] = [HbO2] + [HbCO], we have Kg =[HbO2] + [HbCO] I[1 - ([HbO2] + [HbCO])] [P + K'coo Pco If P =0 [HbO2] Kg= g (1 - [HbO2]) Pon = Ko i.e., Hill's oxygen dissociation constant. Further if P = 0 but since we have by division Kg=K0 = Kl'n (1 - [HbCO] [HbCO]) pno [HbCO1 = (1 - [HbCOI) Pn. Kc K = lrco.o (4) A similar relation involving K,O/O may be derived thermodynamically and independently of any considerations of the kinetics of the reactions concerned. Consider the following equilibrium states:
5 WILLIAM C. STADIE AND KIRBY A. MARTIN 81 (HbO2)n ;t (Hb)n + no2 + AF1 (Hb)n + nco.: (HbCO)n + AF2 nhbo2 + nco.+ nhbco + no2 + naf3 where A F1, A F2 and n A F3 are the free energy changes of the respective reactions. From the known equilibrium constants we have Since AF, + AF2 or - log Ko = AF1 log Kco = AF2 n log Kco/o = n AFs = naf3, we have -log Ko + log Kco = n log Kco/o K. K0 KcO X (4') It follows from 4 and 4'- that K' =0/0 Experimental proof of (4) is offered by the data on the blood of C. A. Douglass (1912). Figures 1, 3 and 4 of their paper give the following data. n is taken as 2.5. Hemoglobin constants of Douglass blood at 40 mm. CO2 and 380C. Kco. Obs Ko.Obs K.o/o. Obs 250 K,0/0 Calculated by equation 4' The experimental and theoretical proof of the identity of the constant K'co/o by which the partial pressure of CO is multiplied to give its combining capacity in oxygen terms with the equilibrium constant Kco/o of the reaction. HbO2 + CO = HbCO + 02 of which equation 2 is the mass action expression permits the introduction of equation 2 into (3). Eliminating Pco we obtain, remembering that Kg = Ko. K ([_2C[HbO] 0 ([O2] + [HbCOI)n-1 (1 - ([HbO,] + [HbCO])) pn ( This equation was tested experimentally on the blood of W. C. S. The blood was equilibrated at 380 with amounts of CO and 02 necessary to give partial
6 82 ELIMINATION OF CARBON MONOXIDE p FIG. 1. SOLID LINE = LOG Ko AT VARYING ph CALCULATED BY EQUATION 5 IN BLOOD PARTIALLY SATURATED WITH CO AND 02 DASHED LINE = Log K. determined at varying ph in the presence of 02 only TABLE 1 -logk0 Date Date Oxygen HbOs2 ~~Tension Satn. HbCO Satn ph O2 CO culated cal lated calcu- by equation mm. vper cme per cent per cen per cent December December February April April A April 11.. i June saturation of the hemoglobin at varyinig ph values. The per cent saturation with 02 and CO, the oxygen tension and ph were determined using the Van Slyke- Stadie, Haldane and Cullen method respectively. The values for Ko thus obtained 5
7 WILLIAM C. STADIE AND KIRBY A. MARTIN 83 are compared with K. determinations using oxygen alone.2 The results are given in figure 1 and table 1. There is excellent agreement. EFFECT OF CHANGE OF ph OF THE BLOOD ON THE OXYGEN DISSOCIATION CURVE IN CARBON MONOXIDE POISONING By means of Equation (5) the oxygen dissociation curve of whole blood may be drawn when the blood is constantly saturated with CO to any definite and fixed degree. Fixing the CO saturation at 50 per cent we have for an average human blood the following normal conditions as contrasted with an acidotic condition easily attained by inhalation of CO2. I II ph Ko K./o * n HbCO...50 per cent 50 per cent In figure 2 are shown the curves for these two conditions calculated from equation 5. Increasing the acidity of the blood changes Ko only according to the well established (over physiological ranges) relation A log K0 = ApH Kco/o and n are unaltered as has been shown by Barcroft and others. Figure 2 shows that the presence of CO has changed markedly the form of the oxygen dissociation curve of that part of the hemoglobin uncombined with CO. The effect of this alteration upon the rate of elimination of CO from the blood as influenced by increasing the hydrogen ion concentration only is clearly seen from the following: At point A we have from figure 2 ph =7.4 HbCO = 50 per cent HbO2 = 40 per cent Pco = mm. Hg P02 =30 mm. Hg 2The control K0 values determined with oxygen alone are taken from published data. Stadie and Martin, Jour. Biol. Chem., 1924, lx, 191.
8 84 ELIMINATION OF CARBON MONOXIDE *Lower the ph to 7.0 and maintain the oxygen tension constant. Then at point B we have Percent ph = 7.0 HbCO = 50 per cent HbO2 = 32 per cent Pco = mm. Hg PO2 = 30 mm. Hg 2i ph 7.4 z to 100 IIhgen tensionmmfa g FIG. 2. OXYGEN DISSOCIATION CURVE OF BLOOD 50 PER CENT SATURATED WITH CO AND ph 7.4 AND 7.0 SHOWING THE EFFECT OF ACIDITY ON THE PARTIAL PRESSURE OF CO At constant oxygen tension a lowering of the ph requires an increase - of * or 20 per cent in the carbon monoxide tension to maintain the blood at 50 per cent saturation with CO.
9 WILLIAM C. STADIE AND KIRBY A. MARTIN 85 If, as is the case in the lungs during recovery from asphyxia, the PCO remains constant it is easy to show from equation 5 that the following would be the approximate conditions. ph = 7.0 HbCO = 40 per cent HbO2 = 40 per cent Pco = mm. Hg PO2 = 30 mm. Hg While we have arbitrarily selected a mid portion of the curves for illustration the same relations must necessarily hold true for any and all equilibrium relations, oxygen, carbon monoxide and hemoglobin. TABLE 2 Elimination of COfrom partially asphyxiated dogs with constant ventilatory rate and varyintg acidity of blood Breathing TeBreathing air C10 pr cent intravenously Hyperventilation Time C02 + Air HbCO ph HbCO ph HbCO ph HbCO ph satn. satn. sah ph satn. minutes per cent per cent per cent per cent Oxygen capacity, volumespercent It is at once evident from the above that increasing acidity of the blood from whatever cause hastens the elimination of carbon monoxide from the blood independently of ventilatory rate. To test this deduction the following experiments on asphyxiated dogs were done. The results are recorded in Table 2. The rate of elimination of CO from the blood was studied under the following conditions: 1. Animal breathing air with constant ventilatory rate. Blood ph normal. 2. Blood ph decreased by breathing 10 per cent CO2 with constant ventilatory rate. HC H
10 86 ELIMINATION OF CARBON MONOXIDE 3. Giving dilute hydrochloric acid intravenously with constant ventilatory rate. 4. Hyperventilation with air. This experimental portion of the work was made possible by the Department of Surgery placing at our disposal their laboratories. We are indebted to Dr. Robert Kapsinow for his kindness in performing the animal surgery necessary. EXPERDIENTAL Medium size dogs were used throughout. Operative technic, anesthesia. gassing, blood sampling and blood analysis were the same in all of the experiments, Except in experiment 4 the ventilatory rate was the same throughout the series. Anesthesia: 30 to 40 cc. of paraldehyde was given by stomach tube three quarters to one hour before the experiment was started. This was found to be sufficient to obtain complete relaxation for two to three hours. Operation: After the animal was completely anesthesized it was placed on the operating table and a trachea cannula was inserted and connected to an artificial respiration machine. The thoracic cavity was opened by making an incision to the left of the sternum and removing portions of three ribs over the heart. A flap was turned back which gave an opening over the heart of about 10 by 14 cm. Respiration was controlled by an artificial respiration apparatus. Great care was taken to prevent loss of blood. The right femoral artery was exposed for blood sampling. The mediastinal partition was punctured. This caused collapse of the lungs. The rate and degree of expansion and contraction of he lungs were set and maintained at a constant throughout experiments 1, 2, 3 by means of a motor driven artificial respiration machine. The pulmonary ventilation was set at about the normal minute volume and was totally independent of the animal's own respiratory effort. Gassing: The animal was given pure illuminating gas through the artificial respiration apparatus at 15 to 20-second intervals until the blood became about 60 to 80 per cent saturated with carbon monoxide. After a little experience the desired saturation could be estimated by the animal's heart action. Blood sampling: Five to 10 cc. samples of blood were drawn from the right femoral artery at intervals with a 10 cc. Luer syringe, and delivered into oxalated sampling tubes under oil. Blood analysis: Oxygen capacity, oxygen and carbon dioxide content, carbon monoxide content were determined by the Van Slyke (1924) method. The hydrogen ion concentration of the blood was determined colorimetrically by Cullen's method (1923). The results are given in tables 1 and 2 and figure 1. In experiment 1 it was found that the blood ph fell slightly during the first half hour of the experiment. To eliminate this complicating factor 10 gm. of NaHCO3
11 WILLIAM C. STADIE AND KIRBY A. MARTIN 87 was given intravenously. limits. This was found to maintain the blood ph within normal DISCUSSION For simplicity of comparison we have plotted the data of table 2 in figure 3, the initial saturation with CO being the same. 1rceit, -3 _ Miin-rtes FIG. 3. CURvEs SHOWING THE RATE OF ELMNATION OF CARBON MONOXDE FROM THE BLOOD OF PARTIALLY ASPHYXIATED DOGS UNDER DIFFERENT CONDITIONS In all cases except one (hyper-ventilation) the ventilatory rate was the same. The rate of elimination of CO at any instant is proportional to the concentration of CO in the blood, i.e., ds S dt 100
12 88 ELIMINATION OF CARBON MONOXIDE where S is the per cent of saturation of blood with CO. Integrating log i = kt Where S is the initial saturation and St the saturation at time t. If the data of table 2 be plotted using log as ordinates and t as abscissae straight line should result. This is exactly the case. The slopes of the lines give k for the four different conditions and with these k values figure 2 is calculated. This allows one to start the curves from a common point and bring out the differences :n a striking fashion. Little comment is needed. It is clear that hyperventilation itself is of minor importance in the elimination of CO. Decreasing ph by any acid, CO2 or HCI causes a great increase in the rate of elimination and is the most important factor. It must be strongly emphasized, however, that CO2 administered to asphyxiated subjects has a powerful stimulating effect on respiration. While hyperventilation plays a minor role yet it is a distinct one. The asphyxiated subject has usually a decreased pulmonary ventilation. This slows CO elimination. Any means of increasing ventilatory rate is beneficial. CO2 has a double function through the same mechanism. It increases blood hydrogen ion concentration which in itself favors CO elimination. This double function makes it the ideal therapy to employ: the addition of pure oxygen while helpful is far less important. The alteration in the oxygen dissociation curve by CO as the cause of the anxoemia An old experiment by Haldane (1895) would force us to believe that CO by itself is non-poisonous. He placed mice in a chamber with sufficient CO to saturate completely the hemoglobin. It could no longer serve to transport oxygen to the tissues and the mice would have died promptly (as did the controls) had it not been for the fact that the chambers also contained oxygen under several atmospheres of pressure. Under these conditions 3 to 4 volumes per cent of oxygen could be carried by the blood in physical solution. This was sufficient for the needs of the animals and they remained perfectly well. While
13 WILLIAM C. STADIE AND KIRBY A. MARTIN 89 it is not entirely dear that these results can be carried over to human beings, since the symptoms of CO poisoning are somewhat anomalous as pure symptoms of anoxemia, yet tentatively we may accept the hypothesis that CO poisoning causes harm solely by depriving the Vols. Per c e-nt Percent _- Q j P 0 tj04 40 H u Cd 41~~~~~o, / /b_. C: )o 2( g W Dxvgen tension mmhg. FIG. 4. OXYGEN DISSOCIATION CURVE OF THE BLOOD OF A CASE OF CARBON MONOXIDE POISONING 60 PER CENT SATURATED WITH CO CONTRASTED WITH THE CURVE OF A CASE OF PERNIcIOUS ANEmIA WITH THE SAME AMOUNT OF AVALABLE OXYHEMOGLOBIN, VIz., 40 PER CENT OF NORMAL tissue of oxygen. There is current, however, a misconprehension of the mechanism of this anoxemia which figure 4 makes clear. By means of equation 5 we have calculated the oxygen dissociation curve of blood when constantly saturated 50 per cent with CO. The form of this curve so calculated is in complete accord with the experi-
14 90 ELIMINATION OF CARBON MONOXIDE mentally determined curve for mouse blood under the same conditions as reported by Haldane (1912). Forty per cent of the total hemoglobin is available for combination with oxygen and the saturation of this residue of hemoglobin with oxygen is plotted against oxygen tension. In contrast the oxygen dissociation curve of a case of pernicious anemia having 40 per cent of the normal amount of hemoglobin is plotted. The curve in the latter case is unaltered as one of us has determined' but the CO case shows a marked alteration in the shape and position of the curve. It is this alteration which causes the anoxemia with its train of profound acute symptoms in marked contrast to the relatively normal condition of the anemia case. In both cases the maximum amount of oxygen available is the same, 8 volumes per cent. In both cases, the physiologic needs of the tissues is the same, say about 4 volumes per cent or half the maximum available. For the anemic case, however, this physiologic oxygen requirement (indicated by the hatched column) is dissociable at a partial pressure greater than 28 mm. of oxygen. Evidently at or above this pressure tissue metabolism is normal. But in the case of CO poisoning, the giving up of 4 volumes of oxygen requires descent to a partial pressure of 12 mm. of oxygen. Indeed three-fourths of the needed oxygen is dissociated between 28 mm. Hg, the minimum for the anemic case, and 12 mm. Now it is quite conceivable that the partial pressure at which oxygen is available in the capillaries for tissue-metabolism is important. Krogh has pictured a single capillary as supplying a cylinder of tissue. In order adequately to supply the periphery of the cylinder with oxygen, the oxygen pressure at th-e capillary wall cannot fall below a certain minimum or else the rate of diffusion of oxygen to the periphery which varies directly as this pressure head will fall below the physiologic requirement. Presumably this partial pressure lies somewhere between 12 and 20 mm. of Hg approximately. Despite the presence in the blood of two or three times the amount of oxygen necessary for normal tissue function profound anoxemia results in CO poisoning. This is due to an alteration of the shape and posi- 8 Stadie: Unpublished data.
15 WILLIAM C. STADIE AND KIRBY A. MARTIN tion of the oxygen dissociation curve causing a marked lowering of the partial pressure at which oxygen is available for tissue metabolism. SUMMARY An equation is derived showing the relation between hemoglobin, oxygen, carbon monoxide and ph at equilibrium. Experiments in vitro are given substantiating the equation. By means of this relation it is possible to predict the effect of increasing acidity on the elimnination of CO from asphyxiated animals. It is shown that increasing hydrogen ion concentration in itself will hasten elimination regardless of the ventilatory rate. Experiments on dogs showed this to be so. It is further shown by the equation that the oxygen dissociation curve of hemoglobin is altered by carbon monoxide. Accepting the hypothesis that carbon monoxide is in itself completely non-poisonous, the altered curve shows that even though twice or three times the physiologic requirements of oxygen are present in the blood there is a marked lowering of the partial pressure of oxygen in the capillaries. This conceivably depresses the rate of diffusion of oxygen to the tissues, thus causing anoxemia. BIBLIOGRAPHY Barcroft, J.: Respiratory Function of the Blood. Cambridge, Douglass, C. A., Haldane, J. H., and Haldane, J. B. S: Jour. Physiol., 1912, xliv, 296. Haldane, J. H.: Jour. Physiol., 1895, xviii, , xlv, Proc. xxii. Hartridge, H., and Roughton, F. J. W.: Proc. Royal Society, 1922, 336. Henderson, Y.: Jour. Amer. Med. Assn., 1921, lxxvii, Hill, A. V.: Biochem. Jour. 1921, xv, 577. Sayers, R. R., and Yant, W. P.: Pub. 2356, U. S. Bureau of Mines. 91
Respiration - Human 1
 Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing
 Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
Douglas and Haldane(2) has shown that the oxygen determinations. since it forms the basis of the "Coefficient of Utilisation" (Krrogh) and
 THE MEASUREMENT OF THE OXYGEN CONTENT OF THE MIXED VENOUS BLOOD, AND OF THE VOLUME OF BLOOD CIRCULATING PER MINUTE. BY J. BARCROFT, F. J. W. ROUGHTON AND R. SHOJI. (From the Physiological Laboratory, Cambridge.)
THE MEASUREMENT OF THE OXYGEN CONTENT OF THE MIXED VENOUS BLOOD, AND OF THE VOLUME OF BLOOD CIRCULATING PER MINUTE. BY J. BARCROFT, F. J. W. ROUGHTON AND R. SHOJI. (From the Physiological Laboratory, Cambridge.)
CHAPTER 3: The respiratory system
 CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
Oxygen convulsions are believed by many workers to be caused by an accumulation
 272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
272 J. Physiol. (I949) I09, 272-280 6I2.223.II:6I2.26I THE ROLE OF CARBON DIOXIDE IN OXYGEN POISONING BY H. J. TAYLOR From the Royal Naval Physiological Laboratory, Alverstoke, Hants (Received 26 March
Lung Volumes and Capacities
 Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
 Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
CHAPTER 3: The cardio-respiratory system
 : The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
: The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
Physiology Unit 4 RESPIRATORY PHYSIOLOGY
 Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
THE literature on this subject, which was reviewed recently (CAMPBELL, doses of amytal, and in addition received A.C.E. mixture during the
 -~~ -v GAS TENSIONS IN THE MUCOUS MEMBRANE OF THE STOMACH AND SMALL INTESTINE. By J. ARGYLL CAMPBELL. From the National Institute for Medical Research, Hampstead. (With six figures in the text.) (Received
-~~ -v GAS TENSIONS IN THE MUCOUS MEMBRANE OF THE STOMACH AND SMALL INTESTINE. By J. ARGYLL CAMPBELL. From the National Institute for Medical Research, Hampstead. (With six figures in the text.) (Received
Section Three Gas transport
 Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
IV. FROM AQUATIC TO ATMOSPHERIC BREATHING: THE TRACHEA & THE LUNG
 GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
RESPIRATORY REGULATION DURING EXERCISE
 RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
P215 Respiratory System, Part 2
 P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
Chapter 11: Respiratory System Review Assignment
 Name: Date: Mark: / 45 Chapter 11: Respiratory System Review Assignment Multiple Choice = 45 Marks Identify the choice that best completes the statement or answers the question. 1. Which of the following
Name: Date: Mark: / 45 Chapter 11: Respiratory System Review Assignment Multiple Choice = 45 Marks Identify the choice that best completes the statement or answers the question. 1. Which of the following
transfer, in part to difference of opinion regarding the mechanics of
 6I2.235 ON THE PARTIAL PRESSURES OF OXYGEN AND CARBON DIOXIDE IN ARTERIAL BLOOD AND ALVEOLAR AIR. By A. V. BOCK, D. B. DILL, H. T. EDWARDS, L. J. HENDERSON AND J. H. TALBOTT. (From the Fatigue Laboratory,
6I2.235 ON THE PARTIAL PRESSURES OF OXYGEN AND CARBON DIOXIDE IN ARTERIAL BLOOD AND ALVEOLAR AIR. By A. V. BOCK, D. B. DILL, H. T. EDWARDS, L. J. HENDERSON AND J. H. TALBOTT. (From the Fatigue Laboratory,
Respiratory System Physiology. Dr. Vedat Evren
 Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
660 mm Hg (normal, 100 mm Hg, room air) Paco, (arterial Pc02) 36 mm Hg (normal, 40 mm Hg) % saturation 50% (normal, 95%-100%)
 148 PHYSIOLOGY CASES AND PROBLEMS Case 26 Carbon Monoxide Poisoning Herman Neiswander is a 65-year-old retired landscape architect in northern Wisconsin. One cold January morning, he decided to warm his
148 PHYSIOLOGY CASES AND PROBLEMS Case 26 Carbon Monoxide Poisoning Herman Neiswander is a 65-year-old retired landscape architect in northern Wisconsin. One cold January morning, he decided to warm his
Oxygen and Carbon dioxide Transport. Dr. Laila Al-Dokhi
 Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
The physiological functions of respiration and circulation. Mechanics. exercise 7. Respiratory Volumes. Objectives
 exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
2. State the volume of air remaining in the lungs after a normal breathing.
 CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
Chapter 4: Ventilation Test Bank MULTIPLE CHOICE
 Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
Respiratory System Study Guide, Chapter 16
 Part I. Clinical Applications Name: Respiratory System Study Guide, Chapter 16 Lab Day/Time: 1. A person with ketoacidosis may hyperventilate. Explain why this occurs, and explain why this hyperventilation
Part I. Clinical Applications Name: Respiratory System Study Guide, Chapter 16 Lab Day/Time: 1. A person with ketoacidosis may hyperventilate. Explain why this occurs, and explain why this hyperventilation
technique which have led to increased precision
 THE ACCURACY OF DIRECT DETERMINATIONS OF OXYGEN AND CARBON DIOXIDE TENSIONS IN HUMAN BLOOD IN VITRO 1 By GILES F. FILLEY, ESTHER GAY, AND GEORGE W. WRIGHT (From the Department of Physiology, the Edwtvard
THE ACCURACY OF DIRECT DETERMINATIONS OF OXYGEN AND CARBON DIOXIDE TENSIONS IN HUMAN BLOOD IN VITRO 1 By GILES F. FILLEY, ESTHER GAY, AND GEORGE W. WRIGHT (From the Department of Physiology, the Edwtvard
Human Biology Respiratory System
 Human Biology Respiratory System Respiratory System Responsible for process of breathing Works in cooperation with Circulatory system Three types: 1. Internal Respiration 2. External Respiration 3. Cellular
Human Biology Respiratory System Respiratory System Responsible for process of breathing Works in cooperation with Circulatory system Three types: 1. Internal Respiration 2. External Respiration 3. Cellular
Circulatory And Respiration
 Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
AIIMS, New Delhi. Dr. K. K. Deepak, Prof. & HOD, Physiology AIIMS, New Delhi Dr. Geetanjali Bade, Asst. Professor AIIMS, New Delhi
 Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
Respiratory physiology II.
 Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation
 Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
ALVEOLAR - BLOOD GAS EXCHANGE 1
 ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
- How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have
 - How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have the highest blood flow of all organs, which makes them
- How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have the highest blood flow of all organs, which makes them
RESPIRATORY GAS EXCHANGE
 RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
Section Two Diffusion of gases
 Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
throughout. The constant-flow respiration was administered through a intravenously at appropriate intervals (in addition to the general
 414 6I2.22I:6I2.2I5.5 GASEOUS INTERCHANGES THROUGH THE VISCERAL PLEURA OF THE CAT. By M. KREMER, A. T. WILSON AND SAMSON WRIGHT. (Department of Physiology, Middlesex Hospital Medical School.) (Received
414 6I2.22I:6I2.2I5.5 GASEOUS INTERCHANGES THROUGH THE VISCERAL PLEURA OF THE CAT. By M. KREMER, A. T. WILSON AND SAMSON WRIGHT. (Department of Physiology, Middlesex Hospital Medical School.) (Received
Essential Skills Course Acute Care Module. Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook
 Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
Gases and Respiration. Respiration Overview I
 Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
Respiration. The resspiratory system
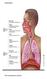 Respiration The resspiratory system The Alveoli The lungs have about 300 million alveoli, with a total crosssec onal area of 50 70 m2.. Each alveolar sac is surrounded by blood capillaries. The walls of
Respiration The resspiratory system The Alveoli The lungs have about 300 million alveoli, with a total crosssec onal area of 50 70 m2.. Each alveolar sac is surrounded by blood capillaries. The walls of
Gas exchange. Tissue cells CO2 CO 2 O 2. Pulmonary capillary. Tissue capillaries
 Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
PICU Resident Self-Study Tutorial The Basic Physics of Oxygen Transport. I was told that there would be no math!
 Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
Respiration (revised 2006) Pulmonary Mechanics
 Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
Breathing: The normal rate is about 14 to 20 times a minute. Taking in of air is called Inspiration and the forcing out of air is called Expiration.
 Biology 12 Respiration Divisions of Respiration Breathing: entrance and exit of air into and out of the lungs External Respiration: exchange of gases(o2 and CO2) between air (in alveoli) and blood Internal
Biology 12 Respiration Divisions of Respiration Breathing: entrance and exit of air into and out of the lungs External Respiration: exchange of gases(o2 and CO2) between air (in alveoli) and blood Internal
(Received 16 January 1946)
 186 J. Physiol. (I946) I05, I86-I90 6I2.2I5.9 THE ABSORPTION OF FLUIDS FROM THE LUNGS BY F. C. COURTICE AND P. J. PHIPPS From the Experimental Station, Porton and the Laboratory of Physiology, Oxford (Received
186 J. Physiol. (I946) I05, I86-I90 6I2.2I5.9 THE ABSORPTION OF FLUIDS FROM THE LUNGS BY F. C. COURTICE AND P. J. PHIPPS From the Experimental Station, Porton and the Laboratory of Physiology, Oxford (Received
Pco2 *20times = 0.6, 2.4, so the co2 carried in the arterial blood in dissolved form is more than the o2 because of its solubility.
 Physiology, sheet #9 Oxygen, is first dissolved in the plasma and the cytosol of the rbc, we have around blood constitutes 7% of our body weight, oxygen, in the capillaries is present in the rbc s and
Physiology, sheet #9 Oxygen, is first dissolved in the plasma and the cytosol of the rbc, we have around blood constitutes 7% of our body weight, oxygen, in the capillaries is present in the rbc s and
Respiratory Pulmonary Ventilation
 Respiratory Pulmonary Ventilation Pulmonary Ventilation Pulmonary ventilation is the act of breathing and the first step in the respiratory process. Pulmonary ventilation brings in air with a new supply
Respiratory Pulmonary Ventilation Pulmonary Ventilation Pulmonary ventilation is the act of breathing and the first step in the respiratory process. Pulmonary ventilation brings in air with a new supply
partial pressure is to be applied to the dissociation curve of fully oxygenated
 6I2. I27. I THE DETERMINATION OF THE CARBON DIOXIDE CONTENT OF THE MIXED VENOUS BLOOD. Part I. The effect of oxygenation and the critical oxygen tension. BY M. C. G. ISRAELS (Platt Physiological Scholar)
6I2. I27. I THE DETERMINATION OF THE CARBON DIOXIDE CONTENT OF THE MIXED VENOUS BLOOD. Part I. The effect of oxygenation and the critical oxygen tension. BY M. C. G. ISRAELS (Platt Physiological Scholar)
respiratory cycle. point in the volumes: 500 milliliters. for men. expiration, up to 1200 milliliters extra makes breathing Respiratory
 10 II. RESPIRATORY VOLUMES, CAPACITIES & PULMONARY FUNCTION TESTS Respiratory volume is the term used for various volumes of air moved by or associated with the lungs at a given point in the respiratory
10 II. RESPIRATORY VOLUMES, CAPACITIES & PULMONARY FUNCTION TESTS Respiratory volume is the term used for various volumes of air moved by or associated with the lungs at a given point in the respiratory
Applied Physics Topics 2
 Applied Physics Topics 2 Dr Andrey Varvinskiy Consultant Anaesthetist Torbay Hospital, UK EDAIC Paper B Lead and Examiner TOPICS 2 Gas Laws Other Laws: Dalton, Avogadro Critical temperature Critical pressure
Applied Physics Topics 2 Dr Andrey Varvinskiy Consultant Anaesthetist Torbay Hospital, UK EDAIC Paper B Lead and Examiner TOPICS 2 Gas Laws Other Laws: Dalton, Avogadro Critical temperature Critical pressure
Respiratory Lecture Test Questions Set 3
 Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
Chapter 23. Gas Exchange and Transportation
 Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity and minor gases argon,
Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity and minor gases argon,
HCO - 3 H 2 CO 3 CO 2 + H H H + Breathing rate is regulated by blood ph and C02. CO2 and Bicarbonate act as a ph Buffer in the blood
 Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
Chapter 13 The Respiratory System
 Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Collin County Community College. Lung Physiology
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/12
 LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
LOW PRESSURE EFFUSION OF GASES revised by Igor Bolotin 03/05/ This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the rates with which selected
Masaji Mochizuki ABSTRACT. ]p(deox). The Haldane effects of [CO2] and [HCO3. ] were obtained by subtracting [CO2]p(ox) from [CO2]p(deox) and [HCO3
![Masaji Mochizuki ABSTRACT. ]p(deox). The Haldane effects of [CO2] and [HCO3. ] were obtained by subtracting [CO2]p(ox) from [CO2]p(deox) and [HCO3 Masaji Mochizuki ABSTRACT. ]p(deox). The Haldane effects of [CO2] and [HCO3. ] were obtained by subtracting [CO2]p(ox) from [CO2]p(deox) and [HCO3](/thumbs/76/73452816.jpg) Yamagata Med J 2006242)51-58 in vivo Masaji Mochizuki Emeritus Professor of Yamagata University, Yamagata, Japan Geriatric Respiratory Research Center, Nishimaruyama Hospital, Chuo-Ku, Sapporo, Japan Accepted
Yamagata Med J 2006242)51-58 in vivo Masaji Mochizuki Emeritus Professor of Yamagata University, Yamagata, Japan Geriatric Respiratory Research Center, Nishimaruyama Hospital, Chuo-Ku, Sapporo, Japan Accepted
UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT
 UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION
UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION MUST, AT ALL TIMES, ACCEPT THE ENTIRE CARDIAC OUTPUT UNIQUE CHARACTERISTICS OF THE PULMONARY CIRCULATION THE PULMONARY CIRCULATION
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D.
 Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Chapter 23. Gas Exchange and Transportation
 Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity other minor gases argon,
Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity other minor gases argon,
CHAPTER 6. Oxygen Transport. Copyright 2008 Thomson Delmar Learning
 CHAPTER 6 Oxygen Transport Normal Blood Gas Value Ranges Table 6-1 OXYGEN TRANSPORT Oxygen Dissolved in the Blood Plasma Dissolve means that the gas maintains its precise molecular structure About.003
CHAPTER 6 Oxygen Transport Normal Blood Gas Value Ranges Table 6-1 OXYGEN TRANSPORT Oxygen Dissolved in the Blood Plasma Dissolve means that the gas maintains its precise molecular structure About.003
6I2.2I6:6I alveolar pressure. It follows that the evident alteration in the respiratory rhythm is an alteration in amplitude.
 6I2.2I6:6I2.223.11 SOME EFFECTS OF CARBONIC ACID ON THE CHARACTER OF HUMAN RESPIRATION. BY J. BARCROFT AND R. MARGARIA' (Turin). (From the Physiological Laboratory, Cambridge.) THE following facts concerning
6I2.2I6:6I2.223.11 SOME EFFECTS OF CARBONIC ACID ON THE CHARACTER OF HUMAN RESPIRATION. BY J. BARCROFT AND R. MARGARIA' (Turin). (From the Physiological Laboratory, Cambridge.) THE following facts concerning
Capnography in the Veterinary Technician Toolbox. Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA
 Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
CARBON DIOXIDE ELIMINATION FROM SEMICLOSED SYSTEMS
 Brit. J. Anaesth. (1956), 28, 196 CARBON DIOXIDE ELIMINATION FROM SEMICLOSED SYSTEMS BY RUSSELL M. DAVIES, I. R. VERNER Queen Victoria Hospital, East Grinstead AND A. BRACKEN Research and Development Centre,
Brit. J. Anaesth. (1956), 28, 196 CARBON DIOXIDE ELIMINATION FROM SEMICLOSED SYSTEMS BY RUSSELL M. DAVIES, I. R. VERNER Queen Victoria Hospital, East Grinstead AND A. BRACKEN Research and Development Centre,
These two respiratory media (air & water) impose rather different constraints on oxygen uptake:
 Topic 19: OXYGEN UPTAKE AND TRANSPORT (lectures 29-30) OBJECTIVES: 1. Be able to compare air vs. water as a respiratory medium with respect to oxygen content, diffusion coefficient, viscosity and water
Topic 19: OXYGEN UPTAKE AND TRANSPORT (lectures 29-30) OBJECTIVES: 1. Be able to compare air vs. water as a respiratory medium with respect to oxygen content, diffusion coefficient, viscosity and water
Physiology of Respiration
 Physiology of Respiration External Respiration = pulmonary ventilation breathing involves 2 processes: inspiration expiration Inspiration an active process involves contraction of diaphragm innervated
Physiology of Respiration External Respiration = pulmonary ventilation breathing involves 2 processes: inspiration expiration Inspiration an active process involves contraction of diaphragm innervated
I Physical Principles of Gas Exchange
 Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Structures of the Respiratory System include:
 Respiratory System Structures of the Respiratory System include: ü Oral Cavity ü Nasal Cavity ü Pharynx ü Epiglottis ü Larynx ü Trachea ü Diaphragm ü Lung ü Bronchus ü Bronchioles ü Alveolus ü Pulmonary
Respiratory System Structures of the Respiratory System include: ü Oral Cavity ü Nasal Cavity ü Pharynx ü Epiglottis ü Larynx ü Trachea ü Diaphragm ü Lung ü Bronchus ü Bronchioles ü Alveolus ü Pulmonary
Respiratory Medicine. A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics. Alveolar Gas Equation. See online here
 Respiratory Medicine A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics See online here Alveolar gas equation helps to calculate the partial pressure of oxygen in alveoli and A-a gradient is the
Respiratory Medicine A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics See online here Alveolar gas equation helps to calculate the partial pressure of oxygen in alveoli and A-a gradient is the
CHAPTER 16 %UHDWKLQJ*DV0L[LQJ3URFHGXUHV
 CHAPTER 16 %UHDWKLQJ*DV0L[LQJ3URFHGXUHV 16-1 INTRODUCTION 16-1.1 Purpose. The purpose of this chapter is to familiarize divers with the techniques used to mix divers breathing gas. 16-1.2 Scope. This chapter
CHAPTER 16 %UHDWKLQJ*DV0L[LQJ3URFHGXUHV 16-1 INTRODUCTION 16-1.1 Purpose. The purpose of this chapter is to familiarize divers with the techniques used to mix divers breathing gas. 16-1.2 Scope. This chapter
J. Physiol. (I941) I00, I98-21I 6I :6I2.825
 198 J. Physiol. (I941) I00, I9821I 6I2.22.02:6I2.825 THE EFFECT OF OXYGEN LACK ON THE CEREBRAL CIRCULATION BY F. C. COURTICE From the Departments of Physiology and of Surgery, Oxford (Received 24 March
198 J. Physiol. (I941) I00, I9821I 6I2.22.02:6I2.825 THE EFFECT OF OXYGEN LACK ON THE CEREBRAL CIRCULATION BY F. C. COURTICE From the Departments of Physiology and of Surgery, Oxford (Received 24 March
BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY
 BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY Dr.R.Selvakumar.M.D.D.A.DNB Professor of Anaesthesiology, K.A.P.Viswanatham Govt medical college, Trichy. The current practice of Anaesthesiology demands knowledge
BASIC PHYSICS APPLIED TO ANAESTHESIOLOGY Dr.R.Selvakumar.M.D.D.A.DNB Professor of Anaesthesiology, K.A.P.Viswanatham Govt medical college, Trichy. The current practice of Anaesthesiology demands knowledge
Lesson 9.1: The Importance of an Organ Delivery System
 Lesson 9.1: The Importance of an Organ Delivery System Animals require a continuous supply of oxygen (O 2 ) for cellular respiration, and they must expel carbon dioxide (CO 2 ), the waste product of this
Lesson 9.1: The Importance of an Organ Delivery System Animals require a continuous supply of oxygen (O 2 ) for cellular respiration, and they must expel carbon dioxide (CO 2 ), the waste product of this
RESPIRATION III SEMESTER BOTANY MODULE II
 III SEMESTER BOTANY MODULE II RESPIRATION Lung Capacities and Volumes Tidal volume (TV) air that moves into and out of the lungs with each breath (approximately 500 ml) Inspiratory reserve volume (IRV)
III SEMESTER BOTANY MODULE II RESPIRATION Lung Capacities and Volumes Tidal volume (TV) air that moves into and out of the lungs with each breath (approximately 500 ml) Inspiratory reserve volume (IRV)
breathing, with the use of initial mixtures in the rebreathing bag which differed in CO2 tensions by 10 mm. or more. By constructing a nomogram
 APPLICABILITY OF REBREATHING METHOD FOR DETERMINING MIXED VENOUS CO2 IN CASES OF CHRONIC PULMONARY DISEASE By DICKINSON W. RICHARDS, JR., ANDRE COURNAND, AND NATALIE A. BRYAN (From the Department of Medicine
APPLICABILITY OF REBREATHING METHOD FOR DETERMINING MIXED VENOUS CO2 IN CASES OF CHRONIC PULMONARY DISEASE By DICKINSON W. RICHARDS, JR., ANDRE COURNAND, AND NATALIE A. BRYAN (From the Department of Medicine
The over-ventilated cat shows a similar adjustment to diminished. being over-ventilated, and he considered that on that account there was
 6I2.235:6I2.26I THE SOURCE OF COa EXPIRED AND THE SITE OF ITS RETENTION. BY LAURENCE IRVING, J. K. W. FERGUSON AND F. B. PLEWES. (From the Department of Physiology, University of Toronto.) AFTER evisceration
6I2.235:6I2.26I THE SOURCE OF COa EXPIRED AND THE SITE OF ITS RETENTION. BY LAURENCE IRVING, J. K. W. FERGUSON AND F. B. PLEWES. (From the Department of Physiology, University of Toronto.) AFTER evisceration
GAS TENSIONS OF THE ABDOMINAL CAVITY, WITH SOME EVIDENCE ON THE DIFFUSION OF GASES WITHIN THE BODY.*
 GAS TENSIONS OF THE ABDOMINAL CAVITY, WITH SOME EVIDENCE ON THE DIFFUSION OF GASES WITHIN THE BODY.* BY HOWARD W. HAGGARD AND YANDELL HENDERSON. WITHTEECOLLABORATIONOF H.H.BEATTY,S.R. TALIAFERRO. DETWILER,
GAS TENSIONS OF THE ABDOMINAL CAVITY, WITH SOME EVIDENCE ON THE DIFFUSION OF GASES WITHIN THE BODY.* BY HOWARD W. HAGGARD AND YANDELL HENDERSON. WITHTEECOLLABORATIONOF H.H.BEATTY,S.R. TALIAFERRO. DETWILER,
VOLUNTARY BREATHHOLDING. I. PULMONARY GAS
 VOLUNTARY BREATHHOLDING. I. PULMONARY GAS EXCHANGE DURING BREATHHOLDING'1 By CHARLES D. STEVENS, EUGENE B. FERRIS, JOSEPH P. WEBB, GEORGE L. ENGEL, AND MYRTLE LOGAN (From the Departments of Internal Medicine
VOLUNTARY BREATHHOLDING. I. PULMONARY GAS EXCHANGE DURING BREATHHOLDING'1 By CHARLES D. STEVENS, EUGENE B. FERRIS, JOSEPH P. WEBB, GEORGE L. ENGEL, AND MYRTLE LOGAN (From the Departments of Internal Medicine
Recitation question # 05
 Recitation and Lab # 05 The goal of this recitations / labs is to review material related to the CV and respiratory lectures for the second test of this course. Info required to answer this recitation
Recitation and Lab # 05 The goal of this recitations / labs is to review material related to the CV and respiratory lectures for the second test of this course. Info required to answer this recitation
Gas Pressure. Pressure is the force exerted per unit area by gas molecules as they strike the surfaces around them.
 Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Chapter 5 Gases Gas Gases are composed of particles that are moving around very fast in their container(s). These particles moves in straight lines until they collides with either the container wall or
Respiratory Physiology. Adeyomoye O.I
 Respiratory Physiology By Adeyomoye O.I Outline Introduction Hypoxia Dyspnea Control of breathing Ventilation/perfusion ratios Respiratory/barometric changes in exercise Intra-pulmonary & intra-pleural
Respiratory Physiology By Adeyomoye O.I Outline Introduction Hypoxia Dyspnea Control of breathing Ventilation/perfusion ratios Respiratory/barometric changes in exercise Intra-pulmonary & intra-pleural
Respiratory system & exercise. Dr. Rehab F Gwada
 Respiratory system & exercise Dr. Rehab F Gwada Objectives of lecture Outline the major anatomical components & important functions of the respiratory system. Describe the mechanics of ventilation. List
Respiratory system & exercise Dr. Rehab F Gwada Objectives of lecture Outline the major anatomical components & important functions of the respiratory system. Describe the mechanics of ventilation. List
Let s talk about Capnography
 Let s talk about Capnography This is one of a series of articles by Keith Simpson BVSc MRCVS MIET (Electronics) discussing the practical aspects of some common monitoring techniques. Capnometry is the
Let s talk about Capnography This is one of a series of articles by Keith Simpson BVSc MRCVS MIET (Electronics) discussing the practical aspects of some common monitoring techniques. Capnometry is the
Using such a method, Morawitz and Siebeck (1) found that the. composition of the alveolar air or of the blood. Unless the obstruc- 483
 THE EFFECT OF SOME PATHOLOGICAL CONDITIONS UPON DYSPNEA DURING EXERCISE I. ARTIFICIAL STENOSIS BY A. W. HEWLETT, J. K. LEWIS AND ANNA FRANKLIN (From the Department of Medicine, Stanford Medical School)
THE EFFECT OF SOME PATHOLOGICAL CONDITIONS UPON DYSPNEA DURING EXERCISE I. ARTIFICIAL STENOSIS BY A. W. HEWLETT, J. K. LEWIS AND ANNA FRANKLIN (From the Department of Medicine, Stanford Medical School)
PROBLEM SET 9. SOLUTIONS April 23, 2004
 Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Some major points on the Effects of Hypoxia
 Some major points on the Effects of Hypoxia Source: Kings College London http://www.kcl.ac.uk/teares/gktvc/vc/dental/year1/lectures/rbmsmajorpoints/effectsofhypoxia.htm Cells obtain their energy from oxygen.
Some major points on the Effects of Hypoxia Source: Kings College London http://www.kcl.ac.uk/teares/gktvc/vc/dental/year1/lectures/rbmsmajorpoints/effectsofhypoxia.htm Cells obtain their energy from oxygen.
2.1.1 List the principal structures of the
 physiology 2.1.1 List the principal structures of the The principle structures of the respiratory are: Nose/Mouth used for inhalation of oxygen-rich air and expelling carbon dioxide rich air Pharynx -
physiology 2.1.1 List the principal structures of the The principle structures of the respiratory are: Nose/Mouth used for inhalation of oxygen-rich air and expelling carbon dioxide rich air Pharynx -
APPENDIX. working blood volume was also rather large; Evans, Grande, and. equilibrated to the new mixture is partially dependent upon the rate
 612.172-5 APPENDIX A SIMPLIFIED HEART OXYGENATOR CIRCUIT FOR BLOOD- FED HEARTS. By J. YULE BOG-UE and R. A. GREGORY.' SINCE 1934 studies on the carbohydrate metabolism of the blood-fed heart without lungs
612.172-5 APPENDIX A SIMPLIFIED HEART OXYGENATOR CIRCUIT FOR BLOOD- FED HEARTS. By J. YULE BOG-UE and R. A. GREGORY.' SINCE 1934 studies on the carbohydrate metabolism of the blood-fed heart without lungs
Alveolus and Respiratory Membrane
 Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Medical Research, Holly Hill, Hampstead, London, N. W. 3 (Received 6 November 1957)
 63 J. Physiol. (958) 42, 63-77 THE CARBON MONOXDE DSSOCATON CURVE OF HUMAN BLOOD BY N. JOELS* AND L. G. C. E. PUGH From the Division of Human Physiology, National nstitute for Medical Research, Holly Hill,
63 J. Physiol. (958) 42, 63-77 THE CARBON MONOXDE DSSOCATON CURVE OF HUMAN BLOOD BY N. JOELS* AND L. G. C. E. PUGH From the Division of Human Physiology, National nstitute for Medical Research, Holly Hill,
Kinetic-Molecular Theory
 GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
GASES Chapter Eleven Kinetic-Molecular Theory! Recall that our only previous description of gases stated that gases completely fill and take the shape of their containers.! The Kinetic-Molecular Theory
BREATHING AND EXCHANGE OF GASES
 96 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 17 BREATHING AND EXCHANGE OF GASES MULTIPLE CHOICE QUESTIONS 1. Respiration in insects is called direct because a. The tissues exchange O 2 directly with the air in
96 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 17 BREATHING AND EXCHANGE OF GASES MULTIPLE CHOICE QUESTIONS 1. Respiration in insects is called direct because a. The tissues exchange O 2 directly with the air in
Chapter 16 Respiratory System
 Introduction Chapter 16 Respiratory System The respiratory system consists of tubes that filter incoming air and transport it to alveoli where gases are exchanged. Think pair share: what organs are associated
Introduction Chapter 16 Respiratory System The respiratory system consists of tubes that filter incoming air and transport it to alveoli where gases are exchanged. Think pair share: what organs are associated
PURE SUBSTANCE. Nitrogen and gaseous air are pure substances.
 CLASS Third Units PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Air is a mixture of several gases, but it is considered to be a pure substance. Nitrogen and
CLASS Third Units PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Air is a mixture of several gases, but it is considered to be a pure substance. Nitrogen and
LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary
 ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
ADH 1/7/014 LOW PRESSURE EFFUSION OF GASES adapted by Luke Hanley and Mike Trenary This experiment will introduce you to the kinetic properties of low-pressure gases. You will make observations on the
Tter person TERM PAPER. Date: 30/4/ MEDICAL VENTILATOR
 Tter person TERM PAPER BM 600 Nuruddin Bahar Date: 30/4/2012 09002040 MEDICAL VENTILATOR It is an artificial ventilating device which is used to mechanically move breathable air in and out of lungs at
Tter person TERM PAPER BM 600 Nuruddin Bahar Date: 30/4/2012 09002040 MEDICAL VENTILATOR It is an artificial ventilating device which is used to mechanically move breathable air in and out of lungs at
BREATHING AND EXCHANGE OF GASES
 96 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 17 BREATHING AND EXCHANGE OF GASES MULTIPLE CHOICE QUESTIONS 1. Respiration in insects is called direct because a. The cell exchange O 2 directly with the air in the
96 BIOLOGY, EXEMPLAR PROBLEMS CHAPTER 17 BREATHING AND EXCHANGE OF GASES MULTIPLE CHOICE QUESTIONS 1. Respiration in insects is called direct because a. The cell exchange O 2 directly with the air in the
Respiratory System. Part 2
 Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Gas Laws. Introduction
 Gas Laws Introduction In 1662 Robert Boyle found that, at constant temperature, the pressure of a gas and its volume are inversely proportional such that P x V = constant. This relationship is known as
Gas Laws Introduction In 1662 Robert Boyle found that, at constant temperature, the pressure of a gas and its volume are inversely proportional such that P x V = constant. This relationship is known as
Prem?ous researches. The previous work on C02 partial pressure in
 THE CARBON DIOXIDE PARTIAL PRESSURE IN BODY CAVITIES AND TISSUE SPACES UNDER VARIOUS CONDITIONS. BY J. ARGYLL CAMPBELL. (From the Department of Applied Physiology, National Institute for Medical Research,
THE CARBON DIOXIDE PARTIAL PRESSURE IN BODY CAVITIES AND TISSUE SPACES UNDER VARIOUS CONDITIONS. BY J. ARGYLL CAMPBELL. (From the Department of Applied Physiology, National Institute for Medical Research,
Chapter 22 The Respiratory System
 Chapter 22 The Respiratory System 1 Respiration Pulmonary ventilation (breathing): movement of air into and out of the lungs External respiration: O 2 and CO 2 exchange between the lungs and the blood
Chapter 22 The Respiratory System 1 Respiration Pulmonary ventilation (breathing): movement of air into and out of the lungs External respiration: O 2 and CO 2 exchange between the lungs and the blood
Rodney Shandukani 14/03/2012
 Rodney Shandukani 14/03/2012 OXYGEN THERAPY Aerobic metabolism accounts for 90% of Oxygen consumption by tissues. generates ATP by oxidative phosphorylation. Oxygen cascade: Oxygen exerts a partial pressure,
Rodney Shandukani 14/03/2012 OXYGEN THERAPY Aerobic metabolism accounts for 90% of Oxygen consumption by tissues. generates ATP by oxidative phosphorylation. Oxygen cascade: Oxygen exerts a partial pressure,
Gas Exchange in Animals. Uptake of O2 from environment and discharge of CO2. Respiratory medium! water for aquatic animals, air for terrestial
 Gas Exchange in Animals Uptake of O2 from environment and discharge of CO2 Respiratory medium! water for aquatic animals, air for terrestial Respiratory surface! skin, gills, lungs Circulatory System O2/CO2
Gas Exchange in Animals Uptake of O2 from environment and discharge of CO2 Respiratory medium! water for aquatic animals, air for terrestial Respiratory surface! skin, gills, lungs Circulatory System O2/CO2
