Understanding Arterial Blood Gases (ABGs)
|
|
|
- Harvey Higgins
- 5 years ago
- Views:
Transcription
1 NYSNA Continuing Education The New York State Nurses Association is accredited as a provider of continuing nursing education by the American Nurses Credentialing Center s Commission on Accreditation. This course has been awarded 2 contact hours. Contact hours will be awarded for this online course until August 31, All American Nurses Credentialing Center (ANCC) accredited organizations' contact hours are recognized by all other ANCC accredited organizations. Most states with mandatory continuing education requirements recognize the ANCC accreditation/approval system. Questions about the acceptance of ANCC contact hours to meet mandatory regulations should be directed to the professional licensing board within that state. NYSNA has been granted provider status by the Florida State Board of Nursing as a provider of continuing education in nursing (Provider number ). 1
2 How to Take This Course Please take a look at the steps below; these will help you to progress through the course material, complete the course examination and receive your certificate of completion. 1. REVIEW THE OBJECTIVES The objectives provide an overview of the entire course and identify what information will be focused on. Objectives are stated in terms of what you, the learner, will know or be able to do upon successful completion of the course. They let you know what you should expect to learn by taking a particular course and can help focus your study. 2. STUDY EACH SECTION IN ORDER Keep your learning "programmed" by reviewing the materials in order. This will help you understand the sections that follow. 3. COMPLETE THE COURSE EXAM After studying the course, click on the "Course Exam" option located on the course navigation toolbar. Answer each question by clicking on the button corresponding to the correct answer. All questions must be answered before the test can be graded; there is only one correct answer per question. You may refer back to the course material by minimizing the course exam window. 4. GRADE THE TEST Next, click on "Submit Test." You will know immediately whether you passed or failed. If you do not successfully complete the exam on the first attempt, you may take the exam again. If you do not pass the exam on your second attempt, you will need to purchase the course again. 5. FILL OUT THE EVALUATION FORM Upon passing the course exam you will be prompted to complete a course evaluation. You will have access to the certificate of completion after you complete the evaluation. At this point, you should print the certificate and keep it for your records. 2
3 Objectives At the completion of this learning activity the learner will: Describe components of the respiratory process. Describe ph and the concept of compensation. Describe the role of the respiratory process in maintaining normal ph. Describe the role of metabolic processes in maintaining normal ph. Identify lab values that indicate compensated/uncompensated respiratory acidosis and alkalosis and metabolic acidosis and alkalosis. Introduction You are caring for a 67-year-old female with breathing difficulties. The treatments you have given her have not helped to improve her breathing status. You return to the physician to update her on your patient. The physician orders an arterial blood gas. You head to the phone and call the Respiratory Therapist. Once you have relayed the order for an arterial blood gas to the Respiratory Therapist, you hang up the phone and return to care for your other patients. You take no part in gathering the blood gas, allowing the respiratory therapist to draw, send and interpret the test results with the physician. If you are lucky, you may be able to overhear a discussion on the ABG results. In the back of your mind you would like to take a more active role in this scenario, if only you were more comfortable with ABGs. If you can relate to the above patient scenario you re not alone. Arterial blood gases can be challenging to all nurses despite their level of expertise. The purpose of this course is to provide nurses with information regarding blood gases and their basic interpretation. An arterial blood gas provides two types of information: acid-base balance and oxygenation. Acid-base balance is measured using a ph scale. The ph is a measurement of the concentration of hydrogen ions (H + ) in a solution. In this case, our solution is the arterial blood. Oxygenation is measured both by the partial pressure of oxygen in the arterial blood (PaO 2 ) and by the arterial oxygen saturation (SaO 2 ). The arterial oxygenation saturation tells us the percentage of heme sites on the hemoglobin molecules that are saturated with oxygen. Arterial oxygen saturation can also be measured non-invasively with a pulse oximeter probe and is then represented by the abbreviation SpO 2 instead of SaO 2. In order for the body to function normally, the ph must be continually maintained within a narrow range. This management is accomplished by the lungs and the kidneys. Before we jump into numbers and attempt to interpret an ABG, let s take some time to understand the fundamentals. We will begin by explaining how we measure gases in the blood and review how we move oxygen and carbon dioxide into and out of our bodies. Following on from this we will review ph balance and the concept of compensation, before continuing on to explain how to take an arterial blood gas. Lastly we will learn to interpret arterial blood gases and work through some examples before completing a mini-quiz to test our newly gained knowledge. Intentionally, this course is designed to review pertinent information without bogging down the reader with technical equations and explanations. Further reading beyond this course is encouraged to build on the basic skills taught. 3
4 Content Outline Introduction Measuring gas Ventilation Diffusion Oxyhemoglobin dissociation curve Carbon dioxide Carbonic acid equation ph Renal compensation Respiratory compensation Base excess/deficit Anion gap Performing ABG Interpretation About the Author David Pickham, MN, RN, began his nursing education at the University of Newcastle in New South Wales, Australia. He has since worked as a registered nurse focusing on emergency medicine in Australia, Canada, and the United States. He has a master s of nursing in advanced practice and, at the time of authoring this course, he was a doctoral candidate at the University of California. He has authored several other courses for e-learn. This course was updated by Sally Dreslin, MA, BS, RN, CEN in September Ms. Dreslin is the Associate Director for the Education, Practice and Research Program of the New York State Nurses Association. Ms. Dreslin has many years of clinical nursing experience in a variety of settings including general medicine, oncology, ambulatory surgery, endoscopy, PACU, US Air Force flight nursing, primary care clinic, corporate occupational clinic, mobile medical van, and the emergency department. As an Education Specialist involved in critical care orientation, she has provided clinical education for ambulance EMT crews, Air Force flight crews, emergency nurses, and cardiac nurses. 4
5 Measuring Gas When dealing with arterial blood gases you will often see measurements stated as millimeters of mercury (mmhg). You may recognize these units from your blood pressure measurements. But what exactly does it represent? The pressure of a gas is measured in terms of the height that a particular fluid is supported by the gas. For example, if we were to measure a fictional gas (for the purpose of this course let s call this gas Lyton), we can do this by pushing it into a tube connected to a fluid measurement. In our example our fluid (in this case we will use soda) rises to a height of 110 centimeters (cm) as a direct result of the pressure of the gas in the tube. In measuring this, we record the height of our fluid and report that the pressure of Lyton in the tube is 110 cm of soda. In another example, water will be supported 10 meters (m) high by 1 atmosphere of pressure. What is one atmosphere of pressure you say? Atmospheric pressure is the pressure exerted by air at sea level. Now it would be difficult to use a 10 m tall water measurement in the hospital as our standard of measure, therefore we use a denser liquid in our measurement: Mercury (Hg). Mercury is much heavier than water and will rise, in response to gas, at a proportionally smaller rate. Years ago someone measured the atmospheric pressure at sea level with Mercury. The result was that one atmosphere of pressure was 760 millimeters of Mercury (mmhg). As you can see, this height is a lot more manageable than 10 m of water. The term millimeters of Mercury (mmhg) was introduced and has since become the international standard unit of pressure (Gas Laws, n.d.). When we relate this to arterial blood gases, the pressure of oxygen within the arterial blood system should raise our mercury filled measure to a height between 80 and 100 millimeters (mm). Likewise, the pressure of carbon dioxide (CO 2 ), another important gas in the body, raises the mercury filled measure to a height of 35 to 45 mm. When you read about blood gases you may see another unit measuring pressure: kilopascals (kpa). Kilopascals (kpa) as a unit of measure is not used in the United States. Countries like the United Kingdom and Australia report pressures in kpa instead of mmhg. You may never see this unless you are from one of these countries, you travel to these countries, or you read international nursing texts or journals. Don t be overwhelmed or confused by this measure. A value reported as kpa can easily be converted to mmhg by multiplying the kpa value by 7.5. For example kpa is converted to mmhg by multiplying 7.5 to give a value of 80 mmhg. Key Points Pressure is measured in mmhg. The pressure of the atmosphere at sea level is 760 mmhg. Normal arterial oxygen pressure (PaO 2 ) is mmhg. Normal arterial carbon dioxide pressure (PaCO 2 ) is mmhg. That s a basic look at how gas is measured, now let s look at how we get these gases into our lungs. 5
6 Respiration Ventilation When we look at air, approximately 21% of it is made up of oxygen, 79% nitrogen, and a tiny fraction (0.04%) is in the form of carbon dioxide and other gases. Knowing these proportions allows us to figure out the pressure of each portion. As we mentioned previously, atmospheric pressure (P) at sea level is approximately 760 mmhg. Oxygen is approximately 21% or 0.21 of the atmospheric pressure. We can therefore find the pressure of oxygen by multiplying the overall atmospheric pressure to the oxygen content. It may help to visualize this in the formula below. Pressure of O 2 in the atmosphere = 760 mmhg x 0.21 = mmhg is the pressure exerted by the portion of oxygen in the atmosphere. The same can be completed for carbon dioxide. Pressure of CO 2 = 760mmHg x = 0.3 mmhg is the pressure exerted by the portion of carbon dioxide in the atmosphere. Why do we need to know this? Well I am glad you asked. Try to recall back to those tedious days of science classes (if recalling these memories has caused any anxiety, stop reading, grab a drink, take a breath, and come back). Do you remember that gases will naturally move from an area of high concentration to an area of lesser concentration? Our bodies move oxygen in and carbon dioxide out through using these gas gradients (difference in gas pressures). During inspiration our thoracic cavity expands increasing the total area within our lungs. This results in the pressure in our lungs becoming lower than the atmospheric pressure. With the lungs expanding, our alveoli are forced open further lowering the pressure in the alveoli. As a response to this gas gradient, air moves into our lungs and does so until the pressure inside the lungs equalizes to the pressure outside the lungs (O Leary, 2002). Seems reasonable enough doesn t it? Let s look at two practical applications of this. The first example will show the effect ventilation changes in the lungs has on the gas gradient, while the second will look at how changes in the atmospheric pressure effects ventilation. Application 1. Application 2. Patients with emphysema lose the ability to expand and contract their lungs and are therefore unable to create large changes in their lung pressure. A decreased amount of air enters their lungs, and to make matters worse, they also have decreased levels of oxygen and carbon dioxide exchange. As a result their lungs are always hyper-inflated with elevated CO 2 levels; they present with hypoxia and often require continuous home oxygen. As we gain altitude (height), the air pressure lowers. On Mount Everest for example, the world s tallest peak, the pressure exerted by air has been measured at 253 mmhg (compared to 760 mmhg at sea level). The pressure of the oxygen portion of air at this altitude has been measured as low as 53 mmhg (compared to mmhg at sea level). Remember, we must have a gas gradient for air to enter our lungs. With only one third of the sea level pressure of oxygen available in the atmosphere, this gas gradient is greatly diminished. Equalizing the pressure inside the lung and in the atmosphere therefore doesn t take long. This explains why climbers use supplemental oxygen when climbing peaks such as Mount Everest. 6
7 Key Points Gases move from high concentration to low concentrations. Pressure inside the lungs is much lower, resulting in air flowing into the lungs. Now we know how gas is measured and how to move air into our lungs, but how do we get oxygen to the tissues? Diffusion Once inside the lungs, oxygen moves through the bronchial branches, slowing until it reaches a standstill in the alveoli. This journey reduces the partial pressure of oxygen from a pressure of mmhg in the atmosphere, to a pressure of 103 mmhg in the alveoli (Barry & Pinard, 2002). On the other side of the alveoli in the capillaries, the partial pressure of oxygen is much lower, measuring 40 mmhg (Note the difference in pressures between the alveoli and the capillaries). As we read previously, oxygen, as all gases do, will move from the higher concentration in the alveoli to the lower concentration in the capillaries in an effort to equalize the gas gradient. To do this, oxygen permeates the surfactant lining the alveoli wall, enters the capillaries, and moves into the plasma. Once in the plasma, oxygen enters an erythrocyte and is finally taken up by the hemoglobin for transportation to the tissues (O Leary, 2002). An illustration of this is provided in Figures 1 and 3. This complex process takes approximately 0.25 seconds to complete (Bullock, Boyle, & Wang, 2001). Figure 1. Alveolar capillary network. (Cuesta Community College, n.d.) Note how the blood moves from the right side of the heart to the lungs, changing color from blue to red as it is oxygenated in the alveolar-capillary network. The binding of oxygen to hemoglobin does not occur by chance. An important aspect in oxygenation is the Oxyhemoglobin dissociation curve. Oxyhemoglobin Dissociation Curve Simply stated, this curve graphs the relationship between two variables. 1. Amount of oxygen available in the arterial blood (PaO 2 ) 2. Percentage of oxygen bound to hemoglobin (SaO 2 ). 7
8 Why is this important to know? Oxygen s willingness to bind to hemoglobin is predictable under certain pressure conditions. Knowing these conditions allows us to predict the amount of oxygen in the artery that is being delivered to the tissues, and this allows us to provide better treatment. Figure 2. Oxyhemoglobin dissociation curve Hemoglobin loves to bind with oxygen. Hemoglobin will bind with up to four oxygen compounds. When all of the heme sites on the hemoglobin modules are bound with oxygen, we say that the hemoglobin is 100% saturated. As mentioned before, this saturation does not occur by chance. It is highly dependent upon the partial pressure of oxygen in the blood. Do you recall what the normal arterial pressure of oxygen is? If you said it was between 80 and 100 mmhg, good job. Now let s see what the corresponding SaO 2 would be. Find the PaO 2 of 80 mmhg on the curve (Figure 2) and landmark the corresponding SaO 2. As you can see on the curve, the hemoglobin saturation would be 96% and above. Can you see what the PaO 2 needs to be to maintain a SaO 2 above 90%? Did you get 60 mmhg? There is a big difference in the PaO 2 of 60 to 100 mmhg; however, the oxygen saturation remains relatively constant (plateau). This is an important aspect of oxygenation. This plateau ensures that our tissues remain oxygenated even during periods of stress where there is a decreased oxygen supply, like during times of exercise. In contrast, a PaO 2 below 40 mmhg will result in a state were the tissues are hungry for oxygen. During this state the tissues would be quick to take up any oxygen available within the blood. Hemoglobin is aware of this and responds by releasing any available oxygen to the tissues, resulting in a decreased oxygen saturation of hemoglobin. Arterial oxygen pressures that are less than 80 mmhg are described as hypoxemia. Below are some partial pressures of oxygen and the corresponding oxygen saturation levels of hemoglobin. PaO 2 of 27 mmhg = hemoglobin is 50% saturated PaO 2 of 40 mmhg = hemoglobin is 75% saturated PaO 2 of 60 mmhg = hemoglobin is 90% saturated PaO 2 of 97 mmhg = hemoglobin is 97% saturated 8
9 Key Points The higher the pressure of oxygen in the blood the greater the hemoglobin saturation will be. The lower the PaO 2, the lower the SaO 2 is likely to be. For a patient example, a SaO 2 below 90% is cause for concern, as the PaO 2 (from the chart) would be below 60 mmhg. A patient in this state needs supplemental oxygen. An illustration of diffusion is provided in Figure 3 on the next page. Okay, we ve reviewed oxygen, now let s review another important gas involved in respiration. Carbon Dioxide Carbon dioxide is a product of cellular metabolism. Once released it travels into the blood stream where it is transported for removal in three different ways. 1. Carbon dioxide (CO 2 ) and water (H 2 0), with the help of the enzyme carbonic anhydrase, combine to form carbonic acid (H 2 CO 3 ). The carbonic acid then breaks down into bicarbonate (HCOˉ3) a base and a hydrogen ion (H + ) an acid. Once formed, the bicarbonate moves out of the red blood cell and into the plasma, where it is ultimately released from the body through respiration. The carbonic acid and bicarbonate/hydrogen ion system is considered a buffer system. As a buffer system, it is a reversible process and can therefore manage significant fluctuations in acid and base levels, without allowing the ph level to dramatically rise or fall. Eighty to 90% of carbon dioxide is removed from the body through this method. 2. Five percent of CO 2 binds to blood proteins. The free hydrogen ion released by the breakdown of carbonic acid binds to hemoglobin in the red blood cell to form a carbaminohemoglobin compound. When oxygen is not bound to the hemoglobin, carbon dioxide binds for transportation back to the lungs. 3. Like oxygen, a small proportion of carbon dioxide (5%) will dissolve directly into the blood. The level of carbon dioxide is closely monitored within the body. Specialized receptors located in the body will send information to the brain regarding any variations in ph status, oxygen and carbon dioxide levels. Change in any of these levels stimulates the body to a response aimed at correcting the imbalance. We will discuss this concept in depth further on. 9
10 Figure 3. Overview of respiratory processes and partial pressures in respiration. (Fox & Brown, 2005) Note how the venous blood (blue) has a decreased PO 2 and a higher PCO 2 than in the alveolar (PaO 2 and PaCO 2 ). As you now know, a gradient allows gas to move from a higher concentration to a lesser concentration. This results in an exchange of CO 2 and O 2. This will decrease the PaCO 2 and increase the PaO 2, in the arterial blood (red). Once in the vicinity of the tissues, the diffusion of CO 2 and PO 2 will occur again. Key Points Carbon dioxide is a product of cell metabolism. Once released from the cells it is transported by the blood for removal; predominately by the lungs. The reversible reactions undertaken by carbon dioxide in the red blood cell are a key element in maintaining a normal environment within the body. This reaction is explained using the Carbonic-acid equation. In an effort not to overburden you with technicalities, we will limit our discussion on this concept. 10
11 Carbonic-acid equation In the previous section we showed that CO 2 is transported in three different ways. To achieve this, the CO 2 released by the tissues needs to undergo changes so that it is more manageable for the body. This change/reaction is represented by the equation below. CO 2 + H 2 0 <--> H 2 CO 3 <--> H + + HCOˉ3 Without getting too technical, note how the arrows point in either direction. This means it is reversible. The body may turn carbon dioxide (CO 2 ) and water (H 2 O) into a hydrogen (H + ) and bicarbonate (HCOˉ3) or likewise H + and HCOˉ3 into CO 2 and H 2 O. The key here is that H + and HCOˉ3 are eliminated or absorbed by the kidneys and CO 2 and H 2 0 is released during expiration by the lungs. The needs of the body will dictate which direction the equation will function. This reaction is very important as it is the main regulator of acid-base balance in the body. This acidbase balance is reflected by the blood s ph level. Key Points Oxygen and carbon dioxide enter and leave the body through a process called diffusion. Diffusion relies entirely on a gas gradient being present at the alveoli/capillary level. PaO 2 is a significant factor in maintaining adequate SaO 2 %. Carbon dioxide is broken down by a reversible process that will adapt depending on the bodies acid-base state. 11
12 ph The ph of the blood is important in maintaining the body s homeostasis. As the level of ph decreases, the solution is more acidotic and conversely, when the solution has a higher ph, the solution is more alkalotic. The ph is measured on a scale from 0 to 14. In the discussion of ph, acids are substances that release hydrogen ions, while alkalis (bases) are substances that absorb hydrogen ions (buffers). Our body will attempt to maintain a ph between 7.35 and This range is considered normal. To do this there must be a balance between the amount of acids and the amount of bases in the body. Simply, the ratio of alkali to acid is maintained at 20 to 1. If the ratio experiences variation, an acid-base imbalance will result. There are two main organs that are responsible for correcting acid-base imbalances, the kidneys and the lungs. When these organs respond to changes in acid-base balance it is known as a compensation mechanism. Each organ is responsible for responding to imbalances in acidbase balance caused by the other organ. Let s look at the organs responses individually. Renal Compensation When you think back, CO 2 is diffused out of the tissues and formed into carbonic acid, where it is broken into bicarbonate and hydrogen. The kidneys will maintain the acid-base balance ratio by continuously absorbing or eliminating bicarbonate and hydrogen compounds. This compensation is slow and can take two to four days for changes to take effect. Below are two responses the kidneys can have in relation to acid-base changes caused by the lungs. In the presence of a high PaCO 2, the kidneys will attempt to buffer the increase in circulating acids by increasing the excretion of hydrogen. This augmented loss of hydrogen normalizes the 20:1 ratio of alkali to acid in the blood. Conversely, in the presence of a low PaCO 2, the kidneys will attempt to adjust to the decrease in circulating acids by increasing re-absorption of hydrogen and increasing excretion of bicarbonate. This will raise the level of acid thus restoring the acid-base balance. Respiratory Compensation The lungs are the organs primarily responsible for removing CO 2. They exert a great deal of control over the acid-base balance within our bodies. Any slight changes in ph can be quickly corrected by variations in the amount of CO 2 the lungs remove from the body (i.e., through hyper- or hypo-ventilation). The mechanism of respiratory compensation is much faster, and will respond to acute changes in ph more effectively than the renal system. Respiratory compensation can occur within seconds or minutes of a metabolic disturbance. This is why a patient s respiratory rate is an important vital sign. The renal system can take days to respond to metabolic disturbances. Let s look at two examples of the lungs correcting for metabolic disturbances. In the presence of increased bicarbonate levels, the brain sends a signal to the lungs to decrease the rate and depth of ventilation. This change will cause CO 2 to be retained, increasing the level of circulating acid, normalizing acid-base balance. As you are probably already hypothesizing, if we have a decreased level of bicarbonate in the blood, the lungs would be signaled to increase the rate and depth of respiration. This will increase the removal of CO 2 from the body, decreasing circulating acids, and correcting acid-base balance. 12
13 Calculation of ph The ph is important and maintained by a constant ratio of bases and acids. Now let s look at calculations that determine the adequacy of this ratio. Base Excess/Deficit Base excess deficit is the difference in the amount of buffers (bases) that are currently in the body compared to the level that is normally expected (Bullock, Boyle, & Wang, 2001). We calculate base excess/deficit to determine the amount of buffers (base) that need to be introduced to the body to restore the blood to a normal ph level. Bicarbonate, plasma proteins, and hemoglobin are the main buffers in the body. As we have learned previously, changes in bicarbonate are primarily the result of non-respiratory causes. Therefore base excess is a measure of metabolic acidosis/alkalosis. Normal base excess is considered to be +/-2 meq/l. Let s see an example of a base deficit and base excess. 1. Accumulation of noncarbonic acid or loss of bicarbonate will result in metabolic acidosis (below 5 meq/l, base deficit). A state where this is not enough base in the blood. Non-carbonic acids are acids that are not associated with the carbonic acid equation (e.g, lactic acid). 2. Loss of acid or accumulation of bicarbonate will result in metabolic alkalosis (greater than 5 meq/l, base excess). A state where there is excess base in the blood. This value allows the physician to calculate what is needed to correct the patient s acid-base imbalance and restore the ph to its normal state (7.35 to 7.45). Now let s look at another important calculation. Anion Gap Cations are ions with a positive charge like sodium and potassium, while anions are ions with a negative charge, such as chloride and bicarbonate. The anion gap is a calculation that estimates the difference between these cations (namely sodium) and anions within the body. The normal anion gap is 12 +/- 4 meq/l. Let s look at an example of this calculation. 140 meq/l of cations (sodium) 128 meq/l of anions (bicarbonate + chloride) = 12 meq/l Therefore each liter of blood contains a gap of 12 meq/l of anions other than chloride and bicarbonate. The anion gap can be used to differentiate the causes of the ph imbalance. A level over 20 meq/l is a cause for concern as this indicates that there is a build up of acid, which may be due to either too much acid being produced or not enough acids being removed through the lungs or kidneys. Although it is not essential for nurses to be able to calculate anion gap or base excess, it is a useful to be aware of what these concepts represent in arterial blood gas interpretation. You will gain a better understanding through continual reading on these values. By now you should have a better working knowledge of the process of ventilation. We have defined mmhg as our measure of pressure, explored ventilation and explained how oxygen and carbon dioxide is diffused and transported. We have looked at ph and discussed the action of the lungs and kidneys in compensating for changes in this value. We have also defined base excess and anion gap. Now that we are through with all the technical aspects, let s review arterial blood gases (ABGs) and begin interpreting some gas values. 13
14 Arterial Blood Gases Background One of the first people to measure the presence of oxygen and carbon dioxide in human blood was Gustav Magnus in the mid-1800s (West, 2004). He used a primitive vacuum pump to discover that venous blood had more carbon dioxide and less oxygen than arterial blood. Soon after this, the first arterial puncture was performed, increasing the ability of researchers to sample blood and study blood gases. Hürter drew the first blood specimen for gas evaluation around 1912 (Martin, 1999; West, 2004). With this, he showed that the arterial oxygen saturation (PO 2 ) was between 93 and 100%. The importance of this work soon subsided and it was not until Stadie (1919) re-introduced arterial sampling to the Rockefeller Institute, that blood sampling became more common. Stadie used Hürters sampling techniques while researching oxygenation in patients with pneumonia. He demonstrated a relationship between patients with cyanosis and incomplete saturation of hemoglobin (Stadie, 1919). Blood gas testing remained in use in laboratories until Clark developed an early form of gas analyzer back in This was used in selected hospitals until 1973, when the first commercially viable automated blood gas analyzer was produced. Since this time, ABG testing has developed into one of the quickest, most accurate and reliable tests performed in health care. Quick question before moving on Do you know who has the authority to perform an arterial blood gas (ABG) in your workplace? Most work sites have protocols on who can and cannot perform an ABG. Some work sites allow nurses to perform ABGs, while others designate the sampling to respiratory therapists and/or physicians. As nurses we need to be aware of our state proscribed scope of practice, but also of our workplace policy. Taking an Arterial Blood Sample Note: To repeat, please follow the policies and procedure in place at your facility regarding taking an arterial blood sample. This is a general overview of accepted technique. Accessing an arterial blood sample may not be allowed by an RN in your facility. 14
15 The first step in taking an ABG is to determine which artery to take a sample from. There are three main arteries to choose from, the radial, femoral and brachial arteries. Of these arteries the radial artery is the preferred site and the artery we will use for discussion purposes. Figure 4. Arterial System The radial artery is preferred due to collateral circulation. Basically both arteries provide circulation to the hand. Therefore if an occlusion were to occur in the radial artery as a result of the ABG, the ulnar artery would remain patent ensuring that circulation to the hand is not compromised. Figures 5a-c. ABC of oxygen are reproduced with permission from the BMJ Publishing Group. Figure 5a. To ensure collateral circulation is present we perform a test named an Allen s test. To perform this test the patient should flex his/her arm above the level of the elbow and squeeze their hand tightly. 15
16 Figure 5b. With your hand, place pressure over both the radial and ulnar arteries and ask the patient to open their hand, it should appear blanched (see Figure 5b). Next remove pressure from the ulnar artery, maintaining pressure on the radial artery. Figure 5c. The circulation and color in the hand should be returned within a few seconds (see Figure 5c). If this occurs it is a positive Allen s test and it is safe to draw your blood sample. Figure 6. Arterial blood sample. (Shea & Martinez, n.d.) Once we have selected the artery of choice and completed an Allen s test (if indicated), we can perform the blood sample. In a common radial artery draw, hyperextending the patient s wrist will allow the artery to be closer to the skin and increase the stabilization of the artery (Newberry, 2003). If you are comfortable with the patient s positioning continue on through these key elements. Your workplace procedures may vary so review the policy of your workplace. 16
17 1. Swab the area with an appropriate skin preparation. 2. Locate the radial artery. 3. Expel any heparin that may be present in syringe. 4. With needle in dominant hand, locate radial artery with non-dominant hand. 5. Insert syringe at 45 degree angle with bevel facing up. 6. Advance syringe away from patient s hand toward fingers, locating the radial artery approximately 5 to 10 mm (see Figure 6). 7. As you feel the needle enter the artery a flash will appear. Do not advance the needle any further; the arterial pressure will fill the syringe. 8. Once sample collected, remove the needle and apply direct firm pressure for 2 to 5 minutes. 9. Place sample on ice and send for analysis. 17
18 Interpreting the Results Now this is what we ve been waiting for! This is by far the easiest part. There are a couple of steps that will help standardize our approach and an easy to remember diagram will be introduced at the end of this section to help remember how to classify blood gases. First though let s standardize our approach. When we receive a blood gas we need to look at the values and determine which values vary from the normal or expected values. Let s look at the main values that we will need to interpret a blood gas and review the corresponding normal values. We will not include base excess or anion gap in our interpretation. ph range Less than 7.4 is acidotic, greater than 7.4 is alkalotic PaCO mmhg > 45 mmhg = more acid < 35 mmhg = less acid PaO mmhg Below 80 mmhg is hypoxemia SaO % Below 90% is hypoxia HCOˉ meq/l > 26 meq/l = more base < 22 meq/l = less base Now that we know what normal values are expected, let s review some examples and become familiar with identifying respiratory and metabolic disturbances. ph 7.3 PaCO 2 39 mmhg PaO 2 85 mmhg SaO 2 97% HCOˉ3 16 meq/l So let s begin by standardizing our approach. We will use the questions below for each interpretation. 1. Is the ph normal? If not, is it acidotic or alkalotic? 2. Is it primarily a metabolic (HCOˉ3) or respiratory (PaCO 2 ) imbalance? 3. Is there any compensation occurring? 4. How is the oxygen status? Consider the PaO 2 and SaO 2. Starting with the above gas analysis, is the ph acidotic or alkalotic? It is below 7.4 so we would consider it to be acidotic. Pretty easy isn t it? (Note: We used 7.4 as our determining value). Now we need to determine if it is a result of a metabolic or a respiratory disturbance. From our earlier discussions we know that acidosis is a result of too many acids in the body. Thinking logically this can be due to either an increased CO 2 (respiratory) or decreased HCOˉ3 (metabolic) level in the blood. Let s look at these two values. The PaCO 2 is normal so that doesn t make sense. What could be causing our imbalance? Now let s look at the HCOˉ3. It is decreased which makes a lot more sense. A decreased HCOˉ3 will result in an increase in the level of acid. So we have a metabolic acidosis. Now we need to consider compensation. When we think back to the discussion on compensation you will recall that the kidney and lungs will respond to imbalances caused by the other organ. We ll recap these quickly. Remember the goal is to return acid-base balance to a normal state. 18
19 Recap Metabolic acidosis. Here we have a decreased HCOˉ3. To compensate, the lungs will increase rate and depth of ventilation blowing off the CO 2. As a result there is a decreased value of PaCO 2. Metabolic alkalosis. Is a state where there is an increased level of HCOˉ3. To compensate the lungs will decreased the rate and depth of ventilation, retaining CO 2 and therefore increasing the value of PaCO 2. Respiratory acidosis. In this imbalance there is an increased PaCO 2. The kidneys compensate by increasing the secretion of acid, which will elevate the level of HCOˉ3. Respiratory alkalosis. By now you can probably figure out what is occurring. There is a decreased value of PaCO 2 in the blood. Therefore the kidneys increase absorption of hydrogen ions, resulting in restoration of acid-base balance. Back to our example, if compensation were occurring we would see an appropriate alteration in the opposite value, which would be the PaCO 2 as it tries to compensate for the decreased HCOˉ3. To do this the lungs would increase their respiratory rate and depth blowing off CO 2 in an effort to decrease PaCO 2. Is this occurring? No, our PaCO 2 is within normal limits. There is no compensation, we simply have a metabolic acidosis with normal oxygenation. Let s do another quick example. ph 7.45 PaCO 2 32 mmhg PaO 2 90 mmhg SaO 2 97% HCOˉ3 18 meq/l What is the ph doing? If you said it is alkalotic, good work. Now we must determine if it is a respiratory or metabolic imbalance. If it is metabolic alkalosis we would have an increase in bases in the blood by either an increase in HCOˉ3 or a decrease in PaCO 2. Now the HCOˉ3 is low which doesn t make sense, so we move on to examine the PaCO 2. It is decreased, which makes sense, respiratory alkalosis is a state where there is decreased acid in the body. So we can contribute the alkalosis to a decrease in PaCO 2. Now we need to determine if compensation is taking place. With a respiratory cause we look for a metabolic response. Is the HCOˉ3 normal? No. It is slightly low. So there is some compensation occurring and the ph is right on the upper side of normal so we can say that the above gas interpretation is a respiratory alkalosis with full metabolic compensation. Good work! Let s keep working through more examples. Try to start the next one by yourself. Be sure to ask yourself the four questions. ph 7.2 PaCO 2 50 mmhg PaO 2 80 mmhg SaO 2 97% HCOˉ3 28 meq/l 1. Is the ph normal? 2. Is it primarily a metabolic (HCOˉ3) or respiratory (PaCO 2 ) imbalance? 3. Is there any compensation occurring? 4. How is the oxygen status? 19
20 Did you answer respiratory acidosis? WELL DONE, you have successfully interpreted an ABG. Now let s look at the example and try to determine if compensation is occurring. Respiratory acidosis has to be compensated by a metabolic process. We should see the kidneys attempt to correct the imbalance by increasing the level of HCOˉ3. Now refer to our example, is the HCOˉ3 elevated above its normal value? It is, so we know that compensation is occurring; however, the ph is still markedly low. So we interpret this arterial blood gas as respiratory acidosis with partial metabolic compensation. Note that we used the word partial, this is important as it indicates that the kidneys are trying to help correct the imbalance but have not quite been able to restore it to its normal state. If the ph were in the normal range we would say that the respiratory acidosis is fully compensated. Knowing the degree of compensation informs us whether the imbalance is acute (uncompensated), relatively acute (partial), or chronic (fully compensated). Let s try two more; this time around you are on your own. Case 1. A 54-year-old female presents to your ER with shortness of breath and a productive cough for 2 weeks. She is febrile with a blood pressure of 133/78, a heart rate of 104 and a respiratory rate of 32. You take blood work, a chest x-ray, and an ABG. The results of the ABG are below. Interpret the ABG for your colleagues. ph 7.44 PaCO 2 28 mmhg PaO 2 66 mmhg SaO 2 91% HCOˉ3 24 meq/l Ask yourself the four questions. 1. Is the ph normal? 2. Is it primarily a metabolic (HCOˉ3) or respiratory (PaCO 2 ) imbalance? 3. Is there any compensation occurring? 4. How is the oxygen status? Case 2. A 67-year-old male presents to the ambulatory care center complaining of chest pain and tingling in his fingers. He appears in distress. His vital signs are: blood pressure of 142/88, heart rate of 122, and respiratory rate of 46. You sense that the man is anxious and attempt to calm him. As you perform your initial workup, the physician asks you to take an ABG, just to see what is happening. You get the results and the physician wants to know what your interpretation is. Perform the interpretation on the following blood gas. ph 7.48 PaCO 2 22 mmhg PaO 2 96 mmhg SaO 2 99% HCOˉ3 24 meq/l Ask yourself the four questions. 1. Is the ph normal? 2. Is it primarily a metabolic (HCOˉ3) or respiratory (PaCO 2 ) imbalance? 3. Is there any compensation occurring? 4. How is the oxygen status? Answers to these two case studies can be found on the next page. 20
21 Answers to Cases 1 and 2 Case 1. Compensated metabolic alkalosis with hypoxemia Case 2. Uncompensated respiratory alkalosis Hopefully you are starting to understand ABGs better. Now for this diagram I have been promising. I find this helps in interpreting blood gases (see Appendix A) as it organizes your thoughts on which value you should expect to be elevated. As you can see in the diagram, the two rings together look a lot like a target. When you get given a blood gas, think about the ph as being above or below 7.4. Now throw your imaginary dart at the target. The inside ring is for a blood gas with a ph below 7.4 and the outside ring is for a ph above 7.4. Once you have thrown your dart and selected either the inside or outside ring, match up the value that corresponds to your blood gas. This will then reveal what sort of blood gas you have. For example, you are given these blood gas values: ph 7.32 PaCO 2 40 mmhg PaO 2 96 mmhg SaO 2 99% HCOˉ3 15 meq/l Throw your dart into the center circle. Now what value matches the direction our values are in the circle? Is it an increased PaCO 2? No, let s check the HCOˉ3. Is the HCO 3 decreased? Yes, so we have a metabolic acidosis. If you are like me, it is much easier to visualize these values on my target than trying to memorize everything. The best thing about this diagram is that it is easily reproduced. Grab a pen and do this with me now as practice. Simply draw two circles. On the inside circle write less than 7.4 or acidotic. The outside circle is therefore alkalotic. Now all you need to remember is that each circle has a PaCO 2 and HCOˉ3 on it and the direction of each value will always be opposite. Let s start at the top, simply remember that the top PaCO 2 is decreased, making the bottom HCOˉ3 increased. Likewise in the inner circle, the PaCO 2 will be opposite the outside circle PaCO 2, so it must be increased and therefore our HCOˉ3 is opposite, it must be decreased. 21
22 Conclusion Many nurses have difficulty interpreting ABGs. Confusion often results when too many pieces of information are analyzed at the same time. Therefore, it is helpful to separate the components of ABGs and categorize the information that they provide. When ABGs are divided into their major components (acid/base balance and oxygenation), they become much easier to understand. The ph tells us if the patient is acidotic or alkalotic. The PaCO 2 and HCOˉ3 tell us where the acid/base abnormality comes from and whether there is compensation. Finally the PaO 2 and O 2 saturation tell us about oxygenation. Now that you ve completed this course and have an understanding of how to interpret blood gases, challenge yourself by completing the course exam. 22
23 References American Association for Respiratory Care. (2001). Blood gas analysis and hemoximetry: 2001 revision & update. Respiratory Care, 46, Barry, B. E., & Pinard, A. E. (2002). Assessing tissue oxygenation. Critical Care Nurse, 22(3), Bullock, J., Boyle, J., & Wang, M. B. (2001). Physiology (4 th ed.). Philadelphia, PA: Lippincott Williams and Wilkins. Cuesta Community College. (n.d.). Alveolar capillary network [Figure 1]. In Smoking and health. Retrieved May 1, 2009, from Fox, S. I., & Brown, C. M. (2005). Overview of respiratory processes and partial pressures in respiration [Figure 3]. In Human physiology (9 th ed.). Illinois: McGraw-Hill College. Gas laws. (n.d.). Retrieved June 20, 2008, from Martin, L. (1999). All you really need to know to interpret arterial blood gases. Philadelphia, PA: Lippincott Williams and Wilkins. Newberry, L. (2003). Sheehy s emergency nursing. Principles and practice (5 th ed.). St. Louis, MO: Mosby. O Leary, J. P. (2002). The physiologic basis of surgery (3 rd ed). Philadelphia, PA: Lippincott Williams and Wilkins. Shea, J. M., & Martinez, C. M. (n.d.). Arterial blood sample [Figure 6]. In Arterial blood gases (ABG s). Retrieved June 20, 2008, from the Pulmonary - Critical Care Associates of East Texas Web site: Stadie, W. C. (1919). The oxygen of the arterial and venous blood in pneumonia and its relationship to cyanosis. Journal of Experimental Medicine, 30, West, J. B. (2004). A century of pulmonary gas exchange. American Journal of Respiratory and Critical Care Medicine, 169, Williams, A. J. (1998). ABC of oxygen [Figure 5a-c]. In Assessing and interpreting arterial blood gases and acid-base balance. British Medical Journal, 317, Woodrow, P. (2004). Arterial blood gas analysis. Nursing Standard, 18(21),
24 Appendix A Illustrating arterial blood gas interpretation. PCO 2 PCO 2 ph > 7.4 ph < 7.4 ph > 7.4 HCO 3 HCO 3 24
25 Course Exam After studying the downloaded course and completing the course exam, you need to enter your answers online. Answers cannot be graded from this downloadable version of the course. To enter your answers online, go to e-learn s Web site, and click on the Login/My Account button. As a returning student, login using the username and password you created, click on the Go to Course link, and proceed to the course exam. Note: Contact hours will be awarded for this online course until August 31, When we take a breath in, our thoracic cavity expands and the pressure in our lungs: a. Decreases until it equalizes with the pressure outside the lungs. b. Increases until it equalizes with the pressure outside the lungs. c. Decreases until it equalizes with atmospheric pressure. d. Increases until it equalizes with atmospheric pressure. 2. The saturation of the oxygen in the blood is determined by the: a. Amount of platelets in the blood. b. Amount of carbon dioxide in the blood. c. Amount of oxygen that combines with hemoglobin. d. Degree of kidney perfusion. 3. Carbon Dioxide is a by-product of: a. Cellular reformation b. Cellular association c. Cellular metabolism d. Cellular diffusion 4. The two organs that are responsible for maintaining a proper ph are the: a. Kidneys and lungs b. Brain and heart c. Lungs and heart d. Tissues and kidneys 5. The act of maintaining the proper ph in the body is called compensation. a. True b. False 6. Hyper-extending the wrist is appropriate when conducting an ABG. a. True b. False 25
26 7. Why is it important to perform an Allen s test prior to performing an arterial blood sample? a. To check for a pulse b. To locate the radial artery c. To ensure collateral circulation d. To assist with locating an insertion site 8. The normal ph for blood is between: a b c d When interpreting an arterial blood gas, what are the normal values? a. ph ; PaCO mmhg; PaO mmhg; SaO %; HCOˉ mmhg b. ph ; PaCO mmhg; PaO mmhg; SaO %; HCOˉ mmhg c. ph ; PaCO mmhg; PaO mmhg; SaO %; HCOˉ mmhg d. ph ; PaCO mmhg; PaO mmhg; SaO %; HCOˉ mmhg 10. The ABG results that come back from the laboratory show: ph = 7.19 PaC0 2 = 67 mmhg HC0ˉ3 = 21 meq/l You interpreted these findings as: a. Compensated Respiratory Acidosis b. Uncompensated Respiratory Acidosis c. Compensated Metabolic Alkalosis d. Uncompensated Metabolic Alkalosis 11. The ABGs results that come back from the laboratory show: ph = 7.38 PaC0 2 = 29 mmhg HC0ˉ3 = 16 meq/l You interpreted these findings as: a. Compensated Respiratory Acidosis b. Uncompensated Respiratory Alkalosis c. Compensated Metabolic Acidoss d. Uncompensated Metabolic alkalosis 26
27 12. The ABGs results that come back from the laboratory show: ph = 7.51 PaC0 2 = 36 mmhg HC0ˉ3 = 28 meq/l You interpreted these findings as: a. Compensated Respiratory Acidosis b. Uncompensated Respiratory Acidosis c. Compensated Metabolic Alkalosis d. Uncompensated Metabolic Alkalosis 13. The ABGs results that come back from the laboratory show: ph = 7.58 PaC0 2 = 26 mmhg HC0ˉ3 = 24 meq/l You interpreted these findings as: a. Compensated Respiratory Acidosis b. Uncompensated Respiratory Alkalosis c. Compensated Metabolic Alkalosis d. Uncompensated Metabolic alkalosis 27
Essential Skills Course Acute Care Module. Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook
 Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
Essential Skills Course Acute Care Module Respiratory Day 2 (Arterial Blood Gases) Pre course Workbook Acknowledgements This pre course workbook has been complied and updated with reference to the original
Section Three Gas transport
 Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
Section Three Gas transport Lecture 6: Oxygen transport in blood. Carbon dioxide in blood. Objectives: i. To describe the carriage of O2 in blood. ii. iii. iv. To explain the oxyhemoglobin dissociation
PICU Resident Self-Study Tutorial The Basic Physics of Oxygen Transport. I was told that there would be no math!
 Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
Physiology of Oxygen Transport PICU Resident Self-Study Tutorial I was told that there would be no math! INTRODUCTION Christopher Carroll, MD Although cells rely on oxygen for aerobic metabolism and viability,
Physiology Unit 4 RESPIRATORY PHYSIOLOGY
 Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
Physiology Unit 4 RESPIRATORY PHYSIOLOGY In Physiology Today Respiration External respiration ventilation gas exchange Internal respiration cellular respiration gas exchange Respiratory Cycle Inspiration
Respiratory System Physiology. Dr. Vedat Evren
 Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
Respiratory System Physiology Dr. Vedat Evren Respiration Processes involved in oxygen transport from the atmosphere to the body tissues and the release and transportation of carbon dioxide produced in
HCO - 3 H 2 CO 3 CO 2 + H H H + Breathing rate is regulated by blood ph and C02. CO2 and Bicarbonate act as a ph Buffer in the blood
 Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
Breathing rate is regulated by blood ph and C02 breathing reduces plasma [CO2]; plasma [CO2] increases breathing. When C02 levels are high, breating rate increases to blow off C02 In low C02 conditions,
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing
 Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing Overview of Pulmonary Circulation o Diffusion of Gases o Exchange of Oxygen and Carbon Dioxide o Transport of Gases in the Blood
The physiological functions of respiration and circulation. Mechanics. exercise 7. Respiratory Volumes. Objectives
 exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
exercise 7 Respiratory System Mechanics Objectives 1. To explain how the respiratory and circulatory systems work together to enable gas exchange among the lungs, blood, and body tissues 2. To define respiration,
Respiration (revised 2006) Pulmonary Mechanics
 Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
Respiration (revised 2006) Pulmonary Mechanics PUL 1. Diagram how pleural pressure, alveolar pressure, airflow, and lung volume change during a normal quiet breathing cycle. Identify on the figure the
Respiratory System Study Guide, Chapter 16
 Part I. Clinical Applications Name: Respiratory System Study Guide, Chapter 16 Lab Day/Time: 1. A person with ketoacidosis may hyperventilate. Explain why this occurs, and explain why this hyperventilation
Part I. Clinical Applications Name: Respiratory System Study Guide, Chapter 16 Lab Day/Time: 1. A person with ketoacidosis may hyperventilate. Explain why this occurs, and explain why this hyperventilation
- How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have
 - How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have the highest blood flow of all organs, which makes them
- How do the carotid bodies sense arterial blood gases? o The carotid bodies weigh 25mg, yet they have their own artery. This means that they have the highest blood flow of all organs, which makes them
Respiration - Human 1
 Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
Respiration - Human 1 At the end of the lectures on respiration you should be able to, 1. Describe events in the respiratory processes 2. Discuss the mechanism of lung ventilation in human 3. Discuss the
 Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Question 1: Define vital capacity. What is its significance? Vital capacity is the maximum volume of air that can be exhaled after a maximum inspiration. It is about 3.5 4.5 litres in the human body. It
Oxygen and Carbon dioxide Transport. Dr. Laila Al-Dokhi
 Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
Oxygen and Carbon dioxide Transport Dr. Laila Al-Dokhi Objectives 1. Understand the forms of oxygen transport in the blood, the importance of each. 2. Differentiate between O2 capacity, O2 content and
CHAPTER 3: The respiratory system
 CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
CHAPTER 3: The respiratory system Practice questions - text book pages 56-58 1) When the inspiratory muscles contract, which one of the following statements is true? a. the size of the thoracic cavity
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation
 Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
Unit II Problem 4 Physiology: Diffusion of Gases and Pulmonary Circulation - Physical principles of gases: Pressure of a gas is caused by the movement of its molecules against a surface (more concentration
2. State the volume of air remaining in the lungs after a normal breathing.
 CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
CLASS XI BIOLOGY Breathing And Exchange of Gases 1. Define vital capacity. What is its significance? Answer: Vital Capacity (VC): The maximum volume of air a person can breathe in after a forced expiration.
Gas exchange. Tissue cells CO2 CO 2 O 2. Pulmonary capillary. Tissue capillaries
 Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
Gas exchange Pulmonary gas exchange Tissue gas exchange CO 2 O 2 O 2 Tissue cells CO2 CO 2 Pulmonary capillary O 2 O 2 CO 2 Tissue capillaries Physical principles of gas exchange Diffusion: continuous
Lung Volumes and Capacities
 Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Lung Volumes and Capacities Normally the volume of air entering the lungs during a single inspiration is approximately equal to the volume leaving on the subsequent expiration and is called the tidal volume.
Respiratory Lecture Test Questions Set 3
 Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
Respiratory Lecture Test Questions Set 3 1. The pressure of a gas: a. is inversely proportional to its volume b. is unaffected by temperature changes c. is directly proportional to its volume d. does not
RESPIRATORY REGULATION DURING EXERCISE
 RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
RESPIRATORY REGULATION DURING EXERCISE Respiration Respiration delivery of oxygen to and removal of carbon dioxide from the tissue External respiration ventilation and exchange of gases in the lung Internal
Section Two Diffusion of gases
 Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
Section Two Diffusion of gases Lecture 5: Partial pressure and the composition of gasses in air. Factors affecting diffusion of gases. Ventilation perfusion ratio effect on alveolar gas concentration.
660 mm Hg (normal, 100 mm Hg, room air) Paco, (arterial Pc02) 36 mm Hg (normal, 40 mm Hg) % saturation 50% (normal, 95%-100%)
 148 PHYSIOLOGY CASES AND PROBLEMS Case 26 Carbon Monoxide Poisoning Herman Neiswander is a 65-year-old retired landscape architect in northern Wisconsin. One cold January morning, he decided to warm his
148 PHYSIOLOGY CASES AND PROBLEMS Case 26 Carbon Monoxide Poisoning Herman Neiswander is a 65-year-old retired landscape architect in northern Wisconsin. One cold January morning, he decided to warm his
Alveolus and Respiratory Membrane
 Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Alveolus and Respiratory Membrane thin membrane where gas exchange occurs in the lungs, simple squamous epithelium (Squamous cells have the appearance of thin, flat plates. They fit closely together in
Arterial Punctures Blood Gas Collections (ABG)
 Arterial Punctures Blood Gas Collections (ABG) Danny Grabau, CPT, (NHA) Clinical Core Laboratory Services Phlebotomy Education Assistant Randy Gruhlke, MS, PBT(ASCP) Associate Program Director, Phlebotomy
Arterial Punctures Blood Gas Collections (ABG) Danny Grabau, CPT, (NHA) Clinical Core Laboratory Services Phlebotomy Education Assistant Randy Gruhlke, MS, PBT(ASCP) Associate Program Director, Phlebotomy
P215 Respiratory System, Part 2
 P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
P15 Respiratory System, Part Gas Exchange Oxygen and Carbon Dioxide constant need for oxygen constant production of carbon dioxide exchange (and movement) lung alveoli pulmonary arteries pulmonary capillaries
CHAPTER 3: The cardio-respiratory system
 : The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
: The cardio-respiratory system Exam style questions - text book pages 44-45 1) Describe the structures involved in gaseous exchange in the lungs and explain how gaseous exchange occurs within this tissue.
ALVEOLAR - BLOOD GAS EXCHANGE 1
 ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
ALVEOLAR - BLOOD GAS EXCHANGE 1 Summary: These notes examine the general means by which ventilation is regulated in terrestrial mammals. It then moves on to a discussion of what happens when someone over
Respiratory physiology II.
 Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
Respiratory physiology II. Learning objectives: 29. Pulmonary gas exchange. 30. Oxygen transport in the blood. 31. Carbon-dioxide transport in the blood. 1 Pulmonary gas exchange The transport mechanism
Breathing: The normal rate is about 14 to 20 times a minute. Taking in of air is called Inspiration and the forcing out of air is called Expiration.
 Biology 12 Respiration Divisions of Respiration Breathing: entrance and exit of air into and out of the lungs External Respiration: exchange of gases(o2 and CO2) between air (in alveoli) and blood Internal
Biology 12 Respiration Divisions of Respiration Breathing: entrance and exit of air into and out of the lungs External Respiration: exchange of gases(o2 and CO2) between air (in alveoli) and blood Internal
Respiratory Physiology. Adeyomoye O.I
 Respiratory Physiology By Adeyomoye O.I Outline Introduction Hypoxia Dyspnea Control of breathing Ventilation/perfusion ratios Respiratory/barometric changes in exercise Intra-pulmonary & intra-pleural
Respiratory Physiology By Adeyomoye O.I Outline Introduction Hypoxia Dyspnea Control of breathing Ventilation/perfusion ratios Respiratory/barometric changes in exercise Intra-pulmonary & intra-pleural
These two respiratory media (air & water) impose rather different constraints on oxygen uptake:
 Topic 19: OXYGEN UPTAKE AND TRANSPORT (lectures 29-30) OBJECTIVES: 1. Be able to compare air vs. water as a respiratory medium with respect to oxygen content, diffusion coefficient, viscosity and water
Topic 19: OXYGEN UPTAKE AND TRANSPORT (lectures 29-30) OBJECTIVES: 1. Be able to compare air vs. water as a respiratory medium with respect to oxygen content, diffusion coefficient, viscosity and water
Recitation question # 05
 Recitation and Lab # 05 The goal of this recitations / labs is to review material related to the CV and respiratory lectures for the second test of this course. Info required to answer this recitation
Recitation and Lab # 05 The goal of this recitations / labs is to review material related to the CV and respiratory lectures for the second test of this course. Info required to answer this recitation
Collin County Community College. Lung Physiology
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 9 Respiratory System 1 Lung Physiology Factors affecting Ventillation 1. Airway resistance Flow = Δ P / R Most resistance is encountered
respiratory cycle. point in the volumes: 500 milliliters. for men. expiration, up to 1200 milliliters extra makes breathing Respiratory
 10 II. RESPIRATORY VOLUMES, CAPACITIES & PULMONARY FUNCTION TESTS Respiratory volume is the term used for various volumes of air moved by or associated with the lungs at a given point in the respiratory
10 II. RESPIRATORY VOLUMES, CAPACITIES & PULMONARY FUNCTION TESTS Respiratory volume is the term used for various volumes of air moved by or associated with the lungs at a given point in the respiratory
Circulatory And Respiration
 Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Circulatory And Respiration Composition Of Blood Blood Heart 200mmHg 120mmHg Aorta Artery Arteriole 50mmHg Capillary Bed Venule Vein Vena Cava Heart Differences Between Arteries and Veins Veins transport
Respiratory Physiology Gaseous Exchange
 Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
Respiratory Physiology Gaseous Exchange Session Objectives. What you will cover Basic anatomy of the lung including airways Breathing movements Lung volumes and capacities Compliance and Resistance in
GAS EXCHANGE & PHYSIOLOGY
 GAS EXCHANGE & PHYSIOLOGY Atmospheric Pressure Intra-Alveolar Pressure Inspiration 760 mm HG at Sea Level (= 1 atm) Pressure due to gases (N2, O2, CO2, Misc.) Pressure inside the alveolus (air sac) Phrenic
GAS EXCHANGE & PHYSIOLOGY Atmospheric Pressure Intra-Alveolar Pressure Inspiration 760 mm HG at Sea Level (= 1 atm) Pressure due to gases (N2, O2, CO2, Misc.) Pressure inside the alveolus (air sac) Phrenic
GASEOUS EXCHANGE 17 JULY 2013
 GASEOUS EXCHANGE 17 JULY 2013 Lesson Description In this lesson we: Discuss what is gaseous exchange? Consider requirements of an efficient gaseous exchange surface. Look at diversity in gas exchange systems.
GASEOUS EXCHANGE 17 JULY 2013 Lesson Description In this lesson we: Discuss what is gaseous exchange? Consider requirements of an efficient gaseous exchange surface. Look at diversity in gas exchange systems.
Respiratory System Lab
 Respiratory System Lab Note: Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before you begin. Take time to organize the materials you will need and set
Respiratory System Lab Note: Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before you begin. Take time to organize the materials you will need and set
I Physical Principles of Gas Exchange
 Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Respiratory Gases Exchange Dr Badri Paudel, M.D. 2 I Physical Principles of Gas Exchange 3 Partial pressure The pressure exerted by each type of gas in a mixture Diffusion of gases through liquids Concentration
Circulation and Respiration: Vital Signs Student Version
 Circulation and Respiration: Vital Signs Student Version In this lab, you will learn about the circulatory and respiratory systems. You will test the capacity of your lungs, measure your blood pressure
Circulation and Respiration: Vital Signs Student Version In this lab, you will learn about the circulatory and respiratory systems. You will test the capacity of your lungs, measure your blood pressure
Lesson 9.1: The Importance of an Organ Delivery System
 Lesson 9.1: The Importance of an Organ Delivery System Animals require a continuous supply of oxygen (O 2 ) for cellular respiration, and they must expel carbon dioxide (CO 2 ), the waste product of this
Lesson 9.1: The Importance of an Organ Delivery System Animals require a continuous supply of oxygen (O 2 ) for cellular respiration, and they must expel carbon dioxide (CO 2 ), the waste product of this
Capnography in the Veterinary Technician Toolbox. Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA
 Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
Capnography in the Veterinary Technician Toolbox Katie Pinner BS, LVT Bush Advanced Veterinary Imaging Richmond, VA What are Respiration and Ventilation? Respiration includes all those chemical and physical
CHAPTER 6. Oxygen Transport. Copyright 2008 Thomson Delmar Learning
 CHAPTER 6 Oxygen Transport Normal Blood Gas Value Ranges Table 6-1 OXYGEN TRANSPORT Oxygen Dissolved in the Blood Plasma Dissolve means that the gas maintains its precise molecular structure About.003
CHAPTER 6 Oxygen Transport Normal Blood Gas Value Ranges Table 6-1 OXYGEN TRANSPORT Oxygen Dissolved in the Blood Plasma Dissolve means that the gas maintains its precise molecular structure About.003
RESPIRATORY GAS EXCHANGE
 RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
RESPIRATORY GAS EXCHANGE Alveolar PO 2 = 105 mmhg; Pulmonary artery PO 2 = 40 mmhg PO 2 gradient across respiratory membrane 65 mmhg (105 mmhg 40 mmhg) Results in pulmonary vein PO 2 ~100 mmhg Partial
AIIMS, New Delhi. Dr. K. K. Deepak, Prof. & HOD, Physiology AIIMS, New Delhi Dr. Geetanjali Bade, Asst. Professor AIIMS, New Delhi
 Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
Course : PG Pathshala-Biophysics Paper 13 : Physiological Biophysics Module 17 : Gas transport and pulmonary circulation Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
82 Respiratory Tract NOTES
 82 Respiratory Tract NOTES RESPIRATORY TRACT The respiratory tract conducts air to the lungs where gaseous exchange occurs. It is separated into air-conducting and respiratory (where gas exchange occurs)
82 Respiratory Tract NOTES RESPIRATORY TRACT The respiratory tract conducts air to the lungs where gaseous exchange occurs. It is separated into air-conducting and respiratory (where gas exchange occurs)
Chapter 13 The Respiratory System
 Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Chapter 13 The Respiratory System by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Atmosphere Tissue cell External respiration Alveoli of lungs 1 Ventilation or gas exchange between the atmosphere
Gases and Respiration. Respiration Overview I
 Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
Respiration Overview I Respiration Overview II Gas Laws Equation of State: PV = nrt Same volumes of different gases have same # of molecules BTPS: body temp, atmospheric pressure, saturated ATPS: ambient
Lab 17. The Respiratory System. Laboratory Objectives
 Lab 17 The Respiratory System Laboratory Objectives Identify and describe the anatomical structures of the respiratory system. Describe the relationship between volume and pressure. Describe changes in
Lab 17 The Respiratory System Laboratory Objectives Identify and describe the anatomical structures of the respiratory system. Describe the relationship between volume and pressure. Describe changes in
RESPIRATION III SEMESTER BOTANY MODULE II
 III SEMESTER BOTANY MODULE II RESPIRATION Lung Capacities and Volumes Tidal volume (TV) air that moves into and out of the lungs with each breath (approximately 500 ml) Inspiratory reserve volume (IRV)
III SEMESTER BOTANY MODULE II RESPIRATION Lung Capacities and Volumes Tidal volume (TV) air that moves into and out of the lungs with each breath (approximately 500 ml) Inspiratory reserve volume (IRV)
Respiratory System Review
 KEY THIS TEST WILL BE COMPLETED IN ONE CLASS PERIOD MONDAY, MARCH 10. 2014 Respiratory System Review Name A. Directions: Fill in the blank with the appropriate vocabulary word or words (several examples
KEY THIS TEST WILL BE COMPLETED IN ONE CLASS PERIOD MONDAY, MARCH 10. 2014 Respiratory System Review Name A. Directions: Fill in the blank with the appropriate vocabulary word or words (several examples
Physiology of Respiration
 Physiology of Respiration External Respiration = pulmonary ventilation breathing involves 2 processes: inspiration expiration Inspiration an active process involves contraction of diaphragm innervated
Physiology of Respiration External Respiration = pulmonary ventilation breathing involves 2 processes: inspiration expiration Inspiration an active process involves contraction of diaphragm innervated
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D.
 Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Physical Chemistry of Gases: Gas Exchange Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Application of the gas laws to pulmonary physiology. 2. How to
Respiratory System. Prepared by: Dorota Marczuk-Krynicka, MD, PhD
 Respiratory System Prepared by: Dorota Marczuk-Krynicka, MD, PhD Lungs: Ventilation Perfusion Gas Exchange - Diffusion 1. Airways and Airway Resistance (AWR) 2. Mechanics of Breathing and Lung (Elastic)
Respiratory System Prepared by: Dorota Marczuk-Krynicka, MD, PhD Lungs: Ventilation Perfusion Gas Exchange - Diffusion 1. Airways and Airway Resistance (AWR) 2. Mechanics of Breathing and Lung (Elastic)
The Continuing Adventures of Mr. O Too
 The Continuing Adventures of Mr. O Too When last we heard from Mr. O Too, he was traveling from the lungs to the leg muscles of an athlete. The unit one observation was a very general overview of the basic
The Continuing Adventures of Mr. O Too When last we heard from Mr. O Too, he was traveling from the lungs to the leg muscles of an athlete. The unit one observation was a very general overview of the basic
Chapter 16 Respiratory System
 Introduction Chapter 16 Respiratory System The respiratory system consists of tubes that filter incoming air and transport it to alveoli where gases are exchanged. Think pair share: what organs are associated
Introduction Chapter 16 Respiratory System The respiratory system consists of tubes that filter incoming air and transport it to alveoli where gases are exchanged. Think pair share: what organs are associated
Chapter 23. Gas Exchange and Transportation
 Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity and minor gases argon,
Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity and minor gases argon,
NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: % of grade in class
 NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: 100 20% of grade in class 1) An arterial blood sample for a patient at sea level is obtained, and the following physiological values
NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: 100 20% of grade in class 1) An arterial blood sample for a patient at sea level is obtained, and the following physiological values
Gas Exchange ACTIVITY OVERVIEW SUMMARY KEY CONCEPTS AND PROCESS SKILLS KEY VOCABULARY. Teacher s Guide B-75 L A B O R ATO R Y
 Gas Exchange 17 40- to 2 50-minute sessions ACTIVITY OVERVIEW L A B O R ATO R Y SUMMARY This activity explores the role of the respiratory system in the regulation of gases in the blood. Students investigate
Gas Exchange 17 40- to 2 50-minute sessions ACTIVITY OVERVIEW L A B O R ATO R Y SUMMARY This activity explores the role of the respiratory system in the regulation of gases in the blood. Students investigate
Respiratory Pulmonary Ventilation
 Respiratory Pulmonary Ventilation Pulmonary Ventilation Pulmonary ventilation is the act of breathing and the first step in the respiratory process. Pulmonary ventilation brings in air with a new supply
Respiratory Pulmonary Ventilation Pulmonary Ventilation Pulmonary ventilation is the act of breathing and the first step in the respiratory process. Pulmonary ventilation brings in air with a new supply
I. Gas Exchange Respiratory Surfaces Respiratory Surface:
 I. Gas Exchange Respiratory Surfaces Respiratory Surface: Common characteristics of respiratory surfaces: a) Moist: allows for the RAPID diffusion of dissolved gasses across its surface. Whereas the respiratory
I. Gas Exchange Respiratory Surfaces Respiratory Surface: Common characteristics of respiratory surfaces: a) Moist: allows for the RAPID diffusion of dissolved gasses across its surface. Whereas the respiratory
PHTY 300 Wk 1 Lectures
 PHTY 300 Wk 1 Lectures Arterial Blood Gas Components The test provides information on - Acid base balance - Oxygenation - Hemoglobin levels - Electrolyte blood glucose, lactate, renal function When initially
PHTY 300 Wk 1 Lectures Arterial Blood Gas Components The test provides information on - Acid base balance - Oxygenation - Hemoglobin levels - Electrolyte blood glucose, lactate, renal function When initially
Some major points on the Effects of Hypoxia
 Some major points on the Effects of Hypoxia Source: Kings College London http://www.kcl.ac.uk/teares/gktvc/vc/dental/year1/lectures/rbmsmajorpoints/effectsofhypoxia.htm Cells obtain their energy from oxygen.
Some major points on the Effects of Hypoxia Source: Kings College London http://www.kcl.ac.uk/teares/gktvc/vc/dental/year1/lectures/rbmsmajorpoints/effectsofhypoxia.htm Cells obtain their energy from oxygen.
Chapter 4: Ventilation Test Bank MULTIPLE CHOICE
 Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
Instant download and all chapters Test Bank Respiratory Care Anatomy and Physiology Foundations for Clinical Practice 3rd Edition Will Beachey https://testbanklab.com/download/test-bank-respiratory-care-anatomy-physiologyfoundations-clinical-practice-3rd-edition-will-beachey/
You Might Also Like. I look forward helping you focus your instruction while saving tons of time. Kesler Science Station Lab Activities 40%+ Savings!
 Thanks Connect Thank you for downloading my product. I truly appreciate your support and look forward to hearing your feedback. You can connect with me and find many free activities and strategies over
Thanks Connect Thank you for downloading my product. I truly appreciate your support and look forward to hearing your feedback. You can connect with me and find many free activities and strategies over
Gas Exchange Respiratory Systems
 alveoli gills Gas Exchange Respiratory Systems elephant seals 2008-2009 Why do we need a respiratory system? respiration for respiration Need O 2 in for aerobic cellular respiration make ATP Need CO 2
alveoli gills Gas Exchange Respiratory Systems elephant seals 2008-2009 Why do we need a respiratory system? respiration for respiration Need O 2 in for aerobic cellular respiration make ATP Need CO 2
Respiratory Medicine. A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics. Alveolar Gas Equation. See online here
 Respiratory Medicine A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics See online here Alveolar gas equation helps to calculate the partial pressure of oxygen in alveoli and A-a gradient is the
Respiratory Medicine A-A Gradient & Alveolar Gas Equation Laboratory Diagnostics See online here Alveolar gas equation helps to calculate the partial pressure of oxygen in alveoli and A-a gradient is the
By: Aseel Jamil Al-twaijer. Lec : physical principles of gas exchange
 By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
By: Aseel Jamil Al-twaijer Lec : physical principles of gas exchange Date:30 /10/2017 this lecture is about the exchange of gases between the blood and the alveoli. I might add some external definitions
Human Biology Respiratory System
 Human Biology Respiratory System Respiratory System Responsible for process of breathing Works in cooperation with Circulatory system Three types: 1. Internal Respiration 2. External Respiration 3. Cellular
Human Biology Respiratory System Respiratory System Responsible for process of breathing Works in cooperation with Circulatory system Three types: 1. Internal Respiration 2. External Respiration 3. Cellular
Experiment B-3 Respiration
 1 Experiment B-3 Respiration Objectives To study the diffusion process of oxygen and carbon dioxide between the alveoli and pulmonary capillaries. To determine the percentage of oxygen in exhaled air while
1 Experiment B-3 Respiration Objectives To study the diffusion process of oxygen and carbon dioxide between the alveoli and pulmonary capillaries. To determine the percentage of oxygen in exhaled air while
HUMAN Sample Experiment High Altitude Simulation (a one variable experiment) (version 5/04/06)
 HUMAN Sample Experiment High Altitude Simulation (a one variable experiment) (version 5/04/06) Students wish to simulate an ascent to the summit of Mount Mckinley (12,500 ft.), perhaps to compare the simulated
HUMAN Sample Experiment High Altitude Simulation (a one variable experiment) (version 5/04/06) Students wish to simulate an ascent to the summit of Mount Mckinley (12,500 ft.), perhaps to compare the simulated
The Physiologic Basis of DLCO testing. Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan
 The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives Review gas transport from inhaled gas to the rest of the
AP Biology. Gas Exchange Respiratory Systems. Gas exchange. Why do we need a respiratory system? Optimizing gas exchange. Gas exchange in many forms
 alveoli Gas Exchange Respiratory Systems gills elephant seals 2008-2009 Why do we need a respiratory system? Need O 2 in food respiration for respiration for aerobic cellular respiration make ATP Need
alveoli Gas Exchange Respiratory Systems gills elephant seals 2008-2009 Why do we need a respiratory system? Need O 2 in food respiration for respiration for aerobic cellular respiration make ATP Need
Exam Key. NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: % of grade in class
 NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: 100 20% of grade in class 1) An arterial blood sample for a patient at sea level is obtained, and the following physiological values
NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 28, 2016 Total POINTS: 100 20% of grade in class 1) An arterial blood sample for a patient at sea level is obtained, and the following physiological values
LAB 7 HUMAN RESPIRATORY LAB. Complete the charts on pgs. 67 and 68 and read directions for using BIOPAC
 66 LAB 7 HUMAN RESPIRATORY LAB Assignments: Due before lab: Quiz: Three Respiratory Interactive Physiology Animations pages 69 73. Complete the charts on pgs. 67 and 68 and read directions for using BIOPAC
66 LAB 7 HUMAN RESPIRATORY LAB Assignments: Due before lab: Quiz: Three Respiratory Interactive Physiology Animations pages 69 73. Complete the charts on pgs. 67 and 68 and read directions for using BIOPAC
Chapter 23. Gas Exchange and Transportation
 Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity other minor gases argon,
Chapter 23 Gas Exchange and Transportation What is air? Mixture of gasses 78.6 % nitrogen 20.9% oxygen 0.04% carbon dioxide 0 4% water vapor depending on temperature and humidity other minor gases argon,
IV. FROM AQUATIC TO ATMOSPHERIC BREATHING: THE TRACHEA & THE LUNG
 GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
GAS EXCHANGE AND TRANSPORT I. INTRODUCTION: Heterotrophs oxidize carbon cmpds using O 2 to generate CO 2 & H 2 O. This is cellular respiration II. HOW GAS ENTERS A CELL A. The composition of air: 79% N
system. and then into the tissues. Diffusion of wastes such as Carbon Dioxide from tissues into blood and out of blood into the lungs.
 Respiratory System 1.Exchange Why do of we gases breathe? into the Think blood of all and the tissues. reasons Diffusion why we of Oxygen need a respiratory into blood from the lungs system. and then into
Respiratory System 1.Exchange Why do of we gases breathe? into the Think blood of all and the tissues. reasons Diffusion why we of Oxygen need a respiratory into blood from the lungs system. and then into
Gas Exchange in Animals. Uptake of O2 from environment and discharge of CO2. Respiratory medium! water for aquatic animals, air for terrestial
 Gas Exchange in Animals Uptake of O2 from environment and discharge of CO2 Respiratory medium! water for aquatic animals, air for terrestial Respiratory surface! skin, gills, lungs Circulatory System O2/CO2
Gas Exchange in Animals Uptake of O2 from environment and discharge of CO2 Respiratory medium! water for aquatic animals, air for terrestial Respiratory surface! skin, gills, lungs Circulatory System O2/CO2
Lab 3. The Respiratory System (designed by Heather E. M. Liwanag with T.M. Williams)
 Name Lab Partners Lab 3. The Respiratory System (designed by Heather E. M. Liwanag with T.M. Williams) Part 1. Lung Volumes and Capacities Objectives 1. Obtain graphical representation of lung capacities
Name Lab Partners Lab 3. The Respiratory System (designed by Heather E. M. Liwanag with T.M. Williams) Part 1. Lung Volumes and Capacities Objectives 1. Obtain graphical representation of lung capacities
Respiration. The resspiratory system
 Respiration The resspiratory system The Alveoli The lungs have about 300 million alveoli, with a total crosssec onal area of 50 70 m2.. Each alveolar sac is surrounded by blood capillaries. The walls of
Respiration The resspiratory system The Alveoli The lungs have about 300 million alveoli, with a total crosssec onal area of 50 70 m2.. Each alveolar sac is surrounded by blood capillaries. The walls of
Respiratory System. Part 2
 Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
Respiratory System Part 2 Respiration Exchange of gases between air and body cells Three steps 1. Ventilation 2. External respiration 3. Internal respiration Ventilation Pulmonary ventilation consists
BIOLOGY 12: UNIT J - CHAPTER 15 - REVIEW WORKSHEET RESPIRATORY SYSTEM
 BIOLOGY 12: UNIT J - CHAPTER 15 - REVIEW WORKSHEET RESPIRATORY SYSTEM A. CHAPTER REVIEW 1. Define the four components of respiration. 2. What happens to the air as it moves along the air passages? What
BIOLOGY 12: UNIT J - CHAPTER 15 - REVIEW WORKSHEET RESPIRATORY SYSTEM A. CHAPTER REVIEW 1. Define the four components of respiration. 2. What happens to the air as it moves along the air passages? What
Respiratory/Pulmonary Laboratory Experimentation
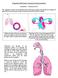 Respiratory/Pulmonary Laboratory Experimentation Introduction Anatomy Review The respiratory system has the dubious honor of being the system that permits the transport of gases from the environment inside
Respiratory/Pulmonary Laboratory Experimentation Introduction Anatomy Review The respiratory system has the dubious honor of being the system that permits the transport of gases from the environment inside
Section 01: The Pulmonary System
 Section 01: The Pulmonary System Chapter 12 Pulmonary Structure and Function Chapter 13 Gas Exchange and Transport Chapter 14 Dynamics of Pulmonary Ventilation HPHE 6710 Exercise Physiology II Dr. Cheatham
Section 01: The Pulmonary System Chapter 12 Pulmonary Structure and Function Chapter 13 Gas Exchange and Transport Chapter 14 Dynamics of Pulmonary Ventilation HPHE 6710 Exercise Physiology II Dr. Cheatham
Module Two. Objectives: Objectives cont. Objectives cont. Objectives cont.
 Transition to the New National EMS Education Standards: EMT-B B to EMT Module Two Objectives: Upon completion, each participant will do the following to a degree of accuracy that meets the Ntl EMS Education
Transition to the New National EMS Education Standards: EMT-B B to EMT Module Two Objectives: Upon completion, each participant will do the following to a degree of accuracy that meets the Ntl EMS Education
Chapter 13 The Respiratory System
 VI edit Pag 451-499 Chapter 13 The Respiratory System V edit. Pag 459-509 Tissue cell Alveoli of lungs Atmosphere 1 External respiration Ventilation or gas exchange between the atmosphere and air sacs
VI edit Pag 451-499 Chapter 13 The Respiratory System V edit. Pag 459-509 Tissue cell Alveoli of lungs Atmosphere 1 External respiration Ventilation or gas exchange between the atmosphere and air sacs
Homeostasis and Negative Feedback Concepts and Breathing Experiments 1
 Homeostasis and Negative Feedback Concepts and Breathing Experiments 1 I. Homeostasis and Negative Feedback Homeostasis refers to the maintenance of relatively constant internal conditions. For example,
Homeostasis and Negative Feedback Concepts and Breathing Experiments 1 I. Homeostasis and Negative Feedback Homeostasis refers to the maintenance of relatively constant internal conditions. For example,
Outline - Respiratory System. Function of the respiratory system Parts of the respiratory system Mechanics of breathing Regulation of breathing
 Respiratory system Function Outline - Respiratory System I. II. III. IV. Respiratory System The function of the respiratory system is to bring in oxygen to the body and remove carbon dioxide. Function
Respiratory system Function Outline - Respiratory System I. II. III. IV. Respiratory System The function of the respiratory system is to bring in oxygen to the body and remove carbon dioxide. Function
Respiratory system & exercise. Dr. Rehab F Gwada
 Respiratory system & exercise Dr. Rehab F Gwada Objectives of lecture Outline the major anatomical components & important functions of the respiratory system. Describe the mechanics of ventilation. List
Respiratory system & exercise Dr. Rehab F Gwada Objectives of lecture Outline the major anatomical components & important functions of the respiratory system. Describe the mechanics of ventilation. List
49 Arterial Blood Gases
 49 Arterial Blood Gases E. P. TRULOCK, III Definition Arterial blood gases (ABGs) is a collective term applied to three separate measurements-ph, Pco 2, and Poe -generally made together to evaluate acid-base
49 Arterial Blood Gases E. P. TRULOCK, III Definition Arterial blood gases (ABGs) is a collective term applied to three separate measurements-ph, Pco 2, and Poe -generally made together to evaluate acid-base
alveoli Chapter 42. Gas Exchange elephant seals gills AP Biology
 alveoli Chapter 42. Gas Exchange gills elephant seals Gas exchange O 2 & CO 2 exchange exchange between environment & cells provides O 2 for aerobic cellular respiration need moist membrane need high
alveoli Chapter 42. Gas Exchange gills elephant seals Gas exchange O 2 & CO 2 exchange exchange between environment & cells provides O 2 for aerobic cellular respiration need moist membrane need high
Monday, ! Today: Respiratory system! 5/20/14! Transport of Blood! What we ve been covering! Circulatory system! Parts of blood! Heart! tubing!
 Monday, 5.19.14! What we ve been covering! Circulatory system! Parts of blood! Heart! tubing! Transport of Blood! What is transported! Nutrients! Oxygen! Carbon Dioxide! Hormones! Antibodies! What it is/does!
Monday, 5.19.14! What we ve been covering! Circulatory system! Parts of blood! Heart! tubing! Transport of Blood! What is transported! Nutrients! Oxygen! Carbon Dioxide! Hormones! Antibodies! What it is/does!
Respiration. Chapter 39
 Respiration Chapter 39 Impacts, Issues Up in Smoke Smoking immobilizes ciliated cells and kills white blood cells that defend the respiratory system; highly addictive nicotine discourages quitting 39.1
Respiration Chapter 39 Impacts, Issues Up in Smoke Smoking immobilizes ciliated cells and kills white blood cells that defend the respiratory system; highly addictive nicotine discourages quitting 39.1
Students measure the change in pressure by varying the volume of trapped air in a syringe while:
 How Does a Trapped Gas Behave? Teacher Information Objective Students investigate the effect of changes in the volume of a confined gas on pressure at constant temperature. Using the pressure sensor, students
How Does a Trapped Gas Behave? Teacher Information Objective Students investigate the effect of changes in the volume of a confined gas on pressure at constant temperature. Using the pressure sensor, students
Pulmonary Circulation Linda Costanzo Ph.D.
 Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
Pulmonary Circulation Linda Costanzo Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The differences between pressures in the pulmonary and systemic circulations. 2. How
Aerobic and Anaerobic Respiration Revision 4
 Aerobic and Anaerobic Respiration Revision 4 64 minutes 64 marks Page of 0 Q. (a) The table shows an athlete s breathing rate after the end of a race. Use the information shown in the table to draw a line
Aerobic and Anaerobic Respiration Revision 4 64 minutes 64 marks Page of 0 Q. (a) The table shows an athlete s breathing rate after the end of a race. Use the information shown in the table to draw a line
Pco2 *20times = 0.6, 2.4, so the co2 carried in the arterial blood in dissolved form is more than the o2 because of its solubility.
 Physiology, sheet #9 Oxygen, is first dissolved in the plasma and the cytosol of the rbc, we have around blood constitutes 7% of our body weight, oxygen, in the capillaries is present in the rbc s and
Physiology, sheet #9 Oxygen, is first dissolved in the plasma and the cytosol of the rbc, we have around blood constitutes 7% of our body weight, oxygen, in the capillaries is present in the rbc s and
